Abstract
Melatonin has not only to be seen as a regulator of circadian clocks. In addition to its chronobiotic functions, it displays other actions, especially in cell protection. This includes antioxidant, anti-inflammatory, and mitochondria-protecting effects. Although protection is also modulated by the circadian system, the respective actions of melatonin can be distinguished and differ with regard to dose requirements in therapeutic settings. It is the aim of this article to outline these differences in terms of function, signaling, and dosage. Focus has been placed on both the nexus and the dissecting properties between circadian and noncircadian mechanisms. This has to consider details beyond the classic view of melatonin's role, such as widespread synthesis in extrapineal tissues, formation in mitochondria, effects on the mitochondrial permeability transition pore, and secondary signaling, for example, via upregulation of sirtuins and by regulating noncoding RNAs, especially microRNAs. The relevance of these findings, the differences and connections between circadian and noncircadian functions of melatonin shed light on the regulation of inflammation, including macrophage/microglia polarization, damage-associated molecular patterns, avoidance of cytokine storms, and mitochondrial functions, with numerous consequences to antioxidative protection, that is, aspects of high actuality with regard to deadly viral and bacterial diseases. Antioxid. Redox Signal. 37, 704–725.
Keywords: circadian, extrapineal, melatonin, miRNAs, mitochondria, sirtuins
Introduction
After the discovery of melatonin as a skin-lightening hormone in frogs and fish (154), the description of high-amplitude circadian rhythms of melatonin synthesis and secretion by vertebrate pineal glands (212) opened a new direction of research that was dominating this field for decades. Contrary to the other vertebrates, melatonin formation in the mammalian pineal gland was shown to be light-dependently controlled by a sympathetic input (277).
Further studies ultimately connected the mammalian pineal to the circadian pacemaker, the suprachiasmatic nucleus (SCN), which receives information on the light–dark cycle from melanopsin-containing retinal ganglion cells (21) and transmits this via the paraventricular nucleus, intermediolateral cell column of the upper thoracic cord, and superior cervical ganglion to the pineal (219). Further details and modulation by additional factors have been summarized elsewhere (83, 112). In the chronobiological context, melatonin was regarded as a “troll” among hormones that mediates the information of darkness and, thereby, contributes to the internal adjustment of body functions to environmental cyclicity, with regard to both circadian and seasonal rhythms (217–219).
This perception of melatonin is widely present in many researchers, although it is widely incomplete. First, melatonin is produced not only by the pineal gland but also by numerous, perhaps, almost all other organs (4, 112). In particular, the frequently read statement that the pineal gland is the main site of melatonin formation has to be dropped, because extrapineal melatonin exceeds the amounts in pineal gland and circulation by orders of magnitude (24, 112, 130). This is in accordance with the finding that melatonin is poorly released from most extrapineal sources, at least, under basal conditions (94).
Moreover, high levels of extrapineal melatonin are not generally cycling in a circadian fashion, and, if so, the amplitude is often too low or poorly phased to allow chronobiological effects. For instance, its temporal pattern in the rodent Harderian gland is almost flat, with only a short and transient drop directly after light onset (127). In the gastrointestinal tract, the amplitude is either much lower than in the pineal gland or almost absent, depending on species (112). Finally, the recent demonstration of intramitochondrial melatonin synthesis in neuronal tissue did not reveal circadian variations (255). Mitochondrial melatonin formation has meanwhile been shown to occur in several different cells and tissues, such as oocytes (123), choroid plexus (213), and even outside the animals (263).
Therefore, the question arises as to whether melatonin biosynthesis has to be seen as not being primarily coupled to circadian functions, but rather originally to redox metabolism. In the latter case, the circadian role of melatonin would appear as a secondary acquisition in the course of evolution. This would not be surprising, since metabolism in general, but also oxidant formation and antioxidative metabolism are often controlled by circadian oscillators (113).
Melatonin's relationship to redox metabolism is meanwhile an established field documented by numerous publications that quantitatively exceed those dealing with circadian issues. In its early beginnings, hints were obtained, showing the nonenzymatic oxidation of melatonin to N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) by photocatalytic mechanisms (110, 114, 118, 119). The topic of melatonin's role in redox metabolism gained considerable attention and developed an enormous drive after this molecule was shown to be an exceptionally potent hydroxyl radical scavenger (258).
However, although melatonin was, thereafter, shown to be highly protective in numerous experimental settings, free radical scavenging did not appear to be physiologically relevant, because of the low levels of this compound in the circulation. At that time, investigators usually believed that such low concentrations were applicable to all tissues and subcellular compartments. To resolve the contradiction between potent antioxidative protection and physiological levels apparently too low for efficient radical scavenging, melatonin's spectrum of actions was investigated with regard to other protective mechanisms.
In fact, melatonin was shown to upregulate antioxidant enzymes (82, 222, 226). Finally, it turned out that various different mechanisms contribute to the reduction of oxidant formation by melatonin, a concept referred to as radical avoidance (82, 112). In terms of organismal metabolic economy, decreasing the production of oxidants may have advantages over detoxification of oxidants already formed. However, the earlier conclusion that melatonin's levels are too small for the latter purpose has turned out to be precocious. First, it was shown that high levels of melatonin that suffice for free-radical scavenging and convey protection do exist in organisms outside the vertebrates, as first demonstrated for a dinoflagellate (11).
Later, even higher concentrations of melatonin were measured in several plants and some nonvertebrate animals (39, 116). A contribution of direct free radical detoxification is, therefore, of biological relevance. To what extent this is also applicable to mammals and other vertebrates may require further investigation. However, findings on higher tissue levels of melatonin and of mitochondrial accumulation (222, 263) let this possibility appear in a new light.
This outline indicates that the roles of melatonin have to be differently judged with regard to low levels in the circulation and higher levels in tissues and mitochondria, and also regarding high-amplitude rhythmicity in the pineal gland and circulation or low amplitudes and virtual absence of rhythmicity in, at least, some tissues and subcellular compartments. It is the aim of this article to discriminate these different roles of melatonin, including the respective mechanisms of action.
Concentration Matters: Receptor Saturation and Oversaturation
Melatonin has been used or tested preclinically and clinically for many different purposes, such as correction of circadian malfunction (12, 50, 57, 246, 272), sleep improvement (26, 233, 238, 303), mood disorders (23, 146, 241, 243, 271), prevention of metabolic syndrome and related pathologies (28, 74, 142, 189, 191), deceleration of aging (27, 89, 90, 253), cancer prevention (126, 159, 223, 256), and, in particular, protection against oxidative stress caused by overshooting inflammation (e.g., sepsis) (6, 71, 72, 59, 62, 105, 124), neuroinflammatory and excitotoxic pathologies (111, 144, 178, 251, 252), stroke and trauma (64, 216, 222, 239, 284), environmental toxins (221), and life-threatening viral diseases (121, 259).
In these highly divergent applications, melatonin was differently dosed, in adult humans, from 1 to 3 mg/day (84, 156) or even less (201) to 300 mg/day (275) or above (260), in newborns or animals in corresponding doses according to dose translation (260). The safety margin for human short-term treatment has been reported to amount to 3750 mg/day for an individual of 75 kg (192).
With regard to this extremely broad spectrum of doses that had been applied for different purposes, it seems necessary to make functional discriminations regarding modes of action. For fundamental reasons, it is immediately clear that several actions characterized by responses to low doses are explained by receptor-mediated signaling, whereas those requiring very high doses have to be explained by entirely different mechanisms. Reliable knowledge on melatonin signaling exists mainly for the G protein-coupled receptors (GPCRs) MT1 and MT2 (231, 232). Variations of signaling pathways regarding G protein subunits and downstream pathways have been summarized elsewhere (85).
Agonist affinities of these receptors are, of course, in accordance with melatonin concentrations in the body fluids. In blood plasma, these amount to 1 nM in some, but not all mammalian species, at the circadian melatonin maximum at night. However, they may be much lower in several species and also in aging humans and animals, whereas daytime values are anyway considerably decreased. According to the still relatively low nocturnal levels, pKi values of the two receptors for melatonin are typically found in the range between 9.5 and slightly above 10, that is, in good correspondence to physiological up- and down-variations around half-saturation (87, 137).
Several other proteins have also been shown to bind melatonin and some of them have been even claimed to represent low-affinity melatonin receptors. Among them, calmodulin (CaM) is the only one that has remained of actual interest. After its discovery as a melatonin-binding protein (19, 20), its capability of interacting with this ligand at reasonable concentrations was vividly discussed. However, it was shown that its affinity to melatonin is strongly increased after its interaction with CaM-activated proteins (145).
Moreover, the relevance of melatonin binding has not to be solely judged on the basis of blood concentrations. Meanwhile, it has been shown that melatonin concentrations in other body fluids and, correspondingly, in the releasing tissues often exceed blood levels by far (4, 222, 229, 257). These findings are in accordance with the demonstration of melatonin synthesis in mitochondria (123, 213, 255), an organelle known since long to also accumulate melatonin from extracellular sources (184). With regard to tissue and subcellular concentrations, melatonin signaling via low-affinity binding sites appears to be possible and should be systematically investigated.
Apart from CaM, several other binding sites have been discussed, but they are devoid of convincingly demonstrated receptor properties. The binding site of NRH:quinone oxidoreductase 2 (NQO2), formerly believed to represent another melatonin receptor (“MT3”), is not specific for melatonin and lacks, according to actual knowledge, any signaling pathway. Therefore, it does meet required criteria of a receptor (112).
Members of the retinoid orphan receptor (ROR) subfamily, which have been considered in numerous publications as “nuclear melatonin receptors,” can presumably be excluded, with certainty in the case of the most frequently investigated RORα, which has been shown to not bind melatonin (248, 249) and whose effects in the context of melatonin require alternate explanations (100). Other putative binding sites are insufficiently characterized, in terms of either protein chemistry or/and binding properties.
As a consequence of the binding properties of MT1 and MT2 receptors, only doses that lead to blood melatonin levels in the physiological range or slightly above can be expected to be suitable for generating meaningful MT1/MT2-mediated effects. This is particularly of importance for actions that affect circadian oscillators, because their dynamics comprises sequential increases and decreases of their intrinsic functions.
However, with regard to the short half-life of melatonin in the circulation, which is mostly between 20 and 45 min (29, 46), but can be lengthened by elevating doses (8), transient receptor oversaturation may still be compatible with circadian MT1/MT2-effects, as far as the dynamics of saturation and desaturation is not heavily disturbed. The possibility of receptor desensitization and internalization has been discussed, but it has not been finally clarified under the various conditions (85). Of course, the duration of melatonin's actions can be extended by using slow-release formulations or choosing nonoral routes of administration (289).
However, doses of, for example, 10 mg/kg that have been frequently used for protecting mice against experimental oxidative stress would be equivalent, after dose translation, to a human dose of about 60 mg/75 kg, that is, 20 or 30 times of the officially approved quantities (3 or 2 mg/pill). Even considerably higher doses have been applied to laboratory animals and to humans (260, 275). The problem has never been the tolerability of melatonin, but one cannot expect a reasonable chronobiological action by continuously oversaturating the melatonin receptors.
In numerous studies, high doses such as those mentioned had been reported to be required. This would be implausible for an MT1/MT2-mediated action, which should reach its maximum at receptor saturation. Therefore, in all the cases in which melatonin treatment by very high doses was found to be required for efficacy, the conclusion can only be that other mechanisms of action beyond these GPCRs have been decisive. This conclusion is of particular importance with regard to the influences on the circadian system. In humans, the entrainment of circadian rhythms was shown to be less efficient with 10 mg melatonin than with doses in the low mg range (156). Thus, increased doses do not enhance effects in the circadian context.
Moreover, if high doses are applied, conclusions should also not be based on effects by MT1 or MT2 antagonists. If a compound such as luzindole is tested, one should be aware of the pKi values that are lower than those of melatonin. To obtain a meaningful effect, the antagonist dose has to be elevated above that of melatonin. In cases in which very high melatonin doses are aimed to be blocked, caution should be due regarding the toxicity of all xenobiotic antagonists, which strongly contrast with the extremely well-tolerated melatonin. Therefore, at high doses, an apparent suppression of a melatonin effect by a receptor blocker may turn out as being toxicity-induced.
These considerations allow discrimination of mechanisms involved in melatonin's antioxidant properties. First, two types of effects that are related to MT1 and/or MT2 signaling can be distinguished: (a) direct effects on gene regulation, for example, regarding antioxidant enzymes, and (b) indirect modulation of antioxidant parameters by influencing circadian oscillators. The circadian multioscillator system controls numerous functions of relevance to antioxidative protection, but also to oxidant generation (113).
Since the influence of melatonin on the circadian oscillator system should mainly, perhaps exclusively, occur via MT1/MT2 signaling, this would only require low doses. The same can be assumed to be the case in the regulation of antioxidant enzymes expression. Regarding the requirement of higher doses for conveying antioxidative protection under severe conditions of exposure to toxins, sepsis, ischemia, or trauma, additional mechanisms are required for explaining melatonin's efficacy. According to actual knowledge, this may concern three different processes: (a) signaling via CaM, (b) binding to the mitochondrial permeability transition pore (mtPTP), and (c) scavenging of free radicals and other oxidants.
The role of CaM in melatonin's actions is only incompletely understood. In relation to anti-excitotoxic, anti-inflammatory, and antioxidant actions, the inhibition of neuronal nitric oxide synthase (nNOS) by melatonin has been interpreted in terms of this mechanism (152). Interestingly, inhibitory effects were also observed with the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK) (58, 151). However, it has remained uncertain as to whether the effect of AMK is related to noncompetitive CaM binding, as assumed earlier (151). Because of the rather unusual kinetics of inhibition, an alternative possibility of protein modification by AMKylation has been suggested (97). The actions of melatonin may extend to other CaM-activated enzymes, such as CaM kinase II and calcineurin, which have been discussed in the context of mitigating endoplasmic reticulum stress (247).
The discovery of melatonin's inhibition of mtPTP opening (9) prompted investigators to determine the inhibition kinetics. The Ki value was reported to be in the range of 0.8 μM, that is, at a concentration much above melatonin's blood levels and MT1/MT2 saturation. The contribution of mtPTP to oxidant release, mitochondrial function and integrity, as well as electron flux would strongly demand a more detailed investigation of the properties of this binding site.
Finally, scavenging of free radicals represents an important field of melatonin's spectrum of actions. After the discovery of highly potent elimination of hydroxyl radicals (258, 264), the detoxification of various other radical species and nonradical oxidants was demonstrated (82, 91). Despite initial skepticism regarding the relevance of radical scavenging by melatonin, with regard to its low levels in the circulation, this mode of action has turned out to be of biological value, since by orders of magnitude higher melatonin concentrations were determined in various nonvertebrate organisms, sometimes up to the millimolar level (39, 66, 116). In vertebrates, a contribution of radical scavenging to antioxidative protection should, at least, be considered at high pharmacological doses.
Melatonin's Role in the Circadian Multioscillator System: Control of Redox-Relevant Rhythms
Without any doubt, melatonin acts on the circadian master clock, the SCN, as can be shown by its capability of phase resetting and synchronizing previously nonentrained rhythms (12–14, 79, 155, 156, 246). The original concept had only assumed circulating melatonin to be effective, which would be in accordance with observations on oral administration. However, after the discovery of melatonin release via the pineal recess into the third ventricle of the brain (267–269), a higher importance of this latter route for the physiological modulation of SCN function has been assumed (225, 262).
In addition to the effects in the SCN, there is substantial evidence that melatonin also influences peripheral circadian oscillators (95, 115), a field that would require more detailed information. With regard to antioxidative protection, it should also be noted that the circadian system is also involved in the avoidance of oxidative damage, even beyond melatonin's direct effects on gene expression.
In organisms as different as fruitflies and hamsters, it was shown that clock mutants led to increased protein and lipid oxidation (48, 49). Conversely, oxidative stress was shown to affect circadian rhythms. In Drosophila, an organism that produces extremely low amounts of melatonin, deficiency of the important radical scavenger urate (rosy mutation ry506) caused changes in circadian patterns of antioxidant enzymes and, much more pronounced, of protein carbonyl, which was elevated by manifold in some but not all circadian phases (53).
