Abstract
Both in the United States and Europe, the number of minors who present at transgender healthcare services before the onset of puberty is rapidly expanding. Many of those who will have persistent gender dysphoria at the onset of puberty will pursue long-term puberty suppression before reaching the appropriate age to start using gender-affirming hormones. Exposure to pubertal sex steroids is thus significantly deferred in these individuals. Puberty is a critical period for bone development: increasing concentrations of estrogens and androgens (directly or after aromatization to estrogens) promote progressive bone growth and mineralization and induce sexually dimorphic skeletal changes. As a consequence, safety concerns regarding bone development and increased future fracture risk in transgender youth have been raised. We here review published data on bone development in transgender adolescents, focusing in particular on differences in age and pubertal stage at the start of puberty suppression, chosen strategy to block puberty progression, duration of puberty suppression, and the timing of re-evaluation after estradiol or testosterone administration. Results consistently indicate a negative impact of long-term puberty suppression on bone mineral density, especially at the lumbar spine, which is only partially restored after sex steroid administration. Trans girls are more vulnerable than trans boys for compromised bone health. Behavioral health measures that can promote bone mineralization, such as weight-bearing exercise and calcium and vitamin D supplementation, are strongly recommended in transgender youth, during the phase of puberty suppression and thereafter.
Keywords: transgender, adolescents, GnRHa treatment, puberty suppression, gender-affirming hormones, bone, bone mineral density
Introduction
Transgender and gender diverse (TGD) adolescents experience a gender identity that is different from the gender assigned at birth and this often results in severe psychological distress, defined as gender dysphoria (GD). Although the spectrum is broad, most TGD youngsters identify as either trans boys (experiencing a male gender identity although being assigned female at birth, AFAB) or trans girls (experiencing a female gender identity although being assigned male at birth, AMAB). Some TGD adolescents experience this binary concept of gender as inappropriate and identify as non-binary or gender fluid (1, 2).
The prevalence of GD among adolescents is difficult to determine. Official reports from the United States estimate that 0.7% of Americans aged 13–24 years are transgender (3), and in many European countries, after the introduction of pediatric gender identity services, the yearly number of referrals has increased manifold in the last decade (4, 5). Pediatric gender identity services are highly specialized clinics dedicated to young people presenting with uncertainties regarding their gender identity, in order to support and help the youngsters and their families to understand and accept the range of gender identity presentations. However, most of the available data derive from self-report surveys (the adolescent autonomously answers the questions, without any external interference or control), which typically results in higher incidence numbers (4). According to a recent systematic review, the prevalence of transgender identity among children and adolescents is estimated between 1.2 and 2.7% and increases to 2.5–8.4% when a broader concept of gender diversity is investigated (6). Also, in the last decades, a predominance of AFAB seeking medical care has been registered, showing an inversion of the previous trend (7). The age of onset of GD is difficult to estimate as in most studies only the mean age of referral at the gender services is reported, being 13.2 years (s.d. ± 0.9), according to a recent systematic review (8).
During the diagnostic phase, a mental health professional will assess the presence of a precise set of diagnostic criteria, specified in the DSM-V manual (9). The subsequent psychosocial and medical management of this condition requires a multidisciplinary team approach. In many TGD adolescents, the initial bodily changes that characterize the onset of puberty are associated with worsening of GD and decreased global functioning and well-being, and as such, this represents an additional diagnostic landmark. In these adolescents, puberty suppression, once the diagnosis of persistent GD is confirmed, serves multiple goals: it can provide time and mental space for the adolescents to explore their gender identity, start a social transition, and live in the social role of the experienced gender. At the same time, the development of secondary sex characteristics is halted. Thus, many TGD adolescents who will seek further transition in the future will not need specific surgeries to reverse physical characteristics they are deeply uncomfortable with (e.g. mammectomy for AFAB or voice feminization surgery for AMAB). Importantly, most of the effects of puberty-suppressing agents are reversible, with sex steroid production and pubertal development resuming once the medication is discontinued.
Several strategies are available to suppress pubertal development and/or the effects of endogenous sex steroids in the context of TGD youth healthcare. Current guidelines recommend the use of GnRH analogs (GnRHa) to suppress puberty and prevent the development of secondary sexual characteristics. At ages 15–16 years, gender-affirming hormones (GAH) are usually added (non-binary adolescents often hesitate whether to start GAH or not) (10). For TGD adolescents presenting at later pubertal stages, anti-androgens (for AMAB, e.g. cyproterone acetate 12.5-25 mg/day) or high dose progestins (for AFAB, e.g. lynestrenol 5-10 mg/day) are sometimes used instead of GnRHa (11, 12), with the choice of the treatment depending on the center and country-specific reimbursement regulations (13, 14).
The increasing number of pre-pubertal children presenting with GD and seeking medical care from the earliest phase of puberty onward has raised questions about the safety of such a prolonged period of unopposed puberty suppression. Puberty occurring at an appropriate time may represent a unique window of opportunity for certain sexually dimorphic traits to develop. Especially pubertal bone mass accrual and adult bone health require further investigation in this context. Some studies in men who had constitutionally delayed puberty suggest that this condition resulted in reduced bone mineralization in adulthood (15). Other studies could not confirm these data (16, 17).
In this review, we will first discuss how sex hormones induce sexually dimorphic pubertal bone mass acquisition. Next, we will review current knowledge on bone development in TGD adolescents who receive puberty-suppressing medication, focusing particularly on data that describe bone health outcomes when puberty suppression is started from the earliest stages of puberty onward.
Effects of sex steroids on bone mass acquisition during puberty
Approximately 95% of the skeletal bone and muscle mass is acquired before the age of 18 years. Thus, childhood and puberty represent an essential time frame to build a strong musculoskeletal system (18). Whereas skeletal growth is heavily influenced by the genetic background, several other factors influence the bone structure and quality (e.g. chronic systemic illnesses, muscular disorders, metabolic disorders, and some medications). Also, a healthy lifestyle, with an adequate calcium intake and regular physical activity, plays an important role.
