Abstract
Biomaterial implants for the sustained delivery of therapeutics can be utilized to deliver drugs at near-constant rates over extended time frames to provide an alternative to daily oral medications. The biomaterials used to construct these systems, however, are often not bioresorbable and thus require a secondary surgery for removal from the body, and fabrication of these systems may require the use of harsh chemical solvents. To address these shortcomings, a fabrication process was developed to generate biodegradable drug reservoir systems from regenerated silk fibroin protein solution (23% w/v). The tubular systems, with an inner diameter of 2.0 mm and wall thickness < 250µm, were developed using an all-aqueous solution-gel-solid phase transition curing process. Two different clinically-relevant therapeutics were released at near-constant rates for 30 days (> 100µg/day). The protein secondary structure of the devices consisted of 40% crystalline beta sheet. Mechanically, radial compression (1mm/min) of unloaded systems demonstrated Young’s moduli similar to cancellous (spongy) bone (100 to 250 MPa) and the systems showed good recovery under cyclic compression (to 17.5% strain). The devices could be generated in complex shapes (e.g., hollow cylinders) via an additive molding process, offering the potential for drug delivery but also for broader applications in tissue engineering and diagnostics.
Keywords: silk fibroin, drug delivery, zero-order release, drug reservoirs, letrozole, rifampicin
I |. Introduction
Implantable drug delivery systems enable sustained therapeutic release over clinically-relevant timescales, providing enhanced utility over oral drug regimens. Indeed, with the appropriate selection of device material, disease state, and relevant drug product, sustained delivery systems offer improved bioavailability1, continuous maintenance of serum drug concentrations within the therapeutic window, and adaptability for localized release2,3. From a provider perspective, implantable systems can reduce regimen compliance issues and abuse while remaining cost effective4, as long as the systems can be fabricated in a cost-effective manner with user acceptability. As a result of these advantages, between 2008 and 2014 the global market for drug delivery systems rose from $134B to nearly $200B5, with implant and reservoir systems comprising roughly one third of that market6. However, significant limitations remain where current implantable delivery systems that are non-biodegradable (such as Surodex®, Supprelin® LA and Implanon®) require a secondary surgery to remove the device from the delivery site, increasing costs as well as the risk of implant site infection. In addition, there is a need to reduce the use of harsh pH conditions and organic solvents often required during implant fabrication, such as with poly(lactic-co-glycolic acid), to avoid degradation to therapeutics7,8. At the same time, expanding the library of bioresorbable materials can address a more diverse set of medical conditions, such as those requiring medium-to-long-term therapeutic treatment.
To address these shortcomings, there has been a significant amount of research focused on silk fibroin and silk fibroin-hybrid drug delivery systems2,9,10. Much of this interest stems from unique properties of silk fibroin related to drug reservoir construction and function, including bioresorbability, tunable degradation, all-aqueous processing and purification, stabilization and preservation of a wide array of clinically-relevant small molecules and macromolecules, and compatibility with manufacturing techniques to support the generation of a wide array of material formats11,12,13,14,15,16,17. The fibroin protein is a block copolymer containing both amorphous and crystalline forming regions, with the crystalline region containing repetitive sequences of amino acids (-Gly-Ala-Gly-Ala-Gly-Ser-)18. The two proteins that comprise the fibroin macrostructure include a heavy chain (~390kDa) and a l light chain (~25kDa)19. The hydrophobic/hydrophilic features of the proteins and their subsequent assembly into different material formats supports diverse therapeutic encapsulation and degradation profiles for these materials.
The structure and versatility of the silk fibroin proteins has been utilized to generate a range of silk-based drug delivery systems with a variety of therapeutics10,11,12,13,14,20,21,22. For example, near-zero-order release of Anastrozole, a non-steroidal aromatase inhibitor, was demonstrated from fiber-spun silk rods over a 30-day period9. Anastrozole is often prescribed orally for breast cancer treatment, as ARMIDEX®. In the silk delivery system, the compound was released from the silk reservoir tubes in a sustained manner both in vitro and in a Sprague-Dawley rat model. While fiber-spinning represents an effective approach to drug encapsulation and release, it is a relatively low-throughput way to produce silk-based reservoir systems. Similarly, approaches that involve machining solid blocks of silk23,24,25,26 to achieve complex geometries necessary for drug reservoir designs results in significant amounts of wasted material, making it difficult to scale in a cost-effective manner.
Here we developed a simple, reproducible process for additive manufacturing of “complex” 3-dimensional monolithic silk drug reservoirs (SDRs) that are reversibly swellable and contain geometries relevant to implantable drug delivery systems. The method involves injecting concentrated silk fibroin solution (22–23% w/v) into customized wax molds, where it undergoes a controlled sol-gel transition27 over several days, increasing the silk II ß-sheet content. After this gradual reduction in water content, the gels are allowed to solidify under ambient conditions and are then re-hydrated in water while still in the original molds. Once the molds are removed from the water bath, they release the gels and re-solidify prior to drug loading. Powdered drug is then loaded into the solidified gels using a polytetrafluoroethylene (PTFE) wire, after which they are capped with a small volume of concentrated silk that solidifies and encapsulates the reservoir.
Letrozole (LET), sold under the brand name Femara®, is prescribed in the treatment of hormonally-responsive breast cancer in postmenopausal women (2.5 mg, oral delivery, daily). In several cases LET has been shown to be more potent in its anti-cancer effects than similarly prescribed aromatase inhibitors such as Anastrozole28,29. However, if prescribed for extended periods of time, LET and other aromatase inhibitors can have undesirable systemic side effects such as increased bone porosity and the associated greater incidence of osteoporotic fractures30. Accordingly, treatment pathways (such as localized delivery) that limit systemic exposure are desirable. Rifampicin (RIF) is an antibiotic used in combating several types of bacterial infections, including tuberculosis31 and methicillin-resistant Staphylococcus aureus (MRSA)32, commonly prescribed at doses between 300 – 600 mg p.o. q.d. While RIF exhibits excellent bioavailability through oral delivery, repeated administration can reduce bioavailability from 80–90% to below 70% in some cases33.
The two small-molecule drugs (LET C17H11N5, m = 285.3 g/mol; RIF C43H58N4O12, m = 822.4 g/mol) differ in water solubility (LET ~150 µg/mL; RIF up to 2.5 g/mL at 25˚C), lipophilicity (LET log P(octanol/water) = 2.5; RIF log P(octanol/water) = 3.85), and ionic character at physiological pH (LET pKa = 2.0 – 3.0; RIF pKa = 7.0 – 8.0). Given the potentially debilitating side effects of LET under extended oral dose regimens, and the previously-demonstrated use of RIF in implantable release systems34,35, these are two viable therapeutic candidates to demonstrate sustained, zero-order release, using silk drug delivery reservoirs reported here, the objective of the present work.
II |. Materials and Methods
2.1. Materials
Bombyx mori silkworm cocoons were purchased from Tajima Shoji Co., LTD (Yokohama, Japan). Letrozole, Rifampicin and other chemical agents were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Regenerated Silk Fibroin Solution
Purified solutions of silk fibroin protein were prepared from cocoons using well-established methods36. Cocoons were manually cut and boiled for 60 minutes in a 0.02 M solution of sodium carbonate. Following a three-step rinsing process in deionized (DI) water, the newly de-gummed silk fiber mats were dried overnight in a fume hood, and then dissolved in a 9.3 M solution of lithium bromide. This protein–salt solution was incubated at 60˚C for 4 hours, resulting in a ~20% w/v solution. The protein–salt solution was then placed dialysis tubing (3.5kDa MWCO, Fisher Scientific, Hampton, NH), comprised of a 28 µm thick semipermeable membrane of regenerated cellulose, and dialyzed against a set volume of DI water for 48 hours with frequent water changes. The resulting purified fibroin solution had a concentration of 5–6% w/v, which was centrifuged in two 20-minute increments at 9,000 rpm and then stored for repeated use as a stock solution at 4˚C. Water-stable silk films were prepared from this stock solution by casting 100 µL aliquots of diluted stock (4% w/v) on 4 cm2 blocks of polydimethylsiloxane (PDMS) (Sylgard 184, Dow Corning, Corp. Midland, MI), which dried over 12 hours. The dried films (4 mg) then underwent a post processing water-annealing treatment for 6 hours, increasing the crystalline character of the films and rendering them water-stable37. Concentrated silk solution was also prepared from the starting 6% w/v stock by placing known volumes into dialysis tubing and exposing the solution to air under a controlled flow rate in the fume hood, allowing the solution to reach concentrations between 11–12% w/v. This semi-concentrated solution was then concentrated to 22–23% w/v using a Centrivap concentrator (Labconco, Kansas City, MO) operating at 20 Hz, 55˚C and connected to a 120 W vacuum pump (Gast Manufacturing, Benton Harbor, MI).
2.3. Fabrication of Silk Drug Reservoirs (SDRs)
A custom setup was developed for fabrication of silk drug reservoirs derived via an all-aqueous process (AP-SDRs), as illustrated in Figure 1. First, modular molds were machined in order to create the thin-walled cylindrical shape reservoir geometry for implant design. Molds consisted of two parts, a machined inner wax insert and an outer polystyrene sheath. The wax insert was machined from a starting wax block (Machinable Wax, Inc., Traverse City, MI), using an A Trak DPM SX3 3-axis bed mill (Southwestern Industries, Shrewsbury, MA). The inserts were 14.0 mm in height, with a minor diameter of 3.00 mm and a major diameter of 4.73 mm. After machining, a hydrophobic mold release coating was applied as an aerosol spray to each insert for 2–3 seconds (CRC Industries, Horseham Township PA). The polystyrene sheaths were cut as open-end segments from 3 mL syringes (BD, Franklin Lakes, NJ). Concentrated (22–23% w/v) silk fibroin solution was warmed to room temperature and injected into prepared molds using a 22G needle affixed to a 3-mL syringe (BD, Franklin Lakes, NJ). The solution was cast radially around the inner 3.0 mm wax axis so as not to create air pockets and filled approximately 2 mm past the terminus of the wax insert for a total fill volume of ~200 µL. Filled molds were then sealed using parafilm tape (Bemis NA, Neenah, WI). Afterwards, the silk solution in the filled mold underwent a sol-gel transition over the course of 7–10 days under near-constant temperature (25˚C). After the sol-gel process was complete, shaped silk gels were removed from their polystyrene sheaths and allowed to cure in open air for 8 hours at ~30% residual humidity, chosen based on prior experimentation with monolithic silk materials23,24, until the water content of the near-solidified silk structures was between 6–8% of system mass. These near-solid silk structures were then placed, still on the inserts, in a DI water bath to soak with subtle agitation for 12 hours. Upon removal from the water bath, the now-swollen systems were able to be easily removed from the wax inserts and placed to dry on a curved polystyrene surface in open-air conditions for 8–10 hours. During the drying process the systems solidified completely into hollow, glassy solids and their water content was reduced to below 5% system mass. Final devices were 8.0 ± 0.5mm in height, O.D. = 2.3 ± 0.05mm, I.D. = 2.0 ± 0.05mm. At this point, powdered drug was loaded directly into the hollow cavity of the systems using a short section of 12G PTFE wire (Bulk Wire, Yorba Linda CA). The end of the PTFE wire was flattened enabling it to be used as a lab utensil thus providing efficient transfer of small mass aliquots of powdered drug into the system cavity. PTFE was selected to minimize any issues that could arise during loading due to static buildup. Mass loading for both therapeutics was 30 ± 2.0g. In order to explore the mechanical properties of the SDR geometry, systems were also fabricated via an alternate solvent processing pathway (SP-SDRs) using the same mold and injection processes as was used to make AP-SDRs. Once the molds were filled with concentrated silk solution, they were placed upright in 2.0 mL Eppendorf tubes. Next, 100% MeOH was injected into the tubes, making contact with the silk solution. The denser silk solution in the mold remained submerged in the methanol bath, and over a period of 1–2 hours the concentrated silk in the mold underwent rapid gelation. The now-gelled systems on the inserts were then soaked in a water bath for 12 hours to remove any methanol under constant stirring and DI replacement, after which they were dried in the same manner as the AP-SDRs and achieved similar dimensions once fully cured.
