Key Points
Question
What is the long-term association of brain radiotherapy (whole-brain radiotherapy [WBRT] or stereotactic radiosurgery [SRS]) with quality of life (QOL) and cognition outcomes for patients with limited brain metastasis following surgical resection?
Findings
This secondary analysis of a randomized phase 3 clinical trial found that more patients declined in 1 or more cognitive tests by 2 standard deviations (SDs) with WBRT than with SRS at 3, 6, and 9 months. A 2 SD decline in at least 2 cognitive tests was associated with worse QOL.
Meaning
Late cognitive effects of WBRT are clinically meaningful and significant, and a significant decline in cognition (2 SD) was associated with worsening overall QOL; this study provides detailed insight into cognitive function over time in this patient population.
This secondary analysis examines the long-term association of whole-brain radiotherapy and stereotactic radiosurgery with quality of life and cognition outcomes for patients with limited brain metastasis following surgical resection.
Abstract
Importance
Long-term outcomes of radiotherapy are important in understanding the risks and benefits of therapies for patients with brain metastases.
Objective
To determine how the use of postoperative whole-brain radiotherapy (WBRT) or stereotactic radiosurgery (SRS) is associated with quality of life (QOL), cognitive function, and intracranial tumor control in long-term survivors with 1 to 4 brain metastases.
Design, Setting, and Participants
This secondary analysis of a randomized phase 3 clinical trial included 48 institutions in the US and Canada. Adult patients with 1 resected brain metastases but limited to those with 1 to 4 brain metastasis were eligible. Unresected metastases were treated with SRS. Long-term survivors were defined as evaluable patients who lived longer than 1 year from randomization. Patients were recruited between July 2011 and December 2015, and data were first analyzed in February 2017. For the present study, intracranial tumor control, cognitive deterioration, QOL, and cognitive outcomes were measured in evaluable patients who were alive at 12 months from randomization and reanalyzed in June 2017.
Interventions
Stereotactic radiosurgery or WBRT.
Main Outcomes and Measures
Intracranial tumor control, toxic effects, cognitive deterioration, and QOL.
Results
Fifty-four patients (27 SRS arm, 27 WBRT arm; female to male ratio, 65% vs 35%) were included for analysis with a median follow-up of 23.8 months. Cognitive deterioration was less frequent with SRS (37%-60%) compared with WBRT (75%-91%) at all time points. More patients declined by 2 or more standard deviations (SDs) in 1 or more cognitive tests for WBRT compared with SRS at 3, 6, and 9 months (70% vs 22%, 46% vs 19%, and 50% vs 20%, respectively). A 2 SD decline in at least 2 cognitive tests was associated with worse 12-month QOL in emotional well-being, functional well-being, general, additional concerns, and total scores. Overall QOL and functional independence favored SRS alone for categorical change at all time points. Total intracranial control for SRS alone vs WBRT at 12 months was 40.7% vs 81.5% (difference, −40.7; 95% CI, −68.1% to −13.4%), respectively. Data were first analyzed in February 2017.
Conclusions and Relevance
The use of SRS alone compared with WBRT resulted in less cognitive deterioration among long-term survivors. The association of late cognitive deterioration with WBRT was clinically meaningful. A significant decline in cognition (2 SD) was associated with overall QOL. However, intracranial tumor control was improved with WBRT. This study provides detailed insight into cognitive function over time in this patient population.
Trial Registration
ClinicalTrials.gov Identifier: NCT01372774; ALLIANCE/CCTG: N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group)
Introduction
Brain metastases are increasingly common in patients with advanced solid cancers.1 The prognosis among patients with brain metastases varies widely and new prognostic indices have demonstrated the ability to identify long-term survivors.2,3 Whole-brain radiotherapy (WBRT) was the historic standard of care after resection of brain metastasis to improve local control; however, there is no proven overall survival benefit and there are concerns about cognitive decline.4,5,6,7,8 To our knowledge, The Alliance N107C/CEC.3 trial was the first prospective randomized cooperative group trial comparing WBRT and stereotactic radiosurgery (SRS) alone following surgical resection. The detailed results have been previously published.9 Briefly, the findings suggest that use of SRS in patients with limited brain metastasis after surgical resection provide acceptable local tumor control with less cognitive decline compared with WBRT.10
The Alliance N107C/CEC.3 trial9 monitored cognitive function and quality of life (QOL) with comprehensive testing. For many patients with brain metastases, cognitive and QOL outcomes are driven by their cancer and the general decline that precedes death from early disease deterioration.11 Long-term survivors are a subset of patients that may potentially be less affected by brain metastases burdens but are at greater risk of late adverse events due to treatment. To evaluate the late effects of SRS and WBRT, a detailed analysis of cognitive functions, QOL, and tumor control among long-term survivors was conducted.
