Abstract
Taraxacum officinale (TO) has been historically used for medicinal purposes due to its biological activity against specific disorders. To investigate the antioxidant and the antiproliferativepotential of TO essential oil in vitro and in vivo, the chemical composition of the essential oil was analyzed by GC-MS. The in vivo antioxidant capacity was assessed on liver and kidney homogenate samples from mice subjected to acetaminophen-induced oxidative stress and treated with TO essential oil (600 and 12,000 mg/kg BW) for 14 days. The in vitro scavenging activity was assayed using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) and the reducing power methods. The cytotoxic effects against the HeLa cancer cell line were analyzed. The GC-MS analysis showed the presence of 34 compounds, 8 of which were identified as major constituents. The TO essential oil protected mice’s liver and kidneys from acetaminophen-induced oxidative stress by enhancing antioxidant enzymes (catalase, superoxide dismutase, and glutathione) and lowering malondialdehyde levels. In vitro, the TO essential oil demonstrated low scavenging activity against DPPH (IC50 = 2.00 ± 0.05 mg/mL) and modest reducing power (EC50 = 0.963 ± 0.006 mg/mL). The growth of the HeLa cells was also reduced by the TO essential oil with an inhibition rate of 83.58% at 95 µg/mL. Current results reveal significant antioxidant and antiproliferative effects in a dose-dependent manner and suggest that Taraxacum officinale essential oil could be useful in formulations for cancer therapy.
Keywords: Taraxacum officinale, cervical cancer (HeLa), antiproliferative activity, antioxidant activity, GC-MS
1. Introduction
Essential oils are volatile fractions of plant materials composed of phytonutrients (amino acids and essential fatty acids), secondary metabolites (polyphenols, flavonoids), antioxidants, vitamins, minerals, and other bioactive compounds. They contain concentrated chemicals that form the ‘essence’ of the plant species. For this reason, it is primarily used in aromatherapy and as flavoring agents. Additionally, due to the presence of concentrated bioactive chemicals, they are regarded as potential medicinal agents [1]. They are commonly used in combination therapies to overcome the side effects of strong medicines (as in case of chemotherapy) [2]. For instance, Mentha spicata and Mentha × piperita essential oils were found to be effective in overcoming chemotherapy-induced nausea and vomiting (CINV) in patients with different types of cancer [3]. After supplementation with Mentha spicata and Mentha x piperita essential oils, a significant reduction (p < 0.05) in the intensity and frequency of emetic events without any side effects in the first 24 h after chemotherapy compared to the control group and a reduction in treatment expenditure in cancer patients undergoing chemotherapy were recorded [3]. Almost every ancient culture and complementary medicinal systems including Ayurveda, Chinese, Korean as well as European origins have described the use of essential oils for over 5000 years [1,4].
Essential oils can be extracted from medicinal plants by a simple distillation process with the use of steam or cold press. For extraction of essential oils that are sensitive to heat, CO2 extraction is used as more a sophisticated technique to get higher yields in less time [5]. Over the years, several plants with significantly higher concentrations of essential oils have been used for medicinal purposes. However, a few well-known medicinal plants contain low concentrations of essential oils and hence, they are not considered as practical for commercial production in spite of their potential medicinal value. One such plant is known as Taraxacum officinale (TO) [6]. It is a perennial herb that belongs to the Asteraceae family and is commonly referred to as ‘dandelion’. Dandelion is a common weed that proliferates immensely in gardens, pastures, wasteland, and forests. In spite of the medicinal properties of this plant, extensive economic damage has been reported due to its weedy nature [7]. Hence, it is possible to overcome the commercial constrains of plant availability for essential oil extraction from this plant. At present, not many studies have analyzed the phytochemical constituents of TO. Among the very few studies reported in literature, gas chromatography–mass spectrometry analysis of TO essential oil obtained by hydrodistillation of flower indicated presence of 25 compounds, which were dominated by 1, 3-dimethylbenzene, 1, 2-dimethylbenzene, 1-ethyl-3-methylbenzene, heneicosane, and tricosane [6].
The Asteraceae family is well known for their antioxidant and antiproliferative properties, which are demonstrated using both in vitro and in vivo models in literature [8,9,10,11,12,13,14]. Globally, TO is one of the most widespread members of this family. The term ‘Taraxacum’ is derived from Greek vocabulary ‘taraxos’ and ‘akos’ which translate to ‘disorder’ and ‘remedy’, respectively [15]. As the name suggests, all members of this genus, consisting of over 2500 species, are botanically recognized for their medicinal properties [15]. The plant is native to Europe and distributed widely in the warm temperate zones of Northern hemisphere. It is also found commonly in regions of Asia and North America. The plant has been utilized in the treatment against various ailments such as cholera, cancer, rheumatism, scurvy, acidosis, headache, collagen buildup, jaundice, and uric acid disorders [16]. The roots and young plants are mainly used for medicinal purposes, showing potent activity against liver damage [17]. The biological properties of TO are attributed to the phytochemicals concentrated in the flowers, leaves, roots, and stem, with every part possessing biological activity against specific disorders [18,19]. The plant is a rich source of sesquiterpene lactones, triterpene, sterols, phenolic acids, and coumarins which are well known for their anticancer properties [20,21,22,23,24,25,26,27]. The phytochemicals in TO stimulate multiple cell signaling pathways by modulating the activities of various cancer-related factors like NFκB, Akt, MEK, ERK, sVCAM-1, MAPK, MMP, TNF, and IL [18,19,21,22,23,24,25,26,27].
Considering the above factors, the present study aims to determine the chemical composition of TO essential oils and evaluate its antioxidant and antiproliferative potential using both in vivo and in vitro models.
2. Results
2.1. Chemical Analysis
The hydrodistillation extraction of the TO provided EO with a light-yellow coloration and a weak odor. The average yield of the oil obtained was (0.071 ± 0.003% (w/w)).
The analysis of the chemical composition of the EO extracted from the TO identified 34 compounds elected between 2.07 and 35.989 min. Based on the intensity of the peaks, it is concluded that among the 34 compounds identified by GC-MS in the EO, 7 compounds compose the majority: n-hexadecanoic acid, 9,12-octadecadienoic acid, octadecadienoic acid, linoelaidic acid, pentadecanoic acid, n-nonadecanol-1, and tetradecanoic acid (Table 1).
Table 1.
Chemical composition of Taraxacum officinale essential oil.
| Name | a RI | b RI | % Area |
|---|---|---|---|
| Pentadecanoic acid | 1762 | 1777 | 2.28 |
| Tetradecanoic acid | 1774 | 1774 | 0.99 |
| n-Hexadecanoic acid | 1987 | 1980 | 26.11 |
| Thunbergol | 2051 | 2047 | 0.66 |
| Heptadecanoic acid | 2081 | 2080 | 0.81 |
| Heptadecanolide | 2094 | 2051 | 0.95 |
| 9,12-Octadecadienoic acid | 2105 | 2152 | 34.19 |
| n-Nonadecanol-1 | 2157 | 2153 | 1.36 |
| Octadecanoic acid | 2205 | 2165 | 1.11 |
| Linoelaidic acid | 2206 | - | 2.57 |
a RI: retention index measured relative to n-alkanes (C6–C30) on the non-polar 123 DB11 column. b Linear retention index taken from the NIST 05 library.
2.2. Total Phenolic Compounds
Based on the measured absorbance value of the EO reacting with Folin–Ciocalteu reagent and comparison to the absorbance values for calibration standards of gallic acid (Y = 0.0133x − 0.01; R² = 0.9902), the amount of total phenolics in EO was calculated to be 4.31 ± 1.68 mg GAE/g extract.
2.3. Antioxidant Activity
2.3.1. DPPH Assay
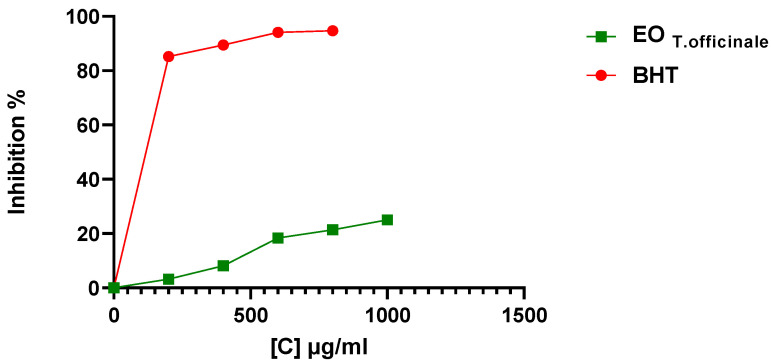
In order to determine the antioxidant power of TO EO, a DPPH free radical scavenging assay was used and scavenging activity was determined on the basis of their percentage of inhibition of the DPPH and their IC50 values. The mean percentage of DPPH free radical scavenging activity at different concentrations of EO are represented in the following histogram (Figure 1).
Figure 1.
Percentage inhibition of DPPH by different concentrations of Taraxacum officinale essential oil and the standard BHT. Results are expressed as means ± SD of three parallel measurements p < 0.01. BHT: butylated hydroxytoluene; EO: essential oil.
