Abstract
Although awareness that air pollution can damage vegetation dates back at least to the 1600s, the processes and mechanisms of damage were not rigorously studied until the late 20th century. In the UK following the Industrial Revolution, urban air quality became very poor, with highly phytotoxic SO2 and NO2 concentrations, and remained that way until the mid-twentieth century. Since then both air quality, and our understanding of pollutants and their impacts, have greatly improved.
Air pollutants remain a threat to natural and managed ecosystems. Air pollution imparts four major threats to vegetation are discussed through a series of case studies. Gas phase effects by the primary emissions of SO2 and NO2 are discussed in the context of impacts on lichens in urban areas. Effects of wet and dry deposited acidity from sulphur and nitrogen compounds are considered with a particular focus on forest decline. Ecosystem eutrophication by nitrogen deposition focuses on heathland decline in the Netherlands, and ground level ozone at phytotoxic concentrations is discussed by considering impacts on semi-natural vegetation. We find that, although air is getting cleaner, there is much room for additional improvement, especially for the effects of eutrophication on managed and natural ecosystems.
Keywords: Acid deposition, ammonia, nitrogen, ozone, sulphur
1. Introduction
There is a very long history of understanding that air pollution is a threat to vegetation. The medieval writer and mystic Hildegard von Bingen (1098 – 1179) noted in her book Causae et Curae [1]that dust within rain was believed to damage crops. In the 1600s developments in early scientific understanding did not miss the importance of pollutants from combustion processes and industry in damaging plants, including the first evidence of air pollution as a transnational concern. In the first decade of the 1600s, King James passed an act “against burning of Ling, and Heath, and other Moorburning…” noting that “some parts even of France itself lying South west of England, did formerly make of being infested with Smoakes driven from our Maritime Coasts, which injur’d their Vines in Flower” [2].
However, prior to the 19th century and the Industrial Revolution, there were no air quality measurement networks, and evidence that air pollution was damaging vegetation was limited to areas in the immediate vicinity of point sources (e.g. lime kilns, charcoal production, early smelting activities). In Sylva [3], John Evelyn recognised the threat to vegetation through being “infected with foggs and poys’nous vapours, or expos’d to sulphurous exhalations”, while Fabri (1670) [4] saw volcanic emanations as a source of acidic damage to fruit. As industrialisation increased, so did reports of environmental damage: in the summer of 1794 a visitor to south Wales observed, “Nearer to Swansea, there are extensive works both of copper and iron, from the malignant influence of which every trace of vegetation in the neighbourhood has from a very short period after their erection, been totally annihilated” [5]. At this time the main phytotoxic pollutant was thought to be SO2 and to a lesser extent HCl and NO2.
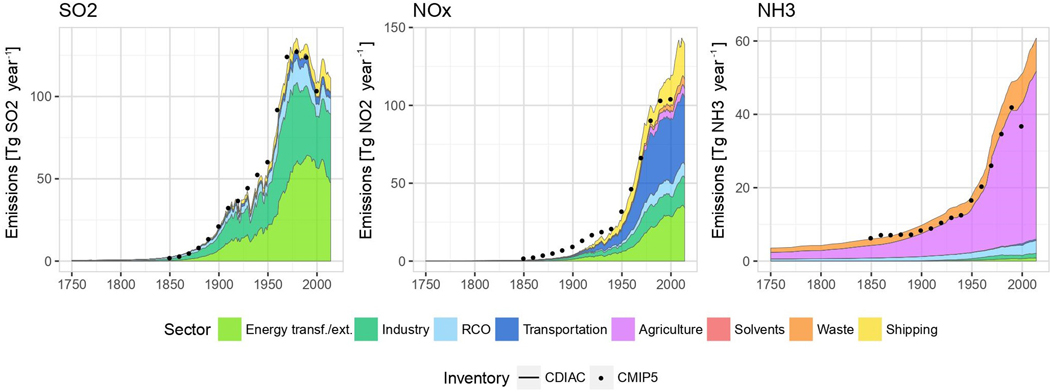
An analysis of global sources of the major gaseous pollutants by Hoesly et al. [6] showed the dominance of sources of SO2 and NOx in Europe and North America from 1880 until 1980 (Figure 1). Thereafter, following the introduction of control measures in Europe and North America and the rapid growth of sources in Asia the main sources of SO2 and NOx have been in East and South Asia (Figure 1). Early records of pollution impacts included observations from both the natural and agricultural environments. From around 1800 the dark or melanic form of some species of moths in England began to increase in frequency. On soot-covered tree trunks, darker moths were better camouflaged and thus more avoided predation than light ones [7]. Sheep fleeces became blackened by smoke [8] and in the industrialising United States bird feathers became covered in black carbon particles [9]. ‘Black rain’ was observed in remote areas of Scotland [10] and soot from Manchester’s factories caused black snow in Scandinavia as described by the playwright Ibsen in his play Brand in 1867. Interestingly, the latter observation shows clear evidence of long-range transport of pollutants in Europe long before the arguments of the 1970s on whether industrial emissions from the UK, Germany and France contributed to acid deposition in Scandinavia.
Figure 1.
Global emissions of SO2, NOx, NH3 and NMVOC between 1750 and 2010. Adapted from Hoesly et al. [6].
The rapid increase in both industrial and domestic SO2 and NO2 emissions in the early 20th century in several European countries, notably the UK, Germany and France, and in North America (Figure 1), generated regional surface concentrations sufficient to damage both crop- and wild plant species as demonstrated by the following examples. An extensive series of field investigations by Cohen and Ruston published in 1925 [11] substantiated the observations of various earlier workers on the effects of coal-smoke polluted atmosphere on city vegetation. Using a transect from the suburbs towards the centre of Leeds, Cohen and Ruston [11] found depressions in yield, various degrees of visible damage and alterations in growth habit of a number of species (e.g. Laurus nobilis), which increased in intensity as pollution levels increased towards the city centre. The ill effects on vegetation were positively correlated with the annual wet deposition of particulate matter and sulphur compounds, but the relative toxicity of the different components of the coal smoke was not determined. In 1928 Pettigrew [12] described extensive damage to plants and the total failure of ornamental trees and shrubs in Manchester parks as a result of atmospheric pollution, while in 1941 Metcalfe [13] described the pollution-induced premature shedding of leaves, flowers and buds on ornamental species (e.g. Begonia foliosa) in towns during periods of foggy weather.
Much of the earlier interest in the effects of air pollution on vegetation in the UK focused on such damage to ornamental species in urban areas, where losses were considered to be more serious from an aesthetic rather than economic viewpoint [e.g. 11, 12, 13]. However in 1952 Bleasdale [14] devised an experiment to show that a coal-smoke polluted atmosphere can considerably depress the yield of the economically important strain of the pasture grass Lolium perenne. When attempts were made to improve the quality of hill pastures in heavily polluted districts of East Lancashire by reseeding with this strain of Lolium perenne, the plants became established successfully, but soon declined in productivity and eventually disappeared. Bleasdale grew the plants in Manchester in two greenhouses: one ventilated with polluted ambient air and the other with air purified by passage through a water scrubber. Significant depressions in yield occurred in the plants in the polluted greenhouse compared with those grown in purified air. Importantly, there were no observable lesions on the leaves, something that had previously been considered an indicator of damage [15].
Metal pollution was a further concern, with hotspots of metal deposition recorded close to smelters in Canada, the United States and at several locations in Europe [16–18]. Effects of metal pollution on vegetation were relatively local, within 30 km of the source, but the scale of emissions and persistent nature of some of the metal species left long-term soil contamination in these areas and damage to some flora and fauna. Research was largely concerned with the possibility of metals entering the human body via ingestion of contaminated crops. Of wider concern was the use of tetraethyl lead in petrol, which had been introduced as an anti-knocking agent in many countries in the 1920s. By the middle of the twentieth century, it became apparent that lead was deposited alongside roads, with levels falling exponentially with distance from the road, but detectable up to 100 m away [19]. The identification of such widespread environmental contamination by lead resulted in massive pressure to reduce the use of tetraethyl lead, leading to a ban throughout much of the world.
Ground level ozone became an important part of the global pollution climate in the mid-20th century. Trends in surface ozone from measurements over the last 6 decades [20] show large, long-term trends in Europe. In presenting trends at remote sites, the effects of local sources are minimised and the regional trend becomes clearer. In the analysis by Cooper et al. [20], annual average surface ozone concentrations increased from 20 to 40 ppb between 1950 and 2000 and changed little since then in Europe. Remote sites in North America and East Asia show increases of typically 10 ppb between 1980 and 2010, whereas the monitoring stations in the remote Pacific, Antarctica, and Tasmania show quite small changes (<10ppb) between 1980 and 2010 [20]. Ozone is produced within the troposphere through photochemical degradation of volatile organic compounds in the presence of NO2. Ozone became recognised as an important regional phytotoxic compound in the 1960s, initially in California [21], but extending to much of the United States and Southern Canada and detected in Europe during the 1970s [22].
