Abstract
Introduction
To evaluate the 6-month efficacy and safety of 650 nm low-level red light (LLRL) for myopia control in children.
Methods
This was a single-center, single-masked randomized controlled trial. A total of 224 children aged 6–12 years with spherical equivalent error (SER) of − 6 diopter (D) to − 0.5 D were enrolled, and were randomized to LLRL group or control group. Children in the LLRL group underwent treatment twice daily, each lasting for 3 min, there was an interval of at least 4 h between treatments. Children in both groups were allowed to wear single-vision spectacles; no additional intervention was given to the control. The primary outcomes included change in cycloplegic SER and change in axial length (AL) during 6 months.
Results
The median 6-month changes in AL of the LLRL and control groups were − 0.06 mm (interquartile range, IQR − 0.15, 0) and 0.14 mm (IQR 0.07, 0.22), respectively. The difference between groups was significant (Z = 10.021, p < 0.001). The median 6-month changes in SER were 0.125 D (IQR 0, 0.375) and − 0.25 D (IQR − 0.5, 0) for the LLRL and control groups, respectively. The difference between groups was significant (Z = 8.827, p < 0.001). Compared with the control, the proportion of children with hyperopic shift in the LLRL group was higher (51.65% vs. 3.41%, p < 0.001), and the proportion of children with shortened AL in the LLRL group was higher (63.74% vs. 2.27%, p < 0.001). No adverse event was observed.
Conclusion
650 nm LLRL significantly slowed down the myopia progression in children aged 6–12 years, and there was no observable side effect in the short term.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-022-00585-w.
Keywords: Myopia, Low-level red light, Children, Randomized controlled trial
Key Summary Points
| Why carry out this study? |
| Myopia is a worldwide public health issue and it is estimated that nearly half of the world’s population could be myopic in 2050. There is no perfect myopia intervention for large populations for the time being. |
| The study asked whether 650 nm LLRL is effective and safe for myopia control in children. |
| What was learned from the study? |
| 650 nm LLRL significantly slowed down the myopia progression in children aged 6–12 years, without any side effect. |
| A large percentage of children treated with 650 nm LLRL showed hyperoptic shift, indicating that 650 nm LLRL has a strong effect on myopia control for children. |
Introduction
Myopia is a worldwide public health issue. China alone has a myopic population that is over 0.4 billion [1]. The prevalence of myopia is as high as 83.2% in Chinese university students aged 16–26 years [2]. An effective and safe intervention for myopia control is of great significance. Currently, there are dozens of myopia interventions, and these can be divided into two categories considering whether they could limit myopia progression.
Single vision spectacle lenses (SVS) [3, 4], refractive surgeries [5–7], and implantable collamer lens (ICL) [8, 9] are widely used for myopia correction but could barely slow down the myopia progression. Interventions limiting myopia progression mainly include orthokeratology [10], different kinds of contact lenses [11, 12], peripheral defocus modifying spectacle lenses [13], pharmacy therapy represented by atropine eye drops [14–16], and more outdoor time [17, 18]. Among these, atropine eye drops might be a better option. Atropine was reported to be most effective [17, 18] among the aforementioned choices, it was not as expensive as orthokeratology or contact lenses [19, 20], and it does not require so much extracurricular time to be effective compared with outdoor activity [20]. However, the main problem with atropine eye drops is that low doses have limited effects while high doses come with rebound effects once the treatment is stopped [21]. Besides, there is a risk of side effects such as photophobia [22].
Recently, a new noninvasive solution was proposed, which is 650 nm low-level red light (LLRL). Its effect on slowing down myopia progression has been preliminarily demonstrated in a randomized controlled trial [23]. However, more evidence is needed to confirm its application prospects. The present study aims to explore the efficacy and safety of the 650 nm LLRL for myopia control in children aged 6–12 years.
Methods
Study Design
This was a single-center, single-masked randomized controlled trial. A total of 224 children were enrolled between August 12, 2021 and September 3, 2021 in Beijing Tongren Hospital in Beijing, China. Children were randomly allocated to the LLRL or the control group at a ratio of 1:1. We used stratified block randomization, with a block length of eight. Because of the distinct difference between LLRL and SVS, participants knew whether they were assigned to the experimental group or not, as did the investigators. Measurement of outcomes was done by independent investigators for masking purposes. The statistician was also masked to allocation. The 6-month measurement was done between February 22 and March 15, 2022.
This study was approved by the Ethics Committee, Beijing Tongren Hospital, Capital Medical University (No. TRECKY2021-239). Informed written consent was obtained from children’s parents. This clinical trial adhered to the tenets of the Declaration of Helsinki.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) age 6–12 years; (2) cycloplegic spherical equivalent error (SER) of − 6 diopter (D) to − 0.5 D (> − 6 D, ≤ − 0.5 D) in both eyes; (3) astigmatism of 2.50 D or less; (4) willingness to participate in the study and sign an informed consent form.
