Abstract
Nanotechnology, the multidisciplinary field based on the exploitation of the unique physicochemical properties of nanoparticles (NPs) and nanoscale materials, has opened a new realm of possibilities for biological research and biomedical applications. The development and deployment of mRNA-NP vaccines for COVID-19, for example, may revolutionize vaccines and therapeutics. However, regulatory and ethical frameworks that protect the health and safety of the global community and environment are lagging, particularly for nanotechnology geared toward biological applications (ie, bionanotechnology). In this article, while not comprehensive, we attempt to illustrate the breadth and promise of bionanotechnology developments, and how they may present future safety and security challenges. Specifically, we address current advancements to streamline the development of engineered NPs for in vivo applications and provide discussion on nano–bio interactions, NP in vivo delivery, nanoenhancement of human performance, nanomedicine, and the impacts of NPs on human health and the environment.
Keywords: Dual-use science, Biotech industry, Code of conduct, Biosafety protection, Nanoparticles
Introduction
The convergence of nanomaterials and technology, inspired by the unique physicochemical properties of nanoparticles (NPs) and materials, has produced the multidisciplinary field of nanotechnology. NPs and nanomaterials range from 1 to 100 nm in at least 1 dimension but may be longer in the other 2.1,2 They can be produced naturally (eg, by degradation, weathering, or human activities such as burning fossil fuels) or synthetically; synthetic NPs and nanomaterials are often referred to as “engineered.”2
Nanomaterials research and development is driven globally by major players, including China, Europe, Russia, and the United States.3-6 In 2000, the United States created a government framework known as the National Nanotechnology Initiative (NNI) to seed the commercialization of nanotechnology.5,7 Since its inception, more than US$35 billion has been invested5,8 and NNI has served as global inspiration for other nations.9 For example, the European Union made a notable investment (US$1.35 billion) in the Graphene Flagship project.3,10,11 Likewise, Russia invested US$2.7 billion as of 2018, with a US$190 million net return; its investments are managed by the RUSNANO group,12 an entity that “implements state policy for the development of the nanoindustry in Russia, acting as a co-investor in nanotechnology projects, which have substantial economic or social potential.”13 The sum of nanotechnology investments in China are less clear,14,15 although between 2012 and 2017, China's Strategic Pioneering Program on Nanotechnology reportedly invested more than US$152 million.14
The increase in research and development funding has fueled extensive global scientific growth: a search for “nanotechnology” in Google Scholar displays over 1 million publications and patents in the last 2 decades. Nanotechnology publications and patents are being collated into publicly available databases to foster collaboration and increase transparency. Journals such as Data in Brief16 and Chemical Data Collections17 have created multiple open access repositories for raw experimental data. In addition, public databases (eg, NBI Knowledgebase,18,19 caNanoLab,18,20 The Nanodatabase,21 and the recently retired Nanomaterial Registry18,22) have been launched to share protocols, data, and literature among a diverse audience.18
The global community has recognized the need for an approach to nanotechnology regulation to supervise the ethical implementation of NPs and protect human health and the environment.23 However, relevant agencies, organizations, councils, and strategies are not globally cooperative and substantial gaps remain within existing national and international policy frameworks24-26 (Table 1). Current NP regulations typically use preexisting standards for microscale and macroscale materials. However, these may not be applicable to NPs57-60 since the physicochemical properties that make nanomaterials useful also make it difficult to extrapolate long-term effects on human and environmental health.1 Additional barriers to NP oversight include disagreements between regulatory committees on the definition of NPs,61 nanomaterial diversity and applications,62,63 and limitations in mass production of NPs that result in poor quality control.64
Table 1.
