Abstract
Cutaneous carcinoma, which has occupied a peculiar place among worldwide populations, is commonly responsible for the considerably increasing morbidity and mortality rates. Currently available medical procedures fail to completely avoid cutaneous carcinoma development or to prevent mortality. Cancer chemoprevention, as an alternative strategy, is being considered to reduce the incidence and burden of cancers through chemical agents. Derived from dietary foods, phytochemicals have become safe and reliable compounds for the chemoprevention of cutaneous carcinoma by relieving multiple pathological processes, including oxidative damage, epigenetic alteration, chronic inflammation, angiogenesis, etc. In this review, we presented comprehensive knowledges, main molecular mechanisms for the initiation and development of cutaneous carcinoma as well as effects of various diet phytochemicals on chemoprevention.
Keywords: Cutaneous carcinoma chemoprevention, Diet phytochemicals, Redox homeostasis, Epigenetic modulators, Cutaneous inflammation, Cancer neovascularization, Metastasis
Graphical Abstract

1. Introduction
Till now, cutaneous carcinoma is the most common human malignancy (especially in the white population) due to steadily rising life expectancy, increasing urbanization and subsequent lifestyle changes, with millions of new cases detected worldwide each year [1-4]. Although the incidence rate for all cancer sites combined is decreasing, the cutaneous carcinoma incidence rate has continued to increase, which is a significant public health problem that exacts a substantial financial and social burden [5].
Cutaneous carcinoma is generally classified as malignant melanoma and non-melanocytic cutaneous carcinoma (NMSC). The latter includes squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) as the main subtypes that are named according to the originating cells and clinical behaviors [6]. NMSC are much more frequent than melanoma, but they have a better prognosis. Both, basal and squamous cell carcinomas, originate from epidermal keratinocytes and both appear mainly in cutaneous areas exposed to sunlight [7]. BCC accounts for the majority of NMSC cases (about 80%–85%) and has a low rate of metastasis to other organs, while 15%–20% of NMSC are SCC with a higher tendency to metastasize and higher mortality than BCC [8,9]. Melanoma, which is derived from epidermal melanocytes, represents only 4% of cutaneous carcinoma cases, yet accounts for 80% of all cutaneous carcinoma-related deaths [6]. Limited progress has been made in the treatment of cutaneous carcinoma over the past 4 decades, through the use of immunotherapy, chemotherapy, radiotherapy, targeted therapy and combinations, which have yielded reduced response rates and low median survival, associated with significant toxic profiles and treatment resistance. Thus, the burden of cutaneous carcinoma remains chronically high [10,11,262].
Development of cutaneous carcinoma is a multistep process that involves initiation, promotion and progression (Graphical abstract). Mutation and abnormalities of key cellular regulators (e.g. RAS, p53, EGFR and p16INK4A), as early events in tumorigenesis, are caused by various carcinogens (eg. UV) directly or through inducing epigenetic alterations and intracellular oxidative stress [12]. Due to subsequent failure of these genes, an accumulating cellular malignant phenotype may appear by activating transcriptional factors such as NF-κB, β-catenin, STAT3, HIF-1α and AP-1, as well as their downstream proteins. As a result, cutaneous carcinoma can be discerned owing to the inflammatory microenvironment together with uncontrollable cell growth and differentiation [13]. Besides, continuous inflammatory conditions and disturbed transcriptional factors activation may further lead to tumor-related angiogenesis and metastasis that are significant pathological indicators of lethal cutaneous carcinoma. Chemopreventive strategies have been reported to control such hyperactive signaling cascades and pathological alterations which may delay and reverse these multistep process of cutaneous carcinoma and lower the burden [14-16].
Over two-thirds of human cancers may be prevented by appropriately modifying the lifestyle, especially by changing the dietary habit [17]. Being responsible for color and other organoleptic properties, diet phytochemicals are chemical compounds naturally constituted in plant-based food [17-19]. Resveratrol, epigallocatechin gallate (EGCG) and curcumin have been reported to directly regulate a variety of molecular signal transduction pathways, which participate in cancer initiation, promotion or progression. Eventually, they can reduce the incidence and death rates of human neoplasms, such as cutaneous carcinoma [20-22].
In this review, we introduced the basic knowledge of cutaneous carcinoma, and highlighted the causative factors and their influences on the intracellular signaling cascades involved in cancer development. In addition, we shed light on some phytochemicals capable of targeting the signaling pathways which make them applicable to cutaneous carcinoma chemoprevention and control of its progression. On this basis, we also expounded the potential correlation between the these compounds and clinical outcome.
2. Risk factors for cutaneous carcinoma
High awareness of the risk factors for cutaneous carcinoma is crucial for prevention. Although why the incidence and death rates of cutaneous carcinoma have ascended so dramatically in recent years remains unclear, they may be attributed to multiple endogenous and exogenous factors. The most common risk factors are discussed as follows.
2.1. UVR
The vast majority of cutaneous carcinoma are caused by UV exposure from the sun and/or indoor tanning devices [23-26,264]. Reducing sun exposure by using sun-protective behaviors (ie, using sunscreen regularly; wearing protective clothing, wide-brimmed hats, and sunglasses; seeking shade; and limiting time outdoors during midday hours when UV radiation from the sun is most intense) can sharply reduce the lifetime risk of developing cutaneous carcinoma [27-29,264]. When UV is transmitted through the skin and absorbed by chromophores therein, a series of photochemical reactions are initiated, inducing direct photic damages to DNA, lipids and proteins. After being absorbed directly by cellular DNA, UV leads to DNA lesions primarily through cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4)-pyrimidone photoproducts, which are able to cause CC to TT or ‘UVR signature’ C to T mutations in multiple suppressor genes or oncogenes, such as p53, p16, Notch1, Nsd1 and mismatch-repair genes [30-33]. Additionally, UV also induces the production of reactive oxygen species (ROS) to cause indirect oxidative damage to DNA and proteins. Moreover, UV also can affect signaling pathways related to protein kinases (MAPKs and Akt), cell-cycle regulatory proteins, inflammatory enzymes and growth factors, which can thus tremendously affect the initiation and promotion of cutaneous carcinoma [34-38].
2.2. Immunosuppression environment
Immune system undergoes key events in detecting and clearing early aberrant cellular and molecular changes that may lower the risk of cutaneous carcinoma, so an opposite result occurs after the system is suppressed [39]. Medically induced immunosuppression, which is common among organ transplant recipients, is well-documented as a significant risk factor for cutaneous carcinoma [40]. Compared with the non-transplanted population, the risk of SCC rises 65- to 250-fold in transplant recipients, and that of BCC increases 10- to 16-fold [41,42]. PUVA light treatment and radiotherapy, which participates in many aspects of medical treatment, exerts suppressive effects on the cutaneous immune system by (1) impairing contact and delayed-type hypersensitivity reactions, (2) weakening antigen-presenting cell function, and (3) inducing the production of immunosuppressive substances such as interleukin (IL)-10, prostaglandins and ROS [43,44].
2.3. Inflammatory cutaneous condition
Inflammation of the skin increases the blood flow, which generates various growth factors and cytokines as well as results in inflammatory cell infiltration, thereby enhancing the growth of SCC and BCC [45]. The infiltrated inflammatory cells cause DNA damage by producing ROS, and the growth factors contribute to the uncontrollable proliferation of cells. Patients with inflammatory bowel disease (IBD) are also reported at increased risk from cutaneous carcinoma [46]. Both preclinical and clinical studies have estimated that anti-inflammatory drugs (e.g. aspirin) could influence on cutaneous carcinoma [47-49].
2.4. Chemical carcinogenic factor
Recently, the number of cutaneous carcinoma cases keeps rising annually owing to exposure of human bodies to chemical carcinogens. With city industrialization, arsenic has contaminated the drinking water for tens of millions of people around the world. In addition to affecting the whole body, chronic arsenic poisoning causes pigmentary changes of the skin, thus disrupting ATP production, hindering DNA repair that highly depends on energy and finally leading to cutaneous carcinoma [50,51].
2.5. Dermatologic diseases
Dysplastic nevus syndrome, which also known as the atypical mole syndrome, is often bound up with the melanoma. Dysplastic nevi raise the malignant melanoma risk 500-fold [52]. As a rare genetic condition affecting skin, endocrine, central nervous, and skeletal systems, basal cell nevus syndrome develops numerous BCCs in a single lifetime, usually beginning from puberty [53-55]. Xeroderma pigmentosum, as another inherited disease that affects the cutaneous ability to recover UVR damage, also elevates the risk of developing cutaneous carcinoma, at an earlier age in particular [56,57].
3. Preventable molecular pathways for cutaneous carcinoma and chemopreventive phytochemicals
Like many other cancer types, cutaneous carcinoma chemoprevention at the molecular level is typified by the disruption of, or at least the delay of, multiple processes and pathways among three carcinogenesis stages, i.e. initiation, promotion and progression [17,58]. According to this, potentially chemopreventive chemicals or biomolecules can be divided into blocking agents and suppressing agents [58].
3.1. Cutaneous carcinoma chemopreventive phytochemicals as blocking agents
By inhibiting the initiation stage, cancer chemopreventive molecules play an important role in the preservation of DNA in its native state. They can circumvent permanent, irreparable DNA damage that takes place during initiation by inactivating or metabolizing carcinogens [59]. Functioning as free-radical scavengers, they physiologically induce antioxidative system and trigger DNA repair [60]. Furthermore, blocking agents are also capable of modifying the epigenetic landscape [61].
3.1.1. Antioxidant activity
Many human epidemiological studies have demonstrated a highly close association between cutaneous carcinoma and chronically oxidative conditions, which caused by UV radiation, infection and chemical agents and can be detected commonly in earlier phase of cancer development [62]. Increased production of oxidative stress has been implicated in cancer development primarily through direct DNA mutation [63,64]. Additionally, this stress can affect protein conformations and functions together with transcription factor DNA binding by modifying the redox status of cells, thus altering gene expression [64,65]. In the meantime, the promotion of angiogenesis and metastasis as well as carcinogen metabolism can also be obviously affected [66]. Consequently, antioxidants can inhibit the onset of cutaneous carcinoma predominantly [64]. This kind of substances exert protective effects not only by directly scavenging ROS, but also by inducing de novo expressions of genes that encode defensive/detoxifying proteins, including phase II enzymes (e.g. γ-GCS, GPx, NQO, GST and HO-1) which is often diminished or become inadequate to alleviate cutaneous cellular canceration induced by radiation or some chemical carcinogens [67,68]. The 5′-flanking regions of these enzyme genes all contain a cis-element, also known as the antioxidant-responsive element (ARE) [69,70]. Many basic leucine zipper transcription factors, can bind ARE sequences and stimulate the expressions of the stress-response genes mentioned above. Notably, Nrf2 is the most important transcription factor that heterodimerizes and binds ARE sequence [71].
Nrf2-knockout mice, which showed greater sensitivity to carcinogenesis, failed to induce the expressions of many genes involved in carcinogen detoxification and protection against oxidative stress/cutaneous cellular vicious transformation [72-75]. Thus, chemopreventive phytochemicals which can eventually protect against cellular oxidative stress directly or activate Nrf2 signaling, could be employed as blocking agents for cutaneous carcinoma [76]. In the Nrf2 pathway, Kelch-like-ECH-associated protein 1 (Keap1), a cytoplasmic repressor of Nrf2, inhibits this transcription factor accumulates into the cell nucleus. Nrf2 is normally sequestered in the cytoplasm through a hydrophilic region in the NEH2 domain and the double glycine-rich domains of Keap1, which basically resembles the proteasome system for proteasome degradation and ubiquitination [77]. The cysteine residues of Keap1 can be oxidized or covalently modified (R) by antioxidant agents, so that Nrf2 is released from the Nrf2-Keap1 complex [78,79]. Sulforaphane, a well-studied isothiocyanate in broccoli, was found to protect cutaneous tissue from UVB-stimulated photodamage and prevent papilloma formation and JB6 P+ cell canceration only with Nrf2wt presence induced by carcinogens through forming a covalent adduct with Keap1 protein at cysteine 151 and accelerating translocation of Nrf2 eventually [80-83]. Additionally, phosphorylation of Nrf2 in serine (S) and threonine (T) residues by upstream protein kinases (e.g. PKC, Akt, MAPKs and AMPK) can facilitate the dissociation of Nrf2 from Keap1 and translocation to the cell nucleus thereafter [84-86]. Lycopene, a carotenoid compound derived from tomato, was found a chemomodulatory efficacy on antioxidant enzymes (CAT, SOD, GR, GPx and HO-1) and DMBA/TPA-induced cutaneous carcinoma in our preliminary studies [87], and the mechanism was related to activate p38 MAPK and PKC (unpublished observation). In addition, direct binding partners other than Keap1 can bind with Nrf2 at the DLG and ETGE motifs to stabilize Nrf2 and thus conferprotection against oxidative stress, such as p21 and caveolin-1 [88]. In our group, one of my colleague has confirmed that DADS (diallyl disulfide), a major garlic derivative can inhibit TPA-induced tumor promotion by means of promoting Nrf2 nuclear localization in mouse skin, which seemed to be mediated through suppressing degradation of Nrf2 via upregulation of p21 protein level [89].
Till to date, numerous additional cutaneous carcinoma prevention studies have been done with a wide variety of anti-oxidant phytochemicals isolated from dietary plants, such as zerumbone from subtropical ginger, curcumin from turmeric, etc. Fig. 1 and Table 1 summarize representative cutaneous carcinoma chemopreventive dietary products capable of activating Nrf2 and inducing the expression of epidermal cytoprotective proteins.
Fig. 1.
Potential molecular targets of anti-oxidant diet phytochemicals for cutaneous carcinoma chemoprevention.
Table 1.
Cutaneum-specific induction of Nrf2 activation and/or its target protein expression/activity by some chemopreventive phytochemicals.
| Phytochemicals | Origin | Cell or animal models | Effect | Mechanism |
|---|---|---|---|---|
| Sulforaphane | Broccoli; cabbage; cauliflower; kale | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | Nrf2 signal pathway↑, HO-1 ↑ [81] |
| UVB-induced cutaneous photodamage in mice HaCaT | Cutaneous inflammation↓ Cellular defense↑ | Nrf2 signal pathway↑ [83] | ||
| Curcumin | Turmeric | Inorganic arsenite-stimulated HaCaT cell | Acute cytotoxicity↓ | Nrf2 signal pathway↑, NQO1↑, HO-1↑, GCL↑, γ-GCS↑ and catalase↑ [82] Nrf2 signal pathway↑, NQO1↑, HO-1↑ and GCLM↑ [90] |
| Lycopene | Tomato | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | Nrf2 signal pathway↑, GSH↑, ROS↓, MDA↓ and anti-oxidant enzymes↑ [87] |
| Resveratrol | Red grape | UVA radiation-induced HaCaT cell photodamage | Cell apoptosis↓ | Nrf2 signal pathway↑, Keap-1↓ and anti-oxidant enzymes↑ [91] |
| Phenylethyl isothiocyanate (PEITC) | Crucifer | UV radiation-induced HaCaT cell photodamage | Cell apoptosis↓ | Nrf2 signal pathway↑, NQO1↑, HO-1↑, GCL↑, γ-GCS↑ and catalase↑ [92] |
| Zerumbone | Tropical ginger | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | Anti-oxidative and phase II metabolizing enzymes↑ [93] |
| TPA-induced JB6P+ cell transformation | Grade malignancy↓ | Nrf2 signal pathway↑ and HO-1↑ [94] | ||
| Ellagic acid | Blackberry, raspberry, strawberry | UVA radiation-induced HaCaT cell photodamage | Cell apoptosis↓ | Nrf2 signal pathway↑, Keap-1↓ and anti-oxidant enzymes↑ [95] |
| Quercitrin | Oak bark | TPA and UVB-induced JB6P+ cell transformation | Grade malignancy↓ | Nrf2 signal pathway↑ MAPKs↓ [96] |
| Quercetin | Onion, apple, red wine, tea | UVA radiation-induced HaCaT cell photodamage | Cell apoptosis↓ | Nrf2 signal pathway↑ [97] |
| Thymoquinone | Black seed | TPA-induced cutaneous injury in mice | Cutaneous inflammation↓ | Nrf2 signal pathway↑, HO-1↑, GST↑ and NQO1↑ [98] |
| Myricetin | Waxberry | UVB-induced cutaneous carcinoma in mice | Cutaneous papillomagenesis↓ | Fyn↓ and Nrf2 signal pathway↑ [99,100] |
| D-limonene | Citrus fruit | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | Anti-oxidant enzymes↑ [101] |
| Caffeic acid | Coffee | TPA-induced cutaneous damage in mice | Edema formation↓ | MDA↓,GSH↑, anti-oxidant enzymes↑ [102] |
| DADS | Garlic | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | p21-dependent Nrf2 signal pathway↑, anti-oxidant enzymes↑ [89] |
3.1.2. Epigenetic modulators
Like other solid tumors, cutaneous carcinoma is thought to arise from a series of genetic and epigenetic events. The contribution of epigenetics to the development of cancers has been well supported, involving reversible heritable changes in gene expressions without changing DNA sequences [103]. Due to early incidence and reversible trait during cancer initiation and development, epigenetic modifications play central roles in cancer prevention [61,104]. The main epigenetic mechanisms by which gene expressions are regulated include DNA methylation, chromatin structure alterations by post-translational modification of histones, and miRNAs (Fig. 2). These processes affect DNA folding, transcript stability, chromatin compaction, nucleosome positioning and complete nuclear organization of genetic materials. They synergistically and cooperatively determine gene silencing, as well as the timing and tissue specificity of the expressions of these genes [105,106]. Numerous cutaneous carcinoma pathogens (UVR and chemicals) play pivotal role in silencing multiple gene expression by inducing the modulation of epigenetics, which be another important pathological factor in the initiation of cutaneous carcinoma, such as tumor suppressor genes (TSG) and cytoprotective genes [107-110]. In addition, these epigenetic changes can also potentially serve as molecular biomarkers in tumor tissues and in blood as circulating DNA, for diagnosing cutaneous carcinoma and predicting outcome and progression [265]. Given that epigenetic modification plays an important role in tumorigenesis, dietary phytochemicals which can modulate one or multiple epigenetic changes could be employed as blocking agents for cutaneous carcinoma.
