Abstract
Laminin, a major component of the basal lamina (BL), is a heterotrimeric protein with many isoforms. In the CNS, laminin is expressed by almost all cell types, yet different cells synthesize distinct laminin isoforms. By binding to its receptors, laminin exerts a wide variety of important functions. However, due to the reciprocal and cell-specific expression of laminin in different cells at the neurovascular unit, its functions in blood-brain barrier (BBB) maintenance and BBB repair after injury are not fully understood. In this review, we focus on the expression and functions of laminin and its receptors in the neurovascular unit under both physiological and pathological conditions. We first briefly introduce the structures of laminin and its receptors. Next, the expression and functions of laminin and its receptors in the CNS are summarized in a cell-specific manner. Finally, we identify the knowledge gap in the field and discuss key questions that need to be answered in the future. Our goal is to provide a comprehensive overview on cell-specific expression of laminin and its receptors in the CNS and their functions on BBB integrity.
Keywords: Neurovascular unit, blood-brain barrier, laminin, integrin, dystroglycan, cell-specific
Introduction
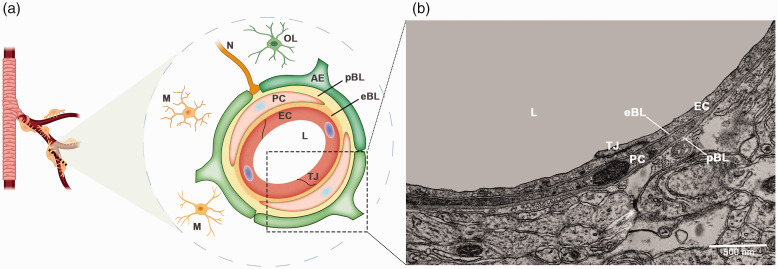
The BBB is a physiological barrier that protects the brain from circulating blood components and toxic compounds. By tightly regulating what enters/exits the brain, the BBB maintains CNS homeostasis and actively modulates the pathogenesis of various neurological disorders. 1 The barrier function of BBB mainly comes from brain microvascular endothelial cells (BMECs), which are held together by specialized tight junctions and adherent junctions. The BMECs are surrounded by pericytes, astrocyte endfeet, microglia, and neurons (Figure 1(a)), which are critical for inducing and/or maintaining the highly restrictive barrier of BMECs. In addition, two layers of basal lamina (BL), known as endothelial BL and parenchymal BL, are also found in the abluminal side of BMECs (Figure 1(a)). The BMECs together with other cells and the BL comprise the neurovascular unit.2,3 While how different cells regulate BBB integrity is well studied, the important function of the BL in BBB permeability has just started to be recognized.4–7
Figure 1.
Diagram illustration of the composition and ultrastructure of the neurovascular unit. (a) Cross-section view of a brain capillary showing the composition of the neurovascular unit. The neurovascular unit is mainly composed of brain microvascular endothelial cells, pericytes, astrocytes, microglia, neurons, and a non-cellular component–the basal lamina (BL). Pericytes separate the BL into endothelial BL and parenchymal BL and (b) Transmission electron microscopy image showing the ultrastructure of the neurovascular unit. AE: astrocyte endfeet; eBL: endothelial BL; EC: endothelial cells; L: lumen; M: microglia; N: neuron; OL: oligodendrocyte; pBL: parenchymal BL; PC: pericyte; TJ: tight junction.
The BL is an amorphous structure composed of highly cross-linked extracellular matrix (ECM) proteins. In the CNS, the BL is separated into endothelial BL and parenchymal BL by pericytes under transmission electron microscopy (Figure 1(b)). In regions without pericytes, however, these two layers are indistinguishable (Figure 1(b)). Although the composition of BL varies depending on tissue type and developmental stage, 8 all BLs contain collagen IV, laminin, nidogen, and heparin sulfate proteoglycans. 9 Among these components, laminin is the only one that is indispensable for the assembly/formation of BL.10–12
In this review, we first briefly introduce the structures of laminin and its receptors. Next, the expression and functions of laminin and its receptors in the CNS are summarized in a cell-specific manner. Last, major challenges and key questions in the field are discussed.
Laminin and laminin receptors
Laminins
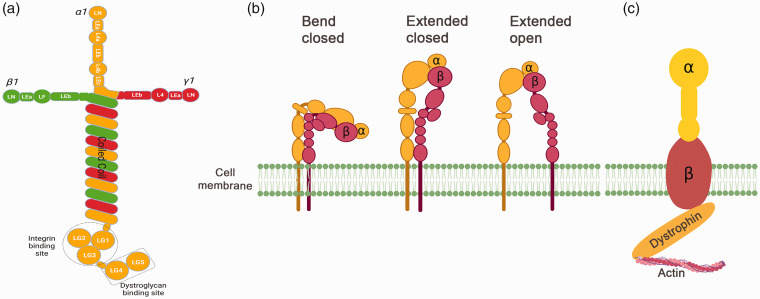
Laminin is a T or cruciform-shaped heterotrimeric glycoprotein composed of α, β, and γ chains (Figure 2(a)). In mammals, five α, four β, and three γ chains have been identified.11,12 Although these genetic variants can generate up to 60 possible laminin isoforms, not all isoforms have been identified. So far, sixteen laminin isoforms have been identified experimentally and four novel combinations have been proposed based on in vivo and in vitro studies.11,12
Figure 2.
Structural illustrations of laminin, integrin, and dystroglycan. (a) Schematic illustration of laminin-111. Laminin-111 is a cross-shaped molecule composed of α1 (orange), β1 (green), and γ1 (red) subunits. Each subunit has multiple globular domains (LN, L4, and LF), rod-like repeats (LE), and a coiled-coil domain. Unique to the α1 subunit are five globular domains (LG1-5) in the C-terminus. The LG1-3 and LG4-5 are integrin- and dystroglycan-binding sites, respectively. (b) Schematic illustration of integrin. Integrin is a heterodimer composed of non-covalently bound α and β subunits. It can adopt three different conformation structures: bend closed, extended closed, and extended open, which have distinct affinity for external ligands. (c) Schematic illustration of dystroglycan. Dystroglycan is a heterodimer composed of two non-covalently bound subunits: an extracellular α subunit and a transmembrane β subunit. The α subunit is responsible for laminin engaging, while the β subunit binds to the intracellular actin network via dystrophin.
Laminin α chains consist of an N terminus with various globular (e.g. LN, L4, LF) domains and/or epidermal growth factor-like (LE) domains, a C-terminus containing five globular (LG1-5) domains, and a coiled-coil domain in the middle (Figure 2(a)).11,12 It should be noted that laminin α3A and α4 have a short N-terminus due to the lack of LN-L4b domains. Laminin β and γ chains have a similar structure as laminin α chains except that they do not have the LG1-5 domains in the C-terminus. These three chains join together via their coiled-coil domains, forming a heterotrimer with a long arm and two or three short arms. The structure of laminin-111 is illustrated in Figure 2(a). Functional studies have shown that laminin short arms are required for its self-polymerization, while its LG domains are involved in receptor interaction.13,14 Laminin receptors are broadly categorized into integrin and non-integrin receptors.
Integrins
Integrins are transmembrane receptors consisting of two (α and β) non-covalently bound subunits. They can adopt three different conformation structures: bend closed, extended closed, and extended open (Figure 2(b)), which have distinct affinity for external ligands. There are 18 α and 8 β genetic variants, which generate 25 distinct integrin heterodimers. 15 Integrins can interact with a variety of ECM proteins and are involved in both outside-in and inside-out signaling pathways.16,17
Previous studies have shown that integrin-α3β1, -α6β1, -α7β1, and -α6β4 are classical laminin-binding integrins, although integrin-α1β1, -α2β1, and -αVβ8 can also interact with laminin.12,14 The integrin-binding site on laminin has been mapped to the first three LG (LG1-3) domains on laminin-α chains. 18 In addition, there is also evidence showing that the glutamic acid (E) residue at the third position from the C-termini of laminin-γ chains is important for laminin-integrin-β1 interaction. 19
Non-integrin receptors
In addition to integrins, laminin can also interact with several non-integrin receptors, including dystroglycan, syndecan, 67 kDa laminin receptor, HNK bearing proteins, the Lutheran antigen, and melanoma cell adhesion molecule. 12 Among these non-integrin receptors, dystroglycan is the most studied and thus the focus of this review. Dystroglycan is a heterodimer composed of two non-covalently bound subunits: an extracellular α subunit that interacts with laminin and a transmembrane β subunit that binds to the intracellular actin network via dystrophin (Figure 2(c)). The dystroglycan-binding site on laminin has been mapped to the last two LG (LG4-5) domains on laminin-α chains. 20
Expression and functions of laminin and its receptors in the BBB
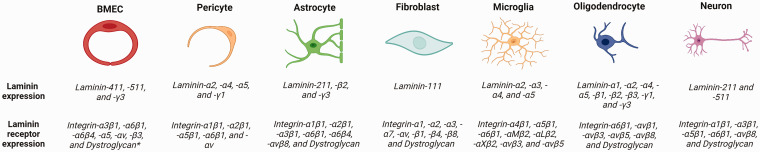
In the CNS, laminin and its receptors are predominantly found in blood vessel wall, with laminin in the BL and its receptors in vascular cells. Although almost all cells in the CNS synthesize laminin and its receptors, their expression exhibits a cell-specific pattern: different cells synthesize distinct laminin isoforms and integrin subtypes.11,12 For example, BMECs make laminin-411 and -511, pericytes synthesize laminin-411/421 and -511/521, while astrocytes mainly generate laminin-211. It is speculated that the expression levels of laminin and its receptors change during aging and in pathological conditions. However, how exactly each laminin isoform and integrin subtype alter remains unclear, partially due to the lack of isoform/subtype-specific antibodies.
Consistent with their close proximity to the BBB, laminin and its receptors have been shown to exert important functions in BBB regulation.11,12 However, the exact role of each laminin isoform or integrin subtype remains largely unknown for three major reasons. First, ablation of many laminin/integrin subunits (e.g. laminin-γ1 and integrin-β1) leads to embryonic lethality, preventing investigation of their functions in BBB integrity. 11 Second, many laminin/integrin subunits are expressed in multiple cell types, which makes it difficult to determine the function of a specific isoform/subtype. For example, laminin-α4 is expressed in both BMECs (laminin-411) and pericytes (laminin-411/421), and thus it is unclear whether the phenotype of laminin-α4 null mice is due to loss of laminin-411 or -421. Third, there may be functional compensation between different laminin isoforms or integrin subtypes. For instance, laminin-411 and -511 can compensate for each other’s loss, which makes it challenging to study their functions with laminin-α4 and -α5 single knockout mice.