Collaborative Global and US Nanoparticle Oversight Frameworks
| International | ||||
|---|---|---|---|---|
| Organization/Council | Committee/Act/Strategy | Responsibilities/ Goals | Participating Countries | References |
| Canada–US Regulatory Cooperation Council | Develop consistent policies on NP oversight | Canada, United States | 27,28 | |
| Organisation for Economic Cooperation and Development | OECD Working Party | Understand properties and risks of NPs | Australia, Austria, Belgium, Canada, Chile, Colombia, Costa Rica, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Japan, Republic of Korea, Latvia, Lithuania, Luxembourg, Mexico, Netherlands, New Zealand, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, United Kingdom, United States | 27-29 |
| International Organization for Standardization | Technical Committee 229 | Establish NP standards | Great Britain, Switzerlanda | 27,30 |
| ASTM International | Committee E56 (Nanotechnology) | Establish NP standards | Canada, India, Italy, United States | 31,32 |
| Federal Ministry for Economic Affairs and Climate Action | Bundesanstalt für Materialforschung und -prüfung | Establish NP standards | Germany | 33 |
| International Electrotechnical Commission | Technical Committee 113 | Standardize nano-based electrotechnical products | Germany, Koreaa | 34,35 |
| Institute of Electrical and Electronics Engineers | Nanotechnology Council | Coordinate and advance nanotechnology | United Statesa | 36,37 |
| United States |
|
|
|
|
|---|---|---|---|---|
| Organization | Acts/Strategies | Responsibilities/ Goals | References | |
| American National Standards Institute Nanotechnology Standards Panel |
ANSI-NSP Nanotechnology Standards Database |
Establish NP standards |
38
|
|
| US Environmental Protection Agency |
Nanomaterial Research Strategy |
Study NPs that pose human and environmental risks |
39,40 |
|
| Toxic Substances and Control Act |
Review safety of new chemicals |
39,41,42 |
||
| Safe Drinking Water Act |
Regulate NPs materials in potable water supplies |
42,43 |
||
| Federal Insecticide, Fungicide, and Rodenticide Act |
Oversee NPs materials used as pesticides |
42,43 |
||
| Comprehensive Environmental Response, Compensation, and Liability Act |
Provide “Superfunds” to remediate hazardous orphan sites |
42,44 |
||
| Resource Conservation and Recovery Act |
Control hazardous waste from inception to grave |
42,46 |
||
| Clean Water Act |
Regulate emissions of materials into surface waters |
42,47 |
||
| Clean Air Act |
Regulate air emission of materials into air |
42,48 |
||
| Nanotechnology Task Force |
Determine regulatory approaches for nano-based products |
49
|
||
| US Food and Drug Administration | Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research; Center for Devices and Radiological Health Federal |
Regulate nano-based therapies, products, and devices |
28,50-52 |
|
| US National Institute for Occupational Safety and Health Administration | NIOSH Nanotechnology Research Center | Lead the health and safety initiative for nanotechnology | 53 |
Denotes countries represented by the committee and council members, where some positions are elected or appointed terms (as of August 2022). The International Organization for Standardization and the International Electrotechnical Commission have members in 16754 and 88 countries,55 respectively, while the Institute of Electrical and Electronics Engineers has chapters in more than 14 countries (as of August 2022).56 Note that this is not a comprehensive list of the international or US nanotechnology oversight frameworks. Abbreviations: ANSI, American National Standards Institute; IEC, International Electrotechnical Commission; NIOSH, National Institute for Occupational Safety and Health; NP, nanoparticle; NSP, Nanotechnology Standards Panel.
Due to the complexity of the problem, the overlap of nanotechnology with biological safety and security is easy to overlook. But nanotechnology is enabling new areas with the potential to directly impact human health, such as platform-based therapeutics and human performance enhancement, which makes it imperative that we consider these gaps in our understanding and regulations. In many ways, the issues associated with the increasing use of nanotechnology are similar to those raised by the increasing use of synthetic biology.65,66 Due to this, and the overlap of safety and security issues, the health security community should be aware of and proactive in addressing these concerns.
The following review is intended to provide a high-level overview of advances in relevant nanobiotechnology (ie, nanotechnology used for biomedical applications) research and development and offer insight into potential hazards that may arise. Given the breadth of current research and development, it is not intended to provide a comprehensive review of nanoscale science but focuses specifically on the promise and concerns associated with NPs for in vivo use, and outlines concerns with respect to biological safety and security. In this review, we refer to NP biological safety (or biosafety) as the risks to human and environmental health associated with unintentional NP exposure67; further, we describe NP biological security (or biosecurity) as the risks to human and environmental health caused by the nefarious application of nano-based technologies.65,68-70
Nanomaterials Are Ideally Suited for Biological Applications
NPs have broadly heterogeneous physicochemical properties such as size, shape, charge, porosity, chemical composition, surface morphology, and stability. They are often classified by their material composition with main classes including carbon-based, lipid-based, polymeric, semiconductor, metallic, and ceramic. This breadth of properties, combined with their small size, makes NPs ideally suited for biological applications (Table 2). NPs are significantly smaller than the average eukaryotic cell and can pass through biological barriers such as cell membranes, tissues, and organs. This is beneficial for applications such as bioimaging, gene therapy, and drug delivery.99
Table 2.
Applications of Nanoparticles for In Vivo Use
| Nanoparticles | Size (nm) | Applications | References |
|---|---|---|---|
| Carbon-based Fullerenes, nanotubes, graphene, carbon black |
0.7-300 | Biosensing, imaging and diagnostics, drug and gene delivery, antivirals, antimicrobial treatment, tissue engineering, therapeutics | 70-75 |
| Ceramic-based Silica, alumina, hydroxyapatite |
<50 | Imaging, drug delivery, catalysis, tissue engineering | 76-79 |
| Metal Gold, silver, iron, cobalt nanoparticles |
1-200 | Drug delivery, biosensing/imaging, therapeutics, biomedical enhancement, antivirals, antimicrobial treatments, antifungal therapies | 80-84 |
| Semiconductor Quantum dots, cadmium-telluride, indium phosphide |
2-50 | Imaging, biosensors | 85 |
| Polymeric Chitosan, dendrimers |
<15 | Imaging and diagnostics, biosensing, therapeutics, drug delivery, tissue engineering, antimicrobial treatments | 86-91 |
| Lipid Micelles, liposomes |
10-500 | Drug and gene delivery, imaging | 92-96 |
| Janusa | 0.7-500 | Drug and gene delivery, bioimaging and sensing, tissue engineering | 97,98 |
Signifies that Janus particles can be a combination of any chemical compositions listed above.
