This secondary analysis of a randomized clinical trial investigates the association of donanemab treatment with plasma biomarkers associated with Alzheimer disease.
Key Points
Question
Does donanemab induce changes in plasma biomarkers?
Findings
In this secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial, plasma levels of phosphorylated tau217 and glial fibrillary acidic protein were significantly lowered with donanemab treatment compared with placebo, whereas no significant changes in plasma levels of amyloid β 42/40 and neurofilament light chain were observed between treatment arms at the end of the study. Changes in plasma phosphorylated tau217 and glial fibrillary acidic protein were positively correlated with change in brain amyloid plaques.
Meaning
These plasma biomarkers are promising candidates for use as pharmacodynamic measures of the effect of donanemab and potentially other anti-amyloid therapies in early symptomatic Alzheimer disease.
Abstract
Importance
Plasma biomarkers of Alzheimer disease may be useful as minimally invasive pharmacodynamic measures of treatment outcomes.
Objective
To analyze the association of donanemab treatment with plasma biomarkers associated with Alzheimer disease.
Design, Setting, and Participants
TRAILBLAZER-ALZ was a randomized, double-blind, placebo-controlled clinical trial conducted from December 18, 2017, to December 4, 2020, across 56 sites in the US and Canada. Exploratory biomarkers were prespecified with the post hoc addition of plasma glial fibrillary acidic protein and amyloid-β. Men and women aged 60 to 85 years with gradual and progressive change in memory function for at least 6 months were included. A total of 1955 participants were assessed for eligibility. Key eligibility criteria include Mini-Mental State Examination scores of 20 to 28 and elevated amyloid and intermediate tau levels.
Interventions
Randomized participants received donanemab or placebo every 4 weeks for up to 72 weeks. The first 3 doses of donanemab were given at 700 mg and then increased to 1400 mg with blinded dose reductions as specified based on amyloid reduction.
Main Outcomes and Measures
Change in plasma biomarker levels after donanemab treatment.
Results
In TRAILBLAZER-ALZ, 272 participants (mean [SD] age, 75.2 [5.5] years; 145 [53.3%] female) were randomized. Plasma levels of phosphorylated tau217 (pTau217) and glial fibrillary acidic protein were significantly lower with donanemab treatment compared with placebo as early as 12 weeks after the start of treatment (least square mean change difference vs placebo, –0.04 [95% CI, –0.07 to –0.02]; P = .002 and –0.04 [95% CI, –0.07 to –0.01]; P = .01, respectively). No significant differences in plasma levels of amyloid-β 42/40 and neurofilament light chain were observed between treatment arms at the end of treatment. Changes in plasma pTau217 and glial fibrillary acidic protein were significantly correlated with the Centiloid percent change in amyloid (Spearman rank correlation coefficient [R] = 0.484 [95% CI, 0.359-0.592]; P < .001 and R = 0.453 [95% CI, 0.306-0.579]; P < .001, respectively) following treatment. Additionally, plasma levels of pTau217 and glial fibrillary acidic protein were significantly correlated at baseline and following treatment (R = 0.399 [95% CI, 0.278-0.508], P < .001 and R = 0.393 [95% CI, 0.254-0.517]; P < .001, respectively).
Conclusions and Relevance
Significant reductions in plasma biomarkers pTau217 and glial fibrillary acidic protein compared with placebo were observed following donanemab treatment in patients with early symptomatic Alzheimer disease. These easily accessible plasma biomarkers might provide additional evidence of Alzheimer disease pathology change through anti-amyloid therapy. Usefulness in assessing treatment response will require further evaluation.
Trial Registration
ClinicalTrials.gov Identifier: NCT03367403
Introduction
The hallmark pathological findings in Alzheimer disease (AD) are amyloid plaques and neurofibrillary tangles due to the accumulation of amyloid-β (Aβ) peptide, and aggregation of tau protein, respectively.1 Extensive research on AD biomarkers aims to allow timely detection and accurate diagnosis of AD.2,3 In addition to imaging and cerebrospinal fluid (CSF) biomarkers, blood measurements, including phosphorylated tau (pTau), glial fibrillary acidic protein (GFAP), neurofilament light chain (NfL), and Aβ, are being explored as biomarkers of AD.3,4 Plasma pTau217 is elevated in pathologically and clinically diagnosed patients with AD and typically not in other common neurodegenerative dementias.5,6,7 GFAP is a marker of glial activation, and plasma levels are elevated in AD, as well as in cognitively normal individuals with amyloid-positive positron emission tomography (PET) results.8,9 Plasma levels of NfL, a marker of neurodegeneration, are correlated with CSF NfL levels in AD and with the severity of postmortem neurofibrillary tangle pathology.10,11 Levels of the Aβ 42/40 ratio in plasma are reduced in AD.12,13 When plasma pTau217 and Aβ 42/40 assay results are combined, the accuracy of identifying amyloid-positive PET results in cognitively unimpaired individuals is improved.14
Donanemab, an antibody specific for the N-terminal pyroglutamate Aβ epitope that is only present in amyloid plaques, is being investigated for the treatment of AD.15,16,17 In the TRAILBLAZER-ALZ phase 2 registration study, donanemab demonstrated robust amyloid plaque reduction as measured on PET in patients with early symptomatic AD.17 Donanemab was superior to placebo in the primary outcome, showing 32% slowing of disease progression from baseline to 76 weeks in the score on the Integrated Alzheimer’s Disease Rating Scale (iADRS).17 The aim of the present exploratory, post hoc analysis is to assess the correlation of donanemab treatment with plasma levels of the biomarkers pTau217, GFAP, NfL, and Aβ 42/40 in patients with early symptomatic AD.
Methods
Patients and Study Design
TRAILBLAZER-ALZ was a randomized, double-blind, placebo-controlled phase 2 study conducted from December 18, 2017, to December 4, 2020, at 56 sites in the US and Canada. Written informed consent was obtained from participants or their legal representatives and from study partners. The protocol (Supplement 1) was reviewed and approved by study site ethical review boards. The study was conducted according to international ethics guidelines, including the Declaration of Helsinki18 and Council for International Organizations of Medical Sciences International Ethical Guidelines and applicable International Council for Harmonization Good Clinical Practice guidelines.
