Abstract
Polydatin is a natural potent stilbenoid polyphenol and a resveratrol derivative with improved bioavailability. Polydatin possesses potential biological activities predominantly through the modulation of pivotal signaling pathways involved in inflammation, oxidative stress, and apoptosis. Various imperative biological activities have been suggested for polydatin towards promising therapeutic effects, including anticancer, cardioprotective, anti-diabetic, gastroprotective, hepatoprotective, neuroprotective, anti-microbial, as well as health-promoting roles on the renal system, the respiratory system, rheumatoid diseases, the skeletal system, and women’s health. In the present study, the therapeutic targets, biological activities, pharmacological mechanisms, and health benefits of polydatin are reviewed to provide new insights to researchers. The need to develop further clinical trials and novel delivery systems of polydatin is also considered to reveal new insights to researchers.
Keywords: polydatin, pharmacological mechanism, therapeutic target, health benefits, novel delivery system
1. Introduction
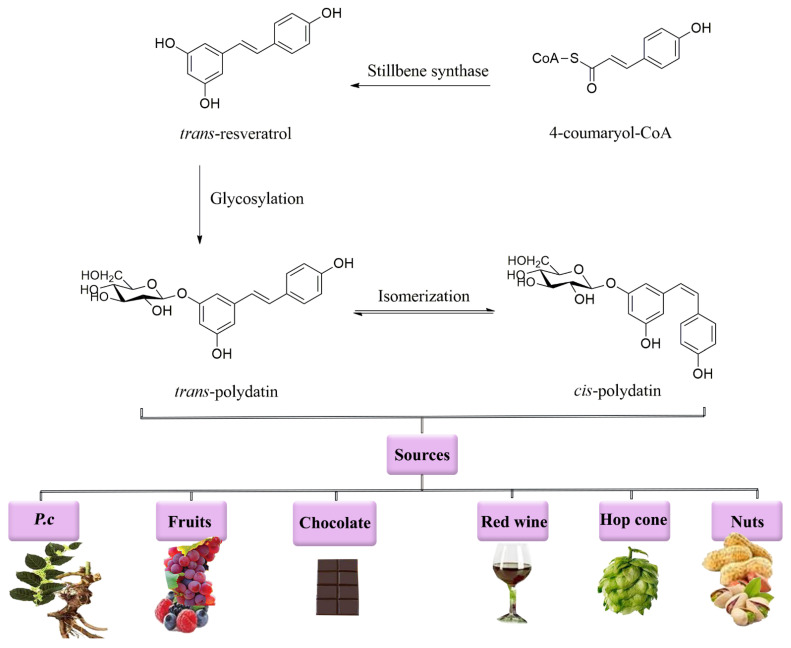
Polydatin is a stilbenoid polyphenol and a monocrystalline natural compound [1,2,3]. The plant families Vitaceae, Liliaceae, and Leguminosae are prominent sources of polydatin extraction [4]. It is primarily isolated from the rhizome and root of Polygonum cuspidatum [5], traditionally used against inflammation, infection, jaundice, skin burns, and hyperlipemia [6]. Reynoutria japonica is an invasive plant from the Far East, and a main source of polydatin in industrial scale [7]. This plant source of polydatin is so invasive that it has reached even the most remote mountain areas where it unbalances the ecosystem including the agricultural potential of mountain areas, being advantaged by the context of climate change [8]. This phytochemical is also found in other plant sources such as red wine [9], nuts, vegetables, fruits [5], hop cones/pellets, and cocoa- and chocolate-containing products [10].
Polydatin is a glucoside derivative of resveratrol. Moreover, polydatin has a higher antioxidant [3] and anti-inflammatory [11] activity compared to resveratrol [1,12,13,14,15,16]. It possesses four leading derivatives in nature, including trans-polydatin, trans-resveratrol, cis-polydatin, and cis-resveratrol [10,17]. trans-polydatin, itself, can be produced from 4-coumaryol-CoA through a stilbene synthase reaction (Figure 1). Several methods have been developed for the isolation of resveratrol from other isomers in P. cuspidatum, including reflux extraction, filtering, hydrolyzing, liquid–liquid extraction, eluting, and high-performance liquid chromatography coupled to an ultraviolet−visible diode array detector [18,19].
Figure 1.
Chemical structure and sources of polydatin. P.c: Polygonum cuspidatum.
The therapeutic and protective effects of polydatin mainly originate from its anti-inflammatory, antioxidant, and anti-apoptotic activities [20]. Previous studies have shown a wide range of therapeutic effects of polydatin in the treatment of several pathological diseases such as cancer [21], cardiovascular diseases [22], diabetes [23], neurodegenerative diseases [24], hepatic/respiratory diseases [25,26], gastrointestinal diseases [27], infectious diseases [28], rheumatoid diseases [29], and skeletal/women disorders [30,31].
Previously, the protective effects of polydatin have been disclosed against neurodegenerative diseases [20,32,33], atherosclerosis [34,35], and multiple organ ischemia/reperfusion injury [36]. The protective effects of polydatin on damaged macrophages were also highlighted by Liu et al. [37]. A more recent study also reviewed the pharmacological effects of polydatin in some diseases [38]. In the current mechanistic review, the therapeutic targets, pharmacological mechanisms, biological activities, and health benefits of polydatin are highlighted for all related biological/pathological conditions. Moreover, the need to provide different novel delivery systems and clinical trials of polydatin is also considered to reveal new insights to researchers.
2. Pharmacological Mechanisms of Polydatin
Considering the critical role of inflammation, oxidative stress, and apoptosis in the progression of several diseases, polydatin could play therapeutic roles by regulating associated signaling pathways.
2.1. Anti-Inflammatory Effects
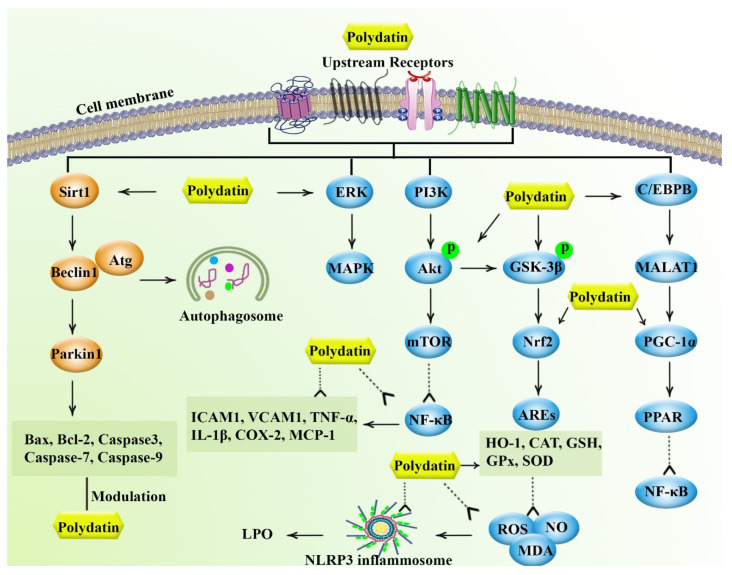
As a protective mechanism, the initiation of inflammation is attributed to the involvement of immune system response against diverse factors such as pathogens, injured tissues, and infectious agents, which is generally attributed to favoring tissue repair and organ healing. This defense process is divided into two major categories known as acute and chronic inflammation. At present, chronic inflammation could be considered a vital cause of human diseases (e.g., atherosclerosis, arthritis, diabetes, neurodegenerative diseases, and cancer) [39,40]. In this context, chronic inflammation is induced via uncontrolled acute inflammatory responses through inflammatory mediators such as cytokine production and immune cell recruitment [39,41]. Reportedly, nuclear factor-kappa B (NF-κB) is considered as the key transcription factor in increasing inflammatory mediators/cascades, including tumor necrosis factor-α (TNF-α), interleukin-1 beta (IL-1β), IL-6, and intercellular adhesion molecules (ICAMs) in various diseases. Such inflammatory mediators and interconnected pathways are mainly involved in the underlying mechanisms of the inflammatory state [42]. Several studies show that phytochemicals are multi-target agents that effectively represent their anti-inflammatory activities through underlying cellular mechanisms against different diseases associated with chronic inflammation [43]. In the phytochemical context, it has been reported that polydatin is a potent anti-inflammatory plant secondary metabolite [44], beneficially promoting miR-200a expression to regulate the Kelch-like ECH-associated protein 1 (Keap1)/nuclear factor E2-related factor 2 (Nrf2) antioxidant axis. This pathway, in turn, suppresses nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome activation against diverse chronic inflammation-related diseases in vivo [25,45]. Furthermore, under inflammatory conditions, polydatin exhibited anti-inflammatory roles by improving AMP-activated protein kinase (AMPK)/sirtuin1 (Sirt1)/Nrf2 signaling expression, leading to the inhibition of the NF-κB/inhibitor of nuclear factor-kappa B α (IκBα)/NLRP3 pathway, as well as the reduction of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [31,46,47]. Meanwhile, polydatin treatment downregulated the expression of cerebral ischemia or spinal cord injury (SCI)-induced inflammatory mediators such as Toll-like receptor 4 (TLR4), NF-κB, cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), nitric oxide (NO), and ICAM-1 [48,49,50]. In line with this, polydatin potentially plays a vital role in preventing the inflammatory action by reducing mitogen-activated protein kinases (MAPKs). This response is mainly orchestrated by extracellular-signal-regulated kinases (ERK1/2), c-Jun N-terminal kinase 1/2 (JNK1/2), and p38 protein kinases, and consequently inhibits NF-κB p65 phosphorylation and the release of inflammatory factors such as xanthine oxidase (XOD), prostaglandin E2 (PGE2), TNF-α, IL-1β, and COX-2 [51,52]. Another mechanism of the neuroprotective effect of polydatin is mediated by the CCAAT/enhancer-binding proteinβ (C/EBPβ)/metastasis-associated lung adenocarcinoma transcript 1 (MALAT1)/cAMP response element binding (CREB)/peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α)/peroxisome proliferative-activated receptor γ (PPARγ) signaling pathway. This process results in silencing NF-κB-associated downstream inflammatory mediators, which could alleviate cerebral infarct volume and ameliorate the integrity of the blood–brain barrier (BBB) [53]. Numerous studies have confirmed that polydatin application could suppress phospholipase A2 (PLA2) against lipopolysaccharide (LPS)-induced lung injury [54], decreasing the serum levels of alanine transaminase (ALT) and aspartate aminotransferase (AST) against fulminant hepatic failure (FHF) [55]. Additional reports also revealed that polydatin downregulated the retinoic acid receptor-related orphan receptor gamma t (RORγt) and signal transducer and activator of transcription 3 (STAT3) gene expression, attenuated IL-17 production against CD3/DC28-induced peripheral blood mononuclear cells and arthritis [11,56], and ultimately reduced both the NF-κB and vascular endothelial growth factor (VEGF) as well as the circulating levels of the downstream inflammatory cascade including IL-6, TNF-α [56].
Altogether, polydatin modulates several inflammatory mediators to show potential anti-inflammatory effects in various diseases.
2.2. Antioxidant Effects
Oxidative stress is characterized by aberrant generation of the reactive oxygen species (ROS) and reactive nitrogen species (RNS) as byproducts of this metabolic process which leads to devastating effects on molecular intracellular signaling pathways and to the development of numerous diseases [57,58]. The antioxidant reaction of polydatin is presented via reducing lipid peroxidation while promoting antioxidant enzymatic activity against hepatotoxicity induction in vivo [59]. Polydatin has also shown modulatory roles on ROS and RNS in peripheral [60] and central diseases [49]. Nrf2 could be the most vital antioxidative response-associated transcription factor and alleviates inflammation [61]. In this regard, polydatin strongly acts as an antioxidant agent on LPS-induced BV2 microglia cells in SCI-induced rat models via promoting the Nrf2/heme oxygenase-1 (HO-1) pathway activity, resulting in improved locomotor performance, suppressing spinal edema, and attenuating neurological deficits [24]. Additionally, the activation of protein kinase B (Akt) phosphorylation may be accelerated through the Nrf2/HO-1/NAD(P)H quinone dehydrogenase 1 (NQO1) expression-mediated antioxidant pathway after polydatin administration [51]. It has also been proposed that polydatin could significantly enhance Nrf2/thioredoxin (TRX) antioxidant signaling, and upregulate the Gli1/patched 1 (Ptch1)/superoxide dismutase 1 (SOD1) pathway, indicating its neuro/nephron-protective action against ischemic brain/renal injury-induced ROS generation [62,63,64]. Further in vivo studies have also indicated that polydatin supplementation provided an antioxidant ability through the Sirt1/Nrf2/antioxidant-responsive element (ARE) signaling pathway to reduce diabetes-induced renal dysfunction [65], as well as through the Notch1/Hes1-Pten/Akt axis to improve ischemic/reperfusion-injured diabetic heart disease [66,67]. Further, polydatin consumption is possibility beneficial against atherosclerosis and cardiovascular disease-induced oxidative stress via the scavenging of hydroxyl, oxygen free radicals, myeloperoxidase (MPO), and ROS [56,68,69], elevating enzymatic antioxidants such as SOD, glutathione peroxidase (GSH-Px), glutathione transferase (GST), catalase (CAT), and glutathione (GSH) [70]. Polydatin also induced protein kinase C (PKC) and the mitochondrial adenosine triphosphate (ATP)-sensitive-K+ (mito-KATP) channel [71] towards the modulation of mitochondrial function and the Akt signaling pathway [35,72]. The body of this evidence strongly supports that the attenuation of Akt is associated to the antioxidant effect of polydatin [50]. In addition, polydatin attenuated the activity of hepatic stellate cells (HSCs) through its antioxidant activity on sphingosine kinase 1 (SphK1) signaling to ameliorate mice liver fibrosis induced by carbon tetrachloride (CCl4) [73].
Overall, polydatin employs several antioxidant mediators while suppressing oxidative pathways towards therapeutic responses in several disorders.
2.3. Anti-Apoptotic Effects
Apoptosis is defined as a programmed model of physiological cell death without the involvement of an inflammatory condition. Nonetheless, the uncontrolled regulation of this mechanism could be engaged in several diseases, including cancer, autoimmune diseases, and neurodegenerative disorders [74]. In this regard, polydatin significantly exhibited anti-apoptotic effects in a mice model of liver injury through the attenuation of Bcl-2-associated x (Bax) expression and enhancing B-cell lymphoma 2 (Bcl-2) expression [59]. In another experimental study, polydatin showed its anti-apoptotic activity by increasing Bcl-2 or D-cyclins and reducing caspase-3 or Bax levels in gastrointestinal injury. Such an effect is made by the activation of Gli1 transcription factor followed by the upregulation of the Sonic hedgehog (Shh) signaling pathway as the major component in repairing dextran sulfate sodium-induced colitis-related damaged tissues [75]. In another study, polydatin exerted its protective effects against acute lung injury-induced mitochondrial apoptosis by increasing Parkin-mediated mitophagy expression via inhibiting Bax, cytochrome c, and caspase-3 activity as well as promoting Bcl-2 and mitochondrial membrane potential proteins [76]. Furthermore, polydatin administration could attenuate neuronal apoptosis through repressing p53/MAPK/JNK signaling activation in a rat model of ischemic brain injury [62]. Consequently, polydatin improved autophagy and apoptosis during osteoarthritis through suppressing the MAPK and phosphoinositide 3-kinases (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway [77]. Taken together, the described anti-apoptotic activities of polydatin indicate that this compound could be considered as a promising agent for apoptosis-induced tissue damage.
3. Biological Activities of Polydatin
As previously mentioned, polydatin exhibits significant pharmacological effects through its anti-inflammatory, antioxidant, and apoptosis-modifying activities. During different physiological/pathological conditions, the activity and expression of involved signaling pathways would change and polydatin plays a critical modulatory effect. We previously described near interconnections between the aforementioned signaling pathways during diseases which support each other [78,79]. Such effects make polydatin a potential therapeutic compound in the treatment of different pathological conditions.
3.1. Anticancer Effects
According to statistics from the World Health Organization, cancers leave the heaviest strain on the worldwide population based on the disability-adjusted life year (DALY) scale [80]. Radiotherapy, surgery, and chemotherapy are the main strategies in treating various cancer types [81,82]; however, associated resistance and side effects limit their applications. In addition, considering the multiple signaling pathways involved in cancer pathogenesis, there is an urgent need to provide novel alternatives and safe multi-target phytochemicals [83,84,85]. In this regard, the anticancer activity of polydatin has been elucidated against different cancer types [86].
From the mechanistic point, polydatin modulates oxidative stress to decrease carcinogenesis and mutagenesis [87]. It is reported that polydatin inhibits tumor growth [88] and improves radiosensitivity via inducing apoptosis in colorectal cancer models [89]. Polydatin also plays therapeutic roles in the treatment of leukemia alone or in combination with Janus kinase (JAK) inhibitors [86,90]. Moreover, it is revealed that polydatin is efficacious in the treatment of laryngeal cancer [91], nasopharyngeal cancer [92], ovarian cancer [93], lung cancer [94,95,96], glioblastoma multiforme [97], multiple myeloma [98], and cervical cancer [99,100]. Consequently, polydatin exerts therapeutic effects against such cancers employing different mechanisms, including the inhibition of proliferation/migration [97]. Several pre-clinical studies demonstrated that polydatin exerted therapeutic effects against an orthotopic metastatic tongue cancer model via inhibiting glucose 6 phosphate dehydrogenase (G6PD), and reduced tumor size and lymph node size and metastases [1]. In another study, the inhibition of G6PD by polydatin reduced tyrosine kinase inhibitor (TKI) resistance in breast cancer models [21]. In addition, polydatin suppressed the growth of MDA-MB-231 and MCF-7 breast cancer cell lines in vitro [101]. This secondary metabolite also induced apoptosis and inhibited cell proliferation/migration in breast cancer cell lines when used in combination with 2-deoxy-D-glucose [102].
Osteosarcoma is a malignant bone tumor; however, its etiology and pathogenesis remain unclear [103]. Prevailing in vitro studies showed that polydatin inhibited cells’ migration [103] and proliferation [103,104,105]. In this regard, inhibition of the β-catenin signaling pathway by polydatin is associated with its anti-proliferative effects [105]. Polydatin also induced apoptosis [103,104] via different mechanisms such as reducing the expression/phosphorylation of STAT3, increasing autophagy-related gene expression [104], and caspase-3 activity [105] in vitro. Polydatin also exerted therapeutic effects against doxorubicin-resistant osteosarcoma by suppressing TUG1/Akt signaling, promoting apoptosis and suppressing cell proliferation. In addition, polydatin reduced tumor size in animal models [106]. On another point, chronic liver disease and cirrhosis lead to hepatocellular carcinoma (HCC). HCC is a common malignancy of the liver and is classified as the third leading cause of cancer-related deaths in the world [107]. Similarly, polydatin showed positive effects against HCC through the inhibition of the Akt/STAT3–forkhead box protein O1 (FOXO1) signaling pathway towards apoptosis induction [108]. It also inhibited proliferation/invasion/migration of cell lines [107], and reduced tumor growth by increasing caspase-3 expression and terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) activity in animal models of HHC [107].
On the other hand, it is worth mentioning that polydatin could remarkably induce apoptosis against acute monocytic leukemia cell proliferation via modulating cyclin D1, Bcl-2, cyclin A, and Bax, accompanied by cell cycle arrest at the S phase [90]. Additionally, polydatin induced apoptosis in human nasopharyngeal carcinoma CNE cells through ROS-mediated mitochondrial dysfunction and endoplasmic reticulum stress. Thus, polydatin might be a promising anti-tumor agent through inducing apoptosis [92]. Additionally, polydatin inhibited the progression of doxorubicin-resistant osteosarcoma by regulating the taurine-upregulated gene 1 (TUG1)/Akt signaling pathway [106]. Furthermore, suppressing the phosphorylation of CREB and cyclin D1 gene expression might be associated with the apoptotic effect of polydatin on human breast cancer cells [101]. Moreover, extensive studies have demonstrated that the suppression of platelet-derived growth factor (PDGF)/Akt signaling [91], stimulation of cytochrome c release, and activation of poly (ADP-ribose) polymerase (PARP) fragmentation [109] could induce cell cycle arrest and apoptosis on polydatin-treated laryngeal/cervical cancer and oral squamous cell carcinoma.
Not only is polydatin efficacious in the treatment of various cancer types, it also reduces adverse effects of some chemotherapeutic agents such as cisplatin [110] and doxorubicin [111]. Polydatin plays a protective role against cisplatin-induced toxicity via improving antioxidant mechanisms and tissue regeneration [110]. It is reported that polydatin attenuated the cardiotoxicity of doxorubicin by increasing heart rate, arterial pressure, and GSH-Px activity while reducing myocardial injury. In addition, polydatin decreased ST, QT, and QRS intervals in an electrocardiogram. Antioxidant effects and the enhancement of metabolism were also associated with the protective effects of polydatin [111].
Taken together, polydatin could be a potential agent in the treatment of different cancer types. Apoptotic and anti-proliferative effects of polydatin, especially, are associated with its anticancer effects. Considering the protective effects of polydatin against the adverse effects of chemotherapy agents, polydatin might be an excellent choice to be co-administered with other agents. In Table 1, several in vitro and in vivo studies on the anticancer properties of polydatin are summarized.
Table 1.
