Abstract
Background:
Patients with chronic kidney disease (CKD) on dialysis (CKD G5D) have worse cardiovascular outcomes than patients with advanced non-dialysis CKD (CKD G4–5: estimated glomerular filtration rate <30 ml/min/1.73m2). Our objective was to evaluate the relationship between achievement of cardiovascular guideline-directed medical therapy (GDMT) goals and clinical outcomes for CKD G5D versus CKD G4–5.
Methods:
This was a subgroup analysis of International Study of Comparative Health Effectiveness of Medical and Invasive Approaches (ISCHEMIA)-CKD participants with CKD G4–5 or CKD G5D and moderate-to-severe myocardial ischemia on stress testing. Exposures included dialysis requirement at randomization and GDMT goal achievement during follow-up. The composite outcome was all-cause mortality or non-fatal myocardial infarction (MI). Individual GDMT goal (smoking cessation, systolic blood pressure <140 mmHg, low-density lipoprotein cholesterol <70 mg/dL, statin use, aspirin use) trajectory was modeled. Percentage point difference was estimated for each GDMT goal at 24 months between CKD G5D and CKD G4–5, and for association with key predictors. Probability of survival free from all-cause mortality or non-fatal MI by GDMT goal achieved was assessed for CKD G5D vs CKD G4–5.
Results:
A total of 415 CKD G5D, and 362 CKD G4–5 participants were randomized. Participants with CKD G5D were less likely to receive statin (−6.9%, 95% CI: −10.3%, −3.7%) and aspirin therapy (−3.0%, 95% CI: −5.6%, −0.6%), with no difference in other GDMT goal attainment. Cumulative exposure to GDMT achieved during follow-up was associated with reduction in all-cause mortality or non-fatal MI (HR 0.88, 95% CI: 0.87, 0.90; per each GDMT goal attained over 60 days), irrespective of dialysis status.
Conclusions:
CKD G5D participants received statin or aspirin therapy less often. Cumulative exposure to GDMT goals achieved was associated with lower incidence of all-cause mortality or non-fatal MI in participants with advanced CKD and chronic coronary disease, regardless of dialysis status.
Keywords: Cardiovascular Disease, Chronic Coronary Disease, Chronic Kidney Disease, Clinical Trial, End Stage Kidney Disease, Guideline-Directed Medical Therapy, Secondary Prevention
Introduction
The evidence for cardiovascular event reduction by single risk factor intervention in patients with advanced chronic kidney disease (CKD) varies by dialysis status. Patients with CKD G3–5 (defined as estimated glomerular filtration rate [eGFR] <60 ml/min/1.73 m2) benefit from two specific risk factor interventions: statin therapy and intensive blood pressure reduction.1–6 However, the evidence for statin therapy in patients receiving dialysis (CKD G5D) is mixed.1,7,8 The evidence base for blood pressure reduction in patients on dialysis is limited. Large randomized controlled trials of intensive versus standard blood pressure control have excluded patients on dialysis.9–11
Multiple risk factor reduction improves outcomes in patients with preserved kidney function and high risk for cardiovascular events; the benefits appear to be directly proportional to the number of risk factors controlled.12–14 The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches—Chronic Kidney Disease (ISCHEMIA-CKD) enrolled participants with CKD G4–5D and chronic coronary disease (CCD), and utilized a multiple risk factor reduction strategy in all participants.15 Our objective was to compare achievement of multiple cardiovascular risk factor goals and associated clinical outcomes between participants with CKD G5D and CKD G4–5.
Methods
The rationale and design of ISCHEMIA-CKD (NCT01985360) have been published previously.16 Briefly, ISCHEMIA-CKD was an investigator-initiated, National Institutes of Health Heart, Lung, and Blood Institute-funded, international randomized comparative effectiveness trial. It was designed to test the hypothesis that an initial invasive strategy when added to guideline-directed medical therapy (GDMT) in participants with CCD and advanced CKD on dialysis (CKD G5D) or not on dialysis (CKD G4–5), will reduce the composite outcome of all-cause mortality or non-fatal myocardial infarction, compared with an initial conservative strategy of GDMT alone. Recruitment occurred between April 29, 2014 and January 31, 2018. The study was approved by the Institutional Review Boards at each participating site. For the current analysis, participants were categorized by their baseline dialysis requirement status (CKD G5D or CKD G4–5).
Risk factor management recommendations were provided to all study sites to guide treatment of study participants, irrespective of treatment strategy. The Clinical Coordinating Center (CCC) developed algorithms for goal attainment in consultation with the trial GDMT and Renal committees. Individual sites were provided with monthly reports of participant-level risk factor control status to identify areas for improvement. Sites with multiple participants not meeting GDMT goals were contacted by CCC faculty and staff members for recommendations to improve out-of-range parameters. To improve medication adherence, the trial provided certain medications at no cost to participants in some countries.16,17 The time points for evaluation were as follows: enrollment/randomization (baseline), 1.5-, 3-, 6-, 12-months and then every 6 months until trial completion; median duration of follow-up was 2.2 years.
GDMT goals included low-density lipoprotein cholesterol (LDL-C) <70 mg/dL (1.8 mmol/L), systolic blood pressure (SBP) <140 mmHg, statin therapy, anti-platelet therapy (monotherapy with aspirin or oral P2Y12 antagonist, or dual antiplatelet therapy following percutaneous coronary intervention [PCI]), and smoking cessation. Participants with CKD G5D were recommended to receive moderate- or high-intensity statin as tolerated and those with CKD 4–5 were to receive high-intensity statins.2,16 The SBP goal was initially <140 mmHg for all participants except for those with known proteinuria, in whom a goal of SBP <130 mmHg was recommended. The goal was changed to <130 mmHg for all participants after April 2018 to be consistent with prevailing guideline changes.15 For the current analysis SBP <140 mmHg was used as the SBP goal. CKD-specific parameters such as management of metabolic bone disease and anemia were left to the participants’ primary nephrologists per local or national guidelines. Site personnel focused on behavioral coaching via the Patient-centered Assessment and Counseling for Exercise and nutrition program.16,18 Briefly, this program focused on counseling and encouraging participants toward achievement of GDMT goals (specifically for smoking cessation, increased physical activity, medication adherence, and reduction of saturated fat intake). Participants were assessed based on the stages of change (precontemplation, contemplation, preparation, action, maintenance, or relapse) and counseled accordingly.
Statistical Analysis
Baseline participant characteristics were described by CKD group. Categorical variables were summarized using counts and percentages and assessed associations with CKD group using the Chi-squared test. Continuously measured variables were summarized with the median and first and third quartiles, with associations with CKD group assessed using the Kruskal-Wallis test.
