Abstract
Posttraumatic stress disorder (PTSD) has long been associated with a heightened risk of cardiovascular disease (CVD). A number of mechanisms have been implicated to underlie this brain-heart axis relationship, such as altered functioning of the autonomic nervous system and increased systemic inflammation. While neural alterations have repeatedly been observed in PTSD, they are rarely considered in the PTSD-CVD link. The brain-heart axis is a pathway connecting frontal and limbic brain regions to the brainstem and periphery via the autonomic nervous system, and it may be a promising model for understanding CVD risk in PTSD given its overlap with PTSD neural deficits. We first provide a summary of the primary mechanisms implicated in the association between PTSD and CVD. We then review the brain-heart axis and its relevance to PTSD, as well as findings from PTSD trials demonstrating that a number of PTSD treatments have effects on areas of the brain-heart axis. Finally, we discuss sex considerations in the PTSD-CVD link. A critical next step in this research is to determine if PTSD treatments that affect the brain-heart axis (e.g., brain stimulation that improves autonomic function) also reduce the risk of CVD.
Keywords: PTSD, cardiovascular disease, autonomic, inflammation, sex, brain-heart axis
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating neuropsychiatric disorder associated with a heightened risk of cardiovascular disease (CVD; Edmondson et al., 2013a; Edmondson & von Känel, 2017; Myers, 2017). A number of physiological mechanisms have been purported to link these disease states, including dysfunction of the autonomic nervous system (e.g., increased heart rate [HR], blood pressure [BP]) and neurohumoral systems (e.g., renin-angiotensin system, HPA-axis, cortisol), as well as heightened systemic inflammation, metabolic dysfunction, and maladaptive health behaviors (e.g., cigarette smoking, poor diet). While impaired top-down brain circuitry and brain connectivity have repeatedly been observed in PTSD (e.g., reduced frontal cortical inhibition and heightened amygdala activity), they are rarely considered in the PTSD-CVD link. However, the brain-heart axis, a pathway connecting frontal and limbic brain regions to the brainstem and periphery via the autonomic nervous system, may be a promising model for understanding CVD risk in PTSD given its overlap with brain regions that have established alterations in PTSD.
We review the evidence for several key mechanisms implicated in the PTSD-CVD link, as also discussed in the recent review by O’Donnell et al. (2021). We build upon their recent summary by discussing the brain-heart axis and its relevance to PTSD, as well as evidence that several PTSD treatments have demonstrated effects on areas of the brain-heart axis. Finally, we review sex considerations in PTSD-CVD risk, which are critical in order to better understand the heightened risk of PTSD in women.
The link between PTSD and CVD
Individuals with trauma exposure and PTSD have higher rates of CVD compared to the general population, such that PTSD is associated with a greater risk of myocardial infarction, stroke, heart failure, congestive heart failure, and peripheral vascular disease, as well as CVD risk factors, such as hypertension and poor endothelial function (for reviews, see Edmondson et al., 2013b and O’Donnell et al., 2021). The evidence for this link is so compelling that the NIH recently convened a working group of experts, including the American Heart Association, to identify the state of the literature on the link between PTSD and CVD, titled “The Cardiovascular Consequences of Post-Traumatic Stress Disorder” (https://www.nhlbi.nih.gov/events/2018/nhlbi-working-group-cardiovascular-consequences-post-traumatic-stress-disorder). While PTSD is typically considered to be a risk factor for CVD, much of the literature is cross-sectional and does not confirm a causal link (see Koenen et al., 2017). Additionally, PTSD can result from cardiac events (e.g., myocardial infarction), and this may further increase subsequent CVD risk (Edmondson et al., 2011, 2012; von Känel et al., 2011). Better characterization of the possible bidirectional relationship between PTSD and CVD is needed, and this should be considered when evaluating the existing literature on underlying mechanisms. Several mechanisms have been implicated in the PTSD-CVD link and are reviewed below.
Autonomic function and the HPA-axis
An altered stress response is the hallmark characteristic of PTSD, represented by autonomic nervous system and HPA-axis dysfunction (Brudey et al., 2015). Studies have repeatedly demonstrated that individuals with PTSD exhibit elevated sympathetic arousal, indicated by higher HR and BP both at rest and in response to fearful stimuli, compared to controls (Buckley & Kaloupek, 2001; Ehlers et al., 2010; Jovanovic et al., 2009; Keane et al., 1998; Orr et al., 1993). PTSD is also associated with decreased parasympathetic activity, such as lower heart rate variability (HRV) at rest (Chang et al., 2013; Hauschildt et al., 2011; Minassian et al., 2014, 2015) and in response to challenge (Jovanovic et al., 2009; Keary et al., 2009; Park et al., 2017; Sahar et al., 2001). As a biomarker of autonomic activity, increased plasma and urine catecholamine levels have also been reported in PTSD (Pan et al., 2018). In terms of the HPA-axis, individuals with PTSD demonstrate lower basal cortisol levels compared to controls, which is thought to be the result of sensitive glucocorticoid receptors that cause excessive negative feedback of cortisol (Daskalakis et al., 2013; Morris et al., 2012; Yehuda et al., 1993). PTSD is also associated with increased secretion of corticotropin-releasing hormone, which ultimately leads to decreased cortisol release as a result of receptor downregulation (Baker et al., 1999; Heim et al., 2001; Yehuda, 2006) There is some evidence for heightened glucocorticoid receptor sensitivity in PTSD as well, but findings are not consistent (Morris et al., 2016; Yehuda, 2006).
The renin-angiotensin system
A related mechanism that has been implicated in the PTSD-CVD link is the renin-angiotensin system (RAS). The RAS is a hormone system that controls BP, fluid regulation, and sodium balance mainly through activity of the liver and kidneys, and it promotes vasoconstriction and sympathetic activity through the synthesis and release of angiotensin II. Several preclinical studies have found that blockade of the angiotensin II type 1 receptor using angiotensin receptor blockers reduces sympathetic activity and improves fear inhibition (Grassi et al., 2003; Klein et al., 2003; Sueta et al., 2014; Wang et al., 2014; Xia et al., 2009). For example, our group demonstrated that mice treated with losartan, an angiotensin receptor blocker, exhibited significantly less freezing (a threat response in rodents) compared to controls (Marvar et al., 2014). This has also been demonstrated in humans, where losartan has been shown to enhance positive learning and to facilitate fear extinction as indexed with skin conductance (Pulcu et al., 2019; Stout & Risbrough, 2019; Zhou et al., 2019). Cross-sectional research in humans has demonstrated that RAS blockade in humans via ace-inhibitors and angiotensin receptor blockers has been associated with decreased likelihood of a PTSD diagnosis (Khoury et al., 2012; Nylocks et al., 2015; Seligowski et al., 2021a), although a recent randomized controlled trial of losartan did not find evidence for the superiority of losartan over placebo for PTSD symptom reduction (Stein et al., 2021). RAS physiology (e.g., renin level) has also been examined and appears to be altered among trauma-exposed individuals and particularly in those with PTSD (Terock et al., 2019a, 2019b). Taken together, RAS activity and its contributions to autonomic pathophysiology may be an important mechanism in further elucidating CVD risk in PTSD.
Inflammation
It is thought that the chronic HPA-axis and autonomic dysfunction in PTSD also strains the immune system and promotes inflammation. Indeed, individuals with PTSD have elevated levels of proinflammatory cytokines compared to trauma-exposed controls, and this inflammation is linked to CVD (Brudey et al., 2015; Kim et al., 2020; O’Donovan et al., 2012). In addition to serum cytokine levels, there is evidence that PTSD is associated with increased concentrations of C-reactive protein, a biomarker of inflammation that can be predictive of CVD (Brudey et al., 2015; Heath, 2013; Mehta et al., 2020; Michopoulos et al., 2015, 2017; Spitzer et al., 2010). Notably, systemic inflammation has been associated with altered neural functioning, such as decreased connectivity between the vmPFC and striatum, and increased connectivity between the dorsomedial PFC and amygdala (Michopoulos et al., 2017). This inflammation underlies not only PTSD but also metabolic disease, pointing towards metabolic dysregulation as an additional mechanism implicated in the connection between PTSD and CVD (Friend et al., 2020; Lindqvist et al., 2014).
Metabolic dysregulation
Highly comorbid with PTSD, metabolic dysregulation is characterized by the presence of phenotypes including increased abdominal fat mass, disrupted glucose regulation, and increased levels of triglycerides (Michopoulos et al., 2016). Like PTSD, metabolic dysregulation is associated with changes in the HPA axis and inflammation, leading to increased abdominal fat mass and potentially exacerbating hyperglycemia and insulin resistance (Michopoulos et al., 2016). A study by Šagud et al. (2017) reported that people with PTSD had a near-double risk for metabolic dysregulation compared to the general population, and metabolic dysregulation is itself a risk factor for CVD (Dedert et al., 2010; Heppner et al., 2009; Kibler et al., 2014; Michopoulos et al., 2016). Stress activates the autonomic nervous system, which triggers the release of catecholamines, thereby increasing the concentration of cholesterol and triglycerides that are integral to metabolic dysregulation (Weiss et al., 2011). It is thus clear that PTSD is related to a high risk of metabolic dysregulation, and to complicate matters, there are multiple health-related behaviors that are linked to both metabolic dysregulation and CVD in people with PTSD (Bartoli et al., 2013).