To answer these important questions, knownledge on cell-specific expression of laminin isoforms/integrin subtypes and sophisticated genetic models, such as conditional knockouts and compound knockouts, are needed. In this section, we summarize the expression of laminin and its receptors in each cell type in the CNS (Figure 3) and discuss their functions in BBB regulation (Tables 1 to 4).
Figure 3.
Expression of laminin isoforms and their receptors in different CNS cell types. Schematic illustration showing the expression of laminin isoforms and their receptors in BMECs, pericytes, astrocytes, fibroblasts, microglia, oligodendrocytes, and neurons. * indicates controversial results.
Table 1.
Expression and functions of endothelium-derived laminin isoforms and their receptors.
| Subunits | Expression | References | Mutations | BBB phenotypes | References |
|---|---|---|---|---|---|
| Laminin-α4 (laminin-411) | Ubiquitously expressed in the vasculature starting from early embryonic stage | 21,22 | Laminin-α4 global knockout | Disrupted vascular integrity at embryonic and neonatal stages; intact BBB in adulthood. | 23 |
| Reduced leukocyte extravasation in a muscle inflammation model | 24 | ||||
| Reduced T lymphocyte extravasation in the EAE model | 22 | ||||
| Laminin-α5 (laminin-511) | Patchy expression in postcapillary venules starting from postnatal stage | 21,22 | Laminin-α5 global knockout | Embryonic lethality at E17 | 25 |
| Laminin-α5 EC-specific knockout | No BBB defects under homeostatic conditions | 24 , 26 , 27 | |||
| Enhanced leukocyte extravasation in a muscle inflammation model | 24 | ||||
| Exacerbated BBB disruption and inflammatory cell infiltration in an ICH model | 26 | ||||
| – | Laminin-511 enhances BBB integrity under hypoxic and inflammatory conditions in an in vitro model | 28 | |||
| – | Laminin-511 improves the barrier properties of pluripotent stem cell-derived BMECs in vitro | 29 | |||
| Laminin-γ3 | Limited distribution compared to laminin-γ1 | 30 | Laminin-γ3 global knockout | Ectopic granule cells in cerebellum and enhanced capillary branching in the outer retina; BBB integrity not studied | 30 |
| Integrin-α3 | Ubiquitously expressed in the vasculature and up-regulated in pathological conditions | 31–33 | Integrin-α3global knockout | Perinatal lethality | 34 |
| Integrin-α3EC-specific knockout | Viable, BBB integrity not studied | 35 | |||
| Integrin-α5 | 33,36–39 | Integrin-α5global knockout | Embryonic lethality at E11 | 40 | |
| Integrin-α5EC-specific knockout | Increased endothelial transcytosis and BBB permeability | 36 | |||
| Enhanced BBB disruption and early onset & faster progression of MS in the EAE model | 41 | ||||
| Decreased BBB permeability after ischemic stroke | 39 | ||||
| Integrin-α6 | 31–33 | Integrin-α6EC-specific knockout | Intact BBB integrity under homeostatic conditions | 42 | |
| Integrin-αv | 36–38,43–45 | Integrin-αvEC-specific knockout | Intact BBB integrity under homeostatic conditions | 37 , 46 | |
| Integrin-αvβ3 | 33,43–45 | Integrin-αvβ3 blockage | Reduced BBB permeability in ischemic stroke | 47 , 48 | |
| Integrin-β1 | 31–33,38,49–51 | Integrin-β1 global knockout | Embryonic lethality at E5.5 | 52 , 53 | |
| Integrin-β1 EC-specific knockout | Embryonic lethality at E10.5-11.5 | 49 , 50 | |||
| Aggravated BBB disruption with 50% reduction in BMECs | 54 | ||||
| Integrin-β1 blockage | Compromisesd vascular integrity | 54 , 55 | |||
| Integrin-β4 | 32,33,56 | Integrin-β4 EC-specific knockout | Intact BBB integrity under homeostatic conditions | 56 , 57 | |
| Aggravated BBB damage and worse MS symptoms in an EAE model | 56 | ||||
| Integrin-β8 | Extremely low expression | 58 | Integrin-β8 EC-specific knockout | Intact BBB integrity under homeostatic conditions | 58 |
| Dystroglycan | Controversial | 59–61 | – | – | – |
BBB: blood-brain barrier; EAE: experimental autoimmune encephalomyelitis; ICH: intracerebral hemorrhage; MS: multiple sclerosis; tMCAO: transient middle cerebral artery occlusion.
Table 2.
Expression and functions of pericyte-derived laminin isoforms and their receptors.
| Subunits | Expression | References | Mutations | BBB phenotypes | References |
|---|---|---|---|---|---|
| Laminin-α2 | Mainly at mRNA level | 38 , 62 , 63 , 64 | – | – | – |
| Laminin-α4 | At both mRNA and protein levels | 63–64 | – | – | – |
| Laminin-α5 | At both mRNA and protein levels | 63–64 | Laminin-α5 mural cell-specific knockout | Intact BBB integrity under homeostatic conditions | 65 |
| Attenuated BBB damage in the tMCAO model | 65 | ||||
| Laminin-γ1 | At both mRNA and protein levels | 63–64 | Laminin-γ1 mural cell-specific knockout | BBB breakdown and hydrocephalus in 11% of mutants in C57Bl6-FVB mixed background | 66 |
| Mild BBB breakdown at old age without hydrocephalus in C57Bl6 dominant background | 67 | ||||
| Exacerbated BBB leakage in an ICH model | 67 | ||||
| Laminin-γ1 vSMC-specific knockout | Grossly normal and intact BBB integrity | 66 | |||
| Integrin-α1 | At both mRNA and protein levels | 38 , 63 , 68 | – | – | – |
| Integrin-α2 | At both mRNA and protein levels | 63,68,69 | Integrin-α2blockage | Pericyte differentiation from the resting stage to the contractile stage | 69 |
| Integrin-α2knockdown | Pericyte differentiation from the resting stage to the contractile stage | 69 | |||
| Integrin-α5 | At mRNA level | 36 , 38 | – | – | – |
| Integrin-α6 | At both mRNA and protein levels | 68,70 | Integrin-α6mural cell-specific knockout | Grossly normal under physiological conditions | 70 |
| Aggravated blood vessel leakage in tumors | 70 | ||||
| Integrin-αv | At mRNA level | 36 , 38 | – | – | – |
| Integrin-αM | At protein level | 71 | – | – | – |
| Integrin-β1 | At both mRNA and protein levels | 38,63,72,73 | Integrin-β1 mural cell-specific knockout | Impaired vascular integrity and pericyte/vSMC defects | 72 |
BBB: blood-brain barrier; ICH: intracerebral hemorrhage; tMCAO: transient middle cerebral artery occlusion.
Table 3.
Expression and functions of astrocyte-derived laminin isoforms and their receptors.
| Subunits | Expression | References | Mutations | BBB phenotypes | References |
|---|---|---|---|---|---|
| Laminin-211 | CNS astrocytes | 69,74,75 | Laminin-γ1 knockout in neurons and glia | Compromised vascular integrity and ICH; pericyte differentiation | 69 , 76 |
| Laminin-γ1 knockout in neurons | Intact BBB integrity | 69 | |||
| Laminin-α2 global knockout | BBB disruption and reduced pericyte coverage | 75 | |||
| – | Laminin-211 fragment enhances the barrier properties of human induced pluripotent stem cell-derived BMECs in vitro | 77 | |||
| Laminin-β2 | Retinal astrocytes | 78 | Laminin-β2 global knockout | Increased glomerular filtration and retinal defects; BBB integrity not studied | 79 |
| Laminin-γ3 | Retinal astrocytes | 78 | Laminin-γ3 global knockout | Defects in cerebellum and outer retina; BBB integrity not studied | 30 |
| Integrin-α1 | Astrocytes | 80,81 | Integrin-α1 global knockout | No BBB disruption | 82 |
| Integrin-α2 | Astrocytes | 33 | Integrin-α2 global knockout | No BBB disruption | 83 |
| Integrin-α3 | Astrocytes | 33,84 | Integrin-α3 global knockout | Perinatal lethality, BBB integrity at embryonic/perinatal stages not studied | 34 |
| Integrin-α6 | Astrocytes | 33,81,84,85 | Integrin-α6 global knockout | Perinatal lethality, intact BBB integrity at embryonic/perinatal stages | 86 , 87 |
| Integrin-αv | Astrocytes | 33,84,88,89 | Integrin-αv knockout in neurons and glia | Vascular defects and cerebral hemorrhage | 46 |
| Integrin-αv knockout in glia | Vascular defects and cerebral hemorrhage | 46 | |||
| Integrin-β1 | Astrocytes | 33,80,84 | Integrin-β1 astrocyte-specific knockout | Intact BBB integrity under homeostatic conditions | 90 , 91 |
| Integrin-β4 | Astrocytes | 33 | Integrin-β4 global knockout | Perinatal lethality, intact BBB integrity at embryonic/perinatal stages | 92 |
| Integrin-β8 | Astrocytes | 33,84,89,93 | Integrin-β8 knockout in neurons and glia | Vascular defects and cerebral hemorrhage at embryonic/perinatal stages | 58 |
| Integrin-β8 knockout in neurons | No BBB defects | 58 | |||
| Dystroglycan | Astrocytes | 94–96 | Dystroglycan knockout in neurons and glia | BBB disruption | 75 |
| Dystroglycan astrocyte-specific knockout | No BBB disruption | 97 , 98 |
BBB: blood-brain barrier; ICH: intracerebral hemorrhage.
Table 4.
Expression and functions of other CNS cell-derived laminin isoforms and their receptors.
| Cell Types | Subunits | Expression | References | Mutations | BBB phenotypes | References |
|---|---|---|---|---|---|---|
| Fibroblasts | Laminin-111 | At both mRNA and protein levels in perivascular fibroblasts | 38,64,74 | Laminin-γ1 fibroblast-specific knockout | Intact BBB integrity under homeostatic conditions; Enhanced BBB damage in an ICH model | Unpublished data |
| Integrin-α1, -α2, -α3, -α7, -αv, -β1, -β4, and -β8 | At mRNA level in perivascular fibroblasts | 64 | – | – | – | |
| Dystroglycan | At mRNA level in fibroblasts | 38 | – | – | – | |
| Microglia | Laminin-α2, -α3, and -α4 | At both mRNA and protein levels in semi-activated primary microglia | Unpublished data | – | – | – |
| Laminin-α5 | At both mRNA and protein levels in semi-activated primary microglia | Unpublished data | Laminin-α5 knockout in microglia & macrophages | No BBB damage under homeostatic conditions; Enhanced BBB leakage and worse stroke outcome in a tMCAO model | Unpublished data | |
| Integrin-α6β1 | Constitutive expression in microglia | 99 | Integrin-β1 knockout in microglia & macrophages | Viable, BBB integrity not studied | 100 | |
| Integrin-αMβ2 | Constitutive expression in microglia | 101 | Integrin-β2 global knockout | Grossly normal under homeostatic conditions; Attenuated BBB leakage and decreased infarct volume after ischemic stroke | 102 | |
| Integrin-αvβ3 | Expression regulated by cytokines and ECM proteins | 33 , 103 | – | – | – | |
| Integrin-αvβ5 | Expression regulated by cytokines and ECM proteins | 33,103 | Integrin-β5 global knockout | Loss of synchronized retinal phagocytosis and age-related blindness; BBB integrity not studied | 104 , 105 | |
| Integrin-α4β1, -α5β1, -αLβ2, and -αXβ2 | Induced by inflammatory cytokines and LPS | 33 , 106 | – | – | – | |
| OLs | Laminin-α1, -α2, -α4, -γ1, and -γ3 | OPCs, at mRNA level | 107 | – | – | – |
| Laminin-α2, -α4, and -γ1 | Pre-myelinating OLs, at mRNA level | 107 | – | – | – | |
| Laminin-α2 and -γ1 | Mature OLs, at mRNA level | 107 | – | – | – | |
| Laminin-α2 and -γ1 | OLs, at mRNA level | 38 , 108 | – | – | – | |
| Laminin-α2, -α4, -α5, -β1, -β2, -β3, -γ1, and -γ2 | Pre-myelinating OLs, at protein level | 109 | – | – | – | |
| Integrin-α6β1 | OPCs > pre-myelinating and mature OLs | 107,108,110,111 | Integrin-β1OL-specific knockout | No BBB damage under homeostatic conditions | 110 | |
| Integrin-αvβ1 | OPCs > pre-myelinating and mature OLs | 112 | ||||
| Integrin-αvβ3 | Pre-myelinating OLs > OPCs and mature OLs | 113 | – | – | – | |
| Integrin-αvβ5 | Pre-myelinating and mature OLs > OPCs | 112 | – | – | – | |
| Integrin-αvβ8 | OPCs and mature OLs > pre-myelinating OLs | 113 | – | – | – | |
| Dystroglycan | OPCs > pre-myelinating and mature OLs | 107 , 108 , 110,111 | – | – | – | |
| Neurons | Laminin-211 | Various neuronal subpopulations | 114,115 | Laminin-γ1 knockout in excitatory neurons | No BBB damage under homeostatic conditions | 69 |
| Laminin-511 | Neurons in dentate gyrus and hippocampus | 116,117 | Laminin-α5 neuron-specific knockout | Age-dependent loss of synapses and behavioral defects; BBB integrity not studied | 117 | |
| Integrin-α1β1 | Neurons | 118,119 | Integrin-β1neuron-specific knockout | Intact BBB integrity under homeostatic conditions | 90 | |
| Integrin-α3β1 | Neurons | 119 | ||||
| Integrin-α5β1 | Dendrites of hippocampal and cortical neurons | 120 | ||||
| Integrin-α6β1 | Neural retinal cells and retinal ganglion cells | 121 | ||||
| Integrin-αv | Neurons | 93 | Integrin-αvneuron-specific knockout | Intact BBB integrity under homeostatic conditions | 46 | |
| Integrin-β8 | Neurons | 93 | Integrin-β8neuron-specific knockout | Intact BBB integrity under homeostatic conditions | 58 | |
| Dystroglycan | Neurons | 96 | – | – | – |
BBB: blood-brain barrier; ICH: intracerebral hemorrhage; OLs: oligodendrocytes; OPC: oligodendrocyte precursor cells; tMCAO: transient middle cerebral artery occlusion.
BMECs
BMECs form the physical barrier of the BBB and are a major component of the neurovascular unit. Unlike peripheral endothelial cells, BMECs display three unique properties that contribute to their barrier function.122,123 First, BMECs have specialized intercellular tight junctions, which limit paracellular transport of molecules. Second, BMECs demonstrate a significantly lower transcytosis rate, which prevents transcellular transport of molecules. Third, BMECs express a large number of efflux and influx transporters, which transport various substances out of and into the brain, respectively.
Laminin expression in BMECs
BMECs predominantly synthesize laminin-411 and -511, which contribute to the formation of endothelial BL.21,24,124 The expression of these laminin isoforms demonstrates different dynamics. Specifically, laminin-411 is ubiquitously expressed in all endothelial cells along the arteriovenous axis starting from early embryonic stage.21,22 Laminin-511, on the other hand, is not expressed in the vasculature until after birth and it has a fragmentary expression pattern in postcapillary venules.21,22 The distinct spatiotemporal expression patterns of laminin-411 and -511 suggest that they may have different functions. In addition, laminin-γ3 is also detected in the brain and retina but with a more limited distribution pattern compared to laminin-γ1. 30 The cellular source of laminin-γ3, however, remains to be determined.
Functions of endothelial laminin
Our knowledge of how laminin regulates BBB integrity mainly comes from loss-of-function studies. An early study reported that laminin-α4 global knockout mice developed hemorrhage at embryonic and neonatal stages, 23 indicating an important role of laminin-α4 in vascular integrity during early development. Since both BMECs and pericytes make laminin-α4, it remains unclear whether this phenotype is due to loss of endothelium- or pericyte-derived laminin-α4. Interestingly, these laminin-α4 global knockout mice failed to show BBB disruption and were grossly normal in adulthood, 23 suggesting that loss of laminin-α4 may be compensated by other laminin isoforms, such as laminin-α5, whose expression in the vasculature starts after birth. Unfortunately, ablation of laminin-α5 leads to embryonic lethality at E17 due to its crucial role in embryonic development. 25 To investigate the function of endothelial laminin-α5 in BBB integrity, we and the Sorokin lab independently generated endothelial laminin-α5 conditional knockout (α5-TKO) mice by crossing laminin-α5flox/flox mice with the Tie2-Cre line. Surprisingly, both groups failed to detect BBB breakdown or other defects in these α5-TKO mice under homeostatic conditions,24,26,27 highlighting a dispensable role of endothelial laminin-α5 in BBB maintenance under physiological conditions. These results suggest that loss of endothelial laminin-α5 may be functionally compensated by other laminin isoforms, such as endothelial laminin-411. Due to the mutual compensation between laminin-411 and -511, the function of endothelial laminin in BBB maintenance remains unknown. To answer this question, we generated a compound mutant line, in which both laminin-411 and -511 are simultaneously deleted in endothelial cells. Our unpublished data showed BBB breakdown in these mutants in adulthood. We are currently characterizing these mutants and exploring the underlying molecular mechanisms.
Since laminin-α4 null and endothelial laminin-α5 knockout mice are grossly normal under homeostatic conditions, these mutants have been studied in pathological conditions. In a muscle inflammation model, laminin-α4 null mice showed reduced leukocyte extravasation, whereas α5-TKO mice displayed enhanced leukocyte extravasation. 24 In a collagenase-induced intracerebral hemorrhage model, α5-TKO mice displayed exacerbated BBB disruption and increased brain infiltration of inflammatory cells (Ly6G+ neutrophils and CD68+ mononuclear cells). 26 Similarly, laminin-α4 null mice exhibited ubiquitous expression of laminin-α5 in the vascular tree and substantially reduced T lymphocyte extravasation in an experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). 22 These findings suggest opposite roles of laminin-α4 and -α5. Consistent with these loss-of-function studies, transmigration of polymorphonuclear leukocytes predominantly occurs in laminin-α4high and laminin-α5low regions. 21 In addition, laminin-511 enhances BBB integrity under hypoxic and inflammatory conditions in an in vitro BBB model. 28 Similarly, laminin-511 but not laminin-411 improves the barrier properties of pluripotent stem cell-derived BMECs in vitro. 29 Together, these results strongly suggest that laminin-α4 and -α5 differentially regulate BBB permeability under pathological conditions, with laminin-α4 promoting and laminin-α5 inhibiting inflammatory cell infiltration into brain parenchyma.
Unlike laminin-γ1−/− mice, laminin-γ3−/− mutants have a normal life span and show ectopic granule cells in cerebellum and enhanced capillary branching in the outer retina. 30 Whether BBB integrity is disrupted in these mutants, however, remains unclear.
Expression of laminin receptors in BMECs
Under physiological conditions, BMECs predominantly express classical laminin-binding integrins, including integrin-α3β1,31–33,49 -α6β1,31–33,49,50 and -α6β4.32,33,56 In addition, there is also evidence showing endothelial expression of integrin-α5, -αv, and -β3.33,36–38,51 Under pathological conditions, not only do BMECs significantly up-regulate the expression of above-mentioned integrin subtypes, but they also start to express new integrin subtypes.39,43–45,56,125
Inconsistent findings exist on whether BMECs express dystroglycan. One study failed to detect dystroglycan expression in BMECs, 94 whereas others showed dystroglycan expression in cultured human BMECs.59–61 Different experimental conditions and systems may be responsible for this discrepancy. Future research should address this controversy.
