Abstract
Background
Severe acute respiratory syndrome coronavirus 2 infection is responsible for the coronavirus disease 2019 (COVID-19) pandemics. Omicron (B.1.1.529) variant is the cause for the surge of the COVID-19 pandemics of the end of 2021 and the beginning of 2022, although its subvariants are responsible for the following daily increase of COVID-19 cases in July 2022. Early reports of Omicron variant confirmed patients indicated less severe disease course compared with the disease caused by previously encountered variants with absence of data regarding cardiac involvement by Omicron.
Case summary
A 42-year-old male who tested positive for Omicron was admitted on January 2022 with chest pain and ST-segment elevation in the inferior leads. Coronary angiography revealed non-significant coronary artery disease. Cardiac magnetic resonance imaging demonstrated features consistent with myocarditis with involvement of 22% of the left ventricular mass by late gadolinium enhancement involving both the lateral and the septal walls. The second patient is a 60-year-old male presented following syncope and palpitations after he was confirmed with Omicron infection. Upon emergency department arrival he had ventricular tachycardia of 250 beats/minute and underwent urgent cardioversion. During his hospitalization, there was no recurrence of malignant arrhythmia, coronary angiography revealed non-obstructive disease. Cardiac magnetic resonance imaging demonstrated imaging features suggesting acute myocarditis with involvement of 19% of the left ventricular mass.
Discussion
This is the first report of myocarditis cases as a possible complication associated with Omicron variant. Despite preliminary reports of less severe disease clinicians should be vigilant for potential deleterious cardiac complications of Omicron.
Keywords: COVID-19, Omicron, Myocarditis, Case report
Learning points.
Coronavirus disease 2019 (COVID-19) pandemic is on the rise due to the recently recognized Omicron (B.1.1.529) variant which is suggested by preliminary reports to cause less severe disease compared to previous variants.
Omicron may cause myocarditis with consequent malignant arrhythmias similarly to previously encountered SARS-CoV2 variants.
Cardiac magnetic resonance imaging may be helpful in the diagnosis of myocarditis in the setting of COVID-19 caused by the Omicron.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has been a major public health issue for the last 2 years, accounting for more than 300 million confirmed cases, causing over 5.5 million deaths. The first four major SARS-CoV2 variants were Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2). They resulted in waves of pandemic and multiorgan involvement with severe lower respiratory tract involvement as the leading determinant of fatality.1 Although myocardial injury is common among patients with COVID-19 with up to 57% in some cohorts,2,3 the incidence of frank clinical myocarditis is probably much lower.3 Yet, these myocarditis cases are clinically important with potential deleterious effects including fulminant myocarditis with clinical deterioration to heart failure and death.4–7 By November 2021, Omicron (B.1.1.529) is designated as the fifth major variant and from December 2021 it has become the dominant variant in many countries worldwide. Omicron’s subvariants (such as the BA.1 and BA.2) are responsible for the increase in daily COVID-19 cases reported in June and July 2022.8 Preliminary reports from South Africa report of different, more subtle clinical course of Omicron variant.9,10 There is scarce data regarding Omicron’s cardiac involvement, here we report for the first time two cases of myocarditis in acutely infected Omicron patient.
