Abstract
Objectives
Few studies have explored correlations between metabolic syndrome (MetS) alterations and renal deterioration in longitudinal cohorts. We aim to investigate associations between MetS recovery/development and rapid estimated glomerular filtration rate (eGFR) decline in the China Health and Retirement Longitudinal Study (CHARLS).
Design
Longitudinal cohort study.
Setting
This study is a secondary analysis of CHARLS.
Participants
After excluding individuals with age <45 years old, eGFR <60 mL/min/1.73 m2 and clinician-reported malignant tumour, heart disease, stroke or kidney disease at baseline, 4142 participants with complete data were selected from the CHARLS during the 4-year follow-up period (2011–2015).
Outcome measures
MetS were measured at 2011 and 2015 in CHARLS. A rapid eGFR decline was defined as an average annual eGFR decline of >3 mL/min/1.73 m2. The associations between rapid eGFR decline and MetS recovery/development were analysed using multivariable adjusted logistic models.
Results
According to MetS baseline status and follow-up, participants were divided into four groups: (1) 2460 (59.4%) in the MetS-free group, (2) 361 (8.7%) in the MetS-developed group, (3) 499 (12.0%) in the MetS recovery group and (4) 822 (19.8%) in the MetS chronic group. When compared with the MetS chronic group, the multivariable adjusted OR of rapid eGFR decline in the MetS recovery group was 0.64 (OR: 0.64; 95% CI 0.45 to 0.90, p=0.01). In contrast, when compared with the MetS-free group, the multivariable adjusted OR of rapid eGFR decline in the MetS-developed group was 1.00 (OR: 1.00; 95% CI 0.73 to 1.38, p=0.98).
Conclusions
Over the 4-year follow-up period, we found that MetS recovery was associated with a reduced risk of rapid eGFR decline in middle-aged and older adults, while MetS occurrence was not related to rapid eGFR decline. Recovery from MetS appeared to protect against a rapid decline in eGFR.
Keywords: Chronic renal failure, Lipid disorders, PUBLIC HEALTH
Strengths and limitations of this study.
A high-quality data from a nationally representative longitudinal cohort was applied to confirm the association between altered metabolic syndrome status and rapid glomerular filtration rate decline.
The metabolic syndrome scores calculated by principal component analysis was applied for model calibration in the study.
Some participants were missing during the follow-up, which biased the results of the study.
Blood tests related to metabolic syndrome and serum creatinine were performed only once, resulting in data inaccuracy.
The unavailability of urine tests and kidney imaging prevented the analysis of the association between metabolic syndrome status and chronic kidney disease.
Introduction
Metabolic syndrome (MetS) is a cluster of clinical characteristics related to abdominal obesity, dyslipidaemia, elevated blood glucose (BG) and elevated blood pressure (BP).1–3 As of 2017, there were approximately 1 billion individuals with MetS around the world, of which China accounted for 21.7%.4 In China, MetS prevalence has been undergoing a steady increase, concomitant with an increasingly aged population, an obesity epidemic and increased diabetes and hypertension levels, which collectively pose a considerable threat to people’s health and impose a heavy burden on healthcare systems.4–6
While investigations of causality relationships between MetS and cardiovascular events have gained considerable traction in recent years,7–9 MetS also impacts the kidneys. It is accepted that the pathological mechanisms underpinning MetS mainly include insulin resistance, increased oxidative stress and a chronic inflammatory state, which may lead to kidney degeneration and chronic kidney disease (CKD) development.5 6 Previously, it was confirmed that MetS and associated components (abdominal obesity, elevated BG, elevated BP and lipid metabolic disorder) are strongly related to CKD and a decreased estimated glomerular filtration rate (eGFR).10–14 Several longitudinal studies reported that MetS and its components were associated with incremental rapid eGFR decline and CKD incidence.15–18 However, these studies failed to articulate the relationship between MetS alterations and renal function changes. This dearth of information on this subject warrants further study, especially within a Chinese population context.
The China Health and Retirement Longitudinal Study (CHARLS) is a prospective cohort study conducted by the National School of Development, Peking University, China.19 The nationwide sample assesses the social, behavioural and health status of individuals aged 45 years and older.19 The CHARLS baseline survey was implemented in 2011 (wave 1), and the samples were followed up every 2 years. Blood samples from populations were only collected in 2011 (wave 1) and 2015 (wave 3). In the current study, we explored the relationship between MetS recovery/occurrence and rapid eGFR decline in middle-aged and older populations in the 4-year follow-up cohort.
Methods
Study population
CHARLS is a nationally representative longitudinal survey on the social, economic and health status of Chinese citizens aged ≥45 years and their spouses in the community.19 In total, 17 708 participants were registered at baseline (wave 1 at 2011), of which 11 847 had blood sample tests.
In this study, our exclusion criteria excluded participants with the following: (1) missing values; (2) without fasting blood values; (3) baseline eGFR <60 mL/min/1.73 m2; (4) clinician-reported malignant tumour, heart disease, stroke or kidney disease; (5) <45 years old; and (6) no follow-up records and related blood examinations in wave 3 at 2015. After applying these criteria, 4142 participants were finally included. The participant screening process is outlined (figure 1).
Figure 1.