We used Bayesian generalized linear mixed models to examine the association between likelihood of attainment of each GDMT goal during follow-up and dialysis status at baseline while accounting for between-participant heterogeneity. We used these models to estimate the difference in the probability of individual goal attainment at 24 months between CKD G5D versus CKD G4–5 and characterized uncertainty in the estimated difference with a 95% credible interval. For each of the GDMT risk factors, a patient may have attained a goal at a given follow-up visit while relapsing at a subsequent visit. The data are in the form of binary risk factors collected at each follow-up visit. To model each longitudinal binary GDMT risk factor, we used generalized linear mixed modeling, which can account for the potential correlation among measurements from the same patient. In other words, for a given patient, it may be reasonable to hypothesize a positive correlation between no smoking goal attainment at different follow-up visits: A patient who attained the no smoking goal at the 6-month visit may be more likely to attain the no smoking goal at the 1-year visit. By averaging over covariate distributions and random effects, inferences pertain to an average participant.
In addition, we defined the total number of GDMT goals attained at each follow-up visit as an ordinal variable with four categories for 0–2, 3, 4, and 5 goals. Using analogous modeling for an ordinal outcome, we modeled the probability of attaining more than 0–2 goals, 3 goals, and 4 goals over follow-up as a function of dialysis status at baseline. All models controlled for age at randomization, sex, treatment strategy, left ventricular ejection fraction, and diabetes status. The coefficients for age at randomization and ejection fraction refer to an interquartile increase from the 25th to the 75th percentile of the distribution. In sensitivity analysis, we evaluated whether estimated associations between GDMT goal attainment and dialysis status at baseline were sensitive to the implicit missing at random assumption about missed GDMT measurements in the mixed modeling. Using pattern mixture models, we hypothesized that missed GDMT measurements may be missing not at random according to whether participants died versus survived to the end of follow-up.19,20
We explored the association of the primary outcome (all-cause mortality or non-fatal myocardial infarction (MI)) with the number of GDMT goals attained at each follow-up visit and dialysis status at baseline. In addition, we examined the number of GDMT goals attained as the running average of goals attained, calculated as an unweighted average across previous visits; and the cumulative exposure to GDMT, calculated as a weighted average of follow-up time with weights as the number of goals attained at each visit. In multivariable analysis, we fit Cox proportional hazards regression models with a time-dependent covariate to assess the association of the primary outcome and GDMT goal attainment over follow-up, and whether this association differed by dialysis status at baseline. We assessed the proportional hazards assumption for the number of GDMT goals attained over follow-up by conducting a score test to test the null hypothesis of independence between the scaled Schoenfeld residuals and time. We examined plots of scaled Schoenfeld residuals against log transformed time to visually assess the proportional hazards assumption. Models controlled for baseline number of GDMT goals attained, dialysis status at baseline, age at randomization, sex, treatment strategy, ejection fraction, and diabetes status. As in the GDMT modeling, the coefficients for age at randomization and ejection fraction refer to an interquartile increase. We used multiple imputation by chained equations to impute missing individual GDMT goals.21 Inferences were pooled across multiply imputed datasets using Rubin’s rules for regression coefficients.22
Detailed methods are provided in the Appendix. All analyses were conducted in R software (The R Foundation for statistical computing, Vienna, Austria); Bayesian modeling was conducted using JAGS.23
Data Sharing
Data will be shared in accordance with the NIH data sharing plan, effective summer 2022.
Results
Baseline characteristics by CKD group
A total of 415 participants with CKD G5D and 362 with CKD G4–5 were enrolled (Table 1). Those with CKD G5D were younger (61 vs. 67 years, P < 0.001), less likely to be diabetic (53 vs. 62%, P = 0.02), more likely to be on the transplant waitlist (22 vs. 3%, P < 0.001), have a lower body mass index (26 vs 28 kg/m2 for CKD G4–5, p < 0.001), and had a higher proportion characterized as Asian or Black/African American (P = 0.03), as compared with participants with CKD G4–5.
Table 1.
Participant Baseline Characteristics by chronic kidney disease (CKD) group
| CKD G4–5† | CKD G5D‡ | P-value | |
|---|---|---|---|
| N | 362 | 415 | |
| Randomized Strategy Arm | 0.209 | ||
| CON | 172/362 (48%) | 217/415 (52%) | |
| INV | 190/362 (52%) | 198/415 (48%) | |
| Female | 115/362 (32%) | 127/415 (31%) | 0.785 |
| Age at randomization, years (Median (Q1, Q3)) | 67 (59, 73) | 61 (54, 67) | <0.001 |
| Race | 0.030 | ||
| Asian | 85/354 (24%) | 106/393 (27%) | |
| Black or African American | 20/354 (6%) | 43/393 (11%) | |
| White | 245/354 (69%) | 236/393 (60%) | |
| Other | 4/354 (1%) | 8/393 (2%) | |
| Comorbidities | |||
| Diabetes | 223/362 (62%) | 221/415 (53%) | 0.023 |
| Hypertension | 330/361 (91%) | 381/412 (92%) | 0.682 |
| Smoking status | 0.823 | ||
| Never smoked | 177/362 (49%) | 194/415 (47%) | |
| Former smoker | 146/362 (40%) | 176/415 (42%) | |
| Current smoker | 39/362 (11%) | 45/415 (11%) | |
| Prior myocardial infarction | 69/362 (19%) | 64/414 (15%) | 0.218 |
| Prior heart failure | 57/362 (16%) | 78/415 (19%) | 0.306 |
| Prior stroke | 30/362 (8%) | 38/415 (9%) | 0.764 |
| Prior peripheral vascular disease | 23/362 (6%) | 25/415 (6%) | 0.967 |
| Renal Parameters | |||
| Dialysis type | |||
| Hemodialysis | 344/409 (84%) | ||
| Peritoneal dialysis | 60/409 (15%) | ||
| Other | 5/409 (1%) | ||
| Duration of dialysis, years | |||
| N | 369 | ||
| Median (Q1, Q3) | 2 (1, 5) | ||
| Prior renal transplant | 7/362 (2%) | 17/415 (4%) | 0.126 |
| Renal transplant waitlist | 10/342 (3%) | 84/382 (22%) | <0.001 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | |||
| N | 362 | ||
| Median (Q1, Q3) | 23 (17, 27) | ||
| Cardiovascular Parameters | |||
| Ejection fraction (%) | 0.458 | ||
| N | 297 | 322 | |
| Median (Q1, Q3) | 58 (50, 64) | 58 (50, 64) | |
| Body mass index, kg/m2 | <0.001 | ||
| N | 362 | 411 | |
| Median (Q1, Q3) | 28 (25, 32) | 26 (23, 30) | |
| Low density lipoprotein-cholesterol, mg/dL | 0.601 | ||
| N | 349 | 378 | |
| Median (Q1, Q3) | 83 (62, 109) | 83 (56, 115) | |
| Prior PCI | 68/362 (19%) | 78/415 (19%) | 1.000 |
| Prior coronary artery bypass grafting | 15/362 (4%) | 13/415 (3%) | 0.575 |
| Guideline-directed medical therapy goals at target at trial entry* | 0.004 | ||
| 0–2 | 42/349 (12%) | 83/378 (22%) | |
| 3 | 117/349 (34%) | 105/378 (28%) | |
| 4 | 133/349 (38%) | 138/378 (37%) | |
| 5 | 57/349 (16%) | 52/378 (14%) | |
| Individual guideline-directed medical therapy goals at target at trial entry | |||
| Not smoking | 323/362 (89%) | 370/415 (89%) | 1.0000 |
| Aspirin | 326/362 (90%) | 326/414 (79%) | <0.001 |
| Low-density lipoprotein cholesterol < 70 mg/dl | 118/349 (34%) | 136/378 (36%) | 0.5929 |
| Systolic blood pressure <140 mmHg | 201/362 (56%) | 228/414 (55%) | 0.9569 |
| On statin | 320/362 (88%) | 309/414 (75%) | <0.001 |
CON: Initial conservative strategy group; INV: initial invasive strategy group; mg/dl: kg: kilograms; m2: meters squared; min: minute; ml: milliliters; mg/dl: milligrams per deciliter; mmHg: millimeters of mercury; N: number; PCI: percutaneous coronary intervention; Q: quartile.
