Abstract
Introduction
Pembrolizumab is a programmed death-ligand 1 inhibitor that was initially indicated for monotherapy in patients with advanced lung cancer. The Japanese Lung Cancer Society conducted an observational study on pembrolizumab using confirmative data obtained through postmarketing all-case surveillance (PMACS), which was performed by a pharmaceutical company under the Japanese law in 2017.
Methods
This multicenter observational study was conducted by the Japanese Lung Cancer Society using PMACS data with the newly created central registration system regarding patients with NSCLC who received pembrolizumab monotherapy between February 1, 2017 and June 30, 2017; a new database was created by adding the clinical information regarding prognosis for 3 years after therapy to the existing data collected by PMACS.
Results
A total of 300 patients from 43 facilities were enrolled in this study. The median overall survival and progression-free survival after pembrolizumab initiation were 558 and 188 days, respectively. Moreover, the 1- and 3-year survival rates were 58.9% and 33.7%, respectively. Results of multivariate analysis revealed performance status (p < 0.0001), histology (p = 0.0118), previous chemotherapy (p = 0.0007), programmed death-ligand 1 expression status (p = 0.0195), and previous steroid use (p = 0.0460) as significant factors that affected overall survival. The toxicity profile was similar to that previously reported.
Conclusions
In this first attempt to use PMACS data, we successfully collected clinical information and found the real-world efficacy and safety of pembrolizumab.
Keywords: Real-world data, Pembrolizumab, Immune checkpoint inhibitor, Immune-related adverse events
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide.1 NSCLC accounts for approximately 80% of all lung cancers, and most cases are unresectable.1
As revealed in previous clinical trials, chemotherapy can improve the prognosis of patients with advanced NSCLC, and new and effective drugs are developed every year.2, 3, 4 Immune checkpoint inhibitors (ICIs) were found to have outstanding efficacy and are recognized as an important treatment option for patients with NSCLC.
ICIs targeting programmed cell death protein-1 (PD-1), programmed death-ligand 1 (PD-L1), and CTLA4 were found to have excellent effects in clinical trials for various types of cancer.5 In 2016, pembrolizumab was approved as an anti–PD-1 antibody for NSCLC treatment by the Food and Drug Administration and Pharmaceuticals and Medical Devices Agency. Pembrolizumab (Keytruda, Merck, Kenilworth, NJ) is a humanized immunoglobulin 4 antibody that inhibits the interaction between PD-1 and PD-L1. PD-L1 is expressed in the tumor tissue and can be detected using the 22C3 antibody, a companion diagnostic test used to evaluate the tumor proportion score (TPS) of PD-L1 expression.
In the KEYNOTE-024 trial,6,7 pembrolizumab was found to have a markedly better survival benefit than that of cisplatin combination chemotherapy in the first-line treatment of patients with NSCLC with PD-L1 expression in tumor cells equals to or greater than 50%. Long-term follow-up data revealed a median overall survival (OS) of 26.3 and 13.4 months in the pembrolizumab and chemotherapy arms, respectively.
Moreover, pembrolizumab has been approved as a second-line treatment for NSCLC in which PD-L1 is expressed in at least 1% of the tumor tissue. This approval was supported by results from the phase 2/3 KEYNOTE-010 study,8,9 in which pembrolizumab was found to have improved OS compared with docetaxel in previously treated patients with NSCLC (11.8 versus 8.4 mo).
New ICIs are also being developed for monotherapy and combination therapy.10 The development of cancer therapies using host immunity is desirable, although the efficacy and safety profiles in clinical practice with long-term follow-up data remain elusive.
Postmarketing all-case surveillance (PMACS) is a unique pharmacovigilance program conducted by a pharmaceutical company under the Japanese law when a drug is newly approved and released for use in clinical practice. In PMACS, a pharmaceutical company works with individual medical institutes to collect clinical data, mainly concerning safety, about all patients who receive a specific drug. PMACS plays a key role in postmarketing activities in Japan and has been implemented for more than 20 years.11,12 Despite tremendous effort and costs incurred for PMACS, the use of these data has been limited. The Japanese Lung Cancer Society planned this observational study on pembrolizumab as a case model for the effective use of PMACS data.