Bone mass accrual is not constant during growth. After a rapid expansion phase in the first 2 years of life, a more steady rate of bone mass acquisition characterizes childhood, followed by a sharp acceleration from the onset of puberty onward, up to early adulthood. During puberty, the growth hormone (GH)/insulin-like growth factor (IGF)-1 axis and sex steroids become the main determinants of bone development, acting on bone both through direct and indirect effects, such as stimulation of muscle mass development. As a result, bone mass doubles and important changes occur in bone geometry and longitudinal growth (19). While bone features do not show significant sex (In this review, we use the term ‘sex’ to refer to biological aspects of male or female development, whereas we use the term ‘gender’ to refer to an individual’s self-reported sense of gender.) differences before puberty, skeletal sexual dimorphism becomes gradually apparent during puberty and, at the end of growth, males display a greater cross-sectional bone area and are on average taller than females. Thus, sex differences in bone parameters are likely regulated by pubertal sex steroids. Although males will reach a higher peak bone mass, a greater bone size, and a stronger skeleton as compared to females (20, 21), the volumetric bone mineral density (vBMD) will not differ between males and females, as bone mineral acquisition in long bones occurs in proportion to the volume of the bone (22). In both sexes, the increase in longitudinal growth during the growth spurt is faster than the mineralization process, resulting in a physiological decrease in lumbar and femoral BMD around mid-puberty, with subsequent temporary increased risk of fractures (23). A higher prevalence of fractures has been reported in males (24).
While serum levels of sex hormones and IGF-1 progressively increase during successive pubertal stages, the concomitant skeletal growth is not linear. Both androgens and estrogens contribute to bone size expansion and mineralization in both sexes, with the first process mainly driven by androgens and the second by estrogens (25). However, since in males, part of the androgens are aromatized into estrogens, the relative contribution of androgens and estrogens to bone expansion is difficult to disentangle and still not completely understood, as will be discussed in more detail later. Furthermore, an important part of the sex steroid effects on bone mass acquisition occurs through interaction with GH/IGF-1 signaling, and also this interaction is complex and still not fully understood (26). Also, the timing of puberty seems to be relevant in this context. Finkelstein et al. reported a significantly lower lumbar and radial areal BMD (aBMD) in men who had a history of pubertal delay compared to men who went through puberty at a physiological age (15). Yap et al. confirmed this finding but reported an adult vBMD within age- and sex-specific references, both at the lumbar spine (LS) and the femoral neck (FN). However, this was associated with reduced limb bone mass and size, suggesting an impaired periosteal expansion during puberty in men with pubertal delay (27). Even lower BMD values were observed in males with congenital hypogonadotropic hypogonadism (28). A reduced peak bone mass was also reported in females with delayed puberty and amenorrhea (29, 30). The importance of appropriate timing of puberty has been emphasized by Gilsanz et al., who showed that healthy girls starting puberty a year earlier had approximately 5% greater bone mineral content and 2.5% greater BMD values at skeletal maturity than girls who had started puberty a year later. Similar findings, although slightly less pronounced, were observed in healthy boys (31).
Women with complete androgen insensitivity syndrome have been studied to better understand androgen actions on bone. These studies show that aBMD and vBMD are reduced in comparison with both female and male reference values, supporting a relevant direct effect of androgens on bone (32, 33). Reduced BMD in men affected by prostatic cancers and treated with androgen receptor antagonists further supports the idea of direct androgen action on the bone (34, 35, 36, 37).
A cross-sectional study performed in 199 healthy boys aged 6–19 years confirmed that estrogens are positively associated with bone mineralization (estimated by both aBMD and vBMD) while being negatively associated with endosteal circumference. On the other hand, testosterone promotes the increase of bone area (both at trabecular and at cortical sites) and periosteal expansion (38).
Bone expansion happens through longitudinal growth at the epiphyseal growth plates (mediated by chondrocytes) and appositional growth at the periosteal surface (mediated by osteoblasts). Concomitantly, the resorption at the endosteal surface (mediated by osteoclasts) leads to the expansion of the marrow cavity. Sex hormones are important regulators of all three of these processes (26).
Early preclinical studies reported that sex steroid deficiency, induced by ovariectomy or orchidectomy, increases vs decreases radial bone growth in female and male rodents, respectively (39). Therefore, androgens have traditionally been considered to promote and estrogens to inhibit periosteal apposition. In line, higher serum levels of testosterone have been associated with a larger periosteal circumference in the long bones of young adult men (40). However, this traditional view has been challenged by the clinical case of a boy with aromatase deficiency. In this patient, estrogen treatment significantly increased cross-sectional bone area (41), implying that both androgens and estrogens contribute to radial bone expansion in males. In females, the situation is even more complex, with the effect of sex hormones on periosteal apposition depending on pubertal stage and crosstalk with the GH/IGF-1 axis (42). Importantly, this crosstalk plays an important role in determining sex differences in bone mass acquisition, as skeletal sexual dimorphism fully disappears in mice with a constitutional deletion of the GH receptor and low circulating IGF-1 (43).
At the end of growth, the greater final bone strength in boys will rely mainly on the achievement of a greater cortical diameter due to more periosteal apposition, while the cortical thickness will be only slightly bigger, because of the parallel greater expansion of the medullary cavity and subsequent greater endocortical diameter (44). Of note, the earlier idea that girls have more endosteal apposition than boys might be erroneous. Indeed, the current consensus is that the medullary cavity enlarges during puberty in both sexes as a result of dominant endosteal resorption, but less so in females, due to the inhibitory effect of estrogens on osteoclast activity (45).
Apart from influencing radial bone expansion, sex steroids are also critical regulators of longitudinal growth. In particular, the current consensus is that estrogens regulate this process in a biphasic mode. During early puberty when estrogen levels are low, they promote linear growth in both sexes by interacting with the GH/IGF-1 axis (26). In contrast, during late puberty when estrogen levels are high, they block longitudinal expansion in both sexes, mainly through direct inhibition of the chondrocytes at the level of the growth plate (46) although more recent work suggests that central estrogen signaling might also be involved (47). The essential role of estrogens in male longitudinal growth is further illustrated by the final tall stature of males with estrogen deficiency due to estrogen receptor defects or aromatase deficiency (48). Of note, androgens also modulate linear growth, in part through GH/IGF-1 stimulation by adrenal androgens (26).
Trabecular bone volume at the end of puberty is higher in males, at least in the appendicular skeleton. This is mainly due to a greater trabecular thickness, although some studies also report a higher trabecular number (26). Rodent studies have shown that both androgens and estrogens promote trabecular bone development during growth in both sexes (42). In fact, trabecular bone is the compartment showing the highest sensitivity to circulating sex steroids (43, 49).
