Abstract
Purpose of Review
Chronic low-grade inflammation may contribute to the onset and progression of communicable and chronic diseases. This review examined the effects and eventual mediation roles of different nutritional factors on inflammation.
Recent Findings
Potential nutritional compounds influencing inflammation processes include macro and micronutrients, bioactive molecules (polyphenols), specific food components, and culinary ingredients as well as standardized dietary patterns, eating habits, and chrononutrition features. Therefore, research in this field is still required, taking into account critical aspects of heterogeneity including type of population, minimum and maximum intakes and adverse effects, cooking methods, physiopathological status, and times of intervention. Moreover, the integrative analysis of traditional variables (age, sex, metabolic profile, clinical history, body phenotype, habitual dietary intake, physical activity levels, and lifestyle) together with individualized issues (genetic background, epigenetic signatures, microbiota composition, gene expression profiles, and metabolomic fingerprints) may contribute to the knowledge and prescription of more personalized treatments aimed to improving the precision medical management of inflammation as well as the design of anti-inflammatory diets in chronic and communicable diseases.
Keywords: Inflammation, Disease, Nutrition, Anti-inflammatory diets, Bioactive compounds, Personalized nutrition
Introduction
Inflammation is a pivotal component of innate immunity in response to endogenous and exogenous harmful stimuli (i.e., toxic chemicals, environmental agents, trauma, and pathogens/viral infection), which is physiologically challenged as a defense mechanism for injury removal and wound healing processes [1].
Based on timing and pathological features, inflammation can be acute and chronic [2]. Acute inflammation is usually of short duration (minutes to days) consisting of the migration of lymphocytes/neutrophils and macrophages to the inflammatory site, which stimulate the release of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and high motility group box-1 (HMGB-1) as well as cell aggregation and enzyme breakdown [3]. Moreover, the activation of NOD-like receptors (NLRs) such as NLRP3, NLRP1, and NLRC4 results in the recruitment of a highly regulated protein complex (known as inflammasome), whose activation initiates downstream inflammatory cytokine production, mainly interleukin 1 beta (IL-1β) and interleukin 18 (IL-18) in response to cellular stress [4]. Other intermediaries encompass chemokines, lipid mediators, acute-phase proteins such as C-reactive protein (CRP), transcriptional factors including the nuclear factor kappa B (NF-κB), and major immune cell types [5].
However, uncontrolled acute inflammation may become a permanent condition leading to expanded tissue damage, hemodynamic changes, and organ failure [6]. In fact, chronic inflammation has been linked to the development of non-communicable diseases such as obesity and associated comorbidities [7]. In this regard, obesity leads to abnormal fat accumulation in adipocytes, immune cell infiltration, and pro-inflammatory milieu that disrupt the insulin signaling cascade inducing insulin resistance [8]. Besides, inflammation and oxidative stress interactions are critical to understand the physiopathology of obesity, involving impairments of endoplasmic reticulum functions, hypoxia in the adipose tissue, mitochondrial alterations, and reactive oxygen species overproduction [9]. Furthermore, gut microbiota seems to be implicated in the development of obesity-related low-grade inflammation involving lipopolysaccharides translocation and toll-like receptor 4 (TLR-4) binding, which trigger a state of blood endotoxemia [10]. The resulting unresolved immune activation not only affects local tissues, but also systemic physiology that is termed meta-inflammation [11].
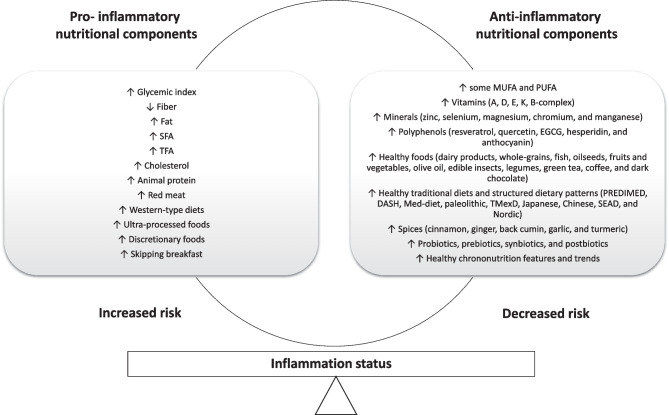
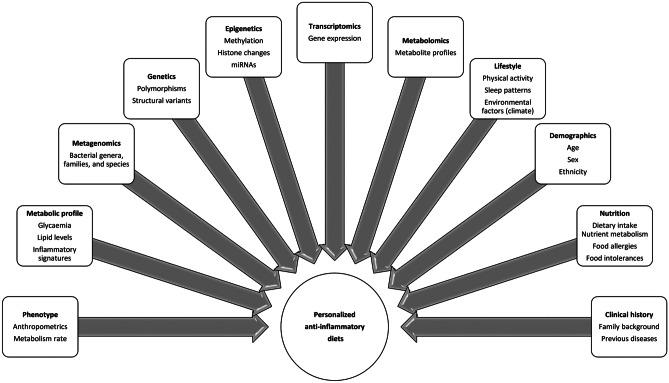
Therefore, it is relevant to identify the triggers that activate pro-inflammatory cascades in order to identify potential targets as well as implement precision intervention strategies for health care [12]. In this context, cumulative population-based evidence supports the role of nutrition on inflammatory pathways [13]. In this critical review, the effects and eventual mediation of different nutritional factors on inflammation are discussed, including specific nutrients (types of carbohydrates, protein sources, structural fatty acids, micronutrients, and trace elements) and bioactive compounds (polyphenols); staple-characterized dietary patterns (i.e., Western, Mediterranean, and Nordic diets); therapeutic diets (i.e., DASH approach); common culinary ingredients (species and herbs); and chrononutrition features (Fig. 1). Moreover, the potential of prescribing personalized anti-inflammatory diets based on social, phenotypical, clinical, genetic/epigenetic, and metabolic/metabolomic characteristics with a precision medicine scope is postulated (Fig. 2).
Fig. 1.
Nutritional factors associated with inflammatory outcomes in humans. DASH, Dietary Approaches to Stop Hypertension; EGCG, epigallocatechin-3-gallate; Med-diet, Mediterranean diet; MUFA, monounsaturated fatty acids; PREDIMED, Prevención con Dieta Mediterránea; PUFA, polyunsaturated fatty acids; SEAD, Southern European Atlantic Diet; SFA, saturated fatty acids; TFA, trans fatty acids; TMexD, traditional Mexican diet
Fig. 2.
Precision information for the prescription of personalized anti-inflammatory nutritional strategies
The Role of Nutrition on Inflammation
Macronutrients
Total Carbohydrates
Dietary carbohydrates exert differential effects on health depending on quantity and quality features [14]. Interestingly, a low-carbohydrate diet (20% of total energy) significantly improved the subclinical inflammatory state (lower serum levels of IL-1Ra and IL-6) in diabetic patients [15]. Notably, the adherence to a low-carbohydrate diet (35% of total energy) reduced the levels of inflammatory markers in women with obesity [16]. Also, it was found an overall favorable effect of a low-carbohydrate diet (≤ 30 g/day) on inflammation in subjects with severe obesity [17]. Additionally, a very low carbohydrate diet (12% of total energy) resulted in relevant reductions in inflammation compared to a low-fat diet (24% of total energy), as reported elsewhere [18].
Glycemic Index
The glycemic index (GI) has been devised to physiologically assess the carbohydrate quality from different foods based on effects on postprandial plasma glucose concentrations [19]. Interestingly, a high-GI diet (based on cooked vermicelli pasta, GI = 35) significantly increased the activation rates of NF-κB in mononuclear cells in lean healthy subjects [20]. Indeed, the negative metabolic and inflammatory responses induced by a high-GI diet (GI > 70) were counteracted by a low-GI diet (GI < 55) in diabetic patients [21]. Furthermore, findings from the DIOGenes trial revealed that low-GI carbohydrates (15 points of difference regarding high-GI carbohydrates) may reduce low-grade inflammation in subjects with overweight or obesity following a weight maintenance diet after weight loss [22].
Fiber
Dietary fiber may provide health benefits involving some immunological mechanisms [23]. Accordingly, fiber intake equal or more than 15 g/1000 kcal has been associated with decreased blood CRP levels in diabetic patients [24]. Accordingly, a randomized intervention trial demonstrated that fiber intake (30 g/day) from a diet naturally rich in fiber or from a supplement can substantially reduce the circulating levels of CRP in lean normotensive participants [25]. Moreover, significant inverse linear associations were detected between dietary fiber intake (mean 16.8 g/day) and CRP serum concentrations in middle age adults [26].
Total Fat
Dietary fat elicit a number of essential functions in the organism; however, excessive fat consumption may lead to obesity and related low-grade inflammatory processes [27]. Indeed, clinical evidence indicates that high-fat diets (i.e., nearly 75% of total energy) cause overproduction of circulating free fatty acids and systemic inflammation [28]. Consistently, a low-fat diet (25% of energy needs) was associated with lower plasma IL-6 levels in diabetic patients [29].
Saturated Fatty Acids
There is increasing evidence concerning the fact that dietary saturated fatty acids (SFAs) act as an important link between obesity and inflammation [30]. Interestingly, subjects consuming more than 10% energy as saturated dietary fat had increased serum levels of CRP compared to subjects having a normal saturated fat intake (< 7% of caloric intake) in young Asian Indians [31]. Likewise, ingestion of SFA (100 mL of dairy cream with 70% saturated fat content) resulted in lipid-induced increases in plasma CRP in women independently of obesity status [32].
Monounsaturated Fatty Acids
Monounsaturated fatty acids (MUFAs) are assumed as a healthy type of fat, being the oleic acid (OA) the most commonly consumed MUFA in daily nutrition [33]. In this context, a cross-sectional epidemiologic study in Japanese population reported a significant inverse relationship between the intake of OA (mean 6.94% of total energy) and serum CRP concentrations [34]. Further controlled trials with different doses of MUFA for the treatment of inflammatory features are warranted.
Polyunsaturated Fatty Acids
In the last years, a plethora of evidence has supported the beneficial effects of polyunsaturated fatty acids (PUFAs) in the prevention of cardiovascular and other chronic diseases with an inflammatory basis [35]. In this context, the intakes of the n-3 PUFA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were inversely associated with plasma levels of soluble TNF receptors 1 and 2 in healthy individuals [36]. Moreover, total dietary n-3 PUFAs inversely correlated with blood levels of CRP and IL-6 in women [37].
Additionally, some clinical trials have evaluated the effects of prescribing diets high in PUFA or through PUFA supplementation on inflammatory outcomes. For instance, fish oil supplementation (38.2 g/day EPA + DHA during 90 days) lowered the blood levels of pro-inflammatory markers in hypertensive patients [38]. Similarly, healthy young adults receiving n-3 PUFA (2.5 g/day, 2085 mg EPA and 348 mg DHA) for 12 weeks underwent a 14% decrease in serum IL-6 levels [39]. Likewise, low (1.25 g/day) or high (2.5 g/day) doses of n-3 PUFA supplementation for 4 months reduced inflammation responses (specifically serum IL-6 and TNF-α concentrations) in overweight adults [40].
Trans Fatty Acids
Trans fatty acids (TFAs) are mainly industrially formed by the hydrogenation of vegetable oils or from ruminant-derived foods including dairy products and meats [41]. TFA intake has been positively associated with plasma biomarkers of inflammation (including CRP, VCAM-1, E-selectin) in women [42]. In this same population, the consumption of TFA was positively associated with the plasma levels of soluble TNF receptors 1 and 2, mainly in women with higher body mass index [43]. Furthermore, serum CRP concentrations were elevated after TFA consumption (8% of total fat) in men [44].
Dietary Cholesterol
Excessive cholesterol may have deleterious effects on health including some processes affecting inflammation status [45]. For example, highest quartile of serum CRP concentrations (5.9 mg/L) was associated with higher intake of dietary cholesterol (189 mg/day) among Iranian adults [46]. Likewise, in a large representative Middle East population, positive correlations between dietary cholesterol and plasma CRP levels were found [47].
Protein Quantity and Quality
Both the quantity and quality of dietary protein are main determinants of nutritional values and body/endocrine homeostasis [48]. Among participants of the Framingham Heart Study Offspring cohort, dietary protein intake (particularly from plant sources) was inversely associated with serum markers of inflammation such as IL-6 and CRP [49]. Moreover, consuming high (30% of total energy) or low (10% of total energy) protein diets resulted in reduced blood CRP concentrations in morbidly obese individuals [50].
Regarding protein sources, diets characterized by higher intakes of animal protein (high levels of fatty and processed meats) were positively associated with certain blood pro-inflammatory markers such as CRP, IL-6, TNF-a, IL-8, serum amyloid A, and glycoprotein acetylation [51]. Furthermore, findings from the RESMENA dietary study (30% energy from protein) revealed positive associations between animal and meat protein intakes and inflammation, whereas vegetable- or fish-derived proteins had no significant influence on inflammatory status [52].
Micronutrients
Vitamins
Longitudinal and observational studies have shown some associations between dietary vitamin intakes and inflammatory features. For instance, consumption of vitamins C and E or carotene were inversely associated with the probability of having serum CRP concentrations > 3 mg/L in American adults [53]. In the cross-sectional KORA study, dose–response analyses revealed that participants, who regularly ingested more than 78 mg vitamin E/day, had 22% lower serum CRP levels than subjects who were not exposed to any extra vitamin E sources [54].
In addition, the intakes of dietary supplements containing vitamin E and C as well as B-complex vitamins (B1, B2, B3, B5, B6, B9, and B12) were associated with lower blood CRP levels in women [55]. Moreover, subjects in the upper tertile of changes in dietary vitamin K1 (phylloquinone) intake (after 1-year of follow-up) showed a greater reduction in IL-6 and TNF-α plasma concentrations than those in the lowest tertile group [56]. Also, dietary B5 vitamin intake was inversely related to serum CRP concentrations in healthy Korean adults [57]. Furthermore, participants who consumed > 310 mg/day of dietary choline (commonly grouped within the B-complex vitamins) had lower blood concentrations of CRP, IL-6, and TNF-α in healthy adults [58]. Findings of clinical trials exploring the impact of vitamins on inflammation status are systematically summarized (Table 1). Some studies have found relevant benefits on lowering inflammation after vitamin supplementation.
Table 1.