Materials and Methods
Data Collection and Patient Enrollment
This multicenter observational study was conducted by the Japanese Lung Cancer Society using a central registry and electronic data capture system developed by the Japan National Clinical Database.
The study design and methodology were approved by the institutional review boards of each participating institution. The requirement for written informed consent was waived by the institutional review boards owing to the retrospective nature of the study and use of anonymized data. Researchers obtained verbal consent from the patients and recorded it in a medical chart. This research was conducted in accordance with the principles of the Declaration of Helsinki and the WHO Guidelines for Good Clinical Practice. The study protocol was registered with the University Hospital Medical Information Network(UMIN) in Japan (number: 000045538).
We enrolled patients with NSCLC who (1) initiated pembrolizumab treatment between February 1, 2017, and June 30, 2017; (2) were treated at a facility that participated in PMACS and belonged to the Japan Lung Cancer Society; and (3) agreed to participate in this study.
Clinical information regarding background characteristics, pretreatment information, pembrolizumab-related adverse events (AEs), post-treatment information, and prognosis at 1 year and 3 years after pembrolizumab treatment was collected. Anonymized registry data regarding basic patient clinical information, AEs, treatment course, and treatment results were automatically collected by uploading the PMACS data. PMACS data were returned to each institution by Merck Sharp & Dohme pharmaceutical company (Merck & Co., Inc., Rahway, NJ) after they were organized. Survival information at 1 year and 3 years after treatment was input directly into the central system by the researchers at each institution. The institutions that participated in this study are listed in Supplementary Table 1.
Statistical Analysis
The Kaplan-Meier method was used to perform time-to-event analyses, including OS and progression-free survival (PFS), from the initiation of pembrolizumab treatment. Univariate and multivariate analyses were performed using Cox proportional hazards regression models. Factors with p less than 0.10 in the univariate analysis were included in the multivariate analyses. All statistical analyses were conducted using SAS software (version 9.4, SAS Institute Inc., Cary, NC). Statistical significance was set at p value less than 0.05.
Results
Baseline Characteristics
A total of 300 patients were enrolled, and their baseline characteristics are summarized in Table 1. Overall, 76.0% were men, 84.0% were smokers, 26.6% were older than 75 years, and 17.0% had a poor performance status (PS ≥2) score.
Table 1.
Patient Background Characteristics
| Characteristics | Patients (N = 300) |
|---|---|
| Age (y) | |
| Mean (range) | 67.1 (28–91) |
| Sex | |
| Male/female | 228/72 |
| Smoking history | |
| Never/former/current/other | 39/199/53/9 |
| Smoking index (N = 253) | |
| Mean (range) | 965.7 (9–3300) |
| Performance status | |
| 0/1/2/3/4 | 88/161/38/11/2 |
| Histology | |
| Squamous/nonsquamous | 79/221 |
| EGFR mutation | |
| Positive/negative/unknown | 22/250/28 |
| ALK fusion | |
| Positive/negative/unknown | 2/257/41 |
| PD-L1 TPS | |
| 1%–49%/≤50%/unknown | 57/242/1 |
| Stage | |
| II–III/IV/recurrence | 44/191/65 |
| Previous chemotherapy | |
| ≤1 line/≤2 lines/unknown | 244/53/3 |
| Liver metastasis | |
| None/metastasis | 277/23 |
| Brain metastasis | |
| None/metastasis | 248/52 |
| Bone metastasis | |
| None/metastasis | 226/74 |
| Malignant pleural effusion | |
| None/metastasis | 286/14 |
| Steroid usage | |
| None/use/unknown | 286/11/3 |
| Radiation (before ICI) | |
| No/yes | 209/91 |
| Surgery (before ICI) | |
| No/yes | 243/57 |
| Body mass index (kg/m2) | |
| Mean (range) | 21.7 (14.3–40.0) |
ICI, immune checkpoint inhibitor; PD-L1 TPS, programmed cell death-ligand 1 tumor proportion score.
Patients with NSCLC harboring EGFR (n = 22) or ALK (n = 2) driver mutations were also included. Most patients received pembrolizumab as an early line treatment (first line, n= 164; second line, n = 82), although 17.7% of the patients were treated with pembrolizumab as a third-line treatment or more.
