Abstract
At present, there are more than 560 million confirmed cases of the coronavirus disease 2019 (COVID-19) worldwide. Although more than 98% of patients with severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infection can survive acute COVID, a significant portion of survivors can develop residual health problems, which is termed as long COVID. Although severe COVID-19 is generally associated with a high risk of long COVID, patients with asymptomatic or mild disease can also show long COVID. The definition of long COVID is inconsistent and its clinical manifestations are protean. In addition to general symptoms, such as fatigue, long COVID can affect many organ systems, including the respiratory, neurological, psychosocial, cardiovascular, gastrointestinal, and metabolic systems. Moreover, patients with long COVID may experience exercise intolerance and impaired daily function and quality of life. Long COVID may be caused by SARS-CoV-2 direct injury or its associated immune/inflammatory response. Assessment of patients with long COVID requires comprehensive evaluation, including history taking, physical examination, laboratory tests, radiography, and functional tests. However, there is no known effective treatment for long COVID. Based on the limited evidence, vaccines may help to prevent the development of long COVID. As long COVID is a new clinical entity that is constantly evolving, there are still many unknowns, and further investigation is warranted to enhance our understanding of this disease.
Keywords: COVID-19, Long COVID, Post-acute COVID, SARS-CoV-2, Vaccine
Introduction
As of July 19, 2022, there have been more than 560 million confirmed cases of the coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2).1 Although most SARS-CoV-2-infected patients are asymptomatic or show mild respiratory symptoms, a small portion of patients can progress to severe COVID-19 or acute respiratory distress syndrome even after vaccination or treatment with antiviral agents.2, 3, 4, 5, 6, 7 COVID-19 has caused more than six million deaths worldwide.1 Some patients may experience various residual health problems after acute COVID-19 illness, a condition termed as long COVID.8 In addition, long COVID is also known as post-COVID-19, long-haul COVID-19, post-acute COVID-19, post-acute sequela of SARS-CoV-2 infection, and chronic COVID-19.8 Long COVID can be defined in many ways, such as the (1) presence of symptoms beyond 3 weeks from the initial onset of symptoms9; (2) development of symptoms, which cannot be explained by an alternative diagnosis, during or after COVID-19 that continue for more than 4 weeks10; or (3) presence of signs and symptoms even after 12 weeks of infection and beyond.11 To better understand long COVID, we have provided comprehensive information about its epidemiology, clinical manifestations, pathogenesis, diagnosis, and treatment in this review. Overall, these findings suggest that vaccines can help prevent the development of long COVID.
Epidemiology
The pooled global prevalence of long COVID at ≥28 days from infection was estimated to be 43% (95% confidence interval [CI], 39–46%) in a meta-analysis involving 50 studies and 1,680,003 patients.12 The estimated prevalence was highest in Asia (51%; 95% CI, 37–65%), followed by Europe (44%; 95% CI, 32–56%) and America (31%; 95% CI, 21–43%).12 Another systematic review involving 57 studies and 250,351 COVID-19 survivors reported that the mean age of survivors was 54.4 ± 8.9 years, and 140,196 (56%) survivors were male. The median rate of long-COVID-19 at 1, 2–5, and ≥6 months was 54.0% (interquartile range [IQR], 46.0–69.0%), 55.0% (IQR, 34.8–65.5%), and 54.0% (IQR, 31.0–67.0%), respectively.13 Another meta-analysis involving 15,244 hospitalized and 9011 non-hospitalized patients reported that 63.2 and 71.9% of patients had ≥1 post-COVID-19 symptom at 30 and 60 d after onset/hospitalization, respectively.14
Ma et al. investigated the long-term consequences of COVID-19 by analyzing 40 studies with 10,945 patients and reported that the pooled prevalence of COVID-19 symptoms at 6–12 months was 63.9% (95% CI, 53.6–74.1%), which further decreased to 58.9% (95% CI, 45.9–71.9%).15 The rate of long COVID was similar in both high-income (54.6%; IQR, 33.0–68.3%) and low- and middle-income (56.0%; IQR, 43.5–67%) countries.13 In this study, 197,777 (79%) patients were hospitalized during acute COVID-19, but the rate of long COVID did not differ according to the percentage of hospitalized patients.13 In contrast, a sizeable difference was observed in the study by Chen et al.,12 where the pooled post-COVID-19 condition prevalence in hospitalized patients (54%; 95% CI, 44–63%) was higher than that in non-hospitalized patients (34%; 95% CI, 25–46%). Among children and adolescents, a meta-analysis involving 21 studies with 80,071 patients reported that the overall prevalence of long COVID, which consisted of both ongoing (4–12 weeks) and post-COVID-19 (≥12 weeks) symptoms, was 25.24% (95% CI, 18.2–33.0%).16 Moreover, the prevalence was increased to 29.19% (95% CI 17.8–42.0%) for hospitalized patients.16
In addition to the evolution of SARS-CoV-2, more and more variants were identified. Recently, one Italian study showed that the prevalence of long COVID among healthcare workers could vary across the pandemic waves, from 48.1% (95% CI, 39.9–56.2%) during wave 1 (February to September 2020 [wild-type variant]) to 35.9% (95% CI, 30.5–41.6%) during wave 2 (October 2020 to July 2021 [Alpha variant]) and 16.5% (95% CI, 12.4–21.4%) during wave 3 (August 2021 to March 2022 [Delta and Omicron variants]).17 However, the association between long COVID and different variant remains unclear. Further study is warranted to investigate whether the risk of long COVID can vary according to different SARS-CoV-2 variants.
