Abstract
Natural antisense transcripts (NATs) are coding or non-coding RNA sequences transcribed on the opposite direction from the same genomic locus. NATs are widely distributed throughout the human genome and seem to play crucial roles in physiological and pathological processes, through newly described and targeted mechanisms. NATs represent the intricate complexity of the genome organization and constitute another layer of potential targets in disease. Here, we focus on the interesting and unique role of non-coding NATs in cancer, paying particular attention to those acting as miRNA sponges.
Keywords: Long non-coding RNAs, lncRNAs, Antisense transcripts, ceRNAs, Cancer
1. Introduction
Over the years, several studies pointed out to the presence of RNA molecules that were transcribed but did not encode proteins, making up the so-called non-coding genome. In fact, comparative analyses carried out between mammalian genomes and transcriptomes led to the important discovery that around three-quarters of genomic DNA were transcribed [1], [2], [3], greatly contrasting with the 2 % that is translated into proteins [1].
Extensive annotation has revealed that about 80 % of the human genome is functional. Indeed, several biochemical functions have been assigned to the non-coding portion of the genome, including transcription, association of transcription factors, chromatin structure and histone modification. This number overwhelmingly exceeds the percentage of the genome assigned to coding proteins, making it clear that the non-coding portion of the genome is not randomly transcribed and is involved in several important biological processes [4].
Non-coding RNAs can be divided according to their length into small and long non-coding RNAs (lncRNAs). The latter constitute a class of RNA molecules of more than 200 nucleotides, and account for the largest, yet less described, class of non-coding RNAs [5], [6]. Many efforts have been applied to distinguish lncRNA from coding RNA transcripts [5], [7], [8], [9]. In some cases, the absence of a detectable ORF is the only biochemical dissimilarity between lncRNAs and messenger RNA (mRNA).
LncRNAs are often classified according to their location relative to neighboring genes [10]. As such, these transcripts can be divided into sense, antisense, intronic, intergenic and bidirectional according to their positioning to proximal protein coding genes [11]. Here, we will focus only on the particular characteristics of antisense lncRNAs. These transcripts overlap at least one exon of another gene, and originate from the antisense strand [11]. Antisense lncRNAs usually overlap protein-coding genes, but some may overlap other lncRNAs [8], [12], [13]. Our main goal is to exemplify antisense transcripts in cancer, with particular emphasis on those acting as microRNA (miRNA) sponges and their role in cancer aggressiveness, metabolism, response to chemotherapy and the epithelial-mesenchymal transition (EMT). miRNA sponges are lncRNAs shown to regulate gene expression through direct binding of specific miRNAs [14], [15], [16], [17], [18]. These lncRNAs have been termed competing endogenous RNAs (ceRNAs), and have been implicated in several biological processes and diseases, including cancer [19], [20], [21], [22].
2. General overview of antisense lncRNAs
Natural antisense transcripts (NATs) have been considered to be extremely common throughout the mouse and human genomes [23]; in fact, around 70 % of mammalian genes are known to produce NATs [24]. Although these RNA molecules may include coding or non-coding sequences that are complementary to either coding or non-coding transcripts [25], [26], here we will specifically describe the biological roles of non-coding NATs (hereafter referred to as ncNATs) complementary to either coding or non-coding sequences.
ncNATs can arise from a variety of promoters, such as independent, bidirectional or cryptic. Bidirectional promoters are shown to generate large numbers of ncRNAs, including in humans. Several factors have been shown to influence promoter bidirectionality, such as chromatin organization and polyadenylation signals that surround the promoter [27], [28]. In fact, bidirectional transcription can also be originated from double strand breaks, demonstrating the interactions between transcription and DNA damage [29]. ncNATs can also be generated from cryptic promoters that are positioned either within the transcribed or from the termination regions of the sense gene [30].. Regarding regulation at the RNA level, several ncNATs, in yeast, for example, are considered cryptic unstable transcripts and are targeted for early degradation by nuclear exosomes. Other ncNATs are degraded by cytoplasmic RNA exonuclease [31] or regulated by 5′ decapping activity [32].
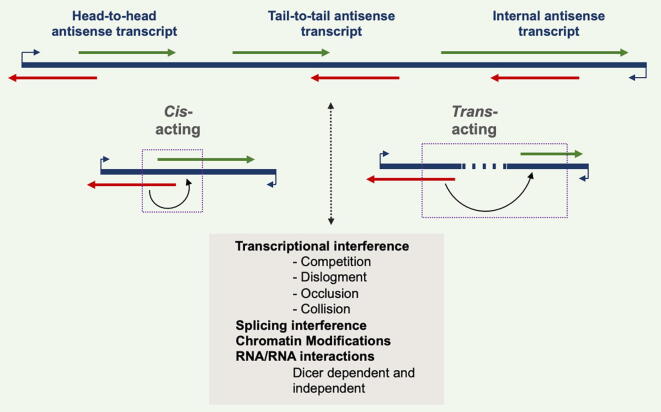
Depending on the location of their target genes, ncNATs can be classified as: cis-NATs, if transcribed from the opposite strand at the same genomic locus (and exhibiting perfect sequence complementarity to their targets); or trans-NATs, if transcribed from different genomic loci (and usually displaying imperfect sequence complementarity) [33], [34]. Stalled RNA polymerases, R-loops and triples helices have been shown to help retain NATs at their site of transcription, allowing cis-acting NATs to exert their function. On the other hand, the three-dimensional organization of chromatin can lead to the interaction of regions of antisense transcription with other loci, thus mediating trans effects (reviewed in [35]). cis-NATs are further classified according to how they overlap with their targets, and are categorized as: head-to-head, whose 5′ end overlaps with the 5′ of the target transcript; tail-to-tail, whose 3′ overlaps with the 3′ of the target gene; and internal, which entirely bind to their target. cis-NATs can also be classified as nearby NATs, which are located very close to their targets, but do not, in fact, overlap [24], [36] (Fig. 1).
Fig. 1.
NAT classification and mechanisms of regulation [35]. Sense and antisense pairs can be head-to-head, tail-to-tail or fully overlapped [26]. NATs can regulate the genes in the vicinity (cis-NATs) or at large distance in the genome (trans-NATs) [82]. A proposed mechanism of action may involve a direct impact on the transcription of the cognate genes [83], through interference with the process of splicing, affecting the DNA or chromatin epigenetic signature or affect mature RNAs by RNA/RNA interactions, through DICER-dependent or -independent mechanisms [84], [85].
ncNATs, just like other lncRNAs, exhibit tissue-specific expression, which suggests that there are many evolutionarily conserved functional roles associated with these transcripts [37]. Regarding the effect in relation to the cognate sequence, their expression may be concordant (associated with an increased expression) [38] or discordant (associated with a decrease in expression levels) [39]. ncNATs may affect negatively or positively the expression of sense transcription, and their expression is closed related with the regulation of their sense gene [40], [41]. Indeed, the regulation of promoter specificities is a potential mechanism of ncNATs in cancer. For instance, HNF4A-AS1L selectively activates the HNF4A P1 promoter via HNF1A, which upregulates the expression of tumor suppressor P1-driven isoforms [42]. RNA-seq data has identified several ncNATs whose expression correlates specifically with the activity of one promoter of their sense gene. Silencing of these ncNATs was shown to alter the promoter usage, demonstrating how these transcripts may regulate promoter-specific programs under different contexts [42].
ncNATs are more frequently located in the nucleus, but they can also be found in the cytoplasm [24], and, like other lncRNAs, contain specific motifs that interact with DNA, RNA and proteins [5], meaning that these transcripts can affect gene expression at the transcriptional, post-transcriptional and translational levels. As such, ncNATs can regulate gene expression by DNA methylation (eg. LUC7L), histone modification (eg. XIST/TSIX, HOTAIR), transcriptional interference (eg. GNG12-AS), regulation of alternative splicing (Zeb2-NAT), and regulation of mRNA stability, either by masking of miRNA binding sites (eg. BACE1-AS) or sponging miRNAs (eg. HOTAIR, NR2F1-AS1) [24], [43]. Interestingly, some ncNATs regulate gene expression through DICER-dependent mechanisms (eg. NAT6531), giving rise to small RNAs with roles in the epigenetic regulation of gene expression. The regulation of mRNA stability through miRNA sponging is of particular interest, as several recently identified ncNATs function as ceRNAs (discussed below). Furthermore, several ncNATs have been detected in both the nucleus and cytoplasm, highlighting their role in the regulation of gene expression through multiple mechanisms.
