Abstract
Early exploratory behaviors have been proposed to facilitate children’s learning, impacting motor, cognitive, language, and social development. This study related the performance of behaviors used to explore oneself to behaviors used to explore objects, and then related both types of exploratory behaviors to motor, language, and cognitive measures longitudinally from 3 through 24 months of age via secondary analysis of an existing dataset. Participants were 52 children (23 full-term, 29 preterm). Previously published results from this dataset documented delays for preterm relative to full-term infants in each assessment. The current results related performance among the assessments throughout the first two years of life. They showed that the developmental trajectories of behaviors children used for self-exploration closely related to the trajectories of behaviors they employed to explore objects. The trajectories of both self and object exploration behaviors significantly related to trajectories of children’s motor, language, and cognitive development. Specifically, significant relations to global development were observed for self-exploratory head lifting, midline head and hand positioning, hand opening, and behavioral variability, as well as for object-oriented bimanual holding, mouthing, looking, banging, manipulating, and transferring of objects, as well as behavioral intensity and variability. These results demonstrate continuity among the early exploratory behaviors infants perform with their bodies alone, exploratory behaviors with portable objects, and global development. The findings identify specific self- and object-exploration behaviors that may serve as early indicators of developmental delay and could be targeted by interventions to advance motor, language, and cognitive outcomes for infants at risk for delay.
Keywords: infancy, exploration, motor, language, cognitive, development
The purpose of this study was to document the dynamic developmental relations among early exploration of self, exploration of objects, and motor, language, and cognitive development during infancy via secondary analysis of an existing dataset. This is one of the few studies to explore these behaviors in combination and longitudinally across the first two years of life. To capture a range of behavioral performance, we included a sample of participants with varying levels of risk for developmental delay (children born full-term or preterm). The results of the self-exploration, object exploration, and developmental assessments have been previously published independently of one another, highlighting developmental delays and learning differences for the preterm group relative to the full-term group in each assessment (Babik, Galloway, & Lobo, 2017; Lobo, Kokkoni, Cunha, & Galloway, 2015; Lobo, Paul, Mackley, Maher, & Galloway, 2014).By contrast, this paper presents these data in relation to one another for the first time. Understanding of the relations among self-exploration behaviors, object exploration behaviors, and global development can help identify specific behaviors that may serve as early indicators of developmental delay to be targeted by interventions aimed at advancing motor, language, and cognitive outcomes for infants at risk for delay.
Theory Underlying This Research
This research was motivated by the dynamic systems theory (DST) and its principle that the behaviors exhibited by a child are the product of a dynamic system constrained and shaped by a variety of factors including the child’s changing body dynamics, the child’s previously developed skills and experiences, environmental affordances and opportunities, and timing effects (Smith & Thelen, 2003; Thelen, 1990; Thelen & Smith, 1994). The DST proposes continuity among behavioral performance in development, as common factors influence behavioral performance at any one point in time and earlier experiences shape the developmental landscape from which future behaviors emerge. For example, crawling infants who spend most of their day moving their arms in an alternating fashion for locomotion tend to grasp objects with one hand, while new walkers, who locomote with their arms symmetrically in a high-guard position, more often grasp objects with two hands during a stationary reaching task (Corbetta & Bojczyk, 2002). The current research proposes that a similar continuity should be observed across the tasks of non-object-oriented and object-oriented exploration.
The current research is also motivated by the principles of grounded cognition (Rakison & Woodward, 2008; Smith & Gasser, 2005). Both DST and grounded cognition suggest that learning across developmental domains occurs through one’s daily perceptual-motor experiences. For example, infants learn to segregate objects and understand causal relations based on their manual experience with objects (Lobo & Galloway, 2008; Needham, 2002); they improve their social and spatial skills through active locomotion (Campos et al., 2000; Clearfield, 2011; Oudgenoeg-Paz & Rivière, 2014). When infants perform exploratory behavior, they have opportunities to gather information and learn about their bodies, objects, events, and others; this knowledge, in turn, should facilitate their motor, language, and cognitive development.
Dynamic Relations Between Self- and Object-Oriented Exploratory Behaviors
When not directly interacting with people or portable objects, children engage in exploration of their bodies (e.g., Babik et al., 2017). For the purpose of this paper, we label exploration of self as non-object-oriented (NOO) exploration and operationally define it as the manual and visual behaviors children engage in when not directly interacting with portable objects (Babik et al., 2017). Note that exploration of self consists not only of tactile, visual, and oral exploration of self (i.e., touching own body with the hand, looking at the hand, mouthing the hand), but also exploration of the affordances of one’s own postural and manual capabilities (i.e., opening and closing the hand, holding the head up and in midline, holding the hands in midline). By contrast, we label exploration of objects as object-oriented (OO) exploration and operationally define that as the manual and visual behaviors children engage in when directly interacting with portable objects. Infants spend more time engaged in NOO exploration in early development and increase their OO exploration with age (Babik et al., 2017; Lobo et al., 2015). It is important to document relations between these behaviors to better understand the processes through which the OO skills that are important for cognitive and language development emerge and develop (Jouen & Molina, 2005; Lobo & Galloway, 2008; Zuccarini et al., 2017).
One important type of NOO exploration involves exploration of one’s body position in space, which facilitates the child’s development of postural control and mobility. Developing postural and motor competencies can impact the child’s perception-action system, resulting in altered patterns of reaching and object exploration. For example, the mastery of sitting and the emergence of crawling shifted children’s reaching preferences from bimanual to unimanual (Goldfield, 1993; Rochat, 1992), whereas the acquisition of walking coincided with an increase in bimanual reaching (Babik, Campbell, & Michel, 2014; Corbetta & Bojczyk, 2002; Corbetta & Thelen, 1999).
Postural advances can also influence the development of visual-manual skills. For example, engaging in NOO exploration to learn to control the head and trunk enables visual fixation and tracking of objects and faces (Bertenthal & von Hofsten, 1998; Bloch & Carchon, 1992; van Beck, Hopkins, Hoeksma, & Samson, 1994; von Hofsten & Rosander, 1997). Postural control also enables visual attention to the hands, which, in turn, allows multimodal visual, tactile, and proprioceptive feedback that stimulates the development of visual-motor coordination, reaching, and object exploration (Barrett, Traupman, & Needham, 2008; Corbetta & Snapp-Childs, 2009; Hopkins & Ronnqvist, 2002; McCarty & Ashmead, 1999; Pogetti, de Souza, Tudella, & Teixeira, 2013; Rochat & Bullinger, 1994; Rochat & Goubet, 1995; Thelen & Spencer, 1998).
Hand position is also imperative for object exploration. Engaging in NOO exploration to learn to manage different hand postures facilitates children’s successful grasping of objects (Lobo & Galloway, 2013; Needham, Barrett, & Peterman, 2002; Thomas et al., 2015). Open hands allow children to gather important haptic information to guide their early reaching behavior (Field, 1977; Lasky, 1977). Children’s early NOO exploration touching surfaces and their bodies provides them with haptic and proprioceptive feedback, increases their body awareness, and is hypothesized to be an important precursor of future reaching and grasping behaviors (Corbetta & Snapp-Childs, 2009; Corbetta, Thurman, Wiener, Guan, & Williams, 2014; DiMercurio, Connell, Clark, & Corbetta, 2018; Thelen et al., 1993; Thomas et al., 2015). In addition, early NOO exploration of hand-to-mouth behavior supports the development of coordination required for future object-directed reaching, grasping, and self-feeding (Lew & Butterworth, 1997; Rochat, 1993). Thus, early NOO exploration allows children to gather information about the capabilities of their bodies, affordances of surrounding surfaces, and possible body-environment interactions, all of which inform children’s early learning and form the foundation for OO exploration (Bertenthal & von Hofsten, 1998; Gibson, 1988; Thelen & Spencer, 1998).
Dynamic Relations Between Exploratory Behavior and Motor, Language, and Cognitive Development
Previous research suggests a strong relation between early exploration and future cognitive outcomes. Object exploration allows for the uptake and comparison of information across sensory modalities which can enhance learning and cognition (Adamson, Bakeman, & Deckner, 2004; Bahrick, Lickliter, & Flom, 2004; Baumgartner & Oakes, 2013; Needham et al., 2002; Soska, Adolph, & Johnson, 2010; Wilcox, Woods, Chapa, & McCurry, 2007). For example, the coupling of manual and visual activity while reaching for and manipulating objects facilitates the development of goal-directedness (Case-Smith, Bigsby, & Clutter, 1998; Gibson & Pick, 2000). Goal-directed multimodal exploration enables learning about object properties, affordances, and relations among objects; this, in turn, facilitates the development of advanced cognitive constructs, including object discrimination and categorization, object permanence, and causal relations (Bahrick et al., 2004; Baumgartner & Oakes, 2013; Bushnell & Boudreau, 1993; Gibson, 1988; Lobo & Galloway, 2008; Needham et al., 2002; Piaget, 1953; Ruff, McCarton, Kurtzberg, & Vaugham, 1984; Smith & Sheya, 2010; Soska et al., 2010; Thelen, 1990; Wilcox et al., 2007).
Positive relations have also been shown between early postural control or object manipulation and language development. The emergence of unsupported sitting corresponded with children’s transition from highly variable vocalizations to patterned speech (Iverson, 2010). Also, object manipulation, especially that involving mouthing, was shown to provide children with opportunities to produce more sophisticated vocalizations (Bates & Dick, 2002; Fagan & Iverson, 2007; Iverson, 2010; Iverson & Thelen, 1999) and improve their language outcomes (Zuccarini et al., 2017). Thus, NOO and OO exploration can provide opportunities for children to experience enriched oral, auditory, and proprioceptive feedback that can facilitate their language development (Iverson, 2010).
