Abstract
Non-alcoholic fatty liver disease is one of the main liver diseases worldwide. The most common cause of death in patients with non-alcoholic fatty liver disease is cardiovascular disease. The relationship between these two conditions has been well established. Indeed, identical reasons may contribute to the development of cardiovascular disease and non-alcoholic fatty liver disease with lifestyle factors such as smoking, sedentariness, poor nutritional habits, and physical inactivity being major aspects. This review focuses on potential pathophysiological mechanisms of cardiovascular disorders in non-alcoholic fatty liver. PubMed, EMBASE, Orphanet, MIDLINE, Google Scholar, and Cochrane Library were searched for articles published between 2006 and 2022. Relevant articles were selected using the following terms: “Non-alcoholic fatty liver disease,” “Сardiovascular diseases,” “Pathophysiological mechanisms.” The reference lists of all identified articles were searched for other relevant publications as well. The pathophysiological mechanisms of cardiovascular disorders in non-alcoholic fatty liver remain largely speculative and may include systemic low-grade inflammation, atherogenic dyslipidemia, abnormal glucose metabolism and hepatic insulin resistance, endothelial dysfunction, gut dysbiosis, as well as the associated cardiac remodeling, which are influenced by interindividual genetic and epigenetic variations. It is clear that the identification of pathophysiological mechanisms underlying cardiovascular disorders in non-alcoholic fatty liver disease will make the selection of therapeutic measures more optimal and effective.
Key Words: Non-alcoholic fatty liver disease, Cardiovascular diseases, Pathophysiological mechanisms
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a main liver disease in Western countries representing approximately 75% of all chronic liver diseases (1). In their meta-analysis, Younossi et al. (2) reported that the global prevalence of NAFLD is 25.24% with the highest prevalence in the Middle East and South America and the lowest in Africa. In the Russian Federation in 2007, the detection rate of NAFLD was 27.0%, and in 2014 this figure reached 37.1%, which placed NAFLD in a leading position among all chronic liver diseases (3). Currently, the most common cause of death in patients with NAFLD is cardiovascular disease (CVD) (4). The problem is aggravated by the growing number of NAFLD patients with CVD, which is supposedly directly associated with cardiovascular risk factors (5).
NAFLD is defined as fatty change (steatosis) affecting ≥5% of hepatocytes, and it has a spectrum of histologic features ranging from simple steatosis without liver fibrosis (nonalcoholic fatty liver) to nonalcoholic steatohepatitis (NASH). In turn, NASH is characterized by not only steatosis, but also hepatocyte ballooning, lobular inflammation, and varying stages of liver fibrosis (6).
All stages of NAFLD are associated with an increased risk of acute coronary syndrome, atherosclerosis, stroke, malignant arrhythmias, etc. (7). The relationship between NAFLD and CVD has been confirmed by numerous experimental and clinical studies (8). In a systematic review and meta-analysis by Wu et al. (9) which included 34 studies (164,494 participants, 21 cross-sectional studies, and 13 cohort studies), although NAFLD was not associated with overall mortality (hazard ratio [HR] 1.14, 95% confidence interval [CI]: 0.99-1.32) or CVD mortality (HR 1.10, 95% CI: 0.86-1.41), NAFLD was accompanied by an increased risk of prevalent (odds ratio [OR] 1.81, 95% CI: 1.23-2.66) and incident (HR 1.37, 95% CI: 1.10-1.72) CVD. For some specific CVDs, NAFLD was associated with an increased risk of prevalent (OR 1.87, 95% CI: 1.47-2.37) and incident (HR 2.31, 95% CI: 1.46-3.65) coronary artery disease, prevalent (OR 1.24, 95% CI: 1.14-1.36) and incident (HR 1.16, 95% CI: 1.06-1.27) hypertension, and prevalent (OR 1.32, 95% CI: 1.07-1.62) atherosclerosis. A meta-analysis including a total of 16 unique, observational prospective and retrospective studies with 34,043 adult individuals (36.3% with NAFLD) and approximately 2600 CVD outcomes (>70% CVD deaths) over a median period of 6.9 years showed that patients with NAFLD had a higher risk of fatal and/or non-fatal cardiovascular events than those without NAFLD (random effect odds ratio [OR] 1.64, 95% CI: 1.26-2.13). Patients with more severe NAFLD were also more likely to develop fatal and non-fatal cardiovascular events (OR 2.58, 95% CI: 1.78-3.75). Sensitivity analyses did not alter these findings. Funnel plot and Egger's test did not reveal any significant publication bias (10).