2.1. Challenges in the detection of ncNATs and how it impacts on their understanding
The overlap of antisense transcripts with their sense counterparts, their relative low expression levels and limited evolutionary conservation, have limited the use of high-throughput approaches to identify these transcripts (reviewed in [35]). In fact, antisense transcription has been carefully exploited at a large scale even before the advent of massive sequencing [44], [45], [46], [47]. Further, detecting active transcription using global run-on sequencing (GRO-seq), native elongating transcript sequencing (NET-seq) or, more recently, POINT-technology, have been shown to be essential to identify antisense transcription and, therefore, antisense transcripts [28], [48], [49]. It is important, however, to discriminate between antisense transcription and antisense transcripts, because the former not always gives rise to functional RNAs, either coding or non-coding. In fact, antisense transcription is known to impact locally on gene expression programs through a variety of mechanisms not always mediated through ncRNAs. The advent of new technologies has been supporting the understanding of transcription complexity (Table 1). Indirect detection of antisense transcripts through chromatin modification states has also been employed, although this approach is rather limited due to the lack of strand specificity of chromatin modification and the generally higher expression levels of sense transcripts [50] (reviewed in [43]). The genomic arrangement of ncNATs has made it difficult to determine their function by loss-of-function studies without affecting the expression of the corresponding sense transcript [35], [51]. To bypass this problem, the analysis of genome-wide gene expression levels at several time points and its comparison to the transcriptional response of cells after modulation of components that are involved in gene regulation by antisense transcription (such as histone-modifying enzymes) has proven to be a more suitable approach [52], [53].
Table 1.
A variety of techniques can be used to detect novel NATs [86]. Most of the methods include sequencing, being RNA-seq the most basic and versatile application of this principle, followed by microarrays. Depending on the length or other specificities of the target RNAs, enrichment or size selection can be done to optimize the screening. A strand-specific protocol should be preferred for the analysis of the antisense strand and its characteristics. It is also possible to analyze specifically the expression of nascent transcripts, i.e., GRO-seq. CAGE and SAGE are examples of methods that sequence the ends of the transcripts. Techniques such as ChIP-seq or ChIRP analyze the indirect effects of their transcription in chromatin signatures, but this might prove a limitation when NATs overlap coding genes. Polyadenylation is also uncommon in NATs so poly(A)-dependent methods, such as 3P-seq, might not detect all the antisense transcripts present in a sample [87], [88]. When NAT structure is already known and annotated, its expression can be further evaluated using RT-qPCR or in situ imaging techniques. There has also been a quick evolution with single-cell adaptations, and it is now already possible to apply several omics at once. Finally, only a handful of the techniques listed have been put to practice in NAT identification, so further advances in this field are to be expected, with the consideration that the best analyses may come from a combined application of several techniques.
| Method | Principle | Advantages | Limitations | Ref Original |
|---|---|---|---|---|
| 4Su-seq | Metabolic labelling of nascent RNA using 4sU-tagging | Changes in RNA kinetics are more visible than with total RNA; more sensitive to transient RNA than RNA-seq | More input required; library preparation may introduce some bias for large transcripts; only a short amount of 3′regions are labelled during the short 4sU exposure leading to 5′ bias | [89] |
| CAGE (Cap Analysis of Gene Expression) | Sequencing method that analyzes 5′ end termini of transcripts | High accuracy and single nucleotide resolution in a high-throughput manner | Not suited for the characterization of novel NATs | [90] |
| ChIP-seq (Chromatin immunoprecipitation) | Detection of chromatin signatures through combination of chromatin immunoprecipitation with RNA-seq, allowing evaluation of expression and primary structure of genes encoding transcripts | High-throughput, sensitivity and specificity, and low cost and input requirement | High complexity; inappropriate to ncRNAs overlapping protein-coding genes and short transcripts; detects all RNA polymerase II (Pol II)-RNA complexes, not just those that are actively engaged | [91] |
| ChIRP-seq (Chromatin Isolation by RNA Purification) | Identification of binding sites and interactions between transcripts and chromatin using tiling oligonucleotides retrieval followed by sequencing | Indicative of putative transcription factor activity for a given ncRNA | Noise caused by precipitation of non-specific DNA fragments from off-target hybridization; prior knowledge of NATs sequence needed | [92] |
| CLIP (Cross-linking immunoprecipitation) | Detects transcripts that interact with RNA Binding Proteins (RBP); RIP followed by sequencing, with variations on the technique depending on the purpose i.e., HITS-CLIP, PAR-CLIP, iCLIP, CRAC | High resolution; can detect unstable transient interactions as well; covalent binding allows washing and purification | UV cross-linking may result in partial degradation of RNA samples; PCR amplification needed to compensate low efficiency if crosslinking | [93] |
| GRO-seq (Global Run-On sequencing) | Sequencing method that analyzes nascent transcripts thus differentiating between transcriptionally active and inactive regions | High resolution and specificity | Detects low but significant amount of antisense transcripts; does not necessarily reflect transcription in vivo | [28] |
| Imaging | Several imaging techniques allow for in situ hybridization of transcripts directly on tissues i.e., smFISH, MERFISH, seqFISH, FISSEQ and SPOTs | Highly specific probes that are easy to use and allow for spatial and contextual information | Small probes can lead to false positives, high background, overcrowding, less sensitivity and spatial resolution | SmFISH- [94], [95], [96] MERFISH- [97] SeqFISH- [98] Fisseq- [99], [100] Spots- [101] |
| Microarrays | Detection of transcripts through hybridization with nucleic acids | Well established, affordable, flexible customization and easy analysis; the use of artificial anti-sense sequence (AFAS) probes identifies unannotated NATs undetectable with cDNA; Affymetrix chips allow a sensitive analysis of most protein coding mRNAs in a more affordable and timely manner | Many non-coding transcripts cannot be detected with standard microarrays due to their design | [102], [103], [104], [105] |
| Tiling microarrays | Detection of transcripts through hybridization with nucleic acids | Identifies more unknown transcripts than RNA-Seq | High false-positive rate; inadequate for low expression transcripts | [106] |
| Nanostring | Directly measures expression of transcripts using probe hybridization and imaging | High automation, specificity, and sensitivity; also detects low expression transcripts without amplification and cDNA production | Requires previous knowledge of the transcript sequence | [107] |
| Nascent-seq | Isolation and sequencing of nascent transcripts | Changes in RNA kinetics are more visible than with total RNA | Many NATs are not polyadenylated and cannot be detected through polyadenylation isolation and sequencing | [108], [109] |
| Northern Blot | Probe labelling of RNAs transferred to a membrane that were size separated using gel electrophoresis | Allows estimation of RNA size; cheap and simple | No signal amplification; larger probes are more specific but are more prone to create background; labor-intensive; poor reproducibility; sensitivity impacted by RNA degradation; poor quantitative technique | [110] |
