Abstract
Background
Severe acute respiratory syndrome Coronavirus-2 invades the cells via ACE2 receptor and damages multiple organs of the human body. Understanding the pathological manifestation is mandatory to endure the rising post-infection sequel reported in patients with or without comorbidities.
Materials and methods
Our descriptive review emphasises the direct, indirect and post-infection damages due to COVID-19. We have performed an electronic database search according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines with selective inclusion and exclusion criteria.
Results
The included studies substantiated the extensive damages in the multiple organs due to direct and indirect consequences of COVID-19. After an apparent recovery, the prolonged presentation of the symptoms manifests as post-COVID that can be related with persisting viral antigens and dysregulated immune response.
Conclusion
A few of the symptoms of respiratory, cardiovascular, and neuropsychiatric systems that persist or reappear as post-COVID manifestations. Vaccination and preventive programs will effectively reduce the prevalence but, the post-COVID, a multisystem manifestation, will be a significant tribulation to the medical profession. However, the issue can be managed by implementing public health programs, rehabilitation services, and telemedicine virtual supports to raise awareness and reduce panic.
Keywords: COVID-19, SARS-CoV-2, Viral pathogenesis, Post-infection damages, Immune dysregulation, Post-COVID, Multiple organ dysfunction
Introduction
Ever since discovering the "filterable agent" tobacco mosaic virus and the first human yellow fever virus, there were 249 (158 RNA + 91 DNA) virus species reported to infect humans [1]. They are responsible for two-thirds of newly emerging and re-emerging human pathogens and accountable for 14 million deaths worldwide [2]. About 58% of new or emerging pathogens are zoonotic that infect humans [3]. Most of these fatal zoonotic viruses belong to the single-strand ribonucleic acid (ssRNA) viruses; the list includes yellow fever virus, West Nile virus, hepatitis C, dengue, zika, rubella, mumps, chikungunya, Ebola, measles, Nipah, rabies, Lassa, influenza, severe acute respiratory syndrome (SARS), middle east respiratory syndrome (MERS), and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [4].
Coronavirus initially reported in bats evolved in few intermediate hosts before infecting humans, including wild animals such as pangolin (anteater), mink, turtle, snake, and ferret [5]. Animals share significant sequence identities with specific receptor complexes of humans that were hijacked by coronavirus's spike protein [5]. The spike protein holds critical information about the host protein and folds separately to spread faster in the ecosystem [6]. The genome of any virus undergoes several mutations to infect another species. An alarming report observed 9653 mutations at 400 mutation sites in human SARS-CoV-2 [7]. The study conveys that the viral receptor-binding domain linked with human angiotensin-converting enzyme-2 (ACE2) comprised 44 distinct mutations [7]. The report raises questions on the reinfecting ability of the virus and the protective capability of the developed vaccines and drugs designed against such mutating viral proteins.
The direct effects of SARS-CoV-2 were associated with the viral targeted cell damages, and the indirect effects were related to collateral viral damages compromising physiological functions [8, 9]. Death and multiple organ dysfunction (MOD) in Coronavirus disease-2019 (COVID-19) patients were caused by both the virus and host defence mechanism [10–12]. Autopsy reports of COVID-19 non-survivors unanimously verified the MOD. [13–19]. The information indirectly conveys that the survivors from critical situations might recover with uncertain internal organ damages manifest as post-COVID [10, 12, 20]. The host conditions such as senility, comorbidities, concurrent or previous infections, antimicrobial resistance, personal habits such as tobacco, alcohol and food choices, obesity, and medicines used for comorbidities, impact the viral pathogenesis and treatment choices during emergencies [9, 21–23]. The recovered patients with mentioned conditions burden a lifetime due to the post-COVID compromised functions of the affected organs [24, 25]. Therefore, it is prudent to recognize the post-infection sequel of the recovered COVID-19 with the evidenced viral damages, to plan and practice public health policies. This review will detail the direct and indirect impacts of the SARS-CoV-2 infection on various organs and post-COVID manifestations to understand the pathological mechanisms that required to manage the post-infection sequel.
Materials and methods
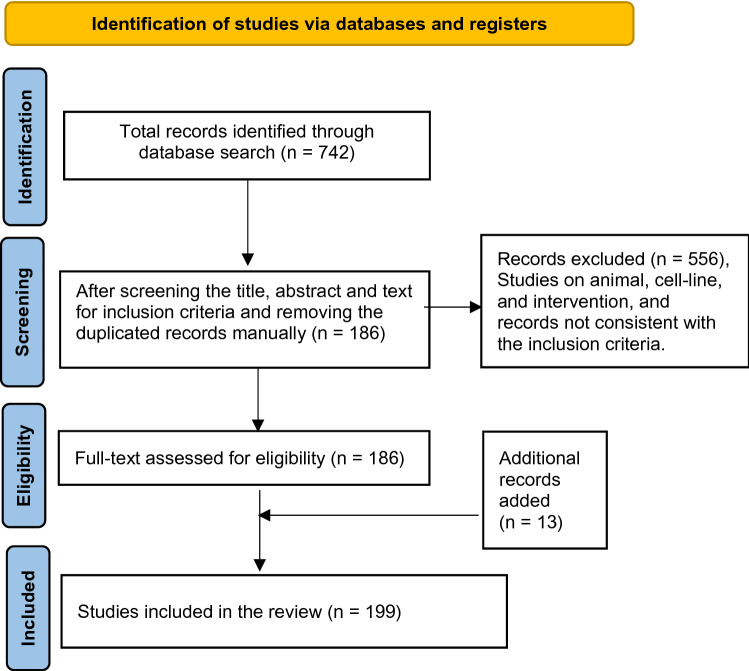
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, records were searched in the electronic database. The following keywords, 'direct damages COVID-19' 'indirect damages COVID-19' 'collateral damages COVID-19' 'post-infection damages COVID-19' 'post-COVID' and 'long-COVID were used to search in the title and abstract fields. The inclusion criteria applied as filters in the search engine PubMed include free full text, Medline, journal articles, clinical studies, meta-analysis, systematic reviews, studies conducted in humans Species and published in English (language), and the publication date from 1st January 2020 to 30th May 2021. The final search reports collected were as of the date 17th July 2021. The results of the total number of records with the applied filters were 742, which included, Direct damages AND COVID-19 (n = 182), Indirect damages AND COVID-19 (n = 34), post-infection damages AND COVID-19 (n = 101), Collateral damages AND COVID-19 (n = 65), post-COVID (n = 347), and long-COVID (n = 13). Animals and cell-line studies, interventional studies, and studies not consistent with the inclusion criteria were excluded. After screening the titles, abstracts, and full text for inclusion criteria, and duplicate records manually about 556 records were removed. The eligible records were about 186. Direct damages AND COVID-19 (n = 86), indirect damages AND COVID-19 (n = 1), post-infection damages AND COVID-19 (n = 37), collateral damages AND COVID-19 (n = 9), post-COVID (n = 46), long-COVID (n = 7). 13 records were manually searched and added in the introduction and discussion to support the manuscript view. The final number of studies recorded in the review was 199 (Fig. 1).
Fig. 1.
PRISMA flow diagram
Results
Direct damages of the SARS-CoV-2
Direct effects were correlated with the SARS-CoV-2 induced changes in the target cells following the viral attachment and replication [26]. As an obligate intracellular parasite with no in-built metabolism, the viruses instruct the host DNA to reprogram host metabolism to work for viral replication utilising the nutrition and energy and release the viral progeny by killing the host cells [24]. The direct cytopathic effects of the virus depend on the viral load and virulence, ability to enter target cells, and severe cell machinery to manufacture, and release the viral progeny [24–27]. Coronavirus tropism of multiple organs in the body by exploiting the ACE2 protein was emphasised in some literature [28–30].
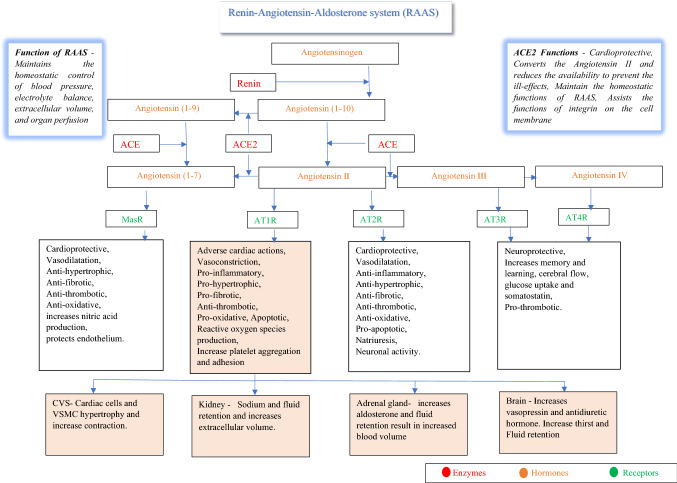
ACE2, an integral protein in the cell membranes, catalyses a group of angiotensin into active substances for specific functions in tissues of the lung, liver, kidney, heart, pancreas, muscles, adipose tissue, reproductive organs, brain, and blood vessels [28, 30, 31]. A brief explanation of the physiological role of ACE2 is explained in Fig. 2. Spike protein of the SARS-CoV-2 binds and blocks the ACE2 protein [28]. The block in the enzymatic actions of ACE2 reduces angiotensin I, the final product and accumulation of the product to be converted, the angiotensin II, causing an altered biochemical milieu that weakens the functions of tissues [28]. The actions of angiotensin II by binding with AT1R are primarily pathological that manifests as MOD if excessively accumulated (Fig. 2) [28].
Fig. 2.
