Abstract
Breast cancer (BrCa) is the most frequent neoplastic disease in female, with high morbidity and mortality. Most of the researches were focused on tumor cells concerning their natural evolution, molecular profile, and potential response to therapy. Few and uncertain data are available about the tumor microenvironment and its impact on the progression of the disease. Mast cells (MCs) associated to BrCa have been reported many years ago, but their real and specific role in the biology of this disease remained elusive. In the current study, we have investigated the predictive role of MCs from the primary tumor on lymph node metastasis on patients stratified based on the molecular classification. We investigated 156 patients with BrCa, stratified as luminal A, luminal B, human epidermal growth factor receptor 2 (HER2) type, basal-like, and unclassified. MCs were identified with anti-MC tryptase antibody in a double immunohistochemical reaction combined with anti-cluster of differentiation 34 (CD34) antibody. Mast cell density (MCD) was calculated based on the hot-spot method, on three fields with maximum density of MCs in each case. The final result was the arithmetic media that was compared with the molecular profile and lymph node metastases. We found no significant correlation between MCD and the molecular profile of the primary tumor, but we noticed a strong correlation between intratumor MCD and lymph node metastases, regardless of the molecular type.
Keywords: breast cancer, immunohistochemistry, lymph node metastasis, mast cell density, molecular classification, prognosis
⧉ Introduction
Breast cancer (BrCa) is the most frequent neoplastic disease in females, and despite significant progress acquired in the field of early diagnosis and therapy, both morbidity and mortality remain high. Although expected, early detection of BrCa is most probably the best method to reduce mortality, as shown by a recent populational study [1]. For many decades, the pathological diagnosis was thought to be the “golden standard” and it had a significant impact in performing a therapeutic strategy. A little bit more than 20 years ago, it was introduced a new classification of BrCa, based on gene analysis and molecular profile [2]. The new molecular classification that includes minimum five different types of BrCa, significantly contributed to our understanding of tumor biology, refined the prognosis, and modified the therapeutic strategy [3].
For more than a century, the main studies on BrCa focused preferentially on malignant cells, and just rarely on the tumor microenvironment. This is somehow surprising, because it was very well known already that the behavior of malignant cells is different, depending on the composition of the medium in which they proliferate [4]. Therefore, the molecular profile governs only in part the natural evolution of tumor cells and their spreading in the lymph nodes and distant organs. Lymph nodes and distant metastases are very important elements of a bad prognosis and many authors tried to investigate the predictive role of some cells, tissues, or biochemical markers. Over the years, there were noticed many changes in the diagnosis, prognosis, and therapy, including primary tumor resection [5]. Despite there were accumulated a lot of solid and convincing data about the individual behavior of BrCa, there are still controversies regarding the relationship between malignant cells and the microenvironment that should be carefully investigated to clarify some controversial issues.
The tumor microenvironment is a complex structure that includes connective fibers, fix and mobile connective cells, nerve fibers, and blood and lymphatic vessels. Most of them are easily identified in routine histological sections, but besides these elements, there is the ground substance with different characteristics from one case to another and it is a basic component of the tumor microenvironment. In the last years, there were accumulated a lot of data that support the determinant role of the micromedium on the evolution of the tumor, particularly in local progression and in lymph node and distant metastases development [6]. It was noticed that some cells and fibers of the microenvironment react by hyperplasia, and in most of the cases, the substrate of this process is unclear or unknown. Elastic fibers, macrophages, fibrocytes, tumor infiltrating lymphocytes, and mast cells (MCs) belong to this group of elements. Although in the last two decades there were accumulated some data regarding these cells and fibers, their prognostic value remains uncertain, and consecutively, they did not become therapeutic targets. Moreover, their value to predict lymph node metastases is uncertain or even controversial.
MCs were reported in the tumor microenvironment more than 100 years ago by Ehrlich & Westphal, but their significance in malignant disease in terms of prognosis is still a matter of controversy [7,8]. The relationships between BrCa and MCs have been investigated by many authors, but the results were significantly different in terms of their number and functional aspects. Some authors reported MCs as elements of good prognosis, and others of bad prognosis. Currently, the MCs reaction associated to BrCa is difficult to explain, despite it is well known a large spectrum of substances stored in the specific granules, which can stimulate or inhibit tumor cell proliferation.