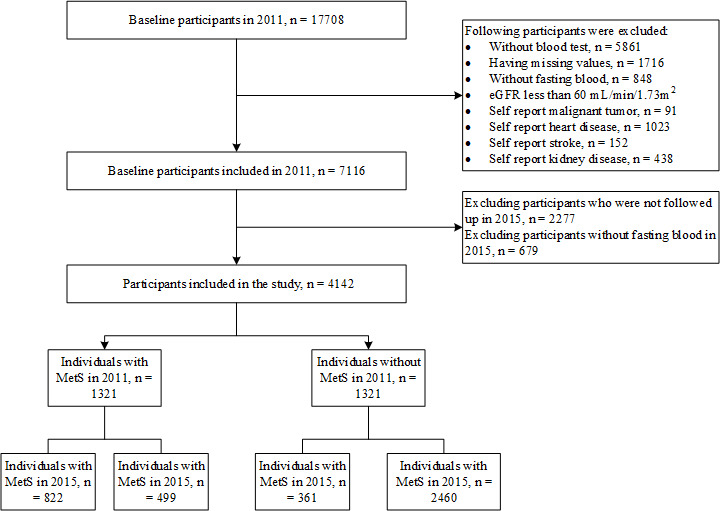
Flow chart of participants selection. eGFR, estimated glomerular filtration rate; MetS, metabolic syndrome.
Blood examinations
At baseline (wave 1), blood measurements and haemoglobin were assayed by the Center for Disease Control and Prevention of the local county, whereas other biochemical indicators were analysed by Youanmen Center for Clinical Laboratory of Capital Medical University, Beijing, China. Serum creatinine (Scr) was measured by the picric acid method; blood urea nitrogen was determined by an enzymatic UV method with urease; blood glucose (BG), total cholesterol, high density lipoprotein (HDL) cholesterol and triglyceride (TG) were assayed by enzymatic colormetric tests; glycosylated haemoglobin (GHbA1c) was determined by high performance liquid chromatography; high-sensitivity C reactive protein (hs-CRP) was examined by immunoturbidimetric assay; and uric acid (UA) was determined by the UA plus method.20 Blood specimen testing in 2015 (wave 3) was completed by KingMed Diagnostics, the leading third-party institution in China, which has testing laboratories in 27 provincial-level cities nationwide. GHbA1c, Scr, HDL, TG and BG were the required blood biomarkers from wave 3. GHbA1c and Scr levels were determined by the same methods as wave 1, while HDL was determined by a direct method, TG by an oxidase method and BG by a hexokinase method.21 The collection, storage, transport, processing and other blood sample details are described elsewhere.20 21 Of note, the models and manufacturer information of blood test instruments in wave 1 and wave 3 were not available. All inspections and calibrations were performed by trained personnel.
Definition and grouping of MetS
Currently, there was no unified definition for MetS. The WHO diagnostic criteria proposed in 1999, the National Cholesterol Education Program Adult Panel Ⅲ (ATP Ⅲ) diagnostic criteria proposed in 2005 and International Diabetes Federation diagnostic criteria proposed in 2006 were commonly used for MetS.22 These diagnostic criteria basically related to abdominal obesity, dyslipidaemia, glucose metabolism disorder and elevated blood pressure. However, these diagnostic criteria had different views and cut-off values for some specific indicators. This study adopted the 2018 China Guidelines for the Prevention and Treatment of Hypertension (CGPTH) definition for MetS, which was similar to the ATP Ⅲ diagnostic criteria.2 Compared with ATP Ⅲ diagnostic criteria, the cut points of waist circumference defined by CGPTH were smaller and more suitable for the Chinese population. According to the 2018 CGPTH definition, MetS was diagnosed when three of the following four conditions were met: (1) central obesity: waist circumference (WC) ≥90 cm in men and ≥85 cm in women; 2) elevated BP: systolic blood pressure (SBP) ≥130 mm Hg or diastolic blood pressure (DBP) ≥85 mm Hg, or diagnosed as hypertension and treated; 3) dyslipidaemia: fasting TG ≥150 mg/dL, or HDL ≤40 mg/dL, or diagnosed as dyslipidaemia and treated; and (4) elevated BG: fasting BG (FBG) ≥100 mg/dL, or 2-hour postprandial BG ≥100 mg/dL, or diagnosed as diabetes and treated.2 Diabetes was defined as fasting BG ≥126 mg/dL, and/or HbA1c ≥6.5%, and/or a self-reported history of diabetes.23 Of note, we did not have 2-hour postprandial BG data.
According to MetS baseline status and follow-up, participants were categorised into: (1) MetS free, (2) MetS developed, (3) MetS recovery and (4) MetS chronic groups.
Study outcomes
We calculated eGFR values using the 2012 Chronic Kidney Disease Epidemiology Collaboration equation based on creatinine levels.24 A rapid eGFR decline was defined as an average annual eGFR decline of >3 mL/min/1.73 m2.16 25 In this study, we defined a rapid eGFR decline as the eGFR in wave 3 minus the eGFR in wave 1, >12 mL/min/1.73 m2.