Timeline patient 1:
| Date | Significant event |
|---|---|
| 8.1.2022 | The patient presented to the emergency department (ED) with squeezing chest pain, ST elevation in the inferior leads of 12 lead ECG, elevated level of troponin I and inferior wall motion hypokinesis on echocardiography |
| 8.1.2022 | Urgent coronary angiography was performed, showing non-significant coronary artery disease |
| 8.1.2022 | A nasopharyngeal swab was for SARS-CoV-2 on real-time reverse transcriptase–polymerase chain reaction (PCR) assay |
| 9.1.2022 | The Omicron variant was confirmed by genotyping |
| 11.1.2022 | Cardiac magnetic resonance imaging (MRI) demonstrated imaging features consistent with acute myocarditis with involvement of 22% of the left ventricular mass by late gadolinium enhancement |
| 13.1.2022 | The patient was discharged from the hospital after being treated with ibuprofen and colchicine with gradual improvement of his symptoms during the hospitalization |
Timeline patient 2:
| Date | Significant event |
|---|---|
| 11.1.2022 | A nasopharyngeal swab was performed due to symptom of chills, with a positive result for SARS-CoV-2 on a PCR assay. The symptoms resolved 2 days later |
| 21.1.2022 | While playing table tennis, the patient had syncope with no prior symptoms. Immediately after he regained consciousness, he complained of palpitations and exercise intolerance |
| 21.1.2022 | The patient presented to the emergency department with monomorphic ventricular tachycardia of 250/per minute. He underwent urgent synchronized cardioversion due to haemodynamic instability. His ECG demonstrated ST elevation in AVR and ST depression in inferior and lateral leads. His PCR assay was still positive with confirmed Omicron variant |
| 22.1.2022 | Coronary angiography was performed, showing non-obstructive coronary artery disease |
| 26.1.2022 | Cardiac MRI demonstrated imaging features consistent with acute myocarditis with involvement of 19% of the left ventricular mass by late gadolinium enhancement with involvement of the inferior and lateral walls |
| 28.1.2022 | The day of the last report of the patient’s chart; he is still hospitalized and is awaiting to receive a wearable defibrillator for the following month |
Case reports
Case 1
A 42-year-old male presented to the emergency department with chest pain (8 January 2022). His medical history was remarkable for prior peri-myocarditis in 2008 without clear aetiology and without recurrence ever since, with no previous diagnosis of autoimmune disorders. The patient received three doses of BNT162b2 mRNA vaccines (third dose on 22 August 2021). He also had hyperlipidaemia treated with ezetimibe as sole chronic medication. Upon emergency department presentation, the patient describes several episodes of a retrosternal squeezing pain, each episode lasting for 3–4 h, without any precipitating or relieving factors. He also described decreased tolerance for exertion during the last three days. The patient denied dyspnoea, fever, cough, and further symptoms.
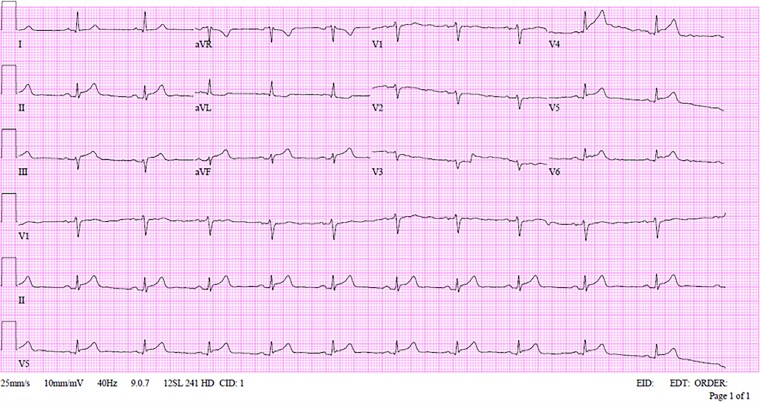
On arrival to the emergency department, the patient complains of ongoing pain that relieved during his stay there, physical examination revealed blood pressure of 131/84 mm/Hg, heart rate of 74 beats per minute, oxygen saturation of 97% while breathing ambient air, and body temperature of 36.7°C. Physical examination was unremarkable. A 12-lead electrocardiogram (ECG) showed sinus rhythm, ST-segment elevation in the inferior and posterior leads, and ST-segment depression in lead AVL (Figure 1). Upon arrival, high-sensitivity Troponin I level was 6309 ng/L (upper normal limit of 20 ng/L), the C-reactive protein level was 10.98 mg/L, the rest of the blood tests were unremarkable.
Figure 1.
First 12 leads electrocardiogram upon presentation to the emergency department of Patient 1.
Given the patients’ complaints, his ECG and the bed side echocardiography that revealed overall preserved left ventricular ejection fraction with regional wall abnormalities of the inferior wall, urgent coronary angiography was performed, demonstrating non-significant coronary artery disease.
The patient was admitted to the intensive care unit with a diagnosis of suspected myocarditis, treatment with ibuprofen (800 mg, three times a day) and colchicine (0.5 mg twice a day) were started. A nasopharyngeal swab was performed upon admission to the intensive care unit (8 January) with a positive result for SARS-CoV-2 on real-time reverse transcriptase–polymerase chain reaction assay. Omicron variant was later determined by genotyping.