Health behaviors
Trauma exposure is associated with increased smoking behavior, which is a major CVD risk factor (de Oliveira et al., 2018; Gilsanz et al., 2017; Lopez et al., 2011). Additionally, individuals with PTSD are more likely to resume smoking after quitting and have a lower tolerance for withdrawal symptoms that are experienced when reducing nicotine intake (Burg & Soufer, 2016; Van den Berk-Clark et al., 2018). Supporting the finding that tobacco use is highly prevalent in those with PTSD are other studies that point to a high comorbidity between substance use disorder and PTSD, with alcohol use in particular being another CVD risk behavior (Berg & Soufer, 2016; Mills et al., 2006). Furthermore, individuals with PTSD may engage less in physical activity and may have poorer diet, which are additional risk factors for poor health outcomes, including CVD (Burg & Soufer, 2016; Dedert et al., 2010; Gilsanz et al., 2017; Hoerster et al., 2019; van den Berk-Clark et al., 2018). PTSD is also associated with disruption in social relationships, such as difficulty maintaining social connection and increased social isolation (Davidson et al., 1991; Platt et al., 2016). Since social isolation is associated with both depression and increased mortality following CVD events, isolation represents an additional health-related behavior that may further increase CVD risk in PTSD (Berkman et al., 1992; Edmondson & Cohen, 2013a).
The confluence of autonomic and RAS dysfunction, as well as inflammation, metabolic dysregulation, and health behaviors, suggests that the PTSD-CVD link is strong but highly complex. It is notable that the mechanisms implicated in the PTSD-CVD link comprise peripheral markers; however, there is a longstanding literature on neural alterations in PTSD. The most-replicated findings are that PTSD is associated with increased activity of the amygdala and dorsal anterior cingulate (dACC), and decreased activity of the ventromedial prefrontal cortex (vmPFC; see Fenster et al., 2018 and Hayes et al., 2012 for reviews). These brain regions affect the peripheral systems mentioned above via innervation of brainstem nuclei that project to the autonomic nervous system. Therefore, the brain-heart axis provides a model of brain-heart interaction that may be useful to apply to the PTSD-CVD link.
The brain-heart axis
The brain-heart axis is a well-established and evolutionarily conserved circuit connecting frontal brain regions to the autonomic nervous system via limbic (i.e., amygdala), hypothalamic, and brainstem structures. Projections from the PFC extend to the insula and cingulate cortex, which project to both the amygdala and hypothalamus, which then project to the solitary nucleus and rostral ventrolateral medulla in the brainstem, regulating HR through sympathetic and parasympathetic projections to the sinoatrial node (the heart’s endogenous pacemaker; Kingma, Simard, & Rouleau, 2018). The solitary nucleus is also a critical hub for integrating bottom up (afferent) inputs from baroreceptors within the carotid bodies and vagal afferents. Thus, the brain-heart axis is directly implicated in cardiovascular and autonomic functioning. No prior studies that we are aware of have directly probed these connections among individuals with PTSD symptoms, but the brain-heart axis clearly has particular relevance to PTSD and trauma-related pathophysiology (see Figure 1 for a depiction of the brain-heart axis and areas implicated in PTSD).
Figure 1. The brain-heart axis and PTSD.
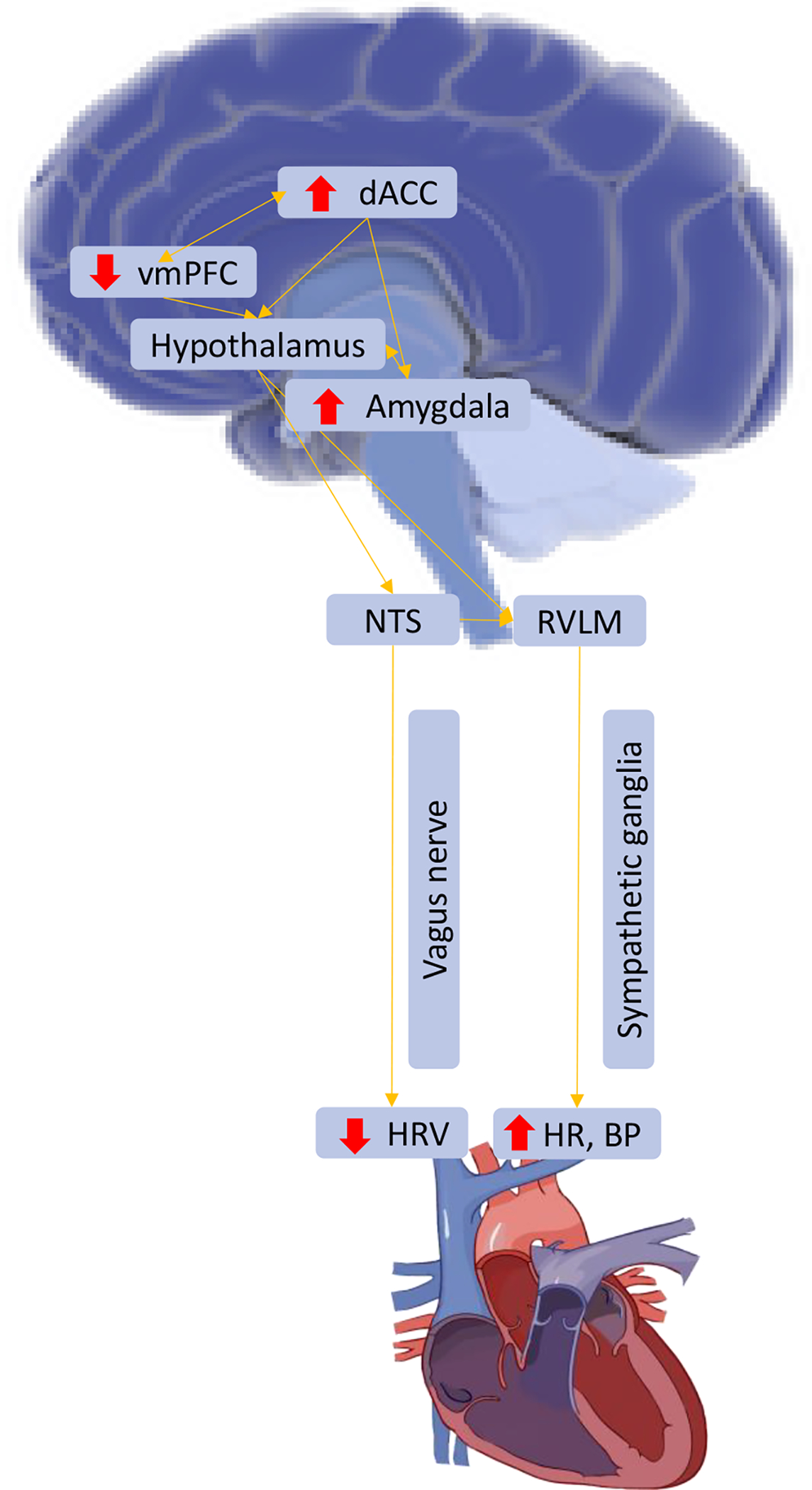
Note. Red arrows indicate areas over- and under-active in PTSD; vmPFC = ventromedial prefrontal cortex; dACC = dorsal anterior cingulate; NTS = nucleus of the solitary tract; RVLM = rostral ventrolateral medulla; HRV = heart rate variability; HR = heart rate; BP = blood pressure.
PTSD is a disorder characterized by poor top-down regulation (e.g., low vmPFC activity) of exaggerated sympathetic responses (e.g., high amygdala, and dACC activity, high HR and BP). In the characteristic fear response, threat perceived by sensory systems stimulates the amygdala and promotes fear learning, and this information is sent to the hypothalamus and brainstem, which contribute to the physiological and cardiovascular response to threat (e.g., increased HR, BP). The hippocampus encodes contextual information about the threat and the vmPFC regulates the response by inhibiting amygdala activation when threat is no longer present. In PTSD, the fear response is typically altered as indicated by hyperactivation of the amygdala combined with hypoactivation of the hippocampus and vmPFC, and these neural deficits may contribute to the peripheral autonomic dysfunction observed in PTSD via the brain-heart axis (for a review, see Ross et al., 2017). Some PTSD symptoms may have greater relevance to this axis than others. For example, a study by Jovanovic et al. (2012) reported that only re-experiencing symptoms of PTSD (e.g., intrusive memories, nightmares) were associated with eye blink startle (a brainstem-mediated reflex) during fear conditioning. Re-experiencing has also been associated with increased amygdala and hippocampus activity (Akiki et al., 2017; Stevens et al., 2017), lower connectivity in the default mode network (Sheynin et al., 2020), decreased cortical thickness in the temporal gyrus (Crombie et al., 2021), and shorter event-related brain potential latencies for safety signals (i.e., decreased processing of safety signals; Seligowski et al., 2021b). Additionally, re-experiencing symptoms have been associated with increased risk for hypertension (Sumner et al., 2020) and plasma-based markers of endothelial dysfunction (von Känel et al., 2008). Thus, re-experiencing symptoms of PTSD may have particular relevance to the brain-heart axis and the link between PTSD and CVD. Studying the connections between central and peripheral aspects of the nervous system may provide greater insight into the PTSD-CVD link, as well as inform newer treatment approaches. For example, better understanding of how established cortical deficits in PTSD contribute to CVD risk via autonomic innervation may suggest that treatments directly targeting cortical function (i.e., neurostimulation) could show promise for reducing CVD risk in PTSD. While the efficacy of PTSD treatments for reducing CVD risk remains unknown, a number of PTSD treatments have already demonstrated effects on the brain-heart axis.