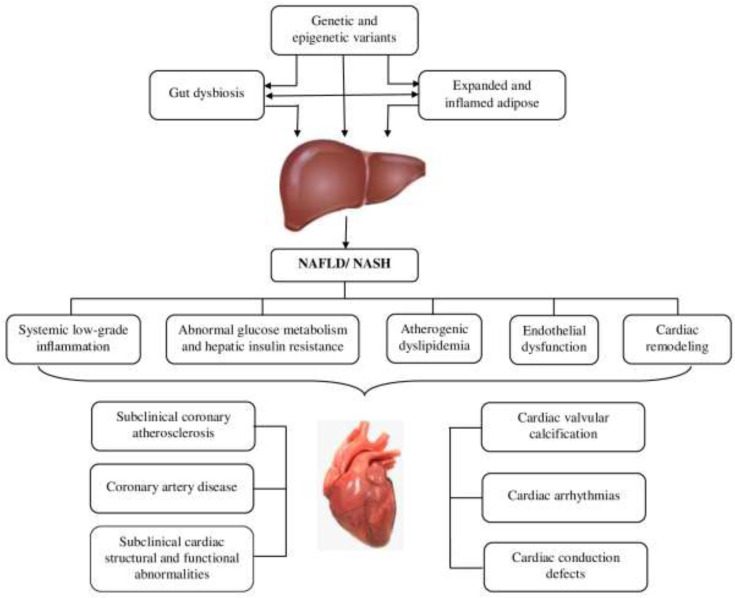
The current review focuses on potential pathophysiological mechanisms of cardiovascular disorders in NAFLD. These mechanisms may include systemic low-grade inflammation, atherogenic dyslipidemia, abnormal glucose metabolism and hepatic insulin resistance, endothelial dysfunction, gut dysbiosis, as well as the associated cardiac remodeling, which are influenced by interindividual genetic and epigenetic variations (11) (Figure 1).
Figure 1.
The potential pathophysiological mechanisms of cardiovascular disorders in non-alcoholic fatty liver disease (NAFLD)
Systemic low-grade inflammation
NAFLD is associated with chronic, low-grade inflammation in the liver that causes systemic effects, which can be detected by revealing systemic alterations in immune cell subsets and humoral factors. In the liver and extrahepatic organs, these signals can promote cellular dysfunction, cell death, and deleterious tissue remodeling in an attempt to maintain structural and functional organ integrity. Importantly, the presence of NASH and advanced liver fibrosis increases the risk for systemic comorbidity in NAFLD. Although the precise nature of the crosstalk between the liver and other organs has not yet been fully elucidated, there is emerging evidence that metabolic inflammation, emanating partly from the fatty liver, is the engine that drives cellular dysfunction, cell death, and deleterious remodeling within various body tissues (12).
The main causes of systemic low-grade inflammation in NAFLD are lipotoxicity, oxidative stress, endoplasmic reticulum stress, and mitochondrial dysfunction (13). Dietary factors may be an important trigger of systemic low-grade inflammation. For example, fatty acids that are contained in food may have a direct effect on immune cells, activate toll-like receptors (TLRs), and induce the cytokine cascade (14). Lipotoxicity activates inflammatory pathways and components of the immune system and has been observed in the liver, arterial vessels, adipose tissue, muscles, pancreas, and the central nervous system. Various compartments such as the liver, the gastrointestinal tract, and adipose tissue are significant sources of proinflammatory drivers including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, C-reactive protein, fibrinogen, and fetuin-A (15). Oxidative stress and endoplasmic reticulum stress have been shown to impact metabolic inflammation by inducing endothelial dysfunction and predisposing patients with NAFLD to the development of cardiovascular disorders (16). In addition, persistent endoplasmic reticulum stress and mitochondrial dysfunction contribute to pathophysiological changes and play an important role in the progression of NALFD to NASH. The endoplasmic reticulum is the most important intracellular Ca2+ storage. It is well known that Ca2+ is a critical and versatile intracellular secondary messenger that is involved in various cellular processes. The abnormal release of endoplasmic reticulum Ca2+ not only induces endoplasmic reticulum stress and mitochondrial dysfunction, but also exacerbates hepatic cell lipotoxicity. The sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) pump, the main regulator of intracellular Ca2+, actively re-accumulates released Ca2+ back into the endoplasmic reticulum thereby maintaining Ca2+ homeostasis. SERCA activity is reduced in NALFD, while enhanced SERCA activity alleviates endoplasmic reticulum stress and apoptosis. It has been shown that the homeostasis of Ca2+ is closely related to the progression of NALFD to NASH (17).
The constant presence and formation of new pro-inflammatory cytokines contribute to such pathological conditions as atherogenesis, cardiomyopathy, and cardiac arrhythmias, among others. For example, systemic low-grade inflammation is involved in all stages of atherogenesis. Activated endothelial cells recruit leukocytes through the expression of adhesion molecules, which are regulated by pro-inflammatory cytokines (18). Chemokines cause the migration of circulating leukocytes, primarily monocytes, into the vascular intima, where they mature into macrophages.
Excessive deposition of cholesterol crystals in the vascular intima activates the NLRP3 (NOD-like receptor family, pyrin domain-containing protein 3) inflammasome, central nucleotide-binding domain NACHT (NOD or NBD), leucine-rich repeat (LRR) C-terminal domain, and N-terminal pyrin domain (PYD), which leads to the release of IL-1 (19).
Atherogenic dyslipidemia
Dyslipidemia is characteristic of NAFLD and closely related to CVD, whereas low-density lipoprotein (LDL) cholesterol is accepted as a key driver of atherosclerosis (20). Typically, dyslipidemia in NAFLD is accompanied by high serum triglycerides and low high-density lipoprotein (HDL) cholesterol levels, with moderately increased serum LDL cholesterol levels (21).