Transthoracic echocardiography revealed involvement of the inferior wall. No pericardial effusion was observed.
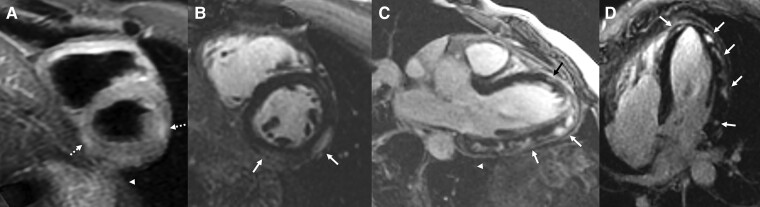
Cardiac magnetic resonance imaging (MRI) was performed using a 1.5T scanner and standard scanning protocol. Both ventricles were normal in size and function (left ventricular ejection fraction = 58%, right ventricular ejection fraction = 52%) with no wall motion abnormality. Hyper-intense signal in the lateral and inferior wall segments on T2 sequence, consistent with oedema was demonstrated. Late gadolinium enhancement (LGE) obtained in the accepted cardiac planes (short-axis, four-chamber, three-chamber, and two-chamber) demonstrated extensive epicardial involvement (sparing the sub-endocardium) of the lateral wall, in a typical non-ischaemic pattern. LGE involvement was quantified as involving 22% of the left ventricular mass, using a dedicated platform (IntelliSpace Portal 11 Philips Healthcare) (Figure 2).
Figure 2.
Cardiac magnetic resonance imaging performed 4 days after his admission demonstrating imaging features consistent with myocarditis for Patient 1. Late Gadolinium enhancement of left ventricular mass = 22%. (A) Short-axis oblique T2-weighted image demonstrating epicardial hyper-intense signal in the lateral and inferior wall (dotted arrows). (B) Short-axis oblique late gadolinium enhancement demonstrating epicardial hyper-intense signal in the lateral and inferior wall (white arrows). (C) Four-chamber late gadolinium enhancement demonstrating epicardial hyper-intense signal in the lateral and apical wall (white arrows). (D) Three-chamber view late gadolinium enhancement demonstrating epicardial hyper-intense signal in the lateral and apical wall (white arrows).
The patient was hemodynamically stable during the stay, without dyspnoea, but continued to complain of intermittent chest pain that relived by the end of his hospitalization. His troponin level increased to maximal level of 10 445 ng/L (on the third day of his hospital stay) and decreased steadily to 190 ng/L upon his discharge, five days after his hospital admission. The rest of his lab tests remained unremarkable. Throughout the hospital stay, no ventricular arrhythmias were recorded. The patient was discharged with recommendation to continue the treatment with colchicine and cardiologic follow-up.
Case 2
A 60-year-old physically active male, with past medical history of controlled asthma currently without the need for chronic therapy and without prior autoimmune disorders, had no chronic medications. He was vaccinated with three doses of BNT 162b2 mRNA COVID-19 vaccine (third dose on 30 August 2021). The patient was confirmed for COVID-19 with Omicron on the 11 January 2022. He have had chills for two days with no other symptoms. On the 21 January, while he was playing table tennis, he had witnessed syncope lasting up to 1 min. He denied any symptoms prior to the syncope including chest pain, palpitations, and dizziness. After he regained consciousness, he had palpitations for the first time in his life with general weakness. While still suffering from palpitations and weakness, he was transferred to the emergency department, where upon presentation he complained of chest pain. He quickly deteriorated and became confused with no pulse palpated and with monomorphic ventricular tachycardia of 250 beats per minute on the monitor. He had synchronized cardioversion with escape junctional rhythm of 40 per minute and after two more minutes sinus rhythm restored. Soon after the cardioversion, he felt well with no recurrence of palpitations, chest pain, or weakness during his hospital stay.
