Abstract
The aim of this study was to search for a specific antibody pattern in sera from patients suffering from Helicobacter pylori-related gastric adenocarcinoma (GAC). The serological response of 22 patients suffering from GAC, 31 patients with gastroduodenal ulcer, and 39 asymptomatic subjects was analyzed using immunoblotting performed with three H. pylori strains: strain ATCC 43579; strain B110, isolated from a patient with ulcers; and strain B225, isolated from a patient with GAC. In addition, the latex agglutination test Pyloriset Dry was used to analyze ambiguous sera. H. pylori seropositivity was 75% in the GAC group, 61.3% in the ulcer group, and 56.4% in the asymptomatic group. Anti-CagA antibodies were found more often in the GAC group (48.8%) and in the ulcer group (47.3%) than in the asymptomatic group (21.2%). These percentages depended on the strain used as an antigen: in the GAC group, the anti-CagA frequencies were 93.3, 40, and 13.3% with strains B225, B110, and ATCC 43579, respectively. Thus the presence of anti-CagA antibodies was increased in patients suffering from H. pylori-related GAC, in particular when the CagA antigen was from a GAC strain. These data suggest the existence of a CagA protein specifically expressed by H. pylori strains isolated from GAC patients.
Gastric adenocarcinoma (GAC) is the second-most-common cancer in the world, with an estimated incidence of 700,000 new cases a year (32). Epidemiological studies suggest that Helicobacter pylori-related gastritis, together with environmental and genetic factors, plays a role in the initiation of GAC (24). H. pylori is a gram-negative, spiral shaped, flagellated microaerophilic bacterium that was identified in the early 1980s (46). It colonizes the stomach of about 50% of all humans and is responsible for the majority of chronic gastritis and peptic ulcer cases (4, 7, 18). A history of H. pylori infection has been found in 50 to 90% of patients with GAC (11, 50). Since 1991, H. pylori has been classified as a group 1 carcinogen, and the relative risk of cancer has been estimated to be 3.6 times higher in patients with H. pylori infection than in noninfected patients (16, 20, 33, 41). Nevertheless, H. pylori infection is more widespread than gastric cancer in Japan, where the prevalence of gastric cancer is high; 0.04% of Japanese subjects who are seropositive for H. pylori suffer from gastric carcinoma (1). Recently, the involvement of H. pylori infection in gastric carcinogenesis has been confirmed in a Mongolian gerbil experimental model (21, 47). Thus, Koch's postulates for H. pylori as a cause of GAC seem to be fulfilled (45).
These results led to research of the specific determinants of both host and bacterium that predispose H. pylori-infected individuals to GAC. Two virulence factors have been found more frequently in H. pylori strains isolated from patients with ulcers or cancer than in strains isolated from patients with gastritis (5, 36, 44, 49). The first one is the vacuolating cytotoxin (VacA); the second one is the cytotoxin-associated antigen (CagA) that reflects the presence of the CagA pathogenicity island, including about 30 genes of unknown function (9).
We previously showed that a specific antibody response pattern is found in the sera from patients suffering from H. pylori-associated peptic ulcer (3). The aim of this study was to extend these data and to search for specific antibody patterns in sera from H. pylori patients suffering from GAC; subjects suffering from peptic ulcer and asymptomatic subjects served as controls. To investigate antibody patterns, immunoblot assays were carried out with three H. pylori strains: one strain isolated from a patient with GAC, one strain isolated from a patient with duodenal ulcers, and the reference strain, ATCC 43579.
MATERIALS AND METHODS
Patients.
Three groups of patients were included in this study. The patients were hospitalized at the University Hospital Center of Brest, France, between 1997 and 1998. The first group included 20 patients (9 men and 11 women) with GAC. The median age was 75.3 years (range, 55 to 95 years) for the men and 73.1 years (range, 53 to 83 years) for the women. Twelve GACs were intestinal-type adenocarcinomas, and eight cancers were diffuse-type adenocarcinomas, according to the Lauren classification (27). The second group included 31 patients (26 men and 5 women) with gastroduodenal ulcers. The median age was 61 years (range, 18 to 80 years) for the men and 72 years (range, 51 to 84 years) for the women. The third group included 39 asymptomatic patients (17 men and 22 women). The median age in this group was 70 years (range, 56 to 87 years) for the men and 76.3 years (range, 63 to 91 years) for the women. No significant demographic differences between the GAC group and the asymptomatic group were present. Serum samples from the 90 patients were collected, aliquoted, and stored frozen at −70°C.