| NET-seq (Native elongating transcript sequencing) | Sequencing of the whole nascent transcriptome that is attached to polymerase | Single-nucleotide resolution of RNA polymerase II (Pol II) associated transcripts | Only detects still attached to RNA polymerase II (Pol II); cannot distinguish between Pol II accumulation and co-transcriptional cleavage | [48] |
| PRO-seq (Precision nuclear Run-On and sequencing assay) | Analyzes nascent RNAs from their 3′ ends that are attached to polymerase | High sensitivity and base-pair resolution | Does not distinguish between Pol I, II and III transcription | [111] |
| POINT (Polymerase intact nascent transcript) | Purification and sequencing of nascent transcripts | Intact nascent RNA and fast | Only detects still attached RNA polymerase II (Pol II); cannot distinguish between Pol II accumulation and co-transcriptional cleavage | [49] |
| PolyA-seq | Sequencing of poly(A)-associated regions of transcripts | High sensitivity; quantitative analysis of novel 3′ UTRs without low false positives; better than RNA-seq to detect short transcripts; already strand-specific | Many NATs are not polyadenylated | [112] |
| Poly(A)-position profiling by sequencing (3P-Seq) | Sequencing of 3′ UTR of polyadenylated transcripts | High-throughput | Many NATs are not polyadenylated | [113] |
| RT-qPCR | PCR amplification of cDNA | Specific and widely used to evaluate expression | Requires previous knowledge of the transcript sequence | [114] |
| RACE (Rapid amplification of cDNA ends) | Sequencing method following PCR amplification | Efficient and precise annotation of single and low abundance transcripts | Less efficiency with larger fragments, in which cases a cDNA library could be done; requires previous knowledge of a short part of the sequence for primer construction | [115] |
| RIP (RNA immunoprecipitation) | Determination of transcript targets of a given RNA-binding protein (RBP) in vivo | Gold standard that also allows to detect nascent NATs | Only captures the relatively stable RNA–protein complexes and may result in under-representation of transient interactions | [116] |
| RNA CaptureSeq | Deep-sequencing method that constructs tiling arrays of regions of interest against which captured cDNAs of transcripts are hybridized | Quantitative analysis with increased sequencing depth, that allows recognition of rare and unannotated NATs | Hybridization might add undesired artifacts | [117] |
| RNA-PET (RNA paired-end ditags) or GIS-PET | Sequencing of 5′ and 3′ end regions of transcripts | Validates and maps boundaries of polyadenylated transcripts | Requires large amount of sample; short tags render less mapping specificity; many NATs are not polyadenylated | [118], [119] |
| RNA-seq | Sequencing method that identifies and characterizes transcripts; adaptations can be made to sequence the wanted targets i.e., Targeted RNA-seq, Strand-specific RNA (ssRNA-seq), Short RNA-seq, Single-cell RNA-seq (scRNA-seq) | Can detect novel transcripts, of various sizes, expression levels and kinds with a high resolution and sequencing depth | Long RNAs need to be fragmented and assembled in libraries, which may lead to unprecise genomic boundaries; needs complementary experimentation to exclude transcriptional noise | [120], [121], [122], [123], [124], [125], [126] |
| RT-PCR-seq | Deep-sequencing method combined with highly multiplexed PCR amplification of cDNA of transcripts | Identifies novel exons of known transcripts | Primer design limits the number of known or predicted testable junctions to be validated | [127] |
| SAGE (Serial Analysis of Gene Expression) | Sequencing method that analyzes 3′ end termini of transcripts (poly(A)-associated regions/sites) | Quantitative and qualitative analysis; can identify novel transcripts | Many NATs are not polyadenylated | [128] |
| Single-cell multiomics | Simultaneous sequencing of the transcriptome and other omics, such as genomics i.e., DR-seq and G&T-seq; epigenomics i.e., scM&T-seq and scMT-seq; and proteomics i.e., PLAYR and SPARC | Allows a contextual analysis of the cell, useful in cancer studies and diagnosis | Expensive; amplification errors; allelic and locus dropout; challenging single-base resolution | [129], [130], [131], [132], [133], [134] |
| TimeLapse-seq | Metabolic labelling of nascent RNA using 4sU-tagging followed by sequencing | Results in temporal information; already has internal normalization; low input required; changes in RNA dynamics are more visible than with total RNA | Reads beyond the poly-A termination signal are rare, and with restricted time, only 3′ ends are labelled, which unables detection of many NATs that are not polyadenylated | [135] |
| TT-seq (Transient transcriptome sequencing) | Metabolic labelling of nascent RNA using 4sU-tagging followed by sequencing | Low input; better than SLAM-seq in analyzing transient RNA species since most ncRNAs are quickly degraded; allows tracking of RNA kinetics; overcomes 4su-seq 5′ bias with RNA fragmentation before isolation thus mapping transcribed regions uniformly | With restricted time, only 3′ end is labelled and many NATs are not polyadenylated | [136] |
| SLAM-seq (Thiol (SH)-Linked Alkylation for the Metabolic sequencing of RNA) | Metabolic labelling of nascent RNA using 4sU-tagging followed by sequencing | Allows tracking of RNA and kinetics; rapid, low input and high throughput | Many NATs are not polyadenylated; the cellular 4sU uptake kinetics, transcriptional activity and library sequencing depth may limit the detection of nascent labelled transcripts; an assessment should be made to know toxic concentrations of 4sU for the cell type | [137] |
3. Selected cancer-related ncNATs
Several ncNATs have been associated to cancer, and their roles have been elucidated in several reviews (see references [24], [43]). For instance, TALAM1 (MALAT1 antisense RNA) is a ncNAT that has been linked to the expression of lncRNA MALAT1 (metastasis-associated lung adenocarcinoma transcript 1). Zong and colleagues revealed that TALAM1 interacts with MALAT1 at its site of transcription, promoting its RNase P-mediated 3′ end cleavage. Interestingly, MALAT1 positively regulates the transcription and RNA stability of TALAM1. The dynamic between these two lncRNAs has been explored in breast cancer. Both MALAT1 and TALAM1 were shown to be upregulated in MCF7 (luminal A) and MDA-MB-231 (triple negative) cell lines. An upregulation of both MALAT1 and TALAM1 was also observed in MCF 10A cells (derived from normal breast epithelium) after exposure to TGF-β. Silencing TALAM1 negatively impacted the ability of breast cancer cells to migrate in vitro and to develop lung metastasis in immunocompromised mice. Ultimately, this study suggests that MALAT1 and TALAM1 act together to regulate breast cancer aggressiveness and malignancy [54].
FGD5-AS1 (FGD5 antisense RNA 1) was shown to be overexpressed in pancreatic cancer and bind to miR-577, which targets β-catenin and LRP6 (low‑density lipoprotein receptor‑related protein 6) [55]. Downregulation of FGD5-AS1 led to increased levels of miR-577, resulting in decreased levels of β-catenin and LRP6. This decrease was associated with a reduction in the Wnt signaling pathway and inhibition of cell proliferation, migration and invasion [55]. CERS6-AS1 (ceramide synthase 6 antisense RNA 1) was also identified as being overexpressed in both pancreatic tissue samples and cell lines [56]. According to study conducted by Gao and colleagues, CERS6-AS1 acts as a ceRNA by interacting with miR-195-5p, resulting in an increase of WIPI2. Silencing this antisense transcript resulted in an increase of miR-195-5p and a decrease of WIPI2, which was accompanied by a decrease of cell proliferation, evidenced by a reduction in EdU positive cells, and an increase of apoptotic cells. Remarkably, overexpression of WIPI2 reversed the effects of CERS6-AS1 silencing. Also in pancreatic cancer, Zhang and colleagues observed increased expression levels of SLCO4A1-AS1 (solute carrier organic anion transporter family member 4A1 antisense RNA 1) in cancer samples. This lncRNA also targeted a miRNA, miR-4673, to derepress KIF21B. Comparable to other antisense transcripts identified in pancreatic cancer, silencing SLCO4A1-AS1 led to a decrease in cell viability and migration, and an increase of apoptosis [57].