Participants included were aged 60 to 85 years, diagnosed with early symptomatic AD (prodromal AD or AD with mild dementia), met elevated amyloid and intermediate tau PET-based criteria, and had Mini-Mental State Examination scores of 20 to 28.17 Participant race and ethnicity were collected by self report and have already been described in the primary publication by Mintun et al.17
Participants included in this analysis were 1:1 randomized to receive intravenous donanemab or placebo every 4 weeks for up to 72 weeks. The first 3 doses of donanemab were given at 700 mg and then increased to 1400 mg. A blinded dose change occurred at weeks 24 and 52 based on amyloid PET scan results.17 If amyloid levels ranged from 11 to 25 Centiloids (CL) on a single scan, donanemab was decreased to 700 mg. If amyloid levels were less than 11 CL on a single scan or less than 25 CL on 2 consecutive scans, donanemab dosing was stopped and participants received placebo. Eligibility criteria and study design of TRAILBLAZER-ALZ were previously reported.17
PET
The effect of donanemab on brain amyloid and tau pathology was assessed using the specific PET tracers 18F-florbetapir (detecting amyloid plaque at baseline and weeks 24, 52, and 76), and 18F-flortaucipir (detecting tau neurofibrillary tangles at baseline and week 76). Additional PET details can be found in the eMethods in Supplement 2.
Plasma Assays
Plasma samples were collected at baseline and weeks 12, 24, 36, 52, 64, and 76. Due to the quantity of plasma collected, not all analyses were able to be completed for every participant at every time point. Plasma pTau217 was measured using a custom Simoa-HD-X assay (Quanterix) made specifically for Eli Lilly and Company. The Simoa Neurology 4-Plex E Advantage Kit (Quanterix) was used for plasma levels of GFAP, NfL, Aβ40, and Aβ42. Additional plasma assay details can be found in the eMethods in Supplement 2.
Statistical Analyses
Plasma pTau217, GFAP, NfL, and Aβ 42/40 values were log10 transformed to normalize the data. Mixed models with repeated measurements were conducted to compare the change from baseline values by treatment arms at each scheduled visit. The mixed models with repeated measurements included fixed categorical effects of treatment, visit, and treatment-by-visit interaction and were adjusted for continuous effect of age and corresponding baseline values. Overall, 74% of participants were apolipoprotein (APOE) ε4 carriers and adding APOE ε4 status to the models produced no evidence of interaction, so APOE ε4 status was not included in the model. Minimal clinically important difference (MCID) on iADRS change has been reported as 5-point or 9-point deterioration for individual participants with mild cognitive impairment and AD with mild dementia, respectively.19 A marginal logistic regression model was conducted to assess the association between pTau217 change with the odds of having MCID across the follow-up visits. The model was adjusted for baseline iADRS scale, pTau217, and age. The intraparticipant correlation was adjusted using general estimating equation method. Scatterplots were generated to describe the relationships and associations between biomarkers, and Spearman rank correlation coefficients were provided to quantify these relationships and associations. All analyses were exploratory or post hoc and not corrected for multiplicity. The data analysis for this paper was generated using SAS software, version 9.4 (SAS Institute). Statistical significance was set at a 2-sided P value of .05.
Results
Participants
Of 1955 participants assessed for eligibility, 272 men and women aged 60 to 85 years were randomized (eFigure 1 in Supplement 2). The mean (SD) age was 75.2 (5.5) years, and 145 (53.3%) were female. Baseline characteristics of participants included in these analyses are listed in eTable 1 in Supplement 2. Additional study details were previously reported.17
Baseline Plasma Biomarker Level Correlation With Baseline Amyloid and Tau PET End Points
Baseline plasma pTau217 levels were positively associated with both baseline amyloid plaque level as measured by amyloid PET imaging (Spearman rank correlation coefficient [R] = 0.148 [95% CI, 0.019-0.271]; P = .02) (Figure 1A), and global tau deposition as measured by tau PET imaging (R = 0.378 [95% CI, 0.262-0.483]; P < .001) (Figure 1B). However, baseline plasma levels of GFAP, NfL, and Aβ 42/40 were not significantly correlated with baseline amyloid or tau PET end points (eFigure 2 in Supplement 2).
Figure 1. Baseline Plasma Phosphorylated Tau217 (pTau217) Levels Correlated With Baseline Amyloid and Tau Positron Emission Tomography (PET) End Points.
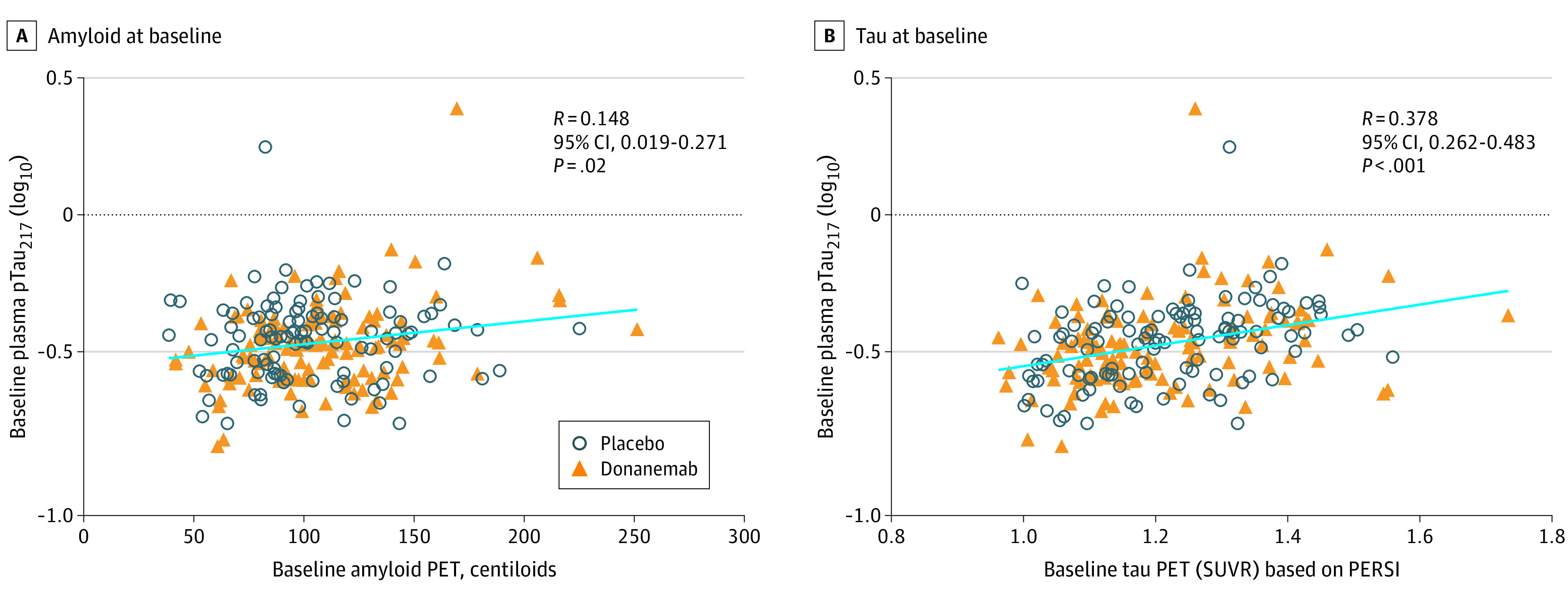
Baseline amyloid PET (placebo n = 111; donanemab n = 121) (A), and tau PET (placebo n = 112; donanemab n = 120) (B) levels compared with baseline plasma pTau217 levels. Plasma levels were log10 transformed. Linear regression of all data points, regardless of treatment, is indicated by the straight line. Spearman rank was used for correlation coefficient. PERSI indicates parametric estimation of reference signal intensity; SUVR, standardized uptake value ratio.
