Abstract
How disrupted sleep contributes to cognitive dysfunction over the dynamic course of Alcohol Use Disorder (AUD) is an emerging topic of investigation. Here, the Pittsburgh Sleep Quality Index (PSQI) was used to evaluate subjective sleep in 90 individuals with AUD sober for an average of 3 months and in 50 healthy controls. Relative to controls, AUD individuals had higher global PSQI scores (worse sleep), higher scores on the Beck Depression Inventory (BDI), worse Quality of Life (QoL) indicators, and poorer performance on cognitive composite tests (executive functioning, attention and working memory, visual and verbal learning or memory). Among AUD individuals, a higher PSQI score correlated with a higher BDI scores and worse QoL, but not with cognitive scales. Also noted in the AUD group were higher global PSQI scores in individuals also diagnosed with major depressive (MDD) or generalized anxiety (GAD) disorders. Together, the 4 variables explained 29.8% of the variance in AUD PSQI scores. In women with AUD, the 4 factors explained 39.3% of the variance in PSQI scores; in AUD men, the 4 measures explained 19.9% of the variance: MDD was salient in women, QoL in men with AUD suggesting differential factors associate with poor sleep in men and women with AUD even with sustained alcohol abstinence. Here, global PSQI scores were related to clinical diagnoses and life functioning but failed to predict cognitive performance in abstinent AUD individuals.
Keywords: Pittsburgh Sleep Quality Index [PSQI], Alcohol Use Disorders Identification Test [AUDIT], Beck Depression Index [BDI], Quality of Life [QOL], cognition, composite scores
INTRODUCTION
Sleep disturbances are a major risk factor for relapse after cessation from chronic alcohol consumption (Brower, 2015; Kolla et al., 2015; Perney, Rigole, Mason, Dematteis, & Lehert, 2015); conversely, chronic alcohol consumption disrupts sleep (Colrain, Nicholas, & Baker, 2014; Feige, Scaal, Hornyak, Gann, & Riemann, 2007; Neu, Sofin, & Danker-Hopfe, 2018). The Pittsburgh Sleep Quality Index [PSQI] is a well-validated measure of subjective sleep quality (Liu, Kahathuduwa, & Vazsonyi, 2021; Mollayeva et al., 2016; Phelps et al., 2020; Buysse, 1989) that has been used in a variety of populations to demonstrate associations between poor sleep and major depressive (MDD), generalized anxiety (GAD), and alcohol use disorders (e.g., Ehlers, Gilder, Criado, & Caetano, 2010; Ehlers, Wills, & Gilder, 2018; Ehlers, Wills, Lau, & Gilder, 2017; Miller et al., 2017). In AUD, high PSQI scores, reflective of poor sleep (Kolla et al., 2014; Perney et al., 2015), are also associated with more symptoms of anxiety and depression (Foster & Peters, 1999; Kolla, Mansukhani, Biernacka, Chakravorty, & Karpyak, 2020) and, in some studies, with alcohol use severity (Hartwell, Bujarski, Glasner-Edwards, & Ray, 2015). Further, health-related quality of life (QoL) – a construct that quantifies the subjective perception of the impact of health, including disease and treatment, on physical, psychosocial, and social well-being (Foster, Marshall, & Peters, 2000) – is compromised and associated with disturbed sleep in AUD (Cohn, Foster, & Peters, 2003; Peters, Millward, & Foster, 2003).
Sleep disruption may contribute to cognitive impairment (Jóhannsdóttir, Ferretti, Árnadóttir, & Jónsdóttir, 2021; Leso et al., 2021). In polysomnography recordings, reduced rapid eye movement (REM) sleep correlated with compromised performance on a backward span task in 20 AUD men in treatment (Benson, Cohen, & Zarcone, 1978). Impaired non-REM sleep and declarative memory consolidation were associated in 36 AUD men in the first several months of abstention (Junghanns, Horbach, Ehrenthal, Blank, & Backhaus, 2009). A recent study discriminated AUD individuals with mild or moderate withdrawal symptoms; only those with moderate withdrawal signs (37 AUD men and women) presented with objective sleep alterations in non-REM sleep associated with short-term memory and executive function deficits (Laniepce et al., 2020). In a large study of the general population, heavy alcohol use was associated with higher PSQI scores that contributed to impaired performance (lower accuracy and longer reaction time) in a relational matching task (Li, Chen, Tang, & Li, 2021). By contrast, AUD inpatients with quantifiable executive deficits scored below 5 (i.e., no sleep disturbances) on the PSQI (Laniepce et al., 2019).
In the current study, PSQI, clinical, and cognitive performance data were collected in 90 individuals with AUD and 50 healthy controls to test the hypothesis that poor subjective sleep would predict impaired performance in working memory and executive cognitive functions in AUD.
METHODS
Study Participants
These data were collected with protocols approved by the Institutional Review Boards of Stanford University and SRI International. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. A total of 140 individuals were administered the PSQI questionnaire between February 2013 and December 2019. The characteristics of individuals with AUD (65 men and 25 women, 53.7±9.6 years) and healthy controls (26 men and 24 women, 56.5±12.1 years) are presented in Table 1. Many of these men and women participated in previously reported studies (Le Berre et al., 2019; E.V. Sullivan, Zahr, Sassoon, & Pfefferbaum, 2020; E. V. Sullivan et al., 2018; Zahr, Pohl, Kwong, Sullivan, & Pfefferbaum, 2021).