Nanoparticles Have Tunable Physicochemical Properties
NP properties can be manipulated and tuned for a desired function, such as the ability to carry and release a therapeutic chemical payload, fluoresce at a particular wavelength, or cross the blood–brain barrier, by utilizing typical design processes.100-102 The development of synthetic nanomaterials, especially those intended for in vivo use, is often lengthy103; however, recent advances may reduce the timescale via sophisticated production and screening techniques. For instance, an autonomous platform that leverages Darwinian evolution has demonstrated great utility in the synthesis of gold NPs with programmable shapes. The platform uses a robotic component to synthesize NPs, with spectroscopic analysis to ascertain shape. The spectroscopic analysis uses a genetic algorithm that makes autonomous decisions for the optimization of synthetic conditions to generate shapes of interest. The platform proceeds through cycles of evolution until the desired NP shape is achieved.104
High-throughput screening using modeling,18 dynamic evolution,105 and libraries103,106,107 are also being investigated. In 2019, researchers at Northwestern University and the Air Force Research Laboratory reported their method to screen megalibraries of millions of NPs with distinct composition and size.103 Gold and silver NPs were formulated into inks that were deposited onto a substrate array using a spray lithography technique. The resulting arrays were used as nanoreactors to catalyze the growth of carbon nanotubes. The catalytic activity of the nanoreactors was screened in a high-throughput fashion using Raman spectroscopy, enabling researchers to identify NPs with optimal catalytic activity based on the composition, size, and spatial distribution of NPs.103 High-throughput screening methods have been investigated for optimizing lipid or polymer nanoparticles for therapeutic delivery of proteins, oligonucleotides, small interfering RNA (siRNA), and messenger RNA (mRNA). These methods will ultimately reduce the time required to develop NP-stabilized therapeutics, such as mRNA and protein vaccines.
Nanoparticle Properties Dictate In Vivo Lifecycle
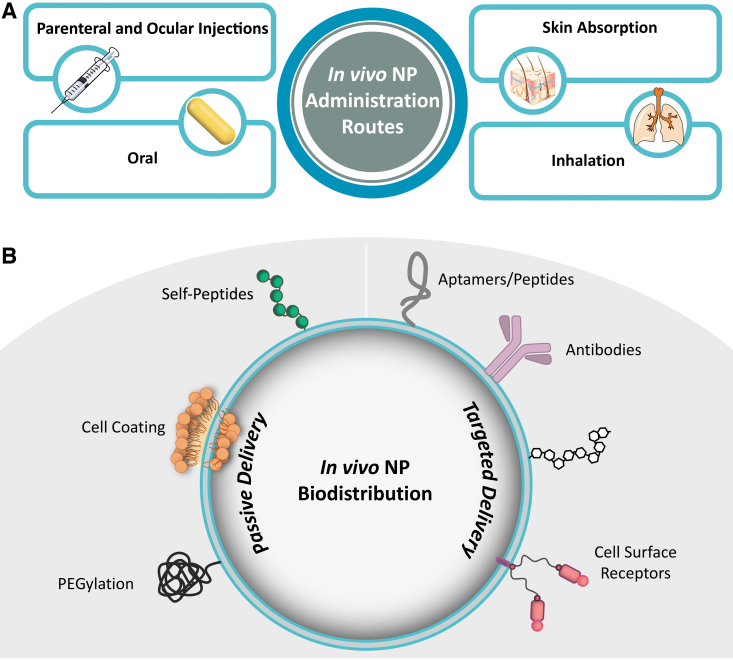
The physicochemical properties of NPs govern in vivo delivery, biodistribution, metabolism, and clearance, and dictate possible therapeutic applications.108 There are numerous reviews that evaluate how the physicochemical properties of nanomaterials influence nano–bio interactions.108-110 Delivery routes into the body for NPs are comparable to traditional routes: parenteral and ocular injections, skin absorption, inhalation, and oral delivery (Figure 1A).108,111-113 NP distribution within the body, or biodistribution, can be accomplished using passive or active delivery. Passive biodistribution relies on undirected (passive) delivery to the target.108,114 It can be enhanced by cloaking the material (eg, coating NPs with polyethylene glycol, also known as PEG)115 to prevent clearance from the body. Active biodistribution methods use targeting mechanisms (eg, carbohydrates or antibodies) to preferentially direct NPs to specific sites (Figure 1).114,116
Figure 1.
Overview of in vivo (A) administration routes of nanoparticles and (B) biodistribution strategies of nanoparticles (ie, passive and targeted delivery) using coatings. Note that passive delivery strategies demonstrated here rely on stealth coatings to bypass in vivo clearance mechanisms to increase circulation time. Abbreviation: NP, nanoparticle.