Efficacy
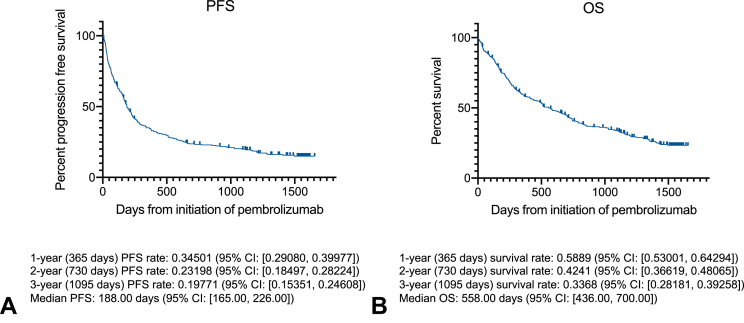
During a median follow-up period of 505 days, 243 (81.0%) and 211 (70.3%) PFS and OS events were observed, respectively (Fig. 1). The median PFS and OS were 188 (95% confidence interval [CI]: 165–226) and 558 (95% CI: 436–700) days, respectively. The median OS in patients who received first-line treatment was 700 (95% CI: 557–843) days and 488 (95% CI: 266–822) days in patients who received second-line treatment. Overall, the efficacy of pembrolizumab was similar to that reported in previous clinical trials (6-996-996-996-9).
Figure 1.
Kaplan-Meier curves of OS and PFS of the patients with prognosis data. Survival curves of (A) OS and (B) PFS, with detailed data at the indicated time points (1 y, 2 y, and 3 y after pembrolizumab initiation). CI, confidence interval; OS, overall survival; PFS, progression-free survival.
In a univariate analysis using a Cox proportional hazards model excluding patients with missing values, the factors related to longer PFS were “good PS (0, 1),” “nonsquamous cell carcinoma,” “Brinkman Index [BI] > 400,” “PD-L1 TPS ≥ 50%,” “number of previous chemotherapies = 0 or 1,” “none or small amount of steroid use (less than or equivalent to 10 mg of prednisolone) before pembrolizumab,” “treatment-related AE (grade ≥3),” and “no previous medical history.” In a multivariate analysis, a PS of 0 or 1 (hazard ratio [HR] = 0.49, 95% CI: 0.33–0.71, p = 0.0002), nonsquamous cell carcinoma (HR = 0.72, 95% CI: 0.53–0.98, p = 0.0349), number of previous chemotherapies more than or equal to two (HR = 1.72, 95% CI: 1.15–2.58, p = 0.0083), BI more than 400 (HR = 0.64, 95% CI: 0.42–0.97, p = 0.0355), and treatment-related AE (grade ≥3) (HR = 0.60, 95% CI: 0.41–0.88, p = 0.0095) were significant independent predictors of PFS (Supplementary Table 2, Supplementary Data 1, Supplementary Fig. 1, and Supplementary Data 2 illustrate the Kaplan-Meier curves for PFS stratified by each predictor).
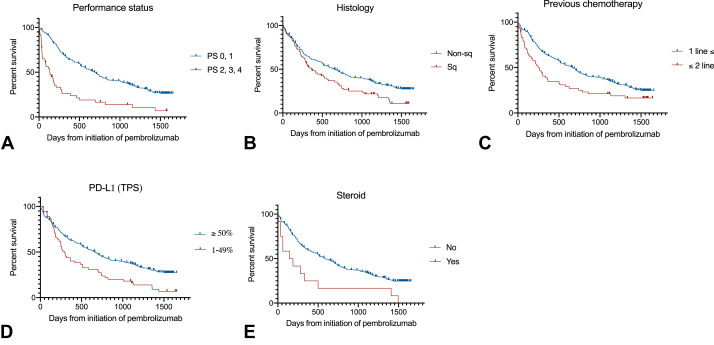
In a univariate analysis using a Cox proportional hazards model excluding patients with missing values, the factors related to longer OS were “good PS (0, 1),” “nonsquamous cell carcinoma,” “BI > 400,” “PD-L1 expression ≥ 50%,” “number of previous chemotherapies = 0 or 1,” “none or small amount of steroid use (less than or equivalent to 10 mg of prednisolone) before pembrolizumab,” and “no previous medical history.” In a multivariate analysis, a PS of 0 or 1 (HR = 0.41, 95% CI: 0.28–0.61, p < 0.0001), nonsquamous cell carcinoma (HR = 0.66, 95% CI: 0.47–0.91, p = 0.0118), number of previous chemotherapies more than or equal to two (HR = 2.01, 95% CI: 1.34–3.01, p = 0.007), PD-L1 TPS more than or equal to 50% (HR = 0.64, 95% CI: 0.43–0.93, p = 0.0195), and steroid use (HR = 2.20, 95% CI: 1.01–4.77, p = 0.0460) were significant independent predictors of OS (Table 2 and Fig. 2 illustrate the Kaplan-Meier curves for OS stratified by each predictor).
