Abstract
Background
The therapeutic role of azathioprine (AZA) and 6‐mercaptopurine (6‐MP) remains controversial due to their relatively slow onset of action and potential for adverse events. An updated meta‐analysis was performed to evaluate the efficacy of these agents for the maintenance of remission in quiescent Crohn's disease.
Objectives
To assess the efficacy of AZA and 6‐MP for maintenance of remission in quiescent Crohn's disease.
Search methods
We searched MEDLINE, EMBASE, and the Cochrane Library from inception to June 30, 2015.
Selection criteria
Randomized controlled trials of oral azathioprine or 6‐mercaptopurine compared to placebo or active therapy involving adult patients (> 18 years) with quiescent Crohn's disease were considered for inclusion. Patients with surgically‐induced remission were excluded.
Data collection and analysis
At least two authors independently extracted data and assessed study quality using the Cochrane risk of bias tool. For dichotomous outcomes, we calculated the risk ratio (RR) and corresponding 95% confidence interval (CI). The primary outcome was maintenance of remission. Secondary outcomes included steroid sparing, adverse events, withdrawals due to adverse events and serious adverse events. All data were analyzed on an intention‐to‐treat basis. The overall quality of the evidence supporting the primary outcome and selected secondary outcomes was assessed using the GRADE criteria.
Main results
Eleven studies (881 participants) were included. Comparisons included AZA versus placebo (7 studies, 532 participants), AZA or 6‐MP versus mesalazine or sulfasalazine (2 studies, 166 participants), AZA versus budesonide (1 study, 77 participants), AZA and infliximab versus infliximab (1 study, 36 patients), 6‐MP versus methotrexate (1 study, 31 patients), and early AZA versus conventional management (1 study, 147 participants). Two studies were rated as low risk of bias. Four studies were rated as high risk of bias for being non‐blinded or single‐blind. Five studies were rated as unclear risk of bias. A pooled analysis of six studies (489 participants) showed that AZA (1.0 to 2.5 mg/kg/day) was significantly superior to placebo for maintenance of remission over a 6 to 18 month period. Seventy‐three per cent of patients in the AZA group maintained remission compared to 62% of placebo patients (RR 1.19, 95% CI 1.05 to 1.34). The number needed to treat for an additional beneficial outcome was nine. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (327 events) and unclear risk of bias. A pooled analysis of two studies (166 participants) showed no statistically significant difference in the proportion of patients who maintained remission between AZA (1.0 to 2.5 mg/kg/day) or 6‐MP (1.0 mg/day) and mesalazine (3 g/day) or sulphasalazine (0.5 g/15 kg/day) therapy. Sixty‐nine per cent of patients in the AZA/6‐MP group maintained remission compared to 67% of mesalazine/sulphasalazine patients (RR 1.09, 95% CI 0.88 to 1.34). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (113 events) and high or unclear risk of bias. One small study found AZA (2.0 to 2.5 mg/kg/day) to be superior to budesonide (6 to 9 mg/day) for maintenance of remission at one year. Seventy‐six per cent (29/38) of AZA patients maintained remission compared to 46% (18/39) of budesonide patients (RR 1.65, 95% CI 1.13 to 2.42). GRADE indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (47 events) and high risk of bias. One small study found no difference in maintenance of remission rates at one year between combination therapy with AZA (2.5 mg/kg) and infliximab (5 mg/kg every 8 weeks) compared to infliximab monotherapy. Eighty‐one per cent (13/16) of patients in the combination therapy group maintained remission compared to 80% (16/20) of patients in the infliximab group (RR 1.02, 95% CI 0.74 to 1.40). GRADE indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (29 events) and high risk of bias. One small study found no difference in maintenance of remission rates at one year between 6‐MP (1 mg/kg/day) and methotrexate (10 mg/week). Fifty per cent (8/16) of 6‐MP patients maintained remission at one year compared to 53% (8/15) of methotrexate patients (RR 0.94, 95% CI 0.47 to 1.85). GRADE indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (16 events) and high risk of bias. One study (147 participants) failed to show any significant benefit for early azathioprine treatment over a conventional management strategy. In the early azathioprine treatment group 67% (11‐85%) of trimesters were spent in remission compared to 56% (29‐73%) in the conventional management group. AZA when compared to placebo had a significantly increased risk of adverse events (RR 1.29, 95% CI 1.02 to 1.64), withdrawal due to adverse events (3.12, 95% CI 1.59 to 6.09) and serious adverse events (RR 2.45, 95% CI 1.22 to 4.90). AZA/6‐MP also demonstrated a significantly higher risk of serious adverse events when compared to mesalazine or sulphasalazine (RR 9.37, 95% CI 1.84 to 47.7). Common adverse events included pancreatitis, leukopenia, nausea, allergic reaction and infection.
Authors' conclusions
Low quality evidence suggests that AZA is more effective than placebo for maintenance of remission in Crohn's disease. Although AZA may be effective for maintenance of remission its use is limited by adverse effects. Low quality evidence suggests that AZA may be superior to budesonide for maintenance of remission but because of small study size and high risk of bias, this result should be interpreted with caution. No conclusions can be drawn from the other active comparator studies because of low and very low quality evidence. Adequately powered trials are needed to determine the comparative efficacy and safety of AZA and 6‐MP compared to other active maintenance therapies. Further research is needed to assess the efficacy and safety of the use of AZA with infliximab and other biologics and to determine the optimal management strategy for patients with quiescent Crohn's disease.
Plain language summary
Azathioprine or 6‐mercaptopurine for maintenance of remission in Crohn's disease
What is Crohn's disease? Crohn's disease is a long‐term chronic inflammatory bowel disease that can affect any part of the gastrointestinal tract from mouth to anus. Symptoms include abdominal pain, diarrhea and weight loss. When people with Crohn's disease are experiencing symptoms of the disease it is said to be ‘active’. Periods when the symptoms stop are called ‘remission’.
What are azathioprine and 6‐mercaptopurine? Azathioprine and 6‐mercaptopurine are oral medications that reduce the body's immune responses and may reduce inflammation associated with Crohn's disease.
What did the researchers investigate? The researchers investigated whether azathioprine or 6‐mercaptopurine maintains remission in people with inactive Crohn's disease and whether these medications cause any harms (side effects). The researchers searched the medical literature up to June 30, 2015.
What did the researchers find? The researchers identified 11 studies that included a total of 881 participants. Seven studies including 532 participants compared azathioprine to a placebo (a fake medicine such as a sugar pill). Two studies including 166 participants compared azathioprine or 6‐mercaptopurine to aminosalicylates (the anti‐inflammatory medications mesalazine or sulfasalazine). One study including 77 participants compared azathioprine to budesonide (a steroid drug). One study including 31 participants compared 6‐mercaptopurine to methotrexate (an immunosuppressive drug). One study including 36 participants compared combination therapy with azathioprine and infliximab (a biologic drug) to infliximab alone. One study with 147 participants compared the early use of azathioprine in patients with recently diagnosed Crohn's disease to a conventional management strategy. Two studies were rated as high quality. Three studies were rated as low quality. Six studies were rated as unclear quality due to a lack of information.
A pooled analysis of six studies (489 participants) suggests that azathioprine (at daily doses of 1.0 to 2.5 mg/kg/day) is superior to placebo for maintenance of remission of Crohn's disease over a 6 to 18 month period. A pooled analysis of two studies (166 participants) showed no difference in the proportion of patients who maintained remission between azathioprine (1.0 to 2.5 mg/kg/day) or 6‐mercaptopurine (1.0 mg/day) and aminosalicylate therapy (mesalazine 3 g/day or sulfasalazine 0.5g/15 kg/day). One small study (77 participants) suggests that azathioprine (2.0 to 2.5 mg/kg/day) may be superior to budesonide (6 to 9 mg/day) for maintenance of remission over a one year period. One small study (36 participants) found no difference in maintenance of remission rates at one year between combination therapy with azathioprine (2.5 mg/kg/day) and infliximab (5 mg/kg every 8 weeks) compared to infliximab alone. One small study (31 participants) found no difference in maintenance of remission rates at one year between 6‐mercaptopurine (1 mg/day) and methotrexate (10 mg/week). One study (147 participants) failed to show any difference in time spent in remission between early azathioprine treatment and a conventional management strategy. An increased risk of side effects was seen in participants who received azathioprine. Some of these side effects such as leukopenia (a reduction in the number of white cells in the blood) were serious in nature. Common side effects included pancreatitis (inflammation of the pancreas), leukopenia, nausea, allergic reaction and infection. The choice to use azathioprine or 6‐mercaptopurine should be made after careful consideration of the risks and benefits of using these drugs. More research is needed to allow conclusions about the comparative effectiveness and side effects of azathioprine and 6‐mercaptopurine compared to other maintenance therapies such as methotrexate. Further research is needed to assess the effectiveness and side effects of the use of azathioprine with infliximab and other biologics and to determine the optimal management strategy for patients with inactive Crohn's disease.
Summary of findings
Background
The antimetabolite 6‐mercaptopurine and its prodrug, azathioprine, are purine analogues that inhibit cell growth by directly interfering with nucleic acid synthesis. Because of their ability to impede the rapid cell proliferation involved in inflammatory processes, these drugs have been shown to demonstrate immuno‐suppressive abilities (Sahasranaman 2008).
There have been many uncontrolled studies evaluating the efficacy of azathioprine for the therapy of Crohn's disease, and the first randomized, double‐blind, placebo‐controlled trials were documented in the early 1970's (Rhodes 1970; Willoughby 1971). Although much experience has been gained with these drugs since then, the therapeutic role of azathioprine and 6‐mercaptopurine remains controversial, in part related to their slow acting therapeutic effect as well as their potential for adverse events, such as leukopenia, pancreatitis, nausea, hypersensitivity, and increased risk of malignancy (Gearry 2004).
Crohn's disease is characterized by its propensity to relapse. Therefore studies were designed to determine the efficacy of azathioprine or 6‐mercaptopurine for maintenance of remission. This systematic review is an update of a previously published Cochrane review (Prefontaine 2009).
Objectives
The primary objective was to assess the efficacy and safety of azathioprine or 6‐mercaptopurine maintenance therapy in patients with quiescent Crohn's disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials were considered for inclusion.
Types of participants
Patients greater than 18 years of age with quiescent Crohn's disease (as defined by the included studies) were considered for inclusion. Quiescent disease, or disease in remission was defined as mild or absent symptoms prior to entering the study or by a validated index (e.g. CDAI < 150), irrespective of the use of prophylactic medication. Patients with surgically‐induced remission were excluded.
Types of interventions
Studies comparing oral azathioprine or 6‐mercaptopurine to placebo or an active therapy for maintenance of remission in Crohn's disease were included.
Types of outcome measures
Most of the included studies used the Crohn's Disease Activity Index (CDAI) to define remission (Best 1976). Using the CDAI, remission is defined as a score of less than 150. The earlier studies utilized other scales such as the Disease Activity Score (DAS) (Dyer 1970), and other less well defined measures such as maintenance of well being. Despite this diversity in outcome measurement, we felt that these earlier studies provided a reasonable measurement of clinically active and inactive disease.
Primary outcomes
The primary outcome was maintenance of remission (as defined by the included studies, e.g. CDAI < 150) at study endpoint.
Secondary outcomes
Secondary outcomes included:
adverse events
withdrawal due to adverse events;
serious adverse events; and
steroid sparing.
Search methods for identification of studies
Electronic searches
We searched the following databases from inception to June 30, 2015:
MEDLINE (Ovid);
EMBASE (Ovid);
Cochrane Library.
Conference proceedings were also searched to identify additional studies.
The electronic search strategies are reported in Appendix 1.
Data collection and analysis
Selection of studies
Study titles and abstracts identified by the literature search were reviewed and potentially relevant studies were identified for full text evaluation. The studies selected for full text review were independently assessed by two authors (PHP and JKM or DJT or BST) and consensus for study inclusion and exclusion was reached through discussion.
Data extraction and management
Each study was reviewed and remission and safety data were independently extracted by at least two authors (PHP and JKM or DJT or BST). If data were missing or unclear, the study authors were contacted for clarification.
Other information extracted from the studies included:
a. Study characteristics and design; b. Characteristics of patients; c. Inclusion and exclusion criteria; d. Interventions; and e. Outcomes scoring methods.
Assessment of risk of bias in included studies
At least two authors (PHP or JKM or DST or BST) independently assessed study quality using the Cochrane risk of bias tool (Higgins 2011a). Items assessed included:
Random sequence generation;
Allocation concealment;
Blinding of participants, personnel and assessment of outcome;
Incomplete outcome data;
Selective reporting;
Other biases.
Each category was evaluated as low, high or unclear risk and judgment justification was provided in the Characteristics of included studies section of the review.
GRADE Analysis
The overall quality of the evidence supporting the primary outcome and selected secondary outcomes was evaluated using the GRADE approach (Guyatt 2008; Schünemann 2011). Using this approach outcome data were rated high, moderate, low or very low quality. Outcome data from randomized controlled trials begins as high quality but can be downgraded based on a number of criteria. These criteria include:
Risk of bias in the included studies;
Indirect evidence (by comparison, population, setting);
Inconsistency (i.e. unexplained heterogeneity);
Imprecise results (i.e. wide confidence intervals); and
Likelihood of publication bias.
Measures of treatment effect
For dichotomous outcomes, we calculated the risk ratio (RR) and corresponding 95% confidence interval.
Unit of analysis issues
For multi‐arm trials with a single placebo group and two treatment dose groups we split the placebo group in half to avoid a unit of analysis error (Higgins 2011b). To avoid potential carry‐over effects we only included the first part of the study (i.e. before the cross‐over) for any cross‐over studies (Higgins 2011b).
Dealing with missing data
Data were analysed on an intention‐to‐treat basis. Studies were evaluated for missing data and where explanations were not provided, the patient outcome was considered to be a failure and counted as a relapse.
Assessment of heterogeneity
Statistical heterogeneity was assessed using the Chi2 test and I2 statistic. For the Chi2 test a P value of less than 0.1 was considered to be statistically significant. The degree of statistical heterogeneity was quantified using the I2 statistic. We investigated heterogeneity by visually inspecting the forest plots to identify outliers. If outliers were identified, we conducted sensitivity analysis to explore potential explanations for the heterogeneity.
Assessment of reporting biases
If protocols were not available for the included studies, we assessed reporting bias by comparing the outcomes specified in the methods section of the manuscript to those reported in the results section. If there was more than 10 included studies in a pooled analysis, we planned to investigate publication bias by constructing funnel plots.
Data synthesis
Data from individual trials were combined for meta‐analysis when the interventions, patient groups and outcomes were sufficiently similar (determined by consensus). We calculated the pooled RR and 95% CI for dichotomous outcomes using a fixed‐effect model. If significant heterogeneity was identified a random‐effects model was used. We performed subgroup analyses by dose of azathioprine or 6‐mercaptopurine (i.e. 1.0, 2.0, and 2.5 mg/kg/day). It was deemed acceptable to pool studies of azathioprine with 6‐mercaptopurine studies, as azathioprine is metabolized to 6‐mercaptopurine.
Results
Description of studies
Results of the search
A literature search conducted on June 30, 2015 identified 1541 studies. After duplicates were removed a total of 1119 studies remained for review of titles and abstracts. Fifty‐seven studies of azathioprine or 6‐mercaptopurine therapy for maintenance of remission in Crohn's were selected for full text review (See Figure 1). Thirty‐five reports of 27 studies were excluded because they did not meet the inclusion criteria. The remaining 22 reports of 11 studies were evaluated for qualitative analysis and 11 studies underwent quantitative analysis.
1.

Study flow diagram.
