Abstract
Traditional serological techniques have some limitations in evaluating the duration of Toxoplasma gondii infection in pregnant women, patients with lymphadenopathy, and older children suspected of having congenital toxoplasmosis. In these three groups of patients, two variants of T. gondii immunoglobulin G (IgG) avidity tests were used: an EIA Kit (Labsystems) and a noncommercial enzyme-linked immunosorbent assay specially elaborated in the laboratory. The avidity of specific IgG in sera from 23 patients with a known recently acquired infection (mainly pregnant women) was low (less than 30%), whereas that in sera from 19 patients with toxoplasmic lymphadenopathy of 3 weeks to 6 months in duration (mean, 8.3 weeks) covered a large range (between 0.2 and 57.8%; mean, 25.7%); high avidity results were observed for 10 of 19 patients (52.6%). The large range of IgG avidity in patients with toxoplasmic lymphadenopathy suggests various durations of infection in these patients, with a tendency for a chronic phase of toxoplasmosis. According to the avidity marker, five patients with lymphadenopathy for less than 3 months did not have a recent Toxoplasma infection. In 6 of 19 patients with lymphadenopathy (31.6%), low IgG avidity values persisted until 5 months after the first serological examination. In all four patients with a documented chronic course of Toxoplasma infection (6 months to 8 years after the first positive serology), high IgG avidity values were observed. Among sera from 10 children and young immunocompetent adults suspected of having ocular reactivation of congenital toxoplasmosis, all had high IgG avidity values (over 40%), suggesting congenitally acquired ocular infection rather than noncongenital infection. In conclusion, the avidity of IgG is a valuable marker of recent toxoplasmosis in pregnant women, suggests the duration of invasion in patients with lymphadenopathy, and may be helpful for differentiation between reactivation of congenital infection and recently acquired ocular toxoplasmosis in immunocompetent patients. A low IgG avidity does not always identify a recent case of toxoplasmosis, but a high IgG avidity can exclude primary infections of less than 5 months’ duration.
In Poland, where the rate of seropositivity for Toxoplasma gondii in the adult population is about 60% (24), the differential diagnosis between recent and chronic infections may be important for a clinician.
Clinical symptoms, if any, are of limited value in evaluation of the duration of T. gondii infection (9, 21). Lymphadenopathy may occur at different times after the initial T. gondii infection, persist, and/or recur for various times independently of the specific antiparasitic treatment. Differentiation between congenital and acquired ocular toxoplasmosis is difficult when some fresh lesions are visible only without retinochoroidal scans (5, 8).
Traditional immunodiagnostic techniques also have some limitations in evaluations of the timing of T. gondii infection. High or increasing titers of specific immunoglobulin G (IgG) antibodies, especially if they are detected by two different techniques, are helpful in the differentiation of recent and late infections (3, 4, 23). However, in patients with reactivation of chronic Toxoplasma infection, a significant rise in the IgG antibody titer is not always observed, especially in older children or adolescents with ocular manifestations of congenital toxoplasmosis. Interpretation of the patterns of other immunoglobulins also has some limitations. For example, IgM antibodies can be present some years after the initial infection (residual IgM) (2, 20). Similarly, specific IgA antibodies can be detected as late as 45 months after a documented seroconversion (6), during a 2-year observation period after their initial detection (18), or in the 8 months after the start of lymphadenopathy (21). Specific IgE antibodies are usually synthesized in a recent stage of infection, but they can also be detected up to 7 months after the onset of clinical symptoms (21).
The measurement of the avidity of anti-T. gondii-specific IgG antibodies for the differentiation of recent and late infections was introduced some time ago but was used mainly with pregnant women to evaluate the risk of congenital toxoplasmosis (9, 13, 17). This study has been undertaken to observe the maturation of the specific IgG avidity in the course of T. gondii lymphadenopathy and to differentiate the recently acquired retinochoroidal lesions from reactivated ones in patients with congenital toxoplasmosis.
MATERIALS AND METHODS
Patients.
In 1995 and 1996, sera from 56 patients were examined in the Clinic of Parasitic and Tropical Diseases in Poznań, Poland. Forty-six patients had acquired toxoplasmosis and had various stages of infection. Ten patients with ocular toxoplasmosis were either children or adolescents with active, fresh foci of the retinochoroiditis type alone (n = 4) or foci that were situated close to the old pigmented lesion and that were strongly suspected of being a reactivation of congenital toxoplasmosis (n = 6). The patients were divided into four groups depending on the clinical expression of toxoplasmosis, duration of symptoms, and the kinetics of specific IgG and IgM antibodies (Table 1).
TABLE 1.
Classification of the 56 patients with acquired or congenital toxoplasmosis according to duration of infection, clinical expression, and results of conventional serological tests
| Patient group | No. of patients | Clinical and/or serological criteria |
|---|---|---|
| Seroconversions or recent infectionsa | 23 | Negative results for IgG and IgM become positve by at least two different techniques and results are confirmed by examination of a third sample, or increasing titers of IgG with the presence of specific IgM (no information about previous negative results available) |
| Toxoplasmic lymphadenopathy | 19 | Lymphadenopathy of 3 wk to 6 mo in duration (mean, 8.3 wk) and presence of specific IgM with increasing or high IgG levels |
| Chronic infections | 4 | Enrolled 6 mo to 8 yr (mean, 13 mo) after the first positive Toxoplasma serology has been documented and stable or decreasing IgG titers with or without IgM |
| Ocular toxoplasmosisb | 10 | Fresh retinochoroidal lesions, recent vision impairment from 2 wk to 3 mo (mean, 6 wks), and low and stable IgG titers |
Twenty asymptomatic pregnant women and three patients with lymphadenitis in the course of coexisting other infectious disease (rubella or varicella).
The patients were suspected of having a reactivation of congenital toxoplasmosis.
Serological tests. (i) Detection of specific IgG and IgM antibodies.
IgG antibodies to T. gondii were detected by an automatic assay (VIDAS TOXO IgG; VITEK system, bioMérieux, Marcy-l’Étoile, France), which is based on an enzyme-linked immunofluorescence assay, and an indirect immunofluorescence assay. Specific IgM titers were measured by VIDAS TOXO IgM, IgM immunosorbent agglutination assay, and an indirect immunofluorescence assay as described previously (1, 25, 26).
(ii) Toxoplasma-specific IgG avidity tests.
IgG avidity was determined (i) with the Toxoplasma gondii IgG avidity EIA Kit (Labsystems, Helsinki, Finland) and (ii) by a noncommercial enzyme-linked immunosorbent assay (ELISA; avidity ELISA) elaborated in 1996 in the Clinic of Parasitic and Tropical Diseases in Poznań, Poland.
For use of the Toxoplasma gondii IgG avidity EIA Kit (Labsystems), each serum sample was examined in increasing serial dilutions (1:50, 1:200, 1:800, 1:3,200, 1:12,800) in a separate row of titration plate wells coated with T. gondii antigen (inactivated tachyzoites from mouse peritoneal fluid) at a volume of 100 μl. The four highest dilutions (starting from 1:200) were placed in wells A to D, respectively, and the four lowest dilutions (starting from 1:50) were placed in wells E to H, respectively. After 60 min of incubation at 37°C, wells A to D were washed with phosphate-buffered saline (PBS)–Tween and wells E to H were washed with 6 M urea in PBS-Tween (three times for 5 min each time), with 150 μl of the washing solution used per well. After the washing step, 100 μl of sheep anti-human IgG alkaline phosphatase conjugate diluted 1:10 in PBS was added to the wells, and the plate was left for 60 min at 37°C. After incubation, all wells were washed in PBS-Tween and 100 μl of p-nitrophenyl phosphate (5 mg of p-nitrophenyl phosphate/2.5 ml) in 1.01 M diethanolamine buffer (pH 9.9) containing 0.5 mM MgCl2 was then added as a substrate solution to each well. After 30 min of incubation in the dark at 37°C, the reaction was stopped by the addition of 100 μl of 1 M NaOH per well and thorough mixing. The optical density (OD) at 405 nm was measured against a blank substrate with an automatic spectrophotometer (Multiscan PLUS; Labsystems).
One-low avidity sample and one-high avidity sample were used as controls for the assay.
Two titration curves, the first obtained from a traditional PBS-Tween washing and the second obtained from a washing with urea solution, were drawn on millimeter paper for each patient’s sample. The distances between the OD of the curve obtained from washing with PBS-Tween and the OD of the curve obtained from avidity washing were measured at a cutoff of 0.2, and the results (percent avidity) were read according to the table included by the manufacturer (curve-shift method).