Figure 1.
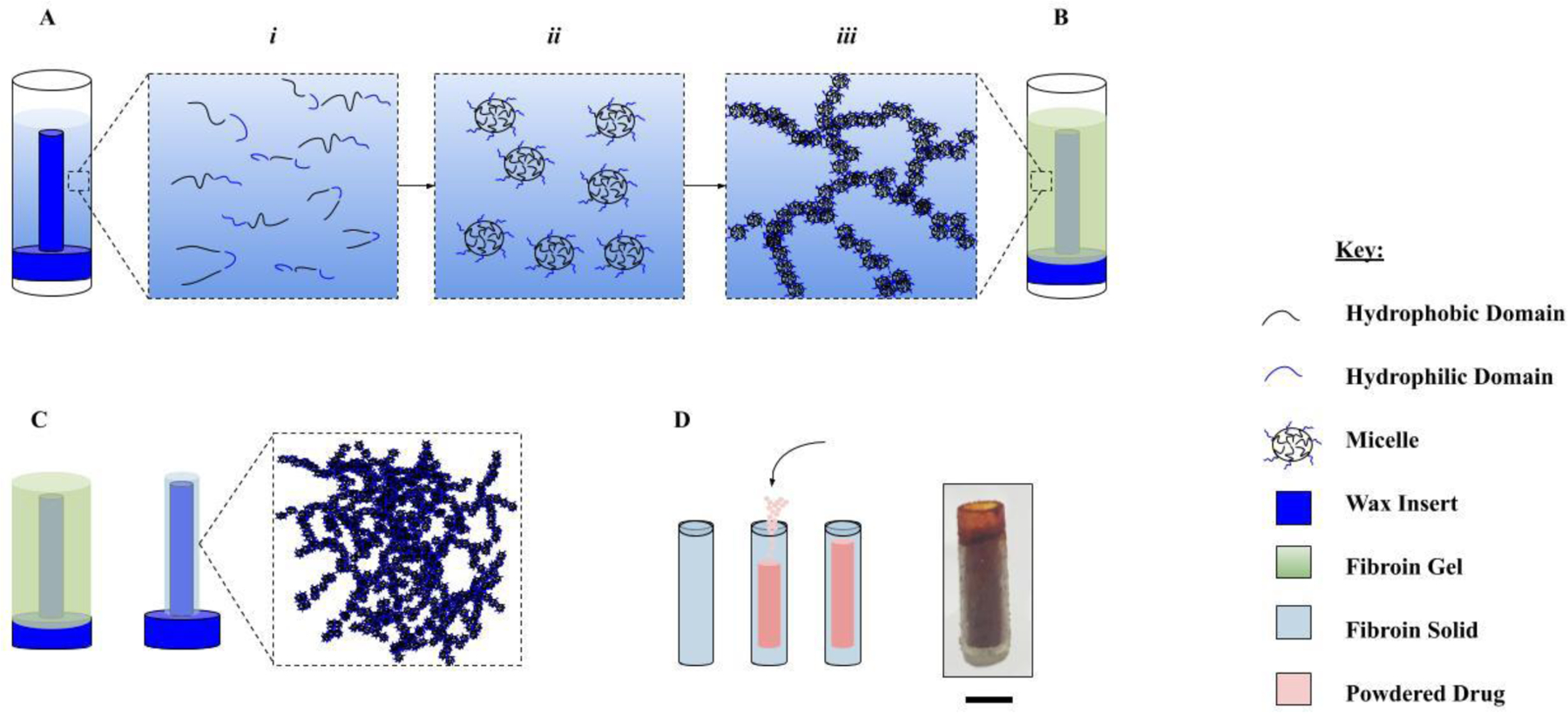
Fabrication of AP-SDRs. Wax inserts are milled from stock wax blocks and sprayed with a hydrophobic mold release. 200µL of concentrated silk solution is then injected around the mold using a 22G syringe needle. [A] Sol-gel transition: i) silk peptide fragments with both hydrophilic and hydrophobic domains arrange into micelles (ii) at high concentrations and as free water is slowly removed from the system via slow dry process. [B] Increased interaction of micelles causes aggregation, packing and formation of nanofibrillar networks (iii) resulting in a stable gel structure. [C] Additional removal of free water results in a densely packed crystalline fibroin solid. [D] This AP-SDR system is loaded directly with powdered drug and the ends are capped with concentrated silk. Scale bar is 2.0mm.
2.4. Scanning Electron Microscopy
Scanning Electron Microscopy (SEM) images of AP-SDR systems were taken using a Field Emission SEM Ultra55 (Zeiss, Oberkochen, Germany) with a tungsten filament in secondary electron imaging mode at 15 kV accelerating voltage, ~300 mA filament current and 39 mm working distance. Cross-sectional morphology and wall thickness measurements were taken to qualify system reproducibility at the microscale.
2.5. Swelling Kinetics
The swell ratios q(t) of AP-SDRs were calculated9,38 using Equation 1:
| Eq. 1 |
Here Mh(t) is the hydrated mass of the system as a function of time while Md is the original dry mass. AP-SDRs were weighed using a Mettler AT20 precision microbalance (Mettler Toledo Solutions, Billerica MA), then immersed in 1.0mL of 1x PBS solution, and weighed at defined time points of 1, 2, 4, 10, 24, 36, and 48 hours to ascertain the time-dependent swell ratios, which were reported as average ± standard deviation for n = 3 systems.
2.6. Fourier Transform Infrared Spectroscopy
Analysis of silk protein secondary structure of reservoir systems was carried out using a Fourier Transform Infrared Spectrophotometer (Alpha-Eco FT-IR, Bruker, Billerica, MA) in the reflectance regime using a Zinc-Selenide (ZnSe) Attenuated Total Reflectance (ATR) setup. A group of n = 6 AP-SDRs were examined, with each sample spectrum given as the Fourier transform of 128 scans (at 4cm−1 resolution) across a wavenumber range of 4–4000 cm-1. An established Fourier Self-Deconvolution (FSD) process39 for quantifying contribution of different silk protein conformations across the amide I (1590–1710 cm−1) region for silk materials was used to further characterize the structure of AP-SDRs. The starting FTIR-ATR spectral data were analyzed using OPUS 5.0 software (Bruker, Billerica, MA). To begin, raw spectra were smoothed using a nine-point smoothing filter (Savitsky-Golay). These smoothed curves then underwent a baseline correction excluding carbon-dioxide (CO2) peaks, after which a Fourier Self-Deconvolution was performed over the amide I region (Lorentzian line shape half-bandwidth 25 cm−1; noise reduction factor 0.3). Spectra were then cut over the desired wavenumber range and curve fit using Gaussian line shapes to generate 12 curves covering the relevant deconvoluted peaks of the cut spectra. The area under these curves was used to quantify the weighted contributions of different silk chain conformations as related to their known positions within the amide I region. Conformation contributions were reported as average ± standard deviation for the n = 6 systems analyzed.
2.7. Protease Degradation
Protease XIV degradation of SDRs was carried out using established methods for the degradation of silk fibroin materials16. AP-SDRs of known dry mass (36 ± 5 mg) were hydrated to equilibrium water uptake, weighed, then subsequently incubated at 37˚C in either a 1.0 mL control solution (n = 3) of 1x PBS, or an experimental solution (n = 3) of 2.0 U/mL Protease XIV (Sigma Aldrich, St. Louis, MO). To ensure continued activity of enzyme, systems in the experimental group were transferred to fresh solutions daily. System mass was recorded at defined time points over the course of a 30-day period. Degradation data are reported as average ± standard deviation for the n = 3 systems across the two groups.
2.8. Mechanical Testing
Static compression testing was performed on both AP-SDRs and SP-SDRs to highlight any differences in mechanical properties, and to compare them to other silk material formats and biological tissues. Compression testing was carried out using an Instron 3366 test frame setup with a 100 N load cell. Testing was performed via BlueHill 3 software (Intstron, Norwood MA) in the compression regime. Raw data gathered included extension (mm) and load (N), which were converted to a measurement of Young’s modulus calculated over the linear strain region. SDR systems underwent a circumferential (transverse) compressive stress response rather than an axial response given how they would likely experience compressive stress in an in vivo setting, and based on where the systems were likely to fail. Compression occurred at a rate of 1mm / min, and all mechanical testing took place on SDRs that had been hydrated to equilibrium in 1x PBS buffer. An equation for Young’s modulus was derived using a specific definition of compressive strain and local bending forces applied to thin-walled cylinders40. While both SDR variants had measured wall thicknesses h slightly larger than one tenth of the swollen radius r (h/r = 0.120), this derivation of the thin-walled assumption was deemed to be the most accurate given the geometry of the systems and the compression regime. Here, a specific expression describing the vertical shortening of the cylindrical shell diameter ∂ due to a transverse distributed compressive force P is defined as:
| Eq. 2 |
Where a is the radius of the mid-thickness of the shell, 2l is the axial length of the cylinder, and D is the flexural rigidity, relating to the bending moment of the material due to compression. D is defined as:
| Eq. 3 |
E is the Young’s Modulus of the material and ν is Poisson’s ratio, which when taken to be ~0.5 as for most incompressible materials simplifies this expression, which can be rearranged and plugged into Eq. 4 to solve for E(a,h,l,dP/d∂) as in:
| Eq. 4 |
Dynamic compression testing was carried out under the same experimental conditions, and the testing regime was chosen as a simple way to mimic compressive loads often experienced by implantable systems in biological soft tissue. Systems underwent cyclic loading for n = 10 cycles at a frequency of 12.5 mHz up to a strain percentage of 17.5%. This value was chosen as it pushed the boundary of the linear strain region as ascertained from static testing. A comparison of moduli of the two SDR types calculated from static compression data was carried out using a Student’s T-Test in MATLAB software (n = 5 SDR systems of each type). Significance was considered for p values < 0.05. Similarly, a comparison of maximum load during cyclical loading of the two fabrication types (n = 5 each) was carried out in a similar manner looking at the load value after the 0.5 cycle mark.
2.9. Model Drug Binding and Release from Silk Fibroin Films
Kinetic binding and release studies of both RIF and LET were conducted to determine their respective association with silk fibroin. Binding took place using DI water as the binding media to prevent any unwanted competitive binding of ionic species in 1x PBS at exposed charged domains present along the fibroin polymer chain. For these studies, 1 mL aliquots of stock solution LET and RIF stock solution (117 and 111 µg/mL respectively) served as the binding media, and binding groups were comprised of n = 6 silk films per drug. Adsorption took place at 37˚C with intermittent time points taken at 1, 5, 24 and 120 hr. At each time point drug concentration was calculated via UV-vis spectroscopy using pre-determined absorbance standards. After each timepoint samples were placed back into the binding media without replacement. Binding took place in 2 mL sample volumes undergoing constant agitation using a 2-D rotary shaker operating at 100 rpm. The mass of total bound drug Mb(t) at each time point was calculated using Equation 5 below2:
| Eq. 5 |
Where Do, D(t) and V are starting concentration of drug solution, time point concentration of drug solution, and volume of drug solution respectively. Drug release from silk films was carried out under the same experimental conditions as for kinetic binding, except that the release media was changed from DI water to 1x PBS (pH 7.4) under sink conditions.
