Abstract
The down regulation of CD4 by cultured monocytes has been observed by our group and by other investigators. Flow cytometric experiments were done to examine which factors might influence this phenomenon. The addition of lipopolysaccharide, granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor, or interleukin-10 to monocyte cultures failed to inhibit the decrease in monocyte CD4 expression routinely observed following overnight culture. The down regulation was an adherence-independent phenomenon and was not influenced by the type of anticoagulant into which the peripheral blood was collected or by the presence or absence of lymphocytes within the cultures. The avoidance of the use of Ficoll-Paque to isolate peripheral blood mononuclear cells did not prevent monocyte CD4 down regulation. Finally, by tagging monocyte CD4 with an anti-CD4 phycoerythrin-conjugated monoclonal antibody prior to culture, we were able to determine that the down regulation observed was the result of the internalization of the molecule. At this time, we conclude that the observed down regulation of monocyte CD4 is probably due to the differentiation of blood monocytes into tissue culture-derived macrophages rather than to some artifact of the isolation procedure.
The CD4 molecule is a 55- to 60-kDa membrane glycoprotein that was first identified on a subset of T cells which usually function as T-helper cells (31). The CD4 molecule consists of four extracellular Ig-like domains, a transmembrane domain, and a cytoplasmic tail (22). These features make CD4 a member of the Ig superfamily (6). In humans, CD4 is also expressed by other cell types, including cells of the dendritic lineage and cells of the monocyte/macrophage lineage (38); CD4 has been identified on virtually all blood monocytes, although at lower levels than expressed by CD4+ T cells (15).
The in vivo role(s) of CD4 on human monocytes and macrophages remains unclear (23). IL-16 is a chemoattractant for human CD4+ monocytes (10) and CD4+ eosinophils (30), as well as for rat and human CD4+ T cells (3, 9). Since IL-16 uses CD4 as a receptor/coreceptor on T cells (11), CD4 probably serves the same function on CD4+ monocytes and eosinophils (4). Other roles for monocyte CD4, if any, remain to be discovered.
Macrophages are important components of the immune system. These cells act as scavengers of invading microorganisms, presenters of antigens to T cells, and secretors of many immune-regulating cytokines (16). The persistence of HIV-infected monocytes and macrophages makes them viral reservoirs capable of infecting other cells (17). HIV-infected monocytes and macrophages have impaired phagocytic and killing abilities, impaired antigen presentation, and altered cytokine profiles, all of which contribute to AIDS pathogenesis (8, 16). Furthermore, HIV-infected macrophages and microglia are thought to produce neurotoxins which contribute to AIDS-related dementia (8). The infection of cells of the monocyte and macrophage lineage requires both the CD4 molecule (21) and the β-chemokine receptor CCR5, an HIV coreceptor (12, 14).
Previous attempts in our laboratory to infect overnight-cultured monocytes with HIV were unsuccessful. We hypothesized that this failure to infect the monocytes was due to their down regulation of CD4, an event which has also been observed by others and which has been attributed to the differentiation of monocytes into tissue culture-derived macrophages (19, 35). In this report, we summarize the findings of experiments in which we used flow cytometric analysis to examine various factors which we hypothesized might play a role in the down regulation of monocyte CD4.
MATERIALS AND METHODS
Abbreviations.
The following abbreviations are used: ACD, acid citrate dextrose; BSA, bovine serum albumin; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; GM-CSF, granulocyte-macrophage colony stimulating factor; HIV, human immunodeficiency virus; Ig, immunoglobulin; IL-10, interleukin-10; LPS, lipopolysaccharide; MAb, monoclonal antibody; M-CSF, macrophage colony-stimulating factor; NaN3, sodium azide; PBMC, peripheral blood mononuclear cells; PBS, phosphate-buffered saline; PE, phycoerythrin.
Ethics approval.
Ethics approval for the following experiments was received from the Ottawa General Hospital Ethics Committee.
Cell cultures.
Buffy coat or whole blood units were obtained from the Ottawa chapter of the Red Cross. Alternatively, peripheral blood was obtained from healthy laboratory personnel following receipt of informed consent. The blood was collected into Vacutainers (Becton Dickinson, San Jose, Calif.) containing either sodium heparin or ACD; for the anticoagulant experiments, blood was collected into separate Vacutainers containing EDTA(K3), heparin, or ACD. PBMC were isolated by using Ficoll-Paque as previously described (20). Cell cultures were established as described below.
(i) Whole-blood cultures.
Whole-blood cultures were established in 6-ml polypropylene tubes (Sarstedt, St. Laurent, Quebec, Canada) by culturing 2.5 ml of whole blood in 1 ml of RPMI 1640 medium (Gibco BRL, Grand Island, N.Y.) containing 2 mM l-glutamine (Gibco BRL), 5 μg of amphotericin B (Fungizone; Bristol-Myers Squibb Canada Inc., Montreal, Quebec, Canada) per ml, 40 μg of gentamicin (Gibco BRL) per ml, 0.01 U of penicillin (Gibco BRL) per ml, and 0.01 μg of streptomycin (Gibco BRL) per ml. The cultures were maintained at 37°C with 5% CO2 until the following day.
(ii) Monocyte cultures.
Monocytes were isolated by adherence on gelatin-fibronectin-coated flasks as previously described (20). The cells were cultured in the same medium as for the whole-blood cultures but with the addition of 10% FBS (Gibco BRL); this medium is referred to as RPMI-FBS. In some experiments, parallel monocyte cultures containing various amounts of recombinant GM-CSF (Genzyme, Cambridge, Mass., or Amersham, Oakville, Ontario, Canada), various amounts of recombinant or purified GM-CSF (kindly provided by Genetics Institute, Cambridge, Mass.), 5 or 10 U of recombinant M-CSF (Genzyme) per ml, 5 μg of LPS (Sigma, St. Louis, Mo.) per ml, or 12 U of recombinant IL-10 (kindly donated by Schering Plough Research Institute) per ml were also established. The units used to characterize cytokine activity were defined by the product manufacturers; the biological activity of the Amersham and Genetics Institute stocks of GM-CSF was confirmed in proliferation assays using GM-CSF-dependent TF-1 cells (data not shown), and the concentrations of these GM-CSF stocks used in the monocyte studies were within the range found to support TF-1 proliferation. The monocyte cultures were maintained overnight and/or for 6 days at 37°C with 5% CO2. In some experiments, some flasks of adherent cells were harvested (as described below) immediately following the 1.5-h incubation of the PBMC and the removal of the nonadherent cells. These harvested adherent cells represent the 1.5-h monocytes.
(iii) Post-Ficoll-Paque PBMC cultures.
PBMC were cultured under various conditions. These conditions involved the culture of 2 × 106 PBMC in uncoated polystyrene T25 flasks (Corning Costar Corp., Cambridge, Mass.), gelatin-fibronectin-coated T25 flasks, polypropylene tubes (Sarstedt), Teflon vials (Savillex Corp., Minnetonka, Minn.), or Nunc six-well plates (Gibco BRL). The PBMC were cultured overnight at 37°C with 5% CO2 in 1 to 10 ml of RPMI-FBS, depending on the size of the culture container.
Harvesting of cells.
PBMC and monocyte cultures were harvested by washing the flasks with ice-cold 10 mM EDTA (BDH Inc., Toronto, Ontario, Canada) in RPMI 1640 and allowing the flasks to incubate on ice for 5-min intervals. The cells were collected into 50-ml polypropylene conical tubes containing 1 to 2 ml of FBS. In experiments in which cells were cultured in both adherent environments (i.e., flasks) and nonadherent environments (i.e., Teflon vials), the cells were also harvested using cold EDTA-RPMI medium to control for harvesting conditions. All cells were washed with PBS (Sigma)–0.1% NaN3 (BDH Inc.) at 200 × g for 5 min at room temperature, resuspended in PBS–0.1% NaN3, blocked, and stained as described below.
Staining of cells for two- and three-color flow cytometric analysis. (i) Whole blood.
One milliliter of whole blood from each unit of Red Cross blood or buffy coat was collected from the bag line, and 500 μl of Hepalean (Organon Teknika, Toronto, Ontario, Canada) was added. Samples from Vacutainer [heparin, EDTA(K3), or ACD) tubes did not receive any additional heparin and were processed as described below. Whole-blood volumes of 100 μl were stained with an optimum dose of mouse anti-human antibodies.