With regard to melatonin's multiplicity of actions in both central and peripheral cellular oscillators, a look at the mechanisms of circadian gene regulation is required. The rhythmic up- and downregulation concerns countless genes, not only by gene-specific mechanisms, but also largely via global chromatin remodeling, which is differentially controlled by the various components of the cellular oscillators. Daily occurring chromatin remodeling is especially achieved by histone modification, such as acetylation/deacetylation and multiple methylation/demethylation of specific lysines, as summarized elsewhere (106).
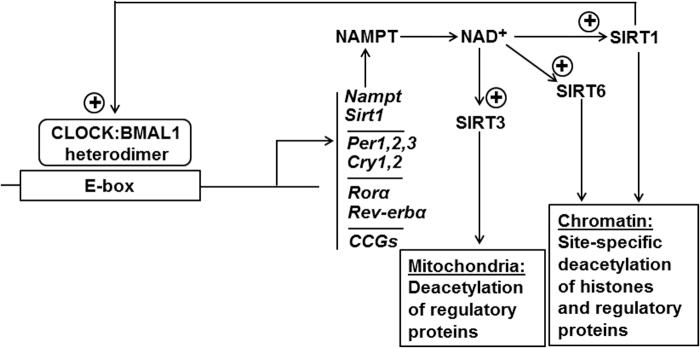
Interestingly, the dynamic balance between histone acetylation and deacetylation is influenced by melatonin at the level of cellular oscillators. There is a sequential interplay between the histone acetyl transferase circadian locomotor output cycles kaput (CLOCK), a core oscillator component that also acetylates various other proteins, and the histone deacetylase sirtuin 1 (SIRT1), an accessory oscillator component that, again, acts on other proteins, too. SIRT1 is interacting with oscillator components, whereas SIRT6, which is constitutively chromatin-associated, has also been reported to interact with CLOCK:BMAL1 heterodimers at E-box containing promoters outside the oscillator (176).
SIRT6 also deacetylates histones and regulatory proteins and can be classified as a circadian output factor, because its activity is dependent, similar to that of SIRT1, on the NAD+ cycle generated by the oscillator (Fig. 1) (175–177). However, SIRT1 and SIRT6 seem to influence different sets of genes (176). The influence of melatonin on these cycles concerns the upregulation of SIRT1 (95, 106), which is of relevance to the anti-inflammatory actions of melatonin (see subsequent section) and also to the properties of circadian oscillators, whose amplitudes are enhanced by SIRT1 (Fig. 2) (37, 240). Melatonin, being anyway a highly pleiotropic agent (112), seems to gain an even higher degree of pleiotropy via the circadian system, because of the multitude of circadian controlled genes (CCGs).
FIG. 1.
E-box-dependent transcription of circadian oscillator genes and CCGs and the oscillator-mediated activation of sirtuins by NAD+. BMAL1, brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1; CCG, circadian-controlled gene; CLOCK, circadian locomotor output cycles kaput; Cry, cryptochrome gene; NAMPT, nicotinamide phosphoribosyltransferase; Per, period gene; Rev-erbα, reverse-related erythroblastoma-α gene (alias Nr1d1, nuclear receptor subfamily 1, group D, member 1); Rorα, retinoic acid receptor-related orphan receptor α; SIRT, sirtuin.
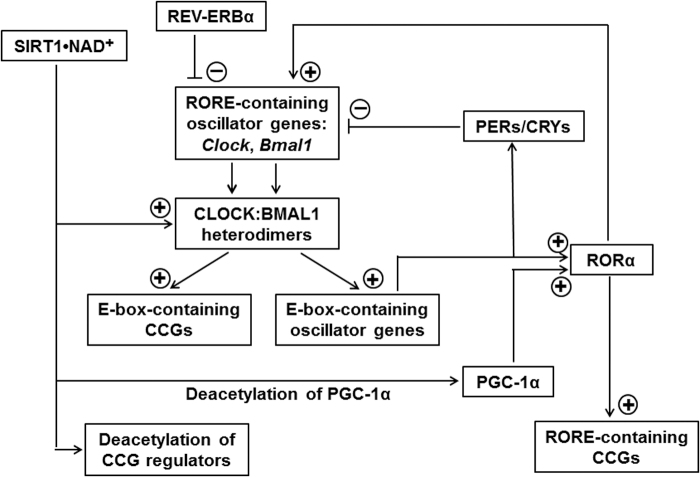
FIG. 2.
The effects of SIRT1 on CCGs with E-box- or RORE-containing promoters or being under control by deacetylatable regulators. RORE, ROR response element; for others see legend Figure 1.
Several of them are co-regulated via response elements such as E-boxes and ROR response elements (ROREs), which are not only present in clock genes but also accessible to the respective oscillator components in various CCGs (Fig. 2) (22). The presence of either E-boxes or ROREs in different genes leads to the important consequence that CCGs with the one or the other response element are controlled by oscillators in different phases, according to the temporal peaking of the respective clock components.
In the case of ROREs, an additional complication results from the fact that ROREs are differently regulated by two oscillator proteins, that is, activation by RORα and suppression by REV-ERBα (alias NR1D1, nuclear receptor subfamily 1, group D, member 1). Promoters of more than 1000 murine genes have been shown to contain binding sites for core oscillator proteins (158). Alternately, they may be controlled via D-boxes, binding sites of circadian output factors such as DBP (albumin D-site-binding protein; D-box positive regulator) or E4BP4 (adenovirus E4 promoter-binding protein; D-box negative regulator, alias NFIL3, nuclear-factor interleukin 3 regulated) (281, 286, 287).
Various other output factors, accessory clock components, and clock-dependent hormones have to be added to the list of global circadian regulators (93, 203, 274, 280), but a complete list would exceed the scope of this article. In this place, it should only be mentioned that additional epigenetic regulation mechanisms have to be considered, too, such as DNA methylation and its erasure, at regulatory CpG islands in promoters but also elsewhere (106), and numerous contributions of noncoding RNAs (63, 92, 101).
These include, apart from microRNAs (miRNAs), various long noncoding RNAs (lncRNAs) of different functions (e.g., miRNA sponging lncRNAs, super enhancer lncRNAs, suppressive lncRNAs such as HOTAIR), circRNAs (miRNA sponging circular RNAs), snoRNAs (small nucleolar RNAs, with contributions to circadian chromatin remodeling), and asRNAs (antisense RNAs), such as the Per2 asRNA, as summarized elsewhere (101). These RNAs are of considerable importance to circadian regulation mechanisms, already from a quantitative point of view. Only in the murine liver, 604 lncRNA clusters were shown to cycle in a circadian fashion, in which a cluster comprises splice variants of the same transcript (63). A broader evaluation of more organs would presumably reveal a much higher number.
Melatonin has been shown to upregulate, in several vertebrate species and tissues, the expression of various antioxidant enzymes, such as glutathione peroxidase (GPx), glutathione reductase (GR), γ-glutamylcysteine synthase, glucose-6-phosphate dehydrogenase, hemoperoxidase/catalase, Cu, Zn- and Mn-superoxide dismutases, as has been multiply reviewed (82, 112, 113, 117, 226). However, a close look at the details reveals that many of these findings cannot be generalized (113), as they are either tissue-, condition-, or species-specific or only demonstrated at mRNA levels, at the borderline of statistical significance.
Moreover, circadian cyclicity has not generally been demonstrated for all these enzymes (113), although the high-amplitude rhythmicity of melatonin may suggest a rhythmic influence on these enzymes' expression. However, one should not forget that antioxidant enzymes have to be controlled by various different factors and routes. In particular, any organism faces the necessity of adapting the expression to needs related to oxidant formation and stressful conditions.
In mammals, melatonin synthesis poorly responds to oxidative stress and is, in this sense, rather nonadaptive. On the contrary, melatonin levels may even decrease under high oxidative pressure, because of consumption by detoxification reactions, an effect first seen in a dinoflagellate that produces melatonin at micromolar levels and above (25). If this effect is already seen in a high-melatonin organism, oxidative melatonin consumption should be ever more relevant in the circulation of vertebrates. Among the antioxidant enzymes, there are especially two that reliably exhibited upregulation by melatonin and circadian rhythmicity, too, namely GPx (16, 18, 73, 78, 141, 180, 196, 198–200, 220, 226, 227, 234, 279) and GR (78, 161, 190, 200, 220, 227).
Additional data on long-term administration and deviating results in cancer will not be discussed here in detail. Circadian rhythms in the activities of these two enzymes have been most impressively demonstrated in chicken brain, in which a temporal sequence became apparent: The peak of the circadian inducer, melatonin, was followed by GPx and, a little bit later, by GR (200). The lag of the GR may reflect a need of GSSG (oxidized glutathione) reduction on increased GSH (reduced glutathione) consumption due to the higher GPx activity. The levels of GSH are of particular importance, under both circadian and toxicological aspects, since GSH is a central antioxidant. Therefore, melatonin hits a major factor by upregulating GPx, GR, and enzymes of GSH synthesis (glucose-6-phosphate dehydrogenase, γ-glutamylcysteine synthase).
In fact, the role of melatonin exceeds the modulation and rhythmicity of antioxidant enzymes. Notably, the immune system is, under inflammatory conditions, a major source of reactive oxygen species, hypochlorite and oxidizing derivatives thereof, such as taurine chloramine, and reactive nitrogen species. The immune system does not only respond to insults or is activated by processes of inflammaging, but also contains numerous factors that are under circadian control (36, 56, 107, 143, 179). Therefore, melatonin can modulate immunological functions via its chronobiotic properties. However, by means of its anti-inflammatory actions, it can also reduce the formation of toxic intermediates, in terms of a contribution to the principle of radical avoidance (82). For details see the subsequent section.
With regard to both antioxidant and anti-inflammatory aspects, melatonin also downregulates enzymes that indirectly contribute to prooxidant and other deleterious effects. In this regard, literature usually refers to the downregulation of lipoxygenase-5 (31, 254). However, these reports were based on actions by CGP 52608, a ligand of RORα, which is meanwhile known to not bind melatonin (100, 248, 249). Another study showed that melatonin does not downregulate lipoxygenase-5 (215). There is no good reason to assume an antioxidant effect of melatonin via suppression of this enzyme.
On the other hand, the lipoxygenase-5 gene was shown to carry an RORE in its promoter, and CGP 52608 was concluded to mediate suppressive effects by RORα (185). This interpretation may be entirely valid, but this means that the circadian system rather than melatonin directly controls the expression of lipoxygenase-5, since RORα is a component of cellular circadian oscillators and CCGs with ROREs are driven by their ligands, RORs and REV-ERBα (Fig. 2). Another action of melatonin was reported for lipoxygenase-12, surprisingly in the rat pineal gland (292), in which some melatonin should be normally present. Exogenous melatonin was reported to suppress 12-lipoxygenation.
However, melatonin was used at a dose of 100 mg i.v., which should have oversaturated the melatonin receptors for the time of the experiments (1 h). Experiments in cultured pineal glands showed that luzindole (0.1 mM) inhibited the effect by melatonin (also 0.1 mM). Although the experimentation may not have been perfect in terms of dosing, a suppression of lipoxygenase-12 by melatonin may still be valid, since untreated animals showed inverse circadian relationships between melatonin and the enzyme (292). Unfortunately, studies on the relationship between melatonin and lipoxygenase-12 have not been extended to other tissues.
Indirectly acting pro- and anti-inflammatory enzymes are also found in immune cells, such as inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2), both of which are downregulated by melatonin. In addition to other functions, •NO is important as a diffusible proinflammatory signal, which is, in this context, produced by iNOS in numerous tissues and various leukocytes, in particular, macrophages, microglia, and neutrophils. In the CNS, both nNOS and iNOS contribute to neuroinflammation by activating microglia and astrocytes (111).
Moreover, •NO combines with O2•− (superoxide) to ONOO− (peroxynitrite), a highly reactive oxidant that can also yield hydroxyl radicals on protonation (ONOOH → •NO2 + •OH) or carbonate radicals on combination with CO2 (ONOOCO2− → •NO2 + CO3•−) and, thereby, contributes via its metabolites to further oxidation and nitration reactions (75, 88, 96). Importantly, iNOS is downregulated by melatonin (2, 60–62, 68, 131, 166, 265) and, thus, decreases these undesired and damaging reactions, in addition to the suppression of the proinflammatory •NO signal. The downregulation of COX-2 in macrophages also reduces proinflammatory signaling, in this case by prostaglandins (51, 181).
Importantly, prooxidant and proinflammatory signaling share the involvement of nuclear factor κB (NF-κB), whereas antioxidant and anti-inflammatory signaling is mediated via nuclear factor erythroid 2-related factor 2 (Nrf2). Melatonin's effects have repeatedly been shown to be characterized by inhibition of NF-κB activation and upregulation of Nrf2, as summarized elsewhere (105, 140).
Melatonin and Inflammation: Circadian and Noncircadian Aspects
As numerous factors of the immune system are under circadian control (36, 56, 107, 112, 125, 143, 179), melatonin can be assumed to partially modulate immunological functions because of its chronobiotic properties. However, the situation is more complex, for two reasons. First, numerous immune cells, perhaps all of them, produce melatonin (32, 34, 47, 76, 112, 148, 171, 188, 242) and the role of leukocytic melatonin as an intracrine, mitocrine, or paracrine agent is still poorly understood, because this cannot be convincingly studied by exposing these cells to exogenous melatonin. In addition to the intracellular production, many leukocytes express melatonin receptors, mostly MT1, and, therefore, respond to extracellular melatonin (33, 70, 139, 112, 147, 171, 210).
The additionally discussed role of RORs should no longer be associated with direct actions of melatonin (cf. Refs. 248, 249), but rather be seen in the circadian context. Second, numerous studies on immunological actions of melatonin have been conducted by using supraphysiological doses and the potent anti-inflammatory actions, which are of practical medical interest (6, 35, 50–62, 71, 72, 105, 121, 124, 166, 259, 260), are beyond the circadian role.
With regard to the circadian control of immune functions and the participation of melatonin, a recently emerging aspect concerns the roles of noncoding RNAs that exceed the already mentioned lncRNAs. In particular, miRNAs are abundantly involved in the post-transcriptional regulation of gene expression. This may be seen as kind of a fine control, but is, sometimes, difficult to judge without detailed analysis, especially as a single miRNA species may have putative interaction sites in numerous mRNAs and, thus, potentially target different mRNAs (98).
Melatonin has been shown to modulate the expression of many miRNAs (102), findings that are not surprising in a highly pleiotropic regulator. The number of melatonin-controlled miRNAs will expectably further rise, as soon as more tissues will be investigated in this regard. Moreover, numerous newly discovered miRNAs have not yet been studied in the context of melatonin. Typically, the melatonin-affected miRNAs are differentially expressed when comparing deviating conditions, for example, cancer versus normal tissue (149).
In such cases, a substantial difficulty of interpretation remains concerning the targeted mRNAs. In the latter article, 22 differentially expressed miRNAs were reported to be putatively capable of targeting 2029 mRNAs, according to their 5′-utr sequences. Therefore, a profound understanding would require determination as to which mRNAs are expressed in which cell type and under which conditions. Another approach of analyzing the roles of miRNAs in the circadian system including the contribution of melatonin is to identify (a) targets within core and accessory clock components and (b) response elements for circadian oscillator proteins including output factors and for agents that are known to cycle with high amplitude, such as glucocorticoids.
In an initial study, this has been done for miRNAs known to be involved in the regulation of inflammation (107). In this study, 28 miRNAs were found to modulate circadian oscillator components. Among them, 10 miRNAs were identified as upregulators, 12 others as downregulators of proinflammatory cytokines. Moreover, nine miRNAs downregulated SIRT1 expression, which was in accordance with proinflammatory roles observed at cytokine levels. Three miRNAs favored macrophage polarization to the proinflammatory type M1, whereas another one favored the anti-inflammatory type M2.
Regarding the circadian control of miRNA expression, 43 miRNAs/miRNA clusters with functions in inflammation control were found to be circadian-regulated. Among them, 24 had binding sites in their promoters for canonical oscillator proteins, 35 for the important circadian output factor DBP, and 15 for the glucocorticoid receptor. Within the selection of miRNAs, nine were modulated by melatonin, five of them downregulated. Many of these miRNAs were multiply controlled by several circadian regulators.