Both kinetic binding and release studies took place using two independent subgroups of n = 3 films per drug (n = 6 replicates total per drug). Statistical differences in LET, RIF binding and release characteristics were determined using a student’s t-test via MATLAB software (Natick, MA) for the combined total n = 6 replicates of the two drugs. Data are expressed as mean ± standard deviation (SD). Significance was determined for p values <0.05.
2.10. SDR Release Model and Partition Coefficient
Release profiles of both drugs from AP-SDR systems were ascertained looking at both cumulative and daily release rate data over a 30-day period. Release profiles were analyzed using a commonly applied controlled release model first obtained by Ritger and Peppas41. This simple model describes diffusion kinetics of small molecules swellable systems, defined in general by equation 6:
| Eq. 6 |
Here, the left-hand side is the time-dependent fraction of drug released from the system, k is the rate constant and n is the diffusion exponent that describes the release kinetics over time-course t. Taking the natural logarithm of both sides yields a linear version of the equation to which in vitro release data was fit42:
| Eq. 7 |
In accordance with the model, plots of vs. ln(t) were generated, with the slope of the line giving the diffusion exponent n. Values for n were averaged across all systems in a given release class (RIF, LET), and used to define the release mechanism along with the R2 statistic for the model, used to judge goodness of fit. The release mechanism as defined by n can vary from diffusion-based release (n = 0.5), complex / amorphous release (0.5 < n < 1) to zero-order (constant) release as n approaches 1. The limitations of the model include its conditional application only after the initial “burst” phase of release has been completed (if one exists), as well as the fact that it should only be applied to the first 60% of all subsequent mass to be released from the system43 given that competing release mechanisms can affect data ascertained at both the beginning and end of the release process. Furthermore, it is important to note that this model does not take into account the complex thin-walled cylindrical geometry of the release systems. This would be relevant to make explicit inferences as to how the system would release in vivo, such as approximating release rate for a model of a tissue region of a specific density having a specific surface area of perfusion. Rather, given that the geometry was held constant for all SDRs, this generalized model is applied simply to understand relative quantitative differences that may exist in the release profiles of both drugs and confirm that the release rate from the system is held constant over the 30-day release window.
The partition coefficient Kd is a useful metric that defines drug distribution between a swellable polymeric release material and the surrounding solvent. Kd values greater than 1 indicate higher solubility of a drug species within the polymeric vehicle, a result of a stronger degree of interaction between the drug and the drug vehicle2,20. Here, the partition coefficients for RIF and LET were calculated to compare their respective distributions within AP-SDR systems and the surrounding 1x PBS solvent. Triplicate AP-SDRs were incubated in 1.0 mL of 111µg / mL solutions of LET and RIF aside a blank sample of each concentrated drug solution to account for drug binding to the sidewalls of the containers. Samples were collected from the drug solutions until apparent equilibrium was achieved, and the partition coefficients9,38 of each drug were calculated using equation 8:
| Eq. 8 |
where Vs is the solution volume, Vsys is the volume of the SDR, CB is the equilibrium concentration of the blank drug sample, and Csys is the equilibrium concentration of the SDR samples incubated in the drug solution.
As a benchmark calculation, a steady-state target release rate of LET R was calculated using a continuous infusion, one-compartment model9:
| Eq. 9 |
In this model, t1/2 is the terminal elimination half-life of the drug compound, V is the volume of distribution, and Css is the steady-state blood plasma concentration of drug. Taking standard values found in literature, R was calculated using t1/2, V and Css values of 42 hr, 1.9 L/kg, and 128 nmol/L44, giving a daily LET release rate of 1.65 mg / day, which for a 70 kg person implies a 34% dose reduction could be expected in the case of zero-order LET release from an implantable system compared to the current 2.5 mg oral dose of Femara tablets.
Kd values of both drugs were calculated as mean ± standard deviation, and a student’s t-test was used to compare Kd values for RIF and LET at the p < 0.05 significance level. Values for the diffusion release exponent n were calculated for both drug compounds and displayed as mean ± standard deviation.
III |. Results and Discussion
3.1. System Morphology
Both the aqueous and solvent-processing approaches to SDR fabrication were successful, yielding reproducible systems in terms of morphology (average radial thickness 260 ± 65µm), micron-scale features improved over machined approaches26 (Figure 2), and were reversibly swellable.
Figure 2.
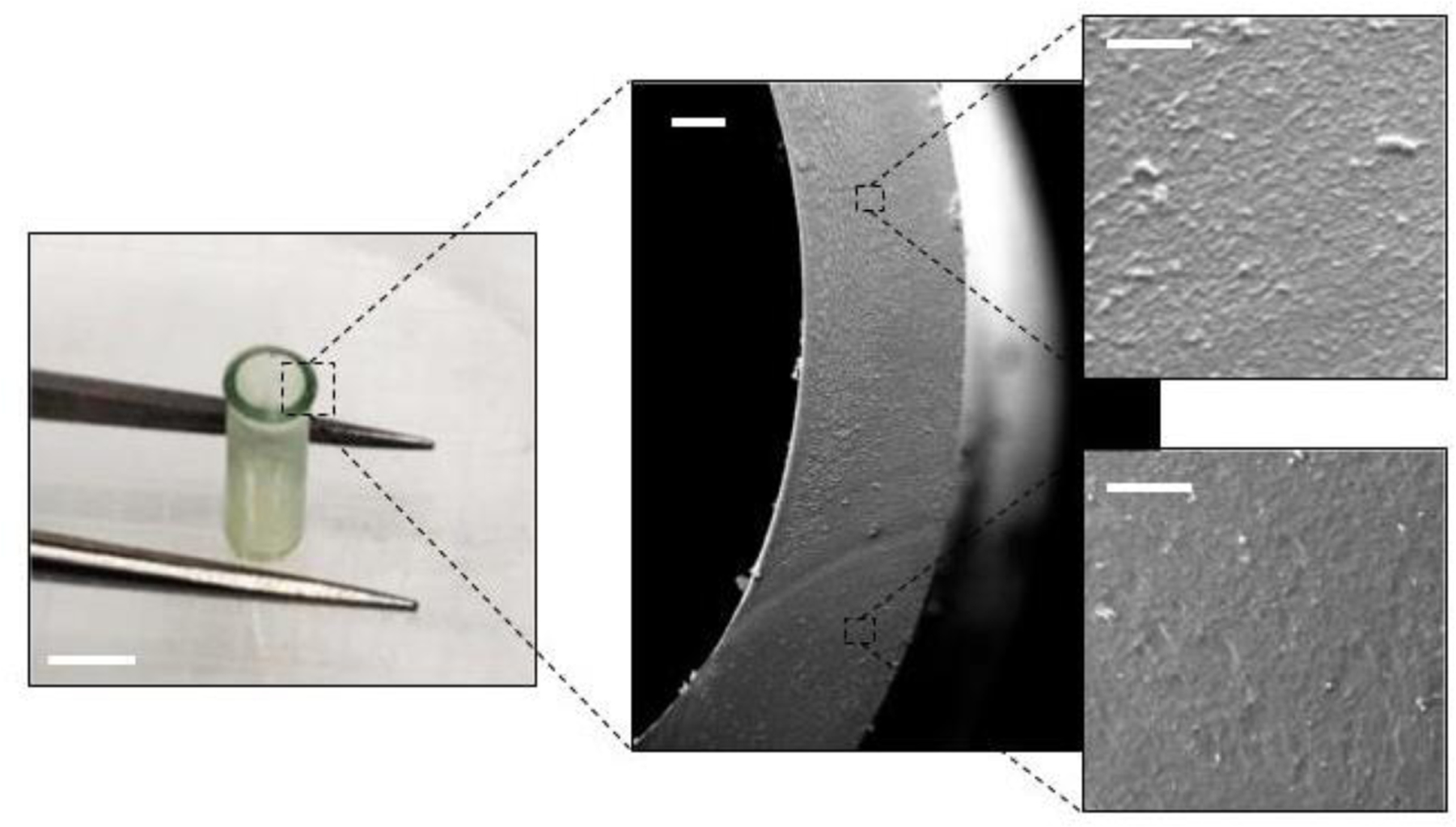
Macroscale (left) and SEM (center, right) images of an AP-SDR reveal consistent features down to the microscale. Consistent wall thickness exhibited by SDRs is a critical attribute of reservoir-type drug implants to ensure uniform drug transport and release across the diffusive membrane. Scale bars: left 2mm, center 100µm, right 20µm.
The effect of reducing the rate of the gel-solid transition on achieving greater reproducibly in SDR fabrication suggests an important contribution of the micro- and nano-scale processes during this material transition. Splitting this process into two steps with an intermediate soaking step also reduces the rate of water removal from the system. As mentioned previously, during the removal of free water there is increased association of nanolattices and nanomatrices as these structures resolve into a solid semi-crystalline matrix. Removing free water too quickly reduces the time for these features to develop homogenously throughout the bulk silk material, resulting in brittle systems that do not cure evenly. The fact that free water acts as a plasticizer in regenerated silk materials45 supports the concept that SDRs formed using an appropriate rate of water removal retain some elasticity and function as compliant materials when hydrated.
The SDR systems resulting from this fabrication process are “hollow silk tubes”, or more specifically, “hollow 3-dimensional monolithic silk structures having orthogonal functions.” While prior work has demonstrated the fabrication of similar systems, the SDRs developed here are differentiated from these prior systems in several ways. From a processing perspective, tubular silk structures have been previously generated via gel-spinning46 and dip-coating methods15. In the case of gel-spinning, silk tubes having excellent fiber alignment and diameters of ~1 mm have been generated. While this is useful for tissue engineering applications, tubes generated in this manner can have a degree of porosity. When these processes were adapted to generate silk tubes able to be loaded with powder drug9, the resulting tubes had excellent uniformity in thickness and were reproducible. However, the fiber-spinning process in general has scaling limitations in that it requires a custom setup involving mechanized and heating components that fabricate rods one at a time. Conversely, the dip-coating method for silk tube fabrication is relatively low-tech in that it involves immersing a Teflon-coated wire into concentrated silk solution, manually twisting the rod to ensure even coating of multiple layers of silk, curing the mandrel in methanol, then allowing the silk to cure overnight into rods47. Again, while this process yielded tubes with mechanical properties and material features useful for blood vessel engineering, the quality of the tubes was not consistent with that needed for a device to be functionalized into a drug reservoir implant. The SDR systems detailed here are most closely associated in structure and form to silk monoliths fabricated previously using the sol-gel-solidification processing approach23. An important novel aspect not present in prior work was the demonstration of 3-dimensional structures that are hollow, and generated under static molding conditions and shrunk to solid form without the use of specialized molds that have to shrink along with the gels during the gel-solid transition. This manner of fabrication allows for the systems to behave as reservoirs rather than bulk-loaded monolithic systems. While bulk-loaded systems are slightly easier to develop (whether solid or hydrogel), in practice they can present the challenge of having a burst release phase early in their release profile, which alters the initial release kinetics and fails to provide a stable concentration of drug available for circulation. Conversely, the geometry of reservoir systems minimizes the burst effect because the drug must diffuse through a fixed thickness of the polymer network at all phases of release. Additionally, reservoir systems are advantageous for delivery of poorly soluble therapeutics because their geometry accommodates greater doses of drug than a monolithic hydrogel or solid film system, which must often be loaded by soaking the systems in concentrated drug solution. These silk tubes are therefore novel in their physical form as well as in their application towards sustained zero-order drug delivery.
3.2. Swelling and Hydration Characteristics
AP-SDRs underwent near-instantaneous hydration (Figure 3), reaching equilibrium swelling after 4 hr. After 48hr, systems were fully saturated at 185 ± 13% original dry mass. In the hydrated state, AP-SDRs maintained features, and were mechanically robust and ductile. The rapid pace of swelling is a critical consideration when designing reservoir systems that modulate release through the physicochemical and material characteristics of the drug barrier (as opposed to osmotic or other means). A gradually swellable system, for example, would be less desirable given that would indicate a longer period during which the system has a variable-length diffusion barrier or variable permeability48. Alternatively, a rapidly-swellable system minimizes this transitory period and arrives at a state of equilibrium conducive to sustained release at a faster pace49.
Figure 3.
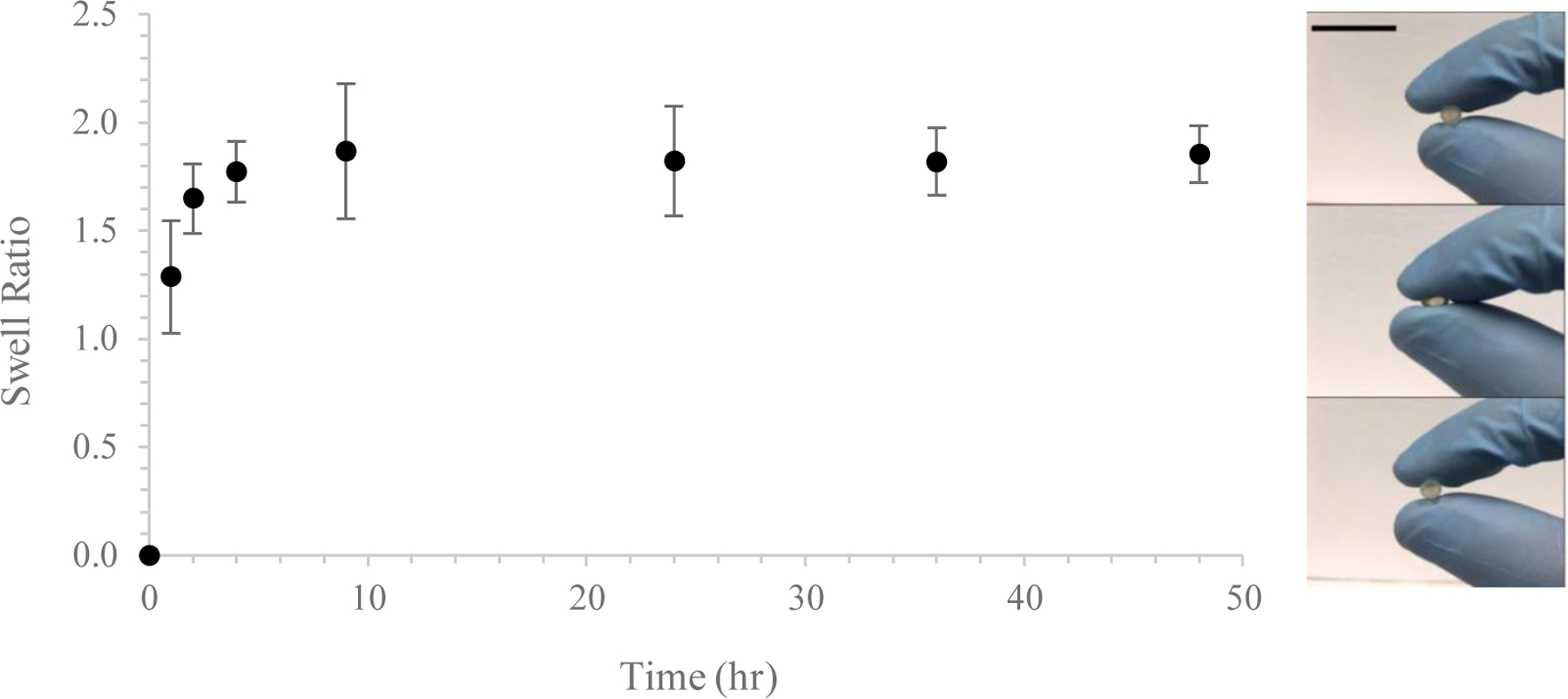
Swelling time course of AP-SDR systems. Swelling took place rapidly in soak media (DI water), with equilibrium hydration reached after roughly 4 hours. Inset: swollen systems exhibited significant ductility and were able to repeatably recover their shape. Scale bar 1.0 cm.
3.3. Protease Degradation
System degradation took place over 30 days. Reduction in system weights for those SDRs in protease solution was evident around the five-day mark, such that by the end of the experimental period those systems had lost nearly 20% of their mass, while the control systems remained unchanged (Figure 4). The degradation time course of AP-SDRs was comparable to that of fiber-spun silk tubes undergoing degradation in vivo9. Nonetheless, it is essential to consider the long-term effects of surface degradation on system mechanical properties, specifically under cyclical transverse loading as could possibly occur in vivo. Losing this amount of mass over a relatively short release period limits the applicability of these systems as implants. However, previous work has shown that subtle variations in ß-sheet content as well as proportions of the two crystalline variants of secondary silk protein structure, silk I and silk II, affect the rate of degradation16, as well as the degree to which any post-processing treatments such as water annealing or methanol treatment are applied to the system50. It’s also feasible to apply a micro or nano-layer coating to the exterior of the SDR systems containing protease-inhibiting agents21 to limit or retard implant surface degradation.
Figure 4.
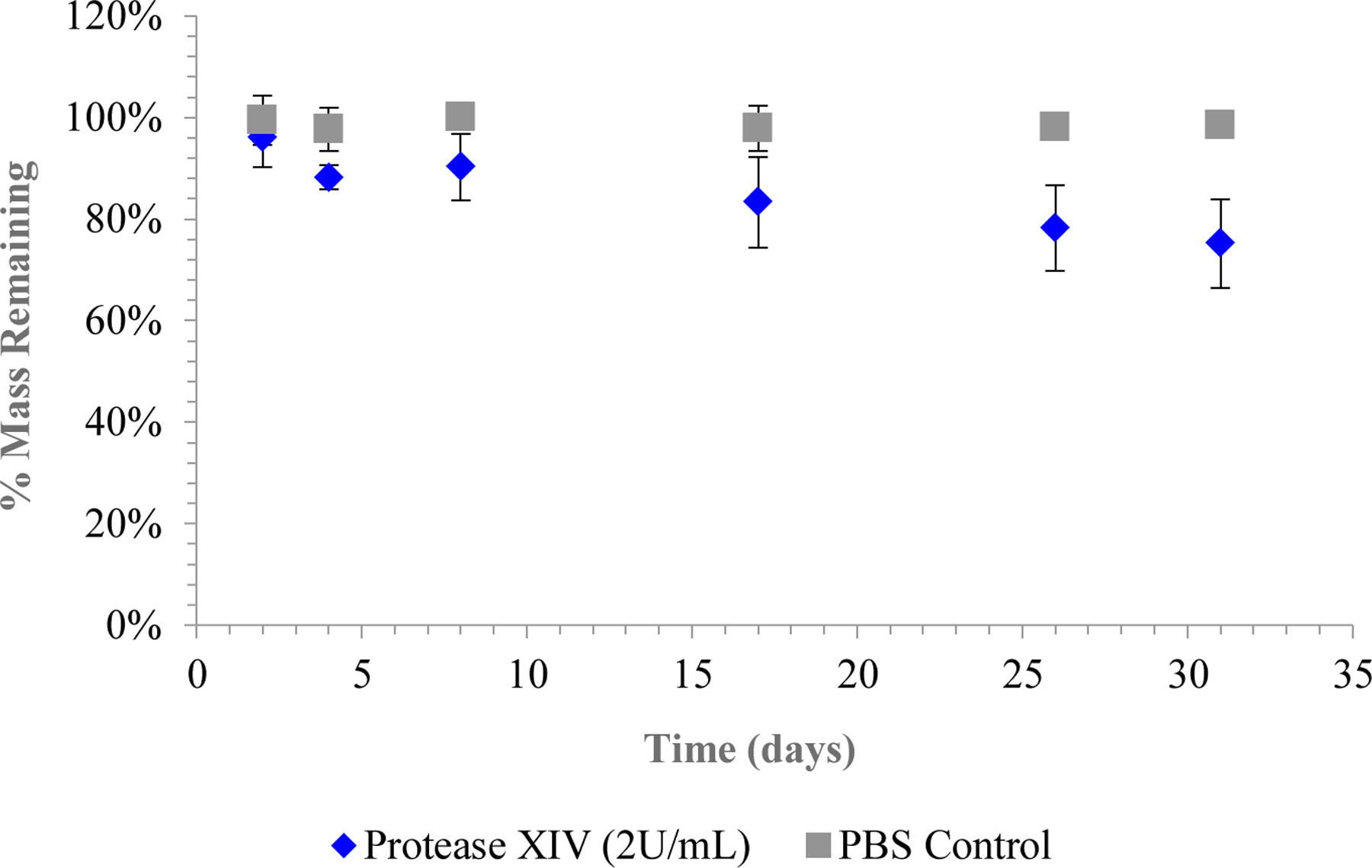
Protease degradation of AP-SDR systems. Change in mass was measured over time under incubation in either 2U/mL Protease XIV solution (blue) or a control solution of 1x PBS at 37˚C (n = 5).
3.4. Secondary Structure of AP-SDRs
Raw ATR-FTIR data was converted into proportional contributions from structural conformations of silk comprising AP-SDRs via Fourier Self-Deconvolution. Beta sheets and turns comprised the majority of the crystalline structure of the systems, with additional contributions by alpha helices. These structural contributions were consistent with previously defined secondary structures of solid silk materials25. Furthermore, the more pronounced presence of ordered structures (ß-turns, ß-sheets, α-helices) compared to random coils and side chains than has been documented in slow-dried silk films51 and underscores the differences in silk concentration and processing parameters used to fabricate these systems. This high degree of crystallinity is desirable as it can be used to further retard drug release given that these dense regions are not easily hydrated and thus less permeable to drug diffusion. Table 1 quantifies the proportional contributions of the various chain conformations as an average of 5 AP-SDR systems tested, an example of which is given by Figure 5.
Table 1.
Summary of proportional contribution of each silk fibroin molecular conformation to aggregate protein secondary structure of AP-SDRs (n = 5).
| Conformation | Contribution (Mean ± S.D.) [%] |
|---|---|
| ß-sheet | 46.0 ± 4.2 |
| ß-turn | 25.3 ± 3.0 |
| α-helix | 15.8 ± 3.8 |
| Random Coil | 9.6 ± 1.1 |
| Side-chain | 5.1 ± 2.5 |
Figure 5.
