Abstract
A metabolically healthy status, whether obese or not, is a transient stage with the potential to develop into metabolic disorders during the course of life. We investigated the incidence of metabolic disorders in 1078 metabolically healthy Chinese adults from the Shanghai Changfeng Study and looked for metabolites that discriminated the participants who would develop metabolic disorders in the future. Participants were divided into metabolically healthy overweight/obesity (MHO) and metabolically healthy normal weight (MHNW) groups according to their body mass index (BMI) and metabolic status. Their serum metabolomic profile was measured using a 1H nuclear magnetic resonance spectrometer (1H-NMR). The prevalence of diabetes, hypertriglyceridemia, hypercholesterolemia and metabolic syndrome was similar between the MHNW and MHO participants at baseline. After a median of 4.2 years of follow-up, more MHO participants became metabolically unhealthy than MHNW participants. However, a subgroup of MHO participants who remained metabolically healthy (MHO → MHO) had a similar prevalence of metabolic disorders as the MHNW participants at the follow-up examination, despite a significant reduction in their serum concentrations of high-density lipoprotein (HDL) and an elevation in valine, leucine, alanine and tyrosine. Further correlation analysis indicated that serum intermediate-density lipoprotein (IDL) and very low-density lipoprotein cholesterol (VLDL-CH) might be involved in the transition from metabolically healthy to unhealthy status and could be valuable to identify the MHNW and MHO with increased metabolic risks.
Keywords: Metabolically healthy normal weight (MHNW), Metabolically healthy overweight/obesity (MHO), 1H nuclear magnetic resonance spectrometer (1H-NMR), Metabolic disorders, Very low-density lipoprotein cholesterol (VLDL-CH), Intermediate-density lipoprotein (IDL)
Introduction
Each metabolically healthy individual could develop a metabolic disorder in the course of their lifetime. Some metabolically healthy participants might become metabolically unhealthy individuals over time, while others might remain metabolically healthy (Feng et al. 2020; Mongraw-Chaffin et al. 2016). Obesity is universally believed to be an important risk factor for metabolic disorders and type 2 diabetes mellitus (T2DM). Even a moderate weight loss of 5–10% of body weight has been proven to improve blood pressure, fasting blood glucose, serum triglycerides, HDL cholesterol and HbA1c, and more weight loss is associated with greater improvements (Wing et al. 2011). An excessive accumulation of fat in adipocytes and the release of cytokines trigger a network of signaling pathways that can induce different levels of inflammation reactions in the human body, which ultimately contributes to insulin resistance, which is involved in the pathophysiology of multiple metabolic disorders in the end (Charles et al. 2011; Saltiel and Olefsky 2017). However, recent studies have indicated that some obese individuals retain insulin sensitivity and are regarded as metabolically healthy obesity individuals. Some studies have shown that insulin sensitivity was relatively preserved in comparison to a group of metabolically unhealthy patients with obesity or metabolic syndrome (Ctoi et al. 2018). Other studies indicated that an increased waist circumference (Feng et al. 2020), fatty liver index (Jung et al. 2016), and a sedentary lifestyle (Navarro-Gonzalez et al. 2016) were related to the risk of metabolic disorders among metabolically healthy obese people. However, there is still a lack of reliable parameters to discriminate among the people with an increased risk of metabolic disorders in the future.
The current methods used for metabolic evaluation and the definitions of metabolic health are not sufficient to distinguish true metabolically healthy people from the people with a tendency to develop metabolic disorders in the future. Several previous studies have indicated that an increased high waist circumference, hepatic fat content and weight gain during follow-up might suggest the potential to develop a metabolically unhealthy status (Espinosa De Ycaza et al. 2018; Hashimoto et al. 2017; Hwang et al. 2019, 2015; Mongraw-Chaffin et al. 2016). However, none of these traditional metabolic parameters could satisfactorily predict the occurrence of all metabolic disorders.
Serum metabolites might be sensitive metabolic biomarkers and be altered prior to the development of metabolic disorders in metabolically healthy people, as indicated in one study reporting that apolipoprotein B-100 and apolipoprotein A4 (Doumatey et al. 2016) were potential prognostic markers of metabolically healthy people. Until now, few studies have systemically compared the serum metabolomic profiles between metabolically healthy people with sustained metabolic healthy status and those with the potential to develop metabolic disorders.
In the current study, we developed a new detection method named 1H nuclear magnetic resonance spectrometry (1H-NMR) which could detect the main fractions, subclasses, and compositional components of all serum lipoproteins and 41 small metabolites involved in different metabolic pathways. Using this novel metabolomics technology, we attempted to find crucial serum metabolites that might be used to discriminate metabolically healthy people with the potential to develop metabolic disorders from traditionally defined “metabolically healthy” people.
Materials and Methods
Participants
The participants came from Shanghai Changfeng Study which was a prospective community-based cohort study focusing on multiple chronic diseases among middle-aged and elderly individuals in Shanghai, China (Gao et al. 2010). Among the Shanghai Changfeng Study population, a total of 1078 metabolically healthy participants at baseline finished a median of 4.2-year follow-up and were enrolled for analysis. Participants were recruited to receive a face-to-face interview using a standard questionnaire on lifestyle, medical history, and demographic characteristics, and they were required to undergo anthropometric measurements and biochemical examinations. The study was approved by the Research Ethics Committee of Zhongshan Hospital, Fudan University. Written informed consent was obtained from each participant.
Anthropometric Measurement and Biochemical Examination
The information of age, gender, history of cigarette smoking, alcohol drinking, and medication use was collected through face-to-face interviews using a questionnaire. Blood pressure (BP) was tested three times after taking a rest for at least 5 min every time in the same arm with an electronic blood pressure monitor (OMRON Model HEM-752 FUZZY, Omron Co., Dalian, China), and the mean value was calculated. Height and weight were measured without shoes and outer clothes. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Waist circumference (WC) was measured in a standing position using a soft tape midway between the lowest rib and iliac crest.
Fasting blood samples were collected after an overnight fast of at least 12 h, and all samples are stored at − 80 °C. Serum cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), fasting plasma glucose (FPG) and 2-h post-challenge glucose (2 h-PG) levels were measured using an automated bioanalyzer (HITACHI 7600, Tokyo, Japan). Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation.
Metabolomics Examination
Serum metabolomics was examined on a 600 MHz AVANCE III NMR spectrometer equipped with a BBI probe (Bruker Biospin GmbH, Germany) using the same methods reported previously (Jimenez et al. 2018). In brief, serum samples stored at − 80 °C were thawed at 24 °C within 30 min, and all subsequent operations were performed upon the ice. 350 μL of each serum sample was mixed with 350 μL phosphate buffer (0.085 M, pH 7.4) containing 10% D2O (Jiang et al. 2012), and 600 μL of such mixture was transferred into a 5 mm diameter NMR tube. Samples were automatically handled by a SampleJet™ (Bruker Biospin) sample changer at 4 °C before inserting into an NMR spectrometer for metabolomic analysis. All NMR spectra were recorded with a standard NOESYGPPR1D pulse sequence into 98 k data points at 310 K with 32 transients. Following the Fourier transform, a total of 112 lipoprotein parameters (including particle number, main fractions, subclasses, and compositional components therein) were quantified using the Bruker IVDr Lipoprotein Subclass Analysis B.I.LISA™ software package (Bruker Biospin). Cholesteryl ester in lipoproteins was calculated by subtracting the free cholesterol component from the total cholesterol in lipoproteins. 41 small metabolites including amino acids, ketone bodies, glucose, carboxylic acids, and ethanol were also quantified from the same spectra using the Bruker IVDr Quantification in Plasma/Serum B.I.Quant-PS™ software package (Bruker Biospin).