MCs are usual components of the connective tissue and they have been constantly reported in the microenvironment of a broad spectrum of malignant tumors. MCs significantly influence the local composition of ground substance, and by growth factors may stimulate the proliferation of tumor cells. The role of MCs in prognosis of BrCa is still a matter of controversy. In a relatively recent study, in which there were analyzed many publications on this topic, and it was shown that the controversy continues [9]. Only three studies signaled a relationship between MCs density and molecular type of BrCa [10,11,12]. Experimental studies signaled the involvement of MCs in tumor cells proliferation and spread. So, in an animal model of BrCa, it was found that MCs stimulate tumor growth and limit the basal cytokeratin (CK) 5 compartment [13]. Accumulation of MCs in the presence of annexin 1 was associated with inflammatory response and angiogenesis in triple negative human BrCa [14]. In some human malignant tumors, it was found an accumulation of MCs at the interface with the stroma. It was suggested that MCs form a barrier that restricts invasion based on their content of glycosaminoglycans. On the other hand, MCs as a bad prognosis element seem to be related by their presence in the tumor area, close to tumor cells. This is supported by the deficient response to chemotherapy, particularly in inflammatory carcinoma [15].
Aim
Although many studies investigated the presence and location of MCs in patients with BrCa, mast cell density (MCD) was not explored yet in terms of the relationship with the molecular types and lymph node metastases. In the current study, we investigated the relationship between MCs density, the molecular profile of BrCa, and their predictive role in lymph node metastases. We have shown that MCs density does not correlate with the molecular profile but is a strong predictor of lymph node metastases.
⧉ Patients, Materials and Methods
In the present study, there were included 156 patients with BrCa. We used the following criteria for inclusion in the study: details on the staging, classification based on the tumor, node, and metastasis (TNM) system, and the status of the lymph nodes. The clinical and pathological characteristics of patients are shown in Table 1. There were selected cases diagnosed as ductal invasive carcinoma not otherwise specified, lobular carcinoma, particular forms (like mucinous, medullary, and papillary), with or without local recurrence, with and without axillary lymph node metastases.
Table 1.
The clinical and pathological characteristics of patients (n=156)
|
Variable |
Values |
Percent [%] |
|
Age at diagnosis [years] |
|
|
|
▪ Mean |
58.9 |
|
|
▪ Range |
34–82 |
|
|
Familial BrCa history |
|
|
|
▪ Yes |
5 |
3.20 |
|
▪ No |
151 |
96.79 |
|
Oral contraceptives |
|
|
|
▪ Never |
62 |
39.74 |
|
▪ Constant |
94 |
60.25 |
|
Menopausal status |
|
|
|
▪ Premenopause |
31 |
19.87 |
|
▪ Postmenopause |
125 |
80.12 |
|
Size of the tumor [cm] |
|
|
|
▪ Mean |
3.8 |
|
|
▪ Variation |
13.5 |
|
|
Lymphovascular invasion |
|
|
|
▪ Present |
65 |
41.66 |
|
▪ Absent |
91 |
58.33 |
|
Lymph node status |
|
|
|
▪ Negative |
76 |
48.71 |
|
▪ 1–3 lymph nodes |
44 |
28.20 |
|
▪ Over three lymph nodes |
30 |
19.23 |
|
Grade |
|
|
|
▪ G1 |
17 |
10.89 |
|
▪ G2 |
81 |
51.92 |
|
▪ G3 |
58 |
37.17 |
|
Histopathology |
|
|
|
▪ Ductal invasive, NOS |
130 |
83.33 |
|
▪ Lobular invasive |
6 |
3.84 |
|
▪ Medullary |
7 |
4.48 |
|
▪ Mucinous |
2 |
1.28 |
|
▪ Metaplastic |
9 |
5.76 |
|
▪ Papillary |
2 |
1.28 |
|
Nottingham Prognostic Index (NPI) |
|
|
|
▪ Less than 3.4 |
15 |
9.61 |
|
▪ 3.4–5.4 |
68 |
43.58 |
|
▪ Over 5.4 |
32 |
20.51 |
|
▪ Not done |
41 |
26.28 |
|
Local recurrence |
|
|
|
▪ Yes |
12 |
7.69 |
|
▪ No |
144 |
92.30 |
BrCa: Breast cancer; NOS: Not otherwise specified
Primary processing
Specimens were fixed in 10% neutral buffered formalin for 24–48 hours, pH 7.2–7.4, and embedded in Paraplast High Melt (Leica Biosystems). Step sections, 3–5 μm thick (Shandon, HM355S automatic microtome, Thermo Scientific, USA), stuck on slides were stained with conventional Hematoxylin–Eosin (HE) for the histopathological (HP) diagnosis and grade. After staining, slides were mounted with Leica CV Mount (Leica Biosystem Newcastle Ltd., Newcastle upon Tyne, UK). The grading was done in accord with World Health Organization (WHO) recommendations [16]. Nottingham Prognostic Index (NPI) was calculated based on the accepted formula, considering the size of the tumor, lymph node status, and grade [17].