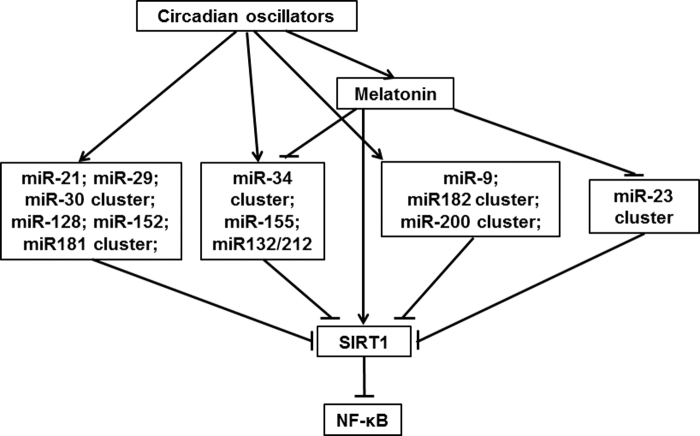
A selection of circadian or melatonin-controlled miRNAs that also downregulate SIRT1 expression is mentioned in Figure 3. The upregulation of SIRT1 by melatonin and the suppression of several of these miRNAs by melatonin, which is also included, seem to antagonize SIRT1 downregulation (Fig. 3). Further analyses regarding miRNA-interacting RNAs revealed that the bifunctional miRNAs with regulatory roles in both immune and circadian systems were sponged by 23 circRNAs.
FIG. 3.
Various inflammation-related microRNAs under circadian control suppress SIRT1, thereby removing the SIRT1-mediated blockade of NF-κB activation, whereas melatonin upregulates SIRT1 and downregulates several of these microRNAs. These relationships are valid for conditions of well-operating circadian oscillators, whereas strongly deviating findings are typically obtained in tumor cells, whose oscillators are often severely dysregulated (92, 95, 108). It should also be noted that many more microRNAs are under circadian control or modulated by melatonin (107). Various others have been shown to stimulate or suppress NF-κB activation or SIRT1 signaling, however, in the absence of information on circadian control (105). Moreover, NF-κB was typically negatively correlated with Nrf2, in many analyzed cases (105) (not incorporated here, for avoiding an overcomplicated scheme). The switching between NF-κB and Nrf2 reflects an important feature of proinflammatory versus anti-inflammatory and prooxidant versus antioxidant regulation. Before the discovery of the melatonin-SIRT1 relationship, it had been assumed that the influence of melatonin on the NF-κB/Nrf2 balance was independent of SIRT1. It may still be that melatonin and SIRT1 additionally act synergistically in parallel. NF-κB, nuclear factor κB; Nrf2, nuclear factor erythroid 2-related factor 2.
Some circRNAs sponged more than one miRNA species, and the number of sponged miRNAs/miRNA clusters amounted to 20. The results of this evaluation show a higher number of RNAs with a role in the circadian system, compared with those that are directly affected by melatonin. This proportion may change in the future, as soon as many more miRNAs have been investigated. In fact, new miRNAs are being continually discovered and the newest of them have not yet been evaluated for roles in both immune and circadian systems.
Although the number of miRNAs that have been shown to be directly controlled by melatonin has still remained rather low, it should be taken into account that the effects of melatonin on this category of molecules have not yet been systematically investigated. Moreover, melatonin may influence more of them indirectly, because of its chronobiotic effects on oscillators. A special aspect of potential future importance concerns the roles of circRNAs, since their sponging of miRNAs is functionally highly relevant to intraorganismal communication by exosomes and ectosomes (also known as microvesicles formed by directly budding from the plasma membrane).
Both these types of extracellular vesicular structures contain numerous regulatory molecules (98, 138, 182, 270, 291). Melatonin has already been shown to influence their contents, especially with regard to macrophage function and, thus, to inflammation control (44): Exosomes from melatonin-treated hepatocellular carcinoma cells downregulated in recipient macrophages the secretion of TNFα, IL-1β, and IL-6, whereas those from untreated cells upregulated these proinflammatory cytokines. Interestingly, melatonin was shown in another study to enhance exosome secretion by adipocytes, along with suppression of adipose inflammation, interaction of exosomes with macrophages, and favoring macrophage polarization toward M2 (164).
This treatment was associated with an SIRT1-dependent increase in the circadian rhythm of isocitrate dehydrogenase 2 (Idh2) mRNA levels in adipocytes. Notably, the favoring of M2 versus M1 polarization by melatonin seems to be a general feature that contributes to the anti-inflammatory actions of this agent, already at physiological levels (278).
Extended Signaling by Melatonin via Sirtuins
Although melatonin's actions via MT1 and MT2 and respective G protein-mediated signaling pathways are well documented, the involvement of sirtuins in melatonin effects has opened new routes of interpretations. Unfortunately, the connections between the GPCR-mediated pathways and sirtuin expression are still poorly understood. However, the repeated demonstration of SIRT1 upregulation by melatonin in nontumor cells substantially expands the spectrum of melatonin's action and has been referred to as “extended signaling” (103).
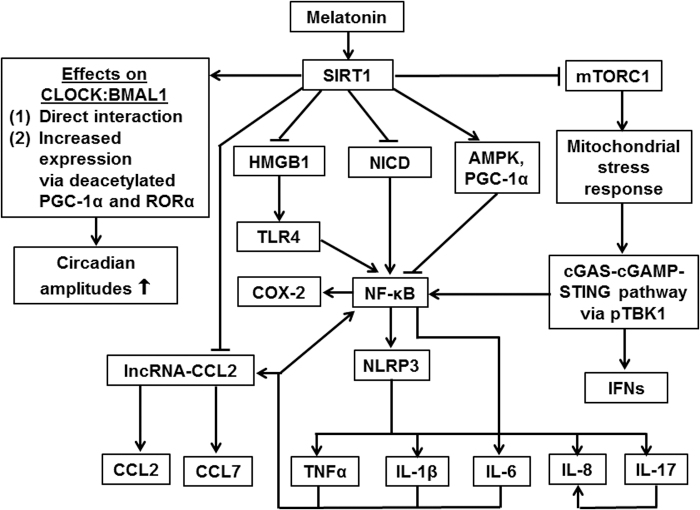
The relationship between melatonin and SIRT1 is of considerable importance, for several reasons: First, SIRT1 is an accessory clock component that increases circadian amplitudes (37, 240), that is, a cellular mechanism also influenced by melatonin. Second, SIRT1 shares with melatonin anti-inflammatory and some antioxidant properties (Fig. 4) (95, 103–105). Third, both melatonin and SIRT1 have been discussed in their roles as potential anti-aging factors (104).
FIG. 4.
Anti-inflammatory and chronobiological actions of SIRT1 under demonstrated or expected upregulation by melatonin, as abolished by SIRT1 inhibition. Arrows indicate positive relationships (activation or upregulation) between connected factors; blunted lines indicate inhibition or downregulation. With regard to the complexity of the immune system, this scheme cannot delineate all possible connections, factors, and differences between cell types. Melatonin may also exert some of these effects both SIRT1-dependetly and -independently. AMPK, adenosine 5′-monophosphate-dependent protein kinase; CCL, CC-chemokine ligand; cGAMP, cyclic 2′,3′ guanosine monophosphate-adenosine monophosphate; cGAS, cyclic GMP-AMP synthase; COX-2, cyclooxygenase-2; HMGB1, high-mobility group box 1; IFN, interferon; IL, interleukin; lncRNA, long noncoding RNA; mTORC1, mechanistic target of rapamycin receptor complex 1; NICD, intracellular domain of Notch; NLRP3, NLR family pyrin domain containing 3; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; pTBK1, phosphorylated TANK-binding kinase 1; RORα, retinoic acid receptor-related orphan receptor α; STING, stimulator of IFN genes; TLR4, toll-like receptor 4; TNFα, tumor necrosis factor-α.
In the beginning of research on the relationship between melatonin and SIRT1, entirely different results had been observed, because SIRT1 was shown to be strongly downregulated by melatonin, an effect that was typical for cancer cells (95, 134, 135). However, this action turned out to be largely cancer-specific and was interpreted on the basis of a profound dysregulation of oscillators in transformed cells, a necessity because of the properties of several core oscillator components as tumor suppressor genes.
The escape of tumor cells from the suppression of tumor properties is based on downregulation of genes with anti-tumor properties such as Per2, but increased levels of Sirt1 and Clock, the latter being a proliferation-promoting factor. Thus, melatonin's action of suppressing SIRT1 may be interpreted as a partial reversal of the tumor-specific dysregulation (92, 95, 108).
However, in nontumor cells, the effects of melatonin on SIRT1 are entirely different and consist, in the majority of studies, in significant upregulations, as summarized elsewhere until 2016 (95). The decisive argument for the melatonin signaling via SIRT1 is based on the repeated findings that various effects by melatonin are suppressed by sirtuin inhibitors such as sirtinol and EX527 or by Sirt1 siRNA. More recent studies have confirmed this observation in various experimental systems (10, 15, 41, 67, 77, 81, 122, 157, 168, 193, 205, 209, 230, 245, 283, 285, 293–295, 297, 298).
In another study on the diabetic heart, melatonin's protective effects were found to be absent in Sirt1−/− (52). In microglia, hypoxia caused by either CoCl2 or carotid occlusion, melatonin suppressed cobalt toxicity, an effect inhibited by EX527, and prevented, in the occlusion model, decreases of nuclear SIRT1 localization (183). Many more publications not summarized in this place have also reported upregulation of SIRT1 by melatonin, but they did not investigate the suppression of melatonin effects by sirtuin inhibition.
Importantly, many of the studies that demonstrated effects of melatonin via SIRT1 also showed upregulation of Nrf2 and downregulation of NF-κB. Collectively, the numerous reports on SIRT1-mediated actions by melatonin that were observed in various tissues or cells and under entirely different conditions constitute an overwhelming body of evidence for the suggested extended signaling of melatonin, which comprises various further details of downstream pathways (Fig. 4) (104, 105). This seems to further extend to the central antagonizing roles of Nrf2 and NF-κB in the control of antioxidant and anti-inflammatory actions known for both melatonin and SIRT1.
One might ask oneself, what the significance of extended signaling via SIRT1 could be, especially under conditions of pharmacological doses. First, one advantage may be sought in the half-lives of the agents (109). Contrary to the short-lived melatonin, SIRT1 exhibits a half-life in the range of several hours, for example, 8 h in glomerular mesangial cells (129), and would, thus, provide longer-lasting actions. Second, especially under physiological conditions, the upregulation of SIRT1 expression may be seen as a mode by which melatonin interacts with cellular circadian oscillators. Both aspects indicate a fundamental relationship in redox biology and inflammation control.
Mitochondrial Protection
Protection of mitochondria by melatonin is well documented and has been multiply reviewed (1–3, 5, 6, 17, 30, 40, 60, 62, 68, 69, 86, 99, 112, 117, 120, 150, 160, 166, 170, 202, 211, 214, 222, 224, 228, 229, 261, 263, 266, 276). Therefore, this important part of melatonin's antioxidant actions will not be discussed in detail, but rather with focus on the distinction between circadian and noncircadian phenomena and on some aspects that have otherwise been poorly considered.
From a fundamental point of view, mitochondrial activity has to be coordinated with circadian rhythms, because of the demands by periodic metabolism resulting from, for example, locomotor activity, but also organ specifically from other metabolic rhythms. This is reflected by circadian rhythms of oxygen consumption (186, 187). This also leads unavoidably to a rhythmic mitochondrial generation of free radicals, due to electron leakage from the electron transport chain (ETC) (113).
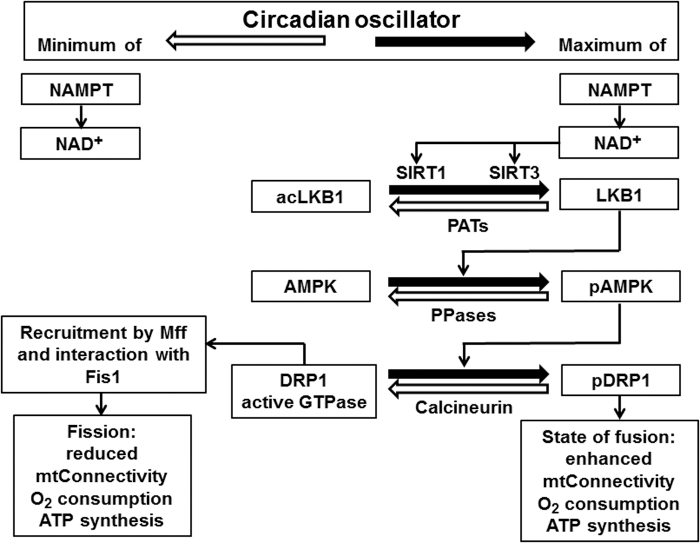
The rhythmicity of mitochondrial activity results, to a certain extent, from the circadian variations of glycolytic and lipolytic precursors fed into the citrate cycle, but it is also a consequence of regulation mechanisms acting on these organelles. In particular, a nexus between the circadian system and mitochondria exists in the rhythmic activity of SIRT3. This latter sirtuin, which is mitochondrially localized and differs in this regard from the aforementioned SIRT1 and SIRT6, cannot directly act on cellular circadian oscillators whose components are present in the nucleus and cytoplasm, but it is driven by the cyclicity of NAD+ (174, 177, 204).
Therefore, the activities of these sirtuins are concomitantly controlled by the same metabolite, however, with the complication that the mitochondrial NAD+/NADH ratio is also influenced by the activity of the mitochondrial citrate cycle. Another circadian regulation mechanism concerns the clock-controlled dynamin-related protein 1 (DRP1), which acts in this way as a circadian output factor (38, 153, 244). DRP1 is a large GTPase, which is recruited to the outer mitochondrial membrane by the mitochondrial fission factor (Mff).
By interacting with fission 1 (Fis1), it promotes fission and can lead, in the extreme, to enhanced mitochondrial fragmentation (132). Under conditions of excessive fragmentation, DRP1 may cause increased electron leakage, mtPTP opening with breakdown of the mitochondrial membrane potential (ΔΨmt), and oxidative stress (132). Moreover, DRP1 was shown to more generally control rates of oxidative phosphorylation and ATP production, since blockade or knockout of DRP1 abolished the circadian rhythms in these parameters (244).
However, DRP1 does not exhibit circadian cycles in protein concentration, but it is rather controlled at the activity level by phosphorylation and dephosphorylation at Ser637 (244). Interestingly, DRP1 phosphorylation seems to be connected to SIRT3 via LKB1 (liver kinase B1) deacetylation, which is followed by AMPK (adenosine 5′-monophosphate-dependent protein kinase) phosphorylation (Fig. 5) (162, 299). Corresponding mechanisms via deacetylation of other metabolic sensors likely exist.
FIG. 5.
The circadian oscillator drives a mitochondrial fusion/fission cycle: a comparison of conditions at circadian minima and maxima of NAD+ levels, O2 consumption, and ATP synthesis. Enhanced fission is associated with low, prevailing fusion with high O2 consumption and ATP formation. ΔΨmt, mitochondrial membrane potential; AMPK, adenosine 5′-monophosphate-dependent protein kinase; DRP1/pDRP1, dynamin-related protein 1/phosphorylated dynamin-related protein 1; Fis1, mitochondrial fission 1 protein; LKB1/acLKB1, liver kinase B1/acetylated liver kinase B1 (LKB1 is part of the AMPKK complex); Mff, mitochondrial fission factor; mt, mitochondrial; PATs, protein acetyltransferases; PPases, protein phosphatases.
With regard to melatonin's role in the interplay of Sirt3, AMPK, and DRP1, several effects including protection have been documented. Activation of SIRT3 by melatonin has been repeatedly reported (65, 80, 163, 169, 208, 228, 250, 282, 288, 290, 293, 302). In many of these studies, no upregulation of SIRT3 protein expression was observed, which is in accordance with the NAD+-dependent activation as the decisive regulation mechanism. Effects of melatonin on AMPK and other metabolic sensors have been summarized elsewhere (90).