Definitions
Metabolic health status was defined if the participants have no more than one metabolic abnormality as follows: (1) waist circumference (WC) ≥ 90 cm in men or ≥ 80 cm in women; (2) systolic blood pressure (SBP) ≥ 130 mmHg and/or diastolic blood pressure (DBP) ≥ 85 mmHg or specific drug treatment; (3) serum triglycerides ≥ 150 mg/dL (1.7 mmol/L) or specific drug treatment; (4) fasting plasma glucose ≥ 100 mg/dL (5.6 mmol/L) or 2-h post-load plasma glucose levels ≥ 140 mg/dL (7.8 mmol/L) or specific drug treatment; (5) serum high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL (1.04 mmol/L) in men or < 50 mg/dL (1.29 mmol/L) in women or specific drug treatment; and (6) homeostasis model assessment insulin resistance (HOMA-IR) score ≥ 2.5 with reference to the diagnostic criteria of metabolic associated fatty liver disease (MAFLD) (Eslam et al. 2020). Metabolically, unhealthy status was defined as if more than 2 above metabolic abnormalities components existed. Normal weight was defined as a BMI of 18.5 to < 23.0 kg/m2, and overweight/obesity was defined as a BMI of ≥ 23.0 kg/m2 to the threshold for Asians (Wen et al. 2009). Individuals were categorized into four groups according to their BMI and metabolic health status: (1) metabolically healthy normal weight (MHNW): BMI < 23 kg/m2, with ≤ 1 metabolic abnormality; (2) metabolically unhealthy normal weight (MUNW): BMI < 23 kg/m2, with > 1 metabolic abnormalities; (3) metabolically healthy overweight/obesity (MHO): BMI ≥ 23 kg/m2, with ≤ 1 metabolic abnormality; (4) metabolically unhealthy overweight/obesity (MUO): BMI ≥ 23 kg/m2, with > 1 metabolic abnormalities.
Diabetes was diagnosed as fasting plasma glucose levels ≥ 7.0 mmol/L, or an OGTT 2-h plasma glucose levels ≥ 11.1 mmol/L, or a previous diagnosis or self-reported current treatment for diabetes with hypoglycemic drugs. Prediabetes was diagnosed as fasting plasma glucose at 6.1–7.0 mmol/L or OGTT 2-h plasma glucose at the level of 7.8–11.1 mmol/L, according to 1999 WHO criteria (Alberti and Zimmet 1998). Metabolic syndrome was defined as central obesity (waist circumference ≥ 90 cm for males and ≥ 80 cm for females) plus two or more of the following conditions: blood pressure ≥ 130/85 mmHg or antihypertensive treatment, FPG ≥ 5.6 mmol/L or antidiabetic treatment, TG ≥ 1.7 mmol/L or antilipidemic treatment, and HDL cholesterol < 1.03 mmol/L for males and < 1.29 mmol/L for females (Alberti et al. 2006). Hypertension was defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or current use of antihypertensive medications (Whelton et al. 2018). Hypertriglyceridemia was defined as serum triglycerides ≥ 200 mg/dL (2.3 mmol/L) or specific drug treatment; and hypercholesterolemia was defined as serum cholesterol ≥ 240 mg/dL (6.2 mmol/L) or LDL-C ≥ 160 mg/dL (4.1 mmol/L) or specific drug treatment (Zhu et al. 2017).
Statistical Analysis
All statistical analyses were performed using SPSS version 18.0 software. The data were presented as mean ± standard deviation (SD). The continuous data were compared using the Student’s t test, whereas the χ2 test was used for comparisons of categorical variables. The prevalence of diabetes, hypertension, hypertriglyceridemia, hypercholesterolemia, and metabolic syndrome in the MHNW → MUNW, MHO → MHO, and MHO → MUO participants was compared with their prevalence in the MHNW → MHNW participants using the χ2 test. The proportion of participants transitioning from metabolically healthy to unhealthy status was also calculated and compared between the MHNW and MHO participants using the χ2 test. Continuous correction was applied when small numbers in the subgroups indicated that this was appropriate. A heatmap was used to represent the value of each serum metabolite in the MHNW → MUNW, MHO → MHO, and MHO → MUO groups relative to those in the MHNW → MHNW group. All metabolites were log-transformed to approximate normality before the analysis and entered into a one-way analysis of variance (ANOVA) to show the differentiated metabolites among the MHNW → MHNW, MHNW → MUNW, MHO → MHO, and MHO → MUO groups. Univariate and multivariate logistic regression models were used to estimate the odds ratios and 95% confidence intervals (CIs) of the risk of transition from MHNW to MUNW or MHO to MUO per-SD increase of each selected metabolite. Age, sex, cigarette smoking, alcohol consumption, BMI, waist circumference, FPG, SBP, serum triglycerides, cholesterol, HDL-C, and LDL-C were adjusted in the multivariate regression model. Finally, the transition rates from metabolically healthy to unhealthy status in the metabolomics-determined high metabolic risk MHNW and low metabolic risk MHO were calculated and compared with the whole MHNW and MHO participants, respectively. All statistical analyses were two-sided, and a value of P < 0.05 was considered statistically significant.
Results
Characteristics of the Baseline Population
The baseline characteristics of the study subjects are shown in Table 1. A total of 701 MHNW and 377 MHO participants who completed the follow-up examination were enrolled for analysis. MHO was more common in male participants, and consistently the proportion of cigarette smoking was higher in MHO. Compared with the MHNW participants, MHO participants had a significantly higher body weight, BMI, waist circumference, and blood pressure levels and lower serum HDL-C levels (all P < 0.001). However, the prevalence of diabetes, hypertriglyceridemia, hypercholesterolemia and metabolic syndrome was similar between the MHO and MHNW.
Table 1.