MetS scores
MetS severity potentially affects the recovery or occurrence of MetS. For instance, individuals with high MetS severity may be less liable to recover. Similarly, for those without MetS, it is not straightforward to progress to severe MetS. Therefore, MetS scores were introduced to assess MetS severity in the study, which was thought to be more sufficient and accurate than other ways using the number of symptoms and complications to reflect MetS severity.26 27 These scores were calculated using principal component (PC) analysis of WC, mean arterial pressure (MAP), FBG, fasting TG and the inverse HDL values. All MetS related variables were normalised by 0–1. According to the PC analysis results, PC1 and PC2 explained 38.9% and 20.9% of the variance, respectively. MetS scores were calculated as follows:
Other covariates
All potential covariates were all collected at baseline in wave 1, including gender (male vs female), age, marital status (married with spouse vs others), education (illiterate, middle school and below or high school and above), household per capita income, smoking (yes vs no), drinking (yes vs no), eGFR, grip strength, height, weight, body mass index (BMI), WC, SBP, DBP, MAP, depressive symptom (yes vs no), self-reporting disease (hypertension, diabetes, dyslipidaemia and arthritis or rheumatism) and corresponding medication. We categorised eGFR into two groups: 60–89 and ≥90. Grip strength was divided into three groups (T1, T2 and T3) according to the one-third percentile. BMI was calculated by weight (kg)/height squared (m2). The BP of each participant was measured three times every 45–60 s with the OmronTM HEM-7112 sphygmomanometer (Omron Co, Ltd, Dalian, China) at rest. Both SBP and DBP were averaged from three measurements. MAP was defined as MAP=1/3×SBP + 2/3×DBP. Previous study demonstrated that depressive symptom was association with baseline eGFR.28 Thus, we should not overlook this variable. The 10-item Center for Epidemiological Studies Depression Scale (CESD-10) was applied in the study.29 A CESD-10 score ≥10 was grouped into the depressive symptom group, and <10 into the non-depressive symptom group. Self-reporting disease was disease diagnosed by a doctor. Medical interventions included taking Chinese traditional and Western modern medicines.
Statistical methods
The Kolmogorov-Smirnov test was used to test the normality of continuous variables. Continuous variables were expressed by the median (IQR) and categorical variables by frequency (%). The Mann-Whitney U test was performed on continuous variables, and categorical variables between the rapid eGFR decline group and the non-rapid eGFR decline group were tested by the χ2 test. In preliminary analyses, variables with p values <0.15 were used to calibrate the logistic model. Continuous variables not presenting a linear relationship with the logit conversion value of the dependent variable were converted to categorical variables. Tolerance and variance inflation factors (VIFs) were used to test for collinearity. This existed if the tolerance was <0.1 or the VIF was >10. Eventually, age, sex, BMI, Scr, haemoglobin, eGFR classification, grip strength classification and MetS scores were selected as confounding variables for model adjustments in this study. Most selected covariates have been reported to be related to renal events.26 30–33 Univariate analysis of variables between eGFR decline group and non-rapid eGFR decline group were carried out. Logistic models were used to test the association between MetS recovery/occurrence and eGFR rapid decline after adjusting for multiple confounding factors. Furthermore, alterations in MetS status were accompanied by changes of diagnostic conditions (elevated blood glucose, elevated blood pressure, central obesity and dyslipidaemia). As a result, logistic models were used to explore the relationship between the recovery/occurrence of MetS components and the rapid decline of eGFR using different adjustments of confounding factors, respectively. P<0.05 was considered statistically significant (two-sided test). Statistics were generated in IBM SPSS V.20.0 software (IBM Corp) and StataMP 16 software (StataCorp, Texas, USA).
Patient and public involvement
There were no participants involved in the development. The results of the survey are disseminated to the public through websites.
Results
Participant characteristics
As shown (figure 1), 4142 participants were selected, including 2460 (59.4%) in the MetS-free group, 361 (8.7%) in the MetS-developed group, 499 (12.0%) in the MetS recovery group and 822 (19.8%) in the MetS chronic group. Comparison of the basic characteristics between the 4142 enrolled participants and 2974 ones that excluded during follow-up were shown in online supplemental table 1.
bmjopen-2021-059504supp001.pdf (153.3KB, pdf)
Participant characteristics were grouped by the eGFR decline rate (table 1). A rapid decline in eGFR developed in 711 (17.2%) participants during the 4-year follow-up. The median age was 58 (52~64) years, and males accounted for 42.5% at baseline. In contrast to rapid eGFR decline group, the non-rapid eGFR decline group was significantly higher with respect to FBG, Scr, haemoglobin, eGFR, weight, BMI, WC, MetS scores, central obesity and elevated BG (all p<0.05). Those in non-rapid eGFR decline group were more likely to be female and younger when compared with the eGFR decline group (both p<0.05).
Table 1.
