Abstract
Photosystem I (PSI) enables photo-electron transfer and regulates photosynthesis in the bioenergetic membranes of cyanobacteria and chloroplasts. Being a multi-subunit complex, its macromolecular organization affects the dynamics of photosynthetic membranes. Here we reveal a chloroplast PSI from the green alga Chlamydomonas reinhardtii that is organized as a homodimer, comprising 40 protein subunits with 118 transmembrane helices that provide scaffold for 568 pigments. Cryogenic electron microscopy identified that the absence of PsaH and Lhca2 gives rise to a head-to-head relative orientation of the PSI–light-harvesting complex I monomers in a way that is essentially different from the oligomer formation in cyanobacteria. The light-harvesting protein Lhca9 is the key element for mediating this dimerization. The interface between the monomers is lacking PsaH and thus partially overlaps with the surface area that would bind one of the light-harvesting complex II complexes in state transitions. We also define the most accurate available PSI–light-harvesting complex I model at 2.3 Å resolution, including a flexibly bound electron donor plastocyanin, and assign correct identities and orientations to all the pigments, as well as 621 water molecules that affect energy transfer pathways.
Subject terms: Cryoelectron microscopy, Photosystem I
This study presents a chloroplast photosystem I structure identified by cryogenic electron microscopy from the green alga Chlamydomonas reinhardtii. In contrast to the cyanobacterial complex, the absence of PsaH and Lhca2 allows a head-to-head orientation of the photosystem I–light-harvesting complex I monomers.
Main
A chloroplast photosystem I (PSI) of green algae consists of a core complex and three antenna modules: inner belt, outer belt and Lhca2-Lhca9 heterodimer, which together comprise 24 subunits1–4. As a short-term light acclimation mechanism in response to fluctuating illumination and anoxia, the algal PSI additionally associates with two light-harvesting complex II (LHCII) trimers5,6. Structural studies have shown that the oligomeric state of a chloroplast PSI is a monomer, due to the presence of the subunit PsaH, whereas in cyanobacteria, structures of dimers7–9 and trimers10,11 were also reported. Cyanobacterial PSI oligomerizes via direct contacts between subunits PsaI and PsaL; however, such an association has been ruled out for chloroplast PSI owing to structural constraints of PsaH presence that impose an apparent rigidity12,13. Yet, recent structural studies of PSI from a chloroplast of a salt-tolerant alga suggested that its functional core may vary more than previously believed14. Functional PsaH-free particles were found, thus showing a potential architectural plasticity of PSI in response to the ecological environment. On the macromolecular level, an atomic force microscopy analysis of a plant thylakoid membrane showed that, when its architecture is altered upon transition from darkness to light, larger inter-membrane contacts are formed, leading to a reduced diffusion distance for the mobile electron carriers15. The membrane architecture in dark- and light-adapted membranes consists of ordered rows of closely packed PSI dimers, which are more abundant in the dark state15. Similarly, closely associated PSI–light-harvesting complex I (LHCI) complexes were detected in plants by negative-stain electron microscopy16, and dimers were found in a subpopulation of PSI from a temperature-sensitive photosystem II mutant alga17. This suggests that reversible PSI dimer formation may have a physiological role in thylakoid membrane structure maintenance in chloroplasts. However, very little is known about PSI–LHCI dimers, and information on their structures is lacking. In the absence of high-resolution data, no evidence is available on composition, elements regulating and mediating dimerization, and how the arrangement would differ from the cyanobacterial counterparts. In this article, we present the structure of a chloroplast PSI-LHCI dimer that suggests a structural mechanism for the regulation of dimerisation.
Structure determination
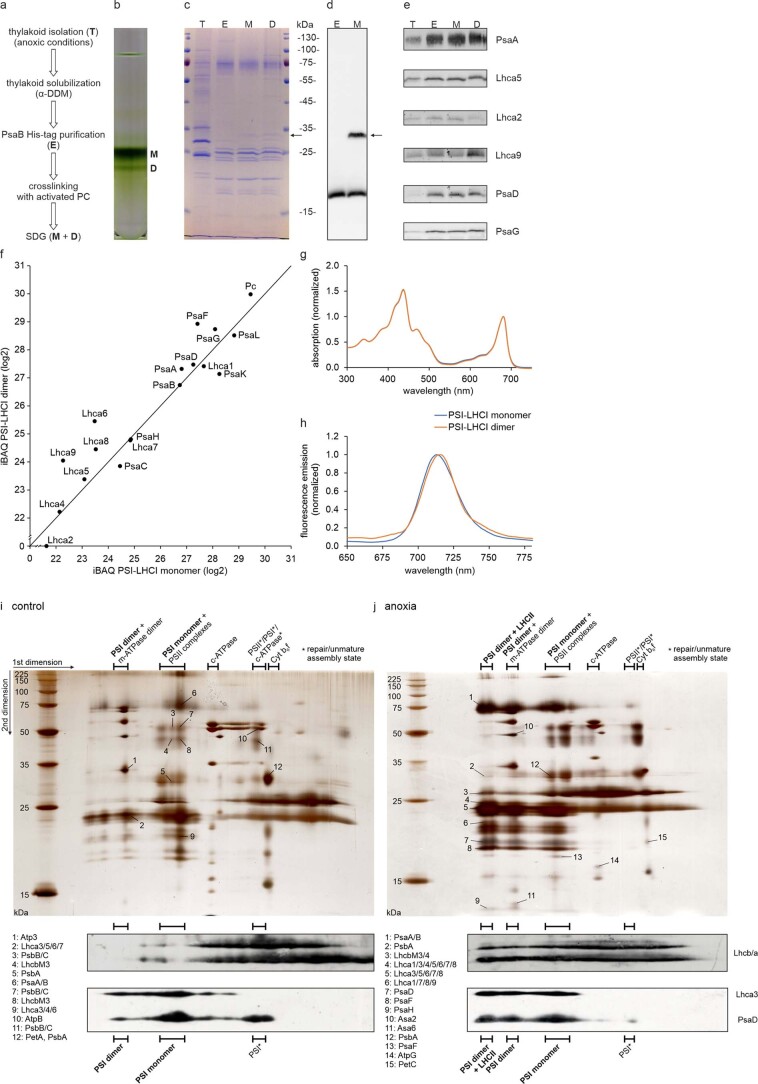
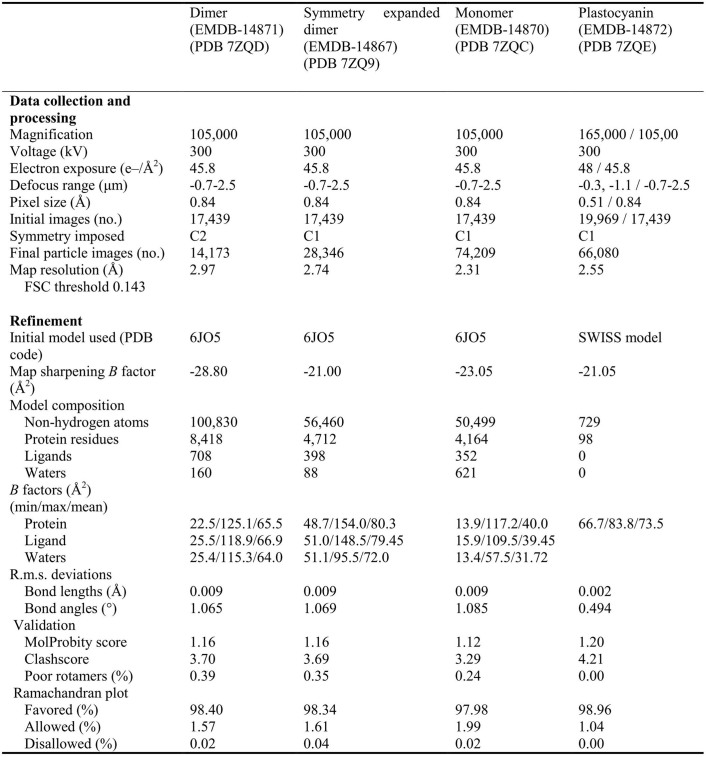
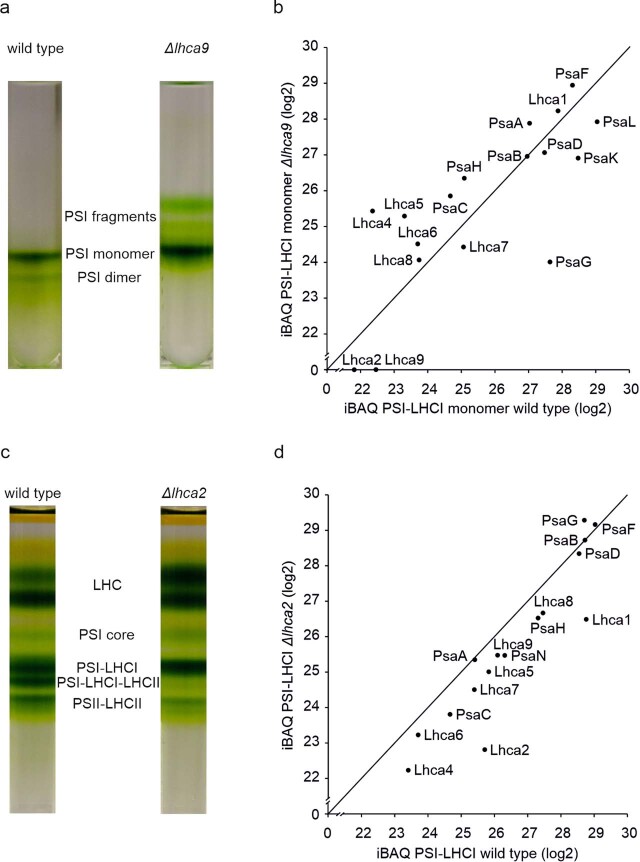
We grew Chlamydomonas reinhardtii cells containing a His-tag at the N-terminus of PsaB in low light and under anoxic conditions (Methods). The thylakoid membranes were solubilized with n-dodecyl-α-D-maltoside (α-DDM), followed by affinity purification, crosslinking via the chemically activated electron donor plastocyanin (Pc) and sucrose density gradient centrifugation (Extended Data Fig. 1a–f). Two PSI fractions were detected on the sucrose gradient, and 2D polyacrylamide gel electrophoresis (native/reducing 2D-PAGE) of isolated thylakoids indicated the presence of PSI dimers (Extended Data Fig. 1i,j). The heavier green band on the gradient was subjected to single-particle cryogenic electron microscopy (cryo-EM) analysis (Extended Data Table 1). We used 2D classification to separate PSI dimers from monomers in a reference-free manner, followed by 3D classification leading to a subset of 14,173 particles, which were refined to an overall resolution of 2.97 Å by applying C2 symmetry (Extended Data Fig. 2). PSI dimers were also found in 2D class averages in a dataset recorded from a sample without the use of crosslinker. Upon symmetry expansion, the resolution was further improved to 2.74 Å (Extended Data Fig. 2). The remaining 74,209 particles containing the monomer were refined to 2.31 Å resolution, representing a considerable improvement on the previously reported maps1–3. A density corresponding to the bound electron donor Pc was found at the lumenal side of both PSI forms, dimer and monomer.