Air pollution issues of the mid-20th century gradually extended to ever-larger areas as global emissions of sulphur approached peaks (Figure 1). In part, these changes were due to the number and scale of sources, but also to the increasing heights of emission, with tall stacks on industrial sources of 200 m or more and large buoyant plumes of pollutant gases effectively injected towards the top of the boundary layer, promoting wide dispersion and long-range transport. These tall stacks, which were deployed to reduce ground-level pollution, essentially turned a local pollution problem into a regional one. Ecological effects of long-range transport of sulphur and nitrogen compounds began to be recognised in countries remote from the sources of the primary pollutants, initially as effects of acid deposition in Scandinavia [23] and in Scottish lochs [24]. By the end of the 20th century, various national air quality laws and programmes had been implemented in many developed countries around the world. These had the effect of reducing emissions and deposition of many air pollutants, though emissions and deposition still remained higher than would be expected in the absence of anthropogenic emissions across much of the world.
Considering this background, we discuss four main threats for the global pollution climate in the late 20th century:
Gas phase effects of the primary emissions of SO2 and NO2 reflecting their emissions in urban areas and industrial regions including substantial tracts of arable cropland in Europe, North America, East and South Asia,
Effects of wet and dry deposited acidity from sulphur and nitrogen compounds, notably in upland areas where inputs are dominated by wet deposition. We give particular attention to the poorly buffered geological regions throughout Northern Europe, Scandinavia, the north-eastern states of the USA and parts of Eastern Canada.
Ecosystem eutrophication by nitrogen deposition in both oxidised and reduced forms over much of northern Europe and large parts of eastern North America,
Ground level ozone present at phytotoxic concentrations over much of the arable cropland of Europe and North America, East and South Asia.
In addition to the four main threats to vegetation more localised effects of metals, especially heavy metals, close to smelters and other metal processing industries, are notable through the 20th century, but severe cases of damage to vegetation were not regional in scale. These four main pollutant threats will be examined via a series of case studies of major pollution impacts.
2. Gas phase effects of SO2, NO2 and NH3: case study lichen decline
In Europe at the beginning of the twentieth century, industrialisation and large populations of cities produced high concentrations of SO2 and NO2 and thus urban air quality was typically very poor. The peaks in emissions and exposure of vegetation were between 1960 and 1970 for SO2 in Europe and North America thus during the middle decades of the 20th century extensive areas of cropland and semi-natural areas of Europe and North America were exposed to damaging concentrations of SO2 [25]. As a consequence of air pollution legislation, concentrations of SO2 declined steadily in many developed countries from about 1970. By 2015, there were no significant areas of Europe or North America experiencing concentrations of SO2 at levels damaging to plants from anthropogenic sources [26]. In East Asia peak emissions were later, but since 2012 emissions of SO2 in China have declined by approximately 40%. Emissions of NO2 peaked around 1990 in Europe, rather later than SO2 due to the rapid growth in vehicle usage, as well as industry and the slower introduction of effective controls on vehicle NO2 emissions. Thus, concentrations of NO2 remained high in urban and many rural areas through to the early years of the 21st century in Europe and North America [26].
Ammonia emissions, mainly from agricultural activities, steadily increased in all industrial countries through the 20th century [27]. Few countries have made significant reductions in emissions, the Netherlands and Denmark being notable exceptions, each of which have reduced emissions over the last two decades by approximately 50% [28, 29]. The overall global emissions of NH3 continue to increase and as emissions of NH3 are closely coupled to ambient temperature, it is likely that changes in climate will further increase emissions [27].
These broad changes in the chemical climate of industrial countries have had widespread effects on crops and natural vegetation, only some of which have been documented in detail. Here, the effects on lichens, which are particularly sensitive to atmospheric composition are used as an example of the changing chemical climate of industrial countries.
Lichens are rarely a dominant feature of terrestrial habitats yet they are a ubiquitous component of most habitats globally, from arctic to desert to tropical forest, and in both managed and natural ecosystems. Although dependent on the substratum for their physical attachment, lichens have no vascular root system: they receive nutrients and water directly from the atmosphere, making them highly susceptible to changing atmospheric conditions.
In 1866 Nylander [30] observed that ‘lichen deserts’ were created around Paris, often extending for considerable distances aligned to the direction of the prevailing wind. Similar patterns were observed around that time across Europe, and in the mid-twentieth century around New York City [31] as well as other parts of the U.S. [32–34]. A cause of these declines was hypothesised as concentrations of acidifying pollutants in urban air. However, there were no systematically-measured air quality data to support the observations. In the UK it took the dramatic loss of life associated with the London smog in the winter of 1952 (estimated 12,000 deaths [35]) to establish a national air quality recording system across both urban and rural areas; by the 1960s this comprised c. 1300 stations [36]. In the late 1960s, Hawksworth and Rose [37] correlated the distribution of lichen species across the UK with recorded levels of SO2 from this network to create a ten-point scale of estimated SO2 pollution based on species’ sensitivity to air pollution. Since lichens absorb pollutants directly from the air, shrubby or hanging filamentous lichens with a large surface area such as Usnea species were at the top of the list of sensitive species, while crustose species associated with naturally acidic habitats such as Lecanora conizaeoides and leprose species of Lepraria, at the bottom of the list, were tolerant and becoming widespread in urban areas [37].
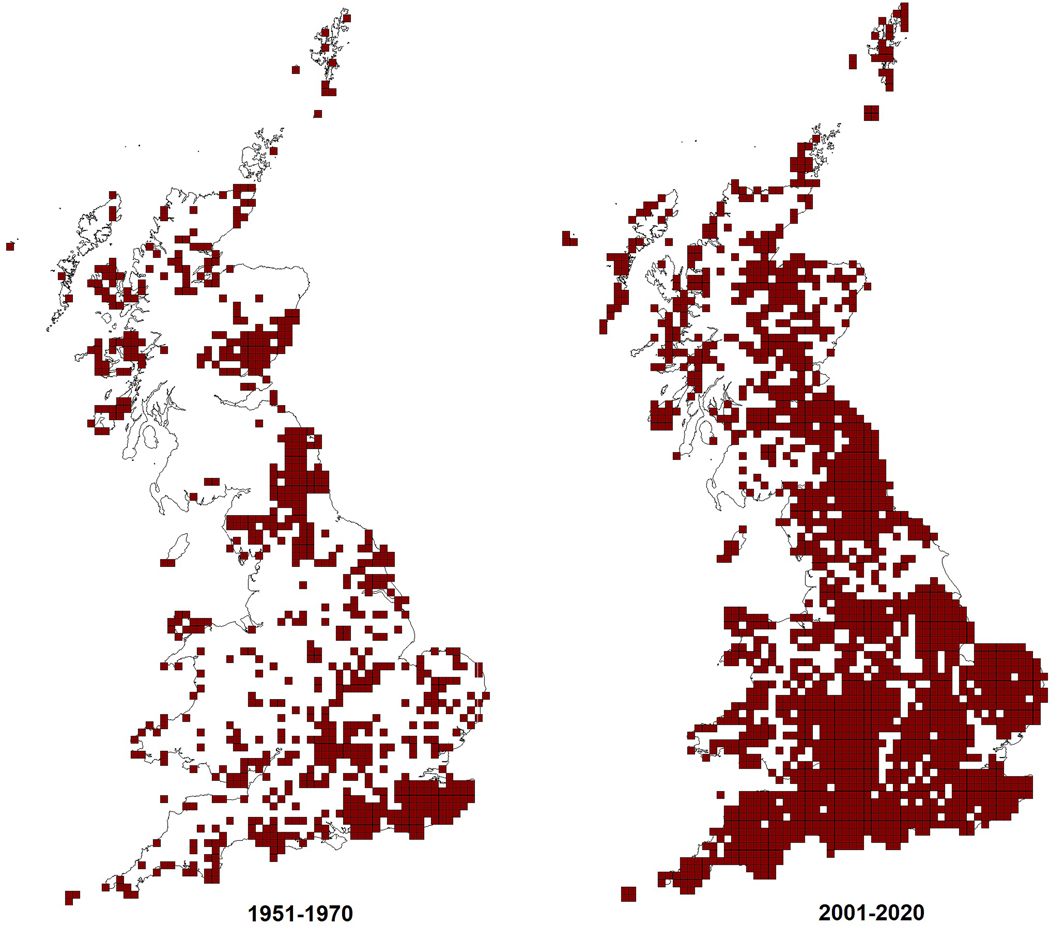
As SO2 pollution declined, reactive nitrogen became the dominant atmospheric pollutant (Figure 1), both as NOx from vehicular and industrial emissions and as NH3 from agricultural processes. The effect on lichen communities was dramatic, as species tolerant of nitrogen (nitrophytes) began to appear, in urban and agricultural areas. Gaseous ammonia deposited onto wet surfaces acts as a base, removing hydrogen ions from water and producing ammonium and hydroxide ions. Thus, in areas receiving high ammonia deposition, formerly common species tolerant of acid (acidophytes) became rare [38]. In areas affected by ammonia deposition, including naturally acidic moorland and heathland vegetation dominated by terricolous species of lichen, Cladonia died back [39, 40]. The effect of ammonia is to increase bark pH, and research in the UK showed a strong correlation between lichen communities and bark pH and ammonia concentrations [41–43]. Quantitative studies of epiphytes on a range of tree species in sites where ammonia was monitored showed that lichen communities varied with tree species as well as air quality, so that acidophytes survived longer on acid-barked trees such as oak and birch, while nitrogen-tolerant species of the Xanthorion alliance appeared earlier on trees with a higher bark pH such as ash or poplar (Figure 2) [41, 43–47]. This shift in lichen communities from acidophyte species to nitrophyte species was demonstrated by a resurvey of twig communities on a nature reserve in Wales after ten years which showed a loss of nitrogen-sensitive species on branches and the appearance of the Xanthorion [48]. A nationwide survey of sensitive and tolerant species of epiphytic macrolichens in the UK was used to test their response to measured ammonia at air pollution monitoring sites across the UK using lichen frequency and bark pH as measured variables. The results showed that a loss of nitrogen-sensitive species was taking place prior to the appearance of nitrophytes, and that this was happening at c. 1µg m3 NH3, well below the current critical level (concentration above which direct adverse effects are thought to occur) of 8µg m3 [49]. Since then 1µg m−3 has been accepted as the critical level for lichens [50].