Included studies
Eleven trials (881 participants) were included (Candy 1995; Cosnes 2013; Lémann 2005; Mantzaris 2004; Mantzaris 2009; Maté‐Jiménez 2000; O'Donoghue 1978; Panes 2013; Rosenberg 1975; Summers 1979; Willoughby 1971). Six of these trials were double‐blind and placebo‐controlled (Candy 1995; Lémann 2005; O'Donoghue 1978; Panes 2013; Rosenberg 1975; Willoughby 1971). Two of these trials were withdrawal studies, where patients who were being maintained in remission by azathioprine were randomized to continue therapy or replace their azathioprine with placebo (O'Donoghue 1978; Lémann 2005). Four trials included an induction phase on the study medication, then followed the patients that achieved remission for the maintenance phase (Candy 1995; Mantzaris 2004; Maté‐Jiménez 2000; Summers 1979). Only one trial studied 6‐mercaptopurine for maintenance of remission (Maté‐Jiménez 2000). Four studies compared azathioprine or 6‐mercaptopurine to an active therapy. Mantzaris 2004 compared combination therapy with azathioprine and infliximab to infliximab. Mantzaris 2009 compared azathioprine to budesonide. Maté‐Jiménez 2000 compared 6‐mercaptopurine to methotrexate and 5‐ASA. Summers 1979 compared azathioprine to sulphasalazine and prednisone. Summers 1979 was the only active comparator study that also included a placebo arm. The Summers 1979 study also known as the National Cooperative Crohn's Disease study contained two parts. Part I had two phases. In phase one, patients with active Crohn's disease were treated with the study drug to induce remission. In phase two, patients who had achieved remission in phase one were followed to see if they maintained remission on 2.5 mg/kg/day of azathioprine. Part II included patients who had quiescent disease and patients were randomized to receive study medication to maintain remission on 1.0 mg/kg/day of azathioprine. This review includes outcome data from part one, phase two and part two. Adverse event data were reported for the entire Summers 1979 study (not by phase). Cosnes 2013 and Panes 2013 evaluated the effect of early administration of azathioprine in patients newly diagnosed with Crohn's disease, and included patients with active disease. Panes 2013 compared azathioprine to placebo. Cosnes 2013 compared early use of azathioprine to conventional management, which could eventually include azathioprine. Both allowed patients to treat flares with steroids and still remain in the study, which was a measure of relapse in all other studies. Two studies included patients aged 15 years old or greater (Candy 1995; Maté‐Jiménez 2000). However the majority of patients in these studies were adults.
Candy 1995 conducted a randomized, double‐blind, placebo‐controlled, trial to assess the efficacy of azathioprine in combination with prednisolone versus placebo in combination with prednisolone for induction and maintenance of remission in 63 patients with active Crohn's Disease (CDAI > 200). Remission was defined as a CDAI score of < 150. Patients (aged 15 to 65 years) were selected from a single site (Groot Schuur Hospital IBD Clinic). Patients were excluded from the study if they had previous surgery for Crohn's disease, symptoms suggestive of mechanical obstruction, recently received immunosuppressives, compromised hepatic function, or if they were pregnant or lactating. After one week of azathioprine 50 mg/day, the dose was held constant at 2.5 mg/kg/day for the remaining 11 weeks of phase 1, and for 12 months of phase 2 (or until the patient relapsed or withdrew from the study). In phase 1, patients (intervention group n = 33 and placebo group n = 30) received a tapering dose of prednisolone (initial dose 1 mg/kg/day tapered to 0 by week 12) in combination with azathioprine or placebo for induction of remission. Twenty out of 63 participants failed to achieve remission (7 in azathioprine group and 9 in placebo group) or withdrew from phase 1. During phase 2, patients (intervention group n = 24 and placebo group n = 19) continued to receive azathioprine or matched placebo for 12 months. The primary outcome was the proportion of patients in clinical remission. Secondary outcomes were median change in CDAI, ESR, serum CRP, serum orosomucoid concentration, and leucocyte count between the first and last visits. Differences in leucocyte count between baseline and final visit were evaluated for responders and non‐responders within the intervention group. Adverse events and compliance were also monitored.
Cosnes 2013 performed a three‐year trial to evaluate the efficacy of early azathioprine treatment within six months of diagnosis versus conventional management of Crohn's disease patients with a high risk of disabling disease. Remission was defined as a trimester spent free of: flare, treatment with steroids or anti‐TNF‐alpha antagonists, active perianal disease (pain or discharge with induration, skin tags, ulceration, fissure or fistula), Crohn's disease‐related hospitalization (including those caused by drug side effects); or surgery. The study was performed at 24 French centres and was a randomized, parallel group, open‐label trial. Patients were at least 18 years of age (N = 147; intervention n= 72); and were classified as high risk if they had been diagnosed when they were under 40 years of age, had received corticosteroids within three months of diagnosis or had active perianal lesions. Patients were excluded if they had previous treatment with immunomodulators or anti–TNF‐alpha therapy; immediate need for surgery or anti‐TNF‐alpha therapy; severe comorbidity; documented infection; renal or liver failure; contraindication to thiopurines according to labelling recommendations; malignancy; history of drug abuse; predictable poor compliance; or if they were pregnant. The primary outcome was the proportion of trimesters spent in remission. The secondary outcomes were the proportion of trimesters in the three year trial period that contain any of the above listed failures of remission; duration of significant corticosteroid exposure per trimester; total exposures (in milligrams) of prednisone and budesonide per trimester; time to first perianal surgery, first intestinal resection, and first anti‐TNF use; median CDAI scores and C‐reactive protein concentrations throughout follow‐up; quality of life (measured by the IBD Questionnaire) at months 12, 24, and 36; total number of days of hospitalization; and total number of days out of work per trimester. Safety data and adverse events were also collected.
Lémann 2005 conducted a randomized, double‐blind, placebo‐controlled trial at 11 French sites and 1 Belgian site. The study evaluated the effectiveness of azathioprine for long term remission of Crohn's disease. Patients were monitored for relapse, (defined as CDAI > 250 or CDAI of 150‐250 on 3 consecutive weeks plus an increase of ≥ 75 points above baseline score, or the need for surgery (except limited perianal surgery)). Patients selected for the study (N = 83, azathioprine group, n = 40) were at least 18 years of age, and had continuous azathioprine treatment for a minimum of 42 months during which they did not experience a flare‐up, receive oral prednisone (>10 mg/day) budesonide or another immunosuppressive or biological agent; ingest artificial nutrition; undergo surgery (except limited perianal surgery). Patients were excluded if they had active disease (CDAI > 150 at entry), disease was limited to the perianal area, or if they had received azathioprine to prevent postoperative recurrence after curative surgical resection. Adverse events were also monitored.
Mantzaris 2004 conducted a randomized, phase II study that evaluated the effectiveness of azathioprine combined with infliximab compared to infliximab monotherapy. Patients (N = 47) were between ages 18 and 57 with active, steroid‐resistant Crohn's disease (CDAI > 150). Patients were ineligible if they had fistulizing or fibrostenotic disease, septic complications, or if they had not received previous treatment with immunomodulators. After a six week induction phase, patients in remission continued their assigned therapies. All patients received either infliximab (5 mg/kg every 8 weeks) or infliximab and azathioprine (2.5 mg/kg/day). The primary outcome was the proportion of patients with maintenance of remission. Adverse effects were also monitored. The study was published as an abstract.
Mantzaris 2009 compared the effectiveness of azathioprine (2 to 2.5 mg/kg/day) to budesonide (6 to 9 mg/day) for maintenance of remission in patients (aged 18‐67 years) with steroid‐dependant Crohn's disease (CDAI < 150) over a one year period, which was extended to 18 months for those maintaining remission at the one year cutoff. Steroid‐dependency was described as individuals having at least one flare in the previous six to twelve months which had been successfully treated with steroids, but returned during steroid tapering or after steroid withdrawal. Included patients had recently flared (within one month of study inclusion) and were receiving a tapered dose of oral prednisolone for at least one month prior to randomization. The primary outcomes were mucosal healing and histological remission. Secondary outcomes included annual relapse rate, time in remission, discontinuation of study medications, changes in CDAI or health‐related quality of life, and safety data. Compliance and the use of any other medications were also monitored.
Maté‐Jiménez 2000 conducted a 106 week, single‐centre, randomized three arm two phase trial with sub‐stratification for ulcerative colitis (n = 34) and Crohn's disease (n = 38). The induction phase was 30 weeks, and was followed by a maintenance phase for 76 weeks to evaluate the efficacy of adding either 6‐mercaptopurine, methotrexate or 5‐aminosalicylic acid to prednisone to induce remission (phase one) and then maintain remission without prednisone (phase two). Patients selected for inclusion (N = 72) were aged 15 to 70 years with steroid‐dependent, radiologically or endoscopically confirmed inflammatory bowel disease from Digestive DIseases Service, Hospital de la Princesa, who had been followed for at least one year and had never previously received either 6‐mercaptopurine or methotrexate. Steroid dependence was defined as patients who could not be weaned off less than 20 mg prednisone without induction of inflammatory activity, and a CDAI > 200. Exclusion criteria included: patients over the age of 70 years or under the age of 15 years; no signed consent; those with clinically significant cardiac, hepatic or renal disease; ongoing bacterial infection; pregnancy; lactating or not using reliable contraceptive techniques; patients using concomitant allopurinol, NSAIDs, tetracyclines or phenytoin in significant doses; those who have had extensive surgery for their Crohn's disease; or those who have symptoms that suggest a potential need for urgent surgery. The induction phase used the individualized dose of prednisone used prior to study inclusion as the starting dose (highest was 1 mg/kg/day prednisone) to be tapered by 8 mg/kg/day prednisone over the 30 weeks to 0 and the following therapies under study, 6‐mercaptopurine 1.5mg/kg/day (group A, n = 16 with Crohn's), methotrexate, 15 mg/week (group B, n = 15 with Crohn's) or 5‐aminosalicyclic acid, 3 g/day (group C, n = 7 with Crohn's). For the maintenance phase, only those who achieved remission after stopping prednisone during the induction phase were included in this portion of the study. In these patients the methotrexate dose was reduced to 10 mg/week and the 6‐mercaptopurine dose reduced to 1 mg/kg/day. The 5‐aminosalicylic acid dose used for maintenance therapy did not change (3g/day). No other medications, except antidiarrhoeals and supplements, were allowed. The primary outcome for phase one was induction of remission and cessation of steroids, while phase 2 was clinical remission. "Relapse was defined as CDAI > 150 and orosomucoid > 100 (N 88 mg/dl) with no response to 6 g daily of 5‐aminosalicylic acid and with need of prednisone therapy in Crohn's disease patients." Adverse events were a secondary outcome.
O'Donoghue 1978 performed a randomized, double‐blind, placebo‐controlled, 52 week, withdrawal study, which initially sub‐stratified participants into those only on azathioprine and those also on other anti‐inflammatories and azathioprine. The study evaluated the efficacy of azathioprine for maintenance of remission, defined as preventing relapse ("significant deterioration in clinical state requiring a change in treatment as judged by two doctors unaware of the patient's treatment"), in patients (N = 51) with quiescent Crohn's disease taking azathioprine (2 mg/kg/day) for at least six months prior to study inclusion. Patients receiving azathioprine combined with sulphasalazine or low dose corticosteroids were included; this treatment remained unchanged throughout study period. Patients were also allowed medications for symptom control and supplements. Patients were monitored for primary outcomes using a modified DAS scoring system completed at each clinical assessment. Adverse events and serious adverse events were also monitored; one patient died during the study.
Panes 2013 conducted a randomized, multi‐centre, double‐blind, placebo‐controlled study over 76 weeks in 31 Spanish hospitals. The study evaluated the efficacy of early administration of azathioprine (2.5 mg/kg/day) in patients with newly diagnosed (< 8 weeks) Crohn's disease for maintenance of corticosteroid‐free clinical remission. Sustained corticosteroid‐free remission was defined as the presence of clinical remission (CDAI score < 150 at each visit) in patients who had not been treated with corticosteroids during the entire study or patients who were treated with corticosteroids at baseline after the scheduled weaning of this treatment. Patients (N= 131; azathioprine, n = 68) aged 18 to 70 years, diagnosed with established Crohn's disease criteria within 8 weeks of screening, "irrespective of disease activity and use of corticosteroids at the time of screening." Exclusion criteria included previous treatment with azathioprine, 6‐mercaptopurine, methotrexate, cyclosporin, tacrolimus, mycophenolate mofetil, infliximab or adalimumab; patients with an immediate need for surgery or patients who had surgery for Crohn's disease; penetrating disease, symptomatic stenosis, perianal fistulas; patients with severe co‐morbidities, documented infection, malignancy, history of drug use; or those who were pregnant. Corticosteroids were allowed to treat flares during the study, (prednisone doses ranged 1 mg/kg/day to a maximum of 60 mg/day; budesonide dosage 9 mg/day), but were tapered using a predetermined schedule. No other medications used to treat Crohn's disease were allowed, except antibiotics for suspected or demonstrated infection. Secondary outcomes included relapse‐free survival, mean CDAI and IBDQ scores, and CRP concentrations at each visit; requirement for corticosteroids; cumulative dose of corticosteroids; development of a fistula; hospitalization; and CD‐related surgery and cumulative prednisone dose. Safety data were also collected.
Rosenberg 1975 conducted a randomized, double‐blind, identically matched placebo‐controlled trial over 26 weeks that evaluated the effect of azathioprine (2.0 mg/kg/day) on reducing the need for steroids in patients > 16 years of age with Crohn's disease who required daily prednisone (minimum dose 10 mg) for symptom control for at least 12 weeks prior to study inclusion (N = 20). Patients were excluded if they had advanced hepatic or renal disease, wished to become pregnant within the study time frame, or were likely to require surgery. The primary outcome was a reduction of prednisone usage over the trial. Prednisone was tapered according to a predetermined schedule when the patient met all of the following criteria: "3 or fewer bowel movements a day, absent to mild pain and malaise, weight loss less than 2 kg, and fever (> 37.5ºC) less than one fourth of the time". Prednisone was increased by 10 mg/day if a patient had > 6 bowel movements per day, severe pain or malaise, > 3 kg weight loss or fever (> 37.5ºC) for greater than half the time over seven days. If the patients condition did not change, the prednisone dosage remained constant. Patients were allowed to continue any other medications prescribed prior to study inclusion. Safety data were also collected.
Summers 1979 conducted two independent trials at the same time to evaluate the efficacy of sulphasalazine, prednisone, and azathioprine against placebo for inducing and maintaining remission in patients with active Crohn's disease (CDAI > 150 with radiologic findings of disease, Part 1, phase 1 and 2) and at maintaining remission in patients already in remission (either medically‐ or surgically‐induced) (Part 2). The studies were randomized, double‐blind, placebo‐controlled trials with patients from 14 centres. Part one was a two phase study, with the first phase (N = 295, placebo n = 77; sulphasalazine n = 74; prednisone n = 85; azathioprine n = 59) inducing remission over 17 weeks using the following identically matched medications and placebo: prednisone: 0.25 mg/kg/day if CDAI < 150; 0.5 mg/kg/day if CDAI = 150 to 300; 0.75 mg/kg/day if CDAI > 300; sulphasalazine: 1 g/15 kg/day; azathioprine: 2.5 mg/kg/day, or placebo. Patients who achieved remission in phase one entered phase two for one to two years of maintenance treatment. These 86 patients included 20 in the placebo group, 19 in the sulphasalazine group, 28 in the prednisone group and 19 in the azathioprine group. Part 2 evaluated maintenance of remission in 226 patients who achieved clinical remission in the previous 2 years or 48 who had undergone complete surgical resection of all diseased bowel within a year of entry. Part 2 included 274 patients: 101 received placebo, 58 received sulphasalazine, 61 received placebo and 54 received azathioprine. Both studies were also sub‐stratified by disease sites, prior steroid usage and disease activity. Safety data were collected throughout both phases.