Dysfunctional visceral adipose tissue and the increased accumulation of dysfunctional, ectopic fat in the liver and other organs such as the pericardium, pancreas, kidneys, or skeletal muscle, are closely related to adverse cardiometabolic outcomes (22). Dysfunctional adipocytes act as antigen-presenting cells and express pro-inflammatory cytokines such as TNF-α, IL-6, Il-1β, monocyte chemoattractant protein (MCP)4/CCL13 regulated on activation, normal T cells expressed and secreted (RANTES)/CCL5 и MCP1/CCL2 (23). The C-C motif chemokine ligand-2 (CCL2) is a key mediator of the crosstalk among adipocytes, macrophages, and endothelial cells and can potentially aggravate the inflammatory state, resulting in increased expression of pro-inflammatory cytokines, chemokines, adipokines, and angiogenic factors (24).
Although the liver is the central organ of lipid metabolism, in a healthy state it does not accumulate them. In NAFLD, hepatic fat accumulation results from an imbalance between lipid acquisition and lipid disposal, which is mediated by inadequate absorption of circulating lipids, enhancement of hepatic de novo lipogenesis (DNL), an insufficient increase of fatty acids compensatory oxidation, and impaired export of lipids as components of very-low-density lipoprotein (VLDL) (25).
Analysis of available genetic data suggests that the altered operation of fatty-acid β-oxidation in liver mitochondria is the key process connecting NAFLD-mediated dyslipidemia and elevated cardiovascular risk (26). In the early stages of NAFLD, an increase in mitochondrial oxidation compensates, at least partially, for the excess of hepatocellular lipids (27). Elevated lipid uptake and enhanced hepatic DNL lead to an increased accumulation of hepatic triglyceride with concomitant overproduction and secretion of large, triglyceride-enriched VLDL particles, which serve to mobilize hepatic fat for transport to peripheral tissues (28). Generally, once secreted into circulation, large VLDL particles lose their triglyceride moiety through lipoprotein lipase activity, turning into an equal number of atherogenic LDL particles with a dominant cholesterol content. LDL particles are taken up into the liver via the LDL receptor pathway (29). The overproduction of VLDL particles in NAFLD causes several lipoprotein disorders that lead to atherogenic dyslipidemia, which is characterized by high serum triglycerides and low HDL cholesterol levels, an atherogenic lipoprotein phenotype including a predominance of small dense LDL particles, an accumulation of triglyceride-rich lipoproteins and their remnants, as well as intermediate-density lipoproteins (30). The severity of these lipid disorders increases with more severe and advanced stages of NAFLD (31). The small dense LDL particles have a special tendency to penetrate through the vascular endothelium into the subendothelial space, which serves as the initiating event of the atherosclerotic plaque formation. In the vascular wall, LDL cholesterol is additionally oxidized and causes an innate immune response through TLRs (32).
Atherosclerosis development is mainly mediated by apolipoprotein B-containing particles. Thus, in addition to LDL cholesterol-mediated risk for atherosclerosis, triglyceride-rich lipoproteins such as VLDL and intermediate-density lipoproteins also contribute to increased cardiovascular risk (33). These particles also contain apolipoprotein C3, which plays an important role in TLRs activation and, consequently, the inflammatory response (34). Lipoproteins containing apolipoprotein C3 activate TLR2 and TLR4 by dimerization, thereby activating the NLRP3 inflammasome (35). Once activated by damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs), or other atherosclerosis-related stimuli, the NLRP3 inflammasome regulates the activity of one of its constituent proteins, i.e. the enzyme caspase-1, also known as IL-1β converting enzyme. Activation of caspase-1 by the NLRP3 inflammasome leads to a proteolytic activation of pro-inflammatory cytokines of the IL-1β family and the subsequent induction of the IL-1 to IL-6 into the CRP inflammatory pathway, which is involved in the development of vascular inflammation and atherosclerotic CVD (36). Thus, TLRs activation by apolipoprotein C3 and subsequent NLRP3 inflammasome activation provide an important link between atherogenic lipoprotein patterns that are commonly observed in patients with NAFLD and activation of vascular immunity (inflammation) (28).
Enhancement of hepatic DNL in NAFLD may also be associated with increased hepatic palmitic acid (16:0) flux and enrichment of palmitic acid in VLDL particles (37). Saturated fatty acids, such as palmitic acid, can induce vascular inflammation by the dimerization and activation of TLR2 and TLR4, representing another mechanism of how NAFLD promotes the development of vascular damage and atherosclerosis (38). It has been noted that higher palmitic acid or palmitoleic acid (16:1n-7) levels were associated with increased risks of all-cause and cardiovascular mortality (39).
