Opioid alkaloids and peptides, such as morphine and the endogenous opioid peptides, including β-endorphin and the dynorphin peptides, modulate the function of lymphocytes and other cells involved in host defense and immunity. In recent years, investigations from several laboratories have indicated that opioids can operate as cytokines, the principal communication signals of the immune system. All of the major properties of cytokines are shared by opioids, i.e., production by immune cells with paracrine, autocrine, and endocrine sites of action, functional redundancy, pleiotropy, and effects that are both dose and time dependent (45). If opioids have a direct effect on immune function, they must act through opioid receptors expressed on immune cells. Evidence indicates that opioid receptors expressed by immune cells are often the same as or similar to neuronal-type opioid receptors, particularly κ- and δ-opioid receptors. Studies also point to the existence of novel opioid receptors or binding sites on lymphocytes that are selective for morphine. Opioids and their receptors appear to function in an autocrine or paracrine manner. For example, opioid peptides generated from immune-derived proenkephalin A may act in a manner similar to that for cytokines, capable of regulating many functions of both granulocytes and mononuclear cells. The immunomodulatory effects of β-endorphin have been shown to depend on both naloxone-sensitive and naloxone-insensitive receptors, suggesting both brain-type and non-neuronal-type opioid receptors on immune cells. Measurements of the mRNAs that encode the neuronal types of opioid receptor have detected rather low levels of receptor mRNA in immune cells. Likewise, the use of radiolabeled binding assays has not been successful in detecting opioid receptors on mixed populations of lymphocytes, probably because the receptor is expressed at a low density on a restricted subpopulation of lymphocytes. Further identification and characterization of receptors and signal tranduction pathways that account for some of the unique properties of opioid binding and immunomodulation represent major research challenges.
FUNCTIONAL EVIDENCE FOR PRESENCE OF κ-OPIOID RECEPTORS ON LYMPHOCYTES
κ opioids modulate both cellular and humoral immune responses. The endogenous κ-opioid-selective peptide dynorphin has been shown to increase macrophage superoxide production (53), modulate macrophage oxidative burst (61), enhance macrophage tumoricidal activity (25, 29), and increase the level of production of the cytokine interleukin-1 (IL-1) from bone marrow macrophages (1). In the macrophage cell line P388D1, the κ-opioid-selective agonist U50,488 inhibited the synthesis of IL-1 and tumor necrosis factor alpha (TNF-α) (4). U50,488 failed to modulate IL-6 production in these cells. Both T cells and macrophages are targets for κ-opioid agonists for production of inhibition of T-cell-mediated antibody production (27). These studies suggest that κ-opioid receptors on T cells and macrophages are involved in maintenance of the homeostasis of the cells. Overstimulation of the κ-opioid receptors on T cells and macrophages by exogenous opioids or endogenous opioid peptides may alter the levels of many cytokines. Changes in cytokine levels may lead to the suppression of antibody production (47, 60).
With cocultures of human fetal brain cells and a chronically human immunodeficiency virus type 1 (HIV-1)-infected promonocytic line, U1, the endogenous κ-opioid peptide dynorphin A(1-13) and the κ-opioid alkaloid U50,488 promoted HIV-1 expression (14). Pretreatment with the κ-opioid-selective antagonist nor-binaltorphimine (nor-BNI) completely blocked this enhancement. The stimulation of HIV-1 expression was largely blocked by antibodies to the cytokines TNF-α and IL-6 but not by IL-10. In addition, dynorphin stimulated TNF-α and IL-6 expression in the brain cell cultures at both the mRNA and the protein levels, suggesting that κ-opioid agonists enhanced HIV-1 expression by increasing the levels of TNF-α and IL-6. In contrast to the chronically infected U1 cells, U50,488, dynorphin A(1-13), and dynorphin A(1-17) inhibited HIV-1 expression in acutely infected human microglial cell cultures (16). This inhibition was blocked by the κ-opioid-selective antagonist nor-BNI. Collectively, these studies strongly suggest the presence of κ-opioid receptors on T cells, macrophages, and microglia.