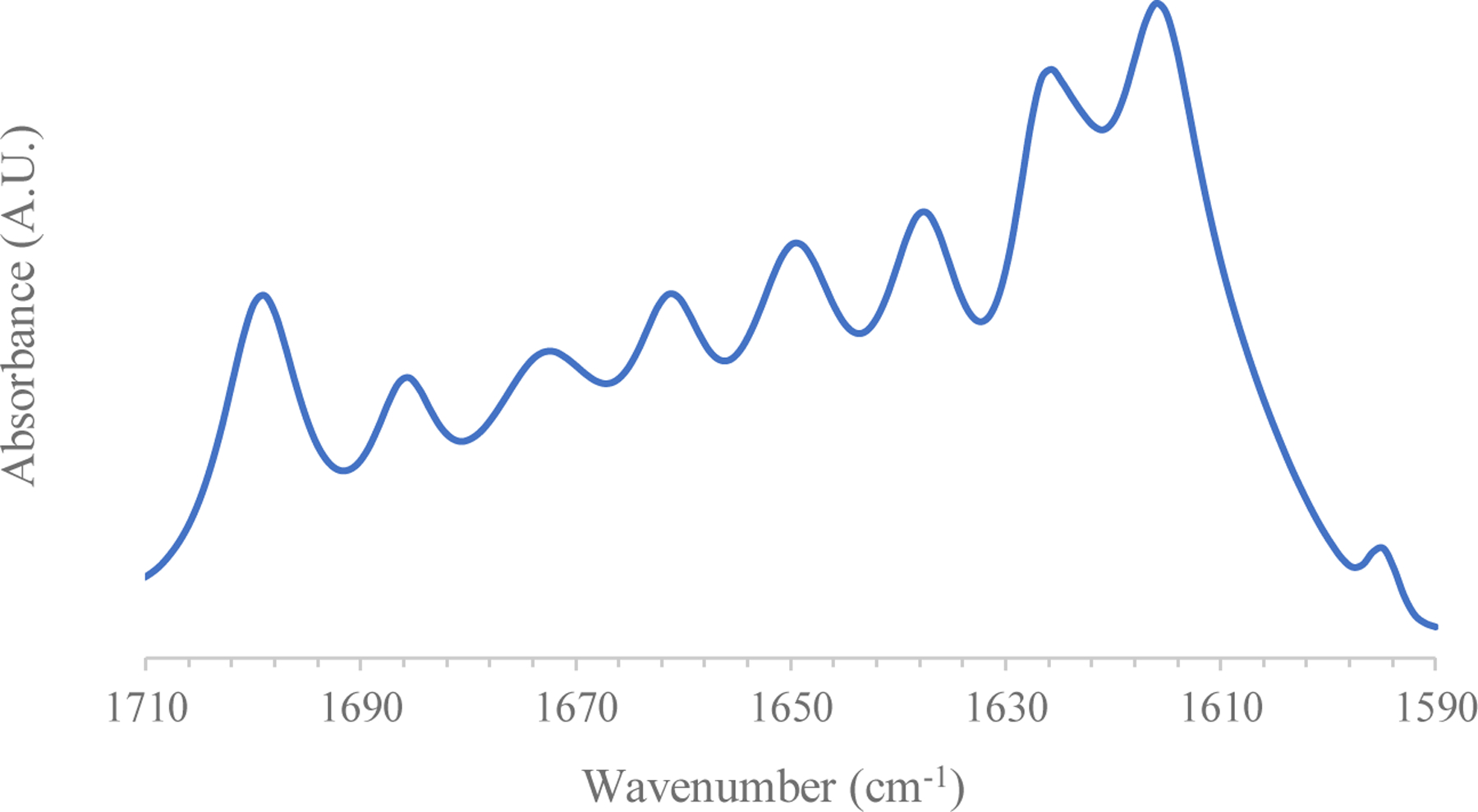
A typical FSD-FTIR Spectrum collected from AP-SDR systems.
3.5. Mechanical Properties
3.5.1. Static Compression
A significant difference (p < 0.01) in Young’s modulus between AP- and SP-SDR systems was observed (Figure 6), indicating their potential to be used as implant systems in tissue environments having different mechanical properties. Aqueously processed systems achieved a modulus of 247.4 ± 53.01 MPa over a linear strain region of 5 – 20%, while solvent processed systems achieved modulus values of 125.2 ± 57.11 MPa over the same linear strain region. This range of linear strain is nearly 50% wider than that of previously generated solid silk materials23. A wide range of linear stress response is desirable for drug implant systems in that they are able to undergo a relatively large degree of loading and still maintain elasticity and therefore system integrity.
Figure 6.
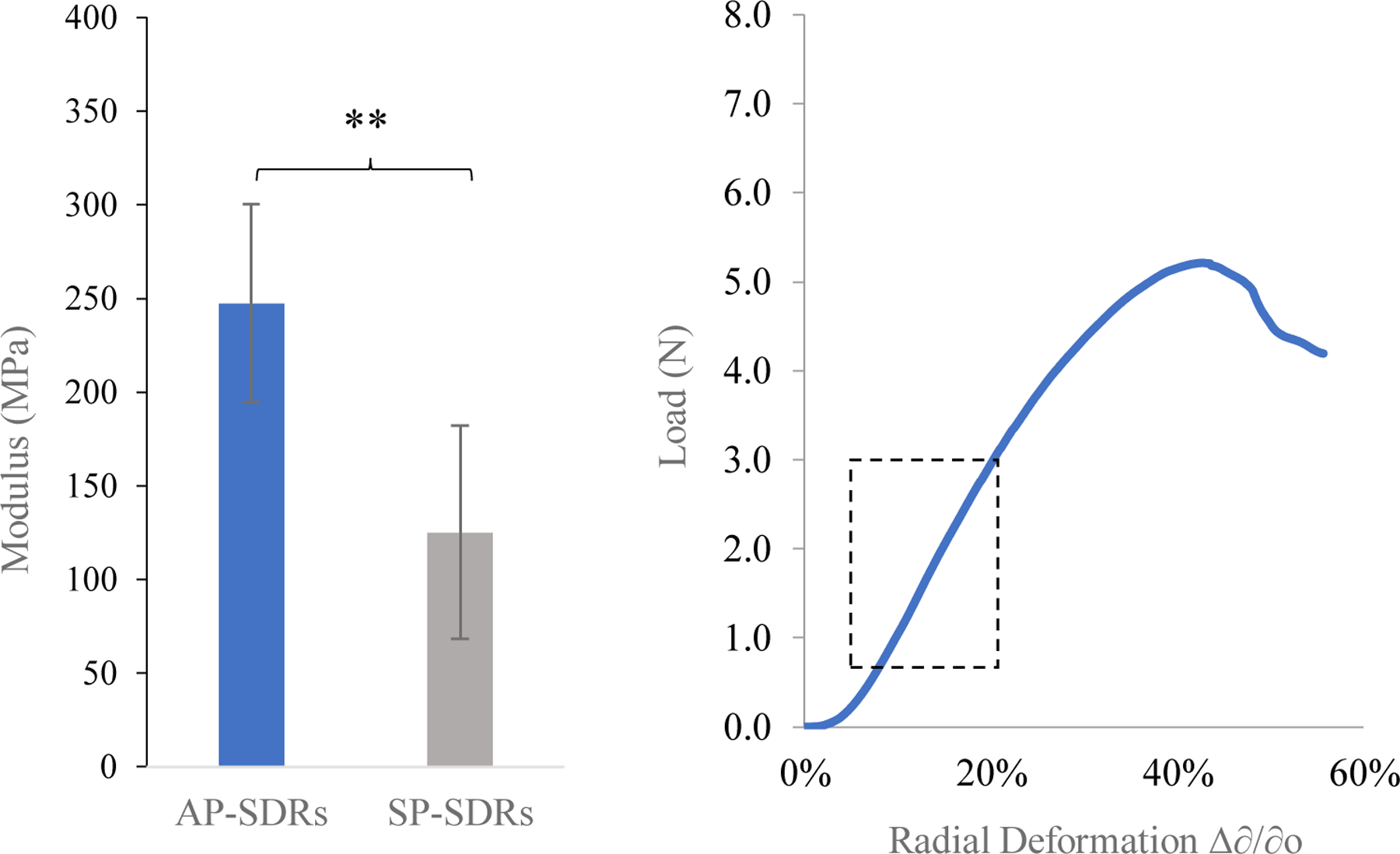
Left Young’s moduli (n = 5) of AP-SDR and SP-SDR systems, calculated using the derived equation described in section 4.8.1. Right Loading curves for AP-SDRs. Dotted rectangle highlights the linear strain region. ** indicates p < 0.01.
The values obtained for modulus were an order of magnitude below those observed for solid silk cylinders under compression23,24. Here, modulus values fall within the range of tested values for Young’s Modulus of cancellous (spongy) bone52, a useful benchmark when considering specific applications of SDRs for use as implants or structural devices. In contrast, both SDR types demonstrated more robust mechanical properties than silk tubes produced via gel or fiber-spinning processes46, further differentiating them from previously generated hollow silk structures. Varied mechanical properties between system types are relevant when considering the theoretical implantation regions of such systems. In using these systems for one or both drug compounds, the implantation region would differ depending on the desired clinical need. In the example case of a patient using an oral RIF dosing regimen as a treatment for peripheral osteoarticular tuberculosis that is multi-drug resistant, the target region for implantation could be the cancellous region proximal to the locus of joint swelling and pain. For applications in soft tissue, minor adjustments in system fabrication could allow the systems to match the moduli of these regions. One approach would be to blend the starting fibroin suspension with plasticizing compounds such as glycerol or polyvinyl alcohol. In one example, incorporation of glycerol at 30% weight ratio of fibroin reduced the modulus of silk films by over 75%59.
3.5.2. Dynamic Compression
A comparison of maximum load achieved between the two system variants after the completion of the 0.5 cycle mark at 17.5% strain did not yield statistically significant results at the p < 0.05 level (p = 0.055). AP-SDRs managed a maximum cyclical loading of 1.42 ± 0.28 N, while SP-SDRs performed at 1.01 ± 0.06 N at maximum strain. Using a max strain rate of 17.5% as the upper cycle limit resulted in a fatigue in stress response for both system types after n = 10 cycles, which was expected considering that the strain was chosen to push the elastic limit of the systems. However, even at this extreme strain value, the system maximum load decreased by a relatively small amount (8.7 ± 5.8% for AP-SDRs, and 10.9 ± 1.5% for SP-SDRs). This small amount of hysteresis occurred over a 14-minute testing cycle. Figure 7 shows example cyclical stress responses for the two system types going up to 17.5% strain.
Figure 7.