PTSD treatments that affect the brain-heart axis
Psychotherapy
The first-line treatment for PTSD is cognitive behavioral therapy, and in particular, Prolonged Exposure and Cognitive Processing Therapy (APA, 2020). There is emerging evidence that cognitive behavioral therapy has effects on areas of the brain-heart axis. Findings from a randomized controlled trial of Prolonged Exposure and Virtual Reality Exposure (versus waitlist control) for PTSD suggested that resting HR and BP were reduced following treatment (Bourassa et al., 2020). Lindauer et al. (2006) reported reduced HR and BP following a randomized controlled trial of Brief Eclectic Psychotherapy for PTSD. Other randomized controlled trials have reported reduced HR in response to trauma-related stressors, such as listening to a script relating to a personal traumatic experience, seeing trauma-related pictures, or interacting with virtual-reality-based trauma cues, following various forms of cognitive behavioral therapy for PTSD (Dunne et al., 2012; Fecteau & Nicki, 1999; Rabe et al., 2006; Wells et al., 2015). Similar findings have been reported from single-arm trials (Griffin et al., 2012; Loucks et al., 2019; Maples-Keller et al., 2019; Wangelin & Tuerk, 2015). In terms of brain-based findings, a systematic review by Manthey et al. (2021) found significant differences in the mPFC, rACC, and amygdala activity following a number of forms of cognitive behavioral therapies. Thus, there is growing support for the potential of psychotherapy to improve aspects of the brain-heart axis. A crucial next step is to determine if CVD risk can be reduced among individuals with PTSD by using these existing gold-standard treatments, as well as by using novel but promising approaches (e.g., TMS, VNS).
Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is a rapidly-evolving form of neurostimulation that uses magnetic pulses to stimulate the cortex. TMS protocols have demonstrated efficacy in reducing PTSD symptoms using a broad range of targets and frequencies, with consistent results indicating efficacy from both randomized controlled/sham-controlled trials (Boggio et al., 2010; Cohen et al., 2004; Watts et al., 2012) and single-arm unblinded trials (Carpenter et al., 2018). Brain-based markers such as network connectivity (including PFC, cingulate, amygdala, insula, hippocampus; Philip et al., 2018; unblinded trial), electroencephalography frequency coherence (Zandvakili et al., 2019; Zandvakili et al., 2020; randomized controlled trial), and white matter tracts (Barredo et al., 2019; sub-analysis from randomized controlled trial) have been implicated in TMS response among those with PTSD. A newer, more rapid TMS protocol is intermittent theta-burst stimulation (iTBS), which provides short bursts of 50 Hz stimulation repeated at 5 Hz (200 ms interval). iTBS is brief (<10 minutes/treatment), highly tolerable, and has recently demonstrated efficacy for PTSD (Philip et al., 2019; randomized controlled/sham-controlled trial) with clinical benefit for up to one year (Petrosino et al., 2020).
There is evidence that TMS and iTBS may improve autonomic functioning (see Makovac et al., 2017 for a review). Among healthy participants, increased HRV has been demonstrated using both TMS (Remue et al., 2016; Yoshida et al., 2001) and iTBS (Poppa et al., 2020) in randomized controlled/sham-controlled trials, and decreased pulse rate and BP have been demonstrated using TMS (Jenkins et al., 2002; non-sham-controlled). In depressed populations, TMS has been associated with reduced sympathetic-to-parasympathetic ratios (Udupa et al., 2007; unblinded trial) and iTBS has been associated with decreased HR and BP, and increased HRV (Iseger et al., 2020; randomized controlled/sham-controlled trial). This circuit has been broadly proposed as a way to optimize TMS treatment for depression (Iseger et al., 2020). Given that autonomic functioning is a proposed mechanism linking PTSD with increased CVD risk, there is reason to suggest that neurostimulation may be appropriate to address CVD risk in PTSD. However, only one study to date has tested the effects of TMS or iTBS on autonomic functioning among individuals with PTSD. In a sample of 50 Veterans with PTSD, we recently demonstrated that those with higher autonomic function exhibited greater PTSD improvement following a randomized controlled/sham-controlled trial of iTBS (Cosmo et al., 2021), suggesting that autonomic function may be a useful biomarker of iTBS response.
Vagal nerve stimulation
While TMS may be considered a top-down neurostimulation approach, a bottom-up approach is non-invasive vagus nerve stimulation (VNS), which involves electrical stimulation of the vagus nerve. There is evidence to show that VNS has an anti-inflammatory effect, modulating the brain-gut axis, and that it can facilitate extinction of the conditioned fear response (Breit et al., 2018; Noble et al., 2017). Further, VNS modulates cardiovascular activity by improving vagal tone (HRV) and reducing heart rate (Koek et al., 2019; Lamb et al., 2017). Few studies have examined VNS in PTSD. In a single-visit pilot study, Lamb et al. (2017) found that Veterans randomized to VNS (versus sham) demonstrated increased HRV and reduced skin conductance response to auditory startle. Using a randomized controlled/sham-controlled design, VNS has also been shown to improve PTSD symptoms, reduce HR, improve vascular function, increase anterior cingulate and hippocampus activity, reduce limbic activity, and reduce inflammatory reactivity during a trauma script (Bremner et al., 2020, 2021; Gurel et al., 2020; Wittbrodt et al., 2020, 2021). VNS may therefore be a highly promising treatment for PTSD and related autonomic deficits, and new devices are currently being developed (e.g., https://www.evrenvns.com/provenresults).
Psychiatric medications
While there are two FDA-approved medications for PTSD (sertraline and paroxetine), different medication classes have demonstrated efficacy for PTSD and impact areas of the brain-heart axis. For example, beta-blockers (e.g., propranolol) impair memory reconsolidation, thus reducing the strength of the conditioned fear response controlled by the autonomic nervous system and brainstem nuclei (for reviews, see McCleery & Harvey, 2004; Noble et al., 2017; Steckler & Risbrough, 2012). Beta-blockers are thought to reduce PTSD symptoms by suppressing the effects of adrenaline and noradrenaline. While the results of one randomized controlled trial suggested that propranolol reduced HR during script-driven imagery (Brunet et al., 2008), findings are generally mixed (Burbiel, 2015; Steckler & Risbrough, 2012). Randomized controlled trials of prazosin, an α1-adrenoreceptor antagonist with efficacy for hypertension, have yielded more mixed outcomes for PTSD (and nightmares, specifically). That said, prazosin has been shown to reduce BP in PTSD populations (Raskind et al., 2018; Reist et al., 2021). SSRIs have demonstrated efficacy in reducing PTSD symptoms and preventing relapse, and multiple classes of antidepressants appear to normalize the HPA axis response to stress, which may be a common mechanism of action among these types of psychiatric medications (Steckler & Risbrough, 2012). They have also been shown to reduce levels of C-reactive protein and interleukin-6, which may result in cardiovascular benefits (Pizzi et al., 2009). While any psychiatric medication may cause unintended side effects, SSRIs generally do not seem to pose serious cardiovascular risk (Andrade et al., 2013) and appear to be safe and effective for patients with acute myocardial infection and unstable angina (Glassman et al., 2002).
Other treatments
Several other treatment approaches have demonstrated efficacy for PTSD, though evidence supporting their use is less strong or in earlier stages of study. For example, acupuncture can reduce PTSD symptoms and improve physical health composite scores in randomized controlled trials, but with mixed findings in terms of long-term benefits (Engel et al., 2014; Hollifield et al., 2007; for a review, see Grant et al., 2018). There is also some evidence that acupuncture may be associated with lower CVD risk, but replication and larger clinical trials are needed (Hao et al., 2014). Another treatment that has been tested in PTSD is stellate ganglion blockade (Rae Olmsted et al., 2020). However, the evidence for its efficacy is mixed and has to date relied on case reports (for a review, see Lipov & Richie, 2015). A review by Krediet et al. (2020) found some support for the efficacy of psychedelics for PTSD (e.g., MDMA, psilocybin, LSD). While there are current trials underway to examine the treatment potential for psychedelics in PTSD, more research is needed to better understand the effects that these drugs might have on the cardiovascular system (Siegel et al., 2021). Given the psychomimetic effects of these compounds, it is reasonable to anticipate some involvement of the cardiovascular system that may have important implications for treatment development.
Consideration of sex
A consistent finding in PTSD is that its prevalence is approximately two times greater in women compared to men (Kilpatrick et al., 2013; Tolin & Foa, 2006); however, few studies have examined mechanisms underlying sex differences in PTSD (for reviews, see Fonkoue et al., 2020; Seligowski et al., 2020). The most robust findings are that women exhibit heightened skin conductance responses to conditioned stimuli compared to men (Inslicht et al., 2013) and that gonadal hormones moderate these responses (e.g., higher progesterone in women with PTSD confers worse extinction retention; Pineles et al., 2016). Other conditioning studies have shown that women with PTSD demonstrate higher HR and lower HRV, but lower BP compared to men, and that women with PTSD and low estradiol (the most common circulating estrogen) demonstrate worse fear inhibition (indexed by acoustic startle paradigms) compared to those with PTSD and high estradiol (Glover et al., 2012, 2013; Seligowski et al., 2021b). Thus, gonadal hormones, in particular estradiol levels, may partially explain differences in PTSD phenomenology among men versus women.