From the results obtained, it can be observed that the radical scavenging activities of the EO and the standard BHT are directly proportional to their concentration. The EO demonstrated a moderate percentage of inhibition of 25 ± 0.64% at a dose of 1 mg/mL compared to the standard antioxidant BHT, which showed a significant DPPH inhibitory power of 94.72 ± 0.03% at 800 μg/mL. The IC50 values found for the DPPH assay corroborate these findings. TO EO has a high IC50 value (2.00 ± 0.05 mg/mL) compared to the standard BHT (0.17 ± 0.01 mg/mL). The higher the IC50 value, the lower the radical scavenging activity of antioxidant potential. When compared to the synthetic standard BHT, the EO had a moderated antioxidant capacity.
A Pearson’s correlation was established between phenolic content and DPPH radical scavenging activity of the TO EO (Table 2).
Table 2.
Pearson’s correlation coefficients between phenolic content and DPPH radical scavenging activity (IC50) of Taraxacum officinale essential oil.
| Correlation Pearson r | Phenolic Content | DPPH |
|---|---|---|
| Phenolic content | 1 | 0.966 |
| DPPH | 0.966 | 1 |
Table 2 reveals a strong positive correlation between phenolic compound content (TPC) and DPPH radical scavenging activity (IC50) with Pearson’s correlation coefficients of r = 0.9669.
2.3.2. Reducing Power Assay
In this experiment, the reducing power capacity of the TO EO and the standard AA was shown to be concentration dependent. An increase in absorbance suggests an increase in the reducing power ability (Table 3). In terms of EC50 value, TO EO showed a good reducing power potential (EC50 = 0.963 ± 0.006 mg/mL) but significantly (p < 0.05) lower than the AA standard (EC50 = 0.034 ± 0.28 mg/mL) (Table 3). According to the data provided here, the significant reducing power of TO EO appears to be due to its antioxidant capacity.
Table 3.
Reducing ability and EC50 values of Taraxacum officinale essential oil and ascorbic acid at different concentrations.
| Concentration (μg/mL) | Ascorbic Acid | EO |
|---|---|---|
| 1000 | 1.52 ± 0.005 | 0.64 ± 0.003 * |
| 800 | 1.21 ± 0.01 | 0.55 ± 0.04 * |
| 600 | 0.95 ± 0.03 | 0.48 ± 0.03 * |
| 400 | 0.71 ± 0.01 | 0.42 ± 0.05 * |
| 200 | 0.43 ± 0.01 | 0.34 ± 0.04 * |
| 0 | 0 | 0 |
| EC50 (mg/mL) | 0.034 ± 0.28 | 0.963 ± 0.006 |
Values are expressed as mean ± SD, n = 3, * p < 0.05 vs. standard. AA: ascorbic acid; EO: essential oil.
2.4. In Vivo Antioxidant Activity
2.4.1. Effects of Treatments on Body Weight of Treated Mice
The body weights of the mice were measured at the beginning (day 0) and at the end of the test (day 14) and the findings are shown in Table 4. For the control groups (C and vehicle CMC), standard AA, and the EO (600 mg/kg and 1200 mg/kg, respectively) groups co-treated with APAP, the body weights recorded slight and normal increases throughout the treatment period, with no statistical difference between day 0 and day 14 (p > 0.05). However, compared to day 0, the APAP group showed a significant drop in body weight at the end of the treatment (day 14) (p < 0.05). It is apparent that EO has an ameliorating effect on the body weight of the mice, as shown by the slight weight gain (p > 0.05) in the batches treated with the combination (EO/APAP). This can be explained by the interesting nutritional value of our EO which is rich in primary metabolites.
Table 4.
Body weight of mice during the treatment period.
| Treatments | Mean Body Weight in Grams ± SD | |
|---|---|---|
| Day 0 | Day 14 | |
| C | 29.39 ± 0.29 | 29.58 ± 0.24 |
| CMC | 30.48 ± 0.31 | 30.71 ± 0.30 |
| APAP | 32.54 ± 0.43 | 29.78 ± 0.65 * |
| AA | 27.47 ± 0.28 | 27.92 ± 0.72 |
| TO 1 | 30.24 ± 0.22 | 31.8 ± 0.71 |
| TO 2 | 23.39 ± 0.27 | 23.81 ± 0.25 |
C: normal control; CMC: vehicle–carboxymethylcellulose group 0.1%; APAP: acetaminophen-treated toxic control 400 mg/kg body weight (ip); TO 1: Taraxacum officinale essential oil 600 mg/kg body weight; TO 2: Taraxacum officinale essential oil 1200 mg/kg body weight; AA: ascorbic acid 200 mg/kg body weight. All data are means ± S.D. (n = 5/group), * p < 0.05 APAP at day 0 vs. APAP at day 14.
The occurrence of body weight variations between treatment and control groups, as seen in Table 4, complicates the interpretation of organ weights. To detect target organ damage, relative organ weight to body weight was used (Table 5). Intact liver and kidney weights were converted to relative weights of 100 g of body weight.
Table 5.
Relative organ weight to body weight of Swiss albino mice receiving Taraxacum officinale essential oil for 14 days.
| Groups/Organs | Relative Weight of Liver and Kidney (g/100 g) | |
|---|---|---|
| Liver | Kidneys | |
| C | 5.26 ± 0.26 | 1.37 ± 0.11 |
| CMC | 5.05 ± 0.11 | 1.36 ± 0.17 |
| APAP | 3.88 ± 0.13 *** | 1.04 ± 0.14 ** |
| AA | 4.66 ± 0.16 **; ### | 1.24 ± 0.08 *; # |
| TO 1 | 4.35 ± 0.55 ****, ### | 1.20 ± 0.13 *; # |
| TO 2 | 4.61 ± 0.07 ***; ### | 1.22 ± 0.09 *; # |
All values are expressed as means ± SD. C: normal control; CMC: vehicle–carboxymethylcellulose group 0.1%; APAP: acetaminophen-treated toxic control 400 mg/kg body weight (ip); TO 1: Taraxacum officinale essential oil 600 mg/kg body weight; TO 2: Taraxacum officinale essential oil 1200 mg/kg body weight; AA: ascorbic acid 200 mg/kg body weight (significant differences from normal control group * p < 0.05; ** p < 0.01; *** p < 0.001; and **** p < 0.001 significant differences from toxic control group # p < 0.05; ### p < 0.001).
Based on the results in Table 5, a statistically significant decrease in organ weights relative to body weight was observed in the kidneys and liver of mice subjected to APAP treatment (AA, APAP alone, TO 1, and TO 2) compared to the control group (**** p < 0.0001). In addition, a significant increase in organ weights relative to body weight was recorded in the kidneys and liver of APAP-exposed mice (AA, TO 1 and TO 2) compared to the APAP toxic group (### p < 0.001). These changes in organ weights relative to the body weight indicate a toxic effect of APAP on the animals’ organs (liver and kidneys). Supplementation with AA and TO EO showed a significant ability to counteract the harmful effect of APAP ### p < 0.001). Interestingly, a higher dose of the TO EO (i.e., 1200 mg/kg PC) showed a protective effect comparable to that of the AA positive control in APAP-treated mouse models (### p < 0.001). The above observations indicate that the hepato/renal protective activity of the studied essential oils act in a dose-dependent manner.
2.4.2. Oxidant Stress Parameters Analysis
To assess the effect of oral administration of TO EO on the biomarkers (enzymes) of oxidative stress in the renal and hepatic tissues of mice models, we quantified the enzymatic activities of catalase (CAT), superoxide dismutase (SOD), and glutathione (GSH) and the levels of malondialdehyde (MDA) in the tissue homogenates.
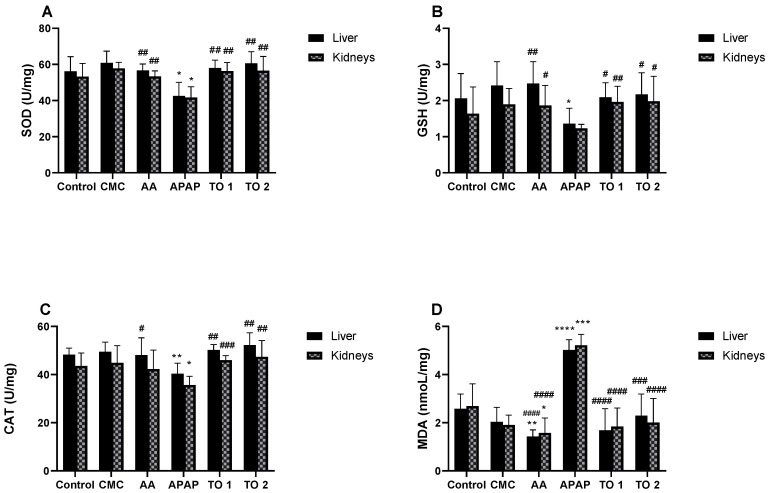
Our findings show that the toxic control group (APAP) has a statistically significant increase in hepatic and renal MDA levels, a lipid peroxidation index reflecting the degree of oxidative stress, and significantly lower levels of SOD, CAT, and GSH compared to the control, standard AA, and EO-treated groups (600 and 1200 mg/kg body weight) (p < 0.05). The increase in MDA could be attributed to the increased generation of reactive oxygen species following APAP administration (Figure 2).