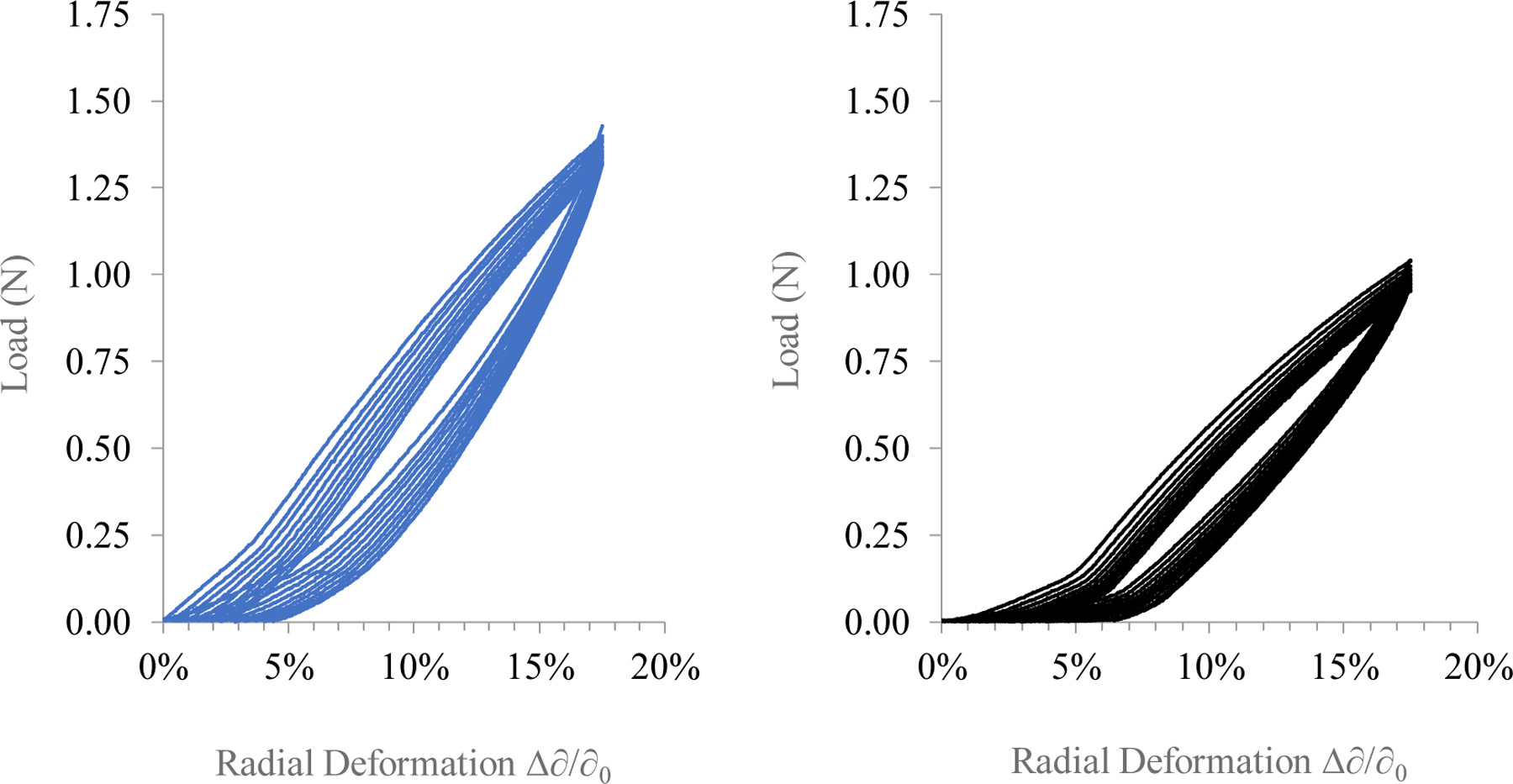
Cyclic loading of AP-SDRs (Left) and SP-SDRs (Right) over a strain range of 0 – 17.5% for n = 10 cycles. Arrows indicate direction of hysteresis during cycle progression. Both systems experienced hysteresis (<10%) over the 14-minute loading cycle (0.7% per minute).
Testing SDRs under cyclic compression conditions provides a quantitative measure of the resilience of these structures and how they fatigue when repeatedly pushed to their strain limits. While compliance testing is more often applied to bulk loaded structures53, it is still useful to assess the unloaded case when considering other applications of swellable silk materials. As an example, cyclical performance of SDRs resulted in less system fatigue over more cycles than for silk elastomeric hydrogels54, which demonstrated cell-seeding and structural applications carrying mesenchymal stem cells (hMSCs).
3.6. Model Drug Binding and Release from Silk Fibroin Films
A minimal amount of LET suspended in binding media bound to the silk film matrix at the end of the 120 hr binding window. Conversely, nearly 20% of RIF initially suspended in binding media was encased in the silk film as the kinetic binding process moved towards equilibrium. The fact that neither drug species bound overwhelmingly within the silk film indicates the potential for a majority of each drug to be available for release when loaded into the full-scale SDR systems. Indeed, at equilibrium the differences in mass binding and encapsulation of the two drug compounds to the silk films were statistically significant at the p << 0.01 level. Given a 4-mg silk fibroin film, RIF bound on average at a drug mass ratio of 6.03 ± 0.64 µg/mg, while LET bound on average at 1.98 ± 0.55 µg/mg. Equilibrium results for the silk film kinetic binding studies can be seen in Figure 8. These trends observed during the binding process can be explained at least in part by the charged state of each drug compound at physiological pH. Specifically, at pH 7.4 LET is largely deprotonated (pKa = 2.0 – 3.0), meaning it would exhibit minimal charge interaction with anionic silk residues, thus preventing significant binding between the drug molecule and the silk matrix (the isoelectric point of silk fibroin, pI, has been found to be 4.522 indicative of an overall negative charge at physiological pH). On the other hand, RIF would exhibit enhanced charge interaction with silk given its pKa = 7.0 – 8.0, as some of the molecular subunits would remain protonated under the experimental conditions. These proposed charge interactions are further supported by consistent zeta potential measurements between −7.0 and −12.0mV for concentrated fibroin solutions at physiological pH55,56.
Figure 8.
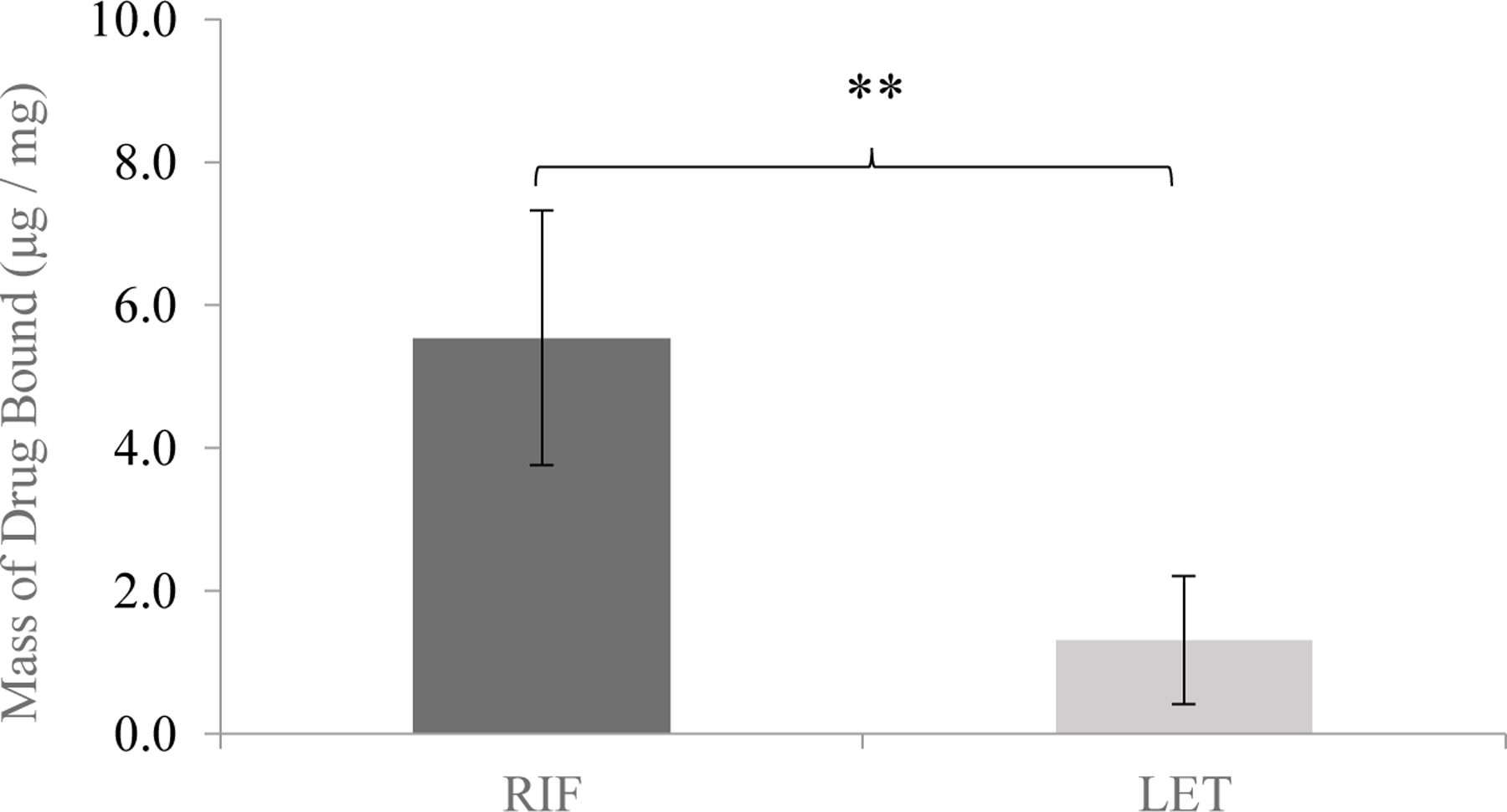
The endpoint mass binding of RIF and LET after expiration of the 120hr binding window. RIF bound far more than LET, explained partially by the charged state of each drug species at physiological pH (** indicates p << 0.01 level of significance).
The kinetic release process was rapid in the case of LET, and barely discernable above baseline for RIF (Figure 9). The total mass of LET encased in the silk films during the kinetic binding time course was released almost immediately, consistent with a loose binding interaction with the silk crystalline matrix. After this quick burst release, virtually no LET was detected in the release media for the duration of the release window. In contrast, almost none of the RIF that had bound to the silk films in the binding study released over the measured time window. Rather, the nominal amount of RIF released underscores the stronger binding interaction between RIF and the silk macrostructure. This result suggests that at the mass proportions of loaded drug in solution and silk protein present in the films used for the study, the mass binding of RIF to silk approached but did not reach its loading limit.
Figure 9.

Release time course of RIF (blue) and LET (black) from 4% w/v silk films. Both compounds underwent a characteristic “burst” release during the first 4hrs of the process, however a detectable amount of RIF remained bound to the silk films over the release window, releasing only basal levels of drug throughout the experiment.
Taking into account the previously-mentioned charged state of each drug molecule and that of the silk fibroin matrix under physiological conditions, the minimal amount of RIF released compared to that which bound to the silk matrix is not surprising – the stronger charge interactions between the silk matrix and RIF would naturally result in more drug mass bound per mass of silk, and that mass bound would release less readily compared to LET, some minimal amount of which was loosely encapsulated in the silk matrix, effectively soaking in drug solution during the binding study. This marginal amount of trapped LET would then release immediately when presented with a strong diffusion gradient. These binding and release trends are consistent with previous work investigating silk interactions with charged drugs, where the more cationic drugs were found to exhibit more robust binding to silk films, especially when those films were modified to have a more anionic character2. In this context, the minimal release of RIF from the films is useful when considering transition to full-scale in vitro release given that the loose binding interaction could play a role in slowing release, providing sustained release of drug for a longer period over a system loaded with LET, which only has the diffusion distance of the swollen silk layer to control the release rate.