EVIDENCE FROM BINDING AND MOLECULAR STUDIES FOR PRESENCE OF κ-OPIOID RECEPTORS ON CELLS FROM IMMUNE SYSTEM
Demonstration of radioligand binding of κ opioids to a mixed population of lymphocytes has been difficult, probably due to the low density of opioid receptors on lymphocytes and the fact that only small subpopulations of lymphocytes may express the receptor. Consequently, cell lines have been used to characterize the presence of κ-opioid receptors on immune cells. Fiorica and Spector (23) identified (−)-[3H]bremazocine binding sites on the EL-4 thymoma cell line; however, this binding site was not stereoselective, and U50,488 concentrations of greater than 1 μM were needed to observe complete inhibition of binding. The macrophage cell line P388D1 expressed binding sites for the κ-opioid agonist [3H]U69,593, but neither the endogenous opioid peptide dynorphin nor the antagonist naltrexone completely displaced binding (12). However, the mouse R1.1 thymoma cell line, derived from a thymoma from a C58/J mouse, expressed a single site with high-affinity binding for [3H]naloxone and [3H]U69,593 (8). The order of potency of competing ligands, including dynorphin peptides, was consistent with the presence of a κ-opioid receptor. This binding site was further characterized with (−)-[3H]bremazocine, which also bound with a high affinity to a single binding site on R1.1 membranes (33). Competition experiments showed that the site was stereoselective and displayed a binding profile consistent with that of the brain κ1-opioid receptor described by Clark et al. (20), particularly because the site had a high affinity for binding for both U50,488 and α-neo-endorphin. In addition, (−)-[3H]bremazocine binding to R1.1 membranes was potently inhibited by both mono- and divalent cations (33), similar to results reported for κ-opioid binding to brain membranes (44). The nucleotides GTP and GDP and the nonhydrolyzable analog guanylyl-5′-imidodiphosphate further reduced the level of (−)-[3H]bremazocine binding in the presence of NaCl, whereas other nucleotides were ineffective (33). That study suggested that the κ-opioid binding site on R1.1 membranes was coupled to a G protein, as has been reported for brain κ-opioid receptors (41). The R1.G1 and the R1EGO cell lines, two derivative cell lines obtained from the mouse R1.1 thymoma, express the κ-opioid receptor at densities that are three- and sixfold greater than the density at which it is expressed by the parent R1.1 cell line, respectively, (34). These three thymoma cell lines were negatively coupled to adenylyl cyclase through a pertussis toxin-sensitive G protein (34). By using the R1.1 and related cell lines, radioligand binding and second messenger studies have demonstrated that a cell derived from the immune system can express a brain-type κ-opioid binding site.
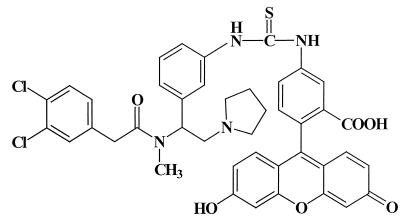
The κ-opioid receptor is a member of the family of seven transmembrane receptors that are coupled to G proteins. A partial κ-opioid receptor amino acid sequence was deduced from cDNA sequences from human and monkey lymphocytes (18). Recently, the full-length nucleotide sequence for the κ-opioid receptor expressed on the R1.1 thymoma cell line was reported (5, 6). The nucleotide sequence shares 99.8% sequence homology and the deduced amino acid sequence shares 100% sequence homology with the reported murine brain κ-opioid receptor (66). Another mRNA population obtained from the R1.1 cells possesses a 30-bp insertion 15 bp upstream of the initiation codon. This 30-bp insertion is also present in the cDNA of the rat brain κ-opioid receptor (38). These results suggest that multiple κ-opioid receptor mRNA species are present in the R1.1 cell line. Splice variants of the κ-opioid receptor may exist on cells from the immune system and may provide a source for protein heterogeneity. The R1.1 cell line is negative for the cell surface phenotypic markers CD4 and CD8, characteristics of thymocytes in one of the early stages of differentiation (6). By cell fractionation techniques, CD4−/CD8− thymocytes were isolated, and analysis by reverse transcription-PCR showed that these primary immature thymocytes also expressed mRNA for the κ-opioid receptor (6). The full-length sequence for the κ-opioid receptor has also been detected on human fetal microglia, the resident macrophages of the brain (16). There was 100% identity between microglial cell cDNA and the human brain κ-opioid receptor gene (67). Human microglia were also shown to express the κ-opioid receptor protein, as detected by flow cytometry with the fluorescent opioid fluorescein isothiocyanate (FITC)–acrylacetamide 2-(3,4-dichlorophenyl-N-methyl-N-[1-3-(aminophenyl)-2-(1-pyrrolidinyl)ethyl]acetamide (AA) (Fig. 1) (16). These studies demonstrate that cDNA for the brain-type κ-opioid receptor is present on cells from the immune system and that these cells express the κ-opioid receptor protein. Thus, a clear molecular basis for the effects of opioid alkaloids and peptides that bind to κ-opioid receptors has been established.
FIG. 1.
Structure of FITC-AA. FITC was condensed with the arylacetamide 2-(3,4-dichlorophenyl)-N-methyl-N-[1-(3-aminophenyl)-2-(1-pyrrolidinyl)ethyl]acetamide as previously described (35). FITC-AA was a κ-opioid-selective ligand used to label the κ-opioid receptor on lymphocytes.