Baseline characteristics of participants between rapid EGFR decline group and non-rapid EGFR decline group
| Characteristics | Overall (n=4142) |
Rapid eGFR decline (n=711) |
Non-rapid eGFR decline (n=3431) |
P value |
| Male (n (%)) | 1874 (45.2) | 351 (49.4) | 1523 (44.4) | 0.02 |
| Age (years) | 58 (52~64) | 59 (52~66) | 58 (52~64) | 0.02 |
| Married with spouse (n (%)) | 3548 (87.5) | 610 (85.8) | 2938 (85.6) | 0.91 |
| Education | ||||
| Illiterate (n (%)) | 1206 (29.1) | 191 (26.9) | 1015 (29.6) | 0.28 |
| Middle school and below (n (%)) | 1770 (41.2) | 309 (43.5) | 1398 (40.7) | |
| High school and above (n (%)) | 1229 (29.7) | 211 (29.7) | 1018 (29.7) | |
| Household per capita income (yuan) | 6461.0 | 6000.0 | 6560.0 | 0.20 |
| M (P25~P75) | (2336.7~13 487.5) | (1866.7~13 490.0) | (2450.0~13 486.7) | |
| Drink (n (%)) | 1470 (32.2) | 234 (32.9) | 1173 (34.2) | 0.51 |
| Smoke (n (%)) | 1567 (37.8) | 272 (38.3) | 1295 (37.7) | 0.80 |
| Blood urea nitrogen (mg/dL) | 15.0 (12.5~17.8) | 15.1 (12.6~18.2) | 15.0 (12.5~17.7) | 0.18 |
| Fasting glucose (mg/dL) | 102.4 (94.9~111.2) | 100.6 (93.4~109.8) | 102.4 (95.2~111.4) | 0.001 |
| Creatinine (mg/dL) | 0.75 (0.64~0.84) | 0.71 (0.60~0.84) | 0.76 (0.64~0.86) | 0.001 |
| Total cholesterol (mg/dL) | 190.6 (168.6~215.8) | 189.8 (164.7~215.3) | 190.6 (169.3~216.1) | 0.20 |
| Triglyceride (mg/dL) | 105.3 (74.3~148.7) | 101.8 (71.7~146) | 106.2 (74.3~148.7) | 0.23 |
| HDL cholesterol (mg/dL) | 49.1 (41.0~59.5) | 49.5 (41.0~59.9) | 49.1 (41.0~59.5) | 0.81 |
| hs-CRP (mg/L) | 1.0 (0.5~2.0) | 1.0 (0.6~2) | 1.0 (0.5~2.0) | 0.43 |
| GHbA1c (%) | 5.1 (4.9~5.4) | 5.1 (4.9~5.4) | 5.1 (4.9~5.4) | 0.36 |
| Uric acid (mg/dL) | 4.2 (3.5~5.0) | 4.2 (3.5~5.0) | 4.2 (3.5~5.0) | 0.83 |
| Haemoglobin (mg/dL) | 14.2 (13.1~15.5) | 14.0 (12.8~15.1) | 14.3 (13.1~15.5) | 0.001 |
| eGFR (mL/min/1.73 m2) | 95.9 (86.4~102.9) | 97.0 (88.3~106.1) | 95.6 (85.9~102.4) | 0.001 |
| eGFR group | ||||
| 60~89 mL/min/1.73 m2 (n (%)) | 1368 (33.0) | 209 (29.4) | 1158 (33.8) | |
| 90~mL/min/1.73 m2 (n (%)) | 2774 (67.0) | 502 (70.6) | 2272 (66.2) | |
| Grip strength (kg) | 29.3 (23.8~36.5) | 29.5 (24.9~36.2) | 29.3 (23.5~36.7) | 0.13 |
| Grip strength group (n (%)) | 0.01 | |||
| T1 | 1386 (33.5) | 209 (29.4) | 1177 (34.3) | |
| T2 | 1387 (33.5) | 268 (37.7) | 1199 (32.6) | |
| T3 | 1369 (33.1) | 234 (32.9) | 1135 (33.1) | |
| Height (cm) | 157.7 (152.0~163.8) | 157.9 (152.0~163.7) | 157.6 (152.0~163.9) | 0.64 |
| Weight (kg) | 58 (51.3~65.5) | 57.1 (50.8~65.1) | 58.2 (51.4~65.7) | 0.08 |
| Body mass index (kg/m2) | 23.2 (21~25.7) | 22.9 (20.8~25.4) | 23.3 (21.1~25.8) | 0.01 |
| Waist circumference (cm) | 84.4 (78.0~92.0) | 83.6 (77.0~90.2) | 84.8 (78.1~92.0) | 0.01 |
| Systolic blood pressure (mm Hg) | 127 (114~141) | 128 (114~142) | 127 (114~141) | 0.72 |
| Diastolic blood pressure (mm Hg) | 75 (67~83) | 74 (66~83) | 75 (67~83) | 0.41 |
| Mean arterial pressure (mm Hg) | 92 (83~102) | 92 (83~103) | 92 (84~102) | 0.76 |
| Depression symptom (n (%)) | 1904 (46.0) | 319 (44.9) | 1585 (46.2) | 0.52 |
| Self-report hypertension (n (%)) | 887 (21.4) | 157 (22.1) | 730 (21.3) | 0.63 |
| Self-report dyslipidaemia (n (%)) | 333 (8.0) | 58 (8.2) | 275 (8.0) | 0.90 |
| Self-report diabetes or HBG (n (%)) | 191 (4.6) | 28 (3.9) | 163 (4.8) | 0.35 |
| Self-report arthritis or rheumatism (n (%)) | 1345 (32.5) | 235 (33.1) | 1110 (32.4) | 0.71 |
| Antihypertensive therapy (n (%)) | 664 (16.0) | 119 (16.7) | 545 (19.5) | 0.57 |
| Lipid-lowering therapy (n (%)) | 187 (4.5) | 37 (5.2) | 150 (4.4) | 0.33 |