Willoughby 1971 performed a 22 patient, randomized, double‐blind, placebo‐controlled study to evaluate the effect that azathioprine has on maintaining remission of Crohn's disease once it has been induced by prednisolone alone or combined with azathioprine. This was completed using two groups of patients, randomizing each to azathioprine or placebo. Group 1 (n = 12, azathioprine n = 6) consisted of patients with first time attacks or relapses of moderate to severe severity not treated with steroids in the previous 3 months, while group 2 (n= 10, azathioprine n =5) studied outpatients maintained on long term prednisolone (7.5 to 15 mg/day) for 5 to 72 months. The first group received prednisolone (initial dose 60 mg/day) until remission was achieved; it was then tapered on a 3‐week schedule to 10 mg and discontinued after hospital discharge if remission was maintained. Azathioprine was prescribed at 4.0 mg/kg/day for the first 10 days and then at 2.0 mg/kg/day until the end of 24 weeks. The second group received 2.0 mg/kg/day azathioprine or placebo and maintained their pre‐study dose of prednisolone for "at least 4 weeks after prednisolone was reduced as for group 1." Participants were also prescribed prochlorperazine for nausea and codeine phosphate was continued if the patient received it prior to study entry. Disease activity was monitored using DAS and relapse was considered the return of previous symptoms.
Excluded studies
Twenty‐seven studies were excluded for various reasons. Nine studies were excluded because they evaluated post‐operative maintenance of remission (Abdelli 2007; Ardizzone 2004; Armuzzi 2013; D'Haens 2008b; Hanauer 2004a; Herfarth 2006; Nos 2000; Reinisch 2010; Savarino 2013). Six studies were excluded because they were not randomized controlled trials (Abdelli 2007; Chebli 2007; Korelitz 1998; Mantzaris 2007; Nos 2000; Treton 2009). Six studies were excluded because the patients had active disease (Colombel 2010; de Souza 2013; Klein 1974; Oren 1997; Present 1980; Reinisch 2008). Three studies were excluded because all of the patients received azathioprine (D'Haens 2008a; Lémann 2006; Manosa 2013). Rhodes 1971 was excluded as it was a cross‐over study that failed to provide analysis before the first cross‐over. Another study did not specify the type of immunosuppressant that was studied (van Assche 2008). Vilien 2004 was excluded because the control group did not receive any treatment. The Watson 1979 study was a randomized, double‐blind placebo‐controlled cross‐over study of azathioprine maintenance therapy which was never published as a full paper. Willoughby 1990 was a randomized trial comparing azathioprine with levamisole. This study did not report any of our pre‐specified outcome variables.
Excluded studies and justification for exclusion are reported in the Characteristics of excluded studies tables.
Risk of bias in included studies
The risk of bias for included studies in summarized Figure 2.
2.
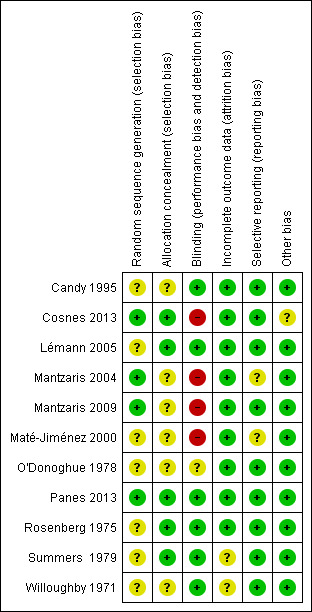
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of randomization was described in four studies (Cosnes 2013; Mantzaris 2004; Mantzaris 2009; Panes 2013), which received a low risk of bias assessment. The remainder of the studies were judged to have an unclear risk of bias for this item. The methods used for allocation concealment were adequately described in six studies (Cosnes 2013; Lémann 2005; Mantzaris 2004; Panes 2013; Rosenberg 1975; Summers 1979). The other six studies were assessed as having an unclear risk of bias with regard to allocation concealment.
Blinding
Six studies described adequate methods for blinding, including who was blinded and how blinding was maintained and these studies were rated as low risk of bias for blinding (Candy 1995; Lémann 2005; Panes 2013; Rosenberg 1975; Summers 1979; Willoughby 1971). Three of the trials were open label (Cosnes 2013; Mantzaris 2009; Maté‐Jiménez 2000), and thus rated as high risk of bias for blinding. Mantzaris 2004 was single‐blind. All assessors were blind to treatment assignment. O'Donoghue 1978 did not describe blinding.
Incomplete outcome data
As a result of some missing data being only vaguely justified or not discussed two studies received an unclear risk of bias assessment for incomplete outcome data (Summers 1979; Willoughby 1971), while the remaining studies were determined to be a low risk for attrition bias.
Selective reporting
One study performed post hoc analyses that were not described in the methods section and this study was rated as unclear risk of bias for selective reporting (Maté‐Jiménez 2000). Panes 2013 reported on all pre‐specified primary and secondary outcomes and a post hoc analysis. However, the authors clearly indicated that they were reporting a post hoc analysis so we rated the study as low risk of bias for selective reporting. Mantzaris 2004 was given an unclear risk of bias assessment as it was an abstract and did not describe all outcomes in the methods section and no protocol could be located. All of the other studies received a low risk of bias assessment for selective reporting.
Other potential sources of bias
All studies were deemed to be at low risk of bias for other potential sources of bias, except Cosnes 2013, which received a unclear risk because this study included patients who had active disease and could change medications from the study medications to secondary study medications, but these changes were not described.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Azathioprine or 6‐mercaptopurine versus placebo for maintenance of remission in Crohn's disease.
| Azathioprine or 6‐mercaptopurine versus placebo for maintenance of remission in Crohn's disease | ||||||
| Patient or population: maintenance of remission in Crohn's disease Setting: Outpatient Intervention: Azathioprine or 6‐mercaptopurine Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with azathioprine or 6‐mercaptopurine | |||||
| Maintenance of remission (sensitivity analysis excluding Candy 1995) | 617 per 10001 | 734 per 1000 (648 to 827) | RR 1.19 (1.05 to 1.34) | 489 (6 RCTs) | ⊕⊕⊝⊝ LOW2,3 | |
| Adverse events | 249 per 10001 | 321 per 1000 (254 to 408) | RR 1.29 (1.02 to 1.64) | 359 (6 RCTs) | ⊕⊕⊝⊝ LOW3,4 | |
| Withdrawals due to adverse events | 25 per 10001 | 78 per 1000 (40 to 152) | RR 3.12 (1.59 to 6.09) | 661 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW3,5 | |
| Serious adverse events | 29 per 10001 | 71 per 1000 (35 to 142) | RR 2.45 (1.22 to 4.90) | 556 (4 RCTs) | ⊕⊕⊝⊝ VERY LOW6,7 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Placebo group risk estimate come from the placebo arm of meta‐analysis, based on included trials 2 Downgraded one level due to sparse data (327 events). 3 Downgraded one level due to unclear risk of bias. Five of the studies in the pooled analysis were rated as unclear risk of bias for random sequence generation, allocation concealment or incomplete outcome data. 4 Downgraded one level due to sparse data (104 events). 5 Downgraded two levels due to very sparse data (37 events). 6 Downgraded one level due to unclear risk of bias. Two of the studies in the pooled analysis were rated as unclear risk of bias for random sequence generation, allocation concealment, blinding or incomplete outcome data. 7 Downgraded two levels due to very sparse data (31 events).
Summary of findings 2. Azathioprine or 6‐mercaptopurine versus mesalazine or sulfasalazine for maintenance of remission in Crohn's disease.
| Azathioprine or 6‐mercaptopurine versus mesalazine or sulfasalazinefor maintenance of remission in Crohn's disease | ||||||
| Patient or population: maintenance of remission in Crohn's disease Setting: Outpatient Intervention: Azathioprine or 6‐mercaptopurine Comparison: Mesalazine or sulfasalazine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with mesalazine or sulfasalazine | Risk with azathioprine or 6‐mercaptopurine | |||||
| Maintenance of remission | 667 per 10001 | 727 per 1000 (587 to 894) | RR 1.09 (0.88 to 1.34) | 166 (2 RCTs) | ⊕⊕⊝⊝ LOW2,3 | |
| Adverse events | 0 per 10001 | 0 per 1000 (0 to 0) | RR 8.77 (0.54 to 142.51) | 45 (1 RCTs) | ⊕⊝⊝⊝ VERY LOW4,5 | |
| Withdrawals due to adverse events | 68 per 10001 | 126 per 1000 (59 to 270) | RR 1.86 (0.87 to 3.97) | 290 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW2,6 | |
| Serious adverse events | 0 per 10001 | 0 per 1000 (0 to 0) | RR 9.37 (1.84 to 47.72) | 235 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW2,7 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk estimate come from the control arm of meta‐analysis, based on included trials 2 Downgraded one level due to unclear and high risk of bias. One study was rated as unclear risk of bias for random sequence generation and incomplete outcome data and the other study was rated as high risk of bias for no blinding. 3 Downgraded one level due to sparse data (113 events). 4 Downgraded one level due to high risk of bias. 5 Downgraded two levels due to very sparse data (8 events). 6 Downgraded two levels due to very sparse data (28 events). 7 Downgraded two levels due to very sparse data (16 events).
Summary of findings 3. Azathioprine versus budesonide for maintenance of remission in Crohn's disease.
| Azathioprine versus budesonide for maintenance of remission in Crohn's disease | ||||||
|
Patient or population: maintenance of remission in Crohn's disease Setting: Outpatient Intervention: Azathioprine Comparison: Budesonide | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with budesonide | Risk with azathioprine | |||||
| Maintenance of remission | 462 per 10001 | 762 per 1000 (522 to 1000) | RR 1.65 (1.13 to 2.42) | 77 (1 RCT) | ⊕⊕⊝⊝ LOW2,3 | |
| Withdrawals due to adverse events | 0 per 10001 | 0 per 1000 (0 to 0) | RR 5.13 (0.25 to 103.43) | 77 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,4 | |
| Serious adverse events | 0 per 10001 | 0 per 1000 (0 to 0) | RR 5.13 (0.25 to 103.43) | 77 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk estimate come from the control arm of meta‐analysis, based on included trials 2 Downgraded one level due to high risk of bias (no blinding). 3 Downgraded one level due to sparse data (47 events). 4 Downgraded two levels due to very sparse data (2 events).
Summary of findings 4. Azathioprine and infliximab versus infliximab for maintenance of remission in Crohn's disease.
| Azathioprine and infliximab versus infliximab for maintenance of remission in Crohn's disease | ||||||
| Patient or population: maintenance of remission in Crohn's disease Setting: Outpatient Intervention: Azathioprine Comparison: Infliximab | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with infliximab |
Risk with azathioprine + infliximab |
|||||
| Maintenance of remission | 800 per 10001 | 816 per 1000 (592 to 1000) | RR 1.02 (0.74 to 1.40) | 36 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,3 | |
| Adverse events | 100 per 10001 | 160 per 1000 (33 to 786) | RR 1.60 (0.33 to 7.86) | 45 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,4 | |
| Withdrawals due to adverse events | 0 per 10001 | 0 per 1000 (0 to 0) | RR 2.42 (0.10 to 56.46) | 45 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,5 | |
| Serious adverse events | 0 per 10001 | 0 per 1000 (0 to 0) | RR 2.42 (0.10 to 56.46) | 45 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,5 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk estimate come from the control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to high risk of bias (single‐blind). 3 Downgraded two levels due to very sparse data (29 events). 4 Downgraded two levels due to very sparse data (6 events). 5 Downgraded two levels due to very sparse data (1 event).
Summary of findings 5. 6‐Mercaptopurine versus methotrexate for maintenance of remission in Crohn's disease.
| 6‐Mercaptopurine versus methotrexate for maintenance of remission in Crohn's disease | ||||||
| Patient or population: maintenance of remission in Crohn's disease Setting: Outpatient Intervention: 6‐mercaptopurine Comparison: Methotrexate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with methotrexate | Risk with 6‐mercaptopurine | |||||
| Maintenance of remission | 533 per 10001 | 501 per 1000 (251 to 987) | RR 0.94 (0.47 to 1.85) | 31 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,3 | |
| Adverse events | 407 per 10001 | 265 per 1000 (126 to 562) | RR 0.65 (0.31 to 1.38) | 57 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,4 | |
| Withdrawals due to adverse events | 148 per 10001 | 101 per 1000 (25 to 407) | RR 0.68 (0.17 to 2.75) | 57 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,5 | |
| Serious adverse events | 148 per 10001 | 101 per 1000 (25 to 407) | RR 0.68 (0.17 to 2.75) | 57 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2,5 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk estimate come from the control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to high risk of bias (no blinding). 3 Downgraded two levels due to very sparse data (16 events). 4 Downgraded two levels due to very sparse data (19 events). 5 Downgraded two levels due to very sparse data (7 events).
Maintenance of remission
Azathioprine versus placebo
Azathioprine was compared to placebo in seven studies (Candy 1995; Lémann 2005; O'Donoghue 1978; Panes 2013; Rosenberg 1975; Summers 1979; Willoughby 1971). Two of the studies included an induction phase prior to the maintenance phase; as per protocol in the studies, only the patients entering remission in the induction phase were included in the maintenance phase (Candy 1995; Summers 1979 Part 1). The pooled analysis contains data from the maintenance phase of these two studies. The pooled analysis contains subgroups based on azathioprine doses used in the various studious; 2.5 mg/kg/day (Candy 1995; Panes 2013; Summers 1979 Part 1), 2.0 mg/kg/day (Lémann 2005; O'Donoghue 1978; Rosenberg 1975; Willoughby 1971) and 1.0 mg/kg/day (Summers 1979 Part 2). In total, 532 patients were included in the pooled analysis. There was a statistically significant difference in the proportion of patients who maintained remission over 6 to 18 months favouring azathioprine over placebo. Seventy‐two per cent (175/244) of azathioprine patients were in remission at study endpoint compared to 58% (168/288) of placebo patients (RR 1.25, 95% CI 1.11 to 1.42). These studies were moderately heterogeneous (I² = 38%). We visually inspected the forest plot and one study appeared to be an outlier (Candy 1995). Further evaluation of the study characteristics showed that Candy 1995 used a CDAI of less than 175 as criteria for remission, which was different from all the other studies using CDAI. A sensitivity analysis excluding Candy 1995 appears to explain the heterogeneity. The pooled analysis included six studies and 489 participants and there was a statistically significant difference in the proportion of patients who maintained remission over 6 to 18 months favouring azathioprine over placebo. Seventy‐three per cent (161/220) of azathioprine remained in remission at study endpoint compared to 62% (166/269) of placebo patients (RR 1.19, 95% CI 1.05 to 1.34; I2 = 0%). The number needed to treat for an additional beneficial outcome was 9. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (327 events) and an unclear risk of bias in 5 of the studies in the pooled analysis. The pooled analysis for the 2.0 mg/kg/day subgroup showed a statistically significant difference favouring azathioprine over placebo (RR 1.33, 95% CI 1.10 to 1.61). There were no statistically significant differences between azathioprine and placebo for the 2.5 mg/kg/day (RR 1.17, 95% CI 0.94 to 1.45) or 1.0 mg/kg/day (RR 1.06, 95% CI 0.84 to 1.34) subgroups.