Functions of laminin receptors in BMECs
Previous studies have shown that ablation of integrin-β1 globally and in endothelial cells specifically (Tie2-Cre and Tie1-Cre) leads to embryonic lethality at E5.552,53 and E10.5 ∼11.5,49,50 respectively, highlighting an indispensable role of endothelial integrin-β1 in embryonic development. The early embryonic lethality of these mutants prevents investigation of endothelial integrin-β1’s function in BBB integrity. To overcome this challenge, the inducible Cre-Lox system was used to ablate integrin-β1 expression in adult mice. One study found that mutants with 50% reduction of integrin-β1 in BMECs exhibited aggravated BBB disruption characterized by significantly increased IgG leakage. 54 Consistent with this finding, pharmacological blockage of integrin-β1 signaling with its function-blocking antibody compromises vascular integrity both in vitro and in vivo. 55 These results highlight a key role of endothelial integrin-β1 in BBB maintenance. In addition, although global knockout of integrin-α5 results in embryonic lethality at E11, 40 its abrogation in endothelial cells (Cdh5-CreERT 2 ) leads to elevated BBB and blood-retina-barrier permeability without affecting vessel morphology and development. 36 Further mechanistic study shows that interaction between pericyte-secreted vitronectin and endothelial integrin-α5 inhibits transcytosis in endothelial cells, contributing to barrier integrity. 36 Together, these findings suggest that endothelial integrin-α5β1 may be involved in BBB maintenance.
Unlike integrin-α5, deletion of integrin-α6, 42 -β4,56,57 -αv,37,46 or -β8 58 in endothelial cells (Tie2-Cre or Cdh5-CreERT 2 ) fails to affect BBB integrity, indicating a dispensable role of integrin-α6β4 and -αvβ8 in BBB maintenance under homeostatic conditions. Currently, the function of integrin-α3 in BBB integrity remains unknown. Loss of integrin-α3 results in perinatal lethality, 34 preventing investigation of its role in BBB integrity postnatally. Although endothelium-specific (Tie1-Cre) integrin-α3 knockout mice are viable and fertile, 35 BBB permeability in these mutants has not been examined. Similarly, the function of endothelial dystroglycan in BBB integrity is also unclear. Future research should elucidate the functional significance of endothelial integrin-α3β1 and dystroglycan in BBB regulation under homeostatic conditions.
Mounting evidence suggests that up-regulated integrin subtypes actively regulate BBB integrity in various disease models. For example, it has been shown that loss of endothelial integrin-β4 leads to aggravated BBB damage and worse MS symptoms in the EAE model. 56 Similarly, ablation of endothelial integrin-α5 enhances BBB disruption and triggers early-onset and faster progression of MS in the EAE model. 41 These findings suggest a BBB-protecting role of endothelial integrin-α6β4 and integrin-α5β1 in MS. In sharp contrast, the up-regulated endothelial integrin-α5β1 seems to impair BBB integrity in global hypoxia or ischemic stroke. It has been reported that loss of endothelial integrin-α5 decreases BBB permeability and increases tight junction protein expression in both in vivo and in vitro ischemic stroke models. 39 In addition, integrin-αvβ3 expression is significantly up-regulated after ischemic injury, 126 and pharmacological inhibition of integrin-αvβ3 reduces BBB permeability in ischemic stroke,47,48 highlighting a BBB-disrupting effect of integrin-αvβ3 in ischemic stroke.
Pericytes
Pericyte together with vSMCs are called mural cells. Unlike vSMCs that reside in large blood vessels, pericytes cover small vascular networks.127,128 Embedded in the BL (Figure 1), pericytes have direct contact with both BMECs and astrocytes. As a cellular component of the neurovascular unit, pericytes play an important role in BBB maintenance: it has been shown that reduced pericyte number or pericyte coverage on endothelial cells leads to BBB disruption.62,129
Laminin expression in pericytes
It has been shown that pericytes predominantly synthesize laminin-α4, -α5, -β1, -β2, and -γ1 mRNA in an endothelium-pericyte co-culture system. 63 Consistent with this finding, we showed that primary mouse brain pericytes expressed laminin-α4 and -α5 but not -α1 or -α2 at the protein level. 66 Interestingly, laminin-α4 and -α5 were absent in laminin-γ1-deficient primary pericytes, 66 suggesting that both α subunits form heterotrimers with γ1 subunit. Together, these findings suggest that brain pericytes mainly synthesize laminin-411/421 and -511/521. In addition, laminin-α2 mRNA was also reported in brain pericytes in recent single-cell RNAseq studies,38,62 suggesting that pericytes may also express α2-containing laminins.
Using single-nucleus RNAseq technique, Yang and colleagues recently identified two subclusters of pericytes in human brains: T-pericytes and M-pericytes, which are involved in small-molecule transport and ECM synthesis, respectively. 64 Echoed with this report, we have identified laminin-expressing and laminin-negative pericytes in mouse brains using a newly generated laminin-reporter mouse line (unpublished data). The laminin-expressing and laminin-negative pericytes may represent M-pericytes and T-pericytes, respectively. These results suggest that different subpopulations of pericytes may synthesize distinct laminin isoforms. Future research should focus on characterizing these pericyte subpopulations.
Functions of pericyte-derived laminin
To study the role of pericyte-derived laminin in BBB integrity, we generated two laminin-γ1 conditional knockout mouse lines: one in mural cells (PDGFRβ-Cre, called PKO) and the other in vSMCs (SM22α-Cre, called SKO). The PKO mice show a genetic background-dependent BBB phenotype. In the C57Bl6-FVB mixed background, approximately 11% of PKO mice displayed BBB breakdown and hydrocephalus, while the rest 89% were grossly normal. 66 To determine if BBB compromise in these mutants is secondary to hydrocephalus or caused by loss of pericyte-derived laminin, we need to separate BBB impairment from hydrocephalus. In the C57Bl6 dominant background, PKO mice failed to show hydrocephalus and developed mild BBB breakdown only at old age, 67 highlighting an age-dependent BBB disruption phenotype. Unlike PKO mice, SKO mice were grossly normal and failed to show BBB damage. 66 These results suggest that pericyte- rather than vSMC-derived laminin contributes to BBB maintenance. To further determine if BBB disruption in PKO mice is caused by loss of α4- or α5-containing laminins, we generated mutant mice with laminin-α5 deficiency in mural cells (α5-PKO). These α5-PKO mutants had intact BBB and failed to develop obvious abnormalities under homeostatic conditions, 65 indicating a dispensable role of mural cell-derived α5-containing laminins in BBB maintenance.
Interestingly, PKO and α5-PKO mice exhibit distinct phenotypes in pathological conditions. Specifically, young PKO mice in C57Bl6 dominant background (before BBB breakdown) showed exacerbated BBB leakage in an intracerebral hemorrhage model, 67 suggesting a beneficial role of mural cell-derived laminin in hemorrhagic stroke. α5-PKO mice, on the other hand, displayed attenuated BBB damage in the transient middle cerebral artery occlusion model, 65 indicating a detrimental role of mural cell-derived laminin-α5 in ischemic stroke. These findings suggest that mural cell-derived laminin-α5 and -α4 may play detrimental and beneficial roles under pathological conditions, respectively. However, we are unable to exclude the possibility that the distinct phenotypes of PKO and α5-PKO mice are caused by different stroke models. We are characterizing the phenotype of PKO mice in ischemic stroke and that of α5-PKO mice in hemorrhagic stroke currently.
Expression of laminin receptors in pericytes
Like BMECs, pericytes primarily express β1-containing integrins. At mRNA level, integrin-β1 was detected in cultured CNS pericytes. 63 At protein level, integrin-β1 was found in pericytes in vivo by immunofluorescence.72,73 Many integrin α subunits have been identified in pericytes. For example, integrin-α1, -α5, -α6, -αv, and -αM mRNAs have been detected in CNS pericytes.36,38,63,68,70,71 Integrin-α6 expression in brain pericytes has been validated by both immunofluorescence 70 and flow cytometry. 68 Similarly, flow cytometry confirmed the expression of integrin-α1 and -α2 in primary mouse brain pericytes and revealed a switch between integrin-α1 and -α2 during inflammation. 68 In addition, the expression of integrin-α2 in primary brain pericytes was also confirmed by western blotting. 69 Currently, there are no reports showing expression of dystroglycan in brain pericytes. Together, these results suggest that brain pericytes predominantly express integrin-α1β1, -α2β1, -α5β1, -α6β1, and αv-containing integrins.
Functions of laminin receptors in pericytes
Consistent with its high expression level, abrogation of integrin-β1 in mural cells (PDGFRβ-Cre) leads to defects in pericytes/vSMCs and impaired vascular integrity. 72 Interestingly, although mice with integrin-α6 knockout in mural cells (PDGFRβ-Cre) are grossly normal under physiological conditions, they demonstrated aggravated blood vessel leakage in tumors. 70 These results suggest that mural cell-derived integrin-α6β1 actively regulates vascular integrity in pathological conditions, albeit dispensable under homeostatic conditions. Using primary brain pericytes, we demonstrated that blockage or knockdown of integrin-α2 induced pericyte differentiation from the resting stage to the contractile stage, switching its function from BBB-protective to BBB-disrupting. 69 These results highlight essential roles of integrin-α2 in pericyte differentiation and BBB integrity. Interestingly, blocking integrin-β1, the only known binding partner for integrin-α2, failed to affect pericyte differentiation, 69 suggesting that integrin-α2 may regulate pericyte differentiation and BBB integrity through a novel/unidentified partner. Currently, there are no pericyte-specific loss-of-function studies on other integrin subunits, such as integrin-α1, -α5, and -αv. Future research should focus on determining key laminin receptors in pericytes that contribute to BBB maintenance and BBB repair after injury.
Astrocytes
Astrocytes cover more than 99% of endothelial cells with their endfeet.130,131 They contribute to BBB integrity via upregulation of tight junction proteins on endothelial cells and polarized expression of specialized transporters and water channels in their endfeet.130,131
Laminin expression in astrocytes
Brain astrocytes primarily produce laminin-211,69,74,75 which is a major component of the parenchymal BL. There is evidence showing that retinal astrocytes make laminin-β2 and -γ3, 78 although the exact laminin isoforms made by these cells remain unknown.
