Supplemental Digital Content is Available in the Text.
It is believed that peripheral neuropathic pain is dependent on activation of microglia in the spinal cord, but human trials targeting these cells have been disappointing. We describe clinically relevant peripheral neuropathic pain hypersensitivity that is microglia-independent in male and female mice.
Keywords: Neuropathic pain, Macrophages, Microglia, Nerve injury, BDNF
Abstract
The dominant view in the field of pain is that peripheral neuropathic pain is driven by microglia in the somatosensory processing region of the spinal dorsal horn. Here, to the contrary, we discovered a form of neuropathic pain that is independent of microglia. Mice in which the nucleus pulposus (NP) of the intervertebral disc was apposed to the sciatic nerve developed a constellation of neuropathic pain behaviours: hypersensitivity to mechanical, cold, and heat stimuli. However, NP application caused no activation of spinal microglia nor was pain hypersensitivity reversed by microglial inhibition. Rather, NP-induced pain hypersensitivity was dependent on cells within the NP which recruited macrophages to the adjacent nerve. Eliminating macrophages systemically or locally prevented NP-induced pain hypersensitivity. Pain hypersensitivity was also prevented by genetically disrupting the neurotrophin brain-derived neurotrophic factor selectively in macrophages. Moreover, the behavioural phenotypes as well as the molecular mechanisms of NP-induced pain hypersensitivity were not different between males and females. Our findings reveal a previously unappreciated mechanism for by which a discrete peripheral nerve lesion may produce pain hypersensitivity, which may help to explain the limited success of microglial inhibitors on neuropathic pain in human clinical trials.
1. Introduction
Chronic pain is a pervasive and often devastating problem that continues to plague modern societies. Indeed, nearly 1 in 5 individuals of the general population report chronic pain.9,13,23,29,85 The enormity of the problem of chronic pain is comparable with that of other major public-health issues, such as cancer, heart disease, and obesity, that imposes an immense personal burden on patients as well as an enormous cost on societies globally. This persistent problem is especially important for neuropathic pain, the most debilitating and most poorly treated form of chronic pain, which affects up to 10% of the population.13,64,78
Neuropathic pain arises as a direct consequence of a lesion or disease of the somatosensory nervous system.53 The most common type of neuropathic pain arises from damage or injury to peripheral nerves—peripheral neuropathic pain. Such pathological pain manifests as hypersensitivity to mechanical, heat, and cold stimuli. A hallmark of pain-producing peripheral nerve injury (PNI) is microgliosis—proliferation, morphological changes, and activation of microglia—in the spinal dorsal horn ipsilateral to the side of the injury. Such microgliosis in the dorsal horn was for a long period of time considered to be an epiphenomenon of PNI.75 Increasingly, microglia are implicated in numerous physiological and pathological conditions throughout the central nervous system.62 For peripheral neuropathic pain, a large body of evidence has accumulated over the past 2 decades indicating that microglia in the spinal cord dorsal horn are not innocent bystanders, rather these cells are critical cellular mediators of pain hypersensitivity caused by PNI.12,28,34,45,65
Microglia are implicated in diverse kinds of preclinical models of neuropathic pain such as traumatic nerve injury in spared nerve injury (SNI),18 spinal nerve ligation,31 and chronic constriction injury (CCI)4 models as well as in the streptozotocin model of diabetic neuropathy81 and in experimental autoimmune neuritis.87 One might wonder therefore whether as a general rule peripheral neuropathic pain depends on the activation of microglia in the spinal dorsal horn. Here, we induced nerve damage by applying material from the intervertebral discs, the nucleus pulposus (NP), to the sciatic nerve which produced profound hypersensitivity to mechanical, heat, and cold stimuli—the constellation of behavioural changes characteristic of neuropathic pain. Applying NP had no effect on number or morphology of microglia in the spinal dorsal horn. Moreover, suppressing microglia function either pharmacologically or genetically had no effect on NP-induced pain hypersensitivity. Rather, we found that pain hypersensitivity induced by NP was driven by peripheral macrophages, which infiltrated around the sciatic nerve where the NP was applied, and by the macrophage mediator brain-derived neurotrophic factor (BDNF). Thus, we conclude that the activation of microglia in the spinal dorsal horn is not a generalized requirement for peripheral neuropathic pain hypersensitivity.
2. Methods
2.1. Animals
C57BL/6 mice (20-25 g, 6-7 weeks) of both sexes were acquired from The Jackson Laboratory for all behavioral studies. For cell type–specific BDNF knock out experiments, we used CX3CR1-specific BDNF transgenic mice expressing tamoxifen-inducible Cre recombinase (CreER) regulated by the endogenous CX3CR1 promoter in microglia (CX3CR1CreER x Bdnffl/fl; 25-30 g, 7-10 weeks; both sexes).51 The transgene encoding CreER is followed by an IRES-eYFP reporter allowing cells expressing CreER to be visualized.51 CX3CR1CreER mice were a generous gift of Dr Wenbiao Gan, New York University School of Medicine. Heterozygous CX3CR1CreER mice (mixed background) were crossed with mice possessing floxed Bdnf coding region (Bdnftm3Jae/J; The Jackson Laboratory, Cat. #00439) to create experimental mice with heterozygous CreER and homozygous floxed Bdnf. CreER null mice were used as controls. Tg(act-EGFP)Y01Osb strain (Cat. # 006567) acquired from The Jackson Laboratory was used as the donor for NP. Mice (5 animals per cage) were housed in polycarbonate cages on a 14:10 hours light:dark cycle (lights on at 06:00 hours) in a temperature-controlled environment with ad libitum access to food and water. Simple randomization was used to allocate animals to treatments. Experimenters were blinded to genotype and drug treatments. In all studies, both the control and experimental groups were assessed on the same day, and experiments were replicated on different days to generate results. All experiments were approved by the Hospital for Sick Children's Animal Care Committee (#46756) and adhered to the Canadian Council on Animal Care (CCAC) guidelines.
2.2. Surgical procedures for nucleus pulposus application
Under 2.5% isoflurane, the sciatic nerve was exposed by blunt dissection through the biceps femoris muscle. Nucleus pulposus, the inner core material of intervertebral discs, was collected from the tail of littermates of the recipient mice to avoid rejection, followed by placing on the exposed common sciatic nerve. In sham operated animals, the sciatic nerve was exposed without the application of NP material.
2.3. Nucleus pulposus irradiation
Nucleus pulposus was harvested from the tail of C57BL/6 mice and placed into PBS. The harvested NP was subsequently exposed to a lethal dose (20 Gy) at a dose rate of 1.0 Gy/min for 20 minutes of γ-irradiation using a 137Cs GammaCell-40 Irradiator. The irradiated NP was rinsed in PBS before applying to sciatic nerve.
2.4. Tamoxifen
Tamoxifen (Sigma) was prepared in corn oil (Sigma) and given to adult mice by oral gavage to induce Cre-mediated recombination in microglia. Animals received 2 doses of 10 mg of tamoxifen in 0.5 mL corn oil 48 hours apart and 5% glucose subcutaneously daily throughout tamoxifen treatment.51 Vehicle controls received corn oil.
2.5. Macrophage depletion by clodronate liposomes
C57BL/6 mice received clodronate liposomes (0.2 mL, 5 mg/mL) or PBS (phosphate buffer saline 1X) liposomes, with 2 intraperitoneal injections 48 hours apart. Efficacy of clodronate liposome depletion was confirmed by macrophage expression (F4/80, 1:2000) in the spleen and nerve 7 days after treatment. In another experiment, 10 µL of clodronate liposomes (5 mg/mL) was applied directly on the sciatic nerve.
2.6. Macrophage migratory inhibition by selenium
C57BL/6 mice received a total 5 intraperitoneal injections of selenium (0.2 mL, 200 mg/mL) or PBS 48 hours apart, except the third injection which is 24 hours apart from the second injection before the NP surgery.
2.7. Sciatic nerve applications
Twenty percent of Pluronic F-127 (Invitrogen) in 10% DMSO and 2% chitosan (Sigma) in 70 mM HCl mixed with 100 mM β-glycerophosphate (Sigma) and heated to 40°C. Y1036 (Calbiochem) was dissolved in PBS to a final concentration of 50 μM. Ten microliter was applied directly on the sciatic nerve for all the compounds.
2.8. Minocycline administration
Minocycline (100 µg, Sigma) was dissolved in distilled water. Intrathecal injections between the L5 and L6 vertebrae were performed under brief 2.5% isoflurane. Five microliter was administered.
2.9. Behaviour testing
2.9.1. Von Frey testing
The ascending method was used to estimate absolute withdrawal thresholds using nylon monofilaments (Stoelting Touch Test). Animals were habituated in Plexiglas cubicles on a perforated metal floor (Ugo Basile) for 1 hour before testing. Starting with the lowest von Frey filament (0.008 g), filaments were applied to the plantar surface of the hind paw until withdrawal threshold was reached. A positive response was considered when there was withdrawal, shake, or lick of the hind paw observed after the application of the filament.
2.9.2. Hargreaves test
Thermal hyperalgesia was assessed by the Hargreaves test.30 In brief, animals were habituated in Plexiglas cubicles on a glass surface. The time (second) for the hind paw to withdraw from a radiant heat stimulus projected to the plantar surface was measured. Three measurements on both ipsilateral and contralateral sides were collected.
2.9.3. Acetone drop test
For cold assessment, 50 µL of acetone was applied to the midplantar surface of the hind paw and animal response was monitored for 20 seconds immediately after acetone application. The time (second) that the animal spent nursing in response to the cooling effect, including withdraw, flick, stamp, or lick, was recorded. Three measurements were collected on both ipsilateral and contralateral sides, with each acetone application 5 minutes apart.
2.9.4. Rotarod
The rotarod was set with a starting speed of 4 rpm and constant acceleration within 30 seconds to a top speed of 40 rpm. The animals were placed on the rotating beam, facing away from the direction of rotation, and the time (second) that animals spent on the beam before falling off was recorded. Three measurements were collected.