Table 1.
Demographic characteristics of the study groups*
| Control n=50 |
AUD n=90 |
p-value^ | |
|---|---|---|---|
| N (men/women) | 26 / 24 | 65 / 25 | 0.02 |
| Age (years) | 56.5±12.1 | 53.7±9.6 | 0.17 |
| Self-Defined Ethnicitya | 30 / 8 / 12 | 41 / 35 / 14 | 0.01 |
| Handedness (right/left) | 46 / 4 | 76 / 14 | 0.31 |
| Body Mass Index (BMI) | 25.8±4.5 | 27.6±4.6 | 0.03 |
| Education (years) | 16.7±2.1 | 13.5±2.7 | <.0001 |
| Socioeconomic Status (SES)b | 22.8±10.9 | 41.4±17.0 | <.0001 |
| WTARc IQ | 109.3±10.0 | 97.8±13.7 | <.0001 |
| Alcohol-Related Variables | |||
| AUD duration (years) | - | 36.3±10.5 | n/a |
| Days since last drink | - | 85.9±103.5 | n/a |
| Lifetime Alcohol Consumption | - | 1257.8±966.4 | n/a |
| Smoker (never/past/current/missing) | 43 / 2 / 1 / 4 | 23 / 18 / 46 / 3 | <.0001 |
| Hepatitis C Virus (+ / − / missing) | 1 / 38 / 11 | 15 / 62 / 13 | 0.005 |
frequency count / mean±SD;
2-group comparisons: t-tests for continuous variables, X2 for categorical variables, bold = signfiicant;
Caucasian / African American / other = Native American, Asian, Islander;
lower score = higher status;
Wechsler Test of Adult Reading
AUD participants were recruited from local outpatient substance abuse treatment programs. Comparison participants comprising the control group were recruited from the local community by referrals and flyers. All participants were screened using the Structured Clinical Interview for DSM-IV or DSM5 (SCID) (First, Spitzer, Gibbon, & Williams, 1998), structured health questionnaires, and a semi-structured timeline follow-back interview to quantify lifetime alcohol consumption (Skinner & Sheu, 1982). Upon initial assessment, participants were excluded if they had a significant history of medical (e.g., epilepsy, stroke, multiple sclerosis, uncontrolled diabetes, or loss of consciousness > 30 minutes), psychiatric (i.e., schizophrenia or bipolar I disorder), or neurological (e.g., neurodegenerative disease) disorders other than alcohol abuse or dependence (DSM-IV) or AUD (DSM5) in the AUD group. Healthy controls were excluded for diagnosis of current major depressive (MDD) or generalized anxiety disorder (GAD); AUD participants were categorized for the presence or absence of these diagnoses. Other exclusionary criteria were recent (i.e., past 3 months) substance dependence other than alcohol in the AUD group or any DSM-IV/DSM5 Axis I disorder or substance dependence in the control group. Socioeconomic status (SES) was derived from the Four-Factor Index of Social Status, which considers education and occupation level and wherein a lower score reflects higher status (Hollingshead, 1975).
Questionnaires
Pittsburgh Sleep Quality Index [PSQI].
This 19-item questionnaire assesses sleep indices over the past month including quality, latency, duration, efficiency, disturbances, use of hypnotics, and daytime dysfunction. A global PSQI score greater than 5 yields a diagnostic sensitivity of 89.6% and specificity of 86.5% in distinguishing good from poor sleepers (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989; also see, Carpenter & Andrykowski, 1998; Knutson, Rathouz, Yan, Liu, & Lauderdale, 2006). A global PSQI score requires completion of all test questions.
Alcohol Use Disorders Identification Test [AUDIT].
The AUDIT was developed by the World Health Organization as a self-report screening test to identify severity of AUD and provides an overall measure of hazardous drinking (Babor, Biddle-Higgins, Saunders, & Monteiro, 2001). Hazardous use, dependence symptoms, and harmful use are the three symptom areas covered by the 10-item scale (Lundin, Hallgren, Balliu, & Forsell, 2015). Total scores range from 0 to 40, and higher scores represent more intense drinking. Indices of internal consistency are generally in the 0.80’s (Allen, Litten, Fertig, & Babor, 1997).
The Beck Depression Inventory [BDI-II].
The BDI is a 21-item, self-report rating inventory that measures characteristic attitudes and symptoms of depression (A.T. Beck, R.A., & G.K., 1996; A. T. Beck, Steer, Ball, & Ranieri, 1996). Each of the 21 items corresponding to a symptom of depression is summed to give a single score with a total score of 0–13 considered minimal, 14–19 mild, 20–28 moderate, and 29–63 indicating severe depression (Storch, Roberti, & Roth, 2004). The BDI-II takes approximately 10 minutes to complete (A. T. Beck et al., 1996) and demonstrates high internal consistency, with alpha coefficients of .86 for psychiatric populations and .81 for non-psychiatric populations (A. T. Beck, Steer, & Carbin, 1988).