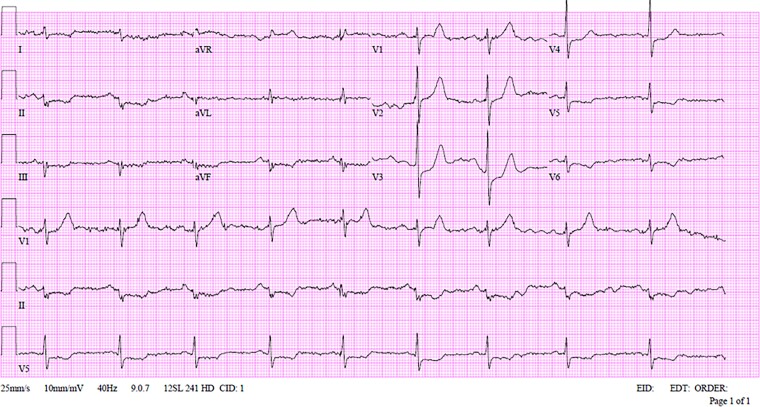
Physical examination after the cardioversion revealed blood pressure of 110/76 mm Hg, heart rate of 58 beats per minute, oxygen saturation of 99% while breathing ambient air. Physical examination was unremarkable. Five minutes after the cardioversion the 12-lead ECG showed sinus rhythm, ST-segment elevation in AVR, ST-segment depression in the inferior and lateral leads (Figure 3). Upon arrival, high-sensitivity Troponin I level was 53 ng/L (upper normal limit of 20 ng/L), with a maximal level of 4400 ng/L on his second test 8 h later, the C-reactive protein level was 5 mg/L (and stayed unchanged during his hospitalization), the rest of the blood tests were unremarkable. The patient was admitted to cardiac intensive unit, where he had no complaints. A nasopharyngeal swab was performed with a still positive result for SARS-CoV-2 of Omicron variant on real-time reverse transcriptase–polymerase chain reaction assay.
Figure 3.
12 leads electrocardiogram after urgent synchronized cardioversion due to monomorphic ventricular tachycardia for Patient 2.
For the first day of his hospitalization, he had several non-sustained ventricular tachycardia of up to five beats. His echocardiography revealed preserved left ventricular ejection fraction of 50%, with posterior wall akinesis and inferior wall hypokinesis, with no pericardial effusion. Coronary angiography was performed demonstrating non-significant coronary artery disease and slight irregularities in the left anterior descending artery and the first diagonal artery.
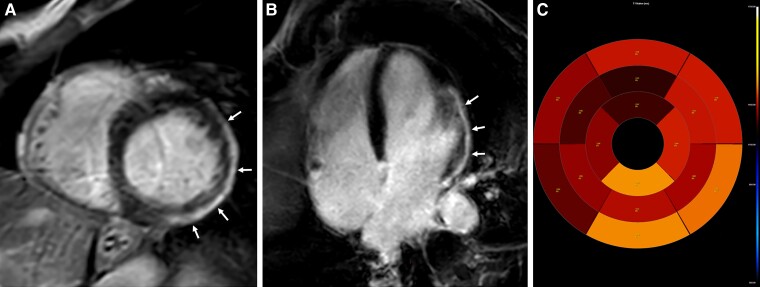
Cardiac MRI was performed using a 3T scanner and standard scanning protocol. The left ventricle was normal in size with mild reduction in function (left ventricular ejection fraction = 51%), The right ventricle was normal in size and function (right ventricular ejection fraction = 48%) with no wall motion abnormality. LGE demonstrated extensive epicardial involvement (sparing the sub-endocardium) of the lateral wall and apex. LGE was calculated to involve 19% of the left ventricular mass. T1 mapping demonstrated increased native T1 values up to 1350–1440 ms (normal values in this 3T scanner are 1200± 50 ms) and an increased extracellular volume value of up to 37% (normal value—up to 28%) (Figure 4).
Figure 4.
Cardiac magnetic resonance imaging demonstrating imaging features consistent with myocarditis for Patient 2. Late gadolinium enhancement (LGE) % of left ventricular mass = 19%. (A) Short-axis oblique late gadolinium enhancement demonstrating epicardial hyper-intense signal in the infero-lateral wall (white arrows). (B) Four-chamber late gadolinium enhancement demonstrating epicardial hyper-intense signal in the lateral wall (white arrows). (C) Native T1 map demonstrating increased T1 values in the lateral and inferior segments with values up to 1400 ms (normal values in this 3T scanner 1200 ± 50 ms).