Immunohistochemistry
All procedures (dewaxing, antigen retrieval, visualization) were performed using Leica Bond-Max (Leica Microsystems GmbH, Wetzlar, Germany). Briefly, slides were dewaxed in two baths of Bond Dewax Solution five minutes each, followed by rehydration with decreasing alcohols for two minutes each, and distilled water. Endogenous peroxidase was blocked with Dako REAL™ Peroxidase-Blocking Solution for five minutes. Nuclei were stained with modified Mayer’s Hematoxylin (HMM500, ScyTek Laboratories, Inc.). Slides were then dehydrated, clarified, and mounted with Leica CV Mount (Leica Biosystems). Details on the primary antibodies, dilution, antigen retrieval, and working system are shown in Table 2.
Table 2.
Antibodies, working system, and expression of the final product
|
Antibody |
Clone |
Dilution |
Antigen retrieval |
Incubation |
Working system/chromogen |
Expression |
|
ER |
1D5 |
RTU |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
LSAB+/HRP, DAB |
Nuclear |
|
PR |
Pgr636 |
RTU |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
LSAB+/HRP, DAB |
Nuclear |
|
Ki67 |
MIB1 |
RTU |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
LSAB+/HRP, DAB |
Nuclear |
|
HER2 |
Rabbit polyclonal |
RTU |
MW, 30 minutes, HercepTest antigen retrieval solution |
30 minutes, RT |
HercepTest™ visualization reagent, DAB |
Membrane pattern |
|
p53 |
DO7 |
RTU |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
LSAB+/HRP, DAB |
Nuclear |
|
Bcl-2 |
124 |
RTU |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
LSAB+/HRP, DAB |
Nuclear, cytoplasmic |
|
E-cadherin |
NCH 38 |
1:100 |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
LSAB+/HRP, DAB |
Membrane/cytoplasmic or both |
|
CK5/6 |
D5/16 B4 |
1:80 |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
LSAB+/HRP, DAB |
Cytoplasmic |
|
CK8 |
35β H11 |
RTU |
Proteinase K, 5 minutes, RT |
30 minutes, RT |
LSAB+/HRP, DAB |
Cytoplasmic |
|
CK18 |
DC10 |
1:25 |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
LSAB+/HRP, DAB |
Cytoplasmic |
|
EGFR |
Polyclonal |
RTU |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
EGFR pharmDx™ visualization reagent, DAB |
Membrane, cytoplasmic |
|
Mast cell tryptase |
AA1 |
1:300 |
MW, 30 minutes citrate buffer, pH 6 |
30 minutes, RT |
LSAB+/HRP, Fast Red |
Cytoplasmic, granular |
Bcl-2: B-cell leukemia/lymphoma-2; CK: Cytokeratin; DAB: 3,3’-Diaminobenzidine (dihydrochloride); EGFR: Epidermal growth factor receptor; ER: Estrogen receptor; HER2: Human epidermal growth factor receptor 2; HRP: Horseradish peroxidase; LSAB: Labeled Streptavidin–Biotin; MW: Microwave; PR: Progesterone receptor; RT: Room temperature; RTU: Ready-to-use.
Interpretation
Slides stained for nuclear markers, like estrogen receptor (ER), progesterone receptor (PR), and Ki67 were evaluated using the semi-automated method proposed by Suciu et al. [18], using the soft NIS-elements D2.30 (Nikon Instruments Europe BV) and the microscope Nikon Eclipse 80i, adjusted with video camera Nikon DS-Fi1 (Nikon Instruments Europe BV). Hormone receptor expression was scored by applying the Allred score [19], which combines the percent of positive nuclei with the intensity of the final product of the reaction. Human epidermal growth factor receptor 2 (HER2) status has been interpreted based on the American Society of Clinical Oncologists (ASCO) recommendations, and only +2 and +3 cases were considered positive. E-cadherin-positive reaction was scored according to the system largely accepted in the literature [20]. Only cases scored as +2 and +3 were considered to be positive. Bcl-2 was scored according to the system proposed by Callagy et al. [21], and p53 was evaluated based on the recommendations of Yamashita et al. [22]. CK5 was performed to characterize basal-like carcinoma, and CK8 and CK18 were done to identify micrometastases in the lymph nodes. Epidermal growth factor receptor (EGFR) was evaluated based on the recommendation of Dako guide (EGFR pharmDX™, Dako, Denmark). The reaction was considered positive if more than 5% of tumor cells were positive.