Anticancer effects of polydatin.
| Study Type | Biological Effects | Cell Lines, Animal Types | Doses | References |
|---|---|---|---|---|
| In vivo, in vitro | ⇅miR-382/PD-L1 axis activity, ┬cell proliferation, ↑cleaved caspase-3 and cleaved caspase-9 expression | Mice, colorectal cancer cell line (Caco-2, HCT 116, HEK293) | 150 mg/kg/day for 16 days, injection into tumor, 50, 100, 150 μM | [88] |
| In vivo, in vitro | ↑BMP signaling pathway activity, radiosensitivity, ┬proliferation | Mice, colon tumor cell line (CT26, HCT116) | 25 mg/kg i.p. once a week for 4 weeks, 0, 40, 60, 80, 100, and 120 μM | [89] |
| In vitro | ↑Bax and cyclin A expression, ↓cyclin D1 and Bc1-2 expression, ┬growth, ↑arresting cells in S phase | Human acute monocytic leukemia cell line (THP-1) |
10, 20, 40, 60, 80, 100, 120, 140, 160 μM for 24 and 48 h |
[90] |
| In vitro | ↑Bcl-2-associated x expression, ↓cyclin D1 and cyclin B1 expression | Leukemia cell line (MOLT-4) | 0.5, 1, 2, 4, 10, or 20 μM | [86] |
| In vivo, in vitro | ┬PDGF/Akt signaling pathway activity, cell proliferation, ↓Bcl-2 expression, ↑Bax expression | Mice, human laryngeal cancer cell line (AMC-HN-8, HeLa Hep-2) | 50 mg/kg 3 times a week for 3 weeks, 2, 4, 6 μM for 24, 48, 72 h | [91] |
| In vitro | ↑ROS level, ER stress, cytosolic cytochrome c level, ↓mitochondrial cytochrome C level, Akt phosphorylation |
Human nasopharyngeal carcinoma cell line (CNE), cervical carcinoma cell line (HeLa), hepatoma cell line (SMMC-7721), epidermal carcinoma cell line (A-431) | - | [92] |
| In vitro | ↓phosphorylated Her-2 and EGFR levels, ERK expression, secretion of VEGF | Human ovarian adenocarcinoma cell line (SKOV-3, OVCAR-8) | 5, 10, 50, 100 μM for 6 days | [93] |
| In vitro | ↓phosphor-NF-κB p6 expression, activation of NF-κB pathway, ┬NLRP3 inflammasome activation, proliferation and migration of NSCLC cells | NSCLC cell line (A549, H1299) | 25, 50, 100 μM for 24 h | [94] |
| In vitro | ↑Bax expression, ↓Bcl-2 and cyclin D1 expression, ⇅mTOR pathway activity | Lung cancer cell line (A549) | 2, 4, 6, 8 μM for 24 and 48 h | [95] |
| In vivo, in vitro | ┬EGFR-Akt/ERK1/2/STAT3-SOX2/Snail signaling pathway activity, ┬proliferation, migration, invasion, stemness, ↑Bax expression, ↓Bcl-2 and cyclin D1 expression | Mice, lung cancer cell lines (NCI-H1975, A549), breast cancer cell lines (MDA-MB-231, MCF-7), cervical cancer cell line (HeLa), ovarian cancer cell line (SKOV-3), liver cancer cell line (SMMC-7721), nasopharyngeal cancer cell line (CNE-1), leukemia cell line (HL-60, K562) | 50 mg/kg/day for 14 days i.p. injection, 0–8 μM for 24 and 48 h | [96] |
| In vitro | ↓phosphorylation of mTOR and p70s6k | Human multiple myeloma cell line (RPMI 8226) | 0, 5, 10, 25, 50, 100, 200, and 400 μM for 24 and 48 h | [98] |
| In vitro | ┬PI3K/Akt/mTOR signaling pathway activity and downstream gene expression | Human cervical cancer cell line (HeLa cells) | 50, 100, 150 μM | [99] |
| In vivo, in vitro | ┬c-Myc expression, ⇅cell cycle-related protein expression (p21, p27, CDK2, CDK4, Cyclin D1, Cyclin E1, EMT, markers (E-cadherin, N-cadherin, Snail and Slug)), ┬proliferation and metastasis | Mice, human cervical cancer cell lines (CaSki, C33A), human embryonic renal cell line (293FT) | 100 mg/kg/day injection for 3 weeks, 0.1, 10, 100, 500 μM for 24 and 48 h | [100] |
| In vivo, in vitro | ┬G6PD activity, ↑ROS level, ER stress | Mice, head, and neck squamous cell carcinoma (HNSCC), breast cancer cell line (MCF-7) | 100 mg/kg 3 times a week for 30 days, 2–100 μM for 24 or 48 h | [1] |
| In vitro | ┬G6PD activity, ↑autophagosomes formation |
Breast cell lines (MCF-7) | 10, 20, 30 μM for 24 h | [21] |
| In vitro | ↓phosphorylated CREB level, cyclin D1 expression, ↑S-phase cell cycle arrest | Breast cancer cell lines (MDA-MB-231, MCF-7) | 0–8 μM for 24 and 48 h | [101] |
| In vivo, in vitro | ┬ROS/PI3K/Akt/HIF-1α/HK2 signaling axis activity | Mice, breast cell lines (4T1, MCF-7) | 100 mg/kg i.p. injection every other day for 3 weeks, 2 μM |
[102] |
| In vitro | ┬Akt activation, ⇅Ki67, p21, cyclin A, cyclin E, CDK2, MMP-2, MMP-9, tissue metalloproteinase-1, PPAR 1, caspase-3, p-glycoprotein 1, lung resistance-related protein 1, growth arrest, and DNA damage-45α, glutathione S-transferase π and heat shock protein 27 expression, ↑chemosensitivity to paclitaxel |
Human osteosarcoma cell lines (U-2OS, MG-63) | 0–400 μM for 24, 48, 72 h | [103] |
| In vitro | ┬STAT3 signaling pathway activity, ↑Atg12, Atg14, BECN, and PIC3K3 expression, autophagic cell death | Human normal osteoblast cell (hFOB1.19), human osteosarcoma cell line (MG-63) | 0–160 μM for 12–72 h | [104] |
| In vitro | ┬β-catenin signaling pathway activity, ↓cell proliferation, ↑Bax expression, ↓Bcl-2 expression, caspase-3 activity | Osteosarcoma cell lines (143B, MG63) | 0, 1, 10, 30, 100 μM for 24, 48, 72 h | [105] |
| In vivo, in vitro | ┬TUG1/Akt signaling pathway activity | Mice, osteosarcoma cell lines (Saos-2, MG-63), normal osteoblast cell line (hFOB), doxorubicin-resistant cell lines (Saos-2/Dox, MG- 63/Dox) |
150 mg/Kg/day i.p. injection for 20 days, 50, 100, 150, 200, 250 μM 24, 48, 72 h | [106] |
| In vivo, in vitro | ↑caspase-3 activity, caspase-3, caspase-9 and Bax expression, TUNEL activity, ↓expression of Bcl-2, Ki-67, ┬Wnt/beta-catenin signaling activity | Mice, normal liver cell lines (HL-7702, HCC), liver cancer cell lines (HepG2, SMMC-7721) | 25, 50, 100 mg/kg/day i.p. injection for 20 days, 1, 3, 10, 30, 100 μM | [107] |
| In vitro | ┬Akt/STAT3–FOXO1 signaling pathway activity | Human hepatocellular carcinoma cell line (HCCLM3), normal hepatic cell line LO2 | 0–800 μM for 24–72 h | [108] |
↓: decrease, ↑: increase, ┬: inhibit, ⇅: regulate, μM: micromole, Akt: protein kinase B, Atg: autophagy-related gene, Bax,: Bcl-2-associated x, Bcl-2: B-cell lymphoma 2, BMP: bone morphogenetic protein, c-Myc: cellular Myc, cAMP: cyclic adenosine monophosphate, CDK: cyclin-dependent kinase, CREB: cAMP response element-binding proteins, E-cadherin: epithelial cadherin, EGFR: epidermal growth factor receptor, EMT: epithelial mesenchymal transition, ER: endoplasmic reticulum, ERK: extracellular signal-regulated kinase, FOXO1: forkhead box protein O1, G6PD: glucose-6-phosphate dehydrogenase, HIF-1α: hypoxia-inducible factor, HK2: hexokinase 2, i.p.: intraperitoneal, kg: kilogram, mg: milligram, mTOR: mammalian target of rapamycin, N-cadherin: neural cadherin, NF-κB: nuclear factor-kappa B, NLRP3: nucleotide-binding domain (NOD)-like receptor protein 3, NSCLC: non-small-cell lung cancer, PI3K: phosphoinositide 3-kinases, ROS: reactive oxygen species, SOX2: sex-determining region Y, STAT: signal transducer and activator of transcription, TUG1: taurine-upregulated gene 1, VEGF: vascular endothelial growth factor.
3.2. Cardioprotective Effects
It is reported that different cardiovascular diseases are associated with stress oxidative and nitrosative processes, such as atherosclerosis and irreversible injury after ischemic reperfusion [112]. In addition, the interconnections between inflammatory processes and the symptoms of cardiovascular diseases have been elucidated [113]. As previously described, polydatin demonstrated potential antioxidant and anti-inflammatory effects. Polydatin has been reported to exert therapeutic effects against cardiovascular diseases such as acute myocardial infarction (AMI) [114], atherosclerosis [115], pulmonary hypertension [116], vascular damages [117], and sepsis-induced cardiac injury [118].
Polydatin consistently inhibited ventricular remodeling through neurohormones, especially in the renin–angiotensin system (RAS) and peroxidation [119,120,121]. In animal models of cardiac remodeling, including mice and rats, polydatin reduced cardiac weight in both animals, and decreased angiotensin II (Ang II) in mice. Polydatin also decreased cardiomyocyte size, aldosterone levels, TNF-α, endothelin-1, Ang II, blood pressure, and ventricular collagen volume [119]. Additionally, polydatin also increased NO, improved CAT and GSH-Px activity, and decreased serum hydroxyproline levels [120].
Insulin deficiency in diabetes causes cardiomyopathy, a disorder of the heart’s ventricles. Polydatin reduced ventricular dysfunction, improved mitochondrial bioenergetics, and increased autophagy flux by upregulating Sirt3 [122]. In addition, the inhibition of NAPDH oxidase and NF-κB is attributed to the cardioprotective effects of polydatin against diabetic cardiomyopathy [35]. Diabetes mellitus during myocardial infarction/reperfusion (MI/R) can increase the severity of oxidative/nitrative injury. Polydatin reduced MI/R cardiac damage during diabetes by activating the Notch1/Hes1-dependent Pten/Akt signaling pathway [36,66,123]. Assessment of the effects of polydatin on diabetes-associated myocardial hypertrophy showed a decrease in fasting blood glucose, insulin, HbA1C, weight gain, and insulin resistance levels by inhibiting NF-κB, COX-2, and the iNOS signaling pathway and activating PPARβ. It also improved hypertrophy factors in animal models of myocardial hypertrophy [124].
Studies have shown that polydatin decreased serum levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), hepatic TG concentrations, and LDL-C/high-density lipoprotein cholesterol (HDL-C) and TC/HDL-C ratios. These effects may indicate the protective effect of polydatin on the liver, coronary heart disease, and atherosclerosis [125,126,127]. Oxidative stress caused by the proliferation of vascular smooth muscle cells (VSMCs) leads to plaque formation and the progression of atherosclerosis. Polydatin inhibited oxidative stress by activating the NOS/Sirt1 pathway [115]. Polydatin also exerted potential protective effects against atherosclerosis by regulating pre-B-cell colony-enhancing factor (PBEF) and the inhibition of cholesterol accumulation in PBEF-induced macrophages. During an in vivo study, polydatin reduced blood lipids and atherosclerotic lesions, regulated the expression of cytokines and genes involved in cholesterol metabolism, and decreased PBEF mRNA and PBEF protein [128]. Moreover, polydatin exerted protective and therapeutic effects against atherosclerosis by inhibiting the PI3K/Akt/mTOR signaling pathway and improving autophagy disorders [129]. Polydatin’s inhibitory effects on β-hydroxy-β-methyl-glutaryl-CoA reductase (HMG-R) and the expression of proprotein convertase subtilisin/kexin type-9 (PCSK-9) are contributed to its anti-atherosclerotic effects [35]. Taken together, polydatin is reported to exert beneficial effects against atherosclerosis mainly through the protection of VSMCs, endothelial cells, mitochondria, and regulatory effects on lipid metabolism and antioxidant and anti-inflammatory effects [34].
In high-fat-diet (HFD)-induced obese mice, polydatin consumption caused weight loss, decreased TC, TG, and LDL, and increased HDL. Polydatin also reduced the size of adipose tissues and the volume of retroperitoneal fat. Polydatin exerted such effects by downregulating PPARγ, upregulating mRNA and leptin expression, and reducing TNF-α/monocyte chemoattractant protein-1 (MCP-1) [130]. Polydatin also inhibited the formation of foam cells caused by peritoneal macrophages via regulating its intracellular metabolism and antioxidant properties by regulating the PPARγ signaling pathway [131].
Polydatin exerted protective effects against cardiac ischemia/reperfusion (I/R) injury through various mechanisms, including the activation of the PKC-KATP-dependent signaling pathway and a direct antioxidative stress effect [71]. Studies have shown that intravenous administration of polydatin before I/R injury limits the size of the infarcted area, creatine phosphokinase, leakage of lactate dehydrogenase from damaged myocardial cells, changes in SOD activity, and malondialdehyde (MDA) levels [132]. Other cardioprotective mechanisms of polydatin include increased Bcl-2 protein expression and decreased Bax protein expression, which inhibits I/R-induced apoptosis in rat myocardium. Additionally, polydatin inhibits the rho kinase (ROCK) system, reducing apoptosis and the size of the infarcted area in animal models [22]. Constitutive nitric oxide synthase (cNOS) and the resulting increase in NO production are other pathways that promote the protective effects of polydatin against I/R injury. The study by Zhang et al. showed that polydatin reduced arrhythmia score and size of myocardial infarction, improved left developed ventricular pressure and coronary flow, increased SOD activity, and decreased MDA compared to the control group [133]. In addition, polydatin limited myocardial damage induced by I/R through increasing and improving autophagic flux [2,134]. Thus, polydatin cleared damaged mitochondria and reduced ROS in cell lines. The results of an in vivo study on cardiac I/R injury showed that polydatin reduced the size of the infarcted area, increased the ejection fraction, and caused higher left fractional ventricular shortening [134].
In the final analysis, polydatin could be considered a potential agent in the treatment of various cardiovascular diseases. Decreasing ROS level, NF-κB activity, VSMC proliferation, and RAS system activity and improving Sirt3 activity and lipid metabolisms contribute to polydatin’s therapeutic and protective cardiovascular effects. The details on the cardioprotective activity of polydatin are presented in Table 2.
Table 2.
Effects of polydatin on cardiovascular activity.
| Study Type | Biological Effects | Cell Lines, Animal Types | Doses | References |
|---|---|---|---|---|
| In vivo, in vitro | ↑Nrf2/HO-1 signaling activity, ↓ROS levels, hypoxia, AMI-induced myocardial damage | Rat, rat embryonic cardiomyocyte cells | 100 mg/kg/day i.p. injection for 30 days, 0, 10, 100, 250, 500 μM for 12 h | [114] |
| In vitro | ↑eNOS/Sirt1 pathway activity, ↓proliferation of VSMCs, ROS | vascular smooth muscle cells (VSMCs) | 10, 50,100 μM for 24 h | [115] |
| In vivo | ⇅NO, Ang II and ET expression, ┬protein kinase C activity | Rat | 5, 10, 20 mg/kg every other day i.p. injection for 3 weeks | [116] |
| In vivo | ↓ICAM-1, V-CAM, TNF-α, IL-1β, iNOS, PARP, and NF-κB expression, Bax and FAS ligand activity, vascular damage | Mice | 30 mg/kg orally for 14 days | [117] |
| In vivo, in vitro | ↑Sirt6-mediated autophagy and Bcl-2 expression, ↓serum TNF-α, IL-1β, IL-6, caspase-3, and Bax expression | Rat, cardiomyoblast cell line (H9c2) | 30 mg/kg i.p. injection once, 100 μM for 30 min | [118] |
| In vivo | ↑reverse cholesterol transport |
Mice | 50, 100 mg/kg/day orally for 12 weeks | [127] |
| In vivo | ↓ROS levels, spark-mediated sarcoplasmic reticulum leak | Rat | 10 mg/kg i.v. injection once | [135] |
| In vivo | ↓size of cardiomyocyte, aldosterone, TNF-α, Ang II, and endothelin-1 expression, blood pressure | Rat, mice | 60, 120 mg/kg/day orally for 30 days | [119] |
| In vivo | ↓renin–angiotensin–aldosterone system activity, ┬peroxidation |
Rat | 25, 50, 100 mg/kg/day orally for 7 weeks | [120] |
| In vivo, in vitro | ┬NADPH oxidase activity, superoxide production, ↓Ang II-mediated cardiac hypertrophy |
Rat, ventricle cells of neonatal rats | 50 mg/kg/day orally for 7 days, 5–50 μM for 60 min | [121] |
| In vitro, in vivo | ↑Sirt3 expression, autophagy flux | Mice, cardiomyocytes of neonates, and adult mice | 7.5 mg/kg/day injection for 28 days | [122] |
| In vivo | ↓NOX4, NOX2, NF-κB expression, NADPH oxidase activity, ROS level, inflammatory cytokines | Rat, embryonic rat cardiac cell line H9c2 | 100 mg/kg/day orally for 8 weeks | [35] |
| In vivo | ↓myocardial superoxide generation, MDA level, and iNOS expression, NO metabolite levels, nitrotyrosine, ↑Notch1/Hes1-Pten/Akt signaling pathway activity, eNOS phosphorylation |
Rat | 20 mg/kg/day orally for 4 days | [36,66,123] |
| In vivo | ↑PPARβ activation, ↓NF-κB p65, COX-2, and iNOS expression | Mice | 50, 100 mg/kg/day | [124] |
| In vivo | ↑LDL-C/HDL-C, TC/HDL-C ratios, ↓serum TC, TG, LDL-C, hepatic TG levels | Hamsters | 25, 50 and 100 mg/kg/day orally for 15 days | [125] |
| In vivo | ↓TC, TG, LDL-C, TC/HDL-C | Rabbit | 25, 50, 100 mg/kg/day orally for 3 weeks | [126] |
| In vitro | ↑eNOS/Sirt1 pathway activity, Kip1/p27 expression, ↓VSMCs proliferation, ROS production | VSMCs of the mouse thoracic aorta | 10, 50,100 μM for 24 h | [115] |
| In vivo, in vitro | ↓PBEF-inducing cholesterol deposition in macrophages | Mice, peritoneal macrophages of mouse | 100 mg/kg/day i.p. injection for 12 weeks, 50, 100, 200 μg/mL for 2 h | [128] |
| In vivo, in vitro | ↓TC, TG, LDL-C, PPARγ expression, MCP-1 and TNF-α expression, ↑HDL, leptin expression | Mice, mouse 3T3-L1 preadipocytes |
100 mg/kg/d orally for 4 weeks, 20 μM for 2 days | [130] |
| In vitro | ⇅PPARγ signaling pathways | Mouse peritoneal macrophages | 8.9 μg/mL | [131] |
| In vivo | ↑PKC--dependent signaling activity, ↓CPK and LDH leakage, ⇅SOD and MDA content | Rat | 20 μg/kg, i.v. once | [71] |
| In vitro | ↑Bcl-2 expression, ↓Bax expression, ↓I/R-induced apoptosis | Rat heart | 50 μM for 10 min | [132] |
| In vivo, in vitro | ┬RAS and the downstream ROCK pathway activity | Rat, ventricular myocytes of rats | 50 mg/kg/day orally for 8 days, 30, 50 μM for 1 h | [22] |
| In vivo, in vitro | ↑SOD activity, NO, NOS activity, cNOS, ↓MDA content | Rat, hearts of rats | 0.2 mL/100 g body weight (0.1% polydatin solution) i.v. injection before model establishment, 0.05 μM 15 min before model establishment | [133] |
| In vivo, in vitro | ↑Sirt3 activity, ↑mitochondrial biogenesis, upregulation of autophagy |
Mice, ventricular cardiomyocytes | 7.5 mg/kg/day i.p. injection for 10 days, 10 μM | [2] |
↓: decrease, ↑: increase, ┬: inhibit, ⇅: regulate, μg: microgram, μM: micromole, Akt: protein kinase B, Ang II: angiotensin II, Bax: Bcl-2-associated x, Bcl-2: B-cell lymphoma 2, cNOS: constitutive nitric oxide synthase, COX-2: cyclooxygenase-2, CPK: creatine phosphokinase, : calcium ion, eNOS: endothelial nitric oxide synthase, ET: endothelin, : glycoprotein P 91 phagocytic oxidase, HDL-C: high-density lipoprotein cholesterol, HO-1: heme oxygenase-1, i.p.: intraperitoneal, i.v.: intravenous, ICAM-1: intercellular adhesion molecule-1, IL-1β: interlukin-1β, IL-6: interleukin-6, iNOS: inducible nitric oxide synthase, kg: kilogram, Kip1/p27: a cyclin–CDK inhibitor, LDL-C: low-density lipoprotein cholesterol, LDH: lactate dehydrogenase, MDA: malondialdehyde, mg: milligram, MCP-1: monocyte chemoattractant protein-1, NADPH: nicotinamide adenine dinucleotide phosphate, NF-κB: nuclear factor-kappa β, NO: nitric oxide, NOS: nitric oxide synthase, NOX4: NADPH oxidase 4, NOX2: NADPH oxidase 2, Notch1: Notch homolog 1 translocation-associated, Nrf2: nuclear factor erythroid 2-related factor 2, PARP: poly (ADP-ribose) polymerase, PBEF: pre-B-cell colony-enhancing factor, PKC: protein kinase C, PPARβ: peroxisome proliferator-activated receptor β, PPARγ: peroxisome proliferator-activated receptor γ, RAS: renin–angiotensin system, ROCK: rho kinase, ROS: reactive oxygen species, Sirt1: sirtuin1, Sirt6: sirtunin6, SOD: superoxide dismutase, TC: total cholesterol, TG: triglyceride, TNF-α: tumor necrosis factor-α, V-CAM: vascular cell adhesion molecule, VSMCs: vascular smooth muscle cells.
3.3. Anti-Diabetic Effects
Pancreatic beta cells are sensitive to oxidative stress and, thus, the development of diabetes. Polydatin has been reported to inhibit inflammation and increase the expression of insulin receptor substrate 2 towards insulin sensitivity, insulin secretion, and the improvement of glucose resistance/fat metabolism. Polydatin exhibits such effects through multiple mechanisms, including ROS scavenging, improving functional cell markers, and modulating anti-apoptotic/antioxidant mediators [136,137].
Diabetic nephropathy is a major cause of end-stage renal disease and one of the most important complications of diabetes progression. Polydatin reduces the expression of fibronectin (FN) protein, an important fibrotic factor, in glomerular mesangial cells (GMCs) by inhibiting the NF-κB signaling pathway. Polydatin also reduced the expression of ICAM-1 and TGF-β in glomerular mesangial cells and, thus, exerted its anti-inflammatory effects [138]. Activation of the SphK1/sphingosine 1-phosphate (S1P) signaling pathway by high blood sugar is known as another factor that leads to an increase in the expression of FN protein in glomerular mesangial cells. Accordingly, polydatin prevented an increase in FN and the expression of ICAM-1 under the diabetes condition [139]. In addition, polydatin reduced oxidative stress in glomerular mesangial cells by upregulating the casein kinase 2 interacting protein-1 (CKIP-1)/Nrf2/ARE signaling pathway [23].
Impaired fat metabolism is another problem observed along with impaired glucose metabolism in diabetic patients. Polydatin improved fat/glucose metabolism by regulating PCSK-9 [140] and the Akt signaling pathway towards decreasing fasting blood glucose, glycosylated hemoglobin, glycosylated serum proteins, TC, TG, and LDL, while increasing serum insulin levels in diabetic rats [141].
Cardiovascular problems (e.g., macrovascular and microvascular diseases) are other complications following diabetes [142]. In this regard, polydatin improved endothelial function [72,142,143] in hyperglycemic conditions by activating the PPARβ/NO signaling pathway. Polydatin also improved endothelial damage in the aorta and vasodilation [142]. Diabetic neuropathy is another problem that affects half of diabetes patients. Mitochondrial dysfunction is closely related to the incidence of diabetic neuropathy. Polydatin improved mitochondrial function through the Sirt3/Nrf2 axis and, thus, reduced thermal and mechanical hyperalgesia in animal models [144].
In conclusion, polydatin could be an effective natural compound in the treatment of diabetes and complications related to diabetes. Activation of anti-fibrotic mechanisms and Nrf2-related signaling pathways and inhibition of NF-κB could improve glucose and lipid metabolism following polydatin administration.
3.4. Effects on Gastrointestinal Tract
Ulcerative colitis and inflammatory bowel disease are caused/characterized by dysfunction in the intestinal epithelial barrier. In addition to the inflammatory mechanisms involved in this disease, oxidative stress and reactive oxygen and nitrogen species could be involved in tissue damage and intestinal fibrosis [47]. Studies have shown that polydatin has an inhibitory effect on the NF-κB signaling pathway towards therapeutic effects against colitis [47,145,146]. In this regard, polydatin has shown therapeutic effects against ulcerative colitis in animal models by reducing NF-κB expression and increasing Sirt1/Nrf2 expression [47]. Polygonum cuspidatum root extract and its main constituent polydatin showed therapeutic responses against ulcerative colitis by inhibiting the NF-κB signaling pathway [145]. Polydatin also suppressed colitis in animal models by regulating the Shh signaling pathway [75].
Severe hemorrhagic shock causes damage to the small intestine. Increasing free radicals and mitochondrial damage caused by gastrointestinal I/R are among the factors influencing small bowel injury in severe hemorrhagic shock. Polydatin acts on the Sirt1/PGC-1α/SOD2 axis in the small intestine, thereby reducing oxidative stress and small intestinal damage [27,147,148].