Functions of astrocyte-derived laminin
To study the function of astrocyte-derived laminin, we generated mutant mice with laminin deficiency in both neurons and glia (Nestin-Cre) and neurons only (CamK2a-Cre). The former mutants displayed age-dependent BBB breakdown and spontaneous intracerebral hemorrhage in deep brain regions, while the latter mutants failed to show gross abnormalities,69,76 strongly indicating that loss of glial rather than neuronal laminin causes this phenotype. Using adenovirus expressing Cre under GFAP promoter, we further determined that loss of astrocyte-derived laminin disrupted vascular integrity, 69 highlighting an indispensable role of astrocytic laminin (laminin-211) in BBB maintenance. A subsequent mechanistic study revealed that astrocytic laminin maintained BBB integrity by inhibiting pericyte differentiation from the resting state to the contractile state. 69 Consistent with our data, laminin-α2 null mice exhibited BBB disruption and reduced pericyte coverage, 75 although a muscular dystrophy phenotype was also observed in these mice.132,133 Consistent with these loss-of-function studies, laminin-211 fragment that contains the full integrin-binding activity enhances the barrier properties of human induced pluripotent stem cell-derived BMECs in vitro. 77 Together, these results suggest that astrocyte-derived laminin-211 actively maintains BBB integrity.
Although laminin-β2−/− mice develop increased glomerular filtration and retinal defects, 79 BBB integrity in these mutants has not been examined. Similarly, it remains unknown if BBB integrity is compromised in laminin-γ3−/− mice, which display mild defects in cerebellum and outer retina. 30 These questions should be answered in the future.
Expression of laminin receptors in astrocytes
Previous studies have shown that astrocytes express various integrin subtypes, including integrin-α1β1, -α2β1, α3β1, -α6β1, -α6β4, and -αvβ8.33,80,81,84,85,88,89,93 It should be noted that integrin-β1 expression is lower in astrocytes compared to BMECs, 55 suggesting a possibly more important role of β1-containing integrins in BMECs than astrocytes. There is also evidence suggesting that astrocytes express dystroglycan in their endfeet.94–96
Functions of laminin receptors in astrocytes
Although astrocytes express integrin-β1, its ablation (hGFAP-Cre) fails to compromise BBB integrity,90,91 indicating a minimal role of astrocyte-derived integrin-β1 in BBB maintenance. Similarly, mice null for integrin-α1 82 and -α2 83 are grossly normal and show no BBB disruption, suggesting a dispensable role of astrocyte-derived integrin-α1β1 and -α2β1 in BBB maintenance. Unlike integrin-α1−/− and -α2−/− mice, integrin-α3−/−, 34 -α6−/−,86,87 and -β4−/−109 mutants die at perinatal stage. Although no BBB damage is observed in integrin-α6−/−87,107 and -β4−/−109 mice at embryonic/perinatal stages, it remains unknown whether integrin-α3−/− mice display BBB impairment at these stages. These results suggest that astrocyte-derived integrin-α6β4 plays a minimal role in BBB formation in early development. How it regulates BBB integrity postnatally remains unclear. This question needs to be answered in future studies.
In contrast to the above-mentioned integrins, integrin-αvβ8 is actively involved in BBB regulation. Vascular defects and cerebral hemorrhage have been reported in mice lacking integrin-β8 in both neurons and glia (Nestin-Cre) at embryonic/perinatal stages, but not in those lacking integrin-β8 in neurons only (NEX-Cre), 58 highlighting a critical role of glial integrin-β8 in BBB integrity. Like integrin-β8 mutants, mice with integrin-αv deficiency in both neurons and glia (Nestin-Cre) or astrocytes (hGFAP-Cre) developed vascular defects and cerebral hemorrhage. 46 These findings suggest that astrocyte-derived integrin-αvβ8 is critical for BBB maintenance. A subsequent mechanistic study revealed that astrocytic integrin-αvβ8 regulated endothelial differentiation and vessel stabilization/homeostasis via transforming growth factor-β. 134
Controversial findings exist on whether astrocytic dystroglycan regulates BBB integrity. On one hand, BBB disruption was found in mutants with dystroglycan deficiency in both neurons and glia (Nestin-Cre). 75 Ablation of dystroglycan in astrocytes (hGFAP-Cre), on the other hand, failed to compromise BBB integrity.97,98 This discrepancy may be caused by different specificity and temporal expression profiles of the Cre lines in the CNS. Astrocyte-specific Cre lines, such as the recently reported Aldh1l1-CreERT 2 mice, 135 may help determine the function of astrocytic dystroglycan in BBB integrity.
Fibroblasts
CNS fibroblasts include meningeal fibroblasts and perivascular fibroblasts, which are located in all three layers of the meninges (pia, arachnoid, and dura) and perivascular space, respectively.136,137 Under physiological conditions, perivascular fibroblasts are sandwiched between vSMCs and astrocytic endfeet in large blood vessels.38,136 Our unpublished data showed that fibroblasts were associated with small blood vessels after hemorrhagic stroke, suggesting a possible role of fibroblasts in BBB regulation under pathological conditions.
Laminin expression in fibroblasts
Although fibroblasts are known to generate ECM proteins, the exact laminin isoforms expressed by fibroblasts remain largely unknown. Previous studies identified laminin-α1, -β1, and -γ1 in brain fibroblasts,38,74 suggesting that they predominantly synthesize laminin-111. It remains unclear whether the expression level of laminin-111 changes or if new laminin isoforms are induced in pathological conditions. Interestingly, a recent single-nucleus RNAseq study found that perivascular fibroblasts synthesized ECM proteins and their receptors, while meningeal fibroblasts expressed high levels of solute transporters. 64 This finding suggests that perivascular fibroblasts and meningeal fibroblasts may have different functions. A thorough understanding of fibroblast heterogeneity will enable an accurate laminin expression profile in fibroblasts.
Functions of fibroblast-derived laminin
To investigate the function of fibroblast-derived laminin in BBB integrity, we generated fibroblast-specific (Col1α1-Cre) laminin-γ1 conditional knockout mice (CKO). These CKO mice were grossly normal and failed to show BBB compromise under homeostatic conditions (unpublished data). In an intracerebral hemorrhage model, however, CKO mice displayed enhanced tracer leakage and exacerbated BBB damage compared to the controls (unpublished data). Together, these results suggest that fibroblast-derived laminin is dispensable for BBB maintenance under physiological conditions, but repairs BBB damage after hemorrhagic stroke.
Expression of laminin receptors in fibroblasts
According to the single-nucleus RNAseq data, fibroblasts synthesize a large number of integrin subunits, including integrin-α1, -α2, -α3, -α7, -αv, -β1, -β4, and -β8. 64 In addition, strong expression of dystroglycan in fibroblasts has also been reported. 38 These results suggest that fibroblasts express multiple integrin subtypes and dystroglycan.
Functions of laminin receptors in fibroblasts
Although multiple integrin subtypes and dystroglycan have been identified in fibroblasts, their functions are still unknown. Fibroblast-specific integrin or dystroglycan conditional knockout mice will enable investigation of their roles in BBB integrity. Future research should focus on generating these valuable tools.
Microglia
Microglia are brain-resident immune cells that play important roles in brain homeostasis and injury. 138 Under homeostatic conditions, microglia exist in a resting state characterized by ramified morphology with long processes. Upon CNS injury, microglia quickly change to an ameboid morphology, alter their gene expression profile, and migrate to the site of injury to resolve/repair brain damage. There is evidence showing that microglia actively communicate with cerebrovasculature and regulate BBB integrity in both physiological and pathological conditions.139,140
Laminin expression in microglia
The exact laminin isoforms synthesized by microglia remain elusive. Our unpublished data showed that freshly isolated semi-activated primary microglia expressed 4 out of 5 laminin α subunits (α2–α5) with laminin-α5 being the most highly expressed subunit, suggesting that microglia can synthesize most laminin isoforms. This finding needs to be validated in vivo in future studies. In addition, whether and how microglial expression of laminin changes in different states (e.g. resting vs. activated) should also be examined.
Functions of microglial laminin
Since laminin-α5 is the most highly expressed α subunit in microglia, we decided to investigate its function in BBB integrity first. To do so, we generated microglia/macrophage-specific laminin-α5 conditional knockout mice. These mutants showed no BBB damage and were grossly normal under physiological conditions (unpublished data), suggesting a minimal role of microglia/macrophage-derived laminin-α5 in BBB maintenance. In a transient middle cerebral artery occlusion model, however, these mutants exhibited enhanced BBB leakage and worse stroke outcome (unpublished data), indicating a neuroprotective role of microglia/macrophage-derived laminin-α5 in ischemic stroke. We are currently exploring how exactly microglia/macrophage-derived laminin-α5 repairs BBB damage and ischemic brain injury. The functions of other microglia-derived laminin isoforms should be investigated in future research.
Expression of laminin receptors in microglia
Microglia express many integrin subtypes. For example, constitutive expression of integrin-αMβ2 101 and -α6β1 99 has been reported in microglia. Integrin-αM is widely used as a microglia/macrophage marker. There is also evidence showing that microglial expression of αv-containing integrins, including integrin-αvβ3 and -αvβ5, is regulated by cytokines and ECM proteins.33,103 In addition, microglia have also been shown to express α4β1, -α5β1, -αLβ2, and -αXβ2.33,106 It should be noted, however, that among these integrin subtypes integrin-α6β1 is the only one that can interact with laminin. 99
Functions of laminin receptors in microglia
A previous loss-of-function study showed that integrin-β2 knockout mice were grossly normal under homeostatic conditions, but displayed attenuated exogenous tracer leakage and decreased infarct volume after ischemic stroke. 102 These results indicate that β2-containing integrins are dispensable for BBB maintenance under normal conditions, but exacerbate BBB disruption and brain injury in ischemic stroke. In addition, although microglia/macrophage-specific integrin-β1 (LysM-Cre) conditional knockout mice have been generated and are viable, 100 BBB integrity in these mutants has not been investigated. Similarly, it remains unclear if BBB integrity is disrupted in integrin-β5 knockout mice, which exhibit loss of synchronized retinal phagocytosis and age-related blindness.104,105 How these and other microglial integrins regulate BBB integrity should be investigated in future research.
Oligodendrocytes
Oligodendrocytes are major glial cells that myelinate neurons and support neuronal conduction in the CNS. 141 They come from differentiation of oligodendrocyte precursor cells (OPCs). A recent study showed that OPCs contributed to BBB integrity during development and after ischemic stroke. 142
Laminin expression in oligodendrocytes
Most results on laminin expression in oligodendrocytes come from RNAseq studies. A previous bulk RNAseq study found that OPCs made laminin-α1, -α2, -α4, -γ1, and -γ3; pre-myelinating oligodendrocytes expressed laminin-α2, -α4, and -γ1; and mature oligodendrocytes synthesized laminin-α2 and -γ1 (https://www.brainrnaseq.org). 107 Two single-cell RNAseq studies revealed that oligodendrocytes predominantly expressed laminin-α2 and -γ1 (https://betsholtzlab.org/VascularSingleCells/database.html).38,108 In addition, a secretome analysis demonstrated that neural stem cell-derived pre-myelinating oligodendrocytes synthesized laminin-α2, -α4, -α5, -β1, -β2, -β3, -γ1, and -γ2. 109 Together, these findings suggest that oligodendrocytes can generate most laminin isoforms.