Change in Plasma Biomarker Levels Following Donanemab Treatment
A significant decrease in plasma pTau217 levels compared with placebo was observed after 12 weeks of donanemab treatment and continued throughout the 76 weeks of the study (Figure 2A and eTable 2 in Supplement 2). Mean plasma pTau217 levels decreased by 23% from baseline after donanemab treatment. In contrast, mean placebo plasma pTau217 levels continued to rise by 6% from baseline to the end of the study. A significant decrease in plasma GFAP levels was observed after 12 weeks of donanemab treatment compared with placebo and continued throughout the 76 weeks of the study (Figure 2B and eTable 2 in Supplement 2). Mean plasma GFAP levels decreased by 12% from baseline after donanemab treatment whereas with placebo, mean levels continued to rise by 15% from baseline to the end of the study. There was no significant difference in plasma NfL levels between treatment arms at the end of the study (Figure 2C and eTable 2 in Supplement 2). At 76 weeks, mean plasma NfL levels were increased 15% from baseline after donanemab treatment compared with 19% for placebo. To determine whether amyloid levels increase in the plasma as donanemab reduces amyloid plaque levels in the brain, we measured plasma Aβ 42/40 levels. However, there was no significant difference in plasma Aβ 42/40 levels between treatment arms at the end of the study (Figure 2D and eTable 2 in Supplement 2). At 76 weeks, mean plasma Aβ 42/40 levels were increased 4% from baseline with donanemab treatment vs 2% for placebo. Of note, there was a significant increase in the Aβ 42/40 ratio with donanemab treatment compared with placebo at week 36 only.
Figure 2. Change in Plasma Levels Following Donanemab Treatment.
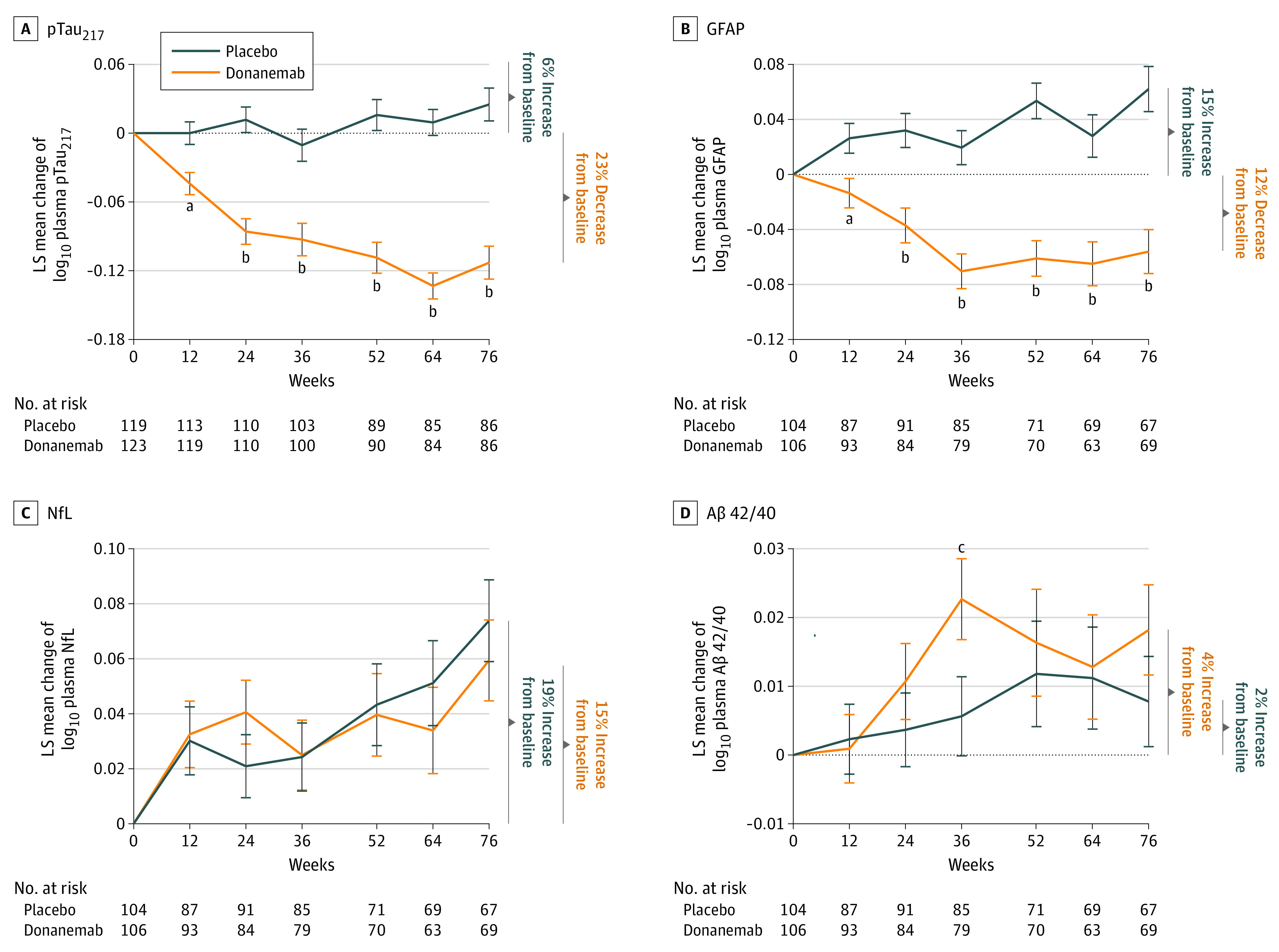
Least square (LS) mean change from baseline in plasma phosphorylated tau217 (pTAU217) (A), glial fibrillary acidic protein (GFAP) (B), neurofilament light chain (NfL) (C), and amyloid-β (Aβ) 42/40 (D) for placebo and donanemab. Plasma values were log10 transformed. Error bars indicate standard error. The dotted line indicates baseline.