Clinical trials analyzing the anti-inflammatory effects of certain vitamins and bioactive compounds
| Vitamin | Study design | Dose/duration | Population | Main finding | Reference |
|---|---|---|---|---|---|
| A (retinyl palmitate) | RCT | 10 000 IU/week or placebo until 6 weeks postpartum |
Pregnant and lactating women: Experimental group (n = 48) Placebo group (n = 50) |
↑ IFN-gamma:IL10 ratio | [59] |
| A (retinyl palmitate) | RCT | 25,000 IU/day or placebo for 6 months |
Patients with multiple sclerosis: Experimental group (n = 18) Placebo group (n = 17) |
NS effects on IL-1β, TNF-α, IFN-γ, IL-2, IL-6, IL-17, IL-10, IL-13, IL-4, and TGF-β | [60] |
| A (retinyl palmitate) | RCT | 25,000 IU/day or placebo for 6 months |
Patients with multiple sclerosis Experimental group (n = 18) Placebo group (n = 17) |
↑ Plasma CRP | [61] |
| B1 | RCT | 3 capsules × 100 mg/day) or placebo for 6 weeks |
Hyperglycemic subjects Experimental group (n = 12) Placebo group (n = 12) |
NS effects on blood CRP | [62] |
| B2 | CIS | 100 mg/day for 3 weeks | Crohn's disease patients with low (n = 40) or high (n = 30) fecal calprotectin (FC) levels |
↓ Plasma IL-2 in low FC ↓ Plasma CRP in high FC |
[63] |
| C | RCT | 500 mg twice a day or free of supplements (control) for 8 weeks |
Adults with obesity and/or hypertension Experimental group (n = 31) Control group (n = 33) |
↓ Serum CRP and IL-6 | [64] |
| C | RCT | 250 mg three times per week or free of supplements (control) for 2 months |
Chronic hemodialysis patients Experimental group (n = 19) Control group (n = 14) |
NS effects on blood CRP | [65] |
| C | RCT |
200 mg/day for 3 months and no treatment in the next 3 months (group 1) No treatment in the first 3 months and 200 mg/day for the next 3 months (group 2) |
Hemodialysis patients Group 1 (n = 48) Group 2 (n = 52) |
↓ Plasma CRP after supplementation, but returned to basal state after supplementation was withdrawn | [66] |
| C | RCT | 1000 mg/day or placebo for 2 months |
Healthy nonsmokers subjects Experimental group (n = 128) Placebo group (n = 138) |
↓ Blood CRP among individuals with CRP > or = 1.0 mg/L | [67] |
| C | RCT | 12 g/50 mL (intravenous) every 12 h or placebo for 7 days |
Critical patients with COVID-19 Experimental group (n = 27) Placebo group (n = 29) |
↓ Serum IL-6 | [68] |
| Choline | RCT | ~ 400 mg choline/day from eggs or supplement sources for 4 weeks | Subjects with metabolic syndrome (n = 23) |
↓ Serum IL-6 with both sources of choline ↓ Blood CRP only with eggs |
[69] |
| D | RCT | 300,000 IU or placebo for 90 days |
Patients with ulcerative colitis Experimental group (n = 46) Placebo group (n = 40) |
↓ Blood CRP and ESR | [70] |
| D | RCT | 100,000 IU bolus followed by 4000 IU daily or matching placebo for 16 weeks |
Adults with overweight or obesity Experimental group (n = 28) Placebo group (n = 26) |
NS effects on serum levels of CRP, TNF, MCP-1, IFN-α, IL-1β, IL-6, IL-8, IL-10, IL-12, IL-18, IL-23, IL-33 NS effects on NFκB activity in PBMCs |
[71] |
| D | RCT | 2000 IU/day plus low-calorie diet or placebo plus low calorie diet for 8 weeks |
Adults with overweight or obesity Experimental group (n = 30) Placebo group (n = 29) |
↓ Plasma CRP and sICAM-1 | [72] |
| D | RCT | 50,000 IU/day plus low-calorie diet or placebo plus low-calorie diet for 12 weeks |
Adults with obesity Experimental group (n = 22) Placebo group (n = 22) |
↓ Serum IL-1β and TLR-4 | [73] |
| D | RCT | 7000 IU/day or placebo for 26 weeks |
Adults with obesity Experimental group (n = 26) Placebo group (n = 26) |
NS effects on blood CRP, IL-6, MCP-1, PAI-1, MMP-9, adiponectin, and leptin | [74] |
| D3 | RCT | 1000 IU/day (group 1); 2000 IU/day (group 2); or 4000 IU/day (group 3); or placebo for 3 months |
African Americans Group 1 (n = 67) Group 2 (n = 76) Group 3 (n = 78) Placebo (n = 71) |
NS effects on blood CRP, IL-6, IL-10, and sTNF-R2 | [75] |
| D3 | RCT | 200,000 IU/day or placebo for 4 weeks |
Women with overweight or obesity Experimental group (n = 14) Placebo group (n = 15) |
↑ Plasma CRP and MDA | [76] |
| D3 | RCT | 200,000 IU/day or placebo for 4 weeks |
Elderly women with vitamin D insufficiency Experimental group (n = 20) Placebo group (n = 20) |
↓ Plasma CRP and AGP-A NS effects on blood MDA |
[77] |
| D3 | RCT | 750 μg/day (group 1); 1500 μg/day (group 2); or placebo for 12 months |
Older adults Group 1 (n = 215) Group 2 (n = 215) Placebo (n = 214) |
NS effects on blood CRP, IL-10, leptin, and adiponectin ↑ IL-6 in group 2 vs. placebo |
[78] |
| E | RCT | 1200 IU/day or placebo for 12 weeks |
Patients with diabetic nephropathy Experimental group (n = 30) Placebo group (n = 30) |
↓ Plasma TNF-α, MDA, MMP-2, MMP-9, and AGEs | [79] |
| E | RCT | 600 IU/day or placebo for 10 weeks |
Hemodialysis patients Experimental group (n = 25) Placebo group (n = 24) |
↓ ICAM-1 and VCAM-1 NS effects on serum CRP and IL-6 |
[80] |
| Folic acid | RCT | 5 mg/day or placebo for 4 weeks |
Healthy cigarette smokers Experimental group (n = 12) Placebo group (n = 12) |
↓ Plasma homocysteine, fibrinogen, and WCC | [81] |
| Folic acid | RCT | 2.5 mg/day or placebo for 3 months |
Healthy overweight volunteers Experimental group (n = 30) Placebo group (n = 30) |
↓ Serum homocysteine, IL-8, MCP-1, and CRP | [82] |
| Folic acid | RCT | 0.8 mg/day for 1 year or placebo |
Men and postmenopausal women with homocysteine concentrations of 1.8 mg/L or higher Experimental group (n = 264) Placebo group (n = 266) |
↓ Serum homocysteine NS effects on plasma CRP and sICAM-1 |
[83] |
| Folic acid | RCT | 1.25 mg/day or free of supplement (control) for 6 months |
Patients with Alzheimer’s disease Experimental group (n = 61) Control group (n = 60) |
↓ TNFα protein and mRNA levels | [84] |
| Folic acid | RCT | 400 μg/day or free of supplement (control) for 12 months |
Patients with mild cognitive impairment Experimental group (n = 84) Control group (n = 84) |
↓ Blood homocysteine, IL-6 and TNF-α | [85] |
| Folic acid plus vitamin B12 | RCT | Folic acid 1.2 mg/day plus vitamin B12 50 μg/day or placebo for 6 months |
Patients with Alzheimer’s disease Experimental group (n = 60) Placebo group (n = 60) |
↓ Serum homocysteine and TNF-α | [86] |
| Folic acid plus vitamin B12 | RCT |
400 µg/day folic acid (group 1); 25 µg/day vitamin B12 (group 2); 400 µg/day folic acid plus 25 µg vitamin B12 (group 3); or free of supplement (control) for 6 months |
Participants with mild cognitive impairment Group 1 (n = 60) Group 2 (n = 60) Group 3 (n = 60) Control (n = 60) |
↓ Blood homocysteine, IL-6, TNF-α, and MCP-1 (group 3 vs. control) | [87] |
| Folic acid plus vitamin B12 | RCT | Folic acid (400 μg/day) plus vitamin B12 (500 μg/day) or placebo for 2 years |
Elderly subjects with hyperhomocysteinemia Experimental group (n = 271) Placebo group (n = 251) |
NS effects on plasma CRP, ICAM-1, VCAM-1, VEGF, and SAA | [88] |
| Folic acid plus vitamin B12 plus vitamin B6 | RCT | Daily folic acid (2.5 mg), vitamin B6 (50 mg), vitamin B12 (1 mg), or matching placebo for 7.3 years |
Women at increased risk of CVD Experimental group (n = 150) Placebo group (n = 150) |
↓ Serum homocysteine NS effects on blood CRP, IL-6, fibrinogen, and ICAM‐1 |
[89] |
| Folic acid plus vitamin B12 plus vitamin B6 | RCT | Group 1: folic acid (0.8 mg/day)/vitamin B12 (0.4 mg/day)/vitamin B6 (40 mg/day); group 2: folic acid (0.8 mg/day)/vitamin B12 (0.4 mg/day); group 3: vitamin B6 (40 mg/day); or placebo for 6 months |
Patients with suspected coronary artery disease Group 1 (n = 22) Group 2 (n = 23) Group 3 (n = 21) Placebo (n = 24) |
↓ Serum homocysteine (groups 1 and 2) NS effects on blood CRP, IL-6, neopterin, and sCD40L |
[90] |
| K1 (phylloquinone) | RCT | 10 mg/day or placebo for 8 weeks |
Patients with definitive rheumatoid arthritis Experimental group (n = 29) Placebo group (n = 29) |
NS effects on plasma IL-6 | [91] |
| K1 (phylloquinone) | RCT | 500 μg/day or placebo during two periods of 6 weeks of duration | Postmenopausal women (n = 31) | NS effects on blood IL-6, CRP, sICAM-1, sVCAM-1 | [92] |
RCT ranzomized controlled trial, CIS clinical intervention study, NS no significant, FC fecal calprotectin, IU international units, MDA malondialdehyde, NF-kB transcription nuclear factor kappa B, CRP C-reactive protein, IL-6 interleukin 6, TNF-α, tumor necrosis factor alpha, IL-8 interleukin 8, IL-4 interleukin 4, IL-10 interleukin 10, IL-2 interleukin 2, IL-1β interleukin-1β, TLR-4 toll-like receptor 4, sTNF-R2 serum soluble tumor necrosis factor receptor 2, IFN-γ interferon gamma; IL-17 interleukin 17, IL-18 interleukin 18, IL-13 interleukin 13, IL-23 interleukin 23, IL-33 interleukin 33, TGF-β transforming growth factor β, ESR erythrocyte sedimentation rate, TNF tumor necrosis factor, MCP-1 monocyte chemoattractant protein-1, IFN-α interferon alpha, PBMCs peripheral blood mononuclear cells, ICAM-1 intercellular adhesion molecule-1, sICAM-1 soluble intercellular adhesion molecule-1, VCAM-1 vascular cell adhesion molecule 1, sVCAM-1 soluble vascular cell adhesion molecule 1, PAI-1 plasminogen activator inhibitor-1, MMP-2 matrix metalloproteinase-2, MMP-9 matrix metalloproteinase-9, AGP-A alpha 1-acid glycoprotein, AGEs advanced glycation endproducts, WCC white cell count, SAA serum amyloid A, VEGF vascular endothelial growth factor; sCD40L soluble CD40 ligand
Minerals and Trace Elements
Minerals and trace elements are essential for structural, immunological, and metabolic functions in the human organism [93]. In this regard, high magnesium consumption was related to lower plasma concentrations of potential markers of systemic inflammation (CRP, sTNF-R2, and IL-6) in postmenopausal women [94]. Similarly, magnesium intake from dietary sources was found to be inversely correlated with plasma IL-6 in women from the Nurses’ Health Study cohort [95]. Likewise, a nested case–control study reported an opposite association concerning dietary manganese and circulating levels of serum pro-inflammatory cytokines in postmenopausal women [96]. Also, changes in lymphocyte proliferation and IL-2R expression have been reported to be early markers of mild zinc dietary deficiency in healthy men [97]. In addition, dietary copper intake has been directly associated with blood CRP concentrations in adults [98]. In turn, iron deficit may be exacerbated by the effects of obesity-related inflammation on gut iron absorption [99]. Furthermore, main outcomes regarding the anti-inflammatory effects of supplementation with certain minerals on humans are shown (Table 2).
Table 2.
Clinical trials analyzing the anti-inflammatory effects of certain minerals
| Mineral | Study design | Dose/duration | Population | Main finding | Reference |
|---|---|---|---|---|---|
| Chromium | RCT | 200 μg/day or placebo for 8 weeks |
Patients with PCOS Experimental group (n = 30) Placebo group (n = 30) |
↓ Plasma CRP and MDA | [100] |
| Chromium | RCT | 200 μg/day or placebo for 8 weeks |
20 patients with PCOS who were candidate for in vitro fertilization Experimental group (n = 20) Placebo group (n = 20) |
↓ Blood CRP ↓ Gene expression of IL-1 in PBMCs NS effects on gene expression of IL-8, TNF-α, TGF-β, and VEGF of in PBMCs |
[101] |
| Chromium | RCT | 400 μg/day or placebo for 3 months |
Patients with NAFLD Experimental group (n = 23) Placebo group (n = 23) |
↓ Serum TNF-α, CRP, and IL-6, and fetuin-A NS effects on blood IL-17 |
[102] |
| Chromium | RCT | 400 μg/day (chromium picolinate); 400 μg/day (chromium dinicocysteinate; or placebo for 3 months |
Patients with T2DM Chromium picolinate (n = 12) Chromium dinicocysteinate (n = 18) Placebo (n = 13) |
↓ Plasma TNF-α (chromium dinicocysteinate vs. placebo) | [103] |
| Chromium | RCT | 1000 μg/day or placebo for 16 weeks |
Patients with metabolic syndrome Experimental group (n = 33) Placebo group (n = 30) |
NS effects on blood CRP | [104] |
| Chromium | RCT | 400 μg/day (chromium picolinate); 400 μg/day (chromium dinicocysteinate; or placebo for 3 months |
Patients with T2DM Chromium picolinate (n = 25) Chromium dinicocysteinate (n = 24) Placebo (n = 25) |
↓ Serum TNF-α (chromium dinicocysteinate vs. baseline) | [105] |
| Chromium + magnesium + zinc | RCT | 300 mg/day magnesium plus 600 μg/day chromium plus 36 mg/day zinc or placebo for 24 weeks |
Adults with metabolic syndrome Experimental group (n = 16) Placebo group (n = 16) |
↓ Plasma CRP | [106] |
| Copper | RCT | 2 mg/day or placebo for 8 weeks |
Adults with moderately high cholesterol Experimental group (n = 35) Placebo group (n = 35) |
NS effects on serum CRP and homocysteine | [107] |
| Iron | RCT | 50 g/day meat; 20 g/day of fortified rice cereal (1.10 mg of iron); or 20 g/day of local rice cereal (0.04 mg of iron) for 1 year |
6-month-old infants Meat group (n = 137) Fortified cereal group (n = 140) Local cereal group (n = 133) |
↑ Plasma CRP and AGP-A | [108] |
| Iron | RCT | 50 mg for 4 day/week or identical placebo for 38 weeks |
Children with iron deficiency Experimental group (n = 22) Placebo group (n = 27) |
NS effects on gut inflammation (measured by fecal calprotectin concentration) | [109] |
| Iron | RCT | 6 mg/kg/day iron plus placebo; or 6 mg/kg/day plus 18 mg/day vitamin E for 8 weeks |
Iron-deficient infants and toddlers Iron plus placebo (n = 22) Iron plus vitamin E (n = 14) |
NS effects on gut inflammation (measured by fecal calprotectin concentration) and blood levels of TNF-α and IL-4 | [110] |
| Magnesium | RCT | 500 mg/day or placebo for 4 weeks |
Healthy overweight volunteers Experimental group (n = 7) Placebo group (n = 7) |
NS effects on plasma CRP, IL-6, and TNF-α, sICAM-1, sVCAM-1, and E-selectin ↓ Gene expression of C1q, C1QTNF9, and PPBP |
[111] |
| Magnesium | RCT | 250 mg/day or placebo for 8 weeks |
Middle-aged overweight women Experimental group (n = 35) Placebo group (n = 34) |
NS effects on serum CRP, IL-6, and fibrinogen | [112] |
| Magnesium | RCT | 320 mg/day or placebo for 7 weeks |
Adults with poor sleep quality Experimental group (n = 46) Placebo group (n = 49) |
↓ Blood CRP in participants with baseline values > 3.0 mg/L | [113] |
| Magnesium | RCT | 300 mg/day or placebo for 6 months |
COPD patients Experimental group (n = 25) Placebo group (n = 24) |
↓ Plasma CRP NS effects on serum TNF-α |
[114] |
| Magnesium | RCT | 30 ml of MgCl(2) 5% solution (equivalent to 382 mg of magnesium) per day or placebo for 3 months |
Subjects with prediabetes and hypomagnesemia Experimental group (n = 13) Placebo group (n = 13) |
NS effects on CRP, IL-6, TNF-α, and IL-10 levels | [115] |
| Magnesium | RCT | 30 mL of MgCl2 5% solution (equivalent to 382 mg of magnesium) per day or placebo for 3 months |
Subjects with new diagnosis of prediabetes and hypomagnesemia Experimental group (n = 29) Placebo group (n = 28) |
↓ Blood CRP | [116] |
| Magnesium and zinc | RCT | 250 mg of magnesium oxide plus 220 mg of zinc sulfate or placebo twice a day for 12 weeks |
Subjects with PCOS Experimental group (n = 30) Placebo group (n = 30) |
↓ Plasma CRP and protein carbonyl ↓ Gene expression of IL-1 and TNF-α |
[117] |
| Na and K | RCT | Supplemental Na (3.0 g/day); supplemental K (2.8 g/day) or placebo for 4 weeks | Pre-hypertensive patients (n = 36) | ↓ Blood IL-8 (K supplementation) | [118] |
| Selenium | RCT | 200 µg/day or placebo for 12 weeks |
Patients with diabetic nephropathy Experimental group (n = 30) Placebo group (n = 30) |
NS effects on plasma CRP, TGF-β, AGEs, protein carbonyl, and MDA | [119] |
| Selenium | RCT | 200 µg/day or placebo for 12 weeks |
Patients with CHF Experimental group (n = 26) Placebo group (n = 27) |
NS effects on serum CRP | [120] |
| Selenium | RCT | 200 µg/day or placebo for 12 weeks |
Hemodialysis patients Experimental group (n = 40) Placebo group (n = 40) |
↓ Blood MDA and IL-6 NS effects on homocysteine, ferritin, and transferrin |
[121] |
| Selenium | RCT | 200 µg/day or placebo for 6 weeks |
Pregnant women with GDM Experimental group (n = 35) Placebo group (n = 35) |
↓ Blood CRP and MDA | [122] |
| Selenium | RCT | 200 μg/day or placebo for 8 weeks |
Women with PCOS Experimental group (n = 32) Placebo group (n = 32) |
↓ Serum CRP and MDA | [123] |
| Selenium | RCT | 200 μg/day or placebo for 4 weeks |
17 patients undergoing CABG surgery Experimental group (n = 17) Placebo group (n = 16) |
↓ Plasma CRP and MDA | [124] |
| Selenium | RCT | 200 μg/day or placebo for 8 weeks |
Patients with T2DM and CHD Experimental group (n = 30) Placebo group (n = 30) |
↓ Blood CRP | [125] |
| Selenium | RCT | 200 μg or placebo twice daily for 14 days |
Patients undergoing HSCT Experimental group (n = 37) Placebo group (n = 37) |
NS effects on serum TNF-α, IL-1, and IL-6 | [126] |
| Zinc | RCT | 30 mg/day or placebo for 12 weeks |
Women with premenstrual syndrome Experimental group (n = 30) Placebo group (n = 30) |
NS effects on serum CRP | [127] |
| Zinc | RCT | 45 mg/day or placebo for 6 months |
Healthy elderly subjects Experimental group (n = 20) Placebo group (n = 20) |
↓ Blood CRP, IL-6, MCP-1, VCAM-1, MDA, and secretory phospholipase A2 ↓ TNF-α, IL-1β, VCAM-1, and NF-kB activity in THP-1 cells and human aortic endothelial cells ↑ Anti-inflammatory proteins A20 and PPAR-α in THP-1 cells and human aortic endothelial cells |
[128] |
| Zinc | RCT | 15 mg/day or placebo for 12 weeks |
Women with migraine Experimental group (n = 30) Placebo group (n = 30) |
NS effects on plasma CRP | [129] |
| Zinc | RCT | 25 mg/day or placebo for 3 months |
18 adult SCD patients Experimental group (n = 18) Placebo group (n = 18) |
↓TNF-α and IL-1β mRNAs in MNCs ↓ NF-kB binding in MNCs ↑ IL-2 and IL-2Ralpha mRNAs in phytohemagglutinin-p-stimulated MNCs |
[130] |
| Zinc | RCT | 25 mg/day or placebo for 12 weeks |
Patients with major depression Experimental group (n = 20) Placebo group (n = 17) |
NS effects on serum IL-6 and TNF-α | [131] |
| Zinc | RCT | 30 mg/day or placebo for 8 weeks |
Women with obesity Experimental group (n = 20) Placebo group (n = 20) |
↓ Blood CRP and IL-6 NS effects on serum leptin and adiponectin levels |
[132] |
| Zinc | RCT | 30 mg/day or placebo for 15 weeks |
Subjects with obesity Experimental group (n = 18) Placebo group (n = 22) |
↓ Plasma CRP | [133] |
| Zinc | RCT | 50 mg/day or placebo for 8 weeks |
24 women with PCOS Experimental group (n = 24) Placebo group (n = 24) |
NS effects on serum CRP | [134] |
| Zinc | RCT | 20 mg/day or placebo for 8 weeks |
Prepubescent children with metabolic syndrome Experimental group (n = 30) Placebo group (n = 30) |
↓ Blood CRP | [135] |
RCT randomized controlled trial, NS no significant, PCOS polycystic ovary syndrome, COPD chronic obstructive pulmonary disease, SCD sickle-cell disease, CHF congestive heart failure, GDM gestational diabetes mellitus, CABG coronary artery bypass grafting surgery, T2DM type 2 diabetes mellitus, CHD coronary heart disease, NAFLD non-alcoholic fatty liver disease, HSCT hematopoietic stem cell transplantation, CRP C-reactive protein, IL-6 interleukin 6, TNF-α tumor necrosis factor alpha, IL-8 interleukin 8, IL-10 interleukin 10, IL-1 interleukin 1, IL-1β interleukin-1β, IL-2 interleukin 2, IL-4 interleukin 4, MDA malondialdehyde, PBMCs peripheral blood mononuclear cells, TGF-β transforming growth factor beta, VEGF vascular endothelial growth factor, IL-17 interleukin 17, AGP-A alpha 1-acid glycoprotein, C1QTNF9 tumor necrosis factor related protein 9, PPBP pro-platelet basic protein, TGF-β transforming growth factor β, AGEs advanced glycation endproducts, MCP-1 monocyte chemoattractant protein-1, VCAM-1 vascular cell adhesion molecule 1, NF-kB transcription nuclear factor kappa B, PPAR-α peroxisome proliferator-activated receptor-alpha, MNCs mononuclear cells
Bioactive Compounds
Polyphenols
Polyphenols are a large family of bioactive molecules widely occurring in plant-based foods, with potent antioxidant and anti-inflammatory properties [136]. In this context, it was reported that total flavonoid intake was inversely associated with serum CRP concentration in American adults [137]. Also, inverse relationships between flavanone consumption and blood IL-6 concentrations were reported in a multiethnic cohort [138]. Likewise, higher isoflavone intake (highest quartile = 1.61–78.8 mg/day) was related to lower plasma CRP in healthy premenopausal women [139]. Besides, lower serum levels of IL-8 were found among women showing high intakes of flavones, flavanones, and total flavonoids (highest quintiles = 264 ng/L, 273 ng/L, and 276 ng/L, respectively) [140]. Moreover, elevated total flavonoids intake and tea consumption inversely correlated with CRP levels in Taiwanese [141]. Of note, a number of randomized clinical trials have tested the anti-inflammatory potential of several polyphenols, whose results are summarized (Table 3).
Table 3.