Table 2.
Univariate and Multivariate Analyses of OS
| N | Median OS (d) | Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |||
| Age | ||||||||
| ≤75 | 78 | 440 | 1.255 | 0.928–1.698 | 0.1397 | |||
| ≤74 | 220 | 614 | ||||||
| Gender | ||||||||
| Male | 226 | 513 | 1.171 | 0.852–1.608 | 0.3303 | |||
| Female | 72 | 626 | ||||||
| Smoking history | ||||||||
| Former or current | 250 | 526 | 1.217 | 0.805–1.838 | 0.3517 | |||
| Never | 39 | 626 | ||||||
| Smoking index | ||||||||
| <400 | 217 | 636 | 0.556 | 0.373–0.830 | 0.0041 | 0.684 | 0.449–1.043 | 0.0777 |
| ≤400 | 33 | 283 | ||||||
| Performance status | ||||||||
| 0, 1 | 248 | 700 | 0.337 | 0.239–0.475 | <0.0001 | 0.409 | 0.276–0.607 | <0.0001 |
| 2, 3, 4 | 50 | 133 | ||||||
| Histology | ||||||||
| Nonsq | 220 | 645 | 0.667 | 0.495–0.899 | 0.0079 | 0.656 | 0.473–0.911 | 0.0118 |
| Squamous | 78 | 366 | ||||||
| PD-L1 TPS | ||||||||
| ≤50% | 240 | 654 | 0.571 | 0.415–0.787 | 0.0006 | 0.635 | 0.433–0.929 | 0.0195 |
| 1%–49% | 57 | 288 | ||||||
| Previous chemotherapy | ||||||||
| ≤2 lines | 53 | 246 | 1.748 | 1.249–2.446 | 0.0011 | 2.009 | 1.342–3.007 | 0.0007 |
| ≤1 line | 244 | 662 | ||||||
| Liver metastasis | ||||||||
| Metastasis | 23 | 420 | 1.401 | 0.852–2.302 | 0.1839 | |||
| None | 275 | 574 | ||||||
| Brain metastasis | ||||||||
| Metastasis | 52 | 585 | 0.894 | 0.616–1.296 | 0.5533 | |||
| None | 246 | 515 | ||||||
| Bone metastasis | ||||||||
| Metastasis | 73 | 420 | 1.214 | 0.891–1.654 | 0.2195 | |||
| None | 225 | 589 | ||||||
| Malignant pleural effusion | ||||||||
| Metastasis | 14 | 294 | 1.411 | 0.769–2.591 | 0.2665 | |||
| None | 284 | 574 | ||||||
| Steroid use | ||||||||
| Use | 11 | 62 | 3.515 | 1.907–6.478 | <0.0001 | 2.200 | 1.014–4.773 | 0.0460 |
| None | 284 | 585 | ||||||
| Radiation (before ICI) | ||||||||
| Yes | 92 | 557 | 0.950 | 0.706–1.277 | 0.7321 | |||
| No | 206 | 558 | ||||||
| Surgery (before ICI) | ||||||||
| Yes | 57 | 717 | 0.930 | 0.661–1.308 | 0.6747 | |||
| No | 241 | 513 | ||||||
| Treatment-related AE (≥grade 3) | ||||||||
| Yes | 60 | 557 | 0.805 | 0.567–1.145 | 0.2276 | |||
| No | 238 | 572 | ||||||
| Previous medical history | ||||||||
| Yes | 194 | 440 | 1.569 | 1.169–2.105 | 0.0027 | 1.395 | 0.996–1.955 | 0.0529 |
| No | 104 | 716 | ||||||
AE, adverse event; CI, confidence interval; HR, hazard ratio; ICI, immune checkpoint inhibitor; PD-L1 TPS, programmed cell death death-ligand 1 tumor proportion score.
