Abstract
Background: Previous observational studies investigated the relationship between coffee and tea intake and the risk of asthma, however, the conclusions were inconsistent. Further, the combined effect of coffee and tea consumption on asthma has rarely been studied. Methods: We examined associations between the self-reported intake of tea and coffee and the risk of incident asthma in a total of 424,725 participants aged from 39 to 73 years old from the UK Biobank. Cox proportional hazards models were used to estimate the associations between coffee/tea consumption and incident adult-onset asthma, adjusting for age, sex, race, smoking status, body mass index (BMI), education, and Townsend deprivation index. Results: Cox models with penalized splines showed J-shaped associations of coffee, tea, caffeinated coffee, and caffeine intake from coffee and tea with the risk of adult-onset asthma (p for nonlinear <0.01). Coffee intake of 2 to 3 cups/d (hazard ratio [HR] 0.877, 95% confidence interval [CI] 0.826–0.931) or tea intake of 0.5 to 1 cups/d (HR 0.889, 95% CI 0.816–0.968) or caffeinated coffee intake of 2 to 3 cups/d (HR 0.858, 95% CI 0.806–0.915) or combination caffeine intake from tea and coffee of 160.0 to 235.0 mg per day (HR 0.899, 95% CI 0.842–0.961) were linked with the lowest hazard ratio of incident asthma after adjustment for age, sex, race, smoking status, BMI, qualification, and Townsend deprivation index. Conclusions: Collectively, the study showed light-to-moderate coffee and tea consumption was associated with a reduced risk of adult-onset asthma and controlling total caffeine intake from coffee and tea for a moderate caffeine dose of 160.0 to 305.0 mg/day may be protective against adult-onset asthma. Further investigation on the possible preventive role of caffeine in asthma is warranted.
Keywords: asthma, coffee, tea, caffeine, UK Biobank
1. Introduction
Asthma is a common chronic respiratory disease that is characterized by airway hyper-responsiveness, inflammation, and remodeling, and it affects more than 339 million people worldwide, which causes a large health and economic burden [1]. Although multiple risk factors for asthma have been identified over the past 30 years, little progress has been made in exploring protective factors for preventing asthma [2].
Coffee and tea are the most popularly consumed beverages worldwide, thus, any biological effects from coffee and tea consumption could have a significant influence on public health [3]. It has been reported that coffee reduces the risks of multiple cancers [4], cardiovascular disease [5], diabetes [6], stroke [7], Parkinson’s disease [8], and gallstones [9]. Similarly, tea has also been proposed to exert a protective role in various cardiopulmonary conditions or other chronic diseases via multiple biologic functions, such as antioxidation, anti-inflammation, and immuno-regulation [10].
Several studies investigated the relationship between coffee and tea consumption and the prevalence and incidence of asthma; however, the conclusions were not consistent. Cross-sectional studies suggested that coffee consumption may link to a low prevalence of asthma [11,12]. Chronic coffee consumption appeared to be associated with improved asthma control [13]. Moreover, another study found an association between tea consumption and prevalence/incidence of asthma in the crude model; however, this association became statistically insignificant in the adjusted model [12]. In addition, a recent cross-sectional/prospective cohort study also found no significant association between coffee or tea consumption and asthma prevalence [14]. Collectively, the above studies with conflicting results lead to the uncertainty of the relationship, and whether asthma benefits from coffee and tea intake remains inconclusive [15].
Coffee and tea are different types of beverages, which contain functional components with both common and distinct contents [16,17,18]. The caffeine is highly enriched in coffee and moderately enriched in tea. Previous studies have reported that the positive effects of coffee are mainly related to caffeine, as its metabolites have greater exposure to tissues than others [19]. Remarkably, caffeine is known as a central nervous system stimulant and bronchodilator and can slightly improve lung function in adults. Furthermore, caffeine could metabolize to theophylline, which is still one of the most widely prescribed drugs for the treatment of asthma [20,21], thus proposing a protective role of caffeine in asthma. However, caffeine at very high levels of intake may also cause multiple side effects that affect asthma in a negative way, such as insomnia, nervousness, reduced quality of sleep, and increased anxiety. Therefore, the possible benefits of caffeine content in coffee and tea drinking must be weighed against potential risks, and a large prospective cohort study to assess the relationship between caffeine and asthma is warranted.
Thus, we used the large population-based cohort study from the UK Biobank to examine the individual effect of coffee- and tea-drinking behaviors on the risk of developing adult-onset asthma, and the effect of caffeine intake in combining coffee and tea consumption on asthma incidence as well.
2. Methods
2.1. Study Design and Population
We used the large-scale cohort study from the UK Biobank that recruited more than 500,000 participants aged 37–73 years from 2006–2010 [22,23]. Prospective participants were identified from National Health Service registers in the United Kingdom, and then were invited to one of 22 assessment centers to fill out a baseline questionnaire about epidemiologic data, undergo a physical examination, and donate biological samples. The UK Biobank was conducted in accordance with the Declaration of Helsinki, and the protocol has already received ethical approval from the Northwest Multi-Center Research Ethics Committee (11/NW/0382) and obtained written informed consent from all participants. Participants with baseline asthma and participants with missing data on coffee or tea intake, sex, age, smoking, or BMI were excluded from the analysis. Data from 424,725 individuals were available for analysis in our present study.
2.2. Exposure Assessment
Coffee and tea intake were assessed by applying questionnaires during the recruitment. Participants were asked the following questions: “How many cups of coffee do you drink each day (including decaffeinated coffee)?”; “How many cups of tea do you drink each day (including black and green tea)?” Participants would select the choice of the number of cups, “Less than one,” “Do not know,” or “Prefer not to answer.” Further, coffee drinkers were further asked the type of coffee they usually drank with the answers of “Decaffeinated coffee (any type)”, “Instant coffee”, “Ground coffee (including espresso and filtered coffee), “other type of coffee”, “Do not know”, or “prefer not to answer”. Caffeine intake (mg/day) was calculated based on self-reported coffee and tea intake from the questionnaire and assumed one cup of coffee to 75 mg of caffeine and one cup of tea as 40 mg of caffeine [24,25,26].
2.3. Ascertainment of Asthma
Asthma events were recorded by the International Classification Disease (ICD-10) coding system: asthma (ICD codes: J45). Incident death events were identified by death registry records. Follow-up began at recruitment and lasted until asthma was diagnosed, death, loss of follow-up, or December 2020.
2.4. Covariates
In the present study, we considered the following potential covariates: age, gender, race, smoking status (never, previous, and current), body mass index (BMI), an education level (college or university degree, professional qualifications, A Levels/AS Levels or equivalent, O Levels/GCSEs or equivalent, and none of the above), Townsend deprivation index. All measures were obtained using the baseline questionnaire.
2.5. Statistical Analyses
The baseline characteristics were summarized across coffee, tea, and combined caffeine intake. Summary statistics for categorical variables are described as frequencies (percentages) and analyzed using a chi-squared test to study differences in population. Continuous variables were expressed as means (SD) and were compared by analysis of the student’s t-test. Restricted cubic spline models were used to assess potential nonlinear associations between coffee, tea, or combined caffeine intake per day with the risk of incident asthma. Those who drank coffee or tea beyond 15 cups per day were recorded as 15 cups/day. Multiple imputations were performed to recover missing data. Cox proportional hazards regression models were also used to investigate the association between coffee, tea, and combined caffeine intake with the risk of incident asthma. Coffee and tea intake were classified into 0, 0.5 to 1, 2 to 3, and ≥4 cups per day. Total caffeine intake of tea and coffee was grouped by quartile into Q1 (≤160.0 mg/day), Q2 (160.0–235.0 mg/day), Q3 (235.0–305.0 mg/day), and Q4 (305.0–390.0 mg/day). All models were adjusted for age, sex, BMI, education level, and Townsend deprivation index, and adjusted for tea in coffee analysis or for coffee in tea analysis. Several sensitivity analyses were performed to check the robustness of the results. Subgroup analyses were stratified by age (<60 versus ≥60 years), sex (male versus female), BMI (<25, 25 to <30 versus ≥30 kg/m2), and smoking status (never, former versus current). Furthermore, we calculated the interaction between coffee, tea, and or combined caffeine intake and subgroups to evaluate the heterogeneity of this association across different levels of these covariates. p values less than 0.05 were statistically significant. Analyses were performed using SPSS version 26.0 (IBM Corporation, Armonk, NY, USA), GraphPad Prism version 9.0 software (GraphPad Software Inc., San Diego, CA, USA), or R-4.1.2 software (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline Characteristics
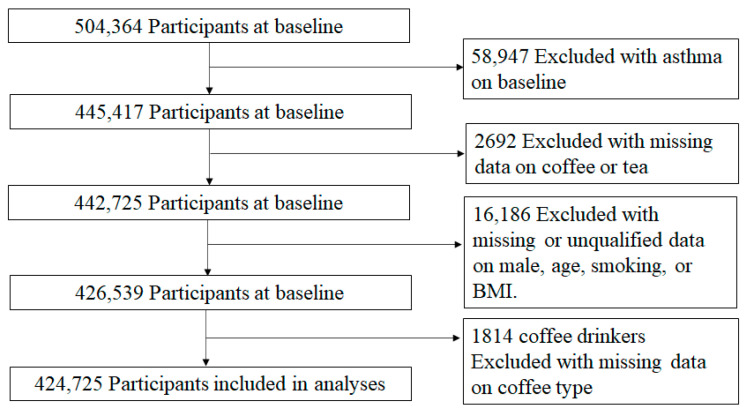
Of the 504,364 baseline participants, 424,725 participants were ultimately included in the analyses (Figure 1). Of 424,725 participants, the mean age was 56.61 ± 8.06 years, and 195,568 (46.0%) were male. Overall, 93,503 (22.0%) were non-coffee drinkers and 61,993 (14.6%) were non-tea drinkers. In total, 331,222 (78.0%) participants were coffee drinkers, with 27.3% of participants reporting reported drinking 0.5 to 1 cup of coffee, 31.2% drinking 2 to 3 cups, and 19.4% drinking ≥4 cups per day. A total of 362,732 (85.4%) participants were tea drinkers, with 11.5% of participants reporting reported drinking 0.5 to 1 cup of tea, 29.5% drinking 2 to 3 cups, and 44.4% drinking ≥4 cups per day. During the follow-up period, 8680 participants (2.0%) developed asthma, and the median time to development of the disease was 5.0 years (range from 3.0 to 7.0 years). Non-coffee drinkers and participants drinking 0.5 to 1 cup of coffee reported higher cups of tea consumption, while non-tea drinkers and participants drinking 0.5 to 1 cup of tea reported higher cups of coffee consumption. Compared to participants drinking 0.5 to 1 or 2 to 3 cups of tea, participants drinking ≥4 cups of tea were more likely to drink decaffeinated coffee (Table 1). To statistically assess the association between daily caffeine intake from coffee and tea with asthma incidence, we then grouped participants in five quartiles by tea and coffee combined caffeine intake. Participants in quartile 2 (take caffeine 160.0–235.0 mg/day) have the lowest incidence rate ratios for asthma (Table 1).
Figure 1.
Flowchart of participant selection.
Table 1.
Baseline characteristics by coffee and tea intake in the UK Biobank cohort.
| Coffee Intake, cups/day, No. (%) | Tea Intake, cups/day, No. (%) | Caffeine Intake, mg/day, No. (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | All Participants | 0 | 0.5 to 1 | 2 to 3 | ≥4 | 0 | 0.5 to 1 | 2 to 3 | ≥4 | Q1(≤160.0) | Q2 (160.0–235.0) | Q3 (235.0–305.0) | Q4 (305.0–390.0) | Q5 (≥390.0) |
| Total number, No. (%) | 424,725 | 93,503 (22.0) | 115,909 (27.3) | 132,751 (31.3) | 82,562 (19.4) | 61,993 (14.6) | 48,852 (11.5) | 125,407 (29.5) | 188,473 (44.4) | 93,308 (22.0) | 84,391 (19.9) | 81,117 (19.1) | 86,317 (20.3) | 79,592 (18.7) |
| Asthma cases, No. (%) | 8680 (2.0) | 2130 (2.3) | 2305 (2.0) | 2476 (1.9) | 1769 (2.1) | 1371 (2.2) | 889 (1.8) | 2417 (1.9) | 4003 (2.1) | 1991 (2.1) | 1602 (1.9) | 1597 (2.0) | 1764 (2.0) | 1757 (2.2) |
| Age, mean (SD), y | 56.61 (8.06) | 55.52 (8.21) | 57.09 (8.02) | 57.16 (7.97) | 56.30 (7.95) | 55.52 (8.22) | 55.60 (8.32) | 56.75 (8.10) | 57.14 (7.85) | 55.29 (8.39) | 57.00 (8.10) | 57.11 (7.90) | 57.34 (7.79) | 56.45 (7.88) |
| Gender | ||||||||||||||
| Male, n (%) | 195,568 (46.0) | 39,726 (42.5) | 50,681 (43.7) | 62,304 (46.9) | 42,857 (51.9) | 27,370 (44.2) | 23,580 (48.3) | 57,911 (46.2) | 86,707 (46.0) | 39,342 (42.2) | 37,471 (44.4) | 36,365 (44.8) | 40,319 (46.7) | 42,071 (52.9) |
| Female, n (%) | 229,157 (54.0) | 53,777 (57.5) | 65,228 (56.3) | 70,712 (53.1) | 39,705 (48.1) | 34,623 (55.8) | 25,272 (51.7) | 67,496 (53.8) | 101,766 (54.0) | 53,966 (57.8) | 46,920 (55.6) | 44,725 (55.2) | 45,998 (53.3) | 37,521 (47.1) |
| Race | ||||||||||||||
| White, n (%) | 385,709 (90.8) | 82,724 (88.5) | 104,622 (90.3) | 121,406 (91.5) | 76,957 (93.2) | 56,422 (91.0) | 42,401 (86.8) | 112,582 (89.8) | 174,304 (92.5) | 79,931 (85.7) | 76,349 (90.5) | 74,983 (92.4) | 80,425 (93.2) | 74,021 (93.0) |
| Non-white, n (%) | 39,016 (9.2) | 10,779 (11.5) | 11,287 (9.7) | 11,345 (8.5) | 5605 (6.8) | 5571 (9.0) | 6451 (13.2) | 12,825 (10.2) | 14,169 (7.5) | 13,377 (14.3) | 8042 (9.5) | 6134 (7.6) | 5892 (6.8) | 5571 (7.0) |
| Coffee intake, mean (SD) | 2.02 (2.07) | 0 (0) | 0.87 (0.22) | 2.39 (0.49) | 5.30 (2.03) | 3.47 (2.77) | 2.78 (2.11) | 1.97 (1.67) | 1.37 (1.69) | 0.50 (0.63) | 1.33 (0.91) | 1.70 (1.37) | 2.42 (1.39) | 4.42 (2.88) |
| Tea intake, mean (SD) | 3.42 (2.86) | 4.52 (3.29) | 4.03 (2.59) | 2.97 (2.36) | 2.03 (2.72) | 0 (0) | 0.87 (0.22) | 2.51 (0.50) | 5.81 (2.54) | 1.80 (1.40) | 2.81 (1.60) | 3.60 (2.23) | 4.11 (2.33) | 5.02 (4.59) |
| Smoking status | ||||||||||||||
| Never, n (%) | 232,699 (54.8) | 55,079 (58.9) | 67,175 (58.0) | 72,692 (54.8) | 37,753 (45.7) | 32,324 (52.1) | 26,598 (54.4) | 71,033 (56.6) | 102,744 (54.5) | 57,178 (61.3) | 47,934 (56.8) | 45,268 (55.8) | 46,339 (53.7) | 35,980 (45.2) |
| Previous, n (%) | 147,129 (34.6) | 29,361 (31.4) | 40,004 (34.5) | 47,618 (35.9) | 30,146 (36.5) | 21,078 (34.0) | 16,857 (34.5) | 43,786 (34.9) | 65,408 (34.7) | 29,346 (31.5) | 30,054 (35.3) | 28,649 (35.3) | 30,854 (35.7) | 28,226 (35.5) |
| Current, n (%) | 44,897 (10.6) | 9063 (9.7) | 8730 (7.5) | 12,441 (9.4) | 14,663 (17.8) | 8591 (13.9) | 5397 (11.0) | 10,588 (8.4) | 20,321 (10.8) | 6784 (7.3) | 6403 (7.6) | 7200 (8.9) | 9124 (10.6) | 15,386 (19.3) |
| Body mass index, mean (SD) | 27.31 (4.69) | 27.37 (4.90) | 26.96 (4.60) | 27.19 (4.53) | 27.92 (4.76) | 27.97 (5.16) | 27.32 (4.78) | 27.10 (4.57) | 27.23 (4.56) | 27.26 (4.99) | 27.07 (4.61) | 27.20 (4.57) | 27.31 (4.50) | 27.74 (4.70) |
| BMI category, n (%) | ||||||||||||||
| <25 | 142,884 (33.6) | 31,945 (34.2) | 42,783 (36.9) | 45,142 (34.0) | 23,014 (27.9) | 18,738 (30.2) | 16,653 (34.1) | 44,083 (35.2) | 63,410 (33.6) | 33,631 (36.0) | 30,291 (35.9) | 27,604 (34.0) | 28,125 (32.6) | 23,233 (29.2) |
| 25 to <30 | 181,689 (42.8) | 38,480 (41.1) | 48,638 (42.0) | 57,957 (43.7) | 36,614 (44.3) | 25,301 (40.8) | 20,541 (42.0) | 53,782 (42.9) | 82,065 (43.5) | 37,265 (39.9) | 35,643 (42.2) | 35,294 (43.5) | 38,153 (44.2) | 35,334 (44.4) |
| ≥30 | 100,152 (23.6) | 23,078 (24.7) | 24,488 (21.1) | 29,652 (22.3) | 22,934 (27.8) | 17,954 (29.0) | 11,658 (23.9) | 27,542 (22.0) | 42,998 (22.8) | 22,412 (24.0) | 18,457 (21.9) | 18,219 (22.5) | 20,039 (23.2) | 21,025 (26.4) |
| Education | ||||||||||||||
| College or University Degree | 137,939 (32.5) | 25,112 (26.9) | 39,162 (33.8) | 47,804 (36.0) | 25,861 (31.3) | 18,838 (30.4) | 19,577 (40.1) | 43,705 (34.9) | 55,819 (29.6) | 30,410 (32.6) | 28,631 (33.9) | 26,548 (32.7) | 28,291 (32.8) | 24,059 (30.2) |
| Professional Qualifications | 50,080 (11.8) | 11,371 (12.2) | 13,369 (11.5) | 15,054 (11.3) | 10,286 (12.5) | 7296 (11.8) | 4937 (10.1) | 14,061 (11.2) | 23,786 (12.6) | 10,226 (11.0) | 9514 (11.3) | 9531 (11.7) | 10,631 (12.3) | 10,178 (12.8) |
| A Levels/AS Levels or Equivalent | 475,04 (11.2) | 9799 (10.5) | 13,452 (11.6) | 15,247 (11.5) | 9006 (10.9) | 7185 (11.6) | 6121 (12.5) | 14,241 (11.4) | 19,957 (10.6) | 10,820 (11.6) | 9583 (11.4) | 9207 (11.4) | 9460 (11.0) | 8434 (10.6) |
| O Levels/GCSEs or Equivalent | 113,778 (26.8) | 26,311 (28.1) | 30,201 (26.1) | 34,284 (25.8) | 22,982 (27.8) | 17,969 (29.0) | 12,180 (24.9) | 33,093 (26.4) | 50,535 (26.8) | 25,308 (27.1) | 22,378 (26.5) | 21,653 (26.7) | 22,911 (26.5) | 21,527 (27.0) |
| None of the above | 75,424 (17.8) | 20,910 (22.4) | 19,725 (17.0) | 20,362 (15.3) | 14,427 (1.7) | 10,704 (17.3) | 6036 (12.4) | 20,307 (16.2) | 38,376 (20.4) | 16,544 (17.7) | 14,285 (16.9) | 14,178 (17.5) | 15,024 (17.4) | 15,393 (19.3) |
| Townsend deprivation, mean (SD) | −1.36 (3.06) | −0.92 (3.25) | −1.45 (3.01) | −1.58 (2.95) | −1.40 (3.02) | −1.14 (3.15) | −1.19 (3.14) | −1.42 (3.04) | −1.44 (3.01) | −0.90 (3.27) | −1.45 (3.00) | −1.58 (2.92) | −1.64 (2.90) | −1.29 (3.09) |
| Townsend deprivation index quartiles | ||||||||||||||
| Q1 (lowest) | 106,212 (25.0) | 20,129 (21.5) | 29,759 (25.7) | 35,562 (26.8) | 20,762 (25.1) | 14,232 (23.0) | 11,680 (23.9) | 32,385 (25.8) | 47,915 (25.4) | 20,285 (21.7) | 21,580 (25.6) | 21,516 (26.5) | 23,309 (27.0) | 19,523 (24.5) |
| Q2 | 106,166 (25.0) | 21,511 (23.0) | 29,219 (25.2) | 34,424 (25.9) | 21,013 (25.5) | 15,010 (24.2) | 11,668 (23.9) | 31,288 (24.9) | 48,200 (25.6) | 20,942 (22.4) | 21,448 (25.4) | 21,178 (26.1) | 23,015 (26.7) | 19,583 (24.6) |
| Q3 | 106,173 (25.0) | 23,525 (25.2) | 29,137 (25.1) | 33,064 (24.9) | 20,447 (24.8) | 15,560 (25.1) | 12,331 (25.2) | 31,156 (24.8) | 47,126 (25.0) | 23,521 (25.2) | 21,228 (25.2) | 20,389 (25.1) | 21,320 (24.7) | 19,714 (24.8) |
| Q4 (highest) | 106,174 (25.0) | 28,338 (30.3) | 27,794 (24.0) | 29,701 (22.4) | 20,340 (24.6) | 17,190 (27.7) | 13,173 (27.0) | 30,578 (24.4) | 45,232 (24.0) | 28,559 (30.6) | 20,135 (23.9) | 18,035 (22.2) | 18,673 (21.6) | 20,772 (26.1) |
| Types of most commonly consumed coffee | ||||||||||||||
| Coffee drinkers, n (%) | 331,222 (78.0) | NA | 115,909 (100.0) | 132,751 (100.0) | 82,562 (100.0) | 52,278 (84.3) | 43,979 (90.0) | 104,787 (83.6) | 130,178 (69.1) | 43,791 (46.9) | 70,465 (83.5) | 63,568 (78.4) | 79,968 (92.6) | 73,430 (92.3) |
| Decaffeinated, n (%) | 63,810 (19.3) | NA | 22,792 (19.7) | 24,755 (18.6) | 16,263 (19.7) | 10,353 (19.8) | 7338 (16.7) | 19,553 (18.7) | 26,566 (20.4) | 8509 (19.4) | 12,579 (17.9) | 11,760 (18.4) | 16,234 (20.3) | 14,728 (20.1) |
| Instant, n (%) | 185,657 (56.1) | NA | 59,075 (51.0) | 73,168 (55.1) | 53,414 (64.7) | 31,757 (60.7) | 24,044 (54.7) | 57,380 (54.8) | 72,476 (55.7) | 20,701 (47.3) | 37,121 (52.7) | 35,252 (19.0) | 46,250 (57.8) | 46,333 (63.1) |
| Ground, n (%) | 75,748 (22.9) | NA | 30,729 (26.5) | 33,009 (24.9) | 12,010 (14.5) | 9371 (17.9) | 11,910 (27.1) | 26,030 (24.8) | 28,437 (21.8) | 13,249 (30.3) | 19,289 (27.4) | 15,497 (20.5) | 16,341 (20.4) | 11,372 (15.5) |
| Other, n (%) | 6007 (1.8) | NA | 3313 (2.9) | 1819 (1.4) | 875 (1.1) | 797 (1.5) | 687 (1.6) | 1824 (1.7) | 2699 (2.1) | 1332 (3.0) | 1476 (2.1) | 1059 (17.6) | 1143 (1.4) | 997 (1.4) |
Abbreviations: A, Advanced; AS, Advanced Subsidiary; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GCSE, General Certificate of Secondary Education; NA, not applicable; No., number; O, Ordinary; Q, quartiles; SD, standard deviation; UK Biobank, United Kingdom Biobank.