Clinical trials analyzing the anti-inflammatory effects of certain polyphenols
| Polyphenol | Study design | Dose/duration | Population | Main finding | Reference |
|---|---|---|---|---|---|
| Anthocyanin | RCT | 40 mg/day (group 1); 80 mg/day (group 2); 320 mg/day (group 3); or placebo for 12 weeks |
Patients with dyslipidemia Group 1 (n = 44) Group 2 (n = 40) Group 3 (n = 42) Placebo (n = 43) |
↓ Blood TNF-α and IL-6 (group 2 vs. baseline) ↓ Blood TNF-α and IL-6, and MDA (group 3 vs. groups 1 and 2) |
[142] |
| Anthocyanin | OLCT | 320 mg/day for 4 weeks |
Patients with T2DM (n = 12) Patients at risk of T2DM (n = 14) Healthy individuals (n = 14) |
↓ Plasma TNF-α, IL-6, and IL-18 (patients with TD2M, pre vs. post intervention) NS effects on IL-1Rα, leptin, IL-8, and CRP in any group (pre vs. post intervention) |
[143] |
| Anthocyanin | RCT | 20 mg/day (group 1); 40 mg/day (group 2); 80 mg/day (group 3); 160 mg/day (group 4); 320 mg/day (group 5); or placebo for 14 days |
Healthy young adults Group 1 (n = 20) Group 2 (n = 19) Group 3 (n = 19) Group 4 (n = 19) Group 5 (n = 19) Placebo (n = 15) |
↓ Blood IL-10 (group 4 and 5 vs. placebo) ↓ IL-6 (groups 2 and 5 vs. placebo) NS effects on TNF-α in any group |
[144] |
| Anthocyanin | RCT | 640 mg/day or placebo for 4 weeks |
Pre-hypertensive men Experimental group (n = 16) Placebo group (n = 15) |
NS effects on serum CRP, IL-6, TNF-α, IL-4, MCP-1, P-selectin, ICAM, VCAM, and CD40L | [145] |
| Anthocyanin | RCT | 320 mg/day or placebo for 28 days |
Sedentary subjects Experimental group (n = 16) Placebo group (n = 16) |
NS effects on blood CRP | [146] |
| EGCG | RCT | 2 tablets 300 mg/day or placebo for 2 months |
Patients with T2DM Experimental group (n = 25) Placebo group (n = 25) |
NS changes in serum IL-6 | [147] |
| EGCG | RCT | 300 mg/day or placebo for 12 weeks |
Pre-menopausal women with obesity Experimental group (n = 43) Placebo group (n = 40) |
NS effects on blood CRP | [148] |
| Hesperidin | RCT | 500 mg or placebo twice daily for 12 weeks |
Patients with metabolic syndrome Experimental group (n = 25) Placebo group (n = 24) |
↓ Plasma TNF-α | [149] |
| Hesperidin | RCT | 600 mg/day or placebo for 4 weeks |
Patients with myocardial infarction Experimental group (n = 38) Placebo group (n = 37) |
NS effects on serum CRP, IL-6, and leptin | [150] |
| Hesperidin | RCT | 500 mg/day or placebo for 6 weeks |
Patients with T2DM Experimental group (n = 32) Placebo group (n = 32) |
↓ Blood TNF-α, CRP, and IL-6 | [151] |
| Hesperidin | RCT | 30 g/day flaxseed plus lifestyle (group 1); 1 g/day hesperidin plus lifestyle (group 2); 30 g/day flaxseed plus 1 g/day hesperidin plus lifestyle (group 3); or lifestyle alone (control) for 12 weeks |
Patients with NAFLD Group 1 (n = 24) Group 2 (n = 22) Group 3 (n = 25) Control (n = 21) |
↓ NF-κB (groups 1 and 2 vs. control) ↓ Plasma CRP (groups 2 and 3 vs. control) |
[152] |
| Hesperidin | RCT | 1 g/day plus lifestyle or placebo (only lifestyle) for 12 weeks |
Patients with NAFLD Experimental group (n = 25) Placebo group (n = 25) |
↓ Serum TNF-α, CRP, and NF-κB | [153] |
| Quercetin | RCT | 500 mg/day or placebo for 8 weeks |
Post-myocardial infarction patients Experimental group (n = 44) Placebo group (n = 44) |
NS changes in blood IL-6, CRP, and TNF-α | [154] |
| Quercetin | RCT | 500 mg/day of quercetin plus 250 mg/day vitamin C (group 1); 500 mg/day of quercetin alone (group 2); 250 mg/day of vitamin C alone (group 3); or placebo for 8 weeks |
Subjects with systematic and regular exercise Group 1 (n = 15) Group 2 (n = 15) Group 3 (n = 15) Placebo (n = 15) |
↓ Plasma IL-6 and F2-isoprostane (group 1 vs. placebo) | [155] |
| Quercetin | RCT |
100 mg/day (-)-epicatechin (group 1); 160 mg/day quercetin-3-glucoside (group 2); or placebo for 4 weeks |
Pre-hypertensive adults Group 1 (n = 37) Group 2 (n = 37) Placebo (n = 37) |
↓ Serum IL-1β (group 2 vs. placebo) | [156] |
| Quercetin | RCT | 500 mg/day or placebo for 8 weeks |
Women with rheumatoid arthritis Experimental group (n = 25) Placebo group (n = 25) |
↓ Blood TNF-α | [157] |
| Quercetin | RCT | 500 mg/day (group 1); 1000 mg/day (group 2); or placebo for 12 weeks |
Women Group 1 (n = 38) Group 2 (n = 40) Placebo (n = 42) |
NS effects on plasma IL-6, TNF-α, and blood leucocyte subsets (groups 1 and 2 vs. placebo) | [158] |
| Quercetin | RCT | 50 mg/day (group 1); 100 mg/day (group 2); or 150 mg/day (group 3) for 2 weeks |
Healthy volunteers Group 1 (n = 11) Group 2 (n = 12) Group 3 (n = 11) |
NS effects on serum TNF-α | [159] |
| Quercetin | RCT | 500 mg/day or placebo for 8 weeks |
Women with rheumatoid arthritis Experimental group (n = 20) Placebo group (n = 20) |
NS effects on blood CRP, MDA, and ox-LDL | [160] |
| Quercetin | RCT | 162 mg/day or placebo for 6 weeks |
Overweight-to-obese subjects with pre-hypertension Experimental group (n = 68) Placebo group (n = 68) |
NS effects on serum CRP and TNF-α | [161] |
| Resveratrol | RCT | 480 mg/day or placebo for 4 weeks |
Patients with T2DM and chronic periodontitis Experimental group (n = 21) Placebo group (n = 22) |
NS effects on blood IL-6 and TNF-α | [162] |
| Resveratrol | RCT | 500 mg/day or placebo for 12 weeks |
Patients with NAFLD Experimental group (n = 25) Placebo group (n = 25) |
↓ Plasma IL-6, CRP, cytokeratin-18, and NF-κB | [163] |
| Resveratrol | RCT | 500 mg/day or placebo for 6 weeks |
Patients with ulcerative colitis Experimental group (n = 25) Placebo group (n = 25) |
↓ Serum TNF-α, CRP, and NF-κB | [164] |
| Resveratrol | RCT | 500 mg/day or placebo for 30 days |
Adult smokers Experimental group (n = 25) Placebo group (n = 25) |
↓ CRP | [165] |
| Resveratrol | RCT | 800 mg/day or placebo for 8 weeks |
Patients with T2DM Experimental group (n = 25) Placebo group (n = 20) |
NS effects on blood TNF-α, IL-6, CRP, and IL-1β NS effects on expression of genes NF-κB, TLR2, and TLR4 |
[166] |
| Resveratrol | RCT | 500 mg/day or placebo for 4 weeks |
CKD patients Experimental group (n = 9) Placebo group (n = 11) |
NS changes in plasma TNF-α, CRP, IL-6, and NF-κB | [167] |
RCT randomized controlled trial, OLCT open-label clinical trial, NS no significant, CKD chronic kidney disease, NAFLD non-alcoholic fatty liver disease, T2DM type 2 diabetes mellitus, EGCG epigallocatechin-3-gallate, CRP C-reactive protein, IL-6 interleukin 6, TNF-α, tumor necrosis factor alpha, IL-4 interleukin 4, IL-8 interleukin 8, IL-18 interleukin 18, IL-1β interleukin-1β, NF-kB transcription nuclear factor kappa B, IL-1Rα interleukin-1R alpha, MCP-1 monocyte chemotactic protein, ICAM intercellular adhesion molecule, VCAM vascular cell adhesion molecule, CD40L CD40 ligand, MDA malondialdehyde, ox-LDL oxidized low density lipoprotein, TLR2 toll-like receptor 2, TLR4 toll-like receptor 4
Specific Foods
Red Meat
Concerning implications on inflammation, greater total (median 54 g/day), unprocessed (median 47 g/day), and processed red meat intakes (median 4 g/day) have been associated with unfavorable plasma concentrations of inflammatory biomarkers (including CRP and ferritin) in women [168]. Within the Multiethnic Cohort Study, red and processed meat consumption positively correlated with serum CRP levels [169]. Likewise, the consumption of processed meat was associated with increased levels of serum CRP (difference of 38% for each 50 g/day higher intake) in British adults [170]. In adjusted models, red meat consumption was significantly associated with blood CRP in a large American sample [171].
Dairy Products
In a cross-sectional study in Brazilians, increased yogurt consumption (median 10 g/day) appeared to exert an anti-inflammatory effect, whereas cheese consumption (median 10.7 g/day) could have potentiated a pro-inflammatory status [172]. In normal-weight adolescents, total dairy product and milk intakes were inversely associated with the serum concentrations of IL-6 [173]. Also, findings from the ATTICA study showed lower blood levels of CRP, IL-6, and TNF-α among individuals weekly consuming between 11 and 14 servings of dairy products compared to those consuming fewer than 8 servings per week [174].
Fish
Findings from the ATTICA study revealed independent associations between habitual fish consumption (> 300 g of fish per week) and lower inflammatory marker levels among healthy adults, including reduced CRP, IL-6, TNF-a, serum amyloid A, and white blood cell counts [175]. During a 6-year follow-up, fish consumption (about 100 g/week) decreased endothelial dysfunction and low-grade inflammation in healthy adults [176]. Furthermore, a high frequency of fish intake was associated with lower peripheral white blood cell counts (a marker of chronic inflammation) in an apparently healthy Japanese population [177]. In fact, the neutrophil/lymphocyte ratio (a marker of systemic inflammation) significantly decreased as the weekly frequency of fish intake (0 day, 1–2 days, 3–4 days, or 5–7 days) increased [178].
Edible Insects
In recent years, edible insects are being recognized as high-value food products with anti-inflammatory and antioxidant properties [179]. For example, cricket consumption (25 g/day) was associated with reduced systemic inflammation via microbiota modulation in healthy adults [180]. However, more research in humans is required to confirm these findings in order to recommend the habitual consumption of edible insects as an anti-inflammatory therapy.
Fruits and Vegetables
Inverse associations were found between the intakes of fruits and vegetables and serum CRP levels in Iranian females [181]. Besides, Chinese women consuming high amounts of cruciferous vegetables (highest quintile > 140.6 g/day) showed decreased circulating levels of TNF-α, IL-1β, and IL-6 [182]. In a randomized crossover trial, the consumption of cruciferous vegetables (14 g/kg body weight) during 14 days consistently reduced circulating IL-6 in in healthy young adults [183].
Oilseeds and Extra Virgin Olive Oil
In the multi-ethnic study of atherosclerosis, frequent nut and seed consumption (especially five or more times/week) was associated with lower levels of inflammatory markers, including IL-6 and CRP [184]. Moreover, in two large cohorts of Americans, subjects with a nut intake of five or more times/week had significantly lower blood concentrations of CRP and IL-6 compared to individuals in the frequency categories of never or almost never [185]. Likewise, a systematic review and meta-analysis of randomized controlled trials revealed that intakes of flaxseed and associated nutritional derivatives systematically reduced circulating CRP levels in obese subjects [186]. Using the same approach, acute high-oleic peanut consumption systematically led to stronger moderation of postprandial TNF-α concentrations in overweight/obese men [187].
Additionally, it has been shown that the incorporation of almonds (56 g/day during 4 weeks) into a healthy diet could improve inflammation and oxidative stress in Chinese diabetic patients [188]. A randomized trial also found that the consumption of almonds (10–20% isoenergetic replacement of control diet with almonds for 4 weeks) lowered serum CRP levels in healthy adults [189]. Indeed, plasma levels of TNF-α and IL-6 decreased after almond feeding (56 g/day for 90 days) in adolescents and young adults [190].
Furthermore, it has been reported that a daily dose of 50 mL of extra virgin olive oil (EVOO), administered over two periods of 3 weeks, decreased the plasma levels of IL-6 and CRP in stable coronary heart disease patients [191]. Besides, EVOO (50 mL) exerted acute postprandial anti-inflammatory and antioxidant effects in normolipemic, healthy subjects [192].
Cereals and Whole Grains
Interestingly, the intake of cereal fiber was inversely associated with lower blood levels of CRP and TNF-R2 in diabetic women [193]. Moreover, concomitant reductions in serum TNF-α and increased plasma IL-10 were found after the consumption of whole-grain wheat (70 g/day during 8 weeks) in subjects with overweight and obesity [194]. In addition, findings from the GRAANDIOOS study showed that whole-grain wheat consumption (98 g/day for 12 weeks) may promote liver and inflammatory resilience in subjects with overweight/obesity and mild hypercholesterolemia [195].
Legumes
Soy food consumption has been inversely related to circulating levels of inflammatory markers such as IL-6, TNFα, and soluble TNF receptors 1 and 2 in middle-aged Chinese women [196]. Consistently, a 6-week nutritional trial with a legume-enriched diet (a total of 24 packs of 65 g were consumed during the all the intervention phase) significantly reduced CRP concentrations in diabetic patients compared with a habitual diet [197]. Moreover, a legume-based hypocaloric diet (160–235 g/day for 8 weeks) consistently reduced pro-inflammatory status and improved metabolic features in overweight/obese subjects [198].
Green Tea and Coffee
In obese women, green tea extract (450 mg/day) supplementation during 8 weeks improved oxidative stress biomarkers and reduced IL-6 circulating levels [199]. Also, green tea consumption (379 mg/day) by 3 months reduced serum CRP and TNF-α concentration in obese, hypertensive patients [200]. Furthermore, high coffee consumption (8 cups/day) exerted beneficial effects on subclinical inflammation in habitual coffee drinkers [201]. Consistently, coffee consumption was inversely associated with markers of inflammation and endothelial dysfunction in healthy and diabetic women [202]. Among older non-Hispanic Whites, heavy coffee drinkers (equal or more than 2.5 cups/day) presented lower systemic inflammation [203]. On the other hand, analyses from the ATTICA study reported an increase on inflammation markers (including IL-6, TNF-α, and CRP) after moderate-to-high coffee consumption (> 200 mL coffee/d), emphasizing the importance of the dose in outcomes [204].
Dark Chocolate
Available evidence suggests that regular consumption of dark may reduce inflammation, especially in consumers of up to 1 serving (20 g) of dark chocolate every 3 days [205]. In a randomized parallel clinical trial, diabetic patients, who received dark chocolate (30 g of 84% dark chocolate during 8 weeks) along with a healthy lifestyle, had lower levels of inflammatory markers (CRP, TNF-α, and IL-6) compared with those subjects who only followed general lifestyle guidelines [206]. Indeed, acute dark chocolate intake (50 g) elicited anti-inflammatory outcomes both by increasing IL-10 expression and by attenuating the intracellular pro-inflammatory stress response [207]. Moreover, healthy women presented lower blood levels of CRP after the ingestion of dark chocolate (100 g per day during one week), which was not detected in men [208].
Spices and Culinary Ingredients
Over the past several decades, several investigations have ascertained the efficacious role of spices and herbs in preventing and combating various chronic diseases [209]. The diverse range of health properties of these culinary ingredients are attributed to bioactive constituents such as sulfur-containing molecules, tannins, alkaloids, and phenolic diterpenes, with potential anti-inflammatory properties [210]. Findings of clinical trials exploring the impact of spices on inflammation status are summarized (Table 4).
Table 4.