Fig. 2.
Potential molecular targets of epigenetic modulated diet phytochemicals for cutaneous carcinoma chemoprevention.
DNA methylation undergoes catalysis that transfer methyl groups from Sadenosyl-L-methionine to a cytosine adjacent to guanine, known as CpG. Short CpG-rich regions, also known as “CpG islands”, exist in over half of human gene promoters, which are unmethylated in most cases [111,112]. However, enhanced methylation of CpG within gene promoters results in transcriptional silencing of TSG in cancer cells [113,114]. Similar to many solid cancers, both melanoma and NMSC are subjected to changes in DNA methylation of tumor-related genes (e.g. MLH-1, BRCA1 and SOCS1), being closely related with poor prognosis and relapse [115,116]. DNA is also methylated in mouse models of UVB- and DMBA/TPA-induced cutaneous carcinoma [107]. Thus, compounds which can downregulate level of DNA methylation could be considered as efficient candidate drugs for cutaneous carcinoma prevention and treatment. DNA methylation is mainly adjusted by methyltransferases (DNMTs), a family of rate-limiting enzymes. Polypeptides derived from Chlamys farreri was found to prevent carcinogen-induced HaCaT cell transformation through reducing DNMT3b and enhancing the level of tumor suppressor genes p16 and RASSF1A [117]. In addition to DNMTs, Tet family proteins are another key regulators, which can promote DNA demethylation by mediating the main intermediate state: DNA hydroxylation. Vitamin C, an essential nutrient for humans and derived from various fruits and vegetables, was demonstrated to protect cells from UV-induced apoptosis via enhancing Tet1/2-dependent DNA demethylation and re-activating suppressor genes (p16 and p21) eventually [118].
In addition to direct methylation of DNA, post-translational modifications of histone proteins on N-terminal histone tails, including methylation, acetylation, phosphorylation, sumoylation, ubiquitylation and ADP ribosylation, can also influence chromatin structure, playing important roles in tumorigenesis and gene regulation [119]. Most of these modifications occur at arginine, lysine and serine residues within the histone tails and regulate critical cellular processes such as replication, transcription and repair, leading to either repression or activation [120]. Histone modifications in cutaneous carcinoma are usually related to the conversion of normal tissues to neoplastic diseases [121]. Like DNA methylation changes, histone modifications are reversible, which can be regulated by enzymes that covalently modify histone proteins or eliminate such modifications (e.g. HATs and HDACs) [122,123]. Clearly, these enzymes are qualified targets for cancer chemoprevention and prevention. For example, ginsenoside Rg3, a monomer extracted from ginseng, can inhibit melanoma cell A375 and C8161 by decreasing HDAC3 and increasing acetylation of p53 on lysine (k373/k382) both in vitro and in vivo [124].
Additionally, miRNAs are also key moderators of epigenetic genes [125]. Although miRNAs are essential to the physiology of normal cells, aberrant expressions of small non-coding RNAs have been linked to carcinogenesis [126-128]. In cutaneous carcinoma, miRNAs, including miR-214, miR-30b, miR-506, miR-21, miR-31 and miR-221 as oncogenes together with miR-137, miR-29c, miR-205, miR034b and miR-375 as tumor suppressors, significantly affect the process of tumorigenesis [129,130]. In short, miRNAs have regulatory effects on key signaling pathways in almost the whole process of cutaneous carcinoma, so particular attention should be paid to miRNA-based cancer prevention and treatment [131]. Curcumin, polyphenol derived from curcuma longa, was found to block tumorigenicity and growth of B78H1 cells mainly through enhancing miR-205-5p level which effecting on cell proliferation and apoptosis eventually [132].
Moreover, interestingly, most of dietary phytochemicals are able to reverse multiple epigenetic changes in a systematic and comprehensive manner and then prevent the initiation and development of cutaneous carcinoma, such as EGCG, proanthocyanidins and apigenin. EGCG, an active and major constituent of green tea, has been shown to have both preventive and therapeutic effect on cutaneous carcinoma through multiple epigenetic modifying and eventually re-expressing tumor suppressor genes, p 16INK4a and Cip1/p21. This compound can not only decrease DNA methylation mediated by inhibiting level and activity of DNMT1, DNMT3a and DNMT3b, but also enhance levels of acetylated lysine 9 and 14 on histone H3 (H3-Lys 9 and 14) and lysine 5, 12 and 16 on histone H4 via decreasing HDAC activity. Besides, this compound can also inhibit levels of trimethylated lysine 27 on histone H3 [133-135]. Table 2 and Fig. 2 show representative dietary agents which have been demonstrated as blocking agents for cutaneous carcinoma through regulating major epigenetic modification mentioned above.
Table 2.
Cutaneous carcinoma-specific epigenetic alteration and related protein expression/activity regulated by some chemopreventive diet phytochemicals.
| Phytochemicals | Origin | Cell or animal models | Effect | Mechanism |
|---|---|---|---|---|
| EGCG | Green tea | UV-induced photo-carcinogenesis in mice | Cutaneous papillomagenesis↓ | UVB-induced global DNA hypomethylation pattern↓ [133] |
| SCC-13 Cutaneous carcinoma cell in vitro | Cell apoptosis↑ | TSG (Cip1/p21)↑, Histone methylation↓ and acetylation↑, PcG protein↓ (Ezh2 and Bmi-1) [134] | ||
| A431 cutaneous carcinoma cell in vitro | Cell survive↓ | TSG (Cip1/p21 and p16ink4a)↑, DNA methylation↓, histones acetylation↑, DNMT1, DNMT3a and DNMT3b↓, HDAC↓ [135] | ||
| Sulforaphane | Broccoli; cabbage; cauliflower; kale | TPA-induced JB6P+ cell transformation | Grade malignancy↓ | Nrf2↑, DNA methylation↓, histones acetylation↑, DNMT1, DNMT3a and DNMT3b↓, HDACs↓ [136] |
| SCC-13 Cutaneous carcinoma cell in vitro | Cell cycle arrest↑, cell apoptosis↑ | TSG (Cip1/p21)↑, Histone methylation↓ and acetylation↑, PcG protein↓ (Ezh2 and Bmi-1) [137]poptosis of skin cancer cell through | ||
| Curcumin | Turmeric | B78H1-tumor-bearing C57BL/6 mouse models | Tumor growth↓ | Anti-apoptosis (Bcl-2) and pro-proliferation protein (PCNA)↓, miR-205-5p↑ [132] |
| Proanthocyanidins | Grape seed | A431 and SCC13 cutaneous carcinoma cell in vitro | Cell survival↓ | TSG (RASSF1A, p16 and Cip1/p21)↑, DNA methylation↓, histones acetylation↑ and histones methylation↓, DNMT1, DNMT3a and DNMT3b↓, HDACs↓, HAT↑ [138] |
| Kaempferol | Tea, broccoli, grapes, apples | UV-induced photo-carcinogenesis in mice | Cutaneous papillomagenesis↓ | AP-1 and NF-κB↓, histone H3 phosphorylation↓, RSK2 and MSK1↓ [139] |
| Polypeptides | Chlamys farreri | UVB- induced HaCaT cell transformation | Grade malignancy↓ | TSG (p16 and RASSF1A)↑, DNA methylation↓, DNMT1, DNMT3a, DNMT3b↓ [117] |
| Apigenin | Caraway | Mouse cutaneous epidermal JB6 P+ cells in vitro | Not mentioned | Nrf2↑, DNA methylation↓, histones acetylation↑, DNMT1, DNMT3a and DNMT3b↓, HDACs↓ [140] |
| Ginsenoside Rg3 | Ginseng | A375 and C8161 Cutaneous carcinoma cell in vitro | Cell cycle arrest↑ | p53↑, histones acetylation↑, HDAC3↓ [124] |
| Butyric acid, nicotinamide and calcium glucarate | Dairy products | DMBA induced cutaneous carcinoma in mice | Cutaneous papillomagenesis↓ | TSG (p16)↑, tumor suppressor (miR203)↑, DNA methylation↓, histones acetylation↑, DNMT1↓ and HDAC1↓ [141] |
| Vitamin C | Vegetables and fruits | UV-induced cell photodamage | Cell apoptosis↓ | TSG (p21 and p16)↑, Tet1/2-dependent DNA demethylation↑ [118] |
3.2. Cutaneous carcinoma chemopreventive phytochemicals as suppressing agents
Compounds that influence later stages (promotion and progression) of carcinogenesis are referred to as “suppressing agents” for being able to decrease the proliferative capacity of initiated cells [142]. They prevent the cutaneous tissue chronic inflammation and proliferation of pre-malignant cells by down-regulating signal transduction pathways such as STAT3 and NF-κB [143-145]. Additionally, suppressing agents may also prevent or delay malignant cancer cells from metastasis by inhibiting pathways inducing angiogenesis, epithelial mesenchymal transition, invasion and dissemination which are mainly responsible for cancer-related death [146,147].
3.2.1. Anti-inflammation activity
“Chronic inflammation”, as suggested by epidemiological and experimental evidences, can be induced by mechanical injuries, physical agents (UVR), chemical agents (arsenic, tar products and immune-modulatory drugs), biological agents (parasites, viruses, bacteria and fungi) and immunological disorders (autoimmunity, hypersensitivity reactions and immune deficiency states) [148]. Accordingly, it is one of the hallmarks of environmental agent-mediated cutaneous carcinomas that affect almost all the important aspects (e.g. survival of cancer cells, responses to hormones and chemotherapeutic drugs, metastasis and angiogenesis) [149,150]. In clinical practice, such pathological phenomenon (e.g. chronic wound sites and dystrophic epidermolysis bullosa) may predispose individuals to augment susceptibility of cutaneous carcinoma [151]. Considering the potential role of inflammation in tumor initiation and its dominant role in promotion/progression, angiogenesis and metastasis, the inflammation-related pathways and factors mentioned above may be attractive targets for the prevention and treatment of cutaneous carcinoma [152,153]. Interestingly, long-term use of aspirin, a traditional non-steroidal anti-inflammatory drugs, has shown obvious chemopreventive effects on cutaneous carcinoma in epidemiological and clinical studies [154].
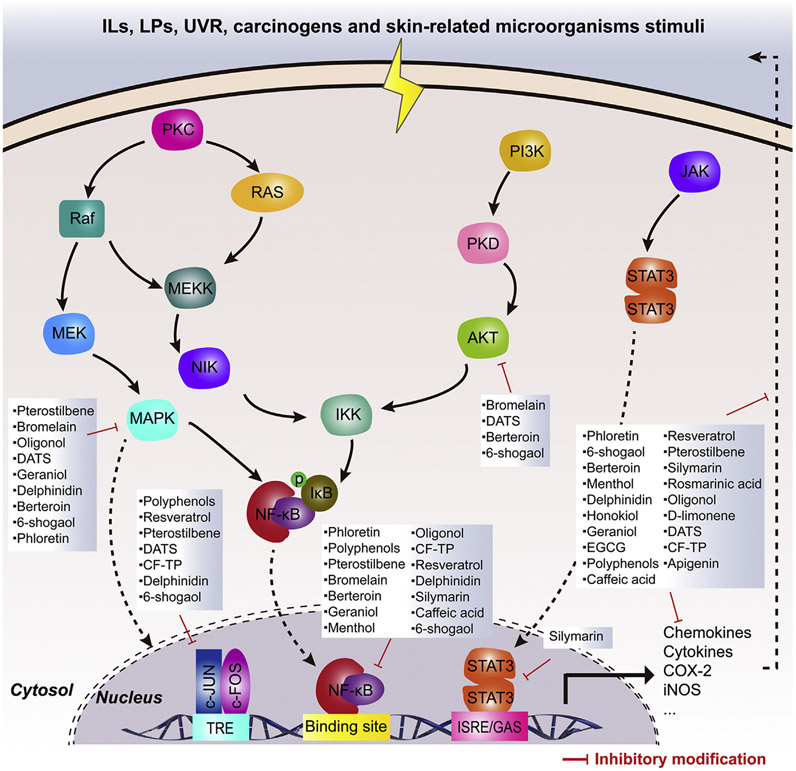
The pathways connecting cancer and inflammation are either intrinsic or extrinsic [155]. Intrinsic pathways are initiated by genetic events, involving inactivation of tumor suppressor genes (p16/INKA4 and p14/ARF) and activation of oncogenes (H-ras, N-ras and c-myc) by mutation, amplification or chromosomal rearrangement [156]. In contrast, extrinsic pathways represent soluble mediators and inflammatory leukocytes caused by mechanical, UVR, ROS, ionizing radiation and skin-related microorganisms (e.g. Neisseria gonorrhoeae, Borrelia burgdorferi, Staphylococcus aureus, measles virus and HIV-1). Moreover, some extrinsic factors also frequently affect gene products such as MC1R and MITF [157-159]. As a result, cooperation between these two pathways stimulates multiple transcription factors (mainly NF-κB, STAT3 and AP-1) in cutaneous cell through activating intracellular kinase system (MAPK, PI3K/Akt, JAK), and then a cancer-related inflammatory microenvironment forms, accompanied by elevated risks of cutaneous carcinoma development and aggravation. Thus, dietary products that can modulate these three transcription factors, which can coordinate the transcription of hundreds of target genes, are generally treated as suppressing agents for cutaneous carcinoma.
Genes for various chemokines (IL-6, IL-1 and TNF), cytokines, VCAM-1, intercellular adhesion molecule-1, E-selectin and urokinase plasminogen activator are essential to the initiation and deterioration of cutaneous inflammation. Geraniol was found to protect cutaneous tissue from TPA-induced damage through downregulating level of multiple chemokines (IL-6, IL-1 and TNF) via inactivating p38-dependent NF-κB [160]. They can also regulate pro-inflammatory enzymes (e.g. COX-2 and iNOS) that participate in chronic inflammation of the skin and subsequently in carcinogenesis. Pterostilbene, a natural analogue of resveratrol from blueberries, was found to prevent DMBA/TPA-induced cutaneous inflammation (iNOS and COX-2) and papillomas through restraining NF-κB and AP-1 via inactivating MAPK and PI3K/Akt [161]. In our lab, we have demonstrated menthol can protect cutaneous tissue from chemical carcinogens via its anti-inflammatory ability by downregulating p38 and ERK-dependent NF-κB pathway [162]. Additionally, these three transcription factors can modulate the expressions of genes controlling cancer cell apoptosis (Bcl-2, Bcl-xL, c-FLIP, XIAP and survivin), proliferation (c-Myc and cyclins), angiogenesis (VEGF), and metastasis (MMPs) [163]. Topical application of honokiol, an active constituent of Magnolia plant, resulted in the prevention of UVB-induced cutaneous papilloma formation through inhibiting proinflammatory disorder (IL-6, IL-1, TNF, COX-2 and PGE2) and cell proliferation (PCNA, cyclin D1, D2, E and CDKs) via inactivating PI3K/Akt [164].
In daily meals, there are various dietary products that can modulate one or multiple signaling pathway (NF-κB, STAT3 and AP-1) and have outstanding, broad-spectrum anti-inflammatory activities, accompanied by chemopreventive proficiency in laboratory models in vivo and in vitro (Table 3 and Fig. 3). Being further associated with lower cutaneous carcinoma risks in human studies, such functional agents may be eligible candidates for development like aspirin.
Table 3.
