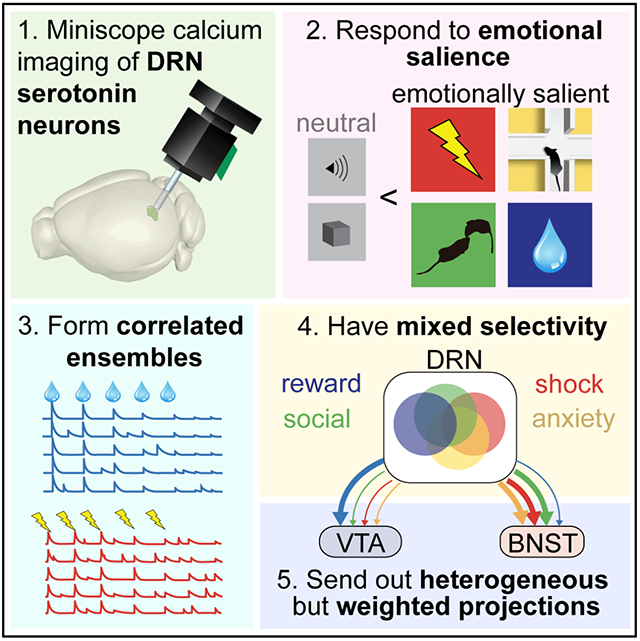
SUMMARY
The serotonin system modulates a wide variety of emotional behaviors and states, including reward processing, anxiety, and social interaction. To reveal the underlying patterns of neural activity, we visualized serotonergic neurons in the dorsal raphe nucleus (DRN5-HT) of mice using miniaturized microscopy during diverse emotional behaviors. We discovered ensembles of cells with highly correlated activity and found that DRN5-HT neurons are preferentially recruited by emotionally salient stimuli as opposed to neutral stimuli. Individual DRN5-HT neurons responded to diverse combinations of salient stimuli, with some preference for valence and sensory modality. Anatomically-defined subpopulations projecting to either a reward-related structure, the ventral tegmental area, or an anxiety-related structure, the bed nucleus of the stria terminalis, contained all response types, but were enriched in reward- and anxiety-responsive cells, respectively. Our results suggest that the DRN serotonin system responds to emotional salience using ensembles with mixed selectivity and biases in downstream connectivity.
Keywords: dorsal raphe nucleus (DRN), serotonin, microendoscopy, calcium imaging, ventral tegmental area (VTA), bed nucleus of the stria terminalis (BNST), salience
eTOC BLURB
Paquelet et al. use miniaturized microscopy to visualize the activity of serotonergic neurons in the dorsal raphe nucleus of mice during emotional behaviors. Their results show that dorsal raphe serotonin neurons are modulated during emotionally salient behaviors using highly correlated ensembles with mixed selectivity and biases in downstream connectivity.
Graphical Abstract
INTRODUCTION
Serotonin is a neuromodulatory neurotransmitter produced in the brainstem raphe nuclei and released throughout the nervous system. The serotonergic system is involved in a wide range of behaviors, including emotional behaviors such as the response to rewards (Liu et al., 2014; Hu, 2016; Xu et al., 2017; Wei et al., 2018), punishments (Cohen et al., 2015), anxiety- and depression-related behaviors (Lowry et al., 2005; Hale et al., 2012; Teissier et al., 2015; Dolzani et al., 2016; Urban et al., 2016; Natarajan et al., 2017; Nishitani et al., 2018), social interaction (Balazsfi et al., 2018; Walsh et al., 2018), and aggression (Nautiyal et al., 2015; Coccaro et al., 2015). The raphe nuclei likewise send projections to nearly every area of the brain. Determining how this complex anatomical system modulates such diverse behaviors is a foundational goal in neuroscience. It is also an essential question in psychiatry, as millions of people worldwide are treated for depression and anxiety with medications that modulate serotonin globally, without regard to the complexity of the system (Cipriani et al., 2018).
The serotonergic system demonstrates considerable molecular and cellular diversity (Okaty et al., 2019). In mammals, it is composed of nine distinct nuclei in the brainstem Müller and Jacobs, 2010), of which the dorsal raphe nucleus (DRN) is the main source of serotonin to the forebrain. Its ~9,000 serotonergic neurons in mice (Ishimura et al., 1988) are diverse in physiology, neurochemistry, developmental trajectory, and downstream targets (Abrams et al., 2006; Calizo et al., 2011; Okaty et al., 2015; Fernandez et al., 2016; Muzerelle et al., 2016; Prouty et al., 2017).
This cellular diversity suggests functional diversity, and in vivo single unit electrophysiology studies have demonstrated heterogenous responses of individual DRN serotonergic neurons (DRN5-HT) (Ranade and Mainen, 2009; Liu et al., 2014; Cohen et al., 2015; Li et al., 2016). Fiber photometry studies have shown that the population-level responses of DRN5-HT neurons to rewards and punishments differ between distinct DRN5-HT projections (Ren et al., 2018), further highlighting functional heterogeneity in the system.
Major gaps remain in our understanding of DRN5-HT network properties during emotional behaviors, and of potential unifying themes. Many of these gaps exist because the DRN5-HT population has not been imaged with single cell resolution across diverse behaviors. We therefore adapted freely behaving microendoscopy to the DRN. This allowed us to image the activity of dozens of DRN5-HT neurons simultaneously and to track their activity across multiple behaviors.
In total, we imaged over 2,000 genetically-identified serotonergic neurons. Applying microendoscopy during emotional behaviors at this scale allowed us to discover four major properties of the serotonergic system across multiple levels of organization: (1) There are distinct DRN5-HT ensembles with highly correlated activity over long timescales. This is a counterpoint to the heterogeneity in the system. (2) Across a range of sensory modalities and valences, the DRN5-HT system responds strongly to emotionally salient stimuli. In all behaviors tested, many more DRN5-HT neurons respond to emotionally salient stimuli than to less salient or valence neutral stimuli. (3) Individual neurons respond to diverse combinations of stimuli with some preference for valence and sensory modality. (4) Two anatomically distinct projections contain the full diversity of functional DRN5-HT subtypes, but in different proportions.
RESULTS
Single-cell imaging of serotonergic neurons in the dorsal raphe nucleus
To visualize activity in DRN5-HT neurons during freely moving behavior, we used 1-photon calcium imaging with miniaturized microscopy (Resendez et al., 2016). In mice expressing Cre-recombinase in serotonergic neurons (Sert-Cre, Zhuang et al., 2005), DRN5-HT neurons virally infected with a Cre-dependent calcium indicator were accessed using a gradient refractive index (GRIN) relay lens implanted at a posterior angle of 32° to avoid major sources of bleeding (Chen et al., 2013, Correia et al., 2017, Fig. 1A–B, S1, see also Table S1 and Methods). While some damage to local circuitry is unavoidable, our approach minimized local tissue damage as the lens sat largely in the fourth ventricle when imaging the dorsal DRN, and posterior to the DRN when imaging ventral subregions. We processed our data using a customized version of a cell segmentation algorithm, constrained non-negative matrix factorization for microendoscopy (CNMF-E, Fig. 1C–D; Zhou et al., 2018; see Methods). CNMF-E is optimized for analyzing phasic increases in activity, so while we did observe phasic decreases in many cases, excitation was the focus of our study.
Figure 1. DRN5-HT neurons are recruited in correlated ensembles during sucrose consumption.
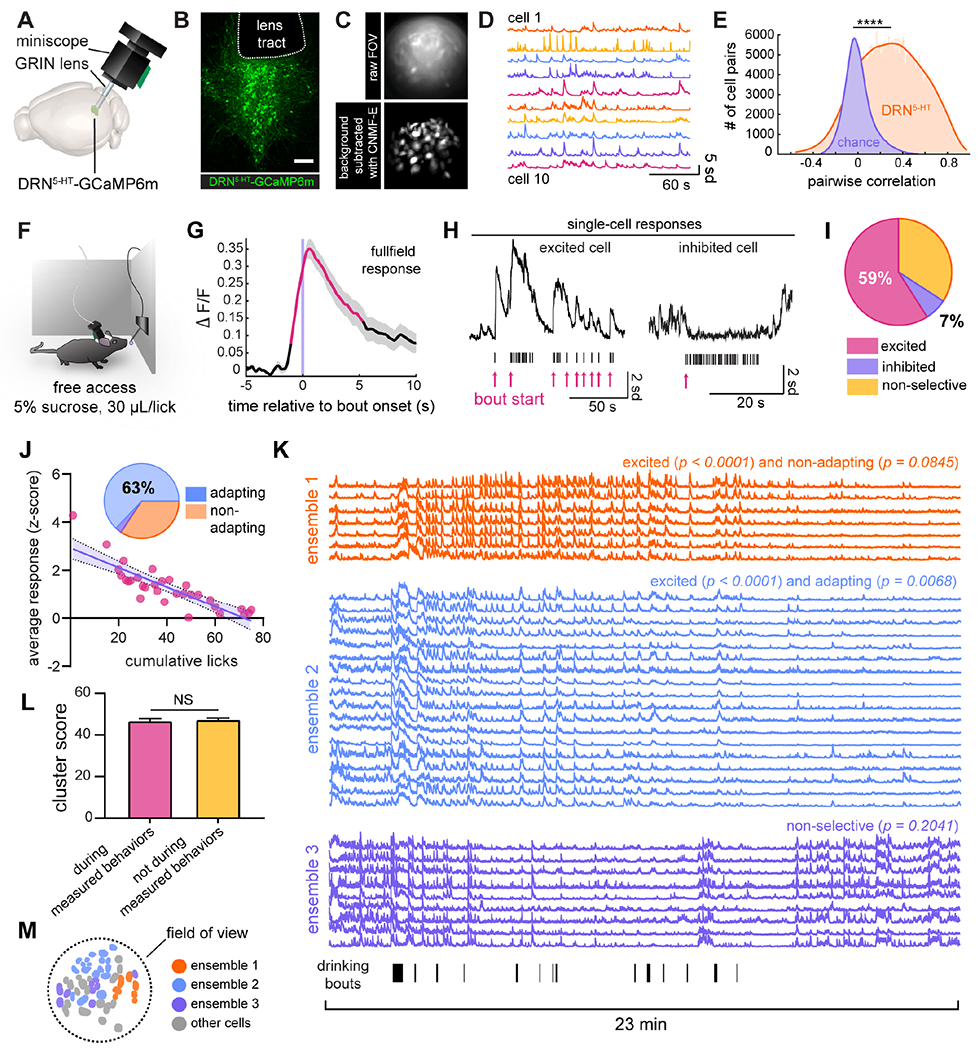
A) Summary of experimental setup: GCaMP6m is expressed in vivo in DRN5-HT neurons and imaged using an angled gradient refractive index (GRIN) lens and miniaturized microscope. Rendering adapted from the Allen Mouse Brain Atlas (Lein et al., 2007; Bakker et al., 2015). See also Fig. S1, Table S1, and Table S2.
B) Confocal image of the DRN, showing GCaMP6m-expressing DRN5-HT neurons and the bottom edge of the tract remaining after removal of the GRIN lens. Scale bar = 200 μm.
C) (top) Single frame from a representative imaging session. (bottom) Standard deviation projection of identified neurons from the same movie as above after background-subtraction with CNMF-E.
D) Example denoised activity traces from cells in (C). Units are standard deviation from each cell’s baseline fluorescence. See also Supplemental Video 1.
E) Distribution of pairwise Pearson’s correlation coefficients of DRN5-HT neuronal activity (n = 48,126 cell pairs) versus chance (n = 1,128 cell pairs x 1,000 simulations). K-S test, D statistic = 0.5543, p <<< 0.0001. See also Fig. S2.
F) Behavioral setup for sucrose consumption. A lick-triggered spout freely dispenses 30 μL drops of 5% sucrose water.
G) Average percent change in fluorescence of the entire field of view relative to drinking bout onset for a representative animal. Red line segments denote timepoints significantly different than baseline (permutation test, α < 0.05), gray shading represents ±SEM, and data is normalized to have a baseline of 0.
H) Responses of individual neurons to sucrose consumption. Shown are examples of raw activity traces. Tick-marks represent licks and red arrows indicate the onsets of separate drinking bouts.
I) Relative proportions of excited (183 cells, 59%), inhibited (21 cells, 7%), and non-selective cells (106 cells, 34%) found across all animals (n = 310 cells in 10 animals, shuffle test to determine significance, α = 0.05).
J) Of excited cells, the majority (63%, pie chart inset, linear regression, α = 0.05) displayed decreasing responses with cumulative licks. The scatter plot depicts this comparison in a representative cell with significant adaptation (linear regression, p = 1.7588 x 10−9, adjusted R2 = 0.7091). Shading represents the 95% confidence interval. See also Fig. S3.
K) The three largest ensembles from a representative animal. Averaged activity of ensemble 1 is excited by sucrose (p < 0.0001) and non-adapting (p = 0.0845), as determined in (I), ensemble 2 is excited (p < 0.0001) and adapting (p = 0.0068), and ensemble 3 is non-selective (p = 0.2041) for sucrose. Licks are plotted as tick marks below ensemble 3. Traces span the complete 23-minute imaging session.
L) For cells in the same ensemble, the average cluster score (the number of times a pair of cells clustered together over 100 iterations of k-means++) during time windows containing measured behaviors is not significantly different than those calculated during time windows that did not contain measured behaviors (n = 212 ensembles across the entire dataset (Fig. 1, 3–5), p = 0.5705, paired t-test).
(M) Spatial distribution of cells in (K).
Using this method, we observed active cells (Fig. 1D) with significantly correlated activity (n = 48,126 cell pairs compared to chance (1,128 cell pairs x 1,000 simulations, each calculating the pairwise Pearson’s correlation between activity traces offset by randomly chosen time windows, K-S test, p <<< 0.0001, Fig. 1E, Supplemental Video 1). Importantly, we performed multiple control experiments and analyses to confirm that this correlated activity was not an artifact of poor cell segmentation or motion in the z-plane (see Fig. S2 and Methods). As detailed in the following sections, we found that this correlated activity among subsets of DRN5-HT neurons is a fundamental property of the network.
DRN5-HT neurons are recruited in ensembles during sucrose consumption
DRN serotonin has been functionally implicated in reward processing (Liu et al., 2014; Hu, 2016; Xu et al., 2017; Wei et al., 2018). To identify DRN5-HT neurons that may mediate this behavioral function, we first imaged DRN5-HT neurons during a simple task to engage reward circuitry: free consumption of sucrose water (5% wt/vol) (Li et al., 2016). For this task, mice were placed in a standard housing cage outfitted with a lick-triggered spout set to deliver a small, fixed volume (30 μL) of sucrose water immediately after every lick (Fig. 1F). Sucrose was available ad libitum without a timeout period or trial structure to simulate natural consumption.