Clinical trials analyzing the anti-inflammatory effects of certain spices and culinary ingredients
| Spice/ingredient | Study design | Dose/duration | Population | Main finding | Reference |
|---|---|---|---|---|---|
| Black cumin (Nigella sativa) | RCT | 2 capsules/day (500 mg each) or placebo for 8 weeks |
Patients with rheumatoid arthritis Experimental group (n = 23) Placebo group (n = 16) |
↑ Serum IL-10 ↓ MDA and nitric oxide NS effects on blood TNF-α |
[211] |
| Black cumin (Nigella sativa) | RCT | 1000 mg oil/day or placebo for 8 weeks |
Patients with Behcet’s disease Experimental group (n = 47) Placebo group (n = 42) |
NS effects on blood TNF-α, IL-10, CRP, and MDA | [212] |
| Black cumin (Nigella sativa) | RCT | 3 g/day plus a low-calorie diet or placebo plus low-calorie diet for 8 weeks |
45 women with obesity Experimental group (n = 45) Placebo group (n = 45) |
↓ Blood CRP and TNF-α NS effects on serum IL-6 |
[213] |
| Cardamom | RCT | 3 g day−1 or identical placebo for 8 weeks |
Pre-diabetic subjects Experimental group (n = 40) Placebo group (n = 40) |
↓ Blood CRP, CRP:IL-6 ratio, and MDA | [214] |
| Cinnamon | RCT | 3 g/day or placebo for 8 weeks |
Adult patients with T2DM Experimental group (n = 20) Placebo group (n = 19) |
NS effects on reduction of SIRT1, IL-6, CRP, and TNF-α levels ↓ Plasma NF-kB |
[215] |
| Cinnamon | RCT | 3 glasses/day of black tea plus either 3 g/day of cardamom (group 1); 3 g/day cinnamon (group 2); 3 g/day ginger (group 3); 3 g/day of saffron (group 4); or control (only 3 glasses of black tea) for 8 weeks |
Adult patients with TD2M Group 1 (n = 42) Group 2 (n = 40) Group 3 (n = 41) Group 4 (n = 42) Control (n = 39) |
NS effects on serum CRP (groups 1, 2, 3, and 4 vs. control) | [216] |
| Cinnamon | RCT | 3 g/day of cinnamon (group 1); 3 g/day of ginger (group 2); or placebo for 6 weeks | Iranian female athletes (n = 60) | NS effects on plasma IL-6 (groups 1 and 2 vs. placebo) | [217] |
| Cinnamon | RCT | 2 capsules/day (each capsule contain 750 mg of cinnamon) or placebo for 12 weeks |
Patients with NAFLD Experimental group (n = 23) Placebo group (n = 22) |
↓ Serum CRP | [218] |
| Cinnamon powder | RCT | 4 capsules/day of 500 mg of cinnamon powder or placebo for 8 weeks |
Women with rheumatoid arthritis Experimental group (n = 18) Placebo group (n = 18) |
↓ Blood CRP and TNF-α | [219] |
| Cinnamon powder | RCT | 3 capsules/day (each containing 600 mg of cinnamon) or placebo |
Patients with migraine Experimental group (n = 21) Placebo group (n = 22) |
↓ Blood IL-6 and nitric oxide | [220] |
| Clove buds | RCT | 1 capsule/day (250 mg of clovinol) or placebo for 2 weeks |
Male social drinkers Experimental group (n = 8) Placebo group (n = 8) |
↓ Serum CRP, IL-6, and lipid peroxidation | [221] |
| Cumin essential oil | RCT | 75-mg cumin essential oil or placebo thrice daily for 8 weeks |
Patients with metabolic syndrome Experimental group (n = 22) Placebo group (n = 22) |
↓ Blood MDA NS effects on CRP and TNF-α |
[222] |
| Garlic extracts | RCT | 3.6 g/day or placebo for 6 weeks |
Healthy adults with obesity Experimental group (n = 24) Placebo group (n = 24) |
↓ Blood IL-6 and TNF-α (borderline) | [223] |
| Garlic extracts | RCT | 400 mg twice a day or placebo for 8 weeks |
Peritoneal dialysis patients Experimental group (n = 19) Placebo group (n = 21) |
↓ Serum IL-6, CRP, and ESR | [224] |
| Garlic powder | RCT | 2.1 g/day or placebo for 3 months |
Overweight and smoking subjects Experimental group (n = 28) Placebo group (n = 26) |
NS effects on serum TNF-α and CRP | [225] |
| Garlic powder | RCT | 300 mg/day or placebo for 8 weeks |
Hemodialysis patients Experimental group (n = 70) Placebo group (n = 70) |
↓ Plasma homocysteine and oxLDL | [226] |
| Garlic powder | RCT | 400 mg/day or placebo for 15 weeks |
Patients with NAFLD Experimental group (n = 47) Placebo group (n = 51) |
↓ Blood CRP | [227] |
| Ginger | RCT | 2 tablets/day (1 g ginger in each) or placebo for 2 months |
Adult patients with T2DM Experimental group (n = 32) Placebo group (n = 32) |
↓ Blood CRP and TNF-α NS effects on serum IL-6 |
[228] |
| Ginger | RCT | 4 tablets 500 mg (2 g) ginger or placebo twice a day for 8 weeks |
Adult patients with T2DM and chronic periodontitis Experimental group (n = 21) Placebo group (n = 21) |
↓ Plasma CRP, IL-6, and TNF-α | [229] |
| Green cardamom | RCT | Two 500 mg capsules three times/day or placebo for 3 months |
Overweight or obese patients with NAFLD Experimental group (n = 43) Placebo group (n = 44) |
↓ Serum CRP, TNF-α, IL-6, and Sirt1 | [230] |
| green cardamom plus low-calorie diet | RCT | 3 g/day plus low-calorie diet or placebo (only low-calorie diet) during 16 weeks |
Obese women with PCOS Experimental group (n = 99) Placebo group (n = 95) |
↓ Serum CRP, TNF-α, and IL-6 ↓ Expression levels of TNF-α and CRP genes |
[231] |
| Green cumin essential oil | RCT | 50 mg/day (group 1); 100 mg/day (group 2); or placebo for 8 weeks |
Adult patients with type 2 diabetes Group 1 (n = 33) Group 2 (n = 33) Placebo group (n = 33) |
NS effects on blood CRP, TNF-α, and adiponectin (groups 1 and 2 vs. placebo) | [232] |
| Herbal formulation | RCT |
Curcumin (300 mg), gingerols (7.5 mg), and piperine (3.75 mg) or naproxen twice a day for 4 weeks |
Patients with knee osteoarthritis Herbal formulation (n = 30) Naproxen (n = 30) |
NS effects on plasma prostaglandin E2 levels | [233] |
| Onion | RCT | 1 capsule of peel extracts (100 mg quercetin/day) or placebo for 12 weeks |
Healthy overweight and obese women Experimental group (n = 18) Placebo group (n = 19) |
NS effects on plasma leptin, TNF-α, IL-4, and visfatin | [234] |
| Turmeric (curcumin) | RCT | 1 g/day or placebo for 4 weeks |
Sulfur mustard-exposed patients Experimental group (n = 46) Placebo group (n = 50) |
↓ Serum IL-8 and CRP NS effects on IL-6 |
[235] |
| Turmeric (curcumin) | RCT | 2 g/day or placebo for 8 weeks |
Patients with NAFLD Experimental group (n = 32) Placebo group (n = 32) |
↓ Plasma MDA | [236] |
| Turmeric (curcumin) | RCT | 500 mg/day or placebo for 8 weeks |
Women with moderate physical activity levels Experimental group (n = 32) Placebo group (n = 33) |
↓ Blood MDA, CRP, and LDH | [237] |
| Turmeric (curcumin) | RCT | 500 mg/day or placebo for 10 weeks |
Overweight and obese adolescent girls Experimental group (n = 30) Placebo group (n = 30) |
↓ Serum IL-6 and CRP | [238] |
| Turmeric (curcumin) | RCT | 1500 mg/day plus lifestyle or placebo (only lifestyle) for 12 weeks |
Patients with NAFLD Experimental group (n = 25) Placebo group (n = 25) |
NS effects on NF-kB activity | [239] |
RCT randomized controlled trial, NS no significant, T2DM type 2 diabetes mellitus, NAFLD non-alcoholic fatty liver disease, PCOS polycystic ovary syndrome, MDA malondialdehyde, NF-kB transcription nuclear factor kappa B, CRP C-reactive protein, IL-6 interleukin 6, TNF-α tumor necrosis factor alpha, IL-8 interleukin 8, IL-4 interleukin 4, IL-10 interleukin 10, SIRT1 sirtuin 1, ESR erythrocyte sedimentation rate, oxLDL oxidized LDL, LDH lactate dehydrogenase
Probiotics, Prebiotics, Synbiotics, and Postbiotics
Probiotics, prebiotics, and synbiotics are either beneficial microorganisms, substrates (polysaccharides and oligosaccharides), or combinations that eventually may also alleviate inflammatory symptoms [240]. In diabetic patients, probiotic and synbiotic supplementation is recommended to decrease inflammatory manifestations by consistently reducing the circulating levels of CRP and TNF-α [241]. Regarding bowel disease, it has been recently reported that the use of probiotics (based on Lactobacillus and Bifidobacterium) and synbiotics can promote anti-inflammatory reactions and balance the intestinal homeostasis [242].
In addition, the anti-inflammatory effects of short-chain fatty acids (nonviable bacterial products known as postbiotics) are mediated by inhibiting the NF-κB pathway, Treg cell differentiation, and pro-inflammatory cytokine blockade in gut epithelial cells [243]. For example, Lactobacillus casei DG and derived postbiotics suppressed IL-8, IL-1α, IL-6, and TLR-4 expression levels in colonic mucosa from patients with irritable bowel syndrome [244].
Dietary Patterns
Traditional Healthy Diets
Overall, plant-based diets have shown to improve the obesity-related inflammation status [245]. Remarkably, the beneficial effect of the vegetarian diet on blood CRP and IL-6 levels was mediated by BMI in North Americans [246]. In addition, a systematic review and meta-analysis revealed that vegetarian-based dietary patterns also lowered immune biomarkers such as fibrinogen and total leukocyte concentrations [247].
A systematic review of observational and intervention trials revealed a beneficial influence of the Nordic diet (based on staple foods from Nordic countries) on low-grade inflammation amelioration [248]. Potential mechanisms include the downregulation of pro-inflammatory genes in individuals with features of the metabolic syndrome, particularly TNFRSF1A and RELA [249].
The Southern European Atlantic Diet (SEAD) is the traditional diet of Northern Portugal and Galicia, Spain, which is characterized by greater intakes of fish, milk, potatoes, fruit, vegetable and olive oil, and red wine [250]. Overall, SEAD adherence has been associated with reduced plasma concentrations of inflammatory markers (predominately CRP) and lowered cardio-metabolic risk [251].
Regarding Asian regions, a healthy Japanese dietary pattern (rich in mushrooms, seaweed, soyabean products and potatoes, vegetables, fish/shellfish, and fruits) seems to exert anti-inflammatory effects and improve mental health in local consumers [252]. Likewise, several herbs from traditional Chinese medicine have shown to suppress pro-inflammatory pathways and control inflammation-associated diseases [253].
Furthermore, the traditional Mexican diet (TMexD) has shown to reduce the risk of systemic inflammation and insulin resistance in women of Mexican descent [254]. Specific foods of the TMexD include maize, beans, chili, squash, tomato, nopal, and onion, which are high in fiber, vitamins, minerals, and capsaicinoids, with potential anti-inflammatory and antioxidant properties [255].
In a pooled cross-sectional study, a Paleolithic diet (based on the consumption of diversity of vegetables and fruits, lean meat, fish, nuts, and sources of calcium) was associated with lower levels of systemic inflammation and oxidative stress in humans [256].
Moreover, the consumption of the DASH eating pattern (characterized by high consumption of fruits and vegetables, low-fat dairy products, and complex carbohydrates) during 6 weeks reduced the circulating levels of CRP among adolescents with metabolic syndrome [257]. In female adults, the DASH diet was cross-sectionally associated with lower serum CRP levels, but not with IL-17A concentrations in Iranians [258]. Quantitative estimations revealed that the DASH diet reduced CRP by 13% after 4 weeks of follow-up [259].
Findings from the PREDIMED trial have shown the anti-inflammatory effect of the Med-diet (rich in vegetables and fruits, fiber, and vitamins C and E) since it downregulates cellular and circulating inflammatory biomarkers involved in atherogenesis development [260]. In this cohort, the Med-Diet reduced the serum CRP and IL-6 levels as well as endothelial and monocytary adhesion molecules and pro-inflammatory chemokines [261]. Additionally, the Med-diet (including EVOO and vegetables) decreased the plasma concentrations of TNFR60 in patients at high cardiovascular risk after 1 year of follow-up [262]. In the long term (3 years), the PREDIMED trial confirmed the anti-inflammatory effect of the Med-diet by decreasing the levels of IL-1β, IL-6, IL-8, and TNF-α compared to a control low-fat diet [263].
Westernized Diets and Ultra-processed/Discretionary Foods
Overall, the adoption of Western-type diets (WTD), with high contents of unhealthy fats, refined grains, sugars, and salt, evokes a state of chronic metabolic inflammation [264]. In this regard, a WTD showed positive associations with markers of inflammation and endothelial dysfunction in women from the Nurses’ Health Study I cohort [265]. Also, a comparable WTD score positively correlated with CRP and IL-6 pro-inflammatory markers among Iranian women [266].
Interestingly, positive associations were found between the consumption of ultra-processed foods (UPF), which contain high amounts of free sugars, total fats, SFA, TFA, and sodium, and serum CRP levels among Brazilian women [267]. Likewise, Brazilian adolescents in the upper tertile of UPF (≥ 30% of total energy) had increased circulating IL-8 concentrations when compared with adolescents in tertile 1 (≤ 15.9% of total energy) of UPF [268].
Furthermore, a poor diet quality (considering habitual discretionary food consumption such as sweets, cakes, soft drinks, and fried potatoes) was associated with increased inflammation measured as plasma CRP and erythrocyte sedimentation rates in Swedish patients with rheumatoid arthritis [269].
Chrononutrition Patterns
Recent progress in the analysis of circadian rhythms and nutrition (referred as “chrononutrition”) has revealed that the time of day when food is consumed may affect metabolic homeostasis and immune function [270]. In this context, a significant association between breakfast skipping and elevated serum CRP concentrations was reported in Chinese adults with poor diet quality [271]. In a randomized controlled crossover trial, breakfast skipping induced a higher activation of the NLRP3 inflammasome in human peripheral blood mononuclear cells and monocytes [272].
Intermittent fasting (IF), where individuals fast on consecutive or alternate days, has improved systemic inflammation in males with obesity [273]. Nevertheless, a transient elevation of biomarkers of macrophage infiltration in adipose tissue (CD40 +) and skeletal muscle (CD163 +) were found after IF in women with overweight or obesity [274].
Available evidence suggests that time-restricted eating (TRE), an alternative chrononutritional approach based on the consolidation of the total calorie intake during the active phase of the day, may modulate a variety of metabolic disease risk factors including exacerbated inflammation [275]. Indeed, it has been postulated that TRE as part of a periodized nutrition plan could be beneficial for reducing inflammation and induce a protective effect on some components of the immune system [276].
Interestingly, greater reductions in blood CRP levels were found in patients with metabolic syndrome after following an alternate day fasting (ADF), which consists of a “fast day,” where caloric intake is limited, alternating with a “feed day,” where food is consumed ad libitum [277]. Moreover, ADF reduced the plasma levels of sICAM-1 (an age-associated inflammatory marker) in healthy non-obese subjects [278].
Additionally, it has been reported that late eating, which refers to delay in the timing of meals (commonly the main meal of the day or the dinner) may increase the risk for developing cardiometabolic diseases [279]. In fact, late eating was associated with abdominal obesity, inflammatory biomarkers such as IL-6 and CRP, and circadian-related disturbances in children [280].
Personalized Anti-inflammatory Nutritional Strategies
The integrative analysis of precision variables (age, sex, body phenotype, habitual dietary intake, physical activity levels, and lifestyle) together with personalized issues (genetic background, epigenetic signatures, microbiota composition, gene expression profiles, and metabolomic fingerprints) may contribute to the prescription of more personalized treatments seeking to improve the nutritional and medical management of inflammation (Fig. 2). For example, there is evidence that genetic variation may predispose to inflammatory conditions through interactions with environmental factors, such as diet, modulating the individual susceptibility to developing inflammation-related chronic and acute diseases [281]. Also, epigenetic signatures (including DNA methylation, miRNA expression, and histone modifications) play a fundamental role in inflammatory gene transcription [282]. Of note, a microbiota-based regression model has been able to predict the obesity-related inflammatory status in humans, which could be a useful tool in the precision management of inflammobesity [283]. Moreover, the expressions of genes with pro- and anti-inflammatory effects ultimately determine the outcome of inflammation [284]. Furthermore, metabolomics is an integrative approach that can be used to dissect the local and systemic metabolic consequences of inflammation, providing novel insights into the regulation of inflammatory diseases [285]. The application of these instruments are helping to elucidate unique and specific inflammo-metabotypes, expanding our understanding of the complexity and diversity of human metabolism [286]. Overall, these novel scientific insights are leading to the design and implementation of precision medicine/nutrition strategies for the prevention and control of prevalent chronic diseases with an inflammatory background [287].
Concluding Remarks
Nutrition is critical for life and well-being not only by contributing to disease prevention, health maintenance, and morbid conditions management, but also as a defense against endogenous and exogenous harmful factors including inflammation/oxidative stress or immune system dysfunctions. On the one hand, pro-inflammatory nutritional factors include the high consumption of foods rich in simple carbohydrates (fructose), SFA, TFA, cholesterol, and animal protein as well as habitually skipping breakfast and late overeating. On the other hand, potential anti-inflammatory compounds encompass MUFA, PUFA, antioxidant vitamins and minerals, bioactive molecules (polyphenols), specific foods (dairy products, whole-grains, fish, oilseeds, fruits and vegetables, edible insects, legumes, green tea, and coffee), culinary spices (cinnamon, ginger, back cumin, garlic, and turmeric) and some chrononutrition features including intermittent fasting and time-restricted eating (Fig. 1). Because inconsistencies and discrepancies between studies exist, further research in this field is still required, taking into account critical aspects of heterogeneity including type of population (ancestry), minimum and maximum levels and adverse effects, cooking methods, physiopathological status, and times of intervention. However, current evidence is contributing to the understanding of the relationship between nutrition and meta-inflammation, providing novel insights and potential targets for the control and management of communicable and non-communicable diseases.
Author Contribution
Conceptualization: Omar Ramos-Lopez and J. Alfredo Martinez; formal analysis and investigation: Diego Martinez-Urbistondo and Juan A. Vargas-Nuñez; writing: Omar Ramos-Lopez and J. Alfredo Martinez. All authors have read and approved the final manuscript.
Funding
Thi study received support from the “FACINGLCOVID-CM project. Funding REACT EU Program (Comunidad de Madrid and The European Regional Development Fund. ERDF. European Union)” and from the “Fundación Española Medicina Interna (FEMI 2020): Nutrición de precisión en pacientes con enfermedades autoinmunes sistémicas y con síndrome metabólico.” Also, Metacategorización personalizada de procesos inflamatorios asociados a síndrome metabólico, enfermedades autoinmunes y virales para medicina de precisión (METAINFLAMACIÓN, Ref: Y2020/BIO-6600) is gratefully acknowledged.
Declarations
Competing Interests
All authors reported no conflict of interest.
