Abstract
Lung tissue-resident memory T cells are crucial mediators of cellular immunity against respiratory viruses; however, their gradual decline hinders the development of T-cell based vaccines against respiratory pathogens. Recently, studies using adenovirus-based vaccine vectors have shown that the number of protective lung-resident CD8+ TRM can be maintained long-term. Here, we show that immunization of mice with a replication-deficient adenovirus expressing influenza nucleoprotein (AdNP) generates a long-lived lung TRM pool that is transcriptionally indistinct from those generated during a primary influenza infection. In addition, we demonstrate that CD4+ T cells contribute to the long-term maintenance of AdNP-induced CD8+ TRM. Using a lineage tracing approach, we identify alveolar macrophages as a cell source of persistent NP antigen following immunization with AdNP. Importantly, depletion of alveolar macrophages following AdNP immunization resulted in significantly reduced numbers of NP-specific CD8+ TRM in the lungs and airways. Combined, our results provide further insight to the mechanisms governing the enhanced longevity of antigen-specific CD8+ lung TRM observed following immunization with recombinant adenovirus.
Introduction:
CD8+ tissue-resident memory T cells (TRM) are a distinct subset of memory T cells that are established within barrier tissues such as the lung, skin, and reproductive tract, where they provide a critical line of local defense against pathogen challenge. Canonically defined as extravascular cells that express surface markers known to promote retention (such as CD69 and/or CD103), TRM share a core transcriptional signature that promotes their longevity and further distinguishes them from effector and central memory T cell subsets (TEM and TCM, respectively) (1–5). Within the lung and airways, CD8+ TRM confer protection against a variety of respiratory pathogens, including influenza virus and SARS-CoV-2 (2–4, 6, 7). Although they do not provide sterilizing immunity, lung resident TRM have been shown to significantly improve the immune response to heterologous influenza infection by rapidly reducing viral loads and limiting immunopathology (1, 8–11). However, while studies of TRM populations in the skin, intestinal tract, and reproductive tract indicate that CD8+ TRM remain relatively stable within these tissues and provide long-lasting protection, the number of virus-specific CD8+ TRM in the lung steadily declines over time to nearly undetectable levels (8, 12–16). The mechanisms behind this loss of TRM are not entirely understood, but it has been well established that the decline in lung TRM greatly diminishes the protective capacity of cellular immunity against influenza virus (17).
Given the demonstrated importance of CD8+ lung TRM in mediating protection against pulmonary challenge, identifying mechanisms governing their formation and longevity within the respiratory tract is of great interest. Despite many gaps in our current knowledge, several key factors such as exposure to TGF-β, IL-15, and recognition of cognate antigen within the lung tissue have been identified as important for the development and long-term survival of CD8+ lung TRM (2, 18–22). Several studies have also investigated the role of co-stimulatory molecules, such as 4–1BB/4–1BBL, in the formation and accumulation of TRM, as well as their inclusion in vaccine platforms designed to target influenza virus (23–28). Virus-based vectors, such as replication-deficient adenoviruses (Ad), are of particular interest as a vaccine platform candidate because they can be easily manipulated and have been shown to induce robust memory CD8+ T cell responses against viral and cancer antigens (29–34). Most recently, Ad vectors have been utilized in the formulation of vaccines against the SARS-CoV-2 pandemic virus (35–38). One key feature of Ad vectors that contributes to their success in inducing long-lasting cellular immunity is the ability of the vector to persist in vivo (39–41). For example, a recent study demonstrated that Ad vectors can generate local antigen depots that support generation of local immunity (42). This finding complements prior work that showed that a combined systemic and local immunization strategy using an adeno-based vector that expresses the influenza A nucleoprotein (AdNP) results in formation of NP-specific CD8+ lung TRM that provide protection against heterologous influenza virus for up to 1-year post-immunization, and that influenza NP antigen persists long-term in the lungs of mice following immunization (31, 43). This starkly contrasts the dynamics of TRM following infection with influenza virus and could provide critical insight to the mechanisms of TRM generation and maintenance within the respiratory tract.
In this present study, we further investigate mechanisms that contribute to the longevity of CD8+ lung TRM and identify the cellular source of persistent antigen in AdNP immunized animals. Prior findings suggested that circulating CD8+ T cells are pulled into the lung TRM pool in AdNP immunized mice, potentially providing an explanation for the enhanced maintenance of lung TRM and duration of protection (43). Here, we find that CD8+ lung TRM generated following infection with influenza or immunization with AdNP are transcriptionally similar, indicating that cell-extrinsic factors are promoting TRM longevity. In addition, we find that help from CD4+ T cells is important for maintaining the TRM pool in the lungs and airways of mice immunized with AdNP. Using a combination of lineage tracing experiments and immunofluorescence microscopy, we identify alveolar macrophages as the cellular source of NP antigen in the lungs following intranasal immunization and confirm that depletion of this cell subset reduces the number of CD8+ lung TRM over time. These results provide further insight into the mechanisms driving enhancement of TRM in the respiratory tract following immunization with replication-deficient adenovirus vectors and will inform future design of vector-based vaccines against respiratory pathogens, including influenza virus.
Materials & Methods:
Mice
C57BL/6J (WT) and B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Ai14) mice were bred in-house or purchased from Jackson Laboratory and were housed at Emory University under specific pathogen-free conditions. Mice were between 8–12 weeks of age at time of infection, after which they were housed in specific animal biosafety level 2 conditions. Both male and female mice were used for experiments. All experiments were conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines of Emory University.
Viral infections
Replication-deficient adenovirus serotype 5 expressing influenza (A/Puerto Rico/8/34) nucleoprotein (AdNP) was produced and titered as previously described (31, 44). Replication-deficient adenovirus serotype 5 expressing Cre recombinase (Ad-Cre) was obtained from SignaGen Laboratories. Prior to all infections, mice were anesthetized using isoflurane (Patterson Veterinary). For primary influenza infection, mice were inoculated intranasally (i.n.) with 30,000 EID50 influenza A/HKx31 (x31) in 30uL volume. For adenovirus immunizations, mice were inoculated with 2×107 plaque-forming units (PFU) of adenovirus via both i.n. and subcutaneous (s.c.) routes each in 30uL volume. For secondary infection experiments, mice received either 500 EID50 Sendai parainfluenza virus or 30,000 PFU x31 NP N370Q (x31 NP−) i.n. in 30uL volume. Control groups for challenge experiments received 30uL i.n. of 1X phosphate buffered saline solution (PBS).
Single cell isolation
To distinguish tissue-resident cells from those in circulation, mice were intravenously (i.v.) labeled via tail vein injection of fluorescent anti-CD3e (1.5 ug) or anti-CD45 antibody (4 ug) in 200uL 1X PBS and rested for 5 minutes. Mice were subsequently euthanized by intraperitoneal (i.p.) injection with Avertin (2,2,2-tribromoethanol) followed by brachial exsanguination. Spleen, lungs, and bronchoalveolar lavage (BAL) were then harvested. Lungs were enzymatically digested in Collagenase D (5g/L, Roche) and DNase (2×106 U/L, Sigma) for 30 minutes at 37C, with occasional mechanical dissociation. To enrich for lymphocytes, lung samples were centrifuged in a 40%/80% Percoll gradient. For Ad-Cre experiments, lungs were digested using Collagenase D (5g/L), DNase (2×106 U/L), and Dispase (15U/mL, Sigma) and then passed through a 70um filter without centrifugation over a Percoll gradient. Spleens were mechanically dissociated and then RBC lysed. For cell sorts, CD8+ CD62L− splenocytes were enriched for using a Miltenyi CD8a+ T cell isolation kit and biotinylated anti-CD62L antibody just prior to staining.
Cell staining and flow cytometry
Single cell suspensions were first FC blocked using murine 2.4G2 antibody. Samples were then stained with influenza-specific tetramer against NP366–374Db (provided by the National Institutes of Health (NIH) Tetramer Core Facility at Emory University) for 1 hour at room temperature, followed by extracellular staining for 30 minutes. Cell viability was determined using either Zombie fixable viability dye (BioLegend) or 7-AAD. All samples were run on either a Fortessa X20 or a Symphony A3 (BD Biosciences) flow cytometer. Flow cytometry data were analyzed using FlowJo v.10 software.