2.9.5. Dynamic weight bearing
The dynamic weight-bearing apparatus (Bioseb, Vitrolles, France) consists of a Plexiglas chamber with a camera attached on the top and a pressure sensitive mat placed at the bottom. The animals were kept in the chamber where they can move freely. Over a 5-minute test period, hind limb weight bearing was continuously monitored through the sensor pad and a video recording was made and used during analysis to recognize the orientation of the animal. Hind paw weight distribution was calculated using Bioseb software (version 1.4.2.92), and a percentage weight borne by the ipsilateral paw was calculated using the following formula: % weight on ipsilateral paw = (weight borne by the ipsilateral paw/weight borne by the ipsilateral paw + weight borne by the contralateral paw).
2.10. Isolation of peritoneal macrophages
Fresh thioglycollate broth (3%, Sigma) was prepared in distilled water and autoclaved. C57BL/6 mice received thioglycollate broth intraperitoneally 72 hours before euthanasia. Peritoneal macrophages were harvested by peritoneal lavage with cold PBS. For in vitro experiment, macrophages were enriched and maintained (37°C, 5% CO2) using Roswell Park Memorial Institute (RPMI)-1640 (Invitrogen) media supplemented with 10% fetal bovine serum (Wisent) and 1% penicillin and streptomycin (Wisent) overnight before stimulation.
2.11. Nucleus pulposus isolation and cell culture
Nucleus pulposus was harvested from the tail of C57BL/6 mice. Annulus fibrosis surrounding the NP tissue was carefully removed during dissection. The NP tissue was rinsed in PBS before culturing for in vitro studies.
The isolated NPs were cultured and maintained for 3 weeks to allow NP cells to expand in a monolayer until confluency in Dulbecco's Modified Eagle Medium (DMEM) media (Wisent) supplemented with 10% fetal bovine serum (Wisent) and 1% penicillin and streptomycin (Wisent). All cell cultures were maintained at 37°C under 5% CO2. The culture medium was changed at a 3-day interval.
To stimulate macrophages, control media or NP cell-conditioned media (NPCM) were used 48 hours after culture media replacement. Media were filtered and applied to macrophages for 48 hours before RNA extraction for quantitative polymerase chain reaction (qPCR).
2.12. Migration assay
Boyden chamber (Fisher Scientific), consisting of an upper transwell insert separated from a bottom chamber by a polycarbonate membrane, was used to assess migration. Six hundred microliter of NP-conditioned media (NPCM), media conditioned without NP cells (RPMI media alone), or fresh RPMI media were added to the lower chamber and stabilized at 37°C for 15 minutes. One hundred microliter of THP1 monocytes (500,000 cells) were subsequently added to the upper transwell followed by cell counting of the migratory THP1 cells in the bottom chamber after 3 hours of incubation at 37°C, 5% CO2.
2.13. RNA extraction and qPCR (macrophage)
RNA was extracted from peritoneal macrophages by RNA Mini Kit (Life Technologies) according to the manufacturer's instructions. cDNA was synthesized using the SuperScript VILO cDNA kit (Life Technologies). 2000 ng per reaction was used for RT-qPCR using predesigned Taqman probes (Life Technologies) to quantify Bdnf (#Mm04230607) and Gapdh (#Mm99999915). qPCR was performed for 40 cycles (95°C for 1 second and 60°C for 20 seconds), and relative quantities of mRNAs were calculated using the comparative ΔΔCt method after normalizing against the housekeeping gene Gapdh.
2.14. Immunohistochemistry
Mice were transcardially perfused with PBS followed by 10% formalin. Sciatic nerve, L4 to L5 DRG, and L4/5 lumbar spinal cords were harvested and postfixed in 10% formalin followed by 30% sucrose solution. Tissues were cryosectioned (sciatic nerve and DRG at 12 µm and spinal cord at 35 µm) for staining. After 3 PBS washes, sections were blocked with 10% normal donkey serum and 0.3% Triton-X in PBS for 1 hour and incubated overnight with primary antibodies against F4/80 (Bio-Rad, 1:1000), ATF3 (Novus Biologicals, 1:1000), Iba1 (Wako, 1:2000), and NeuN (Millipore, 1:1000). Sections were subsequently washed 3 times with PBS and then incubated with secondary antibodies (Jackson ImmunoResearch Labs, 1:1000) in PBS for 2 hours. Sections were washed and mounted onto Superfrost slides (Fisher Scientific) for imaging on Zeiss Epifluorescence Microscope and PerkinElmer Volocity software. Unstained sciatic nerve was imaged on Leica Fluorescence Stereomicroscope for gross anatomy of the nerve.
2.15. Histochemical image analysis and quantification
Serial sections of entire L4/5 DRGs from 4 to 7 animals were cut (14 μm) and placed on Superfrost microslides. For quantitative analysis, 4 to 7 sections from each DRG were used for ATF-3 and NeuN immunoreactive quantification. The number of NeuN+ and ATF3+ neurons was counted in the L4/5 DRGs for sham, NP, SNI, and CCI animals.
F4/80 immunofluorescence intensity in sciatic nerves was quantified by PerkinElmer Volocity software (version 6.5.1). Each group consisted of 4 to 9 mice, and 5 to 10 sections per mouse were quantified. F4/80 immunofluorescence intensity across sections with background subtracted was averaged to produce a final intensity for each animal.
Transverse sections (14 μm) of sciatic nerve were prepared from 4 mice per group, and the diameter of the sciatic nerve was measured using PerkinElmer Volocity software (version 6.5.1). Three measurements were collected and averaged across sections to produce a final average diameter for each animal.
2.16. Isolation of peripheral blood cells and flow cytometry
Generation of single-cell suspension from central nervous system (CNS) cells was performed as previously described.51 To prepare a single-cell suspension from peripheral blood, CX3CR1CreER/+:BDNFfl/fl mice were transcardially perfused and blood was collected from atrium and placed into DPBS with heparin (1 µL/mL). Single-cell suspension was then prepared by RBC lysis buffer (eBioscience) according to the manufacturer's instructions. Single-suspended lymphocytes were washed and stained with CD11b (BD Biosciences). Cells were sorted based on CX3CR1-YFP and CD11b by Beckman Coulter MoFlo or BD Cell AtriaII.
To assess recombination, DNA was extracted from the sorted cells (YFP−/CD11b− or YFP+/CD11b+) from both sexes by GeneJET Genomic DNA Purification Kit (Thermo Fisher). Twenty-five ng per reaction was used for PCR using BDNF primers (forward primer: CAGCGTGTGCAGAGATCATTA and reverse primer: GAACCTCTTTCGAGGGACCTA; IDT) to assess the floxed Bdnf allele at 1381bp or for the recombined allele at 121bp. PCR was performed for 35 cycles (95°C for 15 seconds, 60°C for 15 seconds, and 72°C for 10 seconds), and images were taken on Gel Doc EZ and Image Lab software (version 5.0).
2.17. Statistical analysis
Statistical analysis was performed using GraphPad Software (8.0). All data were tested for normality using the Shapiro–Wilk test. One-way analysis of variance (ANOVA) or the Kruskal–Wallis test was performed when comparisons were made across more than 2 groups. Two-way ANOVA (post hoc Bonferroni) was used to test differences between 2 or more groups across different days. The t test was performed to test differences between 2 groups. Statistical significance refers to *P< 0.05, ** P< 0.01, *** P< 0.001, and ****P < 0.0001; ns>0.05. Data are presented as mean ± SEM.
3. Results
3.1. Nucleus pulposus applied to the sciatic nerve causes pain hypersensitivity
Hypersensitivity to mechanical, cold, and heat stimuli are cardinal signs of neuropathic pain.61,73 Therefore, we assessed the effects of NP applied to the sciatic nerve (Fig. 1A) on responses to each of these forms of peripheral stimulation. In all experiments, the genetic background of the donor mouse was the same as that of the recipient mice. (1) Mechanical: We found that mechanical withdrawal threshold of the ipsilateral hind paw in animals with NP applied to the sciatic nerve was significantly less than the withdrawal threshold of sham-operated animals (Fig. 1B). Applying NP caused a statistically significant decrease in withdrawal threshold beginning on day 1 after NP application and persisting to day 7; withdrawal thresholds recovered to levels indistinguishable from the sham controls by day 15. After recovery, a second application of NP could again cause mechanical hypersensitivity (data not illustrated). No differences were observed in mechanosensitivity of the contralateral hind paw between NP-exposed vs sham-operated animals (Fig. 1B). (2) Cold: To assess responses to cold stimuli, we applied acetone to the plantar surface of the hind paw on day 5 postsurgery. We found that the duration of nocifensive behaviours—licking, shaking, or hind paw withdrawal—evoked by applying acetone to the ipsilateral hind paw was significantly longer in NP-exposed animals as compared with sham controls (Fig. 1C). By contrast, NP exposure had no effect on the duration of nocifensive behaviours to acetone applied to the contralateral hind paw as compared with sham controls. (3) Heat: To assess sensitivity to heat a focal radiant heat, stimulus was applied to the plantar surface of the hind paw, and we found that the withdrawal latency of the ipsilateral hind paw in NP-exposed animals was significantly shorter than that in sham controls (Fig. 1D). However, the heat withdrawal latency of the contralateral hind paw was not different in NP-exposed vs sham-operated animals. Thus, applying NP to sciatic nerve in mice causes mechanical, cold, and heat hypersensitivity of the ipsilateral, but not contralateral, hind paw.
Figure 1.