Guideline-directed medical therapy targets: systolic blood pressure < 140 mmHg; low density lipoprotein-cholesterol < 70 mg/dl; on statin therapy; on antiplatelet therapy; not smoking.
Categories are represented as N (%) except otherwise indicated.
Chronic kidney disease (CKD) G4–5: advanced CKD (estimated glomerular filtration rate < 30 ml/min/1.73m2), but not on dialysis.
CKD G5D: advanced CKD requiring renal replacement therapy (dialysis requirement).
GDMT goal attainment at baseline and 24 months
At baseline there was a higher proportion of CKD G5D participants with only 0–2 GDMT goals achieved as compared with CKD G4–5 (22% versus 12%, respectively; p = 0.004) (Table 1, Figure 1). CKD G5D and CKD G4–5 had similarly low proportions with all 5 GDMT goals at target at baseline (16% vs. 14%, respectively). Among participants with CKD G5D the LDL-C target was met in 36% while 89% were not active smokers (Figure S1a). Those with CKD G4–5 demonstrated achievement of LDL-C target in 34% while the vast majority (90%) were receiving aspirin.
Figure 1.
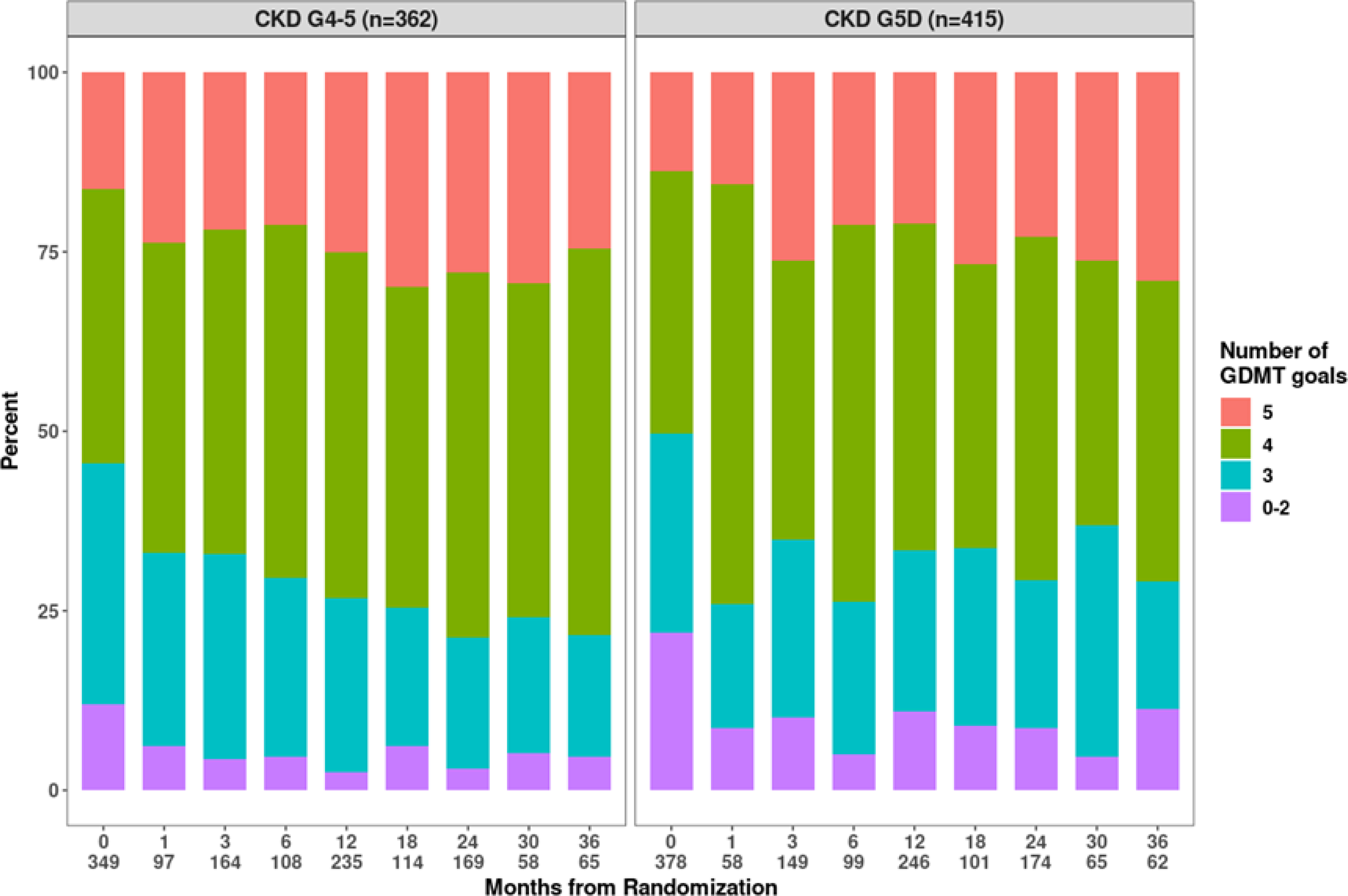
Percent of participants who attained a given number of guideline-directed medical therapy (GDMT) goals over follow-up, by chronic kidney disease (CKD) group. The count of non-missing values at each follow-up visit is appended below the visit month. The 5 GDMT goals include: No smoking; aspirin use; Low Density Lipoprotein-Cholesterol < 70 mg/dl; Systolic Blood Pressure < 140 mmHg; and being on a statin.
At 24 months, GDMT goal attainment of all 5 GDMT goals was 23% in CKD G5D, and 28% in CKD G4–5 (Figure 1). For individual parameters at 24 months (Figure S1b), among participants with CKD G5D, the range of goal attainment was 50% for LDL-C to 91% for not smoking. Among CKD G4–5, goal attainment ranged from 48% for LDL-C target to >90% for being on statins, aspirin and not smoking.
Predicted estimates of GDMT goals attained by CKD Group at 24 months
CKD G5D participants were slightly less likely than CKD G4–5 to attain more than 2 GDMT goals at 24 months following randomization (92% vs. 96%, respectively) (Table 2 and Figure 2). The estimated differences between CKD cohorts for GDMT goal attainment were attenuated for more than 3 or 4 goals, with an estimated difference near null for attaining more than 4 goals (26.8% vs. 26.2%, for CKD G5D vs. CKD G4–5).