Exclusion criteria: (1) underwent refractive surgeries or ICL implantation; (2) undergoing other interventions for myopia, including atropine eye drops and orthokeratology; (3) anisometropia (refractive difference spherical lens > 1.50 D in both eyes), strabismus, and amblyopia; (4) refractive media opacification (keratopathy, lens opacity, etc.); (5) allergic to cycloplegia.
Outcomes
Primary outcomes included change in axial length (AL) and change in the cycloplegic SER. The SER was calculated as sphere + 0.5 × cylinder. In the present study, a positive change was called a hyperopic shift, whereas a negative change was called a myopic shift. Children were classified into three subgroups: improved (equal to AL shortened, or hyperopic shift), worsened (equal to AL elongated, or myopic shift), or unchanged.
Secondary outcomes included change in choroidal thickness (ChT), flat keratometry (K1), and change in steep keratometry (K2). Outcomes were measured at both baseline and 6-month follow-up.
Adverse events referred to any uncomfortable symptom, including but not limited to photophobia, eye itching, burning sensation, dry eye, blurred vision, glare, dazzling, keratitis, and conjunctivitis. Participants were asked to give feedback on any symptom during the treatment. At the time of 6-month follow-up, all participants were contacted to confirm whether they experienced any adverse events during the trial.
Outcomes Measurements
Cycloplegic refraction: The refractive error was measured by cycloplegia. Autorefraction measurement was done with an autorefractor (ARK-510A; Nidek Co. Ltd, Aichi, Japan).
Measurements of AL, central corneal thickness, anterior chamber depth, length thickness, K1, and K2 were done with an optical biometer (Lenstar LS 900; HAAG-STREIT AG, Switzerland). Measurements of intraocular pressure were done with a non-contact tonometer (Canon TX-20; Canon Inc., Tokyo, Japan). Measurements for ChT were done using optical coherence tomography (Spectralis HRA + OCT, Heidelberg Engineering, Germany) at nine locations of the fundus as follows: subfoveal ChT, and locations at 1 mm and 3 mm from the fovea, in four directions. Both baseline and follow-up measurements were done by the same group of trained investigators.
Intervention and Study Procedures
Eligible participants were randomized into the LLRL group or the control group, following a random sequence generated using R software. In the LLRL group, children underwent treatment twice a day, each of which lasted 3 min. The interval between two treatments was 4 h or more. The light source used was a single-wavelength (650 nm) weak red-light laser, with low intensity, and whose radiation category is Class 1 light. Such light can be used safely in eyes and this has been verified by the State Administration for Market Regulation of China. The light source was integrated on a head-mounted device (Product Name: Myopia and Amblyopia Treatment Apparatus. Product Model: YF020A. The Medical Device Registration No. 20212162067. Manufacturer: Hunan EnVan Technology Co.,Ltd. The details are available in Supplementary Material), which is a patented technology of Beijing Tongren Hospital (patent number ZL202022533301.4). In both groups, children with myopia were allowed to wear SVS. For the control group, no additional intervention was given.
The present trial was registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/), registration number ChiCTR2200058963.
Definition of Benefit
In the present study, we estimated the average benefit brought by 650 nm LLRL compared with the control, which was calculated as follows:
where B is the benefit; Mt is the mean value of 6-month change in primary outcomes of the LLRL group if data is normally distributed, otherwise the median value would be used; and Mc is the mean value of six-month change in primary outcomes of the control group if data is normally distributed, otherwise the median value would be used.
Statistical Analysis
The data of the right eye was arbitrarily used for analysis (e.g., comparison of the change in the AL between the LLRL group and the control group). Missing data at the 6-month measurement was not imputed. The Shapiro–Wilk test was used to check for normality of continuous variables. The mean values and standard deviations (SD) were used for the statistical description of normally distributed continuous variables; otherwise, the median values and interquartile range (IQR) were used. Accordingly, the t test or Wilcoxon rank-sum test was used for the comparison of continuous outcomes between groups. Frequency and percentage were used for the statistical description of the categorical variables. The chi-square test or Fisher exact test (if the chi-square test was not applicable) was used for comparison of categorical variables between groups. A linear regression model was used to explore the association between age and change in primary outcomes. Analysis was done using the open-source R program (https://www.r-project.org/, version 4.2.0). The significance level was set to be 0.05, two-tailed. For multiple comparisons, P values were adjusted according to the Bonferroni criteria.