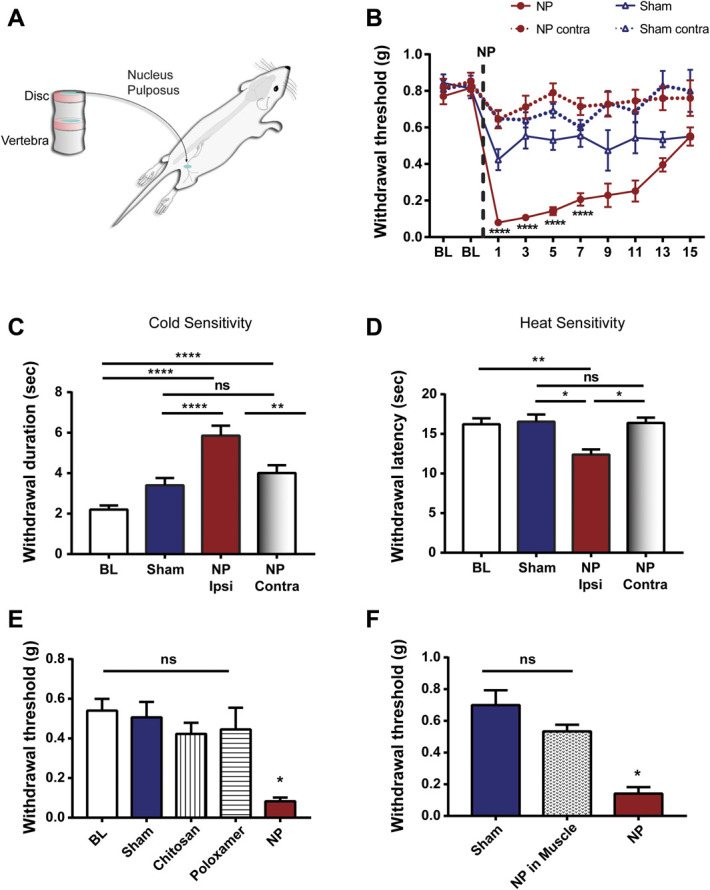
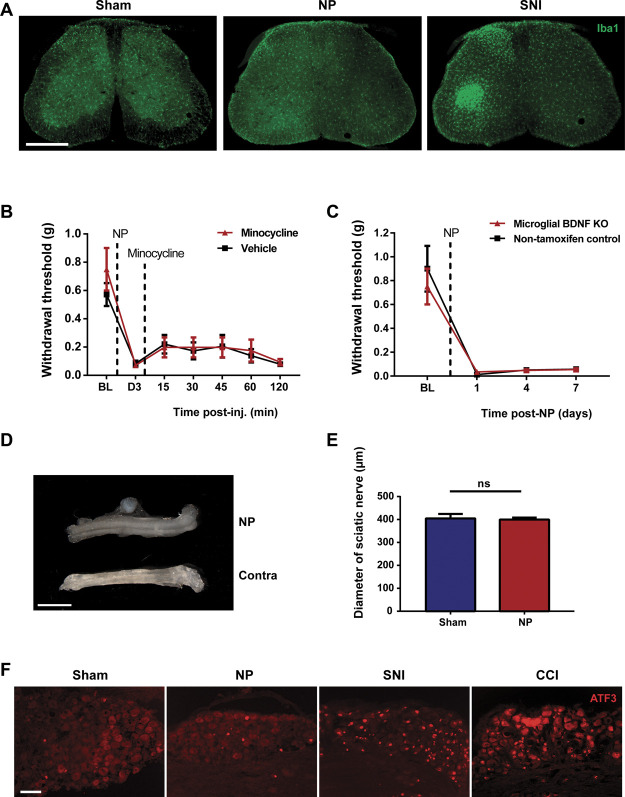
Characterization of NP-induced pain hypersensitivity. (A) Schematic diagram of NP application to the sciatic nerve of a mouse. (B) Paw withdrawal threshold from von Frey filaments on the ipsilateral and contralateral side before (BL) and for 15 days after surgery in animals with NP applied to sciatic nerve (n = 36) or sham controls (n = 23). ****P < 0.0001 comparing NP ipsilateral and sham ipsilateral with two-way repeated-measures ANOVA (Bonferroni post hoc test). (C) Cold sensitivity, measured by withdrawal duration (in seconds) using the acetone test, in the ipsilateral and contralateral paws of NP-exposed mice (n = 28) and sham controls (n = 28) at day 5. **P < 0.01 and ****P < 0.0001; ns>0.05 by 1-way ANOVA with the Bonferroni multiple comparisons post hoc test. (D) Heat hyperalgesia measured by withdrawal latency (in seconds) to nociceptive heat stimulation in the ipsilateral and contralateral paws of NP-exposed mice and sham controls (n = 19 per group) at day 5. (E) Withdrawal threshold from von Frey filaments in ipsilateral paws of sham controls (n = 9) and animals receiving space-filling controls, chitosan and poloxamer (n = 7 per group), or NP (n = 11) at day 5. (F) Withdrawal threshold from von Frey filaments in ipsilateral paws of sham controls (n = 8) and animals receiving NP applied into the neighboring biceps femoris muscle (n = 6) or on sciatic nerve (n = 8) at day 5. Comparisons were made by the Kruskal–Wallis test with the Dunn multiple comparisons test (D–F). Data are mean ± SEM; *P < 0.05, **P < 0.01, *** P < 0.001, ****P < 0.0001; ns>0.05. ANOVA, analysis of variance; NP, nucleus pulposus.
In addition to pain hypersensitivity, motor dysfunction is often associated with peripheral neuropathic pain.61 Therefore, we examined the effect of applying NP to the sciatic nerve on motor function using 2 tests—rotarod and dynamic weight bearing (Supplementary Fig. 1A and Supplementary Fig. 1B, respectively, available at http://links.lww.com/PAIN/B614). In the rotarod test, the latency to fall of the NP-exposed animals was shorter than that of the sham controls (Supplementary Fig. 1A, http://links.lww.com/PAIN/B614). Similarly, the NP-exposed animals showed a significant deficit in dynamic weight bearing compared with sham-operated controls (Supplementary Fig. 1B, http://links.lww.com/PAIN/B614). Together, these data indicate that applying NP to the sciatic nerve induces a constellation of behavioural alterations which are absent in sham animals and which phenocopy those in neuropathic pain.
To determine whether the behavioural changes induced by NP were due to exposure of the sciatic nerve to a space-filling mass, we replaced the NP with 2 biologically inert materials—chitosan or poloxamer—matched in volume to that of the NP. The mechanical withdrawal thresholds of chitosan-exposed and of poloxamer-exposed animals were not significantly different from the withdrawal thresholds of sham-operated controls (Fig. 1E). Moreover, the withdrawal thresholds of the NP-exposed animals were significantly less than those of either the chitosan-exposed or poloxamer-exposed animals. Rotarod and cold testing showed no significant difference between space-filling controls and sham (Supplementary Fig. 2A and supplementary Fig. 2B, available at http://links.lww.com/PAIN/B614). Thus, we conclude that the pain hypersensitivity phenotype caused by applying NP was not due to a nonspecific effect of application of a space-filling mass adjacent to the sciatic nerve.
Moreover, to determine whether the behavioural changes induced by NP were due to placing this material in the hind limb per se, we examined the effect of applying NP into the neighbouring biceps femoris muscle. In animals in which NP was applied into the biceps femoris, we found that the mechanical withdrawal threshold was not different from that in sham-operated animals but was significantly different from that in animals in which NP was applied directly to the sciatic nerve (Fig. 1F). These findings indicate that placing NP into the hind limb is not sufficient to cause mechanical hypersensitivity, but rather, the hypersensitivity requires applying NP directly to the sciatic nerve.
3.2. Nucleus pulposus–induced pain hypersensitivity is not dependent on spinal microglia
A long-recognized hallmark of pain hypersensitivity induced by traumatic PNI is proliferation of microglia and morphological changes of these cells, microgliosis, in the dorsal horn of spinal cord ipsilateral to the injury, and the hypersensitivity is reversed by pharmacological and genetic interventions that suppress microglia function and signaling.2,3,74 By contrast, in this study, we found that applying NP to the sciatic nerve produced neither microgliosis nor morphological changes in dorsal horn microglia (Fig. 2A). As a positive control, SNI produced a readily observable increase in microglia number in the ipsilateral spinal cord (Fig. 2A). Furthermore, NP-induced mechanical hypersensitivity was unaffected by intrathecal administration of minocycline (Fig. 2B), which inhibits microglia function, at a dose (100 µg) known to reliably and reproducibly reverse SNI-induced mechanical hypersensitivity.70 These findings provide evidence that NP-induced pain hypersensitivity is independent of microglia.
Figure 2.
NP-induced pain hypersensitivity is not dependent on spinal microglia. (A) Iba1 staining of transverse sections of the lumbar spinal cord 7 days after sham, NP, or SNI surgery. Scale bar, 500 µm. (B) Withdrawal threshold from von Frey filaments before surgery (BL), 3 days after surgery, (preinjection; D3), and 15 to 120 minutes postinjection of minocycline (100 µg) intrathecal injection (n = 4). n = 7 for vehicle. (C) Withdrawal threshold from von Frey filaments in microglial BDNF KO male mice (n = 5) and in control male mice not receiving tamoxifen (n = 4) before and 1, 4, and 7 days post-NP surgery. Corn oil served as vehicle. (D) Stereoscopic view of ipsilateral sciatic nerve after NP placement (upper) and contralateral sciatic nerve (lower) from same animal. Scale bar, 360 µm. (E) Quantification of the average cross-sectional diameter of sciatic nerve from NP-exposed animals (n = 4) and sham controls (n = 4) in transverse sections. ns>0.05 by the t test. (F) ATF3 staining of L4/L5 DRG neurons 7 days after sham, NP, SNI, or CCI surgery. Scale bar, 90 µm. All numerical data are mean ± SEM. NP, nucleus pulposus; SNI, spared nerve injury.
We examined this possibility by an additional approach with mice in which BDNF was deleted from microglia because microglial BDNF has been shown to be required for PNI-induced pain hypersensitivity.14,70 We used CX3CR1CreER/+:BDNFfl/fl mice in which BDNF is deleted in CX3CR1-expressing cells by treating animals with tamoxifen. We took advantage of the difference in turnover rates between central and peripheral CX3CR1-expressing cells51 and administered NP after a 2-month recovery posttamoxifen so as to allow repopulation of BDNF expression in the circulating cells (Supplementary Fig. 3, available at http://links.lww.com/PAIN/B614) without restoring BDNF in microglia (microglial BDNF KO mice). In microglial BDNF KO mice NP-induced mechanical hypersensitivity, the magnitude of which was not different from that in nontamoxifen treated CX3CR1CreER/+:BDNFfl/fl mice which had received vehicle (Fig. 2C). As a positive control for microglial BDNF-dependent pain,70 mechanical hypersensitivity induced by SNI was significantly reduced in microglial BDNF KO mice than that of mice of the same genotype not receiving tamoxifen (Supplementary Fig. 4, available at http://links.lww.com/PAIN/B614). From these findings, we conclude that pain hypersensitivity induced by NP is independent of dorsal horn microglia.