Values of less than 15% indicated low avidity, values of between 15 and 30% were considered borderline avidity, and values higher than 30% were considered high avidity.
For the noncommercial avidity ELISA, anti-T. gondii-specific IgG antibodies were first detected by indirect ELISA in order to establish the final dilution of each serum that crossed the cutoff line (OD of 0.4). The final dilution for each patient’s sample, defined during multiple preliminary experiments, was then selected for IgG avidity determination (final dilution method) (19).
The wells of flat-bottom polystyrene microtiter plates (Kartell, Noviglio, Italy) were coated with T. gondii antigen, the contents of each well were diluted 1:200 in 50 mM carbonate-bicarbonate buffer (pH 9.6) at a volume of 100 μl per well, and the plates were incubated overnight at 4°C.
A soluble antigen of T. gondii was prepared from lyophilized tachyzoites of the RH strain of T. gondii (bioMérieux). The toxoplasmas were lysed with distilled water (5 × 108 tachyzoites in 1 ml of distilled water) and were then disrupted by six successive freeze-thaw cycles (7). This suspension, consisting of membranous and cytoplasmic antigens, was used for the ELISA.
In the saturation step, which was aimed at the binding of uncoated antigenic sites, the wells were covered with 2% bovine serum albumin (BSA; FR-5, Sigma) in PBS containing 0.05% Tween 20 (PBS-Tween [pH 7.4]), and the plates were incubated for 60 min at room temperature (RT). After three washes with PBS-Tween, 100 μl of the final dilution of each serum sample in 1% BSA–PBS-Tween was added to two separate wells in different plates. After 60 min of incubation at 37°C, the first plate was washed three times with PBS-Tween and the other plate was washed three times with a modified PBS-Tween buffer containing 6 M urea and a fourth time with a traditional PBS-Tween. Alkaline peroxidase-conjugated (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) rabbit anti-human IgG antibody (heavy and light chains) was diluted 1:20,000 in 1% BSA–PBS-Tween at a volume of 100 μl and was added to the wells, and both plates were left for 60 min at RT. After incubation, the plates were washed three times with PBS-Tween and 3,3′,5,5′-tetramethylbenzidine (TMB; 1-mg TMB tablets; Sigma) dissolved in substrate solution containing 0.15 M citrate buffer, and 0.02% H2O2 (1 mg of TMB/ml) was added to each well at a volume of 100 μl. The plates were then incubated for 20 min in the dark at RT. The reaction was stopped by the addition of 50 μl of 2 M H2SO4 to the wells. The OD values at 450 nm were measured by using an automatic spectrophotometer (Multiscan PLUS; Labsystems).
The results were expressed as percent avidity, calculated as a ratio between the OD for the sample washed with urea solution and the OD for the sample washed with a standard PBS-Tween buffer (14). Values of greater than 40% indicated a high avidity of specific IgG, values of between 31 and 40% were considered borderline avidity, and ≤30% indicated low avidity.
Twenty-five serum samples from seronegative healthy patients (as determined with the VIDAS TOXO IgG and VIDAS TOXO IgM Kits) were used as a control group. The cutoff point for IgG ELISA was calculated as the mean OD value for seronegative controls plus 2 standard deviations (cutoff of 0.4).
Statistical analysis of results.
Statistical analysis of the results was done with the Microsoft Excel (version 7.0) program for evaluation of the χ2 test, Student’s t test, and the nonparametric Fisher exact test for small numbers and some general statistical functions such as arithmetic mean, median, standard deviation, and coefficient of variance.
RESULTS
Results obtained with commercial Toxoplasma gondii IgG avidity EIA Kit (Labsystems).
