Summary
Background
In trials conducted in India, recombinant granulocyte colony stimulating factor (GCSF) improved survival in alcohol-associated hepatitis (AH). The aim of this trial was to determine the safety and efficacy of pegfilgrastim, a long-acting recombinant GCSF, in patients with AH in the United States.
Methods
This prospective, randomized, open label trial conducted between March 2017 and March 2020 randomized patients with a clinical diagnosis of AH and a Maddrey discriminant function score ≥32 to standard of care (SOC) or SOC+pegfilgrastim (0.6 mg subcutaneously) on Day 1 and Day 8 (clinicaltrials.gov NCT02776059). SOC was 28 days of either pentoxifylline or prednisolone, as determined by the patient's primary physician. The second injection of pegfilgrastim was not administered if the white blood cell count exceeded 30,000/mm3 on Day 8. Primary outcome was survival at Day 90. Secondary outcomes included the incidence of acute kidney injury (AKI), hepatorenal syndrome (HRS), hepatic encephalopathy, or infections.
Findings
The study was terminated early due to COVID19 pandemic. Eighteen patients were randomized to SOC and 16 to SOC+pegfilgrastim. All patients received prednisolone as SOC. Nine patients failed to receive a second dose of pegfilgrastin due to WBC > 30,000/mm3 on Day 8. Survival at 90 days was similar in both groups (SOC: 0.83 [95% confidence interval [CI]: 0.57–0.94] vs. pegfilgrastim: 0.73 [95% CI: 0.44–0.89]; p > 0.05; CI for difference: -0.18–0.38). The incidences of AKI, HRS, hepatic encephalopathy, and infections were similar in both treatment arms and there were no serious adverse events attributed to pegfilgrastim.
Interpretation
This phase II trial found no survival benefit at 90 days among subjects with AH who received pegfilgrastim+prednisolone compared with subjects receiving prednisolone alone.
Funding
was provided by the United States National Institutes of Health and National Institute on Alcohol Abuse and Alcoholism U01-AA021886 and U01-AA021884.
Keywords: Alcoholic hepatitis, Pegfilgrastim, Phase II, Randomized clinical trial
Abbreviations: ACLF, acute on chronic liver failure; AH, alcohol-related hepatitis; AKI, acute kidney injury; CTCAE, common terminology criteria for adverse events; DF, discriminant function; DSMB, data safety monitoring board; FDA, food and drug administration; FU, follow-up; GCSF, granulocyte colony stimulating factor; HIV, human immunodeficiency virus; HRS, hepatorenal syndrome; INR, international normalized ratio; NIAAA, national institute on alcohol abuse and alcoholism; SD, standard deviation; SOC, standard of care; WBC, white blood cell count
Research in context.
Evidence before this study
We searched the PubMed database for clinical trials published in English between January 1, 2005, and June 30, 2022, using the search terms “granulocyte colony stimulating factor (GCSF) AND (alcoholic hepatitis OR acute on chronic liver failure [ACLF]).” Two trials assessed short-term outcomes of GCSF treatment among patients with alcohol-related hepatitis (AH) receiving standard medical management vs. standard medical therapy+GCSF. The first trial, which enrolled 24 patients with mild AH (only 1 patient died), reported improvement in liver cell regeneration among patients receiving GCSF. The second trial of 46 patients with AH reported approximately 50% reduction in short-term mortality with GCSF+pentoxifylline vs. pentoxifylline monotherapy. Another trial of patients with AH who failed to respond to 7 days of corticosteroids randomized patients to GCSF or placebo for another 28 days reported no difference in 28-day survival but greater improvement in MELD and fewer infections at 90 days among patients receiving GCSF. Two trials compared standard medical therapy vs. SMT+GCSF for patients with ALCF, many of whom had alcohol-related hepatitis. A single-center trial of 47 patients with ACLF (>50% of whom had AH) reported approximately 50% reduction in 60-day mortality. A multicenter trial that randomized 176 patients with ALCF (∼55% alcohol-related) to SMT or SMT+GCSF for 26 days reported no significant difference in 90-day survival or adverse events among patients receiving SMT+GCSF. Adverse events related to GCSF were minimal. Among these publications, clinical trials performed in India reported reduction in mortality with GCSF treatment while studies performed in Europe reported no improvement in outcomes among patients with severe alcoholic liver disease who received GCSF.
Added value of this study
This phase 2 trial is the first RCT in the West to compare corticosteroids (standard medical treatment) to corticosteroids + GCSF among patients with AH. We randomized 34 patients with a clinical diagnosis of AH and a Maddrey discriminant function (mDF) score ≥32 to either prednisolone alone (up to 28 days) or to prednisolone + pegfilgrastim, a long-acting formulation of GCSF that is administered subcutaneously once a week. Survival at Day 90, the primary outcome, was similar in both groups (prednisolone 0.83 vs. GCSF 0.73); survival was similar in both cohorts at 24 weeks (72% vs. 73%, respectively). GCSF did not reduce the incidence of infections, acute kidney injury, or hepatorenal syndrome, common and serious complications of AH. Liver function after one month, as assessed by mDF and the model for end stage liver disease (MELD) score, improved to a greater extent among patients receiving GCSF, although the difference in improvements were not statistically significant between groups. Pegfligrastim increased white blood cell count to greater than 30,000/mm3 one week after administration among more than half of the patients. Adverse event profile was similar in both treatment groups.