LncRNA AFAP1-AS1 (actin filament-associated protein 1 antisense RNA 1) was discovered to play an important role in retinoblastoma, which was shown to be overexpressed in tumor samples and cell lines. AFAP1-AS1 acts as a ceRNA to suppress miR-545-3p, which in turn derepresses its target GNB1. By silencing AFAP1-AS1, miR-545-3p is able to inhibit GNB1, and this was associated with decreased cell proliferation and migration [58].
EMT plays a pivotal role in cancer progression [59]. Recently, Bozgeyik and colleagues identified VIM-AS1 (vimentin antisense RNA 1), which is transcribed opposite of VIMENTIN (VIM), a well-known EMT marker, and both are upregulated in oral cancer. Accordingly, the expression of E-cadherin exhibited an opposite trend, being downregulated in tumor samples. The higher expression of VIM-AS1 was also associated with an advanced clinical stage and the presence of lymph node metastasis [60]. Other crucial EMT-related ncNAT is ZE2-NAT (ZEB2 natural antisense transcript), which was shown to be important for the maintenance of 5′-UTR ZEB2 intron through direct overlapping with the 5′ splice site in the intron [61]. ZEB2 is a transcriptional repressor of E-cadherin and a major activator of EMT, and expression of ZEB2-NAT prevents splicing of the first intron of ZEB2, increasing the levels of ZEB2 protein. Thus, ZEB2-NAT expression favors EMT, and is therefore associated with cancer progression and cellular reprogramming [38], [62]. This ncNAT has been shown to play an important role in the conservation of stemness properties in quiescent cancer stem cells, supporting the existence of a cellular population with chemoresistance potential [63]. In fact, several ncNATs have been shown to be equally important in cancer and stem cells and/or to modulate response of cancer cells to chemotherapeutic agents. One example is a ncNAT occurring in the SOX9 locus (SOX9-NAT). SOX9-NAT expression was significantly lower in cancer tissues or human embryonic stem cells, compared with their matched normal tissues, suggesting that slight modifications in SOX9-NAT may result in remarkable changes in SOX9 expression, making this ncNAT a potential therapeutic target in regenerative medicine and cancer treatment [64]. Another example, TRPM2-AS (transcript receptor potential cation channel subfamily M member 2 antisense RNA), was shown to confer paclitaxel resistance in prostate cancer. Shi and colleagues showed that both prostate cancer tissues and cell lines exhibited higher levels of TRPM2-AS, compared to normal tissues and cells [65]. Moreover, paclitaxel-resistant prostate cancer cell lines demonstrated even higher expression levels of TRPM2-AS. Mechanistically, this transcript was shown to bind to miR-497-5p, whose expression was decreased in prostate cancer tissues, when compared to normal tissues. Furthermore, it was revealed that miR-497-5p negatively regulates FOXK1, whose expression levels are higher in prostate cancer tissues and cell lines. The authors revealed that silencing TRPM2-AS led to increased and decreased levels of miR-497-5p and FOXK1, respectively, resulting in decreased proliferation, migration and invasion and increased apoptosis, and even suppressed tumor growth in vivo [65].
Another example of how ncNATs are associated with chemoresistance was brough to light by Ling and colleagues [66]. They identified FOXD3-AS1 (foxhead box D3 antisense RNA 1) as an overexpressed antisense transcript in glioblastoma associated with a worse prognosis. Furthermore, temozolomide-resistant glioblastoma cell lines evidenced higher FOXD3-AS1 expression levels comparing to those that were sensitive [66]. In fact, overexpression of FOXD3-AS1 increased tolerance of temozolomide in sensitive cells. Additionally, this lncRNA was revealed to interact with miR-128-3p, which in turn was shown to negatively regulate cell cycle protein WEE1. The authors demonstrated that silencing FOXD3-AS1 resulted in decreased and increased expression levels of miR-128-3p and WEE1, respectively. Furthermore, reduced expression of FOXD3-AS1 sensitized resistant glioblastoma cells to temozolomide, inhibiting cell growth and inducing an increase of cleaved caspase-3, resulting in apoptosis [66].
In osteosarcoma, ANRIL (antisense non-coding RNA in the INK4 locus) was identified as a potential biomarker for chemosensitivity and clinical outcome. In a study carried out by Lee et al., ANRIL was shown to be upregulated in osteosarcoma cell lines, which was correlated with resistance to anti-cancer drugs cisplatin and doxorubicin [67]. By silencing the expression of ANRIL, cells became more sensitive to these treatments and less proliferative. On the other hand, overexpression of ANRIL resulted in an opposite outcome: increased cell proliferation was observed, and cells developed further resistance to cisplatin and doxorubicin [67]. ncNATs have also been shown to identically play fundamental roles through the regulation of cancer metabolism. Li and colleagues identified lncRNA OIP5-AS1 in cervical cancer, whose expression was shown to be higher in cancer tissues and cell lines, in comparison to their normal counterparts [68]. Furthermore, patients were stratified, according to OIP5-AS1 expression, into low and high expression levels, and the latter was associated with larger tumor size, lymph node metastasis and poor 5-year overall survival. Interestingly, under hypoxic conditions, this transcript became upregulated, along with HIF-1α, GLUT1 and LDHA. OIP5-AS1 was revealed to bind to miR-124-5p, leading to de-repression of IDH2 [68].
3.1. Therapeutic and prognostic value of ncNATs in cancer?
Although ncNATs have been widely associated to cancer as prognostic and diagnostic markers [43], [69], [70], [71], [72], [73] they are still far from reaching a therapeutic significance. Several clinical trials are in progress to assess their potential role as cancer biomarkers, in particular when explored in circulating particles, such as exosomes (see, for example, [43]). Examples of ncNATs in clinical trials are lncRNA HOTAIR (coexisting in the HOX gene) in thyroid cancers and MFI2-AS1 (melanotransferrin antisense RNA) in localized clear-cell cancers of the kidney. MFI2‐AS1 expression was associated with the recurrence and survival of patients with clear‐cell kidney carcinoma [74]. Interestingly, MFI2‐AS1 may act as a sponge of miR‐574‐5p, a miRNA with potential roles in cancer metastasis [75], [76].
Therapeutics that modulate RNAs in general and ncNATs in particular may be envisioned to play fundamental roles in disease. The development of antisense oligonucleotides (ASOs), duplex RNA technologies (RNAi) or genome editing (e.g. CRISPR), has aided the translational approach of ncNATs to the clinics, in particular when loss of function is projected [77], [78], [79]. One example is the application of NATs therapeutics in the Angelman syndrome. Here, targeting NATs may be used to derepress the normally-repressed paternal copy of the UBE3A gene in patients experiencing absent expression of the UBE3A gene-copy of the maternal allele [79], [80].
Additionally, using the knowledge acquired from how NATs may interfere with the expression of associated genes, new therapies may evolve. Recently, Taekyu Ha and colleagues explored a gene-targeting method to deplete ephrinB2 using an inducible lentiviral vector. EphrinB2 promotes colorectal cancer and predicts poor patient survival. They demonstrated that integration and expression of the lentiviral construct in the host DNA may drive divergent transcription. Antisense transcription was associated with cell death through activation of a stress response providing evidence that divergent gene transcription from lentiviral vector integration may have an impact on the regulation of gene expression [81].