Cutaneous carcinoma-specific inflammatory condition and related protein expression/activity regulated by some chemopreventive diet phytochemicals.
| Phytochemicals | Origin | Cell or animal models | Effect | Mechanism |
|---|---|---|---|---|
| EGCG | Green tea | UV-induced photo-carcinogenesis in mice | Cutaneous papillomagenesis↓ | Pro-inflammatory cytokines↓, COX-2↓, PGE2↓, PCNA↓, and cyclin D1↓ [165] |
| Polyphenols | Black tea | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓ through MAPK↓, AP-1↓ and NF-κB↓ [166] |
| Resveratrol | Red grape | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, IL-6↓, AP-1↓ and NF-κB↓ [167] |
| Pterostilbene | Blueberry | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, iNOS↓, MAPK↓, AP-1↓ and NF-κB↓ [161] |
| Silymarin | Milk thistle | UV-induced photo-carcinogenesis in mice | Cutaneous papillomagenesis↓ | COX-2↓, iNOS↓, STAT3↓ and NF-κB↓ [168] |
| Rosmarinic acid | Rosemary | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | PGE2↓, COX-2↓, chemokines KC↓, macrophage inflammatory protein-2↓ [169] |
| Bromelain | Pineapple | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, NF-κB↓, MAPK↓, Akt↓ [170] |
| Oligonol | Lichee | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, NF-κB↓, MAPK↓ [171] |
| D-limonene | Citrus fruit | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | Cutaneous inflammation↓, COX-2↓ [101] |
| Diallyl trisulfide (DATS) | Garlic | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, iNOS↓, Akt↓, JNK↓, AP-1↓ [172] |
| CF-TP | Pinnatifida | TPA-induced JB6P+ cell transformation | Grade malignancy↓ | COX-2↓, iNOS↓, NF-κB↓, AP-1↓ [173] |
| DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | |||
| Apigenin | Caraway | UVB-induced cutaneous photodamage in mice and in JB6 P+ cell | Inflammatory response↓ | COX-2↓, Src↓ [174] |
| Geraniol | Rose | TPA-induced cutaneous damage in mice | Inflammatory response↓ | NF-κB↓, p38↓, TNF-α↓, IL-1β↓, and IL-6↓ [160] |
| Honokiol | Magnolia officinalis | UV-induced photo-carcinogenesis in mice | Cutaneous papillomagenesis↓ | COX-2↓, PGE2↓, PCNA↓, cyclin Ds↓, CDKs↓, pro-inflammatory cytokines↓ [164] |
| Delphinidin | Berry | TPA-induced JB6P+ cell transformation | Grade malignancy↓ | COX-2↓, PGE2↓, NF-κB↓, AP-1↓ through MEK/ERK↓ [175] |
| Menthol | Mint | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, NF-κB↓, ROS↓ [162] |
| Dehydroglyasperin C | Licorice | TPA-induced JB6P+ cell transformation | Grade malignancy↓ | COX-2↓, NF-κB↓, AP-1↓ through MKK4↓ and PI3K↓ [176] |
| Momordica grosvenori extract | Momordica grosvenori | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, iNOS↓, NF-κB↓, MAPK↓, Akt↓ [177] |
| Berteroin | Cruciferous vegetables | TPA-induced cutaneous damage in mice | Edema formation↓ | COX-2↓, iNOS↓, NF-κB↓, TNF-α↓, IL-6↓, IL-1β↓, Akt↓, ERK↓ [178] |
| Hexahydro-beta-acids | Lupulus | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, iNOS↓, MAPK↓, Akt↓, NF-κB↓ [179] |
| Flavonoid | Artocarpus communis | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, PGE2↓, MAPK↓, Akt↓, NF-κB↓, –AP-1↓, pro-inflammatory cytokines↓ [180] |
| Phloretin | Apple | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, NF-κB↓, ERK↓ [181] |
| Phenethyl isothiocyanate | Cruciferous vegetables | TPA-induced cutaneous damage in mice | Edema formation↓ | COX-2↓, iNOS↓, NF-κB↓, ERK↓, Akt↓, TNF-α↓, IL-6↓, IL-1β↓ [182] |
| 6-shogaol | Ginger | DMBA/TPA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | COX-2↓, iNOS↓, NF-κB↓, MAPK↓, Akt↓ AP-1↓ [183] |
| Caffeic acid | Coffee | TPA-induced cutaneous damage in mice | Edema formation↓ | COX-2↓, NF-κB↓, TNF-α↓ [102] |
Fig. 3.
Potential molecular targets of anti-inflammatory diet phytochemicals for cutaneous carcinoma chemoprevention.
3.2.2. Anti-angiogenesis activity
Pathological angiogenesis, which is regarded as unregulated angiogenesis typically, mainly drives many serious human diseases including cancers [184]. Since angiogenesis delivers nutrients and oxygen to growing tumors, it is an essential pathological feature of cancer, also playing vital roles in enabling other aspects of tumor pathology such as dissemination/metastasis and metabolic deregulation [185,186].
Cancers occurring in the skin, like all solid malignancies, are also highly angiogenic, including benign growth [187,188]. For instance, warts have increasing vascularity that is accompanied with growth and persistence [189]. UVR and chemical carcinogen play vital roles in vascularization-related cutaneous lesion. In normal cutaneous tissues, however, acute damage drastically changes the levels of growth factors and cytokines [190,191]. Angiogenesis stimulators, such as FGF and VEGF, are significantly up-regulated, thus increasing the cutaneous microvessel density. In the meantime, endogenous angiogenesis inhibitors, such as TSP-1, are significantly locally reduced. As mentioned above, carcinogen-induced chronic damage can cause the accumulation of cutaneous tissue mutations, probably leading to neoplastic and hyperplastic transformations. Hyperplastic cutaneous lesions, including atypical melanocytic nevi and AKs, have higher capillary densities than those of surrounding normal tissues [192]. This situation is more obvious during the promotion from hyperplasia to neoplasia (e.g. BCC and SCC) and the progression to malignant melanoma, with higher levels of angiogenesis mediators including FGF-2, VEGF, PIGF, IL-8, MMPs and Ang-2 [193-196]. Above all, angiogenesis is necessarily involved throughout cutaneous carcinoma, especially in promotion and progression, as an available pathological process for preventing cutaneous carcinoma.
The anti-angiogenic properties of many dietary phytochemicals against cutaneous carcinoma have been evaluated in vivo and in vitro. The main mechanisms underlying their anti-angiogenic effects include: (1) inhibition of MMPs: MMPs, a series of zinc-containing endopeptidases, can cleave a wide range of ECM components, promote activated endothelial cells development and invasion. Furthermore, MMPS also play a primary role in angiogenesis by promoting the expression of VEGF, mediating endothelial cell migration and vascular formation processes [197]. Topical application of EGCG, an active constituent of green tea, resulted in the prevention of UV-induced cutaneous papilloma formation partly through inhibiting tumor angiogenesis by decreasing level of MMP-2, MMP-9 and VEGF. (2) modulation of angiogenic related signaling pathways: NF-κB, HIF-1α and AP-1 are the most vital transcription factors in cancer cells, which can control the expression of multiple angiogenesis-related proteins, such as COX-2, growth factors and cytokines [198,199]. These substances cannot only degrade the extracellular matrix, but also activate various growth factor receptors, which can promote the survival, proliferation, metastasis of these tumor endothelial cells and capillary sprout formation. Antiangiogenic activity of berberine in B16F10 xenograft tumor is mediated through the downregulation of HIF1, NF-κB, VEGF, and proinflammatory mediators (ILs, COX-2, iNOS TNF-α) [200]. Most of dietary phytochemicals are able to reverse both two mechanisms mentioned above and then prevent the cutaneous carcinoma angiogenesis and tumor growth. β-carotene has been demonstrated inhibit melanoma inflammatory angiogenesis through decreasing level of serum cytokine (IL-1, IL-6, TNF and GM-CSF), MMP-2, MMP-9 and inactivating NF-κB and AP-1 [201]. Fig. 4 and Table 4 conclude representative cutaneous carcinoma chemopreventive dietary agents capable of blocking tumor angiogenesis and delaying cutaneous carcinoma promotion and progression.
Fig. 4.
Potential molecular targets of anti-angiogenic diet phytochemicals for cutaneous carcinoma chemoprevention.
Table 4.
Cutaneous carcinoma-specific excessive angiogenesis and related protein expression/activity regulated by some chemopreventive diet phytochemicals.
| Phytochemicals | Origin | Cell or animal models | Effect | Mechanism |
|---|---|---|---|---|
| EGCG | Green tea | UV-induced photo-carcinogenesis in mice | Cutaneous papillomagenesis↓ | Cutaneous carcinoma vascular↓ through VEGF↓, MMP-2↓ and MMP-9↓ [202] |
| Resveratrol | Red grape | DMBA-induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | Cutaneous carcinoma vascular↓ through VEGF↓, MMP-2↓ and MMP-9↓ in TLR4-dependent manner [203] |
| Apigenin | Caraway | UV-induced cutaneous photodamage in mice | Epidermal thickness↓, keratinocyte layers↓ | Microvascular density (MVD) in the dermis↓, TSP1↑, VEGF↓, COX-2↓ [204] |
| Myricetin | Waxberry | UV-induced photo-carcinogenesis in mice | Neovascularization in cutaneous↓ | HIF-1↓, VEGF↓, MMP-2↓, MMP-9↓, PI3K/Akt↓ [205] |
| Betulin | Raisins | DMBA/TPA- induced cutaneous tumor in mice | Cutaneous papillomagenesis↓ | Tumor angiogenesis↓, VEGF↓ [206] |
| β-carotene | Green leafy vegetables and orange fruits | B16F10-tumor-bearing mouse models | Tumor growth↓ | Inflammatory angiogenesis↓, serum cytokine levels↓, MMP-2↓, MMP-9↓, TIMP-1↑ by NF-κB↓ and AP-1↓ [201] |
| Ascorbic Acid | Vegetables and fruits | A2058-tumor bearing athymic nude mouse model | Tumor growth↓ | Tumor angiogenesis↓ by VEGF↓ and MMP-9↓ [207] |
| Tocopherol | Vegetable oil, nut | B16F10-tumor bearing mouse models | Tumor growth↓ | Tumor angiogenesis↓ by VEGF↓, VEGFR1↓ and VEGFR2↓ [208] |
| Berberine | Coptis chinensis | B16F10-tumor-bearing mouse models | Neovascularization in melanoma↓ | Pro-angiogenic factors↓ (such as HIF, VEGF, COX-2, NO, NF-κb, and proinflammatory cytokines) [200] |
| Genistein | Soybean | B16-tumor-bearing mouse models | Tumour blood supply↓ | Not mentioned [209] |
| 13-cis-Retinoic acid | Animal foods, carrot, tomato | B16F10-tumor-bearing mouse models | Tumor directed capillaries↓ | Proinflammatory cytokines↓, VEGF↓, TIMP-1↑, NF-κb↓ and AP-1↓ [210] |
3.2.3. Anti-migration activity
Melanoma accounts for merely 4% of all cutaneous carcinoma cases, but it is highly metastatic and most aggressive, causing about 80% of all related deaths [211]. As other metastatic tumors, melanoma cells progress from benign melanocyte hyperplasia into metastatic melanoma by undergoing a remarkable cascade of events. The process of metastasis consists of two steps [212]. Firstly, melanoma cells move and leave their primary sites through multiple courses from extension, adhesion, contraction to detachment. Secondly, melanoma cells that complete the first step are allowed to enter lymphatic or blood vessels (intravasation) and then target organs (extravasation) [213,214]. Given that melanoma therapy is greatly challenged by the metastatic properties of malignant cells, blocking their migratory and invasive capacities by using phytochemicals may pave the way for the prevention and treatment of cutaneous carcinoma (melanoma). Most related studies mainly focuses on the first step that is orchestrated by several external factors and signaling pathways, including proteases, chemokines, growth factors/receptors, extracellular matrix proteins, matrix-degrading enzymes, integrins, cell–cell communication proteins and cell–cell adhesion molecules [215].
First, the migration of melanoma cells begins with the extension of lamellipodium, after escaping the control keratinocytes. Stimulated and regulated by Rac protein, which is overactive in most metastatic melanoma cells, is underpinned by polymerization of actin that provides a traction force for pushing the membrane to move [216]. Capsaicin, the major pungent ingredient of red pepper, has been reported inhibits the migration of B16F10 cells through inhibiting Rac activity by inactivating PI3K/Akt signal pathway [217]. Afterwards, the extended lamellipodium needs to anchor the ECM through adhesion molecules to facilitate cell attachment and spreading [216,218,219]. Oral administration of ursolic acid increased the survival rate of melanoma lung metastasis in C57BL/6 mice through inhibiting focal adhesion signaling pathway, including ICAM-1, VCAM-1, E-selectin, integrin α6β1, etc [220]. Then, to penetrate the matrix, surface proteases, such as MMPs, are recruited toward the attachment sites to hydrolyze adjoining ECM substrates. In invasive melanoma cases, MMP-9, MMP-2 and MT1-MMP are especially up-regulated [221,222]. The regulation of MMPs depends on mutiple intracellular signal transduction processes, such as nuclear transcription factors (e.g. NF-κB, AP-1, ATF-2 and HIF), signaling pathways (e.g. Ras/PI3K/Akt) and inflammatory disorder (e.g. COX-2, EP2, IL-6, IL-1β, TNF-α and GM-CSF) [223,224]. Carnosol, a constant constituent of Rosmarinus, was found to block B16F10 cell invasion by reducing MMP-9 expression and activity through suppressing MAPK and Akt signaling pathway and inhibition of NF-kappaB and AP-1 binding activity [225]. Finally, the trailing edge of cells is detached from ECM and cells now are free to keep moving forward eventually leaving the tumour primary site [212]. We herein reviewed the diet chemical entities with well-documented in vitro and in vivo effects on melanoma migration (Table 5 and Fig. 5).
Table 5.
Chemopreventive diet phytochemicals regulate melanoma metastasis and related protein expression/activity.
| Phytochemicals | Origin | Cell or animal models | Effect | Mechanism |
|---|---|---|---|---|
| Berberine | Coptis chinensis | A375 and Hs294 in vitro | Cell invasion↓, TPA-induced cell migration↓ | COX-2↓, PGE2↓, EP2↓, EP4↓, NF-κb↓ [226] |
| A375 and B16F10 in vitro, B16F10 experimental pulmonary metastasis in mice | Cell invasion and migration↓, lung metastasis↓ | AMPK↑, ERK↓, COX-2↓ [227] | ||
| Proanthocyanidins (PC) | Grape | A375 and Hs294t in vitro, TPA-stimulated A375 in vitro, A375 experimental pulmonary metastasis in mice | Cell invasion and migration↓, lung metastasis↓ | COX-2↓, EP2↓, EP4↓, NF-κb↓, epithelial biomarkers↑ (E-cadherin, cytokeratin), mesenchymal biomarkers↓ (vimentin, fibronectin and N-cadherin) [228] |
| Anthocyanins | Mulberry | B16F1 in vitro, B16F1 spontaneous metastatic model in mice | Cell invasion and migration↓, lung and liver metastasis↓ | MMP-2/9↓, Ras↓, Akt↓, NF-κb↓ [229] |
| Silymarin | Milk thistle | A375 and Hs294t in vitro | Cell invasion and migration↓ | β-catenin↓, MMPs↓ [230] |
| Quercetin | Fennel, caraway, onion | HGF-stimulated A2058 and A375 in vitro | Cell invasion and migration↓ | C-Met↓, Gab1↓, FAK↓, PAK↓ [231] |
| A375 and A2058 in vitro, B16F10 experimental pulmonary metastasis in mice | Cell invasion and migration↓, lung metastasis↓ | STAT3↓, MMP-2↓, MMP-9↓, VEGF↓ [232] | ||
| Apigenin | Caraway | B16F10 experimental pulmonary metastasis in mice | Lung metastasis↓ | STAT3↓, MMP-2↓, MMP-9↓, VEGF↓ and Twist1↓ [233] |
| EGCG | Green tea | A375 and Hs294t in vitro | Cell invasion and migration↓ | COX-2↓, PGE2↓, EP2↓, EP4↓, epithelial biomarkers↑ (E-cadherin, cytokeratin and desmoglein 2), mesenchymal biomarkers↓ (vimentin, fibronectin and N-cadherin) [234] |
| M17 in vitro | Cell migration↓ | MMP-2↓, TIMP-2↑, ERk↓ [235] | ||
| Capsaicin | Pepper | B16F10 in vitro | Cell migration↓ | Akt↓, PI3K↓, Rac1↓ [217] |
| Resveratrol | Red grape | B16F10 in vitro, B16BL6 experimental pulmonary metastasis in mice | Cell invasion and migration↓, lung metastasis↓ | Akt↓ [236] |
| Pterostilbene | Blueberry | B16MF10 experimental liver metastasis in mice | Metastatic growth to the liver↓ | Vascular adhesion molecule 1↓ [237] |
| Gallic acid | Grape, tea | A375. S2 in vitro | Cell invasion and migration↓ | Ras/ERK pathway↓, pathways MMP-2/9↓ [238] |
| Riboflavin | Animal foods, carrot, tomato | B16F10 in vitro, B16F10 experimental pulmonary metastasis in mice | Cell invasion and migration↓, lung metastasis↓ | TIMP↑, MMPs↓, HH pathway↓ [239] |
| Carnosol | Rosemary | B16F10 in vitro | Cell invasion↓ | MMP↓, Akt↓, MAPK↓ NF-κb↓, AP-1↓ [225] |
| Parthenolide | Feverfew | A375 and WM793 in vitro | Cell invasion and migration↓ | NF-κb↓, IL-8↓ and MMP-9↓ [240] |
| Lipid-soluble extract | Ginseng | B16F10 in vitro, B16F10 experimental pulmonary metastasis in mice | Cell migration↓, lung metastasis↓ | MMP-2↓ [241] |
| Ginsenoside Rg3 | Ginseng | B16F10 in vitro | Cell invasion and migration↓ | MMP-13↓ [242] |
| 1-Deoxynojirimycin ursolic acid | Mulberry Berries |
B16F10 in vitro B16F10 experimental pulmonary metastasis in mice |
Cell invasion and migration↓ lung metastasis↓ | MMP-2/9↓, TIMP↑, α-mannose↑ [243] focal adhesion pathway (ICAM-1, VCAM-1, E-selectin, P-selectin, integrins)↓ [220] |
Fig. 5.