Implications of all the available evidence
GCSF is of interest as a treatment for AH because of its excellent safety profile in patients with neutropenia and in patients with AH, its relatively short treatment duration, its marked improvement in survival in trials from India, and the lack of effective alternative treatments for AH. Unlike trials from India, our trial, performed in a Western population, did not demonstrate improvement in 90-day survival with pegfilgrastim + prednisolone among patients with a clinical diagnosis of AH and mDF ≥32. The results of this trial and the results of a trial of GCSF among Western patients with acute on chronic liver failure suggest that GCSF does not improve survival among patients in the West with severe alcohol-related liver disease. The results of this trial should be considered by clinicians who are contemplating GCSF treatment for AH and by investigators who are designing clinical trials evaluating GCSF among patients with AH.
Alt-text: Unlabelled box
Introduction
Alcohol-related hepatitis (AH) is an uncommon liver disease that occurs in a minority of subjects who consume large quantities of alcohol for extended periods of time. AH is characterized by new onset of liver failure with jaundice, often with ascites, fever, and elevated WBC.1,2 AASLD and EASL guidelines recommend corticosteroid treatment, using prednisolone, for patients with Maddrey's discriminant function (DF) ≥32.3,4 Despite these recommendations, use is debated because of increased risks5 among patients who fail to respond to prednisolone. Corticosteroids are usually discontinued after 7 days if the Lille score >0.45.6
Recombinant granulocyte colony stimulating factor (GCSF) has been used to treat AH with excellent safety but uncertain efficacy.7, 8, 9, 10, 11 GCSF injections reduced mortality by >75% in clinical trials conducted in India, associated with a reduction in infections or liver-related complications (eg, encephalopathy, ascites, etc.). The mechanisms by which GCSF might improve survival in AH are poorly understood.12 GCSF is hypothesized to stimulate liver regeneration by repopulating the liver with bone-marrow derived pluripotent stem cells that can differentiate into hepatocytes and potentially other liver cell populations. Receptors for GCSF have been identified on hepatocytes, raising the possibility of direct activation of hepatocyte regeneration.13 Finally, it is postulated that GCSF promotes a neutrophil phenotype that responds more effectively to bacterial translocation and infection.14
We conducted a phase II trial to evaluate the safety and efficacy of pegfilgrastim in the treatment of patients with severe alcoholic hepatitis, defined by Maddrey's discriminant function ≥32. Pegfilgrastim is a long-acting form of filgrastim, a recombinant GCSF, created by covalently linking polyethylene glycol with filgrastim, thereby allowing for once-a-week subcutaneous administration rather the daily administration of filgrastim that was used in prior GCSF clinical trials. Oncologists have used pegfilgrastim to treat chemotherapy-related neutropenia for almost two decades with an excellent safety profile.15
The trial was designed in 2015, after preliminary trials of GCSF from India reported a marked reduction in AH mortality. Given the reported effectiveness and the lack of adverse events, we elected to administer up to two doses of pegfilgrastim, a week apart, with the second dose administered if the WBC did not exceed 30,000/mm3 one week after the initial pegfilgrastim administration. At the time the trial was designed, prednisolone and pentoxifylline were accepted treatments at the participating hospitals; prednisolone, if used, was discontinued among patients with Lille ≥0.45 after one week.
Methods
This was a prospective, randomized, open-label clinical trial conducted between March 2017 and March 2020 at four hospitals in southern California and at the VA Healthcare System, Albuquerque, New Mexico. The IRB at each institution approved the study protocol and all patients provided informed, written consent. All subjects were >18 years of age, had bilirubin >5 mg/dL, Maddrey's DF ≥32, and, in retrospect, met the NIAAA clinical diagnosis of definite or probable AH.16 If liver biopsy was performed for clinical indications, interpretation by the pathologist at the participating clinical site was required to be consistent with the diagnosis of alcoholic hepatitis. Exclusion criteria included white blood cell (WBC) >30,000/mm3, creatinine >2 mg/dL, recent upper gastrointestinal bleeding, uncontrolled infection, known HIV infection, pregnancy, AH treatment for more than 3 days prior to randomization, and a low likelihood to return for follow up. Patients with infections could be enrolled if the infection was controlled with antibiotics for at least 4 days.
This study was registered with clinicaltrials.gov (NCT02776059) and the US Food and Drug Administration (FDA) approved an Investigational New Drug application to use pegfilgrastim (IND#131098). A Certificate of Confidentiality was obtained from the NIAAA (CC-AA-16-046). The study was conducted in accordance with Good Clinical Practices. All authors had access to the study data and reviewed and approved the final manuscript.
Patients were randomized to standard of care (SOC) treatment for 28 days or to SOC + pegfilgrastim (Neulasta®) 0.6 mg subcutaneously on Day 1 and again on Day 8 if the WBC count was <30,000/mm3 on Day 8. At the time the trial was conceived both prednisolone and pentoxifylline were considered acceptable treatments for AH at participating sites. Thus, SOC was 28 days of oral treatment with either prednisolone 40 mg/day or pentoxifylline 400 mg three times a day. SOC was determined by the subject's treating physician and not by the investigators. Lille score was calculated at Day 8 and all treatment (including pegfilgrastim) was stopped if Lille score ≥0.45. The study was not blinded since an elevated WBC in the pegfilgrastim group would have made blinding difficult to maintain and might have caused difficulties when assessing for possible infection.
Study duration was 24 weeks. Clinical assessments were performed at baseline and at Days 8, 15, 29, and at Weeks 12 and 24. In addition, outpatient telephone visits were conducted on Day 4 and Week 8. At each clinic visit, patients were asked about recent alcohol use and counseled to maintain abstinence. Safety assessments included clinical and laboratory evaluations. Adverse events were adjudicated by the site investigator for relatedness and for severity using CTCAE v. 4.03. Events of special interest included infections, acute kidney injury (AKI), hepatorenal syndrome (HRS),17 hepatic encephalopathy, and variceal bleeding.