4. Conclusion
ncNATs are a class of lncRNAs that overlap either protein- or non-coding sequences. Although many of these transcripts can be regarded as junk RNA, several others present a conservation and cellular specificity, guiding them to important roles during normal tissue functions and diseases, including cancer. In particular, ncNATs have been shown to affect eithertheir neighboring genes, and sequences on different genomic loci, thus adding additional layers to the already complex process of gene regulation.
Many recently identified ncNATs have been shown to derepress miRNA targets by functioning as ceRNAs, thus contributing to the regulation of mRNA stability. Interestingly, some of these transcripts also have nuclear functions, making it clear that they can regulate gene expression through several mechanisms.
The advances of gene editing and targeted techniques show great potential for the tissue-specific determination of the role of ncNATs in several biological processes, allowing the characterization of these transcripts as potential therapeutic targets in disease.
Funding
This work was supported by LISBOA-01-0145-FEDER-028534, projeto cofinanciado pelo FEDER através POR Lisboa 2020 - Programa Operacional Regional de Lisboa, do PORTUGAL 2020 e pela Fundação para a Ciência e a Tecnologia (FCT) and Fundação para a Ciência e Tecnologia (FCT) (ERA-CVD 2018 / 3599-PPCDT - ERA-CVD/0001/2018 - INNOVATION). FS has an individual scholarship from FCT (SFRH/BD/146204/2019), S. N-P. has a 2020.00355.CEECIND assistant research contract and AM-C and FM have a Verão com Ciência 2022 research grant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank other members of the iBiMED, Bruno B. de Jesus and Sandrina N-Pereira labs for insightful discussions and advice.
References
- 1.Djebali S., Davis C.A., Merkel A., et al. Landscape of transcription in human cells. Nature. 2012;489(7414):101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carninci P., Kasukawa T., Katayama S., et al. The transcriptional landscape of the mammalian genome. Science (80-) 2005;309(5740):1559–1563. doi: 10.1126/science.1112014. 309/5740/1559 [pii]10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 3.Maeda N., Kasukawa T., Oyama R., et al. Transcript annotation in FANTOM3: Mouse gene catalog based on physical cDNAs. PLoS Genet. 2006;2(4):498–503. doi: 10.1371/journal.pgen.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunham I., Kundaje A., Aldred S.F., et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482(7385):339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batista P.J., Chang H.Y. Long noncoding RNAs: Cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derrien T., Johnson R., Bussotti G., et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L., Bajic V.B., Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):924–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabili M., Trapnell C., Goff L., et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81(1):145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponting C.P., Oliver P.L., Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.T., Davidow L.S., Warshawsky D. Tsix, a gene antisense to Xist at the X-inactivation centre. Nat Genet. 1999;21(4):400–404. doi: 10.1038/7734. [DOI] [PubMed] [Google Scholar]
- 13.Zong X., Nakagawa S., Freier S.M., et al. Natural antisense RNA promotes 3′ end processing and maturation of MALAT1 lncRNA. Nucleic Acids Res. 2016;44(6):2898–2908. doi: 10.1093/nar/gkw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebert M.S., Neilson J.R., Sharp P.A. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4(9):721–726. doi: 10.1038/NMETH1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebert M.S., Sharp P.A. Roles for MicroRNAs in conferring robustness to biological processes. Cell. 2012;149(3):515–524. doi: 10.1016/J.CELL.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert M.S., Sharp P.A. Emerging roles for natural microRNA sponges. Curr Biol. 2010;20(19) doi: 10.1016/J.CUB.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kartha R.V., Subramanian S. Competing endogenous RNAs (ceRNAs): New entrants to the intricacies of gene regulation. Front Genet. 2014;5(JAN):8. doi: 10.3389/FGENE.2014.00008/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karreth F.A., Pandolfi P.P. CeRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3(10):1113–1121. doi: 10.1158/2159-8290.CD-13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Xue M., Du S., et al. Competitive endogenous RNA is an intrinsic component of EMT regulatory circuits and modulates EMT. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-09649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/J.CELL.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ala U., Karreth F.A., Bosia C., et al. Integrated transcriptional and competitive endogenous RNA networks are cross-regulated in permissive molecular environments. Proc Natl Acad Sci U S A. 2013;110(18):7154–7159. doi: 10.1073/PNAS.1222509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poliseno L., Salmena L., Zhang J., Carver B., Haveman W.J., Pandolfi P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033–1038. doi: 10.1038/NATURE09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozsolak F., Kapranov P., Foissac S., et al. Comprehensive polyadenylation site maps in yeast and human reveal pervasive alternative polyadenylation. Cell. 2010;143(6):1018–1029. doi: 10.1016/j.cell.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najafi S., Tan S.C., Raee P., et al. Gene regulation by antisense transcription: A focus on neurological and cancer diseases. Biomed Pharmacother. 2022;145 doi: 10.1016/j.biopha.2021.112265. [DOI] [PubMed] [Google Scholar]
- 25.Balbin O.A., Malik R., Dhanasekaran S.M., et al. The landscape of antisense gene expression in human cancers. Genome Res. 2015;25(7):1068–1079. doi: 10.1101/gr.180596.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latgé G., Poulet C., Bours V., Josse C., Jerusalem G. Natural Antisense Transcripts: Molecular Mechanisms and Implications in Breast Cancers. Int J Mol Sci. 2018;19(1):123. doi: 10.3390/ijms19010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seila A.C., Calabrese J.M., Levine S.S., et al. Divergent transcription from active promoters. Science (80-) 2008;322(5909):1849–1851. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Core LJ, Waterfall JJ, Lis JT. Nascent RNA Sequencing reveals widespread pausing and divergent initiation at human promoters. Science (80-). 2008;322(5909):1845-1848. doi:10.1126/science.11622Nascent. [DOI] [PMC free article] [PubMed]
- 29.Vítor A.C., Sridhara S.C., Sabino J.C., et al. Single-molecule imaging of transcription at damaged chromatin. Sci Adv. 2019;5(1) doi: 10.1126/SCIADV.AAU1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan C.D., Laprade L., Winston F. Transcription elongation factors repress transcription initiation from cryptic sites. Science (80-) 2003;301(5636):1096–1099. doi: 10.1126/science.1087374. [DOI] [PubMed] [Google Scholar]
- 31.Van Dijk E.L., Chen C.L., Daubenton-Carafa Y., et al. XUTs are a class of Xrn1-sensitive antisense regulatory non-coding RNA in yeast. Nature. 2011;475(7354):114–119. doi: 10.1038/nature10118. [DOI] [PubMed] [Google Scholar]
- 32.Geisler S., Lojek L., Khalil A.M., Baker K.E., Coller J. Decapping of long noncoding RNAs regulates inducible genes. Mol Cell. 2012;45(3):279–291. doi: 10.1016/j.molcel.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., Hou Y., Zhao C., et al. Cis-natural antisense transcripts are mainly co-expressed with their sense transcripts and primarily related to energy metabolic pathways during muscle development. Int J Biol Sci. 2016;12(8):1010–1021. doi: 10.7150/ijbs.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao S., Zhang X., Chen S., Zhang S. Natural antisense transcripts in the biological hallmarks of cancer: Powerful regulators hidden in the dark. J Exp Clin Cancer Res. 2020;39(1):1–18. doi: 10.1186/s13046-020-01700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelechano V., Steinmetz L.M. Gene regulation by antisense transcription. Nat Rev Genet. 2013;14(12):880–893. doi: 10.1038/nrg3594. nrg3594 [pii]10.1038/nrg3594. [DOI] [PubMed] [Google Scholar]