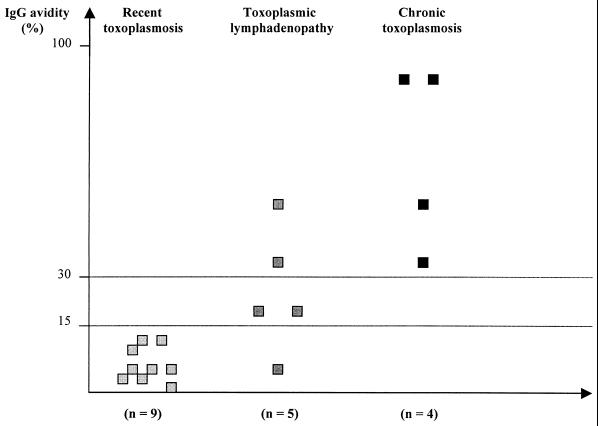
The Labsystems kit was used to test 18 serum samples from 18 patients with various stages of acquired toxoplasmosis. The results are presented in Fig. 1. Nine patients had recent toxoplasmosis of 2 to 6 weeks’ duration (mean, 4.2 weeks), five patients had lymphadenopathy of 2 to 4 months’ duration (mean, 2.6 months), and four patients had chronic toxoplasmosis of 6 months’ to 8 years’ duration (mean, 13 months).
FIG. 1.
Results of IgG avidity for 18 patients with acquired toxoplasmosis examined by using the Toxoplasma gondii IgG avidity EIA Kit (Labsystems).
Sera from all nine patients with recent toxoplasmosis had low avidity values (<15%), i.e., between 1.0 and 13.4% (mean, 6.3% ± 4.5%). Five patients with lymphadenopathy and subacute toxoplasmosis had a wider range of IgG avidity (6.0 to 50%; mean, 25.0% ± 16.9%); four patients had high or borderline results (>15%). All four patients with chronic toxoplasmosis had high avidity values (over 30%, i.e., from 33.0 to 87.1%; mean, 64.3% ± 27.2%). The mean avidity values for low- and high-avidity controls of the test were 2.3 and 71.9%, respectively.
The increase in IgG avidity with the duration of toxoplasmosis is presented in Fig. 1, and its statistical significance was confirmed by the Fisher test for small numbers (P = 0.01).
Results of noncommercial avidity ELISA.
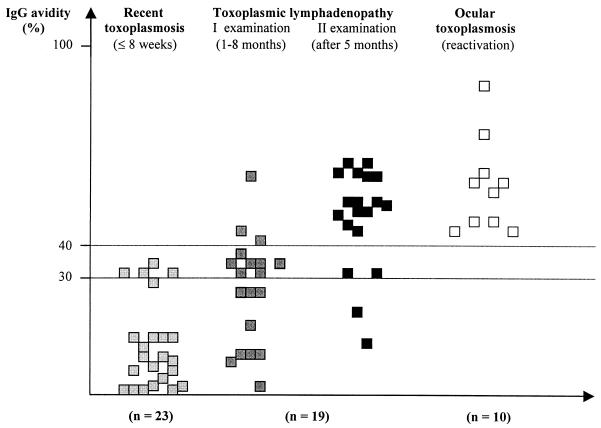
The original noncommercial avidity ELISA was used to test 71 serum samples from 52 patients with various stages of acquired T. gondii infection and ocular toxoplasmosis of unknown origin: acquired or congenital. The results are presented in Fig. 2.
FIG. 2.
Results of IgG avidity for 71 serum samples from 52 patients with recent infection, toxoplasmic lymphadenopathy, or ocular reactivation of congenital toxoplasmosis by a noncommercial ELISA.
For sera from 23 patients with seroconversion or in the early stage of T. gondii infection (less than 8 weeks after an initial infection), IgG avidity values were low (between 0.1 and 35.3%; mean, 13.2% ± 13.3%).