3.3. Nucleus pulposus application does not induce sensory neuron transcriptional reprogramming
Given the lack of involvement of dorsal horn microglia, we investigated the mechanism of NP-induced pain hypersensitivity further. Because pain hypersensitivity can be the result of compression injury to peripheral nerves,4 we examined the possibility that applying NP may cause compression of the sciatic nerve. However, we found no visual evidence that NP applied to the nerve causes nerve compression (Fig. 2D). Furthermore, the average cross-sectional diameter of the sciatic nerve in the region of the nerve adjacent to the NP was not different than that of a corresponding region of the nerve in sham controls (Fig. 2E). By contrast, overly constricting the sciatic nerve with a tight ligature—the chronic constriction injury (CCI) model—produced readily observable swelling around the site of the injury (Supplementary Fig. 5, available at http://links.lww.com/PAIN/B614).
With peripheral nerve compression or transection, the injury to the nerve causes robust changes in gene and protein expression in the soma of primary sensory neurons in the dorsal root ganglia (DRG).42,49,77 Among the most well-characterized of these is a dramatic increase in the expression of activating transcription factor 3 (ATF3)32,39 which drives transcriptional reprogramming and is necessary for axonal regeneration and functional recovery.54 Applying NP to the sciatic nerve produced only a minimal increase in the number of L4-L5 DRG neurons expressing ATF3 (Fig. 2F), whereas CCI or SNI each caused more than a 40-fold increase in the proportion of ATF3+ neurons vs sham controls (Fig. 2F and supplementary Table 1, available at http://links.lww.com/PAIN/B614). Together, our findings indicate that applying NP to the sciatic nerve does not produce indicia of nerve compression injury or of nerve transection.
3.4. Pain hypersensitivity is dependent on macrophages at the site of nucleus pulposus application
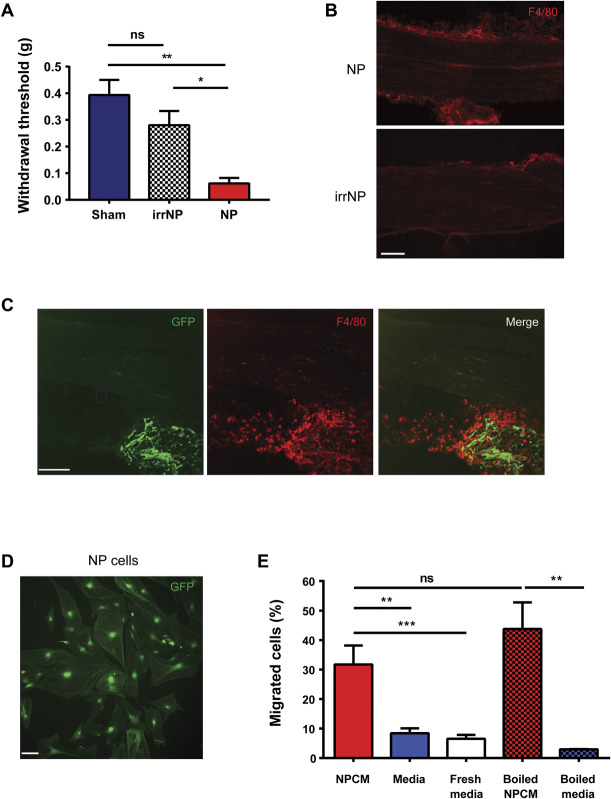
As our findings above indicate that pain hypersensitivity requires NP to be applied directly to the sciatic nerve but does not produce nerve compression, we wondered whether a localized cellular neuro-immune mechanism may drive the hypersensitivity. We first examined possible involvement of macrophages. We found that the sciatic nerve exposed to NP showed dramatically more cells immunoreactive for the macrophage marker F4/8019 than did the sciatic nerve in sham controls (Fig. 3A). The intensity of F4/80 immunofluorescence in the sciatic nerve was significantly increased adjacent to the NP as compared with that in sham animals or in the contralateral sciatic nerve (Fig. 3B). Neither chitosan nor poloxamer caused an increase in F4/80 immunofluorescence (Supplementary Fig. 6, available at http://links.lww.com/PAIN/B614). By 2 weeks after applying NP, a time at which the mechanical hypersensitivity had recovered (Fig. 1B), the F4/80 immunofluorescence had returned to a level indistinguishable from that of sham animals (Fig. 3B).
Figure 3.
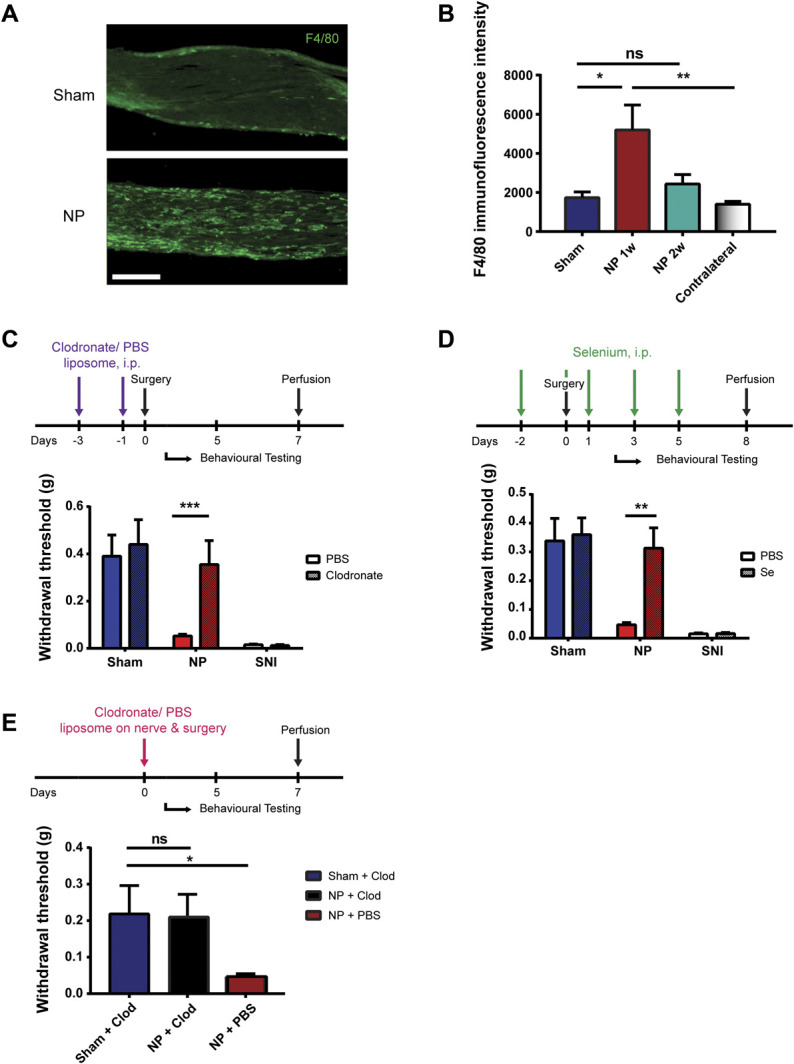
Pain hypersensitivity is dependent on macrophages at the site of NP application. (A) F4/80 staining of sciatic nerve 7 days after sham or NP surgery. Scale bar, 40 µm. (B) Quantification of F4/80 immunofluorescence intensity on sciatic nerves from sham-operated (n = 9) and NP-exposed animals 1 (n = 7) or 2 weeks after surgery (n = 4). *P < 0.05 and **P < 0.01 by the Kruskal–Wallis test with the Dunn multiple comparisons test. (C) Withdrawal threshold from von Frey filaments 5 days after sham (n = 4), NP (n = 8), and SNI (n = 3) surgery after intraperitoneal injections of clodronate liposome (Clod; 2 mg total). ***P< 0.001 comparing NP PBS and NP Clod by the Mann–Whitney test. (D) Withdrawal threshold from von Frey filaments 5 days after sham (n = 7), NP (n = 7), and SNI (n = 3) surgery after intraperitoneal injections of selenium (Se; 200 mg total). **P< 0.01 comparing NP PBS and NP Se by the t test. (E) Withdrawal threshold from von Frey filaments 5 days after sham and NP (n = 6 per group) surgery after the application of clodronate liposome (0.05 mg) local at sciatic nerve at the time of NP application. ns>0.05, *P < 0.05 by the Kruskal–Wallis test with the Dunn multiple comparisons test. All numerical data are mean ± SEM. NP, nucleus pulposus; SNI, spared nerve injury.
That the level of F4/80 immunofluorescence in the nerve adjacent to the NP tracked the mechanical hypersensitivity suggested that macrophages may be required for NP-induced sensitization. We tested this idea by systemic depletion of macrophages using clodronate liposomes, which depletes circulating macrophages6 while not directly affecting other phagocytic cells such as neutrophils.52,79 Animals received intraperitoneal injection of clodronate liposomes or PBS liposomes twice preoperatively, and their mechanical sensitivity was measured on day 5 post-NP administration (Fig. 3C). In NP-treated animals, the paw withdrawal threshold in animals receiving clodronate liposomes was significantly greater than that in animals receiving PBS liposomes (Fig. 3C). By contrast, treating with clodronate liposomes did not prevent the mechanical hypersensitivity induced by SNI (Fig. 3C), indicating that clodronate does not generally prevent pain hypersensitivity caused by manipulations of the sciatic nerve. We confirmed that F4/80 immunofluorescence was greatly reduced in the spleen and the sciatic nerve adjacent to the NP in the animals that had received clodronate liposomes (Supplementary Fig. 7A, available at http://links.lww.com/PAIN/B614). Thus, systemic clodronate treatment, which depleted macrophages, prevented NP-induced mechanical hypersensitivity.
As an independent test of whether macrophages are necessary in the NP-induced pain hypersensitivity, we used selenium, an inhibitor of macrophage activation and migration.17,21 We administered selenium intraperitoneally in NP and sham animals perioperatively (Fig. 3D). We observed no NP-induced hypersensitivity in the animals treated with selenium on day 5 (Fig. 3D), compared with vehicle control animals. Conversely, the SNI-induced mechanical hypersensitivity was not reversed by selenium (Fig. 3D). Taking these findings together, we conclude that peripheral macrophages are necessary for the pain hypersensitivity induced by applying NP to the sciatic nerve.
Thus, systemic depletion or inhibition of macrophages prevented the mechanical hypersensitivity caused by applying NP to the sciatic nerve. To determine whether macrophages at the site of NP application are required for the NP-induced mechanical hypersensitivity, we administered clodronate liposomes at the site of NP at the time of its application. Although animals treated with NP plus vehicle (PBS) showed significant lowering of mechanical withdrawal thresholds (Fig. 3E), the withdrawal thresholds of animals treated with NP plus clodronate liposomes were not significantly different from withdrawal thresholds of sham animals (Fig. 3E). To ensure that clodronate had acted only locally, we verified that clodronate liposomes applied at the site of NP greatly reduced the NP-induced accumulation of macrophages in the sciatic nerve without affecting macrophage levels in the spleen (Supplementary Fig. 7B, available at http://links.lww.com/PAIN/B614). Taken together, we conclude that macrophages at the site of the NP application are necessary for the NP-induced mechanical hypersensitivity.
3.5. Pain hypersensitivity requires nucleus pulposus cells
The NP consists of a small number of cells which produce the extensive matrix of proteoglycans, collagens, and elastin fibres that are characteristic of depictions of the NP.55,56,72 We assessed the possibility that the cells present within the NP may drive pain hypersensitivity. To this end, we irradiated NP discs with γ-radiation to kill the cells within the NP after removal from the donor and immediately before applying NP to the sciatic nerve. Animals that received irradiated NP (irrNP) showed no significant decrease in mechanical paw withdrawal threshold compared with sham animals (Fig. 4A), whereas animals that received nonirradiated NP showed a significantly lower withdrawal threshold as compared with those receiving irrNP (Fig. 4A). In addition, applying irrNP failed to cause macrophage accumulation in the sciatic nerve (Fig. 4B). These findings suggest that cells in the NP must be alive to induce pain hypersensitivity.
Figure 4.
NP-induced mechanical hypersensitivity and macrophage migration requires NP cells. (A) Withdrawal threshold from von Frey filaments 7 days after sham controls and animals receiving irradiated NP (irrNP) or NP (n = 6 per group). *P < 0.05 and **P < 0.01; ns>0.05 by the Kruskal–Wallis test with the Dunn multiple comparisons test. (B) F4/80 staining (shown in red in this figure) of sciatic nerve from animals exposed to NP or irrNP. Scale bar, 100 µm. (C) GFP fluorescence (green) and F4/80 (red) staining of sciatic nerve from C57BL/6 mouse receiving NP from GFP donor. Scale bar, 120 µm. (D) Cultured GFP-expressing NP cells. Scale bar, 120 µm. (E) Transwell migration assay using THP1 cells migrating towards NP-conditioned media (NPCM), media, fresh media, boiled NPCM, or boiled media (n = 3 per group). **P < 0.01, ***P < 0.001; ns>0.05 compared with corresponding media controls by 1-way ANOVA. All numerical data are mean ± SEM.
As live NP cells are required, it is possible that these cells migrate into the sciatic nerve and initiate changes that lead to pain hypersensitivity. To examine this possibility, we harvested NP from constitutively GFP-expressing C57BL/6 mice (Tg(act-EGFP)Y01Osb) and placed the NP (Fig. 4C, GFP) onto the sciatic nerve of wild-type C57BL/6 animals. We found that GFP-expressing cells were abundant in the isografted NP but absent within the sciatic nerve of the recipient. Thus, NP cells appear to not migrate out of NP on being placed adjacent to the sciatic nerve. As described above, placing NP adjacent to the sciatic nerve caused an increase in F4/80-expressing cells in the nerve and, in addition, in the NP disc material itself (Figs. 3B and 4C). However, we found no colocalization of F4/80 signal and GFP (Fig. 4C). These findings suggest that NP cells do not migrate or infiltrate into the sciatic nerve, and they do not colocalize with F4/80-expressing cells, presumed to be macrophages, in the NP disc.
Applying NP adjacent to the sciatic nerve thus attracted macrophages from the recipient into the NP disc material as well as into the nerve. Hence, it is possible that NP cells secrete a diffusible factor, or factors, that attracts these immune cells. We addressed this possibility in vitro by determining whether NP cells release molecules that may pass through a semipermeable membrane and cause migration of cells. To represent potential migrating cells, we used the monocyte/macrophage cell line, THP1, which are widely used in such chemoattractant assays.11,27 We made cultures of NP cells (Fig. 4D) and found that applying conditioned media taken from these cultures (NPCM) caused a significant migration of THP1 cells as compared with that caused by media conditioned without NP cells (Fig. 4E, media alone) or by unconditioned, fresh media (Fig. 4E). To test whether the chemoattractive capacity of the NPCM was due to molecules that were heat-labile, we boiled NPCM at 95°C for 10 minutes before adding to the lower chamber. In this case, boiled NPCM still caused a significant increase in the number of migrating THP1 cells vs boiled media alone (Fig. 4E), and the increase in migration caused by boiled NPCM was not different from that caused by nonboiled NPCM. Thus, NP cells release a heat-stable factor, or factors, that attracts macrophages.
3.6. Nucleus pulposus–induced mechanical sensitization requires brain-derived neurotrophic factor from macrophages
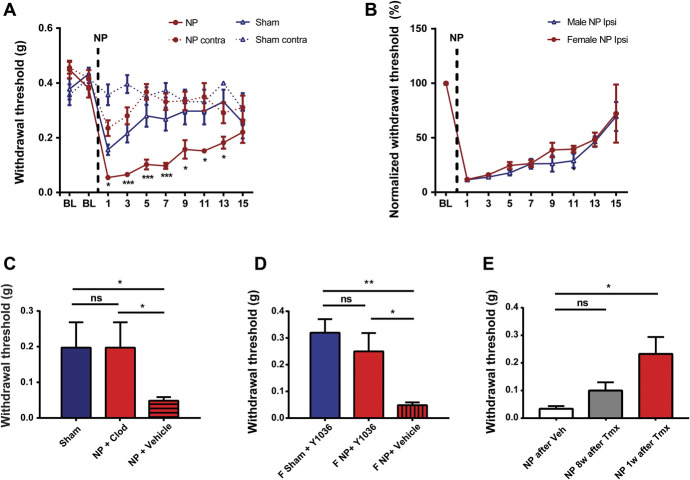
A parsimonious conceptual framework to account for our findings to this point is that living cells in the NP release a heat-stable factor causing local accumulation of macrophages which then produce the behavioural sensitization through acting on the sciatic nerve. Macrophages are known to release effector molecules including neurotrophins, such as NGF and BDNF, which are known to cause pain hypersensitivity in peripheral tissue40 and in the spinal cord.14 To test whether neurotrophins are necessary for pain hypersensitivity caused by applying NP, we locally administered Y1036, a neurotrophin inhibitor that sequesters both NGF and BDNF, preventing them from interacting with their cognate receptors.22 The average withdrawal threshold in animals receiving NP together with Y1036 was indistinguishable from that in sham animals that received Y1036 without NP and was significantly greater than that in animals that received NP together with vehicle (Fig. 5A). Thus, Y1036 prevented NP-induced pain hypersensitivity indicating that local neurotrophin signalling is required.
Figure 5.
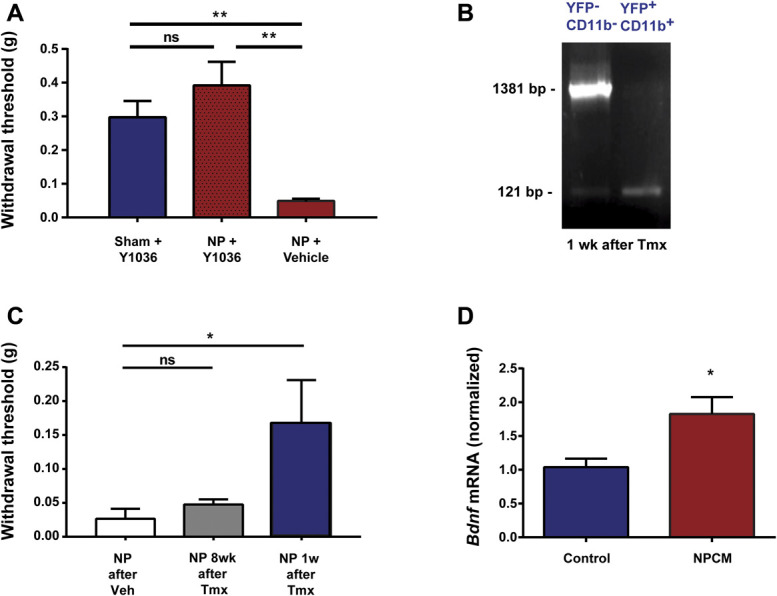
NP-induced mechanical sensitization requires BDNF from macrophages. (A) Withdrawal threshold from von Frey filaments 5 days after sham (n = 7) and NP (n = 5) surgery after the application of Y1036 (50 µM) local at sciatic nerve at the time of NP application. (B) PCR analysis of the YFP−/CD11b− or YFP+/CD11b+ cells sorted from the blood of CX3CR1CreER/+:BDNFfl/fl mice, in which YFP is constitutively expressed in CreER-expressing cells (see Methods), 1 or 8 weeks after tamoxifen treatment. (C) Withdrawal threshold from von Frey filaments on the CX3CR1CreER/+:BDNFfl/fl mice 1 or 8 weeks after tamoxifen treatment followed by NP surgery (n = 4-5 mice/condition). Corn oil served as vehicle. (D) The mRNA expression of Bdnf (normalized to Gapdh) in peritoneal macrophages stimulated with NPCM or control media. n = 6 per group. Comparisons were made by the Kruskal–Wallis test with the Dunn multiple comparisons test (A and C) or t test (D). *P < 0.05, **P < 0.01; ns>0.05. All numerical data are mean ± SEM.
To investigate the possible requirement for BDNF, we used CX3CR1CreER/+:BDNFfl/fl mice51 in which BDNF was deleted in circulating macrophages on recombination elicited by treating with tamoxifen (Fig. 5B). In CX3CR1CreER/+:BDNFfl/fl mice given the vehicle for tamoxifen, which produced no recombination (data not shown), applying NP produced mechanical hypersensitivity (Fig. 5C). By contrast, mechanical hypersensitivity was significantly reduced in NP-exposed CX3CR1CreER/+:BDNFfl/fl mice that had received tamoxifen 1 week before NP (Fig. 5C). As shown above (Fig. 2C) by 8 weeks after tamoxifen treatment, a time at which the circulating population of macrophages had recovered fully (Supplementary Fig. 3, available at http://links.lww.com/PAIN/B614), applying NP elicited pain hypersensitivity in CX3CR1CreER/+:BDNFfl/fl mice. Thus, the capacity for NP to induce pain hypersensitivity was lost when NP was applied at the time when BDNF was depleted from circulating macrophages, and the NP-induced hypersensitivity returned at the time when the peripheral circulation of BDNF-expressing macrophages had been replenished.
As the above experiments indicate that NP-induced behavioural sensitization requires BDNF in circulating peripheral macrophages, we considered the possibility that the expression of BDNF might be induced in macrophages by diffusible factors from NP cells. To test this possibility, we cocultured peritoneal macrophages with NPCM or with control media. We found using real-time PCR analysis that Bdnf mRNA was significantly greater in the macrophages treated with NPCM than in macrophages treated with control media (Fig. 5D). Together, these findings suggest that NP cells release a factor that causes the upregulation of Bdnf gene expression in macrophages and that BDNF from the macrophages is necessary for producing pain hypersensitivity.
3.7. Lack of sex differences in nucleus pulposus–induced pain hypersensitivity
A growing body of evidence indicates that there may be sex differences in behavioural pain phenotypes and in the underlying cellular and molecular mechanisms.41,69,70 We therefore tested for possible male–female differences in the NP-induced pain phenotypes or in the underlying cellular and molecular mechanisms. In female mice, applying NP to the sciatic nerve produced a significant lowering of the paw withdrawal threshold (Fig. 6A). The lowest withdrawal threshold was on day 1, and the threshold recovered to that in sham animals by day 15. Comparing the time courses of mechanical hypersensitivity revealed no significant differences between males and females (Fig. 6B). In addition, we examined the response to acetone applied to the hind paw in females and found that applying NP produced cold sensitization (Supplementary Fig. 8, available at http://links.lww.com/PAIN/B614). Thus, in the tests examined, applying NP to the sciatic nerve induced behavioural sensitization in females that was not phenotypically distinguishable from that induced by NP in males.
Figure 6.
NP-induced pain hypersensitivity in female mice. (A) Paw withdrawal threshold from von Frey filaments on the ipsilateral and contralateral side before (BL) and for 15 days after surgery in female animals with NP applied to sciatic nerve (n = 25) or sham controls (n = 16). *P < 0.05, *** P < 0.001 comparing NP ipsilateral and sham ipsilateral with two-way repeated-measures ANOVA with the Bonferroni multiple comparisons post hoc test. (B) Development of NP-induced mechanical hypersensitivity in male and female mice. Withdrawal threshold on the ipsilateral hind paw normalized to their respective baselines. (C) Withdrawal threshold from von Frey filaments 5 days after sham and NP (n = 4 per group) surgery after intraperitoneal injections of clodronate liposome in female mice (Clod; 2 mg total). (D) Withdrawal threshold from von Frey filaments 5 days after sham and NP (n = 6 per group) surgery after the application of Y1036 (50 µM) local at sciatic nerve in female mice at the time of NP application. (E) Withdrawal threshold from von Frey filaments on the CX3CR1CreER/+:BDNFfl/fl female mice 1 (n = 7) or 8 (n = 3) weeks after tamoxifen treatment followed by NP surgery. Corn oil served as vehicle in this experiment (n = 5). Comparisons were made by the Kruskal–Wallis test with the Dunn multiple comparisons test (C–E). *P < 0.05, **P < 0.01; ns>0.05. All data are mean ± SEM. ANOVA, analysis of variance; ANOVA, analysis of variance; NP, nucleus pulposus.
We probed the underlying mechanisms by determining whether NP-induced pain hypersensitivity in recipient female mice requires macrophages or BDNF. The sciatic nerve exposed to NP showed macrophage accumulation (Supplementary Fig. 9, available at http://links.lww.com/PAIN/B614). For the former, we administered clodronate liposomes to deplete macrophages before the NP application and found that female NP-treated animals receiving clodronate liposomes showed significantly less mechanical sensitivity than animals receiving PBS liposomes (Fig. 6C).
To test the requirement for neurotrophins in female mice, we locally administered Y1036 at the site and time of NP application. We observed no NP-induced hypersensitivity in the female animals treated with Y1036, compared with vehicle control animals (Fig. 6D). Moreover, in female CX3CR1CreER/+:BDNFfl/fl mice, applying NP 1 week after the treatment with tamoxifen did not produce hypersensitivity (Fig. 6E), when BDNF was depleted in circulating macrophages (Supplementary Fig. 10, available at http://links.lww.com/PAIN/B614), whereas NP caused hypersensitivity when applied 8 weeks after tamoxifen, when the macrophages expressing BDNF had repopulated in the circulation (Supplementary Fig. 10, available at http://links.lww.com/PAIN/B614). Together, these results demonstrated that NP-induced behavioural sensitization in female mice requires macrophages and BDNF similar to their male counterparts, indicating that there was no sex difference in the cellular and molecular mechanisms underlying the sensitization.
4. Discussion
Here, we found that apposing NP to the sciatic nerve drives hypersensitivity to mechanical, cold, and heat stimuli and decreased motor coordination, a constellation of behavioural changes indicative of peripheral neuropathic pain hypersensitivity. Although applying NP did not produce signs of compression, it did cause accumulation of macrophages in the nerve nearby indicating there was an immunological lesion to the nerve. Together, the behavioural signs and the presence of this immunological lesion demonstrate that applying NP to the sciatic nerve meets the criteria for peripheral neuropathic pain.64 Consistent with the pain being neuropathic rather than arising from tissue inflammation, there was hypersensitivity elicited by stimulating the paw, which is innervated by the lesioned nerve, but there was no direct damage to the paw itself.
The behavioural changes produced by applying NP phenocopy those in widely accepted models of peripheral neuropathic pain produced by traumatic injury to the sciatic nerve or its branches. Pain hypersensitivity evoked by traumatic PNI is well-known to depend on microglia in the dorsal horn ipsilateral to the side of the injury.33,68,76 However, we found that the immunological lesion caused by applying NP to the sciatic nerve produced no change in the number or morphology of microglia in the spinal cord nor was the NP-induced pain hypersensitivity suppressed by minocycline or in mice in which BDNF was deleted in microglia. Rather, applying NP caused recruitment of macrophages into the sciatic nerve and the pain hypersensitivity was suppressed by depleting macrophages, by blocking BDNF-trkB signaling at the site of NP application, or by deleting BDNF in peripheral macrophages. Thus, our findings demonstrate that pain hypersensitivity produced by a discrete lesion in a peripheral nerve can be induced without involving spinal microglia. The most parsimonious explanation for the totality of our findings is that cells in the NP secrete a diffusible, heat-stable factor, or factors, which recruits macrophages into the sciatic nerve where they release BDNF which acts to elicit the behavioural sensitization as illustrated in the conceptual framework in Figure 7.
Figure 7.
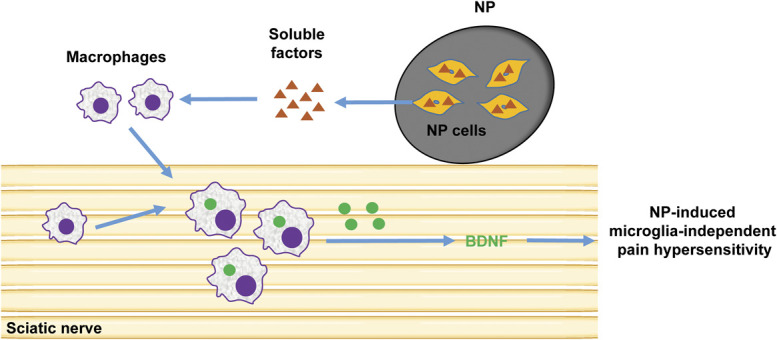
Schematic representation of the mechanism of pain hypersensitivity induced by NP. When NP is apposed to the sciatic nerve, NP cells secrete soluble factors which recruit macrophages into the sciatic nerve where they release BDNF which leads to NP-induced behavioural sensitization. BDNF, brain-derived neurotrophic factor; NP, nucleus pulposus.
Nucleus pulposus has been implicated in pain arising from herniation of spinal discs.36,86 In experimental models of such pain, NP is applied to the spinal dorsal roots. Placing NP in this location may result in surgical damage to the spinal column and may also cause damage or compression of the dorsal roots themselves and dorsal root ganglia.35,50,60 These confounds are obviated by the model we report here which avoids nerve compression and damage to the spinal column, roots, or ganglia. We anticipate, therefore, that the NP—macrophage—BDNF mechanism we have elucidated may contribute to noncompressive aspects of pain from spinal disc herniation.
That BDNF from macrophages drives NP-induced pain hypersensitivity is consistent with previous work showing that macrophages may synthesize and release this neurotrophin.82 Prevention of pain hypersensitivity by NP with local blockade of neurotrophin signaling by Y1036 or by depleting macrophages locally implies that the site of action of BDNF is within the sciatic nerve where the NP is applied. We postulate that BDNF acts within the nerve through its cognate receptor trkB. This receptor is known to be expressed on axons of primary afferents,5,7,25 and thus, activating trkB may lead to enhanced firing of primary afferent neurons which then drive pain hypersensitivity. Alternatively, or in addition, as BDNF bound to trkB is transported anterogradely in neurons,1 it is conceivable that BDNF might be transported transganglionically and released in the spinal dorsal horn. Other cell types in peripheral nerves, such as Schwann cells, express trkB7 and may indirectly mediate the actions of macrophage-derived BDNF. Macrophages are known to release many types of intercellular mediators in addition to BDNF.37,38,58,66 Thus, although our findings indicate that BDNF from macrophages is necessary for NP-induced pain hypersensitivity, we do not rule out the possibility that other mediators released by these cells may also be required. Macrophages are known to have complex and diverse responses.43,48 Thus, defining the response state, or states, induced by NP responsible for producing the pain hypersensitivity will be an important next step after our initial characterization reported here.
From our finding that lethal irradiation of the NP before implantation prevented the development of pain hypersensitivity, we conclude that NP cells are a necessary intermediary cell type. Although NP cells are required, we found that they remain within the NP matrix indicating that these cells act from a distance. These findings together with our experiments showing that NP attracts macrophages in vitro by releasing a heat-stable factor imply that in vivo NP cells may release factor(s) that cause accumulation of macrophages within the nerve. Candidate molecules include heat-stable peptides or small proteins, gases, and lipids, classes of molecules which have been reported to be released from NP cells.15,26,46,57 We speculate that the requisite molecules from the NP drive transmigration of circulating macrophages across the vascular endothelium in the nerve, a process which may involve disrupting the blood–nerve barrier.24,47,63
Nucleus pulposus–induced hypersensitivity to mechanical stimulation resolved approximately 2 weeks after applying NP to the sciatic nerve raising the question of why this behavioural sensitization reverses. The number of macrophages in the sciatic nerve tracked the time course of this recovery. We found that the behavioural changes were recapitulated by reapplying NP after recovery (not illustrated), indicating that the animals had not become resistant, or desensitized, to signaling from the NP. Rather, we propose that the recovery is due to loss of viable NP cells over time and loss of signaling to attract macrophages and that continued signaling by viable NP cells is necessary to maintain pain hypersensitivity.
Pain hypersensitivity produced experimentally by nerve trauma produces similar behavioural changes in males and females but exhibits sex differences in the underlying mechanisms.41,69,70 Here, we found that the constellation of behaviour changes produced by NP in females was not different in magnitude nor in time course from those changes produced in males. However, molecular interventions which prevented NP-induced hypersensitivity in males also prevented hypersensitivity when tested in females. The lack of sex difference in the mechanism is thus a major difference between pain hypersensitivity induced by NP and that induced by traumatic nerve injury.
Another difference between the response to traumatic nerve injury and that to applying NP is that the former causes transcriptional reprogramming of peripheral sensory neurons driven by the rapid induction of the transcription factor ATF3.54 By contrast, we observed no substantive increase in ATF3 when NP was applied to the sciatic nerve. ATF3 is reported to be induced by peripheral nerve compression49 and also by stress loading,83 both of which also drive microgliosis in the dorsal horn. With traumatic and compressive nerve injuries41,70 and with stress loading,84 hypersensitivity is reversed by suppressing microglia. The common link may be the cytokine, CSF-1, which is induced in cell bodies of damaged primary afferent neurons in the dorsal root ganglia.28 CSF-1 is transported to the spinal cord where it is released, activating microglial CSF-1 receptors, leading to microgliosis.28 The upregulation of CSF-1 by nerve injury has recently been shown to be prevented by knocking out ATF3 in primary sensory neurons.54 Thus, a common property of microglia-dependent hypersensitivity is ATF3-mediated transcriptional reprograming in primary sensory neurons. That NP produced no substantive induction of ATF3 nor microgliosis strongly supports the microglia-independence of NP-induced pain hypersensitivity.
From our findings in the mouse, we suspect that there may be microglia-independent forms of neuropathic pain in humans. Clinical trials using treatments directed towards inhibiting microglia have had only limited success,10,16,44,67,71,80 and although there may be various explanations for lack of efficacy, the most straightforward one is that microglia-independent neuropathic pain is relatively common in humans. We note that agents that inhibit microglia do not necessarily also inhibit macrophages.20,59 Minocycline which is touted as a “microglia inhibitor” is reported to have pleiotropic effects on macrophages, for example, inhibiting peritoneal macrophages but activating alveolar macrophages.8 Thus, NP-induced pain hypersensitivity we describe here may model a common form of neuropathic pain in humans. Using the NP model may thus reveal novel therapeutic targets given its distinctive mechanistic framework. Importantly, our findings point to the importance of developing new diagnostic tests in humans to differentiate neuropathic pain that is microglia-dependent from that which is microglia-independent.
In summary, we have developed a model of neuropathic pain hypersensitivity that is not dependent on microglia in either male or female mice. Thus, we speculate that there may be microglia-independent forms of neuropathic pain in humans. As such, our findings help explain the lack of efficacy of microglia inhibitors on neuropathic pain in human clinical trials and reveal new potential targets for developing effective analgesics.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B614.
Supplementary Material
Acknowledgements
The authors thank Yongqian Wang for technical assistance and Janice Hicks for editorial assistance.
Funding: this research was supported by a grant from Canadian Institutes of Health Research (FDN-154336) to MWS. MWS held the Northbridge Chair in Paediatric Research. M.M. Muley was supported by a Pain Scientist Award from the University of Toronto Centre for the Study of Pain and by a Restracomp postdoctoral fellowship from The Hospital for Sick Children Research Training Centre. Author contributions: M.W. Salter conceived the project; M.W. Salter supervised the research; Y. Tu established the methodology; Y. Tu and M.M. Muley designed the experiments and wrote the manuscript with input from all authors; Y. Tu performed imaging analyses and quantification; Y. Tu, M.M. Muley, and S. Beggs collected and analyzed data. All authors read and approved the final manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
YuShan Tu, Email: amy.tu@sickkids.ca.
Milind M. Muley, Email: milind.muley@sickkids.ca.
Simon Beggs, Email: s.beggs@ucl.ac.uk.
References
- [1].Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Menager C, Kawabata S, Fujii K, Iwamatsu A, Segal RA, Fukuda M, Kaibuchi K. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Dev Cell 2009;16:675–86. [DOI] [PubMed] [Google Scholar]
- [2].Beggs S, Salter MW. Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol 2010;20:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci 2012;15:1068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. PAIN 1988;33:87–107. [DOI] [PubMed] [Google Scholar]
- [5].Bhattacharyya A, Watson FL, Bradlee TA, Pomeroy SL, Stiles CD, Segal RA. Trk receptors function as rapid retrograde signal carriers in the adult nervous system. J Neurosci 1997;17:7007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Biewenga J, van der Ende MB, Krist LF, Borst A, Ghufron M, van Rooijen N. Macrophage depletion in the rat after intraperitoneal administration of liposome-encapsulated clodronate: depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of Freund's adjuvant. Cell Tissue Res 1995;280:189–96. [DOI] [PubMed] [Google Scholar]
- [7].Bonalume V, Caffino L, Castelnovo LF, Faroni A, Giavarini F, Liu S, Caruso D, Schmelz M, Fumagalli F, Carr RW, Magnaghi V. Schwann cell autocrine and paracrine regulatory mechanisms, mediated by allopregnanolone and BDNF, modulate PKCepsilon in peripheral sensory neurons. Cells 2020;9:1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bonjoch L, Gea-Sorli S, Jordan J, Closa D. Minocycline inhibits peritoneal macrophages but activates alveolar macrophages in acute pancreatitis. J Physiol Biochem 2015;71:839–46. [DOI] [PubMed] [Google Scholar]
- [9].Breivik H, Eisenberg E, O'Brien T. Openminds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013;13:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Casha S, Zygun D, McGowan D, Yong VW, Hurlbert RJ. Neuroprotection with minocycline after spinal cord injury. Neurosurgery 2009;65:410–11. [Google Scholar]
- [11].Cicha I, Regler M, Urschel K, Goppelt-Struebe M, Daniel WG, Garlichs CD. Resveratrol inhibits monocytic cell chemotaxis to MCP-1 and prevents spontaneous endothelial cell migration through Rho kinase-dependent mechanism. J Atheroscler Thromb 2011;18:1031–42. [DOI] [PubMed] [Google Scholar]
- [12].Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J, Bevan S, Malcangio M. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A 2007;104:10655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain. Nat Rev Dis Primers 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005;438:1017–21. [DOI] [PubMed] [Google Scholar]
- [15].Cunha C, Silva AJ, Pereira P, Vaz R, Goncalves RM, Barbosa MA. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res Ther 2018;20:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Curtin CM, Kenney D, Suarez P, Hentz VR, Hernandez-Boussard T, Mackey S, Carroll IR. A double-blind placebo randomized controlled trial of minocycline to reduce pain after carpal tunnel and trigger finger release. J Hand Surg Am 2017;42:166–74. [DOI] [PubMed] [Google Scholar]
- [17].Dalla Puppa L, Savaskan NE, Brauer AU, Behne D, Kyriakopoulos A. The role of selenite on microglial migration. Ann N Y Acad Sci 2007;1096:179–83. [DOI] [PubMed] [Google Scholar]
- [18].Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. PAIN 2000;87:149–58. [DOI] [PubMed] [Google Scholar]
- [19].Dos Anjos Cassado A. F4/80 as a major macrophage marker: the case of the peritoneum and spleen. Results Probl Cell Differ 2017;62:161–79. [DOI] [PubMed] [Google Scholar]
- [20].Dunston CR, Griffiths HR, Lambert PA, Staddon S, Vernallis AB. Proteomic analysis of the anti-inflammatory action of minocycline. Proteomics 2011;11:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Duntas LH. Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res 2009;41:443–7. [DOI] [PubMed] [Google Scholar]
- [22].Eibl JK, Chapelsky SA, Ross GM. Multipotent neurotrophin antagonist targets brain-derived neurotrophic factor and nerve growth factor. J Pharmacol Exp Ther 2010;332:446–54. [DOI] [PubMed] [Google Scholar]
- [23].Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016;6:e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res 2015;107:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gomes RA, Hampton C, El-Sabeawy F, Sabo SL, McAllister AK. The dynamic distribution of TrkB receptors before, during, and after synapse formation between cortical neurons. J Neurosci 2006;26:11487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disk herniation-associated radiculopathy. Semin Arthritis Rheum 1998;28:60–71. [DOI] [PubMed] [Google Scholar]
- [27].Gouwy M, Struyf S, Berghmans N, Vanormelingen C, Schols D, Van Damme J. CXCR4 and CCR5 ligands cooperate in monocyte and lymphocyte migration and in inhibition of dual-tropic (R5/X4) HIV-1 infection. Eur J Immunol 2011;41:963–73. [DOI] [PubMed] [Google Scholar]
- [28].Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 2016;19:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hagen M, Madhavan T, Bell J. Combined analysis of 3 cross-sectional surveys of pain in 14 countries in Europe, the Americas, Australia, and Asia: impact on physical and emotional aspects and quality of life. Scand J Pain 2020;20:575–89. [DOI] [PubMed] [Google Scholar]
- [30].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. PAIN 1988;32:77–88. [DOI] [PubMed] [Google Scholar]
- [31].Ho Kim S, Mo Chung J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. PAIN 1992;50:355–63. [DOI] [PubMed] [Google Scholar]
- [32].Hunt D, Raivich G, Anderson PN. Activating transcription factor 3 and the nervous system. Front Mol Neurosci 2012;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018;19:138–52. [DOI] [PubMed] [Google Scholar]
- [34].Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? PAIN 2013;154(suppl 1):S10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jin L, Balian G, Li XJ. Animal models for disc degeneration-an update. Histol Histopathol 2018;33:543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kadow T, Sowa G, Vo N, Kang JD. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions?. Clin Orthop Relat Res 2015;473:1903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kiguchi N, Kobayashi D, Saika F, Matsuzaki S, Kishioka S. Pharmacological regulation of neuropathic pain driven by inflammatory macrophages. Int J Mol Sci 2017;18:2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim D, You B, Lim H, Lee SJ. Toll-like receptor 2 contributes to chemokine gene expression and macrophage infiltration in the dorsal root ganglia after peripheral nerve injury. Mol Pain 2011;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Laedermann CJ, Pertin M, Suter MR, Decosterd I. Voltage-gated sodium channel expression in mouse DRG after SNI leads to re-evaluation of projections of injured fibers. Mol Pain 2014;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Luo C, Zhong XL, Zhou FH, Li JY, Zhou P, Xu JM, Song B, Li CQ, Zhou XF, Dai RP. Peripheral brain derived neurotrophic factor precursor regulates pain as an inflammatory mediator. Sci Rep 2016;6:27171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. PAIN 2018;159:1752–63. [DOI] [PubMed] [Google Scholar]
- [42].Martin SL, Reid AJ, Verkhratsky A, Magnaghi V, Faroni A. Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in inflammation, cell death and nociception. Neural Regen Res 2019;14:939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000 Prime Rep 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Martinez V, Szekely B, Lemarie J, Martin F, Gentili M, Ben Ammar S, Lepeintre JF, Garreau de Loubresse C, Chauvin M, Bouhassira D, Fletcher D. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: a randomized, double-blind, controlled study. PAIN 2013;154:1197–203. [DOI] [PubMed] [Google Scholar]
- [45].Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, Mak TW, Tamura T, Tsuda M, Inoue K. Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun 2014;5:3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Molinos M, Almeida CR, Caldeira J, Cunha C, Goncalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interf 2015;12:20150429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 2011;6:323–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguchi K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. PAIN 2003;101:65–77. [DOI] [PubMed] [Google Scholar]
- [50].Ozawa K, Atsuta Y, Kato T. Chronic effects of the nucleus pulposus applied to nerve roots on ectopic firing and conduction velocity. Spine 2001;26:2661–5. [DOI] [PubMed] [Google Scholar]
- [51].Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, III, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013;155:1596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Qian Q, Jutila MA, Van Rooijen N, Cutler JE. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol 1994;152:5000–8. [PubMed] [Google Scholar]
- [53].Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. PAIN 2020;161:1976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Renthal W, Tochitsky I, Yang L, Cheng YC, Li E, Kawaguchi R, Geschwind DH, Woolf CJ. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 2020;108:128–44 e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Richardson SM, Ludwinski FE, Gnanalingham KK, Atkinson RA, Freemont AJ, Hoyland JA. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci Rep 2017;7:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, Iatridis JC, Sakai D, Hoyland JA. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res 2015;33:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014;10:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ristoiu V. Contribution of macrophages to peripheral neuropathic pain pathogenesis. Life Sci 2013;93:870–81. [DOI] [PubMed] [Google Scholar]
- [59].Rothman SM, Guarino BB, Winkelstein BA. Spinal microglial proliferation is evident in a rat model of painful disc herniation both in the presence of behavioral hypersensitivity and following minocycline treatment sufficient to attenuate allodynia. J Neurosci Res 2009;87:2709–17. [DOI] [PubMed] [Google Scholar]
- [60].Sakamoto Y, Nakamura T, Takagi K. Functional and morphological changes of lumbar nerve roots induced by mechanical compression or the nucleus pulposus in contact with the root: analysis of fiber size-dependent vulnerability in rabbits. J Orthop Sci 2004;9:598–604. [DOI] [PubMed] [Google Scholar]
- [61].Salat K, Gawlik K, Witalis J, Pawlica-Gosiewska D, Filipek B, Solnica B, Wieckowski K, Malawska B. Evaluation of antinociceptive and antioxidant properties of 3-[4-(3-trifluoromethyl-phenyl)-piperazin-1-yl]-dihydrofuran-2-one in mice. Naunyn Schmiedebergs Arch Pharmacol 2013;386:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med 2017;23:1018–27. [DOI] [PubMed] [Google Scholar]
- [63].Sano Y, Kanda T. Blood-neural barrier: overview and latest progress. Clin Exp Neuroimmunology 2013;4:220–7. [Google Scholar]
- [64].Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, Benoliel R, Cohen M, Cruccu G, Davis KD, Evers S, First M, Giamberardino MA, Hansson P, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Nurmikko T, Perrot S, Raja SN, Rice ASC, Rowbotham MC, Schug S, Simpson DM, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ, Barke A, Rief W, Treede RD. Classification Committee of the Neuropathic Pain Special Interest G. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 2019;160:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci 2002;5(suppl):1062–7. [DOI] [PubMed] [Google Scholar]
- [66].Segond von Banchet G, Boettger MK, Fischer N, Gajda M, Brauer R, Schaible HG. Experimental arthritis causes tumor necrosis factor-alpha-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. PAIN 2009;145:151–9. [DOI] [PubMed] [Google Scholar]
- [67].Shin DA, Kim TU, Chang MC. Minocycline for controlling neuropathic pain: a systematic narrative review of studies in humans. J Pain Res 2021;14:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sideris-Lampretsas G, Malcangio M. Microglial heterogeneity in chronic pain. Brain Behav Immun 2021;96:279–89. [DOI] [PubMed] [Google Scholar]
- [69].Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci 2011;31:15450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015;18:1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Syngle A, Verma I, Krishan P, Garg N, Syngle V. Minocycline improves peripheral and autonomic neuropathy in type 2 diabetes: MIND study. Neurol Sci 2014;35:1067–73. [DOI] [PubMed] [Google Scholar]
- [72].Tang X, Jing L, Richardson WJ, Isaacs RE, Fitch RD, Brown CR, Erickson MM, Setton LA, Chen J. Identifying molecular phenotype of nucleus pulposus cells in human intervertebral disc with aging and degeneration. J Orthop Res 2016;34:1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Tian DH, Perera CJ, Moalem-Taylor G. Neuropathic pain in animal models of nervous system autoimmune diseases. Mediators Inflamm 2013;2013:298326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig 2016;7:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 2005;28:101–7. [DOI] [PubMed] [Google Scholar]
- [76].Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003;424:778–83. [DOI] [PubMed] [Google Scholar]
- [77].Uttam S, Wong C, Amorim IS, Jafarnejad SM, Tansley SN, Yang J, Prager-Khoutorsky M, Mogil JS, Gkogkas CG, Khoutorsky A. Translational profiling of dorsal root ganglia and spinal cord in a mouse model of neuropathic pain. Neurobiol Pain 2018;4:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. PAIN 2014;155:654–62. [DOI] [PubMed] [Google Scholar]
- [79].Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 1994;174:83–93. [DOI] [PubMed] [Google Scholar]
- [80].Vanelderen P, Van Zundert J, Kozicz T, Puylaert M, De Vooght P, Mestrum R, Heylen R, Roubos E, Vissers K. Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology 2015;122:399–406. [DOI] [PubMed] [Google Scholar]
- [81].Wodarski R, Clark AK, Grist J, Marchand F, Malcangio M. Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats. Eur J Pain 2009;13:807–11. [DOI] [PubMed] [Google Scholar]
- [82].Wong I, Liao H, Bai X, Zaknic A, Zhong J, Guan Y, Li HY, Wang YJ, Zhou XF. ProBDNF inhibits infiltration of ED1+ macrophages after spinal cord injury. Brain Behav Immun 2010;24:585–97. [DOI] [PubMed] [Google Scholar]
- [83].Yasui M, Menjyo Y, Tokizane K, Shiozawa A, Tsuda M, Inoue K, Kiyama H. Hyperactivation of proprioceptors induces microglia-mediated long-lasting pain in a rat model of chronic fatigue syndrome. J Neuroinflammation 2019;16:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yasui M, Yoshimura T, Takeuchi S, Tokizane K, Tsuda M, Inoue K, Kiyama H. A chronic fatigue syndrome model demonstrates mechanical allodynia and muscular hyperalgesia via spinal microglial activation. Glia 2014;62:1407–17. [DOI] [PubMed] [Google Scholar]
- [85].Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. PAIN 2022;163:e328-e332. [DOI] [PubMed] [Google Scholar]
- [86].Zhang X, Wang X, Gao L, Yang B, Wang Y, Niu K, Lai J, Wan S, Luo J. TNF-a induces methylglyoxal accumulation in lumbar herniated disc of patients with radicular pain. Front Behav Neurosci 2021;15:760547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhang Z, Zhang ZY, Fauser U, Schluesener HJ. Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience 2008;152:495–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B614.