Abnormal glucose metabolism and hepatic insulin resistance
Abnormal glucose metabolism and hepatic insulin resistance are other major signs of NAFLD and are crucial in CVD pathogenesis (40). Glucose metabolism disorders in patients with NAFLD can be explained by systemic low-grade inflammation, visceral obesity in combination with usually excess body weight, and increased accumulation of dysfunctional ectopic fatty tissue (41). In addition to the increase in hepatic fat content, the accumulation of dysfunctional ectopic fatty tissue in the pancreas plays an important role in this situation and is essentially related to insulin resistance and β-cell dysfunction (42). Insulin resistance is accompanied by compensatory persistent hyperinsulinemia, which is of central importance for the induction and maintenance of an unfavorable metabolic environment (e.g., increased free fatty acid and glucose levels), as insulin resistance worsens further and subsequently contributes to the development of cardiometabolic disorders. In particular, hyperinsulinemia is associated with the increase in hepatic glucose production, which leads to an increase in plasma glucose levels and a constant increase in insulin levels, representing a self-reinforcing cycle. This condition is aggravated by the fact that NAFLD reduces hepatic insulin clearance. At the same time, high insulin activates two transcription factors, namely sterol regulatory element-binding protein 1c (SREBP-1c) and carbohydrate-responsive element-binding protein (ChREBP), leading to greater expression of various lipogenic enzymes involved in DNL. This causes further hepatic fat accumulation and saturated fatty acids production, which, together with increased plasma glucose levels, maintain a disturbed metabolic environment (43).
Insulin resistance and impaired insulin signaling affect various processes that are associated with atherogenesis, increased progression of atherosclerotic lesions, and the vulnerability of plaques. Persistent hyperglycemia and postprandial glucose spikes contribute to oxidative stress by concomitantly activating inflammatory signaling pathways, inflammasome activation, and vascular inflammation through advanced glycation end products, impaired regulation of lipoprotein metabolism, and ongoing ectopic fat accumulation (44). Furthermore, insulin resistance is associated with dysregulated neurohumoral activation of the renin-angiotensin-aldosterone system (RAAS), may cause fibrinolytic dysfunction through increased plasminogen activator inhibitor-1 levels, and participate in the development of cardiac autonomic neuropathy. In turn, the latter may promote the development of systolic and diastolic dysfunction or cardiac arrhythmias as well as endothelial dysfunction (45).
Endothelial dysfunction
Endothelial dysfunction is an early stage in the pathogenesis of atherosclerosis and, therefore, is also crucial in the development of CVD. It is related to superoxide-associated oxidative stress, lipoprotein (e.g., apolipoprotein C3)-mediated vascular inflammation and selective vascular insulin resistance. Endothelial dysfunction is characterized by decreased bioavailability of nitric oxide (NO), the vascular protective vasodilatory molecule (46). Elevated serum levels of asymmetric dimethylarginine, which is an endogenous antagonist of nitric oxide synthase (NOS), lead to a decrease in NO availability that may contribute to impaired vasomotor regulation or vascular permeability and platelet dysfunction. Elevated serum levels of asymmetric dimethylarginine in patients with NAFLD are primarily a consequence of insulin resistance (47). Another factor involved in the development of endothelial dysfunction is hyperhomocysteinemia. Elevated serum homocysteine levels cause oxidative stress through reduced replication of glutathione storage and, subsequently, impair the formation of NO and increase vascular resistance and platelet hyperactivation. Elevated serum homocysteine levels were found with NAFLD (48). However, patients with NASH had lower serum homocysteine levels (49). Patients with NAFLD may also have an increased risk of developing atherosclerotic CVD due to an imbalance of procoagulants. They frequently have increased serum levels of the coagulation factors FVIII, FIX, FXI, and FXII, which are accompanied by increased circulating concentrations of fibrinogen, von Willebrand factor, and plasminogen activator inhibitor-1, while antithrombin III and protein C are decreased (50). An additional aspect that may affect atherogenesis and plaque instability in patients with NAFLD is altered serum concentrations of vascular endothelial growth factor (VEGF). Elevated serum VEGF levels and signs of active angiogenesis, which indicate vascular remodeling, are observed in patients with NAFLD and are simultaneously associated with both the formation and the instability of plaques (51). However, current data is contradictory, and the atherogenic role of VEGF in NAFLD still needs further research.
Gut dysbiosis
It seems plausible that the gastrointestinal tract may be regarded as an origin of systemic inflammatory changes, thereby playing a crucial role in metabolic diseases such as NAFLD and CVD (52). The common feature of gut dysbiosis is intestinal barrier dysfunction that causes a subsequent increase in mucosal barrier permeability (53). Consequently, intestinal microbes and/or microbial products designated as PAMPs (e.g., lipopolysaccharides or peptidoglycans) and DAMPs (e.g., those that are released from damaged enterocytes) enter the systemic circulation and activate various cellular signaling pathways that induce a systemic inflammatory response associated with gut dysbiosis. This inflammation associated with gut dysbiosis may be a central link between the gut microbiota and the development of CVD (54). In particular, significant alterations in intestinal microbiota were found in patients with coronary artery disease and NAFLD and persisted in a decrease in Colinsella and Parabacterioides. This fact may be a potential explanation for the worse clinical outcome and disease progression in these patients compared to patients with coronary artery disease but no NAFLD (55).
In an experiment on mice, the introduction of pro-inflammatory intestinal microbiota enhanced systemic inflammation and accelerated atherogenesis (56). In this regard, it is interesting that statins, regardless of their effect in reducing LDL levels, can have an anti-inflammatory effect. In addition, their use was associated with a lower prevalence of gut dysbiosis and thereby contributed to the attenuation of systemic inflammation (57).