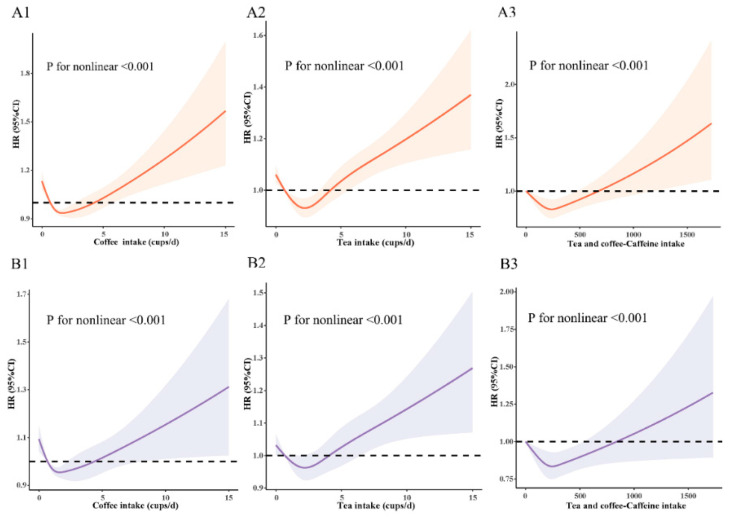
3.2. Nonlinear Association
Restricted cubic spline models were used to evaluate the relationship between coffee, tea, and combined caffeine intake with asthma. Associations between coffee, tea, and combined caffeine intake with risk of asthma were nonlinear (p for nonlinear <0.001), both in unadjusted (Figure 2A) and multiple-adjusted models (Figure 2B). Significant decreases in risk were observed for asthma at light-to-moderate coffee and tea intake, but not at none and high intake. In addition, the low intake of tea and coffee combined with caffeine intake was associated with a lower risk of asthma, while the high intake was not. Additionally, a similar J-shaped association was observed for coffee intake, tea intake, caffeinated coffee intake, and combined caffeine intake from coffee and tea (Figure 2).
Figure 2.
Restricted cubic spline models for the relationship between coffee, tea, and combination caffeine intake from coffee and tea with adult-onset asthma. (A) The restricted cubic spline model is unadjusted: (A1) Coffee and adult-onset asthma; (A2) Tea and adult-onset asthma; (A3) combination caffeine intake from coffee and tea and adult-onset asthma. (B) Restricted cubic spline model is adjusted by age, gender, race, BMI, smoking status, education, and Townsend Index and adjusted for coffee in tea analysis or for tea in coffee analysis: (B1) Coffee and adult-onset asthma, (B2) Tea and adult-onset asthma, (B3) combination caffeine intake from coffee and tea and adult-onset asthma. The 95% CIs of the adjusted HRs are represented by the shaded area. BMI, body mass index; HR: hazard ratio; CI, confidence interval.
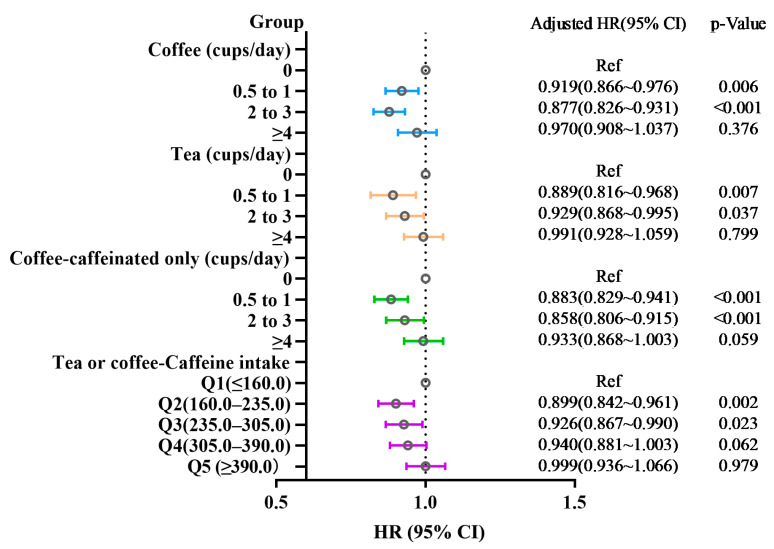
3.3. Coffee Intake, Tea Intake, and Combined Caffeine Intake with Asthma Risk
Univariate and multivariable cox regression analyses showed that compared with noncoffee participants, those who drank 0.5 to 1 (adjusted HR = 0.919, 95%CI 0.866~0.976, p = 0.006) or 2 to 3 cups (adjusted HR = 0.877, 95%CI 0.826~0.931, p < 0.001) coffee per day had a lower risk of asthma. Compared with participants who drank non-tea, participants with 0.5 to 1 (adjusted HR = 0.889, 95%CI 0.816~0.968, p < 0.001) or 2 to 3 cups (adjusted HR = 0.929, 95%CI 0.868~0.995, p < 0.037) of tea per day also had a lower risk of asthma. Further cox regression analyses were taken in caffeinated coffee consumption to assess the effect of caffeine, the results showed that the HR for asthma was reduced slightly both in participants with 0.5 to 1 (adjusted HR = 0.883, 95%CI 0.829~0.941, p < 0.001) and 2 to 3 cups (adjusted HR = 0.858, 95%CI 0.806~0.915, p < 0.001), compared with total coffee. (Table 2; Figure 3). However, participants drinking ≥4 cups of coffee, tea, or caffeinated coffee per day had no decreased risk of asthma. Furthermore, 160.0–235.0 mg/day of combination caffeine intake from coffee and tea intake was associated with a lower risk of asthma (adjusted HR = 0.899, 95%CI 0.842~0.961, p = 0.002) (Table 2; Figure 3).
Table 2.
Coffee and tea consumption in relation to asthma risk: UK Biobank.
| Characteristics | Hazard Ratio for Asthma | |||||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) from Crude Model a | p-Value | HR (95%CI) from Model 1 b | p-Value | HR (95%CI) from Model 2 c | p-Value | HR (95%CI) from Model 3 d | p-Value | |
| Coffee (cups/day) | ||||||||
| 0 | Ref | Ref | Ref | Ref | ||||
| 0.5 to 1 | 0.872 (0.822~0.925) | <0.001 | 0.849 (0.800~0.901) | <0.001 | 0.912 (0.859~0.967) | 0.002 | 0.919 (0.866~0.976) | 0.006 |
| 2 to3 | 0.817 (0.771~0.866) | <0.001 | 0.800 (0.755~0.848) | <0.001 | 0.855 (0.807~0.907) | <0.001 | 0.877 (0.826~0.931) | <0.001 |
| ≥4 | 0.940 (0.882~1.001) | 0.054 | 0.944 (0.886~1.005) | 0.072 | 0.932 (0.874~0.993) | 0.03 | 0.970 (0.908~1.037) | 0.376 |
| Ptrend = 0.002 | Ptrend = 0.003 | Ptrend = 0.002 | Ptrend = 0.090 | |||||
| Tea (cups/day) | ||||||||
| 0 | Ref | Ref | Ref | Ref | ||||
| 0.5 to 1 | 0.822 (0.755~0.894) | <0.001 | 0.827 (0.760~0.900) | <0.001 | 0.891 (0.819~0.970) | 0.008 | 0.889 (0.816~0.968) | 0.007 |
| 2 to3 | 0.870 (0.815~0.930) | <0.001 | 0.856 (0.801~0.914) | <0.001 | 0.935 (0.875~1.000) | 0.049 | 0.929 (0.868~0.995) | 0.037 |
| ≥4 | 0.870 (0.815~0.930) | 0.195 | 0.938 (0.882~0.997) | 0.041 | 1.000 (0.940~1.064) | 0.996 | 0.991 (0.928~1.059) | 0.799 |
| Ptrend = 0.588 | Ptrend = 0.701 | Ptrend = 0.248 | Ptrend = 0.417 | |||||
| Coffee—caffeinated only (cups/day) | ||||||||
| 0 | Ref | Ref | Ref | Ref | ||||
| 0.5 to 1 | 0.837 (0.786~0.892) | <0.001 | 0.820 (0.770~0.870) | <0.001 | 0.876 (0.822~0.933) | <0.001 | 0.883 (0.829~0.941) | <0.001 |
| 2 to3 | 0.796 (0.749~0.846) | <0.001 | 0.911 (0.851~0.975) | <0.001 | 0.837 (0.786~0.890) | <0.001 | 0.858 (0.806~0.915) | <0.001 |
| ≥4 | 0.901 (0.842~0.965) | 0.003 | 0.911 (0.851~0.975) | 0.007 | 0.896 (0.836~0.960) | 0.002 | 0.933 (0.868~1.003) | 0.059 |
| Ptrend < 0.001 | Ptrend < 0.001 | Ptrend < 0.001 | Ptrend = 0.006 | |||||
| Tea and coffee—Caffeine intake quintiles | ||||||||
| Q1(≤160.0) | Ref | Ref | Ref | |||||
| Q2(160.0–235.0) | 0.889 (0.832~0.949) | <0.001 | 0.866 (0.811~0.925) | <0.001 | 0.899 (0.842~0.961) | 0.002 | NA | |
| Q3(235.0–305.0) | 0.922 (0.863~0.985) | 0.016 | 0.898 (0.840~0.959) | 0.001 | 0.926 (0.867~0.990) | 0.023 | NA | |
| Q4 (305.0–390.0) | 0.940 (0.882~1.003) | 0.061 | 0.915 (0.858~0.976) | 0.007 | 0.940 (0.881~1.003) | 0.062 | NA | |
| Q5 (≥390.0) | 1.035 (0.971~1.103) | 0.295 | 1.037 (0.972~1.105) | 0.274 | 0.999 (0.936~1.066) | 0.979 | NA | |
| Ptrend = 0.161 | Ptrend = 0.168 | Ptrend = 0.719 | ||||||
a unadjusted (crude) model; b Adjusted by age and gender; c Adjusted by age, gender, race, BMI, smoking status, education, and Townsend Index; d Adjusted by age, gender, race, body mass index, smoking status, education, and Townsend Index, and adjusted for coffee in tea analysis or for tea in coffee analysis. Abbreviations: HR: hazard ratio; CI, confidence interval; Q, quartiles.
Figure 3.
Summary of hazard ratios and 95% confidence intervals (x-axis) for cups per day categories and linear trend for coffee, tea, caffeinated coffee, and combination caffeine intake from coffee and tea for adult-onset asthma. The model is adjusted by age, gender, race, BMI, smoking status, education, and Townsend Index, and adjusted for coffee in tea analysis or for tea in coffee analysis. Hatched vertical line indicates the null reference value of ‘1′.
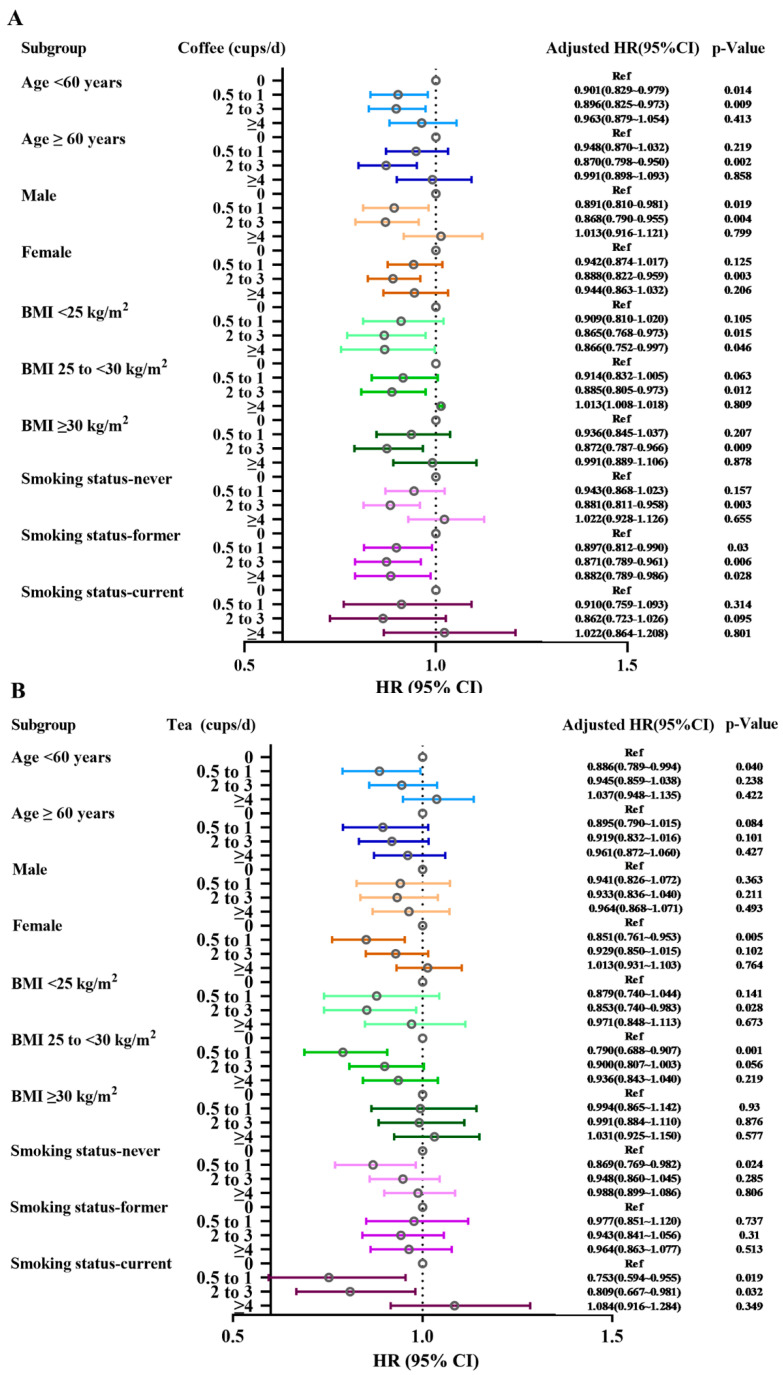
3.4. Subgroup and Sensitivity Analyses
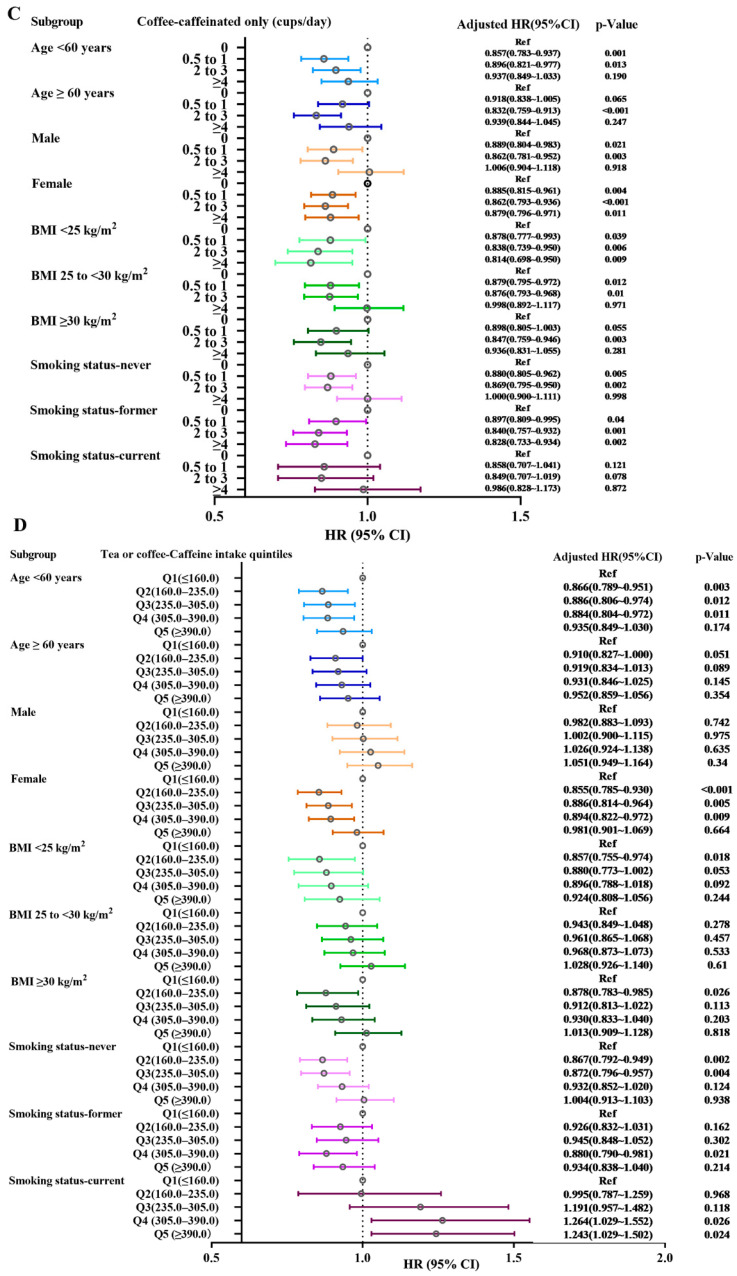
Participants with 160.0–235.0 mg/day of combination caffeine intake with the risk of asthma were more pronounced in individuals aged lower than 60 years old and females. However, participants under 60 years and the male subgroup showed an inverse trend without significance between tea intake and combination caffeine intake with asthma risk for low-to-moderate consumption of tea and combination caffeine intake (Figure 4).
Figure 4.
Subgroup analyses according to age, gender, BMI, and smoking status. Summary of hazard ratios and 95% confidence intervals (x-axis) for cups per day categories and linear trend for (A) coffee, (B) tea, (C) caffeinated coffee, and (D) combination caffeine intake from coffee and tea for adult-onset asthma. The model is adjusted by age, gender, race, BMI, smoking status, education, and Townsend Index and adjusted for coffee in tea analysis or for tea in coffee analysis. Hatched vertical line indicates the null reference value of ‘1′.
Subgroup analyses were conducted according to age, sex, and smoking status (All p values for interaction < 0.001) (Tables S1–S4). In subgroup analyses for all types of coffee and caffeinated coffee consumption, except for the current smoking group, all the others showed a lower risk of incident asthma at a daily consumption level of 0.5 to 1 and/or 2 to 3 cups of coffee (Figure 4A,C). Participants with 160.0–235.0 mg/day of combination caffeine intake with the risk of asthma were more pronounced in individuals aged lower than 60 years old (adjusted HR = 0.866, 95%CI 0.789~0.951, p = 0.003), females (adjusted HR = 0.855, 95%CI 0.785~0.930, p < 0.001), and non-smokers (adjusted HR = 0.867, 95%CI 0.792~0.949, p = 0.002) (Figure 4D). However, participants under 60 years and the male subgroup showed no association between tea intake and combination caffeine intake with asthma risk, although an HR of less than 1 was observed for low-to-moderate consumption of tea and combination caffeine intake (Figure 4B,D). Supplement Tables S5 and S6 presented results from our sensitivity analyses. Similar results were observed when we repeated our analyses in a subset with complete covariates data (Table S5). Moreover, the results were barely changed after excluding participants with incident asthma during the first 2 years of follow-up (Table S6).
4. Discussion
In this prospective cohort study based on the UK Biobank, we found a J-shaped association was observed between coffee, tea intake, caffeinated coffee intake, and caffeine intake with the risk of adult-onset asthma. Specifically, we demonstrated that light-to-moderate coffee and tea separately (0.5 to 3 cups per day) and 160.0 to 305.0 mg/day of combination caffeine intake from coffee and tea were associated with reductions in the risk of incident asthma, compared with nonhabitual drinkers.
Despite the growing concerns arising about the association of coffee and tea intake with health benefits in recent years, limited attention has been played to coffee and tea intake and the risk of asthma. Most studies investigating the relationship between coffee and tea consumption with asthma incidence were stuck 20 years ago with inconsistent results [11,13,14]. Although a new study of 173,209 participants and 3146 cases from Korea reported a relationship between coffee and tea intake and the prevalence of asthma, the cross-sectional design was not sufficient to infer causality [12]. To date, large longitudinal cohort studies were lacking to determine the relationship between coffee and tea intake and asthma. Herein, we observed a J relationship between coffee and tea intake and adult-onset asthma in a large prospective study, which has not been previously reported. Specifically, only regularly light-to-moderate coffee drinkers (0.5 to 3 cups per day) could significantly reduce the risk of adult-onset asthma incidence. The protective effects disappeared in heavy drinkers of coffee and tea in the study, which was inconsistent with the study of Wee et al. [12]. However, the beneficial J-shaped effect of coffee and tea intakes has been extensively reported in other diseases with large prospective cohort studies, such as stroke, dementia [7], and cancer mortality [27].
In addition, we also found that total caffeine intake from coffee and tea may play a central role in the association between coffee and tea consumption and asthma incidence. Caffeine is known to be a common ingredient in bronchodilators that could improve airway function modestly in asthmatics [19,28]. The protective role of caffeine intake may rely on its ability to relax respiratory muscles [29] and its anti-inflammatory and antioxidant properties [28]. Additionally, caffeine and catechin could synergistically potentiate the mast cell-stabilizing property as well [17], and more studies about specific mechanisms of caffeine on asthma are warranted. In the study, a similar J-shaped effect of caffeine intake against asthma was observed as well. Previous studies revealed that a single dose caffeine intake of 200 mg or less is safe, while a caffeine intake over 300 mg could result in toxic effects, such as restlessness, increased urination, gastrointestinal disorders, arrhythmia, and psychomotor agitation [30]. In most cases, a dose caffeine intake of less than 400 mg/day in healthy adults has been suggested [31,32]. Meanwhile, our study also shows the protective dose of caffeine intake from coffee and tea with asthma incidence in the range of 160.0 to 305.0 mg/day and suggests avoiding heavy caffeine consumption.
In the subgroup analysis, age, gender, BMI, and smoking status were noted to have a certain impact on these inverse associations with caffeine intake and asthma incidence. On the one hand, the possible reasons for those differences may be the complex effect on caffeine absorption and caffeine metabolism in heterogeneous participants, the greatest effect may be observed in CYP1A2 activity, a major enzyme that metabolizes caffeine (in about 80%) [33]. Herein, the effect of caffeine consumption from coffee and tea was significant in the females but not in the males. The sex discrepancy was also reported in a previous study showing that coffee consumption was inversely associated with asthma in women but not in men [12], which might be explained by the faster caffeine clearance in males than females due to the inhibited CYP1A2 activity by estradiol. In addition, smoking was also reported to be associated with faster caffeine metabolism and heavy coffee drinking because of the higher CYP1A2 activity in smokers [34]. Our results were significantly affected by the smoking status that the ideal dosage of habitual caffeine consumption from coffee and tea against asthma in former smokers was high doses (305.0 to 390.0 mg/kg per day) and presented no protective effect or even detrimental effect on the incidence of asthma in current smokers. On the other hand, asthmatics could also be influenced by a wide variety of factors, including age [35,36], sex [37], BMI [38,39], and smoking status [40]. In addition to the factors previously discussed, educational background and Townsend deprivation index are socioeconomic factors that exert effects on environmental allergen exposure [41,42] and may lead to variations in coffee and tea consumption [43,44]. Similar to other studies [7,25,45], we adjusted for education and Townsend deprivation index in this study. Although we found coffee and tea intakes were associated with a lower incidence of asthma in the large prospective cohort study, confirming the preventive and therapeutic role of coffee and tea intakes still warrants large well-designed clinical trials.
Strengths and Limitations
This study is the first large prospective cohort study with sufficient follow-up to investigate the relationship between coffee and tea intake with adult-onset asthma; however, there are also certain limitations. Firstly, most participants in UK Biobank were of European ancestry, which would restrict the interpretation of the findings to other ethnic background populations. Secondly, coffee and tea intakes were self-reported based on questionnaires, and the changes in coffee and tea consumption over time were not evaluated after the baseline evaluation. Thirdly, caffeine intake levels were assessed on the self-report coffee and tea intake using the conversion equation provided in the literature without considering some potential confounders, such as chocolate, energy drinks, soft drinks, and medications intake [46,47]. Therefore, further validated tests, containing a precisely controlled content of caffeine, are needed to verify our conclusions. Additionally, the observed associations were slightly modified by genetic factors related to caffeine metabolism in previous studies [25]; however, we did not evaluate the effect of genetic variation in the study. Finally, the ages of participants ranged from 37 to 73 years old, and thus the findings should be deliberately interpreted into children- and adolescent-onset asthma.
5. Conclusions
In conclusion, we found that light-to-moderate coffee and tea consumption (0.5 to 3 cups per day) was associated with a reduced risk of adult-onset asthma and controlling total caffeine intake from coffee and tea for a moderate caffeine dose of 160.0 to 305.0 mg/day may be protective against adult-onset asthma.
Acknowledgments
We thank the participants of the UK Biobank. This research has been conducted using the UK Biobank Resource under the project number of 84979.
Abbreviations
| A | Advanced |
| AS | Advanced Subsidiary |
| BMI | body mass index (calculated as weight in kilograms divided by height in meters squared) |
| CI | confidence interval |
| CSE | Certificate of Secondary Education |
| GCSE | General Certificate of Secondary Education |
| HR | hazard ratio |
| HNC | Higher National Certificate |
| HND | Higher National Diploma |
| ICD-10 | International Classification of Diseases-10th revision |
| NVQ | National Vocational Qualification |
| O | Ordinary |
| Q | quartiles |
| SD | standard deviation |
| UK Biobank | United Kingdom Biobank |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14194039/s1, Table S1: Association of coffee and tea with asthma in the UK Biobank cohort by age; Table S2: Association of coffee and tea with asthma in the UK Biobank cohort by gender; Table S3: Association of coffee and tea with asthma in the UK Biobank cohort by BMI; Table S4: Association of coffee and tea with asthma in the UK Biobank cohort by smoking status; Table S5: Coffee and tea consumption in relation to asthma risk with complete covariates data; Table S6: Coffee and tea consumption in relation to asthma risk after excluding participants with incident asthma during the first 2 years of follow-up.
Author Contributions
Conceptualization: Y.Z. (Yan Zhang), P.P. and H.L. (Hong Liu). Data curation: Y.Z. (Yan Zhang). Formal analysis: F.L. Funding acquisition: Y.Z. (Yan Zhang). Investigation: F.L., Y.Z. (Yan Zhang), P.P. and H.L. (Hong Liu). Methodology: F.L. and Y.Z. (Yan Zhang). Project administration: Y.Z. (Yan Zhang). Resources: Y.Z. (Yan Zhang). Software: F.L., Y.Z. (Yiqun Zhu) and H.L. (Huaying Liang). Supervision: Y.Z. (Yan Zhang). Validation: Y.Z. (Yan Zhang). Visualization: F.L., D.L. and D.J. Writing—original draft: F.L. Writing—review and editing: Y.Z. (Yan Zhang), P.P., H.L. (Hong Liu) and Y.Z. (Yiqun Zhu). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The UK Biobank was conducted in accordance with the Declaration of Helsinki, and the protocol has already received ethical approval from the Northwest Multi-Center Re-search Ethics Committee (11/NW/0382).
Informed Consent Statement
The UK Biobank was obtained written informed consent from all participants.
Data Availability Statement
Data from the UK Biobank cannot be shared publicly, however, data are available from the UK Biobank Institutional Data Access/Ethics Committee (contact via http://www.ukbiobank.ac.uk/ (accessed on 12 September 2022) or contact by email at access@ukbiobank.ac.uk) for researchers who meet the criteria for access to confidential data.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by Natural Science Foundation of China (No. 82100037), National Science Foundation for Post-doctoral Scientists of China (No. 2021TQ0375, No. 2022M713538), Hunan Outstanding Postdoctoral Innovative Talents Program (No. 2021RC2018), and Youth Foundation of Xiangya Hospital (No. 2020Q06).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Asthma Network . The Global Asthma Report 2018. Global Asthma Network; Auckland, New Zealand: 2018. [Google Scholar]
- 2.Soriano J.B., Kendrick P.J., Paulson K.R., Gupta V., Abrams E.M., Adedoyin R.A., Adhikari T.B., Advani S.M., Agrawal A., Ahmadian E., et al. Collaborators GBDCRD: Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020;8:585–596. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paiva I., Cellai L., Meriaux C., Poncelet L., Nebie O., Saliou J.M., Lacoste A.S., Papegaey A., Drobecq H., Le Gras S., et al. Caffeine intake exerts dual genome-wide effects on hippocampal metabolism and learning-dependent transcription. J. Clin. Investig. 2022;132:e149371. doi: 10.1172/JCI149371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poole R., Kennedy O.J., Roderick P., Fallowfield J.A., Hayes P.C., Parkes J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. doi: 10.1136/bmj.j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosso G., Micek A., Godos J., Sciacca S., Pajak A., Martinez-Gonzalez M.A., Giovannucci E.L., Galvano F. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: A dose-response meta-analysis. Eur. J. Epidemiol. 2016;31:1191–1205. doi: 10.1007/s10654-016-0202-2. [DOI] [PubMed] [Google Scholar]
- 6.Ding M., Bhupathiraju S.N., Chen M., van Dam R.M., Hu F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37:569–586. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Yang H., Li S., Li W.D., Wang Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: A cohort study in the UK Biobank. PLoS Med. 2021;18:e1003830. doi: 10.1371/journal.pmed.1003830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi H., Li S. Dose–response meta-analysis on coffee, tea and caffeine consumption with risk of P arkinson’s disease. Geriatr. Gerontol. Int. 2014;14:430–439. doi: 10.1111/ggi.12123. [DOI] [PubMed] [Google Scholar]
- 9.Nordestgaard A.T. Causal relationship from coffee consumption to diseases and mortality: A review of observational and Mendelian randomization studies including cardiometabolic diseases, cancer, gallstones and other diseases. Eur. J. Nutr. 2022;61:573–587. doi: 10.1007/s00394-021-02650-9. [DOI] [PubMed] [Google Scholar]
- 10.Tang G.Y., Meng X., Gan R.Y., Zhao C.N., Liu Q., Feng Y.B., Li S., Wei X.L., Atanasov A.G., Corke H., et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019;20:6196. doi: 10.3390/ijms20246196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pagano R., Negri E., Decarli A., La Vecchia C. Coffee drinking and prevalence of bronchial asthma. Chest. 1988;94:386–389. doi: 10.1378/chest.94.2.386. [DOI] [PubMed] [Google Scholar]
- 12.Wee J.H., Yoo D.M., Byun S.H., Song C.M., Lee H.J., Park B., Park M.W., Choi H.G. Analysis of the relationship between asthma and coffee/green tea/soda intake. Int. J. Environ. Res. Public Health. 2020;17:7471. doi: 10.3390/ijerph17207471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz J., Weiss S.T. Caffeine intake and asthma symptoms. Ann. Epidemiol. 1992;2:627–635. doi: 10.1016/1047-2797(92)90007-D. [DOI] [PubMed] [Google Scholar]
- 14.Annesi I., Kauffmann F., Oryszczyn M.P., Neukirch F., Dore M.F. Coffee drinking and prevalence of bronchial asthma. Chest. 1990;97:1268–1269. doi: 10.1378/chest.97.5.1268c. [DOI] [PubMed] [Google Scholar]
- 15.Alfaro T.M., Monteiro R.A., Cunha R.A., Cordeiro C.R. Chronic coffee consumption and respiratory disease: A systematic review. Clin. Respir. J. 2018;12:1283–1294. doi: 10.1111/crj.12662. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa S., Ohishi T., Miyoshi N., Oishi Y., Nakamura Y., Isemura M. Anti-cancer effects of green tea epigallocatchin-3-gallate and coffee chlorogenic acid. Molecules. 2020;25:4553. doi: 10.3390/molecules25194553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yashima M., Sato Y., Kazama I. Catechin synergistically potentiates mast cell-stabilizing property of caffeine. Allergy Asthma Clin. Immunol. 2021;17:1–7. doi: 10.1186/s13223-020-00502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croft K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. N. Y. Acad. Sci. 1998;854:435–442. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 19.Gong H., Jr., Simmons M.S., Tashkin D.P., Hui K.K., Lee E.Y. Bronchodilator effects of caffeine in coffee. A dose-response study of asthmatic subjects. Chest. 1986;89:335–342. doi: 10.1378/chest.89.3.335. [DOI] [PubMed] [Google Scholar]
- 20.Banner K.H., Page C.P. Theophylline and selective phosphodiesterase inhibitors as anti-inflammatory drugs in the treatment of bronchial asthma. Eur. Respir. J. 1995;8:996–1000. [PubMed] [Google Scholar]
- 21.Bloom C.I., de Preux L., Sheikh A., Quint J.K. Health and cost impact of stepping down asthma medication for UK patients, 2001-2017: A population-based observational study. PLoS Med. 2020;17:e1003145. doi: 10.1371/journal.pmed.1003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox N. UK Biobank shares the promise of big data. Nature. 2018;562:194–195. doi: 10.1038/d41586-018-06948-3. [DOI] [PubMed] [Google Scholar]
- 23.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., Downey P., Elliott P., Green J., Landray M., et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orazi C., Fariello G., Malena S., Caterino S., Ferro F. Torsion of paradidymis or Giraldes’ organ: An uncommon cause of acute scrotum in pediatric age group. J. Clin. Ultrasound. 1989;17:598–601. doi: 10.1002/jcu.1870170811. [DOI] [PubMed] [Google Scholar]
- 25.Zhou A., Hyppönen E. Long-term coffee consumption, caffeine metabolism genetics, and risk of cardiovascular disease: A prospective analysis of up to 347,077 individuals and 8368 cases. Am. J. Clin. Nutr. 2019;109:509–516. doi: 10.1093/ajcn/nqy297. [DOI] [PubMed] [Google Scholar]
- 26.Cornelis M.C., Weintraub S., Morris M.C. Caffeinated Coffee and Tea Consumption, Genetic Variation and Cognitive Function in the UK Biobank. J. Nutr. 2020;150:2164–2174. doi: 10.1093/jn/nxaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu D., Li Z.H., Shen D., Zhang P.D., Song W.Q., Zhang W.T., Huang Q.M., Chen P.L., Zhang X.R., Mao C. Association of sugar-sweetened, artificially sweetened, and unsweetened coffee consumption with all-cause and cause-specific mortality: A large prospective cohort study. Ann. Intern. Med. 2022;175:909–917. doi: 10.7326/M21-2977. [DOI] [PubMed] [Google Scholar]
- 28.Welsh E.J., Bara A., Barley E., Cates C.J. Caffeine for asthma. Cochrane Database Syst. Rev. 2010;2010:CD001112. doi: 10.1002/14651858.CD001112.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Moreno C., Lara B., Brito de Souza D., Gutierrez-Hellin J., Romero-Moraleda B., Cuellar-Rayo A., Del Coso J. Acute caffeine intake increases muscle oxygen saturation during a maximal incremental exercise test. Br. J. Clin. Pharmacol. 2020;86:861–867. doi: 10.1111/bcp.14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musgrave I.F., Farrington R.L., Hoban C., Byard R.W. Caffeine toxicity in forensic practice: Possible effects and under-appreciated sources. Forensic Sci. Med. Pathol. 2016;12:299–303. doi: 10.1007/s12024-016-9786-9. [DOI] [PubMed] [Google Scholar]
- 31.Wikoff D., Welsh B.T., Henderson R., Brorby G.P., Britt J., Myers E., Goldberger J., Lieberman H.R., O’Brien C., Peck J., et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017;109 Pt 1:585–648. doi: 10.1016/j.fct.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Verster J.C., Koenig J. Caffeine intake and its sources: A review of national representative studies. Crit. Rev. Food Sci. Nutr. 2018;58:1250–1259. doi: 10.1080/10408398.2016.1247252. [DOI] [PubMed] [Google Scholar]
- 33.Rodak K., Kokot I., Kratz E.M. Caffeine as a Factor Influencing the Functioning of the Human Body-Friend or Foe? Nutrients. 2021;13:3088. doi: 10.3390/nu13093088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W., Tong Y., Zhao Q., Yu G., Wei X., Lu Q. Coffee consumption and bladder cancer: A meta-analysis of observational studies. Sci. Rep. 2015;5:9051. doi: 10.1038/srep09051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn R.M., Busse P.J., Wechsler M.E. Asthma in the elderly and late-onset adult asthma. Allergy. 2018;73:284–294. doi: 10.1111/all.13258. [DOI] [PubMed] [Google Scholar]
- 36.Shadyab A.H., Manson J.E., Luo J., Haring B., Saquib N., Snetselaar L.G., Chen J.C., Groessl E.J., Wassertheil-Smoller S., Sun Y., et al. Associations of coffee and tea consumption with survival to age 90 years among older women. J. Am. Geriatr. Soc. 2020;68:1970–1978. doi: 10.1111/jgs.16467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dharmage S.C., Perret J.L., Custovic A. Epidemiology of asthma in children and adults. Front. Pediatr. 2019;7:246. doi: 10.3389/fped.2019.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nwaru B.I., Ohlsson C., Bygdell M., Martikainen J., Kindblom J.M. Pubertal BMI change and adult-onset asthma in men: Population-based cohort study in Sweden. Clin. Exp. Allergy. 2020;50:51–60. doi: 10.1111/cea.13534. [DOI] [PubMed] [Google Scholar]
- 39.Guerra S., Wright A.L., Morgan W.J., Sherrill D.L., Holberg C.J., Martinez F.D. Persistence of asthma symptoms during adolescence: Role of obesity and age at the onset of puberty. Am. J. Respir. Crit. Care Med. 2004;170:78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- 40.Coogan P.F., Castro-Webb N., Yu J., O’Connor G.T., Palmer J.R., Rosenberg L. Active and passive smoking and the incidence of asthma in the Black Women’s Health Study. Am. J. Respir. Crit. Care Med. 2015;191:168–176. doi: 10.1164/rccm.201406-1108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jabre N.A., Keet C.A., McCormack M., Peng R., Balcer-Whaley S., Matsui E.C. Material Hardship and Indoor Allergen Exposure among Low-Income, Urban, Minority Children with Persistent Asthma. J. Community Health. 2020;45:1017–1026. doi: 10.1007/s10900-020-00822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y., Jing D., Liang H., Li D., Chang Q., Shen M., Pan P., Liu H., Zhang Y. Vitamin D status and asthma, lung function, and hospitalization among British adults. Front. Nutr. 2022;9:954768. doi: 10.3389/fnut.2022.954768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loftfield E., Freedman N.D., Dodd K.W., Vogtmann E., Xiao Q., Sinha R., Graubard B.I. Coffee Drinking Is Widespread in the United States, but Usual Intake Varies by Key Demographic and Lifestyle Factors. J. Nutr. 2016;146:1762–1768. doi: 10.3945/jn.116.233940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vieux F., Maillot M., Rehm C.D., Drewnowski A. Tea Consumption Patterns in Relation to Diet Quality among Children and Adults in the United States: Analyses of NHANES 2011–2016 Data. Nutrients. 2019;11:2635. doi: 10.3390/nu11112635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creed J.H., Smith-Warner S.A., Gerke T.A., Egan K.M. A prospective study of coffee and tea consumption and the risk of glioma in the UK Biobank. Eur. J. Cancer. 2020;129:123–131. doi: 10.1016/j.ejca.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Mahoney C.R., Giles G.E., Marriott B.P., Judelson D.A., Glickman E.L., Geiselman P.J., Lieberman H.R. Intake of caffeine from all sources and reasons for use by college students. Clin. Nutr. 2019;38:668–675. doi: 10.1016/j.clnu.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Heckman M.A., Weil J., Gonzalez de Mejia E. Caffeine (1,3,7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010;75:R77–R87. doi: 10.1111/j.1750-3841.2010.01561.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the UK Biobank cannot be shared publicly, however, data are available from the UK Biobank Institutional Data Access/Ethics Committee (contact via http://www.ukbiobank.ac.uk/ (accessed on 12 September 2022) or contact by email at access@ukbiobank.ac.uk) for researchers who meet the criteria for access to confidential data.