Table 2.
Table of estimated percentage of higher number of guideline-directed medical therapy (GDMT) goals attained (95% credible interval), and of estimated percentage of guideline-directed medical therapy (GDMT) goal attainment (95% credible interval), at 24m following randomization by chronic kidney disease (CKD) group
| Number of GDMT* Goals Attained | CKD G4–5† | CKD G5D‡ |
|---|---|---|
| > 2 | 96.02 (94.36, 97.38) | 92.32 (90.26, 94.13) |
| > 3 | 73.7 (70, 77.3) | 69.52 (65.83, 73.1) |
| > 4 | 26.15 (22.92, 29.6) | 26.79 (23.33, 30.4) |
| GDMT Goal Attainment | CKD G4–5† | CKD G5D‡ |
| No Smoking | 92.07 (87.53, 96.04) | 93.2 (90.16, 95.92) |
| Aspirin | 96.17 (94.58, 97.32) | 93.13 (91.05, 94.82) |
| LDL-C < 70 mg/dL | 48.98 (44.71, 53.41) | 50.94 (46.59, 55.26) |
| SBP < 140 mmHg | 68.68 (65.22, 72.08) | 70.05 (66.78, 73.26) |
| On Statin | 95.45 (93.58, 96.8) | 88.53 (85.45, 91.14) |
GDMT: guideline-directed medial therapy; LDL-C: low-density lipoprotein cholesterol; mg/dl: milligrams per deciliter; mmHg: millimeters of mercury; SBP: systolic blood pressure.
Guideline-directed medical therapy targets: systolic blood pressure < 140 mmHg; low-density lipoprotein cholesterol <70 mg/dL; on statin therapy; on antiplatelet therapy; not smoking.
Categories are represented as N (%) except otherwise indicated.
Chronic kidney disease (CKD) G4–5: advanced CKD (estimated glomerular filtration rate < 30 ml/min/1.73m2), but not on dialysis.
CKD G5D: advanced CKD requiring renal replacement therapy (dialysis requirement).
Figure 2.
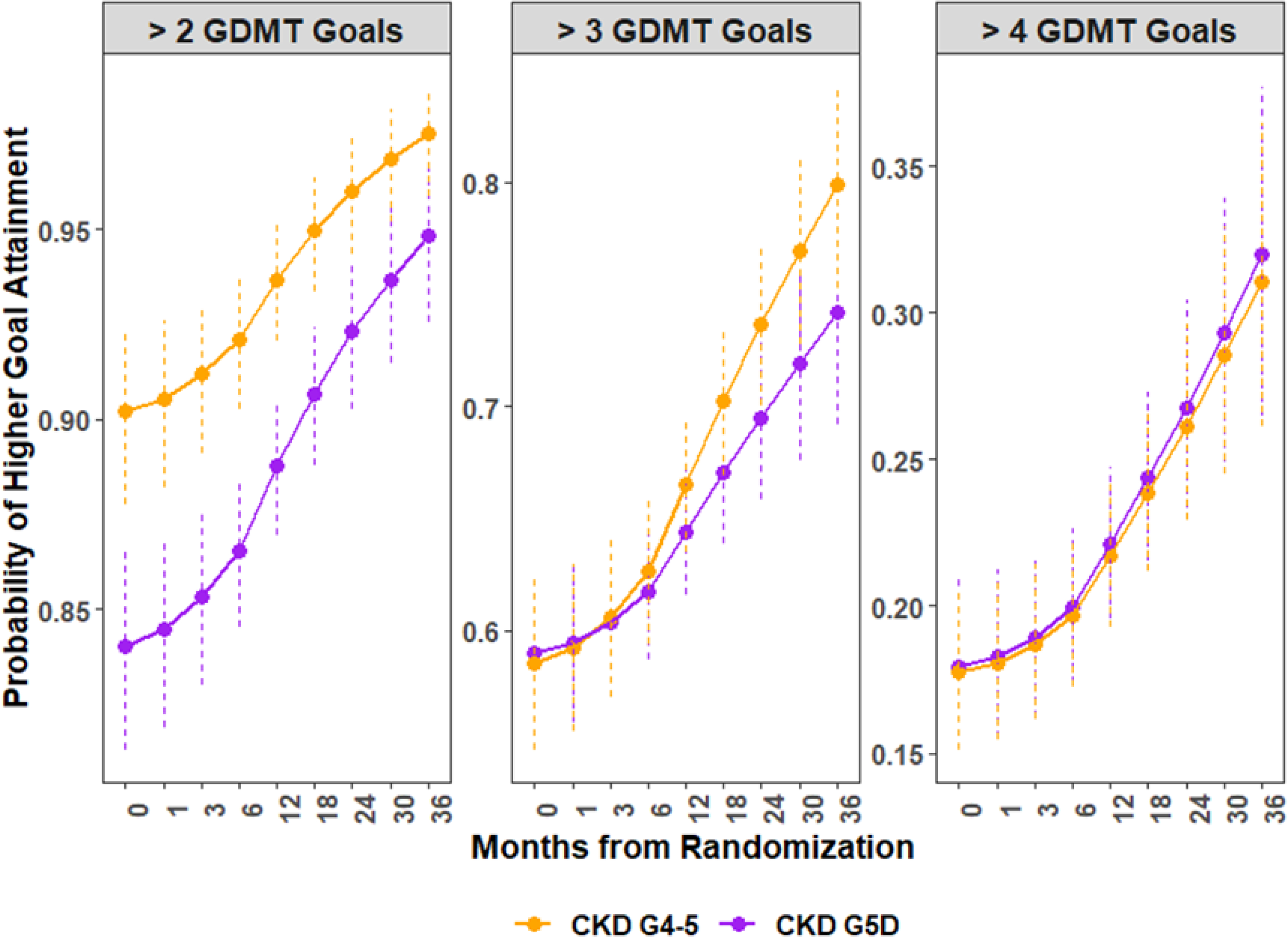
Predicted Probability of higher guideline-directed medical therapy (GDMT) goal attainment by chronic kidney disease (CKD) group. The panels represent the trajectories of attaining the represented total number of GDMT goals at target (>2, > 3, or >4) over study follow-up. The purple lines represent patients with CKD G5D, and the orange represent those with CKD G4–5. The 5 GDMT goals include: No smoking; aspirin use; Low Density Lipoprotein-Cholesterol < 70 mg/dl; Systolic Blood Pressure < 140 mmHg; and being on a statin
CKD G5D participants were less likely than CKD G4–5 participants to be prescribed statin and antiplatelet therapy while accounting for other covariates (Table 2, Figure 3a); treatment strategy assignment was not associated with GDMT goal attainment at 24 months (Figure S2). Sensitivity analyses examining missingness and survival over follow-up demonstrated similar results (Figure 3a, Figure S3a).