Taken together, polydatin is an efficacious compound in the treatment of ulcerative colitis and severe hemorrhagic shock through antioxidant and anti-inflammatory effects. Inhibition of NF-κB is a major mechanism of polydatin towards its gastroprotective effects.
3.5. Anti-Microbial Effects
Although polydatin has not shown promising antibacterial effects, it has been shown that polydatin can exert positive antioxidant and anti-inflammatory effects against Staphylococcus aureus-induced mastitis and related lipoteichoic acid (LTA)-induced injury. The inhibitory effect of polydatin is that of TLR2-mediated activation of the p38MAPK/NF-κB signaling pathway and production of ROS [28,149]. Similarly, polydatin inhibited the NF-κB signaling pathway, contributing to the suppression of inflammatory responses and the development of pulmonary fibrosis induced by Mycoplasma pneumoniae infection. Inhibition of NLRP3 inflammasome by polydatin is the other mechanism that is employed by polydatin against Mycoplasma pneumoniae infection [45,150]. It also has been shown that polydatin exerted anti-inflammatory/protective responses against septic lung injury through the upregulation of HO-1 in lung tissue [151]. Since the coronavirus disease of 2019 (COVID-19) pandemic started, the effects of different natural compounds have been studied. It has been revealed that polydatin has shown inhibitory effects on the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into cells. This originated from binding directly to main protease (Mpro) of SARS-CoV-2. In addition, activation of sirtuin by polydatin may contribute to potential anti-COVID-19 effects [152].
To conclude, polydatin might be a good candidate in the treatment of respiratory diseases such as COVID-19 and Mycoplasma pneumonia-induced pulmonary fibrosis. Antioxidant and anti-inflammatory effects are primarily related to therapeutic anti-microbial effects of polydatin.
3.6. Hepatoprotective Effects
Recent studies have demonstrated polydatin’s hepatoprotective effects against alcoholic liver disease [153], non-alcoholic fatty liver disease (NAFLD) [154], and liver fibrosis [70]. Moreover, polydatin showed beneficial effects in drug-induced liver injury, including acetaminophen-induced injury, through antioxidant and anti-inflammatory effects. In one particular study, polydatin decreased the expression and levels of Bax, cytochrome c, cleaved caspase-3, cleaved caspase-9, and apoptotic protease-activating factor 1, while increasing Bcl-2. Polydatin also reduced oxidative stress factors such as ROS, NO, and MDA [59].
Alcoholic liver disease is one of the problems caused by excessive alcohol consumption, which can occur as a mild steatohepatitis or even severe cirrhosis of the liver [153]. Evaluation of the effect of polydatin on ethanol-induced liver damage in rats showed that polydatin exerted antioxidant and anti-inflammatory effects against ethanol-induced liver damage through downregulation of cytochrome P450 2E1 (CYP2E1), upregulation of Nrf2/HO-1, and modulation of TLR4/NF-κB p65 [48,155].
The mechanisms involved in the most common liver disease, NAFLD, have not been fully identified. Similar to alcoholic liver disease, it can also occur in the form of steatohepatitis in mild cases or in the form of liver cirrhosis in severe cases [156]. Polydatin has shown protective and therapeutic effects against NAFLD via various mechanisms [154]. Further, it reduced animal models’ TG, TC, and free fatty acid (FFA). Polydatin also exerted protective and therapeutic effects against NAFLD by different mechanisms, including lipid peroxidation suppression, reduction of TNF-α, and lipogenesis induced by sterol regulatory element-binding protein (SREBP-1c) [156]. In addition, polydatin decreased serum levels of AST, ALT, caspase-3, TUNEL-positive cells, TG, and oxidative stress in mice. Polydatin downregulated NADPH oxidase 4 (NOX4), inhibited the TLR4/NF-κB p65 signaling pathway, and exerted antioxidant and anti-inflammatory effects in vivo [157].
It has been shown that polydatin can exhibit hepatoprotective effects against carbon tetrachloride and sulfur mustard through antioxidant and anti-inflammatory mechanisms [158,159]. Additional studies elucidated that polydatin has protective and therapeutic effects against liver damage caused by carbon tetrachloride [70,159,160] through reducing liver fibrosis, suppressing inflammatory factors, and improving liver function/histological parameters. It also suppresses hepatic 4-HNE production andNOX4 expression and increases GSH levels, GST activity, SOD activity, GSH superoxidase, and CAT activity [70,159].
FHF is known as a clinical syndrome with high mortality. Consumption of polydatin prior to FHF induction showed protective effects by reducing TNF-α production and inhibiting NF-κB activity. It was revealed that polydatin reduced ALT, AST, mortality, and improved histopathological lesions [55,161].
Taken together, improvement of antioxidant capacity and anti-inflammatory effects are the major reasons of polydatin’s therapeutic effects against liver diseases. These effects are mainly applied through the activation of Nrf2-related signaling and inhibition of NF-κB. Therefore, polydatin is a potential compound in the treatment of liver diseases such as FHF, NAFLD, and alcoholic liver disease. Details on the hepatoprotective activity of polydatin are presented in Table 3.
Table 3.
Hepatoprotective effects of polydatin.
| Study Type | Biological Effects | Animal Types, Cell Lines | Doses | References |
|---|---|---|---|---|
| In vitro, in vivo | ↓CYP2Y3, CYP3A65, HMGCRa, HMGCRb, FASN, lipid and ethanol metabolism, hepatic fat accumulation, oxidative stress, DNA damage↑CHOP and GADD45aa expression | Zebrafish larvae | 6.25, 12.5, 25 μg/mL for 48 h | [153] |
| In vivo | ⇅TLR4 and NF-κB activity, ↓TNF-α, IL-1β, IL-6, CYP2E1, and ROS levels, ↑Nrf2/HO-1, SOD activity, GSH-Px, CAT, ADH, and ALDH activity | Rat | 25, 50, 100 mg/kg/day orally for 7 days | [48,155] |
| In vivo | ↓lipid peroxidation, TNF-α expression, lipogenesis | Rat | 30, 90 mg/kg/day orally for 8 weeks | [156] |
| In vivo, in vitro | ┬mTOR signaling activity, ↑TFEB expression, autophagic flux | Mice, human hepatocyte cell line (LO2) | 100 mg/kg every other day orally for 4 weeks, 24 μM for 24 h | [154] |
| In vivo, in vitro | ↓NOX4 enzymes, oxidative stress, CD68, FAS, SREBP-1c, LDH, cleaved caspase-3, annexin V, ALT, AST levels, ┬TLR-4/NF-κB p65 signaling pathway activity, | Mice, human hepatocellular carcinoma cell line (HepG2) | 5 mg/kg, every other day, i.p. injection for 4 weeks, 5, 10, 20 μM for 24 h | [157] |
| In vivo, in vitro | ↑Sirt1/Nrf2 pathway activity, ↓serum aminotransferase levels, hepatic pathological damage | Mice, human hepatocyte cell line (L02) | 100, 200, 400 mg/kg/day orally for 7 days, 50 μM for 24 h | [158] |
| In vivo | ↓4-HNE production, NOX4 expression | Mice | 5 mg/kg i.p. injection | [159] |
| In vivo | ↓AST, ALT, MDA, TNF-α, IL-1β, COX-2, iNOs, NF-κB, ↑GSH content, GST, SOD, CAT, and GSH-Px activity, hepatic TGF-b1 expression | Mice | 25, 50, 100 mg/kg/day orally for 5 days | [70] |
| In vivo | ┬NF-κB activation, ↓TNF-α expression, MPO activity, ICAM-l and ECAM-l expression, caspase-3 activity | Mice | 10, 30,100 mg/kg i.p. injection | [55,161] |
↓: decrease, ↑: increase, ┬: inhibit, ⇅: regulate, μg: microgram, μM: micromole, μM: microgram, 4-HNE: 4-hydroxy-2-nonenal, ADH: alcohol dehydrogenase, ALDH: aldehyde dehydrogenase, ALT: alanine transaminase, AST: aspartate transaminase, CAT: catalase, CD68: cluster of differentiation 68, COX-2: cyclooxygenase-2, CYP: cytochrome P450, DNA: deoxyribonucleic acid, ECAM-1: epithelial cell adhesion molecule-1, FASN: fatty acid synthase, GADD45αa: growth arrest and DNA-damage-inducible alpha a, GSH: glutathione, GSH-Px: glutathione peroxidase, GST: glutathione transferase, HMGCRa: hydroxymethylglutaryl-CoA reductase a, HMGCRb: hydroxymethylglutaryl-CoA reductase b, HO-1: heme oxygenase-1, i.p.: intraperitoneal, ICAM-1: intercellular adhesion molecule 1, IL-1β: interleukin-1β, IL-6: interleukin-6, iNOs: inducible nitric oxide synthase, kg: kilogram, LDH: lactate dehydrogenase, MDA: malondialdehyde, mg: milligram, MPO: myeloperoxidase, mTOR: mammalian target of rapamycin, NF-κB: nuclear factor-kappa B, ROS: reactive oxygen species, NOX4: NADPH oxidase 4, Nrf2: nuclear factor erythroid 2-related factor 2, Sirt1: sirtuin1, SOD: superoxide dismutase, SREBP-1c: sterol regulatory element-binding protein 1c, TFEB: transcription factor EB, TGF-β1: transforming growth factor-beta1, TNF-α: tumor necrosis factor-α, TLR4: Toll-like receptor 4.
3.7. Neuroprotective Effects
Neurological diseases are known as the second leading cause of death globally. Such disorders are the first cause of severe long-term disability worldwide [162]. Oxidative stress and inflammatory and apoptotic processes play essential roles in the pathogenesis of neurological diseases [112]. Regarding the previously described antioxidant, anti-inflammatory, and anti-apoptotic activities of polydatin, it can be a potential agent in the treatment of neurological diseases. Studies revealed therapeutic and protective effects of polydatin against Parkinson’s disease (PD) [163], intracranial hemorrhage (ICH) [164], cerebral ischemia [5], hemorrhagic shock [165,166], SCI [24], dementia [167], and traumatic brain injury [168].
Oxidative stress and mitochondrial dysfunction are two critical factors involved in PD pathogenesis [163]. It is shown that polydatin has neuroprotective effects against PD through various mechanisms [9,163,169,170]. Polydatin attenuated motor dysfunction in animal models of PD via lowering pro-inflammatory cytokine and microglial suppression. Inhibition of microglial activation leads to the decrement of dopaminergic neurodegeneration. Consequently, polydatin regulated the Akt/glycogen synthase kinase-3β (GSK-3β)/Nrf2/NF-κB signaling axis towards such effects [169]. In addition, polydatin improved cell viability and Sirt1 expression. It also decreased mitochondrial dysfunction and ROS level [170]. Moreover, it was revealed that polydatin exerted therapeutic and protective effects in animal models of PD, including decreasing dopaminergic neuronal degeneration, neural apoptosis, and improving motor function via enhancement of glucose metabolism in neurons [171]. Polydatin also has the ability to pass through the blood–brain barrier [163]. Taken together, polydatin can be a potential therapeutic agent for treatment of PD.
Neurological I/R conditions due to oxygen and glucose deprivation lead to the occurrence of neurotoxicity and apoptosis [53,172]. Production of ROS and the inflammatory process are some of the main factors that contribute to neurological damages of I/R conditions. It has been demonstrated that polydatin, through its antioxidant, anti-inflammatory, and anti-apoptotic activities, exerts neuroprotective effects [53,62,63,167,172,173,174]. It is reported that polydatin improved neurological dysfunction and reduced infarcted area in animal models through inhibition of various CAMs, ameliorating mitochondrial dysfunction and decreasing ROS and pro-inflammatory factors [173,174,175]. Moreover, polydatin causes an enhancement in behavioral scores, reducing brain edema and hemiplegia in animal models [176].
Polydatin can improve cognitive function in conditions such as dementia [167], ethanol toxicity [177], and doxorubicin-induced cognitive impairment [81] via antioxidant [81,167], anti-inflammatory, and anti-apoptotic effects in the hippocampus [167]. Upregulation of the Nrf2/ARE signaling pathway plays an essential role in this polydatin effect [81]. In addition, polydatin ameliorated memory impairment in animal models via upregulation of brain-derived neurotrophic factor (BDNF) [178].
It is reported that polydatin has protective and therapeutic effects on SCI, such as improving motor function, reducing apoptosis, and enhancing neuron and bone marrow stromal cell (BMSC) viability [24,49,179]. Mitochondrial injury plays an essential role in SCI. Polydatin also attenuates mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway [24,180]. In addition, it is revealed that activation of the Nrf2/ARE signaling pathway by polydatin administration and BMSC transplantation led to the improvement of neuronal regeneration in animal models of SCI, facilitating BMSC differentiation and reducing glial scar formation in glial cells [181]. The reduction in inflammatory factors is another mechanism associated with polydatin’s protective and therapeutic effects against SCI [49].
It is demonstrated that polydatin has therapeutic effects against intracranial hemorrhage (ICH) and its complication [164,182]. Polydatin improved neuronal function [164] and inhibited brain edema in animal models [182]. Regulation of the Nrf2/ARE signaling pathway and downstream genes are mechanisms that are associated with the antioxidant activity of polydatin against ICH [164]. In addition, increasing levels of excitatory amino acids by polydatin may be associated with the protective and therapeutic effects of polydatin against ICH [182].
As mentioned in Table 4, activation of Nrf2-related signaling is one of the primary mechanisms involved in polydatin’s therapeutic effects against neurological diseases such as ICH, PD, and cognitive impairment. Moreover, the anti-apoptotic effects play essential roles in this field. Polydatin can be a suitable choice in the treatment of neurological diseases (Table 4).
Table 4.
Neuroprotective effects of polydatin.
| Study Type | Biological Effects | Cell lines, Animal Types | Doses | References |
|---|---|---|---|---|
| In vivo | ↓MDA and activated caspase-3 levels, ↑GSH levels, SOD activity, p-Akt level, motor functions, ⇅TRX and ATP levels | Rat | 20, 40, 80 mg/kg/day orally for 5 weeks | [163] |
| In vivo, in vitro | ↑autophagy-related gene 5 (Atg5) activity, mTOR/Ulk-involved autophagy, ↓PGC1β/mfn2-involved mitochondrial fusion, ⇅autophagic processes, mitochondrial fusion |
Flies, human neuroblastoma cell (SH-SY5Y) | 2 mM orally for 25 days, 1–500 μM for 6 h | [170] |
| In vivo, in vitro | ⇅Akt/GSK3β-Nrf2/NF-κB signaling axis activity | Rat, murine microglia cell line (BV-2 cells) | 25, 50, 100 mg/kg/day orally for 32 days, 100, 200, 400 μM for 1 h | [169] |
| In vitro | ↓caspase-3/7 activity, ↑Bcl-2 expression, ERK1/2, and ERK5 activity | Human dopaminergic neuroblastoma cell line (SH-SY5Y) |
10, 20, 30 μM for 1 h | [9] |
| In vivo | glucose metabolism enhancement | Mice | 20, 80 mg/kg/day orally for 10 days | [171] |
| In vivo | ⇅Nrf2/ARE pathway and downstream gene expression, ↑SOD, GSSG, and GSH levels, ↓NO and MDA levels |
Rat | 50 mg/kg orally | [164] |
| In vivo, in vitro | ↓MDA production,↑SOD and CAT activity | Rat, rat cortical neuron | 12.5, 25, 50 mg/kg/day orally for 30 days, 12.5, 50 μg/mL for 1 h | [167] |
| In vivo | ↓Bax expression, caspase-9 activity, caspase-3 activity, ROS levels, infarcted volume, mitochondrial dysfunction, ↑Bcl-2 expression | Rat | 30 mg/kg i.v. injection | [173] |
| In vivo | ↓infarction volume, neurobehavioral deficits, neuronal apoptosis, ROS level, p38 mitogen-activated protein kinase activity, c-Jun N-terminal kinase activity, ↑Nrf2/HO-1/TRX activity |
Rat | 30 mg/kg i.p. injection twice | [62,167,174] |
| In vivo | ↑Gli1, Ptch1, and SOD1 expression, ↓NF-κB expression, BBB permeability, infarcted volume, brain water content | Rat | 25, 50 mg/kg i.p. injection once or for 2 days | [63] |
| In vivo | ↓brain edema | Rat | 12.5, 50 mg/kg i.p. injection once | [176] |
| In vivo | ↑Nrf2 activity, MDA and GSH levels, ↓hippocampal apoptosis, TUNEL-positive cells, cleaved caspase-3, cleaved caspase-9, NF-κB, TNF-α, PGE-2, and COX-2 levels | Rat | 50 mg/kg/day orally for 3 or 4 weeks | [81] |
| In vivo, in vitro | ↓NO, IL-1β, IL-6, and TNF-α levels, NLRP3 inflammasome activation, ↑motor function | Rat, BV2 mouse microglia | 20, 40 mg/kg i.p. injection once, 1, 2, 4 μM for 24 h | [49] |
| In vivo, in vitro | ↑Nrf2/ARE pathway activity, ↓mitochondrial dysfunction | Mice, spinal cord motor neurons | 30 mg/kg/day orally for 9 days, 3.75, 7.5, 15, 30, or 60 μM | [180] |
| In vitro | ⇅Nrf 2/ARE pathway activity | BMSCs | 3, 10, 30 μM for 2 h | [179] |
| In vivo, in vitro | ↑Nrf2 pathway activity | Mice, BMSCs | 20 mg/kg/day gastrically perfused for 5 days, 3, 10, 30 μM | [181] |
| In vivo | ↑BDNF expression | Rat | 10 mg/kg/day orally for 10 days | [178] |
| In vivo | ↓ICAM-1, VCAM-1, E-selectin, L-selectin, and Integrins levels, ↓neurological deficits, volume of brain infarction | Rat | 7.5, 15, 30 mg/kg i.v. injection once | [175] |
| In vivo, in vitro | ↑MALAT1/CREB/PGC-1α/PPARγ signaling pathway activity, BBB integrity, ↓cerebral infarcted volume, TNF-α, IL-6, IL-1β, COX-2, ICAM-1, VCAM-1, and MCP-1 expression | Rat, human embryonic kidney cell line (HEK-293T), human umbilical vein endothelial cell line (HUVEC) | 30 mg/kg i.v. injection once, 20 μM for 24 h | [53,172] |
| In vivo | ↑intracellular ATP, lysosomal stability, ↓mitochondrial swelling, vasoresponsiveness, arteriolar smooth muscle cell hyperpolarization, KATP channel activity | Rat | 15, 30, and 45 mg/100 g body weight | [166] |
↓: decrease, ↑: increase, ⇅: regulate, μg: microgram, μM: micromole, Akt: protein kinase B, ARE: antioxidant response element, Atg5: autophagy-related gene 5, ATP: adenosine triphosphate, Bax: Bcl-2-associated x, BBB: blood–brain barrier, Bcl-2: B-cell lymphoma 2, BDNF: brain-derived neurotrophic factor, CAT: catalase, COX-2: cyclooxygenase-2, CREB: cAMP-response element-binding protein, E-selectin: CD62 antigen-like family member E, ERK: extracellular signal-regulated kinase, HO-1: heme oxygenase-1, i.v.: intravenous, i.p.: intraperitoneal, IL-1β: interleukin-1β, IL-6: interleukin-6, L-selectin: CD62L, K: potassium, kg: kilogram, g: gram, Gli1: glioma-associated oncogene homologue 1, GSH: glutathione, GSK3β: glycogen synthase kinase-3β, GSSG: glutathione disulfide, ICAM-1: intercellular adhesion molecule 1, MALAT1: metastasis-associated lung adenocarcinoma transcript 1, MCP-1: monocyte chemoattractant protein-1, MDA: malondialdehyde, mTOR: mammalian target of rapamycin, mfn2: mitofusin 2, NF-κB: nuclear factor-kappa B, NLRP3: nucleotide-binding domain (NOD)-like receptor protein 3, NO: nitric oxide, Nrf2: nuclear factor erythroid 2-related factor 2, PGC-1α: peroxisome proliferator-activated receptor γ coactivator 1α, PGC1β: proliferator-activated receptor γ coactivator 1β, PGE-2: prostaglandin E-2, PPARγ: peroxisome proliferator-activated receptor γ, Ptch1: patched 1, ROS: reactive oxygen species, SOD: superoxide dismutase, Ulk: Unc-51-like kinase, TNF-α: tumor necrosis factor-α, TRX: thioredoxin, TUNEL: Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling, VCAM-1: vascular cell adhesion molecule.
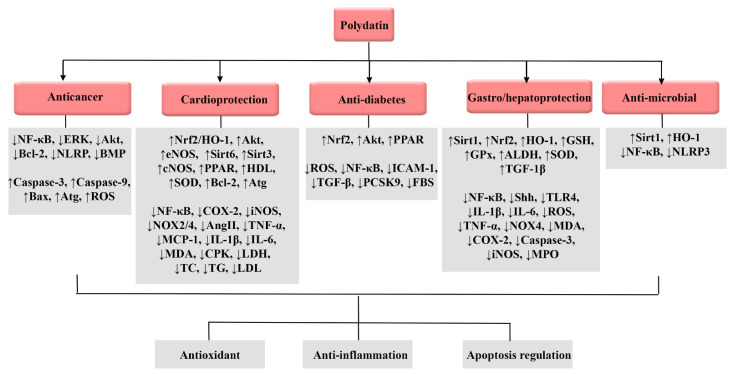
Therapeutic targets of polydatin against cancer, cardiovascular diseases, diabetes, gastric/hepatic failure, and infection are presented in Figure 2.
Figure 2.
Therapeutic targets of polydatin against cancer, cardiovascular diseases, diabetes, gastric/hepatic failure, and infection. ADH: alcohol dehydrogenase, Akt: protein kinase B, ALDH: aldehyde dehydrogenase, ALT: alanine transaminase, AST: aspartate transaminase, Atg: autophagy-related gene, Bax: Bcl-2-associated x, Bcl-2: B-cell lymphoma 2, BMP: bone morphogenetic protein, CAT: catalase, cNOS: constitutive nitric oxide synthase, COX-2: cyclooxygenase-2, CPK: creatine phosphokinase, eNOS: endothelial nitric oxide synthase, ERK: extracellular signal-regulated kinase, FBS: fasting blood sugar, GSH: glutathione, GPx: glutathione peroxidase, HDL: high-density lipoprotein, HO-1: heme oxygenase-1, ICAM-1: intercellular adhesion molecule-1, IL: interleukin, iNOS: inducible nitric oxide synthase, LDH: lactate dehydrogenase, LDL: low-density lipoprotein, MCP-1: monocyte chemoattractant protein-1, MDA: malondialdehyde, MPO: myeloperoxidase, NF-κB: nuclear factor-kappa B, NO: nitric oxide, NOX: NADPH oxidase 4, NLRP3: nucleotide-binding domain (NOD)-like receptor protein 3, NOS: nitric oxide synthase, Nrf2: nuclear factor erythroid 2-related factor 2, PCSK9: proprotein convertase subtilisin/kexin type-9, PPAR: peroxisome proliferator-activated receptor, PBEF: pre-B-cell colony-enhancing factor, ROS: reactive oxygen species, Shh: Sonic hedgehog, Sirt: sirtuin, SOD: superoxide dismutase, TC: total cholesterol, TFEB: transcription factor EB, TG: triglyceride, TGF-β: transforming growth factor-beta1, TLR4: Toll-like receptor 4, TNF-α: tumor necrosis factor-α.
3.8. Effects on Renal System
It has been shown that polydatin exerts therapeutic and protective effects against renal I/R injury, acute kidney injury (AKI), renal fibrosis, sepsis shock, hyperuricemia, and diabetic nephropathy through antioxidant, anti-apoptotic, and anti-inflammatory pathways [65,183,184,185,186,187].