RNA-sequencing
For each population, 100–2000 cells were sorted on a FACSAria II (BD Biosciences) directly into RLT buffer (Qiagen) containing 1% 2-Mercaptoethanol and total RNA isolated using the Quick-RNA Microprep kit (Zymo Research). All resulting RNA was used as input for the SMART-seq v4 cDNA synthesis kit (Takara) with 12 cycles of PCR amplification. cDNA was quantitated and 200 pg of material was used with the NexteraXT kit and NexteraXT Indexing primers (Illumina, Inc) in 12 cycles of PCR to generate libraries. Samples were quality checked on a bioanalyzer, quantitated by Qubit fluorometer, pooled at equimolar ratios, and sequenced on a NextSeq500 using 75 bp paired-end chemistry at the University of Alabama, Birmingham Helfin Genomics Core. Raw sequencing reads were mapped to the mm10 version of the mouse genome using STAR v2.5.3a (45) and duplicate reads flagged using PICARD (http://broadinstitute.github.io/picard/) filtered based on the uniquely mappable and non-redundant reads. Reads mapping to exons for all unique ENTREZ genes was summarized using GenomicRanges v1.34.0 (46) package in R v3.5.2 and data normalized using custom R/Bioconductor scripts. Differentially enriched genes (DEG) were determined using edgeR v3.24.3 (47) and genes that displayed an absolute log2 fold change (log2FC) > l and a Benjamini-Hochberg false-discovery rate (FDR) corrected p-value < 0.05 were considered DEG. Principal component analysis was performed using the vegan package v2.5.6 using the indicated set of DEG. The sequencing dataset can be accessed in the GEO repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE198980) under accession no. GSE198980.
CD4+ T cell depletion
To deplete CD4+ T cells, mice were first injected i.p. with 200ug of anti-CD4 monoclonal antibody (clone GK1.5, Bio X Cell) or isotype control in 1X PBS, and then injected with 100ug i.p. every 3–4 days afterwards for a total of 1 month of treatment.
Depletion of alveolar macrophages
High potency anionic liposomal clodronate and empty liposomes were obtained from FormuMax Scientific. Mice were anesthetized via injection with Avertin and then given 2mg in 100uL volume intra-tracheally (i.t.).
Immunofluorescence microscopy
BAL was collected and re-suspended in 1X PBS. 30,000–50,000 cells were then concentrated onto a glass slide using a Thermo Shandon Cytospin 4 cytocentrifuge. Slides were subsequently H&E stained using standard protocols or fixed using 75:25 acetone/ethanol. Fixed slides were blocked using FACS buffer containing 1ug/mL murine 2.4G2 antibody, 10% mouse serum, 10% rat serum, and 10% donkey serum. Staining was done in blocking buffer using anti-mouse CD11c-A594 (clone N418, BioLegend), anti-mouse influenza A nucleoprotein-FITC (clone 431, Abcam), rabbit anti-fluorescein-A488 (Life Technologies), and DAPI. Coverslips were applied using ProLong Gold antifade reagent and samples were imaged the following day using a Zeiss Axio Observer Z1 immunofluorescence microscope with an Axiocam 506 monochromatic camera. Image processing was performed with Zen 2 software.
Statistical analysis
Cell counts were determined either manually using a hemocytometer or with a LUNA-II automatic cell counter (Logos Biosystems). Statistical analyses were performed using the GraphPad Prism Software.
Results:
CD8+ lung TRM from influenza infected and AdNP immunized mice are transcriptionally indistinct
To determine whether persistent antigen in AdNP immunized mice has any potential cell-intrinsic effects on the genetic program of lung TRM that result in their enhanced longevity, we performed RNA-sequencing to compare the transcriptional profiles of influenza NP-specific lung TRM (CD8+ i.v. antibody− NP+ CD69+ CD103+) and splenic TEM (CD8+ CD62L− NP+) from mice either infected with x31 influenza or immunized with AdNP at 1-month (35 days post-infection (d.p.i.), x31 and AdNP) and 1-year (365 d.p.i., AdNP only) time points (Fig. 1A, B). Principal component analysis (PCA) revealed that TEM and lung TRM cluster separately, as expected, at both 1-month (Fig. 1C) and 1-year (Fig. 1D) post-infection regardless of whether mice were given influenza or AdNP. Interestingly, we identified very few genes that were differentially expressed (DEGs) between lung TRM from AdNP immunized and x31 infected mice at 1-month, suggesting that there is no significant transcriptional difference between lung TRM formed following influenza infection or AdNP immunization (Fig. 1E). In contrast, we identified several DEGs between lung TRM on days 35 and 365 post-immunization with AdNP (Fig. 1F). Notably, lung TRM from AdNP immunized mice had similar expression of genes from a known core TRM transcriptional program, including Itgae, Cdh1, Klf2, and S1pr1, confirming that these cells are bona fide TRM at both timepoints post-immunization (4, 48) (Fig. 1G). However, the DEGs observed at 365 days post-immunization were enriched for TGF-β signaling (including Slc20a1, Smad3, and Cdh1) (Fig. 1G). Nevertheless, overall, we did not identify any transcriptional differences that would suggest the persistence of CD8+ lung TRM in AdNP-immunized mice is due to a distinct genetic program that confers increased durability.
Figure 1. Immunization with AdNP generates CD8+ TRM that are transcriptionally alike those generated during a primary infection with influenza.
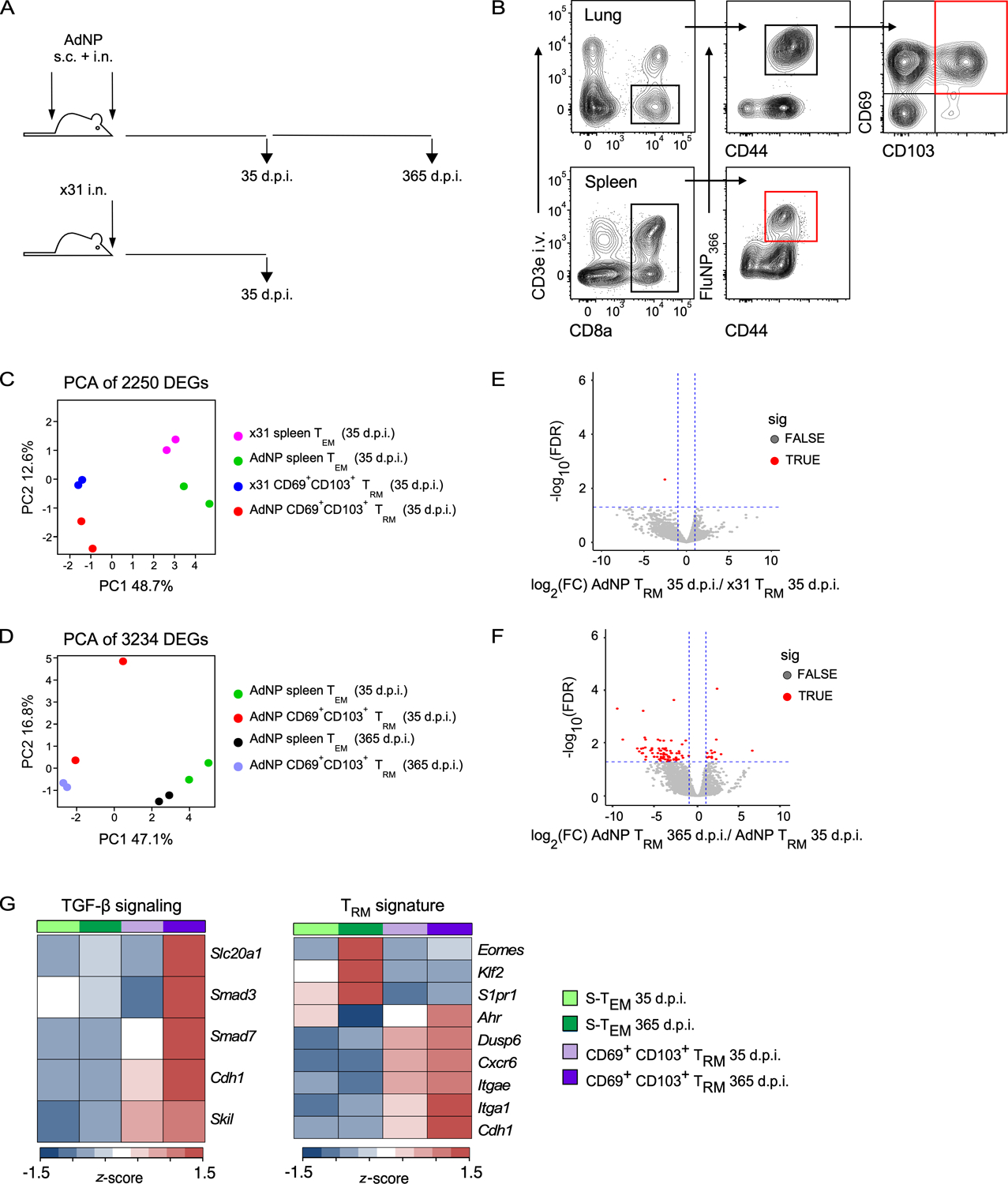
(A) Experimental design. (B) Example gating strategy to sort for influenza NP (FluNP366–374)-specific splenic TEM and CD69+ CD103+ lung TRM from mice either infected with x31 influenza or immunized with AdNP. Final sorted populations are highlighted in red. For x31, n = 10–20 mice per sort, 2 independent sorts. For AdNP, n = 10 mice per sort, 2 independent sorts per timepoint. (C) Principal component analysis (PCA) plot of 2250 differentially expressed genes (DEGs) identified in influenza-infected and AdNP-immunized mice on day 35 post-infection. (D) PCA of 3234 genes identified on day 35 and 365 post-immunization with AdNP. (E) Volcano plot illustrating DEGs identified when comparing CD69+ CD103+ lung TRM from AdNP-immunized mice to those from x31 influenza-infected mice on day 35 post-infection. (F) Volcano plot illustrating DEGs between CD69+ CD103+ lung TRM from AdNP-immunized mice on days 35 and 365 post-immunization. (G) Heatmaps of selected genes from FluNP-specific splenic TEM and CD69+ CD103+ lung TRM from AdNP-immunized mice related to TGF-ß signaling and a core TRM signature.