Functions of oligodendrocyte-derived laminin
Laminin is known to play important roles in oligodendrocyte biology, including survival, migration, proliferation, differentiation, and myelination. 143 However, whether oligodendrocyte-derived laminin isoforms regulate BBB integrity remains unknown. Oligodendrocyte-specific laminin knockout mice will allow us to answer this important question. Future studies should focus on generating these genetic tools.
Expression of laminin receptors in oligodendrocytes
A previous bulk RNAseq study revealed higher levels of integrin-α6 and -β1 as well as dystroglycan in OPCs than pre-myelinating or mature oligodendrocytes. 107 Similarly, expression of integrin-β1 and dystroglycan was found in oligodendrocytes in a recent single-nucleus RNAseq study, although integrin-α6 was not detected possibly due to different experimental approaches and detection limits. 108 Consistent with these reports, expression of integrin-α6β1 and dystroglycan in oligodendrocytes has also been reported at the protein level.110,111,144 In addition, in vitro studies demonstrated that oligodendrocytes also expressed a variety of αv-containing integrins. One study showed that OPC differentiation was associated with down-regulation of integrin-αvβ1 and up-regulation of integrin-αvβ5. 112 Similarly, opposite expression patterns of integrin-αvβ8 and -αvβ3 were observed during OPC differentiation: integrin-αvβ8 was highly expressed on OPCs and mature oligodendrocytes, while integrin-αvβ3 was highly expressed during the intermediate stages of differentiation. 113
Functions of laminin receptors in oligodendrocytes
The functional significance of oligodendrocyte-derived integrins and dystroglycan in BBB maintenance remains largely unknown. One study found no BBB damage in oligodendrocyte-specific (CNP-Cre) integrin-β1 knockout mice, 110 highlighting a dispensable role of oligodendrocyte-derived integrin-β1 in BBB maintenance. How other oligodendrocyte-derived laminin receptors regulate BBB integrity needs to be examined in future research.
Neurons
Neurons are the fundamental units of the CNS. Proper crosstalk between neurons and other cells (e.g. vascular cells) contributes to brain homeostasis. There is evidence suggesting that neurons also contribute to the development, maturation, and maintenance of BBB integrity.145,146
Laminin expression in neurons
Previous studies have identified multiple laminin subunits, including laminin-α2 and -γ1, in various neuronal subpopulations.114–116,147,148 The expression of laminin-β1 and -γ1 in neurons was confirmed in vivo using transgenic mice expressing laminin-reporter fusion proteins, although laminin-α subunits were not detected. 115 In addition, various studies demonstrated that neurons in the hippocampus and dentate gyrus were able to synthesize laminin-511 at both mRNA and protein levels.117,149 These findings suggest that neurons can express laminin-511 and -211. Which other laminin isoforms neurons make and whether different neuronal subpopulations synthesize distinct laminin isoforms remain elusive.
Functions of neuronal laminin
To investigate the function of neuronal laminin in BBB integrity, we generated mice with laminin-γ1 abrogation in forebrain excitatory neurons (CamKII-Cre). These mutants fail to show signs of BBB disruption, 69 highlighting a minimal role of excitatory neuron-derived γ1-containing laminins in BBB maintenance. In addition, although mice with neuronal (Nex-Cre) loss of laminin-α5 show age-dependent loss of synapses and behavioral defects, 117 it remains unclear if BBB integrity is compromised in these mutants. This important question needs to be answered in the future.
Expression of laminin receptors in neurons
Accumulating evidence suggests that neurons can make most integrin subtypes, including integrin-α1β1, -α3β1, -α5β1, -α6β1, and -αvβ8.93,118–121 In addition, there are also reports showing neuronal expression of dystroglycan. 96
Functions of laminin receptors in neurons
Loss-of-function studies showed that ablation of integrin-β1, 90 -αv, 46 and -β8 58 in neurons (Nex-Cre) failed to affect BBB integrity, highlighting a dispensable role of neuronal expression of β1-containing integrins and integrin-αvβ8 in BBB maintenance. The role of neuronal dystroglycan in BBB integrity remains unknown.
Conclusions and future directions
With the development of novel molecular/biochemical techniques and genetic tools (e.g. conditional knockout mice and reporter lines), we have started to understand the expression and functions of laminin and its receptors in each cell type in the CNS. Although considerable progress has been made, several important questions remain unanswered and need to be addressed in the future. Answering these questions will fill the gap of knowledge and significantly move the field forward.
First, cell-specific expression of laminin and its receptors is not fully understood. Although the specific laminin isoforms and integrin subtypes expressed in BMECs and astrocytes have been identified, such expression data in other cell types, such as fibroblasts and microglia, remain largely unknown. This knowledge is essential for their functional research.
Second, the spatiotemporal distribution of laminin and its receptors in different cell types in the CNS is still unclear. Understanding when and where each laminin isoform and integrin subtype are expressed will allow a better understanding of their functional compensation.
Third, although vascular cells synthesize and deposit laminin to the BL, it remains unclear whether non-vascular cell-derived laminin also contributes to BL formation. Additionally, the relative contribution of each cell type-derived laminin in the BL is still unknown. Sophisticated genetic tools, such as cell-specific laminin reporter mice that allow visualization and quantification of laminin expression in a cell-specific manner, will provide answers to these questions.
Fourth, the impact of laminin isoform loss on other laminin isoforms and other BL components (e.g. collagen IV, nidogen, and heparin sulfate proteoglycans) remains largely unknown. Answer to this important question will allow a better understanding of the interplay between laminin isoforms and other BL components.
Fifth, cell-specific functions of laminin and its receptors in BBB integrity are not fully understood. Previous loss-of-function studies mainly focused on vascular cells, leaving the functions of nonvascular cell-derived laminin isoforms and laminin receptors largely unknown. Generating novel conditional knockout mice will enable investigation of their functions.
Sixth, functional compensation among different laminin isoforms and/or integrin subtypes remains elusive. One cell type can synthesize multiple laminin isoforms/integrity subtypes and the same laminin isoform/integrin subtype can be made by multiple cell types. This reciprocal expression pattern suggests that there may be functional compensation among different laminin isoforms and/or integrin subtypes, which poses a challenge for loss-of-function studies using single knockout mice. In this case, compound mutants with simultaneous deletion of multiple laminin isoforms and/or integrin subtypes should be used.
Last, it remains largely unknown how the expression and functions of laminin and its receptors change in pathological conditions. Knowledge on cell-specific expression and functions of laminin and its receptors in different neurological disorders will help identify novel molecular targets with therapeutic potential.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by the National Institute of Health Grants (R01HL146574, RF1AG065345, R21AG073862, and R21AG064422) to YY.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Yao Yao https://orcid.org/0000-0001-8020-9696
References
- 1.Sweeney MD, Zhao Z, Montagne A, et al. Blood-brain barrier: from physiology to disease and back. Physiol Rev 2019; 99: 21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 3.Neuwelt EA, Bauer B, Fahlke C, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 2011; 12: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang M, Yao Y. Basement membrane changes in ischemic stroke. Stroke 2020; 51: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Y. Basement membrane and stroke. J Cereb Blood Flow Metab 2019; 39: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen B, Bix G, Yao Y. Basal lamina changes in neurodegenerative disorders. Mol Neurodegener 2021; 16: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L, Nirwane A, Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc Neurol 2019; 4: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 2004; 20: 255–284. [DOI] [PubMed] [Google Scholar]
- 9.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010; 123: 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbano JM, Torgler CN, Molnar C, et al. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development 2009; 136: 4165–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Y. Laminin: loss-of-function studies. Cell Mol Life Sci 2017; 74: 1095–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nirwane A, Yao Y. Laminins and their receptors in the CNS. Biol Rev 2019; 94: 283–306. [DOI] [PubMed] [Google Scholar]
- 13.Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol 2012; 28: 523–553. [DOI] [PubMed] [Google Scholar]
- 14.Aumailley M. The laminin family. Cell Adh Migr 2013; 7: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 2009; 10: 843–853. [DOI] [PubMed] [Google Scholar]
- 16.Srichai MB, Zent R. Integrin structure and function. In: Zent R and Pozzi A (eds) Cell-extracellular matrix interactions in cancer. 1st ed. New York: Springer, 2010, pp.19–41. [Google Scholar]
- 17.Shen B, Delaney MK, Du X. Inside-out, outside-in, and inside–outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr Opin Cell Biol 2012; 24: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arimori T, Miyazaki N, Mihara E, et al. Structural mechanism of laminin recognition by integrin. Nat Commun 2021; 12: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ido H, Nakamura A, Kobayashi R, et al. The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin γ chains in integrin binding by laminins. J Biol Chem 2007; 282: 11144–11154. [DOI] [PubMed] [Google Scholar]
- 20.Hao Y, Talts JF. Beta1 integrin and alpha-dystroglycan binding sites are localized to different laminin-G-domain-like (LG) modules within the laminin alpha5 chain G domain. Biochem J 2003; 371: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yousif LF, Di Russo J, Sorokin L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adh Migr 2013; 7: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Ivars F, Anderson P, et al. Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain. Nat Med 2009; 15: 519–527. [DOI] [PubMed] [Google Scholar]
- 23.Thyboll J, Kortesmaa J, Cao R, et al. Deletion of the laminin α4 chain leads to impaired microvessel maturation. Mol Cell Biol 2002; 22: 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song J, Zhang X, Buscher K, et al. Endothelial basement membrane laminin 511 contributes to endothelial junctional tightness and thereby inhibits leukocyte transmigration. Cell Rep 2017; 18: 1256–1269. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen NM, Miner JH, Pierce RA, et al. Laminin α5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev Biol 2002; 246: 231–244. [DOI] [PubMed] [Google Scholar]