Cognitive Composite Scores
Cognitive composite scores matched to date of PSQI administration for each individual were extracted from an in-house laboratory release of 3,033 instances. In-house, laboratory cognitive composite scores were derived from the Frascati criteria as described (Antinori et al., 2007) and updated (Gisslén, Price, & Nilsson, 2011). Composites scores were created by averaging age-, education-, and sex- corrected Z-scores of neuropsychological tests based on 102 well-vetted, cognitively intact, laboratory controls (47 women; 47.9±14.9 years; 50 Caucasian, 25 African American, 27 other race; 15.8±2.4 years educated; 27.1±12.9 SES; 90 right handed). Specifically, each test (listed below) in a composite score was age, education, and sex corrected on the 102 controls to create a Z-score, and then mean Z-scores were computed for each composite, which could be based on 2 to 5 measures, depending on the composite, and included executive functioning (EXF), attention and working memory (ATWM), verbal/visual learning (VVL), and verbal/visual memory (VVM).
Quality of Life (QoL)
Similar methods were used to calculate a “quality of life” composite (Antinori et al., 2007) derived from the SCID global assessment of functioning (GAF), assessed by a calibrated research clinicians; the Short Form 21-item (SF-21) QoL total score (Bozzette, Hays, Berry, Kanouse, & Wu, 1995); and Activities of Daily Living (ADL) – instrumental (Lawton & Brody, 1969).
Statistics
Cognitive composite scores were calculated in R 3.5.1 [htpp://www.r-project.org/] (R Core Team, 2013). All remaining statistics were conducted in JMP® Pro 16.0.0 (SAS Institute Inc., Cary, NC, 1989-2021). Analyses used t-tests for continuous variables or χ2 for nominal variables in considering 2 group (i.e., control vs. AUD) comparisons. Within group correlations used Spearman’s ρ for continuous variables and t-tests for nominal variables. Variance explained was derived from linear mixed (i.e., continuous and nominal) REML (restricted maximum likelihood) models. For all statistics, a Bonferroni corrected value of p=.006 was required for significance (derived from Table 1, which includes 8 demographic variables, i.e., p=.05/8).
RESULTS
With respect to demographic variables (Table 1), the AUD relative to the control group had fewer years of education, lower SES, and lower premorbid IQ (Wechsler Test of Adult Reading, predicted full scale IQ). In addition, those in the AUD group were more likely to smoke and be seropositive for the hepatitis C virus, which can independently affect sleep quality (Ichikawa et al., 2020; Ichikawa et al., 2018).
Average global PSQI scores were 7.9±3.5 in the AUD group (80% with PSQI≥5) relative to 4.4±2.2 in the control group (46% with PSQI≥5) (Table 2). Sex did not discriminate global PSQI scores in either participant group (control t=−.05, p=.96; AUD t=−.48, p=.63). PSQI component scores (Table S1) revealed that the AUD relative to the healthy control group had a longer latency to sleep (component 2; χ2=42.8, p<.0001), greater sleep disturbances (component 5; χ2=24.0, p=.004), and more daytime dysfunction (component 7; χ2=27.4, p=.001). Component scores were also not related to sex in either group (p>.06). AUD participants relative to healthy controls (all p<.0001) had higher AUDIT scores, more BDI depressive symptoms, poorer QoL, and performed worse than healthy controls on cognitive composite scores (EXF, ATWM, VVL, VVM) (Table 2).
Table 2.
Behavioral characteristics of the study groups*
| Control | AUD | p-value^ | |
|---|---|---|---|
| Global PSQI Score | 4.4±2.2 | 7.9±3.5 | <0.0001 |
| Questionairres | |||
| AUDIT | 2.1±1.7 | 18.2±10.3 | <0.0001 |
| BDI | 1.7±3.1 | 8.9±7.8 | <0.0001 |
| Quality of Life | −0.0±0.7 | −2.1±1.9 | <0.0001 |
| Cognitive Composites | |||
| EXF | 0.2±0.8 | −0.9±1.4 | <0.0001 |
| ATWM | 0.1±0.8 | −0.8±1.0 | <0.0001 |
| VVL | 0.1±0.8 | −0.8±0.8 | <0.0001 |
| VVM | 0.1±0.8 | −0.7±0.9 | <0.0001 |
frequency count / mean±SD;
2-group comparison: t-tests for continuous variables, X2 for categorical variables, bold=signfiicant; ^none, past/current, missing; GAD=Generalized Anxiety Disorder; MDD=Major Depressive Disorder; AUDIT=AUD Identification Test; BDI=Beck Depression Index; EXF=Executive Function; ATWM=Attention and Working Memory; VVL=Verbal and Visual Learning; VVM=Verbal and Visual Memory
Relations between demographic, alcohol-related, clinical, questionnaire, QoL, cognitive composite scores, and global PSQI scores are presented in Table 3. In the control group, none of the evaluated variables correlated with the global PSQI score. Within the AUD group, the global PSQI score did not correlate with scores on cognitive composites (EXF r=−.00, p=.99; ATWM r=.01, p=.92; VVL r=.05, p=.65; VVM r=−07, p=.49) or with scores on any of the composite component tests (all p>.10). In AUD, however, a high global PSQI score correlated with a higher BDI score (p=.0001, Figure 1a) and worse QoL (p<.0001, Figure 1b). Together, these 2 variables explained 23.1% of the variance in global PSQI scores in the AUD group in a model driven by the contribution of QoL (p=.0004), which alone explained 22.5% of PSQI score variance.
Table 3.
Global PSQI Score correlations*
| Control | AUD | AUD women | AUD men | |||||
|---|---|---|---|---|---|---|---|---|
| ρ / t | p-value | ρ / t | p-value | ρ / t | p-value | ρ / t | p-value | |
| Demographic Variables | ||||||||
| Age | −0.14 | 0.35 | −0.07 | 0.51 | 0.10 | 0.65 | −0.14 | 0.26 |
| Ethnicity | 1.74 | 0.19 | 1.03 | 0.36 | 0.91 | 0.42 | 0.33 | 0.72 |
| BMI | 0.22 | 0.12 | −0.05 | 0.65 | 0.10 | 0.61 | −0.10 | 0.41 |
| Education | −0.03 | 0.85 | 0.07 | 0.53 | 0.40 | 0.05 | −0.06 | 0.66 |
| SES | 0.25 | 0.08 | −0.16 | 0.14 | −0.50 | 0.01 | −0.02 | 0.90 |
| WTAR IQ | −0.08 | 0.57 | 0.12 | 0.26 | 0.17 | 0.42 | 0.10 | 0.44 |
| Alcohol-Related Variables | ||||||||
| AUD duration | −0.06 | 0.68 | −0.12 | 0.25 | −0.07 | 0.76 | −0.13 | 0.29 |
| Days since last drink | −0.06 | 0.69 | −0.13 | 0.23 | −0.02 | 0.94 | −0.18 | 0.15 |
| Lifetime alcohol | −0.24 | 0.09 | −0.16 | 0.13 | −0.24 | 0.25 | −0.10 | 0.44 |
| Smoking^ | 4.03 | 0.02 | 0.16 | 0.88 | −2.42 | 0.03 | 1.60 | 0.12 |
| HCV^ | 0.60 | 0.44 | 0.39 | 0.70 | −0.10 | 0.93 | 0.70 | 0.49 |
| Clinical Variables | ||||||||
| GAD^ | n/a | 4.20 | 0.0002 | 3.30 | 0.003 | 2.49 | 0.03 | |
| MDD^ | n/a | 2.80 | 0.006 | 1.50 | 0.15 | 2.38 | 0.02 | |
| Questionairres | ||||||||
| AUDIT | −0.12 | 0.40 | 0.27 | 0.01 | 0.62 | 0.001 | 0.11 | 0.39 |
| BDI | 0.14 | 0.33 | 0.39 | 0.0001 | 0.49 | 0.01 | 0.35 | 0.004 |
| Quality of Life | −0.07 | 0.63 | −0.49 | <0.0001 | −0.54 | 0.005 | −0.48 | <0.0001 |
| Cognitive Composites | ||||||||
| EXF | −0.12 | 0.4 | 0.04 | 0.75 | −0.02 | 0.92 | −0.03 | 0.84 |
| ATWM | −0.21 | 0.14 | −0.04 | 0.81 | 0.01 | 0.96 | −0.02 | 0.86 |
| VVL | −0.01 | 0.94 | 0.06 | 0.59 | −0.16 | 0.45 | 0.12 | 0.34 |
| VVM | −0.02 | 0.88 | 0.08 | 0.43 | 0.06 | 0.77 | 0.09 | 0.47 |
Spearman’s ρ for continuous variables, F-test for ethnicity, t-tests for nominal variables (^yes/no); bold=significant; BMI=Body Mass Index; SES=Soceoeconomic Status; HCV=hepatitis C virus; GAD=Generalized Anxiety Disorder; MDD=Major Depressive Disorder; AUDIT=AUD Identification Test; BDI=Beck Depression Inventory; EXF=Executive Function; ATWM=Attention and Working Memory; VVL=Verbal and Visual Learning; VVM=Verbal and Visual Memory
Figure 1.
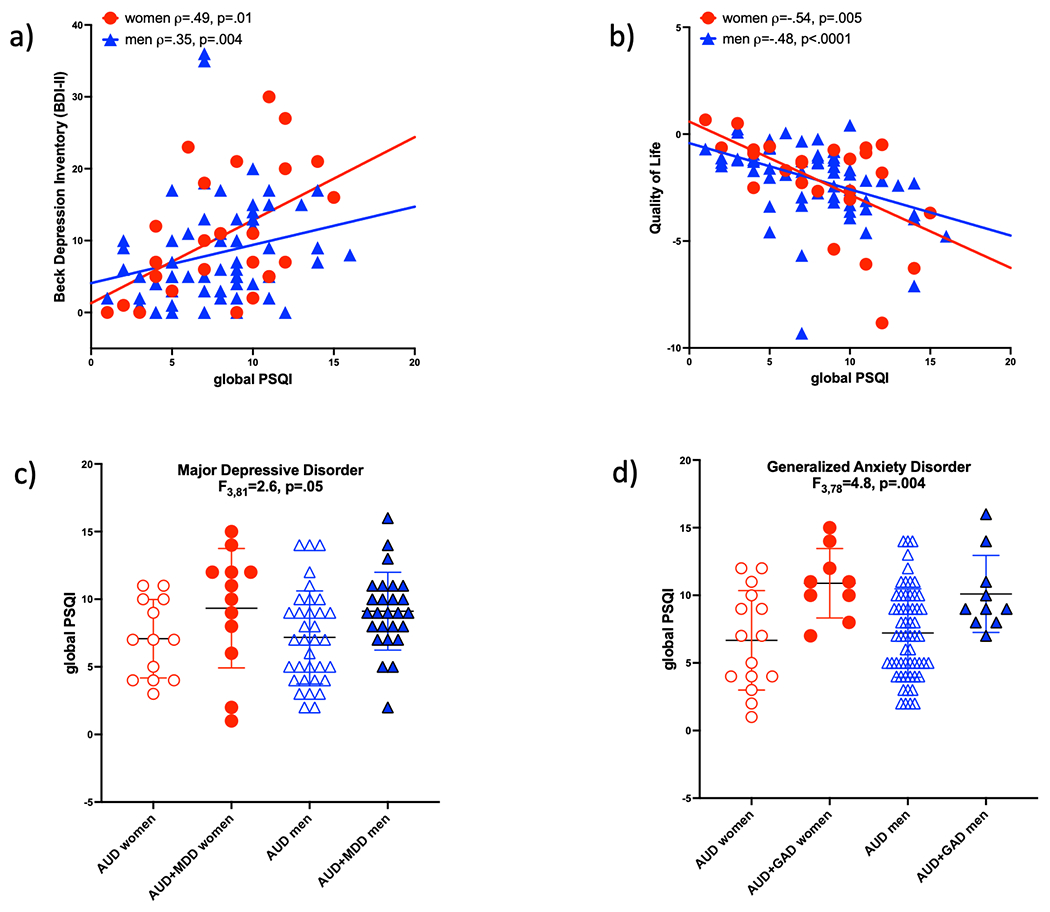
Correlations in the AUD group only between global PSQI scores and BDI-II depressive symptoms (a) and Quality of Life (QoL) indices (b). PSQI scores are higher in AUD men and women with Major Depressive (MDD) (c) or Generalized Anxiety (GAD) (d) disorders than in those without these diagnoses.