The staining of whole-blood cultures took into account the increased volume of blood due to the harvesting medium used in the collection of the culture. We estimated that approximately 140 μl of the diluted whole blood would give the equivalent number of PBMC obtained in the 100-μl sample of undiluted whole blood. This increase in volume was not sufficient to compromise the effectiveness of the Q-Prep procedure (Coulter Corp., Miami, Fla.).
Cells were added to the MAb combinations (Table 1) and incubated in the dark for 10 min at room temperature. The red blood cells were lysed, and the white blood cells were stabilized, using the 35-s cycle on the Q-Prep System. Cells were washed at 200 × g with PBS (optional) and resuspended with PBS to a final volume of 0.5 ml.
TABLE 1.
Summary of the various antibodies used
| Controls, cells, or expt | Antibody(-ies)a | Clone(s) | Company |
|---|---|---|---|
| Autofluorescence control | None added | ||
| Isotype controls | IgG1-PE, IgG2a-FITC | 2T8-2F5, 7T4-1F5 | Coulter Corp. |
| IgG2b-PE | X39 | Becton Dickinson | |
| Color compensation controls | Anti-CD14–FITC, anti-CD4–PE, anti-HLA-DR–TRIb | ||
| Monocytes | Anti-CD14–PE | LeuM3 | Becton Dickinson |
| Mo-2 | Coulter Corp. | ||
| Anti-CD14–FITC | MY4 | Coulter Corp. | |
| Anti-CD4–PE | T4 | Coulter Corp. | |
| Q4120 | Sigma | ||
| Anti-HLA-DR–FITC | L243 | Becton Dickinson | |
| Anti-HLA-DR–TRI | TU-36 | Caltag Laboratories, San Francisco, Calif. | |
| Combinations: two color, anti-CD14–anti-CD4 and anti-CD14–anti-HLA-DR; three-color, anti-CD14–anti-CD4–anti-HLA-DR | |||
| T cells | Anti-CD3–ECD | HIT3A | Coulter Corp. |
| Anti-CD3–FITC | Leu4 | Becton Dickinson | |
| Anti-CD3–TRI | S4.1 | Caltag Laboratories | |
| Anti-CD3–Quantum Red | UCHT-1 | Sigma | |
| Anti-CD4–PE | Various clones as described for monocytes | ||
| Anti-CD3–anti-CD4 combination | |||
| B cells | Anti-CD19–ECD | HD237 | Coulter Corp. |
| Anti-CD19–FITC | SJ25-C1 | Sigma | |
| FMC63 | Serotec, Mississauga, Ontario, Canada | ||
| Anti-CD20–PE | Leu16 | Becton Dickinson | |
| Anti-HLA-DR | Various clones as described for monocytes | ||
| Combinations: anti-CD19–anti-HLA-DR and/or anti-CD20–anti-HLA-DR | |||
| CD4 tagging experiments | Goat anti-mouse Ig–FITC | Not applicable | Biosource International, Camarillo, Calif. |
TRI and Quantum Red are Cy5-PE tandem dyes. ECD, energy-coupled dye.
Color compensation was performed on whole-blood monocytes. For each fluorochrome, the antibody which would result in the highest-intensity stain was used.
(ii) Post-Ficoll-Paque PBMC and adherent monocytes.
Harvested cells were resuspended in PBS–0.1% NaN3 to a concentration of 0.5 × 106 cells/ml and blocked with an equal volume of autologous plasma or with 10 μg of Gamimmune (Canadian Red Cross Society, Ottawa, Ontario, Canada) per ml for 10 min at room temperature. Two hundred microliters of cells was transferred to tubes to which MAb had been added. The cells were incubated in the dark for 10 min at room temperature, washed in PBS–0.1% NaN3 at 200 × g for 5 min at room temperature, and resuspended to a final volume of 0.5 ml with PBS–0.1% NaN3.
(iii) Tagging experiments.
PBMC were placed on ice, and 2 × 106 PBMC were resuspended with sterile PBS to a volume of 1 ml and blocked with 1 ml of 10 μg/ml of Gamimmune. The cells were incubated in the dark for 10 min with 25 μl of anti-CD4–PE mAb and washed in sterile PBS at 300 × g for 5 min at 4°C. The pellet was resuspended in RPMI-FBS, and the cells were cultured overnight in Nunc six-well plates (Gibco BRL). Untagged PBMC cultures were established as controls.
Untagged and CD4-tagged PBMC were harvested with EDTA-RPMI and washed as described above. The cells were blocked with Gamimmune (10 μg/ml), aliquoted, and stained. Untagged PBMC were stained with anti-CD4–PE MAb or with goat anti-mouse Ig–FITC. CD4-tagged PBMC were stained with goat anti-mouse Ig–FITC.
In experiments using antibodies conjugated to Cy5-PE tandem dyes, an additional blocking step was performed (L. G. Filion, unpublished data) to block monocyte receptors specific for the Cy5 component of the conjugate (33). BSA-Cy5 was added prior to the addition of the tandem dye, either simultaneously with the Gamimmune block in the case of PBMC or alone in the case of whole blood. BSA-Cy5 reagent was prepared by conjugating BSA (Sigma) to Fluorolink Cy5 Reactive Dye (Amersham Life Science, Inc., Pittsburgh, Pa.) as per the manufacturer's instructions.
The combinations of antibodies used in the various experiments are summarized in Table 1. Isotype controls were also included initially but were eventually discontinued once it was determined that isotype binding was negligible.
Flow cytometric analysis of cells.
Cells were double or triple stained to assess the relative proportions and the phenotypes of the subpopulations within the whole blood, Ficoll-Paque-isolated PBMC (cultured and uncultured), and monocyte cultures. Autofluorescent controls and the appropriate color compensation controls were also included. Color compensation was performed on whole-blood monocytes; for each fluorochrome, the antibody which would result in the highest intensity stain was used, i.e., anti-CD14–FITC, anti-CD4–PE, and anti-HLA-DR–TRI. Cell surface marker expression was determined in initial experiments using a Profile II flow cytometer and later using an EPICS XL flow cytometer (Coulter Corp.), both of which were equipped with an argon ion laser emitting at 488 nm. In order to compare results obtained at different time points within an experiment, channel targeting was performed at the beginning of each analysis session using Standard Brite beads (Coulter Corp.). Forward- versus side-scatter histograms were established to visualize the cells and to gate out dead cells and debris. In the case of whole-blood and PBMC samples, gates were established around the lymphocytes and monocytes, and these cell subpopulations were analyzed separately. A minimum of 2,000 events was recorded. In the case of the Profile II flow cytometer, data were acquired using the running software and were reanalyzed using the EPICS XL version 1.5 software (Coulter Corp.). In the case of the EPICS XL flow cytometer, data were both acquired and reanalyzed using the Epics XL version 1.5 software. Figures were generated with the WinMDI program (34).
Statistical analysis.
To determine the degree of monocyte CD4 or HLA-DR expression for any particular time point or culture condition, paired t tests were performed using the mean channel number of Ab-stained cells, after correction for autofluorescence. Paired t tests were also performed to compare CD4 or HLA-DR expression between time points or conditions. The word significant is used to indicate a difference at a P value of <0.050.
RESULTS
Monocyte experiments. (i) Kinetics of CD4 expression by freshly isolated and 6-day-cultured monocytes.
The level of CD4 expression by gelatin-fibronectin-isolated monocytes was examined at various time points following their isolation. In the nine isolations in which 1.5-h monocytes were examined, CD4 levels were consistent with the levels observed in whole blood (data not shown). Lymphocyte contamination levels (CD3+ T cells and CD19+ or CD20+ B cells) were determined for all 1.5-h monocyte cultures and ranged from 0.8 to 11.5%.
Monocytes cultured overnight in RPMI-FBS expressed significantly reduced levels of CD4 (Table 2), ranging from undetectable (Fig. 1) (n = 18) to low-level expression (n = 13). Following 6 days of culture in RPMI-FBS, monocyte CD4 expression was variable, ranging from undetectable to significant expression by either the entire population or a subpopulation (Table 2).
TABLE 2.
Monocyte CD4 expression levels before and after overnight or 6 days of culture in RPMI-FBS
| Isolation no. | Monocyte CD4 expression (mean channel no.) in:
|
|||||
|---|---|---|---|---|---|---|
| Whole blood (baseline)
|
Overnight monocyte or PBMC culturesa
|
6-day monocyte cultures
|
||||
| Autob | CD4c | Auto | CD4 | Auto | CD4 | |
| 1 | 0.265 | 6.38 | 0.232 | 0.228 | NDd | ND |
| 2 | 0.216 | 4.07 | 0.266 | 0.405 | ND | ND |
| 3 | 0.193 | 3.60 | 0.223 | 0.367 | ND | ND |
| 4 | 0.239 | 2.54 | ND | ND | 0.869 | 4.64 (63.1%)e |
| 5 | 0.190 | 2.90 | 0.313 | 0.424 | 0.998 | 1.57 |
| 6 | 0.159 | 2.29 | 0.198 | 0.213 | 0.330 | 0.603 |
| 7 | 0.232 | 4.07 | ND | ND | 0.727 | 5.82 (83.6%)e |
| 8 | 0.296 | 4.49 | ND | ND | 0.774 | 1.24 |
| 9 | 0.266 | 2.79 | ND | ND | 0.771 | 2.57 |
| 10 | 0.216 | 2.97 | 0.529 | 0.691 | 3.95 | 5.48 |
| 11 | 0.181 | 2.76 | 0.296 | 0.308 | 2.17 | 2.46 |
| 12 | 0.218 | 3.08 | 0.271 | 0.313 | 2.16 | 3.74 |
| 13 | 0.204 | 2.54 | 0.228 | 0.267 | 1.53 | 2.17 |
| 14 | 0.223 | 2.75 | 0.192 | 0.197 | 2.15 | 5.56 |
| 15 | 0.235 | 3.02 | 0.206 | 0.222 | 1.49 | 2.07 |
| 16 | 0.235 | 3.10 | 0.301 | 0.204 | 1.44 | 1.33 |
| 17 | 0.310 | 2.67 | 0.358 | 0.324 | 1.22 | 1.27 |
| 18 | 0.276 | 5.33 | 0.324 | 0.200 | ND | ND |
| 19 | 0.640 | 8.02 | 0.551 | 0.846 | ND | ND |
| 20 | 0.224 | 4.79 | 0.705 | 0.692 | ND | ND |
| 21 | 0.291 | 5.54 | 0.269 | 0.225 | ND | ND |
| 22 | 0.385 | 6.61 | 0.642 | 0.486 | ND | ND |
| 23 | 0.590 | 9.15 | 0.457 | 0.777 | ND | ND |
| 24 | 0.560 | 3.74 | 0.365 | 0.485 | ND | ND |
| 25 | 0.269 | 3.09 | 0.253 | 0.688 | ND | ND |
| 26 | 0.190f | 4.02f | 0.492 | 1.02 | ND | ND |
| 27 | 0.125f | 3.87f | 0.269 | 0.244 | ND | ND |
| 28 | 0.302 | 6.88 | 0.256 | 0.426 | ND | ND |
| 29 | 0.427 | 4.63 | 0.256 | 0.269 | ND | ND |
| 30 | 0.315 | 4.51 | 0.234 | 0.362 | ND | ND |
| 31 | 0.284 | 4.49 | 0.249 | 0.264 | ND | ND |
| 32 | 0.298 | 5.09 | 0.201 | 0.645 | ND | ND |
| 33 | 0.315g | 7.45g | 0.427g | 1.32g | ND | ND |
| 34 | 0.459g | 2.05g | 0.173g | 0.820g | ND | ND |
| 35 | 0.336g | 5.68g | 0.148g | 0.883g | ND | ND |
| Meanh ± SEM | 4.04 ± 0.29i | 0.159 ± 0.047ij | 1.43 ± 0.44ik | |||
Isolations 1 to 17, monocyte culture; isolations 18 to 35, PBMC culture.
Mean channel number of autofluorescence (FL2 log).
Mean channel number of anti-CD4–PE signal.
ND, not done.
Increased CD4 expression was observed for a subpopulation of monocytes.
Whole-blood data not available due to Q-Prep malfunction; data from post-Ficoll-Paque PBMC are shown.
Data from the citrate condition of the anticoagulant experiments.
Corrected for autofluorescence.
Statistically significant CD4 expression relative to autofluorescence; P < 0.050.
Statistically significant reduction in CD4 expression relative to whole blood; P < 0.050.
Statistically significant increase in CD4 expression relative to overnight culture; P < 0.050.
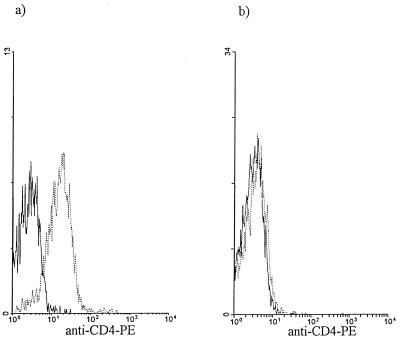
FIG. 1.
Comparison of monocyte CD4 expression after overnight culturing. CD4 expression by monocytes was assessed in whole blood (a) and following monocyte isolation and overnight culture in RPMI-FBS medium (b); monocytes were electronically gated based on their forward- versus side-scatter characteristics. The results of the flow cytometric analysis of a representative experiment in which monocyte CD4 was completely down regulated (n = 18) are shown. ——, autofluorescence; ······, anti-CD4–PE.
The overnight culture of monocytes resulted in significantly increased HLA-DR levels compared to those of whole-blood monocytes (Table 3). HLA-DR was further up regulated following 6-day culturing (Table 3), although the increase did not reach statistical significance; this lack of statistical significance is most likely due to the wide range of expression, from a low of 9.01 mean channel units to a high of 414.7 mean channel units. However, comparison of HLA-DR levels between time points within each isolation reveals that HLA-DR levels did increase with time.
TABLE 3.
Monocyte HLA-DR expression levels before and after overnight or 6 days of culture in RPMI-FBS
| Isolation no. | Monocyte HLA-DR expression in:
|
|||||
|---|---|---|---|---|---|---|
| Whole blood (baseline)
|
Overnight monocyte or PBMC culturesa
|
6-day monocyte cultures
|
||||
| Autob | HLA-DRc | Autob | HLA-DRc | Autob | HLA-DRc | |
| 1 | 0.235 | 18.3 | 0.611 | 86.4 | NDd | ND |
| 2 | 0.618 | 28.4 | 0.289 | 43.6 | ND | ND |
| 3 | 0.489 | 30.4 | 0.191 | 51.7 | ND | ND |
| 4 | 0.232 | 26.0 | ND | ND | 4.58 | 414.7 |
| 5 | 0.622 | 4.85 | 0.566 | 14.9 | 2.82 | 37.7 |
| 6 | 0.290 | 8.17 | 0.389 | 13.7 | 2.85 | 14.9 |
| 7 | 0.329 | 15.5 | ND | ND | 5.10 | 169.3 |
| 8 | 0.255 | 28.0 | ND | ND | 3.59 | 129.4 |
| 9 | 0.377 | 11.8 | ND | ND | 3.46 | 55.9 |
| 10 | 0.976 | 12.8 | 1.49 | 49.7 | 13.4 | 270.7 |
| 11 | 0.265 | 12.6 | 0.369 | 17.7 | 1.33 | 38.7 |
| 12 | 0.354 | 4.66 | 0.340 | 20.0 | 2.03 | 29.9 |
| 13 | 0.304 | 4.35 | 0.295 | 8.46 | 1.41 | 32.7 |
| 14 | 0.358 | 6.18 | 0.637 | 21.4 | 2.08 | 33.7 |
| 15 | 0.340 | 4.60 | 0.338 | 8.87 | 1.37 | 12.2 |
| 16 | 0.359 | 8.38 | 0.514 | 11.3 | 1.48 | 9.01 |
| 17 | 0.480 | 7.13 | 0.458 | 14.6 | 1.56 | 36.6 |
| 18 | 0.299 | 13.3 | 0.378 | 30.9 | ND | ND |
| 19 | 0.439 | 15.8 | 0.353 | 40.0 | ND | ND |
| 20 | 0.244 | 11.0 | 0.681 | 30.0 | ND | ND |
| 21 | 0.599 | 15.1 | 0.594 | 48.5 | ND | ND |
| 22 | 0.702 | 18.9 | 0.895 | 36.1 | ND | ND |
| 23 | 0.630 | 11.9 | 0.548 | 33.6 | ND | ND |
| 24 | 0.488 | 11.6 | 0.583 | 45.5 | ND | ND |
| 25 | 0.599 | 6.65 | 0.505 | 27.4 | ND | ND |
| 26 | 0.205e | 26.8e | 0.532 | 191.7 | ND | ND |
| 27 | 0.141e | 7.97e | 0.275 | 56.5 | ND | ND |
| 28 | 0.216 | 14.0 | 0.239 | 81.0 | ND | ND |
| 29 | 0.289 | 5.96 | 0.447 | 6.97 | ND | ND |
| 30 | 0.447 | 5.46 | 0.366 | 9.54 | ND | ND |
| 31 | 0.486 | 3.17 | 0.348 | 2.54 | ND | ND |
| 32 | 0.428 | 4.01 | 0.305 | 6.56 | ND | ND |
| Meanf ± SEM | 10.33 ± 1.13g | 35.56 ± 7.08gh | 88.45 ± 31.2gi | |||
Isolations 1 to 17, monocyte culture; isolations 18 to 32, PBMC culture.
Mean channel number of autofluorescence (FL1 log or FL4 log).
Mean channel number of anti-HLA-DR–FITC or anti-HLA-DR–TRI staining.
ND, not done.
Whole-blood data not available due to Q-Prep malfunction; data from post-Ficoll-Paque PBMC are shown.
Corrected for autofluorescence.
Statistically significant HLA-DR expression relative to autofluorescence; P < 0.050.
Statistically significant increase in HLA-DR expression relative to whole-blood monocytes; P < 0.050.
Increase in HLA-DR expression by day 6 cultured monocytes relative to overnight-cultured monocytes does not reach statistical significance; P > 0.050.
(ii) Assessment of monocyte CD4 expression following overnight and 6-day culture in the presence or absence of various factors.
Experiments were performed to determine if CD4 expression by gelatin-fibronectin-purified monocytes could be modulated by growth factors or by external stimuli. The addition of 100 U of GM-CSF per ml (n = 4), 10 U of M-CSF per ml (n = 3), 5 μg of LPS per ml (n = 3), or 12 U of IL-10 per ml (n = 3) to monocyte cultures did not prevent CD4 down regulation following overnight culture (Table 4). HLA-DR levels in cultured monocytes were up regulated with respect to those in whole blood but were comparable to those in the RPMI-FBS, GM-CSF, M-CSF, and LPS cultures (data not shown). However, monocytes cultured overnight in the presence of IL-10 showed lower levels of HLA-DR expression than monocytes cultured in RPMI-FBS (Table 5); no statistical analysis was performed, however, because of the bimodal distribution of HLA-DR expression observed for two of the three cultures. Lymphocyte contamination levels (CD3+ T cells and CD19+ or CD20+ B cells) were determined for all overnight monocyte culture conditions and ranged from 0.2 to 12.8%.
TABLE 4.
Monocyte CD4 expression levels before and after overnight culture in RPMI-FBS, with or without various monocyte/macrophage growth factors, suppressors, and activators
| Isolation no. | Monocyte CD4 expression (mean channel no.) in:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole blood (baseline)
|
Overnight monocyte cultures
|
|||||||||||
| RPMI-FBS
|
With GM-CSF
|
With M-CSF
|
With IL-10
|
With LPS
|
||||||||
| Autoa | CD4b | Auto | CD4 | Auto | CD4 | Auto | CD4 | Auto | CD4 | Auto | CD4 | |
| 1 | 0.265 | 6.38 | 0.232 | 0.228 | 0.247 | 0.235 | 0.231 | 0.212 | NDc | ND | 0.231 | 0.277 |
| 2 | 0.216 | 4.07 | 0.266 | 0.405 | 0.286 | 0.497 | 0.366 | 0.574 | ND | ND | 0.369 | 0.373 |
| 3 | 0.193 | 3.60 | 0.223 | 0.367 | 0.243 | 0.344 | 0.233 | 0.349 | ND | ND | 0.248 | 0.296 |
| 12 | 0.218 | 3.08 | 0.271 | 0.313 | 0.362 | 0.397 | ND | ND | 0.503 | 0.525 | ND | ND |
| 14 | 0.223 | 2.75 | 0.192 | 0.197 | ND | ND | ND | ND | 0.224 | 0.317 | ND | ND |
| 16 | 0.235 | 3.10 | 0.301 | 0.204 | ND | ND | ND | ND | 0.358 | 0.190 | ND | ND |
| Meand ± SEM | 3.61 ± 0.58e | 0.038 ± 0.031f | 0.084 ± 0.048g | 0.102 ± 0.066g | −0.018 ± 0.078g | 0.033 ± 0.014g | ||||||
Mean channel number of autofluorescence (FL2 log).
Mean channel number of anti-CD4–PE signal.
ND, not done.
Corrected for autofluorescence.
Statistically significant CD4 expression relative to autofluorescence; P < 0.050.
Statistically significant reduction in CD4 expression relative to whole-blood monocytes; P < 0.050.
No statistically significant difference in CD4 expression between monocytes cultured in RPMI-FBS and cultures supplemented with various factors.
TABLE 5.
Monocyte HLA-DR expression following overnight culture with IL-10
| Isolation no. | Monocyte HLA-DR expression (mean channel no.) in overnight monocyte cultures
|
|||
|---|---|---|---|---|
| RPMI-FBS
|
With IL-10
|
|||
| Autoa | HLA-DRb | Auto | HLA-DRc | |
| 12 | 0.340 | 20.0 | 0.600 | 0.646 (25.0%)d, 10.5 (75.0%)e |
| 14 | 0.637 | 21.4 | 3.68 | 3.50 |
| 16 | 0.514 | 10.8 | 0.575 | 0.908 (20.8%)d, 6.14 (79.2%)e |
| Meanf ± SEM | 16.9 ± 3.3g | 0.066 ± 0.148d, 5.10 ± 2.92e | ||
Mean channel number of autofluorescence (FL1 log).
Mean channel number of anti-HLA-DR–FITC signal.
In isolations 12 and 16, two populations were observed with respect to HLA-DR expression.
Low expression.
High expression.
Corrected for autofluorescence.
Statistically significant HLA-DR expression relative to autofluorescence; P < 0.050.
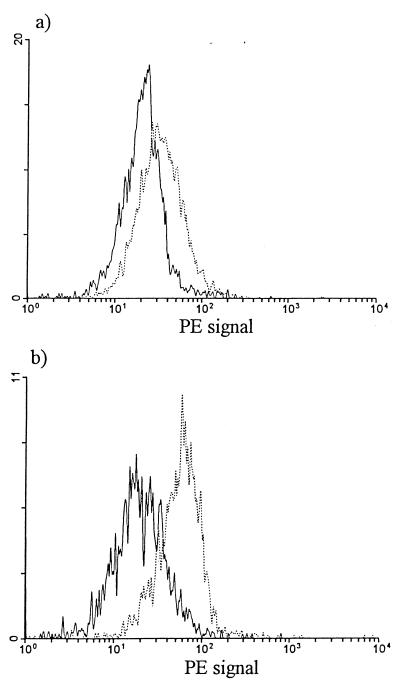
The culture of purified monocytes for 6 days in the presence of LPS (n = 4), M-CSF (5 U/ml [n = 3] or 10 U/ml [n = 4]), or GM-CSF (n = 23; various concentrations ranging from 0.1 to 1,000 U/ml) did not affect CD4 expression relative to CD4 levels for monocytes cultured in RPMI-FBS (data not shown). However, the culture of purified monocytes for 6 days in the presence of 12 U of IL-10 per ml (n = 3) resulted in increased monocyte CD4 reexpression compared to monocytes cultured in RPMI-FBS (Table 6 and Fig. 2). This CD4 reexpression did not quite reach statistical significance, although we expect that significance would have been reached had more experiments been performed.
TABLE 6.