Azathioprine or 6‐mercaptopurine versus mesalazine or sulfasalazine
Maté‐Jiménez 2000 and Summers 1979 (Part 1, phase 2 and Part 2) compared azathioprine or 6‐mercaptopurine to mesalazine or sulfasalazine. A pooled analysis of these studies included 166 patients. Subgroups are reported for the various doses of azathioprine and 6‐mercaptopurine: azathioprine 2 to 2.5 mg/kg/day, azathioprine 1.0 mg/kg/day and 6‐mercaptopurine 50 mg/day. There was no statistically significant difference in the proportion of patients who maintained remission at 12 months. Sixty‐nine percent (61/88) of participants maintained remission on azathioprine or 6‐mercaptopurine compared to 67% (52/78) who maintained remission on mesalamine or sulfasalazine (RR 1.09, 95% CI 0.88 to 1.34). The studies were not found to be heterogeneous (P = 0.72; I² = 0%). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (113 events) and risk of bias. The risk of bias in the Maté‐Jiménez 2000 study was high and the risk of bias in the Summers 1979 study was unclear.
Azathioprine versus budesonide
Azathioprine was compared to budesonide in one small study (Mantzaris 2009). There was a statistically significant difference in the proportion of patients who maintained remission at 12 months favouring azathioprine over budesonide. Seventy‐six per cent (29/38) of patients in the azathioprine group maintained remission compared to 46% (18/39) of budesonide patients (RR 1.65, 95% CI 1.13 to 2.42). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (47 events) and high risk of bias.
Azathioprine + infliximab versus infliximab
One small study compared combination therapy with infliximab and azathioprine to infliximab monotherapy. There was no statistically significant difference in the proportion of patients who maintained remission at 12 months. Eighty‐one per cent (13/16) of patients in the combination therapy group maintained remission compared to 80% (16/20) of patients in the infliximab monotherapy group (RR 1.02, 95% CI 0.74 to 1.40). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (29 events) and high risk of bias.
6‐Mercaptopurine versus methotrexate
Maté‐Jiménez 2000 compared 6‐mercaptopurine to methotrexate. There was no statistically significant difference in the proportion of patients who maintained remission at 76 weeks. Fifty per cent (8/16) of patients in the 6‐mercaptopurine group maintained remission at 76 weeks compared to 53% (8/15) of methotrexate patients (RR 0.94, 95% CI 0.47 to 1.85). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (16 events) and high risk of bias.
Early administration of azathioprine versus conventional management
One study (N = 147 participants) compared the early administration of azathioprine to a conventional management strategy (Cosnes 2013). The study failed to show any significant benefit for early azathioprine treatment over a conventional management strategy. In the early azathioprine treatment group 67% (11‐85%) of trimesters were spent in remission compared to 56% (29‐73%) in the conventional management group.
Adverse events
Azathioprine or 6‐mercaptopurine versus placebo
Safety data were reported in each of the seven studies that compared azathioprine to placebo. Three of the seven studies include data for adverse events, withdrawals due to adverse events and serious adverse events (Lémann 2005; O'Donoghue 1978; Panes 2013). Summers 1979 didn't report on the proportion of patients who experienced at least one adverse event. Three studies did not report on serious adverse events (Candy 1995; Rosenberg 1975; Willoughby 1971). The studies including an induction phase (Candy 1995; Summers 1979), did not describe safety data by trial phase and it is unknown whether the adverse events occurred during the induction or maintenance phase. Similarly, Willoughby 1971, did not separate the reported toxicity data by group, and instead reported it across all study participants. For these studies we report the safety data that were available.
A pooled analysis of six studies (359 participants) showed that a significantly higher proportion of azathioprine patients experienced at least one adverse event compared to placebo (Candy 1995; Lémann 2005; O'Donoghue 1978; Panes 2013; Rosenberg 1975; Willoughby 1971). Thirty‐three per cent of azathioprine patients experienced at least one adverse event compared to 25% of placebo patients (RR 1.29, 95% CI 1.02 to 1.64). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (104 events) and unclear risk of bias. Five of the studies in the pooled analysis were rated as unclear risk of bias for random sequence generation, allocation concealment or incomplete outcome data.
A pooled analysis of seven studies (661 participants) showed that a significantly higher proportion of azathioprine patients withdrew due to adverse events compared to placebo (Candy 1995; Lémann 2005; O'Donoghue 1978; Panes 2013; Rosenberg 1975; Summers 1979; Willoughby 1971). Nine per cent (28/299) of azathioprine patients withdrew due to adverse events compared to 2% (9/362) of placebo patients (RR 3.12, 95% CI 1.59 to 6.09). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (37 events) and unclear risk of bias. Five of the studies in the pooled analysis were rated as unclear risk of bias for random sequence generation, allocation concealment or incomplete outcome data. Common adverse events leading to withdrawal included pancreatitis, leukopenia, nausea, allergic reaction and infection. In a few patients, the adverse event causing withdrawal was not specified.
A pooled analysis of four studies (556 patients) showed that a significantly higher proportion of azathioprine patients experienced a serious adverse event compared to placebo (Lémann 2005; O'Donoghue 1978; Panes 2013; Summers 1979). Nine per cent (22/245) of azathioprine patients experienced a serious adverse event compared to 3% (9/311) of placebo patients (RR 2.45, 95% CI 1.22 to 4.90). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (31 events) and unclear risk of bias.Two of the studies in the pooled analysis were rated as unclear risk of bias for random sequence generation, allocation concealment, blinding or incomplete outcome data. The most common serious adverse event was leukopenia.
Azathioprine or 6‐mercaptopurine versus mesalazine or sulfasalazine
Maté‐Jiménez 2000 found no statistically significant difference in the proportion of patients who experienced at least one adverse event. Twenty‐seven per cent (8/30) of 6‐mercaptopurine patients experienced at least one adverse event compared to 0% (0/15) of mesalazine patients (RR 8.77, 95% CI 0.54 to 142.51). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (8 events) and high risk of bias. The most common adverse events experienced in the 6‐mercaptopurine group were leukopenia, nausea and diarrhoea, melanosis and mild alopecia, with leukopenia being a serious event.
A pooled analysis of two studies (290 participants) showed no statistically significant difference in withdrawals due to adverse events (Maté‐Jiménez 2000; Summers 1979). Thirteen per cent (18/143) of patients in the azathioprine/6‐mercaptopurine group withdrew due to an adverse event compared to 7% (10/147) of patients in the mesalazine/sulfasalazine group (RR 1.86, 95% CI 0.87 to 3.97). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (28 events) and unclear or high risk of bias. One study was rated as unclear risk of bias for random sequence generation and incomplete outcome data and the other study was rated as high risk of bias for no blinding.
A pooled analysis of two studies (235 participants) showed a statistically significant difference in the proportion of patients who experienced a serious adverse event (Maté‐Jiménez 2000; Summers 1979). Thirteen per cent (16/125) of patients in the azathioprine/6‐mercaptopurine group had a serious adverse event compared to 0% (0/110) of patients in the mesalazine/sulfasalazine group (RR 9.37, 95% CI 1.84 to 47.72). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (16 events) and unclear or high risk of bias. One study was rated as unclear risk of bias for random sequence generation and incomplete outcome data and the other study was rated as high risk of bias for no blinding.
Azathioprine versus budesonide
Mantzaris 2009 reported that 112 adverse events occurred in the 38 patients receiving azathioprine compared to 83 adverse events among the 39 patients in the budesonide group. The proportion of patients in each group who experienced at least one adverse event was not reported. Adverse events included pancreatitis, severe leukopenia, infections, transient abdominal pain, and diarrhea. Adverse events thought to be drug‐related included transient paresthesias, hair loss, and elevated transaminases in the azathioprine group and mild acne, moon face, and transient hair loss in the budesonide group. There was no statistically significant difference in the proportion of patients who withdrew due to adverse events. Five per cent (2/38) of azathioprine patients withdrew due to adverse events compared to 0% (0/39) of budesonide patients (RR 5.13, 95% CI 0.25 to 103.43). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (2 events) and high risk of bias. The reported withdrawals were due to serious adverse events including pancreatitis and severe leukopenia. There was no statistically significant difference in the proportion of patients who experienced a serious adverse event (RR 5.13, 95% CI 0.25 to 103.43). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (2 events) and high risk of bias.
Azathioprine + infliximab versus infliximab
Mantzaris 2004 reported that there was no statistically significant difference in the proportion of patients who experienced at least one adverse event. Sixteen per cent (4/25) of patients in the combination therapy group experienced at least one adverse event compared to 10% (2/20) of patients in the infliximab monotherapy group (RR 1.60, 95% CI 0.33 to 7.86). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (6 events) and unclear risk of bias. In the combination therapy group four patients had leukopenia, which was serious in one patient. In the infliximab monotherapy group two patients had acute infusion reactions. The only withdrawal due to an adverse event occurred in the combination therapy group (severe leukopenia also classified as a serious adverse event). There was no statistically significant difference in the proportion of patients who withdrew due to an adverse event or who experienced a serious adverse event (RR 2.42, 95% CI 0.10 to 56.46). GRADE analyses indicated that the overall quality of the evidence supporting these outcomes was very low due to very sparse data (1 event for both comparisons) and unclear risk of bias.
6‐Mercaptopurine versus methotrexate
Maté‐Jiménez 2000 reported no statistically significant differences in the proportion of patients who experienced at least one adverse event. Twenty‐seven per cent (8/30) of 6‐mercaptopurine patients experienced at least one adverse event compared to 41% (11/27) of methotrexate patients (RR 0.65, 95% CI 0.31 to 1.38). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (19 events) and high risk of bias. Adverse events reported in the 6‐mercaptopurine group included leukopenia, nausea and diarrhoea, melanosis and mild alopecia, with leukopenia being a serious adverse event. Adverse events reported in the methotrexate group included nausea and vomiting, mild alopecia, increases in AST, development of perianal abscess, and serious adverse events including hypoalbuminemia, severe rash and atypical pneumonia. Withdrawals from the study were a result of serious adverse events in both arms of the trial. Ten per cent (3/30) of 6‐mercaptopurine patients (3/30) withdrew after a serious adverse event compared to 15% (4/27) of methotrexate patients. There was no statistically significant difference in the proportion of patients who withdrew due to an adverse event or who experienced a serious adverse event (RR 0.68, 95% CI 0.17 to 2.75). GRADE analyses indicated that the overall quality of the evidence supporting these outcomes was very low due to very sparse data (7 events for both comparisons) and high risk of bias.
Steroid sparing
In the two very small studies reporting steroid sparing data (Willoughby 1971; Rosenberg 1975), 87% (13/15) patients receiving maintenance therapy with azathioprine were able to reduce or discontinue steroids (steroid sparing) compared to 53% (8/15) patients receiving placebo (RR 1.59, 95% CI 0.97 to 2.61).
Discussion
This systematic review included 11 randomized controlled trials (881 participants) that evaluated the efficacy and safety of azathioprine or 6‐mercaptopurine for maintaining remission achieved by medical therapy in patients with quiescent Crohn's disease. Seven studies compared azathioprine to placebo (Candy 1995; Lémann 2005; O'Donoghue 1978; Panes 2013; Rosenberg 1975; Summers 1979; Willoughby 1971). Summers 1979 also compared azathioprine to sulfasalazine. Maté‐Jiménez 2000 compared 6‐mercaptopurine to mesalazine or methotrexate. One study compared azathioprine to budesonide (Mantzaris 2009); one study compared the combination of azathioprine and infliximab to infliximab monotherapy (Mantzaris 2004); and one study compared 6‐mercaptopurine to methotrexate (Maté‐Jiménez 2000). One study compared the early administration of azathioprine to a conventional management strategy (Cosnes 2013).
A pooled analysis of six studies (489 participants) suggests that azathioprine (1.0 to 2.5 mg/kg/day) is superior to placebo for maintenance of remission in quiescent Crohn's disease over a 6 to 18 month period. The number needed to treat for an additional beneficial outcome was nine. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (327 events) and unclear risk of bias. Five of the studies in the pooled analysis were rated as unclear risk of bias for random sequence generation, allocation concealment or incomplete outcome data. Although azathioprine may be effective for maintenance of remission it is associated with a significantly increased risk of adverse events, withdrawal due to adverse events and serious adverse events relative to placebo. Common adverse events reported in the placebo‐controlled trials include pancreatitis, leukopenia, nausea, allergic reaction and infection. The adverse effects of azathioprine are well recognized in clinical practice and limit its use. Azathioprine‐related serious adverse events lead to cessation of therapy in 9 to 25% of patients (Gearry 2004; Gearry 2005). Adverse events associated with azathioprine and 6‐mercaptopurine include nausea, allergic reaction, flu‐like illness, malaise, fevers, rash, abdominal pain, pancreatitis, hepatotoxicity, myelosuppression, and an increased risk of lymphoma (Dubinsky 2004; Kandiel 2005; Beaugerie 2008). Advances in the understanding of azathioprine and 6‐mercaptopurine drug metabolism have led to genetic and metabolite tests to help clinicians optimise the use of these drugs. Thiopurine methyltransferase (TPMT) enzyme activity can predict potentially life threatening myelosuppression in patients who are TPMT‐deficient (Dubinsky 2004; Gearry 2005). TPMT may also aid in determining optimal dosing (Dubinsky 2004; Gearry 2005). 6‐Thioguanine nucleotides and 6‐methylmercaptopurine concentrations are helpful in determining why a patient does not respond to a standard dose of azathioprine or 6‐mercaptopurine and may help avoid myelosuppression (Dubinsky 2004; Gearry 2005). The ratio of these metabolites can help differentiate between non‐compliance, under‐dosing, and drug resistance or refractory states (Dubinsky 2004; Gearry 2005). More research is required to determine the optimal use of these tests in patients with Crohn's disease. However, a randomized controlled trial looking at the role of metabolite monitoring proved not to be feasible and was terminated due to slow patient recruitment (NIDDK 2008).
The results of one small study (77 participants) suggest that azathioprine (2.0 to 2.5 mg/kg/day) may be superior to budesonide (6 to 9 mg/day) for maintenance of remission at one year (Mantzaris 2009). However this result should be interpreted with caution as a GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (47 events) and high risk of bias (no blinding). There were no differences in withdrawals due to adverse events or serious adverse events. However, GRADE analyses indicated the quality of the evidence supporting these outcomes was very low due to very sparse data (two events for both comparisons) and high risk of bias. Further research is needed to allow any firm conclusions regarding the comparative efficacy and safety of azathioprine and budesonide for maintenance treatment in quiescent Crohn's disease.
A pooled analysis of two studies (166 participants) found no difference between azathioprine (1.0 to 2.5 mg/kg/day) or 6‐mercaptopurine (1.0 mg/kg/day) and aminosalicylates (mezalazine 3 g/day or sulfasalazine 0.5 g/15 kg) in the proportion of patients who maintained remission at 12 months. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data (113 events) and high or unclear risk of bias. Summers 1979 was rated as unclear risk of bias for random sequence generation and incomplete outcome data and Maté‐Jiménez 2000 was rated as high risk of bias for no blinding. A pooled analysis of two studies showed that antimetabolites (azathioprine or 6‐mercaptopurine) had a significantly increased risk of serious adverse events compared to aminosalicylates (mesalazine or sulphsalazine). However, a GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to sparse data (16 events) and high or unclear risk of bias. Further research with adequately powered trials is needed to allow any definitive conclusions regarding the comparative efficacy and safety of antimetabolites and aminosalicylates for maintenance therapy in Crohn's disease.
One small study (31 participants) found no difference between 6‐mercaptopurine (1 mg/kg/day) and methotrexate (10 mg/week) in the proportion of patients who maintained remission at 72 weeks. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (16 events) and high risk of bias. No differences were found with respect to adverse events, withdrawals due to adverse events, or serious adverse events. GRADE analyses indicated that the overall quality of the evidence supporting these outcomes was very low due to very sparse data (19, 5 and 5 events respectively) and high risk of bias. Moderate quality evidence from a single high quality trial indicates that intramuscular methotrexate at a dose of 15 mg/week is superior to placebo for maintenance of remission in Crohn's disease. The number needed to treat with methotrexate for an additional beneficial outcome was four (Patel 2014). Only two small studies compare 6‐mercaptopurine to methotrexate for maintenance of remission in Crohn's disease (Maté‐Jiménez 2000; Oren 1997), and no studies compare azathioprine to methotrexate. Further research with adequately powered trials is necessary to allow any conclusions regarding the comparative efficacy and safety of antimetabolites (azathioprine or 6‐mercaptopurine) and methotrexate for maintenance therapy in Crohn's disease.
