Abstract
Plant intracellular nucleotide-binding leucine-rich repeat receptors (NLRs) detect pathogen effectors to trigger immune responses1. Indirect recognition of a pathogen effector by the dicotyledonous Arabidopsis thaliana coiled-coil domain containing NLR (CNL) ZAR1 induces the formation of a large hetero-oligomeric protein complex, termed the ZAR1 resistosome, which functions as a calcium channel required for ZAR1-mediated immunity2–4. Whether the resistosome and channel activities are conserved among plant CNLs remains unknown. Here we report the cryo-electron microscopy structure of the wheat CNL Sr355 in complex with the effector AvrSr356 of the wheat stem rust pathogen. Direct effector binding to the leucine-rich repeats of Sr35 results in the formation of a pentameric Sr35–AvrSr35 complex, which we term the Sr35 resistosome. Wheat Sr35 and Arabidopsis ZAR1 resistosomes bear striking structural similarities, including an arginine cluster in the leucine-rich repeats domain not previously recognized as conserved, which co-occurs and forms intramolecular interactions with the 'EDVID' motif in the coiled-coil domain. Electrophysiological measurements show that the Sr35 resistosome exhibits non-selective cation channel activity. These structural insights allowed us to generate new variants of closely related wheat and barley orphan NLRs that recognize AvrSr35. Our data support the evolutionary conservation of CNL resistosomes in plants and demonstrate proof of principle for structure-based engineering of NLRs for crop improvement.
Subject terms: Plant immunity, Cryoelectron microscopy, Plant signalling, Innate immunity
Evolutionary conservation of plant receptor structure allowed for generation of new variants of wheat and barley nucleotide-binding leucine-rich repeat receptors (NLRs) that recognize AvrSr35 of the wheat stem rust pathogen, supporting proof of principle for structure-based engineering of NLRs for crop improvement.
Main
Plant nucleotide-binding leucine-rich repeat receptors (NLRs) are intracellular receptors that play a key role in the plant innate immune system by sensing the presence of pathogen effectors delivered inside plant cells during pathogenesis through direct or indirect recognition1,7. Activation of plant NLRs generally leads to an array of immune responses, often linked to rapid host cell death at sites of attempted pathogen infection. Structural and functional homologues of plant NLRs evolved from independent events for intracellular non-self-perception in animal innate immunity and are characterized by their conserved central nucleotide-binding and oligomerization domains (NODs)8. Plant NLRs can be broadly separated into two classes: CNL with an N-terminal coiled-coil domain and TNL with an N-terminal Toll/interleukin 1 receptor (TIR) domain. Among the flowering plants dicots typically possess both receptor classes, whereas monocots, including cereals, encode only CNL receptors9.
Wheat stem rust caused by fungal infection with Puccinia graminis f. sp. tritici (Pgt) threatens global wheat production10, and the emergence of widely virulent Pgt strains, including the Ug99 lineage, has motivated the search for stem rust resistance in wheat germplasm and wild relatives over the past two decades. This resulted in the isolation of 11 phylogenetically related stem rust resistance (Sr) genes that belong to a clade of grass CNLs, all of which confer strain-specific immunity5,11–18 (‘clade I’ CNLs defined in ref. 18). The mildew resistance locus A (MLA) receptors of the wheat sister species barley also belong to this group of grass CNLs and share strain-specific immunity with Sr genes18. Sr35 was first identified in a landrace of the Triticum urartu relative Triticum monococcum (einkorn) and confers immunity to Pgt Ug99 in bread wheat when transferred as a transgene5. However, because Sr35 was absent in the diploid A genome of the wild ancestor of wheat, T. urartu, it was initially absent in hexaploid bread wheat (Triticum aestivum). Sr35 resistance has been linked to the recognition of the Pgt effector AvrSr356, but until now, it has remained inconclusive whether Sr35 receptor-mediated host cell death is driven by direct physical interaction with the AvrSr35 effector6,19.
Cryo-electron microscopy of the Sr35 resistosome
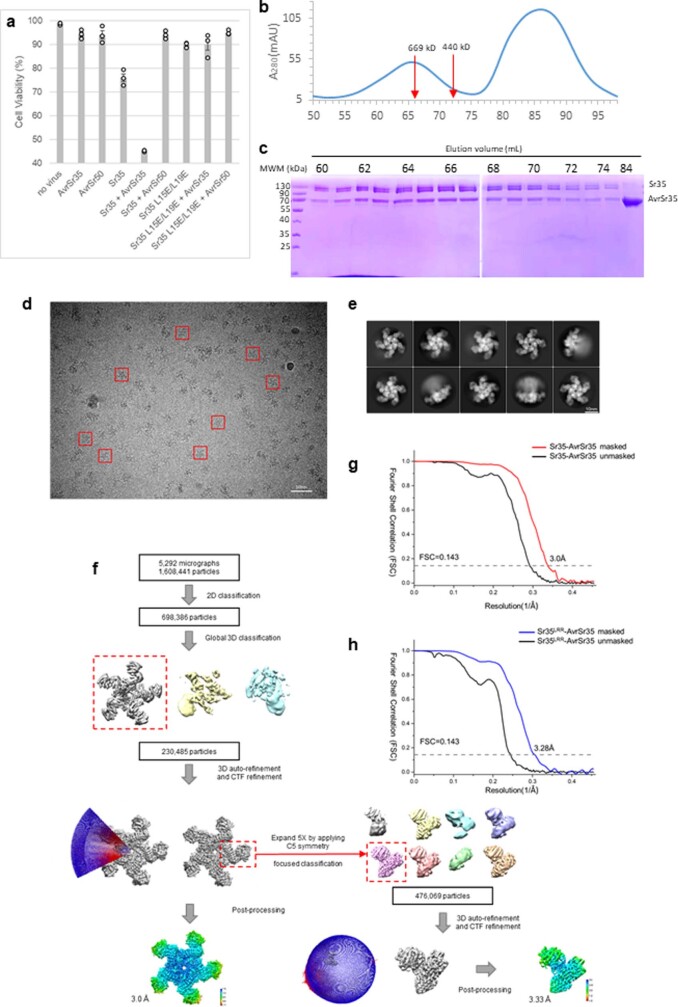
To purify Sr35, we expressed the protein alone or together with AvrSr35 in Sf21 insect cells. Unexpectedly, cell death was observed when the receptor was co-expressed with AvrSr35 (Extended Data Fig. 1a), suggesting that Sr35 and its effector are sufficient to mediate this immunity-associated response in insect cells in the absence of other plant proteins. To circumvent cell death activation for protein purification, we introduced substitutions in the N-terminal residues L15E/L19E (Sr35L15E/L19E), which are predicted to be essential for Sr35 membrane association by analogy with the ZAR1 resistosome3. Mutational analysis of the corresponding N-terminal residues of the tomato CNL NRC4 has been shown to abrogate cell death activity in Nicotiana benthamiana20. Indeed, the Sr35L15E/L19E substitutions markedly reduced Sr35-induced cell death in insect cells (Extended Data Fig. 1a and Supplementary Table 1). Using affinity-tagged Sr35L15E/L19E co-expressed with affinity-tagged AvrSr35, we were able to enrich the Sr35–AvrSr35 complex for subsequent separation of potential receptor complex isoforms and correctly folded receptor complexes by size-exclusion chromatography (Extended Data Fig. 1b,c). The affinity-purified protein complex was eluted with a broad peak with a maximum UV absorbance at 65 ml elution volume exceeding 669 kDa (66 ml) of our largest protein marker. Individual fractions were analysed via negative staining and a large number of star-shaped particles with fivefold symmetry were identified (fractions at 60–69 ml; Fig. 1a). The most monodisperse fractions were pooled and used for cryo-electron microscopy (cryo-EM) analysis.
Extended Data Fig. 1. Sr35-AvrSr35 complex reconstitution in Sf21 insect cells.
a, Cell viability data in Sf21 insect cells. Sr35 constructs carrying amino-terminal 6xHis-Sumo-tag and Avr35 constructs carrying N-terminal GST-tag. Cell viability was determined using trypan blue stain (mean ± SEM; n = 3 technical replicates). Six biological replicates were performed with comparable results. b, Chromatogram of Sr35-AvrSr35 resistosome purification using HiLoad S6 column. Red arrows corresponding to 669 kDa (66 mL) thyroglobulin molecular weight marker, 440 kDa (72 mL) to ferritin. c, SDS–PAGE of individual fractions collected in (b). Numbers represent elution volumes. Molecular weight marker (MWM) on left. d, Representative cryo-EM micrograph of Sr35-AvrSr35 complex. e, Representative 2D class averages of Sr35-AvrSr35 complex. f, Flowchart of cryo-EM data processing and Sr35-AvrSr35 3D reconstruction. g, FSC curves at 0.143 of the final model of Sr35-AvrSr35 complex. h, FSC curves at 0.143 of the final model of Sr35LRR-AvrSr35.
Fig. 1. 3D reconstruction of the Sr35 resistosome.
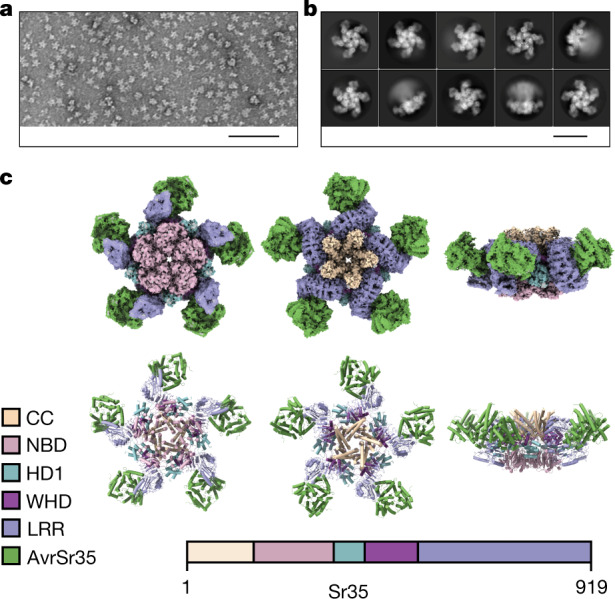
a, Negative staining of purified Sr35 in complex with AvrSr35 (Sr35 resistosome). Star-shaped particles were enriched by affinity purification and size-exclusion chromatography. Monodisperse Sr35 resistosomes have an average size of approximately 24 nm. Scale bar, 100 nm. b, 2D classifications of the Sr35 resistosome particles from the cryo-EM sample. Particles show preferential orientations for bottom and top views. Fewer, but sufficient, particles are in side view. Scale bar, 20 nm. c, Cryo-EM density map with 3 Å resolution (top) and the finally refined structure model (bottom) of the Sr35 resistosome shown in three different orientations. AvrSr35 is coloured green and Sr35 domains are shown according to the colour codes in the inset panel.
We analysed the Sr35–AvrSr35 complex sample by cryo-EM (Extended Data Fig. 1) using a total of 1,608,441 individual particles for reference-free two-dimensional (2D) classification (Fig. 1b). After three-dimensional (3D) classification, a subset of 230,485 particles was used for reconstruction, yielding a density map of 3.0 Å (Fig. 1c, top). Despite the high resolution of up to 2.5 Å in the centre of the complex, the local resolution decreased towards the outer edge to approximately 4 Å (Extended Data Fig. 1f), indicating that the outer region of the complex is more flexible. To compensate for this decreased resolution, a local mask was used for the outer region, yielding a local density map with a resolution of 3.33 Å (Extended Data Fig. 1f). Both density maps were used for model building (Fig. 1c, bottom).