Monocyte CD4 expression following 6 days of culture with IL-10
| Isolation no. | Monocyte CD4 expression (mean channel no.) in overnight monocyte cultures
|
|||
|---|---|---|---|---|
| RPMI-FBS
|
With IL-10
|
|||
| Autoa | CD4b | Auto | CD4 | |
| 12 | 2.16 | 3.74 | 2.10 | 5.85 |
| 14 | 2.15 | 5.56 | 3.50 | 10.7 |
| 16 | 1.44 | 1.33 | 2.52 | 4.17 |
| Meanc ± SEM | 1.63 ± 1.02 | 4.20 ± 1.62d | ||
Mean channel number of autofluorescence (FL2 log).
Mean channel number of anti-CD4–PE signal.
Corrected for autofluorescence.
The increase in CD4 expression does not quite reach statistical significance relative to RPMI-FBS culture; P value, between 0.060 and 0.050.
FIG. 2.
Monocyte CD4 expression after IL-10 treatment. The results of the flow cytometric analysis of a representative experiment (n = 3) in which monocytes were cultured for 6 days in the absence (a) or presence (b) of IL-10 are shown; monocytes were electronically gated based on their forward- versus side-scatter characteristics. ——, autofluorescence; ······, anti-CD4–PE.
Lymphocyte contamination levels (CD3+ T cells and CD19+ and CD20+ B cells) were determined for all day 6 monocyte culture conditions and ranged from 0.2 to 16.8%.
Effect of adherence on monocyte CD4 expression in PBMC cultures.
The role of culture-mediated adherence was assessed by determining monocyte CD4 levels immediately following isolation of PBMC by use of Ficoll-Paque. In 5 of 18 PBMC isolations assessed, down regulation of monocyte CD4 could already be observed prior to the establishment of PBMC cultures (data not shown). Further experiments were performed to examine the effects of specific adherence and nonadherence culture conditions on monocyte CD4 down regulation. The effects of adherence mediated by gelatin-fibronectin-coated polystyrene surfaces (n = 6) and by uncoated polystyrene surfaces (n = 6), were examined; PBMC were used rather than purified monocytes since we had established that the presence or absence of lymphocytes had no effect on monocyte CD4 expression (Table 2). The effect of nonadherence was also examined by culturing PBMC in Teflon vials (n = 3). Following overnight culture, monocyte CD4 expression was significantly and comparably down regulated for all culture conditions (Table 7).
TABLE 7.
Monocyte CD4 expression levels before and after overnight culture in adherent and nonadherent environments
| Isolation no. | Monocyte CD4 expression (mean channel no.) in:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Whole blood (baseline)
|
Overnight PBMC cultures in:
|
|||||||
| Gelatin-fibronectin-coated polystyrene flasks
|
Uncoated polystyrene flasks
|
Teflon vials
|
||||||
| Autoa | CD4b | Auto | CD4 | Auto | CD4 | Auto | CD4 | |
| 18 | 0.276 | 5.33 | 0.324 | 0.200 | 0.440 | 0.253 | NDc | ND |
| 20 | 0.224 | 4.79 | 0.705 | 0.692 | 0.566 | 0.714 | ND | ND |
| 21 | 0.291 | 5.54 | 0.269 | 0.225 | 0.310 | 0.289 | ND | ND |
| 26 | 0.190d | 4.02d | 0.492 | 1.02 | 0.352 | 1.32 | 0.368 | 1.21 |
| 27 | 0.125d | 3.87d | 0.269 | 0.244 | 0.220 | 0.220 | 0.220 | 0.279 |
| 28 | 0.302 | 6.88 | 0.256 | 0.426 | 0.198 | 0.389 | 0.237 | 0.420 |
| Meane ± SEM | 4.84 ± 0.43f | 0.082 ± 0.098g | 0.183 ± 0.166g | 0.361 ± 0.243b | ||||
Mean channel number of autofluorescence (FL2 log).
Mean channel number of anti-CD4–PE signal.
ND, not done.
Whole-blood data not available due to Q-Prep malfunction; data from post-Ficoll-Paque PBMC are shown.
Corrected for autofluorescence.
Statistically significant CD4 expression relative to autofluorescence; P < 0.050.
Statistically significant decrease in CD4 expression relative to whole blood; P < 0.050.
Although the reduction in CD4 expression in Teflon-cultured monocytes compared to whole-blood monocytes did not quite reach statistical significance (P value, between 0.060 and 0.050), no statistically significant difference in CD4 expression between the three types of overnight cultures (P > 0.050) was observed.
Effect of Ficoll-Paque on monocyte CD4 expression.
The effect of Ficoll-Paque isolation of PBMC was examined for its possible involvement in monocyte CD4 down regulation. Whole blood and PBMC were assessed for monocyte CD4 expression before and after overnight culturing.
Following overnight culturing of PBMC, the level of monocyte CD4 expression was significantly down regulated in all four experiments (Table 8), consistent with previous observations. In three of four experiments, monocytes cultured overnight in a whole-blood environment also showed down regulation of CD4 levels (Table 8, isolations 30 to 32; in isolation 30, the down regulation of monocyte CD4 was obvious only by overlaying the anti-CD4–PE histogram for baseline monocyte CD4 expression in whole blood with the anti-CD4–PE histogram of monocyte CD4 expression following overnight culturing of the whole-blood culture). The level of monocyte CD4 expression in these three whole-blood cultures was higher than monocyte CD4 levels observed for overnight PBMC cultures. Monocytes in one of the whole-blood cultures continued to express CD4 levels comparable to those observed in peripheral blood (Table 8, isolation 29). Overall, CD4 expression by monocytes cultured overnight in a whole-blood environment was significantly higher than that by monocytes cultured overnight in RPMI-FBS.
TABLE 8.
Monocyte CD4 expression levels (as a function of Ficoll-Paque isolationa) before and after overnight culture in a whole-blood or RPMI-FBS environment
| Isolation no. | Monocyte CD4 expression (mean channel no.) in:
|
|||||
|---|---|---|---|---|---|---|
| Whole blood (baseline)
|
Overnight cultures
|
|||||
| Whole-blood cultures
|
PBMC cultures in RPMI-FBS
|
|||||
| Autob | CD4c | Auto | CD4 | Auto | CD4 | |
| 29 | 0.427 | 4.63 | 0.253 | 4.93 | 0.256 | 0.269 |
| 30 | 0.315 | 4.51 | 0.266 | 4.02 | 0.234 | 0.362 |
| 31 | 0.284 | 4.49 | 0.233 | 1.82 | 0.249 | 0.264 |
| 32 | 0.298 | 5.09 | 0.275 | 1.98 | 0.201 | 0.645 |
| Meand ± SEM | 4.35 ± 0.15e | 2.93 ± 0.77e | 0.150 ± 0.102fg | |||
For each experiment, monocytes were cultured with and without prior isolation of PBMC by use of Ficoll-Paque.
Mean channel number of autofluorescence (FL2 log).
Mean channel number of anti-CD4–PE signal.
Corrected for autofluorescence.
Statistically significant CD4 expression relative to autofluorescence; P < 0.050.
Statistically significant reduction in CD4 expression relative to whole-blood monocytes; P < 0.050.
Statistically significant reduction in CD4 expression relative to monocytes in whole-blood cultures; P < 0.050.
Effect of anticoagulant on monocyte CD4 expression.
The effects of various anticoagulants on monocyte CD4 expression levels were assessed (n = 3). CD4 expression of monocytes was assessed for PBMC cultures established from peripheral blood collected in Vacutainer tubes containing sodium heparin, ACD, or EDTA(K3). Following overnight culturing, monocyte CD4 levels were equally down regulated, regardless of the anticoagulant used (Table 9).
TABLE 9.