The flow chart details the role of ACE2 in RAAS system. RAAS controls blood pressure and homeostasis. Angiotensinogen secreted by the liver is converted into angiotensin I (1–10) by enzyme renin, secreted by the kidney. The angiotensin I (1–10) is converted into angiotensin II by the enzyme ACE secreted predominately by lungs. The crucial function of the ACE2 is to catalyse the conversion of angiotensin II into angiotensin I (1–7). The converted Angiotensin I (1–7) act by binding with the MasR to maintain the physiological balance by functioning with the RAAS. Angiotensin II bind with the four different types of receptors named angiotensin receptors 1 to 4 (AT1R, AT2R, AT3R and AT4R) expressed by the tissues of RS, CVS, GIT, genitourinary system, nervous system, and endocrine system. The actions of angiotensin II by binding with AT1R are primarily pathological to CVS and alter the RAAS axis. Abbreviations, RAAS-renin-angiotensin-aldosterone system, ACE-angiotensin-converting enzyme, ACE2-angiotensin-converting enzyme2, MasR-mitochondria assembly receptor, and AT1R, AT2R, AT3R and AT4R- angiotensin receptors 1 to 4
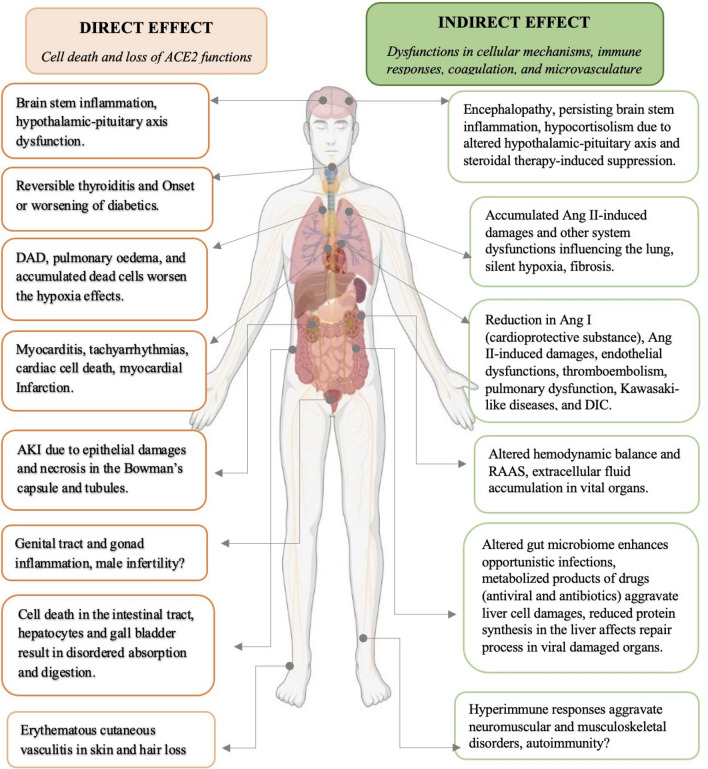
Etiopathogenesis of the direct damages was chiefly due to massive viral-induced cell death and loss of ACE2 actions in the entire body [8, 32, 33]. The loss of primary cells of the lung impedes the exchange of gases that reduced oxygen supply to the whole body [32]. The direct damages at the major organ level included pneumonia, diffuse alveolar damage (DAD), pulmonary oedema, accumulation of desquamated lung cells, reduced oxygenation manifested as hypoxia in the lung; myocarditis, tachyarrhythmias, and loss of cardioprotective functions of angiotensin compromised the functions of the heart [10, 34–37]. The kidneys' filtering ability is altered due to the epithelial cells damages in the Bowman's capsules and tubular necrosis that resulting in acute kidney injury [38–40]. Altered appetite due to loss of taste and smell sensation, colitis, microhaemorrhage, desquamation and necrosis in the lining cells of GIT coerce digestion and absorption, liver and gallbladder cells necrosis resulted in the reduction in synthesis, storage, and delivery of the proteins for defence, repair, and regeneration [22, 41–43]. The inflammation of the brain stem, damages on olfactory, gustatory, and peripheral nerves and their supporting structures, loss of control at the hypothalamus and pituitary axis that regulate the entire endocrine gland have altered the physiological homeostasis [38, 40, 42, 44–46]. Nonspecific skin rashes due to cutaneous vasculitis aggravated pre-existing dermatosis, and hair loss were reported [25, 47]. The direct and indirect SARS-CoV-2 induced damages in the human system are detailed in a flow diagram (Fig. 3).
Fig. 3.
The direct and indirect damages of COVID-19 in the human system
Direct viral involvement demonstrated in the male genital tract and gonads raises the possibility of male infertility [48]. Androgens regulate the receptor expression, such as the transmembrane serine protease 2 (TMPRSS2), a critical receptor arbitrating the entry of SARS-CoV-2 [48]. A higher number of male COVID-19 patients than women and prepubertal children were interrelated with the receptor expression influenced by the androgens [48, 49]. The demonstration of ACE2 and tissue damages in autopsy reports of testis explains the possibility of COVID-19 injuring the various organs [50]. Altered expression of ACE2 were evidenced with diabetics and hypertensive individuals, and gender polymorphism needs consideration during treatment [27, 51, 52].
Indirect damages
Indirect effects are predominantly caused by the defence mechanisms delivered against the virus that collaterally damages the naïve tissues. The indirect effects of SARS-CoV-2 include dysregulation in the cellular mechanisms, host-immune system, biochemistry, coagulation, and microvasculature resulting in loss of hemodynamic equilibria and autoimmunity [9, 10, 22, 53–58]. The reduction in the oxygen intake capacity due to the direct viral damages were aggravated by the indirect effects of cytokine storm, thrombosis, and brain stem dysfunction [45, 59–62]. Microvascular dysregulation hinders the oxygen delivery to the end organs despite the blood saturation being adequate [55, 63]. Studies reveal that acute lung injury, acute respiratory distress, and pulmonary embolism are the initial manifestation of the increased oxygen demand [12, 59, 64]. Surfactant secreted by the type II pneumocytes is vital for recoiling action of the lung to prevent alveolar collapse. The SARS-CoV-2 induced lung cell damages deplete the surfactant [65]. Asymptomatic silent hypoxia, a poor outcome in the COVID-19 patients, are correlated with brain stem inflammation [32, 61, 66].
Cardiopulmonary dysfunction, thromboembolism, and loss of cardioprotective effects of angiotensin II aggravates tachyarrhythmia and heart attack [36, 67, 68]. High viral load in the intestine and cellular damages induce dysbiosis in the microbiome of GIT and massive entry of microbes into the systemic circulation [25]. Drug-induced toxicity further burdens the liver affecting protein synthesis and delivery [41]. Kidneys' workload increases due to cardiopulmonary dysfunction, cytokine accumulation, perfusion dysfunction and microthrombosis [37, 39, 69–72]. A study correlated brainstem inflammation as a principal cause of indirect and post-infection manifestations of COVID-19 [45]. Viral encephalomyelitis, viral influence on the brainstem, hypothalamus, and pituitary axis affects the functions of entire endocrine organs [25, 48, 73]. Lymphopenia, typically reported in COVID-19 patients, was due to the destruction of virus-harbouring lymphocytes destroyed in the spleen, lymph nodes, and other lymphoid tissues that cause immunodeficiency and aids the virus to permeate the organs rapidly [74].
Cytokine storm was reported as one of the crucial causes for the worst clinical manifestations in SARS-CoV-2, like SARS-CoV-1 and MERS [75]. SARS-CoV2 patients exhibit an array of cytokines including TNFα, INFγ, IL-1β, IL-2, IL-4, IL-6, IL-7, IL-9, IL-10, IL-12, IL-13, IL-17, G-CSF, GM-CSF, MCSF, HGF and chemokines CXCL8, MCP1, IP10, MIP1α and MIP1β [53, 76, 77]. The accumulated angiotensin II was responsible for the hyperactivation of transcription factor NF-kappa B to release massive inflammatory cytokines to stimulate cell apoptosis and fibrosis [78]. Overreactive immune system due to systemic viremia similar to viral sepsis affecting the extrapulmonary structures were proven in COVID-19 autopsy specimens [8, 27, 79, 80].
The altered biochemistry and microvascular disorders of COVID-19 manifested as abnormal coagulopathy, endothelial dysfunctions, abnormal platelet activation, fibrotic changes, and altered microRNA functions [30, 35, 41, 59, 81]. Elevated lactate dehydrogenase (LDH) and C-reactive proteins released due to liver cell damages were used to predict the severity of the COVID-19 [27, 82, 83]. The organs are under chemical insult due to the combinations of drugs used for COVID that mandates strict pharmacovigilance because there is lack of standardization in assessing the hepatotoxicity of the drugs [43]. The accumulated drugs and metabolites due to microvascular dysfunctions exaggerate liver damages [83–85]. Steroid induced acute and chronic liver failure and death was never disregarded by the medical professionals during the use of high doses for a longer duration to save life from COVID. Individuals with co-morbidities have already a burdened liver according to the duration of the diseases and drugs intake [22, 84, 85]. Comorbidities such as the hypertension, diabetics and obesity induced pathophysiological changes do augment the COVID induced damages [22, 24]. Elderly individuals having age-related physiological compromises sustain severe pathological damages due to comorbidities that explain the increased death reports in geriatric population after COVID [57]. Therefore, while treating the COVID-19 patients having comorbidities, it is prudent to calculate the chemicals delivered as drugs for the comorbidities, co-infections, and COVID-19, which further aggravate the biochemical alterations [41, 85, 86].
The abnormal chemical storm and endothelial dysfunction stimulate thrombin formation and thromboembolic manifestations [55]. Autopsy specimens of COVID-19 demonstrated both venous and arterial thrombosis, thromboembolism, and microcirculatory damages [55, 56]. SARS-CoV-2 induced hypoxia, cytokine storm, platelet activation, complement activation, stasis, and epithelial dysfunction triggering disseminated intravascular coagulation (DIC) was reported [32, 56, 60, 64, 70, 77, 79, 87]. Viral induced immune dysregulations cross-reacted with self-antigen to induce autoimmunity were reported in COVID-19 [54]. A few autoimmune disease manifestations were correlated with COVID-19 include the Guillain-Barré syndrome, Kawasaki-like manifestations, antiphospholipid syndrome, and autoimmune haemolytic anaemia [25, 88, 89]. Reports evidenced Kawasaki-like diseases in children and adolescents in the SARS CoV-2 positive individuals [63, 90]. Kawasaki-like disease is a systemic vasculitis induced by a delayed hyperimmune response correlated with unclear pathogenesis [83, 90].