Figure 2.
Effect of Taraxacum officinale essential oil on antioxidant enzymes and MDA levels against acetaminophen-induced liver injury in mice. Values are expressed as means ± SD (n = 5), * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 vs. the normal control group and # p < 0.05; ## p < 0.01; ### p < 0.001; #### p < 0.0001 vs. the toxic control group. (A) Effect of Taraxacum officinale EO on SOD level in liver and kidney of APAP-treated mice; (B) effect of Taraxacum officinale EO on GSH level in liver and kidney of APAP-treated mice; (C) effect of Taraxacum officinale EO on CAT level in liver and kidney of APAP-treated mice; (D) effect of Taraxacum officinale EO on MDA level in liver and kidney of APAP-treated mice. SOD: superoxide dismutase; GSH: reduced glutathione; CAT: catalase; MDA: malondialdehyde; AA: ascorbic acid; CMC: sodium carboxymethylcellulose; APAP: acetaminophen; TO 1: Taraxacum officinale essential oil 600 mg/kg body weight; TO 2: Taraxacum officinale essential oil 1200 mg/kg body weight.
The batches treated with TO EO (600 and 1200 mg/kg P.C., respectively) had levels of SOD, GSH, and CAT comparable to the control (C), vehicle (CMC), and standard AA groups (Figure 2). After administration of TO EO, a correction of the antioxidant defense deficit marked by an increase in antioxidant enzymes (CAT, SOD and GSH) at the hepatic and renal level was observed. The APAP-treated group’s kidney homogenate showed a statistically insignificant decrease in GSH level.
Treatment with TO EO preserved the levels of SOD, CAT, and GSH to values comparable to the normal control (p > 0.05) and exerted a protective effect by reducing oxidative damage marked by a significant dose-dependent decrease in the level of MDA in liver and kidney tissue compared to the toxic APAP control (p < 0.05). In light of the findings, the investigated EO could be beneficial in the treatment of disorders caused by oxidative damage.
2.5. Cytotoxic Activity
Human cervical cancer HeLa cells were used to test the cytotoxic effect of different concentrations of TO EO (2–95 µg/mL). The MTT assay was used to measure the degree of cytotoxicity of the tested oil towards HeLa cells (Figure 3). Cytotoxic activity was expressed as percentage inhibition of cell viability compared with the untreated control (100%). The anticancer agent doxorubicin (DOX) was used as a standard (Figure 3).
Figure 3.
Effect of Taraxacum officinale EO on the growth of HeLa cell line. TO: Taraxacum officinale essential oil; DOX: standard doxorubicin (results are expressed as mean ± SD. (n = 5).
The results, presented in Figure 3, show that the studied EO significantly (p < 0.001) and dose-dependently inhibited the growth of HeLa cells compared to untreated cells (100%) (Figure 3).
At the highest applied dose (95 µg/mL), a significant (p < 0.0001) maximum inhibitory effect of TO EO on HeLa cell line growth was recorded (% I: 83.58%; IC50 = 45.56 ± 0.05 µg/mL) but lower than that recorded by the standard DOX (% I = 92.50%; IC50 = 4.34 ± 0.02 µg/mL) (Figure 3). At lower doses (2–10 µg/mL), the oil was still toxic to HeLa cells with comparable inhibition to DOX.
Our essential oil exerted a remarkable antiproliferative effect on the HeLa cell line. Based on these results, it is suggested that the TO essential oil may be useful in the prevention and reduction of cancer occurrence and offer hope for antitumor therapeutic research.
3. Discussion
Essential oils are synthesized throughout the plant, either in specialized secretory channels (present in intercellular spaces) or oil glands located on the outer side of the plants [4]. In rare instances, the highest concentration of essential oil extracted from plants is reported to be 15% [28]. However, generally, the EO content of a plant is approximately 1% or lower [29]. In our study, the yield of TO EO was (0.071 ± 0.003% m/m). The synthesis of volatile and fragrant hydrocarbons takes place in the stem and leaves of plants, from where they migrate towards the flowers. This is because one of the functions of EOs is to attract pollinators. The EOs then accumulate in the fruits and seeds of the plant [4]. A number of studies have reported the efficacy of solvent extracts of test TO plant used in this study. However, very few studies have reported on the yield and composition of EOs obtained from this plant. A comparable yield of 0.08% v/w was recorded by TO EO from fresh flowers [6]. The difference in yield of EOs in reported literature is due to the fact that the concentration and constituents of the EO are influenced by multiple parameters. These include the soil texture, soil nutrient availability, seasonal variations, geographic locations, climatic conditions, and post-harvest storage and treatment conditions. Moreover, they also differ in composition and concentration at different stages of plant and fruit development [30].
The GC-MS analysis of TO EO revealed the presence of 34 components in the current study. Among these compounds, 8 major constituents were identified. Diterpene monocyclic alcohols and fatty acids made up the majority of the chemicals found. The bioactivities of the major constituents identified in the oil of test plant material are represented in Table 6 below.
Table 6.
Bioactivities of the main constituents of the essential oil of the plant reported in the literature.
| Groups | Examples of Compounds Identified in Our Study | Bioactive Potential | Reference |
|---|---|---|---|
| Fatty acid | n-Hexadecanoic acid; 9,12-Octa-decadienoic acid; Octadecanoic acid; Linoelaidic acid; Tetradecanoic acid; Pentadecanoic acid | Anticancer, antioxidative, immunostimulatory, anti-inflammatory, and anti-obesity |
[31,32,33,34,35,36] |
| Fatty alcohol | n-Nonadecanol-1 | Antioxidative and anti-obesity | [37] |
| Diterpene monocyclic alcohol | Thunbergol | Anticancer, antiproliferative, anti-inflammatory, and cardioprotective | [38,39,40] |
The estimation of the total phenols in plant extracts gives a significant impression of their biological activity as well as the suitability in preparations of various medicinal or non-medicinal mixtures. In addition to medicinal properties, the plants with higher phenolic content are more likely to be resistant to changes in temperature, light intensity, pathogenic infections, etc.s [41]. In the present study, the TO EO showed a low concentration of phenol (4.31 ± 1.68 mg GAE/g extract). While the total phenolic content of solvent extracts is commonly reported in literature, no study has documented the total phenolic content of the TO EO. The ethanolic extract of dandelion root from Chirpan, South-Central Bulgaria, using the Soxhlet extraction method, showed a comparable TPC value of 4.5 ± 0.1 mg GAE/g extract to our findings [42]. Conversely, other research has reported a higher total phenolic content of methanolic extract from Bucheon, Korea, and ethylic extract of TO from Erbil, northern Iraq, with TPC values of 176.8 and 10 mg GAE /g dw, respectively, using a large-scale extractor and maceration process, respectively [43,44]. A lower TPC value than ours, ranging from 0.229 ± 0.010 to 0.535 ± 0.033 mg CE/g DW was recorded in the TO leaves and flowers (acetone and triton) extracts from Rzeszów, Poland, using the micelle-mediated extraction method [45]. This difference in the total phenolic values could arise from the genetic and/or growing location, climate, maturity, and harvest season variation [46].
In the present study, the antioxidant activity of essential oil was tested by the DPPH radical scavenging method using BHT as the positive control. The dose-dependent antioxidant activity observed suggests that the DPPH radical scavenging activity is directly proportional with the concentration of essential oil and reference standards. Based on our observations of radical scavenging activity and IC50 values, it was concluded that TO possesses weak antioxidant activity. The values obtained were not significant compared to the reference standards. Similar to our findings, a weak antioxidation potential of TO has been reported by Tettey et al. Hexanic and methylene chloride TO leaf extracts showed a DPPH scavenging of 22.7 ± 0.4% and 23.3 ± 0.4%, respectively [47]. In the same manner, the TO fruit ethanolic extract revealed an inhibition of 37.7% of DPPH at a concentration of 75 µg/mL [48]. Unlike our study, strong antioxidant potential of TO has been also reported in the literature [49]. Paduret et al. found a 80.664% capacity of inhibition of DPPH solution for TO methanolic extract [50]. At a concentration of 100 g/mL, the ethanolic extract of TO flowers recorded an inhibition of 90.27 ± 0.5% [51]. Aqueous extract showed a lower IC50 of 4.48 µg/mL than our finding [52]. The weak activity of the essential oil may be due to comparatively lower concentration of phenols [53]. The statistical findings support this hypothesis; in our investigation, a strong positive correlation was found between the phenol content of essential oil of TO and its antioxidant activity.
In contrast to the DPPH assay, the TO oil showed a modest reducing power with an EC50 of 0.963 mg/mL. A higher reducing power was reported elsewhere for the TO root extract at an IC50 of 0.138 ± 0.001 mg/mL [54]. Lower reducing power of essential oils for other plants within the Asteraceae family was reported in the literature. The reducing power of Silybum marianum seeds oil showed a comparable EC50 to our study of 1 mg/mL [55]. Conversely, the Artemisia herba-alba essential oil exhibited a weak reducing power with an EC50 ranging from 1.2 to 2.9 mg/mL [56]. Recently, Dhouibi et al., 2020 reported a lower reducing power than ours of EC50 > 20 mg/mL for the essential oil of two Centaurea species, C. kroumirensis Coss., and C. sicula L. subsp sicula [57]. The TO essential oil’s reducing power in our investigation appears to be linked to the presence of antioxidant components. Hexadecanoic acid and 9,12-octadecadienoic acid found in the studied oil have been previously described for their antioxidant properties [35].