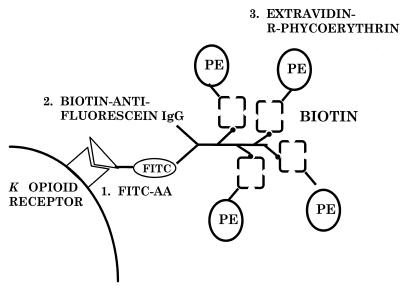
In order to detect the expression of κ-opioid receptors on a mixed lymphocyte population, such as thymocytes and splenocytes, an indirect immunofluorescence method that is more sensitive than radioligand binding assays was developed in this laboratory (35). By using a fluorescein-conjugated arylacetamide, a high-affinity κ-opioid agonist shown in Fig. 1, followed by amplification of FITC-AA binding with biotinylated antifluorescein immunoglobulin G and extravidin-R–phycoerythrin (Fig. 2), specific labeling of κ-opioid receptors on thymocytes from C57BL/6ByJ mice was detected by flow cytometry (31, 35). The κ-opioid selective antagonist nor-BNI inhibited greater than 50% of the phycoerythrin fluorescence, while μ- and δ-opioid-selective antagonists did not inhibit the labeling. Double-labeling experiments with fluorescent antibodies directed against the cell surface markers CD4 (T helper) and CD8 (T cytotoxic) and labeling of the κ-opioid receptor were performed with thymocytes from 6- to 8-week-old C57BL/6ByJ mice. Greater than 80% of the thymocytes were positive for both CD4 and CD8 cell surface markers (31). The κ-opioid receptor was expressed on greater than 60% of the CD4+/CD8+ thymocytes. That study demonstrated that the majority of mouse thymocytes express the κ-opioid receptor, but at a density that is too low to be detected by radioligand binding. These findings are consistent with those obtained with the R1.1 thymoma cell line, which expresses the κ-opioid receptor (8).
FIG. 2.
Amplification procedure used to detect κ-opioid receptors on cells from the immune system. Unfixed mouse thymocytes (31, 35), splenocytes (32), peritoneal macrophages (32), or human microglia (16) were incubated with FITC-AA followed by centrifugal washes to remove unbound FITC-AA. Biotinylated antifluorescein was then added, followed by washing of the cells. Finally, extravidin-R–phycoerythrin was added. Phycoerythrin fluorescence was measured by flow cytometry (35). This procedure amplified the signal by having multiple phycoerythrin molecules for each FITC-AA molecule that bound to the κ-opioid receptor. IgG, immunoglobulin G; PE, phycoerythrin.
To address whether mature lymphocytes expressed the κ-opioid receptor, unfixed primary splenocytes from 6- to 8-week-old C57BL/6ByJ male mice were incubated with the fluorescein-containing κ-opioid-selective ligand FITC-AA, as described above for the labeling of thymocytes. Amplification of FITC-AA binding to the κ-opioid receptor was attained by adding a biotin-conjugated antifluorescein antibody, followed by the addition of extravidin-R–phycoerythrin. As mentioned above, greater than 60% of immature thymocytes (CD4+/CD8+) demonstrated specific κ-opioid receptor labeling. However, less than 25% of either T-helper or T-cytotoxic splenic lymphocytes expressed the κ-opioid receptor (32). Likewise, only 16% of all splenic B lymphocytes expressed the κ-opioid receptor (32). These findings demonstrate a decrease in κ-opioid receptor expression upon maturation of mouse lymphocytes. However, recent studies have shown that mitogen activation of splenocytes increased κ-opioid receptor expression on both CD4+ and CD8+ cells, suggesting that the κ-opioid receptor may modulate the functions of activated T cells (7). Interestingly, resident peritoneal macrophages showed a greater magnitude of specific receptor labeling compared to either thymocytes or splenocytes, and approximately 50% of the resting macrophages expressed the κ-opioid receptor (32). Also, human microglia, the brain's macrophages, possess a high level of κ-opioid receptors, which have been shown to modulate HIV-1 expression in microglia (16). Taken together, these findings demonstrate the diversity in the expression of the κ-opioid receptor on immune cells at various stages of differentiation, with preferential expression demonstrated by thymocytes, resident peritoneal macrophages, and microglia. The detection of high levels of opioid receptors on peritoneal macrophages and microglia correlates with the modulation of TNF-α and IL-1 production by κ opioids (4).
FUNCTIONAL EVIDENCE FOR PRESENCE OF δ-OPIOID RECEPTORS ON LYMPHOCYTES
Methionine-enkephalin stimulated chemotaxis in human peripheral blood mononuclear and polymorphonuclear leukocytes (24, 63). Studies of human T-lymphocyte chemotaxis have shown that both leucine-enkephalin and methionine-enkephalin and the enkephalin analogs [d-Ala2, d-Leu5]enkephalin and [d-Pen2, d-Pen5]enkephalin stimulated chemotaxis (30). The stimulation of chemotaxis was concentration dependent and was inhibited by the opioid antagonist naloxone. Stefano et al. (58) observed that [d-Ala2, d-Met5]enkephalinamide stimulated immunocytes obtained from hemolymphs of the mollusc Mytilas edulis such that the cellular area was increased and the immunocytes clustered, with a peak effect achieved with the opioid peptide at 10 pM. These effects were blocked by naloxone. Similar effects were observed with other δ-selective opioid peptides, but the effects were not concentration dependent (59).
