Abstract
Background
Acute respiratory infections (ARIs) are by far the most common reason for prescribing an antibiotic in primary care, even though the majority of ARIs are of viral or non‐severe bacterial aetiology. It follows that in many cases antibiotic use will not be beneficial to a patient's recovery but may expose them to potential side effects. Furthermore, limiting unnecessary antibiotic use is a key factor in controlling antibiotic resistance. One strategy to reduce antibiotic use in primary care is point‐of‐care biomarkers. A point‐of‐care biomarker (test) of inflammation identifies part of the acute phase response to tissue injury regardless of the aetiology (infection, trauma, or inflammation) and may be used as a surrogate marker of infection, potentially assisting the physician in the clinical decision whether to use an antibiotic to treat ARIs. Biomarkers may guide antibiotic prescription by ruling out a serious bacterial infection and help identify patients in whom no benefit from antibiotic treatment can be anticipated. This is an update of a Cochrane Review first published in 2014.
Objectives
To assess the benefits and harms of point‐of‐care biomarker tests of inflammation to guide antibiotic treatment in people presenting with symptoms of acute respiratory infections in primary care settings regardless of patient age.
Search methods
We searched CENTRAL (2022, Issue 6), MEDLINE (1946 to 14 June 2022), Embase (1974 to 14 June 2022), CINAHL (1981 to 14 June 2022), Web of Science (1955 to 14 June 2022), and LILACS (1982 to 14 June 2022). We also searched three trial registries (10 December 2021) for completed and ongoing trials.
Selection criteria
We included randomised controlled trials (RCTs) in primary care patients with ARIs that compared the use of point‐of‐care biomarkers with standard care. We included trials that randomised individual participants, as well as trials that randomised clusters of patients (cluster‐RCTs).
Data collection and analysis
Two review authors independently extracted data on the following primary outcomes: number of participants given an antibiotic prescription at index consultation and within 28 days follow‐up; participant recovery within seven days follow‐up; and total mortality within 28 days follow‐up. We assessed risk of bias using the Cochrane risk of bias tool and the certainty of the evidence using GRADE. We used random‐effects meta‐analyses when feasible. We further analysed results with considerable heterogeneity in prespecified subgroups of individual and cluster‐RCTs.
Main results
We included seven new trials in this update, for a total of 13 included trials. Twelve trials (10,218 participants in total, 2335 of which were children) evaluated a C‐reactive protein point‐of‐care test, and one trial (317 adult participants) evaluated a procalcitonin point‐of‐care test. The studies were conducted in Europe, Russia, and Asia. Overall, the included trials had a low or unclear risk of bias. However all studies were open‐labelled, thereby introducing high risk of bias due to lack of blinding.
The use of C‐reactive protein point‐of‐care tests to guide antibiotic prescription likely reduces the number of participants given an antibiotic prescription, from 516 prescriptions of antibiotics per 1000 participants in the control group to 397 prescriptions of antibiotics per 1000 participants in the intervention group (risk ratio (RR) 0.77, 95% confidence interval (CI) 0.69 to 0.86; 12 trials, 10,218 participants; I² = 79%; moderate‐certainty evidence).
Overall, use of C‐reactive protein tests also reduce the number of participants given an antibiotic prescription within 28 days follow‐up (664 prescriptions of antibiotics per 1000 participants in the control group versus 538 prescriptions of antibiotics per 1000 participants in the intervention group) (RR 0.81, 95% CI 0.76 to 0.86; 7 trials, 5091 participants; I² = 29; high‐certainty evidence).
The prescription of antibiotics as guided by C‐reactive protein tests likely does not reduce the number of participants recovered, within seven or 28 days follow‐up (567 participants recovered within seven days follow‐up per 1000 participants in the control group versus 584 participants recovered within seven days follow‐up per 1000 participants in the intervention group) (recovery within seven days follow‐up: RR 1.03, 95% CI 0.96 to 1.12; I² = 0%; moderate‐certainty evidence) (recovery within 28 days follow‐up: RR 1.02, 95% CI 0.79 to 1.32; I² = 0%; moderate‐certainty evidence). The use of C‐reactive protein tests may not increase total mortality within 28 days follow‐up, from 1 death per 1000 participants in the control group to 0 deaths per 1000 participants in the intervention group (RR 0.53, 95% CI 0.10 to 2.92; I² = 0%; low‐certainty evidence).
We are uncertain as to whether procalcitonin affects any of the primary or secondary outcomes because there were few participants, thereby limiting the certainty of evidence.
We assessed the certainty of the evidence as moderate to high according to GRADE for the primary outcomes for C‐reactive protein test, except for mortality, as there were very few deaths, thereby limiting the certainty of the evidence.
Authors' conclusions
The use of C‐reactive protein point‐of‐care tests as an adjunct to standard care likely reduces the number of participants given an antibiotic prescription in primary care patients who present with symptoms of acute respiratory infection. The use of C‐reactive protein point‐of‐care tests likely does not affect recovery rates. It is unlikely that further research will substantially change our conclusion regarding the reduction in number of participants given an antibiotic prescription, although the size of the estimated effect may change.
The use of C‐reactive protein point‐of‐care tests may not increase mortality within 28 days follow‐up, but there were very few events. Studies that recorded deaths and hospital admissions were performed in children from low‐ and middle‐income countries and older adults with comorbidities.
Future studies should focus on children, immunocompromised individuals, and people aged 80 years and above with comorbidities. More studies evaluating procalcitonin and potential new biomarkers as point‐of‐care tests used in primary care to guide antibiotic prescription are needed.
Furthermore, studies are needed to validate C‐reactive protein decision algorithms, with a specific focus on potential age group differences.
Plain language summary
Can tests for inflammation help doctors decide whether to use antibiotics for airway infections?
Key messages
1. When a patient presents with symptoms of an airway infection at the doctor's office, the doctor's use of C‐reactive protein point‐of‐care tests during the visit probably reduces the number of patients given an antibiotic prescription, without affecting patient recovery.
2. We do not know if procalcitonin point‐of‐care tests have an effect on antibiotic use or patient recovery.
3. Future studies should focus on children, people with diseases of the immune system, and people aged 80 years and above with comorbidities (additional medical conditions). Studies evaluating procalcitonin and new biomarkers to guide antibiotic prescription are recommended.
What are point‐of‐care tests?
Point‐of‐care tests need only a few drops of blood and are taken during a consultation, providing results within 3 to 20 minutes. This means that blood samples do not need to be transported to a laboratory, and results can be used immediately to make treatment choices during a visit to the doctor. There are point‐of‐care tests that can detect different substances in the blood that your body produces in response to inflammation. These substances are called biomarkers.
What is inflammation and biomarkers?
Inflammation is a reaction in response to injury such as bacterial or viral infections. Your body naturally produces substances in response to inflammation that can be detected in the blood, which are known as biomarkers. Point‐of‐care tests that detect biomarkers are often used when patients have signs of an airway infection. Test results can inform doctors when not to suspect a serious bacterial infection that needs antibiotic treatment to prevent serious illness and possibly death. There are currently three types of biomarkers available as point‐of‐care tests: C‐reactive protein, procalcitonin, and leucocytes.
What are antibiotics?
Antibiotics are medications used to treat bacterial infections, and they are commonly used for airway infections. However, most airway infections are caused by viruses, such as the common cold, against which antibiotics do not work, and can cause harm. Overuse can lead to antibiotic resistance, which means that antibiotics lose their effectiveness and may no longer be effective against serious infections.
Why do we need to investigate whether tests help doctors to decide on antibiotics?
No test can provide absolute certainty regarding when to use antibiotics, but correctly used biomarkers could help doctors make the right decision about when to prescribe antibiotics. We investigated if biomarkers as point‐of‐care tests help doctors reduce antibiotic prescriptions.
What did we want to find out?
We wanted to know whether biomarkers as a point‐of‐care test used by primary care doctors can help decide whether to use antibiotics in people with airway infections.
We were interested in the effect of biomarker guidance on the number of prescriptions of antibiotics, patient recovery, hospital admissions, and risk of death.
What did we do?
We searched for studies that investigated whether biomarkers used as point‐of‐care tests in primary care can be safely used to guide a doctor's decision whether to prescribe antibiotics.
We compared and summarised results of the studies, and rated our confidence of the evidence.
What did we find?
We found 13 studies with a total of 10,535 participants who had symptoms of airway infections and who saw a doctor in a primary care setting for possible treatment.
Twelve studies investigated tests for the biomarker C‐reactive protein, and one study investigated a test for the biomarker procalcitonin.
Use of tests for C‐reactive protein probably reduces the number of patients given an antibiotic prescription, but differences in study design and where the studies took place meant that the precise effect is uncertain. Using these tests probably does not affect the number of patients that recover, and may not reduce the number of patients that feel satisfied with their treatment. C‐reactive protein tests may not lead to an increase in deaths. This means the tests are probably safe when used to guide the prescription of antibiotics.
We do not know if procalcitonin tests have an effect on prescriptions of antibiotics, recovery, hospital admissions, or risk of death.
What are the limitations of the evidence?
We are moderately confident in the evidence for a reduction in antibiotics use with C‐reactive protein tests. However, we are not confident in the evidence for a reduction in antibiotics use with procalcitonin, as we only found one study investigating the effect of procalcitonin in primary care.
New studies are unlikely to change our conclusion regarding the effect of the use of C‐reactive protein on prescribing antibiotics, but more studies are needed to assess the potential for the procalcitonin point‐of‐care test.
How up‐to‐date is this evidence?
The evidence is current to June 2022.
Summary of findings
Summary of findings 1. Point‐of‐care biomarkers (C‐reactive protein) for infection compared with standard of care for guiding antibiotic therapy in acute respiratory infections.
| Point‐of‐care biomarker for infection compared with standard of care for guiding antibiotic therapy in acute respiratory infections | ||||||
|
Patient or population: people with acute respiratory infections Settings: primary care Intervention: point‐of‐care biomarker (C‐reactive protein) test Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | C‐reactive protein | |||||
|
Number of participants given an antibiotic prescription at index consultation |
516 per 1000 |
397 per 1000 (356 to 444) |
RR 0.77 (0.69 to 0.86) |
10218 (12 RCTs) |
⊕⊕⊕⊝ Moderatea | |
|
Number of participants given an antibiotic prescription within 28 days follow‐up |
664 per 1000 |
538 per 1000 (505 to 571) |
RR 0.81 (0.76 to 0.86) |
5091 (7 RCTs) |
⊕⊕⊕⊕
High |
|
|
Clinical recovery within 7 days follow‐up |
567 per 1000 |
584 per 1000 (545 to 636) |
RR 1.03 (0.96 to 1.12) |
3104 (4 RCTs) |
⊕⊕⊕⊝ Moderateb | Defined as number of participants at least substantially improved at 7 days follow‐up |
|
Mortality within 28 days follow‐up |
1 per 1000 |
0 per 1000 (0 to 2) |
RR 0.53 (0.10 to 2.92) | 7737 (9 RCTs) |
⊕⊕⊝⊝ Lowc | 3 studies reported 5 events. 6 studies had no events. 3 studies did not report on death. |
|
Number of participants in need of a hospital admission within 28 days follow‐up |
14 per 1000 |
15 per 1000 (10 to 22) |
RR 1.05 (0.72 to 1.53) |
7514 (10 RCTs) |
⊕⊕⊕⊝ Moderated | 6 studies reported 105 events. 4 studies had no events. 2 studies did not report on hospital admission. |
|
Clinical recovery within 28 days follow‐up |
897 per 1000 |
915 per 1000 (724 to 1000) |
RR 1.02 (0.79 to 1.32) |
2324 (5 RCTs) |
⊕⊕⊕⊝
Moderateb |
Defined as number of participants at least substantially improved at 28 days of follow‐up |
| *The assumed risk was calculated as the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded one level due to substantial heterogeneity. bWe downgraded one level due to risk of bias (lack of blinding). cWe downgraded two levels due to substantial imprecision. dWe downgraded one level due to imprecision.
Summary of findings 2. Point‐of‐care biomarkers (procalcitonin) for infection compared with standard of care for guiding antibiotic therapy in acute respiratory infections.
| Point‐of‐care biomarker for infection compared with standard of care for guiding antibiotic therapy in acute respiratory infections | ||||||
|
Patient or population: people with acute respiratory infections Settings: primary care Intervention: point‐of‐care biomarker (procalcitonin) test Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Procalcitonin | |||||
|
Number of participants given an antibiotic prescription at index consultation |
566 per 1000 |
181 per 1000 (130 to 249) |
RR 0.32 (0.23 to 0.44) |
317 (1 RCT) |
⊕⊝⊝⊝ Very lowa | |
|
Number of participants given an antibiotic prescription within 28 days follow‐up |
70 per 1000 |
74 per 1000 (31 to 174) |
RR 1.05 (0.44 to 2.48) |
277 (1 RCT) |
⊕⊝⊝⊝ Very lowa | |
|
Clinical recovery within 7 days follow‐up |
395 per 1000 |
486 per 1000 (367 to 639) |
RR 1.23 (0.93 to 1.62) |
277 (1 RCT) |
⊕⊝⊝⊝ Very lowa | |
|
Mortality within 28 days follow‐up |
‐ | ‐ | ‐ | 277 (1 RCT) |
‐ | Not estimable due to few participants and events. No events of death occurred in either intervention or control group. |
|
Number of participants in need of a hospital admission within 28 days follow‐up |
35 per 1000 |
49 per 1000 (9 to 264 more) |
RR 1.40 (0.26 to 7.51) |
277 (1 RCT) |
⊕⊝⊝⊝ Very lowa | |
|
Clinical recovery within 28 days follow‐up |
‐ | ‐ | ‐ | ‐ | ‐ | Not estimable, as this outcome was not assessed in the included study |
| *The assumed risk was calculated as the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded one level for study limitations (cluster‐randomised trial), one level for indirectness (study only included adults), and two levels for substantial imprecision (small sample size compared to the optimal information size).
Background
Description of the condition
Since the discovery of the antibacterial effects of penicillins and other antibiotics, they have been one of the most important treatments in modern medicine. Effective antibacterial agents cure bacterial infections, such as pneumonia, and are also essential to ensure that surgery, chemotherapy, and neonatology can be safely delivered. However, antibiotic use can cause antibiotic resistance (Goossens 2005), which leads to ineffective treatments, increased risk of serious complications such as bacterial infections and death, and increased healthcare costs (Carlet 2011; Smith 2013). Combatting antibiotic resistance is therefore a public health priority (WHO 2016). Reducing unneeded antibiotic treatments through evidence‐based antibiotic stewardship programmes is essential to preserve the future effectiveness of antibiotics. However, treating acute respiratory infections (ARIs) with antibiotics is common in primary care settings, despite their predominant self‐limiting nature, and frequent viral origins (Harnden 2007; Pavia 2011; Smieszek 2018), and that antibiotic treatment has been shown to be of marginal benefit in uncomplicated cases (Butler 2009; Butler 2011; Little 2013b; Little 2021; Meropol 2013; Venekamp 2015). Limiting unnecessary antibiotic prescriptions in primary care settings is pivotal to reducing bacterial resistance to antibiotics at both the societal, Bronzwaer 2002; Gonzales 2001; Sande‐Bruinsma 2008, and individual levels (Costelloe 2010), as well as reducing their harmful side effects and drug interactions. A reduction in antibiotic prescriptions in primary care settings will have a large impact on the total use of antibiotics, as the vast majority of antibiotic prescriptions are issued there (Aabenhus 2016; Public Health England 2021). Nevertheless, patient safety must be carefully assessed to minimise the risk of undertreatment of serious bacterial infections.
Interventions to reduce antibiotic use in primary care other than point‐of‐care tests have been studied (Tonkin‐Crine 2017), and educational interventions (Arnold 2009), including the use of multifaceted approaches and communication skills training, have been shown to be effective (Butler 2012; Gjelstad 2013). Letters, with recommendations on how to reduce antibiotic prescription, addressed to primary care physicians with high levels of antibiotic use, have shown an effect on antibiotic prescribing rates (Schwartz 2021). Also, a policy of delayed antibiotic prescription can reduce antibiotic use (Spurling 2017).
The decision whether to prescribe antibiotics for an ARI in primary care settings is challenging. Patients often expect an antibiotic prescription to enhance recovery (Boiko 2020). Besides patient's expectations, diagnostic uncertainty is often present with patients presenting with symptoms of an ARI (Stanton 2010; Wang 2021). Diagnosing solely based on clinical symptoms is known to have both low sensitivity and specificity (Hoare 2006; Metlay 1997), and high interobserver variability (Wipf 1999), and may not reliably differentiate between viral and bacterial aetiologies. In accordance with this, there is evidence of substantial between‐practitioner, Aabenhus 2017; Stocks 2002, and geographical variation in antibiotic prescribing patterns, Curtis 2019; Matthys 2007, related to socioeconomic and cultural differences between communities.
Description of the intervention
Biomarkers of inflammation, such as white blood cell levels, procalcitonin, and C‐reactive protein, form part of the acute immune response and are activated by endogenous and exogenous stimuli following tissue injury due to infectious conditions such as bacteria and viruses, as well as non‐infectious conditions such as connective tissue diseases and trauma. Circulating levels are low in healthy people, but when stimulated synthesis and recruitment is rapid (less than 20 hours). Levels remain high as long as the inflammation and tissue damage persists, and then decline rapidly (Becker 2004; Volanakis 2001). Biomarkers of inflammation act as surrogate measures of the immune response to infection and may reflect the severity of the condition (i.e. degree of tissue damage and immune activation) (Aabenhus 2011; Kruger 2009; Schuetz 2017), but cannot determine aetiology (bacterial versus viral) or predict an infiltrate on chest X‐rays (Holm 2007; van der Meer 2005). No test is able to provide perfect diagnostic accuracy, and false‐negative as well as false‐positive results may occur, leading to possible over‐ or undertreatment of bacterial ARIs. However, in the correct clinical context biomarkers may guide antibiotic prescription in selected cases by ruling out a serious bacterial infection and help identify patients in whom no benefit from antibiotic treatment can be anticipated (Melbye 2011; Schuetz 2017). A point‐of‐care test exists for some of these biomarkers to be performed at, or near, the site of patient care, delivering quick test results that can influence clinical decisions (Table 3).
1. Overview of biomarkers of infection used in acute respiratory infection trials in primary care settings.
| Biomarker | Status | Handling | Biochemistry |
| C‐reactive protein | POC test available | Droplet blood from finger prick. Results in approximately 3 minutes. Uninfected adult controls have levels < 10 mg/L. | Inflammatory cytokines trigger C‐reactive protein release by the liver. Levels of C‐reactive protein increase within 6 to 18 hours, peaking at 48 to 72 hours. |
| Leukocyte count | POC test available | Droplet blood from finger prick. Results in approximately 3 minutes. Uninfected adult controls have leukocyte levels < 9 x 10⁹/L and neutrocyte levels < 7 x 10⁹/L. | Cells of the immune system activated by inflammatory cytokines and foreign antigens. |
| Procalcitonin | POC test available | Uninfected adult controls have levels < 0.05 ng/mL. | Inflammatory cytokines and bacterial endotoxins trigger release of PCT from parenchymal tissues. Levels of PCT increase within 2 to 6 hours, peaking at 24 to 48 hours. |
PCT: procalcitonin POC: point‐of‐care
The decision to prescribe antibiotics for an ARI is guided by prespecified cut‐off values specific to the individual point‐of‐care test, but the test cannot replace clinical skills and expertise, and test results may be overruled on clinical grounds.
How the intervention might work
Following a regular clinical examination that suggests symptoms are indeed compatible with an ARI, a point‐of‐care biomarker may assist the clinician in assessing the likelihood of a serious bacterial infection versus a less severe bacterial or viral infection, thus identifying those patients most likely to benefit from antibiotics (Aabenhus 2011; Hopstaken 2003; Melbye 2011; Schuetz 2017). If after the clinical examination the clinician is confident in the decision to initiate or withhold antibiotic treatment, there is no need for a point‐of‐care test. Possible detrimental effects of point‐of‐care biomarkers include suboptimal use of time, costs, handling errors, patient dissatisfaction, and false‐negative results that can lead to lack of necessary antibiotic treatments, or false‐positive values that may increase inappropriate antibiotic use. Studies indicate that the use of point‐of‐care tests during consultations is acceptable to both physicians and patients (Butler 2008; Wood 2011).
Why it is important to do this review
Avoiding both over‐ and undertreatment with antibiotics in primary care settings is important to limit antibiotic resistance and exposure of patients to unnecessary risks. So far, the evidence of the effect of using point‐of‐care biomarkers to guide antibiotic prescribing in primary care has mainly been from adults and is of moderate certainty (Aabenhus 2014a; Martínez‐González 2020). Evidence regarding children and patients who are older or with comorbidities is lacking (Cals 2018).
We included studies of all available point‐of‐care biomarkers of infection used for ARIs. Updates of this review will therefore include studies of additional point‐of‐care tests as they become available.
Objectives
To assess the benefits and harms of point‐of‐care biomarker tests of inflammation to guide antibiotic treatment in people presenting with symptoms of acute respiratory infections in primary care settings regardless of patient age.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials (RCTs) and cluster‐RCTs.
Types of participants
Primary care patients of all ages with symptoms or a diagnosis of an ARI at study entry. We defined symptoms of ARI as cough, discoloured/increased sputum, fever, runny nose, respiratory distress, feeling unwell, or combinations of focal and systemic symptoms having a duration of less than four weeks. Diagnoses included lower or upper respiratory tract infection, pneumonia, bronchitis, acute exacerbations of chronic obstructive pulmonary disease or asthma, pharyngitis, tonsillitis, laryngitis, rhinosinusitis, common cold, acute otitis media, or influenza.
Types of interventions
Point‐of‐care biomarkers of infection to guide antibiotic treatment for ARI in primary care settings. We only included studies of biomarker point‐of‐care tests for infections available for general use. We did not include specific diagnostic tests like the Strep A test or Monospot in this review. We considered the following biomarkers: C‐reactive protein, procalcitonin, and white blood cell count. The comparator was standard care.
Types of outcome measures
Primary outcomes
Number of participants given an antibiotic prescription at the index consultation and within 28 days follow‐up.
Number of participants with substantial improvement (including full recovery) within seven days follow‐up.
Total mortality within 28 days follow‐up.
Secondary outcomes
Number of participants in need of a reconsultation within 28 days follow‐up.
Number of participants in need of a hospital admission within 28 days follow‐up.
Duration of the ARI (e.g. mean or median days with restrictions in daily activities due to the infection).
Number of satisfied participants.
Number of participants with substantial improvement (including full recovery) within 28 days follow‐up.
As the follow‐up period for specific outcomes may vary between studies, we categorised follow‐up periods as:
0 to 7 days: within 7 days follow‐up.
8 to 28 days: within 28 days follow‐up.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) on 14 June 2022 (2022, Issue 6), MEDLINE (1946 to 14 June 2022), Embase (1974 to 14 June 2022), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1981 to 14 June 2022), Web of Science (1955 to 15 June 2022), and LILACS (Latin American and Caribbean Health Science Information database) (1982 to 14 June 2022).
The search strategy used for CENTRAL and MEDLINE is described in Appendix 1. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2021). We adapted the search strategy to search Embase (Appendix 2), CINAHL (Appendix 3), Web of Science (Appendix 4), and LILACS (Appendix 5). We applied no language or publication type restrictions.
Searching other resources
Trials
We searched the trial registries of the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), the EU Clinical Trials Register (www.clinicaltrialsregister.eu/), and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp) on 10 December 2021 for completed and ongoing trials.
Correspondence
We contacted experts in the field to identify eligible published, non‐published, or ongoing studies. We also contacted companies that manufacture point‐of‐care biomarker tests (Thermo‐Fisher, Hoffmann‐LaRoche, Orion Diagnostica, Axis‐Shield, Hemocue, and Siemens Diagnostica).
Reference lists
We checked the reference lists of included studies.
Data collection and analysis
Selection of studies
Two review authors (SAS and CL) independently assessed the titles and abstracts identified by the searches. We collected and assessed the full‐text copies of potentially eligible articles. Any disagreements were resolved through discussion involving the remaining review authors, when necessary.
Data extraction and management
Two review authors (SAS, CL) independently extracted data and information on study design from the included trials and entered the information onto a data extraction form. We contacted the trial authors for missing outcome data or trial characteristics as necessary. We extracted the following data.
Trial characteristics: geographic location; unit of randomisation; allocation sequence generation; concealment of allocation; blinding; number of participants; number of intervention arms; length of follow‐up.
Participant characteristics: baseline characteristics (mean (or median) age; gender; comorbidities); number of participants randomised to each intervention arm; number of participants completing the trial; inclusion criteria; types of ARIs and duration; exclusion criteria.
Intervention characteristics: type of point‐of‐care biomarker and corresponding specified cut‐off values for guidance of antibiotic prescribing, if any.
Outcome measures: all available primary and secondary outcome measures specified for this review.
We converted ranking scales on recovery and patient satisfaction to dichotomised outcomes by collapsing response categories when needed in order to make a category of substantially recovered versus not substantially recovered.
Assessment of risk of bias in included studies
Two review authors (SAS, CL) independently assessed risk of bias of the included studies using the Cochrane risk of bias tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included assessment of sequence generation, allocation concealment, blinding (participants, personnel, and outcome assessors), incomplete outcome data and selective outcome reporting bias, as well as other sources of bias. We searched for selective outcome reporting by comparing the methods and results section of the study with the trial protocol when available.
For cluster‐RCTs, we specifically checked for other sources of bias including selection bias, baseline imbalance between clusters, loss of clusters, and incorrect analysis (Higgins 2021).
Measures of treatment effect
Dichotomous data
We reported the treatment effect as a risk ratio (RR) with 95% confidence intervals (CIs) for each dichotomised outcome. We estimated the number needed to test (NNT) by using assumed control risk (ACR) calculated from the control groups event rate for C‐reactive protein point‐of‐care tests (Higgins 2021). NNT indicates the number of patients needed to test with a point‐of‐care test to save one patient given an antibiotic prescription. When we could not pool results, we presented them narratively. We did not carry out NNT for procalcitonin, as only one study was included.
Continuous data
For outcomes presented in other forms (e.g. reported as medians, quartiles, etc.) or without consistent statistical information (e.g. standard deviations (SDs), or number of participants), we presented them narratively and included these data in Additional tables (Table 4; Table 5).
2. C‐reactive protein ‐ duration of symptoms.
| Study | Mean (SD) | Median (IQR) | |||
| C‐reactive protein | Control | C‐reactive protein | Control | ||
| Cals 2009a | ‐ | ‐ | 22 (14 to 28) | 22 (14 to 28) | |
| Cals 2010a | LRTI | 17.5 (9.2) | 19.8 (9.5) | 15.5 (9.5 to 28) | 20 (13.5 to > 28) |
| Rhinosinusitis | 17.3 (9.3) | 16.6 (9.9) | 14 (10 to 28) | 14 (7 to > 28) | |
| Do 2016b | ARTI | ‐ | ‐ | 5 (4 to 7) | 5 (4 to7) |
| Little 2013ac | LRTI | ‐ | ‐ | 6 (3 to 9) | 5 (3 to 9) |
| URTI | ‐ | ‐ | 5 (3 to 7) | 4 (3 to 8) | |
| ARI | ‐ | ‐ | 5 (3 to 9) | 5 (3 to 9) | |
Abbreviations: ARI: acute respiratory tract infection (LRTI + URTI); IQR: interquartile range; LRTI: lower respiratory tract infection; SD: standard deviation; URTI: upper respiratory tract infection
aReported as time to full recovery. bReported as time to resolution of symptoms. cReported as resolution of moderately bad or worse symptoms.
3. Procalcitonin ‐ duration of symptoms.
| Study | Mean (SD) | Median (IQR) | ||
| Procalcitonin | Control | Procalcitonin | Control | |
| Lhopitallier 2021a | ‐ | ‐ | 8 | 7 |
Abbreviations: IQR: interquartile range; SD: standard deviation
aBased on total symptoms score reported by participants within 28 days.
We converted ranking scales on recovery and patient satisfaction to dichotomised outcomes by collapsing response categories when needed in order to make a category of substantially recovered versus not substantially recovered.
Unit of analysis issues
The unit of analysis was the individual participant. For cluster‐RCTs, we adjusted the unit of analysis by calculating the design effect to modify sample sizes using intraclass correlation coefficient (ICC) (Higgins 2021).
We analysed multi‐arm trials by combining groups to create a single pair‐wise comparison. We included one factorial trial, and extracted data by including all participants who had received a test compared with all participants who did not receive a test. One trial was a three‐armed study, where one group received both a point‐of‐care test and a lung ultrasound scan. We chose not to include this specific group, as this had too much influence on the antibiotic treatment being given.
Dealing with missing data
We contacted trial authors to obtain any missing data from the included studies. Where possible, we extracted data to permit an intention‐to‐treat (ITT) analysis. We did a worst‐case scenario sensitivity analysis considering missing outcome data as treatment failures in the intervention group and treatment successes in the control group, and not missing at random.
Assessment of heterogeneity
We investigated heterogeneity using the I² statistic, employing the following cut‐off values based on Higgins 2021:
30% to 60%: moderate heterogeneity;
50% to 90%: substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We interpreted the overlapping cut‐off values and the importance of the observed heterogeneity based on the magnitude and direction of effects, and the strength of evidence for heterogeneity.
Assessment of reporting biases
We carried out a funnel plot to assess possible missing results for our primary outcome, which was the number of patients given an antibiotic prescription at index consultation.
Data synthesis
When possible, we calculated a weighted estimate for the selected outcomes by means of a random‐effects meta‐analysis using Review Manager Web software (RevMan Web 2022).
We handled data from studies on C‐reactive protein point‐of‐care test separate from procalcitonin point‐of‐care test, as these two types of tests are two different interventions. They differ in type of biomarker, amount of time used for analysis (2 to 3 minutes versus 20 minutes), and therefore needed to be interpreted differently. We carried out a meta‐analysis on studies that evaluated C‐reactive protein as point‐of‐care test in primary care, but did not carry out a meta‐analysis on procalcitonin as point‐of‐care test, as only one study was included.
We extracted and used ICCs for cluster‐RCTs to avoid a unit of analysis error by calculating the design effect to modify sample sizes.
Subgroup analysis and investigation of heterogeneity
We grouped results from studies according to methodological and clinical aspects.
Planned subgroup analysis
We planned the following subgroup analyses as predefined in the study protocol.
Serious infections (e.g. pneumonia) and less serious infections (e.g. common colds and bronchitis).
Children and adults.
Type of point‐of‐care test.
Trials with low risk of bias and high risk.
Cluster‐RCTs and individually randomised studies.
To examine differences amongst subgroups, we performed a standard test for heterogeneity across subgroups.
Considerations for subgroup analysis
Serious infections and less serious infections
There might be differences in effect estimates between people with severe and people with less severe infection.
Children and adults
There might be a biological variation between adults and children regarding the inflammation and immune system response.
Type of point‐of‐care test
The different types of point‐of‐care test (C‐reactive protein versus procalcitonin versus leucocytes) are different in many aspects. They measure different biomarkers and have different cut‐off values and turnaround times (C‐reactive protein takes 2 to 3 minutes, whilst procalcitonin takes up to 20 minutes). It was therefore essential to keep the type of biomarkers separate in the meta‐analysis, but also to check for possible subgroup differences.
Trials with low risk of bias and high risk of bias
Trials with high risk of bias may overestimate the true effect estimate.
Cluster‐RCTs and individually randomised studies
Although we modified sample sizes for the cluster‐RCTs by using the ICCs to avoid unit of analysis error, we also planned to carry out subgroup analysis on cluster‐RCTs and individually randomised studies. This is particularly of interest, as cluster‐RCTs have a tendency to overestimate the true effect estimate.
Reasons for omitted planned subgroup analysis
Serious infections and less serious infections
Unfortunately the subgroup analysis on serious infections and less serious infections was not possible, as no studies investigated this. However, we did perform a subgroup analysis on similar subgroups between upper respiratory tract infections and lower respiratory tract infections.
Type of point‐of‐care test
A subgroup analysis on different types of point‐of‐care test was not possible, as most studies investigated C‐reactive protein point‐of‐care‐tests, and only one study investigated procalcitonin as a point‐of‐care test in the primary care setting.
Trials with low risk of bias and high risk of bias
All trials were non‐blinded, thereby introducing a high risk of bias, thus we omitted the pre‐planned subgroup analysis on trials with low risk of bias and high risk of bias.
Sensitivity analysis
Planned sensitivity analysis
A per‐protocol sensitivity analysis for our primary outcomes using a fixed‐effect model was planned but was not carried out due to substantial heterogeneity. We carried out a worst‐case sensitivity analysis, considering missing outcome data as treatment failures in the intervention group and treatment successes in the control group.
Post hoc sensitivity analysis
As follow‐up time on specific outcomes varied between studies, we had to specify the follow‐up time, as this was not prespecified in the protocol or the original review. We carried out a sensitivity analysis to investigate whether our new specified follow‐up times would affect which studies to include from the original review and the new search.
To assess the considerable heterogeneity and the subgroup differences detected, we performed a post hoc sensitivity analysis of the newer studies separately, as these studies used specific guidance on antibiotic prescription if C‐reactive protein levels were < 20 mg/L.
Summary of findings and assessment of the certainty of the evidence
We created two summary of findings tables, one for each type of biomarker point‐of‐care‐test: procalcitonin and C‐reactive protein (Table 1; Table 2). Both summary of findings tables used the following outcomes: number of participants given an antibiotic prescription at index consultation; number of participants given an antibiotic prescription within 28 days follow‐up; participant recovery within seven days follow‐up; total mortality within 28 days follow‐up; number of participants in need of a hospital admission within 28 days follow‐up; and participant recovery within 28 days follow‐up. We used the five GRADE domains (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004; Higgins 2021). We assessed the certainty of the evidence for each outcome as high, moderate, low, or very low. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), employing GRADEpro GDT software (GRADEpro GDT). We justified all decisions to down‐ or upgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
A PRISMA flow diagram is presented as Figure 1. We found 13 eligible studies, with 10,535 participants recruited from primary care settings, of which 2335 were children and 8200 were adults. Diagnoses were predominately lower ARIs (75%) (Table 6; Table 7). Twelve studies investigated the point‐of‐care biomarker C‐reactive protein (10,218 participants), and one trial investigated the point‐of‐care biomarker procalcitonin (317 participants). We found no studies comparing different kinds of biomarkers.
1.
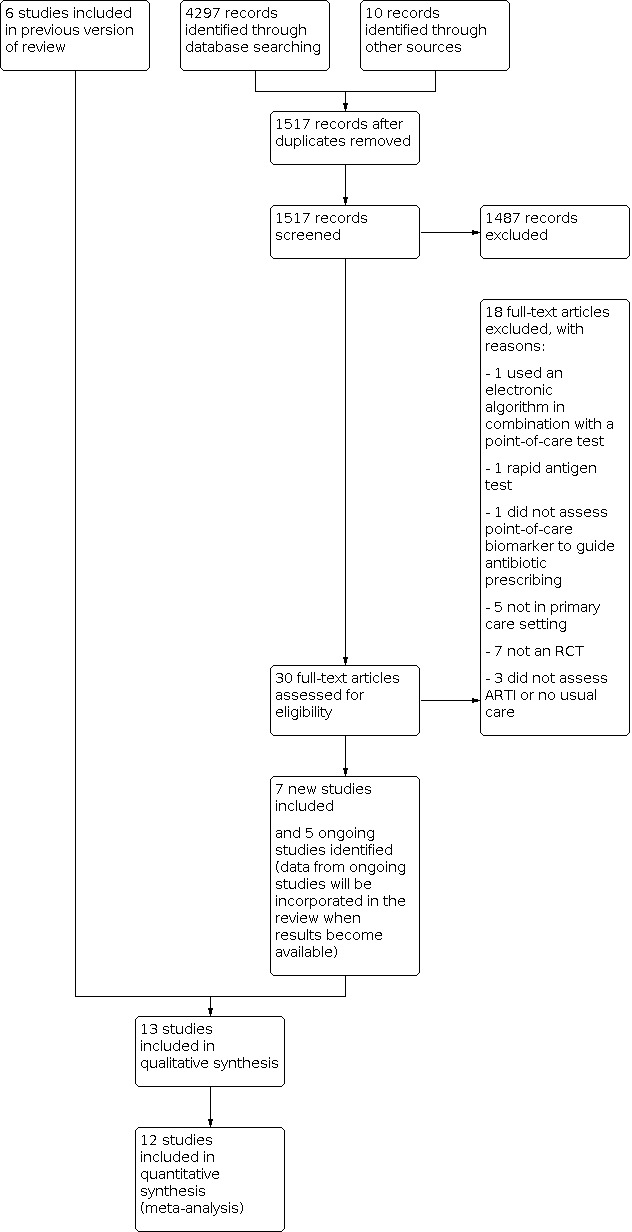
Study flow diagram.
4. C‐reactive protein ‐ baseline characteristics of included participantsa.
| Parameter | Studies | C‐reactive protein group | Control group |
| Age, mean (SD)b | Boere 2021; Butler 2019; Cals 2009; Cals 2010; Diederichsen 2000; Little 2013a; Little 2019 | 62.1 (13.0) | 62.4 (13.1) |
| Gender (female) % (n/N) | All studies | 61.0 (4597/7542) | 60.6 (4258/7030) |
| Current smokers | Andreeva 2013; Butler 2019; Cals 2009; Cals 2010; Little 2013a | 42.5 (1283/3020) | 43.9 (1173/2675) |
| Comorbidityc | Andreeva 2013; Boere 2021; Butler 2019; Cals 2009; Cals 2010; Little 2013a | 32.3 (1014/3139) | 30.7 (862/2806) |
| Primary diagnosis | |||
| Unclassified upper ARId | Andreeva 2013; Little 2013a | 21.5 (499/2325) | 21.1 (446/2118) |
| Otitis media | Diederichsen 2000 | 3.3 (13/394) | 4.5 (17/374) |
| Common cold | Melbye 1995 | 13.9 (15/108) | 16.8 (22/131) |
| Rhinosinusitis | Cals 2010; Diederichsen 2000 | 27.3 (143/523) | 27.2 (137/502) |
| Total upper ARIe | Andreeva 2013; Cals 2010; Diederichsen 2000; Little 2013a; Melbye 1995 | 22.7 (670/2956) | 22.6 (622/2752) |
| Pneumonia | Andreeva 2013; Melbye 1995 | 7.7 (16/209) | 14.4 (30/209) |
| LRTI/acute cough | All studies | 74.3 (2364/3183) | 73.5 (2173/2956) |
| Bronchitis | Melbye 1995 | 37.9 (41/108) | 32.1 (42/131) |
| Exacerbations of COPD or asthma | Butler 2019; Melbye 1995 | 78.8 (341/433) | 76.7 (335/437) |
| Total lower ARIf | All studies | 76.8 (2446/3183) | 70.5 (2271/2956) |
| Influenza | Melbye 1995 | 8.3 (9/108) | 9.2 (12/131) |
| Other respiratory diseases | Diederichsen 2000; Melbye 1995 | 13.3 (67/502) | 13.1 (66/505) |
Abbreviations: ARI: acute respiratory infection; COPD: chronic obstructive pulmonary disease; LRTI: lower respiratory tract infection; SD: standard deviation
aCrude numbers provided from all studies regardless of design. bMelbye 1995 reported the median age: 50 (range 18 to 83) in the C‐reactive protein arm versus 44 (18 to 82) in the control arm. Do 2016 reported median age 16 (8 to 39) in the C‐reactive protein arm versus 15 (8 to 41) in the control arm. Schot 2018 reported the median age 3 (0 to 11) in the C‐reactive protein arm versus 2 (0 to 11) in the control arm. cCOPD; asthma; heart disease; diabetes mellitus. dAcute respiratory infection. eAny upper acute respiratory infections. fAny lower acute respiratory infections.
5. Procalcitonin ‐ baseline characteristics of included participants.
| Parameter | Studies | Procalcitonin group | Control group |
| Age, mean (SD) | Lhopitallier 2021 | 53 (18.0) | 50 (18.0) |
| Gender (female) % (n/N) | Lhopitallier 2021 | 65 (126/195) | 53 (65/122) |
| Current smokers | Lhopitallier 2021 | 23 (44/195) | 25 (31/122) |
| Comorbiditya | Lhopitallier 2021 | 38.5 (75/195) | 42.6 (52/122) |
| Primary diagnosis | |||
| Unclassified upper ARIb | ‐ | ‐ | ‐ |
| Otitis media | ‐ | ‐ | ‐ |
| Common cold | ‐ | ‐ | ‐ |
| Rhinosinusitis | ‐ | ‐ | ‐ |
| Total upper ARIc | ‐ | ‐ | ‐ |
| Pneumonia | ‐ | ‐ | ‐ |
| LRTI/acute cough | Lhopitallier 2021 | 100 (195/195) | 100 (122/122) |
| Bronchitis | ‐ | ‐ | ‐ |
| Exacerbations of COPD or asthma | ‐ | ‐ | ‐ |
| Total lower ARId | ‐ | ‐ | ‐ |
| Influenza | ‐ | ‐ | ‐ |
| Other respiratory diseases | ‐ | ‐ | ‐ |
Abbreviations: ARI: acute respiratory infection; COPD: chronic obstructive pulmonary disease; LRTI: lower respiratory tract infection; SD: standard deviation
aCOPD; asthma; heart disease; diabetes. bAcute respiratory infections. cAny upper respiratory infection. dAny lower acute respiratory infection.
We identified five ongoing studies (ISRCTN01559032; NCT03540706; NCT03855215; NCT03931577; NCT04216277). We will incorporate data from these studies when results are available.
Included studies
The 13 included studies were conducted between 1995 and 2021 in Europe (Boere 2021; Butler 2019; Cals 2009; Cals 2010; Diederichsen 2000; Lhopitallier 2021; Little 2013a; Little 2019; Melbye 1995; Schot 2018), Russia (Andreeva 2013), and Asia (Althaus 2019; Do 2016). Six studies were individually randomised trials (Althaus 2019; Butler 2019; Cals 2010; Diederichsen 2000; Do 2016; Melbye 1995), and seven were cluster‐RCTs (Andreeva 2013; Boere 2021; Cals 2009; Lhopitallier 2021; Little 2013a; Little 2019; Schot 2018). Schot 2018 was originally designed as a cluster‐RCT, but due to insufficient uptake, the study set‐up was changed to include individually randomised participants. We chose to classify and analyse data from Schot 2018 as a cluster‐RCT, as most participants were cluster‐randomised (99 children (32%) were individually randomised, whilst 210 children were cluster randomised).
Inclusion criteria
Inclusion criteria differed amongst studies. Diederichsen 2000, and to a lesser extent Melbye 1995 and Boere 2021, used broad inclusion criteria, whilst Andreeva 2013, Cals 2009, Cals 2010, Do 2016, Little 2013a, and Little 2019 used more narrow diagnostic criteria for lower or upper ARIs, or both. Althaus 2019 included participants with fever in general, but provided detailed information on ARIs.
Population
Participants differed amongst studies. Butler 2019 included patients > 40 years of age with chronic obstructive pulmonary disease (COPD). Boere 2021 included participants from nursing homes. Four studies included children (Althaus 2019; Diederichsen 2000; Do 2016; Schot 2018). Only Schot 2018 included children alone (aged 3 months to 12 years). The remaining three studies included either all age groups or from 1 to 65 years of age.
Setting
All included studies were conducted in a primary healthcare setting. Nine studies included participants from primary care practices (Andreeva 2013; Butler 2019; Cals 2009; Cals 2010; Diederichsen 2000; Lhopitallier 2021; Little 2013a; Little 2019; Melbye 1995), whilst the remaining four studies included participants from other primary healthcare sectors (Althaus 2019; Boere 2021; Do 2016; Schot 2018). Althaus 2019 included participants from nine public primary care units and one outpatient department in Myanmar. Do 2016 included participants from nine primary healthcare centres and one outpatient clinic in Vietnam. The outpatient clinics were manned by nurses and medical doctors, and the clinics form part of the primary healthcare sector in these countries. Schot 2018 included participants in an "out of office hours" facility staffed by general practitioners. Boere 2021 included participants from nursing homes.
C‐reactive protein cut‐off values
We noted appreciable differences between the C‐reactive protein cut‐off values applied to guide antibiotic treatment, which ranged from vague indications of such values to specific numeric recommendations for initiating or withholding antibiotic treatment, or both (Table 8). Test results were made available to the physicians as part of the initial clinical assessment in most studies, except for Melbye 1995, which only made results available to physicians after the initial clinical decision. Althaus 2019 communicated results of C‐reactive protein measurements to the healthcare provider as low C‐reactive protein or high C‐reactive protein using cut‐off thresholds of either 20 mg/L (group A) or 40 mg/L (group B). The exact set‐up was left to the participating physician to accommodate in Diederichsen 2000. The treating physician could overrule C‐reactive protein guidance in all trials.
6. C‐reactive protein ‐ inclusion criteria and C‐reactive protein algorithms of included studies.
| Study | Randomisation | Inclusion criteria | Algorithm used* |
| Melbye 1995 | Individual | Adults (> 18 years) with subjective complaint of i) pneumonia, bronchitis, or asthma (no further description); or ii) 1 of the following symptoms: cough, shortness of breath, chest pain on deep inspiration or when coughing | Duration of illness < 24 hours and C‐reactive protein levels lower than 50 mg/L: no change in clinical decision. C‐reactive protein levels > 50 mg/L: immediate antibiotic prescribing was recommended Duration of illness 1 to 6 days and C‐reactive protein levels < 11 mg/L: no antibiotics recommended. Participants with C‐reactive protein levels between 11 and 49 mg/L: no change in clinical decision. C‐reactive protein levels > 50 mg/L: immediate antibiotic prescribing was recommended Duration of illness > 7 days and C‐reactive protein levels < 11 mg/L: no antibiotics recommended. Participants with C‐reactive protein levels between 11 and 24 mg/L: no change in clinical decision. C‐reactive protein levels > 25 mg/L: immediate antibiotic prescribing was recommended |
| Diederichsen 2000 | Individual | All patients with a respiratory infection (no further description) | Strict cut‐off values were not given, but information was provided that a normal C‐reactive protein level was < 10 mg/L and that C‐reactive protein levels < 50 mg/L were seldom the result of bacterial infection. |
| Cals 2009 | Cluster | Adults (> 18 years) with suspected LRTI (cough < 4 weeks AND 1 focal sign/symptom (shortness of breath, wheezing, chest pain, auscultation abnormalities) AND 1 systemic sign/symptom (fever > 38 °C, perspiring, headache, myalgia, feeling generally unwell) |
C‐reactive protein levels < 20 mg/L: pneumonia extremely unlikely and antibiotic prescribing discouraged C‐reactive protein levels between 20 and 50 mg/L: pneumonia very unlikely C‐reactive protein levels between 50 and 100 mg/L: clear infection. Acute bronchitis most likely, possible pneumonia C‐reactive protein > 100 mg/L: severe infection. Pneumonia more likely. Immediate antibiotic prescribing was recommended. C‐reactive protein levels between 20 and 99 mg/L: consider delayed prescribing |
| Cals 2010 | Individual | Adults (> 18 years) with: i) LRTI (cough < 4 weeks) AND 1 focal sign/symptom (shortness of breath, wheezing, chest pain, auscultation abnormalities) AND 1 systemic sign/symptom (fever > 38 °C, perspiring, headache, myalgia, feeling generally unwell) ii) Rhinosinusitis < 4 weeks AND 1 symptom (history of rhinorrhoea, blocked nose) 1 symptom or sign (purulent rhinorrhoea, unilateral facial pain, headache, teeth pain, pain when chewing, maxillary/frontal pain when bending over, worsening of symptoms after initial improvement) |
C‐reactive protein levels < 20 mg/L: bacterial infection was considered highly unlikely and antibiotic prescribing was discouraged C‐reactive protein levels > 100 mg/L: bacterial infection was considered likely and immediate antibiotic prescribing was recommended C‐reactive protein levels between 20 and 99 mg/L: consider delayed prescribing |
| Little 2013a | Cluster | Adults (> 18 years) with: i) LRTI/acute cough (up to 28 days' duration) as the main symptom, or alternatively where cough was not the most prominent symptom (e.g. fever, malaise), but where the clinician considered acute LRTI to be the main diagnosis. Pneumonia was not an exclusion criterion. ii) URTI: as with LRTI, but judged by the physician to be another acute respiratory infection (sore throat, otitis media, sinusitis, influenza and/or coryzal illness) |
C‐reactive protein ≤ 20 mg/L: self‐limiting ARI, withhold antibiotics C‐reactive protein 21 to 50 mg/L: majority of participants have self‐limiting ARI, withhold antibiotics in most cases C‐reactive protein 51 to 99 mg/L: withhold antibiotics in the majority of cases and consider delayed antibiotics in the minority of cases C‐reactive protein ≥ 100 mg/L: severe infection, prescribe antibiotics |
| Andreeva 2013 | Cluster | Adults (> 18 years) with LRTI/acute cough (including acute bronchitis, pneumonia, and infectious exacerbations of COPD or asthma) for less than 28 days | C‐reactive protein < 20 mg/L: antibiotics usually not needed C‐reactive protein > 50 mg/L: antibiotic prescribing could be indicated taking into account the duration of illness |
| Do 2016 | Individual | All patients aged 1 to 65 years of age presenting with non‐severe acute respiratory tract infection with at least 1 focal and 1 systemic sign or symptom by the treating physician. Focal signs; cough, rhinitis, pharyngitis, shortness of breath, wheezing, chest pain, and auscultation abnormalities Systemic signs and symptoms; fever, perspiration, headache, myalgia, and feeling generally unwell |
C‐reactive protein ≤ 20 mg/L for participants aged 6 to 65 years: prescription of antibiotics not recommended C‐reactive protein ≥ 100 mg/L for participants aged 6 to 65 years: should generally receive antibiotics, and hospital referral should be considered C‐reactive protein ≤ 10 mg/L for children aged 1 to 5 years: prescription of antibiotics not recommended C‐reactive protein ≥ 50 mg/L for children aged 1 to 5 years: should generally receive antibiotics, and hospital referral should be considered. Between these thresholds no specific recommendation was given, and clinicians were advised to use their clinical discretion. |
| Schot 2018 | Cluster | Children between 3 months and 12 years of age with suspicion of lower respiratory tract infection; acute cough < 21 days; reported fever > 38 °C < 5 days | C‐reactive protein levels < 10 mg/L make pneumonia less likely, but should not be used to exclude pneumonia when the GP finds the child ill, or when duration of symptoms is < 6 hours C‐reactive protein between 10 mg/L and 100 mg/L, the likelihood of pneumonia increases with increasing CRP levels C‐reactive protein levels> 100 mg/L make pneumonia much more likely; however, such levels can also be caused by viral infections |
| Althaus 2019 | Individual | Patients aged 1 year or older with: a documented fever (defined as a tympanic temperature of > 37.5 °C according to WHO standards) OR a chief complaint of fever (< 14 days), regardless of previous antibiotic intake and comorbidity other than malignancies |
Intervention group A: the result of CRP measurement was communicated to the healthcare provider as low CRP or high CRP using cut‐off thresholds of 20 mg/L Intervention group B: the result of CRP measurement was communicated to the healthcare provider as low CRP or high CRP using cut‐off thresholds of40 mg/L If CRP measurement was low, antibiotics should be refrained from if no danger signs were present. If CRP measurement was high, antibiotics should be considered on the basis of clinical judgement. |
| Butler 2019 | Individual | Patients aged 40 years or older and diagnosis of COPD in their primary care clinical record; presenting with an acute exacerbation of COPD with at least 1 of AECOPD criteria (increased dyspnoea, increased sputum volume, increased sputum purulence), between 24 hours and 21 days duration | C‐reactive protein ≤ 20 mg/L: antibiotics are unlikely to be beneficial and should usually not be prescribed C‐reactive protein 20 to 40 mg/L: antibiotics may be beneficial, mainly if purulent sputum is present C‐reactive protein ≥ 40 mg/L: antibiotics are likely to be beneficial |
| Little 2019 | Cluster | Adults (> 18 years) with: i) LRTI/acute cough (up to 28 days duration) as the main symptom, or alternatively where cough was not the most prominent symptom (e.g. fever, malaise), but where the clinician considered acute LRTI to be the main diagnosis. Pneumonia was not an exclusion criterion. ii) URTI: as with LRTI, but judged by the physician to be another acute respiratory infection (sore throat, otitis media, sinusitis, influenza and/or coryzal illness) |
C‐reactive protein ≤ 20 mg/L: self‐limiting ARI, withhold antibiotics C‐reactive protein 21 to 50 mg/L: the majority of patients have self‐limiting ARI, withhold antibiotics in most cases C‐reactive protein 51 to 99 mg/L: withhold antibiotics in the majority of cases and consider delayed antibiotics in the minority of cases C‐reactive protein ≥ 100 mg/L: severe infection, prescribe antibiotics |
| Boere 2021 | Cluster | All somatic, psychogeriatric, and short‐stay (geriatric rehabilitation and short‐term residential care) nursing home residents with a suspected LRTI, according to their physician’s assessment | C‐reactive protein levels < 20 mg/L: self‐limiting LRTI. Assessment of signs, symptoms, risk factors, and CRP is important. Withhold antibiotics in most cases C‐reactive protein levels 20 to 60 mg/L: assessment of signs, symptoms, risk factors, and CRP is important. Withhold antibiotics in most cases C‐reactive protein levels > 60 mg/L: severe infection, prescribe antibiotics |
Abbreviations: AECOPD: acute exacerbations of chronic obstructive pulmonary disease; ARI: acute respiratory infection; COPD: chronic obstructive pulmonary disease; CRP: C‐reactive protein; GP: general practitioner; LRTI: lower respiratory tract infection; URTI: upper respiratory tract infection; WHO: World Health Organization
*All studies stated that physicians could deviate from the algorithm at any time.
Procalcitonin
One study investigated the use of procalcitonin as a point‐of‐care biomarker in ARI in primary care (Lhopitallier 2021). The cut‐off value of procalcitonin was 0.25 μg/L (Table 9). The point‐of‐care test provided results in 20 minutes. The study is described in Characteristics of included studies.
7. Procalcitonin ‐ inclusion criteria and procalcitonin algorithms of included studies.
| Study | Randomisation | Inclusion criteria | Algorithm used |
| Lhopitallier 2021 | Cluster | Adults (> 18 years) with acute cough < 21 days and at least 1 of the following signs/symptoms: history of fever for more than 4 days; dyspnoea; tachypnoea (> 22 cycles per minute); abnormal focal findings upon lung auscultation | Procalcitonin ≥ 0.25 μg/L: antibiotics prescribing recommended Antibiotic choice, dose, and duration were left to the discretion of the primary care physician. The physician could also order further diagnostic tests. |
Outcome assessment
Outcome assessment was based on medical records regarding the number of participants given an antibiotic prescription, although Butler 2019 also used participant‐reported antibiotic usage. Secondary outcomes such as clinical recovery were reported by participants using diaries and questionnaires, or during follow‐up visits at the clinics (Althaus 2019; Andreeva 2013; Boere 2021; Butler 2019; Melbye 1995).
Other aspects
One study was terminated by the principal investigator after one year without reaching the target inclusion rate due to an interim analysis that showed no difference between groups and because of lack of interest from the participating general practitioners (Melbye 1995). Little 2019 was a 12‐month follow‐up of the original study, Little 2013a, and included participants from the same practices. The study noted that C‐reactive protein testing was seldom used at 12‐month follow‐up (5.77% in the intervention group) (Little 2019). One study reported frequent violation to adherence to protocol (30 out of 165 participants in the control group had C‐reactive protein measurements) (Schot 2018). Two studies received economic funding from manufacturers of C‐reactive protein point‐of‐care tests (Cals 2010; Melbye 1995). Three studies received test kits or reagents, or both, for the study (Andreeva 2013; Do 2016; Little 2019). On‐site training in C‐reactive protein devices was performed by manufacturers in four studies (Butler 2019; Diederichsen 2000; Little 2013a; Little 2019).
Additional data
We contacted a total of six study authors for additional details. We obtained raw data from Diederichsen 2000 to calculate the number of participants with substantial improvement and to differentiate between children and adults. Althaus 2019 provided specific details and raw data to differentiate participants with symptoms of ARIs to permit inclusion in the meta‐analysis.
Excluded studies
We excluded one study that used an electronic algorithm in combination with a specific point‐of‐care test (C‐reactive protein test) (Keitel 2017), and one study that used a specific antigen test (Little 2014).
Five studies were not conducted in a primary care setting (Ameyaw 2014; Huang 2018; Isa 2022; Montassier 2019; Schechter‐Perkins 2019). We excluded one study that did not assess a point‐of‐care test to guide antibiotic treatment decisions (Mann 2020), but used clinical decision support to guide antibiotic decisions.
We excluded seven studies that were not RCTs (de Lusignan 2020; Eley 2020; Fiore 2017; Meili 2016; Minnaard 2016; Oppong 2018; Stannard 2014).
We contacted three trial authors to obtain additional details on ARIs, as they included acutely ill participants or participants with fever in general (Rebnord 2016a; Van den Bruel 2016; Verbakel 2016). Unfortunately, data regarding specific ARI diagnoses or symptoms were not registered or retrievable, resulting in exclusion of these studies.
Risk of bias in included studies
We assessed the risk of bias for each study and individual outcomes. The risk of bias assessment is presented graphically in Figure 2 and summarised in Figure 3. For further information on included studies, see Characteristics of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The cluster‐RCTs used computer randomisation programs to allocate practices to the intervention or control arms (Andreeva 2013; Boere 2021; Cals 2009; Lhopitallier 2021; Little 2013a; Little 2019; Schot 2018). The individually randomised studies by Cals 2010, Do 2016, and Althaus 2019 used sequentially numbered, opaque, sealed envelopes that were opened on site after participant enrolment. Cals 2010 also had the envelopes prepared in different block sizes by an independent research team. Butler 2019 did not provide information on the allocation concealment in the paper, but in the study protocol described that they used remote allocation and maintained allocation concealment up to the point of the intervention. Diederichsen 2000 provided no information on sequence generation, but stated that they used "pre‐randomised sealed envelopes in blocks of 34". Melbye 1995 did not specify the randomisation procedure, but according to the principal investigator this was adequately done at study sponsor level. Allocation concealment of individual participants does not apply to cluster‐RCTs at practice level, so we assessed Schot 2018, in which most participants were cluster randomised, as at unclear risk of bias.
Blinding
Measurement of C‐reactive protein and procalcitonin does not lend itself to blinding at the clinician level, as the intervention is used in management decisions, therefore lack of blinding for personnel administering the intervention was not a concern. Lack of blinding of participants and assessors may have been a concern for some outcomes, primarily participant‐reported outcomes. Our assessment of the number of participants given an antibiotic prescription was based on electronic or paper records for all studies.
Secondary outcome data assessment procedure varied amongst studies. Seven studies used participant‐reported data on clinical recovery (Althaus 2019; Butler 2019; Cals 2009; Cals 2010; Diederichsen 2000; Lhopitallier 2021; Little 2013a). Two studies used blinded telephone interviews to assess secondary outcome data (Do 2016; Lhopitallier 2021). Five studies assessed secondary outcome data non‐blinded at follow‐up visits (Althaus 2019; Andreeva 2013; Boere 2021; Butler 2019; Melbye 1995). Althaus 2019 did use participant‐reported clinical recovery, but remaining secondary outcome data were assessed non‐blinded by research staff at follow‐up visits. Little 2019 did not present any secondary outcome data.
Incomplete outcome data
We successfully retrieved incomplete outcome data on the number of participants given an antibiotic prescription by contacting the corresponding authors of individual studies when needed. Do 2016 provided missing data on our primary outcome number of participants given an antibiotic prescription (11% in the intervention group and 7% in the control group).
Data on clinical recovery rates ranged from 90% to 98% in completeness for most studies, except for Do 2016 and Schot 2018. Schot 2018 had high rates of missing data for secondary outcomes (58.2%) due to missing consent forms from parents on included children. Do 2016 also reported a high rate of missing data for our secondary outcome number of satisfied participants (56.3%), but the reasons for this remain unclear.
We were unable to obtain information necessary for our planned subgroup analyses comparing serious and non‐serious infections, as this was not reported, and exact diagnoses were not recorded. However, we were able to obtain data on the effect of C‐reactive protein on the number of participants given an antibiotic prescription, which permitted a subgroup analysis of lower and upper ARIs.
Selective reporting
Most studies were analysed as prespecified in study protocols accessed from trial registers. The two oldest studies did not have a published protocol (Diederichsen 2000; Melbye 1995). Schot 2018 changed study set‐up. Although this was not specified in the protocol, we did not consider this change to have introduced reporting bias and it did not affect our risk of bias rating. We did not detect any selective reporting of particular outcome measurement or analysis.
Other potential sources of bias
Selection (recruitment) bias is a risk in cluster‐RCTs, as care providers assigned to the intervention group can select which patients to test (inclusion was at the discretion of the care provider). This means that patients with a higher‐than‐average likelihood that the test might change the clinical decision could preferentially be enrolled, for example those patients that the care provider perceived could be convinced that an intervention was not needed if a test was performed. This may exaggerate the estimated effect relative to more widespread use in clinical practice. However, measures to limit this 'active' recruitment by participating physicians were in place, for example by requirements for consecutive enrolment of the first eligible patients that presented in each practice. In the study by Cals 2009, significantly more participants in the control group had abnormalities on auscultation (60.3% versus 46.7%, P = 0.005), a parameter closely linked to antibiotic prescription (Jakobsen 2010). However, in the larger study by Little 2013a, symptom severity scores were balanced between groups.
Contamination bias is possible in individual RCTs, as the general practitioner may gradually learn to foresee which patients have low C‐reactive protein levels and apply this acquired skill in the control group. As most patients will have low values of C‐reactive protein, this would lead to decreased antibiotic prescription in the control group and underestimate the effect of the test.
Inclusion bias may occur in both trial designs, as general practitioners may be reluctant to include patients with severe disease given the risk that antibiotic treatment is not recommended according to the test result. In individual RCTs, this potential bias would be non‐discriminative, as opposed to in cluster‐RCTs, where this could be a discriminative bias. This may lead to a lower estimate of the effect of biomarkers in individual RCTs (a priori risk of antibiotic treatment is low in both groups) but may overestimate the effect in cluster‐RCTs (a priori risk of antibiotic treatment is different between intervention (low) and control groups (normal)).
Effects of interventions
Defining follow‐up
We changed the wording used in the original review from “at 28 days follow‐up” and “at day seven” to "within 28 days follow‐up” and "within seven days follow‐up” to specify the follow‐up period more precisely. This did not change our decision to include or exclude any specific studies from the review, but it did change which studies were included in our meta‐analysis.
Primary outcomes
1. Number of participants given an antibiotic prescription at the index consultation and within 28 days follow‐up
C‐reactive protein
See Table 1.
All 12 studies, including 10,218 participants (mean age 62), reported point estimates in favour of the C‐reactive protein test to reduce the number of participants given an antibiotic prescription. The pooled result for all included trials showed that C‐reactive protein tests likely reduce the number of participants given an antibiotic prescription (risk ratio (RR) 0.77, 95% confidence interval (CI) 0.69 to 0.86; 12 trials, 10,218 participants; I² = 79%; moderate‐certainty evidence; Analysis 1.1; Figure 4). However, the considerable heterogeneity was not explained through our pre‐planned subgroup analysis of cluster‐RCTs and individual RCTs.
1.1. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 1: CRP ‐ Antibiotics prescribed at index consultation. All trials (cluster‐randomised with modified sample size)
4.

Forest plot of comparison: 1 C‐reactive protein ‐ antibiotic prescribing: all trials, outcome: 1.1 C‐reactive protein ‐ antibiotics prescribed at index consultation. All trials (cluster‐RCTs with modified sample size).
Pooled results from the individual RCTs showed that C‐reactive protein tests likely reduce the number of participants given an antibiotic prescription (RR 0.79, 95% CI 0.70 to 0.89; I² = 75%; 6 trials, 5899 participants; Analysis 1.1; moderate‐certainty evidence; Figure 4), but again with considerable heterogeneity. A similar effect was seen with the cluster‐RCTs (RR 0.73, 95% CI 0.58 to 0.90; 6 trials, 4319 participants; I² = 82%; moderate‐certainty evidence; Analysis 1.1; Figure 4).
The number needed to test (NNT) to avoid one patient being given an antibiotic prescription at the index consultation was 9 (95% CI 7 to 13; Table 10).
8. C‐reactive protein ‐ number needed to test to save 1 antibiotic prescribing.
| NNT | 95% CI | |
| All trials | 9 | 7 to 13 |
| Individual RCT | 10 | 7 to 18 |
| Cluster‐RCT* | 8 | 5 to 20 |
Abbreviations: CI: confidence interval; NNT: number needed to test; RCT: randomised controlled trial
*Cluster‐randomised trials with modified sample size.
Overall, C‐reactive protein tests reduced the number of participants given an antibiotic prescription within 28 days follow‐up (RR 0.81, 95% CI 0.76 to 0.86; 7 trials, 5091 participants; I² = 29%; high‐certainty evidence; Analysis 1.2; Figure 5).
1.2. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 2: CRP ‐ Antibiotics prescribed within 28 days follow‐up (cluster‐randomised trials with modified sample size)
5.

Forest plot of comparison: 1 C‐reactive protein ‐ antibiotic prescribing: all trials, outcome: 1.2 C‐reactive protein ‐ antibiotics prescribed within 28 days (cluster‐RCTs with modified sample size).
Procalcitonin
See Table 2.
One study including 317 participants (median age 53), reported point estimates in favour of the C‐reactive protein test to reduce the number of participants given an antibiotic prescription (RR 0.32, 95% CI 0.23 to 0.44; 1 trial, 317 participants; very low‐certainty evidence; Analysis 2.1). As only one study investigated procalcitonin, the evidence is very uncertain about the effect of procalcitonin on number of participants given an antibiotic prescription.
2.1. Analysis.

Comparison 2: Procalcitonin versus standard care, Outcome 1: Procalcitonin ‐ Antibiotic prescribed at index consultation
2. Number of participants with substantial improvement (including full recovery) within seven days follow‐up
C‐reactive protein
C‐reactive protein tests likely do not reduce clinical recovery (defined as at least substantial improvement) within seven days follow‐up (RR 1.03, 95% CI 0.96 to 1.12; 4 trials, 3104 participants; I² = 0%; moderate‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 3: CRP ‐ Number of participants substantially improved within 7 days follow‐up
Procalcitonin
The evidence is very uncertain about the effect of procalcitonin tests on clinical recovery (RR 1.23 95% CI 0.93 to 1.62; 1 trial, 277 participants; very low‐certainty evidence; Analysis 2.3)
2.3. Analysis.

Comparison 2: Procalcitonin versus standard care, Outcome 3: Procalcitonin ‐ Number of participants substantially improved within 7 days follow‐up
3. Total mortality within 28 days follow‐up
C‐reactive protein
Nine out of the 12 included studies reported information on mortality (Althaus 2019; Boere 2021; Butler 2019; Cals 2009; Cals 2010; Diederichsen 2000; Do 2016; Little 2013a; Schot 2018). Three out of nine studies reported deaths during the follow‐up period (Althaus 2019; Boere 2021; Butler 2019), and six trials reported no deaths (Cals 2009; Cals 2010; Diederichsen 2000; Do 2016; Little 2013a; Schot 2018). The three remaining studies did not report on mortality and were thus not included in the analysis (Andreeva 2013; Little 2019; Melbye 1995).
Pooled analysis showed that C‐reactive protein tests may not increase total mortality within 28 days follow‐up (RR 0.53, 95% CI 0.10 to 2.92; 9 trials, 7737 participants; I² = 0%; low‐certainty evidence; Analysis 1.4), but the number of events was small (2 in 4221 participants in the intervention group versus 3 in 3516 participants in the control group).
1.4. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 4: CRP ‐ Mortality (cluster‐randomised trials with modified sample size)
Causes of death were pneumonia (1), respiratory failure (1), pneumothorax (1), or "unclear reasons" (2). All studies reported that deaths were unrelated to the intervention or procedure as determined by trial investigators. All participants who died received antibiotics at day 0, either at index consultation or upon admission to hospital. All deaths were amongst older adults with comorbidities.
Procalcitonin
No deaths occurred during the trial that investigated procalcitonin.
Secondary outcomes
1. Number of participants in need of a reconsultation within 28 days follow‐up
C‐reactive protein
Use of C‐reactive protein tests results in little or no difference in the number of participants in need of a reconsultation within 28 days follow‐up (RR 1.06, 95% CI 0.91 to 1.24; 7 trials, 6256 participants; I² = 0%; high‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 5: CRP ‐ Number of reconsultations within 28 days follow‐up
Procalcitonin
The evidence is very uncertain about the effect of procalcitonin tests on the number of participants in need of a reconsultation within 28 days follow‐up (RR 1.00, 95% CI 0.69 to 1.46; 1 trial, 317 participants; very low‐certainty evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2: Procalcitonin versus standard care, Outcome 5: Procalcitonin ‐ Number of reconsultations within 28 days follow‐up
2. Number of participants in need of a hospital admission within 28 days follow‐up
C‐reactive protein
Ten of the 12 included studies reported information on hospital admissions (Althaus 2019; Andreeva 2013; Boere 2021; Butler 2019; Cals 2009; Cals 2010; Diederichsen 2000; Do 2016; Little 2013a; Schot 2018). Six of 10 studies reported hospital admissions during the follow‐up period (Althaus 2019; Boere 2021; Butler 2019; Do 2016; Little 2013a; Schot 2018), and four studies reported no hospital admissions amongst the included participants (Althaus 2019; Cals 2009; Cals 2010; Diederichsen 2000). The two remaining studies did not report information on hospital admission and were thus not included in the analysis (Little 2019; Melbye 1995).
C‐reactive protein tests probably do not increase the number of participants in need of a hospital admission within 28 days follow‐up (RR 1.05, 95% CI 0.72 to 1.53; 10 trials, 7514 participants; I² = 0%; moderate‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 6: CRP ‐ Hospital admissions (cluster‐randomised with modified sample size)
Only two studies presented detailed information on the reasons for hospital admission (Boere 2021; Little 2013a). Reasons for hospital admissions were listed as: cardiac (3), respiratory (16), generally unwell/fever (2), gastrointestinal symptoms (2), sinusitis (1), sepsis (1), other (i.e. hyponatraemia, fracture, anaemia) (4).
All hospitalisations were likely not related to the intervention. However, an increase in the risk of hospital admissions in the C‐reactive protein group cannot be ruled out, although the absolute event rate was low (57 in 4728 participants in the intervention group versus 48 in 3386 participants in the control group). Data were not available to determine the number of hospitalised participants who were withheld antibiotic treatment at the index consultation, nor their C‐reactive protein level at the index consultation.
Procalcitonin
Lhopitallier 2021 reported on hospital admissions. There were four hospital admissions in the intervention group and two hospital admissions in the usual care group. Reasons for hospital admissions were: acute psychotic episode (1) and pneumonia (5). Data were not available to determine the number of hospitalised participants who were withheld antibiotic treatment at the index consultation, nor their procalcitonin level at the index consultation.
The evidence is very uncertain about the effect of procalcitonin tests on the number of participants in need of a hospital admission within 28 days follow‐up (RR 1.40, 95% CI 0.26 to 7.51; 1 trial, 277 participants; very low‐certainty evidence; Analysis 2.6).
2.6. Analysis.

Comparison 2: Procalcitonin versus standard care, Outcome 6: Procalcitonin ‐ Hospital admissions
3. Duration of acute respiratory infection
C‐reactive protein
Four studies reported the duration of the ARI as days with restrictions in daily activities due to the infection, but used different measures in their assessment, thereby precluding a pooled analysis (Table 4). Cals 2009 reported no differences in the median symptom duration of the ARI to full recovery, whilst Cals 2010 also provided this measure as a mean number of days. Little 2013a reported the time to resolution of symptoms rated as moderately bad or worse. Do 2016 reported the time to resolution of symptoms. No differences were observed for any of these participant‐reported outcomes.
Procalcitonin
The median duration of the ARI was 8 days in the procalcitonin group and 7 days in the usual care group at 28 days follow‐up (Table 5).
4. Number of satisfied participants
C‐reactive protein
Three out of 12 studies reported information on patient satisfaction (Cals 2009; Cals 2010; Do 2016). All three studies reported satisfaction in terms of satisfaction with care and consultation, either at index consultation, Cals 2009; Cals 2010, or after trial day 14 (Do 2016). C‐reactive protein tests may not reduce the number of satisfied participants (RR 0.81, 95% CI 0.50 to 1.29; 3 trials, 1458 participants; I² = 40%; low‐certainty evidence). However, there was moderate heterogeneity (I² = 40%), possibly related to the use of different assessment scales, and the fact that only three studies reported patient satisfaction reduces the certainty of the effect estimate (Analysis 1.7).
1.7. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 7: CRP ‐ Patient satisfaction
Procalcitonin
The study on procalcitonin reported patient satisfaction regarding the consultation at consultation day (Lhopitallier 2021). Satisfaction was also reported with regard to the diagnosis found, treatment, and the amount of time spent with the primary care physician, using a standardised visit‐specific satisfaction instrument by a blinded phone interview at day 7.
The evidence is very uncertain about the effect of procalcitonin tests on the number of satisfied participants (RR 0.96, 95% CI 0.93 to 1.00; 1 trial, 308 participants; very low‐certainty evidence; Analysis 2.7).
2.7. Analysis.

Comparison 2: Procalcitonin versus standard care, Outcome 7: Procalcitonin ‐ Patient satisfaction
5. Number of participants with substantial improvement (including full recovery) within 28 days follow‐up
C‐reactive protein
Use of C‐reactive protein tests likely does not reduce the number of participants recovered (defined as at least substantial improvement) within 28 days follow‐up (RR 1.02, 95% CI 0.79 to 1.32; 5 trials, 2324 participants; I² = 0%; moderate‐certainty evidence; Analysis 1.8).
1.8. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 8: CRP ‐ Number of participants substantially improved within 28 days follow‐up (cluster‐randomised trials with modified sample size)
Procalcitonin
Lhopitallier 2021 did not report on recovery within 28 days follow‐up.
6. Subgroup analyses
C‐reactive protein
Serious infections and less serious infections
We had to omit our planned analysis of severe and less severe ARI due to lack of data. However, the effect of the C‐reactive protein test on the number of participants given an antibiotic prescription was similar for upper and lower ARIs (Analysis 1.9). Heterogeneity across subgroups was 0%, suggesting no variation in the mean effect in the subgroups.
1.9. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 9: CRP ‐ Subgroup analysis: antibiotics prescribed at index consultation: upper respiratory tract infections and lower respiratory tract infections (cluster‐randomised trials with modified sample size)
Children and adults
Four studies specifically reported on the effect of C‐reactive protein test in children (N = 2335), collectively finding that C‐reactive protein likely reduces the number of children given an antibiotic prescription (RR 0.78, 95% CI 0.67 to 0.91; 4 trials, 2335 participants; I² = 45%; moderate‐certainty evidence; Analysis 1.10) (Althaus 2019; Diederichsen 2000; Do 2016; Schot 2018). The effect was primarily seen in low‐ and middle‐income countries.
1.10. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 10: CRP ‐ Subgroup analysis: children and adults. Antibiotic prescribing at index consultation
The test for subgroup differences (test for heterogeneity across subgroups) suggests there is no variability in the effect estimate between adults and children (I² = 0%) (Analysis 1.10). Points estimates and confidence intervals are also overlapping.
Type of point‐of‐care test
We did not carry out a subgroup analysis on type of point‐of‐care test due to lack of studies on the use of procalcitonin and leukocyte point‐of‐care tests.
Trials with low risk of bias and high risk of bias
As the intervention did not lend itself to blinding, we chose to omit this component when selecting studies for our subgroup analysis comparing trials at low risk of bias and at high risk of bias. Accordingly, Cals 2010 was the only trial with an overall low risk of bias (Figure 3). The primary outcome of the number of participants given an antibiotic prescription at index consultation was RR 0.77, 95% CI 0.60 to 0.98, which was similar to the trials at high risk of bias.
Cluster‐RCTs and individually randomised studies
We carried out a subgroup analysis on cluster‐RCTs and individual RCTs in most analyses. The test for heterogeneity did not show any indication of a variation in the mean effect in the subgroups (I² = 0%). The test for subgroup differences suggests that there is no variation in the mean effect between the cluster RCTs and individual RCTs (I² = 0%). Points estimates and confidence intervals are also overlapping.
Procalcitonin
As only one included study investigated the effect of procalcitonin, we did not carry out any subgroup analysis evaluating procalcitonin as point‐of‐care biomarker.
7. Sensitivity analyses
C‐reactive protein
Planned sensitivity analysis
Due to considerable heterogeneity possibly related to variations in trial designs, we omitted the preplanned sensitivity analysis using a fixed‐effect meta‐analysis.
Sensitivity analyses assuming a worst‐case scenario, where all participants in the C‐reactive protein group lost to follow‐up did not improve, and all participants in the control group lost to follow‐up did substantially improve, showed that C‐reactive protein tests may increase the number of participants given an antibiotic prescription (within 7 days follow‐up: RR 1.11, 95% CI 1.03 to 1.20; 4 trials, 3214 participants; I²= 0%; Analysis 1.11; within 28 days follow‐up: RR 1.37, 95% CI 1.01 to 1.85; 5 trials, 2506 participants; I²= 31%; Analysis 1.12). The sensitivity analysis raised some concerns regarding missing outcome data on recovery (Althaus 2019; Andreeva 2013; Boere 2021; Melbye 1995). Boere 2021 had missing outcome data on recovery that were not equally distributed between the intervention group and the control group (22 participants with missing outcome data items in 162 participants in the intervention group versus 3 participants with missing outcome data items in 79 participants in the control group). Althaus 2019 had missing outcome data on recovery that were equally distributed between groups (22 missing outcome data items in 630 participants in group A, 17 missing outcome data items in 626 participants in group B, and 26 missing outcome data items in 649 participants in the control group). Melbye 1995 had recovery data available in 230 of 239 participants within 7 days follow‐up and in 219 of 239 participants at 21 days follow‐up. Andreeva 2013 had minor missing outcome data (176 out of 179 participants provided outcome data on recovery).
1.11. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 11: CRP ‐ Sensitivity analysis: participant recovery within 7 days follow‐up: missing data in CRP = not recovered
1.12. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 12: CRP ‐ Sensitivity analysis: participant recovery within 28 days follow‐up: missing data in CRP = not recovered (cluster‐randomised trials with modified sample size)
Post hoc sensitivity analysis
We carried out a post hoc sensitivity analysis on follow‐up period, as we had to specify the follow‐up period that was not prespecified in the protocol or the original review. The post hoc sensitivity analysis on follow‐up period did not change which studies to include from the original review, but did change which studies to include from the current search carried out in June 2022 (Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16; Analysis 1.17; Analysis 1.18; Analysis 1.19; Analysis 1.20).
1.13. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 13: CRP ‐ Sensitivity analysis: recovery within 7 days follow‐up when algorithms provide clear cut‐offs to rule out (< 20 mg/L)
1.14. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 14: CRP ‐ Sensitivity analysis: recovery within 28 days follow‐up when algorithms provide clear cut‐offs to rule out (< 20 mg/L)
1.15. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 15: CRP ‐ Sensitivity analysis: antibiotics prescribed, assessed ONLY at day 28
1.16. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 16: CRP ‐ Sensitivity analysis: antibiotics prescribed, assessed WITHIN 28 days
1.17. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 17: CRP ‐ Sensitivity analysis: recovery, assessed ONLY at day 7
1.18. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 18: CRP ‐ Sensitivity analysis: recovery, assessed WITHIN 7 days
1.19. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 19: CRP ‐ Sensitivity analysis: recovery, assessed ONLY at day 28
1.20. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 20: CRP ‐ Sensitivity analysis: recovery, assessed WITHIN 28 days
To assess the considerable heterogeneity, we performed a post hoc sensitivity analysis of newer studies separately (Althaus 2019; Andreeva 2013; Boere 2021; Butler 2019; Cals 2009; Cals 2010; Do 2016; Little 2013a; Little 2019), as these studies used specific guidance on antibiotic prescription if C‐reactive protein levels were < 20 mg/L. This analysis showed that C‐reactive protein tests likely reduce the number of participants given an antibiotic prescription (RR 0.74, 95% CI 0.65 to 0.84; 9 trials, 8341 participants; I²= 82%; moderate‐certainty evidence; Analysis 1.21), but with considerable heterogeneity.
1.21. Analysis.

Comparison 1: C‐reactive protein versus standard care, Outcome 21: CRP ‐ Sensitivity analysis: antibiotic prescribing when algorithms provide clear cut‐offs to rule out (< 20 mg/L)
A summary of the secondary outcomes is presented in Table 11 and Table 12.
9. C‐reactive protein ‐ summary of secondary outcomes.
| Outcome | Studies | Participants |
Pooled resultsa RR (95% CI); I² |
Individually randomised RR (95% CI); I² |
Cluster‐randomised RR (95% CI); I² |
Analysis |
| Reconsultations day 28 | 7 | 6256 | 1.06 (0.91 to 1.24); 0% | 1.31 (0.90 to 1.89); 0% | 1.01 (0.85 to 1.20); 0% | 1.5 |
| Hospital admissions | 10 | 7514 | 1.05 (0.72 to 1.53); 0% | 1.02 (0.69 to 1.51); 0% | 1.48 (0.36 to 6.17); 0% | 1.6 |
| Patient satisfaction | 3 | 1458 | 0.81 (0.50 to 1.29); 40% | 1.15 (0.22 to 6.17); 62% | 0.91 (0.55 to 1.51); 40% | 1.7 |
| Recovery day 28b | 5 | 2324 | 1.02 (0.79 to 1.32); 0% | ‐ | ‐ | 1.8 |
Abbreviations: CI: confidence interval; RR: risk ratio
aWhen I² > 40%, separate analyses of individually and cluster‐randomised trials are presented. bDefined as at least substantial improvement.
10. Procalcitonin ‐ summary of secondary outcomes.
| Outcome | Studies | Participants | RR (95% CI) | Analysis |
| Reconsultations day 28 | 1 | 317 | 1.00 (0.69 to 1.46) | 2.5 |
| Hospital admissions | 1 | 277 | 1.40 (0.26 to 7.51) | 2.6 |
| Patient satisfaction | 1 | 308 | 0.96 (0.93 to 1.00) | 2.7 |
Abbreviations: CI: confidence interval; RR: risk ratio
Procalcitonin
As only one included study investigated the effect of procalcitonin, we did not carry out any sensitivity analysis evaluating procalcitonin as point‐of‐care biomarker.
7. Funnel plot
C‐reactive protein
The funnel plot (Figure 6) refers to the primary outcome of number of participants given an antibiotic prescription. It may be slightly asymmetrical, indicating a possible risk of publication bias which might be due to a lack of smaller, negative studies. Furthermore, the smaller included studies were cluster‐RCTs (Andreeva 2013; Boere 2021; Cals 2009; Schot 2018), with lower methodological rigour (Page 2016).
6.

Funnel plot of risk ratio (RR) from included trials with their standard error (SE) values on a logarithmic scale.
Procalcitonin
As only one included study investigated the effect of procalcitonin, we did not carry out a funnel plot for this study.
Discussion
Summary of main results
We identified seven new studies for this update (Althaus 2019; Boere 2021; Butler 2019; Do 2016; Lhopitallier 2021; Little 2019; Schot 2018), for a total of 13 RCTs with 10,535 participants. Overall, the included studies were at unclear or low risk of bias. However, all studies were open‐labelled, thereby introducing high risk of bias due to lack of blinding.
C‐reactive protein
We found 12 studies that investigated the use of C‐reactive protein point‐of‐care test in ARIs in primary care, and concluded that C‐reactive protein point‐of‐care tests in ARIs in primary care likely reduce the number of participants given an antibiotic prescription, and do not increase the number of participants in need of a reconsultation within 28 days follow‐up. Tests are also unlikely to affect patient safety with regard to clinical recovery within 7 or 28 days follow‐up. Furthermore, C‐reactive protein tests may not increase mortality within 28 days follow‐up, and are unlikely to increase hospital admissions within 28 days follow‐up.
All included studies consistently showed a point estimate indicating a reduction in the number of participants given an antibiotic prescription. However, the precise effect estimate should be interpreted with caution due to the considerable heterogeneity, which may be partly explained by differences in inclusion criteria and design amongst the included studies, and application of C‐reactive protein algorithms for initiating or withholding antibiotics in some trials only. We carried out subgroup analyses on the number of participants given an antibiotic prescription at index consultation for adults and children, which showed C‐reactive protein point‐of‐care tests in both adults and children in primary care likely reduce antibiotic prescriptions at index consultation.
Overall, the number of participants given an antibiotic prescription at 28 days follow‐up was reduced at a similar rate when using C‐reactive protein measurements to guide antibiotic treatment of ARIs in primary care.
Procalcitonin
We found only one study that investigated the use of procalcitonin point‐of‐care test in ARIs in primary care, and the evidence is very uncertain about the effect of procalcitonin for all primary and secondary outcomes.
Overall completeness and applicability of evidence
C‐reactive protein
In general, the available evidence supports the use of C‐reactive protein as a point‐of‐care test in children and adults to guide antibiotic prescriptions for ARIs in primary care. The included trials were from Europe, Russia, and Asia, which are considerably different in terms of antibiotic use, application of C‐reactive protein algorithms, and organisation of primary care. The studies had high levels of completeness of reporting for the primary outcomes (100%); however, secondary outcomes varied in completeness of reporting from moderate (56%) to high (89%). All results were reported as intention‐to‐treat.
Biological variation
Our meta‐analysis included both children and adults. We carried out a subgroup analysis on adults (11 studies, sample size 7883) and children (4 studies, sample size 2335). The respective effect estimates for children and adults both showed a reduction, thus our results support application of C‐reactive protein point‐of‐care tests in both adults and children to guide antibiotic prescription for ARIs in primary care.
The results of this review should not be generalised to include older adults above 80 years of age with severe comorbidities and/or immunocompromised patients, as this group of patients were excluded from the included studies or were underrepresented. It remains unclear how C‐reactive protein should be interpreted in people with severe comorbidities and immunodepression, and no specific algorithms are made for these groups.
Furthermore, this review encompassed different respiratory infections with varying anatomical localisation. We had to omit the pre‐planned subgroup analyses of serious infections and less serious infections, as most studies reported presence and type of symptoms rather than diagnoses or symptom severity. No new studies included in this update reported data for this comparison; however, C‐reactive protein point‐of‐care tests were found likely to reduce the number of participants given an antibiotic prescription for both upper and lower ARIs in older studies.
C‐reactive protein algorithms
The included studies used different algorithms for C‐reactive protein measurements based on different national guidelines or different available evidence. To some extent this can explain the observed heterogeneity. The C‐reactive protein algorithms affect both patient safety and the potential reduction in the number of patients given an antibiotic prescription, as does the a priori likelihood of antibiotic use in any given patient population. By including different algorithms in this review, we regard the findings as a 'proof of concept'; however, identification of an optimal algorithm was not possible.
In studies with specific algorithms (such as C‐reactive protein cut‐off < 20 mg/L for withholding antibiotic treatment), physicians were still able to overrule the algorithm based on clinical assessment. Studies report that variation in adherence to the C‐reactive protein algorithms was present. Do 2016 reported variation in adherence to algorithm across the two included study centres (4% of participants (3/75) in the intervention group in Sai Dong station, and 71% of participants (49/71) in the intervention group in Dong Da Station), suggesting that adherence to protocol was study site‐specific. Mean violation of algorithm in the included studies was 28.6% (range 20% to 35%). This will also be present in practice, as a physician will have to interpret findings as an adjunct to medical history and clinical examination.
Variation in adherence
Two studies had issues regarding adherence to the protocol (Little 2019; Schot 2018). Schot 2018 reported violations to protocol, as one in five (18%) participants randomised to the control group where tested for C‐reactive protein. Little 2019 reported a low (6%) usage of C‐reactive protein measurements in the intervention group. Both studies found a non‐significant reduction in the number of participants given an antibiotic prescription. Little 2019 also demonstrates what may occur when introducing a point‐of‐care test in practice. Initial introduction including training in use and interpretation of C‐reactive protein tests produced a reduction (RR 0.69, 95% CI 0.61 to 0.77) in the number of participants given an antibiotic prescription (Little 2013a). However, subsequent follow‐up without further enforcement, training, or incentives to use the newly acquired skills may lead to discontinuation of the intervention and loss of its initial effect (Little 2019). These interpretations, suggesting a continued support to clinicians upon introducing new interventions, may be relevant to consider if implementing C‐reactive protein testing on a wider scale (Damschroder 2009).
Economic aspects on tests may also be a concern with regard to the implementation of C‐reactive protein tests. Point‐of‐care tests are easily accessible, but kits can be expensive, which may be a barrier for implementation of C‐reactive protein test for primary care in low‐ and middle‐income countries.
Procalcitonin
Biological variation
The study investigating the use of procalcitonin point‐of‐care test in ARI in primary care only included adults, therefore we have no evidence with regards to children. We are uncertain of the effect of procalcitonin on adults due to the level of the evidence.
Algorithms
The algorithm used in the study was to prescribe antibiotics when procalcitonin was > 0.25 μg/L. This cut‐off point was made based on previous RCTs (Briel 2008b; Burkhardt 2010b; Schuetz 2009). The study carried out a per‐protocol characteristics of participants having received antibiotics between day 1 and 28 despite procalcitonin < 0.25 μg/L at day 0. Only presence of chest pain and longer duration of symptoms were associated with antibiotic prescription despite procalcitonin < 0.25 μg/L at day 0.
Variation in adherence
There was no protocol violation with regard to adherence to protocol in the usual care group in the study. There were deviations in 8% in the procalcitonin group, meaning that 8% did not have procalcitonin measured.
Economic aspects on procalcitonin may also be a concern with regard to the implementation of procalcitonin point‐of‐care tests for primary care in low‐ and middle‐income countries.
Procalcitonin as point‐of‐care test provides results in 20 minutes. This may also be a concern with regard to implementation of the test in primary care.
Certainty of the evidence
C‐reactive protein
The overall certainty of the evidence was moderate to high according to our GRADE assessment, except for mortality, which we downgraded by two levels due to substantial imprecision (Table 1). Further research is unlikely to alter our overall conclusions, but may change the effect estimates due to the current heterogeneity.
Considerable heterogeneity in the pooled analysis of the number of participants given an antibiotic prescription at the index consultation may well be explained by differences in study setting (geographical locations and different primary care settings), as well as the application of different C‐reactive protein algorithms (Table 8). However, we downgraded the certainty of the evidence due to the considerable, unexplained heterogeneity.
We downgraded the certainty of the evidence for the outcomes total mortality and hospital admissions within 28 days follow‐up due to imprecision. We chose to downgrade hospital admissions by one level, but downgraded mortality by two levels as there were few events and wide confidence intervals, where the upper limit of the confidence interval could in theory include clinically meaningful differences. The low event rates for these safety outcomes prevented us from drawing any firm conclusions, although it seems unlikely that the intervention substantially increases mortality or hospital admission based on the available data.
Clinical recovery rates were participant‐reported outcomes, and we chose to downgrade the certainty of the evidence to moderate due to lack of blinding of participants. The rating of high risk of bias will likely not be changed by future research, as blinding is impossible due to the nature of the intervention. We chose not to downgrade for missing data on recovery, despite the fact that our worst‐case sensitivity analysis showed differences in favour of standard care. The worst‐case analysis might not be the best way to handle missing data (Higgins 2008), but this specific analysis does remind us to be aware of missing data, which could potentially change the overall effect estimate on recovery.
Procalcitonin
As already mentioned, the evidence is very uncertain about the effect of procalcitonin on all primary and secondary outcomes. More studies are needed to evaluate the use of procalcitonin point‐of‐care tests in ARIs in primary care.
We downgraded the certainty of the evidence for the use of procalcitonin in general because we identified only one study, with a small study sample. We chose to downgrade two levels due to imprecision. Another reason for downgrading the certainty of the evidence for the use of procalcitonin in ARI in primary care was indirectness, as only adults were included. This could be discussed, but we are interested in the effect of the use of procalcitonin in the population in primary care, not only in adults.
We chose to downgrade the certainty of the evidence by one level due to study limitations, because the study was a cluster‐randomised trial. Only one study was included, and we are uncertain whether individually randomised trials will provide the same effect estimate as this cluster‐randomised trial.
We were unable to assess the certainty of the evidence with regard to inconsistency of results, as only one study was included.
Potential biases in the review process
To the best of our knowledge, no bias was introduced in the review process.
Agreements and disagreements with other studies or reviews
Three systematic reviews studied the evidence for C‐reactive protein point‐of‐care tests to guide antibiotic prescription in primary care (Engel 2011; Huang 2013; Martínez‐González 2020). Engel 2011 concluded that the current evidence does not support the use of C‐reactive protein in primary care for this purpose. However, no meta‐analyses were performed, with no reason for this provided. Huang 2013, like us, reported a reduction in the number of participants given an antibiotic prescription for ARIs (RR 0.75, 95% CI 0.67 to 0.83), but with considerable heterogeneity (I² = 76%). However, the main meta‐analysis included both RCTs and observational studies. Both Engel 2011 and Huang 2013 did not include the eight latest trials, which added 8785 participants (Althaus 2019; Andreeva 2013; Boere 2021; Butler 2019; Do 2016; Little 2013a; Little 2019; Schot 2018).
A more recent systematic review reported a reduction in the number of participants given an antibiotic prescription in ARI (RR 0.79, 95% CI 0.79 to 0.90), also with considerable heterogeneity (I² = 76%) (Martínez‐González 2020). The review included five studies that we chose to exclude (Gonzales 2011; Rebnord 2016b; Takemura 2005; Van den Bruel 2016; Verbakel 2016). Gonzales 2011 was conducted in an emergency department, whereas our review focused on primary care. Three trials included participants with acute illness (fever) that were not specifically ARIs, which were the conditions of interest in our review (Rebnord 2016b; Takemura 2005; Van den Bruel 2016). We excluded Verbakel 2016 as this study had no usual care group and carried out a more complex intervention. Martínez‐González 2020 also conducted a subgroup analysis comparing age groups, but only found a likely reduction in the number of participants given an antibiotic prescription amongst adults (n = 3729), not children (n = 2755). Martínez‐González 2020 detected greater heterogeneity in the subgroup analysis of children (I² = 67% versus I² = 45% in our subgroup analysis), and some included children (n = 1684) had acute illness rather than ARIs (Van den Bruel 2016; Verbakel 2016). Another concern was the study by Rebnord 2016b, which combined two settings, an out‐of‐hours primary care clinic and a paediatric emergency department.
Two systematic reviews studied the evidence of point‐of‐care tests in ambulatory care (Van Hecke 2020; Verbakel 2019). Both reviews included studies conducted not only in primary care but also emergency departments, ambulatory care, and outpatient clinics. The evidence for both reviews was in concordance to ours. Verbakel 2019 reported a likely reduction of the number of participants given an antibiotic prescription (RR 0.81, 95% CI 0.71 to 0.92). When including guidance on antibiotic prescriptions in accordance to C‐reactive protein level, a further reduction was found (RR 0.68, 95% CI 0.63 to 0.74 in adults and RR 0.56, 95% CI 0.33 to 0.95 in children), also with substantial heterogeneity (I² = 72%). Van Hecke 2020 concluded that point‐of‐care C‐reactive protein tests may reduce immediate antibiotic prescribing amongst children by a third (risk difference ‐0.29, 95% CI ‐0.47 to ‐0.11; n = 2747) in ARI in low‐ and middle‐income (LMIC) countries. This difference was seen in RCTs in LMICs that included guidance on interpretation of point‐of‐care C‐reactive protein tests, specific training, or employed a diagnostic algorithm prior to point‐of‐care testing. Our subgroup analysis of children also found a more pronounced effect in LMIC countries (Althaus 2019; Do 2016). Together, these findings suggest that the impact of point‐of‐care C‐reactive protein tests is context‐specific. Settings with high baseline antibiotic prescription rates might benefit more from an implementation of point‐of‐care C‐reactive protein test than settings with low baseline antibiotic prescription rates. These observations likely contribute to the substantial heterogeneity detected.
According to Verbakel 2019 mortality was generally underreported, and this review called for safety assessments for implementing point‐of‐care C‐reactive protein test in ambulatory care. To our knowledge, no reviews and only a few randomised studies have assessed the use of point‐of care C‐reactive protein tests in primary care amongst older adults with frailty and severe comorbidities (Boere 2021; Butler 2019). This is a concern as frail older adults with comorbidity contributes with a high amount of consultations due to acute respiratory infection in primary care (Childs 2019; Cillóniz 2018; Stupka 2009). More studies are needed to assess the safety of using C‐reactive protein tests to guide antibiotic prescriptions in frail, multimorbid older adults in primary care.
One study systematically screened the evidence for interventions that reduce antibiotic prescriptions in primary care amongst people suffering from ARIs (Köchling 2018). The review included 17 studies, and concluded that communication skills training and point‐of‐care tests were the most effective interventions, in combination or alone. Observed reductions in antibiotic use ranged from 1.5% to 23.3% depending on intervention type (Köchling 2018). These findings are similar to ours, and some studies included in our meta‐analysis also compared communication training to C‐reactive protein point‐of‐care testing and showed similar potential to reduce antibiotic use, whilst an additive effect was observed when both C‐reactive protein tests and training sessions in communication skills were combined (Cals 2009; Little 2013a; Little 2019).
During the last decade, several cost‐effectiveness studies on C‐reactive protein have been conducted in European settings (Cals 2011; Fawsitt 2021; Hunter 2015; Lingervelder 2021; Oppong 2013). A majority of the studies concluded that C‐reactive protein testing increases healthcare costs. When calculating quality‐adjusted life‐years (QALYs), C‐reactive protein is cost‐effective alone or in combination with communication skills training, and is likely to provide a cost‐effective diagnostic intervention in terms of reducing prescription of antibiotics as well as in QALYs gained. To our knowledge, no cost‐effectiveness studies have included cost savings from potential reduction of antibiotic resistance, as no generally accepted threshold of a particular quantum of antibiotic prescribing has been accepted as cost‐effective.
C‐reactive protein guidance in primary care has primarily been implemented in high‐income countries (NICE Clinical Guidelines 2019; Woodhead 2011), even though evidence suggests that settings with high baseline antibiotic prescription rates might benefit more from an implementation of point‐of‐care C‐reactive protein test (Van Hecke 2020). Furthermore, no specific C‐reactive protein cut‐off threshold exists. European guidelines follow recommendations to delay prescription of antibiotics when C‐reactive protein levels are < 20 mg/L and prescribe when > 100 mg/L (NICE Clinical Guidelines 2019; Woodhead 2011), although several studies suggest that different thresholds are needed depending on age (Nouvenne 2016; Van den Bruel 2011; Van Hecke 2020; Verbakel 2019). Studies have reported that the C‐reactive protein test may not be sufficiently sensitive and specific to be of diagnostic value in primary care, where the incidence of serious bacterial infection is low (Falk 2009; van der Meer 2005). Still, it may be used as a prognostic marker to determine the likelihood of a non‐serious infection. Low C‐reactive protein levels may assist in ruling out serious bacterial infections and withhold unnecessary antibiotic treatments that are not likely to benefit the patient. Of note, C‐reactive protein is not a perfect test and should only be used in the relevant clinical context, as risks exist for over‐ as well as undertreatment with antibiotics.
C‐reactive protein is the only point‐of‐care test widely used in primary care. However, procalcitonin, another biomarker of infection, has been developed as a point‐of‐care‐test. We only found one study carried out in primary care that investigated the use of procalcitonin point‐of‐care test to guide decisions about antibiotic treatment of ARIs. Primarily due to few participants, the certainty of evidence for procalcitonin as a test to guide antibiotic treatment is too low to permit any definitive conclusions. A Cochrane Review from 2017 suggests that procalcitonin is a safe and effective tool to guide decisions about antibiotic treatment of ARIs (Schuetz 2017), but only one out of the 17 studies included in Schuetz 2017 used procalcitonin as a point‐of‐care test, and the study was conducted in an emergency department. Safety aspects could not be assessed in primary care due to low mortality rates.
To our knowledge, no new biomarkers as point‐of‐care test have been evaluated in an RCT in primary care.
Authors' conclusions
Implications for practice.
Use of C‐reactive protein point‐of‐care tests as an adjunct to taking a medical history and clinical examination of patients with symptoms of acute respiratory infections (ARIs) likely reduces the number of participants given an antibiotic prescription in primary care, and is unlikely to affect recovery within 7 or 28 days follow‐up, or duration of the ARI. C‐reactive protein point‐of‐care tests may not increase mortality or risk of hospital admissions within 28 days follow‐up; however, event numbers for these outcomes were very low, thereby limiting the certainty of evidence.
Clinicians must be aware of the different algorithms/cut‐off points when using C‐reactive protein tests. C‐reactive protein values less than 20 mg/L suggest that antibiotics may be initially withheld, but this cut‐off is not currently validated in children and older adults with comorbidities.
At present, C‐reactive protein is the only biomarker available for point‐of‐care test in primary care settings, where evidence supports that it can guide antibiotic prescriptions for ARIs. Furthermore, C‐reactive protein point‐of‐care tests are applicable in primary care, as test results are provided within 2 to 3 minutes.
The evidence of is very uncertain about the effect of procalcitonin point‐of‐care test on the number of participants given an antibiotic prescription, recovery, mortality, and hospital admissions. Clinicians must also be aware that procalcitonin point‐of‐care tests currently provide results in 20 minutes. Time to results may therefore limit widespread implementation of this test in primary care.
Implications for research.
C‐reactive protein point‐of‐care tests in children and adults likely reduce the number of participants given an antibiotic prescription for ARIs in primary care. It is unlikely that further research will change our conclusion, although the size of the estimated effect may change.
C‐reactive protein point‐of‐care tests may not increase mortality within 28 days follow‐up, but this assessment was based on very few events and thus low‐certainty evidence. C‐reactive protein point‐of‐care tests are unlikely to affect hospital admissions within 28 days follow‐up. Studies that recorded deaths and hospital admissions were performed in children and older adults with comorbidities in low‐ and middle‐income countries. Future studies should focus on children, immunocompromised individuals, and people aged 80 years and older with comorbidities, and should assess mortality and hospital admission as critically important outcomes.
Furthermore, algorithms/cut‐off points for C‐reactive protein to rule out serious disease are not validated and were used differently in studies included in this review. Future studies are needed to validate C‐reactive protein algorithms/cut‐off points, with specific focus on potential age group differences.
The evidence of is very uncertain about the effect of procalcitonin point‐of‐care test, as only one study investigating procalcitonin point‐of‐care test was identified. More studies are needed to evaluate procalcitonin as well as potential new biomarkers as point‐of‐care tests used in primary care to guide antibiotic prescription.
What's new
| Date | Event | Description |
|---|---|---|
| 19 October 2022 | Amended | Review republished to correct author byline order. |
History
Protocol first published: Issue 10, 2012 Review first published: Issue 11, 2014
| Date | Event | Description |
|---|---|---|
| 14 June 2022 | New citation required but conclusions have not changed | The previous version of this review did not register any events of death and was not able to carry out an analysis on mortality. This update includes four studies with events, and we were able to carry out an analysis and added conclusions on mortality. We concluded that C‐reactive protein tests may not increase mortality, but because there were few events, the certainty of the effect estimate is low. The evidence is uncertain as to whether procalcitonin point‐of‐care tests affect primary and secondary outcomes, as data were sparse. More studies are needed to evaluate the use of procalcitonin on antibiotic prescriptions, recovery, hospital admission, and mortality. |
| 14 June 2022 | New search has been performed | We updated the searches for this review. We included seven new studies (Althaus 2019; Boere 2021; Butler 2019; Do 2016; Lhopitallier 2021; Little 2019; Schot 2018), and excluded 18 new studies (Ameyaw 2014; de Lusignan 2020; Eley 2020; Fiore 2017; Huang 2018; Isa 2022; Keitel 2017; Little 2014; Mann 2020; Meili 2016; Minnaard 2016; Montassier 2019; Oppong 2018; Rebnord 2016a; Schechter‐Perkins 2019; Stannard 2014; Van den Bruel 2016; Verbakel 2016). We identified five ongoing studies (ISRCTN01559032; NCT03540706; NCT03855215; NCT03931577; NCT04216277). |
Acknowledgements
For the previous version of this review, we are grateful for the help provided by Liz Dooley, Managing Editor. We also wish to thank Sarah Thorning from the Cochrane Acute Respiratory Infections Group for assistance with the electronic searches in the previous review, and Marta Perpiñan for assistance with the electronic searches for this updated review. We also wish to thank the following people for commenting on the draft protocol and the previous review: Amanda Roberts, Janet Wale, Anette Holm, Renato Seligman, Robert Ware, Mark Jones, and Inge Axelsson. We wish to thank Jens‐Ulrik S Jensen, Asbjørn Hrobjartsson, and Lars Bjerrum for contributing to the first review.
The following people conducted the editorial process for this review update.
Sign‐off Editors (final editorial decision): Mark Jones (Bond University, Australia); Mieke van Driel (The University of Queensland, Australia).
Managing Editors (provided editorial guidance to authors, edited the review, selected peer reviewers, and collated peer‐reviewer comments): Liz Dooley (Bond University, Australia); Fiona Russell (Bond University, Australia).
Contact Editor (assessed peer‐review comments and recommended an editorial decision): Tom Fahey (HRB Centre for Primary Care Research, Department of General Practice, Royal College of Surgeons in Ireland).
Statistical Editor (provided comments): Teresa Neeman, PhD statistics, AStat (Biology Data Science Institute, Australian National University).
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Copy Edit Support.
Peer reviewers (provided comments and recommended an editorial decision):
Clinical/content review: Sandra Arnold, MD, MSc (University of Tennessee Health Science Center Le Bonheur Children’s Hospital, Memphis, TN, USA).
Consumer review: Amanda Roberts; Janet Wale (independent consumer representative).
Methods review: Rachel Richardson (Associate Editor, Cochrane, UK).
Search review: Justin Clark (Institute for Evidence‐Based Healthcare, Bond University, Australia); Ina Monsef (Cochrane Haematology, Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany).
Appendices
Appendix 1. CENTRAL and MEDLINE (Ovid) search strategy
1 exp Respiratory Tract Infections/ 2 (respiratory* adj3 (inflam* or infect*)).tw. 3 (ari or urti or lrti).tw. 4 (pneumon* or bronchopneumon* or pleuropneumon*).tw. 5 exp Otitis media/ 6 (otitis media or aom).tw. 7 (bronchit* or bronchiolit*).tw. 8 (pharyngit* or laryngit* or tonsillit* or sore throat* or cough*).tw. 9 (nasopharyngit* or rhinopharyngit*).tw. 10 (sinusit* or rhinosinusit* or nasosinusit*).tw. 11 (common cold* or coryza).tw. 12 ((acute or viral or bacter*) adj2 rhinit*).tw. 13 (influenza* or flu or ili).tw. 14 (severe acute respiratory syndrome or sars).tw. 15 croup.tw. 16 Pulmonary Disease, Chronic Obstructive/ 17 ((acute or exacerbation*) adj3 (copd or coad or chronic obstructive pulmonary disease or chronic obstructive airway* disease or chronic obstructive lung disease)).tw. 18 or/1‐17 19 Point‐of‐Care Systems/ 20 (("point of care" or "point‐of‐care" or "near patient" or poc or rapid or bedside) adj5 (test* or analys* or immunoassay* or technique* or immunofluorescence or "fluorescent antibody" or fluorescent antibodies)).tw. 21 exp Biological Markers/ 22 (biomarker* or (biological adj3 (marker* or indicator*))).tw. 23 Calcitonin/ 24 calcitonin*.tw,nm. 25 procalcitonin*.tw,nm. 26 pct.tw. 27 C‐Reactive Protein/ 28 (c reactive protein or c‐reactive protein or C‐reactive protein).tw,nm. 29 exp Leukocyte Count/ 30 ((leucocyte or leukocyte) adj1 count*).tw. 31 (neutrophil* adj1 count*).tw. 32 (white blood cell count* or wbc or wbcc).tw. 33 or/19‐32 34 18 and 33 35 exp Anti‐Bacterial Agents/ 36 antibiotic*.tw. 37 exp Penicillins/ 38 penicillin*.tw. 39 exp Macrolides/ 40 macrolide*.tw,nm. 41 exp Amoxicillin/ 42 (amoxicillin* or amoxycillin*).tw,nm. 43 amoxacillin*.tw,nm. 44 exp Tetracyclines/ 45 tetracycline*.tw,nm. 46 exp Quinolones/ 47 quinolone*.tw,nm. 48 ciprofloxacin*.tw,nm. 49 exp Ciprofloxacin/ 50 or/35‐49 51 34 and 50
Appendix 2. Embase (Elsevier) search strategy
#47 #38 AND #46 #46 #41 NOT #45 #45 #42 NOT #44 #44 #42 AND #43 #43 'human'/de #42 'animal'/de OR 'animal experiment'/de OR 'nonhuman'/de #41 #39 OR #40 #40 random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti #39 'randomised controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #38 #20 AND #32 AND #37 #37 #33 OR #34 OR #35 OR #36 #36 penicillin*:ab,ti OR macrolide*:ab,ti OR amoxacillin*:ab,ti OR amoxicillin*:ab,ti OR amoxycillin*:ab,ti OR tetracycline*:ab,ti OR quinolone*:ab,ti OR ciprofloxacin*:ab,ti #35 'quinolone derivative'/exp OR 'ciprofloxacin'/exp #34 antibiotic*:ab,ti #33 'antibiotic agent'/exp #32 #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 #31 'neutrophil count':ab,ti #30 'white blood cell count':ab,ti OR wbc:ab,ti OR wbcc:ab,ti #29 ((leukocyte OR leucocyte) NEXT/1 count*):ab,ti #28 'leukocyte count'/exp #27 'c reactive protein':ab,ti OR 'c‐reactive protein':ab,ti OR C‐reactive protein:ab,ti #26 'c reactive protein'/de #25 procalcitonin:ab,ti OR calcitonin:ab,ti OR pct:ab,ti #24 'procalcitonin'/de #23 biomarker*:ab,ti OR (biological NEAR/2 (marker* OR indicator*)):ab,ti #22 'biological marker'/de OR 'pharmacological biomarker'/de #21 (('point of care' OR 'point‐of‐care' OR 'near patient' OR poc OR rapid OR bedside) NEAR/5 (test* OR analys* OR immunoassay* OR technique* OR immunofluores* OR 'fluorescent antibody' OR 'fluorescent antibodies')):ab,ti #20 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 #19 ((acute OR exacerbation*) NEAR/3 (copd OR coad OR 'chronic obstructive pulmonary disease' OR 'chronic obstructive airways disease' OR 'chronic obstructive lung disease')):ab,ti OR aecb:ab,ti #18 'chronic obstructive lung disease'/de #17 croup:ab,ti #16 'severe acute respiratory syndrome':ab,ti OR sars:ab,ti #15 influenza*:ab,ti OR flu:ab,ti OR ili:ab,ti #14 ((acute OR viral OR bacter*) NEAR/2 rhinit*):ab,ti #13 'common cold':ab,ti OR 'common colds':ab,ti OR coryza:ab,ti #12 rhinosinusit*:ab,ti OR nasosinusit*:ab,ti #11 nasopharyngit*:ab,ti OR rhinopharyngit*:ab,ti #10 'sore throat'/de #9 pharyngit*:ab,ti OR laryngit*:ab,ti OR tonsillit*:ab,ti OR 'sore throat':ab,ti OR 'sore throats':ab,ti OR cough* #8 bronchit*:ab,ti OR bronchiolit*:ab,ti #7 'otitis media':ab,ti OR aom:ab,ti #6 'otitis media'/de OR 'acute otitis media'/exp #5 pneumon*:ab,ti OR bronchopneumon*:ab,ti OR pleuropneumon*:ab,ti #4 ari:ab,ti OR urti:ab,ti OR lrti:ab,ti #3 (respiratory NEAR/2 (infect* OR inflam*)):ab,ti #2 'respiratory tract inflammation'/exp #1 'respiratory tract infection'/exp
Appendix 3. CINAHL (EBSCO) search strategy
S49 S38 and S48 S48 S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 S47 (MH "Quantitative Studies") S46 TI placebo* OR AB placebo* S45 (MH "Placebos") S44 (MH "Random Assignment") S43 TI random* OR AB random* S42 TI ( (singl* or doubl* or tripl* or trebl*) N1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) N1 (blind* or mask*)) S41 TI clinical* trial* OR AB clinical* trial* S40 PT clinical trial S39 (MH "Clinical Trials+") S38 S18 and S32 and S37 S37 S33 or S34 or S35 or S36 S36 TI (penicillin* or macrolide* or amoxicillin* or amoxycillin* or amoxacillin* or tetracyclin* or quinolon* or ciprofloxacin*) OR AB (penicillin* or macrolide* or amoxicillin* or amoxycillin* or amoxacillin* or tetracyclin* or quinolon* or ciprofloxacin*) S35 (MH "Antiinfective Agents, Quinolone+") S34 TI antibiotic* OR AB antibiotic* S33 (MH "Antibiotics+") S32 S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 S31 TI neutrophil* N1 count* OR AB neutrophil* N1 count* S30 TI ("white blood cell count" or wbc or wbcc) OR AB ("white blood cell count" or wbc or wbcc) S29 TI ( (leukocyte or leucocyte) N1 count*) OR AB ((leukocyte or leucocyte) N1 count*) S28 (MH "Leukocyte Count") S27 TI ("c reactive protein" or c‐reactive protein or C‐reactive protein) OR AB ("c reactive protein" or c‐reactive protein or C‐reactive protein) S26 (MH "C‐Reactive Protein") S25 TI (procalcitonin or calcitonin or pct) OR AB (procalcitonin or calcitonin or pct) S24 (MH "Calcitonin") S23 TI (biological N2 (marker* or indicator*)) OR AB (biological N2 (marker* or indicator*)) S22 TI biomarker* OR AB biomarker* S21 (MH "Biological Markers") S20 TI (("point of care" or point‐of‐care or poc or "near patient" or rapid or bedside*) N5 (test* or analys* or immunoass* or technique* or immunofluores* or "fluorescent antibody")) OR AB (("point of care" or point‐of‐care or poc or "near patient" or rapid or bedside*) N5 (test* or analys* or immunoass* or technique* or immunofluores* or "fluorescent antibody")) S19 (MH "Point‐of‐Care Testing") S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 S17 TI ((acute or exacerbation) N3 (copd or coad or chronic obstructive pulmonary disease or chronic obstructive airway* disease or chronic obstructive lung disease)) OR AB ((acute or exacerbation) N3 (copd or coad or chronic obstructive pulmonary disease or chronic obstructive airway* disease or chronic obstructive lung disease)) S16 (MH "Pulmonary Disease, Chronic Obstructive+") S15 TI croup OR AB croup S14 TI (severe acute respiratory syndrome or sars) OR AB (severe acute respiratory syndrome or sars) S13 TI (influenza* or flu or ili) OR AB (influenza* or flu or ili) S12 TI ((acute or viral or bacter*) N2 rhinit*) OR AB ((acute or viral or bacter*) N2 rhinit*) S11 TI (common cold* or coryza) OR AB (common cold* or coryza) S10 TI (sinusit* or rhinosinusit* or nasosinusit*) OR AB (sinusit* or rhinosinusit* or nasosinusit*) S9 TI (nasopharyngit* or rhinopharyngit*) OR AB (nasopharyngit* or rhinopharyngit*) S8 TI (pharyngit* or laryngit* or tonsillit* or sore throat* or cough*) OR AB (pharyngit* or laryngit* or tonsillit* or sore throat* or cough*) S7 TI (otitis media or aom) OR AB (otitis media or aom) S6 (MH "Otitis Media+") S5 TI (bronchit* or bronchiolit*) OR AB (bronchit* or bronchiolit*) S4 TI (pneumon* or bronchopneumon* or pleuropneumon*) OR AB (pneumon* or bronchopneumon* or pleuropneumon) S3 TI (ari or arti or urti or lrti) OR AB (ari or arti or urti or lrti) S2 TI (respiratory N3 (inflam* or infect*)) OR AB (respiratory N3 (inflam* or infect*)) S1 (MH "Respiratory Tract Infections+")
Appendix 4. Web of Science (Thomson Reuters) search strategy
| # 6 | 347 | #5 AND #4 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
| # 5 | 1,313,934 | Topic=(random* or rct or placebo* or allocat* or crossover* or "cross over" or "clinical trial" or ((singl* or doubl*) NEAR/1 blind*)) OR Title=(trial) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
| # 4 | 1,358 | #3 AND #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
| # 3 | 255,737 | Topic=(antibiotic* or penicillin* or macrolide* or amoxicillin* or amoxycillin* or amoxacillin* or tetracyclin* or quinolone* or ciprofloxacin*) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
| # 2 | 241,330 | Topic=(("point of care" or "point‐of‐care" or "near patient" or poc or rapid or bedside) NEAR/5 (test* or analys* or immunoassay* or technique* or immunofluorescence or "fluorescent antibody")) OR Topic=(biomarker* or (biological NEAR/3 (marker* or indicator*)) or calcitonin* or procalcitonin* or pct or "c‐reactive protein" or "c reactive protein" or C‐reactive protein or ((leukocyte or leucocyte or neutrophil* or "white blood cell" or wbc) NEAR/2 count*) or wbcc) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
| # 1 | 286,470 | Topic=((respiratory NEAR/3 (infect* or inflam*)) or pneumon* or broncopneumon* or pleuropneumon* or "otitis media" or bronchit* or bronchiolit* or pharyngit* or laryngit* or tonsilit* or "sore throat" or "sore throats" or cough* or nasopharyngit* or rhinopharyngit* or sinusit* or rhinosinusit* or nasosinusit* or "common cold" or "common colds" or coryza or ((acute or viral or bacter*) NEAR/2 rhinit*) or influenza* or flu or ili or "severe acute respiratory syndrome" or sars or croup) OR Topic=((acute or exacerbation*) NEAR/3 ("chronic obstructive pulmonary disease" or "chronic obstructive airway disease" or "chronic obstructive airways disease" or "chronic obstructive lung disease" or copd or coad)) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
Appendix 5. LILACS (BIREME) search strategy
> Search > (MH:"Point‐of‐Care Systems" OR "Bedside Testing" OR "Point of Care Technology" OR "Computación de Cabecera" OR "Diagnóstico de Cabecera" OR "Tecnología de Atención de Punto" OR "Computação Junto ao Leito" OR "Testes Junto ao Leito" OR "Tecnologia de Assistência" OR "Junto ao Leito" OR "point of care" OR "point‐of‐care" OR poc OR "near patient" OR bedside OR rapid OR immunoassay$ OR immunofluorescence OR "fluorescent antibody" OR MH:"Biological Markers" OR MH:D23.101$ OR "Marcadores Biológicos" OR "Biochemical Markers" OR "Clinical Markers" OR biomarker$ OR "biological marker" OR "biological markers" OR "biological indicator" OR "biological indicators" OR "Immunologic Markers" OR "surrogate markers" OR "viral markers" OR "Marcadores Bioquímicos" OR "Marcadores Clínicos" OR "Marcadores Inmunológicos" OR "Marcadores Sustitutos" OR "Marcadores Virales" OR "Marcadores Bioquímicos" OR "Marcadores Clínicos" OR "Marcadores Imunológicos" OR "Marcadores Substitutos" OR "Marcadores Séricos" OR "Marcadores Virais" OR MH:Calcitonin OR calcitonin$ OR procalcitonin$ OR pct OR MH:"C‐Reactive Protein" OR "c‐reactive protein" OR "c reactive protein" OR C‐reactive protein OR "Proteína C‐Reactiva" OR "Proteína C‐Reativa" OR MH:"Leukocyte Count" OR "white blood cell count" OR "leukocyte count" OR "leucocyte count" OR "Recuento de Leucocitos" OR "Contagem de Leucócitos" OR "Número de Leucocitos" OR "Recuento de Células Blancas Sanguíneas" OR "Número de Leucócitos" OR "Contagem de Células Brancas do Sangue" OR "neutrophil count" OR wbcc OR wbc) AND (MH:"Anti‐Bacterial Agents" OR antibiotic$ OR Antibacterianos OR MH:D27.505.954.122.085$ OR MH:Penicillins OR penicil$ OR MH:D02.065.589.099.750$ OR MH:D02.886.108.750$ OR MH:D03.438.260.825$ OR MH:D03.605.084.737$ OR MH:D04.075.080.875.099.221.750$ OR MH:Macrolides OR macrolid$ OR MH:D02.540.505$ OR MH:D02.540.576.500$ OR MH:D04.345.674.500$ OR MH:Amoxicillin OR amoxicil$ OR amoxacillin$ OR amoxycillin$ OR MH:D02.065.589.099.750.750.050.050$ OR MH:D02.886.108.750.750.050.050$ OR MH:D03.438.260.825.750.050.050$ OR MH:D03.605.084.737.750.050.050$ OR MH:D04.075.080.875.099.221.750.750.050.050$ OR MH:Tetracyclines OR tetracyclin$ OR Tetraciclin$ OR MH:D02.455.426.559.847.562.900$ OR MH:D04.615.562.900$ OR MH:Quinolones OR MH:quinolon$ OR MH:D03.438.810.835$ OR MH:Ciprofloxacin OR ciprofloxacin$ OR MH:D03.438.810.835.322.186$) > clinical_trials
Data and analyses
Comparison 1. C‐reactive protein versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 CRP ‐ Antibiotics prescribed at index consultation. All trials (cluster‐randomised with modified sample size) | 12 | 10218 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.69, 0.86] |
| 1.1.1 Individually randomised trials | 6 | 5899 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.70, 0.89] |
| 1.1.2 Cluster‐randomised trials (modified sample size) | 6 | 4319 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.58, 0.90] |
| 1.2 CRP ‐ Antibiotics prescribed within 28 days follow‐up (cluster‐randomised trials with modified sample size) | 7 | 5091 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.76, 0.86] |
| 1.2.1 Individually randomised trials | 5 | 4880 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.77, 0.87] |
| 1.2.2 Cluster‐randomised trials (modified sample size) | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.51, 0.91] |
| 1.3 CRP ‐ Number of participants substantially improved within 7 days follow‐up | 4 | 3104 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.12] |
| 1.3.1 Individually randomised trials | 4 | 3104 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.12] |
| 1.4 CRP ‐ Mortality (cluster‐randomised trials with modified sample size) | 9 | 7737 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.10, 2.92] |
| 1.4.1 Individually randomised trials | 5 | 5660 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.06, 4.76] |
| 1.4.2 Cluster‐randomised trials (modified sample size) | 4 | 2077 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.04, 7.97] |
| 1.5 CRP ‐ Number of reconsultations within 28 days follow‐up | 7 | 6256 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 1.5.1 Individually randomised trials | 3 | 4199 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.90, 1.89] |
| 1.5.2 Cluster‐randomised trials (modified sample size) | 4 | 2057 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 1.6 CRP ‐ Hospital admissions (cluster‐randomised with modified sample size) | 10 | 7514 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.72, 1.53] |
| 1.6.1 Individually randomised trials | 5 | 5350 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.69, 1.51] |
| 1.6.2 Cluster‐randomised trials (modified sample sizes) | 5 | 2164 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.36, 6.17] |
| 1.7 CRP ‐ Patient satisfaction | 3 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.50, 1.29] |
| 1.7.1 Individually randomised trials | 2 | 1334 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.22, 6.17] |
| 1.7.2 Cluster‐randomised trials (modified sample size) | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.55, 1.51] |
| 1.8 CRP ‐ Number of participants substantially improved within 28 days follow‐up (cluster‐randomised trials with modified sample size) | 5 | 2324 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.32] |
| 1.9 CRP ‐ Subgroup analysis: antibiotics prescribed at index consultation: upper respiratory tract infections and lower respiratory tract infections (cluster‐randomised trials with modified sample size) | 2 | 2024 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.63, 0.78] |
| 1.9.1 Upper respiratory tract infections | 2 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.90] |
| 1.9.2 Lower respiratory tract infections | 2 | 1514 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.62, 0.78] |
| 1.10 CRP ‐ Subgroup analysis: children and adults. Antibiotic prescribing at index consultation | 12 | 10218 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.70, 0.85] |
| 1.10.1 Children (cluster‐randomised trials with modified sample size) | 4 | 2335 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.91] |
| 1.10.2 Adults | 11 | 7883 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.68, 0.86] |
| 1.11 CRP ‐ Sensitivity analysis: participant recovery within 7 days follow‐up: missing data in CRP = not recovered | 4 | 3214 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.03, 1.20] |
| 1.12 CRP ‐ Sensitivity analysis: participant recovery within 28 days follow‐up: missing data in CRP = not recovered (cluster‐randomised trials with modified sample size) | 5 | 2506 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.01, 1.85] |
| 1.13 CRP ‐ Sensitivity analysis: recovery within 7 days follow‐up when algorithms provide clear cut‐offs to rule out (< 20 mg/L) | 2 | 1522 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.93, 1.12] |
| 1.14 CRP ‐ Sensitivity analysis: recovery within 28 days follow‐up when algorithms provide clear cut‐offs to rule out (< 20 mg/L) | 4 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.87, 1.39] |
| 1.15 CRP ‐ Sensitivity analysis: antibiotics prescribed, assessed ONLY at day 28 | 5 | 1364 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.88] |
| 1.15.1 Individually randomised trials | 3 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.70, 0.93] |
| 1.15.2 Cluster‐randomised trials (modified sample size) | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.08] |
| 1.16 CRP ‐ Sensitivity analysis: antibiotics prescribed, assessed WITHIN 28 days | 7 | 5091 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.72, 0.84] |
| 1.16.1 Individually randomised trials | 5 | 4880 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.73, 0.85] |
| 1.16.2 Cluster‐randomised trials (modified sample size) | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.51, 0.91] |
| 1.17 CRP ‐ Sensitivity analysis: recovery, assessed ONLY at day 7 | 3 | 1264 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.05] |
| 1.17.1 Individually randomised trials | 3 | 1264 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.05] |
| 1.18 CRP ‐ Sensitivity analysis: recovery, assessed WITHIN 7 days | 4 | 3169 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 1.18.1 Individually randomised trials | 4 | 3169 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 1.19 CRP ‐ Sensitivity analysis: recovery, assessed ONLY at day 28 | 4 | 2371 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.69, 1.26] |
| 1.20 CRP ‐ Sensitivity analysis: recovery, assessed WITHIN 28 days | 5 | 2422 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.25] |
| 1.21 CRP ‐ Sensitivity analysis: antibiotic prescribing when algorithms provide clear cut‐offs to rule out (< 20 mg/L) | 9 | 8341 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.65, 0.84] |
| 1.21.1 Individually randomised trials | 4 | 4222 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.66, 0.82] |
| 1.21.2 Cluster‐randomised trials (modified sample size) | 5 | 4119 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.56, 0.91] |
Comparison 2. Procalcitonin versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Procalcitonin ‐ Antibiotic prescribed at index consultation | 1 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.23, 0.44] |
| 2.2 Procalcitonin ‐ Antibiotic prescribed within 28 days follow‐up | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.44, 2.48] |
| 2.3 Procalcitonin ‐ Number of participants substantially improved within 7 days follow‐up | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.93, 1.62] |
| 2.4 Procalcitonin ‐ Mortality | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.5 Procalcitonin ‐ Number of reconsultations within 28 days follow‐up | 1 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.69, 1.46] |
| 2.6 Procalcitonin ‐ Hospital admissions | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.26, 7.51] |
| 2.7 Procalcitonin ‐ Patient satisfaction | 1 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 1.00] |
2.2. Analysis.

Comparison 2: Procalcitonin versus standard care, Outcome 2: Procalcitonin ‐ Antibiotic prescribed within 28 days follow‐up
2.4. Analysis.

Comparison 2: Procalcitonin versus standard care, Outcome 4: Procalcitonin ‐ Mortality
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Althaus 2019.
| Study characteristics | ||
| Methods | Non‐blinded, randomised controlled trial in 6 public primary care units in Thailand, 3 primary care clinics, and 1 outpatient department in Myanmar | |
| Participants |
Inclusion criteria Aged 1 year or older; a documented fever (defined as a tympanic temperature of > 37.5 °C according to WHO standards); or a chief complaint of fever (< 14 days), regardless of previous antibiotic intake, and comorbidities other than malignancies Exclusioncriteria Infants younger than 1 year; symptoms requiring hospital referral, defined as either impaired consciousness, inability to take oral medication, or convulsions; a positive malaria test; the main complaint being trauma or injury; suspicion of either tuberculosis, urinary tract infection, or local skin or dental abscess or infection; any symptom present for more than 14 days; any bleeding; and an inability to comply with the follow‐up visit at day 5 Included in this analysis 1905 participants with ARIs (1256 in the intervention group; 649 in the control group) |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein 3‐armed study: 1) usual care, 2) intervention group A: the result of C‐reactive protein measurement was communicated to the healthcare provider as low C‐reactive protein or high C‐reactive protein using cut‐off thresholds of 20 mg/L; 3) intervention group B: the result of C‐reactive protein measurement was communicated to the healthcare provider as low C‐reactive protein or high C‐reactive protein using cut‐off thresholds of 40 mg/L |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Starting date: June 2016 to August 2017 Contact information: Dr Yoel Lubell, Mahidol‐Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand yoel.lubell@ndm.ox.ac.uk Trial registration: NCT02758821 Funding: public funding (Wellcome Trust Institutional Strategic Support Fund grant (105605/Z/14/Z) and Foundation for Innovative New Diagnostics (FIND) funding from the Australian Government) Kit used: NycoCard II Reader, Axis Shield, Oslo, Norway CRP measurements: participants from the intervention groups had a capillary blood sample analysed for CRP on site by the study staff who used a CRP reader. A brief educational video on antimicrobial resistance and CRP was shown to participants in the intervention groups with the intention of ensuring patients’ understanding of the test. The participants were then provided with a card specifying whether their CRP concentrations were high or low in relation to their intervention group and referred to the healthcare provider. Usual care: healthcare providers were asked to manage febrile participants as per standard of care. A venous blood sample was collected by study staff, stored at 4 °C, and retrospectively tested for CRP concentrations. Adherence to C‐reactive protein suggested cut‐offs (20% of participants with C‐reactive protein < 20 mg/L were prescribed antibiotics) Researchers recruited 2410 participants with fever, of whom 803 participants were randomly assigned to CRP group A (threshold of 20 mg/L), 800 to CRP group B (threshold of 40 mg/L), and 807 to the control group. In this review we provide information on the number of participants with ARTIs, which are 630, 649, and 626, respectively; information provided by study authors. Regarding CRP thresholds, a higher proportion of participants with elevated C‐reactive protein concentrations were prescribed more antibiotics in the intervention groups than in the control group. Conversely, in participants with low C‐reactive protein concentrations, antibiotic prescription was lower in the intervention groups than in the control group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated individual randomisation 1:1:1 ratio carried out by the trial statistician (MM) using ralloc command in STATA version 14. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed, opaque envelopes used and opened sequentially on site after participants were enrolled. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions are targeted at the level of the general practitioner. Non‐blinded trial where personnel and participants were aware if the participant was in the intervention group or control group, though masked to allocation between the 2 intervention groups. |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on prescribing were recorded independently on site, and the outcome was assessed centrally. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | High risk | Assessment of secondary outcome data was carried out at follow‐up visit by non‐blinded research staff. Quote: “Secondary outcomes included the proportion of patients prescribed an antibiotic from day 0 to day 14 at the health facility. Clinical outcomes included patient self‐reported recovery at each follow‐up visit, duration and severity of symptoms, frequency of un‐ planned re‐consultation within the 14 days of follow‐up, temperature and CRP concentrations at day 5 as objective measures of clinical recovery, and occurrence of serious adverse events, defined as events requiring admission to hospital or death within 14 days of enrolment.” |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Immediate prescribed antibiotic 2410/2410 (100%) |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | Low risk | Information was complete for the antibiotic prescribing within the first 2 weeks but not fully complete for clinical recovery, 2300 for day 5 (95.4%), and 2311 for day 14 (95.9%). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are reported as per study protocol. |
| Other bias | Unclear risk | The authors mention a potential observation bias due to the presence of research staff and a possible contamination effect on prescribing the control group. |
Andreeva 2013.
| Study characteristics | ||
| Methods | Non‐blinded cluster‐RCT, multicentre in 8 general practice offices with a total of 18 doctors in Arkhangelsk and Murmansk regions, Russia | |
| Participants |
Inclusion criteria Adult participants (> 18 years) with index case of lower respiratory tract infection/acute cough for less than 28 days Exclusion criteria Previously seen by GP for infection in question, immunocompromised status, ongoing treatment with oral corticosteroids Included in this analysis 179 (48% and 39% were upper respiratory tract infections in intervention and control arm) (number tested for eligibility not stated) |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein Algorithm used in this study: C‐reactive protein level < 20 mg/L, antibiotics usually not needed. C‐reactive protein levels > 50 mg/L, antibiotic prescribing could be indicated taking into account the duration of illness. Doctors were given training sessions in lower respiratory tract infection/acute cough and C‐reactive protein testing. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Clinical trials reg. NCT01794819 Clinical recovery was assessed with a 5‐point scale at a follow‐up visit after 14 days. Adherence to C‐reactive protein suggested cut‐offs (28% of participants with C‐reactive protein < 20 mg/L were prescribed antibiotics) Before‐and‐after study was simultaneously performed and used as a sensitivity analysis. Funding: none stated. Kits were provided by manufacturer, and C‐reactive protein readers were acquired at a reduced price. Kit used: the Afinion test system (Axis‐Shield, Norway) Training sessions on the use of C‐reactive protein were given over 2 sessions including practical and theoretical information. A sample size calculation was performed: "The sample sizes were based on a hypothesis of 20% reduction in antibiotic prescribing in the intervention group compared to the control group." Power 90% and false‐positive difference < 5%. The sample size of 72 in each group was reached. 20 control participants from 2 GPs were excluded due to incomplete registrations. Intracluster coefficients were not provided. Symptom severity was similar between groups, but feeling unwell and experiencing interference with daily activities was more common in the intervention group. Wheeze and perceived patient preference for antibiotics occurred more often in the control group. The primary outcome was number of antibiotic prescriptions, and the study reported a significant reduction in the number of antibiotic prescriptions in the intervention arm at index consultation (37.6% versus 58.9%; P = 0.006) and after 14 days (40.6% versus 71.8%; P < 0.001). Also, the number of referrals for chest radiography was significantly lower in the C‐reactive protein group (P = 0.004). No difference was seen in reconsultation rates or the recovery rate between groups as determined on follow‐up at day 14 on a 5‐point scale (fully recovered; almost recovered; slightly improved; unchanged or worse). Sensitivity analysis performed as a before‐and‐after study with 11 of the 18 participating GPs found significant reductions due to introduction of C‐reactive protein testing. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomisation into 2 groups was performed with GPs as units. Allocation sequence was performed by computer‐generated numbers by second author. |
| Allocation concealment (selection bias) | Unclear risk | Individual participant allocation concealment was not performed as the unit of randomisation was doctors and/or practices. Quote "based on this list [of clusters] and using the allocations sequence, the first author assigned clusters to interventions." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions are targeted at the level of the GP. Cluster‐randomisation was performed at the GP office level. Non‐blinded trial where physicians and participants knew which treatment modality was used. |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing were obtained from medical records at end of study. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Unclear risk | Unclear who (GPs, clinic personnel, or study group) performed this assessment, but participant recovery was determined at a follow‐up consultation on day 14 using a 5‐item recovery scale |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Follow‐up for antibiotic prescribing and use 179/179 (100%) |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | Unclear risk | Recovery data were available in 176/179 (98%) on day 14. However, there are inconsistencies in Table 2, as % are calculated on enrolled patients and not on patients providing data. Data not reported for reconsultations in final publication but were provided in draft version of published paper. No data collected on patient satisfaction. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes and all secondary outcomes reported are in accordance with study protocol, but primary outcome not precisely defined (within 14 days). However, data from index consultation and after 14 days provided after correspondence with investigators. |
| Other bias | High risk | Risk of selection bias due to cluster‐randomised design. Baseline characteristics identified differences between groups. 20 participants (20%) were omitted postrandomisation from 2 GPs in the control arm due to incomplete case report forms. |
Boere 2021.
| Study characteristics | ||
| Methods | Non‐blinded cluster‐RCT carried out in 11 nursing homes in the Netherlands | |
| Participants |
Inclusion criteria All somatic, psychogeriatric, and short‐stay (geriatric rehabilitation and short‐term residential care) nursing home residents with a suspected lower respiratory tract infection, according to their physician’s assessment, were eligible for participation. Exclusioncriteria Current or recent (in the past week) infection or use of antibiotics, or a recorded statement to withhold antibiotic treatment Included in this analysis 241 participants (162 in the intervention group, 79 in the control group) |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein 2‐armed study: 1) usual care in 5 nursing homes; 2) use of C‐reactive protein point‐of‐care test in 6 nursing homes, based on available evidence and the current Dutch LRTI guideline recommendations: < 20 mg/L, 20 to 60 mg/L, and > 60 mg/L |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Starting date: 2018 to the end of March 2020 Contact information: LW van Buul l.vanbuul@amsterdamumc.nl, Department of General Practice & Old Age Medicine, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, location VU University Medical Center, Amsterdam, the Netherlands Trial registration: Netherlands Trial Register (trial No NL5054) Funding: Public. Grant from The Netherlands Organisation for Health Research and Development (ZonMw) Kit used: QuikRead Go C‐reactive protein, Aidian, Espoo, Finland Training session: experts from the primary care laboratory provided the technical training. The CRP POCT devices were installed to enable the trained group to immediately start familiarising themselves with CRP POCT in routine practice. During the trial, decisions on the use and interpretation of CRP POCT were informed by the medical training knowledge and guideline recommendations, but remained at the discretion of the physicians. Adherence to C‐reactive protein suggested cut‐offs (6.38% of participants with C‐reactive protein < 20 mg/L were prescribed antibiotics) A 2‐phase informed consent process was used. The first phase allowed all residents to opt out of participation. In the second phase, physicians asked for written informed consent only from patients who were eligible for participation, during or shortly after initial consultation. The physician asked the patient’s representative for consent if the patient definitely did not have decision‐making capacity. Deferred consent (informed consent requested after the use of CRP POCT) was obtained when the patient was critically ill or the patient’s representative was not available during the initial consultation. Intervention group doctors received a medical training session from the research team in the correct use and interpretation of C‐reactive protein, based on available evidence and the current Dutch LRTI guideline recommendations: < 20 mg/L, 20 to 60 mg/L, and > 60 mg/L. A select group of physicians and/or nurses from each nursing home received technical training in use of CRP POCT devices by experts from the primary care laboratory. The sample size calculation that adjusted for an intracluster correlation coefficient of 0.06 and resulted in a total of 671 participants The main analysis was performed using intention‐to‐treat analysis. Intracluster correlation coefficient was calculated (0.175), and sizes of clusters were 6 for intervention group and 5 for the usual care group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation procedure with a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Individual participant allocation concealment was not performed, as the unit of randomisation was nursing homes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions are targeted at the level of the general practitioner. Non‐blinded trial where participants and personnel knew if C‐reactive protein levels were used for guidance of antibiotic treatment |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing were obtained from case report forms. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | High risk | Outcome assessment carried out by healthcare personnel who were not blinded to intervention. Data on antibiotic prescribing were obtained from medical records at day 7 and day 21. |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Follow‐up for antibiotic prescribing was nearly complete: 241/242. |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | High risk | A total of 22 participants in the intervention group (13.6%) and 3 participants in the control group dropped out for recovery. Unbalanced dropout percentages in the 2 groups |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | High risk | Risk of selection bias present due to lack of individual randomisation. |
Butler 2019.
| Study characteristics | ||
| Methods | Non‐inferiority, open‐label, randomised controlled trial carried out in 86 general medical practices in the UK | |
| Participants |
Inclusion criteria 40 years of age or older; diagnosis of COPD in their primary care clinical record; presenting with an acute exacerbation of COPD with at least 1 of AECOPD criteria (with at least 1 of: increased dyspnoea, increased sputum volume, increased sputum purulence), between 24 hours and 21 days duration, and informed, written consent Exclusioncriteria Urgent hospital admission; had severe illness (e.g. suspected pneumonia, tachypnoea > 30 breaths per minute); had a concurrent infection at another site (e.g. urinary tract infection); had a past history of respiratory failure or mechanical ventilation; were currently taking antibiotics or had already taken antibiotics for this AECOPD; had an active inflammatory condition; had cystic fibrosis, tracheostomy, or bronchiectasis; were immunocompromised; pregnancy; previously participated in the study Included in this analysis 649 (325 in the intervention group and 324 in the control group) |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein 2‐armed study: 1) usual care; 2) use of C‐reactive protein point‐of‐care test Algorithm used in this study Participants with a CRP level lower than 20 mg/L, antibiotics are unlikely to be beneficial and usually should not be prescribed; for those with a CRP level from 20 to 40 mg/L, antibiotics may be beneficial, mainly if purulent sputum is present; and for those with a CRP level higher than 40 mg/L, antibiotics are likely to be beneficial |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Starting date: January 2015 to September 2017 Contact information: Christopher C. Butler, the Nuffield Department of Primary Care Health Sciences, University of Oxford, christopher.butler@phc.ox.ac.uk Trial registration: ISRCTN24346473 Public funding (funded by the National Institute for Health Research Health Technology Assessment Programme). Staff members at the GPs were trained in the use, care, and calibration of the devices by representative of Alere (at no cost to the trial or to the practices) or by members of the trial team. Quote: "The company had no role in the design of the trial; in the accrual, analysis, or interpretation of the data; or in the preparation of the manuscript.” Kit used: Afinion desktop devices for CRP point‐of‐care testing (Alere, now Abbott) Adherence to C‐reactive protein suggested cut‐offs (32.8% of participants with C‐reactive protein < 20 mg/L were prescribed antibiotics) Sample size calculation: Quote ”… to detect a 15% absolute reduction from an estimated 70% of patients consuming antibiotics for AECOPD during the four weeks following randomization. Detecting a difference in proportions between 0.70 and 0.55 at the 5% significance level and with 90% power requires a total of 434 participants, inflated to 544 to account for loss 20% to follow‐up.” Clinicians were provided with guidance on the interpretation of CRP test results emphasising that decisions about antibiotic prescribing should be based on a comprehensive assessment of likely risks and benefits, given a patient’s underlying health status and clinical features. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated individual randomisation 1:1:1 ratio carried out by the Centre for Trials Research at Cardiff University. The number of Anthonisen criteria was used as a minimisation variable with a random element set at 80%. |
| Allocation concealment (selection bias) | Low risk | From the published protocol: "Remote allocation will maintain allocation concealment from both the participant and the recruiting clinician up to the point of intervention, as this is an open study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions are targeted at the level of the GP. Non‐blinded trial where participants and personnel knew if C‐reactive protein levels were used for guidance of antibiotic treatment. |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing were obtained from case report forms registered by the clinicians after randomisation. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | High risk | Secondary variables were registered by the clinicians themselves after randomisation on the case report form. Follow‐up visits were carried out non‐blinded by clinicians. |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Immediate prescribed antibiotic 648/649 Quote: ”The antibiotic prescribing decisions made by clinicians at the initial consultation were ascertained for all but 1 patient, and antibiotic prescriptions issued over the first 4 weeks of follow‐up were ascertained for 96.9% of the patients.” |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | High risk | Follow‐up data on reconsultation and recovery were 282/325 (86.7%) in the intervention group and 283/324 (87.3%) in the control group. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | ‐ |
Cals 2009.
| Study characteristics | ||
| Methods | Non‐blinded, cluster‐randomised (practice level) clinical trial, multicentre in 20 primary care practices in the Netherlands | |
| Participants |
Inclusion criteria Adults (> 18 years) with suspected lower respiratory tract infection (cough < 4 weeks, + 1 focal and + 1 systemic symptom or sign) Exclusion criteria Aged under 18 years, current antibiotic use or usage within previous 2 weeks. Hospitalisation in past 6 weeks, non‐fluent in Dutch, previous participation in the study and the need for immediate hospitalisation Included in this analysis 431 participants with lower respiratory tract infection, 110 C‐reactive protein; 84 communication skills training; 117 C‐reactive protein + communication skills training; 120 control. Total of 227 C‐reactive protein group versus 204 no‐test group |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein, communication skills training, or a combination thereof Algorithm used in this study: recommended cut‐off values: participants with C‐reactive protein levels lower than 20 mg/L: bacterial infection was considered highly unlikely and antibiotic prescribing was discouraged. Participants with C‐reactive protein levels higher than 100 mg/L: bacterial infection was considered likely and immediate antibiotic prescribing was recommended. Participants with C‐reactive protein levels between 20 and 99 mg/L: delayed prescribing was recommended. Physician could deviate from algorithm at any time. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Trial registration: ISRCTN85154857 Cluster‐randomised at practice level as general practitioners trained in communication skills were unable to shift at random between using new skills and usual care 8‐week run‐in to enable familiarisation Participant diary to assess clinical recovery Funding: public Kit used: Nycocard II Reader (Axis‐Shield, Norway) 4 groups were compared: C‐reactive protein testing (1), communication training (2), communication training and C‐reactive protein testing (3) and usual care (4). A factorial analysis plan was prespecified: C‐reactive test (cells 1 + 3) compared with no test (cells 2 + 4) whilst controlling for the effect of communication training. No statistically significant interaction (P = 0.78) was found between the interventions. Half an hour of guidance and training on the use of C‐reactive protein testing in the consultation was given by the study team, including C‐reactive protein cut‐off values for recommending or withholding antibiotic treatment. An 8‐week run‐in period to ensure familiarisation with the C‐reactive protein test was done prior to recruitment. Sample size calculations allowed for detection of a reduction in antibiotic use from 80% to 60% (power 80%, follow‐up 90%), and target inclusion (400) was reached. Clinical recovery was assessed by a 28‐day diary (on day 4, 14, and 28 a postcard or telephone reminder was sent to ensure completion of diaries). Primary analysis was intention‐to‐treat. A significantly reduced use of antibiotics was found in the C‐reactive protein group at index consultation (RR 0.58, 95% CI 46 to 0.74) and day 28 (RR 0.77, 95% CI 0.64 to 0.93). No difference in patient recovery rate was observed at day 7 or day 28. Participant satisfaction and number of reconsultations was comparable between groups. Intracluster coefficients provided. Significant differences between auscultation abnormalities in the 2 groups. Sensitivity analysis showed no differences in previous antibiotic treatment of subsample (14 general practitioners), but participants enrolled in study were younger than registered patients. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices were randomised by a computer program balancing for recruitment potential. Random permuted blocks of 4 were generated. |
| Allocation concealment (selection bias) | Unclear risk | Individual participant allocation concealment was not performed as the unit of randomisation was doctors or practices, or both. No information on how doctors were allocated into the generated groups was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions are targeted at the level of the general practitioner. Cluster‐randomised at practice level as general practitioners trained in communication skills were unable to shift at random between using new skills and usual care Non‐blinded trial where physicians and participants knew which treatment modality was used |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing and reconsultations were obtained from medical records after 28 days. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Data on antibiotic prescribing were obtained from medical records at day 28. Participant reminders (phone or mail) to complete the diary were sent on day 4, 14, 28. |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Follow‐up for antibiotic prescribing: C‐reactive protein versus control 431/431 (100%) |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | Low risk | Participant recovery assessed as median scores of illness duration and median daily symptom scores provided, but it was not possible to calculate substantial improvement at specific time points. Follow‐up for reconsultations and participant satisfaction ranged from 88% to 93%. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | High risk | Risk of selection bias due to lack of individual randomisation |
Cals 2010.
| Study characteristics | ||
| Methods | Open randomised clinical trial, multicentre in 11 primary care practices in the Netherlands | |
| Participants |
Inclusion criteria Adult (> 18 years) with index case of:
Exclusion criteria Aged under 18 years, antibiotic use or hospitalisation within the previous 14 days, non‐fluent in Dutch, immunocompromised status or need for immediate hospitalisation Included in this analysis 258 (107 lower respiratory tract infection, 151 rhinosinusitis) out of 258 randomised participants (tested for eligibility 270). Follow‐up 100% on primary outcome |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein Algorithm used in this study: recommended cut‐off values: participants with C‐reactive protein levels lower than 20 mg/L: bacterial infection was considered highly unlikely and antibiotic prescribing was discouraged. Participants with C‐reactive protein levels higher than 100 mg/L: bacterial infection was considered likely and immediate antibiotic prescribing was recommended. Participants with C‐reactive protein levels between 20 and 99 mg/L: delayed prescribing was recommended Physician could deviate from algorithm at any time. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Netherlands national trials register (NTR 1112) Intention‐to‐treat analysis Funding: Orion Diagnostica Espoo, Finland The C‐reactive protein test was performed by nurses and made available to the general practitioner to be used in addition to clinical assessment. Practice nurses were instructed in the use of C‐reactive protein testing, and a 30‐minute practice‐based seminar on the use of C‐reactive protein and C‐reactive protein cut‐off values for immediate antibiotics, delayed antibiotics, or withhold antibiotic treatment was given by the study team. A 4‐week run‐in period was done prior to start of inclusion to allow for familiarisation with the C‐reactive protein test. A sample size calculation was performed to allow detection of a 20% reduction with a power of 80%, allowing for a 5% loss to follow‐up, resulting in a total of 200 participants to be recruited. Clinical recovery was measured by a patient diary to be completed for the first 7 days. Participants not recovered by day 7 were followed up by phone interview on day 14 or 28. Study results indicated a significant reduction in the number of antibiotic prescriptions in the C‐reactive protein group at index consultation (RR 0.77, 95% CI 0.56 to 0.98) and at 28 days (RR 0.81, 95% CI 0.62 to 0.99). This effect was primarily due to fewer fillings of delayed prescriptions. Clinical recovery rates were similar across groups. Patient satisfaction was more pronounced in the C‐reactive protein group (P = 0.03). Sensitivity analysis was performed to evaluate clustering by way of a multilevel analysis. The effect size remained significant. Baseline characteristics were balanced. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by "remote independent research team, using permuted block randomisation to ensure similar enrolment in both groups." |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes (SNOSE). Different block sizes were chosen to prevent the allocation sequence from being anticipated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded trial where physicians and participants knew if C‐reactive protein levels were used for guidance of antibiotic treatment. C‐reactive protein levels were only communicated in the intervention arm. In 13 participants allocated to the control arm, the C‐reactive protein level was revealed after the consultation. In 1 case the C‐reactive protein level of a participant in the control arm was revealed to the physician with no implications for the management. The impact of using C‐reactive protein levels to guide antibiotic prescribing was the intervention being tested and as such could not be blinded. |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing and reconsultations was obtained from electronic medical records accessed on day 28. Participant‐reported outcomes were assessed by clinical diaries, and: "Patients who indicated they had not recovered from their illness on day 7 were contacted by the research team by telephone to follow‐up and record whether they had recovered on day 14 or day 28." |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Reconsultations documented from electronic medical records on day 28. Participants not recovered at day 7 (when diary was handed in) were contacted by the research team by telephone on day 14 and day 28. |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Follow‐up for antibiotic prescribing and use 258/258 (100%) |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | Low risk | Recovery was assessed at day 7 (243/258; 94%), also assessed as median scores of illness duration, and median daily symptom scores provided. Reconsultation data were complete. Participant‐reported outcomes were available on recovery and satisfaction in the range of 91% to 97%. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | ‐ |
Diederichsen 2000.
| Study characteristics | ||
| Methods | Open randomised clinical trial, multicentre in 35 single‐handed primary care practices in Denmark | |
| Participants |
Inclusion criteria All patients with index case of respiratory infection Exclusion criteria Previously seen by general practitioner for infection in question, patients who had streptococcal rapid testing performed, patients with chronic inflammatory diseases Included in this analysis 812 (30 acute otitis media, 129 rhinosinusitis, 507 chest, 102 other) out of 812 randomised participants (number of participants tested for eligibility not stated) |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein Algorithm used in this study: strict cut‐off values were not given, but information was provided that a normal C‐reactive protein level was below 10 mg/L, and that C‐reactive protein levels below 50 mg/L were seldom the result of bacterial infection |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | No strict inclusion and exclusion criteria but dependent on physician's opinion, may lack generalisability Kit used: Nycocard II Reader (Axis‐Shield, Norway). On‐site training in the use of CRP device provided by manufacturer. Funding: none stated It is unclear when and how the C‐reactive protein values were made available to the doctors. No direct recommendations of antibiotic treatment according to a C‐reactive protein cut‐off value were given, but normal values were communicated to GPs (< 11 mg/L) and that results < 50 mg/L seldom were the result of bacterial infection A sample size calculation was not described. Clinical recovery was assessed by a self‐reported questionnaire chart that was returned to the project leader after 7 days. No significant difference in the use of antibiotic prescriptions was found; children and adults combined (RR 0.94, 95% CI 0.80 to 1.09). Clinical improvement on day 7 in the C‐reactive protein group stated "unchanged or increased morbidity" more frequently than controls (OR 1.6, 95% CI 1.0 to 2.6). This was especially the case for participants not prescribed antibiotics and with normal C‐reactive protein values (OR 2.2, 95% CI 1.1 to 4.4). 25% (57/233) of participants with C‐reactive protein < 11 mg/L received antibiotic treatment, as did 51% (50/98) of participants with values between 11 mg/L and 25 mg/L. We obtained raw data to calculate participants substantially improved on day 7. We also attempted to include data from 7‐day antibiotic description (authors state that no added antibiotic consumption was noted), however even with raw data it was not possible to be entirely sure which data to include; we have done an analysis including the presumed antibiotic use at 7 days (CRP 190/407 versus 186/384); an extra 13 scripts in the 7 days which did not change the interpretation of the meta‐analysis on antibiotic use at day 28 (OR 0.85, 95% CI 0.73 to 0.98), I² = 47%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information given on the randomisation process in publication. Authors state randomisation was adequately done using a computer program. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "each patient drew one of 34 pre‐randomised sealed envelopes..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded trial where physicians and participants knew if C‐reactive protein levels were used for guidance of antibiotic treatment. The impact of using C‐reactive protein levels to guide antibiotic prescribing was the intervention being tested and as such could not be blinded. |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Unclear risk | Registration and consent chart sent to project leader with details on treatment from index consultation. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Participants handed in diary on day 7. Participants with missing or incomplete diaries were contacted by research team by telephone or letter on day 14. |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Follow‐up for antibiotic prescribing at index consultation 812/812 participants (100%). Antibiotic use at day 7 was assessed but not provided in publication. Quote: "No statistically significant differences were found." |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | Low risk | Participant‐reported outcomes of recovery day 7 were available in 792/812 participants (98%). Reconsultation data not provided in publication, but: "No statistically significant differences were found." Satisfaction was not assessed. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Unclear risk | Recruitment was not strictly regulated, but baseline characteristics of participants were balanced. Quote: "Each day during the study period the first or the first 2 patients, whichever was more practical, who consulted the general practitioner because of respiratory infection were asked to participate in the study." This limits externalisation of results. |
Do 2016.
| Study characteristics | ||
| Methods | Multicentre, open‐label, randomised controlled trial conducted in Northern Vietnam in 10 primary healthcare centres | |
| Participants | Patients aged 1 to 65 years of age presenting with non‐severe acute respiratory tract infection Inclusion criteria At least 1 focal and 1 systemic sign or symptom by the treating physician.
Exclusioncriteria
Included in this analysis 2036 (1017 in intervention group, 1019 in the control group) |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein 2‐armed study: 1) usual care; 2) use of C‐reactive protein point‐of‐care test Algorithm used in this study The cutoffs used to recommend that antibiotics not be prescribed were a CRP of 20 mg/L or less for participants aged 6 to 65 years, and a CRP of 10 mg/L or less for participants aged to 5 years. Doctors were advised that adults with a CRP of 100 mg/L or more and children with a CRP of 50 mg/L or more should generally receive antibiotics and hospital referral should be considered. Between these thresholds no specific recommendation was given, and clinicians were advised to use their clinical discretion. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Starting date: March 2014 to July 2015 Contact information: Prof Heiman FL Wertheim, Department of Medical Microbiology, Radboudumc, Nijmegen, the Netherlands, heiman.wertheim@gmail.com Trial registration number: NCT01918579 Funding: public (Wellcome Trust Major Overseas Programme, UK, and the Center for Disease Dynamics, Economics and Policy (CDDEP), Washington, DC, USA, as part of the Global Antibiotic Resistance Partnership (GARP)). Alere Technologies (Ais Sheield); providing reagents and equipment for CRP testing Adherence to C‐reactive protein suggested cut‐offs (35% of participants with C‐reactive protein < 20 mg/L were prescribed antibiotics), but was setting‐specific: "For patients aged 6–65 years with a CRP value at day 0 of 20 mg/L or less, the immediate antibiotic prescription rate ranged from three (4%) of 75 patients (in Sai Dong station) to 49 (71%) of 69 patients (in Dong Da station)." Kit used: Nycocard analyser (Nycocard II Reader, Alere Technologies, Norway) Training session: an initial training workshop was held centrally. Further training was implemented on site during visits at the 10 health centres by the study team. Training followed a model developed for a similar study in Maastricht, Netherlands, contextualised to the Vietnamese setting and carried out in Vietnamese. Training materials were both verbal and written, consisting of oral presentations and written information leaflets for the doctors and health centres to keep for future reference. The health centres and doctors were given a telephone number to contact should any queries arise during the study. Laminated posters and desk reminders with recommended cut‐off values for the specific age groups were provided. Sample size calculations: the trial was powered to detect a reduction of antibiotic prescription rate from 70% to 60%, based on antibiotic use data from communities in Vietnam. With a power of 90% and 2‐sided 5% significance, a total of 477 participants were required per arm. To analyse adults and children separately, the target sample size was set at 2000 participants (50% children and 50% adults). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated individual randomisation 1:1 ratio using variable block lengths of 4 (with probability 0.75) and 6 (with probability 0.25), and stratified by health station and age category (child and adult) |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed, opaque envelopes used and opened in strict chronological sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions are targeted at the level of the general practitioners. Non‐blinded trial where personnel and participants were aware if the participant was in the intervention group or control group |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing were obtained from case report forms after index consultation. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Reconsultations, recovery, and satisfaction were obtained through telephone interviews, blinded to the intervention. |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | High risk | A total of 115 of 1017 (11%) participants assigned to the intervention group and 72 of 1019 (7%) participants assigned to the control group had missing data on antibiotic prescribing. |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | Unclear risk | Data on satisfaction were reported in 1091 of 2036 (53.6%) participants. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. The authors carried out post hoc analysis due to heterogeneity in the primary endpoint. Quote: “However, for the primary outcome, we also did an additional, alternative analysis based on multiple imputation of outcomes for those patients. Moreover, the analysis of the primary endpoint was repeated in the per‐protocol population that included only patients for whom all components of the primary endpoint as mentioned above were non‐missing. Because we saw considerable heterogeneity in the primary endpoint between health‐care centres, we decided post hoc to visualise results by site using forest plots and to do a standard random effects meta‐analysis…” |
| Other bias | Low risk | ‐ |
Lhopitallier 2021.
| Study characteristics | ||
| Methods | Non‐blinded cluster‐RCT in 60 primary care practices in Switzerland | |
| Participants |
Inclusion criteria Adults above 18 years of age with acute cough < 21 days and at least 1 of the following signs/symptoms: history of fever for more than 4 days, dyspnoea, tachypnoea (> 22 cycles per minute), abnormal focal findings upon lung auscultation Exclusion criteria Previous prescription of antibiotics for the current episode; working diagnosis of acute sinusitis or of a non‐infective disorder; previous episode of chronic obstructive pulmonary disease exacerbation treated with antibiotics during the last 6 months; known pregnancy; severe immunodeficiency (untreated HIV infection with CD4 count < 200 cells/mm³, solid organ transplant receiver, neutropenia (< 1000 cells/µL), treatment with a corticosteroid dose equivalent to 20 mg prednisone/day for > 28 days); decision by the GP to admit the participant; GP not available for performing study due to time constraints; patient unable to provide informed consent |
|
| Interventions | Guiding antibiotic decisions in primary care with point‐of‐care biomarker measurement of procalcitonin and point‐of‐care lung ultrasonography 3‐armed study: 1) usual care; 2) procalcitonin‐guided antibiotics (PCT group); 3) sequential procalcitonin and lung ultrasonography point‐of‐care tests (UltraPro group) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Starting date: September 2018 to March 2020 Contact information: Nóemi Boillat‐Blanco, Infectious Diseases Service, Lausanne University Hospital, Lausanne, Switzerland; noemie.boillat@chuv.ch Trial registration: NCT03191071 Funding: public. Supported in part by the Swiss National Science Foundation (grant 407240_167133) and by an academic award of the Leenaards Foundation (to NB‐B) Kit used: BRAHMS PCT direct point‐of‐care test. They provided the BRAHMS direct Reader, BRAHMS PCT direct tests, and quality controls free of charge. Training session: GPs in the UltraPro and PCT arms participated in a half‐day training session. Topics included antibiotic resistance, epidemiology of pneumonia in Switzerland, management of CAP in primary care, the use of PCT and lung ultrasound to guide antibiotic prescription, the rationale for the UltraPro algorithm, and the procedures of the study. GPs in the UltraPro arm also received sessions in ultrasound physics, ultrasound equipment, probe positioning, image recording and interpretation using a phantom simulator. The algorithm recommended prescribing antibiotics only in the presence of an elevated procalcitonin (≥ 0.25 μg/L). In all groups, antibiotic choice, dose, and duration were left to the discretion of the GPs, who could also order further diagnostic tests. Adherence to procalcitonin cut‐offs: 77 participants with levels < 0.25 received antibiotics on day 0 (out of 283) in the 2 intervention groups. Researchers expected to recruit 600 participants, but they only recruited 469 participants due to the COVID‐19 outbreak: usual care (n = 122), procalcitonin‐guided antibiotics (n = 195), and sequential procalcitonin and lung ultrasonography point‐of‐care tests (UltraPro) (n = 152). Participants included in the protocol analysis: 114, 163, and 131, respectively Sample size calculation: assumed 60% of participants would receive antibiotics with usual care. Expected an absolute difference in antibiotic prescriptions of at least 15% between procalcitonin group and UltraPro group. A study sample of 60 general practitioners and a mean of 10 participants per general practitioners (200 participants per group for a total of 600 participants) gives a power of 80% to detect the expected difference in antibiotic prescription with 5% level of significance when adjusting for clustering at practice level. Intracluster correlation coefficient: 0.06 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list assigned at a 1:1:1 ratio between arms. |
| Allocation concealment (selection bias) | Unclear risk | Individual participant allocation concealment was not performed as unit of randomisation was practices. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions are targeted at the level of the general practitioner. Non‐blinded trial where participants and personnel knew if C‐reactive protein levels were used for guidance of antibiotic treatment |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing were obtained from electronic case report forms. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Outcome assessment carried out by a blinded research member. Members of the study team, blinded to study group, conducted standardised phone interviews of all participants on day 7. Participants were asked to fill a previously validated daily symptom diary until resolution of symptoms or day 28. |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Follow‐up for antibiotic prescribing was nearly complete: 420/443. |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | Low risk | Participant satisfaction completed in 119/122 (usual care) and 189/195 (procalcitonin). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | High risk | Risk of selection bias present due to lack of individual randomisation. |
Little 2013a.
| Study characteristics | ||
| Methods | Non‐blinded cluster‐randomised (practice level) clinical trial, multinational with 246 primary care practices in Spain, England, Wales, Poland, Belgium, the Netherlands | |
| Participants |
Inclusion criteriafor practices No prior participation in interventions to reduce antibiotic use; recruited more than 10 patients in the baseline audit Inclusion criteriaforpatients Adults (18 years or older) with LRTI or URTI.
Exclusioncriteriafor patients A non‐infective working diagnosis (e.g. pulmonary embolus, heart failure, oesophageal reflux, allergy); antibiotic use in the previous month; unable to provide informed consent (dementia, psychosis, severe depression); pregnant; immunological deficiencies Patients with lower respiratory tract infection (up to the first 30 presenting in each practice) and upper respiratory tract infection (up to the first 5 presenting) were recruited following informed consent. Included in this analysis 4264 participants at follow‐up: 2224 to the C‐reactive protein group versus 2040 to the no‐test group 80% of participants had lower respiratory tract infections, whilst the remainder had upper respiratory tract infections. |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein, communication skills training, or a combination thereof Algorithm used in this study: recommended cut‐off values: C‐reactive protein ≤ 20 mg/L: self‐limiting ARI, withhold antibiotics; C‐reactive protein 21 to 50 mg/L: majority of patients have self‐limiting ARI, withhold antibiotics in most cases; C‐reactive protein 51 to 99 mg/L: withhold antibiotics in the majority of cases and consider delayed antibiotics in the minority of cases; C‐reactive protein ≥ 100 mg/L: severe infection, prescribe antibiotics Physicians could deviate from algorithm at any time. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Trial registration: ISRCTN99871214 Cluster‐randomised at practice level as general practitioners trained in communication skills were unable to shift at random between using new skills and usual care Funding: public Kit used: QuikRead C‐reactive protein, Orion Diagnostica (Espoo, Finland). On‐site training to practices in kit use provided by manufacturer. Following training, prior to data collection, there was a run‐in period for physicians to practice using the device. A baseline audit (October 2010 to December 2010) functioned to characterise patients and the 'everyday' prescribing behaviour of clinicians. A cluster‐randomised design was chosen to minimise contamination within practices (since more than 1 physician per practice could participate) and because a practice‐based meeting was part of the intervention. Following a baseline audit to determine the antibiotic prescription rate, 4 groups were compared: C‐reactive protein testing (1), communication training (2), communication training and C‐reactive protein testing (3), and usual care (4). A factorial analysis plan was prespecified where groups were combined: C‐reactive test (cells 1 + 3) compared with no test (cells 2 + 4) whilst controlling for the effect of communication training. No statistically significant interaction was found between the interventions, although a synergistic effect was noted. 446 practices approached; 259 agreed to participate; 228 practices contributed with data. Compliance with the intervention (training) was good, with completion of all the training modules in 99/113 (88%) of the C‐reactive protein group, 94/108 (87%) of the communication group, and 116/127 (91%) of the combined group. The intervention consisted of an estimated 30‐minute internet training module on the use of C‐reactive protein to target antibiotics for serious infections and providing C‐reactive protein cut‐off values for recommending or withholding antibiotic treatment. Compliance with the intervention was good with 90% (215/240) of participating doctors having completed the internet training module. The interaction term between C‐reactive protein and communication training on the primary outcome (number of antibiotic prescriptions) was not significant (P = 0.41). Sample size calculations were done to allow detection of a reduction in antibiotic use of 10% (50% to 40%) (power 80%) and adjusting for clustering with intracluster coefficients of 0.16 and 0.06 determined a sample size of minimum 2600 participants and maximum 5400. The primary outcome of antibiotic prescribing was assessed at index consultation. Secondary outcomes were reconsultations (including hospitalisations) with new and worsening symptoms documented by medical notes review. Symptom severity and duration: a) severity 2 to 4 days after index consultation; and b) duration of symptoms rated moderately bad or worse. These outcomes were assessed by participant diary and mailed to study team upon completion. A telephone reminder was given to postal non‐responders. The study reported a significantly reduced use of antibiotics in the C‐reactive protein group at index consultation (33% versus 58%) (RR 0.54, 95% CI 0.42 to 0.69) (adjustment for baseline antibiotic prescribing, GP and practice). No significant difference in the number of patient reconsultations was recorded (RR 1.06, 95% CI 0.80 to 1.40), however an increase in hospital admissions was present in the C‐reactive protein group that disappeared with adjustment for various potential confounders, including clinical presentation, which weakened the association to be of borderline significance (P = 0.06). Information on hospital admissions was available in 15 cases, the stated reasons being cardiac (2), respiratory (8), generally unwell/pyrexia (2), gastrointestinal symptoms (2), sinusitis (1). We were unable to obtain the percentages of hospital admissions in the C‐reactive protein not initially prescribed an antibiotic. No study‐related deaths were reported. A similar resolution of symptoms rated moderately bad or worse was observed (median 5 days, IQR 3 to 9 days), as was symptom severity 2 to 4 days after index consultation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of practices was performed by study team, stratified by network (country) by computer‐generated random numbers, balanced for recruitment potential. |
| Allocation concealment (selection bias) | Unclear risk | Centralised randomisation. Quote: "... physicians and patients were blind to initial group allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions are targeted at the level of the general practitioners. Cluster‐randomised at practice level, as GP trained in communication skills were unable to shift at random between using new skills and usual care. Non‐blinded trial where physicians and participants knew which treatment modality was used |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing were obtained from case report forms after index consultation. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Reconsultations documented by medical notes review. Symptom severity and duration by participant diaries with reminders (phone or mail, or both) |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Follow‐up for antibiotic prescribing and use was complete: 4264/4264 (100%). |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | Low risk | The study reports on the time to resolution of symptoms rated moderately bad or worse, and recovery could not be assessed at specific time points. Participant satisfaction was not reported. Data on reconsultations were available in 4121/4264 (97%). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | High risk | Risk of selection bias present due to lack of individual randomisation. |
Little 2019.
| Study characteristics | ||
| Methods | Non‐blinded, cluster‐randomised (practice level) clinical trial, multinational with 168 primary care practices in Belgium, the Netherlands, Poland, Spain, and the UK Antwerp (Belgium), Barcelona (Spain), Cardiff (Wales), Łódź (Poland), Southampton (UK), Szczecin (Poland), Utrecht (the Netherlands), and the Spanish Society of Family Medicine (Spain) |
|
| Participants |
Inclusion criteria for practices No prior participation in interventions to reduce antibiotic use; recruited more than 10 patients in the baseline audit Inclusion criteria forpatients Adults (18 years or older) with LRTI or URTI
Exclusioncriteria for patients A non‐infective working diagnosis (e.g. pulmonary embolus, heart failure, oesophageal reflux, allergy); antibiotic use in the previous month; unable to provide informed consent (dementia, psychosis, severe depression); pregnant; immunological deficiencies Patients with lower respiratory tract infection (up to the first 30 presenting in each practice) and upper respiratory tract infection (up to the first 5 presenting) were recruited following informed consent. |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein 4‐armed trial:
CRP algorithm used
|
|
| Outcomes |
Primary outcomes
Secondary outcomes None |
|
| Notes | Starting date: October 2010 until May 2012 Contact information: Paul Little, MBBS, FRCGP, University of Southampton Aldermoor Health Centre Aldermoor Close, Southampton, SO16 5ST, UK; p.little@soton.ac.uk Trial registration number: ISRCTN99871214 Funding: public, supported by the European Commission Framework 6 Programme (grant 518226). The work in the UK was also supported by the National Institute for Health Research and the Research Foundation Flanders (grant G.0274.08N). Orion Diagnostica supplied all the equipment and consumables for CRP testing. Kit used: QuikRead CRP kits (Orion Diagnostica, Espoo, Finland) after on‐site training by the manufacturer Intraclass correlation coefficient was provided (0.06 to 0.16), size of clusters (40 for the usual group and 39 for the intervention group). Training session: the test device was demonstrated by company representatives, and an internet training module was provided on CRP use. The device and testing materials were provided free. Sample size calculation: "The sample size was calculated for an α of 0.025 and a β of 0.2. We assumed that 30 patients per practice would be recruited; that a 50% to 40% reduction in antibiotic prescribing would be achieved for at least 1of the interventions; and that the intra‐cluster correlation coefficient would range from 0.16 to 0.06. We therefore required 2,600 patients (intra‐cluster correlation coefficient=0.06) to 5,400 patients (intra‐cluster correlation coefficient=0.16)." Adherence to protocol: by 12 months, clinicians in all groups had seldom used CRP testing in patient care, even though they were given free access to CRP diagnostic kits (62 of 1075 (5.77%) and 85 of 1419 (5.99%)). Prescribing in the CRP group rose almost 9% from 35% (368 of 1062) at 3 months (Little 2013a) to 43% (456 of 1052) at 12 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation carried out centrally stratified by network. Quote: “Practices were remotely randomized using the minimisation approach, based on practice characteristics (baseline prescribing, number of clinicians, number of patients at baseline) and with stratification by network” |
| Allocation concealment (selection bias) | Unclear risk | Individual participant allocation concealment was not performed, as the unit of randomisation was doctors or practices, or both. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions were targeted at the level of the general practitioners. Non‐blinded trial where personnel and participants were aware if the participant was in the intervention group or control group, although masked to allocation between the 2 intervention groups |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing were obtained from case report forms after index consultation. |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Unclear risk | Follow‐up for antibiotic prescribing and use was nearly complete: 4822/4830 (99.8%). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | High risk | High risk of selection bias due to lack of individual randomisation |
Melbye 1995.
| Study characteristics | ||
| Methods | Open randomised clinical trial, multicentre in 10 primary care practices in Norway | |
| Participants |
Inclusion criteria Adult (> 18 years) with subjective complaint of i) pneumonia, bronchitis, or asthma or ii) 1 of the following symptoms: cough, shortness of breath, chest pain on deep inspiration or cough Exclusion criteria Aged under 18 years, patients with sore throat, blocked nose, pain in ears or sinuses. Patients with angina‐like chest pain were also excluded. Included in this analysis 239 (108 C‐reactive protein group, 131 controls) out of 239 randomised participants (245 eligible participants) |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement at the end of consultation Algorithm used in this study: recommended cut‐off values:
Physicians could deviate from algorithm at any time, but reasons for doing so should be stated. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Study was stopped after 1 year and prior to estimated power calculation of 260 participants had been included due to lack of interest from participating doctors, and interim analysis showed that the null hypothesis was not subject to change regardless. Kit used: Nycocard II Reader (Axis‐Shield, Norway) Funding: Nycomed Pharma A sample size calculation was performed to allow detection of 20% difference in the number of antibiotic prescriptions with 90% power (target inclusion of 260 participants). The study was terminated after 1 year by the principal investigator without reaching the target inclusion due to an interim analysis that found no difference between groups, as well as lack of interest from participating GPs. Low adherence to protocol, and C‐reactive protein values only available after initial decision on clinical management. Baseline characteristics of participants were balanced. Clinical recovery was assessed at a follow‐up visit by health personnel preferably at the practice, alternatively on phone. No significant difference was found in the number of antibiotic prescriptions between groups (RR 0.96, 95% CI 0.75 to 1.24). No difference in patient recovery rate or rate of improvement was observed on day 7 (RR 0.94, 95% CI 0.75 to 1.18) or day 21 (RR 0.85, 95% CI 0.57 to 1.29). Management decisions were changed by C‐reactive protein testing in 10% (11/108) of cases; estimated algorithm adherence 42%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. Correspondence with principal investigators stated that randomisation was adequate and performed by sponsor at sponsor level. No additional details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stated, but participants were unaware of group allocation until after consent to participate in study was obtained; however, study personnel are not accounted for |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded trial where physicians were only communicated the C‐reactive protein results in the intervention arm. The impact of using C‐reactive protein levels to guide antibiotic prescribing was the intervention being tested and as such could not be blinded. Participants were not informed of the C‐reactive protein results. |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic use were obtained from medical records. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | High risk | Health personnel responsible for C‐reactive protein testing and randomisation performed follow‐up interviews with participants at day 7 and 21 in health clinic or on phone. |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Follow‐up for antibiotic prescribing and use 239/239 (100%) |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | Low risk | Recovery data were available in 230/239 (96%) at day 7 and 219/239 (92%) at day 21. Reconsultations and patient satisfaction were not assessed. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes are reported, but no study protocol available. |
| Other bias | Unclear risk | Premature study stop guided by preliminary study results before target inclusion was met, indicating that principal investigator had access to data. |
Schot 2018.
| Study characteristics | ||
| Methods | Non‐blinded randomised clinical trial in 28 primary care practices and 4 out‐of‐hours services in the Netherlands Cluster‐RCT in the primary care practices; individual RCT in the out‐of‐hours services |
|
| Participants |
Inclusion criteria Children between 3 months and 12 years of age with suspicion of lower respiratory tract infection; acute cough < 21 days; reported fever > 38 °C < 5 days; written informed consent Exclusioncriteria Impaired immunity; severe pulmonary disease; serious congenital defects; use of systemic antibiotics or corticosteroids, or both, in past 4 weeks; judged severely ill by the GP based on symptoms and signs; highly suspected of having pneumonia by the GP; referral to specialist or emergency department deemed necessary by GP Included in this analysis 301 participants (136 in the intervention group and 165 in the control group) |
|
| Interventions | Guiding antibiotic decisions in primary care with a single point‐of‐care measurement of C‐reactive protein 2‐armed study: 1) usual care; 2) use of C‐reactive protein point‐of‐care test performed after clinical assessment Algorithm used in this study
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Starting date: December 2013 and May 2016 Contact information: Marjolein JC Schot, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, Netherlands; m.j.c.schot‐3@umcutrecht.nl Trial registration: The Netherlands National Trial Register (trial identifier NTR4399) Funding: public (The Netherlands Organisation for Health Research and Development) and Alere Technologies AS. 2 participating laboratories, SALTRO and Star Medical Diagnostic Center, provided the reagents and equipment for the CRP test. 2 study authors were employed at SALTRO (funding agreement was carried out to ensure the authors’ independence in designing the study, interpreting the data, writing, and publishing the report). Kit used: Afinion point‐of‐care testing, Alere Technologies AS, Oslo, Norway Training session: not specified in the published paper General practitioners were not provided with strict decision rules based on C‐reactive protein levels, but were given the following guidance.
Adherence to protocol: antibiotic was prescribed in 14% of children with a CRP level < 10 mg/L Sample size calculation was based on: “... the expectation that POC CRP testing would reduce antibiotic prescribing by at least 20%, from 70% to 50%. To detect such a difference with 80% power and two‐sided 5% significance, and considering a cluster size of 16 and an intra‐cluster correlation coefficient of 0.06, a total of 354 patients were required”. A total of 46% of children had C‐reactive protein levels < 10 mg/L, 51% 10 to 100 mg/L, and 4% > 100 mg/L. Children were more likely to get an antibiotic prescription with increasing C‐reactive protein levels, ranging from 14% in children with a level < 10 mg/L; 44% with levels between 10 and 100 mg/L; and more than 50% with levels > 40 mg/L. A total of 210 children were recruited in primary care and 99 in the out‐of‐hours services. 5 children in the intervention group and 3 children in the control group were excluded from the analysis. Quote: “POC CRP was not measured in two children in the intervention group (1.4%), and in the control group point‐of care CRP was measured 30 times (18.2%), in violation of protocol” Intracluster coefficient provided (0.06), as well as size of clusters (16). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation stratified by region and practice type (academic versus non‐academic) for children recruited in primary care practices. Permuted block randomisation in out‐of‐hours services |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment of the individual participant is not applicable for the cluster‐randomised part of this study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Interventions are targeted at the level of the general practitioner. For the cluster‐randomised part of the study: non‐blinded trial where personnel and participants were aware if the participant was in the intervention group or control group, although masked to allocation between the 2 intervention groups For the individually randomised part of the study: sequentially numbered, opaque, sealed envelopes (SNOSE) prepared by a member of the research team using permuted block randomisation for out‐of‐hours services Quote: “After the treating GP checked eligibility, an onsite research assistant, blinded to the clinical evaluation of the child, opened the envelope.” |
| Blinding of outcome assessment (detection bias) Antibiotic prescribing | Low risk | Data on antibiotic prescribing were obtained from medical records. |
| Blinding of outcome assessment (detection bias) Secondary outcomes | Low risk | Reconsultations documented from electronic medical records 3 months after inclusion. Quote: “Three months after inclusion, children’s medical records were reviewed to collect data on secondary outcomes” |
| Incomplete outcome data (attrition bias) Antibiotic prescriptions | Low risk | Follow‐up for antibiotic prescribing and use was nearly complete: 301/309 (97.4%). |
| Incomplete outcome data (attrition bias) Other outcomes: recovery, re‐consultations, satisfaction | High risk | Due to missing consent of the parents for follow‐up, follow‐up data on secondary outcomes were only available for 180 children (58.2%). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | High risk | This study is a combined cluster‐ and individually randomised trial. We have chosen to characterise it as a cluster‐randomised trial. The study had frequent protocol violations, and 30 out of 165 participants in the control group had CRP measurements. |
AECOPD: acute exacerbations of chronic obstructive pulmonary disease ARI: acute respiratory infection ARTI: acute respiratory tract infection CAP: community acquired pneumonia CI: confidence interval COPD: chronic obstructive pulmonary disease CRP: C‐reactive protein CRQ‐SAS: Chronic Respiratory Disease Questionnaire GP: general practitioner IQR: interquartile range LRTI: lower respiratory tract infection OR: odds ratio POCT: point‐of‐care test RCT: randomised controlled trial RR: risk ratio URTI: upper respiratory tract infection WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ameyaw 2014 | Not a primary care setting |
| Briel 2008a | Not a point‐of‐care biomarker (procalcitonin). Procalcitonin was analysed at hospital ‐ not as a point‐of‐care test. |
| Burkhardt 2010a | Not a point‐of‐care biomarker (procalcitonin). Procalcitonin was analysed at hospital ‐ not as a point‐of‐care test. |
| Dahler‐Eriksen 1999 | Did not assess C‐reactive protein to guide antibiotic prescriptions |
| de Lusignan 2020 | Not a randomised controlled trial, not a point‐of‐care biomarker (molecular rapid test for influenza) |
| Eley 2020 | Not a classic randomised controlled trial, but rather a service evaluation using elements of randomisation. However, the study was not disclosed as an RCT to either healthcare providers or patients. The study used register‐based data. |
| Fiore 2017 | Not a randomised controlled trial |
| Gonzales 2011 | Not a primary care setting |
| Huang 2018 | Not a primary care setting |
| Isa 2022 | Not a primary care setting |
| Kavanagh 2011 | Not a randomised controlled trial |
| Keitel 2017 | Not a point‐of‐care biomarker (electronic algorithm including point‐of‐care biomarker) |
| Little 2014 | Not a point‐of‐care biomarker (rapid antigen detection test) |
| Llor 2012 | Not a randomised controlled trial |
| Mann 2020 | Did not assess C‐reactive protein to guide antibiotic prescriptions |
| Meili 2016 | Not a randomised controlled trial |
| Minnaard 2016 | Not a randomised controlled trial |
| Montassier 2019 | Not a primary care setting, not a point‐of‐care biomarker (procalcitonin) |
| Oppong 2018 | Not a randomised controlled trial |
| Rebnord 2016a | Did not include acute respiratory tract infections, but rather fever in general; data sought specifically for ARIs but not possible to access |
| Schechter‐Perkins 2019 | Not a primary care setting, not a point‐of‐care biomarker (influenza rapid test) |
| Stannard 2014 | Not a randomised controlled trial |
| Takemura 2005 | Not a primary care setting |
| Van den Bruel 2016 | Did not assess ARTI |
| Verbakel 2016 | Did not include acute respiratory tract infections, but rather acutely ill children |
ARIs: acute respiratory infections ARTI: acute respiratory tract infection RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ISRCTN01559032.
| Study name | Converting habits of antibiotic prescribing for respiratory tract infections in German primary care (CHANGE‐2) |
| Methods | 3‐armed cluster‐RCT with units being general practitioners and practice‐based paediatricians. Sample size was calculated to detect a relative reduction of 30% between groups: target inclusion 480 participants, but inflating for clustering yields a sample size of 13,160 in 188 practices. |
| Participants | Eligible participants are health‐insured in the same company (AOK), a minimum age of 3 months, first visit due to an acute respiratory infection (both upper and lower), and otherwise healthy. |
| Interventions | The interventions are: 1) communication training; 2) communication training and point‐of‐care test (C‐reactive protein test or rapid antigen detection testing, or both); 3) usual care. Communication training will be given at to small groups in 1 seminar‐based session. All tests are provided free of charge, and staff and physicians will receive training on performing the test and when to use point‐of‐care tests. |
| Outcomes | Physicians' antibiotic prescription rates over 3 winters. Secondary outcomes include reconsultation rates, complications (including hospital admissions). |
| Starting date | 10 September 2012 |
| Contact information | Annette Diener, Institute of General Practice, Rostock University Medical Centre, 18055 Rostock, Germany. Email: anette.diener@med.uni.rostock.de |
| Notes | Completed. We contacted the authors for data. A paper is currently in production. No data available up to 2022. |
NCT03540706.
| Study name | Impact of the use of CRP on the prescription of antibiotics in general practitioners |
| Methods | Open, parallel randomised clinical trial |
| Participants | Eligible participants are French health‐insured patients with a clinical suspicion of a respiratory infection defined by the presence of at least 1 respiratory sign amongst cough, dyspnoea, chest pain, and auscultatory abnormality and at least 1 general sign amongst fever, sweat, headache, myalgia, impairment of general condition of more than 1 day of symptoms, with a minimum age of 3 years. Planned sample size: 406 |
| Interventions | The interventions include: 1) C‐reactive protein assay in micro method; 2) usual care. |
| Outcomes | Antibiotic therapy prescribed within the first 10 days. Secondary outcomes include antibiotic therapy in participants aged 3 to 17, 18 to 64, and ≥ 65 years old; type of antibiotic prescribed; complementary exams ordered and types; participants referred to emergency; participants with delayed antibiotic therapy; number of prescriptions following the recommended algorithms; adequacy between the proposed decision algorithm according to the CRP and the antibiotic prescription; concordance between the prescription proposed by the decision algorithm as a function of the micro‐CRP and the prescription realised; and number of COVID‐19‐positive patients (included from October 2020). |
| Starting date | 1 May 2018 |
| Contact information | Robert Touitou. Centre Hospitalier Intercommunal Creteil, France. Email: robtouitou@gmail.com |
| Notes | Recruiting |
NCT03855215.
| Study name | Implementation of CRP point‐of‐care testing in primary care to improve antibiotic targeting in respiratory illness (ICAT) |
| Methods | Open, parallel randomised clinical trial |
| Participants | Patients with an acute respiratory infection aged from 1 to less than 65 years old, with at least 1 focal sign or symptom lasting less than 7 days: (1) cough, (2) rhinitis (sneezing, nasal congestion, or runny nose), (3) pharyngitis (sore throat), (4) shortness of breath, (5) wheezing, (6) chest pain, or (7) auscultation abnormalities. Planned sample size: 24,960 |
| Interventions | The interventions include: 1) ACTIM C‐reactive protein rapid test provided to healthcare workers at the commune health centres for use in patients in the target population; 2) usual care. |
| Outcomes | Prescription of antibiotics. Secondary outcomes include participants that consulted with an acute respiratory infection in the preceding year; prescription rates for these participants in the preceding year; participants indicated C‐reactive protein test according to diagnosis, age, and gender; participants prescribed an antibiotic in the intervention arm with a CRP < 10 mg/L, CRP 10 to 40 mg/L, and CRP > 40 mg/L; participants receiving an antibiotic with the denominator being all attendances, and all non‐routine attendances; participants receiving an antibiotic prescription by diagnosis, age, season, recorded fever, and sex; participants referred to a higher‐level facility at the initial consultation by checking the e‐database of health insurance reimbursement; participants in whom the test is indicated who re‐attend the health facility within a 30‐day period, and whether antibiotics were prescribed; participants in whom the test is indicated who were hospitalised within a 30‐day period; participants in whom use of the test was recommended that received the test; usability, acceptability, and views of C‐reactive protein test among healthcare workers; and cost‐effectiveness. |
| Starting date | 26 February 2019 |
| Contact information | Sonia Lewycka, Oxford University Clinical Research Unit, Vietnam Email: slewycka@oucru.org |
| Notes | Recruiting |
NCT03931577.
| Study name | Effectiveness of improving diagnostic and communication skills on antibiotic prescribing appropriateness in acute cough (ISAAC‐CAT) |
| Methods | Open cluster‐randomised factorial controlled trial |
| Participants | Patients with a first consultation for acute cough of up to 3 weeks' duration (new cough or worsening of a previous cough) aged 18 or older, which the clinician believes to be an infectious acute lower respiratory tract infection. Planned sample size: 24,960 |
| Interventions | The interventions include: 1) continuous (workshop and monthly web‐based training) disease‐focused intervention with the use of C‐reactive protein rapid testing; 2) continuous (on‐site and monthly online training) illness‐focused intervention with enhancement of communication skills to optimise doctor‐patient consultations and share decision making with the aid of patient‐centred leaflets; 3) both interventions; 4) usual care. |
| Outcomes | Participant‐reported consumption of antibiotics and health status by means of the EuroQol questionnaire. Secondary outcomes include reconsultations and complications; duration of symptoms and duration of severe symptoms; antibiotic prescription at the baseline visit; drugs other than antibiotics; number of non‐antibiotic prescriptions; tests ordered by clinicians; number of tests ordered by clinicians; participant satisfaction with care; participant satisfaction score; participant perception of the usefulness of the information received; participant perception score about the usefulness of the information received; participant future consulting intention; participant future consulting intention score; serious adverse events; number of serious adverse events; absenteeism. |
| Starting date | 30 April 2019 |
| Contact information | Carl Llor, University Primary Care Research Institute Jordi Gol i Gurina, Spain Email: carles.llor@gmail.com |
| Notes | Recruiting |
NCT04216277.
| Study name | The procalcitonin guided antibiotics in respiratory infections in general practice (PARI) |
| Methods | Parallel randomised clinical trial |
| Participants | Patients older than 18 years with acute cough, e.g. less than 2 weeks with probable acute upper or lower respiratory tract infection (pharyngitis, tonsillitis, otitis media, sinusitis, exacerbations of asthma or chronic obstructive pulmonary disease, bronchitis, or pneumonia), with a C‐reactive protein level > 20 mg/L. Patients with severe symptoms present for more than 2 weeks, immunocompromised, sore throat with a positive test for Group A streptococcus, or those with prior antibiotic exposure the last 14 days up to inclusion were excluded. |
| Interventions | The interventions include: 1) procalcitonin values disclosed to the attending physician assisting in antibiotic guidance in addition to usual care; 2) usual care without procalcitonin values disclosed. |
| Outcomes | Participant‐reported duration of illness and symptoms from acute respiratory tract infections. Secondary outcomes include antibiotic treatments; side effects from antibiotic treatment; reconsultations; severe adverse effects; biomarker levels. |
| Starting date | 2 January 2020 |
| Contact information | Rune Munck Aabenhus. Research Unit of General Practice, Copenhagen, Denmark Email: runa@sund.ku.dk |
| Notes | Active, not recruiting |
CRP: C‐reactive protein GP: general practitioner RCT: randomised controlled trial
Differences between protocol and review
Changes from the 2014 review
Types of interventions
We have changed the wording in the Types of interventions section to clarify the interventions eligible for this review. These changes did not impact on the decision to include or exclude any specific studies.
Follow‐up period
We changed the wording used in the original review and protocol from “at 28 days follow‐up” and “at day seven” to "within 28 days follow‐up” and "within seven days follow‐up” in the Methods to specify the follow‐up period more precisely. We chose to change the wording in order to avoid misinterpretation. We also specified the follow‐up period to clarify the follow‐up periods for future updates. This did not change our decision to include or exclude any specific studies from the review, but it did change which studies were included in our meta‐analysis.
Search procedure
We did not search conference abstracts. We carried out a search in the EU Clinical Trials Register to identify further studies or ongoing studies.
Changes from the protocol
Types of interventions
The requirement for specific cut‐off points in the intervention group was removed in the original review, and we kept to the methods as carried out in the original review.
Methods
We extracted and used intraclass correlation coefficients (ICCs) for cluster‐randomised trials in order to avoid a unit of analysis error.
Plans for dichotomising recovery and patient satisfaction data were introduced in the original review, and we kept this plan.
Plans for performing a power calculation to determine the sample size of future studies were removed in the original review, and we kept this plan.
Subgroup analysis
We were unable to compare studies performed in participants with suspected serious infections (e.g. pneumonia) and suspicion of less serious infections (e.g. common cold and bronchitis) due to lack of data on specific diagnoses. However, we reported reductions in antibiotic use by C‐reactive protein guidance in upper acute respiratory infections (ARI) and lower ARI.
We were unable to compare different biomarkers, as only one study was identified evaluating procalcitonin as a point‐of‐care biomarker in ARI in primary care.
As the intervention did not lend itself to blinding, we chose to omit conducting the subgroup analysis on trials at low risk of bias and at high risk of bias.
We have included a post hoc analysis of C‐reactive protein algorithms of newer studies with a clear cut‐off of 20 mg/L to withhold antibiotic treatment and older studies without a clear cut‐off to withhold antibiotic treatment.
Sensitivity analysis
We did not carry out the planned fixed‐effect meta‐analysis as a sensitivity analysis due to the considerable heterogeneity of data.
We added a post hoc sensitivity analysis on follow‐up period, as we had to specify the follow‐up period that was not prespecified in the protocol or the original review. The post hoc sensitivity analysis on follow‐up period did not change which studies to include from the original review, but did change which studies to include in the meta‐analysis from the current search carried out in June 2022.
Previous changes from the protocol
Methods
We removed the requirement for specific cut‐off‐points in the intervention group.
We extracted and used ICCs for cluster‐randomised trials in order to avoid a unit of analysis error.
We removed plans to use continuous measures, and introduced plans to dichotomise recovery and patient satisfaction data.
We removed plans for performing a power calculation to determine the sample size of future studies.
Subgroup analysis
We were unable to compare studies performed in participants with suspected serious infections (e.g. pneumonia) and suspicion of less serious infections (e.g. common cold and bronchitis) due to lack of data on specific diagnoses. However, we have reported reductions in antibiotic use by C‐reactive protein guidance in upper ARI and lower ARI.
We were unable to compare different biomarkers, as most studies investigated C‐reactive protein point‐of‐care tests, and only one study investigated procalcitonin as a point‐of‐care test in primary care.
As the intervention did not lend itself to blinding, we chose to omit conducting the subgroup analysis on studies at low risk of bias and at high risk of bias.
We have included a post hoc analysis of C‐reactive protein algorithms of newer studies with a clear cut‐off of 20 mg/L to withhold antibiotic treatment and older studies without a clear cut‐off to withhold antibiotic treatment.
Sensitivity analysis
We did not carry out the planned fixed‐effect meta‐analysis as a sensitivity analysis due to the considerable heterogeneity of data.
Contributions of authors
Protocol stage
Rune Aabenhus (RA) was responsible for drafting the protocol.
Review stage
RA and Jens‐Ulrik S Jensen (J‐U SJ) were responsible for selecting trials for inclusion and data extraction. RA was responsible for entering data into RevMan Web and analysing data. RA was responsible for drafting the final review. All authors were responsible for interpreting the analyses.
Update stage
Siri Aas Smedemark (SAS) and Carl Llor (CL) were responsible for selecting trials for inclusion and data extraction for the current update. SAS was responsible for entering data into RevMan Web and analysing data. SAS was responsible for drafting the final review. All authors were responsible for interpreting the analyses and revising the text of the review.
Next update stage
SAS will be responsible for updating this review.
Sources of support
Internal sources
-
University of Copenhagen, Denmark
Administrative support
-
Nordic Cochrane Centre, Denmark
Methodological support
External sources
-
Marta Perpiñan, Barcelona Official Nursing College, Spain
Marta Perpiñan carried out the search for the updated version in 2022.
Declarations of interest
Siri Aas Smedemark: declared that she has no conflict of interest.
Carl Llor: the public institution CL works for has received funding in the form of grants from a company producing C‐reactive protein tests and products.
Rune Aabenhus: RA has received speaker fees from two companies, including one company producing procalcitonin tests. Additionally, RA was primary investigator in a trial receiving funding in the form of procalcitonin kits and assays from the manufacturer. The trial was closed down (October 2021) due to delays related to the COVID‐19 pandemic. RA is involved in a trial using C‐reactive protein to guide antibiotic treatment in children in Kyrgyz Republic. The trial is estimated to start in the second half of 2022.
Anders Fournaise: declared that he has no conflict of interest.
Ole Olsen: declared that he has no conflict of interest.
Karsten Juhl Jørgensen: declared that he has no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Althaus 2019 {published and unpublished data}
- Althaus T, Greer RC, Swe MM, Cohen J, Tun NN, Heaton J, et al. Effect of point-of-care C-reactive protein testing on antibiotic prescription in febrile patients attending primary care in Thailand and Myanmar: an open-label, randomised, controlled trial. Lancet Global Health 2019;7:e119-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreeva 2013 {published and unpublished data}
- Andreeva E, Melbye H. Usefulness of C-reactive protein testing in acute cough/respiratory tract infection: an open cluster-randomised clinical trial with CRP testing in the intervention group. BMC Family Practice 2014;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boere 2021 {published data only}
- Boere TM, Buul LW, Hopstaken RM, Tulder MW, Twisk JW, Verheij TJ, et al. C-reactive protein point-of-care testing reduces antibiotic prescribing for lower respiratory tract infections in nursing home residents: results of a cluster randomized, controlled trial. BMJ 2021;374:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler 2019 {published data only}
- Butler CC, Gillespie D, White P, Bates J, Lowe R, Thomas-Jones E, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. PACE Study. New England Journal of Medicine 2019;381:111-20. [DOI] [PubMed] [Google Scholar]
Cals 2009 {published data only}85154857
- Cals JW, Butler CC, Hopstaken RM, Hood K, Dinant GJ. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ 2009;338:b1374. [DOI: 10.1136/bmj.b1374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cals 2010 {published data only}
- Cals JW, Schot MJ, Jong SA, Dinant GJ, Hopstaken RM. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: a randomized controlled trial. Annals of Family Medicine 2010;8(2):124-33. [DOI: 10.1370/afm.1090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diederichsen 2000 {published and unpublished data}
- Diederichsen HZ, Skamling M, Diederichsen A, Grinsted P, Antonsen S, Petersen PH, et al. Randomised controlled trial of CRP rapid test as a guide to treatment of respiratory infections in general practice. Scandinavian Journal of Primary Health Care 2000;18:39-43. [DOI] [PubMed] [Google Scholar]
Do 2016 {published data only}
- Do NT, Ta NT, Tran NT, Than HM, Vu BT, Hoang LB, et al. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: a randomised controlled trial. Lancet Global Health 2016;4:e633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lhopitallier 2021 {published data only}
- Lhopitallier L, Kronenberg A, Meuwly J, Locatelli I, Mueller Y, Senn N, et al. Procalcitonin and lung ultrasonography point-of-care testing to determine antibiotic prescription in patients with lower respiratory tract infection in primary care: pragmatic cluster randomised trial. BMJ 2021;374:n2132. [DOI: 10.1136/bmj.n2132] [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2013a {published data only (unpublished sought but not used)}99871214
- Little P, Stuart B, Francis N, Tonkin-Crine S, Douglas E, Anthierens S, et al. The effect of web-based training in communication skills and an interactive patient booklet and the use of a CRP point of care test in acute respiratory tract infection (RTI): a multi-national cluster randomised factorial controlled trial. Lancet 2013;382(9899):1175-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2019 {published data only}
- Little P, Stuart B, Francis N, Douglas E, Tonkin-Crine S, Anthierens S, et al, GRACE consortium. Antibiotic prescribing for acute respiratory tract infections 12 months after communication and CRP training: a randomized trial. Annals of Family Medicine 2019;17(2):125-32. [DOI: 10.1370/afm.2356] [DOI] [PMC free article] [PubMed] [Google Scholar]
Melbye 1995 {published and unpublished data}
- Melbye H, Aaraas I, Fleten N, Kolstrup N, Mikalsen J-I. The value of C-reactive protein testing in suspected lower respiratory tract infections. A study from general practice on the effect of a rapid test on antibiotic research and course of the disease in adults [Nytten av å teste C-reaktivt protein ved mulig nedre luftveisinfeksjon. En undersøkelse fran allmennpraksis av en hurtigtests innvirkning på antibiotikaforskrivning og sykdomsforløp hos voksne]. Tidsskrift for Norske Laegeforeningen 1995;115(13):1610-5. [PubMed] [Google Scholar]
Schot 2018 {published data only}
- Schot MJ, Van den Bruel A, Broekhuizen BD, Cals JW, Noteboom EA, Balemans W, et al. Point-of-care C-reactive protein to assist in primary care management of children with suspected non-serious lower respiratory tract infection: a randomised controlled trial. BJGP Open 2018;2(3):1-10. [DOI: 10.3399/bjgpopen18X101600] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ameyaw 2014 {published data only}
- Ameyaw E, Nguah SB, Ansong D, Page I, Guillerm M, Bates I. The outcome of a test-treat package versus routine outpatient care for Ghanaian children with fever: a pragmatic randomized control trial. Malaria Journal 2014;13:461. [DOI: 10.1186/1475-2875-13-461] [DOI] [PMC free article] [PubMed] [Google Scholar]
Briel 2008a {published data only}73182671
- Briel M, Schuertz P, Mueller B, Young J, Schild U, Nusbarmer C, et al. Procalcitonin-guided antibiotic use vs. standard approach for acute respiratory tract infections in primary care. Archives of Internal Medicine 2008;168(18):2000-7. [DOI] [PubMed] [Google Scholar]
Burkhardt 2010a {published data only}
- Burkhardt O, Ewig S, Haagen U, Giersdorf S, Hartmann O, Wescheider K, et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory care. European Respiratory Journal 2010;36(3):601-7. [DOI] [PubMed] [Google Scholar]
Dahler‐Eriksen 1999 {published data only}
- Dahler-Eriksen BS, Lauritzen T, Lassen JF, Lund ED, Brandslund I. Near-patient test for C-reactive protein in general practice: assessment of clinical, organizational, and economic outcomes. Clinical Chemistry 1999;45(4):478-85. [PubMed] [Google Scholar]
de Lusignan 2020 {published data only}
- Lusignan S, Hoang U, Liyanage H, Tripathy M, Yonova I, Byford R, et al. Integrating molecular point-of-care testing for influenza into primary care: a mixed-methods feasibility study. British Journal of General Practice 2020;70(697):e555-62. [DOI: 10.3399/bjgp20X710897] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eley 2020 {published data only}
- Eley CV, Sharma A, Lee H, Charlett A, Owens R, McNulty CA. Effects of primary care C-reactive protein point-of-care testing on antibiotic prescribing by general practice staff: pragmatic randomised controlled trial, England, 2016 and 2017. Euro Surveillance 2020;25(44):1-11. [DOI: 10.2807/1560-7917.ES.2020.25.44.1900408] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 2017 {published data only}
- Fiore D. Procalcitonin to guide antibiotic therapy for acute respiratory infections. Internal Medicine Alert 2017;39:1-2. [Google Scholar]
Gonzales 2011 {published data only}
- Gonzales R, Aagaard EM, Camargo CA Jr, Ma OJ, Plautz M, Maselli JH, et al. C-reactive protein testing does not decrease antibiotic use for acute cough illness when compared to a clinical algorithm. Journal of Emergency Medicine 2011;41(1):1-7. [DOI] [PubMed] [Google Scholar]
Huang 2018 {published data only}
- Huang DT, Yealy DM, Filbin MR, Brown AM, Chang CH, Doi Y, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. New England Journal of Medicine 2018;379(3):236-49. [DOI: 10.1056/NEJMoa1802670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Isa 2022 {published data only}
- Isa HM, Mohroofi AD, Alkhan FN, Hasan AZ, Alkubisi MM, Alhewaizem SS, et al. C-reactive protein levels in children with acute bronchiolitis. International Journal of Pediatrics 23 May 2022;eCollection:1311936. [DOI: 10.1155/2022/1311936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2011 {published data only}
- Kavanagh KE, O'Shea E, Halloran R, Cantillon P, Murphy AW. A pilot study of the use of near-patient C-reactive protein testing in the treatment of adult respiratory tract infections in one Irish general practice. BMC Family Practice 2011;12:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keitel 2017 {published data only}
- Keitel K, Kagoro F, Samaka J, Masimba J, Said Z, Temba H, et al. A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): a randomized, controlled non-inferiority trial. PLOS Medicine 2017;23(14):e1002411. [DOI: 10.1371/journal.pmed.1002411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2014 {published data only}
- Little P, Hobbs R, Moore M, Mant D, Williamson I. PRImary Care Streptococcal Management Study (PRISM): in vitro study, diagnostic cohorts, and a pragmatic adaptive randomised controlled trial with nested qualitative study and cost-effectiveness study. Health Technology Assessement 2014;18(6):1-101. [DOI: 10.3310/hta18060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Llor 2012 {published data only}
- Llor C, Bjerrum L, Arranz J, Garcia G, Cots JM, Gonzalez BL-V, et al. C-reactive protein testing in patients with acute rhinosinusitis leads to a reduction in antibiotic use. Family Practice 2012;29(6):653-8. [DOI] [PubMed] [Google Scholar]
Mann 2020 {published data only}
- Mann D, Hess R, McGinn T, Richardson S, Jones S, Palmisano J, et al. Impact of clinical decision support on antibiotic prescribing for acute respiratory infections: a cluster randomized implementation trial. Journal of General Internal Medicine 2020;35:788-95. [DOI: 10.1007/s11606-020-06096-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meili 2016 {published data only}
- Meili M, Kutz A, Briel M, Christ-Crain M, Bucher HC, Mueller B, et al. Infection biomarkers in primary care patients with acute respiratory tract infections - comparison of procalcitonin and C-reactive protein. BMC Pulmonary Medicine 2016;16:Article number: 43. [DOI: 10.1186/s12890-016-0206-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Minnaard 2016 {published data only}
- Minnaard MC, de Pol AC, Hopstaken RM, Delft S, Broekhuizen BD, Verheij TJ, et al. C-reactive protein point-of-care testing and associated antibiotic prescribing. Family Practice 2016;33(4):408-13. [DOI: 10.1093/fampra/cmw039] [DOI] [PubMed] [Google Scholar]
Montassier 2019 {published data only}
- Montassier E, Javaudin F, Moustafa F, Nandjou D, Maignan M, Hardouin JB, et al. Guideline-based clinical assessment versus procalcitonin-guided antibiotic use in pneumonia: a pragmatic randomized trial. Annals of Emergency Medicine 2019;74(4):580-91. [DOI: 10.1016/j.annemergmed.2019.02.025.] [DOI] [PubMed] [Google Scholar]
Oppong 2018 {published data only}
- Oppong R, Smith RD, Little P, Verheij T, Butler CC, Goossens H, et al. Cost-effectiveness of internet-based training for primary care clinicians on antibiotic prescribing for acute respiratory tract infections in Europe. Journal of Antimicrobial Chemotherapy 2018;17(11):3189-98. [DOI: 10.1093/jac/dky309.] [DOI] [PubMed] [Google Scholar]
Rebnord 2016a {published data only (unpublished sought but not used)}
- Rebnord IK, Sandvik H, Mjelle AB, Hunskaar S. Out-of-hours antibiotic prescription after screening with C reactive protein: a randomised controlled study. BMJ Open 2016;6(5):e011231. [DOI: 10.1136/bmjopen-2016-011231] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schechter‐Perkins 2019 {published data only}
- Schechter-Perkins EM, Mitchell PM, Nelson KP, Liu JH, Shannon A, Ahern J, et al. Point-of-care influenza testing does not significantly shorten time to disposition among patients with an influenza-like illness. American Journal of Emergency Medicine 2019;37(5):873-8. [DOI: 10.1016/j.ajem.2018.08.005.] [DOI] [PubMed] [Google Scholar]
Stannard 2014 {published data only}
- Stannard D. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Critical Care Nurse 2014;34(2):75-6. [DOI: 10.4037/ccn2014559] [DOI] [PubMed] [Google Scholar]
Takemura 2005 {published data only}
- Takemura Y, Ebisawa K, Kakoi H, Saitoh H, Kure H, Ishida H, et al. Antibiotic selection patterns in acutely febrile new outpatients with or without immediate testing for C reactive protein and leucocyte count. Journal of Clinical Pathology 2005;58(7):729-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Bruel 2016 {published data only (unpublished sought but not used)}
- Van den Bruel A, Jones C, Thompson M, Mant D. C-reactive protein point-of-care testing in acutely ill children: a mixed methods study in primary care. Archive of Diseases in Childhood 2016;101(4):382-5. [DOI: 10.1136/archdischild-2015-309228] [DOI] [PubMed] [Google Scholar]
Verbakel 2016 {published data only (unpublished sought but not used)}
- Lemiengre MB, Verbakel JY, Colman R, De Burghgraeve T, Buntinx F, Aertgeerts B, et al. Reducing inappropriate antibiotic prescribing for children in primary care: a cluster randomised controlled trial of two interventions. British Journal of General Practice 2018;68(668):e204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemiengre MB, Verbakel JY, Colman R, Van Roy K, De Burghgraeve T, Buntinx F, et al. Point-of-care CRP matters: normal CRP levels reduce immediate antibiotic prescribing for acutely ill children in primary care: a cluster randomized controlled trial. Scandinavian Journal of Primary Health Care 2018;36(4):423-36. [DOI: 10.1080/02813432.2018.1529900] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbakel JY, Lemiengre MB, De Burghgraeve T, De Sutter A, Aertgeerts B, Shinkins B, et al. Should all acutely ill children in primary care be tested with point-of-care CRP: a cluster randomised trial. BMC Medicine 2016;14(1):131. [DOI: 10.1186/s12916-016-0679-2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN01559032 {published and unpublished data}01559032
- ISRCTN01559032. Respiratory tract infections in primary care [Converting habits of antibiotic prescribing for respiratory tract infections in German primary care - the cluster-randomized controlled CHANGE-2 trial]. www.isrctn.com/ISRCTN01559032 (first received 24 August 2012). [DOI] [PMC free article] [PubMed]
NCT03540706 {published data only}NCT03540706
- NCT03540706. Impact of the use of CRP on the prescription of antibiotics in General Practitioners (VIP). clinicaltrials.gov/ct2/show/NCT03540706 (first received 30 May 2018).
NCT03855215 {published data only}NCT03855215
- NCT03855215. Implementation of CRP point-of-care testing in primary care to improve antibiotic targeting in respiratory illness (ICAT). clinicaltrials.gov/ct2/show/study/NCT03855215 (first received 26 February 2019).
NCT03931577 {published data only}NCT03931577
- NCT03931577. Effectiveness of improving diagnostic and communication skills on antibiotic prescribing appropriateness in acute cough (ISAAC-CAT). clinicaltrials.gov/ct2/show/NCT03931577 (first received 30 April 2019).
NCT04216277 {published data only}
- NCT04216277. The procalcitonin guided antibiotics in respiratory infections in General Practice (PARI). clinicaltrials.gov/ct2/show/NCT04216277 (first received 2 January 2020).
Additional references
Aabenhus 2011
- Aabenhus R, Jensen JU. Procalcitonin-guided antibiotic treatment of respiratory tract infections in a primary care setting: are we there yet? Primary Care Respiratory Journal 2011;20(4):360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aabenhus 2014a
- Aabenhus R, Jensen JU, Jørgensen KJ, Hróbjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD010130. [DOI: 10.1002/14651858.CD010130] [DOI] [PubMed] [Google Scholar]
Aabenhus 2016
- Aabenhus R, Siersma V, Hansen MP, Bjerrum L. Antibiotic prescribing in Danish general practice 2004-13. Journal of Antimicrobial Chemotherapy 2016;71(8):2286-94. [DOI: 10.1093/jac/dkw117] [DOI] [PubMed] [Google Scholar]
Aabenhus 2017
- Aabenhus R, Siersma V, Sandholdt H, Køster-Rasmussen R, Hansen MP, Bjerrum L. Identifying practice-related factors for high-volume prescribers of antibiotics in Danish general practice. Journal of Antimicrobial Chemotherapy 2017;72(8):2385-91. [DOI: 10.1093/jac/dkx115.] [DOI] [PubMed] [Google Scholar]
Arnold 2009
- Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD003539. [DOI: 10.1002/14651858.CD003539.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2004
- Becker KL, Nylen ES, White JC, Muller B, Snider RH Jr. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. Journal of Clinical Endocrinology and Metabolism 2004;89(4):1512-25. [DOI] [PubMed] [Google Scholar]
Boiko 2020
- Boiko O, Gulliford MC, Burgess C. Revisiting patient expectations and experiences of antibiotics in an era of antimicrobial resistance: qualitative study. Health Expectations 2020;23:1250–8. [DOI: 10.1111/hex.13102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Briel 2008b
- Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Archives of Internal Medicine 2008;168(18):2000-7. [DOI: 10.1001/archinte.168.18.2000.] [DOI] [PubMed] [Google Scholar]
Bronzwaer 2002
- Bronzwaer SL, Cars O, Buchholz U, Molstad S, Goettsch W, Veldhuijzen IK, et al. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerging Infectious Diseases 2002;8(3):278-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burkhardt 2010b
- Burkhardt O, Ewig S, Haagen U, Giersdorf S, Hartmann O, Wegscheider K, et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. European Respiratory Journal 2010;36(3):601-7. [DOI: 10.1183/09031936.00163309] [DOI] [PubMed] [Google Scholar]
Butler 2008
- Butler CC, Simpson S, Wood F. General practitioners' perceptions of introducing near-patient testing for common infections into routine primary care: a qualitative study. Scandinavian Journal of Primary Health Care 2008;26:17-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler 2009
- Butler CC, Hood K, Verheij T, Little P, Melbye H, Nuttall J, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ 2009;338:b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler 2011
- Butler CC, Kelly MJ, Hood K, Schaberg T, Melbye H, Serra-Prat M, et al. Antibiotic prescribing for discoloured sputum in acute cough/lower respiratory tract infection. European Respiratory Journal 2011;38(1):119-25. [DOI] [PubMed] [Google Scholar]
Butler 2012
- Butler CC, Simpson SA, Dunstan F, Rollnick S, Cohen D, Gillespie D, et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ 2012;344:d8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cals 2011
- Cals JW, Ament AJ, Hood K, Butler CC, Hopstaken RM, Wassink GF, et al. C-reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: economic evaluation of a cluster randomized trial. Journal of Evaluation in Clinical Practice 2011;17(6):1059-69. [DOI: 10.1111/j.1365-2753.2010.01472.x.] [DOI] [PubMed] [Google Scholar]
Cals 2018
- Cals JW, Ebell MH. C-reactive protein: guiding antibiotic prescribing decisions at the point of care. British Journal of General Practice 2018;68(668):112-3. [DOI: 10.3399/bjgp18X694901] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlet 2011
- Carlet J, Collignon P, Goldmann D, Goossens H, Gyssens IC, Harbarth S, et al. Society's failure to protect a precious resource: antibiotics. Lancet 2011;378(9788):369-71. [DOI] [PubMed] [Google Scholar]
Childs 2019
- Childs A, Zullo AR, Joyce NR, McConeghy KW, Aalst R, Moyo P, et al. The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatrics 2019;19(1):210. [DOI: 10.1186/s12877-019-1236-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cillóniz 2018
- Cillóniz C, Rodríguez-Hurtado D, Torres A. Characteristics and management of community-acquired pneumonia in the era of global aging. Medical Sciences (Basel, Switzerland) 2018;6(2):35. [DOI: 10.3390/medsci6020035] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costelloe 2010
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340:c2096. [DOI] [PubMed] [Google Scholar]
Curtis 2019
- Curtis HJ, Walker AJ, Mahtani KR, Goldacre B. Time trends and geographical variation in prescribing of antibiotics in England 1998-2017. Journal of Antimicrobial Chemotherapy 2019;74(1):242-50. [DOI: 10.1093/jac/dky377.] [DOI] [PubMed] [Google Scholar]
Damschroder 2009
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science 2009;4:50. [DOI: 10.1186/1748-5908-4-50] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engel 2011
Falk 2009
- Falk G, Fahey T. C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies. Family Practice 2009;26(1):10-21. [DOI] [PubMed] [Google Scholar]
Fawsitt 2021
- Fawsitt CG, Lucey D, Harrington P, Jordan K, Marshall L, O'Brien KK, et al. A cost-effectiveness and budget impact analysis of C-reactive protein point-of-care testing to guide antibiotic prescribing for acute respiratory tract infections in primary care settings in Ireland: a decision-analytic model. Family Practice 2022;39:389-97. [DOI: 10.1093/fampra/cmab123.] [DOI] [PubMed] [Google Scholar]
Gjelstad 2013
- Gjelstad S, Hoye S, Straand J, Brekke M, Dalen I, Lindbaek M. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing (Rx-PAD) study). BMJ 2013;347:f4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzales 2001
- Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clinical Infectious Diseases 2001;33(6):757-62. [DOI] [PubMed] [Google Scholar]
Goossens 2005
- Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365(9459):579-87. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 2 January 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Harnden 2007
- Harnden A, Perera R, Brueggemann AB, Mayon-White R, Crook DW, Thomson A, et al. Respiratory infections for which general practitioners consider prescribing an antibiotic: a prospective study. Archives of Diseases in Childhood 2007;92(7):594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JP, White IR, Wood AM. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clinical Trials 2008;5(3):225-39. [DOI: 10.1177/1740774508091600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Hoare 2006
- Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332(7549):1077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holm 2007
- Holm A, Pedersen SS, Nexoe J, Obel N, Nielsen LP, Koldkjaer O, et al. Procalcitonin versus C-reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. British Journal of General Practice 2007;57(540):555-60. [PMC free article] [PubMed] [Google Scholar]
Hopstaken 2003
- Hopstaken RM, Muris JW, Knottnerus JA, Kester AD, Rinkens PE, Dinant GJ. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C-reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. British Journal of General Practice 2003;53(490):358-64. [PMC free article] [PubMed] [Google Scholar]
Huang 2013
- Huang Y, Chen R, Wu T, Wei X, Guo A. Association between point-of-care CRP testing and antibiotic prescribing in respiratory tract infections: a systematic review and meta-analysis of primary care studies. British Journal of General Practice 2013;63(616):e787-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunter 2015
- Hunter R. Cost-effectiveness of point-of-care C-reactive protein tests for respiratory tract infection in primary care in England. Advances in Therapy 2015;32(1):69-85. [DOI: 10.1007/s12325-015-0180-x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2010
- Jakobsen KA, Melbye H, Kelly MJ, Ceynowa C, Molstad S, Hood K, et al. Influence of CRP testing and clinical findings on antibiotic prescribing in adults presenting with acute cough in primary care. Scandinavian Journal of Primary Health Care 2010;28(4):229-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Köchling 2018
- Köchling A, Löffler C, Reinsch S, Hornung A, Böhmer F, Altiner A, et al. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: a systematic review. Implementation Science 2018;13(1):47. [DOI: 10.1186/s13012-018-0732-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kruger 2009
- Kruger S, Ewig S, Papassotiriou J, Kunde J, Marre R, Baum H, et al. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: results from the German competence network CAPNETZ. Respiratory Research 2009;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Lingervelder 2021
- Lingervelder D, Koffijberg H, Kusters RI, Jzerman MJ. Health economic evidence of point-of-care testing: a systematic review. PharmacoEconomics - Open 2021;5(2):157-73. [DOI: 10.1007/s41669-020-00248-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2013b
- Little P, Stuart B, Moore M, Coenen S, Butler CC, Godycki-Cwirko M, et al. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial. Lancet Infectious Disease 2013;13(2):123-9. [DOI] [PubMed] [Google Scholar]
Little 2021
- Little P, Francis NA, Stuart B, O'Reilly G, Thompson N, Becque T, et al. Antibiotics for lower respiratory tract infection in children presenting in primary care in England (ARTIC PC): a double-blind, randomised, placebo-controlled trial. Lancet 2021;16:1417-26. [DOI: 10.1016/S0140-6736(21)01431-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martínez‐González 2020
- Martínez-González N, Keizer A, Plate E, Coenen A, Valeri S, Verbakel F, et al. Point-of-care C-reactive protein testing to reduce antibiotic prescribing for respiratory tract infections in primary care: systematic review and meta-analysis of randomised controlled trials. Antibiotics (Basel, Switzerland) 2020;9(9):610. [DOI: 10.3390/antibiotics9090610] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthys 2007
- Matthys J, De Meyere M, Driel ML, De Sutter A. Differences among international pharyngitis guidelines: not just academic. Annals of Family Medicine 2007;5(5):436-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melbye 2011
- Melbye H, Francis NA, Butler CC. Inflammatory markers are helpful when treating LRTI in primary care. Primary Care Respiratory Journal 2011;20(4):367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meropol 2013
- Meropol SB, Localio AR, Metlay JP. Risks and benefits associated with antibiotic use for acute respiratory infections: a cohort study. Annals of Family Medicine 2013;11(2):165-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metlay 1997
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278(17):1440-5. [PubMed] [Google Scholar]
NICE Clinical Guidelines 2019
- National Institute for Health and Care Excellence (NICE). Pneumonia in adults: diagnosis and management. www.ncbi.nlm.nih.gov/books/NBK552669/ (accessed prior to 27 September 2022). [PubMed]
Nouvenne 2016
- Nouvenne A, Ticinesi A, Folesani G, Cerundolo N, Prati B, Morelli I, et al. The association of serum procalcitonin and high-sensitivity C-reactive protein with pneumonia in elderly multimorbid patients with respiratory symptoms: retrospective cohort study. BMC Geriatrics 2016;15(16):16. [DOI: 10.1186/s12877-016-0192-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oppong 2013
- Oppong R, Jit M, Smith RD, Butler CC, Melbye H, Mölstad S, et al. Cost-effectiveness of point-of-care C-reactive protein testing to inform antibiotic prescribing decisions. British Journal of General Practice 2013;63(612):e465-71. [DOI: 10.3399/bjgp13X669185.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016
- Page MJ, Higgins JP, Clayton G, Sterne JA, Hróbjartsson A, Savović J. Empirical evidence of study design biases in randomized trials: systematic review of meta-epidemiological studies. PLOS ONE 2016;11(7):e0159267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pavia 2011
- Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clinical Infectious Diseases 2011;52(Suppl 4):284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Public Health England 2021
- Public Health England. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2020 to 2021. assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1069632/espaur-report-2020-to-2021-16-Nov-FINAL-v2.pdf (accessed prior to 8 October 2022).
Rebnord 2016b
- Rebnord IK, Sandvik H, Mjelle AB, Hunskaar S. Out-of-hours antibiotic prescription after screening with C reactive protein: a randomised controlled study. BMJ Open 2016;12(6):e011231. [DOI: 10.1136/bmjopen-2016-011231] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Sande‐Bruinsma 2008
- Sande-Bruinsma N, Grundmann H, Verloo D, Tiemersma E, Monen J, Goossens H, et al. Antimicrobial drug use and resistance in Europe. Emerging Infectious Diseases 2008;14(11):1722-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuetz 2009
- Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al, ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302(10):1059-66. [DOI: 10.1001/jama.2009.1297] [DOI] [PubMed] [Google Scholar]
Schuetz 2017
- Schuetz P, Muller B, Christ-Crain M, Stolz D, Tamm M, Bouadma L, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No: CD007498. [DOI: 10.1002/14651858.CD007498.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 2021
- Schwartz KL, Ivers N, Langford BJ, Taljaard M, Neish D, Brown KA, et al. Effect of antibiotic-prescribing feedback to high-volume primary care physicians on number of antibiotic prescriptions: a randomized clinical trial. JAMA Internal Medicine 2021;181(9):1165-73. [DOI: 10.1001/jamainternmed.2021.2790.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smieszek 2018
- Smieszek T, Pouwels KB, Dolk FC, Smith DR, Hopkins S, Sharland M, et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. Journal of Antimicrobial Chemotherapy 2018;73:ii36-43. [DOI: 10.1093/jac/dkx500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013
- Smith R, Coast J. The true cost of antimicrobial resistance. BMJ 2013;346:f1493. [DOI] [PubMed] [Google Scholar]
Spurling 2017
- Spurling GK, Del Mar CB, Dooley L, Foxlee R, Farley R. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD004417. [DOI: 10.1002/14651858.CD004417.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanton 2010
- Stanton N, Francis NA, Butler CC. Reducing uncertainty in managing respiratory tract infections in primary care. British Journal of General Practice 2010;60(581):e466-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stocks 2002
- Stocks N, Faher T. Labelling of acute respiratory illness: evidence of between-practitioner variation in the UK. Family Practice 2002;19(4):375-7. [DOI] [PubMed] [Google Scholar]
Stupka 2009
- Stupka JE, Mortensen EM, Anzueto A, Restrepo MI. Community-acquired pneumonia in elderly patients. Aging Health 2009;5(6):763-74. [DOI: 10.2217/ahe.09.74] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tonkin‐Crine 2017
- Tonkin-Crine SK, Tan PS, Hecke O, Wang K, Roberts NW, McCullough A, et al. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012252. [DOI: 10.1002/14651858.CD012252.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Bruel 2011
- Van den Bruel A, Thompson MJ, Haj-Hassan T, Stevens R, Moll H, Lakhanpaul M, et al. Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. BMJ 2011;342:d3082. [DOI: 10.1136/bmj.d3082.] [DOI] [PubMed] [Google Scholar]
van der Meer 2005
- Meer V, Neven AK, den Broek PJ, Assendelft WJ. Diagnostic value of C reactive protein in infections of the lower respiratory tract: systematic review. BMJ 2005;331(7507):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Hecke 2020
- Van Hecke O, Raymond M, Lee JJ, Turner P, Goyder CR, Verbakel JY, et al. In-vitro diagnostic point-of-care tests in paediatric ambulatory care: a systematic review and meta-analysis. PLOS ONE 2020;15(7):0235605. [DOI: 10.1371/journal.pone.0235605] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venekamp 2015
- Venekamp RP, Sanders S, Glaszioiu PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD000219. [DOI: 10.1002/14651858.CD000219.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verbakel 2019
- Verbakel JY, Lee JJ, Goyder C, Tan PS, Ananthakumar T, Turner PJ, et al. Impact of point-of-care C reactive protein in ambulatory care: a systematic review and meta-analysis. BMJ Open 2019;9(1):e025036. [DOI: 10.1136/bmjopen-2018-025036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volanakis 2001
- Volanakis JE. Human C-reactive protein: expression, structure, and function. Molecular Immunology 2001;38(2-3):189-97. [DOI] [PubMed] [Google Scholar]
Wang 2021
- Wang D, Liu C, Zhang X, Liu C. Does diagnostic uncertainty increase antibiotic prescribing in primary care? NPJ Primary Care Respiratory Medicine 2021;31(1):17. [DOI: 10.1038/s41533-021-00229-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2016
- World Health Organization. Global action plan on antimicrobial resistance. www.who.int/publications/i/item/9789241509763 (accessed prior to 8 October 2022).
Wipf 1999
- Wipf JE, Lipsky BA, Hirschmann JV, Boyko EJ, Takasugi J, Peugeot RL, et al. Diagnosing pneumonia by physical examination: relevant or relic? Archives of Internal Medicine 1999;159(10):1082-7. [DOI] [PubMed] [Google Scholar]
Wood 2011
- Wood F, Brookes-Howell L, Hood K, Cooper L, Verheij T, Goossens H, et al. An ideal test? A multi-country qualitative study of clinicians' and patients' views of point of care tests for lower respiratory tract infection in primary care. Family Practice 2011;28(6):661-9. [DOI] [PubMed] [Google Scholar]
Woodhead 2011
- Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clinical Microbiology and Infection 2011;17(Suppl 6):E1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Aabenhus 2012
- Aabenhus R, Jensen J-US, Jørgensen KJ, Hróbjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD010130. [DOI: 10.1002/14651858.CD010130] [10.1002/14651858.CD010130] [DOI] [PubMed] [Google Scholar]
Aabenhus 2014b
- Aabenhus R, Jensen J-US, Jørgensen KJ, Hróbjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD010130. [DOI: 10.1002/14651858.CD010130.pub2] [DOI] [PubMed] [Google Scholar]