Risk factors
A study analyzing the US Department of Veterans Affairs National Healthcare databases showed that breakthrough infection (BTI) in vaccinated people exhibited a higher risk of post-acute sequelae (hazard ratio [HR] = 1.50; 95% CI = 1.46–1.54), including cardiovascular, coagulation and hematological, gastrointestinal, kidney, mental health, metabolic, musculoskeletal, and neurological disorders, than those with no evidence of SARS-CoV-2 infection.18 Another study using unvaccinated females in wave 1 with no allergies or comorbidities as a reference group found that old age (odds ratio [OR] = 1.23; 95% CI, 1.01–1.49), allergies (OR = 1.50; 95% CI, 1.06–2.11), and an increasing number of comorbidities (OR = 1.32; 95% CI, 1.04–1.68) were associated with a risk of long COVID.17
One retrospective, multicenter cohort study of 2433 patients at 1-year follow-up found that the most common symptoms included fatigue, sweating, chest tightness, anxiety, and myalgia.19 In this study, old age (OR = 1.02; 95% CI, 1.01–1.03) and severe disease (OR = 1.51; 95% CI, 1.14–1.99; P = 0.004) were associated with an increased risk of having at least three symptoms.19 Moreover, fatigue was associated with the following risk factors: old age (OR = 1.02; 95% CI, 1.01–1.02), female sex (OR = 1.27; 95% CI, 1.06–1.52), and severe disease during hospital stay (OR = 1.43; 95% CI, 1.18–1.74).19
Long COVID can be associated with poor quality of life, and one study reported that the median 36-Item Short-Form Health Survey score among 130 patients at four months follow-up after hospitalization for COVID-19 was 25 (IQR, 25.0–75.0) for the subscale “role limited owing to physical problems,” 46.9 (IQR, 31.2–68.8) for “vitality,” and 57.5 (IQR, 40.0–75.0) for “general health” (potential best score, 100; worst score, 0).20 Further meta-analysis including 12 studies patients found that the pooled prevalence of poor quality of life was 59% (95% CI, 42–75%) among 4828 patients with long COVID.21 Another meta-regression analysis showed that the poor quality of life was significantly high among post-COVID-19 patients who underwent intensive care unit (ICU) admission (p = 0.004) and showed fatigue (p = 0.0015).21
Clinical manifestation
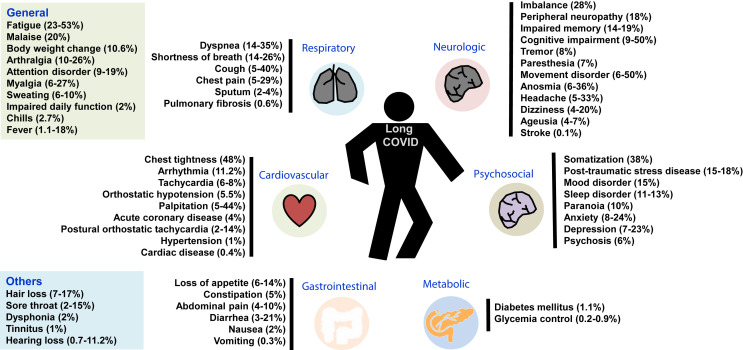
In addition to the general symptoms, long COVID can involve many organ systems (Fig. 1 ).16 , 22 , 23 One study consisting of 538 COVID-19 survivors in Wuhan reported that general symptoms were the most common (n = 267, 49.6%), followed by respiratory (n = 210, 39%), cardiovascular-related (n = 70, 13%), and neuropsychosocial (n = 122, 22.7%) symptoms.24 Moreover, Daugherty et al. conducted a retrospective cohort study in US and found that the risk of new clinical sequelae after the acute phase (>21 d), including chronic respiratory failure, cardiac arrythmia, hypercoagulability, encephalopathy, peripheral neuropathy, amnesia (impaired memory), diabetes, liver test abnormalities, myocarditis, anxiety, and fatigue, was significantly greater in the patients infected by SARS-CoV-2 than in the three comparative groups (2020, 2019, and viral low respiratory tract illness groups).25 Similar findings were observed in children and young adults, and those with SARS-CoV-2 infection exhibited a higher risk of persistent dyspnea, anosmia/ageusia, and/or fever compared to controls.16
Figure 1.
Clinical manifestations of long coronavirus disease (COVID).12,22,23
Fatigue was the most common systemic symptom, which could be observed in more than 10% of patients with long COVID and lasted for ≥2 months.12 , 13 , 19 , 20 , 22 , 24 , 27, 28, 29, 30, 31, 32 In some studies, the prevalence of fatigue was more than 50%.13 , 33 A surveillance using the 20-item Multidimensional Fatigue Inventory questionnaire for 145 patients found that the median score was 4.5 (IQR, 3.0–5.0) for reduced motivation and 3.7 (IQR, 3.0–4.5) for mental fatigue (potential best score, 1; worst score, 5).20 In addition, other symptoms included joint pain, myalgia, insomnia, sweating, body weight changes, pain, poor attention span, impaired daily function, hair loss, sexual dysfunction, and asthenia.16 , 19 , 26 , 27 , 34 In children and young adults, mood swings (16.50%) was the most prevalent clinical manifestation, followed by fatigue (9.66%), sleep disorders (8.42%), headache (7.84%), and loss of appetite (6.27%).16 Other less common symptoms included swollen lymph nodes (2.58%), dysphonia (1.89%), fever (1.87%), changes in menstruation (1.27%), dysphagia (0.446%), and speech disturbance (0.44%).16
Respiratory manifestations
Respiratory system was the most commonly involved organ system in long COVID.19 , 20 , 22, 23, 24 , 26, 27, 28, 29, 30, 31, 32 , 34 One meta-analysis reported that shortness of breath was the most common respiratory symptom with a prevalence of 25.6% (95% CI 15.1–39.8%), followed by post-activity polypnea (29.8%; 95% CI 20.5–41.3%), dyspnea (15.5%; 95% CI 11.3–20.9%), pain on breathing (13.9%; 95% CI, 11.9–16.2%), chest distress (10.8%; 95% CI, 11.9–16.2%), cough (9.8%; 95% CI, 7.8–12.4%), sputum production (9.3%; 95% CI, 3.2–24.1%), polypnea (7.9%; 95% CI, 3.8–15.7%), chest pain (6.9%; 95% CI, 5.3–9.0%), and globus (5.9%; 95% CI, 4.5–7.6%).23 In children and young adults, approximately 7.62% patients had persistent respiratory symptoms, 4.62% had chest pain, and 3.80% had cough.16
Neurological manifestations