Figure 2.
Kaplan-Meier curves of survival stratified by prognostic factors identified by multivariate analysis. Survival data were stratified by (A) performance status, (B) histology, (C) number of previous chemotherapies, (D) TPS of PD-L1, and (E) steroid use before treatment. PD-L1, programmed death-ligand 1; PS, performance status; Sq, squamous; TPS, tumor proportion score.
Toxicity
Overall, 55 patients (18%) had more than or equal to grade 3 AEs (Table 3, Supplementary Table 3, and Supplementary Data 1 illustrate the treatment-related AEs). Five patients (1.7%) died of treatment-related AEs attributed to pembrolizumab, including pneumonitis (n = 3) and pneumonia (n = 2). Among the patients who developed grade 3 or higher nonhematologic AEs, the most frequent AE was pneumonitis (n = 17, 5.7% of all patients) (Table 3).
Table 3.
List of Adverse Events (Grade ≥3) Observed in This Study
| Drug-Related Adverse Events | Grades 3–5 |
|---|---|
| Overall | 74 |
| Pneumonitis | 17 |
| Hepatotoxicity | 8 |
| Skin toxicity | 5 |
| Pneumonia | 5 |
| Colitis | 3 |
| Diabetes mellitus | 3 |
| Hypopituitarism | 3 |
| Hyperglycemia | 2 |
| Herpes zoster | 2 |
| Encephalitis | 2 |
| Respiratory failure | 2 |
| Dyspnea | 2 |
| Arthritis | 2 |
| Hyponatremia | 1 |
| Decreased appetite | 1 |
| Pleural effusion | 1 |
| Empyema | 1 |
| Uveitis | 1 |
| Myasthenia gravis | 1 |
| Optic neuritis | 1 |
| Cerebeller ataxia | 1 |
| Cerebral infarction | 1 |
| Bronchial fistula | 1 |
| Oral mucositis | 1 |
| Pericardial effusion | 1 |
| Pericarditis | 1 |
| AV block | 1 |
| Cardiac tamponade | 1 |
| Myocardial infarction | 1 |
| Intestinal obstruction | 1 |
| Digestive tract perforation | 1 |
AV, atrioventricular.
Pseudoprogression
Pseudoprogression is an uncommon phenomenon observed in patients treated with ICIs. The actual frequency of this phenomenon and its effect on patients’ prognoses are not well understood. We have collected data on pseudoprogression in this study and found seven patients who experienced pseudoprogression (Supplementary Fig. 2). Pseudoprogression did not reveal great effect on patients’ prognoses in this study; however, a larger sample size is necessary to clarify the clinical effect of pseudoprogression.
Discussion
This is the first observational study to use Japanese PMACS data. Although collected and validated by pharmaceutical companies, the data are mainly used to report unexpected AEs observed in clinics and are not used to evaluate the efficacy of the drug. In this study, we established a new system for collecting and analyzing the clinical information of patients who were administered newly approved drugs and enrolled in PMACS in Japan. We recycled the collected data from each hospital and collected and incorporated additional original follow-up data to evaluate the efficacy and safety of the study drug during a long-term follow-up period. A strength of this study is that we were able to conduct the analysis independently from the pharmaceutical companies, as they only provided PMACS data. Notably, with the exception of requesting the addition of simple prognostic information, this study reduced the burden of case report forms usually placed on researchers.
PMACS is a unique surveillance system in Japan and is often required by the Pharmaceutical and Medical Devices Agency when a new drug is approved, especially an anticancer drug.
Recently, multiple reports on real-world data analysis have been published,13, 14, 15, 16 among which ICIs were often analyzed.17, 18, 19 Regarding pembrolizumab monotherapy, Cramer-van der Welle et al.18 compared efficacy data from electronic health records and clinical trials. In first-line treatment settings, the OS was significantly lower in real-world compared with that in clinical trial settings (HR = 1.55, 95% CI: 1.07–2.25, p = 0.018).18 The authors analyzed the data and suggested that the reason for the difference in efficacy was that fewer subsequent treatments were administered in real-world settings than in clinical trials.