This was the first clinical trial of pegfilgrastim in the United States for patients with AH, prompting the FDA to request additional assurances of drug safety. The FDA recommended limiting pegfilgrastim use to two injections. To avoid markedly elevated WBC, pegfilgrastim was not administered if the WBC >30,000/mm3 at baseline or on Day 8. Finally, because rare spleen rupture has been reported among patients with hematologic malignancies18 receiving filgrastim, spleen size (greatest length in one dimension on ultrasound) was measured at Day 15, Day 29, and Week 24.
Endpoints
Study duration was 24 weeks. Primary outcome was time to death through Day 90. Secondary outcomes included adverse events, change in MELD score and Maddrey's DF at Day 30, and survival at one month and at Week 24. The National Death Index was accessed a year after the study ended to determine survival among participants who did not return for protocol-defined study visits. Data collection for events not related to survival (e.g., adverse events, alcohol use, spleen size, etc.) ended at Week 24, at the time patient failed to come to clinic visits, or death.
Randomization
Patients were randomly assigned in a 1:1 ratio to receive either SOC or SOC+pegfilgrastim; randomization was stratified by study site. Three sites initially participated in the clinical trial; two sites were added after ∼2 years. The study statistician created a randomization schedule with permuted blocks of 2 or 4 using a standard software randomization program, which was entered into REDCap database, and concealed from study personnel (investigators, coordinators, etc.). Randomization occurred by entering inclusion and exclusion data. After three years and enrollment of 30 patients, the DSMB recommended reconsideration of the randomization scheme. Following their recommendation, the protocol was modified, and randomization was changed to 2:1 (pegfilgrastim: SOC) with a total enrollment goal of 45 subjects.
Statistical methods
This was a phase II trial. The power analysis for the primary outcome was based on a one-sided, two sample proportion Z-test with pooled variance at level of 0.05 using NCSS PASS software (NCSS, Kaysville, Utah, USA). Survival in the SOC arm was estimated to be 65% at 90 days based on data from more than 100 patients with AH treated with prednisolone at one of the participating clinical sites. We estimated a 25% improvement in absolute survival in the pegfilgrastim arm (assumed survival of 90% at 90 days) based on reduction in mortality among patients receiving GCSF in trials from India. With these assumptions, 35 subjects per treatment group had a power of 81%. A sample size of 78 was approved for randomization with an anticipated lack of survival data on 10% (eight patients) for a final assessment of 70 patients. Power was not calculated for secondary outcomes; given the sample size of this phase II trial, the power for each secondary outcome was assumed to be <80%. Coded data at each site was entered into a REDCap database. The intention to treat survival analysis included all randomized patients through Week 24 using their known date of death as obtained from the National Death Index or from medical records at the participating clinical site. We performed another analysis that censored patients who failed to complete the trial visits per protocol. The Kaplan-Meier (KM) survival curves were compared using a log-rank test. The primary comparison was survival at Day 90. Secondary survival outcomes included survival at Week 24 and survival at one month, which we report as 31-day survival rather than 30-day survival to account for one patient in the pegfilgrastim group with HRS who died at Day 31 and one patient in the SOC group with liver failure who died at Day 30. Secondary outcomes included all randomized patients and are reported as means and standard deviations (SD) or as median. For continuous data, a two-sided, two sample t-test (after assessing homogeneity of variance) or a Mann-Whitney U test (if normality assumption is not tenable; assessed via QQ-plot), whichever was appropriate, were conducted to compare the SOC group and the SOC+pegfilgrastim group at a significance level of 0.05. Statistical analyses were performed using the SAS™ (version 9.4) software program. A Data Safety Monitoring Board reviewed the data throughout the study.
Role of funding
This investigation was funded by the National Institutes of Health and National Institute on Alcohol Abuse and Alcoholism U01-AA021886 and U01-AA021884. The funds were used for recruitment of participants, as well as for support of researcher/nurse time, data and sample collection, and analyses. We confirm the independence of researchers from funders and that all authors, external and internal, had access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The funders had no role in writing the manuscript or the decision to submit for publication.
Results
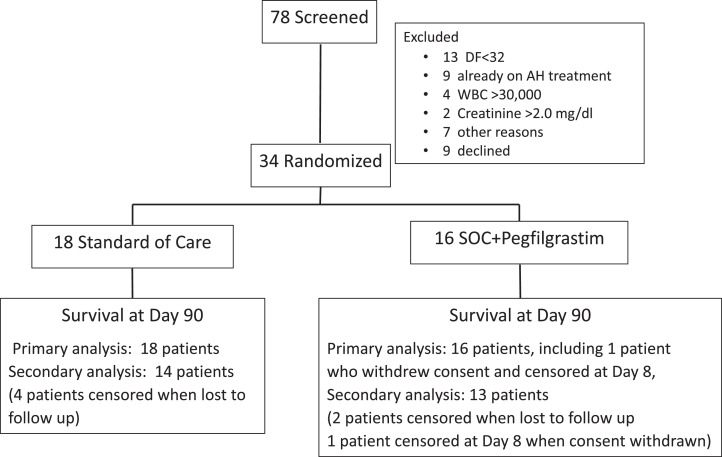
Seventy-eight patients were screened and 44 were excluded; 13 because of DF < 32, nine due to current AH treatment, 13 for other reasons, and nine patients who declined. The remaining 34 patients were randomized: 18 to SOC and 16 to SOC + pegfilgrastim (hereafter referred to as the pegfilgrastim group)(Figure 1). One patient in the pegfilgrastim group withdrew consent on Day 8 and was censored at that time point. The study was stopped early because of the COVID19 pandemic. At baseline, the average age of participants was approximately 50 years, all were male, >80% were Caucasian, 68% were Hispanic, and average MELD was approximately 25 (Table 1). Standard liver blood tests, WBC, hemoglobin, sodium, creatinine, and INR are reported in Table 1. The median Maddrey's DF was lower (45.3 v. 55.8, p = 0.32) and the number of days between hospital admission and randomization was shorter (3.9 ± 1.9 days vs. 5.7 ± 2.1 days) in the SOC group.