Studies demonstrated that polydatin can decrease uric acid levels and creatinine in serum and urine [46,188,189] via the inhibition of XOD [52] in animal models of hyperuricemia. In addition, polydatin attenuated oxidative stress induced by inflammation and urates [52]. Polydatin can also ameliorate renal inflammation by activating the AMPK/Sirt1 pathway [46,189]. Consequently, polydatin showed positive effects against AKI in animal models through different mechanisms such as enhancing mitochondrial function (176) and inhibiting inflammatory and oxidative pathways [186,190]. Polydatin also decreased blood urea nitrogen (BUN) and serum creatinine in AKI animal models [186,191]. Improvement of mitophagy, inhibition of mitochondrial dysfunction [187,191], and NLRP3 inflammasome activation are related to protective effects of polydatin against AKI [187].
Oxidative stress and autophagy imbalance are two important factors associated with high-fructose-induced nephropathy. It has been depicted that polydatin activating the Nrf2 pathway leads to the attenuation of autophagy imbalance in a mTORC1-dependent manner [192]. In addition, activation of the Sirt1/Nrf2/ARE signaling pathway exerts protective effects against diabetic nephropathy. By activating this pathway, polydatin inhibited upregulation of fibronectin and TGF-β1 induced by advanced glycation end-products [65].
In conclusion, polydatin exerts therapeutic/protective effects in the treatment of renal diseases, primarily via the activation of the Nrf2 signaling pathway and inhibition of NF-κB/NLRP3 inflammasome activity (Table 5).
Table 5.
Effect of polydatin on the renal system.
| Study Type | Biological Effects | Cell Lines, Animal Types | Doses | References |
|---|---|---|---|---|
| In vivo | ⇅renal expression of mURAT1, mGLUT9, mAGCG2, mOAT1, mOCT1, mOCTN1, mOCTN2, mUMOD, ↑antihyperuricemic, nephroprotective effect |
Mice | 20, 40 mg/kg/day orally for 7 days | [183] |
| In vivo | ↑PI3K/Akt pathway activity, SOD, GST, GSH-Px, and CAT activity, GSH level, ↓TNF-α, IL-1, COX-2, iNOS, PGE-2, NO, and MDA levels | Mice | 10,20,40 mg/kg/day i.p. injection for 6 days | [10,50,184] |
| In vivo | ↓NF-κB p65, COX-2, and iNOS expression, ┬TNF-α, PGE-2, and IL-1β production | Mice | 12.5, 25.0, 50.0 mg/kg/day orally for 4 weeks | [52] |
| In vivo | ↑AMPK activity, Sirt1 expression, ┬NF-κB/NLRP3 inflammasome activity | Rats | 25, 50 mg/kg/day orally for 7 days | [46,189] |
| In vivo, in vitro | ↑Cx32 expression, K48-linked polyubiquitination activity, ↓NOX4, ROS, FN, and ICAM-1 levels | Mice, primary GMCs of rat | 100 mg/k/day orally 6 days a week for 12 weeks, 5, 10, 20, 40, 80, 160 μM for 24 h | [185] |
| In vivo | ┬NF-κB signaling, ↑Nrf2 signaling pathway activity, ↓TNF-α, IL-1β, and IL-6 expression, MPO activity, MDA content | Mice | 20, 40, 80 mg/kg i.p. injection once | [186] |
| In vivo, in vitro | ↑Sirt1/Nrf2/ARE pathway activity, ↓ROS, FN, and TGF-β1 levels | Rats, GMCs of rat | 150 mg/kg/day orally for 12 weeks, 5, 10, 20 μM | [65] |
| In vivo, in vitro | ↓AMPK/p38 MAPK signaling pathway activity, autophagy imbalance, ↑Nrf2 signaling pathway activity | Rat, conditionally immortalized human podocytes (HPCs) | 7.5, 15, 30 mg/kg/day orally for 6 weeks, 5, 10, 20 μM for 24 h | [192] |
| In vivo, in vitro | ↑Sirt1–p53 expression, ↓mitochondrial dysfunction | Rat, human renal proximal tubular epithelial cell line (HK-2) |
30 mg/kg infusion for 2 h, various concentrations for 2 h and 50 μM for 48 h |
[191] |
| In vivo | ↓IL-6 level, lipid peroxide, lysosomal instability, mitochondrial swelling, ↑ATP level, mitochondrial membrane potential | Rats | 30 mg/kg i.v. injection three times (6, 12, and 18 h after model establishment) |
[190] |
| In vivo | ↑Sirt1 activity, Parkin-dependent mitophagy, ↓NLRP3 inflammasome activity, mitochondrial dysfunction | mice | 30 mg/kg i.v. injection once | [187] |
↓: decrease, ↑: increase, ┬: inhibit, ⇅: regulate, μg: microgram, μM: micromole, Akt: protein kinase B, AMPK: adenosine 5′-monophosphate-activated protein kinase, ARE: antioxidant response element, ATP: adenosine triphosphate, CAT: catalase, COX-2: cyclooxygenase-2, FN: fibronectin, GMCs: glomerular mesenchymal cells, GSH: glutathione, GSH-Px: glutathione peroxidase, GST: glutathione transferase, ICAM-1: intercellular adhesion molecule 1, i.p.: intraperitoneal, i.v.: intravenous, IL-1β: interleukin-1β, IL-6: interleukin-6, iNOS: inducible nitric oxide synthase, kg: kilogram, MAPK: mitogen-activated protein kinase, MDA: malondialdehyde, mg: milligram, MPO: myeloperoxidase, NF-κB: nuclear factor-kappa B, NLRP3: nucleotide-binding domain (NOD)-like receptor protein 3, NO: nitric oxide, NOX4: NADPH oxidase 4, Nrf2: Nuclear factor erythroid 2-related factor 2, PGE-2: prostaglandin E-2, PI3K: phosphatidylinositol 3-kinase, ROS: reactive oxygen species, Sirt1: sirtuin1, TGF-β1: transforming growth factor-beta1, TNF-α: tumor necrosis factor-α.
3.9. Effects on Respiratory System
Inflammatory and oxidative processes are associated with respiratory diseases such as asthma and radiation-induced lung injury. Polydatin shows protective and therapeutic effects against such diseases through antioxidant, anti-inflammatory, and anti-fibrotic mechanisms [193,194,195]. Moreover, it is shown that polydatin exerts protective and therapeutic effects against fetal conditions such as pulmonary fibrosis [196], acute respiratory distress syndrome (ARSD) [26], and lung I/R injury [197,198].
In various animal models of lung injury, polydatin showed protective and therapeutic effects such as reducing mortality, pathological alternation [151,199], lung water content [199], and formation of fibrotic tissue [193]. It also improved the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (PaO2/FIO2) [199]. In burning-induced lung injury models, polydatin reduced the thickness of alveolar walls, neutrophil accumulation, microvascular hyper-permeability, and polymorphonuclear leukocytes (PMNs) in bronchoalveolar lavage fluid (BAFL). In addition, polydatin reduced wet-to-dry lung weight (W/D) ratio and improved histopathological lung damage [200]. Results of radiation-induced and LPS-induced lung injury models demonstrated that polydatin exerts such effects through upregulation of HO-1 [151], club cell secretory protein (CCSP) [54,169,201], Sirt3 expression, and inhibition of epithelial–mesenchymal transition (EMT) [193]. In addition, polydatin caused anti-inflammatory effects that lead to decreases in W/D, MPO, and the number of neutrophils via regulation of the TLR4/MyD88/NF-κB signaling pathway in animal models [202]. Studies showed that polydatin exerts therapeutic effects against asthma through antioxidant and anti-inflammatory pathways [194,195]. Polydatin reduced inflammatory factors, oxidative and nitrosative stresses, fibroblasts levels, and histopathological factors via the activation of Nrf2, and reduced ROS and TGF-β levels [195]. In addition, it is revealed that the attenuation of surfactant-d (SP-D) and urocortin (UCN) is another mechanism that is associated with the anti-asthma effects of polydatin [194].
Taken together, polydatin can be a potential agent in the treatment of asthma, lung injuries, and other conditions that can lead to lung fibrosis. Anti-inflammatory, anti-apoptotic, and antioxidant activities of polydatin are obtained through decreasing PLA2 and NF-κB activity, increasing Nrf2-related signaling activity, and improving antioxidant capacities (Table 6).
Table 6.
Effect of polydatin on respiratory system.
| Study Type | Biological Effects | Cell Lines, Animal Types | Doses | References |
|---|---|---|---|---|
| In vivo, in vitro | ┬TGF-β1/Smad3 signaling pathway activity, epithelial–mesenchymal transition, ↓IL-4 and IL-3 levels, ↑Sirt3 expression, Nrf2 and PGC1a levels, INF-γ activity | Mice, human bronchial epithelial cell line (BEAS-2B) | 100 mg/kg/day i.p. injection for 31 days | [193] |
| In vivo | ↓MDA level, W/D, ultrastructure injuries, ↑SOD content | Rabbit | - | [198] |
| In vivo | ↓TLR4 and NF-κB expression, ┬inflammatory mediators | Rabbit | - | [197] |
| In vivo | ↓TNF-α, IL-1β, and IL-6 levels, MPO activity, total cells, PMNs in BALF, Bcl-xl expression, ↑Bax and caspase-3 activity | Rat | 15, 30, 45 mg/kg via caudal vein once | [199] |
| In vivo, in vitro | ↑mitophagy via Parkin, Bcl-2 expression, ↓mitochondrial-dependent apoptosis, Bax expression, cytochrome c release, caspase-3 activity | Mice, human bronchial epithelial cell line (BEAS 2B cells) | 45 mg/kg once, 50 μM for 6 h | [26] |
| In vivo | ┬PLA2 | Rat | 1 mg/kg i.v. injection once | [203] |
| In vitro, in vivo | ┬TGF-β/Smad/ERK pathway activity, ↓α-smooth muscle actin, collagen I, TNF-α, IL-6, IL-13, MPO, and MDA expression, ↑epithelial cell cadherin expression, SOD activity | Rat | 10, 40, and 160 mg/kg/day for 28 days | [196] |
| In vivo | ↓PLA2 activity, hydroxyproline, PGE-2, LTC4, and TGF-β1 levels in BALF | Rat | 10, 20, 40 mg/kg intraperitoneal injection once before model establishment | [204] |
| In vivo, in vitro | ┬TGF-β1 activity, ↓ROS activity, ↑Nrf2 signaling pathway activity | Mice, human lung epithelial cell line (BEAS-2B) | 100 mg/kg at days 0, 7, 14, 100 μM for up to 48 h | [195] |
| In vivo | ⇅SP-D and UCN expression, ↓serum IgE, IL-4, IL-13, IL-5, MDA, TNF-α, IFN-γ, iNOS, and eosinophil levels, ↑SOD activity, GSH level | Rats | 200 mg/kg/day orally for 14 days | [194] |
| In vitro | ↑GSH-Px and SOD content, ↓apoptosis, MDA content, activation-related proteins of the NLRP3 inflammasome, TNF-α, TGF-β, IL-1β, IL-6 | Embryonic lung fibroblast MRC-5 | 12.5, 25, 50, 100, 200, 400 μmol/L for 24 h | [205] |
| In vitro, in vivo | ↑CCSP expression, ┬PLA2 activation | Rats, human bronchial epithelia cells (BEAS-2B cells) | 1, 5, 10, 30 mg/kg i.v. injection once, 0.5 m mol/L for 4 h |
[54,169,201] |
| In vivo | ↓TUNEL-positive lung cells, TNF-α, IL-1, and IL-6 levels, lung MPO activity, ┬Bax upregulation, caspase-3 activity, Bcl-xl downregulation | Rat | 45 mg/kg i.v. injection once | [200] |
| In vitro, in vivo | ┬TLR4/MyD88/NF-κB signaling pathway activity, W/D, MPO, neutrophil number, ↓IL-6, IL-1β, TNF-α, IL-8 levels | Mice, human bronchial epithelial cell line (BEAS-2B cells) | 20, 80 mg/kg i.p. injection once, 2 μM, 4 μM, 8 μM for 2 h | [202] |
| In vivo | ┬NF-κB activity, ↓TNF-α and IL-6 levels, lung COX-2 and iNOS expression | Mice | 15, 45, 100 mg/kg i.p. injection once | [151] |
↓: decrease, ↑: increase, ┬: inhibit, ⇅: regulate, μg: microgram, μM: micromole, BALF: bronchoalveolar lavage fluid, Bax: Bcl-2-associated x, Bcl: B-cell lymphoma 2, CCSP: club cell secretory protein, COX-2: cyclooxygenase-2, ERK: extracellular signal-regulated kinase, GSH: glutathione, GSH-Px: glutathione peroxidase, i.p.: intraperitoneal, i.v.: intravenous, IgE: immunoglobulin E, INF-γ: interferon-γ, iNOS: inducible nitric oxide synthase, IL-3: interleukin-3, IL-4: interleukin-4, IL-13: interleukin-13, kg: kilogram, LTC4: leukotriene C4, MDA: malondialdehyde, mg: milligram, MPO: myeloperoxidase, MyD88: myeloid differentiation factor 88, NF-κB: nuclear factor-κB, NLRP3: nucleotide-binding domain (NOD)-like receptor protein 3, Nrf2: nuclear factor erythroid 2-related factor 2, PGC1a: peroxisome proliferator-activated receptor γ coactivator 1α, PLA2: phospholipase A2, ROS: reactive oxygen species, Sirt3: sirtuin3, Smad3: mothers against decapentaplegic homolog 3, SOD: superoxide dismutase, SP-D: surfactant-d, TGF-β1: transforming growth factor-β1, TLR4: Toll-like receptor 4, TNF-α: tumor necrosis factor-α, TUNEL: terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling, UCN: urocortin, W/D: wet-to-dry.
3.10. Effects on Rheumatoid Diseases
Systematic lupus erythematosus and rheumatoid arthritis are two autoimmune diseases that can be present in various organs and systems such as the cardiovascular system, kidneys, joints, and eyes. Inflammatory and oxidative processes have essential roles in the pathogenesis of such autoimmune disorders [56,206,207]. It is revealed that polydatin has therapeutic effects against rheumatoid arthritis and its progression [56,206,208]. In vitro and in vivo studies showed that polydatin ameliorated arthritis severity and neutrophil extracellular trap (NET) accumulation in the ankles of mice [208]. In addition, it reduced matrix metalloproteinase-9 (MMP-9) [206] and ankle/hind paw diameter [56]. Regulation of the IL-6/STAT3/IL-17/NF-κB pathway is attributed to polydatin’s effects against rheumatoid arthritis [56].
The NET formation is also associated with systematic lupus erythematous pathogenesis. It is shown that polydatin inhibits ROS-mediated NET formation and NET release in vitro and in vivo. Polydatin reduced lupus markers as much as chemical drugs such as mycophenolate mofetil and cyclophosphamide. Polydatin also reduced proteinuria, circulating autoantibody levels, and the immune complex in the kidney [207].
To conclude, polydatin can be a potential compound in treating rheumatoid diseases. Lowering NET formation and decreasing inflammatory cytokines are two main mechanisms involved in such effects (Table 7).
Table 7.
Effects of polydatin on rheumatoid diseases.
| Study Type | Biological Effects | Cell Lines, Animal Types | Doses | References |
|---|---|---|---|---|
| In vivo, in vitro | ┬NET formation | Mice, neutrophils isolated from RA patients and mice | 45 mg/kg/day i.p. injection for 24 days, 0, 50, 75, 100, and 125 μg/mL | [208] |
| In vivo | ↓TNF-α, IL-6, IL-17, and MMP levels, MPO activity, MDA level, ↓RANKL and STAT3 expression, ┬VEGF, NF-κB, ↑GSH content | Rats | 200 mg/kg/day orally for 21 days | [56] |
| In vivo | ↓IL-1β, TNF-α, MDA, and SOD expression, ↑Bcl-2/Bax pathway expression, MMP-9 activity, ┬caspase-3/9 activity | Mice | 15, 30, 45 mg/kg i.d. injection for 24 h | [206] |
| In vivo, in vitro | ┬ROS-mediated NET formation | Mice, neutrophils from systematic lupus erythematous patients and healthy people | 45 mg/kg/day i.p. injection for 8 or 16 weeks, 50, 75, 100, 125, and 150 μg/mL | [207] |
↓: decrease, ↑: increase, ┬: inhibit, μg: microgram, μM: micromole, Bax: Bcl-2-associated x, Bcl-2: B-cell lymphoma 2, GSH: glutathione, i.d: intradermal, IL-1β: interleukin-1β, IL-6: interleukin-6, IL-17: interleukin-17, i.p.: intraperitoneal, kg: kilogram, MDA: malondialdehyde, mg: milligram, MMP: matrix metalloprotease, NET: neutrophil extracellular trap, NF-κB: nuclear factor-κB, RANKL: receptor activator of nuclear factor-kappa Β ligand, ROS: reactive oxygen species, SOD: superoxide dismutase, STAT3: signal transducer and activator of transcription, TNF-α: tumor necrosis factor-α, VEGF: vascular endothelial growth factor.
3.11. Effects on the Skeletal System
Osteoporosis is one of the most common diseases among the elderly population of societies. Because of its complications such as hip fracture, it can impose much cost on the healthcare system [209]. Studies showed that polydatin exerts therapeutic and protective effects through various mechanisms in the field of osteoporosis [209,210,211], such as activation of tafazzin (TAZ) [210] and regulation of osteoprotegerin, the receptor activator of NF-κB ligand (RANKL), and B-catenin [209]. Moreover, polydatin improved osteogenic differentiation, migration of hBMSCs, and protection of the bone matrix [210,211,212]. In animal models of osteoporosis, polydatin increased ALP serum levels, calcium, ferrous, W/D, and osteoprotegerin [209]. In addition to osteoporosis, polydatin showed therapeutic effects on other skeletal diseases, including intervertebral disc degeneration and ankylosing spondylitis [30,213]. Endplate chondrocyte apoptosis is associated with the degeneration of the cartilaginous endplate (CEP) that leads to intervertebral disc degeneration [30]. Polydatin ameliorated mitochondrial injury and oxidative stress, leading to protective effects on CEP and discs against degeneration via upregulation of Nrf2 [30,214]. In addition, activation of Nrf2 caused protective effects on nucleus pulposus cells. Nucleus pulposus cells play an essential role in the inhibition/degradation of the extracellular matrix [214].
Increasing the proliferation of fibroblasts is a crucial factor in manifesting ankylosing spondylitis. In vitro studies revealed that polydatin can increase fibroblast apoptosis via upregulation of active caspase-3 expression and Bax and decreasing Bcl2. It also increased autophagy mechanisms and therapeutic effects on ankylosing spondylitis [213].
Taken together, we can consider polydatin as an efficacious agent in the treatment of osteoporosis, ankylosing spondylitis, and intervertebral disc degeneration; therefore, it could be an interesting subject for further studies in this field (Table 8).
Table 8.
Effects of polydatin on skeletal system.
| Study Type | Biological Effects | Cell Lines, Animal Types | Doses | References |
|---|---|---|---|---|
| In vivo, in vitro | ↑Nrf2 signaling pathway activity, Parkin expression | Rats, human endplate chondrocytes | 50 mg/kg/day orally for 4 weeks, 200 μM for 2 h |
[30] |
| In vivo, in vitro | ⇅BMP2–Wnt/β-catenin, ↑TAZ, mRNAs RUNX2, osteopontin, DLX5, β-catenin, and osteocalcin expression | Mice, hBMSCs | 3 mg/kg every 2 days i.p. injection for 12 weeks, 0, 30, 10, 100 μM for 1, 2, 3, 7, 14 days | [210] |
| In vitro | ↑ERK ½ activity | Mouse BMSCs | 5, 10, 15, 30, 100 μM for 24 h | [212] |
| In vitro | ↑active caspase-3, Bax, LC3II, Beclin 1, and Atg5 expression, ↓Bcl2 expression | Fibroblasts of patients with AS | 0, 0.33, 1, 3, 10 μM for 24, 48, or 72 h | [213] |
| In vivo, In vitro | ↑aggrecan and collagen II levels, Nrf2 signaling pathway activity, ↓TNF-α, p53, p16, MMP-3, MMP-13, and ADAMTS-4 expression | Rats, nucleus pulposus cells of rats | 50 mg/kg/day orally for 4 weeks, 0, 200, 400 μmol/L for 24 h |
[214] |
| In vivo, in vitro | ⇅osteoprotegerin, RANKL, β-catenin expression | Mice, mouse bone marrow stromal cell line ST2 | 10, 20, 40, mg/kg/day i.p. injection for 12 weeks, 20, 40 μg/mL for 48 h | [209] |
↓: decrease, ↑: increase, ⇅: regulate, μg: microgram, μM: micromole, ADAMTS-4: a disintegrin and metalloproteinase with thrombospondin motif 4, Atg5: autophagy-related gene, Bax: Bcl-2-associated x, Bcl2: B-cell lymphoma 2, BMP2: bone morphogenetic protein 2, DLX5: distal-less homeobox 5, ERK: extracellular signal-regulated kinase, hBMSCs: human bone marrow stromal cells, i.p.: intraperitoneal, kg: kilogram, LC3II: microtubule-associated protein 1A/1B-light chain 3 ll, mg: milligram, MMP: matrix metalloprotease, Nrf2: nuclear factor erythroid 2-related factor 2, RANKL: receptor activator of nuclear factor-kappa Β ligand, RUNX2: runt-related transcription factor 2, TAZ: tafazzin, TNF-α: tumor necrosis factor-α.
3.12. Effects on Women’s Health
Endometriosis targets the female population with a prevalence of 10% [215]. There is a connection between endometriosis and inflammatory processes [31]. Mast cells that are close to nerve fibers in endometrial lesions play an essential role in regulating inflammatory processes [215]. Mast cell activation leads to the aggravation of pain and hyperalgesia in endometriosis [216]. In different studies, the effectiveness of palmitoylethanolamide (PEA)/polydatin combination was evaluated on endometriosis. PEA showed anti-inflammatory and inhibitory effects on mast cells. On the other hand, polydatin is known for its antioxidant and anti-inflammatory effects in different diseases [216]. Results in animal models of endometriosis showed that PEA/polydatin combination reduced cyst diameter, mast cell number, uterus impairment, inflammatory factors, and inflammatory cell infiltration [31,217]. In addition, clinical studies showed that PEA/polydatin combination improved chronic pelvic pain, dysmenorrhea, deep dyspareunia, and dyschezia [215,216,218]. It also improved life quality and psychological condition [218]. In addition to endometriosis, polydatin showed therapeutic and protective effects against hemorrhagic shock during pregnancy in vivo. It also improved survival time and microcirculation in animal models [219]. Table 9 shows the main benefits of polydatin on women’s health.
Table 9.
Effects of polydatin on women’s health.
| Study Type | Biological Effects | Cell Lines, Animal Types | Doses | References |
|---|---|---|---|---|
| In vivo | ↓VEGF, nerve growth factor, ICAM and MMP-9 expression, lymphocyte accumulation, peroxynitrite formation, PARP activation, IkBa phosphorylation, NF-κB translocation | Rats | 10 mg/kg/day orally for 14 days | [217] |
| In vivo | ↑capillary perfusion, functional capillary density, survival time | Rabbits | single bolus infusion 4 mL/kg | [219] |
| In vivo | ↓NF-κB activity, IL-6, IL-1β, 8-OHdG, 4-HNE, IL-6, γ-H2AX, Bax, cleaved-caspase-3, IL-6, caspase-1, phosphorylated p6 levels | Mice | 50, 100 mg/kg/day orally for 28 days | [220] |
| In vivo | ┬NF-κB pathway activity, ↑Nrf2 signaling pathway activity, ↓TNF-α, IL-1β, and IL-6 expression | Mice | 20, 40, 80 mg/kg i.p. injection once | [31] |
↓: decrease, ↑: increase, ┬: inhibit, μg: microgram, μM: micromole, γ-H2AX: γ-H2A histone family member X, 4-HNE: 4-hydroxy-2-nonenal, 8-OHdG: 8-hydroxydeoxyguanosine, Bax: Bcl-2-associated x, i.p.: intraperitoneal, ICAM: intercellular adhesion molecule, IkBa: inhibitory kappa B kinases a, IL-1β: interleukin-1β, IL-6: interleukin-6, kg: kilogram, mg: milligram, mL: milliliter, MMP-9: matrix metalloprotease-9, NF-κB: nuclear factor-κB Nrf2: nuclear factor erythroid 2-related factor 2, PAR: poly-ADP ribose, VEGF: vascular endothelial growth factor.
Therapeutic targets of polydatin against kidney injury, neurodegeneration, respiratory dysfunction, skeletal problems, and women’s disorders are presented in Figure 3.
Figure 3.
Therapeutic targets of polydatin against kidney injury, neurodegeneration, respiratory dysfunction, skeletal problems, and women’s disorders. Akt: protein kinase B, AMPK: adenosine 5′-monophosphate-activated protein kinase, Atg: autophagy-related gene, Bax: Bcl-2-associated x, Bcl-2: B-cell lymphoma 2, BDNF: brain-derived neurotrophic factor, CAT: catalase, COX-2: cyclooxygenase-2, CREB: cAMP response element-binding proteins, ERK: extracellular signal-regulated kinase, GSH: glutathione, GPx: glutathione peroxidase, ICAM-1: intercellular adhesion molecule-1, IgE: immunoglobulin E, IL: interleukin, INF-γ: interferon-γ, iNOS: inducible nitric oxide, MALAT1: metastasis-associated lung adenocarcinoma transcript 1, MCP-1: monocyte chemoattractant protein-1, MDA: malonaldehyde, MMP: matrix metalloprotease, MPO: myeloperoxidase, NO: nitric oxide, NF-κB: nuclear factor-kappa B, NOX4: NADPH oxidase 4, NLRP3: nucleotide-binding domain (NOD)-like receptor protein 3, Nrf2: nuclear factor erythroid 2-related factor 2, PGE2: prostaglandin E2, PLA2: phospholipase A2, RANKL: receptor activator of nuclear factor-kappa Β ligand, ROS: reactive oxygen species, SOD: superoxide dismutase, STAT: signal transducer and activator of transcription, TGF-β1: transforming growth factor-beta1, TLR4: Toll-like receptor 4, TNF-α: tumor necrosis factor-α, CAM-1: vascular cell adhesion molecule-1, VEGF: vascular endothelial growth factor.
3.13. Miscellaneous Effects
Polydatin is reported to have protective and therapeutic effects on other diseases and pathological conditions, including allergies [221,222,223], benign prostatic hyperplasia (BPH) [224], anxiety [225], Graves’ orbitopathy [226], pain [227], and age-related diseases [228]. Moreover, it is revealed that polydatin exerts protective effects in skin-related problems [229,230], such as facilitating epidermal growth factor receptor (EGFR) and inhibiting cutaneous side effects [230]. Studies showed that polydatin reduced pigmentation and histamine serum levels in passive cutaneous anaphylaxis and passive systemic anaphylaxis, respectively [221]. Mast cell stabilization [221,222] and decreasing inflammatory cytokines [221] are associated with such effects. In addition, polydatin attenuated food allergies via mast cell stabilization, reducing immunoglobulin E (IgE) production and improving integrity of the mucosal barrier [223]. Using polydatin in combination with PEA reduced dihydrotestosterone production and prostate weight through anti-apoptotic and anti-inflammatory mechanisms in animal models of BPH [224].
As previously mentioned, polydatin exhibited analgesic effects on endometriosis, chronic pelvic pain, and dysmenorrhea [215,216,218]. In addition, polydatin ameliorated osteoarthritis [227], cystitis/bladder pain syndrome [231], and diabetic neuropathy [232]. Polydatin reduced inflammatory factors and matrix-degrading protease in osteoarthritis through anti-inflammatory and chondroprotective effects [227]. Activation of the Nrf2 pathway is related to therapeutic and protective effects of polydatin on osteoarthritis [227] and diabetic neuropathy [232]. Polydatin also demonstrated protective effects on sciatic neurons and Schwann cells in animal models of diabetic neuropathy via inhibition of Keap 1, and activation of Nrf2/glyoxalase 1 [232].
4. Clinical Trials on Polydatin
Therapeutic and protective effects of polydatin have been evaluated in different clinical trials involving disorders such as chronic pelvic pain, inflammatory bowel syndrome (IBS), liver disease, and EGFR TKI-related rashes. As previously described, the effectiveness of polydatin on chronic pelvic pain related to endometriosis has been elucidated. Loi et al. assessed the effects of polydatin against chronic pelvic pain related to endometriosis in thirty symptomatic women desiring pregnancy. Patients administered 600 mg ultramicronized PEA (um-PEA) twice a day for 10 days followed by 400/40 mg co-micronized PEA/polydatin twice a day for 80 days demonstrated protective responses. The um-PEA and PEA/polydatin regimen improved pain symptoms, psychological condition, and the quality of life effectively. In addition, no adverse events were reported by this regimen, so it can be considered a potential regimen in the therapy of endometriosis symptoms [218]. Soave et al. showed that taking PEA/polydatin 400/40 mg twice a day for 90 days decreased pelvic pain and dysmenorrhea/dyspareunia. It also improved the quality of life in the 24 patients with suspected endometriosis suffering from severe pelvic pain who were involved in this study [233]. In addition, results of a review study showed that administration of PEA/polydatin 400 mg/40 mg twice a day exerted positive effects against endometriotic chronic pelvic pain, including improving chronic pelvic pain, dysmenorrhea, and deep dyspareunia [215].
Moreover, the assessment of PEA/polydatin effects against IBS on 54 patients with IBS and 12 healthy people showed potential approaches toward pain management in the IBS patients. Consequently, PEA/polydatin 200 mg/20 mg twice a day for 12 weeks reduced the fatty acid amide oleoylethanolamide. It also increased the expression of cannabinoid receptor 2, thereby significantly decreasing abdominal pain severity [234].
The protective and therapeutic effects of polydatin were investigated in 20 alcoholic patients hospitalized for rehabilitative therapy. They were given one of two oral nutritional supplements containing vitamin C or vitamin C/polydatin. It was shown that the later regiment reduced levels of AST, ALT, and lipid peroxidation in patients. Moreover, the polydatin-receiving group improved cognitive performance. Taken together, polydatin administration can be considered as a protective and therapeutic strategy in liver disease and cognitive impairment in alcoholic patients [235]. In another study, dietary supplements including polydatin and precursors for the endogenous synthesis of glutathione in healthy people are reported to be more effective on the redox status than dietary supplements containing precursors for the endogenous synthesis of glutathione alone. In the first supplement group, levels of vitamins C, E, and A are increased more than in the second group. Neopterin levels also decreased more effectively in the first group [236].
Fuggetta et al. showed that topical administration of 1.5% polydatin-based cream twice a day lowered the incidence of grade ≥2 skin toxicities in patients with mutated non-small-cell lung cancer (NSCLC) taking afatinib (40 mg/day) [230]. In addition, administration of 1.5% polydatin cream in patients with papulopustular adverse effects improved cutaneous adverse effects. Plydatin 0.8% also prevented the development of cutaneous adverse effects in patients without papulopustular adverse effects, and was used twice a day for 6 months [237].
Therefore, polydatin’s efficacy has been proven in clinical trials. Polydatin can be a good choice in the management of chronic pelvic pain, IBS, liver disease, and EGFR TKI-related rashes. Moreover, the therapeutic effects of polydatin can be considered as rational subjects for clinical trials (Table 10).
Table 10.
Clinical trials on polydatin.
| Clinical Study Type | Health Benefits | Gender | Dose | Study Type | References |
|---|---|---|---|---|---|
| Women’s health | ↓chronic pelvic pain, deep dyspareunia, dysmenorrhea, dyschezia, ↑life quality | Symptomatic women with endometriosis desiring pregnancy | 600 mg um-PEA orally twice a day for 10 days followed by 400/40 mg m(PEA/polydatin) orally twice a day for another 80 days | single-arm, non-randomized, open-label clinical study | [218] |
| Gastrointestinal | ↓abdominal pain severity | IBS patients and healthy people | 200/20 mg PEA/polydatin twice a day for 12 weeks | randomized, double-blind, placebo-controlled, multi-center clinical study | [234] |
| Liver disease | ↓AST and ALT levels, lipid peroxidation, ↓cognitive impairment | Alcoholic patients | 40 mg orally twice a day for 2 weeks | A pilot study | [235] |
| Antioxidant and anti-inflammatory activity | ↓oxidized species levels, neopterin levels,↑GSH level, other thiol species, vitamins C, E, and A | Healthy people | 35 mg orally twice a day for 8 weeks | randomized clinical study | [236] |
| Skin | ↓pruritus, skin manifestation such as papulopustular rash,↑quality of life | Cancer patients taking anti-EGFR treatment regimen with or without cutaneous adverse events | Cream (1.5% or 0.8%) twice a day topically for 6 months | prospective pilot study | [237] |
| Skin | ↓incidence of rash | Patients with mutated NSCLC taking afatinib | 1.5% cream twice a day topically for 6.4 months | pilot study | [230] |
↓: decrease, ↑: increase, ALT: alanine transaminase, AST: aspartate transaminase, EGFR: epidermal growth factor receptor, GSH: glutathione, IBS: irritable bowel syndrome, mg: milligram, NSCLC: non-small-cell lung cancer, PEA: palmitoylethanolamide.
5. Novel Delivery Systems of Polydatin
Various novel drug delivery systems, including nanoparticles [238], liposomes [239], micelles [240], quantum dots [241], and polymeric nanocapsules [242], are designed to enhance polydatin pharmacodynamics and pharmacokinetics [243].
ZnO#ZnS quantum dot (QD) heterojunctions (QDHJs) have been reported to lower free concentration of stilbenes such as polydatin and their pharmacological effects through increasing the affinity of polydatin for common bovine plasma proteins [241]. Polydatin-loaded chitosan nanoparticles (polydatin-CSNPs) showed better efficacy than free polydatin against type 2 diabetes [244], diabetic nephropathy [245], and diabetic cardiomyopathy [238]. Polydatin-CSNPs also showed a prolonged profile release [238,244]. In animal models, polydatin-CSNPs exerted better attenuating effects against diabetic cardiomyopathy than metformin [238]. Moreover, it has been demonstrated that polydatin-loaded chitosan nanoparticles (polydatin-CSNPs) attenuated diabetic liver damage through different mechanisms, including anti-inflammatory and antioxidant effects, regulating glucose transporter 2 expression, and influencing carbohydrate metabolism enzymes. Polydatin-CSNPs had higher bioavailability than naïve polydatin, which also showed a prolonged release pattern. Therefore, polydatin-CSNPs showed more efficacy than naïve polydatin [243]. In addition to polydatin-CSNPs, the effects of a polydatin-loaded micelle (polydatin-MC) were assessed against liver fibrosis. Polydatin-MC is designed based on the ROS and pH dual-sensitive block polymer PEG-P (PBEM-co-DPA). The micelle enhanced the biocompatibility of polydatin, and it also improved drug release in the liver in response to the fibrotic microenvironment. Interestingly, blank micelles exerted anti-inflammatory effects by improving hepatic ROS consumption and enhancing polydatin’s therapeutic effects [240]. Polydatin-loaded liposome is another drug delivery system designed to improve polydatin’s release profile. This drug delivery system improved the oral bioavailability of polydatin and prolonged drug circulation time remarkably [239].
Polydatin-loaded polymeric nanocapsules are reported to have the same therapeutic effects as naïve polydatin against anti-inflammatory and antioxidant activities on LPS-induced changes in hippocampal organotypic cultures. On the other hand, the encapsulation procedure enhanced polydatin bioavailability and stability [242]. Polydatin-encapsulated poly [lactic-co-glycolic acid] (polydatin-PLGA-NPs) showed preventive effects against oral squamous cell carcinoma (OSCC) in animal models. It also inhibited lipid peroxidation, oxidative stress, and modulated phase I and phase II of the detoxification system [246].
Polydatin gold nanoparticles (polydatin-AuNP) significantly reduced dense tumor cells in Ehrlich ascites carcinoma animal models. Polydatin-AuNPs also exerted protective effects against doxorubicin’s cardiovascular side effects [247]. Moreover, polydatin-modified Au@AgNPs exerted positive effects in topical wound healing. Administration of topical polydatin-modified Au@AgNPs led to higher migration of keratinocytes [248].
To sum up, novel drug delivery systems have improved the pharmacokinetics and pharmacodynamics properties of polydatin. Improvements in bioavailability, biocompatibility, and efficacy are the main beneficial effects of polydatin’s novel drug delivery systems.
6. Conclusions
The complex pathophysiological mechanisms behind chronic diseases urge the need for providing novel multi-targeting agents with higher efficacy/bioavailability and lower side effects. The plant kingdom is now a great source of natural secondary metabolites with the potential of targeting multiple dysregulated pathways in diseases. Polydatin is a glucoside derivative of resveratrol possessing better bioavailability and higher health-promoting/disease-modifying activities. The anti-inflammatory, antioxidant, and apoptotic-modifying properties of polydatin provide protective effects against inflammatory, oxidative stress, and apoptotic responses. Prevailing evidence reveals the biological activities and health-promoting effects of polydatin in various conditions, including cancer, cardiovascular diseases, diabetes, gastrointestinal/hepatic diseases, neurodegeneration, and infectious conditions, as well as protective roles in the respiratory system, the renal system, rheumatoid diseases, the skeletal system, and women’s health. Polydatin applies such effects through multiple mediators (Figure 4). As such, the broad therapeutic potential of polydatin urges the need for finding potential sources to promote the application of polydatin in industrial, commercial, and research sectors. Of polydatin sources, Reynoutria japonica is a useful highly invasive plant and a rich source in the pharmaceutical industry [7]. However, to better improve the bioavailability and efficacy of polydatin in clinical trials, investigating appropriate delivery systems in order to solve the pharmacokinetic limitation is of great importance.
Figure 4.
Major pharmacological mechanisms and therapeutic targets of polydatin towards biological activities and health benefits. ┬: inhibition or downregulation, →: increase or upregulation, Akt: protein kinase B, AREs: antioxidant response elements, Bax: Bcl-2-associated x, Bcl-2: B-cell lymphoma 2, C/EBPB: CCAAT/enhancer-binding proteinβ, CAT: catalase, COX-2: cyclooxygenase-2, Creb: cAMP response element-binding proteins, ERK: extracellular signal-regulated kinase, GSH: glutathione, GPx: glutathione peroxidase, GSK-3β: glycogen synthase kinase-3β, HO-1: heme oxygenase-1, IL-1β: interleukin-1β, ICAM-1: intercellular adhesion molecule-1, MALAT1: metastasis-associated lung adenocarcinoma transcript 1, MAPK: mitogen-activated protein kinases, MDA: malondialdehyde, MMP: mitochondrial membrane potential, mTOR: mammalian target of rapamycin, MCP-1: monocyte chemoattractant protein-1, NLRP3: nucleotide-binding domain (NOD)-like receptor protein 3, NF-κB: nuclear factor-kappa B, NO: nitric oxide, Nrf2: nuclear factor erythroid 2-related factor 2, PGC-1α: peroxisome proliferator-activated receptor γ coactivator 1α, PGE2: prostaglandin E-2, PPARγ: peroxisome proliferator-activated receptor γ, ROS: reactive oxygen species, Sirt1: sirtuin1, SOD: superoxide dismutase, TLR: Toll-like receptor, TNF-α: tumor necrosis factor-α, VCAM-1: vascular cell adhesion molecule-1.
Today, pan-assay interference compounds (PAINS) have been proposed as a threat to identifying the bioactivity of natural compounds, including compounds such as resveratrol [249]. PAINS cause disruption in the membrane by reducing the membrane dipole potential and, as a result, can affect intracellular signaling pathways. PAINS-type behavior can affect intracellular signaling pathways in a non-specific way. The cell-based data of some phytochemicals might be a product of such effects and not caused by specific binding to therapeutic targets. Recent studies have shown that C-glucosylation of polyphenol compounds causes the PAINS effect, while O-glucosylation has no effect on this behavior [250]. As per previous data [250], polydatin, an O-glucosylated derivative of resveratrol, is expected to present the same concerns as the aglycone with regards to inducing non-specific reductions in the membrane dipole potential. Therefore, in order to assess if C-glucosylation of resveratrol is able to preserve the promising bioactivities of polydatin described in this review while addressing the mentioned PAINS-related issue, the biological evaluation of this C-glucosyl analog of polydatin is of utmost importance. All biological activities, health benefits, and pharmacological mechanisms of action of polydatin have been highlighted in the current study. A further area of research should include extensive pre-clinical studies to reveal the precise pharmacological mechanisms of polydatin followed by well-controlled clinical trials towards therapeutic potentials. Such reports will help to provide more biological applications of polydatin and other stilbenoids in the prevention, management, and treatment of several diseases.