- 26.Gautam J, Miner JH, Yao Y. Loss of endothelial laminin α5 exacerbates hemorrhagic brain injury. Transl Stroke Res 2019; 10: 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J, Lokmic Z, Lämmermann T, et al. Extracellular matrix of secondary lymphoid organs impacts on B-cell fate and survival. Proc Natl Acad Sci U S A 2013; 110: E2915–E2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kangwantas K, Pinteaux E, Penny J. The extracellular matrix protein laminin-10 promotes blood-brain barrier repair after hypoxia and inflammation in vitro. J Neuroinflammation 2016; 13: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motallebnejad P, Azarin SM. Chemically defined human vascular laminins for biologically relevant culture of hiPSC-derived brain microvascular endothelial cells. Fluids Barriers CNS 2020; 17: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li YN, Radner S, French MM, et al. The γ3 chain of laminin is widely but differentially expressed in murine basement membranes: expression and functional studies. Matrix Biol 2012; 31: 120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujiwara H, Kikkawa Y, Sanzen N, et al. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J Biol Chem 2001; 276: 17550–17558. [DOI] [PubMed] [Google Scholar]
- 32.Kikkawa Y, Sanzen N, Fujiwara H, et al. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J Cell Sci 2000; 113: 869–876. [DOI] [PubMed] [Google Scholar]
- 33.Paulus W, Baur I, Schuppan D, et al. Characterization of integrin receptors in normal and neoplastic human brain. Am J Pathol 1993; 143: 154–163. [PMC free article] [PubMed] [Google Scholar]
- 34.Kreidberg JA, Donovan MJ, Goldstein SL, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 1996; 122: 3537–3547. [DOI] [PubMed] [Google Scholar]
- 35.Da Silva RG, Tavora B, Robinson SD, et al. Endothelial α3β1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am J Pathol 2010; 177: 1534–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayloo S, Lazo CG, Sun S, et al. Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier. Neuron 2022; 110: 1641–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Flier A, Badu-Nkansah K, Whittaker CA, et al. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development 2010; 137: 2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanlandewijck M, He L, Mäe MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018; 554: 475–480. [DOI] [PubMed] [Google Scholar]
- 39.Roberts J, De Hoog L, Bix GJ. Mice deficient in endothelial α5 integrin are profoundly resistant to experimental ischemic stroke. J Cereb Blood Flow Metab 2017; 37: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Dev 1993; 119: 1093–1105. [DOI] [PubMed] [Google Scholar]
- 41.Kant R, Halder SK, Bix GJ, et al. Absence of endothelial α5β1 integrin triggers early onset of experimental autoimmune encephalomyelitis due to reduced vascular remodeling and compromised vascular integrity. Acta Neuropathol Commun 2019; 7: 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouvard C, De Arcangelis A, Dizier B, et al. Tie2-dependent knockout of alpha6 integrin subunit in mice reduces post-ischaemic angiogenesis. Cardiovasc Res 2012; 95: 39–47. [DOI] [PubMed] [Google Scholar]
- 43.Friedlander M, Theesfeld CL, Sugita M, et al. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A 1996; 93: 9764–9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada Y, Copeland BR, Hamann GF, et al. Integrin alphavbeta3 is expressed in selected microvessels after focal cerebral ischemia. Am J Pathol 1996; 149: 37. [PMC free article] [PubMed] [Google Scholar]
- 45.Abumiya T, Lucero J, Heo JH, et al. Activated microvessels express vascular endothelial growth factor and integrin αvβ3 during focal cerebral ischemia. J Cereb Blood Flow Metab 1999; 19: 1038–1050. [DOI] [PubMed] [Google Scholar]
- 46.McCarty JH, Lacy-Hulbert A, Charest A, et al. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 2005; 132: 165–176. [DOI] [PubMed] [Google Scholar]
- 47.Shimamura N, Matchett G, Solaroglu I, et al. Inhibition of integrin αvβ3 reduces blood–brain barrier breakdown in focal ischemia in rats. J Neurosci Res 2006; 84: 1837–1847. [DOI] [PubMed] [Google Scholar]
- 48.Shimamura N, Matchett G, Yatsushige H, et al. Inhibition of integrin αvβ3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke 2006; 37: 1902–1909. [DOI] [PubMed] [Google Scholar]
- 49.Carlson TR, Hu H, Braren R, et al. Cell-autonomous requirement for beta1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice. Development 2008; 135: 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei L, Liu D, Huang Y, et al. Endothelial expression of beta1 integrin is required for embryonic vascular patterning and postnatal vascular remodeling. Mol Cell Biol 2008; 28: 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milner R, Campbell IL. Developmental regulation of beta1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci 2002; 20: 616–626. [DOI] [PubMed] [Google Scholar]
- 52.Fassler R, Pfaff M, Murphy J, et al. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol 1995; 128: 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephens LE, Sutherland AE, Klimanskaya IV, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev 1995; 9: 1883–1895. [DOI] [PubMed] [Google Scholar]
- 54.Izawa Y, Gu YH, Osada T, et al. beta1-integrin-matrix interactions modulate cerebral microvessel endothelial cell tight junction expression and permeability. J Cereb Blood Flow Metab 2018; 38: 641–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osada T, Gu Y-H, Kanazawa M, et al. Interendothelial claudin-5 expression depends on cerebral endothelial cell–matrix adhesion by β1-integrins. J Cereb Blood Flow Metab 2011; 31: 1972–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welser JV, Halder SK, Kant R, et al. Endothelial alpha6beta4 integrin protects during experimental autoimmune encephalomyelitis-induced neuroinflammation by maintaining vascular integrity and tight junction protein expression. J Neuroinflammation 2017; 14: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welser-Alves JV, Boroujerdi A, Tigges U, et al. Endothelial beta4 integrin is predominantly expressed in arterioles, where it promotes vascular remodeling in the hypoxic brain. Arterioscler Thromb Vasc Biol 2013; 33: 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proctor JM, Zang K, Wang D, et al. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci 2005; 25: 9940–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimizu H, Hosokawa H, Ninomiya H, et al. Adhesion of cultured bovine aortic endothelial cells to laminin-1 mediated by dystroglycan. J Biol Chem 1999; 274: 11995–12000. [DOI] [PubMed] [Google Scholar]
- 60.Uchino M, Hara A, Mizuno Y, et al. Distribution of dystrophin and dystrophin-associated protein 43DAG (beta-dystroglycan) in the central nervous system of normal controls and patients with duchenne muscular dystrophy. Intern Med 1996; 35: 189–194. [DOI] [PubMed] [Google Scholar]
- 61.Belkin AM, Smalheiser NR. Localization of cranin (dystroglycan) at sites of cell-matrix and cell-cell contact: recruitment to focal adhesions is dependent upon extracellular ligands. Cell Adhes Commun 1996; 4: 281–296. [DOI] [PubMed] [Google Scholar]
- 62.Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood–brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 63.Stratman AN, Malotte KM, Mahan RD, et al. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009; 114: 5091–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang AC, Vest RT, Kern F, et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 2022; 603: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nirwane A, Johnson J, Nguyen B, et al. Mural cell-derived laminin-α5 plays a detrimental role in ischemic stroke. Acta Neuropathol Commun 2019; 7: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gautam J, Zhang X, Yao Y. The role of pericytic laminin in blood brain barrier integrity maintenance. Sci Rep 2016; 6: 36450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gautam J, Cao Y, Yao Y. Pericytic laminin maintains blood-brain barrier integrity in an age-dependent manner. Transl Stroke Res 2020; 11: 228–242. [DOI] [PubMed] [Google Scholar]
- 68.Tigges U, Boroujerdi A, Welser-Alves JV, et al. TNF-α promotes cerebral pericyte remodeling in vitro, via a switch from α1 to α2 integrins. J Neuroinflammation 2013; 10: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao Y, Chen ZL, Norris EH, et al. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun 2014; 5: 3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reynolds LE, D'Amico G, Lechertier T, et al. Dual role of pericyte alpha6beta1-integrin in tumour blood vessels. J Cell Sci 2017; 130: 1583–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balabanov R, Washington R, Wagnerova J, et al. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res 1996; 52: 127–142. [DOI] [PubMed] [Google Scholar]
- 72.Abraham S, Kogata N, Fassler R, et al. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res 2008; 102: 562–570. [DOI] [PubMed] [Google Scholar]
- 73.Turlo KA, Noel OD, Vora R, et al. An essential requirement for beta1 integrin in the assembly of extracellular matrix proteins within the vascular wall. Dev Biol 2012; 365: 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hannocks M-J, Pizzo ME, Huppert J, et al. Molecular characterization of perivascular drainage pathways in the murine brain. J Cereb Blood Flow Metab 2018; 38: 669–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menezes MJ, McClenahan FK, Leiton CV, et al. The extracellular matrix protein laminin α2 regulates the maturation and function of the blood–brain barrier. J Neurosci 2014; 34: 15260–15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen ZL, Yao Y, Norris EH, et al. Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J Cell Biol 2013; 202: 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aoki H, Yamashita M, Hashita T, et al. Laminin 221 fragment is suitable for the differentiation of human induced pluripotent stem cells into brain microvascular endothelial-like cells with robust barrier integrity. Fluids Barriers CNS 2020; 17: 25. 20200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gnanaguru G, Bachay G, Biswas S, et al. Laminins containing the beta2 and gamma3 chains regulate astrocyte migration and angiogenesis in the retina. Development 2013; 140: 2050–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Libby RT, Lavallee CR, Balkema GW, et al. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J Neurosci 1999; 19: 9399–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tawil N, Wilson P, Carbonetto S. Expression and distribution of functional intergrins in rat CNS glia. J Neurosci Res 1994; 39: 436–447. [DOI] [PubMed] [Google Scholar]
- 81.Tawil N, Wilson P, Carbonetto S. Integrins in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs. focal contacts. J Cell Biol 1993; 120: 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gardner H, Kreidberg J, Koteliansky V, et al. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol 1996; 175: 301–313. [DOI] [PubMed] [Google Scholar]
- 83.Chen J, Diacovo TG, Grenache DG, et al. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol 2002; 161: 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.del Zoppo GJ, Milner R. Integrin–matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol 2006; 26: 1966–1975. [DOI] [PubMed] [Google Scholar]