The ingestion of TO EO was accompanied by a slight weight gain in the mice. Although the weight gain was not significant, the short time span of the trial distinctly highlights the nutritional value of the EO. This may suggest that the EO exerted a protective effect on liver and kidney, more pronounced for the higher dose (i.e., 1200 mg/kg PC). This would indicate that the nutritive and hepato/renal protective activity of essential oils is dose dependent.
Regarding the study of oxidative stress biomarkers in the renal and hepatic tissues, we found decreased SOD, CAT, and GSH levels and increased MDA levels in the APAP group. The resulting oxidative stress was mitigated in the mice groups treated with TO EO with enzyme levels comparable to those of standard controls. The co-administration of TO oil in mice groups with APAP-induced oxidative stress significantly decreased the MDA level and restored the antioxidant enzymes (CAT, SOD, and GSH) to levels that were comparable to controls.
Antioxidant potential is a major part of the innate immune system in humans and animals [58,59]. The reactive oxygen species generated in different tissues as a result of metabolic activity are counteracted by the antioxidant enzymes and neutralized [60]. Each antioxidant enzyme specifically neutralizes a type of ROS contributing to the overall activity of the immune system [61]. SOD, CAT, and GSH act on superoxide, peroxide, and singlet oxygen radicals, respectively, to convert them into molecular oxygen and/or water [62]. In the absence of an effective antioxidant system, the ROSs cause the peroxidation of the membrane polyunsaturated fatty acids [63]. The kidney and liver are the main centers of metabolic activity, and hence the changes in enzyme activity are more apparent in these tissues. The increase in the levels of antioxidant enzymes after administration of EO in our study clearly demonstrates its role in the inhibition of ROS. As observed in the APAP group, an eventual inability of the EO to overcome the oxidative stress would have resulted in a similar triggering of the inflammatory response in the test groups, atrophy of hepatic and renal tissues, and significant reduction in the level of antioxidant enzymes. However, despite the moderate antioxidant activity observed in the in vitro experimental conditions, the TO EO demonstrated overall a significant antioxidant activity in the APAP-induced oxidative stress mice model.
SOD is primarily distributed in the cytosol, but it is also detected in lysosomes, peroxisomes, mitochondria, and the nucleus [64]. Several subtypes of SOD also exist, and a high degree of specificity for each subtype of SOD is recorded in the extracellular matrix of specialized tissues including that of lung, heart, kidney, plasma, lymph, ascites, and cerebrospinal fluid [65]. Interestingly, a specific type of SOD (known as MnSOD) is reported to decrease in cancer cells, suggesting its critical role in cancer prevention [66]. Moreover, studies have reported reversal of cancer pathology to normal cell physiology upon administration of MnSOD in vivo models [67]. Similar anticancer effects are also reported for CAT and GSH [68,69]. The CAT enzyme is largely located in the subcellular organelles known as peroxisomes [70]. CAT has been found to have anti-tumor properties [71,72]. It catalyzes the dismutation of hydrogen peroxide, which is a cell growth stimulator and a secondary messenger of mitogenic signaling cascades [73]. It also inhibits cell proliferation and the activation of growth factor-dependent mitogen-activated protein kinases ‘MAPKs’ [74]. Gal-catalase (galactosylated) and Suc-catalase (succinylated), catalase derivatives, have been discovered to reduce liver surface metastasis by suppressing nuclear factor B (NF-B) activity in liver and tumor cells [75].
Another marker of oxidative stress is MDA, which indicates lipid peroxidation of membrane structure and cellular injury due to depletion of the endogenous antioxidant enzymes [17]. Patients with breast, laryngeal, lung, and oral cancers have been reported to have high levels of MDA [76,77]. This byproduct has been described as a co-carcinogen and tumor promoter due to its high reactivity and cytotoxicity, and as a result, destabilizes the membrane structure and formation of carcinogen–DNA adducts due to its reaction with cellular components including DNA [75,78,79].
The in vivo antioxidant results of this article corroborate with the findings of Colle et al. on ethanolic TO extract administration to mice preventing liver tissue damage and alterations in biochemical parameters caused by APAP [48]. Colle et al. attributed the antioxidant activity of TO to the electron transfer ability of its phenolic compounds [48]. In our study, the capacity of TO oil to thwart the APAP-induced oxidative stress in the liver and kidney could be attributed to its chemical composition, which has previously documented for its potent antioxidant activity (Table 6). The hepatoprotective effects of plant essential oils are frequently reported on in the literature due to their antioxidant properties. Essential oils extracted from fennel, cumin, and flower buds of clove the level of antioxidant enzymes in cyclophosphamide-induced hepatotoxic mice models were shown to restore the function of liver cells [80]. The mechanism of antioxidant enzyme induction was described in a study of Zou et al. on the efficacy of oregano essential oil to overcome the deleterious cellular effects of the specifical induction of SOD1 and GSH occurring following the activation of the nuclear factor-erythroid 2-related factor-2 after oil exposure in peroxide-induced oxidative damage models of porcine small intestinal epithelial cells [81].
The cytotoxic activities of TO EO were determined by MTT assay using HeLa (cervical cancer) cell lines. The antiproliferative effects of the oil were significantly visible in the HeLa cell lines. TO oil demonstrated the best antiproliferative activity at a concentration of 95 µg/mL, with an inhibition percentage of 83.53% and an IC50 of 45.56 ± 0.05 µg/mL. Since MTT reduction indicates mitochondrial/non-mitochondrial dehydrogenase activity, in showing a positive MTT assay, the essential oil (as an indication of cytotoxic activity) also indicates a potential to overwhelm the enzymatic activity of mitochondria. This insight is indicative of substantial injury to the cancer cells and the efficacy of test samples [82]. Previous research has shown that TO possesses anticancer properties, which supports our findings. Aqueous dandelion root extract was shown to have anticancer activity in numerous carcinoma cell types in vitro, with no damage to non-cancer cells [83]. At a concentration of 50 g/mL, the aqueous TO root extract inhibited and reduced the cell viability of MCF-7/AZ breast cancer cells by 50% via inhibition of the dual kinase complex focal adhesion kinase (FAK)-steroid receptor coactivator (Src) and downregulation of matrix metalloproteinases MMP-2 and MMP-9 [84]. In human hepatocellular carcinoma cells, the methanolic extract of TO demonstrated significant anti-carcinoma activity [85]. Huh7 cell viability was reduced significantly when TO and the TNF-related apoptosis-inducing ligand ‘TRAIL’ (a cytokine that promotes apoptosis in cancer cells) were combined when compared to TRAIL treatment alone, with no effect on the viability of cells [85]. This suggests that TO may act as a novel TRAIL, sensitizing carcinoma cells to TRAIL-induced apoptosis by blocking mitogen protein kinase kinase 7-TOR signaling pathway regulator-like (MKK7-TIPRL) interaction and activating JNK [85]. The root extracts of TO induce apoptosis and autophagy in BxPC-3 and PANC-1 pancreatic cancer cells with no effect on non-cancerous cells [86]. Furthermore, Hexadecanoic acid, a key component of our EO, has been shown to induce apoptosis in HT–29 colon cancer cells [31].
In general, the cytotoxic effects of essential oils are mediated through the induction of cell death [87]. This mechanism is activated by sequential activation of molecules that trigger apoptosis/necrosis, cell cycle arrest, and loss of function of essential organelles [88]. However, the most recognized benefit of essential oils in exhibiting cytotoxic effects can be attributed to its lipophilic nature and biochemical makeup of low-molecular-weight components [89]. These properties of essential oil readily allow the alteration in membrane composition, which in turn, increases the fluidity of the cell membrane and allows the individual bioactive molecules of essential oils to enter the cell. Consequently, this results in the leakage of ions and cytoplasmic components. Moreover, the essential oil also causes alterations in the pH gradient and loss of mitochondrial/cellular redox potential that compromise ATP production in cell membranes. Ultimately, this results in cell death [88,90].
Existing literature has highlighted the mechanism and activity of very few essential oils as anti-proliferative. The fact that the essential oil used in our study exhibits both antiproliferative and antioxidant activities indicates a high possibility of its use in cancer treatment. Together, these two properties may also enhance cellular immunity by activation of detoxification systems and DNA repair [2]. Several studies have also identified diverse pathways of anti-proliferative mechanisms of key oil components in cancer cell lines [91,92,93,94]. The most common mechanism is the induction of apoptosis leading to cytoskeletal alterations, plasma membrane damage, mitochondrial dysfunction, DNA fragmentation, caspase-3 activation, and cleavage of pro-survival proteins [91,92,93,94]. Although these mechanisms are not entirely understood, they seem to be effective against glioblastoma, melanoma, leukemia, bone, breast, lung, ovary, pancreas, and prostate cancers [95]. The anti-proliferative and apoptotic effects of the thunbergol diterpene present in TO EO was confirmed in J82 (Blc) cell lines and human melanoma and renal UO-31 cancer cells [39,96]. Additionally, the plant used in our studies is considered to be an adjuvant drug in traditional therapies to enhance immunity [97]. In addition, their anti-tumor and anticancer activities are also extensively reported [84,97,98]. These factors collectively favor the potential use of essential oils in cancer therapies.