Unusual microbleeding and occlusive disorders were noticed in the brain of a few critically ill patients [91, 92]. Individual reports of oral manifestation of salivary gland ectasia, ophthalmic manifestations, histiocytic hyperplasia in the lung, pneumatosis intestinalis, intestinal ischemia, bleeding, and necrotic changes in the small bowel, non-alcoholic fatty changes in the liver, retinal microhaemorrhages, and the increased risk in smokers, were witnessed in the literature [42, 85, 93–101]. Increased tubal ectopic pregnancies and fetal distress with MOD was reported in an asymptomatic SARS-CoV-2 positive pregnant woman with infected placenta [102, 103]. The impact of lethal and unexplored co-infections during SARS-CoV2, treatment modalities, and antimicrobial resistance also observed [104].
Post-infection manifestations (Post-COVID)
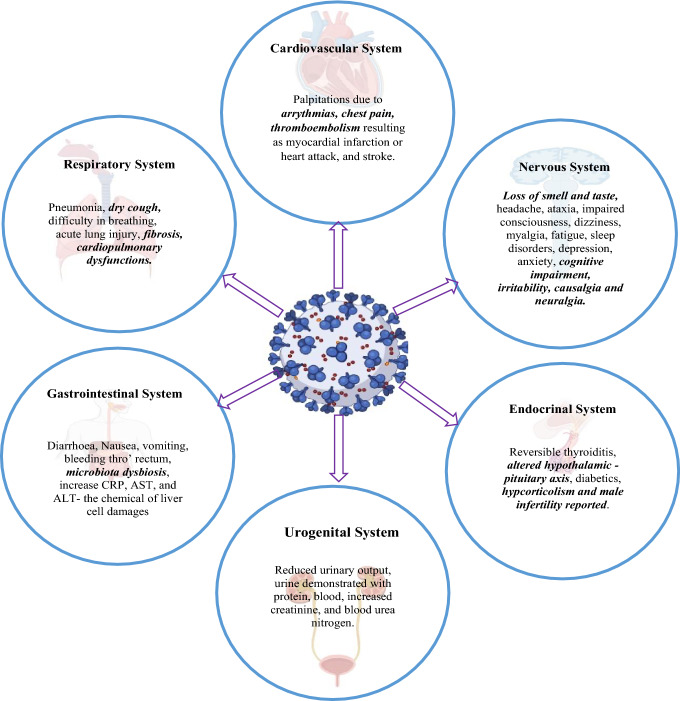
The term post-COVID correlated to manifestations of clinical symptoms after evidenced recovery from COVID-19. The term post-COVID in this review denotes the persistent or recurrence of clinical symptoms within six months following an apparent recovery with no positive isolation of virus [24, 25]. Sever post-Covid manifestation can be associated with persisting viral antigens and re-infection of a mutant variant [59, 105]. A few symptoms of the respiratory, cardiovascular, and neuropsychiatric systems commonly persist as post-COVID (Fig. 4) [106, 107]. Fibrosis of the lung and persistent brainstem inflammation were blamed for hypoxia, and the patients were reported with exertional dyspnea reducing exercise capacity, apart from persistent dry cough [24, 45, 108–110]. The pulmonary dysfunction, myocardial fibrosis and enduring coagulopathy manifest as palpitations, arrhythmias, cardiac arrest, and stroke [71, 111–119]. An unusual increase in cardiac complications, including acute myocardial injury, atrial fibrillation, mitral valve regurgitation and acute coronary events, were reported [67, 120–125].
Fig. 4.
The common signs and symptoms of COVID-19. The highlighted were reported as the persisting or reappearing symptoms during post-COVID manifestation
The altered hypothalamic–pituitary–adrenal axis weakens the control over the endocrine system influence the diabetic and adrenal manifestations [21, 24, 25]. The reports about genital systems have yet to strengthen the evidence of male infertility [48, 50]. The microbiota dysbiosis influencing various physiological functions were extensively narrated [126]. Human coronavirus was reported as neuroinvasive, and cytokine surge from the immune cells of local and peripheral circulation penetrating the blood–brain barrier can precipitate neurodegeneration [127–132]. The post-COVID effects on the brain were recounted more frequently among other systems because of prolonged hypoxia and the inability of the nervous system to repair [128, 133, 134]. Brain, the most metabolically active organ demands continuous oxygen supply for survival. Many reports observed continued loss of taste and smell sensation and hearing and supporting tissue functions in the resulted oropharyngeal dysphagia [129, 135–139]. The hypothesis of autonomic nervous system disturbance by the coronavirus manifesting as long-COVID was also debated [140–143].
Apart from Corona-phobia increasing psychological stress, neuropsychiatric symptoms include insomnia, depression, anxiety, obsessive–compulsive disorder, irritability, behavioural and mood changes, anhedonia, neuromuscular dysfunction, intrusive thoughts, maladaptive beliefs, suicidal risk, and psychosis, were reported [144–157]. In addition, chronic post-COVID muscular weakness, sarcopenia, and fatigue linked with the hindered flow of cerebrospinal fluid were reported [158–160]. Follow-up studies advised rehabilitation programs to support the recovered patients with a multidisciplinary approach, including psychological support [129, 144, 145, 161, 162].
Discussion
Studies revealed that COVID-19 death was due to viral damages, while the comorbidities were aggravating factors that enforced the importance of autopsies to understand the pathological mechanisms [9, 21, 24]. After the COVID-19 pandemic, individuals reporting complications of comorbidities or a new pandemic will significantly burden the physicians at a higher level [10]. The evidenced viral tropism for ACE2 protein explains the MOD that demands the importance of preventing the spread of the virus in the initial phase, which is possible only with vaccines and antiviral drugs [28]. The pathological mechanisms of post-infection damages were primarily due to prolonged dysregulated immune reactions, circulating virus antigens, re-infection with the same or mutant virus or similar infections and post-traumatic stress [10, 59, 163, 164]. The concept of prolonged hyperactive dysregulated immune responses was extensively investigated [24, 25]. The accumulated counteracting biological chemicals such as the pro-inflammatory, antiinflammatory, and cell-death related cytokines coerce the interior and exterior milieu of the host cells. Therefore, treatment modalities to control and suppress cytokine storm's ill effects need to be considered pivotal [165, 166]. Extracorporeal blood purification methods to remove the excess cytokines were suggested for critical cases with provisional approval granted by FDA [166]. Nevertheless, considering the controversial outcomes and treatment expenses compared with the benefits of dexamethasone treatment, the use of blood purification procedures was restricted only for randomised clinical trials [167].
Vaccine protection is questionable within the six weeks of vaccination because the immunity is not entirely developed. Prolonged viral shedding for 24 days and a 17% possibility of re-infection were reported in a study [168]. Infections or re-infection within this period will be worst in individuals having comorbidities. Emerging variants of mutated SARS-CoV-2 have demonstrated the ability to spread faster and manifest disease with less sensitivity to the conventional diagnostic tests and therapy together with the ability to evade the vaccine-induced immunity [www.cdc.gov]. The theory of antigenic sin explains the rapid spread during re-infection [169]. The SARS-CoV-2 antigens remaining inside the tissues need investigations adequately; however, many viruses reside dormant and re-emerge, such as the herpes virus, following a 'slow and low tactic' mechanism [59, 170]. Hypothetically the virus or viral antigen trapped in the dead and damaged tissues and immune complex might re-emerge during the repair and removal by the scavenger cells. The dormant residing ability of SARS-CoV-2 requires future research to accept yet, the left-over viral antigens, antibodies, and immune complexes were evidenced [59]. The primed immune cells initiate a chain of immune reactions by secreting the cytokines and antibodies until their removal; even though most of the immune cells' lifespan was limited, clearance of the cytokines requires stopping the priming action [171].
Silent hypoxia could be monitored with the pulse oximeters connected through mobile applications directly delivering messages to the hospital monitoring system via telemedicine. Currently operating selected COVID-centres need to be improved as rehabilitation centres for the post-COVID situations [172]. Health policies to monitor and support the recovered individuals with the help of various professionals through telemedicine and telerehabilitation are required [172]. Understanding the SARS-CoV-2 induced endocrinal disturbances will assist the physicians in recognising the manifestation to decide the clinical management at acute, convalescent, and post-infection stages.
A study reported an increase in Kawasaki-like diseases with multiple-organ failure in children diagnosed with SARS-CoV2 [173]. Health authorities should warn the healthcare workers and the infected public to prevent viral transmission to their children. Since systemic vasculitis might result in critical situations, careful observation of children is mandated during the pandemic [173]. The non-survivors of the SARS-CoV2 infected were confirmed with secondary infection and sepsis due to co-infections [79]. In addition, a sudden increase in cases of mycotic infections such as mucormycosis, an opportunistic fungal infection caused due to careless use of steroids, uncontrolled diabetics, microbial dysbiosis, and antimicrobial resistance was noticed [174].
The evidenced hypoxia, cytokine storm, microvascular dysfunctions, and related tissue necrosis need considerable time to restore physiological homeostasis [9]. While considering the whole body, sparing the brain, major organs have repeated functional cellular units and possess stem cell niches to regenerate. If the damaged cells were not renewed, tissue integrity was maintained by a non-functional fibrous tissue named scarring, which is one of the post-infection damages of viral infections, including COVID-19 [162, 175]. The lung has 480 million repeated alveoli units, the liver has 140 million cells per gram, the kidney has one million repeated units in each, and the pancreas has more than one million islets [176–178]. The loss of functional units in these organs may not be evident until a rise in physiological demand. The nerve and muscles have permanent cells with minimal potential to repair [179]. Avascular structures such as cartilages and tendons in joints deposited with antigen–antibody complexes pave a pathway for inflammation resulting in enduring damages like arthritis [180]. The nervous and muscular injuries aggravate muscle and joint pain like causalgia [181, 182]. Post-COVID manifestations related to neuromuscular disorders will increase unless an essential rehabilitation program is implemented [158, 163, 183, 184].
