Abstract
An evaluation of total immunoglobulin E (IgE) and dengue serostatus in 168 subjects from San Andrés Island, Colombia, revealed altered levels of IgE in 89% of the population. IgE levels were higher in patients with a history of dengue or with a current secondary or current primary infection than in subjects with no exposure (P = 0.01). Dengue infection accounted for 23% of the variation in IgE levels.
At the end of 1995, over 200,000 cases of dengue and more than 5,500 cases of dengue hemorrhagic fever were reported in Central and South America. Colombia, with 107 new cases of dengue hemorrhagic fever, ranked third in Central and South America (2) in occurrence of the disease. In light of reports demonstrating a relationship between immunoglobulin (IgE) levels and infectious diseases (3, 10–14, 17), the present study evaluated total IgE levels and dengue serostatus in a probability sample of individuals on San Andrés Island, Colombia. San Andrés Island is located in the Caribbean Sea approximately 150 miles northwest of the Colombian mainland. Although the island is located in an area where dengue fever is considered endemic, only a low incidence of dengue serotype 2 cases has been documented by the Colombian Ministry of Health (7).
Based on the total island population, a cross-sectional, geographically stratified, proportionally representative, and randomly selected group of subjects was used to measure baseline IgE levels and dengue serostatus in 1996. The population (n = 168) included 78 men and 90 women ranging in age from 13 to 76 years (mean, 32 ± 14 years). The participants were from different locations on San Andrés Island, as follows: 43% (n = 72) came from El Centro, 25% (n = 42) came from La Rocosa, 19% (n = 22) came from La Loma, and 13% (n = 32) came from San Luis. Sociodemographic data and past and present medical histories were collected from all participants. None of the study subjects reported gastrointestinal symptoms or any classical signs or symptoms of dengue during the months prior to the examination.
The study was performed in accordance with the human subject policies of the National Health Institute of Colombia as well as the U.S. Department of Health and Human Services. Informed consent was obtained from all individuals who participated in this study.
Serum samples from an age (13 to 78 years)- and gender (nine women and eight men)-matched group were obtained from a serum bank established during a 1995 dengue fever outbreak in La Guajira, Colombia. Only dengue serotype 2 has been identified in La Guajira, which is located in the northernmost part of Colombia.
Single venous blood samples from the San Andrés Island study subjects were centrifuged immediately after collection. All serum samples were aliquoted and stored at −70°C until immune assessments (using 10-μl samples) were made in duplicate at 37°C. Total IgE levels were assessed with the Enzymun test (Boehringer Mannheim, Mannheim, Germany). The reportable ranges of the test are between 1.5 and 500 IU/ml. IgE levels of >100 IU/ml are considered elevated (4).
Dengue serostatus was determined with UMELISA IgG and IgM antibody detection kits (TecnoSuma, Havana, Cuba), a double-sandwich immunoenzyme assay in which a mouse monoclonal antibody conjugate with alkaline phosphatase serves as a marker for an antigen-antibody binding reaction. In this procedure, a fluorogenic substrate (4-methylumbelliferyl phosphate) is added in the final step. A Suma reader was used to measure the intensity of the fluorescence signal (60 to 180 U), which indicates the presence of dengue antibodies in the patient’s serum.
All statistics were calculated with SAS software (15). A logarithmic transformation was used to correct nonnormal distribution for IgE. One-way comparisons were evaluated with the Wilcoxon rank sum test. Comparisons of the four subject groups were evaluated with Duncan’s multiple-range test. Multivariate analysis employed analysis of variance and analysis of covariance. A stepwise procedure was used to provide a final analytic model.
One-third (33%) of the San Andrés Island study population tested positive for dengue IgM (n = 55), while the remaining 67% (n = 113) were IgM seronegative. Table 1 illustrates the distribution of dengue infection within the four geographical areas of San Andrés Island. The highest number of dengue IgM-seropositive participants were from San Luis (n = 26; 47%), with the second-highest concentration from La Loma (n = 16; 29%). Statistically higher dengue IgM seropositivity was found among subjects residing in San Luis (26 of 32; 81%) than in those living in El Centro (2 of 72; P = 0.001) or La Rocosa (11 of 42; 20%; P = 0.0003).
TABLE 1.
Prevalence of dengue-specific antibodies in individuals from different regions of San Andrés Island
| Island locale (no. of subjects) | No. (%) of
patients with indicated dengue infection type and antibody status
|
|||
|---|---|---|---|---|
| Acute
|
Secondary
|
|||
| IgM+ | IgM− | IgG+ | IgG− | |
| El Centro (72) | 2 (3) | 70 (97) | 64 (89) | 8 (11) |
| La Rocosa (42) | 11 (26) | 31 (74) | 35 (83) | 7 (7) |
| La Loma (22) | 16 (73) | 6 (27) | 12 (55) | 10 (45) |
| San Luis (32) | 26 (81) | 6 (19) | 25 (78) | 7 (22) |
IgG-seropositive results were observed in the majority of participants (81%). In contrast to the geographical IgM seropositive distribution, most of the dengue IgG-seropositive individuals were from El Centro (n = 64) and La Rocosa (n = 35) (Table 1). A total of 21 subjects (13%) were negative for both IgG and IgM antibodies, with 48 participants (29%) having both IgG and IgM antibodies. Fifty percent (n = 8) of the La Guajira serum samples were IgM seropositive, but IgG seronegative, for dengue virus. The remaining serum samples (n = 8) were both IgM and IgG seronegative.