| Hypoglycaemic therapy (n (%)) | 127 (3.1) | 19 (2.7) | 108 (3.1) | 0.50 |
| Therapy for arthritis or rheumatism (n (%)) | 643 (15.5) | 116 (16.3) | 527 (15.4) | 0.52 |
| MetS (n (%)) | 1321 (31.9) | 207 (29.1) | 1114 (32.5) | 0.08 |
| MetS scores | −0.1 (−0.4~0.3) | −0.1 (−0.5~0.3) | 0 (−0.4~0.3) | 0.02 |
| MetS components (n (%)) | ||||
| Central obesity | 1726 (41.7) | 264 (37.1) | 1462 (42.6) | 0.01 |
| Elevated blood pressure | 2099 (50.7) | 368 (51.8) | 1731 (50.5) | 0.52 |
| Dyslipidaemia | 1595 (38.5) | 278 (39.1) | 1317 (38.4) | 0.72 |
| Elevated blood glucose | 2456 (59.3) | 383 (53.9) | 2073 (60.4) | <0.01 |
| Baseline non-MetS group (n (%)) | ||||
| MetS free | 2460 (59.4) | 444 (62.4) | 2016 (58.8) | |
| MetS developed | 361 (8.7) | 60 (8.4) | 301 (8.8) | |
| Baseline MetS group (n (%)) | ||||
| MetS recovery | 499 (12.0) | 64 (9.0) | 435 (12.7) | |
| MetS chronic | 822 (19.8) | 143 (20.1) | 679 (19.8) |
Data are n (%) or median (IQR).
Grip strength is divided into T1, T2 and T3 groups by one-third percentile.
eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C reactive protein; MetS, metabolic syndrome.
Rapid EGFR decline odds based on MetS recovery or occurrence
Univariate analysis was conducted to select covariates for correction (online supplemental table 2). As shown (table 2), after adjustment for age, sex, BMI, Scr, haemoglobin, eGFR classification, grip strength classification, and MetS scores, the OR of rapid eGFR decline in the MetS recovery group was 0.64 (OR: 0.64; 95% CI 0.45 to 0.90, p=0.01) when compared with the MetS chronic group. In contrast, after adjustment for age, sex, BMI, serum creatinine, haemoglobin, eGFR classification, grip strength classification and MetS score, the OR of rapid eGFR decline in the MetS-developed group was 1.00 (OR: 1.00; 95% CI 0.73 to 1.38, p=0.98) when compared with the MetS-free group.
Table 2.
Multivariate logistic regression of rapid EGFR decline between study groups
| Model 1 | Model 2 | |||
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Baseline MetS groups | ||||
| MetS chronic | ref | ref | ||
| MetS recovery | 0.68 (0.50–0.95) | 0.02 | 0.64 (0.45–0.90) | 0.01 |
| Baseline non-MetS groups | ||||
| MetS free | ref | ref | ||
| MetS developed | 0.93 (0.69–1.25) | 0.64 | 1.00 (0.73–1.38) | 0.98 |
Model 1: additional adjusted for age and sex.
Model 2: additional adjusted for age, sex, serum creatinine, eGFR classification, grip strength classification, haemoglobin, MetS scores and body mass index.
eGFR, estimated glomerular filtration rate; MetS, metabolic syndrome.
MetS components and rapid EGFR decline odds
The association of changes in the composition of MetS groups with rapid eGFR decline is shown (table 3). In the baseline MetS population, after adjustment for age, sex, BMI, serum creatinine, haemoglobin, eGFR classification, grip strength classification and MetS score, the OR of rapid eGFR decline in the population recovered from central obesity was 0.31 (OR: 0.31; 95% CI 0.15 to 0.65, p<0.01) when compared with chronic central obesity, whereas recovery from elevated BP, dyslipidaemia and elevated BG did not show statistically significant differences when compared with the corresponding population (all p>0.05). In the baseline population without MetS, we observed no statistical difference in the rapid decline of eGFR between the occurrence of all MetS component groups and corresponding contrast groups (all p>0.05). This was consistent with the overall trend.
Table 3.