Figure 2.
Distribution of the nitrogen tolerant lichen Xanthoria parietina showing records in 10 km squares between 1951–1970 and between 2001–2020 (British Lichen Society).
3. Deposited acidity in upland areas: case study forest decline
Prior to the late 1970s, concerns about acid deposition focused primarily on aquatic ecosystems. However, in the late 1970s and early 1980s, foresters and scientists began to notice widespread diebacks of forest trees in both the north-eastern US and Europe, and suspicion mounted that acid deposition was the cause. The European decline was popularly known as “Waldsterben” (forest death): it affected both hardwood and softwood trees and was characterized by extreme thinning of the crown, premature senescence, discolouration and loss of foliage, active casting of green foliage, and loss of fine root biomass [51]. The North American decline was most severe in red spruce (Picea rubens) in mountainous locations of New York and New England [52]. Red spruce decline symptoms were the same on both continents and included reddening of needles in the early spring, a condition usually associated with winter freezing damage.
Most researchers on both continents pointed the finger at air pollution as the driving force behind the forest declines. Large concentrations of acidity in orographic cloud and a windy upland climate for many forests leads to high deposition rates of the pollutants contained within the cloud water, which were shown to reduce the frost hardiness of red spruce [53]. Furthermore, Cape et al. [50] showed that it was the acidity and SO42− ions rather than NO3− that were responsible for the observed reduction in frost hardiness. In Europe two major sets of hypotheses around the mechanisms gained prominence [51, 54, 55], The ‘top-down’ hypotheses focused on the direct impacts to leaves and needles of high levels of pollutants, particularly oxides of sulphur and nitrogen, and ozone [e.g. 51, 56]. Other pollutants such as metals and organic compounds were also implicated. The ‘bottom-up’ hypotheses proposed that the primary causal element of forest decline lies in the soil, through acidification and depletion of basic cations from soil exchange sites, leading to mobilization of toxic aluminium, as well as the excess enrichment by reactive nitrogen, leading to nutrient imbalances and acidifying nitrification pulses [e.g. 57, 58]. Soluble aluminium causes a range of physiological damage, including direct mortality to fine roots and mycorrhizae, impaired ability to transport calcium and magnesium at the soil-root interface and a loss of the soil fauna that transport oxygen and nutrients to the deeper soil [59]. These two groups of hypotheses were generally not considered mutually exclusive, but which were the primary and which were the contributory factors was hotly debated. Since forest decline damage was ultimately recorded across a large number of hardwood and conifer species over a wide area and range of environmental conditions in Europe, it is highly likely that the primary and secondary drivers of damage also varied. However, to our knowledge there has been no comprehensive analysis of the literature to provide an overview of the relative roles of these different mechanisms across European forests.
The cause of declines in red spruce in the north-eastern US was identified as foliar leaching of calcium, specifically membrane-bound calcium, causing reduced frost hardiness that could lead to winter damage [53, 60, 61]. The problem was particularly acute for red spruce because it occurred primarily in montane forests where it was exposed to cold winter temperatures and high deposition of acid rain and also to extremely acidic cloud droplets which, are very effectively scavenged by conifer needles [62]. Low calcium availability in the soil could exacerbate the problem, as indicated by a study that showed that, compared to a reference catchment, frost damage was much reduced in a catchment in which calcium was added experimentally to the soil [63]. The same study showed a major improvement in the health of sugar maple (Acer saccharum) in the calcium-treated catchment, underscoring that it had also been affected by acid rain in the north-eastern U.S. [64]. Researchers have since reported that many other species likely show similar effects, though possibly not as severe and not as widespread because they are less common across the landscape [65].
In recent decades, the decline in acid deposition in the eastern US has led to a resurgence of red spruce, which is growing well throughout the region and expanding its range into lower-elevation forests [66, 67]. Similarly the International Co-operative Programme on the Assessment and Monitoring of Air Pollution Effects on Forests (ICP Forests) showed no major changes in the proportion of damaged trees in Europe overall from the mid-1990s to the early 2010s, with conifers showing an improvement in condition and broadleaves showing a decline. Much of this was due to the new inclusion of Mediterranean ecosystems, where oak have shown a strong decline in recent years, mainly attributed to drought and insect damage [68]. In 2018 and 2019 a new decline in forest condition was reported in central and northern Europe; it is thought the primary driver is a widespread, persistent drought [69].
As sulphur emissions began to decline in North America and Europe, research focused increasingly on nitrogen deposition. The concept of “nitrogen saturation” was advanced by Ågren and Bosatta [70] and Aber et al. [71] to indicate deposition of nitrogen exceeding the biological capacity of the ecosystem to retain it. According to these studies, this leads to a sequence of changes in ecosystem function including increased plant tissue nitrogen concentrations, increased nitrogen cycling in the soil, increased nitrification, and ultimately elevated nitrogen leaching and soil acidification. Elevated nitrogen leaching was found to occur in many catchments in the eastern US where deposition was above 8 kg N ha−1 y−1 [72] and in many forest plots in Europe where deposition was above about 10 kg N ha−1 y−1, especially if in the soil pH was low and % organic nitrogen was high [73]. The nitrogen saturation theory was further refined by Lovett and Goodale [74] who noted that the impacts of excess nitrogen deposition were not necessarily sequential, but rather all could occur simultaneously and their expression was controlled by the relative strength of the vegetation and soil sinks for nitrogen in the ecosystem. The nitrogen saturation theory marked a major shift in thinking about nitrogen by forest ecologists: previously nitrogen was primarily considered a key limiting nutrient, but the nitrogen saturation theory argued that when chronic deposition levels are high, it can also have adverse effects.
The impact of air pollution on forest health is now considered as a part of, or an acceleration to, a combination of stresses, especially drought [75, 76]. Past management is also clearly a contributing factor: many parts of central Europe had a history of poor silviculture practices including even-aged, monocultural plantations with little genetic variability and litter-raking which removed valuable nutrients [75]. Thus the extreme acid deposition of the late 1970s may have been a ‘final straw’ for a large area of European forest already damaged and predisposed to further stress, triggering a widespread decline.
As pollutant emissions continue to decline, there may be a long-term legacy of damage done to soils by decades of acid deposition. Key studies show that forests where acidification is reduced can undergo major shifts in ecosystem pools and processes, including reductions in forest floor and upper mineral soil carbon and nitrogen pools and mobilization of nitrogen into deeper soil levels or export from the ecosystem [55, 64, 77, 78]. The impact and duration of legacy effects in the ecosystem for both nitrogen and sulphur are determined by the extent to which the “slow pools” in the ecosystem have been affected. These slow pools could include the available base cation pools that are replenished primarily by mineral weathering, or the stable soil organic matter pools that turn over very slowly [79, 80]. As a result, we see some impacts of acid deposition reversing quickly (e.g., lower foliar leaching of nutrients, improved health of trees and declining stream nitrate fluxes and concentrations [81–83]) while other impacts, such as the depleted base saturation of acidified soils, may take decades or centuries to recover [84]. Thus, although the forests have appeared to stabilize and even improve in some areas, reducing acid deposition has also not removed all of the stresses.
4. Ecosystem eutrophication by nitrogen deposition: case study heathland decline and biodiversity losses
As described above, air pollution in Europe during the second half of the 20th century is dominated by the gradual reduction in sulphur emissions and deposition and the slower decline in emissions and deposition of oxidized nitrogen. Notably, the deposition of nitrogen has been largely unchanged as emissions of NH3 continued largely unabated [38]. The European hot spots for nitrogen deposition have been, and largely remain, the low countries of northern mainland Europe and the Netherlands in particular. Parts of the intensive livestock farming areas of the UK France and Denmark also receive high levels of nitrogen deposition and gas phase NH3 [42].
The most dramatic impacts of nitrogen deposition have been large community shifts in nutrient-poor terrestrial habitats such as grassland, heathland and bogs. In the Netherlands during the 1970’s and 80’s, large areas of heathland transitioned to grassland. This typically occurred quite rapidly at a site (one to two years as documented in one study [85]) and by 1983 80 km2 of heathland in the Netherlands, nearly 20% of the total, had become dominated by grasses [86, 87]. The grasses that became dominant were typically Molinea caerulea and Deschampsia flexusosa. It was observed that outbreaks of heather beetle (Lochmaea suturalis) caused serious damage to Calluna vulgaris which led to the death of heather plants and a replacement by grasses. This was first linked to eutrophication in 1977 by de Smit [88] and early experiments pointed to the existence of relationships between eutrophication, heather beetle infestation and grass encroachment [86].
In the 1970’s nitrogen deposition in the Netherlands ranged from around 40 to 80 kg N ha−1 y−1 [89]. Experiments demonstrated, however, that even at N addition levels much higher than ambient (200 kg N ha−1 yr−1), M. caerulea did not directly outcompete Calluna vulgaris [90]. Instead, fertilisation by atmospheric deposition of N increased the quality of C. vulgaris as food plant for heather beetles. Heather beetle infestations on C. vulgaris cause many individual plants to die, which opens up the canopy and allows vigorously-growing grasses to become dominant. During a heavy infestation of heather beetles little nitrogen is lost from the system: almost none is leached and only about 1 kg N ha−1 is lost with dispersing insects [86]. Soils under dead C. vulgaris were found to have high mineralisation and large N pools and low NH4+ immobilisation, these conditions led to ammonium accumulation under dead heather, facilitating grass invasion [91].
Heather beetle larvae are able to detect and feed preferentially on the most N-rich shoots [92] and experiments showed that the beetles from fertilised plots showed higher growth rates compared to unfertilised plots and were the heaviest adults [85, 93, 94]. This was a consequence of better survival rates at larval stage and through hibernation induced by high nitrogen in the larval food supply [92]. The increased survival rates led to an increase in the severity (outbreak densities can be as high as 2000 beetles m−2 [95]) and frequency of beetle outbreaks [93] with the frequency of outbreaks increasing from every 20 years to every 8 years [96]. It has since been shown that population numbers are highest after long-term nitrogen addition, indicating that impacts are likely to be exacerbated over time [92].
The conversion of heathland to grassland is very visible, but other impacts of nitrogen deposition on biodiversity are less apparent without surveys. One of the most reported of these is wide-spread losses of plant species richness. Declines in species richness with increasing nitrogen deposition had been observed in experimental situations for many years [e.g. 97, 98]. In well-buffered prairie grasslands long-term N-addition experiments in the field showed declines in species richness as a consequence of competition for other resources [99, 100]. In 2004 it was demonstrated that the plant species richness of acidic grasslands was negatively correlated with ambient levels of nitrogen deposition across the UK. In acidic grasslands the decline equated to one plant species lost for every additional 2.5 kg N ha−1 y−1 [101] of long-term N deposition. Forbs in particular declined in species richness and cover, whilst cover of grasses tended to increase.
Since 2004 negative correlations between nitrogen deposition and plant species richness have been reported in a wide range of habitats [e.g. 102, 103] and in different parts of the world [e.g. 104, 105, 106]. These changes in species richness are accompanied by changes in the chemistry of soils, microbial communities [e.g. 107] and soil processes [e.g. 108]. It is likely that changes in plant species richness and composition are driven by a combination of mechanisms including acidification, eutrophication and subsequent plant competition and interactions with secondary drivers. The relative importance of these drivers is likely influenced by habitat type, soil properties and climate as well as other variables. Such widespread changes in plant species richness are probably having impacts on invertebrate communities and higher up the food chain. There is growing evidence of these impacts [109] although this is an area where further research is needed. N deposition in some regions of the world continues to increase, but in parts of Europe it is beginning to decline [26]. However, evidence from some long-term experiments indicates plant species richness and composition in many damaged areas are unlikely to recover without active management [e.g. 110, 111-113].
5. Ground-level ozone at phytotoxic concentrations
Ground level ozone is formed in a series of photochemically driven reactions between oxides of nitrogen and volatile organic compounds. Unlike sources of SO2 and NO2, ozone is produced within the atmosphere, through the photochemical degradation of VOC in the presence of NO2 [114]. The rate of production varies between the organic compounds as well as NO2 mixing ratios and the VOC emitted by traffic provide some of the largest ozone production rates. Ozone production continues throughout the troposphere where the reactants and solar radiation are present and methane is a major contributor to tropospheric ozone production in remote regions [115]. Ozone is a hemispheric scale pollutant [114] among the gaseous pollutants its effects on vegetation may occur much further from the sources of the precursor pollutants than the effects of SO2 and NO2. Ground level ozone pollution is an issue in much of the mid-latitudes of the northern hemisphere as well as parts of the southern hemisphere, with highest levels during summer in Southern Europe, South and East Asia and large areas of the southern states of the USA and Mexico [116]. Concentrations of ozone in the Northern Hemisphere mid-latitudes doubled between the late 19th century and 1980 and have continued to increase more slowly, reaching current levels of 35–40 ppb [116]. Ozone is a toxic pollutant to both agricultural crops and semi-natural vegetation. As a powerful oxidant it exerts its effects primarily following stomatal uptake and the extent of injury is proportional to the absorbed stomatal dose [117]. Ozone impacts on crops are discussed in depth by Emberson et al. [118].
Ozone impacts on vegetation were first recognised in Los Angeles, California in the 1950s. At the time poor air quality in the region was characterised by dense smogs which led to eye irritation and had a distinctive odour. These smogs were first linked to ozone by the chemist Arie Haagen-Smit, who described the process of ozone formation by photochemical oxidation of hydrocarbons and nitrogen oxides [119]. The impacts of the smog on crops were already well described, but Haagen-Smit isolated ozone as the cause using laboratory fumigation experiments with spinach, beets, endive, oats and alfalfa. The results showed damage from ozone that was indistinguishable from that produced by the smog [119]. Whilst the impacts of ozone on crops had already been described [120] this was the first time they were linked to smog.
Until the early 1970s it had not been considered that ozone could ever become a problem in Britain due to the cooler climate, but then cases of extensive plant damage in the Rotterdam district of Holland occurred as a result of photochemical pollutants. Atkins, Cox and Eggleton [22] subsequently detected summertime elevated ozone levels in a rural district of Berkshire UK, remote from any large conurbations. Bell and Cox [121] followed up these measurements by recording visible ozone damage on an ozone-sensitive cultivar of tobacco at the same location and showed that the injury correlated with ambient levels.
For the next two decades research into the impacts of ozone on plants focussed on damage to crops, with many species identified as showing visible damage and yield reductions [122]. Forest damage in the San Bernadino Mountains, California, in the early 1970s and visible signs of ozone stress in central European forests led to research into the impacts of ozone on young trees. This work led to clear evidence that ozone can affect the chlorophyll content, photosynthetic rate [123] and carbon allocation [124] of trees. Despite this, separating out the effects of ozone, acid deposition and other stressors on crown condition was a challenge. A number of regional- and continental-scale studies were devised to address this [125]. However, it was not until the 1990s that interest in impacts of ozone widened from crops and trees. In 1998 a thorough review of ozone impacts on wild species highlighted the urgent need to investigate ozone impacts widely on semi-natural vegetation [125].
It remains the case that less is known about ozone impacts on semi-natural plants than agricultural crops, but understanding has developed considerably over the last two decades. Understanding the mechanisms of ozone impacts in semi-natural ecosystems is complicated by the need to account for species interactions which can lead to shifts in species composition and losses of biodiversity [126]. Wide variation in the responses of semi-natural species have been reported, as well as intra-specific variability in ozone sensitivity and heritable differences in ozone responses [126, 127]. These heritable differences are likely a consequence of previous exposure [126]. Legumes [128] and summer annual plants [127] have been identified as particularly sensitive to ozone, but beyond this there remains debate about whether sensitivity can be predicted from plant traits [126].