CD4+ T cells are important for the maintenance of CD8+ lung TRM following immunization with AdNP
CD4+ T cells are important for proper maintenance and recall of influenza-specific CD8+ memory T cells in the lungs and airways (49, 50). Furthermore, IFN-γ produced by CD4+ T cells is critical for formation of protective CD103+ CD8+ TRM in the lung following infection with influenza virus (51). To investigate whether CD4+ T cell-dependent signals are required for long-term maintenance of CD8+ TRM in AdNP immunized mice, we treated mice with anti-CD4 depleting antibody starting 30 days post-immunization (Fig. 2A). After administering depleting antibody for a total of 1 month, we confirmed depletion of CD4+ T cells in all tissues (data not shown) and evaluated the number of influenza NP-specific CD8+ TRM (Fig. 2B). As expected, there was no change in the number of CD8+ splenic TEM upon depletion of CD4+ T cells. However, within the lung and airways, depletion of CD4+ T cells resulted in a significant reduction in the number of NP-specific CD8+ TRM when compared to mice that received an isotype control antibody. Furthermore, the decrease in the overall number of CD8+ TRM in the lungs and airways correlated in both tissues with a reduction in CD69+ CD103+ NP-specific CD8+ TRM (Fig. 2C, D). These results show that help from CD4+ T cells plays an important role in the long-term maintenance of CD8+ TRM in the lungs and airways of mice immunized with AdNP.
Figure 2. CD4+ T cells are important for the maintenance of CD8+ TRM following immunization with AdNP.
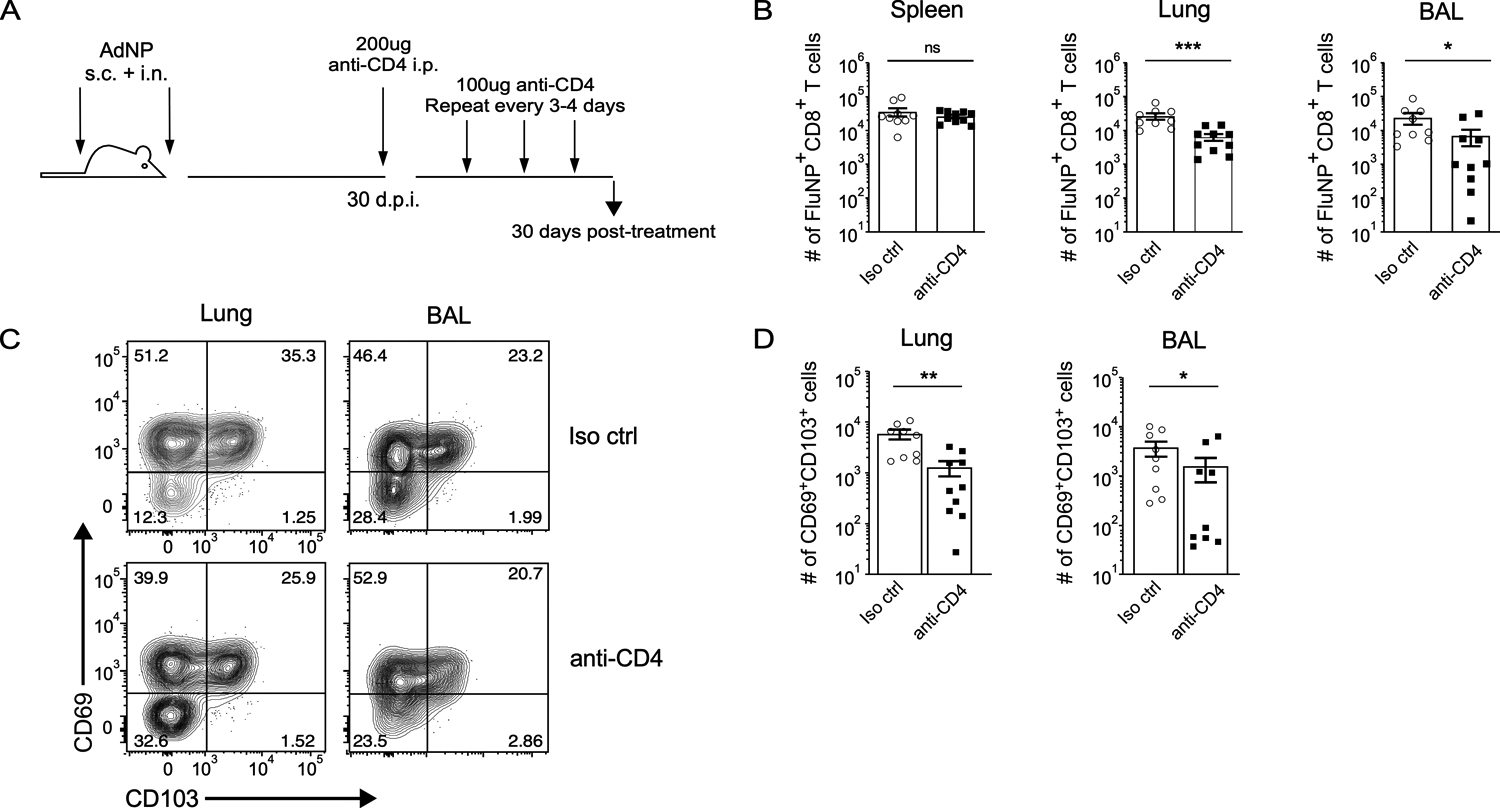
(A) Experimental design. (B) Number of FluNP-specific CD8+ TRM in the spleen, lung, and bronchoalveolar lavage (BAL) following depletion of CD4+ T cells. For isotype control, n = 9 mice total, 2 independent experiments. For anti-CD4, n = 10 mice total, 2 independent experiments. (C) Example staining for CD69 and CD103 subsets amongst FluNP-specific CD8+ TRM in the lung and BAL. (D) Number of CD69+ CD103+ FluNP-specific CD8+ TRM in the lung and BAL following depletion of CD4+ T cells. n = 4–5 mice per group, 2 independent experiments. Significance was determined using a Mann-Whitney test. Data represent mean ± SEM. P values are as follows: * = p<0.05, ** = p<0.01, *** = p<0.001, ns= not significant.
Alveolar macrophages harbor persistent influenza NP antigen in AdNP immunized mice
Although it has been established that influenza NP antigen is still present in mice immunized with AdNP for at least several months following immunization, the cellular source of this persistent antigen reservoir has not yet been identified (43). To investigate this, we used a replication-deficient adenovirus that expresses Cre recombinase protein (Ad-Cre) to immunize Ai14 (tdTomato) reporter mice, in which cells express the reporter protein tdTomato following Cre-mediated recombination. Within the lung, tdTomato fluorescence was predominantly observed in alveolar macrophages up to at least a year post-immunization with Ad-Cre (Fig. 3A, B). Minimal, if any, fluorescence was observed in dendritic cells (Fig. 3B). Even as early as day 4 post-immunization with Ad-Cre, tdTomato fluorescence was mostly limited to alveolar macrophages and was not detected in any other cell type, including fibroblasts, epithelial cells, and monocytes (Supplementary Fig. 1). In addition, the frequency of tdTomato+ alveolar macrophages was varied at all timepoints examined (Fig. 3C). To confirm this finding, we obtained a cytospin of BAL samples from naïve and AdNP-immunized mice (90 d.p.i.). Immunofluorescent staining revealed co-localization of CD11c and influenza nucleoprotein at memory following immunization with AdNP (Fig. 3D). Combined, these experiments identify alveolar macrophages as the cellular source of persistent influenza NP antigen following intranasal immunization with recombinant adenovirus.
Figure 3. Alveolar macrophages are the cell source of persistent influenza NP antigen in AdNP-immunized mice.