3.7. SDR Partition Coefficient and Release Model
Values for partition coefficient (KD) for LET and RIF followed trends observed in kinetic film binding results for the two drugs. Values for KD were statistically significant (p << 0.01), with LET achieving an apparent equilibrium partition value of 5.87 ± 0.18, and RIF achieving a higher KD value of 10.93 ± 0.46. The degree to which higher partitioning of RIF in the SDR matrix was observed was consistent with the higher mass binding values observed for the drug over LET in section 3.6.
The partition coefficient values of KD > 1 obtained for both drugs indicated that both were relatively soluble within the swollen silk matrix and thus able to be dispersed effectively throughout the SDRs. The higher partitioning of RIF within the film is likely due to its larger molecular size and its stronger charge interactions with negatively-charged domains present on the silk chain superstructure. Both partition coefficients being slightly greater than but not orders of magnitude different than 1 are desirable for sustained release, as it indicates both drugs are not extremely soluble in and thus not able to permeate too quickly through the silk matrix57, or conversely not soluble at all in the silk matrix making drug transport across the diffusion barrier infeasible. Rather, their marginal solubility and, in the case of RIF, binding interactions with silk help to control the release profile.
Table 2 summarizes calculated values for the diffusion exponent n and associated R2 statistics for both drugs based on the cumulative release rate data (falling within the first 60% of total mass released from the system), following the linear model described by Eq. 7. Both drugs exhibited release characteristics consistent with a zero-order release mechanism. While the release mechanism can be described as near-zero order, the model fit data suggest a complex interaction that draws on elements of both diffusive and solvent-limited release mechanisms. This is especially relevant when considering the difference in acid dissociation constants and solubility of both drug species. Figure 10 shows both cumulative release and daily release rate profiles of LET and RIF, respectively. As a check, cumulative mass release data for both drug compounds were compared to physical mass measurements of dried samples of all SDR release systems. In the case of both drugs, cumulative mass release values attained by spectroscopic methods were well within 20% of those data obtained by physical weighing of dried systems. For LET, physical weighing of systems indicated cumulative release of 3.91 ± 0.56 mg, while for RIF this process yielded cumulative release values of 4.55 ± 0.81 mg. These values compare well to those calculated release values via spectroscopic methods (4.33 ± 0.23 mg, 5.21 ± 0.44 mg for LET and RIF respectively).
Table 2.
in-vitro release diffusion exponent and R2 statistic values for LET, RIF.
| Drug | Release Medium | n (AVG ± S.D.) | R2 |
|---|---|---|---|
| LET | 1x PBS | 0.92 ± 0.05 | 0.996 |
| RIF | 1x PBS | 0.87 ± 0.02 | 0.985 |
Figure 10.
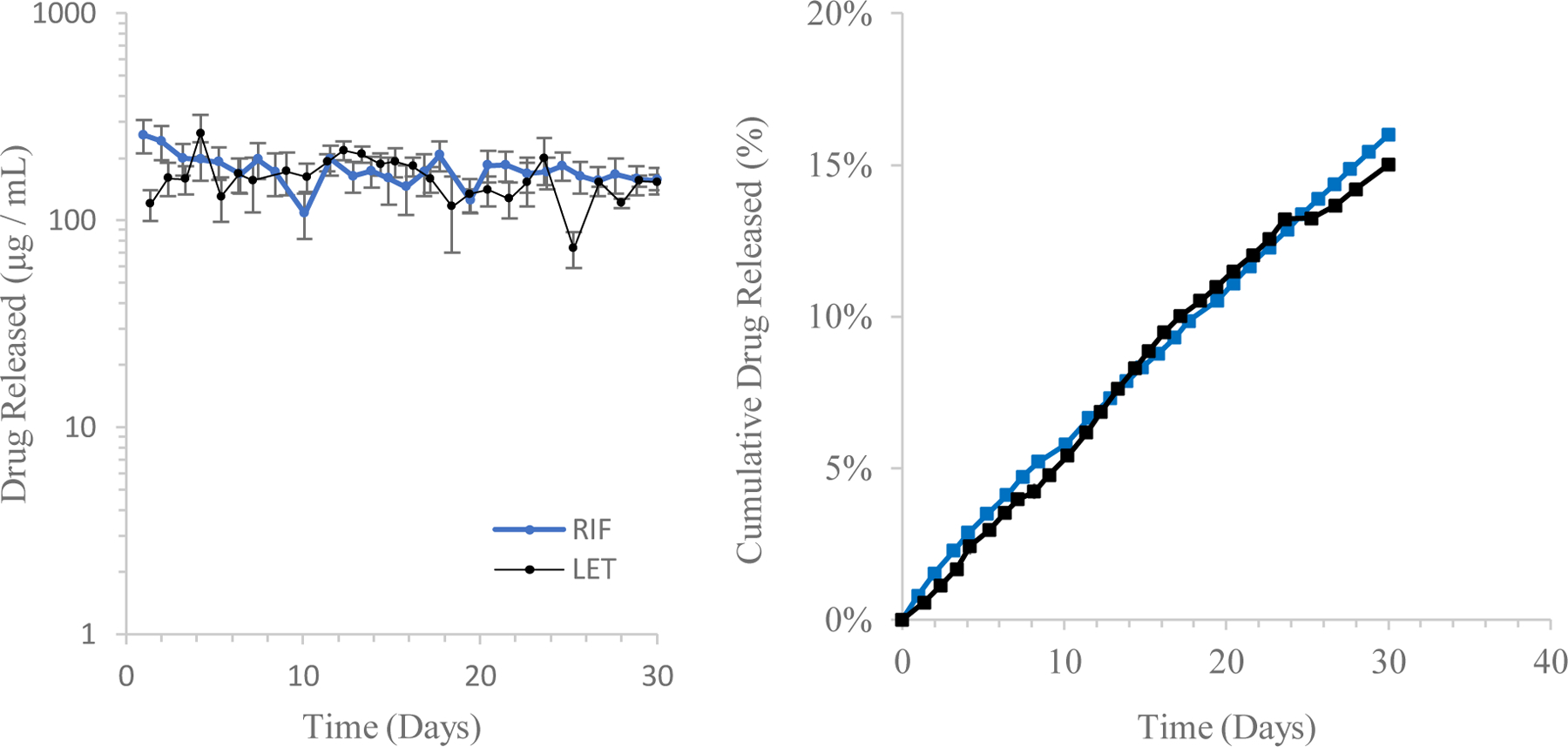
Daily release rate (left) and cumulative mass release profile (right) of both LET- and RIF- loaded AP-SDR systems over a 30-day incubation period (n = 9 replicates per group). Total drug mass released over this period (based on spectroscopic methods) was 4.33 ± 0.23 mg, or 15% of loaded mass for LET and 5.21 ± 0.44 mg, or 16% of loaded mass for RIF.
The partitioning of each drug and their respective physicochemical properties explains much of the sustained release behavior observed during in vitro release. The limiting factor of LET was most likely its sparse solubility, while for RIF the enhanced bonding interaction and larger absolute particle size retarded its release from the encapsulating reservoir. Both of these characteristics resulted in near-zero order (constant) release profiles over the full 30-day release period, with both loaded systems releasing less than 20% of encapsulated drug. The daily released mass, however, holds different implications based on the clinical applications of each drug. SDRs loaded with LET, for example, released ~100µg of drug per day, which represents more absolute mass of drug released over a longer period than prior systems42, and furthermore approaches clinical relevance based on dose values and bioavailability of orally-administered LET58. In the case of RIF, loaded SDRs achieved higher daily doses of released drug than in prior work14, but further work should be done in vivo to address how these systems, which release less drug than is currently required for oral regimens of RIF, can most effectively be used for local therapeutic delivery. Regardless, characterizing the release of a compound having mild charge interactions with silk is a useful standard case to consider when testing other compounds having similar physicochemical characteristics. Thus, this in vitro testing yielded a useful base of examination for LET as a locally-delivered anti-cancer therapeutic, and RIF as a locally-administered antiretroviral for adjuvant treatment of bone tuberculosis. Furthermore, extrapolating the proportion of mass of released drug (15% and 16% for LET and RIF respectively over 30 days) up to the 60% threshold that is one assumption of the linear release model yields a potential constant release period of ~120 days for both drugs. For LET specifically, this would mean at the current size and loading volume a patient could have near-constant infusion of a clinically effective dose of drug for up to 4 months. Alternatively, this indicates that there is a real potential to shrink a silk implant device such as this to a size where it could be injected subcutaneously rather than inserted via surgical incision while still delivering drug for a shorter period of time. This would enhance the clinical argument for such a system given that there is no need for either a primary or secondary surgery to insert nor remove the device.
IV |. Conclusion
The sustained release of two clinically relevant therapeutics, Letrozole and Rifampicin, from “complex” hollow 3D solid silk structures was demonstrated. The in vitro release time course of loaded SDR systems exhibited zero-order characteristics, and in the case of LET was a near direct match to the daily oral dosage administered in a clinical setting. This indicates that a sustained release biodegradable reservoir for localized delivery of LET could be presented as an alternative to oral regimens. Furthermore, two fabrication methods, solvent-process and aqueous-process pathways, both successfully yielded reproducible SDRs that followed precise morphology, resolved micron-scale features and were reversibly swellable and compliant. These systems demonstrated robust mechanical properties, including calculated values for Young’s modulus similar to that of certain biological tissues and ability to undergo cyclic loading with minimal fatigue.
V |. Acknowledgements
We thank the NIH (R01NS094218, P41EB002520) for support of this work. We also thank Jeannine Coburn, Megan McGill, Eliad Cohen, Burcin Yavuz, and Nicole Raia for various inputs, and a special thanks to Scott MacCorkle for machine shop support for this research.
Footnotes
Supporting Information. Compressive loading curves of AP-SDR systems.