Methods
Study Design
This secondary analysis was based on the N107C/CEC.3 randomized clinical trial comparing SRS with WBRT for patients with 1 to 4 brain metastases after surgical resection of 1 brain metastasis. Eligibility criteria included an ECOG performance status of 0 to 2, a single resected brain metastasis with a resection cavity of no more than 5.0 cm, and 3 or fewer unresected brain metastases (each with a maximum diameter of 3.0 cm or less). The Trial Protocol is available in Supplement 1.
All study participants provided written informed consent; the protocol was approved by 51 institutional review boards. Patients were recruited between July 2011 and December 2015, and data were first analyzed in February 2017.
For the present study, intracranial tumor control, cognitive deterioration, QOL, and cognitive outcomes were measured in evaluable patients who were alive at 12 months from randomization and reanalyzed in June 2017.
Treatments
The conventional WBRT schedule was 3000 cGy in 10 fractions or 3750 cGy in 15 fractions with the WBRT fractionation schedule predetermined by the treating institution. Unresected metastases were treated with SRS in the WBRT arm (13 [25%] cases). Radiosurgery dose to the surgical cavity varied based on the cavity volume; dose to intact metastasis was based on size and treatment arm.9
Assessments
Cognitive testing covered various domains1: immediate/delayed memory using the Hopkins Verbal Learning Test-Revised (HVLT-R) immediate, delayed, and recognition tests,2 verbal fluency using the Controlled Oral Word Association test (COWAT), and3 visual attention and executive function using the Trail Making Tests A/B (TMT-A/B). Participant QOL was measured using the Functional Assessment of Cancer Therapy-Brain (FACT-Br), and fatigue was measured with QOL/Uniscale and the Linear Analog Self-Assessment (LASA). Functional independence was assessed using the Barthel Index of Activities of Daily Living (ADL). Cognitive testing was performed by a trained, certified team member at each study site. Adverse events regardless of attribution were recorded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE 4.0).
Statistical Analysis
Continuous variables were summarized as median (IQR) and categorical variables as frequency (percent). Long-term cognitive status, functional independence, and QOL were secondary end points of the original trial. Cognitive deterioration was defined as a drop of greater than 1 standard deviation (SD) from baseline in at least 1 of the 6 cognitive tests at the time of evaluation, and all tests were standardized based on published norms. Overall, QOL scores were transformed to a 0 to 100 point scale (100 being most favorable). We defined a decline in functional dependence as a 10% decline below baseline. We compared the proportion of patients with cognitive decline using the Newcombe-Wilson score interval.12 Linear models for 12-month cognitive outcomes included baseline cognitive score, age, sex, and treatment arm as predictor variables. Linear models for 12-month QOL outcomes included baseline QOL score, age, sex, an indicator variable for patients with at least 2 cognitive scores with 2 SDs or greater decline from baseline, and treatment arm as predictor variables. Estimated differences between treatment arms and their 95% CIs were summarized. Complete case analysis was used for instances of missing data. The P values are presented without correction for multiplicity. Analyses were conducted using R statistical software (version 3.6.1; R Foundation, Inc).
Results
Patient Characteristics
The CONSORT diagram of long-term survivor patients is provided in eFigure 1 in Supplement 2. There were 54 (27.8%) long-term survivors with 27 in the SRS group and 27 in the WBRT group. The baseline characteristics of the long-term survivors in the treatment arms are described in Table 1. The median (range) follow-up was 23.8 (12.0-49.0) months for living patients.