Footnotes
This article is part of the Topical Collection on Metabolism
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004;18:385–405. doi: 10.1016/j.bpa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. J Theor Biol. 2004;230:145–155. doi: 10.1016/j.jtbi.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 4.Lukens JR, Dixit VD, Kanneganti TD. Inflammasome activation in obesity-related inflammatory diseases and autoimmunity. Discov Med. 2011;12:65–74. [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett JM, Reeves G, Billman GE, Sturmberg JP. Inflammation-nature's way to efficiently respond to all types of challenges: implications for understanding and managing "the epidemic" of chronic diseases. Front Med (Lausanne) 2018;5:316. doi: 10.3389/fmed.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015:508409. doi: 10.1155/2015/508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. 2012;68:701–711. doi: 10.1007/s13105-012-0154-2. [DOI] [PubMed] [Google Scholar]
- 10.Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. 2020;17:7618. doi: 10.3390/ijerph17207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta-inflammation. Transl Res. 2018;191:29–44. doi: 10.1016/j.trsl.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi JF, Lu ZY, Massart C, Levon K. Dynamic immune/inflammation precision medicine: the good and the bad inflammation in infection and cancer. Front Immunol. 2021;12:595722. doi: 10.3389/fimmu.2021.595722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricordi C, Garcia-Contreras M, Farnetti S. Diet and inflammation: possible effects on immunity, chronic diseases, and life span. J Am Coll Nutr. 2015;34(Suppl 1):10–13. doi: 10.1080/07315724.2015.1080101. [DOI] [PubMed] [Google Scholar]
- 14.Slavin J, Carlson J. Carbohydrates. Adv Nutr. 2014;5:760–761. doi: 10.3945/an.114.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonasson L, Guldbrand H, Lundberg AK, Nystrom FH. Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann Med. 2014;46:182–187. doi: 10.3109/07853890.2014.894286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavakoli A, Mirzababaei A, Sajadi F, Mirzaei K. Circulating inflammatory markers may mediate the relationship between low carbohydrate diet and circadian rhythm in overweight and obese women. BMC Womens Health. 2021;21:87. doi: 10.1186/s12905-021-01240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seshadri P, Iqbal N, Stern L, Williams M, Chicano KL, Daily DA, McGrory J, Gracely EJ, Rader DJ, Samaha FF. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. Am J Med. 2004;117:398–405. doi: 10.1016/j.amjmed.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, Kraemer WJ, Feinman RD, Volek JS. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43:65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- 19.Esfahani A, Wong JM, Mirrahimi A, Srichaikul K, Jenkins DJ, Kendall CW. The glycemic index: physiological significance. J Am Coll Nutr. 2009;28(Suppl):439S–445S. doi: 10.1080/07315724.2009.10718109. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson S, Hancock DP, Petocz P, Ceriello A, Brand-Miller J. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean healthy subjects. Am J Clin Nutr. 2008;87:1188–1193. doi: 10.1093/ajcn/87.5.1188. [DOI] [PubMed] [Google Scholar]
- 21.Gomes JMG, Fabrini SP, Alfenas RCG. Low glycemic index diet reduces body fat and attenuates inflammatory and metabolic responses in patients with type 2 diabetes. Arch Endocrinol Metab. 2017;61:137–144. doi: 10.1590/2359-3997000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gögebakan O, Kohl A, Osterhoff MA, van Baak MA, Jebb SA, Papadaki A, Martinez JA, Handjieva-Darlenska T, Hlavaty P, Weickert MO, Holst C, Saris WH, Astrup A, Pfeiffer AF, DiOGenes Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the diet, obesity, and genes (DiOGenes) study: a randomized, controlled trial. Circulation. 2011;124:2829–38. doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JW, Baird P, Davis RH, Jr, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 24.Vitale M, Masulli M, Rivellese AA, Babini AC, Boemi M, Bonora E, Buzzetti R, Ciano O, Cignarelli M, Cigolini M, Clemente G, Citro G, Corsi L, Dall'Aglio E, Del Prato S, Di Cianni G, Dolci MA, Giordano C, Iannarelli R, Iovine C, Lapolla A, Lauro D, Leotta S, Mazzucchelli C, Montani V, Perriello G, Romano G, Romeo F, Santarelli L, di Cola RS, Squatrito S, Tonutti L, Trevisan R, Turco AA, Zamboni C, Riccardi G, Vaccaro O. Influence of dietary fat and carbohydrates proportions on plasma lipids, glucose control and low-grade inflammation in patients with type 2 diabetes-The TOSCA.IT Study. Eur J Nutr. 2016;55:1645–51. doi: 10.1007/s00394-015-0983-1. [DOI] [PubMed] [Google Scholar]
- 25.King DE, Egan BM, Woolson RF, Mainous AG, 3rd, Al-Solaiman Y, Jesri A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med. 2007;167:502–506. doi: 10.1001/archinte.167.5.502. [DOI] [PubMed] [Google Scholar]
- 26.Ning H, Van Horn L, Shay CM, Lloyd-Jones DM. Associations of dietary fiber intake with long-term predicted cardiovascular disease risk and C-reactive protein levels (from the National Health and Nutrition Examination Survey Data [2005-2010]) Am J Cardiol. 2014;113:287–291. doi: 10.1016/j.amjcard.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein AH, Kennedy E, Barrier P, Danford D, Ernst ND, Grundy SM, Leveille GA, Van Horn L, Williams CL, Booth SL. Dietary fat consumption and health. Nutr Rev. 1998;56:S3–19. doi: 10.1111/j.1753-4887.1998.tb01728.x. [DOI] [PubMed] [Google Scholar]
- 28.Tan BL, Norhaizan ME. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients. 2019;11:2579. doi: 10.3390/nu11112579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis NJ, Crandall JP, Gajavelli S, Berman JW, Tomuta N, Wylie-Rosett J, Katz SD. Differential effects of low-carbohydrate and low-fat diets on inflammation and endothelial function in diabetes. J Diabetes Complications. 2011;25:371–376. doi: 10.1016/j.jdiacomp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H, Urso CJ, Jadeja V. Saturated fatty acids in obesity-associated inflammation. J Inflamm Res. 2020;13:1–14. doi: 10.2147/JIR.S229691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arya S, Isharwal S, Misra A, Pandey RM, Rastogi K, Vikram NK, Dhingra V, Chatterjee A, Sharma R, Luthra K. C-reactive protein and dietary nutrients in urban Asian Indian adolescents and young adults. Nutrition. 2006;22:865–871. doi: 10.1016/j.nut.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 32.González F, Considine RV, Abdelhadi OA, Acton AJ. Inflammation triggered by saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2020;105:e2152–e2167. doi: 10.1210/clinem/dgaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014;13:154. doi: 10.1186/1476-511X-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoneyama S, Miura K, Sasaki S, Yoshita K, Morikawa Y, Ishizaki M, Kido T, Naruse Y, Nakagawa H. Dietary intake of fatty acids and serum C-reactive protein in Japanese. J Epidemiol. 2007;17:86–92. doi: 10.2188/jea.17.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. 2018;9:345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- 36.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, Willett WC, Hu FB. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134:1806–1811. doi: 10.1093/jn/134.7.1806. [DOI] [PubMed] [Google Scholar]
- 38.Yang B, Ren XL, Li ZH, Shi MQ, Ding F, Su KP, Guo XJ, Li D. Lowering effects of fish oil supplementation on proinflammatory markers in hypertension: results from a randomized controlled trial. Food Funct. 2020;11:1779–1789. doi: 10.1039/c9fo03085a. [DOI] [PubMed] [Google Scholar]
- 39.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26:988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allison DB, Egan SK, Barraj LM, Caughman C, Infante M, Heimbach JT. Estimated intakes of trans fatty and other fatty acids in the US population. J Am Diet Assoc. 1999;99:166–174. doi: 10.1016/S0002-8223(99)00041-3. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–566. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 43.Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004;79:606–612. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79:969–973. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 45.Andersen CJ. Impact of dietary cholesterol on the pathophysiology of infectious and autoimmune disease. Nutrients. 2018;10:764. doi: 10.3390/nu10060764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazidi M, Heidari-Bakavoli A, Khayyatzadeh SS, Azarpazhooh MR, Nematy M, Safarian M, Esmaeili H, Parizadeh SM, Ghayour-Mobarhan M, Kengne AP, Ferns GA. Serum hs-CRP varies with dietary cholesterol, but not dietary fatty acid intake in individuals free of any history of cardiovascular disease. Eur J Clin Nutr. 2016;70:1454–1457. doi: 10.1038/ejcn.2016.92. [DOI] [PubMed] [Google Scholar]
- 47.Khayyatzadeh SS, Kazemi-Bajestani SMR, Bagherniya M, Mehramiz M, Tayefi M, Ebrahimi M, Ferns GA, Safarian M, Ghayour-Mobarhan M. Serum high C reactive protein concentrations are related to the intake of dietary macronutrients and fiber: findings from a large representative Persian population sample. Clin Biochem. 2017;50:750–755. doi: 10.1016/j.clinbiochem.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Wu G. Dietary protein intake and human health. Food Funct. 2016;7:1251–1265. doi: 10.1039/c5fo01530h. [DOI] [PubMed] [Google Scholar]
- 49.Hruby A, Jacques PF. Dietary protein and changes in biomarkers of inflammation and oxidative stress in the Framingham Heart Study Offspring Cohort. Curr Dev Nutr. 2019;3:nzz019. doi: 10.1093/cdn/nzz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koelman L, Markova M, Seebeck N, Hornemann S, Rosenthal A, Lange V, Pivovarova-Ramich O, Aleksandrova K. Effects of high and low protein diets on inflammatory profiles in people with morbid obesity: a 3-week intervention study. Nutrients. 2020;12:3636. doi: 10.3390/nu12123636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeh KL, Kautz A, Lohse B, Groth SW. Associations between dietary patterns and inflammatory markers during pregnancy: a systematic review. Nutrients. 2021;13:834. doi: 10.3390/nu13030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Legarrea P, de la Iglesia R, Abete I, Navas-Carretero S, Martinez JA, Zulet MA. The protein type within a hypocaloric diet affects obesity-related inflammation: the RESMENA project. Nutrition. 2014;30:424–429. doi: 10.1016/j.nut.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 53.Floegel A, Chung SJ, von Ruesten A, Yang M, Chung CE, Song WO, Koo SI, Pischon T, Chun OK. Antioxidant intake from diet and supplements and elevated serum C-reactive protein and plasma homocysteine concentrations in US adults: a cross-sectional study. Public Health Nutr. 2011;14:2055–2064. doi: 10.1017/S1368980011000395. [DOI] [PubMed] [Google Scholar]
- 54.Schwab S, Zierer A, Schneider A, Heier M, Koenig W, Kastenmüller G, Waldenberger M, Peters A, Thorand B. Vitamin E supplementation is associated with lower levels of C-reactive protein only in higher dosages and combined with other antioxidants: The Cooperative Health Research in the Region of Augsburg (KORA) F4 study. Br J Nutr. 2015;113:1782–1791. doi: 10.1017/S0007114515000902. [DOI] [PubMed] [Google Scholar]
- 55.Scheurig AC, Thorand B, Fischer B, Heier M, Koenig W. Association between the intake of vitamins and trace elements from supplements and C-reactive protein: results of the MONICA/KORA Augsburg study. Eur J Clin Nutr. 2008;62:127–137. doi: 10.1038/sj.ejcn.1602687. [DOI] [PubMed] [Google Scholar]
- 56.Juanola-Falgarona M, Salas-Salvadó J, Estruch R, Portillo MP, Casas R, Miranda J, Martínez-González MA, Bulló M. Association between dietary phylloquinone intake and peripheral metabolic risk markers related to insulin resistance and diabetes in elderly subjects at high cardiovascular risk. Cardiovasc Diabetol. 2013;12:7. doi: 10.1186/1475-2840-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung S, Kim MK, Choi BY. The long-term relationship between dietary pantothenic acid (vitamin B5) intake and C-reactive protein concentration in adults aged 40 years and older. Nutr Metab Cardiovasc Dis. 2017;27:806–816. doi: 10.1016/j.numecd.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87:424–430. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- 59.Cox SE, Arthur P, Kirkwood BR, Yeboah-Antwi K, Riley EM. Vitamin A supplementation increases ratios of proinflammatory to anti-inflammatory cytokine responses in pregnancy and lactation. Clin Exp Immunol. 2006;144:392–400. doi: 10.1111/j.1365-2249.2006.03082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bitarafan S, Mohammadpour Z, Jafarirad S, Harirchian MH, Yekaninejad MS, Saboor-Yaraghi AA. The effect of retinyl-palmitate on the level of pro and anti-inflammatory cytokines in multiple sclerosis patients: a randomized double blind clinical trial. Clin Neurol Neurosurg. 2019;177:101–105. doi: 10.1016/j.clineuro.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Jafarirad S, Siassi F, Harirchian MH, Amani R, Bitarafan S, Saboor-Yaraghi A. The effect of vitamin a supplementation on biochemical parameters in multiple sclerosis patients. Iran Red Crescent Med J. 2013;15:194–198. doi: 10.5812/ircmj.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alaei-Shahmiri F, Soares MJ, Zhao Y, Sherriff J. The impact of thiamine supplementation on blood pressure, serum lipids and C-reactive protein in individuals with hyperglycemia: a randomised, double-blind cross-over trial. Diabetes Metab Syndr. 2015;9:213–217. doi: 10.1016/j.dsx.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 63.von Martels JZH, Bourgonje AR, Klaassen MAY, Alkhalifah HAA, Sadaghian Sadabad M, Vich Vila A, Gacesa R, Gabriëls RY, Steinert RE, Jansen BH, Bulthuis MLC, van Dullemen HM, Visschedijk MC, Festen EAM, Weersma RK, de Vos P, van Goor H, Faber KN, Harmsen HJM, Dijkstra G. Riboflavin supplementation in patients with Crohn's disease [the RISE-UP study] J Crohns Colitis. 2020;14:595–607. doi: 10.1093/ecco-jcc/jjz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ellulu MS, Rahmat A, Patimah I, Khaza'ai H, Abed Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des Devel Ther. 2015;9:3405–3412. doi: 10.2147/DDDT.S83144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fumeron C, Nguyen-Khoa T, Saltiel C, Kebede M, Buisson C, Drüeke TB, Lacour B, Massy ZA. Effects of oral vitamin C supplementation on oxidative stress and inflammation status in haemodialysis patients. Nephrol Dial Transplant. 2005;20:1874–1879. doi: 10.1093/ndt/gfh928. [DOI] [PubMed] [Google Scholar]
- 66.Zhang K, Li Y, Cheng X, Liu L, Bai W, Guo W, Wu L, Zuo L. Cross-over study of influence of oral vitamin C supplementation on inflammatory status in maintenance hemodialysis patients. BMC Nephrol. 2013;14:252. doi: 10.1186/1471-2369-14-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Block G, Jensen CD, Dalvi TB, Norkus EP, Hudes M, Crawford PB, Holland N, Fung EB, Schumacher L, Harmatz P. Vitamin C treatment reduces elevated C-reactive protein. Free Radic Biol Med. 2009;46:70–77. doi: 10.1016/j.freeradbiomed.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, Luo G, Meng Z, De Backer D, Xiang H, Peng Z. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11:5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DiBella M, Thomas MS, Alyousef H, Millar C, Blesso C, Malysheva O, Caudill MA, Fernandez ML. Choline intake as supplement or as a component of eggs increases plasma choline and reduces interleukin-6 without modifying plasma cholesterol in participants with metabolic syndrome. Nutrients. 2020;12:3120. doi: 10.3390/nu12103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharifi A, Hosseinzadeh-Attar MJ, Vahedi H, Nedjat S. A randomized controlled trial on the effect of vitamin D3 on inflammation and cathelicidin gene expression in ulcerative colitis patients. Saudi J Gastroenterol. 2016;22:316–323. doi: 10.4103/1319-3767.187606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mousa A, Naderpoor N, Johnson J, Sourris K, de Courten MPJ, Wilson K, Scragg R, Plebanski M, de Courten B. Effect of vitamin D supplementation on inflammation and nuclear factor kappa-B activity in overweight/obese adults: a randomized placebo-controlled trial. Sci Rep. 2017;7:15154. doi: 10.1038/s41598-017-15264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheshmazar E, Hosseini AF, Yazdani B, Razmpoosh E, Zarrati M. Effects of vitamin D supplementation on omentin-1 and spexin levels, inflammatory parameters, lipid profile, and anthropometric indices in obese and overweight adults with vitamin D deficiency under low-calorie diet: a randomized placebo controlled trial. Evid Based Complement Alternat Med. 2020;2020:3826237. doi: 10.1155/2020/3826237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lotfi-Dizaji L, Mahboob S, Aliashrafi S, Vaghef-Mehrabany E, Ebrahimi-Mameghani M, Morovati A. Effect of vitamin D supplementation along with weight loss diet on meta-inflammation and fat mass in obese subjects with vitamin D deficiency: a double-blind placebo-controlled randomized clinical trial. Clin Endocrinol (Oxf) 2019;90:94–101. doi: 10.1111/cen.13861. [DOI] [PubMed] [Google Scholar]
- 74.Wamberg L, Kampmann U, Stødkilde-Jørgensen H, Rejnmark L, Pedersen SB, Richelsen B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels - results from a randomized trial. Eur J Intern Med. 2013;24:644–649. doi: 10.1016/j.ejim.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Chandler PD, Scott JB, Drake BF, Ng K, Manson JE, Rifai N, Chan AT, Bennett GG, Hollis BW, Giovannucci EL, Emmons KM, Fuchs CS. Impact of vitamin D supplementation on inflammatory markers in African Americans: results of a four-arm, randomized, placebo-controlled trial. Cancer Prev Res (Phila) 2014;7:218–225. doi: 10.1158/1940-6207.CAPR-13-0338-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pessoa Mamede LCG, de Lima RLFC, Silva AS, Rodrigues Pita JCL, Galdino Gomes NI, de Sena EA, Moraes Nobrega RP, Scarano Alcântara JO, Fontes de Souza JH, Cardoso GA, de Brito Alves JL, Rodrigues Gonçalves MDC. Effects of a single oral megadose of vitamin D3 on inflammation and oxidative stress markers in overweight and obese women: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Metab Syndr Obes. 2021;14:525–534. doi: 10.2147/DMSO.S285597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Medeiros Cavalcante IG, Silva AS, Costa MJ, Persuhn DC, Issa CT, de Lunafreire TL, da Conceição Rodrigues Gonçalves M. Effect of vitamin D3 supplementation and influence of BsmI polymorphism of the VDR gene of the inflammatory profile and oxidative stress in elderly women with vitamin D insufficiency: vitamin D3 megadose reduces inflammatory markers. Exp Gerontol. 2015;66:10–6. doi: 10.1016/j.exger.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 78.Waterhouse M, Tran B, Ebeling PR, English DR, Lucas RM, Venn AJ, Webb PM, Whiteman DC, Neale RE. Effect of vitamin D supplementation on selected inflammatory biomarkers in older adults: a secondary analysis of data from a randomised, placebo-controlled trial. Br J Nutr. 2015;114:693–699. doi: 10.1017/S0007114515002366. [DOI] [PubMed] [Google Scholar]
- 79.Khatami PG, Soleimani A, Sharifi N, Aghadavod E, Asemi Z. The effects of high-dose vitamin E supplementation on biomarkers of kidney injury, inflammation, and oxidative stress in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. J Clin Lipidol. 2016;10:922–929. doi: 10.1016/j.jacl.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 80.Pirhadi-Tavandashti N, Imani H, Ebrahimpour-Koujan S, Samavat S, Hakemi MS. The effect of vitamin E supplementation on biomarkers of endothelial function and inflammation among hemodialysis patients: a double-blinded randomized clinical trial. Complement Ther Med. 2020;49:102357. doi: 10.1016/j.ctim.2020.102357. [DOI] [PubMed] [Google Scholar]