Estradiol is well-established as protective against CVD, such that it is associated with lower BP, lower cholesterol, and better endothelial function (Charkoudian et al., 2017; Hashimoto et al., 1995, 2002; Mendelsohn & Karas, 1999). The primary explanation for the decreased incidence of CVD in pre-menopausal women (compared to men) is that they have higher circulating levels of estradiol, as this sex difference no longer exists in older age groups with post-menopausal women (Kannel et al., 1976; Vitale et al., 2009). Further, sex differences within the RAS in the context of cardiovascular regulation are well studied (see Medina et al., 2020 for a review). The RAS consists of two axes: one that leads to vasoconstriction and sympathetic activation, and one that leads to vasodilation and sympathoinhibition. Estradiol shifts the balance towards the vasodilation axis by lowering the production of renin, which subsequently decreases sympathetic activity, and by reducing angiotensin II activity (the primary vasoconstrictive peptide in the RAS; Medina et al., 2020). In contrast, testosterone shifts the balance towards the vasoconstriction axis. Much less is known about how sex differences in the RAS impact fear learning in PTSD, however, a recent pre-clinical study found that female rats with low estradiol exhibited worse fear extinction compared to those with high estradiol (Parrish et al., 2019). This could suggest that RAS-estradiol interactions are relevant to fear learning and that low estradiol in humans confers increased PTSD risk through impaired RAS regulation.
The effect of estradiol on metabolic and inflammatory indices is unknown among women with PTSD, however, there is research supporting a protective role of estradiol on these systems in other populations (for a review, see Taylor & Sullivan, 2016). Considering that CVD is the leading cause of death among women and that women are more likely to have PTSD, determining the role of estradiol on the mechanisms implicated in the PTSD-CVD link will be an essential step in identifying and reducing CVD risk in women with PTSD.
Conclusions
The brain-heart axis is a well-established neural pathway connecting frontal and limbic brain regions to the autonomic nervous system via brainstem projections. Given that it connects several brain regions and peripheral systems implicated in both PTSD and CVD, the brain-heart axis offers a model to study the link between these sets of diseases and better understand their interrelations. Further, as PTSD treatments have demonstrated effects on multiple areas of the brain-heart axis, an area for future research will be to test whether such treatments reduce the risk of CVD in PTSD populations. Clinical trials that would be particularly useful are those that test the effects of PTSD treatments (e.g., TMS) on subsequent CVD development among those with pre-existing risk, such as individuals with elevated BP, heightened RAS activity, and/or poor endothelial function. Further, the established effects of estradiol on the RAS and fear learning necessitate that future trials account for sex as a biological variable.
Acknowledgments
AVS supported by NIH K23MH125920-01 and AHA 20CDA35310031 (AS PI), and ORWH-NIMH U54 MH118919 (Goldstein /Tobet Multi-PIs). PJM is supported by NIH 5R01HL137103-03. KJR supported by NIH (R21MH112956, P50MH115874, R01MH094757 and R01MH106595), and the Frazier Foundation Grant for Mood and Anxiety Research. NSP is supported by VA (RR&D Center for Neurorestoration and Neurotechnology, I01 RX002450, and I01 HX002572) and NIH (P20 GM130452, R01 MH120126).
Footnotes
Disclosures
AVS, TKW, PJM, have no biomedical financial interests or conflicts of interest. KJR has performed scientific consultation for Bioxcel, Bionomics, Acer, Takeda, and Jazz Pharma; serves on Scientific Advisory Boards for Sage and the Brain Research Foundation, and he has received sponsored research support from Takeda, Brainsway and Alto Neuroscience. NSP has received clinical trial support, through VA contracts, from Wave Neuro and Neurolief. None of this work is directly related to the work presented here.
References
- Akiki TJ, Averill CL, Wrocklage KM, Schweinsburg B, Scott JC, Martini B, Averill LA, Southwick SM, Krystal JH, & Abdallah CG (2017). The association of PTSD symptom severity with localized hippocampus and amygdala abnormalities. Chronic Stress, 1, 2470547017724069. 10.1177/2470547017724069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C, Kumar C, & Surya S (2013). Cardiovascular mechanisms of SSRI drugs and their benefits and risks in ischemic heart disease and heart failure. International Clinical Psychopharmacology, 28(3), 145–155. doi: 10.1097/YIC.0b013e32835d735d [DOI] [PubMed] [Google Scholar]
- American Psychological Association (2020). https://www.apa.org/ptsd-guideline/treatments
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, & Geracioti TD, Jr (1999). Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. The American Journal of Psychiatry, 156(4), 585–588. 10.1176/ajp.156.4.585 [DOI] [PubMed] [Google Scholar]
- Barredo J, Bellone JA, Edwards M, Carpenter LL, Correia S, & Philip NS (2019). White matter integrity and functional predictors of response to repetitive transcranial magnetic stimulation for posttraumatic stress disorder and major depression. Depression and Anxiety, 36(11), 1047–1057. 10.1002/da.22952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli F, Carrà G, Crocamo C, Carretta D, & Clerici M (2013). Metabolic syndrome in people suffering from posttraumatic stress disorder: A systematic review and meta-analysis. Metabolic Syndrome and Related Disorders, 11(5), 301–308. 10.1089/met.2013.0010 [DOI] [PubMed] [Google Scholar]
- Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanhã C, Ferreira-Santos E, Meleiro A, Corchs F, Zaghi S, Pascual-Leone A, & Fregni F (2010). Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. The Journal of Clinical Psychiatry, 71(8), 992–999. doi: 10.4088/JCP.08m04638blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa KJ, Stevens ES, Katz AC, Rothbaum BO, Reger GM, & Norr AM (2020). The impact of exposure therapy on resting heart rate and heart rate reactivity among active-duty soldiers with post-traumatic stress disorder. Psychosomatic Medicine, 82(1), 108–114. 10.1097/PSY.0000000000000758 [DOI] [PubMed] [Google Scholar]
- Breit S, Kupferberg A, Rogler G, & Hasler G (2018). Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Frontiers in Psychiatry, 9, 44. 10.3389/fpsyt.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Park J, Bikson M, & Inan OT (2020). Application of noninvasive vagal nerve stimulation to stress-related psychiatric disorders. Journal of Personalized Medicine, 10(3). 10.3390/jpm10030119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Wittbrodt MT, Gurel NZ, Shandhi MH, Gazi AH, Jiao Y, Levantsevych OM, Huang M, Beckwith J, Herring I, Murrah N, Driggers EG, Ko YA, Alkhalaf ML, Soudan M, Shallenberger L, Hankus AN, Nye JA, Park J, … Inan OT (2021). Transcutaneous cervical vagal nerve stimulation in patients with posttraumatic stress disorder (PTSD): A pilot study of effects on PTSD symptoms and interleukin-6 response to stress. Journal of Affective Disorders Reports, 6, 100190. 10.1016/j.jadr.2021.100190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, & Marvar PJ (2015). Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 309(4), R315–R321. 10.1152/ajpregu.00343.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, & Pitman RK (2008). Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. Journal of Psychiatric Research, 42(6), 503–506. 10.1016/j.jpsychires.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Buckley TC, & Kaloupek DG (2001). A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic Medicine, 63(4), 585–594. [DOI] [PubMed] [Google Scholar]
- Burbiel JC (2015). Primary prevention of posttraumatic stress disorder: drugs and implications. Military Medical Research, 2, 24. 10.1186/s40779-015-0053-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MM, & Soufer R (2016). Post-traumatic stress disorder and cardiovascular disease. Current Cardiology Reports, 18(10), 94. 10.1007/s11886-016-0770-5 [DOI] [PubMed] [Google Scholar]
- Berkman LF, Leo-Summers L, & Horwitz RI (1992). Emotional support and survival after myocardial infarction. A prospective, population-based study of the elderly. Annals of Internal Medicine, 117(12), 1003–1009. 10.7326/0003-4819-117-12-1003 [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Conelea C, Tyrka AR, Welch ES, Greenberg BD, Price LH, Niedzwiecki M, Yip AG, Barnes J, & Philip NS (2018). 5 Hz Repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. Journal of Affective Disorders, 235, 414–420. 10.1016/j.jad.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H-A, Chang C-C, Tzeng N-S, Kuo TB, Lu R-B, & Huang S-Y (2013). Decreased cardiac vagal control in drug-naïve patients with posttraumatic stress disorder. Psychiatry Investigation, 10(2), 121–130. doi: 10.4306/pi.2013.10.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Hart E, Barnes JN, & Joyner MJ (2017). Autonomic control of body temperature and blood pressure: influences of female sex hormones. Clinical Autonomic Research : Official Journal of the Clinical Autonomic Research Society, 27(3), 149–155. 10.1007/s10286-017-0420-z [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kotler M, Kouperman I, Moisa R, & Grisaru N (2004). Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. The American Journal of Psychiatry, 161(3), 515–524. 10.1176/appi.ajp.161.3.515 [DOI] [PubMed] [Google Scholar]