As a function of study design (i.e., inclusion in the control group precluded the presence of an MDD and GAD diagnosis), the effects of MDD and GAD on PSQI scores could only be evaluated in the AUD group. In the AUD group, higher PSQI scores were observed in individuals also diagnosed with an MDD (p=.006, Figure 1c) or GAD (p=.0002, Figure 1d). Together with BDI and QoL scores, an MDD or GAD diagnoses added about 7% to the variance in global PSQI score explained in AUD (total variance explained 29.8%, driven by QoL p=.008). In women with AUD, the 4 factors (i.e., BDI and QoL scores, MDD and GAD diagnoses) explained 39.3% of the variance in PSQI scores, driven by an MDD diagnosis (p=04); in AUD men, the 4 measures explained 19.9% of the variance, with a significant contribution from QoL (p=.05).
In the AUD group, PSQI component scores showed similar relational patterns as the global PSQI score (Table S2). Longer latency to sleep (component 2) was related to higher incidence of GAD (χ2=22.5, p<.0001) and impaired QoL (F3,86=3.8, p=.01). Similarly, greater sleep disturbances (component 5) were related to worse QoL (F3,86=3.8, p=.01). Greater daytime dysfunction (component 7), in addition to replicating previously demonstrated relations (i.e., BDI F3,86=5.5, p=.002; QoL F3,86=5.0, p=.003; MDD χ2=10.6, p=.01; GAD χ2=11.8, p=.008), was also associated with alcohol-related variables (i.e., days since last drink [F3,86=4.8, p=.004] and AUDIT scores [F3,86=5.6, p=.002]).
DISCUSSION
The results of the current study support the presence of poor, self-reported sleep in AUD (Hartwell et al., 2015; Kolla et al., 2020) even with upwards of 3 months of sobriety (Cohn et al., 2003; Currie, Clark, Rimac, & Malhotra, 2003; Foster & Peters, 1999; Gann et al., 2001; Neu et al., 2018; Perney & Lehert, 2018). Also comporting with the literature are relations in AUD between poor sleep and the presence of anxiety, depression (Blümel et al., 2012; Kolla et al., 2020; Wallen, Park, Krumlauf, & Brooks, 2019), and reduced quality of life (Cohn et al., 2003; Foster, Marshall, Hooper, & Peters, 1998; Mackenzie, Funderburk, & Allen, 1999; Wallen et al., 2014). Of note, although sex did not discriminate PSQI scores, the variables evaluated herein described nearly 40% of the variance in PSQI scores in AUD women but only about 20% of the variance in AUD men, suggesting that additional factors not here considered are relevant to poor sleep in men with AUD.
The current data contribute to an emerging literature on the impact of sleep disturbances on cognitive functioning over the dynamic course of AUD (Laniepce, Lahbairi, Cabé, Pitel, & Rauchs, 2021). To our knowledge, the extant studies evaluating objective sleep measures (i.e., polysomnography) and cognition in AUD were conducted in residential treatment facilities during early abstinence (Benson et al., 1978; Junghanns et al., 2009; Laniepce et al., 2020). Across those 3 studies, a total of 93 individuals in treatment for AUD were found to have REM or non-REM sleep impairments associated with memory or executive dysfunction (Benson et al., 1978; Junghanns et al., 2009; Laniepce et al., 2020). While objective sleep measures do not necessarily correlate with PSQI scores (Mairesse & Neu, 2016), polysomnography determined sleep efficiency (Brooks, Krumlauf, Whiting, Clark, & Wallen, 2012) and latency (Conroy et al., 2006) were significantly correlated with self-reported indices of sleep in AUD, especially in early recovery (Brooks et al., 2021). Thus, the failure of the current study to identify relations between poor subjective sleep and cognitive dysfunction in AUD may be related to use of the PSQI rather than objective sleep measures or could be related to the longer length of sobriety (i.e., ≥3 months) of the current study participants than those included in previous studies. The only preceding study utilizing the PSQI in AUD that also evaluated cognitive functioning found that inpatient AUD participants with quantifiable executive deficits reported a score below 5 (i.e., no sleep disturbances) on the PSQI (Laniepce et al., 2019), a result suggesting that those in early recovery may be unable to report sleep quality accurately.