Results
Characteristics of Participants
Initially, 293 children were assessed for eligibility between August 12 and September 3, 2021, in Beijing Tongren Hospital in Beijing, China. The 32 children who were undergoing treatment with low-dose atropine eye drops or orthokeratology were excluded. The same applied to 16 with ineligible SER, 8 who refused to sign the informed consent form, 6 who were not within the required age range, 6 with anisometropia, as well as one with allergic history of cycloplegia. Finally, 224 children were enrolled and randomized to either the LLRL group or the control group. The participant selection process is shown in Fig. 1. The average age of the participants was 9.57 ± 1.62 years (median 9 years, range 6–12 years). Boys accounted for 50% (112/224). The baseline information, including age, gender, body mass index, SER, AL, intraocular pressure, central corneal thickness, anterior chamber depth, length thickness, K1, K2, and astigmatism, is shown in Table 1. There was no significant difference in baseline information between the two groups.
Fig. 1.
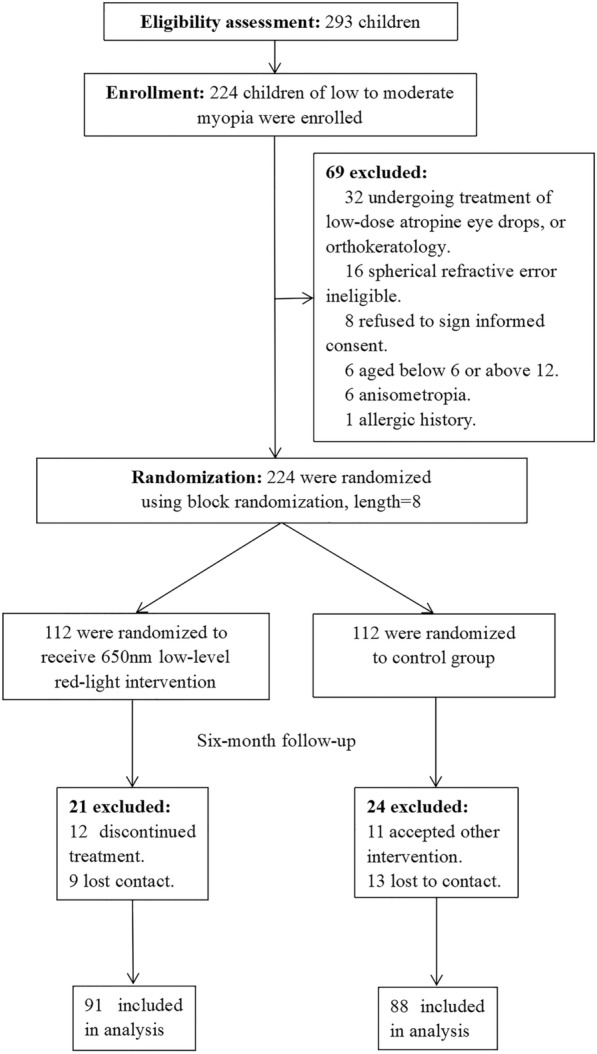
Flowchart of the process of participant selection
Table 1.
Children’s demographic characteristics and ocular parameters at baseline
| Variables | Control | Treatment | Statistics | p |
|---|---|---|---|---|
| Gender | χ2 = 0.071 | 0.789 | ||
| Male | 57 (50.89%) | 55 (49.11%) | ||
| Female | 55 (49.11%) | 57 (50.89%) | ||
| Age (years) | 9.47 ± 1.59 | 9.66 ± 1.65 | t = − 0.867 | 0.387 |
| Body mass index (kg/m2) | 17.97 ± 3.89 | 17.63 ± 3.62 | t = 0.682 | 0.496 |
| Spherical equivalent error (D) | − 2 (− 2.75, − 1.25) | − 2 (− 3.25, − 1.25) | Z = 0.364 | 0.716 |
| Axial length (mm) | 24.20 ± 0.85 | 24.31 ± 0.92 | t = − 0.901 | 0.369 |
| Intraocular pressure (mmHg) | 16 (14, 18) | 16 (14, 17) | Z = − 0.499 | 0.618 |
| Central corneal thickness (μm) | 550.34 ± 30.96 | 546.81 ± 31.42 | t = 0.846 | 0.398 |
| Anterior chamber depth (mm) | 3.22 ± 0.22 | 3.23 ± 0.22 | t = − 0.416 | 0.678 |
| Length thickness (mm) | 3.36 (3.28, 3.44) | 3.32 (3.24, 3.46) | Z = − 1.119 | 0.263 |
| Subfoveal choroid thickness (μm) | 296 (244, 352) | 290.5 (242, 352.5) | Z = − 0.626 | 0.531 |
| K1 (D) | 43 ± 1.29 | 43.02 ± 1.43 | t = − 0.119 | 0.905 |
| K2 (D) | 44.26 ± 137 | 44.22 ± 1.59 | t = 0.196 | 0.845 |
| Astigmatism (D) | 1.27 (0.90, 1.56) | 1.16 (0.82, 1.48) | Z = 1.332 | 0.183 |
For normally distributed continuous variables, data are expressed as mean ± SD, otherwise data are expressed as median and interquartile range
Six-Month Changes in Primary Outcomes
During follow-up, 18.8% (21/112) of participants in the LLRL group and 21.4% (24/112) of participants in the control group were lost. There was no significant difference in the follow-up rate between the two groups (p = 0.617).