Children with poor postural and visual-manual control often show delayed object exploration and missed opportunities to explore and learn, placing them at risk for motor, language, and cognitive developmental delays (Cioni et al., 1997; Dusing & Harbourne, 2010; Gibson, 1988; Heathcock, Bhat, Lobo, & Galloway, 2004; Landry & Chapieski, 1988; Lobo et al., 2015; Soska & Adolph, 2014; Wijnroks & van Veldhoven, 2003). For example, children born preterm showed delays in their postural control and multimodal exploration of self (Babik et al., 2017; Cioni et al., 1997; Dusing & Harbourne, 2010; Fetters, Sapir, Chen, Kubo, & Tronick, 2010). They also displayed reduced understanding of object affordances and less variability of their OO exploration behaviors compared to their full-term peers (Bos, van Braeckel, & Hitzert, 2013; Grönqvist, Strand-Brodd, & von Hofsten, 2011; Lobo et al., 2015). Importantly, early delays in OO exploration in children born preterm have been related to poorer future language and cognitive outcomes (Bertenthal & Clifton, 1998; Gibson, 1988; Ruff et al., 1984; Zuccarini et al., 2017).
Study Hypotheses
The current study tested the following hypotheses: 1) the developmental trajectories of the exploratory behaviors children perform with their own bodies (NOO) would positively relate to the developmental trajectories of the associated behaviors they perform with portable objects (OO); and 2) the developmental trajectories of self and object exploration behaviors would positively relate to the trajectories of motor, language, and cognitive development.
Method
Participants
The current sample consisted of 23 children born full-term (FT; 14 males; 37-42 weeks gestational age, Mean=39.4; SD=1.1) recruited from the community and 29 children born preterm (PT; 10 males; 22-30 weeks gestational age, Mean=26.5; SD=1.7) recruited from a regional neonatal intensive care unit. The sample was 57.4% Caucasian, 29.6% African-American, 13.0% Asian; 9.3% Hispanic; 11.1% reported $0-14,999 gross household income, 9.2% reported $15,000-24,999, 1.9% reported $25,000-34,999, 9.3% reported $35,000-44,999, 9.3% reported $45,000-59,999, 22.2% reported $60,000-79,999, and 37.0% reported greater than $80,000 income. One full-term participant left the study at 4-months of age due to scheduling conflicts; data for 2.7% of the visits were missing. Recruitment of participants, informed consent, and data collection were done in accordance with the regulations set by the University of Delaware’s (UD) and Christiana Care Health System’s (CCC) Institutional Review Boards (“The Relationship Between Early Brain Structure and Development in Full-term and Pre-term Infants”, UD#128785/CCC#27122). Families received monetary compensation for their participation in the study.
Procedure
Children’s NOO exploratory behavior, OO exploratory behavior, and global development were assessed longitudinally in the home environment. During all the assessments, children were in an alert, neutral or positive state.
To evaluate NOO exploratory behavior, children were observed without direct social interaction or portable objects within reach for three minutes (Figure 1A-B for setup; for details see Babik et al., 2017). NOO exploration was assessed in supine, sitting, and then prone to account for varied affordances associated with each posture, with the order of postures held constant. The actual average durations of data collected were 2.70±0.60 minutes in supine, 2.53±0.74 minutes in prone, and 2.83±0.47 minutes in sitting. As children do not typically maintain stationary supine or prone positions after 9 months of age, NOO was assessed in all three positions at 3, 4, 6, and 9 months of corrected age but only in sitting at 12, 18, and 24 months of corrected age. A portable high chair was used for children before they demonstrated upper trunk and head control (typically at the age of 9 months); after this point the testing was conducted with infants in a booster seat.
Figure 1.
The frontal and side camera views for the non-object-oriented exploration (A & B) and object-oriented exploration testing procedures (C & D). The authors received signed consent for the child’s likeness to be published in this article.
OO exploration was assessed in supported sitting at 3, 4, 6, 9, 12, 18, and 24 months of corrected age since sitting is a common position in which young children experience early object play. This assessment was performed directly after the NOO exploration assessment. Children were observed during exploration of seven objects (4" set of plastic keys, 5.5" beaded ring, 5" maraca rattle, 4" smooth plastic ring, 6" soft frog ring, 3" soft crab toy, and 2.5" soft spiky ball) presented in random order, one at a time, for up to 30 seconds each (resulting in up to 3.5 total minutes of OO exploration). Each object was presented once within the child’s reach, in midline. If the child did not attempt to reach for the object, it was placed directly into the child’s hand. The presentation of objects was alternated between the two hands, with the starting hand being randomized (Figure 1C-D for setup; for details see Lobo et al., 2015). OO exploration behavior was only coded while infants were grasping the object. If an infant dropped the object, the experimenter placed it in the child’s same hand up to three times in the 30-second period; if the object was dropped three times before 30 seconds elapsed, the experimenter ended that trial early. Due to trials ending early at times, the actual average duration of data collected was 2.67±0.74 minutes. Note that the NOO and OO exploration were tested at the same time points (i.e., age), but in separate assessments (Figure 1) to avoid the confounding effects of concurrent measurement.
Global development was assessed using the Bayley Scales of Infant & Toddler Development, Third Edition (Bayley, 2006) at 3, 4, 6, 9, 12, 18, and 24 months after the NOO and OO assessments. All assessments were performed by a trained experimenter with expertise in child development and video recorded using two synchronized cameras for a frontal and side view.
Measures
All NOO and OO behaviors were coded in a manner that provided data on both frequency of occurrence and duration; frequency was used to calculate the number of exploration bouts, whereas duration was used to calculate the percentages of assessment time for all other variables (see details below).
Non-Object-Oriented Exploratory Behavior.
Different behaviors were coded in each position. In prone, Head up behavior was coded when no part of the child’s head, chin, or face was touching the support surface. In supine, Head in midline behavior was coded when the head was not turned more than a third of the range to the right or left. In sitting, the following behaviors were coded: 1) Both hands in midline – both of the child’s hands were positioned within the limits of the trunk; 2) One hand fisted – at least four of the child’s fingers were flexed completely into the palm on only one hand; 3) Hand in the mouth – any part of either hand was in contact with the child’s mouth, tongue, or lips; 4) Hand touching own body – the child’s hand contacted a part of his body, such as his head, trunk, or arm; 5) Looking at the hand – the child’s eyes were directed at either hand. Using Filemaker Pro Advanced custom programs (Filemaker, Inc., Santa Clara, CA), the coded data were then converted to percentages of assessment time, dividing each behavior’s cumulative duration by the total assessment time for the position in which the behavior was coded. The obtained percentages of assessment time were then used in current statistical analyses. Note that different behaviors could be performed with each hand, so we coded the right and left hands separately. When accounting for overall performance of a behavior, the behavior was considered irrespective of the hand performing it (i.e., the cumulative duration of the behavior would be calculated including performance with the right or left hand, counting durations where the behavior occurred with both hands only once).
For the sitting data, temporally overlapping occurrences of all coded behaviors were evaluated using Filemaker Pro Advanced custom programs to create the following variables: 1) Bouts of NOO exploration (measure of exploration intensity) – number of times per minute when the child switched from performing one or a combination of behaviors to another, such as looking at the hand and then looking at the hand while touching own body with that hand; 2) Variability of individual NOO behaviors (basic measure of behavioral variability) – of the five individual behaviors coded (listed above), the percentage of those behaviors the child was observed performing at each assessment; and 3) Variability of combined NOO behaviors (more complex measure of behavioral variability and multimodality) – of the potential combinations of the five behaviors coded, the percentage of the behavioral combinations the child was observed performing at each assessment. In total, ten NOO behaviors were analyzed.
Object-Oriented Exploratory Behavior.
The following object-oriented exploratory behaviors were coded during periods of time when the child was holding an object: 1) Holding the object unimanually – the child held the object with only one hand; 2) Holding the object bimanually – the child held the object with both hands; 3) Object in the mouth – the object was in contact with the mouth, tongue, or lips; 4) Object touching own body – the child brought the object into contact with a part of her body, including the head, face, trunk, arms, and legs, but excluding the mouth and other hand; 5) Looking at the object in the hand – the child’s eyes were directed toward the object; 6) Fingering the object – the child’s fingers moved over the surface of the object for at least 2 sec.; 7) Banging the object – the object contacted a surface or the child’s body in a repetitive manner; 8) Manipulating the object – the child’s one hand moved part(s) of the object as it was supported by the other hand; 9) Transferring the object from one hand to the other – the child moved the object from one hand to the other. Using Filemaker Pro, the coded data were then converted to percentages of assessment time, dividing each behavior’s cumulative duration by the total assessment time. Temporally overlapping occurrences of all behaviors coded were identified to create the variables: 1) Bouts of OO exploration; 2) Variability of individual OO behaviors; and 3) Variability of combined OO behaviors (defined as above). In total, twelve OO behaviors were analyzed. Again, although we coded behaviors performed by each hand (right vs. left), the analysis of each behavior was conducted irrespective of the performing hand: the cumulative duration of the behavior across the two hands was calculated while counting any overlaps between the two hands only once. See the coding protocol as an example of our methods in the supplementary materials. Figure S1 shows an example of the data after overlaps in behavioral occurrence were identified in the database. Note that each procedure (NOO in supine, NOO in prone, NOO in sitting, OO) was administered, coded, and processed independently of one another.
Bayley Scales of Infant & Toddler Development, Third Edition (Bayley-III).
Children’s motor, language, and cognitive development was assessed using the Bayley-III Gross and Fine Motor, Receptive and Expressive Language, as well as Cognitive subscales. The Bayley-III (Bayley, 2006) is a norm-referenced assessment commonly used in research and clinical practice to monitor children’s development and to detect delays (Weiss, Oakland, & Aylward, 2010). Raw, rather than standardized, scores for each subscale were analyzed because of fluctuations in the standardized scores across this age range (see Lobo et al., 2014).