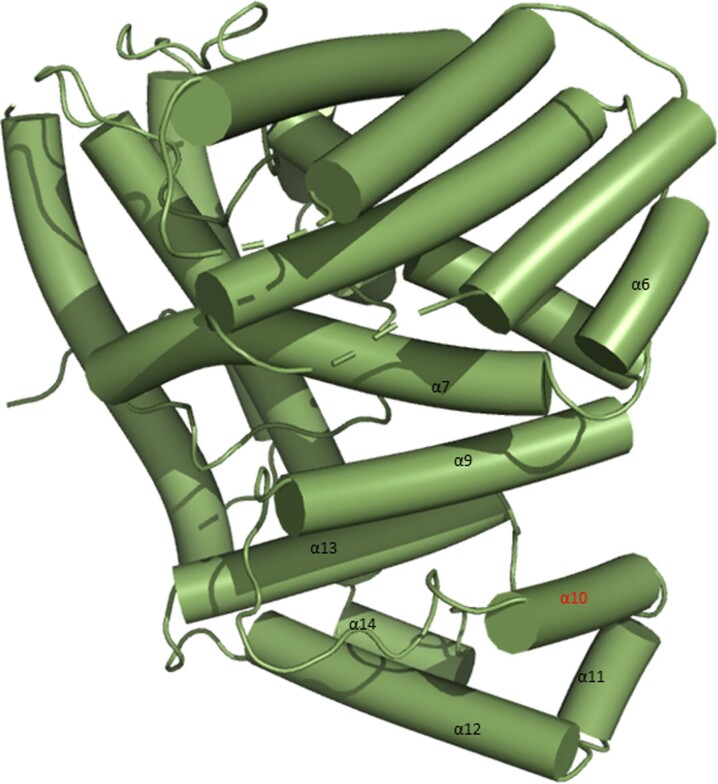
The final 3D reconstruction of the Sr35–AvrSr35 complex contains five receptor protomers, each bound to one effector molecule. The reconstruction revealed a star-shaped structure, similar to the ZAR1 resistosome3, that we termed the Sr35 resistosome. As in the ZAR1 resistosome, five Sr35 NOD modules define the base of the circular protomer arrangement, and a helical barrel formed by the fivecoiled-coil domains is buried at the centre. Unlike ZAR1, the leucine-rich repeat (LRR) domains at the outer region do not pack against each other in the Sr35 resistosome, which might explain why this region is more flexible. AvrSr35 adopts an exclusively helical fold (Extended Data Fig. 2). A 3D structure homology search using the server DALI21 showed that there are no other known protein structures sharing the AvrSr35 fold. Five AvrSr35 proteins bind exclusively to the C-terminal part of the LRR domains in the complex.
Extended Data Fig. 2. AvrSr35 structure from the Sr35 resistosome.
α10-helix (red) is involved in most extensive contacts with Sr35 LRR.
Oligomerization of the Sr35 resistosome
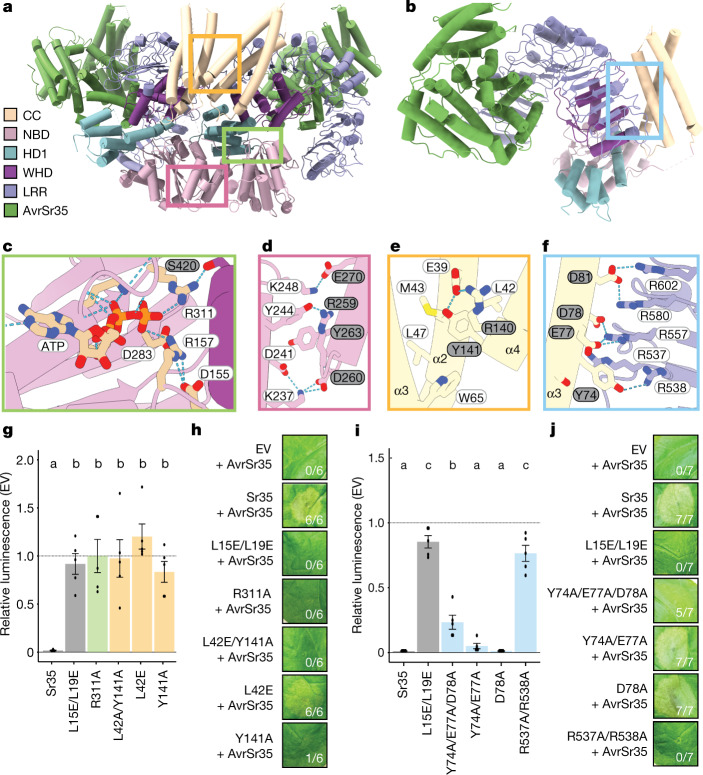
The central NOD module of plant NLRs is subdivided into a nucleotide-binding domain (NBD), helical domain 1 (HD1) and winged helical domain (WHD). ATP/dATP has been shown to be important for ZAR1 oligomerization as it stabilizes the active conformation of ZAR1 via its interaction with the WHD in the NOD module. There is an unambiguous cryo-EM density at the predicted nucleotide-binding site between the HD1 and NBD domains that is unfilled by Sr35 and AvrSr35. An ATP molecule fits well into this cryo-EM density. The modelled ATP is nested in a groove formed by HD1 and NBD. The short α-helix that mediates interprotomer interaction (Fig. 2a,c) also caps the ATP molecule. In contrast to that of ZAR1, the ATP in Sr35 does not directly contact the WHD. Instead, the γ-phosphate group of the ATP forms a bidentate hydrogen bond with Sr35 NBDR157 and NBDR311 (Fig. 2c). The latter also forms a hydrogen bond with Sr35 WHDS420 (Fig. 2c), showing an indirect coupling of the ATP γ-phosphate group with the WHD of Sr35.
Fig. 2. Assembly of the Sr35 resistosome.
a, Sr35 resistosome showing a lateral dimer. Boxes in green, yellow and pink indicate positions of the zoomed views in c–f. Sr35 domains and AvrSr35 coloured according to the inset panel. b, Structure of one Sr35 protomer in complex with AvrSr35. Colour codes as in a. The blue box indicates position of structural detail in f. c, Structural detail of ATP binding in one protomer. Note the specific hydrogen bond of R311 with the γ-phosphate group of ATP at a 2.8 Å distance. Grey and white residue labels correspond to NBD and WHD residues, respectively. d, Structural detail of the interface between NBDs of a lateral dimer. Dashed lines represent polar interactions. Grey and white residue labels correspond to two neighbouring protomers from the pentamer. e, Structural detail of interface between two coiled-coil (CC) protomers. f, Structural detail of coiled-coil and LRR domain intramolecular packing in one Sr35 protomer. Acidic residues in the CCEDIVD form salt-bridges with basic Arg (R) residues of the LRRR-cluster. g, Cotransfection of Sr35 and Sr35 mutants with AvrSr35 in wheat protoplasts. Relative luminescence as readout for cell death. EV treatment defined the relative baseline (mean ± s.e.m.; n = 5). Test statistics derived from analysis of variance (ANOVA) and Tukey post hoc tests (P < 0.05). Exact P values for all protoplast plots are provided in Supplementary Table 3. Bar colours as box colours in c and d. h, Tobacco cell death data of Sr35 and Sr35 mutants with AvrSr35. i, Wheat protoplast data of EDIVD and R-cluster mutants. Experiment and statistics as in g. Bar colours as box colour in f. j, Nicotiana benthamiana cell death data of EDVID and R-cluster mutants. Representative data in h and j shown from seven replicates and scored for leaf cell death.
Similar to the ZAR1 resistosome, NBD–NBD contacts contribute to Sr35 protomer packing (Fig. 2a,d). Sr35 NBDY244 from one protomer packs tightly against Sr35 NBDR259 and Sr35 NBDY263 from an adjacent protomer (Fig. 2d). Additionally, a hydrogen bond is established between Sr35 NBDY244 and Sr35 NBDR259. Of note, the coiled-coil domain of Sr35 contributes considerably to the interprotomer interactions (Fig. 2a): the C-terminal half of α4-helix from one protomer packs against the C-terminal sides of α2- and α4-helices of the neighbouring coiled-coil protomer. At the centre of this interface in the coiled-coil is Tyr141 (CCY141), which makes extensive hydrophobic contacts with Sr35 CCL42, CCM43, CCL47 and CCW65 (Fig. 2e). Moreover, CCY141 participates in a hydrogen bonding triad together with CCR140 from the same and CCE39 from the neighbouring protomer (Fig. 2e). As previously reported22, the long linker region between the coiled-coil and NBD domain is also involved in mediating oligomerization of the Sr35 resistosome.
To functionally test the requirements for these interactions in mediating the assembly of the Sr35 hetero-oligomeric complex, we introduced amino acid substitutions into the receptor and assessed their impact on Sr35-mediated cell death using a luciferase (LUC) activity assay in wheat protoplasts23 prepared from a genotype that does not recognize AvrSr35 (cultivar ‘Chinese Spring’). In this protoplast transfection assay, the relative (to empty vector, EV) luminescence of the LUC reporter is an indicator of cell viability. Cotransfection of Sr35, AvrSr35 and the LUC reporter resulted in a near complete loss of luminescence signal, indicating massive cell death of the protoplasts and suggesting receptor activation by AvrSr35 (Fig. 2g). Consistent with the insect cell data described above, wheat protoplasts co-expressing Sr35L15E/L19E and AvrSr35 displayed luminescence levels that were comparable to those co-expressing EV and AvrSr35 constructs, indicating that the cell death activity of the Sr35L15E/L19E receptor is substantially impaired (Fig. 2g). A similarly drastic loss of receptor-mediated cell death activity was observed with substitutions predicted to affect coiled-coil interprotomer interactions (Y141A, L42E and L42E/Y141A) or the ATP-binding site (R311A) (Fig. 2g) (raw data of all protoplast measurements are provided in Supplementary Table 2).
To corroborate the data from wheat protoplasts, we used Agrobacterium tumefaciens-mediated transient gene expression of Sr35 and AvrSr35 in N. benthamiana leaves. Co-expression of Sr35 and AvrSr35, but not AvrSr35 plus EV, resulted in cell death in the Agrobacterium-infiltrated area (Fig. 2h). By contrast, cell death was abolished when AvrSr35 was co-expressed with the Sr35 mutants predicted to perturb Sr35 oligomerization (Fig. 2h), with the exception of Sr35L42E, which showed residual cell death activity only in N. benthamiana (full versions of all tobacco agroinfiltrations are provided in Supplementary Figs. 1–8). In planta, protein levels of wild-type Sr35 and all receptor mutants tested were comparable, indicating that these substitutions do not render the receptor unstable (Extended Data Fig. 3a, and full versions of all blots are provided in Supplementary Figs. 9–11). Together, these data strongly suggest that the residues mediating Sr35 oligomerization in the cryo-EM structure are necessary for cell death activity in wheat and heterologous N. benthamiana.
Extended Data Fig. 3. Western blot of N. benthamiana experiments.
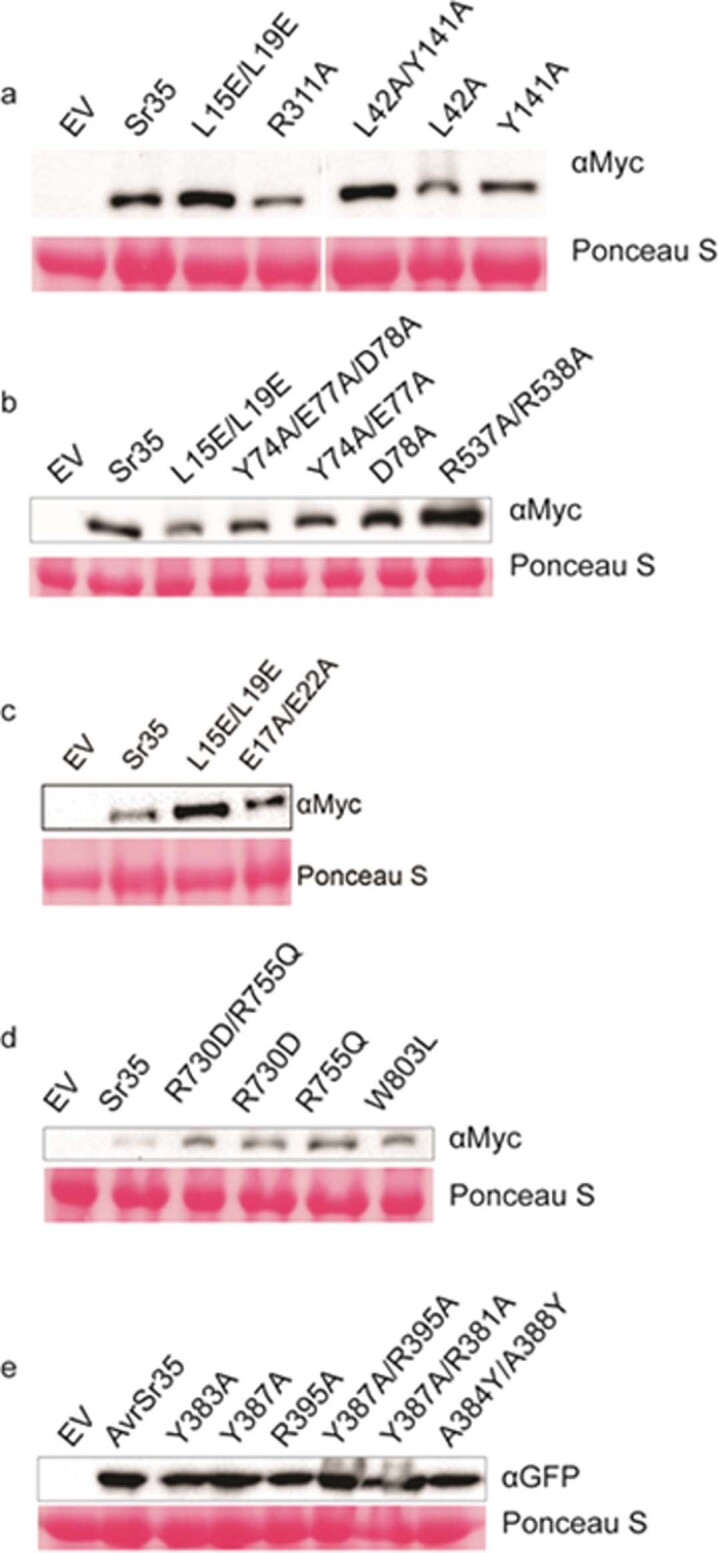
Pooled samples from 3 technical replicates. Ponceau S staining as a loading control. a, Sr35 NBD ATP-binding and coiled-coil protomer interface mutants. Myc-tagged protein. Left and right side merged from the same blot. b, Sr35 EDVID and arginine-cluster mutants. Myc-tagged protein. Last lane cropped from the same blot. c, Sr35 channel mutants. Myc-tagged protein. d, Sr35 LRR mutants. Myc-tagged protein. e, AvrSr35 mutants. YFP-tagged protein detected by GFP antibody.