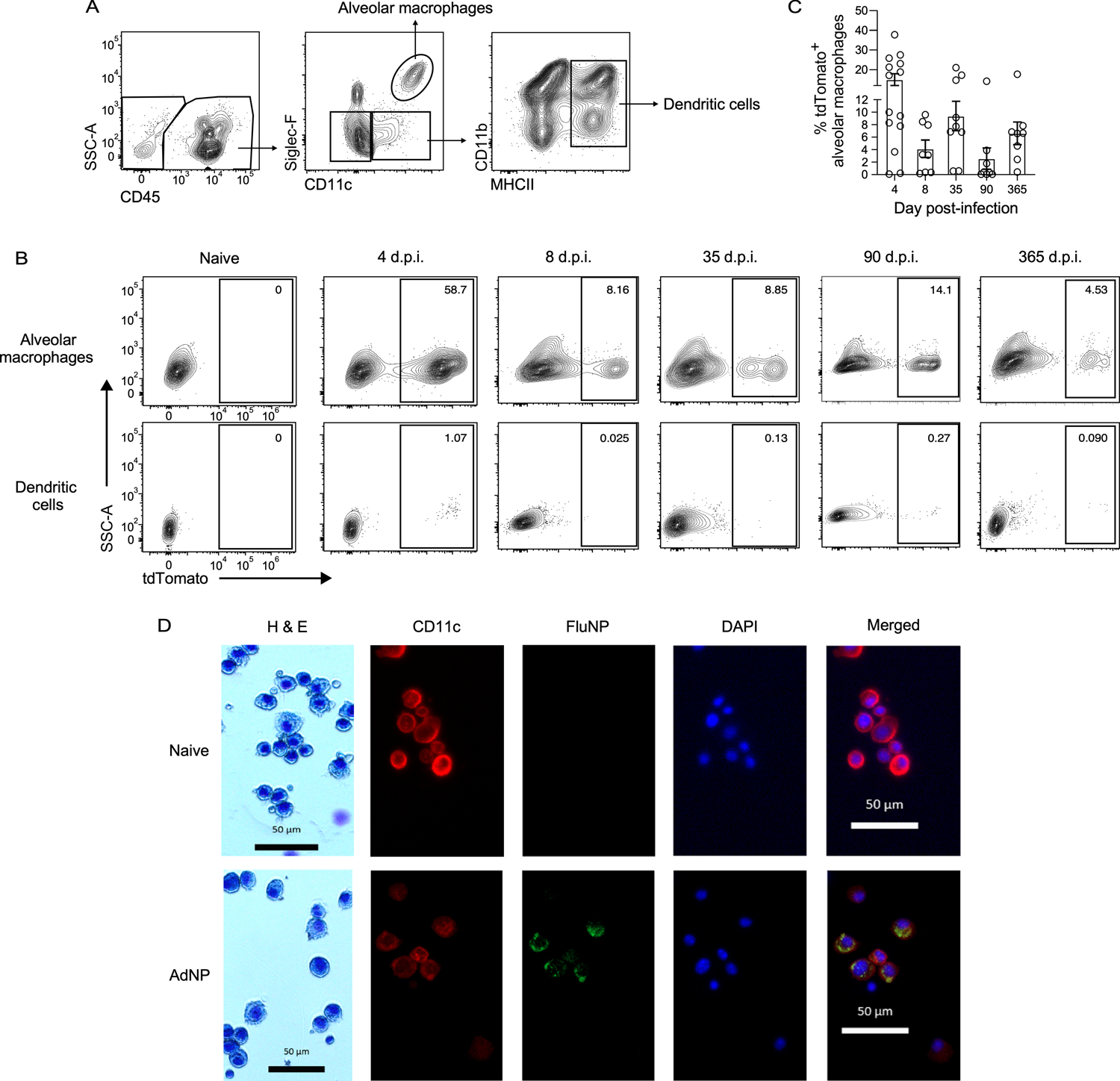
(A) Example gating strategy for alveolar macrophages and dendritic cells in the lung of Ai14 reporter mice immunized with Ad-Cre. (B) Expression of tdTomato by alveolar macrophages (top row) and dendritic cells (bottom row) from mice immunized with either PBS (naïve) or Ad-Cre at indicated timepoints. (C) Frequency of tdTomato+ alveolar macrophages at indicated timepoints. n = 3–5 mice per timepoint, 2 experiments per timepoint. (D) H&E staining and immunofluorescence microscopy of BAL samples from mice that were naïve (top row) or 90 days post-immunization with AdNP (bottom row) showing CD11c (red), influenza nucleoprotein (FluNP, green), and DAPI (blue). Original images were taken at 100X magnification.
Depletion of alveolar macrophages impairs the longevity of CD8+ TRM in the airways
Alveolar macrophages are among the first responders to influenza infection, and their depletion has been shown to result in increased morbidity and mortality during infection (52, 53).
Given our data shows that alveolar macrophages are a source of prolonged influenza NP antigen following intranasal immunization with AdNP, we hypothesized that depletion of this population would result in decreased maintenance of NP-specific CD8+ TRM over time. We therefore depleted alveolar macrophages by administering liposomal clodronate at 1-month post-immunization with AdNP (Fig. 4A). Treatment resulted in a significant reduction of alveolar macrophages in both lung and airways, when compared to injection of empty liposomes or mock treatment (Supplementary Fig. 2). Although depletion of alveolar macrophages had no effect on the number of influenza NP-specific CD8+ TRM in the lung, we did observe a significant decrease in the number of TRM within the airways, including CD69− CD103−, CD69+ CD103−, and CD69+ CD103+ NP-specific CD8+ TRM (Fig. 4B, C). These data further support the observations that alveolar macrophages provide a source of persistent influenza NP antigen in animals following immunization with recombinant adenovirus.
Figure 4. Depletion of alveolar macrophages results in reduced longevity of influenza NP-specific CD8+ TRM.
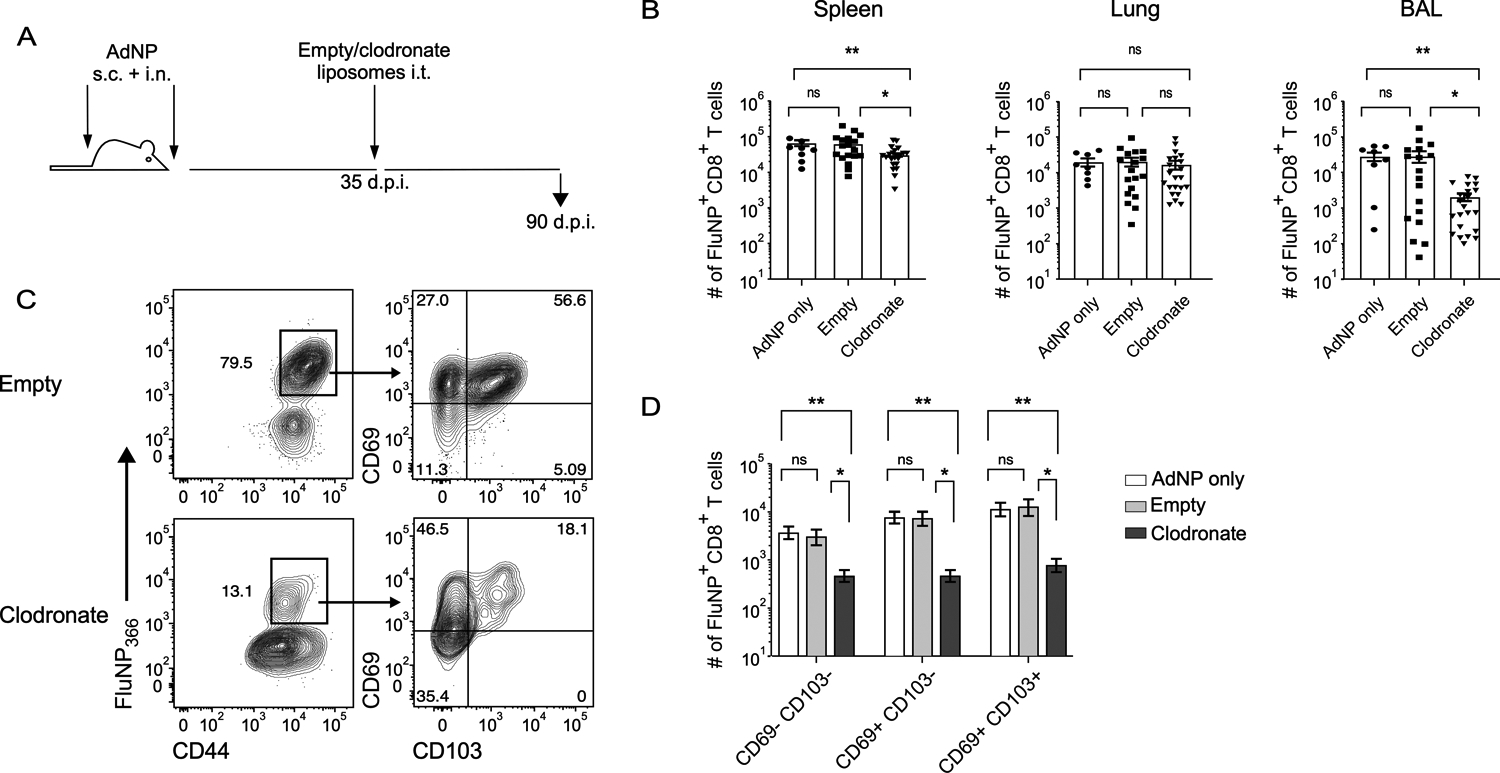
(A) Experimental design. (B) Number of influenza NP-specific CD8+ TRM in the spleen, lung, and BAL in mice immunized with AdNP and then intra-tracheally administered empty liposomes or liposomes containing clodronate. (C) Example staining of influenza NP-specific CD8+ TRM based on expression of CD69 and CD103 from the BAL of mice immunized with AdNP and then treated with empty or clodronate liposomes. (D) Number of CD69− CD103−, CD69+ CD103−, and CD69+ CD103+ influenza NP-specific CD8+ TRM in the BAL. n = 3–8 mice per group, 3 independent experiments. Significance was determined using a Mann-Whitney test. Data represent mean ± SEM. P values are as follows: * = p<0.05, ** = p<0.01, *** = p<0.001, ns= not significant.