Mast cell density
MCD was evaluated using a system similar to that proposed by Weidner to count microvessels. There were chosen three microscopic fields with a maximum density of MCs in the tumor area, at a magnification of ×400. There were counted MCs stained for MC tryptase and the arithmetic media was the final result for each case. Overestimation of MCD values is less probable, based on the high specificity of the antibody used in the current study. Values of MCD were correlated then with the HP form, grade, molecular types of carcinoma and lymph node metastases.
Statistical analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) 22.0 software (SPSS Inc., Chicago, IL, USA). Student’s t-test was applied and a value of p<0.05 was considered significant.
⧉ Results
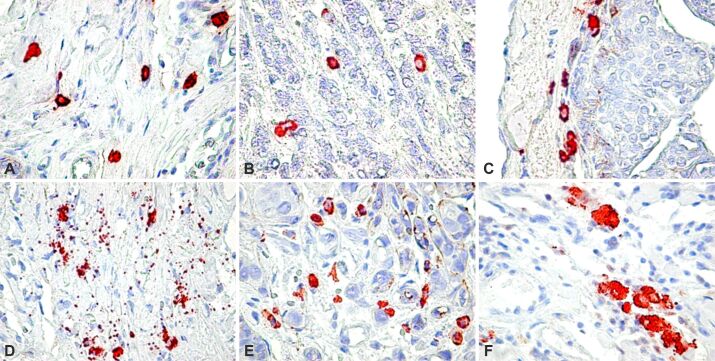
MCs were found in all the cases included in the present study, but their density was significantly different from one case to another. MCs were noticed in both intratumor and peritumor areas but associated with different components of the tumor. In the peritumor area, MCs were frequently observed in the perivascular space with limited variation in number from one field to another (Figure 1A). MCs in the tumor area were often found in close contact with tumor cells and just rarely located in the perivascular space. In some cases, few MCs were found in the tumor area (Figure 1B), but in most of the cases, their number was higher than in the peritumor area. On occasion, we found numerous MCs agglomerated as a continuous line at the interface between neoplastic cells and the connective tissue of the microenvironment (Figure 1C). The release of specific granules of MCs in the pericellular space, known as degranulation, was noticed in many cases (Figure 1D), particularly in the peritumoral space and never in the intratumor area. No significant correlation was found between degranulation and lymph node metastases (p<0.22) or the molecular profile of the primary tumor (p<0.71). The highest value for MCD in the intratumor area was found in cases in which tumor cells were arranged in small groups, usually discohesive (Figure 1, E and F). We found no relationship between the number of intratumor and peritumor MCs and the conventional HP type of carcinoma. Although MCs were most numerous in ductal invasive carcinoma, and just rare in mucinous carcinoma, they were not good indicators of the pathological form. We found a slight correlation with the grade, with values of MCD increasing as G increased (p<0.047).
Figure 1.
(A) MCD in the peritumor microenvironment; (B) Low MCD in the tumor area; (C) Borderline MC barrier; (D) Release of MC granules in the peritumor area; (E) High MCD in the tumor area; (F) Details, MCs agglomeration. MC tryptase immunoreaction: (A–E) ×400; (F) ×900. MC: Mast cell; MCD: Mast cell density
We then analyzed the relationship between MCD and the molecular types of BrCa. Luminal A was found in 73 of the cases, based on the strong expression of ER and PR, Bcl-2, low Ki67 expression, and negative reaction for HER2. Luminal B cases (n=26) showed expression for hormone receptors, usually lower than in luminal A, with a high Ki67 index and negative reaction for HER2 protein. Basal-like carcinoma (16 cases) was characterized by the expression of CK5, EGFR and p53, and negative reaction for hormone receptors and HER2. Finally, unclassified cases (n=8) were negative for all markers included in this study. We found the highest values for MCD in luminal A and HER2 types of BrCa, but without a correlation with the global prognostic impact of the molecular type (Table 3). The lowest value for MCD was found in the basal-like carcinoma, well-known for the reduced rate of metastases in the axillary lymph nodes.
Table 3.
Correlation between MCD and molecular types of breast carcinoma
|
Molecular type (n=156) |
MCD/mm2 |
p-value |
|
|
Intratumor MCD |
Peritumor MCD |
||
|
Luminal A (n=73) |
120.09 |
83.16 |
0.025 |
|
Luminal B (n=28) |
111.84 |
73.17 |
0.797 |
|
HER2 (n=31) |
129.69 |
71.94 |
0.904 |
|
Basal-like (n=16) |
57.84 |
105.33 |
0.029 |
|
Unclassified (n=8) |
74.13 |
74.55 |
0.987 |
HER2: Human epidermal growth factor receptor 2; MCD: Mast cell density
An aspect with significant clinical importance resulted from the analysis of the relationship between MCD and axillary lymph node metastases. We found a strongly statistically significant relation between the high number of MCs in the tumor area only and lymph node metastases (Table 4). No correlation was noticed between the peritumor MCD and lymph node metastases.
Table 4.
Correlation between MCD and axillary lymph node status
|
Lymph node status |
MCD in the primary/HPF |
p-value |
|
Positive lymph nodes |
56.47 |
<0.0001 |
|
Negative lymph nodes |
16.22 |
<0.0001 |
HPF: High-power field (×400); MCD: Mast cell density
⧉ Discussions
In the current work, we have shown that MCs are important players in the behavior of BrCa. Their heterogeneous distribution and morphology most probably reflect the ability of these cells to secrete a broad spectrum of biologically active substances. Therefore, MCs should be considered an essential component of the tumor microenvironment. The tumor microenvironment is a complex structure that includes fixed and wandering connective tissue cells, fibers, blood, and lymphatic vessels. In the last years, there were accumulated many data that support the determinant role of the microenvironment in the tumor evolution, contributing to both local progression, lymph node, and distant metastases [23]. We have investigated the MCs because of their high capacity to react to pathological conditions.
MCs are usual components of the loose connective tissue, and they were constantly reported in the tumor microenvironment. It is difficult to discriminate between “good MCs” and “bad MCs”, because the active biological substances contained in the specific granules are different. Therefore, MCs form a heterogeneous cell population that influences not only the vascular dynamic and composition of the ground substance, but also stimulates the proliferation of tumor cells by secreting growth factors.
The prognostic role of MCs in BrCa is very controversial. So, in a relatively recent study on this topic, analyzing many publications, it was concluded that is some conditions MCs are of good prognosis, and in others, of bad prognosis [9, 24]. Few studies investigated the relationship between the MCD and molecular type of BrCa. We found no relationship between the molecular type and MCD. Therefore, we believe it is important to identify a particular signature of BrCa. On the other hand, some studies signal the involvement of MCs in tumor cell proliferation and spreading. In an animal model of BrCa, it was noticed that MCs stimulate tumor growth and limit the development of the basal CK5 compartment [25].
These data are supported by our findings, which signal the predictive role of MCs number in the primary tumor to lymph node metastases. In their turn, these data are supported on triple-negative tumors, in which MCs infiltrates in the presence of annexin 1 is associated with inflammation and angiogenesis [26]. In some human malignant tumors, it was reported the agglomeration of MCs at the front of tumor proliferation. It was thought that MCs have the tendency to form a barrier against tumor invasion, particularly using secreted glycosaminoglycans. Our results do not confirm this hypothesis, although we found some aspects of this kind. The location of MCs in the immediate vicinity of tumor cells is most likely a local adaptation to the release of some growth factors, like vascular endothelial growth factor (VEGF). It is well-known that in an advanced stage, BrCa tumor cells do not express VEGF, and we believe that this growth factor is secreted by cells of the microenvironment, like MCs.
A bad prognosis is suggested by the accumulation of MCs in the intratumor area is given by the lack of response to chemotherapy, particularly in inflammatory BrCa [15, 27]. The unfavorable prognosis of tumors with MCs infiltrates has been demonstrated in a large variety of human cancers [28,29]. Based on these data, we believe that MCD is a useful method to predict lymph node metastases and bad prognosis in patients with BrCa. In these conditions, MCs become interesting candidates for target and personalized therapy.
MCs and conventional clinicopathological data
We did not find a correlation between MCD and the HP form, and just a weak correlation with the grade of the tumor. Our data confirm previous findings related to this topic that show the increased number of MCs in high-grade BrCa [30]. This could be indirect support for the hypothesis that a high number of MCs in the intratumor area is of bad prognosis.
MCs and molecular classification
Very few data are available about the relationship between MCs and the molecular type of BrCa. In the current study, we found no correlation between these parameters, and we cannot confirm previous data published on this topic [12, 31,32,33]. This could be due to the limited number of patients included in these studies and the use of a restricted panel of antibodies to characterize the molecular types of BrCa.
MCs and tumor cells
The role of MCs in the tumor microenvironment is still elusive. The association between MCs and tumor cells has been reported by many authors [34,35,36]. The close apposition of MCs to tumor cells could be the morphological expression of a very important step in the evolution of neoplasia: the release of growth factors that MCs is able to secrete. This could be an explanation for the location of MCs in the tumor area in the proximity of tumor cells but not around blood vessels. This is supported by the lack of degranulation in the tumor area because growth factors, like VEGF, are released usually by diffusion. MCs are known for their pivotal role in tumor-associated angiogenesis, as they secrete several angiogenic factors, we hypothesize that MCs secrete an important amount of VEGF and contribute to the formation of new blood vessels. This is also supported by the fact that BrCa tumor cells do not secrete VEGF in an advanced stage, as it was the case in the current study. Taken together, these data suggest that intratumor MCs are of bad prognosis, as also has been shown by other publications [8, 37]. In this condition, MCs inhibitors could be of interest in the clinical practice of patients with BrCa [38].
To the best of our knowledge, it is the first demonstration of the predictive role of MCs from the intratumor area on axillary lymph node metastases. MCs could be the source of growth factors that can induce lymphangiogenesis in the tumor area and thus facilitating the spread of malignant cells on the lymphatic route. In such a condition, MCs became an attractive therapeutic target, and inhibitors of MCs degranulation could be considered as adjuvant therapy in BrCa.
⧉ Conclusions
In the present study, we have investigated the distribution location and number of MCs in 156 specimens of BrCa. MCD from the tumor and the peritumoral area does not correlate with the conventional HP diagnosis, the molecular profile of BrCa included in the current study and shows just a slight correlation with the grade of the primary tumor. On the other hand, the MCD in the tumor, but not in the peritumoral area, statistically and significantly correlated with lymph node metastases, regardless of the molecular type of the tumor. Based on our data, the MCD from the tumor area is a good predictor for lymph node metastases.
Conflict of interests
The authors declare that they have no conflict of interests.
Authors’ contribution
EF, design of the study, analysis, and specimen collection; ICNT, specimen collection; ARC, RMC, performed immunohistochemistry and quality assurance; RAP, writing the manuscript; NPG, MR, supervisor, and writing the manuscript.
Acknowledgments
The authors are grateful to Emanuel-Ciprian Onica for his excellent technical support. Thanks to the Angiogenesis Research Center Timişoara, which provided the financial support for this work. The authors thank all colleagues from Timişoara, Arad, and Cluj-Napoca that contributed with cases to this study.
References
- 1.Duggan C, Trapani D, Ilbawi AM, Fidarova E, Laversanne M, Curigliano G, Bray F, Anderson BO. National health system characteristics, breast cancer stage at diagnosis, and breast cancer mortality: a population-based analysis. Lancet Oncol. 2021;22(11):1632–1642. doi: 10.1016/S1470-2045(21)00462-9. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh AA, Ross DT, Perou CM, van de Rijn M. Towards a novel classification of human malignancies based on gene expression patterns. J Pathol. 2001;195(1):41–52. doi: 10.1002/path.889. [DOI] [PubMed] [Google Scholar]
- 3.Steindl A, Brunner TJ, Heimbach K, Schweighart K, Moser GM, Niziolek HM, Moor E, Kreminger J, Starzer AM, Dieckmann K, Gatterbauer B, Widhalm G, Preusser M, Berghoff AS. Changing characteristics, treatment approaches and survival of patients with brain metastasis: data from six thousand and thirty-one individuals over an observation period of 30 years. Eur J Cancer. 2022;162:170–181. doi: 10.1016/j.ejca.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Derakhshan F, Reis-Filho JS. Pathogenesis of triple-negative breast cancer. Annu Rev Pathol. 2022;17:181–204. doi: 10.1146/annurev-pathol-042420-093238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Y, Hu X, Deng X. Distant lymph node metastases from breast cancer - is it time to review TNM Cancer Staging. JAMA Netw Open. 2021;4(3):e212026–e212026. doi: 10.1001/jamanetworkopen.2021.2026. [DOI] [PubMed] [Google Scholar]
- 6.Natale G, Stouthandel MEJ, Van Hoof T, Bocci G. The lymphatic system in breast cancer: anatomical and molecular approaches. Medicina (Kaunas) 2021;57(11):1272–1272. doi: 10.3390/medicina57111272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simu G, Csaba G. Mast cells in tumour-bearing patients. Acta Morphol Acad Sci Hung. 1972;20(3):327–338. [PubMed] [Google Scholar]
- 8.Xiang M, Gu Y, Zhao F, Lu H, Chen S, Yin L. Mast cell tryptase promotes breast cancer migration and invasion. Oncol Rep. 2010;23(3):615–619. doi: 10.3892/or_00000676. [DOI] [PubMed] [Google Scholar]
- 9.Aponte-López A, Fuentes-Pananá EM, Cortes-Muñoz D, Muñoz-Cruz S. Mast cell, the neglected member of the tumor microenvironment: role in breast cancer. J Immunol Res. 2018;2018:2584243–2584243. doi: 10.1155/2018/2584243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raica M, Cîmpean AM, Ceauşu RA, Fulga V, Nica C, Rudico L, Saptefrati L. Hormone receptors and HER2 expression in primary breast carcinoma and corresponding lymph node metastasis: do we need both. Anticancer Res. 2014;34(3):1435–1440. [PubMed] [Google Scholar]
- 11.Raica M, Cimpean AM, Ceauşu R, Ribatti D, Gaje P. Interplay between mast cells and lymphatic vessels in different molecular types of breast cancer. Anticancer Res. 2013;33(3):957–963. [PubMed] [Google Scholar]
- 12.Carpenco E, Ceauşu RA, Cîmpean AM, Gaje PN, Şaptefraţi L, Fulga V, David V, Raica M. Mast cells as an indicator and prognostic marker in molecular subtypes of breast cancer. In Vivo. 2019;33(3):743–748. doi: 10.21873/invivo.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majorini MT, Cancila V, Rigoni A, Botti L, Dugo M, Triulzi T, De Cecco L, Fontanella E, Jachetti E, Tagliabue E, Chiodoni C, Tripodo C, Colombo MP, Lecis D. Infiltrating mast cell-mediated stimulation of estrogen receptor activity in breast cancer cells promotes the luminal phenotype. Cancer Res. 2020;80(11):2311–2324. doi: 10.1158/0008-5472.CAN-19-3596. [DOI] [PubMed] [Google Scholar]
- 14.Okano M, Oshi M, Butash AL, Katsuta E, Tachibana K, Saito K, Okayama H, Peng X, Yan L, Kono K, Ohtake T, Takabe K. Triple-negative breast cancer with high levels of annexin A1 expression is associated with mast cell infiltration, inflammation, and angiogenesis. Int J Mol Sci. 2019;20(17):4197–4197. doi: 10.3390/ijms20174197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy SM, Reuben A, Barua S, Jiang H, Zhang S, Wang L, Gopalakrishnan V, Hudgens CW, Tetzlaff MT, Reuben JM, Tsujikawa T, Coussens LM, Wani K, He Y, Villareal L, Wood A, Rao A, Woodward WA, Ueno NT, Krishnamurthy S, Wargo JA, Mittendorf EA. Poor response to neoadjuvant chemotherapy correlates with mast cell infiltration in inflammatory breast cancer. Cancer Immunol Res. 2019;7(6):1025–1035. doi: 10.1158/2326-6066.CIR-18-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dooijeweert C, van Diest PJ, Ellis IO. Grading of invasive breast carcinoma: the way forward. Virchows Arch. 2022;480(1):33–43. doi: 10.1007/s00428-021-03141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AHS, Ellis IO. The Nottingham prognostic index for invasive carcinoma of the breast. Path Oncol Res. 2008;14(2):113–115. doi: 10.1007/s12253-008-9067-3. [DOI] [PubMed] [Google Scholar]
- 18.Suciu C, Muresan A, Cornea R, Suciu O, Dema A, Raica M. Semi-automated evaluation of Ki-67 index in invasive ductal carcinoma of the breast. Oncol Lett. 2014;7(1):107–114. doi: 10.3892/ol.2013.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–168. [PubMed] [Google Scholar]
- 20.Qureshi HS, Linden MD, Divine G, Raju UB. E-cadherin status in breast cancer correlates with histologic type but does not correlate with established prognostic parameters. Am J Clin Pathol. 2006;125(3):377–385. [PubMed] [Google Scholar]