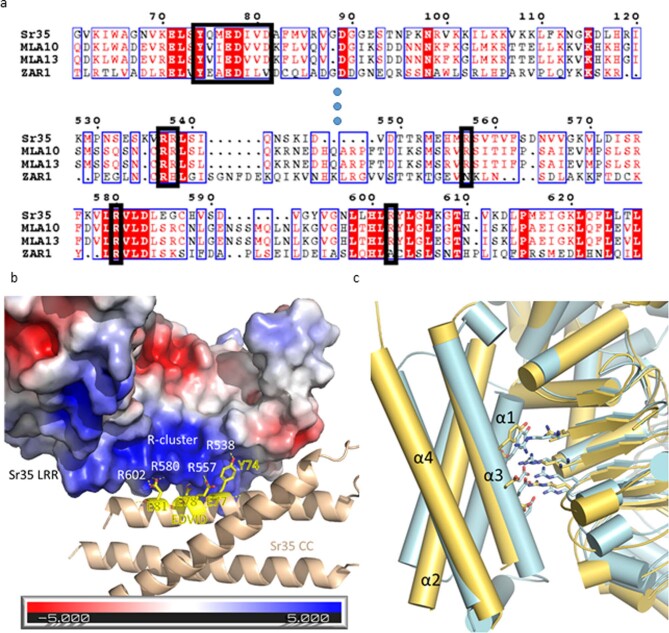
A conserved sequence in the coiled-coil domain, long known as the ‘EDVID (Glu-Asp-Val-Ile-Asp) motif’ that is present in approximately 38% of Arabidopsis CNLs24 and first described in the potato CNL Rx, is used to group CNLs with or without this motif24,25. In the cryo-EM structure of the Sr35 resistosome, the EDIVD motif (Glu-Asp-Ile-Val-Asp) and the adjacent Sr35 Y74 mediate the packing of the LRR domain against the coiled-coil domain. Acidic residues from the motif form strong contacts with five arginine residues in the LRR domain (LRRR537, LRRR538, LRRR557, LRRR580 and LRRR602). These contacts comprise two bidentate salt bonds and a cation–π interaction (Fig. 2b,f). The extensive contacts in this region are further reinforced by hydrogen bonding and van der Waals contacts. These arginine residues are each separated by one iteration of the LRR motif, resulting in their spatial separation along the Sr35 amino acid sequence (Extended Data Fig. 4a). As previously noted26, the cryo-EM structure of the ZAR1 resistosome shows that similar intramolecular interactions exist between arginine residues in the ZAR1 LRR and ‘EDVID’. In both resistosomes the respective arginine residues cluster together and form a positively charged surface patch (Extended Data Fig. 4b). We therefore term this resistosome region LRRR-cluster. Location of the arginine residues in different repeats of the LRR domain explains why the conservation of the LRRR-cluster had remained unnoticed. A sequence alignment of CNLs shows that the LRRR-cluster is conserved and co-occurs with the EDVID motif (Extended Data Fig. 4a).
Extended Data Fig. 4. Details of EDIVD and R-cluster.
a, Multiple protein sequence alignment of HvMLA10, HvMLA13, Sr35 and ZAR1. Amino acids highlighted in red and in red text are identical and possess similar properties, respectively. Alignment of the EDIVD motif and arginine cluster are boxed in black (Robert and Gouet 2014). b, Electrostatic surface charge of Sr35 LRR around the EDVID motif. c, Structural alignment of Sr35 inactive structure prediction (cyan) and one protomer (yellow) from Sr35 resistosome. Detailed view of EDVID and arginine cluster interactions. In analogy to ZAR1, the Sr35 coiled-coil (CC) α1-helix might undergo structural rearrangement, which likely requires EDVID with arginine cluster interactions to transiently resolve.
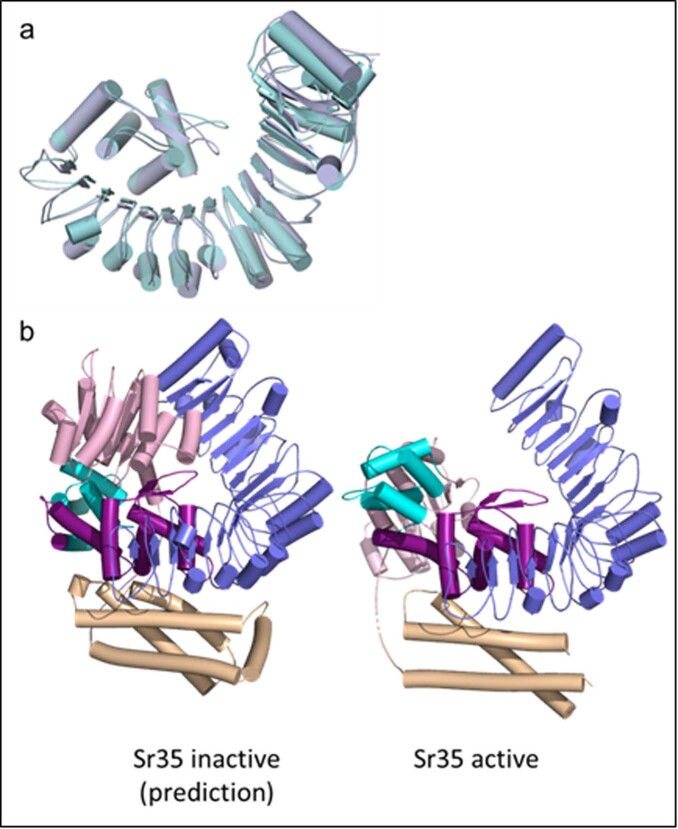
To test whether the LRRR-cluster is necessary for Sr35-mediated cell death, we substituted residues from the interface between the arginine cluster and the EDIVD motif and assessed the impact of these mutations on cell death activity using the wheat protoplast and N. benthamiana leaf assays described above. Simultaneous mutations of LRRR537A/R538A in the LRRR-cluster essentially abolished cell death activity (Fig. 2i,j). Similarly, a triple substitution in the Sr35 EDIVD motif, including the adjacent Sr35 Y74, (Y74A/E77A/D78A) reduced or abolished Sr35 cell death activity in protoplasts and N. benthamiana, without affecting NLR stability (Extended Data Fig. 3b). These observations suggest that the co-occurrence of the EDVID motif and LRRR-cluster is an evolutionarily conserved stabilization mechanism of CNL resistosomes. As the EDVID–LRRR-cluster interactions are also present in the inactive ZAR1 and AlphaFold2-modelled Sr35 monomers and an extensive fold switching occurs in the coiled-coil domain during receptor activation (Extended Data Fig. 4c), these intramolecular interactions may be transiently disrupted to allow α1-helix to flip.
Channel activity of the Sr35 resistosome
Albeit having only 28.4% sequence conservation and although the α1-helix region of Sr35, whose equivalent in ZAR1 resistosome forms a funnel-shaped structure, is not well defined, the structures of the wheat Sr35 and Arabidopsis ZAR1 resistosomes are highly similar (Extended Data Fig. 5). We thus reasoned that the two complexes might share channel activity. To test this conjecture we used an assay previously established in Xenopus laevis (Xenopus) oocytes4 to assess potential channel activity of the Sr35 resistosome. Co-expression of Sr35 and AvrSr35, but not either alone, generated currents as recorded by two-electrode voltage-clamp (Fig. 3a,b), suggesting that assembly of the Sr35 resistosome is required for the currents. In strong support of this conclusion, two Sr35 mutants that impaired the interaction with AvrSr35 and abolished AvrSr35-dependent cell death activity of the receptor in planta (Sr35R730D/R755Q and Sr35W803L/K754G; see below), lost their ability to produce currents in oocytes (Fig. 3c). In agreement with the data on cell death in planta and insect cells, co-expression of the α1-helix substitution mutant Sr35L15E/L19E with AvrSr35 did not mediate currents in oocytes (Fig. 3c). Substitutions affecting the acidic inner lining of the funnel formed by α1-helices in ZAR1E11A have been shown to abolish cell death in planta and channel activities in oocytes3,4. Unexpectedly, both Sr35 resistosome channel and cell death activities were tolerant to these analogous acidic residue substitutions (Sr35E17A/E22A) (Fig. 3c–e and Extended Data Fig. 3c) (raw data of all oocytes measurements are provided in Supplementary Table 4).
Extended Data Fig. 5. CNL resistosome structural conservation.
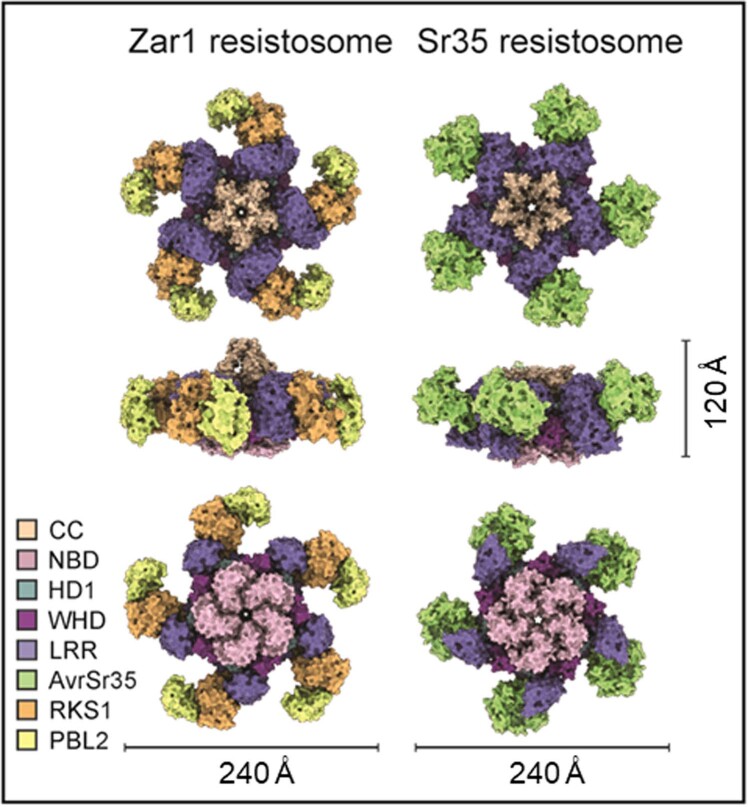
The structures (in surface representation) of the ZAR1 resistosome and the Sr35 resistosome are shown. Zar1 is indirectly activated by the host proteins PBL2 and RKS1. Sr35 is directly activated by the fungal effector AvrSr35. The first, second, and third row show the top, side, and bottom views of these structures, respectively. Domains are coloured according to in-figure legend. Sizes are indicated by scale bar.
Fig. 3. The Sr35 resistosome forms a Ca2+-permeable non-selective cation channel.