Data Coding and Scoring.
NOO and OO behaviors were coded in a frame-by-frame manner using OpenSHAPA software by research assistants blind to participants’ age and birth status. The Bayley-III was scored by two researchers with graduate education in child development. Coding reliability was established across 20% of the re-coded data for both the cumulative frequency of occurrence and duration of the behaviors by visit. The following equation was used: [Agreed / (Agreed + Disagreed)] * 100. For NOO exploration, intra-rater agreement was 87.5±2.8% and inter-rater agreement with a primary coder was 86.8±0.4%, when averaged across variables. For OO exploration, intra-rater agreement was 88.7±3.3% and inter-rater agreement with a primary coder was 87.0±1.5%, when averaged across variables. For the Bayley-III, intra-rater agreement was 98.4±0.4% and inter-rater agreement was 96.2±0.8% between the two coders.
Statistical Analyses
Analyses were performed using Hierarchical Linear Modeling Software (HLM; Raudenbush, Bryk, Cheong, Congdon, & du Toit, 2004). HLM is the most appropriate and recommended technique for longitudinal designs, allowing for a hierarchical data structure in which observations across age are nested within participants, thus, accounting for non-independence of multiple observations for each participant. Moreover, to evaluate dynamic relations among NOO exploration, OO exploration, and developmental outcomes, we used HLM to model trajectories of each behavior and then to relate those trajectories to one another.
To model change in each behavior across age, we used the AGE variable. Since many early behaviors exhibited by children have a quadratic trend of change across age, with some leveling off or even showing a change in the trajectory (e.g., from incline to decline), both linear and quadratic trends of change (AGE and AGE2) were entered as independent variables in each statistical model. To evaluate whether developmental trends of change depend on the birth status of the child (PT vs. FT), an independent dummy-coded STATUS variable marking each child’s birth status (0 = PT; 1 = FT) was included in each statistical model. While children’s birth status was considered in this manuscript, the primary focus was not on the direct comparison between children born preterm or full-tem, but rather on relating self-exploration and object exploration developmental trajectories to each other and to trajectories for children’s global development, as well as evaluating whether the relations between these trajectories changed based on the birth status of the children.
Statistically non-significant variables were eliminated from the final statistical models. Statistical effects with p≤.05 were considered significant. To ensure that observed effects were not only statistically significant, but also meaningful, effect sizes are reported for each target variable as Cohen’s d with 0.2 signifying small, 0.5 medium, 0.8 large, and ≥1.2 very large effects (Cohen, 1988; Sawilowsky, 2009).
Relating Non-Object-Oriented to Object-Oriented Exploration.
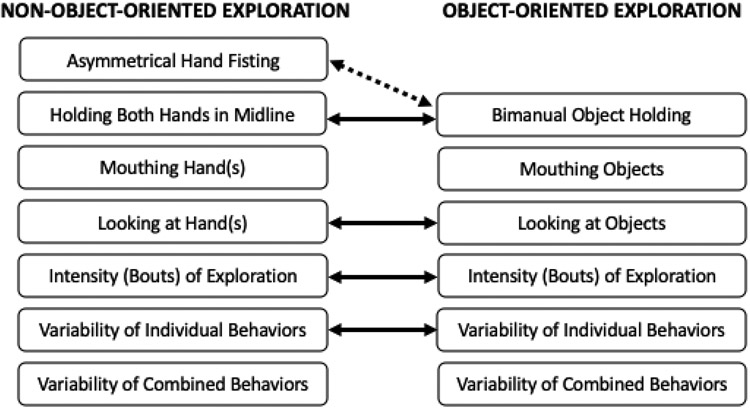
We tested whether the trajectory of change across age for NOO exploratory behaviors related to the trajectory of change for OO exploratory behaviors for the NOO-OO behavioral pairings: 1) Holding the hands in midline to Holding the object bimanually; 2) Hand in the mouth to Object in the mouth; 3) Looking at the hand to Looking at the object in the hand; 4) Hand touching own body to Object touching own body; 5) Bouts of NOO exploration to Bouts of OO exploration; 6) Variability of individual NOO behaviors to Variability of individual OO behaviors; and 7) Variability of combined NOO behaviors to Variability of combined OO behaviors. Most of the NOO-OO behavioral pairs represent similar behaviors performed without an object vs. with an object, allowing us to examine behavioral continuity across contexts within the theoretical framework of this study. To test the hypothesis that unilateral hand fisting might impede bimanual exploration, we also related the One hand fisted NOO variable to the Holding the object bimanually OO variable. Each OO behavior was entered into the statistical model as the dependent variable, whereas each NOO behavior was entered as an independent variable, along with the AGE, AGE2, and STATUS independent variables. In addition, we evaluated developmental trends of change across age for each behavior in the NOO-OO pairs, entering each behavior into the model as a dependent variable, with AGE and AGE2 serving as independent variables.
Relating Non-Object-Oriented and Object-Oriented Exploration to Motor, Language, and Cognitive Development.
In these multilevel analyses, we related developmental trajectories for the NOO or OO exploratory behaviors to the trajectories for the developmental outcomes measured with each Bayley scale. Each Bayley outcome was entered into the statistical model as the dependent variable, whereas each NOO or OO behavior was entered as an independent variable, along with the AGE, AGE2, and STATUS independent variables.
This study was not preregistered. The deidentified data used in the current analyses are available upon request.
Results
Only 4.40% of the data were missing for the NOO assessment, 4.95% for the OO assessment, and 0% for the Bayley assessment. Descriptive statistics (Mean and SE) for the NOO exploration, OO exploration, and Bayley assessments are presented in online supplementary materials Tables S1-S3. Participants’ ages (Mean and SD; corrected ages for preterm participants) at each visit were 3.0±0.2 months, 4.0±0.3 months, 6.0±0.3 months, 9.1±0.2 months, 12.1±0.3 months, 18.1±0.3 months, and 24.0±0.4 months.
Relating Non-Object-Oriented to Object-Oriented Exploration
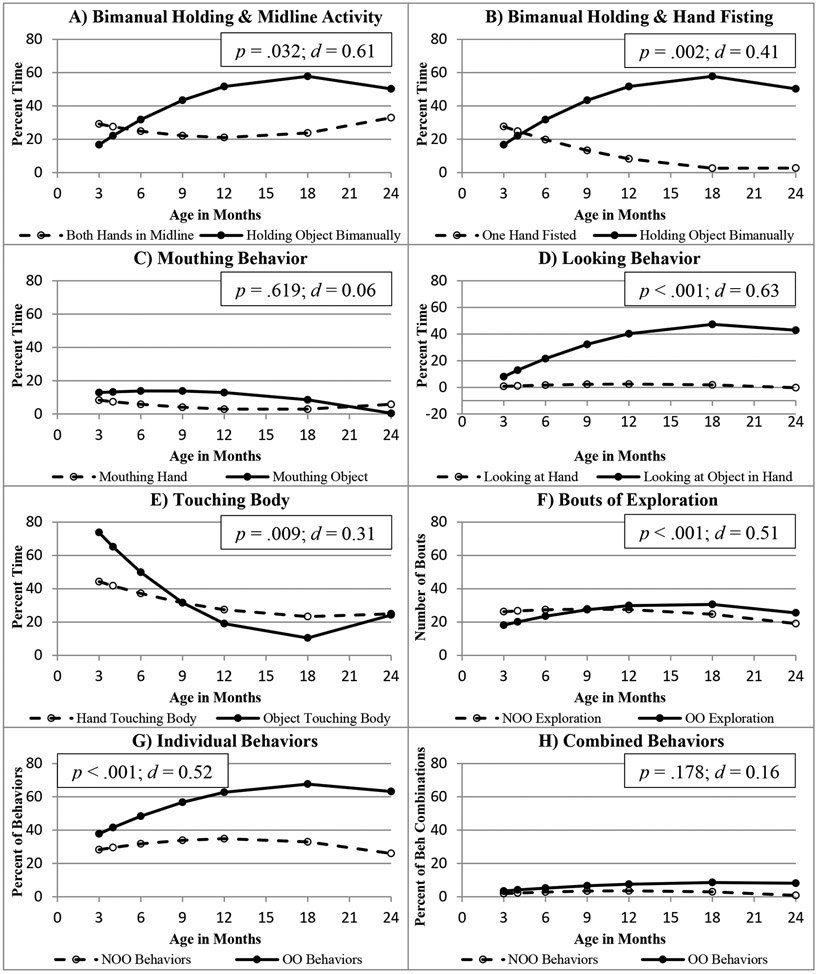
Detailed information on statistical parameters of the following analyses is provided in online supplementary materials Table S5. The developmental trajectory for holding both hands in midline during NOO exploration positively predicted the trajectory of change for holding objects during OO exploration (t(52)=2.21, SE=0.04, p=.032, d=0.61). Conversely, the developmental trajectory for keeping one hand fisted during NOO exploration was negatively related to the trajectory for bimanual holding of objects during OO exploration (t(234)=−3.11, SE=0.05, p=.002, d=0.41).
No significant relation was found between the trajectory for children’s mouthing their hand(s) during NOO exploration and that for mouthing objects during OO exploration (t(286)=0.50, SE=0.06, p=.619, d=0.06). The developmental trajectory for touching one’s own body with the hand(s) during NOO exploration positively predicted that for touching one’s own body with an object during OO exploration (t(286)=2.64, SE=0.03, p=.009, d=0.31). A positive relation was found between the trajectory for looking at the hand(s) during NOO exploration and that for looking at an object in the hand during OO exploration (t(286)=5.35, SE=0.15, p<.001, d=0.63).