Potential molecular targets of anti-migratory diet phytochemicals for cutaneous carcinoma chemoprevention.
4. Cutaneous carcinoma chemoprevention using dietary phytochemicals: clinical studies
Although the preventive effects of diverse bioactive phytochemical constituents on cutaneous carcinogenesis have been well-documented, their efficacy, safety and dose adjustment for human remain largely unknown. It is crucial to develop cutaneous chemoprotective or chemopreventive agents based on natural compounds by translating these preclinical findings into clinical settings. Some well-defined chemopreventive phytochemicals (curcumin, green tea polyphenols, sulforaphane and resveratrol) have been verified to affect various biological activities such as inactivation of protein kinases (PI3K/Akt and MAPK), modulation of the activities of transcriptional factors (AP-1, NF-κB, STAT3 and Nrf2), decrease of inflammatory and growth factors (TNF-α, VEGF and IL-1β) and regulation of epigenetic alterations (HDACs and DNMTs). Accordingly, multi-stages of cutaneous cell cancerization and deterioration may be better inhibited by such versatile chemicals via modulating key cellular proteins participating in different signaling transduction pathways and thus changing the expressions of genes involved in epigenetic modification, antioxidation and cancer-related angiogenesis and inflammation. Up to now, human intervention trials that target cutaneous carcinoma prevention are still lacking, cutaneous damages and diseases have been treated by these versatile chemicals though. As indicated by the limited human trials, systemic administration of these phytochemicals has protective benefits, barely causing adverse effects also.
4.1. Green tea polyphenols
Polyphenols derived from green tea, also known as green tea polyphenols (GTPs), have attracted global attention recently because of beneficial effects, especially significant roles in cancer chemoprevention and chemotherapy. GTPs predominantly comprise (−)-epigallocatechin, (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin and (−)-epicatechin-3-gallate, among which EGCG is most abundant and associated with the majority of green tea intake-related health benefits [21]. Although GTPs have been widely proven as promising chemopreventive agents in preclinical studies, their clinical benefits for human subjects remain elusive. As a comprehensive and noninvasive biomarker for evaluating UV radiation (UVR) damage, erythema has been used as a quantifiable surrogate end point for cutaneous damage and risk of cutaneous carcinoma [244]. Topical application of GTPs can reduce the number of sunburnt cells, photoprotect against UVR-induced erythema response and DNA damage, and protect epidermal Langerhans cells [245]. Similarly, Heinrich U et al. validated the photoprotective bioactivities of orally administered polyphenols in a 12-week, double-blinded, placebo-controlled study [246]. In a phase I clinical study, topical use of 660 μM EGCG for two weeks during radiotherapy was non-toxic for patients with non-inflammatory breast cancer, which managed to prevent radiation-induced dermatitis effectively and to lower the symptom scores of burning, pain, tenderness and itching significantly [247]. Therefore, GTPs/EGCG can relieve carcinogen-induced cutaneous damages and may then help prevent cutaneous carcinogenesis. In-depth studies are still in need to further demonstrate the relationships between these compounds and cutaneous cancer or precancerous lesions.
4.2. Curcumin
As a component of turmeric, curcumin is a strong antioxidant and anti-inflammatory agent and has been employed to treat cutaneous ailments such as acne, scabies, wound, eczema and wrinkled skin. Due to low toxicity and broad-spectrum applications, curcumin has been extensively studied, including in cancer prevention and treatment. In a phase I clinical trial of Chen et al., curcumin that was orally taken at up to 8 g/day for 3 months was non-toxic to human and improved the histological characteristics of arsenic Bowen’s disease of the skin, one of the main precancerous symptoms [248]. To exert pharmacological effects on tissues by augmenting the absorption and bioavailability of curcumin, several formulation techniques, such as combination with a naturally bioavailable enhancer (curcumin C3 complex combining curcumin with piperine), have been developed [249]. A randomized, double-blinded, placebo-controlled clinical trial demonstrated that oral administration of curcumin C3 complex (equivalent to 2 g curcumin) three times a day effectively prevented radiation-induced dermatitis during radiotherapy for breast cancer [250]. Additionally, continuously using this formulation for 4 weeks also enhanced the activities of various antioxidant enzymes, and alleviated sulfur mustard-induced atopic dermatitis and cutaneous pruritus [251]. Moreover, some percutaneous absorption preparations for topical administration, such as curcumin cream [252], have been developed to maintain a biologically effective concentration in cutaneous tissues. Thus, given either systemically or topically, curcumin can change the cutaneous biochemistry in human body and prevent cutaneous carcinogenesis.
4.3. Sulforaphane
Sulforaphane, naturally occurring in cruciferous vegetables such as broccoli and cabbage, has been highlighted recently. Preclinical studies have verified that it inhibited tumor development and progression by preventing inflammation, oxidative stress and carcinogenesis. Still, the cancer preventive effects of sulforaphane on human have not yet been well validated. A randomized, double-blinded, and placebo-controlled study demonstrated that topical application of sulforaphane-containing extracts (200 nmol/cm2, 3 times/day) protected healthy human subjects against development of cutaneous erythema induced by solar-simulated UVR through activation of the Nrf2 signaling pathway [253]. Likewise, Talalay et al. found that after topical application of sulforaphane-rich extracts from 3-day-old broccoli sprouts, phase 2 enzyme (NQO1) in the homogenates of human cutaneous punch biopsies with 3-mm full thickness was up-regulated, and the susceptibility to erythema resulting from narrow-band (311 nm) UVR was attenuated, without any adverse reactions [254]. Additionally, sulforaphane (5 and 10 μM) can alleviate UVR-induced structural damages in sunburnt cells and apoptosis by activating the Nrf2 pathway and raising the levels of several antioxidant enzymes (CAT, HO-1, NQO1 and γGCS) in the full-thickness skin explant samples collected from patients receiving abdominoplastic surgery [92]. Since all the studies focused only on whether sulforaphane protected cutaneous tissues from carcinogen (UVR)-induced damages hitherto, the applicability of this compound to cutaneous cancer prevention in human body still needs further research.
4.4. Resveratrol
With well-established effects on inflammation, oxidative stress, angiogenesis and metastasis, resveratrol has become a feasible chemopreventive agent for cutaneous carcinoma, but its clinical benefits for human remain unclear. Topically applying a formulation containing 1% resveratrol, 0.5% baicalin and 1% vitamin E for 12 weeks can mildly modulate photodamaged skin, thereby being suitable for cutaneous rejuvenation [255]. Given the low bioavailability of this compound when administrated either orally or topically, novel formulation strategies have been attempted. For example, topically treating human volunteers with resveratrol-containing cream improved skin-related parameters including sharply decreased aging signs [256]. Researchers have endeavored to realize dermal resveratrol delivery into human skin by using formulation techniques such as optimized microemulsions [257] and lipid-core nanocapsules [258]. In addition, Amiot MJ et al. developed a soluble resveratrol formulation that had an 8.8-fold higher plasma concentration in healthy volunteers than that of powders [259]. Based on these pharmaceutical achievements in human subjects, it is necessary to further confirm the chemopreventive activities of resveratrol against cutaneous carcinoma.
4.5. Apigenin
As a flavone, apigenin has shown antioxidant, anti-inflammatory and anti-angiogenic effects in experimental studies, being closely associated with its prevention properties against cutaneous carcinoma. However, developing apigenin for clinical use is also obstructed by its low permeability and bioavailability. The concentration of apigenin in cutaneous tissues has been tentatively elevated by liposomes, etc. Topically applying liposomal creams containing apigenin can effectively reverse the inflammatory state in patients suffering from contact dermatitis [260]. A similar formulation is also capable of suppressing UV-induced cytotoxicity and preventing skin aging signs in clinical practice [261]. Hence, apigenin has outstanding biological activities and clinical efficacy, allowing it a potential cutaneous carcinoma-preventive compound, which, however, needs to be further validated.
5. Conclusion and future prospects
The incidence and death rates of cutaneous carcinoma have skyrocketed compared with those of other cancers in recent years. Even if effective cutaneous carcinoma treatment modalities (including chemo-, radio-, photodynamic-, immunotherapies and selective inhibitors) have been developed, the burden of cutaneous carcinoma remains chronically high as their potential treatment-resistant and serious toxic effect. The upgraded efficacy of treatment commonly arises with the potential expense of toxicity, which depending on the specific agents used as adjuvants, as well as on the dose used, the extent of treatment and the route of administration [10,11]. As an example, BRAF inhibitors show ability as a potentially effective treatment in BRAFV600 mutated metastatic melanoma patients. However, major limitations include an dermatological “off target” effects and serious adverse reactions, as numerous cutaneous toxicities [263].
Nowadays, chemoprevention by using edible diet phytochemicals has become an inexpensive, easily applicable, accessible and acceptable approach to control and to manage cutaneous carcinoma. With healthcare costs being a key issue, consumption of phytochemicals would be a more cost-effective cancer-preventive strategy than use of clinical drugs. These compounds may not be so effective as chemotherapeutic or pharmaceutical agents, but they have clear potentials. Furthermore, diet phytochemicals may play exciting roles in preventive care, especially because they only need to be supplemented in the daily diet and lifestyle regimens with skyscraping safety and wide human acceptance in a relative long-term use process. Phytochemicals have lower specificities to single target proteins than synthetic inhibitors do, which may reduce the toxicities of the former. In addition, as the speciality of a native compound, each one of them has versatile multproperties (e.g. anti-inflammation, antioxidation, anti-metastasis, anti-angiogenesis and epigenetic modulation) through which they exert anti-carcinogenic effects to regulate multiple different molecular signaling pathways and biochemical processes mediated or induced by carcinogens. Based on maxi-multiple epidemiological and laboratory evidences, routine application or consumption of these natural agents may sufficiently shield the damaging effects of solar UVR and other cutaneous carcinogens in the environment. Ultimately, agents combined with cutaneous care cosmetics or sunscreens may provide a reasonable strategy for reducing the incidence rates of cutaneous carcinomas and other cutaneous diseases.
Still, the relationship between cutaneous carcinoma and intake of dietary phytochemicals remains largely unknown and should be further explored. Despite abundant laboratory and epidemiological evidences, more human randomized controlled trials (RCTs) are still needed to further assess the preventive effects of these phytochemicals on cutaneous carcinoma. Human beings have a big difference from experimental animals in pharmacokinetic characteristics. Thus, both pharmacokinetic properties and bioavailability are key issues in investigating the dietary prevention of cancer and should be assessed carefully before undertaking human RCTs.
In recent years it has become more and more obvious that experimental figures obtained in vivo are very often followed by unacceptable results in clinic. A hopeful strategy to overcome this problem includes the development of suitable carrier systems. The key advantage is that the in clinic fate of the drug is no longer merely determined by the characteristics of the drug, but by the carrier system, which must permit a localized and controlled drug release achieved through topical and systemic delivery systems. Thus, series of drug carrier system (such as nanocarriers nanoparticles, nanoemulsions, liposomes, micelles, vesicles, soluble polymer–drug conjugates, etc) need to be further exploit and utilize, which may improve the effect of diet phytochemicals on cutaneous carcinoma prevention in clinic.
In addition, while it is encouraging that single dietary phytochemicals have meglio chemopreventative properties in a range of different models in most experimental study, it is worth noting that the combined effects of multiple dietary chemopreventative compounds are scarcely reported. Cutaneous carcinoma development is a multistep process and diverse pathological conditions and signaling pathways are involved in. Thus, whether using a combination of phytochemicals can provide synergistic or additive preventive effects through the different pathway in the complicated process of cancerization need to be further demonstrated in both laboratory and clinical studies.
Moreover, cancer incidence risk, prognosis and response to treatment differ among individuals, so they can be effectively treated only by utilizing individualized regimens. Till now, a personalized diet for preventing cancers, although feasible in theory, has not been well documented. Thus, to control cutaneous carcinoma through chemoprevention, we need to identify the right patient population and the right agents simultaneously. In the future, we will endeavor to find out risk factors of cutaneous carcinoma, gene signatures and corresponding pathological reaction relationships to identify the high-risk population, to propose targeted preventive therapies with diverse dietary phytochemicals, and to finally benefit each individual. To summarize, to make chemoprevention a successful strategy for cutaneous carcinoma in use of dietary phytochemicals, aimed at identifying the right patient population, the right agents and the right drug carrier systems need to be investigated simultaneously.