- 21.Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, Ellis IO, Huntsman D, Caldas C. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res. 2006;12(8):2468–2475. doi: 10.1158/1078-0432.CCR-05-2719. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita H, Nishio M, Toyama T, Sugiura H, Zhang Z, Kobayashi S, Iwase H. Coexistence of HER2 over-expression and p53 protein accumulation is a strong prognostic molecular marker in breast cancer. Breast Cancer Res. 2004;6(1):R24–R30. doi: 10.1186/bcr738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanes MR, Giacomantonio CA, Marshall JS. Mast cells and skin and breast cancers: a complicated and microenvironment-dependent role. Cells. 2021;10(5):986–986. doi: 10.3390/cells10050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aponte-López A, Enciso J, Muñoz-Cruz S, Fuentes-Pananá EM. An in vitro model of mast cell recruitment and activation by breast cancer cells supports anti-tumoral responses. Int J Mol Sci. 2020;21(15):5293–5293. doi: 10.3390/ijms21155293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majorini MT, Colombo MP, Lecis D. Few, but efficient: the role of mast cells in breast cancer and other solid tumors. Cancer Res. 2022;82(8):1439–1447. doi: 10.1158/0008-5472.CAN-21-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fereydouni M, Ahani E, Desai P, Motaghed M, Dellinger A, Metcalfe DD, Yin Y, Lee SH, Kafri T, Bhatt AP, Dellinger K, Kepley CL. Human tumor targeted cytotoxic mast cells for cancer immunotherapy. Front Oncol. 2022;12:871390–871390. doi: 10.3389/fonc.2022.871390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribatti D, Annese T, Tamma R. Controversial role of mast cells in breast cancer tumor progression and angiogenesis. Clin Breast Cancer. 2021;21(6):486–491. doi: 10.1016/j.clbc.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Molderings GJ, Zienkiewicz T, Homann J, Menzen M, Afrin LB. Risk of solid cancer in patients with mast cell activation syndrome: results from Germany and USA. F1000Res. 2017;6:1889–1889. doi: 10.12688/f1000research.12730.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu G, Wang S, Cheng P. Tumor-infiltrating tryptase+ mast cells predict unfavorable clinical outcome in solid tumors. Int J Cancer. 2018;142(4):813–821. doi: 10.1002/ijc.31099. [DOI] [PubMed] [Google Scholar]
- 30.Fakhrjou A, Naghavi-Behzad M, Montazeri V, Karkon-Shayan F, Norouzi-Panahi L, Piri R. The relationship between histologic grades of invasive carcinoma of breast ducts and mast cell infiltration. South Asian J Cancer. 2016;5(1):5–7. doi: 10.4103/2278-330X.179699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glajcar A, Szpor J, Pacek A, Tyrak KE, Chan F, Streb J, Hodorowicz-Zaniewska D, Okoń K. The relationship between breast cancer molecular subtypes and mast cell populations in tumor microenvironment. Virchows Arch. 2017;470(5):505–515. doi: 10.1007/s00428-017-2103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyla RD, Potekar RM, Patil VS, Reddy AK, Sathyashree KV. Quantitative mast cell analysis and hormone receptor study (ER, PR and HER2/neu) in invasive carcinoma of breast. Indian J Pathol Microbiol. 2020;63(2):200–204. doi: 10.4103/IJPM.IJPM_155_19. [DOI] [PubMed] [Google Scholar]
- 33.Sang J, Yi D, Tang X, Zhang Y, Huang T. The associations between mast cell infiltration, clinical features and molecular types of invasive breast cancer. Oncotarget. 2016;7(49):81661–81669. doi: 10.18632/oncotarget.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueshima C, Kataoka TR, Hirata M, Furuhata A, Suzuki E, Toi M, Tsuruyama T, Okayama Y, Haga H. The killer cell Ig-like receptor 2DL4 expression in human mast cells and its potential role in breast cancer invasion. Cancer Immunol Res. 2015;3(8):871–880. doi: 10.1158/2326-6066.CIR-14-0199. [DOI] [PubMed] [Google Scholar]
- 35.Yuan H, Hsiao YH, Zhang Y, Wang J, Yin C, Shen R, Su Y. Destructive impact of T-lymphocytes, NK and mast cells on basal cell layers: implications for tumor invasion. BMC Cancer. 2013;13:258–258. doi: 10.1186/1471-2407-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hugo HJ, Lebret S, Tomaskovic-Crook E, Ahmed N, Blick T, Newgreen DF, Thompson EW, Ackland ML. Contribution of fibroblast and mast cell (afferent) and tumor (efferent) IL-6 effects within the tumor microenvironment. Cancer Microenviron. 2012;5(1):83–93. doi: 10.1007/s12307-012-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen J, Luk K, Vang D, Soto W, Vincent L, Robiner S, Saavedra R, Li Y, Gupta P, Gupta K. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth. 2014;113(Suppl 1):i4–i13. doi: 10.1093/bja/aeu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batista J, Friedrichson T, Schlechtingen G, Braxmeier T, Jennings G, Bajorath J. Computational screening for membrane-directed inhibitors of mast cell activation. Eur J Med Chem. 2010;45(6):2700–2704. doi: 10.1016/j.ejmech.2010.01.061. [DOI] [PubMed] [Google Scholar]