The developmental trajectory for bouts of NOO exploratory behavior per minute positively predicted the pattern of change in bouts of OO exploratory behavior (t(336)=4.67, SE=0.03, p<.001, d=0.51). Similarly, the trajectory for the variability of individual behaviors performed during NOO exploration positively predicted that for variability of individual behaviors manifested during OO exploration (t(286)=4.38, SE=0.05, p<.001, d=0.52). However, no relation was found between the trajectories for the variability of combined behaviors demonstrated during NOO vs. OO exploration (t(286)=1.35, SE=0.07, p=.178, d=0.16). Relations between developmental trajectories for NOO and OO variables are illustrated in Figure 2. Figure 3 (see supplementary materials Table S4 for statistical parameters) shows the estimated amount of performance of these behaviors across time.
Figure 2.
Positive (solid arrow) and negative (dashed arrow) relations between the behaviors children performed without portable objects (non-object-oriented exploration) and with portable objects (object-oriented exploration); non-connected behaviors were not significantly related.
Figure 3.
Estimated trajectories for self- (NOO) and object (OO) exploration behaviors across time, with corresponding p-values and Cohen’s d effect sizes.
For most analyses relating trajectories for NOO exploration to those for OO exploration, the infant status variable was not statistically significant and was dropped from the final models. Infant status significantly affected only the relation between the trajectories for bouts of NOO and OO exploration, with PT infants showing a significantly lower number of bouts of OO exploration in relation to bouts of NOO exploration at the beginning of the study, but a higher rate of change (steeper trajectory) of OO exploration bouts in relation to NOO exploration bouts across the study compared to FT infants.
Relating Non-Object-Oriented Exploration to Motor, Language, and Cognitive Development
Detailed information on statistical parameters of the following analyses is provided in online supplementary materials Table S6, while a more concise summary of the data is presented in Table 1. The developmental trajectory for the Gross Motor scale was positively predicted by the trajectories for holding the head up in prone (t(57)=5.01, SE=0.01, p<.001, d=1.33) and holding the head in midline in supine (t(55)=2.41, SE=0.01, p=.019, d=0.65), but negatively predicted by the trajectory for touching one’s own body with the hand (t(238)=−3.29, SE=0.01, p=.001, d=0.43). The trajectory for the Fine Motor scale was positively predicted by that for holding the head up in prone (t(56)=2.88, SE=0.01, p=.006, d=0.77).
Table 1.
Summarized Statistically Significant (p≤.05) Results Relating Children’s Non-Object-Oriented (NOO) Exploration to Bayley-III Outcomes, Presenting p-Values and the Direction of the Effect: (+) Positive Relation; (−) Negative Relation; GM = Gross Motor; FM = Fine Motor; RL = Receptive Language; EL = Expressive Language; CG = Cognitive.
| NOO Exploratory Behaviors (Independent Variable) |
Bayley Scales of Infant and Toddler Development (Dependent Variable) |
||||
|---|---|---|---|---|---|
| GM | FM | RL | EL | CG | |
| Head Up | p<.001 (+) | p=.006 (+) | |||
| Head in Midline | p=.019 (+) | p=.026 (+) | |||
| Both Hands in Midline | p=.028 (−) | ||||
| One Hand Fisted | p=.033 (−) | ||||
| Hand in Mouth | |||||
| Hand Touching Body | p=.001 (−) | ||||
| Looking at Hand | |||||
| Bouts of Exploration | |||||
| Variability of Individual Behaviors | |||||
| Variability of Combined Behaviors | p=.038 (+) | ||||
No relations were found between the developmental trajectory for the Receptive Language scale and trajectories for NOO exploratory behaviors. The developmental trajectory for the Expressive Language scale was positively predicted by that for holding the head in midline (t(54)=2.30, SE=0.003, p=.026, d=0.63) and negatively predicted by the trajectory for asymmetrical one-hand fisting (t(238)=−2.15, SE=0.01, p=.033, d=0.28). The developmental trajectory for the Cognitive scale was positively predicted by the trajectory for the variability of combined behaviors (t(238)=2.09, SE=0.07, p=.038, d=0.27) and negatively predicted by the trajectory for holding both hands in midline (t(53)=−2.05, SE=0.01, p=.028, d=0.62).
In terms of infant birth status, for the Gross Motor scale, PT infants showed lower initial gross motor scores in relation to all the tested NOO variables except holding the head up in prone and holding the head in midline while in supine; no difference was found between FT and PT infants in their rate of change in gross motor skills in relation to the NOO variables. For the Fine Motor scale, there was no difference in the initial scores between FT and PT infants, whereas PT infants showed a lower rate of change in fine motor skills in relation to the NOO variables. For the Receptive Language scale, PT infants showed lower initial scores and a lower rate of change in receptive language in relation to all the tested NOO variables except holding the head up in prone and holding the head in midline while in supine. For the Expressive Language scale, PT infants showed lower initial expressive language scores only in relation to holding the head in midline variable, and a lower rate of change in expressive language skills in relation to all the tested NOO variables except holding the head up in prone and holding the head in midline while in supine. For the Cognitive scale, FT and PT infants did not differ in their initial cognitive scores in relation to NOO exploration scores, whereas the rate of change in cognitive skills was lower for PT infants in relation to all the tested NOO variables except holding the head up in prone.
Relating Object-Oriented Exploration to Motor, Language, and Cognitive Outcomes
Detailed information on statistical parameters of the following analyses is provided in online supplementary materials Table S7; a more concise summary of the data is presented in Table 2. No statistically significant relations were found between the developmental trajectory for children’s performance on the Gross Motor scale and trajectories for OO exploratory behaviors. The developmental trajectory for the Fine Motor scale was negatively predicted by that of unimanual holding of objects (t(289)=−4.28, SE=0.02, p<.001, d=0.50), but positively predicted by the trajectories for bimanual holding of objects (t(289)=4.24, SE=0.01, p<.001, d=0.50), mouthing objects (t(289)=2.65, SE=0.01, p=.008, d=0.31), looking at objects in the hand (t(289)=2.10, SE=0.01, p=.037, d=0.25), banging objects (t(289)=3.84, SE=0.03, p<.001, d=0.45), manipulating objects (t(289)=2.01, SE=0.05, p=.046, d=0.24), transferring objects between the hands (t(289)=2.41, SE=0.10, p=.017, d=0.28), bouts of OO behavior per minute (t(289)=4.70, SE=0.01, p<.001, d=0.55), as well as the trajectories for variability of both individual (t(289)=5.69, SE=0.01, p<.001, d=0.67) and combined behaviors (t(289)=5.85, SE=0.05, p<.001, d=0.69).
Table 2.
Summarized Statistically Significant (p≤.05) Results Relating Children’s Object-Oriented (OO) Exploration to Bayley-III Outcomes, Presenting p-Values and the Direction of the Effect: (+) Positive Relation; (−) Negative Relation; GM = Gross Motor; FM = Fine Motor; RL = Receptive Language; EL = Expressive Language; CG = Cognitive.
| OO Exploratory Behaviors (Independent Variable) |
Bayley Scales of Infant and Toddler Development (Dependent Variable) |
||||
|---|---|---|---|---|---|
| GM | FM | RL | EL | CG | |
| Holding Object Unimanually | p<.001 (−) | p=.021 (−) | |||
| Holding Object Bimanually | p<.001 (+) | p=.021 (+) | |||
| Object in Mouth | p=.008 (+) | p=.008 (+) | |||
| Object Touching Body | p=.028 (−) | p=.001 (−) | p=.004 (−) | ||
| Looking at Object in Hand | p=.037 (+) | p=.043 (+) | p=.035 (+) | ||
| Fingering Object | |||||
| Banging Object | p<.001 (+) | p=.003 (+) | |||
| Object Manipulation | p=.046 (+) | ||||
| Transferring Object | p=.017 (+) | p=.004 (+) | |||
| Bouts of Exploration | p<.001 (+) | p<.001 (+) | |||
| Variability of Individual Behaviors | p<.001 (+) | p<.001 (+) | |||
| Variability of Combined Behaviors | p<.001 (+) | p=.021 (+) | |||
The developmental trajectory for the Receptive Language scale was positively predicted by the trajectories for mouthing objects (t(289)=2.65, SE=0.01, p=.008, d=0.31) and looking at objects in the hand (t(289)=2.03, SE=0.01, p=.043, d=0.24), but negatively predicted by the trajectory for touching one’s own body with objects (t(289)=−2.20, SE=0.01, p=.028, d=0.26). The trajectory for the Expressive Language scale was negatively predicted by that for touching one’s own body with objects (t(289)=−3.26, SE=0.01, p=.001, d=0.38).
The developmental trajectory for the Cognitive scale was negatively predicted by the trajectories for unimanual holding of objects (t(289)=−2.32, SE=0.02, p=.021, d=0.27) and touching one’s own body with objects (t(289)=−2.92, SE=0.01, p=.004, d=0.34), but was positively predicted by the trajectories for bimanual holding of objects (t(289)=2.32, SE=0.01, p=.021, d=0.27), looking at objects in the hand (t(289)=2.12, SE=0.01, p=.035, d=0.25), banging objects (t(289)=2.96, SE=0.05, p=.003, d=0.35), transferring objects between the hands (t(289)=2.87, SE=0.14, p=.004, d=0.34), bouts of OO behavior per minute (t(289)=3.65, SE=0.02, p<.001, d=0.43), as well as the trajectories for the variability of both individual (t(289)=4.02, SE=0.02, p<.001, d=0.47) and combined behaviors (t(289)=2.32, SE=0.07, p=.021, d=0.27).
In terms of infant birth status, for the Gross Motor scale, PT infants showed lower initial gross motor scores in relation to all the tested OO variables; no differences between FT and PT infants were found in their rate of change in gross motor skills in relation to the observed OO variables. For the Fine Motor and Expressive Language scales, there was no difference between FT and PT infants in their initial scores, whereas PT infants showed a lower rate of developmental change in relation to all the tested OO variables. For the Receptive Language scale, PT infants showed lower initial scores and a lower rate of change in receptive language skills in relation to all the tested OO variables. For the Cognitive scale, PT infants showed lower initial cognitive scores than FT infants only in relation to the fingering variable, and a lower rate of change in cognitive skills in relation to all the tested OO variables.