Intestinal commensals convert nutrients such as choline or L-carnitine into trimethylamine, which is then transformed into trimethylamine N-oxide by hepatic flavin monooxygenases. Trimethylamine N-oxide production is less pronounced in vegans or vegetarians, whereas its formation is facilitated by a diet enriched with L-carnitine (58). A decrease in trimethylamine N-oxide levels was observed within 4 weeks of stopping long-term consumption of red meat (59). Many studies (60) and several meta-analyses (61) have shown an adverse effect of elevated circulating trimethylamine N-oxide levels on the outcome of CVD. These studies have made it possible to predict mortality in patients with CVD and coexisting peripheral arterial disease (62). In addition, the harmful effect of trimethylamine N-oxide was noted in patients with ischemic stroke (63). Despite a small number of studies examining the role of trimethylamine N-oxide in NAFLD, some have shown a correlation of its serum levels with NAFLD severity, specifically due to concomitant CVD (64). Due to its ability to change the calcium signaling in platelets, trimethylamine N-oxide is associated with an increased risk of blood clot formation (65). At the same time, inhibitors of trimethylamine-producing enzymes significantly reduced serum trimethylamine N-oxide levels for up to 3 days and prevented an increase in diet-induced platelet reaction and blood clot formation (66). In another study, a U-shaped relationship was observed between serum trimethylamine N-oxide levels and the risk of death in patients with recurrent venous thromboembolism (67).
Genetic variants
The five key genes involved in the NAFLD pathogenesis have been thoroughly studied. These include Patatin Like Phospholipase Domain Containing 3 (PNPLA3), Transmembrane 6 Superfamily Member 2 (TM6SF2), Glucokinase Regulator (GCKR), Membrane-Bound O-acyltransferase Domain Containing 7 (MBOAT7), and Hydroxysteroid 17-Beta Dehydrogenase 13 (HSD17B13) (68).
Genetic variants, especially single-nucleotide polymorphisms, affect the flow of free fatty acids, oxidative stress, reactions to endotoxin production and cytokine activity, and determine the development and progression of NAFLD (69). Additionally, the following have been linked to NAFLD and coronary artery disease: the genetic polymorphisms of adiponectin rs266729, adiponectin-encoding gene (ADIPOQ), leptin receptor (LEPR), apolipoprotein C3, peroxisome proliferator-activated receptors (PPARs), sterol regulatory element-binding proteins (SREBPs), transmembrane 6 superfamily member 2 (TM6SF2), microsomal triglyceride transfer protein (MTTP), TNF-α, and manganese superoxide dismutase (MnSOD) (70).
Some genetic polymorphisms, such as PNPLA3 (rs738409 C>G) and TM6SF2 (rs58542926 C>T), may worsen liver diseases but also attenuate the strength of the association between NAFLD and CVD, possibly by their effects on lipoprotein metabolism (71). For example, Lauridsen et al. (72) demonstrated that the risk of coronary artery disease gradually rose with an increase in hepatic fat content. However, when using Mendelian randomization, PNPLA3 polymorphism was not causally associated with coronary artery disease. In addition, the genetic variants PNPLA3 and TM6SF2 were not only strongly associated with NAFLD and its progression to NASH, liver cirrhosis, and hepatocellular carcinoma, but also with lower blood triglycerides and LDL-cholesterol concentrations as well as protection from coronary artery disease. However, further clarification is required as to what extent these genetic modifications affect the development of other CVD (73).
Although MBOAT7 seems to have a neutral effect on atherosclerotic CVD, future studies are needed to confirm this and to investigate whether HSD17B13 influences atherosclerotic cardiovascular risk (74).
Overall, genetic studies support the relationship between NAFLD and atherosclerotic CVD, notably by altered VLDL secretion and mixed hyperlipidemia. In particular, these studies support the notion that genetic variants altering both NAFLD/hepatic fat and plasma lipid levels affect atherosclerotic cardiovascular risk. Some genetic variants cause VLDL overproduction, while others create VLDL retention, which affects lipids opposingly. Future genome-wide association studies involving liver biopsies are expected to provide novel NAFLD loci from large international patient cohorts. This will aid in elucidating the relation between NAFLD, atherosclerotic CVD, plasma lipids, and other coincident metabolic factors (75).
Cardiac remodeling
Cardiac remodeling is defined as a group of molecular, cellular and interstitial changes that manifest clinically as changes in size, mass, geometry, and function of the heart (76). This is the most common and complex response to injury causing cardiac mechanical, inflammatory, and neurohumoral stress. The process results in poor prognosis because of its association with ventricular dysfunction and malignant arrhythmias, which eventually lead to irreversible heart failure and mortality (77).
Abundant clinical evidence supports close associations between NAFLD and cardiac remodeling (78). The potential mechanisms facilitating cardiac remodeling in NAFLD patients involve multistep processes associated with insulin resistance, RAAS and sympathetic nervous system, systemic inflammation, oxidative stress, gut microbiota, and genetic and epigenetic variations (79). VanWagner et al. (80) showed that NAFLD is related to increased body surface area and an increase in cardiac output and left ventricular filling pressures that may, over time, lead to the development of clinical heart failure. In the study by Styczynski et al. (81), the patients with NASH demonstrated significantly increased cardiac output and the echocardiographic signs of left ventricular concentric remodeling when compared with the isolated steatosis and no steatosis groups.