One small study (36 participants) found no difference in maintenance of remission rates at one year between combination therapy with azathioprine (2.5 mg/kg/day) and infliximab (5 mg/kg every 8 weeks) compared to infliximab monotherapy. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to very sparse data (29 events) and high risk of bias. An adequately powered trial would be necessary to allow for any conclusions regarding the role of azathioprine as an adjunctive to infliximab maintenance therapy in Crohn's disease. There is moderate quality evidence that combination therapy with azathioprine and infliximab is superior to infliximab monotherapy for induction of remission in moderate to severe Crohn's disease (Chande 2013; Colombel 2010). Furthermore, therapy with azathioprine and 6‐mercaptopurine may help to prevent the development of antibodies to infliximab. The development of antibodies to infliximab has been associated with infusion reactions and loss of response to infliximab. An analysis of the ACCENT I induction trial data by Hanauer 2004b found that patients who received therapy with azathioprine, 6‐mercaptopurine or methotrexate in conjunction with infliximab had a significantly lower chance of developing antibodies to infliximab than patients who received infliximab monotherapy. Future maintenance trials assessing combination therapy should evaluate the interaction between antimetabolite (azathioprine or 6‐mercaptopurine) therapy and infliximab with respect to antibody formation and efficacy.
One study (147 participants) failed to show any significant benefit for early azathioprine treatment over a conventional management strategy. In the early azathioprine treatment group 67% (11‐85%) of trimesters were spent in remission compared to 56% (29‐73%) in the conventional management group. The results of this trial need to be confirmed by another study. Further research is required to determine optimal management strategies for patients with quiescent Crohn's disease.
The current review only included two studies where existing therapy was discontinued (Lémann 2005; O'Donoghue 1978). Vilien 2004 which was excluded from this review, randomized patients to continue azathioprine therapy or to a no treatment control. The findings of this trial demonstrated that azathioprine was superior to no treatment for maintenance of remission (Vilien 2004). Future studies could randomize patients started on a maintenance regimen to discontinue therapy after a given amount of time spent in remission (i.e. randomize to groups based on when they stop taking azathioprine or 6‐mercaptopurine). These patients could then be analysed on time in remission on azathioprine or 6‐mercaptopurine and risk of relapse thereafter. Such trials would enable physicians to better explain to their patients their likelihood of relapsing if they stop therapy. Moreover, such trials would enable physicians to better weigh the risks of adverse events with therapy to the risk of relapse without therapy.
We attempted to limit bias by performing a comprehensive literature search to identify all applicable studies. At least two review authors independently assessed studies for inclusion, extracted data and assessed study quality. However, the review does have some limitations. Only two of the included studies were rated as low risk of bias (Lémann 2005; Panes 2013). Three studies were rated as high risk of bias because they were not blinded (Cosnes 2013; Mantzaris 2009; Maté‐Jiménez 2000). The remaining six studies were rated as unclear risk of bias for random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other bias. Furthermore, GRADE analyses indicated that the overall quality of the evidence supporting the outcomes in this review ranged from low to very low quality.
Authors' conclusions
Implications for practice.
Low quality evidence suggests that azathioprine is more effective than placebo for maintenance of remission in Crohn's disease. Although azathioprine may be effective for maintenance of remission its use may be limited by the potential for adverse events. Low quality evidence suggests that azathioprine may be superior to budesonide for maintenance of remission but because of small study size and high risk of bias, this result should be interpreted with caution. No conclusions can be drawn from the other active comparator studies because of low and very low quality evidence. The choice to use antimetabolite therapy should be individualized, and therapy should be undertaken only after a careful discussion of the risks and benefits with the patient.
Implications for research.
Adequately powered trials are needed to determine the comparative efficacy and safety of azathioprine and 6‐mercaptopurine compared to other active maintenance therapies. Further research is needed to assess the efficacy and safety of the use of azathioprine with infliximab and other biologics and to determine the optimal management strategy for patients quiescent Crohn's disease.
What's new
| Date | Event | Description |
|---|---|---|
| 5 May 2016 | Amended | New risk of bias data added for included study |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1997
| Date | Event | Description |
|---|---|---|
| 30 June 2015 | New citation required and conclusions have changed | Substantively updated review with new conclusions and authors. |
| 30 June 2015 | New search has been performed | New literature searches conducted on June 30, 2015. New studies added. |
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
The authors would like to thank Gerassimos Mantzaris for providing additional information about his 2004 study
Appendices
Appendix 1. Electronic search strategy
1. EMBASE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. exp Crohn disease/
22. Crohn.mp.
23. (inflammatory bowel disease* or IBD).mp.
24. 21 or 22 or 23
25. 20 and 24
26. (anti‐metabolite* or anti‐metabolite* or antimetabolite*).mp.
27. (6‐mercaptopurine or mercaptopurine or 6‐MP or 6MP).mp.
28. (AZA or azathioprine).mp.
29. exp azathioprine/
30. or/26‐29
31. 25 and 30
2. MEDLINE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. exp Crohn disease/
22. Crohn.mp.
23. (inflammatory bowel disease* or IBD).mp.
24. 21 or 22 or 23
25. 20 and 24
26. (anti‐metabolite* or anti‐metabolite* or antimetabolite*).mp.
27. (6‐mercaptopurine or mercaptopurine or 6‐MP or 6MP).mp.
28. (AZA or azathioprine).mp.
29. exp azathioprine/
30. or/26‐29
31. 25 and 30
3. Cochrane Library
#1 crohn #2 crohn's or crohn's disease #3 inflammatory bowel disease or IBD #4 MeSH descriptor Crohn Disease explode all trees #5 MeSH descriptor Inflammatory Bowel Diseases explode all trees #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 anti‐metabolite* or anti‐metabolite* or antimetabolite* #8 MeSH descriptor Antimetabolites explode all trees #9 6‐mercaptopurine or mercaptopurine or 6‐MP #10 MeSH descriptor 6‐Mercaptopurine explode all trees #11 AZA or azathioprine #12 MeSH descriptor Azathioprine explode all trees #13 (#7 OR #8 OR #9 OR #10 OR #11 OR #12) #14 (#6 AND #13)
4. IBD Group Specialized Register
1. (title or abstract) antimetabol*
2. (title or abstract) 6‐MP
3. (title or abstract) AZA*
4. (title or abstract) 6MP
5. (title or abstract) 6‐mercaptopurine
6. (title or abstract) mercaptopurine
Data and analyses
Comparison 1. Azathioprine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of remission | 7 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.11, 1.42] |
| 1.1 Azathioprine dose 2.5 mg/kg/day | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.08, 1.68] |
| 1.2 Azathioprine dose 2.0 mg/kg/day | 4 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.10, 1.61] |
| 1.3 Azathioprine dose 1.0 mg/kg/day | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 2 Maintenance of remission (sensitivity analysis excluding Candy 1995) | 6 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.05, 1.34] |
| 2.1 Azathioprine dose 2.5 mg/kg/day | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.94, 1.45] |
| 2.2 Azathioprine dose 2.0 mg/kg/day | 4 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.10, 1.61] |
| 2.3 Azathioprine dose 1.0 mg/kg/day | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 3 Adverse events | 6 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.02, 1.64] |
| 4 Withdrawals due to adverse events | 7 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [1.59, 6.09] |
| 5 Serious adverse events | 4 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.22, 4.90] |
| 6 Steroid sparing effect | 2 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.97, 2.61] |
1.1. Analysis.
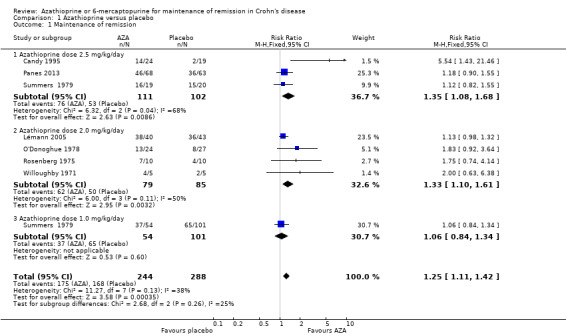
Comparison 1 Azathioprine versus placebo, Outcome 1 Maintenance of remission.
1.2. Analysis.

Comparison 1 Azathioprine versus placebo, Outcome 2 Maintenance of remission (sensitivity analysis excluding Candy 1995).
1.3. Analysis.
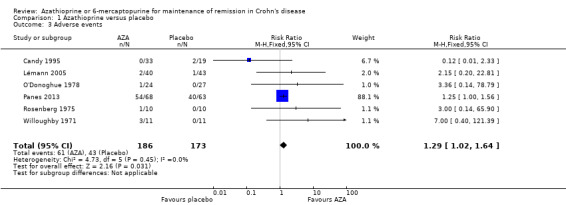
Comparison 1 Azathioprine versus placebo, Outcome 3 Adverse events.
1.4. Analysis.
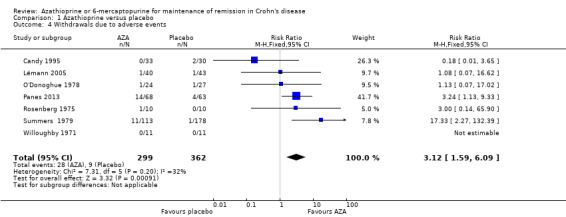
Comparison 1 Azathioprine versus placebo, Outcome 4 Withdrawals due to adverse events.
1.5. Analysis.

Comparison 1 Azathioprine versus placebo, Outcome 5 Serious adverse events.
1.6. Analysis.

Comparison 1 Azathioprine versus placebo, Outcome 6 Steroid sparing effect.
Comparison 2. Azathioprine or 6‐mercaptopurine versus mesalazine or sulfasalazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of remission | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.88, 1.34] |
| 1.1 Azathioprine 2‐2.5mg/kg/day | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.76, 1.32] |
| 1.2 Azathioprine 1.0 mg/kg/day | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.45] |
| 1.3 6‐mercaptopurine 1.5mg/kg | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.18, 24.48] |
| 2 Adverse events | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.77 [0.54, 142.51] |
| 2.1 Azathioprine 2‐2.5mg/kg/day | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Azathioprine 1.0 mg/kg/day | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 6‐Mercaptopurine 50mg/day | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.77 [0.54, 142.51] |
| 3 Withdrawals due to adverse events | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.87, 3.97] |
| 3.1 Azathioprine 2‐2.5mg/kg/day | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.35, 2.56] |
| 3.2 Azathioprine 1.0mg/kg/day | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.95, 19.34] |
| 3.3 6‐mercaptopurine 50mg/day | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.65 [0.27, 81.01] |
| 4 Serious adverse events | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.37 [1.84, 47.72] |
| 4.1 Azathioprine 2‐2.5mg/kg/day | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.0 [1.15, 313.35] |
| 4.2 Azathioprine 1mg/kg/day | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.36 [0.26, 109.26] |
| 4.3 6‐mercaptopurine 50mg/day | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.65 [0.27, 81.01] |
2.1. Analysis.
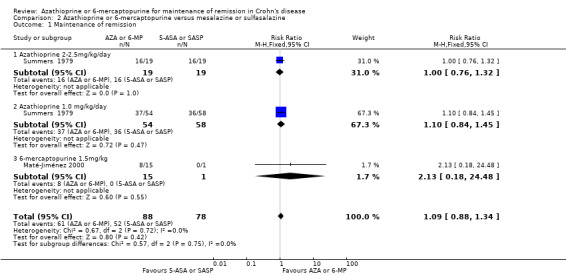
Comparison 2 Azathioprine or 6‐mercaptopurine versus mesalazine or sulfasalazine, Outcome 1 Maintenance of remission.
2.2. Analysis.
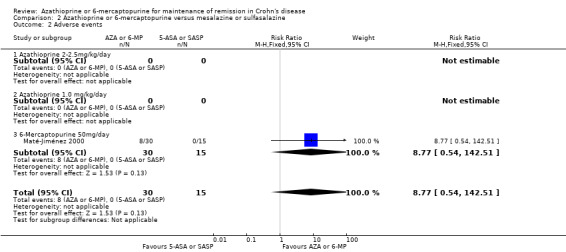
Comparison 2 Azathioprine or 6‐mercaptopurine versus mesalazine or sulfasalazine, Outcome 2 Adverse events.
2.3. Analysis.
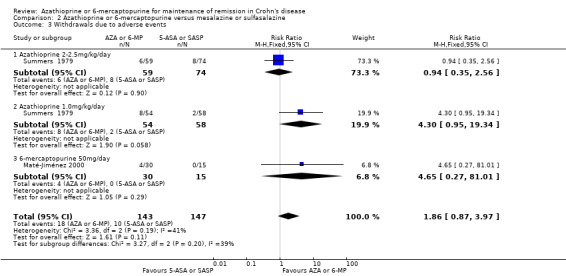
Comparison 2 Azathioprine or 6‐mercaptopurine versus mesalazine or sulfasalazine, Outcome 3 Withdrawals due to adverse events.
2.4. Analysis.

Comparison 2 Azathioprine or 6‐mercaptopurine versus mesalazine or sulfasalazine, Outcome 4 Serious adverse events.
Comparison 3. Azathioprine versus budesonide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3 Azathioprine versus budesonide, Outcome 1 Maintenance of remission.
3.2. Analysis.

Comparison 3 Azathioprine versus budesonide, Outcome 2 Withdrawals due to adverse events.
3.3. Analysis.

Comparison 3 Azathioprine versus budesonide, Outcome 3 Serious adverse events.
Comparison 4. Azathioprine + infliximab versus infliximab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.

Comparison 4 Azathioprine + infliximab versus infliximab, Outcome 1 Maintenance of remission.
4.2. Analysis.
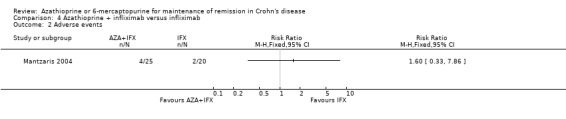
Comparison 4 Azathioprine + infliximab versus infliximab, Outcome 2 Adverse events.
4.3. Analysis.
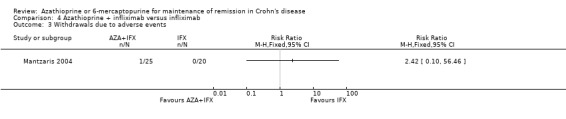
Comparison 4 Azathioprine + infliximab versus infliximab, Outcome 3 Withdrawals due to adverse events.
4.4. Analysis.

Comparison 4 Azathioprine + infliximab versus infliximab, Outcome 4 Serious adverse events.
Comparison 5. 6‐Mercaptopurine versus methotrexate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of remission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.

Comparison 5 6‐Mercaptopurine versus methotrexate, Outcome 1 Maintenance of remission.
5.2. Analysis.
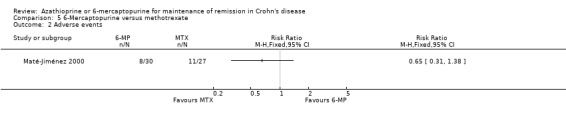
Comparison 5 6‐Mercaptopurine versus methotrexate, Outcome 2 Adverse events.
5.3. Analysis.
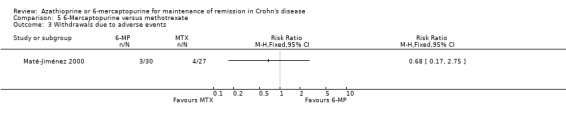
Comparison 5 6‐Mercaptopurine versus methotrexate, Outcome 3 Withdrawals due to adverse events.
