Abstract
Among the treatments for malignant tumors, radiotherapy is of great significance both as a main treatment and as an adjuvant treatment. Radiation therapy damages cancer cells with ionizing radiation, leading to their death. However, radiation-induced toxicity limits the dose delivered to the tumor, thereby constraining the control effect of radiotherapy on tumor growth. In addition, the delayed toxicity caused by radiotherapy significantly harms the physical and mental health of patients. FLASH-RT, an emerging class of radiotherapy, causes a phenomenon known as the ‘FLASH effect’, which delivers radiotherapy at an ultra-high dose rate with lower toxicity to normal tissue than conventional radiotherapy to achieve local tumor control. Although its mechanism remains to be fully elucidated, this modality constitutes a potential new approach to treating malignant tumors. In the present review, the current research progress of FLASH-RT and its various particular effects are described, including the status of research on FLASH-RT and its influencing factors. The hypothetic mechanism of action of FLASH-RT is also summarized, providing insight into future tumor treatments.
Keywords: flash, radiation, cancer, diagnosis, dose
1. Introduction
Malignant tumors threaten human life worldwide. Treating malignant tumors includes surgery, chemotherapy, targeted therapy, immunotherapy and, importantly, radiation therapy. In recent years, the effect of radiation therapy has been demonstrated repeatedly. Radiotherapy is mainly applied to local solid tumors, such as head and neck tumors, skin cancer, lymphoma, lung cancer, esophageal cancer, etc. It may also be used as an adjuvant treatment, combined with chemotherapy and surgery, for the treatment of breast cancer, cervical cancer, gastrointestinal tumors, etc. For cancers of the blood, radiotherapy has little effect. However, its further clinical application has been substantially restricted as it damages normal tissues. Radiation therapy uses an external radiation beam, which sends radiation into the body tissue and causes damage (1). When deep tumors are irradiated, healthy pre-tumor normal tissue receives a higher dose of ionizing radiation than tumor tissue, resulting in extra damage. In addition, for certain superficial tumors, if the radiation passes directly through the surface of the tumor, normal tissue behind the tumor may also be exposed to radiation, although the tumor is directly irradiated. In either case, the normal tissues, particularly those tissues or organs sensitive to radiation, are severely damaged. Thus, the dose-limiting toxicity of normal tissues is one of the significant obstacles to the development of tumor radiotherapy (2).
Current directions of clinical radiotherapy technology developments are to improve the ability of radiotherapy machines and change the local mode of radiotherapy to maximize the accuracy of irradiation of tumor tissue, avoiding damage to normal tissue. It is exciting that FLASH radiotherapy (FLASH-RT), as an ultra-high dose rate (UHDR) radiotherapy, has been proposed as a new method in recent years. The FLASH effect was first reported in 1959. However, due to technical limitations, it was relatively challenging to translate the results into clinical practice and further research on this new radiotherapy was severely limited (3,4). However, due to the improvement of radiotherapy equipment technology, in-depth progression of radiation source research and advances in radiobiology research, the great potential of FLASH-RT has been recognized again as a tool for clinical radiotherapy. FLASH-RT is a new non-invasive external irradiation radiotherapy technology that is able to extend patients' treatment window and significantly change the radiotherapy and tumor treatment pattern by delivering ultra-high dose rays at an UHDR (5). Compared with conventional radiotherapy (Con-RT), FLASH-RT delivers doses higher than 8 Gy in a short time (<1 sec) and the dose rate may exceed 50 Gy/sec (1). In several studies, radiation toxicity to normal surrounding healthy tissues was significantly reduced and tumor growth was inhibited, with a degree of tumor control similar to that of traditional dose rate irradiation. While certain researchers remain skeptical about the effectiveness of FLASH-RT in cancer patients, it is widely thought that FLASH irradiation holds substantial promise for the future and is perhaps the most significant recent discovery in the history of radiation therapy (5). In the present review, the research progress of FLASH-RT is summarized and its various special influences, including the research status of FLASH-RT and its influencing factors, are outlined. At the same time, the mechanism of FLASH-RT is also summarized, which provides insight for future cancer treatment.
2. Effect of FLASH
Normal tissue responses to FLASH-RT
UHDR irradiation damages normal tissues to a lesser extent than Con-RT. In 1959, the FLASH effect was observed for the first time. Dewey and Boag (6) found that when bacteria were subjected to large-pulse electron radiation, radiosensitivity was reduced as compared to ordinary dose rate irradiation. In 1967, Town (7) obtained the corresponding rays in the form of pulse waves and improved the radiation dose rate by shortening the time. These results were consistent with Dewey and Boag (6). They also indicated that damage to normal tissue decreased with increasing and constant total doses. This reduction was confirmed in the 1970s by Field and Bewley (8), as well as Hornsey and Alper (4), using mouse intestines. In 1969, Berry et al (9) demonstrated that normal mammalian cells exposed to UHDR irradiation had more robust viability than those exposed to conventional dose rate irradiation. In 1971, Hornsey and Bewley (10) reported that a high dose rate electron beam (60 Gy/min and above) caused tissue hypoxia, reducing the radiosensitivity of tissues. The research on the FLASH effect in the 1960s and 1970s was not successfully translated into clinical applications and research stagnated again.
In 2014, Favaudon et al (11) evaluated the effects of FLASH UHDR or conventional dose rate irradiation on lung tissue by local irradiation of mice. The results indicated that all mice irradiated at the conventional dose rate of 17 Gy developed severe pneumonia and fibrosis. This was the opposite of the result in FLASH-irradiated mice; no pneumonia or fibrosis was observed in these mice after the same dose of FLASH. When the dose was increased to 30 Gy, FLASH began to induce pneumonia and fibrosis. The authors found that 17-Gy FLASH irradiation prevented the activation of TGF-β and acute apoptosis of bronchi and blood vessels.
In 2017, Montay-Gruel et al (12) performed several studies on the effects of FLASH-RT on brain tissues and found that spatial memory was significantly impaired after irradiation with a total dose of 10 Gy at a conventional dose rate of 0.1 Gy/sec. However, the spatial memory was significantly protected when the average dose rate of radiation was >100 Gy/sec. Even 2 months after the mice received radiotherapy, the animals' ability to recognize new objects was significantly better in the FLASH-RT group than in the conventional dose rate group. In addition, the discrimination of newborn neurons in the mouse hippocampus indicated that the protective effect of FLASH-RT on nerve regeneration depended on the protective effect of neural stem cells. Further studies were performed in 2018 and 2019; these concluded that FLASH-RT had a more substantial protective effect on normal brain tissue than conventional dose rate radiotherapy (13,14).
Alaghband et al (15) indicated that, compared with conventional dose rate (0.077 Gy/sec, 6 MeV electron) irradiation, irradiation of the whole brain of mice with an UHDR (4.4×106 Gy/sec; 6 MeV beam) had an evident radiation protection FLASH effect. As the animals passed further cognitive tests, the authors observed no significant difference in the brains of the FLASH-irradiated mice compared to the control group, while the brains of the mice exposed to conventional dose rates were significantly damaged. Mice irradiated at conventional dose rates has considerably lower levels of immature and mature neurons after 4 months. Regarding pituitary function, the authors found a two-fold decrease in plasma growth hormone in mice exposed to conventional dose rates but not in mice exposed to FLASH. These findings illustrate the benefits of FLASH irradiation over conventional dose rate irradiation. In large mammals, a phase I single-dose escalation trial (25–41 Gy) studied six cats with locally advanced T2/T3N0M0 nasal plane squamous cell carcinoma with hair loss and fibrinoid necrosis as acute and late endpoints and observed a ‘protective effect’ (damage to normal tissue is less than that of Con-RT) of FLASH-RT (5). Further histological analysis revealed no acute toxicity in three cats, moderate/mild transient mucositis in three cats and depilation in all cats. The 16-month progression-free survival in the experimental group was 84%. This finding confirmed the potential benefits of FLASH-RT and provided a basis for further evaluation of FLASH-RT effects in humans. Numerous studies have examined the effects of FLASH in normal tissues, which are summarized in Table I.
Table I.
Studies examining the effects of different modes of FLASH irradiation on normal tissue.
| Author, year | System | Dose, Gy | Dose rate, Gy/sec | Assay | (Refs.) |
|---|---|---|---|---|---|
| Hornsey and Bewley, 1971 | Mouse intestine | 11.9 | 17-83 | LD50/5 | (10) |
| Field and Bewley, 1974 | Mouse foot skin | 24 | 56-83 | Early and late reactions | (8) |
| Hendry et al, 1982 | Mouse tail skin | 50 | 17-170 | Necrosis ND50 | (95) |
| Favaudon et al, 2014 | Mouse lung | 15-17 | 40-60 | Lung fibrosis | (11) |
| Montay-Gruel et al, 2017 | Mouse brain | 10 | 100-106 | Memory tests | (12) |
| Vozenin et al, 2019 | Mouse intestine | 14.7 | 70-210 | LD50/5 (survival) | (56) |
| Montay-Gruel et al, 2018 | Mouse brain | 10 | 37 | Neurocognitive tests | (13) |
| Simmons et al, 2019 | Mouse brain | 30 | 200/300 | Neurocognitive tests | (71) |
| Montay-Gruel et al, 2019 | Mouse brain | 10 | >100 | Neurocognitive tests | (14) |
| Abel et al, 2019 | Mouse lung | 15/17.5/20 | 40 | Survival, dermatitis, breathing function | (96) |
| Girdhani et al, 2019 | Mouse lung | 15/17.5/20 | 40 | Lung fibrosis, skin dermatitis | (35) |
| Vozenin et al, 2019 | Mini-pig skin | 22-34 | 300 | Skin toxicity/injury | (5) |
| Montay-Gruel et al, 2019 | Zebrafish embryo | 8 | >100 | Morphology | (14) |
| Alaghband et al, 2020 | Mouse brain | 8 | 4.4×106 | Neurocognitive tests | (15) |
| Fouillade et al, 2020 | Mouse lung | 17 | 40-60 | Cellular proliferation, inflammation | (76) |
| Levy et al, 2020 | Mouse abdomen | 12-16 | 216 | Crypt cells, stool production, survival, regeneration | (57) |
| Diffenderfer et al, 2020 | Mouse abdomen | 15 | 78 | Intestinal crypt cell proliferation | (38) |
| Diffenderfer et al, 2020 | Mouse intestine | 18 | 78 | Fibrosis | (38) |
| Cao et al, 2021 | Mouse mammary gland | 20 | 300 | Oxygen depletion test | (60) |
| Liew et al, 2021 | Mouse skin | 30 | 125 | Survival | (32) |
| Cunningham et al, 2021 | Mouse skin | 15,35 | 57,115 | Plasma and skin levels of TGF-β1 and skin | (97) |
| Velalopoulou et al, 2021 | Mouse skin, muscle, bone | 30,45 | 69-124 | Survival, histology, pathology | (98) |
| Montay-Gruel et al, 2021 | Mouse brain | 10-30 | 1.8×106 | Survival, neurocognitive tests | (99) |
Tumor control effect
Numerous studies indicated that FLASH reduces irradiation damage in normal tissues; however, its therapeutic effect on tumor tissues remained to be determined. Favaudon et al (11) observed no difference in anti-tumor efficiency when an orthotopic mouse lung cancer model was exposed to FLASH-RT or Con-RT. They also indicated that only 20% of the mice irradiated at the conventional dose rate of 15 Gy were tumor-free at weeks 8–9 as opposed to 70% of the mice treated with 28 Gy FLASH-RT. These findings suggest that FLASH irradiation may enhance tumor inhibition. Numerous studies suggested that flash-RT and Con-RT have almost the same therapeutic effect on tumors when used at equal doses (5,11,16). For instance, the first pre-clinical study of nasal plane spontaneous squamous cell carcinoma in felines indicated no significant difference in the efficacy of FLASH compared with Con-RT (5).
