Abstract
Calcification is an independent predictor of atherosclerosis-related cardiovascular events. Microcalcification is linked to inflamed, unstable lesions, in comparison to the fibrotic stable plaque phenotype generally associated with advanced calcification. This paradox relates to recognition that calcification presents in a wide spectrum of manifestations that differentially impact plaque’s fate. Macrophages, the main inflammatory cells in atherosclerotic plaque, have a multifaceted role in disease progression. They crucially control the mineralization process, from microcalcification to the osteoid metaplasia of bone-like tissue. It is a bilateral interaction that weighs heavily on the overall plaque fate but remains rather unexplored. This review highlights current knowledge about macrophage phenotypic changes in relation to and interaction with the calcifying environment. On the one hand, macrophage-led inflammation kickstarts microcalcification through a multitude of interlinked mechanisms, which in turn stimulates phenotypic changes in vascular cell types to drive microcalcification. Macrophages may also modulate the expression/activity of calcification inhibitors and inducers, or eliminate hydroxyapatite nucleation points. Contrarily, direct exposure of macrophages to an early calcifying milieu impacts macrophage phenotype, with repercussions for plaque progression and/or stability. Macrophages surrounding macrocalcification deposits show a more reparative phenotype, modulating extracellular matrix, and expressing osteoclast genes. This phenotypic shift favours gradual displacement of the pro-inflammatory hubs; the lipid necrotic core, by macrocalcification. Parallels to bone metabolism may explain many of these changes to macrophage phenotype, with advanced calcification able to show homeostatic osteoid metaplasia. As the targeted treatment of vascular calcification developing in atherosclerosis is thus far severely lacking, it is crucial to better understand its mechanisms of development.
Keywords: Macrophages, Inflammation, Calcification, Vascular remodelling, Atherosclerosis
1. Introduction
Atherosclerosis is a slowly progressing, chronic inflammatory disease that affects large- and middle-size arteries,1 featuring the accumulation of fatty and fibrous elements together with immune cells, and structural vascular smooth muscle cells (VSMCs) in the intimal layer of the arterial wall. During disease progression, atherosclerotic plaques develop regions of mineralization, a process which has been traditionally linked to an increased risk for heart disease, atherosclerotic plaque rupture, and stroke.2,3 Rather than a mere by-product of the development and changing inflammatory environment of the plaque, calcification impacts grievously on disease progression and pathogenesis, particularly through mediating biomechanical destabilization and directly impacting plaque inflammation. Calcification, be it bone related or ectopic, is an active process involving interplay between multiple cell types,4 with an important role for osteoclast-like macrophages in bone. Osteoclasts, the specialized resorptive cells found in bone, derived from a common myeloid progenitor with macrophages. In plaque, both multinucleated giant cells and macrophages are observed to emulate osteoclast traits5–9 induced through the RANK/RANK-L/OPG signalling axis,10,11 as present in late-stage calcification.
Although micro- and macrocalcification often occur side-by-side during plaque progression, microcalcification is largely observed in earlier-stage lesions,12 while the latter predominates in late-stage plaque.13 Microcalcification particles, defined as <50 μm in size,14 are developed in a four-stage process, involving calcifying extracellular vesicle (cEV) accumulation, aggregation, membrane fusion, and finally, mineralization.15 During mineralization, amorphous calcium phosphate transforms into mature crystal-like form hydroxyapatite ‘microcalcification’ particles, present in spherical and needle-like morphology types, 0.5–15 µm in size.16 They become larger as lesions progress, as ‘speckled calcification’ (≥15 µm to ≤2 mm in diameter).17 Microcalcification particles in the fibrous cap increase the risk of plaque rupture.14,18 These particles coalesce into larger sheet-like or nodular structures, up to several millimetres in diameter. Such macrocalcification has been linked with healing response and plaque stability.19,20 However, increased coronary artery calcification (CAC) score is related to atherosclerotic plaque burden, has been linked with all-cause mortality and is a broadly adopted predictor of cardiovascular events.21,22
Like Janus, the Roman God of duality, macrophages in the atherosclerotic plaque are seen to have both accelerative and decelerative, bilateral relationship with calcification. On the one hand, they may trigger and exacerbate vascular calcification onset, as calcification first develops in inflammatory hotspots throughout the plaque23; whereas on the other hand, macrophages may limit calcification by encapsulation, internalization, and resorption of macro24 and microdeposits.25
Macrophages co-localize with calcium phosphate crystals in developing atherosclerotic lesions.26–28 The presence of inflammatory macrophages has even been used as a surrogate marker for early microcalcification.29 Vice versa, a high score of intimal microcalcification can help to pinpoint the most inflamed28 and likely to rupture plaque areas.30 Multimodal 18F-NaF and 18F-Fluorodeoxyglucose PET imaging of both measures allows detection of highly metabolically active inflamed areas and microcalcified areas in plaque in one shot.31 In contrast, areas of macrocalcification have been largely observed to feature fewer inflammatory cells, more reparative macrophages, including osteoclast-like cells,6 more fibrosis,32 and presentation of osteoid metaplasia.33 This was confirmed by transcriptional analysis of human high- vs. low-calcified carotid atherosclerotic plaques, showing repressed inflammation, lipid transport, and chemokine signalling pathways.13 Hence, a better comprehension of exactly how macrophages engage with calcification throughout disease progression will offer more opportunities for highly necessary, novel therapeutics. In this review, we will outline current literature on macrophage crosstalk with intimal calcification in atherosclerosis, including both direct and indirect interactions, and its impact on disease progression.
2. Macrophage phenotypic plasticity in response to a calcified microenvironment
Macrophages’ remarkable plasticity and functional heterogeneity render them adaptive, according to specific microenvironment stimuli, to different subsets or phenotypes.34 To understand how macrophages behave in calcified plaques, in vitro assessment of macrophage response to individual calcifying stimuli has been performed. In this, it is important to note that the outcome appears to greatly depend on the initial phenotype of the cells being studied, but this is not regularly factored into account. The M1/M2 macrophage model,35 whilst being now considered a too-broad descriptor of macrophage’s full phenotypic spectrum, has been shown to have opposing effects on extracellular endogenous mechanisms of calcification as further elaborated on below and may respond differently to a calcified/calcifying environment.
Attempting to mimic macrophage responses to a microcalcified environment, some in vitro studies have shown M2-like phenotypic shift in hyperphosphataemia. Macrophages had increased phosphate-handling ability and enhanced arginine hydrolysis, which both may dampen crystal nucleation within the plaque.36 These phosphate-polarized cells produced higher levels of secreted adenosine triphosphate (ATP) and increased pyrophosphate (PPi) synthesis, inhibiting calcium phosphate deposition.37 PPi is produced by the enzyme ectonucleotide pyrophosphatase/phosphodiesterase 1 (eNPP), which hydrolyses extracellular ATP to generate PPi and adenosine monophosphate. Therefore, PPi inhibits the precipitation of calcium phosphate, preventing the formation of hydroxyapatite and favouring its dissolution. Phosphate-polarized cells also showed enrichment in oxidative stress handling genes.36
Inversive to the response seen to hyperphosphataemia, incubation of macrophages with calcium phosphate-supplemented medium could induce the release of calcifying matrix extracellular vesicles, and increased interleukin (IL)-6 expression in M1 polarized cells, while M2 polarized cells had reduced induction of Arginase-1 expression upon the same stimulation,38 both pointing to net M1 skewing. Alone, increased extracellular Ca2+ could trigger NLR family pyrin domain containing 3 (NLRP3) activation in monocytes and increase IL-1β secretion after lipopolysaccharide stimulation.39 However, the combined exposure to the pro-inflammatory cytokine tumour necrosis factor-alpha (TNF-α), plus CaPO4 stimulated the transformation of macrophages into osteoclast-like cells in vitro, in an RANK-L independent manner.40 Importantly, in vitro cell culture models reliant on supplementing additional calcium phosphate to alter normal equilibrium may better reflect a medial calcification environment, as it is observed during kidney dysfunction.41
Calcium phosphate crystals can be internalized actively by human monocyte-derived macrophages through phagocytosis, induce a pro-inflammatory M1 phenotype, and activate the NLRP3 inflammasome complex to release IL-1β, amongst others.25,42,43 IL-1 molecule release in response to cholesterol crystal phagocytosis and NLRP3 activation drives the recruitment of neutrophils, and early lesion formation.44 This pro-inflammatory response to calcium phosphate particles could be reversed/dampened by co-incubation with Fetuin A or Gla-Rich Protein (GRP), both natural calcification inhibitors.45,46 Stimulation of THP-1 derived macrophages with hydroxyapatite nanoparticles, the naturally occurring mineral form of calcium phosphate, alone could also induce the expression of GRP and Matrix Gla Protein (MGP),46 a potent vitamin K-dependent protein inhibitor of vascular calcification produced by VSMCs and chondrocytes. The mechanism behind the hydroxyapatite induced pro-inflammatory response is not fully understood, nor is it known if macrophages can directly sense and respond to hydroxyapatite particles, or if pro-inflammatory responses are instead a by-product of frustrated phagocytosis due to the inability to effectively breakdown ingested hydroxyapatite particles. However, the physicochemical properties of hydroxyapatite particles are highly variable, with sizes ranging from 0.1 to 100 µm and needle-shaped/spherical morphology and smooth/rough surface topology, all factors that can modulate the degree of inflammatory response.47
As the atherosclerotic plaque milieu is so complex and difficult to model in culture, it is still largely unclear how direct macrophage-calcification stimulation seen in vitro is taking place in the inflammatory state of the plaque itself. Particularly, as plaque macrophages are likely to be highly inflammatory, this may skew responses to calcification stimuli in vivo. Deep phenotyping studies of plaque macrophages in proximity to early and advanced plaque calcification would help mapping this causality dilemma. Several ground-breaking single-cell sequencing studies in atherosclerotic plaque have helped to highlight the macrophage spectrum in this disease,48–53 but as of yet, studies comparing cellular heterogeneity of calcified vs. non-calcified plaques are lacking. A second outstanding question is to what extent the calcification-related macrophage phenotype in vivo is dependent on the physicochemical features of the calcium phosphate particle (e.g. charge, size, composition). The distinct response of macrophages to hydroxyapatite particles and inorganic minerals suggests that pathological atherosclerotic calcification is not merely a passive consequence of chronic inflammatory disease but may lead to a positive feedback loop as a result of the active interplay between calcification and inflammation during the disease progression.
3. Macrophage contribution to intimal calcification
The critical step in the formation of an atherosclerotic plaque is the infiltration of macrophages in the subendothelial space. In this sense, macrophage infiltration is a sine qua non for vascular calcification. Direct causal involvement of macrophages in vascular calcification is conceivable. This section will review the diverse mechanisms in microcalcification initiation (i) cEV release, (ii) apoptotic body nucleation, (iii) endogenous inhibitor dysregulation, and (iv) osteogenic transdifferentiation, involving both direct and indirect macrophage engagement. All these mechanisms, occurring simultaneously in actively calcifying plaques, have been shown to be initiated and driven by macrophage interaction with the microenvironment and contained cells.
3.1 Macrophage extracellular vesicles
Macrophages can directly contribute to atherosclerotic plaque calcification through the release of cEVs.38,54 These macrophage extracellular vesicles are characterized by markers CD9, CD63, CD81, TSG101, and CD68,38,55 externalized phosphatidylserine, and are loaded with S100A9 and Annexin-5 proteins. As well as possessing high calcification potential, accumulation and aggregation of cEVs initiate nucleation of hydroxyapatite particles, promoting the mineralization process within plaques.56
Parallels between extracellular vesicles released in the pro-inflammatory atherosclerotic plaque milieu, and matrix vesicles in bone formation can be drawn, since they share many commonalities; high mineralization potential, annexin expression, and acidic lipids such as phosphatidylserine.57,58 However, extracellular vesicles are highly variable; recent high-throughput technologies highlighted phenotypic differences, consistent with their originating cell type; including immunopositivity for cell markers, protein, and RNA content.59 Furthermore, differences between vesicles from the same origin cells can be seen in an altered microenvironment. Comparative proteomic profiling analysis of extracellular vesicles released from primary mouse aortic smooth muscle cells upon different pro-osteogenic conditions demonstrated significant differences in protein composition, such as endocytosis-associated proteins reduced vesicles released from phosphate-stimulated cells.60 In agreement, proteomic analysis of cEVs from human VSMCs and valvular interstitial cells cultured in osteogenic media revealed an enrichment of annexins including ANXA1 and its calcium-dependent binding partner, S100 calcium-binding protein A11 (S100A11) that could tether extracellular vesicles.61 Interestingly, ANXA1 knockdown attenuated extracellular vesicle microcalcification and therefore human SMCs and VICs calcification.61 More research to that direction is needed in the developing multi-omics era, for further characterization of these vesicles, their loading molecules as well as their emerging role in and beyond the vascular calcification pathology. Extracellular vesicles’ ability to contain proteins, lipids, nucleic acids, and other signalling molecules, as well as their capability to circulate and transmit specific molecular information to other cell types influencing their function, is of great interest for potential and promising diagnostic and prognostic biomarker evaluation.62
3.2 Macrophage lipid handling and cell death driving microcalcification
Lipid infiltration and modification in early atherosclerosis trigger an inflammatory response, monocyte recruitment, and macrophage differentiation, as well as foam cell generation.63,64 The lipid-rich necrotic core is a key site of early calcification, with high hydroxyapatite nucleation potential.65,66 Lipids may enhance the deposition of calcium crystals serving as an extra scaffold for calcification, by triggering osteogenic differentiation of VSMCs. Similarly, by affecting foam cell efferocytosis and stimulating inflammation, the lipid core increases the calcification propensity of surrounding cells and extracellular matrix. Apoptosis of VSMCs in culture was shown to be a key regulator of the initiation of vascular calcification with apoptotic bodies acting as nucleation sites for calcification.67 Parallels have also been drawn between apoptotic bodies and matrix microvesicles that induce calcification in bone.68 Failure of macrophages to clear apoptotic bodies, as observed in advanced atherosclerosis,69,70 allows calcium crystal growth to progress, and may also be a significant inflammatory spur leading to the release of cytokines such as TNF-α, also a potent inducer of osteogenic gene expression in VSMCs.71
Uptake of ox-LDL triggers apoptosis in macrophages and VSMCs.72,73 In early lesions, macrophage apoptosis can reduce overall plaque size and lesion inflammation,74 however, in more advanced lesions, with compromised efferocytosis, apoptosis will transition to secondary necrosis, which is detrimental to plaque development and increases calcification.75,76 Macrophages are highly effective in efferocytosis and have a high capacity for continued clearance of apoptotic cells in the plaque; upon uptake of apoptotic cells, they release anti-inflammatory cytokines IL-10 and transforming growth factor-beta (TGF-β).77 Thus, targeting efferocytosis in macrophages may have promise in reducing vascular calcification, as well as overall plaque progression. Efferocytosis-targeting strategies such as blockage of CD47 ‘don’t eat me’ signalling dramatically reduced atherosclerosis in ApoE−/− through the improvement of debris clearance by macrophages.78 CD47 inhibition is already considered for cancer therapy, making clinical translation to atherosclerosis potentially easier79 and recently CD47-interference nanotherapy was shown to have a favourable outcome in atherosclerotic ApoE−/− mice.80
Beyond influencing cell death, macrophage lipid handling also impacts the inflammatory nature of the plaque. OxLDL has a chemotactic effect on monocytes, and is a TLR4 agonist81; stimulation of both macrophages and VSMCs with oxLDL increases their expression of TLR4.82,83 It activates NF-kB signalling, producing a pro-inflammatory phenotype in macrophages, and increasing osteoblastic differentiation and calcification in VSMCs,84 as well as increasing foam cell formation in both. Moreover, oxLDL uptake and subsequent lysosomal cholesterol crystal generation are inflammasome activating factors in macrophages, allowing maturation and secretion of IL-1β and IL-18.85,86 IL-1β production is a key factor in perpetuated atherosclerotic calcification, as mentioned, as it is also induced in response to hydroxyapatite stimulation,87 suggestive of positive feedback during microcalcification establishment. Indeed therapeutic inhibition of IL-1β in Ldlr−/− mice using a monoclonal antibody showed greatly diminished calcification burden within plaques.88 Inflammasome associated IL-1β production is kept in check by Rho GTPases RAC1 and 2, the expression of which was seen to be down-regulated with plaque progression, potentially accelerating atherosclerotic calcification.87 A neutralizing IL-1β antibody increased macrophage presence within the fibrous cap and promoted M2 macrophage polarization; whereas IL-1 signalling in VSMCs is essential for their migration and collagen secretion into the fibrous cap in advanced atherosclerotic plaques.89 Of interest, no difference in the lesion calcification was observed, compared to IL-1 signalling and inflammasome modulation in early plaques.90
3.3 Macrophage impact on endogenous calcification inhibitors
Several endogenous mechanisms exist throughout the body to prevent ectopic calcification. Macrophage-driven inflammation causes several vascular cell types—including smooth muscle cells, endothelial cells and pericytes—to undergo phenotypic changes resulting in altered expression of calcification modulating factors.91–93 Macrophage-produced inflammatory drivers initiate simultaneous loss of VSMC-expressed calcification inhibitors, such as MGP, osteopontin (OPN) and PPi, and gain of inducers such as osteoprotegerin (OPG).2 Stimulation of VSMCs with macrophage conditioned medium simultaneously increased bone morphogenic protein-2 (BMP-2) and inhibited MGP expression.94
As in macrophage response to a calcified microenvironment, polarized macrophages can exert opposing pro- and anti-calcifying activity via endogenous inhibitors. M1 macrophages have higher expression and activity of the enzyme ectonucleoside triphosphate diphosphohydrolase 1 (eNTPD1, a.k.a. CD39), which hydrolyses ATP to AMP and Pi.95 Thus, macrophages may promote calcification by not only producing Pi, a calcification substrate, but also lowering ATP availability for eNPP1 to produce PPi,96 a potent calcification inhibitor. In the aortic wall, more than 90% of extracellular ATP is degraded to Pi,95 at a rate 10 times more rapid than the rate of PPi synthesis and insufficient for the inhibition of hydroxyapatite formation.97 Co-culture of M1 macrophages, or M1-derived TNF-α enhances the TNAP activity of VSMCs; augmenting calcification in vitro.98 Contrastingly, co-culture of VSMCs with M2 macrophages stimulated the synthesis of extracellular ATP and PPi and enhanced the activity of eNPP1 in VSMCs.37
Macrophages secrete large amounts of the inhibitors OPN and Fetuin-A in calcified plaques, which have been suggested to enhance microcalcification opsonization for the purposes of phagocytosis.99 Although OPN can have pro-atherogenic effects,100 it has been shown to be anti-calcifying in atherosclerosis, and specifically in macrophages can induce carbonic anhydrase II expression, attenuate inflammatory activation, and regulate osteoclast formation.101,102 Exogenous OPN exerts a significant role as an inflammatory mediator of vascular injury; it is induced in the differentiation of peripheral monocytes into an M2-like phenotype.102
M2 macrophages release anti-inflammatory mediators and phagocytize necrotic fragments or apoptotic cells to prevent the formation of calcified nucleation sites.103 Of interest, macrophage-derived OPN binding to calcium phosphate or hydroxyapatite particles functions as an opsonin104 and facilitates their ingestion through the phagocytosis process. In accordance, fetuin/α2-HS glycoprotein, another vascular calcification inhibitor, enhances phagocytosis of apoptotic cells and macropinocytosis by macrophages,105 reducing the accumulation of pro-calcifying apoptotic vesicles.
3.4 Macrophage impact on smooth muscle cell osteogenic transdifferentiation
Macrophage interaction with VSMCs heavily contributes to plaque calcification and is perhaps their most impactful indirectly calcifying activity. Macrophages release a vast variety of pro-osteogenic cytokines71,106,107 that stimulate smooth muscle cells to transdifferentiate into an osteogenic phenotype. VSMC-osteo/chondrogenic phenotype94 is accompanied by genetic lineage reprogramming involving up-regulation of osteochondrogenic markers (RUNX2, SOX9 ALP, osteocalcin, osterix, type II, and X collagen), down-regulation of VSMC markers (SM22a, SMa actin, etc.),108 and secretion of calcifying microvesicles.109
It was shown that co-culture of macrophages with VSMCs profoundly affected the ability of the latter to calcify. Inflammatory macrophages especially induced VSMC chondrogenic switch, as well as active calcification.110 Co-culture of VSMCs with murine M2 macrophages, however, inhibited calcification.37 Paradoxically, M2 hallmark cytokines such as TGF-β have also been reported to have pro-calcifying effects: direct stimulation of VSMCs with TGF-β increases calcification, as well as increased VSMC migration and foam cell generation.111 Moreover, TGF-β1 osteo-inductive signalling involves the Smad2/3 pathway112 and SOX9-mediated113 up-regulation of RUNX2 in VSMCs. The up-regulation of osteoblast markers, such as Runx2, in VSMCs can be induced through incubation with many M1 inflammatory stimuli, including TNF-α, IL-6, and IL-18.114–116 Similarly, products of oxidative stress such as reactive oxygen species, a hallmark of the M1 macrophage phenotype, can also induce VSMC phenotypic switching to pro-calcifying.117 In addition, M1 macrophages can directly secrete oncostatin M, contributing to the development of atherosclerotic calcification by inducing osteoblastic transdifferentiation of VSMCs through the JAK3-STAT3 pathway.118 An auto/paracrine mechanism of M1-released BMP-2119 may have implications in VSMCs calcification via BMP-2 receptor/Smad1/5 signalling axis. The activation of Runx2, along with its chondrogenic downstream targets, can also induce VSMC apoptosis, as positive feedback for calcification nucleation, and is linked mechanistically to the DNA damage response.120 Hence, several interlinked mechanisms exist, in which macrophage-led inflammation synergistically intensifies active intimal vascular calcification.
4. Observed macrophage phenotype in macrocalcified plaque areas
In advanced calcified atherosclerotic plaques, macrophages surrounding areas of macrocalcification generally have acquired markedly less inflamed, more reparative phenotypes.33 The transition from inflamed micro- to stable macrocalcification is highly understudied. Macrocalcified plaque environments have not yet been successfully modelled in culture. Also, little research has been documented on how microcalcification in the arteries can transition into nodular or sheet-like structures, and whether this is influenced by the inflammatory state of the plaque, or if the structures themselves are influential factors. Certainly, differing macrocalcification structures contribute varyingly to the overall risk of rupture.4 CD68+ Mannose Receptor+ (M2-like) macrophages were stated to co-localize with cell-rich stable plaque areas, particularly away from the lipid core and rupture-prone shoulder regions of the plaque where M1-like cells tend to dominate.69,121 Furthermore, macrophages near macrocalcified deposits showed an M2-like phenotype.122,123 As stated, in vitro M2 macrophages are engaged in the healing response103 to plaque inflammation; and through the induction of VSMC osteoblastic differentiation,19 mediated mainly, but not exclusively by TGF-β signalling124 may be facilitating the macrocalcification process.19 But moreover, they can also reflect a level of calcification inhibiting activity.37
Overall, macrophage co-localization with calcification is reduced with larger calcifications and higher in microcalcified areas.27 Macrocalcified plaque appears to be enriched in tartrate resistant acid phosphatase (TRAP)-positive multinucleated giant cells and CD68+/Carbonic Anhydrase II/TRAP-positive osteoclast-like macrophages.6,125 These CD68+ MR+, CAII+, Cathepsin K (CATK)low macrophages had minimal resorptive activity, indicating that RANK-L-led osteoclast-like changes within the plaque may not produce efficient osteoclasts from macrophages.7,122 Bone marrow-derived osteoclast-like cells could reduce calcified elastin mineral content in vitro by 80% whereas in vivo, osteoclasts induced elastin demineralization by 50%, without altering elastin integrity.126 This lack of efficiency may be caused by vascular cell production of soluble factors such as OPG and IL-18—shown to inhibit normal osteoclast generation and resorptive capability.127 Recent reports have shown that macrophage multinucleated giant cell formation in chronic inflammatory disease and osteoclast fusion in bone mass regulation display a common molecular signature.128 Research into the plaque proteome showed cartilage oligomeric matrix protein, a musculoskeletal and cardiovascular non-collagenous glycoprotein, can regulate macrophage phenotype within the atherosclerotic plaque and skew towards an alternatively activated and osteoclast-like phenotype.129
Macrophage–osteoclast interrelationship has been described in bone formation; a distinct population of osteolineage-associated resident macrophages, termed ‘osteal macrophages’ or osteomacs, has been recently described in mice; classified as F4/80+, TRAPc−.130 Interestingly, it was reported that osteomacs are injury-associated macrophage cells. They are present in high numbers in areas of bone matrix deposition during fracture healing processes. Their depletion suppressed bone healing in vivo131,132 and they were able to differentiate to multinucleated TRAP+ osteoclasts capable of bone resorption.133 Of note, true vascular ossification, with the presence of an established bone marrow-like region is greatly influenced by the anatomical location of the plaque and its originating arterial bed.134,135 For example, plaques in femoral arteries show a higher propensity for osteoid metaplasia than carotid plaques. Interestingly, a distinct myeloid origin circulating cell fraction expressing osteocalcin and bone alkaline phosphatase has pro-calcific activity in vitro and in vivo, contributing to ectopic vascular calcification in type 2 diabetes.136 While osteoclast-like cells in plaque may provide an interesting therapeutic potential due to their mineral removal and remodelling capability, it is yet unclear if exacerbating their presentation would be of benefit, since extreme enrichment of cathepsin K in atherosclerotic plaques could lead to redundant proteolytic plaque remodelling and plaque rupture.137,138 In line, calcified nodule formation is initiated in the regions of elastin degradation, therefore, a balance between osteo-immune cells is critical, yet subverted by aberrant and/or unresolved immune responses in atherosclerosis.139
5. Macrophage-osteoclast phenotypic switch
As mentioned above, lipids are important factors in the biomineralization process140–142; histologically, early calcification can be detected in acellular lipid pools in bone and ectopic mineralization.143 The lipid-rich necrotic core is the highest risk area to precursor micro- and macrocalcification deposits, and undergo long-term transformation into dense calcium phosphate.65,66,144 This notion is enhanced from studies showing that lesions with a higher load of calcification contain less lipid core.145 High serum LDL-cholesterol is highly correlated to vascular calcification,146,147 and both serum LDL and total cholesterol have been independently associated with CAC incidence.148 However, it remains elusive whether lipids are causative in atherosclerotic calcification or just represent an epiphenomenon, although they have been linked to calcification-associated phenotypes in macrophages.
Lipid handling equivalent processes can be drawn between macrophages and observed osteoclast-like traits. Although it is vastly under-investigated, this parallel may help to better understand the origin and role of osteoclast-like cells in the plaque. More specifically, foam cells, expressing the lysosomal protease CATK, have been shown to contribute to plaque remodelling,149 much like activated macrophages and osteoclast-like cells.150 Plaque multinucleated giant cells express markers such as TRAP and CATK along with their distinctive osteoclast morphological overlap.6 In culture, lipids and modified lipids have been shown to promote osteoclastogenesis through VSMC RANK-L up-regulation, direct macrophage osteoclast gene up-regulation, and promoting osteoclast survival.151,152 Similarly, lipid exposure in murine bone marrow-derived macrophages could trigger multinucleated giant cell formation in culture, a phenotype that could be greatly exacerbated by myeloid Mcl-1 depletion in Ldlr−/− mice where a lipid accumulating, giant cell forming and apoptosis prone phenotype in macrophages was demonstrated.153 Furthermore, hyperlipidaemia in Ldlr−/− mice, which is associated with increased plasma oxLDL levels, was seen to increase osteoclastogenesis potential in pre-osteoclasts ex vivo.154 Cochain et al.48 reported a triggering receptor expressed on myeloid cells 2 (TREM2) high, OPN expressing macrophage subset, probably foam cells, in single-cell sequencing of CD45+ cells isolated from the atherosclerotic aorta of Ldlr−/− mice fed a western-type diet. This subset showed gene enrichment for lipid handling as well as osteoclast function and osteoclastogenesis. These cells were confirmed in meta-analysis with single-cell data from Kim et al. to be indistinguishable from foam cells and possess low inflammatory gene expression.52,155
As with the lipid crossover between ectopic calcification and bone, developing intimal calcification acquires the RANK/RANK-L/OPG axis, greatly influencing intimal calcification development and macrophage phenotypic switch. RANK-L drives osteoclastogenesis.156 VSMCs, stimulated by conditioned medium from inflammatory macrophages, up-regulate Runx2-controlled RANK-L production and secretion.8 Next to supporting osteoclast differentiation, RANK-L has been shown to induce IL-6 and TNF-α secretion in macrophages, and thus can reinforce the VSMC pro-calcifying phenotype.157 Partial deletion of Runx2 in VSMC in ApoE-/- mice causing an alternative functional truncated Runx2 protein, showed reduced vascular calcification, with RANK-L expression reduction, reduced macrophage infiltration to the lesion, and reduced macrophage to osteoclast-like phenotypic switch.158 Further study with a VSMC-specific runx2 deletion model showed reduced calcification, but no change in lipid metabolism, lesion size, or macrophage recruitment.159 This highlights a mechanism of vascular calcification possibly separable from the inflammatory and lipid-driven mechanisms.
It is possible that the osteoid metaplasia, driven by the pathways here highlighted, acts as a compensatory mechanism to control calcification progression, and moreover, as a mechanism of inflammation control and wound healing. The observations linking lipid handling to osteoclastogenesis may help to bridge the gap between highly inflammatory microcalcification-associated macrophages, and the osteoclastogenic switch capable of taking place. Understanding this association, and how it affects calcification development and progression, may be critical in future efforts to clinically modulate plaque inflammation and calcification.
6. Conclusions and future perspectives
Whilst calcification is an independent predictor of clinical cardiovascular events, the overall risk to plaque rupture or stability critically depends on the actual calcification phenotype. Bone-like vascular calcification has been shown to be a typical feature of more stable plaques and asymptomatic disease.160,161 However, even fibrocalcific plaques have an associated risk of adverse events, such as rupture, occlusion, or thrombosis through calcified nodules.4 Plaque regression studies and meta-analysis showed that a common feature of a regressing plaque is an increase in dense calcium volume and CAC score, which is inversely correlated to event risk.22,162 Treatment and rupture prevention, largely relies on aggressive lipid-lowering statin therapy, shown to stabilize plaques but also increase calcification. However, combination therapy with protein convertase subtilisin/kexin type 9 (PCSK9) could inhibit statin-induced calcification progression in 16 subjects, compared to statin monotherapy (n = 15) in a paired longitudinal study.163
Drugs to specifically treat or reverse atherosclerotic calcification are still currently missing. However, treating atherosclerotic calcification effectively at later stages will likely also not rely on purely targeting calcification, but through a better understanding of the greatest risks at each stage of the disease, so that treatment can be more targeted. Reducing inflammation and improving beneficial macrophage functions could represent a more powerful tailored strategy to prevent and reduce microcalcification, particularly in atherosclerosis patients with a high degree of vascular calcification, unresponsive to regular lipid-lowering therapy.
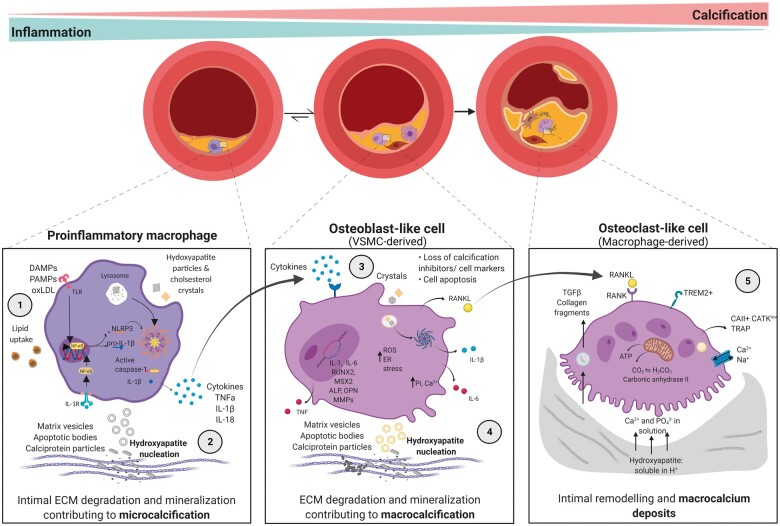
A research explosion has occurred based on the role of macrophages in the process of vascular calcification and is summarized in Figure 1. The phenotypic plasticity and functional heterogeneity of macrophages according to the microenvironment variables led to the understanding of their pleiotropic effects in the atherosclerotic plaque calcification. Inflammatory macrophage activity accelerates plaque calcification profoundly, through many mechanisms that also couple to plaque growth and risk of rupture. Meanwhile in-kind, a calcified microenvironment reinforces these processes and produces calcification-associated macrophage phenotypes linked to macrocalcification. As such, net M2 skewing in atherosclerosis through clinical intervention may reduce not only plaque progression but also calcification growth, both in early and late stages. Reducing microcalcification generation, and enhancing fibrotic activity associated with established stable calcification; specifically through inflammation reduction, direct calcification inhibition activity and regulation of plaque cell death. The precise modulation mechanisms that allow for in vivo differentiation of macrophages into a phenotype in a manner that is more protective for the patient is still an unmet need and an urgent problem to be solved. Therefore, deep phenotyping of macrophages subsets with high-resolution omic methodologies like single-cell technology in calcified plaques is still an open research area and represents a clear benefit for better disease understanding and assessment of clinical risk. However, the relative contribution of macrophages to late-stage calcification and disease state is also yet to be comprehensively elucidated. Comparative assessment of cellular phenotypes presenting at all stages of vascular calcification can help to fill in many gaps in understanding of how this contributes to disease progression, risk of plaque rupture (i.e. clinical events) and how current therapeutic strategies may be improved. Conclusively, additional investigation of the potential molecular mechanism and function of how macrophages modulate the progression and regression of vascular calcification is expected not only to bridge the gap between in vitro and in vivo observations but also to uncover a new notion for the prevention and treatment of vascular calcification.
Figure 1.
Interplay between macrophages and calcification in atherosclerotic plaque from early to late disease stage. Pro-inflammatory macrophages in the atherosclerotic plaque milieu undergo hydroxyapatite crystal and modified lipid uptake (1), as well as extracellular matrix vesicle and cytokine release (2). This establishes microcalcification in the plaque and induces an osteoblast-like phenotype in VSMCs (3). A pro-calcifying microenvironment and the osteochondrogenic switch of VSMCs, driven by macrophages, increase inflammatory calcification deposition and establish more densely calcified nodules (macrocalcification) (4). Macrophage engagement in the receptor activator of nuclear factor kappa B (RANK)/RANK-Ligand axis results in alternatively activated, remodelling-associated, and osteoclast-like macrophages surrounding macrocalcification deposits (5). As such, the inflammatory burden in this plaque microenvironment falls, with increased calcification and remodelling. ALP, alkaline phosphatase; ATP, adenosine triphosphate; Ca2+, calcium; CAII, carbonic anhydrase 2; CATK, cathepsin K; DAMPs, damage-associated molecular patterns; ECM, extracellular matrix; ER, endoplasmic reticulum; IL-1R, interleukin-1 receptor; IL-1β, interleukin 1 beta; IL-6, interleukin 6; IL-18: interleukin 18; MMPs, matrix metalloproteinases; MSX2, Msh Homeobox 2; NLRP3, NLR family pyrin domain containing 3; OPN, osteopontin; PAMPs, pathogen-associated molecular patterns; Pi, phosphate; ROS, reactive oxygen species; RUNX2, runt-related transcription factor 2; SASP, senescence-associated secretory phenotype; TGFβ, transforming growth factor-beta; TLRs, toll-like receptors; TNF-α, tumour necrosis factor-alpha; TRAP, tartrate resistant acid phosphatase; TREM2, triggering receptor expressed on myeloid cells 2.
Acknowledgements
The figure was created with BioRender.com
Contributor Information
Olivia J Waring, Department of Pathology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, P. Debyelaan 25, 6229 HX, Maastricht, The Netherlands.
Nikolaos T Skenteris, Department of Pathology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, P. Debyelaan 25, 6229 HX, Maastricht, The Netherlands; Cardiovascular Medicine Unit, Department of Medicine, Karolinska Institutet, Visionsgatan 4, 171 64, Solna, Sweden.
Erik A L Biessen, Department of Pathology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, P. Debyelaan 25, 6229 HX, Maastricht, The Netherlands; Institute for Molecular Cardiovascular Research, RWTH Aachen University, Templergraben 55, 52062, Aachen, Germany.
Marjo M P C Donners, Department of Pathology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, P. Debyelaan 25, 6229 HX, Maastricht, The Netherlands.
Authors’ contributions
O.J.W. and N.T.S. wrote and revised the manuscript, M.M.P.C.D. and E.A.L.B. revised the manuscript.
Funding
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 722609.
Data availability
This review article does not contain new original data.
References
- 1. Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, Ibañez B, López-Melgar B, Laclaustra M, Oliva B, Mocoroa A, Mendiguren J, Vega VD, García L, Molina J, Sánchez-González J, Guzmán G, Alonso-Farto JC, Guallar E, Civeira F, Sillesen H, Pocock S, Ordovás JM, Sanz G, Jiménez-Borreguero LJ, Fuster V.. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation 2015;131:2104–2113. [DOI] [PubMed] [Google Scholar]
- 2. Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM.. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res 2018;114:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicoll R, Henein MY.. The predictive value of arterial and valvular calcification for mortality and cardiovascular events. Int J Cardiol Heart Vessel 2014;3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R.. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging 2018;11:127–142. [DOI] [PubMed] [Google Scholar]
- 5. Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res 2004;95:1046–1057. [DOI] [PubMed] [Google Scholar]
- 6. Qiao JH, Mishra V, Fishbein MC, Sinha SK, Rajavashisth TB.. Multinucleated giant cells in atherosclerotic plaques of human carotid arteries: identification of osteoclast-like cells and their specific proteins in artery wall. Exp Mol Pathol 2015;99:654–662. [DOI] [PubMed] [Google Scholar]
- 7. Davaine JM, Quillard T, Chatelais M, Guilbaud F, Brion R, Guyomarch B, Brennan MA, Heymann D, Heymann MF, Gouëffic Y.. Bone like arterial calcification in femoral atherosclerotic lesions: prevalence and role of osteoprotegerin and pericytes. Eur J Vasc Endovasc Surg 2016;51:259–267. [DOI] [PubMed] [Google Scholar]
- 8. Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, Anderson PG, Tintut Y, Demer LL, Wang D, Chen Y, Byon C, H, Sun Y, Chen J, Yuan K, Mao X, Heath JM, Anderson PG, Tintut Y, Demer LL, Wang D, Chen Y.. Runx2-upregulated receptor activator of nuclear factor κb ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol 2011;31:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deneke T, Langner K, Grewe PH, Harrer E, Müller KM.. Ossification in atherosclerotic carotid arteries. Z Kardiol Springer 2001;90:106–115. [DOI] [PubMed] [Google Scholar]
- 10. Xu F, Teitelbaum SL.. Osteoclasts: new insights. Bone Res 2013;1:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang D, Wan Y.. Molecular determinants for the polarization of macrophage and osteoclast. Semin Immunopathol 2019;41:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roijers RB, Debernardi N, Cleutjens JPM, Schurgers LJ, Mutsaers PHA, Vusse GVD.. Microcalcifications in early intimal lesions of atherosclerotic human coronary arteries. Am J Pathol 2011;178:2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karlöf E, Seime T, Dias N, Lengquist M, Witasp A, Almqvist H, Kronqvist M, Gådin JR, Odeberg J, Maegdefessel L, Stenvinkel P, Matic LP, Hedin U.. Correlation of computed tomography with carotid plaque transcriptomes associates calcification with lesion-stabilization. Atherosclerosis 2019;288:175–185. [DOI] [PubMed] [Google Scholar]
- 14. Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S.. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci U S A 2013;110:10741–10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, Yabusaki K, Faits T, Bouten C, Franck G, Quillard T, Libby P, Aikawa M, Weinbaum S, Aikawa E.. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater 2016;15:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perrotta I, Perri E.. Ultrastructural, elemental and mineralogical analysis of vascular calcification in atherosclerosis. Microsc Microanal 2017;23:1030–1039. [DOI] [PubMed] [Google Scholar]
- 17. Jinnouchi H, Sato Y, Sakamoto A, Cornelissen A, Mori M, Kawakami R, Gadhoke NV, Kolodgie FD, Virmani R, Finn AV.. Calcium deposition within coronary atherosclerotic lesion: implications for plaque stability. Atherosclerosis 2020;306:85–95. [DOI] [PubMed] [Google Scholar]
- 18. Shi X, Gao J, Lv Q, Cai H, Wang F, Ye R, Liu X.. Calcification in atherosclerotic plaque vulnerability: friend or foe? Front Physiol 2020;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shioi A, Ikari Y.. Plaque calcification during atherosclerosis progression and regression. J Atheroscler Thromb 2018;25:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwee RM. Systematic review on the association between calcification in carotid plaques and clinical ischemic symptoms. J Vasc Surg 2010;51:1015–1025. [DOI] [PubMed] [Google Scholar]
- 21. Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P.. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3:1229–1236. [DOI] [PubMed] [Google Scholar]
- 22. Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA.. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014;311:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shanahan CM. Inflammation ushers in calcification: a cycle of damage and protection? Circulation 2007;116:2782–2785. [DOI] [PubMed] [Google Scholar]
- 24. Kusmartsev S, Dominguez-Gutierrez PR, Canales BK, Bird VG, Vieweg J, Khan SR.. Calcium oxalate stone fragment and crystal phagocytosis by human macrophages. J Urol 2016;195:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pazár B, Ea H-K, Narayan S, Kolly L, Bagnoud N, Chobaz V, Roger T, Lioté F, So A, Busso N.. Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J Immunol 2011;186:2495–2502. [DOI] [PubMed] [Google Scholar]
- 26. Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R.. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007;116:2841–2850. [DOI] [PubMed] [Google Scholar]
- 27. Burgmaier M, Milzi A, Dettori R, Burgmaier K, Marx N, Reith S.. Co-localization of plaque macrophages with calcification is associated with a more vulnerable plaque phenotype and a greater calcification burden in coronary target segments as determined by OCT. PLoS One 2018;13:e0205984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reith S, Milzi A, Dettori R, Marx N, Burgmaier M.. Predictors for target lesion microcalcifications in patients with stable coronary artery disease: an optical coherence tomography study. Clin Res Cardiol 2018;107:763–771. [DOI] [PubMed] [Google Scholar]
- 29. Derlin T, Tóth Z, Papp L, Wisotzki C, Apostolova I, Habermann CR, Mester J, Klutmann S.. Correlation of inflammation assessed by18F-FDG PET, active mineral deposition assessed by18F-fluoride PET, and vascular calcification in atherosclerotic plaque: a dual-tracer PET/CT study. J Nucl Med 2011;52:1020–1027. [DOI] [PubMed] [Google Scholar]
- 30. Joshi NV, Vesey AT, Williams MC, Shah ASV, Calvert PA, Craighead FHM, Yeoh SE, Wallace W, Salter D, Fletcher AM, Beek EJR, Van Flapan AD, Uren NG, Behan MWH, Cruden NLM, Mills NL, Fox KAA, Rudd JHF, Dweck MR, Newby DE.. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–713. [DOI] [PubMed] [Google Scholar]
- 31. Creager MD, Hohl T, Hutcheson JD, Moss AJ, Schlotter F, Blaser MC, Park MA, Ho Lee L, Singh SA, Alcaide-Corral CJ, Tavares AAS, Newby DE, Kijewski MF, Aikawa M, Carli MD, Dweck MR, Aikawa E.. 18F-fluoride signal amplification identifies microcalcifications associated with atherosclerotic plaque instability in positron emission tomography/computed tomography images. Circ Cardiovasc Imaging 2019;12:e007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Menini S, Iacobini C, Ricci C, Fantauzzi CB, Salvi L, Pesce CM, Relucenti M, Familiari G, Taurino M, Pugliese G.. The galectin-3/RAGE dyad modulates vascular osteogenesis in atherosclerosis. Cardiovasc Res 2013;100:472–480. [DOI] [PubMed] [Google Scholar]
- 33. Chinetti-Gbaguidi G, Daoudi M, Rosa M, Vinod M, Louvet L, Copin C, Fanchon M, Vanhoutte J, Derudas B, Belloy L, Haulon S, Zawadzki C, Susen S, Massy ZA, Eeckhoute J, Staels B.. Human alternative macrophages populate calcified areas of atherosclerotic lesions and display impaired RANKL-induced osteoclastic bone resorption activity. 2017;121:19–30. [DOI] [PubMed] [Google Scholar]
- 34. Ginhoux F, Guilliams M.. Tissue-resident macrophage ontogeny and homeostasis. Immunity 2016;44:439–449. [DOI] [PubMed] [Google Scholar]
- 35. Murray PJ. Macrophage polarization. Annu Rev Physiol 2017;79:541–566. [DOI] [PubMed] [Google Scholar]
- 36. Villa-Bellosta R, Hamczyk MR, André V, Andrés V.. Novel phosphate-activated macrophages prevent ectopic calcification by increasing extracellular ATP and pyrophosphate. PLoS One 2017;12:e0174998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villa-Bellosta R, Hamczyk MR, Andrés V.. Alternatively activated macrophages exhibit an anticalcifying activity dependent on extracellular ATP/pyrophosphate metabolism. Am J Physiol Cell Physiol 2016;310:C788–C799. [DOI] [PubMed] [Google Scholar]
- 38. New SEP, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E.. Macrophage-derived matrix vesicles : an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res 2013;113:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jäger E, Murthy S, Schmidt C, Hahn M, Strobel S, Peters A, Stäubert C, Sungur P, Venus T, Geisler M, Radusheva V, Raps S, Rothe K, Scholz R, Jung S, Wagner S, Pierer M, Seifert O, Chang W, Estrela-Lopis I, Raulien N, Krohn K, Sträter N, Hoeppener S, Schöneberg T, Rossol M, Wagner U.. Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis. Nat Commun 2020;11:4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takei Y, Tanaka T, Kent KC, Yamanouchi D.. Osteoclastogenic differentiation of macrophages in the development of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2016;36:1962–1971. [DOI] [PubMed] [Google Scholar]
- 41. Bundy JD, Chen J, Yang W, Budoff M, Go AS, Grunwald JE, Kallem RR, Post WS, Reilly MP, Ricardo AC, Rosas SE, Zhang X, He J.. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: the CRIC study. Atherosclerosis 2018;271:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laquerriere P, Grandjean-Laquerriere A, Jallot E, Balossier G, Frayssinet P, Guenounou M.. Importance of hydroxyapatite particles characteristics on cytokines production by human monocytes in vitro. Biomaterials 2003;24:2739–2747. [DOI] [PubMed] [Google Scholar]
- 43. Nadra I, Mason JC, Philippidis P, Florey O, Smythe CDWW, McCarthy GM, Landis RC, Haskard DO.. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways. Circ Res 2005;96:1248–1256. [DOI] [PubMed] [Google Scholar]
- 44. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E.. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith ER, Hanssen E, McMahon LP, Holt SG.. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS One 2013;8:e60904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Viegas CSB, Costa RM, Santos L, Videira PA, Silva Z, Araújo N, Macedo AL, Matos AP, Vermeer C, Simes DC.. Gla-rich protein function as an anti-inflammatory agent in monocytes/macrophages: implications for calcification-related chronic inflammatory diseases. PLoS One 2017;12:e0177829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lebre F, Sridharan R, Sawkins MJ, Kelly DJ, O'Brien FJ, Lavelle EC.. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci Rep 2017;7:2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, Zernecke A.. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res 2018;122:1661–1674. [DOI] [PubMed] [Google Scholar]
- 49. Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, Zernecke A, Pramod AB, Ghosh AK, Michel NA, Hoppe N, Hilgendorf I, Zirlik A, Hedrick CC, Ley K, Wolf D.. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res 2018;122:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin J, Da Nishi H, Poles J, Niu X, Mccauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, Ramsey SA, Fisher EA, Loke P.. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 2019;4:e124574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir E-AD, Amadori L, Khan NS, Wong CK, Shamailova R, Hill CA, Wang Z, Remark R, Li JR, Pina C, Faries C, Awad AJ, Moss N, Bjorkegren JLM, Kim-Schulze S, Gnjatic S, Ma'ayan A, Mocco J, Faries P, Merad M, Giannarelli C.. Single-cell immune landscape of human atherosclerotic plaques. Nat Med 2019;25:1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zernecke A, Winkels H, Cochain C, Williams JW, Wolf D, Soehnlein O, Robbins CS, Monaco C, Park I, McNamara CA, Binder CJ, Cybulsky MI, Scipione CA, Hedrick CC, Galkina EV, Kyaw T, Ghosheh Y, Dinh HQ, Ley K.. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ Res 2020;127:402–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McArdle S, Buscher K, Ghosheh Y, Pramod AB, Miller J, Winkels H, Wolf D, Ley K.. Migratory and dancing macrophage subsets in atherosclerotic lesions. Circ Res 2019;125:1038–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hutcheson JD, Blaser MC, Aikawa E.. Giving calcification its due: recognition of a diverse disease: a first attempt to standardize the field. Circ Res 2017;120:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boulanger CM, Loyer X, Rautou PE, Amabile N.. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 2017;14:259–272. [DOI] [PubMed] [Google Scholar]
- 56. He S, Wu C, Xiao J, Li D, Sun Z, Li M.. Endothelial extracellular vesicles modulate the macrophage phenotype: potential implications in atherosclerosis. Scand J Immunol 2018;87:e12648. [DOI] [PubMed] [Google Scholar]
- 57. Shapiro IM, Landis WJ, Risbud MV.. Matrix vesicles: are they anchored exosomes? Bone 2015;79:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cui L, Houston DA, Farquharson C, MacRae VE.. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 2016;87:147–158. [DOI] [PubMed] [Google Scholar]
- 59. Bakhshian Nik A, Hutcheson JD, Aikawa E.. Extracellular vesicles as mediators of cardiovascular calcification. Front Cardiovasc Med 2017;4:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chaudhary SC, Khalid S, Smethurst V, Monier D, Mobley J, Huet A, Conway JF, Napierala D.. Proteomic profiling of extracellular vesicles released from vascular smooth muscle cells during initiation of phosphate-induced mineralization. Connect Tissue Res 2018;59:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rogers MA, Buffolo F, Schlotter F, Atkins SK, Lee LH, Halu A, Blaser MC, Tsolaki E, Higashi H, Luther K, Daaboul G, Bouten CVC, Body SC, Singh SA, Bertazzo S, Libby P, Aikawa M, Aikawa E.. Annexin A1-dependent tethering promotes extracellular vesicle aggregation revealed with single-extracellular vesicle analysis. Sci Adv 2020;6:eabb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bank IE, Timmers L, Gijsberts CM, Zhang Y-N, Mosterd A, Wang J-W, Chan MY, Hoog V, De Lim SK, Sze SK, Lam CS, Kleijn DD.. The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Expert Rev Mol Diagn 2015;15:1577–1588. [DOI] [PubMed] [Google Scholar]
- 63. Li AC, Glass CK.. The macrophage foam cell as a target for therapeutic intervention. Nat Med 2002;8:1235–1242. [DOI] [PubMed] [Google Scholar]
- 64. Wang Y, Dubland JA, Allahverdian S, Asonye E, Sahin B, Jaw JE, Sin DD, Seidman MA, Leeper NJ, Francis GA.. Smooth muscle cells contribute the majority of foam cells in ApoE (apolipoprotein E)-deficient mouse atherosclerosis. Arterioscler Thromb Vasc Biol 2019;39:876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zeng Y, Tateishi H, Cavalcante R, Tenekecioglu E, Suwannasom P, Sotomi Y, Collet C, Nie S, Jonker H, Dijkstra J, Radu MD, Räber L, McClean DR, Geuns RJ, van, Christiansen EH, Fahrni T, Koolen J, Onuma Y, Bruining N, Serruys PW.. Serial assessment of tissue precursors and progression of coronary calcification analyzed by fusion of IVUS and OCT: 5-year follow-up of scaffolded and nonscaffolded arteries. JACC Cardiovasc Imaging 2017;10:1151–1161. [DOI] [PubMed] [Google Scholar]
- 66. Maldonado N, Kelly-Arnold A, Laudier D, Weinbaum S, Cardoso L.. Imaging and analysis of microcalcifications and lipid/necrotic core calcification in fibrous cap atheroma. Int J Cardiovasc Imaging 2015;31:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL.. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res 2000;87:1055–1062. [DOI] [PubMed] [Google Scholar]
- 68. Faggiano P, Dasseni N, Gaibazzi N, Rossi A, Henein M, Pressman G.. Cardiac calcification as a marker of subclinical atherosclerosis and predictor of cardiovascular events: a review of the evidence. Eur J Prev Cardiol 2019;26:1191–1204. [DOI] [PubMed] [Google Scholar]
- 69. Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, Derudas B, Mayi T, Bories G, Tailleux A, Haulon S, Zawadzki C, Jude B, Staels B.. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ Res 2011;108:985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Doran AC, Yurdagul A, Tabas I.. Efferocytosis in health and disease. Nat Rev Immunol 2020;20:254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tintut Y, Patel J, Parhami F, Demer LL.. Tumor necrosis factor-α promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000;102:2636–2642. [DOI] [PubMed] [Google Scholar]
- 72. Chen JH, Riazy M, Smith EM, Proud CG, Steinbrecher UP, Duronio V.. Oxidized LDL-mediated macrophage survival involves elongation factor-2 kinase. Arter Thromb Vasc Biol 2009;29:92–98. [DOI] [PubMed] [Google Scholar]
- 73. Hsieh CC, Yen MH, Yen CH, Lau YT.. Oxidized low density lipoprotein induces apoptosis via generation of reactive oxygen species in vascular smooth muscle cells. Cardiovasc Res 2001;49:135–145. [DOI] [PubMed] [Google Scholar]
- 74. Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS.. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol 2005;25:174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thorp E, Tabas I.. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol 2009;86:1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kayashima Y, Makhanova N, Maeda N.. DBA/2J haplotype on distal chromosome 2 reduces Mertk expression, restricts efferocytosis, and increases susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol 2017;37:e82–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM.. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest 1998;101:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ.. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016;536:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Veillette A, Chen J.. SIRPα–CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol 2018;39:173–184. [DOI] [PubMed] [Google Scholar]
- 80. Flores AM, Hosseini-Nassab N, Jarr KU, Ye J, Zhu X, Wirka R, Koh AL, Tsantilas P, Wang Y, Nanda V, Kojima Y, Zeng Y, Lotfi M, Sinclair R, Weissman IL, Ingelsson E, Smith BR, Leeper NJ.. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat Nanotechnol 2020;15:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Miller YI, Choi S-H, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw Px Tsimikas S, Binder CJ, Witztum JL.. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res 2011;108:235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu X-P, Rajavashisth TB, Yano J, Kaul S, Arditi M.. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 2001;104:3103–3108. [DOI] [PubMed] [Google Scholar]
- 83. Yang K, Zhang XJ, Cao LJ, Liu XH, Liu ZH, Wang XQ, Chen QJ, Lu L, Shen WF, Liu Y.. Toll-like receptor 4 mediates inflammatory cytokine secretion in smooth muscle cells induced by oxidized low-density lipoprotein. PLoS One 2014;9:e95935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Song Y, Hou M, Li Z, Luo C, Ou JS, Yu H, Yan J, Lu L.. TLR4/NF-κB/Ceramide signaling contributes to Ox-LDL-induced calcification of human vascular smooth muscle cells. Eur J Pharmacol 2017;794:45–51. [DOI] [PubMed] [Google Scholar]
- 85. Rajamäki K, Lappalainen J, Öörni K, Välimäki E, Matikainen S, Kovanen PT, Eklund KK.. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 2010;5:e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jiang Y, Wang M, Huang K, Zhang Z, Shao N, Zhang Y, Wang W, Wang S.. Oxidized low-density lipoprotein induces secretion of interleukin-1β by macrophages via reactive oxygen species-dependent NLRP3 inflammasome activation. Biochem Biophys Res Commun 2012;425:121–126. [DOI] [PubMed] [Google Scholar]
- 87. Ceneri N, Zhao L, Young BD, Healy A, Coskun S, Vasavada H, Yarovinsky TO, Ike K, Pardi R, Qin L, Qin L, Tellides G, Hirschi K, Meadows J, Soufer R, Chun HJ, Sadeghi MM, Bender JR, Morrison AR.. Rac2 modulates atherosclerotic calcification by regulating macrophage interleukin-1β production. Arterioscler Thromb Vasc Biol 2017;37:328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Awan Z, Denis M, Roubtsova A, Essalmani R, Marcinkiewicz J, Awan A, Gram H, Seidah NG, Genest J.. Reducing vascular calcification by anti-IL-1β monoclonal antibody in a mouse model of familial hypercholesterolemia. Angiology 2016;67:157–167. [DOI] [PubMed] [Google Scholar]
- 89. Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, Hilaire CS, Müller W, Waisman A, Francis SE, Pinteaux E, Randolph GJ, Gram H, Owens GK.. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med 2018;24:1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Grebe A, Hoss F, Latz E.. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res 2018;122:1722–1740. [DOI] [PubMed] [Google Scholar]
- 91. Jiang W, Zhang Z, Li Y, Chen C, Yang H, Lin Q, Hu M, Qin X.. The cell origin and role of osteoclastogenesis and osteoblastogenesis in vascular calcification. Front Cardiovasc Med 2021;8:639740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sánchez-Duffhues G, García de Vinuesa A, van de Pol V, Geerts ME, de Vries M, Janson SG, van Dam H, Lindeman JH, Goumans M-J, Ten Dijke P.. Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J Pathol 2019;247:333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Leszczynska A, O'Doherty A, Farrell E, Pindjakova J, O'Brien FJ, O'Brien T, Barry F, Murphy M.. Differentiation of vascular stem cells contributes to ectopic calcification of atherosclerotic plaque. Stem Cells 2016;34:913–923. [DOI] [PubMed] [Google Scholar]
- 94. Ikeda K, Souma Y, Akakabe Y, Kitamura Y, Matsuo K, Shimoda Y, Ueyama T, Matoba S, Yamada H, Okigaki M, Matsubara H.. Macrophages play a unique role in the plaque calcification by enhancing the osteogenic signals exerted by vascular smooth muscle cells. Biochem Biophys Res Commun 2012;425:39–44. [DOI] [PubMed] [Google Scholar]
- 95. Villa-Bellosta R. Synthesis of extracellular pyrophosphate increases in vascular smooth muscle cells during phosphate-induced calcification. Arterioscler Thromb Vasc Biol 2018;38:2137–2147. [DOI] [PubMed] [Google Scholar]
- 96. Hamczyk MR, Villa-Bellosta R.. Pyrophosphate metabolism and calcification. Aging (Albany NY) 2018;10:3652–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Villa-Bellosta R, Wang X, Millán JL, Dubyak GR, O’Neill WC.. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am J Physiol Heart Circ Physiol 2011;301:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee HL, Woo KM, Ryoo HM, Baek JH.. Tumor necrosis factor-α increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem Biophys Res Commun 2010;391:1087–1092. [DOI] [PubMed] [Google Scholar]
- 99. Jahnen-Dechent W, Schäfer C, Ketteler M, Mckee MD.. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med 2008;86:379–389. [DOI] [PubMed] [Google Scholar]
- 100. Chiba S, Okamoto H, Kon S, Kimura C, Murakami M, Inobe M, Matsui Y, Sugawara T, Shimizu T, Uede T, Kitabatake A.. Development of atherosclerosis in osteopontin transgenic mice. Heart Vessels 2002;16:111–117. [DOI] [PubMed] [Google Scholar]
- 101. Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM.. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol 2002;161:2035–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ge Q, Ruan CC, Ma Y, Tang XF, Wu QH, Wang JG, Zhu DL, Gao PJ.. Osteopontin regulates macrophage activation and osteoclast formation in hypertensive patients with vascular calcification. Sci Rep 2017;7:40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Braga TT, Agudelo JSH, Camara NOS.. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol 2015;6:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pedraza CE, Nikolcheva LG, Kaartinen MT, Barralet JE, McKee MD.. Osteopontin functions as an opsonin and facilitates phagocytosis by macrophages of hydroxyapatite-coated microspheres: implications for bone wound healing. Bone 2008;43:708–716. [DOI] [PubMed] [Google Scholar]
- 105. Jersmann HPA, Dransfield I, Hart SP.. Fetuin/α2-HS glycoprotein enhances phagocytosis of apoptotic cells and macropinocytosis by human macrophages. Clin Sci (Lond) 2003;105:273–278. [DOI] [PubMed] [Google Scholar]
- 106. Watson KE, Boström K, Ravindranath R, Lam T, Norton B, Demer LL.. TGF-β1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest 1994;93:2106–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, Nishizawa Y.. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-α and oncostatin M derived from macrophages. Circ Res 2002;91:9–16. [DOI] [PubMed] [Google Scholar]
- 108. Leopold JA. Vascular calcification: mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med 2015;25:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kapustin AN, Chatrou MLL, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, Rosales RTM, De Alvarez-Hernandez D, Shroff R, Yin X, Muller K, Skepper JN, Mayr M, Reutelingsperger CP, Chester A, Bertazzo S, Schurgers LJ, Shanahan CM.. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res 2015;116:1312–1323. [DOI] [PubMed] [Google Scholar]
- 110. Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL.. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 2002;105:650–655. [DOI] [PubMed] [Google Scholar]
- 111. Borland SJ, Morris TG, Borland SC, Morgan MR, Francis SE, Merry CLR, Canfield AE.. Regulation of vascular smooth muscle cell calcification by syndecan-4/FGF-2/PKCα signalling and cross-talk with TGFβ. Cardiovasc Res 2017;113:1639–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhao F, Wu Y, Yang W, Wu D, Wang C, Zhang F.. Inhibition of vascular calcification by microRNA-155-5p is accompanied by the inactivation of TGF-β1/Smad2/3 signaling pathway. Acta Histochem 2020;122:151551. [DOI] [PubMed] [Google Scholar]
- 113. Alesutan I, Musculus K, Castor T, Alzoubi K, Voelkl J, Lang F.. Inhibition of phosphate-induced vascular smooth muscle cell osteo-/chondrogenic signaling and calcification by bafilomycin A1 and methylamine. Kidney Blood Press Res 2015;40:490–499. [DOI] [PubMed] [Google Scholar]
- 114. Kurozumi A, Nakano K, Yamagata K, Okada Y, Nakayamada S, Tanaka Y.. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone 2019;124:53–61. [DOI] [PubMed] [Google Scholar]
- 115. Lee G-L, Yeh C-C, Wu J-Y, Lin H-C, Wang Y-F, Kuo Y-Y, Hsieh Y-T, Hsu Y-J, Kuo C-C.. TLR2 promotes vascular smooth muscle cell chondrogenic differentiation and consequent calcification via the concerted actions of osteoprotegerin suppression and IL-6–mediated RANKL induction. Arterioscler Thromb Vasc Biol 2019;39:432–445. [DOI] [PubMed] [Google Scholar]
- 116. Zhang K, Zhang Y, Feng W, Chen R, Chen J, Touyz RM, Wang J, Huang H.. Interleukin-18 enhances vascular calcification and osteogenic differentiation of vascular smooth muscle cells through TRPM7 activation. Arterioscler Thromb Vasc Biol 2017;37:1933–1943. [DOI] [PubMed] [Google Scholar]
- 117. Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y, Chang HB, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y.. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 2008;283:15319–15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kakutani Y, Shioi A, Shoji T, Okazaki H, Koyama H, Emoto M, Inaba M.. Oncostatin M promotes osteoblastic differentiation of human vascular smooth muscle cells through JAK3-STAT3 pathway. J Cell Biochem 2015;116:1325–1333. [DOI] [PubMed] [Google Scholar]
- 119. Dube PR, Birnbaumer L, Vazquez G.. Evidence for constitutive bone morphogenetic protein-2 secretion by M1 macrophages: constitutive auto/paracrine osteogenic signaling by BMP-2 in M1 macrophages. Biochem Biophys Res Commun 2017;491:154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cobb AM, Yusoff S, Hayward R, Ahmad S, Sun M, Verhulst A, D’Haese PC, Shanahan CM.. Runx2 (runt-related transcription factor 2) links the DNA damage response to osteogenic reprogramming and apoptosis of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2021;41:1339–1357. [DOI] [PubMed] [Google Scholar]
- 121. Stöger JL, Gijbels MJJ, Velden S. V D, Manca M, Loos CM, van der Biessen EAL, Daemen MJAP, Lutgens E, Winther M. D.. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012;225:461–468. [DOI] [PubMed] [Google Scholar]
- 122. Chinetti-Gbaguidi G, Daoudi M, Rosa M, Vinod M, Louvet L, Copin C, Fanchon MM, Vanhoutte J, Derudas B, Belloy L, Haulon S, Zawadzki C, Susen S, Massy ZA, Eeckhoute J, Staels B.. Human alternative macrophages populate calcified areas of atherosclerotic lesions and display impaired RANKL-induced osteoclastic bone resorption activity. Circ Res 2017;121:19–30. [DOI] [PubMed] [Google Scholar]
- 123. Montanaro M, Scimeca M, Anemona L, Servadei F, Giacobbi E, Bonfiglio R, Bonanno E, Urbano N, Ippoliti A, Santeusanio G, Schillaci O, Mauriello A.. The paradox effect of calcification in carotid atherosclerosis: microcalcification is correlated with plaque instability. Int J Mol Sci 2021;22:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Erlebacher A, Filvaroff EH, Ye JQ, Derynck R.. Osteoblastic responses to TGF-β during bone remodeling. Mol Biol Cell 1998;9:1903–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Oksala N, Levula M, Pelto-Huikko M, Kytömäki L, Soini JT, Salenius J, Kähönen M, Karhunen PJ, Laaksonen R, Parkkila S, Lehtimäki T.. Carbonic anhydrases II and XII are up-regulated in osteoclast-like cells in advanced human atherosclerotic plaques—Tampere Vascular Study. Ann Med 2010;42:360–370. [DOI] [PubMed] [Google Scholar]
- 126. Simpson CLS, Lindley S, Eisenberg C, Basalyga DM, Starcher BC, Simionescu DT, Vyavahare NR.. Toward cell therapy for vascular calcification: osteoclast-mediated demineralization of calcified elastin. Cardiovasc Pathol 2007;16:29–37. [DOI] [PubMed] [Google Scholar]
- 127. Tintut Y, Abedin M, Cho J, Choe A, Lim J, Demer LL.. Regulation of RANKL-induced osteoclastic differentiation by vascular cells. J Mol Cell Cardiol 2005;39:389–393. [DOI] [PubMed] [Google Scholar]
- 128. Pereira M, Petretto E, Gordon S, Bassett JHD, Williams GR, Behmoaras J.. Common signalling pathways in macrophage and osteoclast multinucleation. J Cell Sci 2018;131:jcs216267. [DOI] [PubMed] [Google Scholar]
- 129. Fu Y, Gao C, Liang Y, Wang M, Huang Y, Ma W, Li T, Jia Y, Yu F, Zhu W, Cui Q, Li Y, Xu Q, Wang X, Kong W.. Shift of macrophage phenotype due to cartilage oligomeric matrix protein deficiency drives atherosclerotic calcification. Circ Res 2016;119:261–276. [DOI] [PubMed] [Google Scholar]
- 130. Michalski MN, McCauley LK.. Macrophages and skeletal health. Pharmacol Ther 2017;174:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chang MK, Raggatt L-J, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR.. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 2008;181:1232–1244. [DOI] [PubMed] [Google Scholar]
- 132. Vi L, Baht GS, Whetstone H, Ng A, Wei Q, Poon R, Mylvaganam S, Grynpas M, Alman BA.. Macrophages promote osteoblastic differentiation in vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res 2015;30:1090–1102. [DOI] [PubMed] [Google Scholar]
- 133. Mohamad SF, Xu L, Ghosh J, Childress PJ, Abeysekera I, Himes ER, Wu H, Alvarez MB, Davis KM, Aguilar-Perez A, Hong JM, Bruzzaniti A, Kacena MA, Srour EF.. Osteomacs interact with megakaryocytes and osteoblasts to regulate murine hematopoietic stem cell function. Blood Adv 2017;1:2520–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Steenman M, Espitia O, Maurel B, Guyomarch B, Heymann MF, Pistorius MA, Ory B, Heymann D, Houlgatte R, Gouëffic Y, Quillard T.. Identification of genomic differences among peripheral arterial beds in atherosclerotic and healthy arteries. Sci Rep 2018;8:3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Herisson F, Heymann MF, Chétiveaux M, Charrier C, Battaglia S, Pilet P, Rouillon T, Krempf M, Lemarchand P, Heymann D, Gouëffic Y.. Carotid and femoral atherosclerotic plaques show different morphology. Atherosclerosis 2011;216:348–354. [DOI] [PubMed] [Google Scholar]
- 136. Fadini GP, Albiero M, Menegazzo L, Boscaro E, Vigili De Kreutzenberg S, Agostini C, Cabrelle A, Binotto G, Rattazzi M, Bertacco E, Bertorelle R, Biasini L, Mion M, Plebani M, Ceolotto G, Angelini A, Castellani C, Menegolo M, Grego F, Dimmeler S, Seeger F, Zeiher A, Tiengo A, Avogaro A.. Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ Res 2011;108:1112–1121. [DOI] [PubMed] [Google Scholar]
- 137. Lutgens E, Lutgens SPM, Faber BCG, Heeneman S, Gijbels MMJ, de Winther MPJ, Frederik P, van der Made I, Daugherty A, Sijbers AM, Fisher A, Long CJ, Saftig P, Black D, Daemen MJAP, Cleutjens KBJM.. Disruption of the Cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation 2006;113:98–107. [DOI] [PubMed] [Google Scholar]
- 138. Liu CL, Guo J, Zhang X, Sukhova GK, Libby P, Shi GP.. Cysteine protease cathepsins in cardiovascular disease: from basic research to clinical trials. Nat Rev Cardiol 2018;15:351–370. [DOI] [PubMed] [Google Scholar]
- 139. Simionescu A, Simionescu DT, Vyavahare NR.. Osteogenic responses in fibroblasts activated by elastin degradation products and transforming growth factor-β1: role of myofibroblasts in vascular calcification. Am J Pathol 2007;171:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tintut Y, Hsu JJ, Demer LL.. Lipoproteins in cardiovascular calcification: potential targets and challenges. Front Cardiovasc Med 2018;5:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rendina-Ruedy E, Rosen CJ.. Lipids in the bone marrow: an evolving perspective. Cell Metab 2020;31:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Reid DG, Shanahan CM, Duer MJ, Arroyo LG, Schoppet M, Brooks RA, Murray RC.. Lipids in biocalcification: contrasts and similarities between intimal and medial vascular calcification and bone by NMR. J Lipid Res 2012;53:1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R.. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 2002;105:297–303. [DOI] [PubMed] [Google Scholar]
- 144. Ishiwata Y, Kaneta T, Nawata S, Hino-Shishikura A, Yoshida K, Inoue T.. Quantification of temporal changes in calcium score in active atherosclerotic plaque in major vessels by 18F-sodium fluoride PET/CT. Eur J Nucl Med Mol Imaging 2017;44:1529–1537. [DOI] [PubMed] [Google Scholar]
- 145. van den Bouwhuijsen QJA, Bos D, Ikram MA, Hofman A, Krestin GP, Franco OH, van der Lugt A, Vernooij MW.. Coexistence of calcification, intraplaque hemorrhage and lipid core within the asymptomatic atherosclerotic carotid plaque: the Rotterdam study. Cerebrovasc Dis 2015;39:319–324. [DOI] [PubMed] [Google Scholar]
- 146. Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd-Jones DM.. Discordance between apolipoprotein B and LDL-cholesterol in young adults predicts coronary artery calcification the CARDIA study. J Am Coll Cardiol 2016;67:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kindi M, Al Bélanger AM, Sayegh K, Senouci S, Aljenedil S, Sivakumaran L, Ruel I, Rasadi K, Al Waili K, Al Awan Z, Valenti D, Genest J.. Aortic calcification progression in heterozygote familial hypercholesterolemia. Can J Cardiol 2017;33:658–665. [DOI] [PubMed] [Google Scholar]
- 148. Diederichsen SZ, Grønhøj MH, Mickley H, Gerke O, Steffensen FH, Lambrechtsen J, R, Sand NP, Rasmussen LM, Olsen MH, Diederichsen A.. CT-detected growth of coronary artery calcification in asymptomatic middle-aged subjects and association with 15 biomarkers. JACC Cardiovasc Imaging 2017;10:858–866. [DOI] [PubMed] [Google Scholar]
- 149. Barascuk N, Skjøt-Arkil H, Register TC, Larsen L, Byrjalsen I, Christiansen C, Karsdal MA.. Human macrophage foam cells degrade atherosclerotic plaques through cathepsin K mediated processes. BMC Cardiovasc Disord 2010;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bühling F, Reisenauer A, Gerber A, Krüger S, Weber E, Brömme D, Roessner A, Ansorge S, Welte T, Röcken C.. Cathepsin K—a marker of macrophage differentiation? J Pathol 2001;195:375–382. [DOI] [PubMed] [Google Scholar]
- 151. Sinningen K, Rauner M, Goettsch C, Al-Fakhri N, Schoppet M, Hofbauer LC.. Monocytic expression of osteoclast-associated receptor (OSCAR) is induced in atherosclerotic mice and regulated by oxidized low-density lipoprotein in vitro. Biochem Biophys Res Commun 2013;437:314–318. [DOI] [PubMed] [Google Scholar]
- 152. Luegmayr E, Glantschnig H, Wesolowski GA, Gentile MA, Fisher JE, Rodan GA, Reszka AA.. Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death Differ 2004;11(Suppl. 1):S108–S118. [DOI] [PubMed] [Google Scholar]
- 153. Fontaine MAC, Westra MM, Bot I, Jin H, Franssen AJPM, Bot M, de Jager SCA, Dzhagalov I, He Y-W, van Vlijmen BJM, Gijbels MJJ, Reutelingsperger CP, van Berkel TJC, Sluimer JC, Temmerman L, Biessen EAL.. Low human and murine Mcl-1 expression leads to a pro-apoptotic plaque phenotype enriched in giant-cells. Sci Rep 2019;9:14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tintut Y, Morony S, Demer LL.. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol 2004;24:e6–e10. [DOI] [PubMed] [Google Scholar]
- 155. Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, Jang MY, Jang HS, Yun TJ, Lee SH, Yoon WK, Prat A, Seidah NG, Choi J, Lee SP, Yoon SH, Nam JW, Seong JK, Oh GT, Randolph GJ, Artyomov MN, Cheong C, Choi JH.. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ Res 2018;123:1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM.. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999;397:315–323. [DOI] [PubMed] [Google Scholar]
- 157. Deuell KA, Callegari A, Giachelli CM, Rosenfeld ME, Scatena M.. RANKL enhances macrophage paracrine pro-calcific activity in high phosphate-treated smooth muscle cells: dependence on IL-6 and TNF-α. J Vasc Res 2012;49:510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y.. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res 2012;111:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lin ME, Chen TM, Wallingford MC, Nguyen NB, Yamada S, Sawangmake C, Zhang J, Speer MY, Giachelli CM.. Runx2 deletion in smooth muscle cells inhibits vascular osteochondrogenesis and calcification but not atherosclerotic lesion formation. Cardiovasc Res 2016;112:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Davaine J-M, Quillard T, Brion R, Lapérine O, Guyomarch B, Merlini T, Chatelais M, Guilbaud F, Brennan MÁ, Charrier C, Heymann D, Gouëffic Y, Heymann M-F.. Osteoprotegerin, pericytes and bone-like vascular calcification are associated with carotid plaque stability. PLoS One 2014;9:e107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hunt JL, Fairman R, Mitchell ME, Carpenter JP, Golden M, Khalapyan T, Wolfe M, Neschis D, Milner R, Scoll B, Cusack A, Mohler ER.. Bone formation in carotid plaques. Stroke 2002;33:1214–1219. [DOI] [PubMed] [Google Scholar]
- 162. Forbang NI, Michos ED, McClelland RL, Remigio-Baker RA, Allison MA, Sandfort V, Ix JH, Thomas I, Rifkin DE, Criqui MH.. Greater volume but not higher density of abdominal aortic calcium is associated with increased cardiovascular disease risk: MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging 2016;9:e005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ikegami Y, Inoue I, Inoue K, Shinoda Y, Iida S, Goto S, Nakano T, Shimada A, Noda M.. The annual rate of coronary artery calcification with combination therapy with a PCSK9 inhibitor and a statin is lower than that with statin monotherapy. NPJ Aging Mech Dis 2018;4:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review article does not contain new original data.