In conclusion, sex hormones interact with the GH/IGF-1 axis to regulate different steps in the process of bone growth and bone mass acquisition in boys and girls. These combined actions result in an important skeletal sexual dimorphism at the end of puberty, with men having longer and wider bones compared to women, mostly due to sex differences in periosteal apposition and endosteal resorption, but with similar bone mineralization in both sexes (Fig. 1).
Figure 1.
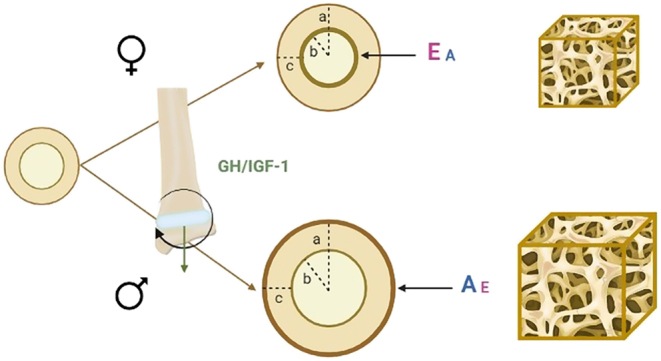
Skeletal sexual dimorphism at the end of puberty. Before puberty, male and female bone geometry is similar. The GH/IGF-1 axis, active from infancy to adulthood, stimulates mainly longitudinal growth at the level of the epiphyseal plates. At puberty, sex hormones play a key role in skeletal growth. Both estrogens (E) and androgens (A) contribute to bone size expansion and mineralization in both sexes, although estrogens action is predominant in females and androgens action in males. In females, endosteal resorption occurs at a lower rate; in males, periosteal apposition is more pronounced, with consequent achievement of a bigger cortical diameter (a). Because of the concomitant greater endosteal resorption, the endocortical diameter (b) is also greater in males than in females. Consequently, cortical thickness (c) is similar in both sexes. At the end of growth, males will have bigger bones (longer and wider) than females, although the volumetric bone mineral density will not be significantly different. Trabecular bone volume, regulated by both estrogens and androgens, will be greater in males, mainly due to a greater trabecular thickness.
Thus, based on the aforementioned impact of sex steroids and pubertal timing on bone development, bone health in transgender adolescents has been questioned in several studies.
Gender-affirming treatment and bone health
The effects of puberty suppression and GAH on bone mineralization and growth are not fully understood. Current guidelines for the treatment of TGD youth highlight the importance of monitoring BMD, at baseline and every 1–2 years during puberty suppression and GAH, until the age of 25–30 years or until attainment of peak bone mass (10). Also the International Society of Clinical Densitometry (ISCD) recommends to perform dual-energy X-ray absorptiometry (DXA) at baseline in all transgender individuals who use treatments that lower their endogenous sex steroid levels, irrespective of age. Particular attention should be given to those who have co-morbidities impairing bone mineralization (e.g. chronic corticosteroid therapy, hyperparathyroidism, chronic illness, etc.) and to individuals who do not intend to initiate GAH or have suboptimal compliance for GAH (50). Interestingly, the ISCD recommends expression of BMD as T-scores, for all transgender adults, using normative ranges for a Caucasian female healthy population and using a cut-off of <−2.5 s.d. for the diagnosis of osteoporosis. This derives from the observation that BMD in transgender women is closer to female than male values, while for transgender men the evidence is more contradictory, and also because some manufacturers use outdated reference values for men (51). Therefore, the use of female reference values for all transgender adults would reduce heterogeneity. Nonetheless, experts rely on local autonomy in the choice of the database to use. In addition, T-scores should always be interpreted in the light of personal and family medical history and the presence of other risk factors, considering that the particular hormonal milieu of transgender people differs from both cis-females and cis-males (52). In TGD youth, the use of Z-scores is recommended, based on comparison with a normative population matched for age and for the experienced gender in trans adolescents, and for the sex assigned at birth in non-binary youth. A Z-score of ≤−2.0 is ‘below the expected range for age’. The ISCD argues that in particular for adolescents who had puberty suppression from an early Tanner stage onward, matching with the sex assigned at birth could result in non-reliable Z-scores, as these individuals did not acquire the characteristic changes in bone mass and bone geometry that are typical for pubertal development in line with the chromosomal sex. Currently, however, most studies on bone health in TGD youth have reported BMD Z-scores for the sex assigned at birth.
Although DXA is considered the golden standard technique for the evaluation of BMD, it has the limitations of being a 2D technique, thus providing an areal density that can result in an underestimation of BMD in short bones. To overcome this limitation, the bone mineral apparent density (BMAD) can be calculated and represents an estimated volumetric BMD; however, it is not commonly used in clinical practice (53, 54).
Another relevant limitation of DXA is the inability to differentiate the trabecular bone compartment from the cortical one; however, it is known that vertebrae are mainly made by trabecular bone while the hip rather represents the cortical bone compartment. As a consequence, measurements performed at the LS spine are used to estimate trabecular bone density while measurements performed at the hip (total hip or femoral neck depending on the study) mainly reflect cortical bone density.
Prior to start of any treatment and irrespective of age, lower aBMD Z-scores at lumbar spine (aBMD-LS) and femoral neck or total hip (aBMD-FN/TH) have been reported in trans girls as compared to trans boys in almost all studies performed to date. Moreover, the mean aBMD Z-score (for AMAB) in trans girls at the start of treatment is consistently reported to be below 0 (range −0.84 to −0.33 for aBMD-LS and −0.81 to −0.43 for aBMD at the hip) (55, 56, 57, 58). Similar findings have been reported in adult trans women (59). In the absence of studies that provide mechanistic insight, one can only speculate that trans girls may be less physically active and may have less exposure to sunlight (many trans adolescents prefer to wear body-covering clothes) as compared to their cis-peers. Other involved factors could be unhealthy food behaviors and low intake of calcium, but a genetic predisposition or the need for specific reference values for transgender people cannot be excluded and remain open questions. Adequate intake of calcium with the diet (at least 1000 mg per day), vitamin D supplementation, an active lifestyle, and weight-bearing physical activity is advised for all TGD youth (10).
Several studies indicate that, during GnRHa treatment for a mean period of 1.5 years (range 0.97–2.5 years), Z-scores at both LS and hip further decline in trans girls (55, 56, 57, 60). However, only very few studies provide data on TGD youth who start GnRHa at the beginning of puberty (Tanner II–early III) and/or use GnRHa as monotherapy for a duration of more than 2 years. These studies show that the decrease in BMD does not stop after the first year of treatment but progresses as long as the puberty suppression continues, although the further decline after the second year of treatment is not always significant and stabilization of BMD Z-scores may occur at a certain point (57, 60, 61). In particular, the study by Vlot and colleagues divides the study population into young (bone age below 15 years) and older (bone age above 15 years) trans girls. After GnRHa administration for a mean duration of 2.5 years, a significant decrease in BMAD-LS Z-scores was registered only in the group of ‘young trans girls’, but this was not associated with a significant decrease in BMAD-TH Z-scores (61).
As an alternative to GnRHa, anti-androgens such as cyproterone acetate can be administered to trans girls to decrease androgen production and its effects. The impact of these drugs on bone health has also been investigated, showing a significant decrease in aBMD Z-scores at the hip and LS, similar to GnRHa. Hence, it is hypothesized that cyproterone acetate impairs pubertal periosteal bone expansion and trabecular bone acquisition to the same extent as GnRHa (58).
Some studies have investigated the potential of estrogens administered following puberty suppression, to restore bone mineralization Although an increasing trend in aBMD or BMAD Z-scores after the first 2–3 years of estrogen therapy has been observed, the increase is not always significant and Z-scores (for the sex assigned at birth) often remain below baseline values (57, 61, 62). However, it can be argued that this time frame of GAH administration is too short to elicit a maximal promoting effect on bone mineralization, especially considering that GAHs are administered in TGD youth generally starting with low doses, and trans girls will only receive an adult estradiol dose of 2-4 mg/day after at least 1-2 years. Nevertheless, this hypothesis is not supported by the study of Klink et al., who evaluated aBMD at the age of 22 (mean duration of GAH in trans girls 5.8 years, following 1.3 years of puberty suppression with GnRHa). The authors showed aBMD-LS Z-scores after GAH were still well below the values at baseline, while the aBMD-FN Z-scores were almost restored (56).
In trans boys, aBMD/BMAD Z-scores at LS and hip assessed at baseline seem to be usually within the age-specific reference range (for sex assigned at birth), around 0 (from −0.40 to +0.38 for the LS and from −0.86 to +0.93 for the hip) (55, 56, 57, 58, 60, 63). As in trans girls, also in trans boys, GnRHa administration for a mean period of 1.2 years (range 0.67–1.8) results in a significant decrease of aBMD-LS and hip Z-scores (55, 56, 57, 60, 63). In line with what has been reported in trans girls, very few studies have included trans boys starting puberty suppression at an early Tanner stage and/or receiving GnRHa for more than 2 years. Also in trans boys, a more reduced aBMD/BMAD is seen in association with prolonged puberty suppression, with a similar or even greater decrease in Z-scores than in trans girls (57, 60, 61, 63). For example in the study by Vlot et al., a significant decrease in BMAD-LS and TH Z-scores is seen in the end-pubertal trans boys during GnRHa treatment but not in the group of end-pubertal trans girls, for whom Z-scores remained stable (although lower than in trans boys) (61).
Different from trans girls, the use of progestins in trans boys (e.g. lynestrenol) as an alternative treatment to GnRHa does not impact bone health (58). If confirmed by future independent studies, lynestrenol could represent a good alternative to GnRHa in trans boys starting the transition at late pubertal stages, especially when risk factors for bone health impairment and/or low BMD at baseline are present.
Testosterone administration significantly increases aBMD/BMAD Z-scores after at least 2 years of treatment, although in most cases the baseline values are not restored (56, 57, 61, 63), with the exception of the study by Schagen et al., in which the early-pubertal trans boys who had long-term GnRHa followed by 3 years of GAH had improved aBMD/BMAD-LS and FN as compared to the baseline (57).
A detailed overview of the cited studies is offered in Table 1 and Supplementary Table 1 (see section on supplementary materials given at the end of this article). These studies investigated mainly the decrease of BMD; however, it is important to keep in mind that a reduced BMD in the absence of a history of fractures occurring without a major trauma is insufficient to diagnose osteoporosis in the pediatric population (64). Moreover, as mentioned, bone strength relies also on bone geometry. Therefore, long-term follow-up studies are needed to assess the risk of fractures. To date, only studies performed in adults can offer a few insights into the fracture risk among transgender individuals. However, the fracture risk can differ between transgender persons starting the transition as adults and those who start medical treatments already in adolescence. Dobrolinska et al. reported a significant decrease in BMD-TH (but not in BMD-LS) for both trans men and trans women after 15 years of GAH (65). On the other hand, Wiepjes and colleagues showed an increase in BMD-LS Z-score by +0.22 in trans women and +0.34 in trans men after 10 years of GAH (66). The occurrence of low BMD in adult trans women has been associated with low compliance for estrogen therapy and consequently low serum estradiol levels (67).
Table 1.
Overview of studies evaluating the effect of puberty suppression on bone mineral density in TGD youth.