We first measured the mean population response to a rapid series of licks, termed a bout, by calculating the relative change in fluorescence (ΔF/F) of the entire field of view across time (see Methods). We found a large increase in activity whose onset preceded the first lick of the bout by 1-2 seconds (Fig. 1G, S3B), suggesting anticipation. This effect is consistent with previous experiments measuring DRN5-HT activity (Nakamura et al., 2008; Liu et al., 2014; Li et al., 2016; Zhong et al., 2017). Since mice were naïve to the task, we also noted that their first-ever lick did not elicit such anticipatory activity (Fig. S3A–B).
We then examined the underlying single-cell responses to sucrose, and we found a mix of DRN5-HT responses underlying the population average signal (Fig. 1H). Most DRN5-HT neurons were excited during sucrose consumption (183/310, 59%, in n = 10 animals, Fig. 1I), as defined by an average activity increase at bout onset significantly more than chance (shuffle test, α = 0.05). Non-selective DRN5-HT neurons accounted for a smaller fraction (106/310, 34%), defined as cells whose responses did not meet the significance threshold (shuffle test, α = 0.05). A small fraction of DRN5-HT neurons was inhibited (21/310, 7%, shuffle test, α = 0.05). Of the excited cells, we further found that the majority (63%, linear regression, α = 0.05, Fig. 1J, see also Fig. S3C) had progressively smaller responses correlating with cumulative licks (see also Fig. S3F–E). Anticipatory activity also adapted (43%, linear regression, α = 0.05, Fig. S3D).
We then examined the degree of coordination between DRN5-HT neurons during sucrose consumption sessions. We observed that the DRN5-HT population contains discrete ensembles with highly correlated activity. This feature of the DRN5-HT system was present in each behavior we examined. To identify these groups quantitatively, we clustered denoised activity traces using an iterative k-means approach (Barbera et al., 2016). We consistently identified 2-4 ensembles in each imaging session that contained a substantial number of cells that were correlated over long timescales (e.g., Fig. 1K and Fig. S3I). Importantly, the ensembles are defined by activity alone, irrespective of behavior. This clustering could be an ongoing feature of DRN5-HT neurons, or it could be driven primarily by specific measured behaviors and presented stimuli. To distinguish between these possibilities, we calculated a cluster score for each pair of neurons in an ensemble within 30 second time windows that spanned the entire imaging session. These cluster scores were not significantly different between time windows containing measured behaviors and those that did not (Fig. 1L, n = 212 ensembles). This indicates that the ensembles are correlated over an extended period, and not only when the mice are presented with a stimulus or exhibiting a measured behavior.
We then further characterized the activity of ensembles during measured behaviors. In the example shown in Figure 1, the first ensemble is composed of excited cells (shuffle test on mean activity, p < 0.0001) whose response does not adapt to continued sucrose consumption (linear regression, p = 0.0845); the second and largest ensemble includes excited cells (p < 0.0001) which do adapt (p = 0.0068); and the third contains cells that have no temporally-specific response to bout onset (p = 0.2041, Fig. 1J). Across all animals, we found that 64% of identified ensembles were excited by sucrose consumption and 23% were both excited and adapt (39/61 and 14/61 ensembles, respectively, in 10 animals, shuffle test, α = 0.05). Interestingly, although these ensembles are defined solely by correlated activity, many also appear to be loosely organized in space (Fig. 1M and Fig. S3J). 57% (n = 54 pairs) of sucrose-responsive ensembles were spatially segregated from non-responsive ensembles and 61% (n = 23 pairs) of adapting ensembles were segregated from non-adapting ensembles (2-dimensional K-S test, Fasano and Franceschini, 1987, n = 10 animals).
We further characterized ensembles across our dataset. As with the dataset as a whole, there was no correlation between pairwise distance and correlated activity of cells within the ensembles, which argues against segmentation artifact (Fig. S2C). Ensembles ranged in size, with the maximum and median distance between cells of 100-500 um. We then assessed whether cells of the same ensemble, which were highly correlated over long timescales, were synchronous, which could be an artifact of poor cell segmentation or z-motion. This was not the case; in randomly chosen 20-second time bins, activity was not synchronous and correlation between cell pairs of an ensemble varied across time bins (Fig. S2Q–R).
Responses of DRN5-HT neurons to gustatory stimuli of varying valence
To determine whether sucrose-responsive DRN5-HT neurons respond specifically to the positive valence of sucrose, or more generally to emotional salience (of either valence), we randomly presented mice with one of five solutions using a gustometer (Fig. 2A). The solutions included 0.1 mM quinine (a bitter tastant), water, and ascending concentrations of sucrose. Mice consumed significantly less quinine than water (p < 0.05, post hoc one-sample t-test, n = 7 mice), and significantly more 2.5% (p < 0.05) and 5% sucrose (p < 0.005) (ANOVA, p < 0.0001, Fig. 2B). If neurons respond to the positive valence of the stimulus, their responses would scale with this demonstrated palatability. In contrast, if these DRN5-HT neurons are responding to salience, they would respond more to both quinine and sucrose than to water, as the addition of either a bitter or a sweet tastant would increase the salience of the plain water stimulus. We discovered that DRN5-HT responses were higher to both quinine and sucrose than to plain water (Fig. 2C–D). This dissociation between palatability and DRN5-HT response is consistent with these DRN5-HT neurons responding to emotional salience.
Figure 2. Responses of DRN5-HT neurons to gustatory stimuli of varying valence.
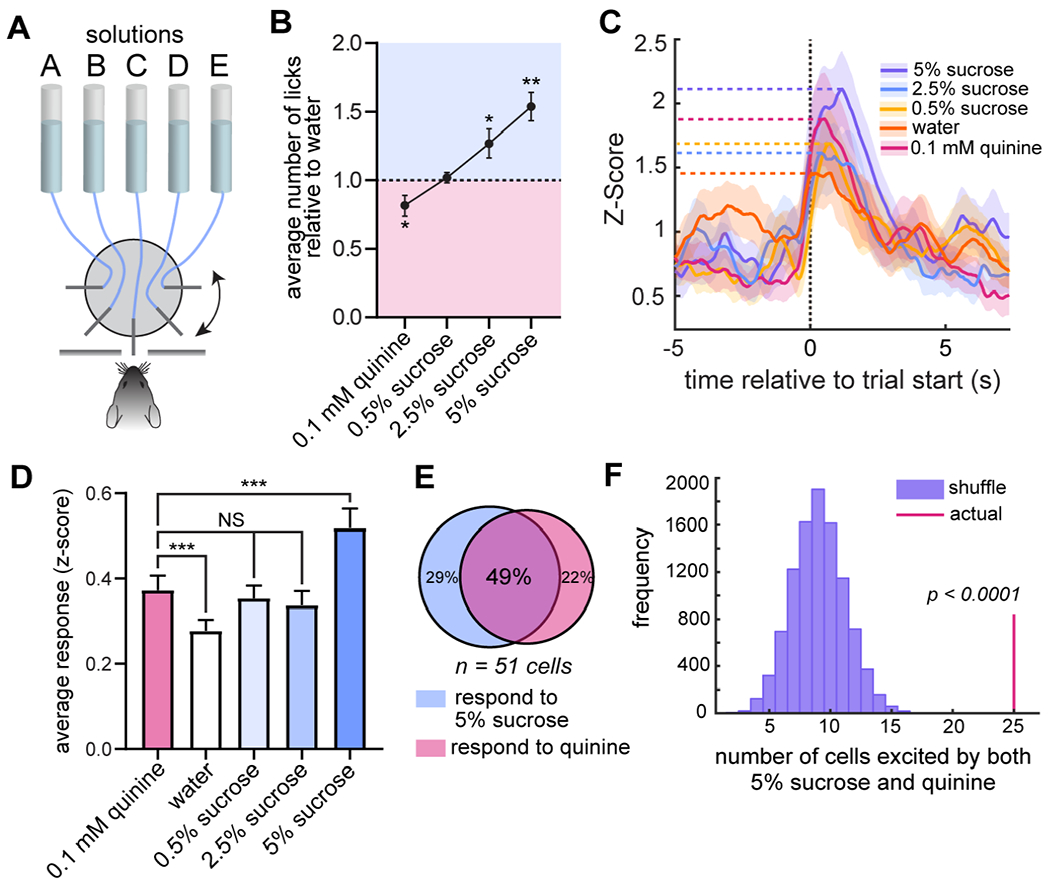
A) Behavioral setup showing five spouts randomly delivering five different solutions one-at-a-time to a mouse through a slot in the wall using a rotating wheel.
B) Licking behavior of mice (n = 7) for each solution relative to water. One-way ANOVA, p < 0.0001; one-sample t-test comparing each solution to a theoretical mean of 1: 0.5% sucrose, p > 0.05, 2.5% sucrose, p < 0.05, 5% sucrose, p < 0.005, quinine, p < 0.05. Data are shown as mean ±SEM.
C) Representative average responses of a single cell to each solution. Shading represents ±SEM.
D) Data subset of 59 cells that are significantly excited (shuffle test, ± = 0.05) by at least one solution, from n = 5 mice. Repeated-measures one-way ANOVA, p < 0.0001. Paired t-tests: quinine vs. water, p = 0.0004; 0.5% sucrose vs. water, p = 0.0057; quinine vs. 5% sucrose, p = 0.0006; quinine vs. 0.5% sucrose, p > 0.05; quinine vs. 2.5% sucrose, p > 0.05. Data are shown as mean ±SEM.
E) Overlap in populations significantly excited by 5% sucrose or quinine.
F) Overlap is significantly greater than chance (shuffle test, p = 4.6 x 10−13).
We confirmed that the majority of these neurons respond to both quinine and sucrose, and that this overlap is far more than expected by chance (p = 4.6 x 10−13, shuffle test, Fig. 2E–F). Interestingly, there are smaller fractions of DRN5-HT neurons that responded to only quinine or sucrose, highlighting the heterogeneity of the system (Fig. 2E). We further characterized DRN5-HT gustatory responses by calculating single-cell selectivity to 2.5% sucrose and quinine, which had similar averaged responses (Fig. 2D), compared to water (Fig. S4A). Most neurons (80%, 80/100 neurons that responded significantly (shuffle test, p < 0.05) to water, 2.5% sucrose, or quinine) had greater modulation by both sucrose and quinine than to plain water. This is again consistent with a response to emotional salience. The varied responses in the remaining 20% of neurons highlight the heterogeneity of the system.
We then examined responses to a valence neutral stimulus. Auditory stimuli are known to excite DRN5-HT neurons (Ranade and Mainen, 2009). We presented animals with a series of neutral auditory cues (Fig. S3K) and found that only 16% were significantly excited (n = 221 cells in 6 animals, Fig. S3K–L), and of those, the vast majority did not adapt their responses over time (Fig. S3L–M). The proportion of DRN5-HT neurons modulated by this valence neutral stimulus was significantly smaller than that modulated by sucrose (Fig. S3O). Interestingly, a small fraction was selective for one frequency of tone over another (Fig. S3N).
DRN5-HT neurons develop responses to a neutral stimulus after it is paired with an emotionally salient stimulus
We next determined how DRN5-HT responses to a neutral stimulus change after it becomes emotionally salient through associative conditioning. The gustometer contains a rotating wheel that acts as an audiovisual cue indicating spout availability. This is a neutral cue on the first day of exposure to the gustometer before mice have learned this association. To determine how DRN5-HT responses to this cue change with learning, we imaged DRN5-HT neurons during the two-day learning period and compared it to the third day of gustometry (test day). Learning was evident behaviorally as the latency to lick after the cue significantly decreased over the course of the three days (Fig. S4B). On day 1, very few DRN5-HT cells were modulated by the neutral cue (Fig. S4C). Over the course of learning, the cue recruited a progressively larger fraction of the DRN5-HT population (Fig. S4C), and individual cells were seen to develop a cue response (Fig. S4D). The majority of DRN5-HT neurons that became cue-responsive after learning also responded to the gustatory stimulus (p < 0.0001 for overlap between cue- and reward-responsive cells, shuffle test, Fig. S4E).
Ensembles of DRN5-HT neurons adapt to repeated footshock
We next examined calcium activity in DRN5-HT neurons during a series of footshocks, an emotionally salient stimulus of strong negative valence. Mice were subjected to ten shocks, randomly timed between 20 and 40 seconds apart to prevent anticipation (Fig. 3A, Li et al., 2016). We first noticed that population-level activity differed from one shock to the next. The response to the first shock included a large increase in activity, while that to the following two shocks was progressively smaller. Shocks 4-10 elicited the same response as shock 3: little to no increase in activity (Fig. 3B). Thus, the overall DRN5-HT response declined with repeated presentations of this aversive stimulus. This is similar to the decline we found with repeated presentations of appetitive stimuli.
Figure 3. DRN5-HT ensembles adapt to repeated footshocks.
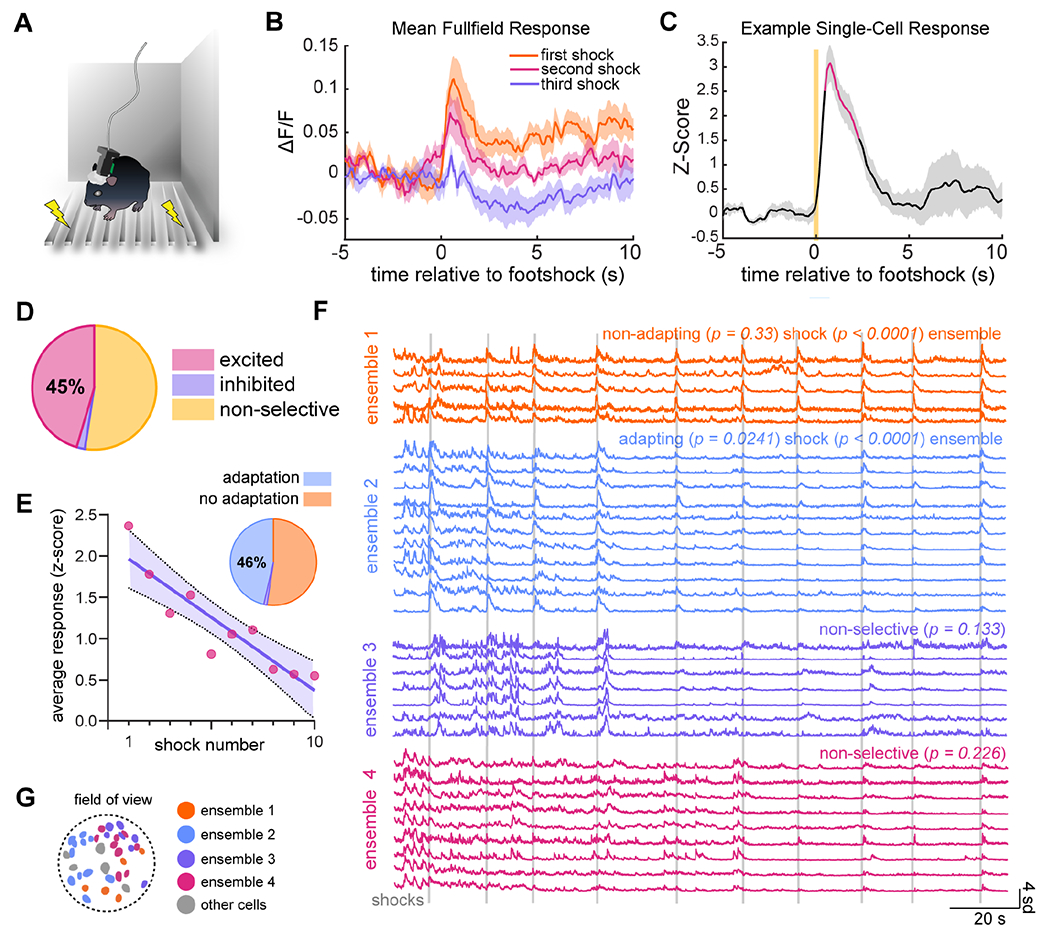
A) Experimental setup showing a mouse with attached miniscope in a small, enclosed arena, standing on metal bars wired to deliver shocks.
B) Population-level response to first, second, and third shocks in the series of ten, averaged across animals (n = 8). Shading represents ±SEM.
C) Example single-cell response as in Fig. 1G.
D) 45% of cells were excited as in (C), 2% were inhibited, and 52% were non-selective (n = 185 cells in 8 animals, shuffle test, α = 0.05).
E) Inverse correlation between response size and shock number in a representative cell. The purple line is a best-fit from linear regression (p = 0.0003, adjusted R2 = 0.826). Shading represents 95% confidence interval. Of excited cells, 46% display this trend (pie chart inset, n = 84 cells in 8 mice, α = 0.05).
F) Four ensembles from a representative animal. Ensemble 1 is excited by footshock (p < 0.0001) and does not significantly adapt to repeated shocks (p = 0.33); ensemble 2 is excited (p < 0.0001) but does adapt (p = 0.0241); and ensembles 3 and 4 are non-selective for footshock (p = 0.226). Vertical gray lines represent individual shocks.
G) Spatial distribution of ensembles shown in (F). Other detected cells are in gray. See also Fig. S5N–O.
At the single cell level, we found that a high fraction (45%) of DRN5-HT neurons were significantly excited by footshock (n = 185 cells in 8 animals, shuffle test, α = 0.05, Fig. 3C–D). We then found that 46% of these responding cells also had a significant reduction in response size across the series of shocks (linear regression, α = 0.05, Fig. 3E, see also Fig. S5M).
As with sucrose consumption, we found distinct ensembles with highly correlated activity during footshock sessions (Fig. 3F and Fig. S5N). These ensembles also included cells whose response size decreases with shock number (ensemble 2, p < 0.0001 for excitation, p = 0.0241 for adaptation), cells whose response size is consistent (ensemble 1, p < 0.0001 for excitation, p = 0.33 for adaptation), and cells that do not specifically respond to the onset of the shock (ensembles 3-4, p = 0.133 and p = 0.226, respectively) (Fig. 3F). Across animals, 52% of ensembles were excited by footshock (16/31 in 7 animals, shuffle test, α = 0.05). As in the sucrose task, ensembles were loosely organized in space (Fig. 3G and Fig. S5O). 57% of excited and non-selective ensembles were (2-D K-S test, n = 30 ensemble pairs in n = 7 mice). This and the previously observed responses to sucrose consumption further demonstrate that the existence of these ensembles is a general property of the DRN5-HT network across diverse behaviors and emotional states.
We next determined whether changes in DRN5-HT responses to successive footshocks are due to changes in locomotion. Several studies have found a negative correlation between DRN5-HT activity and locomotion (Correia et al., 2017, Seo et al., 2019). We also observed that population-level activity decreased with increasing velocity when mice explored an open field arena (Fig. S5G–I). Interestingly, 38% of individual cells were positively correlated with velocity, compared to 18% that were negatively correlated, demonstrating both heterogeneity and a disconnect between population- and single cell-level data due to differential contributions of single cells (n = 495 cells in 19 mice, Fig. S5I).
We quantified the velocity of mice immediately after the shock and compared the size of the locomotor response with that of the neural response. There was neither a correlation between shock number and locomotor response (linear regression, p > 0.05, Fig. S5J) nor a correlation between single-cell responses and locomotor response (linear regression, n = 84 cells in 8 animals, 5% positively correlated, α = 0.05, Fig. S5K). Thus, the adapting DRN5-HT responses are not secondary to changes in locomotion.
We next determined if a neutral stimulus could recruit a larger fraction of DRN5-HT neurons after it had been paired with an aversive stimulus. To do so, we trained mice to associate an auditory cue with a footshock using a two-day cued fear conditioning protocol (Fig. S5A–B). On day 1, a small fraction of DRN5-HT neurons was modulated by the neutral tone (Fig. S5C), and this was significantly less than the proportion responding to the footshock (p = 0.0075). On day 2, after animals learned the association, the fraction of DRN5-HT neurons responding to the tone significantly increased (9/171 cells on day 1 vs. 66/207 cells on day 2, Chi-squared test of proportions, p = 1.1 x 10−10, Fig. S5C). There was no significant difference between the size of the populations responding to the shock on day 1 and the tone on day 2 (p = 0.52, Fig. S5F), reflecting the tone’s newfound emotional salience. Interestingly, the cells responding to the tone after pairing were largely non-overlapping with those responding to the footshock (Fig. S5D–E).
DRN5-HT neurons are recruited during exploratory behavior in the elevated plus maze
We next investigated DRN5-HT activity during elevated plus maze (EPM) exploration and social interaction, two complex emotionally salient behaviors. In the EPM, the drive to explore the potentially threatening center and open arms of the maze intermittently outweighs the self-protective instinct to remain in the walled, safe closed arms (Fig. 4A, Waif and Frye, 2007, David et al., 2009, Likhtik and Gordon, 2014, Jimenez et al., 2018) The degree of open arm exploration can be modulated by acute manipulations of serotonergic neurons (Teissier et al., 2015; Marcinkiewcz et al., 2016; Garcia-Garcia et al., 2018; Ren et al., 2018, Nishitani et al., 2018). However, it is unknown how DRN5-HT neurons respond, either at the population- or single-cell level, during this conflict test. We therefore imaged DRN5-HT activity as mice explored the EPM. Expectedly, mice spent significantly less time in the open arms and center of the arena than in the closed arms (n = 13 mice, one-sample t-test comparing open arm and center time to a theoretical mean of 50%, p < 0.0001, Fig. 4B).
Figure 4. DRN5-HT ensembles are excited during exploration under potential threat.
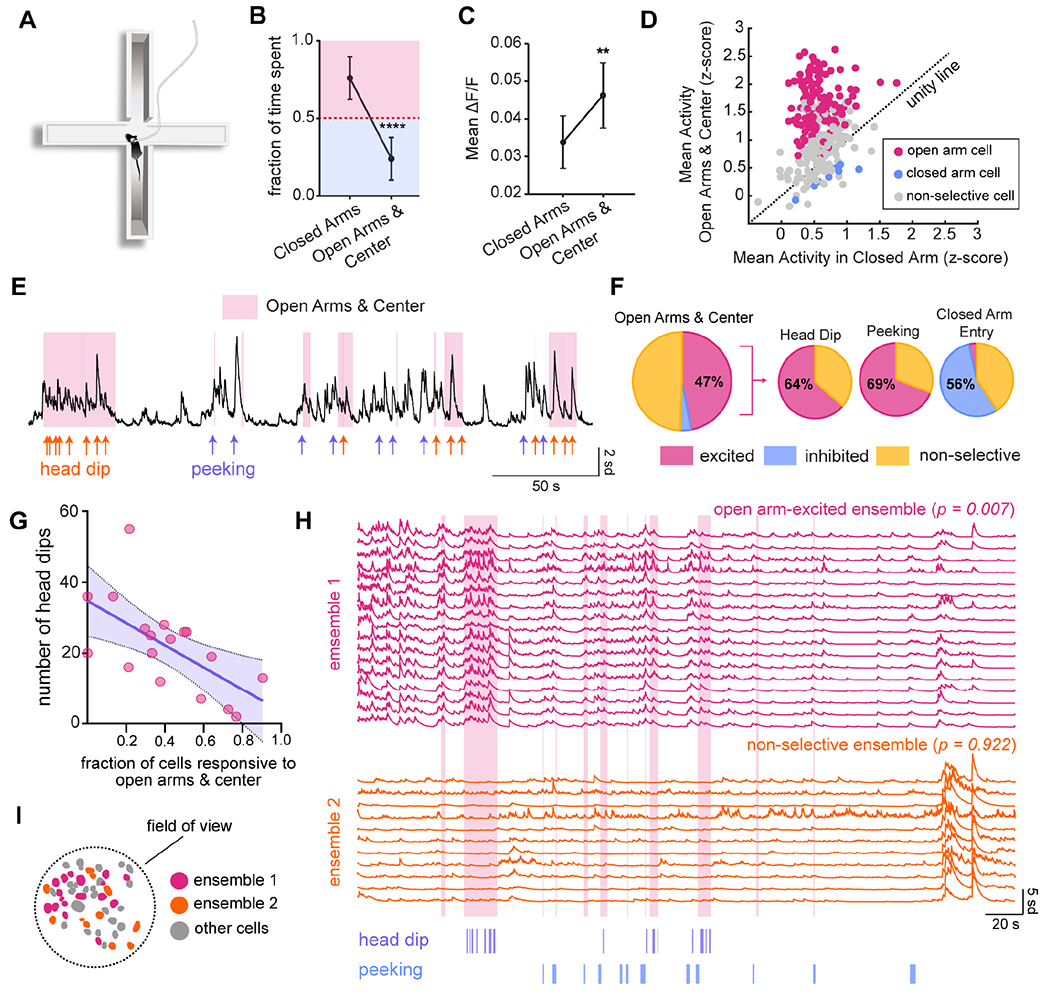
A) Diagram of an elevated plus maze showing a mouse engaged in peeking behavior.
B) Fraction of the total session time spent in open arms/center versus closed arms. The red dotted line indicates no preference for either zone, and error bars represent ±SEM. N = 13 mice. One-sample t-test comparing open arm and center time to a theoretical mean of 50%, p < 0.0001.
C) Comparison of population-level activity between open arms/center and closed arms. Error bars represent ±SEM. p = 0.0054, two-tailed paired t-test, n = 13 mice.
D) Comparison of activity in open arms/center and closed arms for individual cells. Cells with a significant preference are color-coded (shuffle test, α < 0.05). The dotted line represents no difference in activity between zones. n = 304 cells in 12 mice.
E) Calcium activity from an example cell indicating relevant behavioral events: head dips (orange) and peeking (purple). Epochs during which the mouse occupied the open arms or center are shaded pink.
F) Relative proportion of open arm-excited cells across all animals (47%, n = 304 cells in 12 mice, shuffle test, α < 0.05), and the fraction of open arm-excited cells (n = 143) that are also excited by head dip (64%) and peeking (69%), and inhibited by closed arm re-entry (56%).
G) Relationship between an animal’s proportion of open arm-excited cells and time spent in the open arms and center. The purple line is a best-fit by linear regression (p = 0.0058, R2 = 0.387, n = 18 mice). Shading represents 95% confidence interval.
H) The two largest ensembles from a representative animal. Ensemble 1 is significantly excited in the open arms and center (p = 0.007), while ensemble 2 is non-selective for arm type (p =0.922). Occupancy of the open arms and center is indicated in pink, head dips in purple, and peeking in blue.
I) Spatial distribution of ensembles in (H).
DRN5-HT population activity was significantly higher during exploration of the open arms and center than in the closed arms (paired t-test, p = 0.0054, n = 13 mice, Fig. 4C). Likewise, at the single-cell level there were vastly more DRN5-HT neurons recruited during open arm exploration than in the safe closed arms (47% versus 4%, n = 304 cells in 12 mice, shuffle test, α = 0.05, Fig. 4D).
If these neurons are indeed recruited during exploration under potential threat, we would expect their activity to be positively correlated with specific exploratory behaviors. We identified time-locked responses to three such behaviors (Fig. 4E): (i) head dip, the most pronounced exploratory behavior when the mouse extends its head over the edge of the open arms, (ii) peeking, when the mouse’s body is in the closed arm while its head looks out to the open arms and center (shown in Fig. 4A), and, (iii) re-entry into the closed arms. Of the open arm-excited cells, 64% were excited by head dips and 69% by peeking, while 56% were inhibited during closed arm entry (Fig. 4F, S6F).
We then leveraged interindividual differences in EPM exploration to further probe the association between DRN5-HT neurons and emotional behavior. Strikingly, we found that all three behavioral measures of EPM exploration were inversely correlated with the degree of DRN5-HT excitation. The amount of time spent in the open arms, the number of head dips, and the number of peeks were all inversely correlated with the fraction open arm-excited DRN5-HT neurons (n = 18 mice, linear regression, p = 0.036, 0.0058, and 0.0193, respectively, Fig. 4G and Fig. S6A–B). Thus, animals that treated the open arms as more of a threat (as evidenced by their higher levels of avoidance) had higher levels of DRN5-HT excitation.
We also found a significant relationship between DRN subregion and DRN5-HT activity during open arm and center exploration (see Supplementary Table 2). Excited cells were preferentially located posteriorly and laterally within the DRN (Fig. S6L–M).
Importantly, there was no significant difference in velocity between open and closed arms (paired t-test, p > 0.05, n = 13 mice, Fig. S6C), and DRN5-HT preference for open over closed arms was maintained from low to high velocities in the population as a whole (Fig. S6D), and even more so in individual cells (Fig. S6E).
We then examined the composition of ensembles detected during EPM exploration. Open arm-excited cells typically comprised a single, large ensemble amongst others that did not respond specifically to the task (Fig. 4H–I, Fig. S6J–K). Across animals, 34% of ensembles were significantly recruited during open arm exploration (22/58 ensembles in 12 mice, shuffle test, α = 0.05). Of excited and non-selective ensembles, 48% of pairs were significantly segregated in space (n = 12 mice, 2D-K-S test, α < 0.05,).
DRN5-HT neurons are recruited during the initial investigation of a novel mouse
Social interaction is another emotionally salient behavior in which DRN5-HT activity has been implicated (Balázsfi et al., 2018; Walsh et al., 2018). We therefore determined how individual DRN5-HT neurons respond during social interaction. Male mice were placed in an open field arena, and allowed to explore alone for several minutes before a novel, juvenile male was introduced (Fig. 5A). Behaviorally, we found that mice spent double the time interacting with the novel mouse during the initial interaction period than in subsequent interactions, suggesting that this is the most emotionally salient period (Fig. 5B). We found that the DRN5-HT system was specifically recruited by the first social interaction, eliciting a time-locked DRN5-HT response from the population as a whole (Fig. 5C), and from a significant fraction of individual cells (53%, n = 404 cells across 13 mice, shuffle test, α = 0.05, Fig. 5D–E, Fig. S7A–D).
Figure 5. DRN5-HT ensembles are specifically excited by the first interaction with a novel mouse.
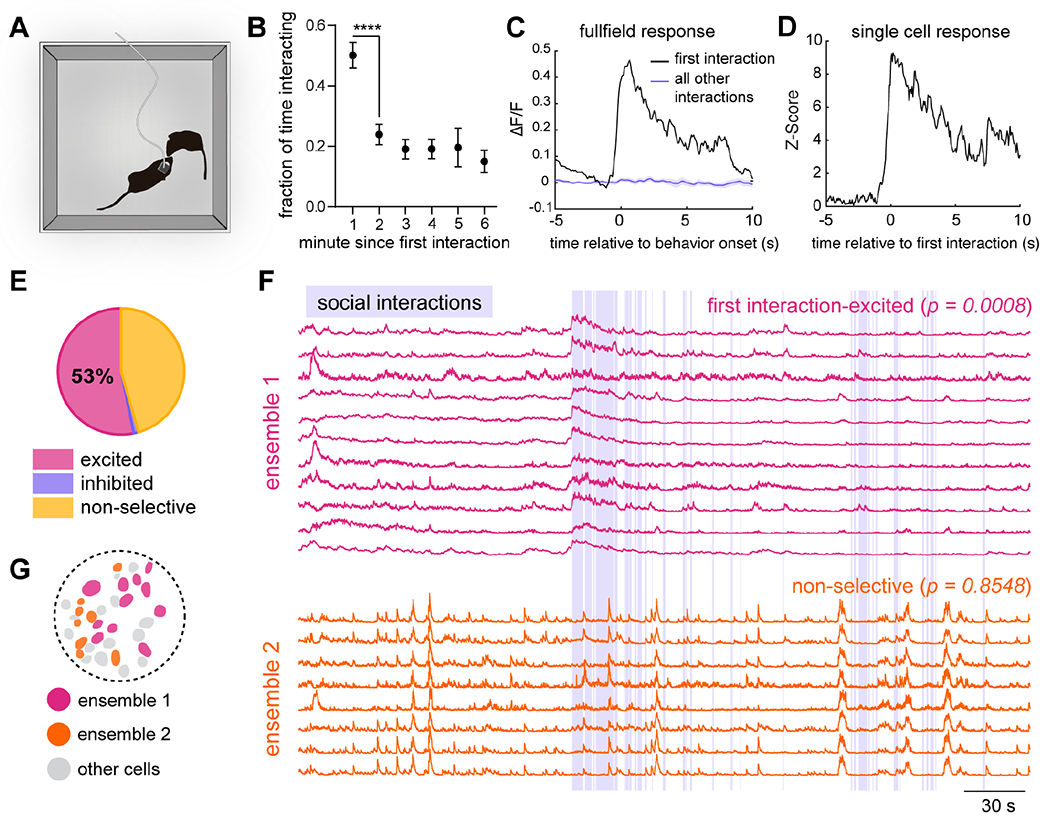
A) Diagram of the social interaction paradigm.
B) Fraction of time mice spend interacting. n = 13 mice (minutes 5 and 6, n = 12 and 11, respectively). Mixed-effects analysis, p = 0.0035. Paired t-test between 1 and 2 minutes, p < 0.0001.
C) Population-level response of a representative animal to the first interaction (black) compared all other interactions (purple). Shading represents ±SEM.
D) Example single cell response to first interaction.
E) Relative proportion of cells with a significant response to the first interaction (53% excited, 215/404 cells, shuffle test, α = 0.05, n = 13 mice).
F) Example ensembles. Ensemble 1 is excited by first interaction (p = 0.0008), while ensemble 2 is non-selective (p = 0.8548). Purple shading represents epochs in which mice are in close physical contact.
G) Spatial distribution of cells in (F).
We assessed the possibility that the DRN5-HT response to the first interaction was due to a specific behavioral type of interaction regardless of whether it is the first interaction. We found that the first interaction was typically face sniffing (11/13 mice), and 16% of neurons were exclusively excited by face sniffing. However, when the first interaction was excluded from the analysis, this proportion became negligible (5%, Fig. S7E). Thus, individual DRN5-HT neurons are specifically excited during the first period of intense social investigation.
We next determined if exploration of a neutral object would also recruit a large fraction of DRN5-HT neurons. Mice were allowed to explore an open field arena containing a novel object in one quadrant (Fig. S7H). The object was neither appetitive nor aversive, as mice spent the same amount of time in each quadrant of the arena (n = 7 mice, repeated-measures two-way ANOVA, p = 0.65, Fig. S7I). As with other neutral stimuli, approximately ¼ of cells were modulated during interaction with the object (Fig. S7J). In contrast to interaction with a novel mouse, the DRN5-HT response did not decrease after the first interaction (Fig. S7K–L), and there was no correlation between DRN5-HT activity and periods of increased interaction with the object (Fig. S7M). Furthermore, the proportion of cells across mice that responded to neutral object interaction was significantly smaller than that responding to the first social interaction (Fig. S7N). Thus, both the magnitude and temporal characteristics of the DRN5-HT response were distinct between social interaction and neutral object interaction.
As with cells detected during EPM exploration, the first interaction-excited cells formed highly correlated ensembles, alongside comparable subsets of cells that did not respond to social stimuli (Fig. 5F–G, Fig. S7F–G). Across animals, 52% of ensembles were excited by the first interaction (32/61 ensembles in 10 mice, shuffle test, α = 0.05). 56% of first interaction-excited ensembles were spatially segregated from non-selective ensembles (n = 54 pairs in 10 mice, 2D K-S test). Our results indicate that a discrete ensemble of first interaction-excited neurons is involved in social processing in the DRN.
Mixed selectivity of DRN5-HT neurons across behavioral tasks
To understand the logic of the DRN5-HT network, it is necessary to determine how individual DRN5-HT neurons respond across multiple behaviors. To address this, we tracked single cells across behaviors using the method described by Sheintuch et al. (2017) (Fig. S8A–B, see Methods). Many factors affect the ability to confidently detect the same cells across days (Fig. S8C), and our highest yield of tracked cells was between pairs of behaviors. This was best exemplified by repeating the sucrose consumption task with the same mice. Cells that were trackable maintained their responsiveness to sucrose (52/55 trackable neurons that were excited by sucrose on day 2, shuffle test to determine significance of overlap, p < 0.0001, Fig. 6A and H), and formed ensembles with the same cells in each session (see Fig. S8E), demonstrating a stable population. Nevertheless, only half of the total number of detected cells in each session were trackable across both days (94/184 (51%) of day 1, 94/167 (56%) of day 2). We therefore considered behaviors two-at-a-time and restricted our analyses to the trackable cells.
Figure 6. Mixed selectivity of individual DRN5-HT neurons across behavioral tasks.
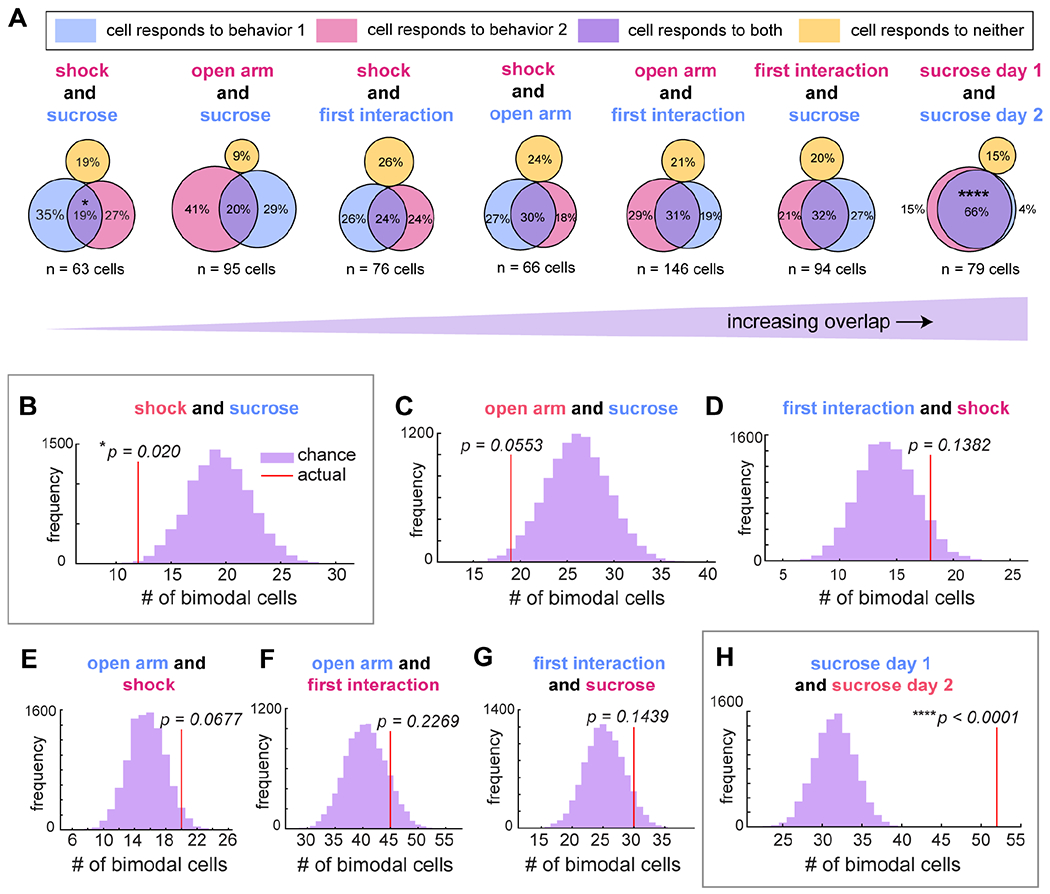
A) Relative proportions of cells tracked across each pair of behaviors that respond only to one (pink or blue) or to both (purple). Responsiveness was determined as in Fig. 1–5 (shuffle test, α < 0.05). Between sucrose consumption and footshock, overlap was 19% (n = 63 cells, 6 animals); open arm exploration and sucrose consumption, 20% (n = 95 cells in 8 mice); first social interaction and footshock, 24% (n = 76 cells in 6 mice); open arm exploration and footshock, 30% (n = 66 cells in 6 mice); open arm exploration and first interaction, 31% (n = 146 cells in 10 mice); first interaction and sucrose consumption, 32% (n = 94 cells in 8 mice); and two days of sucrose consumption, 66% (n = 79 cells in 6 mice).
B-H) Histograms indicating the likelihood of any number of cells, compared to the actual number observed (red line), responding to both sucrose consumption and footshock ((B), shuffle test, p = 0.020), open arm exploration and sucrose consumption ((C), p = 0.0553), first interaction and footshock ((D), p = 0.1382), open arm exploration and footshock ((E), p = 0.0677), open arm exploration and first interaction ((F), p = 0.2269), first interaction and sucrose consumption ((G), p = 0.1439), and sucrose consumption on two separate days ((H), p < 0.0001). P-values in (B), (C), (D), and (H) have been corrected for 4 comparisons between sucrose consumption and other behaviors using the Bonferroni method. Significantly separate or overlapping populations are highlighted in gray boxes.
For each behavior pair, we counted the number of tracked cells that responded to one behavior, to the other behavior, or to both, and we determined whether the fraction of cells responding to more than one behavior reflected statistically significant overlap. If two behaviors independently recruit a subset of cells from the population, an intermediate fraction would be expected to respond to both behaviors by chance. Significantly lower or greater overlap would indicate a mechanism that separates or combines the two populations (Fig. S8D). To establish the chance number of bimodal cells in each comparison, we iteratively shuffled preference assignments for all cells in each behavior, and then counted the number of bimodal cells in the tracked population (Fig. S8D). We then compared the actual number of bimodal cells to the resulting null distribution.
This analysis revealed a large degree of mixed selectivity and functional heterogeneity among DRN5-HT neurons, with most behavior pairs recruiting an intermediate fraction of bimodal DRN5-HT neurons at chance levels (Fig. 6). There were individual DRN5-HT neurons that responded to each possible combination of footshock, sucrose consumption, EPM exploration, and social interaction. Thus, DRN5-HT neurons that respond during emotionally salient behaviors are not strictly subdivided by behavior type, sensory modality, or valence. The exception to chance overlap levels was at the extreme comparison between highly aversive (footshock) and highly appetitive (sucrose consumption) behaviors. Overlap between footshock responsive cells and sucrose responsive cells was 19%, which is less than expected by chance (p = 0.02, n = 63 cells in 6 animals, p-value corrected for 4 comparisons between sucrose consumption and other behaviors using the Bonferroni method). Cells responding to sucrose consumption and open arm exploration also showed a trend towards being separate populations, with only 20% overlap (p = 0.0553, n = 95 cells in 8 mice, p-value corrected for 4 comparisons).
We lastly compared the behavioral preferences of the 43 cells that we could track across all four behaviors (Fig. S8F). The results likewise highlight the overlap between responses to multiple behaviors, the distinction at the extreme end of footshock and sucrose, and the high degree of heterogeneity in the population.
VTA- and BNST-projecting DRN5-HT neurons are heterogenous but functionally biased
DRN5-HT neurons exert their influence through their widespread projections. We next examined whether projection-specific neurons in the DRN correspond to activity-based ensembles, or if they are more heterogeneous. We chose to investigate two strong projections, both of which mediate behaviors affected by DRN5-HT afferents: the BNST, a region associated with anxiety behavior and modulated by serotonergic input (Marcinkiewcz et al., 2016; Garcia-Garcia et al., 2018), and the VTA, a region involved in reward processing (Hu, 2016).
To image these populations, we injected retrograde viruses encoding Cre-dependent GCaMP6m into either structure of Sert-Cre mice (Fig. 7A–C, Fig. S9A–F), and confirmed immunohistochemically that both populations were serotonergic (Fig. S9C–D). Because DRN5-HT axons are known to collateralize to multiple targets (Waselus et al., 2012), we further determined whether the populations are anatomically distinct. Using two retrograde viruses of the same serotypes as in this experiment (Fig. 7), but encoding different fluorophores, we saw very little overlap in the DRN, confirming that the populations are non-overlapping (2%, n = 2706 cells in 3 mice, Fig. S9G–H).
Figure 7. BNST- and VTA-projecting DRN5-HT neurons respond to diverse behaviors in complementary proportions.
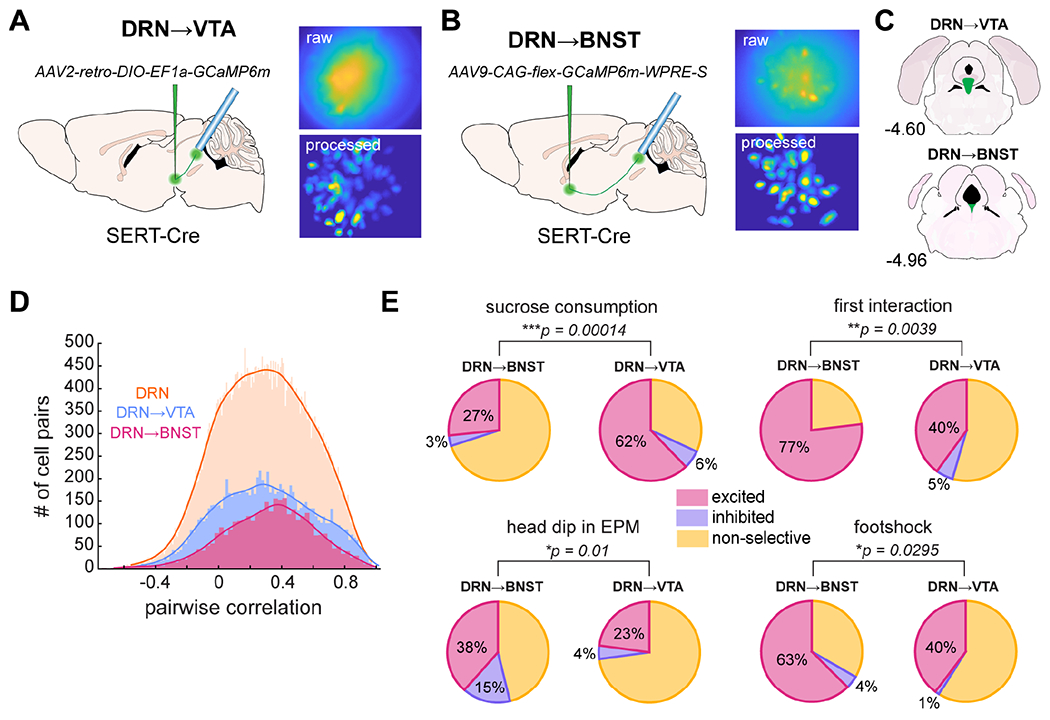
A-B) Experimental setup to target VTA- (A) or BNST-projectors (B) showing the viral injection of retrograde Cre-dependent GCaMP6m and lens placement in the DRN. A sample field of view is shown on the upper right, and a standard deviation projection of the same field after CNMF-E background subtraction on the lower right.
C) Location of VTA- (upper) and BNST- (lower) projectors in the DRN with respect to the anteroposterior axis. Figures in (A-C) were adapted from the Allen Mouse Brain Atlas (Lein et al., 2007; Bakker et al., 2015). See also Fig. S9A–F.
D) Comparisons of pairwise Pearson’s correlations of cells’ activity in each of the three populations described in this paper: DRN5-HT as a whole (n = 48, 126 cell pairs, same data as Fig. 1E), DRN5-HT➔VTA (n = 9,918 cell pairs), and DRN5-HT➔BNST (n = 3,506 cell pairs). K-S test to compare subpopulations to the whole (DRN5-HT➔VTA, D-stat = 0.0319, DRN5-HT➔BNST, D-stat = 0.0702).
E) Summary of cell selectivity across behaviors and subpopulations. Asterisks denote the results from a χ2 test of proportions between each pair of datasets. Percentages of excited and inhibited cells are indicated on the figure. VTA, n = 5 mice, BNST, n = 2 mice. Sucrose consumption: p = 0.00014 (BNST, n = 30 cells, VTA, n = 119 cells); first interaction: p = 0.0039 (BNST, n = 26 cells, VTA, n = 110 cells); head dip, p = 0.01 (BNST, n = 26 cells, VTA, n = 70 cells); footshock, p = 0.0295 (BNST, n = 24 cells, VTA, n = 73 cells).
Because a large volume of virus was required to visualize these populations, we assessed viral spread to nearby structures. We found minimal spread outside of the BNST, and some spread outside of the VTA to the interfascicular nucleus (IFN) (Fig. S9I–N). As such, the DRN5-HT→VTA population may also contain some cells that project to the IFN, or to both the IFN and VTA via collaterals. Any significant infection of axons on route to other brain areas is unlikely, as we did not observe GCaMP-expressing axons outside of the IFN or VTA (Fig. S9N).
We first investigated whether these projection-specific DRN5-HT neurons were more correlated than the DRN5-HT population as a whole. Surprisingly, we found varied levels of correlation in each projection similar to that in the DRN as a whole (Fig. 7D). Although they did have slightly more correlated activity, the effect size was extremely small (K-S test, DRN→VTA, D-stat = 0.0319, DRN→BNST, D-stat = 0.0702, Fig. 7D). This shows that anatomically-distinct projections within the DRN5-HT network are themselves heterogeneous.
We then characterized the nature of this heterogeneity by determining each projection’s responses to different emotional behaviors (Fig. 7E). We found that both projections contained DRN5-HT neurons that respond to each of the wide range of emotional behaviors we tested. Thus, the projections are not strictly segregated by behavioral responses. However, the relative fractions of cells responsive to each behavior were biased (Fig. 7E, Fig. S9O). The composition of these subpopulations was complementary and consistent with the roles of the VTA and BNST in behavior: VTA-projectors responded more to sucrose reward, while BNST-projectors responded more to head dips in the EPM, footshock, and the first social interaction with a novel mouse (Fig. 7E, Fig. S9O).
DISCUSSION
Imaging over 2,000 serotonergic neurons during emotionally salient behaviors revealed several key principles of the DRN5-HT system (Fig. 8).
Figure 8. Proposed model of DRN5-HT network at the population- and single-cell level.
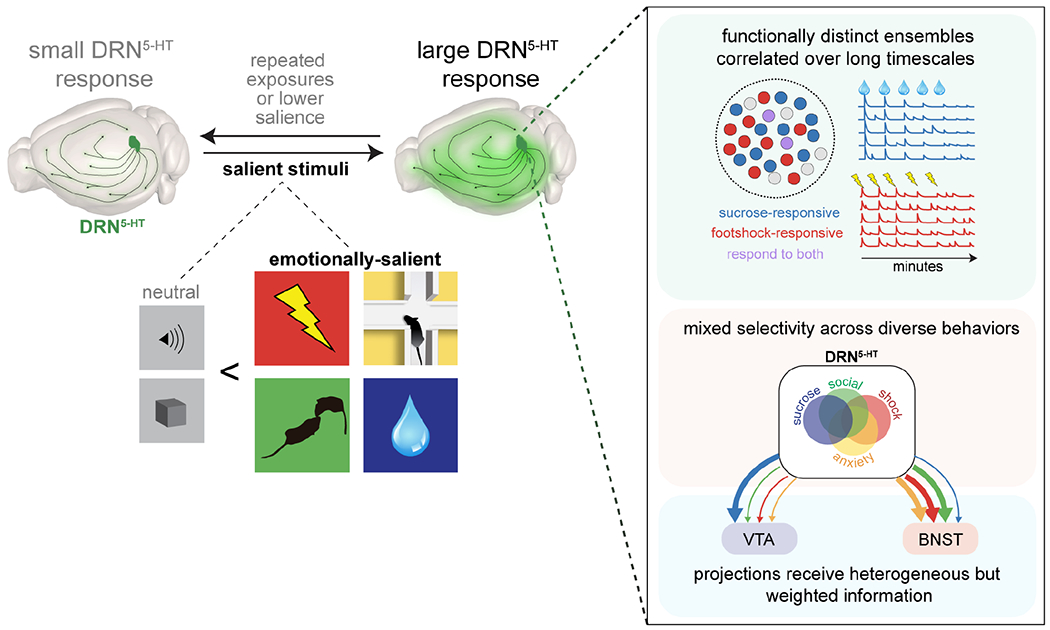
A large fraction of serotonergic neurons in the dorsal raphe nucleus is modulated by emotional salience. More cells respond to emotionally salient stimuli than to neutral stimuli, and activity scales with relative salience. At the network level, discrete ensembles are highly correlated over long timescales. Single cells respond to diverse combinations of individual stimuli with some constraints by valence and sensory modality. Projection-specific subpopulations contain the full complement of response types but in weighted proportions. Arrows represent the populations of cells responding to sucrose (blue), first social interaction (green), exploratory behavior in the elevated plus maze (yellow), and footshock (red).
DRN5-HT neurons are organized into highly correlated ensembles
By imaging numerous DRN5-HT neurons simultaneously, we found evidence of both heterogeneity and coordination. Despite their heterogeneity, DRN5-HT neurons cluster into discrete ensembles with highly correlated activity. A future systematic comparison of the results of these analyses in different brain regions would put the degree of correlation in the DRN serotonin system in context. In cortical areas and the hippocampus, for example, correlated activity is much lower (Miller et al., 2014; Jimenez et al., 2020).
Activity within ensembles was correlated during behavioral bouts, at baseline outside of any specific behavioral epochs, and across multiple days. During behavioral bouts, DRN5-HT ensembles often reflected features of the behavior. For instance, during sucrose consumption, some ensembles were excited by sucrose consumption and habituated, some did not habituate, and some were not excited by sucrose at all.
Correlated activity could arise from shared inputs or interconnectivity within ensembles. Ensembles could serve several functions. Projections from a DRN5-HT ensemble that converge on a single target would release serotonin in a highly coordinated fashion and this may intensify the downstream response. Alternatively, neurons in a DRN5-HT ensemble that project to different circuits could coordinate their activity.
We performed several experimental and analytical controls to determine if brain motion led to spurious correlations, and we did not find evidence of this (Fig. S2). However, it will be important to further rule out this potential limitation in future studies using independent methods.
Emotionally salient stimuli strongly modulate the DRN5-HT system
We found that a wide range of emotionally salient stimuli strongly recruit the DRN5-HT system. In each behavioral paradigm we tested, a large proportion, approximately 50%, of detected DRN5-HT cells were modulated by an emotionally salient stimulus. This feature spans both valence and sensory modality. Across paradigms, DRN5-HT activity was highest during the most salient periods. Furthermore, we found that DRN5-HT responses in the gustometry paradigm scaled with salience rather than valence.
In contrast, neutral stimuli activated a smaller fraction of DRN5-HTneurons and the responses did not adapt. However, after associative conditioning with emotionally salient stimuli, previously neutral cues recruited a comparable fraction of DRN5-HT neurons.
Importantly, although emotionally salient stimuli strongly recruit the DRN5-HT system, our study and others have found that locomotion and neutral stimuli also modulate DRN5-HT neurons (Ranade and Mainen, 2009, Correia et al., 2017, Seo et al., 2019). This highlights the heterogeneity of the system.
Mixed selectivity of DRN5-HT neurons
Electrophysiological studies have shown that DRN neuron responses are heterogeneous (Ranade and Mainen, 2009). Thus, there is not a unitary DRN5-HT response profile, and this has been borne out by further electrophysiology and fiber photometry studies (Liu et al., 2014; Cohen et al., 2015; Hayashi et al., 2015; Li et al., 2016; Ren et al., 2018). This could reflect a heterogeneous population of individually well-tuned DRN5-HT neurons (e.g., tuned by modality or valence). Alternatively, it could reflect heterogenous DRN5-HT neurons with mixed selectivity. We addressed this by imaging DRN5-HT neurons across a range of modalities and valence, and found that most tracked DRN5-HT neurons had mixed selectivity. Importantly, most DRN5-HT neurons were not broadly tuned to respond in all of these contexts. Instead, most showed overlap between a subset of stimuli at levels expected by chance. The exception to this was the response to highly appetitive sucrose versus highly aversive footshock. However, this was not absolute, and there were several DRN5-HT neurons that responded to both.
Functionally-biased projections
How do the single-cell response properties that we identified map onto different circuits? To address this, we specifically imaged cells targeting the VTA, a reward-related structure, or the BNST, an anxiety-related structure, and report for the first time DRN5-HT single-cell responses with known targets. Surprisingly, we found that all response types were represented in each projection, and that the extent of their heterogeneity was similar to that of the entire DRN. Thus, diverse DRN5-HT responses are broadcast to multiple projections at least to some degree. These populations did, however, differ in relative proportions of response types. The VTA projection contains a higher proportion of sucrose-responsive cells, while the BNST projection has a higher proportion of cells that respond to footshock, potential threat, and social interaction (Fig. 7). This demonstrates a middle ground between completely heterogeneous projections and distinct homogenous subsystems. Our findings are consistent with the fiber photometry results of Ren et al. (2018) that show that the population average responses between two DRN5-HT projections differ. However, we now show that the heterogeneity of the DRN5-HT system is still present within projections.
A balance of heterogeneity and coordination, within the DRN itself and between its outgoing projections, thus underlies its role in a wide array of emotionally salient behaviors.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Bradley Miller (bradley.miller@nyspi.columbia.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal Subjects
All procedures were conducted in accordance with the U.S. NIH Guide for the Care and Use of Laboratory Animals and the New York State Psychiatric Institute Institutional Animal Care and Use Committees at Columbia University. Adult male Sert-Cre mice (Zhuang et al., 2005) were bred in-house on a C57BL/6J background and used from approximately 10-14 weeks of age. Mice were given unrestricted access to food and water, except during the 24-hour period before the sucrose consumption task (Fig. 1), for which they were water-restricted. They were on a 12-hour light cycle, and experiments were conducted during the light portion.
Viral Constructs
For use in calcium imaging in the DRN, AAVDJ-DIO-EF1α-GCaMP6m was packaged and supplied by the UPenn Vector Core at a titer of ~6x1012 vg/ml, and aliquots were diluted prior to use with artificial cortex buffer to ~2x1012 vg/ml. For the z-motion GFP control (Fig. S2D–I), AAV5-flex-GFP was packaged and supplied by the UNC Vector Core at a titer of ~8x1012 vg/ml and diluted prior to use to ~2x1012 vg/ml. Viruses for projection-specific imaging (AAV9-CAG-flex-GCaMP6m-WPRE-S and AAV2-retro-DIO-EF1α-GCaMP6m, Fig. 6, Fig. S9) were packaged and supplied by the Stanford Vector Core at ~6x1012 vg/ml and aliquots were diluted prior to use with artificial cortex buffer to ~2x1012 vg/ml. For the comparison of projection-specific populations (Fig. S9G), AAV2-retro-DIO-EF1α-GCaMP6m was sourced and prepared as described, and AAV9-CAG-flex-tdTomato-WPRE was supplied by the UPenn Vector Core at a titer of ~8x1012 vg/ml and diluted to ~2x1012 before use. For histological analysis of viral spread (Fig S9I–L), AAVDJ-hSyn-GCaMP6m was obtained from the Stanford Vector Core at ~1x1013 vg/ml and diluted to 2x1012 vg/ml before use.
METHOD DETAILS
Stereotactic Surgeries
For all surgical procedures, anesthesia was induced with 3.0% isoflurane, and then maintained with 1.5% isoflurane at an oxygen flow rate of 1 L/min. Mice were head-fixed in a stereotactic frame (David Kopf, Tujunga, CA). Eyes were lubricated with Vaseline, and body temperature maintained at 37°C with a T/pump warm water recirculator (Stryker, Kalamazoo, MI). Fur was shaved and incision site sterilized with betadine and ethanol before surgical procedures, and subcutaneous Carpofen and saline were provided peri-operatively. For 1 week post-operatively, mice were fed MediGel CPF (Carpofen at ~5 mg/kg/day, ClearH2O) for analgesia and given subcutaneous injections of saline as needed to prevent dehydration.
For in vivo Ca2+ imaging, mice underwent two surgeries. Serotonergic neurons were first labeled for in vivo Ca2+ imaging by injecting 800 nL of Cre-dependent calcium indicator GCaMP6m packaged in an adeno-associated virus (AAVDJ-DIO-EF1α-GCaMP6m) into the dorsal raphe nucleus (coordinates from bregma: −4.5 AP, −1.12 ML, & −3.1 DV at a 22° lateral angle) with a syringe (World Precision Instruments, Sarasota, FL). All viruses were injected at a rate of 100 nL/min. For z-motion control experiments (Fig. S2D), virus expressing Cre-dependent GFP (rAAV5-flex-GFP) was injected into the DRN using the same protocol as for Cre-dependent GCaMP6m.
BNST-projecting DRN5-HT cells were targeted using two injections of 600 nL each of Cre-dependent GCaMP6m (AAV9-CAG-flex-GCaMPM6m-WPRE-S) bilaterally into the BNST. Injections targeted both the dorsal and ventral subdomains of the structure (coordinates from bregma: +0.3 AP, ±0.9 ML, −4.7 (vBNST) and −4.2 (dBNST) DV). VTA-projecting neurons were similarly targeted using a retrograde adeno-associated Cre-dependent virus (AAV2-retro-DIO-EF1α-GCaMP6m) into the VTA (coordinates from bregma: −3.3 AP, ±0.3 ML, −4.7 & −4.2 DV). The respective serotypes were chosen empirically by surveying those with retrograde activity, and selecting for each target structure the serotype that yielded the highest expression of GCaMP6m in DRN5-HT cell bodies. The relatively large volume of virus used was determined empirically to be required for sufficient visualization of cell bodies in the DRN.
For the anatomical analysis of VTA- and BNST-projecting subpopulations (Fig. S9G), Cre-dependent retrograde virus encoding GCaMP6m as in the corresponding imaging experiment (AAV2-retro-DIO-EF1α-GCaMP6m) was injected into the VTA and Cre-dependent tdTomato (AAV9-CAG-flex-tdTomato-WPRE) was injected into the BNST. Experimental parameters were the same as used in the projection-specific imaging experiment.
For imaging experiments, GRIN lenses were implanted into the DRN during a second surgery using adapted methods two weeks after viral injection of Cre-dependent GCaMP6m (Resendez et al., 2016). A vertical incision was made using a No.10 scalpel blade (Fine Science Tools (FST) Foster City, CA) to expose the skull. The vertical path above the DRN contains a confluence of blood vessels, which, when pierced by a lens, irreparably obscures the field of view. To avoid this, we inserted the lens at a posterior angle, which required us to sever the trapezius and occipitalis from the occipital bone to accommodate the craniotomy at −6.53 mm AP and 0 mm ML. After the craniotomy, the dura was removed from the brain surface and cleaned with a stream of sterile saline and absorptive spears (FST, Foster City, CA). A GRIN lens (~0.5 mm diameter and −6.1 mm long) was lowered at a 32° posterior angle until an adequate plane of cell bodies and/or diffuse fluorescence were visible with the miniscope using the Proview system (Inscopix, Palo Alto, CA). The DV position of the lens thus varied by animal and was ultimately assessed histologically. The lens was then fixed to the skull with dental cement (Parkell, Edgewood, NY). At the completion of surgery, the lens was protected with liquid mold rubber (World Precision Instruments, Sarasota FL).
One week later, mice were again anesthetized and head-fixed into a stereotactic frame. The protective rubber mold was removed from the lens using fine-tipped forceps (FST, Foster City, CA). A magnetic baseplate was gradually lowered towards the lens while the imaging field of view was monitored using the attached miniscope. The final position of the baseplate was chosen to optimize the focal plane of the miniscope. The baseplate was secured above the lens with dental cement to provide a stable attachment point for the miniscope. The focal plane was fixed throughout the entire experiment, ensuring a stable field of view across sessions.
Freely-Moving Calcium Imaging
Awake-behaving imaging sessions were commenced approximately 1 week after baseplating. On each imaging day, mice were briefly anesthetized (< 5 minutes) with 3% isoflurane in order to attach the miniscope to the baseplate. Mice were allowed to recover from anesthesia in their home cage for 20 minutes before imaging. Ca2+ videos were recorded using nVista acquisition software (Inscopix, Palo Alto, CA), and triggered with a TTL pulse from EthoVision XT 11 via the Noldus IO box system to allow for simultaneous acquisition of Ca2+ and behavioral videos. To later account for any lag between the onset of behavior and Ca2+ movies, a continuous train of TTL pulses was sent from Ethovision XT 11 to nVista acquisition software at 0.5 Hz and a 50% duty cycle for the duration of the session for running synchronization of the two datasets.
Ca2+ videos were acquired at 17 frames per second with an automatic exposure length. An optimal LED power was selected for each mouse to optimize the dynamic range of pixel values in the field of view, and the same LED settings were used for each mouse throughout the series of imaging sessions. The maximum LED power used was 85% and movies were collected without gain.
Behavioral Tasks
For each imaging session, behavioral data was recorded at 30 frames per second using a webcam input to EthoVision XT 11. The estimated center of mass of the mouse was used to automatically track the mouse within custom-defined arenas and their sub-zones. Tracking data was routinely corrected manually to best match the position of the mouse at each frame of the behavioral movie.
Order of tests.
Behavioral tests were typically ordered from neutral or positive to negative valence. Half of the mice performed the open field test, sucrose consumption, social interaction, the elevated plus maze, and footshock, in that order. The other half performed the open field test, then social interaction, the elevated plus maze, sucrose consumption, and footshock. To test for sequence effects, we compared the proportions of sucrose- and open arm/center-responsive neurons between these groups, which differ in the relative order of the sucrose consumption task and the elevated plus maze. We found no significant difference (sucrose-responders, p = 0.7287; open arm and center-responders, p = 0.1432, unpaired t-tests). Separate cohorts of a total of seven mice underwent the open field test, neutral object, social interaction, auditory cue, gustometer (3 training days and 1 test day), elevated plus maze, and cued fear conditioning (2 sessions), in that order.
Sucrose Consumption.
Mice were placed in a standard housing cage in dim light (< 20 lux) with fresh bedding and were allowed at least 5 minutes to habituate to the cage before imaging. Just prior to imaging, a spout was attached with tape to the inside wall of the cage. The spout was connected to an MPR-121 capacitive touch sensor breakout board (SparkFun Electronics) to trigger opening of a liquid solenoid valve via an Arduino Uno. As the mouse touched its tongue to the spout, 30 μL of aqueous sucrose (5% wt/vol) was immediately dispensed and the lick was recorded using a TTL pulse transmitted from the Arduino to the nVista data acquisition box for automatic synchronization with imaging data. The volume of liquid dispensed was set by the open time of the solenoid valve (30 ms) and was consistent across animals, days, and individual licks. Sucrose was delivered ad libitum, without trial structure or a delay between lick and reward; i.e., for every lick, the mouse was delivered sucrose water.
Social Interaction.
Mice were placed in an arena (18 x 18 x 12” length-width-height; Kinder Scientific, Poway, CA) in dim light (~20 lux) and allowed to interact with a novel, juvenile male mouse of the same strain for 11 minutes while behavior was recorded and analyzed with EthoVision software. Anogenital sniffing, body sniffing, face sniffing, flank-to-flank positioning (see Li et al., 2016), and aggressive interactions were hand-scored with Observer XT software.
Elevated Plus Maze.
Mice were placed in a standard EPM maze (13.5” height of the maze from the floor, 25” full length of each arm-type, 2” arm width, 7” tall closed arm walls, with 0.5” tall/wide ledges on the open arms), at ~400 lux centered over the open arms to promote avoidance. Mice were initially placed in the center region of the maze and were allowed to explore for 11 minutes while recording behavior with a webcam and tracking the mouse using EthoVision XT 11 (Noldus, Leesburg, VA). Head dip and peeking behaviors were hand-scored with Observer XT software (Noldus, Leesburg, VA).
Footshock.
Mice were placed in a standard fear conditioning shock box (Coulbourn Instruments, Holliston, MA) and allowed to habituate for 5 minutes. Ten shocks were delivered at 0.7 mA and approximately 0.5 seconds in length. The interval between shocks was between 20 and 40 seconds, decided using a pseudorandom number generator.
Auditory Cue.
Mice were allowed to freely explore a standard housing cage with bedding while exposed to a series of tones played using a speaker and controlled by an Arduino Uno. A total of 30 2-second tones were played every 30 seconds at 80 dB as follows: 3.5-minute habituation to the cage, 10 2 kHz tones, 10 10 kHz tones, 4-minute break, 10 2 kHz tones.
Neutral Object Interaction.
Mice were placed in a standard open field (18 x 18 x 12” length-width-height; Kinder Scientific, Poway, CA) in dim light (~20 lux) with a neutral object (upside-down plastic funnel) placed in a randomly chosen quadrant of the arena.
Cued Fear Conditioning.
On day 1, mice were placed in a standard fear conditioning shock box (Coulbourn Instruments, Holliston, MA) with the following contextual cues: bright light on (~700 lux), fan on, banana scent (isoamyl acetate), doors closed, metal bar floor. After a 3-minute habituation period, mice were played a series of three tones at 2 kHz and 90 dB, each lasting 20 seconds with 40 seconds in between tones. Then, mice were presented with another five tones with the same specifications and schedule, each co-terminating with a 2-second footshock of 0.7 mA. On day 2, mice were placed in the same box with the following contextual cues: dim light (~20 lux), no fan, fruity scent (methyl butyrate), doors open, smooth plastic floor. After 3 minutes of habituation, five tones were played as on day 1, but with no co-terminating shock. Freezing was scored semi-automatically using custom code written in MATLAB and based on the differences in pixel values between successive frames of the behavioral video. A threshold was manually chosen and any series of frames with subthreshold variance (i.e., less movement of the mouse) that lasted at least 2 seconds was considered freezing behavior.
Open Field Test.
Mice were placed in a standard open field arena (18 x 18 x 12” length-width-height; Kinder Scientific, Poway, CA) in dim light (~20 lux) and allowed to explore the space for 11 minutes.
Gustometry.
Water-deprived mice were placed in a custom-built operant box (8 x 10”) with a slot in one of the narrower walls at eye-level. Through this space, one of five spouts was presented at a time. Using custom Arduino code, an MPR121 touch capacitor, and solenoid valves as detailed above, mice received a 20 μL drop of a solution upon licking. Spouts were alternated using a rotating wheel and a generic high torque servo motor (SparkFun Electronics). On the first day of training mice were habituated to the box, spout, and rotating wheel. Two spouts, each delivering water regardless of licking, were alternated every 10 seconds. On the second day of training, all five spouts were used, each delivering water. For the first 95 trials, the spouts each delivered water immediately, regardless of licking, and changed every 10 seconds. For the remaining trials, the drop of water was delivered only upon licking and the spout was changed 10 seconds after this delivery. The next day, the same protocol was followed as the latter trials of training day 2. On test day, the protocol was once again the same, except the spouts each delivered a different solution: water, 0.5% (wt/vol) sucrose, 2.5% sucrose, 5% sucrose, or 0.1 mM quinine.
Histology and Microscopy
For all histology, mice were perfused transcardially with 4% (wt/vol) paraformaldehyde in phosphate buffered saline (PBS). Brains were removed and drop-fixed in 4% PFA for 24 hours and transferred to 30% sucrose solution in PBS until equilibrated (2-3 days). For mice used in calcium imaging experiments, heads with head-caps and lenses still in place were first drop-fixed in 4% PFA for 72 hours to achieve a clear lens tract. Sucrose-saturated brains were then frozen on dry ice and sliced into 35 μm-thick coronal sections on a cryostat (Leica CM 3050S). Sections were collected in individual culture wells to maintain anterior-posterior order.
For both standard and projection-specific imaging studies, sections were mounted in anterior-posterior order and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA) 24 hours after slicing. GCaMP6m expression was typically confirmed using endogenous viral expression. In some cases, and for images in this report, this signal was boosted and stabilized using an anti-GFP antibody pre-conjugated to AlexaFluor™-488 (Invitrogen, 1:500). To ensure specific expression of GCaMP in serotonergic neurons, sections were counterstained with Anti-Tryptophan Hydroxylase/Tyrosine Hydroxylase/Phenylalanine Hydroxylase Antibody, clone PH8 (Millipore Sigma, 1:10000) and visualized using the secondary antibody Cy5 AffiniPure Goat Anti-Rabbit IgG (H+L) (Jackson ImmunoResearch Laboratories). For the assessment of GCaMP-expressing axon terminals in the BNST and VTA of projection-specific imaging mice, sections were counterstained with anti-5-HTT (Millipore Sigma, 1:500) and visualized using the secondary antibody goat anti-rabbit IgG AlexaFluor-555 (Invitrogen, 1:1000). GRIN lens placement was determined by inspecting sections relative to a mouse brain atlas (Paxinos and Franklin, 2001) using an epifluorescent microscope (Zeiss Axiovert 200). To generate images for cell counting, and for those used in this report, histology slides were imaged on a Leica TCS SP8 confocal microscope using a 10x or 20x objective.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data Processing
Data Pre-processing.
Each movie was manually cropped to the field of view and spatially downsampled by a factor of four to reduce the computational power required for motion correction and cell segmentation. The dimensions of each preprocessed movie were approximately 256 x 256 pixels.
Calculation of ΔF/F (Population-level Activity).
The mean fluorescence over time of each movie was calculated from a large circular ROI hand-drawn over the entire field of view in FIJI. Raw fluorescence traces were then processed using custom code in MATLAB for conversion to ΔF/F. At each timepoint, ΔF/F was calculated as the percent change from baseline, with the baseline calculated as the average of the bottom 20% of values in the preceding 40 seconds.
Behavioral and Calcium Data Alignment.
Behavioral data was aligned to calcium data using the record of a synchronization signal between the two computers used for data collection as described above. Behavioral data, recorded at 30 fps, was downsampled to match calcium imaging data, recorded at 17 fps.
Motion Correction.
Due to its position in the brainstem, and immediately beneath the fourth ventricle, the DRN is subject to a high degree of motion during freely-moving behavior. To accommodate this, we took advantage of our cells’ baseline fluorescence. Likely the result of tonic firing, a well-known characteristic of DRN5-HT neurons (Müller and Jacobs, 2010), this baseline activity allowed a large number of putative neurons to be spatially resolvable in every frame of our movies. We were consequently able to motion correct our data using a simple, frame-by-frame normalized, two-dimensional cross-correlation after crudely subtracting the background with a high-pass filter. For the filter, each frame was morphologically opened using a disk-shaped structuring element approximately twice the size of a typical, in-focus cell. This was subtracted from the raw movie to create the ‘tophat-filtered’ movie which was used to calculate optimal translations. The first frame was typically considered the reference. The (x,y) translations that optimized the correlation between each frame and a reference were then applied to the raw, unfiltered movie using a custom program in MATLAB. When necessary, the tophat-filtered movie was cropped to an approximately 300 x 300 μm field at the center of the field of view to prevent interference from the stationary lens edge. On our data, this method outperformed other algorithms, including TurboReg (Thévenaz et al., 1998, via Inscopix data processing software) and NoRMCorre (Pnevmatikakis and Giovannucci, 2017).
Cell Segmentation.
In the DRN, we experienced both residual motion, decreasing the stability of spatial components, and highly correlated activity between cells and their background. We were ultimately able to segment cells using CNMF-E, a method that models both global and local background variants to identify cells even in high-noise environments (Zhou et al., 2018). Because residual (x,y) motion often results in single cells being interpreted as multiple units, we selected regions of interest (ROIs) on the CNMF-E background-subtracted movie based on visual inspection of both the raw data and a temporal projection of the processed data. An alternative method, PCA/ICA (Mukamel et al., 2009), failed to reliably identify cells. It should be noted that we are unable to characterize baseline firing rates of individual cells because of low temporal resolution and are therefore blind to tonic modulation of activity. Because CNMF-E is optimized to detect phasic increases in activity, it is also theoretically more difficult to detect phasic decreases in activity. Nevertheless, our results are consistent with electrophysiology reporting little time-locked inhibition, except in reward tasks (Ranade and Mainen, 2009) in which we also observed the incidence of inhibited cells (Fig. 1G, Fig S4C). We also observed large populations of cells inhibited upon closed arm reentry in the elevated plus maze (Fig. 4F).
CNMF-E was run using single-stream processing on the full field of view to reduce cell-splitting across patches processed in parallel. The movie was temporally downsampled by a factor of three for initialization only. The maximum allowed neuron diameter was optimized for each movie, and was typically in the range of 15-25 pixels with a Gaussian kernel width one third of this value. Similarly, the minimum local correlation and peak-to-noise ratio used for seeding were adjusted manually for each movie, and were at least 0.90 and 12, respectively. Two iterations of updates to the estimated background, spatial, and temporal components were run unsupervised. Results were then applied to the full resolution movie, and neurons were manually sorted. In anticipation of ROI selection, large components were trimmed so that any pixels that overlapped with another component would be subtracted from that component and therefore not aberrantly included in its final trace. After a third and final iteration of CNMF-E, the neurons were sorted and trimmed again.
ROIs were then drawn and managed using CalTracer3beta from a standard deviation Z-projection of the denoised movie, reconstituted from the spatial footprints and their deconvolved temporal components (Y = AC). Traces were generated by applying these ROIs to the background-subtracted movie (Ysignal) output by CNMF-E. For each cell, the value at each timepoint was calculated as the mean pixel value within the ROI. Resulting traces were then normalized by converting them into units of standard deviation, as previously described (Jimenez et al., 2018).
Quality Control.
As is custom, cells were then manually sorted to identify units with low signal to noise ratio for exclusion. Importantly, we did include these neurons when tracking cells across multiple days (Fig. S6), with the interpretation that they are present but inactive during the task. To further confirm that identified neurons were not dendrites, we modeled their sphericity using a standard Gaussian mixture model in a randomly sampled subset of our data (Fig. S2F). Spatial footprints were converted to x-y coordinates in units of micrometers, and pixel intensity was coded as the number of individual datapoints.
We performed several analyses to confirm that activity, and especially correlated activity, was not an artifact of neuropil contamination or z-motion. We first compared the distance between cells with their correlation in activity. If correlated activity is highest in cells that are close together, that may indicate insufficient background subtraction or contamination of one cell’s activity by another. Instead, we found no correlation between these two metrics (n = 21,297 cell pairs, adjusted R2 = 0.0684, Fig. S2B), and we observed many cases of adjacent cells with no apparent signal contamination (Fig. S2A–B). We further compared pairwise distance measurements between data subsets with varying degrees of correlated activity, and found little difference (Fig. S2C (top), K-S test, all cells vs. cells with Pearson’s correlation coefficient (r) > 0.3, D-stat = 0.0664, all cells vs. r > 0.6, D-stat = 0.1466, r > 0.3 vs. r > 0.6, D-stat = 0.0841; n = 21,297 (all), 12,662 (r > 0.3), and 5,077 (r > 0.6) cell pairs).
To determine if correlation was due to a residual neuropil signal, we gathered fluorescence timeseries from ROIs selected in the neuropil between identified cells and then assessed their correlation. We found significantly less correlation in neuropil ROIs than between actual cells, demonstrating adequate subtraction of both local and global background signals (n = 3,815 neuropil ROI pairs and 6,626 cell pairs, K-S test, D-stat = 0.5630, p <<< 0.0001, Fig. S2D–E).
We then assessed whether the observed DRN5-HT activity was an artifact of motion in the z-plane by repeating our experiments with a non-dynamic indicator, GFP (Fig. S2G). This is a good indicator of z-motion artifact because changes in detected GFP fluorescence reflect z-motion. Despite comparable numbers of cells in the field of view (Fig. S2H), no nominally active cells were detectable by CNMF-E, which only returns units with dynamic fluorescence (Zhou et al., 2018). Population-level analysis revealed that population-level GFP responses to our considered behaviors, including those that involve head motion, are undetectable compared to analogous responses observed in experimental mice expressing GCaMP6m (Fig. S2I). To confirm that correlated activity and behavioral responses in single cells were not the result of z-motion, we first used Inscopix Data Processing Software to spatially bandpass each movie, then motion corrected and selected ROIs as above. For each cell, fluorescence traces were modeled by x- and y-motion of the field of view (computed during motion correction) using linear regression and allowing interactions. Residuals were computed as the raw fluorescence traces minus the best-fit timeseries.
This single cell modeling of z-motion was very effective as shown by the high correlation between modeled z-motion and z-motion derived fluorescence fluctuations in GFP-expressing cells (Fig. S2J–K, n = 97 cells in 2 mice). To determine if z-motion could recapitulate the correlated activity we discovered in DRN5-HT neurons, we compared pairwise activity correlations between pairs of GFP-expressing cells (caused by z-motion) and pairs of GCaMP-expressing cells. We found that the correlation patterns are highly distinct (Fig. S2L, n = 97 GFP-expressing cells in 2 mice and 135 cells in GCaMP-expressing cells in 2 randomly selected mice during the same behavior, K-S test, D-stat = 0.4321, p = 4.5 x 10−170). Thus, z-motion does not underlie the correlated activity we found between DRN5-HT neurons. We then examined if z-motion artifact drives the signal we found in DRN5-HT neurons during specific behaviors. To do so, we subtracted the fitted z-motion signal from single-cell fluorescence traces (Fig. S2J–M). After subtraction of z-motion, GCaMP responses remained, while the relatively small GFP responses essentially disappeared (Fig. S2O–P, same dataset as in Fig. S2L, K-S test, D-statistic = 0.3608, p = 3.98 x 10−6). We found that GCaMP fluorescence was markedly distinct from modeled z-motion during all behaviors (examples in Fig. S2N, S5L, 6G–I). Thus, z-motion does not underlie the DRN5-HT activity we observed during behavior.
Correlation Analysis.
To determine whether overall correlation between DRN5-HT neurons was significant, we computed pairwise Pearson’s correlation coefficients across the entire dataset and compared the distribution to that generated using shuffled data (Fig. 1E). To generate this chance distribution, we offset traces from each other by a randomly chosen number of frames and then calculated the pairwise correlation coefficients. This process was repeated 1,000 times.
Multi-Session Cell Tracking
Cells were tracked across sessions using CellReg (Sheintuch et al., 2017). Briefly, rigid alignment with both translations and rotations was performed on spatial footprint projections of each session and manually inspected for quality. To improve performance with our data, we modified the CellReg source code to consider complete spatial footprints instead of centroids during alignment. The spatial correlation and centroid distance between neighbors were then calculated and used to create a probabilistic model that estimated the expected error rate at different thresholds. The optimal threshold was chosen by the algorithm and used to match cells. A clustering algorithm then refined these decisions previously made using pairwise comparisons. In cases in which the model itself was poor (common when few or sparse cells), a fixed threshold of 0.65 spatial correlation was used.
Following cell registration, tracked cells were matched with their corresponding cell selectivities. All analyses carried out were pairwise, to maximize the number of cells in each comparison and to minimize the total number of comparisons. For the analyses presented, pairs were counted as being significantly excited by both behaviors, by one or the other, or by neither. At least one cell of the pair had to have been classified as a good cell during the quality control step, i.e., active in one of the behaviors, and as previously discussed, ‘bad’ cells excluded from other analyses were treated as present but inactive. We did not restrict our statistical analysis to tracked cells, nor did we treat untrackable cells as inactive, as this would bias our results towards overlapping or segregated populations, respectively. Instead, for each pairwise comparison, we iteratively shuffled preference assignments in each population and counted the number of bimodal cells in the tracked population (nsim = 10,000, Fig. S8D). We then assessed whether the actual number of bimodal cells was significantly more or less than expected by chance. Because the resulting distributions were of numbers of cells, and therefore discrete rather than continuous, we calculated p-values by comparing the actual data to a normal distribution with mean and standard deviation computed from the shuffled distribution.
Cell Selectivity Analysis
Two tests were used to determine whether each cell’s activity was significantly correlated with a behavior. The first was used for temporally-specific behaviors, such as sucrose consumption, footshock, head dip, and first interaction. For this test, we calculated the mean difference in z-score during the first three seconds following each event onset and 5 to 2 seconds before the event onset from raw traces. The period from −2 to 0 seconds was excluded to accommodate both anticipatory activity and variability in manually-scored behavior onsets. These mean differences were themselves averaged, and compared to a null distribution of the same metric generated by shuffling the onsets across the entire session for 10,000 separate iterations. P-values were calculated as twice the fraction of simulated values more extreme than the actual value, and therefore reflect a two-tailed test. This process, and that described below, was repeated for each cell, as baseline activity affects the shape of the null distribution. To visualize heterogeneity in single-cell responses to different gustatory stimuli (Fig. 2, Fig. S4), we calculated a selectivity index for one solution (A) compared to water (B) with the formula (A−B)/(A+B), and using the same mean difference in z-score values.
The second test for determining cell selectivity was used when assessing behaviors that occurred over long periods of time, with no temporally-specific component, such as the exploration of the open arms of the elevated plus maze. We entirely preserved the temporal dynamics of the calcium activity trace, and instead shuffled behavioral epochs to generate the null distribution. Epochs were thus fixed in length to account for bias in occupancy. In each of 10,000 iterations, the difference in average activity between the behavioral epoch and all other timepoints was calculated for comparison to the actual difference in activity. P-values were again calculated as twice the fraction of simulated values more extreme than the actual value.
Activity-Based Clustering
Before clustering, raw traces were denoised using OASIS (Friedrich et al., 2017) to minimize the effects of correlations in noise. Traces for each session were then clustered as previously described (Barbera et al., 2016). K-means++ clustering was run iteratively on randomly chosen 30 second windows of each session. In each performance of k-means, the prior number of clusters was determined by taking the square root of the number of neurons. One-thousand windows were generated for each session, and each was clustered 100 times. Time windows were used to allow post hoc identification of highly correlated time series within and between the clusters determined using summed data. All clustering presented in the paper, however, reflects only the sum, i.e., the total number of times that two neurons were clustered across all iterations, ranging between 0 and 1000 x 100 = 100,000. The threshold used to sort neurons into their final clusters was customized for each session to maximize the ratio of clusters to unclustered cells.
To assess the stability of clusters across days, we used three independent assessments. First, we compared for each tracked cell pair the number of times they clustered together in our method on one day versus another. Second, we computed the correlation in this cluster score for all neurons across days and compared it to a simulated null distribution generated by randomizing cluster assignments for all cells (nsimulations = 10,000). Finally, we considered pairwise Pearson’s correlations instead of cluster scores. For each cell pair in each session, we determined whether their activity was significantly correlated by comparing the actual Pearson’s coefficient to those generated when traces were shifted in time (nsimulations = 10,000). The number of cell pairs that were significantly correlated on both days was calculated, and compared to that generated after randomizing assignments (nsimulations = 10,000).
To characterize each cluster by its behavioral selectivity, all traces from cells in each cluster were summated and subjected to the same selectivity analysis as individual cells.
To determine whether clustering was driven primarily or exclusively by responses to measured behaviors, we utilized the clustering data from individual 30-second windows. We defined the total number of times cells clustered together during the 100 iterations of k-means++ as the cluster score (see Fig. 1L). We then classified these time windows as either during or not during measured behaviors. For sucrose consumption, elevated plus maze exploration, and social interaction, 30% of the time window needed to contain measured behaviors (drinking bouts, open arm and center exploration, and any social interaction) to be classified as during a measured behavior. For footshock, the window was required to contain at least one shock. All other windows were classified as not being during measured behaviors. The average cluster score of cells within each ensemble was then calculated for each classification.
Spatial Analysis
To determine the statistical significance of the spatial segregation of ensembles, a 2-dimensional K-S test was applied to the centroids of cells in each pair of ensembles. To obtain a p-value, we used a Monte Carlo simulation as described by Fasano and Franceschini (1987). The actual K-S statistic was compared to the distribution calculated by comparing the same numbers of datapoints with values randomly generated, but within the field of view, over 10,000 simulations. P-values reflect a two-tailed comparison against the shuffled distribution and were corrected for the number of comparisons made per ensemble using the Bonferroni method.
Histological Analysis
Lens placement was determined using coronal sections of the DRN through which the lens tract was visible, and was considered to be at the center of the end of the lens. Because the lens was implanted at an angle relative to our coronal sections, we determined anteroposterior position by locating the section with the broadest base of the lens tract and comparing visible histological landmarks with those documented in a mouse brain atlas (Paxinos and Franklin, 2001). The dorsoventral and mediolateral coordinates were considered to be at the center of the base of the lens tract in the same tissue section.
Co-expression of fluorescent markers in brain sections (Fig. S1C–D, Fig. S9C–D, H) was quantified manually in Fiji (Schindelin et al., 2012; Rueden et al., 2017) using the Cell Counter plugin.
Figure Preparation
Plots were generated in MATLAB and Prism and edited in Adobe Illustrator CC. Diagrams were created in Adobe Illustrator. Images were generated in FIJI (Schindelin et al., 2012; Rueden et al., 2017) and edited in Adobe Photoshop CC. Permutation tests were used as previously described (Groppe et al., 2011) to indicate significant timepoints in event-triggered averages for presentation purposes only. Several schematics were generated using data from the Allen Mouse Brain Atlas compiled by www.scalablebrainatlas.incf.org (Bakker et al., 2015; Lein et al., 2007) and then adapted in Adobe Illustrator.
EXCLUSIONS
Data were excluded rarely and only for technical reasons.
Supplementary Material
Supplemental Video 1. Related to Figure 1. Example of DRN5-HT single-cell activity during behavior
Single-cell calcium activity extracted and denoised using CNMF-E (right) aligned to behavior (left). In this example, there is a large increase in activity in most visible neurons during the first interaction between the novel juvenile and the imaging mouse (which has a miniscope attached).
Supplemental Table 1. Related to Figure 1. Details of the experimental conditions for individual imaging mice.
For each mouse used in calcium imaging experiments, this table lists the experimental group; lens placement coordinates determined histologically and referring to the point at the center of the bottom of the lens; the general subregion of the DRN containing these coordinates; the behaviors completed; the corresponding numbers of cells detected, associated figure panels; and relevant notes. For clarity, the listed figure panels are those specific to behavioral paradigms, and for analyses using randomly sampled imaging sessions. Panels without statistics or showing representative data of a single animal or cell are excluded. Several figures included data from all sessions: Fig. 1E, 1L, S2B–C, and S3P use data from all sucrose, EPM, social, and shock sessions from mice DRN57-88. Fig. 7D uses data from all sucrose, EPM, social, and shock sessions from mice DRN57-88, 96-97, and 111-120.
Abbreviations: ID, identification; AP, anteroposterior; ML, mediolateral; DV, dorsoventral; FOV, field of view; EPM, elevated plus maze.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-GFP rabbit polyclonal Alexa Fluor™-488 conjugate | Invitrogen | Cat#A-21311 |
| Anti-Tryptophan Hydroxylase/Tyrosine Hydroxylase/Phenylalanine Hydroxylase Antibody, clone PH8 | Millipore Sigma | Cat#MAB5278 |
| Cy5 AffiniPure Goat Anti-Rabbit IgG (H+L) (min X Hu, Ms, Rat Sr Prot) | Jackson ImmunoResearch Laboratories | Cat#111-175-144 |
| goat anti-rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody AlexaFluor 555 | Invitrogen | Cat#A-21428 |
| anti-serotonin (5-HT) transporter (602-622) rabbit polyclonal antibody | Millipore Sigma | Cat#PC177L-100UL |
| Bacterial and Virus Strains | ||
| AAVDJ-DIO-EF1a-GCaMP6m | Stanford | Cat#GVVC-AAV-092 |
| AAV9-CAG-flex-GCaMP6m-WPRE-S | U Penn Vector Core | Cat#AV-9-PV2817 |
| AAV2-retro-DIO-EF1a-GCaMP6m | Stanford | N/A, custom preparation |
| AAV5-flex-GFP | UNC Gene Therapies | Cat#AV4531 |
| AAV9-CAG-flex-tdTomato-WPRE | U Penn Vector Core | Cat#AV-9-ALL864 |
| AAVDJ-hSyn-GCaMP6m | Stanford | Cat#GVCC-AAV-095 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Sucrose | Sigma-Aldrich | Cat#S7903-5KG |
| Quinine hydrochloride dihydrate | Sigma-Aldrich | Cat#Q1125-10G |
| Vectashield Antifade Mounting Medium | Vector Laboratories | Cat#H-1000 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | Jackson Laboratory | Cat#000664; RRID:SCR_004633; https://www.jax.org/index.html |
| B6.129(Cg)-Slc6a4tm1(cre)Xz/J (SERT-Cre) mice | Jackson Laboratory | Cat#014554; https://www.jax.org/index.html |
| Software and Algorithms | ||
| Ethovision XT11 | Noldus | http://www.noldus.com; RRID:SCR_000441 |
| Inscopix Data Processing Software | Inscopix | https://www.inscopix.com |
| Observer XT | Noldus | http://www.noldus.com/human-behavior-research/products/the-observer-xt |
| MATLAB | Mathworks | https://www.mathworks.com/products/matlab/; RRID:SCR_001622 |
| CNMF-E | GitHub | https://github.com/zhoupc/CNMFE |
| Image J | Fiji | https://imagej.nih.gov/ij/ |
| CalTracer 3 (beta) | Yuste Lab | http://www.columbia.edu/cu/biology/faculty/yuste/methods |
HIGHLIGHTS.
Dorsal raphe serotonin neurons (DRN5-HT) are modulated by emotionally salient stimuli
DRN5-HT ensembles have highly correlated activity
Individual DRN5-HT neurons respond to diverse combinations of emotional behaviors
Projection-specific subpopulations contain biased proportions of response types
ACKNOWLEDGEMENTS
This work was funded by the NSF Graduate Research Fellowship DGE-16-44869 (G.E.P.), the Leon Levy Neuroscience Fellowship (B.R.M.), NARSAD Young Investigator Grant (B.R.M.), NIMH T32 MH015144 (B.R.M.), K08 MH116368 (B.R.M.), NIMH R01 MH068542 (R.H.), and the Hope for Depression Research Foundation (R.H., B.R.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
INCLUSION AND DIVERSITY
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science.
REFERENCES
- Abrams JK, Johnson PL, Hollis JH, and Lowry CA (2006). Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci 1018: 46–57. [DOI] [PubMed] [Google Scholar]
- Bakker R, Tiesinga P, and Kötter R (2015). The scalable brain atlas: instant web-based access to public brain atlases and related content. Neuroinformatics 13(3): 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázsfi D, Zelena D, Demeter K, Miskolczi C, Varga ZK, Nagyváradi Á, Nyíri G, Cserép C, Baranyi M, Sperlágh B, and Haller J (2018). Differential roles of the two raphe nuclei in amiable social behavior and aggression – an optogenetic study. Front Behav Neurosci 12: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera G, Liang B, Zhang L, Gerfen CR, Culurciello E, Chen R, Li Y, and Lin D-T. (2016). Spatially compact neural clusters in the dorsal striatum encode locomotion relevant information. Neuron 92(1): 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, Ma X, Pan Y, Lemos J, Craige C, Heemstra L, and Beck SG (2011). Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology 61(3): 524–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, and Kim DS (2013). Ultra-sensitive fluorescent proteins for imaging neuronal activity. Nature 499(7458): 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, loannidis JPA, and Geddes JR (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391(10128): 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Fanning JR, Phan KL, and Lee R (2015). Serotonin and impulsive aggression. CNS Spectrums 20: 295–302. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Amoroso MW, and Uchida N (2015). Serotonergic neurons signal reward and punishment on multiple timescales. Elife e06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia PA, Matias S, and Mainen ZF (2017). Stereotaxic adeno-associated virus injection and cannula implantation in the dorsal raphe nucleus of mice. Bio Protoc 7(18): e2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang J, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux J, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, and Hen R (2009). Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62(4): 479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzani SD, Baratta MV, Amat J, Agster KL, Saddoris MP, Watkins LR, and Maier SF (2016). Activation of a habenulo-raphe circuit is critical for the behavioral and neurochemical consequences of uncontrollable stress in the male rat. eNeuro 3(5): ENEURO.0229-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano G and Franceschini A (1987). A multidimensional version of the Kolmorogov-Smirnov test. Mon Not R astr Soc 225: 155–170. [Google Scholar]
- Fernandez SP, Cauli B, Cabezas C, Muzerelle A, Poncer J-C, and Gaspar P (2016). Multiscale single-cell analysis reveals unique phenotypes of raphe 5-HT neurons projecting to the forebrain. Brain Struct and Fun 221(8): 4007–4025. [DOI] [PubMed] [Google Scholar]
- Friedrich J, Zhou P, and Paninski L (2017). Fast online deconvolution of calcium imaging data. PLoS Comput Biol 13(3): e1005423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Canetta S, Stujenske JM, Burghardt NS, Ansorge MS, Dranovsky A, and Leonardo ED (2018). Serotonin inputs to the dorsal BNST modulate anxiety in a 5-HT1A receptor-dependent manner. Mol Psychiatry 23: 1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe DM, Urbach TP, and Kutas M (2011). Mass univariate analysis of event-related brain potentials/fields I: A critical tutorial review. Psychophysiology 48(12): 1711–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, and Lowry CA (2012). Stress-related serotonergic systems: implications for symptomology of anxiety and affective disorders. Cell Mol Neurobiol 32(5): 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Nakao K, and Nakamura K (2015). Appetitive and aversive information coding in the primate dorsal raphe nucleus. J Neurosci 35(15): 6195–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H (2016). Reward and aversion. Ann Rev Neurosci 39: 297–324. [DOI] [PubMed] [Google Scholar]
- Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, Paninski L, Hen R, and Kheirbek MA (2018). Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97(3): 670–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez JC, Berry JE, Lim SC, Ong SK, Kheirbek MA, and Hen R (2020). Contextual fear memory retrieval by correlated ensembles of ventral CA1 neurons. Nat Comm 11(1): 3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimura K, Takeuchi Y, Fujiwara K, Tominaga M, Yoshioka H, and Sawada T (1988). Quantitative analysis of the distribution of serotonin-immunoreactive cell bodies in the mouse brain. Neuroscience Letters 91(3): 265–270. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, et al. , (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, Fu L, and Luo M (2016). Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Comm 28(7): 10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, and Gordon JA (2014). Circuits in sync: decoding theta communication in fear and safety. Neuropsychopharmacology 39(1): 235–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y Ma M, Feng Q, Zhang J, Wang D, Zeng J, Bao J, Kim J-Y, Chen Z-F, El Mestikawy S, and Luo M (2014). Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81(6): 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, and Shekhar A (2005). Modulation of anxiety circuits by serotonergic systems. Stress 8(4): 233–46. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier, … Kash TL (2016). Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Neuron 537(7618): 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Ayzenshtat I, Carrillo-Reid L, and Yuste R (2014). Visual stimuli recruit intrinsically generated cortical subpopulations. Proc Natl Acad Sci USA 111(38): E4053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP, and Jacobs BL, eds. (2010). Handbook of the behavioral neurobiology of serotonin. London: Academic Press. [Google Scholar]
- Mukamel EA, Nimmerjahn A, and Schnitzer MJ (2009). Automated analysis of cellular signals from large-scale calcium imaging data. Neuron 63(6): 747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, and Hikosaka O (2008). Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci 28(20): 5331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan R, Forrester L, Chiaia NL, and Yamamoto BK (2017). Chronic-stress-induced behavioral changes associated with subregion-specific serotonin cell death in the dorsal raphe. J Neurosci 37(26): 6214–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Tanaka KF, Barr MM, Tritschler L, Le Dantec Y, David DJ, Gardier AM, Blanco C, Hen R, and Ahmari SE (2015). Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron 86(3): 813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Andoh C, Nagai Y, Kinoshita H, Kawai H, Shibui N, Liu B, … Kaneko S (2018). Manipulation of dorsal raphe serotonergic neurons modulates active coping to inescapable stress and anxiety-related behaviors in mice and rats. Neuropsychopharmacology 0: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Commons KG, and Dymecki SM (2019). Embracing diversity in the 5-HT neuronal system. Nat Rev Neurosci 20(7): 397–424. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Freret ME, Rood BD, Brust RD, Hennessy ML, deBairos D, Kim JC, Cook MN, and Dymecki SM (2015). Multi-scale molecular deconstruction of the serotonin neuron system. Neuron 88(4): 774–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G and Franklin KBJ (2001). The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press. [Google Scholar]
- Pnevmatikakis EA and Giovannucci A (2017). NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. J Neurosci Methods 291(1): 83–94. [DOI] [PubMed] [Google Scholar]
- Prouty EW, Chandler DJ, and Waterhouse BD (2017). Neurochemical differences between target-specific populations of rat dorsal raphe projection neurons. Brain Res 1675: 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SP and Mainen ZF (2009). Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J Physiol 102(5): 3026–3037. [DOI] [PubMed] [Google Scholar]
- Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, DeLoach KE, Ran C, Pun A, Sun Y, Weissbourd B, Neve RL, Huguenard J, Horowitz MA, and Luo L (2018). Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175(2): 472–487.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Jennings JH, Ung RL, Namboodiri VMK, Zhou ZC, Otis JM, Nomura H, McHenry JA, Kosyk O, and Stuber GD (2016). Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nature Protocols 11: 566–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, and Eliceiri KW (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J, White DW, Hartenstein V, Eliceiri K, Tomancak P, and Cardona A (2012). Fiji: an open-source platform for biological-image analysis. Nature Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo C, Guru A, Jin M, Ito B, Sleezer BJ, Ho Y, Wang E, Boada C, Krupa NA, Kullakanda DS, Shen C, and Warden MR (2019). Intense threat switches dorsal raphe serotonin neurons to a paradoxical operational mode. Science 363(6426): 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheintuch L, Rubin A, Brande-Eilat N, Geva N, Sadeh N, Pinchasof O, and Ziv Y (2017). Tracking the same neurons across multiple days in Ca2+ imaging data. Cell Reports 21(4), 1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier A, Chemiakine A, Inbar B, Bagchi S, Ray RS, Palmiter RD, Dymecki SM, Moore H, and Ansorge M (2015). Activity of raphe serotonergic neurons controls emotional behaviors. Cell Rep 13(9): 1965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenaz P, Ruttimann UE, and Unser M (1998). A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7(1): 27–41. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D, Aryal DK, Farrell MS, Lowery-Gionta E, Olsen RH, Wetsel WC, Kash TL, Hurd YL, Tecott LH, and Roth BL (2016). Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology 41(5): 1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, and Frye CA (2007). The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2): 322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW, Deisseroth K, and Malenka RC (2018). 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 560: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, and Van Bockstaele EJ (2012). Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J Chem Neuroanat 41(4): 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Han X, Weng D, Feng Q, Qi X, Li J, and Luo M (2018). Response dynamics of midbrain dopamine neurons and serotonin neurons to heroin, nicotine, cocaine, and MDMA. Cell Discov 4: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Das G, Hueske E, and Tonegawa S (2017). Dorsal raphe serotonergic neurons control intertemporal choice under trade-off. Curr Biol 27(20): 3111–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Li Y, Feng Q, and Luo M (2017). Learning and stress shape the reward response patterns of serotonin neurons. J Neurosci 37(37): 8863–8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Resendez SL, Rodriguez-Romaguera J, Jimenez JC, Neufeld SQ, Giovannucci A, Friedrich J, Pnevmatikakis EA, Stuber GD, Hen R, et al. (2018). Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 7, e28728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, and Hen R (2005). Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods 143(1):27–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1. Related to Figure 1. Example of DRN5-HT single-cell activity during behavior
Single-cell calcium activity extracted and denoised using CNMF-E (right) aligned to behavior (left). In this example, there is a large increase in activity in most visible neurons during the first interaction between the novel juvenile and the imaging mouse (which has a miniscope attached).
Supplemental Table 1. Related to Figure 1. Details of the experimental conditions for individual imaging mice.
For each mouse used in calcium imaging experiments, this table lists the experimental group; lens placement coordinates determined histologically and referring to the point at the center of the bottom of the lens; the general subregion of the DRN containing these coordinates; the behaviors completed; the corresponding numbers of cells detected, associated figure panels; and relevant notes. For clarity, the listed figure panels are those specific to behavioral paradigms, and for analyses using randomly sampled imaging sessions. Panels without statistics or showing representative data of a single animal or cell are excluded. Several figures included data from all sessions: Fig. 1E, 1L, S2B–C, and S3P use data from all sucrose, EPM, social, and shock sessions from mice DRN57-88. Fig. 7D uses data from all sucrose, EPM, social, and shock sessions from mice DRN57-88, 96-97, and 111-120.
Abbreviations: ID, identification; AP, anteroposterior; ML, mediolateral; DV, dorsoventral; FOV, field of view; EPM, elevated plus maze.
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