The suppression of DRP1 activation by melatonin has also been reported, sometimes showing the involvement of AMPK, but without demonstration of participation of SIRT3 (7, 43, 45, 136, 296, 300, 301). Another study reported the involvement of SIRT1 instead of SIRT3 as the deacetylating agent (52). This is also possible as an alternate route, since SIRT1 is present in the cytosol, where it can likewise deacetylate AMPK, with same consequences to DRP1. Notably, most studies mentioned in this paragraph have shown, in addition to the prevention of excessive mitochondrial fission, protection against oxidative stress, often associated with the prevention of apoptosis and, sometimes, anti-inflammatory actions.
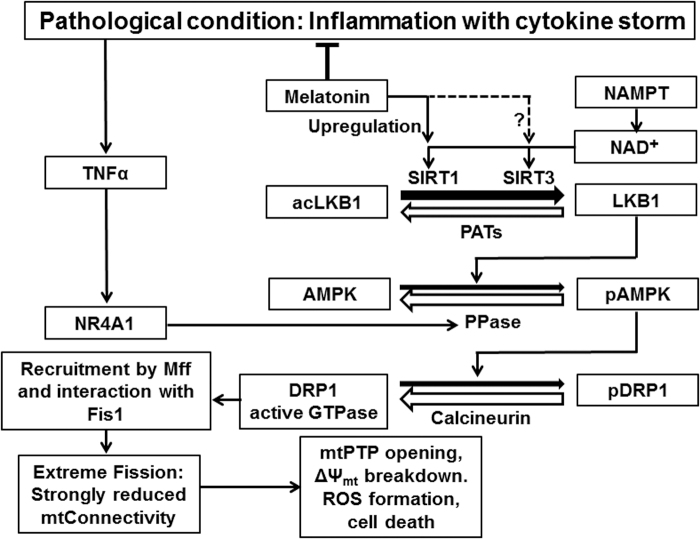
Strong signaling toward mitochondrial fission, with consequences to ΔΨmt breakdown and cell death, is especially observed under conditions of inflammation with cytokine storm, in which TNFα leads to activation of NR4A1 (nuclear receptor subfamily 4 group A member 1) (299), which initiates pAMPK dephosphorylation and, thereby, favors pDRP1 dephosphorylation by calcineurin (Fig. 6). The protective action of melatonin may be mainly attributed to the multiple anti-inflammatory effects of this agent (104, 105). The contributions of SIRT1 and, even more, SIRT3 signaling may deserve further clarification with regard to changes in NAD+ levels, since upregulations of sirtuin expression are not per se indicative of higher activities.
FIG. 6.
Inflammation can drive mitochondria toward fission, with severe consequences to mitochondrial function and cell survival: a simplified model. The protein phosphatase upregulated via NR4A1 has not yet been identified; pAMPK dephosphorylation is known for PP1, PP2A, and PP2C. The model is restricted to the best understood details regarding fission and may have to be expanded by additional routes and factors. Additional mitochondrial effects of inflammation regarding •NO and its derivatives, ROS, the GSSG/GSH balance, and DAMP factors have been omitted. In this model, the counteraction by melatonin has been restricted to the mitigation of the cytokine storm, which is sufficiently documented. Upregulation of SIRT1 and, perhaps, SIRT3 by melatonin is indicated, but it remains uncertain whether this effect can prevail over the NAD+-dependent regulation. DAMP, damage-associated molecular pattern; GSH, reduced glutathione; GSSG, oxidized glutathione; mtPTP, mitochondrial permeability transition pore; NR4A1, nuclear receptor subfamily 4, group A, member 1; ROS, reactive oxygen species.
Several additional mechanisms contribute to mitochondrial protection. The aforementioned downregulation of iNOS by melatonin is not only important for avoiding excessive peroxynitrite formation (120), but it has also been found to be decisive for the maintenance of mitochondrial function under conditions of sepsis or endotoxemia (3, 60, 62, 68, 166, 197). Recent evidence indicates that the protective effects in mitochondria are, at least partially, mediated by SIRT1 (208, 295). The maintenance of electron flux by melatonin also comprises upregulation of proteins of the ETC complexes (172, 173, 194, 195), prevention of cytochrome c-independent nonenzymatic cardiolipin peroxidation (128, 167, 202, 206, 207), and upregulation of the mitochondrial GPx subform, GPx4 (60, 62, 166, 235–237), which may be related to the observed reduction of cardiolipin peroxidation (99).
Another aspect of mitochondrial protection concerns the direct effects of melatonin on mtPTP opening. Apart from its inhibition via binding to a low-affinity mitochondrial site (9), more recent experiments revealed that melatonin, at reasonable concentrations, did not permanently block the mtPTP, but rather reduced the duration of opening and, thus, allowed superoxide flashes with a soon return to normal ΔΨmt values and, thus, avoided apoptosis induction (99, 133).
Much of the data regarding mitochondrial protection have been obtained with supraphysiological doses of melatonin. Therefore, these effects are not easily attributed to the chronobiological role of melatonin. Moreover, the intracellular role of mitochondrially produced melatonin does not indicate a circadian relationship. In the seminal article on mitochondrial synthesis in neurons, the formation rates did not show any circadian fluctuation (255).
However, the consequences of chronodisruption by activation of the innate immune system with inflammation induction and resulting mitochondrial damage have indicated another, more indirect relationship to the circadian system, in terms of requirements of well-operating oscillators for preserving mitochondrial function (6). Another nexus between the circadian system, melatonin, and mitochondrial protection may be deduced from the involvement of sirtuins, as discussed in the preceding section.
Mitochondrial protection under conditions of disease-related overshooting inflammation has recently gained considerable actuality in the context of COVID-19, although the required melatonin doses are far beyond the physiological range (260). Melatonin is remarkably efficient in the treatment of various deadly viral and bacterial diseases (121, 259) and also targets mitochondria, which are subject to pathogen-associated molecular pattern- and damage-associated molecular pattern-associated cytokine storms and to hijacking by viruses that alter mitochondrial metabolism.
This may be seen as a good reason for applying melatonin with the purpose of protecting mitochondria in such diseases (261). Notably, the virus-induced Warburg effect also leads to a depletion of the cofactors needed for melatonin synthesis. Repletion of melatonin by exogenous administration is, therefore, a requirement for readjusting the availability of melatonin (261).
Conclusion
Melatonin can be applied, experimentally or therapeutically, within a remarkably broad range of concentrations. Notably, the highly divergent doses can be justified for the specific applications and all of them have been shown to exert effects in the field of antioxidative protection. However, it is important to distinguish between different mechanisms of action that are based on their own concentration requirements. From the mechanistic point of view, lowest concentrations as attained, in humans, by doses in the low mg range are sufficient for actions via the MT1 and MT2 receptors. Somewhat higher levels may be appropriate for effects via complexes of CaM with CaM-activated proteins.
Concentrations in the millimolar range seem to be required for actions via mtPTP-associated binding site (9). The highest concentrations (daily doses of several 100 mg in humans, or 10–20 mg/kg in rodents), which may include direct scavenging of free radicals, are needed for efficiently rescuing from several acute diseases, such as viral infections, sepsis, stroke, or brain trauma (42, 121, 165, 260, 273), and they have been also applied in a chronic neurodegenerative disease (275).
These differences in dosage are often poorly considered in articles, and it seems necessary to pay more attention to this point in the future. Researchers should be aware of the problems regarding signaling mechanisms, if very high doses are required that are oversaturating MT1 and MT2 receptors for extended periods of time. If in such studies arguments on MT1 and/or MT2 involvement are forwarded, one would expect efficacy already at much lower doses.
Receptor-mediated effects of melatonin, for example, via G protein components such as αi2/3, αq, or βγ (85), can be of a direct or an indirect nature. Direct effects via MT1 or MT2 are typical for melatonin's chronobiotic actions and various other well-documented effects in numerous tissues (54, 55) and can also be inferred for the upregulation of antioxidant enzymes, such as GPx. However, numerous pathways other than the primary decrease of cAMP or activation of mitogen-activated protein kinase cascades have been suggested for numerous protective effects, without connecting them to the G protein-mediated changes.
As far as the circadian system is involved, the influence of melatonin on cellular oscillators may allow many additional actions by circadian output factors and various clock-controlled proteins that are acting via other signaling mechanisms. The multitude of miRNAs that are regulated by the circadian system and/or by melatonin introduce another degree of complexity into the signaling matrix, both intracellularly and, via exosomes and ectosomes, intercellularly (98, 102, 103, 105, 107, 149).
Another route of secondary signaling by melatonin concerns the actions mediated by sirtuins, especially SIRT1, with regard to the roles of this protein as a circadian oscillator component and as an anti-inflammatory and antioxidant agent (95, 103–105, 107, 109). Moreover, the influence of melatonin toward macrophage and microglial M2 polarization deserves (278) particular attention in the future, because of its numerous consequences to inflammation regulation.
Abbreviations Used
- AFMK
N1-acetyl-N2-formyl-5-methoxykynuramine
- AMK
N1-acetyl-5-methoxykynuramine
- AMPK
adenosine 5′-monophosphate-dependent protein kinase
- AMPKK
adenosine 5′-monophosphate-dependent protein kinase kinase
- asRNA
antisense RNA
- BMAL1
brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1
- CaM
calmodulin
- CCG
circadian-controlled gene
- CCL
CC-chemokine ligand
- cGAMP
cyclic 2′,3′ guanosine monophosphate-adenosine monophosphate
- cGAS
cyclic GMP-AMP synthase
- circRNA
circular RNA
- CLOCK
circadian locomotor output cycles kaput
- COX-2
cyclooxygenase-2
- Cry
cryptochrome
- DAMP
damage-associated molecular pattern
- DBP
albumin D-site-binding protein
- DRP1/pDRP1
dynamin-related protein 1/phosphorylated dynamin-related protein 1
- E4BP4
adenovirus E4 promoter-binding protein
- ETC
electron transport chain
- Fis1
mitochondrial fission 1 protein
- GPCR
G protein-coupled receptor
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HMGB1
high-mobility group box 1
- Idh2
isocitrate dehydrogenase 2
- IFN
interferon
- LKB1/acLKB1
liver kinase B1/acetylated liver kinase B1
- IL
interleukin
- iNOS
inducible NO synthase
- lncRNA
long noncoding RNA
- Mff
mitochondrial fission factor
- miRNA
microRNA
- MT1
melatonin receptor 1
- MT2
melatonin receptor 2
- mTORC1
mechanistic target of rapamycin receptor complex 1
- mtPTP
mitochondrial permeability transition pore
- NAMPT
nicotinamide phosphoribosyltransferase
- NF-κB
nuclear factor κB
- NFIL3
nuclear-factor interleukin 3
- NICD
intracellular domain of Notch
- NLRP3
NLR family pyrin domain containing 3
- nNOS
neuronal nitric oxide synthase
- NQO2
NRH:quinone oxidoreductase 2
- NR1D1
nuclear receptor subfamily 1, group D, member 1
- NR4A1
nuclear receptor subfamily 4, group A, member 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- pAMPK
phosphorylated adenosine 5′-monophosphate-dependent protein kinase
- PAT
protein acetyltransferase
- Per
period
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator-1α
- PPase
protein phosphatase; PP1, PP2A, PP2C, protein phosphatase subforms 1, 2A, 2C
- pTBK1
phosphorylated TANK-binding kinase 1
- REV-ERBα
reverse-related erythroblastoma-α
- RORα
retinoic acid receptor-related orphan receptor α
- RORE
ROR response element
- ROS
reactive oxygen species
- SCN
suprachiasmatic nucleus
- SIRT
sirtuin
- snoRNA
small nucleolar RNA
- STING
stimulator of IFN genes
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor-α
- ΔΨmt
mitochondrial membrane potential
Author's Contribution
This article was written by R.H., who served as the sole author.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. Acuña-Castroviejo D, Escames G, León J, Carazo A, and Khaldy H. Mitochondrial regulation by melatonin and its metabolites. Adv Exp Med Biol 527: 549–557, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Acuña-Castroviejo D, Escames G, López LC, Hitos AB, and León J. Melatonin and nitric oxide: two required antagonists for mitochondrial homeostasis. Endocrine 27: 159–168, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Acuna-Castroviejo D, Escames G, Rodriguez MI, and Lopez LC. Melatonin role in the mitochondrial function. Front Biosci 12: 947–963, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan D-X, and Reiter RJ. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71: 2997–3025, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Acuña Castroviejo D, López LC, Escames G, López A, García JA, and Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem 11: 221–240, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Acuña-Castroviejo D, Rahim I, Acuña-Fernández C, Fernández-Ortiz M, Solera-Marín J, Sayed RKA, Díaz-Casado ME, Rusanova I, López LC, and Escames G. Melatonin, clock genes and mitochondria in sepsis. Cell Mol Life Sci 74: 3965–3987, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agil A, Chayah M, Visiedo L, Navarro-Alarcon M, Rodríguez Ferrer JM, Tassi M, Reiter RJ, and Fernández-Vázquez G. Melatonin improves mitochondrial dynamics and function in the kidney of Zucker diabetic fatty rats. J Clin Med 9: 2916, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersen LPH, Werner MU, Rosenkilde MM, Harpsøe NG, Fuglsang H, Rosenberg J, and Gögenur I. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol Toxicol 17: 8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrabi SA. Sayeed I, Siemen D, Wolf G, and Horn TF. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J 18: 869–871, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Ansari Dezfouli M, Zahmatkesh M, Farahmandfar M, and Khodagholi F. Melatonin protective effect against amyloid β-induced neurotoxicity mediated by mitochondrial biogenesis; involvement of hippocampal Sirtuin-1 signaling pathway. Physiol Behav 204: 65–75, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Antolín I, Obst B, Burkhardt S, and Hardeland R. Antioxidative protection in a high-melatonin organism: the dinoflagellate Gonyaulax polyedra is rescued from lethal oxidative stress by strongly elevated, but physiologically possible concentrations of melatonin. J Pineal Res 23: 182–190, 1997. [DOI] [PubMed] [Google Scholar]
- 12. Arendt J and Deacon S. Treatment of circadian rhythm disorders—melatonin. Chronobiol Int 14: 185–204, 1997. [DOI] [PubMed] [Google Scholar]
- 13. Arendt J and Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev 9: 25–39, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Arendt J, Skene DJ, Middleton B, Lockley SW, and Deacon S. Efficacy of melatonin treatment in jet lag, shift work, and blindness. J Biol Rhythms 12: 604–617, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Arioz BI, Tastan B, Tarakcioglu E, Tufekci KU, Olcum M, Ersoy N, Bagriyanik A, Genc K, and Genc S. Melatonin attenuates LPS-induced cute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front Immunol 10: 1511, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balkan J, Sener G, Cevikbas U, Keyser-Uysal M, and Uysal M. Melatonin improved the disturbances in hepatic prooxidant and antioxidant balance and hepatotoxicity induced by a cholesterol diet in C57BL/6J mice. Int J Vitam Nutr Res 74: 349–354, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Baltatu OC, Amaral FG, Campos LA, and Cipolla-Neto J. Melatonin, mitochondria and hypertension. Cell Mol Life Sci 74: 3955–3964, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barlow-Walden LR, Reiter RJ, Abe M, Pablos M, Menendez-Pelaez A, Chen LD, and Poeggeler B. Melatonin stimulates brain glutathione peroxidase activity. Neurochem Int 26: 497–502, 1995. [DOI] [PubMed] [Google Scholar]
- 19. Benítez-King G and Antón-Tay F. Calmodulin mediates melatonin cytoskeletal effects. Experientia 49: 635–641, 1993. [DOI] [PubMed] [Google Scholar]
- 20. Benítez-King G, Huerto-Delgadillo L, and Antón-Tay F. Binding of 3H-melatonin to calmodulin. Life Sci 53: 201–207, 1993. [DOI] [PubMed] [Google Scholar]
- 21. Berson DM, Dunn FA, and Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295: 1070–1073, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Bozek K, Kiełbasa SM, Kramer A, and Herzel H. Promoter analysis of Mammalian clock controlled genes. Genome Inform 18: 65–74, 2007. [PubMed] [Google Scholar]
- 23. Brown GM, McIntyre RS, Rosenblat J, and Hardeland R. Depressive disorders: processes leading to neurogeneration and potential novel treatments. Prog Neuropsychopharmacol Biol Psychiatry 80(Pt C): 189–204, 2018. [DOI] [PubMed] [Google Scholar]
- 24. Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 47, 2336–2348, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Burkhardt S, Hardeland R, and Poeggeler B. Various forms of oxidative stress strongly diminish 5-methoxylated indoleamines in Gonyaulax polyedra. In: Biological Rhythms and Antioxidative Protection, edited by Hardeland R. Göttingen, Germany: Cuvillier, 1997, pp. 98–102. [Google Scholar]
- 26. Cajochen C, Kräuchi K, and Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol 15: 432–437, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Cardinali DP. Melatonin: clinical perspectives in neurodegeneration. Front Endocrinol (Lausanne) 10: 480, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cardinali DP and Hardeland R. Inflammaging, metabolic syndrome and melatonin: a call for treatment studies. Neuroendocrinology 104: 382–397, 2017. [DOI] [PubMed] [Google Scholar]
- 29. Cardinali DP, Srinivasan V, Brzezinski A, and Brown GM. Melatonin and its analogs in insomnia and depression. J Pineal Res 52: 365–375, 2012. [DOI] [PubMed] [Google Scholar]
- 30. Cardinali DP and Vigo DE. Melatonin, mitochondria, and the metabolic syndrome. Cell Mol Life Sci 74: 3941–3954, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carlberg C and Wiesenberg I. The orphan receptor family RZR/ROR, melatonin and 5-lipoxygenase: an unexpected relationship. J Pineal Res 18: 171–178, 1995. [DOI] [PubMed] [Google Scholar]
- 32. Carrillo-Vico A, Calvo JR, Abreu P, Lardone PJ, García-Mauriño S, Reiter RJ, and Guerrero JM. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J 18: 537–539, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Carrillo-Vico A, García-Pergañeda A, Naji L, Calvo JR, Romero MP, and Guerrero JM. Expression of membrane and nuclear melatonin receptor mRNA and protein in the mouse immune system. Cell Mol Life Sci 60: 2272–2278, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carrillo-Vico A, Lardone PJ, Fernandez-Santos JM, Martín-Lacave I, Calvo JR, Karasek M, and Guerrero JM. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J Clin Endocrinol Metab 90: 992–1000, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Carrillo-Vico A, Lardone PJ, Naji L, Fernández-Santos JM, Martín-Lacave I, Guerrero JM, and Calvo JR. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J Pineal Res 39: 400–408, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Cermakian N, Lange T, Golombek D, Sarkar D, Nakao A, Shibata S, and Mazzoccoli G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int 30: 870–888, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang HC and Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 15: 1448–1460, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang JY, Shi L, Ko ML, and Ko GY. Circadian regulation of mitochondrial dynamics in retinal photoreceptors. J Biol Rhythms 33: 151–165, 2018. [DOI] [PubMed] [Google Scholar]
- 39. Chen G, Huo Y, Tan DX, Liang Z, Zhang W, and Zhang Y. Melatonin in Chinese medicinal herbs. Life Sci 73: 19–26, 2003. [DOI] [PubMed] [Google Scholar]
- 40. Chen J, Wang L, Wu C, Hu Q, Gu C, Yan F, Li J, Yan W, and Chen G. Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J Pineal Res 56: 12–19, 2014. [DOI] [PubMed] [Google Scholar]
- 41. Chen J, Xia H, Zhang L, Zhang H, Wang D, and Tao X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed Pharmacother 117: 109150, 2019. [DOI] [PubMed] [Google Scholar]
- 42. Chen KH, Lin KC, Ko SF, Chiang JY, Guo J, and Yip HK. Melatonin against acute ischaemic stroke dependently via suppressing both inflammatory and oxidative stress downstream signallings. J Cell Mol Med 24: 10402–10419, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen WR, Zhou YJ, Sha Y, Wu XP, Yang JQ, and Liu F. Melatonin attenuates vascular calcification by inhibiting mitochondria fission via an AMPK/Drp1 signalling pathway. J Cell Mol Med 24: 6043–6054, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng L, Liu J, Liu Q, Liu Y, Fan L, Wang F, Yu H, Li Y, Bu L, Li X, Wei W, Wang H, and Sun G. Exosomes from melatonin treated hepatocellularcarcinoma cells alter the immunosupression status through STAT3 pathway in macrophages. Int J Biol Sci 13: 723–734, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chuang JI, Pan IL, Hsieh CY, Huang CY, Chen PC, and Shin JW. Melatonin prevents the dynamin-related protein 1-dependent mitochondrial fission and oxidative insult in the cortical neurons after 1-methyl-4-phenylpyridinium treatment. J Pineal Res 61: 230–240, 2016. [DOI] [PubMed] [Google Scholar]
- 46. Claustrat B, Brun J, and Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev 9: 11–24, 2005. [DOI] [PubMed] [Google Scholar]
- 47. Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, and Maestroni GJM. Evidence for melatonin synthesis in mouse and human bone marrow cells. J Pineal Res 28: 193–202, 2000. [DOI] [PubMed] [Google Scholar]
- 48. Coto-Montes A and Hardeland R. Diurnal rhythm of protein carbonyl as an indicator of oxidative damage in Drosophila melanogaster: influence of clock gene alleles and deficiencies in the formation of free-radical scavengers. Biol Rhythm Res 30: 383–391, 1999. [Google Scholar]
- 49. Coto-Montes A, Tomás-Zapico C, Rodríguez-Colunga MJ, Tolivia-Cadrecha D, Martínez-Fraga J, Hardeland R, and Tolivia D. Effects of the circadian mutation ‘tau’ on the Harderian glands of Syrian hamsters. J Cell Biochem 83: 426–434, 2001. [DOI] [PubMed] [Google Scholar]
- 50. Culnan E, McCullough LM, and Wyatt JK. Circadian rhythm sleep-wake phase disorders. Neurol Clin 37: 527–543, 2019. [DOI] [PubMed] [Google Scholar]
- 51. Deng WG, Tang ST, Tseng HP, and Wu KK. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 108: 518–524, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ding M, Feng N, Tang D, Feng J, Li Z, Jia M, Liu Z, Gu X, Wang Y, Fu F, and Pei J. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J Pineal Res 65: e12491, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dittrich M, Coto-Montes A, and Hardeland R. The Drosophila melanogaster urate null mutant rosy: chronobiology reveals complexity of changes in the antioxidative protection system. In: Studies on Antioxidants and their Metabolites, edited by Hardeland R. Göttingen, Germany: Cuvillier, 1999, pp. 120–123. [Google Scholar]
- 54. Dubocovich ML and Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 27: 101–110, 2005. [DOI] [PubMed] [Google Scholar]
- 55. Dubocovich MI, Rivera-Bermúdez MA, Gerdin MJ, and Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci 8: d1093–d1108, 2003. [DOI] [PubMed] [Google Scholar]
- 56. Dumbell R, Matveeva O, and Oster H. Circadian clocks, stress, and immunity. Front Endocrinol (Lausanne) 7: 37, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Emens JS and Burgess HJ. Effect of light and melatonin and other melatonin receptor agonists on human circadian physiology. Sleep Med Clin 10: 435–453, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Entrena A, Camacho ME, Carrión MD, López-Cara LC, Velasco G, León J, Escames G, Acuña-Castroviejo D, Tapias V, Gallo MA, Vivó A, and Espinosa A. Kynurenamines as neural nitric oxide synthase inhibitors. J Med Chem 48: 8174–8181, 2005. [DOI] [PubMed] [Google Scholar]
- 59. Escames G, Acuña-Castroviejo D, López LC, Tan D-X, Maldonado MD, Sánchez-Hidalgo M, León J, and Reiter RJ. Pharmacological utility of melatonin in the treatment of septic shock: experimental and clinical evidence. J Pharm Pharmacol 58: 1153–1165, 2006. [DOI] [PubMed] [Google Scholar]
- 60. Escames G, López LC, Ortiz F, López A, García JA, Ros E, and Acuña-Castroviejo D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J 274: 2135–2147, 2007. [DOI] [PubMed] [Google Scholar]
- 61. Escames G, López LC, Ortiz F, Ros E, and Acuña-Castroviejo D. Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: effects of melatonin treatment. Exp Gerontol 41: 1165–1173, 2006. [DOI] [PubMed] [Google Scholar]
- 62. Escames G, López LC, Tapias V, Utrilla P, Reiter RJ, Hitos AB, León J, Rodríguez MI, and Acuña-Castroviejo D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J Pineal Res 40: 71–78, 2006. [DOI] [PubMed] [Google Scholar]
- 63. Fan Z, Zhao M, Joshi PD, Li P, Zhang Y, Guo W, Xu Y, Wang H, Zhao Z, and Yan J. A class of circadian long non-coding RNAs mark enhancers modulating long-range circadian gene regulation. Nucleic Acids Res 45: 5720–5738, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Feng D, Wang B, Wang L, Abraham N, Tao K, Huang L, Shi W, Dong Y, and Qu Y. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J Pineal Res 62: e12395, 2017. [DOI] [PubMed] [Google Scholar]
- 65. Feng J, Chen X, Liu R, Cao C, Zhang W, Zhao Y, and Nie S. Melatonin protects against myocardial ischemia-reperfusion injury by elevating Sirtuin3 expression and manganese superoxide dismutase activity. Free Radic Res 52: 840–849, 2018. [DOI] [PubMed] [Google Scholar]
- 66. Fuhrberg B, Hardeland R, Poeggeler B, and Behrmann G. Dramatic rises of melatonin and 5-methoxytryptamine in Gonyaulax exposed to decreased temperature. Biol Rhythm Res 28: 144–150, 1997. [Google Scholar]
- 67. Gao K, Niu J, and Dang X. Neuroprotection of melatonin on spinal cord injury by activating autophagy and inhibiting apoptosis via SIRT1/AMPK signaling pathway. Biotechnol Lett 42: 2059–2069, 2020. [DOI] [PubMed] [Google Scholar]
- 68. García JA, Ortiz F, Miana J, Doerrier C, Fernández-Ortiz M, Rusanova I, Escames G, García JJ, and Acuña-Castroviejo D. Contribution of inducible and neuronal nitric oxide synthases to mitochondrial damage and melatonin rescue in LPS-treated mice. J Physiol Biochem 73: 235–244, 2017. [DOI] [PubMed] [Google Scholar]
- 69. García S, Martín Giménez VM, Mocayar Marón FJ, Reiter RJ, and Manucha W. Melatonin and cannabinoids: mitochondrial-targeted molecules that may reduce inflammaging in neurodegenerative diseases. Histol Histopathol 35: 789–800, 2020. [DOI] [PubMed] [Google Scholar]
- 70. García-Pergañeda A, Pozo D, Guerrero JM, and Calvo JR. Signal transduction for melatonin in human lymphocytes: involvement of a pertussis toxin-sensitive G protein. J Immunol 159: 3774–3781, 1997. [PubMed] [Google Scholar]
- 71. Gitto E, Aversa S, Reiter RJ, Barberi I, and Pellegrino S. Update on the use of melatonin in pediatrics. J Pineal Res 50: 21–28, 2011. [DOI] [PubMed] [Google Scholar]
- 72. Gitto E, Karbownik M, Reiter RJ, Tan D-X, Cuzzocrea S, Chiurazzi P, Cordaro S, Corona G, Trimarchi G, and Barberi I. Effects of melatonin treatment in septic newborns. Pediatr Res 50: 756–760, 2001. [DOI] [PubMed] [Google Scholar]
- 73. Gómez M, Esparza JL, Nogués MR, Giralt M, Cabré M, and Domingo JL. Pro-oxidant activity of aluminum in the rat hippocampus: gene expression of antioxidant enzymes after melatonin administration. Free Radic Biol Med 38: 104–111, 2005. [DOI] [PubMed] [Google Scholar]
- 74. Goyal A, Terry PD, Superak HM, Nell-Dybdahl CL, Chowdhury R, Phillips LS, and Kutner MH. Melatonin supplementation to treat the metabolic syndrome: a randomized controlled trial. Diabetol Metab Syndr 6: 124, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guenther AL, Schmidt SI, Laatsch H, Fotso S, Ness H, Ressmeyer A-R, Poeggeler B, and Hardeland R. Reactions of the melatonin metabolite AMK (N1-acetyl-5-methoxykynuramine) with reactive nitrogen species: formation of novel compounds, 3-acetamidomethyl-6-methox-ycinnolinone and 3-nitro-AMK. J Pineal Res 39: 251–260, 2005. [DOI] [PubMed] [Google Scholar]
- 76. Guerrero JM and Reiter RJ. Melatonin-immune system relationships. Curr Top Med Chem 2: 167–179, 2000. [DOI] [PubMed] [Google Scholar]
- 77. Guo Y, Sun J, Bu S, Li B, Zhang Q, Wang Q, and Lai D. Melatonin protects against chronic stress-induced oxidative meiotic defects in mice MII oocytes by regulating SIRT1. Cell Cycle 19: 1677–1695, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gupta M, Gupta YK, Agarwal S, Aneja S, and Kohli K. A randomized, double-blind, placebo controlled trial of melatonin add-on therapy in epileptic children on valproate monotherapy: effect on glutathione peroxidase and glutathione reductase enzymes. Br J Clin Pharmacol 58: 542–547, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hack LM, Lockley SW, Arendt J, and Skene DJ. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J Biol Rhythms 18: 420–429, 2003. [DOI] [PubMed] [Google Scholar]
- 80. Han L, Wang H, Li L, Li X, Ge J, Reiter RJ, and Wang Q. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J Pineal Res 63: e12431, 2017. [DOI] [PubMed] [Google Scholar]
- 81. Han N, Wang Z, and Li X. Melatonin alleviates d-galactose-decreased hyaluronic acid production in synovial membrane cells via Sirt1 signalling. Cell Biochem Funct 39: 488–495, 2021. [DOI] [PubMed] [Google Scholar]
- 82. Hardeland R. Antioxidative protection by melatonin—multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 27: 119–130, 2005. [DOI] [PubMed] [Google Scholar]
- 83. Hardeland R. Melatonin, hormone of darkness and more—occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci 65: 2001–2018, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hardeland R. New approaches in the management of insomnia: weighing the advantages of prolonged release melatonin and synthetic melatoninergic agonists. Neuropsychiatr Dis Treat 5: 341–354, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hardeland R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors 35: 183–192, 2009. [DOI] [PubMed] [Google Scholar]
- 86. Hardeland R. Melatonin, mitochondrial electron flux and leakage: recent findings and resolution of contradictory results. Adv Stud Biol 1: 207–230, 2009. [Google Scholar]
- 87. Hardeland R. Investigational melatonin receptor agonists. Expert Opin Investig Drugs 19: 747–764, 2010. [DOI] [PubMed] [Google Scholar]
- 88. Hardeland R. Melatonin and its metabolites as anti-nitrosating and anti-nitrating agents. J Exp Integ Med 1: 67–81, 2011. [Google Scholar]
- 89. Hardeland R. Melatonin in aging and disease—multiple consequences of reduced secretion, options and limits of treatment. Aging Dis 3: 194–225, 2012. [PMC free article] [PubMed] [Google Scholar]
- 90. Hardeland R. Melatonin and the theories of aging: a critical appraisal of melatonin's role in antiaging mechanisms. J Pineal Res 55: 325–356, 2013. [DOI] [PubMed] [Google Scholar]
- 91. Hardeland R. Melatonin's antioxidant properties: molecular mechanisms. In: Melatonin and Melatonergic Drugs in Clinical Practice, edited by Srinivasan V, Brzezinski A, Oter S, and Shillcutt SD. New Delhi, India: Springer India, 2014, pp. 17–26. [Google Scholar]
- 92. Hardeland R. Melatonin, noncoding RNAs, messenger RNA stability and epigenetics—evidence, hints, gaps and perspectives. Int J Mol Sci 15: 18221–18252, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hardeland R. Melatonin and circadian oscillators in aging—a dynamic approach to the multiply connected players. Interdisc Top Gerontol 40, 128–140, 2015. [DOI] [PubMed] [Google Scholar]
- 94. Hardeland R. Melatonin—more than just a pineal hormone. Biomed J Sci Tech Res 1, 351, 2017. [Google Scholar]
- 95. Hardeland R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J Pineal Res 62: e12377, 2017. [DOI] [PubMed] [Google Scholar]
- 96. Hardeland R. The underrated carbonate radical (CO3•–)—detoxification and reduced formation by melatonin. Biomed J Sci Tech Res 1: 264, 2017. [Google Scholar]
- 97. Hardeland R. Focus on the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK). Biomed J Sci Tech Res 1: 318, 2017. [Google Scholar]
- 98. Hardeland R. Intercellular communication via exosomal and ectosomal microRNAs: Facing a jungle of countless microRNAs and targets. In: Mini-Reviews in Recent Melatonin Research, edited by Hardeland R. Göttingen, Germany: Cuvillier, 2017, pp. 109–122. [Google Scholar]
- 99. Hardeland R. Melatonin and the electron transport chain. Cell Mol Life Sci 74: 3883–3896, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hardeland R. Melatonin and retinoid orphan receptors: demand for new interpretations after their exclusion as nuclear melatonin receptors. Melatonin Res 1: 77–92, 2018. [Google Scholar]
- 101. Hardeland R. On the relationships between lncRNAs and other orchestrating regulators: role of the circadian system. Epigenomes 2: 9, 2018. [Google Scholar]
- 102. Hardeland R. Interactions of melatonin and microRNAs. Biochem Mol Biol J 4: 7, 2018. [Google Scholar]
- 103. Hardeland R. Extended signaling by melatonin. Cell Cell Life Sci J 3: 123, 2018. [Google Scholar]
- 104. Hardeland R. Melatonin and inflammation—story of a double-edged blade. J Pineal Res 65: e12525, 2018. [DOI] [PubMed] [Google Scholar]
- 105. Hardeland R. Aging, melatonin and the pro- and anti-inflammatory networks. Int J Mol Sci 20, 1223, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hardeland R. Melatonin and chromatin. Melatonin Res 2: 67–93, 2019. [Google Scholar]
- 107. Hardeland, R. Noncoding RNAs: bridging regulation of circadian rhythms and inflammation. Adv Neuroimmune Biol 7: 155–177, 2019. [Google Scholar]
- 108. Hardeland R. Dysregulation of sirtuins in cancer. Oncol Res Rev 2: 1–4, 2019. [Google Scholar]
- 109. Hardeland R. Sirtuins, melatonin, and the relevance of circadian oscillators. In: Sirtuin Biology in Medicine. Targeting New Avenues of Care in Development, Aging, and Disease, edited by Maiese K. London, United Kingdom: Academic Press, 2021, pp. 137–151. [Google Scholar]
- 110. Hardeland R, Balzer I, Poeggeler B, Fuhrberg B, Uría H, Behrmann G, Wolf R, Meyer TJ, and Reiter RJ. On the primary functions of melatonin in evolution: mediation of photoperiodic signals in a unicell, photooxidation and scavenging of free radicals. J Pineal Res 18: 104–111, 1995. [DOI] [PubMed] [Google Scholar]
- 111. Hardeland R, Cardinali DP, Brown GM, and Pandi-Perumal SR. Melatonin and brain inflammaging. Prog Neurobiol 127–128: 46–63, 2015. [DOI] [PubMed] [Google Scholar]
- 112. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, and Pandi-Perumal SR. Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93: 350–384, 2011. [DOI] [PubMed] [Google Scholar]
- 113. Hardeland R, Coto-Montes A, and Poeggeler B. Circadian rhythms, oxidative stress and antioxidative defense mechanisms. Chronobiol Int 20: 921–962, 2003. [DOI] [PubMed] [Google Scholar]
- 114. Hardeland R, Fuhrberg B, Behrmann G, and Balzer I. Sleep-latency reducing pineal hormone melatonin as a scavenger of free radicals: hemin-catalysed formation of N1-acetyl-N2-formyl-5-methoxykynuramine. Sleep Res 22: 621, 1993. [Google Scholar]
- 115. Hardeland R, Madrid JA, Tan, D-X, and Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res 52: 139–166, 2012. [DOI] [PubMed] [Google Scholar]
- 116. Hardeland R, Pandi-Perumal SR, and Poeggeler B. Melatonin in plants—focus on a vertebrate night hormone with cytoprotective properties. Funct Plant Sci Biotechnol 1: 32–45, 2007. [Google Scholar]
- 117. Hardeland R and Poeggeler B. Melatonin beyond its classical functions. Open Physiol J 1: 1–23, 2008. [Google Scholar]
- 118. Hardeland R, Poeggeler B, Balzer I, and Behrmann G. Common basis of photoperiodism in phylogenetically distant organisms and its possible origins. J Interdiscipl Cycle Res 22: 122–123, 1991. [Google Scholar]
- 119. Hardeland R, Poeggeler B, Balzer I, and Behrmann G. A hypothesis on the evolutionary origins of photoperiodism based on circadian rhythmicity of melatonin in phylogenetically distant organisms. In: Chronobiology & Chronomedicine, edited by Gutenbrunner C, Hildebrandt G, and Moog R. Frankfurt/M., Germany: Lang, 1993, pp. 113–120. [Google Scholar]
- 120. Hardeland R, Poeggeler B, and Pappolla MA. Mitochondrial actions of melatonin—an endeavor to identify their adaptive and cytoprotective mechanisms. Proc Saxon Acad Sci 65(Pt 3): 14–31, 2009. [Google Scholar]
- 121. Hardeland R and Tan D-X. Protection by melatonin in respiratory diseases: valuable information for the treatment of COVID-19. Melatonin Res 3: 264–275, 2020. [Google Scholar]
- 122. He B, Zhang W, Qiao J, Peng Z, and Chai X. Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can J Physiol Pharmacol 97: 386–391, 2019. [DOI] [PubMed] [Google Scholar]
- 123. He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, and Liu G. Mitochondria synthesize melatonin to ameliorate its junction and improve mice oocyte's quality under in vitro conditions. Int J Mol Sci 17: 939, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Henderson R, Kim S, and Lee E. Use of melatonin as adjunctive therapy in neonatal sepsis: a systematic review and meta-analysis. Complement Ther Med 39: 131–136, 2018. [DOI] [PubMed] [Google Scholar]
- 125. Hergenhan S, Holtkamp S, and Scheiermann C. Molecular interactions between components of the circadian clock and the immune system. J Mol Biol 432: 3700–3713, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hill SM, Blask DE, Xiang S, Yuan L, Mao L, Dauchy RT, Dauchy EM, Frasch T, and Duplesis T. Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia 16: 235–245, 2011. [DOI] [PubMed] [Google Scholar]
- 127. Hoffman RA, Johnson LB, and Reiter RJ. Harderian glands of golden hamsters: temporal and sexual differences in immunoreactive melatonin. J Pineal Res 2: 161–168, 1985. [DOI] [PubMed] [Google Scholar]
- 128. Hsiao CW, Peng TI, Peng AC, Reiter RJ, Tanaka M, Lai YK, and Jou MJ. Long-term Aβ exposure augments mCa2+-independent mROS-mediated depletion of cardiolipin for the shift of a lethal transient mitochondrial permeability transition to its permanent mode in NARP cybrids: a protective targeting of melatonin. J Pineal Res 54: 107–125, 2013. [DOI] [PubMed] [Google Scholar]
- 129. Huang KP, Chen C, Hao J, Huang JY, Liu PQ, and Huang HQ. AGEs-RAGE system down-regulates Sirt1 through the ubiquitin-proteasome pathway to promote FN and TGF-β1 expression in male rat glomerular mesangial cells. Endocrinology 156: 268–279, 2015. [DOI] [PubMed] [Google Scholar]
- 130. Huether G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49: 665–670, 1993. [DOI] [PubMed] [Google Scholar]
- 131. Jiménez-Ortega V, Cano P, Cardinali DP, and Esquifino AI. 24-Hour variation in gene expression of redox pathway enzymes in rat hypothalamus: effect of melatonin treatment. Redox Rep 14: 132–138, 2009. [DOI] [PubMed] [Google Scholar]
- 132. Joshi AU, Ebert AE, Haileselassie B, and Mochly-Rosen D. Drp1/Fis1-mediated mitochondrial fragmentation leads to lysosomal dysfunction in cardiac models of Huntington's disease. J Mol Cell Cardiol 27: 125–133, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jou MJ. Melatonin preserves the transient mitochondrial transition for protection during mitochondrial Ca2+ stress in astrocyte. J Pineal Res 50: 427–435, 2011. [DOI] [PubMed] [Google Scholar]
- 134. Jung-Hynes B, Reiter RJ, and Ahmad N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J Pineal Res 48: 9–19, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jung-Hynes B, Schmit TL, Reagan-Shaw SR, Siddiqui IA, Mukhtar H, and Ahmad N. Melatonin, a novel Sirt1 inhibitor, imparts proliferative effects against prostate cancer cells in vitro culture and in vivo in TRAMP model. J Pineal Res 50: 140–149, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kang JW, Hong JM, and Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res 60: 383–393, 2016. [DOI] [PubMed] [Google Scholar]
- 137. Kato K, Hirai K, Nishiyama K, Uchikawa O, Fukatsu K, Ohkawa S, Kawamata Y, Hinuma S, and Miyamoto M. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 48: 301–310, 2005. [DOI] [PubMed] [Google Scholar]
- 138. Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, and Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA 8: 1413, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Konakchieva R, Kyurkchiev S, Kehayov I, Taushanova P, and Kanchev L. Selective effect of methoxyindoles on the lymphocyte proliferation and melatonin binding to activated human lymphoid cells. J Neuroimmunol 63: 125–132, 1995. [DOI] [PubMed] [Google Scholar]
- 140. Korkmaz A, Rosales-Corral S, and Reiter RJ. Gene regulation by melatonin linked to epigenetic phenomena. Gene 503: 1–11, 2012. [DOI] [PubMed] [Google Scholar]
- 141. Kotler M, Rodríguez C, Sáinz RM, Antolín I, and Menéndez-Peláez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res 24: 83–89, 1998. [DOI] [PubMed] [Google Scholar]
- 142. Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, and Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res 50: 261–266, 2011. [DOI] [PubMed] [Google Scholar]
- 143. Labrecque N and Cermakian N. Circadian clocks in the immune system. J Biol Rhythms 30: 277–290, 2015. [DOI] [PubMed] [Google Scholar]
- 144. Lahiri DK, Chen D, Ge YW, Bondy SC, and Sharman EH. Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J Pineal Res 36: 224–231, 2004. [DOI] [PubMed] [Google Scholar]
- 145. Landau M and Zisapel N. The low affinity binding of melatonin to calmodulin: use of computational methods to explain its physiological relevance. In: Melatonin—From Molecules to Therapy, edited by Pandi-Perumal SR and Cardinali DP. Nova Science New York, USA: Nova Science, 2007. pp. 69–79. [Google Scholar]
- 146. Lanfumey L, Mongeau R, and Hamon M. Biological rhythms and melatonin in mood disorders and their treatments. Pharmacol Ther 138: 176–184, 2013. [DOI] [PubMed] [Google Scholar]
- 147. Lardone PJ, Carrillo-Vico A, Molinero P, Rubio A, and Guerrero JM. A novel interplay between membrane and nuclear melatonin receptors in human lymphocytes: significance in IL-2 production. Cell Mol Life Sci 66: 516–525, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lardone PJ, Carrillo-Vico A, Naranjo MC, De Felipe B, Vallejo A, Karasek M, and Guerrero JM. Melatonin synthesized by Jurkat human leukemic T cell line is implicated in IL-2 production. J Cell Physiol 206: 273–279, 2006. [DOI] [PubMed] [Google Scholar]
- 149. Lee SE, Kim SJ, Youn J-P, Hwang SY, Park C-S, and Park YS. MicroRNA and gene expression analysis of melatonin-exposed breast cancer cell lines indicating involvement of the anticancer effect. J Pineal Res 51: 345–352, 2011. [DOI] [PubMed] [Google Scholar]
- 150. Leon J, Acuña-Castroviejo D, Sainz RM, Mayo JC, Tan D-X, and Reiter RJ. Melatonin and mitochondrial function. Life Sci 75: 765–790, 2004. [DOI] [PubMed] [Google Scholar]
- 151. León J, Escames G, Rodríguez MI, López LC, Tapias V, Entrena A, Camacho E, Carrión MD, Gallo MA, Espinosa A, Tan D-X, Reiter RJ, and Acuña-Castroviejo D. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J Neurochem 98: 2023–2033, 2006. [DOI] [PubMed] [Google Scholar]
- 152. León J, Macías M, Escames G, Camacho E, Khaldy H, Martín M, Espinosa A, Gallo MA, and Acuña-Castroviejo, D. Structure-related inhibition of calmodulin-dependent neuronal nitric-oxide synthase activity by melatonin and synthetic kynurenines. Mol Pharmacol 58: 967–975, 2000. [DOI] [PubMed] [Google Scholar]
- 153. Leong I. Metabolism: DRP1 links mitochondrial dynamics to the clock. Nat Rev Endocrinol 14: 252, 2018. [DOI] [PubMed] [Google Scholar]
- 154. Lerner AB, Case JD, and Takahashi Y. Isolation of melatonin, a pineal factor that lightens melanocytes. J Am Chem Soc 80: 2587, 1958. [Google Scholar]
- 155. Lewy AJ, Emens JS, Lefler BJ, Yuhas K, and Jackman AR. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol Int 22: 1093–1106, 2005. [DOI] [PubMed] [Google Scholar]
- 156. Lewy AJ, Emens JS, Sack RL, Hasler BP, and Bernert RA. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol Int 19: 649–658, 2002. [DOI] [PubMed] [Google Scholar]
- 157. Li J, Liu L, Zhou X, Lu X, Liu X, Li G, and Long J. Melatonin attenuates sepsis-induced acute lung injury through improvement of epithelial sodium channel-mediated alveolar fluid clearance via activation of SIRT1/SGK1/Nedd4-2 signaling pathway. Front Pharmacol 11: 590652, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Li S and Zhang L. Circadian control of global transcription. Biomed Res Int 2015: 187809, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Li Y, Li S, Zhou Y, Meng X, Zhang JJ, Xu DP, and Li HB. Melatonin for the prevention and treatment of cancer. Oncotarget 8: 39896–39921, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Liu D, Ma Z, Di S, Yang Y, Yang J, Xu L, Reiter RJ, Qiao S, and Yuan J. AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic Biol Med 129: 59–72, 2018. [DOI] [PubMed] [Google Scholar]
- 161. Liu F and Ng TB. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem Cell Biol 78: 447–453, 2000. [DOI] [PubMed] [Google Scholar]
- 162. Liu J, Yan W, Zhao X, Jia Q, Wang J, Zhang H, Liu C, He K, and Sun Z. Sirt3 attenuates post-infarction cardiac injury via inhibiting mitochondrial fission and normalization of AMPK-Drp1 pathways. Cell Signal 53: 1–13, 2019. [DOI] [PubMed] [Google Scholar]
- 163. Liu L, Chen H, Jin J, Tang Z, Yin P, Zhong D, and Li G. Melatonin ameliorates cerebral ischemia/reperfusion injury through SIRT3 activation. Life Sci 239: 117036, 2019. [DOI] [PubMed] [Google Scholar]
- 164. Liu Z, Gan L, Zhang T, Ren Q, and Sun C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res 64: e12455, 2018. [DOI] [PubMed] [Google Scholar]
- 165. Liu ZJ, Ran YY, Qie SY, Gong WJ, Gao FH, Ding ZT, and Xi JN. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther 25: 1353–1362, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. López LC, Escames G, Tapias V, Utrilla P, León J, and Acuña-Castroviejo D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: its relation with mitochondrial dysfunction and prevention by melatonin. Int J Biochem Cell Biol 38: 267–278, 2006. [DOI] [PubMed] [Google Scholar]
- 167. Luchetti F, Canonico B, Mannello F, Masoni C, D'Emilio A, Battistelli M, Papa S, and Falcieri E. Melatonin reduces early changes in intramitochondrial cardiolipin during apoptosis in U937 cell line. Toxicol In Vitro 21: 293–301, 2007. [DOI] [PubMed] [Google Scholar]
- 168. Ma M, Chen X-Y, Li B, and Li X-T. Melatonin protects premature ovarian insufficiency induced by tripterygium glycosides: role of SIRT1. Am J Transl Res 9: 1580–1602, 2017. [PMC free article] [PubMed] [Google Scholar]
- 169. Ma S, Chen J, Feng J, Zhang R, Fan M, Han D, Li X, Li C, Ren J, Wang Y, and Cao F. Melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxid Med Cell Longev 2018: 9286458, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ma Z, Xin Z, Di W, Yan X, Li X, Reiter RJ, and Yang Y. Melatonin and mitochondrial function during ischemia/reperfusion injury. Cell Mol Life Sci 74: 3989–3998, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Maldonado MD, Mora-Santos M, Naji L, Carrascosa-Salmoral MP, Naranjo MC, and Calvo JR. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol Res 62: 282–287, 2010. [DOI] [PubMed] [Google Scholar]
- 172. Martín M, Macías M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, and Acuña-Castroviejo D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res 28: 242–248, 2000. [DOI] [PubMed] [Google Scholar]
- 173. Martín M, Macías M, León J, Escames G, Khaldy H, and Acuña-Castroviejo D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol 34: 348–357, 2002. [DOI] [PubMed] [Google Scholar]
- 174. Masri S. Sirtuin-dependent clock control: new advances in metabolism, aging and cancer. Curr Opin Clin Nutr Metab Care 18: 521–527, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Masri S, Orozco-Solis R, Aguilar-Arnal L, Cervantes M, and Sassone-Corsi P. Coupling circadian rhythms of metabolism and chromatin remodelling. Diabetes Obes Metab 17(Suppl 1): 17–22, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Masri S, Rigor P, Cervantes M, Ceglia N, Sebastian C, Xiao C, Roqueta-Rivera M, Deng C, Osborne TF, Mostoslavsky R, Baldi P, and Sassone-Corsi P. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158: 659–762, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Masri S and Sassone-Corsi P. Sirtuins and the circadian clock: bridging chromatin and metabolism. Sci Signal 7: re6, 2014. [DOI] [PubMed] [Google Scholar]
- 178. Matsubara E, Bryant-Thomas T, Pacheco Quinto J, Henry TL, Poeggeler B, Herbert D, Cruz-Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke-Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA, Chain DG, and Neria E. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer's disease. J Neurochem 85: 1101–1108, 2003. [DOI] [PubMed] [Google Scholar]
- 179. Mavroudis PD, Scheff JD, Calvano SE, and Androulakis IP. Systems biology of circadian-immune interactions. J Innate Immun 5: 153–162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, and Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci 59: 1706–1713, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Mayo JC, Sainz RM, Tan D-X, Hardeland R, Leon J, Rodriguez C, and Reiter RJ. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-me-thoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol 165: 139–149, 2005. [DOI] [PubMed] [Google Scholar]
- 182. Meldolesi J. Ectosomes and exosomes—two extracellular vesicles that differ only in some details. Biochem Mol Biol J 2: 3, 2016. [Google Scholar]
- 183. Merlo S, Luaces JP, Spampinato SF, Toro-Urrego N, Caruso GI, D'Amico F, Capani F, and Sortino MA. SIRT1 mediates melatonin's effects on microglial activation in hypoxia: in vitro and in vivo evidence. Biomolecules 10: 364, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Messner M, Hardeland R, Rodenbeck A, and Huether G. Tissue retention and subcellular distribution of continuously infused melatonin in rats under near physiological conditions. J Pineal Res 25: 251–259, 1998. [DOI] [PubMed] [Google Scholar]
- 185. Moretti RM, Montagnani Marelli M, Sala A, Motta M, and Limonta P. Activation of the orphan nuclear receptor RORα counteracts the proliferative effect of fatty acids on prostate cancer cells: crucial role of 5-lipoxygenase. Int J Cancer 112: 87–93, 2004. [DOI] [PubMed] [Google Scholar]
- 186. Mortola JP. Breathing around the clock: an overview of the circadian pattern of respiration. Eur J Appl Physiol 91: 119–129, 2004. [DOI] [PubMed] [Google Scholar]
- 187. Mortola JP and Seifert EL. Circadian patterns of breathing. Respir Physiol Neurobiol 131: 91–100, 2002. [DOI] [PubMed] [Google Scholar]
- 188. Naranjo MC, Guerrero JM, Rubio A, Lardone PJ, Carrillo-Vico A, Carrascosa-Salmoral MP, Jiménez-Jorge S, Arellano MV, Leal-Noval SR, Leal M, Lissen E, and Molinero P. Melatonin biosynthesis in the thymus of humans and rats. Cell Mol Life Sci 64: 781–790, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Navarro-Alarcón M, Ruiz-Ojeda FJ, Blanca-Herrera RM, A-Serrano MM, Acuña-Castroviejo D, Fernández-Vázquez G, and Agil A. Melatonin and metabolic regulation: a review. Food Funct 5: 2806–2832, 2014. [DOI] [PubMed] [Google Scholar]
- 190. NaveenKumar SK, Hemshekhar M, Jagadish S, Manikanta K, Vishalakshi GJ, Kemparaju K, and Girish KS. Melatonin restores neutrophil functions and prevents apoptosis amid dysfunctional glutathione redox system. J Pineal Res 69: e12676, 2020. [DOI] [PubMed] [Google Scholar]
- 191. Nduhirabandi F and Maarman GJ. Melatonin in heart failure: a promising therapeutic strategy? Molecules 23: 1819, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Nickkholgh A, Schneider H, Sobirey M, Venetz WP, Hinz U, Pelzl LH, Gotthardt DN, Cekauskas A, Manikas M, Mikalauskas S, Mikalauskene L, Bruns H, Zorn M, Weigand MA, Büchler MW, and Schemmer P. The use of high-dose melatonin in liver resection is safe: first clinical experience. J Pineal Res 50: 381–388, 2011. [DOI] [PubMed] [Google Scholar]
- 193. Nopparat C, Sinjanakhom P, and Govitrapong P. Melatonin reverses H2O2-induced senescence in SH-SY5Y cells by enhancing autophagy via sirtuin 1 deacetylation of the RelA/p65 subunit of NF-κB. J Pineal Res 63: e12407, 2017. [DOI] [PubMed] [Google Scholar]
- 194. Okatani Y, Wakatsuki A, and Reiter RJ. Melatonin protects hepatic mitochondrial respiratory chain activity in senescence-accelerated mice. J Pineal Res 32: 143–148, 2002. [DOI] [PubMed] [Google Scholar]
- 195. Okatani Y, Wakatsuki A, Reiter RJ, and Miyahara Y. Acutely administered melatonin restores hepatic mitochondrial physiology in old mice. Int J Biochem Cell Biol 35: 367–375, 2003. [DOI] [PubMed] [Google Scholar]
- 196. Okatani Y, Wakatsuki A, Shinohara K, Kaneda C, and Fukaya T. Melatonin stimulates glutathione peroxidase activity in human chorion. J Pineal Res 30: 199–205, 2001. [DOI] [PubMed] [Google Scholar]
- 197. Ortiz F, García JA, Acuña-Castroviejo D, Doerrier C, López A, Venegas C, Volt H, Luna-Sánchez M, López LC, and Escames G. The beneficial effects of melatonin against heart mitochondrial impairment during sepsis: inhibition of iNOS and preservation of nNOS. J Pineal Res 56: 71–81, 2014. [DOI] [PubMed] [Google Scholar]
- 198. Pablos MI, Agapito MT, Gutierrez R, Recio JM, Reiter RJ, Barlow-Walden L, Acuña-Castroviejo D, and Menendez-Pelaez A. Melatonin stimulates the activity of the detoxifying enzyme glutathione peroxidase in several tissues of chicks. J Pineal Res 19: 111–115, 1995. [DOI] [PubMed] [Google Scholar]
- 199. Pablos MI, Chuang J, Reiter RJ, Ortiz GG, Daniels WM, Sewerynek E, Melchiorri D, and Poeggeler B. Time course of the melatonin-induced increase in glutathione peroxidase activity in chick tissues. Biol Signals 4: 325–330, 1995. [DOI] [PubMed] [Google Scholar]
- 200. Pablos MI, Reiter RJ, Ortiz GG, Guerrero JM, Agapito MT, Chuang JI, and Sewerynek E. Rhythms of glutathione peroxidase and glutathione reductase in brain of chick and their inhibition by light. Neurochem Int 32: 69–75, 1998. [DOI] [PubMed] [Google Scholar]
- 201. Pandi-Perumal SR, Srinivasan V, Poeggeler B, Hardeland R, and Cardinali DP. Drug insight: the use of melatonergic agonists for the treatment of insomnia—focus on ramelteon. Nat Clin Pract Neurol 3: 221–228, 2007. [DOI] [PubMed] [Google Scholar]
- 202. Paradies G, Petrosillo G, Paradies V, Reiter RJ, and Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res 48: 297–310, 2010. [DOI] [PubMed] [Google Scholar]
- 203. Pastore N, Vainshtein A, Herz NJ, Huynh T, Brunetti L, Klisch TJ, Mutarelli M, Annunziata P, Kinouchi K, Brunetti-Pierri N, Sassone-Corsi P, and Ballabio A. Nutrient-sensitive transcription factors TFEB and TFE3 couple autophagy and metabolism to the peripheral clock. EMBO J 38: e101347, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, and Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342: 1243417, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Peng Z, Zhang W, Qiao J, and He B. Melatonin attenuates airway inflammation via SIRT1 dependent inhibition of NLRP3 inflammasome and IL-1β in rats with COPD. Int Immunopharmacol 62: 23–28, 2018. [DOI] [PubMed] [Google Scholar]
- 206. Petrosillo G, Di Venosa N, Pistolese M, Casanova G, Tiravanti E, Colantuono G, Federici A, Paradies G, and Ruggiero FM. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia-reperfusion: role of cardiolipin. FASEB J 20: 269–276, 2006. [DOI] [PubMed] [Google Scholar]
- 207. Petrosillo G, Fattoretti P, Matera M, Ruggiero FM, Bertoni-Freddari C, and Paradies G. Melatonin prevents age-related mitochondrial dysfunction in rat brain via cardiolipin protection. Rejuvenation Res 11: 935–943, 2008. [DOI] [PubMed] [Google Scholar]
- 208. Pi H, Xu S, Reiter RJ, Guo P, Zhang L, Li Y, Li M, Cao Z, Tian L, Xie J, Zhang R, He M, Lu Y, Liu C, Duan W, Yu Z, and Zhou Z. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 11: 1037–1051, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Pi QZ, Wang XW, Jian ZL, Chen D, Zhang C, and Wu QC. Melatonin alleviates cardiac dysfunction via increasing Sirt1-mediated beclin-1 deacetylation and autophagy during sepsis. Inflammation 44: 1184–1193, 2021. [DOI] [PubMed] [Google Scholar]
- 210. Pozo D, García-Mauriño S, Guerrero JM, and Calvo JR. mRNA expression of nuclear receptor RZR/RORα, melatonin membrane receptor MT1, and hydroxindole-O-methyltransferase in different populations of human immune cells. J Pineal Res 37: 48–54, 2004. [DOI] [PubMed] [Google Scholar]
- 211. Proietti S, Cucina A, Minini M, and Bizzarri M. Melatonin, mitochondria, and the cancer cell. Cell Mol Life Sci 74: 4015–4025, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Quay WB. Circadian and estrous rhythms in pineal melatonin and 5-hydroxy indole-3-acetic acid. Proc Soc Exp Biol 115: 710–713, 1964. [DOI] [PubMed] [Google Scholar]
- 213. Quintela T, Gonçalves I, Silva M, Duarte AC, Guedes P, Andrade K, Freitas F, Talhada D, Albuquerque T, Tavares S, Passarinha LA, Cipolla-Neto J, and Santos CRA. Choroid plexus is an additional source of melatonin in the brain. J Pineal Res 65: e12528, 2018. [DOI] [PubMed] [Google Scholar]
- 214. Radogna F, Cristofanon S, Paternoster L, D'Alessio M, De Nicola M, Cerella C, Dicato M, Diederich M, and Ghibelli L. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J Pineal Res 44: 316–325, 2008. [DOI] [PubMed] [Google Scholar]
- 215. Radogna F, Sestili P, Martinelli C, Paolillo M, Paternoster L, Albertini MC, Accorsi A, Gualandi G, and Ghibelli L. Lipoxygenase-mediated pro-radical effect of melatonin via stimulation of arachidonic acid metabolism. Toxicol Appl Pharmacol 238: 170–177, 2009. [DOI] [PubMed] [Google Scholar]
- 216. Rehman SU, Ikram M, Ullah N, Alam SI, Park HY, Badshah H, Choe K, and Kim MO. Neurological enhancement effects of melatonin against brain injury-induced oxidative stress, neuroinflammation, and neurodegeneration via AMPK/CREB signaling. Cells 8: 760, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol 79: C153–C159, 1991. [DOI] [PubMed] [Google Scholar]
- 218. Reiter RJ. Pineal gland: interface between the photoperiodic environment and the endocrine system. Trends Endocrinol Metab 2: 13–19, 1991. [DOI] [PubMed] [Google Scholar]
- 219. Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia 49: 654–664, 1993. [DOI] [PubMed] [Google Scholar]
- 220. Reiter RJ, Garcia JJ, and Pie J. Oxidative toxicity in models of neurodegeneration: responses to melatonin. Restor Neurol Neurosci 12: 135–142, 1998. [PubMed] [Google Scholar]
- 221. Reiter RJ, Korkmaz A, Paredes SD, Manchester LC, and Tan D-X. Melatonin reduces oxidative/nitrosative stress due to drugs, toxins, metals, and herbicides. Neuro Endocrinol Lett 29: 609–613, 2008. [PubMed] [Google Scholar]
- 222. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, and Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res 61: 253–278, 2016. [DOI] [PubMed] [Google Scholar]
- 223. Reiter RJ, Rosales-Corral SA, Tan D-X, Acuna-Castroviejo D, Qin L, Yang SF, and Xu K. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci 18: 843, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Reiter RJ, Rosales-Corral S, Tan D-X, Jou MJ, Galano A, and Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol Life Sci 74: 3863–3881, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Reiter RJ, Tan DX, Kim SJ, and Cruz MHC. Delivery of pineal melatonin to the brain and SCN: role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct Funct 219: 1873–1887, 2014. [DOI] [PubMed] [Google Scholar]
- 226. Reiter RJ, Tan D-X, Mayo JC, Sainz RM, Leon J, and Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol 50: 1129–1146, 2003. [PubMed] [Google Scholar]
- 227. Reiter RJ, Tan D-X, Osuna C, and Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci 7: 444–458, 2000. [DOI] [PubMed] [Google Scholar]
- 228. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Jou M-J, and Acuna-Castroviejo D. Melatonin mitigates mitochondrial meltdown: interactions with SIRT3. Int J Mol Sci 19: 2439, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, and Xu B. Mitochondria: central organelles for melatonin's antioxidant and anti-aging actions. Molecules 23; 509, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Ren J, Jin M, You ZX, Luo M, Han Y, Li GC, and Liu HG. Melatonin prevents chronic intermittent hypoxia-induced injury by inducing sirtuin 1-mediated autophagy in steatotic liver of mice. Sleep Breath 23: 825–836, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, and Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A 92: 8734–8738, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Reppert SM, Weaver DR, and Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 13: 1177–1185, 1994. [DOI] [PubMed] [Google Scholar]
- 233. Riha RL. The use and misuse of exogenous melatonin in the treatment of sleep disorders. Curr Opin Pulm Med 24: 543–548, 2018. [DOI] [PubMed] [Google Scholar]
- 234. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, and Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36: 1–9, 2004. [DOI] [PubMed] [Google Scholar]
- 235. Rodríguez MI, Carretero M, Escames G, López LC, Maldonado MD, Tan DX, Reiter RJ, and Acuña-Castroviejo D. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic Res 41: 15–24, 2007. [DOI] [PubMed] [Google Scholar]
- 236. Rodriguez MI, Escames G, López LC, García JA, Ortiz F, López A, and Acuña-Castroviejo D. Melatonin administration prevents cardiac and diaphragmatic mitochondrial oxidative damage in senescence-accelerated mice. J Endocrinol 194: 637–643, 2007. [DOI] [PubMed] [Google Scholar]
- 237. Rodríguez MI, Escames G, López LC, López A, García JA, Ortiz F, Sánchez V, Romeu M, and Acuña-Castroviejo D. Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp Gerontol 43: 749–756, 2008. [DOI] [PubMed] [Google Scholar]
- 238. Sack RL, Lewy AJ, and Hughes RJ. Use of melatonin for sleep and circadian rhythm disorders. Ann Med 30: 115–121, 1998. [DOI] [PubMed] [Google Scholar]
- 239. Sadanandan N, Cozene B, Cho J, Park YJ, Saft M, Gonzales-Portillo B, and Borlongan CV. Melatonin-A potent therapeutic for stroke and stroke-related dementia. Antioxidants (Basel) 9: 672, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Sahar S and Sassone-Corsi P. The epigenetic language of circadian clocks. Handb Exp Pharmacol: 29–44, 2013. [DOI] [PubMed] [Google Scholar]
- 241. Sanchez-Barcelo EJ, Rueda N, Mediavilla MD, Martinez-Cue C, and Reiter RJ. Clinical uses of melatonin in neurological diseases and mental and behavioural disorders. Curr Med Chem 24: 3851–3878, 2017. [DOI] [PubMed] [Google Scholar]
- 242. Sanchez-Hidalgo M, Alarcon de la Lastra C, Carrascosa-Salmoral MP, Naranjo MC, Gomez-Corvera A, Caballero B, and Guerrero JM. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp Gerontol 44: 328–334, 2009. [DOI] [PubMed] [Google Scholar]
- 243. Satyanarayanan SK, Su H, Lin YW, and Su KP. Circadian rhythm and melatonin in the treatment of depression. Curr Pharm Des 24: 2549–2555, 2018. [DOI] [PubMed] [Google Scholar]
- 244. Schmitt K, Grimm A, Dallmann R, Oettinghaus B, Restelli LM, Witzig M, Ishihara N, Mihara K, Ripperger JA, Albrecht U, Frank S, Brown SA, and Eckert A. Circadian control of DRP1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metab 27: 657–666, 2018. [DOI] [PubMed] [Google Scholar]
- 245. Shi S, Lei S, Tang C, Wang K, and Xia Z. Melatonin attenuates acute kidney ischemia/reperfusion injury in diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling pathway. Biosci Rep 39: BSR20181614, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Skene DJ, Deacon S, and Arendt J. Use of melatonin in circadian rhythm disorders and following phase shifts. Acta Neurobiol Exp (Wars) 56: 359–362, 1996. [DOI] [PubMed] [Google Scholar]
- 247. Slominski A, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, and Paus R. Melatonin: a cutaneous perspective on its production, metabolism, and functions. J Invest Dermatol 138: 490–499, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, and Jetten AM. RORα and RORγ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J 28: 2775–2789, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Slominski AT, Zmijewski MA, and Jetten AM. RORα is not a receptor for melatonin (response to DOI 10.1002/bies.201600018). Bioessays 38: 1193–1194, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Song J, Lu C, Zhao W, and Shao X. Melatonin attenuates TNF-α-mediated hepatocytes damage via inhibiting mitochondrial stress and activating the Akt-Sirt3 signaling pathway. J Cell Physiol 234: 20969–20979, 2019. [DOI] [PubMed] [Google Scholar]
- 251. Srinivasan V, Pandi-Perumal SR, Cardinali DP, Poeggeler B, and Hardeland R. Melatonin in Alzheimer's disease and other neurodegenerative disorders. Behav Brain Funct 2: 5, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Srinivasan V, Pandi-Perumal SR, Maestroni GJM, Esquifino AI, Hardeland R, and Cardinali DP. Role of melatonin in neurodegenerative diseases. Neurotox Res 7: 293–318, 2005. [DOI] [PubMed] [Google Scholar]
- 253. Stacchiotti A, Favero G, and Rodella LF. Impact of melatonin on skeletal muscle and exercise. Cells 9: 288, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Steinhilber D, Brungs M, Werz O, Wiesenberg I, Danielsson C, Kahlen JP, Nayeri S, Schräder M, and Carlberg, C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human B lymphocytes. J Biol Chem 270: 7037–7040, 1995. [DOI] [PubMed] [Google Scholar]
- 255. Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, Baranov SV, Leronni D, Mihalik AC, He Y, Cecon E, Wehbi VL, Kim J, Heath BE, Baranova OV, Wang X, Gable MJ, Kretz ES, Di Benedetto G, Lezon TR, Ferrando LM, Larkin TM, Sullivan M, Yablonska S, Wang J, Minnigh MB, Guillaumet G, Suzenet F, Richardson RM, Poloyac SM, Stolz DB, Jockers R, Witt-Enderby PA, Carlisle DL, Vilardaga JP, and Friedlander RM. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci U S A 114: E7997–E8006, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Talib WH. Melatonin and cancer hallmarks. Molecules 23: 518, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Tan D, Manchester LC, Reiter RJ, Qi W, Hanes MA, and Farley NJ. High physiological levels of melatonin in the bile of mammals. Life Sci 65: 2523–2529, 1999. [DOI] [PubMed] [Google Scholar]
- 258. Tan D-X, Chen L-D, Poeggeler B, Manchester LC. and Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J 1: 57–60, 1993. [Google Scholar]
- 259. Tan D-X and Hardeland R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: focus on COVID-19. Melatonin Res 3: 120–143, 2020. [Google Scholar]
- 260. Tan D-X and Hardeland R. Estimated doses of melatonin for treating deadly virus infections: focus on COVID-19. Melatonin Res 3: 276–296, 2020. [Google Scholar]
- 261. Tan D-X and Hardeland R. Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 25: 4410, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Tan D-X, Manchester LC, and Reiter RJ. CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal. Med Hypotheses 86: 3–9, 2016. [DOI] [PubMed] [Google Scholar]
- 263. Tan D-X and Reiter, RJ. Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res 2: 44–66, 2019. [Google Scholar]
- 264. Tan D-X, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, and Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2: 181–197, 2002. [DOI] [PubMed] [Google Scholar]
- 265. Tapias V, Escames G, López LC, López A, Camacho E, Carrión MD, Entrena A, Gallo MA, Espinosa A, and Acuña-Castroviejo D. Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J Neurosci Res 87: 3002–3010, 2009. [DOI] [PubMed] [Google Scholar]
- 266. Tobore TO. On the central role of mitochondria dysfunction and oxidative stress in Alzheimer's disease. Neurol Sci 40: 1527–1540, 2019. [DOI] [PubMed] [Google Scholar]
- 267. Tricoire H, Locatelli A, Chemineau P, and Malpaux B. Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology 143: 84–90, 2002. [DOI] [PubMed] [Google Scholar]
- 268. Tricoire H, Malpaux B, and Møller M. Cellular lining of the sheep pineal recess studied by light-, transmission-, and scanning electron microscopy: morphologic indications for a direct secretion of melatonin from the pineal gland to the cerebrospinal fluid. J Comp Neurol 456: 39–47, 2003. [DOI] [PubMed] [Google Scholar]
- 269. Tricoire H, Møller M, Chemineau P, and Malpaux B. Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod Suppl 61: 311–321, 2003. [PubMed] [Google Scholar]
- 270. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, and Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. [DOI] [PubMed] [Google Scholar]
- 271. Valdés-Tovar M, Estrada-Reyes R, Solís-Chagoyán H, Argueta J, Dorantes-Barrón AM, Quero-Chávez D, Cruz-Garduño R, Cercós MG, Trueta C, Oikawa-Sala J, Dubocovich ML, and Benítez-King G. Circadian modulation of neuroplasticity by melatonin: a target in the treatment of depression. Br J Pharmacol 175: 3200–3208, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Vural EM, van Munster BC, and de Rooij SE. Optimal dosages for melatonin supplementation therapy in older adults: a systematic review of current literature. Drugs Aging 31: 441–451, 2014. [DOI] [PubMed] [Google Scholar]
- 273. Wang X, Figueroa BE, Stavrovskaya IG, Zhang Y, Sirianni AC, Zhu S, Day AL, Kristal BS, and Friedlander RM. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke 40: 1877–1885, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Wang Y, Song L, Liu M, Ge R, Zhou Q, Liu W, Li R, Qie J, Zhen B, Wang Y, He F, Qin J, and Ding C. A proteomics landscape of circadian clock in mouse liver. Nat Commun 9: 1553, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Weishaupt JH, Bartels C, Pölking E, Dietrich J, Rohde G, Poeggeler B, Mertens N, Sperling S, Bohn M, Hüther G, Schneider A, Bach A, Sirén A-L, Hardeland R, Bähr M, Nave K-A, and Ehrenreich H. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res 41: 313–323, 2006. [DOI] [PubMed] [Google Scholar]
- 276. Wu J, Bai Y, Wang Y, and Ma J. Melatonin and regulation of autophagy: mechanisms and therapeutic implications. Pharmacol Res 163: 105279, 2021. [DOI] [PubMed] [Google Scholar]
- 277. Wurtman RJ, Axelrod J, and Fischer JE. Melatonin synthesis in the pineal gland: effect of light mediated by the sympathetic nervous system. Science 143: 1328–1329, 1964. [DOI] [PubMed] [Google Scholar]
- 278. Xia Y, Zeng S, Zhao Y, Zhu C, Deng B, Zhu G, Yin Y, Wang W, Hardeland R, and Ren W. Melatonin in macrophage biology: current understanding and future perspectives. J Pineal Res 66: e12547, 2019. [DOI] [PubMed] [Google Scholar]
- 279. Xu G, Zhao J, Liu H, Wang J, and Lu W. Melatonin inhibits apoptosis and oxidative stress of mouse Leydig cells via a SIRT1-dependent mechanism. Molecules 24: 3084, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Yamada K and Miyamoto K. Basic helix-loop-helix transcription factors, BHLHB2 and BHLHB3; their gene expressions are regulated by multiple extracellular stimuli. Front Biosci 10: 3151–3171, 2005. [DOI] [PubMed] [Google Scholar]
- 281. Yamajuku D, Shibata Y, Kitazawa M, Katakura T, Urata H, Kojima T, Takayasu S, Nakata O, and Hashimoto S. Cellular DBP and E4BP4 proteins are critical for determining the period length of the circadian oscillator. FEBS Lett 585: 2217–2222, 2011. [DOI] [PubMed] [Google Scholar]
- 282. Yang S, Chen X, Li S, Sun B, and Hang C. Melatonin treatment regulates SIRT3 expression in early brain injury (EBI) due to reactive oxygen species (ROS) in a mouse model of subarachnoid hemorrhage (SAH). Med Sci Monit 24: 3804–3814, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Yang W, Kang X, Qin N, Li F, Jin X, Ma Z, Qian Z, and Wu S. Melatonin protects chondrocytes from impairment induced by glucocorticoids via NAD+-dependent SIRT1. Steroids 126: 24–29, 2017. [DOI] [PubMed] [Google Scholar]
- 284. Yawoot N, Govitrapong P, Tocharus C, and Tocharus J. Ischemic stroke, obesity, and the anti-inflammatory role of melatonin. Biofactors 47: 41–58, 2021. [DOI] [PubMed] [Google Scholar]
- 285. Yi S, Zheng B, Zhu Y, Cai Y, Sun H, and Zhou J. Melatonin ameliorates excessive PINK1/Parkin-mediated mitophagy by enhancing SIRT1 expression in granulosa cells of PCOS. Am J Physiol Endocrinol Metab 319: E91–E101, 2020. [DOI] [PubMed] [Google Scholar]
- 286. Yoshitane H, Asano Y, Sagami A, Sakai S, Suzuki Y, Okamura H, Iwasaki W, Ozaki H, and Fukada Y. Functional D-box sequences reset the circadian clock and drive mRNA rhythms. Commun Biol 2: 300, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Yu F, Zhang T, Zhou C, Xu H, Guo L, Chen M, and Wu B. The circadian clock gene Bmal1 controls intestinal exporter MRP2 and drug disposition. Theranostics 9: 2754–2767, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Yu L, Gong B, Duan W, Fan C, Zhang J, Li Z, Xue X, Xu Y, Meng D, Li B, Zhang M, Bin Zhang, Jin Z, Yu S, Yang Y, and Wang H. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1alpha-SIRT3 signaling. Sci Rep 7: 41337, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Zetner D, Andersen LPK, Alder R, Jessen ML, Tolstrup A, and Rosenberg J. Pharmacokinetics and safety of intravenous, intravesical, rectal, transdermal, and vaginal melatonin in healthy female volunteers: a cross-over study. Pharmacology 16: 1–8, 2020. [DOI] [PubMed] [Google Scholar]
- 290. Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M, Yu L, Liu Z, Yu B, Ren K, Gao E, Yang Y, Liang H, Jin Z, and Yu S. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res 63: e12419, 2017. [DOI] [PubMed] [Google Scholar]
- 291. Zhang D, Lee H, Zhu Z, Minhas JK, and Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 312: L110–L121, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Zhang H, Akbar M, and Kim HY. Melatonin: an endogenous negative modulator of 12-lipoxygenation in the rat pineal gland. Biochem J 344(Pt 2): 487–493, 1999. [PMC free article] [PubMed] [Google Scholar]
- 293. Zhang M, Lin J, Wang S, Cheng Z, Hu J, Wang T, Man W, Yin T, Guo W, Gao E, Reiter RJ, Wang H, and Sun D. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J Pineal Res 63: e12418, 2020. [DOI] [PubMed] [Google Scholar]
- 294. Zhang M, Lu Y, Chen Y, Zhang Y, and Xiong B. Insufficiency of melatonin in follicular fluid is a reversible cause for advanced maternal age-related aneuploidy in oocytes. Redox Biol 28: 101327, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Zhang WX, He BM, Wu Y, Qiao JF, and Peng ZY. Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci 217: 8–15, 2019. [DOI] [PubMed] [Google Scholar]
- 296. Zhang X, Zhao D, Wu W, Ali Shah SZ, Lai M, Yang D, Li J, Guan Z, Li W, Gao H, Zhao H, Zhou X, and Yang L. Melatonin regulates mitochondrial dynamics and alleviates neuron damage in prion diseases. Aging (Albany NY) 12: 11139–11151, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Zhang Y, Zhu X, Wang G, Chen L, Yang H, He F, and Lin J. Melatonin rescues the Ti particle-impaired osteogenic potential of bone marrow mesenchymal stem cells via the SIRT1/SOD2 signaling pathway. Calcif Tissue Int 107: 474–488, 2020. [DOI] [PubMed] [Google Scholar]
- 298. Zhang Z, Lin J, Tian N, Wu Y, Zhou Y, Wang C, Wang Q, Jin H, Chen T, Nisar M, Zheng G, Xu T, Gao W, Zhang X, and Wang X. Melatonin protects vertebral endplate chondrocytes against apoptosis and calcification via the Sirt1-autophagy pathway. J Cell Mol Med 23: 177–193, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Zheng Z, Xiang S, Wang Y, Dong Y, Li Z, Xiang Y, Bian Y, Feng B, Yang B, and Weng X. NR4A1 promotes TNF-α-induced chondrocyte death and migration injury via activating the AMPK/Drp1/mitochondrial fission pathway. Int J Mol Med 45: 151–161, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 300. Zhou H, Cheang T, Su F, Zheng Y, Chen S, Feng J, Pei Z, and Chen L. Melatonin inhibits rotenone-induced SH-SY5Y cell death via the downregulation of Dynamin-Related Protein 1 expression. Eur J Pharmacol 819: 58–67, 2018. [DOI] [PubMed] [Google Scholar]
- 301. Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, and Chen Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res 63: e12413, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Zhou W, Liu Y, Shen J, Yu B, Bai J, Lin J, Guo X, Sun H, Chen Z, Yang H, Xu Y, and Geng D. Melatonin increases bone mass around the prostheses of OVX rats by ameliorating mitochondrial oxidative stress via the SIRT3/SOD2 signaling pathway. Oxid Med Cell Longev 2019: 4019619, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol 175: 3190–3199, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]