Predicting community responses remains a challenge. Mills et al. [129] used EUNIS (European Nature Information System) level 4 communities to predict community-wide ozone sensitivity from the published responses of individual species. They found all 54 of the EUNIS communities they considered had six or more sensitive species and were thus defined by the authors to be ozone sensitive. Grasslands had the most sensitive communities followed by heathland, scrub and tundra and mires, bogs and fens. However, Bassin et al. [130] caution that the risk to low-productivity perennial grasslands is commonly less than predicted from risk assessments based on individual species or immature mixtures in chambers.
6. Learning from the past and looking to the future
It is clear that we have come a long way in our understanding of the effects of atmospheric pollution on managed and natural vegetation. Pollution effects on plants have been observed for hundreds of years, but the identity of the pollutants responsible, the dose response relationships, and causal mechanisms were unknown until the mid-20th century.
This paper has reviewed the evidence for four main threats from air pollution: (1) direct phytotoxic effects from gas phase constituents, (2) indirect effects from deposition of acidifying agents, (3) indirect effects from deposition of nutrients and (4) direct toxicity of ozone. The evidence suggests that each of these is not controlled by a single mechanism, nor do they operate in isolation. Rather, there are multiple pathways through which each mechanism may operate, many of which are occurring simultaneously, but to different degrees, within the same ecosystem [131, 132]. For SO2, direct phytotoxic effects appear to mostly be localized phenomena around point sources, although historically, when emissions were less regulated, direct effects were likely more widespread [11, 12]. Acidifying and eutrophicating effects appear to be widespread and simultaneous phenomena, but differ in relative magnitude based on a host of factors including the composition of the atmosphere. Ozone interactions are commonplace because ozone usually occurs in concert with other air pollutants such as sulphur and nitrogen oxides, which may affect plants simultaneously.
Although we have learned much about the possible outcomes related to increasing pollution deposition, we are only beginning to understand the reversibility of effects [79, 113]. Pollution deposition has been decreasing through much of Europe and North America since the 1980s and 1990s and much more recently in parts of Asia [133]. These patterns provide an opportunity for “natural experiments” to learn about the reversibility of these effects, in addition to the few controlled experiments. Early signs indicate that “fast cycling” processes may recover fairly quickly (e.g. foliar nutrient balances, nitrate leaching, etc.), while “slow cycling” processes may take several decades or more to recover (e.g. soil N pools, base cation availability, plant communities) [55, 64, 77, 78]. In some cases, an alternative stable state may be reached and recovery may not occur over timescales relevant to public decision-making (e.g. several decades) without management intervention [79, 134].
Looking forward, it is clear that there are multiple paths that different countries may follow either intentionally or unintentionally. Many developed nations are beginning to reduce pollution emissions and deposition through successful environmental policies, although the ultimate outcomes of many of those national policies on the environment are undetermined because the timescales of some anticipated changes are expected to take many decades and so far, insufficient time has passed. Developing nations may tread the same paths as developed nations, increasing both economic activity and air pollution emissions leading to regional reductions in biodiversity, soil acidification and eutrophication, only later to consider recovery, or they may evade the worst by learning from history. The axiom that “an ounce of prevention is worth a pound of cure” is especially germane, as these developing nations, with the right international support and domestic incentives, have the opportunity to avoid many of these adverse effects of air pollution; for example the costs of renewable energy have reached grid parity with many fossil fuels in many nations [135]. Regardless, the global scientific community has made significant strides in understanding and addressing the ecological effects from air pollution, though much work remains as we assess the reversibility of these effects and the transferability of these lessons to understudied ecosystems.
7. Acknowledgements
We thank the Royal Society for the invitation to write this manuscript, Janet Simkin of the British Lichen Society for the maps in Figure 2, and Henning Meesenburg (Nordwestdeutsche Forstliche Versuchsanstalt) for helpful input on European forest decline. We also thank US EPA reviewers, two anonymous reviewers and editor Mark Sutton who provided valuable comments on earlier drafts. The views expressed in this manuscript are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
8. References
- [1].Von Bingen H. 1903. Causae et Curae. Leipzig, P. Keiser. [Google Scholar]
- [2].Evelyn J. 1661. Fumifugium. London, UK. [Google Scholar]
- [3].Evelyn J. 1664. Sylva, or A Discourse of Forest-Trees. London. [Google Scholar]
- [4].Fabri H. 1670. Physica, id est Scientia Rerum Corporearum, 3. Anisson. Lyon. [Google Scholar]
- [5].Clutterbuck R. 1794. Journal of a Tour From Cardiff, Glamorganshire through South and North Wales. In the summer of 1794. In company with Taylor Combe Esquire Cardiff Public Library, MS 3.277 [Google Scholar]
- [6].Hoesly RM, Smith SJ, Feng L, Klimont Z, Janssens-Maenhout G, Pitkanen T, Seibert JJ, Vu L, Andres RJ, Bolt RM, et al. 2018 Historical (1750–2014) anthropogenic emissions of reactive gases and aerosols from the Community Emissions Data System (CEDS). Geoscientific Model Development 11, 369–408. [Google Scholar]
- [7].Kettlewell B. 1973. The evolution of melanism: the study of a recurring necessity. Oxford, Clarendon Press. [Google Scholar]
- [8].Burritt E. 1868. Walks in the black country its green border-land. London, Sampson Low, Son, and Marston. [Google Scholar]
- [9].DuBay SG & Fuldner CC. 2017. Bird specimens track 135 years of atmospheric black carbon and environmental policy. Proceedings of the National Academy of Sciences 114, 11321–11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brimblecombe P, Davies T & Tranter M. 1986. Nineteenth century black Scottish showers. Atmospheric Environment 20, 1053–1057. [Google Scholar]
- [11].Cohen JB & Ruston AG. 1925. Smoke: a study of town air. London, Edward Arnold. [Google Scholar]
- [12].Pettigrew WN. 1928. The influence of air pollution on vegetation. Gardeners Chronicle 292, 308–309. [Google Scholar]
- [13].Metcalfe CR. 1941. Damage to greenhouse plants by town fogs with special reference to sulphur dioxide and light. Annals of Applied Biology 28, 301–315. [Google Scholar]
- [14].Bleasdale JKA. 1952. Atmospheric pollution and plant growth [PhD Thesis], University of Manchester. [DOI] [PubMed] [Google Scholar]
- [15].Thomas MD & Hill GR. 1937. Relation of sulphur dioxide in the atmosphere to photosynthesis and respiration of alfalfa. Plant Physiology 12, 309–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hutchinson TC & Whitby LM. 1974. Heavy-metal pollution in the Sudbury mining and smelting region of Canada, I. Soil and vegetation contamination by nickel, copper, and other metals. Environmental Conservation 7, 123–132. [Google Scholar]
- [17].Nriagu JO. 1996. A History of Global Metal Pollution Science 272, 223. [Google Scholar]
- [18].Nriagu JO & Pacyna JM. 1988. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333, 134–139. [DOI] [PubMed] [Google Scholar]
- [19].Zimdahl RL, Arvik JH & Hammond PB. 1973. Lead in soils: A literature review. CRC Critical reviews in Environmental Control 3, 213. [Google Scholar]
- [20].Cooper OR, Parrish DD, Ziemke J, Balashov NV, Cupeiro M, Galbally IE, Gilge S, Horowitz L, Jensen NR, Lamarque J-F, et al. 2014. Global distribution and trends of tropospheric ozone: An observation-based review. Science of the Anthropocene 2, 000029. [Google Scholar]