Table 1. Baseline Characteristics in 54 Long-term Survivors.
| Demographic and baseline clinical characteristics | No. (%) | P valuea | Overall, No. (%) | |
|---|---|---|---|---|
| SRS (n = 27) | WBRT (n = 27) | |||
| Age, median (IQR), y | 61 (53-64) | 59 (53-65) | >.99 | 60 (52-64) |
| <60 | 11 (41) | 14 (52) | .60 | 25 (46) |
| ≥60 | 16 (59) | 13 (48) | 29 (54) | |
| Sex | ||||
| Female | 14 (52) | 21 (78) | .09 | 35 (65) |
| Male | 13 (48) | 6 (22) | 19 (35) | |
| Education level | ||||
| K-12 | 12 (44) | 15 (58) | .50 | 27 (51) |
| College/vocational 1 y | 13 (48) | 10 (38) | 23 (43) | |
| Postgraduate (MA, MS) | 0 | 1 (3.8) | 1 (1.9) | |
| Professional degree (MD, PhD) | 1 (3.7) | 0 | 1 (1.9) | |
| Other | 1 (3.7) | 0 | 1 (1.9) | |
| Missing, No. | 0 | 1 | 1 | |
| Disease controlled, mo | ||||
| ≤3 | 15 (56) | 14 (52) | >.99 | 29 (54) |
| >3 | 12 (44) | 13 (48) | 25 (46) | |
| No. of brain metastases | ||||
| 1 | 21 (78) | 20 (74) | >.99 | 41 (76) |
| 2-4 | 6 (22) | 7 (26) | 13 (24) | |
| Histology | ||||
| Lung | 19 (70) | 16 (59) | .50 | 35 (65) |
| Other | 7 (26) | 7 (26) | 14 (26) | |
| Radioresistant | 1 (3.7) | 4 (15) | 5 (9.3) | |
| Resection cavity diameter, cm | ||||
| ≤3 | 17 (63) | 18 (67) | >.99 | 35 (65) |
| >3 | 10 (37) | 9 (33) | 19 (35) | |
| ECOG Performance Score | ||||
| 0 | 11 (41) | 11 (41) | >.99 | 22 (41) |
| 1 | 16 (59) | 15 (56) | 31 (57) | |
| 2 | 0 | 1 (3.7) | 1 (1.9) | |
| Anticonvulsant use at study entry | 12 (44) | 10 (37) | .80 | 22 (41) |
| Corticosteroid use | 15 (56) | 13 (48) | .80 | 28 (52) |
| Memantine use | 0 | 0 | >.99 | 0 |
| Missing, No. | 3 | 1 | 4 | |
| Extent of resection | ||||
| Subtotal | 1 (3.7) | 4 (15) | .40 | 5 (9.3) |
| Gross total | 26 (96) | 23 (85) | 49 (91) | |
| Surgical approach | ||||
| En bloc resection | 20 (74) | 17 (63) | .60 | 37 (69) |
| Piecemeal | 7 (26) | 10 (37) | 17 (31) | |
| Physical Well-Being Subscore [0-100], median (IQR) | 86 (70-91) | 82 (71-86) | .70 | 82 (71-89) |
| Missing, No. | 0 | 1 | 1 | |
| Social/Family Well-Being Subscore [0-100], median (IQR) | 86 (79-93) | 91 (80-96) | .20 | 89 (79-96) |
| Missing, No. | 0 | 1 | 1 | |
| Emotional Well-Being Subscore [0-100], median (IQR) | 79 (70-86) | 75 (48-81) | .06 | 75 (62-83) |
| Missing, No. | 1 | 0 | 1 | |
| Functional Well-Being Subscore [0-100], median (IQR) | 64 (44-78) | 57 (46-64) | .40 | 57 (46-68) |
| Missing, No. | 1 | 0 | 1 | |
| Additional Concerns Section Score [0-100], median (IQR) | 73 (60-84) | 70 (62-81) | >.99 | 71 (62-84) |
| Missing, No. | 1 | 0 | 1 | |
| Total Score FACT-Br [0-100], median (IQR) | 74 (64-83) | 70 (63-77) | .60 | 73 (63-81) |
| Missing, No. | 2 | 1 | 3 | |
| General Section Score [0-100], median (IQR) | 78 (67-84) | 71 (66-79) | .20 | 74 (66-82) |
| Missing, No. | 1 | 1 | 2 | |
| Z Score: HVLT Total Recall, median (IQR) | −1.05 (−2.01 to −0.23) | −1.05 (−2.01 to −0.03) | .70 | −1.05 (−2.03 to −0.14) |