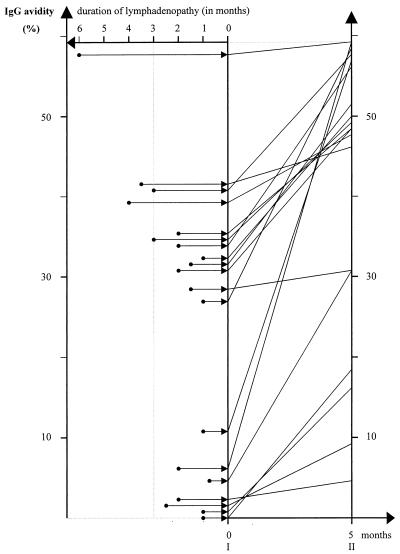
For sera from 19 patients with toxoplasmic lymphadenitis who were examined for the first time 3 weeks to 6 months (mean, 8.3 weeks) after their lymphadenopathy occurred, the range of IgG avidity was wide (between 0.2 and 57.8%; mean, 25.7% ± 16.6%); a low IgG avidity (<30%) was observed for sera from 9 patients (47.4%). The positive correlation between an increasing IgG avidity value and a longer duration of lymphadenopathy was statistically significant (P = 0.02). Upon reexamination of sera from these 19 patients after 5 months, IgG avidity results were between 6.6 and 59.8% (mean, 41.5% ± 17.5%), with a dominance of values over 40% for 13 patients (68.4%). The results are presented at Fig. 3. For all patients, over the 5 months of observation an increase in the IgG avidity values of from 1.7 to 52.4% (mean, 17.3%) was observed. A lower increase was observed for patients with lymphadenopathy of ≥3 months’ duration (P = 0.01). The positive predictive value of high avidity results at the first examination in relation to the results at the reexamination 5 months later was 100% for 10 patients. The positive predictive value of low avidity results at the reexamination in relation to the results at the first serological examination 5 months earlier was 100% for six patients.
FIG. 3.
Maturation of IgG avidity in 19 patients depending on the duration of lymphadenopathy. Paired sera from individual patients are connected by lines. I and II, first and second examinations, respectively.
Sera from all 10 patients with ocular toxoplasmosis showed high avidity results (between 40.7 and 86.8%; mean, 52.8% ± 15.0%). This fact suggests a congenital origin of the eye lesions, which were reactivated 10 to 24 years (median, 16.5 years) after birth.
In summary, the IgG avidity statistically correlated with the duration of T. gondii infection (P = 0.001). IgG avidity values increased with time after the initial infection; the best correlation was observed (P = 0.0001) between low avidity and recent toxoplasmosis of less than 8 weeks’ duration.
DISCUSSION
The determination of IgG avidity started in the 1980s in the diagnosis of rubella, hepatitis C, and other viral infections (10, 12, 28). In pregnant women with antibodies to the rubella virus, the measurement of IgG avidity was used to differentiate subclinical primary infections characterized by low avidity from reinfections characterized by high avidity and thus to evaluate the risk of congenital rubella for the fetus (22, 27). Later, the avidity test was introduced to differentiate recent and chronic T. gondii infections in pregnant women (9, 14, 17, 19). The value of using an avidity test to evaluate the risk of congenital toxoplasmosis has been confirmed by the present study with 20 pregnant women who were close to seroconversion for toxoplasmosis and who had low avidity values.
For the 19 patients with toxoplasmic lymphadenopathy, the avidity values covered a wide range by both commercial and noncommercial avidity assays. However, the distribution of avidity values had a tendency to cluster for patients with similar durations of infections, as established by the kinetics of the IgG and IgM antibodies. On the contrary, sera from patients with similar durations of lymphadenopathy showed different patterns of increases in IgG avidity (Fig. 3).
The wide range of avidity values suggests that, at least in some patients, lymphadenopathy may not be related to the early, acute stage of infection only, as proposed by Kocięcka (15) and Kocięcka et al. (16), but may be a clinical sign that occurs in patients with an advanced course of infection; one cannot exclude the possibility that this is provoked by a superinfection. This suggestion is also supported by an observation that three patients with lymphadenopathy of more than 3 months’ duration had high avidity values, which was similar to the case for seven of 16 patients with lymphadenopathy of less than 3 months’ duration. These results indicate that lymphadenopathy of ≤3 months’ duration may not be a valuable clinical marker of a recent stage of infection (Fig. 3).
The duration of low avidity values in patients with lymphadenopathy is not well defined. Holliman et al. (11) suggested that low IgG avidity occurs during less than 3 months of lymphadenopathy. Lecolier and Pucheu (19) observed patients whose sera had a low IgG avidity for as long as 20 weeks after the acquisition of infection. In the present study, low IgG avidity values were still observed 5 months after the first serological examination in 6 of 19 patients with lymphadenopathy (31.6%). In conclusion, one can accept the fact that although a high IgG avidity value strongly excludes a recent infection, that is, one that was acquired during the previous 5 months, a low avidity is not a safe marker of an early stage of infection.