The median 6-month changes in AL of the LLRL and control groups were − 0.06 mm (IQR − 0.15, 0) and 0.14 mm (IQR 0.07, 0.22), respectively (Fig. 2). The difference between groups was significant (Z = 10.021, p < 0.001). The median 6-month changes in SER were 0.125 D (IQR 0, 0.375) and − 0.25 D (IQR − 0.5, 0) for the LLRL and control groups, respectively. The difference between groups was significant (Z = 8.827, p < 0.001).
Fig. 2.
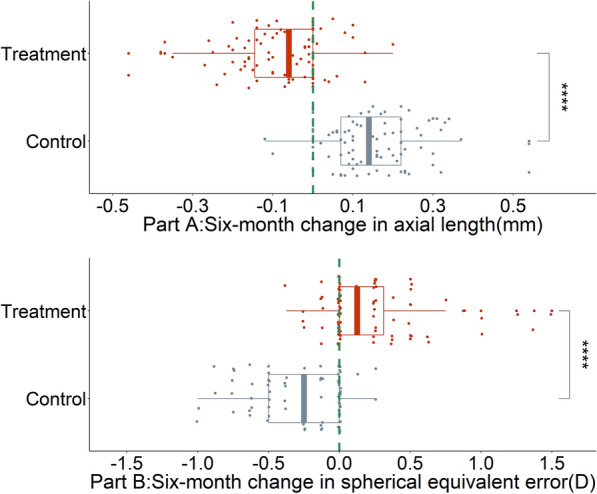
Changes in primary outcomes in 6 months. Six-month change in a axial length and b spherical equivalent error
Proportions of Children with Improved, Worsened, or Unchanged Primary Outcomes
A hyperopic shift was observed in 51.65% (47/91) of children in the LLRL group and 3.41% (3/88) in the control group (Table 2), the difference was significant (χ2 = 51.715, p < 0.001). On the other hand, a myopic shift was noted in 65.91% (58/88) of participants in the control group and 10.99% (10/91) participants in the LLRL group. Again, the difference was significant (χ2 = 57.281, p < 0.001).
Table 2.
Proportions of children with improved, worsened, or unchanged primary outcomes
| Outcomes | Control | LLRL | χ2 | p | padjusted |
|---|---|---|---|---|---|
| Spherical equivalent error | |||||
| Improved (hyperopic shift) | 3 (3.41%) | 47 (51.65%) | 51.715 | < 0.001 | < 0.001 |
| Unchanged | 27 (30.68%) | 34 (37.36%) | 0.889 | 0.346 | 0.99 |
| Worsened (myopic shift) | 58 (65.91%) | 10 (10.99%) | 57.281 | < 0.001 | < 0.001 |
| Axial length | |||||
| Improved (shortened) | 2 (2.27%) | 58 (63.74%) | 75.843 | < 0.001 | < 0.001 |
| Unchanged | 11 (12.50%) | 21 (23.08%) | 3.409 | 0.065 | 0.195 |
| Worsened (elongated) | 75 (85.23%) | 12 (13.19%) | 92.944 | < 0.001 | < 0.001 |
Data are expressed as no. (%)
LLRL 650 nm low-level red light
P values were adjusted according to Bonferroni criteria because of multiple comparisons
In the LLRL group, the AL of 63.74% (58/91) of children was shortened (Table 2), while this proportion was 2.27% (2/88) in the control group; the difference was significant (χ2 = 75.843, p < 0.001). The AL of 85.23% (75/88) of children in the control group was elongated, while the proportion was 13.19% (12/91) in the LLRL group; the difference was significant (χ2 = 92.944, p < 0.001).
Degree of Hyperopic Shift and Myopic Shift
Further, we classified the progression on SER into seven different subgroups at an interval of 0.5 D: hyperopic shift of 1 D or greater (≥ 1 D), hyperopic shift of 0.5 D to 1 D (≥ 0.5 D, < 1 D), hyperopic shift of 0 D to 0.5 D (> 0 D, < 0.5 D), unchanged (0 D), myopic shift within 0.5 D (> − 0.5 D, < 0 D), myopic shift of 0.5 D to 1 D (> − 1 D, ≤ − 0.5 D), and myopic shift of 1 D or more (≤ − 1 D). The results of the LLRL group and control group following the aforementioned order were as follows: 5.49% (5/91) vs. 0%, 14.29% (13/91) vs. 0%, 31.87% (29/91) vs. 3.41% (3/88), 37.36% (34/91) vs. 30.68% (27/88), 10.99% (10/91) vs. 37.50% (33/88), 0% vs. 26.14% (23/88), 0% vs. 2.27% (2/88) (Fig. 3).
Fig. 3.