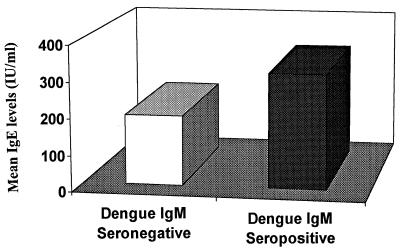
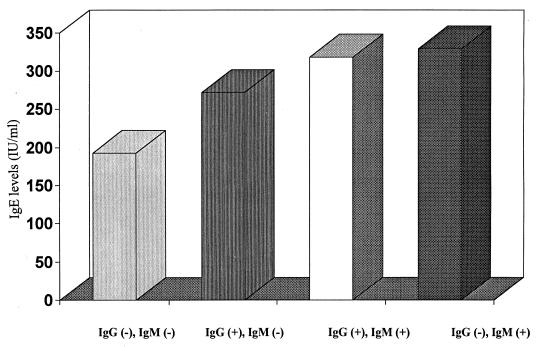
In the San Andrés Island subject group, abnormally high IgE levels (>100 IU/ml) were prevalent, occurring in 89% (n = 150) of the participants (279.3 ± 12.8 IU/ml [mean ± standard deviation]). As indicated in Fig. 1, significantly higher levels of IgE were observed in subjects who were IgM seropositive for dengue (315.4 ± 19.9) than in IgM-seronegative individuals (261.6 ± 5.9; P = 0.016). The IgG-seropositive subjects also exhibited significantly higher serum IgE levels than dengue IgG-seronegative participants (288.4 ± 5.9 versus 192.6 ± 49.3; P = 0.01). Additional analyses revealed a significant difference between IgE levels in subjects who had never been exposed to dengue virus (192.6 ± 34) and participants who had a history of dengue (271.9 ± 18.5), a current secondary infection as determined by positive IgG and IgM responses (318.7 ± 21.3), or a primary infection in the acute phase (329.3 ± 21.3; P = 0.01) (Fig. 2).
FIG. 1.
Comparison of mean IgE levels between dengue IgM-seropositive and -seronegative individuals on San Andrés Island. P = 0.016.
FIG. 2.
Total IgE levels and dengue infection status. The results were statistically significant (P = 0.01).
Acute dengue infection and past exposure to dengue were significantly associated with higher IgE levels. These two independent variables accounted for 23% of the variation in IgE levels; 18% was associated with a positive IgM response and an additional 5% was associated with a positive IgG response. Results from La Guajira similarly demonstrated significantly higher levels of IgE in subjects who were dengue IgM-seropositive (372 ± 48.5) than in participants who were IgM and IgG seronegative (48.6 ± 11; P = 0.001).
This is the first study, to our knowledge, to describe elevated IgE levels in a population exposed to dengue infection. IgE levels were significantly higher in subjects with a history of dengue infection and strikingly elevated in those subjects in the primary or secondary acute phase of dengue infection, compared to individuals with no dengue exposure.
The relationship between elevated IgE levels and dengue infection may reflect the role of IgE in immunohomeostasis. IgE elevation has been attributed mainly to allergic reactions and parasitic infections and classified as a TH2 response. The immune response directed to viral infection, in contrast, has been classically described as TH1 mediated (1, 4). Despite this differentiation of TH1 and TH2 responses, IgE elevation and specific IgE response in viral infections have been increasingly reported in past years (3, 8, 10–17); consequently, the understanding of immunity has radically changed.
Abnormally high IgE levels in individuals with current or past exposure to the dengue virus suggest that a balanced immune response may lead to disease resolution, rather than the induction of a particular TH cell pathway. In addition, the dengue virus has demonstrated the capacity to induce interleukin 6 production (9), which is involved in IgE synthesis in humans (16). The increase in serum IgE during the acute stage of dengue infection and the persistence of elevated IgG levels after recuperation from dengue disease (IgG response) may indicate the presence of a specific IgE response and possibly reflect immune memory. Results from La Guajira confirm that the IgE elevation in dengue-infected subjects on San Andrés Island is not a unique phenomenon.
Our data indicate that two recent outbreaks of dengue have occurred on the island in which most of the cases were apparently asymptomatic. Studies in Thailand have similarly shown that clinically undetected infection can be prevalent, thus permitting the widespread dissemination of dengue (5, 6). An understanding of the nature of the antibody response in dengue infection may be particularly helpful for improving dengue serodiagnosis. Immunoassays of IgE responses to infectious agents, including viruses, have been shown to be more specific and sensitive than those of IgG, IgM, or IgA antibody responses (3, 8, 12–14, 17), suggesting that measuring IgE levels may be useful in disease evaluation. Thus, we conclude that further studies are warranted to better define the nature of elevated IgE response during dengue virus infection.