Monocyte CD4 expression levels (as a function of anticoagulanta) before and after overnight culture in RPMI-FBS
| Isolation no. | Monocyte CD4 expression (mean channel no.) in:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole blood (baseline) with:
|
Overnight PBMC cultures with:
|
|||||||||||
| Citrate
|
EDTA
|
Heparin
|
Citrate
|
EDTA
|
Heparin
|
|||||||
| Autob | CD4c | Auto | CD4 | Auto | CD4 | Auto | CD4 | Auto | CD4 | Auto | CD4 | |
| 33 | 0.315 | 7.45 | 0.445 | 3.70 | 0.456 | 6.51 | 0.427 | 1.32 | 0.353 | 0.956 | 0.334 | 0.943 |
| 34 | 0.459 | 2.05 | 0.408 | 4.24 | 0.371 | 2.61 | 0.173 | 0.820 | 0.140 | 0.922 | 0.174 | 0.954 |
| 35 | 0.336 | 5.68 | 0.353 | 5.79 | 0.359 | 3.95 | 0.148 | 0.883 | 0.143 | 0.721 | 0.147 | 0.726 |
| Meand ± SEM | 4.69 ± 1.63e | 4.17 ± 0.65e | 3.96 ± 1.12e | 0.758 ± 0.072f | 0.654 ± 0.064f | 0.656 ± 0.063f | ||||||
For each isolation, whole blood was collected in three different anticoagulants and assessed for CD4 expression. PBMC were subsequently isolated from each whole-blood–anticoagulant combination, and the overnight PBMC cultures were assessed for monocyte CD4 expression.
Mean channel number of autofluorescence (FL2 log).
Mean channel number of anti-CD4–PE signal.
Corrected for autofluorescence.
No statistically significant difference in CD4 expression between any of the whole-blood conditions; P > 0.050.
No statistically significant difference in CD4 expression between any of the overnight cultures; P > 0.050.
Determination of the fate of down-regulated monocyte CD4.
The fate of monocyte CD4 was determined in tagging experiments (n = 3) in which PBMC cultures were stained with anti-CD4–PE MAb prior to overnight culture; untagged PBMC cultures were established as controls.
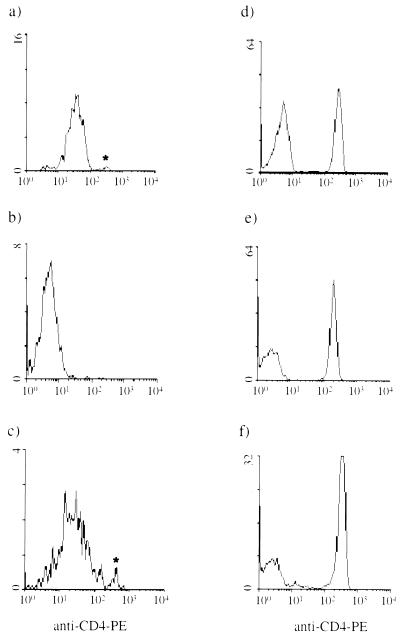
Untagged and anti-CD4–PE-tagged PBMC cultures were harvested following overnight culture. The untagged PBMC cultures were stained with anti-CD4–PE MAb, whereas the tagged PBMC cultures were not. Flow cytometric analysis of the anti-CD4–PE-tagged cultures revealed a PE signal for the monocyte (Table 10 and Fig. 3c) and lymphocyte (Fig. 3f) populations. The untagged PBMC cultures, which were stained with anti-CD4–PE MAb upon harvesting, showed staining of CD4+ lymphocytes (Fig. 3e), consistent with the levels observed for whole blood (Fig. 3d), but negligible staining of the monocytes (Table 10 and Fig. 3b).
TABLE 10.
Monocyte CD4 tagging experiments: anti-CD4-PE signal
| Isolation no. | Monocyte CD4 expression (mean channel no.) in:
|
||||
|---|---|---|---|---|---|
| Whole blood (baseline)
|
Overnight PBMC cultures
|
||||
| Untagged cultures
|
CD4-tagged cultures (CD4c) | ||||
| Autoa | CD4b | Auto | CD4b | ||
| 19 | 0.640 | 8.02 | 0.551 | 0.846 | 1.68 |
| 24 | 0.560 | 3.74 | 0.365 | 0.485 | 2.47 |
| 25 | 0.269 | 3.09 | 0.253 | 0.688 | 4.52 |
| Meand ± SEM | 4.46 ± 1.46 | 0.283 ± 0.091 | 2.89 ± 0.85e | ||
Mean channel number of autofluorescence (FL2 log).
Mean channel number of anti-CD4–PE signal.
Mean channel number of anti-CD4–PE signal, with MAb added prior to overnight culturing.
Corrected for autofluorescence (except for CD4-tagged culture).
The difference between the anti-CD4–PE signals for untagged and tagged cultures does not reach statistical difference; P > 0.050.
FIG. 3.
Tagging of monocyte CD4. The level of CD4 expression by untagged and CD4-tagged PBMC following overnight culture was assessed; monocytes and lymphocytes were electronically gated based on their respective forward- versus side-scatter characteristics. The results of a representative experiment (n = 3) are shown. (a) Baseline CD4 expression by monocytes in whole blood; (b) CD4 expression of untagged monocytes following overnight culture with subsequent staining for CD4; (c) CD4-tagged monocytes following overnight culture (no additional anti-CD4–PE MAb was added to the cells after overnight culturing); (d) baseline CD4 expression by CD4+ T cells in whole blood; (e) CD4 expression by untagged lymphocytes following overnight culture with subsequent staining for CD4; (f) CD4-tagged lymphocytes following overnight culture. The asterisks indicate contaminating CD4+ T cells in the monocyte gate, as confirmed by the CD3+ status of this population.
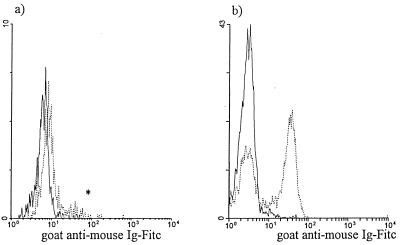
Following overnight culture, the location of the CD4—anti-CD4–PE MAb complex in the monocyte and lymphocyte subpopulations was determined using goat anti-mouse Ig–FITC. The untagged cultures were used to control for nonspecific binding by the goat antibody. Negligible binding by the goat antibody was observed for the tagged and untagged monocytes (Fig. 4a and Table 11), as well as for the untagged lymphocytes (Fig. 4b), as determined by comparison to the FITC autofluorescence signal for each of these conditions (autofluorescence data not shown). However, the goat antibody did bind the tagged lymphocytes (Fig. 4b).
FIG. 4.
Localization of the CD4—anti-CD4–PE MAb complex with goat anti-mouse Ig–FITC. The experiment was performed as outlined in Fig. 3. Goat anti-mouse Ig–FITC was used to stain cultures harvested following overnight culture to assess the presence of the CD4—anti-CD4–PE MAb complex on the cell surface; monocytes and lymphocytes were electronically gated based on their respective forward- versus side-scatter characteristics. The goat anti-mouse Ig–FITC signal was assessed for untagged (——) and CD4-tagged (······) monocytes (a) and lymphocytes (b) (n = 3). The asterisk indicates a goat anti-mouse Ig–FITC signal most likely due to the presence of contaminating CD4+ T cells in the monocyte gate.
TABLE 11.
Monocyte CD4 tagging experiments: use of goat anti-mouse Ig–FITC to localize CD4—anti-CD4–PE MAb complex of tagged monocytes
| Isolation no. | Monocyte CD4 expression (mean channel no.) in overnight PBMC cultures
|
|||
|---|---|---|---|---|
| Untagged cultures
|
CD4-tagged cultures
|
|||
| Autoa | GaM-FITCb | Auto | GaM-FITC | |
| 19 | 0.357 | 0.367 | 0.372 | 0.367 |
| 24 | 0.510 | 0.664 | 0.654 | 0.898 |
| 25 | 0.505 | 0.561 | 0.763 | 0.717 |
| Meanc ± SEM | 0.073 ± 0.042d | 0.064 ± 0.091de | ||
Mean channel number of autofluorescence (FL1 log).
Mean channel number of goat anti-mouse Ig (GaM)–FITC signal.
Corrected for autofluorescence.
No statistically significant binding of goat anti-mouse Ab to monocytes; P > 0.050.
No statistically significant difference in levels of goat anti-mouse Ab between untagged and tagged cultures; P > 0.050.
To control for the possibility that the increased monocyte PE signal observed in the tagged PBMC cultures was actually due to internalization of the anti-CD4–PE MAb, as opposed to some effect of the antibody on the autofluorescence of the cells following overnight culture, experiments were performed (n = 2) in which PBMC were tagged prior to culture with either anti-CD4–PE MAb or unconjugated/cold anti-CD4 MAb; a control consisting of untagged PBMC was also included. Following overnight culture, the monocytes from each PBMC culture were compared with respect to their FL2 log signals. Whereas the monocyte FL2 log signals for the untagged PBMC and PBMC tagged with unconjugated anti-CD4 MAb were comparable, the monocytes in the PBMC cultures tagged with anti-CD4–PE MAb showed an increase in FL2 log signal (data not shown). These results confirm our previous conclusion that the increased FL2 log signal observed in the earlier tagging experiments was in fact due to the internalization of the anti-CD4–PE MAb.
DISCUSSION
Attempts in our laboratory to infect overnight-cultured monocytes with HIV were unsuccessful. We hypothesized that the absence of CD4 might explain the refractoriness of these cultured cells to infection. The down regulation of CD4 by cultured monocytes has been reported by others and has been proposed to be the result of monocyte differentiation into macrophages (19, 35). Other investigators (7) have failed to observe down regulation of monocyte CD4 upon culture. Our study, which includes 35 isolations, represents a comprehensive analysis of monocyte CD4 expression by healthy donors under most experimental conditions employed in the laboratory.
Monocyte CD4 levels in whole blood were assessed within hours of their isolation from the donor, in post-Ficoll-Paque PBMC fractions, and following overnight and 6 days of culture. Down regulation was immediately observed following the isolation of PBMC by use of Ficoll-Paque in 5 of 18 experiments. Following overnight culture under various conditions (including adherent and nonadherent environments and in the presence or absence of lymphocytes), monocyte CD4 was consistently down regulated, either completely or to negligible levels. Analysis of monocytes cultured for 6 days showed variable CD4 expression levels, ranging from undetectable to high. We speculate that these variations may be at least partially attributed to donor variability in response to numerous factors, including variable sensitivity to trace levels of LPS in the culture environment (26), variable sensitivity to mediators or factors involved in the collection of blood or the manipulation of the cells (26), or possible differences in the cytokine responses of cells from the various donors to their in vitro environment.
Various monocyte/macrophage growth factors, activators, or suppressors were tested for their ability to maintain monocyte CD4 following overnight culture or to enhance reexpression following 6 days in culture. HIV infection and replication in monocyte cultures supplemented with GM-CSF (29) or M-CSF (2) have been reported; the addition of M-CSF to macrophage cultures resulted in increased CD4 expression, although the quantities used were in excess of those used in our experiments (2). The addition of either GM-CSF or M-CSF to our cultures failed to inhibit monocyte CD4 expression, nor did it enhance reexpression in day 6 cultures.
LPS, a potent activator of monocytes/macrophages (26), has been shown to down regulate monocyte CD4 expression (18, 26). In our experiments, LPS-treated monocyte cultures showed CD4 levels comparable to those in untreated overnight and day 6 cultures.
HLA-DR expression in the GM-CSF-, M-CSF-, or LPS-supplemented cultures was also assessed and was found to be variable compared to that for monocytes cultured in control media (data not shown).
IL-10, an immunosuppressive cytokine able to suppress T-cell and macrophage cytokine production (36), is expressed by T cells, B cells, and macrophages (24). In HIV infection, elevated IL-10 levels (1, 36) play an important role in the pathogenesis of HIV and AIDS by causing more efficient infection of macrophages (32) and increased viral replication in T cells and macrophages (37) and by impairing antigen presentation by macrophages (25). Overnight culture of monocytes in the presence of IL-10 had no effect on monocyte CD4 but did result in decreased HLA-DR expression. Similar observations with respect to HLA-DR have been made by other investigators (13). These results could be explained by the observation made by Chang et al. (5) that IL-10 inhibited the maturation of monocytes in their cultures.
In three of four experiments in which the role of Ficoll-Paque was assessed, monocytes in whole-blood cultures did show decreased CD4 expression; however, the CD4 levels were higher than those for monocytes cultured as a PBMC fraction. In the remaining isolation, monocytes in the whole-blood culture did not show a loss of CD4. Our results suggest that whole blood may contain some factor which stabilizes monocyte CD4 expression and/or inhibits monocyte differentiation to macrophages.
Differential regulation of CD4 on monocytes and T cells was observed in overnight PBMC cultures; while monocyte CD4 was routinely down regulated, T-cell CD4 levels were fairly consistent, most likely due to the association of T-cell CD4 with p56lck, which keeps CD4 anchored on the cell surface (27). Because of the lack of p56lck expression by monocytes, monocyte CD4 is not anchored to the cell membrane, thus explaining the ability of monocytic cell lines and monocytes to constitutively endocytose and recycle their CD4 molecules (27, 28).
We have shown, albeit indirectly, that internalization is the process by which monocyte CD4 was down regulated in our experiments. Monocytes and lymphocytes from cultures tagged with anti-CD4–PE MAb prior to culture continued to display a PE signal following overnight culture and harvesting. The addition of goat anti-mouse Ig–FITC to these harvested cells revealed binding to the tagged lymphocytes but negligible binding to the tagged monocytes, indicating that the CD4—anti-CD4–PE MAb complex remained external to the tagged lymphocytes but was almost completely internalized in the case of the tagged monocytes. Attempts to stain PBMC from the untagged overnight culture revealed that the majority of monocyte CD4 had been down regulated. We conclude that the internalization of monocyte CD4 observed in these experiments reflects the down regulation we routinely observe following overnight culturing and is not an artifact of our tagging procedure. Other processes, such as protein shedding from the cell surface, may also be involved in CD4 down regulation, although we have not investigated such mechanisms.
At this time, we conclude that the observed down regulation of monocyte CD4 is probably due to the differentiation of blood monocytes into tissue culture-derived macrophages (35) rather than to some artifact of the isolation procedure. Despite its regular use in the immunophenotyping of monocytes, the physiological role of CD4 in this cell population has been poorly studied and is poorly understood (23). The results of our study suggest that monocyte CD4 may play a role in the monocyte/macrophage differentiation process. It is possible that as monocytes migrate from the circulation to certain tissues, the cells may down regulate CD4 expression by a process similar to that responsible for the down regulation of monocyte CD4 observed in our cultures. Thus, our in vitro observations may reflect the naturally occurring in vivo processes responsible for the lower CD4 expression by tissue macrophages (39). Cytokines (such as IL-10), adhesion factors, and/or other cell types in the microenvironment may play important roles in the regulation of monocyte CD4 expression in vivo.
ACKNOWLEDGMENTS
We thank Jean-Marc Renaud for assistance with the statistical analysis and William Ross and Ashok Kumar for assistance with the preparation of the manuscript. We also thank Michelle Jaynes for technical assistance.
This work was supported by an Ontario Graduate Scholarship (OGS) from the Ontario Ministry of Education and Training, by a National Health Ph.D. Fellowship (AIDS) from Health Canada granted to G.M.G.-B., and by an AIDS grant from Health Canada and the Ontario Ministry of Health granted to L.G.F.
REFERENCES
- 1.Barcellini W, Rizzardi G P, Marriott J B, Fain C, Shattock R J, Meroni P L, Poli G, Dalgleish A G. Interleukin-10-induced HIV-1 expression is mediated by induction of both membrane-bound tumour necrosis factor (TNF)-alpha and TNF-receptor type 1 in a promonocytic cell line. AIDS. 1996;10:835–842. doi: 10.1097/00002030-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bergamini A, Perno C F, Dini L, Capozzi M, Pesce C D, Ventura L, Cappannoli L, Falasca L, Milanese G, Calio R, Rocchi G. Macrophage colony-stimulating factor enhances the susceptibility of macrophages to infection by human immunodeficiency virus and reduces the activity of compounds that inhibit virus binding. Blood. 1994;84:3405–3412. [PubMed] [Google Scholar]
- 3.Center D M, Cruikshank W W. Modulation of lymphocyte migration by human lymphokines. I. Identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J Immunol. 1982;128:2562–2568. [PubMed] [Google Scholar]
- 4.Center D M, Berman J S, Kornfeld H, Theodore A C, Cruikshank W W. The lymphocyte chemoattractant factor. J Lab Clin Med. 1995;125:167–172. [PubMed] [Google Scholar]
- 5.Chang J, Naif H M, Li S, Jozwiak R, Ho-Shon M, Cunningham A L. The inhibition of HIV replication in monocytes by interleukin 10 is linked to cell differentiation. AIDS Res Hum Retroviruses. 1996;12:1227–1235. doi: 10.1089/aid.1996.12.1227. [DOI] [PubMed] [Google Scholar]
- 6.Clark S J, Jefferies W A, Barclay A N, Gagnon J, Williams A F. Peptide and nucleotide sequences of rat CD4 (W3/25) antigen: evidence for derivation from a structure with four immunoglobulin-related domains. Proc Natl Acad Sci USA. 1987;84:1649–1653. doi: 10.1073/pnas.84.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collman R, Godfrey B, Cutilli J, Rhodes A, Hassan N F, Sweet R, Douglas S D, Friedman H, Nathanson N, Gonzalez-Scarano F. Macrophage-tropic strains of human immunodeficiency virus type 1 utilize the CD4 receptor. J Virol. 1990;64:4468–4476. doi: 10.1128/jvi.64.9.4468-4476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowe S M. Role of macrophages in the pathogenesis of human immunodeficiency virus (HIV) infection. Aust NZ J Med. 1995;25:777–783. doi: 10.1111/j.1445-5994.1995.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 9.Cruikshank W W, Center D M. Modulation of lymphocyte migration by human lymphokines. II. Purification of a lymphotactic factor (LCF) J Immunol. 1982;128:2569–2574. [PubMed] [Google Scholar]
- 10.Cruikshank W W, Berman J S, Theodore A C, Bernardo J, Center D M. Lymphokine activation of T4+ T lymphocytes and monocytes. J Immunol. 1987;138:3817–3823. [PubMed] [Google Scholar]
- 11.Cruikshank W W, Center D M, Nisar N, Wu M, Natke B, Theodore A C, Kornfeld H. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci USA. 1994;91:5109–5113. doi: 10.1073/pnas.91.11.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 13.de Waal Malefyt R, Haanen J, Spits H, Roncarolo M G, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries J E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 15.Filion L G, Izaguirre C A, Garber G E, Huebsh L, Aye M T. Detection of surface and cytoplasmic CD4 on blood monocytes from normal and HIV-1 infected individuals. J Immunol Methods. 1990;135:59–69. doi: 10.1016/0022-1759(90)90256-u. [DOI] [PubMed] [Google Scholar]
- 16.Filion L G. Master lectures in opportunistic infections, monograph 2. Milan, Italy: Intramed; 1993. HIV immunocytopathology in the development of opportunistic infections: cellular dysfunction in macrophages; pp. 25–47. [Google Scholar]
- 17.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 18.Herbein G, Doyle A G, Montaner L J, Gordon S. Lipopolysaccharide (LPS) down-regulates CD4 expression in primary human macrophages through induction of endogenous tumour necrosis factor (TNF) and IL-1 beta. Clin Exp Immunol. 1995;102:430–437. doi: 10.1111/j.1365-2249.1995.tb03801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazazi F, Mathijs J-M, Foley P, Cunningham A. Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus. J Gen Virol. 1989;70:2661–2672. doi: 10.1099/0022-1317-70-10-2661. [DOI] [PubMed] [Google Scholar]
- 20.LaCroix F, Zhao D Y, Izaguirre C A, Filion L G. Suppression by HIV of IL-1 and IL-6 secretion in accessory cells: AC function defect partially corrected with exogenous IL-1 and IL-6. Clin Immunol Immunopathol. 1993;67:109–116. doi: 10.1006/clin.1993.1052. [DOI] [PubMed] [Google Scholar]
- 21.Levy J A, Shimabukuro J, McHugh T, Casavant C, Stites D, Oshiro L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology. 1985;147:441–448. doi: 10.1016/0042-6822(85)90146-1. [DOI] [PubMed] [Google Scholar]
- 22.Maddon P J, Littman D R, Godfrey M, Maddon D E, Chess L, Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985;42:93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- 23.Marsh M, Pelchen-Matthews A. Endocytic and exocytic regulation of CD4 expression and function. Curr Top Microbiol Immunol. 1996;205:107–135. doi: 10.1007/978-3-642-79798-9_6. [DOI] [PubMed] [Google Scholar]
- 24.Montaner L J, Griffin P, Gordon S. Interleukin-10 inhibits initial reverse transcription of human immunodeficiency virus type I and mediates a virostatic latent state in primary blood-derived human macrophages in vitro. J Gen Virol. 1994;75:3393–3400. doi: 10.1099/0022-1317-75-12-3393. [DOI] [PubMed] [Google Scholar]
- 25.Muller F, Aukrust P, Lien E, Haug C J, Froland S S. Enhanced interleukin-10 production to Mycobacterium avium products in mononuclear cells from patients with human immunodeficiency virus infection. J Infect Dis. 1998;177:586–594. doi: 10.1086/514222. [DOI] [PubMed] [Google Scholar]
- 26.Neate E V, Greenhalgh A M, McPhee D A, Crowe S M. Bacterial lipopolysaccharide mediates the loss of CD4 from the surface of purified peripheral blood monocytes. Clin Exp Immunol. 1992;90:539–544. doi: 10.1111/j.1365-2249.1992.tb05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelchen-Matthews A, Boulet I, Littman D R, Fagard R, Marsh M. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J Cell Biol. 1992;117:279–290. doi: 10.1083/jcb.117.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelchen-Matthews A, Da Silva R P, Bijlmakers M-J, Signoret N, Gordon S, Marsh M. Lack of p56lck expression correlates with CD4 endocytosis in primary lymphoid and myeloid cells. Eur J Immunol. 1998;28:3639–3647. doi: 10.1002/(SICI)1521-4141(199811)28:11<3639::AID-IMMU3639>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Perno C F, Cooney D A, Currens M J, Rocchi G, Johns D G, Broder S, Yarchoan R. Ability of anti-HIV agents to inhibit HIV replication in monocyte/macrophages or U937 monocytoid cells under conditions of enhancement by GM-CSF or anti-HIV antibody. AIDS Res Hum Retroviruses. 1990;6:1051–1055. doi: 10.1089/aid.1990.6.1051. [DOI] [PubMed] [Google Scholar]
- 30.Rand T H, Cruikshank W W, Center D M, Weller P F. CD4-mediated stimulation of human eosinophils: lymphocyte chemoattractant factor and other CD4-binding ligands elicit eosinophil migration. J Exp Med. 1991;173:1521–1528. doi: 10.1084/jem.173.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinherz E L, Kung P C, Goldstein G, Schlossman S F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci USA. 1979;76:4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, Polentarutti N, Sica A, Locati M, Mackay C, Wells T N, Biswas P, Vicenzi E, Poli G, Mantovani A. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439–444. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart C C, Stewart S J. Cell preparation for the identification of leukocytes. Methods Cell Biol. 1994;41:52. doi: 10.1016/s0091-679x(08)61708-2. [DOI] [PubMed] [Google Scholar]
- 34.Trotter J. Purdue cytometry. Vol. 2. West Lafayette, Ind: Purdue University; 1996. . [CD-ROM.] [Google Scholar]
- 35.Valentin A, Von Gegerfelt A, Matsuda S, Nilsson K, Asjo B. In vitro maturation of mononuclear phagocytes and susceptibility to HIV-1 infection. J Acquired Immune Defic Syndr. 1991;4:751–759. [PubMed] [Google Scholar]
- 36.Weissman D, Poli G, Fauci A S. Interleukin-10 blocks HIV replication in macrophages by inhibiting the autocrine loop of tumor necrosis factor alpha and interleukin 6 induction of virus. AIDS Res Hum Retroviruses. 1994;10:199–206. doi: 10.1089/aid.1994.10.1199. [DOI] [PubMed] [Google Scholar]
- 37.Weissman D, Poli G, Fauci A S. IL-10 synergizes with multiple cytokines in enhancing HIV production in cells of monocytic lineage. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;15:442–449. [PubMed] [Google Scholar]
- 38.Wood G S, Warner N L, Warnke R N. Anti-Leu3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983;131:212–216. [PubMed] [Google Scholar]
- 39.Wood G S, Turner R R, Shiurba R A, Eng L, Warnke R A. Human dendritic cells and macrophages. In situ immunophenotypic definition of subsets that exhibit specific morphologic and microenvironmental characteristics. Am J Pathol. 1985;119:73–82. [PMC free article] [PubMed] [Google Scholar]