Cognitive disorders, including brain fog, difficulty in thinking, poor attention span, and memory impairment, were the most common neurological manifestations with a prevalence of 35.4% (95% CI, 2.1–81.7%) reported in an analysis involving 3305 patients.33 Additionally, bradykinesia, inability to focus vision, imbalance, paresthesia, vertigo, headache, dysnomia, dysgeusia, hypomnesia, anguish, and anhedonia were other common symptoms.12 , 13 , 23 , 32 , 33 Other complications, including tremors, muscle atrophy, anosmina, dysmetria, altered mental status, movement disorder, cranial and peripheral nerve disorders, and encephalitis, were rarely reported.23 , 33 In children and young adults, neurological abnormalities, including abnormal sensation, tremors, and numbness, were rarely reported in 0.86% patients.16
Psychosocial manifestations
Psychosocial sequelae are not uncommon in patients with long COVID.13 The associated presentations, including somatization, phobic-anxiety, somnipathy, anxiety, sadness, post-traumatic stress disorder (PTSD), anger, depression, paranoia, psychosis, behavioral disorder, obsessive-compulsive nature, phobia, dysphoria, interpersonal sensibility, and inferiority complex, are protean.13 , 16 , 22 , 23 Han et al. performed a meta-analysis and reported that depression and anxiety were the most common presentations, which accounted for the pooled prevalence of 23% (95% CI: 12–34) and 22% (95% CI: 15–29), respectively.22 In addition, memory loss/memory complaints/forgetfulness, concentration, and insomnia/sleep difficulties can be found in 19% (95% CI: 7–31%), 18% (95% CI: 2–35%), and 12% (95% CI: 7–17%) patients, respectively.22
Cardiovascular manifestations
Several cardiovascular sequelae, including the increase in resting heart rate, tachycardia, palpitation, hypotension, syncope, discontinuous flushing, orthostatic tachycardia, newly diagnosed hypertension, angina pectoris, and heart attack, have been reported, but most of them are observed in less than 10% of patients.22 , 23 , 32 , 35 Similar findings were observed in children and young adults, and orthostatic intolerance (6.92%) was the most prevalent symptom, followed by arrhythmia (2.29%) and palpitation (1.27%).16 Although one cohort study showed that acute COVID-19 can be associated with increased risk of cardiovascular disease (CVD) (RR = 5.82; 95% CI, 4.82–7.03), including pulmonary embolism (RR = 11.5; 95% CI, 7.07–18.73), atrial arrythmias (RR = 6.44; 95% CI, 4.17–9.96), and venous thromboses (RR = 5.43, 95% CI, 3.27–9.01), the incidence of CVD decreases from 13 to 52 weeks (RR = 0.80; 95% CI, 0.73–0.88).36
Gastrointestinal manifestations
Yang et al. reported that 15.3% (95% CI, 2.1–59.8%) of patients had bloated stomach after meals, and approximately <10% may exhibit hypophagia, poor appetite, constipation, abdominal pain, diarrhea, nausea, and vomiting.22 , 23 , 32 Similarly, Chen et al. reported that only 4 and 3% of patients exhibit abdominal pain and diarrhea, respectively.12 For children and young adults, abdominal pain (2.91%) is the most common gastrointestinal manifestation, followed by constipation (2.05%), diarrhea (1.65%), and nausea/vomiting (1.53%).16
Metabolic manifestations
Since SARS-CoV-2 can adversely affect the β-cells of pancreatic tissues, the development of new diabetes mellitus (DM) following acute COVID-19 is another serious concern.37, 38, 39 A meta-analysis based on the pooled analysis of 5,787,027 subjects reported that the risk of developing incident DM was significantly higher in post-acute COVID-19 phase than that in healthy controls (HR = 1.59; 95% CI, 1.40–1.81).40 Additionally, high risk of incident DM was also observed following COVID-19 versus severity matched non-COVID-19 respiratory tract infections (moderate-severe/hospitalized cases, HR = 1.52; 95% CI: 1.36–1.70; mild cases, HR = 1.22; 95% CI: 1.14–1.31).40 Compared with the influenza cohort using 1:1 propensity match method, patients with mild COVID-19 showed 1.54 (95% CI, 1.46–1.62) times higher risk of new-onset diabetes than mild influenza controls.41 A similar higher risk was observed in those with moderate/severe COVID-19 compared to those with moderate/severe influenza (RR = 1.4; 95% CI 1.26–1.69).41
Cytokines and biomarkers
Several studies evaluated the cytokine and biomarker profiles of patients with long COVID.42, 43, 44, 45 QueirozIn et al. found that compared with 90 patients without sequelae, 135 cases with long COVID had higher levels of interleukin (IL)-17 and IL-2 (p < 0.05), and lower levels of IL-10, IL-6, and IL- 4 (p < 0.05).42 Further studies also reported the potential roles of biomarkers according to the specific manifestations. Several markers, including SLC6A19, ACE2, TMRSS2, TMPRSS4, IFN-γ, IL-17A, and zonulin, were found to be associated with neurological sequelae.43 For pulmonary fibrosis in post-COVID-19 patients, several biomarkers, such as TNF-α, IL-17A, IL-17D, VCAM-1, ICAM-1, PIGF, or KL-6, may be associated with a high risk of susceptibility.44 For exercise endurance, three inflammatory markers, including hsCRP, IL-6, and TNF-α, and the SARS-CoV-2 receptor-binding domain (RBD) immunoglobulin G, were negatively correlated with exercise capacity (peak VO 2) more than 1 year later.45 However, no specific circulating, coagulation, and inflammatory markers, were highly predictive of the cardiovascular outcome of long COVID when measured 3 months after SARS-CoV-2 infection.46 Therefore, further studies are needed to identify specific biomarkers to guide future preventive or treatment strategies for long COVID.