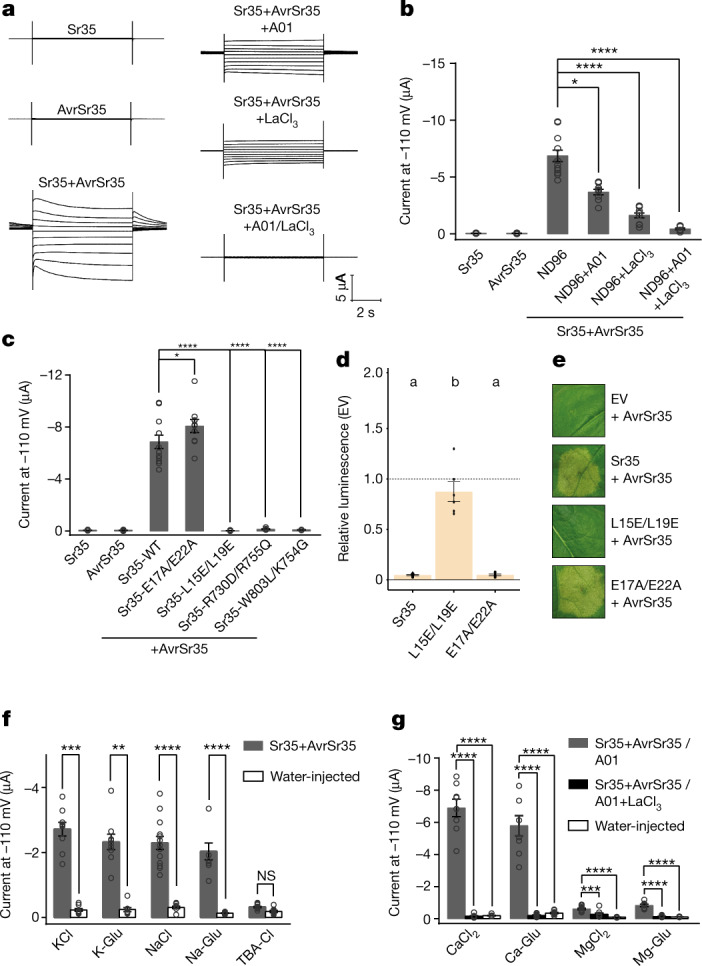
a, Representative measurements from two-electrode (TEVC) recordings from Xenopus oocytes expressing Sr35, AvrSr35 and Sr35/AvrSr35. Effects of CaCCinh-A01 (Ca2+-activated chloride channel inhibitor) and LaCl3 (Ca2+ channel blocker) on the Sr35-mediated currents in ND96 solution (96 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES, pH 7.6). Current traces shown at different voltages from −110 mV to +70 mV in 20 mV increments and current amplitudes at −110 mV. b, Quantitative measurements of data as in a. c, Structure-based mutagenesis of Sr35 residues at the interface between the LRR domain of Sr35 and AvrSr35, and Sr35 α1-helix. TEVC recordings in ND96 solution, and current amplitudes at −110 mV. d, Wheat protoplast data of Sr35 mutations at α1-helix. Relative luminescence as readout for cell death. EV treatment defined the relative baseline (mean ± s.e.m.; n = 5). Test statistics derived from ANOVA and Tukey post hoc tests (P < 0.05). Exact P values are provided in Supplementary Table 3. e, Tobacco cell death data of Sr35 and Sr35 channel mutants. Representative data shown from a minimum of three replicates. f, The Sr35 channel is selective for cations. TEVC recordings performed in various solutions, including KCl (96 mM), K-gluconate (96 mM), NaCl (96 mM), Na-gluconate (96 mM) and TBA-Cl (96 mM). g, Cationic currents of CaCl2, Ca-Glu, MgCl2 and Mg-Glu in the presence of CaCCinh-A01 and CaCCinh-A01+LaCl3. Data are mean ± s.e.m., n ≥ 8 (b,c,f,g). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001, one-way ANOVA analyses and Tukey post hoc test in b, c and g, and two-sided Student’s t-tests in f.
Xenopus oocytes express endogenous calcium-gated chloride channels (CaCC); thus, the currents detected in this assay could be confounded by the activity of these native channels. However, the addition of the CaCC inhibitor A01 only partially blocked the currents in Xenopus oocytes (Fig. 3b) and cotreatment with A01 and the calcium channel blocker LaCl3 was required for complete inhibition of the electrical activity (Fig. 3b). Together, these results indicate that the Sr35 resistosome may contribute to mixed currents in Xenopus oocytes, possibly via Sr35 resistosome calcium channel activity.
To test whether the Sr35 resistosome can function as a non-selective cation channel, we tested cation flux in the presence of monovalent solutions of potassium and sodium chloride salts (KCl, NaCl). Similar to the ZAR1 resistosome, co-expression of Sr35 and AvrSr35 increased cation flux in oocytes, which was retained for potassium and sodium salts with the immobile gluconate counter-ion (K-Glu, Na-Glu). By contrast, we observed only residual ion flux when a chloride salt of the immobile tetrabutylammonium was used (TBA-Cl) (Fig. 3f). A comparison of the divalent ions Ca2+ and Mg2+ (MgCl2, CaCl2) combined with the Sr35 and AvrSr35 co-expression in oocytes, showed that ion flux was significant for calcium but not magnesium (Fig. 3g). This finding supports the conclusion that the Sr35 resistosome is permeable to calcium. Although our collective data strongly suggest that the Sr35 resistosome functions similarly to the ZAR1 resistosome by forming a non-selective calcium channel, the channel activity of the Sr35 resistosome is tolerant to substitutions of acidic residues predicted to line the inner surface of the channel. Thus, we cannot exclude the possibility that the very N terminus of the Sr35 resistosome (residues 1–21) is structurally and functionally distinct from that of the ZAR1 resistosome.
Direct recognition of AvrSr35 by Sr35
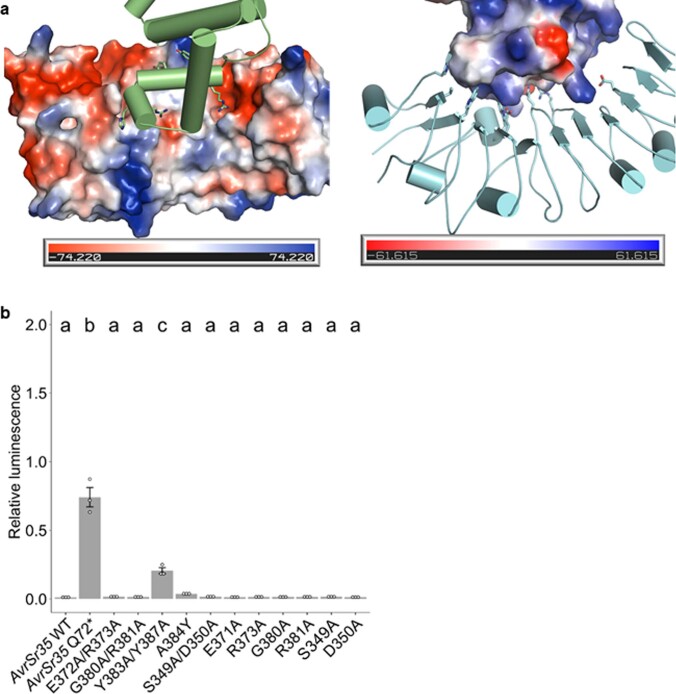
In the cryo-EM structure, AvrSr35 binds to the very C-terminal part of the Sr35 LRR domain, supporting a direct recognition mechanism of AvrSr35 by Sr35 (Fig. 4a). AvrSr35 is much larger than many other pathogen effectors, but only a small portion of the protein is in contact with the Sr35 LRR through charge and shape complementarity (Fig. 4a and Extended Data Fig. 6a,b). Nearly all residues that contribute to recognizing AvrSr35 are from the ascending lateral side of the last eight LRRs, and many of the residues interact with a single helix (α10) of AvrSr35. AvrSr35Y383, AvrSr35A384, AvrSr35Y387 and AvrSr35A388 from one α10 side are located at the centre of the Sr35–AvrSr35 interface and make extensive contacts with their respective neighbouring residues in Sr35 (Fig. 4b). Several residues in the loop region located C-terminal to α10 form hydrophobic contacts with Sr35W919. Similar interactions are also made between AvrSr35R381 in the N-terminal side of α10 and Sr35. In addition to the hydrophobic and van der Waals interactions, a large network of hydrogen bonds also mediates the Sr35–AvrSr35 interface, supporting specific recognition of AvrSr35 by Sr35 (individual contacts provided in Fig. 4b).
Fig. 4. Direct AvrSr35 effector recognition is mediated by the Sr35 LRR domain.
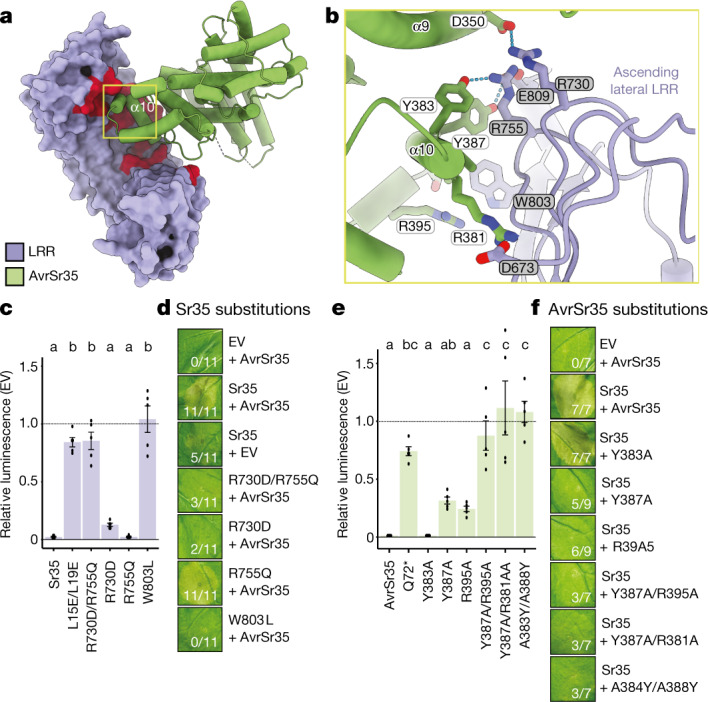
a, Interface between Sr35 LRR and AvrSr35. Red colour indicates the critical LRR residues within 5 Å from AvrSr35. b, Structural detail of Sr35 receptor and AvrSr35 effector interface. Dashed lines represent polar interactions. Grey and white residue label boxes correspond to Sr35 and AvrSr35 sidechains, respectively. c, Cotransfection of Sr35 LRR mutants with AvrS35 in wheat protoplasts. Relative luminescence as readout for cell death. EV treatment defined the relative baseline (mean ± s.e.m.; n = 5). Test statistics derived from ANOVA and Tukey post hoc tests (P < 0.05). Exact P values for all protoplast plots are provided in Supplementary Table 3. Bar colours correspond to box colours in b. d, Nicotiana benthamiana cell death data of Sr35 LRR mutations at the receptor–effector interface. Representative data are shown from 11 replicates and scored for leaf cell death. e, Cotransfection of Sr35 with AvrS35 mutants in wheat protoplasts. Experimental layout and statistics as in c (mean ± s.e.m.; n = 5). Bar colours as domain colours in a. f, Nicotiana benthamiana cell death data of AvrSr35 mutants co-expressed with Sr35. Representative data are shown from nine replicates and scored for leaf cell death.
Extended Data Fig 6. Recognition of AvrSr35 effector by Sr35 LRR domain.
a, Shape and charge complementarity of Sr35 LRR and AvrSr35 at their interface. (Left) AvSr35 shown as cartoon (lime) and Sr35 as electrostatics surface model. (Right) Sr35 LRR shown as cartoon (cyan) and AvrSr35 as electrostatics surface model. b, Wheat protoplast data of AvrSr35 mutants predicted to impair Sr35 recognition. Relative luminescence as readout for cell death. Empty vector treatment defined the relative baseline (mean ± SEM; n = 3). Test statistics derived from ANOVA and Tukey post hoc tests (P <0.05). Exact p values provided in Supplementary Table 3.
To functionally verify the Sr35–AvrSr35 interaction, we first substituted R730, R755 and W803 in Sr35 with their counterparts in the Sr35 homologue of wheat cultivar Chinese Spring27 (here denoted TaSh1), which shares 86.5% sequence identity with Sr35 but is derived from a wheat cultivar susceptible to Pgt strains encoding AvrSr3514. These W803L or R730D substitutions strongly and weakly suppressed Sr35-mediated cell death activity, respectively, when co-expressed with AvrSr35 in wheat protoplasts (Fig. 4c). By contrast, R755Q had no detectable effect on Sr35-induced cell death, but its combination with R730D resulted in a complete loss of cell death in wheat protoplasts (Fig. 4c). Similar results were obtained when these Sr35 mutants were assayed in N. benthamiana (Fig. 4d and Extended Data Fig. 3d). These data support the Sr35–AvrSr35 interaction in the cryo-EM structure and explain why TaSh1 in susceptible cultivar Chinese Spring is unable to recognize AvrSr35. To further verify specific AvrSr35 recognition by Sr35, we made the following substitutions in the fungal effector at their interface: Y383A, Y387A, R395A, Y387A/R395A, Y387A/R381A, A384Y/A388Y, all of which either form hydrogen bonds or salt-bridges with the Sr35 LRR (Fig. 4b). Similar to the Q72* premature stop codon mutant of AvrSr35 (Fig. 4e)6, the mutations Y387A/R395A, Y387A/R381A and A384Y/A388Y abolished Sr35-induced cell death in wheat protoplasts and N. benthamiana (Fig. 4e,f and Extended Data Fig. 3e). By contrast, single mutations of Y387A and R395A only partially abolished effector-triggered receptor activation (Fig. 4e,f and Extended Data Fig. 3e), and several other single mutations of AvrSr35 (Extended Data Fig. 6b) had no effect, suggesting that much of the AvrSr35–Sr35 interface is resilient to disruption by single amino acid substitutions.