(This study was presented in part at the International Conference on Emerging Infectious Diseases, Atlanta, Ga., March 1998 [12a].)
Acknowledgments
This work was supported by NIH grant 5D43TW00017-08.
We are most grateful to Nohora Charry, Ma Camila Garcia, and Sheila Garnica for excellent recruitment support.
REFERENCES
- 1.Allen J E, Maizels R. TH1-TH2: reliable paradigm or dangerous dogma? Immunol Today. 1997;18:387–392. doi: 10.1016/s0167-5699(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Dengue and dengue hemorrhagic fever 1990–1994. Weekly Epidemiol Rec. 1995;70:334–335. [PubMed] [Google Scholar]
- 3.Bahana S L, Horwitz C A, Fiala M, Heiner D C. IgE response in heterophil positive infectious mononucleosis. J Allergy Clin Immunol. 1978;62:167–173. doi: 10.1016/0091-6749(78)90102-1. [DOI] [PubMed] [Google Scholar]
- 4.Bierman C, Pearlman D. Allergic diseases from infancy to adulthood. 2nd ed. Vol. 1. Philadelphia, Pa: W. B. Saunders Company; 1988. pp. 1–19. [Google Scholar]
- 5.Burke D S, Nisalak A, Johnson D E, Scott R M. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 6.Eamchan P, Nisalak A, Foy H M, Chateonsookoa O A. Epidemiology and control of dengue virus infection in Thai villages in 1987. Am J Trop Med Hyg. 1989;41:95–101. [PubMed] [Google Scholar]
- 7.Jaramillo C, Alvarez G, Granados R. Dengue and hemorrhagic dengue in Colombia since colonialization to 1995. Trib Medica. 1997;95:45–54. [Google Scholar]
- 8.Krivitskaia V Z, Aleksandrova N A, Pokhaznikova M A. Anti-RS viral IgE in respiratory syncytial viral infection in adult patients with a complicated course of bronchitis. Mikrobiol Epidemiol Immunobiol. 1998;4:56–61. . (In Russian.) [PubMed] [Google Scholar]
- 9.Kuno G, Bailey R E. Cytokine responses to dengue infection among Puerto Rican patients. Mem Inst Oswaldo Cruz. 1994;89:179–182. doi: 10.1590/s0074-02761994000200010. [DOI] [PubMed] [Google Scholar]
- 10.Maggi E, Macchia D, Parronchi P, Fauger M W, Ennis F A. The IgE response in atopy and infections. Clin Exp Allergy. 1991;21:72–81. doi: 10.1111/j.1365-2222.1991.tb01709.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez F D, Stern D A, Wright A L, Taussig L M. Differential immune response to acute lower respiratory illness in early life and subsequent development of persistent wheezing and asthma. J Allergy Clin Immunol. 1998;102:915–920. doi: 10.1016/s0091-6749(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 12.Míguez-Burbano M J, Hutto C, Shor-Posner G, Del Prete G, DeCorli M, Piccinni M P, Simonelli C, Biswes P. An IgE based assay for early diagnosis of HIV-1 infection in infants. Lancet. 1997;350:782–783. doi: 10.1016/S0140-6736(05)62566-4. [DOI] [PubMed] [Google Scholar]
- 12a.Míguez-Burbano M J, Jaramillo C A, Palmer C J, Shor-Posner G, Velásquez L S, Lai H, Braun M K. Abstracts of the International Conference on Emerging Infectious Diseases. 1998. , abstr. p9.38. [Google Scholar]
- 13.Nielsen S L, Sørensen I, Andersen H K. Kinetics of specific immunoglobulins M, E, A, and G in congenital, primary, and secondary cytomegalovirus infection studied by antibody-capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1988;26:654–661. doi: 10.1128/jcm.26.4.654-661.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinon J M, Toubas D, Marx C, Mougeot G, Bonnin A, Bonhomme A, Villaume M, Foudrinier F, Lepan H. Detection of specific immunoglobulin E in patients with toxoplasmosis. J Clin Microbiol. 1990;28:1739–1743. doi: 10.1128/jcm.28.8.1739-1743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SAS Institute. SAS/STAT software: changes and enhancement, release 6.07. SAS technical report P-229. Cary, N.C: SAS Institute; 1992. [Google Scholar]
- 16.Vercelli D, Jabara H H, Geha R S. Regulation of human IgE synthesis. Int Rev Immunol. 1989;5:11–15. doi: 10.3109/08830188909061977. [DOI] [PubMed] [Google Scholar]
- 17.Weber B, Stemmler A, Ernst W, Schenerman E H, Braun W, Doerr H W. Improvement of serological diagnosis of human cytomegalovirus infection in renal transplant recipients by testing for specific immunoglobulin E by ELISA. Infection. 1993;21:158–163. doi: 10.1007/BF01710536. [DOI] [PubMed] [Google Scholar]