Stem cells have proved their efficiency in treating diseases that have no approved treatment [185]. Clinical trials reporting the success of cell transplantation or cell-derived products such as extracellular vesicles for treating infectious diseases, including COVID-19, were recounted [185]. The therapeutic ability of the stem cells was correlated with the antiinflammatory, immunomodulatory, antiapoptotic, antifibrotic, pro-proliferative, and pro-angiogenic actions. Our preclinical study on treating dengue with stem cells reported the possible antiviral ability of the stem cells [186]. Murine hepatitis virus-1 infected mice influencing the stem cell niches to reprogram the stemness to fight against the virus and secrete antiviral substances were observed in a preclinical study and recommended the possible role of stem cells in vaccine developments [187]. The tissues recovering after viral damages with MOD are expected to manifest widespread micro-necrotic environments raising the cellular stress, including the stem cell niches. Damages of stem cell niche due to microcirculation involvement and DIC resulting as fibrosis were reported with SARS and MERS [188, 189]. While stem cell treatment for infections prevails, preserving the naïve stem cells in our body is the best [175]. Nevertheless, viable stem cells population at the tissue level after viral infections were difficult to conceive unless special investigations were performed.
Also, further studies acknowledging the functional ability of the ACE2 receptors after vaccination may unravel more vital information. For this purpose, prospective studies could be done on the vaccinated individuals diagnosed with secondary infections or with the human on-chip design. The microvascular malfunctions due to obesity and lack of exercise influence the recovery. Social groups promoting the importance of balanced food, exercises, yoga, and meditation, to improve health in a holistic approach need to be encouraged [190]. Adopting harmless immunity-boosting herbal preparations, herbal therapy, aromatherapy, and sound therapy could be encouraged regardless of clinical studies [191].
Although the reduction in mortality and improved life expectancy was the success of medical fraternities, life after severe MOD might mitigate the quality of life, increase dependency, and health care consumption, causing a substantial rise in economic burden after the pandemic [162, 192]. Patients treated in ICU are prone to physical and mental problems [162]. Specific post-traumatic longitudinal research needs to be carried for these patients [151, 193]. Public health strategies must be advocated to allocate funds to manage post-COVID issues [194, 195]. The pandemic situation strongly impacted the mental health of the global population due to a drop in the economics involved in sectors including agriculture, education, research, and the medical industry that may be moderated by implementing policies minimising the burden designed for the country [196]. Fighting pandemics should include identifying the hidden dangerous outcomes and how best to overcome them [197, 198]. The learning health system (LHS) comprehensively integrating the collective hospital data, treatment protocol, vaccine efficiency, and morbidity on COVID-19 to discuss the disease's improvement with the lessons learned from pandemic situations is mandatory.
Scientists developed the COVID-19 vaccines within a year, unlike other vaccines. Similar efforts need to be directed to prevent infections at the zoonotic level. An antiviral drug or a vaccine arresting the folding ability of the viral protein can deliver a better solution [6]. Interventions targeting the viral proteins at the entry level might aid the immune responses to initiate antibodies against the invaders with minimum damage to native tissue. Also, predicting the threat before becoming pandemic by studying the virus at zoonosis level to design a vaccine for animals and humans and planning essential prophylaxis might offer better infection control [199]. Permitting and aiding crucial researches such as gene editing, stem cells, and virus manipulations with human and mammalian cell lines should be restricted to labs with a high level of control. Finally, each country must study the drug, vaccine, and post-COVID effects in their population.
Conclusion
The prevalence of COVID-19 can be reduced by vaccination and community preventive measures. However, the post-infection burden will downgrade future developments unless health policies are instituted. The message emphasised in this review is that the recovered individuals, even though considered normal, requires continuous monitoring to prevent re-hospitalisation and better living. Patient education to increase awareness will reduce the post-COVID symptoms. Future studies planned to collect data from clinical, laboratory, and epidemiological presentations of post-COVID will confirm the pathogenesis. Developing the multidisciplinary post-COVID centre to help recovered patients, especially in underdeveloped areas, needs to be considered. Stem cell therapy to boost the viable stem cell niches might improve the recovery of the infected and alleviate post-COVID issues.
Acknowledgements
Author Contributions as follow, SPP outlined the concept and drafted the manuscript. CB and RPRK designed the figures. SPP, CB, ASR and RPRK performed the article search. SPM, SV, MFARI, JJA, SSK, reviewed, and edited the manuscript. All authors have contributed and approved the article.
Abbreviations
- ACE
Angiotensin-converting enzyme
- ACE2
Angiotensin-converting enzyme 2
- ARB
Angiotensin receptor blockers
- AT1R, AT2R, AT3R and AT4R
Angiotensin receptors 1 to 4
- CVS
Cardiovascular system
- COVID-19
Coronavirus disease 2019
- DAD
Diffuse alveolar damage
- DIC
Disseminated intravascular coagulation
- DNA
Deoxyribonucleic acid
- FDA
Food and Drug Administration
- GIT
Gastrointestinal system
- LHS
Learning health system
- MasR
Mitochondria assembly receptor
- MERS
Middle east respiratory syndrome
- miRNA
MicroRNA
- MOD
Major organ dysfunction
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- RAAS
Renin-angiotensin-aldosterone system
- RS
Respiratory system
- SARS
Severe acute respiratory syndrome
- SARS-CoV
Severe acute respiratory syndrome Coronavirus
- SARS-CoV-2
Severe acute respiratory syndrome Coronavirus-2
- ssRNA
Single-strand ribonucleic acid
- TMPRSS2
Transmembrane serine protease 2
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Woolhouse ME, Adair K. The diversity of human RNA viruses. Future Virol 2013;8. 10.2217/fvl.12.129. [DOI] [PMC free article] [PubMed]
- 2.Woolhouse M, Gaunt E. Ecological origins of novel human pathogens. Crit Rev Microbiol 2007;33. 10.1080/10408410701647560. [DOI] [PubMed]
- 3.Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005 doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelemans T, Kikkert M. Viral innate immune evasion and the pathogenesis of emerging RNA virus infections. Viruses 2019;11. 10.3390/v11100961. [DOI] [PMC free article] [PubMed]
- 5.Zhao J, Cui W, Tian BP. The Potential Intermediate Hosts for SARS-CoV-2. Front Microbiol 2020;11. 10.3389/fmicb.2020.580137. [DOI] [PMC free article] [PubMed]
- 6.Huang Y, Yang C, Xu X feng, Xu W, Liu S wen. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin 2020;41. 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed]
- 7.Guruprasad L. Human SARS CoV-2 spike protein mutations. Proteins: Struct Funct Bioinformatics 2021;89. 10.1002/prot.26042. [DOI] [PMC free article] [PubMed]
- 8.Sarkesh A, Sorkhabi AD, Sheykhsaran E, Alinezhad F, Mohammadzadeh N, Hemmat N, et al. Extrapulmonary clinical manifestations in COVID-19 Patients. Am J Trop Med Hyg. 2020;103:1783–1796. doi: 10.4269/ajtmh.20-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, Zhang Q, Ye G, Zhang H, Heng BC, Fei Y, et al. Structural and physiological changes of the human body upon SARS-CoV-2 infection. J Zhejiang Univ Sci B. 2021;22:310–317. doi: 10.1631/jzus.B2000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021;11. 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed]
- 11.Carsetti R, Zaffina S, Piano Mortari E, Terreri S, Corrente F, Capponi C, et al. Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases. Front Immunol 2020;11. 10.3389/fimmu.2020.610300. [DOI] [PMC free article] [PubMed]
- 12.Calabrese F, Pezzuto F, Fortarezza F, Hofman P, Kern I, Panizo A, et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Archiv. 2020;477:359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deinhardt-Emmer S, Wittschieber D, Sanft J, Kleemann S, Elschner S, Haupt KF, et al. Early postmortem mapping of sars-cov-2 rna in patients with covid-19 and the correlation with tissue damage. Elife 2021;10. 10.7554/eLife.60361. [DOI] [PMC free article] [PubMed]
- 14.Ducloyer M, Gaborit B, Toquet C, Castain L, Bal A, Arrigoni PP, et al. Complete post-mortem data in a fatal case of COVID-19: clinical, radiological and pathological correlations. Int J Legal Med. 2020;134:2209–2214. doi: 10.1007/s00414-020-02390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adachi T, Chong JM, Nakajima N, Sano M, Yamazaki J, Miyamoto I, et al. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19. Japan Emerg Infect Dis. 2020;26:2157–2161. doi: 10.3201/eid2609.201353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chmielik E, Jazowiecka-Rakus J, Dyduch G, Nasierowska-Guttmejer A, Michalowski L, Sochanik A, et al. COVID-19 Autopsies: A Case Series from Poland. Pathobiology 2021;88. 10.1159/000512768. [DOI] [PMC free article] [PubMed]
- 17.Fassan M, Mescoli C, Sbaraglia M, Guzzardo V, Russo FP, Fabris R, et al. Liver histopathology in COVID-19 patients: A mono-Institutional series of liver biopsies and autopsy specimens. Pathol Res Pract 2021;221. 10.1016/j.prp.2021.153451. [DOI] [PMC free article] [PubMed]
- 18.Nienhold R, Ciani Y, Koelzer VH, Tzankov A, Haslbauer JD, Menter T, et al. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat Commun 2020;11. 10.1038/s41467-020-18854-2. [DOI] [PMC free article] [PubMed]
- 19.Remmelink M, de Mendonça R, D’Haene N, de Clercq S, Verocq C, Lebrun L, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care 2020;24. 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed]
- 20.Raveendran A v., Jayadevan R, Sashidharan S. Long COVID: An overview. Diabetes Metabolic Syndrome: Clin Res Rev 2021;15. 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed]
- 21.de Lorenzo R, Conte C, Lanzani C, Benedetti F, Roveri L, Mazza MG, et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS One 2020;15. 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed]