| Z Score: HVLT Delayed Recall, median (IQR) | −1.18 (−2.07 to −0.44) | −1.00 (−1.94 to −0.31) | .80 | −1.00 (−1.94 to −0.44) |
| Missing, No. | 1 | 0 | 1 | |
| Z Score: HVLT Delayed Recognition, median (IQR) | −0.57 (−1.16 to 0.24) | 0.14 (−1.29 to 0.79) | .30 | −0.57 (−1.29 to 0.73) |
| Missing, No. | 1 | 0 | 1 | |
| Z Score: TM-A time to complete, median (IQR) | −1.24 (−3.27 to 0.16) | −0.63 (−1.91 to 0.38) | .40 | −0.86 (−2.44 to 0.22) |
| Z Score: TM-B time to complete, median (IQR) | −2.55 (−5.83 to −0.61) | −1.86 (−3.53 to −0.42) | .50 | −2.39 (−4.84 to −0.43) |
| Missing, No. | 0 | 1 | 1 | |
| Z Score: COWAT Total, median (IQR) | −0.95 (−1.53 to −0.44) | −1.34 (−1.82 to −0.59) | .30 | −1.12 (−1.75 to −0.56) |
| Missing, No. | 0 | 1 | 1 | |
Abbreviations: Barthel ADL, Barthel Index of Activities of Daily Living; COWAT, Controlled Oral Word Association; ECOG, Eastern Cooperative Oncology Group; FACT-BR, Functional Assessment of Cancer Therapy-Brain; HVLT, Hopkins Verbal Learning Test; SRS, stereotactic radiosurgery; TM-A, Trail Making Test Part A; TM-B, Trail Making Test Part B; WBRT, whole brain radiotherapy.
Wilcoxon rank sum test; Fisher exact test.
Intracranial Tumor Control and Adverse Events
The rates of surgical bed control, local control, and distant control favored WBRT across the time points (eTable 1 in Supplement 2). Total intracranial control rates for SRS alone vs WBRT at 3, 6, and 12 months were 88.9% vs 100%, 70.4% vs 92.6%, and 40.7% vs 81.5%, respectively (log-rank P = .003). No patients developed leptomeningeal disease. There were 26 patients who underwent WBRT and 22 who received SRS who experienced grade 1 or higher adverse events of any attribution. There were 8 (29.6%) patients with a grade 3 or higher adverse event of any attribution in the WBRT group, and 5 patients with a grade 3 or higher adverse event of any attribution (18.5%) in the SRS alone group (eTable 2 in Supplement 2).
Cognitive Deterioration and Function Over Time
Change from baseline cognitive deterioration was less frequent in the SRS group at all time points (Table 2). The WBRT group showed high rates of deterioration from 88.9% at 3 months to 91.3% at 12 months, whereas the SRS group increased in cognitive deterioration over time, from 37% at 3 months to 60% at 1 year.
Table 2. Cognitive Deterioration Over Timea.
| Time variable | Patients, No. (%) | Difference % (95% CI)b | |
|---|---|---|---|
| SRS (n = 27) | WBRT (n = 27) | ||
| Cognitive deterioration | |||
| 3 mo | 10/27 (37.0) | 24/27 (88.9) | −51.9 (−69.0 to −26.6) |
| 6 mo | 12/26 (46.2) | 23/26 (88.5) | −42.3 (−61.3 to −17.0) |
| 9 mo | 12/25 (48.0) | 21/26 (80.8) | −32.8 (−53.7 to −6.5) |
| 12 mo | 15/24 (62.5) | 21/23 (91.3) | −28.8 (−49.6 to −4.42) |
| 16 mo | 12/21 (57.1) | 16/19 (84.2) | −27.1 (−50.1 to 1.43) |
Abbreviations: SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
1 SD decrease from baseline in 1 or more cognitive tests.
Newcombe-Wilson score interval.
