Abstract
We analyzed the seasonal dynamics of lipid profile, glucose, and insulin in healthy subjects from 29 studies conducted in 23 regions, located in different climate zones ranging from subarctic to tropical. Our meta-analysis showed that people have higher the level of TC (total cholesterol), LDL (low-density lipoprotein), HDL (high-density lipoprotein), FBG (fasting blood glucose) in winter than in summer regardless of gender. Regional climate had a significant impact on the seasonal dynamics of lipid profile and glucose. TC, HDL, FBG seasonal fluctuations were more prominent in a climate that had a marked increase in average monthly atmospheric pressure in winter compared with summer as opposed to a climate where atmospheric pressure did not vary significantly in winter and summer. In a climate with humid winters, TC seasonal changes were significantly greater than in the regions with humid summers, most likely due to LDL seasonal changes, since HDL seasonal dynamics with peaks in winter were more prominent in the regions with humid summers. The level of triglycerides had prominent seasonal dynamics with peak values in winter only in the regions with a large difference in winter and summer air temperatures. The results of our current and prior meta-analysis allow for the conclusion that the seasonal dynamics of circulating lipids and glucose are frequently linked to the seasonal dynamics of thyroid-stimulating hormone and hematocrit. Dependence of the seasonal changes in the biochemical parameters on annual fluctuations in air temperature, atmospheric pressure and relative humidity is more obvious than on photoperiod changes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12291-022-01064-6.
Keywords: Triglycerides, Cholesterol, Lipoproteins, Glucose, Season, Climate
Introduction
Cardiovascular diseases are known to flare up more often in winter rather than in summer. Winters are also associated with higher occurrence of the metabolic syndrome [1, 2] and manifestations of type 1 diabetes [3]. These regular patterns are usually linked to winter increase in blood pressure, as well as an increase in the level of circulating lipids during the coldest season [1]. It is still not completely clear whether seasonal functional and biochemical changes in modern humans are based on changes in their general metabolism or are merely a reaction to the sedentary lifestyle and seasonal dietary habits.
Metabolism of lipids and carbohydrates is regulated by many hormones, including thyroid hormones, cortisol, catecholamines, and insulin. Thyroid hormones stimulate metabolism and food intake, lipolysis and lipogenesis, both absorption of cholesterol and its synthesis, and also increase gluconeogenesis, among other things, by regulating the release of insulin and sensitivity to it [4]. In vertebrates, the level of thyroid hormones is regulated by TSH (thyroid-stimulating hormone of the pituitary). Several studies show that higher TSH values correspond to higher total cholesterol (TC), lipoprotein and glucose values in humans [5–8]. Activity of thyroid hormones is influenced by environmental factors (daytime length, air temperature, atmospheric pressure, partial density of oxygen in the air), as well as one’s diet content and energy value [4, 9, 10]. Activity thyroid hormones increases under the effect of a decrease in melatonin levels and air temperature. An increase in melatonin levels and air temperature, as well as a decrease in calorie intake, reduce the activity of thyroid hormones.
Insulin is the main hormone of carbohydrate metabolism; it enhances cell uptake of glucose, stimulates glycogen synthesis, and also promotes the conversion of glucose to triglycerides (TG) [11, 12]. Sympathetic nervous system activity suppresses insulin secretion and stimulates the release of cortisol, which can stimulate lipolysis at low insulin levels and lipogenesis at elevated insulin levels [13, 14]. Higher cortisol levels are associated with higher TC and lipoprotein values [15, 16]. Moreover, cortisol stimulates gluconeogenesis and plays an important role in glycogenolysis [17, 18]. Adverse conditions (heat, cold, dietary deficiency, etc.) cause an increase in the level of circulating catecholamines and cortisol [19–21].
In addition to hormones, the concentration of circulating lipids and glucose can be influenced by hematocrit level. There are observations of a direct relationship between circulating lipids, glucose and hematocrit [22–24]. It is known that an increase in hematocrit contributes to an increase in flow-related insulin resistance, diffusional transport of cholesterol between erythrocytes and plasma lipoproteins, and concentration of substances in a smaller plasma volume [23, 24]. Hyperthermia and hypothermia cause an increase in hematocrit, but this can be compensated by sufficient water intake [25].
Several studies have shown that the relationship between air temperature and lipid profile indicators is not linear. Both higher and lower air temperature compared against the threshold level cause an increase in TG, low-density lipoproteins (LDL) and high-density lipoproteins (HDL) [26, 27]. The lowest glucose levels were observed in a thermoneutral environment (22 ºC), and the highest were recorded under hyperthermia (43 ºC). Insulin was at its maximum value under hyperthermia and minimum under hypothermia (7 ºC). Still, adaptation to temperature conditions was accompanied by normalization of glucose and insulin levels [28].
There have been reports that melatonin treatment or exposure to short photoperiod resulted in lower TG, TC, and glucose levels [29–33]. On the other hand, in the lack of sunlight may increase TC levels in blood and allow the metabolism of 7-dehydrocholesterol to shift to cholesterol synthesis rather than vitamin D synthesis, which would have occurred in case of greater exposure to sunlight [34]. Vitamin D deficiency is associated with decreased insulin synthesis and increased insulin resistance [35].
Besides daytime length and meteorological factors which determine hormone activity and general metabolism, physical activity and diet can affect the level of lipids and carbohydrates. Many researchers point out that an average person usually makes more steps in summer than in winter [36]. Regular walking has been found to lower TC and glucose [37, 38]. There are reports of seasonal variations in the consumption of saturated/unsaturated fats, carbohydrates, and vitamins [39, 40]. In the coldest season, consumption of saturated fats is recorded at peak levels, while consumption of unsaturated fats peaks in the warm season, and consumption of carbohydrates is at its highest in spring. The intake of vitamins C and E increases in summer, and the intake of vitamin A is at its highest in spring. While consumption of unsaturated fats is associated with higher HDL and lower TC and insulin resistance, consumption of saturated fats is linked to an increase in the concentration of TC and LDL and higher insulin resistance. An increase in carbohydrate intake (especially processed carbohydrates) affects not only glucose levels, but also causes an increase in the concentration of circulating TG and TC [11, 41–43]. By contrast, daily intake of vitamins C and E promotes lower TG, TC, and LDL [44, 45].
The seasonal dynamics of lipids and carbohydrates are ultimately determined by various influences that sometimes have the opposite effect. In the prior meta-analyses, we showed that modern healthy people have higher body mass index, hematocrit, circulating T3 (total triiodothyronine) and norepinephrine levels in winter than in summer, and TSH is higher in winter than in other seasons [9, 25, 46, 47]. However, the level of cortisol in winter and summer did not differ significantly [46]. The purpose of this meta-analysis was to study the seasonal dynamics of circulating lipids and carbohydrates in healthy adults, as well as the dynamics’ dependence on gender and special characteristics of the regional climate.
Methods
Meta-analysis complied with PRISMA guidelines (http://www.prisma-statement.org). Search for publications was performed independently by two researchers using PubMed, Scopus, and Google Scholar databases. The search was done in September 2021 in English and in Russian without limitations on the publication period. The search strategies were based on combinations of keywords related to biochemical parameters (lipids, carbohydrates, cholesterol, triglycerides, lipoproteins, glucose, insulin) and to climate (season, climate, weather, winter, summer, spring, autumn, fall, temperature, heat, hot, warm, cold, atmospheric pressure, barometric pressure, air humidity, relative humidity). We used the “human subject” filter. As a part of our secondary search, we explored references cited in included studies.
We selected panel and cross-sectional studies for our meta-analysis. We relied on methodology description to determine the design of each study. Publications were selected based on ethical and methodological standards for conducting research on biological rhythms [48].
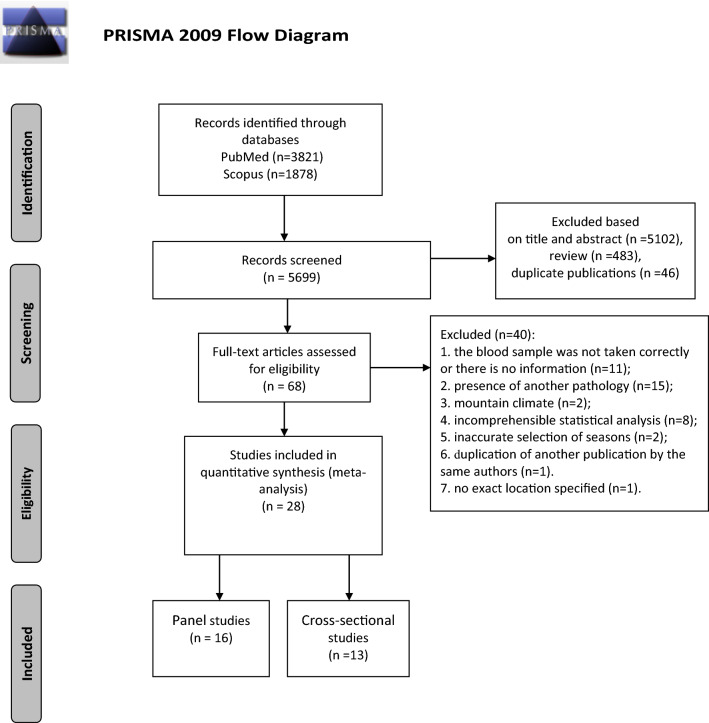
We selected publications which studied the seasonal dynamics of TG, TC, LDL, HDL, FBG (fasting blood glucose), and insulin levels in blood. Testing of circulating lipids and carbohydrates was supposed to be performed in morning under fasting conditions. Studies were excluded from the meta-analysis if the time of blood sampling was not specified or was incorrect or was not on an empty stomach. Our selection included only those studies that evaluated healthy adults who were not receiving any treatment. We did not account for gender and age of participating subjects, but excluded studies involving: pregnant women and children; seasonal workers, members of polar expeditions, as well as other cases of people’s temporary exposure to climatic conditions different from the region of their permanent residency; professional athletes because their training and competition schedule could have a major effect on their hormone levels; people working night shifts. The study location was also taken into account with studies conducted in a mountainous regions or for which the precise geographical location was not identified being excluded from the meta-analysis (Fig. 1).
Fig. 1.