Multivariate logistic regression of rapid EGFR decline between study groups according the changes of MetS components
| Model 1 | Model 2 | |||
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Recovered components in baseline MetS groups (chronic MetS components as reference) | ||||
| Central obesity | 0.29 (0.15 to 0.59) | 0.01 | 0.31 (0.15 to 0.65) | <0.01 |
| Elevated blood pressure | 0.80 (0.50 to 1.26) | 0.33 | 0.79 (0.49 to 1.27) | 0.33 |
| Elevated SBP | 0.89 (0.61 to 1.31) | 0.56 | 0.88 (0.59 to 1.30) | 0.51 |
| Elevated DBP | 0.75 (0.46 to 1.23) | 0.26 | 0.68 (0.41 to 1.15) | 0.15 |
| Dyslipidaemia | 1.09 (0.82 to 1.44) | 0.57 | 1.05 (0.78 to 1.40) | 0.77 |
| Elevated TG | 1.22 (0.87 to 1.72) | 0.26 | 1.14 (0.79 to 1.36) | 0.50 |
| Decreased HDL | 0.84 (0.59 to 1.12) | 0.32 | 0.85 (0.59 to 1.22) | 0.38 |
| Elevated blood glucose | 1.08 (0.87 to 1.34) | 0.49 | 1.08 (0.86 to 1.36) | 0.52 |
| Elevated fasting glucose | 1.14 (0.91 to 1.43) | 0.25 | 1.13 (0.89 to 1.43) | 0.32 |
| Developed components in baseline non-MetS groups (free MetS components as reference) | ||||
| Central obesity | 1.21 (0.92 to 1.59) | 0.16 | 1.32 (0.97 to 1.77) | 0.74 |
| Elevated blood pressure | 0.84 (0.63 to 1.13) | 0.26 | 0.87 (0.64 to 1.18) | 0.37 |
| Elevated SBP | 0.88 (0.66 to 1.17) | 0.37 | 0.92 (0.68 to 1.23) | 0.56 |
| Elevated DBP | 0.88 (0.62 to 1.24) | 0.46 | 0.91 (0.63 to 1.30) | 0.59 |
| Dyslipidaemia | 0.92 (0.69 to 1.22) | 0.54 | 0.96 (0.72 to 1.30) | 0.81 |
| Elevated TG | 0.93 (0.70 to 1.25) | 0.64 | 1.02 (0.75 to 1.37) | 0.91 |
| Decreased HDL | 1.02 (0.65 to 1.59) | 0.95 | 0.97 (0.61 to 1.55) | 0.91 |
| Elevated blood glucose | 1.07 (0.76 to 1.50) | 0.71 | 1.07 (0.75 to 1.52) | 0.71 |
| Elevated fasting glucose | 1.06 (0.74 to 1.51) | 0.76 | 1.09 (0.76 to 1.57) | 0.64 |
Model 1: additional adjusted for age and sex.
Model 2: additional adjusted for age, sex, serum creatinine, eGFR classification, grip strength classification, haemoglobin, MetS score and body mass index.
Each MetS component runs in their own model to predict rapid eGFR decline.
DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; MetS, metabolic syndrome; SBP, systolic blood pressure; TG, triglyceride.
Discussion
We examined the relationship between MetS changes and rapid eGFR decline in a large nationwide cohort. At the 4-year follow-up, MetS recovery was significantly associated with a reduced risk of rapid eGFR decline in the middle-aged and elderly, with only waist circumference recovery consistent with the overall trend. The occurrence of MetS and its components did not significantly increase the risk of rapid eGFR decline. Further follow-up is required to elucidate the relationship between MetS dynamics and the rapid decline in eGFR.
Longitudinal cohort studies in several Asian countries concluded that MetS increased the risk of CKD, although follow-up times varied from study to study.15–18 34 However, the effect of MetS on the rapid decline of eGFR remains controversial. In a 3-year cohort, Cheng et al34 found no significant correlations between MetS and eGFR rapid decline in the elderly. However, other studies reported that baseline MetS was associated with a decline in eGFR and even acted as an independent predictor of eGFR decline.16–18 Wu et al26 investigated the association between the MetS severity score and kidney function and found that the MetS severity score was an independent risk factor for the CKD development and progressive eGFR decline, although the definition of rapid eGFR decline was different from this study. Here, the MetS severity score was a continuous variable that was primarily used to calibrate the MetS (yes vs no). We noted that none of the aforementioned studies accounted for the MetS status of participants during follow-up periods. In a 4-year follow-up cohort, Park et al35 explored the relationship between MetS status change and CKD events and concluded that MetS recovery was associated with a decreased risk of CKD incidence, but the occurrence of MetS increased the risk of CKD incidence. One of the highlights of the article was to observe the status of MetS three times over a 4-year period, thereby making the MetS diagnosis more robust. However, Park et al did not discuss the association with the rapid eGFR decline. In this study, we concluded that MetS recovery was associated with a reduced risk of rapid eGFR decline, while MetS occurrence was not related to rapid eGFR decline. It should be emphasised that we need to be cautious about the conclusion between the MetS occurrence and the rapid eGFR decline in this study. Because the follow-up time was short and the timing of MetS onset was unknown, the impairment of renal function caused by MetS may not have occurred in some populations. To sum up, studies exploring the relationship between MetS dynamic changes and the rapid decline of eGFR in the Chinese population are rare. Our investigation of the relationship between MetS recovery/occurrence and eGFR rapid decline in a large nationwide cohort may support renal function management in individuals with MetS.
The effect of MetS on renal function is complex, thus, no definitive mechanisms can explain our study observations. The evidence suggests that every component of MetS is associated with adverse renal events (10-14). It is accepted that hypertension and diabetes play pivotal roles in CKD development and progression.36–38 Also, lipid metabolism dysregulation and abnormal lipid distribution can lead to lipotoxicity-related renal damage.39 40 Thus, MetS may result from the combined effects of central obesity, increased BP, insulin resistance and blood lipid disorder, leading to physiopathological lipotoxicity, oxidative stress increments, endothelial dysfunction, elevated inflammation and apoptosis, which would contribute to kidney dysfunction.5 39 However, the relationship between MetS components and the weight of each factor on kidney injury remain unclear.