aP < .01 vs placebo.
bP < .001 vs placebo.
cP < .05 vs placebo.
We further explored the significant reductions in plasma levels of pTau217 and GFAP by separating data from participants who were switched from donanemab to placebo at 24 weeks due to amyloid levels below 11 CL and those who continued receiving donanemab treatment. At the end of the study, plasma levels of pTau217 (eFigure 3A and eTable 3 in Supplement 2) and GFAP (eFigure 3B and eTable 3 in Supplement 2) were significantly lower in participants treated with donanemab compared with placebo, regardless of stopping or continuing treatment. The results from participants who stopped donanemab treatment at 24 weeks reveal that the effect of donanemab on plasma levels of pTau217 and GFAP persist up to 1 year beyond the end of treatment (eFigure 3A-B in Supplement 2). Further, the decrease in plasma levels of pTau217 and GFAP in participants who continued treatment was not significantly greater than those who stopped treatment at 24 weeks (eFigure 3A-B and eTable 3 in Supplement 2). The reductions in plasma levels of pTau217 and GFAP observed with donanemab treatment are independent of amyloid-related imaging abnormalities–E; significant reductions compared with placebo were still observed when those with amyloid-related imaging abnormalities–E were removed from analysis (eFigure 3C-D and eTable 3 in Supplement 2).
Correlations of Change in Plasma Biomarker Levels With Change in Amyloid PET End Point
The change in plasma pTau217 was positively correlated with the percent change in amyloid plaque (CL units) observed at 76 weeks (R = 0.484 [95% CI, 0.359-0.592]; P < .001) (Figure 3A). Additionally, the change in plasma GFAP was positively correlated with the percent change in amyloid plaque observed at 76 weeks (R = 0.453 [95% CI, 0.306-0.579]; P < .001) (Figure 3B).
Figure 3. Correlations of Change in Plasma Phosphorylated tau217 (pTau217) and Glial Fibrillary Acidic Protein (GFAP) With Change in Amyloid Positron Emission Tomography (PET) End Points.
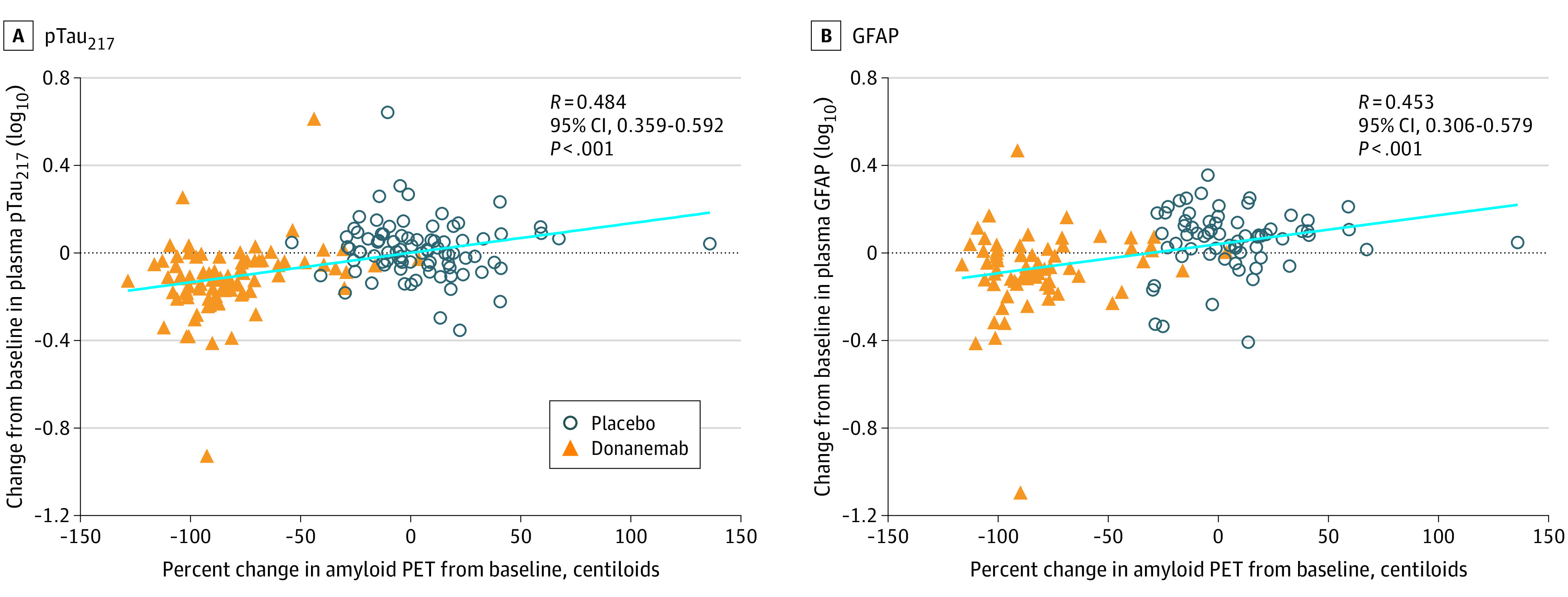
Percent change in amyloid PET levels from baseline to 76 weeks compared with change in plasma levels of pTau217 (placebo n = 85; donanemab n = 84) (A) and GFAP (placebo n = 66; donanemab n = 66) (B) from baseline to 76 weeks. Plasma levels were log10 transformed. Linear regression of all data points, regardless of treatment, is shown in light blue. Spearman rank was used for correlation coefficient.