Metabolic parameters of the participants at baseline
| MHNW | MHO | P value | |
|---|---|---|---|
| No. of participants | 701 | 377 | – |
| Male, n (%) | 237 (33.8%) | 193 (51.2%) | < 0.001 |
| Age, year | 60.2 ± 8.4 | 60.6 ± 7.8 | 0.452 |
| Cigarette Smoking, n (%) | 124 (17.7%) | 91 (24.1%) | 0.012 |
| Alcohol drinking, n (%) | 71 (10.1%) | 69 (18.3%) | < 0.001 |
| Body weight, kg | 54.5 ± 6.7 | 65.4 ± 7.1 | < 0.001 |
| BMI, kg/m2 | 20.8 ± 1.5 | 24.8 ± 1.7 | < 0.001 |
| Waist circumference, cm | 74 ± 6 | 83 ± 6 | < 0.001 |
| Systolic BP, mmHg | 122 ± 16 | 127 ± 16 | < 0.001 |
| Diastolic BP, mmHg | 71 ± 9 | 74 ± 9 | < 0.001 |
| FPG, mmol/L | 5.0 ± 0.9 | 5.0 ± 0.6 | 0.853 |
| OGTT 2hPG, mmol/L | 5.8 ± 1.5 | 6.1 ± 1.4 | 0.004 |
| TG, mmol/L | 1.1 ± 0.7 | 1.2 ± 0.4 | 0.741 |
| TC, mmol/L | 5.1 ± 0.9 | 5.0 ± 0.9 | 0.058 |
| HDL cholesterol, mmol/L | 1.7 ± 0.4 | 1.5 ± 0.3 | < 0.001 |
| LDL cholesterol, mmol/L | 2.9 ± 0.8 | 2.9 ± 0.7 | 0.498 |
| Diabetes, n(%) | 22(3.1%) | 12(3.2%) | 0.892 |
| Hypertension, n(%) | 83(11.8%) | 70(18.6%) | 0.003 |
| Hypertriglyceridemia, n(%) | 19(2.7%) | 4(1.1%) | 0.074 |
| Hypercholesterolemia, n(%) | 79(11.3%) | 33(8.8%) | 0.197 |
MHNW, metabolically healthy normal weight; MHO, metabolically healthy overweight/obesity; BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; OGTT 2hPG, oral glucose tolerance test 2-h plasma glucose; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Metabolic Health Status of the MHNW and MHO at Follow-up
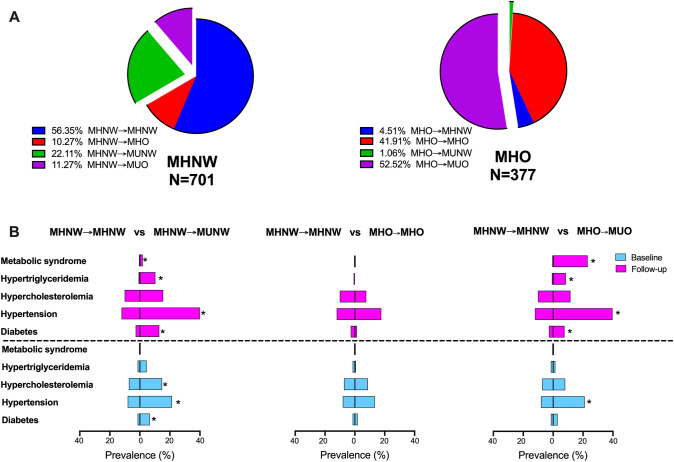
As shown in Fig. 1A, more MHO participants developed a metabolically unhealthy status than comparing to MHNW participants (53.58% vs 33.38%, P < 0.001) after a median of 4.2 years of follow-up. Although MHNW and MHO participants had a similar prevalence of diabetes, metabolic syndrome, and hyperlipidemia (hypertriglyceridemia and hypercholesterolemia) at baseline, MHO had a higher prevalence of metabolic syndrome and hypertriglyceridemia at follow-up (not shown). Compared with the MHNW participants who remained metabolically healthy at follow-up (MHNW → MHNW), those who developed metabolic disorders (MHNW → MUNW) had a significantly higher prevalence of diabetes, hypertension, hypertriglyceridemia, and hypercholesterolemia at baseline and a higher prevalence of diabetes, hypertension, hypertriglyceridemia and metabolic syndrome at follow-up (all P < 0.05) (Fig. 1B, left). There seemed to be two subgroups of MHO, including the MHO with and without the occurrence of metabolic disorders during the longitudinal follow-up. For the MHO participants who remained metabolically healthy (MHO → MHO), the prevalence of all metabolic diseases was similar to that of MHNW participants at both the baseline and follow-up examination (all P > 0.05), but for those who became metabolically unhealthy overweight/obesity (MHO → MUO), the prevalence of diabetes, hypertension, hypertriglyceridemia and metabolic syndrome at follow-up examination was significantly higher than that of the MHNW participants (all P < 0.05) (Fig. 1B, middle and right panels).
Fig. 1.
A Metabolic health status of MHNW and MHO patients at follow-up examination and B Comparison of the prevalence of multiple metabolic diseases between MHNW → MHNW and MHNW → MUNW (left), MHNW → MHNW and MHO → MHO (middle), and MHNW → MHNW and MHO → MUO (right) participants, repectively. *P < 0.05 compared with MHNW → MHNW group. MHNW, metabolically healthy normal weight; MUNW, metabolically unhealthy normal weight; MHO, metabolically healthy overweight/obesity; MUO, metabolically unhealthy overweight/obesity; MHNW→MHNW, remaining in MHNW; MHNW→MHO, MHNW transition to MHO; MHNW→MUNW, MHNW transition to MUNW; MHNW→MUO, MHNW transition to MUO; MHO→MHNW, MHO transition to MHNW; MHO→MHO, remaining in MHO; MHO→MUNW, MHO transition to MUNW; MHO→MUO, MHO transition to MUO
Predictors of the Development of Metabolic Disorders in MHNW and MHO
To investigate the clinical predictors of the transition from the metabolically healthy status to metabolically unhealthy status, the baseline metabolic parameters were compared between the participants with MHNW → MHNW and MHNW → MUNW and the participants with MHO → MHO and MHO → MUO (Table 2). Compared with the MHNW participants who remained metabolically healthy at follow-up, the baseline prevalence of diabetes and hypertension was significantly higher in the MHNW → MHUW participants. However, for the MHO participants, there was no significant difference in the baseline metabolic disorders between the MHO → MHO and MHO → MUO subgroups (Table 2).
Table 2.
Baseline metabolic status of the MHO participants divided by follow-up metabolic status
| MHNW → MHNW | MHNW → MUNW | P value | MHO → MHO | MHO → MUO | P value | |
|---|---|---|---|---|---|---|
| Diabetes, n(%) | 6 (1.5%) | 10 (6.5%) | 0.004 | 3 (1.9%) | 6 (3.0%) | 0.738 |
| Hypertension, n(%) | 32 (8.1%) | 33 (21.3%) | < 0.001 | 21 (13.3%) | 42 (21.2%) | 0.052 |
| Hypertriglyceridemia, n(%) | 6 (1.5%) | 7 (4.5%) | 0.077 | 1 (0.6%) | 3 (1.5%) | 0.781 |
| Hypercholesterolemia, n(%) | 29 (7.3%) | 23 (14.8%) | 0.007 | 15 (9.5%) | 16 (8.1%) | 0.774 |
| Metabolic syndrome, n(%) | 0 (0.0%) | 0 (0.0%) | – | 0 (0.0%) | 0 (0.0%) | – |
MHNW, metabolically healthy normal weight; MUNW, metabolically unhealthy normal weight; MHO, metabolically healthy overweight/obesity; MUO, metabolically unhealthy overweight/obesity; MHNW→MHNW, remaining in MHNW; MHNW→MUNW, MHNW transition to MUNW; MHO→MHO, remaining in MHO; MHO→MUO, MHO transition to MUO
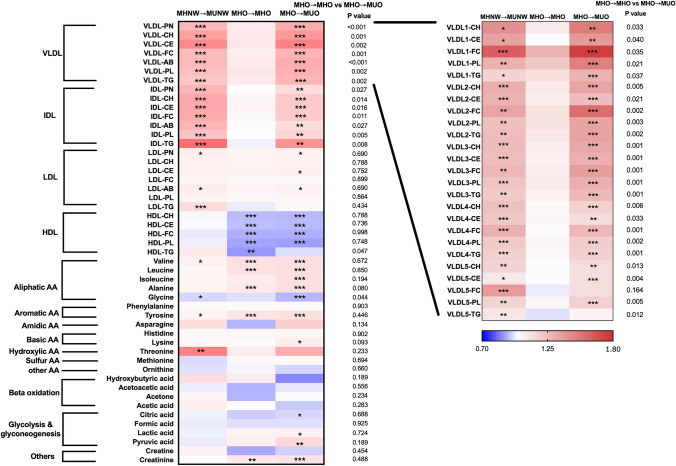
The serum metabolomic profiles were examined and compared among MHNW → NHNW, MHNW → MUNW, MHO → MHO, and MHO → MUO participants, as shown in Fig. 2. Compared with the MHNW → MHNW participants, the MHO participants, whether they developed MUO or not, had significantly lower concentrations of HDL components and glycine and higher concentrations of other aliphatic amino acids (valine, leucine, isoleucine, alanine) and tyrosine, which indicated that HDL components and aliphatic amino acids might be directly correlated with adiposity, independent of metabolic status. Moreover, compared with the participants with a sustained metabolically healthy status, both the MHNW and MHO participants who developed two or more metabolic disorders at follow-up had higher VLDL and IDL particle numbers and components at the baseline examination. Further analysis of the relationship between the different subtypes of VLDLs (VLDL1–VLDL5) and the metabolic status at follow-up indicated that all subtypes of VLDL at baseline were positively associated with the development of metabolic disorders at follow-up. The strength of the correlation gradually weakened from VLDL1 to VLDL5. Moreover, the concentrations of several amino acids (valine, glycine, tyrosine, and threonine) were significantly different between the MHNW → MHNW and MHNW → MUNW participants.