Author Contributions
Conceptualization: S.F. and H.K.; drafting of the manuscript: A.K., L.K. and S.F.; software: S.F.; reviewing and editing: S.F. and H.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that no conflict of interest could be perceived as prejudicing the impartiality of the research reported.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mele L., Paino F., Papaccio F., Regad T., Boocock D., Stiuso P., Lombardi A., Liccardo D., Aquino G., Barbieri A. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 2018;9:572. doi: 10.1038/s41419-018-0635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M., Zhao Z., Shen M., Zhang Y., Duan J., Guo Y., Zhang D., Hu J., Lin J., Man W. Polydatin protects cardiomyocytes against myocardial infarction injury by activating Sirt3. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017;1863:1962–1972. doi: 10.1016/j.bbadis.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Şöhretoğlu D., Baran M.Y., Arroo R., Kuruüzüm-Uz A. Recent advances in chemistry, therapeutic properties and sources of polydatin. Phytochem. Rev. 2018;17:973–1005. doi: 10.1007/s11101-018-9574-0. [DOI] [Google Scholar]
- 4.Mei X., Wang Y., Li J., Liu Z., Lang S., Ouyang W., Zhang J. Comprehensive metabolism study of polydatin in rat plasma and urine using ultra-high performance liquid chromatography coupled with high-resolution mass spectrometry. J. Chromatogr. B. 2019;1117:22–35. doi: 10.1016/j.jchromb.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 5.San Tang K., Tan J.S. The protective mechanisms of polydatin in cerebral ischemia. Eur. J. Pharmacol. 2019;842:133–138. doi: 10.1016/j.ejphar.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Peng W., Qin R., Li X., Zhou H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb. et Zucc.: A review. J. Ethnopharmacol. 2013;148:729–745. doi: 10.1016/j.jep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Negrea B.-M., Stoilov-Linu V., Pop C.-E., Deák G., Crăciun N., Făgăraș M.M. Expansion of the Invasive Plant Species Reynoutria japonica Houtt in the Upper Bistrița Mountain River Basin with a Calculus on the Productive Potential of a Mountain Meadow. Sustainability. 2022;14:5737. doi: 10.3390/su14095737. [DOI] [Google Scholar]
- 8.Neag M.A., Mocan A., Echeverría J., Pop R.M., Bocsan C.I., Crişan G., Buzoianu A.D. Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018;9:557. doi: 10.3389/fphar.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potdar S., Parmar M.S., Ray S.D., Cavanaugh J.E. Protective effects of the resveratrol analog piceid in dopaminergic SH-SY5Y cells. Arch. Toxicol. 2018;92:669–677. doi: 10.1007/s00204-017-2073-z. [DOI] [PubMed] [Google Scholar]
- 10.Du Q.-H., Peng C., Zhang H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013;51:1347–1354. doi: 10.3109/13880209.2013.792849. [DOI] [PubMed] [Google Scholar]
- 11.Lanzilli G., Cottarelli A., Nicotera G., Guida S., Ravagnan G., Fuggetta M.P. Anti-inflammatory effect of resveratrol and polydatin by in vitro IL-17 modulation. Inflammation. 2012;35:240–248. doi: 10.1007/s10753-011-9310-z. [DOI] [PubMed] [Google Scholar]
- 12.Muzio L.L., Bizzoca M.E., Ravagnan G. New intriguing possibility for prevention of coronavirus pneumonitis: Natural purified polyphenols. Oral Dis. 2020;28:899–903. doi: 10.1111/odi.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao G., Yang L., Zhong W., Hu Y., Tan Y., Ren Z., Ban Q., Yang C.S., Wang Y., Wang Z. Polydatin, A Glycoside of Resveratrol, Is Better Than Resveratrol in Alleviating Non-alcoholic Fatty Liver Disease in Mice Fed a High-Fructose Diet. Front. Nutr. 2022;9:857879. doi: 10.3389/fnut.2022.857879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bokkenheuser V.D., Shackleton C., Winter J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987;248:953–956. doi: 10.1042/bj2480953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marín L., Miguélez E.M., Villar C.J., Lombó F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015;2015:905215. doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim M., Lee J., Han J. Deglycosylation of isoflavone C-glycosides by newly isolated human intestinal bacteria. J. Sci. Food Agric. 2015;95:1925–1931. doi: 10.1002/jsfa.6900. [DOI] [PubMed] [Google Scholar]
- 17.de Lima M.T.R., Waffo-Téguo P., Teissedre P.L., Pujolas A., Vercauteren J., Cabanis J.C., Mérillon J.M. Determination of stilbenes (trans-astringin, cis-and trans-piceid, and cis-and trans-resveratrol) in Portuguese wines. J. Agric. Food Chem. 1999;47:2666–2670. doi: 10.1021/jf9900884. [DOI] [PubMed] [Google Scholar]
- 18.Wang D.-G., Liu W.-Y., Chen G.-T. A simple method for the isolation and purification of resveratrol from Polygonum cuspidatum. J. Pharm. Anal. 2013;3:241–247. doi: 10.1016/j.jpha.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero-Pérez A.I., Lamuela-Raventós R.M., Andrés-Lacueva C., de la Torre-Boronat M.C. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 2001;49:210–215. doi: 10.1021/jf000745o. [DOI] [PubMed] [Google Scholar]
- 20.Fakhri S., Gravandi M.M., Abdian S.A.-O., Akkol E.A.-O., Farzaei M.A.-O., Sobarzo-Sánchez E.A.-O. The Neuroprotective Role of Polydatin: Neuropharmacological Mechanisms, Molecular Targets, Therapeutic Potentials, and Clinical Perspective. Molecules. 2021;26:5985. doi: 10.3390/molecules26195985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mele L., la Noce M., Paino F., Regad T., Wagner S., Liccardo D., Papaccio G., Lombardi A., Caraglia M., Tirino V. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation. J. Exp. Clin. Cancer Res. 2019;38:1–13. doi: 10.1186/s13046-019-1164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ming D., Songyan L., Yawen C., Na Z., Jing M., Zhaowen X., Ye L., Wa D., Jie L. trans-Polydatin protects the mouse heart against ischemia/reperfusion injury via inhibition of the renin–angiotensin system (RAS) and Rho kinase (ROCK) activity. Food Funct. 2017;8:2309–2321. doi: 10.1039/C6FO01842D. [DOI] [PubMed] [Google Scholar]
- 23.Gong W., Li J., Chen Z., Huang J., Chen Q., Cai W., Liu P., Huang H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating CKIP-1 to resist HG-induced up-regulation of FN and ICAM-1 in GMCs and diabetic mice kidneys. Free Radic. Biol. Med. 2017;106:393–405. doi: 10.1016/j.freeradbiomed.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Lv R., Du L., Zhang L., Zhang Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci. 2019;217:119–127. doi: 10.1016/j.lfs.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X.-J., Yu H.-W., Yang Y.-Z., Wu W.-Y., Chen T.-Y., Jia K.-K., Kang L.-L., Jiao R.-Q., Kong L.-D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018;18:124–137. doi: 10.1016/j.redox.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T., Liu Y., Xu W., Dai X., Liu R., Gao Y., Chen Z., Li Y. Polydatin mediates Parkin-dependent mitophagy and protects against mitochondria-dependent apoptosis in acute respiratory distress syndrome. Lab. Investig. 2019;99:819–829. doi: 10.1038/s41374-019-0191-3. [DOI] [PubMed] [Google Scholar]
- 27.Zeng Z., Chen Z., Xu S., Song R., Yang H., Zhao K.-S. Polydatin alleviates small intestine injury during hemorrhagic shock as a SIRT1 activator. Oxidative Med. Cell. Longev. 2015;2015:965961. doi: 10.1155/2015/965961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang K.-F., Zhao G., Deng G.-Z., Wu H.-C., Yin N.-N., Chen X.-Y., Qiu C.-W., Peng X.-L. Polydatin ameliorates Staphylococcus aureus-induced mastitis in mice via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB pathway. Acta Pharmacol. Sin. 2017;38:211–222. doi: 10.1038/aps.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masodsai K., Lin Y.Y., Chaunchaiyakul R., Su C.T., Lee S.D., Yang A.L. Twelve-Week Protocatechuic Acid Administration Improves Insulin-Induced and Insulin-Like Growth Factor-1-Induced Vasorelaxation and Antioxidant Activities in Aging Spontaneously Hypertensive Rats. Nutrients. 2019;11:699. doi: 10.3390/nu11030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang L., Liu S., Li J., Tian Y., Xue Y., Liu X. Parkin and Nrf2 prevent oxidative stress-induced apoptosis in intervertebral endplate chondrocytes via inducing mitophagy and anti-oxidant defenses. Life Sci. 2020;243:117244. doi: 10.1016/j.lfs.2019.117244. [DOI] [PubMed] [Google Scholar]
- 31.Li R., Maimai T., Yao H., Liu X., He Z., Xiao C., Wang Y., Xie G. Protective effects of polydatin on LPS-induced endometritis in mice. Microb. Pathog. 2019;137:103720. doi: 10.1016/j.micpath.2019.103720. [DOI] [PubMed] [Google Scholar]
- 32.Tang K.S. Protective Effects of Polydatin Against Dementia-Related Disorders. Curr. Neuropharmacol. 2020;19:127–135. doi: 10.2174/1570159X18666200611144825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H., Li Y., Xun Y., Liu H., Wei C., Wang H., Yang X., Yuan S., Liu N., Xiang S. Polydatin protects neuronal cells from hydrogen peroxide damage by activating CREB/Ngb signaling. Mol. Med. Rep. 2022;25:1–10. doi: 10.3892/mmr.2021.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu M., Li X., Wang S., Yang S., Zhao R., Xing Y., Liu L. Polydatin for treating atherosclerotic diseases: A functional and mechanistic overview. Biomed. Pharmacother. 2020;128:110308. doi: 10.1016/j.biopha.2020.110308. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad P., Alvi S.S., Iqbal D., Khan M.S. Insights into pharmacological mechanisms of polydatin in targeting risk factors-mediated atherosclerosis. Life Sci. 2020;254:117756. doi: 10.1016/j.lfs.2020.117756. [DOI] [PubMed] [Google Scholar]
- 36.Sun Z., Wang X. Protective effects of polydatin on multiple organ ischemia-reperfusion injury. Bioorganic Chem. 2019;94:103485. doi: 10.1016/j.bioorg.2019.103485. [DOI] [PubMed] [Google Scholar]
- 37.Liu C., Wang W., Zhang K., Liu Q., Ma T., Tan L., Ma L. Protective Effects of Polydatin from Grapes and Reynoutria japonica Houtt. on Damaged Macrophages Treated with Acetaminophen. Nutrients. 2022;14:2077. doi: 10.3390/nu14102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salem M.A., Serag A., El-Seedi H.R., Hamdan D.I., Ezzat S.M., Zayed A. Phytochemistry, the Military and Health. Elsevier; Amsterdam, The Netherlands: 2021. Identification and analysis of toxic phytochemicals; pp. 443–479. [Google Scholar]
- 39.Rajendran P., Chen Y.F., Chen Y.F., Chung L.C., Tamilselvi S., Shen C.Y., Day C.H., Chen R.J., Viswanadha V.P., Kuo W.W. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018;233:6458–6471. doi: 10.1002/jcp.26479. [DOI] [PubMed] [Google Scholar]
- 40.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell S., Vargas J., Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016;8:227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R.X., Zhou M., Ma H.L., Qiao Y.B., Li Q.S. The Role of Chronic Inflammation in Various Diseases and Anti-inflammatory Therapies Containing Natural Products. ChemMedChem. 2021;16:1576–1592. doi: 10.1002/cmdc.202000996. [DOI] [PubMed] [Google Scholar]
- 44.Mikulski D., Molski M. Quantitative structure–antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-β-D-glucopyranoside. Eur. J. Med. Chem. 2010;45:2366–2380. doi: 10.1016/j.ejmech.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Tang J., Li Y., Wang J., Wu Q., Yan H. Polydatin suppresses the development of lung inflammation and fibrosis by inhibiting activation of the NACHT domain-, leucine-rich repeat-, and pyd-containing protein 3 inflammasome and the nuclear factor-κB pathway after Mycoplasma pneumoniae infection. J. Cell. Biochem. 2019;120:10137–10144. doi: 10.1002/jcb.28297. [DOI] [PubMed] [Google Scholar]
- 46.Chen L., Lan Z. Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-κB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food Funct. 2017;8:1785–1792. doi: 10.1039/C6FO01561A. [DOI] [PubMed] [Google Scholar]
- 47.Peritore A.F., D’Amico R., Cordaro M., Siracusa R., Fusco R., Gugliandolo E., Genovese T., Crupi R., Di Paola R., Cuzzocrea S. PEA/Polydatin: Anti-Inflammatory and Antioxidant Approach to Counteract DNBS-Induced Colitis. Antioxidants. 2021;10:464. doi: 10.3390/antiox10030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Q.-H., Xu L.-Q., Liu Y.-H., Wu J.-Z., Wu X., Lai X.-P., Li Y.-C., Su Z.-R., Chen J.-N., Xie Y.-L. Polydatin Protects Rat Liver against Ethanol-Induced Injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-κB p65 Pathway. Evid. Based Complement. Altern. Med. 2017;2017:7953850. doi: 10.1155/2017/7953850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv R., Du L., Liu X., Zhou F., Zhang Z., Zhang L. Polydatin alleviates traumatic spinal cord injury by reducing microglial inflammation via regulation of iNOS and NLRP3 inflammasome pathway. Int. Immunopharmacol. 2019;70:28–36. doi: 10.1016/j.intimp.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Liu H.-B., Meng Q.-H., Huang C., Wang J.-B., Liu X.-W. Nephroprotective Effects of Polydatin against Ischemia/Reperfusion Injury: A Role for the PI3K/Akt Signal Pathway. Oxidative Med. Cell. Longev. 2015;2015:362158. doi: 10.1155/2015/362158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen G., Yang Z., Wen D., Guo J., Xiong Q., Li P., Zhao L., Wang J., Wu C., Dong L. Polydatin has anti-inflammatory and antioxidant effects in LPS-induced macrophages and improves DSS-induced mice colitis. Immun. Inflamm. Dis. 2021;9:959–970. doi: 10.1002/iid3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L., Lan Z., Lin Q., Mi X., He Y., Wei L., Lin Y., Zhang Y., Deng X. Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem. Toxicol. 2013;52:28–35. doi: 10.1016/j.fct.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 53.Ruan W., Li J., Xu Y., Wang Y., Zhao F., Yang X., Jiang H., Zhang L., Saavedra J.M., Shi L., et al. MALAT1 Up-Regulator Polydatin Protects Brain Microvascular Integrity and Ameliorates Stroke Through C/EBPβ/MALAT1/CREB/PGC-1α/PPARγ Pathway. Cell. Mol. Neurobiol. 2019;39:265–286. doi: 10.1007/s10571-018-00646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiyu S., Zhiyu L., Mao Y., Lin B., Lijia W., Tianbao Z., Jie C., Tingyu L. Polydatin up-regulates Clara cell secretory protein to suppress phospholipase A2 of lung induced by LPS in vivo and in vitro. BMC Cell Biol. 2011;12:31. doi: 10.1186/1471-2121-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu M.J., Gong X., Jiang R., Zhang L., Li X.H., Wan J.Y. Polydatin Protects against Lipopolysaccharide-Induced Fulminant Hepatic Failure in D-Galactosamine-Sensitized Mice. Int. J. Immunopathol. Pharmacol. 2012;25:923–934. doi: 10.1177/039463201202500410. [DOI] [PubMed] [Google Scholar]
- 56.Kamel K.M., Gad A.M., Mansour S.M., Safar M.M., Fawzy H.M. Novel anti-arthritic mechanisms of Polydatin in complete Freund’s adjuvant-induced arthritis in rats: Involvement of IL-6, STAT-3, IL-17, and NF-κB. Inflammation. 2018;41:1974–1986. doi: 10.1007/s10753-018-0841-4. [DOI] [PubMed] [Google Scholar]
- 57.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Storz G., Imlayt J.A. Oxidative stress. Curr. Opin. Microbiol. 1999;2:188–194. doi: 10.1016/S1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y.-H., Huang Q.-H., Wu X., Wu J.-Z., Liang J.-L., Lin G.-S., Xu L.-Q., Lai X.-P., Su Z.-R., Chen J.-N. Polydatin protects against acetaminophen-induced hepatotoxicity in mice via anti-oxidative and anti-apoptotic activities. Food Funct. 2018;9:5891–5902. doi: 10.1039/C8FO01078A. [DOI] [PubMed] [Google Scholar]
- 60.Liu M., Huang Q., Zhu Y., Chen L., Li Y., Gong Z., Ai K. Harnessing reactive oxygen/nitrogen species and inflammation: Nanodrugs for liver injury. Mater. Today Bio. 2022;13:100215. doi: 10.1016/j.mtbio.2022.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi E., Suzuki T., Yamamoto M. Roles Nrf2 Plays in Myeloid Cells and Related Disorders. Oxidative Med. Cell. Longev. 2013;2013:529219. doi: 10.1155/2013/529219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah F.A., Kury L.A., Li T., Zeb A., Koh P.O., Liu F., Zhou Q., Hussain I., Khan A.U., Jiang Y., et al. Polydatin Attenuates Neuronal Loss via Reducing Neuroinflammation and Oxidative Stress in Rat MCAO Models. Front. Pharmacol. 2019;10:663. doi: 10.3389/fphar.2019.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ji H., Zhang X., Du Y., Liu H., Li S., Li L. Polydatin modulates inflammation by decreasing NF-κB activation and oxidative stress by increasing Gli1, Ptch1, SOD1 expression and ameliorates blood–brain barrier permeability for its neuroprotective effect in pMCAO rat brain. Brain Res. Bull. 2012;87:50–59. doi: 10.1016/j.brainresbull.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 64.Meng Q.-h., Liu H.-b., Wang J.-b. Polydatin ameliorates renal ischemia/reperfusion injury by decreasing apoptosis and oxidative stress through activating sonic hedgehog signaling pathway. Food Chem. Toxicol. 2016;96:215–225. doi: 10.1016/j.fct.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 65.Huang K., Chen C., Hao J., Huang J., Wang S., Liu P., Huang H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol. Cell. Endocrinol. 2015;399:178–189. doi: 10.1016/j.mce.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 66.Yu L., Li Z., Dong X., Xue X., Liu Y., Xu S., Zhang J., Han J., Yang Y., Wang H. Polydatin Protects Diabetic Heart against Ischemia-Reperfusion Injury via Notch1/Hes1-Mediated Activation of Pten/Akt Signaling. Oxidative Med. Cell. Longev. 2018;2018:2750695. doi: 10.1155/2018/2750695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan Y.-Y., Chen L.-X., Fang L., Zhang Q. Cardioprotective effects of polydatin against myocardial injury in diabetic rats via inhibition of NADPH oxidase and NF-κB activities. BMC Complement. Med. Ther. 2020;20:12. doi: 10.1186/s12906-020-03177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dan S., Bangle Z., Menglei H., Qiuju H., Chunlai Z., Siyuan Z. Comparison of Resveratrol and Polydatin on Anti-oxidative Activities. China Pharm. 2010;4:471–473. [Google Scholar]
- 69.Chai Y.-y., Wang F., Li Y.-l., Liu K., Xu H. Antioxidant Activities of Stilbenoids from Rheum emodi Wall. Evid. Based Complement. Altern. Med. 2012;2012:603678. doi: 10.1155/2012/603678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H., Yu C.-H., Jiang Y.-P., Peng C., He K., Tang J.-Y., Xin H.-L. Protective effects of polydatin from Polygonum cuspidatum against carbon tetrachloride-induced liver injury in mice. PLoS ONE. 2012;7:e46574. doi: 10.1371/journal.pone.0046574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miao Q., Wang S., Miao S., Wang J., Xie Y., Yang Q. Cardioprotective effect of polydatin against ischemia/reperfusion injury: Roles of protein kinase C and mito KATP activation. Phytomedicine. 2011;19:8–12. doi: 10.1016/j.phymed.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 72.Pang N., Chen T., Deng X., Chen N., Li R., Ren M., Li Y., Luo M., Hao H., Wu J., et al. Polydatin Prevents Methylglyoxal-Induced Apoptosis through Reducing Oxidative Stress and Improving Mitochondrial Function in Human Umbilical Vein Endothelial Cells. Oxidative Med. Cell. Longev. 2017;2017:7180943. doi: 10.1155/2017/7180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lan T., Zhuang L., Li S., Yang G., Xuan Y., Guo J. Polydatin attenuates hepatic stellate cell proliferation and liver fibrosis by suppressing sphingosine kinase 1. Biomed. Pharmacother. 2020;130:110586. doi: 10.1016/j.biopha.2020.110586. [DOI] [PubMed] [Google Scholar]
- 74.Carson D.A., Ribeiro J.M. Apoptosis and disease. Lancet. 1993;341:1251–1254. doi: 10.1016/0140-6736(93)91154-E. [DOI] [PubMed] [Google Scholar]
- 75.Lv T., Shen L., Yang L., Diao W., Yang Z., Zhang Y., Yu S., Li Y. Polydatin ameliorates dextran sulfate sodium-induced colitis by decreasing oxidative stress and apoptosis partially via Sonic hedgehog signaling pathway. Int. Immunopharmacol. 2018;64:256–263. doi: 10.1016/j.intimp.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 76.Yu H.I., Shen H.C., Chen S.H., Lim Y.P., Chuang H.H., Tai T.S., Kung F.P., Lu C.H., Hou C.Y., Lee Y.R. Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment. Int. J. Mol. Sci. 2019;20:5315. doi: 10.3390/ijms20215315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Z., Luan Z., Zhang X., Zou K., Ma S., Yang Z., Feng W., He M., Jiang L., Li J., et al. Retraction Note: Chondro-protective effects of polydatin in osteoarthritis through its effect on restoring dysregulated autophagy via modulating MAPK, and PI3K/Akt signaling pathways. Sci. Rep. 2020;10:11511. doi: 10.1038/s41598-020-68880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fakhri S., Abbaszadeh F., Moradi S.Z., Cao H., Khan H., Xiao J. Effects of Polyphenols on Oxidative Stress, Inflammation, and Interconnected Pathways during Spinal Cord Injury. Oxidative Med. Cell. Longev. 2022;2022:8100195. doi: 10.1155/2022/8100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fakhri S., Piri S., Moradi S.Z., Khan H. Phytochemicals Targeting Oxidative Stress, Interconnected Neuroinflammatory, and Neuroapoptotic Pathways Following Radiation. Curr. Neuropharmacol. 2022;20:836–856. doi: 10.2174/1570159X19666210809103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mattiuzzi C., Lippi G. Current cancer epidemiology. J. Epidemiol. Glob. Health. 2019;9:217. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tong Y., Wang K., Sheng S., Cui J. Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats. Biosci. Biotechnol. Biochem. 2020;84:1201–1210. doi: 10.1080/09168451.2020.1722057. [DOI] [PubMed] [Google Scholar]
- 82.Schirrmacher V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019;54:407–419. doi: 10.3892/ijo.2018.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fakhri S., Abbaszadeh F., Jorjani M., Pourgholami M.H. The effects of anticancer medicinal herbs on vascular endothelial growth factor based on pharmacological aspects: A review study. Nutr. Cancer. 2021;73:1–15. doi: 10.1080/01635581.2019.1673451. [DOI] [PubMed] [Google Scholar]
- 84.Fakhri S., Khodamorady M., Naseri M., Farzaei M.H., Khan H. The ameliorating effects of anthocyanins on the cross-linked signaling pathways of cancer dysregulated metabolism. Pharmacol. Res. 2020;159:104895. doi: 10.1016/j.phrs.2020.104895. [DOI] [PubMed] [Google Scholar]
- 85.Nouri Z., Fakhri S., Nouri K., Wallace C.E., Farzaei M.H., Bishayee A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers. 2020;12:2276. doi: 10.3390/cancers12082276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao W.J., Wu K., Wang C., Wan D.M. Polydatin-induced cell apoptosis and cell cycle arrest are potentiated by Janus kinase 2 inhibition in leukemia cells. Mol. Med. Rep. 2016;13:3297–3302. doi: 10.3892/mmr.2016.4909. [DOI] [PubMed] [Google Scholar]
- 87.Quagliariello V., Berretta M., Buccolo S., Iovine M., Paccone A., Cavalcanti E., Taibi R., Montopoli M., Botti G., Maurea N. Polydatin reduces cardiotoxicity and enhances the anticancer effects of sunitinib by decreasing pro-oxidative stress, pro-inflammatory cytokines, and NLRP3 inflammasome expression. Front. Oncol. 2021;11:2188. doi: 10.3389/fonc.2021.680758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin Y., Zhan X., Zhang B., Chen Y., Liu C., Yu L. Polydatin exerts an antitumor effect through regulating the miR-382/PD-L1 axis in colorectal cancer. Cancer Biother. Radiopharm. 2020;35:83–91. doi: 10.1089/cbr.2019.2999. [DOI] [PubMed] [Google Scholar]