4. Material and Methods
4.1. Plant Material
TO samples were collected from El-Jadida, Morocco. For future reference, a specimen was deposited at the Faculty of Sciences and Technologies, Hassan 1st University, Settat, Morocco (Voucher n° 0358/M). The plant was carefully cleaned with sterile distilled water to remove dust and foreign matter, then shade dried and processed to a fine powder using an electric grinder.
4.2. Isolation of Essential Oil
The hydrodistillation was performed as previously demonstrated by our group [99].
4.3. Chemicals and Reagents
Doxorubicin, superoxide dismutase (SOD), iron chloride (FeCl3), nitro blue tetrazolium (NBT), ascorbic acid (AA), catalase (CAT) from bovine liver, acetaminophen (APAP), 2,2′-Diphenyl-1-picrylhydrazyl (DPPH), n-alkanes (C6–C30), thiobarbituric acid (TBA), trichloroacetic acid (TCA), ethylenediaminetetra acetic acid (EDTA), nicotinamide-adenine dinucleotide phosphate (NADPH), butylated hydroxytoluene, gallic acid, sodium carboxymethyl cellulose (CMC), phenazine methosulfate, Folin–Ciocalteu reagent, reduced glutathione (GSH), and 1,2-dithio-bis nitro benzoic acid (DTNB) were purchased from Sigma Co. (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO) was purchased from Merck (Merck, Darmstadt, Germany). Streptomycin, Dulbecco’s modified Eagle’s medium (DMEM), and streptomycin were purchased from Biochrom (Biochrome AG, Berlin, Germany, A 321-44). Fetal bovine serum was purchased from Sigma (Sigma, Darmstadt, Germany). Potassium phosphate monobasic, sodium pyrophosphate dibasic, sodium carbonate (Na2CO3), disodium hydrogen phosphate (Na2HPO4), hydrogen peroxide (H2O2), potassium ferricyanide (K3Fe(CN)6), sodium sulphate anhydrous (Na2SO4), acetic acid (ACA), and n-butanol (99.8%) were of analytical grade and purchased from Merck (Nottingham, UK).
4.4. Gas Chromatography–Mass Spectrometry of Essential Oil
The GC-MS was conducted as previously described by our group [99].
4.5. Determination of Total Phenolic Content
The total phenolic was evaluated as previously described by our group [99].
4.6. In Vitro Antioxidant Activity
DPPH and Reducing Power (RP) Assays
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) and reducing power (RP) assays were conducted as previously described by our group [99].
4.7. In Vivo Antioxidant Activity
4.7.1. Animal Models and Induction of Oxidative Stress
Animal models and the induction of oxidative stress were previously described by our group [99].
4.7.2. Experimental Design
The experimental design was evaluated as previously mentioned by our group [99]. For this purpose, mice were randomly divided into 5 groups (n = 5):
Group I was designated as vehicle and was treated with 0.1% CMC;
Group II (negative control) received no treatment but had free access to water and food;
Groups III (toxic control), IV, V, and VI received a single intraperitoneal injection of acetaminophen (APAP) (400 mg/kg, ip) before the start of the experiment to induce hepato-renal oxidative injury;
Group IV served as the standard and received AA, 200 mg/kg body weight;
Groups V and VI received TO EO at doses of 600 and 1200 mg/kg body weight.
These doses were chosen following a screening procedure in which we tested three doses (600, 1200, and 1600 mg/kg) of the EO by oral administration to mice for 2 weeks (unpublished results). At the highest concentration (1600 mg/kg), we observed the installation of the LD50, whereas the other two doses did not induce signs or symptoms of toxicity and were selected for further antioxidant studies. Animals were treated orally once daily (at 9 am) for two weeks (14 days). A 10-day quarantine was observed before treatment [100].
The experiment was conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Bioethics Advisory Commission of the Faculty of Sciences of Agadir (CCBE-FSA Ref. Ref. No: Ref. No: ER-BS-04/2022-0001).
4.7.3. Preparation of Tissue Homogenates
The preparation of tissue homogenates was performed as previously described by our group [99].
4.7.4. Body and Organ Weights
The body and organ weights were evaluated following the protocol previously described by our group [99].
4.7.5. Quantification of Oxidative Stress Biomarkers in Tissue Homogenates
The enzymatic activities of catalase, superoxide dismutase (SOD), glutathione GSH, and malondialdehyde MDA were assessed following the protocol previously described by our group [99].
4.8. Cytotoxic Assay
4.8.1. Sample Preparation
The essential oil was resuspended in 0.1% DMSO at a final concentration of (1.0 mg/mL). The positive control doxorubicin (DOX) 1MG (44583, Sigma-Aldrich) was prepared as stock solutions (1 mg/mL) in Nacl 0.9%. The working solutions of the oil and DOX were prepared by further dilution of the stock with the Dulbecco’s modified Eagle’s medium to concentrations of 2, 5, 10, 25, 45, 55, 75, 85, and 95 µg/mL. The samples were filtered using 0.22 µm Syringe Filter, PVDF (Sterile) (Starlab Scientific, Cape Town, South Africa).
4.8.2. Cellular Assays
Cell Lines
The human cervical epithelioid carcinoma HeLa (CCL-2, ATCC) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, T043-10, Biochrom AG), supplemented with fetal bovine serum 10 % (v/v) (FBS, Sigma, Darmstadt Germany, F9665), penicillin 100 IU/mL (Biochrom AG, Berlin, Germany, A 331-26) and streptomycin 100 μg/mL (Biochrom AG, Berlin, Germany, A 321-44). The cell cultures were seeded at 10.000 cells/cm2 and maintained in a CO2 incubator (Nuve EC 160, Ankara, Turkey) at 37 °C in 5% CO2. Cell viability was determined using the trypan blue dye exclusion method.
4.8.3. Cytotoxic Assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to evaluate the cytotoxic effects of TO EO against Hela cell line [101]. The cell cultures (cells/mL) were plated in 96-well flat-bottomed plates (92096, TPP, Switzerland) overnight. After the medium was discarded and cells were washed, the renewal complete medium was supplemented with different concentrations of the tested oil and the positive control doxorubicin (DOX) in doses of 2, 5, 10, 25, 45, 55, 75, 85, and 95 µg/mL and incubated for 24 h (the well containing the complete medium with 0.1 % DMSO was used as a control group). After treatment time expired, the medium was discarded, cells were washed with PBS (phosphate-buffered saline), and 100 µL of fresh medium containing 10 µL of MTT (5 mg/mL) was added in each well and incubated for 3 h. To solubilize the formed formazan crystals, 100 µL DMSO (dimethyl sulfoxide, 1029521000, Merck, Darmstadt, Germany) was added and the absorbance was read at 540 nm (after 10 min of shaking at 250 rpm) by an ELISA reader microplate (EZ400 reader, Biochrom, Cambridge, UK). All experiments were performed in triplicate (n = 5). Cellular viability (%) and inhibition (%) were calculated according to the formula:
| % cell viability = (Absorbancesample/Absorbancecontrol) × 100. |
| %I = 100 [1 − (Absorbancesample/Absorbancecontrol)] |
The IC50 value of the essential oil was determined by curve fitting and was used as a criterion to judge cytotoxicity.
4.9. Statistical Analysis
The experiments were replicated three times. The data were statistically analyzed using one way analysis of variance (ANOVA) followed by Student’s t-test analysis using GraphPad Prism 8.4.3 (GraphPad Software Inc., San Diego, CA, USA), and the values were expressed as mean ± SD. Linear regression analysis was used to calculate the IC50 values. Pearson’s correlation coefficient was calculated using GraphPad Prism 8.4.3 (GraphPad Software Inc., San Diego, CA, USA). Values of p < 0.0001 were considered statistically significant.
Acknowledgments
The authors would like to thank Latifa Ez-zaher of the Laboratory of Cell Biology and Molecular Genetics, Ibn Zohr University, Faculty of Sciences, Agadir, Morocco for her invaluable assistance in carrying out this research.