More patients had a 1.5, 2.0 and 3.0 SD decline in 1, 2 and 3 or more cognitive measures in the WBRT arm compared with the SRS arm (Figure 1). A 1.5 SD decline in 1 or more cognitive tests per patient was less likely in the SRS arm compared with the WBRT arm; 30% vs 74% (difference, −44%; 95% CI, −63% to −18%), 35% vs 77% (difference, −42%; 95% CI, −62% to −15%), 50% vs 83% (difference, −33%; 95% CI, −54% to −5.5%), and 43% vs 74% (difference, −31%; 95% CI, −54% to −0.3%) at 3, 6, 12, and 16 months, respectively. A 1.5 SD decline in 2 or more cognitive tests was less likely in the SRS arm compared with the WBRT arm at 6 months, 12% vs 50% (difference, −38%; 95% CI, −58% to −13%). A 2.0 SD decline in 1 or more cognitive tests was less likely in the SRS arm compared with the WBRT arm; 22% vs 70% (difference, −48%; 195% CI, −66% to −22%), 20% vs 46% (difference, −27%; 95% CI, −48% to −1.4%), 20% vs 50% (difference, −30%; 95% CI, −51% to −3.8%) at 3, 6, and 9 months, respectively. A 2.0 SD decline in 2 or more cognitive tests was more likely in the SRS vs WBRT arms at 6 months; 0% vs 31% (difference, −31%; −50% to −12%). A 2.0 SD decline in 3 or more cognitive tests was more likely in the SRS vs WBRT arms at 6 months, 0% vs 15% (difference, −15%; −34% to 0.5%). A 3.0 SD decline in 1 or more cognitive tests was more likely in the SRS vs WBRT arms; 4% vs 35% (difference, −31%; 95% CI, −50% to −9.4%), 16% vs 38% (difference, −22%; 95% CI, −44% to 2%), at 6 and 9 months, respectively.
Figure 1. The Difference in Patients Who Had a 1.5, 2.0, or 3.0 Standard Deviation (SD) Decline in Cognitive Measures.
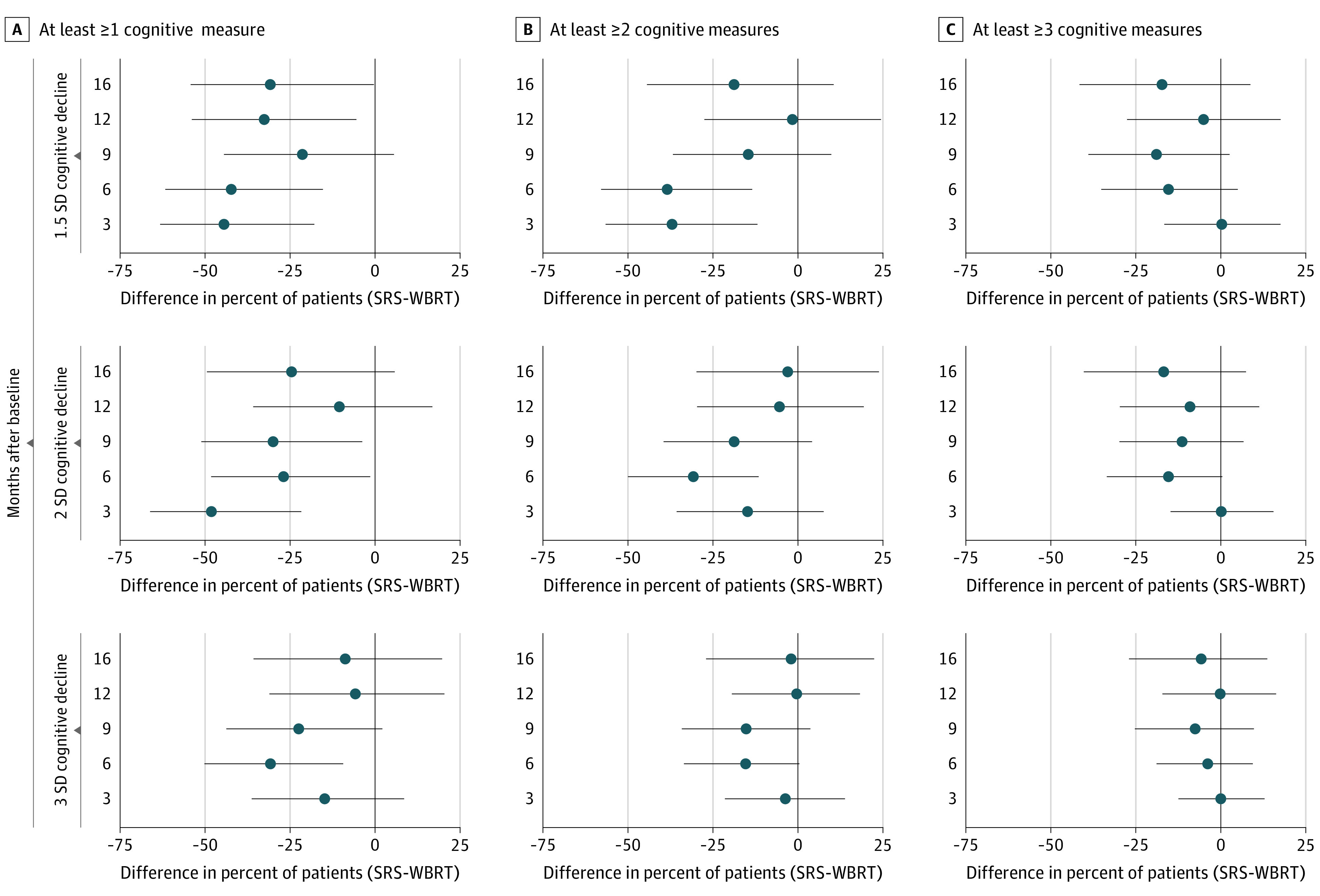