Correlations of Change in Plasma pTau217 and GFAP Levels With Change in Tau PET Imaging
Regionally, we found significant positive correlations between change in plasma pTau217 and change in frontal tau standardized uptake value ratio (SUVR) (R = 0.243 [95% CI, 0.092-0.383]; P = .002) (Figure 4A) and change in temporal tau SUVR (R = 0.177 [95% CI, 0.023-0.323]; P = .02) (Figure 4B). There were no significant correlations between change in plasma pTau217 and change in parietal tau SUVR (Figure 4C) or occipital tau SUVR (Figure 4D). Globally, there was a significant positive correlation between change in plasma pTau217 and change in tau SUVR (R = 0.170 [95% CI, 0.016-0.317]; P = .03) (Figure 4E). Correlations between change in plasma GFAP and change in tau PET imaging were not statistically significant (eFigure 4 in Supplement 2).
Figure 4. Correlations of Change in Plasma Phosphorylated Tau217 (pTau217) With Change in Tau Positron Emission Tomography (PET) End Points.
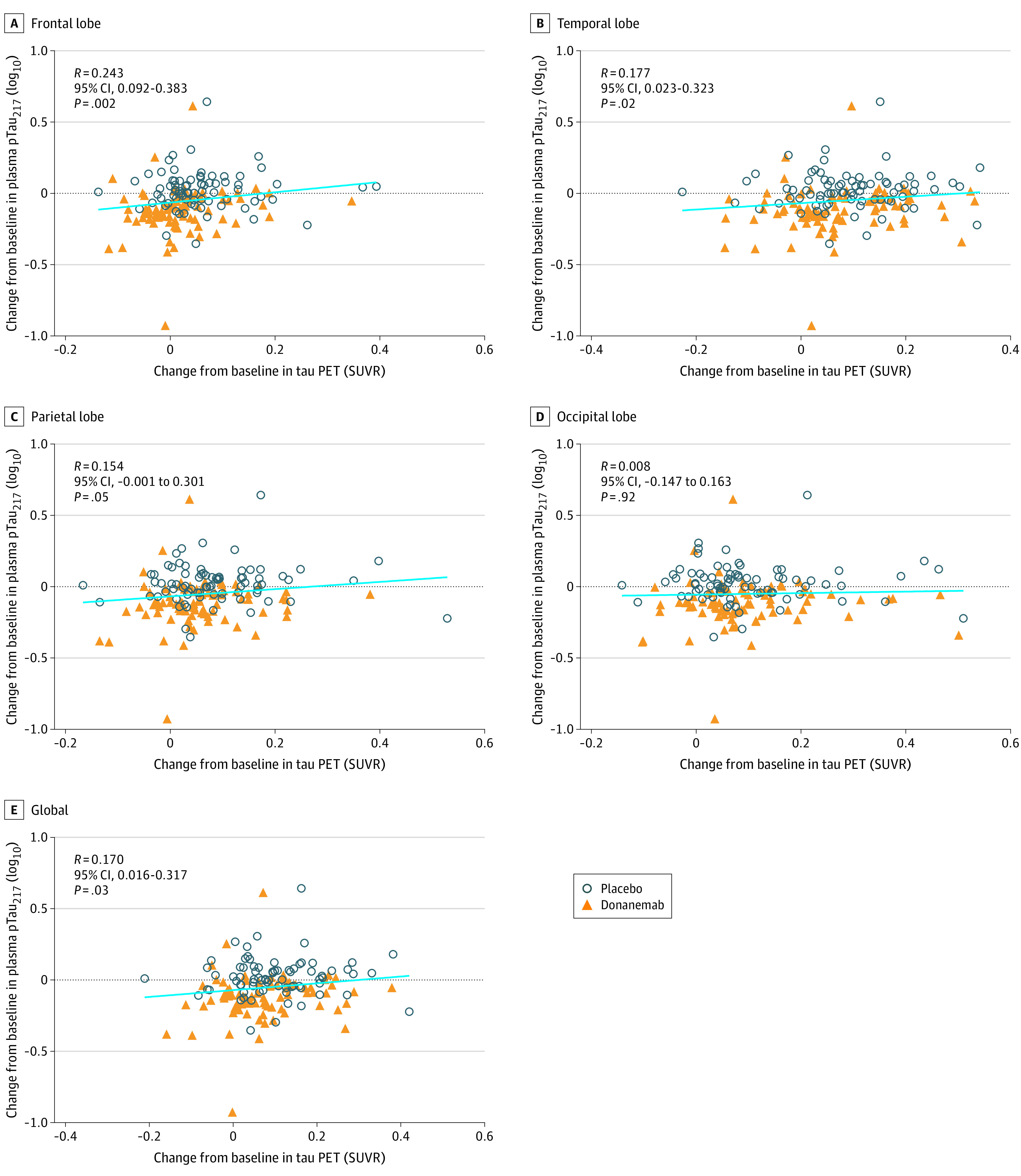
Change in tau PET levels compared with change in plasma pTau217 levels from baseline to 76 weeks in frontal (A), temporal (B), parietal (C), and occipital (D) lobes as well as globally (E). Plasma levels were log10 transformed. Linear regression of all data points, regardless of treatment, is shown in light blue. Spearman rank was used for correlation coefficient. There were 78 individuals in the placebo group and 83 in the donanemab group. SUVR indicates standardized uptake value ratio.
Correlations of Plasma pTau217 Levels With Plasma GFAP Levels
At baseline, plasma levels of pTau217 and GFAP were significantly correlated (R = 0.399 [95% CI, 0.278-0.508]; P < .001) (Figure 5A). The positive correlation at baseline persisted through week 76, the end of the study (R = 0.393 [95% CI, 0.254-0.517]; P < .001) (Figure 5B). Additionally, the changes in plasma levels of pTau217 and GFAP from baseline to 76 weeks were also significantly correlated (R = 0.475 [95% CI, 0.334-0.596]; P < .001) (Figure 5C).
Figure 5. Correlations of Plasma Phosphorylated tau217 (pTau217) Levels With Plasma Glial Fibrillary Acidic Protein (GFAP) Levels.