- [21].Parmeter JRJ & Miller PR. 1968. Studies relating to the cause of decline and death of ponderosa pine in southern California. Plant Disease Reporter 52, 707–711. [Google Scholar]
- [22].Atkins DHF, Cox RA & Eggleton AEJ. 1972. Photochemical ozone and sulphuric acid formation in the atmosphere over southern England. Nature 235, 372–376. [DOI] [PubMed] [Google Scholar]
- [23].Oden S. 1968. Nederbördens och Luftens Försurning-dess Orsaker, Förlopp och Verkan I Olika Miljöer. Statens Naturvetenskapliga Forskningsrad Stockholm Bulletin. [Google Scholar]
- [24].Bartarbee RW, Flower RJ, Stevenson AC, Jones VJ, Harriman R & Appleby PG. 1988. Diatom and chemical evidence for reversibility of acidification of Scottish lochs. Nature 332, 530–532. [Google Scholar]
- [25].Fowler D & Cape JN. 1982. Air pollutants in agriculture and horticulture. In Effects of Gaseous Air Pollution in Agriculture and Horticulture (eds. Unsworth MH& Ormerod DP). London, Butterworth Scientific. [Google Scholar]
- [26].Fowler D, Brimblecombe P, Burrows J, Heal MR, Grennfelt P, Stevenson DS, Jowett RA, Coyle M, Lui X, Chang Y, et al. In Press A chronology of global air quality Philosophical Transactions of the Royal Society A This volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sutton MA, Reis S, Riddick SN, Dragosits U, Nemitz E, Theobald MR, Tang YS, Braban CF, Vieno M, Dore AJ, et al. 2013. Towards a climate-dependent paradigm of ammonia emission and deposition. Philosophical Transactions of the Royal Society B-Biological Sciences 368. (doi: 10.1098/rstb.2013.0166). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Erisman JW, Van Eekeren N, Koopmans C, De Wit J, Cuijpers W, Oerlemans N & Koks B. 2016. Agriculture and biodiversity: a better balance benefits both. AIMS Agriculture and Food 1, 157–174. [Google Scholar]
- [29].EMEP. 2019 Transboundary particulate matter, photo-oxidants, acidifying and eutrophying components. Status Report 2019. (Norwegian Meteorological Institute. [Google Scholar]
- [30].Nylander W. 1866. Les lichens du Jardin du Luxembourg. Bulletin de la Société Botanique de France 13, 364–372. [Google Scholar]
- [31].Brodo IM. 1966. Lichen growth and cities: a study on Long Island, New York. The Bryologist 69, 427–429. [Google Scholar]
- [32].Sigal LL & Nash THI. 1983. Lichen communities on conifers in Southern California mountains: An ecological survey relative to oxidant air pollution Ecology 64, 1343–1354. [Google Scholar]
- [33].Showman R. 1975. Lichens as indicators of air quality around a coal-fired power generating station. The Bryologist 78, 1–6. [Google Scholar]
- [34].McCune R. 1988. Lichen communities along 03 and SO2 gradients in Indianapolis. The Bryologist 9, 223–228. [Google Scholar]
- [35].Bell ML, Davis DL & Fletcher T. 2004. A retrospective assessment of mortality from the London smog episode of 1952: the role of influenza and pollution. Environmental Health Perspectives 112, 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Malley CS, Heal MR & Braban CF. 2016. Insights from a chronology of the development of atmoshpheric composition monitoring networks since the 1800s. Atmosphere 7, 160–182. [Google Scholar]
- [37].Hawksworth DL & Rose F. 1970. Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature 227, 145–148. [DOI] [PubMed] [Google Scholar]
- [38].Sutton MA, Van Dijk N, Levy PE, Jones MR, Leith ID, Sheppard LJ, Leeson S, Tang YS, Stephens A, Braban CF, et al. Under Review Alkaline Air: changing perspectives on nitrogen and air pollution in an ammonia-rich world. Philosophical Transactions of the Royal Society A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hogan EJ, Minnullina G, SmithR I & Crittenden PD. 2010. Effects of nitrogen enrichment on phosphatase activity and N/P relationships in Cladonia portentosa. New Phytologist 186, 911–925. [DOI] [PubMed] [Google Scholar]
- [40].Crittenden PD. 1989. Nitrogen relations of mat-forming lichens. In Nitrogen Phosphorus and Sulphur Utilization by Fungi (ed. Boddy L, Marchant R, Read DJ), pp. 243–268. Cambridge, Cambridge University Press. [Google Scholar]
- [41].Sutton MA, Pitcairn CER & C.P. W. 2004. Bioindicator and biomonitoring methods for assessing the effects of atmospheric nitrogen on statutory nature conservation sites In JNCC report 356 (Peterborough, JNCC. [Google Scholar]
- [42].Sutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt P, Van Grinsven H & Grizzetti B. 2011. The European Nitrogen Assessment: Sources, effects and policy perspectives. Cambridge, Cambridge University Press. [Google Scholar]
- [43].Wolseley PA, Leith ID, Van Dijk N & M.A. 2009. Macrolichens on twigs and trunks as insicators of ammonia concentrations across the UK – a practical method. In Atmospheric Ammonia–detecting emission changes and environmental impacts (eds. Sutton MA, Reis S & Baker SMH), Springer Science [Google Scholar]
- [44].van Herk CM. 1999. Mapping of ammonia pollution with epiphytic lichens in the Netherlands. Lichenologist 31, 9–20. [Google Scholar]
- [45].Sparrius LB. 2007. Response of epiphytic lichen communities to decreasing ammonia air concentrations in a moderately polluted area of The Netherlands. Environmental Pollution 146, 375–379. [DOI] [PubMed] [Google Scholar]
- [46].Sutton MA, Leith ID, Pitcairn CER, van Dijk N, Tang YS, Sheppard LJ, Dragosits U, Fowler D, James PW & Wolseley PA. 2004. Exposure of ecosystems to atmospheric ammonia in the UK and the development of practical bioindicator methods. In Lichens in a changing pollution environment (eds. P.A. Wolseley & Lambley PW), pp. 51–62. Peterborough, English Nature. [Google Scholar]
- [47].Wolseley P, Sutton M, Leith ID & van Dijk N. 2010. Epiphytic lichens as indicators of ammonia concentrations across the UK. In Biology of Lichens: Symbiosis, Ecology, Environmental Monitoring, Systematics and Cyber Applications. (eds. Nash THI, Geiser L, McCune B, Triebel D, Tomescu AMF& Sanders WB), pp. 75–85. Stuttgart, Cramer in der Gebrüder Borntraeger Verlagsbuchhandlung. [Google Scholar]
- [48].Larsen Vilsholm R, Wolseley PA, Søchting U & J. C. 2009. Biomonitoring with lichens on twigs. Lichenologist 41, 189–202. [Google Scholar]
- [49].Sutton MA, Wolseley PA, Leith ID, van Dyk N, Sim Tang Y, James PW, Theobald M & Whitfield C. 2009. Estimation of the Ammonia critical level for epiphytic lichens based on observations at Farm, Landscape and National Scales. In Atmospheric Ammonia– detecting emission changes and environmental impacts (eds. Sutton MA, Reis S & Baker SMH). London, Springer Science. [Google Scholar]
- [50].Cape JN, van der Eerden LJ, Sheppard LJ, Leith ID & Sutton MA. 2009. Evidence for changing the critical level for ammonia. Environmental Pollution 157, 1033–1037. [DOI] [PubMed] [Google Scholar]
- [51].Schütt P & Cowling EB. 1985. Waldsterben, a general decline of forests in central Europe: symptoms, development and possible causes. Plant Disease 69, 548–558. [Google Scholar]
- [52].Peart DR, Nicholas NS, Zedaker SM, Miller-Weeks MM & Siccam a.T. 1992. Condition and recent trends in high-elevation red spruce populations. In Ecology and Decline of Red Spruce in the Eastern United States (eds. Eagar C& Adams MB), pp. 125–191. New York, Springer-Verlag [Google Scholar]
- [53].Fowler D, Cape JN, Deans JD, Leith ID, Murray MB, Smith RI, Sheppard LJ & Unsworth MH. 1989. Effects of acid mist on the frost hardiness of red spruce seedlings. New Phytologist 113, 321–335. [DOI] [PubMed] [Google Scholar]
- [54].Krahl-Urban B, Papke HE & Peters K. 1988. Forest Decline: Cause-Effect Research in the United States of North America and Federal Republic of Germany. Germany, Assessment Group for Biology, Ecology and Energy of the Julich Nuclear Research Center. [Google Scholar]
- [55].Johnson AH, McLaughlin SB, Adams MB, Cook ER, DeHayes DH, Eagar C, Fernandez IJ, Johnson DW, Kohut RJ, Mohnen VA, et al. 1992. Synthesis and conclusions from epidemiological and mechanistic studies of red spruce decline. In Ecology and Decline of Red Spruce in the Eastern United States (eds. Eagar C & Adams MB), pp. 385–411. New York, Springer-Verlag [Google Scholar]