Acknowledgements
This project was supported in part by National Natural Science Foundation of China (81403260, 81573859), China Postdoctoral Science Foundation (2014M551639), Postdoctoral funding in Jiangsu Province (1401138C), Jiangsu College graduate research and innovation projects (KYZZ15_0271), 2013′ Program for Excellent Scientific and Technological Innovation Team of Jiangsu Higher Education, A Project Funded by the Flagship Major Development of Jiangsu Higher Education Institutions (PPZY2015A070), and a project of the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Abbreviations:
- Akt
protein kinase B
- AMPK
adenosine 5′-monophosphate (AMP)-activated protein kinase
- Ang
angiopoietin
- AP-1
activator protein 1
- ARE
antioxidant-responsive element
- ATF-2
activating transcription factor 2
- BCC
basal cell carcinoma
- Bcl-2
B-cell lymphoma 2
- Bcl-Xl
B-cell lymphoma-extra large
- Bmi-1
B lymphoma Mo-MLV insertion region 1 homolog
- CCL2
chemokine (C-C motif) ligand 2
- CDK
cyclin-dependent kinases
- c-FLIP
cellular Fas-associated death domain-like interleulin-1 β converting enzyme inhibitory protein
- CF-TP
polyphenol fraction of Crataegus pinnatifida
- COX-2
cyclooxygenase-2
- CPDs
cyclobutane pyrimidine dimers
- CREB
cAMP response element-binding protein
- CXCL8
chemokine (C-X-C motif) ligand 8
- DATS
diallyl trisulfide
- DMBA
7,12-dimethylbenz[a]anthracene
- DNMTs
DNA methyltransferases
- EC
endothelial cells
- ECM
extracellular matrix
- EGCG
epigallocatechin gallate
- EP
prostaglandin E2 receptor
- ERK
extracellular signal–regulated kinases
- Ezh2
enhancer of zeste homolog 2
- FAK
focal adhesion kinase
- FGF
fibroblast growth factors
- GCL
glutamate cysteine ligase
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GPx
glutathione peroxidase
- GSH
glutathione
- GST
glutathione S-transferases
- HATs
histone acetyltransferase
- HDACs
histone deacetylase
- HDMs
histone demethylase
- HH
hedgehog
- HIF-1α
hypoxia-inducible factor-1 alpha
- HMTs
histone methyltransferases
- HO-1
heme oxygenase 1
- HPVs
human papilloma viruses
- IBD
inflammatory bowel disease
- IKK
IκB kinase
- IL
interleukin
- iNOS
inducible nitric oxide synthases
- JNK
c-Jun N-terminal kinases
- Keap1
kelch-like-ECH-associated protein 1
- LPSs
lipopolysaccharides
- MAPK
mitogen-activated protein kinases
- MC1R
melanocortin 1 receptor
- MDA
malonaldehyde
- MITF
microphthalmia-associated transcription factor
- MMP
matrix metalloproteinase
- m-TOR
mammalian target of rapamycin
- TXA2
thromboxane A2
- MVD
microvascular density
- NF-κB
nuclear factor kappa-light-chain enhancer of activated B cells
- NMSC
non-melanocytic cutaneous carcinoma
- NO
nitric oxide
- NQO
NAD(P)H:quinoneoxidoreductase
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- PCNA
proliferating cell nuclear antigen
- PDGF
platelet-derived growth factor
- PGE2
prostaglandin E2
- PGI2
prostaglandin I2
- PI3K
phosphatidylinositol-3-kinases
- PKC
protein kinase C
- PLGF
Placental growth factor
- PTEN
phosphatase and tensin homolog
- ROS
reactive oxygen species
- SCC
squamous cell carcinoma
- STAT3
signal transducer and activator of transcription 3
- TIMP-1
tissue inhibitor of metalloproteinases 1
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor alpha
- TPA
12-O-Tetradecanoylphorbol-13-acetate
- TSP-1
thrombospondin 1
- UVR
ultraviolet radiation
- VEGF
vascular endothelial growth factor
- XIAP
X-linked inhibitor of apoptosis protein
- γ-GCS
γ-glucocorticoids
- TSG
tumor suppressor genes
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- [1].Zuluaga-Sepulveda MA, Arellano-Mendoza I, Ocampo-Candiani J, Update on surgical treatment of primary and metastatic cutaneous melanoma, Cir. Cir 84 (2016) 77–84. [DOI] [PubMed] [Google Scholar]
- [2].Deady S, Sharp L, Comber H, Increasing skin cancer incidence in young, affluent, urban populations: a challenge for prevention, Br. J. Dermatol 171 (2014) 324–331. [DOI] [PubMed] [Google Scholar]
- [3].Lomas A, Leonardi-Bee J, Bath-Hextall F, A systematic review of worldwide incidence of nonmel-anoma skin cancer, British J. Dermatol 166 (2012) 1069–1080. [DOI] [PubMed] [Google Scholar]
- [4].Whiteman DC, Green AC, Olsen CM, The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031, J. Invest. Dermatol 136 (2016) 1161–1171. [DOI] [PubMed] [Google Scholar]
- [5].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2016, CA: Cancer J. Clin 66 (2016) 7–30. [DOI] [PubMed] [Google Scholar]
- [6].Gordon R, Skin cancer: an overview of epidemiology and risk factors, Semin. Oncol. Nurs 29 (2013) 160–169. [DOI] [PubMed] [Google Scholar]
- [7].McCormack CJ, Kelly JW, Dorevitch AP, Differences in age and body site distribution of the histo-logical subtypes of basal cell carcinoma. A possible indicator of differing causes, Arch. Dermatol 133 (1997) 593–596. [PubMed] [Google Scholar]
- [8].Madan V, Lear JT, Szeimies RM, Non-melanoma skin cancer, Lancet 375 (2010) 673–685. [DOI] [PubMed] [Google Scholar]
- [9].Koyuncuer A, Histopathological evaluation of non-melanoma skin cancer, World J. Surg. Oncol 12 (2014) 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Savoia P, Cremona O, Fava P, New perspectives in the pharmacological treatment of non-melanoma skin cancer, Curr. Drug Targets 17 (2016) 353–374. [DOI] [PubMed] [Google Scholar]
- [11].Simoes MC, Sousa JJ, Pais AA, Skin cancer and new treatment perspectives: a review, Cancer Lett. 357 (2015) 8–42. [DOI] [PubMed] [Google Scholar]
- [12].Emmert S, Schon MP, Haenssle HA, Molecular biology of basal and squamous cell carcinomas, Adv. Exp. Med. Biol 810 (2014) 234–252. [DOI] [PubMed] [Google Scholar]
- [13].Seebode C, Lehmann J, Emmert S, Photocarcinogenesis and skin cancer prevention strategies, Anticancer Res. 36 (2016) 1371–1378. [PubMed] [Google Scholar]
- [14].Skin cancer: prevention is better than cure, Lancet 384 (2014) 470. [DOI] [PubMed] [Google Scholar]
- [15].Uzarska M, Czajkowski R, Schwartz RA, Bajek A, Zegarska B, Drewa T, Chemoprevention of skin melanoma: facts and myths, Melanoma Res. 23 (2013) 426–433. [DOI] [PubMed] [Google Scholar]
- [16].Chen AC, Halliday GM, Damian DL, Non-melanoma skin cancer: carcinogenesis and chemopre-vention, Pathology 45 (2013) 331–341. [DOI] [PubMed] [Google Scholar]
- [17].Surh YJ, Cancer chemoprevention with dietary phytochemicals, Nat. Rev. Cancer 3 (2003) 768–780. [DOI] [PubMed] [Google Scholar]
- [18].Kotecha R, Takami A, Espinoza JL, Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence, Oncotarget (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee KW, Bode AM, Dong Z, Molecular targets of phytochemicals for cancer prevention, Nat. Rev. Cancer 11 (2011) 211–218. [DOI] [PubMed] [Google Scholar]
- [20].Shukla Y, Singh R, Resveratrol and cellular mechanisms of cancer prevention, Ann. N. Y. Acad. Sci 1215 (2011) 1–8. [DOI] [PubMed] [Google Scholar]
- [21].Chen D, Wan SB, Yang H, Yuan J, Chan TH, Dou QP, Egcg, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment, Adv. Clin. Chem 53 (2011) 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Park W, Amin AR, Chen ZG, Shin DM, New perspectives of curcumin in cancer prevention, Cancer Prev. Res 6 (2013) 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moan J, Grigalavicius M, Baturaite Z, Dahlback A, Juzeniene A, The relationship between uv ex-posure and incidence of skin cancer, Photodermatol. Photoimmunolo. Photomed 31 (2015) 26–35. [DOI] [PubMed] [Google Scholar]
- [24].Narayanan DL, Saladi RN, Fox JL, Ultraviolet radiation and skin cancer, Int. J. Dermatol 49 (2010) 978–986. [DOI] [PubMed] [Google Scholar]
- [25].Zhang M, Qureshi AA, Geller AC, Frazier L, Hunter DJ, Han J, Use of tanning beds and incidence of skin cancer, J. Clin. Oncol 30 (2012) 1588–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF, Meta-analysis of risk factors for cutaneous melanoma: II: Sun exposure, Eur. J. Cancer 41 (2005) 45–60. [DOI] [PubMed] [Google Scholar]
- [27].Geller AC, Colditz G, Oliveria S, Emmons K, Jorgensen C, Aweh GN, Frazier AL, Use of sunscreen, sunburning rates, and tanning bed use among more than 10 000 us children and adolescents, Pediatrics 109 (2002) 1009–1014. [DOI] [PubMed] [Google Scholar]
- [28].Hirst NG, Gordon LG, Scuffham PA, Green AC, Lifetime cost-effectiveness of skin cancer preven-tion through promotion of daily sunscreen use, Value Health 15 (2012) 261–268. [DOI] [PubMed] [Google Scholar]
- [29].Green AC, Williams GM, Point: sunscreen use is a safe and effective approach to skin cancer pre-vention, Cancer Epidemiol. Biomark. Prev 16 (2007) 1921–1922. [DOI] [PubMed] [Google Scholar]
- [30].Quintana RM, Dupuy AJ, Bravo A, Casanova ML, Alameda JP, Page A, Sanchez-Viera M, Ramirez A, Navarro M, A transposon-based analysis of gene mutations related to skin cancer development, J. Invest. Dermatol 133 (2013) 239–248. [DOI] [PubMed] [Google Scholar]
- [31].Lubbe J, Reichel M, Burg G, Kleihues P, Absence of p53 gene mutations in cutaneous melanoma, J. Invest. Dermatol 102 (1994) 819–821. [DOI] [PubMed] [Google Scholar]
- [32].Hussein MR, Wood GS, Hmlh1 and hmsh2 gene mutations are present in radial growth-phase cutaneous malignant melanoma cell lines and can be induced further by ultraviolet-b irradiation, Exp. Dermatol 12 (2003) 872–875. [DOI] [PubMed] [Google Scholar]
- [33].Piccinin S, Doglioni C, Maestro R, Vukosavljevic T, Gasparotto D, D’Orazi C, Boiocchi M, P16/cdkn2 and cdk4 gene mutations in sporadic melanoma development and progression, Int. J. Cancer 74 (1997) 26–30. [DOI] [PubMed] [Google Scholar]
- [34].Godic A, Poljsak B, Adamic M, Dahmane R, The role of antioxidants in skin cancer prevention and treatment, Oxid. Med. Cell. Longev 2014 (2014) 860479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen H, Weng QY, Fisher DE, Uv signaling pathways within the skin, J. Invest. Dermatol 134 (2014) 2080–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu K, Yu D, Cho YY, Bode AM, Ma W, Yao K, Li S, Li J, Bowden GT, Dong Z, Dong Z, Sunlight uv-induced skin cancer relies upon activation of the p38alpha signaling pathway, Cancer Res. 73 (2013) 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pfeifer GP, Besaratinia A, Uv wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer, Photochem. Photobiol. Sci 11 (2012) 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Elmets CA, Ledet JJ, Athar M, Cyclooxygenases: mediators of uv-induced skin cancer and potential targets for prevention, J. Invest. Dermatol 134 (2014) 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gerlini G, Romagnoli P, Pimpinelli N, Skin cancer and immunosuppression, Crit. Rev. Oncol. Hematol 56 (2005) 127–136. [DOI] [PubMed] [Google Scholar]
- [40].Neuburg M, Transplant-associated skin cancer: role of reducing immunosuppression, J. Natl. Compr. Cancer Netw.: JNCCN 5 (2007) 541–549. [DOI] [PubMed] [Google Scholar]
- [41].Firnhaber JM, Diagnosis and treatment of basal cell and squamous cell carcinoma, Am. Fam. Phys 86 (2012) 161–168. [PubMed] [Google Scholar]
- [42].Burke MT, Isbel N, Barraclough KA, Jung JW, Wells JW, Staatz CE, Genetics and nonmelanoma skin cancer in kidney transplant recipients, Pharmacogenomics 16 (2015) 161–172. [DOI] [PubMed] [Google Scholar]
- [43].Granstein RD, Matsui MS, Uv radiation-induced immunosuppression and skin cancer, Cutis 74 (2004) 4–9. [PubMed] [Google Scholar]
- [44].Noonan F, De Fabo EC, Uv immunosuppression and skin cancer, J. Investig. Dermatol 111 (1998) 706–708. [DOI] [PubMed] [Google Scholar]
- [45].Baykan H, Cihan YB, Ozyurt K, Roles of white blood cells and subtypes as inflammatory markers in skin cancer, Asian Pac. J. Cancer Prev.: APJCP 16 (2015) 2303–2306. [DOI] [PubMed] [Google Scholar]
- [46].Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD, Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease, Gastroenterology 143 (2012) 390–399, e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Reinau D, Surber C, Jick SS, Meier CR, Nonsteroidal anti-inflammatory drugs and the risk of non-melanoma skin cancer, Int. J. Cancer 137 (2015) 144–153. [DOI] [PubMed] [Google Scholar]
- [48].Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, Sorensen HT, Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: a population-based case-control study, Cancer 118 (2012) 4768–4776. [DOI] [PubMed] [Google Scholar]
- [49].Abbadessa G, Spaccamiglio A, Sartori ML, Nebbia C, Dacasto M, Di Carlo F, Racca S, The aspirin metabolite, salicylate, inhibits 7,12-dimethylbenz[a]anthracene-DNA adduct formation in breast cancer cells, Int. J. Oncol 28 (2006) 1131–1140. [PubMed] [Google Scholar]
- [50].Surdu S, Non-melanoma skin cancer: occupational risk from uv light and arsenic exposure, Rev. Environ. Health 29 (2014) 255–264. [DOI] [PubMed] [Google Scholar]
- [51].Oberoi S, Barchowsky A, Wu F, The global burden of disease for skin, lung, and bladder cancer caused by arsenic in food, Cancer Epidemiol. Biomark. Prev 23 (2014) 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Green A, MacLennan R, Siskind V, Common acquired naevi and the risk of malignant melanoma, Int. J. Cancer 35 (1985) 297–300. [DOI] [PubMed] [Google Scholar]
- [53].Runger TM, Epe B, Moller K, Dekant B, Hellfritsch D, Repair of directly and indirectly uv-induced DNA lesions and of DNA double-strand breaks in cells from skin cancer-prone patients with the disor-ders dysplastic nevus syndrome or basal cell nevus syndrome, Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer 143 (1997) 337–351. [DOI] [PubMed] [Google Scholar]
- [54].Nikolaou V, Stratigos AJ, Tsao H, Hereditary nonmelanoma skin cancer, Semin. Cutan. Med. Surg 31 (2012) 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Halpern AC, Altman JF, Genetic predisposition to skin cancer, Curr. Opin. Oncol 11 (1999) 132–138. [DOI] [PubMed] [Google Scholar]
- [56].Lambert WC, Lambert MW, Development of effective skin cancer treatment and prevention in xeroderma pigmentosum, Photochem. Photobiol 91 (2015) 475–483. [DOI] [PubMed] [Google Scholar]
- [57].Marchetto MC, Muotri AR, Burns DK, Friedberg EC, Menck CF, Gene transduction in skin cells: preventing cancer in xeroderma pigmentosum mice, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 17759–17764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wattenberg LW, Chemoprevention of cancer, Cancer Res. 45 (1985) 1–8. [PubMed] [Google Scholar]
- [59].Landis-Piwowar KR, Iyer NR, Cancer chemoprevention: current state of the art, Cancer Growth Metastasis 7 (2014) 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Collins AR, Antioxidant intervention as a route to cancer prevention, Eur. J. Cancer 41 (2005) 1923–1930. [DOI] [PubMed] [Google Scholar]
- [61].Romani M, Pistillo MP, Banelli B, Environmental epigenetics: crossroad between public health, lifestyle, and cancer prevention, BioMed Res. Int 2015 (2015) 587983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Toyokuni S, Molecular mechanisms of oxidative stress-induced carcinogenesis: from epidemiology to oxygenomics, IUBMB Life 60 (2008) 441–447. [DOI] [PubMed] [Google Scholar]
- [63].Evans MD, Dizdaroglu M, Cooke MS, Oxidative DNA damage and disease: induction, repair and significance, Mutat. Res 567 (2004) 1–61. [DOI] [PubMed] [Google Scholar]
- [64].Prasad S, Gupta SC, Tyagi AK, Reactive oxygen species (ros) and cancer: role of antioxidative nutraceuticals, Cancer Lett. (2016). [DOI] [PubMed] [Google Scholar]
- [65].Manson MM, Cancer prevention—the potential for diet to modulate molecular signalling, Trends Mol. Med 9 (2003) 11–18. [DOI] [PubMed] [Google Scholar]
- [66].Wu WS, The signaling mechanism of ros in tumor progression, Cancer Metastasis Rev. 25 (2006) 695–705. [DOI] [PubMed] [Google Scholar]
- [67].Moon EJ, Giaccia A, Dual roles of nrf2 in tumor prevention and progression: possible implications in cancer treatment, Free Rad. Biol. Med 79 (2015) 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dhar SK, Xu Y, Noel T, St Clair DK, Chronic exposure to 12-o-tetradecanoylphorbol-13-acetate represses sod2 induction in vivo: the negative role of p50, Carcinogenesis 28 (2007) 2605–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB, Active defense under oxidative stress: the antioxidant responsive element, Biochem. Biokhimiia 71 (2006) 962–974. [DOI] [PubMed] [Google Scholar]
- [70].Hayes JD, McMahon M, Molecular basis for the contribution of the antioxidant responsive ele-ment to cancer chemoprevention, Cancer Lett. 174 (2001) 103–113. [DOI] [PubMed] [Google Scholar]
- [71].Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y, An nrf2/small maf heterodimer mediates the induction of phase ii detox-ifying enzyme genes through antioxidant response elements, Biochem. Biophys. Res. Commun 236 (1997) 313–322. [DOI] [PubMed] [Google Scholar]
- [72].Khor TO, Huang MT, Prawan A, Liu Y, Hao X, Yu S, Cheung WK, Chan JY, Reddy BS, Yang CS, Kong AN, Increased susceptibility of nrf2 knockout mice to colitis-associated colorectal cancer, Cancer Prev. Res 1 (2008) 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cheung KL, Lee JH, Khor TO, Wu TY, Li GX, Chan J, Yang CS, Kong AN, Nrf2 knockout enhances intestinal tumorigenesis in apc(min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation, Mol. Carcinog 53 (2014) 77–84. [DOI] [PubMed] [Google Scholar]
- [74].Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW, Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcrip-tion factor-deficient mice, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 3410–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Becks L, Prince M, Burson H, Christophe C, Broadway M, Itoh K, Yamamoto M, Mathis M, Orchard E, Shi R, McLarty J, Pruitt K, Zhang S, Kleiner-Hancock HE, Aggressive mammary carcinoma progression in nrf2 knockout mice treated with 7,12-dimethylbenz[a]anthracene, BMC Cancer 10 (2010) 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Beyer TA, Auf dem Keller U, Braun S, Schafer M, Werner S, Roles and mechanisms of action of the nrf2 transcription factor in skin morphogenesis, wound repair and skin cancer, Cell Death Differ. 14 (2007) 1250–1254. [DOI] [PubMed] [Google Scholar]
- [77].Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M, Keap1 is a redox-regulated substrate adaptor protein for a cul3-dependent ubiquitin ligase complex, Mol. Cell Biol 24 (2004) 10941–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P: Direct evidence that sulfhydryl groups of keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants, Proc. Natl. Acad. Sci. U. S. A 99 (2002) 11908–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P, Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the keap1 sensor modified by inducers, Proc. Natl. Acad. Sci. U. S. A 101 (2004) 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hu C, Eggler AL, Mesecar AD, van Breemen RB, Modification of keap1 cysteine residues by sul-foraphane, Chem. Res. Toxicol 24 (2011) 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, Conney AH, Kong AN, Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in c57bl/6 mice by sulforaphane is me-diated by nuclear factor e2-related factor 2, Cancer Res. 66 (2006) 8293–8296. [DOI] [PubMed] [Google Scholar]
- [82].Wagner AE, Ernst I, Iori R, Desel C, Rimbach G, Sulforaphane but not ascorbigen, in-dole-3-carbinole and ascorbic acid activates the transcription factor nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture, Exp. Dermatol 19 (2010) 137–144. [DOI] [PubMed] [Google Scholar]
- [83].Saw CL, Huang MT, Liu Y, Khor TO, Conney AH, Kong AN, Impact of nrf2 on uvb-induced skin in-flammation/photoprotection and photoprotective effect of sulforaphane, Mol. Carcinog 50 (2011) 479–486. [DOI] [PubMed] [Google Scholar]
- [84].Lin M, Zhai X, Wang G, Tian X, Gao D, Shi L, Wu H, Fan Q, Peng J, Liu K, Yao J, Salvianolic acid b protects against acetaminophen hepatotoxicity by inducing nrf2 and phase ii detoxification gene ex-pression via activation of the pi3k and pkc signaling pathways, J. Pharmacol. Sci 127 (2015) 203–210. [DOI] [PubMed] [Google Scholar]
- [85].Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A, Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/akt pathway and the nrf2 transcription factor in response to the antioxidant phytochemical carnosol, J. Biol. Chem 279 (2004) 8919–8929. [DOI] [PubMed] [Google Scholar]
- [86].Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, Han X, Li J, Yang M, Wang Z, Wei D, Xiao H, The crosstalk between nrf2 and ampk signal pathways is important for the anti-inflammator effect of berberine in lps-stimulated macrophages and endotoxin-shocked mice, Antioxid. Redox Signal 20 (2014) 574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shen C, Wang S, Shan Y, Liu Z, Fan F, Tao L, Liu Y, Zhou L, Pei C, Wu H, Tian C, Ruan J, Chen W, Wang A, Zheng S, Lu Y, Chemomodulatory efficacy of lycopene on antioxidant enzymes and carcino-gen-induced cutaneum carcinoma in mice, Food Funct. 5 (2014) 1422–1431. [DOI] [PubMed] [Google Scholar]
- [88].Bryan HK, Olayanju A, Goldring CE, Park BK, The nrf2 cell defence pathway: keap1-dependent and -independent mechanisms of regulation, Biochem. Pharmacol 85 (2013) 705–717. [DOI] [PubMed] [Google Scholar]
- [89].Shan Y, Wei Z, Tao L, Wang S, Zhang F, Shen C, Wu H, Liu Z, Zhu P, Wang A, Chen W, Lu Y, Prophy-laxis of diallyl disulfide on skin carcinogenic model via p21-dependent nrf2 stabilization, Sci. Rep 6 (2016) 35676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhao R, Yang B, Wang L, Xue P, Deng B, Zhang G, Jiang S, Zhang M, Liu M, Pi J, Guan D, Curcumin protects human keratinocytes against inorganic arsenite-induced acute cytotoxicity through an nrf2-dependent mechanism, Oxid. Med. Cell. Longev 2013 (2013) 412576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Liu Y, Chan F, Sun H, Yan J, Fan D, Zhao D, An J, Zhou D, Resveratrol protects human keratinocytes hacat cells from uva-induced oxidative stress damage by downregulating keap1 expression, Eur. J. Pharmacol 650 (2011) 130–137. [DOI] [PubMed] [Google Scholar]
- [92].Kleszczynski K, Ernst IM, Wagner AE, Kruse N, Zillikens D, Rimbach G, Fischer TW, Sulforaphane and phenylethyl isothiocyanate protect human skin against uvr-induced oxidative stress and apoptosis: role of nrf2-dependent gene expression and antioxidant enzymes, Pharmacol. Res 78 (2013) 28–40. [DOI] [PubMed] [Google Scholar]
- [93].Murakami A, Tanaka T, Lee JY, Surh YJ, Kim HW, Kawabata K, Nakamura Y, Jiwajinda S, Ohigashi H, Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in icrmice, Int. J. Cancer 110 (2004) 481–490. [DOI] [PubMed] [Google Scholar]
- [94].Shin JW, Ohnishi K, Murakami A, Lee JS, Kundu JK, Na HK, Ohigashi H, Surh YJ, Zerumbone induces heme oxygenase-1 expression in mouse skin and cultured murine epidermal cells through activation of nrf2, Cancer Prev. Res 4 (2011) 860–870. [DOI] [PubMed] [Google Scholar]
- [95].Hseu YC, Chou CW, Senthil Kumar KJ, Fu KT, Wang HM, Hsu LS, Kuo YH, Wu CR, Chen SC, Yang HL, Ellagic acid protects human keratinocyte (hacat) cells against uva-induced oxidative stress and apopto-sis through the upregulation of the ho-1 and nrf-2 antioxidant genes, Food Chem. Toxicol 50 (2012) 1245–1255. [DOI] [PubMed] [Google Scholar]
- [96].Ding M, Zhao J, Bowman L, Lu Y, Shi X, Inhibition of ap-1 and mapk signaling and activation of nrf2/are pathway by quercitrin, Int. J. Oncol 36 (2010) 59–67. [PubMed] [Google Scholar]
- [97].Kimura S, Warabi E, Yanagawa T, Ma D, Itoh K, Ishii Y, Kawachi Y, Ishii T, Essential role of nrf2 in keratinocyte protection from uva by quercetin, Biochem. Biophys. Res. Commun 387 (2009) 109–114. [DOI] [PubMed] [Google Scholar]
- [98].Kundu JK, Liu L, Shin JW, Surh YJ, Thymoquinone inhibits phorbol ester-induced activation of nf-kappab and expression of cox-2, and induces expression of cytoprotective enzymes in mouse skin in vivo, Biochem. Biophys. Res. Commun 438 (2013) 721–727. [DOI] [PubMed] [Google Scholar]
- [99].Jung SK, Lee KW, Byun S, Kang NJ, Lim SH, Heo YS, Bode AM, Bowden GT, Lee HJ, Dong Z, Myricetin suppresses uvb-induced skin cancer by targeting fyn, Cancer Res. 68 (2008) 6021–6029. [DOI] [PubMed] [Google Scholar]
- [100].Jain AK, Jaiswal AK, Gsk-3beta acts upstream of fyn kinase in regulation of nuclear export and degradation of nf-e2 related factor 2, J. Biol. Chem 282 (2007) 16502–16510. [DOI] [PubMed] [Google Scholar]
- [101].Chaudhary SC, Siddiqui MS, Athar M, Alam MS, D-Limonene modulates inflammation, oxidative stress and ras-erk pathway to inhibit murine skin tumorigenesis, Hum. Exp. Toxicol 31 (2012) 798–811. [DOI] [PubMed] [Google Scholar]
- [102].Khan AQ, Khan R, Qamar W, Lateef A, Ali F, Tahir M, Muneeb UR, Sultana S, Caffeic acid attenuates 12-o-tetradecanoyl-phorbol-13-acetate (tpa)-induced nf-kappab and cox-2 expression in mouse skin: abrogation of oxidative stress, inflammatory responses and proinflammatory cytokine production, Food Chem. Toxicol 50 (2012) 175–183. [DOI] [PubMed] [Google Scholar]
- [103].Esteller M, Epigenetics in cancer, N. Engl. J. Med 358 (2008) 1148–1159. [DOI] [PubMed] [Google Scholar]
- [104].Shinjo K, Kondo Y, Targeting cancer epigenetics: linking basic biology to clinical medicine, Adv. Drug Deliv. Rev 95 (2015) 56–64. [DOI] [PubMed] [Google Scholar]
- [105].Ellis L, Atadja PW, Johnstone RW, Epigenetics in cancer: targeting chromatin modifications, Mol. Cancer Ther 8 (2009) 1409–1420. [DOI] [PubMed] [Google Scholar]
- [106].Jaenisch R, Bird A, Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals, Nat. Genet 33 (Suppl) (2003) 245–254. [DOI] [PubMed] [Google Scholar]
- [107].Yang AY, Lee JH, Shu L, Zhang C, Su ZY, Lu Y, Huang MT, Ramirez C, Pung D, Huang Y, Verzi M, Hart RP, Kong AN, Genome-wide analysis of DNA methylation in uvb- and dmba/tpa-induced mouse skin cancer models, Life Sci. 113 (2014) 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kraemer A, Chen IP, Henning S, Faust A, Volkmer B, Atkinson MJ, Moertl S, Greinert R, Uva and uvb irradiation differentially regulate microrna expression in human primary keratinocytes, PLoS One 8 (2013) e83392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Katiyar SK, Singh T, Prasad R, Sun Q, Vaid M, Epigenetic alterations in ultraviolet radiation-induced skin carcinogenesis: interaction of bioactive dietary components on epigenetic targets, Photochem. Photobiol 88 (2012) 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sigalotti L, Covre A, Fratta E, Parisi G, Colizzi F, Rizzo A, Danielli R, Nicolay HJ, Coral S, Maio M, Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies, J. Transl. Med 8 (2010) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bird A, DNA methylation patterns and epigenetic memory, Genes. Dev 16 (2002) 6–21. [DOI] [PubMed] [Google Scholar]
- [112].Wang Y, Leung FC, An evaluation of new criteria for cpg islands in the human genome as gene markers, Bioinformatics 20 (2004) 1170–1177. [DOI] [PubMed] [Google Scholar]
- [113].Herman JG, Baylin SB, Gene silencing in cancer in association with promoter hypermethylation, N. Engl.J. Med 349 (2003) 2042–2054. [DOI] [PubMed] [Google Scholar]
- [114].Torano EG, Petrus S, Fernandez AF, Fraga MF, Global DNA hypomethylation in cancer: review of validated methods and clinical significance, Clin. Chem. Lab. Med 50 (2012) 1733–1742. [DOI] [PubMed] [Google Scholar]
- [115].Tanemura A, Terando AM, Sim MS, van Hoesel AQ, de Maat MF, Morton DL, Hoon DS, Cpg island methylator phenotype predicts progression of malignant melanoma, Clin. Cancer Res 15 (2009) 1801–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Fraga MF, Herranz M, Espada J, Ballestar E, Paz MF, Ropero S, Erkek E, Bozdogan O, Peinado H, Niveleau A, Mao JH, Balmain A, Cano A, Esteller M, A mouse skin multistage carcinogenesis model reflects the aberrant DNA methylation patterns of human tumors, Cancer Res. 64 (2004) 5527–5534. [DOI] [PubMed] [Google Scholar]
- [117].Han Y, Zhao H, Jiang Q, Gao H, Wang C, Chemopreventive mechanism of polypeptides from chlamy farreri (pcf) against uvb-induced malignant transformation of hacat cells, Mutagenesis 30 (2015) 287–296. [DOI] [PubMed] [Google Scholar]
- [118].Lin JR, Qin HH, Wu WY, He SJ, Xu JH, Vitamin c protects against uv irradiation-induced apoptosis through reactivating silenced tumor suppressor genes p21 and p16 in a tet-dependent DNA demeth-ylation manner in human skin cancer cells, Cancer Biother. Radiopharm 29 (2014) 257–264. [DOI] [PubMed] [Google Scholar]
- [119].Fullgrabe J, Kavanagh E, Joseph B, Histone onco-modifications, Oncogene 30 (2011) 3391–3403. [DOI] [PubMed] [Google Scholar]
- [120].Gelato KA, Fischle W, Role of histone modifications in defining chromatin structure and function, Biol. Chem 389 (2008) 353–363. [DOI] [PubMed] [Google Scholar]
- [121].Fan T, Jiang S, Chung N, Alikhan A, Ni C, Lee CC, Hornyak TJ, Ezh2-dependent suppression of a cellular senescence phenotype in melanoma cells by inhibition of p21/cdkn1a expression, Mol. Cancer Res.: MCR 9 (2011) 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K, Combinatorial patterns of histone acetylations and methylations in the human genome, Nat. Genet 40 (2008) 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chen YP, Hou XY, Yang CS, Jiang XX, Yang M, Xu XF, Feng SX, Liu YQ, Jiang G, DNA methylation and histone acetylation regulate the expression of mgmt and chemosensitivity to temozolomide in malig-nant melanoma cell lines, Tumour Biol. (2016). [DOI] [PubMed] [Google Scholar]
- [124].Shan X, Fu YS, Aziz F, Wang XQ, Yan Q, Liu JW, Ginsenoside rg3 inhibits melanoma cell proliferation through down-regulation of histone deacetylase 3 (hdac3) and increase of p53 acetylation, PLoS One 9 (2014) e115401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Sato F, Tsuchiya S, Meltzer SJ, Shimizu K, Micrornas and epigenetics, FEBS J. 278 (2011) 1598–1609. [DOI] [PubMed] [Google Scholar]
- [126].Kala R, Peek GW, Hardy TM, Tollefsbol TO, Micrornas: an emerging science in cancer epigenetics, J. Clin. Bioinform 3 (2013) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Saito Y, Alterations of epigenetics and micrornas in cancer and cancer stem cell, Front. Genet 5 (2014) 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Abba ML, Patil N, Leupold JH, Moniuszko M, Utikal J, Niklinski J, Allgayer H, Micrornas as novel targets and tools in cancer therapy, Cancer Lett. (2016). [DOI] [PubMed] [Google Scholar]
- [129].Balci S, Ayaz L, Gorur A, Yildirim Yaroglu H, Akbayir S, Dogruer Unal N, Bulut B, Tursen U, Tamer L, Microrna profiling for early detection of nonmelanoma skin cancer, Clin. Exp. Dermatol 41 (2016) 346–351. [DOI] [PubMed] [Google Scholar]
- [130].Mirzaei H, Gholamin S, Shahidsales S, Sahebkar A, Jaafari MR, Mirzaei HR, Hassanian SM, Avan A, Micrornas as potential diagnostic and prognostic biomarkers in melanoma, Eur. J. Cancer 53 (2016) 25–32. [DOI] [PubMed] [Google Scholar]
- [131].Luo C, Weber CE, Osen W, Bosserhoff AK, Eichmuller SB, The role of micrornas in melanoma, Eur. J. Cell Biol 93 (2014) 11–22. [DOI] [PubMed] [Google Scholar]
- [132].Dahmke IN, Backes C, Rudzitis-Auth J, Laschke MW, Leidinger P, Menger MD, Meese E, Mahlk-necht U, Curcumin intake affects mirna signature in murine melanoma with mmu-mir-205-5p most significantly altered, PLoS One 8 (2013) e81122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Mittal A, Piyathilake C, Hara Y, Katiyar SK, Exceptionally high protection of photocarcinogenesis by topical application of (−)-epigallocatechin-3-gallate in hydrophilic cream in skh-1 hairless mouse model: relationship to inhibition of uvb-induced global DNA hypomethylation, Neoplasia 5 (2003) 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Balasubramanian S, Adhikary G, Eckert RL, The bmi-1 polycomb protein antagonizes the (−)-epigallocatechin-3-gallate-dependent suppression of skin cancer cell survival, Carcinogenesis 31 (2010) 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [135].Nandakumar V, Vaid M, Katiyar SK, (−)-epigallocatechin-3-gallate reactivates silenced tumor sup-pressor genes, cip1/p21 and p16ink4a, by reducing DNA methylation and increasing histones acetyla-tion in human skin cancer cells, Carcinogenesis 32 (2011) 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Su ZY, Zhang C, Lee JH, Shu L, Wu TY, Khor TO, Conney AH, Lu YP, Kong AN, Requirement and epi-genetics reprogramming of nrf2 in suppression of tumor promoter tpa-induced mouse skin cell trans-formation by sulforaphane, Cancer Prev. Res 7 (2014) 319–329. [DOI] [PubMed] [Google Scholar]
- [137].Balasubramanian S, Chew YC, Eckert RL, Sulforaphane suppresses polycomb group protein level via a proteasome-dependent mechanism in skin cancer cells, Mol. Pharmacol 80 (2011) 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Vaid M, Prasad R, Singh T, Jones V, Katiyar SK, Grape seed proanthocyanidins reactivate silenced tumor suppressor genes in human skin cancer cells by targeting epigenetic regulators, Toxicol. Appl. Pharmacol 263 (2012) 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Yao K, Chen H, Liu K, Langfald A, Yang G, Zhang Y, Yu DH, Kim MO, Lee MH, Li H, Bae KB, Kim HG, Ma WY, Bode AM, Dong Z, Dong Z, Kaempferol targets rsk2 and msk1 to suppress uv radiation-induced skin cancer, Cancer Prev. Res 7 (2014) 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Paredes-Gonzalez X, Fuentes F, Su ZY, Kong AN, Apigenin reactivates nrf2 anti-oxidative stress signaling in mouse skin epidermal jb6 p + cells through epigenetics modifications, AAPS J. 16 (2014) 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Tiwari P, Gupta KP, Modulation of mir-203 and its regulators as a function of time during the de-velopment of 7, 12 dimethylbenz [a] anthracene induced mouse skin tumors in presence or absence of the antitumor agents, Toxicol. Appl. Pharmacol 278 (2014) 148–158. [DOI] [PubMed] [Google Scholar]
- [142].Steward WP, Brown K, Cancer chemoprevention: a rapidly evolving field, Br. J. Cancer 109 (2013) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lin Y, Bai L, Chen W, Xu S, The nf-kappab activation pathways, emerging molecular targets for cancer prevention and therapy, Expert Opin. Ther. Targets 14 (2010) 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Chai EZ, Shanmugam MK, Arfuso F, Dharmarajan A, Wang C, Kumar AP, Samy RP, Lim LH, Wang L, Goh BC, Ahn KS, Hui KM, Sethi G, Targeting transcription factor stat3 for cancer prevention and therapy, Pharmacol. Ther 162 (2016) 86–97. [DOI] [PubMed] [Google Scholar]
- [145].Puntoni M, Marra D, Zanardi S, Decensi A, Inflammation and cancer prevention, Ann. Oncol 19 (Suppl. 7) (2008) vii225–vii229. [DOI] [PubMed] [Google Scholar]
- [146].Sohal SS, Mahmood MQ, Walters EH, Clinical significance of epithelial mesenchymal transition (emt) in chronic obstructive pulmonary disease (copd): Potential target for prevention of airway fibro-sis and lung cancer, Clin. Transl. Med 3 (2014) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Albini A, Tosetti F, Li VW, Noonan DM, Li WW, Cancer prevention by targeting angiogenesis, Nat. Rev. Clin. Oncol 9 (2012) 498–509. [DOI] [PubMed] [Google Scholar]
- [148].Mueller MM, Inflammation in epithelial skin tumours: old stories and new ideas, Eur. J. Cancer 42 (2006) 735–744. [DOI] [PubMed] [Google Scholar]
- [149].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144 (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [150].Maru GB, Gandhi K, Ramchandani A, Kumar G, The role of inflammation in skin cancer, Adv. Exp. Med. Biol 816 (2014) 437–469. [DOI] [PubMed] [Google Scholar]
- [151].Nickoloff BJ, Ben-Neriah Y, Pikarsky E, Inflammation and cancer: is the link as simple as we think, J. Invest. Dermatol 124 (2005) x–xiv. [DOI] [PubMed] [Google Scholar]
- [152].Ohshima H, Tazawa H, Sylla BS, Sawa T, Prevention of human cancer by modulation of chronic inflammatory processes, Mutat. Res 591 (2005) 110–122. [DOI] [PubMed] [Google Scholar]
- [153].Kim YS, Young MR, Bobe G, Colburn NH, Milner JA, Bioactive food components, inflammatory targets, and cancer prevention, Cancer Prev. Res 2 (2009) 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Zhu Y, Cheng Y, Luo RC, Li AM, Aspirin for the primary prevention of skin cancer: a meta-analysis, Oncol. Lett 9 (2015) 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Diakos CI, Charles KA, McMillan DC, Clarke SJ, Cancer-related inflammation and treatment effec-tiveness, Lancet Oncol. 15 (2014) e493–503. [DOI] [PubMed] [Google Scholar]
- [156].Hocker TL, Singh MK, Tsao H, Melanoma genetics and therapeutic approaches in the 21 st century: moving from the benchside to the bedside, J. Invest. Dermatol 128 (2008) 2575–2595. [DOI] [PubMed] [Google Scholar]
- [157].Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A, Molecular pathways in cancer-related inflammation, Biochem. Med 21 (2011) 264–275. [DOI] [PubMed] [Google Scholar]
- [158].Scott MC, Wakamatsu K, Ito S, Kadekaro AL, Kobayashi N, Groden J, Kavanagh R, Takakuwa T, Virador V, Hearing VJ, Abdel-Malek ZA, Human melanocorti 1 receptor variants, receptor function and melanocyte response to uv radiation, J. Cell Sci 115 (2002) 2349–2355. [DOI] [PubMed] [Google Scholar]
- [159].Liu JJ, Fisher DE, Lighting a path to pigmentation: mechanisms of mitf induction by uv, Pigm. Cell Melanoma Res 23 (2010) 741–745. [DOI] [PubMed] [Google Scholar]
- [160].Khan AQ, Khan R, Qamar W, Lateef A, Rehman MU, Tahir M, Ali F, Hamiza OO, Hasan SK, Sultana S, Geraniol attenuates 12-o-tetradecanoylphorbol-13-acetate (tpa)-induced oxidative stress and in-flammation in mouse skin: possible role of p38 map kinase and nf-kappab, Exp. Mol. Pathol 94 (2013) 419–429. [DOI] [PubMed] [Google Scholar]
- [161].Tsai ML, Lai CS, Chang YH, Chen WJ, Ho CT, Pan MH, Pterostilbene, a natural analogue of resvera-trol, potently inhibits 7,12-dimethylbenz[a]anthracene (dmba)/12-o-tetradecanoylphorbol-13-acetate (tpa)-induced mouse skin carcinogenesis, Food Funct. 3 (2012) 1185–1194. [DOI] [PubMed] [Google Scholar]
- [162].Liu Z, Shen C, Tao Y, Wang S, Wei Z, Cao Y, Wu H, Fan F, Lin C, Shan Y, Zhu P, Sun L, Chen C, Wang A, Zheng S, Lu Y, Chemopreventive efficacy of menthol on carcinogen-induced cutaneous carcinoma through inhibition of inflammation and oxidative stress in mice, Food Chem. Toxicol 82 (2015) 12–18. [DOI] [PubMed] [Google Scholar]
- [163].Hoesel B, Schmid JA, The complexity of nf-kappab signaling in inflammation and cancer, Mol. Cancer 12 (2013) 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Vaid M, Sharma SD, Katiyar SK, Honokiol, a phytochemical from the magnolia plant, inhibits pho-tocarcinogenesis by targeting uvb-induced inflammatory mediators and cell cycle regulators: devel-opment of topical formulation, Carcinogenesis 31 (2010) 2004–2011. [DOI] [PubMed] [Google Scholar]
- [165].Meeran SM, Akhtar S, Katiyar SK, Inhibition of uvb-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation, J. Invest. Dermatol 129 (2009) 1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Patel R, Krishnan R, Ramchandani A, Maru G, Polymeric black tea polyphenols inhibit mouse skin chemical carcinogenesis by decreasing cell proliferation, Cell Prolif. 41 (2008) 532–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Kowalczyk MC, Junco JJ, Kowalczyk P, Tolstykh O, Hanausek M, Slaga TJ, Walaszek Z, Effects of combined phytochemicals on skin tumorigenesis in sencar mice, Int. J. Oncol 43 (2013) 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R, Silibinin inhibits inflammatory and angi-ogenic attributes in photocarcinogenesis in skh-1 hairless mice, Cancer Res. 67 (2007) 3483–3491. [DOI] [PubMed] [Google Scholar]
- [169].Osakabe N, Yasuda A, Natsume M, Yoshikawa T, Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of perilla frutescens extract in the murine two-stage skin model, Carcinogenesis 25 (2004) 549–557. [DOI] [PubMed] [Google Scholar]
- [170].Kalra N, Bhui K, Roy P, Srivastava S, George J, Prasad S, Shukla Y, Regulation of p53, nuclear factor kappab and cyclooxygenase-2 expression by bromelain through targeting mitogen-activated protein kinase pathway in mouse skin, Toxicol. Appl. Pharmacol 226 (2008) 30–37. [DOI] [PubMed] [Google Scholar]
- [171].Kundu JK, Hwang DM, Lee JC, Chang EJ, Shin YK, Fujii H, Sun B, Surh YJ, Inhibitory effects of oligonol on phorbol ester-induced tumor promotion and cox-2 expression in mouse skin: nf-kappab and c/ebp as potential targets, Cancer Lett. 273 (2009) 86–97. [DOI] [PubMed] [Google Scholar]
- [172].Shrotriya S, Kundu JK, Na HK, Surh YJ, Diallyl trisulfide inhibits phorbol ester-induced tumor pro-motion, activation of ap-1, and expression of cox-2 in mouse skin by blocking jnk and akt signaling, Cancer Res. 70 (2010) 1932–1940. [DOI] [PubMed] [Google Scholar]
- [173].Kao ES, Wang CJ, Lin WL, Chu CY, Tseng TH, Effects of polyphenols derived from fruit of crataegus pinnatifida on cell transformation, dermal edema and skin tumor formation by phorbol ester applica-tion, Food Chem. Toxicol 45 (2007) 1795–1804. [DOI] [PubMed] [Google Scholar]
- [174].Byun S, Park J, Lee E, Lim S, Yu JG, Lee SJ, Chen H, Dong Z, Lee KW, Lee HJ, Src kinase is a direct target of apigenin against uvb-induced skin inflammation, Carcinogenesis 34 (2013) 397–405. [DOI] [PubMed] [Google Scholar]
- [175].Kang NJ, Lee KW, Kwon JY, Hwang MK, Rogozin EA, Heo YS, Bode AM, Lee HJ, Dong Z, Delphinidin attenuates neoplastic transformation in jb6 cl41 mouse epidermal cells by blocking raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling, Cancer Prev. Res 1 (2008) 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Lee JH, Kim JE, Jang YJ, Lee CC, Lim TG, Jung SK, Lee E, Lim SS, Heo YS, Seo SG, Son JE, Kim JR, Lee CY, Lee HJ, Lee KW, Dehydroglyasperin c suppresses tpa-induced cell transformation through direct inhibition of mkk4 and pi3k, Mol. Carcinog 55 (2016) 552–562. [DOI] [PubMed] [Google Scholar]
- [177].Weerawatanakorn M, Yang JR, Tsai ML, Lai CS, Ho CT, Pan MH, Inhibitory effects of momordica grosvenori swingle extracts on 12-o-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mouse skin, Food Funct. 5 (2014) 257–264. [DOI] [PubMed] [Google Scholar]
- [178].Jung YJ, Jung JI, Cho HJ, Choi MS, Sung MK, Yu R, Kang YH, Park JH, Berteroin present in cruciferous vegetables exerts potent anti-inflammatory properties in murine macrophages and mouse skin, Int. J. Mol. Sci 15 (2014) 20686–20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Hsu CH, Ho YS, Lai CS, Hsieh SC, Chen LH, Lin E, Ho CT, Pan MH, Hexahydro-beta-acids potently inhibit 12-o-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mice, J. Agric. Food. Chem 61 (2013) 11541–11549. [DOI] [PubMed] [Google Scholar]
- [180].Lin JA, Chen HC, Yen GC, The preventive role of breadfruit against inflammation-associated epi-thelial carcinogenesis in mice, Mol. Nutr. Food Res 58 (2014) 206–210. [DOI] [PubMed] [Google Scholar]
- [181].Shin JW, Kundu JK, Surh YJ, Phloretin inhibits phorbol ester-induced tumor promotion and ex-pression of cyclooxygenase-2 in mouse skin: extracellular signal-regulated kinase and nuclear fac-tor-kappab as potential targets, J. Med. Food 15 (2012) 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Lee YM, Cho HJ, Ponnuraj SP, Kim J, Kim JS, Kim SG, Park JH, Phenethyl isothiocyanate inhibits 12-o-tetradecanoylphorbol-13-acetate-induced inflammatory responses in mouse skin, J. Med. Food 14 (2011) 377–385. [DOI] [PubMed] [Google Scholar]
- [183].Wu H, Hsieh MC, Lo CY, Liu CB, Sang S, Ho CT, Pan MH, 6-Shogaol is more effective than 6-gingerol and curcumin in inhibiting 12-o-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice, Mol. Nutr. Food Res 54 (2010) 1296–1306. [DOI] [PubMed] [Google Scholar]
- [184].Chung AS, Ferrara N, Developmental and pathological angiogenesis, Annu. Rev. Cell Dev. Biol 27 (2011) 563–584. [DOI] [PubMed] [Google Scholar]
- [185].Fidler IJ, Angiogenesis and cancer metastasis, Cancer J. 6 (Suppl. 2) (2000) S134–141. [PubMed] [Google Scholar]
- [186].Wang Z, Dabrosin C, Yin X, Fuster MM, Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B, Ribatti D, Chen YC, Honoki K, Fujii H, Georgakilas AG, Nowsheen S, Amedei A, Niccolai E, Amin A, Ashraf SS, Helferich B, Yang X, Guha G, Bhakta D, Ciriolo MR, Aquilano K, Chen S, Halicka D, Mohammed SI, Azmi AS, Bilsland A, Keith WN, Jensen LD, Broad targeting of angiogenesis for cancer prevention and therapy, Semin. Cancerbiol 35 (Suppl) (2015) S224–S243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Weninger W, Uthman A, Pammer J, Pichler A, Ballaun C, Lang IM, Plettenberg A, Bankl HC, Sturzl M, Tschachler E, Vascular endothelial growth factor production in normal epidermis and in benign and malignant epithelial skin tumors, Lab. Investig 75 (1996) 647–657. [PubMed] [Google Scholar]
- [188].Li VW, Li WW, Antiangiogenesis in the treatment of skin cancer, J. Drugs Dermatol.: JDD 7 (2008) s17–24. [PubMed] [Google Scholar]
- [189].Harada K, Lu S, Chisholm DM, Syrjanen S, Schor AM, Angiogenesis and vasodilation in skin warts: association with hpv infection, Anticancer Res. 20 (2000) 4519–4523. [PubMed] [Google Scholar]
- [190].Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M, Ultraviolet b irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1, Br. J. Dermatol 152 (2005) 115–121. [DOI] [PubMed] [Google Scholar]
- [191].Bielenberg DR, Bucana CD, Sanchez R, Donawho CK, Kripke ML, Fidler IJ, Molecular regulation of uvb-induced cutaneous angiogenesis, J. Invest. Dermatol 111 (1998) 864–872. [DOI] [PubMed] [Google Scholar]
- [192].Newell B, Bedlow AJ, Cliff S, Drysdale SB, Stanton AW, Mortimer PS, Comparison of the micro-vasculature of basal cell carcinoma and actinic keratosis using intravital microscopy and immuno-histochemistry, Br. J. Dermatol 149 (2003) 105–110. [DOI] [PubMed] [Google Scholar]
- [193].Nijsten T, Colpaert CG, Vermeulen PB, Harris AL, Van Marck E, Lambert J, Cyclooxygenase-2 ex-pression and angiogenesis in squamous cell carcinoma of the skin and its precursors: a paired immunohistochemical study of 35 cases, Br. J. Dermatol 151 (2004) 837–845. [DOI] [PubMed] [Google Scholar]
- [194].Jour G, Ivan D, Aung PP, Angiogenesis in melanoma: an update with a focus on current targeted therapies, J. Clin. Pathol 69 (2016) 472–483. [DOI] [PubMed] [Google Scholar]
- [195].Danielsen T, Rofstad EK, Vegf, bfgf and egf in the angiogenesis of human melanoma xenografts, Int. J. Cancer 76 (1998) 836–841. [DOI] [PubMed] [Google Scholar]
- [196].Lattanzio L, Tonissi F, Torta I, Gianello L, Russi E, Milano G, Merlano M, Lo Nigro C, Role of il-8 induced angiogenesis in uvea melanoma, Invest. New Drugs 31 (2013) 1107–1114. [DOI] [PubMed] [Google Scholar]
- [197].Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M, Role of matrix metallopro-teinases in photoaging and photocarcinogenesis, Int. J. Mol. Sci (2016) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Konstantina A, Lazaris AC, Ioannidis E, Liossi A, Aroni K, Immunohistochemical expression of vegf, hif1-a, and plgf in malignant melanomas and dysplastic nevi, Melanoma Res. 21 (2011) 389–394. [DOI] [PubMed] [Google Scholar]