- 22.Ahmad A, Ishtiaq SM, Khan JA, Aslam R, Ali S, Arshad MI. COVID-19 and comorbidities of hepatic diseases in a global perspective. World J Gastroenterol 2021;27. 10.3748/wjg.v27.i13.1296. [DOI] [PMC free article] [PubMed]
- 23.Buja LM, Wolf D, Zhao B, Akkanti B, McDonald M, Lelenwa L, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovascular Pathol 2020;48. 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed]
- 24.Wang F, Kream RM, Stefano GB. Long-term respiratory and neurological sequelae of COVID-19. Med Sci Monitor 2020;26. 10.12659/MSM.928996. [DOI] [PMC free article] [PubMed]
- 25.Andrade BS, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, dos Santos Freitas A, et al. Long-covid and post-covid health complications: An up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021;13. 10.3390/v13040700. [DOI] [PMC free article] [PubMed]
- 26.Mohanty SK, Satapathy A, Naidu MM, Mukhopadhyay S, Sharma S, Barton LM, et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19)—anatomic pathology perspective on current knowledge. Diagn Pathol 2020;15. 10.1186/s13000-020-01017-8. [DOI] [PMC free article] [PubMed]
- 27.Fiel MI, el Jamal SM, Paniz-Mondolfi A, Gordon RE, Reidy J, Bandovic J, et al. Findings of Hepatic Severe Acute Respiratory Syndrome Coronavirus-2 Infection. CMGH 2021;11. 10.1016/j.jcmgh.2020.09.015. [DOI] [PMC free article] [PubMed]
- 28.Datta PK, Liu F, Fischer T, Rappaport J, Qin X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020;10. 10.7150/thno.48076. [DOI] [PMC free article] [PubMed]
- 29.Skok K, Stelzl E, Trauner M, Kessler HH, Lax SF. Post-mortem viral dynamics and tropism in COVID-19 patients in correlation with organ damage. Virchows Archiv 2021;478. 10.1007/s00428-020-02903-8. [DOI] [PMC free article] [PubMed]
- 30.Trougakos IP, Stamatelopoulos K, Terpos E, Tsitsilonis OE, Aivalioti E, Paraskevis D, et al. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J Biomed Sci 2021;28. 10.1186/s12929-020-00703-5. [DOI] [PMC free article] [PubMed]
- 31.Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun 2021;12. 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed]
- 32.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep 2021;9. 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed]
- 33.Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and Multiorgan Response. Curr Probl Cardiol 2020;45. 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed]
- 34.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020;77. 10.1111/his.14134. [DOI] [PMC free article] [PubMed]
- 35.Aguiar D, Lobrinus JA, Schibler M, Fracasso T, Lardi C. Inside the lungs of COVID-19 disease. Int J Legal Med 2020;134. 10.1007/s00414-020-02318-9. [DOI] [PMC free article] [PubMed]
- 36.Magadum A, Kishore R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020;9. 10.3390/cells9112508. [DOI] [PMC free article] [PubMed]
- 37.Dou Q, Wei X, Zhou K, Yang S, Jia P. Cardiovascular manifestations and mechanisms in Patients with COVID-19. Trends Endocrinol Metabolism 2020;31. 10.1016/j.tem.2020.10.001. [DOI] [PMC free article] [PubMed]
- 38.Kellum JA, Olivier van Till JW, Mulligan G. Targeting acute kidney injury in COVID-19. Nephrol Dial Transplantation 2020;35. 10.1093/ndt/gfaa231. [DOI] [PMC free article] [PubMed]
- 39.Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, Abediazar S, Shoja MM, Ardalan M, et al. Covid-19 and kidney injury: pathophysiology and molecular mechanisms. Rev Med Virol 2021;31. 10.1002/rmv.2176. [DOI] [PMC free article] [PubMed]
- 40.Prasad N, Agrawal S. COVID 19 and acute kidney injury. Indian J Nephrol 2020;30. 10.4103/ijn.IJN_120_20. [DOI] [PMC free article] [PubMed]
- 41.Vinken M. COVID-19 and the liver: an adverse outcome pathway perspective. Toxicology 2021;455. 10.1016/j.tox.2021.152765. [DOI] [PMC free article] [PubMed]
- 42.Mönkemüller K, Fry LC, Rickes S. COVID-19, coronavirus, SARS-CoV-2 and the small bowel. Rev Espanola Enfermedades Digestivas 2020;112. 10.17235/reed.2020.7137/2020. [DOI] [PubMed]
- 43.Chenlu Huang. Molecular and cellular mechanisms of liver dysfunction in COVID-19. Discov Med (nd). [PubMed]
- 44.Hill AR, Spencer-Segal JL. Glucocorticoids and the Brain after critical illness. Endocrinology (United States) 2021;162. 10.1210/endocr/bqaa242. [DOI] [PMC free article] [PubMed]
- 45.Yong SJ. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem Neurosci 2021;12. 10.1021/acschemneuro.0c00793. [DOI] [PubMed]
- 46.Pennisi M, Lanza G, Falzone L, Fisicaro F, Ferri R, Bella R. Sars-cov-2 and the nervous system: From clinical features to molecular mechanisms. Int J Mol Sci 2020;21. 10.3390/ijms21155475. [DOI] [PMC free article] [PubMed]
- 47.Killion L, Beatty PE, Salim A. Rare cutaneous manifestation of COVID-19. BMJ Case Rep 2021;14. 10.1136/bcr-2020-240863. [DOI] [PMC free article] [PubMed]
- 48.Seymen CM. The other side of COVID-19 pandemic: Effects on male fertility. J Med Virol 2021;93. 10.1002/jmv.26667. [DOI] [PMC free article] [PubMed]
- 49.Sheikhzadeh Hesari F, Hosseinzadeh SS, Asl Monadi Sardroud MA. Review of COVID-19 and male genital tract. Andrologia 2021;53. 10.1111/and.13914. [DOI] [PMC free article] [PubMed]
- 50.Huang C, Ji X, Zhou W, Huang Z, Peng X, Fan L, et al. Coronavirus: A possible cause of reduced male fertility. Andrology 2021;9. 10.1111/andr.12907. [DOI] [PMC free article] [PubMed]
- 51.Pal R, Bhadada SK. COVID-19 and diabetes mellitus: An unholy interaction of two pandemics. Diab Metabolic Syndrome: Clin Res Rev 2020;14. 10.1016/j.dsx.2020.04.049. [DOI] [PMC free article] [PubMed]
- 52.Lima-Martínez MM, Carrera Boada C, Madera-Silva MD, Marín W, Contreras M. COVID-19 and diabetes: A bidirectional relationship. ClinInvestigacion En Arteriosclerosis 2021;33. 10.1016/j.arteri.2020.10.001. [DOI] [PMC free article] [PubMed]
- 53.Bülow Anderberg S, Luther T, Berglund M, Larsson R, Rubertsson S, Lipcsey M, et al. Increased levels of plasma cytokines and correlations to organ failure and 30-day mortality in critically ill Covid-19 patients. Cytokine 2021;138. 10.1016/j.cyto.2020.155389. [DOI] [PMC free article] [PubMed]
- 54.Salvetti M, Bellucci G, Ballerini C, Mechelli R, Bigi R, Rinaldi V, et al. SARS-CoV-2 meta-interactome suggests disease-specific, autoimmune pathophysiologies and therapeutic targets. F1000Res 2020;9. 10.12688/f1000research.25593.1. [DOI] [PMC free article] [PubMed]
- 55.Gąsecka A, Borovac JA, Guerreiro RA, Giustozzi M, Parker W, Caldeira D, et al. Thrombotic Complications in Patients with COVID-19: pathophysiological mechanisms, Diagnosis, and Treatment. Cardiovasc Drugs Ther 2021;35. 10.1007/s10557-020-07084-9. [DOI] [PMC free article] [PubMed]
- 56.Kipshidze N, Dangas G, White CJ, Kipshidze N, Siddiqui F, Lattimer CR, et al. Viral Coagulopathy in Patients With COVID-19: Treatment and Care. Clin Appl Thrombosis/Hemostasis 2020;26. 10.1177/1076029620936776. [DOI] [PMC free article] [PubMed]
- 57.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med 2020;173. 10.7326/M20-2003. [DOI] [PMC free article] [PubMed]
- 58.Cañas CA. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med Hypotheses 2020;145. 10.1016/j.mehy.2020.110345. [DOI] [PMC free article] [PubMed]
- 59.Bussani R, Schneider E, Zentilin L, Collesi C, Ali H, Braga L, et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine 2020;61. 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed]
- 60.Janardhan V, Janardhan V, Kalousek V. COVID-19 as a blood clotting disorder Masquerading as a respiratory illness: A cerebrovascular perspective and therapeutic Implications for stroke thrombectomy. J Neuroimaging 2020;30. 10.1111/jon.12770. [DOI] [PMC free article] [PubMed]
- 61.Nouri-Vaskeh M, Sharifi A, Khalili N, Zand R, Sharifi A. Dyspneic and non-dyspneic (silent) hypoxemia in COVID-19: Possible neurological mechanism. Clin Neurol Neurosurg 2020;198. 10.1016/j.clineuro.2020.106217. [DOI] [PMC free article] [PubMed]
- 62.Verkhratsky A, Li Q, Melino S, Melino G, Shi Y. Can COVID-19 pandemic boost the epidemic of neurodegenerative diseases? Biol Direct 2020;15. 10.1186/s13062-020-00282-3. [DOI] [PMC free article] [PubMed]
- 63.Diorio C, McNerney KO, Lambert M, Paessler M, Anderson EM, Henrickson SE, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv 2020;4. 10.1182/bloodadvances.2020003471. [DOI] [PMC free article] [PubMed]
- 64.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and agiogenesis in Covid-19. New Engl J Med 2020;383. 10.1056/nejmoa2015432. [DOI] [PMC free article] [PubMed]
- 65.Wu MA, Fossali T, Pandolfi L, Carsana L, Ottolina D, Frangipane V, et al. Hypoalbuminemia in COVID-19: assessing the hypothesis for underlying pulmonary capillary leakage. J Intern Med 2021;289. 10.1111/joim.13208. [DOI] [PubMed]
- 66.Tan BH, Zhang Y, Gui Y, Wu S, Li YC. The possible impairment of respiratory-related neural loops may be associated with the silent pneumonia induced by SARS-CoV-2. J Med Virol 2020;92. 10.1002/jmv.26158. [DOI] [PMC free article] [PubMed]
- 67.Sheth AR, Grewal US, Patel HP, Thakkar S, Garikipati S, Gaddam J, et al. Possible mechanisms responsible for acute coronary events in COVID-19. Med Hypotheses 2020;143. 10.1016/j.mehy.2020.110125. [DOI] [PMC free article] [PubMed]
- 68.Zeng JH, Wu WB, Qu JX, Wang Y, Dong CF, Luo YF, et al. Cardiac manifestations of COVID-19 in Shenzhen, China. Infection 2020;48. 10.1007/s15010-020-01473-w. [DOI] [PMC free article] [PubMed]
- 69.Lau WL, Zuckerman JE, Gupta A, Kalantar-Zadeh K. The COVID-kidney controversy: Can SARS-CoV-2 cause direct renal infection? Nephron 2021;145. 10.1159/000513789. [DOI] [PMC free article] [PubMed]
- 70.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020;98. 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed]
- 71.Samidurai A, Das A. Cardiovascular complications associated with COVID-19 and potential therapeutic strategies. Int J Mol Sci 2020;21. 10.3390/ijms21186790. [DOI] [PMC free article] [PubMed]
- 72.Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, et al. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020;17. 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed]
- 73.Lopes CCB, Brucki SMD, Passos Neto CEB, Corazza LA, Baima JPS, Fiorentino MD, et al. Acute disseminated encephalomyelitis in COVID-19: Presentation of two cases and review of the literature. Arq Neuropsiquiatr 2020;78. 10.1590/0004-282X20200186. [DOI] [PubMed]
- 74.Xiang Q, Feng Z, Diao B, Tu C, Qiao Q, Yang H, et al. SARS-CoV-2 Induces lymphocytopenia by promoting inflammation and decimates secondary lymphoid organs. Front Immunol 2021;12. 10.3389/fimmu.2021.661052. [DOI] [PMC free article] [PubMed]
- 75.Zhang JW, Hu X, Jin PF. Cytokine Storm Induced by SARS-CoV-2 and the Drug Therapy. Chin Pharmaceutical J 2020;55. 10.11669/cpj.2020.05.001.