Sr35 receptor activation by steric clash
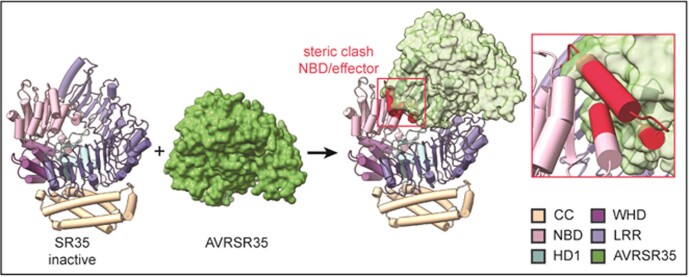
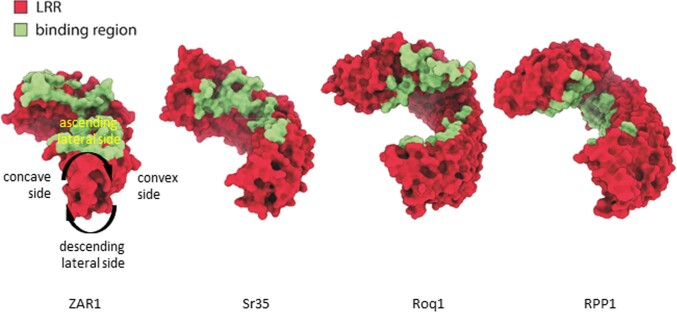
We made structural predictions of inactive, monomeric Sr35 using AlphaFold2 (ref. 28). In these predictions, structures of all individual domains were highly similar to those in the Sr35 resistosome and the LRR domain in particular was accurately predicted (Extended Data Fig. 7a). Although some predictions were a close match with the domain organization of Sr35 in the resistosome, other individual predictions showed striking differences in the domain organization of NOD module (NBD–HD1 relative to WHD) (Extended Data Fig. 7b). These predictions shared the relative domain organization of inactive, monomeric ZAR1 and other inactive NLR structures29, and most likely represent an inactive Sr35 structure. Modelling of AvrSr35 onto the LRR domain of the predicted structure of inactive Sr35 shows substantial overlap between the effector and Sr35 NBD (Extended Data Fig. 8). This is reminiscent of ZAR1 activation, which occurs through an allosteric mechanism involving a ‘steric clash’ with the NBD2,3. Comparison of Sr35 and ZAR1 resistosomes suggests that this ‘steric clash’ mechanism is likely to be conserved in CNLs. AvrSr35 binding dislodges the NBD, allowing subsequent nucleotide exchange for further ATP-triggered allosteric changes in the receptor and assembly of the Sr35 resistosome. Together, these results support a conserved allosteric mechanism underlying activation of the Sr35 and ZAR1 resistosomes. Ligand binding to the ascending lateral side of the LRR domain was also seen in the structures of the TNL RPP1 (ref. 30) and Roq1 (ref. 31) resistosomes (Extended Data Fig. 9), suggesting that the ligand binding mechanism may be conserved in plant NLRs29.
Extended Data Fig. 7. Comparison of the Sr35 prediction (AlphaFold2) with the Sr35 protomer from the cryo-EM structure.
a, Structural alignment of WHD and LRR domains from Sr35 AlphaFold2 prediction (cyan) and from Sr35 resistosome Cryo-EM structure (blue). b, Structural comparison of monomeric Sr35 from prediction (left) and from Cryo-EM structure (right). Substantial differences exist highlighting the structural re-organization within the NOD module (NBD-HD1 relative to WHD). Domain color code: coiled-coil (yellow), NBD (light pink), HD1 (cyan), WHD (purple), and LRR (blue).
Extended Data Fig. 8. Steric clash between AvrSr35 and Sr35 NBD mediates Sr35 receptor activation.
Inactive Sr35 inside the cell comes in contact with Pgt effector AvrSr35. In avoidance of a steric clash (red) between AvrSr35 and the Sr35 NBD domain, the Sr35 NBD domain is forced to structurally rearrange and a ‘primed’ receptor-effector complex is formed. Full activation and oligomerization requires subsequent ADP release, ATP binding and, NOD module rearrangement and coiled-coil (CC) domain structural rearrangement. Sr35 domains and AvrSr35 are coloured according to in-figure legend.
Extended Data Fig. 9. Comparison of ZAR1, Sr35, ROQ1, RPP1 ligand binding sites.
Ligand binding to LRR of CNLs (Zar1, Sr35) and LRR-CJID of TNLs (Roq1, RPP1) occurs in equivalent region in the ascending lateral side of the LRR domain (compare concave, convex, ascending and descending lateral sides defined on Zar1).
Engineering of CNLs for effector recognition specificity
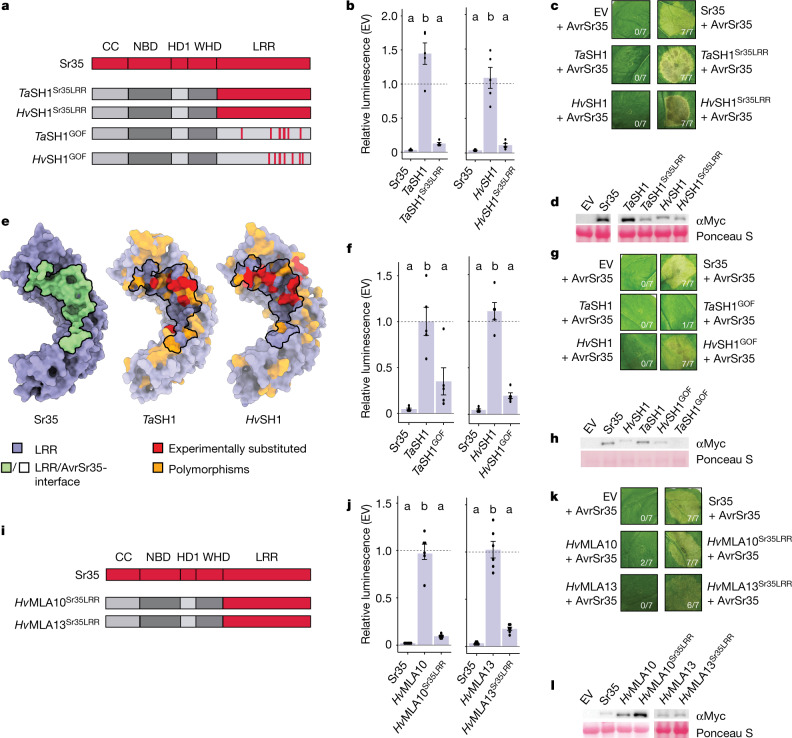
To test whether the evolutionary conservation of CNL resistosomes can be harnessed for the design of new receptors with altered function, we first chose two closely related Sr35 homologues (Sh) of unknown resistance function in bread wheat (Triticumaestivum; TaSh1) and in the sister species barley (Hordeumvulgare; HvSh1). We generated hybrid receptors of TaSh1 and HvSh1 in which the LRR domain, including the highly conserved WHD α4-helix, was substituted by the equivalent fragment of Sr35 (termed TaSh1Sr35LRR and HvShSr35LRR) (Fig. 5a and Extended Data Fig. 10a). Unlike wild-type TaSh1 or HvSh1 genes, both hybrid receptors mediated AvrSr35-dependent cell death in wheat leaf protoplasts prepared from cultivar Chinese Spring and when expressed in leaves of N. benthamiana (Fig. 5b–d), indicating neofunctionalization of the orphan receptors.
Fig. 5. Structure-guided neofunctionalization of orphan CNLs and MLA receptor hybrids.
a, Illustration of Sr35 domain structure and hybrid receptors made from Sr35 homologues (Sh) in bread wheat (Triticum aestivum; TaSh1) and barley (Hordeum vulgare; HvSh1). Sr35LRR (red) substitutes TaSH1LRR and HvSH1LRR (TaSH1Sr35LRR and HvSH1Sr35LRR). GOF receptor variants (TaSh1GOF and HvSh1GOF) were derived from sequence polymorphisms between Sr35, TaSH1 and HvSH1. b, Wheat protoplast transfections of TaSh1Sr35LRR, HvSh1Sr35LRR and controls co-expressed with AvrSr35. EV treatment defined the relative baseline (mean ± s.e.m.; n = 6). Test statistics derived from ANOVA and Tukey post hoc tests (P < 0.05). Exact P values for all protoplast plots are provided in Supplementary Table 3. c, Tobacco cell death of TaSh1Sr35LRR and HvSh1Sr35LRR. Representative data shown from seven replicates and scored for cell death. d, Western blot of hybrid receptors tested in tobacco. Pooled three replicates. Ponceau S staining as loading control. Composite image of same blot. e, Cryo-EM structure of Sr35 and structural predictions of TaSH1LRR and HvSH1LRR (ref. 28). Polymorphisms between TaSH1 or HvSH1, and Sr35 are shown (orange). Residues mutated are shown red. f, Wheat protoplast transfections of TaSh1GOF, HvSh1GOF and controls co-expressed with AvrSr35. Experiment and statistics as in b. g, Tobacco cell death of TaSh1GOF and HvSh1GOF. Replicates and scoring as in c. h, Western blot of GOF experiment in tobacco. Replicates and loading control as in d. i, Graphical illustration of MLA hybrid receptors; design as in a (HvMla10Sr35LRR and HvMla13Sr35LRR). j, Wheat protoplast transfections of HvMla10Sr35LRR, HvMla13Sr35LRR and controls co-expressed with AvrSr35. Experiment and statistics as in b. k, Tobacco cell death of HvMla10Sr35LRR and HvMla13Sr35LRR. Replicates and scoring as in c. l, Western blot of MLA hybrid receptors in tobacco. Replicates and loading control as in d. Composite image of two independent blots.
Extended Data Fig. 10. Rationale for hybrid receptor design.
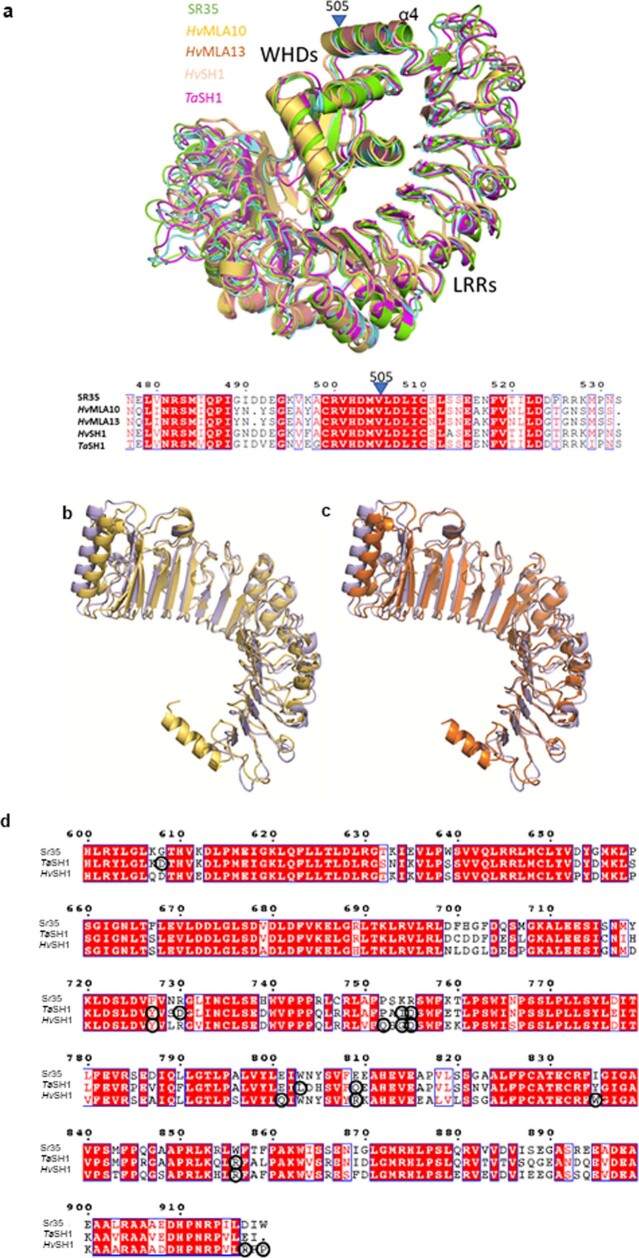
a, Structural (top) and sequence (bottom) alignment of Sr35, HvMLA10, HvMLA13, TaSH1 and HvSH1. Amino acid 505 in the structurally and sequence conserved α4-helix of the WHD of Sr35 was included in hybrid CNL receptors. Structure of Sr35 is isolated from the cryo-EM Sr35 resistosome structure, while HvMLA10, HvMLA13, TaSH1 and HvSH1 were predicted using AlphaFold2. b, Structural alignment of Sr35 LRR (light blue) with structural prediction of TaSh1 (yellow) and c, HvSh1 (orange). d, Multiple protein sequence alignment of Sr35, TaSH1 and HvSH1. Circled amino acids were substituted to corresponding amino acids in the Sr35 sequence for the generation of TaSh1GOF and HvSh1GOF constructs. Amino acids highlighted in red and in red text are identical and possess similar properties, respectively.
Owing to the high sequence similarity of TaSH1 and HvSH1 with T. monococcum Sr35 (86.5% and 86.4% amino acid sequence identity to Sr35, respectively), we reasoned that targeted amino acid substitutions in the LRR domains of the homologues might be sufficient to enable detection of AvrSr35. Combined structural model (Extended Data Fig. 10b,c) and protein sequence alignments indicated that the AvrSr35-interacting residues of Sr35 are polymorphic in TaSH1 and HvSH1 (Fig. 5e and Extended Data Fig. 10d). The alignments identified several residues in the LRR domains of TaSH1 and HvSH1 that are likely to hinder effector binding at the modelled interface. Accordingly, we generated TaSh1 and HvSh1 variants encoding receptors with eight and ten substitutions in the LRR, respectively (TaSH1D609G/Y728F/D731R/I754K/Q755R/L804W/Q810E/R857W and HvSH1Y727F/Q801E/G754K/Q752P/Q755R/R809E/W835I/R856W/917D/P919W; designated for simplicity TaSH1GOF and HvSH1GOF, respectively, in which GOFdenotes gain-of-function) (Fig. 5a). Unlike wild-type HvSh1, HvSh1GOF mediated a clear cell death response in wheat protoplasts and N. benthamiana when co-expressed with the effector AvrSr35 (Fig. 5f–h). TaSh1GOF induced a notable cell death phenotype in wheat protoplasts, but not in N. benthamiana (Fig. 5f–h), which is probably due to undetectable TaSH1GOF protein in the heterologous N. benthamiana expression system (Fig. 5h). These findings suggest that targeted amino acid substitutions that mimic the effector binding region of Sr35 are sufficient for neofunctionalization of these orphan receptors. The relatively small number of nucleotide changes needed to enable TaSH1 to detect AvrSr35 makes it feasible to introduce such changes in elite bread wheat by gene editing. In this way, generating varieties that are resistant to Pgt Ug9932–36 provides an alternative strategy to transgene-mediated Sr35 transfer from T. monococcum to bread wheat5,37.
Next, we investigated whether the Sr35 LRR domain, transferred to more distant CNLs (approximately 45% amino acid sequence identity) in the sister species barley, can generate functional hybrid receptors. We chose barley HvMLA10 and HvMLA13, known to confer isolate-specific immunity against the barley powdery mildew fungus38, Blumeria graminis f. sp. hordei (Bgh), as templates to engineer AvrSr35 recognition (Fig. 5i). The ascomycete Bgh effectors recognized by HvMLA10 and HvMLA13 lack sequence similarity to AvrSr35 from the basidiomycete Pgt. Using the above reasoning for hybrid receptor generation of Sr homologues, the LRR domains of HvMLA10 and HvMLA13 were replaced by the Sr35 LRR. The two resulting hybrid receptors, HvMla10Sr35LRR and HvMla13Sr35LRR, induced cell death when co-expressed with Pgt AvrSr35 in wheat protoplasts and N. benthamiana (Fig. 5j–l). This finding supports our hypothesis that a combination of effector binding to the LRR and steric clash of the effector with the NBD is needed for CNL activation, as exemplified here for hybrid receptors where the AvrSr35 effector is predicted to clash with the MLA NBD.
Discussion
Our results, together with earlier data, strongly suggest that the activation and signalling mechanisms of CNLs are evolutionarily conserved. Three independent lines of evidence support this idea: (1) our structural elucidation of the wheat Sr35 resistosome and its similarity to the previously reported Arabidopsis ZAR1 resistosome structure3; (2) the functional interspecies hybrid receptors generated from the non-orthologous CNLs wheat Sr35 and barley MLAs; and (3) the conservation extends to the non-selective cation flux across membranes enabled by pentamerization, although it is possible that ion selectivity and channel dynamics differ between individual CNLs, including the channel activity of so-called helper NLRs acting downstream of canonical plant NLRs39. Reconstitution of effector-dependent Sr35-triggered cell death in insect cells indicates that regulated channel activity is sufficient to recapitulate plant CNL-mediated cell death in eukaryotic cells of another kingdom. It is possible that plant CNL pore formation and ion flux trigger and intersect with intrinsic cell death pathways in animals, for example, Apaf-1 apoptosome-mediated developmental and stress-induced cell death40,41. Although the components needed for cell death downstream of CNL channel activity in plants remain to be identified, the evolutionary conservation of channel activity rationalizes how highly diverse pathogen signals activate a shared set of downstream responses. This is reminiscent of the highly conserved NADase activity encoded by the TIR domain, which converts the presence or activity of diverse pathogen molecules into TNL-triggered immune signals30,31.
Our study also uncovers the mechanism by which direct or indirect recognition of pathogen effectors results in the formation of the conserved pentameric scaffold that facilitates channel activity. Indirect recognition of a bacterial pathogen effector by ZAR1 results in a conformational change of the NBD, allosterically promoting exchange of ADP with ATP/dATP for full receptor activation. Our data support a similar mechanism for Sr35 activation by direct recognition of AvrSr35. These results lend further support to the notion that the exchange of ADP with ATP/dATP is widely involved in the activation of NLRs. Although AvrSr35 is essential for the activation of Sr35, the effector makes no contribution to oligomerization of the Sr35 resistosome, which is principally mediated by the conserved NBD. This is also true for the assembly of the ZAR1 resistosome in Arabidopsis and the Apaf-1 apoptosome in animals3,42. It seems that recognition of diverse pathogen effectors by the polymorphic LRRs release the conserved NBD to mediate NLR oligomerization.
A third plant NLR recognition mechanism involves a combination of direct and indirect recognition through the incorporation of effector target domains (integrated decoys) into the NLR domain architecture, termed NLR-IDs, representing approximately 10% of all NLR-encoding genes of a plant species43. Crystal structures of the ID in complex with the bound pathogen effector have been resolved for two NLR-IDs, enabling structure-informed ID engineering to extend pathogen strain-specific NLR recognition44–46. How the corresponding full-length NLR-ID receptors are activated, including a potential steric clash with their NBD is unclear owing to a lack of full-length receptor structures. This is further complicated as NLR-IDs, which directly recognize effectors, typically function with canonical NLRs as interacting pairs43. Direct recognition of pathogen effectors by plant NLRs can be rapidly circumvented by polymorphisms of effector residues at the effector–NLR interface, particularly as a pathogen and its host plant typically evolve at different time scales. Virulent isolates of Pgt within and beyond the Ug99 lineage have escaped the recognition of at least one of the recently cloned Sr genes, including single amino acid changes in the effector47. For example, a Pgt isolate with combined virulence against Sr35 and Sr50 caused an epidemic in Sicily in 201748. Our findings allow the prediction not only of AvrSr35 substitutions that might escape Sr35 recognition, but also substitutions in the Sr35 LRR that can physically ‘re-capture’ such effector variants. More generally, the evolutionarily conserved plant CNL resistosome architecture with its conserved function highlights the future potential of structure-guided NLR engineering for crop improvement. Note added in proof: After completion of this work, the Sr35 resistosome structure was confirmed in an independent study49.
Methods
Protein expression, purification and negative staining
Codon optimized Sr35L15E/L19E and AvrSr35 genes were cloned into the pFastBac1 vector (Invitrogen) with an N-terminal 6×His-SUMO tag and an N-terminal glutathione S-transferase (GST) tag, respectively. The constructs were transformed into EMBacY50 competent cells for recombinant bacmid DNA generation. Recombinant baculovirus was generated by initial lipofection with Xtreme gene reagent (Roche) of Sf21 insect cells (Invitrogen). Baculovirus was generally amplified to the P2 generation before protein expression. Sr35L15E/L19E and AvrSr35 were co-expressed in Sf21 insect cells, 50 ml of each virus was used per 1 l of culture. After expression of protein at 28 °C for 48 h, the insect cells were ollected and resuspended with buffer containing 50 mM Tris pH 8.0, 150 mM NaCl, 0.05% Triton X-100 and 5% glycerol. The cell lysates generated by sonication were centrifuged at 13,000 r.p.m. for 1.5 h, and then the supernatant was collected. The protein complex was purified with glutathione Sepharose 4B (GS4B) resin. After binding to the glutathione agarose twice, the agarose was washed with three column volumes of resuspension buffer, and the tagged protein complex was treated with GST-tagged PreScission protease at 4 °C overnight to remove GST and 6xHis-SUMO tags simultaneously. The digested protein complex in the flow-through was concentrated and subjected to HiLoad superpose 6 column (GE) in buffer containing 50 mM Tris pH 8.0, 100 mM NaCl and 0.01% Triton X-100. Pooled peak fractions were used for cryo-EM sample preparation.
Cryo-EM sample preparation and data collection
The Sr35–AvrSr35 complex grids were prepared for cryo-EM analysis. Holy carbon grids (Quantifoil Au 1.2/1.3, 300 mesh) were glow-discharged for 30 s at medium level in HarrickPlasma after 2 min evacuation. The purified Sr35–AvrSr35 protein was concentrated to approximately 0.5 mg ml–1 and 3 µl of sample were applied to the grid. The grids were blotted for 2–3 s using a pair of filter papers (55 mm, Ted Pella Inc.) at 8 °C with 100% humidity and flash-frozen in liquid ethane using a FEI Vitrobot Marked IV. Stacks of Sr35–AvrSr35 cryo-EM samples were collected by a Titan Krios microscope operated at 300 kV, equipped with a K3 Summit direct electron detection camera (Gatan) using EPU 2 (Thermo Fisher Scientific, 2.8.1.10REL) at Zhengzhou University. Micrographs were recorded at 81,000× magnification corresponding to 1.1 Å per pixel. The defocus ranged from −1.5 µm to −2.0 µm. Each image stack contains 32 frames recorded every 0.11 s for an accumulated dose of approximately 50 e− per Å2 and a total exposure time of 3.5 s. A second dataset from an independent protein purification was recorded at EMBL Heidelberg with the following parameters: Titan Krios microscope operated at 300 kV, equipped with a K3 Summit direct electron detection camera (Gatan), 50 e per Å2, 40 frames per stack.
Image processing and 3D reconstruction
All micrographs of the Sr35–AvrSr35 complex were 2 × 2 binned, generating a pixel size of 1.1 Å. The MotionCor2 program was used to perform Motion correction51. Contrast transfer function (CTF) parameters were estimated by CTFFIND452. On the basis of the CTF estimations, 5,292 micrographs were manually picked and were further processed in RELION3.153.
1,608,441 particles were picked using Laplacian-of-Gaussian auto picking and then subjected to several rounds of 2D classification54,55. Every round of 2D classification performed 25 iterations with regularisation parameter T = 2 and number of classes = 100 to remove bad particles. The particles with the best quality were used to generate the initial model using ab initio calculation from RELION3.1. Then 698,386 particles were imported into 3D classification with C1 symmetry. There were five Sr35 molecules in the complex, each of which was bound to one AvrSr35 molecule. C5 symmetry was used in the following 3D refinement. After CTF refinement and postprocessing, the resolution of the Sr35–AvrSr35 complex reconstruction was 3.0 Å. The resolution was estimated by the gold-standard Fourier shell correlation = 0.143 criterion56. Local resolution distribution was evaluated using RELION 3.1 (ref. 57).
In the reconstruction above, the LRR and AvrSr35 portions were more flexible than the other parts of the Sr35–AvrSr35 complex. To improve the density of the more flexible portions, we used a procedure as previously described58. The final refined particles were expanded with C5 symmetry. A local mask was generated using USCF Chimera59. Expanded particles and local mask were subjected to 3D classification without alignment. Finally, 476,069 particles were used for 3D auto-refinement and CTF refinement. A final resolution of 3.33 Å was achieved after postprocessing. For the second dataset, one third of the micrographs were analysed the same way and resulted in the same overall structure at a resolution of 3.4 Å. The resulting model was not used further for model building.
Model building and refinement
The final density map was obtained by merging the global map and the local map which contained LRR and AvrSr35, using a ‘combine_focused_map’ in PHENIX 1.18.2 (ref. 60). The model of the Sr35–AvrSr35 complex was manually built in COOT 0.9 (ref. 61) based on the global and the local maps. The generated model was refined against the combined Sr35–AvrSr35 EM density using real space refinement in PHENIX with secondary structure and geometry restraints61. Model statistics can be found in Extended Data Table 1. USCF Chimera 1.15 and ChimeraX 1.15 were used to visualize models and density maps.
Extended Data Table 1.
Cryo-EM data collection, refinement and validation statistics
Transient gene expression assays in wheat protoplasts
Seedlings of the wheat cultivar. Chinese Spring were grown at 19 °C, 70% humidity and under a 16 h photoperiod. Protoplasts were isolated from the leaves and transfected as previously described23. The coding sequences of TaSh1 (NCBI XP_044359492.1) and HvSh1 (NCBI KAE8803279.1) were generated by gene synthesis based on wild-type codons (GeneArt, Invitrogen). The coding sequence of all tested receptor constructs, or an EV as negative control, were expressed from pIPKb002 vector62 containing the strong ubiquitin promoter. Receptors were co-expressed with AvrSr35 in pIPKb002. In addition, cotransfection of pZmUBQ:LUC63 facilitated the expression of the LUC reporter construct. Each treatment was transfected with 4.5 µg of pZmUBQ:LUC and 5 µg of pIPKb002:AvrSr35. Quantities of receptor-encoding pIPKb002 plasmid were varied for each construct in an effort to minimize cell death due to (receptor) toxicity-mediated cell death (EV 8 µg; Sr35 and Sr35 mutants 2 µg; AvrSr35 and AvrSr35 mutants 5 ug; HvMla10, HvMla13, HvMla10Sr35LRR, HvMla13Sr35LRR, TaSh1, TaSh1, TaSh1GOF, TaSh1GOF 8 µg; TaSh1Sr35LRR and TaSh1Sr35LRR 2 µg). A maximum of two technical replicates were completed with the same batch of wheat seedlings. Luminescence was measured using a luminometer (Centro, LB960). Relative luminescence was calculated by dividing the absolute luminescence value by that of the corresponding EV treatment (EV = 1).
Transient gene expression and western blotting in tobacco
For N. benthamiana transient gene expression, Sr35 and Sr35 mutants, AvrSr35 and AvrSr35 mutants were cloned into the pDONR vector (Invitrogen). The obtained plasmids of Sr35 and Sr35 mutants were recombined by an LR clonase II (Thermo Fisher Scientific) reaction into pGWB517-4×Myc with a C-terminally fused 4×Myc epitope tag64, while AvrSr35 and AvrSr35 mutants were recombined into the pXCSG-mYFP65 vector with a C-terminally fused mYFP epitope tag. After being verified by Sanger sequencing, all the constructs were transformed into Agrobacterium tumefaciens GV3101 pMP90RK by electroporation. Transformants were grown on LB media selection plates containing rifampicin (15 mg ml–1), gentamycin (25 mg ml–1), kanamycin (50 mg ml–1), and spectinomycin (50 mg ml–1) for transformants harbouring pGWB517-4×Myc or carbenicillin (50 mg ml–1) for pXCSG-mYFP.
Individual Agrobacterium transformants were picked and cultured in LB medium containing respective antibiotics in the abovementioned concentration. After shaking culture at 28 °C for 16 h, the culture was harvested at 3,800 r.p.m. for 10 min and resuspended with infiltration buffer containing 10 mM MES pH 5.6, 10 mM MgCl2 and 150 μM acetosyringone. The OD600 of AvrSr35 and AvrSr35 mutant strains was adjusted to 1.0. For Sr35 and Sr35 substitution mutants, the OD600 was adjusted to 0.15. Hybrid receptor bacterial strains (HvMla10Sr35LRR, HvMla13Sr35LRR, TaShSr35LRR, HvShSr35LRR) were adjusted to an OD600 of 0.6. In the hybrid receptor gain-of-function experiment, the OD600 of TaSh1, HvSh1, TaSh1GOF and HvSh1GOF bacterial strains was adjusted to 1.8 without resulting in cell death in co-expression of TaSh1 and HvSh1 when co-expressed with AvrSr35. After dilution, all the cell suspensions were incubated at 28 °C for 1 h at 200 rpm. Construct expression was conducted in leaves of four-week-old N. benthamiana plants via Agrobacterium-mediated transient expression assays. For phenotypic experiments, Agrobacteria cultures expressing receptor constructs, or the respective receptor mutants, were co-infiltrated with AvrSr35, or its mutants, at 1:1 ratio using a syringe. As a control, either receptor or effector bacterial strains were replaced with Agrobacteria transformed with EVs. Phenotypic data were recorded at day 3 after infiltration.
Agrobacterium-mediated transient expression assays for protein detection were conducted as described above. The infiltrated leaves were harvested at 24 h after infiltration, flash-frozen in liquid nitrogen and ground to powder using a Retsch grinder. Plant powder was mixed with 4xLämmli buffer in a 1:2 ratio. Five microlitres was loaded onto 10% SDS–PAGE. After transfer to PVDF membrane, protein was detected using monoclonal mouse anti-MYC (1:3,000; R950-25, Thermofisher), polyclonal rabbit anti-GFP (1:3,000; pabg1, Chromotek), polyclonal goat anti-mouse IgG-HRP (1:7,500; ab6728, Abcam) and polyclonal swine anti-rabbit IgG-HRP (1:5,000; PO399, Agilent DAKO) antibodies. Protein was detected using SuperSignal West Femto:SuperSignal substrates (ThermoFisher Scientific) in a 1:1 ratio.
Electrophysiology
The TEVC recordings were conducted as previously described4. The cDNAs of Sr35, or Sr35 mutants, and AvrSr35 were cloned into the pGHME2 plasmid for expression in Xenopus oocytes. cRNAs for all constructs were transcribed using T7 polymerase. Ovarian lobes were obtained from adult Xenopus laevis under anaesthesia. Both the amount of cRNA injected and the oocyte incubation time were optimized to minimize toxicity caused by the assembled Sr35 resistosome. Isolated oocytes were co-injected with 0.5 ng cRNA of Sr35 (WT and mutants) and AvrSr35. Oocytes were then incubated at 18 °C for approximately 4 h in ND96 buffer (96 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES pH 7.6) supplemented with 10 μg l−1 penicillin and 10 μg l−1 streptomycin. TEVC measurements were performed between 4–7 h later after injection. Water-injected oocytes served as controls.
Two-electrode voltage-clamp recordings were performed using an OC-725C oocyte clamp amplifier (Warner Instruments) and a Digidata 1550 B low-noise data acquisition system with pClamp 10.6 software (Molecular Devices). Data were analysed using OriginPro, 2022 (OriginLab). The microelectrode solutions contained 3 M KCl (electrical resistance of 0.5–1 MΩ), and the bath electrode was a 3 M KCl agar bridge. To eliminate the chloride currents mediated by endogenous Ca2+-activated chloride channels in Xenopus oocytes, the ND96 recording solution was supplemented with 200 µM CaCC inhibitor (CaCCinh)-A01, and the oocytes were pre-incubated 5–10 min before measurement. To test the channel blocking effect of LaCl3, the oocytes were pre-incubated for 5–10 min in the recording solutions supplemented with 200 µM CaCCinh-A01 and 100 µM LaCl3 before measurement. For the recordings in Fig. 3g, the various recording solutions were as follows: KCl (96 mM), K-gluconate (96 mM), NaCl (96 mM), Na-gluconate (96 mM) and TBA-Cl (96 mM). All solutions contained 5 mM HEPES pH 7.6, and 1 mM MgCl2 or Mg-gluconate. For the recordings in Fig. 3h, the various recording solutions were as follows: CaCl2 (12 mM), Ca-gluconate (12 mM), MgCl2 (12 mM) and Mg-gluconate (12 mM). All solutions contain 5 mM HEPES pH 7.6, and 1 mM MgCl2 or Mg-gluconate. The treatments of CaCCinh-A01 and LaCl3 were conducted as above. Voltage-clamp currents were measured in response to voltage steps lasting 7.5 s and to test potentials ranging from −110 mV to +70 mV, in 20 mV increments. Before each voltage step, the membrane was held at 0 mV for 1.60 s, and following each voltage step, the membrane was returned to 0 mV for 2 s. I–V relations for Sr35 resistosome channels were generated from currents that were measured 0.2 s by the end of each test voltage step. Three independent batches of oocytes were investigated and showed consistent findings. Data from one representative oocyte batch are shown.
Statistics and reproducibility
No statistical method was used to predetermine sample size. Sample size was chosen in accordance with the generally accepted standards of the resprective scientific field. Data distribution for each protoplast transfection experiment was subjected to the Shapiro-Wilk normality test. All experiments were found to be normally distributed. An ANOVA and subsequent Tukey post hoc test was completed for each experiment. Treatments found to be significantly different were labelled with different letters (α = 0.05). All statistical output is listed in Supplementary Information.
Purification of the Sr35 resistosome was performed more than 10 times. Pull-down and SDS analysis were highly reproducible between biological replicates and comparable with Extended Data Fig. 1b,c. Negative staining was performed for each protein preparation and showed some variability compared to Fig. 1a, but generally yielded >20% star-shaped particles. Cryo-EM datasets were recorded twice from independent protein preparations (micrograph of one cryo-EM sample preparation shown in Extended Data Fig. 1d) and yielded highly similar cryo-EM density maps.
Insect cell death data were performed with six biological replicates and yielded comparable results to Extended Data Fig. 1a.
Tobacco agroinfiltration data was performed with at least two biological replicates for each substitution mutant and always simultaneously with western blot analysis. Technical replicates of one dataset are shown as raw image data. Western blot samples were always obtained from the same biological replicate as the phenotypic data. Only phenotypic data for which the western blot gave a clear signal are shown.
Ethics declarations
The animal study (Xenopus laevis) was reviewed and approved by the Laboratory Animal Ethics Committee at Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China with the approval ID AP2020029.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-022-05231-w.
Supplementary information
Supplementary Figs.1–11.
Raw data of insect cell viability corresponding to Extended Data Figure 1a.
Raw data for all protoplast measurements presented in the main figures.
Statistics for all protoplast measurements presented in the main figures.
Raw data of the electrophysiology data shown in Fig. 3.
AlphaFold2 structure predictions of Sr35, TaSH1, HvSH1, HvMLA10 and HvMLA13. All structure predictions are presumed to represent inactive conformations.
Acknowledgements
We acknowledge Zhengzhou University and EMBL Heidelberg for their assistance with cryo-EM data acquisition. We thank U. Neumann (Max Planck Institute for Plant Breeding Research, Cologne, MPIPZ) and F. Babatz (CECAD, Cologne) for TEM support, J. Jirschitzka for technical support (MPIPZ) and N. Donnelly (MPIPZ) for manuscript polishing. We thank our funding bodies: the Alexander von Humboldt Foundation (a Humboldt professorship to J. Chai), the Max-Planck-Gesellschaft (P.S.-L. and a Max Planck fellowship to J. Chai), Deutsche Forschungsgemeinschaft SFB-1403-414786233 (J. Chai and P.S.-L.) and Germany’s Excellence Strategy CEPLAS (EXC-2048/1, Project 390686111) (J. Chai and P.S.-L.), iNEXT-Discovery for funding Cryo-EM dataset collection at EMBL Heidelberg (PID 16414 to A.F. and J. Chai), the National Key Research and Development Program of China 2021YFA1300701 (Z.H.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA24020305 to Y.C.) and the National Key Research and Development Program of China (2020YFA0509903 to Y.C.).
Extended data figures and tables
Author contributions
A.F. and J. Chai conceived the study. A.F., Y.C., P.S.-L. and J. Chai conceptualized the study. A.F., Y.C., P.S.-L. and J. Chai acquired funding. A.F., E. Li, A.W.L., Y. D., and J. Chang designed the experiments. A.F. and E. Li performed the biochemistry; and A.F., E.Li and Y.S. acquired the structure. J.W. and X.Z. assisted during structure study. A.F., E. Li, A.W.L. and E. Logemann performed plant functional studies. Y.D. performed electrophysiology study. A.F., E. Li, A.W.L., Y.D., Z.H. and Y.C. analysed the data. A.F., P.S.-L., J. Chai and Y.C. wrote the manuscript with input from all authors.
Peer review
Peer review information
Nature thanks Peter Dodds, Bostjan Kobe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Funding
Open access funding provided by Max Planck Society.