Figure 1.
Consort flow diagram.
Table 1.
Demographics and baseline laboratory tests.a
| SOC (n=18) | Pegfilgrastim (n=16) | |
|---|---|---|
| Age (years/SD) | 50 (10.9) | 49.9 (12.7) |
| Gender (male:female) | 18:0 | 16:0 |
| Race (n, %) | ||
| Caucasian | 17 (94.4) | 13 (81.3) |
| Black/AA | 0 (0%) | 1 (6.3%) |
| Asian | 0 (0%) | 1 (6.3%) |
| Native American | 0 (0%) | 1 (6.3%) |
| Refused | 1 (5.6%) | 0 (0%) |
| Ethnicity | ||
| Hispanic | 13 (72.2%) | 10 (62.5%) |
| Non-Hispanic | 5 (27.8%) | 6 (37.5%) |
| Bilirubin (mg/dL) (median (IQR)) |
12.8 (7.4) | 17.7 (11.45) |
| Albumin (g/dL) (Mean (SD)) |
2.3 (0.5) | 2.3 (0.4) |
| AST (IU/L) (median (IQR)) |
156 (84) | 131.5 (63) |
| ALT (IU/L) (median (IQR)) |
50 (64) | 51.5 (25.5) |
| Creatinine (mg/dL) (mean (SD)) |
0.8 (0.2) | 0.8 (0.2) |
| Sodium (mEq/L) (mean (SD)) |
133.1 (3.1) | 131.8 (2.8) |
| WBC (x103/µL) (median (IQR)) |
9,700 (12,300) | 9,600 (9,560) |
| INR (median (IQR)) |
1.8 (1.08) | 1.8 (0.48) |
| Maddrey DF | ||
| Mean (SD) | 53.7 (23.6) | 55.2 (15.9) |
| Median (IQR) | 45.3 (32.98) | 55.8 (17.63) |
| MELD | ||
| Mean (SD) | 25.6 (3.8) | 26.2 (2.2) |
| Median (IQR) | 26 (5) | 27 (3) |
| Days between hospitalization and randomization (mean (SD)) | 3.9 (1.9) | 5.7 (2.1) |
Data that are not normally distributed as assessed by QQ-plots are reported as median and interquartile range (IQR). Other data is reported as mean and standard deviation (SD).
All patients in both groups received prednisolone as SOC. All patients randomized to pegfilgrastim received pegfilgrastim on Day 1. Ten patients had Lille score ≥0.45 (SOC = 5/16 and pegfilgrastim = 5/14) at Day 8 and therefore stopped prednisolone; Lille score was >0.60 in 9 of these 10 patients. Lille score was not obtained for two SOC patients (death [1] and blood tests not obtained [1]) and for two pegfilgrastim patients (withdrew consent [1] and blood tests not obtained [1]). Prednisolone was stopped in three patients because of adverse events (presumed perforated diverticulum (Day 8), abdominal pain (Day 20), and a “cold” (Day 27)). Nine patients receiving pegfilgrastim had WBC >30,000/mm3 on Day 8; the second injection of pegfilgrastim was not administered in these patients (two of the nine also had Lille score ≥0.45). Two of these patients had WBC >60,000/mm3 at Day 8. No patient had an adverse event related to elevated WBC.
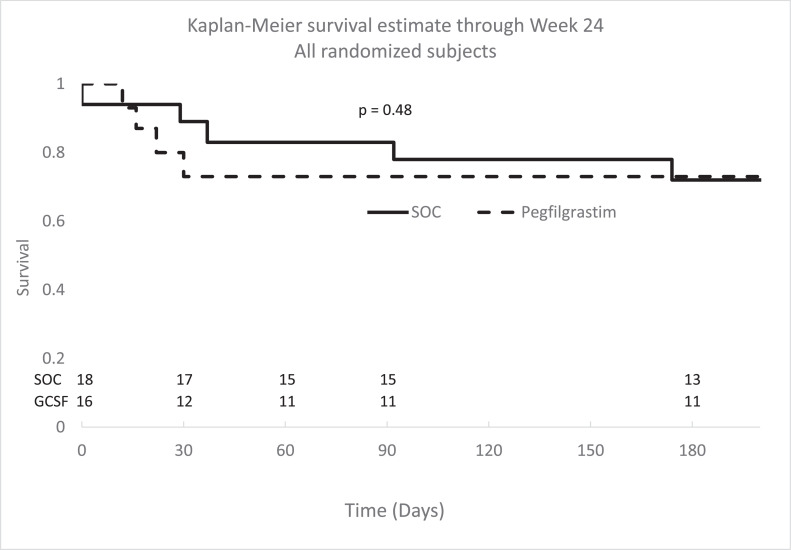
Survival at Week 24 was ascertained for all patients based on follow-up clinic visits, subsequent visits to the healthcare system, or the National Death Index. When all randomized patients (18 to SOC and 16 to pegfilgrastim) were included in the Kaplan-Meier estimates, survival at Day 90 was similar in both groups (SOC: 0.83 [95% confidence interval [CI]: 0.57–0.94] vs. pegfilgrastim: 0.73 [95% CI: 0.44–0.89], Figure 2; 95% CI for the difference: -0.18–0.38). A sensitivity analysis was conducted using a Cox model to estimate treatment effect adjusted for site. The effect of site is not significant (P = 0.4966) and the effect of treatment groups (SOC and GCSF arms) on survival remained not significant (P = 0.6272), consistent with the primary analysis results. Survival was similar in both treatment groups at Day 31 (SOC: 0.89 [95% CI: 0.62–0.97] vs. pegfilgrastim: 0.73 [95% CI: 0.44–0.89]; 95% CI for difference: -0.11–0.42) and at Week 24 (SOC: 0.72 [95% CI: 0.46–0.87] vs. pegfilgrastim: 0.73 [95% CI: 0.44–0.89]; 95% CI for difference: -0.32–0.29). By Day 90, two pegfilgrastim treated patients with Lille score <0.45 died and four patients with Lille score ≥0.45 (SOC = 2 vs. pegfilgrastim = 2) died. Lille score was not available for the SOC patient who died before Day 8. Kaplan-Meier survival estimates that included all randomized patients categorized according to Lille score, and for patients who completed protocol-defined visits through Day 90, are shown in the Supplemental figures.