Subsequent respiratory infections impact the longevity of CD8+ TRM
Given our identification of alveolar macrophages as an antigen source following intranasal immunization with AdNP, we next investigated the impact of subsequent, antigenically distinct, respiratory infections known to deplete alveolar macrophages on the maintenance of pre-existing NP-specific CD8+ TRM. To do so, we first infected AdNP immunized mice with Sendai virus, a murine parainfluenza virus, and then subsequently infected them with an x31 influenza strain that does not present NP antigen on MHC class I (x31 NP−) and would therefore not boost the pre-existing influenza NP-specific TRM population (54) (Fig. 5A). We then examined the impact of these infections on the number of NP-specific CD8+ TRM generated during the initial AdNP immunization. Following initial infection of AdNP immunized mice with Sendai virus, we observed no significant effect on the number of NP-specific CD8+ TRM (Fig. 5B, D). However, the number of NP-specific CD8+ TRM was significantly reduced in the lungs and airways following the second unrelated infection with x31 NP− influenza when compared to mock infection (Fig. 5C). Unsurprisingly, subsequent infection with x31 NP− had no effect on the number of NP-specific memory CD8+ T cells in the spleen (Fig. 5C). Following both Sendai virus and x31 NP− infections, the number of CD69+ CD103+ NP-specific CD8+ TRM also declined in both the lung and airways (Fig. 5E). Lastly, infecting Ad-Cre-immunized reporter mice with Sendai virus and x31 NP− also resulted in an overall decline in the percentage of tdTomato+ alveolar macrophages when compared to animals that were mock infected (Fig. 5F). These findings underscore the importance of alveolar macrophages for the long-term maintenance of NP-specific CD8+ TRM generated following intranasal immunization with AdNP.
Figure 5. Subsequent respiratory viral infections impact the maintenance of influenza NP-specific CD8+ TRM in AdNP-immunized mice.
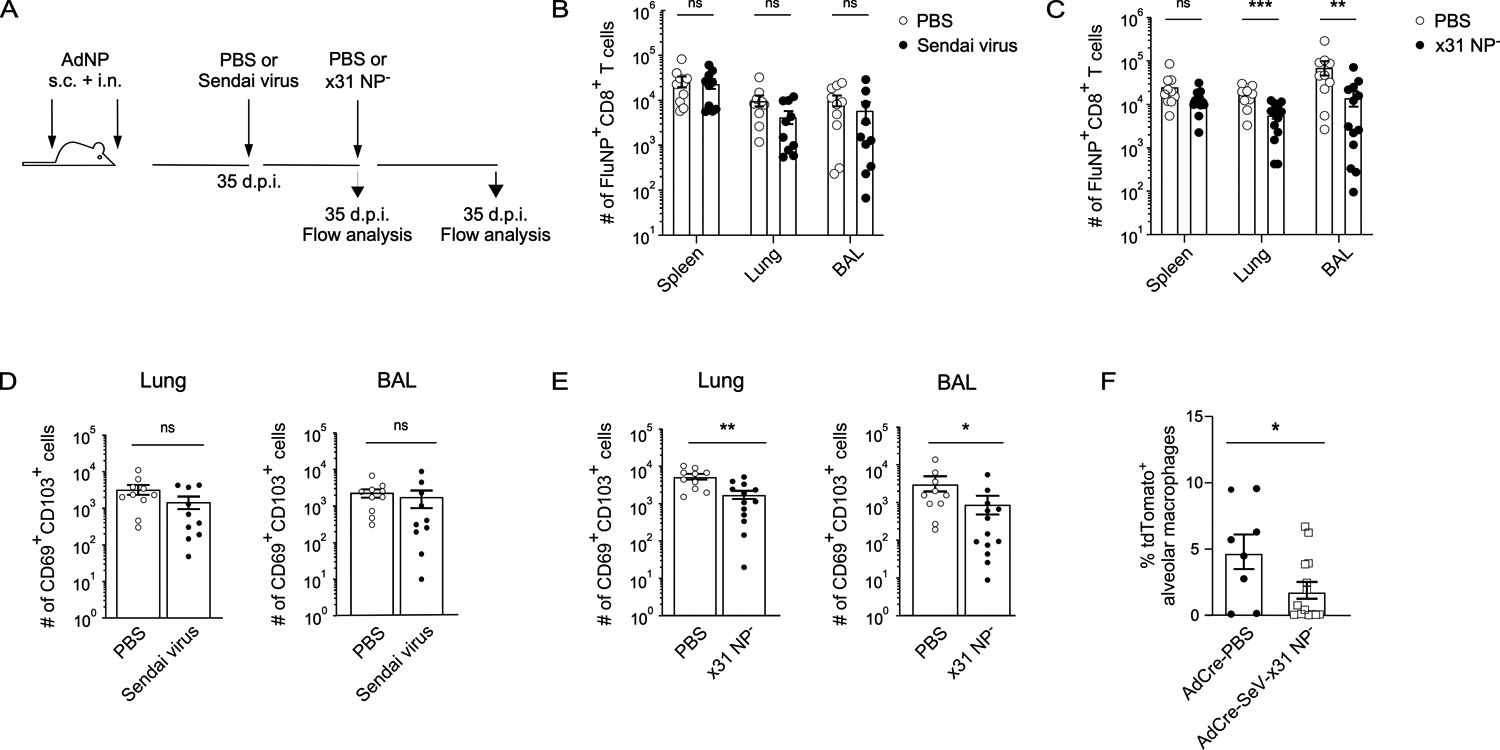
(A) Experimental design. (B) Number of influenza NP-specific CD8+ TRM in the spleen, lung, and BAL of AdNP-immunized mice subsequently infected with Sendai parainfluenza or mock infected with PBS. n = 5 mice per group, 2 independent experiments. (C) Number of influenza NP-specific CD8+ TRM in the spleen, lung, and BAL of AdNP-immunized mice subsequently infected with Sendai parainfluenza followed by x31 NP− influenza. n = 5–8 mice per group, 2 independent experiments. (D) Number of CD69+ CD103+ influenza NP-specific CD8+ TRM in the lung and BAL following Sendai parainfluenza infection of AdNP-immunized mice. (E) Number of CD69+ CD103+ influenza NP-specific CD8+ TRM in the lung and BAL following infection of AdNP-immunized mice with both Sendai parainfluenza and x31 NP− influenza. (F) Frequency of tdTomato+ alveolar macrophages in Ai14 reporter mice immunized with Ad-Cre and then either mock infected (PBS) or infected with Sendai parainfluenza and x31 NP− influenza as described in part A. n = 8–14 mice per group, 2 independent experiments. Significance was determined using a Mann-Whitney test. Data represent mean ± SEM. P values are as follows: * = p<0.05, ** = p<0.01, *** = p<0.001, ns= not significant.
Discussion:
Establishment of a robust memory T cell response is critical to the success of T-cell based vaccines. However, in the case of respiratory infections, the steady decline of lung resident CD8+ TRM over time presents a concern in generating long-term immunity. Although the mechanism behind this decline is not entirely understood, a recent study has shown that the harsh environment of the lung and airways leads to a high rate of apoptosis amongst CD8+ TRM (55). However, when mice are immunized with a replication-deficient adenovirus 5 vector that expresses the influenza nucleoprotein (AdNP) using a combination of intranasal and systemic routes, the number of pulmonary CD8+ TRM is maintained long-term (31). These CD8+ lung TRM are protective for up to at least a year post-immunization and are replenished by circulating TEM being recruited in to the TRM pool after encountering antigen locally within the lung tissue (43). In this present study, we demonstrate that CD8+ lung TRM generated following immunization with AdNP or infection with influenza virus have similar transcriptional profiles, indicating that immunization with AdNP does not result in cell-intrinsic differences responsible for the improved longevity of CD8+ lung TRM. In addition, we find further support for the model of TEM recruitment to the lungs following immunization with AdNP when we deplete CD4+ T cells post-immunization and observe a significant decrease in the number of CD8+ TRM in the lungs and airways. A prior study showed that CD4+ T cells are required for generation of CD103+ CD8+ lung TRM and promote their migration to the airways via an interferon-γ-dependent mechanism (51). CD4+ T cell help was also found to be associated with lower expression of the transcription factor T-bet, thereby allowing for TGF-β mediated induction of CD103 (51). We hypothesize a similar mechanism is occurring in our model. Lastly, lineage tracing using an adenovirus vector expressing Cre recombinase identified alveolar macrophages as a primary cell source of antigen in the respiratory tract. We were able to confirm this finding by observing colocalization of CD11c and influenza nucleoprotein in airway cells isolated from mice immunized with AdNP. Combined with prior findings, our data support a model in which an antigen reservoir maintained in long-lived alveolar macrophages helps promote differentiation of CD8+ lung TRM from the circulating effector memory T cell pool.