- [199].Kim A, Im M, Yim NH, Ma JY, Reduction of metastatic and angiogenic potency of malignant cancer by eupatorium fortunei via suppression of mmp-9 activity and vegf production, Sci. Rep 4 (2014) 6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Hamsa TP, Kuttan G, Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor-1, vegf, and proinflammatory mediators, Drug Chem. Toxicol 35 (2012) 57–70. [DOI] [PubMed] [Google Scholar]
- [201].Guruvayoorappan C, Kuttan G, Beta-carotene inhibits tumor-specific angiogenesis by altering the cytokine profile and inhibits the nuclear translocation of transcription factors in b16f-10 melanoma cells, Integr. Cancer Ther 6 (2007) 258–270. [DOI] [PubMed] [Google Scholar]
- [202].Mantena SK, Roy AM, Katiyar SK, Epigallocatechin-3-gallate inhibits photocarcinogenesis through inhibition of angiogenic factors and activation of cd8+ t cells in tumors, Photochem. Photobiol 81 (2005) 1174–1179. [DOI] [PubMed] [Google Scholar]
- [203].Yusuf N, Nasti TH, Meleth S, Elmets CA, Resveratrol enhances cell-mediated immune response to dmba through tlr4 and prevents dmba induced cutaneous carcinogenesis, Mol. Carcinog 48 (2009) 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Tong X, Mirzoeva S, Veliceasa D, Bridgeman BB, Fitchev P, Cornwell ML, Crawford SE, Pelling JC, Volpert OV, Chemopreventive apigenin controls uvb-induced cutaneous proliferation and angiogenesis through hur and thrombospondin-1, Oncotarget 5 (2014) 11413–11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Jung SK, Lee KW, Byun S, Lee EJ, Kim JE, Bode AM, Dong Z, Lee HJ, Myricetin inhibits uvb-induced angiogenesis by regulating pi-3 kinase in vivo, Carcinogenesis 31 (2010) 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Dehelean CA, Feflea S, Gheorgheosu D, Ganta S, Cimpean AM, Muntean D, Amiji MM, An-ti-angiogenic and anti-cancer evaluation of betulin nanoemulsion in chicken chorioallantoic membrane and skin carcinoma in balb/c mice, J. Biomed. Nanotechnol 9 (2013) 577–589. [DOI] [PubMed] [Google Scholar]
- [207].Roomi MW, Ivanov V, Netke S, Kalinovsky T, Niedzwiecki A, Rath M, In vivo and in vitro antitumor effect of ascorbic acid, lysine, proline and green tea extract on human melanoma cell line a2058, In Vivo 20 (2006) 25–32. [PubMed] [Google Scholar]
- [208].Malafa MP, Fokum FD, Smith L, Louis A, Inhibition of angiogenesis and promotion of melanoma dormancy by vitamin e succinate, Ann. Surg. Oncol 9 (2002) 1023–1032. [DOI] [PubMed] [Google Scholar]
- [209].Wietrzyk J, Boratynski J, Grynkiewicz G, Ryczynski A, Radzikowski C, Opolski A, Antiangiogenic and antitumour effects in vivo of genistein applied alone or combined with cyclophosphamide, Anticancer Res. 21 (2001) 3893–3896. [PubMed] [Google Scholar]
- [210].Guruvayoorappan C, Kuttan G, 13 cis-retinoic acid regulates cytokine production and inhibits angiogenesis by disrupting endothelial cell migration and tube formation, J. Exp. Therap. Oncol 7 (2008) 173–182. [PubMed] [Google Scholar]
- [211].Kuphal S, Bosserhoff A, Recent progress in understanding the pathology of malignant melanoma, J. Pathol 219 (2009) 400–409. [DOI] [PubMed] [Google Scholar]
- [212].Gaggioli C, Sahai E, Melanoma invasion – current knowledge and future directions, Pigm. Cell Res 20 (2007) 161–172. [DOI] [PubMed] [Google Scholar]
- [213].Friedl P, Wolf K, Tumour-cell invasion and migration: diversity and escape mechanisms, Nat. Rev. Cancer 3 (2003) 362–374. [DOI] [PubMed] [Google Scholar]
- [214].McGary EC, Lev DC, Bar-Eli M, Cellular adhesion pathways and metastatic potential of human melanoma, Cancer. Biol. Ther 1 (2002) 459–465. [DOI] [PubMed] [Google Scholar]
- [215].AlQathama A, Prieto JM, Natural products with therapeutic potential in melanoma metastasis, Nat. Prod. Rep 32 (2015) 1170–1182. [DOI] [PubMed] [Google Scholar]
- [216].Parri M, Chiarugi P, Rac and rho gtpases in cancer cell motility control, Cell Commun. Signal.: CCS 8 (2010) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Shin DH, Kim OH, Jun HS, Kang MK, Inhibitor effect of capsaicin on b16-f10 melanoma cell mi-gration via the phosphatidylinositol 3-kinase/akt/rac1 signal pathway, Exp. Mol. Med 40 (2008) 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Haass NK, Smalley KS, Li L, Herlyn M, Adhesion, migration and communication in melanocytes and melanoma, Pigm. Cell Res. 18 (2005) 150–159. [DOI] [PubMed] [Google Scholar]
- [219].Kuphal S, Bosserhoff AK, E-cadherin cell–cell communication in melanogenesis and during devel-opment of malignant melanoma, Arch. Biochem. Biophys 524 (2012) 43–47. [DOI] [PubMed] [Google Scholar]
- [220].Xiang L, Chi T, Tang Q, Yang X, Ou M, Chen X, Yu X, Chen J, Ho RJ, Shao J, Jia L, A pentacyclic triterpene natural product, ursolic acid and its prodrug us597 inhibit targets within cell adhesion pathway and prevent cancer metastasis, Oncotarget 6 (2015) 9295–9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Ranjan A, Kalraiya RD, Invasive potential of melanoma cells correlates with the expression of mt1-mmp and regulated by modulating its association with motility receptors via n-glycosylation on the receptors, BioMed Res. Int 2014 (2014) 804680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Redondo P, Lloret P, Idoate M, Inoges S, Expression and serum levels of mmp-2 and mmp-9 during human melanoma progression, Clin. Exp. Dermatol 30 (2005) 541–545. [DOI] [PubMed] [Google Scholar]
- [223].Chen YJ, Chang LS, Nfkappab- and ap-1-mediated DNA looping regulates matrix metalloprotein-ase-9 transcription in tnf-alpha-treated human leukemia u937 cells, Biochim. Biophys. Acta 1849 (2015) 1248–1259. [DOI] [PubMed] [Google Scholar]
- [224].Shi H, Wu Y, Wang Y, Zhou M, Yan S, Chen Z, Gu D, Cai Y, Liquiritigenin potentiates the inhibitory effects of cisplatin on invasion and metastasis via downregulation mmp-2/9 and pi3k/akt signaling pathway in b16f10 melanoma cells and mice model, Nutr. Cancer 67 (2015) 761–770. [DOI] [PubMed] [Google Scholar]
- [225].Huang SC, Ho CT, Lin-Shiau SY, Lin JK, Carnosol inhibits the invasion of b16/f10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa b and c-jun, Biochem. Pharmacol 69 (2005) 221–232. [DOI] [PubMed] [Google Scholar]
- [226].Singh T, Vaid M, Katiyar N, Sharma S, Katiyar SK, Berberine, an isoquinoline alkaloid, inhibits melanoma cancer cell migration by reducing the expressions of cyclooxygenase-2, prostaglandin e(2) and prostaglandin e(2) receptors, Carcinogenesis 32 (2011) 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [227].Kim HS, Kim MJ, Kim EJ, Yang Y, Lee MS, Lim JS, Berberine-induced ampk activation inhibits the metastatic potential of melanoma cells via reduction of erk activity and cox-2 protein expression, Biochem. Pharmacol 83 (2012) 385–394. [DOI] [PubMed] [Google Scholar]
- [228].Vaid M, Singh T, Katiyar SK, Grape seed proanthocyanidins inhibit melanoma cell invasiveness by reduction of pge2 synthesis and reversal of epithelial-to-mesenchymal transition, PLoS One 6 (2011) e21539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [229].Huang HP, Shih YW, Chang YC, Hung CN, Wang CJ, Chemoinhibitory effect of mulberry anthocya-nins on melanoma metastasis involved in the ras/pi3k pathway, J. Agric. Food Chem 56 (2008) 9286–9293. [DOI] [PubMed] [Google Scholar]
- [230].Vaid M, Prasad R, Sun Q, Katiyar SK, Silymarin targets beta-catenin signaling in blocking migra-tion/invasion of human melanoma cells, PLoS One 6 (2011) e23000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [231].Cao HH, Cheng CY, Su T, Fu XQ, Guo H, Li T, Tse AK, Kwan HY, Yu H, Yu ZL, Quercetin inhibits hgf/c-met signaling and hgf-stimulated melanoma cell migration and invasion, Mol. Cancer 14 (2015) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Cao HH, Tse AK, Kwan HY, Yu H, Cheng CY, Su T, Fong WF, Yu ZL, Quercetin exerts anti-melanoma activities and inhibits stat3 signaling, Biochem. Pharmacol 87 (2014) 424–434. [DOI] [PubMed] [Google Scholar]
- [233].Cao HH, Chu JH, Kwan HY, Su T, Yu H, Cheng CY, Fu XQ, Guo H, Li T, Tse AK, Chou GX, Mo HB, Yu ZL, Inhibition of the stat3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma, Sci. Rep 6 (2016) 21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Singh T, Katiyar SK, Green tea catechins reduce invasive potential of human melanoma cells by targeting cox-2, pge2 receptors and epithelial-to-mesenchymal transition, PLoS One 6 (2011) e25224. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [235].Chang CW, Hsieh YH, Yang WE, Yang SF, Chen Y, Hu DN, Epigallocatechingallate inhibits migration of human uveal melanoma cells via downregulation of matrix metalloproteinase-2 activity and erk1/2 pathway, BioMed Res. Int 2014 (2014) 141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Bhattacharya S, Darjatmoko SR, Polans AS, Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic pro-tooncogenic protein akt/pkb, Melanoma Res. 21 (2011) 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Ferrer P, Asensi M, Segarra R, Ortega A, Benlloch M, Obrador E, Varea MT, Asensio G, Jorda L, Estrela JM, Association between pterostilbene and quercetin inhibits metastatic activity of b16 mela-noma, Neoplasia 7 (2005) 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Lo C, Lai TY, Yang JS, Yang JH, Ma YS, Weng SW, Lin HY, Chen HY, Lin JG, Chung JG, Gallic acid inhibits the migration and invasion of a375: S2 human melanoma cells through the inhibition of matrix metalloproteinase-2 and ras, Melanoma Res. 21 (2011) 267–273. [DOI] [PubMed] [Google Scholar]
- [239].Machado D, Shishido SM, Queiroz KC, Oliveira DN, Faria AL, Catharino RR, Spek CA, Ferreira CV, Irradiated riboflavin diminishes the aggressiveness of melanoma in vitro and in vivo, PLoS One 8 (2013) e54269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Czyz M, Lesiak-Mieczkowska K, Koprowska K, Szulawska-Mroczek A, Wozniak M, Cell con-text-dependent activities of parthenolide in primary and metastatic melanoma cells, Br. J. Pharmacol 160 (2010) 1144–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Yun J, Kim BG, Kang JS, Park SK, Lee K, Hyun DH, Kim HM, In MJ, Kim DC, Lipid-soluble ginseng extract inhibits invasion and metastasis of b16f10 melanoma cells, J. Med. Food 18 (2015) 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Lee SG, Kang YJ, Nam JO, Anti-metastasis effects of ginsenoside rg3 in b16f10 cells, J. Microbiol. Biotechnol 25 (2015) 1997–2006. [DOI] [PubMed] [Google Scholar]
- [243].Wang RJ, Yang CH, Hu ML, 1-Deoxynojirimycin inhibits metastasis of b16f10 melanoma cells by attenuating the activity and expression of matrix metalloproteinases-2 and -9 and altering cell surface glycosylation, J. Agric. Food Chem 58 (2010) 8988–8993. [DOI] [PubMed] [Google Scholar]
- [244].de Gruijl FR, Van der Leun JC, Estimate of the wavelength dependency of ultraviolet carcinogene-sis in humans and its relevance to the risk assessment of a stratospheric ozone depletion, Health Phys. 67 (1994) 319–325. [DOI] [PubMed] [Google Scholar]
- [245].Elmets CA, Singh D, Tubesing K, Matsui M, Katiyar S, Mukhtar H, Cutaneous photoprotection from ultraviolet injury by green tea polyphenols, J. Am. Acad. Dermatol 44 (2001) 425–432. [DOI] [PubMed] [Google Scholar]
- [246].Heinrich U, Moore CE, De Spirt S, Tronnier H, Stahl W, Green tea polyphenols provide photopro-tection, increase microcirculation, and modulate skin properties of women, J. Nutr 141 (2011) 1202–1208. [DOI] [PubMed] [Google Scholar]
- [247].Zhao H, Zhu W, Jia L, Sun X, Chen G, Zhao X, Li X, Meng X, Kong L, Xing L, Yu J, Phase i study of topical epigallocatechin-3-gallate (egcg) in patients with breast cancer receiving adjuvant radiotherapy, Br. J. Radiol 89 (2016) 20150665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY, Phase i clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions, Anticancer Res. 21 (2001) 2895–2900. [PubMed] [Google Scholar]
- [249].Ranjan AP, Mukerjee A, Helson L, Vishwanatha JK, Scale up, optimization and stability analysis of curcumin c3 complex-loaded nanoparticles for cancer therapy, J. Nanobiotechnol 10 (2012) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, Morrow GR, Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients, Radiat. Res 180 (2013) 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Panahi Y, Sahebkar A, Parvin S, Saadat A, A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications, Ann. Clin. Biochem 49 (2012) 580–588. [DOI] [PubMed] [Google Scholar]
- [252].Afshariani R, Farhadi P, Ghaffarpasand F, Roozbeh J, Effectiveness of topical curcumin for treat-ment of mastitis in breastfeeding women: a randomized, double-blind, placebo-controlled clinical trial, Oman. Med. J 29 (2014) 330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Knatko EV, Ibbotson SH, Zhang Y, Higgins M, Fahey JW, Talalay P, Dawe RS, Ferguson J, Huang JT, Clarke R, Zheng S, Saito A, Kalra S, Benedict AL, Honda T, Proby CM, Dinkova-Kostova AT, Nrf2 activation protects against solar-simulated ultraviolet radiation in mice and humans, Cancer Prev. Res 8 (2015) 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT, Sulforaphane mobilizes cellular defenses that protect skin against damage by uv radiation, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 17500–17505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Farris P, Yatskayer M, Chen N, Krol Y, Oresajo C, Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin, J. Drugs Dermatol.: JDD 13 (2014) 1467–1472. [PubMed] [Google Scholar]
- [256].Moyano-Mendez JR, Fabbrocini G, De Stefano D, Mazzella C, Mayol L, Scognamiglio I, Carnuccio R, Ayala F, La Rotonda MI, De Rosa G, Enhanced antioxidant effect of trans-resveratrol: potential of binary systems with polyethylene glycol and cyclodextrin, Drug Dev. Ind. Pharm 40 (2014) 1300–1307. [DOI] [PubMed] [Google Scholar]
- [257].Juskaite V, Ramanauskiene K, Briedis V, Design and formulation of optimized microemulsions for dermal delivery of resveratrol, Evidence-based Complem. Alternative Med.: eCAM 2015 (2015) 540916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Friedrich RB, Kann B, Coradini K, Offerhaus HL, Beck RC, Windbergs M, Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin, Eur. J. Pharm. Sci 78 (2015) 204–213. [DOI] [PubMed] [Google Scholar]
- [259].Amiot MJ, Romier B, Dao TM, Fanciullino R, Ciccolini J, Burcelin R, Pechere L, Emond C, Savouret JF, Seree E, Optimization of trans-resveratrol bioavailability for human therapy, Biochimie 95 (2013) 1233–1238. [DOI] [PubMed] [Google Scholar]
- [260].Arsic I, Tadic V, Vlaovic D, Homsek I, Vesic S, Isailovic G, Vuleta G, Preparation of novel apigenin-enriched, liposomal and non-liposomal, antiinflammatory topical formulation as substitutes for corticosteroid therapy, Phytother. Res.: PTR 25 (2011) 228–233. [DOI] [PubMed] [Google Scholar]
- [261].Choi S, Youn J, Kim K, Joo da H, Shin S, Lee J, Lee HK, An IS, Kwon S, Youn HJ, Ahn KJ, An S, Cha HJ, Apigenin inhibits uva-induced cytotoxicity in vitro and prevents signs of skin aging in vivo, Int. J. Mol. Med 38 (2016) 627–634. [DOI] [PubMed] [Google Scholar]
- [262].Ulrich J, Hartmann JT, Dorr W, Ugurel S, Skin toxicity of anti-cancer therapy, J. Dtsch. Dermatol. Ges 6 (2008) 959–977. [DOI] [PubMed] [Google Scholar]
- [263].Mandala M, Massi D, De Giorgi V, Cutaneous toxicities of braf inhibitors: clinical and pathological challenges and call to action, Crit. Rev. Oncol. Hematol 88 (2013) 318–337. [DOI] [PubMed] [Google Scholar]
- [264].Tripp MK, Watson M, Balk SJ, Swetter SM, Gershenwald JE, State of the science on prevention and screening to reduce melanoma incidence and mortality: the time is now, CA: Cancer J. Clin (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Greenberg ES, Chong KK, Huynh KT, Tanaka R, Hoon DS, Epigenetic biomarkers in skin cancer, Cancer Lett. 342 (2014) 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]