Imaging characteristics
Regarding chest imaging findings, Mandal et al. evaluated the follow-up radiographs (4–6 weeks after COVID-19) of 244 patients and found that 151 (62%) radiographs were normal, 66 (27%) demonstrated significant improvement, 4 (2%) remained unchanged, and 23 (9%) showed significant deterioration.30 Of the 23 patients who had deteriorated radiographs at follow-up, 10 (43%) were typical for COVID-19 and 11 (48%) were indeterminate for or unlikely to represent COVID-19.30 The study by Arnold et al. showed similar findings that only 14% (15/110) patients had abnormal follow-up radiographs (n = 10 moderate group; n = 5 severe group), and two had worsened after hospital admission with high radiographic severity scores.31 The abnormal radiographic findings included reticulation (n = 8), atelectasis (n = 5), consolidation (n = 1), and pleural effusion (n = 1). In addition, abnormal high-resolution CT findings, including fibrotic changes (n = 2), minor persistent ground glass changes (n = 2), and pleural effusion (n = 1), were found in 5/9 patients.31 In contrast, one study including 171 patients who underwent CT scanning found that 63.2% (n = 108) had abnormal image findings, in which persistent ground glass opacity was the most common abnormality (n = 72, 42.4%).20 Another study of 60 patients showed that ground glass abnormality was more common than reticulation, with 83% patients having ground glass, 65% having reticulation, and only 12% with no imaging abnormality.47 In rare cases, pulmonary embolus or lung infarcts could be found.48 Fortunately, one sequential follow-up evaluation at 60 and 100 d after COVID-19 onset demonstrated that these CT abnormalities can be significantly improved over time.34
Functional tests
Abnormal pulmonary function can be found in >50% of patients during the follow-up after COVID-10-related hospitalization,47 and pulmonary diffusion capacity is the most commonly impaired lung function in recovered patients with COVID-19.20 , 34 , 47 , 49 , 50 Shah reported that 52 of 60 patients had an abnormal diffusion lung capacity for carbon monoxide (DLCO) at 12 weeks,47 and Bellan et al. showed that DLCO was reduced to <80% of the estimated value in 113 patients (51.6%) and <60% in 34 patients (15.5%) among 219 survivors after severe COVID-19.49 Another study reported that impaired DLCO can be associated with the following risk factors: female sex (OR = 4.011; 95% CI: 2.928–5.495), altered chest CT (OR = 3.002; 95% CI: 1.319–6.835), age (OR = 1.018; 95% CI, 1.007–1.030), high D-dimer levels (OR = 1.012; 95% CI, 1.001–1.023), and high urea nitrogen levels (OR = 1.004; 95% CI, 1.002–1.007).51 Similarly, Wu et al. identified that increasing odds of impaired DLCO was associated with female sex (OR = 8.61; 95% CI, 2.83–26.2).50
In the cardiovascular surveillance of 106 patients by the Yale Heart and Vascular Center, Wang et al. found that 11 patients (9%) had left ventricular systolic dysfunction, 10 patients (8%) had left ventricular diastolic dysfunction, and 9 patients (7%) had right ventricular systolic dysfunction.32 Among 48 patients receiving Holter monitoring, 43 had symptomatic sinus tachycardia, 3 had new atrial fibrillation, and 2 had supraventricular tachycardia.32 Finally, 4 of 53 patients undergoing stress test showed evidence of new ischemia and 19 (76%) of 25 patients showed evidence of late gadolinium enhancement and/or T2 inflammation on a cardiac magnetic resonance imaging (MRI) examination.32
Exercise intolerance was not uncommon in patients with long-term COVID. One study involving 1733 discharged patients with COVID-19 at 6 months reported that the proportions of median 6-min walking distance at 6 months less than the lower limit of the normal range were 24, 22, and 29% for those at severity scale of 3, 4, and 5–6, respectively.29 Another study reported that 20% (34/170) patients had an oxygen desaturation of greater than 4% while undergoing the 6-min walking test.48 Furthermore, Skjørten et al. assessed the cardiopulmonary exercise capacity of 156 patients using a cardiopulmonary function and found peak oxygen uptake <80% than predicted in 31% (n = 49) patients 3 months after discharge.52 They also found that there were no differences in the ventilation, breathing reserve, and ventilatory efficiency between the ICU and non-ICU groups.52
Mechanisms
As long COVID has recently developed in the past two years and its disease pattern is still evolving, its exact mechanisms are poorly understood at present.53 Several possible mechanisms have been proposed: (1) direct cell damage of ACE2-expressing organ system by the entry of SARS-CoV-2 through ACE-2 receptors; (2) the inflammation related to persistent viral reservoir and antigen even after infection resolution; (3) activation of immune system causing autoimmunity because of the cross-reacting antibodies against SARS-CoV-2; (4) host’s counter response including overproduction of counter-regulatory hormones and cytokines; (5) delayed resolution of inflammation altering the homeostatic milieu of the organ, increasing the amount of proinflammatory cells, and altering the cytokine production and immunometabolic pathway; and (6) the damage of vascular endothelial system enhancing platelet adhesion and coagulation, and resulting in the impairment of various organ functions.53 , 54 Several response signaling mechanisms, such as the increased phosphorylation of nuclear factor-kβ and Janus kinase–signal transducer and activator of transcription pathway molecules and cytokines, including type I and type III interferons, interleukin (IL)-1β, IL-6, and transforming growth factor-β, are involved in this process.53 , 55 In addition, residual organ damage in acute COVID-19, an adverse effect of anti-COVD-19 treatment, and exacerbation of underlying medical and psychiatric illnesses are also observed.53 , 56
Vaccine effects
Many randomized controlled trials have demonstrated that COVID-19 vaccines are effective in treating SARS-CoV-2 infection and decreasing its progression.57, 58, 59, 60, 61, 62, 63, 64, 65 However, the effect of COVID-19 vaccine on the development of long COVID has rarely been investigated.17 , 18 , 66 A prospective, community-based, case–control study in UK found that compared with the unvaccinated controls, individuals after their second vaccine dose were less likely to have persistent (≥28 d) symptoms (all age group: OR = 0.51; 95% CI, 0.32–0.82; adults [18–59 years]: OR = 0.37; 95% CI, 0.16–0.88; elderly [≥ 60 years]: OR = 0.56; 95% CI, 0.31–0.98).66 A study of old US veterans reported that people with BTI (n = 33,940) exhibited lower risks of incident post-acute sequelae (HR = 0.85, 95% CI: 0.82, 0.89) than those with SARS-CoV-2 infection who were not previously vaccinated (n = 113,474).18 However, the greatest benefit appeared to be in reducing blood clotting and lung complications. By contrast, there was no difference between the vaccinated and unvaccinated people in terms of longer-term sequala of neurological issues, gastrointestinal symptoms, kidney failure and other conditions.18 An observational cohort study of 2560 healthcare workers in Italy demonstrated that BNT162b2 vaccination was associated with decreased long COVID prevalence of 41.8% (95% CI, 37.0–46.7%), 30.0% (95% CI, 6.7–65.2%), 17.4% (95% CI, 7.8–31.4%), and 16.0% (95% CI, 11.8–21.0%) in those without vaccination, with one, two, and three doses, respectively.17 Compared to unvaccinated females in wave 1 with no allergies or comorbidities, the risk of long COVID was significantly lower in those receiving two (OR = 0.25; 95% CI, 0.07–0.87), and three (OR = 0.16; 95% CI, 0.03–0.84) BNT162b2 vaccine doses.17 Further subgroup analysis of 265 vaccinated individuals found that the time between the second vaccination dose and infection was not associated with long COVID (OR = 0.66; 95% CI, 0.34–1.29).17 Although mask and other control measures are still needed against ongoing new omicron variants, these findings suggest the importance of vaccination in the prevention of long COVID.
Diagnostic tools
Since long COVID can present with only non-specific symptoms or involve multiorgan systems, the appropriate evaluation criteria for patients with suspected long COVID remain unclear. An appropriate diagnostic strategy should be guided by the history, physical examination results, and clinical manifestations of the patient. Basic diagnostic laboratory tests determine the blood count, electrolyte levels, renal function, liver function, inflammatory markers (C-reactive protein and ferritin), thyroid function, and vitamin D and B12 levels. Specialized tests include the determination of autoantibody (for rheumatologic conditions), D-dimer and fibrinogen (for coagulation disorder), troponin (for myocardial injury), and B-type natriuretic peptide (for differentiating the cause of dyspnea) levels.
Specific functional assessment tools can be applied according to the patient’s condition. Patient-reported outcome measurement information system, post-COVID-19 function status scale, and EuroQol-5D can be used to evaluate the functional status or quality of life of the patient. The modified Medical Research Council dyspnea scale can be used in patients with dyspnea. For neurological conditions, Montreal cognitive assessment, mini mental status examination, Compass 31, and neurobehavioral system inventory are useful tools. For psychiatric conditions, general anxiety disorder-7, patient health questionnaire-9, PTSD symptom scale, screen for posttraumatic stress symptoms, PTSD checklist for DSM-5, impact of event scale-revised, hospital anxiety, and depression scale, may be used.
Additionally, chest radiography, pulmonary function tests, electrocardiograms, or echocardiograms are used for persistent or new respiratory or cardiac concerns. The diagnostic value of computed tomography (CT) images is low in patients with normal chest X-rays and normal oxygen saturation. Similarly, the yield of CT pulmonary angiography for diagnosing pulmonary embolisms is low in patients without elevated D-dimer levels and compatible symptoms, and brain MRI may not reveal any pathological findings in the absence of focal neurological deficits in patients with brain fog symptoms.
Potential treatment options
The symptoms and functional impairment in patients with long COVID symptoms can be multidimensional, as this disease can be episodic and unpredictable.67 The management of long COVID requires prompt multidisciplinary assessment and its treatment should be focused on excluding serious complications, managing specific symptom clusters, and supporting whole-person rehabilitation.9 , 68 , 69 In addition to specific treatment according to specific organ disorder and rehabilitation for general symptoms, hyperbaric oxygen therapy (HBOT) can be used as a potential treatment modality.70 One randomized, sham-control, double-blind trial involving 73 post-COVID-19 patients with ongoing symptoms for at least 3 months demonstrated that following HBOT, there were significant group-by-time interactions among the global cognitive function (d = 0.495, p = 0.038), attention (d = 0.477, p = 0.04), and executive function (d = 0.463, p = 0.05) as well as in the energy domain (d = 0.522, p = 0.029), sleep (d = −0.48, p = 0.042), psychiatric symptoms (d = 0.636, p = 0.008), and pain interference (d = 0.737, p = 0.001).70 Moreover, these clinical improvements were associated with brain MRI perfusion and microstructural changes.70 These findings suggest the promising role of HBOT for long COVID. Additionally, many registered trials are being conducted to investigate the effects of cognitive and neurorehabilitation interventions, physiotherapy, and physical rehabilitation in patients. More conclusive evidence can be obtained in the near future.
Although oral antivirals expand armament against COVID-19, their effects on long COVID remain unknown. A small series involving four cases reported the effect of nirmatrelvir on the symptoms of long COVID, in which two cases reported improvement in persistent COVID symptoms when nirmatrelvir was taken 25 and 60 days following initial symptom onset.71 Moreover, one case with presumed long COVID for 2 years reported substantial improvement when taking nirmatrelvir following SARS-CoV-2 re-infection.71 Despite these findings suggest the promising role of oral antivirals, further study is warranted to assess their clinical efficacy and safety for long COVID.
Outcomes
A large cohort study consisting of 47,780 patients discharged after COVID-19-related hospitalization found that 29.4% (n = 14,060) required readmission and 12.3% (n = 5875) died during the mean follow-up of 140 d, and the risks of readmission and death were 3.5 (95% CI, 3.4–3.6) and 7.7 (95% CI, 7.2–8.3) times greater, respectively, than those of the matched controls.34 In addition, new onset respiratory diseases were diagnosed in 6085 patients, and the rate of new onset of respiratory disease was 539 (95% CI, 525–553) per 1000 people, which was 27.3 (95% CI, 24.0–31.2) times greater than that in the controls.34 Moreover, new onset of diabetes, a major cardiovascular event disease, chronic kidney disease, and chronic liver disease were found in 36,100, 36,130, 41,705, and 46,395 patients after discharge, respectively, and their was 1.5–3.0 times more than that of the control group.34 Finally, the subgroup analysis disclosed that rate ratios comparing patients with covid-19 and matched controls were greater in individuals aged less than 70 than those aged 70 or more for all these outcomes, especially for death (14.1% vs 7.7%) and respiratory disease (10.5% vs 4.6%).34
Conclusions
In addition to the increase in the number of confirmed cases of COVID-19,72, 73, 74 the number of cases with long COVID are expected to increase in the future. Based on the current knowledge, we can state that: (1) long COVID can develop in all patients with COVID-19 with different severities, although an increased risk is associated with severe COVID-19, (2) long COVID can have various clinical manifestations, and many of them may be non-specific, (3) the patients with long COVID can be associated with a worse clinical outcome than those without, and (4) based on limited evidence, vaccination is the only way to prevent long COVID. However, there are many unknowns regarding long COVID. Despite long COVID likes acute COVID, which can involve many organ systems and has various presentation in different populations during different wave,75, 76, 77, 78, 79, 80 but long COVID may be more complicated. Because of the various definitions of long COVID and its protean clinical manifestations, the epidemiology of this disease remains unclear. In addition, the diagnostic criteria are vague, and possible mechanisms and effective management of this disease require further investigation. Further studies using an established definition and diagnostic criteria for long COVID can help in better understanding this disease.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- 1.World Health Organization https://covid19.who.int/
- 2.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao W., Zhang W., Fang X., Yu D., Wang X. Challenges of SARS-CoV-2 omicron variant and appropriate countermeasures. J Microbiol Immunol Infect. 2022;55:387–394. doi: 10.1016/j.jmii.2022.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng M.Y., Hsih W.H., Ho M.W., Lai Y.C., Liao W.C., Chen C.Y., et al. Younger adults with mild-to-moderate COVID-19 exhibited more prevalent olfactory dysfunction in Taiwan. J Microbiol Immunol Infect. 2021;54:794–800. doi: 10.1016/j.jmii.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin K.Y., Wu P.Y., Liu W.D., Sun H.Y., Hsieh S.M., Sheng W.H., et al. Effectiveness of COVID-19 vaccination among people living with HIV during a COVID-19 outbreak. J Microbiol Immunol Infect. 2022;55:535–539. doi: 10.1016/j.jmii.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai C.C., Chao C.M., Hsueh P.R. Clinical efficacy of antiviral agents against coronavirus disease 2019: a systematic review of randomized controlled trials. J Microbiol Immunol Infect. 2021;54:767–775. doi: 10.1016/j.jmii.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai C.C., Chen I.T., Chao C.M., Lee P.I., Ko W.C., Hsueh P.R. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expert Rev Vaccines. 2021;20:1013–1025. doi: 10.1080/14760584.2021.1949293. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
- 9.Greenhalgh T., Knight M., A'Court C., Buxton M., Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 10.Sivan M., Taylor S. NICE guideline on long covid. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 11.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C., Haupert S.R., Zimmermann L., Shi X., Fritsche L.G., Mukherjee B. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022:jiac136. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groff D., Sun A., Ssentongo A.E., Ba D.M., Parsons N., Poudel G.R., et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández-de-Las-Peñas C., Palacios-Ceña D., Gómez-Mayordomo V., Florencio L.L., Cuadrado M.L., Plaza-Manzano G., et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021;92:55–70. doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y., Deng J., Liu Q., Du M., Liu M., Liu J. Long-term consequences of COVID-19 at 6 months and above: a systematic review and meta-analysis. Int J Environ Res Publ Health. 2022;19:6865. doi: 10.3390/ijerph19116865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Leon S., Wegman-Ostrosky T., Ayuzo Del Valle N.C., Perelman C., Sepulveda R., Rebolledo P.A., et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12:9950. doi: 10.1038/s41598-022-13495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azzolini E., Levi R., Sarti R., Pozzi C., Mollura M., Mantovani A., et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022 doi: 10.1001/jama.2022.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Aly Z., Bowe B., Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X., Wang F., Shen Y., Zhang X., Cen Y., Wang B., et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morin L., Savale L., Pham T., Colle R., Figueiredo S., Harrois A., et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik P., Patel K., Pinto C., Jaiswal R., Tirupathi R., Pillai S., et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J Med Virol. 2022;94:253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Q., Zheng B., Daines L., Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11:269. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang T., Yan M.Z., Li X., Lau E.H.Y. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: a systematic review and meta-analysis. Infection. 2022:1–43. doi: 10.1007/s15010-022-01862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong Q., Xu M., Li J., Liu Y., Zhang J., Xu Y., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daugherty S.E., Guo Y., Heath K., Dasmariñas M.C., Jubilo K.G., Samranvedhya J., et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise J. Long covid-19: hair loss and sexual dysfunction are among wilder symptoms, study finds. BMJ. 2022;27:378. doi: 10.1136/bmj.o1887. [DOI] [PubMed] [Google Scholar]
- 27.Goërtz Y.M.J., Van Herck M., Delbressine J.M., Vaes A.W., Meys R., Machado F.V.C., et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):542. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 29.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., et al. 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold D.T., Hamilton F.W., Milne A., Morley A.J., Viner J., Attwood M., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S.Y., Adejumo P., See C., Onuma O.K., Miller E.J., Spatz E.S. Characteristics of patients referred to a cardiovascular disease clinic for post-acute sequelae of SARS-CoV-2 infection. Am Heart J. 2022;18 doi: 10.1016/j.ahjo.2022.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinzon R.T., Wijaya V.O., Jody A.A., Nunsio P.N., Buana R.B. Persistent neurological manifestations in long COVID-19 syndrome: a systematic review and meta-analysis. J Infect Public Health. 2022;15:856–869. doi: 10.1016/j.jiph.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayoubkhani D., Khunti K., Nafilyan V., Maddox T., Humberstone B., Diamond I., et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ormiston C.K., Świątkiewicz I., Taub P.R. Postural orthostatic tachycardia syndrome as a sequela of COVID-19. Heart Rhythm. 2022;S1547–5271(22):2185–2193. doi: 10.1016/j.hrthm.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rezel-Potts E., Douiri A., Sun X., Chowienczyk P.J., Shah A.M., Gulliford M.C. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PLoS Med. 2022;19 doi: 10.1371/journal.pmed.1004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burekovic A., Asimi Z.V., Divanovic A., Halilovic D. Diabetes - a consequence of COVID-19 infection. Mater Sociomed. 2022;34:4–7. doi: 10.5455/msm.2022.33.4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jedrzejak A.P., Urbaniak E.K., Wasko J.A., Ziojla N., Borowiak M. Diabetes and SARS-CoV-2-is there a mutual connection? Front Cell Dev Biol. 2022;10 doi: 10.3389/fcell.2022.913305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khunti K., Del Prato S., Mathieu C., Kahn S.E., Gabbay R.A., Buse J.B. COVID-19, Hyperglycemia, and new-onset diabetes. Diabetes Care. 2021;44:2645–2655. doi: 10.2337/dc21-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee M., Pal R., Dutta S. Risk of incident diabetes post-COVID-19: a systematic review and meta-analysis. Prim Care Diabetes. 2022;S1751-S9918(22) doi: 10.1016/j.pcd.2022.05.009. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birabaharan M., Kaelber D.C., Pettus J.H., Smith D.M. Risk of new-onset type 2 diabetes in 600 055 people after COVID-19: a cohort study. Diabetes Obes Metabol. 2022;24:1176–1179. doi: 10.1111/dom.14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Queiroz M.A.F., Neves P., Lima S.S., Lopes J.D.C., Torres M., Vallinoto I., et al. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.922422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wais T., Hasan M., Rai V., Agrawal D.K. Gut-brain communication in COVID-19: molecular mechanisms, mediators, biomarkers, and therapeutics. Expet Rev Clin Immunol. 2022 doi: 10.1080/1744666X.2022.2105697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vianello A., Guarnieri G., Braccioni F., Lococo S., Molena B., Cecchetto A., et al. The pathogenesis, epidemiology and biomarkers of susceptibility of pulmonary fibrosis in COVID-19 survivors. Clin Chem Lab Med. 2022;60:307–316. doi: 10.1515/cclm-2021-1021. [DOI] [PubMed] [Google Scholar]
- 45.Durstenfeld M.S., Peluso M.J., Kaveti P., Hill C., Li D., Sander E., et al. Inflammation during early post-acute COVID-19 is associated with reduced exercise capacity and Long COVID symptoms after 1 year. medRxiv. 2022 doi: 10.1101/2022.05.17.22275235. [DOI] [Google Scholar]
- 46.Gyöngyösi M., Alcaide P., Asselbergs F.W., Brundel B., Camici G.G., da Costa Martins P., et al. Long COVID and the cardiovascular system - elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial & Pericardial Diseases. Cardiovasc Res. 2022 doi: 10.1093/cvr/cvac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah A.S., Wong A.W., Hague C.J., Murphy D.T., Johnston J.C., Ryerson C.J., et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76:402–404. doi: 10.1136/thoraxjnl-2020-216308. [DOI] [PubMed] [Google Scholar]
- 48.Hall J., Myall K., Lam J.L., Mason T., Mukherjee B., West A., et al. Identifying patients at risk of post-discharge complications related to COVID-19 infection. Thorax. 2021;76:408–411. doi: 10.1136/thoraxjnl-2020-215861. [DOI] [PubMed] [Google Scholar]
- 49.Bellan M., Soddu D., Balbo P.E., Baricich A., Zeppegno P., Avanzi G.C., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhi H., Ji X., Zhao Z., Liang H., Zhong S., Luo Y., et al. Risk factors for impaired pulmonary diffusion function in convalescent COVID-19 patients: a systematic review and meta-analysis. EClinicalMedicine. 2022;49 doi: 10.1016/j.eclinm.2022.101473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skjørten I., Ankerstjerne O.A.W., Trebinjac D., Brønstad E., Rasch-Halvorsen Ø., Einvik G., et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J. 2021;58 doi: 10.1183/13993003.00996-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newell K.L., Waickman A.T. Inflammation, immunity, and antigen persistence in post-acute sequelae of SARS-CoV-2 infection immunity and inflammation in post-acute sequelae of SARS-CoV-2 infection. Curr Opin Immunol. 2022;77 doi: 10.1016/j.coi.2022.102228. 2022;23:194-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C., Yu C., Jing H., Wu X., Novakovic V.A., Xie R., et al. Long COVID: the nature of thrombotic sequelae determines the necessity of early anticoagulation. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.861703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X., Shao M., Zeng X., Qian P., Huang H. Signaling pathways in the regulation of cytokine release syndrome in human diseases and intervention therapy. Signal Transduct Targeted Ther. 2021;6:367. doi: 10.1038/s41392-021-00764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holmes E., Wist J., Masuda R., Lodge S., Nitschke P., Kimhofer T., et al. Incomplete systemic recovery and metabolic phenoreversion in post-acute-phase nonhospitalized COVID-19 patients: implications for assessment of post-acute COVID-19 syndrome. J Proteome Res. 2021;20:3315–3329. doi: 10.1021/acs.jproteome.1c00224. [DOI] [PubMed] [Google Scholar]
- 57.Creech C.B., Anderson E., Berthaud V., Yildirim I., Atz A.M., Melendez Baez I., et al. Evaluation of mRNA-1273 covid-19 vaccine in children 6 to 11 years of age. N Engl J Med. 2022;386:2011–2023. doi: 10.1056/NEJMoa2203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunkle L.M., Kotloff K.L., Gay C.L., Áñez G., Adelglass J.M., Barrat Hernández A.Q., et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levin M.J., Ustianowski A., De Wit S., Launay O., Avila M., Templeton A., et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of covid-19. N Engl J Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreira E.D., Jr., Kitchin N., Xu X., Dychter S.S., Lockhart S., Gurtman A., et al. Safety and efficacy of a third Dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022;386:1910–1921. doi: 10.1056/NEJMoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N Engl J Med. 2022;386:847–860. doi: 10.1056/NEJMoa2117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., et al. Effect of 2 Inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reindl-Schwaighofer R., Heinzel A., Mayrdorfer M., Jabbour R., Hofbauer T.M., Merrelaar A., et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: a randomized clinical trial. JAMA Intern Med. 2022;182:165–171. doi: 10.1001/jamainternmed.2021.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stephenson K.E., Le Gars M., Sadoff J., de Groot A.M., Heerwegh D., Truyers C., et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA. 2021;325:1535–1544. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown D.A., O'Brien K.K. Conceptualising Long COVID as an episodic health condition. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nurek M., Rayner C., Freyer A., Taylor S., Järte L., MacDermott N., et al. Recommendations for the recognition, diagnosis, and management of long COVID: a Delphi study. Br J Gen Pract. 2021;71:e815–e825. doi: 10.3399/BJGP.2021.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 70.Zilberman-Itskovich S., Catalogna M., Sasson E., Elman-Shina K., Hadanny A., Lang E., et al. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: randomized controlled trial. Sci Rep. 2022;12 doi: 10.1038/s41598-022-15565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peluso M.J., Anglin K., Durstenfeld M.S., Martin J.N., Kelly J.D., Hsue P.Y., et al. Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies. Pathog Immun. 2022;7:95–103. doi: 10.20411/pai.v7i1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ota S., Sugawa S., Suematsu E., Shinoda M., Izumizaki M., Shinkai M. Possibility of underestimation of COVID-19 prevalence by PCR and serological tests. J Microbiol Immunol Infect. 2021;S1684–1182(21):192–194. doi: 10.1016/j.jmii.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shastri J., Yadav P.D., Agrawal S., Shete A.M., Nyayanit D.A., Parikh S., et al. Community transmission of SARS-CoV-2 with B.1.1.7 lineage in Mumbai, India. J Microbiol Immunol Infect. 2021;S1684–1182(21) doi: 10.1016/j.jmii.2021.10.004. 00232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong N.S., Chung S.L., Lee S.S. The challenges of enhancing global preparedness in response to the impending Omicron pandemic. J Microbiol Immunol Infect. 2022;55:549–551. doi: 10.1016/j.jmii.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chao C.M., Lai C.C., Yu W.L. COVID-19 associated mucormycosis - an emerging threat. J Microbiol Immunol Infect. 2022;55:183–190. doi: 10.1016/j.jmii.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang K.M., Epstein M.E., Kennedy W.A., 3rd, Niknam N. Supraglottitis as the sole manifestation of COVID-19 in a patient who received two doses of mRNA vaccine. J Microbiol Immunol Infect. 2022;S1684–1182(22) doi: 10.1016/j.jmii.2022.04.010. 00064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sim J.Y., Wu P.S., Cheng C.F., Yiang G.T., Yu C.H. Characteristics, contacts, and relative risk of SARS-CoV-2 infection among children during school closures. J Microbiol Immunol Infect. 2021;S1684–1182(21):274–277. doi: 10.1016/j.jmii.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao T.S., Zeng H.L., Zhang X., Chen X., Jiang W.L., Du J., et al. Neurological manifestations in COVID-19 patients and their application in predicting fatal disease: a retrospective cohort study. J Microbiol Immunol Infect. 2022;55:445–453. doi: 10.1016/j.jmii.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cardoso Dos Santos K., Rosa da Costa E Silva G., Alves Moura W., Magalhães L.S., Rodrigues de Oliveira B., Dos Santos Carvalho P.M.R., et al. SARS-CoV-2 infection in vulnerable population in Goiania, Central Brazil. J Microbiol Immunol Infect. 2022;55:552–553. doi: 10.1016/j.jmii.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang T., Liu D., Tian D., Xia L. The roles of nausea and vomiting in COVID-19: did we miss something? J Microbiol Immunol Infect. 2021;54:541–546. doi: 10.1016/j.jmii.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]