- 76.Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine 2020;57. 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed]
- 77.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; What we know so far. Front Immunol 2020;11. 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed]
- 78.Carcaterra M, Caruso C. Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-Kb pathway deregulation: A physio-pathological theory. Med Hypotheses 2021;146. 10.1016/j.mehy.2020.110412. [DOI] [PMC free article] [PubMed]
- 79.Maiese A, Manetti AC, la Russa R, di Paolo M, Turillazzi E, Frati P, et al. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol 2021;17. 10.1007/s12024-020-00310-8. [DOI] [PMC free article] [PubMed]
- 80.Ozieranski K, Tyminska A, Jonik S, Marcolongo R, Baritussio A, Grabowski M, et al. Clinically suspected myocarditis in the course of severe acute respiratory syndrome novel Coronavirus-2 infection: fact or fiction? J Card Fail 2021;27. 10.1016/j.cardfail.2020.11.002. [DOI] [PMC free article] [PubMed]
- 81.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res 2020;69. 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed]
- 82.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol 2020;73. 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed]
- 83.Luglio M, Tannuri U, de Carvalho WB, Bastos KL de M, Rodriguez IS, Johnston C, et al. COVID-19 and liver damage: Narrative review and proposed clinical protocol for critically ill pediatric patients. Clinics (Sao Paulo) 2020;75. 10.6061/clinics/2020/e2250. [DOI] [PMC free article] [PubMed]
- 84.Portincasa P, Krawczyk M, Machill A, Lammert F, di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med 2020;77. 10.1016/j.ejim.2020.05.035. [DOI] [PMC free article] [PubMed]
- 85.Portincasa P, Krawczyk M, Smyk W, Lammert F, di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest 2020;50. 10.1111/eci.13338. [DOI] [PMC free article] [PubMed]
- 86.Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int 2020;40. 10.1111/liv.14601. [DOI] [PMC free article] [PubMed]
- 87.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 2020;396. 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed]
- 88.Nanda S, Handa R, Prasad A, Anand R, Zutshi D, Dass SK, et al. Covid-19 associated Guillain-Barre Syndrome: Contrasting tale of four patients from a tertiary care centre in India. Am J Emerg Med 2021;39. 10.1016/j.ajem.2020.09.029. [DOI] [PMC free article] [PubMed]
- 89.Raahimi MM, Kane A, Moore CE, Alareed AW. Late onset of Guillain-Barré syndrome following SARS-CoV-2 infection: part of “long COVID-19 syndrome”? BMJ Case Rep 2021;14. 10.1136/bcr-2020-240178. [DOI] [PMC free article] [PubMed]
- 90.Larenas-Linnemann D, Luna-Pech J, Navarrete-Rodríguez EM, Rodríguez-Pérez N, Arias-Cruz A, Blandón-Vijil MV, et al. Cutaneous manifestations related to COVID-19 immune dysregulation in the pediatric age group. Curr Allergy Asthma Rep 2021;21. 10.1007/s11882-020-00986-6. [DOI] [PMC free article] [PubMed]
- 91.Fitsiori A, Pugin D, Thieffry C, Lalive P, Vargas MI. COVID-19 is associated with an unusual pattern of brain microbleeds in critically ill patients. J Neuroimaging 2020;30. 10.1111/jon.12755. [DOI] [PMC free article] [PubMed]
- 92.Saitta L, Molin A, Villani F, Insorsi A, Roccatagliata L, Inglese M, et al. Brain microvascular occlusive disorder in COVID-19: a case report. Neurol Sci 2020;41. 10.1007/s10072-020-04795-7. [DOI] [PMC free article] [PubMed]
- 93.Gherlone EF, Polizzi E, Tetè G, de Lorenzo R, Magnaghi C, Rovere Querini P, et al. Frequent and Persistent Salivary Gland Ectasia and Oral Disease After COVID-19. J Dent Res 2021;100. 10.1177/0022034521997112. [DOI] [PMC free article] [PubMed]
- 94.Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and eye: A review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol 2021;69. 10.4103/ijo.IJO_297_21. [DOI] [PMC free article] [PubMed]
- 95.Kabbani N, Olds JL. Does COVID19 Infect the Brain? If So, Smokers might be at a higher risk. Mol Pharmacol 2020;97. 10.1124/MOLPHARM.120.000014. [DOI] [PMC free article] [PubMed]
- 96.Prieto-Pérez L, Fortes J, Soto C, Vidal-González Á, Alonso-Riaño M, Lafarga M, et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Modern Pathol 2020;33. 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed]
- 97.Meini S, Zini C, Passaleva MT, Frullini A, Fusco F, Carpi R, et al. Pneumatosis intestinalis in COVID-19. BMJ Open Gastroenterol 2020;7. 10.1136/bmjgast-2020-000434. [DOI] [PMC free article] [PubMed]
- 98.Cheung S, Quiwa JC, Pillai A, Onwu C, Tharayil ZJ, Gupta R. Superior mesenteric artery thrombosis and acute intestinal ischemia as a consequence of COVID-19 infection. Am J Case Reports 2020;21. 10.12659/AJCR.925753. [DOI] [PMC free article] [PubMed]
- 99.Lani-Louzada R, do Val Ferreira Ramos C, Cordeiro RM, Sadun AA. Retinal changes in COVID-19 hospitalized cases. PLoS One 2020;15. 10.1371/journal.pone.0243346. [DOI] [PMC free article] [PubMed]
- 100.Bhagali R, Prabhudesai N, Prabhudesai M. Post COVID-19 opportunistic candida retinitis: A case report. Indian J Ophthalmol 2021;69. 10.4103/ijo.IJO_3047_20. [DOI] [PMC free article] [PubMed]
- 101.Kumar MA, Krishnaswamy M, Arul JN. Post COVID-19 sequelae: Venous thromboembolism complicated by lower GI bleed. BMJ Case Rep 2021;14. 10.1136/bcr-2020-241059. [DOI] [PMC free article] [PubMed]
- 102.Schoenmakers S, Snijder P, Verdijk RM, Kuiken T, Kamphuis SSM, Koopman LP, et al. Severe acute respiratory syndrome Coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. J Pediatric Infect Dis Soc 2021;10. 10.1093/jpids/piaa153. [DOI] [PMC free article] [PubMed]
- 103.Dvash S, Cuckle H, Smorgick N, Vaknin Z, Padoa A, Maymon R. Increase rate of ruptured tubal ectopic pregnancy during the COVID-19 pandemic. Euro J Obstet Gynecol Reprod Biol 2021;259. 10.1016/j.ejogrb.2021.01.054. [DOI] [PMC free article] [PubMed]
- 104.Lai CC, Chen SY, Ko WC, Hsueh PR. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents 2021;57. 10.1016/j.ijantimicag.2021.106324. [DOI] [PMC free article] [PubMed]
- 105.Jacobs JJL. Persistent SARS-2 infections contribute to long COVID-19. Med Hypotheses 2021;149. 10.1016/j.mehy.2021.110538. [DOI] [PMC free article] [PubMed]
- 106.Yelin D, Margalit I, Yahav D, Runold M, Bruchfeld J. Long COVID-19—it’s not over until? Clin Microbiol Infect 2021;27. 10.1016/j.cmi.2020.12.001. [DOI] [PMC free article] [PubMed]
- 107.Iwu CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J 2021;38. 10.11604/pamj.2021.38.65.27366. [DOI] [PMC free article] [PubMed]
- 108.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 2020;19. 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed]
- 109.Rai DK, Sharma P, Kumar R. Post covid 19 pulmonary fibrosis. Is it real threat? Indian J Tuberculosis 2021;68. 10.1016/j.ijtb.2020.11.003. [DOI] [PMC free article] [PubMed]
- 110.Fernández-de-las-Peñas C, Guijarro C, Plaza-Canteli S, Hernández-Barrera V, Torres-Macho J. Prevalence of post-COVID-19 cough one year after SARS-CoV-2 infection: a multicenter study. Lung 2021;199. 10.1007/s00408-021-00450-w. [DOI] [PMC free article] [PubMed]
- 111.Lazaridis C, Vlachogiannis NI, Bakogiannis C, Spyridopoulos I, Stamatelopoulos K, Kanakakis I, Involvement of cardiovascular system as the critical point in coronavirus disease et al. (COVID-19) prognosis and recovery. Hellenic J Cardiol. 2019;2020:61. doi: 10.1016/j.hjc.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Special Article—acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog Cardiovasc Dis 2020;63. 10.1016/j.pcad.2020.05.013. [DOI] [PMC free article] [PubMed]
- 113.Bandyopadhyay D, Akhtar T, Hajra A, Gupta M, Das A, Chakraborty S, et al. COVID-19 pandemic: Cardiovascular complications and future implications. Am J Cardiovascular Drugs 2020;20. 10.1007/s40256-020-00420-2. [DOI] [PMC free article] [PubMed]
- 114.Zhu H, Rhee JW, Cheng P, Waliany S, Chang A, Witteles RM, et al. Cardiovascular complications in patients with COVID-19: Consequences of viral toxicities and host immune response. Curr Cardiol Rep 2020;22. 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed]
- 115.Biscetti F, Rando MM, Nardella E, Cecchini AL, Bruno P, Landolfi R, et al. Cardiovascular disease and SARS-CoV-2: The role of host immune response versus direct viral injury. Int J Mol Sci 2020;21. 10.3390/ijms21218141. [DOI] [PMC free article] [PubMed]
- 116.Sarfraz Z, Sarfraz A, Barrios A, Garimella R, Dominari A, KC M, et al. Cardio-pulmonary sequelae in recovered COVID-19 patients: Considerations for primary care. J Prim Care Community Health 2021;12. 10.1177/21501327211023726. [DOI] [PMC free article] [PubMed]