The expression of proenkephalin A mRNA by concanavalin A-stimulated thymocytes was modulated in a biphasic manner by the δ-opioid agonist deltorphin I (40). Deltorphin I concentrations between 10−13 and 10−11 M increased the level of proenkephalin A mRNA expression, while concentrations of 10−9 to 10−7 M inhibited proenkephalin A mRNA expression. The δ-opioid antagonists naltrindole and naltriben blocked both the enhancing and inhibiting effects of deltorphin I, suggesting the direct involvement of δ-opioid receptors. IL-2 secretion from CD4+ cells was also suppressed by δ-opioid agonists (52).
Both the endogenous enkephalin-like agonists produced by thymic T cells (39, 40) and the addition of δ-opioid selective peptides have been shown to exert complex effects on T-cell proliferation. Deltorphin at picomolar concentrations enhanced concanavalin A-stimulated splenocyte proliferation, an effect blocked by the δ-opioid selective antagonist naltrindole (11, 46). In contrast, three enkephalin analogs, including deltorphin, inhibited the proliferation of highly purified CD4+ and CD8+ murine T cells that were activated by cross-linking the T-cell receptor complex with anti-CD3-ɛ (52). This effect was blocked by the δ-opioid-selective antagonist naltrindole. In order to observe inhibition of T-cell proliferation, it was necessary to pretreat the purified lymphocytes with the δ-opioid peptides before the activation of the cells with anti-CD3-ɛ. In summary, these investigations suggest that murine T cells express the δ-opioid receptor and that activation of these receptors may enhance or inhibit T-cell proliferation, depending on the conditions, such as conditions in which purified cells versus accessory cells are present.
EVIDENCE FROM BINDING AND MOLECULAR STUDIES FOR PRESENCE OF δ-OPIOID RECEPTORS ON CELLS FROM IMMUNE SYSTEM
As with the κ-opioid receptor, the observation of classical brain-type δ opioid binding of a 3H-labeled δ-selective opioid ligand to a mixed population of lymphocytes has not been achieved. [3H]deltorphin binding to a single high-affinity binding site on membranes from human peripheral blood polymorphonuclear leukocytes has been reported (59). This high-affinity binding site for δ opioids also had a high affinity for the μ-opioid-selective peptide [d-Ala2, (Me)Phe4, Gly-(ol)5]enkephalin. This result brings into question whether the [3H]deltorphin binding site was the classical brain-type δ-opioid receptor binding site or a unique binding site.
Simian peripheral blood mononuclear cells express the δ-opioid receptor mRNA identical to the δ-opioid receptor mRNA expressed by brain cells (17). Another laboratory has reported that δ-opioid receptor transcripts were undetectable in human peripheral blood lymphocyte and monocyte populations after PCR amplification but were found at low levels in human T-cell, B-cell, and monocyte cell lines (26). In addition, δ-opioid receptor transcripts were found in murine splenocytes and on some B- and T-cell lines. Human peripheral blood lymphocytes and several human lymphoid cell lines expressed δ-opioid receptor transcripts that were nearly identical to the known sequence from the human brain (65). Sharp et al. (55) have reported that the sequence of a PCR transcript amplified from enriched mouse splenic and lymph node T cells had 98% identity with the mouse brain δ-opioid receptor (21).
FUNCTIONAL EVIDENCE FOR PRESENCE OF μ-OPIOID RECEPTORS ON LYMPHOCYTES
Many studies have used the prototypic ligand morphine to study the effect of this clinically relevant opiate on immune function. While being a μ-opioid-preferring ligand, morphine is not selective for the μ-opioid receptor. Morphine increased the rate of mortality among infected mice (15, 62). Also, morphine inhibited the cytolytic activity of natural killer cells and mitogen-stimulated proliferation (2, 3, 22, 64; Y. Shavit, F. C. Martin, L. H. Angarita, R. P. Gale, and J. C. Liebeskind, Soc. Neurosci. Abstr. 12:339, 1986). Morphine was shown to affect the brain-immune axis by modulating an IL-1β-dependent pathway (13). After chronic exposure in vivo, morphine attenuated lymphocyte proliferation (9), natural killer cell cytotoxicity (37; Shavit et al., Soc. Neurosci. Abstr. 12:339, 1986), antibody and serum hemolysin formation (28), and the phagocytic properties of peripheral mononuclear leukocytes (62). Morphine is known to activate the hypothalamic-pituitary-adrenal axis and release glucocorticoid, which is immunosuppressive (10). Therefore, not knowing if an effect is centrally mediated or peripherally mediated, or both, has complicated studies of chronic morphine administration.
EVIDENCE FROM BINDING AND MOLECULAR STUDIES FOR PRESENCE OF μ-OPIOID RECEPTORS ON CELLS FROM IMMUNE SYSTEM
Binding studies with lymphocytes suggest that morphine may bind to a site that is not the classical brain μ-opioid receptor (42, 50, 57). Morphine receptors expressed on resting thymocytes have a low affinity for morphine, with a Kd value of approximately 100 nM (48, 50). IL-1 activation of thymocytes increased the level of [3H]morphine binding to the thymocytes. The existence of a low-affinity, naloxone-insensitive morphine binding site designated μ3 on human peripheral blood macrophages has been reported by Makman et al. (43). Two morphine binding sites have been observed on the murine macrophage/monocyte cell line Bac 1.2F5 (50).