Metabolism and clearance of NPs is not completely understood, but is known to be facilitated by the kidneys, liver, mucosa, and so on.108,113 The ability of NPs to stabilize molecules is attractive for therapeutic delivery and in vivo applications; however, some NPs appear to persist indefinitely through encapsulation in tissues.113,117,118 As in vivo monitoring methods evolve, a better understanding of metabolism and clearance will be essential to enable efficient targeted distribution, and to ensure biosafety and biosecurity.
In Vivo Nanotechnology Holds Promise and Potential Concerns
Adoption of nano-based products for in vivo use has been slowed by the difficulty of assuring long-term safety. To date, long-term compatibility studies on the metabolism of NPs are sparse, stemming from issues with NP production and detection.108,119 Precise control of NP production is a challenge, resulting in NPs with heterogenous physicochemical properties.108 The range of properties complicates biocompatibility studies because different combinations of NPs in a payload could produce vastly different behavior in vivo. Further, traditional methods to study the metabolism of drugs are not sufficient to analyze the metabolic cycle of NPs. Carbon-based NPs, although attractive for in vivo applications, are arduous to differentiate from the carbon-rich environment of the surrounding tissue, complicating the study of biodistribution, metabolism, and clearance. In addition, nanomaterials often persist much longer than in vitro cell culture experiments and the lifetimes of model organisms, so full clearance from the model system may not be observed.119
Nanoparticle Technology to Advance Treatment of Human Disease
Nanomaterial development for in vivo applications has already generated sophisticated technology, such as NP-stabilized mRNA and protein vaccines for COVID-19. NPs have also been applied in research settings to facilitate genomic editing, alter drug potency, and manipulate the immune response.1,2 Here, we intend to highlight some interesting current and future nano-based technologies for nanomedicine, but this discussion is not intended to provide a comprehensive list of technologies, which are covered in other reviews.
Nanoparticles Can Enhance the Precision of Genomic Editing
Genomic editing offers the promise of a permanent solution to disease or disability as an alternative to surgery or medication. However, some clinical applications have been hindered by inefficient in vivo delivery of the gene editing machinery. Gene editing machinery, such as Clustered Regularly Interspaced Palindromic Repeats (CRISPR), consists of a large protein and nucleic acid component, neither of which readily cross cell membranes.120-123 Because NPs readily cross cell membranes, researchers have begun to use NPs to deliver gene editing constituents with greater efficacy.
Several comprehensive reviews detail the state of the art of NP-mediated gene editing.123-126 To briefly summarize, lipid-, polymeric-, and gold-based NPs are the most widely studied in vivo CRISPR delivery systems. Targeted delivery strategies have proven to be effective, and in some cases, have been designed so that stimuli (eg, magnetic fields) trigger the release of the CRISPR payload.124
NP-CRISPR systems have been designed to target specific cell-types, tissues, and organs in animal models.127 Strategies using NP-CRISPR systems have been developed to understand disease and improve treatment methods for genetic disorders,128 certain cancers,128,129 and other conditions. An interesting example of recent achievements in the NP-mediated delivery of CRISPR is a promising intrauterine gene editing method to treat mice that model human β-thalassemia (a blood disorder). The study demonstrated that gene editing using poly(lactic-co-glycolic acid) NPs encapsulating therapeutic payloads could treat disease even before birth.127
Nanoparticles Can Enhance or Decrease Drug Potency
Improving drug delivery systems via NPs is a major area of research. Multitudes of studies have demonstrated the use of NPs for superior, targeted delivery of therapeutics to enhance drug potency; the diversity of NPs used for therapeutic delivery is too extensive to summarize in a review, but include NP classes such as carbon-based, lipid-based, polymeric, and ceramic.128-135
One of the more interesting areas of nanobiotechnology drug delivery may be the use of NPs that can cross the blood–brain barrier, an inherently difficult endeavor. Most research in this area has focused on using polymeric or magnetic NPs to translocate therapeutics into the brain136-142 for greater efficacy in treating neurodegenerative diseases.140-142 The ability of NPs to cross the blood–brain barrier could revolutionize brain imaging and treatment for diseases like Alzheimer's disease and glioblastoma. However, the development of NPs to circumvent a relatively impermeable biological barrier raises significant peripheral concerns.
Nanoparticles Can Be Used to Modulate Immune Response
NPs can be engineered to avoid recognition from the immune system or to directly influence an immune response.143,144 For example, researchers have identified a way to slow the response of macrophages to prevent rapid clearance of foreign, polystyrene nanobeads.145 Tagging the NPs with peptides recognized by phagocytes as “self” allowed the NPs to evade immune system and exhibit greater persistence.
The NVX-CoV2373 vaccine pioneered by Novavax for COVID-19 was developed using a proprietary NP-mediated delivery system known as Matrix-M to enhance the immune response.146,147 These types of immune system modulation could ultimately be used to enhance drug delivery efficiency and imaging.
“Switchable” Nanoparticles
Numerous researchers have been investigating possibilities for “switching” the activity of an NP on or off in vivo. Methods that induce an NP to switch behavior between active and inactive states have been developed using intrinsic or extrinsic stimuli. Intrinsic switching methods include changes in internal homeostasis such as variations in pH, osmolarity, permeability, and enzymatic activity.148 Extrinsic switching can be achieved by thermal regulation, ultraviolet radiation, ultrasounds, or proximity to a magnetic source.149 Switching is an attractive feature for therapeutics but raises concerns, including unintended switching or malicious switch “hacking.”
Nanoparticle-Enabled Enhancement of Human Performance
Due to their tunable properties, NPs have been used to enhance chemical reactions and physical properties of materials in laboratory settings for decades. Their ability to cross biological membranes makes them equally promising for human performance enhancement. Researchers have been using NPs to enhance human senses and initiate cellular actions with great success. In an earlier section, we also discussed advances in “switching” nanoparticle activity, which may ultimately enable temporary or reversible enhancement.
Nanoparticles Can Facilitate Physiological Enhancements
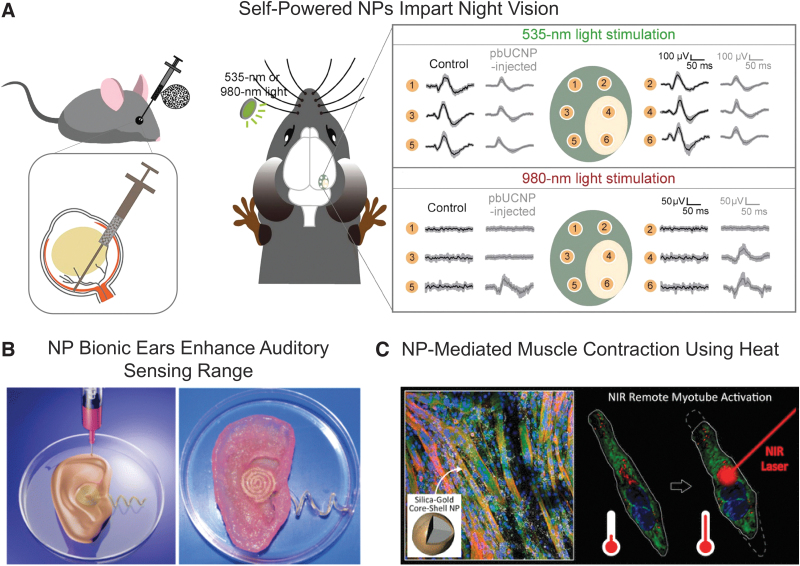
Enhancing or repairing damaged human senses (ie, smell, taste, touch, sight, hearing) is an active area of research. Multiple groups are investigating the use of NPs to enhance or repair vision. One set of researchers successfully imparted “night vision” to mice by injecting engineered NPs into the eye, where they bound to the photoreceptor cells in the retina. In vivo, the bound NPs acted as self-powered antennae that converted infrared light into perceptible vision with no impact upon day vision (Figure 2A).150 In 2018, a team at Bar-Ilan University reported nanomaterial-mediated vision repair: drops directly applied to the eye that repair near- and farsighted vision by altering the corneal refractive index, creating an alternative, possibly permanent, method to replace glasses, contact lenses, or surgery.151
Figure 2.
Nanomaterial studies that have enhanced physiological performance. (A) An engineered nanoparticle (pbUCNP) that imparts night vision in mice. Representation of pbUCNP injection into the eye, where it binds with photoreceptor cells in the retina. pbUCNP serves as a self-powered antenna that can be stimulated at 535 nm (day vision) and 980 nm (night vision). Reprinted with permission from Ma Y.150 (B) A 3D printed bionic ear composed of a hydrogel laden with cells and conductive nanoparticles coupled to electrodes for auditory sensing. The bionic ear demonstrated enhanced auditory sensing when compared with human hearing. Reprinted with permission from the American Chemical Society.152 (C) In vitro study with muscle cells doped with gold nanoshells (ie, NPs consisting of silica coated with a thin layer of gold), where the NPs induced muscle contraction with an externally applied heat source. Reprinted with permission from the American Chemical Society.156 Abbreviations: NP, nanoparticle; pbUCNP, photoreceptor-binding upconversion nanoparticle.
NPs are also being used to enhance hearing. A collaborative group at 2 academic universities developed a bionic ear with superior auditory sensing (Figure 2B).152 The researchers 3D-printed the ear using a cell- and conductive NP-laden hydrogel integrated with electrodes. The ear was able to detect radio frequencies well outside the normal range of human hearing. The team was also able to create complementary ears (right and left) that cooperated to listen to audible music. The bionic ears, while a proof-of-concept demonstration, could be used in the future for organ replacement or to enhance the range of auditory communication.