- 36.Zinad H.S., Natasya I., Werner A. Natural antisense transcripts at the interface between host genome and mobile genetic elements. Front Microbiol. 2017;8(NOV):1–9. doi: 10.3389/fmicb.2017.02292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling M.H.T., Ban Y., Wen H., Wang S.M., Ge S.X. Conserved expression of natural antisense transcripts in mammals. BMC Genomics. 2013;14(1):1–9. doi: 10.1186/1471-2164-14-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Jesus B.B., Marinho S.P., Barros S., et al. Silencing of the lncRNA Zeb2-NAT facilitates reprogramming of aged fibroblasts and safeguards stem cell pluripotency. Nat Commun. 2018;9:1–11. doi: 10.1038/s41467-017-01921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modarresi F., Faghihi M.A., Lopez-Toledano M.A., et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat Biotechnol. 2012;30(5):453–459. doi: 10.1038/NBT.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin S., Zhang L., Luo W., Zhang X. Characteristics of antisense transcript promoters and the regulation of their activity. Int J Mol Sci. 2015;17(1):9. doi: 10.3390/ijms17010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozsolak F., Goren A., Gymrek M., et al. Digital transcriptome profiling from attomole-level RNA samples. Genome Res. 2010;20(4):519–525. doi: 10.1101/gr.102129.109. gr.102129.109 [pii]10.1101/gr.102129.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molias F.B., Sim A., Leong K.W., et al. Antisense RNAs influence promoter usage of their counterpart sense genes in cancer. Cancer Res. 2021;81(23):5849–5861. doi: 10.1158/0008-5472.CAN-21-1859/670645/AM/ANTISENSE-RNAS-INFLUENCE-PROMOTER-USAGE-OF-THEIR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krappinger J.C., Bonstingl L., Pansy K., et al. Non-coding natural antisense transcripts: analysis and application. J Biotechnol. 2021;340:75–101. doi: 10.1016/j.jbiotec.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Shendure J, Church GM. Computational discovery of sense-antisense transcription in the human and mouse genomes. Genome Biol 2002 39. 2002;3(9):1-14. doi:10.1186/GB-2002-3-9-RESEARCH0044. [DOI] [PMC free article] [PubMed]
- 45.Chen J., Sun M., Kent W.J., et al. Over 20% of human transcripts might form sense-antisense pairs. Nucleic Acids Res. 2004;32(16):4812–4820. doi: 10.1093/NAR/GKH818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiyosawa H., Yamanaka I., Osato N., et al. Antisense transcripts with FANTOM2 clone set and their implications for gene regulation. Genome Res. 2003;13(6B):1324–1334. doi: 10.1101/GR.982903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katayama S., Tomaru Y., Kasukawa T., et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309(5740):1564–1566. doi: 10.1126/SCIENCE.1112009. [DOI] [PubMed] [Google Scholar]
- 48.Churchman L.S., Weissman J.S. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature. 2011;469(7330):368–373. doi: 10.1038/nature09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sousa-Luís R., Dujardin G., Zukher I., et al. POINT technology illuminates the processing of polymerase-associated intact nascent transcripts. Mol Cell. 2021;81(9):1935. doi: 10.1016/J.MOLCEL.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guttman M., Amit I., Garber M., et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Z., Wei W., Gagneur J., et al. Antisense expression increases gene expression variability and locus interdependency. Mol Syst Biol. 2011;7(468) doi: 10.1038/msb.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margaritis T., Oreal V., Brabers N., et al. Two distinct repressive mechanisms for histone 3 lysine 4 methylation through promoting 3′-end antisense transcription. PLoS Genet. 2012;8(9) doi: 10.1371/journal.pgen.1002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim T., Xu Z., Clauder-Münster S., Steinmetz L.M., Buratowski S. Set3 HDAC mediates effects of overlapping noncoding transcription on gene induction kinetics. Cell. 2012;150(6):1158–1169. doi: 10.1016/j.cell.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomes C.P., Nóbrega-Pereira S., Domingues-Silva B., et al. An antisense transcript mediates MALAT1 response in human breast cancer. BMC Cancer. 2019;19(1):1–11. doi: 10.1186/s12885-019-5962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W.-T., Zhang J.-J., Shao Q., et al. FGD5-AS1 is an oncogenic lncRNA in pancreatic cancer and regulates the Wnt/β-catenin signaling pathway via miR-577. Oncol Rep. 2022;47(1):1–11. doi: 10.3892/or.2021.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao K.F., Zhao Y.F., Liao W.J., Xu G.L., Zhang J.D. CERS6-AS1 promotes cell proliferation and represses cell apoptosis in pancreatic cancer via miR-195-5p/WIPI2 axis. Kaohsiung J Med Sci. 2022;(January):1–12. doi: 10.1002/kjm2.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J., Shen Y., Ma D., Li Z., Zhang Z., Jin W. SLCO4A1-AS1 mediates pancreatic cancer development via miR-4673/KIF21B axis. Open Med. 2022;17(1):253–265. doi: 10.1515/med-2022-0418. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Tang W., Zhang L., Li J., Guan Y. AFAP1 antisense RNA 1 promotes retinoblastoma progression by sponging microRNA miR-545-3p that targets G protein subunit beta 1. Bioengineered. 2022;13(3):5638–5652. doi: 10.1080/21655979.2022.2033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De C.B., Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 60.Bozgeyik E., Ege B., Koparal M., Ceylan O. Clinical significance of Vimentin Antisense RNA 1 and its correlation with other epithelial to mesenchymal transition markers in oral cancers. Pathol - Res Pract. 2021;2022(232) doi: 10.1016/j.prp.2022.153807. [DOI] [PubMed] [Google Scholar]
- 61.Beltran M., Puig I., Pena C., et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008;22(6):756–769. doi: 10.1101/gad.455708. 22/6/756 [pii]10.1101/gad.455708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eroğlu Güneş C., Güçlü E., Vural H., Kurar E. Knockdown of lncRNA ZEB2NAT suppresses epithelial mesenchymal transition, metastasis and proliferation in breast cancer cells. Gene. 2021;805 doi: 10.1016/j.gene.2021.145904. [DOI] [PubMed] [Google Scholar]
- 63.Francescangeli F., Contavalli P., De Angelis M.L., et al. A pre-existing population of ZEB2+ quiescent cells with stemness and mesenchymal features dictate chemoresistance in colorectal cancer. J Exp Clin Cancer Res. 2020;39(1):2. doi: 10.1186/s13046-019-1505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eftekhary M., Mohammadi-Yeganeh S., Bolandi Z., et al. A novel natural antisense transcript at human SOX9 locus is down-regulated in cancer and stem cells. Biotechnol Lett. 2020;42(2):329–339. doi: 10.1007/S10529-019-02774-3/FIGURES/5. [DOI] [PubMed] [Google Scholar]
- 65.Shi T., Li R., Duan P., et al. TRPM2-AS promotes paclitaxel resistance in prostate cancer by regulating FOXK1 via sponging miR-497-5p. Drug Dev Res. 2021;2022:1–12. doi: 10.1002/ddr.21924. [DOI] [PubMed] [Google Scholar]
- 66.Ling Z., Zhang J., Liu Q. Oncogenic Forkhead box D3 antisense RNA 1 promotes cell survival and confers temozolomide resistance in glioblastoma cells through the miR-128-3p/WEE1 G2 checkpoint kinase axis. Bioengineered. 2022;13(3):6012–6023. doi: 10.1080/21655979.2022.2042133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee A.M., Ferdjallah A., Moore E., et al. Long non-coding rna anril as a potential biomarker of chemosensitivity and clinical outcomes in osteosarcoma. Int J Mol Sci. 2021;22(20) doi: 10.3390/ijms222011168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L., Ma Y., Maerkeya K., Reyanguly D., Han L. LncRNA OIP5-AS1 regulates the warburg effect through miR-124-5p/IDH2/HIF-1α pathway in cervical cancer. Front Cell Dev Biol. 2021;9(August):1–14. doi: 10.3389/fcell.2021.655018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Annie D, Serena D, Sandrine C, et al. LADON, a natural antisense transcript of NODAL, promotes an invasive behaviour in melanoma cells. bioRxiv. Published online September 30, 2021:2020.04.09.032375. doi:10.1101/2020.04.09.032375.