So far the avidity test has not been used for the differentiation of primary and reactivated chronic infections. Holliman et al. (11) showed no significant difference between avidity values in human immunodeficiency virus-positive patients with toxoplasmic encephalitis and those without clinical signs of reactivation; IgG avidity was high (>50%) in both groups. Among other infectious diseases, in patients with rubella reinfection, high IgG avidity levels were always detected (22, 27). These observations suggest that in patients with reactivated infection or reinfection, the IgG avidity values remain high and constant, similar to those in the chronic stage of infection.
For patients with ocular toxoplasmosis an avidity test may be important for the differentiation of the fresh lesions in recently acquired toxoplasmosis resulting from systemic parasitemia from the lesions that occur in children and/or adolescents due to a reactivation of congenital toxoplasmosis that was not previously diagnosed. In 10 patients examined in the present study, the high values of the IgG avidity favor a congenital origin of ocular toxoplasmosis; a reactivation of chronic acquired infection in immunocompetent patients is less likely.
In summary, the IgG avidity test is a valuable diagnostic method for differentiation of a recent infection and a chronic infection, especially in patients with lymphadenopathy, and confirmation of the congenital origin of toxoplasmosis in older children with active retinochoroidal lesions. Therefore, low IgG avidity values are not sufficient to identify an acute case of toxoplasmosis, but high avidity results can exclude a primary infection acquired during the previous 5 months.
ACKNOWLEDGMENTS
I thank Zbigniew S. Pawlowski from the University of Medical Sciences in Poznań, Poland, for kind help during the study and the preparation of the manuscript.
This work was supported by The Polish-American Research Program Maria Skłodowska-Curie Joint Fund II (grant MZ-HHS-134/93).
REFERENCES
- 1.Ambroise-Thomas P, Garin J P, Rigaud A. Amélioration de la technique d’immunofluorescence par l’emploi de contre-colorants. Application à la toxoplasmose. Presse Med. 1966;74:2215–2216. [PubMed] [Google Scholar]
- 2.Bessières M H, Roques C, Berrebi A, Barre V, Cazaux M, Séguéla J P. IgA antibody response during acquired and congenital toxoplasmosis. J Clin Pathol. 1992;45:605–608. doi: 10.1136/jcp.45.7.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cazenave J, Bessières M H. Le diagnostic anténatal de toxoplasmose. Aspects récents de la biologie. Rev Fr Lab. 1992;240:95–102. [Google Scholar]
- 4.Chumpitazi B F F, Boussaid A, Pelloux H, Racinet C, Bost M, Goullier-Fleuret A. Diagnosis of congenital toxoplasmosis by immunoblotting and relationship with other methods. J Clin Microbiol. 1995;33:1479–1485. doi: 10.1128/jcm.33.6.1479-1485.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couvreur J, Bloch-Michel E. Toxoplasmose oculaire: traitement préventif et curatif. Med Hyg. 1985;43:2216–2220. [Google Scholar]
- 6.Decoster A, Darcy F, Caron A, Vinatier D, Houze de l’Aulnoit D, Vittu G, Niel G, Heyer F, Lecolier B, Delcroix M, Monnier J C, Duhamel M, Capron A. Anti-P30 IgA antibodies as prenatal markers of congenital Toxoplasma infection. Clin Exp Immunol. 1992;87:310–315. doi: 10.1111/j.1365-2249.1992.tb02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derouin F, Sulcebe G, Ballet J J. Sequential determination of IgG subclasses and IgA specific antibodies in primary and reactivating toxoplasmosis. Biomed Pharmacother. 1987;41:429–433. [PubMed] [Google Scholar]
- 8.François J. La toxoplasmose oculaire congénitale et ses récidives tardives. J Fr Ophtalmol. 1981;4:157–165. [PubMed] [Google Scholar]
- 9.Hedman K, Lappalainen M, Seppäiä I, Mäkelä O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- 10.Hedman K, Lappalainen M, Söderlund M, Hedman L. Avidity of IgG in serodiagnosis of infectious diseases. Rev Med Microbiol. 1993;4:123–129. [Google Scholar]
- 11.Holliman R E, Raymond R, Renton N, Johnson J D. The diagnosis of toxoplasmosis using IgG avidity. Epidemiol Infect. 1994;112:399–408. doi: 10.1017/s0950268800057812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inouye S, Hasegawa A, Matsuno S, Katow S. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J Clin Microbiol. 1984;20:525–529. doi: 10.1128/jcm.20.3.525-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenum P A, Stray-Pedersen B, Gundersen A G. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J Clin Microbiol. 1997;35:1972–1977. doi: 10.1128/jcm.35.8.1972-1977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joynson D H M, Payne R A, Rawal B K. Potential role of IgG avidity for diagnosing toxoplasmosis. J Clin Pathol. 1990;43:1032–1033. doi: 10.1136/jcp.43.12.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocięcka W. Kliniczne kryteria rozpoznawania i zasady postępowania w niektórych postaciach toksoplazmozy. Pol Tyg Lek. 1989;XLIV(34–35):809–812. . (In Polish.) [PubMed] [Google Scholar]
- 16.Kocięcka W, Mrozewicz B, Simon E, Pakuła M. Kryteria kliniczne i ocena obrazu oraz przebiegu toksoplazmozy węzłowej (Clinical criteria and evaluation of lymphonodular toxoplasmosis) Wiad Parazytol. 1990;36(4):99–119. . (In Polish.) [PubMed] [Google Scholar]
- 17.Lappalainen M, Koskela P, Koskiniemi M, Ämmälä P, Hiilesmaa V, Teramo K, Raivio K O, Remington J S, Hedman K. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J Infect Dis. 1993;167:691–697. doi: 10.1093/infdis/167.3.691. [DOI] [PubMed] [Google Scholar]
- 18.Lecolier B. Toxoplasmose au cours de la grossesse. Lett Presse Med. 1993;22:1324. [PubMed] [Google Scholar]
- 19.Lecolier B, Pucheu B. Intérêt de l’étude de l’avidité des IgG pour le diagnostic de la toxoplasmose. Pathol Biol (Paris) 1993;41:155–158. [PubMed] [Google Scholar]
- 20.Le Fichoux Y, Marty P, Chan H, Doucet J. Détection des IgM anti-toxoplasmiques par I.S.Ag.A. A propos de 3786 sérologies. Bull Soc Fr Parasitol. 1984;3:13–18. [Google Scholar]
- 21.Montoya J G, Remington J S. Studies on the serodiagnosis of toxoplasmic lymphadenitis. Clin Infect Dis. 1995;20:781–789. doi: 10.1093/clinids/20.4.781. [DOI] [PubMed] [Google Scholar]
- 22.Morgan-Capner P, Thomas H I J. Serological distinction between primary rubella and reinfection. Lancet. 1988;i:1397. doi: 10.1016/s0140-6736(88)92209-x. [DOI] [PubMed] [Google Scholar]
- 23.Paul M. M.D. thesis. Poznań, Poland: University of Medical Sciences; 1998. [Google Scholar]
- 24.Pawłowski Z, Mrozewicz B, Kacprzak E, Pisarski T, Szczapa J, Rybakowski Ł, Tomaszewski S, Święcicka-Konarska T, Rokossowski H, Moczko J. Toksoplazmoza wrodzona w województwie poznańskim (Congenital toxoplasmosis in Poznań region) Gin Pol. 1994;65:409–412. . (In Polish.) [PubMed] [Google Scholar]
- 25.Pelloux H, Ciapa P, Goullier-Fleuret A, Ambroise-Thoms P. Évaluation du systeme Vidas pour le diagnostic sérologique de la toxoplasmose. Ann Biol Clin. 1993;50:875–878. [PubMed] [Google Scholar]
- 26.Pinon J M, Toubas D, Marx C, Mougeot G, Bonnin A, Bonhomme A, Villaume M, Foudrinier F, Lepan H. Detection of specific immunoglobulin E in patients with toxoplasmosis. J Clin Microbiol. 1990;28:1739–1743. doi: 10.1128/jcm.28.8.1739-1743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rousseau S, Hedman K. Rubella infection and reinfection distinguished by avidity of IgG. Lancet. 1988;i:1108–1109. doi: 10.1016/s0140-6736(88)91926-5. [DOI] [PubMed] [Google Scholar]
- 28.Wreghitt T G, Gray J J, Aloyisus S, Contreras M, Barbara J G J. Antibody avidity test for recent infection with hepatitis C virus. Lancet. 1990;335:789. doi: 10.1016/0140-6736(90)90902-h. [DOI] [PubMed] [Google Scholar]