Data availability
The atomic coordinates of the Sr35 resistosome have been deposited in the Protein Data Bank (PDB) with the accession code 7XC2. The EM map for the local mask of Sr35 LRR in complex with AvrSr35 has been deposited in the Electron Microscopy Data Bank (EMDB) with the accession code EMD-33111. Sequences of TaSh1 and HvSh1 are available at NCBI under accession codes XP_044359492.1 and KAE8803279.1, respectively. Source data of tobacco agroinfiltrations, western blots, insect cell viability and wheat protoplast cell death are provided with this manuscript. All plasmids are available from the authors.
Code availability
No custom codes were generated for this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alexander Förderer, Ertong Li, Aaron W. Lawson, Ya-nan Deng
Contributor Information
Yuhang Chen, Email: yuhang.chen@genetics.ac.cn.
Paul Schulze-Lefert, Email: schlef@mpipz.mpg.de.
Jijie Chai, Email: chai@mpipz.mpg.de.
Extended data
are available for this paper at 10.1038/s41586-022-05231-w.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-022-05231-w.
References
- 1.Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 2.Wang JZ, et al. Ligand-triggered allosteric ADP release primes a plant NLR complex. Science. 2019;364:eaav5868. doi: 10.1126/science.aav5868. [DOI] [PubMed] [Google Scholar]
- 3.Wang JZ, et al. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science. 2019;364:eaav5870. doi: 10.1126/science.aav5870. [DOI] [PubMed] [Google Scholar]
- 4.Bi GZ, et al. The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling. Cell. 2021;184:3528–352. doi: 10.1016/j.cell.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Saintenac C, et al. Identification of wheat gene Sr35 that confers resistance to Ug99 stem rust race group. Science. 2013;341:783–786. doi: 10.1126/science.1239022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salcedo A, et al. Variation in the AvrSr35 gene determines Sr35 resistance against wheat stem rust race Ug99. Science. 2017;358:1604–1606. doi: 10.1126/science.aao7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 8.Urbach JM, Ausubel FM. The NBS-LRR architectures of plant R-proteins and metazoan NLRs evolved in independent events. Proc. Natl Acad. Sci. USA. 2017;114:1063–1068. doi: 10.1073/pnas.1619730114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johanndrees, O. et al. Differential EDS1 requirement for cell death activities of plant TIR-domain proteins. Preprint at bioRxiv10.1101/2021.11.29.470438 (2021).
- 10.Singh RP, et al. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu. Rev. Phytopathol. 2011;49:465–481. doi: 10.1146/annurev-phyto-072910-095423. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, et al. Identification and characterization of Sr13, a tetraploid wheat gene that confers resistance to the Ug99 stem rust race group. Proc. Natl Acad. Sci. USA. 2017;114:E9483–E9492. doi: 10.1073/pnas.1706277114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Periyannan S, et al. The gene Sr33, an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99. Science. 2013;341:786–788. doi: 10.1126/science.1239028. [DOI] [PubMed] [Google Scholar]
- 13.Mago R, et al. The wheat Sr50 gene reveals rich diversity at a cereal disease resistance locus. Nat. Plants. 2015;1:15186. doi: 10.1038/nplants.2015.186. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Zhang W, Bolus S, Rouse MN, Dubcovsky J. Identification and characterization of wheat stem rust resistance gene Sr21 effective against the Ug99 race group at high temperature. PLoS Genet. 2018;14:e1007287. doi: 10.1371/journal.pgen.1007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steuernagel B, et al. Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 2016;34:652–655. doi: 10.1038/nbt.3543. [DOI] [PubMed] [Google Scholar]
- 16.Arora S, et al. Resistance gene cloning from a wild crop relative by sequence capture and association genetics. Nat. Biotechnol. 2019;37:139–143. doi: 10.1038/s41587-018-0007-9. [DOI] [PubMed] [Google Scholar]
- 17.Gaurav K, et al. Population genomic analysis of Aegilops tauschii identifies targets for bread wheat improvement. Nat. Biotechnol. 2022;40:422–431. doi: 10.1038/s41587-021-01058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, et al. A recombined Sr26 and Sr61 disease resistance gene stack in wheat encodes unrelated NLR genes. Nat. Commun. 2021;12:3378. doi: 10.1038/s41467-021-23738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolus S, Akhunov E, Coaker G, Dubcovsky J. Dissection of cell death induction by wheat stem rust resistance protein Sr35 and its matching effector AvrSr35. Mol. Plant Microbe Interact. 2020;33:308–319. doi: 10.1094/MPMI-08-19-0216-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi H, et al. An N-terminal motif in NLR immune receptors is functionally conserved across distantly related plant species. eLife. 2019;8:e49956. doi: 10.7554/eLife.49956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holm, L. in Structural Bioinformatics: Methods and Protocols (ed. Zoltán Gáspári) 29–42 (Springer US, 2020).
- 22.Xiong Y, Han Z, Chai J. Resistosome and inflammasome: platforms mediating innate immunity. Curr. Opin. Plant Biol. 2020;56:47–55. doi: 10.1016/j.pbi.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Saur IML, Bauer S, Lu X, Schulze-Lefert P. A cell death assay in barley and wheat protoplasts for identification and validation of matching pathogen AVR effector and plant NLR immune receptors. Plant Methods. 2019;15:118. doi: 10.1186/s13007-019-0502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wróblewski T, et al. Genome-wide functional analyses of plant coiled–coil NLR-type pathogen receptors reveal essential roles of their N-terminal domain in oligomerization, networking, and immunity. PLoS Biol. 2018;16:e2005821. doi: 10.1371/journal.pbio.2005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rairdan GJ, et al. The coiled-coil and nucleotide binding domains of the potato Rx disease resistance protein function in pathogen recognition and signaling. Plant Cell. 2008;20:739–751. doi: 10.1105/tpc.107.056036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burdett H, et al. The plant 'resistosome': structural insights into immune signaling. Cell Host Microbe. 2019;26:193–201. doi: 10.1016/j.chom.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Jia H, et al. A semi-dominant NLR allele causes whole-seedling necrosis in wheat. Plant Physiol. 2021;186:483–496. doi: 10.1093/plphys/kiab058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Förderer A, Yu D, Li E, Chai J. Resistosomes at the interface of pathogens and plants. Curr. Opin. Plant Biol. 2022;67:102212. doi: 10.1016/j.pbi.2022.102212. [DOI] [PubMed] [Google Scholar]
- 30.Ma S, et al. Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science. 2020;370:eabe3069. doi: 10.1126/science.abe3069. [DOI] [PubMed] [Google Scholar]
- 31.Martin R, et al. Structure of the activated ROQ1 resistosome directly recognizing the pathogen effector XopQ. Science. 2020;370:eabd9993. doi: 10.1126/science.abd9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishida, Y., Tsunashima, M., Hiei, Y. & Komari, T. in Agrobacterium Protocols: Volume 1 (ed. Kan Wang) 189–198 (Springer, 2015).
- 33.Lowe K, et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell. 2016;28:1998–2015. doi: 10.1105/tpc.16.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson T, Thistleton J, Higgins TJ, Howitt C, Ayliffe M. Efficient Agrobacterium transformation of elite wheat germplasm without selection. Plant Cell Tiss. Org. 2014;119:647–659. doi: 10.1007/s11240-014-0564-7. [DOI] [Google Scholar]
- 35.Sánchez-León S, et al. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018;16:902–910. doi: 10.1111/pbi.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jouanin, A. et al. CRISPR/Cas9 gene editing of gluten in wheat to reduce gluten content and exposure—reviewing methods to screen for coeliac safety. Front. Nutr.10.3389/fnut.2020.00051 (2020). [DOI] [PMC free article] [PubMed]
- 37.Luo M, et al. A five-transgene cassette confers broad-spectrum resistance to a fungal rust pathogen in wheat. Nat. Biotechnol. 2021;39:561–566. doi: 10.1038/s41587-020-00770-x. [DOI] [PubMed] [Google Scholar]
- 38.Lu X, et al. Allelic barley MLA immune receptors recognize sequence-unrelated avirulence effectors of the powdery mildew pathogen. Proc. Natl Acad. Sci. USA. 2016;113:E6486–E6495. doi: 10.1073/pnas.1612947113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob P, et al. Plant 'helper' immune receptors are Ca2+-permeable nonselective cation channels. Science. 2021;373:420–425. doi: 10.1126/science.abg7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chai J, Shi Y. Apoptosome and inflammasome: conserved machineries for caspase activation. Natl Sci. Rev. 2014;1:101–118. doi: 10.1093/nsr/nwt025. [DOI] [Google Scholar]
- 41.Cerella C, Diederich M, Ghibelli L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int. J. Cell Biol. 2010;2010:546163–546163. doi: 10.1155/2010/546163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou M, et al. Atomic structure of the apoptosome: mechanism of cytochrome c-and dATP-mediated activation of Apaf-1. Genes Dev. 2015;29:2349–2361. doi: 10.1101/gad.272278.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cesari S. Multiple strategies for pathogen perception by plant immune receptors. New Phytol. 2018;219:17–24. doi: 10.1111/nph.14877. [DOI] [PubMed] [Google Scholar]
- 44.Cesari, S. et al. New recognition specificity in a plant immune receptor by molecular engineering of its integrated domain. Nat. Commun.13, 1524 (2022). [DOI] [PMC free article] [PubMed]
- 45.De la Concepcion JC, et al. Protein engineering expands the effector recognition profile of a rice NLR immune receptor. eLife. 2019;8:e47713. doi: 10.7554/eLife.47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, et al. A designer rice NLR immune receptor confers resistance to the rice blast fungus carrying noncorresponding avirulence effectors. Proc. Natl Acad. Sci. USA. 2021;118:e2110751118. doi: 10.1073/pnas.2110751118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortiz D, et al. The stem rust effector protein AvrSr50 escapes Sr50 recognition by a substitution in a single surface-exposed residue. New Phytol. 2022;234:592–606. doi: 10.1111/nph.18011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattacharya S. Deadly new wheat disease threatens Europe’s crops. Nature. 2017;542:145–146. doi: 10.1038/nature.2017.21424. [DOI] [PubMed] [Google Scholar]
- 49.Zhao, Y.-B. et al. Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism. Sci. Adv.8, eabq5108 (2022). [DOI] [PMC free article] [PubMed]
- 50.Trowitzsch S, Bieniossek C, Nie Y, Garzoni F, Berger I. New baculovirus expression tools for recombinant protein complex production. J. Struct. Biol. 2010;172:45–54. doi: 10.1016/j.jsb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 2003;142:334–347. doi: 10.1016/S1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 53.Scheres SHW. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheres SHW. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 2012;415:406–418. doi: 10.1016/j.jmb.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheres, S. H. W. in Methods in Enzymology Vol. 579 (ed. R. A. Crowther) 125–157 (Academic Press, 2016).
- 56.Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Kucukelbir A, Sigworth FJ, Tagare HD. Quantifying the local resolution of cryo-EM density maps. Nat. Methods. 2014;11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai X-c, Rajendra E, Yang G, Shi Y, Scheres SHW. Sampling the conformational space of the catalytic subunit of human γ-secretase. eLife. 2015;4:e11182. doi: 10.7554/eLife.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pettersen EF, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 60.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Himmelbach A, et al. A set of modular binary vectors for transformation of cereals. Plant Physiol. 2007;145:1192–1200. doi: 10.1104/pp.107.111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Müller J, et al. Conserved ERAD-like quality control of a plant polytopic membrane protein. Plant Cell. 2005;17:149–163. doi: 10.1105/tpc.104.026625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakagawa T, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- 65.Wiermer, M. Molecular and spatial characterisation of Arabidopsis EDS1 defence regulatory complexes. Inaugural dissertation, Universität zu Köln (2005).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs.1–11.
Raw data of insect cell viability corresponding to Extended Data Figure 1a.
Raw data for all protoplast measurements presented in the main figures.
Statistics for all protoplast measurements presented in the main figures.
Raw data of the electrophysiology data shown in Fig. 3.
AlphaFold2 structure predictions of Sr35, TaSH1, HvSH1, HvMLA10 and HvMLA13. All structure predictions are presumed to represent inactive conformations.
Data Availability Statement
The atomic coordinates of the Sr35 resistosome have been deposited in the Protein Data Bank (PDB) with the accession code 7XC2. The EM map for the local mask of Sr35 LRR in complex with AvrSr35 has been deposited in the Electron Microscopy Data Bank (EMDB) with the accession code EMD-33111. Sequences of TaSh1 and HvSh1 are available at NCBI under accession codes XP_044359492.1 and KAE8803279.1, respectively. Source data of tobacco agroinfiltrations, western blots, insect cell viability and wheat protoplast cell death are provided with this manuscript. All plasmids are available from the authors.
No custom codes were generated for this study.