It has been well established that anatomic location directly impacts the development and maintenance of TRM (56). Since generation of CD8+ TRM in the lung is largely dependent on recognition of local cognate antigen, establishing a reliable antigen depot within the tissue can be critical to the success of immunization against respiratory pathogens. Interestingly, a recent report showed fibroblastic stromal cells in the lung can also serve as long-lived antigen depots following intravenous administration of an Ad5-based vector and support inflationary memory CD8+ T cells in an IL-33-dependent manner (42). Although this seemingly contrasts our finding, we believe both studies emphasize the importance of the route of immunization in defining unique mechanisms and cell types that can support the maintenance of lung TRM by acting as antigen depots. For example, alveolar macrophages may be shielded from infection by blood-borne vectors, whereas intranasal delivery of the vectors does not allow for efficient infection of cells within the lung parenchyma, such as fibroblastic stromal cells. In addition to lung FSCs, IL-33 is produced by activated macrophages in both humans and mice and has been shown to influence CD8+ TRM formation by downregulating expression of KLF2 and inducing expression of CD69 and CD103 (57–60). It is therefore conceivable that IL-33 could also be playing a role in our system. Importantly, intramuscular injection remains the most popular route of vaccination and is currently in use for the mRNA- and Ad-vector-based SARS-CoV-2 vaccines, but it can induce suboptimal mucosal immune responses (61). Alternatively, intranasal administration is widely accepted as the ideal route for targeting the respiratory tract since it most accurately mimics the natural route of infection. However, prior work showed that combined intranasal and subcutaneous injections of AdNP were superior compared to intranasal immunization alone in establishing a long-lived CD8+ lung TRM population, suggesting that local antigen supply on its own is not sufficient for maximal T cell responses (31).
As some of the first immune cells to encounter pathogens within the airways, alveolar macrophages have long been appreciated as important players in respiratory immunity, with their depletion resulting in increased viral load, pulmonary damage, and mortality following infection with influenza virus (52, 53, 62, 63). They have also been shown to be important for the establishment and reactivation of memory CD8+ T cells in the lung (21, 64, 65). One likely reason why intranasal immunization with AdNP results in prolonged maintenance of the CD8+ lung TRM pool is the longevity of alveolar macrophages (66, 67). In both humans and mice, macrophage populations are maintained for several months to years following formation (68–71). However, following infection with influenza virus, alveolar macrophages undergo high levels of cell death (72, 73). In contrast, replication-deficient adenovirus vectors are capable of transducing macrophages without causing their elimination (74–80). Indeed, we show here that Ad-Cre transduced alveolar macrophages persist in the lung at varying frequencies for up to a year post-immunization. Furthermore, loss of alveolar macrophages through depletion using liposomal clodronate resulted in a significant decline in the number of CD8+ TRM in the airways, despite no significant change in the number of CD8+ TRM within the lung tissue. We hypothesize that this is likely due to alveolar macrophages residing predominantly within the airway spaces. Another possibility is that a small frequency of interstitial macrophages harbors persistent antigen and support maintenance of CD8+ TRM within the lung interstitium, however, we are not able to distinguish this using our system. Lastly, it is also important to note that although the use of liposomal clodronate is widely considered the standard method of depleting alveolar macrophages, its effects are not exclusive to this population and is known to target dendritic cells as well. Although we cannot ensure that dendritic cells were not impacted during our depletion experiments, our Ad-Cre data clearly demonstrates that dendritic cells are transduced by Ad-Cre at very low frequency and do not persist over time.
Recombinant adenovirus vectors (rAd) display broad tissue tropism and have been shown to transduce a variety of immune and non-immune cell types both in vitro and in vivo. Although murine macrophages do not express the classical Coxsackie and adenovirus receptor (CAR), rAd vectors including human Ad5 and Ad26, two vectors currently being developed as vaccines for SARS-CoV-2, have been shown to transduce macrophages using scavenging receptors (38, 81–85). Less is known about the entry methods utilized by non-human rAd vectors; nevertheless, given the ease of altering rAd vectors, targeting adenovirus vectors to specific tissues and cell types is possible and an important consideration for future vaccine design (86). However, given the sensitive nature of the lung tissue, careful consideration must also be taken when designing any immunization strategy that creates a persistent antigen source. Firstly, although i.n. + s.c. immunization with AdNP results in prolonged protection against challenge with influenza virus, we show here that subsequent unrelated infection(s) also result in a decline in the number of NP-specific CD8+ TRM. This presents a potential limitation to targeting antigen to alveolar macrophages, since recurrent respiratory infections from diverse pathogens are likely to deplete the reservoir. In addition, the possibility of prolonged inflammation and immunopathology that accompany persistent antigen must be more thoroughly evaluated.
In summary, our results identify alveolar macrophages as a persistent cellular source of antigen following intranasal immunization with a recombinant Ad5-based vector that expresses influenza nucleoprotein. Transduced alveolar macrophages are maintained for at least a year post-immunization and are essential for continual replenishment of the CD8+ TRM pool. Furthermore, we show that persistent antigen does not induce T cell-intrinsic changes that account for the longevity of CD8+ TRM. These results further define mechanisms that promote CD8+ lung TRM generation and maintenance and have important implications for the design of T-cell based vaccines against respiratory pathogens.
Supplementary Material
Key points:
Alveolar macrophages harbor antigen following intranasal adenovirus immunization
Depletion of this antigen depot impacts the longevity of respiratory CD8+ TRM
Acknowledgments
We thank the NIH Tetramer Core Facility (contract number 75N93020D00005) for providing class I tetramers. We acknowledge that this research project was supported in part by the Emory University School of Medicine Flow Cytometry Core and the Pediatric/Winship Flow Cytometry Core. This project was supported by NIH grant R35HL150803. J.L.L. was supported by NIH grant F31HL156639.