Plasma GFAP levels compared with plasma pTau217 levels at baseline (placebo n = 103, donanemab n = 104) (A), 76 weeks (placebo n = 78, donanemab n = 83) (B), and change from baseline to 76 weeks (placebo n = 67, donanemab n = 69) (C). Plasma levels were log10 transformed. Linear regression of all data points, regardless of treatment, is shown in light blue. Spearman rank was used for correlation coefficient.
Correlations of Plasma Biomarkers With Volumetric Magnetic Resonance Imaging
There were no significant correlations between baseline pTau217, GFAP, NfL, or Aβ 42/40 with baseline whole brain, ventricular, or hippocampal volumes (eTable 4 in Supplement 2). When looking at change in plasma biomarker vs change in volumetric magnetic resonance imaging (MRI), only NfL showed a significant correlation with change in whole brain volume (R = –0.1710 [95% CI, –0.3205 to –0.0132]; P = .03) (eTable 4 in Supplement 2).
Relationship of Plasma Biomarkers With iADRS
There were no significant correlations between plasma pTau217, GFAP, NfL, or Aβ 42/40 and baseline iADRS (eTable 5 in Supplement 2). Only change in plasma NfL showed a significant correlation with change in iADRS (R = –0.182 [95% CI, –0.322 to –0.035]; P = .02) (eTable 5 in Supplement 2). However, the marginal logistical analysis, classifying individual participants as exhibiting a meaningful worsening (MCID) on iADRS, or not, showed that pTau217 change at 24 weeks was positively associated with the odds of exhibiting an iADRS MCID (coefficient estimate, 0.704 [95% CI, 0.022-1.386]; P = .04) (eTable 6 in Supplement 2). That is, the decline in pTau217 values is associated with lower odds of within-patient change in iADRS consistent with meaningful worsening. The analyses on GFAP did not show any association (eTable 6 in Supplement 2).
Discussion
Exploratory, post hoc analysis of plasma biomarkers from the TRAILBLAZER-ALZ study demonstrated that donanemab treatment reduces plasma levels of pTau217 and GFAP compared with placebo. Further, the changes in plasma pTau217 and GFAP significantly correlate with percent change in amyloid plaque level as measured by amyloid PET imaging. These results are consistent with, and extend, findings of changes in CSF and plasma biomarkers with other anti-Aβ antibodies.20,21,22 They add weight to the hypothesis that reduction of amyloid by anti-Aβ antibodies could have downstream effects consistent with disease modification and suggest that fluid biomarkers could have a role in monitoring response to treatments (at least as a group measure of target engagement/amyloid clearance) in future clinical trials.
Using blood-based biomarkers as a minimally invasive way to assess disease is an emerging topic in AD research.3 In a 2020 report, plasma pTau217 was able to distinguish between AD and other neurodegenerative diseases with similar accuracy to CSF and PET imaging measures.7 Our results showing baseline plasma pTau217 levels are correlated with both amyloid and tau PET end points are in line with previously published results. These associations demonstrating statistical significance in our data are especially striking considering the study enrollment criteria restricted participants to those who had elevated amyloid and intermediate tau level determined by PET, thereby limiting the dynamic range of the data. Our analyses further revealed plasma pTau217 levels were significantly reduced with donanemab treatment compared with placebo, and the reduction persisted to the end of the study even in participants who switched to placebo at 24 weeks after achieving the target level of amyloid reduction.
GFAP is an important regulator of astrocytic function that is associated with activated astrocytes in AD and other inflammatory conditions.23 Previous studies using a similar Simoa-based assay found plasma GFAP levels are positively associated with amyloid PET.9,24 Although we saw plasma GFAP levels decrease with donanemab treatment, we did not see a significant correlation between baseline plasma GFAP levels and baseline amyloid or tau PET end points. However, as with plasma pTau217, changes in plasma GFAP significantly correlated with change in amyloid PET level at 76 weeks. Unlike plasma pTau217, changes in plasma GFAP were not significantly correlated with changes in tau PET signal, but there were correlations between plasma GFAP and plasma pTau217 both at baseline and end point/change from baseline. These results are consistent with studies suggesting GFAP reflects activation of astrocytes in response to amyloid in AD.25
In a head-to-head comparison of different plasma Aβ 42/40 assays, Simoa immunoassays, as used in this study, were less accurate than mass spectrometry-based assays.26 However, even when using a mass spectrometry-based assay, there was not a good correlation between plasma Aβ 42/40 and amyloid PET results within the subset of amyloid-positive participants.27 Therefore, it is not surprising that we did not see significant baseline correlations since our study includes only amyloid PET–positive participants. Further, lack of assay precision could hinder our ability to detect small changes in plasma Aβ 42/40 levels following treatment. While we do see greater normalization of plasma Aβ 42/40 levels with donanemab compared with placebo, significant differences only occurred at 1 time point. Using a more precise assay in future studies may reveal significant drug effects on plasma Aβ 42/40 levels.
Plasma NfL levels continued to increase in both groups with no significant differences in plasma NfL levels between placebo and donanemab treatment arms. One possible explanation for the lack of significant effect of donanemab/amyloid reduction on plasma NfL is that factors unrelated to amyloid/AD may contribute significantly to plasma NfL values. Plasma NfL is a nonspecific marker of neurodegeneration and elevated levels can be attributed to many neurological conditions.28 Plasma NfL levels also increase with age in cognitively normal individuals as well as those with AD.29 Further, plasma NfL levels increase with age, independent of Aβ status, and among individuals with cognitive impairment, NfL was not significantly associated with amyloid PET status but did increase in association with myocardial infarction and hypertension.30 Similarly, longitudinal change was increased in patients with elevated tau or neurodegeneration at baseline, regardless of baseline amyloid status.31 Thus, changes in NfL may reflect the intensity of neuronal injury that may occur independent of Aβ pathology31 or as a downstream effect of Aβ pathology. A second consideration relevant to the present finding is that we do not presently know the time lag between onset of Aβ pathology and downstream neurodegeneration, but it may be significant. Thus, NfL changes over a 76-week trial, such as the present, may reflect the impact of the amyloid pathology that was present at baseline rather than the impact of removing amyloid over the course of the trial.