- 81.Mangoni AA, Arya R, Ford E, Asonganyi B, Sherwood RA, Ouldred E, Swift CG, Jackson SH. Effects of folic acid supplementation on inflammatory and thrombogenic markers in chronic smokers. A randomised controlled trial. Thromb Res. 2003;110:13–17. doi: 10.1016/s0049-3848(03)00295-0. [DOI] [PubMed] [Google Scholar]
- 82.Solini A, Santini E, Ferrannini E. Effect of short-term folic acid supplementation on insulin sensitivity and inflammatory markers in overweight subjects. Int J Obes (Lond) 2006;30:1197–1202. doi: 10.1038/sj.ijo.0803265. [DOI] [PubMed] [Google Scholar]
- 83.Durga J, van Tits LJ, Schouten EG, Kok FJ, Verhoef P. Effect of lowering of homocysteine levels on inflammatory markers: a randomized controlled trial. Arch Intern Med. 2005;165:1388–1394. doi: 10.1001/archinte.165.12.1388. [DOI] [PubMed] [Google Scholar]
- 84.Chen H, Liu S, Ji L, Wu T, Ji Y, Zhou Y, Zheng M, Zhang M, Xu W, Huang G. Folic acid supplementation mitigates Alzheimer’s disease by reducing inflammation: a randomized controlled trial. Mediators Inflamm. 2016;2016:5912146. doi: 10.1155/2016/5912146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma F, Wu T, Zhao J, Song A, Liu H, Xu W, Huang G. Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Sci Rep. 2016;6:37486. doi: 10.1038/srep37486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen H, Liu S, Ge B, Zhou D, Li M, Li W, Ma F, Liu Z, Ji Y, Huang G. Effects of folic acid and vitamin B12 supplementation on cognitive impairment and inflammation in patients with Alzheimer's disease: a randomized, single-blinded, placebo-controlled trial. J Prev Alzheimers Dis. 2021;8:249–256. doi: 10.14283/jpad.2021.22. [DOI] [PubMed] [Google Scholar]
- 87.Ma F, Zhou X, Li Q, Zhao J, Song A, An P, Du Y, Xu W, Huang G. Effects of Folic Acid and Vitamin B12, Alone and in combination on cognitive function and inflammatory factors in the elderly with mild cognitive impairment: a single-blind experimental design. Curr Alzheimer Res. 2019;16:622–632. doi: 10.2174/1567205016666190725144629. [DOI] [PubMed] [Google Scholar]
- 88.van Dijk SC, Enneman AW, Swart KM, van Wijngaarden JP, Ham AC, de Jonge R, Blom HJ, Feskens EJ, Geleijnse JM, van Schoor NM, Dhonukshe-Rutten RA, de Jongh RT, Lips P, de Groot LC, Uitterlinden AG, van den Meiracker TH, Mattace-Raso FU, van der Velde N, Smulders YM. Effect of vitamin B12 and folic acid supplementation on biomarkers of endothelial function and inflammation among elderly individuals with hyperhomocysteinemia. Vasc Med. 2016;21:91–98. doi: 10.1177/1358863X15622281. [DOI] [PubMed] [Google Scholar]
- 89.Christen WG, Cook NR, Van Denburgh M, Zaharris E, Albert CM, Manson JE. Effect of combined treatment with folic acid, vitamin B6, and vitamin B12 on plasma biomarkers of inflammation and endothelial dysfunction in women. J Am Heart Assoc. 2018;7:e008517. doi: 10.1161/JAHA.117.008517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bleie Ø, Semb AG, Grundt H, Nordrehaug JE, Vollset SE, Ueland PM, Nilsen DW, Bakken AM, Refsum H, Nygård OK. Homocysteine-lowering therapy does not affect inflammatory markers of atherosclerosis in patients with stable coronary artery disease. J Intern Med. 2007;262:244–253. doi: 10.1111/j.1365-2796.2007.01810.x. [DOI] [PubMed] [Google Scholar]
- 91.Shishavan NG, Gargari BP, Jafarabadi MA, Kolahi S, Haggifar S, Noroozi S. Vitamin K1 supplementation did not alter inflammatory markers and clinical status in patients with rheumatoid arthritis. Int J Vitam Nutr Res. 2018;88:251–257. doi: 10.1024/0300-9831/a000276. [DOI] [PubMed] [Google Scholar]
- 92.Kristensen M, Kudsk J, Bügel S. Six weeks phylloquinone supplementation produces undesirable effects on blood lipids with no changes in inflammatory and fibrinolytic markers in postmenopausal women. Eur J Nutr. 2008;47:375–379. doi: 10.1007/s00394-008-0737-4. [DOI] [PubMed] [Google Scholar]
- 93.Mehri A. Trace elements in human nutrition (II) - an update. Int J Prev Med. 2020;11:2. doi: 10.4103/ijpvm.IJPVM_48_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chacko SA, Song Y, Nathan L, Tinker L, de Boer IH, Tylavsky F, Wallace R, Liu S. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care. 2010;33:304–310. doi: 10.2337/dc09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song Y, Li TY, van Dam RM, Manson JE, Hu FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr. 2007;85:1068–1074. doi: 10.1093/ajcn/85.4.1068. [DOI] [PubMed] [Google Scholar]
- 96.Gong JH, Lo K, Liu Q, Li J, Lai S, Shadyab AH, Arcan C, Snetselaar L, Liu S. Dietary manganese, plasma markers of inflammation, and the development of type 2 diabetes in postmenopausal women: findings from the Women's Health Initiative. Diabetes Care. 2020;43:1344–1351. doi: 10.2337/dc20-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pinna K, Kelley DS, Taylor PC, King JC. Immune functions are maintained in healthy men with low zinc intake. J Nutr. 2002;132:2033–2036. doi: 10.1093/jn/132.7.2033. [DOI] [PubMed] [Google Scholar]
- 98.Bo S, Durazzo M, Gambino R, Berutti C, Milanesio N, Caropreso A, Gentile L, Cassader M, Cavallo-Perin P, Pagano G. Associations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adults. J Nutr. 2008;138:305–310. doi: 10.1093/jn/138.2.305. [DOI] [PubMed] [Google Scholar]
- 99.Cepeda-Lopez AC, Osendarp SJ, Melse-Boonstra A, Aeberli I, Gonzalez-Salazar F, Feskens E, Villalpando S, Zimmermann MB. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr. 2011;93:975–983. doi: 10.3945/ajcn.110.005439. [DOI] [PubMed] [Google Scholar]
- 100.Jamilian M, Bahmani F, Siavashani MA, Mazloomi M, Asemi Z, Esmaillzadeh A. The effects of chromium supplementation on endocrine profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2016;172:72–78. doi: 10.1007/s12011-015-0570-6. [DOI] [PubMed] [Google Scholar]
- 101.Amiri Siavashani M, Zadeh Modarres S, Mirhosseini N, Aghadavod E, Salehpour S, Asemi Z. The effects of chromium supplementation on gene expression of insulin, lipid, and inflammatory markers in infertile women with polycystic ovary syndrome candidate for in vitro fertilization: a randomized, double-blinded, placebo-controlled trial. Front Endocrinol (Lausanne) 2018;9:726. doi: 10.3389/fendo.2018.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moradi F, Kooshki F, Nokhostin F, Khoshbaten M, Bazyar H, Pourghassem GB. A pilot study of the effects of chromium picolinate supplementation on serum fetuin-A, metabolic and inflammatory factors in patients with nonalcoholic fatty liver disease: a double-blind, placebo-controlled trial. J Trace Elem Med Biol. 2021;63:126659. doi: 10.1016/j.jtemb.2020.126659. [DOI] [PubMed] [Google Scholar]
- 103.Saiyed ZM, Lugo JP. Impact of chromium dinicocysteinate supplementation on inflammation, oxidative stress, and insulin resistance in type 2 diabetic subjects: an exploratory analysis of a randomized, double-blind, placebo-controlled study. Food Nutr Res. 2016;60:31762. doi: 10.3402/fnr.v60.31762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iqbal N, Cardillo S, Volger S, Bloedon LT, Anderson RA, Boston R, Szapary PO. Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metab Syndr Relat Disord. 2009;7:143–150. doi: 10.1089/met.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jain SK, Kahlon G, Morehead L, Dhawan R, Lieblong B, Stapleton T, Caldito G, Hoeldtke R, Levine SN, Bass PF., 3rd Effect of chromium dinicocysteinate supplementation on circulating levels of insulin, TNF-α, oxidative stress, and insulin resistance in type 2 diabetic subjects: randomized, double-blind, placebo-controlled study. Mol Nutr Food Res. 2012;56:1333–1341. doi: 10.1002/mnfr.201100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim HN, Kim SH, Eun YM, Song SW. Effects of zinc, magnesium, and chromium supplementation on cardiometabolic risk in adults with metabolic syndrome: a double-blind, placebo-controlled randomised trial. J Trace Elem Med Biol. 2018;48:166–171. doi: 10.1016/j.jtemb.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 107.DiSilvestro RA, Joseph EL, Zhang W, Raimo AE, Kim YM. A randomized trial of copper supplementation effects on blood copper enzyme activities and parameters related to cardiovascular health. Metabolism. 2012;61:1242–1246. doi: 10.1016/j.metabol.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 108.Ma J, Sun Q, Liu J, Hu Y, Liu S, Zhang J, Sheng X, Hambidge KM. The effect of iron fortification on iron (Fe) status and inflammation: a randomized controlled trial. PLoS ONE. 2016;11:e0167458. doi: 10.1371/journal.pone.0167458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dostal A, Baumgartner J, Riesen N, Chassard C, Smuts CM, Zimmermann MB, Lacroix C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: a randomised, placebo-controlled intervention trial in South African children. Br J Nutr. 2014;112:547–556. doi: 10.1017/S0007114514001160. [DOI] [PubMed] [Google Scholar]
- 110.Tang M, Frank DN, Sherlock L, Ir D, Robertson CE, Krebs NF. Effect of vitamin E with therapeutic iron supplementation on iron repletion and gut microbiome in US iron deficient infants and toddlers. J Pediatr Gastroenterol Nutr. 2016;63:379–385. doi: 10.1097/MPG.0000000000001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chacko SA, Sul J, Song Y, Li X, LeBlanc J, You Y, Butch A, Liu S. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: a randomized, double-blind, controlled, crossover trial in overweight individuals. Am J Clin Nutr. 2011;93:463–473. doi: 10.3945/ajcn.110.002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moslehi N, Vafa M, Rahimi-Foroushani A, Golestan B. Effects of oral magnesium supplementation on inflammatory markers in middle-aged overweight women. J Res Med Sci. 2012;17:607–614. [PMC free article] [PubMed] [Google Scholar]
- 113.Nielsen FH, Johnson LK, Zeng H. Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Magnes Res. 2010;23:158–168. doi: 10.1684/mrh.2010.0220. [DOI] [PubMed] [Google Scholar]
- 114.Zanforlini BM, Ceolin C, Trevisan C, Alessi A, Seccia DM, Noale M, Maggi S, Guarnieri G, Vianello A, Sergi G. Clinical trial on the effects of oral magnesium supplementation in stable-phase COPD patients. Aging Clin Exp Res. 2021 doi: 10.1007/s40520-021-01921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simental-Mendía LE, Rodríguez-Morán M, Reyes-Romero MA, Guerrero-Romero F. No positive effect of oral magnesium supplementation in the decreases of inflammation in subjects with prediabetes: a pilot study. Magnes Res. 2012;25:140–146. doi: 10.1684/mrh.2012.0322. [DOI] [PubMed] [Google Scholar]
- 116.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. Oral magnesium supplementation decreases C-reactive protein levels in subjects with prediabetes and hypomagnesemia: a clinical randomized double-blind placebo-controlled trial. Arch Med Res. 2014;45:325–330. doi: 10.1016/j.arcmed.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 117.Afshar Ebrahimi F, Foroozanfard F, Aghadavod E, Bahmani F, Asemi Z. The effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress, and gene expression related to inflammation in polycystic ovary syndrome: a randomized controlled clinical trial. Biol Trace Elem Res. 2018;184:300–307. doi: 10.1007/s12011-017-1198-5. [DOI] [PubMed] [Google Scholar]
- 118.Gijsbers L, Dower JI, Schalkwijk CG, Kusters YH, Bakker SJ, Hollman PC, Geleijnse JM. Effects of sodium and potassium supplementation on endothelial function: a fully controlled dietary intervention study. Br J Nutr. 2015;114:1419–1426. doi: 10.1017/S0007114515002986. [DOI] [PubMed] [Google Scholar]
- 119.Bahmani F, Kia M, Soleimani A, Mohammadi AA, Asemi Z. The effects of selenium supplementation on biomarkers of inflammation and oxidative stress in patients with diabetic nephropathy: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2016;116:1222–1228. doi: 10.1017/S0007114516003251. [DOI] [PubMed] [Google Scholar]
- 120.Raygan F, Behnejad M, Ostadmohammadi V, Bahmani F, Mansournia MA, Karamali F, Asemi Z. Selenium supplementation lowers insulin resistance and markers of cardio-metabolic risk in patients with congestive heart failure: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2018;120:33–40. doi: 10.1017/S0007114518001253. [DOI] [PubMed] [Google Scholar]
- 121.Salehi M, Sohrabi Z, Ekramzadeh M, Fallahzadeh MK, Ayatollahi M, Geramizadeh B, Hassanzadeh J, Sagheb MM. Selenium supplementation improves the nutritional status of hemodialysis patients: a randomized, double-blind, placebo-controlled trial. Nephrol Dial Transplant. 2013;28:716–723. doi: 10.1093/ndt/gfs170. [DOI] [PubMed] [Google Scholar]
- 122.Asemi Z, Jamilian M, Mesdaghinia E, Esmaillzadeh A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: randomized, double-blind, placebo-controlled trial. Nutrition. 2015;31:1235–1242. doi: 10.1016/j.nut.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 123.Razavi M, Jamilian M, Kashan ZF, Heidar Z, Mohseni M, Ghandi Y, Bagherian T, Asemi Z. Selenium supplementation and the effects on reproductive outcomes, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome. Horm Metab Res. 2016;48:185–190. doi: 10.1055/s-0035-1559604. [DOI] [PubMed] [Google Scholar]
- 124.Kamali A, Amirani E, Asemi Z. Effects of selenium supplementation on metabolic status in patients undergoing for coronary artery bypass grafting (CABG) surgery: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2019;191:331–337. doi: 10.1007/s12011-019-1636-7. [DOI] [PubMed] [Google Scholar]
- 125.Farrokhian A, Bahmani F, Taghizadeh M, Mirhashemi SM, Aarabi MH, Raygan F, Aghadavod E, Asemi Z. Selenium supplementation affects insulin resistance and serum hs-CRP in patients with type 2 diabetes and coronary heart disease. Horm Metab Res. 2016;48:263–268. doi: 10.1055/s-0035-1569276. [DOI] [PubMed] [Google Scholar]
- 126.Daeian N, Radfar M, Jahangard-Rafsanjani Z, Hadjibabaie M, Ghavamzadeh A. Selenium supplementation in patients undergoing hematopoietic stem cell transplantation: effects on pro-inflammatory cytokines levels. Daru. 2014;22:51. doi: 10.1186/2008-2231-22-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jafari F, Amani R, Tarrahi MJ. Effect of zinc supplementation on physical and psychological symptoms, biomarkers of inflammation, oxidative stress, and brain-derived neurotrophic factor in young women with premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2020;194:89–95. doi: 10.1007/s12011-019-01757-9. [DOI] [PubMed] [Google Scholar]
- 128.Bao B, Prasad AS, Beck FW, Fitzgerald JT, Snell D, Bao GW, Singh T, Cardozo LJ. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am J Clin Nutr. 2010;91:1634–1641. doi: 10.3945/ajcn.2009.28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mazaheri M, Aghdam AM, Heidari M, Zarrin R. Assessing the effect of zinc supplementation on the frequency of migraine attack, duration, severity, lipid profile and hs-CRP in adult women. Clin Nutr Res. 2021;10:127–139. doi: 10.7762/cnr.2021.10.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bao B, Prasad AS, Beck FW, Snell D, Suneja A, Sarkar FH, Doshi N, Fitzgerald JT, Swerdlow P. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl Res. 2008;152:67–80. doi: 10.1016/j.trsl.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 131.Ranjbar E, Shams J, Sabetkasaei M, M-Shirazi M, Rashidkhani B, Mostafavi A, Bornak E, Nasrollahzadeh J. Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr Neurosci. 2014;17:65–71. doi: 10.1179/1476830513Y.0000000066. [DOI] [PubMed] [Google Scholar]
- 132.Kim J, Ahn J. Effect of zinc supplementation on inflammatory markers and adipokines in young obese women. Biol Trace Elem Res. 2014;157:101–106. doi: 10.1007/s12011-013-9885-3. [DOI] [PubMed] [Google Scholar]
- 133.Khorsandi H, Nikpayam O, Yousefi R, Parandoosh M, Hosseinzadeh N, Saidpour A, Ghorbani A. Zinc supplementation improves body weight management, inflammatory biomarkers and insulin resistance in individuals with obesity: a randomized, placebo-controlled, double-blind trial. Diabetol Metab Syndr. 2019;11:101. doi: 10.1186/s13098-019-0497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jamilian M, Foroozanfard F, Bahmani F, Talaee R, Monavari M, Asemi Z. Effects of zinc supplementation on endocrine outcomes in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2016;170:271–278. doi: 10.1007/s12011-015-0480-7. [DOI] [PubMed] [Google Scholar]
- 135.Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, Poursafa P, Rouzbahani A. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab Syndr Relat Disord. 2010;8:505–510. doi: 10.1089/met.2010.0020. [DOI] [PubMed] [Google Scholar]
- 136.Landete JM. Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit Rev Food Sci Nutr. 2012;52:936–948. doi: 10.1080/10408398.2010.513779. [DOI] [PubMed] [Google Scholar]
- 137.Chun OK, Chung SJ, Claycombe KJ, Song WO. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 2008;138:753–60. doi: 10.1093/jn/138.4.753. [DOI] [PubMed] [Google Scholar]
- 138.Rohrmann S, Shvetsov YB, Morimoto Y, Wilkens LR, Monroe KR, Le Marchand L, Franke AA, Kolonel LN, Maskarinec G. Self-reported dietary flavonoid intake and serum markers of inflammation: the multiethnic cohort. Cancer Causes Control. 2018;29:601–607. doi: 10.1007/s10552-018-1034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Filiberto AC, Mumford SL, Pollack AZ, Zhang C, Yeung EH, Perkins NJ, Wactawski-Wende J, Schisterman EF. Habitual dietary isoflavone intake is associated with decreased C-reactive protein concentrations among healthy premenopausal women. J Nutr. 2013;143:900–906. doi: 10.3945/jn.112.173187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Landberg R, Sun Q, Rimm EB, Cassidy A, Scalbert A, Mantzoros CS, Hu FB, van Dam RM. Selected dietary flavonoids are associated with markers of inflammation and endothelial dysfunction in U.S. women. J Nutr. 2011;141:618–25. doi: 10.3945/jn.110.133843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hsieh CT, Wang J, Chien KL. Association between dietary flavonoid intakes and C-reactive protein levels: a cross-sectional study in Taiwan. J Nutr Sci. 2021;10:e15. doi: 10.1017/jns.2021.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang H, Xu Z, Zhao H, Wang X, Pang J, Li Q, Yang Y, Ling W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 2020;32:101474. doi: 10.1016/j.redox.2020.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nikbakht E, Singh I, Vider J, Williams LT, Vugic L, Gaiz A, Kundur AR, Colson N. Potential of anthocyanin as an anti-inflammatory agent: a human clinical trial on type 2 diabetic, diabetic at-risk and healthy adults. Inflamm Res. 2021;70:275–284. doi: 10.1007/s00011-021-01438-1. [DOI] [PubMed] [Google Scholar]
- 144.Guo Y, Zhang P, Liu Y, Zha L, Ling W, Guo H. A dose-response evaluation of purified anthocyanins on inflammatory and oxidative biomarkers and metabolic risk factors in healthy young adults: a randomized controlled trial. Nutrition. 2020;74:110745. doi: 10.1016/j.nut.2020.110745. [DOI] [PubMed] [Google Scholar]
- 145.Hassellund SS, Flaa A, Kjeldsen SE, Seljeflot I, Karlsen A, Erlund I, Rostrup M. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens. 2013;27:100–106. doi: 10.1038/jhh.2012.4. [DOI] [PubMed] [Google Scholar]
- 146.Thompson K, Hosking H, Pederick W, Singh I, Santhakumar AB. The effect of anthocyanin supplementation in modulating platelet function in sedentary population: a randomised, double-blind, placebo-controlled, cross-over trial. Br J Nutr. 2017;118:368–374. doi: 10.1017/S0007114517002124. [DOI] [PubMed] [Google Scholar]
- 147.Bazyar H, Hosseini SA, Saradar S, Mombaini D, Allivand M, Labibzadeh M, Alipour M. Effects of epigallocatechin-3-gallate of Camellia sinensis leaves on blood pressure, lipid profile, atherogenic index of plasma and some inflammatory and antioxidant markers in type 2 diabetes mellitus patients: a clinical trial. J Complement Integr Med. 2020;18:405–411. doi: 10.1515/jcim-2020-0090. [DOI] [PubMed] [Google Scholar]
- 148.Mielgo-Ayuso J, Barrenechea L, Alcorta P, Larrarte E, Margareto J, Labayen I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br J Nutr. 2014;111:1263–1271. doi: 10.1017/S0007114513003784. [DOI] [PubMed] [Google Scholar]
- 149.Yari Z, Movahedian M, Imani H, Alavian SM, Hedayati M, Hekmatdoost A. The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Eur J Nutr. 2020;59:2569–2577. doi: 10.1007/s00394-019-02105-2. [DOI] [PubMed] [Google Scholar]
- 150.Haidari F, Heybar H, Jalali MT, Ahmadi Engali K, Helli B, Shirbeigi E. Hesperidin supplementation modulates inflammatory responses following myocardial infarction. J Am Coll Nutr. 2015;34:205–211. doi: 10.1080/07315724.2014.891269. [DOI] [PubMed] [Google Scholar]
- 151.Homayouni F, Haidari F, Hedayati M, Zakerkish M, Ahmadi K. Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes; a randomized double-blind controlled clinical trial. Phytother Res. 2018;32:1073–1079. doi: 10.1002/ptr.6046. [DOI] [PubMed] [Google Scholar]
- 152.Yari Z, Cheraghpour M, Alavian SM, Hedayati M, Eini-Zinab H, Hekmatdoost A. The efficacy of flaxseed and hesperidin on non-alcoholic fatty liver disease: an open-labeled randomized controlled trial. Eur J Clin Nutr. 2021;75:99–111. doi: 10.1038/s41430-020-0679-3. [DOI] [PubMed] [Google Scholar]
- 153.Cheraghpour M, Imani H, Ommi S, Alavian SM, Karimi-Shahrbabak E, Hedayati M, Yari Z, Hekmatdoost A. Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled, double-blind clinical trial. Phytother Res. 2019;33:2118–2125. doi: 10.1002/ptr.6406. [DOI] [PubMed] [Google Scholar]
- 154.Dehghani F, Sezavar Seyedi Jandaghi SH, Janani L, Sarebanhassanabadi M, Emamat H, Vafa M. Effects of quercetin supplementation on inflammatory factors and quality of life in post-myocardial infarction patients: a double blind, placebo-controlled, randomized clinical trial. Phytother Res. 2021;35:2085–2098. doi: 10.1002/ptr.6955. [DOI] [PubMed] [Google Scholar]
- 155.Askari G, Ghiasvand R, Feizi A, Ghanadian SM, Karimian J. The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J Res Med Sci. 2012;17:637–641. [PMC free article] [PubMed] [Google Scholar]
- 156.Dower JI, Geleijnse JM, Gijsbers L, Schalkwijk C, Kromhout D, Hollman PC. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: a randomized double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145:1459–1463. doi: 10.3945/jn.115.211888. [DOI] [PubMed] [Google Scholar]
- 157.Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, Jazayeri S. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J Am Coll Nutr. 2017;36:9–15. doi: 10.1080/07315724.2016.1140093. [DOI] [PubMed] [Google Scholar]
- 158.Heinz SA, Henson DA, Nieman DC, Austin MD, Jin F. A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br J Nutr. 2010;104:849–857. doi: 10.1017/S000711451000156X. [DOI] [PubMed] [Google Scholar]
- 159.Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, Wagner AE, Frank J, Rimbach G, Mueller MJ. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 160.Javadi F, Eghtesadi S, Ahmadzadeh A, Aryaeian N, Zabihiyeganeh M, Foroushani AR, Jazayeri S. The effect of quercetin on plasma oxidative status, C-reactive protein and blood pressure in women with rheumatoid arthritis. Int J Prev Med. 2014;5:293–301. [PMC free article] [PubMed] [Google Scholar]
- 161.Brüll V, Burak C, Stoffel-Wagner B, Wolffram S, Nickenig G, Müller C, Langguth P, Alteheld B, Fimmers R, Stehle P, Egert S. No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in overweight-to-obese patients with (pre-)hypertension: a randomized double-blinded, placebo-controlled crossover trial. Eur J Nutr. 2017;56:2265–2275. doi: 10.1007/s00394-016-1267-0. [DOI] [PubMed] [Google Scholar]
- 162.Javid AZ, Hormoznejad R, Yousefimanesh HA, Haghighi-Zadeh MH, Zakerkish M. Impact of resveratrol supplementation on inflammatory, antioxidant, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Diabetes Metab Syndr. 2019;13:2769–2774. doi: 10.1016/j.dsx.2019.07.042. [DOI] [PubMed] [Google Scholar]
- 163.Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837–843. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 164.Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch Med Res. 2015;46:280–285. doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 165.Bo S, Ciccone G, Castiglione A, Gambino R, De Michieli F, Villois P, Durazzo M, Cavallo-Perin P, Cassader M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem. 2013;20:1323–1331. doi: 10.2174/0929867311320100009. [DOI] [PubMed] [Google Scholar]
- 166.Khodabandehloo H, Seyyedebrahimi S, Esfahani EN, Razi F, Meshkani R. Resveratrol supplementation decreases blood glucose without changing the circulating CD14+CD16+ monocytes and inflammatory cytokines in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Nutr Res. 2018;54:40–51. doi: 10.1016/j.nutres.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 167.Saldanha JF, Leal VO, Rizzetto F, Grimmer GH, Ribeiro-Alves M, Daleprane JB, Carraro-Eduardo JC, Mafra D. Effects of resveratrol supplementation in Nrf2 and NF-κB expressions in nondialyzed chronic kidney disease patients: a randomized, double-blind, placebo-controlled, crossover clinical trial. J Ren Nutr. 2016;26:401–406. doi: 10.1053/j.jrn.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 168.Ley SH, Sun Q, Willett WC, Eliassen AH, Wu K, Pan A, Grodstein F, Hu FB. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr. 2014;99:352–360. doi: 10.3945/ajcn.113.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chai W, Morimoto Y, Cooney RV, Franke AA, Shvetsov YB, Le Marchand L, Haiman CA, Kolonel LN, Goodman MT, Maskarinec G. Dietary red and processed meat intake and markers of adiposity and inflammation: the multiethnic cohort study. J Am Coll Nutr. 2017;36:378–385. doi: 10.1080/07315724.2017.1318317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Papier K, Hartman L, Tong TYN, Key TJ, Knuppel A. Higher meat intake is associated with higher inflammatory markers, mostly due to adiposity: results from UK Biobank. J Nutr. 2021:nxab314. 10.1093/jn/nxab314. [DOI] [PMC free article] [PubMed]
- 171.Mazidi M, Kengne AP, George ES, Siervo M. The association of red meat intake with inflammation and circulating intermediate biomarkers of type 2 diabetes is mediated by central adiposity. Br J Nutr. 2021;125:1043–1050. doi: 10.1017/S0007114519002149. [DOI] [PubMed] [Google Scholar]
- 172.Gadotti TN, Norde MM, Rogero MM, Fisberg M, Fisberg RM, Oki E, Martini LA. Dairy consumption and inflammatory profile: a cross-sectional population-based study, São Paulo, Brazil. Nutrition. 2018;48:1–5. doi: 10.1016/j.nut.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 173.Abreu S, Agostinis-Sobrinho C, Santos R, Moreira C, Lopes L, Gonçalves C, Oliveira-Santos J, Sousa-Sá E, Rodrigues B, Mota J, Rosário R. Association of dairy product consumption with metabolic and inflammatory biomarkers in adolescents: a cross-sectional analysis from the LabMed study. Nutrients. 2019;11:2268. doi: 10.3390/nu11102268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Panagiotakos DB, Pitsavos CH, Zampelas AD, Chrysohoou CA, Stefanadis CI. Dairy products consumption is associated with decreased levels of inflammatory markers related to cardiovascular disease in apparently healthy adults: the ATTICA study. J Am Coll Nutr. 2010;29:357–364. doi: 10.1080/07315724.2010.10719852. [DOI] [PubMed] [Google Scholar]
- 175.Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y, Stefanadis C. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005;46:120–124. doi: 10.1016/j.jacc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 176.van Bussel BC, Henry RM, Schalkwijk CG, Ferreira I, Feskens EJ, Streppel MT, Smulders YM, Twisk JW, Stehouwer CD. Fish consumption in healthy adults is associated with decreased circulating biomarkers of endothelial dysfunction and inflammation during a 6-year follow-up. J Nutr. 2011;141:1719–1725. doi: 10.3945/jn.111.139733. [DOI] [PubMed] [Google Scholar]
- 177.Tani S, Kawauchi K, Atsumi W, Matsuo R, Ashida T, Imatake K, Suzuki Y, Yagi T, Takahashi A, Matsumoto N, Okumura Y. Association among daily fish intake, white blood cell count, and healthy lifestyle behaviors in an apparently healthy Japanese population: implication for the anti-atherosclerotic effect of fish consumption. Heart Vessels. 2021;36:924–933. doi: 10.1007/s00380-020-01769-9. [DOI] [PubMed] [Google Scholar]
- 178.Tani S, Matsuo R, Atsumi W, Kawauchi K, Ashida T, Yagi T, Imatake K, Suzuki Y, Takahashi A, Matsumoto N, Okumura Y. Higher frequency of fish intake may be associated with a lower neutrophil/lymphocyte ratio: anti-atherosclerotic effects of fish consumption. Ann Nutr Metab. 2021;77:146–153. doi: 10.1159/000515915. [DOI] [PubMed] [Google Scholar]
- 179.Acosta-Estrada BA, Reyes A, Rosell CM, Rodrigo D, Ibarra-Herrera CC. Benefits and challenges in the incorporation of insects in food products. Front Nutr. 2021;8:687712. doi: 10.3389/fnut.2021.687712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stull VJ, Finer E, Bergmans RS, Febvre HP, Longhurst C, Manter DK, Patz JA, Weir TL. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci Rep. 2018;8:10762. doi: 10.1038/s41598-018-29032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Khatibi N, Shahvazi S, Nadjarzadeh A, Samadi M, Zare F, Salehi-Abargouei A. Empirically derived dietary patterns and serum inflammatory markers in Iranian female teachers: a cross-sectional study. Nutr Diet. 2019;76:462–471. doi: 10.1111/1747-0080.12463. [DOI] [PubMed] [Google Scholar]
- 182.Jiang Y, Wu SH, Shu XO, Xiang YB, Ji BT, Milne GL, Cai Q, Zhang X, Gao YT, Zheng W, Yang G. Cruciferous vegetable intake is inversely correlated with circulating levels of proinflammatory markers in women. J Acad Nutr Diet. 2014;114:700–8.e2. doi: 10.1016/j.jand.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Navarro SL, Schwarz Y, Song X, Wang CY, Chen C, Trudo SP, Kristal AR, Kratz M, Eaton DL, Lampe JW. Cruciferous vegetables have variable effects on biomarkers of systemic inflammation in a randomized controlled trial in healthy young adults. J Nutr. 2014;144:1850–1857. doi: 10.3945/jn.114.197434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jiang R, Jacobs DR, Jr, Mayer-Davis E, Szklo M, Herrington D, Jenny NS, Kronmal R, Barr RG. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:222–231. doi: 10.1093/aje/kwj033. [DOI] [PubMed] [Google Scholar]
- 185.Yu Z, Malik VS, Keum N, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS, Bao Y. Associations between nut consumption and inflammatory biomarkers. Am J Clin Nutr. 2016;104:722–728. doi: 10.3945/ajcn.116.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ren GY, Chen CY, Chen GC, Chen WG, Pan A, Pan CW, Zhang YH, Qin LQ, Chen LH. Effect of flaxseed intervention on inflammatory marker C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8:136. doi: 10.3390/nu8030136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moreira Alves RD, Boroni Moreira AP, Macedo VS, Bressan J, de Cássia Gonçalves Alfenas R, Mattes R, Brunoro Costa NM. High-oleic peanuts: new perspective to attenuate glucose homeostasis disruption and inflammation related obesity. Obesity (Silver Spring) 2014;22:1981–8. doi: 10.1002/oby.20825. [DOI] [PubMed] [Google Scholar]
- 188.Liu JF, Liu YH, Chen CM, Chang WH, Chen CY. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: a randomized crossover controlled feeding trial. Eur J Nutr. 2013;52:927–935. doi: 10.1007/s00394-012-0400-y. [DOI] [PubMed] [Google Scholar]
- 189.Rajaram S, Connell KM, Sabaté J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. Br J Nutr. 2010;103:907–912. doi: 10.1017/S0007114509992480. [DOI] [PubMed] [Google Scholar]
- 190.Madan J, Desai S, Moitra P, Salis S, Agashe S, Battalwar R, Mehta A, Kamble R, Kalita S, Phatak AG, Udipi SA, Vaidya RA, Vaidya AB. Effect of almond consumption on metabolic risk factors-glucose metabolism, hyperinsulinemia, selected markers of inflammation: a randomized controlled trial in adolescents and young adults. Front Nutr. 2021;8:668622. doi: 10.3389/fnut.2021.668622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fitó M, Cladellas M, de la Torre R, Martí J, Muñoz D, Schröder H, Alcántara M, Pujadas-Bastardes M, Marrugat J, López-Sabater MC, Bruguera J, Covas MI, SOLOS Investigators Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr. 2008;62:570–4. doi: 10.1038/sj.ejcn.1602724. [DOI] [PubMed] [Google Scholar]
- 192.Bogani P, Galli C, Villa M, Visioli F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis. 2007;190:181–186. doi: 10.1016/j.atherosclerosis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 193.Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29:207–211. doi: 10.2337/diacare.29.02.06.dc05-1903. [DOI] [PubMed] [Google Scholar]
- 194.Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, Gibbons SM, La Storia A, Gilbert JA, Jonnalagadda S, Thielecke F, Gallo MA, Scalfi L, Fogliano V. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr. 2015;101:251–261. doi: 10.3945/ajcn.114.088120. [DOI] [PubMed] [Google Scholar]
- 195.Hoevenaars FPM, Esser D, Schutte S, Priebe MG, Vonk RJ, van den Brink WJ, van der Kamp JW, Stroeve JHM, Afman LA, Wopereis S. Whole grain wheat consumption affects postprandial inflammatory response in a randomized controlled trial in overweight and obese adults with mild hypercholesterolemia in the Graandioos Study. J Nutr. 2019;149:2133–2144. doi: 10.1093/jn/nxz177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu SH, Shu XO, Chow WH, Xiang YB, Zhang X, Li HL, Cai Q, Ji BT, Cai H, Rothman N, Gao YT, Zheng W, Yang G. Soy food intake and circulating levels of inflammatory markers in Chinese women. J Acad Nutr Diet. 2012;112(996–1004):1004.e1–4. doi: 10.1016/j.jand.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Saraf-Bank S, Esmaillzadeh A, Faghihimani E, Azadbakht L. Effect of non-soy legume consumption on inflammation and serum adiponectin levels among first-degree relatives of patients with diabetes: a randomized, crossover study. Nutrition. 2015;31:459–465. doi: 10.1016/j.nut.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 198.Hermsdorff HH, Zulet MÁ, Abete I, Martínez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. 2011;50:61–69. doi: 10.1007/s00394-010-0115-x. [DOI] [PubMed] [Google Scholar]
- 199.Noronha NY, Pinhel MAS, Nicoletti CF, Quinhoneiro DCG, Pinhanelli VC, Oliveira BAP, Cortes-Oliveira C, Delfino HBP, Wolf LS, Frantz FG, Marchini JS, Nonino CB. Green tea supplementation improves oxidative stress biomarkers and modulates IL-6 circulating levels in obese women. Nutr Hosp. 2019;36:583–588. doi: 10.20960/nh.2159. [DOI] [PubMed] [Google Scholar]
- 200.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32:421–427. doi: 10.1016/j.nutres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 201.Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, Koenig W, Sundvall J, Bidel S, Kuha S, Tuomilehto J. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91:950–957. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 202.Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–893. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- 203.Loftfield E, Shiels MS, Graubard BI, Katki HA, Chaturvedi AK, Trabert B, Pinto LA, Kemp TJ, Shebl FM, Mayne ST, Wentzensen N, Purdue MP, Hildesheim A, Sinha R, Freedman ND. Associations of coffee drinking with systemic immune and inflammatory markers. Cancer Epidemiol Biomarkers Prev. 2015;24:1052–1060. doi: 10.1158/1055-9965.EPI-15-0038-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zampelas A, Panagiotakos DB, Pitsavos C, Chrysohoou C, Stefanadis C. Associations between coffee consumption and inflammatory markers in healthy persons: the ATTICA study. Am J Clin Nutr. 2004;80:862–867. doi: 10.1093/ajcn/80.4.862. [DOI] [PubMed] [Google Scholar]
- 205.di Giuseppe R, Di Castelnuovo A, Centritto F, Zito F, De Curtis A, Costanzo S, Vohnout B, Sieri S, Krogh V, Donati MB, de Gaetano G, Iacoviello L. Regular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy Italian population. J Nutr. 2008;138:1939–1945. doi: 10.1093/jn/138.10.1939. [DOI] [PubMed] [Google Scholar]
- 206.Jafarirad S, Ayoobi N, Karandish M, Jalali MT, Haghighizadeh MH, Jahanshahi A. Dark chocolate effect on serum adiponectin, biochemical and inflammatory parameters in diabetic patients: a randomized clinical trial. Int J Prev Med. 2018;9:86. doi: 10.4103/ijpvm.IJPVM_339_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kuebler U, Arpagaus A, Meister RE, von Känel R, Huber S, Ehlert U, Wirtz PH. Dark chocolate attenuates intracellular pro-inflammatory reactivity to acute psychosocial stress in men: a randomized controlled trial. Brain Behav Immun. 2016;57:200–208. doi: 10.1016/j.bbi.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 208.Hamed MS, Gambert S, Bliden KP, Bailon O, Singla A, Antonino MJ, Hamed F, Tantry US, Gurbel PA. Dark chocolate effect on platelet activity, C-reactive protein and lipid profile: a pilot study. South Med J. 2008;101:1203–1208. doi: 10.1097/SMJ.0b013e31818859eb. [DOI] [PubMed] [Google Scholar]
- 209.Kunnumakkara AB, Sailo BL, Banik K, Harsha C, Prasad S, Gupta SC, Bharti AC, Aggarwal BB. Chronic diseases, inflammation, and spices: how are they linked? J Transl Med. 2018;16:14. doi: 10.1186/s12967-018-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jiang TA. Health benefits of culinary herbs and spices. J AOAC Int. 2019;102:395–411. doi: 10.5740/jaoacint.18-0418. [DOI] [PubMed] [Google Scholar]
- 211.Hadi V, Kheirouri S, Alizadeh M, Khabbazi A, Hosseini H. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J Phytomed. 2016;6:34–43. [PMC free article] [PubMed] [Google Scholar]
- 212.Amizadeh S, Rashtchizadeh N, Khabbazi A, Ghorbanihaghjo A, Ebrahimi AA, Vatankhah AM, Malek Mahdavi A, Taghizadeh M. Effect of Nigella sativa oil extracts on inflammatory and oxidative stress markers in Behcet’s disease: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J Phytomed. 2020;10:181–189. [PMC free article] [PubMed] [Google Scholar]
- 213.Mahdavi R, Namazi N, Alizadeh M, Farajnia S. Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: a randomized double-blind, placebo-controlled clinical trial. J Clin Lipidol. 2016;10:1203–1211. doi: 10.1016/j.jacl.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 214.Kazemi S, Yaghooblou F, Siassi F, Rahimi Foroushani A, Ghavipour M, Koohdani F, Sotoudeh G. Cardamom supplementation improves inflammatory and oxidative stress biomarkers in hyperlipidemic, overweight, and obese pre-diabetic women: a randomized double-blind clinical trial. J Sci Food Agric. 2017;97:5296–5301. doi: 10.1002/jsfa.8414. [DOI] [PubMed] [Google Scholar]
- 215.Davari M, Hashemi R, Mirmiran P, Hedayati M, Sahranavard S, Bahreini S, Tavakoly R, Talaei B. Effects of cinnamon supplementation on expression of systemic inflammation factors, NF-kB and Sirtuin-1 (SIRT1) in type 2 diabetes: a randomized, double blind, and controlled clinical trial. Nutr J. 2020;19:1. doi: 10.1186/s12937-019-0518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Azimi P, Ghiasvand R, Feizi A, Hariri M, Abbasi B. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev Diabet Stud. 2014;11:258–266. doi: 10.1900/RDS.2014.11.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mashhadi NS, Ghiasvand R, Askari G, Feizi A, Hariri M, Darvishi L, Barani A, Taghiyar M, Shiranian A, Hajishafiee M. Influence of ginger and cinnamon intake on inflammation and muscle soreness endued by exercise in Iranian female athletes. Int J Prev Med. 2013;4:S11–S15. [PMC free article] [PubMed] [Google Scholar]