Limitations of the current study include the relatively small sample of AUD women, the potential for a lack of generalizability of the enrolled sample, and other cited issues with the PSQI (i.e., lack of correlation with objective sleep measures). For example, women relative to men are more likely to report disturbed sleep although men may have objectively worse sleep (van den Berg et al., 2009). Similarly, QoL measures are subjective and rely on individual expectations and experiences (Longabaugh, Mattson, Connors, & Cooney, 1994; Muldoon, Barger, Flory, & Manuck, 1998).
In summary, this study extends the literature by providing further evidence for relationships among poor sleep, clinical anxiety and depression, and impaired QoL in AUD, but does not support relationships between subjective sleep measures and cognitive impairment in AUD with 3 months of sobriety. These findings suggest that poor sleep persists in later recovery and that sleep, MDD, and GAD may be considered as clinical targets of intervention to improve quality of life in AUD. Future studies utilizing actigraphy, for example, will permit the evaluation of sleep in larger populations in more natural settings (Tracy, Reid, & Baron, 2021) to better understand relations between poor sleep and cognitive dysfunction over the course of AUD.
Supplementary Material
Funding:
This study was supported with grant funding from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) including R01 AA005965 and R01 AA010723.
Footnotes
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of SRI International Protocol ID SRI-40008 2/3/2020, Protocol ID SRI-40045 6/30/20, and Stanford University Protocol ID IRB-26710 1/31/2021, Protocol ID IRB-22487 11/30/2020.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
- Allen JP, Litten RZ, Fertig JB, & Babor T (1997). A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res, 21(4), 613–619. [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69(18), 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor T, Biddle-Higgins J, Saunders J, & Monteiro M (2001). AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Beck AT, R.A. S, & G.K. B (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, Ball R, & Ranieri W (1996). Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess, 67(3), 588–597. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Carbin MG (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–100. [Google Scholar]
- Benson K, Cohen M, & Zarcone V Jr. (1978). REM sleep time and digit span impairment in alcoholics. J Stud Alcohol, 39(9), 1488–1498. [DOI] [PubMed] [Google Scholar]
- Blümel JE, Cano A, Mezones-Holguín E, Barón G, Bencosme A, Benítez Z, et al. (2012). A multinational study of sleep disorders during female mid-life. Maturitas, 72(4), 359–366. [DOI] [PubMed] [Google Scholar]
- Bozzette SA, Hays RD, Berry SH, Kanouse DE, & Wu AW (1995). Derivation and properties of a brief health status assessment instrument for use in HIV disease. J Acquir Immune Defic Syndr Hum Retrovirol, 8(3), 253–265. [DOI] [PubMed] [Google Scholar]
- Brooks AT, Kazmi N, Yang L, Tuason RT, Krumlauf MC, & Wallen GR (2021). Sleep-Related Cognitive/Behavioral Predictors of Sleep Quality and Relapse in Individuals with Alcohol Use Disorder. Int J Behav Med, 28(1), 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AT, Krumlauf MC, Whiting BP, Clark RJ, & Wallen GR (2012). Are you Sleeping? Pilot Comparison of Self-Reported and Objective Measures of Sleep Quality and Duration in an Inpatient Alcoholism Treatment Program. Subst Abuse, 6, 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ (2015). Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol, 49(4), 417–427. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, & Andrykowski MA (1998). Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res, 45(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Chueh KH, Yang MS, Chen CS, & Chiou SM (2009). Poor sleep quality and alcohol use problems among elderly Taiwanese aboriginal women. Int Psychogeriatr, 21(3), 593–599. [DOI] [PubMed] [Google Scholar]
- Cohn TJ, Foster JH, & Peters TJ (2003). Sequential studies of sleep disturbance and quality of life in abstaining alcoholics. Addict Biol, 8(4), 455–462. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Nicholas CL, & Baker FC (2014). Alcohol and the sleeping brain. Handb Clin Neurol, 125, 415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DA, Todd Arnedt J, Brower KJ, Strobbe S, Consens F, Hoffmann R, et al. (2006). Perception of sleep in recovering alcohol-dependent patients with insomnia: relationship with future drinking. Alcohol Clin Exp Res, 30(12), 1992–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SR, Clark S, Rimac S, & Malhotra S (2003). Comprehensive assessment of insomnia in recovering alcoholics using daily sleep diaries and ambulatory monitoring. Alcohol Clin Exp Res, 27(8), 1262–1269. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Criado JR, & Caetano R (2010). Sleep quality and alcohol-use disorders in a select population of young-adult Mexican Americans. J Stud Alcohol Drugs, 71(6), 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills D, & Gilder DA (2018). A history of binge drinking during adolescence is associated with poorer sleep quality in young adult Mexican Americans and American Indians. Psychopharmacology (Berl), 235(6), 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Lau P, & Gilder DA (2017). Sleep Quality in an Adult American Indian Community Sample. J Clin Sleep Med, 13(3), 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B, Scaal S, Hornyak M, Gann H, & Riemann D (2007). Sleep electroencephalographic spectral power after withdrawal from alcohol in alcohol-dependent patients. Alcohol Clin Exp Res, 31(1), 19–27. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1998). Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. New York, NY.: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Foster JH, Marshall EJ, Hooper R, & Peters TJ (1998). Quality of life measures in alcohol dependent subjects and changes with abstinence and continued heavy drinking. Addict Biol, 3(3), 321–332. [DOI] [PubMed] [Google Scholar]