Figure 2.
Kaplan Meier survival estimates for all enrolled subjects through Week 24. Survival was not significantly different between SOC and SOC + GCSF at Day 31 (p = 0.27), Day 90 (p = 0.48), or Week 24 (p = 0.97)(log-rank test). SOC: standard of care (solid line); pegfilgrastim (dotted line).
Seven patients died by Day 90 (3 SOC and 4 pegfilgrastim); two additional patients in the SOC group died between Day 90 and Week 24 (Table 2). Six of the nine patients died with liver failure without an identifiable precipitating event (Table 2; Supplemental Table 1). The three remaining patients died within the first 30 days following a precipitating event. One SOC patient developed presumed respiratory infection (cultures negative) followed by respiratory and liver failure (death at Day 6), a 70-year-old pegfilgrastim treated patient developed presumed perforated diverticulum at Day 8 followed by sepsis and renal failure (death at Day 13), and a second pegfilgrastim treated patient was found unconscious at home and developed respiratory failure followed by liver failure and death over 48 hours in the hospital (death at Day 17). The relationship of the precipitating events to the administration of prednisolone with or without pegfilgrastim is uncertain. Four patients (1 SOC and 3 pegfilgrastim) were hospitalized within the first 31 days and survived (Table 2). Three additional patients were hospitalized and survived between Day 32 and Day 90 (1 SOC and 2 pegfilgrastim).
Table 2.
Hospitalizations and deaths through Week 24 (each patient listed once).
| Day 2–Day 31 |
Day 32–Day 90 |
Day 91–Week 24 |
||||
|---|---|---|---|---|---|---|
| SOC | Pegfilgrastim | SOC | Pegfilgrastim | SOC | Pegfilgrastim | |
| Death | • Liver failure • Respiratory infection → liver failure |
• Liver failure • HRS • Perforated diverticulum → sepsis → liver failure • Found unconscious at home, respiratory failure → liver failure |
• Liver failure | • Liver failure (2) | ||
| Hospitalizationa | • UTI | • Abdominal pain (3) | • Alcohol withdrawal | • Alcohol withdrawal • SBP |
• Alcohol withdrawal with abdominal pain | • Alcohol withdrawal • Ascites |
Hospitalized and survived.
Events of special interest included infections, acute kidney injury, hepatorenal syndrome, worsening hepatic encephalopathy, and variceal bleeding. Most of the events of special interest occurred during the first 30 days. Overall, the incidences of infection, AKI, and HRS were similar in both groups; no patient developed variceal bleeding or worsening hepatic encephalopathy (Table 3; Supplemental Table 2). During the first 30 days, four patients in the SOC group and five patients in the pegfilgrastim group reported at least one AE not related to an event of special interest. Two of these AE's were assessed as probably or definitely related to prednisolone: dyspepsia at Day 2 in a SOC patient and abdominal pain at Day 20 in a pegfilgrastim treated patient. No AE was assessed as related to pegfilgrastim.
Table 3.
Events of special interest through Week 12.
| Day 2 through Day 30 |
Day 2 through Week 12 |
|||
|---|---|---|---|---|
| SOC (N = 18) | Pegfilgrastim (N = 16) | SOC (N = 18) | Pegfilgrastim (N = 16) | |
| Acute kidney injury | 2 | 4 | 3 | 4 |
| Hepatorenal syndrome | 2 | 2 | 2 | 2 |
| Infections | 4 | 3 | 5 | 4 |
| Hepatic encephalopathy (worse than baseline) | 0 | 0 | 0 | 0 |
| Variceal bleeding | 0 | 0 | 0 | 0 |
At Day 30, median Maddrey's DF improved 10.5 points in the SOC group and 19.5 points in the pegfilgrastim group; median MELD improved two points and five points, respectively, in these groups (Supplemental Table 3; both p > 0.05). MELD improved by three or more points in 5/13 patients receiving SOC and 7/10 patients receiving pegfilgrastim (Supplemental Table 3). Two patients in the SOC group and three patients in the pegfilgrastim group resumed alcohol consumption within the first 90 days; one additional subject in the SOC group resumed alcohol use between Day 90 and Week 24 (Supplemental Table 4). None of these patients died by Week 24. Compared with baseline, ultrasound-determined maximal spleen length during the first month significantly increased in the pegfilgrastim group (p < 0.05) but not in SOC group. When compared between groups, maximal change in spleen size during the first month did not differ (p > 0.05; Supplemental Table 5). During the first month, spleen length increased ≥1.5 cm in three patients receiving SOC and four patients receiving pegfilgrastim. The pegfilgrastim treated patient with the largest increase in spleen length during the first month (3.7 cm at Day 15) had a baseline spleen measurement performed at an outside hospital (ultrasound not available for quality assessment); he was asymptomatic, and no subsequent ultrasounds were obtained. At Week 24, two patients receiving SOC and no patients receiving pegfilgrastim had spleen size increase ≥1.5 cm when compared with baseline.