- 218.Askari F, Rashidkhani B, Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr Res. 2014;34:143–148. doi: 10.1016/j.nutres.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 219.Shishehbor F, Rezaeyan Safar M, Rajaei E, Haghighizadeh MH. Cinnamon consumption improves clinical symptoms and inflammatory markers in women with rheumatoid arthritis. J Am Coll Nutr. 2018:1–6. 10.1080/07315724.2018.1460733. [DOI] [PubMed]
- 220.Zareie A, Sahebkar A, Khorvash F, Bagherniya M, Hasanzadeh A, Askari G. Effect of cinnamon on migraine attacks and inflammatory markers: a randomized double-blind placebo-controlled trial. Phytother Res. 2020;34:2945–2952. doi: 10.1002/ptr.6721. [DOI] [PubMed] [Google Scholar]
- 221.Mammen RR, Natinga Mulakal J, Mohanan R, Maliakel B, Illathu MK. Clove bud polyphenols alleviate alterations in inflammation and oxidative stress markers associated with binge drinking: a randomized double-blinded placebo-controlled crossover study. J Med Food. 2018;21:1188–1196. doi: 10.1089/jmf.2017.4177. [DOI] [PubMed] [Google Scholar]
- 222.Morovati A, Pourghassem Gargari B, Sarbakhsh P, Azari H, Lotfi-Dizaji L. The effect of cumin supplementation on metabolic profiles in patients with metabolic syndrome: a randomized, triple blind, placebo-controlled clinical trial. Phytother Res. 2019;33:1182–1190. doi: 10.1002/ptr.6313. [DOI] [PubMed] [Google Scholar]
- 223.Xu C, Mathews AE, Rodrigues C, Eudy BJ, Rowe CA, O'Donoughue A, Percival SS. Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr ESPEN. 2018;24:148–155. doi: 10.1016/j.clnesp.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 224.Zare E, Alirezaei A, Bakhtiyari M, Mansouri A. Evaluating the effect of garlic extract on serum inflammatory markers of peritoneal dialysis patients: a randomized double-blind clinical trial study. BMC Nephrol. 2019;20:26. doi: 10.1186/s12882-019-1204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.van Doorn MB, Espirito Santo SM, Meijer P, Kamerling IM, Schoemaker RC, Dirsch V, Vollmar A, Haffner T, Gebhardt R, Cohen AF, Princen HM, Burggraaf J. Effect of garlic powder on C-reactive protein and plasma lipids in overweight and smoking subjects. Am J Clin Nutr. 2006;84:1324–1329. doi: 10.1093/ajcn/84.6.1324. [DOI] [PubMed] [Google Scholar]
- 226.Asgharpour M, Khavandegar A, Balaei P, Enayati N, Mardi P, Alirezaei A, Bakhtiyari M. Efficacy of oral administration of Allium sativum powder "garlic extract" on lipid profile, inflammation, and cardiovascular indices among hemodialysis patients. Evid Based Complement Alternat Med. 2021;2021:6667453. doi: 10.1155/2021/6667453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Soleimani D, Parisa Moosavian S, Zolfaghari H, Paknahad Z. Effect of garlic powder supplementation on blood pressure and hs-C-reactive protein among nonalcoholic fatty liver disease patients: a randomized, double-blind, placebo-controlled trial. Food Sci Nutr. 2021;9:3556–3562. doi: 10.1002/fsn3.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mahluji S, Ostadrahimi A, Mobasseri M, Ebrahimzade Attari V, Payahoo L. Anti-inflammatory effects of zingiber officinale in type 2 diabetic patients. Adv Pharm Bull. 2013;3:273–276. doi: 10.5681/apb.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zare Javid A, Bazyar H, Gholinezhad H, Rahimlou M, Rashidi H, Salehi P, Haghighi-Zadeh MH. The effects of ginger supplementation on inflammatory, antioxidant, and periodontal parameters in type 2 diabetes mellitus patients with chronic periodontitis under non-surgical periodontal therapy. A double-blind, placebo-controlled trial. Diabetes Metab Syndr Obes. 2019;12:1751–1761. doi: 10.2147/DMSO.S214333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Daneshi-Maskooni M, Keshavarz SA, Qorbani M, Mansouri S, Alavian SM, Badri-Fariman M, Jazayeri-Tehrani SA, Sotoudeh G. Green cardamom increases Sirtuin-1 and reduces inflammation in overweight or obese patients with non-alcoholic fatty liver disease: a double-blind randomized placebo-controlled clinical trial. Nutr Metab (Lond) 2018;15:63. doi: 10.1186/s12986-018-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cheshmeh S, Ghayyem M, Khamooshi F, Heidarzadeh-Esfahani N, Rahmani N, Hojati N, Mosaieby E, Moradi S, Pasdar Y. Green cardamom plus low-calorie diet can decrease the expression of inflammatory genes among obese women with polycystic ovary syndrome: a double-blind randomized clinical trial. Eat Weight Disord. 2021:1–10. 10.1007/s40519-021-01223-3. [DOI] [PMC free article] [PubMed]
- 232.Jafari S, Sattari R, Ghavamzadeh S. Evaluation the effect of 50 and 100 mg doses of Cuminum cyminum essential oil on glycemic indices, insulin resistance and serum inflammatory factors on patients with diabetes type II: a double-blind randomized placebo-controlled clinical trial. J Tradit Complement Med. 2016;7:332–338. doi: 10.1016/j.jtcme.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Heidari-Beni M, Moravejolahkami AR, Gorgian P, Askari G, Tarrahi MJ, Bahreini-Esfahani N. Herbal formulation "turmeric extract, black pepper, and ginger" versus Naproxen for chronic knee osteoarthritis: a randomized, double-blind, controlled clinical trial. Phytother Res. 2020;34:2067–2073. doi: 10.1002/ptr.6671. [DOI] [PubMed] [Google Scholar]
- 234.Kim KA, Yim JE. The effect of onion peel extract on inflammatory mediators in korean overweight and obese women. Clin Nutr Res. 2016;5:261–269. doi: 10.7762/cnr.2016.5.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Panahi Y, Sahebkar A, Parvin S, Saadat A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann Clin Biochem. 2012;49:580–588. doi: 10.1258/acb.2012.012040. [DOI] [PubMed] [Google Scholar]
- 236.Jarhahzadeh M, Alavinejad P, Farsi F, Husain D, Rezazadeh A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: a randomized double blind clinical trial. Diabetol Metab Syndr. 2021;13:112. doi: 10.1186/s13098-021-00731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Salehi M, Mashhadi NS, Esfahani PS, Feizi A, Hadi A, Askari G. The effects of curcumin supplementation on muscle damage, oxidative stress, and inflammatory markers in healthy females with moderate physical activity: a randomized, double-blind, placebo-controlled clinical trial. Int J Prev Med. 2021;12:94. doi: 10.4103/ijpvm.IJPVM_138_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Saraf-Bank S, Ahmadi A, Paknahad Z, Maracy M, Nourian M. Effects of curcumin supplementation on markers of inflammation and oxidative stress among healthy overweight and obese girl adolescents: a randomized placebo-controlled clinical trial. Phytother Res. 2019;33:2015–2022. doi: 10.1002/ptr.6370. [DOI] [PubMed] [Google Scholar]
- 239.Saadati S, Sadeghi A, Mansour A, Yari Z, Poustchi H, Hedayati M, Hatami B, Hekmatdoost A. Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019;19:133. doi: 10.1186/s12876-019-1055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Saier MH, Jr, Mansour NM. Probiotics and prebiotics in human health. J Mol Microbiol Biotechnol. 2005;10:22–25. doi: 10.1159/000090345. [DOI] [PubMed] [Google Scholar]
- 241.Tabrizi R, Ostadmohammadi V, Lankarani KB, Akbari M, Akbari H, Vakili S, Shokrpour M, Kolahdooz F, Rouhi V, Asemi Z. The effects of probiotic and synbiotic supplementation on inflammatory markers among patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Eur J Pharmacol. 2019;852:254–264. doi: 10.1016/j.ejphar.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 242.Zhang XF, Guan XX, Tang YJ, Sun JF, Wang XK, Wang WD, Fan JM. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: a systematic review and meta-analysis. Eur J Nutr. 2021;60:2855–2875. doi: 10.1007/s00394-021-02503-5. [DOI] [PubMed] [Google Scholar]
- 243.Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22:849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 244.Compare D, Rocco A, Coccoli P, Angrisani D, Sgamato C, Iovine B, Salvatore U, Nardone G. Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: an ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2017;17:53. doi: 10.1186/s12876-017-0605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Eichelmann F, Schwingshackl L, Fedirko V, Aleksandrova K. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes Rev. 2016;17:1067–1079. doi: 10.1111/obr.12439. [DOI] [PubMed] [Google Scholar]
- 246.Jaceldo-Siegl K, Haddad E, Knutsen S, Fan J, Lloren J, Bellinger D, Fraser GE. Lower C-reactive protein and IL-6 associated with vegetarian diets are mediated by BMI. Nutr Metab Cardiovasc Dis. 2018;28:787–794. doi: 10.1016/j.numecd.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Craddock JC, Neale EP, Peoples GE, Probst YC. Vegetarian-based dietary patterns and their relation with inflammatory and immune biomarkers: a systematic review and meta-analysis. Adv Nutr. 2019;10:433–451. doi: 10.1093/advances/nmy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lankinen M, Uusitupa M, Schwab U. Nordic diet and inflammation-a review of observational and intervention studies. Nutrients. 2019;11:1369. doi: 10.3390/nu11061369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ulven SM, Holven KB, Rundblad A, Myhrstad MCW, Leder L, Dahlman I, Mello VD, Schwab U, Carlberg C, Pihlajamäki J, Hermansen K, Dragsted LO, Gunnarsdottir I, Cloetens L, Åkesson B, Rosqvist F, Hukkanen J, Herzig KH, Savolainen MJ, Risérus U, Thorsdottir I, Poutanen KS, Arner P, Uusitupa M, Kolehmainen M. An isocaloric nordic diet modulates RELA and TNFRSF1A gene expression in peripheral blood mononuclear cells in individuals with metabolic syndrome-a SYSDIET sub-study. Nutrients. 2019;11:2932. doi: 10.3390/nu11122932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Calvo-Malvar Mdel M, Leis R, Benítez-Estévez AJ, Sánchez-Castro J, Gude F. A randomised, family-focused dietary intervention to evaluate the Atlantic diet: the GALIAT study protocol. BMC Public Health. 2016;16:820. doi: 10.1186/s12889-016-3441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Guallar-Castillón P, Oliveira A, Lopes C, López-García E, Rodríguez-Artalejo F. The Southern European Atlantic Diet is associated with lower concentrations of markers of coronary risk. Atherosclerosis. 2013;226:502–509. doi: 10.1016/j.atherosclerosis.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 252.Konishi K. Associations between healthy Japanese dietary patterns and depression in Japanese women. Public Health Nutr. 2021;24:1753–1765. doi: 10.1017/S1368980020001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pan MH, Chiou YS, Tsai ML, Ho CT. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complement Med. 2011;1:8–24. doi: 10.1016/s2225-4110(16)30052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Santiago-Torres M, Tinker LF, Allison MA, Breymeyer KL, Garcia L, Kroenke CH, Lampe JW, Shikany JM, Van Horn L, Neuhouser ML. Development and use of a traditional Mexican diet score in relation to systemic inflammation and insulin resistance among women of Mexican descent. J Nutr. 2015;145:2732–2740. doi: 10.3945/jn.115.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Valerino-Perea S, Lara-Castor L, Armstrong MEG, Papadaki A. Definition of the traditional Mexican diet and its role in health: a systematic review. Nutrients. 2019;11:2803. doi: 10.3390/nu11112803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Whalen KA, McCullough ML, Flanders WD, Hartman TJ, Judd S, Bostick RM. Paleolithic and Mediterranean diet pattern scores are inversely associated with biomarkers of inflammation and oxidative balance in adults. J Nutr. 2016;146:1217–1226. doi: 10.3945/jn.115.224048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Saneei P, Hashemipour M, Kelishadi R, Esmaillzadeh A. The Dietary Approaches to Stop Hypertension (DASH) diet affects inflammation in childhood metabolic syndrome: a randomized cross-over clinical trial. Ann Nutr Metab. 2014;64:20–27. doi: 10.1159/000358341. [DOI] [PubMed] [Google Scholar]
- 258.Sakhaei R, Shahvazi S, Mozaffari-Khosravi H, Samadi M, Khatibi N, Nadjarzadeh A, Zare F, Salehi-Abargouei A. The Dietary Approaches to Stop Hypertension (DASH)-style diet and an alternative mediterranean diet are differently associated with serum inflammatory markers in female adults. Food Nutr Bull. 2018;39:361–376. doi: 10.1177/0379572118783950. [DOI] [PubMed] [Google Scholar]
- 259.Juraschek SP, Kovell LC, Appel LJ, Miller ER, 3rd, Sacks FM, Chang AR, Christenson RH, Rebuck H, Mukamal KJ. Effects of diet and sodium reduction on cardiac injury, strain, and inflammation: the DASH-Sodium trial. J Am Coll Cardiol. 2021;77:2625–2634. doi: 10.1016/j.jacc.2021.03.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Urpi-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, Valderas-Martínez P, Arranz S, Andres-Lacueva C, Llorach R, Medina-Remón A, Lamuela-Raventos RM, Estruch R. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol Res. 2012;65:577–583. doi: 10.1016/j.phrs.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 261.Estruch R. Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc. 2010;69:333–340. doi: 10.1017/S0029665110001539. [DOI] [PubMed] [Google Scholar]
- 262.Urpi-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, Valderas-Martínez P, Salas-Salvadó J, Covas MI, Toledo E, Andres-Lacueva C, Llorach R, García-Arellano A, Bulló M, Ruiz-Gutierrez V, Lamuela-Raventos RM, Estruch R. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J Nutr. 2012;142:1019–1025. doi: 10.3945/jn.111.148726. [DOI] [PubMed] [Google Scholar]
- 263.Urpi-Sarda M, Casas R, Sacanella E, Corella D, Andrés-Lacueva C, Llorach R, Garrabou G, Cardellach F, Sala-Vila A, Ros E, Ruiz-Canela M, Fitó M, Salas-Salvadó J, Estruch R. The 3-year effect of the mediterranean diet intervention on inflammatory biomarkers related to cardiovascular disease. Biomedicines. 2021;9:862. doi: 10.3390/biomedicines9080862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51:794–811. doi: 10.1016/j.immuni.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 265.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 266.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137:992–998. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 267.Lopes AEDSC, Araújo LF, Levy RB, Barreto SM, Giatti L. Association between consumption of ultra-processed foods and serum C-reactive protein levels: cross-sectional results from the ELSA-Brasil study. Sao Paulo Med J. 2019;137:169–176. doi: 10.1590/1516-3180.2018.0363070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Martins GMDS, França AKTDC, Viola PCAF, Carvalho CA, Marques KDS, Santos AMD, Batalha MA, Alves JDA, Ribeiro CCC. Intake of ultra-processed foods is associated with inflammatory markers in Brazilian adolescents. Public Health Nutr. 2021:1–9. 10.1017/S1368980021004523. [DOI] [PMC free article] [PubMed]
- 269.Bärebring L, Winkvist A, Gjertsson I, Lindqvist HM. Poor dietary quality is associated with increased inflammation in Swedish patients with rheumatoid arthritis. Nutrients. 2018;10:1535. doi: 10.3390/nu10101535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291–315. doi: 10.1146/annurev-nutr-082018-124320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhu S, Cui L, Zhang X, Shu R, VanEvery H, Tucker KL, Wu S, Gao X. Habitually skipping breakfast is associated with chronic inflammation: a cross-sectional study. Public Health Nutr. 2021;24:2936–2943. doi: 10.1017/S1368980020001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nas A, Mirza N, Hägele F, Kahlhöfer J, Keller J, Rising R, Kufer TA, Bosy-Westphal A. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am J Clin Nutr. 2017;105:1351–1361. doi: 10.3945/ajcn.116.151332. [DOI] [PubMed] [Google Scholar]
- 273.Zouhal H, Bagheri R, Ashtary-Larky D, Wong A, Triki R, Hackney AC, Laher I, Abderrahman AB. Effects of Ramadan intermittent fasting on inflammatory and biochemical biomarkers in males with obesity. Physiol Behav. 2020;225:113090. doi: 10.1016/j.physbeh.2020.113090. [DOI] [PubMed] [Google Scholar]
- 274.Liu B, Hutchison AT, Thompson CH, Lange K, Heilbronn LK. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes Res Clin Pract. 2019;13:408–415. doi: 10.1016/j.orcp.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 275.Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72:308–318. doi: 10.1111/nure.12104. [DOI] [PubMed] [Google Scholar]
- 276.Moro T, Tinsley G, Longo G, Grigoletto D, Bianco A, Ferraris C, Guglielmetti M, Veneto A, Tagliabue A, Marcolin G, Paoli A. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: a randomized controlled trial. J Int Soc Sports Nutr. 2020;17:65. doi: 10.1186/s12970-020-00396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Razavi R, Parvaresh A, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N, Clark CCT, Morteza SS. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int J Vitam Nutr Res. 2021;91:242–250. doi: 10.1024/0300-9831/a000623. [DOI] [PubMed] [Google Scholar]
- 278.Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J, Schroeder S, Tadic J, Eisenberg T, Magnes C, Stumpe M, Zuegner E, Bordag N, Riedl R, Schmidt A, Kolesnik E, Verheyen N, Springer A, Madl T, Sinner F, de Cabo R, Kroemer G, Obermayer-Pietsch B, Dengjel J, Sourij H, Pieber TR, Madeo F. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019;30:462–476.e6. doi: 10.1016/j.cmet.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 279.Kinsey AW, Ormsbee MJ. The health impact of nighttime eating: old and new perspectives. Nutrients. 2015;7:2648–2662. doi: 10.3390/nu7042648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Martínez-Lozano N, Tvarijonaviciute A, Ríos R, Barón I, Scheer FAJL, Garaulet M. Late eating is associated with obesity, inflammatory markers and circadian-related disturbances in school-aged children. Nutrients. 2020;12:2881. doi: 10.3390/nu12092881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Curti ML, Jacob P, Borges MC, Rogero MM, Ferreira SR. Studies of gene variants related to inflammation, oxidative stress, dyslipidemia, and obesity: implications for a nutrigenetic approach. J Obes. 2011;2011:497401. doi: 10.1155/2011/497401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ramos-Lopez O, Milagro FI, Riezu-Boj JI, Martinez JA. Epigenetic signatures underlying inflammation: an interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm Res. 2021;70:29–49. doi: 10.1007/s00011-020-01425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Aranaz P, Ramos-Lopez O, Cuevas-Sierra A, Martinez JA, Milagro FI, Riezu-Boj JI. A predictive regression model of the obesity-related inflammatory status based on gut microbiota composition. Int J Obes (Lond) 2021;45:2261–2268. doi: 10.1038/s41366-021-00904-4. [DOI] [PubMed] [Google Scholar]
- 284.Saklatvala J, Dean J, Clark A. Control of the expression of inflammatory response genes. Biochem Soc Symp. 2003;70:95–106. doi: 10.1042/bss0700095. [DOI] [PubMed] [Google Scholar]
- 285.Fitzpatrick M, Young SP. Metabolomics–a novel window into inflammatory disease. Swiss Med Wkly. 2013;143:w13743. doi: 10.4414/smw.2013.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Palmnäs M, Brunius C, Shi L, Rostgaard-Hansen A, Torres NE, González-Domínguez R, Zamora-Ros R, Ye YL, Halkjær J, Tjønneland A, Riccardi G, Giacco R, Costabile G, Vetrani C, Nielsen J, Andres-Lacueva C, Landberg R. Perspective: metabotyping-a potential personalized nutrition strategy for precision prevention of cardiometabolic disease. Adv Nutr. 2020;11:524–532. doi: 10.1093/advances/nmz121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ramos-Lopez O, Milton-Laskibar I, Martínez JA, Collaborators: Rodrigo San-Cristobal and Maria P. Portillo Precision nutrition based on phenotypical traits and the (epi)genotype: nutrigenetic and nutrigenomic approaches for obesity care. Curr Opin Clin Nutr Metab Care. 2021;24:315–325. doi: 10.1097/MCO.0000000000000754. [DOI] [PubMed] [Google Scholar]