Flow diagram according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (http://prisma-statement.org/)
Upon extracting data on biochemical indicators considered herein from publications, we used online calculators (http://unitslab.com/en) to convert all results into the same units of measure. We used the following units: mg/dl for TG, TC, LDL, HDL, FBG, and mIU/l for insulin. Data were extracted independently by two researchers and were used for the meta-analysis following their comparison and verification, including compliance checks for the norm. In addition to absolute values of parameters provided in the text or tables, we used data presented as graphs if they clearly displayed mean values and standard deviations/standard errors of mean. If data on gender/age groups were provided separately, we calculated arithmetic mean values. In some cases (when a publication listed lipid profile parameters, but missed some of them), we used the Friedewald equation [49] to calculate the missing data in mg/dl: LDL = TC – (TG/5)–HDL.
When there were a sufficient number of studies, we performed a meta-analysis of dependence of circulating lipids’ and carbohydrates’ seasonal dynamics on gender and age. This analysis relied only on research that simultaneously studied different age/gender groups and provided data separately for each group.
Moreover, when there were a sufficient number of publications, we analyzed dependence of circulating lipids’ and carbohydrates’ seasonal dynamics (winter vs. summer) on regional climate’s special characteristics (geographical latitude and amplitude of seasonal fluctuations of such meteorological parameters as temperature, atmospheric pressure, relative humidity, and partial density of oxygen in the air (ρO2)). If a study contained meteorological data, we used them. In the absence of such data, we used archival data to calculate meteorological parameters, as we described in our earlier published work [9]. Depending on annual fluctuations in a meteorological factor in a region of a study, publications were divided into two subgroups: one with the maximum amplitude of change and another with the minimum amplitude of change of a meteorological factor.
Statistics
The meta-analysis of published results was performed using Review Manager 5.3 (Cochrane Library) statistical program. We used an inverse variance test (mean difference) in our analysis. Criterion I2 was used to establish heterogeneity of studies included in the meta-analysis. The choice of fixed- or randomized-effects model was made in accordance with recommendations of Borenstein et al. (2009) [50]. We used Z-test to assess the statistical significance of overall results. The confidence interval was 95%. Differences were considered statistically significant at p < 0.05. The funnel plot was used to detect publication bias.
Results
Database search yielded 5699 publications on the subject of our meta-analysis, 483 of them overviews. We selected 29 publications for the meta-analysis [51–78]: 16 panel studies and 13 cross-sectional studies (Fig. 1). Koono’s publication (1980) reported the results of a panel and cross-functional study [62]. Tables 1 presents characteristics of studies included in the meta-analysis. Only average and high quality studies were selected for our meta-analysis (Table S1). 39 publications were excluded for various reasons (Fig. 1, Table S2). Evaluation of funnel plots demonstrated the absence of significant bias in the selected publications (Fig. S1).
Table 1.
Publications selected for meta-analysis
| Publication | Study period | Biochemical parameter |
Design | Sex, male (%) |
Average age | Blood sampling | Number of persons in group | Research location | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bio-material | Morning fasting |
Winter | Spring | Summer | Autumn | |||||||
| Anthanont P., 2017 [51] | 1995–2012 | TG, TC, LDL, HDL, FBG, IN | CS | 54 | 41 | Pl | + | 220 | − | 214 | − | Rochester |
| Boĭko E.R., 1994 [52] | – | TG, TC | CS | 100 | 33 | S | + | 58 | 51 | 75 | 40 | Naryan-Mar |
| Calton E., 2017 [53] | 2015–2016 | TG, TC, LDL, HDL, FBG, IN | P | 50 | 45 | − | + | 30 | − | 30 | − | Perth |
| Doyle J., 1965 [54] | 1961–1962 | TC | P | 100 | 54 | S | + | 52 | 52 | 52 | 52 | Albany |
| Fuller J., 1974 [55] | 1971–1972 | TG, TC | CS | 48 | 41.5 | Pl | + | 73 | 80 | 73 | 80 | London |
| Hattori T., 2015 [56] | 2010–2011 | LDL, HDL, FBG | P | 100 | 37.6 | Pl | + | 79 | − | 79 | − | Sendai |
| Hoffman A., 1967 [57] | – | TC | CS | 100 | 48 | P | + | 101 | − | 125 | − | Washington |
| Jarrett R., 1984 [58] | 1975–1978 | FBG | CS | 84* | 58 | C | + | 460 | 455 | 282 | 497 | London |
| Kamezaki F., 2010 (a) [59] | 2008 | TG, LDL, HDL | P | 90 | 43 | S | + | 1331 | − | 1331 | − | Nagoya |
| Kamezaki F., 2010 (b) [60] | 2008 | FBG | P | 100 | 43 | S | + | 1202 | − | 1202 | − | Nagoya |
| Kochan T.I., 2009 [61] | – | TG, TC, FBG | CS | − | 39** | Pl | + | 44 | 68 | 20 | 51 | Syktyvkar |
| Koono N., 1980 (1) [62] | 1978–1979 | TC | P | 100 | 42 | S | + | 15 | − | 15 | − | Sapporo |
| Koono N., 1980 (2) [62] | 1978–1979 | TC | CS | 100 | 42 | S | + | 49 | − | 58 | − | Sapporo |
| Kreindl C., 2014 [63] | 2005–2006 | TG, TC, LDL, HDL, FBG | P | 47* | 40 | − | + | 43 | 43 | 43 | 43 | Santiago |
| Kristal-Boneh E., 1993 [64] | 1985–1987 | TC | CS | 71* | 42 | S | + | 1607 | 1475 | 1191 | 944 | Ra'anana |
| Kuroshima A., 1979 [65] | – | FBG | P | 62* | 31 | Pl | + | 21 | 21 | 21 | 21 | Asahikawa |
| Levy S., 2016 [66] | 2009–2011 | TG, TC, LDL, HDL, FBG | P | 38* | 50 | S | + | 115 | − | 115 | − | Berdigestyakh |
| Lucas J., 2005 [67] | 1997–2000 | FBG | CS | 0 | 74 | − | + | 386 | 396 | 382 | 442 | Auckland |
| Murciano Revert J., 2000 [68] | 1999 | TG, TC, LDL, HDL, FBG | P | 39 | 63 | Pl | + | 86 | − | 86 | − | Valencia |
| Ockene I., 2004 [69] | 1994–1998 | TG, TC, LDL, HDL | P | 51* | 49 | S | + | 476 | 476 | 476 | 476 | Worcester |
| Otto C., 1996 [70] | 1994 | TG, TC, LDL, HDL | P | 21 | 37.9 | Pl | + | 14 | − | 14 | − | Munich |
| Pham D., 2020 [71] | 2009–2017 | FBG | CS | 44* | 44.5 | Pl | + | 169 | 330 | 148 | 220 | Seoul |
| Sasaki J., 1983 [72] | 1979–1980 | TG, TC, LDL, HDL | P | 100 | 39 | S | + | 31 | 31 | 31 | 31 | Fukuoka |
| Shahar D., 1999 [73] | – | TG, TC, LDL, HDL | P | 100 | 43 | S | + | 94 | − | 94 | − | Ra'anana |
| Solonin I.G., 2014 [74] | – | TG, TC, LDL, HDL, FBG | P | 100 | 32 | Pl | + | 17 | 17 | 17 | 17 | Syktyvkar |
| Suarez L., 1982 [75] | 1972–1974 | FBG | CS | 56* | 50** | Pl | + | 924 | 952 | 589 | 1169 | San Diego |
| Sung K.C., 2006 [76] | 2002–2003 | TG, TC, LDL, HDL, FBG | CS | 65 | 47 | − | + | 2631 | 2177 | 3445 | 3632 | Seoul |
| Walker B.R., 1997 [77] | – | FBG, IN | CS | 100 | 28.5 | Pl | + | 41 | 16 | 25 | 23 | Edinburgh |
| Wood A., 2012 [78] | 2009–2010 | TG, TC, LDL, HDL, FBG, IN | P | 0 | 64 | S | + | 84 | 84 | 84 | 84 | Aberdeen |
TG is triglycerides.TC is total cholesterol. LDL is low-density lipoprotein. HDL is high-density lipoprotein. FBG is fasting blood glucose. IN is insulin. P is panel study. CS is cross-sectional study. S is serum. Pl is plasma. C is capillary blood. * is investigated separately men and women. ** is investigated separately young and old people. ( −) is no information
We used only TC data from study [57] because the it provided only mean values for TG without SD or SEM. We used FBG data for men and women who were not on hormone therapy from Suarez and Barrett-Connor study [75].
According to the results of our meta-analysis, the level of circulating TG did not exhibit prominent seasonal dynamics. TC, LDL levels were higher in the colder season compared to the warmer one. The differences were statistically significant when comparing winter against summer levels; TC levels were statistically significant when comparing winter and autumn as well (Table 2). HDL levels were higher in winter compared to summer (Table 2). FBG levels were higher in winter compared against summer and autumn, while insulin levels were not significantly different in winter and summer (Table 2). A study by Sung (2006) [76] was excluded from the following comparisons due to excessive weight: for TG (spring vs. summer), for TC (autumn vs. summer, spring vs. summer, spring vs. autumn), and for LDL (autumn vs. summer, spring vs. autumn).
Table 2.
The seasonal dynamics of circulating lipids and carbohydrates
| Compared seasons | Number of studies | Mean difference | I2% | Test for overall effect | |||
|---|---|---|---|---|---|---|---|
| Season 1/total | Season 2/total | Rand. or Fix | Z | P | |||
| Triglycerides, mg/dl | |||||||
| Winter/5283 | Summer/6109 | 16 | − 1.41 [− 9.61, 6.79] | 80 | R | 0.34 | 0.74 |
| Winter/3455 | Spring/3025 | 9 | − 0.43 [− 14.91, 14.05] | 81 | R | 0.06 | 0.95 |
| Winter/3455 | Autumn/4452 | 9 | − 6.35 [− 19.38, 6.68] | 76 | R | 0.96 | 0.34 |
| Autumn/4452 | Summer/4262 | 9 | 0.59 [− 11.84, 13.03] | 72 | R | 0.09 | 0.93 |
| Spring/848 | Summer/817 | 8 | − 4.90 [− 12.10, 2.29] | 0 | F | 1.34 | 0.18 |
| Spring/3025 | Autumn/4452 | 9 | − 6.95 [− 18.60, 4.71] | 71 | R | 1.17 | 0.24 |
| Total cholesterol, mg/dl | |||||||
| Winter/7107 | Summer/7550 | 21 | 6.10 [3.10, 9.11] | 50 | R | 3.98 | 0.0001 |
| Winter/5114 | Spring/4552 | 11 | 4.12 [− 1.31, 9.56] | 59 | R | 1.49 | 0.14 |
| Winter/5114 | Autumn/5448 | 11 | 4.97 [0.21, 9.74] | 49 | R | 2.05 | 0.04 |
| Autumn/1816 | Summer/2060 | 10 | 2.17 [− 1.97, 6.30] | 0 | F | 1.03 | 0.30 |
| Spring/2375 | Summer/2060 | 10 | 3.08 [− 1.10, 7.27] | 0 | F | 1.44 | 0.15 |
| Spring/2375 | Autumn/1816 | 10 | 0.96 [− 3.22, 5.13] | 0 | F | 0.45 | 0.65 |
| Low-density lipoproteins, mg/dl | |||||||
| Winter/5183 | Summer/6011 | 14 | 8.76 [3.98, 13.55] | 87 | R | 3.59 | 0.0003 |
| Winter/3279 | Spring/2825 | 6 | 6.23 [− 2.07, 14.54] | 84 | R | 1.47 | 0.14 |
| Winter/3279 | Autumn/4280 | 6 | 7.11 [− 2.51, 16.73] | 88 | R | 1.45 | 0.15 |
| Autumn/648 | Summer/648 | 5 | 3.27 [− 1.22, 7.76] | 0 | R | 1.43 | 0.15 |
| Spring/2825 | Summer/4093 | 6 | 5.00 [− 0.78, 10.78] | 66 | R | 1.69 | 0.09 |
| Spring/648 | Autumn/648 | 5 | 0.42 [− 4.17, 5.01] | 0 | R | 0.18 | 0.86 |
| High-density lipoproteins, mg/dl | |||||||
| Winter/5188 | Summer/6023 | 14 | 1.73 [0.42, 3.04] | 58 | R | 2.58 | 0.01 |
| Winter/3282 | Spring/2828 | 6 | 0.27 [− 1.68, 2.21] | 51 | R | 0.27 | 0.79 |
| Winter/3282 | Autumn/4283 | 6 | − 0.19 [− 2.84, 2.46] | 73 | R | 0.14 | 0.89 |
| Autumn/4283 | Summer/4096 | 6 | 0.50 [− 0.69, 1.69] | 20 | R | 0.82 | 0.41 |
| Spring/2828 | Summer/4096 | 6 | 0.03 [− 1.63, 1.69] | 41 | R | 0.03 | 0.97 |
| Spring/2828 | Autumn/4283 | 6 | − 0.17 [− 2.68, 2.35] | 70 | R | 0.13 | 0.90 |
| Fasting blood glucose, mg/dl | |||||||
| Winter/6488 | Summer/6730 | 17 | 2.46 [0.76, 4.16] | 80 | R | 2.84 | 0.005 |
| Winter/4814 | Spring/4553 | 11 | 1.24 [− 0.75, 3.23] | 73 | R | 1.22 | 0.22 |
| Winter/4814 | Autumn/6193 | 11 | 2.42 [0.50, 4.35] | 72 | R | 2.46 | 0.01 |
| Autumn/6193 | Summer/5050 | 11 | − 0.03 [− 1.43, 1.37] | 48 | R | 0.04 | 0.97 |
| Spring/4553 | Summer/5050 | 11 | 1.19 [− 1.57, 3.95] | 87 | R | 0.85 | 0.40 |
| Spring/4553 | Autumn/6193 | 11 | 1.19 [− 1.15, 3.52] | 83 | R | 1.00 | 0.32 |
| Insulin, mU/l | |||||||
| Winter/344 | Summer/326 | 4 | − 0.04 [− 0.51, 0.44] | 0 | F | 0.16 | 0.88 |
According to studies which simultaneously considered male and female subjects, gender did not affect the seasonal dynamics of TG, TC, LDL, HDL, and FBG (Table 3). It should be noted that, according to the results of studies included herein, men and women did not exhibit significant differences in the studied parameters, with the exception of a slight increase in the TG levels for men (136 ± 48 mg/dl vs. 109 ± 22 mg/dl, p = 0.25).
Table 3.
Associations of circulating lipids and glucose seasonal dynamics (winter versus summer) with gender
| Subgroup | N study | Total | Mean difference (winter vs summer) | I2% | Z | P | Test for subgroup differences, P | The presence of an effect | Diversity in the representation of climatic zones | |
|---|---|---|---|---|---|---|---|---|---|---|
| winter | summer | |||||||||
| Triglycerides, mg/dl | ||||||||||
| Men | 3 | 308 | 308 | 8.93 [− 3.49, 21.36] | 0 | 1.41 | 0.16 | 0.88 | No | Yes |
| Women | 3 | 326 | 326 | 7.82 [0.09, 15.56] | 0 | 1.98 | 0.05 | Yes | ||
| Total cholesterol, mg/dl | ||||||||||
| Men | 4 | 1338 | 1031 | 10.21 [1.60, 18.81] | 49 | 2.32 | 0.02 | 0.62 | No | Yes |
| Women | 4 | 903 | 794 | 7.64 [2.41, 12.86] | 0 | 2.86 | 0.004 | Yes | ||
| Low-density lipoproteins, mg/dl | ||||||||||
| Men | 3 | 308 | 308 | 10.66 [− 5.10, 26.43] | 78 | 1.33 | 0.19 | 0.75 | No | Yes |
| Women | 3 | 326 | 326 | 7.50 [− 4.35, 19.35] | 66 | 1.24 | 0.21 | Yes | ||
| High-density lipoproteins, mg/dl | ||||||||||
| Men | 3 | 308 | 308 | 2.33 [− 2.70, 7.37] | 62 | 0.91 | 0.36 | 0.93 | No | Yes |
| Women | 3 | 326 | 326 | 1.99 [− 3.39, 7.36] | 77 | 0.72 | 0.47 | Yes | ||
| Fasting blood glucose, mg/dl | ||||||||||
| Men | 6 | 1093 | 686 | 2.88 [− 1.06, 6.82] | 78 | 1.43 | 0.15 | 0.92 | No | Yes |
| Women | 6 | 639 | 512 | 3.20 [− 1.65, 8.05] | 79 | 1.29 | 0.20 | Yes | ||
The seasonal dynamics of lipids and carbohydrates were studied in 23 regions of the world (20 regions in the Northern Hemisphere and 3 regions of the Southern Hemisphere) with different types of climate ranging from subarctic to tropical. Main climate characteristics of the studied regions are presented in Table 4.
Table 4.
Annual fluctuations of meteorological factors in the studied regions (regions are arranged in decreasing order of geographical latitude)
| Regions | Air temperature °C | Atmospheric pressure hPa | Atmospheric pressure variability hPa | Humidity % | ρO2 g/m3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | |
| North Hemisphere | ||||||||||
| Naryan-Mar, Russia, 67.4° N, 53° E, 7 m asl | − 16.0 | 11.0 | 1004 | 1006 | 11.9 | 6.5 | 95 | 75 | 315 | 284 |
| Berdigestyakh, Sakha, Russia, 62° N, 126.4°, 212 m asl | − 33.6 | 19.5 | 993 | 976 | 9.7 | 4.1 | 68 | 61 | 335 | 266 |
| Syktyvkar, Russia, 61.4°N, 50.5°E, 172 m asl | − 25.8 | 18.0 | 1000 | 993 | 12.9 | 5.9 | 83 | 72 | 328 | 273 |
| Aberdeen, UK, 57° N, 2° W, 13 m asl | 2.9 | 14.7 | 999 | 1002 | 14.0 | 6.5 | 88 | 85 | 291 | 278 |
| Edinburgh, Scotland, UK, 55.6° N, 3.1° W, 47 m asl | 5.2 | 12.3 | 1000 | 1003 | 14.0 | 6.4 | 83 | 78 | 289 | 281 |
| London, UK, 51.3º'N, 00ºW, 22 m asl | 5.1 | 17.0 | 1010 | 1010 | 11.8 | 5.9 | 83 | 70 | 292 | 278 |
| Munich, Germany, 48°N, 11.3°E, 519 m asl | − 1.0 | 17.1 | 953 | 952 | 9.0 | 3.9 | 88 | 80 | 282 | 261 |
| Asahikawa, Japan, 43.5° N, 142.2°E, 121 m asl | − 5.7 | 21.4 | 995 | 992 | 7.3 | 4.1 | 70 | 77 | 300 | 267 |
| Sapporo, Japan, 43° N, 141.2°E, 17 m asl | − 3.6 | 22.9 | 1007 | 1003 | 7.6 | 4.5 | 70 | 77 | 301 | 268 |
| Albany, New York, USA, 42.4º'N, 73.4ºW, 60 m asl | − 3.0 | 21.7 | 1013 | 1010 | 8.8 | 4.8 | 68 | 72 | 302 | 272 |
| Worcester, Massachusetts, USA, 42.2° N 71.5°W, 146 m asl | 0.1 | 23.1 | 997 | 998 | 9.0 | 5.1 | 66 | 73 | 294 | 267 |
| Rochester, Minnesota, USA, 40° N, 92° 27′W, 312 m asl | − 4.6 | 23.0 | 965 | 965 | 7.7 | 4.5 | 78 | 78 | 290 | 258 |
| Valencia, Spain, 39.3° N, 00.2° W, 15 m asl | 12.8 | 25.8 | 1011 | 1012 | 7.9 | 3.4 | 61 | 67 | 283 | 268 |
| Washington, District of Columbia, USA, 38.9° N, 77° W, 125 m asl | 3.5 | 25.4 | 1004 | 1001 | 8.0 | 4.3 | 65 | 67 | 291 | 264 |
| Sendai, Japan, 38.1° N, 140.5° E, 57 m asl | 5.7 | 28.1 | 1008 | 1006 | 5.8 | 5.1 | 61 | 77 | 291 | 262 |
| Seoul, South Korea, 37.3° N, 127° E, 38 m asl | 0.9 | 23.9 | 1008 | 993 | 5.0 | 4.3 | 60 | 73 | 297 | 265 |
| Nagoya, Japan, 35° N, 136.5°E, 75 m asl | 5.6 | 26.1 | 1009 | 999 | 5.3 | 3.6 | 59 | 65 | 291 | 264 |
| Fukuoka, Japan, 33° N, 130° E, 3 m asl | 8.7 | 26.9 | 1016 | 1003 | 4.9 | 3.8 | 63 | 74 | 289 | 263 |
| San Diego, California, USA, 33° N 116° W, 22 m asl | 14.0 | 21.0 | 1015 | 1010 | 3.3 | 2.0 | 68 | 76 | 283 | 272 |
| Ra'anana, Israel, 32° N, 34.5°E, 40 m asl | 16.7 | 29.0 | 1011 | 1002 | 4.2 | 1.6 | 73 | 68 | 278 | 261 |
| Southern hemisphere | ||||||||||
| Auckland, New Zealand, 36.5° S, 174.5° E, 196 m asl | 11.3 | 19.0 | 1012 | 1012 | 8.9 | 5.5 | 85 | 77 | 283 | 275 |
| Santiago, Chile, 33.3°S, 70.4°W, 520 m asl | 10.3 | 20.6 | 954 | 950 | 2.7 | 2.1 | 83 | 53 | 269 | 258 |
| Perth, Australia, 31.6° S, 115.5° E, 2 m asl | 14.0 | 26.0 | 1015 | 1007 | 6.0 | 3.3 | 71 | 56 | 283 | 267 |
The trend in the seasonal dynamics of glucose and lipid profile did not vary significantly for the Northern and Southern Hemispheres. In the Southern Hemisphere, according to the results of three studies [53, 63, 67], the level of TG, TC, LDL, HDL, and FBG was higher in winter than in summer.
