Abstract
Synucleinopathies are characterized by the deposition of α-synuclein (α-syn) aggregates before the onset of clinical symptoms. Therefore, in vivo imaging of α-syn may contribute to early diagnosis of these diseases and has attracted much attention in recent years. However, no clinically useful probes have been reported. In the present study, 16 quinoline/quinoxaline derivatives with different styryl and fluorine groups were evaluated in order to develop α-syn imaging probes. Among them, SQ3, which is a quinoline analogue with a p-(dimethylamino)styryl group and fluoroethoxy group at the 2- and 7- positions of the skeleton, displayed moderate selectivity for α-syn aggregates over β-amyloid (Aβ) aggregates (Ki = 230 nM), while maintaining high binding affinity for α-syn aggregates (Ki = 39.3 nM). In a biodistribution study, [18F]SQ3 exhibited high uptake (2.08% ID/g at 2 min after intravenous injection) into a normal mouse brain. Taken together, we demonstrate that [18F]SQ3 has basic properties as a lead compound for the development of a useful α-syn imaging probe.
Keywords: α-Synuclein, PET probe, Styrylquinoline, Styrylquinoxaline
In recent years, with the advent of an aging society, the increase in the number of patients suffering from synucleinopathies, including Parkinson’s disease, dementia with Lewy bodies, and multiple-system atrophy, has been a concern. However, even the method of definite diagnosis has not been established, let alone radical treatment. Abnormal depositions of Lewy bodies, Lewy neurites, and glial cytoplasmic inclusions are observed in the brains of patients suffering from these diseases before the onset of clinical symptoms.1 α-Synuclein (α-syn) aggregates are major constituents of these hallmarks and have been gathering attention as biomarkers of synucleinopathies. However, the association between the progress of synucleinopathies and amount of α-syn aggregates in the brain is unclear. Therefore, in vivo imaging of α-syn is considered to contribute to early diagnosis and elucidation of the pathophysiology of synucleinopathies.
Among several imaging methods, positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are excellent tools for non-invasive and quantitative imaging of biomolecules with high sensitivity. Based on this, several kinds of nuclear medicine imaging probes targeting α-syn aggregates have been reported over the past few years.2−5 However, the detection of α-syn aggregates in vivo remains elusive. There are two major problems regarding the in vivo imaging of α-syn aggregates. The first problem is low brain permeability. Many α-syn imaging probes with high binding affinity for α-syn aggregates generally have a large molecular size (molecular weight (MW) > 430) and high lipophilicity (CLogP > 4.0), markedly decreasing brain permeability. The second problem is selectivity for α-syn aggregates over β-amyloid (Aβ) aggregates. It is well-known that α-syn aggregates are colocalized with Aβ—which is a major biomarker of Alzheimer’s disease (AD)—aggregates in some synucleinopathy patients’ brains.6 Since both proteins form aggregates with β-sheet structures, most of the probes with high affinity for α-syn aggregates also exhibit high affinity for Aβ aggregates. In addition, the concentration of α-syn aggregates is much lower than that of Aβ aggregates in the brain.7 Taken together, α-syn imaging probes must show selectivity for α-syn aggregates versus Aβ aggregates. Therefore, it is necessary to identify a compound showing three properties: high binding affinity for α-syn aggregates, high brain uptake, and selective binding for α-syn aggregates.
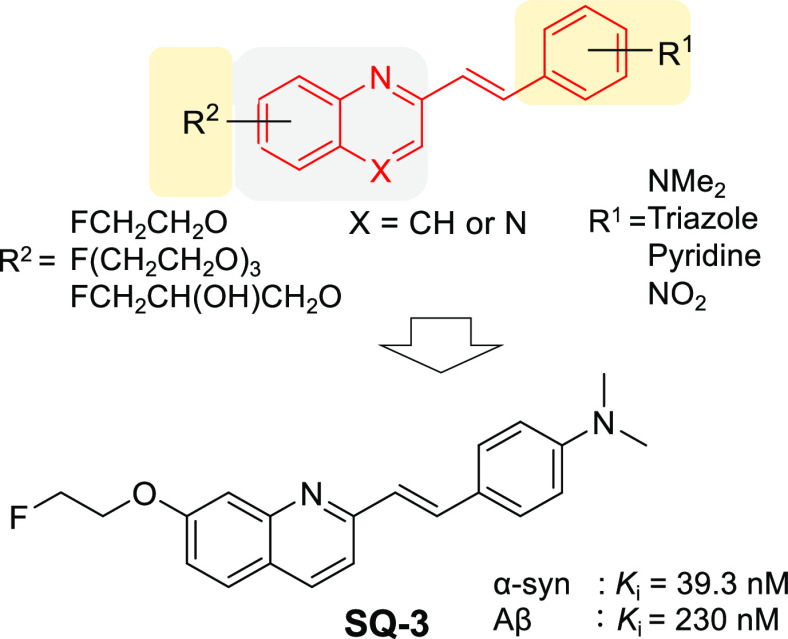
Various kinds of quinoline and quinoxaline analogues were reported as amyloid imaging probes.8−13 Some reports suggested that binding affinities for Aβ aggregates change, depending on the position of the substitution group on the quinoxaline scaffold.11,13 Furthermore, there was a report that the quinoline scaffold with a styryl moiety at the 2-position displayed a high binding affinity for α-syn aggregates ([18F]14: inhibition constant Ki = 18 nM, dissociation constant Kd = 79 nM) in vitro.14 This report also suggested that the double bond between quinoline and the aromatic ring may be important to enhance the affinity for α-syn aggregates. This study was focused on the styrylquinoline/quinoxaline backbone, and structure–activity relationship studies were performed on 16 derivatives for the development of α-syn imaging probes.
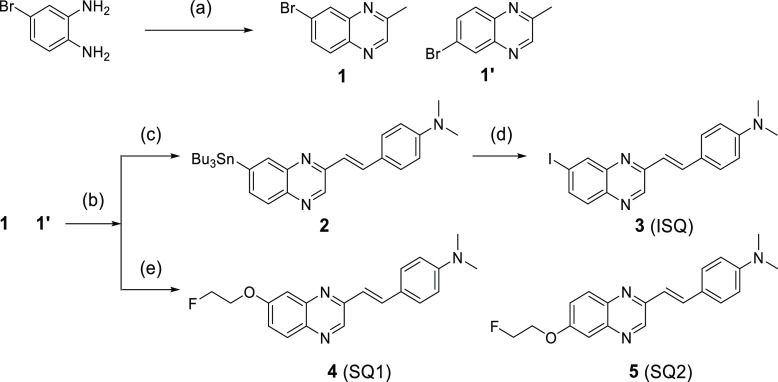
Quinoxaline derivatives were synthesized according to Scheme 1. After a mixture of 1 and 1′ was synthesized according to a method reported previously,15 styrylquinoxaline scaffolds were obtained by a condensation reaction. In the case of 3 (ISQ), the 7-tributyltin quinoxaline scaffold was prepared from a mixture of bromo compounds using a bromo-to-tributyltin exchange reaction catalyzed by Pd(0) and isolated. Thereafter, the 7-tributyltin scaffold was reacted with I2 in chloroform at 25 °C to give 3. In the case of 4 (SQ1) and 5 (SQ2), crude phenol scaffolds were reacted with 2-fluoroethyl p-toluenesulfonate in N,N-dimethylformamide (DMF) to give 4 and 5 after preparing the crude phenol scaffolds from a mixture of bromo compounds using a method reported previously.16 Compounds 4 (SQ1) and 5 (SQ2) could be separated by column chromatography. Finally, the structures of quinoxaline derivatives were determined by X-ray crystallography. The total yields from the materials were 6–16%.
Scheme 1. Synthesis Route of Quinoxaline Derivatives.
Reagents and conditions: (a) pyruvic aldehyde, EtOH, 25 °C; (b) p-dimethylaminobenzaldehyde, piperidine, AcOH, toluene, reflux; (c) Bu4Sn, Pd(Ph3)4, toluene, reflux; (d) I2, CHCl3, 25 °C; (e) (1) Cu(acac)2, LiOH·H2O, N1,N2-bis(4-hydroxy-2,6-dimethylphenyl)oxalamide, dimethyl sulfoxide (DMSO)/H2O, 80 °C, (2) 2-fluoroethyl p-toluenesulfonate, Cs2CO3, DMF, 95 °C.
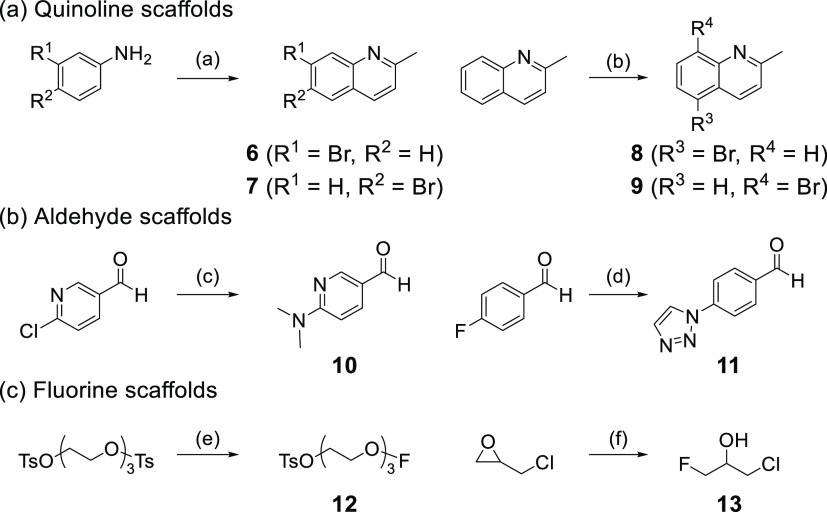
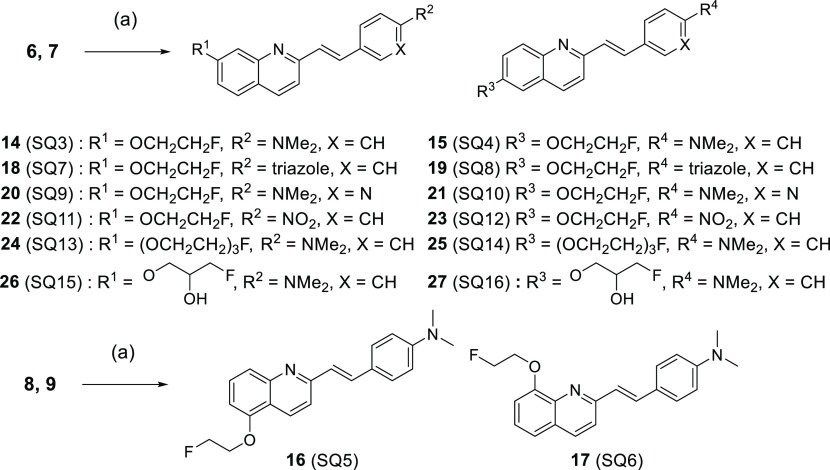
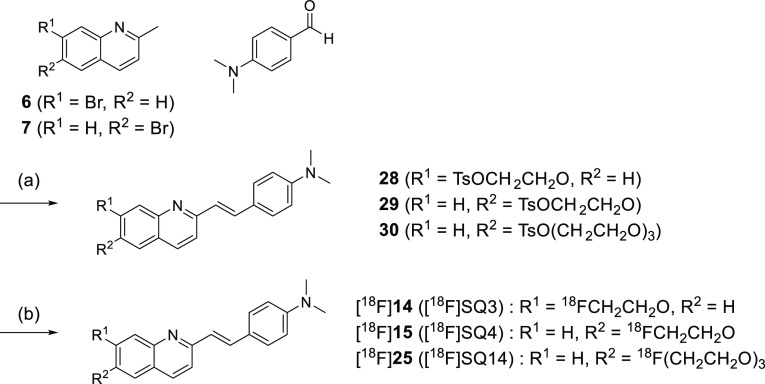
Next, quinoline derivatives were synthesized according to Schemes 2-1 and 2-2. Quinoline,17,18 aldehyde,19,20 and fluorine21,22 scaffolds were synthesized using methods reported previously. Styrylquinoline scaffolds were obtained by condensation reaction from quinoline scaffolds (6, 7, 8, and 9) and aldehyde scaffolds (p-dimethylaminobenzaldehyde, p-nitrobenzaldehyde, 10, and 11). Thereafter, the phenol scaffolds were prepared from styryl scaffolds using the same method as for quinoxaline derivatives. The mixture of phenol scaffolds was reacted with fluorine scaffolds (2-fluoroethyl p-toluenesulfonate, 12, and 13) in DMF to give SQ derivatives (14–27, SQ3–16). The total yields from the materials were 0.4–17%.
Scheme 2-1. Synthesis Routes of Quinoline, Aldehyde, and Fluorine Scaffolds.
Reagents and conditions: (a) ethyl vinyl ether, AcOH, 25 °C → 100 °C; (b) N-bromosuccinimide (NBS), conc. H2SO4, 25 °C; (c) NHMe2, H2O, 80 °C; (d) 1,2,3-triazole, K2CO3, DMF, 100 °C; (e) tetrabutylammonium fluoride (TBAF), tetrahydrofuran (THF), 70 °C; (f) TREAT HF, 130 °C.
Scheme 2-2. Synthesis Routes of Quinoline Derivatives.
Reagents and conditions: (a) (1) aldehyde scaffolds, piperidine, AcOH, toluene, reflux, (2) Cu(acac)2, LiOH·H2O, N1,N2-bis(4-hydroxy-2,6-dimethylphenyl)oxalamide, DMSO/H2O, 80 °C, (3) fluorine scaffolds, NaH or Cs2CO3, DMF, 95 °C.
The main reason for the low yields of styrylquinoline/quinoxaline derivatives was the low yields of the condensation reaction in the first step. The amount of aldehyde was increased and the reaction time was extended, but no significant improvement in yield was observed. In addition, many inseparable byproducts were produced. Therefore, there is room for improvement in the method of this reaction.
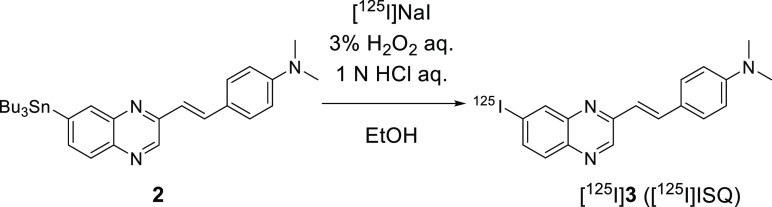
Since 125I has a longer half-life time (T1/2 = 60 days) than 18F (T1/2 = 109.8 min), we used a 125I-labeled compound as a competitive inhibitor. Accordingly, [125I]ISQ was designed, synthesized, and evaluated. This compound is suitable as a competitive inhibitor because the structure is similar to those of the SQ derivatives. [125I]ISQ was obtained from tributyltin precursor 2 by the iododestannylation reaction (Scheme 3). The radiochemical identity of the radioiodinated ligands was confirmed by co-injection with non-radioiodinated compounds from their high-performance liquid chromatography (HPLC) profiles. [125I]ISQ was obtained in a radiochemical yield of 19.4% with radiochemical purities of over 95% after HPLC purification.
Scheme 3. Radiosynthesis of [125I]ISQ.
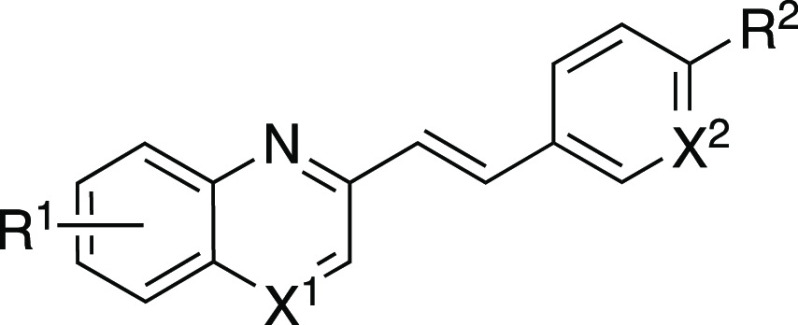
The affinities of ISQ were evaluated for both α-syn and Aβ aggregates. From the results of binding saturation assays using recombinant α-syn and Aβ aggregates, ISQ displayed high binding affinities for both of them (α-syn: Kd = 25.1 nM, Aβ: Kd = 8.53 nM). Considering these results, a binding inhibition assay was performed using [125I]ISQ as a competitive inhibitor for both α-syn and Aβ aggregates. Table 1 summarizes the Ki value of all SQ derivatives for α-syn and Aβ aggregates.
Table 1. Ki Values of SQ Derivatives for Recombinant α-Syn and Aβ Aggregates.
|
Ki (nM)a |
||||||
|---|---|---|---|---|---|---|
| compd | X1 | X2 | R1 | R2 | α-syn | Aβ |
| SQ1 | N | CH | 7-OCH2CH2F | N(Me)2 | 75.6 ± 43.1 | 83.6 ± 59.2 |
| SQ2 | N | CH | 6-OCH2CH2F | N(Me)2 | 11.4 ± 8.97 | 11.7 ± 0.97 |
| SQ3 | CH | CH | 7-OCH2CH2F | N(Me)2 | 39.3 ± 17.6 | 230 ± 49.1 |
| SQ4 | CH | CH | 6-OCH2CH2F | N(Me)2 | 4.38 ± 2.81 | 7.69 ± 0.86 |
| SQ5 | CH | CH | 5-OCH2CH2F | N(Me)2 | 29.2 ± 18.5 | 6.26 ± 3.83 |
| SQ6 | CH | CH | 8-OCH2CH2F | N(Me)2 | >1000 | >1000 |
| SQ7 | CH | N | 7-OCH2CH2F | N(Me)2 | >1000 | >1000 |
| SQ8 | CH | N | 6-OCH2CH2F | N(Me)2 | 56.5 ± 20.0 | 47.8 ± 4.84 |
| SQ9 | CH | CH | 7-OCH2CH2F | triazole | 586 ± 162 | >1000 |
| SQ10 | CH | CH | 6-OCH2CH2F | triazole | 185 ± 123 | 21.0 ± 2.20 |
| SQ11 | CH | CH | 7-OCH2CH2F | NO2 | 361 ± 180 | >1000 |
| SQ12 | CH | CH | 6-OCH2CH2F | NO2 | 275 ± 105 | 31.7 ± 11.3 |
| SQ13 | CH | CH | 7-(OCH2CH2)3F | N(Me)2 | >1000 | >1000 |
| SQ14 | CH | CH | 6-(OCH2CH2)3F | N(Me)2 | 10.8 ± 5.53 | 9.56 ± 1.57 |
| SQ15 | CH | CH | 7-OCH2CH(OH)CH2F | N(Me)2 | 618 ± 252 | >1000 |
| SQ16 | CH | CH | 6-OCH2CH(OH)CH2F | N(Me)2 | 85.3 ± 26.1 | 200 ± 22.8 |
Values are the mean ± standard deviation of the mean for 6–9 independent experiments.
First, four compounds (SQ1–4) were evaluated to determine which backbone (quinoline or quinoxaline) is suitable for α-syn imaging probes. A dimethylamino group was introduced in the p-position on the styryl moiety because many α-syn imaging probes with such designs have been reported.23,24 SQ4 displayed the highest binding affinity for α-syn aggregates (Ki = 4.38 nM) of the four compounds but also bound to Aβ aggregates (Ki = 7.69 nM). SQ3 displayed moderate selectivity for α-syn aggregates over Aβ aggregates (Ki = 230 nM) while maintaining high binding affinity for α-syn aggregates (Ki = 39.3 nM). On the other hand, the fact that the quinoxaline derivatives (SQ1 and SQ2) displayed lower binding affinity for α-syn aggregates than the corresponding quinoline derivatives (SQ3 and SQ4) revealed that the quinoline backbone may be preferable to the quinoxaline backbone for α-syn imaging probes.
Next, SQ5 and SQ6 had a fluoroethoxy moiety introduced at the 5- and 8-positions of quinoline, respectively, in order to evaluate the effect of the introduction site of a substituent on binding affinities for α-syn and Aβ aggregates. Interestingly, SQ5 displayed high binding affinities for both protein aggregates (α-syn: Ki = 29.2 nM, Aβ: Ki = 6.26 nM), although SQ6 displayed low binding affinities for them (α-syn: Ki > 1000 nM, Aβ: Ki > 1000 nM). It was suggested that there is much space near the 5- and 6-positions of the quinoline skeleton at the binding site of the SQ compounds for both protein aggregates. In addition, since the space near the 7-position at the binding site for α-synuclein aggregates of the probe might be wider than that of Aβ aggregates, it is considered that SQ3 showed higher affinity for α-syn aggregates than Aβ aggregates.
SQ4, with the highest affinity for α-syn aggregates, and SQ3, with the highest selectivity for α-syn aggregates, against Aβ aggregates of 4 compounds (SQ3–6) were selected as lead compounds for further structure–activity relationship study. Next, the brain permeability of styrylquinoline derivatives was the focus. CNS MPO SCORE is an index of brain permeability that is calculated from six elements (CLogP, MW, pKa, topological polar surface area (TPSA), hydrogen bond donor (HBD), and ClogD).25 CNS MPO SCORE of most compounds showing brain permeability is equivalent to or higher than 4 (Table 2). Although SQ3 and SQ4 meet this standard, six compounds (SQ7–12) were designed and evaluated with a modified styryl moiety in order to identify better α-syn imaging probes. These six compounds showed a higher MPO SCORE than SQ3 and SQ4 (Table 2). In particular, CLogP has been greatly reduced, and they were designed to improve water solubility. Moreover, it was reported that kinetics in the normal rat brain were improved by the introduction of a triazole group or pyridine ring in IMPY (N,N-dimethyl-4-(6-(methylthio)imixazo[1,2-a]pyridinyl)aniline) derivatives, Aβ imaging probes, instead of a dimethylamine group or benzene ring.26 It was also reported that chalcone derivatives detect α-syn aggregates with high affinity and selectivity by replacing a p-dimethylamino group with a p-nitro group.5 However, SQ7–12 displayed lower affinity for α-syn aggregates (Ki ≥ 185 nM) than the corresponding p-dimethylamino SQ derivatives (SQ3 and SQ4). Although there are several possible causes for this result, the dimethylamino group and benzene ring are largely involved in the hydrophobic binding site of the probe for protein aggregates. For that reason, it is considered that the hydrophobic interaction would be diminished by the design to increase water solubility in order to improve brain permeability.
Table 2. MPO Score and Its Component Parameter Values.
| compd | CLogP | CLogD | MW | TPSA | HBD | CNS MPO score |
|---|---|---|---|---|---|---|
| ISQ | 4.735 | 2.74 | 401.2 | 29.02 | 0 | 3.83 |
| SQ1,2 | 4.301 | 2.734 | 337.4 | 38.25 | 0 | 4.77 |
| SQ3,4 | 4.207 | 3.207 | 336.4 | 25.36 | 0 | 4.17 |
| SQ5,6 | 4.207 | 3.207 | 336.4 | 25.36 | 0 | 4.17 |
| SQ7,8 | 3.564 | 3.564 | 360.4 | 52.83 | 0 | 4.94 |
| SQ9,10 | 3.023 | 2.678 | 337.4 | 38.25 | 0 | 5.32 |
| SQ11,12 | 3.987 | 3.987 | 338.3 | 67.94 | 0 | 4.60 |
| SQ13,14 | 3.924 | 3.481 | 424.5 | 43.82 | 0 | 4.58 |
| SQ15,16 | 3.321 | 2.555 | 366.4 | 45.59 | 1 | 5.10 |
Next, the study focused on the fluoroethoxy moiety, and four compounds (SQ13–16) modified with this moiety in order to improve brain permeability were evaluated. These compounds also showed a higher CNS MPO SCORE than SQ3 and SQ4 (Table 2). Moreover, a previous study indicated that brain permeability was improved by the introduction of fluorotriethylene glycol or (3-fluoro-2-hydroxy)propoxyl instead of a fluoroethoxy moiety.12 However, most of them displayed lower affinity for α-syn aggregates than the corresponding fluoroethoxy SQ derivatives (SQ3 and SQ4). Only SQ14 displayed high binding affinity for α-syn aggregates (Ki = 10.8 nM) but also showed high binding affinity for Aβ aggregates (Ki = 9.56 nM). It was suggested that long linkers at the 7-position of the quinoline backbone and linkers with hydroxyl groups may reduce the binding affinity for α-syn aggregates. To summarize the results for SQ7–14, although we used CNS MPO SCORE to increase brain permeability, it is suggested that the improvement of water solubility markedly reduced the binding affinity for α-syn aggregates, as for styrylquinoline derivatives.
Summarizing the results of SQ1–14, SQ3 had the highest selectivity for α-syn aggregates against Aβ aggregates. Efficacious α-syn imaging agents show Ki ≤ 1 nM for α-syn,27 although it is not possible to establish a clear standard because the quantitative value varies greatly depending on the kind of inhibitor in the inhibition assay. In addition, since α-syn aggregates have a lower concentration in the brain than Aβ aggregates and the size of the aggregates is smaller, the selectivity against Aβ aggregates should be 10 times higher or more. Considering the results from these points of view, the binding affinity and selectivity versus Aβ aggregates of SQ3 are considered to be moderate.
CNS MPO SCORE is only a predictive index, and there is no correlation between CNS MPO SCORE and brain uptake for some compounds. From the results of the binding assay, both SQ4 and SQ14 showed much higher affinity for α-syn aggregates than SQ3. Therefore, three compounds (SQ3, SQ4, and SQ14) were labeled with 18F to evaluate brain uptake and the correlation between brain uptake and CNS MPO SCORE. The tosyl precursors (28, 29, and 30) of [18F]SQ3, [18F]SQ4, and [18F]SQ14 were prepared using the same method as for non-radioactive SQ3 (Scheme 4). [18F]SQ3, [18F]SQ4, and [18F]SQ14 were obtained from 28, 29, and 30 by a nucleophilic substitution reaction. The radiochemical identity of the radiofluorinated ligands was confirmed by the same method of 125I radiolabeling. [18F]SQ3, [18F]SQ4, and [18F]SQ14 were obtained in radiochemical yields of 20.0, 30.2, and 2.2%, respectively, with radiochemical purities of over 99% after HPLC purification. Specific activities of [18F]SQ3, [18F]SQ4, and [18F]SQ14 were 4260, 23.4, and 298 MBq/mmol, respectively.
Scheme 4. Synthesis Route of Precursors 28, 29, and 30 and Radiosynthesis of [18F]SQ3, [18F]SQ4, and [18F]SQ14.
Reagents and conditions: (a) (1) quinoline scaffolds (6 or 7), piperidine, AcOH, toluene, reflux, (2) Cu(acac)2, LiOH·H2O, N1,N2-bis(4-hydroxy-2,6-dimethylphenyl)oxalamide, DMSO/H2O, 80 °C, (3) tosyl scaffolds (1,2-bis(tosyloxy)ethane or triethylene glycol bis(p-toluenesulfonate), Cs2CO3, DMF, 100 °C; (b) Kryptofix 222, [18F]KF, DMF, 120 °C.
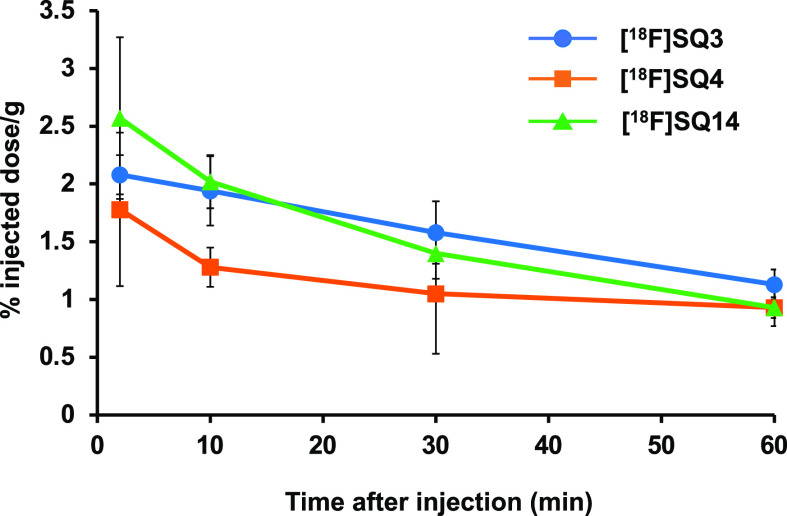
To evaluate brain uptake and washout, a biodistribution experiment using normal mice was performed (Table 3). In order to specifically detect α-syn aggregates in the brain, it is desirable for α-syn imaging probes to be rapidly taken up at an early time-point after injection and rapidly washed out from the brain because normal mice do not have α-syn aggregates in the brain. The radioactivity accumulation of [18F]SQ3, [18F]SQ4, and [18F]SQ14 in the brain was 2.08, 1.78, and 2.57 percentage injected dose per gram (% ID/g) at 2 min post injection, respectively. There was a positive correlation between CNS MPO SCORE and brain uptake. Thereafter, it was dispersed with time, decreasing to 1.05–1.58 and 0.93–1.13% ID/g at 30 and 60 min post injection, respectively. These uptakes were higher than those with [18F]2FBox3 (0.47% ID/g peak at 12 min after intravenous injection), which detected α-syn aggregates in an ex vivo autoradiography study, and [123/125I]PHNP-35 (0.78% ID/g peak at 2 min after intravenous injection), which detected α-syn aggregates selectively in fluorescence staining. However, these values were lower than those of Aβ and tau probes, which are used clinically.28,29 Washout from the brain was confirmed for all compounds (Figure 1). However, the radioactivity accumulation after 60 min exceeded 1% ID/g for all compounds, indicating that a part of these compounds tended to remain in the brain. The difference between SQ3 and SQ4 was not clear, but it is predicted that the difference in metabolic rate may have led to the difference in washout.
Table 3. Biodistribution of Radioactivity after Intravenous Injection of [18F]SQ3, [18F]SQ4, and [18F]SQ14 in Normal Micea.
| time after
injection (min) |
||||
|---|---|---|---|---|
| tissue | 2 | 10 | 30 | 60 |
| [18F]SQ3 | ||||
| blood | 4.04 (0.40) | 2.29 (0.53) | 1.97 (0.31) | 1.97 (0.22) |
| liver | 16.07 (2.37) | 16.51 (1.60) | 11.26 (1.23) | 7.83 (0.86) |
| kidney | 10.41 (0.59) | 7.87 (0.87) | 5.51 (0.68) | 4.19 (0.46) |
| intestine | 2.08 (0.44) | 3.81 (0.38) | 7.97 (0.93) | 10.22 (2.07) |
| spleen | 2.47 (0.29) | 2.70 (0.29) | 2.29 (0.25) | 1.79 (0.62) |
| pancreas | 4.57 (0.58) | 3.25 (0.45) | 2.32 (0.27) | 1.94 (0.23) |
| heart | 6.98 (1.09) | 3.37 (0.37) | 2.56 (0.30) | 2.21 (0.33) |
| lung | 6.55 (1.14) | 3.86 (0.49) | 2.83 (0.41) | 2.29 (0.44) |
| stomachb | 1.00 (0.16) | 2.26 (0.28) | 3.38 (0.84) | 4.22 (0.93) |
| brain | 2.08 (0.17) | 1.94 (0.30) | 1.58 (0.27) | 1.13 (0.13) |
| bone | 1.67 (0.51) | 2.11 (0.55) | 2.36 (0.64) | 2.56 (0.52) |
| [18F]SQ4 | ||||
| blood | 10.93 (1.50) | 4.34 (0.49) | 2.16 (0.74) | 1.55 (0.11) |
| liver | 19.72 (2.48) | 35.82 (3.71) | 34.00 (4.63) | 31.57 (4.21) |
| kidney | 13.17 (1.59) | 16.62 (2.63) | 13.12 (2.67) | 11.57 (2.30) |
| intestine | 1.98 (0.44) | 3.39 (0.29) | 4.92 (0.94) | 7.69 (0.65) |
| spleen | 2.99 (0.53) | 5.65 (1.02) | 5.13 (1.19) | 4.97 (1.19) |
| pancreas | 2.93 (0.84) | 2.31 (0.24) | 1.84 (0.45) | 2.06 (0.60) |
| heart | 6.33 (1.21) | 3.10 (1.68) | 2.54 (0.23) | 2.03 (1.05) |
| lung | 10.15 (0.59) | 6.78 (0.78) | 4.11 (0.74) | 3.49 (0.73) |
| stomachb | 1.50 (0.39) | 2.70 (0.78) | 3.11 (1.51) | 3.89 (1.10) |
| brain | 1.78 (0.64) | 1.28 (0.17) | 1.05 (0.52) | 0.93 (0.09) |
| bone | 1.45 (0.29) | 1.48 (0.35) | 2.08 (0.68) | 3.36 (0.84) |
| [18F]SQ14 | ||||
| blood | 5.88 (2.31) | 2.51 (0.78) | 1.89 (0.13) | 1.80 (0.30) |
| liver | 9.67 (0.80) | 15.08 (3.08) | 13.23 (1.41) | 12.92 (2.31) |
| kidney | 12.91 (1.35) | 11.89 (2.00) | 7.71 (0.82) | 6.80 (2.14) |
| intestine | 2.40 (0.40) | 3.40 (0.79) | 7.43 (0.91) | 10.11 (1.90) |
| spleen | 2.94 (0.36) | 3.49 (0.66) | 2.40 (0.31) | 1.96 (0.47) |
| pancreas | 3.85 (0.77) | 2.63 (0.35) | 1.80 (0.18) | 1.39 (0.08) |
| heart | 5.55 (0.61) | 3.22 (0.31) | 2.23 (0.17) | 1.97 (0.35) |
| lung | 6.44 (0.96) | 3.73 (0.83) | 2.63 (0.16) | 2.35 (0.34) |
| stomachb | 1.10 (0.14) | 2.01 (0.32) | 3.14 (0.64) | 3.04 (0.64) |
| brain | 2.57 (0.70) | 2.02 (0.23) | 1.40 (0.22) | 0.93 (0.16) |
| bone | 2.44 (0.50) | 6.35 (3.52) | 6.76 (1.40) | 7.48 (2.36) |
Expressed as % injected dose per gram. Each value represents the mean (SD) of 5 animals.
Expressed as % injected dose per organ.
Figure 1.
Washout from brains of normal mice of each compound ([18F]SQ3, [18F]SQ4, and [18F]SQ14).
Marked accumulation of [18F]SQ3 and [18F]SQ4 in the bone was not observed (1.67, 1.45 and 2.56, 3.36% ID/g at 2 and 60 min post injection, respectively), indicating that they may exhibit high stability against defluorination in vivo until 60 min post injection. On the other hand, defluorination of [18F]SQ14 with fluorotriethylene glycol was observed (2.44 and 7.48% ID/g at 2 and 60 min post injection, respectively).
Based on these results, [18F]SQ3 also exhibited the most favorable pharmacokinetics in terms of brain permeability and stability against defluorination. In addition, considering the results of SQ4 and SQ14, a compound design which reduces lipophilicity while maintaining the molecular size is needed to achieve more favorable brain pharmacokinetics than with SQ3. However, from a clinical point of view, there are still points to be improved regarding the slow clearance rate and moderate brain permeability.
Sixteen styrylquinoline/quinoxaline derivatives were newly designed, synthesized, and evaluated to identify a novel in vivo α-syn imaging probe. This study revealed that a quinoline backbone is more preferable than a quinoxaline backbone in light of binding affinity for α-syn aggregates. In addition, binding affinities for both α-syn and Aβ aggregates changed markedly depending on both the sites and kinds of substituent. In particular, SQ3, with a fluoroethoxy group at the 7-position, showed good binding affinity for α-syn aggregates and moderate selectivity for α-syn aggregates over Aβ aggregates in vitro among these compounds. Moreover, [18F]SQ3 also exhibited favorable brain pharmacokinetics in normal mice. These encouraging in vitro and in vivo results suggest that [18F]SQ3 has basic properties as a lead compound for the development of a useful α-syn imaging probe.
Acknowledgments
We thank Dr. Shimpei Iikuni for his helpful discussion. This research was supported by the Naito Foundation and JSPS KAKENHI grant number 20H03622.
Glossary
Abbreviations
- α-syn
α-synuclein
- AD
Alzheimer’s disease
- Aβ
β-amyloid
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- HBD
hydrogen bond donor
- HPLC
high-performance liquid chromatography
- MW
molecular weight
- NBS
N-bromosuccinimide
- %ID/g
percentage injected dose per gram
- PET
positron emission tomography
- SPECT
single-photon emission computed tomography
- TBAF
tetrabutylammonium fluoride
- THF
tetrahydrofuran
- TPSA
topological polar surface area
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00279.
Full experiments methods and 1H and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Goedert M.; Jakes R.; Spillantini M. G. The Synucleinopathies: Twenty Years On. J. Parkinsons Dis. 2017, 7 (s1), S51–S69. 10.3233/JPD-179005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler L.; Buss S.; Leonov A.; Ryazanov S.; Schmidt F.; Maurer A.; Weckbecker D.; Landau A. M.; Lillethorup T. P.; Bleher D.; Saw R. S.; Pichler B. J.; Griesinger C.; Giese A.; Herfert K. [11C]MODAG-001-towards a PET tracer targeting α-synuclein aggregates. Eur. J. Nucl. Med. Mol. Imaging 2021, 48 (6), 1759–1772. 10.1007/s00259-020-05133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdurand M.; Levigoureux E.; Zeinyeh W.; Berthier L.; Mendjel-Herda M.; Cadarossanesaib F.; Bouillot C.; Iecker T.; Terreux R.; Lancelot S.; Chauveau F.; Billard T.; Zimmer L. In Silico, in Vitro, and in Vivo Evaluation of New Candidates for alpha-Synuclein PET Imaging. Mol. Pharmaceutics 2018, 15 (8), 3153–3166. 10.1021/acs.molpharmaceut.8b00229. [DOI] [PubMed] [Google Scholar]
- Miranda-Azpiazu P.; Svedberg M.; Higuchi M.; Ono M.; Jia Z.; Sunnemark D.; Elmore C. S.; Schou M.; Varrone A. Identification and in vitro characterization of C05–01, a PBB3 derivative with improved affinity for α-synuclein. Brain Res. 2020, 1749, 147131. 10.1016/j.brainres.2020.147131. [DOI] [PubMed] [Google Scholar]
- Kaide S.; Watanabe H.; Iikuni S.; Hasegawa M.; Itoh K.; Ono M. Chalcone Analogue as New Candidate for Selective Detection of α-Synuclein Pathology. ACS Chem. Neurosci. 2022, 13 (1), 16–26. 10.1021/acschemneuro.1c00441. [DOI] [PubMed] [Google Scholar]
- Kotzbauer P. T.; Cairns N. J.; Campbell M. C.; Willis A. W.; Racette B. A.; Tabbal S. D.; Perlmutter J. S. Pathologic accumulation of α-synuclein and Aβ in Parkinson disease patients with dementia. Arch Neurol. 2012, 69 (10), 1326–1331. 10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling J. L.; Dave K. D.; Frasier M. A. α-synuclein imaging: a critical need for Parkinson’s disease research. J. Parkinsons Dis. 2013, 3 (4), 565–567. 10.3233/JPD-130247. [DOI] [PubMed] [Google Scholar]
- Fodero-Tavoletti M. T.; Okamura N.; Furumoto S.; Mulligan R. S.; Connor A. R.; McLean C. A.; Cao D.; Rigopoulos A.; Cartwright G. A.; O’Keefe G.; Gong S.; Adlard P. A.; Barnham K. J.; Rowe C. C.; Masters C. L.; Kudo Y.; Cappai R.; Yanai K.; Villemagne V. L. 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer’s disease. Brain 2011, 134 (4), 1089–1100. 10.1093/brain/awr038. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Jia H. M.; Liu B. L. (E)-5-styryl-1H-indole and (E)-6-styrylquinoline derivatives serve as probes for β-amyloid plaques. Molecules 2012, 17 (4), 4252–4265. 10.3390/molecules17044252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M.; Ono M.; Kimura H.; Liu B.; Saji H. Novel quinoxaline derivatives for in vivo imaging of β-amyloid plaques in the brain. Bioorg. Med. Chem. Lett. 2011, 21 (14), 4193–4196. 10.1016/j.bmcl.2011.05.079. [DOI] [PubMed] [Google Scholar]
- Yoshimura M.; Ono M.; Matsumura K.; Watanabe H.; Kimura H.; Cui M.; Nakamoto Y.; Togashi K.; Okamoto Y.; Ihara M.; Takahashi R.; Saji H. Structure-Activity Relationships and in Vivo Evaluation of Quinoxaline Derivatives for PET Imaging of β-Amyloid Plaques. ACS Med. Chem. Lett. 2013, 4 (7), 596–600. 10.1021/ml4000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago T.; Furumoto S.; Okamura N.; Harada R.; Adachi H.; Ishikawa Y.; Yanai K.; Iwata R.; Kudo Y. Structure-Activity Relationship of 2-Arylquinolines as PET Imaging Tracers for Tau Pathology in Alzheimer Disease. J. Nucl. Med. 2016, 57 (4), 608–614. 10.2967/jnumed.115.166652. [DOI] [PubMed] [Google Scholar]
- Zhou K.; Yang F.; Li Y.; Chen Y.; Zhang X.; Zhang J.; Wang J.; Dai J.; Cai L.; Cui M. Synthesis and Evaluation of Fluorine-18 Labeled 2-Phenylquinoxaline Derivatives as Potential Tau Imaging Agents. Mol. Pharmaceutics 2021, 18 (3), 1176–1195. 10.1021/acs.molpharmaceut.0c01078. [DOI] [PubMed] [Google Scholar]
- Yue X.; Dhavale D. D.; Li J.; Luo Z.; Liu J.; Yang H.; Mach R. H.; Kotzbauer P. T.; Tu Z. Design, synthesis, and in vitro evaluation of quinolinyl analogues for α-synuclein aggregation. Bioorg. Med. Chem. Lett. 2018, 28 (6), 1011–1019. 10.1016/j.bmcl.2018.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürli R.; et al. Fused pyrazine derivatives as kinase inhibitors. WO2010052448A2, 2010.
- Xia S.; Gan L.; Wang K.; Li Z.; Ma D. Copper-Catalyzed Hydroxylation of (Hetero)aryl Halides under Mild Conditions. J. Am. Chem. Soc. 2016, 138 (41), 13493–13496. 10.1021/jacs.6b08114. [DOI] [PubMed] [Google Scholar]
- Chandrashekarappa K. K. H.; Mahadevan K. M.; Manjappa K. B. High throughput one pot synthesis of 2-methylquinolines. Tetrahedron Lett. 2013, 54 (11), 1368–1370. 10.1016/j.tetlet.2012.12.094. [DOI] [Google Scholar]
- Eros G.; Nagy K.; Mehdi H.; Papai I.; Nagy P.; Kiraly P.; Tarkanyi G.; Soos T. Catalytic hydrogenation with frustrated Lewis pairs: selectivity achieved by size-exclusion design of Lewis acids. Chemistry. 2012, 18 (2), 574–585. 10.1002/chem.201102438. [DOI] [PubMed] [Google Scholar]
- Zhou K.; Bai H.; Feng L.; Dai J.; Cui M. Smart D-π-A Type Near-Infrared Abeta Probes: Effects of a Marked pi Bridge on Optical and Biological Properties. Anal. Chem. 2017, 89 (17), 9432–9437. 10.1021/acs.analchem.7b02246. [DOI] [PubMed] [Google Scholar]
- Turner W. W.Hepatitis B core protein allosteric modulators. WO2015138895A1, 2015.
- Moldovan R. P.; Teodoro R.; Gao Y.; Deuther-Conrad W.; Kranz M.; Wang Y.; Kuwabara H.; Nakano M.; Valentine H.; Fischer S.; Pomper M. G.; Wong D. F.; Dannals R. F.; Brust P.; Horti A. G. Development of a High-Affinity PET Radioligand for Imaging Cannabinoid Subtype 2 Receptor. J. Med. Chem. 2016, 59 (17), 7840–7855. 10.1021/acs.jmedchem.6b00554. [DOI] [PubMed] [Google Scholar]
- Chaabouni M. M.; Baklouti A. Ring-Cleavage Reactions of F-Alkyl and Cl-Alkyl Epoxides by Action of Amines Hydrofluorides. Bull. Soc. Chim. Fr. 1989, (4), 549–553. [Google Scholar]
- Ono M.; Doi Y.; Watanabe H.; Ihara M.; Ozaki A.; Saji H. Structure–activity relationships of radioiodinated diphenyl derivatives with different conjugated double bonds as ligands for α-synuclein aggregates. RSC Adv. 2016, 6, 44305–44312. 10.1039/C6RA02710E. [DOI] [Google Scholar]
- Fodero-Tavoletti M. T.; Mulligan R. S.; Okamura N.; Furumoto S.; Rowe C. C.; Kudo Y.; Masters C. L.; Cappai R.; Yanai K.; Villemagne V. L. In vitro characterisation of BF227 binding to α-synuclein/Lewy bodies. Eur. J. Pharmacol. 2009, 617 (1–3), 54–58. 10.1016/j.ejphar.2009.06.042. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Villalobos A.; Beck E. M.; Bocan T.; Chappie T. A.; Chen L.; Grimwood S.; Heck S. D.; Helal C. J.; Hou X.; Humphrey J. M.; Lu J.; Skaddan M. B.; McCarthy T. J.; Verhoest P. R.; Wager T. T.; Zasadny K. Design and selection parameters to accelerate the discovery of novel central nervous system positron emission tomography (PET) ligands and their application in the development of a novel phosphodiesterase 2A PET ligand. J. Med. Chem. 2013, 56 (11), 4568–4579. 10.1021/jm400312y. [DOI] [PubMed] [Google Scholar]
- Okumura Y.; Maya Y.; Onishi T.; Shoyama Y.; Izawa A.; Nakamura D.; Tanifuji S.; Tanaka A.; Arano Y.; Matsumoto H. Design, Synthesis, and Preliminary Evaluation of SPECT Probes for Imaging β-Amyloid in Alzheimer’s Disease Affected Brain. ACS Chem. Neurosci. 2018, 9 (6), 1503–1514. 10.1021/acschemneuro.8b00064. [DOI] [PubMed] [Google Scholar]
- Korat Š.; Bidesi N. S.; Bonanno F.; Di Nanni A.; Hoàng A. N.; Herfert K.; Maurer A.; Battisti U. M.; Bowden G. D.; Thonon D.; Vugts D.; Windhorst A. D.; Herth M. M. α-Synuclein PET Tracer Development-An Overview about Current Efforts. Pharmaceuticals (Basel). 2021, 14 (9), 847. 10.3390/ph14090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman A.; Rokka J.; Lopez-Picon F. R.; Eskola O.; Wilson I.; Farrar G.; Scheinin M.; Solin O.; Rinne J. O.; Haaparanta-Solin M. Pharmacokinetics of [18F]flutemetamol in wild-type rodents and its binding to β amyloid deposits in a mouse model of Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging. 2012, 39 (11), 1784–1795. 10.1007/s00259-012-2178-9. [DOI] [PubMed] [Google Scholar]
- Xia C. F.; Arteaga J.; Chen G.; Gangadharmath U.; Gomez L. F.; Kasi D.; Lam C.; Liang Q.; Liu C.; Mocharla V. P.; Mu F.; Sinha A.; Su H.; Szardenings A. K.; Walsh J. C.; Wang E.; Yu C.; Zhang W.; Zhao T.; Kolb H. C. [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013, 9 (6), 666–676. 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.