VI | References
- [1].Gupta R; Bansal S; Aqil F; Jeyabalan J; Cao P; Kausar H; Russell G; Munagala R; Ravoori S; Vadhanam M Controlled-release systemic delivery – a new concept in cancer chemoprevention. Carcinogenesis 2012, 33(8), 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coburn J; Na E; Kaplan D Modulation of vincristine and doxorubicin binding and release from silk films. J. Control. Rel 2015, 220, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Overstreet D; McLaren A; Calara F; Vernon B; McLemore R Local gentamicin delivery from resorbable viscous hydrogels is therapeutically effective. Clin. Orthop. Relat. Res 2015, 473(1), 348–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tiihonen J; Krupitsky E; Verbitskaya E; Mamontova O; Föhr J; Tuomola P; Kuoppasalmi K; Kiviniemi V; Zwartau E Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. Am. J. Psychiatry 2012, 169(5), 531–6. [DOI] [PubMed] [Google Scholar]
- [5].Stevenson C; Santini J; Langer R Reservoir-based drug delivery systems utilizing microtechnology. Adv. Drug Deliv. Rev 2012, 64(14), 1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anselmo A; Mitragotri S An overview of the clinical and commercial impact of drug delivery systems. J. Control. Rel 2014, 190(28), 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Niwa T; Takeuchi H; Hino T; Kunou N; Kawashima Y Preparations of biodegradable nanospheres of water-soluble and -insoluble drugs with D, L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior. J. Control. Rel 1993, 25(1), 89–98. [Google Scholar]
- [8].Makadia H; Siegel S Poly lactic-co-glycolic acid (PLGA) as a biodegradable controlled drug delivery carrier. Polymers (Basel) 2011, 3(3), 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yucel T; Lovett M; Giangregorio R; Coonahan E; Kaplan D Silk fibroin rods for sustained delivery of breast cancer therapeutics. Biomaterials 2014, 35, 8613–8620. [DOI] [PubMed] [Google Scholar]
- [10].Lovett M; Wang X; Yucel T; York L; Keirstead M; Haggerty L; Kaplan D Silk hydrogels for sustained ocular delivery of anti-vascular endothelial growth factor (anti-VEGF) therapeutics. Eur. J. Pharm. Biopharm 2015, 95, 271–278. [DOI] [PubMed] [Google Scholar]
- [11].Tsioris K; Raja W; Pritchard E; Panilaitis B; Kaplan D; Omenetto F Fabrication of silk microneedles for controlled-release drug delivery. Adv. Func. Mater 2012, 22, 330–335. [Google Scholar]
- [12].Wang X; Wenk E; Matsumoto A; Meinel L; Li C; Kaplan D Silk microspheres for encapsulation. J. Control. Rel 2007, 117(3), 360–370. [DOI] [PubMed] [Google Scholar]
- [13].Pritchard E; Szybala C; Boison D; Kaplan D Silk Fibroin Encapsulated Powder Reservoirs for Sustained Release of Adenosine. J. Control. Rel 2010, 144(2), 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pritchard E; Valentin T; Panilaitis B; Omenetto F; Kaplan D Antibiotic-releasing silk biomaterials for infection prevention and treatment. Adv. Func. Mater 2013, 23, 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rockwood D; Preda R; Yücel T; Wang X; Lovett M; Kaplan D Materials fabrication from Bombyx mori silk fibroin. Nature Protocols 2011, 6, 1612–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lu Q; Zhang B; Li M; Zuo B; Huang Y; Zhu H; Kaplan D Degradation mechanism and control of silk fibroin. Biomacromolecules 2011, 12, 1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu J; Zhang Y; Huili S; Hu X Electrospinning and rheology of regenerated Bombyx mori silk fibroin aqueous solutions: the effects of pH and concentration. Polymer 2008, 49, 2880–2885. [Google Scholar]
- [18].Cao Y; Wang B Biodegradation of silk biomaterials. Int. J. Mol. Sci 2009, 10(4), 1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zafar M; Belton D; Hanby B; Kaplan D; Perry C Functional Material Features of Bombyx mori Silk Light vs. Heavy Chain Proteins. Biomacromolecules 2015, 16(2), 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Seib F; Kaplan D Doxorubicin-loaded silk films: drug-silk interactions and in vivo performance in human orthotopic breast cancer. Biomaterials 2012, 33, 8442–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pritchard E; Valentin T; Boison D; Kaplan D Incorporation of Proteinase Inhibitors into Silk-Based Delivery Devices for Enhanced Control of Degradation and Drug Release. Biomaterials 2011, 32(3), 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lammel A; Hu X; Park S; Kaplan D; Scheibel T Controlling silk fibroin features for drug delivery. Biomaterials 2010, 31(16), 4583–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marelli B; Patel N; Duggan T; Perotto G; Shirman E; Kaplan D; Omenetto F Programming function into mechanical forms by directed assembly of silk bulk materials. Proc. Natl. Acad. Sci 2017, 114(3), 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li C; Hotz B; Ling S; Guo J; Haas D; Marielli B; Omenetto F; Lin S; Kaplan D Regenerated silk materials for functionalized silk orthopedic devices by mimicking natural processing. Biomaterials 2016, 110, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Perrone G; Leisk G; Lo T; Moreau J; Haas D; Papenburg B; Golden B; Partlow B; Fox S; Ibrahim A; Lin S; Kaplan D The use of silk-based devices for fracture fixation. Nature Comms 2014, 5, 1–9. [DOI] [PubMed] [Google Scholar]
- [26].Hartnick C; Whalen M; Kaplan D World Patent No. WO2017015387 A1 Alexandria, VA: United States Patent and Trademark Office. [Google Scholar]
- [27].Matsumoto A; Chen J; Collette A; Kim U; Altman G; Cebe P; Kaplan D Mechanisms of silk fibroin sol-gel transitions. J. Phys. Chem 2006, 110, 21630–21638. [DOI] [PubMed] [Google Scholar]
- [28].Bhatnager A The discovery of the mechanism of action of letrozole. Breast Cancer Res. Treat 2007, 105(1), 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Geisler J Differences between nonsteroidal aromatase inhibitors anastrozole and letrozole – of clinical importance?. Br. J. Cancer 2011, 104(7), 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Perez E; Serene M; Durling F; Weilbaecher K Aromatase inhibitors and bone loss. Oncology 2006, 20(9), 1029–1048. [PMC free article] [PubMed] [Google Scholar]
- [31].Abdool Karim S; Naidoo K; Grobler A; Padayatchi N; Baxter C; Gray A; Gengiah T; Nair G; Bamber S; Singh A; Khan M; Pienaar J; El-Sadr W; Friedland G; Karim Q Timing of initiation of antiretroviral drugs during tuberculosis therapy. N. Engl. J. Med 2010, 362, 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lefebvre M; Jacqueline C; Amador V; Le Mabecque V; Miegeville A; Potel G; Caillon J; Asseray N Efficacy of daptomycin combined with rifampicin for the treatment of experimental meticillin-resistant Staphylococcus aureus (MRSA) acute osteomyelitis. Int. J. Antimicrob. Agents 2010, 36(6), 542–544. [DOI] [PubMed] [Google Scholar]
- [33].Loos U; Musch E; Jensen J; Mikus G; Schwabe H; Eichelbaum M Pharmacokinetics of oral and intravenous rifampicin during chronic administration. Klin Wochensch 1985, 63(23), 1205–1211. [DOI] [PubMed] [Google Scholar]
- [34].Wu W; Zheng Q; Guo X; Sun J; Liu Y A programmed release multi-drug implant fabricated by three-dimensional printing technology for bone tuberculosis therapy. Biomed. Mater 2009, 4(6), 1748–1758. [DOI] [PubMed] [Google Scholar]
- [35].Sanz-Ruiz P; Carbó-Laso E; Del Real-Romero J; Arán-Ais F; Ballesteros-Iglesias Y; Paz-Jiménez E; Sánchez-Navarro M; Pérez-Limiñana M; Vaquero-Martín J Microencapsulation of rifampicin: A technique to preserve the mechanical properties of bone cement. J. Ortho. Res 2017, 23, 1–8. [DOI] [PubMed] [Google Scholar]
- [36].Vepari C; Kaplan D Silk as a biomaterial. Prog. Polym. Sci 2007, 32(8–9), 991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lawrence B; Omenetto F; Chui K; Kaplan D Processing methods to control silk fibroin film biomaterial features. J. Mater. Sci 2008, 43, 6967–6985. [Google Scholar]
- [38].Karve K; Gil E; McCarthy S; Kaplan D Effect of ß-sheet crystalline content on mass transfer in silk films. J. Memb. Sci 2011, 383(1–2), 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hu X; Kaplan D; Cebe P Determining Beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar]
- [40].Timoshenko S; Woinowsky-Krieger S Inextensional Deformation of a Circular Cylindrical Shell. Theory of Plates and Shells 1956, (pp. 501–507). New York, NY: McGraw-Hill. [Google Scholar]
- [41].Ritger P; Peppas N A simple equation for description of solute release II. Fickian and non-Fickian release from swellable devices in the form of slabs, spheres, cylinders or disks. J. Control. Rel 1987, 5, 23–36. [PubMed] [Google Scholar]
- [42].Siddiqa A; Chaudhury K; Adhikari B Letrozole dispersed on poly (vinyl alcohol) anchored maleic anhydride grafted low-density polyurethane: A controlled drug delivery system for treatment of breast cancer. Colloids and Surfaces B 2014, 116, 169–175. [DOI] [PubMed] [Google Scholar]
- [43].Hines D; Kaplan D Mechanisms of controlled release from silk fibroin films. Biomacromolecules 2011, 12, 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Williams G; Rahman A Clinical Pharmacology and Biopharmaceuticals Review: Letrozole (Femara) 1997. Report prepared for the Center for Drug Evaluation and Research, Federal Drug Administration, Silver Spring MD. [Google Scholar]
- [45].Brenckle M; Tao H; Kim S; Paquette M; Kaplan D; Omenetto F Protein-protein nanoimprinting of silk fibroin films. Adv. Func. Mater 2013, 25, 2409–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lovett M; Cannizzaro C; Vunjak-Novakovic G; Kaplan D Gel spinning of silk tubes for tissue engineering. Biomaterials 2008, 29(35), 4650–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lovett M; Cannizzaro C; Daheron L; Messmer B; Vunjak-Novakovic G; Kaplan D Silk fibroin microtubes for blood vessel engineering. Biomaterials 2007, 28(35), 5271–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liechty W; Kryscio D; Slaughter B; Peppas N Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng 2010, 1, 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sung K; Topp E Effect of drug hydrophilicity and membrane hydration on diffusion in hyaluronic acid ester membranes. J. Control. Rel 1995, 37(2), 95–104. [Google Scholar]
- [50].Jin H; Park J; Karageorgiou V; Kim U; Valluzzi R; Cebe P; Kaplan D Water-stable silk films with reduced ß-sheet content. Adv. Func. Mater 2005, 15, 1241–1247. [Google Scholar]
- [51].Lu Q; Hu X; Wang X; Kluge J; Lu S; Cebe P; Kaplan D Water-Insoluble silk films with silk I structure. Acta Biomater 2010, 6(4), 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pal S Mechanical Properties of Biological Materials. Design of Artificial Human Joints & Organs 2014, (pp 23–40). New York, NY: Springer US. [Google Scholar]
- [53].Cezar C; Kennedy S; Mehta M; Weaver J; Gu L; Vandenburgh H; Mooney D Biphasic ferrogels for triggered drug and cell delivery. Adv. Health. Mater 2014, 3, 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Partlow B; Hanna C; Rnjak-Kovacina J; Moreau J; Applegate M; Burke K; Marelli B; Mitropoulos A; Omenetto F; Kaplan D Highly tunable elastomeric silk biomaterials. Adv. Func. Mater 2014, 24, 4615–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shen G; Hu X; Guan G; Wang L Surface Modification and Characterisation of Silk Fibroin Fabric Produced by the Layer-by-Layer Self-Assembly of Multilayer Alginate/Regenerated Silk Fibroin. PLoS ONE 2015, 10(4), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lu Q; Huang Y; Li M; Zuo B; Lu S; Wang J; Zhu H; Kaplan D Silk fibrion electrogelation mechanisms. Acta Biomater 2011, 7, 2394–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kwon Y 4.2.4: Partition and Distribution Coefficients. Handbook of Essential Pharmacokinetics, Pharmacodynamics and Drug Metabolism for Industrial Scientists 2001. (pp. 44). New York: Kluwer Academic/Plenum Publishers. [Google Scholar]
- [58].U.S. Department of Health and Human Services. National Cancer Institute: Letrozole 2017 Retrieved from https://www.cancer.gov/about-cancer/treatment/drugs/letrozole
- [59].Lu S; Wang X; Lu Q; Zhang X; Kluge J; Uppal N; Omenetto F; Kaplan D Insoluble and flexible silk films containing glycerol. Biomacromol 2010, 11, 143–150. [DOI] [PubMed] [Google Scholar]