Based on the results of our meta-analysis, seasonal TG dynamics (winter vs. summer) did not significantly depend on geographical latitude, amplitude of circannual changes in atmospheric pressure, relative humidity, and ρO2 in the air (Table 5). But annual fluctuations in air temperature affected seasonal TG dynamics. In a climate with significant annual changes in air temperature, TG exhibited prominent seasonal dynamics with maximum values in winter and minimum values in summer; in the regions where the difference between winter and summer temperatures was small, seasonal TG dynamics were not prominent (Fig. 2, Table 5).
Table 5.
Associations of circulating lipids and glucose seasonal dynamics (winter versus summer) with latitude and annual changes in meteorological factors
| Meteorological parameter | N study | Total | Mean difference (winter vs summer) | I2% | Z | P | Test for subgroup differences, P | The presence of an effect | Diversity in the representation of climatic zones | |
|---|---|---|---|---|---|---|---|---|---|---|
| Winter | Summer | |||||||||
| Triglycerides, mg/dl | ||||||||||
| above 50º latitude | 6 | 389 | 382 | − 2.97 [− 26.57, 20.64] | 91 | 0.25 | 0.81 | 0.67 | No | Yes |
| below 36º latitude | 5 | 1529 | 1529 | 2.25 [− 2.75, 7.24] | 0 | 0.88 | 0.38 | Yes | ||
| ∆t > 25ºC | 5 | 392 | 404 | 11.36 [1.78, 20.95] | 39 | 2.32 | 0.02 | 0.08 | Yes | Yes |
| ∆t < 13ºC | 6 | 408 | 408 | − 10.51 [− 32.76, 11.74] | 83 | 0.93 | 0.35 | Yes | ||
| ∆P ≥ 8 hPa | 6 | 4232 | 5046 | 1.25 [− 5.84, 8.35] | 63 | 0.35 | 0.73 | 0.85 | No | Yes |
| ∆P < 4 hPa | 7 | 947 | 983 | − 1.00 [− 22.68, 20.67] | 90 | 0.09 | 0.93 | Yes | ||
| max ϕ in winter | 8 | 394 | 387 | − 2.52 [− 23.43, 18.39] | 88 | 0.24 | 0.81 | 0.91 | No | Yes |
| max ϕ in summer | 5 | 4555 | 5369 | − 1.35 [− 5.95, 3.26] | 20 | 0.57 | 0.57 | Yes | ||
| ∆ρO2 > 29 g/m3 | 6 | 3023 | 3849 | 6.75 [− 4.98, 18.49] | 80 | 1.13 | 0.26 | 0.18 | No | Yes |
| ∆ρO2 < 18 g/m3 | 6 | 408 | 408 | − 10.51 [− 32.76, 11.74] | 83 | 0.93 | 0.35 | Yes | ||
| Total cholesterol, mg/dl | ||||||||||
| above 50º latitude | 6 | 389 | 382 | 12.59 [0.76, 24.43] | 72 | 2.09 | 0.04 | 0.39 | No | Yes |
| below 36º latitude | 6 | 3136 | 2720 | 7.24 [4.56, 9.93] | 0 | 5.29 | 0.00001 | Yes | ||
| ∆t > 23ºC | 10 | 3615 | 4450 | 8.52 [2.76, 14.28] | 66 | 2.90 | 0.004 | 0.55 | No | Yes |
| ∆t < 13ºC | 7 | 2015 | 1599 | 6.24 [1.46, 11.02] | 0 | 2.56 | 0.01 | Yes | ||
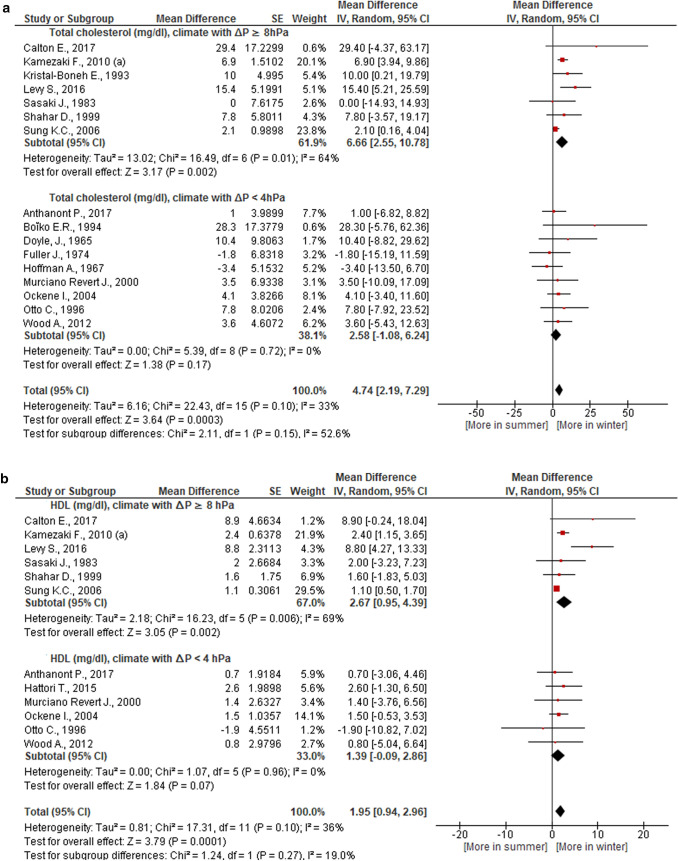
| ∆P ≥ 8 hPa | 7 | 5839 | 6237 | 6.66 [2.55, 10.78] | 64 | 3.17 | 0.002 | 0.15 | Yes | Yes |
| ∆P < 4 hPa | 9 | 1100 | 1160 | 2.58 [− 1.07, 6.24] | 0 | 1.38 | 0.17 | Yes | ||
| max ϕ in winter | 8 | 394 | 387 | 15.50 [5.13, 25.86] | 57 | 2.93 | 0.003 | 0.04 | Yes | Yes |
| max ϕ in summer | 7 | 4619 | 5442 | 4.10 [1.74, 6.46] | 22 | 3.40 | 0.0007 | Yes | ||
| ∆ρO2 > 29 g/m3 | 9 | 3139 | 3974 | 9.85 [2.83, 16.87] | 70 | 2.75 | 0.006 | 0.41 | No | Yes |
| ∆ρO2 < 18 g/m3 | 7 | 2015 | 1599 | 6.24 [1.46, 11.02] | 0 | 2.56 | 0.01 | Yes | ||
| Low-density lipoproteins, mg/dl | ||||||||||
| above 50º latitude | 3 | 213 | 213 | 22.96 [− 9.28, 55.20] | 97 | 1.40 | 0.16 | 0.37 | No | Yes |
| below 36º latitude | 5 | 1529 | 1529 | 7.93 [0.86, 15.01] | 46 | 2.20 | 0.03 | Yes | ||
| ∆t > 23ºC | 7 | 3473 | 4301 | 11.26 [1.11, 21.40] | 94 | 2.17 | 0.03 | 0.76 | No | Yes |
| ∆t < 13ºC | 5 | 334 | 334 | 9.21 [1.00, 17.43] | 39 | 2.20 | 0.03 | Yes | ||
| ∆P ≥ 8 hPa | 6 | 4232 | 5046 | 2.72 [− 0.11, 5.55] | 48 | 1.88 | 0.06 | 0.74 | No | Yes |
| ∆P < 4 hPa | 6 | 891 | 905 | 3.46 [0.01, 6.91] | 0 | 1.97 | 0.05 | Yes | ||
| max ϕ in winter | 5 | 219 | 219 | 24.57 [− 1.19, 50.33] | 93 | 1.87 | 0.06 | 0.09 | Yes | Yes |
| max ϕ in summer | 6 | 4634 | 5448 | 2.35 [− 0.01, 4.71] | 40 | 1.96 | 0.05 | Yes | ||
| ∆ρO2 > 29 g/m3 | 5 | 2997 | 3825 | 13.66 [0.53, 26.78] | 95 | 2.04 | 0.04 | 0.57 | No | Yes |
| ∆ρO2 < 18 g/m3 | 5 | 334 | 334 | 9.21 [1.00, 17.43] | 39 | 2.20 | 0.03 | Yes | ||
| High-density lipoproteins, mg/dl | ||||||||||
| above 50º latitude | 3 | 216 | 216 | − 2.23 [− 14.71, 10.25] | 90 | 0.35 | 0.73 | 0.49 | Yes/No | Yes |
| below 36º latitude | 5 | 1529 | 1529 | 2.20 [0.79, 3.60] | 7 | 3.06 | 0.002 | Yes | ||
| ∆t > 23ºC | 7 | 3475 | 4301 | 1.70 [− 0.81, 4.20] | 79 | 1.33 | 0.19 | 0.87 | No | Yes |
| ∆t < 13ºC | 5 | 337 | 337 | 1.41 [− 0.97, 3.80] | 2 | 1.16 | 0.25 | Yes | ||
| ∆P ≥ 8 hPa | 6 | 4232 | 5046 | 2.67 [0.95, 4.39] | 69 | 3.05 | 0.002 | 0.27 | Yes/No | Yes |
| ∆P < 4 hPa | 6 | 896 | 917 | 1.39 [− 0.09, 2.86] | 0 | 1.84 | 0.07 | Yes | ||
| max ϕ in winter | 5 | 219 | 219 | − 0.65 [− 9.49, 8.19] | 84 | 0.14 | 0.88 | 0.65 | Yes/No | Yes |
| max ϕ in summer | 6 | 4634 | 5448 | 1.38 [0.87, 1.89] | 0 | 5.27 | 0.00001 | Yes | ||
| ∆ρO2 > 29 g/m3 | 5 | 2999 | 3834 | 1.33 [− 2.53, 5.20] | 83 | 0.68 | 0.50 | 0.97 | No | Yes |
| ∆ρO2 < 18 g/m3 | 5 | 337 | 337 | 1.41 [− 0.97, 3.80] | 2 | 1.16 | 0.25 | Yes | ||
| Fasting blood glucose, mg/dl | ||||||||||
| above 50º latitude | 6 | 755 | 537 | 3.63 [− 1.74, 8.99] | 89 | 1.32 | 0.19 | 0.68 | No | Yes |
| below 36º latitude | 5 | 2585 | 2246 | 5.03 [0.93, 9.13] | 67 | 2.41 | 0.02 | Yes | ||
| ∆t > 23ºC | 7 | 3159 | 3934 | 3.12 [0.04, 6.19] | 89 | 1.98 | 0.05 | 0.85 | No | Yes |
| ∆t < 13ºC | 8 | 2048 | 1515 | 2.71 [− 0.31, 5.72] | 65 | 1.76 | 0.08 | Yes | ||
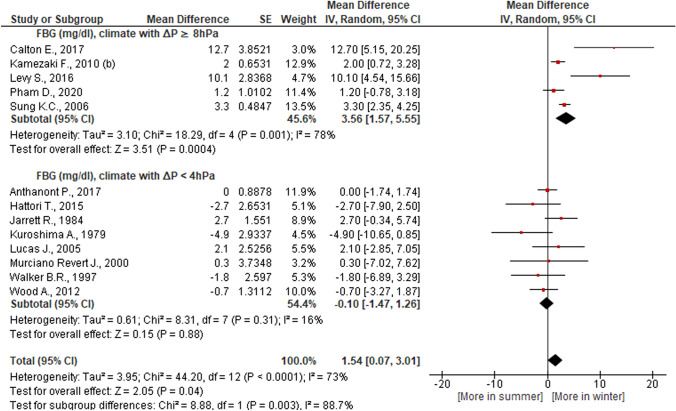
| ∆P ≥ 8 hPa | 5 | 4147 | 4940 | 3.56 [1.57, 5.55] | 78 | 3.51 | 0.0004 | 0.003 | Yes | Yes |
| ∆P < 4 hPa | 8 | 1313 | 1121 | − 0.10 [− 1.47, 1.26] | 16 | 0.15 | 0.88 | Yes | ||
| max ϕ in winter | 8 | 750 | 532 | 5.39 [− 0.41, 11.20] | 84 | 1.82 | 0.07 | 0.23 | No | Yes |
| max ϕ in summer | 7 | 5112 | 5570 | 1.71 [− 0.05, 3.47] | 70 | 1.90 | 0.06 | Yes | ||
| ∆ρO2 > 29 g/m3 | 8 | 3238 | 4013 | 2.46 [− 0.45, 5.36] | 88 | 1.66 | 0.10 | 0.91 | No | Yes |
| ∆ρO2 < 18 g/m3 | 8 | 2048 | 1515 | 2.71 [− 0.31, 5.72] | 65 | 1.76 | 0.08 | Yes | ||
∆t is amplitude of annual fluctuations in air temperature. ∆P is amplitude of annual fluctuations in mean monthly atmospheric pressure. ϕ is relative humidity. ∆ρO2 is amplitude of annual fluctuations in partial density of oxygen in air
Fig. 2.
Dependence of triglycerides seasonal dynamics (winter versus summer) on the amplitude of annual fluctuations in air temperature
We did not establish the existence of a dependence of seasonal TC and FBG dynamics (winter vs. summer) on geographic latitude, as well as on the amplitude of circannual fluctuations in air temperature and ρO2 in the air (Table 5). As for seasonal LDL and HDL dynamics, they were more prominent in the regions located below 36 latitude, but the compared subgroup (above 50 latitude) comprised only 3 studies with high data heterogeneity (Table 5). Elimination of the study with great weight [59] canceled this pattern for HDL.
Seasonal TC, HDL, and FBG fluctuations were more prominent in a climate that had a pronounced increase in average monthly atmospheric pressure in winter compared to summer as compared against a climate where atmospheric pressure in winter and in summer did not vary significantly (Table 5, Figs. 3 and 4). The first type of climate is typical for the regions of East Asia, the Middle East, South Australia, the second type of climate is typical for the temperate zones of Europe and America, New Zealand (Table 4). Elimination of great weight studies [59, 76] from our meta-analysis did not change these patterns.
Fig. 3.
Dependence of total cholesterol and high density lipoproteins (HDL) seasonal dynamics (winter versus summer) on the amplitude of annual fluctuations in atmospheric pressure
Fig. 4.
Dependence of glucose (FBG) seasonal dynamics (winter versus summer) on the amplitude of annual fluctuations in atmospheric pressure
In the regions with wet winters, seasonal TC changes were significantly higher than in the regions with wet summers, most likely due to seasonal changes in LDL, because seasonal HDL dynamics with a winter maximum were more prominent in the regions with wet summers (Fig. 5, Table 5). Extractions from great weight studies (Kamezaki et al. 2010 (a); Sung et al. 2006 [59, 76]) did not change these patterns. Relative air humidity did not affect seasonal FBG dynamics (Table 5).
Fig. 5.
Dependence of total cholesterol, low and high density lipoproteins seasonal dynamics (winter versus summer) on annual fluctuations in relative humidity (ϕ)
A complete statistical analysis is presented in the Supplementary Materials (Fig. S1-S7).
Discussion
Our meta-analysis showed that healthy individuals in both the Northern and Southern hemispheres have higher TC, LDL, HDL, and FBG levels in winter than in summer. Insulin had no prominent seasonal dynamics. According to the results of our meta-analysis, the level of circulating TG had prominent seasonal dynamics with maximum values during winter only in the regions with a large difference in winter and summer air temperatures. Such seasonal fluctuations in air temperature are typical of a continental climate. It is possible that people living in such conditions experience maximum seasonal lifestyle changes. Moreover, seasonal TG dynamics are likely to be affected by an increase in sebum secretion in warm weather and a decrease in cold weather. Sebum is known to consist of 34% TG and only 3% cholesterol [79, 80].
According to our earlier meta-analysis, levels of circulating T3, TSH and noradrenaline, as well as hematocrit [9, 25, 46], increase in winter compared to summer. It can also contribute to seasonal changes in lipid profile and glucose. Seasonal TSH, cortisol and hematocrit dynamics were not associated with geographical latitude and the amplitude of circannual fluctuations in air temperature [9, 25, 46]. Similarly, according to the results of this meta-analysis, seasonal TC, LDL, HDL, and FBG dynamics did not depend on latitude and the amplitude of circannual fluctuations in air temperature. Latitude also did not affect seasonal TG dynamics. On the other hand, circannual fluctuations of TC, HDL, and FBG were more prominent in a climate where atmospheric pressure in winter is significantly higher than in summer. Earlier, we established that seasonal TSH and hematocrit dynamics were also more pronounced in regions with this type of climate [9, 25]. It is known that the values of TSH and hematocrit are associated with the level of circulating lipids and glucose [5–8, 22–24].
We have previously shown that high air humidity in summer is associated with prominent seasonal TSH dynamics with a nadir in summer [9]. As a result of this meta-analysis, it was found that in the regions with a similar type of climate there are pronounced seasonal HDL dynamics with a nadir in summer. On the other hand, in the regions where summers are drier than winters, there is a prominent drop in TC over summer as compared with winter, most likely due to a decrease in LDL. In the regions where air humidity is higher in summer than in winter, the amplitude of seasonal TC fluctuations was significantly lower. This may be due to the fact that an increase in air humidity during heat is associated with a decrease in sweat evaporation and compromises body cooling, which can cause heat stress symptoms [81, 82]. It was shown that an increase in circulating TC is observed under heat stress mainly due to an increase in LDL [26, 27, 83]. Moreover, high humidity in winter, especially in the absence of central heating, can exacerbate the discomfort from cold weather.
The results of our prior meta-analysis [9], as well as studies carried out in a mountainous climates and experimental work on the effects of hypoxia [10, 84, 85], show that TSH depends on air ρO2. There were observations of a decrease in the corpuscular volume of erythrocytes and hematocrit during mild hypoxia [86]. A number of studies have shown that moderate hypoxia led to a decrease in the level of TC, lipoproteins and FBG [87, 88]. According to the results of this meta-analysis, the seasonal dynamics of lipid profile and glucose were not associated with ρO2 in the air calculated according to the Mendeleev-Clapeyron equation [89]. This may be due to the fact that ρO2 does not affect these parameters directly, but through modulation of TSH levels. In addition, Mendeleev-Clapeyron equation takes into account atmospheric pressure, temperature, and air humidity [89], but the partial pressure of alveolar gas mainly depends on atmospheric pressure [90]. Moreover, the influence of air temperature can be minimized by heating and air conditioning, but the influence of atmospheric pressure cannot be changed. As we discussed above, in the regions where atmospheric pressure is significantly lower in summer than in winter, summer decrease in the level of TSH, TC, HDL, FBG and hematocrit is more pronounced than in a climate with the same atmospheric pressure in winter and summer. Interesting to note, that low atmospheric pressure contributes to an increase in the risk of ischemic strokes in the hot season [91], despite a decrease in TC, FBG, hematocrit and blood pressure in summer [25, 92].
We have previously found that seasonal TSH dynamics (with a maximum in winter and a nadir in summer) are prominent in women, but not in men [9]. On the other hand, we did not find dependence of cortisol’s and hematocrit’s seasonal dynamics on gender [25, 46]. Many authors report metabolism differences between men and women [93]. Some studies show differences in levels of circulating lipids and glucose in men and women [94–97]. This meta-analysis found no differences in the seasonal dynamics of lipid profile and glucose of healthy men and women.
Aging is known to be associated with major changes in metabolism. A number of publications report significant increases in lipid profile and glucose indicators in older subjects [61, 64, 75, 98]. Still, there are very few studies that explore the seasonal dynamics of these parameters simultaneously on different age groups for us to perform comprehensive statistical analysis. Therefore, this issue was not considered in this meta-analysis. In the study [75], seasonal FBG dynamics did not depend on age. The study [61] showed that in a group aged 18–20 years FBG levels were higher in summer than in winter, while in subjects aged 30–59 years the seasonal dynamics of FBG were inversed. In this study [61], age did not affect the seasonal dynamics of TG and TC. We have previously shown that TSH seasonal dynamics did not depend on age [9].
Based on the results hereof and our earlier meta-analyses [9, 25], we can conclude that the seasonal dynamics of circulating lipids and glucose depend on special characteristics of regional climates and are also frequently associated with the seasonal dynamics of TSH and hematocrit. The results of our meta-analyses did not show an association of geographic latitude with the severity of the seasonal fluctuations in the parameters, which calls into question the influence of photoperiod on the functioning of the body of a modern person. This is probably due to the use of artificial lighting throughout the year. Despite the fact that the amplitude of annual fluctuations in air temperature was associated only with the seasonal dynamics of TG, the level of TSH, TC, LDL was higher in the colder season compared to the warmer one. This indicates that air temperature has an impact on the biochemical parameters of a modern person, but its effect can be offset by the use of heating and air conditioning. For example, in Syktyvkar, in winter, outdoor air temperature dropped to -25 ºC, but at the same time, inside air temperature was 21–22 ºC throughout the year [74]. In the work [99], an inverse relationship between indoor temperature and lipid profile indicators was observed in winter. In contrast, the effect of relative humidity is more difficult to decrease (especially, to reduce high humidity in hot weather), the effect of atmospheric pressure cannot be reduced. According to the results of our meta-analyses, annual fluctuations of these meteorological factors have a great influence on the seasonal dynamics of the studied biochemical parameters. The results of our work indicate that the annual dynamics of atmospheric pressure and relative humidity must be taken into account when studying seasonal rhythms. The most likely mechanism of action of atmospheric pressure on the body is its effect on blood oxygenation. High relative humidity and heat increase the hypoxic effect of low atmospheric pressure. In addition, high humidity increases the feeling of cold in winter and contributes to heat stress in hot weather.
Limitations
Meta-analysis has some constraints. Compared subgroups sometimes varied significantly by the number of included studies and sampling size; studies selected for meta-analysis had different observation periods, which may have influenced their results. We would also like to point out the lack of accuracy in the processing of meteorological data when archives do not have data for the period under consideration and missing data are replaced with 10-year statistics, but circannual trends are rather stable, and we focused on maximum climatic differences when comparing subgroups. When studying dependence of seasonal parameter fluctuations on geographic latitude and circannual changes in meteorological factors, we compared winter and summer as seasons with the most contrasting and stable weather conditions. We did not take into account the month of registration of the parameters, although it is known that the weather can be different even during one season.
The selected publications did not take into account the laboratory method for determining the studied indicators.
It should be noted that studies of seasonal insulin dynamics comprised only four works, so it is not possible to draw a definitive conclusion about seasonal fluctuations of insulin based on the results of our meta-analysis.
This meta-analysis was carried out without taking into account ethnic characteristics and seasonal changes in subjects’ lifestyles (diet, holidays, fasting, prevalence of seasonal sports and participation in seasonal, e.g., agricultural, work, etc.). However, according to the results of our meta-analysis results, the vector of seasonal lipid profile and glucose dynamics coincided in the Northern and Southern Hemispheres, which indicates that, for example, Christmas holidays do not affect seasonal fluctuations of these parameters.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- TC
Total cholesterol
- LDL
Low-density lipoprotein
- HDL
High-density lipoprotein
- FBG
Fasting blood glucose
- TG
Triglycerides
- TSH
Thyroid-stimulating hormone of pituitary
- ρO2
Partial density of oxygen in the air
Funding
The authors received no financial support for research, authorship, and/or publication of this article.
Declaration
Conflict of interest
The authors declare that there is no potential conflict of interest regarding research, authorship, and/or publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marti-Soler H, Gubelmann C, Aeschbacher S, Alves L, Bobak M, Bongard V, et al. Seasonality of cardiovascularrisk factors: an analysis including over 230 000 participants in 15 countries. Heart. 2014;100:1517–1523. doi: 10.1136/heartjnl-2014-305623. [DOI] [PubMed] [Google Scholar]
- 2.Turner JB, Kumar A, Koch CA. The effects of indoor and outdoor temperature on metabolic rate and adipose tissue - the Mississippi perspective on the obesity epidemic. Rev Endocr Metab Disord. 2016;17(1):61–71. doi: 10.1007/s11154-016-9358-z. [DOI] [PubMed] [Google Scholar]
- 3.Patterson CC, Gyürüs E, Rosenbauer J, Cinek O, Neu A, Schober E, et al. Seasonal variation in month of diagnosis in children with type 1 diabetes registered in 23 European centers during 1989–2008: little short-term influence of sunshine hours or average temperature. Pediatr Diabetes. 2015;16(8):573–580. doi: 10.1111/pedi.12227. [DOI] [PubMed] [Google Scholar]
- 4.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos-Palacios S, Brugos-Larumbe A, Guillén-Grima F, Galofré JC. A cross-sectional study of the association between circulating TSH level and lipid profile in a large Spanish population. Clin Endocrinol (Oxf) 2013;79(6):874–881. doi: 10.1111/cen.12216. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Tan Y, Wang C, Zhang X, Zhao Y, Song X, et al. Thyroid-stimulating hormone levels within the reference range are associated with serum lipid profiles independent of thyroid hormones. J Clin Endocrinol Metab. 2012;97(8):2724–2731. doi: 10.1210/jc.2012-1133. [DOI] [PubMed] [Google Scholar]
- 7.Wanjia X, Chenggang W, Aihong W, Xiaomei Y, Jiajun Z, Chunxiao Y, et al. A high normal TSH level is associated with an atherogenic lipid profile in euthyroid non-smokers with newly diagnosed asymptomatic coronary heart disease. Lipids Health Dis. 2012;11:44. doi: 10.1186/1476-511X-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laclaustra M, Hurtado-Roca Y, Sendin M, Leon M, Ledesma M, Andres E, et al. Lower-normal TSH is associated with better metabolic risk factors: A cross-sectional study on Spanish men. Nutr Metab Cardiovasc Dis. 2015;25(12):1095–1103. doi: 10.1016/j.numecd.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Kuzmenko NV, Tsyrlin VA, Pliss MG, Galagudza MM. Seasonal variations in levels of human thyroid-stimulating hormone and thyroid hormones: a meta-analysis. Chronobiol Int. 2021;38(3):1–17. doi: 10.1080/07420528.2020.1865394. [DOI] [PubMed] [Google Scholar]
- 10.Sarne D. Effects of the Environment, Chemicals and Drugs on Thyroid Function. 2016 Sep 27. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000–. https://www.ncbi.nlm.nih.gov/books/NBK285560/ [PubMed]
- 11.Bjornstad P, Eckel RH. Pathogenesis of lipid disorders in insulin resistance: a brief review. Curr Diab Rep. 2018;18(12):127. doi: 10.1007/s11892-018-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thevis M, Thomas A, Schänzer W. Insulin. Handb Exp Pharmacol. 2010;195:209–226. doi: 10.1007/978-3-540-79088-4_10. [DOI] [PubMed] [Google Scholar]
- 13.Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol. 2017;234(1):R1–R21. doi: 10.1530/JOE-16-0513. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman RP. Sympathetic mechanisms of hypoglycemic counterregulation. Curr Diabetes Rev. 2007;3(3):185–193. doi: 10.2174/157339907781368995. [DOI] [PubMed] [Google Scholar]
- 15.Maduka IC, Neboh EE, Ufelle SA. The relationship between serum cortisol, adrenaline, blood glucose and lipid profile of undergraduate students under examination stress. Afr Health Sci. 2015;15(1):131–136. doi: 10.4314/ahs.v15i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varma VK, Rushing JT, Ettinger WH., Jr High density lipoprotein cholesterol is associated with serum cortisol in older people. J Am Geriatr Soc. 1995;43(12):1345–1349. doi: 10.1111/j.1532-5415.1995.tb06612.x. [DOI] [PubMed] [Google Scholar]
- 17.Coderre L, Srivastava AK, Chiasson JL. Role of glucocorticoid in the regulation of glycogen metabolism in skeletal muscle. Am J Physiol. 1991;260(6 Pt 1):E927–E932. doi: 10.1152/ajpendo.1991.260.6.E927. [DOI] [PubMed] [Google Scholar]
- 18.Oh KJ, Han HS, Kim MJ, Koo SH. Transcriptional regulators of hepatic gluconeogenesis. Arch Pharm Res. 2013;36(2):189–200. doi: 10.1007/s12272-013-0018-5. [DOI] [PubMed] [Google Scholar]
- 19.de Bruijn R, Romero LM. The role of glucocorticoids in the vertebrate response to weather. Gen Comp Endocrinol. 2018;269:11–32. doi: 10.1016/j.ygcen.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul. 2008; 42(4):111–9. PMID: 18999898 PMCID: PMC5522726 [PMC free article] [PubMed]
- 21.Greaney JL, Kenney WL, Alexander LM. Sympathetic regulation during thermal stress in human aging and disease. Auton Neurosci. 2016;196:81–90. doi: 10.1016/j.autneu.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adlercreutz H, Tallqvist G. Variations in the serum total cholesterol and hematocrit values in normal women during the menstrualcycle. Scand J Clin Lab Invest. 1959;11:1–9. doi: 10.3109/00365515909060400. [DOI] [PubMed] [Google Scholar]
- 23.Lopes GPR, Munhoz MAG, Antonangelo L. Evaluation of relationship between hematocrit and lipid profile in adults. J Bras Patol Med Lab. 2018;54(3):146–152. doi: 10.5935/1676-2444.20180027. [DOI] [Google Scholar]
- 24.Tamariz LJ, Young JH, Pankow JS, Yeh HC, Schmidt MI, Astor B, Brancati FL. Blood viscosity and hematocrit as risk factors for type 2 diabetes mellitus: the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. 2008;168(10):1153–1160. doi: 10.1093/aje/kwn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzmenko NV, Tsyrlin VA, Pliss MG. Seasonal dynamics of red blood parameters in healthy people in regions with different types of climate: a meta-analysis. Izv Atmos Ocean Phys. 2021;57(10):1271–1292. doi: 10.1134/S0001433821100078. [DOI] [Google Scholar]
- 26.Girard-Globa A, Schutz AM. Enlargement of the high density lipoprotein pool in rats by exposure to cold and by feeding a high fat diet. Horm Metab Res. 1981;13(4):214–218. doi: 10.1055/s-2007-1019224. [DOI] [PubMed] [Google Scholar]
- 27.Madaniyazi L, Guo Y, Williams G, Jaakkola JK, Wu S, Li S. The nonlinear association between outdoor temperature and cholesterol levels, with modifying effect of individual characteristics and behaviors. Int J Biometeorol. 2020;64(3):367–375. doi: 10.1007/s00484-019-01816-9. [DOI] [PubMed] [Google Scholar]
- 28.Dumke CL, Slivka DR, Cuddy JS, Hailes WS, Rose SM, Ruby BC. The Effect of Environmental Temperature on Glucose and Insulin After an Oral Glucose Tolerance Test in Healthy Young Men. Wilderness Environ Med. 2015;26(3):335–342. doi: 10.1016/j.wem.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Hauton D, Richards SB, Egginton S. The role of the liver in lipid metabolism during cold acclimation in non-hibernator rodents. Comp Biochem Physiol B Biochem Mol Biol. 2006;144(3):372–381. doi: 10.1016/j.cbpb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi-Sartang M, Ghorbani M, Mazloom Z. Effects of melatonin supplementation on blood lipid concentrations: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;37(6 Pt A):1943–1954. doi: 10.1016/j.clnu.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan MK, Brainard GC, Reiter RJ. The influence of natural short photoperiodic and temperature conditions on plasma thyroid hormones and cholesterol in male Syrian hamsters. Int J Biometeorol. 1984;28(3):201–210. doi: 10.1007/BF02187960. [DOI] [PubMed] [Google Scholar]
- 32.Doosti-Irani A, Ostadmohammadi V, Mirhosseini N, Mansournia MA, Reiter RJ, Kashanian M, et al. The effects of melatonin supplementation on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2018;50(11):783–790. doi: 10.1055/a-0752-8462. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, McFadden JW, Yang G, Zhu H, Lian H, Fu T, et al. Effect of melatonin on visceral fat deposition, lipid metabolism and hepatic lipo-metabolic gene expression in male rats. J Anim Physiol Anim Nutr (Berl) 2021;105(4):787–796. doi: 10.1111/jpn.13497. [DOI] [PubMed] [Google Scholar]
- 34.Grimes DS, Hindle E, Dyer T. Sunlight, cholesterol and coronary heart disease. QJM. 1996;89(8):579–589. doi: 10.1093/qjmed/89.8.579. [DOI] [PubMed] [Google Scholar]
- 35.Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and meta-analysis. Clin Chem. 2013;59(2):381–91. doi: 10.1373/clinchem.2012.193003. [DOI] [PubMed] [Google Scholar]
- 36.Shephard RJ, Aoyagi Y. Seasonal variations in physical activity and implications for human health. Eur J Appl Physiol. 2009;107(3):251–271. doi: 10.1007/s00421-009-1127-1. [DOI] [PubMed] [Google Scholar]
- 37.Hanson S, Jones A. Is there evidence that walking groups have health benefits? A systematic review and meta-analysis. Br J Sports Med. 2015;49(11):710–715. doi: 10.1136/bjsports-2014-094157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oja P, Kelly P, Murtagh EM, Murphy MH, Foster C, Titze S. Effects of frequency, intensity, duration and volume of walking interventions on CVD risk factors: a systematic review and meta-regression analysis of randomised controlled trials among inactive healthy adults. Br J Sports Med. 2018;52(12):769–775. doi: 10.1136/bjsports-2017-098558. [DOI] [PubMed] [Google Scholar]
- 39.Fyfe CL, Stewart J, Murison SD, Jackson DM, Rance K, Speakman JR, et al. Evaluating energy intake measurement in free-living subjects: when to record and for how long? Public Health Nutr. 2010;13(2):172–180. doi: 10.1017/S1368980009991443. [DOI] [PubMed] [Google Scholar]
- 40.Ma Y, Olendzki BC, Li W, Hafner AR, Chiriboga D, Hebert JR, et al. Seasonal variation in food intake, physical activity, and body weight in a predominantly overweight population. Eur J Clin Nutr. 2006;60(4):519–528. doi: 10.1038/sj.ejcn.1602346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016;13(7):e1002087. doi: 10.1371/journal.pmed.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr. 2014;100(1):65–79. doi: 10.3945/ajcn.113.081521. [DOI] [PubMed] [Google Scholar]
- 43.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 44.Asbaghi O, Choghakhori R, Abbasnezhad A. Effect of Omega-3 and vitamin E co-supplementation on serum lipids concentrations in overweight patients with metabolic disorders: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2019;13(4):2525–2531. doi: 10.1016/j.dsx.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Dobson HM, Muir MM, Hume R. The effect of ascorbic acid on the seasonal variations in serum cholesterol levels. Scott Med J. 1984;29(3):176–182. doi: 10.1177/003693308402900308. [DOI] [PubMed] [Google Scholar]
- 46.Kuzmenko NV, Tsyrlin VA, Pliss MG. Seasonal dynamics of melatonin, prolactin, sex hormones and adrenal hormones in healthy people: a meta-analysis. J Evol Biochem Phys. 2021;57:451–472. doi: 10.1134/S0022093021030029. [DOI] [Google Scholar]
- 47.Kuzmenko NV, Tsyrlin VA, Pliss MG, Galagudza MM. Seasonal body weight dynamics in healthy people: a meta-analysis. Hum Physiol. 2021;47(6):676–689. doi: 10.1134/S0362119721060062. [DOI] [Google Scholar]
- 48.Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27(9–10):1911–1929. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- 49.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 50.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009. p. 421. [Google Scholar]
- 51.Anthanont P, Levine JA, McCrady-Spitzer SK, Jensen MD. Lack of seasonal differences in basal metabolic rate in humans: a cross-sectional study. Horm Metab Res. 2017;49(1):30–35. doi: 10.1055/s-0042-107793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boĭko ER, Tkachev AV. Kharakteristika lipidnogo obmena u postoiannykh zhiteleĭ Severa [The characteristics of lipid metabolism in permanent residents of the North] Fiziol Cheloveka. 1994;20(2):136–142. [PubMed] [Google Scholar]
- 53.Calton EK, Keane KN, Raizel R, Rowlands J, Soares MJ, Newsholme P. Winter to summer change in vitamin D status reduces systemic inflammation and bioenergetic activity of human peripheral blood mononuclear cells. Redox Biol. 2017;12:814–820. doi: 10.1016/j.redox.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doyle JT, Kinch SH, Brown DF. Seasonal variation in serum cholesterol concentration. J Chron Dis. 1965;18:657–664. doi: 10.1016/0021-9681(65)90067-6. [DOI] [Google Scholar]
- 55.Fuller JH, Grainger SL, Jarrett RJ, Keen H. Possible seasonal variation of plasma lipids in a healthy population. Clin Chim Acta. 1974;52(3):305–310. doi: 10.1016/0009-8981(74)90115-6. [DOI] [PubMed] [Google Scholar]
- 56.Hattori T, Munakata M. Blood pressure measurement under standardized indoor condition may mask seasonal blood pressure variation in men with mildly elevated blood pressure. Clin Exp Hypertens. 2015;37(4):317–22. doi: 10.3109/10641963.2014.960975. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman AA, Nelson WR, Goss FA. Effects of an exercise program on plasma lipids of senior air force officers. Am J Cardiol. 1967;20(4):516–524. doi: 10.1016/0002-9149(67)90029-x. [DOI] [PubMed] [Google Scholar]
- 58.Jarrett RJ, Murrells TJ, Shipley MJ, Hall T. Screening blood glucose values: effects of season and time of day. Diabetologia. 1984;27(6):574–577. doi: 10.1007/BF00276970. [DOI] [PubMed] [Google Scholar]
- 59.Kamezaki F, Sonoda S, Tomotsune Y, Yunaka H, Otsuji Y. Seasonal variation in serum lipid levels in Japanese workers. J Atheroscler Thromb. 2010;17(6):638–43. doi: 10.5551/jat.3566. [DOI] [PubMed] [Google Scholar]
- 60.Kamezaki F, Sonoda S, Tomotsune Y, Yunaka H, Otsuji Y. Seasonal variation in metabolic syndrome prevalence. Hypertens Res. 2010;33(6):568–572. doi: 10.1038/hr.2010.32. [DOI] [PubMed] [Google Scholar]
- 61.Kochan TI, Eseva TV. Seasonal dynamics of clinically significant metabolic parameters in northern residents of different age. Bull Exp Biol Med. 2009;147(6):757–759. doi: 10.1007/s10517-009-0615-y. [DOI] [PubMed] [Google Scholar]
- 62.Koono N. Reciprocal changes in serum concentrations of triiodothyronine and reverse triiodothyronine between summer and winter in normal adult men. Endocrinol Jpn. 1980;27(4):471–476. doi: 10.1507/endocrj1954.27.471. [DOI] [PubMed] [Google Scholar]
- 63.Kreindl C, Olivares M, Brito A, Araya M, Pizarro F. Variación estacional del perfil lipídico en adultos aparentemente sanos de Santiago, Chile [Seasonal variations in the lipid profile of apparently healthy young adults living in Santiago, Chile] Arch Latinoam Nutr. 2014;64(3):145–152. [PubMed] [Google Scholar]
- 64.Kristal-Boneh E, Harari G, Green MS. Circannual variations in blood cholesterol levels. Chronobiol Int. 1993;10(1):37–42. doi: 10.3109/07420529309064480. [DOI] [PubMed] [Google Scholar]
- 65.Kuroshima A, Doi K, Ohno T. Seasonal variation of plasma glucagon concentrations in men. Jpn J Physiol. 1979;29(6):661–8. doi: 10.2170/jjphysiol.29.661. [DOI] [PubMed] [Google Scholar]
- 66.Levy SB, Leonard WR, Tarskaia LA, Klimova TM, Fedorova VI, Baltakhinova ME, Josh SJ. Lifestyle mediates seasonal changes in metabolic health among the yakut (sakha) of northeastern siberia. Am J Hum Biol. 2016;28(6):868–878. doi: 10.1002/ajhb.22879. [DOI] [PubMed] [Google Scholar]
- 67.Lucas JA, Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, et al. Determinants of vitamin D status in older women living in a subtropical climate. Osteoporos Int. 2005;16(12):1641–1648. doi: 10.1007/s00198-005-1888-2. [DOI] [PubMed] [Google Scholar]
- 68.Murciano Revert J, Martínez-Lahuerta JJ, Aleixandre Porcar L. Seasonal change in blood concentration of uric acid and its potential clinical implications. Aten Primaria. 2000;26(7):468–71. doi: 10.1016/s0212-6567(00)78705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ockene IS, Chiriboga DE, Stanek EJ, 3rd, Harmatz MG, Nicolosi R, Saperia G, et al. Seasonal variation in serum cholesterol levels: treatment implications and possible mechanisms. Arch Intern Med. 2004;164(8):863–870. doi: 10.1001/archinte.164.8.863. [DOI] [PubMed] [Google Scholar]
- 70.Otto C, Donner MG, Schwandt P, Richter WO. Seasonal variations of hemorheological and lipid parameters in middle-aged healthy subjects. Clin Chim Acta. 1996;256(1):87–94. doi: 10.1016/s0009-8981(96)06414-5. [DOI] [PubMed] [Google Scholar]
- 71.Pham DD, Lee JH, Hong KH, Jung YJ, Kim SJ, Leem CH. Seasonal effects on resting energy expenditure are dependent on age and percent body fat. Clin Nutr. 2020;39(4):1276–1283. doi: 10.1016/j.clnu.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 72.Sasaki J, Kumagae G, Sata T, Ikeda M, Tsutsumi S, Arakawa K. Seasonal variation of serum high density lipoprotein cholesterol levels in men. Atherosclerosis. 1983;48(2):167–172. doi: 10.1016/0021-9150(83)90103-x. [DOI] [PubMed] [Google Scholar]
- 73.Shahar DR, Froom P, Harari G, Yerushalmi N, Lubin F, Kristal-Boneh E. Changes in dietary intake account for seasonal changes in cardiovascular disease risk factors. Eur J Clin Nutr. 1999;53(5):395–400. doi: 10.1038/sj.ejcn.1600761. [DOI] [PubMed] [Google Scholar]
- 74.Solonin IuG, Markov AL, Boĭko ER, Potolitsyna NN, Parshukova OI. Functional indices of the participants of the satellite experiments of the "Mars-500" project in the north of Russia in different seasons of a year. Fiziol Cheloveka. 2014;40(6):58–66. doi: 10.1134/S0362119714050156. [DOI] [PubMed] [Google Scholar]
- 75.Suarez L, Barrett-Connor E. Seasonal variation in fasting plasma glucose levels in man. Diabetologia. 1982;22(4):250–253. doi: 10.1007/BF00281300. [DOI] [PubMed] [Google Scholar]
- 76.Sung KC. Seasonal variation of C-reactive protein in apparently healthy Koreans. Int J Cardiol. 2006;107(3):338–342. doi: 10.1016/j.ijcard.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 77.Walker BR, Best R, Noon JP, Watt GC, Webb DJ. Seasonal variation in glucocorticoid activity in healthy men. J Clin Endocrinol Metab. 1997;82(12):4015–4019. doi: 10.1210/jcem.82.12.4430. [DOI] [PubMed] [Google Scholar]
- 78.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97(10):3557–3568. doi: 10.1210/jc.2012-2126. [DOI] [PubMed] [Google Scholar]
- 79.Cunliffe WJ, Burton JL, Shuster S. The effect of local temperature variations on the sebum excretion rate. Br J Dermatol. 1970;83(6):650–654. doi: 10.1111/j.1365-2133.1970.tb15759.x. [DOI] [PubMed] [Google Scholar]
- 80.De Luca C, Valacchi G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediators Inflamm. 2010 doi: 10.1155/2010/321494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moyen NE, Ellis CL, Ciccone AB, Thurston TS, Cochrane KC, Brown LE, et al. Increasing relative humidity impacts low-intensity exercise in the heat. Aviat Space Environ Med. 2014;85(2):112–119. doi: 10.3357/asem.3787.2014. [DOI] [PubMed] [Google Scholar]
- 82.Moyen NE, Mündel T, Du Bois AM, Ciccone AB, Morton RH, Judelson DA. Increasing humidity affects thermoregulation during low-intensity exercise in women. Aviat Space Environ Med. 2014;85(9):905–911. doi: 10.3357/ASEM.3993.2014. [DOI] [PubMed] [Google Scholar]
- 83.Keatinge WR, Coleshaw SR, Easton JC, Cotter F, Mattock MB, Chelliah R. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. Am J Med. 1986;81(5):795–800. doi: 10.1016/0002-9343(86)90348-7. [DOI] [PubMed] [Google Scholar]
- 84.Fathzadeh M, Seyedna Y, Khazali H, Sheidai M, Farhud DD. Epidemiological study of T4, T3 and TSH mean concentrations in four Iranian populations. Iranian J Publ Health. 2005; 34(1):74–79. http://ijph.tums.ac.ir/index.php/ijph/article/view/1884
- 85.Surks MI. Effect of thyrotropin on thyroidal iodine metabolism during hypoxia. Am J Physiol. 1969;216(2):436–439. doi: 10.1152/ajplegacy.1969.216.2.436. [DOI] [PubMed] [Google Scholar]
- 86.Ruíz-Argüelles GJ, Sánchez-Medal L, Loría A, Piedras J, Córdova MS. Red cell indices in normal adults residing at altitude from sea level to 2670 meters. Am J Hematol. 1980;8(3):265–271. doi: 10.1002/ajh.2830080304. [DOI] [PubMed] [Google Scholar]
- 87.Wee J, Climstein M. Hypoxic training: clinical benefits on cardiometabolic risk factors. J Sci Med Sport. 2015;18(1):56–61. doi: 10.1016/j.jsams.2013.10.247. [DOI] [PubMed] [Google Scholar]
- 88.Woolcott OO, Ader M, Bergman RN. Glucose homeostasis during short-term and prolonged exposure to high altitudes. Endocr Rev. 2015;36(2):149–173. doi: 10.1210/er.2014-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ginzburg AS, Vinogradova AA, Fedorova EI. Content of oxygen in the atmosphere over large cities and respiratory problems. Izv Atmos Ocean Phys. 2014;50(8):782–792. doi: 10.1134/S0001433814080040. [DOI] [Google Scholar]
- 90.Sharma S, Hashmi MF. Partial Pressure Of Oxygen. 2021 Sep 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. https://www.ncbi.nlm.nih.gov/books/NBK493219/ [PubMed]
- 91.Kuzmenko NV, Galagudza MM. Dependence of seasonal dynamics of hemorrhagic and ischemic strokes on the climate of a region: A meta-analysis. Int J Stroke. 2022;17(2):226–235. doi: 10.1177/17474930211006296. [DOI] [PubMed] [Google Scholar]
- 92.Kuzmenko NV, Tsyrlin VA, Pliss MG, Galagudza MM. Seasonal fluctuations of blood pressure and heart rate in healthy people: a meta-analysis of panel studies. Hum Physiol. 2020;48(3):313–327. doi: 10.1134/S0362119722030100. [DOI] [Google Scholar]
- 93.Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex differences in lipid and lipoprotein metabolism. Mol Metab. 2018;15:45–55. doi: 10.1016/j.molmet.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eades CE, France EF, Evans JM. Prevalence of impaired glucose regulation in Europe: a meta-analysis. Eur J Public Health. 2016;26(4):699–706. doi: 10.1093/eurpub/ckw085. [DOI] [PubMed] [Google Scholar]
- 95.Faerch K, Borch-Johnsen K, Vaag A, Jørgensen T, Witte DR. Sex differences in glucose levels: a consequence of physiology or methodological convenience? The Inter99 study. Diabetologia. 2010;53(5):858–865. doi: 10.1007/s00125-010-1673-4. [DOI] [PubMed] [Google Scholar]
- 96.Hosseini M, Yousefifard M, Taslimi S, Sohanaki H, Nourijelyani K, Asgari F, et al. Trend of blood cholesterol level in Iran: results of four national surveys during 1991–2008. Acta Med Iran. 2013; 51(9):642–51. https://acta.tums.ac.ir/index.php/acta/article/view/4360 [PubMed]
- 97.Lohner S, Fekete K, Marosvölgyi T, Decsi T. Gender differences in the long-chain polyunsaturated fatty acid status: systematic review of 51 publications. Ann Nutr Metab. 2013;62(2):98–112. doi: 10.1159/000345599. [DOI] [PubMed] [Google Scholar]
- 98.Ko GT, Wai HP, Tang JS. Effects of age on plasma glucose levels in non-diabetic Hong Kong Chinese. Croat Med J. 2006; 47(5):709–13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2080461/ [PMC free article] [PubMed]
- 99.Umishio W, Ikaga T, Kario K, Fujino Y, Suzuki M, Hoshi T, et al. on beharf of the smart wellness housing survey group association between indoor temperature in winter and serum cholesterol: a cross-sectional analysis of the smart wellness housing survey in Japan. J Atheroscler Thromb. 2022 doi: 10.5551/jat.63494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.