The results of FLASH irradiation have also been validated in humans; a recent study by Bourhis et al (17) reported the first patient receiving FLASH-RT. A 75-year-old patient presented with a multiresistant CD30+ T-cell cutaneous lymphoma disseminated throughout the whole skin surface. Prior to this treatment, Localized skin RT has been previously used over 110 times for various ulcerative and/or painful cutaneous lesions progressing despite systemic treatments. However, due to the emergence of a new skin tumor (3.5 cm in diameter), FLASH-RT was administered for a total dose of 15 Gy. The purpose of FLASH irradiation is to minimize the toxicity of surrounding normal tissues. After 3 weeks of treatment, the toxicity of normal tissues decreased significantly and the tumor was well controlled. This was the world's first clinical report of FLASH applied to humans, providing a stimulus for further basic research and clinical applications of FLASH. Subsequently, further studies on the clinical application of FLASH radiotherapy were published. Van de Water et al (16) conducted a clinical study on four patients with head-and-neck cancer who received four treatment plans (the clinical treatment plan, a ‘standard’ spot-reduced plan, an ‘arc’ spot-reduced plan and an ‘arc-shoot-through’ spot-reduced plan). They indicated that FLASH dose rates were not achieved for conventional planning and clinical spot-scanning machines. As such, increased spot-wise beam intensities, spot-reduced planning, hypofractionation and arc-shoot-through plans were required to achieve FLASH-compatible dose rates. In addition, Wei et al (18) performed a dosimetric clinical study on two patients with lung cancer and observed that the single-energy Bragg peak plans achieve superior dosimetry performances in organ-at-risk sparing (OARs) to transmission plans with comparable dose rate performances for lung cancer FLASH therapy. Beam angle optimization may further improve the OAR dosimetry parameters with similar 3D FLASH dose rate coverage.
3. Factors affecting FLASH irradiation
Dose effect
Earlier FLASH studies used monopulse and nanosecond X-ray irradiation, and the instantaneous dose rate was up to 7×108 Gy/sec (9). The dose rate mentioned in FLASH-RT studies is the average dose rate of the whole irradiation process. Smyth et al (19) found that, compared with a cathode ray tube, synchrotron broad-beam radiation therapy with a high dose rate (37–41 Gy/sec) and an equivalent dose did not provide normal tissue protection. These results suggest that the protective effect of FLASH-RT on normal tissues may not be universal and the dose rate required to induce the FLASH effect may not be universal. Montay-Gruel et al (12) studied whole brains irradiated with 10 Gy of 4.5 and 6 MeV electrons at dose rates from 0.1 to 500 Gy/sec. When the dose rate was ≥30 Gy/sec, the neuroprotective FLASH effect was significant; when the dose rate was ≥100 Gy/sec, the maximum FLASH effect was induced; however, when the dose rate was <30 Gy/sec, the neuroprotective effect disappeared. Another study indicated that FLASH-X-ray whole-brain irradiation of 37 Gy/sec has a more significant memory protection effect than Con-RT (13). Contrary to these results, Venkatesulu et al (20) suggested that FLASH-RT at 3 5Gy/sec gave higher toxicity than Con-RT at 0.1 Gy/sec.
The total radiation dose used in pre-clinical FLASH-RT studies is not uniform. Bourhis et al (21) suggested using low-segmented FLASH delivery because low-segmented FLASH has the same efficacy as Con-RT in controlling orthotopic glioma in mice. By contrast, Vozenin et al (5) used FLASH-RT to treat cats with locally advanced nasal squamous cell carcinoma and found that a single dose of up to 41 Gy failed to reach the maximum tolerated dose; no dose-limiting toxicity was observed under a single dose of 25–41 Gy. Meanwhile, normal tissue had good tolerance.
Although there has been much research on FLASH-RT, the optimal dose rate for FLASH-RT remains undefined. Zhou et al (22) found that FLASH-RT-induced transient hypoxia protected normal tissue better than Con-RT; they analyzed the order of magnitude of the minimum dose rate required by ultra-short radiation pulse FLASH-RT through dimensionality. The results indicated that the lower limit of the dose rate of FLASH-RT may be very close to the minimum dose rate in pre-clinical trials (>40 Gy/sec). In addition, if FLASH-RT is used in the clinical treatment of deep tumors while delivering an UHDR for deep tissues, normal tissues along the radiation beam trajectory may receive a low dose rate (lower than the minimum dose rate to induce the FLASH effect) and the damage of normal tissues along the radiation path also requires to be considered.
The linear quadratic formula (L-Q model) (or α/β equation) was proposed by Kellerer and has been used in radiobiology research and clinical radiotherapy, where it had a profound influence on theoretical research and clinical application of radiobiology (23). According to the linear quadratic model, cells are killed if two strands of a DNA or two arms of a chromosome are damaged simultaneously (24). There are two mechanisms of damage in this event: i) Cell death caused by radiation hitting two strands of DNA at once and ii) the single-target multiple-hit effect, with cell death caused by radiation hitting two strands of DNA separately (Fig. 1). Rays with energy particles that accomplish this effect alone are called high-let rays, while particles that require multiple particles to cause DNA damage are called low-let rays. However, certain authors were skeptical about the L-Q model. Steel et al (25) indicated that the L-Q model was not able to predict the cell survival rate when the cell population received a fractional irradiation dose, suggesting that factors other than the local dose affected the survival rate after irradiation. Hadrons are being used for clinical cancer treatments, predominantly with proton and carbon-ion beams but with increasing interest in the application of other ions. As particles slow down in the body, they become more densely ionized, eventually losing all energy and stopping moving. One result of this is that as the particles decelerate and the LET of these beams increases, resulting in an increase in relative bioavailability (RBE), depending on the energy and particle type (26,27). In radiobiology, the increase in RBE is driven by the production of more complex or clustered DNA damage that cells find increasingly difficult to repair. With the increase of LET (28,29), the dose savings of the graded treatment decreased and the low dose rate savings also decreased.
Figure 1.
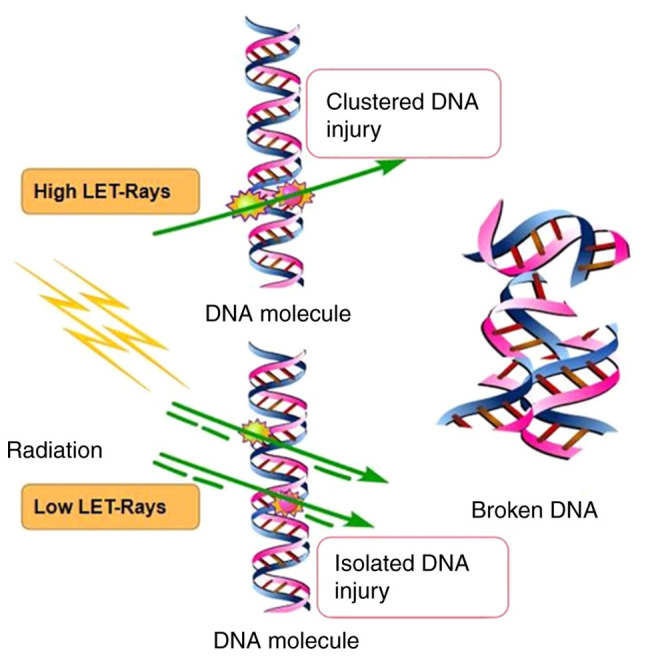
Schematic illustrating the single-hit model and double-click model. LET, linear energy transfer.
Therefore, it is necessary to consider the dose. Venkatesulu et al (20) observed that FLASH-RT (35 Gy/sec) was more likely to induce acute gastrointestinal syndrome than Con-RT (0.1 Gy/sec) for the same single 16 Gy abdominal irradiation. Tissues with low α/β values (such as the spinal cord, lung, kidney, liver, bone and the vascular system) are sensitive to the fractional dose and the late response of tissues is aggravated when the fractional dose is increased in Con-RT. However, a single high dose of FLASH-RT significantly protected normal brain and lung tissues (10,21). Tissues with high α/β values (such as small intestine and skin) were more sensitive to the total treatment time and premature tissue response was aggravated when the total treatment time was shortened. This finding suggests that the efficacy of FLASH-RT is associated with tissue specificity and has considerable complexity. Rothwell et al (30) analyzed the effects of different doses and dose rates on FLASH. The authors indicated that within a specific range (<1,000 Gy/sec), a higher dose rate was associated with a greater ∆OER, which was consistent with previous studies (22,31). The authors also found that, even at these high dose rates, no significant change in ∆OER was observed without a sufficiently high dose. This finding reaffirms the importance of the dose and dose rate for the FLASH effect.