Figure 3: Differences in individual goal attainment between chronic kidney disease groups.
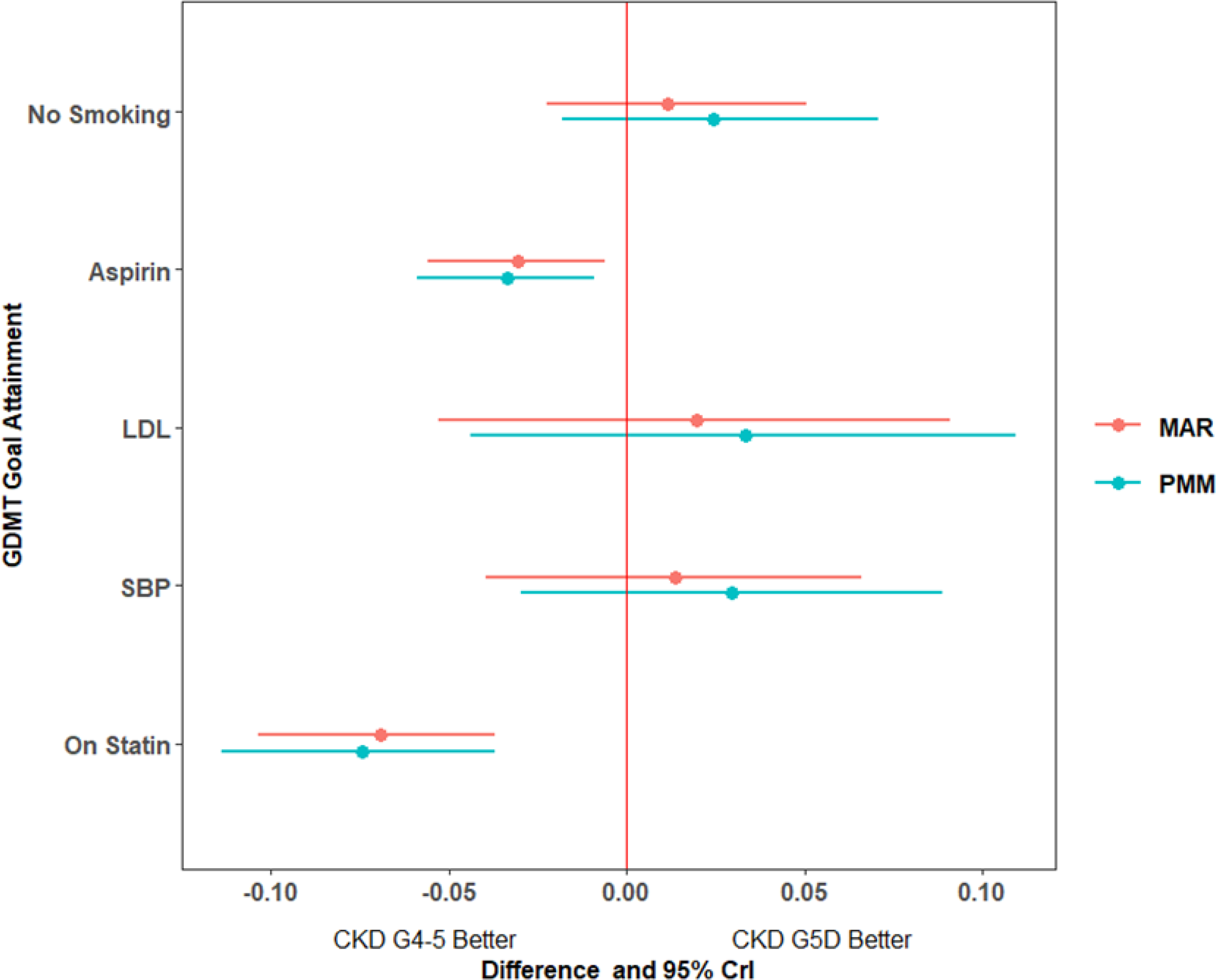
Figure 3a. Differences in probability of guideline-directed medical therapy (GDMT) goal attainment and 95% credible intervals at 24m for chronic kidney disease (CKD) G5D versus CKD G4–5. Estimates are displayed under two separate assumptions for missing values: missing at random (MAR) and missing not at random according to a pattern mixture model (PMM) approach. For example, compared to CKD G4–5, CKD G5D were approximately 7 percentage points less likely to receive statin therapy by 24 months. 95% CrI: 95% credible interval; LDL: low density lipoprotein; SBP: systolic blood pressure.
Both CKD G5D and CKD G4–5 demonstrated similar trends of increasing proportion at control from baseline to 24 months, though CKD G5D participants were less often prescribed statins over the course of follow-up (Figure 3b). In sensitivity analysis, there was no statistical difference between CKD cohorts over time in statin therapy among those who died, whereas there was statistical evidence of separation for statin therapy between CKD cohorts (lower prescription in CKD G5D versus CKD G4–5) among those who survived. (Figure S3b)
Figure 3b.
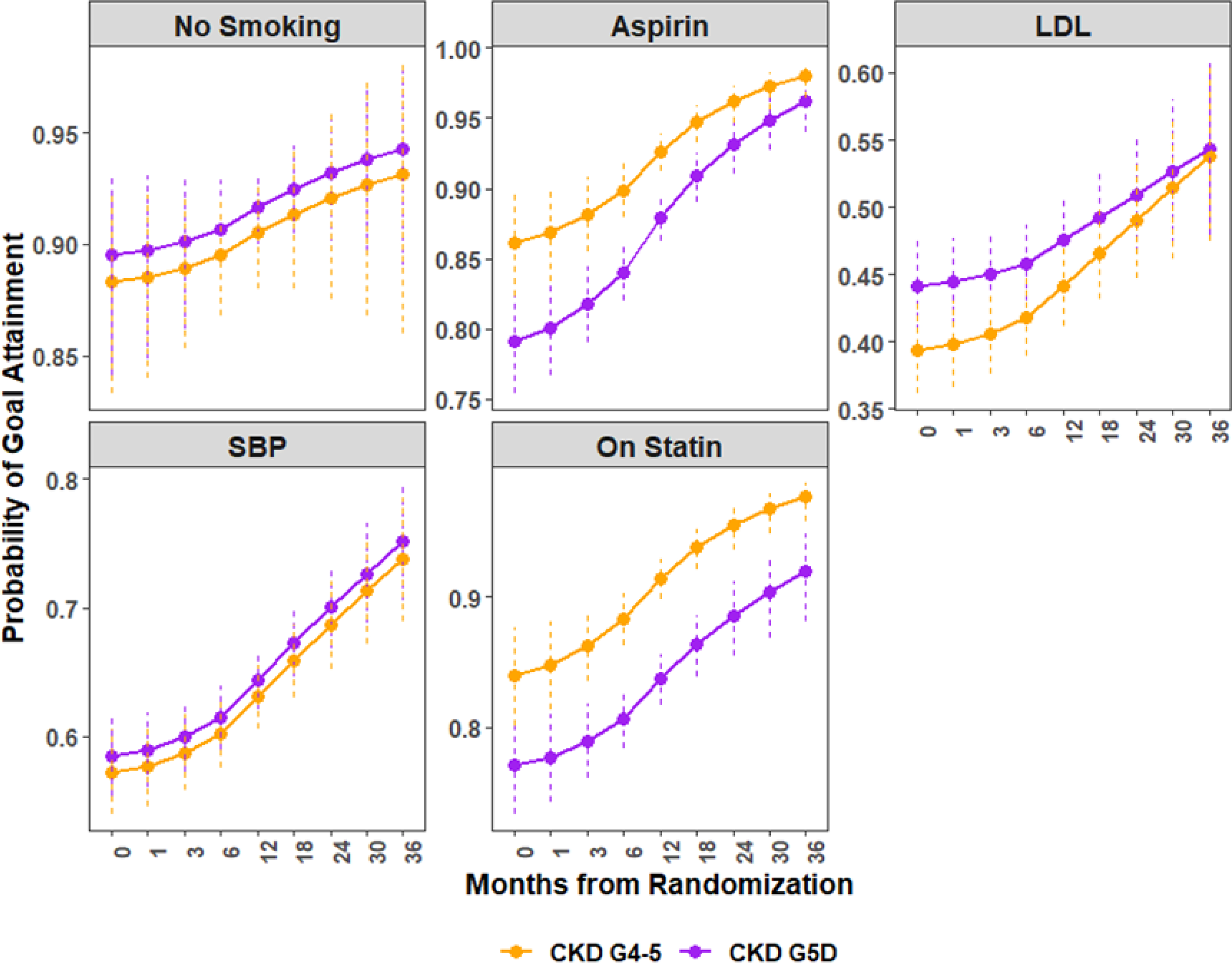
Predicted probability of individual guideline-directed medical therapy (GDMT) goal attainment over follow up by chronic kidney disease (CKD) group. The purple lines represent patients with CKD G5D, and the orange represent those with CKD G4–5. LDL: low density lipoprotein; SBP: systolic blood pressure.