Fig. 2.
The heatmap represents the relative value of each serum metabolite in the MHNW → MUNW, MHO → MHO, and MHO → MUO groups in comparison to that in the MHNW → MHNW group. *P < 0.05, **P < 0.01, ***P < 0.001 compared with MHNW → MHNW participants. MHNW, metabolically healthy normal weight; MUNW, metabolically unhealthy normal weight; MHO, metabolically healthy overweight/obesity; MUO, metabolically unhealthy overweight/obesity; MHNW→MHNW, remaining in MHNW; MHNW→MUNW, MHNW transition to MUNW; MHO→MHO, remaining in MHO; MHO→MUO, MHO transition to MUO; VLDL-PN, very low-density lipoprotein particle numbers; VLDL-CH, very low-density lipoprotein cholesterol; VLDL-CE, very low-density lipoprotein cholesteryl ester; VLDL-FC, very low-density lipoprotein free cholesterol; VLDL-AB, very low-density lipoprotein apolipoprotein B-100; VLDL-PL, very low-density lipoprotein phospholipid; VLDL-TG, very low-density lipoprotein triglycerides; IDL-PN, intermediate-density lipoprotein particle numbers; IDL-CH, intermediate-density lipoprotein cholesterol; IDL-CE, intermediate-density lipoprotein cholesteryl ester; IDL-FC, intermediate-density lipoprotein free cholesterol; IDL-AB, intermediate-density lipoprotein apolipoprotein B-100; IDL-PL, intermediate-density lipoprotein phospholipid; IDL-TG, intermediate-density lipoprotein triglyceride; HDL-CH, high-density lipoprotein cholesterol; HDL-CE, high-density lipoprotein cholesteryl ester; HDL-FC, high-density lipoprotein free cholesterol; HDL-PL, high-density lipoprotein phospholipid; HDL-TG, high-density lipoprotein triglycerides; AA, amino acid; VLDL1-CH, very low-density lipoprotein-1 cholesterol; VLDL1-CE, very low-density lipoprotein-1 cholesteryl ester; VLDL1-FC, very low-density lipoprotein-1 free cholesterol; VLDL1-PL, very low-density lipoprotein-1 phospholipid; VLDL1-TG, very low-density lipoprotein-1 triglycerides; VLDL2-CH, very low-density lipoprotein-2 cholesterol; VLDL2-CE, very low-density lipoprotein-2 cholesteryl ester; VLDL2-FC, very low-density lipoprotein-2 free cholesterol; VLDL2-PL, very low-density lipoprotein-2 phospholipid; VLDL2-TG, very low-density lipoprotein-2 triglycerides; VLDL3-CH, very low-density lipoprotein-3 cholesterol; VLDL3-CE, very low-density lipoprotein-3 cholesteryl ester; VLDL3-FC, very low-density lipoprotein-3 free cholesterol; VLDL3-PL, very low-density lipoprotein-3 phospholipid; VLDL3-TG, very low-density lipoprotein-3 triglycerides; VLDL4-CH, very low-density lipoprotein-4 cholesterol; VLDL4-CE, very low-density lipoprotein-4 cholesteryl ester; VLDL4-FC, very low-density lipoprotein-4 free cholesterol; VLDL4-PL, very low-density lipoprotein-4 phospholipid; VLDL4-TG, very low-density lipoprotein-4 triglycerides; VLDL5-CH, very low-density lipoprotein-5 cholesterol; VLDL5-CE, very low-density lipoprotein-5 cholesteryl ester; VLDL5-FC, very low-density lipoprotein-5 free cholesterol; VLDL5-PL, very low-density lipoprotein-5 phospholipid; VLDL5-TG, very low-density lipoprotein-5 triglycerides
Considering the significant alterations of VLDL, IDL, and several amino acids in individuals with a transition from metabolically healthy to unhealthy status, the independent predictive values of these metabolites for the development of metabolic disorders were further detected using multivariate logistic regression models. As shown in Table 3, univariate analysis showed that the baseline serum VLDL and IDL particle number and components were consistently positively associated with the risk of a transition from metabolically healthy to unhealthy status in both in the MHNW and MHO participants. After multivariate adjustment for age, sex, cigarette smoking, alcohol consumption, BMI, waist circumference, FPG, SBP, serum triglycerides, cholesterol, HDL-C and LDL-C, the IDL but not the VLDL components remained significantly associated with a metabolically unhealthy phenotype at follow-up in MHNW participants. The multivariate ORs and 95% CIs for the metabolically unhealthy phenotype at follow-up per-SD increase in IDL particle number were 2.28 (1.51–3.44) in the MUNW participants. However, in the MHO participants, the VLDL but not the IDL components that were independently associated with a metabolically unhealthy phenotype at follow-up after multiple adjustments. Among all the VLDL components, VLDL-CH showed the strongest association with the transition from metabolically healthy to unhealthy status (multivariate OR: 6.36, 95% CI 1.77–22.81, P = 0.005). In addition, all the selected serum amino acids showed no significant association with the metabolically unhealthy phenotype at follow-up after multiple adjustments.
Table 3.