Our study had some limitations. First, MetS diagnoses were not comprehensively checked (using multiple tests), and the exact timing of the MetS alteration is unknown. Second, renal stone disease, epiculopathy, epiculoepicardial disease or acute urinary tract infection are related to the occurrence and development of renal disease. Unfortunately, urine or kidney ultrasound results were unavailable in CHARLS cohort. Third, CKD occurrence was not included as a study outcome because of the lack of urine test results, which would underestimate the CKD incidence. Fourth, blood analyses from wave 1 and wave 3 were performed at a different testing centre, with inconsistent HDL, TG and BG measurement methods; therefore, measurement errors may have occurred. Fifth, a large proportion of individuals were excluded due to exclusion criteria or missing values, and the basic characteristics between the 4142 enrolled participants and 2974 ones that excluded during follow-up might have biased some of our results. Sixth, we did not establish a model with all four MetS change groups included in the study.
Conclusions
Over a 4-year follow-up, we observed that MetS recovery, including recovery of central obesity, was associated with a reduced risk of rapid eGFR decline in middle-aged and older adults, while MetS occurrence was not related to rapid eGFR decline. Reversing MetS, especially central obesity, might benefit the kidney function in MetS population. However, further follow-up studies are required to observe the relationship between MetS alterations and adverse renal events.
Supplementary Material
Acknowledgments
The authors are grateful to the China Health and Retirement Longitudinal Study (CHARLS) team for providing the data
Footnotes
PL and LT contributed equally.
Contributors: PL, LT and JF: analysis and interpretation of data and preparation of the manuscript. XL: study concept and design, and preparation and critical review of the manuscript. CC: critical review and statistical guidance of the revised manuscript. All authors read, provided feedback and approved the final manuscript.
Funding: This work was supported by Guangzhou Municipal Science and Technology Bureau (202002020047) and Legal and Ethical Compliance Standards for Data Use in Clinical Research on Major Chronic Diseases Based on Data Security (2018YFC1315403).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
CHARLS data of the study will be available to investigators at the CHARLS website (http://charls.pku.edu.cn/en).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The Medical Ethics Review Committee of Peking University approved this study, and all participants provided written informed consent before participating. This study is a secondary analysis of a public dataset and does not require ethics approval again. Participants gave informed consent to participate in the study before taking part.
References
- 1.Alberti KGMM, Zimmet P, Shaw J, et al. The metabolic syndrome--a new worldwide definition. Lancet 2005;366:1059–62. 10.1016/S0140-6736(05)67402-8 [DOI] [PubMed] [Google Scholar]
- 2.Joint Committee for Guideline Revision . 2018 Chinese guidelines for prevention and treatment of Hypertension-A report of the revision Committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol 2019;16:182–241. 10.11909/j.issn.1671-5411.2019.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009;2:231–7. 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep 2018;20:12. 10.1007/s11906-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol 2018;36:14–20. 10.1016/j.clindermatol.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Lerman LO. The metabolic syndrome and chronic kidney disease. Transl Res 2017;183:14–25. 10.1016/j.trsl.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EY, Han K, Kim DH, et al. Exposure-weighted scoring for metabolic syndrome and the risk of myocardial infarction and stroke: a nationwide population-based study. Cardiovasc Diabetol 2020;19:153. 10.1186/s12933-020-01129-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guembe MJ, Fernandez-Lazaro CI, Sayon-Orea C, et al. Risk for cardiovascular disease associated with metabolic syndrome and its components: a 13-year prospective study in the RIVANA cohort. Cardiovasc Diabetol 2020;19:195. 10.1186/s12933-020-01166-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoang K, Zhao Y, Gardin JM, et al. LV Mass as a Predictor of CVD Events in Older Adults With and Without Metabolic Syndrome and Diabetes. JACC Cardiovasc Imaging 2015;8:1007–15. 10.1016/j.jcmg.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie K, Bao L, Jiang X, et al. The association of metabolic syndrome components and chronic kidney disease in patients with hypertension. Lipids Health Dis 2019;18:229. 10.1186/s12944-019-1121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viazzi F, Piscitelli P, Giorda C, et al. Metabolic syndrome, serum uric acid and renal risk in patients with T2D. PLoS One 2017;12:e0176058. 10.1371/journal.pone.0176058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Kong X, Jia X, et al. Association between metabolic syndrome and chronic kidney disease in a Chinese urban population. Clin Chim Acta 2017;470:103–8. 10.1016/j.cca.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 13.Thomas G, Sehgal AR, Kashyap SR, et al. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2011;6:2364–73. 10.2215/CJN.02180311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang IH, Han JH, Myung SC, et al. Association between metabolic syndrome and chronic kidney disease in the Korean population. Nephrology 2009;14:321–6. 10.1111/j.1440-1797.2009.01091.x [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Shi L-X, Zhang Q, et al. Increased risk of chronic kidney diseases in patients with metabolic syndrome: a 3-year prospective cohort study. Curr Med Sci 2019;39:204–10. 10.1007/s11596-019-2020-8 [DOI] [PubMed] [Google Scholar]