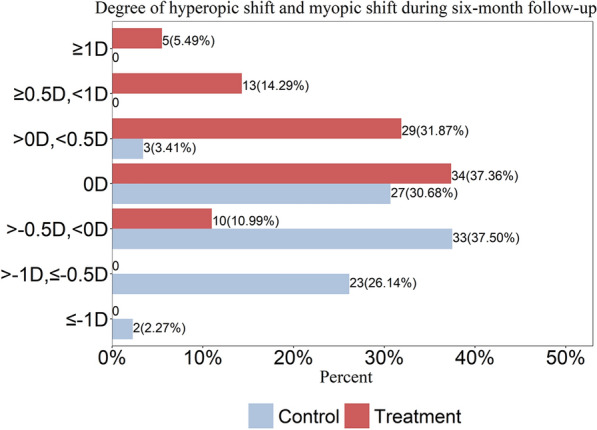
Degree of hyperopic shift and myopic shift during 6-month follow-up
Six-Month Changes in Secondary Outcomes
The 6-month changes in subfoveal ChT of the LLRL group and control group were 15 μm (IQR − 3, 34.5) and − 7 μm (IQR − 28, 14.5), respectively. The difference was significant (Z = 4.085, p < 0.001). The 6-month changes in ChT at eight other locations are shown in Table 3; all locations showed a significant difference between groups except for 1 mm in the inferior direction from the fovea (padjusted = 0.747).
Table 3.
Six-month changes of secondary outcomes in LLRL group and control group
| Change in secondary outcomes | Control | LLRL | Z | p | padjusted |
|---|---|---|---|---|---|
| ChT (μm; location, subfoveal) | − 7 (− 28, 14.5) | 15 (− 3, 34.5) | 4.085 | < 0.001 | < 0.001 |
| ChT (μm; location 3 mm temporal) | − 5 (− 31.5, 15) | 14 (− 9, 37.5) | 3.410 | < 0.001 | 0.006 |
| ChT (μm; location, 1 mm temporal) | − 4 (− 25, 11.5) | 13 (− 8, 37.5) | 3.552 | < 0.001 | 0.004 |
| ChT (μm; location, 1 mm nasal) | − 9 (− 31, 14) | 10.5 (− 8, 26) | 3.602 | < 0.001 | 0.003 |
| ChT (μm; location, 3 mm nasal) | − 5 (− 19, 7) | 4.5 (− 7.5, 17.5) | 2.865 | 0.004 | 0.036 |
| ChT (μm; location, 3 mm inferior) | 0 (− 21.5, 26) | 17 (0.5, 35) | 3.088 | 0.002 | 0.018 |
| ChT (μm; location, 1 mm inferior) | − 2 (− 31, 21) | 6 (− 16, 31.5) | 1.732 | 0.083 | 0.747 |
| ChT (μm; location, 1 mm superior) | − 2.5 (− 24, 22) | 12 (− 8, 35) | 2.848 | 0.004 | 0.036 |
| ChT (μm; location, 3 mm superior) | 2.5 (− 21.5, 26) | 17 (2, 39.5) | 2.723 | 0.005 | 0.045 |
| K1 (D) | − 0.02 (− 0.11, 0.06) | − 0.06 (− 0.14, 0.04) | − 1.442 | 0.149 | – |
| K2 (D) | − 0.06 (− 0.20, 0.05) | 0.01 (− 0.17, 0.14) | 0.422 | 0.673 | – |
Data are described as median value (interquartile range)
LLRL 650 nm low-level red light, ChT choroidal thickness, K1 flat keratometry, K2 steep keratometry
The 6-month changes in K1 of the LLRL and control groups were − 0.06 D (IQR − 0.14, 0.04) and − 0.02 D (IQR − 0.11, 0.06), respectively. The difference was not significant (Z = − 1.442, p = 0.149).
The 6-month changes in K2 of the LLRL and control groups were 0.01 D (IQR − 0.17, 0.14) and − 0.06 D (IQR − 0.20, 0.05), respectively. The difference was not significant (Z = 0.422, p = 0.673).
Stratification Analysis by Gender and Age
Among the children who underwent LLRL intervention, the median changes in SER were 0 D (IQR 0, 0.375) and 0.125 D (IQR 0, 0.25) for boys and girls, respectively; the difference between gender was not significant (Z = 0.614, p = 0.539). The 6-month changes in AL were − 0.03 mm (IQR − 0.14, 0) and − 0.07 mm (IQR − 0.16, 0) for boys and girls, respectively; the difference between gender was not significant either (Z = − 0.459, p = 0.646).
Among the children who underwent LLRL intervention, there was no statistical difference in 6-month changes in SER (H = 6.717, p = 0.348) or AL (H = 9.306, p = 0.157) among different age groups either.
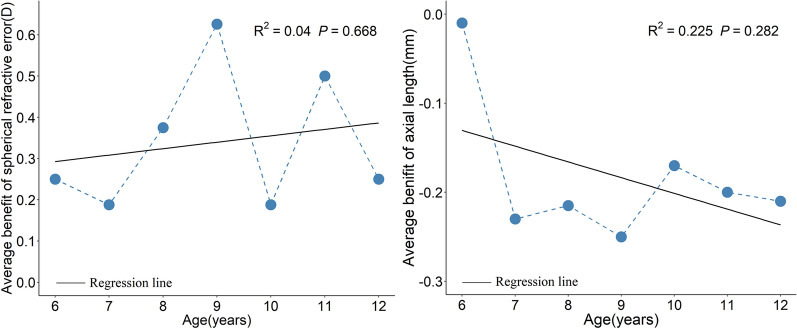
We further explored the association between age and the 6-month benefit brought by LLRL compared with the control. Overall, the 6-month benefits on AL and SER were 0.20 mm and 0.375 D. There was no significant association between the benefit on SER with age (β = 0.199, R2 = 0.04, p = 0.668), nor between the benefit on AL with age (β = − 0.024, R2 = 0.225, p = 0.282) (Fig. 4).
Fig. 4.
Six-month benefit on axial length and spherical refractive error brought by 650 nm low-level red light
Discussion
The present study revealed that a twice-daily, 3-min 650 nm LLRL intervention significantly slowed down the progression of myopia. On average, the AL of children who underwent 650 nm LLRL treatment during 6 months was shortened by 0.08 mm (median 0.06 mm); the SER showed a hyperopic shift of 0.20 D (median 0.125 D).
The effect of 650 nm LLRL for myopia control might be more powerful than other currently available non-invasive interventions, such as more outdoor activity, orthokeratology, and atropine eye drops. Outdoor time has been receiving wide attention in recent years. He et al. [17] reported that more outdoor time indeed slowed down the myopia progression compared with the control. However, on average, children still experienced a − 1.42 D myopic shift during the 3-year follow-up, and a 0.95 mm elongation of AL, despite an additional 40 min of outdoor activity being added to each school day. In comparison, outdoor activity seemed less effective than 650 nm LLRL, let alone the challenge to implement 40 min of additional outdoor activity given the pressure of students’ academic work. Orthokeratology is another common choice for myopia control, but its effect seemed inferior to 650 nm LLRL, too. A review [24] based on 13 studies summarized that the AL of children treated with orthokeratology typically elongated by approximately 0.15 mm/year. A network meta-analysis (NMA) [24] compared 16 myopia interventions, including atropine eye drops, orthokeratology, outdoor activity, peripheral defocus modifying contact lenses, bifocal spectacle lenses, and so on. This NMA deduced that high-dose atropine eye drops (1% and 0.5%) showed the strongest control effect for myopia, followed by moderate dose and low dose atropine eye drops. 650 nm LLRL was not evaluated in this NMA since no relative study was published at that time. However, although atropine eye drops ranked first for myopia control, studies reported that most children undergoing such treatment experienced myopia progression [25–29]. It was estimated that children treated with atropine eye drops showed a myopic shift of between − 0.63 and − 0.16 D/year on average. In comparison, among children treated with 650 nm LLRL in the present study, only 13.19% experienced AL elongation after 6 months treatment, only 10.99% experienced SER progression. On average, the shortened AL was equal to 0.16 mm/year, the hyperopic shift was equal to 0.40 D/year. Moreover, in the LLRL group, nearly 20% of children showed a hyperopic shift of ≥ 0.5 D, nearly 50% of children’s AL was shortened by 0.05 mm or more. The benefit brought by 650 nm LLRL was inspiring. Similarly, another randomized controlled trial reported that 32.9% of children treated with 650 nm LLRL showed clinically significant AL shortening (> 0.05 mm) after 6 months [23]. Zhou et al. [30] followed 105 myopic children aged 9.19 ± 2.40 years treated with 650 nm LLRL or SVS for 9 months. The average SER of the LLRL group increased from − 3.09 to − 2.87 D, while for the SVS group the average SER decreased from − 3.04 to − 3.57 D. Xiong et al. [31] reported that after 6 months treatment of 650 nm LLRL, the AL of children (aged 7–15) was shortened by 0.06 mm on average, while in the SVS group and the orthokeratology group, the average AL was elongated by 0.23 mm and 0.06 mm, respectively. On the basis of the results of short-term follow-up, 650 nm LLRL intervention not only had a promising control effect for myopia but also seemed to be safe at the same time. No complication was reported during 650 nm LLRL treatment by a previous study [23] or by the present study. In comparison, it is well known that orthokeratology comes with the risk of adverse events like infective keratitis [32]. Atropine eye drops come with the risk of photophabia, and so on [15, 25, 26].