Physiological enhancements using NPs are not limited to the senses. NPs have been used to promote muscle recovery in in vivo animal models153-155 and enhance existing muscle function in vitro.156 Muscle recovery studies in mice have typically focused on using NPs as carriers and delivery systems for therapeutics that induce muscle repair such as mRNA,153 cytokines,154 and growth factors.155 However, a team of researchers used an in vitro muscle cell line to enhance muscle function using the intrinsic properties of gold nanoshells (NPs with a silica core coated in a thin layer of gold). The team showed that exposing nanoshell-doped muscle cells to near-infrared light (ie, heat) within physiological temperature ranges induced muscle contraction (Figure 2C). This wireless stimulation of muscle cells used a unique mechanism distinct from natural muscle contraction.156
Nanoparticles May Be Developed to Facilitate Cognitive Enhancements
The development and implementation of NPs that enhance cognitive function has yet to be realized. However, recent advances on the micro- and macro-level with neural–machine interfacing provide the building blocks necessary to develop this technology on the nanoscale. A noninvasive brain–computer interface to control a robotic arm was developed by teams at 2 universities.157 A US-based company, Neuralink, is at the forefront of implementing implantable, intracortical microelectrodes that provide an interface between the human brain and technology.158,159 Utilization of intracortical microelectrodes may ultimately provide thought-initiated access and control of computers and mobile devices, and possibly expand cognitive function by accessing underutilized areas of the brain.158
Nanobiotechnology Raises Biosafety and Biosecurity, Ethical, and Environmental Quandaries
Nanobiotechnology is enabling advances in genome editing and therapeutics stabilization and delivery and may one day enable modulation of the immune response and human performance enhancement. These are enormous scientific accomplishments; however, these technologies generate a litany of biological safety and security concerns as well as ethical issues. The scientific community is still grappling with the biosafety, biosecurity, ethics, and legality of CRISPR-enabled performance enhancement and “biohacking.” For example, many nations are apprehensive of human genome editing and ban its practice160,161; yet in 2018, a Chinese scientist announced the birth of 2 babies whose genomes were edited to be more resistant to HIV infection.161-163 In response, the World Health Organization called for development of an international governance framework for human genome editing.164 NP-enabled human biohacking brings a new aspect to this type of problem. Considering the breadth and complexity of the ethical concerns regarding in vivo use of NPs, we refer readers to the NanoEthics journal,165 which provides a multidisciplinary platform to discuss the ethical and social implications of NP technologies.
Nanobiotechnology Raises Biological Safety and Security Concerns
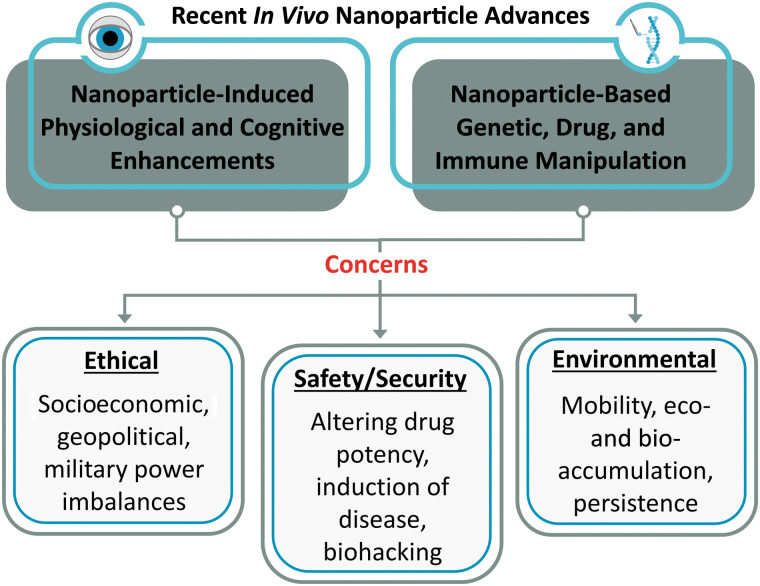
NPs have intrinsic properties that enable them to pass through biological barriers including cell membranes, organs, and the blood–brain barrier, and many researchers are working to enhance and direct these properties. While this work will enable advanced biomedical applications, it significantly increases biosafety concerns (Figure 3). Researchers handling NP-enhanced therapeutics should consider short-term and long-term effects of exposure to both the individual and combined materials. The mechanisms by which NPs are removed from the body are not completely understood; but, because NPs are often picked up by phagocytic cells, they have been postulated to produce unintended effects such as immunostimulation or immunosuppression, which could result in allergic reactions, chronic inflammation, and potential disease.166
Figure 3.
Ethical, safety and security, and environmental concerns arising from NP-mediated physiological or cognitive enhancements and NP-mediated manipulation of genetics, drugs, and the immune system. Abbreviation: NP, nanoparticle.
NPs that facilitate delivery of therapeutics across the blood–brain barrier also present unique biosafety security concerns. The molecular pathways that dictate cognition and memory formation are not completely understood, but research has implicated that small molecules (eg, formaldehyde) influence these pathways.167,168 Human health could be negatively impacted by unintentional or nefarious exposure to chemicals that inhibit memory167 or sequester chemicals needed for memory formation.168,169 NPs may also increase the permeability of the blood–brain barrier, which is associated with neurological disorders.
