Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused substantial global morbidity and deaths, leading governments to turn to non-pharmaceutical interventions to slow down the spread of infection and lessen the burden on health care systems. These policies have evolved over the course of the COVID-19 pandemic, including after the availability of COVID-19 vaccines, with regional and country-level differences in their ongoing use. The COVID-19 pandemic has been associated with changes in respiratory virus infections worldwide, which have differed between virus types. Reductions in respiratory virus infections, including by influenza virus and respiratory syncytial virus, were most notable at the onset of the COVID-19 pandemic and continued in varying degrees through subsequent waves of SARS-CoV-2 infections. The decreases in community infection burden have resulted in reduced hospitalizations and deaths associated with non-SARS-CoV-2 respiratory infections. Respiratory virus evolution relies on the maintaining of a diverse genetic pool, but evidence of genetic bottlenecking brought on by case reduction during the COVID-19 pandemic has resulted in reduced genetic diversity of some respiratory viruses, including influenza virus. By describing the differences in these changes between viral species across different geographies over the course of the COVID-19 pandemic, we may better understand the complex factors involved in community co-circulation of respiratory viruses.
Subject terms: Epidemiology, Infectious-disease diagnostics, Viral infection
The COVID-19 pandemic has had a considerable impact on respiratory virus infections worldwide. In this Review, Chu and colleagues discuss the changes in community spread and consequent infections by respiratory viruses other than severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) throughout the course of the pandemic, and describe the impact on the evolution and genetic diversity of these viruses.
Introduction
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has upended the daily life of individuals around the world, with historic numbers of cases and deaths1. Early pandemic public health responses focused on non-pharmaceutical interventions (NPIs), actions that can be taken at the individual, environmental, community and country level to limit the spread of infection, given the significant health care burden of acute COVID-19 and the lack of COVID-19 vaccines or virus-specific therapeutics. NPIs have been the mainstay of pandemic influenza planning guidance issued by the World Health Organization (WHO)2, the Pan-American Health Organization3 and the US Centers for Disease Control and Prevention4. The goal of near-simultaneous global implementation of NPIs (Table 1) during the COVID-19 pandemic was to slow down the community transmission of SARS-CoV-2, mitigate the burden of disease and the need for health care resources, and allow time to develop vaccines and treatments.
Table 1.
Community non-pharmaceutical interventions used during the COVID-19 pandemic
| Category of NPI | Description | Mitigation objective |
|---|---|---|
| Individual or personal level | ||
| Face masks | Use of masks that cover the nose and mouth. Effectiveness depends on proper use and fitting, the particle size filtering efficiency of the material used, the amount of use and reuse, and virus levels in the respiratory tract of the symptomatic or asymptomatic infected wearer (for source control) or in expelled particles of the infected individuals (for prevention) | Reduce virus transmission to and from individuals by mitigating dispersal of respiratory droplets and aerosols containing infectious virus |
| Respiratory etiquette | Cover coughs and sneezes of symptomatic persons with tissues, sleeves and elbows and avoid the use of hands | Limit virus transmission by reducing suspension and dispersal of respiratory droplets and aerosols containing infectious virus expelled by symptomatic infected persons to the surrounding environment, hands, and high-touch surfaces |
| Hand hygiene | Hand washing with soap and water or hand sanitation with an alcohol-based hand sanitizer | Reduce virus transmission through contact with surfaces and fomites |
| Social distancing | Maintain a separation of 2 m or more from others and avoid crowds | Reduce likelihood of virus transmission through respiratory droplets and aerosols from infected persons to exposed persons |
| Screening and isolation of sick individuals | Physically separate ill individuals from others at home, in public, at school and at work, combined with virus testing | Reduce virus transmission from infected symptomatic persons during the infectious period to close contacts (does not identify infected persons who are asymptomatic/presymptomatic) |
| Quarantine of exposed individuals in the community | Identify exposed individuals and encourage or require them to stay at home. Monitor them for the onset of symptoms, combined with virus testing | Identify high-risk exposures early and mitigate virus transmission to others before a potentially infected individual is contagious. Identify infected contacts and isolate them early in the infection course to further reduce spread to their contacts |
| Community | ||
| Face mask mandates in public spaces | Require the use of face masks in closed public settings and on public transportation | Limit virus transmission in situations with limited ability for social distancing |
| School and childcare facility closures | Close childcare facilities and limit social gatherings outside school and childcare facilities | Reduce virus infections among members of vulnerable age groups that may have difficulty with implementation of individual or personal NPIs and reduce virus introduction into households and the risk of secondary transmission |
| Close schools, colleges and universities; implement distance learning | Reduce virus infections among paediatric and young adult populations in locations where social distancing may be difficult to implement and reduce virus introduction into households and the risk of secondary transmission | |
| Postponing or cancelling gatherings and events | Limit large gatherings, particularly in enclosed spaces | Reduce community virus transmission and burden of infection |
| Stay-at-home and lockdown measures |
Close non-essential businesses and prohibit indoor dining at restaurants (with the option to offer takeaway orders only) Implement stay-at-home measures and limit movement in the community to essential workers |
Reduce community virus transmission and density of people in public spaces Maximize protection of essential workers to ensure essential businesses remain functional |
| Encourage teleworking in professions where in-person attendance is not essential | Reduce workplace virus transmission and encourage workers to stay at home when ill by allowing them to work as they are able. Minimize the impact on businesses | |
| Home delivery of necessities including groceries, food and medications | Reduce community spread of virus and protect essential workers in these fields. Reduce virus transmission in enclosed settings | |
| Contact tracing |
Identify and test exposed close contacts combined with quarantine Educate and promote individual or personal-level NPIs. Identify symptomatic high-risk individuals for early initiation of targeted pharmaceutical interventions |
Identify, evaluate, quarantine and monitor close contacts with high-risk exposures to reduce further virus transmission among first-responders, providers and patients in health care settings, residents of congregate settings, household members and individuals at high risk of disease complications. Provides surveillance data to quickly identify locations of outbreaks to provide appropriate education to mitigate further transmission |
| Reduction in public transport availability and use | Limit public transportation use except when essential. Limit availability of public transportation during periods of high community burden of infection | Minimize exposure and virus transmission in enclosed spaces where social distancing is difficult, and ventilation or air exchanges may be inadequate |
| Public health education | Distribute educational materials on individual and community mitigation strategies including updated information on virus transmission routes and dynamics. Use of multiple communication modalities, including social media | Provide individuals and the community with current information to prevent and control virus spread for themselves and others, and to combat misinformation |
| Community surveillance | Routine screening and virus testing for communicable disease in specific community populations | Assess community burden of infection and reduce further virus spread, allowing rapid implementation of other community measures to mitigate community disease burden |
| Environmental | ||
| Air quality improvement | Upgrade and improve ventilation systems in homes and buildings in consultation with heating, ventilation and air conditioning professionals. Enhance air filtration, including the use of portable air filters, HEPA filters, improvements to central air filtration and the use of restroom exhaust fans | Reduce the concentration of viral particles in the air in enclosed spaces to reduce transmission in enclosed spaces, including workplaces, health care settings, public indoor spaces and congregate settings |
| Increase air exchanges through opening of windows and doors and the use of fans particularly when indoor social distancing may not be possible | Reduce the concentration of viral particles in the air at home to reduce transmission, particularly in situations where social distancing may be difficult and in homes with a high density of people | |
| Use of ultraviolet germicidal irradiation where other systems may not be available | Reduce the concentration of viable viral particles in the air capable of causing infection to reduce transmission where other forms of air filtration are not available | |
| Disinfection of high-touch surfaces | Routine surface cleaning of high-touch objects, including toys, refrigerator handles, desks, doorknobs, railings, bathroom fixtures | Reduce transmission of virus from fomites, including in community health care settings |
| Country policies | ||
| Border closures | Restrict travel into countries and between political borders | Reduce the introduction of virus from geographic locations with a high burden of infections. Limit the introduction of asymptomatic and symptomatic infected people. Slow down the introduction of virus and variants of concern |
| Health screening at points of entry/exit | Identify infected individuals through various screening methods before they leave or enter a country. Screening and virus testing of symptomatic persons or testing of all persons | Reduce or slow down international spread of virus in or out of a country, including for variants of concern |
| Quarantine measures for inbound travel | Quarantine of inbound travellers to certain countries and locations upon entry | Reduce the introduction of virus into a country, including from infected persons in their incubation period who are asymptomatic/presymptomatic or who have not yet yielded a positive test result |
| Travel advisories, mandates and restrictions | Require negative test results and/or up-to-date vaccination | Reduce the spread of virus from infected individuals |
| Travel alerts to visiting locations with a high burden of infection | Educate individuals before travel, including to practise precautions or to avoid non-essential travel, particularly to locations with a high burden of infection, and provide educational materials to visitors to these locations when travel is necessary | |
| Face mask mandates for air travel | Reduce the spread of virus when social distancing may not be possible. Limit virus transmission from asymptomatic and minimally symptomatic infected individuals | |
| Zero COVID-19 policy | NPIs and vaccination implemented at all community levels. Isolation of ill individuals and quarantine of exposed close contacts. Restriction of movement in the community | Contain and suppress levels of community infections to prevent sustained virus spread |
HEPA, high-efficiency particulate air; NPI, non-pharmaceutical intervention.
Since 2020, the COVID-19 pandemic and public health implementation of NPIs have had broad effects on individuals, communities and governments. Given that NPIs were designed to mitigate respiratory virus transmission, there have been notable disruptions to the typical seasonal circulation patterns of common respiratory virus infections, including by influenza virus and respiratory syncytial virus (RSV). The reduction in community respiratory virus activity, in turn, has led to other downstream effects, including an associated decreased prevalence of invasive Streptococcus pneumoniae infections (Box 1). These changes to the community prevalence of non-SARS-CoV-2 respiratory viruses are undoubtedly multifactorial, influenced in part by the collective implementation and ongoing use of NPIs, changes in health behaviours, reductions in travel, virus-specific transmission factors (Supplementary Table 1) and shifts in testing priorities and surveillance systems5,6. Virus–virus interactions, including viral interference, have been hypothesized as a factor explaining some of the changes in the community circulation of respiratory viruses7 (Box 2). Public health responses have varied since the start of the pandemic as COVID-19 vaccinations became available and hospitalizations declined, resulting in a patchwork of community, regional and country-level differences in the ongoing use of NPIs, with corresponding effects on the circulation of respiratory viruses in the community. Documenting and understanding these changes in respiratory virus circulation during the COVID-19 pandemic can inform future public health responses.
In this Review, we summarize the epidemiology of common respiratory virus infections and then focus on the effect of SARS-CoV-2 and the use of NPIs in modifying the circulation, clinical burden and evolution of endemic respiratory virus infections.
Box 1 Changes in invasive pneumococcal disease during the COVID-19 pandemic.
In addition to changes in viral pathogen activity, there have been reported global reductions in invasive pneumococcal disease (IPD) caused by Streptococcus pneumoniae, particularly among children younger than 5 years149–156. S. pneumoniae is a clinically important bacterial pathogen spread by respiratory droplets and can cause significant morbidity and death. Transmission can lead to colonization and a high carriage density157, which can in turn affect the risk of progression to invasive disease, including sepsis, meningitis and lower respiratory tract infections such as pneumonia158. Colonization of the respiratory tract by S. pneumoniae is influenced by vaccination159–161, host immunity162, respiratory microbiota interactions163 and viral co-infections157,163. Specifically, influenza virus and other respiratory virus co-infections have been associated with increased S. pneumoniae carriage density through modifications of the microenvironment and facilitating S. pneumoniae growth in respiratory sites of colonization157,163. Furthermore, influenza virus co-infection has been associated with increased IPD risk164–166; however, this synergistic relationship may differ across species of respiratory viruses that cause co-infection with S. pneumoniae157.
The decline in IPD during the pandemic is likely multifactorial, owing to factors such as reduction of person-to-person bacterial transmission through face mask mandates and social distancing156, reduction of bacterial burden among children from school closures151,154 and changes in the circulation of viruses other than severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which decreased the risk of co-infection153,156. The reduction of influenza virus infection and RSV infection cases mirrored reductions in IPD cases153. Although people with SARS-CoV-2-associated IPD experience severe disease outcomes152, S. pneumoniae and SARS-CoV-2 co-infections are uncommon167,168.
However, these reductions in IPD cases may not be sustained, as reports have shown increased incidence of IPD where non-pharmaceutical intervention measures were removed169. Furthermore, reduced paediatric vaccination rates during the COVID-19 pandemic170, including S. pneumoniae immunizations, may result in long-term increases in the community burden of IPD171.
Box 2 Viral interference.
Respiratory viral infections can involve complex viral pathogen interactions when a host is infected with more than one type of respiratory virus or when a host is sequentially infected with different respiratory viruses. Among the different virus–virus interactions, viral interference is a direct or indirect antagonistic interaction between respiratory viruses that affects one virus’s ability to infect and cause disease in the host. Evidence of such inhibitory interactions was reported during the 2009 H1N1 influenza pandemic in Europe, during which preceding regional rhinovirus epidemics were temporally associated with unexplained and abrupt declines of influenza cases172,173. In turn, annual respiratory syncytial virus activity in France was delayed while infections due to pandemic influenza A(H1N1)pdm09 virus became widespread174. These early observations laid the foundation for subsequent studies exploring viral interference. Negative virus interactions have also been reported in other viral pairings175,176, and viral interference involving severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is also thought to occur given the number of cases of SARS-CoV-2 but relatively few viral co-infections during the COVID-19 pandemic177.
Laboratory-based models have demonstrated plausible mechanisms by which viral interference could exist. For instance, infection with one virus stimulates a broad innate immune response and interferon release, which may inhibit subsequent respiratory viral infections65,178. Alternatively, simultaneous respiratory viral infections may lead to antagonistic effects; for example, viruses may compete for resources179, or the presence of one virus may drive the downregulation of cellular receptors that are necessary for viral entry in the host180. These effects can result in inhibition or delay of secondary infections, or the reduction of severe disease risk associated with subsequent infection.
Community circulation of non-SARS-CoV-2 respiratory viruses
The community co-circulation of respiratory viruses was common before the emergence of SARS-CoV-2. Established local, national and global surveillance systems, many of which measured medically attended respiratory virus infection cases, have helped monitor seasonal changes in circulation before the COVID-19 pandemic8–10. While influenza viruses and RSV have been prioritized for surveillance, the expanded use of multiplex molecular respiratory virus assays and the increased awareness of the public health burden of respiratory viruses have renewed interest in characterizing the epidemiology of respiratory viruses. Furthermore, the COVID-19 pandemic has highlighted the importance of community-based surveillance as a means to assess the burden of infection11–13.
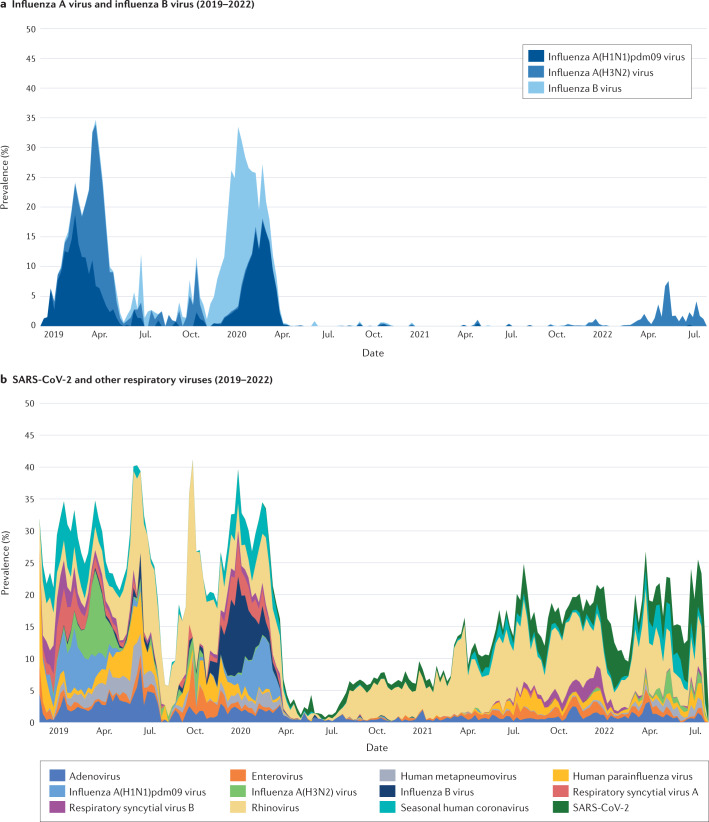
Through the Seattle Flu Study (a community surveillance consortium in the Seattle, WA, USA, metropolitan area) community respiratory virus detection was monitored year-round14. With the community spread of SARS-CoV-2, there were associated circulation changes across the tested non-SARS-CoV-2 viral pathogens from March 2020 onwards, coinciding with community implementation of NPIs, including a stay-at-home ordinance implemented at the onset of the COVID-19 pandemic15,16 (Fig. 1). Over time, the prevalence of SARS-CoV-2 and other respiratory viruses varied throughout the different phases of the COVID-19 pandemic, with changes in the detection of influenza virus, RSV, rhinovirus and respiratory enterovirus infections most commonly described. Characterizing these changes during the COVID-19 pandemic may help to shape public health recommendations to mitigate infection, especially in those most vulnerable to severe disease, and help our understanding of the interviral complexities that govern community co-circulation.
Fig. 1. Prevalence of community respiratory viruses and SARS-CoV-2 from the Seattle Flu Study 2019–2022.
a | Prevalence of influenza A virus A and influenza B virus (2019–2022). b | Prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other respiratory viruses. Included are respiratory specimens from 2018 epidemiological week 47 to 2022 epidemiological week 30; a total of 154,740 specimens were tested: 957 in 2018, 25,019 in 2019, 64,869 in 2020, 48,458 in 2021 and 15,437 in 2022. Respiratory specimens were tested with use of a custom-arrayed reverse transcription–PCR platform (Thermo Fisher)16. Adapted with permission from ref.16, Seattle Flu alliance.
Influenza virus circulation during the COVID-19 pandemic
Perhaps documented more than for any other respiratory virus, global influenza virus activity decreased markedly with the implementation of NPIs early in the COVID-19 pandemic17–20. Since 1952, the WHO has coordinated a global influenza surveillance system through the Global Influenza Surveillance and Response System, with 124 WHO member states21,22. The Centers for Disease Control and Prevention conducts seasonal influenza surveillance through syndromic surveillance for influenza-like illness (ILI) in laboratory, outpatient and hospital-based platforms, as well as through monitoring for deaths due to influenza and pneumonia in adult and paediatric populations23. The combined use of these and other systems around the globe has helped to clarify regional and temporal factors that affect the circulation of influenza virus, which are critical to influenza pandemic planning4.
Across different regions and hemispheres, there have been reduced cases of influenza since the start of the COVID-19 pandemic. While declines in the numbers of influenza cases may be due in part to changes in health care-seeking behaviours and influenza testing practices, specimen positivity rates have also shown an associated decline over this period18. Influenza vaccination coverage rates increased in adults and adolescents at the onset of the COVID-19 pandemic24 while influenza vaccine uptake in those younger than 5 years decreased over the same period24. Influenza vaccination campaigns historically have focused on the youngest age groups25–27 as children account for a substantial burden of influenza virus infections and are important contributors to influenza virus transmission in the community25–29. However, reductions in influenza virus activity reported by surveillance systems occurred despite evidence of decreased influenza vaccination coverage in children24,30.
Infections during the influenza season were reduced across a range of countries in the Northern Hemisphere. Influenza virus activity levels between October 2020 to early 2021 in the USA and Canada showed historically low levels, with US cases at their lowest since influenza surveillance data were first publicly available in 1997 (refs.31,32). Similarly, in Mexico, where bimodal influenza seasons occur, national indicators of influenza virus activity showed a decline of influenza cases after broad NPI implementation began on 23 March 2020 (ref.33). This included decreases in the frequency of ILI (see later), of clinical specimens that tested positive for influenza viruses, and of the numbers of severe influenza cases. In Bangladesh, the 2020–2021 influenza season was delayed and was shorter than prior seasons, with the start of the annual influenza epidemic occurring soon after NPIs were removed34. A modelling study of the timing of NPI implementation on influenza virus activity in China and the USA showed an associated decrease in influenza virus infections at the onset of the COVID-19 pandemic19, with influenza virus activity remaining suppressed in China throughout the remainder of 2020 (refs.35,36). Surveillance reports in China from 2020–2021 show increased influenza virus activity relative to the previous year, with regional differences in the onset of peak activity; however, the degree of activity remained lower than in seasons before the COVID-19 pandemic37.
Similarly, data from the Southern Hemisphere showed much lower levels of influenza virus activity than in previous seasons during the months that typically mark the beginning of the influenza season18. Influenza surveillance sites in Australia, Chile and South Africa found that the influenza virus per cent positivity of clinical specimens was markedly lower from April to July 2020 compared with data from the 2017–2019 influenza seasons18. In Australia, this pattern of low influenza virus activity continued through to September 2020, despite variation in the implemented NPI strategies across the country6.
Global influenza virus activity remained low during the 2021–2022 influenza season, with low influenza virus numbers (as well as of most viruses) detected overall in northern temperate zone countries and countries in the tropics and subtropics38. However, there was a relative increase in influenza virus activity, particularly in temperate zone countries, compared with the 2020–2021 influenza season38. In China, influenza B virus predominated during the 2021–2022 influenza season, while other Asian countries reported cases of both influenza A virus and influenza B virus38.
How influenza virus activity will change over the remainder of the COVID-19 pandemic is unknown, especially with the variable lifting of travel restrictions, changes in NPI implementation policies, return to in-person learning, behavioural changes (such as variable compliance with masking), waning population immunity to influenza viruses, the emergence of new SARS-CoV-2 variants and subvariants, the evolving epidemiology of COVID-19 and challenges associated with influenza vaccine strain selection with low levels of circulating virus39.
Community circulation is thought to be driven in part by household transmission of influenza viruses40, with school-aged children playing a significant role in these infections41. However, despite the reopening of schools in some communities, early surveillance data during the 2021–2022 influenza season in the Northern Hemisphere indicated a return of only sporadic cases of influenza virus infections but not the same degree of activity observed before the COVID-19 pandemic in Canada42 and the USA8. These examples show that personal-level NPIs, such as social distancing or face mask use, are associated with low influenza virus community circulation.
But even in communities where virus circulation is low, influenza outbreaks may still occur. An influenza A(H3N2) outbreak on a US university campus highlighted the effect of an abrupt return of community influenza virus circulation43. Data from the Seattle Flu Study identified a spike in influenza A(H3N2) virus prevalence in the spring of 2022, coinciding with the easing of pandemic NPIs16. This occurred at a time typically associated with the end of the traditional annual influenza season.
Moreover, several countries in the Southern Hemisphere reported influenza virus activity that was different from that in the pre-COVID-19 pandemic period. In Brazil, an unusual interseasonal influenza A(H3N2) epidemic occurred between October and November 2021 (ref.44), while in Argentina influenza virus activity was increased in 2022, as reported in epidemiological week 17 (ref.45). In June 2022, Australia experienced an early epidemic surge of seasonal influenza virus activity that peaked in May and June, with higher numbers of reported cases than during the three influenza seasons before the emergence of SARS-CoV-2 (ref.46). It is unclear whether this is a reflection of the removal of COVID-19 pandemic NPIs or whether it was related to factors specific to that region.
RSV circulation during the COVID-19 pandemic
With COVID-19 NPIs in place, case counts decreased significantly across geographic regions and climate zones31,32,47–50. The effect of stay-at-home orders during the early pandemic decreased non-household social interactions, thereby reducing viral introductions and secondary household transmission. Because RSV is often transmitted through close contact in a childcare facility or school, the effect of these stay-at-home orders was likely to have had a more pronounced effect on the spread of RSV in paediatric populations, as well as onward transmission to groups at increased risk of infection, such as infants and adults aged 65 and older.
In Tokyo, where RSV outbreaks occur in summer and autumn months, no RSV outbreaks were observed in 2020 (ref.51). However, in 2021, the Tokyo metropolitan area had the largest increase in RSV cases since RSV surveillance was established in 2003 (ref.51). The rapid resurgence of RSV infections was thought to be due in part to the reduced population-level immunity as well as children’s re-engagement in social activities51.
Interseasonal RSV circulation has occurred during the COVID-19 pandemic, with uncharacteristic peaks during times of the year not typical of RSV seasonality51. In Australia and South Africa, where seasonal peaks generally occur during the winter, peaks of RSV cases were noted in the spring and summer of 2020, coinciding with the implementation and subsequent easing of community NPIs48,52,53. US cases of RSV infection decreased rapidly after NPI implementation and remained at historical low levels into early 2021 (ref.54). However, RSV activity peaked in the USA during the spring and summer months of 2021, with variations by geographic region likely attributed to differences in implementation of community NPIs by states55.
Rhinovirus and respiratory enterovirus circulation during the COVID-19 pandemic
The case counts of rhinoviruses and respiratory enteroviruses decreased at the start of the pandemic. However, unlike for most of the other endemic respiratory viruses, the prevalence of rhinoviruses and respiratory enteroviruses quickly rebounded and persisted despite ongoing community NPI implementation.
Rhinoviruses and respiratory enteroviruses, which are non-enveloped viruses that are members of the same viral genus and are thus indistinguishable by most molecular tests, have been observed across multiple studies from different countries and regions. US national surveillance of respiratory viruses showed that rhinovirus and respiratory enterovirus detections decreased substantially between March and May 2020 but then returned to the seasonal levels of previous years31. In California, a sentinel surveillance system reported levels of rhinovirus and respiratory enterovirus activity that were similar to the levels in pre-COVID-19 pandemic seasons, with typical seasonal-level changes observed beginning in the autumn of 2021, after lower activity at the onset of the COVID-19 pandemic56. Persistence of non-enveloped respiratory viruses, including rhinovirus and respiratory enterovirus, was also seen in South Korea despite stringent social distancing practices during the pandemic57,58. The authors of these studies suggested that this was due to the non-enveloped virus structure’s resistance to environmental factors and prolonged shedding. Similarly, in a nationwide analysis of paediatric data in a Finnish infectious disease registry, rhinovirus and respiratory enterovirus detection initially decreased with early lockdown procedures and school closures but returned to typical levels as social distancing measures were eased59. During the winter season in Australia over the same period6, rhinovirus and respiratory enterovirus were noted to be present at above-average levels, and the levels increased as schools reopened60. Studies from tropical regions, including Brazil61 and the Philippines62, where seasonality of respiratory viruses may differ from that in temperate regions, reported continued detection of rhinovirus and respiratory enterovirus among respiratory samples tested during the pandemic.
Collectively, these studies suggest that the inherent properties of rhinoviruses and respiratory enteroviruses, including the absence of a viral envelope, their stability on surfaces and the genomic diversity and number of strains that co-circulate, may allow infections to quickly increase and persist when the implementation of NPIs is relaxed. This is despite the continued suppression of other respiratory viruses by the remaining NPIs. A greater proportion of rhinovirus infections are minimally symptomatic or asymptomatic, compared with most other respiratory viruses, which may lead to ongoing transmission in settings where symptom screening was used as part of the criteria for school or work attendance. Given the sensitivity of rhinovirus and respiratory enterovirus to changes in implementation of NPIs, some have proposed using rhinovirus and respiratory enterovirus as a sentinel indicator of the stringency and effectiveness of NPI use63.
Interestingly, studies have identified an inverse relationship between levels of SARS-CoV-2 and rhinovirus and respiratory enterovirus in some locations. As rhinovirus and respiratory enterovirus cases returned to near pre-pandemic levels in both California56 and South Korea57, their peaks and troughs were the inverse of those of SARS-CoV-2. While this finding may be incidental, viral interference7 (Box 2) between the three viruses may be possible64. A similar virus–virus interaction has been reported at the host and population level between rhinovirus and influenza virus65.
Circulation of other non-SARS-CoV-2 respiratory viruses during the COVID-19 pandemic
Among other non-SARS-CoV-2 respiratory viruses, there has been variation in circulation changes by viral species during the COVID-19 pandemic.
Like influenza virus and RSV, seasonal human coronavirus16,31,32,66,67, human parainfluenza virus16,32,60,67,68 and human metapneumovirus16,32,60,67,68 experienced marked reductions in circulation at the onset of the COVID-19 pandemic when NPIs were in place. All three are negative-stranded RNA viruses in the family Paramyxoviridae, and share the features of having a viral envelope, circulating primarily in paediatric populations and having a clear seasonality. These viruses all rebounded as pandemic NPIs were lifted, thought to be due to school reopening and relaxation of NPIs leading to transmission16,69.
Adenoviruses and respiratory enteroviruses have both persisted despite NPIs. Although the levels of both decreased at the onset of the COVID-19 pandemic16,32,67,70, there were fewer fluctuations in case counts of these viruses compared with influenza virus and RSV despite shifting community policies in some localities60. In part, the persistence of adenovirus may be attributable to its lack of a viral envelope, and environmental stability, leading to persistent detection on surfaces, and extended shedding among infected individuals71. In Europe, relaxation of NPIs was associated with a rapid increase in enterovirus D68 cases between July and October 2021 (ref.72).
Reports on human bocavirus early in the COVID-19 pandemic demonstrated different circulation patterns, with some reporting increasing positive testing rates66 and others showing decreased frequencies67,70. Studies from China and South Korea found initial decreases in cases of human bocavirus35,57 early in 2020, followed by a later resurgence beyond what was expected from previous season patterns in China35.
Data on human parechovirus circulation patterns during the COVID-19 pandemic are limited, with one study showing a decrease in human parechovirus detection in young children early in 2020 (ref.67).
Health care burden of non-SARS-CoV-2 viruses
The health care burden of respiratory virus infections during the COVID-19 pandemic reflected the changes seen in respiratory virus community circulation.
From a health care utilization standpoint, the volume of emergency department (ED) visits and hospital admissions associated with non-SARS-CoV-2 respiratory virus infections decreased at the onset of the COVID-19 pandemic as broad NPIs were first implemented. One study estimated that community face mask use was associated with a significant decrease in ED visits for diseases associated with non-SARS-CoV-2 respiratory infections, including exacerbations of asthma and chronic obstructive pulmonary disease (COPD)73. As public health measures were lifted and some viruses returned, a corresponding increase in health care utilization was also seen.
While NPIs likely played an important part in medical visits, other factors may have also affected these measures of health care burden. Human behaviour and health care attendance changed during the COVID-19 pandemic, along with increases in telemedicine consultations, contributing to reports of fewer in-person clinic appointments74,75 and ED visits76–79. Supply chain shortages and changes in respiratory virus testing priorities may have led to a prioritization of testing for SARS-CoV-2 in some localities until reagents became available, which may have been associated with less testing and therefore less detection of other respiratory viruses20. But even as patients returned for in-person care and health care systems increased their capacity to test for non-SARS-CoV-2 respiratory viruses, there were fewer ED visits and hospital admissions associated with non-SARS-CoV-2 respiratory viruses early in the COVID-19 pandemic.
ED visits associated with respiratory illnesses and non-SARS-CoV-2 viruses
The weekly number of US ED visits associated with respiratory illnesses and non-SARS-CoV-2 infections decreased early in the COVID-19 pandemic compared with the corresponding weeks of 2017–2019 (ref.80). The number of ED visits associated primarily with respiratory concerns (including fever, cough and shortness of breath) initially increased between March and April 2020, but by the end of 2020, there were fewer non-SARS-CoV-2 respiratory ED visits than in previous years. The start of the COVID-19 pandemic was also associated with a decline in US paediatric ED visits compared with 2019, with the greatest decreases observed for non-SARS-CoV-2-associated respiratory illnesses81.
France82, Israel83, South Africa84, Turkey85 and Finland76 also reported decreases in ED visits associated with respiratory illness at the start of the COVID-19 pandemic. However, there was considerable variability in the number of ED visits for respiratory illnesses as the COVID-19 pandemic progressed, likely related to differences in country-specific implementation of NPIs.
Changes to ED visits associated with specific viral pathogens have also been noted, despite variations in testing patterns and implementation of COVID-19 NPIs. Sharp declines of both influenza virus-related ED visits76,80,86–88 and RSV-related ED visits76,80,88 were reported as NPIs were implemented, consistent with the decline in the community circulation of influenza virus and RSV. In addition, ED visits in the USA associated with other enveloped respiratory viruses, such as human metapneumovirus and human parainfluenza virus, showed a similar decline and remained low for the duration of 2020 (ref.80). By contrast, the numbers of ED visits associated with the non-enveloped rhinovirus and respiratory enterovirus were similar and sometimes higher than what was observed in previous years, which is consistent with their persistence in the community80.
Hospital admissions associated with non-SARS-CoV-2 respiratory virus infections
Hospital admissions associated with non-SARS-CoV-2 respiratory virus infections showed declines similar to those of other measures of health care burden early in the COVID-19 pandemic. Hospitalization rates are an important public health measure of disease severity, and population-level hospital-based surveillance systems were established before the COVID-19 pandemic, particularly to monitor seasonal influenza virus activity.
As SARS-CoV-2 community spread began early in 2020, influenza-associated hospitalizations decreased internationally and continued to be at historically low levels until the 2021–2022 influenza season worldwide. Hospital-based surveillance early in the COVID-19 pandemic in Australia6, South Africa48 and South Korea58 showed reductions in influenza-associated hospitalizations throughout the remainder of 2020.
In the USA, where surveillance of influenza-associated hospitalization is conducted through the Influenza Hospitalization Surveillance Network (FluSurv-NET)89, the rates of influenza-associated hospitalization between February and March 2020 plateaued following community spread of SARS-CoV-2 (ref.90). During the 2020–2021 influenza season, US surveillance sites reported a cumulative influenza-associated hospitalization rate of 0.8 per 100,000 population, the lowest rate of any season since 2005, when data collection began91. US hospitalization rates by age group in that year were not calculated given the low numbers of influenza-associated hospitalizations. Through the subsequent waves of SARS-CoV-2, there was regional variation in the ongoing use of COVID-19 NPIs, and by the 2021–2022 influenza season, influenza-associated hospitalization rates had returned to the levels seen in previous influenza seasons. As of 11 June 2022, the cumulative influenza-associated hospitalization rate in the US for all ages for the 2021–2022 influenza season was 17.1 per 100,000 population, compared with the pre-COVID-19 pandemic rate of 62.0 per 100,000 population during the 2016–2017 influenza season and 102.9 per 100,000 population during the 2019–2020 influenza season8.
Early in the COVID-19 pandemic, RSV-associated hospitalizations declined in countries in both the Northern Hemisphere58,92–96 and the Southern Hemiphere48,53,97, where the typical RSV season was ending or just beginning, respectively. However, unlike influenza-associated hospitalizations, there has been variation in the trends of RSV-associated hospitalizations as COVID-19 NPIs were lifted. Many countries reported delayed returns of RSV-associated hospitalizations in comparison with pre-COVID-19 pandemic seasons, corresponding to community circulation patterns. In the USA, between October 2020 and April 2021, the Respiratory Syncytial Virus Hospitalization Surveillance Network98 reported a lower RSV-associated hospitalization rate (0.3 per 100,000 persons) than in the two prior seasons (27.1 per 100,000 persons and 33.4 per 100,000 persons)54. Of the RSV-associated hospitalizations between October 2020 and May 2021, 77% were reported during April and May 2021, illustrating a shift in the typical peak of RSV-associated hospitalizations, which usually peak in the winter months31. One New York City area hospital reported a delay in its expected winter peak of RSV-associated hospitalizations during the 2020-2021 RSV season to the spring and summer months99. Potential reasons for this shift may be lower population-level immunity from lack of infections because of reduced circulation during the early days of the pandemic, leading to a larger susceptible pool of individuals who can be infected and transmit RSV.
Shifts in hospitalizations associated with the RSV season have also been seen in other countries, with variations in the degree of delay relative to previous seasons. In France, the 2020–2021 RSV season was characterized by a 3–4-month delay in the seasonal peak of RSV-associated hospitalizations100,101. Moreover, one Spanish hospital reported that RSV-associated bronchiolitis admissions were delayed until June 2021; in previous seasons, RSV-associated hospitalization peaks typically occurred between November and February95.
In the Southern Hemisphere, countries also experienced delays in RSV-associated hospitalizations during the 2020 winter months. Regional reports from Western Australia noted significant reductions in RSV-associated infections during the winter months47,53. However, an uncharacteristic interseasonal increase of RSV-associated ED visits and hospitalizations occurred in the spring and continued into the early summer months following the lifting of social distancing policies but before relaxation of restrictions on interstate travel53. In 2020, RSV-associated hospitalizations in Buenos Aires, Argentina, were very low during the winter months, when hospitalizations typically peak. In the winter season of 2021, a seasonal peak of RSV-associated hospitalizations returned following the reopening of schools in the community, but was delayed by 10 weeks compared with the 2016–2019 seasons97. The unpredictable onset and duration of the RSV season after initial COVID-19 pandemic NPIs were implemented complicated decisions around administration of palivizumab, a monoclonal antibody given to infants at increased risk of severe illness during the RSV season102.
In addition to the variation in RSV incidence shifts, there has been considerable geographic variation in the proportion of RSV infections requiring hospitalization, with some countries reporting higher rates and others lower rates as RSV re-emerged100. Moreover, one US study reported that children hospitalized with RSV during the 2020–2021 season required higher levels of care (81% versus 45%, respectively, admitted to an intensive care unit) and had a longer median length of hospitalization (4 days versus 3 days, respectively) than children hospitalized with RSV during the 2019–2020 season99.
Why these changes in the epidemiology of RSV occurred is not known, although these shifts may be in part due to waning population-level immunity. Infants are protected through transplacentally derived maternal antibody in the first several weeks of life. Low levels of RSV circulation will lead to waning population RSV antibody titres, and subsequently lead to lower levels of maternal antibody being transferred to infants during gestation103. This has the potential to result in a birth cohort with low levels of maternally derived antibody to experience their first RSV season, at a time when RSV circulation is much higher than in prior years. This would lead to both increased risk of infection and minimal neutralizing antibody titres to protect against severe disease. Simultaneously, there will also be older children who will also encounter their first RSV infection, and infections in young and older children could strain hospital capacity. Modelling studies have estimated that RSV hospitalizations in the post-pandemic period were twice as great as in a normal RSV season for children aged 1 year or younger, but to be highly dependent on NPIs in place and introductions of the virus from other regions104.
Data on hospital admissions due to other non-SARS-CoV-2 respiratory viruses are limited. Several reports demonstrate that rhinovirus-associated and respiratory enterovirus-associated hospitalizations in adults and children persisted or appeared to increase as the COVID-19 pandemic progressed, coinciding in part with the return of in-person school instruction for children58,92,105,106. A retrospective study of paediatric hospitalizations in one US children’s hospital reported a persistence of rhinovirus and respiratory enterovirus detection, while the number of other viruses decreased92. In a report on hospitalized adult patients from the UK, there was an initial decrease in rhinovirus-positive and respiratory enterovirus-positive test results between March and September 2020 (ref.105). However, 2 weeks after the reopening of primary and secondary schools, there was a notable increase in the detection of rhinovirus and respiratory enterovirus in these hospitalized patients, reaching levels seen during the 2019 season105. In both China106 and South Korea58, the numbers of rhinovirus-associated hospitalizations in the summer months of 2020 were higher than the numbers reported in previous seasons.
Whether increases in rhinovirus and respiratory enterovirus circulation reflect decreased interactions with other respiratory viruses, alteration of predominant rhinovirus species or serotypes, or an opportunistic change where rhinovirus infections are filling a niche left by decreases in the numbers of enveloped viruses is unknown. However, rhinovirus sequencing data early in the COVID-19 pandemic showed no significant changes in rhinovirus species predominance in comparison with previous seasons57,106.
Similar persistence patterns were also observed for other non-enveloped viruses, including adenovirus and human bocavirus, which demonstrated persistent but oscillating detections as the COVID-19 pandemic unfolded58.
Reports on hospitalizations associated with other non-SARS-CoV-2 respiratory viruses are limited, but some studies have shown similar declines at the start of the COVID-19 pandemic. In one US paediatric hospital, there was an abrupt decrease in non-SARS-CoV-2-associated hospitalizations after 21 April 2020 that continued through to December 2020 (ref.92). In addition to no reported cases of influenza virus-associated or RSV-associated hospitalization, there were no hospitalizations for human metapneumovirus, human parainfluenza or seasonal human coronavirus infections through to 31 December 2020 (ref.92). Hospital surveillance data from South Korea also demonstrated decreases in the monthly rate of detection of enveloped viruses, including human parainfluenza virus, seasonal human coronavirus and human metapneumovirus58.
Respiratory disease associated with non-SARS-CoV-2 viruses
The landscape of clinical respiratory disease burden not associated with SARS-CoV-2 infection has also changed during the COVID-19 pandemic. Respiratory syndromes and conditions such as otitis media, pneumonia and invasive bloodstream infections have been affected by shifts in viral pathogens, showing initial declines in frequency and alterations that match the clinical epidemiology of community-acquired respiratory viruses.
Acute respiratory illnesses and ILI
Declines in cases of acute respiratory illnesses (ARIs) and ILI were associated with the mitigation of non-SARS-CoV-2 respiratory viruses and have been reported most prominently among children. One multicentre ARI surveillance study in the USA showed a sharp decline in paediatric ARI cases in April 2020 after many communities had implemented stay-at-home orders107. This was largely due to the decline of influenza-associated and RSV-associated ARI.
In a multicentre prospective study of primary care paediatric practices in Germany between November 2020 and April 2021, rhinovirus was the most commonly detected respiratory virus among children with ARI, while SARS-CoV-2 and influenza virus infections were minimally detected108. Among children in Thailand seeking care, there was a notable increase in cases of ILI caused by rhinovirus in July 2020, which were replaced by RSV-associated ILI cases later in the season109. This suggests that although overall numbers of ILI cases declined, rhinovirus and RSV were important contributors to those cases that did require hospitalization.
All-cause outpatient ILI surveillance in the USA, conducted through ILI-Net, has shown general fluctuating patterns between the 2019–2020 and 2021–2022 influenza seasons8. The changes in the percentages of ILI outpatient visits are likely due in large part to the waves of SARS-CoV-2 infections in adults, but are possibly also due in part to non-SARS-CoV-2 respiratory viruses as endemic respiratory virus infections increased during 2021–2022 with the relaxation of NPIs. In a study of ILI in South Korea, the levels of respiratory virus causes fluctuated with community circulation, including initially lower levels of human bocavirus followed by increases in the detection rate towards the end of 2020 (ref.57).
Community-acquired pneumonia incidence
Community-acquired pneumonia (CAP) causes substantial clinical burden and has both viral and bacterial aetiologies110,111. Many early COVID-19 pandemic reports showed a decrease in the incidence of non-COVID-19 CAP that was associated with the implementation of community NPIs112–120. Comparisons of these CAP cases early in the COVID-19 pandemic with CAP cases in periods during the pandemic but before NPI implementation and with CAP cases in previous seasons show a change in both bacterial and viral aetiologies118,121.
One multicentre study in Germany observed a seasonal decline in the frequencies of CAP cases associated with non-SARS-CoV-2 respiratory viruses, and these corresponded to a reduction in the diversity of the causative viral pathogen (that is, there were fewer types of respiratory viruses associated with CAP)121. For instance, from May 2020 through the remainder of the year, there was an abrupt reduction in the diversity of pathogens, with only rhinovirus-associated CAP as a persistent and lingering viral cause of non-COVID-19 CAP.
Bronchiolitis incidence
The burden and epidemiology of bronchiolitis, a common lower respiratory tract disease diagnosis in young children, has also been impacted by the effects of the COVID-19 pandemic.
While bronchiolitis can be associated with any respiratory virus infection, including SARS-CoV-2 infection and influenza virus infection, cases are commonly associated with RSV infection122. Many countries reported changes in the frequency of bronchiolitis cases that mirrored trends in community circulation of RSV during the COVID-19 pandemic. Bronchiolitis cases decreased in Northern Hemisphere countries, with low numbers of cases reported throughout much of the 2020–2021 respiratory virus season94–96,120,123–126.
The implementation of NPIs early in 2020 occurred at a time when seasonal cases of bronchiolitis were expected in the Southern Hemisphere. However, acute lower respiratory tract infections, including bronchiolitis, in children in Argentina remained low for the duration of the 2020 season, when bronchiolitis and other paediatric lower respiratory tract infection peaks were expected127. In Brazil, infant hospitalizations for respiratory disease, including bronchiolitis, declined during April and June 2020 (typically seasonal peaks occur from March to July)128. In Australia, fewer cases of hospitalized bronchiolitis and fewer bronchiolitis admissions to paediatric intensive care units were observed than in previous seasons129. However, sentinel surveillance of Australian hospital records when RSV epidemics reoccurred in late 2020 to early 2021 showed numbers similar to or higher than the numbers in previous seasons, confirming the mirrored incidence of bronchiolitis and RSV community transmission130.
Chronic respiratory disease incidence
Chronic respiratory diseases have also been impacted by the effects of the COVID-19 pandemic. Exacerbations of asthma and COPD are frequently associated with respiratory virus infections, and national lockdowns and pandemic NPIs were associated with fewer asthma hospital admissions in the early COVID-19 pandemic period120,124,125,131. In the UK, in addition to fewer asthma admissions, there were reductions in severe asthma exacerbations, with no significant changes in deaths due to asthma132. Frequencies of hospital admissions for exacerbations of COPD have also declined in South Korea and Slovenia133,134.
Several explanations have been proposed to explain these findings, including infections and non-infectious causes. Respiratory virus infections are frequently associated with exacerbations of chronic respiratory diseases. Therefore, the decreased community circulation, which is associated with behavioural changes and NPIs, may have led to fewer exacerbations attributed to respiratory viruses. One report noted that there was an increase in the number of prescriptions for inhaled and oral corticosteroid before lockdown was implemented, possibly due to concern about medication shortages or access during lockdown, and higher adherence to controller medication use132. Fewer hospitalizations due to asthma and COPD may also be due to improved home management of exacerbations. Other factors associated with fewer chronic respiratory disease exacerbations include additional changes in health-seeking behaviour, such as the use of telehealth visits that allowed management of exacerbations at home and reduction in air pollution from stay-at-home measures135.
Virus-associated severe illnesses and deaths
Severe illnesses associated with non-SARS-CoV-2 respiratory virus infections declined during some periods of the COVID-19 pandemic. For instance, only one influenza-associated paediatric death was reported in the USA during the 2020–2021 season, in contrast to144 deaths during the 2018–2019 season and 199 deaths during the 2019–2020 season. From October 2021 to April 2022, 24 influenza-associated paediatric deaths were reported in the USA, corresponding to increased influenza virus activity during the 2021–2022 season8.
Evolution of influenza viruses and other non-SARS-CoV-2 respiratory viruses
The global implementation of NPIs, broad community spread of SARS-CoV-2 and individual behaviour changes during the COVID-19 pandemic seem to have dramatically reduced the circulation of common respiratory viruses. However, many respiratory viruses returned as these measures were lifted, with the frequency of infections and circulation patterns influenced by regional differences in community NPIs. A hallmark of respiratory viruses is their ability to continue circulating despite the accumulated population immunity from infections, and in the case of influenza virus infections, the variable use of vaccines. Evolution is central to immune evasion by respiratory viruses, and is facilitated by genetic mutations from a diverse viral gene pool. The sudden drop in respiratory virus transmission may have affected the genetic diversity of many viral species by creating an evolutionary bottleneck, but the lasting effects of this evolutionary pressure will vary and will be impacted by many factors, such as changing population-level susceptibility, the introduction of strains through increased international travel and the development of novel vaccines.
Impact of changes in viral transmission
Both baseline pre-pandemic genetic diversity and specific viral characteristics may influence a virus’s ability to resist the selection pressure associated with NPI use. RSV, for example, circulates in the community as two subtypes, RSV-A and RSV-B, that can alternate in predominance in many regions. In Australia, both subtypes frequently co-circulate with similar prevalence130. Before the emergence of SARS-CoV-2, RSV genomic diversity was maintained by sources both within and outside Australia136. However, genomic analyses of samples obtained before and after the onset of the COVID-19 pandemic showed both a change in the RSV subtype predominance (with RSV-A predominating after community NPIs were implemented) and a reduction in RSV-A sublineages and the emergence of novel lineages, which were genomically linked to frequent domestic importations as well as rarer importations from Washington state in the USA130. Whether similar changes in RSV subtype predominance or genomic diversity were experienced in other regions is not known, and subsequent analyses will be needed to understand whether regional RSV diversity could be restored as global travel increases.
By contrast, limited genomic analyses of rhinoviruses suggested a different outcome during the COVID-19 pandemic. Rhinovirus includes several species that are genetically diverse and are able to continuously circulate in the community despite NPIs. Although differentiating rhinovirus types through genomic sequencing is not always possible, the predominance of rhinovirus species in South Korea did not seem to change during the COVID-19 pandemic despite community SARS-CoV-2 mitigation efforts57.
Similarly, although there was an initial reduction in the detection of seasonal human coronavirus throughout 2020, epidemics did occur early in 2021 in the USA as all four seasonal human coronavirus species reappeared, coinciding with the relaxing of NPIs later in the COVID-19 pandemic137. Epidemic peaks in early 2021 occurred at different time points, differing by geographic region and viral species, with most regions experiencing increased prevalence of seasonal human coronavirus that continued throughout early summer137. Data are limited on the effects of the COVID-19 pandemic on seasonal human coronavirus genomic diversity. One study comparing the genomic diversity of seasonal human coronavirus infections in homeless shelters before and during the COVID-19 pandemic showed similar diversity between the two periods138.
Impact of changes in immunity
Changes in immunity may also influence the evolution of some viruses. For influenza viruses, for instance, population-level immunity is developed from prior infections and vaccination. With the decline in levels of circulating influenza viruses, lower vaccination coverage and antigenic drift, defined as small changes in the viral genome associated with errors in viral replication, leading to vaccine subtype mismatch39,139, waning humoral immunity, as defined by lower antibody titres, may make infection more likely, leading to more infections, increased viral replication and additional opportunities for mutations leading to emergence of new variants. It is also possible that in locations where influenza vaccination is not widely administered, waning immunity from recent infections can result in high infection attack rates despite limited influenza virus diversity. Moreover, the strain selection process for the influenza vaccine depends on an evaluation of humoral immunity, specifically haemagglutinin inhibition antibody titres against the circulating virus strains from influenza sentinel surveillance sites in the Northern Hemisphere and the Southern Hemisphere. Low levels of circulating influenza virus may also create challenges for the annual vaccine strain selection process because the smaller number of characterized viruses identified through surveillance makes it more difficult to predict the predominant influenza virus strains for the upcoming season for inclusion in the vaccine, with unknown effect on influenza vaccine effectiveness.
Impact of travel
International travel, which has facilitated the spread of respiratory viruses, may also influence the evolution of certain viruses. Global circulation and seasonal epidemic patterns of influenza virus vary by species and type. Influenza A(H3N2) virus continuously circulated in a complex network of regional epidemics in eastern Asia and Southeast Asia that subsequently facilitated the seeding of temperate regions outside this area, leading to annual seasonal epidemics140.
By contrast, during the pandemic, influenza A(H1N1) virus and influenza B virus experienced relatively slower rates of evolution and were less reliant on viral seeding from Asia, with variants continuing to circulate across multiple seasons141. It is possible that changes in travel behaviour during the COVID-19 pandemic may have had differential effects on viral species and types. The resumption of global travel is likely to facilitate the spread and evolution of influenza viruses and other respiratory viruses by enhancing the diversity of the viral genetic pool.
The broad application of genomic sequencing to study SARS-CoV-2 molecular epidemiology has enhanced our understanding of respiratory virus evolution. Expanded sequencing of non-SARS-CoV-2 viruses will be useful to monitor viral diversity and evolutionary changes during and after the COVID-19 pandemic, and with the introduction of vaccines, antiviral, and monoclonal antibodies for prevention and treatment.
Changes in the influenza B/Yamagata virus lineage and observations from the influenza A(H1N1)pdm09 pandemic
One evolutionary change observed during the COVID-19 pandemic is the possible extinction of the influenza B/Yamagata virus lineage, with no confirmed detections from March 2020 onward142. The 2019–2020 influenza season in the USA was characterized by influenza B/Victoria virus predominance, with a later predominance of influenza A(H1N1)pdm09 virus143. During the US 2020–2021 season, low levels of influenza-positive test results were reported, with influenza B virus predominating144. Influenza virus activity resumed during 2021–2022 but was lower than in most seasons before the COVID-19 pandemic.
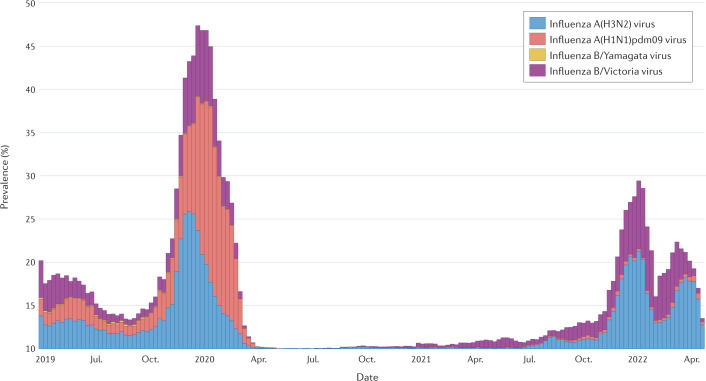
Interestingly, Nextstrain last reported influenza B/Yamagata lineage virus in March 2020 (ref.145). Similarly, FluNet, a WHO global influenza virological surveillance tool started in 1997 through the Global Influenza Surveillance and Response System, has shown a dramatic reduction in influenza B/Yamagata lineage virus detections reported, with none confirmed by sequencing after March 2020146 (Fig. 2). Ongoing global influenza surveillance will determine whether the influenza B/Yamagata virus lineage is no longer circulating and whether this viral antigen no longer needs to be included in the composition of quadrivalent influenza vaccines used worldwide.
Fig. 2. Surveillance of influenza viruses by the World Health Organization Global Influenza Surveillance and Response System 2019–2022.
The prevalence of influenza A(H3N2) virus, influenza A(H1N1)pdm09 virus, influenza B/Yamagata virus and influenza B/Victoria virus is shown. The date range of the data extends from 2019 epidemiological week 17 to 2022 epidemiological week 16. The last confirmed report of influenza B/Yamagata virus was in March 2020 (ref.146). Adapted with permission from ref.146, WHO.
Recent influenza pandemics offer few clues as to how COVID-19 will affect the circulation of seasonal influenza viruses. In 2009, the emergence of the pandemic influenza A(H1N1)pdm09 virus was associated with a marked decline in seasonal influenza A and influenza B viruses147 and replacement of seasonal influenza A(H1N1) virus in subsequent seasons148. Although low numbers of circulating seasonal influenza viruses were reported while COVID-19 pandemic NPIs were in place, the longer-term effects of the COVID-19 pandemic may not be apparent until future influenza seasons.
Conclusions
The COVID-19 pandemic has led to unprecedented societal and human behaviour changes that have altered the community circulation of non-SARS-CoV-2 respiratory viruses. Although changes in testing priorities and available resources may have influenced the earliest reports of non-SARS-CoV-2 respiratory virus activity, subsequent studies across multiple geographic locations have shown consistent reductions in the activity of most respiratory virus species. However, as the COVID-19 pandemic progressed, there were increasing differences in the application of NPIs and community circulation of SARS-CoV-2 that led to variability in the return of some respiratory viruses. Most notably, influenza virus activity, including the clinical burden of disease and paediatric mortality, remained low through the 2020–2021 season before influenza virus activity increased in 2021–2022. While influenza A and influenza B viruses have begun circulating more widely, influenza B/Yamagata viruses have hardly been detected, and ongoing surveillance will inform whether these viruses might be extinct. Detections of RSV infections initially remained low, with the resurgence of interseasonal circulation in many locations since 2021. There is also evidence that influenza virus diversity and RSV diversity have been reduced as a result of the COVID-19 pandemic, but whether these changes are maintained requires continued surveillance and genomic analyses.
Unlike many of the enveloped viruses, non-enveloped viruses, including rhinovirus, demonstrated remarkable persistence through NPIs, with early evidence showing similar species circulation before and during the COVID-19 pandemic. In part, the persistence of non-enveloped viruses despite NPI implementation may be due to prolonged shedding, their greater diversity and their persistence on environmental surfaces.
These collective findings and lessons learned during the COVID-19 pandemic have important implications for pandemic preparedness. Although the changes in circulation of non-SARS-CoV-2 respiratory viruses cannot be attributed to any one factor, the near-simultaneous implementation of NPIs at individual, community, environmental and country levels, in addition to behavioural changes, seems to have been an important contributor to reducing community circulation, decreasing the associated health care burden and blunting the evolution of non-SARS-CoV-2 respiratory viruses. As such, these collective NPI strategies and global collaboration will be important public health considerations for the next pandemic.
Supplementary information
Acknowledgements
This work was supported in part by the US National Institutes of Health National Institute of Allergy and Infectious Diseases (grant number T32 AI007044 to E.J.C.).
Glossary
- Syndromic surveillance
Nonspecific symptom-based monitoring used with case definitions to detect early and unusual events or changes from the baseline; requires laboratory testing to provide disease specificity.
- Influenza-like illness
(ILI). Fever (temperature of 37.8 °C or greater) and a cough and/or a sore throat.
- Sentinel surveillance system
Disease monitoring by use of specific case definitions at health care facilities to assess disease trends; requires defined a catchment population to generate disease incidence or prevalence rates.
- Influenza Hospitalization Surveillance Network (FluSurv-NET)
US surveillance system that collects population-level data on laboratory-confirmed influenza-associated hospitalizations across 14 states.
- Respiratory Syncytial Virus Hospitalization Surveillance Network
US surveillance system that collects data on respiratory syncytial virus-associated hospitalizations across 12 states each year.
- Acute respiratory illnesses
(ARIs). New respiratory symptoms with or without fever caused by infection of the respiratory tract.
- Community-acquired pneumonia
(CAP). Pneumonia caused by respiratory pathogens circulating among the community, not health care associated.
- Antigenic drift
Genetic mutations that result in changes to key viral antigens that allow respiratory viruses to escape humoral immunity conferred by prior infection or vaccination.
- Humoral immunity
Adaptive immune response to generate antibodies (antibody-mediated immunity) to the key viral epitopes to prevent infection or reduce the severity of disease.
- Nextstrain
An open-source scientific platform to visualize pathogen genomic data from GISAID that has included influenza genomic sequences since 2015.
Author contributions
All authors researched data for the article. All authors substantially contributed to the discussion of the content. All authors contributed to the writing of the manuscript. All authors reviewed/edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Microbiology thanks Aubree Gordon, who co-reviewed with John Kubale, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
E.J.C. has received honoraria from Providence Health & Services, Seattle, WA, USA, for presentations on COVID-19. H.Y.C. has provided consulting services for Ellume, Pfizer, the Bill and Melinda Gates Foundation, GlaxoSmithKline and Merck. H.Y.C. has received research funding from Gates Ventures and Sanofi Pasteur and has received support and reagents from Ellume and Cepheid outside the submitted work. T.M.U. reports no competing interests.
Footnotes
Disclaimer
The views expressed are those of the authors and do not necessarily reflect the official policy of the Centers for Disease Control and Prevention.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41579-022-00807-9.
References
- 1.Collaborators C-EM. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Non-pharmaceutical public health measures for mitigating the risk and impact of epidemic and pandemic influenzahttps://www.who.int/publications/i/item/non-pharmaceutical-public-health-measuresfor-mitigating-the-risk-and-impact-of-epidemic-and-pandemic-influenza (2019).
- 3.Pan American Health Organization. Non-pharmaceutical interventions (NPIs): actions to limit the spread of the pandemic in your municipalityhttps://www.paho.org/disasters/dmdocuments/RespToolKit_11_Tool%2004_NonPharmaceuticalInterventions(NPIs).pdf (2004).
- 4.Qualls N, et al. Community mitigation guidelines to prevent pandemic influenza - United States, 2017. MMWR Recomm. Rep. 2017;66:1–34. doi: 10.15585/mmwr.rr6601a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bish DR, Bish EK, El-Hajj H, Aprahamian H. A robust pooled testing approach to expand COVID-19 screening capacity. PLoS ONE. 2021;16:e0246285. doi: 10.1371/journal.pone.0246285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan SG, et al. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Eur. Surveill. 2020 doi: 10.2807/1560-7917.ES.2020.25.47.2001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piret J, Boivin G. Viral interference between respiratory viruses. Emerg. Infect. Dis. 2022;28:273–281. doi: 10.3201/eid2802.211727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Weekly U.S. influenza surveillance report: FluViewhttps://www.cdc.gov/flu/weekly/index.htm (2022).
- 9.Centers for Disease Control and Prevention. The National Respiratory and Enteric Virus Surveillance System (NREVSS)https://www.cdc.gov/surveillance/nrevss/index.html (2022).
- 10.World Health Organization. Global Influenza Programmehttps://www.who.int/tools/flunet (2022).
- 11.Centers for Disease Control and Prevention. COVID Data Trackerhttps://covid.cdc.gov/covid-data-tracker/#cases-deaths-testing-trends (2022).
- 12.Johns Hopkins University Coronavirus Resource Center. COVID-19 United States cases 2020https://coronavirus.jhu.edu/us-map (2020).
- 13.World Health Organization. WHO Coronavirus (COVID-19) Dashboardhttps://covid19.who.int/ (2022).
- 14.Chu HY, et al. The Seattle Flu Study: a multiarm community-based prospective study protocol for assessing influenza prevalence, transmission and genomic epidemiology. BMJ Open. 2020;10:e037295. doi: 10.1136/bmjopen-2020-037295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.State of Washington: Office of the Governor. Proclamation by the Governor Amending Proclamation 20-05: Stay Home-Stay Healthyhttps://www.governor.wa.gov/sites/default/files/proclamations/20-25%20Coronovirus%20Stay%20Safe-Stay%20Healthy%20%28tmp%29%20%28002%29.pdf (2020).
- 16.Seattle Flu Alliance. Pathogenshttps://seattleflu.org/pathogens (2022).
- 17.Laurie KL, Rockman S. Which influenza viruses will emerge following the SARS-CoV-2 pandemic? Influenza Other Respir. Viruses. 2021;15:573–576. doi: 10.1111/irv.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen SJ, et al. Decreased influenza activity during the COVID-19 pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng L, et al. Impact of COVID-19 outbreaks and interventions on influenza in China and the United States. Nat. Commun. 2021;12:3249. doi: 10.1038/s41467-021-23440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hills T, Kearns N, Kearns C, Beasley R. Influenza control during the COVID-19 pandemic. Lancet. 2020;396:1633–1634. doi: 10.1016/S0140-6736(20)32166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hay AJ, McCauley JW. The WHO global influenza surveillance and response system (GISRS) — a future perspective. Influenza Other Respir. Viruses. 2018;12:551–557. doi: 10.1111/irv.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Global Influenza Surveillance and Response System (GISRS)https://www.who.int/initiatives/global-influenza-surveillance-and-response-system (2022).
- 23.Centers for Disease Control and Prevention. Fluview: flu activity & surveillancehttps://www.cdc.gov/flu/weekly/fluactivitysurv.htm (2022).
- 24.Roman PC, et al. Influenza vaccinations during the COVID-19 pandemic - 11 U.S. jurisdictions, September–December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1575–1578. doi: 10.15585/mmwr.mm7045a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weycker D, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23:1284–1293. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Anderson EJ, Daugherty MA, Pickering LK, Orenstein WA, Yogev R. Protecting the community through child vaccination. Clin. Infect. Dis. 2018;67:464–471. doi: 10.1093/cid/ciy142. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SA, Chui KK, Naumova EN. Influenza vaccination in young children reduces influenza-associated hospitalizations in older adults, 2002–2006. J. Am. Geriatr. Soc. 2011;59:327–332. doi: 10.1111/j.1532-5415.2010.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tinoco YO, et al. Burden of Influenza in 4 ecologically distinct regions of Peru: household active surveillance of a community cohort, 2009–2015. Clin. Infect. Dis. 2017;65:1532–1541. doi: 10.1093/cid/cix565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen C, et al. Asymptomatic transmission and high community burden of seasonal influenza in an urban and a rural community in South Africa, 2017-18 (PHIRST): a population cohort study. Lancet Glob. Health. 2021;9:e863–e874. doi: 10.1016/S2214-109X(21)00141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogel B, Schaefer EW, Hicks SD. Early influenza vaccination rates decline in children during the COVID-19 pandemic. Vaccine. 2021;39:4291–4295. doi: 10.1016/j.vaccine.2021.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen SJ, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic - United States, 2020–2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1013–1019. doi: 10.15585/mmwr.mm7029a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park KY, Seo S, Han J, Park JY. Respiratory virus surveillance in Canada during the COVID-19 pandemic: an epidemiological analysis of the effectiveness of pandemic-related public health measures in reducing seasonal respiratory viruses test positivity. PLoS ONE. 2021;16:e0253451. doi: 10.1371/journal.pone.0253451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arellanos-Soto D, et al. Decline in influenza cases in Mexico after the implementation of public health measures for COVID-19. Sci. Rep. 2021;11:10730. doi: 10.1038/s41598-021-90329-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhtar Z, et al. Seasonal influenza during the COVID-19 pandemic in Bangladesh. PLoS ONE. 2021;16:e0255646. doi: 10.1371/journal.pone.0255646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li ZJ, et al. Broad impacts of COVID-19 pandemic on acute respiratory infections in China: an observational study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu P, et al. Impact of COVID-19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China. Virol. J. 2021;18:159. doi: 10.1186/s12985-021-01627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang W, et al. Epidemiological and virological surveillance of seasonal influenza viruses-China, 2020–2021. China CDC Wkly. 2021;3:918–922. doi: 10.46234/ccdcw2021.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. Weekly Epidemiological Record (WER), 25 March 2022, Vol. 97, No. 12, https://reliefweb.int/report/world/weekly-epidemiological-record-wer-25-march-2022-vol-97-no-12-pp109-132-enfr (2022).
- 39.Dhanasekaran V, et al. Human seasonal influenza under COVID-19 and the potential consequences of influenza lineage elimination. Nat. Commun. 2022;13:1721. doi: 10.1038/s41467-022-29402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson NM, et al. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viboud C, et al. Risk factors of influenza transmission in households. Br. J. Gen. Pract. 2004;54:684–689. [PMC free article] [PubMed] [Google Scholar]
- 42.Bancej C, et al. National FluWatch mid-season report, 2021–2022: sporadic influenza activity returns. Can. Commun. Dis. Rep. 2022;48:39–45. doi: 10.14745/ccdr.v48i01a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delahoy MJ, et al. Influenza A(H3N2) outbreak on a university campus-Michigan, October–November 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1712–1714. doi: 10.15585/mmwr.mm7049e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nott R, Fuller TL, Brasil P, Nielsen-Saines K. Out-of-season influenza during a COVID-19 void in the State of Rio de Janeiro, Brazil: temperature matters. Vaccines. 2022;10:821. doi: 10.3390/vaccines10050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan American Health Organization. Regional update, influenza. epidemiological week 17 (10 May 2022)https://iris.paho.org/handle/10665.2/55976 (2022).
- 46.Australian Government Department of Health. Australian Influenza Surveillance Reporthttps://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-ozflu-flucurr.htm/$File/flu-08-2022.pdf (2022).
- 47.Yeoh DK, et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin. Infect. Dis. 2021;72:2199–2202. doi: 10.1093/cid/ciaa1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tempia S, et al. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Eur. Surveill. 2021 doi: 10.2807/1560-7917.ES.2021.26.29.2001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varela FH, et al. Absence of detection of RSV and influenza during the COVID-19 pandemic in a Brazilian cohort: likely role of lower transmission in the community. J. Glob. Health. 2021;11:05007. doi: 10.7189/jogh.11.05007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casalegno JS, et al. Characteristics of the delayed respiratory syncytial virus epidemic, 2020/2021, Rhone Loire, France. Eur. Surveill. 2021 doi: 10.2807/1560-7917.ES.2021.26.29.2100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ujiie M, Tsuzuki S, Nakamoto T, Iwamoto N. Resurgence of respiratory syncytial virus infections during COVID-19 pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021;27:2969–2970. doi: 10.3201/eid2711.211565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saravanos GL, et al. RSV epidemiology in australia before and during COVID-19. Pediatrics. 2022 doi: 10.1542/peds.2021-053537. [DOI] [PubMed] [Google Scholar]
- 53.Foley DA, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019-related public health measures. Clin. Infect. Dis. 2021;73:e2829–e2830. doi: 10.1093/cid/ciaa1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olsen SJ, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic-United States, 2020–2021. Am. J. Transpl. 2021;21:3481–3486. doi: 10.1111/ajt.16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. The National Respiratory and Enteric Virus Surveillance System (NREVSS): RSV census regional trendshttps://www.cdc.gov/surveillance/nrevss/rsv/region.html (2021).
- 56.Cooksey GLS, et al. Severe acute respiratory syndrome coronavirus 2 and respiratory virus sentinel surveillance, California, USA, May 10, 2020–June 12, 2021. Emerg. Infect. Dis. 2022;28:9–19. doi: 10.3201/eid2801.211682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HM, et al. Impact of coronavirus disease 2019 on respiratory surveillance and explanation of high detection rate of human rhinovirus during the pandemic in the Republic of Korea. Influenza Other Respir. Viruses. 2021;15:721–731. doi: 10.1111/irv.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park S, Michelow IC, Choe YJ. Shifting patterns of respiratory virus activity following social distancing measures for coronavirus disease 2019 in South Korea. J. Infect. Dis. 2021;224:1900–1906. doi: 10.1093/infdis/jiab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuitunen I, Artama M, Haapanen M, Renko M. Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions — a nationwide register study in Finland. J. Med. Virol. 2021;93:6063–6067. doi: 10.1002/jmv.27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Heneidy A, et al. Respiratory virus detection during the COVID-19 pandemic in Queensland, Australia. Aust. N. Z. J. Public Health. 2022;46:10–15. doi: 10.1111/1753-6405.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eisen AKA, et al. Low circulation of Influenza A and coinfection with SARS-CoV-2 among other respiratory viruses during the COVID-19 pandemic in a region of southern Brazil. J. Med. Virol. 2021;93:4392–4398. doi: 10.1002/jmv.26975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agrupis KA, et al. If not COVID-19 what is it? Analysis of COVID-19 versus common respiratory viruses among symptomatic health care workers in a tertiary infectious disease referral hospital in Manila, Philippines. Trop. Med. Infect. Dis. 2021 doi: 10.3390/tropicalmed6010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kitanovski S, et al. Rhinovirus prevalence as indicator for efficacy of measures against SARS-CoV-2. BMC Public Health. 2021;21:1178. doi: 10.1186/s12889-021-11178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dee K, et al. Human rhinovirus infection blocks severe acute respiratory syndrome Coronavirus 2 replication within the respiratory epithelium: implications for COVID-19 epidemiology. J. Infect. Dis. 2021;224:31–38. doi: 10.1093/infdis/jiab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu A, Mihaylova VT, Landry ML, Foxman EF. Interference between rhinovirus and influenza A virus: a clinical data analysis and experimental infection study. Lancet Microbe. 2020;1:e254–e262. doi: 10.1016/S2666-5247(20)30114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yum S, Hong K, Sohn S, Kim J, Chun BC. Trends in viral respiratory infections during COVID-19 pandemic, South Korea. Emerg. Infect. Dis. 2021;27:1685–1688. doi: 10.3201/eid2706.210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takashita E, et al. Increased risk of rhinovirus infection in children during the coronavirus disease-19 pandemic. Influenza Other Respir. Viruses. 2021;15:488–494. doi: 10.1111/irv.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang K, et al. Rapid disappearance of influenza following the implementation of COVID-19 mitigation measures in Hamilton, Ontario. Can. Commun. Dis. Rep. 2021;47:202–209. doi: 10.14745/ccdr.v47i04a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention. The National Respiratory and Enteric Virus Surveillance System (NREVSS): national trends for common human coronaviruseshttps://www.cdc.gov/surveillance/nrevss/coronavirus/natl-trends.html (2021).
- 70.Ippolito G, et al. Disappearance of seasonal respiratory viruses in children under two years old during COVID-19 pandemic: a monocentric retrospective study in Milan, Italy. Front. Pediatr. 2021;9:721005. doi: 10.3389/fped.2021.721005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Russell KL, et al. Transmission dynamics and prospective environmental sampling of adenovirus in a military recruit setting. J. Infect. Dis. 2006;194:877–885. doi: 10.1086/507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benschop KS, et al. Re-emergence of enterovirus D68 in Europe after easing the COVID-19 lockdown, September 2021. Eur. Surveill. 2021 doi: 10.2807/1560-7917.ES.2021.26.45.2100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dezman ZDW, et al. Masking for COVID-19 is associated with decreased emergency department utilization for non-COVID viral illnesses and respiratory conditions in Maryland. Am. J. Med. 2021;134:1247–1251. doi: 10.1016/j.amjmed.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wood BR, et al. Visit trends and factors associated with telemedicine uptake among persons with HIV during the COVID-19 pandemic. Open Forum Infect. Dis. 2021;8:ofab480. doi: 10.1093/ofid/ofab480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel SY, et al. Trends in outpatient care delivery and telemedicine during the COVID-19 pandemic in the US. JAMA Intern. Med. 2021;181:388–391. doi: 10.1001/jamainternmed.2020.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuitunen I, et al. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr. Infect. Dis. J. 2020;39:e423–e427. doi: 10.1097/INF.0000000000002845. [DOI] [PubMed] [Google Scholar]
- 77.Sun H, et al. The influence of coronavirus disease 2019 on emergency department visits in Nanjing, China: a multicentre cross-sectional study. Am. J. Emerg. Med. 2020;38:2101–2109. doi: 10.1016/j.ajem.2020.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeffery MM, et al. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern. Med. 2020;180:1328–1333. doi: 10.1001/jamainternmed.2020.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adjemian J, et al. Update: COVID-19 pandemic-associated changes in emergency department visits - United States, December 2020–January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:552–556. doi: 10.15585/mmwr.mm7015a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodgers L, et al. Changes in seasonal respiratory illnesses in the United States during the coronavirus disease 2019 (COVID-19) pandemic. Clin. Infect. Dis. 2021;73:S110–S117. doi: 10.1093/cid/ciab311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Radhakrishnan L, et al. Pediatric emergency department visits before and during the COVID-19 pandemic - United States, January 2019–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:313–318. doi: 10.15585/mmwr.mm7108e1. [DOI] [PubMed] [Google Scholar]
- 82.Angoulvant F, et al. Coronavirus disease 2019 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and nonviral infections-a time series analysis. Clin. Infect. Dis. 2021;72:319–322. doi: 10.1093/cid/ciaa710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Be’er M, et al. Unforeseen changes in seasonality of pediatric respiratory illnesses during the first COVID-19 pandemic year. Pediatr. Pulmonol. 2022 doi: 10.1002/ppul.25896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perofsky AC, et al. The direct and indirect effects of the COVID-19 pandemic on private healthcare utilization in South Africa, March 2020–September 2021. Clin. Infect. Dis. 2022 doi: 10.1093/cid/ciac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Onay ZR, et al. Did hospital admissions caused by respiratory infections and asthma decrease during the COVID-19 pandemic? Medeni. Med. J. 2022;37:92–98. doi: 10.4274/MMJ.galenos.2022.02779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.COVID-19 Stats. COVID-19* and Influenza(dagger) discharge diagnoses as a percentage of emergency department (ED) visits,(section sign) by year - United States, June 2018–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:573. doi: 10.15585/mmwr.mm7015a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Casalino E, et al. Analysis of emergency department visits and hospital activity during Influenza season, COVID-19 epidemic, and lockdown periods in view of managing a future disaster risk: a multicenter observational study. Int. J. Environ. Res. Public Health. 2020;17:8302. doi: 10.3390/ijerph17228302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stamm P, et al. Influenza and RSV incidence during COVID-19 pandemic-an observational study from in-hospital point-of-care testing. Med. Microbiol. Immunol. 2021;210:277–282. doi: 10.1007/s00430-021-00720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Centers for Disease Control and Prevention. Influenza Hospitalization Surveillance Network (FluSurv-NET)https://www.cdc.gov/flu/weekly/influenza-hospitalization-surveillance.htm (2021).
- 90.Centers for Disease Control and Prevention. Influenza: US virologic surveillancehttps://www.cdc.gov/flu/weekly/weeklyarchives2019-2020/Week17.htm (2020).
- 91.Centers for Disease Control and Prevention. FluView summary ending on April 24, 2021https://www.cdc.gov/flu/weekly/weeklyarchives2020-2021/week16.htm (2021).
- 92.Song X, et al. Common seasonal respiratory viral infections in children before and during the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control. Hosp. Epidemiol. 2021 doi: 10.1017/ice.2021.430. [DOI] [PubMed] [Google Scholar]
- 93.Nolen LD, et al. Impact of social distancing and travel restrictions on non-coronavirus disease 2019 (Non-COVID-19) respiratory hospital admissions in young children in rural Alaska. Clin. Infect. Dis. 2021;72:2196–2198. doi: 10.1093/cid/ciaa1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stera G, et al. Impact of SARS-CoV-2 pandemic on bronchiolitis hospitalizations: the experience of an Italian tertiary center. Children. 2021 doi: 10.3390/children8070556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bermudez Barrezueta L, et al. Variation in the seasonality of the respiratory syncytial virus during the COVID-19 pandemic. Infection. 2022 doi: 10.1007/s15010-022-01794-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torres-Fernandez D, Casellas A, Mellado MJ, Calvo C, Bassat Q. Acute bronchiolitis and respiratory syncytial virus seasonal transmission during the COVID-19 pandemic in Spain: a national perspective from the pediatric Spanish Society (AEP) J. Clin. Virol. 2021;145:105027. doi: 10.1016/j.jcv.2021.105027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferrero F, Ossorio MF, Rial MJ. The return of RSV during the COVID-19 pandemic. Pediatr. Pulmonol. 2022;57:770–771. doi: 10.1002/ppul.25802. [DOI] [PubMed] [Google Scholar]
- 98.Centers for Disease Control and Prevention. Respiratory Syncytial Virus (RSV) Hospitalization Surveillance Network (RSV-NET)https://www.cdc.gov/rsv/research/rsv-net.html (2021).
- 99.Agha R, Avner JR. Delayed seasonal RSV surge observed during the COVID-19 pandemic. Pediatrics. 2021 doi: 10.1542/peds.2021-052089. [DOI] [PubMed] [Google Scholar]
- 100.Williams TC, Sinha I, Barr IG, Zambon M. Transmission of paediatric respiratory syncytial virus and influenza in the wake of the COVID-19 pandemic. Eur. Surveill. 2021 doi: 10.2807/1560-7917.ES.2021.26.29.2100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fourgeaud J, et al. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:2389–2395. doi: 10.1007/s10096-021-04323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weinberger Opek M, et al. Delayed respiratory syncytial virus epidemic in children after relaxation of COVID-19 physical distancing measures, Ashdod, Israel, 2021. Eur. Surveill. 2021 doi: 10.2807/1560-7917.ES.2021.26.29.2100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reicherz F, et al. Waning immunity against respiratory syncytial virus during the COVID-19 pandemic. J. Infect. Dis. 2022 doi: 10.1093/infdis/jiac192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng Z, Pitzer VE, Shapiro ED, Bont LJ, Weinberger DM. Estimation of the timing and intensity of reemergence of respiratory syncytial virus following the COVID-19 pandemic in the US. JAMA Netw. Open. 2021;4:e2141779. doi: 10.1001/jamanetworkopen.2021.41779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poole S, Brendish NJ, Tanner AR, Clark TW. Physical distancing in schools for SARS-CoV-2 and the resurgence of rhinovirus. Lancet Respir. Med. 2020;8:e92–e93. doi: 10.1016/S2213-2600(20)30502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang RX, et al. Surges of hospital-based rhinovirus infection during the 2020 coronavirus disease-19 (COVID-19) pandemic in Beijing, China. World J. Pediatr. 2021;17:590–596. doi: 10.1007/s12519-021-00477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haddadin Z, et al. Acute respiratory illnesses in children in the SARS-CoV-2 pandemic: prospective multicenter study. Pediatrics. 2021 doi: 10.1542/peds.2021-051462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Engels G, et al. Very low incidence of SARS-CoV-2, influenza and RSV but high incidence of rhino-, adeno- and endemic coronaviruses in children with acute respiratory infection in primary care pediatric practices during the second and third wave of the SARS-CoV-2 pandemic. Pediatr. Infect. Dis. J. 2022;41:e146–e148. doi: 10.1097/INF.0000000000003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thongpan I, Vichaiwattana P, Vongpunsawad S, Poovorawan Y. Upsurge of human rhinovirus infection followed by a delayed seasonal respiratory syncytial virus infection in Thai children during the coronavirus pandemic. Influenza Other Respir. Viruses. 2021;15:711–720. doi: 10.1111/irv.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jain S, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jain S, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lai YH, Lin KY, Chang CH, Chang SC. Collateral benefit of non-pharmacological interventions against COVID-19 to prevent community-acquired pneumonia in Jin-Shan, New Taipei, April to December 2020. J. Formos. Med. Assoc. 2021;120:2195–2196. doi: 10.1016/j.jfma.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lastrucci V, et al. The indirect impact of COVID-19 large-scale containment measures on the incidence of community-acquired pneumonia in older people: a region-wide population-based study in Tuscany, Italy. Int. J. Infect. Dis. 2021;109:182–188. doi: 10.1016/j.ijid.2021.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nagano H, et al. Hospitalization of mild cases of community-acquired pneumonia decreased more than severe cases during the COVID-19 pandemic. Int. J. Infect. Dis. 2021;106:323–328. doi: 10.1016/j.ijid.2021.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamamoto T, et al. COVID-19 pandemic and the incidence of community-acquired pneumonia in elderly people. Respir. Investig. 2020;58:435–436. doi: 10.1016/j.resinv.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang C. Pediatric Non-COVID-19 community-acquired pneumonia in COVID-19 pandemic. Int. J. Gen. Med. 2021;14:7165–7171. doi: 10.2147/IJGM.S333751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yan Y, Tomooka K, Naito T, Tanigawa T. Decreased number of inpatients with community-acquired pneumonia during the COVID-19 pandemic: a large multicenter study in Japan. J. Infect. Chemother. 2022;28:709–713. doi: 10.1016/j.jiac.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang C. The COVID-19 Pandemic and the incidence of the non-COVID-19 pneumonia in adults. Front. Med. 2021;8:737999. doi: 10.3389/fmed.2021.737999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rybak A, et al. Shift in clinical profile of hospitalized pneumonia in children in the non-pharmaceutical interventions period during the COVID-19 pandemic: a prospective multicenter study. Front. Pediatr. 2022;10:782894. doi: 10.3389/fped.2022.782894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilder, J. L., Parsons, C. R., Growdon, A. S., Toomey, S. L. & Mansbach, J. M. Pediatric hospitalizations during the COVID-19 pandemic. Pediatrics14610.1542/peds.2020-005983 (2020). [DOI] [PubMed]
- 121.Dahne T, et al. The impact of the SARS-CoV-2 pandemic on the prevalence of respiratory tract pathogens in patients with community-acquired pneumonia in Germany. Emerg. Microbes Infect. 2021;10:1515–1518. doi: 10.1080/22221751.2021.1957402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hasegawa K, et al. Respiratory virus epidemiology among us infants with severe bronchiolitis: analysis of 2 multicenter, multiyear cohort studies. Pediatr. Infect. Dis. J. 2019;38:e180–e183. doi: 10.1097/INF.0000000000002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guitart C, et al. Bronchiolitis, epidemiological changes during the SARS-CoV-2 pandemic. BMC Infect. Dis. 2022;22:84. doi: 10.1186/s12879-022-07041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pelletier JH, et al. Trends in US pediatric hospital admissions in 2020 compared with the decade before the COVID-19 Pandemic. JAMA Netw. Open. 2021;4:e2037227. doi: 10.1001/jamanetworkopen.2020.37227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zee-Cheng JE, et al. Changes in pediatric ICU utilization and clinical trends during the coronavirus pandemic. Chest. 2021;160:529–537. doi: 10.1016/j.chest.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Brusselen D, et al. Bronchiolitis in COVID-19 times: a nearly absent disease? Eur. J. Pediatr. 2021;180:1969–1973. doi: 10.1007/s00431-021-03968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ferrero F, Ossorio MF. Is there a place for bronchiolitis in the COVID-19 era? Lack of hospitalizations due to common respiratory viruses during the 2020 winter. Pediatr. Pulmonol. 2021;56:2372–2373. doi: 10.1002/ppul.25391. [DOI] [PubMed] [Google Scholar]
- 128.Friedrich F, et al. Early impact of social distancing in response to coronavirus disease 2019 on hospitalizations for acute bronchiolitis in infants in Brazil. Clin. Infect. Dis. 2021;72:2071–2075. doi: 10.1093/cid/ciaa1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Britton PN, et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child. Adolesc. Health. 2020;4:e42–e43. doi: 10.1016/S2352-4642(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eden JS, et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat. Commun. 2022;13:2884. doi: 10.1038/s41467-022-30485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shah SA, Quint JK, Nwaru BI, Sheikh A. Impact of COVID-19 national lockdown on asthma exacerbations: interrupted time-series analysis of English primary care data. Thorax. 2021;76:860–866. doi: 10.1136/thoraxjnl-2020-216512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Davies GA, et al. Impact of COVID-19 lockdown on emergency asthma admissions and deaths: national interrupted time series analyses for Scotland and Wales. Thorax. 2021;76:867–873. doi: 10.1136/thoraxjnl-2020-216380. [DOI] [PubMed] [Google Scholar]
- 133.Huh K, et al. Decrease in hospital admissions for respiratory diseases during the COVID-19 pandemic: a nationwide claims study. Thorax. 2021;76:939–941. doi: 10.1136/thoraxjnl-2020-216526. [DOI] [PubMed] [Google Scholar]
- 134.Sarc I, et al. Mortality, seasonal variation, and susceptibility to acute exacerbation of COPD in the pandemic year: a nationwide population study. Ther. Adv. Respir. Dis. 2022;16:17534666221081047. doi: 10.1177/17534666221081047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ulrich L, Macias C, George A, Bai S, Allen E. Unexpected decline in pediatric asthma morbidity during the coronavirus pandemic. Pediatr. Pulmonol. 2021;56:1951–1956. doi: 10.1002/ppul.25406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Robertson M, et al. The spatial-temporal dynamics of respiratory syncytial virus infections across the east-west coasts of Australia during 2016-17. Virus Evol. 2021;7:veab068. doi: 10.1093/ve/veab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Centers for Disease Control and Prevention. Census regional trends for common human coronaviruseshttps://www.cdc.gov/surveillance/nrevss/coronavirus/region.html (2021).
- 138.Chow EJ, et al. The clinical and genomic epidemiology of seasonal human coronaviruses in congregate homeless shelter settings: A repeated cross-sectional study. Lancet Reg. Health Am. 2022;15:100348. doi: 10.1016/j.lana.2022.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chung JR, et al. Interim estimates of 2021–22 seasonal influenza vaccine effectiveness - United States, February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:365–370. doi: 10.15585/mmwr.mm7110a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Russell CA, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science. 2008;320:340–346. doi: 10.1126/science.1154137. [DOI] [PubMed] [Google Scholar]
- 141.Bedford T, et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature. 2015;523:217–220. doi: 10.1038/nature14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2022-2023 northern hemisphere influenza seasonhttps://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-northern-hemisphere-recommendation-2022-2023/202202_recommendation.pdf?sfvrsn=5c88e006_13&download=true (2022).
- 143.Centers for Disease Control and Prevention. FluView summary ending on September 26, 2020https://www.cdc.gov/flu/weekly/weeklyarchives2019-2020/Week39.htm (2020).
- 144.Centers for Disease Control and Prevention. FluView summary ending on October 2, 2021https://www.cdc.gov/flu/weekly/weeklyarchives2020-2021/week39.htm (2021).
- 145.Nextstrain. Real-time tracking of influenza B/Yam evolutionhttps://nextstrain.org/flu/seasonal/yam/ha/2y (2022).
- 146.World Health Organization. FluNet: influenza laboratory surveillance information by the Global Influenza Surveillance and Response System (GISRS)https://apps.who.int/flumart/Default?ReportNo=10 (2022).
- 147.Centers for Disease Control and Prevention. FluView 2009-2010 influenza season summary, https://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/09-10summary.htm (2011).
- 148.Centers for Disease Control and Prevention. FluView 2010-2011 influenza season summaryhttps://www.cdc.gov/flu/weekly/weeklyarchives2010-2011/10-11summary.htm (2010).
- 149.Teng JLL, et al. Substantial decline in invasive pneumococcal disease during Coronavirus disease 2019 pandemic in Hong Kong. Clin. Infect. Dis. 2022;74:335–338. doi: 10.1093/cid/ciab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brueggemann AB, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit. Health. 2021;3:e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Van Groningen KM, Dao BL, Gounder P. Declines in invasive pneumococcal disease (IPD) during the COVID-19 pandemic in Los Angeles county. J. Infect. 2022 doi: 10.1016/j.jinf.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amin-Chowdhury Z, et al. Impact of the Coronavirus disease 2019 (COVID-19) Pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): prospective National Cohort Study, England. Clin. Infect. Dis. 2021;72:e65–e75. doi: 10.1093/cid/ciaa1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Danino D, et al. Decline in pneumococcal disease in young children during the COVID-19 pandemic in Israel associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Janapatla RP, et al. Serotype transmission dynamics and reduced incidence of invasive pneumococcal disease caused by different serotypes after implementation of non-pharmaceutical interventions during COVID-19 pandemic. Eur. Respir. J. 2021 doi: 10.1183/13993003.00978-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chan KF, Ma TF, Ip MS, Ho PL. Invasive pneumococcal disease, pneumococcal pneumonia and all-cause pneumonia in Hong Kong during the COVID-19 pandemic compared with the preceding 5 years: a retrospective observational study. BMJ Open. 2021;11:e055575. doi: 10.1136/bmjopen-2021-055575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sarmiento Clemente A, et al. Decrease in pediatric invasive pneumococcal disease during the COVID-19 pandemic. J. Pediatr. Infect. Dis. Soc. 2022 doi: 10.1093/jpids/piac056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wolter N, et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J. Infect. Dis. 2014;210:1649–1657. doi: 10.1093/infdis/jiu326. [DOI] [PubMed] [Google Scholar]
- 158.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 159.Valente C, et al. Decrease in pneumococcal co-colonization following vaccination with the seven-valent pneumococcal conjugate vaccine. PLoS ONE. 2012;7:e30235. doi: 10.1371/journal.pone.0030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dunn MG, et al. Impact of 13-valent pneumococcal conjugate vaccine on nasopharyngeal carriage rates of streptococcus pneumoniae in a rural community in the Dominican Republic. J. Infect. Dis. 2021;224:S237–S247. doi: 10.1093/infdis/jiab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Branche AR, et al. Effect of prior vaccination on carriage rates of Streptococcus pneumoniae in older adults: a longitudinal surveillance study. Vaccine. 2018;36:4304–4310. doi: 10.1016/j.vaccine.2018.05.107. [DOI] [PubMed] [Google Scholar]
- 162.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol. 2018;16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.de Steenhuijsen Piters WAA, et al. Interaction between the nasal microbiota and S. pneumoniae in the context of live-attenuated influenza vaccine. Nat. Commun. 2019;10:2981. doi: 10.1038/s41467-019-10814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Berry I, et al. Association of influenza activity and environmental conditions with the risk of invasive pneumococcal disease. JAMA Netw. Open. 2020;3:e2010167. doi: 10.1001/jamanetworkopen.2020.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuster SP, et al. Evaluation of coseasonality of influenza and invasive pneumococcal disease: results from prospective surveillance. PLoS Med. 2011;8:e1001042. doi: 10.1371/journal.pmed.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weinberger DM, et al. Pneumococcal disease seasonality: incidence, severity and the role of influenza activity. Eur. Respir. J. 2014;43:833–841. doi: 10.1183/09031936.00056813. [DOI] [PubMed] [Google Scholar]
- 167.Russell CD, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2:e354–e365. doi: 10.1016/S2666-5247(21)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dirkx KKT, et al. The drop in reported invasive pneumococcal disease among adults during the first COVID-19 wave in the Netherlands explained. Int. J. Infect. Dis. 2021;111:196–203. doi: 10.1016/j.ijid.2021.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Casanova C, Kuffer M, Leib SL, Hilty M. Re-emergence of invasive pneumococcal disease (IPD) and increase of serotype 23B after easing of COVID-19 measures, Switzerland, 2021. Emerg. Microbes Infect. 2021;10:2202–2204. doi: 10.1080/22221751.2021.2000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.DeSilva MB, et al. Association of the COVID-19 pandemic with routine childhood vaccination rates and proportion up to date with vaccinations across 8 US health systems in the vaccine safety datalink. JAMA Pediatr. 2022;176:68–77. doi: 10.1001/jamapediatrics.2021.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Choi YH, Miller E. Impact of COVID-19 social distancing measures on future incidence of invasive pneumococcal disease in England and Wales: a mathematical modelling study. BMJ Open. 2021;11:e045380. doi: 10.1136/bmjopen-2020-045380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Linde A, Rotzen-Ostlund M, Zweygberg-Wirgart B, Rubinova S, Brytting M. Does viral interference affect spread of influenza? Eur. Surveill. 2009;14:19354. doi: 10.2807/ese.14.40.19354-en. [DOI] [PubMed] [Google Scholar]
- 173.Casalegno JS, et al. Rhinoviruses delayed the circulation of the pandemic influenza A (H1N1) 2009 virus in France. Clin. Microbiol. Infect. 2010;16:326–329. doi: 10.1111/j.1469-0691.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- 174.Casalegno JS, et al. Impact of the 2009 influenza A(H1N1) pandemic wave on the pattern of hibernal respiratory virus epidemics, France, 2009. Eur. Surveill. 2010;15:19485. doi: 10.2807/ese.15.06.19485-en. [DOI] [PubMed] [Google Scholar]
- 175.Achten NB, et al. Interference between respiratory syncytial virus and human rhinovirus infection in infancy. J. Infect. Dis. 2017;215:1102–1106. doi: 10.1093/infdis/jix031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Opatowski L, Baguelin M, Eggo RM. Influenza interaction with cocirculating pathogens and its impact on surveillance, pathogenesis, and epidemic profile: a key role for mathematical modelling. PLoS Pathog. 2018;14:e1006770. doi: 10.1371/journal.ppat.1006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nowak MD, Sordillo EM, Gitman MR, Paniz Mondolfi AE. Coinfection in SARS-CoV-2 infected patients: where are influenza virus and rhinovirus/enterovirus? J. Med. Virol. 2020;92:1699–1700. doi: 10.1002/jmv.25953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Essaidi-Laziosi M, et al. Interferon-dependent and respiratory virus-specific interference in dual infections of airway epithelia. Sci. Rep. 2020;10:10246. doi: 10.1038/s41598-020-66748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shinjoh M, Omoe K, Saito N, Matsuo N, Nerome K. In vitro growth profiles of respiratory syncytial virus in the presence of influenza virus. Acta Virol. 2000;44:91–97. [PubMed] [Google Scholar]
- 180.Huang IC, et al. Influenza A virus neuraminidase limits viral superinfection. J. Virol. 2008;82:4834–4843. doi: 10.1128/JVI.00079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.