Decline in at least 1, 2, or 3 cognitive measures. Negative values indicate stereotactic radiosurgery (SRS) had a favorable outcome compared with whole brain radiotherapy (WBRT). Circles indicate point estimates and bars indicate 95% CIs.
In patients with a decline of 3.0 SD in 3 or more cognitive tests, the cognitive tests included: delayed recall, delayed recognition, trail making test A/B, and total recall (eFigure 2 in Supplement 2).
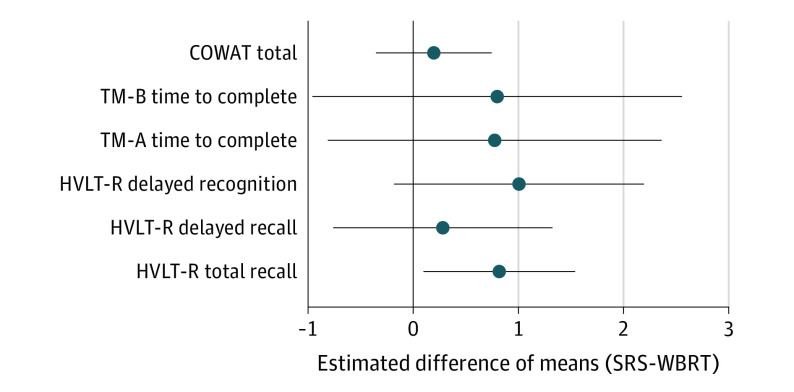
The baseline adjusted linear model for 12-month total recall was found to have higher estimated mean standardized cognitive scores among patients in the SRS arm vs the WBRT arm (difference, 0.82; 95% CI, 0.10-1.50). Patients in the SRS arm had consistently higher estimated 12-month standardized scores compared with the WBRT arm for each of the 6 measures; however, no other significant differences between these 2 groups were observed (Figure 2).
Figure 2. Baseline-Adjusted Estimates of Difference in Mean 12-Month Standardized Cognition Score Between Treatment Arms.
Circles indicate point estimates and bars indicate 95% CIs. HVLT-R indicates the Hopkins Verbal Learning Test-Revised; SD, standard deviation; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy; TM-A, Trail Making Test Part A; TM-B, Trail Making Test Part B.
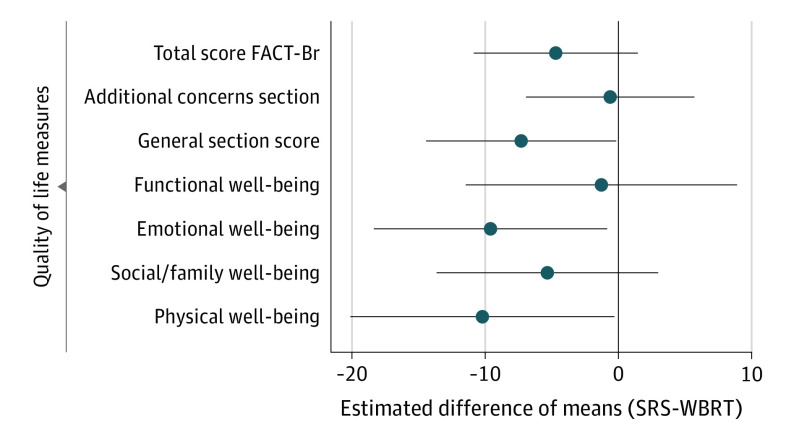
QOL at 12 Months
The baseline adjusted difference in estimated mean QOL scores, by treatment group, and are shown in Figure 3. Overall, SRS had lower mean QOL for physical well-being (PWB) (difference, −10; 95% CI, −20 to −0.3), emotional well-being (EWB) (difference, −9.6; 95% CI, −18 to −0.84), and the general score (difference, −7.3; 95% CI, −14 to −0.16). Other measures showed lower QOL scores for the SRS group, though not significantly.