References:
- 1.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, and Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10: 524–530. [DOI] [PubMed] [Google Scholar]
- 2.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, and Carbone FR. 2015. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43: 1101–1111. [DOI] [PubMed] [Google Scholar]
- 3.Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, and Bevan MJ. 2012. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol 189: 3462–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho SH, Lerner H, Friedman AL, Shen Y, and Farber DL. 2017. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep 20: 2921–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, and Masopust D. 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grau-Exposito J, Sanchez-Gaona N, Massana N, Suppi M, Astorga-Gamaza A, Perea D, Rosado J, Falco A, Kirkegaard C, Torrella A, Planas B, Navarro J, Suanzes P, Alvarez-Sierra D, Ayora A, Sansano I, Esperalba J, Andres C, Anton A, Ramon YCS, Almirante B, Pujol-Borrell R, Falco V, Burgos J, Buzon MJ, and Genesca M. 2021. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat Commun 12: 3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon MML, Rybkina K, Kato Y, Kubota M, Matsumoto R, Bloom NI, Zhang Z, Hastie KM, Grifoni A, Weiskopf D, Wells SB, Ural BB, Lam N, Szabo PA, Dogra P, Lee YS, Gray JI, Bradley MC, Brusko MA, Brusko TM, Saphire EO, Connors TJ, Sette A, Crotty S, and Farber DL. 2021. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci Immunol: eabl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, and Cauley LS. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMaster SR, Wilson JJ, Wang H, and Kohlmeier JE. 2015. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J Immunol 195: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergsbaken T, and Bevan MJ. 2015. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol 16: 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, and Ahmed R. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 207: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilchuk P, Hill TM, Guy C, McMaster SR, Boyd KL, Rabacal WA, Lu P, Shyr Y, Kohlmeier JE, Sebzda E, Green DR, and Joyce S. 2016. A Distinct Lung-Interstitium-Resident Memory CD8(+) T Cell Subset Confers Enhanced Protection to Lower Respiratory Tract Infection. Cell Rep 16: 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan RJ, Usherwood EJ, Zhong W, Roberts AD, Dutton RW, Harmsen AG, and Woodland DL. 2001. Activated Antigen-Specific CD8+ T Cells Persist in the Lungs Following Recovery from Respiratory Virus Infections. The Journal of Immunology 166: 1813–1822. [DOI] [PubMed] [Google Scholar]
- 14.Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, and Doherty PC. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci U S A 98: 6313–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohlmeier JE, Miller SC, and Woodland DL. 2007. Cutting Edge: Antigen Is Not Required for the Activation and Maintenance of Virus-Specific Memory CD8+ T Cells in the Lung Airways. The Journal of Immunology 178: 4721–4725. [DOI] [PubMed] [Google Scholar]
- 16.Kohlmeier JE, and Woodland DL. 2009. Immunity to respiratory viruses. Annu Rev Immunol 27: 61–82. [DOI] [PubMed] [Google Scholar]
- 17.Slutter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, and Harty JT. 2017. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheridan BS, and Lefrancois L. 2011. Regional and mucosal memory T cells. Nat Immunol 12: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, and Cauley L. 2013. Antigen and transforming growth factor Beta receptors contribute to long term functional and phenotypic heterogeneity of memory CD8 T cells. Front Immunol 4: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, Umemura M, Kyusai A, Tomura M, Nakayama T, Woodland DL, Kohlmeier JE, and Miyazawa M. 2016. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med 213: 3057–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakim LM, Smith J, Caminschi I, Lahoud MH, and Villadangos JA. 2015. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 8: 1060–1071. [DOI] [PubMed] [Google Scholar]
- 22.Takamura S, and Kohlmeier JE. 2019. Establishment and Maintenance of Conventional and Circulation-Driven Lung-Resident Memory CD8(+) T Cells Following Respiratory Virus Infections. Front Immunol 10: 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moraes TJ, Lin GH, Wen T, and Watts TH. 2011. Incorporation of 4–1BB ligand into an adenovirus vaccine vector increases the number of functional antigen-specific CD8 T cells and enhances the duration of protection against influenza-induced respiratory disease. Vaccine 29: 6301–6312. [DOI] [PubMed] [Google Scholar]
- 24.Chu KL, Batista NV, Wang KC, Zhou AC, and Watts TH. 2019. GITRL on inflammatory antigen presenting cells in the lung parenchyma provides signal 4 for T-cell accumulation and tissue-resident memory T-cell formation. Mucosal Immunol 12: 363–377. [DOI] [PubMed] [Google Scholar]
- 25.Lapuente D, Ruzsics Z, Thirion C, and Tenbusch M. 2018. Evaluation of adenovirus 19a as a novel vector for mucosal vaccination against influenza A viruses. Vaccine 36: 2712–2720. [DOI] [PubMed] [Google Scholar]
- 26.Lapuente D, Storcksdieck Genannt Bonsmann M, Maaske A, Stab V, Heinecke V, Watzstedt K, Hess R, Westendorf AM, Bayer W, Ehrhardt C, and Tenbusch M. 2018. IL-1beta as mucosal vaccine adjuvant: the specific induction of tissue-resident memory T cells improves the heterosubtypic immunity against influenza A viruses. Mucosal Immunol 11: 1265–1278. [DOI] [PubMed] [Google Scholar]
- 27.Zhou AC, Batista NV, and Watts TH. 2019. 4–1BB Regulates Effector CD8 T Cell Accumulation in the Lung Tissue through a TRAF1-, mTOR-, and Antigen-Dependent Mechanism to Enhance Tissue-Resident Memory T Cell Formation during Respiratory Influenza Infection. J Immunol 202: 2482–2492. [DOI] [PubMed] [Google Scholar]
- 28.Zhou AC, Wagar LE, Wortzman ME, and Watts TH. 2017. Intrinsic 4–1BB signals are indispensable for the establishment of an influenza-specific tissue-resident memory CD8 T-cell population in the lung. Mucosal Immunol 10: 1294–1309. [DOI] [PubMed] [Google Scholar]
- 29.Coughlan L, Sridhar S, Payne R, Edmans M, Milicic A, Venkatraman N, Lugonja B, Clifton L, Qi C, Folegatti PM, Lawrie AM, Roberts R, de Graaf H, Sukhtankar P, Faust SN, Lewis DJM, Lambe T, Hill A, and Gilbert SC. 2018. Heterologous Two-Dose Vaccination with Simian Adenovirus and Poxvirus Vectors Elicits Long-Lasting Cellular Immunity to Influenza Virus A in Healthy Adults. EBioMedicine 29: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell TJ, Peng Y, Berthoud TK, Blais ME, Lillie PJ, Hill AV, Rowland-Jones SL, McMichael AJ, Gilbert SC, and Dong T. 2013. Examination of influenza specific T cell responses after influenza virus challenge in individuals vaccinated with MVA-NP+M1 vaccine. PLoS One 8: e62778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uddback IE, Pedersen LM, Pedersen SR, Steffensen MA, Holst PJ, Thomsen AR, and Christensen JP. 2016. Combined local and systemic immunization is essential for durable T-cell mediated heterosubtypic immunity against influenza A virus. Sci Rep 6: 20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vitelli A, Quirion MR, Lo CY, Misplon JA, Grabowska AK, Pierantoni A, Ammendola V, Price GE, Soboleski MR, Cortese R, Colloca S, Nicosia A, and Epstein SL. 2013. Vaccination to conserved influenza antigens in mice using a novel Simian adenovirus vector, PanAd3, derived from the bonobo Pan paniscus. PLoS One 8: e55435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klenerman P 2018. The (gradual) rise of memory inflation. Immunol Rev 283: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Gracht ET, Schoonderwoerd MJ, van Duikeren S, Yilmaz AN, Behr FM, Colston JM, Lee LN, Yagita H, van Gisbergen KP, Hawinkels LJ, Koning F, Klenerman P, and Arens R. 2020. Adenoviral vaccines promote protective tissue-resident memory T cell populations against cancer. J Immunother Cancer 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, Botikov AG, Izhaeva FM, Popova O, Ozharovskaya TA, Esmagambetov IB, Favorskaya IA, Zrelkin DI, Voronina DV, Shcherbinin DN, Semikhin AS, Simakova YV, Tokarskaya EA, Egorova DA, Shmarov MM, Nikitenko NA, Gushchin VA, Smolyarchuk EA, Zyryanov SK, Borisevich SV, Naroditsky BS, Gintsburg AL, and Gam C-VVTG. 2021. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397: 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephenson KE, Le Gars M, Sadoff J, de Groot AM, Heerwegh D, Truyers C, Atyeo C, Loos C, Chandrashekar A, McMahan K, Tostanoski LH, Yu J, Gebre MS, Jacob-Dolan C, Li Z, Patel S, Peter L, Liu J, Borducchi EN, Nkolola JP, Souza M, Tan CS, Zash R, Julg B, Nathavitharana RR, Shapiro RL, Azim AA, Alonso CD, Jaegle K, Ansel JL, Kanjilal DG, Guiney CJ, Bradshaw C, Tyler A, Makoni T, Yanosick KE, Seaman MS, Lauffenburger DA, Alter G, Struyf F, Douoguih M, Van Hoof J, Schuitemaker H, and Barouch DH. 2021. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 325: 1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, and Oxford CVTG. 2020. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, Liu J, Peter L, McMahan K, Tostanoski LH, He X, Martinez DR, Rutten L, Bos R, van Manen D, Vellinga J, Custers J, Langedijk JP, Kwaks T, Bakkers MJG, Zuijdgeest D, Rosendahl Huber SK, Atyeo C, Fischinger S, Burke JS, Feldman J, Hauser BM, Caradonna TM, Bondzie EA, Dagotto G, Gebre MS, Hoffman E, Jacob-Dolan C, Kirilova M, Li Z, Lin Z, Mahrokhian SH, Maxfield LF, Nampanya F, Nityanandam R, Nkolola JP, Patel S, Ventura JD, Verrington K, Wan H, Pessaint L, Van Ry A, Blade K, Strasbaugh A, Cabus M, Brown R, Cook A, Zouantchangadou S, Teow E, Andersen H, Lewis MG, Cai Y, Chen B, Schmidt AG, Reeves RK, Baric RS, Lauffenburger DA, Alter G, Stoffels P, Mammen M, Van Hoof J, Schuitemaker H, and Barouch DH. 2020. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586: 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, Lin SW, Bian A, Xiang ZQ, Iparraguirre A, Lopez-Camacho C, Wherry EJ, and Ertl HC. 2007. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 110: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehrhardt A, Xu H, and Kay MA. 2003. Episomal persistence of recombinant adenoviral vector genomes during the cell cycle in vivo. J Virol 77: 7689–7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson JE, and Kay MA. 1997. Persistence of recombinant adenovirus in vivo is not dependent on vector DNA replication. J Virol 71: 8902–8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cupovic J, Ring SS, Onder L, Colston JM, Lutge M, Cheng HW, De Martin A, Provine NM, Flatz L, Oxenius A, Scandella E, Krebs P, Engeler D, Klenerman P, and Ludewig B. 2021. Adenovirus vector vaccination reprograms pulmonary fibroblastic niches to support protective inflating memory CD8(+) T cells. Nat Immunol 22: 1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uddback I, Cartwright EK, Scholler AS, Wein AN, Hayward SL, Lobby J, Takamura S, Thomsen AR, Kohlmeier JE, and Christensen JP. 2021. Long-term maintenance of lung resident memory T cells is mediated by persistent antigen. Mucosal Immunol 14: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker TC, Noel RJ, Coats WS, Gomez-Foix AM, Alam T, Gerard RD, and Newgard CB. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol 43 Pt A: 161–189. [DOI] [PubMed] [Google Scholar]