As mentioned in a recent report, Japanese patients were only assessed as a small subpopulation in global, large-scale, phase 3 trials, and the evaluation of efficacy and safety specific to the Japanese population may be insufficient.14 Because it is extremely difficult to conduct another phase 3 trial in a Japanese subpopulation after approval of innovative new treatments, assessments using postmarketing data become important.
This study had some limitations. Although more than 3000 patients were originally included in PMACS for pembrolizumab, only 300 ultimately participated in this study, thus failing to reveal a sufficient number for real-data analysis. Furthermore, as the PMACS system was created by a pharmaceutical company to collect data of unexpected AEs in clinics, it did not contain enough information to assess the real-world effectiveness of drugs.
Theoretically, our system may be applied to other newly approved drugs, and our experience may be useful for the establishment of a more efficient and informative system for using PMACS data. Indeed, several ongoing studies intend to use PMACS data in Japan (UMIN000044375, UMIN000033133, UMIN000041263, and UMIN000037090), although most of these projects are funded by pharmaceutical companies. Collaboration among academic societies, pharmaceutical companies, and clinicians is required in modern health care to build a system that effectively uses valuable patient medical information, as new drugs are being developed every day.
Japanese PMACS is useful for assessing real-world data, as revealed with our new system. In the first trial of this system, we successfully collected and used a combination of PMACS and direct input information for real-world data analysis of pembrolizumab in Japan. In the future, a more sophisticated system should be established to collect more valuable data from PMACS efficiently.
CRediT Authorship Contribution Statement
Hideki Terai: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing—original draft, Writing—review and editing.
Kenzo Soejima: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing—original draft, Writing—review and editing.
Asanao Shimokawa: Formal analysis, Methodology, Writing—original draft, Writing—review and editing.
Hidehito Horinouchi: Conceptualization, Data curation, Investigation, Methodology, Writing—review and editing.
Junichi Shimizu, Tetsunari Hase, Ryota Kanemaru, Kana Watanabe, Kiichiro Ninomiya, Naoko Aragane, Noriko Yanagitani, Yoshihiko Sakata, Masahiro Seike, Daichi Fujimoto, Masashi Kasajima, Akihito Kubo, Sojiro Kusumoto, Yoshitaka Oyamada, Keiichi Fujiwara, Masahide Mori, Midori Hashimoto, Shingyoji Masato, Masahiro Kodani, Takashige Kuraki, Toshihiko Agatsuma, Kosuke Kashiwabara, Minehiko Inomata, Motoko Tachihara, Kazuhisa Tanaka, Kenji Hayashihara, Nobuyuki Koyama, Kaoru Matsui, Koichi Minato, Jingu Daisuke, Hiroyuki Sakashita, Satoshi Hara, Tomoyuki Naito, Asuka Okada, Masayuki Tanahashi, Yuki Sato, Koichiro Asano, Takayuki Takeda, Kensuke Nakazawa, Toshiyuki Harada, Kazuhiko Shibata, Tatsuo Kato: Data curation, Investigation, Writing—review and editing.
Etsuo Miyaoka: Formal analysis, Methodology, Writing—review and editing.
Ichiro Yoshino: Conceptualization, Funding acquisition, Data curation, Investigation, Methodology, Project administration, Writing—original draft, Writing—review and editing.
Akihiko Gemma, Tetsuya Mitsudomi: Funding acquisition, Project administration, Writing—original draft, Writing—review and editing.
Acknowledgments
This work was supported by the Japan Agency for Medical Research and Development (grant number 17lk1010016h0002), the Ministry of Health, Labour and Welfare Science Research Grant (grant number 201708003B), the Japan Science and Technology Agency (grant number JPMJRC1503), and the Japan Lung Cancer Society. This research is neither intended for the benefit of any particular private company nor conducted under any restrictions from these sources. The authors thank Dr. Arata Takahashi, Department of Health Policy and Management, Faculty of Medicine, Keio University, for his effort of data management. We thank Dr. Tomohiro Takehara, Department of Respiratory Medicine, Tokyo Saiseikai Central Hospital, for his effort in project administration. The authors thank Editage (http://www.editage.com) for editing and reviewing this manuscript for English language.