Based on these results, further studies analyzed the influencing factors of FLASH irradiation and established models to predict the effects of FLASH irradiation under various influencing factors. To facilitate the radiobiological investigation of FLASH phenomena and the assessment of clinical applicability, Liew et al (32) presented an extension of the mechanistic radiobiological model ‘UNified and VERSatile Bio Response Engine’ (UNIVERSE), which reproduces the dose-, dose rate and oxygen tension-dependent influence on cell killing. For these systems, the findings suggest that the extent of the cell/tissue sparing effect, if present, strongly depends on beam quality used for reference conventional irradiation. For instance, the dose rate effects are associated with survival and survival was observed from doses and dose rates of ~8 Gy and ~40 Gy/sec, respectively. Although this model may predict the response to FLASH radiotherapy, it is difficult to estimate the dose and dose rate accurately. In addition, other models may help us understand and predict the FLASH effect. These include the model developed by Rothwell et al (30) using parameters involved in oxygen consumption, including those related to dose transfer, radiochemical oxygen consumption dynamics and tissue inherent biological characteristics. Although the FLASH study indicates the need for low initial oxygen concentrations and high doses, this study provides a quantitative tool to determine more precise values for different situations. After establishing a clinically relevant parameter space for flash radiotherapy, the model may be used to answer complex questions surrounding the mechanism of action.
Influence of radiation sources
The FLASH effect is thought to exist in electron-wire radiotherapy, which has been preliminarily verified in small animal models (33,34); relevant human experiments are in progress (17). In addition to electron wires, FLASH effects have also been observed for X-irradiation (13). Due to its physical properties, proton beam radiotherapy has a protective effect on normal tissue and the proton FLASH effect may add additional protection to normal tissue. Girdhani et al (35) demonstrated for the first time that proton FLASH-RT reduced normal tissue toxicity in a mouse model. Beyreuther et al (36) irradiated zebrafish embryos with a conventional dose rate proton beam of 5 Gy/min and a proton FLASH beam of 100 Gy/sec and observed no difference in toxicity. However, Buonanno et al (37) indicated that proton FLASH irradiation in vitro did not increase the survival rate of normal human lung fibroblasts. The reason for these findings may be that the pulse rate of radiation affected the FLASH effect. This finding suggests that the role of protons in FLASH mode requires to be further studied. Several studies discussed the design, implementation and in vivo verification of a new proton FLASH-RT system (38), clarifying the importance and clinical significance of FLASH-RT research in proton plasma radiotherapy (39). UHDR proton beam therapy is already under consideration (40).
The correlation between the FLASH proton and the FLASH effect has been confirmed in numerous studies. Several studies examined the effect of proton transport in FLASH mode (36–39,41). The proton relative bioavailability values using the point scanning system are almost identical to those using the passive scattering system. In a recent review of the data from these studies, Colangelo and Azzam (39) provided corresponding evidence for the FLASH effect in their study. These studies were performed at aerobic levels; therefore, the results were limited. Certain experiments under very low oxygen tension established the relationship between the FLASH effect and proton FLASH in vitro (42–44). Buonanno et al (37) conducted experiments using normal non-fibrocytes and compared conventional dose rate and proton FLASH irradiation. The expression of TGF-β in pre-senescent cells decreased with increasing dose rate. These findings provide evidence for the long-term effects of proton-derived FLASH. A colony-forming cell assay indicated that the cell survival index at a constant dose rate had an exponential relationship with the increased dose. The ability of proton-derived FLASH-RT to reduce long-term radiotoxicity in normal tissue compared with conventional dose rate radiotherapy may be due to differences in the types or amounts of DNA damage. These findings provide another aspect of the proton-derived FLASH effect. Proton beam therapy (PBT) may be the ideal solution for treating certain deep tumors. Due to its high energy and dose rate, FLASH irradiation has been applied in clinical practice (16). The PBT beam has also been applied to other devices for testing innovations in equipment and devices (38,45). However, the limitations of PBT hamper its further popularization. Proton scattering is necessary for patients with large tumors, resulting in the loss of scattered particles and reducing the total delivery dose. Furthermore, the total duration increases to provide UHDR at each point; therefore, the total dose rates decrease and may not be sufficient to cause FLASH effects (21,46).
Although the majority of current radiation treatments are performed using X-rays, preclinical studies examining results of exposure to X-rays are rare. Montay-Gruel et al (47) have summarized the different methods that may be used to generate X-rays, their beam properties and their effects. Schüler et al (33) provided a comprehensive review of the numerous results generated from electron FLASH experiments. They suggest the following set to be at a minimum in terms of dose parameter: The dose and dose parameters should be defined to a dose specification point (DSP) at the center of the irradiated volume of interest. If a highly irregular volume is considered, a representative DSP in this volume should be defined and used. The reporting of the dose parameters should be accompanied by the coordinates of the DSP as well as dose profile measurements along the lateral and axial directions, centered on the DSP.
Weber et al (48) discussed the technical challenges in beam delivery and provided a promising solution using 3D range-modulators in order to apply UHDRs compatible with FLASH with carbon ions. Next, they also discussed the possible outcome of C-ion therapy at UHDR on the level of the radiobiological and radiochemical effects. Although a large number of studies have assessed the preclinical FLASH status of different radiation types, there is still no consensus on the normalization and standardization of radiation types, which still requires to be confirmed by further studies.
4. FLASH-RT biological mechanism
Oxygen consumption hypothesis
Traditional radiotherapy is based on the classical ‘4R’ theories of radiobiology (repair, reoxygenation, redistribution of cell cycle and repopulation) (49). Regarding the biological mechanism of FLASH-RT, early studies focused on the possibility of oxygen metabolism in the environment surrounding cells (50). FLASH-RT irradiation results in radiochemical oxygen depletion, which leads to an acute period of hypoxia in the irradiated tissue and transient radiation resistance. Oxygen is a critical factor affecting the FLASH effect and a physical parameter that evaluates the FLASH effect. There is evidence that numerous normal tissues are able to maintain a small number of cell populations for continuous renewal/regeneration at low physiological oxygenation levels (51). High doses rapidly deplete oxygen at high dose rates, allowing it to diffuse to maintain adequate oxygenation levels, and normal tissue responds as hypoxic tissue. As a result, UHDRs deplete oxygen, mimicking hypoxia and increasing tissue resistance to radiation. In the case of hypoxic (radiation-resistant) tumors surrounded by oxidized normal tissue (radiosensitivity), UHDRs increase the radiation resistance of normal tissue with minimal effects on already hypoxic tumor tissue (52). In common ionizing radiation reactions, water in cells breaks down as a result of radiation and produces reactive oxygen species (ROS), which indirectly damage DNA, including the common process of hydroxyl radicals attacking DNA (53). In low-linear-energy transfer radiation, DNA damage caused by ROS may account for up to 70% and the remainder is caused by direct interactions between DNA and radiation (31,54,55). According to the oxygen fixation hypothesis, if oxygen radicals cause this indirect DNA damage, the damage is fixed by the presence of molecular oxygen by forming more damaging peroxy radicals (56).
After FLASH-RT, radiochemical depletion of oxygen occurs in tumor tissue, leading to radiation resistance of the tumor. Petersson et al (42) provided a reliable quantitative model for understanding the biological effects of FLASH-RT that was compatible with experimental observations of FLASH effects. The model suggests that oxygen levels may be depleted at moderate oxygen concentrations (but not at high or very low concentrations); oxygen levels may be depleted sufficiently to affect radiosensitivity. Under physiologically relevant oxygen concentrations (relative partial pressure 1.6–20%), Adrian et al (43) reported that the FLASH effect depended on the oxygen concentration in vitro and the survival rate of hypoxic prostate cancer cells (1.6%) was significantly improved by FLASH irradiation. However, numerous studies suggested that FLASH-RT was able to maintain an anti-tumor response similar to Con-RT (21,57); in certain cases, FLASH-RT may have generated anti-tumor responses better than those to Con-RT (34,58). Several studies on oxygen in FLASH-RT indicated that oxygen and hypoxic environments may have a critical role in the FLASH effect. There are numerous descriptions of the traditional mechanics of the FLASH effect (Table II). For instance, Petersson et al (42) used a model of oxygen dynamics during irradiation to develop a time-dependent model of the oxygen enhancement ratio in mammalian cells, which includes oxygen depletion. Then the characteristics of the model were discussed in terms of dose and dose rate dependence of the oxygen enhancement ratio. Eventually, they determined that only under moderate oxygen tension, oxygen levels were able to be depleted in quantities sufficient to affect radiation sensitivity. Boscolo et al (59) studied the yield as a function of time and LET for all radioactive substances simulated for different ionic radiation and different oxygenation levels at different energies within 1 µsec after radiation passage. Under oxygenated conditions, high production of two highly toxic species, O2•− and HO2•, was predicted, particularly at low LET radiation. All of these studies have further promoted the mechanistic research progress on FLASH-RT.
Table II.
Studies exploring the mechanisms of FLASH radiotherapy.
| Author, year | Dose rate, Gy/sec | Dose, Gy | Type of ray | Core theory | System | (Refs.) |
|---|---|---|---|---|---|---|
| Petersson et al, 2020 | 0-100 | 0-30 | Electrons | Oxygen consumption | In vitro and in vivo | (42) |
| Labarbe et al, 2020 | 10−3−107 | 10 | Electrons or photons | ROS | In vitro | (78) |
| Boscolo et al, 2020 | - | - | Ion and proton | Oxygen consumption | In vitro | (59) |
| Liew et al, 2021 | 0.01-104 | 2-32 | Electrons and x-rays | ‘UNIVERSE’ | In vitro and in vivo | (32) |
| Cao et al, 2021 | 0-300 | 0-30 | Electrons | Oxygen consumption | In vitro and in vivo | (60) |
| Boscolo et al, 2021 | 109 | 0-150 | Electrons | Oxygen consumption | In vitro | (64) |
| Jansen et al, 2021 | 0-340 | 10 | X-rays, protons and carbon ions | Oxygen consumption | In vitro | (63) |
| Rothwell et al, 2021 | 40–150+ | 2-50 | - | Oxygen consumption | - | (30) |
| Tinganelli et al, 2022 | 0-70 | 0-7.5 | Ions | Oxygen consumption | In vitro | (61) |
UNIVERSE, UNified and VERSatile Bio Response Engine (mechanistic radiobiological model); ROS, reactive oxygen species.