The presence of an increased cardiac output and cardiac index in patients with NASH is especially intriguing, because NASH is regarded as an early step in the development of liver cirrhosis, the disease that is classically associated with the presence of hyperdynamic systemic circulation and portal hypertension (82). However, several reports have shown that portal hypertension may be present in patients with significant steatosis even before the development of evident liver fibrosis (83). Thus, more studies are needed to elucidate the presence of hyperdynamic systemic circulation in NASH. If confirmed, it could explain one of the potential mechanisms facilitating the development of heart failure or atherosclerotic complications in this important group of patients.
Conclusions
The relationship between NAFLD and CVD has been confirmed by numerous experimental and clinical studies. Moreover, CVD is the main cause of death in patients with NAFLD, which makes the problem extremely relevant. Indeed, identical reasons may contribute to the development of CVD and NAFLD, with lifestyle factors such as smoking, sedentariness, poor nutritional habits, and physical inactivity being major aspects. It is clear that the identification of pathophysiological mechanisms underlying cardiovascular disorders in NAFLD will make the choice of therapeutic measures more optimal and effective.
Conflict of interests
The authors have no conflicts of interest to declare.
References
- 1.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–30. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Ivashkin VT, Mayevskaya MV, Pavlov ChS, Tikhonov IN, Shirokova YeN, Buyeverov AO, et al. Diagnostics and treatment of non-alcoholic fatty liver disease: clinical guidelines of the Russian Scientific Liver Society and the Russian gastroenterological association. Rus J gastroenterol hepatol coloproctol. 2016;26:24–42. [Google Scholar]
- 4.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Polyzos SA, Kechagias S, Tsochatzis EA. Review article: non-alcoholic fatty liver disease and cardiovascular diseases: associations and treatment considerations. Aliment Pharmacol Ther. 2021;54:1013–1025. doi: 10.1111/apt.16575. [DOI] [PubMed] [Google Scholar]
- 6.Lackner C. Prospects for a better diagnosis and prognosis of NAFLD: a pathologist´s view. Hepatoma Res. 2021;7:27. [Google Scholar]
- 7.Shroff H, VanWagner LB. Cardiovascular disease in nonalcoholic steatohepatitis: Screening and management. Curr Hepatol Rep. 2020;19:315–26. doi: 10.1007/s11901-020-00530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jichitu A, Bungau S, Stanescu AMA, Vesa CM, Toma MM, Bustea C, et al. Non-Alcoholic fatty liver disease and cardiovascular comorbidities: pathophysiological links, diagnosis, and therapeutic management. Diagnostics (Basel) 2021;11:689. doi: 10.3390/diagnostics11040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386. doi: 10.1038/srep33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Bisaccia G, Ricci F, Mantini C, Tana C, Romani GL, Schiavone C, et al. Nonalcoholic fatty liver disease and cardiovascular disease phenotypes. SAGE Open Med. 2020;8:2050312120933804. doi: 10.1177/2050312120933804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehrke N, Schattenberg JM. Metabolic inflammation-A role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology. 2020;158:1929–47. doi: 10.1053/j.gastro.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–46. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 14.Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol. 2011;668:50–8. doi: 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 15.Niederseer D, Wernly B, Aigner E, Stickel F, Datz C. NAFLD and cardiovascular diseases: Epidemiological, mechanistic and therapeutic considerations. J Clin Med. 2021;10:467. doi: 10.3390/jcm10030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirase T, Node K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am J Physiol Heart Circ Physiol. 2012;302:499–505. doi: 10.1152/ajpheart.00325.2011. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Guo S, Zhang S, Liu A, Shi L, Zhang Y. Matrine attenuates endoplasmic reticulum stress and mitochondrion dysfunction in nonalcoholic fatty liver disease by regulating SERCA pathway. J Transl Med. 2018;16 doi: 10.1186/s12967-018-1685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dusi V, Ghidoni A, Ravera A, De Ferrari GM, Calvillo L. Chemokines and heart disease: A network connecting cardiovascular biology to immune and autonomic nervous systems. Mediators Inflamm. 2016;2016:5902947. doi: 10.1155/2016/5902947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease Evidence from genetic epidemiologic, and clinical studies A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–72. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halcox JP, Banegas JR, Roy C, Dallongeville J, De Backer G, Guallar E, et al. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc Disord. 2017;17:160. doi: 10.1186/s12872-017-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–25. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Tourniaire F, Romier-Crouzet B, Lee JH, Marcotorchino J, Gouranton E, Salles J, et al. Chemokine expression in inflamed adipose tissue is mainly mediated by NF-κB. PLoS One. 2013;8:66515. doi: 10.1371/journal.pone.0066515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313–27. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dabravolski SA, Bezsonov EE, Baig MS, Popkova TV, Orekhov AN. Mitochondrial Lipid Homeostasis at the Crossroads of Liver and Heart Diseases. Int J Mol Sci. 2021;22:6949. doi: 10.3390/ijms22136949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson RE, Kalavalapalli S, Williams CM, Nautiyal M, Mathew JT, Martinez J, et al. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am J Physiol Endocrinol Metab. 2016;310:484–94. doi: 10.1152/ajpendo.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechner K, McKenzie AL, Kränkel N, Von Schacky C, Worm N, Nixdorff U, et al. High-risk atherosclerosis and metabolic phenotype: The roles of ectopic adiposity, atherogenic dyslipidemia, and inflammation. Metab Syndr Relat Disord. 2020;18:176–85. doi: 10.1089/met.2019.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones JG. Hepatic glucose and lipid metabolism. Diabetologia. 2016;59:1098–103. doi: 10.1007/s00125-016-3940-5. [DOI] [PubMed] [Google Scholar]