Predictive value of lipidomics components for the transition from metabolically healthy to unhealthy status
| MHNW → MUNW | MHO → MUO | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate OR per SD | P value | Multivariate OR per SD | P value | Univariate OR per SD | P value | Multivariate OR per SD | P value | |
| VLDL-PN | 1.41 (1.19–1.68) | < 0.001 | 1.40 (0.75–2.61) | 0.286 | 1.66 (1.28–2.15) | < 0.001 | 3.67 (1.24–10.83) | 0.019 |
| VLDL-CH | 1.38 (1.16–1.64) | < 0.001 | 1.38 (0.57–3.32) | 0.479 | 1.60 (1.23–2.08) | < 0.001 | 6.36 (1.77–22.81) | 0.005 |
| VLDL-CE | 1.36 (1.15–1.61) | < 0.001 | 1.26 (0.58–2.75) | 0.556 | 1.56 (1.20–2.03) | 0.001 | 3.70 (1.22–11.28) | 0.021 |
| VLDL-FC | 1.40 (1.17–1.66) | < 0.001 | 1.37 (0.61–3.09) | 0.448 | 1.62 (1.25–2.10) | < 0.001 | 3.66 (1.21–11.07) | 0.022 |
| VLDL-AB | 1.41 (1.19–1.68) | < 0.001 | 1.40 (0.75–2.61) | 0.286 | 1.66 (1.28–2.15) | < 0.001 | 3.67 (1.24–10.84) | 0.019 |
| VLDL-PL | 1.39 (1.16–1.65) | < 0.001 | 1.25 (0.64–2.44) | 0.516 | 1.56 (1.21–2.01) | 0.001 | 1.60 (0.68–3.73) | 0.281 |
| VLDL-TG | 1.34 (1.11–1.61) | 0.002 | 0.48 (0.18–1.25) | 0.130 | 1.63 (1.24–2.14) | < 0.001 | 1.20 (0.37–3.89) | 0.761 |
| IDL-PN | 1.65 (1.36–1.99) | < 0.001 | 2.28 (1.51–3.44) | < 0.001 | 1.42 (1.08–1.88) | 0.013 | 1.48 (0.90–2.42) | 0.122 |
| IDL-CH | 1.55 (1.29–1.88) | < 0.001 | 2.46 (1.38–4.37) | 0.002 | 1.49 (1.13–1.97) | 0.005 | 2.02 (1.01–4.04) | 0.046 |
| IDL-CE | 1.56 (1.29–1.88) | < 0.001 | 2.40 (1.38–4.16) | 0.002 | 1.48 (1.12–1.95) | 0.006 | 2.02 (1.03–3.95) | 0.041 |
| IDL-FC | 1.54 (1.28–1.86) | < 0.001 | 2.49 (1.34–4.62) | 0.004 | 1.51 (1.14–2.01) | 0.004 | 1.97 (0.95–4.12) | 0.070 |
| IDL-AB | 1.65 (1.36–1.99) | < 0.001 | 2.28 (1.51–3.44) | < 0.001 | 1.42 (1.08–1.88) | 0.012 | 1.48 (0.90–2.42) | 0.122 |
| IDL-PL | 1.47 (1.21–1.78) | < 0.001 | 1.88 (0.68–5.22) | 0.227 | 1.51 (1.15–1.98) | 0.003 | 1.80 (0.51–6.44) | 0.364 |
| IDL-TG | 1.38 (1.12–1.71) | 0.003 | 0.77 (0.26–2.27) | 0.638 | 1.67 (1.23–2.28) | 0.001 | 0.11 (0.02–0.87) | 0.036 |
| Valine | 1.27 (1.05–1.53) | 0.014 | 1.25 (0.99–1.58) | 0.063 | 1.05 (0.85–1.30) | 0.651 | 1.08 (0.83–1.41) | 0.564 |
| Glycine | 0.80 (0.66–0.98) | 0.030 | 0.87 (0.70–1.08) | 0.212 | 0.80 (0.64–0.99) | 0.041 | 0.85 (0.66–1.09) | 0.206 |
| Tyrosine | 1.21 (1.01–1.46) | 0.044 | 1.13 (0.91–1.39) | 0.271 | 1.08 (0.88–1.31) | 0.463 | 1.02 (0.83–1.26) | 0.840 |
| Threonine | 1.37 (1.09–1.73) | 0.007 | 1.29 (1.00–1.68) | 0.054 | 1.16 (0.91–1.47) | 0.230 | 1.17 (0.88–1.55) | 0.281 |
Adjusted for age, sex, smoking, alcohol drinking, BMI, waist circumference, FPG, SBP, serum triglycerides, cholesterol, HDL cholesterol, LDL cholesterol
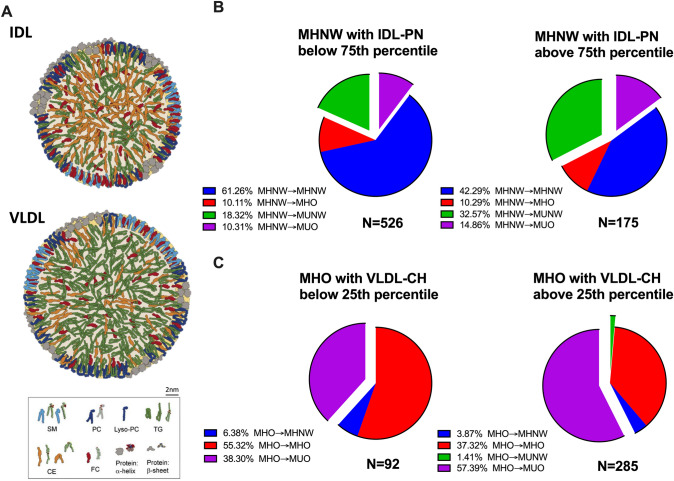
As shown in Fig. 3, if we further divided the MHNW and MHO participants into quartiles according to their baseline IDL particle number and VLDL-CH, respectively, we found that the MHNW participants with an IDL particle number above the 75th percentile had a risk of developing metabolic disorders that was 1.7 times higher than those with an IDL particle number below the 75th percentile (47.43% vs 28.63%, P < 0.001), and the metabolic risk was similar to that of the MHO (47.43% vs 53.58%, P = 0.170). Meanwhile, the MHO participants with VLDL-CH below the 25th percentile showed a significantly lower risk of developing metabolic disorders than those with VLDL-CH above the 25th percentile (38.3% vs 58.8%, P < 0.001), which was similar to the MHNW participants (38.3% vs 33.38%, P = 0.359).
Fig. 3.
A Size and structure of VLDL and IDL. B Metabolic health status of MHNW with IDL-PN below the 75th percentile (n = 526) and above the 75th percentile (n = 175) at the follow-up examination. C Metabolic health status of MHO with VLDL-CH below the 25th percentile (n = 92) and above the 25th percentile (n = 285) at the follow-up examination. IDL, intermediate-density lipoprotein; VLDL, very low-density lipoprotein; SM, sphingomyelin; PC, phosphatidylcholine; Lyso-PC, lysophosphatidylcholine; TG, triglyceride; CE, cholesteryl ester; FC, free cholesteryl; MHNW, metabolically healthy normal weight; MUNW, metabolically unhealthy normal weight; MHO, metabolically healthy overweight/obesity; MUO, metabolically unhealthy overweight/obesity; MHNW→MHNW, remaining in MHNW; MHNW→MHO, MHNW transition to MHO; MHNW→MUNW, MHNW transition to MUNW; MHNW→MUO, MHNW transition to MUO; MHO→MHNW, MHO transition to MHNW; MHO→MHO, remaining in MHO; MHO→MUNW, MHO transition to MUNW; MHO→MUO, MHO transition to MUO; IDL-PN, intermediate-density lipoprotein particle numbers; VLDL-CH, very low-density lipoprotein cholesterol
Discussion
The main findings of this study are as follows: (1) Not all “metabolically healthy” people are truly healthy. A significant proportion of metabolically healthy people will acquire metabolically unhealthy phenotypes in the course of their lifetime; (2) Traditional metabolic parameters are not accurate enough to predict the development of metabolic disorders in metabolically healthy people, especially MHO; (3) Serum metabolomic examination indicated that IDL components and VLDL-CH might be valuable in discriminating MHNW and MHO people with the potential to transition to metabolically unhealthy status.
It is of great importance to investigate the predictors of the development of metabolic disorders in the metabolically healthy population. Conventionally, obesity is thought to be a reliable and direct predictor of metabolic disorders in “metabolically healthy” people. It has been fully demonstrated that obesity is an independent risk factor for metabolic disorders in metabolically healthy populations (Eckel et al. 2018; Espinosa De Ycaza et al. 2018). The presence of obesity in metabolically healthy people is associated with a 44% risk of transitioning to a metabolically unhealthy phenotype over the 6-years follow-up, and the proportion increased to 62% over a 12-year follow-up (Gilardini et al. 2018). MHO was not a permanent situation, just a transient state, and the increased risk of diabetes in MHO is attributed to its progression to an unhealthier state (Navarro-Gonzalez et al. 2016; Wang et al. 2018). Several researchers even held the opinion that all MHO individuals have an increased risk of developing metabolic disorders at some point during the follow-up (Chang et al. 2016; Feng et al. 2020).
However, there are exceptions for the relationship between BMI and the risk of incident metabolic disorders. The MUNW and MHO individuals accounted for a nonnegligible proportion of the population, with an estimated prevalence ranging from 10 to 37% in lean individuals and 13–29% in obese individuals (Badoud et al. 2015). Despite their normal body weight, MUNW people have a greater than threefold higher risk of all-cause mortality or cardiovascular events (Kramer et al. 2013). Insulin resistance, nonalcoholic fatty liver disease, visceral adiposity, and increased carotid intima-media thickness have been used to distinguish MHNW people with sustained metabolic health status and with a potential risk for developing metabolic abnormalities (Stefan et al. 2017). A low percentage of subcutaneous leg fat mass has also been found in MUNW people (Karpe and Pinnick 2015).
In the current study, we found that MHNW → MUNW patients had higher blood glucose, blood pressure, and serum cholesterol than MHNW → MHNW patients, which might contribute to the development of metabolic disorders at follow-up. Intriguingly, we also found that serum IDL and its components could predict a metabolically unhealthy phenotype after a 4.2-year follow-up, and the prediction effect was independent of all conventional metabolic parameters (including BMI, waist circumference, serum triglycerides, cholesterol, HDL-C, and LDL-C). IDL is generated from the degradation of VLDL and HDL, cleared in the liver through the LDL receptor or further degraded to form LDL particles. The independent association of a metabolically unhealthy phenotype with IDL but not VLDL or HDL indicated that the elevation of IDL in MHNW → MUNW participants might be related to its reduced clearance in the liver. Since serum IDL and its components are rarely measured in routine examinations, the causal relationship between IDL and metabolic diseases as well as its underlying mechanism have not been fully investigated and it requires further investigation.
Compared with the MHNW participants, the predictors of the metabolically unhealthy phenotype in MHO participants seem to be more important clinically, because none of the conventional metabolic parameters could be used to predict the transition from MHO to MUO. We found that although the majority of individuals with MHO would transition to a metabolically unhealthy status, there was still a nonnegligible portion (approximately 40%) of MHO with sustained metabolically healthy status, and their risk of multiple metabolic diseases was similar to that of the MHNW participants during the longitudinal follow-up. Consistent with our findings, Bell et al. reported that among 66 healthy obese adults, 68.2% of these subjects remained metabolically healthy after 5 years, and 59.1%, 34.8%, and 48.5% of them were healthy obese after 10, 15, and 20 years, respectively (Bell et al. 2015). Another study found that MHO showed a similar risk to MHNW for developing cardiovascular diseases throughout life (Mongraw-Chaffin et al. 2018). However, the current criteria for metabolically healthy status were not sufficient to discriminate true MHO from MHO with the potential to turn into MUO. By taking a large number of “MHOs” into consideration, it will be interesting to explore novel predictors for the transition from MHO to MUO in a large number of MHO participants.
Several previous studies have investigated clinical predictors of the transition from MHO to MUO during longitudinal follow-up. They reported that the transition from MHO to MUO and the subsequent development of diabetes was linked to having a higher baseline BMI as well as being male and a smoker (Heianza et al. 2014). Body composition was another factor resulting in an increased risk for MUO. The body fat mass, arm fat mass, and total fat mass measured by DEXA were critically higher among individuals with the MUO phenotype than among those with the MHO phenotype in Korean women (Kang and Yim 2019). Lifestyle, including various levels of physical activity and dietary habits, were associated with this as well, with sedentary habits and unhealthier eating having a higher risk of developing metabolically unhealthy status (Kanagasabai et al. 2015; Kang and Yim 2019; Slagter et al. 2018). It was found that weight gain could increase the risk of developing cardiometabolic complications in the MHO group if individuals with the MHO phenotype gained > 15% of their body weight (Espinosa De Ycaza et al. 2018). A favorable lifestyle also benefited MHO through weight loss, as one study among adolescents showed that the difference in sedentary habits between the MHO and MUO phenotypes was absent after adjusting for BMI (Cadenas-Sanchez et al. 2017). Intensive lifestyle modification inducing clinically significant weight loss in the MHO phenotype could lead to a reduction of serum adipokines and inflammatory biomarkers, which play important roles in the pathological mechanism of obesity and insulin resistance (Gomez-Huelgas et al. 2019). However, none of these body mass-related metabolic parameters is sufficient to discriminate MHO with or without an increased risk of MHO to MUO transition.
Conventional serum biochemical parameters have also been evaluated for the possibility of predicting the transition from MHO to MUO. Gilardini et al. (2018) found that LDL-C was an independent factor that predicted the transition to metabolically unhealthy status. Another study also found that ApoB-100 might predict the conversion from MHO to the MUO phenotype (Doumatey et al. 2016). However, their prediction value was limited due to either their small sample size or the relatively weak correlation of MHO to MUO, and few previous studies have systemically investigated novel metabolomic biomarkers for the prediction of the development of metabolic disorders in MHO.
In our current study, we investigated the association between hundreds of serum metabolites and the clinical transition from MHO to MUO through a large-scale Chinese community cohort. We found that most metabolites related to VLDL, especially VLDL-CH, showed a consistent and remarkable relationship with the MHO–MUO transition in MHO participants. VLDL represents the influence of hepatic lipid metabolism on whole-body metabolism. Al-Mrabeh et al. (2020) found that VLDL could directly regulate insulin secretion. In addition, VLDL reflects the hepatic steatosis grades and insulin resistance level. While in a state of hepatic insulin resistance, the serum VLDL concentration would increase by stimulating VLDL assembly, enlarging the VLDL particle size, increasing VLDL secretion, and decreasing VLDL catabolism (Hirano 2018). Thus, our study agreed well with previous studies on the function of VLDL and indicated that VLDL might be a useful serum biomarker for the prediction of MHO to MUO. Unexpectedly, we found that VLDL-CH had the strongest predictive value for the development of metabolic disorders among all VLDL components. The effect of VLDL-TG was adjusted and masked by the effect of serum triglycerides, but the independent strong correlation between VLDL-CH and metabolic disorders is still worthy of further study.
Conclusion
Current criteria for metabolic health will lead to the missed identification of a nonnegligible proportion of people with an increased risk of metabolic disorders from the perspective of long-term follow-up. Measurement of serum IDL components and VLDL-CH might help to identify MHNW/MHO with the potential to develop a metabolically unhealthy status. It might also be worthwhile to test the effect of a pharmaceutical reduction of serum VLDL-CH and IDL on the risk of transitioning from metabolically healthy to unhealthy status.
Acknowledgements
We acknowledge the financial support of the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), and the Science and Technology Commission of Shanghai Municipality (16JC1400500).
Abbreviations
- MHO
Metabolically healthy overweight/obesity
- MUO
Metabolically unhealthy overweight/obesity
- MHNW
Metabolically healthy normal weight
- MUNW
Metabolically unhealthy normal weight
- MHNW → MHNW
Remaining in MHNW
- MHNW → MHO
MHNW transition to MUO
- MHNW → MUNW
MHNW transition to MUNW
- MHNW → MUO
MHNW transition to MUO
- MHO → MHNW
MHO transition to MHNW
- MHO → MHO
Remaining in MHO
- MHO → MUNW
MHO transition to MUNW
- MHO → MUO
MHO transition to MUO
- BMI
Body mass index
- BP
Blood pressure
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- FBG
Fasting blood glucose
- TC
Total cholesterol
- TG
Triglycerides
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- 2hPG
2-H Post-challenge plasma glucose
- OGTT
Oral glucose tolerance test
- VLDL-PN
Very low-density lipoprotein particle numbers
- VLDL-CH
Very low-density lipoprotein cholesterol
- VLDL-CE
Very low-density lipoprotein cholesteryl ester
- VLDL-FC
Very low-density lipoprotein free cholesterol
- VLDL-AB
Very low-density lipoprotein apolipoprotein B-100
- VLDL-PL
Very low-density lipoprotein phospholipid
- VLDL-TG
Very low-density lipoprotein triglycerides
- IDL-PN
Intermediate-density lipoprotein particle numbers
- IDL-CH
Intermediate-density lipoprotein cholesterol
- IDL-CE
Intermediate-density lipoprotein cholesteryl ester
- IDL-FC
Intermediate-density lipoprotein free cholesterol
- IDL-AB
Intermediate-density lipoprotein apolipoprotein B-100
- IDL-PL
Intermediate-density lipoprotein phospholipid
- IDL-TG
Intermediate-density lipoprotein triglyceride
- HDL-CH
High-density lipoprotein cholesterol
- HDL-CE
High-density lipoprotein cholesteryl ester
- HDL-FC
High-density lipoprotein free cholesterol
- HDL-PL
High-density lipoprotein phospholipid
- HDL-TG
High-density lipoprotein triglycerides
- AA
Amino acid
- VLDL1-CH
Very low-density lipoprotein-1 cholesterol
- VLDL1-CE
Very low-density lipoprotein-1 cholesteryl ester
- VLDL1-FC
Very low-density lipoprotein-1 free cholesterol
- VLDL1-PL
Very low-density lipoprotein-1 phospholipid
- VLDL1-TG
Very low-density lipoprotein-1 triglycerides
- VLDL2-CH
Very low-density lipoprotein-2 cholesterol
- VLDL2-CE
Very low-density lipoprotein-2 cholesteryl ester
- VLDL2-FC
Very low-density lipoprotein-2 free cholesterol
- VLDL2-PL
Very low-density lipoprotein-2 phospholipid
- VLDL2-TG
Very low-density lipoprotein-2 triglycerides
- VLDL3-CH
Very low-density lipoprotein-3 cholesterol
- VLDL3-CE
Very low-density lipoprotein-3 cholesteryl ester
- VLDL3-FC
Very low-density lipoprotein-3 free cholesterol
- VLDL3-PL
Very low-density lipoprotein-3 phospholipid
- VLDL3-TG
Very low-density lipoprotein-3 triglycerides
- VLDL4-CH
Very low-density lipoprotein-4 cholesterol
- VLDL4-CE
Very low-density lipoprotein-4 cholesteryl ester
- VLDL4-FC
Very low-density lipoprotein-4 free cholesterol
- VLDL4-PL
Very low-density lipoprotein-4 phospholipid
- VLDL4-TG
Very low-density lipoprotein-4 triglycerides
- VLDL5-CH
Very low-density lipoprotein-5 cholesterol
- VLDL5-CE
Very low-density lipoprotein-5 cholesteryl ester
- VLDL5-FC
Very low-density lipoprotein-5 free cholesterol
- VLDL5-PL
Very low-density lipoprotein-5 phospholipid
- VLDL5-TG
Very low-density lipoprotein-5 triglycerides
Authors' contributions
Study conception and design: XG, HT. Data collection: HL. Sample examination: QW, SM, HZ. Data management: QH. Data analysis: MX. Manuscript preparation: QW. Manuscript revision: GX and MX.
Funding
This work was supported by the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01) and the Science and Technology Commission of Shanghai Municipality (16JC1400500).
Availability of data and materials
The data generated and analyzed during the current study are not publicly available due to the relevant policy of data management from the sponsors in the Chinese national and local government, but the data are available from the corresponding authors upon reasonable request with the permission of the Chinese national and local government.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflicts of interest. HT is the associate editor of Phenomics, and he was not involved in reviewing this paper.
Ethics approval
This prospective cohort study from Shanghai Changfeng Community was approved by the Research Ethics Committee of the Shanghai Health Bureau, China.
Consent to participate
Each participant provided written informed consent.
Consent for publication
Not applicable.
Footnotes
Qi Wu and Qing-xia Huang contributed equally to this work.
Contributor Information
Ming-feng Xia, Email: dr_xiamingfeng@163.com.
Hui-ru Tang, Email: huiru_tang@fudan.edu.cn.
Xin Gao, Email: zhongshan_endo@126.com, Email: happy20061208@126.com.
References
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications part 1—diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Al-Mrabeh A, Zhyzhneuskaya SV, Peters C, Barnes AC, Melhem S, Jesuthasan A, Aribisala B, Hollingsworth KG, Lietz G, Mathers JC, et al. Hepatic lipoprotein export and remission of human type 2 diabetes after weight loss. Cell Metab. 2020;31:233–249. doi: 10.1016/j.cmet.2019.11.018. [DOI] [PubMed] [Google Scholar]
- Badoud F, Perreault M, Zulyniak MA, Mutch DM. Molecular insights into the role of white adipose tissue in metabolically unhealthy normal weight and metabolically healthy obese individuals. FASEB J. 2015;29:748–758. doi: 10.1096/fj.14-263913. [DOI] [PubMed] [Google Scholar]
- Bell JA, Hamer M, Sabia S, Singh-Manoux A, Batty GD, Kivimaki M. The natural course of healthy obesity over 20 years. J Am Coll Cardiol. 2015;65:101–102. doi: 10.1016/j.jacc.2014.09.077. [DOI] [PubMed] [Google Scholar]
- Cadenas-Sanchez C, Ruiz JR, Labayen I, Huybrechts I, Manios Y, Gonzalez-Gross M, Breidenassel C, Kafatos A, De Henauw S, Vanhelst J, et al. Prevalence of metabolically healthy but overweight/obese phenotype and its association with sedentary time, physical activity, and fitness. J Adolesc Health. 2017;61:107–114. doi: 10.1016/j.jadohealth.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Chang Y, Jung HS, Yun KE, Cho J, Ahn J, Chung EC, Shin H, Ryu S. Metabolically healthy obesity is associated with an increased risk of diabetes independently of nonalcoholic fatty liver disease. Obesity (silver Spring) 2016;24:1996–2003. doi: 10.1002/oby.21580. [DOI] [PubMed] [Google Scholar]
- Charles BA, Doumatey A, Huang H, Zhou J, Chen G, Shriner D, Adeyemo A, Rotimi CN. The roles of IL-6, IL-10, and IL-1RA in obesity and insulin resistance in African-Americans. J Clin Endocrinol Metab. 2011;96:E2018–2022. doi: 10.1210/jc.2011-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ctoi AF, Parvu AE, Andreicut AD, Mironiuc A, Crciun A, Ctoi C, Pop ID. Metabolically healthy versus unhealthy morbidly obese: chronic inflammation, nitro-oxidative stress, and insulin resistance. Nutrients. 2018 doi: 10.3390/nu10091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumatey AP, Zhou J, Zhou M, Prieto D, Rotimi CN, Adeyemo A. Proinflammatory and lipid biomarkers mediate metabolically healthy obesity: a proteomics study. Obesity (silver Spring) 2016;24:1257–1265. doi: 10.1002/oby.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90,257 women (the Nurses' Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6:714–724. doi: 10.1016/s2213-8587(18)30137-2. [DOI] [PubMed] [Google Scholar]
- Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- Espinosa De Ycaza AE, Donegan D, Jensen MD. Long-term metabolic risk for the metabolically healthy overweight/obese phenotype. Int J Obes (lond) 2018;42:302–309. doi: 10.1038/ijo.2017.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Gong X, Liu H, Lu R, Duan T, Wang M, Wang J, Wang H, Chen J, Liu Y, et al. The diabetes risk and determinants of transition from metabolically healthy to unhealthy phenotypes in 49,702 older adults: 4-year cohort study. Obesity (silver Spring) 2020;28:1141–1148. doi: 10.1002/oby.22800. [DOI] [PubMed] [Google Scholar]
- Gao X, Hofman A, Hu Y, Lin H, Zhu C, Jeekel J, Jin X, Wang J, Gao J, Yin Y, et al. The Shanghai Changfeng Study: a community-based prospective cohort study of chronic diseases among middle-aged and elderly: objectives and design. Eur J Epidemiol. 2010;25:885–893. doi: 10.1007/s10654-010-9525-6. [DOI] [PubMed] [Google Scholar]
- Gilardini L, Zambon A, Soranna D, Croci M, Invitti C. Predictors of the transition from metabolically healthy obesity to unhealthy obesity. Eat Weight Disord. 2018;23:739–744. doi: 10.1007/s40519-018-0600-4. [DOI] [PubMed] [Google Scholar]
- Gomez-Huelgas R, Ruiz-Nava J, Santamaria-Fernandez S, Vargas-Candela A, Alarcon-Martin AV, Tinahones FJ, Bernal-Lopez MR. Impact of intensive lifestyle modification on levels of adipokines and inflammatory biomarkers in metabolically healthy obese women. Mediators Inflamm. 2019;2019:4165260. doi: 10.1155/2019/4165260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Hamaguchi M, Fukuda T, Ohbora A, Kojima T, Fukui M. Fatty liver as a risk factor for progression from metabolically healthy to metabolically abnormal in non-overweight individuals. Endocrine. 2017;57:89–97. doi: 10.1007/s12020-017-1313-6. [DOI] [PubMed] [Google Scholar]
- Heianza Y, Kato K, Kodama S, Suzuki A, Tanaka S, Hanyu O, Sato K, Sone H. Stability and changes in metabolically healthy overweight or obesity and risk of future diabetes: Niigata wellness study. Obesity (silver Spring) 2014;22:2420–2425. doi: 10.1002/oby.20855. [DOI] [PubMed] [Google Scholar]
- Hirano T. Pathophysiology of diabetic dyslipidemia. J Atheroscler Thromb. 2018;25:771–782. doi: 10.5551/jat.RV17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, Boyko EJ. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes (lond) 2015;39:1365–1370. doi: 10.1038/ijo.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YC, Ahn HY, Park CY. Association between nonalcoholic fatty liver disease and future deterioration of metabolic health: a cohort study. Obesity (silver Spring) 2019;27:1360–1366. doi: 10.1002/oby.22536. [DOI] [PubMed] [Google Scholar]
- Jiang L, Huang J, Wang Y, Tang H. Eliminating the dication-induced intersample chemical-shift variations for NMR-based biofluid metabonomic analysis. Analyst. 2012;137:4209–4219. doi: 10.1039/c2an35392j. [DOI] [PubMed] [Google Scholar]
- Jimenez B, Holmes E, Heude C, Tolson RF, Harvey N, Lodge SL, Chetwynd AJ, Cannet C, Fang F, Pearce JTM, et al. Quantitative lipoprotein subclass and low molecular weight metabolite analysis in human serum and plasma by (1)H NMR spectroscopy in a multilaboratory trial. Anal Chem. 2018;90:11962–11971. doi: 10.1021/acs.analchem.8b02412. [DOI] [PubMed] [Google Scholar]
- Jung CH, Kang YM, Jang JE, Hwang JY, Kim EH, Park JY, Kim HK, Lee WJ. Fatty liver index is a risk determinant of incident type 2 diabetes in a metabolically healthy population with obesity. Obesity (silver Spring) 2016;24:1373–1379. doi: 10.1002/oby.21483. [DOI] [PubMed] [Google Scholar]
- Kanagasabai T, Thakkar NA, Kuk JL, Churilla JR, Ardern CI. Differences in physical activity domains, guideline adherence, and weight history between metabolically healthy and metabolically abnormal obese adults: a cross-sectional study. Int J Behav Nutr Phys Act. 2015;12:64. doi: 10.1186/s12966-015-0227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EY, Yim JE. Differences in dietary intakes, body compositions, and biochemical indices between metabolically healthy and metabolically abnormal obese Korean women. Nutr Res Pract. 2019;13:488–497. doi: 10.4162/nrp.2019.13.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- Kramer CK, Zinman B, Retnakaran R. Review 21.317 Ann Intern Med. Are metabolically healthy overweight and obesity benign conditions?—A systematic review and meta-analysis. Ann Intern Med. 2013;159:758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- Mongraw-Chaffin M, Foster MC, Kalyani RR, Vaidya D, Burke GL, Woodward M, Anderson CA. Obesity severity and duration are associated with incident metabolic syndrome: evidence against metabolically healthy obesity from the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2016;101:4117–4124. doi: 10.1210/jc.2016-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongraw-Chaffin M, Foster MC, Anderson CAM, Burke GL, Haq N, Kalyani RR, Ouyang P, Sibley CT, Tracy R, Woodward M, et al. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2018;71:1857–1865. doi: 10.1016/j.jacc.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Gonzalez D, Sanchez-Inigo L, Fernandez-Montero A, Pastrana-Delgado J, Alfredo Martinez J. Are all metabolically healthy individuals with obesity at the same risk of diabetes onset? Obesity (silver Spring) 2016;24:2615–2623. doi: 10.1002/oby.21667. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter SN, Corpeleijn E, van der Klauw MM, Sijtsma A, Swart-Busscher LG, Perenboom CWM, de Vries JHM, Feskens EJM, Wolffenbuttel BHR, Kromhout D, et al. Dietary patterns and physical activity in the metabolically (un)healthy obese: the Dutch Lifelines cohort study. Nutr J. 2018;17:18. doi: 10.1186/s12937-018-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N, Schick F, Haring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26:292–300. doi: 10.1016/j.cmet.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhang M, Wang S, Wang C, Wang J, Li L, Zhang L, Ren Y, Han C, Zhao Y, et al. Dynamic status of metabolically healthy overweight/obesity and metabolically unhealthy and normal weight and the risk of type 2 diabetes mellitus: a cohort study of a rural adult Chinese population. Obes Res Clin Pract. 2018;12:61–71. doi: 10.1016/j.orcp.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Wen CP, David Cheng TY, Tsai SP, Chan HT, Hsu HL, Hsu CC, Eriksen MP. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr. 2009;12:497–506. doi: 10.1017/S1368980008002802. [DOI] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey Jr DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065/. [DOI] [PubMed] [Google Scholar]
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Gao R, Zhao S, Lu G, Zhao D, Li J. Guidelines for the prevention and treatment of dyslipidemia in adults in china (2016 revised edition) Chin J Health Manag. 2017;11:7–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and analyzed during the current study are not publicly available due to the relevant policy of data management from the sponsors in the Chinese national and local government, but the data are available from the corresponding authors upon reasonable request with the permission of the Chinese national and local government.
Not applicable.