- [56].Prinz B, Krause GHM & Jung K-D. 1987. Development and Causes of Novel Forest Decline in Germany. In Effects of Atmospheric Pollutants on Forests, Wetlands and Agricultural Ecosystems (eds. Hutchinson TC & Meema KM), pp. 1–24. Heidelberg, Springer-Verlag [Google Scholar]
- [57].Ulrich B. 1984. Effects of air pollution on forest ecosystems and waters—the principles demonstrated at a case study in Central Europe. Atmospheric Environment 18, 621–628. [Google Scholar]
- [58].Tamm CO. 1991. Nitrogen in terrestrial ecosystems: Questions of productivity, vegetational changes, and ecosystem stability. Berlin and New York, Springer-Verlag. [Google Scholar]
- [59].Andersson M. 1988. Toxicity and tolerance of aluminium in vascular plants. Water, Air and Soil Pollution 39, 439–462. [Google Scholar]
- [60].DeHayes DH, Schaberg PG, Hawley GJ & G.R. S. 1999. Acid rain impacts on calcium nutrition and forest health. Bioscience 39, 378–386. [Google Scholar]
- [61].DeHayes DH, Thornton FC, Waite CE & Ingle MA. 1991. Ambient cloud deposition reduces cold tolerance of red spruce seedlings. Canadian Journal of Forestry Research 21, 1292–1295. [Google Scholar]
- [62].Lovett GM & Kinsman JD. 1990. Atmospheric pollutant deposition to high-elevation ecosystems. Atmospheric Environment 24A, 2767–2786. [Google Scholar]
- [63].Hawley GJ, Schaberg PG, Eagar C & C.H. B. 2006. Calcium addition at the Hubbard Brook Experimental Forest reduced winter injury to red spruce in a high-injury year. Canadian Journal of Forestry Research 36, 2544–2549. [Google Scholar]
- [64].Battles JJ, Fahey TJ, Driscoll CT, Blum JD & Johnson CE. 2014. Restoring soil calcium reverses forest decline. Environmental Science & Technology Letters 1, 15–19. [Google Scholar]
- [65].Horn KJ, Thomas RQ, Clark CM, Pardo LH, Fenn ME, Lawrence GB, Perakis PG, Smithwick EAH, Baldwin D, Braun S, et al. 2018. Growth and survival relationships of 71 tree species with nitrogen and sulfur deposition across the conterminous U.S. PloS one 13, e0205296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].van Doorn NS, Battles JJ, Fahey TJ, Siccama TG & P.A. S. 2011. Links between biomass and tree demography in a northern hardwood forest: a decade of stability and change in Hubbard Brook Valley, New Hampshire. Canadian Journal of Forestry Research 41, 1369–1379. [Google Scholar]
- [67].Kosiba AM, Schaberg PG, Rayback SA & G.J. H. 1989. The surprising recovery of red spruce growth shows links to decreased acid deposition and elevated temperature. Science of the Total Environment 637, 1480–1491. [DOI] [PubMed] [Google Scholar]
- [68].Fischer R, Beck W, Calatayud V, Cools N, De Vos B, Dobbertin M, Fleco S, Giordani P, Granke O, Kindermann G, et al. 2011. The condition of forests in Europe. 2011 Executive Report. ICP Forests and European Commission. Hamburg and Brussels. [Google Scholar]
- [69].Schuldt B, Buras A, Arend M, Vitasse Y, Beierkuhnlein C, Damm A, Gharun M, Grams TEE, Hauck M, Hajek P, et al. In Press A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic and Applied Ecology. [Google Scholar]
- [70].Ågren GI & E., B. 1988. Nitrogen saturation of terrestrial ecosystems. Environmental Pollution 54, 185–197. [DOI] [PubMed] [Google Scholar]
- [71].Aber JD, Nadelhoffer KJ, Steudler P & Melillo JM. 1989. Nitrogen saturation in northern forest ecosystems. BioScience 39, 378–386. [Google Scholar]
- [72].Aber JD, Goodale CL, Ollinger SV, Smith M, Magill A, Martin ME, Hallett RA & Stoddard JL. 2003. Is nitrogen deposition altering the nitrogen status of northeastern forests? Bioscience 53, 375–389. [Google Scholar]
- [73].Dise NB & Wright RF. 1995. Nitrogen leaching from European forests in relation to nitrogen deposition. Forest Ecology and Management 71, 153–161. [Google Scholar]
- [74].Lovett GM & C.L. G. 2011. A new conceptual model of nitrogen saturation based on experimental nitrogen addition to an oak forest. Ecosystems 14, 615–631. [Google Scholar]
- [75].Kandler O & J.L. I. 1995. Air pollution and forest decline in central Europe. Environmental Pollution 90, 171–180. [DOI] [PubMed] [Google Scholar]
- [76].Rehfuess KE. 1991. Review of forest decline research activities and results in the federal republic of Germany, Journal of Environmental Science and Health. Environmental Science and Engineering and Toxicology 26, 415–445. [Google Scholar]
- [77].Oulehle F, Evans CD, Hofmeister J, Krejci R, Tahovska K, Persson T, Cudlin P & J. H. 2011. Major changes in forest carbon and nitrogen cycling caused by declining sulphur deposition. Global Change Biology 17, 3115–3129. [Google Scholar]
- [78].Lawrence GB, Scanga SE & Sabo RD. 2019. Recovery of soils from acidic deposition may exacerbate nitrogen export from forested watersheds. JGR Biogeosciences 125, e2019JG005036. [Google Scholar]
- [79].Stevens CJ. 2016. How long do ecosystems take to recover from atmospheric nitrogen deposition? Biological Conservation 200, 160–167. [Google Scholar]
- [80].Gilliam FS, Burns DA, Driscoll CT, Frey SD, Lovett GM & Watmough SA. 2019. Decreased atmospheric nitrogen deposition in eastern North America: Predicted responses of forest ecosystems. Environmental Pollution 244, 560–574. [DOI] [PubMed] [Google Scholar]
- [81].Eshleman KN, Sabo RD & Kline KM. 2013. Surface water quality is improving due to declining atmospheric N deposition. Environmental science & technology 47, 12193–12200. [DOI] [PubMed] [Google Scholar]
- [82].Gundersen P, Callesen I & de Vries W. 1998. Nitrate leaching in forest ecosystems is related to forest floor C/N ratios. Environmental Pollution 102, 403–407. [Google Scholar]
- [83].Gundersen P, Emmett BA, Kjonaas OL, Koopmans CJ & Tietema A. 1998. Imapact of nitrogen deposition on nitrogen cycling in forests: a synthesis of NITREX data. Forest Ecology and Management 101, 37–55. [Google Scholar]
- [84].Likens GE, Driscoll CY & Buso DC. 1996. Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272, 244–246. [Google Scholar]
- [85].Van der Eerden LJ, Dueck THA, Berdowski JJM, Greven H & Van Dobben HF. 1991. Influence of NH3 and (NH4)2SO4 on heathland vegetation. Acta Botanica Neerlandica 40, 281–296. [Google Scholar]
- [86].Heil GW & Diemont WH. 1983. Raised nutrient levels change heathland into grassland. Vegetatio 53, 113–120. [Google Scholar]
- [87].Van Dobben HF. 1991. Inegrated effects (low vegetation). In Acidification research in The Netherlands: Final report of the Dutch priority programme on acidification (eds. Heij GJ & Schneider T), pp. 465–523. The Netherlands, Elsevier. [Google Scholar]
- [88].de Smit JT. 1977. Interaction of Calluna vulgaris and the heather beetle (Lochmaea suturalis). In Vegetation und Fauna, 1976. Berichte uber die internationalen Symposien der Internationalen Vereinigung fur Vegetationskunde in Stolzenau und Rinteln (ed. Thxen R). Vaduz, J. Cramer. [Google Scholar]
- [89].van Breemen N & van Dijk HFG. 1988. Ecosystem effects of atmospheric deposition of nitrogen in The Netherlands. Environmental Pollution 54, 249–274. [DOI] [PubMed] [Google Scholar]
- [90].Aerts R. 1990. Nutrient use efficiency in evergreen and deciduous species from heathlands. Oecologia 84, 391–397. [DOI] [PubMed] [Google Scholar]
- [91].Kristensen HL & McCarty GW. 1999. Mineralization and immobilization of nitrogen in heath soil under intact Calluna, after heather beetle infestation and nitrogen fertilization. Applied Soil Ecology 13, 187–198. [Google Scholar]
- [92].Taboda A, Marcos E & Calvo L. 2016. Disruption of trophic interactions involving the heather beetle by atmospheric nitrogen deposition. Environmental Pollution 218, 436–445. [DOI] [PubMed] [Google Scholar]
- [93].Brunsting AMH & Heil GW. 1985. The role of nutrients in the interactions between a herbivorous beetle and some competing plant species in heathlands. Oikos 44, 23–26. [Google Scholar]
- [94].Power SA, Ashmore MR & Cousins DA. 1998. Impacts and fate of experimentally enhanced nitrogen deposition on a British lowland heath. Environmental Pollution 102, 27–34. [Google Scholar]
- [95].Berdowski JJM & Zeilinga R. 1987. Transition from heathland to grassland: Damaging effects of the heather beetle. Journal of Ecology 75, 159–175. [Google Scholar]
- [96].Heil GW & Bobbink R. 1993. Impact of atmospheric nitrogen deposition on dry heathlands. A stochastic model simulating competition between Calluna vulgaris and two grass species. In Heathlands: Patterns and Processes in a Changing Environment (eds. Aerts R & Heil GW). London, Kluwer Academic Publishers. [Google Scholar]