Serological assays.
The presence of antibodies to H. pylori in serum was determined using the rapid latex agglutination test Pyloriset Dry (Orion Diagnostica, Fumouze, France) in accordance with the manufacturer's instructions and a home-made immunoblot assay with saline extracts from three strains: ATCC 43579, B110, and B225 (29). Strain B110 was isolated from a patient with a duodenal ulcer, and strain B225 was isolated from a patient with GAC. The three strains were cagA positive and had the s1 vacA signal sequence (2, 38). They were consequently considered virulent. For the preparation of antigens, H. pylori strains were cultivated on Pylori agar (bioMérieux, Marcy l'Etoile, France) and incubated at 37°C under microaerobic conditions for 3 to 4 days. Saline extracts corresponding to the water-soluble and surface-exposed antigens were prepared according to a previously described method (3). Briefly, bacterial cells were harvested in sterile 0.15 M NaCl, vortexed for 5 min, and centrifuged (10,000 × g for 10 min at 4°C). The protein concentration of supernatants was determined, and the saline extracts were stored frozen at −70°C. Using a mini-gel apparatus (Bio-Rad, Richmond, Calif.), we carried out sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 50 μg of saline extracts per gel (26). Molecular mass markers ranging from 14 to 94 kDa (Pharmacia, Uppsala, Sweden) were included on each gel. After migration, proteins were electrotransferred to a nitrocellulose membrane. The membranes were cut into strips and incubated for 1 h at room temperature with serum samples or with monospecific serum (see below) at a 1:100 dilution. They were then incubated for 1 h at room temperature with alkaline phosphatase-conjugated anti-human, anti-rabbit, or anti-mouse immunoglobulin G (Dakopatts, Copenhagen, Denmark). Color reactions were developed with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (Sigma, St. Quentin Fallavier, France).
To aid identification of the immunoreactive bands detected by the patient's serum, a set of eight monospecific polyclonal rabbit sera raised to the 20-kDa ferritin-like protein (Felp), the 26- and 35-kDa antigens, the 54-kDa catalase, the 60-kDa HspB, the 76-kDa fumarate reductase, the 87-kDa VacA, and the 125 kDa CagA antigens and two murine monoclonal antibodies raised to the 30-kDa UreA and 66-kDa UreB antigens were used. These sera were generously supplied by AVENTIS Pasteur (Marcy l'Etoile, France). Immunoreactive bands were also identified with a calibration curve constructed by plotting the migration distance of the various markers versus their respective molecular masses. Immunoblots were considered to be positive for antibodies to H. pylori when three or more immunoreactive bands were present and when at least one of these three bands corresponded to CagA (125 kDa), HspB (60 kDa), UreB (66 kDa), or UreA (30 kDa) antigens (3). The Pyloriset Dry test was used to analyze ambiguous sera: those sera that were positive by Western blotting with only one H. pylori strain were considered to be positive if positive with the Pyloriset Dry test also.
Statistical analysis.
The chi-square test was used to compare the frequencies of the immunoreactive bands. Factorial analysis of correspondence was done for every immunoblot series, one series for each of the three H. pylori strains (19, 28, 42). This analysis was used to compare entire immunoblot patterns. The data of the immunoblots were transferred into three two-way tables, one table for each strain. Each table had 90 rows, with 1 row for each serum, and 10 columns, with 1 column for each immunoreactive band. The presence or the absence of each band was encoded as follows: present = 2, absent = 1. Factorial analysis of correspondence (SPAD.N software; Cisia, Saint-Mandé, France) was performed from each table, first considering all sera and then only the seropositive sera.
RESULTS
Protein profiles of the saline extracts used as antigens in immunoblots.
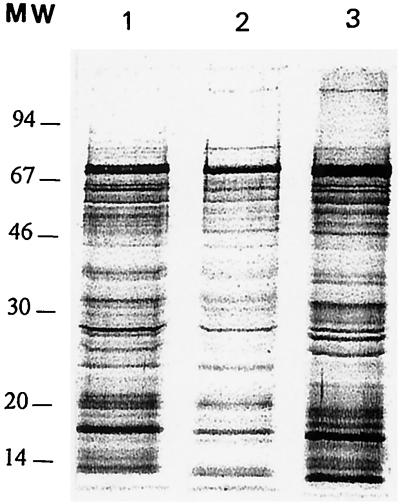
Saline extracts prepared from the three H. pylori strains included in the study were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 12% acrylamide gel. They showed qualitative and quantitative differences for numerous proteins (Fig. 1). In particular, the CagA protein was present in a larger amount in strain ATCC 43579 than in strains B225 and B110.
FIG. 1.
Electrophoretic profiles of the saline extracts prepared from the three H. pylori strains: B110 (lane 1), B225 (lane 2), and ATCC 43759 (lane 3). Proteins were resolved in a 12% acrylamide gel and silver stained. MW, molecular weight markers in thousands.
H. pylori serological status of the 90 patients.
The sera of 31 subjects (34.4%) were negative by both serological tests, the agglutination assay and immunoblotting. The agglutination assay was positive in the sera of 44 (48.9%) of the 90 subjects. Depending on the H. pylori strain used as an antigen in the immunoblot, the number of positive sera ranged from 49 (54.5%) with strain B225, 51 (56.7%) with strain ATCC 43579, to 52 (57.8%) with strain B110. The sera of 42 (46.7%) of the 90 subjects were positive with all three strains; the sera of 11 of 90 subjects (12.2%) were positive with two strains. Among these 53 subjects, the sera of 41 were positive by the agglutination assay. These 53 subjects were considered seropositive. The sera of six subjects (6.7%) were positive with only one strain; of these six subjects, three had sera positive by the agglutination test and were therefore considered seropositive; the three others were considered seronegative.
Thus, 56 of 90 (62.2%) subjects were considered H. pylori seropositive: 15 of the 20 patients belonging to the GAC group (75.0%), 19 of the 31 patients belonging to the ulcer group (61.3%), and 22 of the 39 subjects belonging to the control group (56.4%). The prevalence of seropositive subjects in the three groups was not significantly different. There was no influence of sex on seropositivity: 60.5% of the women and 63.5% of the men were seropositive. H. pylori seropositivity increased with age: 25% of those under 50 and 64% of those over 50 were seropositive. In the GAC group, there was no significant difference in seropositivity between patients with diffuse-type tumors and those with intestinal-type tumors.
Antibody patterns of the 90 patients.
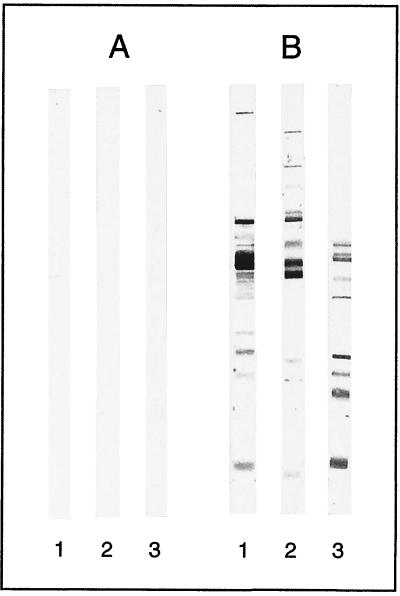
For each serum sample, the number and nature of the immunoreactive bands observed on the blots varied substantially according to the H. pylori strain used to prepare the antigen. The average number of bands was 5.5, 10.5 and 11.75 with B225, B110, and ATCC 43579, respectively. Figure 2 shows the reactivity patterns of one negative and one positive serum sample tested by immunoblotting with the three H. pylori strains.
FIG. 2.
Variability of the immunoblots obtained with one seronegative patient (A) and one seropositive patient (B) according to the three H. pylori stains used as antigens: B110 (lanes 1), B225 (lanes 2), and ATCC 43759 (lanes 3).
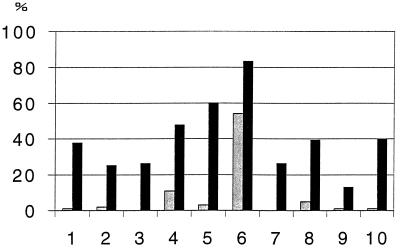
We focused our study on the 10 bands which were most often encountered on the blots. These bands, identified by their molecular mass and by reactivity with monospecific antisera, corresponded to eight antigens identified as CagA, VacA, fumarate reductase, UreB, HspB, catalase, UreA, and ferritin-like protein and to two unidentified antigens of 35 and 26 kDa, respectively. For each patient serum sample, the frequencies of each band obtained with each of the three antigenic preparations were established, and the mean frequency of each band was calculated (Fig. 3). Significant differences of mean frequencies between seropositive and seronegative subjects were found for all antibodies. Catalase, HspB, and UreB were the antigens that were most often recognized by sera from seropositive subjects. However, antibody to catalase had a low level of specificity, since it was detected in 83.3% of the seropositive subjects but also in 55% of the seronegative subjects. Antibody to HspB had the best ratio of sensitivity to specificity.
FIG. 3.
Mean frequencies (as percentages) of the 10 immunoreactive bands detected by immunoblot assay in the sera of 56 seropositive subjects (columns in black) and the 34 seronegative subjects (columns in grey). Antibodies were directed to CagA (columns 1), VacA (columns 2), fumarate reductase (columns 3), UreB (columns 4), HspB (columns 5), catalase (columns 6), p35 (columns 7), UreA (columns 8), p26 (columns 9), and ferritin like protein (columns 10).
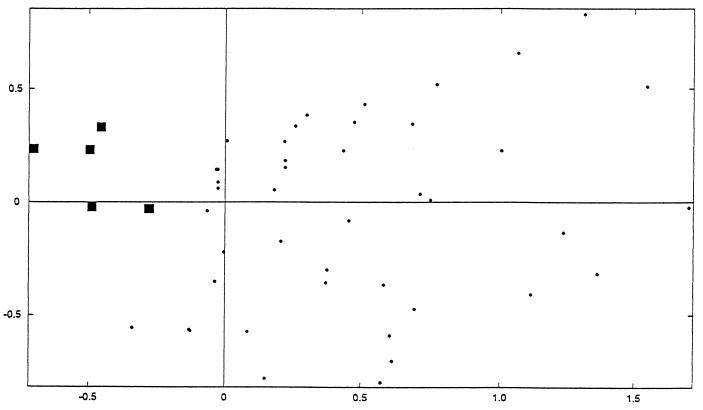
Factorial analysis of correspondence performed with the data of the immunoblots obtained with the three strains confirmed the distinction between the immunoblots of seropositive and seronegative subjects. Data points for the latter were clearly focused on the factorial plane by the negative values of the F1 axis (Fig. 4).
FIG. 4.
Factorial analysis of correspondence of the 90 immunoblots performed with strain B110. ■, projection of the 34 seronegative subjects; ●, projection of the 56 seropositive subjects.
Antibody patterns of seropositive subjects.
We next compared the antibody patterns of the seropositive subjects, including 15 GAC patients, 19 ulcer subjects, and 22 asymptomatic control subjects, to search for a specific pattern of GAC. Of the 10 antibodies studied, the mean frequencies of only 3 antibodies depend on the clinical origin of the serum. Anti-CagA antibodies were found more often in sera from the GAC patients (48.8%) and the ulcer subjects (47.3%) than in sera from asymptomatic subjects (21.2%) (P < 0.0001). Anti-VacA antibodies were more frequent in the asymptomatic control subjects (37.8%) than in the ulcer group (14%) and in the GAC group (20%) (P < 0.0001). Anti-ferritin-like protein antibodies were more frequent in the ulcer group (68.8%) than in the GAC group (31%) and in the asymptomatic control group (33.3%) (P < 0.00001).
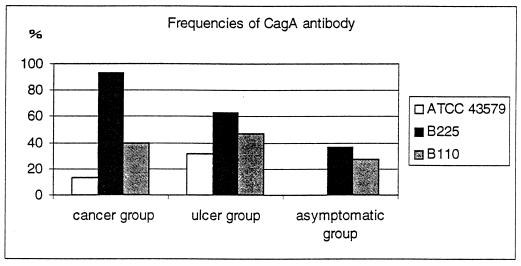
There was no statistical association between the frequencies of the antibodies and the strain used to prepare the antigen, except for anti-CagA antibodies, which were statistically more frequently detected in the three groups of patients when GAC strain B225 was used as an antigen (Fig. 5). In particular in the GAC group, anti-cagA antibodies were present in 93.3, 40, and 13% of the sera when strains B225, B110, and ATCC 43579 were used as antigens, respectively. Thus, anti-CagA antibodies of subjects suffering from GAC seemed to be more easily detected when immunoblotting was performed with a strain isolated from a patient suffering from the same disease.
FIG. 5.
Frequency (as percentages) of anti-CagA antibody in the three groups according to the H. pylori strain used as an antigen to perform the immunoblots.
Finally, factorial analysis of correspondence restricted to seropositive subjects was realized to analyze entire immunoblot patterns. Data points for seropositive patients were widely scattered on the plan, and the data did not focus according to the clinical origin of the patients, whatever the strain used as an antigen (data not shown).
DISCUSSION
In this study, we analyzed the serological responses of patients suffering from H. pylori-related GAC. The age and sex distributions of GAC patients studied were representative of our area. The risk that asymptomatic subjects would be affected by GAC was very low; hence, our control group can be considered free from cancer, despite the fact that no upper gastroduodenal endoscopy was performed.
The seroprevalence of H. pylori and its epidemiological characteristics were similar to the data reported by others (13, 14). We found no significant difference in H. pylori seroprevalence between GAC patients with diffuse-type tumors and those with intestinal-type tumors, in agreement with some studies but not with others (8, 10, 22, 34). The seroprevalences in the three clinical groups were also similar to the results of other studies (10, 13, 40).
Serology is recognized as one of the most reliable methods for diagnosis of H. pylori infection. Therefore, the seropositive patients may be considered H. pylori infected, and the seronegative may be considered H. pylori free (23, 35, 43). Among the serological methods used to study the antibody response to H. pylori, immunoblotting is certainly one of the most powerful (3, 43). This method allows the detection of antibodies and a determination of the specificity of these antibodies. The interpretation of the antibody patterns in H. pylori-infected patients has not been well established so far, even if some attempts have already been made (6, 15). Nevertheless, because the antibody response reflects the features of both the infecting strain and the host response, it may be a helpful tool to predict the risk of an H. pylori-infected patient developing severe disease. For many years, several studies had sought correlations between antibodies against H. pylori and diseases related to this bacterium, using enzyme-linked immunosorbent assay or immunoblot assay, but only one considered all antibodies on immunoblot patterns of GAC patients (25). In our study, we analyzed all antibody patterns by immunoblotting using the factorial analysis of correspondence method. We did not find an antibody pattern specific for GAC. Nevertheless, we found an association between antibodies to ferritin-like protein and ulcer disease (12, 17). To our knowledge, this association has never been reported. In contrast to the majority of the studies, we found that anti-VacA antibodies were more often present in sera from asymptomatic subjects than in sera from the two other patient groups (3, 37, 39, 50). At present, we have no explanation for this finding. As with the majority of the studies, we found an association between anti-CagA antibodies and GAC or gastroduodenal ulcer (11, 25, 37, 39, 48). There was no correlation between the expression of the CagA proteins and their recognition by human serum. Strain ATCC 43579 expresses high CagA protein levels, and strain B225 expresses lower levels. The CagA protein was not observed with strain B110 under our analysis conditions (Fig. 1). However, CagA was more frequently recognized when strain B225 was used as an antigen, a finding which indicates qualitative differences in the CagA proteins.
The most adequate immunoblotting assay would probably be realized with an antigenic extract prepared from the patient strain. However, the infecting strains are not always available and, practically speaking, such an assay would not be feasible. Thus, it may be helpful to define what kind of strain is more relevant in obtaining an antigenic preparation designed for immunoblot assays. Our results suggest that in searching for anti-CagA antibodies in the sera of patients suffering from GAC, the best choice would be a strain isolated from a patient suffering from the same disease. Moreover, the specific recognition of anti-CagA antibodies present in sera from GAC subjects and the CagA antigen of strain B225 could suggest that GAC-associated H. pylori strains express a specific type of CagA protein. To confirm these findings, it would be interesting to test more strains isolated from patients in the three clinical groups included in the study and also to analyze and compare the sequence of the cagA genes in the three strains. The structure of the cagA gene of H. pylori strain B225 is currently under investigation.
In conclusion, we confirmed the high diversity of the antibody response to H. pylori and the strong association between anti-CagA and the presence of GAC. This association is stronger if the CagA protein used as an antigen comes from a strain isolated from a patient with GAC, suggesting the existence of a type of CagA protein more implicated in gastric carcinogenesis.
ACKNOWLEDGMENTS
We thank the Ligue contre le Cancer and the Université de Poitiers for their financial support.
We are very grateful to B. J. Appelmek for his help in the improvement of the manuscript.
REFERENCES
- 1.Asaka M, Kudo M, Kato M, Sugiyama T, Takeda H. Long term Helicobacter pylori infection from gastritis to gastric cancer. Aliment Pharmacol Ther. 1998;12:9–15. doi: 10.1111/j.1365-2036.1998.00007.x. [DOI] [PubMed] [Google Scholar]
- 2.Atherton J C, Cao P, Peek R M, Jr, Tummuru M K, Blaser M J, Cover T L. Mosaicism in vacuolation cytotoxin alleles of Helicobacter pylori. Association of vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 3.Aucher P, Petit M L, Mannant P R, Pezennec L, Babin P, Fauchère J L. Use of immunoblot assay to define serum antibody patterns associated with Helicobacter pylori infection and with H. pylori-related ulcers. J Clin Microbiol. 1998;36:931–936. doi: 10.1128/jcm.36.4.931-936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron J H, Logan R P H. Infection by Helicobacter pylori is the major cause of duodenal ulcer. Proc R Coll Physicians Edinb. 1994;24:21–36. [Google Scholar]
- 5.Basso D, Navaglia F, Brigato L, Piva M G, Toma A, Greco E, Di Mario F, Galeotti F, Roveroni G, Corsini A, Plebani M. Analysis of Helicobacter pylori vacA and cagA genotypes and serum antibody profile in benign and malignant gastroduodenal diseases. Gut. 1998;43:182–186. doi: 10.1136/gut.43.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazillou M, Fendri C, Castel O, Ingrand P, Fauchère J L. Serum antibody response to the superficial and released components of Helicobacter pylori. Clin Diagn Lab Immunol. 1994;1:310–317. doi: 10.1128/cdli.1.3.310-317.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser M J, Chyou P H, Nomura A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res. 1995;55:562–565. [PubMed] [Google Scholar]
- 8.Blaser M J, Perez-Perez G I, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stermmermann G N, Nomura A. Infection with strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 9.Censini S, Lange C, Xiang Z, Crabtree J, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cagA, a pathogenicity island of Helicobacter pylori, encodes type-I specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craanen M E, Blok P, Dekker W, Tytgat G N J. Helicobacter pylori and early gastric cancer. Gut. 1994;35:1372–1374. doi: 10.1136/gut.35.10.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtee J E, Wyatt J I, Sobala G M, Miller G, Tompkins D S, Primrose J N, Morgan A G. Systemic and mucosal humoral responses to Helicobacter pylori in gastric cancer. Gut. 1993;34:1339–1343. doi: 10.1136/gut.34.10.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doig P, Austin J W, Trust T J. The Helicobacter pylori 19.6-kilodalton protein is an iron-containing protein resembling ferritin. J Bacteriol. 1993;175:557–560. doi: 10.1128/jb.175.2.557-560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eurogast Study Group. An international association between Helicobacter pylori and gastric cancer. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 14.Eurogast Study Group. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut. 1993;34:1672–1676. doi: 10.1136/gut.34.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulde M, Schrôder J P, Sobe D. Serodiagnosis of Helicobacter pylori infections by detection of immunoglobulin G antibodies using an immunoblot technique and enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1992;11:589–594. doi: 10.1007/BF01961664. [DOI] [PubMed] [Google Scholar]
- 16.Forman D, Newell D G, Fullerton F, Yarnell J G W, Stacey A R, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazier B A, Pfeifer J D, Russel D G, Falk P, Olsen A N, Hammar M, Westblom T U, Normark S J. Paracrystalline inclusions of a novel ferritin containing nonheme iron, produced by the human gastric pathogen Helicobacter pylori: evidence for a third class of ferritins. J Bacteriol. 1993;175:966–972. doi: 10.1128/jb.175.4.966-972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham D Y. Helicobacter pylori: its epidemiology and its role in duodenal ulcer disease. J Gastroenterol Hepatol. 1991;6:105–113. doi: 10.1111/j.1440-1746.1991.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 19.Greenacre M J. Theory and applications of correspondences. London, England: Academic Press; 1984. p. 364. [Google Scholar]
- 20.Hansson L E, Engstrand L, Nyren O, Evans D J, Lindgren A, Bergstrôm R, Anderson B, Athlin L, Bendtsen O, Tracz P. Helicobacter pylori infection: independent risk factor of gastric adenocarcinoma. Gastroenterology. 1993;105:1098–1103. doi: 10.1016/0016-5085(93)90954-b. [DOI] [PubMed] [Google Scholar]
- 21.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 22.Huang J Q, Sridhar S, Chen Y, Hunt R H. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 23.Jensen A K V, Andersen L P, Wachman C H. Evaluation of eight commercial kits for Helicobacter pylori IgG antibody detection. APMIS. 1993;101:795–801. [PubMed] [Google Scholar]
- 24.Joossens J, Geboers J. Diet and environment in the etiology of gastric cancer. In: Levin B, Riddel R H, editors. Frontiers in gastrointestinal cancer. New York, N.Y: Elsevier; 1984. pp. 168–183. [Google Scholar]
- 25.Klaamas K, Held M, Wadstrôm T, Lipping A, Kurtenkov O. IgG immune response to Helicobacter pylori antigens in patients with gastric cancer as defined by elisa and immunoblotting. Int J Cancer. 1996;67:1–5. doi: 10.1002/(SICI)1097-0215(19960703)67:1<1::AID-IJC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 28.Lebart L, Morineau A, Warwick K M. Multivariate descriptive analysis and related techniques for large matrices. New York, N.Y: Wiley Interscience; 1984. [Google Scholar]
- 29.Midolo P D, Lambert J R, Russel E G, Lin S K. A practical single sample dry latex agglutination test for Helicobacter pylori antibody detection. J Clin Pathol. 1995;48:969–971. doi: 10.1136/jcp.48.10.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell H A, Hazell S L, Kolesnikow T, Mitchell J, Frommer D. Antigen recognition during progression from acute to chronic infection with a cagA-positive strain of Helicobacter pylori. Infect Immun. 1996;64:1166–1172. doi: 10.1128/iai.64.4.1166-1172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson I, Ljungh A, Aleljung P, Wadstrôm T. Immunoblot assay for serodiagnosis of Helicobacter pylori infections. J Clin Microbiol. 1997;35:427–432. doi: 10.1128/jcm.35.2.427-432.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkin P, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1993. Int J Cancer. 1995;54:594–606. doi: 10.1002/ijc.2910540413. [DOI] [PubMed] [Google Scholar]
- 33.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 34.Parsonnet J, Vandersteen D, Goates J, Sibley R K, Pritikin J, Chang Y. Helicobacter pylori infection in intestinal and diffuse type gastric adenocarcinomas. J Natl Cancer Inst. 1991;83:640–643. doi: 10.1093/jnci/83.9.640. [DOI] [PubMed] [Google Scholar]
- 35.Pronovost A D, Rose S L, Pawlak J W, Robin H, Schneider R. Evaluation of a new immunodiagnostic assay for Helicobacter pylori antibody detection: correlation with histopathological and microbiological results. J Clin Microbiol. 1994;32:46–50. doi: 10.1128/jcm.32.1.46-50.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudi J, Kolb C, Maiwald M, Kuck D, Sieg A, Galle P R, Stremmel W. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J Clin Microbiol. 1998;36:944–948. doi: 10.1128/jcm.36.4.944-948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudi J, Kolb C, Maiwald M, Zuna I, von Herbay A, Galle P R, Stremmel W. Serum antibodies against Helicobacter pylori proteins VacA and CagA are associated with increased risk for gastric cancer. Dig Dis Sci. 1997;42:1652–1659. doi: 10.1023/a:1018849112533. [DOI] [PubMed] [Google Scholar]
- 38.Salaün L, Audibert C, Le Lay G, Burucoa C, Fauchere J L, Picard B. Panmictic structure of Helicobacter pylori demonstrated by the comparative study of six genetic markers. FEMS Microbiol Lett. 1998;161:231–239. doi: 10.1111/j.1574-6968.1998.tb12953.x. [DOI] [PubMed] [Google Scholar]
- 39.Shimoyama T, Fukuda S, Tanaka M, Mikami T, Munakata A, Crabtree J E. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol. 1998;51:225–228. doi: 10.1136/jcp.51.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siman J H, Forsgren A, Berglund G, Floren C H. Association between Helicobacter pylori and gastric cancer in the city of Malmö, Sweden. Scand J Gastroenterol. 1997;32:1215–1221. doi: 10.3109/00365529709028150. [DOI] [PubMed] [Google Scholar]
- 41.Talley N J, Zinsmeister A R, Weaver A, Dimagno E P, Carpenter H A, Perez-Perez G I, Blaser M J. Gastric adenocarcinoma and Helicobacter pylori infection. J Natl Cancer Inst. 1991;83:1734–1739. doi: 10.1093/jnci/83.23.1734. [DOI] [PubMed] [Google Scholar]
- 42.Tenehaus M, Young F W. An analysis and synthesis of multiple correspondence analysis, optimal scaling, dual scaling, homogeneity analysis, and other methods for quantifying categorical multivariate data. Psychometrika. 1985;50:91–119. [Google Scholar]
- 43.Vaira D, Holton J, Menegatti M, Landi F, Ricci C, Ali A, Landi F, Moretti C, Miglioli M. Blood tests in the management of Helicobacter pylori infection. Gut. 1998;43(Suppl. 1):S39–S46. doi: 10.1136/gut.43.2008.s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Doorn L J, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 45.Wang T C, Fox J G. Helicobacter pylori and gastric cancer: Koch's postulates fullfilled? Gastroenterology. 1998;115:780–783. doi: 10.1016/s0016-5085(98)70159-3. [DOI] [PubMed] [Google Scholar]
- 46.Warren J B, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 47.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 48.Webb P M, Forman D, Newell D, Covacci A, Crabtree J E. European Study Group. An international association between prevalence of infection with cagA positive strains of H. pylori and mortality from gastric cancer. Gut. 1996;39(Suppl. 2):A1. [Google Scholar]
- 49.Weel J F L, van der Hulst R W M, Gerrits Y, Roorda P, Feller M, Dankert J, Tytgat G N, van der Ende A. The relationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996;173:1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- 50.Whiting J L, Hallissey M T, Fielding J W L, Dunn J. Screening for gastric cancer by Helicobacter pylori serology: a retrospective study. Br J Surg. 1998;85:408–411. doi: 10.1046/j.1365-2168.1998.00581.x. [DOI] [PubMed] [Google Scholar]