- 117.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging 2020;13. 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed]
- 118.Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Euro Respiratory J 2021;57. 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed]
- 119.Gasecka A, Pruc M, Kukula K, Gilis-Malinowska N, Filipiak KJ, Jaguszewski MJ, et al. Post-covid-19 heart syndrome. Cardiol J 2021;28. 10.5603/CJ.a2021.0028. [DOI] [PMC free article] [PubMed]
- 120.Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci 2020;253. 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed]
- 121.Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol 2020;31. 10.1111/jce.14479. [DOI] [PMC free article] [PubMed]
- 122.Khanduri A, Anand U, Doss M, Lovett L. Severe acute mitral valve regurgitation in a COVID-19-infected patient. BMJ Case Rep 2021;14. 10.1136/bcr-2020-239782. [DOI] [PMC free article] [PubMed]
- 123.Holt A, Gislason GH, Schou M, Zareini B, Biering-Sørensen T, Phelps M, et al. New-onset atrial fibrillation: Incidence, characteristics, and related events following a national COVID-19 lockdown of 5.6 million people. Eur Heart J 2020;41. 10.1093/eurheartj/ehaa494. [DOI] [PMC free article] [PubMed]
- 124.Ritt LEF, Viana MS, Feitosa GF, de Oliveira AM, Souza FS, Darzé ES. Covid-19 and acute coronary events—collateral damage. A case report. Arq Bras Cardiol 2020;114. 10.36660/abc.20200329. [DOI] [PMC free article] [PubMed]
- 125.Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, et al. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int J Cardiol 2020;311. 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed]
- 126.de Oliveira AP, Lopes ALF, Pacheco G, de Sá Guimarães Nolêto IR, Nicolau LAD, Medeiros JVR. Premises among SARS-CoV-2, dysbiosis and diarrhea: Walking through the ACE2/mTOR/autophagy route. Med Hypotheses 2020;144. 10.1016/j.mehy.2020.110243. [DOI] [PMC free article] [PubMed]
- 127.Nuzzo D, Picone P. Potential neurological effects of severe COVID-19 infection. Neurosci Res 2020;158. 10.1016/j.neures.2020.06.009. [DOI] [PMC free article] [PubMed]
- 128.Bougakov D, Podell K, Goldberg E. Multiple Neuroinvasive Pathways in COVID-19. Mol Neurobiol 2021;58. 10.1007/s12035-020-02152-5. [DOI] [PMC free article] [PubMed]
- 129.Kanjanaumporn J, Aeumjaturapat S, Snidvongs K, Seresirikachorn K, Chusakul S. Smell and taste dysfunction in patients with SARS-CoV-2 infection: A review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac J Allergy Immunol 2020;38. 10.12932/AP-030520-0826. [DOI] [PubMed]
- 130.Azizi SA, Azizi SA. Neurological injuries in COVID-19 patients: direct viral invasion or a bystander injury after infection of epithelial/endothelial cells. J Neurovirol 2020;26. 10.1007/s13365-020-00903-7. [DOI] [PMC free article] [PubMed]
- 131.Singh AK, Bhushan B, Maurya A, Mishra G, Singh SK, Awasthi R, Novel coronavirus disease (COVID-19) and neurodegenerative disorders. Dermatol Ther. 2019;2020:33. doi: 10.1111/dth.13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sieracka J, Sieracki P, Kozera G, Szurowska E, Gulczynski J, Sobolewski P, et al. Covid-19 - neuropathological point of view, pathobiology, and dilemmas after the first year of the pandemic struggle. Folia Neuropathol 2021;59. 10.5114/fn.2021.105128. [DOI] [PubMed]
- 133.Song WJ, Hui CKM, Hull JH, Birring SS, McGarvey L, Mazzone SB, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med 2021;9. 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed]
- 134.Frontera JA, Yang D, Lewis A, Patel P, Medicherla C, Arena V, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci 2021;426. 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed]
- 135.Gómez-Iglesias P, Porta-Etessam J, Montalvo T, Valls-Carbó A, Gajate V, Matías-Guiu JA, et al. An online observational study of patients with olfactory and gustory alterations secondary to SARS-CoV-2 infection. Front Public Health 2020;8. 10.3389/fpubh.2020.00243. [DOI] [PMC free article] [PubMed]
- 136.Kavaz E, Tahir E, Bilek HC, Kemal Ö, Deveci A, Aksakal Tanyel E. Clinical significance of smell and taste dysfunction and other related factors in COVID-19. Euro Arch Oto-Rhino-Laryngol 2021;278. 10.1007/s00405-020-06503-9. [DOI] [PMC free article] [PubMed]
- 137.Cavalagli A, Peiti G, Conti C, Penati R, Vavassori F, Taveggia G. Cranial nerves impairment in post-acute oropharyngeal dysphagia after COVID-19. Eur J Phys Rehabil Med 2020;56. 10.23736/S1973-9087.20.06452-7. [DOI] [PubMed]
- 138.Murta V, Villarreal A, Ramos AJ. Severe acute respiratory syndrome Coronavirus 2 impact on the central nervous system: Are astrocytes and microglia main players or merely bystanders? ASN Neuro 2020;12. 10.1177/1759091420954960. [DOI] [PMC free article] [PubMed]
- 139.Koumpa FS, Forde CT, Manjaly JG. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep 2020;13. 10.1136/bcr-2020-238419. [DOI] [PMC free article] [PubMed]
- 140.Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med J Roy College Phys Lond 2021;21. 10.7861/CLINMED.2020-0896. [DOI] [PMC free article] [PubMed]
- 141.Shouman K, Vanichkachorn G, Cheshire WP, Suarez MD, Shelly S, Lamotte GJ, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Autonomic Res 2021;31. 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed]
- 142.Versace V, Sebastianelli L, Ferrazzoli D, Romanello R, Ortelli P, Saltuari L, et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin Neurophysiol 2021;132. 10.1016/j.clinph.2021.03.001. [DOI] [PMC free article] [PubMed]
- 143.Townsend L, Moloney D, Finucane C, McCarthy K, Bergin C, Bannan C, et al. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS One 2021;16. 10.1371/journal.pone.0247280. [DOI] [PMC free article] [PubMed]
- 144.Steardo L, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry 2020;10. 10.1038/s41398-020-00949-5. [DOI] [PMC free article] [PubMed]
- 145.Ryoo N, Pyun JM, Baek MJ, Suh J, Kang MJ, Wang MJ, et al. Coping with dementia in the middle of the COVID-19 pandemic. Int J Agric Biol 2020;35. 10.3346/jkms.2020.35.e383. [DOI] [PMC free article] [PubMed]
- 146.Maury A, Lyoubi A, Peiffer-Smadja N, de Broucker T, Meppiel E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians. Rev Neurol (Paris) 2021;177. 10.1016/j.neurol.2020.10.001. [DOI] [PMC free article] [PubMed]
- 147.Sher L. Post-COVID syndrome and suicide risk. QJM Int J Med 2021;114. 10.1093/qjmed/hcab007. [DOI] [PMC free article] [PubMed]
- 148.Miners S, Kehoe PG, Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther 2020;12. 10.1186/s13195-020-00744-w. [DOI] [PMC free article] [PubMed]
- 149.Simon FAJ, Schenk M, Palm D, Faltraco F, Thome J. The collateral damage of the covid-19 outbreak on mental health and psychiatry. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18094440. [DOI] [PMC free article] [PubMed]
- 150.Islam MS, Ferdous MZ, Islam US, Mosaddek ASM, Potenza MN, Pardhan S. Treatment, persistent symptoms, and depression in people infected with covid-19 in bangladesh. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18041453. [DOI] [PMC free article] [PubMed]
- 151.Simani L, Ramezani M, Darazam IA, Sagharichi M, Aalipour MA, Ghorbani F, et al. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J Neurovirol 2021;27. 10.1007/s13365-021-00949-1. [DOI] [PMC free article] [PubMed]
- 152.Ren FF, Guo RJ. Public mental health in post-covid-19 era. Psychiatr Danub 2020;32. 10.24869/PSYD.2020.251. [DOI] [PubMed]
- 153.el Sayed S, Shokry D, Gomaa SM. Post-COVID-19 fatigue and anhedonia: A cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacol Rep 2021;41. 10.1002/npr2.12154. [DOI] [PMC free article] [PubMed]
- 154.Ortelli P, Ferrazzoli D, Sebastianelli L, Engl M, Romanello R, Nardone R, et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J Neurol Sci 2021;420. 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed]
- 155.Weerahandi H, Hochman KA, Simon E, Blaum C, Chodosh J, Duan E, et al. Post-Discharge Health Status and Symptoms in Patients with Severe COVID-19. J Gen Intern Med 2021;36. 10.1007/s11606-020-06338-4. [DOI] [PMC free article] [PubMed]
- 156.Mahmud R, Rahman MM, Rassel MA, Monayem FB, Sayeed SKJB, Islam MS, et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: A prospective cohort study in a tertiary care center of Bangladesh. PLoS One 2021;16. 10.1371/journal.pone.0249644. [DOI] [PMC free article] [PubMed]