The patient has been haemodynamically stable during his stay, without chest pain, palpitations and dyspnoea during his hospitalization. After the first day of his hospitalization, he had no recurrences of arrhythmias recorded on continuous monitoring, treated only with bisoprolol 1.25 mg and without any treatment with anti-arrhythmic medications. He was discharged with wearable cardioverter-defibrillator until repeated cardiac MRI and arrythmias specialist visit follow-up 3 months following hospital discharge for the decision regarding permanent implantable cardioverter-defibrillator (ICD). On April 2022, the patient had additional MRI with LGE involving 16% of the LV and consequently was recommended permanent ICD.
Discussion
To the best of our knowledge this is the first report of cases of myocarditis among COVID-19, Omicron confirmed patient, with malignant life dangerous arrhythmia among one of them. These patients underwent through work up with confirmation of extensive acute myocarditis diagnosis in cardiac MRI.
Cardiac involvement in COVID-19 has been reported previously with conflicting data regarding its prevalence depending on the severity of the disease.3,11,12 The largest registry up to date was based on The Israeli Clalit Health Maintenance Organization registry describing that myocarditis following SARS-CoV2 was prevalent in older adults (>40 years) similarly to younger patients and was as common in both sexes with overall 11.5 excess events per 100 000 persons.13 On the contrary, the widely described mRNA COVID-19 vaccine induced myocarditis is more common in young male adults and adolescents with a lower incidence of 8.6 excess events per 100 000 persons.14 Cardiac involvement in COVID-19 patients is well established, especially in hospitalized patients with prognostic value for these patients.3 Most of the previous reports addressed the cardiac involvement solely as troponin elevation. However, it was shown that most of these patients are likely to have ongoing inflammatory process within their myocardial tissue.12 Several possible mechanisms were suggested to explain the SARS-Cov2 myocarditis:11 (i) direct viral injury to the myocardial injury; (ii) CD8 T lymphocytes mediated cytotoxicity by migration to cardiomyocytes and induction of local inflammation; (iii) hyperactivation of the innate and adaptive autoimmune system as part of a systemic inflammation.
Omicron is a new variant of the SARS-CoV2 with numerous recognized mutations in the coding gene of the receptor binding domain and the N-terminal domain in the viral spike protein responsible for the efficient cell entry through the ACE2 receptor. Consequently, Omicron variant is estimated to be 2–3 times more transmissible compared to the Delta variant and thus explaining the quick spread and the peak number of daily new cases of new COVID-19 in most of western countries.10 This phenomenon is observed despite the increasing rate of vaccinated persons around the globe. Meanwhile, the published reports of the new variant severity from South Africa, where Omicron was first recognized, describe less severe clinical course with lower hospitalization rates by up to 50% compared with the delta variant.15,16 Other reports from the United Kingdom and Denmark report of similar hospitalization rate and disease severity caused by Omicron compared with previously encountered SARS-CoV2 variants.10 However, none of previous reports addressed the cardiovascular effect of Omicron variant and myocarditis specifically. The aforementioned cases suggest that the COVID-19 of Omicron variant may involve the heart, leading to myocarditis in particular, with further complications such as malignant arrhythmia with haemodynamic instability as observed in the second patient.
Limitations
Endomyocardial biopsy was not conducted as suggested traditionally for definitive myocarditis diagnoses despite its low sensitivity.17 However, we based our diagnosis on cardiac MRI which is the test of choice in most of myocarditis diagnosed cases of the overall population18 and those with COVID-19 in particular.12
In conclusion, we believe that this report of myocarditis among Omicron COVID-19 patients, highlights the need to account for the possibility of this diagnosis in patients presenting with cardiac related symptoms such as angina, syncope and palpitations in the following period of the sharp rise in the COVID-19 cases. This is of importance especially in light of suggested less severe, flu-like, clinical course of the COVID-19 caused by Omicron variant.