Although the oxygen consumption mechanism of FLASH irradiation is widely known, this mechanism is being challenged due to progress in-depth research. FLASH irradiation is unlikely to consume oxygen to radiation-related levels of hypoxia in large tissues. At the same dose, FLASH irradiation led to less oxygen consumption than conventional in vitro irradiation, which may be related to the protective effect of FLASH. However, the difference in oxygen consumption between FLASH and conventional exposure cannot be quantified in vivo because measurements of oxygen consumption under conventional exposure are hampered by oxygen supplementation in the blood (60). Tinganelli et al (61) studied the effect of FLASH irradiation on CHO-K1 cells in oxygenation content ranging from 0 to 21%. The authors determined that the FLASH protection effect was oxygenation-dependent and the protective effect was more significant in the presence of lower oxygen content. This finding affirms the opinion of researchers who were skeptical about the mechanism of oxygen consumption (43,62). These studies (60–62) are significant to the research on the mechanisms of FLASH. Studies reported a slight reduction in oxygenation after FLASH irradiation, claiming a negligible effect on radiosensitivity (32,63,64). Cao et al (60) used a fluorescence quenching method and a water-soluble molecular probe to measure oxygen in vivo and in vitro, and quantified the change of oxygen per unit dose by irradiation with a 10 MeV electron beam. In in vitro experiments, the oxygen consumption g values of conventional irradiation ranged from 0.19 to 0.21 mm Hg/Gy (0.34 to 0.37 µM/Gy) and that of UHDR irradiation ranged from 0.16 to 0.17 mm Hg/Gy (0.28 to 0.30 µM/Gy). In vivo, the oxygen content decreased by 2.3±0.3 mm Hg in normal tissues and 1.0±0.2 mm Hg in tumor tissues after a single dose of 20 Gy FLASH irradiation, while no reduction in oxygen was observed with a single fraction of 20 Gy applied in the conventional mode. Therefore, it is indicated that FLASH irradiation induces less oxygen consumption than conventional irradiation at the same dose, which may be related to the retention effect of FLASH. However, the difference in oxygen consumption between FLASH and conventional irradiation cannot be quantified in vivo, as measurements of oxygen consumption under conventional irradiation is replenished by oxygen supplementation in the blood. Jansen et al (63) experimentally investigates whether oxygen depletion occurs during FLASH irradiation by measuring the oxygen concentration in vitro during irradiation of water by photons, protons and carbon ions. They observed that oxygen consumption in water was only related to radiation dose, dose rate and linear energy transfer, with a higher dose rate associated with lower oxygen consumption. They also found no clinically relevant oxygen consumption limits. Eventually, they concluded that the FLASH did consume oxygen, but not a sufficient amount to use up all of it. For high dose rates, less oxygen was consumed than for the standard radiotherapy dose rate and loss of oxygen of any analyzed radiation type was found when using FLASH for 10-Gy dose delivery. These studies also challenge the traditional hypothesis of oxygen consumption.
ROS-mediated cell damage
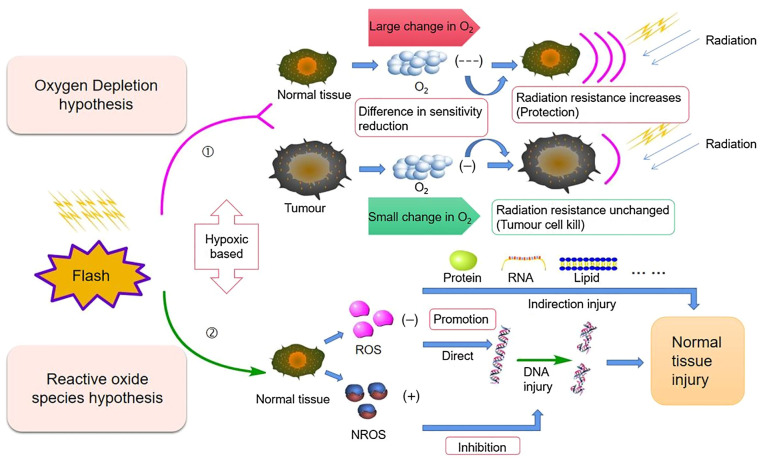
Other biochemical mechanisms are thought to be responsible for the FLASH effect on ROS and free radicals. A study indicated that the electronic irradiation of zebrafish embryos with Con-RT and FLASH-RT only had a minor effect on their morphology 5 days after fertilization due to the low production of ROS, suggesting that the radiation resistance to FLASH of normal tissues was significantly associated with reduced ROS levels (14). Abolfath et al (65) performed molecular dynamics simulations to study the generation of reactive species around DNA at various dose rates and oxidation levels. Under normoxic conditions at high dose rates, individual ROS aggregate to form resonant or metastable molecular states linked by hydrogen bonds. The resulting clusters have low diffusivity and are known as non-reactive oxygen species (NROS), which, unlike ROS, have a limited biological damage potential. At low dose rates and low oxygen tension, the production of NROS is reduced, resulting in a higher proportion of free ROS. Montay-Gruel et al (14) indicated that oxygen consumption of UHDR promotes the protection of normal tissues by inhibiting ROS production. The authors irradiated water with 4% oxygen (to simulate physiological oxygen tension) with FLASH-RT or Con-RT. After FLASH irradiation, the concentration of H2O2 in an aqueous solution was significantly decreased. Spitz et al (31) indicated that the FLASH effect was related to the instantaneous generation of free radicals and inherent differences in redox and free radical chemistry between normal and tumor tissue. The study suggested that the content of unstable iron in normal tissue cells was less than that in tumor tissue cells; therefore, the further reaction of ROS was more easily restricted, reducing cell damage (31). Tumor tissue cells contain a large amount of unstable iron ions and internal reactions are more likely to occur. This phenomenon results in a similar reduction in radiation sensitivity in FLASH-RT with less reduction in tumor cell sensitivity and a greater reduction in normal cell sensitivity. The difference in sensitivity of tumor cells and normal cells to FLASH-RT resulted in different tissue responses. Both Con-RT and Flash-RT mechanisms are related to oxygen and their related mechanisms will be discussed below (Fig. 2).
Figure 2.
Mechanistic diagram of the oxygen consumption hypothesis and ROS hypothesis: ①Oxygen consumption hypothesis: High-dose transient irradiation reduces the presence of oxygen, and this effect is greater on normal cells, resulting in stronger radiation resistance; ROS hypothesis: In normal cells in this hypoxic environment, there is a decrease in ②ROS levels that causes DNA, RNA, protein and lipid injury, and an increase in the protective NROS levels that inhibits DNA injury. NROS, non-reactive oxygen species.
Favaudon et al (66) proposed three hypotheses and compared them with the current results. The radiation-induced transient oxygen depletion (TOD) hypothesis suggests that normal tissue preservation at UHDRs is the result of transient hypoxic radiation protection due to oxygen depletion. Although in vivo data (14) suggested that local oxygen tension had a strong effect on the final results, the isoefficiency of tumor cell killing under normoxic and hypoxic conditions was less supportive. In addition, both direct measurement of oxygen consumption during FLASH irradiation by optical methods in vitro and in vivo and observations of the FLASH effect in aerated cultured cells with DNA damage and survival as endpoints support the TOD hypothesis, and free radical self-destruction appears to be a more plausible explanation (66).
Immune and inflammatory hypothesis
Another explanation is that chromatin remodeling is mediated by poly (adenosine diphosphate ribose) polymerase and inflammatory/anti-inflammatory cell signaling may depend on the duration of treatment. As certain circulating blood cells are irradiated, they protect the immune system better than under traditional dose irradiation. The chromosomal aberrations of circulating blood lymphocytes depend strongly on the amount and duration of irradiation. In FLASH exposure, time reduction allows more circulating immune cells to survive. In this case, the efficacy of fractionated irradiation is lost (67). It has been reported that the TGF-β signaling pathway is lower in FLASH-irradiated mice than in mice subjected to Con-RT (11). One study reported that the key to radiation resistance of tumor-infiltrating T cells is TGF-β (68), while other studies indicated that TGF-β signaling inhibits the immune system and promotes cancer progression, concluding that inhibitors of the TGF-β pathway may enhance the treatment of malignant tumors (69). Rama et al (58) reported that FLASH proton radiation improved lung tumor control, possibly due to the recruitment of CD3+ T lymphocytes into the tumor. In several studies, FLASH-RT and Con-RT were compared in immunocompromised animals; however, no differences in tumor response were observed. Girdhani et al (35) found that proton FLASH-RT had lower toxicity to normal tissue in pre-clinical mouse models than Con-RT. Subsequent genome-wide microarray analysis suggested that extensive activation and maturation of the immune system were inhibited in FLASH-RT mice (10,13,70). FLASH may provide better immune responses due to reduced exposure of circulating immune cells because of the short exposure time, although segmental FLASH-RT may reduce this effect (38). A study on whole-brain irradiation in C57BL/6J mice indicated lower levels of pro-inflammatory cytokines in the hippocampus after FLASH than conventional dose rate irradiation (71). A significant increase in five of 10 cytokines (IL-6, IL-1β, TNF-α, KC/GRO and IL-4) measured at conventional dose rates was reported 10 weeks after irradiation, whereas FLASH only produced increases in three cytokines (IL-1β, TNF-α and KC/GRO).