- 85.Wagner S, Tagaya M, Koziol JA, et al. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin α6β4 during focal cerebral ischemia/reperfusion. Stroke 1997; 28: 858–865. [DOI] [PubMed] [Google Scholar]
- 86.Georges-Labouesse E, Mark M, Messaddeq N, et al. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol 1998; 8: 983–986. [DOI] [PubMed] [Google Scholar]
- 87.Georges-Labouesse E, Messaddeq N, Yehia G, et al. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 1996; 13: 370–373. [DOI] [PubMed] [Google Scholar]
- 88.Milner R, Relvas JB, Fawcett J, et al. Developmental regulation of alphav integrins produces functional changes in astrocyte behavior. Mol Cell Neurosci 2001; 18: 108–118. [DOI] [PubMed] [Google Scholar]
- 89.Milner R, Huang X, Wu J, et al. Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J Cell Sci 1999; 112: 4271–4279. [DOI] [PubMed] [Google Scholar]
- 90.Robel S, Mori T, Zoubaa S, et al. Conditional deletion of beta1-integrin in astroglia causes partial reactive gliosis. Glia 2009; 57: 1630–1647. [DOI] [PubMed] [Google Scholar]
- 91.Venkatesan C, Birch D, Peng C-y, et al. Astrocytic β1-integrin affects cellular composition of murine blood brain barrier in the cerebral cortex. Int J Dev Neurosci 2015; 44: 48–54. [DOI] [PubMed] [Google Scholar]
- 92.van der Neut R, Krimpenfort P, Calafat J, et al. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet 1996; 13: 366–369. [DOI] [PubMed] [Google Scholar]
- 93.Nishimura SL, Boylen KP, Einheber S, et al. Synaptic and glial localization of the integrin alphavbeta8 in mouse and rat brain. Brain Res 1998; 791: 271–282. [DOI] [PubMed] [Google Scholar]
- 94.Tian M, Jacobson C, Gee SH, et al. Dystroglycan in the cerebellum is a laminin alpha 2-chain binding protein at the glial-vascular interface and is expressed in Purkinje cells. Eur J Neurosci 1996; 8: 2739–2747. [DOI] [PubMed] [Google Scholar]
- 95.Milner R, Hung S, Wang X, et al. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab 2008; 28: 812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zaccaria ML, Di Tommaso F, Brancaccio A, et al. Dystroglycan distribution in adult mouse brain: a light and electron microscopy study. Neurosci 2001; 104: 311–324. [DOI] [PubMed] [Google Scholar]
- 97.Noell S, Wolburg-Buchholz K, Mack AF, et al. Evidence for a role of dystroglycan regulating the membrane architecture of astroglial endfeet. Eur J Neurosci 2011; 33: 2179–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moore SA, Saito F, Chen J, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 2002; 418: 422–425. [DOI] [PubMed] [Google Scholar]
- 99.Milner R, Campbell IL. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the alpha6beta1 integrin. J Neurosci 2002; 22: 1562–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hawk CS, Coelho C, de Oliveira DSL, et al. Integrin β1 promotes the interaction of murine IgG3 with effector cells. J Immunol 2019; 202: 2782–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol 1990; 30: 81–93. [DOI] [PubMed] [Google Scholar]
- 102.Su EJ, Cao C, Fredriksson L, et al. Microglial-mediated PDGF-CC activation increases cerebrovascular permeability during ischemic stroke. Acta Neuropathol 2017; 134: 585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milner R. Microglial expression of αvβ3 and αvβ5 integrins is regulated by cytokines and the extracellular matrix: β5 integrin null microglia show no defects in adhesion or MMP‐9 expression on vitronectin. Glia 2009; 57: 714–723. [DOI] [PubMed] [Google Scholar]
- 104.Huang X, Griffiths M, Wu J, et al. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol 2000; 20: 755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nandrot EF, Kim Y, Brodie SE, et al. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med 2004; 200: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Milner R, Campbell IL. The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res 2002; 69: 286–291. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014; 34: 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He L, Vanlandewijck M, Mäe MA, et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci Data 2018; 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim WK, Kim D, Cui J, et al. Secretome analysis of human oligodendrocytes derived from neural stem cells. PloS One 2014; 9: e84292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benninger Y, Colognato H, Thurnherr T, et al. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J Neurosci 2006; 26: 7665–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Colognato H, Galvin J, Wang Z, et al. Identification of dystroglycan as a second laminin receptor in oligodendrocytes, with a role in myelination. Development 2007; 134: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 112.Milner R, Edwards G, Streuli C, et al. A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors. J Neurosci 1996; 16: 7240–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Milner R, Frost E, Nishimura S, et al. Expression of αvβ3 and αvβ8 integrins during oligodendrocyte precursor differentiation in the presence and absence of axons. Glia 1997; 21: 350–360. [PubMed] [Google Scholar]
- 114.Hagg T, Portera-Cailliau C, Jucker M, et al. Laminins of the adult mammalian CNS; laminin-α2 (merosin M-) chain immunoreactivity is associated with neuronal processes. Brain Res 1997; 764: 17–27. [DOI] [PubMed] [Google Scholar]
- 115.Yin Y, Kikkawa Y, Mudd JL, et al. Expression of laminin chains by central neurons: analysis with gene and protein trapping techniques. Genesis 2003; 36: 114–127. [DOI] [PubMed] [Google Scholar]
- 116.Grimpe B, Dong S, Doller C, et al. The critical role of basement membrane-independent laminin γ1 chain during axon regeneration in the CNS. J Neurosci 2002; 22: 3144–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Omar MH, Campbell MK, Xiao X, et al. CNS neurons deposit laminin alpha5 to stabilize synapses. Cell Rep 2017; 21: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tawil NJ, Houde M, Blacher R, et al. Alpha. 1. beta. 1 integrin heterodimer functions as a dual laminin/collagen receptor in neural cells. Biochemistry 1990; 29: 6540–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tomaselli K, Hall D, Flier L, et al. A neuronal cell line (PC12) expresses two β 1-class integrins-α1β1, and -α3β1 that recognize different neurite outgrowth-promoting domains in laminin. Neuron 1990; 5: 651–662. [DOI] [PubMed] [Google Scholar]
- 120.Bi X, Lynch G, Zhou J, et al. Polarized distribution of α5 integrin in dendrites of hippocampal and cortical neurons. J Comp Neurol 2001; 435: 184–193. [DOI] [PubMed] [Google Scholar]
- 121.De Curtis I, Reichardt LF. Function and spatial distribution in developing chick retina of the laminin receptor alpha 6 beta 1 and its isoforms. Development 1993; 118: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Langen UH, Ayloo S, Gu C. Development and cell biology of the blood-brain barrier. Annu Rev Cell Dev Biol 2019; 35: 591–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaplan L, Chow BW, Gu C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci 2020; 21: 416–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sixt M, Engelhardt B, Pausch F, et al. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood–brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol 2001; 153: 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Edwards DN, Bix GJ. Roles of blood-brain barrier integrins and extracellular matrix in stroke. Am J Physiol Cell Physiol 2019; 316: C252–C263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abumiya T, Lucero J, Heo JH, et al. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab 1999; 19: 1038–1050. [DOI] [PubMed] [Google Scholar]
- 127.Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des 2008; 14: 1581–1593. [DOI] [PubMed] [Google Scholar]
- 128.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. [DOI] [PubMed] [Google Scholar]
- 129.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 2010; 468: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 2010; 119: 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia 2013; 61: 1939–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuang W, Xu H, Vachon PH, et al. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J Clin Invest 1998; 102: 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miyagoe Y, Hanaoka K, Nonaka I, et al. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett 1997; 415: 33–39. [DOI] [PubMed] [Google Scholar]
- 134.Cambier S, Gline S, Mu D, et al. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: an angiogenic control switch. Am J Pathol 2005; 166: 1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Srinivasan R, Lu TY, Chai H, et al. New transgenic mouse lines for selectively targeting astrocytes and studying calcium signals in astrocyte processes in situ and in vivo. Neuron 2016; 92: 1181–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu L, Yao Y. Central nervous system fibroblast-like cells in stroke and other neurological disorders. Stroke 2021; 52: 2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.DeSisto J, O'Rourke R, Jones HE, et al. Single-cell transcriptomic analyses of the developing meninges reveal meningeal fibroblast diversity and function. Dev Cell 2020; 54: 43–59. e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kettenmann H, Hanisch U-K, Noda M, et al. Physiology of microglia. Physiol Rev 2011; 91: 461–553. [DOI] [PubMed] [Google Scholar]
- 139.Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun 2019; 10: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mehrabadi AR, Korolainen MA, Odero G, et al. Poly (ADP-ribose) polymerase-1 regulates microglia mediated decrease of endothelial tight junction integrity. Neurochem Int 2017; 108: 266–271. [DOI] [PubMed] [Google Scholar]
- 141.Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol 2010; 119: 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang L, Geng J, Qu M, et al. Oligodendrocyte precursor cells transplantation protects blood–brain barrier in a mouse model of brain ischemia via Wnt/β-catenin signaling. Cell Death Dis 2020; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kang M, Yao Y. Laminin regulates oligodendrocyte development and myelination. Glia 2022; 70: 414–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Milner R, Ffrench-Constant C. A developmental analysis of oligodendroglial integrins in primary cells: changes in alpha v-associated beta subunits during differentiation. Development 1994; 120: 3497–3506. [DOI] [PubMed] [Google Scholar]
- 145.Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood–brain barrier. Neurobiol Dis 2010; 37: 13–25. [DOI] [PubMed] [Google Scholar]
- 146.He Y, Yao Y, Tsirka SE, et al. Cell-culture models of the blood-brain barrier. Stroke 2014; 45: 2514–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Väänänen AJ, Rauhala P, Tuominen RK, et al. KDI tripeptide of γ1 laminin protects rat dopaminergic neurons from 6‐OHDA induced toxicity. J Neurosci Res 2006; 84: 655–665. [DOI] [PubMed] [Google Scholar]
- 148.Powell SK, Kleinman HK. Neuronal laminins and their cellular receptors. Int J Biochem Cell Biol 1997; 29: 401–414. [DOI] [PubMed] [Google Scholar]
- 149.Indyk J, Chen Z, Tsirka S, et al. Laminin chain expression suggests that laminin-10 is a major isoform in the mouse hippocampus and is degraded by the tissue plasminogen activator/plasmin protease Cascade during excitotoxic injury. Neurosci 2003; 116: 359–371. [DOI] [PubMed] [Google Scholar]