| Study | Number of participants | Medication used | Duration of treatment | Age (years) at the start of PS | Tanner stage at the start of PS | Outcome (Z-scores) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Start PS | After PS | Delta | P value | ||||||
| Carmichael et al. (62) | 19 FtM 25 MtF (outcome analyzed as one group) |
Triptorelin: 3.75 mg/4 wk i.m. | 2.58 years (1.67–3.5) | 13.6 (12.8–14.6) | Tanner 3–5 | aBMD-LS | −0.5 (−1.1, 0.0) | −1.5 (−2.1, −0.8) | −1 | N.A. |
| aBMD-TH | −0.5 (−1.1, 0.1) | −1.4 (−2.0, −0.9) | −0.9 | N.A. | ||||||
| Joseph et al. (60) | 39 FtM | GnRHa (scheme N.A.) | 1 year | 12.9 (3.0) | 95% post-menarchal | aBMD-LS | −0.40 ± 1.43 | −1.28 ± 1.41 | −0.88 | 0.000 |
| BMAD-LS | −0.19 ± 1.23 | −0.54 ± 1.40 | −0.35 | 0.006 | ||||||
| aBMD-TH | −0.86 ± 1.22 | −1.44 ± 1.08 | −0.58 | 0.000 | ||||||
| 31 MtF | 13.0 (1.1) | 57% Tanner 2–3 43% Tanner 4–5 |
aBMD-LS | −0.02 ± 1.11 | −0.46 ± 1.12 | −0.44 | 0.003 | |||
| BMAD-LS | 0.86 ± 0.15 | −0.23 ± 1.03 | −1.09 | 0.000 | ||||||
| aBMD-TH | 0.16 ± 0.91 | −0.34 ± 0.82 | −0.5 | 0.002 | ||||||
| Navabi et al. (55) | 119 FtM | Leuprolide acetate: 3 × 7.5 mg/4 wk i.m., then 11.25 mg/12 wk i.m. | 355.2 ± 96.7 days | 15.2 ± 1.8 | 90.7% Tanner 4–5 |
aBMD-LS | 0.04 ± 1.10 | −0.72 ± 0.97 | −0.76 | <0.001 |
| BMAD-LS | −0.10 ± 1.00 | −0.76 ± 0.93 | −0.66 | <0.001 | ||||||
| aBMD-TH | 0.10 ± 1.06 | −0.31 ± 0.99 | −0.41 | <0.001 | ||||||
| 51 MtF | 15.4 ± 2.0 | 80.3% Tanner 4–5 |
aBMD-LS | −0.84 ± 1.29 | −1.33 ± 1.39 | −0.49 | <0.001 | |||
| BMAD-LS | −0.22 ± 1.41 | −0.76 ± 1.48 | −0.54 | N.S. | ||||||
| aBMD-TH | −0.44 ± 1.39 | −1.03 ± 1.64 | −0.59 | <0.001 | ||||||
| Tack et al. (58) | 44 FtM | Lynestrenol 5–10 mg/day | 11.6 (4–40) months | 16.2 ± 1.05 | Tanner 4–5 | aBMD-LS | −0.32 ± 1.09 | −0.32 ± 1.09 | 0 | 0.938 |
| aBMD-TH | −0.16 ± 0.89 | −0.02 ± 0.89 | −0.14 | <0.001 | ||||||
| 21 MtF | Cyproterone acetate 50 mg/day | 10.6 (5–31) months | 16.3 ± 1.21 | Tanner 4–5 | aBMD-LS | −0.77 ± 1.08 | −1.15 ± 0.94 | −0.38 | 0.002 | |
| aBMD-TH | −0.81 ± 0.99 | -1.01 ± 0.98 | −0.2 | 0.006 | ||||||
Values are expressed as mean ± s.d.; median (IQR) or median (range). Z-scores are calculated using a normative population matched for age and sex assigned at birth.
aBMD, areal bone mineral density; BMAD, apparent bone mineral density; FN, femoral neck; LS, lumbar spine; N:A., not available; N.S., not significant; PS, puberty suppression; s.c., subcutaneously; TH, total hip.
No differences in bone geometry have been found in both trans men and trans women after GAH of variable duration, while the trabecular bone score (an index of trabecular microarchitecture) was increased in trans women and decreased in trans men, supporting the promoting role of estrogens on trabecular bone (68).
The effect of 18 years of testosterone administration was evaluated by Broulik et al. in 35 trans men, with BMD at the hip statistically higher than female values and equal to male values. The T-score at the lumbar spine was not significantly different from both female and male controls matched by age (69). No such long-term outcome studies have been reported so far in individuals who started GAH during puberty.
In the light of the presented literature data, we believe that long-term follow-up studies in TDG youth are needed to fully understand the effects of GAT on bone growth and mineralization, assessing the changes in bone parameters after the peak bone mass is reached and GAHs have been taken at an adult dose for several years. In doing so, it is of particular importance in our view to discriminate between puberty suppression started in (almost) puberty-naïve children, as compared to adolescents who had endogenous sex steroids acting on bone prior to puberty suppression. In the meanwhile, and in order to gain further mechanistic insights, the use of specific animal models could support the expansion of this research field (49).
Conclusions
Puberty represents a window of opportunity to build a strong skeleton. In TGD youth, the particular hormonal milieu and the altered timing of puberty can have a negative impact on bone growth and mineralization. To date, available literature data suggest to monitor BMD in order to protect bone health in all TGD adolescents undergoing puberty suppression for several years. In particular, trans girls present with BMD Z-scores below zero already at the start of gender transition and have a higher risk for impaired bone mass accrual. A calcium-rich diet, physical activity,, and weight-bearing exercise are encouraged for all TGD adolescents, and particular attention should be paid to those adolescents who have other risk factors for bone fragility or an unhealthy lifestyle.
After the start of GAH, bone mineral density increases, although the negative effect of prolonged puberty suppression is not always fully restored. In this respect, the recently proposed induction of puberty at a younger age, e.g. at the age of 15 years (10), in those adolescents who are mentally ready for it, and who have clearly persistent GD, could reduce the gap between BMD Z-scores at baseline and BMD Z-scores at the end of the growth.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
S C and M C are supported by a grant from the Flanders Research Foundation (FWO grant G044119N). This publication has been supported by Endo-ERN, which is co-funded by the European Union’s 3rd Health Programme (CHAFEA Framework Partnership Agreement No 739527).
Author contribution statement
S C: Study design, data analysis, manuscript drafting, manuscript review and approval of the final version. V D: Data analysis, manuscript drafting, manuscript review and approval of the final version. M C: Funding, study design, data analysis, manuscript drafting, manuscript review and approval of the final version, overall coordination.