5.4. Analysis.

Comparison 5 6‐Mercaptopurine versus methotrexate, Outcome 4 Serious adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Candy 1995.
| Methods | Disease‐site stratified and randomized, double‐blind, placebo‐controlled, two phase trial performed at a single centre. Analysed by intention to treat | |
| Participants | Patients (aged 15‐65), N=63 (45 women, 18 men), with active Crohn's Disease (CDAI > 200) confirmed by standard radiological or endoscopic evaluation. Study participant exclusionary criteria: previous extensive surgery for Crohn's disease or symptoms suggestive of mechanical obstruction (might require imminent surgery), recently received immunosuppressors, had compromised hepatic function, or were pregnant or lactating Phase 1: Intervention group (n = 33) and placebo group (n = 30) Phase 2: Patients in remission at 12 weeks, CDAI < 150 Intervention group (n = 24) and placebo group (n = 19) |
|
| Interventions | Azathioprine, 50 mg/day for the first week to prevent idiosyncratic leucopenia, increased to 2.5 mg/Kg if patient's white count was normal for remainder of the 15 months (if white count was < 4x109, dose after 1 week was increased to 2 mg/Kg (or at physician's discretion)) or matched placebo. Prednisolone, 1 mg/kg/day with a 5 mg/wk taper over 12 weeks Phase 1: prednisolone tapered over 12 weeks + azathioprine or matched placebo Phase 2: patients continued on azathioprine (2.5 mg/kg/day) or placebo for additional 12 months All other medications for Crohn's were ceased with the exception of antidiarrhoeals, which use was monitored at each visit |
|
| Outcomes | Primary outcomes: Remission induction (phase 1) and maintenance of remission (phases 2); with remission defined as CDAI < 150, and relapse defined as CDAI > 175 Secondary outcomes: median change in CDAI, ESR, serum CRP, serum orosomucoid concentration, and leucocyte count between the first and last visits Differences in leucocyte count between baseline and final visit were evaluated for responders versus non‐responders within the intervention group |
|
| Notes | Advese events and compliance were also monitored. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not described. Contains sub‐stratification for disease site; method not described, but matched in control and intervention groups |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded, control and intervention groups matched for treatment regime (taken once daily), including azathioprine and matching placebo Physician who monitored hematological and biochemical data was independent of the trial Nurse practitioner relayed dose adjustment information to patient, sampled blood, monitored compliance via pill count and assessed symptoms Physical exam physician monitored for adverse events and concomitant therapies taken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were approximately balanced between intervention and placebo groups across the 2 phases of the trial, with similar reasons, other than phase failure, provided, including side effects, loss to follow‐up or requested withdrawal |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes described in the methods section were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Cosnes 2013.
| Methods | 3‐year, randomized, parallel, open‐label trial performed at 24 centers in France | |
| Participants | Patients (aged ≥ 18 years) (N = 147; intervention n = 72) diagnosed with CD within 6 months of study entry High risk patients (for disabling disease) were those with ≥ 2 of the following: ‐ age < 40 years ‐ active perianal lesions ‐ corticosteroid use within 3 months of dx Patient exclusionary criteria: previous treatment with immunomodulators or anti–TNF therapy; immediate need for surgery or anti‐TNF therapy; severe comorbidity; documented infection; renal or liver failure; contraindication to thiopurines according to labelling recommendations; malignancy; history of drug abuse; predictable poor compliance; or pregnancy |
|
| Interventions | Intervention group: 2.5 mg/kg/day azathioprine, using a step‐up adaptation dosing schedule (switched to mercaptopurine if tolerance was poor; switched to methotrexate (25 mg/wk sc) if pancreatitis developed Control group: conventional management For management of flares (CDAI > 150), either group received prednisone (40 mg to 1 mg/kg daily for 3 weeks tapered within 2 months) or budesonide (9 mg/day for 1 month tapered within 2 months) If this therapy failed or was refused, patients received anti‐TNF medication |
|
| Outcomes | Primary outcome: proportion of trimesters spent in remission over the 3‐year trial Remission defined as a trimester spent free of disease flare, treatment with steroid or anti‐TNF drugs, active perianal disease, CD‐related hospitalization (including those caused by drug side effects); or surgery Secondary outcomes: duration of significant corticosteroid exposure (number of days with daily dose >10 mg of prednisone or >3 mg of budesonide) per trimester; total exposures (in milligrams) of prednisone and budesonide per trimester; time to first perianal surgery, first intestinal resection, and first anti‐TNF use; median CDAI scores and C‐reactive protein concentrations at follow‐up; IBDQ (IBD Questionnaire) scores at months 12, 24, and 36; total number of days of hospitalisation; and total number of days out of work per trimester |
|
| Notes | Safety data was also monitored | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed centrally with computer‐derived permutation tables of size 4 and stratified by centre. Patients were assigned identification codes, which were sent to the investigator after receipt and validation of the inclusion form by the statistical centre (this centre was independent from the funding source) |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients and investigators were not blind to the therapeutic strategy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were approximately balanced between intervention and control groups. Reasons for each withdrawal were appropriately described. Intention to treat analysis performed |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes described in the methods section were reported |
| Other bias | Unclear risk | This study included patients who had active disease and could change medications from the study medications to secondary study medications, but these changes were not described |
Lémann 2005.
| Methods | Randomized, double‐blind, placebo‐controlled withdrawal study conducted for 18 months at 11 French and 1 Belgian site. Analysised with intention to treat | |
| Participants | Patients (N = 83), aged ≥18 years, with Crohn's disease, confirmed by standard clinical, endoscopic, radiological, and histological criteria, that is in remission and have been treated with azathioprine for at least 42 months Specific inclusion criteria: continuous AZA treatment for minimum 42 months during which there was no: flare‐up; oral prednisone (>10 mg/day), budesonide or other immunosuppressive or biological agents; artificial nutrition; surgery (except limited perianal surgery); and during the preceding 6 months there was no treatment with rectal steroids, aminosalicylates, metronidazole, or ciprofloxacin Specific exclusion criteria: patient had active disease (CDAI>150 at entry); disease was limited to the perianal area; or if patient received AZA to prevent postoperative recurrence after curative surgical resection |
|
| Interventions | Azathioprine, continued at pre‐study individualized doses (mean 1.7 mg/kg/day) versus identically matched placebo (pre‐study AZA ceased) for 18 months | |
| Outcomes | Proportion of patients to experience clinical relapse in 18 months, relapse defined as CDAI > 250 or CDAI of 150‐250 on 3 consecutive weeks + an increase of ≥ 75 points above baseline score, or the need for surgery for Crohn’s disease (except limited perianal surgery) | |
| Notes | Adverse events were also monitored | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not adequately described |
| Allocation concealment (selection bias) | Low risk | Randomization was performed centrally by using permutation tables of size 2 or 4 after stratification by centre according to the number of patients anticipated to be enrolled at each centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, matched placebo for dose regime, appearance and taste to AZA (25 mg tablets). PI blinding maintained at each site by a co‐investigator, who did not have physical contact with the patients, reviewing biochemical data and reporting back to PI on a case form. Any dosing changes were completed on a pre‐specified schedule based on hematologic results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/83 patients withdrew from the study with reasons given (2 from azathioprine group, 3 from placebo group) Data was analyzed by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Relaspe rates and adverse events were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mantzaris 2004.
| Methods | Randomized, controlled two phase trial Induction phase (6 weeks) and maintenance phase (54 weeks) |
|
| Participants | Patients (N = 47), aged 18‐57 years, with active Crohn's disease (CDAI > 150) unable to taper off steroids Exclusion criteria: patients with fibrostenotic or fistulizing CD; septic complications; previous treatment with immunomodulators Phase 1: Combination group (n = 25) and IFX only group (n = 20) Phase 2: Combination group (n = 20) and IFX only group (n = 16) |
|
| Interventions | Phase 1: Induction of remission with tapered steroids with Infliximab (5 mg/kg at week 0,2,6) or infliximab and AZA (2.5 mg/kg/day) for 6 weeks Phase 2: Maintenance of remission phase with infliximab (5mg/kg every 8 weeks) or infliximab and AZA (2.5 mg/kg/day) |
|
| Outcomes | Primary outcomes: Phase 1‐ induction of remission at week 6; only those achieving remission continued in the maintenance phase Phase 2‐ maintenance of remission at an additional 12 months |
|
| Notes | Only the abstract was available
Adverse effects were also monitored The first author provided additional information which allowed us to assess the risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind All assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seven patients were withdrawn from the study: 3 in each group because of relapse of CD and 1 on AZA because of severe leucopenia Intention to treat analyses were performed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described were reported No access to protocol or methods section |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mantzaris 2009.
| Methods | Prospective, randomized, controlled, 1‐year trial Only the endoscopist and pathologist were blinded Analyzed by the intention to treat principle |
|
| Participants | Patients (N = 77, AZA n = 37 and budesonide n = 38) aged 18‐67 years, with steroid dependant Crohn's ileocolitis or proximal colitis in remission (CDAI < 150)
Steroid‐dependency was described as individuals having at least 1 flare in the previous 6‐12 months which was successfully treated with steroids, but had relapsed again during steroid tapering or just after steroid withdrawal Patients included in the study must have recently flared (within 1 month of study inclusion) and were receiving a starting dose of oral prednisolone of 0.75 mg/kg a day that was tapered gradually to the lowest dose that effectively maintained remission for at least 1 month prior to randomization |
|
| Interventions | Azathioprine 2‐2.5 mg/kg/day or budesonide 6‐9 mg/day controlled‐release tablets (dose was dictated by prednisolone dependence, such that patients being maintained with 15‐30 mg/day of prednisolone would receive 9 mg/day BUD and those maintained on < 15 mg/day prednisolone would receive 6 mg/day BUD) for 1 year with extension out to 18 months in those still in remission at the 1 year mark | |
| Outcomes | Primary outcome: mucosal healing and histologic remission Secondary outcomes: annual relapse rate, time in remission, discontinuation of study medications, changes in CDAI or health‐related quality of life, safety Treatment failure was defined as "withdrawal of a patient from the study irrespective of cause" Relapse classification: Mild (CDAI 150–219), moderate (CDAI 220–450) and severe (CDAI > 450) |
|
| Notes | Compliance and use of other medications were also monitored | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not adequately described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was limited to the endoscopist and pathologist Patients were aware of the intervention they were receiving, but were unaware of the second study arm |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data was accounted for with explanation 8 patients (6 due to relapse and 2 from adverse events) from the AZA arm and 14 patients from the budesonide arm (all due to relapse) failed Analysis was performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Primary outcome and some secondary outcomes described in the methods section were reported as results |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Maté‐Jiménez 2000.
| Methods | Single‐centre, randomized, controlled, 2 phase trial, including an induction phase for 30 weeks, followed by a maintenance phase for 76 weeks studying 3 drugs without placebo Sub‐stratification was based on disease: UC (n = 34) vs CD (n = 38) |
|
| Participants | Patients (N = 72), aged 15‐70 years with steroid‐dependent, radiologically or endoscopically confirmed inflammatory bowel disease (CD or UC) who had been followed for at least 1 year and had never previously received either 6‐MP or methotrexate Steroid dependence was defined as patients who could not be weaned off less than 20 mg prednisone without induction of inflammatory activity Exclusion criteria: patients aged > 70 and < 15 years; those with "clinically significant cardiac, hepatic or renal disease; ongoing bacterial infection; pregnancy; lactating women; women not using reliable contraceptive techniques; patients using concomitant allopurinol, NSAIDs, tetracyclines or phenytoin in significant doses; those who have had extensive surgery for CD; or those who have symptoms that suggest a potential need for urgent surgery For the maintenance phase, only those who achieved remission after stopping prednisone during the induction phase were included in this portion of the study |
|
| Interventions | 6‐mercaptopurine 1.5 mg/kg/day (group A, n = 16 with Crohn's), methotrexate, 15 mg/week (group B, n = 15 with Crohn's) or 5‐aminosalicyclic acid, 3g/day (group C, n = 7 with Crohn's) Induction phase (weeks 0‐30): prednisone + study arm drug (group A, B or C) After the week 2 assessment, prednisone dose was decreased by 8 mg/wk Decreases were maintained until dose of 0 was achieved, as long as patients maintained stable condition Prednisone was discontinued upon clinical remission Maintenance phase (weeks 31‐76): once in remission, methotrexate dose was reduced to 10 mg/wk and 6‐MP dose reduced to 1 mg/kg/day; 5‐ASA dose did not change All other treatment medications except prednisone were ceased at least 6 months prior to study entry Antidiarrhoeal agents and folic acid supplements were allowed for symptom control |
|
| Outcomes | Primary outcome for phase 1 was induction of remission and cessation of steroids Primary outcome for phase 2 was clinical remission |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not adequately described |
| Allocation concealment (selection bias) | Unclear risk | Not adequately described |
| Blinding (performance bias and detection bias) All outcomes | High risk | The use of blinding was not described in the manuscript We assume that blinding was not used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data was accounted for; 24/72 patients withdrew in the first 30 weeks, reasons described (side effects and treatment failure) Intention to treat analysis used |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome described in the methods section was reported, as well as a number of other post hoc outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
O'Donoghue 1978.
| Methods | Sub‐stratified for AZA and AZA + other anti‐inflammatory drug users, randomized, double‐blind, placebo‐controlled withdrawal study Analyzed on intention‐to‐treat basis |
|
| Participants | Patients (N = 51) with Crohn's disease taking AZA (2 mg/kg/day) for at least 6 months prior to study inclusion, who were in remission or in stable good health (as agreed by patient and physician) Those receiving AZA combined with sulphasalazine or low dose corticosteroids were included; this treatment remained unchanged throughout study period |
|
| Interventions | AZA 2 mg/kg/day (n = 24) versus placebo (n = 27) for 52 weeks Patients were also allowed medications for symptom control and supplements |
|
| Outcomes | Primary outcome was maintenance of remission, defined as not relapsing Relapse was defined as a significant deterioration in clinical state requiring a change in treatment as judged by two doctors unaware of the patient's treatment | |
| Notes | Patients were monitored using a modified DAS scoring system completed at each clinical assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not described in the published study |
| Allocation concealment (selection bias) | Unclear risk | Not described in the published study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in the published study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10/51 patients withdrew because of relapse which was classified as a end point and a reason for withdrawal 5/51 patients withdrew for other reasons (3 taking azathioprine and 2 taking placebo) It was not clear how these patients were classified with regards to reason for withdrawal |
| Selective reporting (reporting bias) | Low risk | Primary outcome described in the methods section was reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Panes 2013.