- Foster JH, Marshall EJ, & Peters TJ (2000). Application of a quality of life measure, the life situation survey (LSS), to alcohol-dependent subjects in relapse and remission. Alcohol Clin Exp Res, 24(11), 1687–1692. [PubMed] [Google Scholar]
- Foster JH, & Peters TJ (1999). Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcohol Clin Exp Res, 23(6), 1044–1051. [PubMed] [Google Scholar]
- Gann H, Feige B, Hohagen F, van Calker D, Geiss D, & Dieter R (2001). Sleep and the cholinergic rapid eye movement sleep induction test in patients with primary alcohol dependence. Biol Psychiatry, 50(5), 383–390. [DOI] [PubMed] [Google Scholar]
- Gisslén M, Price RW, & Nilsson S (2011). The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis, 11, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Hu H, Liu Y, Leng Y, Gao X, Cui Q, et al. (2018). Gender differences in the relationship between alcohol consumption and insomnia in the northern Chinese population. PLoS One, 13(12), e0207392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell EE, Bujarski S, Glasner-Edwards S, & Ray LA (2015). The Association of Alcohol Severity and Sleep Quality in Problem Drinkers. Alcohol Alcohol, 50(5), 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A (1975). Four-factor index of social status. New Haven, CT: Department of Sociology, Yale University. [Google Scholar]
- Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Yamashima M, Yamamichi S, et al. (2020). Direct-acting Antivirals Improved the Quality of Life, Ameliorated Disease-related Symptoms, and Augmented Muscle Volume Three Years Later in Patients with Hepatitis C Virus. Intern Med, 59(21), 2653–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Miyaaki H, Miuma S, Taura N, Motoyoshi Y, Akahoshi H, et al. (2018). Hepatitis C virus-related symptoms, but not quality of life, were improved by treatment with direct-acting antivirals. Hepatol Res, 48(3), E232–e239. [DOI] [PubMed] [Google Scholar]
- Jóhannsdóttir KR, Ferretti D, Árnadóttir BS, & Jónsdóttir MK (2021). Objective Measures of Cognitive Performance in Sleep Disorder Research. Sleep Med Clin, 16(4), 575–593. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Horbach R, Ehrenthal D, Blank S, & Backhaus J (2009). Chronic and high alcohol consumption has a negative impact on sleep and sleep-associated consolidation of declarative memory. Alcohol Clin Exp Res, 33(5), 893–897. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Rathouz PJ, Yan LL, Liu K, & Lauderdale DS (2006). Stability of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Questionnaires over 1 year in early middle-aged adults: the CARDIA study. Sleep, 29(11), 1503–1506. [DOI] [PubMed] [Google Scholar]
- Kolla BP, Mansukhani MP, Biernacka J, Chakravorty S, & Karpyak VM (2020). Sleep disturbances in early alcohol recovery: Prevalence and associations with clinical characteristics and severity of alcohol consumption. Drug Alcohol Depend, 206, 107655. [DOI] [PubMed] [Google Scholar]
- Kolla BP, Schneekloth T, Biernacka J, Mansukhani M, Geske J, Karpyak V, et al. (2014). The course of sleep disturbances in early alcohol recovery: an observational cohort study. Am J Addict, 23(1), 21–26. [DOI] [PubMed] [Google Scholar]
- Kolla BP, Schneekloth T, Mansukhani MP, Biernacka JM, Hall-Flavin D, Karpyak V, et al. (2015). The association between sleep disturbances and alcohol relapse: A 12-month observational cohort study. Am J Addict, 24(4), 362–367. [DOI] [PubMed] [Google Scholar]
- Laniepce A, Cabé N, André C, Bertran F, Boudehent C, Lahbairi N, et al. (2020). The effect of alcohol withdrawal syndrome severity on sleep, brain and cognition. Brain Commun, 2(2), fcaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laniepce A, Lahbairi N, Cabé N, Pitel AL, & Rauchs G (2021). Contribution of sleep disturbances to the heterogeneity of cognitive and brain alterations in alcohol use disorder. Sleep Med Rev, 58, 101435. [DOI] [PubMed] [Google Scholar]
- Laniepce A, Segobin S, Lannuzel C, Boudehent C, Ritz L, Urso L, et al. (2019). Neuropsychological and Neuroimaging Examinations of Self-Reported Sleep Quality in Alcohol Use Disorder With and Without Korsakoff’s Syndrome. Alcohol Clin Exp Res, 43(5), 952–964. [DOI] [PubMed] [Google Scholar]
- Lawton MP, & Brody EM (1969). Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist, 9(3), 179–186. [PubMed] [Google Scholar]
- Le Berre AP, Fama R, Sassoon SA, Pfefferbaum A, Sullivan EV, & Zahr NM (2019). Cognitive and Motor Impairment Severity Related to Signs of Subclinical Wernicke’s Encephalopathy in HIV Infection. J Acquir Immune Defic Syndr, 81(3), 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leso V, Fontana L, Caturano A, Vetrani I, Fedele M, & Iavicoli I (2021). Impact of Shift Work and Long Working Hours on Worker Cognitive Functions: Current Evidence and Future Research Needs. Int J Environ Res Public Health, 18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Chen Y, Tang X, & Li CR (2021). Alcohol use severity and the neural correlates of the effects of sleep disturbance on sustained visual attention. J Psychiatr Res, 142, 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Kahathuduwa C, & Vazsonyi AT (2021). The Pittsburgh Sleep Quality Index (PSQI): Psychometric and clinical risk score applications among college students. Psychol Assess, 33(9), 816–826. [DOI] [PubMed] [Google Scholar]
- Longabaugh R, Mattson ME, Connors GJ, & Cooney NL (1994). Quality of life as an outcome variable in alcoholism treatment research. J Stud Alcohol Suppl, 12, 119–129. [DOI] [PubMed] [Google Scholar]
- Lundin A, Hallgren M, Balliu N, & Forsell Y (2015). The use of alcohol use disorders identification test (AUDIT) in detecting alcohol use disorder and risk drinking in the general population: validation of AUDIT using schedules for clinical assessment in neuropsychiatry. Alcohol Clin Exp Res, 39(1), 158–165. [DOI] [PubMed] [Google Scholar]
- Mackenzie A, Funderburk FR, & Allen RP (1999). Sleep, anxiety, and depression in abstinent and drinking alcoholics. Subst Use Misuse, 34(3), 347–361. [DOI] [PubMed] [Google Scholar]
- Mairesse O, & Neu D (2016). Tired of blunt tools? Sharpening the clinical assessment of fatigue and sleepiness. Psychiatry Res, 238, 100–108. [DOI] [PubMed] [Google Scholar]
- Miller MB, Van Reen E, Barker DH, Roane BM, Borsari B, McGeary JE, et al. (2017). The impact of sleep and psychiatric symptoms on alcohol consequences among young adults. Addict Behav, 66, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, & Colantonio A (2016). The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev, 25, 52–73. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Barger SD, Flory JD, & Manuck SB (1998). What are quality of life measurements measuring? Bmj, 316(7130), 542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu P, Sofin Y, & Danker-Hopfe H (2018). The Effect of Detoxification on Sleep: How Does Sleep Quality Change during Qualified Detoxification Treatment? J Addict, 2018, 9492453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Cho MJ, Chang SM, Bae JN, Jeon HJ, Cho SJ, et al. (2010). Relationships of sleep duration with sociodemographic and health-related factors, psychiatric disorders and sleep disturbances in a community sample of Korean adults. J Sleep Res, 19(4), 567–577. [DOI] [PubMed] [Google Scholar]
- Park SY, Oh MK, Lee BS, Kim HG, Lee WJ, Lee JH, et al. (2015). The Effects of Alcohol on Quality of Sleep. Korean J Fam Med, 36(6), 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perney P, & Lehert P (2018). Insomnia in Alcohol-Dependent Patients: Prevalence, Risk Factors and Acamprosate Effect: An Individual Patient Data Meta-Analysis. Alcohol Alcohol, 53(5), 611–618. [DOI] [PubMed] [Google Scholar]
- Perney P, Rigole H, Mason B, Dematteis M, & Lehert P (2015). Measuring sleep disturbances in patients with alcohol use disorders: a short questionnaire suitable for routine practice. J Addict Med, 9(1), 25–30. [DOI] [PubMed] [Google Scholar]
- Peters TJ, Millward LM, & Foster J (2003). Quality of life in alcohol misuse: comparison of men and women. Arch Womens Ment Health, 6(4), 239–243. [DOI] [PubMed] [Google Scholar]
- Phelps C, Bellon S, Hinkey M, Nash A, Boyd J, Cook CE, et al. (2020). Measurement properties of Patient-Reported Outcome Measures used to assess the sleep quality in adults with high prevalence chronic pain conditions: a systematic review. Sleep Med, 74, 315–331. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- Skinner HA, & Sheu WJ (1982). Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol, 43(11), 1157–1170. [DOI] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, & Roth DA (2004). Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory-Second Edition in a sample of college students. Depress Anxiety, 19(3), 187–189. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Sassoon SA, & Pfefferbaum A (2020). Disturbed Sensory Physiology Underlies Poor Balance and Disrupts Activities of Daily Living in Alcohol Use Disorder. Addiction Biology, in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, et al. (2018). The Role of Aging, Drug Dependence, and Hepatitis C Comorbidity in Alcoholism Cortical Compromise. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy EL, Reid KJ, & Baron KG (2021). The relationship between sleep and physical activity: the moderating role of daily alcohol consumption. Sleep, 44(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, & Tiemeier H (2009). Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep, 32(10), 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen GR, Brooks AT, Whiting B, Clark R, Krumlauf MC, Yang L, et al. (2014). The prevalence of sleep disturbance in alcoholics admitted for treatment: a target for chronic disease management. Fam Community Health, 37(4), 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen GR, Park J, Krumlauf M, & Brooks AT (2019). Identification of Distinct Latent Classes Related to Sleep, PTSD, Depression, and Anxiety in Individuals Diagnosed With Severe Alcohol Use Disorder. Behav Sleep Med, 17(4), 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pohl KM, Kwong AJ, Sullivan EV, & Pfefferbaum A (2021). Preliminary Evidence for a Relationship between Elevated Plasma TNFα and Smaller Subcortical White Matter Volume in HCV Infection Irrespective of HIV or AUD Comorbidity. International Journal of Molecular Sciences, submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