- 30.Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41:2313–30. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiqui MS, Fuchs M, Idowu MO, Luketic VA, Boyett S, Sargeant C, et al. Severity of nonalcoholic fatty liver disease and progression to cirrhosis are associated with atherogenic lipoprotein profile. Clin Gastroenterol Hepatol. 2015;13:1000–8. doi: 10.1016/j.cgh.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzo M, Berneis K, Corrado E, Novo S. The significance of low-density-lipoproteins size in vascular diseases. Int Angiol. 2006;25:4–9. [PubMed] [Google Scholar]
- 33.Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, et al. Remnant-like particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol. 2018;72:156–69. doi: 10.1016/j.jacc.2018.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dittrich J, Beutner F, Teren A, Thiery J, Burkhardt R, Scholz M, et al. Plasma levels of apolipoproteins C-III, A-IV, and E are independently associated with stable atherosclerotic cardiovascular disease. Atherosclerosis. 2019;281:17–24. doi: 10.1016/j.atherosclerosis.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Zewinger S, Reiser J, Jankowski V, Alansary D, Hahm E, Triem S, et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat Immunol. 2020;21:30–41. doi: 10.1038/s41590-019-0548-1. [DOI] [PubMed] [Google Scholar]
- 36.Libby P, Everett BM. Novel antiatherosclerotic therapies. Arterioscler Thromb Vasc Biol. 2019;39:538–45. doi: 10.1161/ATVBAHA.118.310958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afonso MS, Lavrador MS, Koike MK, Cintra DE, Ferreira FD, Nunes VS, et al. Dietary interesterified fat enriched with palmitic acid induces atherosclerosis by impairing macrophage cholesterol efflux and eliciting inflammation. J Nutr Biochem. 2016;32:91–100. doi: 10.1016/j.jnutbio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Hwang DH, Kim JA, Lee JY. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur J Pharmacol. 2016;785:24–35. doi: 10.1016/j.ejphar.2016.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai HTM, de Oliveira Otto MC, Lee Y, Wu JHY, Song X, King IB, et al. Serial plasma phospholipid fatty acids in the de novo lipogenesis pathway and total mortality, cause-specific mortality, and cardiovascular diseases in the cardiovascular health study. J Am Heart Assoc. 2019;8:012881. doi: 10.1161/JAHA.119.012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. 2016;65:425–43. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131–41. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 42.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98:2133–223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worm N. Beyond body weight-loss: Dietary strategies targeting intrahepatic fat in NAFLD. Nutrients. 2020;12:1316. doi: 10.3390/nu12051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: Cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. 2016;133:2459–502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 46.Scholz GH, Hanefeld M. Metabolic vascular syndrome: New insights into a multidimensional network of risk factors and diseases. Visc Med. 2016;32:319–26. doi: 10.1159/000450866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasumov T, Edmison JM, Dasarathy S, Bennett C, Lopez R, Kalhan SC. Plasma levels of asymmetric dimethylarginine in patients with biopsy-proven nonalcoholic fatty liver disease. Metabolism. 2011;60:776–81. doi: 10.1016/j.metabol.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Carvalho SC, Muniz MT, Siqueira MD, Siqueira ER, Gomes AV, Silva KA, et al. Plasmatic higher levels of homocysteine in non-alcoholic fatty liver disease (NAFLD) Nutr J. 2013;12 doi: 10.1186/1475-2891-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Y, Guan Y, Yang X, Xia Z, Wu J. Association of serum homocysteine levels with histological severity of NAFLD. J Gastrointestin Liver Dis. 2020;29:51–8. doi: 10.15403/jgld-529. [DOI] [PubMed] [Google Scholar]
- 50.Tripodi A, Fracanzani AL, Primignani M, Chantarangkul V, Clerici M, Mannucci PM, et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;61:148–54. doi: 10.1016/j.jhep.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Coulon S, Francque S, Colle I, Verrijken A, Blomme B, Heindryckx F, et al. Evaluation of inflammatory and angiogenic factors in patients with non-alcoholic fatty liver disease. Cytokine. 2012;59:442–9. doi: 10.1016/j.cyto.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20:40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 53.Garbuzenko DV. The role of intestinal microflora in the development of complications of hepatic cirrhosis-associated portal hypertension. Klin Med (Mosk) 2007;85:15–9. [PubMed] [Google Scholar]
- 54.Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2089–105. doi: 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Xu J, Wang X, Ren X, Liu Y. Changes of intestinal bacterial microbiota in coronary heart disease complicated with nonalcoholic fatty liver disease. BMC Genomics. 2019;20:862. doi: 10.1186/s12864-019-6251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandsma E, Kloosterhuis NJ, Koster M, Dekker DC, Gijbels MJJ, van der Velden S, et al. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ Res. 2019;124:94–100. doi: 10.1161/CIRCRESAHA.118.313234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–5. doi: 10.1038/s41586-020-2269-x. [DOI] [PubMed] [Google Scholar]
- 58.Koeth RA, Lam-Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. 2019;129:373–87. doi: 10.1172/JCI94601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–94. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi J, You T, Li J, Pan T, Xiang L, Han Y, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22:185–94. doi: 10.1111/jcmm.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roncal C, Martínez-Aguilar E, Orbe J, Ravassa S, Fernandez-Montero A, Saenz-Pipaon G, et al. Trimethylamine-N-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease. Sci Rep. 2019;9:15580. doi: 10.1038/s41598-019-52082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu C, Xue F, Lian Y, Zhang J, Wu D, Xie N, et al. Relationship between elevated plasma trimethylamine N-oxide levels and increased stroke injury. Neurology. 2020;94:667. doi: 10.1212/WNL.0000000000008862. [DOI] [PubMed] [Google Scholar]
- 64.Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. doi: 10.1038/srep19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite tmao enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–24. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407–17. doi: 10.1038/s41591-018-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reiner MF, Müller D, Gobbato S, Stalder O, Limacher A, Bonetti NR, et al. Gut microbiota-dependent trimethylamine-N-oxide (TMAO) shows a U-shaped association with mortality but not with recurrent venous thromboembolism. Thromb Res. 2019;174:40–7. doi: 10.1016/j.thromres.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Carlsson B, Lindén D, Brolén G, Liljeblad M, Bjursell M, Romeo S, et al. Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2020;51:1305–20. doi: 10.1111/apt.15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Del Campo JA, Gallego-Durán R, Gallego P, Grande L. Genetic and epigenetic regulation in nonalcoholic fatty liver disease (NAFLD) Int J Mol Sci. 2018;19:911. doi: 10.3390/ijms19030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li XL, Sui JQ, Lu LL, Zhang NN, Xu X, Dong QY, et al. Gene polymorphisms associated with non-alcoholic fatty liver disease and coronary artery disease: a concise review. Lipids Health Dis. 2016;15:53. doi: 10.1186/s12944-016-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byrne CD, Targher G. Non-alcoholic fatty liver disease-related risk of cardiovascular disease and other cardiac complications. Diabetes Obes Metab. 2021:29. doi: 10.1111/dom.14484. [DOI] [PubMed] [Google Scholar]
- 72.Lauridsen BK, Stender S, Kristensen TS, Kofoed KF, Køber L, Nordestgaard BG, et al. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J. 2018;39:385–93. doi: 10.1093/eurheartj/ehx662. [DOI] [PubMed] [Google Scholar]
- 73.Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921–37. doi: 10.1007/s00392-020-01709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simons N, Isaacs A, Koek GH, Kuč S, Schaper NC, Brouwers MCGJ. PNPLA3, TM6SF2, and MBOAT7 genotypes and coronary artery disease. Gastroenterology. 2017;152:912–3. doi: 10.1053/j.gastro.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 75.Stols-Gonçalves D, Hovingh GK, Nieuwdorp M, Holleboom AG. NAFLD and atherosclerosis: Two sides of the same dysmetabolic coin? Trends Endocrinol Metab. 2019;30:891–902. doi: 10.1016/j.tem.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA. Cardiac remodeling: Concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol. 2016;106:62–9. doi: 10.5935/abc.20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou J, Bai L, Zhang XJ, Li H, Cai J. Nonalcoholic fatty liver disease and cardiac remodeling risk: Pathophysiological mechanisms and clinical implications. Hepatology. 2021;74:2839–47. doi: 10.1002/hep.32072. [DOI] [PubMed] [Google Scholar]
- 78.Cai J, Zhang XJ, Ji YX, Zhang P, She ZG, Li H. Nonalcoholic fatty liver disease pandemic fuels the upsurge in cardiovascular diseases. Circ Res. 2020;126:679–704. doi: 10.1161/CIRCRESAHA.119.316337. [DOI] [PubMed] [Google Scholar]
- 79.Garbuzenko DV, Belov DV. Non-alcoholic fatty liver disease as an independent factor of cardiometabolic risk of cardiovascular diseases. Exp Clin Gastroenterol. 2021;194:22–34. [Google Scholar]
- 80.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology. 2015;62:773–83. doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Styczynski G, Kalinowski P, Michałowski Ł, Paluszkiewicz R, Ziarkiewicz-Wróblewska B, Zieniewicz K, et al. Cardiac morphology, function, and hemodynamics in patients with morbid obesity and nonalcoholic steatohepatitis. J Am Heart Assoc. 2021;10:017371. doi: 10.1161/JAHA.120.017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garbuzenko DV, Arefyev NO, Belov DV. Restructuring of the vascular bed in response to hemodynamic disturbances in portal hypertension. World J Hepatol. 2016;8:1602–9. doi: 10.4254/wjh.v8.i36.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baffy G, Bosch J. Overlooked subclinical portal hypertension in non-cirrhotic NAFLD: Is it real and how to measure it? J Hepatol. 2022;76:458–63. doi: 10.1016/j.jhep.2021.09.029. [DOI] [PubMed] [Google Scholar]