- 45.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawrence M, Huber W, Pages H, Aboyoun P, Carlson M, Gentleman R, Morgan MT, and Carey VJ. 2013. Software for computing and annotating genomic ranges. PLoS Comput Biol 9: e1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson MD, McCarthy DJ, and Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, Braun A, Wynne-Jones E, Behr FM, Stark R, Pellicci DG, Godfrey DI, Belz GT, Pellegrini M, Gebhardt T, Busslinger M, Shi W, Carbone FR, van Lier RA, Kallies A, and van Gisbergen KP. 2016. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352: 459–463. [DOI] [PubMed] [Google Scholar]
- 49.Ballesteros-Tato A, Leon B, Lund FE, and Randall TD. 2013. CD4+ T helper cells use CD154-CD40 interactions to counteract T reg cell-mediated suppression of CD8+ T cell responses to influenza. J Exp Med 210: 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belz GT, Wodarz D, Diaz G, Nowak MA, and Doherty PC. 2002. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol 76: 12388–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, and Kaech SM. 2014. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41: 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardani A, Boulton A, Kim TS, and Braciale TJ. 2017. Alveolar Macrophages Prevent Lethal Influenza Pneumonia By Inhibiting Infection Of Type-1 Alveolar Epithelial Cells. PLoS Pathog 13: e1006140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, van Rooijen N, Vogel J, and Kopf M. 2014. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog 10: e1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webby RJ, Andreansky S, Stambas J, Rehg JE, Webster RG, Doherty PC, and Turner SJ. 2003. Protection and compensation in the influenza virus-specific CD8+ T cell response. Proc Natl Acad Sci U S A 100: 7235–7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayward SL, Scharer CD, Cartwright EK, Takamura S, Li ZT, Boss JM, and Kohlmeier JE. 2020. Environmental cues regulate epigenetic reprogramming of airway-resident memory CD8(+) T cells. Nat Immunol 21: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schenkel JM, and Masopust D. 2014. Tissue-resident memory T cells. Immunity 41: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, and Jameson SC. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, and Masopust D. 2012. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 188: 4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLaren JE, Clement M, Marsden M, Miners KL, Llewellyn-Lacey S, Grant EJ, Rubina A, Gimeno Brias S, Gostick E, Stacey MA, Orr SJ, Stanton RJ, Ladell K, Price DA, and Humphreys IR. 2019. IL-33 Augments Virus-Specific Memory T Cell Inflation and Potentiates the Efficacy of an Attenuated Cytomegalovirus-Based Vaccine. J Immunol 202: 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, and Kastelein RA. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23: 479–490. [DOI] [PubMed] [Google Scholar]
- 61.Su F, Patel GB, Hu S, and Chen W. 2016. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum Vaccin Immunother 12: 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wein AN, Dunbar PR, McMaster SR, Li ZT, Denning TL, and Kohlmeier JE. 2018. IL-36gamma Protects against Severe Influenza Infection by Promoting Lung Alveolar Macrophage Survival and Limiting Viral Replication. J Immunol 201: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghoneim HE, Thomas PG, and McCullers JA. 2013. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol 191: 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macdonald DC, Singh H, Whelan MA, Escors D, Arce F, Bottoms SE, Barclay WS, Maini M, Collins MK, and Rosenberg WM. 2014. Harnessing alveolar macrophages for sustained mucosal T-cell recall confers long-term protection to mice against lethal influenza challenge without clinical disease. Mucosal Immunol 7: 89–100. [DOI] [PubMed] [Google Scholar]
- 65.Low JS, Farsakoglu Y, Amezcua Vesely MC, Sefik E, Kelly JB, Harman CCD, Jackson R, Shyer JA, Jiang X, Cauley LS, Flavell RA, and Kaech SM. 2020. Tissue-resident memory T cell reactivation by diverse antigen-presenting cells imparts distinct functional responses. J Exp Med 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy J, Summer R, Wilson AA, Kotton DN, and Fine A. 2008. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol 38: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel AA, Ginhoux F, and Yona S. 2021. Monocytes, macrophages, dendritic cells and neutrophils: an update on lifespan kinetics in health and disease. Immunology 163: 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, and Jung S. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, and Merad M. 2013. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38: 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nayak DK, Zhou F, Xu M, Huang J, Tsuji M, Hachem R, and Mohanakumar T. 2016. Long-Term Persistence of Donor Alveolar Macrophages in Human Lung Transplant Recipients That Influences Donor-Specific Immune Responses. Am J Transplant 16: 2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan SY, and Krasnow MA. 2016. Developmental origin of lung macrophage diversity. Development 143: 1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fesq H, Bacher M, Nain M, and Gemsa D. 1994. Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology 190: 175–182. [DOI] [PubMed] [Google Scholar]
- 73.Laghlali G, Lawlor KE, and Tate MD. 2020. Die Another Way: Interplay between Influenza A Virus, Inflammation and Cell Death. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan Q, Zhao F, Chung SW, Fan P, Sultzer BM, Kan YW, and Wong PM. 2000. Dominant negative down-regulation of endotoxin-induced tumor necrosis factor alpha production by Lps(d)/Ran. Proc Natl Acad Sci U S A 97: 2852–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang S, Endo RI, and Nemerow GR. 1995. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol 69: 2257–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haddada H, Lopez M, Martinache C, Ragot T, Abina MA, and Perricaudet M. 1993. Efficient adenovirus-mediated gene transfer into human blood monocyte-derived macrophages. Biochem Biophys Res Commun 195: 1174–1183. [DOI] [PubMed] [Google Scholar]
- 77.Wan Y, Bramson J, Carter R, Graham F, and Gauldie J. 1997. Dendritic cells transduced with an adenoviral vector encoding a model tumor-associated antigen for tumor vaccination. Hum Gene Ther 8: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 78.Zhong L, Granelli-Piperno A, Choi Y, and Steinman RM. 1999. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur J Immunol 29: 964–972. [DOI] [PubMed] [Google Scholar]
- 79.Tippimanchai DD, Nolan K, Poczobutt J, Verzosa G, Li H, Scarborough H, Huang J, Young C, DeGregori J, Nemenoff RA, and Malkoski SP. 2018. Adenoviral vectors transduce alveolar macrophages in lung cancer models. Oncoimmunology 7: e1438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chu Y, Sperber K, Mayer L, and Hsu MT. 1992. Persistent infection of human adenovirus type 5 in human monocyte cell lines. Virology 188: 793–800. [DOI] [PubMed] [Google Scholar]
- 81.Bos R, Rutten L, van der Lubbe JEM, Bakkers MJG, Hardenberg G, Wegmann F, Zuijdgeest D, de Wilde AH, Koornneef A, Verwilligen A, van Manen D, Kwaks T, Vogels R, Dalebout TJ, Myeni SK, Kikkert M, Snijder EJ, Li Z, Barouch DH, Vellinga J, Langedijk JPM, Zahn RC, Custers J, and Schuitemaker H. 2020. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 5: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Langel SN, Johnson S, Martinez CI, Tedjakusuma SN, Peinovich N, Dora EG, Kuehl PJ, Irshad H, Barrett EG, Werts A, and Tucker SN. 2022. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci Transl Med: eabn6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maler MD, Nielsen PJ, Stichling N, Cohen I, Ruzsics Z, Wood C, Engelhard P, Suomalainen M, Gyory I, Huber M, Muller-Quernheim J, Schamel WWA, Gordon S, Jakob T, Martin SF, Jahnen-Dechent W, Greber UF, Freudenberg MA, and Fejer G. 2017. Key Role of the Scavenger Receptor MARCO in Mediating Adenovirus Infection and Subsequent Innate Responses of Macrophages. mBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stichling N, Suomalainen M, Flatt JW, Schmid M, Pacesa M, Hemmi S, Jungraithmayr W, Maler MD, Freudenberg MA, Pluckthun A, May T, Koster M, Fejer G, and Greber UF. 2018. Lung macrophage scavenger receptor SR-A6 (MARCO) is an adenovirus type-specific virus entry receptor. PLoS Pathog 14: e1006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Custers J, Kim D, Leyssen M, Gurwith M, Tomaka F, Robertson J, Heijnen E, Condit R, Shukarev G, Heerwegh D, van Heesbeen R, Schuitemaker H, Douoguih M, Evans E, Smith ER, Chen RT, and G. Brighton Collaboration Viral Vector Vaccines Safety Working. 2021. Vaccines based on replication incompetent Ad26 viral vectors: Standardized template with key considerations for a risk/benefit assessment. Vaccine 39: 3081–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beatty MS, and Curiel DT. 2012. Chapter two--Adenovirus strategies for tissue-specific targeting. Adv Cancer Res 115: 39–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