The ability to manipulate drug potency with NPs raises biological safety and security concerns. Drug delivery methods developed for therapeutics, such as pain relief, 170-172 ultimately could be adapted to trigger side effects or increase potency of commonly abused substances such as narcotics and opioids. With the drug epidemic at an all-time high, NP technology could be used to increase addiction numbers and the severity of a user's dependency, resulting in more overdose-related deaths. NPs could also be used to alter over-the-counter products to increase potency and the possibility of side effects.
Novel technologies are vulnerable to ethical asymmetries and unintended use, creating concerns that nanobiotechnology could be used to cause deliberate damage to human health. The capability to hack human health-related technology has already been demonstrated on the macro- and microscale. “White hacker” proofs by government agencies and academic institutions of medical devices such as magnetic resonance imaging (MRI) machines173 and implantables (eg, pacemakers, insulin pumps,173 and neurostimulators)174 enabled with wireless technology have created disquiet in the medical and security communities. Deliberate “hacking” of NPs that can be switched by thermal regulation, ultraviolet radiation, ultrasounds, or proximity to a magnetic source could be used to activate or deactivate a critical medical function. These types of biosecurity risks will continue to increase as NP-enabled medical technologies come to market.
Nanobiotechnology Raises Ethical Concerns
Numerous researchers have raised ethical issues associated with in vivo nanobiotechnology. In 2019, a special edition of the AMA Journal of Ethics explored a variety of issues associated with nanomedicine, ranging from helping patients understand the unknowns associated with NP-enabled medicines to identifying violations of individual privacy (eg, use of nanomedicine to track prescription drug compliance).175 More recently, the concentrated distribution of NP-stabilized COVID-19 vaccines in wealthy countries has been questioned.176 Like any advanced technology, nanobiotechnology has the potential to exacerbate socioeconomic imbalances. If used nefariously or to enhance human performance, it might also create significant geopolitical or military power imbalances (Figure 3).
Nanobiotechnology Raises Environmental Concerns
NPs are common constituents in cosmetic, electronic,177 optic, automotive,178 wound dressing,176,179 surgical equipment,179 and food products.180 As a result, they are commonly distributed throughout the environment. The extensive use of NPs in consumer goods raises concerns about mobility, accumulation, and persistence of nanomaterials in the environment (Figure 3).177 These concerns will be exacerbated by the use of NPs in in vivo applications.
Sunscreen has become a major source of unintentional NP pollution. Sunscreen contains titanium dioxide (TiO2) and zinc oxide (ZnO) NPs that reflect, scatter, and/or absorb ultraviolet rays.181 TiO2 and ZnO are considered safe for topical use182 because they are not soluble and do not absorb through the skin. However, the increasing use of NPs in sunscreens has resulted in the distribution and accumulation of TiO2 and ZnO in water and soils.183 While TiO2 and ZnO are considered safe for topical usage, chronic exposure to animals through inhalation and ingestion causes an onset of health issues that can lead to aggregation into tissues.184,185 TiO2 particles have been shown to cause oxidative stress that damages brain cells in model organisms.
In part due to their ubiquity and ease of production, ZnO and TiO2 NPs also are being considered for in vivo applications. There is great interest in using TiO2 as a photosensitizer for photodynamic therapy for diseases ranging from cancer to psoriasis.186 ZnO is being considered as an antitumor therapeutic, although the mechanism of toxicity in tumor cells is not well understood.187 While in vivo applications of these NPs are unlikely to drive pollution compared with sunscreen, these uses highlight both growing interest and the uncertainties associated with environmental accumulation of biologically active NPs.
Silver NPs have a long history of use for their biological activity, specifically their antimicrobial properties, and are in widespread use in products185,186 such as water filters,185 cosmetics,188,189 toothpaste,188 wound dressings, and surgical instruments.189 To date, silver NP safety has not been properly established,188 although an increasing number of studies have emphasized their toxicity189 and associated silver NP exposure with health risks188-191 such as encapsulation in lung tissue,189 oxidative stress, DNA damage,191 inflammation,189 and cognitive impairment.188
The increasing risk of engineered nanomaterial accumulation in the environment and unintended exposure has galvanized policymakers to begin the inception of regulatory NP policies. France banned the use of TiO2 in food products beginning in January 2020.192 In October 2021, the European Commission amended certification of certain TiO2 powders as a Category 2 suspected carcinogen.193 The Canadian General Standards Board implemented a more comprehensive approach to NP regulations, banning all NPs from the production and preparation processes of organic food.194 These policies do not address other families of nanomaterials or other consumer products.59
Ultimately, NP environmental pollution or deliberate contamination are also biosecurity concerns. Because NPs can aggregate in water and sediment, aquatic organisms used as food sources may accumulate contaminants, creating short- and long-term deleterious effects on the food chain and ecosystem. Accidental or deliberate environmental dispersal of NPs could also render areas unsafe for agricultural use.