- 16.Huh JH, Yadav D, Kim JS, et al. An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism 2017;67:54–61. 10.1016/j.metabol.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K, Takayama M, Abe T, et al. Investigation of metabolic factors associated with eGFR decline over 1 year in a Japanese population without CKD. J Atheroscler Thromb 2017;24:863–75. 10.5551/jat.38612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamoto R, Akase T, Ninomiya D, et al. Metabolic syndrome is a predictor of decreased renal function among community-dwelling middle-aged and elderly Japanese. Int Urol Nephrol 2019;51:2285–94. 10.1007/s11255-019-02320-0 [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Hu Y, Smith JP, et al. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol 2014;43:61–8. 10.1093/ije/dys203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Crimmins E, Hu P. ChinaHealth and Retirement Longitudinal Study: 2011–2012 National BaselineUsers’ Guide. Beijing, China: National School of Development, Peking University, 2013. [Google Scholar]
- 21.Chen X, Crimmins E, Hu PP, et al. Venous blood-based biomarkers in the China health and retirement longitudinal study: rationale, design, and results from the 2015 wave. Am J Epidemiol 2019;188:1871–7. 10.1093/aje/kwz170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International diabetes Federation Task force on epidemiology and prevention; National heart, lung, and blood Institute; American heart association; world heart Federation; international atherosclerosis Society; and international association for the study of obesity. Circulation 2009;120:1640–5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association . 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44:S15–33. 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 24.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985;33:278–85. 10.1111/j.1532-5415.1985.tb07117.x [DOI] [PubMed] [Google Scholar]
- 26.Wu M, Shu Y, Wang L, et al. Metabolic syndrome severity score and the progression of CKD. Eur J Clin Invest 2022;52:e13646. 10.1111/eci.13646 [DOI] [PubMed] [Google Scholar]
- 27.Wijndaele K, Beunen G, Duvigneaud N, et al. A continuous metabolic syndrome risk score: utility for epidemiological analyses. Diabetes Care 2006;29:2329. 10.2337/dc06-1341 [DOI] [PubMed] [Google Scholar]
- 28.Jia F, Li X, Liu F, et al. Association of renal function and depressive symptoms: evidence from the China health and retirement longitudinal study. J Psychosom Res 2020;137:110224. 10.1016/j.jpsychores.2020.110224 [DOI] [PubMed] [Google Scholar]
- 29.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item center for epidemiological studies depression scale (CES-D). Arch Intern Med 1999;159:1701–4. 10.1001/archinte.159.15.1701 [DOI] [PubMed] [Google Scholar]
- 30.Ma X, Zhang C, Su H, et al. Increasing body mass index predicts rapid decline in renal function: a 5 year retrospective study. Horm Metab Res 2018;50:556–61. 10.1055/a-0599-6360 [DOI] [PubMed] [Google Scholar]
- 31.Meguro S, Tomita M, Kabeya Y, et al. Factors associated with the decline of kidney function differ among eGFR strata in subjects with type 2 diabetes mellitus. Int J Endocrinol 2012;2012:687867. 10.1155/2012/687867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deicher R, Hörl WH. Anaemia as a risk factor for the progression of chronic kidney disease. Curr Opin Nephrol Hypertens 2003;12:139–43. 10.1097/00041552-200303000-00003 [DOI] [PubMed] [Google Scholar]
- 33.Young BA, Katz R, Boulware LE, et al. Risk factors for rapid kidney function decline among African Americans: the Jackson heart study (JHS). Am J Kidney Dis 2016;68:229–39. 10.1053/j.ajkd.2016.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng H-T, Huang J-W, Chiang C-K, et al. Metabolic syndrome and insulin resistance as risk factors for development of chronic kidney disease and rapid decline in renal function in elderly. J Clin Endocrinol Metab 2012;97:1268–76. 10.1210/jc.2011-2658 [DOI] [PubMed] [Google Scholar]
- 35.Park S, Lee S, Kim Y, et al. Reduced risk for chronic kidney disease after recovery from metabolic syndrome: a nationwide population-based study. Kidney Res Clin Pract 2020;39:180–91. 10.23876/j.krcp.20.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet 2017;389:1238–52. 10.1016/S0140-6736(16)32064-5 [DOI] [PubMed] [Google Scholar]
- 37.Tonneijck L, Muskiet MHA, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 2017;28:1023–39. 10.1681/ASN.2016060666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz-Ortega M, Rayego-Mateos S, Lamas S, et al. Targeting the progression of chronic kidney disease. Nat Rev Nephrol 2020;16:269–88. 10.1038/s41581-019-0248-y [DOI] [PubMed] [Google Scholar]
- 39.Kim Y, Park CW. Can management of the components of metabolic syndrome modify the course of chronic kidney disease? Kidney Res Clin Pract 2020;39:118–20. 10.23876/j.krcp.20.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Agati VD, Chagnac A, de Vries APJ, et al. Obesity-Related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 2016;12:453–71. 10.1038/nrneph.2016.75 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059504supp001.pdf (153.3KB, pdf)
Data Availability Statement
CHARLS data of the study will be available to investigators at the CHARLS website (http://charls.pku.edu.cn/en).