Relationship of GDMT goal attainment and CKD group with the primary outcome of all-cause death or non-fatal myocardial infarction
In multivariable analysis (Table 3) a 1-goal increase in the number of GDMT goals attained at each visit and the running average of number of GDMT goals demonstrated protective associations with the primary outcome but these associations were marked by greater uncertainty as indicated by the wide 95% confidence interval (CI). Cumulative exposure to GDMT was associated with the primary outcome: A 1-goal increase over a 60-day period was associated with a 12% reduction in the hazard of the primary outcome at any time point (hazard ratio [HR] = 0.88 95% CI: 0.87, 0.90), after controlling for dialysis status, baseline number of GDMT goals, sex, age, presence of diabetes, ejection fraction, and randomized strategy arm. The point estimates for the hazard ratios and 95% CI were similar to the overall analysis for participants with CKD G5D and CKD G4–5 (p value for interaction between CKD group and primary outcome based on cumulative GDMT exposure: 0.49) (Tables S1 and S2, Figure S4).
Table 3:
Hazard ratios and 95% confidence intervals for assessing the relationship of all-cause death/MI and number of guideline-directed medical therapy (GDMT) goals attained over follow-up in all participants, Cox regression models with GDMT goals as a time-dependent covariate.
| Number of GDMT Goals | Running average of GDMT goals | Cumulative exposure to GDMT* | |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Primary Outcome (all-cause mortality or non-fatal myocardial infarction) | 0.93 (0.8, 1.08) | 0.84 (0.63, 1.11) | 0.88 (0.87, 0.9) |
| Dialysis (chronic kidney disease stage 5D) | 1.55 (1.19, 2.02) | 1.55 (1.19, 2.03) | 1.56 (1.18, 2.07) |
| Baseline number of GDMT goals | 1.14 (0.99, 1.31) | 1.22 (0.99, 1.51) | 1.81 (1.55, 2.1) |
| Female | 0.86 (0.65, 1.14) | 0.86 (0.65, 1.14) | 0.83 (0.62, 1.11) |
| Age† | 1.47 (1.22, 1.78) | 1.48 (1.22, 1.79) | 1.77 (1.46, 2.16) |
| INV | 1.02 (0.79, 1.3) | 1.02 (0.8, 1.31) | 1.05 (0.81, 1.36) |
| Diabetes | 1.89 (1.44, 2.49) | 1.9 (1.45, 2.5) | 2.1 (1.56, 2.84) |
| Ejection fraction† | 0.7 (0.59, 0.83) | 0.7 (0.59, 0.83) | 0.75 (0.63, 0.89) |
CI: confidence interval; GDMT: guideline directed medical therapy; HR: hazard ratio; INV: Initial invasive strategy.
Per 1 goal increase over 60 days. The 5 GDMT goals include: No smoking; aspirin use; Low Density Lipoprotein-Cholesterol < 70 mg/dl; Systolic Blood Pressure < 140 mmHg; being on a statin.
Per interquartile increase in variable.
The proportional hazards assumption was not violated for the number of GDMT goals attained or the running average of GDMT goals. The assumption, however, was rejected for the cumulative exposure to GDMT (P-value < 0.001). In non-proportional hazard Cox modeling with an interaction term between cumulative exposure to GDMT and an indicator for before versus after 1 year of follow-up, the protective association of cumulative exposure to GDMT was more pronounced before compared to after 1 year.
Discussion
ISCHEMIA-CKD is the largest trial to prospectively implement a multiple risk factor intervention strategy in participants with CKD G4–5D and CCD. With the implementation of multiple risk factor intervention focusing on GDMT goals, both CKD cohorts had improvements in the total number of GDMT targets achieved and individual goal attainment over the course of follow-up. However, CKD G5D were less likely to receive statin and aspirin therapy than CKD G4–5, regardless of assigned strategy. Irrespective of dialysis status, achieving a greater cumulative exposure (greater number over time) to GDMT goals was associated with a lower risk of the primary composite outcome of all-cause mortality or non-fatal myocardial infarction.
The current analysis sought to examine the value of improving multiple risk factors simultaneously. In the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial of participants with stable ischemic heart disease, most of whom had preserved renal function, a graded positive association with survival was noted with increasing number of risk factor goals attained.12 Even in that largely non-CKD population, it was difficult to achieve all risk factor goals (3% of the total population at 12 months following randomization). Similarly, in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial, controlling only 0–2 risk factors was associated with nearly doubling of the risk of death, MI or stroke as compared with controlling 6 risk factors.14
Data in patients with advanced CKD not yet on dialysis are limited, and are primarily observational analyses of non-randomized cohorts24,25, but generally support the notion of multiple risk factor control improving outcomes. Our data in a cohort of patients with advanced CKD and CCD with moderate or severe myocardial ischemia support better outcomes with greater goal attainment. Even fewer data are available for a multifactorial risk factor intervention in patients with CKD G5D. A single-center randomized controlled trial in patients with CKD G4–5D found no significant reduction in carotid intima media thickness (−0.00 vs. −0.01 mm) with intensive risk factor control vs. standard care, respectively.26 Challenges with that analysis included the modest sample size and targeting of a surrogate outcome, as well as an inability to fully assess the effect in CKD G5D due to small subgroup sample size. In ISCHEMIA-CKD, a large multinational cohort of participants with advanced CKD and CCD, including those on dialysis, we observed a 12% relative risk reduction in the primary outcome with each GDMT goal achieved; with the assumption that risk factor control was maintained over the course of follow-up.
Several limitations of this analysis should be noted. As this was a strategy trial testing the effect of invasive management added to GDMT, the study was not designed to assess the efficacy of any single risk factor intervention on outcomes. The current subgroup analysis was non-randomized, and our findings are subject to residual confounding. Individual risk factor goal attainment, specifically for statin use in CKD G5D, may have been influenced by prevailing medical society recommendation against initiating statins in patients with CKD G5D not already on them (Kidney Disease: Improving Global Outcomes Cholesterol guidelines27). The setting of a randomized controlled trial may limit generalizability to the real-world setting. However, the follow-up regimen and monitoring was similar to current guideline-based recommendations for patients with advanced CKD and CKD G5D.28–29 Finally, long term follow-up (ISCHEMIA-EXTEND) will provide additional insights into whether these findings are sustained during extended follow up.
Despite these limitations, this analysis demonstrates the longitudinal feasibility and tolerability of a multiple risk factor reduction strategy in participants with advanced/end-stage CKD, as recommended by the Kidney Disease: Improving Global Outcomes initiative 2020 Clinical Practice Guideline to reduce cardiovascular disease burden in diabetic CKD patients.30,31
In conclusion, this is the first large-scale analysis of a multiple risk factor secondary prevention strategy in patients with CKD G5D or CKD 4–5 and CCD. A majority of participants in both CKD cohorts achieved 3 or more out of 5 total GDMT targets over a median follow-up of 2.2 years. There was an associated improvement in the primary outcome in both cohorts with every GDMT goal achieved over time.
Supplementary Material
What is known:
Advanced chronic kidney disease is associated with heightened cardiovascular risk, and implementation of dialysis for renal replacement further increases this risk.
Single cardiovascular risk reduction has primarily been successful in the non-dialytic advanced chronic kidney disease stage, with the exception of a possible benefit for statin (+/− ezetimibe) use in patients on dialysis.
The incremental outcomes benefit with successive number of cardiovascular risk factors controlled has been demonstrated from secondary analyses of randomized controlled trials in patients without advanced chronic kidney disease.
What this study adds:
Controlling multiple cardiovascular risk factors is achievable in patients with advanced chronic kidney disease on or not on dialysis.
Greater number of cardiovascular risk factors controlled over time is associated with reduced incidence of death or non-fatal myocardial infarction in those with advanced chronic kidney disease irrespective of dialysis status.
Acknowledgments:
Sources of Funding
The trial was funded by the National Heart, Lung, and Blood institute of the National Institutes of Health. NIH Grants U01HL117904 and U01HL117905
Other Support:
This project was supported by grants from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. Devices or medications were provided by Abbott Vascular (previously St. Jude Medical, Inc); Medtronic, Inc.; Phillips (previously Volcano Corporation); and Omron Healthcare, Inc.; medications provided by Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Espero Pharmaceuticals; Merck, Sharp & Dohme Corp. and Sunovion Pharmaceuticals
Disclosures:
RO Mathew reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
DJ Maron reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
R Anthopolos reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
JL Fleg reports employment by the National Heart, Lung, and Blood Institute during the conduct of the study;
SM O’Brien reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
FW Rockhold reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; grants and personal fees from Janssen, personal fees from Merck HeathCare KGaA, personal fees from Merck Research Labs, personal fees from Novo Nordisk, personal fees from KLSMC, personal fees from Aldeyra, personal fees from Rhythm, personal fees from Phathom, grants and personal fees from AstraZeneca, personal fees from Complexa, grants and personal fees from Eidos, other from Athira, other from Spencer Healthcare, outside the submitted work;
C Briguori reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
MF Roik reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
T Mazurek reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
M Demkow reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; personal fees from ABBOTT, personal fees from MEDTRONIC, personal fees from BOSTON SCIENTIFIC, outside the submitted work;
Z Ye reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
U Kaul reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
GW Stone reports grants and personal fees from the National Heart, Lung, and Blood Institute during the conduct of the study; personal fees from Terumo, Amaranth, and Shockwave; personal fees and other from Valfix; personal fees from TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor, Neovasc, V-wave, Abiomed, Claret, and Sirtex; personal fees and other from Ancora and Qool Therapeutics; other from Cagent, Applied Therapeutics, Biostar family of funds, and MedFocus family of funds; personal fees and other from SpectraWave; personal fees from MAIA Pharmaceuticals; personal fees and other from Orchestra Biomed; other from Aria; personal fees from Vectorious; and other from Cardiac Success, outside the submitted work;
R Wald reports grants from National Heart, Lung, and Blood Institute during the conduct of the study;
DM Charytan reports personal fees from Jannssen, personal fees from PLC Medical, grants from bioporto, personal fees from Merck, grants and personal fees from NovoNordisk, personal fees and other from Jannssen, grants and personal fees from Gilead, personal fees from AstraZeneca, grants and personal fees from Medtronic, grants and personal fees from Amgen, personal fees from GSK, personal fees from Fresenius, other from Daichi-Sankyo, personal fees from Douglas and London, personal fees from Zoll, outside the submitted work;
MS Sidhu reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; personal fees from Astra Zeneca, personal fees from Sanofi-Regeneron, outside the submitted work;
JS Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular; Medtronic, Inc.; Abbott Laboratories (formerly St. Jude Medical, Inc); Royal Philips NV (formerly Volcano Corporation); Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc, Sunovion Pharmaceuticals, Inc. Espero BioPharma; and Amgen, Inc; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP;
S Bangalore reports Research Grant NHLBI and Abbott Vascular Advisory board- Abbott Vascular, Pfizer, Amgen, Biotronik, Meril and Reata.
R Wald reports the receipt of unrestricted research funding and speaker fees from Baxter.
Non-Standard Abbreviations
- CCD
chronic coronary disease
- CI
confidence interval
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- GDMT
guideline-directed medical therapy
- HR
hazard ratio
- ISCHEMIA-CKD
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches—Chronic Kidney Disease
- LDL-C
low-density lipoprotein cholesterol
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- SBP
systolic blood pressure
Footnotes
Disclaimer:
The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the official views of the Veterans Health Administration, National Heart, Lung, and Blood Institute, National Institutes of Health or the United States Department of Health and Human Services.
Trial Registration: NCT01985360; ISCHEMIA-ChronicKidneyDiseaseTrial-FullTextView-ClinicalTrials.gov
References
- 1.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrington WG, Emberson J, Mihaylova B, Blackwell L, Reith C, Solbu MD, Mark PB, Fellström B, Jardine AG, Wanner C, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4:829–839. [DOI] [PubMed] [Google Scholar]
- 3.Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV., Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A randomized trial of intensive versus standard blood-pressure control. New England Journal of Medicine. 2015; 373: 2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhotra R, Nguyen HA, Benavente O, Mete M, Howard BV., Mant J, Odden MC, Peralta CA, Cheung AK, Nadkarni GN, et al. Association between more intensive vs less intensive blood pressure lowering and risk of mortality in chronic kidney disease stages 3 to 5: A systematic review and meta-analysis. JAMA Internal Medicine. 2017;177:1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Der Mesropian PJ, Shaikh G, Cordero Torres E, Bilal A, Mathew RO. Antihypertensive therapy in nondiabetic chronic kidney disease: a review and update. Journal of the American Society of Hypertension. 2018;12:154–181. [DOI] [PubMed] [Google Scholar]
- 6.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, Cushman WC, Hawfield AT, Johnson KC, Lewis CE, et al. Effects of intensive BP control in CKD. Journal of the American Society of Nephrology. 2017;28:2812–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 8.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. [DOI] [PubMed] [Google Scholar]
- 9.Cushman WC, Evans GW, Byington RP, Goff DCJ, Grimm RHJ, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. The New England journal of medicine. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek DA, Douglas-Baltimore JG, Gassman J, Glassock R, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. Journal of the American Medical Association. 2002;288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 11.Group SR, Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maron DJ, Mancini GBJ, Hartigan PM, Spertus JA, Sedlis SP, Kostuk WJ, Berman DS, Teo KK, Weintraub WS, Boden WE. Healthy Behavior, Risk Factor Control, and Survival in the COURAGE Trial. Journal of the American College of Cardiology. 2018;72:2297–2305. [DOI] [PubMed] [Google Scholar]
- 13.Gæde P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. New England Journal of Medicine. 2008;358:580–591. [DOI] [PubMed] [Google Scholar]
- 14.Bittner V, Bertolet M, Barraza Felix R, Farkouh ME, Goldberg S, Ramanathan KB, Redmon JB, Sperling L, Rutter MK. Comprehensive cardiovascular risk factor control improves survival: The BARI 2D trial. Journal of the American College of Cardiology. 2015;66:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bangalore S, Maron DJ, O’Brien SM, Fleg JL, Kretov EI, Briguori C, Kaul U, Reynolds HR, Mazurek T, Sidhu MS, et al. Management of coronary disease in patients with advanced kidney disease. New England Journal of Medicine. 2020;382:1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangalore S, Maron DJ, Fleg JL, O’Brien SM, Herzog CA, Stone GW, Mark DB, Spertus JA, Alexander KP, Sidhu MS, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches–Chronic Kidney Disease (ISCHEMIA-CKD): Rationale and design. American Heart Journal. 2018;205:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman JD, Alexander KP, Gu X, O’Brien SM, Boden WE, Govindan SC, Senior R, Moorthy N, Rezende PC, Demkow M, et al. Baseline predictors of low-density lipoprotein cholesterol and systolic blood pressure goal attainment after 1 year in the ISCHEMIA trial. Circulation: Cardiovascular Quality and Outcomes. 2019;12: e006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrick K, Sallis JF, Long B, Calfas KJ, Wooten W, Heath G, Pratt M. A new tool for encouraging activity: Project PACE. Physician and Sportsmedicine. 1994; 22:45–55. [DOI] [PubMed] [Google Scholar]
- 19.Chang TI, Streja E, Ko GJ, Naderi N, Rhee CM, Kovesdy CP, Kashyap ML, Vaziri ND, Kalantar-Zadeh K, Moradi H. Inverse association between Serum non-high-density lipoprotein cholesterol levels and mortality in patients undergoing incident hemodialysis. Journal of the American Heart Association. 2018. Jun 9; 7:e009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrington W, Staplin N, Judge PK, Mafham M, Emberson J, Haynes R, Wheeler DC, Walker R, Tomson C, Agodoa L, et al. Evidence for Reverse Causality in the Association between Blood Pressure and Cardiovascular Risk in Patients with Chronic Kidney Disease. Hypertension. 2017;69:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Annals of Translational Medicine. 2016;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin Donald B. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons Ltd, New York, NY USA; 1987. [Google Scholar]
- 23.Plummer M. JAGS : A Program for Analysis of Bayesian Graphical Models. Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003); 125.10.2003. [Google Scholar]
- 24.Hamada S, Gulliford MC. Multiple risk factor control, mortality and cardiovascular events in type 2 diabetes and chronic kidney disease: a population-based cohort study. BMJ open. 2018;8:e019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrauben SJ, Hsu JY, Amaral S, Anderson AH, Feldman HI, Dember LM. Effect of Kidney Function on Relationships between Lifestyle Behaviors and Mortality or Cardiovascular Outcomes: A Pooled Cohort Analysis. Journal of the American Society of Nephrology. 2021;32:ASN.2020040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isbel NM, Haluska B, Johnson DW, Beller E, Hawley C, Marwick TH. Increased targeting of cardiovascular risk factors in patients with chronic kidney disease does not improve atheroma burden or cardiovascular function. American Heart Journal. 2006;151:745–753. [DOI] [PubMed] [Google Scholar]
- 27.Tonelli M, Wanner C. Lipid management in chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2013 clinical practice guideline. Ann Intern Med. 2014;160:182. [DOI] [PubMed] [Google Scholar]
- 28.Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, Griffith KE, Hemmelgarn BR, Iseki K, Lamb EJ, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Workgroup. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements. 2013; 3:1–50 [Google Scholar]
- 29.National Kidney Foundation. KDOQI Clinica Practice Guideline For Hemodialysis Adequacy: 2015 Update. Am J Kidney Dis. 2015; 66: 884–930. [DOI] [PubMed] [Google Scholar]
- 30.de Boer IH, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, Liew A, Michos ED, Navaneethan SD, Olowu WA, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney International. 2020;98:839–848. [DOI] [PubMed] [Google Scholar]
- 31.Navaneethan SD, Zoungas S, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Liew A, Michos ED, Olowu WA, Sadusky T, et al. Diabetes Management in Chronic Kidney Disease: Synopsis of the 2020 KDIGO Clinical Practice Guideline. Annals of Internal Medicine. 2020:M20–5938. [DOI] [PubMed] [Google Scholar]
- 32.R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org. [Google Scholar]
- 33.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis, Third Edition. Chapman and Hall/CRC. 1995. Jun 1. [Google Scholar]
- 34.Li Q, Su L. Accommodating informative dropout and death: a joint modelling approach for longitudinal and semicompeting risks data. Journal of the Royal Statistical Society. Series C: Applied Statistics. 2018;67:145–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little RJA. Pattern-Mixture Models for Multivariate Incomplete Data. Journal of the American Statistical Association. 1993;88:125–134. [Google Scholar]
- 36.Pauler DK, McCoy S, Moinpour C. Pattern mixture models for longitudinal quality of life studies in advanced stage disease. Statistics in Medicine. 2003; 22:795–809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