References
- 1.Rosenthal SM.Challenges in the care of transgender and gender-diverse youth: an endocrinologist’s view. Nature Reviews: Endocrinology 202117581–591. ( 10.1038/s41574-021-00535-9) [DOI] [PubMed] [Google Scholar]
- 2.Chew D, Tollit MA, Poulakis Z, Zwickl S, Cheung AS, Pang KC. Youths with a non-binary gender identity: a review of their sociodemographic and clinical profile. Lancet: Child and Adolescent Health 20204322–330. ( 10.1016/S2352-4642(1930403-1) [DOI] [PubMed] [Google Scholar]
- 3.Herman JL, Flores AR, Brown TNT, Wilson BDM, Conron KJ. Age of Individuals Who Identify as Transgender in the United States. Los Angeles,CA, USA: The Williams; Institute, 2017. [Google Scholar]
- 4.Kaltiala-Heino R, Lindberg N. Gender identities in adolescent population: methodological issues and prevalence across age groups. European Psychiatry 20195561–66. ( 10.1016/j.eurpsy.2018.09.003) [DOI] [PubMed] [Google Scholar]
- 5.Butler G, De Graaf N, Wren B, Carmichael P. Assessment and support of children and adolescents with gender dysphoria. Archives of Disease in Childhood 2018103631–636. ( 10.1136/archdischild-2018-314992) [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Goodman M, Adams N, Corneil T, Hashemi L, Kreukels B, Motmans J, Snyder R, Coleman E. Epidemiological considerations in transgender health: a systematic review with focus on higher quality data. International Journal of Transgender Health 202021125–137. ( 10.1080/26895269.2020.1753136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiniara LN, Bonifacio HJ, Palmert MR. Characteristics of adolescents referred to a gender clinic: are youth seen now different from those in initial reports? Hormone Research in Paediatrics 201889434–441. ( 10.1159/000489608) [DOI] [PubMed] [Google Scholar]
- 8.Thompson L, Sarovic D, Wilson P, Sämfjord A, Gillberg C. A PRISMA systematic review of adolescent gender dysphoria literature: 1) epidemiology. PLoS Global Public Health 20222 e0000245. ( 10.1371/journal.pgph.0000245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Association American Psychiatry. Diagnostic and Statistical Manual of Mental Disorders. Association American Psychiatry, 2013. [Google Scholar]
- 10.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GG. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 20171023869–3903. ( 10.1210/jc.2017-01658) [DOI] [PubMed] [Google Scholar]
- 11.Tack LJ, Craen M, Dhondt K, Vanden Bossche H, Laridaen J, Cools M. Consecutive lynestrenol and cross-sex hormone treatment in biological female adolescents with gender dysphoria: a retrospective analysis. Biology of Sex Differences 20167 14. ( 10.1186/s13293-016-0067-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tack LJW, Heyse R, Craen M, Dhondt K, Bossche HV, Laridaen J, Cools M. Consecutive cyproterone acetate and estradiol treatment in late-pubertal transgender female adolescents. Journal of Sexual Medicine 201714747–757. ( 10.1016/j.jsxm.2017.03.251) [DOI] [PubMed] [Google Scholar]
- 13.T’Sjoen G, Arcelus J, Gooren L, Klink DT, Tangpricha V. Endocrinology of transgender medicine. Endocrine Reviews 20194097–117. ( 10.1210/er.2018-00011) [DOI] [PubMed] [Google Scholar]
- 14.Tangpricha V, den Heijer M. Oestrogen and anti-androgen therapy for transgender women. Lancet: Diabetes and Endocrinology 20175291–300. ( 10.1016/S2213-8587(1630319-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelstein JS, Klibanski A, Neer RM. A longitudinal evaluation of bone mineral density in adult men with histories of delayed puberty. Journal of Clinical Endocrinology and Metabolism 1996811152–1155. ( 10.1210/jcem.81.3.8772591) [DOI] [PubMed] [Google Scholar]
- 16.Bertelloni S, Baroncelli GI, Ferdeghini M, Perri G, Saggese G. Normal volumetric bone mineral density and bone turnover in young men with histories of constitutional delay of puberty. Journal of Clinical Endocrinology and Metabolism 1998834280–4283. ( 10.1210/jcem.83.12.5348) [DOI] [PubMed] [Google Scholar]
- 17.Darelid A, Ohlsson C, Nilsson M, Kindblom JM, Mellstrom D, Lorentzon M. Catch up in bone acquisition in young adult men with late normal puberty. Journal of Bone and Mineral Research 2012272198–2207. ( 10.1002/jbmr.1675) [DOI] [PubMed] [Google Scholar]
- 18.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. Journal of Bone and Mineral Research 2011261729–1739. ( 10.1002/jbmr.412) [DOI] [PubMed] [Google Scholar]
- 19.Saggese G, Baroncelli GI, Bertelloni S. Puberty and bone development. Best Practice and Research: Clinical Endocrinology and Metabolism 20021653–64. ( 10.1053/beem.2001.0180) [DOI] [PubMed] [Google Scholar]
- 20.Garn S.The Earlier Gain and Later Loss of Cortical Bone, in Nutritional Perspective. Springfield, IL, USA: Charles C.Thomas, 1970. [Google Scholar]
- 21.Seeman E.Pathogenesis of bone fragility in women and men. Lancet 20023591841–1850. ( 10.1016/S0140-6736(0208706-8) [DOI] [PubMed] [Google Scholar]
- 22.Zamberlan N, Radetti G, Paganini C, Gatti D, Rossini M, Braga V, Adami S. Evaluation of cortical thickness and bone density by roentgen microdensitometry in growing males and females. European Journal of Pediatrics 1996155377–382. ( 10.1007/BF01955265) [DOI] [PubMed] [Google Scholar]
- 23.Alffram P-A, Bauer GCH. Epidemiology of fractures of the forearm: a biomechanical investigation of bone strength. Bone and Joint Surgery 196244-A105–114. [PubMed] [Google Scholar]
- 24.Bailey DA, Wedge JH, McCulloch RG, Martin AD, Bernhardson SC. Epidemiology of fractures of the distal end of the radius in children as associated with growth. Journal of Bone and Joint Surgery: American Volume 1989711225–1231. ( 10.2106/00004623-198971080-00016) [DOI] [PubMed] [Google Scholar]
- 25.Banica T, Vandewalle S, Zmierczak HG, Goemaere S, De Buyser S, Fiers T, Kaufman JM, De Schepper J, Lapauw B. The relationship between circulating hormone levels, bone turnover markers and skeletal development in healthy boys differs according to maturation stage. Bone 2022158 116368. ( 10.1016/j.bone.2022.116368) [DOI] [PubMed] [Google Scholar]