Extended Data Fig. 1. Sample preparation and characterization.
a, The experimental workflow. b, Sucrose density gradient affinity purified PSI (~ 60 µg chl). Monomer (M) and dimer (D) fractions are indicated. c, SDS-PAGE analysis (1 µg chl). T: thylakoids; E: PsaB-His elution, M: PSI monomer, D: PSI dimer. The arrow indicates a putative PsaF-Pc crosslinked product. d, SDS-PAGE and Western Blot against PsaF confirming the cross-linking with Pc. e, SDS-PAGE and Western Blot against PsaA, Lhca5, Lhca2, Lhca9, PsaD and PsaG confirming the presence of small PSI subunits as well as LHCI subunits from both sides of the PSI core. f, Quantitative mass spectrometry analysis of the PSI fractions from (b). iBAQ values are normalized to the PSI core subunit PsaB and values below 21 are excluded as they represent already low intensity values, which might not be reliable. PSI-LHCI subunits PsaE and Lhca3 were not detected at quantifiable intensities in this experiment. Detection of these small PSI and Lhca subunits via mass spectrometry depends on the presence of only a few proteotypic tryptic peptides, which could be missed, as measurements were done in a data dependent fashion (12 MS2 per MS1). g, Room temperature absorption spectra of PSI monomer and dimer fractions. The spectra are normalized to the red region. h, Low temperature (77 K) fluorescence emission spectra of PSI monomer and dimer fractions upon excitation of chlorophyll a at 436 nm. The spectra are normalized to the maximum of the emission peaks and smoothed according to Savitzky-Golay78 using the Jasco spectra analysis program. Data in panel b to h is representative of n = 3 independently performed experiments with similar results. Notably, the presence of PsaH in the dimer sample, but its absence in the cryo-EM structure, indicates that PsaH is only loosely attached to the PSI dimer and readily lost during the cryo-EM analyses. i, 2D-PAGE of β-DDM solubilized C. reinhardtii wild type thylakoids, control conditions. j, low light and anoxic conditions. In the first dimension, multi-protein complexes were separated in their native form by blue native PAGE. In the second dimension, subunits of these protein complexes were separated by SDS-PAGE. “PSII complexes” refers to PSII complexes of different molecular weight due to varying extents of LHCII association. Labelled silver-stained spots were subjected to LC-MS/MS analysis. For convenience, the figure includes a condensed list of representative subunits. For a list of proteins identified in the respective spots, refer to Supplementary Information Dataset 1. Western Blots of Lhcb/a, Lhca3 and PsaD indicate the distribution of LHCII, LHCI and the PSI core. Data in panels i and j is representative of n = 3 independently performed experiments with similar results.
Extended Data Table 1.
Cryo-EM statistics. Data collection, processing, model refinement and validation statistics
Extended Data Fig. 2. Cryo-EM processing workflow and local resolution.
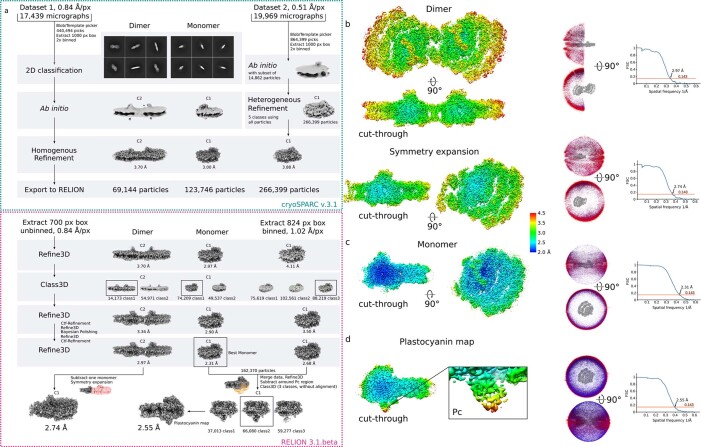
a, Flowchart of the data processing for the PSI dimer and monomer. The processing steps performed in cryoSPARC and RELION are indicated. To increase the numbers of Pc bound particles Dataset 2 was added for focused classification with signal subtraction. b, The map of the dimer and symmetry expanded dimer colored by local resolution in two orientations (left). Angular distribution of the dimer and symmetry expanded dimer and the FSC-curves are shown. c, The map of the monomer, angular distribution and FSC-curve. d, The map with the best density for plastocyanin, angular distribution and FSC-curve.
Overall structure
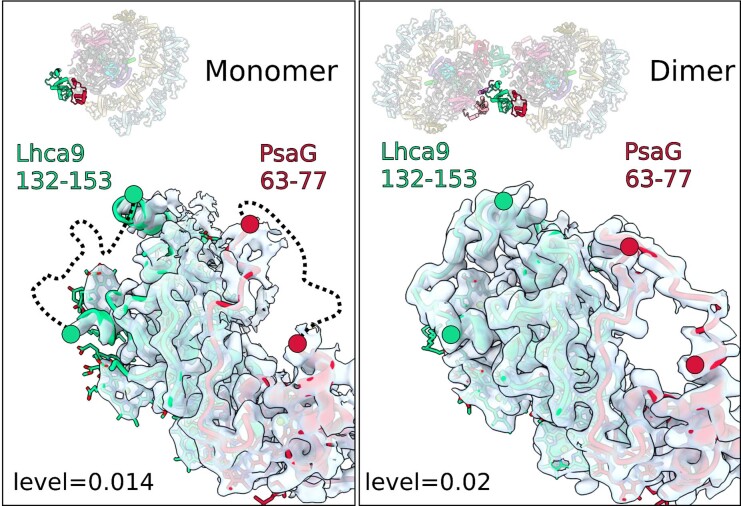
To derive a structure of the chloroplast PSI dimer, we first built an accurate model of one monomer using the 2.74 Å resolution map and then fitted it into the cryo-EM density of the C2 refined dimer. Compared with the monomer, all but two core subunits (PsaH and PsaO) and one light-harvesting protein (Lhca2) are found in the dimer (Fig. 1 and Supplementary Video 1). The structure contains 40 protein subunits, 398 chlorophyll a (Chl a), 60 chlorophyll b (Chl b), 56 β-carotenes, 54 luteins, 2 violaxanthins, 2 neoxanthins, 4 phylloquinones, 6 iron–sulfur clusters and 32 lipids (Fig. 1). In addition, two unaccounted densities corresponding to two loops on the stromal side of Lhca9 and PsaG could be interpreted in the dimer, due to the stabilization by the adjacent monomer (Extended Data Fig. 3). The first better-defined density is the Lhca9 loop region 132–153, which is stabilized owing to a direct interaction with PsaL of the second monomer within the dimer. As a result, the area is closely packed with PsaG, and therefore also the PsaG loop region 63–77 is better resolved in the dimer (Extended Data Fig. 3). Finally, co-factors of Lhca9 could be modelled at the interface between the monomers. No density for the His-tag on PsaB could be detected. Overall, taking into account the challenges of modelling photosynthetic complexes18, we were able to further improve the quality of the structure, resulting in better validation statistics, compared with the most recent cryo-EM17 and X-ray crystallography studies19,20 (Extended Data Table 1). Thus, the current study represents the most complete reference model of PSI.
Fig. 1. Overall structure of the PSI dimer.
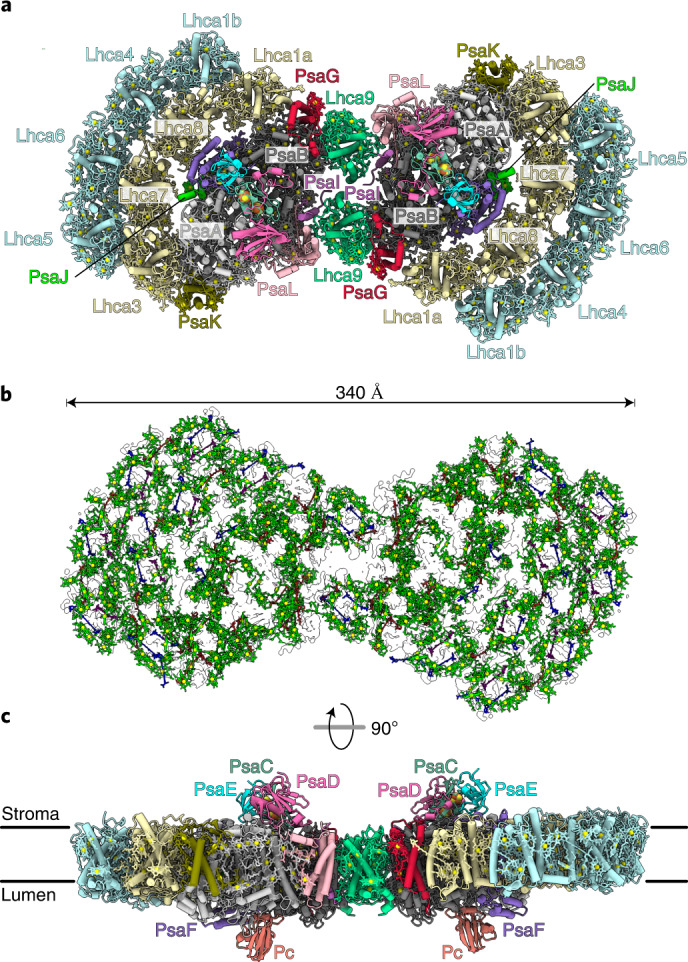
a, View of individual proteins from stroma. b, Arrangement of the pigments in the outline of the map: chlorophylls green (Mg yellow), luteins blue, β-carotenes red, violaxanthin purple and neoxanthin pink. c, Overall view along the membrane.
Extended Data Fig. 3. Stabilization of loops in Lhca9 and PsaG in PSI dimer.
Left, in the monomer, the Lhca9 loop 132–153 and PsaG loop 63–77 are disordered (dashed lines). Right, in the dimer, both loop regions are ordered due to a stabilization by the neighbouring monomer.
Structural basis for PSI dimerization
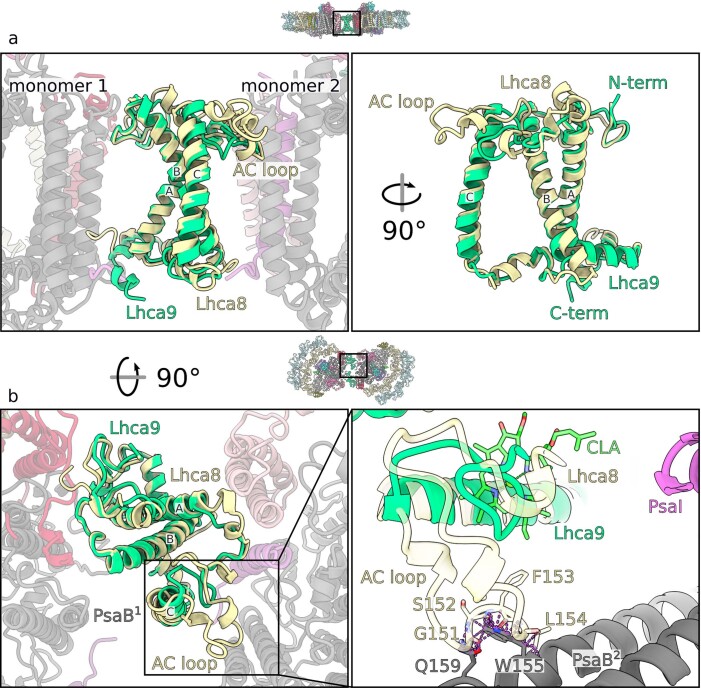
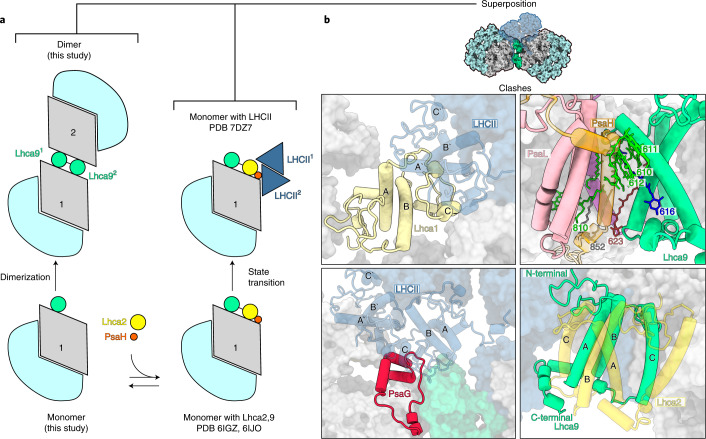
The structural basis for the algal chloroplast PSI dimerization is fundamentally different from cyanobacteria (Fig. 2a). In cyanobacteria, PSI dimerizes via the stromal region of PsaL7–9 and trimerizes via the lumenal C-terminus of PsaL, assisted by PsaI11. In our structure of the chloroplast PSI–LHCI dimer, neither PsaL nor PsaI interacts with each other between the neighbouring units. Instead, PsaH, which normally preserves a monomer, is not present, and Lhca9 with its associated co-factors acts as a symmetrical linker between the monomers, highlighting the importance of the light-harvesting antenna proteins for regulation of the macro-organization. Lhca9 is distinct among the light-harvesting proteins in our structure owing to a truncated loop between helices A and C and lack of the associated chlorophyll6. As a result, it contains the fewest chlorophylls among Lhcas (Supplementary Table 1). On the basis of this difference, we rationalized how Lhca9 allows for dimerization, as a longer AC loop would clash with the neighbouring PsaB (Extended Data Fig. 4).
Fig. 2. Dimerization of PSI.
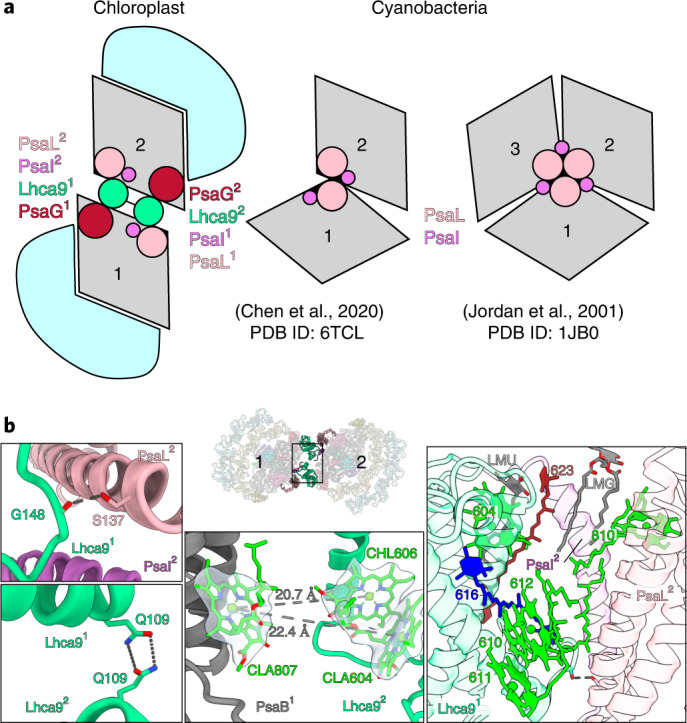
a, Schematic representation: chloroplast PSI–LHCI dimer associated via Lhca9, cyanobacterial PSI dimer (PDB ID: 6TCL, ref. 7) and trimer (PDB ID: 1JB0, ref. 11) associated via PsaI/L. The PSI core is grey and LHCI light blue. b, The dimer interface is formed by: hydrogen bonds between PsaL and Lhca9, and between two Lhca9 copies (left); potential energy transfer paths between the two monomers (centre); pigments and lipids (right).
Extended Data Fig. 4. Lhca9 short AC-loop contributes to the dimerization.
a, Superposition of Lhca8 and Lhca9, highlighting the difference in the AC-loop. b, The 90°-rotated view illustrates a clash between the longer AC-loop version and PsaB of monomer 2. Clashes between atoms with a VdW overlap > 0.6 Å are highlighted by the dashed lines.
The two Lhca9 copies tether the PSI monomers in a head-to-head fashion, resulting in a 340-Å-long structure (Figs. 1 and 2b, and Supplementary Video 1). They form interactions of four types covering the entire membrane span: (1) a hydrogen bond of the backbone carbonyl of G148 with S137 of PsaL in the stroma; (2) a hydrogen bond between the two Q109 of the Lhca9 copies; (3) hydrophobic contacts via coordinated co-factors in the membrane that include a newly modelled β-carotene 623 (N2 in nomenclature according to ref. 6) and five chlorophylls (604, 610, 611, 612 and 810); (4) lipid-mediated hydrophobic interactions via monogalactosyl diglyceride LMG852 and LMU624. One acyl chain of lipid 852 associates with chlorophyll 810 from monomer 2, while the other acyl chain associates with β-carotene BCR623 from monomer 1 (Fig. 2b). Thus, lipids contribute to the oligomerization of PSI, meaning that the membrane itself plays a role in the association. The finding that specific carotenes and lipids enable inter-molecular contacts that bridge the PSI monomers is of a particular interest, as it can be detected only by high-resolution structural studies. Similarly, a recently determined structure of the reaction centre dimer from Rhodobacter sphaeroides revealed a bound sulfoquinovosyldiacylglycerol that brings together each monomer forming an S-shaped array21. The involvement of lipids in the oligomerization is consistent with the formation of supercomplexes in other bioenergetic membranes22–25.
To solidify the structural observations, we engineered an lhca9 insertional mutant having the His-tag at the N-terminus of PsaB and repeated the purification procedure in the same way as for the wild type. This time, no PSI dimer band could be found in the sucrose density gradient (Extended Data Fig. 5). Notably, Lhca9 is present in Δlhca2, while Lhca2 is absent from Δlhca9 (ref. 26). Moreover, Lhca9 stably associates with PSI–LHCI after sucrose density gradient centrifugation of solubilized thylakoids isolated from lhca2 insertional mutant (Extended Data Fig. 5).
Extended Data Fig. 5. Analysis of Δlhca9 and Δlhca2.
a, Sucrose density gradient of affinity purified PSI from lhca9 insertional mutant (~ 60 µg chl) compared to wild type. b, Quantitative mass spectrometry analysis of the PSI-LHC monomer fraction. iBAQ values are normalized to the PSI core subunit PsaB and values below 21 are excluded as they represent already low intensity values, which might not be reliable. PSI-LHCI subunits PsaE and Lhca3 were not detected at quantifiable intensities in this experiment. Detection of these small PSI and Lhca subunits via mass spectrometry depends on the presence of only a few proteotypic tryptic peptides, which could be missed, as measurements were done in a data dependent fashion (12 MS2 per MS1). c, Sucrose density gradients of α-DDM solubilized thylakoids (~ 250 µg chl) isolated from wild type and lhca2 insertional mutant. d, Quantitative mass spectrometry analysis of the PSI-LHCI fraction in lhca2 insertional mutant. iBAQ values are normalized to the PSI core subunit PsaB and values below 21 are excluded as they represent already low intensity values, which might not be reliable. PSI-LHCI subunits PsaE, PsaK and Lhca3 were not detected at quantifiable intensities in this experiment. Detection of these small PSI and Lhca subunits via mass spectrometry depends on the presence of only a few proteotypic tryptic peptides, which could be missed, as measurements were done in a data dependent fashion (12 MS2 per MS1). Gradients in panel a and c are representative of n = 3 independently performed respective experiments with similar results.
Implications of PSI dimerization
The specific interactions between the monomers are enabled owing to unoccupied positions of PsaH and Lhca2. As PsaH is also required for the lateral binding of LHCII to the PSI core in state transitions5,6, we next compared the structure of PSI–LHCI dimer to the state transition complex (Fig. 3). The superposition shows that Lhca9 from the neighbouring monomer is positioned in the membrane, where Lhca2 resides in PSI–LHCI–LHCII, and their three transmembrane helices would overlap with each other (Fig. 3b). The presence of the PsaH transmembrane helix is not compatible with the Lhca92-associated co-factors CLA610-612, LMG852 and BCR623 that extend from the neighbouring monomer into the dimer. In addition, the superposition shows that there would be a clash between PsaG and Lhca1 of the inner belt with one of the LHCII trimers, but not the other (Fig. 3b). As Lhca2 and PsaH are absent, the structure of the algal PSI dimer would not facilitate LHCII binding at this position. However, our 2D-PAGE indicated a co-migration of LHCII polypeptides with the dimer fraction, and therefore a structural adaptation cannot be excluded (Extended Data Fig. 1). The antagonistic relationship of Lhca92 and Lhca2 and the assembly state of PsaH might further reflect a regulation of PSI dimerization (Fig. 3a).
Fig. 3. PSI dimer and LHCII association in C. reinhardtii.
a, Schematic view of PSI divarication. The pathway towards state transition or dimer is dependent on presence/absence of PsaH and Lhca2. b, Superposition of PSI dimer with PSI–LHCII shows that state transition (PDB ID: 7DZ8, transparent) would result in clashes of Lhca1 (top left) and PsaG (bottom left) with LHCII. PsaH (top right) and Lhca2 (bottom right) would clash with the Lhca9 from the neighbouring PSI monomer.
PsaH is an 11 kDa transmembrane protein that is imported into chloroplasts and peripherally associates with the PSI reaction centre on the opposite side to the LHCI belt27. A recent study of PSI biogenesis apparatus showed that PsaH is assembled in a separate protein module that comes before Lhca2 assembly28. Arabidopsis mutants of PsaH can grow photoautotrophically29, and structures of functional algal PSI particles that lack PsaH have been determined2,14. In addition, RNA sequencing analysis in algae further confirmed that expression of psaH is regulated upon physiological stimuli30. It is also of note that PsaH, unlike other PSI subunits, was found to be specifically enriched within the pyrenoid tubules31. Together, these data suggest that PsaH-lacking complexes represent a previously overlooked functional form of PSI, where PsaH is either downregulated or could not be assembled. Building on these data, our structure of PSI–LHCI dimer further shows that PsaH/Lhca2-lacking particles can associate with each other to form larger complexes (Figs. 1 and 4). This feature is likely to be conserved in plants, as larger-than-monomer fractions of a plant PSI have been reported32, and 2D projections from negative stain images of Arabidopsis PSI suggest the presence of a putative Lhca1/Lhca4 heterodimer at the PsaL pole that is analogous to alga33. The dense organization would be beneficial for membrane crowding and compartmentalization of PSI, even if the two monomers are not energetically coupled.
Fig. 4. High-resolution features of co-factors and hydration.
a, Energy transfer pathways. Distribution of chlorophylls indicated by Mg atoms. Chl a is grey, Chl b blue and newly identified Chl b cyan. Pathways within 23 Å are connected by lines, and the line width reflects the distance. The most likely inter-subunit pathways are red. b, Water molecules modelled in PSI are shown as red spheres (oxygen atoms) in the outline of the map, chlorophylls green, phylloquinones purple and iron–sulfur clusters yellow-red.
For other model organisms, such as Saccharomyces cerevisiae, whole-cell modelling revealed that metabolic strategies are driven by specific protein expression profiles and compartment-specific proteome constraints34. This allows eukaryotic cells to adapt metabolically to nutrient and proteome limitations. Crowding of large complexes in the membrane systems of thylakoid35,36 and mitochondrial cristae37, as well as in the cytosol, for example, ribosome dimers38, is an established and conserved mechanism for a more efficient differentiation of functions, ecological adaptation and material storage during less active periods. As the basic structure of the PSI core is rigid and it operates according to conserved mechanisms, the regulation by PsaH/Lhca2 provides a degree of flexibility on the macromolecular level that would allow the photosynthetic apparatus to adapt to stresses and tolerate changes in the range of light intensities that might involve membrane re-organization15,39. Thus, PsaH/Lhca2 appears to be a regulator that defines a late assembly pathway and coordinates the macromolecular organization of PSI in chloroplasts (Fig. 3 and Extended Data Fig. 6). This regulatory role could also be mediated through post-translational modifications such as phosphorylation in C. reinhardtii40 or acetylation in Arabidopsis41. In C. reinhardtii, a consequence of PsaH regulation would be the differential binding of Lhca2. Reduction of Lhca2 alters regulation of photosynthetic electron transfer and hydrogen production, suggesting further potential functional consequences of membrane re-organization and PSI remodelling26.
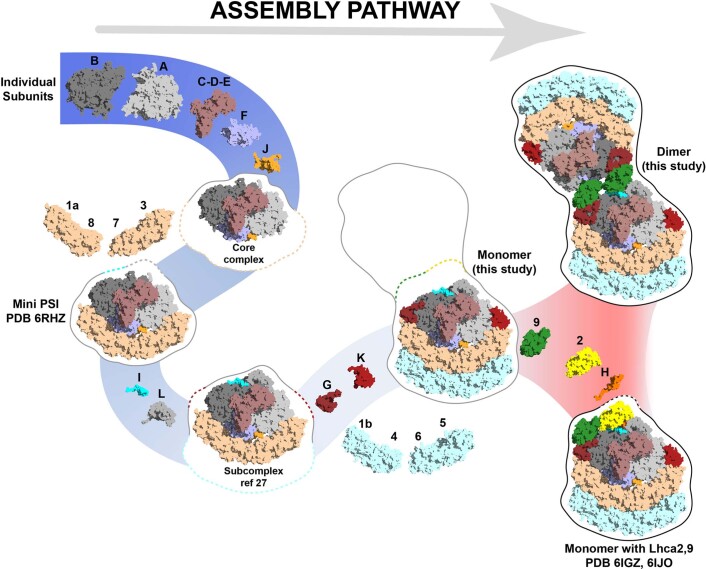
Extended Data Fig. 6. Assembly pathway of PSI towards dimer.
a, Schematic view of known biochemically and structurally defined PSI states and assembling protein subunits. Subunit composition for each state is outlined with the shape of a following state and corresponding color. The divaricating of the assembly pathway takes place at the last stage (red background). The path towards PSI monomer or dimer is dependent on presence/absence of PsaH and Lhca2.
Coupling of PSI monomers in the dimer
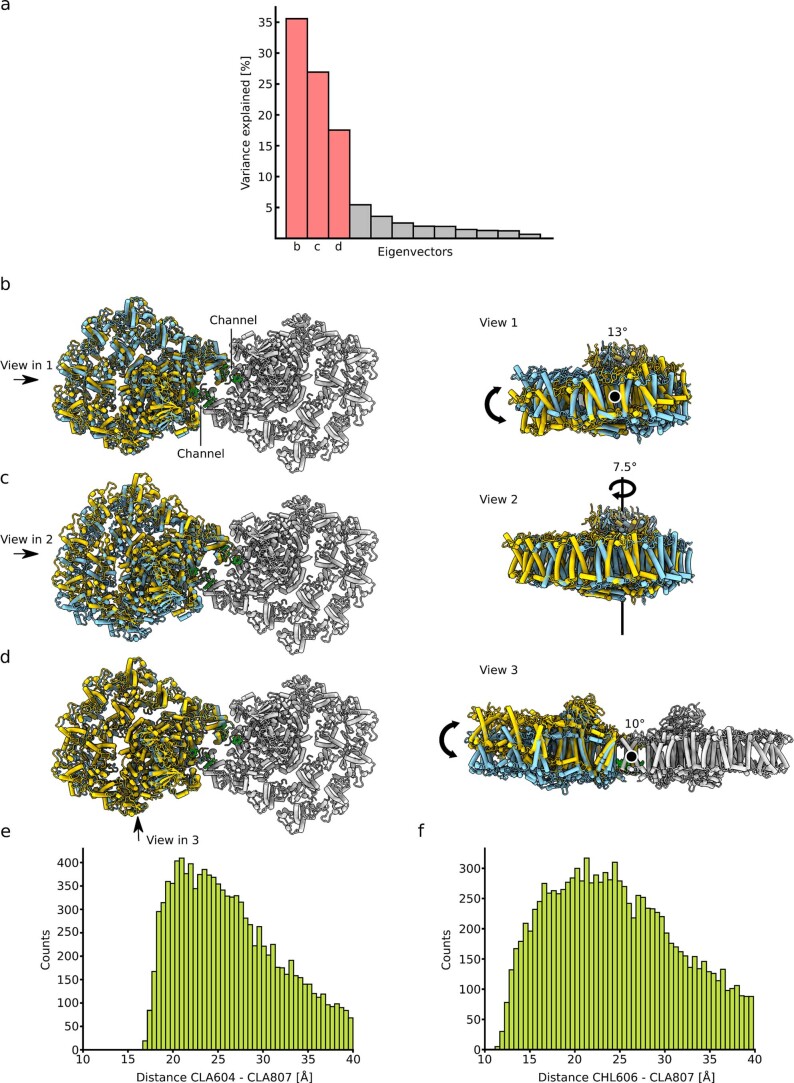
To further extrapolate potential conformational changes during the dimerization of PSI, we applied multi-body refinement analysis of the PSI dimer using the two monomers as bodies (Extended Data Fig. 7). The analysis indicated no distinct conformational states, but instead revealed continuous motions in the three eigenvectors describing a relative movement of the monomers in relation to each other (Extended Data Fig. 7a). The intrinsic flexibility is dominated by combinations of all three rotations of one monomer with respect to the other up to 13° (Extended Data Fig. 7b–d). Therefore, excitation energy transfer between the PSI monomers in the dimeric scaffold would also depend on degrees of rotation around the identified pivot points. Specifically, three chlorophylls are found within a potential cross-monomer excitation sharing: CLA807 (PsaB), CLA604 (Lhca92) and CHL606 (Lhca92), and the distance between them is ~20 Å in the consensus map (Fig. 2). While such a positioning might suggest direct coupling, the multi-body analysis indicates considerable variability (Extended Data Fig. 7e,f). Therefore, similarly to the cyanobacterial PSI dimer, an excitation coupling between the two monomers is less favourable in vitro, and this is consistent with measurements of room-temperature and 77 K fluorescence spectra that showed only a minor shift between monomer and dimer (Extended Data Fig. 1g,h). However, in vivo the observed PSI–LHCI dimer conformation, and therefore the distance between the chlorophylls at the interface, could also be affected by a local membrane curvature.
Extended Data Fig. 7. Multi-body refinement analysis.
a, Eigenvectors that explain the variability of the data. The three major eigenvectors account for ~78% of the motion in the PSI dimer. b-d, Stromal and side view of the model, showing the maximal motion along the three vectors. e, f, The distance between the chlorophylls is plotted based on the relative motion from the multi-body analysis. The y-axis shows the counts of particles that exhibit a certain distance.
High-resolution features and hydration of PSI
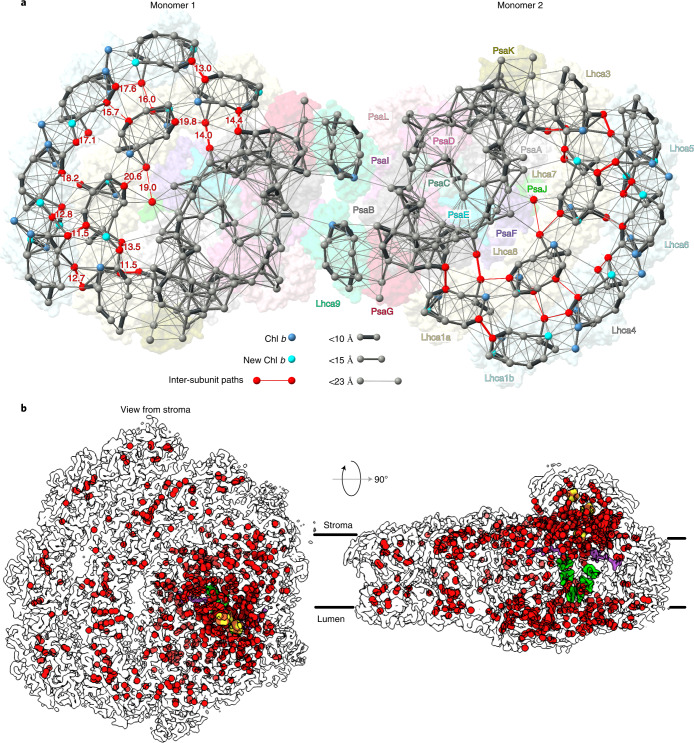
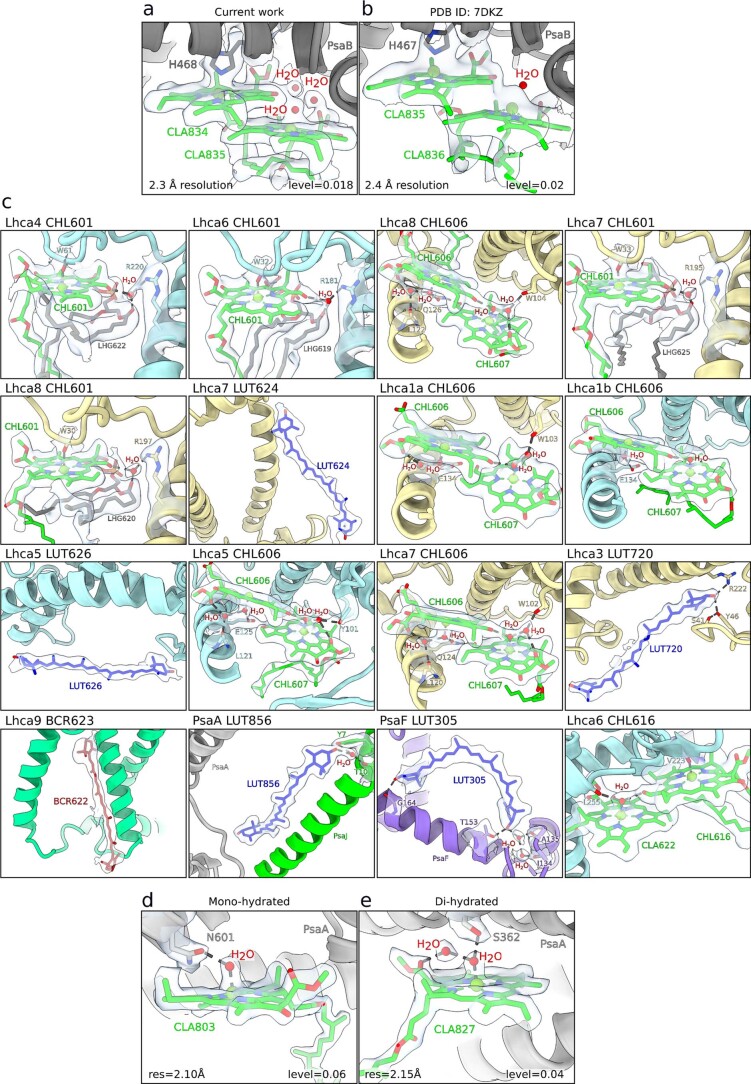
In our PSI monomer reconstruction, the resolution in the core is ~2.1 Å, and in the LHCI inner belt 2.1–2.5 Å, revealing additional structural details of chlorophylls, carotenoids and 621 water molecules (Fig. 4, Extended Data Fig. 8 and Extended Data Table 1). The map can improve the level of detail not only compared with the previous cryo-EM studies of algal PSI1–3,17, but also the plant PSI maps obtained by X-ray crystallography19,20 (Extended Data Fig. 8a,b). The quality of the data aided in improving the previous models in functionally important regions. This includes the identification of nine Chl b molecules, two newly modelled luteins, a β-carotene and more accurate estimation of the coordination of 53 chlorophylls (Extended Data Figs. 8c and 9, Supplementary Table 1). Particularly, Chl b molecules are identified at positions 601 and 606 in Lhca4, Lhca5, Lhca6, Lhca7 and Lhca8. The two newly modelled luteins 720 and 626 are in the N-terminus of Lhca3 next to Chl a 614, and in Lhca5 next to Chl a 617, respectively (Extended Data Figs. 8c). The newly modelled β-carotene 622 is in Lhca9 and could be identified owing to structural stabilization of the interface region in the dimer (Extended Data Figs. 8c). As Chl b limits free diffusion of excitation energy42, some of the new assignments affect the energy pathways between the antenna proteins. Together with the new structural data, this allowed us to produce a more accurate map of the energy channelling in PSI based on the new model (Fig. 4a).