- 89.Chen Q., Zeng Y.-N., Zhang K., Zhao Y., Wu Y.-Y., Li G., Cheng H.-Y., Zhang M., Lai F., Wang J.-B. Polydatin increases radiosensitivity by inducing apoptosis of stem cells in colorectal cancer. Int. J. Biol. Sci. 2019;15:430. doi: 10.7150/ijbs.27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang C., Luo Y., Lu J., Wang Y., Sheng G. Polydatin induces apoptosis and inhibits growth of acute monocytic leukemia cells. J. Biochem. Mol. Toxicol. 2016;30:200–205. doi: 10.1002/jbt.21779. [DOI] [PubMed] [Google Scholar]
- 91.Li H., Shi B., Li Y., Yin F. Polydatin inhibits cell proliferation and induces apoptosis in laryngeal cancer and HeLa cells via suppression of the PDGF/AKT signaling pathway. J. Biochem. Mol. Toxicol. 2017;31:e21900. doi: 10.1002/jbt.21900. [DOI] [PubMed] [Google Scholar]
- 92.Liu H., Zhao S., Zhang Y., Wu J., Peng H., Fan J., Liao J. Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE cells. J. Cell. Biochem. 2011;112:3695–3703. doi: 10.1002/jcb.23303. [DOI] [PubMed] [Google Scholar]
- 93.Hogg S.J., Chitcholtan K., Hassan W., Sykes P.H., Garrill A. Resveratrol, acetyl-resveratrol, and polydatin exhibit antigrowth activity against 3D cell aggregates of the SKOV-3 and OVCAR-8 ovarian cancer cell lines. Obstet. Gynecol. Int. 2015;2015:279591. doi: 10.1155/2015/279591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zou J., Yang Y., Yang Y., Liu X. Polydatin suppresses proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via NF-κB pathway. Biomed. Pharmacother. 2018;108:130–136. doi: 10.1016/j.biopha.2018.09.051. [DOI] [PubMed] [Google Scholar]
- 95.Verma N., Tiku A.B. Polydatin-Induced Direct and Bystander Effects in A549 Lung Cancer Cell Line. Nutr. Cancer. 2021;74:237–249. doi: 10.1080/01635581.2020.1870705. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y., Zhuang Z., Meng Q., Jiao Y., Xu J., Fan S. Polydatin inhibits growth of lung cancer cells by inducing apoptosis and causing cell cycle arrest. Oncol. Lett. 2014;7:295–301. doi: 10.3892/ol.2013.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y., Niu J., Li L., Li Z., Jiang J., Zhu M., Dong T., Zhang J., Shi C., Xu P. Polydatin executes anticancer effects against glioblastoma multiforme by inhibiting the EGFR-AKT/ERK1/2/STAT3-SOX2/Snail signaling pathway. Life Sci. 2020;258:118158. doi: 10.1016/j.lfs.2020.118158. [DOI] [PubMed] [Google Scholar]
- 98.Yang B., Zhao S. Polydatin regulates proliferation, apoptosis and autophagy in multiple myeloma cells through mTOR/p70s6k pathway. OncoTargets Ther. 2017;10:935. doi: 10.2147/OTT.S123398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pan J.-H., Wang H.-B., Du X.-F., Liu J.-Y., Zhang D.-J. Polydatin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling pathway. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2017;42:2345–2349. doi: 10.19540/j.cnki.cjcmm.2017.0111. [DOI] [PubMed] [Google Scholar]
- 100.Bai L., Ma Y., Wang X., Feng Q., Zhang Z., Wang S., Zhang H., Lu X., Xu Y., Zhao E. Polydatin Inhibits Cell Viability, Migration, and Invasion Through Suppressing the c-Myc Expression in Human Cervical Cancer. Front. Cell Dev. Biol. 2021;9:587218. doi: 10.3389/fcell.2021.587218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen S., Tao J., Zhong F., Jiao Y., Xu J., Shen Q., Wang H., Fan S., Zhang Y. Polydatin down-regulates the phosphorylation level of Creb and induces apoptosis in human breast cancer cell. PLoS ONE. 2017;12:e0176501. doi: 10.1371/journal.pone.0176501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang T., Zhu X., Wu H., Jiang K., Zhao G., Shaukat A., Deng G., Qiu C. Targeting the ROS/PI3K/AKT/HIF-1α/HK2 axis of breast cancer cells: Combined administration of Polydatin and 2-Deoxy-d-glucose. J. Cell. Mol. Med. 2019;23:3711–3723. doi: 10.1111/jcmm.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao W., Chen Z., Guan M. Polydatin enhances the chemosensitivity of osteosarcoma cells to paclitaxel. J. Cell. Biochem. 2019;120:17481–17490. doi: 10.1002/jcb.29012. [DOI] [PubMed] [Google Scholar]
- 104.Jiang C.-q., Ma L.-l., Lv Z.-d., Feng F., Chen Z., Liu Z.-D. Polydatin induces apoptosis and autophagy via STAT3 signaling in human osteosarcoma MG-63 cells. J. Nat. Med. 2020;74:533–544. doi: 10.1007/s11418-020-01399-5. [DOI] [PubMed] [Google Scholar]
- 105.Xu G., Kuang G., Jiang W., Jiang R., Jiang D. Polydatin promotes apoptosis through upregulation the ratio of Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin signaling in human osteosarcoma cells. Am. J. Transl. Res. 2016;8:922. [PMC free article] [PubMed] [Google Scholar]
- 106.Hu T., Fei Z., Su H., Xie R., Chen L. Polydatin inhibits proliferation and promotes apoptosis of doxorubicin-resistant osteosarcoma through LncRNA TUG1 mediated suppression of Akt signaling. Toxicol. Appl. Pharmacol. 2019;371:55–62. doi: 10.1016/j.taap.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 107.Jiao Y., Wu Y., Du D. Polydatin inhibits cell proliferation, invasion and migration, and induces cell apoptosis in hepatocellular carcinoma. Braz. J. Med. Biol. Res. 2018;51:e6867. doi: 10.1590/1414-431x20176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang J., Chen Y., Dong T., Yue M., Zhang Y., An T., Zhang J., Liu P., Yang X. Polydatin inhibits hepatocellular carcinoma via the AKT/STAT3-FOXO1 signaling pathway Corrigendum in /10.3892/ol.2019.10856. Oncol. Lett. 2019;17:4505–4513. doi: 10.3892/ol.2019.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bang T.-H., Park B.-S., Kang H.-M., Kim J.-H., Kim I.-R. Polydatin, a Glycoside of Resveratrol, Induces Apoptosis and Inhibits Metastasis Oral Squamous Cell Carcinoma Cells In Vitro. Pharmaceuticals. 2021;14:902. doi: 10.3390/ph14090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ince S., Acaroz D.A., Neuwirth O., Demirel H.H., Denk B., Kucukkurt I., Turkmen R. Protective effect of polydatin, a natural precursor of resveratrol, against cisplatin-induced toxicity in rats. Food Chem. Toxicol. 2014;72:147–153. doi: 10.1016/j.fct.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 111.Xu Z., Feng W., Shen Q., Yu N., Yu K., Wang S., Chen Z., Shioda S., Guo Y. Rhizoma Coptidis and Berberine as a Natural Drug to Combat Aging and Aging-Related Diseases via Anti-Oxidation and AMPK Activation. Aging Dis. 2017;8:760–777. doi: 10.14336/AD.2016.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fakhri S., Abbaszadeh F., Dargahi L., Jorjani M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018;136:1–20. doi: 10.1016/j.phrs.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 113.Goldstein L., Brown S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu. Rev. Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 114.Chen G., Liu G., Cao D., Jin M., Guo D., Yuan X. Polydatin protects against acute myocardial infarction-induced cardiac damage by activation of Nrf2/HO-1 signaling. J. Nat. Med. 2019;73:85–92. doi: 10.1007/s11418-018-1241-7. [DOI] [PubMed] [Google Scholar]
- 115.Ma Y., Gong X., Mo Y., Wu S. Polydatin inhibits the oxidative stress-induced proliferation of vascular smooth muscle cells by activating the eNOS/SIRT1 pathway. Int. J. Mol. Med. 2016;37:1652–1660. doi: 10.3892/ijmm.2016.2554. [DOI] [PubMed] [Google Scholar]
- 116.Miao Q., Shi X.-P., Ye M.-X., Zhang J., Miao S., Wang S.-W., Li B., Jiang X.-X., Zhang S., Hu N. Polydatin attenuates hypoxic pulmonary hypertension and reverses remodeling through protein kinase C mechanisms. Int. J. Mol. Sci. 2012;13:7776–7787. doi: 10.3390/ijms13067776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gugliandolo E., Fusco R., Biundo F., D’Amico R., Benedetto F., Di Paola R., Cuzzocrea S. Palmitoylethanolamide and Polydatin combination reduces inflammation and oxidative stress in vascular injury. Pharmacol. Res. 2017;123:83–92. doi: 10.1016/j.phrs.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 118.Yuan X., Chen G., Guo D., Xu L., Gu Y. Polydatin alleviates septic myocardial injury by promoting SIRT6-mediated autophagy. Inflammation. 2020;43:785–795. doi: 10.1007/s10753-019-01153-4. [DOI] [PubMed] [Google Scholar]
- 119.Gao J.P., Chen C.X., Gu W.L., Wu Q., Wang Y., Lü J. Effects of polydatin on attenuating ventricular remodeling in isoproterenol-induced mouse and pressure-overload rat models. Fitoterapia. 2010;81:953–960. doi: 10.1016/j.fitote.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 120.Gao Y., Gao J., Chen C., Wang H., Guo J., Wu R. Cardioprotective effect of polydatin on ventricular remodeling after myocardial infarction in coronary artery ligation rats. Planta Med. 2015;81:568–577. doi: 10.1055/s-0035-1545907. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Q., Tan Y., Zhang N., Yao F. Polydatin prevents angiotensin II-induced cardiac hypertrophy and myocardial superoxide generation. Exp. Biol. Med. 2015;240:1352–1361. doi: 10.1177/1535370214561958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang M., Wang S., Cheng Z., Xiong Z., Lv J., Yang Z., Li T., Jiang S., Gu J., Sun D. Polydatin ameliorates diabetic cardiomyopathy via Sirt3 activation. Biochem. Biophys. Res. Commun. 2017;493:1280–1287. doi: 10.1016/j.bbrc.2017.09.151. [DOI] [PubMed] [Google Scholar]
- 123.Pessina A., Di Vincenzo M., Maradonna F., Marchegiani F., Olivieri F., Randazzo B., Gioacchini G., Carnevali O. Polydatin Beneficial Effects in Zebrafish Larvae Undergoing Multiple Stress Types. Int. J. Environ. Res. Public Health. 2021;18:1116. doi: 10.3390/ijerph18031116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang B., Xue L., Wu Y., Jiang Q. Effect and mechanism of polydatin on diabetic myocardial hypertrophy in mice. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2015;40:4256–4261. [PubMed] [Google Scholar]
- 125.Du J., Sun L.-N., Xing W.-W., Huang B.-K., Jia M., Wu J.-Z., Zhang H., Qin L.-P. Lipid-lowering effects of polydatin from Polygonum cuspidatum in hyperlipidemic hamsters. Phytomedicine. 2009;16:652–658. doi: 10.1016/j.phymed.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 126.Xing W.-W., Wu J.-Z., Jia M., Du J., Zhang H., Qin L.-P. Effects of polydatin from Polygonum cuspidatum on lipid profile in hyperlipidemic rabbits. Biomed. Pharmacother. 2009;63:457–462. doi: 10.1016/j.biopha.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 127.Peng Y., Xu J., Zeng Y., Chen L., Le Xu X. Polydatin attenuates atherosclerosis in apolipoprotein E-deficient mice: Role of reverse cholesterol transport. Phytomedicine. 2019;62:152935. doi: 10.1016/j.phymed.2019.152935. [DOI] [PubMed] [Google Scholar]
- 128.Huang Z., Tian G., Cheng S., Zhao D., Zhang Y., Jia Y., Zhou F. Polydatin attenuates atherosclerosis in ApoE−/− mice through PBEF mediated reduction of cholesterol deposition. Am. J. Chin. Med. 2018;46:1841–1859. doi: 10.1142/S0192415X18500921. [DOI] [PubMed] [Google Scholar]
- 129.Xiong Q., Yan Z., Liang J., Yuan J., Chen X., Zhou L., Hu Y., Wu J., Jing Y., Zhang Q. Polydatin alleviates high-fat diet induced atherosclerosis in apolipoprotein E-deficient mice by autophagic restoration. Phytomedicine. 2021;81:153301. doi: 10.1016/j.phymed.2020.153301. [DOI] [PubMed] [Google Scholar]
- 130.Zheng L., Wu J., Mo J., Guo L., Wu X., Bao Y. Polydatin inhibits adipose tissue inflammation and ameliorates lipid metabolism in high-fat-fed mice. BioMed Res. Int. 2019;2019:7196535. doi: 10.1155/2019/7196535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu M., Liu M., Guo G., Zhang W., Liu L. Polydatin inhibits formation of macrophage-derived foam cells. Evid. Based Complement. Altern. Med. 2015;2015:729017. doi: 10.1155/2015/729017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang L.-P., Ma H.-J., Bu H.-M., Wang M.-L., Li Q., Qi Z., Zhang Y. Polydatin attenuates ischemia/reperfusion-induced apoptosis in myocardium of the rat. Sheng Li Xue Bao. 2009;61:367–372. [PubMed] [Google Scholar]
- 133.Zhang L.-P., Yang C.-Y., Wang Y.-P., Cui F., Zhang Y. Protective effect of polydatin against ischemia/reperfusion injury in rat heart. Sheng Li Xue Bao [Acta Physiol. Sin.] 2008;60:161–168. [PubMed] [Google Scholar]
- 134.Ling Y., Chen G., Deng Y., Tang H., Ling L., Zhou X., Song X., Yang P., Liu Y., Li Z. Polydatin post-treatment alleviates myocardial ischaemia/reperfusion injury by promoting autophagic flux. Clin. Sci. 2016;130:1641–1653. doi: 10.1042/CS20160082. [DOI] [PubMed] [Google Scholar]
- 135.Jiang X., Liu W., Deng J., Lan L., Xue X., Zhang C., Cai G., Luo X., Liu J. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic. Biol. Med. 2013;60:292–299. doi: 10.1016/j.freeradbiomed.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 136.Yousef A.I., Shawki H.H., El-Shahawy A.A., Abd El-Twab S.M., Abdel-Moneim A., Oishi H. Polydatin mitigates pancreatic β-cell damage through its antioxidant activity. Biomed. Pharmacother. 2021;133:111027. doi: 10.1016/j.biopha.2020.111027. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Q., Tan Y., Zhang N., Yao F. Polydatin supplementation ameliorates diet-induced development of insulin resistance and hepatic steatosis in rats. Mol. Med. Rep. 2015;11:603–610. doi: 10.3892/mmr.2014.2708. [DOI] [PubMed] [Google Scholar]
- 138.Xie X., Peng J., Huang K., Huang J., Shen X., Liu P., Huang H. Polydatin ameliorates experimental diabetes-induced fibronectin through inhibiting the activation of NF-κB signaling pathway in rat glomerular mesangial cells. Mol. Cell. Endocrinol. 2012;362:183–193. doi: 10.1016/j.mce.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 139.Chen C., Huang K., Hao J., Huang J., Yang Z., Xiong F., Liu P., Huang H. Polydatin attenuates AGEs-induced upregulation of fibronectin and ICAM-1 in rat glomerular mesangial cells and db/db diabetic mice kidneys by inhibiting the activation of the SphK1-S1P signaling pathway. Mol. Cell. Endocrinol. 2016;427:45–56. doi: 10.1016/j.mce.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 140.Wang Y., Ye J., Li J., Chen C., Huang J., Liu P., Huang H. Polydatin ameliorates lipid and glucose metabolism in type 2 diabetes mellitus by downregulating proprotein convertase subtilisin/kexin type 9 (PCSK9) Cardiovasc. Diabetol. 2016;15:1–13. doi: 10.1186/s12933-015-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hao J., Chen C., Huang K., Huang J., Li J., Liu P., Huang H. Polydatin improves glucose and lipid metabolism in experimental diabetes through activating the Akt signaling pathway. Eur. J. Pharmacol. 2014;745:152–165. doi: 10.1016/j.ejphar.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 142.Wu Y., Xue L., Du W., Huang B., Tang C., Liu C., Qiu H., Jiang Q. Polydatin restores endothelium-dependent relaxation in rat aorta rings impaired by high glucose: A Novel insight into the PPARβ-NO signaling pathway. PLoS ONE. 2015;10:e0126249. doi: 10.1371/journal.pone.0126249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang D., Zhou Z., Yuan L. Polydatin reverses oxidation low lipoprotein (oxLDL)-induced apoptosis of human umbilical vein endothelial cells via regulating the miR-26a-5p/BID axis. Eur. J. Histochem. 2022;66 doi: 10.4081/ejh.2022.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bheereddy P., Yerra V.G., Kalvala A.K., Sherkhane B., Kumar A. SIRT1 activation by polydatin alleviates oxidative damage and elevates mitochondrial biogenesis in experimental diabetic neuropathy. Cell. Mol. Neurobiol. 2020;41:1563–1577. doi: 10.1007/s10571-020-00923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu B., Li S., Sui X., Guo L., Liu X., Li H., Gao L., Cai S., Li Y., Wang T. Root extract of Polygonum cuspidatum Siebold & Zucc. ameliorates DSS-induced ulcerative colitis by affecting NF-kappaB signaling pathway in a mouse model via synergistic effects of polydatin, resveratrol, and emodin. Front. Pharmacol. 2018;9:347. doi: 10.3389/fphar.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yao J., Wang J.-Y., Liu L., Zeng W.-S., Li Y.-X., Xun A.-Y., Zhao L., Jia C.-H., Feng J.-L., Wei X.-X. Polydatin ameliorates DSS-induced colitis in mice through inhibition of nuclear factor-kappaB activation. Planta Med. 2011;77:421–427. doi: 10.1055/s-0030-1250462. [DOI] [PubMed] [Google Scholar]
- 147.Zeng Z., Yang Y., Dai X., Xu S., Li T., Zhang Q., Zhao K.-s., Chen Z. Polydatin ameliorates injury to the small intestine induced by hemorrhagic shock via SIRT3 activation-mediated mitochondrial protection. Expert Opin. Ther. Targets. 2016;20:645–652. doi: 10.1080/14728222.2016.1177023. [DOI] [PubMed] [Google Scholar]
- 148.Li P., Wang X., Zhao M., Song R., Zhao K.-s. Polydatin protects hepatocytes against mitochondrial injury in acute severe hemorrhagic shock via SIRT1-SOD2 pathway. Expert Opin. Ther. Targets. 2015;19:997–1010. doi: 10.1517/14728222.2015.1054806. [DOI] [PubMed] [Google Scholar]
- 149.Zhao G., Jiang K., Wu H., Qiu C., Deng G., Peng X. Polydatin reduces Staphylococcus aureus lipoteichoic acid-induced injury by attenuating reactive oxygen species generation and TLR 2-NF κB signalling. J. Cell. Mol. Med. 2017;21:2796–2808. doi: 10.1111/jcmm.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qiu Y., Pan X., Hu Y. Polydatin ameliorates pulmonary fibrosis by suppressing inflammation and the epithelial mesenchymal transition via inhibiting the TGF-β/Smad signaling pathway. RSC Adv. 2019;9:8104–8112. doi: 10.1039/C8RA08659A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li X.-h., Gong X., Zhang L., Jiang R., Li H.-z., Wu M.-j., Wan J.-y. Protective effects of polydatin on septic lung injury in mice via upregulation of HO-1. Mediat. Inflamm. 2013;2013:354087. doi: 10.1155/2013/354087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ricordi C., Pacifici F., Lanzoni G., Palamara A.T., Garaci E., Della-Morte D. Dietary and protective factors to halt or mitigate progression of autoimmunity, COVID-19 and its associated metabolic diseases. Int. J. Mol. Sci. 2021;22:3134. doi: 10.3390/ijms22063134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lai Y., Zhou C., Huang P., Dong Z., Mo C., Xie L., Lin H., Zhou Z., Deng G., Liu Y. Polydatin alleviated alcoholic liver injury in zebrafish larvae through ameliorating lipid metabolism and oxidative stress. J. Pharmacol. Sci. 2018;138:46–53. doi: 10.1016/j.jphs.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 154.Chen X., Chan H., Zhang L., Liu X., Ho I.H., Zhang X., Ho J., Hu W., Tian Y., Kou S. The phytochemical polydatin ameliorates non-alcoholic steatohepatitis by restoring lysosomal function and autophagic flux. J. Cell. Mol. Med. 2019;23:4290–4300. doi: 10.1111/jcmm.14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luo J.-L., Chen S., Wang L., Zhao X.-H., Piao C.-L. Pharmacological effects of polydatin in the treatment of metabolic diseases: A review. Phytomedicine. 2022:154161. doi: 10.1016/j.phymed.2022.154161. [DOI] [PubMed] [Google Scholar]
- 156.Zhang J., Tan Y., Yao F., Zhang Q. Polydatin alleviates non-alcoholic fatty liver disease in rats by inhibiting the expression of TNF-α and SREBP-1c. Mol. Med. Rep. 2012;6:815–820. doi: 10.3892/mmr.2012.1015. [DOI] [PubMed] [Google Scholar]
- 157.Li R., Li J., Huang Y., Li H., Yan S., Lin J., Chen Y., Wu L., Liu B., Wang G. Polydatin attenuates diet-induced nonalcoholic steatohepatitis and fibrosis in mice. Int. J. Biol. Sci. 2018;14:1411. doi: 10.7150/ijbs.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang H., Chen Y., Pei Z., Gao H., Shi W., Sun M., Xu Q., Zhao J., Meng W., Xiao K. Protective effects of polydatin against sulfur mustard-induced hepatic injury. Toxicol. Appl. Pharmacol. 2019;367:1–11. doi: 10.1016/j.taap.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 159.Zhao X., Li R., Liu Y., Zhang X., Zhang M., Zeng Z., Wu L., Gao X., Lan T., Wang Y. Polydatin protects against carbon tetrachloride-induced liver fibrosis in mice. Arch. Biochem. Biophys. 2017;629:1–7. doi: 10.1016/j.abb.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 160.Huang Z.-S., Wang Z.-W., Liu M.-P., Zhong S.-Q., Li Q.-M., Rong X.-L. Protective effects of polydatin against CCl4-induced injury to primarily cultured rat hepatocytes. World J. Gastroenterol. 1999;5:41. doi: 10.3748/wjg.v5.i1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zeng Z., Chen Z., Li T., Zhang J., Gao Y., Xu S., Cai S., Zhao K.S. Polydatin: A new therapeutic agent against multiorgan dysfunction. J. Surg. Res. 2015;198:192–199. doi: 10.1016/j.jss.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 162.Dumurgier J., Tzourio C. Epidemiology of neurological diseases in older adults. Rev. Neurol. 2020;176:642–648. doi: 10.1016/j.neurol.2020.01.356. [DOI] [PubMed] [Google Scholar]
- 163.Chen Y., Zhang D.-q., Liao Z., Wang B., Gong S., Wang C., Zhang M.-z., Wang G.-h., Cai H., Liao F.-F. Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease. Mol. Neurodegener. 2015;10:1–14. doi: 10.1186/1750-1326-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao X., Qin J., Li H., Feng X., Lv Y., Yang J. Effect of Polydatin on Neurological Function and the Nrf2 Pathway during Intracerebral Hemorrhage. J. Mol. Neurosci. 2020;70:1332–1337. doi: 10.1007/s12031-020-01546-3. [DOI] [PubMed] [Google Scholar]
- 165.Wang X., Song R., Chen Y., Zhao M., Zhao K.-s. Polydatin–a new mitochondria protector for acute severe hemorrhagic shock treatment. Expert Opin. Investig. Drugs. 2013;22:169–179. doi: 10.1517/13543784.2013.748033. [DOI] [PubMed] [Google Scholar]
- 166.Wang X., Song R., Bian H.N., Brunk U.T., Zhao M., Zhao K.-s. Polydatin, a natural polyphenol, protects arterial smooth muscle cells against mitochondrial dysfunction and lysosomal destabilization following hemorrhagic shock. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R805–R814. doi: 10.1152/ajpregu.00350.2011. [DOI] [PubMed] [Google Scholar]
- 167.Li R.-P., Wang Z.-Z., Sun M.-X., Hou X.-L., Sun Y., Deng Z.-F., Xiao K. Polydatin protects learning and memory impairments in a rat model of vascular dementia. Phytomedicine. 2012;19:677–681. doi: 10.1016/j.phymed.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 168.Huang L., He S., Cai Q., Li F., Wang S., Tao K., Xi Y., Qin H., Gao G., Feng D. Polydatin alleviates traumatic brain injury: Role of inhibiting ferroptosis. Biochem. Biophys. Res. Commun. 2021;556:149–155. doi: 10.1016/j.bbrc.2021.03.108. [DOI] [PubMed] [Google Scholar]
- 169.Huang B., Liu J., Meng T., Li Y., He D., Ran X., Chen G., Guo W., Kan X., Fu S. Polydatin prevents lipopolysaccharide (LPS)-induced Parkinson’s disease via regulation of the AKT/GSK3β-Nrf2/NF-κB signaling axis. Front. Immunol. 2018;9:2527. doi: 10.3389/fimmu.2018.02527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bai H., Ding Y., Li X., Kong D., Xin C., Yang X., Zhang C., Rong Z., Yao C., Lu S. Polydatin protects SH-SY5Y in models of Parkinson’s disease by promoting Atg5-mediated but parkin-independent autophagy. Neurochem. Int. 2020;134:104671. doi: 10.1016/j.neuint.2020.104671. [DOI] [PubMed] [Google Scholar]
- 171.Zhang S., Wang S., Shi X., Feng X. Polydatin alleviates parkinsonism in MPTP-model mice by enhancing glycolysis in dopaminergic neurons. Neurochem. Int. 2020;139:104815. doi: 10.1016/j.neuint.2020.104815. [DOI] [PubMed] [Google Scholar]