Footnotes
Disclosure: Sojiro Kusumoto reports receiving personal fees from AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Kracie Pharmaceutical Co. Ltd. and Novartis Pharma KK outside of the submitted work. Noriko Yanagitani reports receiving personal fees from Chugai Pharmaceutical Co. Ltd., Pfizer Inc., Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly Japan KK, AstraZeneca KK, Bayer Yakuhin, Ltd. outside of the submitted work. Yoshihiko Sakata reports receiving personal fees from Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan KK, Merck Sharp & Dohme KK, Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co., Ltd., Akihiko Gemma reports receiving personal fees from Merck Sharp & Dohme KK, Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd. outside of the submitted work. Motoko Tachihara reports receiving grants and personal fees from AstraZeneca KK and personal fees from Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan KK, Merck Sharp & Dohme KK, Nippon Kayaku Co. Ltd., Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Taiho Pharmaceutical Co. Ltd., Pfizer Inc. outside of the submitted work. Daichi Fujimoto reports receiving grants and personal fees from AstraZeneca KK and Boehringer Ingelheim Japan Inc. and personal fees from Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Merck Sharp & Dohme KK, Eli Lilly Japan KK, Novartis Pharma KK and Takeda Pharmaceutical Co., Ltd., outside of the submitted work. Junichi Shimizu personal fees from Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Merck Sharp & Dohme KK, Novartis Pharma KK, Takeda Pharmaceutical Co. MSD K.K., Amgen Co. Ltd., Novartis Pharma K.K. and Pfizer Japan Inc. outside of the submitted work. Kiichiro Ninomiya personal fees from AstraZeneca KK, Boehringer Ingelheim Japan Inc., Kyowa Kirin Co. Ltd., Eli Lilly Japan KK, Chugai Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Taiho Pharmaceutical Co. Ltd., Merck Sharp & Dohme KK, Ono Pharmaceutical Co. Ltd., Novartis Pharma KK, Takeda Pharmaceutical Co., Pfizer Japan Inc. and Bristol-Myers Squibb Co. Ltd. outside of the submitted work. Koichi Minato reports receiving grants from Taiho Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd. and Parexel International outside of the submitted work. Hiroyuki Sakashita reports receiving grants from Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., Boehringer Ingelheim Japan Inc., Novartis Pharma KK, Merck & Co. Inc. and SBI Pharmaceuticals outside of the submitted work. Masahiro Seike reports receiving grants and personal fees from Merck Sharp & Dohme KK, Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan KK and Nippon Kayaku Co. Ltd. and personal fees from AstraZeneca KK, Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan KK, Nippon Kayaku Co. Ltd., Daiichi Sankyo Co. Ltd., Kyowa Kirin Co. Ltd. and Takeda Pharmaceutical Co., Ltd. outside of submitted work. Tetsuya Mitsudomi reports receiving grants and personal fees from AstraZeneca KK, Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., receiving grants from Merck Sharp & Dohme KK, and personal fees from Bristol-Myers Squibb Co. Ltd outside of submitted work. Masahide Mori reports receiving grants and personal fees from Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., grant from Delta-fly Pharma Inc., and personal fees from AstraZeneca KK, Eli Lilly Japan KK, Nippon Kayaku Co. Ltd., Novartis Pharma KK, Takeda Pharmaceutical Co., Pfizer Japan Inc. , Boehringer Ingelheim Japan Inc., Kyowa Kirin Co. Ltd., Daiichi Sankyo Co. Ltd., Taiho Pharmaceutical Co. Ltd., Shionogi Pharmaceutical Co. Ltd. outside of the submitted work. Yuki Sato reports receiving personal fees from AstraZeneca KK, Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan KK, Nippon Kayaku Co. Ltd., Novartis Pharma KK, Takeda Pharmaceutical Co., Pfizer Japan Inc. , Kyowa Kirin Co. Ltd., Bristol-Myers Squibb Co. Ltd., Merck Sharp & Dohme KK, Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. outside of the submitted work. Keiichi Fujiwara reports receiving personal fees from Merck Sharp & Dohme KK, AstraZeneca KK, Ono Pharmaceutical Co. Ltd., Sanofi KK and Eli Lilly Japan KK outside of the submitted work. Tetsunari Hase reports grant and personal fees from Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., grant from Novartis Pharma KK, personal fees from Merck Sharp & Dohme KK, Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan KK, Ono Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd., Nippon Kayaku Co. Ltd., Boehringer Ingelheim Japan Inc., Pfizer Japan Inc. and Takeda Pharmaceutical Co. Hidehito Horinouchi reports grant and personal fees from Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Novartis Pharma KK, Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Merck Sharp & Dohme KK, Daiichi Sankyo Co. Ltd., Roche, receiving grant from Merck Biopharma Co. Ltd., AbbVie Inc., and Genomic Health Inc., receiving personal fee from Eli Lilly Japan KK, Ono Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd. outside of submitted work. Kazuhiko Shibata reports receiving personal fees from AstraZeneca KK, Chugai Pharmaceutical Co. Ltd. and Bristol-Myers Squibb Co. Ltd. outside of the submitted work. The remaining authors declare no conflict of interest.