Extended Data Fig. 8. Identification of new elements and map quality.
a, The current density around CLA834-835 is shown in iso-surface representation at a local resolution of 2.3 Å. Three water molecules involved in chlorophyll coordination are identified. Updated libraries are used for correct chlorophyll modeling. b, The same region from X-ray study at 2.4 Å resolution20 lacks the density for waters, whereas the modeled water molecule has no density, and Mg atoms are not in the correct plane. c, Close up views of newly identified elements, coordination and chemical environment with the density map. d, An example of a mono-hydrated chlorophyll with the corresponding density. e, An example of a di-hydrated chlorophyll with the corresponding density. Local resolution and map levels are indicated.
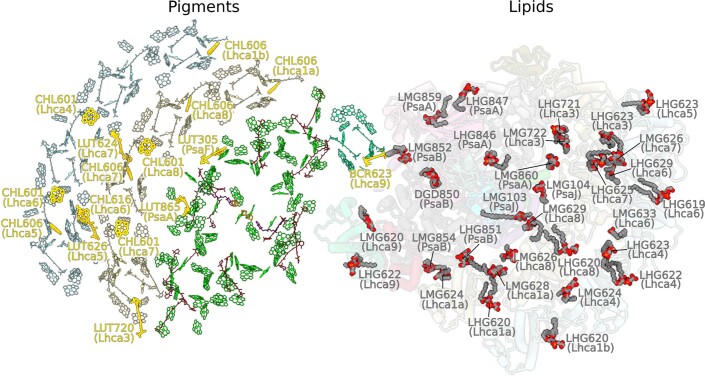
Extended Data Fig. 9. Overview of the pigments and lipids.
Pigments (left) and lipids (right) are shown from stroma. The newly identified pigments are highlighted in bold gold.
Another striking feature of the high-resolution cryo-EM map is resolvable density for multiple newly detected water molecules, which particularly aided in modelling the coordination of chlorophylls (Fig. 4b and Supplementary Video 1). Thus, we report the most complete available experimental picture of a chemical environment for chlorophyll binding (Supplementary Table 1). Particularly, it allows one to distinguish between mono- and di-hydrated forms, which largely escaped detection by X-ray crystallography (Extended Data Fig. 8d,e). This is mechanistically important because the di-hydrated derivative is chemically more stable, as illustrated by quantum chemical calculations43. We observe that, other than the previously reported CLA824 (ref. 20), only two water molecules can be involved in penta-coordinated Mg for all the chlorophylls. Remarkably, water molecules play a coordinative role for most of Chl b, for which the relative ratio of water coordination is four times higher than for Chl a (Supplementary Table 1). The difference between Chl a and Chl b is a methyl versus a formyl group; thus, water serves as a hydrogen bond donor to the latter, while it also interacts with charged/polar protein residues or lipids. Therefore, the immediate surrounding of Chl b molecules is more enriched with non-protein material than previously thought, which plays a role in tuning the photophysics and the transport properties of excitation energy in PSI. Together, the presented model now allows for comparison of PSI phylogenetic conservation also on the level of chlorophyll coordination and solvent positioning.
Structure of PSI-Pc complex
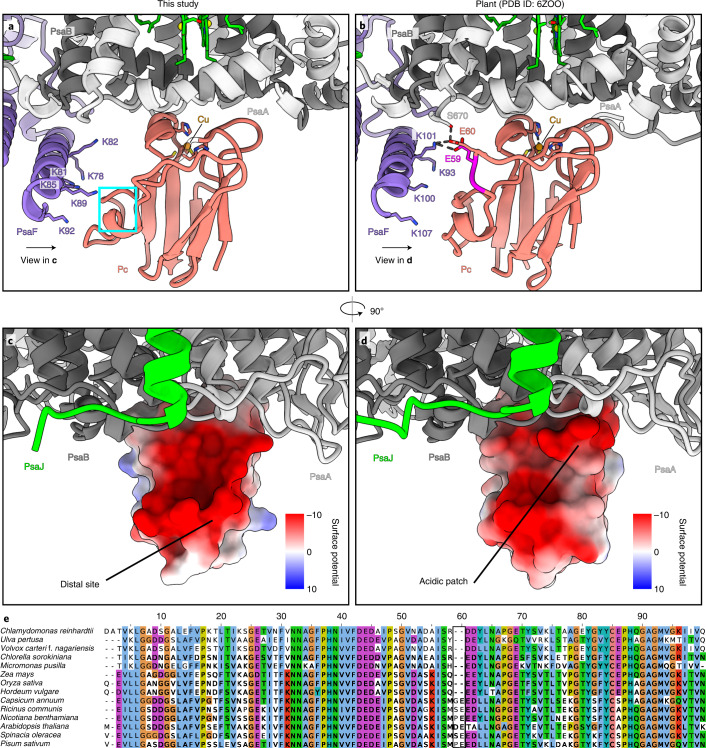
On the lumenal side of PSI, we observed a density corresponding to the associated electron donor Pc, whose binding has been stabilized by crosslinking (Methods). The bound Pc is found on PSI monomers and dimers. Signal subtraction, followed by focused 3D classification, allowed us to rigid body fit a model for Pc into the density at a local resolution of ~3.5 Å (Extended Data Fig. 2). We then performed flexible fitting using self-similarity restraints in Coot44,45. With respect to the PSI–Pc interactions, comparison between our model with a plant counterpart46,47 revealed two main differences (Fig. 5). In C. reinhardtii, the negatively charged residues of the Pc acidic patch are shifted by ~5 Å owing to the missing residues P58–E59, and therefore, the interaction with K101 of PsaF is weakened (Fig. 5a,b). Instead, the binding strength is compensated with the PsaF region K78–K92, which has six lysines (78, 81, 82, 85, 89 and 92) increasing a positively charged concentration at a distal site (Fig. 5c–e), thus supporting additional electrostatic interactions with the acidic residues of Pc. The importance of the distal lysine residues is supported by site-directed mutagenesis of the C. reinhardtii N-terminal PsaF domain and functional analyses of electron transfer between Pc and mutant as well as wild-type PSI48. Thus, algal and plant Pc have adapted their slightly different interfaces for optimal interactions with PSI.
Fig. 5. Pc binding site.
a, Pc binding in C. reinhardtii (current work). The positively charged residues of PsaF stabilize the interactions. The corresponding Pc region that deviates from the plant counterpart is cyan. b, Pc binding in plants29. The two inserted residues are magenta. c, Ninety-degree rotated view with Pc surface shown with Coulomb potential from the interface. d, The same view for a plant counterpart (PDB ID: 6ZOO), illustrating that the acidic patch is shifted. e, Multiple sequence alignment of Pc from different species of the green lineage (algae and plants) showing that the inserted residues 58 and 59 occur in a subset of plants and do not represent a general case.
In summary, this study illustrates that PSI–LHCI can dimerize and explains how the process is structurally regulated by subunits PsaH, Lhca2 and Lhca9 (Fig. 3a and Extended Data Fig. 6). It remains to be seen how thylakoid regulatory networks manage to implement PsaH allocation strategies. The data provide the most complete description so far of the structure of PSI, including newly identified co-factors that play specific roles in accommodating the light harvesting and excitation transfer functions, as well as water molecules involved in chlorophyll coordination. Finally, the binding of electron donor Pc is resolved. Together, the data explain how the PSI is modulated to perform its functional and structural roles in a chloroplast.
Methods
Strains and growth conditions
Experiments were performed using a strain expressing PsaB fused with His20-tag after the third residue from the N-terminus2 and an Δlhca9 insertional mutant49 transformed with the corresponding PsaB His-tag plasmid. Chloroplast transformation was performed using a particle gun50, and transformants were selected on Tris-acetate-phosphate (TAP) medium containing 150 µg spectinomycin ml−1 and recloned three to four times until they were homoplasmic2. Further experiments were performed with the C. reinhardtii wild-type strain 137c as well as Δlhca2 insertional mutant49 back-crossed into wild-type 137c. All strains were maintained on TAP medium, solidified with 1.5% w/v agar at 25 °C in the presence of ~20 μmol photons m−2 s−1 photosynthetically active, continuous illumination. For experiments, strains were cultured in TAP medium on a rotary shaker (120 rpm) at 25 °C in the presence of ~20 μmol photons m−2 s−1 photosynthetically active, continuous illumination.
Purification of PSI
C. reinhardtii cells were incubated in anoxic conditions (~108 cells ml−1 in TAP medium + 10 mM glucose, 40 U ml−1 glucose oxidase and 50 U ml−1 catalase) and dim light (~20 µmol photons m−2 s−1) for 60 min. All following steps were performed at 4 °C and dim light. Cells were disrupted in 0.33 M sucrose, 25 mM HEPES–KOH pH 7.5, 5 mM MgCl2, 1 mM PMSF, 1 mM benzamidine and 5 mM aminocaproic acid with a nebulizer (2 bar, two passages). Broken cells were centrifuged at 32,800g for 10 min (Beckman Coulter JA-25.50 rotor, 20,000 rpm). The pellet was carefully resuspended in 0.5 M sucrose, 5 mM HEPES–KOH pH 7.5, 10 mM EDTA, 1 mM benzamidine and 5 mM aminocaproic acid with a potter homogenizer. The resuspended material was layered on top of a sucrose density step gradient (1.8 M and 1.3 M sucrose, 5 mM HEPES–KOH pH 7.5, 10 mM EDTA, 1 mM benzamidine and 5 mM aminocaproic acid). Thylakoid membranes were extracted via ultracentrifugation at 70,800g for 1 h and 20 min (Beckman Coulter SW 32 Ti rotor, 24,000 rpm). Thylakoids were collected from the step gradient interphases with a Pasteur pipet, diluted four times with 5 mM HEPES–KOH pH 7.5 and centrifuged at 37,900g for 20 min (Beckman Coulter JA 25.50 rotor, 21,500 rpm).
Isolated thylakoids were set to 1 mg chlorophyll ml−1 in 5 mM HEPES–KOH pH 7.5 and solubilized by addition of an equal volume of 2% α-DDM for 10 min. Unsolubilized material was separated by centrifugation. The supernatant was diluted four times to 125 µg chlorophyll ml−1 and 0.25% α-DDM. The sample was loaded onto a TALON metal affinity column (1 ml resin mg chlorophyll−1) in 5 mM HEPES–KOH pH 7.5, 100 mM NaCl, 5 mM MgSO4 and 10 % glycerol at a flow rate of ~0.5 ml min−1. The column was washed with ten times the bed volume of 5 mM HEPES–KOH pH 7.5, 100 mM NaCl, 5 mM MgSO4, 10% glycerol and 0.02% α-DDM at a flow rate of ~1 ml min−1. A second wash was performed with ten times the bed volume of 5 mM HEPES–KOH pH 7.5, 100 mM NaCl, 5 mM MgSO4, 10% glycerol, 0.02% α-DDM and 5 mM imidazole at a flow rate of ~1 ml min−1. The PSI was eluted with 5 mM HEPES–KOH pH 7.5, 100 mM NaCl, 5 mM MgSO4, 10% glycerol, 0.02% α-DDM and 150 mM imidazole. The PSI was concentrated with a spin column (regenerated cellulose: 100,000 molecular weight cut-off (MWCO)) to ~ 1.5 mg chlorophyll ml−1, diluted five times with 30 mM HEPES–KOH pH 7.5 and 0.02% α-DDM and reconcentrated twice.
PSI-Pc crosslinking was performed in 30 mM HEPES–KOH pH 7.5, 1 mM ascorbate, 0.1 mM diaminodurene (a redox mediator between ascorbate and Pc), 3 mM MgCl2 with 0.1 mg chlorophyll ml−1 PSI particles and 20 µM activated Pc for 45 min at room temperature. Pc activation was performed in 10 mM MOPS–KOH pH 6.5 with 100 µM recombinant Pc, 5 mM 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride and 10 mM sulfo-N-hydroxysuccinimide for 20 min at room temperature. The crosslinker was removed and the buffer exchanged to 30 mM HEPES–KOH pH 7.5 via a PD G25 desalting column followed by ultrafiltration with a centricon (regenerated cellulose: 10,000 MWCO).
The crosslinked PSI-Pc particles (~ 60 µg chlorophyll per gradient) or solubilized thylakoids of non-tagged strains (~ 250 µg chlorophyll per gradient) were loaded onto a 1.3 M to 0.1 M sucrose density gradient including 5 mM HEPES–KOH pH 7.5 and 0.02% α-DDM. PSI fractions were collected after ultracentrifugation at 134,400g (Beckman Coulter SW 41 Ti rotor, 33,000 rpm) for 14 h (Extended Data Figs. 1b and 5). Before further analysis, sucrose was removed via a PD G25 desalting column followed by concentration with a spin column (regenerated cellulose: 100,000 MWCO).
Biochemical analysis of PSI
For SDS–PAGE (Extended Data Fig. 1c–e), samples were adjusted to 1 µg chlorophyll, supplemented with loading buffer and incubated at 65 °C for 15 min. Proteins were separated by 13% (w/v) SDS–PAGE51. Gels were stained with Coomassie Brilliant Blue R-250 or blotted onto nitrocellulose membranes (Amersham) and detected by specific primary antibodies: PsaF (ref. 52), Pc (Agrisera), PsaA, Lhca5 (ref. 4), Lhca2 (ref. 4), Lhca9 (ref. 4), Lhca3 (ref. 53), PsaD (ref. 54), PsaG (ref. 55), Lhcb/a (ref. 56), PsbA (Agrisera) and LhcSR3 (ref. 77). The antibody against PsaA was raised using the peptides STPEREAKKVKIAVDR and VKIAVDRNPVETSFEK and was obtained from Eurogentec. All primary antibodies were used at a 1:1,000 dilution, except for anti-Lhcb/a (1:2,500) and anti-PsbA (1:10,000). Secondary antibodies for ECL detection were used at a 1:10,000 dilution (goat anti-rabbit IgG (H + L)-HRP conjugate, Bio-Rad).
For quantitative analysis by mass spectrometry, removal of sucrose and protein digestion was carried out following the filter-aided sample preparation, using 2 µg of sequencing grade trypsin (Promega) per fraction57. Iodoacetamide and dithiothreitol used in the original protocol were replaced by chloroacetamide and tris(2-carboxyethyl)phosphine, respectively. After overnight digestion at 37 °C, samples were acidified by adding trifluoroacetic acid to a final volume of 0.1%. Five percent of the peptide solution was desalted using self-made StageTips according to established protocols58. Desalted peptides were dried by vacuum centrifugation and stored at −20 °C until further use. The liquid chromatography (LC)–tandem mass spectrometry (MS/MS) system consisted of an Ultimate 3000 RSLC nanoLC System (Thermo Fisher Scientific) coupled via a Nanospray Flex ion source (Thermo Fisher Scientific) to a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific). Samples were reconstituted in 5 µl of 2% (v/v) acetonitrile/0.05% (v/v) trifluoroacetic acid in ultrapure water (eluent A1), loaded on a trap column (C18 PepMap 100, 300 µM × 5 mm, 5 µm particle size, 100 Å pore size; Thermo Fisher Scientific) and desalted for 3 min at a flow rate of 15 µl min−1 using eluent A1. Subsequently, the trap column was switched in-line with an Acclaim PepMap100 reversed phase column (75 µm × 50 cm, 2 µm particle sizes, 100 Å pore size; Thermo Fisher Scientific) for peptide separation. The mobile phases were composed of 0.1% (v/v) formic acid in ultrapure water (eluent A2) and 80% (v/v) acetonitrile/0.08% (v/v) formic acid in ultrapure water (B). Flow rate was 250 nl min−1. The following gradient was applied: 5–35% B over 105 min, 35–99% B over 5 min and 99% B for 20 min. Mass spectrometry full scans (scan range m/z 350–1,400, resolution 70,000 at m/z 200, automatic gain control) target value 3 × 106, maximum injection time 50 ms) were acquired in data-dependent mode, dynamically selecting the 12 most abundant precursor ions for fragmentation by higher-energy C-trap dissociation (27% normalized collision energy, resolution 17,500 at m/z 200, precursor isolation window 1.5 m/z). Dynamic exclusion was set to ‘auto’ (chromatographic peak width 15 s). AGC target value and intensity threshold for MS/MS were 5 × 104 and 1 × 104, respectively, at 80 ms maximum ion fill time. Singly charged ions, ions with charge state 5 or above and ions with unassigned charge states were rejected. Internal lock mass calibration was enabled on m/z 445.12003. LC–MS/MS data were processed in MaxQuant 1.6.14 for protein identification and label-free quantification59. Default settings were used, except for calculation of intensity-based absolute quantitation (iBAQ) values and ‘second peptide search’, which were enabled and disabled, respectively. iBAQ values were normalized to the PSI core subunit PsaB, and values below 21 were excluded as they represent already low intensity values, which might not be reliable. Spectra were searched against a concatenated database containing protein sequences based on the Chlamydomonas v5.6 gene models (Joint Genome Institute, www.phytozome.org), as well as sequences of chloroplast- and mitochondrial-encoded proteins (GenBank BK000554.2 and NC_001638.1). Carbamidomethylation of cysteines was set as a fixed modification. Oxidation of methionine and acetylation of protein N-termini were considered as variable modifications. A false discovery rate of 1% was applied to peptide and protein identifications. Label-free quantitation data were imported into Perseus (version 1.6.15.0)60 for log2 transformation, and contaminants, proteins identified only by site and reverse hits, were removed.
Room-temperature absorption spectra (300–750 nm) of PSI monomer and dimer fractions (Extended Data Fig. 1g) were measured with an ultraviolet–visible spectrophotometer (V-650, Jasco) at 10 µg chlorophyll ml−1 and normalized to the red region. Fluorescence emission spectra (650–780 nm) of PSI fractions (Extended Data Fig. 1h) were recorded at 77 K at 1 µg chlorophyll ml−1 with a spectrofluorometer (P-6500, Jasco) upon excitation at 436 nm. Spectra were normalized to the maximum of the emission peaks and smoothed according to Savitzky-Golay77 using the Jasco spectra analysis program (Spectra Manager II). For 2D-PAGE, thylakoids (0.8 mg chlorophyll ml−1) isolated from control and anoxic 137c wild-type cells were solubilized with 0.9% β-DDM for 20 min. Two-dimensional PAGE61 and silver staining52 were performed as described. Excised spots from silver-stained blue native PAGE gels were destained by incubation with 15 mM potassium hexacyanoferrate (III)/50 mM sodium thiosulfate for 8 min and then submitted to tryptic in-gel digestion62. No reduction and alkylation of cysteines was performed. LC–MS/MS was implemented54, where an Ultimate 3000 nano-LC system was coupled via a nanospray source to an LCQ Deca XP Plus mass spectrometer (Thermo Finnigan).
LC–MS/MS data were processed with Proteome Discoverer (Thermo Fisher Scientific, version 2.4). Raw files were searched using the SequestHT and MS Amanda algorithms against a concatenated database containing sequences of nuclear- (Chlamydomonas v5.6 gene models, www.phytozome.org), chloroplast- (GenBank BK000554.2) and mitochondrial-encoded (NC_001638.1) proteins. Search settings were: precursor and fragment mass tolerances: 250 ppm and 0.25 Da, respectively; minimum peptide length: 6; maximum of missed cleavages: 2; variable modifications: oxidation of methionine, N-acetylation of protein N-termini. Identifications were filtered to achieve a peptide and protein level false discovery rate of 0.01.
Cryo-EM data collection and processing
Three microlitres of purified PSI complex at 1 mg chlorophyll ml−1 was applied to glow-discharged (GloQube Quorum, 40 s, 20 mA) holey carbon grids (Quantifoil 300 Au R1.2/R1.3, Electron Microscopy Sciences) and vitrified using a Vitrobot MKIV (2 s blotting time, 4 °C, 100% humidity). The data collection was carried out using a 300KV Titan Krios G2 Microscope (Thermo Fisher Scientific) equipped with a Gatan Bioquantum energy filter and a K3 Summit direct electron detector (Ametek). Movies were recorded using counting mode at a magnification of ×105,000 corresponding to a calibrated pixel size of 0.84 Å. The dose rate was 15.27 e pixel−1 s−1 and the exposure time was 3 s divided into 45 frames, leading to a total dose of 45.8 e Å−2. Software E Pluribus Unum (EPU) was used to collect 17,439 movies with a defocus range from −0.7 µm to −2.5 µm. Data statistics are shown in Extended Data Table 1.
Extended Data Fig. 2 shows the processing scheme applied. The pre-processing steps were performed using cryoSPARC 3.1.0 (ref. 63). Movie stacks were motion corrected and dose weighted using MotionCor2 (ref. 64). Contrast transfer function (CTF) of the motion-corrected micrographs was estimated using CTFFIND4 (ref. 65). Blob picker and then template picker were used to pick 440,494 particles, and 2D classification in cryoSPARC was performed. Dimeric particles were separated from monomeric by inspection of the 2D-class averages, and for each subpopulation an ab initio model was generated using cryoSPARC applying C2 and C1 symmetry, respectively. For each model homogeneous refinement was performed, leading to a nominal resolution of 3.7 Å for the dimer and 3.0 Å for the monomer. Particles (dimer, 69,144; monomer, 123,746) were converted into a Star file format66 and imported into RELION 3.1.beta67,68. Particles were re-extracted (un-binned) and processed in RELION using a box size of 700 pixel and 500 pixel for the dimer and monomer, respectively. Three-dimensional refinement followed by 3D classification was performed imposing C2 symmetry for the dimer and C1 for the monomer. A subset of high-quality particles was selected for the dimer and monomer and subjected to 3D refinement, which resulted in a resolution of 3.3 Å for the dimer and 2.9 Å for the monomer. CTF refinement69,70 followed by 3D refinement and Bayesian polishing followed by another round of CTF refinement was performed for the dimer as well as for the monomer. A final 3D refinement resulted in an overall resolution of 2.97 Å for the dimer and 2.31 Å for the monomer. The resolution of the dimer could be further improved to 2.74 Å by using signal subtraction of one monomer followed by symmetry expansion and 3D refinement applying C1 symmetry.
To increase the number of particles for classification on the Pc region, dataset 2 was collected from the same dimer band, but with a pixel size of 0.51 Å. The dataset was processed with cryoSPARC 3.1.0 (ref. 63). After template picking, 864,399 particles were extracted. With a small subset of the extracted particles an ab initio reconstruction was generated followed by heterogeneous refinement using five classes, one of which contained ab initio reconstruction as reference. The class containing the PSI monomer was then subjected to homogeneous refinement in cryoSPARC 3.1.0, resulting in a reconstruction at 3.88 Å resolution. The particles were then exported to RELION71, and 3D classification was performed. The class that contained 88,219 good particles was used for further refinement, which improved the overall resolution to 3.5 Å. Applying CTF refinement and Bayesian polishing resulted in further improvement, and the final nominal resolution is 2.68 Å. The data were then merged with the monomer (dataset 1), and signal subtraction followed by focused classification using a mask around the Pc region was performed. A class with 66,080 particles showed the best density for Pc, which was used for model building. The workflow is further illustrated in Extended Data Fig. 2a.
For analysis of the motion between the two monomers of the dimer, we performed a multi-body refinement in RELION71 followed by a principal component analysis using the program relion_flex_analyse. Two bodies were chosen, one for each monomer, resulting in 12 eigenvectors describing the motion. Ten maps for each of the three eigenvectors that describe about 78% of the motion in the data were printed out, and the maps with the extreme positions (maps 1 and 10) were used to fit the models that are shown in Extended Data Fig. 6. A Python script was used to estimate the distances between the chlorophylls at the dimer interface for each particle in the data and to plot the results as histograms as depicted in Extended Data Fig. 7e,f.
Model building and refinement
Initially, the available model of the PSI structure (Protein Data Bank (PDB) ID: 6JO5) of C. reinhardtii was rigid body fitted into the 2.74 Å map of the symmetry expanded dimer using Chimera v 1.14 (ref. 72). Model building and real-space refinement was then carried out using Coot v9.1.4 (refs. 44,45) to complete one monomer. Two copies of the completed monomeric model were then rigid body fitted into the C2 generated 2.97 Å dimer map using Chimera. The model of the monomer was then fitted separately in the highest-resolution 2.3 Å map of the monomer. All protein residues as well as pigments were fitted using Coot44,45 with locally optimized map weights. The cis–trans isomerism of each pigment was judged on the basis of density and modelled accordingly. Newly identified chlorophylls and carotenoids were modelled, when the experimental evidence (density map) supported and the chemical environment matched the surrounding of the pigments. For carotenoids, the density that clearly showed the characteristics of an elongated tetraterpenoid with densities for the four methyl groups, two sticking out on each side of the chain, was identified as a new carotenoid binding site. To further analyse the identity of the corresponding pigment, possible candidates were fitted and compared. A carotenoid that fitted best in terms of density and chemical environment was then selected. In case of luteins, the oxygen of the cyclohexane ring was the main criterion for pigment identity because it is involved in hydrogen bonding. For Chl b identification, the densities for the aldehyde group needed to be present as well as the hydrogen bonding occurring with a water molecule that are usually stabilized by other chlorophylls, lipids and protein side chains. Water molecules involved in pigment interactions were placed manually. All other water molecules were picked by Coot44,45 with the autopicking function followed by manual inspection and correction. All high-resolution features were modelled using Coot44,45 until the model was completed. For all modelling steps, restraint files for pigments and ligands were used that were generated using the Grade server (http://grade.globalphasing.org). Restraint files were adopted manually if it was required.
For Pc, a model was generated using SWISS model73. The model was then rigid body fitted using Chimera. Rotamers were corrected for the residues that were allowed owing to the better local densities. Self-restraints in Coot were activated followed by flexible fitting into the density. All models were refined using Real-Space-Refine from the PHENIX suite74 using the Grade server restraint files for the ligands and a distance.edit file that was generated by Ready-set in PHENIX. Further, hydrogen atoms were added for refinement to the model using Ready-set. The refinement protocol was optimized using different weight parameters. The refinement statistics are presented in Extended Data Table 1. Multiple rounds of validation and model building were carried out using MolProbity75 and Coot44,45. For further validation, the PDB Validation server was used (https://validate-rcsb-2.wwpdb.org). The structure was analysed using Coot and Chimera. Figures were prepared using ChimeraX76.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Algal PSI dimer and high-resolution model of PSI-Pc complex.
Mass spectrometry dataset.
Chemical moieties involved in coordination of chlorophyll a/b.
Source data
Unmodified Coomassie stains, western blots and silver stains for Fig. 1.
Acknowledgements
The cryo-EM data were collected at the SciLifeLab facility (funded by the KAW, EPS and Kempe foundations) and the Karolinska Institutet 3D-EM facility. We thank D. Kimanius for providing a script for conversion of poses from Euler space into an orientation matrix in Cartesian space, S. Hawat for mass spectrometric analyses, V. Adlfar for isolation of Pc. Funding: Swedish Foundation for Strategic Research (ARC19–0051), Knut and Alice Wallenberg Foundation (2018.0080), Deutsche Forschungsgemeinschaft (739/13-2), Federal states (NRW 313-WO44A) and German-Israeli Foundation for Scientific Research and Development (G-1483-207/2018). A.A. is supported by the EMBO Young Investigator Programme, and M.H. is supported by the RECTOR programme (University of Okayama, Japan).
Extended data
Author contributions
M.H. and A.A. designed the project. L.M., A.P.-B. and A.N. prepared the sample for cryo-EM and performed initial screening. A.N. collected and processed the cryo-EM data and built the model. L.M., S.K., M.S. and A.V.-M. performed biochemical analysis. V.T. performed computational analysis. Y.T. provided antibodies. T.T.H.H. produced mutant strains. A.N. and A.A. analysed the structure and wrote the manuscript with contributions from L.M. and M.H. All authors contributed to the analysis and the final version of the manuscript.
Peer review
Peer review information
Nature Plants thanks Matthew Johnson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Funding
Open access funding provided by Stockholm University.
Data availability
The atomic coordinates have been deposited in the RCSB PDB, and EM maps in the Electron Microscopy Data Bank under accession codes: 7ZQD and EMD-14871 (dimer), 7ZQ9 and EMD-14867 (symmetry expanded dimer), 7ZQC and EMD-14870 (monomer), and 7ZQE and EMD-14872 (PSI–Pc). Mass spectrometry datasets: DOI: 10.6019/PXD026990; project accession ID: PXD027067. The following atomic coordinates were used in this study: 1JB0 (PSI trimer from Synechococcus elongatus), 6JO5 (PSI from C. reinhardtii), 6TCL (PSI tetramer from Anabaena), 6ZOO (PSI-Pc from Pisum sativum) and 7DZ8 (PSI-LHCII from C. reinhardtii). Source data are provided with this paper.
Code availability
All the codes that were used in this study are publicly accessible, and the script for conversion of poses from Euler space into an orientation matrix in Cartesian space is available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Andreas Naschberger, Laura Mosebach.
Contributor Information
Michael Hippler, Email: mhippler@uni-muenster.de.
Alexey Amunts, Email: amunts@scilifelab.se.
Extended data
is available for this paper at 10.1038/s41477-022-01253-4.
Supplementary information
The online version contains supplementary material available at 10.1038/s41477-022-01253-4.
References
- 1.Qin X, et al. Structure of a green algal photosystem I in complex with a large number of light-harvesting complex I subunits. Nat. Plants. 2019;5:263–272. doi: 10.1038/s41477-019-0379-y. [DOI] [PubMed] [Google Scholar]
- 2.Suga M, et al. Structure of the green algal photosystem I supercomplex with a decameric light-harvesting complex I. Nat. Plants. 2019;5:626–636. doi: 10.1038/s41477-019-0438-4. [DOI] [PubMed] [Google Scholar]
- 3.Su X, et al. Antenna arrangement and energy transfer pathways of a green algal photosystem-I–LHCI supercomplex. Nat. Plants. 2019;5:273–281. doi: 10.1038/s41477-019-0380-5. [DOI] [PubMed] [Google Scholar]
- 4.Ozawa S-I, et al. Configuration of ten light-harvesting chlorophyll a/b complex I subunits in Chlamydomonas reinhardtii photosystem I. Plant Physiol. 2018;178:583–595. doi: 10.1104/pp.18.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Z, et al. Structure of photosystem I-LHCI–LHCII from the green alga Chlamydomonas reinhardtii in state 2. Nat. Commun. 2021;12:1100. doi: 10.1038/s41467-021-21362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan X, et al. Structural basis of LHCBM5-mediated state transitions in green algae. Nat. Plants. 2021;7:1119–1131. doi: 10.1038/s41477-021-00960-8. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, et al. Distinct structural modulation of photosystem I and lipid environment stabilizes its tetrameric assembly. Nat. Plants. 2020;6:314–320. doi: 10.1038/s41477-020-0610-x. [DOI] [PubMed] [Google Scholar]
- 8.Kato K, et al. Structure of a cyanobacterial photosystem I tetramer revealed by cryo-electron microscopy. Nat. Commun. 2019;10:4929. doi: 10.1038/s41467-019-12942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng L, et al. Structural and functional insights into the tetrameric photosystem I from heterocyst-forming cyanobacteria. Nat. Plants. 2019;5:1087–1097. doi: 10.1038/s41477-019-0525-6. [DOI] [PubMed] [Google Scholar]
- 10.Kato K, et al. Structural basis for the absence of low-energy chlorophylls in a photosystem I trimer from Gloeobacter violaceus. eLife. 2022;11:e73990. doi: 10.7554/eLife.73990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan P, et al. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature. 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Shem A, Frolow F, Nelson N. Crystal structure of plant photosystem I. Nature. 2003;426:630–635. doi: 10.1038/nature02200. [DOI] [PubMed] [Google Scholar]
- 13.Amunts A, Nelson N. Plant photosystem I design in the light of evolution. Structure. 2009;17:637–650. doi: 10.1016/j.str.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Boerema A, et al. Structure of a minimal photosystem I from the green alga Dunaliella salina. Nat. Plants. 2020;6:321–327. doi: 10.1038/s41477-020-0611-9. [DOI] [PubMed] [Google Scholar]
- 15.Wood WH, et al. Dynamic thylakoid stacking regulates the balance between linear and cyclic photosynthetic electron transfer. Nat. Plants. 2018;4:116–127. doi: 10.1038/s41477-017-0092-7. [DOI] [PubMed] [Google Scholar]
- 16.Yadav KNS, et al. Supercomplexes of plant photosystem I with cytochrome b6f, light-harvesting complex II and NDH. Biochim. Biophys. Acta. 2017;1858:12–20. doi: 10.1016/j.bbabio.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Caspy I, et al. Dimeric and high-resolution structures of Chlamydomonas photosystem I from a temperature-sensitive photosystem II mutant. Commun. Biol. 2021;4:1380. doi: 10.1038/s42003-021-02911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amunts A. The revolution evolution. Nat. Plants. 2021;8:14–17. doi: 10.1038/s41477-021-01050-5. [DOI] [PubMed] [Google Scholar]
- 19.Mazor Y, Borovikova A, Caspy I, Nelson N. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat. Plants. 2017;3:17014. doi: 10.1038/nplants.2017.14. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, et al. Structure of plant photosystem I–light harvesting complex I supercomplex at 2.4 Å resolution. J. Integr. Plant Biol. 2021;63:1367–1381. doi: 10.1111/jipb.13095. [DOI] [PubMed] [Google Scholar]
- 21.Qian P, et al. Cryo-EM structure of the dimeric Rhodobacter sphaeroides RC–LH1 core complex at 2.9 Å: the structural basis for dimerisation. Biochem. J. 2021;478:3923–3937. doi: 10.1042/BCJ20210696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mühleip A, McComas SE, Amunts A. Structure of a mitochondrial ATP synthase with bound native cardiolipin. eLife. 2019;8:e51179. doi: 10.7554/eLife.51179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flygaard RK, Mühleip A, Tobiasson V, Amunts A. Type III ATP synthase is a symmetry-deviated dimer that induces membrane curvature through tetramerization. Nat. Commun. 2020;11:5342. doi: 10.1038/s41467-020-18993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mühleip A, et al. ATP synthase hexamer assemblies shape cristae of Toxoplasma mitochondria. Nat. Commun. 2021;12:120. doi: 10.1038/s41467-020-20381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett CF, et al. Peroxisomal-derived ether phospholipids link nucleotides to respirasome assembly. Nat. Chem. Biol. 2021;17:703–710. doi: 10.1038/s41589-021-00772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho TT, et al. Photosystem I light-harvesting proteins regulate photosynthetic electron transfer and hydrogen production. Plant Physiol. 2022;189:329–343. doi: 10.1093/plphys/kiac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amunts A, Drory O, Nelson N. The structure of a plant photosystem I supercomplex at 3.4 Å resolution. Nature. 2007;447:58–63. doi: 10.1038/nature05687. [DOI] [PubMed] [Google Scholar]
- 28.Nellaepalli S, Ozawa S-I, Kuroda H, Takahashi Y. The photosystem I assembly apparatus consisting of YCF3–Y3IP1 and YCF4 modules. Nat. Commun. 2018;9:2439. doi: 10.1038/s41467-018-04823-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen PE, Haldrup A, Rosgaard L, Scheller HV. Molecular dissection of photosystem I in higher plants: topology, structure and function. Physiol. Plant. 2003;119:313–321. doi: 10.1034/j.1399-3054.2003.00157.x. [DOI] [Google Scholar]
- 30.Binte Safie SR, Ng YK, Yao L, Lee YK. Growth bottlenecks of microalga Dunaliella tertiolecta in response to an up-shift in light intensity. Eur. J. Phycol. 2018;53:509–519. doi: 10.1080/09670262.2018.1478131. [DOI] [Google Scholar]
- 31.Mackinder LCM, et al. A spatial interactome reveals the protein organization of the algal CO2-concentrating mechanism. Cell. 2017;171:133–147.e14. doi: 10.1016/j.cell.2017.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amunts A, Toporik H, Borovikova A, Nelson N. Structure determination and improved model of plant photosystem I. J. Biol. Chem. 2010;285:3478–3486. doi: 10.1074/jbc.M109.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crepin A, Kučerová Z, Kosta A, Durand E, Caffarri S. Isolation and characterization of a large photosystem I–light‐harvesting complex II supercomplex with an additional lhca1–A4 dimer in Arabidopsis. Plant J. 2020;102:398–409. doi: 10.1111/tpj.14634. [DOI] [PubMed] [Google Scholar]
- 34.Elsemman IE, et al. Whole-cell modeling in yeast predicts compartment-specific proteome constraints that drive metabolic strategies. Nat. Commun. 2022;13:801. doi: 10.1038/s41467-022-28467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen L, et al. Architecture of the chloroplast psi–NDH supercomplex in Hordeum vulgare. Nature. 2021;601:649–654. doi: 10.1038/s41586-021-04277-6. [DOI] [PubMed] [Google Scholar]
- 36.Su X, et al. Supramolecular assembly of chloroplast NADH dehydrogenase-like complex with photosystem I from Arabidopsis thaliana. Mol. Plant. 2022;15:454–467. doi: 10.1016/j.molp.2022.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Gahura, O. et al. An ancestral interaction module promotes oligomerization in divergent mitochondrial ATP synthases. Preprint at bioRxiv10.1101/2021.10.10.463820 (2022). [DOI] [PMC free article] [PubMed]
- 38.Matzov D, et al. The cryo-EM structure of hibernating 100s ribosome dimer from pathogenic Staphylococcus aureus. Nat. Commun. 2017;8:723. doi: 10.1038/s41467-017-00753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amunts A, Nelson N. Functional organization of a plant photosystem I: evolution of a highly efficient photochemical machine. Plant Physiol. Biochem. 2008;46:228–237. doi: 10.1016/j.plaphy.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Bergner SV, et al. State transition7-dependent phosphorylation is modulated by changing environmental conditions, and its absence triggers remodeling of photosynthetic protein complexes. Plant Physiol. 2015;168:615–634. doi: 10.1104/pp.15.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bienvenut WV, et al. Dual lysine and N‐terminal acetyltransferases reveal the complexity underpinning protein acetylation. Mol. Syst. Biol. 2020;16:e9464. doi: 10.15252/msb.20209464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Croce R, van Amerongen H. Light harvesting in oxygenic photosynthesis: structural biology meets spectroscopy. Science. 2020;369:e9464. doi: 10.1126/science.aay2058. [DOI] [PubMed] [Google Scholar]
- 43.Fredj, A. B., Lakhdar, Z. B. & Ruiz-López, M. F. The structure of chlorophyll a–water complexes: insights from quantum chemistry calculations. Chem. Commun. 10.1039/b716800d (2008). [DOI] [PubMed]
- 44.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 45.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caspy I, Borovikova-Sheinker A, Klaiman D, Shkolnisky Y, Nelson N. The structure of a triple complex of plant photosystem I with ferredoxin and plastocyanin. Nat. Plants. 2020;6:1300–1305. doi: 10.1038/s41477-020-00779-9. [DOI] [PubMed] [Google Scholar]
- 47.Caspy I, et al. Structure of plant photosystem I–plastocyanin complex reveals strong hydrophobic interactions. Biochem. J. 2021;478:2371–2384. doi: 10.1042/BCJ20210267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hippler M, Drepper F, Haehnel W, Rochaix J-D. The N-terminal domain of psaf: precise recognition site for binding and fast electron transfer from cytochrome c 6 and plastocyanin to photosystem I of Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA. 1998;95:7339–7344. doi: 10.1073/pnas.95.13.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, et al. A genome-wide algal mutant library and functional screen identifies genes required for eukaryotic photosynthesis. Nat. Genet. 2019;51:627–635. doi: 10.1038/s41588-019-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boynton JE, et al. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988;240:1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- 51.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 52.Hippler M, Drepper F, Farah J, Rochaix J-D. Fast electron transfer from cytochrome c6 and plastocyanin to photosystem I of Chlamydomonas reinhardtii requires psaf. Biochemistry. 1997;36:6343–6349. doi: 10.1021/bi970082c. [DOI] [PubMed] [Google Scholar]
- 53.Hippler M, Klein J, Fink A, Allinger T, Hoerth P. Towards functional proteomics of membrane protein complexes: analysis of thylakoid membranes from Chlamydomonas reinhardtii. Plant J. 2001;28:595–606. doi: 10.1046/j.1365-313X.2001.01175.x. [DOI] [PubMed] [Google Scholar]
- 54.Naumann B, Stauber EJ, Busch A, Sommer F, Hippler M. N-terminal processing of LHCA3 is a key step in remodeling of the photosystem I–light-harvesting complex under iron deficiency in Chlamydomonas reinhardtii. J. Biol. Chem. 2005;280:20431–20441. doi: 10.1074/jbc.M414486200. [DOI] [PubMed] [Google Scholar]
- 55.Ozawa S-ichiro, Onishi T, Takahashi Y. Identification and characterization of an assembly intermediate subcomplex of photosystem I in the green alga Chlamydomonas reinhardtii. J. Biol. Chem. 2010;285:20072–20079. doi: 10.1074/jbc.M109.098954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michel H, Tellenbach M, Boschetti A. A chlorophyll B-less mutant of Chlamydomonas reinhardii lacking in the light-harvesting chlorophyll ab-protein complex but not in its apoproteins. Biochim. Biophys. Acta. 1983;725:417–424. doi: 10.1016/0005-2728(83)90182-2. [DOI] [Google Scholar]
- 57.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 58.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 59.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 60.Tyanova S, et al. The Perseus Computational Platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016;13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- 61.Busch A, Nield J, Hippler M. The composition and structure of photosystem I-associated antenna from Cyanidioschyzon merolae. Plant J. 2010;62:886–897. doi: 10.1111/j.1365-313X.2010.04202.x. [DOI] [PubMed] [Google Scholar]
- 62.Shevchenko A, Tomas H, Havli J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 63.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 64.Zheng SQ, et al. Motioncor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rohou A, Grigorieff N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 2015;192:216–221. doi: 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pisoni, E. Code on Zenodo. Zenodo10.5281/zenodo.3576630 (2020).
- 67.Scheres SHW. Amyloid structure determination in RELION-3.1. Acta Crystallogr D Struct Biol. 2020;76:94–101. doi: 10.1107/S2059798319016577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zivanov J, Nakane T, Scheres SH. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ. 2020;7:253–267. doi: 10.1107/S2052252520000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zivanov J, et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018;7:e42166. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zivanov J, Nakane T, Scheres SH. A bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ. 2019;6:5–17. doi: 10.1107/S205225251801463X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakane T, Kimanius D, Lindahl E, Scheres SHW. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. eLife. 2018;7:e36861. doi: 10.7554/eLife.36861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 73.Waterhouse A, et al. Swiss-model: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Afonine PV, et al. Real-space refinement in phenix for cryo-EM and crystallography. Acta Crystallogr D Struct Biol. 2018;74:531–544. doi: 10.1107/S2059798318006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams CJ, et al. Molprobity: more and better reference data for improved all-atom structure validation. Protein Sci. 2017;27:293–315. doi: 10.1002/pro.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goddard TD, et al. UCSF Chimerax: meeting modern challenges in visualization and analysis. Protein Sci. 2017;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bianca N, et al. Comparative quantitative proteomics to investigate the remodeling of bioenergetic pathways under iron deficiency in Chlamydomonas reinhardtii. PROTEOMICS. 2007;7:3964–3979. doi: 10.1002/pmic.200700407. [DOI] [PubMed] [Google Scholar]
- 78.Abraham S, Golay MJE. Smoothing and Differentiation of Data by simplified least squares procedures. Analytical Chemistry. 1964;36:1627–1639. doi: 10.1021/ac60214a047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Algal PSI dimer and high-resolution model of PSI-Pc complex.
Mass spectrometry dataset.
Chemical moieties involved in coordination of chlorophyll a/b.
Unmodified Coomassie stains, western blots and silver stains for Fig. 1.
Data Availability Statement
The atomic coordinates have been deposited in the RCSB PDB, and EM maps in the Electron Microscopy Data Bank under accession codes: 7ZQD and EMD-14871 (dimer), 7ZQ9 and EMD-14867 (symmetry expanded dimer), 7ZQC and EMD-14870 (monomer), and 7ZQE and EMD-14872 (PSI–Pc). Mass spectrometry datasets: DOI: 10.6019/PXD026990; project accession ID: PXD027067. The following atomic coordinates were used in this study: 1JB0 (PSI trimer from Synechococcus elongatus), 6JO5 (PSI from C. reinhardtii), 6TCL (PSI tetramer from Anabaena), 6ZOO (PSI-Pc from Pisum sativum) and 7DZ8 (PSI-LHCII from C. reinhardtii). Source data are provided with this paper.
All the codes that were used in this study are publicly accessible, and the script for conversion of poses from Euler space into an orientation matrix in Cartesian space is available upon request.