Figure 3. Baseline-Adjusted Estimates of Difference in Mean 12-Month Quality of Life Between Treatment Arms.
Circles indicate point estimates and bars indicate 95% CIs. FACT-Br indicates the Functional Assessment of Cancer Therapy-Brain test; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
Association of Cognitive Decline With 12-Month QOL
At least 2 cognitive measures with 2.0 or more SD decline was associated with lower mean QOL for EWB (difference, −10; 95% CI, −20 to −0.30), functional well-being (difference, −16; 95% CI, −27 to −3.9), general score (difference, −8.9; 95% CI, −17 to −0.70), additional concerns (difference, −11; 95% CI, −18 to −4.5), and total score (difference, −12; 95% CI, −19 to −4.5). Physical well-being and social/family well-being each estimated a decrease in mean QOL score when a patient had 2 cognitive measures with 2.0 or more SD decline; however, the variability in those estimates did not exclude no effect.
Discussion
In the current study, cognitive function and QOL following radiotherapy for brain metastasis in long-term survivors was generally superior in the SRS arm compared with the WBRT arm. However, there are components of cognition and more notably with patient-reported outcomes that improved with time from WBRT including physical well-being, social and emotional well-being, fatigue, and functional well-being. Also, intracranial tumor control favored WBRT at all time points. Our data support the use of SRS alone after surgical resection as the standard of care, as was previously reported; however, our data suggest that the additional local and distant control gained by the use of WBRT may be important if the long-term toxic effects of WBRT is mitigated.
Limitations
Our study is limited by the small number of patients surviving after 1 year who completed testing. Also, clinicians and patients were not blinded to treatment, which may have affected symptom reporting. In addition, cognitive and QOL decline are likely multifactorial processes comprised of treatment-related toxic effects, intracranial progression, systemic disease progression, intervening behavior/mood changes (anxiety, depression, and coping), and financial burden of therapy.
Prior studies have suggested that intracranial disease control is important and may be associated with long-term survival. In the JROSG 99-1 randomized clinical trial2 the small number of long-term survivors limited our ability to draw conclusions in this regard. Our findings are consistent with prior clinical trials assessing long-term outcomes of WBRT, which reported initial cognitive decline to be associated with long-term decline in cognition.13,14,15
The international Cognition and Cancer Task Force determined that important measures of significant cognitive decline include assessing the number of patients with a decline of 1.5 SD from baseline in 2 or more cognitive tests or a single test with a decline of 2.0 SD.16 Notably, this study is the first to demonstrate, to our knowledge, that along with the known cognitive deterioration (as measured by a 1.0 SD decline in 1 or more cognitive tests), which favors the use SRS alone, a more substantial 1.5, 2.0, and 3.0 SD decline from baseline in 1, 2, and 3 or more cognitive tests was more likely among patients who received WBRT. This was noted early and persisted with time through 24 months posttreatment. We also demonstrated for the first time to our knowledge that a 2.0 SD decline from baseline in 2 or more cognitive tests was associated with a worsening QOL in the functional well-being, general score, additional concerns, and overall QOL domains. However, the long-term effect of SRS and WBRT is not consistently in favor of 1 modality over the other for each patient-reported outcome or cognitive test, which may mean that there is a population of patients who still may benefit from early use of WBRT. Future study with hippocampal avoidance (HA) and concomitant neurostabilizing agents like memantine may improve toxic effects, allowing for more personalized treatment for patients with brain metastasis to determine the optimal timing of WBRT.17,18,19 The NRG Oncology BN-009 study, a phase 3 trial of salvage SRS or SRS plus HA-WBRT for the first or second distant brain relapse for patients with more than 4 new brain metastases per year, may help to further define the proper timing of HA-WBRT. In addition, the CCTG/Alliance CE.7 study,9 was a phase 3 trial using SRS or HA-WBRT with memantine for patients with 5 to 15 brain metastases.