Cite this article as: Terai H, Soejima K, Shimokawa A, et al. Real-world data analysis of pembrolizumab monotherapy for NSCLC using Japanese postmarketing all-case surveillance data. JTO Clin Res Rep. XXXX;X:XXXXXX.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100404.
Supplementary Data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Grant M.J., Herbst R.S., Goldberg S.B. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol. 2021;18:625–644. doi: 10.1038/s41571-021-00520-1. [DOI] [PubMed] [Google Scholar]
- 3.Kerr K.M., Bibeau F., Thunnissen E., et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154:161–175. doi: 10.1016/j.lungcan.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Arbour K.C., Riely G.J. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322:764–774. doi: 10.1001/jama.2019.11058. [DOI] [PubMed] [Google Scholar]
- 5.Doroshow D.B., Sanmamed M.F., Hastings K., et al. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res. 2019;25:4592–4602. doi: 10.1158/1078-0432.CCR-18-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 8.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 9.Herbst R.S., Baas P., Perez-Gracia J.L., et al. Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial. Ann Oncol. 2019;30:281–289. doi: 10.1093/annonc/mdy545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murciano-Goroff Y.R., Warner A.B., Wolchok J.D. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30:507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiramatsu K., Barrett A., Miyata Y. PhRMA Japan Medical Affairs Committee Working Group 1. Current status, challenges, and future perspectives of real-world data and real-world evidence in Japan. Drugs Real World Outcomes. 2021;8:459–480. doi: 10.1007/s40801-021-00266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondo H., Masamune K. Effectiveness of drug postmarketing all-case surveillance as a safety measure in Japan. Ther Adv Drug Saf. 2021;12 doi: 10.1177/20420986211065215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamiya M., Tamiya A., Hosoya K., et al. Efficacy and safety of pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: a multicenter retrospective cohort study (HOPE-001) Investig New Drugs. 2019;37:1266–1273. doi: 10.1007/s10637-019-00843-y. [DOI] [PubMed] [Google Scholar]
- 14.Velcheti V., Chandwani S., Chen X., Pietanza M.C., Piperdi B., Burke T. Outcomes of first-line pembrolizumab monotherapy for PD-L1-positive (TPS >/=50%) metastatic NSCLC at US oncology practices. Immunotherapy. 2019;11:1541–1554. doi: 10.2217/imt-2019-0177. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K., Nakachi I., Naoki K., et al. Real-world efficacy and safety of nivolumab for advanced non-small-cell lung cancer: a retrospective multicenter analysis. Clin Lung Cancer. 2018;19:e349–e358. doi: 10.1016/j.cllc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Ksienski D., Wai E.S., Croteau N., et al. Pembrolizumab for advanced nonsmall cell lung cancer: efficacy and safety in everyday clinical practice. Lung Cancer. 2019;133:110–116. doi: 10.1016/j.lungcan.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Pasello G., Pavan A., Attili I., et al. Real world data in the era of immune checkpoint inhibitors (ICIs): increasing evidence and future applications in lung cancer. Cancer Treat Rev. 2020;87 doi: 10.1016/j.ctrv.2020.102031. [DOI] [PubMed] [Google Scholar]
- 18.Cramer-van der Welle C.M., Verschueren M.V., Tonn M., et al. Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci Rep. 2021;11:6306. doi: 10.1038/s41598-021-85696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jemielita T., Li X.N., Piperdi B., Zhou W., Burke T., Chen C. Overall survival with second-line pembrolizumab in patients with non-small-cell lung cancer: randomized phase III clinical trial versus propensity-adjusted real-world data. JCO Clin Cancer Inform. 2021;5:56–65. doi: 10.1200/CCI.20.00099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.