Discussion
This open-label, prospective, randomized trial found no survival benefit at 90 days for pegfilgrastim + SOC vs. SOC alone among patients with AH and DF ≥32. Although the protocol allowed for treatment with pentoxifylline, the supervising physicians chose prednisolone 40 mg/day for each patient. We administered pegfilgrastim, a long-acting form of recombinant GCSF that has an excellent safety profile when given as a subcutaneous injection for chemotherapy-related neutropenia. Adverse events were similar in the pegfilgrastim and the SOC groups, including adverse events frequently associated with AH such as acute kidney injury and infections.
Most deaths in both groups were from liver failure without an identified precipitating event. The three patients who did not directly die from progressive liver failure and/or hepatorenal syndrome had clinical courses commonly found in patients dying with AH. Two of these patients had presumed infections that precipitated renal failure and death; the third patient was found unconscious at home and subsequently developed respiratory and renal failure in the hospital. The relationship of these deaths to study medicines is uncertain. Prednisolone administration in AH has been associated with an increased incidence of infections; we have found no association of increased infections following GCSF administration. Clinical events that result in poor outcomes in AH, such as infection and acute kidney injury, were similar among patients receiving SOC and patients receiving pegfilgrastim. Thus, there was no evidence that pegfilgrastim treatment either increased or decreased complications of AH.
GCSF has been used as a treatment for AH or ACLF (often due to AH) with mixed effectiveness.7 Meta-analysis of five prospective randomized trials of GCSF from India reported ∼75% reduction in 90-day mortality and ∼85% reduction in infections among patients with AH8, 9, 10, 11 or ACLF.19 The current study differs from the trials in India in several ways. First, the mortality in the SOC arms was approximately 70% in the trials from India, significantly higher than the ∼20–30% observed in this and other trials. Increased mortality could be related to degree of liver injury in trials from India, with MELD at entry often ∼25–30. Second, in India the SOC for AH consisted of pentoxifylline. Third, the Indian AH trials administered recombinant GCSF twice a day for 5 days; the study of ACLF continued to administer GCSF every third day through day 30.19 Other differences, such as age of patients, supportive care, and genetic factors, might also have contributed to differences in survival between the trials in India and this trial. The outcomes of our trial are similar to two trials from Europe, in which GCSF administration did not improve liver function, survival, or reduce infections. However, the trials from Europe were limited by small size and less severe AH20 or by enrollment of patients with ACLF (many with alcohol-related ACLF).21
We did not observe safety concerns with pegfilgrastim administration. The FDA requested withholding the second pegfilgrastim injection among patients with an elevated WBC, which was set at >30,000/mm3. Using this criterion, 9 of 14 patients were excluded from receiving a second injection at Day 8. As a safety concern in this phase II trial, the FDA also requested that we monitor spleen size. Compared with baseline, spleen size increased during the first month among patients receiving pegfilgrastim although the increase in spleen size during the first month did not significantly differ compared with SOC patients. Several caveats are important. First, relatively few patients received an ultrasound during the first month. Second, by convention we measured longest spleen length in one dimension. This technique may fail to adequately assess changes in spleen volume. Third, spleen size increased ≥1.5 cm within the first 30 days in four patients receiving pegfilgrastim vs. three patients receiving SOC. Spleen size at 6 months remained ≥1.5 cm above baseline length in two patients receiving SOC and in no patients receiving pegfilgrastim. The question of whether GCSF causes clinically significant increase in spleen size was not resolved by this trial.
An increase in WBC is a pharmacological effect of filgrastim and a theoretical surrogate marker of therapeutic effect in AH. Mean WBC in the pegfilgrastim group increased from ∼11,000/mm3 at entry to ∼36,000/ mm3 at Day 8, with 9 of 14 patients having a WBC > 30,000/mm3, two of whom had WBC > 60,000/mm3. The increase in WBC with pegfilgrastim was similar to, or greater than, the WBC increase with GCSF in clinical trials from India, suggesting that pegfilgrastim has similar or greater biological effect as compared with daily GCSF administration. The actual mechanism of action of GCSF in reducing liver injury and improving survival in AH is not well established and the assumption an increase in WBC is a surrogate marker of beneficial effect in AH is unproven.