The pathogenesis of myopia is a complex process involving genetic and environmental factors, as well as gene–environment interactions. To date, we know relatively little about how LLRL affects refractive development in humans. In the present study, we found no difference in changes in K1 and K2 between the LLRL and control groups, indicating that slowed myopia progression was mainly due to AL shortening, instead of corneal curvature flattening. One widely accepted hypothesis suggests that bright light increases the synthesis and release of dopamine in the retina [33]. Dopamine acts as a termination signal in refractive ocular development [34]. Besides, the dopamine in the retina may induce choroidal thickening and ocular growth inhibition via the release of nitric oxide from the retina or choroid [35, 36]. This slows the development of myopia. Previous studies reported a significant increase in choroidal thickness after 650 nm LLRL treatment. Similarly, Gawne et al. [37] observed a refractive shift produced by a reduction in the depth of the vitreous chamber, coupled with an increase in the thickness of the choroid. The present study showed similar findings. However, it was worth mentioning that by the present study the AL of children in the LLRL group was thickened by 60 μm, while the choroidal thickness was only thickened 4.5–17 μm at different locations. This indicates that the growth of choroidal thickness could only partially explain the AL shortening during 650 nm LLRL treatment.
Conclusion
Overall, current evidence suggested that 650 nm LLRL is an effective and safe solution for myopia control in the short term. The present study revealed the clinical significance of 650 nm LLRL for myopia control—it not only slowed down the myopia progression but also reversed the myopia progression in a large proportion of children, without any adverse event. In the future, continuous longitudinal follow-up is still required to evaluate the long-term efficacy and safety of this treatment.
The strengths of this study included a single-masked randomized controlled trial design, the standardized measurement of refraction with cycloplegia, and the inclusion of primary outcome of change in the AL. Moreover, this is the first trial that estimates the benefit on AL and SER brought by 650 nm LLRL among children aged 6–12 years. One shortcoming of our study is that the follow-up duration was short, so the long-term efficacy and safety of 650 nm LLRL for myopia control remains to be estimated. Besides, we do not know whether there is a rebound effect similar to atropine eye drops once treatment is stopped, which is a knowledge gap for future study.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the participants for their involvement and the investigators for their diligence.
Funding
The present study was funded by Capital Health Research and Development of Special (Ying Jie 2022-1-1081). The study sponsor is also funding the journal’s Rapid Service Fee.
Author Contributions
All authors contributed to the material preparation and data collection. Data analysis was performed by Kai Cao. The first draft of the manuscript was written by Kai Cao and Lei Tian. All authors read and approved the final manuscript.
Disclosures
Lei Tian, Kai Cao, Dong-Li Ma, Shi-Qiang Zhao, Li-Xin Lu, Ao Li, Chang-Xi Chen, Chun-Rong Ma, Zhang-Fang Ma, Ying Jie have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee, Beijing Tongren Hospital, Capital Medical University (No. TRECKY2021-239). Informed written consent was obtained from children’s parents. This clinical trial adhered to the tenets of the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Lei Tian and Kai Cao contributed equally to this study.
References
- 1.Dolgin E. The myopia boom. Nature. 2015;519(7543):276–278. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 2.Wei S, Sun Y, Li S, et al. Refractive errors in university students in central China: the Anyang University students eye study. Invest Ophthalmol Vis Sci. 2018;59(11):4691–4700. doi: 10.1167/iovs.18-24363. [DOI] [PubMed] [Google Scholar]
- 3.Qin Z, Peng T, Zhang Z, et al. Myopia progression and stabilization in school-aged children with single-vision lenses. Acta Ophthalmol. 2022;100(4):e950–e956. doi: 10.1111/aos.15038. [DOI] [PubMed] [Google Scholar]
- 4.Bao J, Huang Y, Li X, et al. Spectacle lenses with aspherical lenslets for myopia control vs single-vision spectacle lenses: a randomized clinical trial. JAMA Ophthalmol. 2022;140(5):472–478. doi: 10.1001/jamaophthalmol.2022.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang B, Valerio GS, Manche EE. Prospective, randomized contralateral eye comparison of wavefront-guided laser in situ keratomileusis and small incision lenticule extraction refractive surgeries. Am J Ophthalmol. 2022;237:211–220. doi: 10.1016/j.ajo.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Wen D, McAlinden C, Flitcroft I, et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: a network meta-analysis. Am J Ophthalmol. 2017;178:65–78. doi: 10.1016/j.ajo.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya K, Igarashi A, Hayashi K, et al. A multicenter prospective cohort study on refractive surgery in 15 011 eyes. Am J Ophthalmol. 2017;175:159–168. doi: 10.1016/j.ajo.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Plaza E, Lopez-Miguel A, Lopez-de LRA, et al. Effect of the EVO+ Visian phakic implantable collamer lens on visual performance and quality of vision and life. Am J Ophthalmol. 2021;226:117–125. doi: 10.1016/j.ajo.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Ghoreishi M, Kashfi A, Peyman M, et al. Comparison of TORIC implantable collamer lens and toric artiflex phakic IOLs in terms of visual outcome: a paired contralateral eye study. Am J Ophthalmol. 2020;219:186–194. doi: 10.1016/j.ajo.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Cho P, Tan Q. Myopia and orthokeratology for myopia control. Clin Exp Optom. 2019;102(4):364–377. doi: 10.1111/cxo.12839. [DOI] [PubMed] [Google Scholar]
- 11.Sankaridurg P. Contact lenses to slow progression of myopia. Clin Exp Optom. 2017;100(5):432–437. doi: 10.1111/cxo.12584. [DOI] [PubMed] [Google Scholar]
- 12.Logan N. Myopia control and contact lenses. Cont Lens Anterior Eye. 2020;43(1):3. doi: 10.1016/j.clae.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Sankaridurg P, Donovan L, Varnas S, et al. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optom Vis Sci. 2010;87(9):631–641. doi: 10.1097/OPX.0b013e3181ea19c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yam JC, Jiang Y, Lee J, et al. The association of choroidal thickening by atropine with treatment effects for myopia: two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study. Am J Ophthalmol. 2022;237:130–138. doi: 10.1016/j.ajo.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Chia A, Chua WH, Wen L, et al. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5% Am J Ophthalmol. 2014;157(2):451–457. doi: 10.1016/j.ajo.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Uzun S, Uzun F. Comment on: The association of choroidal thickening by atropine with treatment effects for myopia: two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study. Am J Ophthalmol. 2022;241:290. [DOI] [PubMed]
- 17.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314(11):1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 18.Cao K, Wan Y, Yusufu M, et al. Significance of outdoor time for myopia prevention: a systematic review and meta-analysis based on randomized controlled trials. Ophthalmic Res. 2020;63(2):97–105. doi: 10.1159/000501937. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123(4):697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Foo LL, Lanca C, Wong CW, et al. Cost of myopia correction: a systematic review. Front Med (Lausanne) 2021;8:718724. doi: 10.3389/fmed.2021.718724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran H, Tran YH, Tran TD, et al. A review of myopia control with atropine. J Ocul Pharmacol Ther. 2018;34(5):374–379. doi: 10.1089/jop.2017.0144. [DOI] [PubMed] [Google Scholar]
- 22.Wu PC, Chuang MN, Choi J, et al. Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond) 2019;33(1):3–13. doi: 10.1038/s41433-018-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y, Zhu Z, Tan X, et al. Effect of repeated low-level red-light therapy for myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2021 doi: 10.2139/ssrn.3800007. [DOI] [PubMed] [Google Scholar]
- 24.VanderVeen DK, Kraker RT, Pineles SL, et al. Use of orthokeratology for the prevention of myopic progression in children: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(4):623–636. doi: 10.1016/j.ophtha.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Q, Tang Y, Guo L, et al. Efficacy and safety of 1% atropine on retardation of moderate myopia progression in Chinese school children. Int J Med Sci. 2020;17(2):176–181. doi: 10.7150/ijms.39365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2) Ophthalmology. 2012;119(2):347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Fu A, Stapleton F, Wei L, et al. Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol. 2020;104(11):1535–1541. doi: 10.1136/bjophthalmol-2019-315440. [DOI] [PubMed] [Google Scholar]
- 28.Wei S, Li SM, An W, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138(11):1178–1184. doi: 10.1001/jamaophthalmol.2020.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yam JC, Jiang Y, Tang SM, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113–124. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Xing C, Qiang W, et al. Low-intensity, long-wavelength red light slows the progression of myopia in children: an Eastern China-based cohort. Ophthalmic Physiol Opt. 2022;42(2):335–344. doi: 10.1111/opo.12939. [DOI] [PubMed] [Google Scholar]
- 31.Xiong F, Mao T, Liao H, et al. Orthokeratology and low-intensity laser therapy for slowing the progression of myopia in children. Biomed Res Int. 2021;2021:8915867. doi: 10.1155/2021/8915867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leo SW. Current approaches to myopia control. Curr Opin Ophthalmol. 2017;28(3):267–275. doi: 10.1097/ICU.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 33.Cohen Y, Peleg E, Belkin M, et al. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–119. doi: 10.1016/j.exer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Nickla DL, Damyanova P, Lytle G. Inhibiting the neuronal isoform of nitric oxide synthase has similar effects on the compensatory choroidal and axial responses to myopic defocus in chicks as does the non-specific inhibitor L-NAME. Exp Eye Res. 2009;88(6):1092–1099. doi: 10.1016/j.exer.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekaran S, Cunningham J, Neal MJ, et al. Nitric oxide release is induced by dopamine during illumination of the carp retina: serial neurochemical control of light adaptation. Eur J Neurosci. 2005;21(8):2199–2208. doi: 10.1111/j.1460-9568.2005.04051.x. [DOI] [PubMed] [Google Scholar]
- 37.Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017;140:55–65. doi: 10.1016/j.visres.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.