Sedqi et al. (51) were the first to report the existence of mRNA for the μ-opioid receptor on rat peritoneal macrophages. Chuang et al. (19) reported the presence of mRNA for the μ-opioid receptor in human T- and B-cell lines, CD4+ T cells, monocytes, macrophages, and granulocytes. In addition, transcripts have been found in simian peripheral blood mononuclear cells and granulocytes (19). Collectively, these investigations demonstrate that mRNAs for the κ-, μ-, and δ-opioid receptors are expressed on cells from the immune system.
CONCLUSION
By understanding the synthesis of opioid peptides by lymphocytes and the localization of the multiple opioid receptors on lymphocytes, the mechanisms involved in opioid-mediated regulation of immunocompetence will be determined. Although the roles of opiates and opioids in the physiological and pathological functions of the immune system are only beginning to be unraveled, multiple lines of evidence indicate that the opioid receptors expressed by immune cells are often the same or identical to the neuronal opioid receptors. Further identification and characterization of the receptors and the signal transduction pathways that account for some of the unique properties of opioid binding and immunomodulation represent major research challenges that lie ahead (56). Elucidation of mechanisms such as these may provide unique therapeutic opportunities through the application of opioid immunopharmacology.
ACKNOWLEDGMENTS
This work was supported by grants K05-DA00360 and DA04355 from the National Institute on Drug Abuse.
REFERENCES
- 1.Apte R N, Durum S K, Oppenheim J J. Opioids modulate interleukin-1 production and secretion by bone-marrow macrophages. Immunol Lett. 1990;24:141–148. doi: 10.1016/0165-2478(90)90026-m. [DOI] [PubMed] [Google Scholar]
- 2.Bayer B M, Daussin S, Hernandez M, Irvin L. Morphine inhibition of lymphocyte activity is mediated by an opioid dependent mechanism. Neuropharmacology. 1990;29:369–374. doi: 10.1016/0028-3908(90)90096-a. [DOI] [PubMed] [Google Scholar]
- 3.Bayer B M, Gastonguay M R, Hernandez M C. Distinction between the in vitro and in vivo inhibitory effects of morphine on lymphocyte proliferation based on agonist selectivity and naltrexone reversibility. Immunopharmacology. 1992;23:117–124. doi: 10.1016/0162-3109(92)90035-b. [DOI] [PubMed] [Google Scholar]
- 4.Belkowski S M, Alicea C, Eisenstein T K, Adler M W, Rogers T J. Inhibition of interleukin-1 and tumor necrosis factor-α synthesis following treatment of macrophages with the kappa opioid agonist U50,488H. J Pharmacol Exp Ther. 1995;273:1491–1496. [PubMed] [Google Scholar]
- 5.Belkowski S M, Zhu J M, Liu-Chen L-Y, Eisenstein T K, Adler M W, Rogers T J. Sequence of kappa-opioid receptor cDNA in the R1.1 thymoma cell line. J Neuroimmunol. 1995;62:113–117. doi: 10.1016/0165-5728(95)00116-j. [DOI] [PubMed] [Google Scholar]
- 6.Belkowski S M, Zhu J, Liu-Chen L-Y, Eisenstein T K, Adler M W, Rogers T J. Detection of kappa-opioid receptor mRNA in immature T-cells. Adv Exp Med Biol. 1995;373:11–16. doi: 10.1007/978-1-4615-1951-5_2. [DOI] [PubMed] [Google Scholar]
- 7.Bidlack, J. M., and M. K. Abraham. Mitogen-induced activation of mouse T cells increases kappa opioid receptor expression. Adv. Exp. Med. Biol., in press. [DOI] [PubMed]
- 8.Bidlack J M, Saripalli L D, Lawrence D M P. κ-Opioid binding sites on a murine lymphoma cell line. Eur J Pharmacol. 1992;227:257–265. doi: 10.1016/0922-4106(92)90003-e. [DOI] [PubMed] [Google Scholar]
- 9.Bryant H U, Bernton E W, Holaday J W. Immunosuppressive effects of chronic morphine treatment in mice. Life Sci. 1987;41:1731–1738. doi: 10.1016/0024-3205(87)90601-1. [DOI] [PubMed] [Google Scholar]
- 10.Bryant H U, Bernton E W, Kenner J R, Holaday J W. Role of adrenal cortical activation in the immunosuppressive effects of chronic morphine treatment. Endocrinology. 1991;128:3253–3258. doi: 10.1210/endo-128-6-3253. [DOI] [PubMed] [Google Scholar]
- 11.Caroleo M C, Arbitrio M, Melchiorri D, Nistico G. A reappraisal of the role of the various opioid receptor subtypes in cell-mediated immunity. Neuroimmunomodulation. 1994;1:141–147. doi: 10.1159/000097148. [DOI] [PubMed] [Google Scholar]