| Methods | Randomized, multi‐center, double‐blind, placebo‐controlled study performed in 31 Spanish hospitals over 76 weeks
Data was analysed using an intention‐to‐treat approach The study was stopped because it met pre‐established futility criteria in the interim analysis |
|
| Participants | Patients (N = 131) aged 18‐70 years, diagnosed with established CD criteria within 8 weeks of screening irrespective of disease activity and use of corticosteroids at the time of screening Exclusion criteria: patients who had previously been treated with AZA, 6‐MP, methotrexate, cyclosporine, tacrolimus, mycophenolate mofetil, infliximab or adalimumab; an immediate need for surgery; previous surgery for CD; symptomatic stenosis; internal penetrating or perianal fistulizing disease; severe comorbidity; documented infection; malignancy; or history of drug abuse and pregnancy |
|
| Interventions | AZA 2.5 mg/kg/day, oral (n = 68) versus identically‐matched placebo (n = 63) Those receiving corticosteroids at randomization point had doses decreased by 10 mg/wk until a dosage of 20 mg/day was achieved; it was then decreased by 5 mg/wk until cessation Those previously receiving budesonide were tapered according to the following schedule: budesonide was tapered after completion of 6 weeks of treatment with 9 mg/day to 6 mg/day for 3 weeks and to 3 mg/day for 3 weeks thereafter Corticosteroids were allowed to treat flares during the study, (prednisone doses ranged 1 mg/kg/day to a maximum of 60 mg/day; budesonide dosage 9 mg/day), but were tapered using the above schedule No other medications used to treat CD were allowed, except antibiotics for suspected or demonstrated infection |
|
| Outcomes | Primary outcome was the rate of corticosteroid free clinical remission (CDAI < 150) at week 76 Secondary outcomes were rates at different time points; relapse‐free survival, mean CDAI and IBDQ scores, and CRP concentrations at each visit; requirement for corticosteroids; cumulative dose of corticosteroids; development of a fistula; requirements for hospitalization; and CD‐related surgery; and cumulative prednisone dose Safety data was also collected |
|
| Notes | Sustained corticosteroid‐free remission was defined as the presence of clinical remission (CDAI score <150 at each visit) in patients who had not been treated with corticosteroids during the entire study or patients who were treated with corticosteroids at baseline after the scheduled weaning of this treatment Relapse was defined as a CDAI score > 175 at week 12 or later Patients who did not achieve remission after randomization with a CDAI score > 175 at week 12 were categorized as in relapse |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed centrally with the use of an adaptive randomization procedure stratified according to age (< 40 years) and use of systemic corticosteroids at the time of inclusion (yes or no) |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind Patients, study site personnel, and study investigators were unaware of the treatment assignment The local investigator was not blinded; performed dose adjustment if bone marrow suppression was observed in the blood tests |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Any missing patient data is described and justified throughout the screening and study period Description of analysis as intention‐to‐treat, with missing data accounted for |
| Selective reporting (reporting bias) | Low risk | Primary and secondary endpoints described in the methods section were reported in the results section Post hoc analyses evaluating requirement of corticosteroids in patients under 40 years and the presence or perianal disease as predictors of disabling disease |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rosenberg 1975.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | Patients (N = 20) > 16 years with Crohn's disease requiring daily prednisone (minimum dose 10 mg) for symptom control for the 12 weeks prior to study inclusion Exclusion Criteria: advanced hepatic or renal disease, wished to become pregnant within study time frame, or were likely to require an operation during study |
|
| Interventions | AZA (2.0 mg/kg/day) or placebo for 26 weeks while tapering prednisone according to a schedule once specific symptom criteria were met (3 or fewer bowel movements a day, absent to mild pain and malaise, weight loss less than 2 kg, and fever (> 37.5ºC) less than one fourth of the time) Prednisone was increased by 10 mg when one of the following symptoms occurred within 7 days on the interview: > 6 bowel movements/day, severe pain/malaise, > 3 kg weight loss or fever (> 37.5ºC) for > 7 days If patients condition did not change, the prednisone dosage remained constant All other medications prior to study inclusion were continued |
|
| Outcomes | Reduction of steroid (prednisone) dosage Safety data were also collected | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Patients were allocated randomly to each group by the pharmacist such that of every 10 patients entering the study half were in each group |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded, with an identically‐matched placebo Physicians were blinded to the drug administration; two physicians working independently of one another assumed responsibility for the total care of each patient: one physician managed the clinical course, recorded data, and adjusted the dose of prednisone, while the other monitored blood counts for evidence of toxicity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient failed to complete the study |
| Selective reporting (reporting bias) | Low risk | Primary outcome described in the methods section was reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Summers 1979.
| Methods | Two independent, randomized, double‐blind, placebo‐controlled trial with patients from 14 centres Part 1, phase 1: 17 week, 4 arm Part 1, phase 2: extends phase 1 out to 1 or 2 years, 4 arm Part 2: 1‐2 years, 4 arm |
|
| Participants | N = 604, patients with typical histology of bowel resection or typical appearance of Crohn's on barium x‐ray examination Patients analysed N = 569 Part 1, phase 1: patients with radiologically and clinically active Crohn's disease, n = 295 (placebo n = 77; sulfasalazine n = 74; prednisone n = 85; AZA n = 59) Part I, phase 2: patients from Part 1, Phase 1 who achieved a CDAI <150 after 17 weeks; n = 86 (placebo n = 20; sulfasalazine n = 19; prednisone n = 28; AZA n = 19) Part 2: Patients with inactive disease (CDAI <150) established by medical (n = 226) or surgical intervention (n = 48); n = 274 (placebo n = 101; sulfasalazine n = 58; prednisone n = 61; AZA n = 54) |
|
| Interventions |
Part 1 Daily Dosing: Prednisone: CDAI < 150 = 0.25 mg/kg; CDAI = 150‐300 = 0.5 mg/kg; CDAI > 300 = 0.75 mg/kg Sulphasalazine: 1 g/15 kg AZA: 2.5 mg/kg Placebo Part 2 Daily Dosing: Prednisone: CDAI = 150‐300 = 0.25 mg/kg Sulphasalazine: 0.5 g/15 kg AZA: 1 mg/kg Placebo |
|
| Outcomes | Part 1, phase 1: induction of remission, efficacy of sulphasalazine, prednisone or AZA versus placebo in patients with active disease. Patients that entered remission (CDAI < 150) with no radiographic progression entered phase 2 Part I, phase 2: maintenance of remission using the same medications to which the patients were assigned in phase 1 Part 2: maintenance of remission after remission was induced medically or with surgical resection within the year before study entry |
|
| Notes | Cumulative dose: 612.5 mg/kg for part I phase 2; 364 mg/kg for part 2 Data from Part 2 censored at 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, identically matched "all prepared in uncoated tablets of identical external and internal appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition appears to be low (possible secondary to the fact that reasons for exiting the study were included in the "Outcome Ranking Scheme") A subset of patients were randomized and then excluded after study completion (justification was wrong diagnosis or inappropriate entry) |
| Selective reporting (reporting bias) | Low risk | All outcomes described for each part and phase were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Willoughby 1971.
| Methods | Randomized, double‐blind, placebo‐controlled. | |
| Participants | Patients with Crohn's disease, N = 22, 2 groups, each randomized to AZA or placebo Group 1: n = 12 (AZA n = 6), patients with first time attacks/relapses of moderate to severe severity not treated with steroids in the previous 3 months Group 2; n = 10 (AZA n = 5), outpatients maintained on long term prednisolone (7.5‐15 mg/day) for 5‐72 months |
|
| Interventions | AZA 2.0 mg/kg/day or placebo for 24 weeks Group 1: prednisolone (initial dose 60 mg/day) until remission achieved; tapered on a 3‐week schedule to 10mg. Prednisolone discontinued after hospital discharge if remission maintained. AZA was prescribed at 4.0 mg/kg/day for the first 10 days and then at 2.0 mg/kg/day until the end of 24 weeks Group 2: Patients received 2.0 mg/kg/day AZA or placebo and maintained their pre‐study dose of prednisolone for "at least 4 weeks after prednisolone was reduced as for group 1" Prochlorperazine for nausea Codeine phosphate was continued if receiving it prior to study induction |
|
| Outcomes | Determine the effect that AZA has on maintaining remission of CD once it has been induced by prednisolone alone or combined with AZA Disease activity monitored by DAS Relapse as defined as the recurrence of previous symptoms. Patients completing 6 months without recurrence were classified as being in remission Toxicity was also monitored |
|
| Notes | Withdrawal occurred at study completion or prematurely if patient's condition deteriorated, requiring different treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized according to a scheme, actual method of scheme not discussed |
| Allocation concealment (selection bias) | Unclear risk | Allocation performed by pharmacist, such that half received AZA and half placebo Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded "Only the pharmacist dispensing the tablets knew whether azathioprine or placebo had been selected for a given case" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients that withdrew from the study prematurely were not discussed |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section were reported in the results |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelli 2007 | Study followed patients with surgically induced remission Not a randomized controlled trial Retrospective study of 17 patients that received AZA for the first time to prevent relapse, after undergoing curative surgical treatment Moderate clinical relapse in 3 patients; no surgical relapse observed |
| Ardizzone 2004 | Study followed patients with surgically induced remission |
| Armuzzi 2013 | Study followed patients with surgically induced remission |
| Chebli 2007 | Non‐randomized open label study 69 corticosteroid‐dependent patients were enrolled in a prospective, non‐randomized, open‐label study to receive AZA (2‐3 mg/kg/day) for 7 years, for maintenance of steroid‐free remission Steroid‐free remission was maintained in 68‐81% of patients |
| Colombel 2010 | Pateints had active disease Study was an induction of remission trial |
| D'Haens 2008a | Randomized trial where patients received either infliximab with AZA (and possibly steroids) or steroids followed by infliximab with AZA Therefore not a placebo‐controlled trial, everyone received AZA |
| D'Haens 2008b | Study followed patients with surgically induced remission |
| de Souza 2013 | Patients had active disease |
| Hanauer 2004a | Study followed patients with surgically induced remission |
| Herfarth 2006 | Study followed patients with surgically induced remission |
| Klein 1974 | Patients had active disease |
| Korelitz 1998 | Study followed patients with surgically induced remission |
| Lémann 2006 | All patients received AZA or 6‐MP |
| Manosa 2013 | Drug of study was metronidazole, all patients received AZA |
| Mantzaris 2007 | Not a randomized controlled trial No placebo control Patients treated with AZA for 2‐4 years (n = 58) were compared to patients treated with AZA for 4‐8 years |
| Nos 2000 | Study followed patients with surgically induced remission Not a randomized controlled trial. 39 patients were enrolled in a prospective study and received either 5‐ASA (3 mg/day) or AZA (50 mg/day) following surgical resection for maintenance of remission |
| Oren 1997 | Patients had active disease Patients entered remission at various time points throughout the trial They could have entered remission with one month remaining in the study and been counted as maintaining remission if they were still in remission at the end of the study |
| Present 1980 | Patients had active disease |
| Reinisch 2008 | There were patients with active disease included in the analysis of patients in remission Interm‐analysis study, which was stopped during the maintenance phase |
| Reinisch 2010 | Study followed patients with surgically induced remission |
| Rhodes 1971 | Cross‐over trial that did not perform analysis before the first cross‐over |
| Savarino 2013 | Study followed patients with surgically induced remission |
| Treton 2009 | Not an RCT Cohort extension trial observing relapse in 66 patients who had stopped AZA |
| van Assche 2008 | Patients treated with infliximab were randomized to continue or stop other immunosuppressives Does not specify the type of immunosuppressant used Study evaluated withdrawal, not treatment effect |
| Vilien 2004 | Patients in the control group received no intervention (i.e. placebo medication) |
| Watson 1979 | Randomized, double‐blind placebo‐controlled cross‐over study of maintenance therapy (n = 11), 1 year per arm Intervention: AZA 50 mg TID Never published as a full paper, so unable to properly evaluate the methods and data |
| Willoughby 1990 | Randomized, double blind, controlled trial with long term follow up Study used median disease activity score as its primary outcome, not maintenance of remission |
Differences between protocol and review
Previous versions of this review included patients with surgically‐induced remission. We have since decided that pooling patients who were induced by medical therapy with patients who were surgically‐induced may be inappropriate. The use of azathioprine and 6‐mercaptopurine for maintenance of surgically‐induced remission in Crohn's disease is now covered by a separate review (Gordon 2014).
Declarations of interest
Nilesh Chande has received fees for consultancy from Abbott/AbbVie, Ferring, and Actavis; fees for lectures from Abbott, travel expenses from Merck and has stock/stock options in Pfizer, Glaxo Smith Kline, Proctor and Gamble and Johnson and Johnson. All of these financial activities are outside the submitted work.