Conclusions
This secondary analysis of a phase 3 randomized clinical trial found that in patients with resected, limited number of brain metastases, the use of SRS alone compared with WBRT resulted in less cognitive deterioration among long-term survivors. Both local and distant intracranial tumor controls were improved with WBRT. A significant decline in cognitive scores (2.0 SD) was associated with worsening QOL. Mean cognition scores were higher for patients treated with SRS at 12 months. Each QOL measure showed higher estimated mean scores for WBRT at 12 months posttreatment, possibly related to the improved intracranial control associated with WBRT.
Trial Protocol
eTable 1. Intracranial Control Rates Based on Treatment Arm
eTable 2. Adverse Events Summary for Long-term Survivors
eTable 3. Changes in Quality of Life From Baseline for Long-term Survivors Comparing SRS vs WBRT
eFigure 1. Consort Diagram
eFigure 2. Number of Patients With a 3 SD. Decline in 3 or More Cognitive Tests Based on Treatment Arm
eFigure 3A. Categorical Change in QOL From Baseline Comparing SRS vs WBRT
eFigure 3B. Categorical Change From Baseline for QOL and Functional Independence Comparing SRS vs WBRT
Data Sharing Statement
References
- 1.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54. doi: 10.1007/s11912-011-0203-y [DOI] [PubMed] [Google Scholar]
- 2.Aoyama H, Tago M, Shirato H; Japanese Radiation Oncology Study Group 99-1 (JROSG 99-1) Investigators . Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464. doi: 10.1001/jamaoncol.2015.1145 [DOI] [PubMed] [Google Scholar]
- 3.Sperduto PW, Mesko S, Li J, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38(32):3773-3784. doi: 10.1200/JCO.20.01255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672. doi: 10.1016/S0140-6736(04)16250-8 [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387-395. doi: 10.1016/S1470-2045(14)70061-0 [DOI] [PubMed] [Google Scholar]
- 6.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489. doi: 10.1001/jama.280.17.1485 [DOI] [PubMed] [Google Scholar]
- 7.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044. doi: 10.1016/S1470-2045(09)70263-3 [DOI] [PubMed] [Google Scholar]
- 8.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141. doi: 10.1200/JCO.2010.30.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049-1060. doi: 10.1016/S1470-2045(17)30441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040-1048. doi: 10.1016/S1470-2045(17)30414-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159-168. doi: 10.1016/S1474-4422(04)00680-5 [DOI] [PubMed] [Google Scholar]
- 12.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873-890. doi: [DOI] [PubMed] [Google Scholar]
- 13.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401-409. doi: 10.1001/jama.2016.9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onodera S, Aoyama H, Tha KK, et al. The value of 4-month neurocognitive function as an endpoint in brain metastases trials. J Neurooncol. 2014;120(2):311-319. doi: 10.1007/s11060-014-1550-y [DOI] [PubMed] [Google Scholar]
- 15.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65-72. doi: 10.1200/JCO.2011.41.0639 [DOI] [PubMed] [Google Scholar]
- 16.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703-708. doi: 10.1016/S1470-2045(10)70294-1 [DOI] [PubMed] [Google Scholar]
- 17.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816. doi: 10.1200/JCO.2014.57.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) . Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437. doi: 10.1093/neuonc/not114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown PD, Gondi V, Pugh S, et al. ; for NRG Oncology . Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III Trial NRG Oncology CC001. J Clin Oncol. 2020;38(10):1019-1029. doi: 10.1200/JCO.19.02767 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Intracranial Control Rates Based on Treatment Arm
eTable 2. Adverse Events Summary for Long-term Survivors
eTable 3. Changes in Quality of Life From Baseline for Long-term Survivors Comparing SRS vs WBRT
eFigure 1. Consort Diagram
eFigure 2. Number of Patients With a 3 SD. Decline in 3 or More Cognitive Tests Based on Treatment Arm
eFigure 3A. Categorical Change in QOL From Baseline Comparing SRS vs WBRT
eFigure 3B. Categorical Change From Baseline for QOL and Functional Independence Comparing SRS vs WBRT
Data Sharing Statement