- 172.Schimith L.E., Dos Santos M.G., Arbo B.D., André-Miral C., Muccillo-Baisch A.L., Hort M.A. Polydatin as a therapeutic alternative for central nervous system disorders: A systematic review of animal studies. Phytother. Res. PTR. 2022;36:2852–2877. doi: 10.1002/ptr.7497. [DOI] [PubMed] [Google Scholar]
- 173.Gao Y., Chen T., Lei X., Li Y., Dai X., Cao Y., Ding Q., Lei X., Li T., Lin X. Neuroprotective effects of polydatin against mitochondrial-dependent apoptosis in the rat cerebral cortex following ischemia/reperfusion injury. Mol. Med. Rep. 2016;14:5481–5488. doi: 10.3892/mmr.2016.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bonucci M., Raggi R., Vacca R.A. Polydatin and its potential protective effect on COVID-19. Clin. Nutr. 2020;39:3850–3851. doi: 10.1016/j.clnu.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cheng Y., Zhang H.-T., Sun L., Guo S., Ouyang S., Zhang Y., Xu J. Involvement of cell adhesion molecules in polydatin protection of brain tissues from ischemia–reperfusion injury. Brain Res. 2006;1110:193–200. doi: 10.1016/j.brainres.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 176.Chen F., Fang X., Zhang H. Effect of polydatin on expression of p53 and Notch1 in brain tissue of ischemic cerebrovascular disease. J. Biol. Regul. Homeost. Agents. 2018;32:133–138. [PubMed] [Google Scholar]
- 177.Xu C.-Y., Li S., Hao W., Zhang R.-L. Effect of polydatin on expression of CDK5 in the prefrontal cortex of rats with chronic alcoholism. Zhongguo Ying Yong Sheng Li Xue Za Zhi Chin. J. Appl. Physiol. 2012;28:158–188. [PubMed] [Google Scholar]
- 178.Sun J., Qu Y., He H., Fan X., Qin Y., Mao W., Xu L. Protective effect of polydatin on learning and memory impairments in neonatal rats with hypoxic-ischemic brain injury by up-regulating brain-derived neurotrophic factor. Mol. Med. Rep. 2014;10:3047–3051. doi: 10.3892/mmr.2014.2577. [DOI] [PubMed] [Google Scholar]
- 179.Chen M., Hou Y., Lin D. Polydatin protects bone marrow stem cells against oxidative injury: Involvement of Nrf 2/ARE pathways. Stem Cells Int. 2016;2016:9394150. doi: 10.1155/2016/9394150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhan J., Li X., Luo D., Yan W., Hou Y., Hou Y., Chen S., Luan J., Zhang Q., Lin D. Polydatin Attenuates OGD/R-Induced Neuronal Injury and Spinal Cord Ischemia/Reperfusion Injury by Protecting Mitochondrial Function via Nrf2/ARE Signaling Pathway. Oxidative Med. Cell. Longev. 2021;2021:6687212. doi: 10.1155/2021/6687212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhan J., Li X., Luo D., Hou Y., Hou Y., Chen S., Xiao Z., Luan J., Lin D. Polydatin promotes the neuronal differentiation of bone marrow mesenchymal stem cells in vitro and in vivo: Involvement of Nrf2 signalling pathway. J. Cell. Mol. Med. 2020;24:5317–5329. doi: 10.1111/jcmm.15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu H., Zhang G., Bie X., Liu M., Yang J., Wan H., Zhang Y. Effect of polydatin on dynamic changes of excitatory amino acids in cerebrospinal fluid of cerebral hemorrhage rats. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2010;35:3038–3042. [PubMed] [Google Scholar]
- 183.Maoka T., Yasui H., Ohmori A., Tokuda H., Suzuki N., Osawa A., Shindo K., Ishibashi T. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J. Oleo Sci. 2012;62:181–186. doi: 10.5650/jos.62.181. [DOI] [PubMed] [Google Scholar]
- 184.Wiwanitkit V. Polydatin and COVID-19. Clin. Nutr. 2021 doi: 10.1016/j.clnu.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen Z.-q., Sun X.-h., Li X.-j., Xu Z.-c., Yang Y., Lin Z.-y., Xiao H.-m., Zhang M., Quan S.-j., Huang H.-q. Polydatin attenuates renal fibrosis in diabetic mice through regulating the Cx32-Nox4 signaling pathway. Acta Pharmacol. Sin. 2020;41:1587–1596. doi: 10.1038/s41401-020-0475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gu L., Liu J., Xu D., Lu Y. Polydatin prevents LPS-induced acute kidney injury through inhibiting inflammatory and oxidative responses. Microb. Pathog. 2019;137:103688. doi: 10.1016/j.micpath.2019.103688. [DOI] [PubMed] [Google Scholar]
- 187.Gao Y., Dai X., Li Y., Li G., Lin X., Ai C., Cao Y., Li T., Lin B. Role of Parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J. Transl. Med. 2020;18:114. doi: 10.1186/s12967-020-02283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Han B., Gong M., Li Z., Qiu Y., Zou Z. NMR-based metabonomic study reveals intervention effects of polydatin on potassium oxonate-induced hyperuricemia in rats. Oxidative Med. Cell. Longev. 2020;2020:6943860. doi: 10.1155/2020/6943860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sun Z., Wang X., Xu Z. SIRT1 provides new pharmacological targets for polydatin through its role as a metabolic sensor. Biomed. Pharmacother. 2021;139:111549. doi: 10.1016/j.biopha.2021.111549. [DOI] [PubMed] [Google Scholar]
- 190.Gao Y., Zeng Z., Li T., Xu S., Wang X., Chen Z., Lin C. Polydatin inhibits mitochondrial dysfunction in the renal tubular epithelial cells of a rat model of sepsis-induced acute kidney injury. Anesth. Analg. 2015;121:1251–1260. doi: 10.1213/ANE.0000000000000977. [DOI] [PubMed] [Google Scholar]
- 191.Zeng Z., Chen Z., Xu S., Zhang Q., Wang X., Gao Y., Zhao K.-s. Polydatin protecting kidneys against hemorrhagic shock-induced mitochondrial dysfunction via SIRT1 activation and p53 deacetylation. Oxidative Med. Cell. Longev. 2016;2016:1737185. doi: 10.1155/2016/1737185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gu T.-T., Zhang D.-M., Wan Z.-Y., Li T.-S., Jiao R.-Q., Chen T.-Y., Zhao X.-J., Kong L.-D. Polydatin enhances glomerular podocyte autophagy homeostasis by improving Nrf2-dependent antioxidant capacity in fructose-fed rats. Mol. Cell. Endocrinol. 2021;520:111079. doi: 10.1016/j.mce.2020.111079. [DOI] [PubMed] [Google Scholar]
- 193.Cao K., Lei X., Liu H., Zhao H., Guo J., Chen Y., Xu Y., Cheng Y., Liu C., Cui J. Polydatin alleviated radiation-induced lung injury through activation of Sirt3 and inhibition of epithelial–mesenchymal transition. J. Cell. Mol. Med. 2017;21:3264–3276. doi: 10.1111/jcmm.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hanna D.A., Khalaf M.M., Abo-Saif A.A. Polydatin protects against ovalbumin-induced bronchial asthma in rats; involvement of urocortin and surfactant-D expression. Immunopharmacol. Immunotoxicol. 2019;41:403–412. doi: 10.1080/08923973.2018.1536985. [DOI] [PubMed] [Google Scholar]
- 195.Zeng H., Wang Y., Gu Y., Wang J., Zhang H., Gao H., Jin Q., Zhao L. Polydatin attenuates reactive oxygen species-induced airway remodeling by promoting Nrf2-mediated antioxidant signaling in asthma mouse model. Life Sci. 2019;218:25–30. doi: 10.1016/j.lfs.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 196.Liu Y.-L., Chen B.-Y., Nie J., Zhao G.-H., Zhuo J.-Y., Yuan J., Li Y.-C., Wang L.-L., Chen Z.-W. Polydatin prevents bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β/Smad/ERK signaling pathway. Exp. Ther. Med. 2020;20:1. doi: 10.3892/etm.2020.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jin X.-F., Xu Z.-J., Wang W.-T., Xu Y.-X., Zhang X.-L. The regulative effects of polydatin on toll-like receptor 4 signal transduction pathway in lung ischemia/reperfusion injury in rabbits. Zhongguo Ying Yong Sheng Li Xue Za Zhi Chin. J. Appl. Physiol. 2009;25:41–44. [PubMed] [Google Scholar]
- 198.Wang F.-Y., Xu Z.-J., Zhang X.-L., Wang W.-T., Ha M.-L., Wang Y. Protective effects of polydatin against lung ischemia/reperfusion injury and the initial exploration for its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi Chin. J. Appl. Physiol. 2008;24:62–65. [PubMed] [Google Scholar]
- 199.Li T., Liu Y., Li G., Wang X., Zeng Z., Cai S., Li F., Chen Z. Polydatin attenuates ipopolysaccharide-induced acute lung injury in rats. Int. J. Clin. Exp. Pathol. 2014;7:8401. [PMC free article] [PubMed] [Google Scholar]
- 200.Li T., Cai S., Zeng Z., Zhang J., Gao Y., Wang X., Chen Z. Protective effect of polydatin against burn-induced lung injury in rats. Respir. Care. 2014;59:1412–1421. doi: 10.4187/respcare.02831. [DOI] [PubMed] [Google Scholar]
- 201.Matacchione G., Gurău F., Silvestrini A., Tiboni M., Mancini L., Valli D., Rippo M.R., Recchioni R., Marcheselli F., Carnevali O., et al. Anti-SASP and anti-inflammatory activity of resveratrol, curcumin and β-caryophyllene association on human endothelial and monocytic cells. Biogerontology. 2021;22:297–313. doi: 10.1007/s10522-021-09915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jiang Q., Yi M., Guo Q., Wang C., Wang H., Meng S., Liu C., Fu Y., Ji H., Chen T. Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-κB pathway. Int. Immunopharmacol. 2015;29:370–376. doi: 10.1016/j.intimp.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 203.Shu S., Wang X., Ling Z., Lu Z. Effect of polydatin on phospholipase A2 in lung tissues in rats with endotoxic shock. Chin. J. Traumatol. 2004;7:239–243. [PubMed] [Google Scholar]
- 204.Zhang Y., Chen L., Liang B. Effects of polydatin on bleomycin-induced pulmonary fibrosis in rats. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2011;36:3528–3534. [PubMed] [Google Scholar]
- 205.Fu Y., Yan M., Xie C., Hu J., Zeng X., Hu Q. Polydatin relieves paraquat-induced human MRC-5 fibroblast injury through inhibiting the activation of the NLRP3 inflammasome. Ann. Transl. Med. 2020;8:765. doi: 10.21037/atm-20-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li B., Wang X.L. Effective treatment of polydatin weakens the symptoms of collagen-induced arthritis in mice through its anti-oxidative and anti-inflammatory effects and the activation of MMP-9. Mol. Med. Rep. 2016;14:5357–5362. doi: 10.3892/mmr.2016.5903. [DOI] [PubMed] [Google Scholar]
- 207.Liao P., He Y., Yang F., Luo G., Zhuang J., Zhai Z., Zhuang L., Lin Z., Zheng J., Sun E. Polydatin effectively attenuates disease activity in lupus-prone mouse models by blocking ROS-mediated NET formation. Arthritis Res. Ther. 2018;20:1–11. doi: 10.1186/s13075-018-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang F., Luo X., Luo G., Zhai Z., Zhuang J., He J., Han J., Zhang Y., Zhuang L., Sun E. Inhibition of NET formation by polydatin protects against collagen-induced arthritis. Int. Immunopharmacol. 2019;77:105919. doi: 10.1016/j.intimp.2019.105919. [DOI] [PubMed] [Google Scholar]
- 209.Zhou Q.L., Qin R.Z., Yang Y.X., Huang K.B., Yang X.W. Polydatin possesses notable anti-osteoporotic activity via regulation of OPG, RANKL and β-catenin. Mol. Med. Rep. 2016;14:1865–1869. doi: 10.3892/mmr.2016.5432. [DOI] [PubMed] [Google Scholar]
- 210.Shen Y.-S., Chen X.-J., Wuri S.-N., Yang F., Pang F.-X., Xu L.-L., He W., Wei Q.-S. Polydatin improves osteogenic differentiation of human bone mesenchymal stem cells by stimulating TAZ expression via BMP2-Wnt/β-catenin signaling pathway. Stem Cell Res. Ther. 2020;11:204. doi: 10.1186/s13287-020-01705-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 211.Di Benedetto A., Posa F., De Maria S., Ravagnan G., Ballini A., Porro C., Trotta T., Grano M., Muzio L.L., Mori G. Polydatin, natural precursor of resveratrol, promotes osteogenic differentiation of mesenchymal stem cells. Int. J. Med. Sci. 2018;15:944. doi: 10.7150/ijms.24111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen Z., Wei Q., Hong G., Chen D., Liang J., He W., Chen M.H. Polydatin induces bone marrow stromal cells migration by activation of ERK1/2. Biomed. Pharmacother. 2016;82:49–53. doi: 10.1016/j.biopha.2016.04.059. [DOI] [PubMed] [Google Scholar]
- 213.Ma C., Wen B., Zhang Q., Shao P., Gu W., Qu K., Shi Y., Wang B. Polydatin regulates the apoptosis and autophagy of fibroblasts obtained from patients with ankylosing spondylitis. Biol. Pharm. Bull. 2018;42:50–56. doi: 10.1248/bpb.b18-00522. [DOI] [PubMed] [Google Scholar]
- 214.Wang J., Huang C., Lin Z., Pan X., Chen J., Zheng G., Tian N., Yan Y., Zhang Z., Hu J. Polydatin suppresses nucleus pulposus cell senescence, promotes matrix homeostasis and attenuates intervertebral disc degeneration in rats. J. Cell. Mol. Med. 2018;22:5720–5731. doi: 10.1111/jcmm.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Indraccolo U., Indraccolo S.R., Mignini F. Micronized palmitoylethanolamide/trans-polydatin treatment of endometriosis-related pain: A meta-analysis. Ann. Dell’istituto Super. Sanita. 2017;53:125–134. doi: 10.4415/ANN_17_02_08. [DOI] [PubMed] [Google Scholar]
- 216.Indraccolo U., Barbieri F. Effect of palmitoylethanolamide–polydatin combination on chronic pelvic pain associated with endometriosis: Preliminary observations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010;150:76–79. doi: 10.1016/j.ejogrb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 217.Di Paola R., Fusco R., Gugliandolo E., Crupi R., Evangelista M., Granese R., Cuzzocrea S. Co-micronized palmitoylethanolamide/polydatin treatment causes endometriotic lesion regression in a rodent model of surgically induced endometriosis. Front. Pharmacol. 2016;7:382. doi: 10.3389/fphar.2016.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Loi E.S., Pontis A., Cofelice V., Pirarba S., Fais M.F., Daniilidis A., Melis I., Paoletti A.M., Angioni S. Effect of ultramicronized-palmitoylethanolamide and co-micronized palmitoylethanolamide/polydatin on chronic pelvic pain and quality of life in endometriosis patients: An open-label pilot study. Int. J. Women’s Health. 2019;11:443. doi: 10.2147/IJWH.S204275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sheng C., Yu Y.-h., Zhao K.-s., Qin W., Wang C.-h. Hypotensive resuscitation combined with polydatin improve microcirculation and survival in a rabbit model of uncontrolled hemorrhagic shock in pregnancy. J. Surg. Res. 2011;168:103–110. doi: 10.1016/j.jss.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 220.Ou C., Song H., Zhou Y., Peng J., Peng Q. Exploring the Molecular Mechanism of Qing Guang An Granule in Treating Glaucoma Using Network Pharmacology and Molecular Docking. Evid. Based Complement. Altern. Med. Ecam. 2020;2020:8824150. doi: 10.1155/2020/8824150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ye J., Piao H., Jiang J., Jin G., Zheng M., Yang J., Jin X., Sun T., Choi Y.H., Li L. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci. Rep. 2017;7:11895. doi: 10.1038/s41598-017-12252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yuan M., Li J., Lv J., Mo X., Yang C., Chen X., Liu Z., Liu J. Polydatin (PD) inhibits IgE-mediated passive cutaneous anaphylaxis in mice by stabilizing mast cells through modulating Ca2+ mobilization. Toxicol. Appl. Pharmacol. 2012;264:462–469. doi: 10.1016/j.taap.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 223.Yang B., Li J.-J., Cao J.-J., Yang C.-B., Liu J., Ji Q.-M., Liu Z.-G. Polydatin attenuated food allergy via store-operated calcium channels in mast cell. World J. Gastroenterol. WJG. 2013;19:3980. doi: 10.3748/wjg.v19.i25.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cordaro M., Impellizzeri D., Siracusa R., Gugliandolo E., Fusco R., Inferrera A., Esposito E., Di Paola R., Cuzzocrea S. Effects of a co-micronized composite containing palmitoylethanolamide and polydatin in an experimental model of benign prostatic hyperplasia. Toxicol. Appl. Pharmacol. 2017;329:231–240. doi: 10.1016/j.taap.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 225.Guan S.-Y., Zhang K., Wang X.-S., Yang L., Feng B., Tian D.-D., Gao M.-R., Liu S.-B., Liu A., Zhao M.-G. Anxiolytic effects of polydatin through the blockade of neuroinflammation in a chronic pain mouse model. Mol. Pain. 2020;16:1744806919900717. doi: 10.1177/1744806919900717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li H., Min J., Chen Y., Li H., Zhang Y. Polydatin attenuates orbital oxidative stress in Graves’ orbitopathy through the NRF2 pathway. Chem. Biol. Interact. 2020;315:108894. doi: 10.1016/j.cbi.2019.108894. [DOI] [PubMed] [Google Scholar]
- 227.Tang S., Tang Q., Jin J., Zheng G., Xu J., Huang W., Li X., Shang P., Liu H. Polydatin inhibits the IL-1β-induced inflammatory response in human osteoarthritic chondrocytes by activating the Nrf2 signaling pathway and ameliorates murine osteoarthritis. Food Funct. 2018;9:1701–1712. doi: 10.1039/C7FO01555K. [DOI] [PubMed] [Google Scholar]
- 228.Xu L.-Q., Xie Y.-L., Gui S.-H., Zhang X., Mo Z.-Z., Sun C.-Y., Li C.-L., Luo D.-D., Zhang Z.-B., Su Z.-R. Polydatin attenuates d-galactose-induced liver and brain damage through its anti-oxidative, anti-inflammatory and anti-apoptotic effects in mice. Food Funct. 2016;7:4545–4555. doi: 10.1039/C6FO01057A. [DOI] [PubMed] [Google Scholar]
- 229.Ravagnan G., De Filippis A., Cartenì M., De Maria S., Cozza V., Petrazzuolo M., Tufano M.A., Donnarumma G. Polydatin, a natural precursor of resveratrol, induces β-defensin production and reduces inflammatory response. Inflammation. 2013;36:26–34. doi: 10.1007/s10753-012-9516-8. [DOI] [PubMed] [Google Scholar]
- 230.Fuggetta M.P., Migliorino M.R., Ricciardi S., Osman G., Iacono D., Leone A., Lombardi A., Ravagnan G., Greco S., Remotti D. Prophylactic dermatologic treatment of afatinib-induced skin toxicities in patients with metastatic lung cancer: A pilot study. Scientifica. 2019;2019:9136249. doi: 10.1155/2019/9136249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cervigni M., Nasta L., Schievano C., Lampropoulou N., Ostardo E. Micronized palmitoylethanolamide-polydatin reduces the painful symptomatology in patients with interstitial cystitis/bladder pain syndrome. BioMed Res. Int. 2019;2019:9828397. doi: 10.1155/2019/9828397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen L., Chen Z., Xu Z., Feng W., Yang X., Qi Z. Polydatin protects Schwann cells from methylglyoxal induced cytotoxicity and promotes crushed sciatic nerves regeneration of diabetic rats. Phytother. Res. 2021;35:4592–4604. doi: 10.1002/ptr.7177. [DOI] [PubMed] [Google Scholar]
- 233.Soave I., Marci R. Administration of micronized palmitoylethanolamide (PEA)-transpolydatin in the treatment of chronic pelvic pain in women affected by endometriosis: Preliminary results. Minerva Ginecol. 2013;65:453–463. [PubMed] [Google Scholar]
- 234.Cremon C., Stanghellini V., Barbaro M., Cogliandro R., Bellacosa L., Santos J., Vicario M., Pigrau M., Alonso Cotoner C., Lobo B. Randomised clinical trial: The analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2017;45:909–922. doi: 10.1111/apt.13958. [DOI] [PubMed] [Google Scholar]
- 235.Pace M.C., Passavanti M.B., Aurilio C., Sansone P., Aurilio R., De Maria S., Lama S., Federico A., Ravagnan G., Caraglia M. Polydatin administration improves serum biochemical parameters and oxidative stress markers during chronic alcoholism: A pilot study. Vivo. 2015;29:405–408. [PubMed] [Google Scholar]
- 236.Biswas P., Dellanoce C., Vezzoli A., Mrakic-Sposta S., Malnati M., Beretta A., Accinni R. Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a Combination of Glutathione and Resveratrol Precursors. Nutrients. 2020;12:3224. doi: 10.3390/nu12113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bavetta M., Silvaggio D., Campione E., Sollena P., Formica V., Coletta D., Graziani G., Romano MC P., Roselli M., Peris K. The Effects of Association of Topical Polydatin Improves the Preemptive Systemic Treatment on EGFR Inhibitors Cutaneous Adverse Reactions. J. Clin. Med. 2021;10:466. doi: 10.3390/jcm10030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mostafa F., Abdel-Moneim A., Abdul-Hamid M., Galaly S.R., Mohamed H.M. Polydatin and polydatin-loaded chitosan nanoparticles attenuate diabetic cardiomyopathy in rats. J. Mol. Histol. 2021;52:135–152. doi: 10.1007/s10735-020-09930-4. [DOI] [PubMed] [Google Scholar]
- 239.Wang X., Guan Q., Chen W., Hu X., Li L. Novel nanoliposomal delivery system for polydatin: Preparation, characterization, and in vivo evaluation. Drug Des. Dev. Ther. 2015;9:1805. doi: 10.2147/DDDT.S77615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lin L., Gong H., Li R., Huang J., Cai M., Lan T., Huang W., Guo Y., Zhou Z., An Y. Nanodrug with ROS and pH Dual-Sensitivity Ameliorates Liver Fibrosis via Multicellular Regulation. Adv. Sci. 2020;7:1903138. doi: 10.1002/advs.201903138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xiao J., Wang F., Liu J., Wang L., Kai G., Yu X. Effect of ZnO# ZnS QDs heterojunctures on the stilbenes–plasma proteins interactions. Mol. BioSystems. 2011;7:2452–2458. doi: 10.1039/c1mb05087g. [DOI] [PubMed] [Google Scholar]
- 242.Basta-Kaim A., Ślusarczyk J., Szczepanowicz K., Warszyński P., Leśkiewicz M., Regulska M., Trojan E., Lasoń W. Protective effects of polydatin in free and nanocapsulated form on changes caused by lipopolysaccharide in hippocampal organotypic cultures. Pharmacol. Rep. 2019;71:603–613. doi: 10.1016/j.pharep.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 243.Abd El-Hameed A.M., Yousef A.I., Abd El-Twab S.M., El-Shahawy A.A., Abdel-Moneim A. Hepatoprotective Effects of Polydatin-Loaded Chitosan Nanoparticles in Diabetic Rats: Modulation of Glucose Metabolism, Oxidative Stress, and Inflammation Biomarkers. Biochemistry. 2021;86:179–189. doi: 10.1134/S0006297921020061. [DOI] [PubMed] [Google Scholar]
- 244.Abdel-Moneim A., El-Shahawy A., Yousef A.I., Abd El-Twab S.M., Elden Z.E., Taha M. Novel polydatin-loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: In silico, in vitro and in vivo approaches. Int. J. Biol. Macromol. 2020;154:1496–1504. doi: 10.1016/j.ijbiomac.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 245.Abd El-Hameed A.M. Polydatin-loaded chitosan nanoparticles ameliorates early diabetic nephropathy by attenuating oxidative stress and inflammatory responses in streptozotocin-induced diabetic rat. J. Diabetes Metab. Disord. 2020;19:1599. doi: 10.1007/s40200-020-00699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Vijayalakshmi S., Mariadoss AV A., Ramachandran V., Shalini V., Agilan B., Sangeetha C.C., Balu P., Kotakadi V.S., Karthikkumar V., Ernest D. Polydatin encapsulated poly [lactic-co-glycolic acid] nanoformulation counteract the 7, 12-dimethylbenz [a] anthracene mediated experimental carcinogenesis through the inhibition of cell proliferation. Antioxidants. 2019;8:375. doi: 10.3390/antiox8090375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Samra Y.A., Abdelghany A.M., Zaghloul R.A. Polydatin gold nanoparticles potentiate antitumor effect of doxorubicin in Ehrlich ascites carcinoma-bearing mice. J. Biochem. Mol. Toxicol. 2021;35:e22869. doi: 10.1002/jbt.22869. [DOI] [PubMed] [Google Scholar]
- 248.Orlowski P., Zmigrodzka M., Tomaszewska E., Ranoszek-Soliwoda K., Pajak B., Slonska A., Cymerys J., Celichowski G., Grobelny J., Krzyzowska M. Polyphenol-conjugated bimetallic Au@ AgNPs for improved wound healing. Int. J. Nanomed. 2020;15:4969. doi: 10.2147/IJN.S252027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Magalhães P.R., Reis P.B., Vila-Viçosa D., Machuqueiro M., Victor B.L. Computational Design of Membrane Proteins. Springer; Berlin/Heidelberg, Germany: 2021. Identification of Pan-Assay INterference compoundS (PAINS) Using an MD-Based Protocol; pp. 263–271. [DOI] [PubMed] [Google Scholar]
- 250.de Matos A.M., Blázquez-Sánchez M.T., Sousa C., Oliveira M.C., de Almeida R.F., Rauter A.P. C-Glucosylation as a tool for the prevention of PAINS-induced membrane dipole potential alterations. Sci. Rep. 2021;11:4443. doi: 10.1038/s41598-021-83032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.