The trial had limitations and caveats. First, the power of the study is limited by the relatively small number of subjects, due, in part, to the COVID19 pandemic. One of the purposes of a phase II trial is to estimate the effect size for planning a phase III trial. In this respect, the outcome of this trial is useful despite premature termination. Our survival data do not suggest a clinically significant survival benefit at 90 days or at 24 weeks among patients receiving pegfilgrastim. However, the confidence intervals of the KM survival curves are large, limiting definitive assessments of differences in survival between the two treatment arms. Second, the mortality in the SOC group at 90 days was less than predicted from our preliminary survival data or from older clinical trials but similar to the survival rates in more recent trials from the UK and France.22,23 Third, prednisolone was used as standard of care. Although prednisolone is the most frequently used treatment for AH in the United States, its effectiveness has been challenged.24 Investigators felt that a clinical trial without a SOC treatment arm for AH was not justified and the patients’ supervising physician chose prednisolone as SOC. Fourth, four patients in each group failed to complete the trial visits per protocol. Despite failing to attend all clinic visits, we ascertained survival status, the primary outcome, in all patients through Week 24. Fifth, the study was unblinded. Because of the expected (and observed) increase in WBC, blinding would have been difficult and could have led to mismanagement of the patients, who are at risk of infections. Sixth, liver biopsy was not required for entry. Seventh, all randomized patients were male, and steroids may be less effective among males (although studies of GCSF in India were among males with AH). Also, the process of randomization resulted in a shorter pre-randomization interval as well as a lower median (but not mean) DF score among patients randomized to SOC. It is unclear whether the extra ∼2 days in the hospital prior to treatment in the pegfilgrastim treated group was clinically significant for the primary outcome. All but one patient in each group was randomized within the first 7 days of hospitalization. The lower median DF score in the SOC group might have contributed to reduced early mortality in this group. Finally, we note methodological limitations, including measurement bias inherent in ITT analysis and potential selection bias in the per-protocol analysis which censored 6 patients who failed to complete trial visits per protocol.
In summary, this unblinded, phase II, randomized clinical trial found no survival benefit at 90 days among patients with AH who received pegfilgrastim+prednisolone as compared with those receiving prednisolone alone. Treatment with pegfilgrastim increased WBC > 30,000/mm3 during the first week in more than one half of treated patients, suggesting a significant biological effect. Pegfilgrastim treatment was not associated with a decrease (or increase) in adverse events commonly associated with AH, such as infections and renal injury. Spleen size during the first month increased by ≥1.5 cm in four patients receiving pegfilgrastim and three patients receiving prednisolone; there were no complications or symptoms related to the increased spleen size.
Contributors
Conceptualization: TRM; Data curation: TRM, DN, AA; Study design: DN, TRM, AAS, AA; Subject enrollment and data collection: JAT, AAS, DC-KC, JAD, MWF, JMA, AA, TRM; access to primary data and supervised the data analyses: TRM, AA; manuscript writing: TRM, AA; manuscript review and editing: JAT, AAS, DN, DC-KC, JAD, MWF, JMA, AA, TRM; Project administration: AA; Supervision: AA, TRM; Funding acquisition: TRM.
Data sharing statement
Deidentified summary data are available from the corresponding author for reasonable requests of approved scientific studies.
Declaration of interests
TRM reports grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), personal fees from the University of California, Irvine, during the conduct of the study. JAT and AAS received funding from the NIAAA to perform this clinical trial. AAS received funding from the NIDDK to conduct the Drug Induced Liver Injury (DILI) clinical center. TRM received funding to his institution to perform clinical trials by AbbVie, Gilead, Merck and Genfit (not related to alcoholic liver disease). JAD is on the governing board of the Southern California Society of Gastroenterology, a non-profit organization focused on education of gastroenterologists.
Acknowledgements
This investigation was funded by the Unites States National Institutes of Health and National Institute on Alcohol Abuse and Alcoholism U01-AA021886 and U01-AA021884.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101689.
Contributor Information
Timothy R. Morgan, Email: timothy.morgan@va.gov.
Southern California Alcoholic Hepatitis (SCAH) Consortium:
Divya Birudaraju, Greg Botwin, Hema Buddha, Lavanya Cherukuri, Sheena Cruz, Monique French, Rachel Gonzalez, Jessica Gozum, Rebecca Gutierrez, Sajad Hamal, Preston Head, Carol Jones, Neil Kaplowitz, Robert Lee, Lauren MacHarg, Susan Milstein, Yuxin Ouyang, Christy Rico, and Cory Zarick
Appendix. Supplementary materials
Supplemental Figure 1: Kaplan Meier survival estimates through Week 24 for subjects with Lille score <0.45. Survival was not significantly different between SOC and SOC+GCSF at Day 31 (p=0.11), Day 90 (p=0.11), or Week 24 (p=0.39)(log-rank test). SOC: standard of care (solid line); pegfilgrastim (dotted line).
Supplemental Figure 2: Kaplan Meier survival estimates through Week 24 for subjects with Lille score ≥0.45. Survival was not significantly different between SOC and SOC+GCSF at Day 31 (p=0.52), Day 90 (p=0.90), or Week 24 (p=0.74)(log-rank test). SOC: standard of care (solid line); pegfilgrastim (dotted line).
Supplemental Figure 3: Kaplan Meier survival estimates through Week 24 among subjects completing the protocol-defined study visits through Week 24 (or died). By Day 90, six patients failed to complete protocol-defined study visits and were removed from this KM survival estimate when compared with the KM survival estimate in Figure 2. Subjects removed from this Day 90 KM survival analysis consisted of four patients in the SOC arm (solid down arrows; failure occurred at Days 5, 32, 40, and 89) and two patients in the pegfilgrastim arm (dashed up arrows; failure occurred at Days 5 and 44). One additional patient in the pegfilgrastim arm stopped attending protocol-defined study visits after Day 101 and was removed from the Week 24 KM survival analysis. Survival was not significantly different between SOC and SOC+GCSF at Day 31 (p=0.26), Day 90 (p=0.47), or Week 24 (p=0.97)(log-rank test). SOC: standard of care (solid line); pegfilgrastim (dotted line).