In contrast to the associations among changes in plasma pTau217 and GFAP with PET, the only significant relationship between plasma biomarkers and MRI measures was a modest correlation between increasing NfL and decreasing whole brain volume. The absence of other correlations may in fact reflect weaknesses in global MRI measures; regional measures, eg, of cortical thickness, might be more sensitive. MRI volume results may also be confounded by decreased amyloid plaque volume.17 The association of MRI volume change with NfL and lack of relationship with the presumed amyloid-driven plasma markers (pTau217, GFAP) again suggests that increases in NfL and atrophy may be sufficiently downstream that the 76-week NfL/MRI result is reflective of amyloid status at the start of the trial, rather than amyloid removal during the trial. Future studies will need to investigate later time points after the initiation of treatment.
PET and plasma biomarker data each provide unique information on insoluble pathology and soluble proteoforms.32 Understanding how the information from each relates to each other is of great importance to the field in how they should be used in the future. The results of the present study align with recent studies supporting the hypothesis that Aβ accumulation facilitates an increase in plasma pTau217, which reflects an ongoing process of tau misfolding, mislocalization, and hyperphosphorylation that are precursors to tau aggregation and deposition in neurofibrillary tangles.33 Increased pTau217 levels have been detected early in disease in cognitively unimpaired, Aβ-positive individuals, even before tau is detectable on PET imaging.5,34 The present results suggest Aβ may be important in facilitating the increase in plasma pTau217 prior to tau aggregation. Thus, we see a relatively modest correlation between plasma pTau217 and amyloid PET at baseline, since all participants were amyloid PET positive as defined by inclusion criteria. Plasma pTau217 levels drop rapidly, by 12 weeks, following donanemab treatment. As Aβ is removed and plasma pTau217 generation is impeded, tau deposition is expected to slow, but deposition from tau fibrils already present may continue for some time after Aβ reduction. Hence, it could be expected that the correlation between change in tau PET and change in plasma pTau217 is weaker than correlation between change in amyloid PET and change in plasma pTau217.
We see plasma GFAP levels drop with similar timing as plasma pTau217 levels and plasma levels of both are significantly correlated with each other at the beginning and end of the study. Therefore, our GFAP results seem to support a similar hypothesis in which plasma GFAP is elevated in response to Aβ accumulation. In AD, amyloid plaques are infiltrated by reactive astrocytes, which have increased expression of GFAP.35 Accordingly, removal of amyloid plaques should reduce the activation state of the astrocytes, leading to decreased plasma GFAP levels with donanemab treatment. We observed a weaker correlation between change in plasma GFAP and change in tau PET compared with the correlation between plasma GFAP and change in amyloid PET, consistent with the hypothesis that elevations of GFAP in AD are more directly related to the presence of pathological amyloid than to presence of aggregated deposited tau, which may be further downstream of Aβ and on a separate parallel pathway from GFAP.
The primary outcome of the TRAILBLAZER-ALZ clinical trial was change from baseline in the iADRS score. As previously reported, donanemab significantly slowed disease progression as measured by the iADRS score.17 We do not see associations between change in iADRS and change in plasma pTau217 or GFAP, except a borderline significant association between plasma pTau217 change at 24 weeks with the marginal odds of exhibiting a meaningful worsening (MCID) on iADRS. The lack of significant associations may be due in part to the small number of participants and the variability of the assays. Systematic factors may contribute to the observed absence of correlation. Specifically, the participants with the highest baseline assay values have the largest room to show reductions, eg, in plasma pTau217 and GFAP, but these same participants also should be expected to have generally higher levels of ongoing pathology (ie, abnormal tau phosphorylation and inflammation) and thus would be expected to have a greater degree of cognitive decline, confounding potential correlation between reduction in plasma pTau217 or GFAP levels and slower cognitive decline. Additionally, in a relatively short trial, rate of pathology reduction/time to clearance may be more important than the amount of change seen at end point. Finally, despite our best efforts, change in cognitive functional scales may not be linearly related to clinical impact; a given amount of change may have greater clinical importance to a patient earlier in disease than a patient later in disease.19 Modeling the relationship between plasma pTau217 and MCID iADRS score showed a modest but statistically significant association between of plasma pTau217 change at 26 weeks and the odds of an individual achieving a minimally clinically important decline. Given that this analysis was performed post hoc, and the result is not corrected for multiplicity, it should be considered only as hypothesis generating. However, it serves as a reminder of the potential complexity in interpreting correlations between biomarker and clinical change. Longer follow-up and larger studies are needed to further explore whether changes in the plasma biomarkers are associated with slowing of cognitive decline.
Limitations
The exploratory, post hoc analyses reported here have several additional limitations. Importantly, the small trial size limits the power of the analyses and further makes it difficult to interpret effects in subgroups, eg, as a function of age, APOE ε4 status, or early stopping vs continuation of donanemab therapy. The conclusions drawn from this data need to be confirmed in a larger trial. Additional analyses such as the impact of race and ethnicity on plasma biomarkers also needs to be addressed in larger trials.
There are caveats to our correlation analyses as well. The biomarker analyses are exploratory and thus not corrected for multiple testing. Correlation coefficients are small as the dynamic range of the data are limited due to inclusion criteria limiting participants to those who had elevated amyloid and intermediate tau levels. Data from the phase 3 study, TRAILBLAZER-ALZ2, of donanemab in early symptomatic AD will be important to confirm the results for all the plasma biomarkers analyzed here with a greater sample size.
Conclusions
In summary, the present study showed plasma biomarker improvement following treatment in exploratory analyses and suggests that amyloid reduction driven by anti-Aβ antibodies influences downstream effects potentially associated with disease modification.
Trial protocol and statistical analysis plan
eMethods
eFigure 1. Participant flow diagram
eFigure 2. Correlations between baseline plasma GFAP, NfL, Aβ 42/40, and baseline amyloid and tau PET endpoints
eFigure 3. Change in plasma levels after discontinuing donanemab treatment
eFigure 4. Correlations of change in plasma GFAP with change in tau PET imaging
eTable 1. Baseline characteristics and biomarker levels
eTable 2. Plasma biomarker change from baseline
eTable 3. Plasma biomarker change from baseline after discontinuing donanemab treatment
eTable 4. Correlations of plasma biomarkers with vMRI
eTable 5. Correlations of plasma biomarkers with iADRS
eTable 6. Effect of plasma biomarker change at weeks 24 on iADRS MCID
eReferences
Data sharing statement
References
- 1.2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327-406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 2.Lloret A, Esteve D, Lloret MA, et al. When does Alzheimer’s disease really start? the role of biomarkers. Int J Mol Sci. 2019;20(22):E5536. doi: 10.3390/ijms20225536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leuzy A, Mattsson-Carlgren N, Palmqvist S, Janelidze S, Dage JL, Hansson O. Blood-based biomarkers for Alzheimer’s disease. EMBO Mol Med. 2022;14(1):e14408. doi: 10.15252/emmm.202114408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66-77. doi: 10.1016/S1474-4422(21)00361-6 [DOI] [PubMed] [Google Scholar]
- 5.Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho-tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021;78(2):149-156. doi: 10.1001/jamaneurol.2020.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattsson-Carlgren N, Janelidze S, Palmqvist S, et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain. 2020;143(11):3234-3241. doi: 10.1093/brain/awaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772-781. doi: 10.1001/jama.2020.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee P, Pedrini S, Stoops E, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry. 2021;11(1):27. doi: 10.1038/s41398-020-01137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira JB, Janelidze S, Smith R, et al. Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease. Brain. 2021;144(11):3505-3516. doi: 10.1093/brain/awab223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashton NJ, Leuzy A, Lim YM, et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun. 2019;7(1):5. doi: 10.1186/s40478-018-0649-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattsson N, Andreasson U, Zetterberg H, Blennow K; Alzheimer’s Disease Neuroimaging Initiative . Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74(5):557-566. doi: 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thijssen EH, Verberk IMW, Vanbrabant J, et al. Highly specific and ultrasensitive plasma test detects Abeta(1-42) and Abeta(1-40) in Alzheimer’s disease. Sci Rep. 2021;11(1):9736-9736. doi: 10.1038/s41598-021-89004-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related β-amyloid status. JAMA Neurol. 2019;76(9):1060-1069. doi: 10.1001/jamaneurol.2019.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janelidze S, Palmqvist S, Leuzy A, et al. Detecting amyloid positivity in early Alzheimer’s disease using combinations of plasma Aβ42/Aβ40 and p-tau. Alzheimers Dement. 2021. doi: 10.1002/alz.052117 [DOI] [PubMed] [Google Scholar]
- 15.Lowe SL, Willis BA, Hawdon A, et al. Donanemab (LY3002813) dose-escalation study in Alzheimer’s disease. Alzheimers Dement (N Y). 2021;7(1):e12112. doi: 10.1002/trc2.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowe SL, Duggan Evans C, Shcherbinin S, et al. Donanemab (LY3002813) phase 1b study in Alzheimer’s disease: rapid and sustained reduction of brain amyloid measured by florbetapir F18 imaging. J Prev Alzheimers Dis. 2021;8(4):414-424. doi: 10.14283/jpad.2021.56 [DOI] [PubMed] [Google Scholar]
- 17.Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704. doi: 10.1056/NEJMoa2100708 [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 19.Wessels AM, Rentz DM, Case M, Lauzon S, Sims JR. Integrated Alzheimer’s Disease Rating Scale: Clinically meaningful change estimates. Alzheimers Dement (N Y). 2022;8(1):e12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):197-210. doi: 10.14283/jpad.2022.30 [DOI] [PubMed] [Google Scholar]
- 21.Ostrowitzki S, Lasser RA, Dorflinger E, et al. ; SCarlet RoAD Investigators . A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther. 2017;9(1):95. doi: 10.1186/s13195-017-0318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salloway S, Honigberg LA, Cho W, et al. Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer’s disease (BLAZE). Alzheimers Res Ther. 2018;10(1):96. doi: 10.1186/s13195-018-0424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Liu X, Liu T, et al. Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia. 2020;68(5):878-897. doi: 10.1002/glia.23734 [DOI] [PubMed] [Google Scholar]
- 24.Benedet AL, Milà-Alomà M, Vrillon A, et al. ; Translational Biomarkers in Aging and Dementia (TRIAD) study, Alzheimer’s and Families (ALFA) study, and BioCogBank Paris Lariboisière cohort . Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 2021;78(12):1471-1483. doi: 10.1001/jamaneurol.2021.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost GR, Li YM. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017;7(12):170228. doi: 10.1098/rsob.170228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janelidze S, Teunissen CE, Zetterberg H, et al. Head-to-head comparison of 8 plasma amyloid-β 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78(11):1375-1382. doi: 10.1001/jamaneurol.2021.3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West T, Kirmess KM, Meyer MR, et al. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener. 2021;16(1):30. doi: 10.1186/s13024-021-00451-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577-589. doi: 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 29.Zhou W, Zhang J, Ye F, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Plasma neurofilament light chain levels in Alzheimer’s disease. Neurosci Lett. 2017;650:60-64. doi: 10.1016/j.neulet.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 30.Syrjanen JA, Campbell MR, Algeciras-Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2022;18(6):1128-1140. doi: 10.1002/alz.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791-799. doi: 10.1001/jamaneurol.2019.0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ossenkoppele R, van der Kant R, Hansson O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 2022;21(8):726-734. doi: 10.1016/S1474-4422(22)00168-5 [DOI] [PubMed] [Google Scholar]
- 33.Wennström M, Janelidze S, Nilsson KPR, et al. ; Netherlands Brain Bank . Cellular localization of p-tau217 in brain and its association with p-tau217 plasma levels. Acta Neuropathol Commun. 2022;10(1):3. doi: 10.1186/s40478-021-01307-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattsson-Carlgren N, Andersson E, Janelidze S, et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Sci Adv. 2020;6(16):eaaz2387. doi: 10.1126/sciadv.aaz2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7-35. doi: 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eMethods
eFigure 1. Participant flow diagram
eFigure 2. Correlations between baseline plasma GFAP, NfL, Aβ 42/40, and baseline amyloid and tau PET endpoints
eFigure 3. Change in plasma levels after discontinuing donanemab treatment
eFigure 4. Correlations of change in plasma GFAP with change in tau PET imaging
eTable 1. Baseline characteristics and biomarker levels
eTable 2. Plasma biomarker change from baseline
eTable 3. Plasma biomarker change from baseline after discontinuing donanemab treatment
eTable 4. Correlations of plasma biomarkers with vMRI
eTable 5. Correlations of plasma biomarkers with iADRS
eTable 6. Effect of plasma biomarker change at weeks 24 on iADRS MCID
eReferences
Data sharing statement