Supplementary Material
Contributor Information
Boris Fishman, Sheba Medical Center, Tel Hashomer, affiliated to Sackler Medical School, Tel Aviv University, Israel.
Orly Goitein, Sheba Medical Center, Tel Hashomer, affiliated to Sackler Medical School, Tel Aviv University, Israel.
Anat Berkovitch, Sheba Medical Center, Tel Hashomer, affiliated to Sackler Medical School, Tel Aviv University, Israel.
Galia Rahav, Sheba Medical Center, Tel Hashomer, affiliated to Sackler Medical School, Tel Aviv University, Israel.
Shlomi Matetzky, Sheba Medical Center, Tel Hashomer, affiliated to Sackler Medical School, Tel Aviv University, Israel.
Lead author biography
 Boris Fishman is a second year cardiology fellow at the Leviev Heart institute, in Sheba Medical Center, Tel-Hashomer, Israel, where he also finished his residency in internal medicine. He received his medical degree from the Goldman School of Medicine, Ben Gurion University, Beer-Shev, Israel, where he also completed his MPH degree. He has special interest in heart failure and cardiology of the older adult population.
Boris Fishman is a second year cardiology fellow at the Leviev Heart institute, in Sheba Medical Center, Tel-Hashomer, Israel, where he also finished his residency in internal medicine. He received his medical degree from the Goldman School of Medicine, Ben Gurion University, Beer-Shev, Israel, where he also completed his MPH degree. He has special interest in heart failure and cardiology of the older adult population.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm patients’ consent for submission and publication of these case reports including images of their ECG and cardiac MRI in accordance with COPE guidelines.
Funding: None declared.
Data availability
The data included in this manuscript is a case report rather than a database.
References
- 1. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021;19:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khaloo P, Shaqdan A, Ledesma PA, et al. Distinct etiologies of high-sensitivity troponin T elevation predict different mortality risks for patients hospitalized with COVID-19. Int J Cardiol 2022;351:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernal-Torres W, Herrera-Escandón Á, Hurtado-Rivera M, et al. COVID-19 fulminant myocarditis: a case report. S Eur Heart J Case Rep 2020;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiore G, Sanvito F, Fragasso G, et al. Case report of cardiogenic shock in COVID-19 myocarditis: peculiarities on diagnosis, histology, and treatment. Eur Heart J Case Rep 2021;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dyer O. COVID-19: omicron is causing more infections but fewer hospital admissions than delta, South African data show. BMJ. 2021:n3104. [DOI] [PubMed] [Google Scholar]
- 7. Papageorgiou J-M, Almroth H, Törnudd M, et al. Fulminant myocarditis in a COVID-19 positive patient treated with mechanical circulatory support – a case report. Eur Heart J Case Rep 2021;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hachmann NP, Miller J, Collier AY, et al. Neutralization Escape by SARS-CoV;2, omicron subvariants BA.2.12.1, BA.4, and BA.5. N Eng J Med 2022;387:86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022;399:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. del Rio C, Omer SB, Malani PN. Winter of omicron—the evolving COVID-19 pandemic. JAMA 2022;327:319–320. [DOI] [PubMed] [Google Scholar]
- 11. Carretta DM, Silva AM, D’Agostino D, et al. Cardiac involvement in COVID-19 patients: a contemporary review. Infect Dis Rep 2021;13:494–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daniels CJ, Rajpal S, Greenshields JT, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 cardiac registry. JAMA Cardiol 2021;6:1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dagan N, Barda N, Balicer RD. Adverse effects after BNT162b2 vaccine and SARS-CoV-2 infection, according to age and sex. N Eng J Med 2021;385:2299–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Eng J Med 2021;385:2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kleynhans J, Tempia S, Wolter N, et al. SARS-CoV-2 seroprevalence in a rural and urban household cohort during first and second waves of infections. South Africa July 2020-march 2021. Emerging Infect Dis. 2021;27:3020–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahase E. COVID-19: hospital admission 50-70% less likely with omicron than delta, but transmission a major concern. BMJ 2021;375:n3151. [DOI] [PubMed] [Google Scholar]
- 17. Ammirati E, Frigerio M, Adler ED, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail 2020:663–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data included in this manuscript is a case report rather than a database.