- 70.Braicu C, Zimta AA, Harangus A, et al. The Function of non-coding RNAs in lung cancer tumorigenesis. Cancers 2019, 11, Page 605. 2019;11(5):605. doi:10.3390/CANCERS11050605. [DOI] [PMC free article] [PubMed]
- 71.Shademan M., Salanghuch A.N., Zare K., et al. Expression profile analysis of two antisense lncRNAs to improve prognosis prediction of colorectal adenocarcinoma. Cancer Cell Int. 2019;19(1):1–12. doi: 10.1186/S12935-019-1000-1/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wenric S, Elguendi S, Caberg JH, et al. Transcriptome-wide analysis of natural antisense transcripts shows their potential role in breast cancer. Sci Reports 2017 71. 2017;7(1):1-12. doi:10.1038/s41598-017-17811-2. [DOI] [PMC free article] [PubMed]
- 73.Ros G., Pegoraro S., De Angelis P., et al. HMGA2 antisense long non-coding RNAs as new players in the regulation of HMGA2 expression and pancreatic cancer promotion. Front Oncol. 2020;9:1526. doi: 10.3389/FONC.2019.01526/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flippot R., Mouawad R., Spano J.-P., et al. Expression of long non-coding RNA MFI2-AS1 is a strong predictor of recurrence in sporadic localized clear-cell renal cell carcinoma. Sci Rep. 2017;7(1):8540. doi: 10.1038/s41598-017-08363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ji S., Ye G., Zhang J., et al. miR-574-5p negatively regulates Qki6/7 to impact β-catenin /Wnt signalling and the development of colorectal cancer. Gut. 2013;62(5):716–726. doi: 10.1136/gutjnl-2011-301083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li C., Tan F., Pei Q., et al. Non-coding RNA MFI2-AS1 promotes colorectal cancer cell proliferation, migration and invasion through miR-574-5p/MYCBP axis. Cell Prolif. 2019;52(4) doi: 10.1111/cpr.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J., Hu C., Moufawad El Achkar C., et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med. 2019;381(17):1644–1652. doi: 10.1056/NEJMOA1813279/SUPPL_FILE/NEJMOA1813279_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Messina S, Sframeli M. New treatments in spinal muscular atrophy: positive results and new challenges. J Clin Med 2020, Vol 9, Page 2222. 2020;9(7):2222. doi:10.3390/JCM9072222. [DOI] [PMC free article] [PubMed]
- 79.Quemener A.M., Bachelot L., Forestier A., Donnou-Fournet E., Gilot D., Galibert M.D. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip Rev RNA. 2020;11(5):e1594. doi: 10.1002/wrna.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meng L., Ward A.J., Chun S., Bennett C.F., Beaudet A.L., Rigo F. Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature. 2015;518(7539):409–412. doi: 10.1038/nature13975. nature13975 [pii]10.1038/nature13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ha T, Diprima M, Koparde V, et al. Antisense transcription from lentiviral gene targeting linked to an integrated stress response in colorectal cancer cells. Published online 2022. doi:10.1016/j.omtn.2022.05.029. [DOI] [PMC free article] [PubMed]
- 82.Sun Y., Li D., Zhang R., et al. Strategies to identify natural antisense transcripts. Biochimie. 2017;132:131–151. doi: 10.1016/j.biochi.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Vance K.W., Ponting C.P. Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends Genet. 2014;30(8):348–355. doi: 10.1016/j.tig.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faghihi M.A., Wahlestedt C. RNA interference is not involved in natural antisense mediated regulation of gene expression in mammals. Genome Biol. 2006;7(5):R38. doi: 10.1186/gb-2006-7-5-r38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pastori C., Peschansky V.J., Barbouth D., Mehta A., Silva J.P., Wahlestedt C. Comprehensive analysis of the transcriptional landscape of the human FMR1 gene reveals two new long noncoding RNAs differentially expressed in Fragile X syndrome and Fragile X-associated tremor/ataxia syndrome. Hum Genet. 2014;133(1):59–67. doi: 10.1007/s00439-013-1356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levin J.Z., Yassour M., Adiconis X., et al. Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nat Methods. 2010;7(9):709–715. doi: 10.1038/nmeth.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wanowska E., Kubiak M.R., Rosikiewicz W., Makałowska I., Szcześniak M.W. Natural antisense transcripts in diseases: From modes of action to targeted therapies. Wiley Interdiscip Rev RNA. 2018;9(2):1–16. doi: 10.1002/wrna.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kung J.T.Y., Colognori D., Lee J.T. Long noncoding RNAs: Past, present, and future. Genetics. 2013;193(3):651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Windhager L., Bonfert T., Burger K., et al. Ultrashort and progressive 4sU-tagging reveals key characteristics of RNA processing at nucleotide resolution. Genome Res. 2012;22(10):2031–2042. doi: 10.1101/gr.131847.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shiraki T., Kondo S., Katayama S., et al. Cap analysis gene expression for high-throughput analysis of transcriptional starting point and identification of promoter usage. Proc Natl Acad Sci U S A. 2003;100(26):15776–15781. doi: 10.1073/pnas.2136655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robertson G., Hirst M., Bainbridge M., et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods. 2007;4(8):651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- 92.Chu C., Qu K., Zhong F.L., Artandi S.E., Chang H.Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell. 2011;44(4):667–678. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ule J., Jensen K.B., Ruggiu M., Mele A., Ule A., Darnell R.B. CLIP identifies nova-regulated RNA networks in the brain. Science (80-) 2003;302(5648):1212–1215. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- 94.Raj A., Van Oudenaarden A. Stochastic gene expression and its consequences. Cell. 2008;135(2):216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zenklusen D., Larson D.R., Singer R.H. Single-RNA counting reveals alternative modes of gene expression in yeast. Nat Struct Mol Biol. 2008;15(12):1263–1271. doi: 10.1038/nsmb.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan R.Z., Van Oudenaarden A. Transcript counting in single cells reveals dynamics of rDNA transcription. Mol Syst Biol. 2010;6(358):1–7. doi: 10.1038/msb.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen K.H., Boettiger A.N., Moffitt J.R., Wang S., Zhuang X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348(6233) doi: 10.1126/SCIENCE.AAA6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shah S., Takei Y., Zhou W., et al. Dynamics and spatial genomics of the nascent transcriptome by intron seqFISH. Cell. 2018;174(2):363–376. doi: 10.1016/j.cell.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee J.H., Daugharthy E.R., Scheiman J., et al. Highly multiplexed subcellular RNA sequencing in situ. Science (80-) 2014;343(6177):1360–1363. doi: 10.1126/science.1250212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J.H., Daugharthy E.R., Scheiman J., et al. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat Protoc. 2015;10(3):442–458. doi: 10.1038/nprot.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eng C.H.L., Shah S., Thomassie J., Cai L. Profiling the transcriptome with RNA SPOTs. Nat Methods. 2017;14(12):1153–1155. doi: 10.1038/nmeth.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schena M., Shalon D., Davis R.W., Brown P.O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science (80-) 1995;270(5235):467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 103.Numata K., Osada Y., Okada Y., et al. Identification of novel endogenous antisense transcripts by DNA microarray analysis targeting complementary strand of annotated genes. BMC Genomics. 2009;10:392. doi: 10.1186/1471-2164-10-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oeder S., Mages J., Flicek P., Lang R. Uncovering information on expression of natural antisense transcripts in Affymetrix MOE430 datasets. BMC Genomics. 2007;8(1):1–8. doi: 10.1186/1471-2164-8-200/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Werner A., Schmutzler G., Carlile M., Miles C.G., Peters H. Expression profiling of antisense transcripts on DNA arrays. Physiol Genomics. 2007;28(3):294–300. doi: 10.1152/PHYSIOLGENOMICS.00127.2006. [DOI] [PubMed] [Google Scholar]
- 106.Shoemaker D.D., Schadt E.E., Armour C.D., et al. Experimental annotation of the human genome using microarray technology. Nature. 2001;409(6822):922–927. doi: 10.1038/35057141. [DOI] [PubMed] [Google Scholar]
- 107.Geiss G.K., Bumgarner R.E., Birditt B., et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26(3):317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 108.Khodor Y.L., Rodriguez J., Abruzzi K.C., Tang C.H.A., Marr M.T., Rosbash M. Nascent-seq indicates widespread cotranscriptional pre-mRNA splicing in Drosophila. Genes Dev. 2011;25(23):2502–2512. doi: 10.1101/gad.178962.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin F.R., Niparko J.K., Ferrucci L. Nascent-SEQ indicates widespread cotranscriptional rna editing in drosophila. Mol Cell. 2012;47(1):27–37. doi: 10.1016/j.molcel.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alwine J.C., Kemp D.J., Stark G.R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kwak H., Fuda N.J., Core L.J., Lis J.T. Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science (80-) 2013;339(6122):950–953. doi: 10.1126/science.1229386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Derti A., Garrett-Engele P., MacIsaac K.D., et al. A quantitative atlas of polyadenylation in five mammals. Genome Res. 2012;22(6):1173–1183. doi: 10.1101/gr.132563.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jan C.H., Friedman R.C., Ruby J.G., Bartel D.P. Formation, regulation and evolution of Caenorhabditis elegans 3’UTRs. Nature. 2011;469(7328):97–103. doi: 10.1038/nature09616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. 1986. Biotechnology. 1992;24(Table 1):17-27. [PubMed]
- 115.Frohman M.A., Dush M.K., Martin G.R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gilbert C., Kristjuhan A., Winkler G.S., Svejstrup J.Q. Elongator interactions with nascent mRNA revealed by RNA immunoprecipitation. Mol Cell. 2004;14(4):457–464. doi: 10.1016/S1097-2765(04)00239-4. [DOI] [PubMed] [Google Scholar]
- 117.Mercer T.R., Gerhardt D.J., Dinger M.E., et al. Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat Biotechnol. 2012;30(1):99–104. doi: 10.1038/nbt.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ng P., Wei C.L., Sung W.K., et al. Gene identification signature (GIS) analysis for transcriptome characterization and genome annotation. Nat Methods. 2005;2(2):105–111. doi: 10.1038/nmeth733. [DOI] [PubMed] [Google Scholar]
- 119.Ng P, Wei C-L, Ruan Y. Paired-end ditagging for transcriptome and genome analysis; 2007. doi:10.1002/0471142727.mb2112s79. [DOI] [PubMed]
- 120.Nagalakshmi U., Wang Z., Waern K., et al. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science (80-) 2008;320(5881):1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilhelm B.T., Marguerat S., Watt S., et al. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature. 2008;453(7199):1239–1243. doi: 10.1038/nature07002. [DOI] [PubMed] [Google Scholar]
- 122.Mortazavi A., Williams B.A., McCue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 123.Lister R., O’Malley R.C., Tonti-Filippini J., et al. Highly integrated single-base resolution maps of the epigenome in arabidopsis. Cell. 2008;133(3):523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cloonan N., Forrest A.R.R., Kolle G., et al. Stem cell transcriptome profiling via massive-scale mRNA sequencing. Nat Methods. 2008;5(7):613–619. doi: 10.1038/nmeth.1223. [DOI] [PubMed] [Google Scholar]
- 125.Marioni J.C., Mason C.E., Mane S.M., Stephens M., Gilad Y. RNA-seq: An assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008;18(9):1509–1517. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morin R.D., Bainbridge M., Fejes A., et al. Profiling the HeLa S3 transcriptome using randomly primed cDNA and massively parallel short-read sequencing. Biotechniques. 2008;45(1):81–94. doi: 10.2144/000112900. [DOI] [PubMed] [Google Scholar]
- 127.Howald C., Tanzer A., Chrast J., et al. Combining RT-PCR-seq and RNA-seq to catalog all genic elements encoded in the human genome. Genome Res. 2012;22(9):1698–1710. doi: 10.1101/gr.134478.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu M., Polyak K. Serial analysis of gene expression. Nat Protoc. 2006;1(4):1743–1760. doi: 10.1038/nprot.2006.269. [DOI] [PubMed] [Google Scholar]
- 129.Frei A.P., Bava F.A., Zunder E.R., et al. Highly multiplexed simultaneous detection of RNAs and proteins in single cells. Nat Methods. 2016;13(3):269–275. doi: 10.1038/nmeth.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reimegård J., Tarbier M., Danielsson M., et al. A combined approach for single-cell mRNA and intracellular protein expression analysis. Commun Biol. 2021;4(624):1–11. doi: 10.1038/s42003-021-02142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Macaulay I.C., Haerty W., Kumar P., et al. G&T-seq: Parallel sequencing of single-cell genomes and transcriptomes. Nat Methods. 2015;12(6):519–522. doi: 10.1038/nmeth.3370. [DOI] [PubMed] [Google Scholar]
- 132.Hu Y., Fan J., Zhang H., Chen X., Dai G. An estimated method of urban PM2.5 concentration distribution for a mobile sensing system. Pervasive Mob Comput. 2016;25:88–103. doi: 10.1016/j.pmcj.2015.06.004. [DOI] [Google Scholar]
- 133.Dey S.S., Kester L., Spanjaard B., Bienko M., Van Oudenaarden A. Integrated genome and transcriptome sequencing of the same cell. Nat Biotechnol. 2015;33(3):285–289. doi: 10.1038/nbt.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Angermueller C., Clark S.J., Lee H.J., et al. Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nat Methods. 2016;13(3):229–232. doi: 10.1038/nmeth.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schofield JA, Duffy EE, Kiefer L, et al. Sequencing through nucleoside recoding. 2018;15(3):221-225. doi:10.1038/nmeth.4582.TimeLapse-seq. [DOI] [PMC free article] [PubMed]
- 136.Schwalb B., Michel M., Zacher B., et al. TT-seq maps the human transient transcriptome. Science (80-) 2016;352(6290):1225–1228. doi: 10.1126/science.aad9841. [DOI] [PubMed] [Google Scholar]
- 137.Herzog V.A., Reichholf B., Neumann T., et al. Thiol-linked alkylation of RNA to assess expression dynamics. Nat Methods. 2017;14(12):1198–1204. doi: 10.1038/nmeth.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]