- 157.Wong TL, Weitzer DJ. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-A systemic review and comparison of clinical presentation and symptomatology. Medicina (Lithuania) 2021;57. 10.3390/medicina57050418. [DOI] [PMC free article] [PubMed]
- 158.Wostyn P. COVID-19 and chronic fatigue syndrome: Is the worst yet to come? Med Hypotheses 2021;146. 10.1016/j.mehy.2020.110469. [DOI] [PMC free article] [PubMed]
- 159.Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract 2021;75. 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed]
- 160.Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One 2020;15. 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed]
- 161.Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol 2021;93. 10.1002/jmv.26368. [DOI] [PubMed]
- 162.Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers 2020;6. 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed]
- 163.Camargo-Martínez W, Lozada-Martínez I, Escobar-Collazos A, Navarro-Coronado A, Moscote-Salazar L, Pacheco-Hernández A, et al. Post-COVID 19 neurological syndrome: Implications for sequelae’s treatment. J Clin Neurosci 2021;88. 10.1016/j.jocn.2021.04.001. [DOI] [PMC free article] [PubMed]
- 164.Davido B, Seang S, Tubiana R, de Truchis P. Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect 2020;26. 10.1016/j.cmi.2020.07.028. [DOI] [PMC free article] [PubMed]
- 165.Khadke S, Ahmed N, Ahmed N, Ratts R, Raju S, Gallogly M, et al. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents. Virol J 2020;17. 10.1186/s12985-020-01415-w. [DOI] [PMC free article] [PubMed]
- 166.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol 2020;16. 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed]
- 167.Clark EG, Hiremath S, McIntyre L, Wald R, Hundemer GL, Joannidis M. Haemoperfusion should only be used for COVID-19 in the context of randomized trials. Nat Rev Nephrol 2020;16. 10.1038/s41581-020-00352-9. [DOI] [PMC free article] [PubMed]
- 168.Gao C, Zhu L, Jin CC, Tong YX, Xiao AT, Zhang S. Prevalence and impact factors of recurrent positive SARS-CoV-2 detection in 599 hospitalized COVID-19 patients. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brown EL, Essigmann HT. Original Antigenic Sin: the Downside of Immunological Memory and Implications for COVID-19. MSphere 2021;6. 10.1128/msphere.00056-21. [DOI] [PMC free article] [PubMed]
- 170.Laval K, Enquist LW. The potential role of Herpes Simplex Virus Type 1 and Neuroinflammation in the pathogenesis of Alzheimer’s Disease. Front Neurol 2021;12. 10.3389/fneur.2021.658695. [DOI] [PMC free article] [PubMed]
- 171.Zito A, Alfonsi E, Franciotta D, Todisco M, Gastaldi M, Cotta Ramusino M, et al. COVID-19 and Guillain–Barré syndrome: A case report and review of literature. Front Neurol 2020;11. 10.3389/fneur.2020.00909. [DOI] [PMC free article] [PubMed]
- 172.Xie P, Ma W, Tang H, Liu D. Severe COVID-19: A review of recent progress with a look toward the future. Front Public Health 2020;8. 10.3389/fpubh.2020.00189. [DOI] [PMC free article] [PubMed]
- 173.Kohli U, Lodha R. Cardiac Involvement in Children With COVID-19. Indian Pediatr 2020;57. 10.1007/s13312-020-1998-0. [DOI] [PMC free article] [PubMed]
- 174.Chakraborty T, Jamal RF, Battineni G, Teja KV, Marto CM, Spagnuolo G. A review of prolonged post-covid-19 symptoms and their implications on dental management. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18105131. [DOI] [PMC free article] [PubMed]
- 175.Ojo AS, Balogun SA, Williams OT, Ojo OS. Pulmonary fibrosis in COVID-19 survivors: Predictive factors and risk reduction strategies. Pulm Med 2020;2020. 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed]
- 176.Sohlenius-Sternbeck AK. Determination of the hepatocellularity number for human, dog, rabbit, rat and mouse livers from protein concentration measurements. Toxicol in Vitro 2006;20. 10.1016/j.tiv.2006.06.003. [DOI] [PubMed]
- 177.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: Implications for health and disease. Pediatric Nephrol 2011;26. 10.1007/s00467-011-1843-8. [DOI] [PubMed]
- 178.Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004 doi: 10.1164/rccm.200308-1107oc. [DOI] [PubMed] [Google Scholar]
- 179.Zoghi A, Ramezani M, Roozbeh M, Darazam IA, Sahraian MA. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Scler Relat Disord 2020;44. 10.1016/j.msard.2020.102324. [DOI] [PMC free article] [PubMed]
- 180.Gasparotto M, Framba V, Piovella C, Doria A, Iaccarino L. Post-COVID-19 arthritis: a case report and literature review. Clin Rheumatol. 2021 doi: 10.1007/s10067-020-05550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fisicaro F, di Napoli M, Liberto A, Fanella M, di Stasio F, Pennisi M, et al. Neurological sequelae in patients with covid-19: A histopathological perspective. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18041415. [DOI] [PMC free article] [PubMed]
- 182.Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 symptom burden: What is long-COVID and how should we manage it? Lung 2021;199. 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed]
- 183.Sisó-Almirall A, Brito-Zerón P, Ferrín LC, Kostov B, Moreno AM, Mestres J, et al. Long covid-19: Proposed primary care clinical guidelines for diagnosis and disease management. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18084350. [DOI] [PMC free article] [PubMed]
- 184.Nakamura ZM, Nash RP, Laughon SL, Rosenstein DL. Neuropsychiatric complications of COVID-19. Curr Psychiatry Rep 2021;23. 10.1007/s11920-021-01237-9. [DOI] [PMC free article] [PubMed]
- 185.Hashemian SMR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther 2021;12. 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed]
- 186.Sakinah S, Priya SP, Mok PL, Munisvaradass R, Teh SW, Sun Z, et al. Stem Cell Therapy in Dengue Virus-Infected BALB/C Mice Improves Hepatic Injury. Front Cell Dev Biol 2021;9. 10.3389/fcell.2021.637270. [DOI] [PMC free article] [PubMed]
- 187.Pathak L, Gayan S, Pal B, Talukdar J, Bhuyan S, Sandhya S, et al. Coronavirus activates an Altruistic stem cell–mediated defense mechanism that reactivates dormant tuberculosis. Am J Pathol 2021;191. 10.1016/j.ajpath.2021.03.011. [DOI] [PMC free article] [PubMed]
- 188.Cheung CKM, Law MF, Lui GCY, Coronavirus WSH Disease (COVID-19): A haematologist’s perspective. Acta Haematol. 2019;2021:144. doi: 10.1159/000510178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Julius MA, Cantrell D, Sharif S, Zelnik-Yovel D, Rapoport MJ. The first fatal post-COVID-19 adult patient with multi-system inflammatory syndrome in Israel. Israel Med Assoc J 2021;23. [PubMed]
- 190.Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, Martínez-Cava A, Franco-López F, Sánchez-Alcaraz Martínez BJ, et al. Post-covid-19 syndrome and the potential benefits of exercise. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18105329. [DOI] [PMC free article] [PubMed]
- 191.Laksemi DAAS, Sukrama DM, Sudarmaja M, Damayanti PAA, Swastika K, Diarthini NLPE, et al. Medicinal plants as recent complementary and alternative therapy for covid-19: A review. Trop J Natural Product Res 2020;4. 10.26538/tjnpr/v4i12.1.
- 192.Korompoki E, Gavriatopoulou M, Hicklen RS, Ntanasis-Stathopoulos I, Kastritis E, Fotiou D, et al. Epidemiology and organ specific sequelae of post-acute COVID19: A narrative review. J Infect. 2021 doi: 10.1016/j.jinf.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open 2020;3. 10.1001/jamanetworkopen.2020.19686. [DOI] [PMC free article] [PubMed]
- 194.Sigfrid L, Drake TM, Pauley E, Jesudason EC, Olliaro P, Shen Lim W, et al. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. MedRxiv 2021. [DOI] [PMC free article] [PubMed]
- 195.Galván-Tejada CE, Herrera-García CF, Godina-González S, Villagrana-Bañuelos KE, Amaro JDDL, Herrera-García K, et al. Persistence of covid-19 symptoms after recovery in mexican population. Int J Environ Res Public Health 2020;17. 10.3390/ijerph17249367. [DOI] [PMC free article] [PubMed]
- 196.Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg 2020;78. 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed]
- 197.Bhavana V, Thakor P, Singh SB, Mehra NK. COVID-19: Pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci 2020;261. 10.1016/j.lfs.2020.118336. [DOI] [PMC free article] [PubMed]
- 198.Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ 2021;372. 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed]
- 199.Morse SS, Mazet JAK, Woolhouse M, Parrish CR, Carroll D, Karesh WB, et al. Prediction and prevention of the next pandemic zoonosis. Lancet 2012;380. 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed]