INTRODUCTION
Heart failure (HF) is an important public health problem, with high mortality and morbidity. Its prevalence has increased due to the aging of the population, once the disease affects approximately 1–2% of the adult population in developed countries, rising to more than 10% among people over 70 years of age. In Brazil, according to DATA-SUS, an organ of the Ministry of Health, more than 26000 patients died due to HF in 2012 1 .
HF patients are recognized by a progressive increase in congestion that is associated with an elevation of circulating biomarkers of inflammation, a condition that is associated with impairment in functional capacity and predicts poor clinical outcomes. Inflammation in HF patients is a frequent condition, contributing to the pathogenesis and progression of the disease through diverse mechanistic pathways that culminate with increased levels of pro-inflammatory cytokines, especially interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α) 2 .
Although inflammation is a common condition in HF patients, it is still poorly understood what the origin of the inflammatory process in these patients is 3 . Recent evidence suggested that gut microbiome plays a major role in both health maintenance and disease. The imbalance of microbial communities in the gut, named gut dysbiosis, seems to be a potential contributor to HF progression by activating inflammatory pathways 4 .
Thus, the possible cross talk between gut dysbiosis and HF severity is intriguing and has the potential to identify new pathways and treatment strategies for HF. So, the aim of this revision was to clarify the possible association of gut dysbiosis, inflammation, and HF, and possible diagnosis, prevention, and treatment strategies.
Gut microbiota
The human gut microbiota is a complex ecological community that has likely coevolved with humans for millions of years, resulting in reciprocal physiological changes. The colonization of gut bacteria begins at birth and gradually becomes more diverse by 2–3 years of age, when it begins to resemble the adult gut microbiota 4 . It has been established that >10 14 (>100 trillion) microorganisms (e.g., bacteria, archaea, yeast, and viruses) inhabit the human intestine, with differences in numbers of microbes and microbiota composition along the digestive tract 5 .
At the moment, four main bacterial phyla have been identified in the human gut: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, with the phyla Firmicutes and Bacteroidetes being the most characteristic in the healthy gut (>90%) 5 . However, the composition of human microbiota is subjected to a number of changes during health and disease, being influenced by stress, diet, exercise, disease, and medications, and becoming less diverse again toward extreme old age 4,6 .
Thus, the effect of gut microbiota on host physiology is not limited to processing food nutrients otherwise indigestible, but promotes the host’s health in a number of other ways, which include a local protective function regulating mucosal barriers and the immune system preventing the proliferation of pathogens 5 . Therefore, the effects of gut flora on host metabolism and immunity might be considered a key mechanism in human physiology.
Gut dysbiosis and HF severity
Gut dysbiosis has generally been described as a significant deviation from the functional microbiome 4 . Each of the following three conditions can be considered as dysbiosis:
loss of valuable microbial organisms,
expression of pathobionts of possibly beneficial microorganisms, and
loss of general microbial variet1y 7 .
The literature already describes the possible association between gut dysbiosis and the manifestation or worsening of several diseases, e.g., HF 4,8 .
HF is a disease characterized by a state of chronic inflammation with elevated circulating levels of pro-inflammatory cytokines, such as TNF-α, as originally described by Levine et al. in 1990 9 . These circulating cytokines act as cardiosuppressors via different pathways that include alterations in myocardial intracellular calcium homeostasis, reduction in mitochondrial activity, and alterations in matrix metalloproteinase expression, resulting in an adverse response from myocardial, which includes negative inotropism, cardiomyocyte hypertrophy, and apoptosis 10 . However, the origin of inflammation in patients with HF is still controversial and includes different hypotheses, highlighting a decrease in intestinal perfusion and mucosal ischemia, resulting in gut disruption with increased gut permeability, and subsequently enhancing the translocation of bacteria and bacterial toxins in the blood, which can contribute to systemic inflammation and then to HF exacerbations 10,11 .
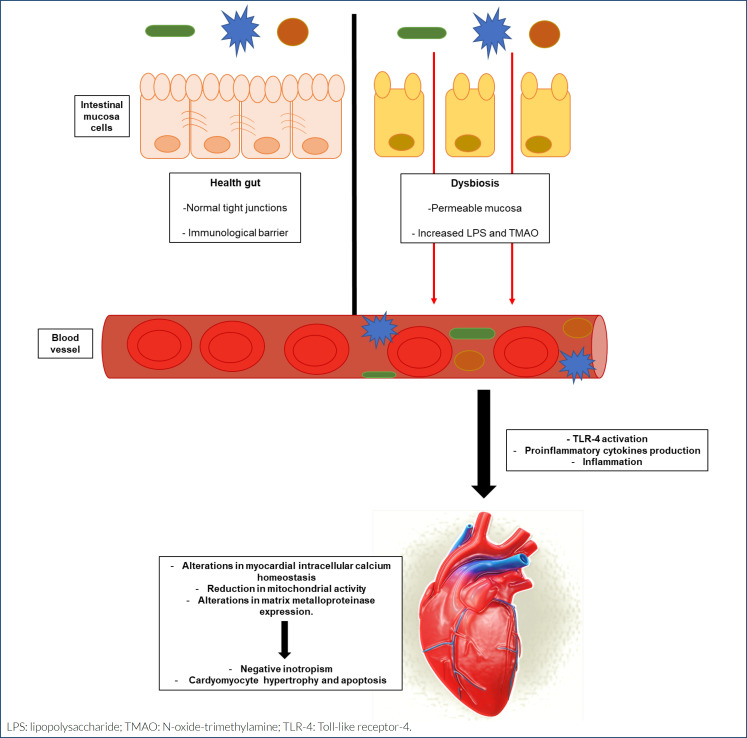
The intestinal epithelium acts as an impervious barrier to prevent lipopolysaccharide (LPS) translocation. However, in a dysbiosis condition, the intestinal barrier increases in permeability as a result of a disruption to the regulation of the epithelial cell-to-cell tight junction protein network. A compromised intestinal barrier can be associated with bacterial translocation from the gut into the systemic circulation increasing the risk of inflammation and metabolic endotoxemia (ME), and may represent an important mediator of low-grade systemic inflammation 7,12 . Figure 1 summarizes the possible relationship between gut dysbiosis and HF.
Figure 1. Relationship between gut dysbiosis and heart failure. Under health conditions, gut mucosa has normal tight junctions and works as an immunological barrier. In dysbiosis, mucosa becomes permeable and the levels of lipopolysaccharide and N-oxide-trimethylamine increase. This condition induces an inflammatory response by Toll-like receptor-4 activation, resulting in negative inotropism and cardiomyocyte hypertrophy and apoptosis.
LPS is the major component of the outer membrane of Gram-negative bacteria. Under septic circumstances, circulating LPS acts as a pathogen-associated molecular pattern, being able to stimulate the innate immune system, mediating a local or systemic inflammatory response. LPS can also stimulate nonimmune cells and initiate the inflammatory process. The literature reports that an innate LPS-pattern recognition receptor, the Toll-like receptor-4 (TLR-4) is widely expressed in the body, including cardiac tissue 13 . Thus, the innate inflammatory response can be induced in cardiomyocytes by LPS independently of the immune cell involvement 14 .
Biomarkers of intestinal dysbiosis
Given the relevance of gut-associated inflammation in HF patients, the early identification of this condition is fundamental for the treatment and aggravation of this disease 15 . Thus, in the face of dysbiosis, some metabolites, including N-oxide-trimethylamine (TMAO), short-chain fatty acids (SCFAs), circulating LPS, and zonulin primary and secondary bile acid, are generated and may act as biomarkers of intestinal dysbiosis, predicting inflammation in HF 16 .
TMAO is a urine toxin stimulated by choline, phosphatidylcholine, and L-carnitine fermentation that occurs biologically in the intestinal microbiota 17 . However, in conditions of gut dysbiosis, the levels of TMAO are elevated in the circulation, which can contribute to the severity of heart disease, especially by stimulating chronic inflammation 7 . The literature reports that increased levels of TMAO contribute to overexpression of pro-inflammatory cytokines, such as TNF-α and Il-1β, and also the attenuation of anti-inflammatory cytokines such as IL-10 18 . Recent evidence has suggested the TMAO level as a biomarker to assess gut barrier permeability 19 .
Zonulin is a family peptide produced in the intestinal and hepatic cells that regulate a protein complex named tight junctions. The literature has reported that high levels of zonulin are associated with increased intestinal permeability 20 , a condition that allows the translocation of LPS from the intestinal lumen into circulation, resulting in endotoxemia and a low-grade chronic inflammation through the activation of Toll-like receptors 21 .
The SCFAs acetate, propionate, and butyrate are the main metabolites produced in the colon by bacterial fermentation of dietary fibers and resistant starch, exerting effects on the colon as energy supply and trophic factors 22 . SCFAs improve gut health through a number of local effects, ranging from maintenance of intestinal barrier integrity, mucus production, to protection against inflammation 22 . Higher fecal SCFAs are also associated with central obesity, hypertension, and subclinical measures of cardiometabolic disease (e.g., inflammation, glycemia, and dyslipidemia) 23 .
LPS is the major component of the outer membrane of Gram-negative bacteria. Increased gut permeability enhances the penetration of gut microbiota-derived LPS from the intestine into the bloodstream 24 . High levels of serum LPS have been associated with pathological processes, including diabetes, the progression of kidney disease, obesity, and inflammation. LPS induces inflammation via a cascade of inflammatory responses following the recognition of lipid A by immune cells. Lipid A is the toxic component of LPS and serves as the microbe-specific molecular signal that binds to the surface receptor complexes of immune cells, which comprise TLR-4 25 .
Modulation of dysbiosis as a potential target in heart failure
Once dysbiosis may contribute to the pathogenesis and progression of HF, modulation of this condition could be an effective therapeutic target. Among the main interventions, the literature reports diet modification, including high intake of fruits and vegetables and low consumption of red meat and simple carbohydrate, is well-documented 26 .
Probiotics are live beneficial bacteria that re-establish an appropriate intestinal balance by different mechanisms, including pH modulation, antibacterial compound production, and competition with pathogens. Probiotics mainly include bifidobacteria, yeasts, and lactic acid bacteria 26,27 . Prebiotics are non-digestible carbohydrates used as fermentation substrates and stimulate the proliferation and activity of beneficial intestinal bacteria. It includes oligofructose administered by supplements or consumed in foods, such as asparagus, sugar beet, garlic, chicory, onion, banana, etc 28 .
Fecal microbiota transplantation (FMT) is a method of treating intestinal microecological imbalance and reconstructing normal intestinal function by introducing bacteria or metabolites from donor feces into diseased receptors. It is used to treat Clostridium difficile. There are no clinical studies that evaluate FMT in HF patients 27 .
Antibiotic treatment destroys the balance of intestinal flora, leading to a decrease in flora abundance and changes in composition 27 . A study conducted by Zhou et al. has shown that antibiotics injected to eliminate intestinal bacterial translocation are able to alleviate systemic inflammation and myocardial cell damage in mice with myocardial infarction 29 . It is important to emphasize that improper use of antibiotics can kill beneficial bacteria in the body, making pathogens resistant and causing various adverse reactions. Thus, the positive and negative effects of the use of antibiotics have to be considered.
FINAL CONSIDERATIONS
Gut dysbiosis can be both a cause and consequence of inflammation in HF and plays a central role in disease pathogenesis and progression. Although some studies have suggested the association among gut dysbiosis, inflammation, and HF, more studies are necessary to elucidate the involved mechanisms. Additionally, the modulation of gut dysbiosis is an important strategy to be tested in clinical studies as a possible intervention to reduce the inflammation and HF severity.
Footnotes
Funding: none.
REFERENCES
- 1.Albuquerque DC, Souza JD, Neto, Bacal F, Rohde LEP, Bernardez-Pereira S, Berwanger O, et al. I registro Brasileiro de insuficiência Cardíaca – Aspectos clínicos, qualidade assistencial e desfechos hospitalares. Arq Bras Cardiol. 2015;104(6):433–442. doi: 10.5935/abc.20150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy SP, Kakkar R, McCarthy CP, Januzzi JL., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(11):1324–1340. doi: 10.1016/j.jacc.2020.01.0143. [DOI] [PubMed] [Google Scholar]
- 3.Yuzefpolskaya M, Bohn B, Nasiri M, Zuver AM, Onat DD, Royzman EA, et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. J Hear Lung Transplant. 2020;39(9):880–890. doi: 10.1016/j.healun.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madan S, Mehra MR. Gut dysbiosis and heart failure: navigating the universe within. Eur J Heart Fail. 2020;22(4):1–8. doi: 10.1002/ejhf.1792. [DOI] [PubMed] [Google Scholar]
- 5.Schiattarella GG, Sannino A, Esposito G, Perrino C. Diagnostics and therapeutic implications of gut microbiota alterations in cardiometabolic diseases. Trends Cardiovasc Med. 2019;29(3):141–147. doi: 10.1016/j.tcm.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moludi J, Maleki V, Jafari-Vayghyan H, Vaghef-Mehrabany E, Alizadeh M. Metabolic endotoxemia and cardiovascular disease: a systematic review about potential roles of prebiotics and probiotics. Clin Exp Pharmacol Physiol. 2020;47(6):927–939. doi: 10.1111/1440-1681.13250. [DOI] [PubMed] [Google Scholar]
- 8.Novakovic M, Rout A, Kingsley T, Kirchoff R, Singh A, Verma V, et al. Role of gut microbiota in cardiovascular diseases. World J Cardiol. 2020;12(4):110–122. doi: 10.4330/wjc.v12.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323(4):236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 10.Sandek A, Anker SD, von Haehling S. The gut and intestinal bacteria in chronic heart failure. Curr Drug Metab. 2009;10(1):22–28. doi: 10.2174/138920009787048374. [DOI] [PubMed] [Google Scholar]
- 11.Van Linthout S, Tschöpe C. Inflammation – cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14(4):251–265. doi: 10.1007/s11897-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madan S, Mehra MR. Gut dysbiosis and heart failure: navigating the universe within. Eur J Heart Fail. 2020;22(4):629–637. doi: 10.1002/ejhf.1792. [DOI] [PubMed] [Google Scholar]
- 13.Alves PHR, Ferron AJT, Costa MR, Hasimoto FK, Gregolin CS, Garcia JL, et al. Relationship between Innate Immune Response Toll-Like Receptor 4 (TLR-4) and the Pathophysiological Process of Obesity Cardiomyopathy. Arq Bras Cardiol. 2021;117(1):91–99. doi: 10.36660/abc.20190788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowman JD, Surani S, Horseman MA. Endotoxin, Toll-like Receptor-4, and Atherosclerotic Heart Disease. Curr Cardiol Rev. 2017;13(2):86–93. doi: 10.2174/1573403X12666160901145313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Rodriguez E, Egea-Zorrilla A, Plaza-Díaz J, Aragón-Vela J, Muñoz-Quezada S, Tercedor-Sánchez L, et al. The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients. 2020;12(3):1–24. doi: 10.3390/nu12030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang WHW, Kitai T, Hazen SL, Clinic C, Clinic C, Clinic C. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hairrman RS, Gouveia CG, Sichinel ÂH, Silva LSA, Oliveira TSS, Farias MN, et al. Tmao and the relationship with cardiovascular disease: the elderly and their physiological aspects. Brazilian J Dev. 2021;7(1):6971–6982. doi: 10.34117/bjdv7n1-472. [DOI] [Google Scholar]
- 18.Tang WH, Hazen SL. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl Res. 2017;179:108–115. doi: 10.1016/j.trsl.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ufnal M, Pham K. The gut-blood barrier permeability – a new marker in cardiovascular and metabolic diseases? Med Hypotheses. 2017;98:35–37. doi: 10.1016/j.mehy.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Carpes LS, Nicoletto BB, Canani LH, Rheinhemer J, Crispim D, Souza GC. Could serum zonulin be an intestinal permeability marker in diabetes kidney disease? PLoS One. 2021;16(6):e0253501. doi: 10.1371/journal.pone.0253501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrera-Bastos P, Óscar Picazo, Fontes-Villalba M, Pareja-Galeano H, Lindeberg S, Martínez-Selles M, et al. Serum Zonulin and endotoxin levels in exceptional longevity versus precocious myocardial infarction. Aging Dis. 2018;9(2):317–321. doi: 10.14336/AD.2017.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 2020;11:25–25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, et al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients. 2018;11(1):51–51. doi: 10.3390/nu11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita T, Yoshida N, Emoto T, Saito Y, Hirata KI. Two gut microbiota-derived toxins are closely associated with cardiovascular diseases: a review. Toxins (Basel) 13(5):297–297. doi: 10.3390/toxins13050297. 20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salguero MV, Al-Obaide MAI, Singh R, Siepmann T, Vasylyeva TL. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med. 2019;18(5):3461–3469. doi: 10.3892/etm.2019.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitai T, Kirsop J, Tang WH. Exploring the Microbiome in Heart Failure. Curr Heart Fail Rep. 2016;13(2):103–109. doi: 10.1007/s11897-016-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia Q, Li H, Zhou H, Zhang X, Zhang A, Xie Y, et al. Role and effective therapeutic target of gut microbiota in heart failure. Cardiovasc Ther. 2019;2019:5164298–5164298. doi: 10.1155/2019/5164298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92–92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X, Li J, Guo J, Geng B, Ji W, Zhao Q, et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. 2018;6(1):66–66. doi: 10.1186/s40168-018-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]