- 12.Carr D J J, DeCosta B R, Kim C-H, Jacobson A E, Guarcello V, Rice K C, Blalock J E. Opioid receptors on cells of the immune system: evidence for δ- and κ-classes. J Endocrinol. 1989;122:161–168. doi: 10.1677/joe.0.1220161. [DOI] [PubMed] [Google Scholar]
- 13.Chang S L, Moldow R L, House S D, Zadina J E. Morphine affects the brain-immune axis by modulating an interleukin-1 beta dependent pathway. Adv Exp Med Biol. 1996;402:35–42. doi: 10.1007/978-1-4613-0407-4_6. [DOI] [PubMed] [Google Scholar]
- 14.Chao C C, Gekker G, Hu S, Sheng W S, Portoghese P S, Peterson P K. Upregulation of HIV-1 expression in co-cultures of chronically infected promonocytes and human brain cells by dynorphin. Biochem Pharmacol. 1995;50:715–722. doi: 10.1016/0006-2952(95)00176-z. [DOI] [PubMed] [Google Scholar]
- 15.Chao C C, Sharp B M, Pomeroy C, Filice G A, Peterson P K. Lethality of morphine in mice infected with Toxoplasma gondii. J Pharmacol Exp Ther. 1990;252:605–609. [PubMed] [Google Scholar]
- 16.Chao C C, Gekker G, Hu S, Sheng W S, Bu D-F, Archer S, Bidlack J M, Peterson P K. Kappa opioid receptors in human microglia downregulate human immunodeficiency virus-1 expression. Proc Natl Acad Sci USA. 1996;93:8051–8056. doi: 10.1073/pnas.93.15.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang L F, Chuang T K, Killam K F, Jr, Chuang A J, Kung H, Yu L, Chuang R Y. Delta opioid receptor gene expression in lymphocytes. Biochem Biophys Res Commun. 1994;202:1291–1299. doi: 10.1006/bbrc.1994.2071. [DOI] [PubMed] [Google Scholar]
- 18.Chuang L F, Chuang T K, Killam K F, Jr, Qui Q, Wang X R, Lin J J, Kung H F, Sheng W, Chao C, Yu L, Chuang R Y. Expression of kappa opioid receptors in human and monkey lymphocytes. Biochem Biophys Res Commun. 1995;209:1003–1010. doi: 10.1006/bbrc.1995.1597. [DOI] [PubMed] [Google Scholar]
- 19.Chuang T K, Killam K F, Jr, Chuang L F, Kung H F, Sheng W S, Chao C C, Yu L, Chuang R Y. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995;216:922–930. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- 20.Clark J A, Liu L, Price M, Hersh B, Edelson M, Pasternak G W. Kappa opioid receptor multiplicity: evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J Pharmacol Exp Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- 21.Evans C J, Keith D E, Jr, Morrison H, Magendzo K, Edwards R H. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- 22.Fecho K, Dykstra L A, Lysle D T. Evidence for β-adrenergic receptor involvement in the immunomodulatory effects of morphine. J Pharmacol Exp Ther. 1993;265:1079–1087. [PubMed] [Google Scholar]
- 23.Fiorica E, Spector S. Opioid binding site in EL-4 thymoma cell line. Life Sci. 1988;42:199–206. doi: 10.1016/0024-3205(88)90683-2. [DOI] [PubMed] [Google Scholar]
- 24.Foris G, Medgyesi G A, Nagy J T, Varga Z. Concentration-dependent effect of met-enkephalin on human polymorphonuclear leukocytes. Ann N Y Acad Sci. 1987;496:151–157. doi: 10.1111/j.1749-6632.1987.tb35758.x. [DOI] [PubMed] [Google Scholar]
- 25.Foster J S, Moore R N. Dynorphin and related opioid peptides enhance tumoricidal activity mediated by murine peritoneal macrophages. J Leukoc Biol. 1987;42:171–174. doi: 10.1002/jlb.42.2.171. [DOI] [PubMed] [Google Scholar]
- 26.Gaveriaux C, Peluso J, Simonin F, LaForet J, Kieffer B. Identification of kappa- and delta-opioid receptor transcripts in immune cells. FEBS Lett. 1995;369:272–276. doi: 10.1016/0014-5793(95)00766-3. [DOI] [PubMed] [Google Scholar]
- 27.Guan L, Townsend R, Eisenstein T K, Adler M W, Rogers T J. Both T-cells and macrophages are target of κ-opioid-induced immunosuppression. Brain Behav Immun. 1994;8:229–240. doi: 10.1006/brbi.1994.1021. [DOI] [PubMed] [Google Scholar]
- 28.Gungor M, Genc E, Sogduyu H, Eroglu L, Koyuncuoglu H. Effect of chronic administration of morphine on primary immune response in mice. Experientia. 1980;36:1309–1310. doi: 10.1007/BF01969606. [DOI] [PubMed] [Google Scholar]
- 29.Hagi K, Uno K, Inaba K, Muramatsu S. Augmenting effect of opioid peptides on murine macrophage activation. J Neuroimmunol. 1994;50:71–76. doi: 10.1016/0165-5728(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 30.Heagy W, Laurance M, Cohen E, Finberg R. Neurohormones regulate T-cell function. J Exp Med. 1990;171:1625–1633. doi: 10.1084/jem.171.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ignatowski T A, Bidlack J M. Detection of kappa opioid receptors on mouse thymocyte phenotypic subpopulations as assessed by flow cytometry. J Pharmacol Exp Ther. 1998;284:298–306. [PubMed] [Google Scholar]
- 32.Ignatowski T A, Bidlack J M. Differential κ-opioid receptor expression on mouse lymphocytes at varying stages of maturation and on mouse macrophages after selective elicitation. J Pharmacol Exp Ther. 1999;290:863–870. [PubMed] [Google Scholar]
- 33.Lawrence D M P, Bidlack J M. Kappa opioid binding sites on the R1.1 murine lymphoma cell line: sensitivity to cations and guanine nucleotides. J Neuroimmunol. 1992;41:223–230. doi: 10.1016/0165-5728(92)90073-t. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence D M P, Joseph D B, Bidlack J M. Kappa opioid receptors expressed on three related thymoma cell lines. Differences in receptor-effector coupling. Biochem Pharmacol. 1995;49:81–89. doi: 10.1016/0006-2952(94)00440-w. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence D M P, El-Hamouly W, Archer S, Leary J F, Bidlack J M. Identification of κ opioid receptors in the immune system by indirect immunofluorescence. Proc Natl Acad Sci USA. 1995;92:1062–1066. doi: 10.1073/pnas.92.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence D M P, Hutchinson I, Seyed-Mozaffari A, Archer S, Bidlack J M. Fluorescent staining of kappa opioid receptors in the immune system using naltrexamine derivatives and phycoerythrin. J Immunol Methods. 1997;201:173–181. doi: 10.1016/s0022-1759(96)00223-2. [DOI] [PubMed] [Google Scholar]
- 37.Lefkowiz S S, Chiang C Y. Effects of certain abused drugs on hemolysin forming cells. Life Sci. 1975;17:1763–1768. doi: 10.1016/0024-3205(75)90458-0. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Zhu J, Chen C, Chen Y-W, DeRiel J K, Ashby B, Liu-Chen L-Y. Molecular cloning and expression of a rat kappa opioid receptor. Biochem J. 1993;295:629–633. doi: 10.1042/bj2950629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linner K M, Beyer H S, Sharp B M. Induction of the messenger ribonucleic acid for proenkephalin A in cultured murine CD4-positive thymocytes. Endocrinology. 1991;128:717–724. doi: 10.1210/endo-128-2-717. [DOI] [PubMed] [Google Scholar]
- 40.Linner K M, Quist H E, Sharp B M. Met-enkephalin-containing peptides encoded by proenkephalin A mRNA expressed in activated murine thymocytes inhibit thymocyte proliferation. J Immunol. 1995;154:5049–5060. [PubMed] [Google Scholar]
- 41.Mack K J, Lee M F, Weyhenmeyer J A. Effects of guanyl nucleotides and ions on kappa opioid binding. Brain Res Bull. 1985;14:301–306. doi: 10.1016/0361-9230(85)90189-3. [DOI] [PubMed] [Google Scholar]
- 42.Madden J J, Whaley W L, Ketelsen D. Opiate binding sites in the cellular immune system: expression and regulation. J Neuroimmunol. 1998;83:57–62. doi: 10.1016/s0165-5728(97)00221-x. [DOI] [PubMed] [Google Scholar]
- 43.Makman M H, Dvorkin B, Stefano G B. Murine macrophage cell lines contain μ3-opiate receptors. Eur J Pharmacol. 1995;273:5–6. doi: 10.1016/0014-2999(95)00002-3. [DOI] [PubMed] [Google Scholar]
- 44.Paterson S J, Robson L E, Kosterlitz H W. Control by cations of opioid binding in guinea pig brain membranes. Proc Natl Acad Sci USA. 1986;83:6216–6220. doi: 10.1073/pnas.83.16.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson P K, Molitor T W, Chao C C. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- 46.Portoghese P S, Sultana M, Takemori A E. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- 47.Radulovic J, Miljevic C, Djergovic D, Vujic V, Antic J, Von Hsrstein S, Jankovic B D. Opioid receptor-mediated suppression of humoral immune response in vivo and in vitro: involvement of κ opioid receptors. J Neuroimmunol. 1995;57:55–62. doi: 10.1016/0165-5728(94)00161-g. [DOI] [PubMed] [Google Scholar]
- 48.Roy S, Ge B L, Ramakrishan S, Lee N M, Loh H H. [3H]-morphine binding to thymocytes is enhanced by IL-1 stimulation. FEBS Lett. 1991;287:93–96. doi: 10.1016/0014-5793(91)80023-v. [DOI] [PubMed] [Google Scholar]
- 49.Roy S, Ramakrishnan S, Loh H H, Lee N M. Chronic morphine treatment selectively suppresses macrophage colony formation in bone marrow. Eur J Pharmacol. 1991;195:359–363. doi: 10.1016/0014-2999(91)90476-7. [DOI] [PubMed] [Google Scholar]
- 50.Roy S, Sedqi M, Ramakrishnan S, Barke R A, Loh H H. Differential effects of opioids on the proliferation of a macrophage cell line. Cell Immunol. 1996;169:271–277. doi: 10.1006/cimm.1996.0118. [DOI] [PubMed] [Google Scholar]
- 51.Sedqi M, Roy S, Ramakrishnan S, Elde R, Loh H H. Complementary DNA cloning of a μ-opioid receptor from rat peritoneal macrophages. Biochem Biophys Res Commun. 1995;209:563–574. doi: 10.1006/bbrc.1995.1538. [DOI] [PubMed] [Google Scholar]
- 52.Shahabi N A, Sharp B M. Antiproliferative effects of delta opioids on highly purified CD4(+) and CD8(+) murine T cells. J Pharmacol Exp Ther. 1995;273:1105–1113. [PubMed] [Google Scholar]
- 53.Sharp B M, Keane W F, Suh H J, Gekker G, Tsukayama D, Peterson P K. Opioid peptides rapidly stimulate superoxide production by human polymorphonuclear leukocytes and macrophages. Endocrinology. 1985;117:793–795. doi: 10.1210/endo-117-2-793. [DOI] [PubMed] [Google Scholar]
- 54.Sharp B M, Shahabi N A, Heagy W, McAllen K, Bell M, Huntoon C, McKeane D J. Dual signal transduction through delta opioid receptors in a transfected human T-cell line. Proc Natl Acad Sci USA. 1996;93:8294–8299. doi: 10.1073/pnas.93.16.8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharp B M, Shahabi N, McKean D, Li M D, McAllen K. Detection of basal levels and induction of delta opioid receptor mRNA in murine splenocytes. J Neuroimmunol. 1997;78:198–202. doi: 10.1016/s0165-5728(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 56.Sharp B M, Roy S, Bidlack J M. Evidence for opioid receptors on cells involved in host defense and the immune system. J Neuroimmunol. 1998;83:45–56. [PubMed] [Google Scholar]
- 57.Sibinga N E S, Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]
- 58.Stefano G B, Cadet P, Scharrer B. Stimulatory effects of opioid neuropeptides on locomotory activity and conformational changes in invertebrate and human immunocytes: evidence for a subtype of delta receptor. Proc Natl Acad Sci USA. 1989;86:6307–6311. doi: 10.1073/pnas.86.16.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stefano G B, Melchiorri P, Negri L, Hughes T K, Jr, Scharrer B. [d-Ala2]deltorphin I binding and pharmacological evidence for a special subtype of delta opioid receptor on human and invertebrate immune cells. Proc Natl Acad Sci USA. 1992;89:9316–9320. doi: 10.1073/pnas.89.19.9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taub D D, Eisenstein T K, Geller E B, Adler M W, Rogers T J. Immunomodulatory activity of μ- and κ-selective opioid agonists. Proc Natl Acad Sci USA. 1991;88:360–364. doi: 10.1073/pnas.88.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tosk J M, Grim J R, Kinback K M, Sale E J, Bozetti L P, Will A D. Modulation of chemiluminescence in a murine macrophage cell line by neuroendocrine hormones. Int J Immunopharmacol. 1993;15:615–620. doi: 10.1016/0192-0561(93)90079-e. [DOI] [PubMed] [Google Scholar]
- 62.Tubaro E, Borelli G, Croce C, Cavallo G, Santiangeli C. Effect of morphine on resistance to infection. J Infect Dis. 1983;148:656–666. doi: 10.1093/infdis/148.4.656. [DOI] [PubMed] [Google Scholar]
- 63.VanEpps D E, Saland L. β-Endorphin and met-enkephalin stimulate human peripheral blood mononuclear cell chemotaxis. J Immunol. 1984;132:3046–3053. [PubMed] [Google Scholar]
- 64.Weber R J, Pert A. The periaqueductal gray matter mediates opiate-induced immunosuppression. Science. 1989;245:188–190. doi: 10.1126/science.2749256. [DOI] [PubMed] [Google Scholar]
- 65.Wick M J, Minnerath S R, Roy S, Ramakrishnan S, Loh H H. Expression of alternate forms of brain opioid orphan receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Mol Brain Res. 1995;32:342–347. doi: 10.1016/0169-328x(95)00096-b. [DOI] [PubMed] [Google Scholar]
- 66.Yasuda K, Raynor K, Kong H, Breder C D, Takeda J, Reisine T, Bell G I. Cloning and functional comparison of κ and δ opioid receptors from mouse brain. Proc Natl Acad Sci USA. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu J, Chen C, Xue J C, Kunapuli S, DeRiel J K, Liu-Chen L-Y. Cloning of a human kappa opioid receptor from the brain. Life Sci. 1995;56:201–207. doi: 10.1016/0024-3205(94)00507-o. [DOI] [PubMed] [Google Scholar]