The inclusion of the infant birth status variable (FT vs. PT) in the statistical analyses highlighted differences between infants born full-term vs. preterm in their developmental trajectories for NOO/OO exploration and global developmental outcomes, while supporting the conclusion that the observed relations between the trajectories of NOO exploration behaviors, OO exploration behaviors, and global development remain true for infants with either birth status.
Summary of the Findings
Better head control (holding the head up and in midline) was associated with advanced motor and expressive language skills. Better upper extremity control (unfisted hands held in midline) was associated with more bimanual object manipulation, which, in turn, was related to better fine motor and cognitive outcomes. Looking at the empty hand was predictive of looking at an object in the hand, which, in turn, was positively related to fine motor, receptive language, and cognitive skills. Finally, greater variability of both NOO and OO exploration was associated with better cognitive skills, whereas only intensity of OO exploration was positively associated with children’s fine motor and cognitive outcomes.
Discussion
Overall, the findings of these analyses supported our hypothesis that trajectories of NOO and OO exploration behaviors would relate to one another; this was true for all but two of the pairs of behaviors analyzed. The results also identified a number of exploration behaviors whose trajectories predicted trajectories of motor, language, and cognitive development throughout the first two years of life (Tables 1 & 2). Below we discuss the findings in more detail, highlighting their implications in the context of the existing literature and developmental theory.
Dynamic Relations Between Self- and Object-Oriented Exploratory Behaviors
Positive relations were found between most of the behaviors that children performed to explore their bodies and the corresponding behaviors they performed to explore objects. For instance, children who held their hands in midline more during NOO showed more bimanual manipulation of objects. By contrast, asymmetrical one-handed fisting during NOO exploration was related to less bimanual manipulation during OO exploration, highlighting the role of hand position for grasp and bimanual object manipulation. Children who looked at their hands more during NOO exploration looked more at objects in their grasp. Similar positive relations were also found for touching one’s own body with the hands or objects and for intensity and variability of the behaviors performed between NOO and OO exploration. Only mouthing behavior and combined behaviors demonstrated during NOO vs. OO exploration were not related. Examination of the developmental trajectories for the exploratory behaviors in NOO-OO pairs showed that most trajectories are closely associated, either changing in the same direction or complementing each other (Figure 3). By contrast, whereas combined behaviors in NOO exploration declined after the age of 12 months, the OO combined behaviors continued to increase. This trend could be explained by children’s increased skill and motivation for manipulating objects during the second year of life.
We also noticed a considerable mismatch between the hand mouthing and object mouthing trajectories. Hand mouthing behavior decreased from 3 to 12 months, leveled off until the age of 18 months, and then increased from 18 to 24 months, whereas object mouthing behavior decreased steadily from 3 through 24 months. We propose that the mouthing results may represent the fact that mouthing serves multiple purposes for infants. Infants likely mouthed their hands often in early development to sooth themselves and explore their hands (Anderson, 2004). In contrast, the increase in hand mouthing from 18 to 24 months might have been connected to pain management, as infants were teething, and to children’s experimentation with sounds during this period of active language development and vocabulary growth (e.g., Goldfield & Reznick, 1990).
These findings closely align with the dynamic systems approach, suggesting continuity in the dynamic co-development of NOO and OO exploratory behaviors (Corbetta & Thelen, 1996; Goldfield, 1993; Thelen, 1990). Specifically, NOO exploration behaviors likely allow children to understand the foundational affordances of their bodies and to establish the repertoire of behaviors that can be amplified and applied in novel combinations when children are provided opportunities to explore objects (Lobo & Galloway, 2013). OO exploration behaviors may emerge from NOO exploration behaviors in the same way that arm flapping transforms into controlled reaching behavior (Thelen et al., 1993) or leg kicking cascades into walking behavior (Smith, Trujillo-Priego, Lane, Finley, & Horak, 2015; Thelen, Ulrich, & Wolff, 1991). Note that the current study explored developmental trajectories for different skills and relations between them; age is embedded in those trajectories and the current results do not allow us to disentangle the effects of maturation from the effects of self-organization in these dynamic developing systems (Kelso & Tuller, 1984; Kugler & Turvey, 1987; Thelen, 1990).
Dynamic Relations of Exploratory Behavior to Motor and Cognitive Development
Greater performance of certain exploratory behaviors was related to better motor and cognitive development. Children with better control to lift the head up in prone had more advanced fine and gross motor skills. Holding the head in midline while in supine was also associated with more advanced gross motor development. These findings are in agreement with previous research reporting that postural control develops in a cephalocaudal manner, with head and neck control being important precursors to improved stability in the shoulders, waist, and hips that supports the development of independent sitting, crawling, and locomotion (e.g., Adolph & Franchak, 2017). Head control may, therefore, be an early indicator of broader future gross motor ability. Moreover, the current results support previous research suggesting that advances in head and trunk control strongly facilitate visual-motor coordination, reaching, and object exploration (Bertenthal & von Hofsten, 1998; Hopkins & Rönnqvist, 2002; Rochat & Bullinger, 1994; Rochat & Goubet, 1995; Thelen & Spencer, 1998), thus, advancing the child’s fine motor development. The findings of this study support the DST proposal that motor ability, or, specifically, postural control here, is a key organizer of developmental change (Thelen & Smith, 1994).
The results also suggested that children who spent more time touching their body with their hands had poorer gross motor development. Similarly, children who spent more time touching their body with objects had poorer language (both receptive and expressive) and cognitive outcomes. While supporting the head and holding it in midline are indicators of better postural control, touching one’s own body may indicate an attempt to compensate for poor postural control (Butler & Major, 2003; Butler, Saavedra, Sofranac, Jarvis, & Woollacott, 2010). Poor postural control, potentially signaled by touching one’s own body, might negatively affect children’s respiration, communicative gestures, and visual exploration, which, in turn, could result in poorer motor, language, and cognitive outcomes (Bahrick et al., 2004; Bushnell & Boudreau, 1993; Gibson, 1988; Iverson, 2010).
Furthermore, children who demonstrated more bimanual holding of objects, visual attention to objects, oral exploration of objects, banging and transferring objects between hands, and greater intensity, variability, and multimodality of exploratory behavior in this study had more advanced fine motor and cognitive development. Thus, as children engaged in object exploration using different modalities (e.g., visual, tactile, oral, somatosensory, and proprioceptive), used two hands for sophisticated bimanual manipulation, and were variable in their behavioral performance, not only did their fine motor skills improve, but they also likely gathered enriched information about objects that informed their cognitive development (Adamson et al., 2004; Babik & Michel, 2016; Bahrick et al., 2004; Gibson, 1988; Kimmerle, Ferre, Kotwica, & Michel, 2010; Lobo & Galloway, 2008; Needham et al., 2002; Soska et al., 2010; Thelen, 1990; Wilcox et al., 2007).
Greater variability in both the NOO and OO exploratory behaviors performed was related to better cognitive development. Variability is acknowledged as an essential characteristic of early development (Hadders-Algra, 2002; Piek, 2002; Touwen, 1978). Indeed, decreased levels of variability in postural control, spontaneous movement, and object exploration have been found for children born preterm and at risk for motor delays (Babik et al., 2017; Cunha et al., 2018; Dusing & Harbourne, 2010; Lobo et al., 2015; Prechtl, 1990). Our findings highlight a potential consequence of diminished variability of exploratory behaviors – that of suboptimal cognitive outcomes. Specifically, reduced variability of exploratory behavior may result in a narrower range of learning opportunities and long-term suboptimal cognition. Previous research has made similar conclusions (e.g., Einspieler, Bos, Libertus, & Marschik, 2016; Gibson, 1988; Hadders-Algra, 2002; Lobo & Galloway, 2013) after studying either general movements (NOO here) or object exploration alone, without a comprehensive account of the co-development of these skills across the first two years of life.
Dynamic Relations Between Exploratory Behavior and Language Development
Greater performance of key exploratory behaviors was also related to better language development. Children who visually attended to objects more had more advanced receptive language development. It may be that children who are able to successfully direct and sustain their gaze to target objects for longer durations are provided more opportunities to hear their parents label those objects and their features. It may also be that those children have the necessary motor control and interest in objects to follow parental cues, such as pointing gestures and looking, directed towards objects being discussed. These experiences would facilitate the development of language (Iverson, 2010; Mundy & Newell, 2007).
More mouthing of an object was associated with better receptive language. Previous research also related oral exploration of objects to better word comprehension and first vocalizations (Fagan & Iverson, 2007; Iverson, 2010; Iverson & Thelen, 1999; Zuccarini et al., 2018). Note that we did not find a relation between oral exploration of an object and the child’s expressive language development. Having an object in the mouth may advance initial vocalizations, rather than production of words and gesture-word combinations important for the development of expressive language (Capirci, Contaldo, Caselli, & Volterra, 2005; Mayberry & Nicoladis, 2000).
Children who demonstrated better midline head control had more advanced expressive language development. Head and trunk control can help children attend to people, objects, and events, facilitating communicative learning opportunities through joint attention and verbal exchange, thus, improving expressive language skills. These findings align well with previous research on the development of joint attention and language skills (Brooks & Meltzoff, 2005; Iverson, 2010; Libertus & Violi, 2016; Oudgenoeg-Paz, Volman, & Leseman, 2012; Yu & Smith, 2013).
Asymmetrical fisting of one hand also negatively related to expressive language development. This finding corresponds with previous research showing positive relations of children’s fine motor and motor planning skills to their expressive language development (Bishop & Edmunson, 1987; Iverson & Thelen, 1999; LeBarton & Iverson, 2013; Lifter & Bloom, 1989; Stone & Yoder, 2001). Previous research proposed the following possible explanations for such a relation: 1) both fine motor skills and expressive language might require sophisticated motor coordination and planning, and difficulties with the latter skills might affect the development of the former; or 2) fine motor skills allow sophisticated object manipulation, which provides learning opportunities that facilitate the development of expressive language (Iverson, 2010; Iverson & Thelen, 1999; LeBarton & Iverson, 2013). Both explanations are viable, and it was not possible to tease them apart in the context of the current study.
Asymmetrical hand fisting might be perceived as a soft sign of neurologic dysfunction or a motor control deficit, as a result of perturbations related to preterm birth (Babik et al., 2017). The current results showed that asymmetrical fisting negatively affected both bimanual manipulation and expressive language skills. Spending more time with one hand fisted may prevent children from executing complex bimanual exploratory behaviors, thus, impeding fine motor development, which, in turn, would negatively affect children’s engagement with objects and development of expressive language skills (Babik & Michel, 2016; Bates & Dick, 2002; Fagan & Iverson, 2007; Iverson, 2010; Iverson & Thelen, 1999; Kimmerle et al., 2010).
Birth Status as a Factor in Child Development
Although children’s birth status was not the primary focus of this study, it was included in the statistical analyses to ensure that the obtained relations between the different developmental outcomes were not restricted to only one group of children. The results suggested that the FT children had better NOO and OO exploration, as well as global development relative to children born PT. The advantage was observed either in a higher initial skill level, in a higher rate of change over time, or in both. Importantly, the observed relations between NOO and OO exploration, as well as between NOO/OO exploration and Bayley outcomes could be generalized to all children, irrespective of birth status.
Strengths and Limitations of the Current Study
This study implemented a longitudinal design, systematically assessing different aspects of child development during the first two years of life. A diverse sample of FT and PT infants was included to represent a wide range of skills and allow generalization of the results to a wider population. NOO and OO exploration were tested in separate assessments, rather than simultaneously. The data were analyzed using a method that accounted for the non-independence of longitudinal observations while modeling and relating the developmental trajectories of different behaviors. This study is limited in that it cannot identify causal or directional relations among the behaviors evaluated.
Summary and Significance
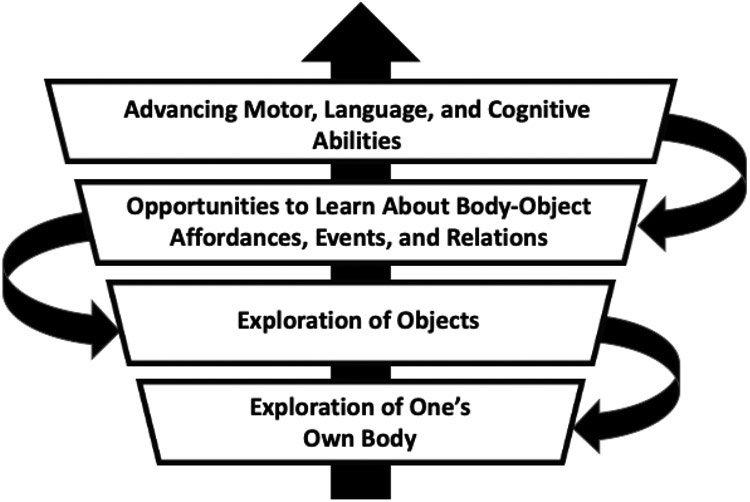
Early self and object exploration behaviors are closely related: the repertoire of object exploration behaviors develops in close relation with the existing repertoire of non-object-oriented behaviors. Furthermore, the amount of self and object exploration behavior performed relates significantly to children’s motor, language, and cognitive development throughout the first two years of life (Figure 4). These findings highlight dynamic interrelations and continuity among motor, language, and cognitive abilities in early childhood. This study is one of the few to longitudinally and comprehensively evaluate the dynamic co-development of motor, language, and cognitive skills in a diverse sample of children throughout the first two years of life.
Figure 4.
The model underlying this study suggests that early exploratory behavior with objects emerges from the behavior children use to explore their own bodies. Performance of those combined exploratory behaviors provides opportunities for children to learn about body-object affordances, object properties, events, and relations, thus, advancing children’s global development.
This research is significant because it highlights developmental continuity and relations that could be utilized for early identification and intervention purposes. Standardized developmental assessments are limited in their ability to identify delays early and consistently throughout the first two years of life. Even with the Bayley III assessment, when scores are categorized for service qualification based on early intervention cut-off values, children’s classifications fluctuate in a manner that is not meaningful throughout the first two years of life (Lobo et al., 2014). In the current study, we used raw Bayley scores to avoid this limitation and allow more accurate charting of developmental trajectories.
Behavioral analysis of early self- and object-oriented exploration behaviors may provide an effective means for very early prediction of developmental delays. Early interventions may target these behaviors with the aim of advancing future motor, language, and cognitive outcomes. Therefore, an understanding of developmental relations like the ones documented in this study is imperative to improve early assessment and intervention practices.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the children and their parents for their participation in making the current longitudinal study possible. Also, we would like to thank the research assistants who helped with the coding of data. This research was supported by the National Institute of Health, Eunice Kennedy Shriver National Institute of Child Health & Human Development (1R01HD051748).
Footnotes
This study was not preregistered. The deidentified data used in the current analyses are available upon request.
References
- Adamson LB, Bakeman R, & Deckner DF (2004). The development of symbol-infused joint engagement. Child Development, 75, 1171–1187. doi: 10.1111/j.1467-8624.2004.00732.x [DOI] [PubMed] [Google Scholar]
- Adolph KE, & Franchak JM (2017). The development of motor behavior. WIREs Cognitive Science, 8, 1–2. doi: 10.1002/wcs.1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE (2004). "Nothing but the tooth": Dispelling myths about teething. Contemporary Pediatrics, 21(7), 75–83. [Google Scholar]
- Atkinson J, & Braddick O (2007). Visual and visuocognitive development in children born very prematurely. From Action to Cognition, 164, 123–149. doi: 10.1016/S0079-6123(07)64007-2 [DOI] [PubMed] [Google Scholar]
- Babik I, Campbell JM, & Michel GF (2014). Postural influences on the development of infant lateralized and symmetric hand-use. Child Development, 85, 294–307. doi: 10.1111/cdev.12121 [DOI] [PubMed] [Google Scholar]
- Babik I, Galloway JC, & Lobo MA (2017). Infants born preterm demonstrate impaired exploration of their bodies and surfaces throughout the first two years of life. Physical Therapy, 97, 915–925. doi: 10.1093/ptj/prx064 [DOI] [PubMed] [Google Scholar]
- Babik I, & Michel GF (2016). Development of role-differentiated bimanual manipulation in infancy: Part 1. The emergence of the skill. Developmental Psychobiology, 58, 243–256. doi: 10.1002/dev.21382 [DOI] [PubMed] [Google Scholar]
- Bahrick LE, Lickliter R, & Flom R (2004). Intersensory redundancy guides the development of selective attention, perception, and cognition in infancy. Current Directions in Psychological Science, 13(3), 99–102. doi: 10.1111/j.0963-7214.2004.00283.x [DOI] [Google Scholar]
- Barrett TM, Traupman E, & Needham A (2008). Infants’ visual anticipation of object structure in grasp planning. Infant Behavior and Development, 31, 1–9. doi: 10.1016/j.infbeh.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Bates E, & Dick F (2002). Language, gesture, and the developing brain. Developmental Psychobiology, 40, 293–310. doi: 10.1002/dev.10034 [DOI] [PubMed] [Google Scholar]
- Bayley N (2006). Bayley scales of infant and toddler development (3rd ed.). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Baumgartner HA, & Oakes LM (2013). Investigating the relation between infants’ manual activity with objects and their perception of dynamic events. Infancy, 18, 983–1006. doi: 10.1111/infa.12009 [DOI] [Google Scholar]
- Bertenthal BI, & Clifton RK (1998). Perception and action. In Damon W, Kuhn D. & Siegler RS (Eds.), Handbook of child psychology : Vol. 2. Cognition, perception, and language (5th ed., pp. 51–102). New York: Wiley. [Google Scholar]
- Bertenthal BI, & von Hofsten C (1998). Eye, head and trunk control: The foundation for manual development. Neuroscience and Biobehavioral Reviews, 22, 515–520. doi: 10.1016/S0149-7634(97)00038-9 [DOI] [PubMed] [Google Scholar]
- Bishop DVM, & Edmundson A (1987). Specific language impairment as a maturational lag: Evidence from longitudinal data on language and motor development. Developmental Medicine and Child Neurology, 29, 442–459. doi: 10.1111/j.1469-8749.1987.tb02504.x [DOI] [PubMed] [Google Scholar]
- Bloch H, & Carchon I (1992). On the onset of eye-head coordination in infants. Behavioral Brain Research, 49(1), 85–90. doi: 10.1016/S0166-4328(05)80197-4 [DOI] [PubMed] [Google Scholar]
- Bos AF, van Braeckel KNJA, Hitzert MM, Tanis JC, & Roze E (2013). Development of fine motor skills in preterm infants. Developmental Medicine and Child Neurology, 55(4), 1–4. doi: 10.1111/dmcn.12297 [DOI] [PubMed] [Google Scholar]
- Brooks R, & Meltzoff AN (2005). The development of gaze following and its relation to language. Developmental Science, 8(6), 535–543. doi: 10.1111/j.1467-7687.2005.00445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell EW, & Boudreau JP (1993). Motor development and the mind: The potential role of motor abilities as a determinant of aspects of perceptual development. Child Development, 64, 1005–1021. doi: 10.1111/j.1467-8624.1993.tb04184.x [DOI] [PubMed] [Google Scholar]
- Butler PB, & Major RE (2003). The Missing Link?: Therapy issues of open and closed chains. Physiotherapy, 89(8), 465–470. doi: 10.1016/S0031-9406(05)60003-X [DOI] [Google Scholar]
- Butler P, Saavedra MS, Sofranac MM, Jarvis MS, & Woollacott M (2010). Refinement, reliability and validity of the segmental assessment of trunk control (SATCo). Pediatric physical therapy: The official publication of the Section on Pediatrics of the American Physical Therapy Association, 22(3), 246. doi: 10.1097/PEP.0b013e3181e69490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, & Witherington D (2000). Travel broadens the mind. Infancy, 1, 149–219. doi: 10.1207/S15327078IN0102_1 [DOI] [PubMed] [Google Scholar]
- Capirci O, Contaldo A, Caselli MC, & Volterra V (2005). From action to language through gesture: A longitudinal perspective. Gesture, 5(1), 155–177. doi: 10.1075/gest.5.1.12cap [DOI] [Google Scholar]
- Case-Smith J, Bigsby R, & Clutter J (1998). Perceptual-motor coupling in the development of grasp. American Journal of Occupational Therapy, 52(2), 102–110. doi: 10.5014/ajot.52.2.102 [DOI] [PubMed] [Google Scholar]
- Cioni G, Ferrari F, Einspieler C, Paolicelli PB, Barbani T, & Prechtl HFR (1997). Comparison between observation of spontaneous movements and neurologic examination in preterm infants. Journal of Pediatrics, 130(5), 704–711. doi: 10.1016/S0022-3476(97)80010-8 [DOI] [PubMed] [Google Scholar]
- Clearfield MW (2011). Learning to walk changes infants’ social interactions. Infant Behavior and Development, 34(1), 15–25. doi: 10.1016/j.infbeh.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Corbetta D, & Bojczyk KE (2002). Infants return to two-handed reaching when they are learning to walk. Journal of Motor Behavior, 34, 83–95. doi: 10.1080/00222890209601933 [DOI] [PubMed] [Google Scholar]
- Corbetta D, & Snapp-Childs W (2009). Seeing and touching: The role of sensory-motor experience on the development of infant reaching. Infant Behavior and Development, 32, 44–58. doi: 10.1016/j.infbeh.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Corbetta D, & Thelen E (1996). The developmental origins of bimanual coordination: A dynamic perspective. Journal of Experimental Psychology-Human Perception and Performance, 22(2), 502–522. doi: 10.1037/0096-1523.22.2.502 [DOI] [PubMed] [Google Scholar]
- Corbetta D, & Thelen E (1999). Lateral biases and fluctuations in infants' spontaneous arm movements and reaching. Developmental Psychobiology, 34, 237–255. doi: 10.1002/(SICI)1098-2302(199905)34:2 [DOI] [PubMed] [Google Scholar]
- Corbetta D, & Thelen E (2002). Behavioral fluctuations and the development of manual asymmetries in infancy: Contributions of the dynamic systems approach. Handbook of neuropsychology, 8(1), 311–330. [Google Scholar]
- Corbetta D, Thurman SL, Wiener RF, Guan Y, & Williams JL (2014). Mapping the feel of the arm with the sight of the object: On the embodied origins of infant reaching. Frontiers in Psychology, 5, 576. doi: 10.3389/fpsyg.2014.00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha AB, Babik I, Ross SM, Logan SW, Galloway JC, Clary E, & Lobo MA (2018). Prematurity may negatively impact means-end problem solving across the first two years of life. Research in Developmental Disabilities, 81, 24–36. doi: 10.1016/j.ridd.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMercurio AJ, Connell JP, Clark M, & Corbetta D (2018). A naturalistic observation of spontaneous touches to the body and environment in the first 2 months of life. Frontiers in Psychology, 9, 2613. doi: 10.3389/fpsyg.2018.02613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusing SC, & Harbourne RT (2010). Variability in postural control during infancy: Implications for development, assessment, and intervention. Physical Therapy, 90, 1838–1849. doi: 10.2522/ptj.2010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspieler C, Bos AF, Libertus ME, & Marschik PB (2016). The general movement assessment helps us to identify preterm infants at risk for cognitive dysfunction. Frontiers in Psychology, 7, 406. doi: 10.3389/fpsyg.2016.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan MK, & Iverson JM (2007). The influence of mouthing on infant vocalization. Infancy, 11, 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetters L, Sapir I, Chen YP, Kubo M, & Tronick E (2010). Spontaneous kicking in full-term and preterm infants with and without white matter disorder. Developmental Psychobiology, 52, 524–536. doi: 10.1002/dev.20455 [DOI] [PubMed] [Google Scholar]
- Field J (1977). Coordination of vision and prehension in young infants. Child Development, 48, 97–103. doi : 10.2307/1128886 [DOI] [PubMed] [Google Scholar]
- Gibson EJ (1988). Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annual Review of Psychology, 39(1), 1–41. doi: 10.1146/annurev.ps.39.020188.000245 [DOI] [Google Scholar]
- Gibson EJ, & Pick AD (2000). An ecological approach to perceptual learning and development. Oxford, UK: Oxford University Press. [Google Scholar]
- Goldfield BA, & Reznick JS (1990). Early lexical acquisition: Rate, content, and the vocabulary spurt. Journal of Child Language, 17, 171–183. doi: 10.1017/S0305000900013167 [DOI] [PubMed] [Google Scholar]
- Goldfield EC (1993). Dynamic systems in development: Action systems. In Thelen E & Smith L (Eds.), Dynamic approaches to development: Applications. Cambridge, MA: MIT Press. [Google Scholar]
- Grönqvist H, Strand-Brodd K, & von Hofsten C (2011). Reaching strategies of very preterm infants at 8 months corrected age. Experimental Brain Research, 209(2), 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadders-Algra M (2002). Variability in infant motor behavior: A hallmark of the healthy nervous system. Infant Behavior and Development, 25, 433–451. doi: 10.1016/S0163-6383(02)00144-3 [DOI] [Google Scholar]
- Heathcock JC, Bhat AN, Lobo MA, & Galloway JC (2004). The performance of infants born preterm and full-term in the mobile paradigm: Learning and memory. Physical Therapy, 84(9), 808–821. doi: 10.1093/ptj/84.9.808 [DOI] [PubMed] [Google Scholar]
- Hopkins B, & Rönnqvist L (2002). Facilitating postural control: Effects on the reaching behavior of 6-month-old infants. Developmental Psychobiology, 40, 168–182. doi: 10.1002/dev.10021 [DOI] [PubMed] [Google Scholar]
- Iverson JM (2010). Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language, 37, 229–261. doi: 10.1017/S0305000909990432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, & Thelen E (1999). Hand, mouth, and brain: The dynamic emergence of speech and gesture. Journal of Consciousness Studies, 6, 19–40. [Google Scholar]
- Jouen F, & Molina M (2005). Exploration of the newborn’s manual activity: A window onto early cognitive processes. Infant Behavior and Development, 28(3), 227–239. doi: 10.1016/j.infbeh.2005.05.001 [DOI] [Google Scholar]
- Kelso JAS, & Tuller B (1984). A dynamical basis for action systems. In Gazzaniga MS (Ed.), Handbook of cognitive neuroscience (pp. 321–356). New York: Plenum Press. [Google Scholar]
- Kimmerle M, Ferre CL, Kotwica KA, & Michel GF (2010). Development of role-differentiated bimanual manipulation during the infant’s first year. Developmental Psychobiology, 52, 168–180. doi: 10.1002/dev.20428 [DOI] [PubMed] [Google Scholar]
- Kugler PN, & Turvey MT (1987). Information, natural law, and the self-assembly of rhythmic movement. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Landry SH, & Chapieski ML (1988). Visual attention during toy exploration in preterm infants: Effects of medical risk and maternal interactions. Infant Behavior and Development, 11, 187–204. doi: 10.1016/S0163-6383(88)80005-5 [DOI] [Google Scholar]
- Lasky RE (1977). The effect of visual feedback of the hand on the reaching and retrieval behavior of young infants. Child Development, 48, 112–117. doi: 10.2307/1128888 [DOI] [PubMed] [Google Scholar]
- LeBarton ES, & Iverson JM (2013). Fine motor skill predicts expressive language in infant siblings of children with autism. Developmental Science, 16(6), 815–827. doi: 10.1111/desc.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew AR, & Butterworth G (1997). The development of hand-mouth coordination in 2- to 5-month-old infants: Similarities with reaching and grasping. Infant Behavior and Development, 20, 59–69. doi: 10.1016/S0163-6383(97)90061-8 [DOI] [Google Scholar]
- Libertus K, & Violi DA (2016). Sit to talk: Relation between motor skills and language development in infancy. Frontiers in Psychology, 7, 475. doi: 10.3389/fpsyg.2016.00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifter K, & Bloom L (1989). Object knowledge and the emergence of language. Infant Behavior and Development, 12, 395–423. doi: 10.1016/0163-6383(89)90023-4 [DOI] [Google Scholar]
- Lobo MA, & Galloway JC (2008). Postural and object-oriented experiences advance early reaching, object exploration, and means-end behavior. Child Development, 79, 1869–1890. doi: 10.1111/j.1467-8624.2008.01231.x [DOI] [PubMed] [Google Scholar]