Petrease H Patton: None known
David J Tsoulis: None known
Benson S Thomas: None known
John K MacDonald: None known
Edited (no change to conclusions)
References
References to studies included in this review
Candy 1995 {published data only}
- Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn's disease. Gut 1995;37(5):674‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candy S, Wright JP, Gerber M, Adams G, Gerig M, Goodman R. A double blind controlled study of azathioprine in the treatment and maintenance of remission in Crohn's disease [abstract]. Gastroenterology 1994;106:A659. [Google Scholar]
Cosnes 2013 {published data only}
- Cosnes J, Bourrier A, Bouhnik Y, Laharie D, Nahon S, Bonnet J, et al. Accelerated step‐care therapy with early azathioprine (AZA) vs. conventional step‐care therapy in Crohn's disease: a randomized study. Gastroenterology 2012;142(5 Suppl 1):S161. [Google Scholar]
- Cosnes J, Bourrier A, Laharie D, Nahon S, Bouhnik Y, Carbonnel F, et al. Early administration of azathioprine vs conventional management of Crohn's Disease: a randomized controlled trial. Gastroenterology 2013;145(4):758‐65.e2; quiz e14‐5. [DOI] [PubMed] [Google Scholar]
Lémann 2005 {published data only}
- Colombel JF, Lemann M, Bouhnik Y, Duclos B, Soule JC, Lerebours E, et al. Endoscopic healing of Crohn's ileo‐colitis with azathioprine. Gastroenterology 2003;124(4 Suppl 1):A196‐7. [Google Scholar]
- Lemann M, Bouhnik Y, Colombel JF, Duclos B, Soule JC, Lerebours E, et al. Randomized, double‐blind, placebo‐controlled, multicenter, azathioprine (AZA) withdrawal trial in Crohn’s disease (CD). Gastroenterology 2002;122(4 Suppl 1):A23. [DOI] [PubMed] [Google Scholar]
- Lémann M, Mary JY, Colombel JF, Duclos B, Soule JC, Lerebours E, et al. A randomized, double‐blind, controlled withdrawal trial in Crohn's disease patients in long‐term remission on azathioprine. Gastroenterology 2005;128(7):1812‐8. [DOI] [PubMed] [Google Scholar]
Mantzaris 2004 {published data only}
- Mantzaris GJ, Ployzou P, Karagiannidis A, Christidou A, Koilakou S, Tsounis D, et al. A prospective, randomized trial of infliximab and azathioprine for the induction and maintenance of remission of steroid‐dependent Crohn's disease. Gastroenterology 2004;126:A54. [Google Scholar]
Mantzaris 2009 {published data only}
- Mantzaris G, Christidou A, Sfakianakis M, Roussos A, Koilakou S, Petraki K, et al. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid‐dependent Crohn's disease. Inflammtory Bowel Diseases 2009;15:375‐82. [DOI] [PubMed] [Google Scholar]
- Mantzaris GJ, Petraki K, Chadio‐Iordanides H, Christidou A, Karagiannidis A, Glarakis J, et al. Maintenance therapy with azathioprine is superior to budesonide in healing endoscopic lesions and improving histology in clinically quiescent Crohn's disease. Gastroenterology 2002;122(4 Suppl 1):A81. [Google Scholar]
Maté‐Jiménez 2000 {published data only}
- Hermida C, Cantero J, Moreno‐Otero R, Mate‐Jimenez J. Methotrexate and 6‐mercaptopurine in steroid‐dependent inflammatory bowel disease patients: A randomized controlled clinical trial. Gut 1999;45(Suppl V):A132. [Google Scholar]
- Maté‐Jiménez J, Hermida C, Cantero Pernoa J, Moreno Otero R. 6‐mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid‐dependent inflammatory bowel disease. European Journal of Gastroenterology and Hepatology 2000;12:1227‐33. [DOI] [PubMed] [Google Scholar]
O'Donoghue 1978 {published data only}
- O'Donoghue DP, Dawson AM, Powell‐Tuck J. Azathioprine as a maintenance treatment for Crohn's disease: a double blind withdrawal trial. Gastroenterology 1978;72(5 II):1138. [DOI] [PubMed] [Google Scholar]
- O'Donoghue DP, Dawson AM, Powell‐Tuck J, Bown RL, Lennard‐Jones JE. Double‐blind withdrawal trial of azathioprine as maintenance treatment for Crohn's disease. Lancet 1978;2:955‐7. [DOI] [PubMed] [Google Scholar]
Panes 2013 {published data only}
- Panés J, López‐Sanromán A, Bermejo F, García‐Sánchez V, Esteve M, Torres Y, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn's disease. Gastroenterology 2013;145(4):766‐74. [DOI] [PubMed] [Google Scholar]
- Sans M, López‐San Romain A, Esteve M, Bermejo F, Garcia‐Sánchez V, Torres Y, et al. Early use of azathioprine has a steroid sparing effect on recently diagnosed Crohn's disease patients. Gastroenterology 2012;142(5 Suppl 1):S109. [Google Scholar]
Rosenberg 1975 {published data only}
- Rosenberg JL, Levin B, Wall AJ, Kirsner JB. A controlled trial of azathioprine in Crohn's disease. American Journal of Digestive Diseases 1975;20:721‐6. [DOI] [PubMed] [Google Scholar]
Summers 1979 {published data only}
- Singleton J, Law D, Kelley M, Mekhjian H, Sturdevant R. National Cooperative Crohn's Disease Study: Adverse reactions to study drugs. Gastroenterology 1979;77:870‐82. [PubMed] [Google Scholar]
- Summers RW, Singleton JW. National Cooperative Crohn's Disease Study (NCCDS): a controlled prospective trial of three drugs vs placebo. Gut 1977;18(11):A972‐3. [Google Scholar]
- Summers RW, Switz DM, Sessions JT, Becktel JM, Best WR, Kern F, et al. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology 1979;77:847‐69. [PubMed] [Google Scholar]
Willoughby 1971 {published data only}
- Willoughby JM, Beckett J, Kumar PJ, Dawson AM. Controlled trial of azathioprine in Crohn's disease. Lancet 1971;2:944‐7. [DOI] [PubMed] [Google Scholar]
- Willoughby JM, Kumar P, Beckett J, Dawson AM. A double‐blind trial of azathioprine in Crohn's disease. Gut 1971;12(10):864. [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelli 2007 {published data only}
- Abdelli MN, Ben Abdallah H, Houissa F, Bouali MR, Khediri MF. Azathioprine for prevention of postoperative recurrence in Crohn's disease. Tunisie Médicale 2007;85:569‐72. [PubMed] [Google Scholar]
Ardizzone 2004 {published data only}
- Ardizzone S, Maconi G, Sampietro GM, Russo A, Radice E, Colombo E, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease.. Gastroenterology 2004;127:730‐40. [DOI] [PubMed] [Google Scholar]
Armuzzi 2013 {published data only}
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Prevention of postoperative recurrence with azathioprine or anti‐TNF alpha in patients with Crohn's disease: A pilot study. Digestive and Liver Disease 2012;44:S194. [Google Scholar]
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Prevention of postoperative recurrence with azathioprine or anti‐TNF alpha in patients with Crohn's disease: An open‐label pilot study. Gastroenterology 2012;142(5 Suppl 1):S780. [DOI] [PubMed] [Google Scholar]
- Armuzzi A, Felice C, Papa A, Marzo M, Pugliese D, Andrisani G, et al. Prevention of postoperative recurrence with azathioprine or infliximab in patients with Crohn's disease: an open‐label pilot study. Journal of Crohn's and Colitis 2013;7(12):e623‐9. [DOI] [PubMed] [Google Scholar]
Chebli 2007 {published data only}
- Chebli JM, Gaburri PD, Souza AF, Pinto AL, Chebli LA, Felga GE, et al. Long‐term results with azathioprine therapy in patients with corticosteroid‐dependent Crohn's disease: open‐label prospective study. Journal of Gastroenterology and Hepatology 2007;22:268‐74. [DOI] [PubMed] [Google Scholar]
Colombel 2010 {published data only}
- Colombel J, Sandborn W, Reinisch W, Mantzaris G, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. New England Journal of Medicine 2010;362(15):1383‐95. [DOI] [PubMed] [Google Scholar]
D'Haens 2008a {published data only}
- D’Haens G, Baert F, Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660‐7. [DOI] [PubMed] [Google Scholar]
D'Haens 2008b {published data only}
- D'Haens G, Vermeire S, Assche G, Noman M. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s disease: a controlled randomized trial. Gastroenterology 2008;135(4):1123–29. [DOI] [PubMed] [Google Scholar]
- D'Haens GR, Noman M, Assche GA, Olmen G, Aerden I, Wermeire S, et al. Combination therapy with metronidazole and azathioprine reduces severe postoperative recurrence of Crohn's disease: a double‐blind controlled randomized trial. Gastroenterology 2007;132(4 Suppl 2):A52. [DOI] [PubMed] [Google Scholar]
de Souza 2013 {published data only}
- Souza GS, Vidigal FM, Chebli LA, Rocha Ribeiro TC, Furtado MC, Lima Pace FH, et al. Effect of azathioprine or mesalazine therapy on incidence of re‐hospitalization in sub‐occlusive ileocecal Crohn's disease patients. Medical Science Monitor 2013;19:716‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanauer 2004a {published data only}
- Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, et al. Postoperative maintenance of Crohn's disease remission with 6‐mercaptopurine, mesalamine, or placebo: a 2‐year trial. Gastroenterology 2004;127(3):723‐9. [DOI] [PubMed] [Google Scholar]
Herfarth 2006 {published data only}
- Herfarth H, Obermeier F, Tjaden C, Lukas M, Serclova Z, Dignass AU, et al. Double‐blind, double dummy, randomized, multicentre, comparative study on the efficacy and safety of azathioprine (AZA) versus mesalazine (5‐ASA) for prevention of postoperative endoscopic recurrence in Crohn's disease. Gastroenterology 2006;130(4 Suppl 2):A480. [Google Scholar]
Klein 1974 {published data only}
- Klein M, Binder HJ, Mitchell M, Aaronson R, Spiro H. Treatment of Crohn's disease with azathioprine: a controlled evaluation. Gastroenterology 1974;66(5):916‐22. [PubMed] [Google Scholar]
Korelitz 1998 {published data only}
- Korelitz B, Hanauer S, Rutgeerts P, Present D, Peppercorn M. Post‐operative prophylaxis with 6‐MP, 5‐ASA or placebo in Crohn’s Disease: a 2 year multicenter trial. Gastroenterology 1998;114(4 Pt 2):A‐486. [Google Scholar]
Lémann 2006 {published data only}
- Lémann M, Mary JY, Duclos B, Veyrac M, Dupas JL, Delchier JC, et al. Infliximab plus azathioprine for steroid‐dependent Crohn’s disease patients: a randomized placebo‐controlled trial. Gastroenterology 2006;130:1054‐61. [DOI] [PubMed] [Google Scholar]
Manosa 2013 {published data only}
- Manosa M, Cabre E, Bernal I, Esteve M, Garcia‐Planella E, Ricart E, et al. Addition of metronidazole to azathioprine for the prevention of postoperative recurrence of Crohn's disease: a randomized, double‐blind, placebo‐controlled trial. Inflammatory Bowel Diseases 2013;19(9):1889‐95. [DOI] [PubMed] [Google Scholar]
- Manosa M, Cabre E, Bernal I, Esteve M, Garcia‐Planella E, Ricart E, et al. Azathioprine versus azathioprine plus metronidazole for the prevention of postoperative endoscopic recurrence of Crohn's disease: a randomized, placebo‐controlled trial. Journal of Crohn's and Colitis 2012;6:S93. [DOI] [PubMed] [Google Scholar]
Mantzaris 2007 {published data only}
- Mantzaris GJ, Roussos A, Christidou A, Koilakou S, Kalantzis CN, Petraki K, et al. The long‐term efficacy of azathioprine does not wane after four years of continuous treatment in patients with steroid‐dependent luminal Crohn's disease. Journal of Crohn's and Colitis 2007;1(1):28‐34. [DOI] [PubMed] [Google Scholar]
Nos 2000 {published data only}
- Nos P, Hinojosa J, Aguilera V, Moles JR, Pastor M, Ponce J, et al. Azathioprine and 5‐ASA in the prevention of postoperative recurrence of Crohn's disease. Gastroenterología y Hepatología 2000;23:374‐8. [PubMed] [Google Scholar]
Oren 1997 {published data only}
- Oren R, Moshkowitz M, Odes S, Becker S, Keter D, Pomeranz I, et al. Methotrexate in chronic active Crohn's disease: a double‐blind, randomized, Israeli multicenter trial. American Journal of Gastroenterology 1997;92(12):2203‐9. [PubMed] [Google Scholar]
Present 1980 {published data only}
- Present DH, Korelitz BI, Wisch N. Treatment of Crohn's disease with 6‐mercatopurine ‐ a long‐term, randomized, double‐blind study. Innere Medizin 1980;7(4):147‐8. [DOI] [PubMed] [Google Scholar]
- Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn's disease with 6‐mercaptopurine. A long‐term, randomized, double‐blind study. New England Journal of Medicine 1980;302(18):981‐7. [DOI] [PubMed] [Google Scholar]
Reinisch 2008 {published data only}
- Reinisch W, Panés J, Lémann M, Schreiber S, Feagan B, Schmidt S, et al. A multicenter, randomized, double‐blind trial of everolimus versus azathioprine and placebo to maintain steroid‐induced remission in patients with moderate‐to‐severe active Crohn's disease. American Journal of Gastroenterology 2008;103:2284‐93. [DOI] [PubMed] [Google Scholar]
Reinisch 2010 {published data only}
- Reinisch W, Angelberger S, Herrlinger K, Shonova O, Lukas S, Bar‐Meir S, et al. A double‐blind, double‐dummy, randomized, controlled, multicenter trial on the efficacy and safety of azathioprine vs mesalamine for prevention of clinical relapses in Crohn's disease patients with postoperative moderate or severe endoscopic recurrence. Gastroenterology 2008;134(4 Suppl 1):A‐70. [Google Scholar]
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar‐Meir S, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn's disease with endoscopic recurrence: efficacy and safety results of a randomised, double‐blind, double‐dummy, multicentre trial. Gut 2010;59:752‐9. [DOI] [PubMed] [Google Scholar]
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Greinwald R, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in Crohn's disease patients with endoscopic recurrence: Follow‐up data of a randomised, double‐blind, double‐dummy, 1‐year, multicentre trial. Journal of Crohn's and Colitis 2013;7:S254. [DOI] [PubMed] [Google Scholar]
Rhodes 1971 {published data only}
- Rhodes J, Bainton D, Beck P. Azathioprine in Crohn's disease. Lancet 1970;2(7683):1142. [DOI] [PubMed] [Google Scholar]
- Rhodes J, Bainton D, Beck P, Campbell H. Controlled trial of azathioprine in Crohn's disease. Lancet 1971;2(7737):1273‐6. [DOI] [PubMed] [Google Scholar]
Savarino 2013 {published data only}
- Savarino E, Bodini G, Dulbecco P, Assandri L, Bruzzone L, Mazza F, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease: a randomized controlled trial. The American journal of gastroenterology 2013;108(11):1731‐42. [DOI] [PubMed] [Google Scholar]
Treton 2009 {published data only}
- Treton X, Bouhnik Y, Mary J, Colombel J, Duclos B, Soule J, et al. Azathioprine withdrawal in patients with Crohn's disease (CD) maintained on prolonged remission under treatment is associated with a high risk of relapse. Gastroenterology 2004;126(4 Suppl 2):A113. [DOI] [PubMed] [Google Scholar]
- Treton X, Bouhnik Y, Mary J, Colombel J, Duclos B, Soule J, et al. Azathioprine withdrawal in patients with Crohn's disease maintained on prolonged remission: a high risk of relapse. Clinical Gastroenterology and Hepatology 2009;7(1):80‐5. [DOI] [PubMed] [Google Scholar]
van Assche 2008 {published data only}
- Assche G, Magdelaine‐Beuzelin C, D'Haens G, Baert F, Noman M, Vermeire S, et al. Withdrawal of immunosuppression in crohn’s disease treated with scheduled infliximab maintenance: A randomized trial. Gastroenterology 2008;134(7):1861‐8. [DOI] [PubMed] [Google Scholar]
Vilien 2004 {published data only}
- Vilien M, Dahlerup JF, Munck LK, Norregaard P, Gronbaek K, Falligborg J. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn's disease: increased relapse rate the following year. Alimentary Pharmacology and Therapeutics 2004;19(11):1147‐52. [DOI] [PubMed] [Google Scholar]
Watson 1979 {published data only}
- Watson WC, Bukosdky M. Azathioprine in management of Crohn's disease: a randomized cross‐over study. Gastroenterology 1974;66:796. [Google Scholar]
Willoughby 1990 {published data only}
- Willoughby JM, Thomas JM, Sudweeks DM. Azathioprine and levamisole in Crohn's disease: a double blind controlled trial of one year's treatment with long follow up. Gut 1990;31(Suppl 10):A1193. [Google Scholar]
Additional references
Beaugerie 2008
- Beaugerie L, Carrat F, Bouvier AM, Brousse N, Carbonnel F, Colombel JF, et al. Excess risk of lymphoproliferative disorders (Lpd) in inflammatory bowel diseases (IBD): Interim results of the Cesame cohort. Gastroenterology 2008;134(4 Suppl 1):A116. [Google Scholar]
Best 1976
- Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
Chande 2013
- Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6‐mercaptopurine for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD000545.pub4] [DOI] [PubMed] [Google Scholar]
Dubinsky 2004
- Dubinsky MC. Azathioprine, 6‐mercaptopurine in inflammatory bowel disease: Pharmacology, efficacy, and safety. Clinical Gastroenterology and Hepatology 2004;2(9):731‐43. [DOI] [PubMed] [Google Scholar]
Dyer 1970
- Dyer N. Studies on Crohn's disease. MD Thesis University of Cambridge 1970.
Gearry 2004
- Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiology and Drug Safety 2004;13(8):563‐7. [DOI] [PubMed] [Google Scholar]
Gearry 2005
- Gearry RB, Barclay ML. Azathioprine and 6‐mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. Journal of Gastroenterology and Hepatology 2005;20(8):1149‐57. [DOI] [PubMed] [Google Scholar]
Gordon 2014
- Gordon M, Taylor K, Akobeng AK, Thomas AG. Azathioprine and 6‐mercaptopurine for maintenance of surgically‐induced remission in Crohn's disease. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD010233.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanauer 2004b
- Hanauer SB, Wagner CL, Bala M, Mayer L, Travers S, Diamond RH, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clinical Gastroenterology and Hepatology 2004;2(7):542‐53. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Kandiel 2005
- Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6‐mercaptopurine. Gut 2005;54(8):1121‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
NIDDK 2008
- National Institute of Diabetes and Digestive and Kidney Diseases. A multi‐site trial of azathioprine dosing in Crohn's disease. ClinicalTrials.gov, NCT00113503, 2008. [NCT00113503]
Patel 2014
- Patel V, Wang Y, MacDonald JK, McDonald JWD, Chande N. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD006884.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rhodes 1970
- Rhodes J, Bainton D, Beck P. Azathioprine in Crohn's disease. Lancet 1970;2:1142. [DOI] [PubMed] [Google Scholar]
Sahasranaman 2008
- Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. European Journal of Clinical Pharmacology 2008;64:753‐67. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
References to other published versions of this review
Pearson 1995
- Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6‐mercaptopurine in Crohn disease: a meta‐analysis. Annals of Internal Medicine 1995;122:132‐42. [DOI] [PubMed] [Google Scholar]
Pearson 1998
- Pearson DC, May GR, Fick G, Sutherland LR. Azathioprine for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD000067] [DOI] [PubMed] [Google Scholar]
Prefontaine 2009
- Prefontaine E, Sutherland LR, MacDonald JK, Cepoiu M. Azathioprine or 6‐mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000067.pub2] [DOI] [PubMed] [Google Scholar]