- Cosmo C, Seligowski AV, Aiken EM, Van’t Wout-Frank M, & Philip NS (2021). Heart rate variability features as predictors of intermittent theta-burst stimulation response in posttraumatic stress disorder. Neuromodulation: Journal of the International Neuromodulation Society, 10.1111/ner.13529. Advance online publication. 10.1111/ner.13529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie KM, Ross MC, Letkiewicz AM, Sartin-Tarm A, & Cisler JM (2021). Differential relationships of PTSD symptom clusters with cortical thickness and grey matter volumes among women with PTSD. Scientific Reports, 11(1), 1825. 10.1038/s41598-020-80776-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Lehrner A, & Yehuda R (2013). Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinology and Metabolism Clinics of North America, 42(3), 503–513. 10.1016/j.ecl.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Davidson JR, Hughes D, Blazer DG, & George LK (1991). Post-traumatic stress disorder in the community: An epidemiological study. Psychological Medicine, 21(3), 713–721. 10.1017/s0033291700022352 [DOI] [PubMed] [Google Scholar]
- Dedert EA, Calhoun PS, Watkins LL, Sherwood A, & Beckham JC (2010). Posttraumatic stress disorder, cardiovascular, and metabolic disease: A review of the evidence. Annals of Behavioral Medicine : A Publication of the Society of Behavioral Medicine, 39(1), 61–78. 10.1007/s12160-010-9165-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira JF, Wiener CD, Jansen K, Portela LV, Lara DR, Souza L, da Silva RA, Moreira FP, & Oses JP (2018). Serum levels of interleukins IL-6 and IL-10 in individuals with posttraumatic stress disorder in a population-based sample. Psychiatry Research, 260, 111–115. 10.1016/j.psychres.2017.11.061 [DOI] [PubMed] [Google Scholar]
- Dunne RL, Kenardy J, & Sterling M (2012). A randomized controlled trial of cognitive-behavioral therapy for the treatment of PTSD in the context of chronic whiplash. The Clinical Journal of Pain, 28(9), 755–765. 10.1097/AJP.0b013e318243e16b [DOI] [PubMed] [Google Scholar]
- Edmondson D, & Cohen BE (2013a). Posttraumatic stress disorder and cardiovascular disease. Progress in Cardiovascular Diseases, 55(6), 548–556. 10.1016/j.pcad.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, Shaffer JA, Falzon L, & Burg MM (2013b). Posttraumatic stress disorder and risk for coronary heart disease: A meta-analytic review. American Heart Journal, 166(5), 806–814. 10.1016/j.ahj.2013.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, & Neria Y (2012). Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: A meta-analytic review. PloS one, 7(6), e38915. 10.1371/journal.pone.0038915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Rieckmann N, Shaffer JA, Schwartz JE, Burg MM, Davidson KW, Clemow L, Shimbo D, & Kronish IM (2011). Posttraumatic stress due to an acute coronary syndrome increases risk of 42-month major adverse cardiac events and all-cause mortality. Journal of Psychiatric Research, 45(12), 1621–1626. 10.1016/j.jpsychires.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, & von Känel R (2017). Post-traumatic stress disorder and cardiovascular disease. The Lancet: Psychiatry, 4(4), 320–329. 10.1016/S2215-0366(16)30377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Suendermann O, Boellinghaus I, Vossbeck-Elsebusch A, Gamer M, Briddon E, Martin MW, & Glucksman E (2010). Heart rate responses to standardized trauma-related pictures in acute posttraumatic stress disorder. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 78(1), 27–34. 10.1016/j.ijpsycho.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel CC, Cordova EH, Benedek DM, Liu X, Gore KL, Goertz C, Freed MC, Crawford C, Jonas WB, & Ursano RJ (2014). Randomized effectiveness trial of a brief course of acupuncture for posttraumatic stress disorder. Medical Care, 52(12), S57–S64. doi: 10.1097/MLR.000000000000023 [DOI] [PubMed] [Google Scholar]
- Fecteau G, & Nicki R (1999). Cognitive behavioural treatment of post traumatic stress disorder after motor vehicle accident. Behavioural and Cognitive Psychotherapy, 27(3), 201–214. 10.1017/S135246589927302X [DOI] [Google Scholar]
- Fenster RJ, Lebois L, Ressler KJ, & Suh J (2018). Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nature Reviews: Neuroscience, 19(9), 535–551. 10.1038/s41583-018-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonkoue IT, Michopoulos V, & Park J (2020). Sex differences in post-traumatic stress disorder risk: autonomic control and inflammation. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society, 30(5), 409–421. 10.1007/s10286-020-00729-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend SF, Nachnani R, Powell SB, & Risbrough VB (2020). C-Reactive Protein: Marker of risk for post-traumatic stress disorder and its potential for a mechanistic role in trauma response and recovery. The European Journal of Neuroscience. Advance online publication. 10.1111/ejn.15031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz P, Winning A, Koenen KC, Roberts AL, Sumner JA, Chen Q, Glymour MM, Rimm EB, & Kubzansky LD (2017). Post-traumatic stress disorder symptom duration and remission in relation to cardiovascular disease risk among a large cohort of women. Psychological Medicine, 47(8), 1370–1378. 10.1017/S0033291716003378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, Mclvor M, & Sertraline Antidepressant Heart Attack Randomized Trial (SADHEART) Group (2002). Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA, 288(6), 701–709. 10.1001/jama.288.6.701 [DOI] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, & Norrholm SD (2012). Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological Psychiatry, 72(1), 19–24. 10.1016/j.biopsych.2012.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, Ressler KJ, & Jovanovic T (2013). Inhibition of fear is differentially associated with cycling estrogen levels in women. Journal of Psychiatry & Neuroscience: JPN, 38(5), 341–348. doi: 10.1503/jpn.120129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Colaiaco B, Motala A, Shanman R, Sorbero M, & Hempel S (2018). Acupuncture for the treatment of adults with posttraumatic stress disorder: A systematic review and meta-analysis. Journal of Trauma & Dissociation, 19(1), 39–58. 10.1080/15299732.2017.1289493 [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell’Oro R, Trevano FQ, Bombelli M, Scopelliti F, Facchini A, Mancia G, & CROSS Study. (2003). Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. Journal of Hypertension, 21(9), 1761–1769. DOI: 10.1097/00004872-200309000-00027 [DOI] [PubMed] [Google Scholar]
- Griffin MG, Resick PA, & Galovski TE (2012). Does physiologic response to loud tones change following cognitive-behavioral treatment for posttraumatic stress disorder?. Journal of Traumatic Stress, 25(1), 25–32. 10.1002/jts.21667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel NZ, Wittbrodt MT, Jung H, Shandhi M, Driggers EG, Ladd SL, Huang M, Ko YA, Shallenberger L, Beckwith J, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Inan OT, & Bremner JD (2020). Transcutaneous cervical vagal nerve stimulation reduces sympathetic responses to stress in posttraumatic stress disorder: A double-blind, randomized, sham controlled trial. Neurobiology of Stress, 13, 100264. 10.1016/j.ynstr.2020.100264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao PP, Jiang F, Chen YG, Yang J, Zhang K, Zhang MX, Zhang C, Zhao YX, & Zhang Y (2015). Traditional Chinese medication for cardiovascular disease. Nature Reviews Cardiology, 12(2), 115–122. DOI: 10.1038/nrcardio.2014.177 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, & Ouchi Y (1995). Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation, 92(12), 3431–3435. 10.1161/01.cir.92.12.3431 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Miyao M, Akishita M, Hosoi T, Toba K, Kozaki K, Yoshizumi M, & Ouchi Y (2002). Effects of long-term and reduced-dose hormone replacement therapy on endothelial function and intima-media thickness in postmenopausal women. Menopause, 9(1), 58–64. 10.1097/00042192-200201000-00009 [DOI] [PubMed] [Google Scholar]
- Hauschildt M, Peters MJV, Moritz S, & Jelinek L (2011). Heart rate variability in response to affective scenes in posttraumatic stress disorder. Biological Psychology, 88(2–3), 215–222. 10.1016/j.biopsycho.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, & Mikedis AM (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2(9). 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath NM, Chesney SA, Gerhart JI, Goldsmith RE, Luborsky JL, Stevens NR, & Hobfoll SE (2013). Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine, 63(2), 172–178. 10.1016/j.cyto.2013.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, & Nemeroff CB (2001). Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry, 158, 575–581. [DOI] [PubMed] [Google Scholar]
- Heppner PS, Crawford EF, Haji UA, Afari N, Hauger RL, Dashevsky BA, Horn PS, Nunnink SE, & Baker DG (2009). The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Medicine, 7, 1. 10.1186/1741-7015-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollifield M, Sinclair-Lian N, Warner TD, & Hammerschlag R (2007). Acupuncture for posttraumatic stress disorder: A randomized controlled pilot trial. The Journal of Nervous and Mental Disease, 195(6), 504–513. doi: 10.1097/NMD.0b013e31803044f8 [DOI] [PubMed] [Google Scholar]
- Hoerster KD, Campbell S, Dolan M, Stappenbeck CA, Yard S, Simpson T, & Nelson KM (2019). PTSD is associated with poor health behavior and greater Body Mass Index through depression, increasing cardiovascular disease and diabetes risk among US veterans. Preventive Medicine Reports, 15, 100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, Marmar CR, & Neylan TC (2013). Sex differences in fear conditioning in posttraumatic stress disorder. Journal of Psychiatric Research, 47(1), 64–71. 10.1016/j.jpsychires.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseger TA, Arns M, Downar J, Blumberger DM, Daskalakis ZJ, & Vila-Rodriguez F (2020). Cardiovascular differences between sham and active iTBS related to treatment response in MDD. Brain Stimulation, 13(1), 167–174. 10.1016/j.brs.2019.09.016 [DOI] [PubMed] [Google Scholar]
- Jenkins J, Shajahan PM, Lappin JM, & Ebmeier KP (2002). Right and left prefrontal transcranial magnetic stimulation at 1 Hz does not affect mood in healthy volunteers. BMC Psychiatry, 2, 1. 10.1186/1471-244X-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, & Davis M (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62(2), 695–704. doi: 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, & Kozarić-Kovacić D (2009). Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 71(3), 264–268. 10.1016/j.ijpsycho.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Hjortland MC, McNamara PM, & Gordon T (1976). Menopause and risk of cardiovascular disease: the Framingham study. Annals of Internal Medicine, 85(4), 447–452. 10.7326/0003-4819-85-4-447 [DOI] [PubMed] [Google Scholar]
- Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, Hsieh FY, & Lavori PW (1998). Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. Journal of Consulting and Clinical Psychology, 66(6), 914–923. 10.1037/0022-006X.66.6.914 [DOI] [PubMed] [Google Scholar]
- Keary TA, Hughes JW, & Palmieri PA (2009). Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 73(3), 257–264. 10.1016/j.ijpsycho.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, & Ressler KJ (2012). The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. The Journal of Clinical Psychiatry, 73(6), 849–855. 10.4088/JCP.11m07316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibler JL, Tursich M, Ma M, Malcolm L, & Greenbarg R (2014). Metabolic, autonomic and immune markers for cardiovascular disease in posttraumatic stress disorder. World Journal of Cardiology, 6(6), 455–461. 10.4330/wjc.v6.i6.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, & Friedman MJ (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress, 26, 537–547. 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TD, Lee S, & Yoon S (2020). Inflammation in post-traumatic stress disorder (PTSD): A review of potential correlates of PTSD with a neurological perspective. Antioxidants (Basel, Switzerland), 9(2), 107. 10.3390/antiox9020107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma JG, Simard D, & Rouleau JR (2018). Autonomic nervous system and neurocardiac physiopathology. In Svorc P (Ed.), Autonomic Nervous System. Rijeka: IntechOpen. DOI: 10.5772/intechopen.77087 [DOI] [Google Scholar]
- Klein IHHT, Ligtenberg G, Oey PL, Koomans HA, & Blankestijn PJ (2003). Enalapril and losartan reduce sympathetic hyperactivity in patients with chronic renal failure. Journal of the American Society of Nephrology: JASN, 14(2), 425–430. 10.1097/01.asn.0000045049.72965.b7 [DOI] [PubMed] [Google Scholar]
- Koek RJ, Roach J, Athanasiou N, van ‘t Wout-Frank M, & Philip NS (2019). Neuromodulatory treatments for post-traumatic stress disorder (PTSD). Progress in Neuro-Psychopharmacology & Biological Psychiatry, 92, 148–160. 10.1016/j.pnpbp.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Koenen KC, Sumner JA, Gilsanz P, Glymour MM, Ratanatharathorn A, Rimm EB, Roberts AL, Winning A, & Kubzansky LD (2017). Post-traumatic stress disorder and cardiometabolic disease: Improving causal inference to inform practice. Psychological Medicine, 47(2), 209–225. doi: 10.1017/S0033291716002294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krediet E, Bostoen T, Breeksema J, van Schagen A, Passie T, & Vermetten E (2020). Reviewing the potential of psychedelics for the treatment of PTSD. International Journal of Neuropsychopharmacology, 23(6), 385–400. doi: 10.1093/ijnp/pyaa018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb DG, Porges EC, Lewis GF, & Williamson JB (2017). Non-invasive vagal nerve stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic brain injury: Preliminary evidence. Frontiers in Medicine, 4, 124. 10.3389/fmed.2017.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer RT, van Meijel EP, Jalink M, Olff M, Carlier IV, & Gersons BP (2006). Heart rate responsivity to script-driven imagery in posttraumatic stress disorder: specificity of response and effects of psychotherapy. Psychosomatic Medicine, 68(1), 33–40. 10.1097/01.psy.0000188566.35902.e7 [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer LM, Abu-Amara D, Coy M, Neylan TC, Makotkine I, Reus VI, Yan X, Taylor NM, Marmar CR, & Dhabhar FS (2014). Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain, Behavior, and Immunity, 42, 81–88. 10.1016/j.bbi.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Lipov E, & Ritchie EC (2015). A review of the use of stellate ganglion block in the treatment of PTSD. Current Psychiatry Reports, 17(8), 1–5. DOI 10.1007/s11920-015-0599-4 [DOI] [PubMed] [Google Scholar]
- Lopez WD, Konrath SH, & Seng JS (2011). Abuse-related post-traumatic stress, coping, and tobacco use in pregnancy. Journal of Obstetric, Gynecologic, and Neonatal Nursing : JOGNN, 40(4), 422–431. 10.1111/j.1552-6909.2011.01261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks L, Yasinski C, Norrholm SD, Maples-Keller J, Post L, Zwiebach L, Fiorillo D, Goodlin M, Jovanovic T, Rizzo AA, & Rothbaum BO (2019). You can do that?!: Feasibility of virtual reality exposure therapy in the treatment of PTSD due to military sexual trauma. Journal of Anxiety Disorders, 61, 55–63. 10.1016/j.janxdis.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Makovac E, Thayer JF, & Ottaviani C (2017). A meta-analysis of non-invasive brain stimulation and autonomic functioning: Implications for brain-heart pathways to cardiovascular disease. Neuroscience and Biobehavioral Reviews, 74(Pt B), 330–341. 10.1016/j.neubiorev.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Manthey A, Sierk A, Brakemeier EL, Walter H, & Daniels JK (2021). Does trauma-focused psychotherapy change the brain? A systematic review of neural correlates of therapeutic gains in PTSD. European Journal of Psychotraumatology, 12(1), 1929025. 10.1080/20008198.2021.1929025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples-Keller JL, Rauch SA, Jovanovic T, Yasinski CW, Goodnight JM, Sherrill A, Black K, Michopoulos V, Dunlop BW, Rothbaum BO, & Norrholm SD (2019). Changes in trauma-potentiated startle, skin conductance, and heart rate within prolonged exposure therapy for PTSD in high and low treatment responders. Journal of Anxiety Disorders, 68, 102147. 10.1016/j.janxdis.2019.102147 [DOI] [PubMed] [Google Scholar]
- Marvar PJ, Goodman J, Fuchs S, Choi DC, Banerjee S, & Ressler KJ (2014). Angiotensin type 1 receptor inhibition enhances the extinction of fear memory. Biological Psychiatry, 75(11), 864–872. 10.1016/j.biopsych.2013.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery JM, & Harvey AG (2004). Integration of psychological and biological approaches to trauma memory: implications for pharmacological prevention of PTSD. Journal of Traumatic Stress, 17(6), 485–496. 10.1007/s10960-004-5797-5 [DOI] [PubMed] [Google Scholar]
- Medina D, Mehay D, & Arnold AC (2020). Sex differences in cardiovascular actions of the renin-angiotensin system. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society, 30(5), 393–408. 10.1007/s10286-020-00720-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Stevens JS, Li Z, Gillespie CF, Fani N, Michopoulos V, & Felger JC (2020). Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Social Cognitive and Affective Neuroscience, 15(10), 1046–1055. 10.1093/scan/nsz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn ME, & Karas RH (1999). The protective effects of estrogen on the cardiovascular system. The New England Journal of Medicine, 340(23), 1801–1811. 10.1056/NEJM199906103402306 [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, & Jovanovic T (2017). Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 42(1), 254–270. 10.1038/npp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, & Ressler KJ (2015). Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. The American Journal of Psychiatry, 172(4), 353–362. 10.1176/appi.ajp.2014.14020263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Vester A, & Neigh G (2016). Posttraumatic stress disorder: A metabolic disorder in disguise?. Experimental Neurology, 284(Pt B), 220–229. 10.1016/j.expneurol.2016.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Teesson M, Ross J, & Peters L (2006). Trauma, PTSD, and substance use disorders: Findings from the Australian national survey of mental health and well-being. American Journal of Psychiatry, 163(4), 651–658. 10.1176/ajp.2006.163.4.652 [DOI] [PubMed] [Google Scholar]
- Minassian A, Geyer MA, Baker DG, Nievergelt CM, O’Connor DT, Risbrough VB, & Marine Resiliency Study Team. (2014). Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosomatic Medicine, 76(4), 292–301. doi: 10.1097/PSY.0000000000000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB, & Marine Resiliency Study Team. (2015). Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiatry, 72(10), 979–986. doi: 10.1001/jamapsychiatry.2015.0922 [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, & Garber J (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review, 32, 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Hellman N, Abelson JL, & Rao U (2016). Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clinical Psychology Review, 49, 79–91. 10.1016/j.cpr.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B (2017). Corticolimbic regulation of cardiovascular responses to stress. Physiology & Behavior, 172, 49–59. 10.1016/j.physbeh.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Gonzalez IJ, Meruva VB, Callahan KA, Belfort BD, Ramanathan KR, Meyers E, Kilgard MP, Rennaker RL, & McIntyre CK (2017). Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Translational Psychiatry, 7(8), e1217. 10.1038/tp.2017.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylocks KM, Michopoulos V, Rothbaum AO, Almli L, Gillespie CF, Wingo A, Schwartz AC, Habib L, Gamwell KL, Marvar PJ, Bradley B, & Ressler KJ (2015). An angiotensin-converting enzyme (ACE) polymorphism may mitigate the effects of angiotensin-pathway medications on posttraumatic stress symptoms. American Journal of Medical Genetics, 168B(4), 307–315. 10.1002/ajmg.b.32313 [DOI] [PubMed] [Google Scholar]
- O’Donnell CJ, Schwartz Longacre L, Cohen BE, Fayad ZA, Gillespie CF, Liberzon I, Pathak GA, Polimanti R, Risbrough V, Ursano RJ, Vander Heide RS, Yancy CW, Vaccarino V, Sopko G, & Stein MB (2021). Posttraumatic stress disorder and cardiovascular disease: State of the science, knowledge gaps, and research opportunities. JAMA Cardiology, 6(10), 1207–1216. 10.1001/jamacardio.2021.2530 [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Neylan TC, Metzler T, & Cohen BE (2012). Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain, Behavior, and Immunity, 26(4), 642–649. 10.1016/j.bbi.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, & Herz LR (1993). Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology, 102(1), 152–159. 10.1037/0021-843X.102.1.152 [DOI] [PubMed] [Google Scholar]
- Pan X, Kaminga AC, Wen SW, & Liu A (2018). Catecholamines in post-traumatic stress disorder: A systematic review and meta-analysis. Frontiers in Molecular Neuroscience, 11, 450. 10.3389/fnmol.2018.00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Lee JY, Kang S-H, Choi JH, Kim TY, So HS, & Yoon I-Y (2017). Heart rate variability of chronic posttraumatic stress disorder in the Korean veterans. Psychiatry Research, 255, 72–77. 10.1016/j.psychres.2017.05.011 [DOI] [PubMed] [Google Scholar]
- Parrish JN, Bertholomey ML, Pang HW, Speth RC, & Torregrossa MM (2019). Estradiol modulation of the renin-angiotensin system and the regulation of fear extinction. Translational Psychiatry, 9(1), 36. 10.1038/s41398-019-0374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino NJ, Wout-Frank M. van ‘t, Aiken E, Swearingen HR, Barredo J, Zandvakili A, & Philip NS (2020). One-year clinical outcomes following theta burst stimulation for post-traumatic stress disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 10.1038/s41386-019-0584-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Barredo J, Aiken E, Larson V, Jones RN, Shea MT, Greenberg BD, & van ‘t Wout-Frank M (2019). Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. The American Journal of Psychiatry, 176(11), 939–948. 10.1176/appi.ajp.2019.18101160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Barredo J, van ‘t Wout-Frank M, Tyrka AR, Price LH, & Carpenter LL (2018). Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biological Psychiatry, 83(3), 263–272. 10.1016/j.biopsych.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Nillni YI, King MW, Patton SC, Bauer MR, Mostoufi SM, Gerber MR, Hauger R, Resick PA, Rasmusson AM, & Orr SP (2016). Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. Journal of Abnormal Psychology, 125(3), 349–355. 10.1037/abn0000138 [DOI] [PubMed] [Google Scholar]
- Pizzi C, Mancini S, Angeloni L, Fontana F, Manzoli L, & Costa GM (2009). Effects of selective serotonin reuptake inhibitor therapy on endothelial function and inflammatory markers in patients with coronary heart disease. Clinical Pharmacology and Therapeutics, 86(5), 527–532. 10.1038/clpt.2009.121 [DOI] [PubMed] [Google Scholar]
- Platt JM, Lowe SR, Galea S, Norris FH, & Koenen KC (2016). A longitudinal study of the bidirectional relationship between social support and posttraumatic stress following a natural disaster. Journal of Traumatic Stress, 29(3), 205–213. 10.1002/jts.22092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppa T, de Witte S, Vanderhasselt M-A, Bechara A, & Baeken C (2020). Theta-burst stimulation and frontotemporal regulation of cardiovascular autonomic outputs: The role of state anxiety. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 149, 25–34. 10.1016/j.ijpsycho.2019.12.011 [DOI] [PubMed] [Google Scholar]
- Pulcu E, Shkreli L, Holst CG, Woud ML, Craske MG, Browning M, & Reinecke A (2019). The effects of the angiotensin II receptor antagonist losartan on appetitive versus aversive learning: A randomized controlled trial. Biological Psychiatry, 86(5), 397–404. 10.1016/j.biopsych.2019.04.010 [DOI] [PubMed] [Google Scholar]
- Rabe S, Dörfel D, Zöllner T, Maercker A, & Karl A (2006). Cardiovascular correlates of motor vehicle accident related to posttraumatic stress disorder and its successful treatment. Applied Psychophysiology and Biofeedback, 31(4), 315–330. 10.1007/s10484-006-9027-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae Olmsted KL, Bartoszek M, Mulvaney S, McLean B, Turabi A, Young R, Kim E, Vandermaas-Peeler R, Kelley Morgan J, Constantinescu O, Kane S, Nguyen C, Hirsch S, Munoz B, Wallace D, Croxford J, Lynch JH, White R, &Walters, B. B. (2020). Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: A randomized clinical trial. JAMA Psychiatry, 77(2), 130–138. doi: 10.1001/jamapsychiatry.2019.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Chow B, Harris C, Davis-Karim A, Holmes HA, Hart KL, McFall M, Mellman TA, Reist C, Romesser J, Rosenheck R, Shih MC, Stein MB, Swift R, Gleason T, Lu Y, & Huang GD (2018). Trial of prazosin for post-traumatic stress disorder in military Veterans. The New England Journal of Medicine, 378(6), 507–517. 10.1056/NEJMoa1507598 [DOI] [PubMed] [Google Scholar]
- Reist C, Streja E, Tang CC, Shapiro B, Mintz J, & Hollifield M (2021). Prazosin for treatment of post-traumatic stress disorder: A systematic review and meta-analysis. CNS Spectrums, 26(4), 338–344. 10.1017/S1092852920001121 [DOI] [PubMed] [Google Scholar]
- Remue J, Vanderhasselt M-A, Baeken C, Rossi V, Tullo J, & De Raedt R (2016). The effect of a single HF-rTMS session over the left DLPFC on the physiological stress response as measured by heart rate variability. Neuropsychology, 30(6), 756–766. DOI: 10.1037/neu0000255 [DOI] [PubMed] [Google Scholar]
- Ross DA, Arbuckle MR, Travis MJ, Dwyer JB, van Schalkwyk GI, & Ressler KJ (2017). An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: An educational review. JAMA Psychiatry, 74(4), 407–415. doi: 10.1001/jamapsychiatry.2016.3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šagud M, Jakšić N, Vuksan-Ćusa B, Lončar M, Lončar I, Peleš AM, Miličić D, & Jakovljević M (2017). Cardiovascular disease risk factors in patients with posttraumatic stress disorder (PTSD): A narrative review. Psychiatria Danubina, 29(4), 421–430. 10.24869/psyd.2017.421 [DOI] [PubMed] [Google Scholar]
- Sahar T, Shalev AY, & Porges SW (2001). Vagal modulation of responses to mental challenge in posttraumatic stress disorder. Biological Psychiatry, 49(7), 637–643. 10.1016/S0006-3223(00)01045-3 [DOI] [PubMed] [Google Scholar]
- Seligowski AV, Duffy LA, Merker JB, Michopoulos V, Gillespie CF, Marvar PJ, Stein MB, & Ressler KJ (2021a). The renin-angiotensin system in PTSD: A replication and extension. Neuropsychopharmacology. 10.1038/s41386-020-00923-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligowski AV, Harnett NG, Merker JB, & Ressler KJ (2020). Nervous and endocrine system dysfunction in posttraumatic stress disorder: An overview and consideration of sex as a biological variable. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(4), 381–391. 10.1016/j.bpsc.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligowski AV, Reffi AN, Phillips KA, Orcutt HK, Auerbach RP, Pizzagalli DA, & Ressler KJ (2021b). Neurophysiological responses to safety signals and the role of cardiac vagal control. Behavioural Brain Research, 396, 112914. 10.1016/j.bbr.2020.112914 [DOI] [PubMed] [Google Scholar]