- Lobo MA, & Galloway JC (2013). The onset of reaching significantly impacts how infants explore both objects and their bodies. Infant Behavior and Development, 36, 14–24. doi: 10.1016/j.infbeh.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Lobo MA, Kokkoni E, Cunha AB, & Galloway JC (2015). Infants born preterm demonstrate impaired object exploration behaviors throughout infancy and toddlerhood. Physical Therapy, 95, 51–64. doi: 10.2522/ptj.20130584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MA, Paul DA, Mackley A, Maher J, & Galloway JC (2014). Instability of delay classification and determination of early intervention eligibility in the first two years of life. Research in Developmental Disabilities, 35(1), 117–126. doi: 10.1016/j.ridd.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry RI, & Nicoladis E (2000). Gesture reflects language development: Evidence from bilingual children. Current Directions in Psychological Science, 9(6), 192–196. doi: 10.1111/1467-8721.00092 [DOI] [Google Scholar]
- McCarty ME, & Ashmead DH (1999). Visual control of reaching and grasping in infants. Developmental Psychology, 35, 620–631. doi: 10.1037/0012-1649.35.3.620 [DOI] [PubMed] [Google Scholar]
- Mundy P, & Newell L (2007). Attention, joint attention, and social cognition. Current Directions in Psychological Science, 16(5), 269–274. doi: 10.1111/j.1467-8721.2007.00518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham A (2000). Improvements in object exploration skills may facilitate the development of object segregation in early infancy. Journal of Cognition and Development, 1(2), 131–156. doi: 10.1207/S15327647JCD010201 [DOI] [Google Scholar]
- Needham A, Barrett T, & Peterman K (2002). A pick-me-up for infants’ exploratory skills: Early simulated experiences reaching for objects using ‘sticky mittens’ enhances young infants’ object exploration skills. Infant Behavior and Development, 25(3), 279–295. doi: 10.1016/S0163-6383(02)00097-8 [DOI] [Google Scholar]
- Oudgenoeg-Paz O, & Rivière J (2014). Self-locomotion and spatial language and spatial cognition: Insights from typical and atypical development. Frontiers in Psychology, 5, 521. doi: 10.3389/fpsyg.2014.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudgenoeg-Paz O, Volman MCJM, & Leseman PPM (2012). Attainment of sitting and walking predicts development of productive vocabulary between ages 16 and 28 months. Infant Behavior and Development, 35, 733–736. doi: 10.1016/j.infbeh.2012.07.010 [DOI] [PubMed] [Google Scholar]
- Piaget J (1953). The origin of intelligence in the child. London: Routledge & Kegan Paul. [Google Scholar]
- Piek JP (2002). The role of variability in early motor development. Infant Behavior and Development, 25(4), 452–465. doi: 10.1016/S0163-6383(02)00145-5 [DOI] [Google Scholar]
- Pogetti LS, de Souza RM, Tudella E, & Teixeira LA (2013). Early infant’s use of visual feedback in voluntary reaching for spatial target. Frontiers in Psychology, 4, 520. doi: 10.3389/fpsyq.2013.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechtl HFR (1990). Qualitative changes of spontaneous movements in fetus and preterm infants are a marker of neurological dysfunction. Early Human Development, 23, 151–159. doi: 10.1016/0378-3782(90)90011-7 [DOI] [PubMed] [Google Scholar]
- Rakison DH, & Woodward AL (2008). New perspectives on the effects of action on perceptual and cognitive development. Developmental Psychology, 44(5), 1209–1213. doi: 10.1037/a0012999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Cheong YF, Congdon R, & du Toit M (2004). HLM 6: Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International, Inc. [Google Scholar]
- Rochat P (1992). Self-sitting and reaching in 5-to 8-month-old infants: The impact of posture and its development on early eye-hand coordination. Journal of Motor Behavior, 24(2), 210–220. doi: 10.1080/00222895.1992.9941616 [DOI] [PubMed] [Google Scholar]
- Rochat P (1993). Hand-mouth coordination in the newborn: Morphology, determinants, and early development of a basic act. In Savelsbergh GJP (Ed.), The development of coordination in infancy (pp. 265–288). Amsterdam, Netherlands: North-Holland/Elsevier Science Publishers. [Google Scholar]
- Rochat P, & Bullinger A (1994). Posture and functional action in infancy. In Vyt A, Bloch H, & Bornstein MH (Eds.), Early child development in the French tradition: Contributions from current research (pp. 15–34). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Rochat P, & Goubet N (1995). Development of sitting and reaching in 5-6-month-old infants. Journal of Motor Behavior, 24, 210–220. doi: 10.1016/0163-6383(95)90007-1 [DOI] [PubMed] [Google Scholar]
- Ruff HA, McCarton C, Kurtzberg D, & Vaughan HG (1984). Preterm infants' manipulative exploration of objects. Child Development, 55, 1166–1173. doi: 10.2307/1129985 [DOI] [PubMed] [Google Scholar]
- Sawilowsky SS (2009). New effect size rules of thumb. Journal of Modern Applied Statistical Methods, 8, 467–474. [Google Scholar]
- Smith LB, & Gasser M (2005). The development of embodied cognition: Six lessons from babies. Artificial Life, 11(1–2), 13–29. doi: 10.1162/1064546053278973 [DOI] [PubMed] [Google Scholar]
- Smith LB, & Sheya A (2010). Is cognition enough to explain cognitive development? Trends in Cognitive Science, 2, 725–735. doi: 10.1111/j.1756-8765.2010.01091.x [DOI] [PubMed] [Google Scholar]
- Smith LB, & Thelen E (2003). Development as a dynamic system. Trends in Cognitive Sciences, 7(8), 343–348. [DOI] [PubMed] [Google Scholar]
- Smith BA, Trujillo-Priego IA, Lane CJ, Finley JM, & Horak FB (2015). Daily quantity of infant leg movement: Wearable sensor algorithm and relationship to walking onset. Sensors, 15(8), 19006–19020. doi: 10.3390/s150819006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soska KC, & Adolph KE (2014). Postural position constrains multimodal object exploration in infants. Infancy, 19, 138–161. doi: 10.1111/infa.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soska KC, Adolph KE, & Johnson SP (2010). Systems in development: Motor skill acquisition facilitates three-dimensional object completion. Developmental Psychology, 46, 129–138. doi: 10.1037/a0014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone W, & Yoder P (2001). Predicting spoken language level in children with autism spectrum disorders. Autism, 5, 341–361. doi: 10.1177/1362361301005004002 [DOI] [PubMed] [Google Scholar]
- Thelen E (1990). Coupling perception and action in the development of skill: A dynamic approach. In Bloch H & Bertenthal BI (Eds.), Sensory-motor organizations and development in infancy and early childhood. Boston: Kluwer Academic. [Google Scholar]
- Thelen E, Corbetta D, Kamm K, Spencer JP, Schneider K, & Zernicke RF (1993). The transition to reaching: Mapping intention and intrinsic dynamics. Child Development, 64, 1058–1098. doi: 10.1111/j.1467-8624.1993.tb04188.x [DOI] [PubMed] [Google Scholar]
- Thelen E, & Smith LB (1994). A dynamic systems approach to the development of cognition and action. Cambridge, MA: Bradford Books/MIT Press. [Google Scholar]
- Thelen E, & Spencer JP (1998). Postural control during reaching in young infants: A dynamic systems approach. Neuroscience and Biobehavioral Reviews, 22(4), 507–514. doi: 10.1016/S0149-7634(97)00037-7 [DOI] [PubMed] [Google Scholar]
- Thelen E, Ulrich BD, & Wolff PH (1991). Hidden skills: A dynamic systems analysis of treadmill stepping during the first year. Monographs of the Society for Research in Child Development, i–103. doi: 10.2307/1166099 [DOI] [PubMed] [Google Scholar]
- Thomas BL, Karl JM, & Whishaw IQ (2015). Independent development of the Reach and the Grasp in spontaneous self-touching by human infants in the first 6 months. Frontiers in Psychology, 5, 1–11. doi: 10.3389/fpsyg.2014.01526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touwen BCL (1978). Variability and stereotypy in normal and deviant development. In Apley J (Ed.), Care of the Handicapped Child. Clinics in Developmental Medicine No. 67 (pp. 99–110). London: Heinemann Medical Books. [Google Scholar]
- van Beck Y, Hopkins B, Hoeksma J, & Samson JF (1994). Prematurity, posture, and the development of looking behavior during early communication. Journal of Child Psychology and Psychiatry, 35, 1093–1107. doi: 10.1111/j.1469-7610.1994.tb01811.x [DOI] [PubMed] [Google Scholar]
- von Hofsten C, & Rosander K (1997). Development of smooth pursuit tracking in young infants. Vision Research, 37(13), 1799–1810. doi: 10.1016/S0042-6989(96)00332-X [DOI] [PubMed] [Google Scholar]
- Weiss LG, Oakland T, & Aylward GP (2010). Bayley-III clinical use and interpretation. Academic Press. [Google Scholar]
- Wilcox T, Woods R, Chapa C, & McCurry S (2007). Multisensory exploration and object individuation in infancy. Developmental Psychology, 43(2), 479–495. doi: 10.1037/0012-1649.43.2.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnroks L, & van Veldhoven N (2003). Individual differences in postural control and cognitive development in preterm infants. Infant Behavior and Development, 26, 14–26. doi: 10.1016/S0163-6383(02)00166-2 [DOI] [Google Scholar]
- Yu C, & Smith LB (2013). Joint attention without gaze following: Human infants and their parents coordinate visual attention to objects through eye-hand coordination. PLOS ONE, 8(11), e79659. doi: 10.1371/journal.pone.0079659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccarini M, Guarini A, Iverson JM, Benassi E, Savini S, Alessandorni R, … Sansavini A (2018). Does early object exploration support gesture and language development in extremely preterm infants and full-term infants? Journal of Communication Disorders, 76, 91–100. doi: 10.1016/j.jcomdis.2018.09.004 [DOI] [PubMed] [Google Scholar]
- Zuccarini M, Guarini A, Savini S, Iverson JM, Aureli T, Alessandorni R, … Sansavini A (2017). Object exploration in extremely preterm infants between 6 and 9 months and relation to cognitive and language development at 24 months. Research in Developmental Disabilities, 68, 140–152. doi: 10.1016/j.ridd.2017.06.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.