References
- 1.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360(26):2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 2.Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease: a review. JAMA. 2021;326(2):165–176. doi: 10.1001/jama.2021.7683. [DOI] [PubMed] [Google Scholar]
- 3.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306–333. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Vergis N, Atkinson SR, Knapp S, et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology. 2017;152(5):1068–1077.e4. doi: 10.1053/j.gastro.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45(6):1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 7.Marot A, Singal AK, Moreno C, Deltenre P. Granulocyte colony-stimulating factor for alcoholic hepatitis: a systematic review and meta-analysis of randomised controlled trials. JHEP Rep. 2020;2(5) doi: 10.1016/j.jhepr.2020.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A, Setia A, RR R. Effect of granulocyte colony-stimulating factor (G-CSF) on mortality and complications viz. sepsis, encephalopathy, hepatorenal syndrome, and gastrointestinal bleed in severe alcoholic hepatitis: a randomized controlled study. United Eur Gastroenterol J. 2017;5:A17. [Google Scholar]
- 9.Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of granulocyte colony-stimulating factor in the management of steroid-nonresponsive severe alcoholic hepatitis: a double-blind randomized controlled trial. Hepatology. 2019;70(3):802–811. doi: 10.1002/hep.30516. [DOI] [PubMed] [Google Scholar]
- 10.Singh V, Keisham A, Bhalla A, et al. Efficacy of granulocyte colony stimulating factor and N-acetyl cysteine therpies in patients with severe alcoholic hepatitis. Clin Gastroenterol Hepatol. 2018;16:1650–1656. doi: 10.1016/j.cgh.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol. 2014;109(9):1417–1423. doi: 10.1038/ajg.2014.154. [DOI] [PubMed] [Google Scholar]
- 12.Rathi S, Hussaini T, Yoshida EM. Granulocyte colony stimulating factor: a potential therapeutic rescue in severe alcoholic hepatitis and decompensated cirrhosis. Ann Hepatol. 2021;20 doi: 10.1016/j.aohep.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan P, Sharma Y, Jaber FL, Tchaikovskaya T, Gupta S. Transplanted hepatocytes rescue mice in acetaminophen-induced acute liver failure through paracrine signals for hepatic ATM and STAT3 pathways. FASEB J. 2021;35(4):e21471. doi: 10.1096/fj.202002421R. [DOI] [PubMed] [Google Scholar]
- 14.Moreau R, Rautou PE. G-CSF therapy for severe alcoholic hepatitis: targeting liver regeneration or neutrophil function? Am J Gastroenterol. 2014;109(9):1424–1426. doi: 10.1038/ajg.2014.250. [DOI] [PubMed] [Google Scholar]
- 15.Parker SD, King N, Jacobs TF. StatPearls; Treasure Island (FL): 2021. Pegfilgrastim. [Google Scholar]
- 16.Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology. 2016;150(4):785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56(9):1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuamah NM, Goker H, Kilic YA, Dagmoura H, Cakmak A. Spontaneous splenic rupture in a healthy allogeneic donor of peripheral-blood stem cell following the administration of granulocyte colony-stimulating factor (g-csf). A case report and review of the literature. Haematologica. 2006;91(5 suppl):ECR08. [PubMed] [Google Scholar]
- 19.Garg V, Garg H, Khan A, et al. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142(3):505–512.e1. doi: 10.1053/j.gastro.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Spahr L, Lambert JF, Rubbia-Brandt L, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48(1):221–229. doi: 10.1002/hep.22317. [DOI] [PubMed] [Google Scholar]
- 21.Engelmann C, Herber A, Franke A, et al. Granulocyte-colony stimulating factor (G-CSF) to treat acute-on-chronic liver failure: A multicenter randomized trial (GRAFT study) J Hepatology. 2021;71(6):1346–1354. doi: 10.1016/j.jhep.2021.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372(17):1619–1628. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 23.Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310(10):1033–1041. doi: 10.1001/jama.2013.276300. [DOI] [PubMed] [Google Scholar]
- 24.Sehrawat TS, Liu M, Shah VH. The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol Hepatol. 2020;5(5):494–506. doi: 10.1016/S2468-1253(19)30326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Kaplan Meier survival estimates through Week 24 for subjects with Lille score <0.45. Survival was not significantly different between SOC and SOC+GCSF at Day 31 (p=0.11), Day 90 (p=0.11), or Week 24 (p=0.39)(log-rank test). SOC: standard of care (solid line); pegfilgrastim (dotted line).
Supplemental Figure 2: Kaplan Meier survival estimates through Week 24 for subjects with Lille score ≥0.45. Survival was not significantly different between SOC and SOC+GCSF at Day 31 (p=0.52), Day 90 (p=0.90), or Week 24 (p=0.74)(log-rank test). SOC: standard of care (solid line); pegfilgrastim (dotted line).
Supplemental Figure 3: Kaplan Meier survival estimates through Week 24 among subjects completing the protocol-defined study visits through Week 24 (or died). By Day 90, six patients failed to complete protocol-defined study visits and were removed from this KM survival estimate when compared with the KM survival estimate in Figure 2. Subjects removed from this Day 90 KM survival analysis consisted of four patients in the SOC arm (solid down arrows; failure occurred at Days 5, 32, 40, and 89) and two patients in the pegfilgrastim arm (dashed up arrows; failure occurred at Days 5 and 44). One additional patient in the pegfilgrastim arm stopped attending protocol-defined study visits after Day 101 and was removed from the Week 24 KM survival analysis. Survival was not significantly different between SOC and SOC+GCSF at Day 31 (p=0.26), Day 90 (p=0.47), or Week 24 (p=0.97)(log-rank test). SOC: standard of care (solid line); pegfilgrastim (dotted line).