- Sheynin J, Duval ER, King AP, Angstadt M, Phan KL, Simon NM, Rauch S, & Liberzon I (2020). Associations between resting-state functional connectivity and treatment response in a randomized clinical trial for posttraumatic stress disorder. Depression and Anxiety, 37(10), 1037–1046. 10.1002/da.23075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AN, Meshkat S, Benitah K, Lipsitz O, Gill H, Lui LMW, Teopiz KM, McIntyre RS, & Rosenblat JD (2021). Registered clinical studies investigating psychedelic drugs for psychiatric disorders. Journal of Psychiatric Research, 139, 71–81. 10.1016/j.jpsychires.2021.05.019 [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, Löwe B, & Grabe HJ (2010). Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. Journal of Psychiatric Research, 44(1), 15–21. 10.1016/j.jpsychires.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Steckler T, & Risbrough V (2012). Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology, 62(2), 617–627. 10.1016/j.neuropharm.2011.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Jain S, Simon NM, West JC, Marvar PJ, Bui E, He F, Benedek DM, Cassano P, Griffith JL, Howlett J, Malgaroli M, Melaragno A, Seligowski AV, Shu IW, Song S, Szuhany K, Taylor CT, Ressler KJ, & LOSe-PTSD Investigators (2021). Randomized, placebo-controlled trial of the angiotensin receptor antagonist losartan for posttraumatic stress disorder. Biological Psychiatry, 90(7), 473–481. 10.1016/j.biopsych.2021.05.012 [DOI] [PubMed] [Google Scholar]
- Stevens JS, Reddy R, Kim YJ, van Rooij S, Ely TD, Hamann S, Ressler KJ, & Jovanovic T (2017). Episodic memory after trauma exposure: Medial temporal lobe function is positively related to re-experiencing and inversely related to negative affect symptoms. NeuroImage: Clinical, 17, 650–658. 10.1016/j.nicl.2017.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout DM, & Risbrough VB (2019). [Review of Angiotensin II Signaling and Fear Extinction: Translational Evidence and Novel Receptor Targets]. Biological Psychiatry, 86(12), 874–876. DOI: 10.1016/j.biopsych.2019.09.026 [DOI] [PubMed] [Google Scholar]
- Sueta D, Koibuchi N, Hasegawa Y, Toyama K, Uekawa K, Katayama T, Uekewa K, Katayama T, Jie Ma M, Nakagawa T, Ogawa H, & Kim-Mitsuyama S (2014). Telmisartan exerts sustained blood pressure control and reduces blood pressure variability in metabolic syndrome by inhibiting sympathetic activity. American Journal of Hypertension, 27(12), 1464–1471. DOI: 10.1093/ajh/hpu076 [DOI] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Roberts AL, Chen Q, Rimm EB, & Koenen KC (2020). Not all posttraumatic stress disorder symptoms are equal: Fear, dysphoria, and risk of developing hypertension in trauma-exposed women. Psychological Medicine, 50(1), 38–47. 10.1017/S0033291718003914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LE, & Sullivan JC (2016). Sex differences in obesity-induced hypertension and vascular dysfunction: a protective role for estrogen in adipose tissue inflammation?. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 311(4), R714–R720. 10.1152/ajpregu.00202.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terock J, Hannemann A, Janowitz D, Freyberger HJ, Felix SB, Dörr M, Nauck M, Völzke H, & Grabe HJ (2019a). Associations of trauma exposure and post-traumatic stress disorder with the activity of the renin-angiotensin-aldosterone-system in the general population. Psychological Medicine, 49(5), 843–851. doi: 10.1017/S0033291718001496 [DOI] [PubMed] [Google Scholar]
- Terock J, Hannemann A, Janowitz D, Van der Auwera S, Bahls M, Völzke H, & Grabe HJ (2019b). Differential activation of the renin-angiotensin-aldosterone-system in response to childhood and adulthood trauma. Psychoneuroendocrinology, 107, 232–240. 10.1016/j.psyneuen.2019.05.026 [DOI] [PubMed] [Google Scholar]
- Tolin DF, & Foa EB (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132, 959–992. 10.1037/1942-9681.S.1.37 [DOI] [PubMed] [Google Scholar]
- Tovote P, Meyer M, Ronnenberg A, Ogren SO, Spiess J, & Stiedl O (2005). Heart rate dynamics and behavioral responses during acute emotional challenge in corticotropin-releasing factor receptor 1-deficient and corticotropin-releasing factor-overexpressing mice. Neuroscience, 134(4), 1113–1122. 10.1016/j.neuroscience.2005.05.027 [DOI] [PubMed] [Google Scholar]
- Udupa K, Sathyaprabha TN, Thirthalli J, Kishore KR, Raju TR, & Gangadhar BN (2007). Modulation of cardiac autonomic functions in patients with major depression treated with repetitive transcranial magnetic stimulation. Journal of Affective Disorders, 104(1–3), 231–236. 10.1016/j.jad.2007.04.002 [DOI] [PubMed] [Google Scholar]
- van den Berk-Clark C, Secrest S, Walls J, Hallberg E, Lustman PJ, Schneider FD, & Scherrer JF (2018). Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring [sic.] smoking: A systematic review and meta-analysis. Health Psychology : Official Journal of the Division of Health Psychology, American Psychological Association, 37(5), 407–416. 10.1037/hea0000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale C, Mendelsohn ME, & Rosano GM (2009). Gender differences in the cardiovascular effect of sex hormones. Nature Reviews: Cardiology, 6(8), 532–542. 10.1038/nrcardio.2009.105 [DOI] [PubMed] [Google Scholar]
- von Känel R, Hari R, Schmid JP, Wiedemar L, Guler E, Barth J, Saner H, Schnyder U, & Begré S (2011). Non-fatal cardiovascular outcome in patients with posttraumatic stress symptoms caused by myocardial infarction. Journal of Cardiology, 58(1), 61–68. 10.1016/j.jjcc.2011.02.007 [DOI] [PubMed] [Google Scholar]
- von Känel R, Hepp U, Traber R, Kraemer B, Mica L, Keel M, Mausbach BT, & Schnyder U (2008). Measures of endothelial dysfunction in plasma of patients with posttraumatic stress disorder. Psychiatry Research, 158(3), 363–373. 10.1016/j.psychres.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Wang Y, Seto S-W, & Golledge J (2014). Angiotensin II, sympathetic nerve activity and chronic heart failure. Heart Failure Reviews, 19(2), 187–198. 10.1007/s10741-012-9368-1 [DOI] [PubMed] [Google Scholar]
- Wangelin BC, & Tuerk PW (2015). Taking the pulse of prolonged exposure therapy: Physiological reactivity to trauma imagery as an objective measure of treatment response. Depression and Anxiety, 32(12), 927–934. 10.1002/da.22449 [DOI] [PubMed] [Google Scholar]
- Watts BV, Landon B, Groft A, & Young-Xu Y (2012). A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimulation, 5(1), 38–43. 10.1016/j.brs.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Weiss T, Skelton K, Phifer J, Jovanovic T, Gillespie CF, Smith A, Umpierrez G, Bradley B, & Ressler KJ (2011). Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. General Hospital Psychiatry, 33(2), 135–142. 10.1016/j.genhosppsych.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A, Walton D, Lovell K, & Proctor D (2015). Metacognitive therapy versus prolonged exposure in adults with chronic post-traumatic stress disorder: A parallel randomized controlled trial. Cognitive Therapy and Research, 39(1), 70–80. 10.1007/s10608-014-9636-6 [DOI] [Google Scholar]
- Wittbrodt MT, Gurel NZ, Nye JA, Ladd S, Shandhi M, Huang M, Shah AJ, Pearce BD, Alam ZS, Rapaport MH, Murrah N, Ko YA, Haffer AA, Shallenberger LH, Vaccarino V, Inan OT, & Bremner JD (2020). Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimulation, 13(5), 1333–1348. 10.1016/j.brs.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittbrodt MT, Gurel NZ, Nye JA, Shandhi M, Gazi AH, Shah AJ, Pearce BD, Murrah N, Ko YA, Shallenberger LH, Vaccarino V, Inan OT, & Bremner JD (2021). Noninvasive cervical vagal nerve stimulation alters brain activity during traumatic stress in individuals with posttraumatic stress disorder. Psychosomatic Medicine, 83(9), 969–977. 10.1097/PSY.0000000000000987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Feng Y, Obr TD, Hickman PJ, & Lazartigues E (2009). Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension, 53(2), 210–216. 10.1161/HYPERTENSIONAHA.108.123844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Mason JW, & Giller EL (1993). Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders. Biological Psychiatry, 34(1–2), 18–25. 10.1016/0006-3223(93)90252-9 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Yang RK, Buchsbaum MS, & Golier JA (2006). Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology, 31(4), 447–451. 10.1016/j.psyneuen.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yoshino A, Kobayashi Y, Inoue M, Kamakura K, & Nomura S (2001). Effects of slow repetitive transcranial magnetic stimulation on heart rate variability according to power spectrum analysis. Journal of the Neurological Sciences, 184(1), 77–80. 10.1016/S0022-510X(00)00505-0 [DOI] [PubMed] [Google Scholar]
- Zandvakili A, Philip NS, Jones SR, Tyrka AR, Greenberg BD, & Carpenter LL (2019). Use of machine learning in predicting clinical response to transcranial magnetic stimulation in comorbid posttraumatic stress disorder and major depression: A resting state electroencephalography study. Journal of Affective Disorders, 252, 47–54. 10.1016/j.jad.2019.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandvakili A, Swearingen HR, & Philip NS (2020). Changes in functional connectivity after theta-burst transcranial magnetic stimulation for post-traumatic stress disorder: A machine-learning study. European Archives of Psychiatry and Clinical Neuroscience, 271, 29–37. 10.1007/s00406-020-01172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Geng Y, Xin F, Li J, Feng P, Liu C, Zhao W, Feng T, Guastella AJ, Ebstein RP, Kendrick KM, & Becker B (2019). Human extinction learning is accelerated by an angiotensin antagonist via ventromedial prefrontal cortex and its connections with basolateral amygdala. Biological Psychiatry, 86(12), 910–920. 10.1016/j.biopsych.2019.07.007 [DOI] [PubMed] [Google Scholar]