- [97].Mountford JO, Lakhani KH & Kirkham FW. 1993. Experimental assessment of the effects of nitrogen addition under hay-cutting and aftermath grazing on the vegetation of meadows on a Somerset peat moor. Journal of Applied Ecology 30, 321–332. [Google Scholar]
- [98].Silvertown J, Poulton P, Johnston E, Edwards G, Heard M & Biss PM. 2006. The Park Grass Experiment 1856–2006: its contribution to ecology. Journal of Ecology 94, 801–814. [Google Scholar]
- [99].Tilman D & Olff H. 1991. An experimental study of the effects of pH and nitrogen on grassland vegetation. Acta Oecologica 12, 427–441. [Google Scholar]
- [100].Wedin D & Tilman D. 1993. Competition amoung grasses along a nitrogen gradient: Initial conditions and mechanisms of competition. Ecological Monographs 63, 199–219. [Google Scholar]
- [101].Stevens CJ, Dise NB, Mountford JO & Gowing DJ. 2004. Impact of nitrogen deposition on the species richness of grasslands. Science 303, 1876–1879. [DOI] [PubMed] [Google Scholar]
- [102].Field C, Dise NB, Payne RJ, Britton A, Emmett BA, Helliwell R, Hughes S, Jones LM, Leake JR, Phoenix G, et al. 2014. Nitrogen drives plant community change across semi-natural habitats. Ecosystems 17, 864–877. [Google Scholar]
- [103].Maskell LC, Smart SM, Bullock JM, Thompson K & Stevens CJ. 2010. Nitrogen Deposition causes widespread species loss in British Habitats. Global Change Biology 16, 671–679. [Google Scholar]
- [104].Stevens CJ, Duprè C, Dorland E, Gaudnik C, Gowing DJG, Bleeker A, Diekmann M, Alard D, Bobbink R, Fowler D, et al. 2010. Nitrogen deposition threatens species richness of grasslands across Europe. Environmental Pollution 158, 2940–2945. [DOI] [PubMed] [Google Scholar]
- [105].Simkin SM, Allen EB, Bowman WD, Clark CM, Belnap J, Brooks ML, Cade BS, Collins SL, Geiser LH, Gilliam FS, et al. 2016. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proceedings of the National Academy of Sciences of the United States of America 113, 4086–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Clark CM, Simkin SM, Allan EB, Bowman WD, Belnap J, Brooks ML, Collins SL, Geiser LH, Gilliam FS, Jovan SE, et al. 2019. Potential vulnerability of 348 herbaceous species to atmospheric deposition of nitrogen and sulfur in the U.S. Nature Plants 5, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Frey SD, Knorr M, Parrent JL & Simpson RT. 2004. Chronic nitrogen enrichment affects the structure and function of the soil microbial community intemperate hardwood and pine forests. Forest Ecology and Management 196, 159–171. [Google Scholar]
- [108].Manning P, Saunders M, Bardgett RD, Bonkowski M, Bradford MA, Ellis RJ, Kandeler E, Marhan S & Tscherko D. 2008. Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biology and Biochemistry 40, 688–698. [Google Scholar]
- [109].Stevens CJ, david TI & Storkey J. 2018. Atmospheric nitrogen deposition in terrestrial ecosystems: Its impact on plant communities and consequences across trophic levels. Functional Ecology 32, 1757–1769. [Google Scholar]
- [110].Strengbom J, Nordin A, Nasholm T & Ericson L. 2001. Slow recovery of boreal forest ecosystem following decreased nitrogen input. Functional Ecology 15, 451–457. [Google Scholar]
- [111].Shi S, Yu Z & Zhao Q. 2014. Responses of plant diversity and species composition to the cessation of fertilization in a sandy grassland. Journal of Forestry Research 25, 337–342. [Google Scholar]
- [112].Isbell F, Reich PB, Tilman D, Hobbie SE, Polasky S & Binder S. 2013. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proceedings of the National Academy of Sciences of the United States of America 16, 11911–11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Clark CM & Tilman D. 2010. Recovery of plant diversity following N cessation: effects of recruitment, litter, and elevated N cycling. Ecology 91, 3620–3630. [DOI] [PubMed] [Google Scholar]
- [114].Monks PS, Archibald AT, Colette A, Cooper O, Coyle M, Derwent R, Fowler D, Granier C, Law KS, Mills GE, et al. 2015. Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer Atmospheric Chemistry and Physics 15, 8889–8973. [Google Scholar]
- [115].Jacob DJ, J.A. L & J.J. M. 1999. Effect of rising Asian emissions on surface ozone in the United States. Geophysical Research Letters 26, 2175–2178. [Google Scholar]
- [116].Fowler D, Amann M, Anderson R, Ashmore M, Cox P, Depledge M, Derwent D, Grennfelt P, Hewitt N, Hov O, et al. 2008. Ground-level ozone in the 21st century: Future trends, impacts and policy implications, Royal Society Science Policy Report, 15 (08). [Google Scholar]
- [117].Tingey DT & Hogsett WE. 1985. Water stress reduces ozone injury via a stomatal mechanism. Plant Physiology 77, 944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Emberson L. In press Effects of ozone on food supply and natural ecosystems. Philosophical Transactions of the Royal Society A This volume. [Google Scholar]
- [119].Haagen-Smit AJ. 1952. Chemistry and physiology of Los Angeles smog. Industrial and Engineering Chemistry Research 44, 1342–1346. [Google Scholar]
- [120].Thomas MD. 1951. Gas damage to plants. Annual Review of Plant Physiology and Plant Molecular Biology 2, 293–322. [Google Scholar]
- [121].Bell JNB & Cox RA. 1975. Atmospheric ozone and plant damage in the United Kingdom. Environmental Pollution 8, 163–170. [Google Scholar]
- [122].Bobbink R. 1998. Impacts of tropospheric ozone and airborne nitrogenous pollutants on natural and semi-natural ecosystems : a commentary. New Phytologist 139, 161–168. [Google Scholar]
- [123].Darrall NM. 1989. The effect of air pollutants on physiological processes in plants. Plant Cell and Environment 12, 1–30. [Google Scholar]
- [124].Laurence JA, Amundson RG, Friend AL, Pell EJ & Temple PJ. 1994. Allocation of carbon in plants under stress: an analysis of the ROPIS experiments. Journal of Environmental Quality 23, 412–417. [Google Scholar]
- [125].Davison AW & Barnes JD. 1998. Effects of ozone on wild plants. New Phytologist 139, 131–151. [Google Scholar]
- [126].Fuhrer J, Skärby L & Ashmore MR. 1997. Critical levels for ozone effects on vegetation in Europe. Environmental Pollution 97, 91–106. [DOI] [PubMed] [Google Scholar]
- [127].Hayes F, Jones MLM, Mills G & Ashmore M. 2007. Meta-analysis of the relative sensitivity of semi-natural vegetation species to ozone. Environmental Pollution 146, 754–762. [DOI] [PubMed] [Google Scholar]
- [128].Gimeno BS, Bermejo V, Sanz J, De la Torre D & Elvira S. 2004. Growth response to ozone of annual species from Mediterranean pastures. Environmental Pollution 132, 297–306. [DOI] [PubMed] [Google Scholar]
- [129].Mills G, Hayes F, Jones MLM & Cinderby S. 2007. Identifying ozone-sensitive communities of (semi-)natural vegetationsuitable for mapping exceedance of critical levels. Environmental Pollution 146, 739–743. [DOI] [PubMed] [Google Scholar]
- [130].Bassin S, Volk M & Fuhrer J. 2007. Factors affecting the ozone sensitivity of temperate European grasslands: An overview. Environmental Pollution 146, 678–691. [DOI] [PubMed] [Google Scholar]
- [131].Sutton M, Oenema O, Erisman JW, Leip A, van Grinsven H & Winiwarter W. 2011. Too much of a good thing. Nature 472, 159–161. [DOI] [PubMed] [Google Scholar]
- [132].Clark CM, Cleland EE, Collins SL, Fargione JE, Gough L, Gross KL, Pennings SC, Suding KN & Grace JB. 2007. Environmental and plant community determinants of species loss following nitrogen enrichment. Ecology Letters 10, 596–607. [DOI] [PubMed] [Google Scholar]
- [133].Zhu J, Chen Z, Wang Q, Xu L, He N, Jia Y, Zhang Q & Yu G. 2019. Potential transition in the effects of atmospheric nitrogen deposition in China. Environmental Pollution 258, 113739. [DOI] [PubMed] [Google Scholar]
- [134].Isbell F, Craven D, Connolly J, Loreau M, Schmid B, Beierkuhnlein C, Bezemer TM, Bonin C, Bruelheide H, de Luca E, et al. 2015. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–U263. [DOI] [PubMed] [Google Scholar]
- [135].Shah V, Booream-Phelps J & Min S. 2014. 2014 Outlook: Let the Second Gold Rush Begin, Deutche Bank Markets Research https://www.deutschebank.nl/nl/docs/Solar_-_2014_Outlook_Let_the_Second_Gold_Rush_Begin.pdf?dbiquery=null%3Avishal+shah