There is evidence that radiation may lead to pro-inflammatory immune stimulation and anti-inflammatory immunosuppressive responses, and the potential of this immunomodulatory response is the main theoretical basis for numerous recent clinical trials (72). Durante et al (73) noted that the difference in the high dose rate and total treatment time may reduce the proportion of circulating blood cells irradiated, thereby protecting the immune system, and predicted that this would be more effective than subconventional dosing. They indicated that chromosomal aberrations detected in circulating lymphocytes following radiation exposure depend on exposure time and volume (73), but this has not been confirmed for flash exposure. Rama et al (58) demonstrated that Flash-RT improves T cell infiltration in irradiated tumors. It is speculated that routine and flash irradiation may directly alter the expression levels of immune factors and active immune cells, but indirectly affect immune reactivity by affecting DNA damage or the microenvironment. It has also been suggested that radiation exposure leads to the expression of a range of other immune factors, including interleukins, interferons, immune checkpoint ligands and other cytokines. These may induce more than direct radiation responses (74,75).
DNA damage, senescence and fibrosis
There is also considerable evidence of differences in DNA damage in normal tissues after Con-RT and FLASH irradiation. Fouillade et al (76) studied the phosphorylation of histone H2AX and recruitment of cohesion protein 53BP1 at DNA damage sites in MRC5 and IMR90 normal human fibroblasts and A549 human lung adenocarcinoma cells and indicated that after FLASH (5×106 Gy/sec) and Con-RT (5 Gy) irradiation, no significant difference was observed in the number of H2AX lesions between the three cell lines and the two irradiation modes. However, it was noteworthy that flash-RT-induced 53BP1 lesions were significantly fewer than CONV lesions in the two fibroblast cell lines. It has been observed that tumor cells are frequently deficient in radiation-induced G1 arrest and that pathologic DNA repair features and defects are present in double-strand breaks reconnected at G0/G1 (77). Reduced production of the 53BP1 substrate DNA damage subset through free radical recombination and defective repair of G1 tumor cells are also considered to be two important processes in the differential response of normal and tumor cells to FLASH-RT (78).
FLASH irradiation has been indicated to protect tissues from radiation-induced fibrosis similar to that seen in Con-RT. This has been demonstrated in a large number of animal studies (5,11,76). Fouillade et al (76) analyzed the characteristics of radiation-induced lung senescence in mice using immunofluorescence and transcriptome techniques and observed that FLASH-RT was less efficient than Con-RT in inducing p53BP1 lesions. The persistent focal accumulation of 53BP1 at chromosomal damage sites was associated with DNA fragments and chromatin-enhanced senescence. Furthermore, they demonstrated that FLASH-RT was able to prevent the induction of senescent cells in irradiated lungs. They also pioneered the use of single-cell RNA sequencing in radiation biology, allowing the identification of cell-type-specific transcriptional changes (79).
5. Pre-clinical application prospect of FLASH
FLASH-RT is as lethal to tumors as conventional dose rate radiotherapy and has low side effects on normal tissue. Therefore, FLASH-RT will likely have a considerable clinical impact in the future. This inference is supported by the researchers who studied the protection of mouse brain tissue during FLASH-RT (80), thereby suggesting that FLASH-RT modifies a common initial event that may control the development of both acute and delayed toxicity. To date, the FLASH effect has been demonstrated in several in vivo models, mostly in wild-type mice, and in several organ systems. These organs comprise the so-called acute reaction organs, such as the gut and hematopoietic system, (81,82), and delayed reaction organs such as the brain, lung and skin (76,83–87). In 2019, researchers from Stanford University, the SLAC National Accelerator Laboratory, and Indiana University solved the problem of FLASH-RT device architecture (88). FLASH-RT has two primary clinical purposes. First, the FLASH effect may increase the total dose for treating radio-resistant tumors associated with poor outcomes (5). Larger doses of radiation may be delivered to the tumor without the severe toxicity to surrounding normal tissue that Con-RT is expected to cause. Furthermore, analysis of the distribution of initial DNA damage after a high dose rate irradiation showed that the distribution of DNA damage after high-dose rate irradiation shifted in the direction of severe damage and the distribution range widened (89,90). These findings suggest that increasing the dose rate and changing the pulse frequency of ultrafast electrons increases the complexity of DNA damage and reduces its repairability (91). A modified UHDR electron FLASH-RT (eFLASH-RT) linear accelerator was proposed by Rahman et al (92). This device was the first functional beam model commissioned in a clinical treatment planning system for eFLASH-RT, enabling planning and evaluation with minimal deviation from the Con-RT workflow. The device facilitates clinical translation because eFLASH-RT and Con-RT plan quality were comparable for humans with complex geometries and tissue heterogeneity. The methods may be expanded to model other eFLASH irradiators with different beam characteristics. The differences between conventional dose rate radiotherapy and FLASH-RT are significant in numerous aspects, such as in the dose rate. Compared with Con-RT, the dose rate of FLASH-RT may be >40 Gy/sec and the irradiation time may be <1 sec. At the same time, the irradiation source, the equipment used and the corresponding mechanism also exhibit certain differences. The most important difference, however, is in the extent of damage to normal tissue (Table III).
Table III.
Comparison of Con-RT and FLASH-RT.
| Item | Con-RT | FLASH |
|---|---|---|
| Equipment | Proton and ion accelerator, X-knife, γ-knife, | Proton accelerators, linear accelerator |
| Cost | Proven equipment, technology, research and clinical translation | Higher technology cost, equipment cost, personnel cost, research cost, automation and clinical efficiency |
| Dose rate, Gy/sec | 0.002-0.017 | >40 |
| Ray | X- and γ-ray, proton, heavy ion, electron | Proton, X-ray, electron |
| Time, sec | ≥120 | <1 |
| Tumor control effect | Efficient | Efficient |
| Damage degree of normal tissue | High | Low |
| Factors | Tissue radiosensitivity, dose | Dose, dose segmentation, oxygen content, pulse |
| Mechanism | Oxygen depletion hypothesis, ROS, immunoinflammatory hypothesis | Repair, reoxygenation, redistribution, repopulation, oxygen depletion hypothesis, ROS |
Con-RT, conventional radiotherapy; ROS, reactive oxygen species.
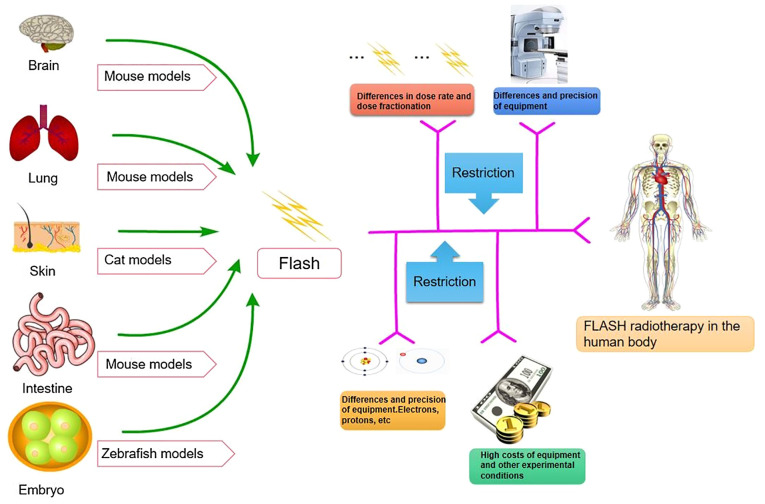
Although several FLASH-related animal studies have been performed, the limitations of FLASH in human studies remain evident (Fig. 3). First, FLASH-RT systems are scarce and high-energy electron or proton FLASH-RT is required for deep tumor radiotherapy. Safety is paramount when patients are exposed to such high dose rates. Developing a comprehensive dose monitoring system is necessary to ensure safety (17,93). The issue of fractional treatment is also an area to be further explored; the correct dose of therapeutic irradiation must be determined in fractional treatment and single fractional treatment within 1 sec (89). Furthermore, treatment equipment limits FLASH-RT. The linear accelerator cannot generate the irradiation dose at the required rate. In addition, pre-clinical studies lack relevant information, such as radiobiology studies to ensure that their findings may be replicated in different settings and to assess potential long-term effects while further exploring specific mechanisms (89). Furthermore, financial factors limit FLASH-RT. It is essential to reduce the cost of technology and make equipment more economical, compact and compatible with existing facilities. Taylor et al (94) warn that although many studies have reported the efficacy of FLASH (95–99), it is essential to conduct prospective clinical trials safely and effectively for FLASH. In this paper, the status of FLASH quality assurance and safety system is reviewed in the aspects of FLASH standards, beam monitoring, calibration and machine quality assurance, external peer review of programs and system dose. For instance, in the clinical trial of FLASH-RT, challenges include no charge to current standards, multiple datasets required for trial submission when it appeared relevant, end-to-end testing required, motion phantom when relevant, planning goals, special guidance on beam arrangements by modality, detailed delivery log files, minimum of a registry for all patients. Further questions that need to be addressed are how well we can select, measure, optimize and reproduce the fine structure of the UHDR dose delivery process; whether it is possible to measure if the biological response to FLASH treatment varies from patient to patient and from tumor to tumor from day to day, in particular if medications or drugs vary the critical biology of FLASH therapy; if FLASH treatment may be delivered across realistic, deep and/or larger volumes, how easy it will be to introduce FLASH therapy into the overall care matrix of a patient; and whether FLASH irradiation causes any long-term damage to tissues. These points all require to be addressed prior to the clinical implementation of FLASH-RT. In addition, further clinical trials of FLASH-RT have been proposed (100,101); for instance, it has been suggested that organizations such as The American Association of Physicists in Medicine, European Society Therapeutic Radiation Oncology and European Federation of Organizations For Medical Physics continue to collaborate on guidance and collect time-structure information in clinical trials to ensure a balanced and retrospective analysis; late effects occur at a later time and clinical access is equitable. In conclusion, the clinical trials and applications of FLASH-RT face numerous challenges.
Figure 3.
FLASH-related animal experiments and limitations in human experiments: Studies in animals (mice, cats and zebrafish) have promoted the further development of FLASH radiotherapy. However, there remain various difficulties in studying its application in humans, in terms of dosage, equipment, radiation source and economical factors.
6. Conclusion
FLASH-RT is a particular irradiation method that has been in development for ~60 years. The modality has been studied from bacteria to cells in vitro, from mice to small animals, and eventually in patients. FLASH-RT began with UHDR radiotherapy and the FLASH effect was discovered in the development process of UHDR radiotherapy. Subsequently, scholars continued in-depth research, hypothesized the possible radiobiological mechanism of FLASH-RT and designed experiments to verify it. The essence of FLASH-RT lies in its robust tumor control and specific protective effect on normal tissues. The success of FLASH-RT in the first patient substantially increased confidence in applications for clinical patients. In the future, FLASH-RT may eventually have a critical role in the treatment of various liver, pancreatic and colon tumors, and the improvement and popularization of radiotherapy equipment. FLASH-RT may even replace certain surgical treatments, significantly reducing the pain and economic pressure, improving survival rates and reducing the side effects of radiotherapy. FLASH-RT has considerable potential for tumor treatment.
Several countries are at the forefront of FLASH-RT research. FLASH-RT may fundamentally overturn the current theoretical system of radiotherapy in the future. There is much speculation about the biological mechanisms that support the FLASH effect. Radiation leads to radiochemical depletion of oxygen and this is particularly evident at high dose rates. It may be safely concluded that oxygen consumption is at least partly responsible for the FLASH effect. Based on available data, the extent of its contribution remains unclear and requires further investigation. In addition to oxygen consumption, the FLASH effect is involved in immune regulation; however, the evidence supporting this view is scarce and preliminary. Similarly, any potential contribution of FLASH-mediated immune effects requires to be explored. In addition to mechanistic insight, the most important question remains the clinical translational potential of FLASH-RT.
In conclusion, FLASH-RT is expected to serve as an example of radiation therapy innovation that improves the therapeutic index. Despite the complexity of its technology and the uncertainty of its efficacy, future studies on the mechanism will facilitate translation to clinical practice for the benefit of patients. In the previous special issue (33,46–48), relevant reviews frequently describe in detail a specific point about FLASH radiotherapy, such as FLASH effects, mechanisms, or potential and obstacles to clinical application. However, the present review provided a unified and comprehensive overview of FLASH in numerous aspects. It may also serve as an introduction to those researchers who are not familiar with FLASH-RT. In particular, for non-radiology researchers, such as surgical researchers, it may provide new ideas for the interdisciplinary treatment of malignant tumors.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- Con-RT
conventional radiotherapy
- PBT
proton beam therapy
- ROS
reactive oxygen species
- LET
linear energy transfer
- NROS
non-reactive oxygen species
- PFS
progression-free survival
- CRT
cathode ray tube
Funding Statement
This work was supported by grants from the National Key Technologies R&D Program (grant no. 2018YFC1106800).
Availability of data and materials
Not applicable.
Authors' contributions
YHL, ZW and YL conceived the study. YHL, TL, XPF and JZ conducted the literature search. YHL, HC, XM and WWL wrote and prepared the original draft of the manuscript. YHL, YL, ZW, TL, XPF, HC, JL, XM, JPD, GMH, WWL, KFY and HW participated in the writing and reviewing the article. YHL, JPD, GMH, KFY and HW edited the article. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kurup A, Pasternak J, Taylor R, Murgatroyd L, Ettlinger O, Shields W, Nevay L, Gruber S, Pozimski J, Lau HT, et al. Simulation of a radiobiology facility for the centre for the clinical application of particles. Phys Med. 2019;65:21–28. doi: 10.1016/j.ejmp.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Durante M, Bräuer-Krisch E, Hill M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br J Radiol. 2018;91:20170628. doi: 10.1259/bjr.20170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry RJ. Effects of radiation dose-rate from protracted, continuous irradiation to ultra-high dose-rates from pulsed accelerators. Br Med Bull. 1973;29:44–47. doi: 10.1093/oxfordjournals.bmb.a070955. [DOI] [PubMed] [Google Scholar]
- 4.Hornsey S, Alper T. Unexpected dose-rate effect in the killing of mice by radiation. Nature. 1966;210:212–213. doi: 10.1038/210212a0. [DOI] [PubMed] [Google Scholar]
- 5.Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, Petit B, Burki M, Ferrand G, Patin D, et al. The Advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res. 2019;25:35–42. doi: 10.1158/1078-0432.CCR-17-3375. [DOI] [PubMed] [Google Scholar]
- 6.Dewey DL, Boag JW. Modification of the oxygen effect when bacteria are given large pulses of radiation. Nature. 1959;183:1450–1451. doi: 10.1038/1831450a0. [DOI] [PubMed] [Google Scholar]
- 7.Town CD. Radiobiology. Effect of high dose rates on survival of mammalian cells. Nature. 1967;215:847–848. doi: 10.1038/215847a0. [DOI] [PubMed] [Google Scholar]
- 8.Field SB, Bewley DK. Effects of dose-rate on the radiation response of rat skin. Int J Radiat Biol Relat Stud Phys Chem Med. 1974;26:259–267. doi: 10.1080/09553007414551221. [DOI] [PubMed] [Google Scholar]
- 9.Berry RJ, Hall EJ, Forster DW, Storr TH, Goodman MJ. Survival of mammalian cells exposed to × rays at ultra-high dose-rates. Br J Radiol. 1969;42:102–107. doi: 10.1259/0007-1285-42-494-102. [DOI] [PubMed] [Google Scholar]
- 10.Hornsey S, Bewley DK. Hypoxia in mouse intestine induced by electron irradiation at high dose-rates. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19:479–483. doi: 10.1080/09553007114550611. [DOI] [PubMed] [Google Scholar]
- 11.Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, Poupon MF, Brito I, Hupé P, Bourhis J, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6:245ra93. doi: 10.1126/scitranslmed.3008973. [DOI] [PubMed] [Google Scholar]
- 12.Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, Doenlen R, Favaudon V, Bochud F, Bailat C, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol. 2017;124:365–369. doi: 10.1016/j.radonc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, Petersson K, Petit B, Bailat C, Bourhis J, et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol. 2018;129:582–588. doi: 10.1016/j.radonc.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, Ollivier J, Petit B, Jorge PG, Syage AR, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci USA. 2019;116:10943–10951. doi: 10.1073/pnas.1901777116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alaghband Y, Cheeks SN, Allen BD, Montay-Gruel P, Doan NL, Petit B, Jorge PG, Giedzinski E, Acharya MM, Vozenin MC, Limoli CL. Neuroprotection of radiosensitive juvenile mice by ultra-high dose rate FLASH irradiation. Cancers (Basel) 2020;12:1671. doi: 10.3390/cancers12061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Water S, Safai S, Schippers JM, Weber DC, Lomax AJ. Towards FLASH proton therapy: The impact of treatment planning and machine characteristics on achievable dose rates. Acta Oncol. 2019;58:1463–1469. doi: 10.1080/0284186X.2019.1627416. [DOI] [PubMed] [Google Scholar]
- 17.Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, Patin D, Ozsahin M, Bochud F, Germond JF, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18–22. doi: 10.1016/j.radonc.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Wei S, Lin H, Choi JI, Simone CB, II, Kang M. A novel proton pencil beam scanning FLASH RT delivery method enables optimal OAR sparing and ultra-high dose rate delivery: A comprehensive dosimetry study for lung tumors. Cancers (Basel) 2021;13:5790. doi: 10.3390/cancers13225790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smyth LML, Donoghue JF, Ventura JA, Livingstone J, Bailey T, Day LRJ, Crosbie JC, Rogers PAW. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci Rep. 2018;8:12044. doi: 10.1038/s41598-018-30543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatesulu BP, Sharma A, Pollard-Larkin JM, Sadagopan R, Symons J, Neri S, Singh PK, Tailor R, Lin SH, Krishnan S. Ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Sci Rep. 2019;9:17180. doi: 10.1038/s41598-019-53562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourhis J, Montay-Gruel P, Gonçalves Jorge P, Bailat C, Petit B, Ollivier J, Jeanneret-Sozzi W, Ozsahin M, Bochud F, Moeckli R, et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother Oncol. 2019;139:11–17. doi: 10.1016/j.radonc.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Zhou S, Zheng D, Fan Q, Yan Y, Wang S, Lei Y, Besemer A, Zhou C, Enke C. Minimum dose rate estimation for pulsed FLASH radiotherapy: A dimensional analysis. Med Phys. 2020;47:3243–3249. doi: 10.1002/mp.14181. [DOI] [PubMed] [Google Scholar]
- 23.Fowler JF, Stern BE. Dose-rate effects: Some theoretical and practical considerations. Br J Radiol. 1960;33:389–395. doi: 10.1259/0007-1285-33-390-389. [DOI] [PubMed] [Google Scholar]
- 24.Orton CG. A unified approach to dose-effect relationships in radiotherapy. II: Inhomogeneous dose distributions. Int J Radiat Oncol Biol Phys. 1988;14:557–560. doi: 10.1016/0360-3016(88)90274-X. [DOI] [PubMed] [Google Scholar]
- 25.Steel H, Brüningk SC, Box C, Oelfke U, Bartzsch SH. Quantification of differential response of tumour and normal cells to microbeam radiation in the absence of FLASH effects. Cancers (Basel) 2021;13:3228. doi: 10.3390/cancers13133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaub L, Harrabi SB, Debus J. Particle therapy in the future of precision therapy. Br J Radiol. 2020;93:20200183. doi: 10.1259/bjr.20200183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blakely EA. The 20th Gray lecture 2019: Health and heavy ions. Br J Radiol. 2020;93:20200172. doi: 10.1259/bjr.20200172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ando K, Kase Y. Biological characteristics of carbon-ion therapy. Int J Radiat Biol. 2009;85:715–728. doi: 10.1080/09553000903072470. [DOI] [PubMed] [Google Scholar]
- 29.Parodi K. The biological treatment planning evolution of clinical fractionated radiotherapy using high LET. Int J Radiat Biol. 2018;94:752–755. doi: 10.1080/09553002.2018.1427904. [DOI] [PubMed] [Google Scholar]
- 30.Rothwell BC, Kirkby NF, Merchant MJ, Chadwick AL, Lowe M, Mackay RI, Hendry JH, Kirkby KJ. Determining the parameter space for effective oxygen depletion for FLASH radiation therapy. Phys Med Biol. 2021;66:055020. doi: 10.1088/1361-6560/abe2ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitz DR, Buettner GR, Petronek MS, St-Aubin JJ, Flynn RT, Waldron TJ, Limoli CL. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol. 2019;139:23–27. doi: 10.1016/j.radonc.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liew H, Mein S, Dokic I, Haberer T, Debus J, Abdollahi A, Mairani A. Deciphering time-dependent DNA damage complexity, repair, and oxygen tension: A mechanistic model for FLASH-dose-rate radiation therapy. Int J Radiat Oncol Biol Phys. 2021;110:574–586. doi: 10.1016/j.ijrobp.2020.12.048. [DOI] [PubMed] [Google Scholar]
- 33.Schüler E, Acharya M, Montay-Gruel P, Loo BW, Jr, Vozenin MC, Maxim PG. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med Phys. 2022;49:2082–2095. doi: 10.1002/mp.15442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correction to lancet diabetes endocrinol. Lancet Diabetes Endocrinol. 2019;2019;77:288–99. e5. doi: 10.1016/S2213-8587(19)30110-X. [DOI] [PubMed] [Google Scholar]
- 35.Girdhani S, Abel E, Katsis A, Rodriquez A, Senapati S, KuVillanueva A, Jackson IL, Eley J, Vujaskovic Z, Parry R. Abstract LB-280: FLASH: A novel paradigm changing tumor irradiation platform that enhances therapeutic ratio by reducing normal tissue toxicity and activating immune pathways. Cancer Res. 2019;79((Suppl 13)):LB–280. doi: 10.1158/1538-7445.AM2019-LB-280. [DOI] [Google Scholar]
- 36.Beyreuther E, Brand M, Hans S, Hideghéty K, Karsch L, Leßmann E, Schürer M, Szabó ER, Pawelke J. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother Oncol. 2019;139:46–50. doi: 10.1016/j.radonc.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Buonanno M, Grilj V, Brenner DJ. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother Oncol. 2019;139:51–55. doi: 10.1016/j.radonc.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diffenderfer ES, Verginadis II, Kim MM, Shoniyozov K, Velalopoulou A, Goia D, Putt M, Hagan S, Avery S, Teo K, et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int J Radiat Oncol Biol Phys. 2020;106:440–448. doi: 10.1016/j.ijrobp.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colangelo NW, Azzam EI. The importance and clinical implications of FLASH ultra-high dose-rate studies for proton and heavy ion radiotherapy. Radiat Res. 2020;193:1–4. doi: 10.1667/RR15537.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Marlen P, Dahele M, Folkerts M, Abel E, Slotman BJ, Verbakel WFAR. Bringing FLASH to the clinic: Treatment planning considerations for ultrahigh dose-rate proton beams. Int J Radiat Oncol Biol Phys. 2020;106:621–629. doi: 10.1016/j.ijrobp.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Diffenderfer ES, Sørensen BS, Mazal A, Carlson DJ. The current status of preclinical proton FLASH radiation and future directions. Med Phys. 2022;49:2039–2054. doi: 10.1002/mp.15276. [DOI] [PubMed] [Google Scholar]
- 42.Petersson K, Adrian G, Butterworth K, McMahon SJ. A quantitative analysis of the role of oxygen tension in FLASH radiation therapy. Int J Radiat Oncol Biol Phys. 2020;107:539–547. doi: 10.1016/j.ijrobp.2020.02.634. [DOI] [PubMed] [Google Scholar]
- 43.Adrian G, Konradsson E, Lempart M, Bäck S, Ceberg C, Petersson K. The FLASH effect depends on oxygen concentration. Br J Radiol. 2020;93:20190702. doi: 10.1259/bjr.20190702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MM, Verginadis II, Goia D, Haertter A, Shoniyozov K, Zou W, Maity A, Busch TM, Metz JM, Cengel KA, et al. Comparison of FLASH proton entrance and the spread-out bragg peak dose regions in the sparing of mouse intestinal crypts and in a pancreatic tumor model. Cancers (Basel) 2021;13:4244. doi: 10.3390/cancers13164244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patriarca A, Fouillade C, Auger M, Martin F, Pouzoulet F, Nauraye C, Heinrich S, Favaudon V, Meyroneinc S, Dendale R, et al. Experimental set-up for FLASH proton irradiation of small animals using a clinical system. Int J Radiat Oncol Biol Phys. 2018;102:619–626. doi: 10.1016/j.ijrobp.2018.06.403. [DOI] [PubMed] [Google Scholar]
- 46.Nesteruk KP, Togno M, Grossmann M, Lomax AJ, Weber DC, Schippers JM, Safai S, Meer D, Psoroulas S. Commissioning of a clinical pencil beam scanning proton therapy unit for ultra-high dose rates (FLASH) Med Phys. 2021;48:4017–4026. doi: 10.1002/mp.14933. [DOI] [PubMed] [Google Scholar]
- 47.Montay-Gruel P, Corde S, Laissue JA, Bazalova-Carter M. FLASH radiotherapy with photon beams. Med Phys. 2022;49:2055–2067. doi: 10.1002/mp.15222. [DOI] [PubMed] [Google Scholar]
- 48.Weber UA, Scifoni E, Durante M. FLASH radiotherapy with carbon ion beams. Med Phys. 2022;49:1974–1992. doi: 10.1002/mp.15135. [DOI] [PubMed] [Google Scholar]
- 49.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: The 4 R's of radiobiology revisited. Stem Cells. 2010;28:639–648. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson JD, Hammond EM, Higgins GS, Petersson K. Corrigendum: Ultra-high dose rate (FLASH) radiotherapy: Silver bullet or fool's gold? Front Oncol. 2020;10:210. doi: 10.3389/fonc.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Wilson P, Jones B, Yokoi T, Hill M, Vojnovic B. Revisiting the ultra-high dose rate effect: Implications for charged particle radiotherapy using protons and light ions. Br J Radiol. 2012;85:e933–e939. doi: 10.1259/bjr/17827549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan WF, Sowa MB. Effects of ionizing radiation in nonirradiated cells. Proc Natl Acad Sci USA. 2005;102:14127–14128. doi: 10.1073/pnas.0507119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santivasi WL, Xia F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal. 2014;21:251–259. doi: 10.1089/ars.2013.5668. [DOI] [PubMed] [Google Scholar]
- 55.Grimes DR, Partridge M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed Phys Eng Express. 2015;1:045209. doi: 10.1088/2057-1976/1/4/045209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vozenin MC, Hendry JH, Limoli CL. Biological benefits of ultra-high dose rate FLASH radiotherapy: Sleeping beauty awoken. Clin Oncol (R Coll Radiol) 2019;31:407–415. doi: 10.1016/j.clon.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levy K, Natarajan S, Wang J, Chow S, Eggold JT, Loo P, Manjappa R, Lartey FM, Schüler E, Skinner L, et al. FLASH irradiation enhances the therapeutic index of abdominal radiotherapy for the treatment of ovarian cancer. bioRxiv. 2020 doi: 10.1038/s41598-020-78017-7. 2019.2012.2012.873414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rama N, Saha T, Shukla S, Goda C, Milewski D, Mascia AE, Vatner RE, Sengupta D, Katsis A, Abel E, et al. Improved tumor control through T-cell infiltration modulated by ultra-high dose rate proton FLASH using a clinical pencil beam scanning proton system. Int J Radiat Oncol Biol Phys. 2019;105((Suppl 1)):S164–S165. doi: 10.1016/j.ijrobp.2019.06.187. [DOI] [Google Scholar]
- 59.Boscolo D, Krämer M, Fuss MC, Durante M, Scifoni E. Impact of target oxygenation on the chemical track evolution of ion and electron radiation. Int J Mol Sci. 2020;21:424. doi: 10.3390/ijms21020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao X, Zhang R, Esipova TV, Allu SR, Ashraf R, Rahman M, Gunn JR, Bruza P, Gladstone DJ, Williams BB, et al. Quantification of oxygen depletion during FLASH irradiation in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2021;111:240–248. doi: 10.1016/j.ijrobp.2021.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tinganelli W, Sokol O, Quartieri M, Puspitasari A, Dokic I, Abdollahi A, Durante M, Haberer T, Debus J, Boscolo D, et al. Ultra-high dose rate (FLASH) carbon ion irradiation: Dosimetry and first cell experiments. Int J Radiat Oncol Biol Phys. 2022;112:1012–1022. doi: 10.1016/j.ijrobp.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 62.Kranzer R, Poppinga D, Weidner J, Schüller A, Hackel T, Looe HK, Poppe B. Ion collection efficiency of ionization chambers in ultra-high dose-per-pulse electron beams. Med Phys. 2021;48:819–830. doi: 10.1002/mp.14620. [DOI] [PubMed] [Google Scholar]
- 63.Jansen J, Knoll J, Beyreuther E, Pawelke J, Skuza R, Hanley R, Brons S, Pagliari F, Seco J. Does FLASH deplete oxygen? Experimental evaluation for photons, protons, and carbon ions. Med Phys. 2021;48:3982–3990. doi: 10.1002/mp.14917. [DOI] [PubMed] [Google Scholar]
- 64.Boscolo D, Scifoni E, Durante M, Krämer M, Fuss MC. May oxygen depletion explain the FLASH effect? A chemical track structure analysis. Radiother Oncol. 2021;162:68–75. doi: 10.1016/j.radonc.2021.06.031. [DOI] [PubMed] [Google Scholar]
- 65.Abolfath R, Grosshans D, Mohan R. Oxygen depletion in FLASH ultra-high-dose-rate radiotherapy: A molecular dynamics simulation. Med Phys. 2020;47:6551–6561. doi: 10.1002/mp.14548. [DOI] [PubMed] [Google Scholar]
- 66.Favaudon V, Labarbe R, Limoli CL. Model studies of the role of oxygen in the FLASH effect. Med Phys. 2022;49:2068–2081. doi: 10.1002/mp.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernet M, Ponette V, Deniaud-Alexandre E, Ménissier-De Murcia J, De Murcia G, Giocanti N, Megnin-Chanet F, Favaudon V. Poly(ADP-ribose) polymerase, a major determinant of early cell response to ionizing radiation. Int J Radiat Biol. 2000;76:1621–1629. doi: 10.1080/09553000050201118. [DOI] [PubMed] [Google Scholar]
- 68.Arina A, Beckett M, Fernandez C, Zheng W, Pitroda S, Chmura SJ, Luke JJ, Forde M, Hou Y, Burnette B, et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat Commun. 2019;10:3959. doi: 10.1038/s41467-019-11906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, Inigo I, Dobkin J, Manro JR, Iversen PW, et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer. 2018;6:47. doi: 10.1186/s40425-018-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zlobinskaya O, Siebenwirth C, Greubel C, Hable V, Hertenberger R, Humble N, Reinhardt S, Michalski D, Röper B, Multhoff G, et al. The effects of ultra-high dose rate proton irradiation on growth delay in the treatment of human tumor xenografts in nude mice. Radiat Res. 2014;181:177–183. doi: 10.1667/RR13464.1. [DOI] [PubMed] [Google Scholar]
- 71.Simmons DA, Lartey FM, Schüler E, Rafat M, King G, Kim A, Ko R, Semaan S, Gonzalez S, Jenkins M, et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol. 2019;139:4–10. doi: 10.1016/j.radonc.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Friedl AA, Prise KM, Butterworth KT, Montay-Gruel P, Favaudon V. Radiobiology of the FLASH effect. Med Phys. 2022;49:1993–2013. doi: 10.1002/mp.15184. [DOI] [PubMed] [Google Scholar]
- 73.Durante M, Yamada S, Ando K, Furusawa Y, Kawata T, Majima H, Nakano T, Tsujii H. Measurements of the equivalent whole-body dose during radiation therapy by cytogenetic methods. Phys Med Biol. 1999;44:1289–1298. doi: 10.1088/0031-9155/44/5/314. [DOI] [PubMed] [Google Scholar]
- 74.Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fouillade C, Curras-Alonso S, Giuranno L, Quelennec E, Heinrich S, Bonnet-Boissinot S, Beddok A, Leboucher S, Karakurt HU, Bohec M, et al. FLASH irradiation spares lung progenitor cells and limits the incidence of radio-induced senescence. Clin Cancer Res. 2020;26:1497–1506. doi: 10.1158/1078-0432.CCR-19-1440. [DOI] [PubMed] [Google Scholar]
- 77.Scully R, Panday A, Elango R, Willis NA. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Biol. 2019;20:698–714. doi: 10.1038/s41580-019-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Labarbe R, Hotoiu L, Barbier J, Favaudon V. A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother Oncol. 2020;153:303–310. doi: 10.1016/j.radonc.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Kulkarni A, Anderson AG, Merullo DP, Konopka G. Beyond bulk: A review of single cell transcriptomics methodologies and applications. Curr Opin Biotechnol. 2019;58:129–136. doi: 10.1016/j.copbio.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lohse I, Lang S, Hrbacek J, Scheidegger S, Bodis S, Macedo NS, Feng J, Lütolf UM, Zaugg K. Effect of high dose per pulse flattening filter-free beams on cancer cell survival. Radiother Oncol. 2011;101:226–232. doi: 10.1016/j.radonc.2011.05.072. [DOI] [PubMed] [Google Scholar]
- 81.Levy K, Natarajan S, Wang J, Chow S, Eggold JT, Loo PE, Manjappa R, Melemenidis S, Lartey FM, Schüler E, et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci Rep. 2020;10:21600. doi: 10.1038/s41598-020-78017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chabi S, To THV, Leavitt R, Poglio S, Jorge PG, Jaccard M, Petersson K, Petit B, Roméo PH, Pflumio F, et al. Ultra-high-dose-rate FLASH and conventional-dose-rate irradiation differentially affect human acute lymphoblastic leukemia and normal hematopoiesis. Int J Radiat Oncol Biol Phys. 2021;109:819–829. doi: 10.1016/j.ijrobp.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 83.Vozenin MC, Montay-Gruel P, Limoli C, Germond JF. All irradiations that are ultra-high dose rate may not be FLASH: The critical importance of beam parameter characterization and in vivo validation of the FLASH effect. Radiat Res. 2020;194:571–572. doi: 10.1667/RADE-20-00141.1. [DOI] [PubMed] [Google Scholar]
- 84.Jorge PG, Jaccard M, Petersson K, Gondré M, Durán MT, Desorgher L, Germond JF, Liger P, Vozenin MC, Bourhis J, et al. Dosimetric and preparation procedures for irradiating biological models with pulsed electron beam at ultra-high dose-rate. Radiother Oncol. 2019;139:34–39. doi: 10.1016/j.radonc.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 85.Ko RB, Soto LA, von Eyben R, Melemenidis S, Rankin EB, Maxim PG, Graves EE, Loo BW. Evaluating the reproducibility of mouse anatomy under rotation in a custom immobilization device for conformal FLASH radiotherapy. Radiat Res. 2020;194:600–606. doi: 10.1667/RADE-20-00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soto LA, Casey KM, Wang J, Blaney A, Manjappa R, Breitkreutz D, Skinner L, Dutt S, Ko RB, Bush K, et al. FLASH irradiation results in reduced severe skin toxicity compared to conventional-dose-rate irradiation. Radiat Res. 2020;194:618–624. doi: 10.1667/RADE-20-00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan S, Bassenne M, Wang J, Manjappa R, Melemenidis S, Breitkreutz DY, Maxim PG, Xing L, Loo BW, Jr, Pratx G. Multicellular spheroids as in vitro models of oxygen depletion during FLASH irradiation. Int J Radiat Oncol Biol Phys. 2021;110:833–844. doi: 10.1016/j.ijrobp.2021.01.050. [DOI] [PubMed] [Google Scholar]
- 88.Maxim PG, Keall P, Cai J. FLASH radiotherapy: Newsflash or flash in the pan? Med Phys. 2019;46:4287–4290. doi: 10.1002/mp.13685. [DOI] [PubMed] [Google Scholar]
- 89.Prempree T, Michelsen A, Merz T. The repair time of chromosome breaks induced by pulsed x-rays on ultra-high dose-rate. Int J Radiat Biol Relat Stud Phys Chem Med. 1969;15:571–574. doi: 10.1080/09553006914550871. [DOI] [PubMed] [Google Scholar]
- 90.Evans SE, Grigoryan A, Szalai VA. Oxidation of guanine in double-stranded DNA by [Ru(bpy)2dppz]Cl2 in cationic reverse micelles. Inorg Chem. 2007;46:8349–8361. doi: 10.1021/ic0700708. [DOI] [PubMed] [Google Scholar]
- 91.Lapi A, Pratviel G, Meunier B. Guanine oxidation in double-stranded DNA by MnTMPyP/KHSO(5): At least three independent reaction pathways. Met Based Drugs. 2001;8:47–56. doi: 10.1155/MBD.2001.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahman M, Ashraf MR, Gladstone DJ, Bruza P, Jarvis LA, Schaner PE, Cao X, Pogue BW, Hoopes PJ, Zhang R. Treatment planning system for electron FLASH radiation therapy: Open-source for clinical implementation. Int J Radiat Oncol Biol Phys. 2022;112:1023–1032. doi: 10.1016/j.ijrobp.2021.10.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin B, Gao F, Yang Y, Wu D, Zhang Y, Feng G, Dai T, Du X. FLASH radiotherapy: History and future. Front Oncol. 2021;11:644400. doi: 10.3389/fonc.2021.644400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taylor PA, Moran JM, Jaffray DA, Buchsbaum JC. A roadmap to clinical trials for FLASH. Med Phys. 2022;49:4099–4108. doi: 10.1002/mp.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hendry JH, Moore JV, Hodgson BW, Keene JP. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat Res. 1982;92:172–181. doi: 10.2307/3575852. [DOI] [PubMed] [Google Scholar]
- 96.Abel E, Girdhani S, Jackson I, Eley J, Katsis A, Marshall A, Rodriguez A, Senapati S, Bentzen SM, Vujaskovic Z, et al. Characterization of radiation-induced lung fibrosis and mode of cell death using single and multi-pulsed proton flash irradiation. Int J Radiat Oncol Biol Phys. 2019;105((Suppl 1)):E652–E653. doi: 10.1016/j.ijrobp.2019.06.1033. [DOI] [Google Scholar]
- 97.Cunningham S, McCauley S, Vairamani K, Speth J, Girdhani S, Abel E, Sharma RA, Perentesis JP, Wells SI, Mascia A, Sertorio M. FLASH proton pencil beam scanning irradiation minimizes radiation-induced leg contracture and skin toxicity in mice. Cancers (Basel) 2021;13:1012. doi: 10.3390/cancers13051012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Velalopoulou A, Karagounis IV, Cramer GM, Kim MM, Skoufos G, Goia D, Hagan S, Verginadis II, Shoniyozov K, Chiango J, et al. FLASH proton radiotherapy spares normal epithelial and mesenchymal tissues while preserving sarcoma response. Cancer Res. 2021;81:4808–4821. doi: 10.1158/0008-5472.CAN-21-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Montay-Gruel P, Acharya MM, Gonçalves Jorge P, Petit B, Petridis IG, Fuchs P, Leavitt R, Petersson K, Gondré M, Ollivier J, et al. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin Cancer Res. 2021;27:775–784. doi: 10.1158/1078-0432.CCR-20-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.AAPM, corp-author. PUBLICATIONS-REPORTS. https://www.aapm.org/pubs/reports/ [ August 26; 2021 ]; [Google Scholar]
- 101.ESTRO, corp-author. ESTRO Guidelines. https://www.estro.org/Science/Guidelines. [ August 26; 2021 ]; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.