Author Contributions
Conceptualization, F.Z.K., A.C., I.R. and C.-T.M.; data curation, R.L., C.-T.M., I.M. and F.Z.K.; formal analysis, F.Z.K., R.L., C.-T.M. and A.C.; investigation, F.Z.K.; methodology, F.Z.K., C.-T.M., A.C., R.L. and I.R.; project administration, F.Z.K., R.L. and A.C.; supervision, C.-T.M., A.A., I.M., H.F. and A.E.-C.; writing—original draft, F.Z.K.; writing—review and editing, F.Z.K., I.M., A.A. and R.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Bioethics Advisory Commission of the Faculty of Sciences of Agadir (CCBE-FSA Ref. Ref. No: Ref. No: ER-BS-04/2022-0001).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used are available on request.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Juliani H.R., Koroch A.R., Simon J.E. Essential Oils and Aromas: Green Extractions and Applications. Har Krishan Bhalla & Sons; Dehradun, India: 2009. Chemical diversity of essential oils of Ocimum species and their associated antioxidant and antimicrobial activity. [Google Scholar]
- 2.Gautam N., Mantha A.K., Mittal S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014;2014:154106. doi: 10.1155/2014/154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tayarani-Najaran Z., Talasaz-Firoozi E., Nasiri R., Jalali N., Hassanzadeh M.K. Antiemetic activity of volatile oil from Mentha spicata and Mentha× piperita in chemotherapy-induced nausea and vomiting. Ecancermedicalscience. 2013;7:290. doi: 10.3332/ecancer.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharifi-Rad J., Sureda A., Tenore G.C., Daglia M., Sharifi-Rad M., Valussi M., Tundis R., Sharifi-Rad M., Loizzo M.R., Ademiluyi A.O., et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules. 2017;22:70. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TNAU Agritech Extraction Methods of Natural Essential Oils. [(accessed on 7 September 2022)]. Available online: https://agritech.tnau.ac.in/horticulture/extraction_methods_natural_essential_oil.pdf.
- 6.Bylka W., Matlawska I., Frański R. Essential oil composition of Taraxacum officinale. Acta Physiol. Plant. 2010;32:231–234. doi: 10.1007/s11738-009-0381-5. [DOI] [Google Scholar]
- 7.Mingarro D.M., Plaza A., Galán A., Vicente J.A., Martínez M.P., Acero N. The effect of five Taraxacum species on in vitro and in vivo antioxidant and antiproliferative activity. Food Funct. 2015;6:2787–2793. doi: 10.1039/C5FO00645G. [DOI] [PubMed] [Google Scholar]
- 8.Réthy B., Csupor-Löffler B., Zupkó I., Hajdú Z., Máthé I., Hohmann J., Rédei T., Falkay G. Antiproliferative activity of Hungarian Asteraceae species against human cancer cell lines. Part I. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007;21:1200–1208. doi: 10.1002/ptr.2240. [DOI] [PubMed] [Google Scholar]
- 9.Mondino A., Yaneselli K., Ingold A., Echeverry C., Raffaelli S., Vázquez Á., García y Santos C. Cytotoxic effect of Senecio madagascariensis (Asteraceae) extracts on cancer derived cell lines. Agrociencia Urug. 2022;26:e425. doi: 10.31285/AGRO.26.425. [DOI] [Google Scholar]
- 10.Aghraz A., Gonçalves S., Rodríguez-Solana R., Dra L.A., Di Stefano V., Dugo G., Cicero N., Larhsini M., Markouk M., Romano A. Antioxidant activity and enzymes inhibitory properties of several extracts from two Moroccan Asteraceae species. S. Afr. J. Bot. 2018;118:58–64. doi: 10.1016/j.sajb.2018.06.017. [DOI] [Google Scholar]
- 11.Vidic D., Ćavar Zeljković S., Dizdar M., Maksimović M. Essential oil composition and antioxidant activity of four Asteraceae species from Bosnia. J. Essent. Oil Res. 2016;28:445–457. doi: 10.1080/10412905.2016.1150216. [DOI] [Google Scholar]
- 12.Abdolmaleki Z., Arab H.A., Amanpour S., Muhammadnejad S. Anti-angiogenic effects of ethanolic extract of Artemisia sieberi compared to its active substance, artemisinin. Rev. Bras. Farmacogn. 2016;26:326–333. doi: 10.1016/j.bjp.2015.11.008. [DOI] [Google Scholar]
- 13.Asif M., Saadullah M., Yaseen H.S., Saleem M., Yousaf H.M., Khan I.U., Yaseen M., Shams M.U. Evaluation of in vivo anti-inflammatory and anti-angiogenic attributes of methanolic extract of Launaea spinosa. Inflammopharmacology. 2020;28:993–1008. doi: 10.1007/s10787-020-00687-6. [DOI] [PubMed] [Google Scholar]
- 14.Shakeri A., Amini E., Asili J., Masullo M., Piacente S., Iranshahi M. Screening of several biological activities induced by different sesquiterpene lactones isolated from Centaurea behen L. and Rhaponticum repens (L.) Hidalgo. Nat. Prod. Res. 2018;32:1436–1440. doi: 10.1080/14786419.2017.1344661. [DOI] [PubMed] [Google Scholar]
- 15.Wirngo F.E., Lambert M.N., Jeppesen P.B. The physiological effects of Dandelion (Taraxacum officinale) in type 2 Diabetes. Rev. Diabet. Stud. 2016;13:113–131. doi: 10.1900/RDS.2016.13.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brock M.T. The potential for genetic assimilation of a native dandelion species, Taraxacum ceratophorum (Asteraceae), by the exotic congener T. officinale. Am. J. Bot. 2004;91:656–663. doi: 10.3732/ajb.91.5.656. [DOI] [PubMed] [Google Scholar]
- 17.Escudero N.L., de Arellano M.L., Fernandez S., Albarracin G., Mucciarelli S. Taraxacum officinale as a food source. Plant Foods Hum. Nutr. 2003;58:1–10. doi: 10.1023/B:QUAL.0000040365.90180.b3. [DOI] [Google Scholar]
- 18.Choi U.K., Lee O.H., Yim J.H., Cho C.W., Rhee Y.K., Lim S.I., Kim Y.C. Hypolipidemic and antioxidant effects of dandelion (Taraxacum officinale) root and leaf on cholesterol-fed rabbits. Int. J. Mol. Sci. 2010;11:67–78. doi: 10.3390/ijms11010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma K., Zafar R. Simultaneous estimation of taraxerol and taraxasterol in root callus cultures of Taraxacum officinale Weber. Int. J. Pharmacogn. Phytochem. Res. 2014;6:540–546. [Google Scholar]
- 20.González-Castejón M., Visioli F., Rodriguez-Casado A. Diverse biological activities of dandelion. Nutr. Rev. 2012;70:534–547. doi: 10.1111/j.1753-4887.2012.00509.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S., Won Y.K., Ong C.N., Shen H.M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. Anti-Cancer Agents. 2005;5:239–249. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- 22.Chen J., Wu W., Zhang M., Chen C. Taraxasterol suppresses inflammation in IL-1β-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice. Int. Immunopharmacol. 2019;70:274–283. doi: 10.1016/j.intimp.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Moon D.O., Lee K.J., Choi Y.H., Kim G.Y. β-Sitosterol-induced-apoptosis is mediated by the activation of ERK and the downregulation of Akt in MCA-102 murine fibrosarcoma cells. Int. Immunopharmacol. 2007;7:1044–1053. doi: 10.1016/j.intimp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Jin U.H., Lee J.Y., Kang S.K., Kim J.K., Park W.H., Kim J.G., Moon S.-K., Kim C.-H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Dandriyal J., Singla R., Kumar M., Jaitak V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur. J. Med. Chem. 2016;119:141–168. doi: 10.1016/j.ejmech.2016.03.087. [DOI] [PubMed] [Google Scholar]
- 26.Han S., Zhou V., Pan S., Liu Y., Hornsby M., McMullan D., Klock H.E., Haugen J., Lesley S.A., Gray N., et al. Identification of coumarin derivatives as a novel class of allosteric MEK1 inhibitors. Bioorganic Med. Chem. Lett. 2005;15:5467–5473. doi: 10.1016/j.bmcl.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 27.Serreli G., Deiana M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019;10:6999–7021. doi: 10.1039/C9FO01733J. [DOI] [PubMed] [Google Scholar]
- 28.Butnariu M., Sarac I. Essential oils from plants. J. Biotechnol. Biomed. Sci. 2018;1:35–43. doi: 10.14302/issn.2576-6694.jbbs-18-2489. [DOI] [Google Scholar]
- 29.Ngan T., Muoi N., Quan P., Cang M. Huynh CM. Evaluation of physical and chemical properties of Pomelo (Citrus grandis L.) essential oil using steam distillation process. Asian J. Chem. 2020;32:1433. doi: 10.14233/ajchem.2020.22234. [DOI] [Google Scholar]
- 30.Fernández-Sestelo M., Carrillo J.M. Environmental effects on yield and composition of essential oil in wild populations of spike lavender (Lavandula latifolia Medik.) Agriculture. 2020;10:626. doi: 10.3390/agriculture10120626. [DOI] [Google Scholar]
- 31.Bharath B., Perinbam K., Devanesan S., AlSalhi M.S., Saravanan M. Evaluation of the anticancer potential of Hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J. Mol. Struct. 2021;1235:130229. doi: 10.1016/j.molstruc.2021.130229. [DOI] [Google Scholar]
- 32.Bradberry J.C., Hilleman D.E. Overview of omega-3 fatty acid therapies. P and T. Peer Rev. J. Formul. Med. 2013;38:681–691. [PMC free article] [PubMed] [Google Scholar]