- 26.Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and androgens in skeletal physiology and pathophysiology. Physiological Reviews 201797135–187. ( 10.1152/physrev.00033.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yap F, Hogler W, Briody J, Moore B, Howman-Giles R, Cowell CT. The skeletal phenotype of men with previous constitutional delay of puberty. Journal of Clinical Endocrinology and Metabolism 2004894306–4311. ( 10.1210/jc.2004-0046) [DOI] [PubMed] [Google Scholar]
- 28.Varimo T, Miettinen PJ, Laine T, Salonen P, Tenhola S, Voutilainen R, Huopio H, Hero M, Raivio T. Bone structure assessed with pQCT in prepubertal males with delayed puberty or congenital hypogonadotropic hypogonadism. Clinical Endocrinology 202195107–116. ( 10.1111/cen.14466) [DOI] [PubMed] [Google Scholar]
- 29.Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. Journal of Clinical Investigation 199493799–808. ( 10.1172/JCI117034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hergenroeder AC.Bone mineralization, hypothalamic amenorrhea, and sex steroid therapy in female adolescents and young adults. Journal of Pediatrics 1995126683–689. ( 10.1016/s0022-3476(9570393-4) [DOI] [PubMed] [Google Scholar]
- 31.Gilsanz V, Chalfant J, Kalkwarf H, Zemel B, Lappe J, Oberfield S, Shepherd J, Wren T, Winer K. Age at onset of puberty predicts bone mass in young adulthood. Journal of Pediatrics 20111581, 00,–105, 10, 5.e1–10. ( 10.1016/j.jpeds.2010.06.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertelloni S, Baroncelli GI, Federico G, Cappa M, Lala R, Saggese G. Altered bone mineral density in patients with complete androgen insensitivity syndrome. Hormone Research 199850309–314. ( 10.1159/000023296) [DOI] [PubMed] [Google Scholar]
- 33.Zachmann M, Prader A, Sobel EH, Crigler JF, Ritzén EM, Atarés M, Ferrandez A. Pubertal growth in patients with androgen insensitivity: indirect evidence for the importance of estrogens in pubertal growth of girls. Journal of Pediatrics 1986108694–697. ( 10.1016/s0022-3476(8681043-5) [DOI] [PubMed] [Google Scholar]
- 34.Hussain A, Tripathi A, Pieczonka C, Cope D, McNatty A, Logothetis C, Guise T. Bone health effects of androgen-deprivation therapy and androgen receptor inhibitors in patients with nonmetastatic castration-resistant prostate cancer. Prostate Cancer and Prostatic Diseases 202124290–300. ( 10.1038/s41391-020-00296-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura Het al. Apalutamide treatment and metastasis-free survival in prostate cancer. New England Journal of Medicine 20183781408–1418. ( 10.1056/NEJMoa1715546) [DOI] [PubMed] [Google Scholar]
- 36.Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, Jievaltas M, Luz M, Alekseev B, Kuss Iet al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. New England Journal of Medicine 20193801235–1246. ( 10.1056/NEJMoa1815671) [DOI] [PubMed] [Google Scholar]
- 37.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, Ivashchenko P, Demirhan E, Modelska K, Phung Det al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. New England Journal of Medicine 20183782465–2474. ( 10.1056/NEJMoa1800536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandewalle S, Taes Y, Fiers T, Toye K, Van Caenegem E, Roggen I, De Schepper J, Kaufman JM. Associations of sex steroids with bone maturation, bone mineral density, bone geometry, and body composition: a cross-sectional study in healthy male adolescents. Journal of Clinical Endocrinology and Metabolism 201499E1272–E1282. ( 10.1210/jc.2013-3887) [DOI] [PubMed] [Google Scholar]
- 39.Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. Journal of Endocrinology 2010207127–134. ( 10.1677/JOE-10-0209) [DOI] [PubMed] [Google Scholar]
- 40.Lorentzon M, Swanson C, Andersson N, Mellstrom D, Ohlsson C. Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD study. Journal of Bone and Mineral Research 2005201334–1341. ( 10.1359/JBMR.050404) [DOI] [PubMed] [Google Scholar]
- 41.Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. Journal of Clinical Endocrinology and Metabolism 2004896025–6029. ( 10.1210/jc.2004-0602) [DOI] [PubMed] [Google Scholar]
- 42.Laurent M, Antonio L, Sinnesael M, Dubois V, Gielen E, Classens F, Vanderschueren D. Androgens and estrogens in skeletal sexual dimorphism. Asian Journal of Andrology 201416213–222. ( 10.4103/1008-682X.122356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callewaert F, Venken K, Kopchick JJ, Torcasio A, van Lenthe GH, Boonen S, Vanderschueren D. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. Journal of Bone and Mineral Research 201025617–626. ( 10.1359/jbmr.090828) [DOI] [PubMed] [Google Scholar]
- 44.Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. Journal of Bone and Mineral Research 201227273–282. ( 10.1002/jbmr.552) [DOI] [PubMed] [Google Scholar]
- 45.Gabel L, Nettlefold L, Brasher PM, Moore SA, Ahamed Y, Macdonald HM, McKay HA. Reexamining the surfaces of bone in boys and girls during adolescent growth: a 12-year mixed longitudinal pQCT study. Journal of Bone and Mineral Research 2015302158–2167. ( 10.1002/jbmr.2570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borjesson AE, Lagerquist MK, Liu C, Shao R, Windahl SH, Karlsson C, Sjogren K, Moverare-Skrtic S, Antal MC, Krust Aet al. The role of estrogen receptor alpha in growth plate cartilage for longitudinal bone growth. Journal of Bone and Mineral Research 2010252690–2700. ( 10.1002/jbmr.156) [DOI] [PubMed] [Google Scholar]
- 47.Kim NR, Jardi F, Khalil R, Antonio L, Schollaert D, Deboel L, van Lenthe GH, Decallonne B, Carmeliet G, Gustafsson JÅet al. Estrogen receptor alpha signaling in extrahypothalamic neurons during late puberty decreases bone size and strength in female but not in male mice. FASEB Journal 2020347118–7126. ( 10.1096/fj.202000272R) [DOI] [PubMed] [Google Scholar]
- 48.Lee PA, Witchel SF. The influence of estrogens on growth. Current Opinion in Pediatrics 19979431–436. ( 10.1097/00008480-199708000-00020) [DOI] [PubMed] [Google Scholar]
- 49.Kim NR, Khalil R, David K, Antonio L, Schollaert D, Deboel L, Van Herck E, Wardenier N, Cools M, Decallonne Bet al. Novel model to study the physiological effects of temporary or prolonged sex steroid deficiency in male mice. American Journal of Physiology: Endocrinology and Metabolism 2021320E415–E424. ( 10.1152/ajpendo.00401.2020) [DOI] [PubMed] [Google Scholar]
- 50.Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, Rosen HN, Weber DR, Zemel BS, Shepherd JA. Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. Journal of Clinical Densitometry 201922453–471. ( 10.1016/j.jocd.2019.07.001) [DOI] [PubMed] [Google Scholar]
- 51.Watts NB, Leslie WD, Foldes AJ, Miller PD. 2013 International Society for Clinical Densitometry Position Development Conference: task force on normative databases. Journal of Clinical Densitometry 201316472–481. ( 10.1016/j.jocd.2013.08.001) [DOI] [PubMed] [Google Scholar]
- 52.Rosen HN, Hamnvik OR, Jaisamrarn U, Malabanan AO, Safer JD, Tangpricha V, Wattanachanya L, Yeap SS. Bone densitometry in transgender and gender non-conforming (TGNC) individuals: 2019 ISCD official position. Journal of Clinical Densitometry 201922544–553. ( 10.1016/j.jocd.2019.07.004) [DOI] [PubMed] [Google Scholar]
- 53.Bachrach LK, Gordon CM. & Section on Endocrinology. Bone densitometry in children and adolescents. Pediatrics 2016138 e20162398. ( 10.1542/peds.2016-2398) [DOI] [PubMed] [Google Scholar]
- 54.Ciancia S, van Rijn RR, Hogler W, Appelman-Dijkstra NM, Boot AM, Sas TCJ, Renes JS. Osteoporosis in children and adolescents: when to suspect and how to diagnose it. European Journal of Pediatrics 20221812549–2561. ( 10.1007/s00431-022-04455-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navabi B, Tang K, Khatchadourian K, Lawson ML. Pubertal suppression, bone mass, and body composition in youth with gender dysphoria. Pediatrics 2021148 e2020039339. ( 10.1542/peds.2020-039339) [DOI] [PubMed] [Google Scholar]
- 56.Klink D, Caris M, Heijboer A, van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin-releasing hormone analog treatment and cross-sex hormone treatment in adolescents with gender dysphoria. Journal of Clinical Endocrinology and Metabolism 2015100E270–E275. ( 10.1210/jc.2014-2439) [DOI] [PubMed] [Google Scholar]
- 57.Schagen SEE, Wouters FM, Cohen-Kettenis PT, Gooren LJ, Hannema SE. Bone development in transgender adolescents treated with gnrh analogues and subsequent gender-affirming hormones. Journal of Clinical Endocrinology and Metabolism 2020105e4252–e4263. ( 10.1210/clinem/dgaa604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tack LJW, Craen M, Lapauw B, Goemaere S, Toye K, Kaufman JM, Vandewalle S, T’Sjoen G, Zmierczak HG, Cools M. Proandrogenic and antiandrogenic progestins in transgender youth: differential effects on body composition and bone metabolism. Journal of Clinical Endocrinology and Metabolism 20181032147–2156. ( 10.1210/jc.2017-02316) [DOI] [PubMed] [Google Scholar]
- 59.Van Caenegem E, Taes Y, Wierckx K, Vandewalle S, Toye K, Kaufman JM, Schreiner T, Haraldsen I, T’Sjoen G. Low bone mass is prevalent in male-to-female transsexual persons before the start of cross-sex hormonal therapy and gonadectomy. Bone 20135492–97. ( 10.1016/j.bone.2013.01.039) [DOI] [PubMed] [Google Scholar]
- 60.Joseph T, Ting J, Butler G. The effect of GnRH analogue treatment on bone mineral density in young adolescents with gender dysphoria: findings from a large national cohort. Journal of Pediatric Endocrinology and Metabolism 2019321077–1081. ( 10.1515/jpem-2019-0046) [DOI] [PubMed] [Google Scholar]
- 61.Vlot MC, Klink DT, den Heijer M, Blankenstein MA, Rotteveel J, Heijboer AC. Effect of pubertal suppression and cross-sex hormone therapy on bone turnover markers and bone mineral apparent density (BMAD) in transgender adolescents. Bone 20179511–19. ( 10.1016/j.bone.2016.11.008) [DOI] [PubMed] [Google Scholar]
- 62.Carmichael P, Butler G, Masic U, Cole TJ, De Stavola BL, Davidson S, Skageberg EM, Khadr S, Viner RM. Short-term outcomes of pubertal suppression in a selected cohort of 12 to 15 year old young people with persistent gender dysphoria in the UK. PLoS ONE 202116 e0243894. ( 10.1371/journal.pone.0243894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoffers IE, de Vries MC, Hannema SE. Physical changes, laboratory parameters, and bone mineral density during testosterone treatment in adolescents with gender dysphoria. Journal of Sexual Medicine 2019161459–1468. ( 10.1016/j.jsxm.2019.06.014) [DOI] [PubMed] [Google Scholar]
- 64.Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, Makitie O, Munns CF, Shaw N. & International Society of Clinical Densitometry. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. Journal of Clinical Densitometry 201417275–280. ( 10.1016/j.jocd.2014.01.004) [DOI] [PubMed] [Google Scholar]
- 65.Dobrolinska M, van der Tuuk K, Vink P, van den Berg M, Schuringa A, Monroy-Gonzalez AG, Garcia DV, Schultz WCMW, Slart RHJA. Bone mineral density in transgender individuals after gonadectomy and long-term gender-affirming hormonal treatment. Journal of Sexual Medicine 2019161469–1477. ( 10.1016/j.jsxm.2019.06.006) [DOI] [PubMed] [Google Scholar]
- 66.Wiepjes CM, de Jongh RT, de Blok CJ, Vlot MC, Lips P, Twisk JW, den Heijer M. Bone safety during the first ten years of gender-affirming hormonal treatment in transwomen and transmen. Journal of Bone and Mineral Research 201934447–454. ( 10.1002/jbmr.3612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Motta G, Marinelli L, Barale M, Brustio PR, Manieri C, Ghigo E, Procopio M, Lanfranco F. Fracture risk assessment in an Italian group of transgender women after gender-confirming surgery. Journal of Bone and Mineral Metabolism 202038885–893. ( 10.1007/s00774-020-01127-9) [DOI] [PubMed] [Google Scholar]
- 68.Wiepjes CM, Vlot MC, de Blok CJM, Nota NM, de Jongh RT, den Heijer M. Bone geometry and trabecular bone score in transgender people before and after short- and long-term hormonal treatment. Bone 2019127280–286. ( 10.1016/j.bone.2019.06.029) [DOI] [PubMed] [Google Scholar]
- 69.Broulik PD, Urbanek V, Libansky P. Eighteen-year effect of androgen therapy on bone mineral density in trans(gender) men. Hormone and Metabolic Research 201850133–137. ( 10.1055/s-0043-118747) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a