- 33.Marathe S.J., Hamzi W., Bashein A.M., Deska J., Seppänen-Laakso T., Singhal R.S., Shamekh S. Anti-angiogenic effect of Cantharellus cibarius extracts, its correlation with lipoxygenase inhibition, and role of the bioactives therein. Nutr. Cancer. 2021;74:1–11. doi: 10.1080/01635581.2021.1909739. [DOI] [PubMed] [Google Scholar]
- 34.Loffredo S., Borriello F., Iannone R., Ferrara A.L., Galdiero M.R., Gigantino V., Esposito P., Varricchi G., Lambeau G., Cassatela M.A., et al. Group V secreted phospholipase A2 induces the release of proangiogenic and antiangiogenic factors by human neutrophils. Front. Immunol. 2017;8:443. doi: 10.3389/fimmu.2017.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei L.S., Wee W., Siong J.Y.F., Syamsumir D.F. Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Med. Iran. 2011;49:670–674. [PubMed] [Google Scholar]
- 36.Premathilaka R., Silva M. Bioactive compounds and antioxidant activity of Bunchosia armeniaca. World J. Pharm. Pharm. Sci. 2016;5:1237–1247. [Google Scholar]
- 37.Cotrim B.A., Joglar J., Rojas M.J.L., Del Olmo J.M.D., Macias-González M., Cuevas M.R., Fito M., Munoz-Aguayo D., Covas Planells M.I., Farre I., et al. Unsaturated fatty alcohol derivatives of olive oil phenolic compounds with potential low-density lipoprotein (LDL) anti-oxidant and anti-obesity properties. J. Agric. Food Chem. 2012;60:1067–1074. doi: 10.1021/jf203814r. [DOI] [PubMed] [Google Scholar]
- 38.Vasas A., Hohmann J. Euphorbia Diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012) Chem. Rev. 2014;114:8579–8612. doi: 10.1021/cr400541j. [DOI] [PubMed] [Google Scholar]
- 39.Frank M.B., Yang Q., Osban J., Azzarello J.T., Saban M.R., Saban R., Ashley R.A., Welter J.C., Fung K.-M., Lin H.-K. Frankincense oil derived from Boswellia carteri induces tumor cell specific cytotoxicity. BMC Complement. Altern. Med. 2009;9:6. doi: 10.1186/1472-6882-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Lima E.J., Alves R.G., Anunciação T.A.D., Silva V.R., Santos L.D.S., Soares M.B., Cardozo N.M.D., Costa E.V., da Silva F.M.A., Koolen H.H.F., et al. Antitumor effect of the essential oil from the leaves of Croton matourensis Aubl. (Euphorbiaceae) Molecules. 2018;23:2974. doi: 10.3390/molecules23112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aryal S., Baniya M.K., Danekhu K., Kunwar P., Gurung R., Koirala N. Total phenolic content, flavonoid content and anti-oxidant potential of wild vegetables from western Nepal. Plants. 2019;8:96. doi: 10.3390/plants8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petkova N., Ivanov I., Topchieva S., Denev P., Pavlov A. Biologically active substances and in vitro antioxidant activity of different extracts from dandelion (Taraxacum officinale) roots. Sci. Bulletin. Ser. F Biotechnic. 2015;19:190–197. [Google Scholar]
- 43.Yan L., Meng Q.W., Kim I.H. The effects of dietary Houttuynia cordata and Taraxacum officinale extract powder on growth performance, nutrient digestibility, blood characteristics and meat quality in finishing pigs. Livest. Sci. 2011;141:188–193. doi: 10.1016/j.livsci.2011.05.017. [DOI] [Google Scholar]
- 44.Ghaima K.K., Hashim N.M., Ali S.A. Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale) J. Appl. Pharm. Sci. 2013;3:96. [Google Scholar]
- 45.Miłek M., Marcinčáková D., Legáth J. Polyphenols content, antioxidant activity, and cytotoxicity assessment of Taraxacum officinale extracts prepared through the micelle-mediated extraction method. Molecules. 2019;24:1025. doi: 10.3390/molecules24061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J., Meyers K.J., van der Heide J., Liu R.H. Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J. Agric. Food Chem. 2004;52:6787–6793. doi: 10.1021/jf0307144. [DOI] [PubMed] [Google Scholar]
- 47.Tettey C.O., Ocloo A., Nagajyothi P.C., Lee K.D. Antioxidant Activity of Solvent Fractions of Taraxacum officinale (Dandelion) Leaves. J. Herbs Spices Med. Plants. 2014;20:329–340. doi: 10.1080/10496475.2013.871382. [DOI] [Google Scholar]
- 48.Colle D., Arantes L.P., Gubert P., da Luz S.C.A., Athayde M.L., Teixeira Rocha J.B., Soares F.A.A. Antioxidant Properties of Taraxacum officinale Leaf Extract Are Involved in the Protective Effect Against Hepatoxicity Induced by Acetaminophen in Mice. J. Med. Food. 2012;15:549–556. doi: 10.1089/jmf.2011.0282. [DOI] [PubMed] [Google Scholar]
- 49.Ishfaq S., Sabir S.M., Khurshid H., Zaman T., Ahmad Z. Antioxidant activities and inhibitory effect of Taraxacum officinale, Cichorium intybus and Lectuca sativa on prooxidant induced lipid peroxidation in mice liver. Croat. J. Food Sci. Technol. 2018;10:16–22. doi: 10.17508/CJFST.2018.10.1.03. [DOI] [Google Scholar]
- 50.Pădureţ S.E.R.G.I.U., Amariei S., Gutt G., Piscuc B. The evaluation of dandelion (Taraxacum officinale) properties as a valuable food ingredient. Rom. Biotechnol. Lett. 2016;21:11569. [Google Scholar]
- 51.Hu C., Kitts D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine. 2005;12:588–597. doi: 10.1016/j.phymed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Thu K., Myint P.P. Pharmacological activities of Cuscuta reflexa (Shwe-nwe) stem and Taraxacum officinale Weber ex FH Wigg. (Dai-Si) leaf extracts. J. Med. Plants. 2019;7:109–112. [Google Scholar]
- 53.Matthaus B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002;50:3444–3452. doi: 10.1021/jf011440s. [DOI] [PubMed] [Google Scholar]
- 54.Ghorbel Koubaa F., Chaâbane M., Choura B., Turki M., Makni-Ayadi F., El Feki A. Hepatoprotective effects of Taraxacum officinale root extract on permethrin-induced liver toxicity in adult mice. Pharm. Biomed. Res. 2020;6:223–236. doi: 10.18502/pbr.v6i3.4649. [DOI] [Google Scholar]
- 55.Mhamdi B., Abbassi F., Smaoui A., Abdelly C., Marzouk B. Fatty acids, essential oil and phenolics composition of Silybum marianum seeds and their antioxidant activities. Pak. J. Pharm. Sci. 2016;29:951–959. [PubMed] [Google Scholar]
- 56.Bourgou S., Tammar S., Salem N., Mkadmini K., Msaada K. Phenolic composition, essential oil, and antioxidant activity in the aerial part of Artemisia herba-alba from several provenances: A comparative study. Int. J. Food Prop. 2016;19:549–563. doi: 10.1080/10942912.2015.1040495. [DOI] [Google Scholar]
- 57.Dhouibi N., Manuguerra S., Arena R., Mahdhi A., Messina C.M., Santulli A., Dhaouadi H. Screening of antioxidant potentials and bioactive properties of the extracts obtained from two Centaurea L. Species (C. kroumirensis Coss. and C. sicula L. subsp sicula) Appl. Sci. 2020;10:2267. doi: 10.3390/app10072267. [DOI] [Google Scholar]
- 58.Boukes G.J., van de Venter M. Rooperol as an antioxidant and its role in the innate immune system: An in vitro study. J. Ethnopharmacol. 2012;144:692–699. doi: 10.1016/j.jep.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Diotallevi M., Checconi P., Palamara A.T., Celestino I., Coppo L., Holmgren A., Abbas K., Peyrot F., Mengozzi M., Ghezzi P. Glutathione fine-tunes the innate immune response toward antiviral pathways in a macrophage cell line independently of its antioxidant properties. Front. Immunol. 2017;8:1239. doi: 10.3389/fimmu.2017.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012;2012:217037. doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- 61.Vodjgani M., Salehi Z., Izad M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020;2020:5793817. doi: 10.1155/2020/5793817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marrocco I., Altieri F., Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxidative Med. Cell. Longev. 2017;2017:6501046. doi: 10.1155/2017/6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halliwell B., Gutterdige J.M.C. Free Radicals in Biology and Medicine. Oxford University Press; Oxford, UK: 1999. [Google Scholar]
- 64.Wang Y., Branicky R., Noë A., Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okado-Matsumoto A., Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J. Biol. Chem. 2001;27:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 66.Wong H.W.G., Elwell J.H., Oberley L.W., Goeddel D.V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 67.Weydert C., Roling B., Liu J., Hinkhouse M.M., Ritchie J.M., Oberley L.W., Cullen J.J. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol. Cancer Ther. 2003;2:361–369. [PubMed] [Google Scholar]
- 68.Liu J., Du J., Zhang Y., Sun W., Smith B.J., Oberley L.W., Cullen J.J. Suppression of the malignant phenotype in pancreatic cancer by overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum. Gene Ther. 2006;17:105–116. doi: 10.1089/hum.2006.17.105. [DOI] [PubMed] [Google Scholar]
- 69.Liu J., Hinkhouse M.M., Sun W., Weydert C.J., Ritchie J.M., Oberley L.W., Cullen J.J. Redox regulation of pancreatic cancer cell growth: Role of glutathione peroxidase in the suppression of the malignant phenotype. Hum. Gene Ther. 2004;15:239–250. doi: 10.1089/104303404322886093. [DOI] [PubMed] [Google Scholar]
- 70.Cullen J.J., Mitros F.A., Oberley L.W. Expression of anti-oxidant enzymes in diseases of the human pancreas: Another link between chronic pancreatitis and pancreatic cancer. Pancreas. 2003;26:23–27. doi: 10.1097/00006676-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Glorieux C., Dejeans N., Sid B., Beck R., Calderon P.B., Verrax J. Catalase overexpression in mammary cancer cells leads to a less aggressive phenotype and an altered response to chemotherapy. Biochem. Pharmacol. 2011;82:1384–1390. doi: 10.1016/j.bcp.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Glorieux C., Calderon P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017;398:1095–1108. doi: 10.1515/hsz-2017-0131. [DOI] [PubMed] [Google Scholar]
- 73.Kodydková J., Vávrová L., Kocík M., Zak A. Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biol. 2014;60:153. [PubMed] [Google Scholar]
- 74.Nishikawa M., Hashida M., Takakura Y. Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv. Drug Deliv. Rev. 2009;61:319–326. doi: 10.1016/j.addr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Chole R.H., Patil R.N., Basak A., Palandurkar K., Bhowate R. Estimation of serum malondialdehyde in oral cancer and precancer and its association with healthy individuals, gender, alcohol, and tobacco abuse. J. Cancer Res. Ther. 2010;6:487. doi: 10.4103/0973-1482.77106. [DOI] [PubMed] [Google Scholar]
- 76.Gönenç A., Özkan Y., Torun M., Şimşek B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J. Clin. Pharm. Ther. 2001;26:141–144. doi: 10.1046/j.1365-2710.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 77.Taysi S., Uslu C., Akcay F., Sutbeyaz M.Y. Malondialdehyde and nitric oxide levels in the plasma of patients with advanced laryngeal cancer. Surg. Today. 2003;33:651–654. doi: 10.1007/s00595-002-2562-3. [DOI] [PubMed] [Google Scholar]
- 78.Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang M., Dhingra K., Hittelman W.N., Liehr J.G., De Andrade M., Li D. Lipid peroxidation-induced putative malondialdehyde-DNA adducts in human breast tissues. Cancer Epidemiol. Prev. Biomark. 1996;5:705–710. [PubMed] [Google Scholar]
- 80.Sheweita S.A., El-Hosseiny L.S., Nashashibi M.A. Protective effects of essential oils as natural anti-oxidants against hepatotoxicity induced by cyclophosphamide in mice. PLoS ONE. 2016;11:e0165667. doi: 10.1371/journal.pone.0165667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zou Y., Wang J., Peng J., Wei H. Oregano essential oil induces SOD1 and GSH expression through Nrf2 activation and alleviates hydrogen peroxide-induced oxidative damage in IPEC-J2 cells. Oxidative Med. Cell. Longev. 2016;2016:5987183. doi: 10.1155/2016/5987183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Sousa A.C., Gattass C.R., Alviano D.S., Alviano C.S., Blank A.F., Alves P.B. Melissa officinalis L. essential oil: Anti-tumoral and anti-oxidant activities. J. Agric. Food Chem. 2004;52:2485–2489. [Google Scholar]
- 83.Ovadje P., Ammar S., Guerrero J.A., Arnason J.T., Pandey S. Dandelion root extract affects colorectal cancer proliferation and survival through the activation of multiple death signalling pathways. Oncotarget. 2016;7:73080. doi: 10.18632/oncotarget.11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sigstedt S.C., Hooten C.J., Callewaert M.C., Jenkins A.R., Romero A.E., Pullin M.J., Kornienko A., Lowrey T.K., Van Slambrouck S., Steelant W.F. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int. J. Oncol. 2008;32:1085–1090. doi: 10.3892/ijo.32.5.1085. [DOI] [PubMed] [Google Scholar]
- 85.Yoon J.Y., Cho H.S., Lee J.J., Lee H.J., Jun S.Y., Lee J.H., Song H.-H., Choi S., Saloura V., Gil Park C., et al. Novel TRAIL sensitizer Taraxacum officinale FH Wigg enhances TRAIL-induced apoptosis in Huh7 cells. Mol. Carcinog. 2016;55:387–396. doi: 10.1002/mc.22288. [DOI] [PubMed] [Google Scholar]
- 86.Ovadje P., Chochkeh M., Akbari-Asl P., Hamm C., Pandey S. Selective induction of apoptosis and autophagy through treatment with dandelion root extract in human pancreatic cancer cells. Pancreas. 2012;41:1039–1047. doi: 10.1097/MPA.0b013e31824b22a2. [DOI] [PubMed] [Google Scholar]
- 87.Ahn C., Lee J.H., Park M.J., Kim J.W., Yang J., Yoo Y.M., Jeung E.B. Cytostatic effects of plant essential oils on human skin and lung cells. Exp. Ther. Med. 2020;19:2008–2018. doi: 10.3892/etm.2020.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russo R., Corasaniti M.T., Bagetta G., Morrone L.A. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid.-Based Complement. Altern. Med. 2015;2015:397821. doi: 10.1155/2015/397821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loizzo M.R., Tundis R., Menichini F., Saab A.M., Statti G.A., Menichini F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anti-Cancer Res. 2007;27:3293–3299. [PubMed] [Google Scholar]
- 90.Sertel S., Eichhorn T., Plinkert P.K., Efferth T. Cytotoxicity of Thymus vulgaris essential oil towards human oral cavity squamous cell carcinoma. Anti-Cancer Res. 2011;31:81–87. [PubMed] [Google Scholar]
- 91.Park K.R., Nam D., Yun H.M., Lee S.G., Jang H.J., Sethi G., Cho S.K., Ahn K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011;312:178–188. doi: 10.1016/j.canlet.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 92.Xiao Y., Feng-Qing Y., Shao-Ping L., Jian-Li G., Guang H., Sin-Cheng L., Conceição Leong E., Fung K.-P., Wang Y.-T., Simon L.M.Y. Furanodiene induces G2/M cell cycle arrest and apoptosis through MAPK signaling and mitochondria-caspase pathway in human hepatocellular carcinoma cells. Cancer Biol. Ther. 2007;6:1044–1050. doi: 10.4161/cbt.6.7.4317. [DOI] [PubMed] [Google Scholar]
- 93.Carnesecchi S., Bradaia A., Fischer B., Coelho D., Schöller-Guinard M., Gosse F., Raul F. Perturbation by geraniol of cell membrane permeability and signal transduction pathways in human colon cancer cells. J. Pharmacol. Exp. Ther. 2002;303:711–715. doi: 10.1124/jpet.102.039263. [DOI] [PubMed] [Google Scholar]
- 94.Wang H.Y., Cai B., Cui C.B., Zhang D.Y., Yang B.F. Vitexicarpin, a flavonoid from Vitex trifolia L., induces apoptosis in K562 cells via mitochondria-controlled apoptotic pathway. Yao Xue Xue Bao. 2005;40:27–31. [PubMed] [Google Scholar]
- 95.Bayala B., Bassole I.H., Scifo R., Gnoula C., Morel L., Lobaccaro J.M.A., Simpore J. Anti-cancer activity of essential oils and their chemical components—A review. Am. J. Cancer Res. 2014;4:591–607. [PMC free article] [PubMed] [Google Scholar]
- 96.Salikhov S.M., Faizullina L.K., Valeev F.A. Synthesis and cytotoxic activity of isocembrol and its hydroxy derivatives. Russ. Chem. Bull. 2020;69:1933–1937. doi: 10.1007/s11172-020-2981-6. [DOI] [Google Scholar]
- 97.Jalili C., Taghadosi M., Pazhouhi M., Bahrehmand F., Miraghaee S., Pourmand D., Rashidi I. An overview of therapeutic potentials of Taraxacum officinale (Dandelion): A traditionally valuable herb with a reach historical background. World Cancer Res. J. 2020;7:e1679. [Google Scholar]
- 98.Menke K., Schwermer M., Felenda J., Beckmann C., Stintzing F., Schramm A., Zuzak T.J. Taraxacum officinale extract shows antitumor effects on pediatric cancer cells and enhance mistletoe therapy. Complement. Ther. Med. 2018;40:158–164. doi: 10.1016/j.ctim.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 99.Kamal F.Z., Stanciu G.D., Lefter R., Cotea V.V., Niculaua M., Ababei D.C., Ciobica A., Ech-Chahad A. Chemical Composition and Antioxidant Activity of Ammi visnaga L. Essential Oil. Antioxidants. 2022;11:347. doi: 10.3390/antiox11020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y., Huang B., He J., Han L., Zhan Y., Wang Y. In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J. Ethnopharmacol. 2011;136:309–315. doi: 10.1016/j.jep.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 101.Barbarić M., Mišković K., Bojić M., Lončar M.B., Smolčić-Bubalo A., Debeljak Ž., Medić-Šarić M. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J. Ethnopharmacol. 2011;135:772–778. doi: 10.1016/j.jep.2011.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used are available on request.