Conclusion
The convergence of nanomaterials, technology, and biology holds tremendous promise. NPs enable superior strategies compared with traditional microscale materials for many biomedical applications, including gene therapy, drug delivery, and bioimaging/biosensing. Many of the same properties that make nanomaterials excellent candidates for in vivo use also raise biological safety and security concerns and could be intentionally exploited for harmful activities. Because of this potential for harm, awareness of concerns and threats arising from nano-based research is becoming increasingly important.
The recent widespread use of NP-stabilized vaccines for COVID-19 is just one example of the utility of nanobiotechnology to transform human health. However, the long-term biological safety and security effects of these technologies should be an active area of consideration, specifically in the realm of policy regulation. Unfortunately, regulation of nano-based technologies is not sufficient. The policy framework is often segmented by locale (eg, policies in the United States differ greatly from those in European countries), and within these segmented frameworks, there is a lack of interagency overlap to address the regulation of NPs. For example, in the United States, nanomedicines are regulated by the US Food and Drug Administration, but the persistence of these therapies and their effects on the environment is often not considered or regulated by the US Environmental Protection Agency. Thus, we suggest the development of a more cooperative, global policy framework that considers the heterogeneity and persistence of NP-based technologies.
The development of a global policy framework for nanobiotechnology will not be an easy feat and requires a proactive approach to continually identify short- and long-term biosafety and biosecurity risks associated with NP usage. The synthetic biology community has developed a unique approach to identify biological safety and security issues associated with new technologies using the International Genetically Engineered Machine (iGEM) competition. iGEM is set up broadly to cover synthetic biology as a whole and is governed by an experienced panel of judges and coaches and defined rules. Medical- and pharmaceutical-focused nanobiotechnology is a smaller field than what iGEM encompasses, and therefore adoption of a similar approach would need to be scaled and focused to be most effective.
References
- 1. Resnik DB. How should engineered nanomaterials be regulated for public and environmental health? AMA J Ethics. 2019;21(4):363-369. [DOI] [PubMed] [Google Scholar]
- 2. Singh AK. Structure, synthesis, and application of nanoparticles. In: Singh AK, ed. Engineered Nanoparticles. Boston: Academic Press; 2016:19-76. [Google Scholar]
- 3. Jackman JA, Cho DJ, Lee J, et al. Nanotechnology education for the global world: training the leaders of tomorrow. ACS Nano. 2016;10(6):5595-5599. [DOI] [PubMed] [Google Scholar]
- 4. Porter AL, Garner J, Newman NC, et al. National nanotechnology research prominence. Technol Anal Strateg Manag. 2019;31(1):25-39. [Google Scholar]
- 5. Sargent JF Jr. Nanotechnology: A Policy Primer. Washington DC: Congressional Research Service; 2016. Accessed July 11, 2022. https://sgp.fas.org/crs/misc/RL34511.pdf
- 6. European Commission. Advanced Technologies for Industry: Report on Technology Trends and Technology Adoption – Final Report. Luxembourg: Publications Office of the European Union; 2021 Accessed July 22, 2022. https://ati.ec.europa.eu/sites/default/files/2021-10/ATI%20Final%20Report%20on%20technology%20trends%20and%20technology%20adoption.pdf
- 7. National Science and Technology Council. National Nanotechnology Initiative: The Initiative and its Implementation Plan. Washington DC: Office of Science and Technology Policy; 2000. Accessed August 4, 2022. https://www.nano.gov/sites/default/files/pub_resource/nni_implementation_plan_2000.pdf
- 8. Subcommittee on Nanoscale Science, Engineering, and Technology. The National Nanotechnology Initiative Supplement to the President's 2022 Budget. Washington, DC: National Science and Technology Council; 2022. Accessed July 22, 2022. https://www.nano.gov/sites/default/files/pub_resource/NNI-FY22-Budget-Supplement.pdf
- 9. Roco MC, Hersam MC, Mirkin CA. Nanotechnology Research Directions for Societal Needs in 2020: Retrospective and Outlook. New York: Springer; 2011. [Google Scholar]
- 10. Peplow M. Graphene: the quest for supercarbon. Nature. 2013;503(7476):327-329. [DOI] [PubMed] [Google Scholar]
- 11. Graphene Flagship. Funding. Accessed August 2, 2022. https://graphene-flagship.eu/research/funding/
- 12. KPMG. JSC RUSNANO International Financial Reporting Standards Consolidated Financial Statements and Independent Auditor's Report. Moscow: KPMG; 2018. Accessed August 4, 2022. https://www.rusnano.com/upload/normativedocs/RUSNANO_IFRS_2018_ENG.pdf
- 13. Zvonareva O. Risky economies: innovation of medical devices in Russia. In: Zvonareva O. Health, Technologies, and Politics in Post-Soviet Settings: Navigating Uncertainties. London: Palgrave Macmillan; 2018:89-116. [Google Scholar]
- 14. Qiu J. Nanotechnology development in China: challenges and opportunities. Natl Sci Rev. 2016;3(1):148-152. [Google Scholar]
- 15. O'Meara S. Small science grows large in new hands. Nature. 2018;564(7735):S65-S66. [DOI] [PubMed] [Google Scholar]
- 16. Data in Brief. Accessed September 19, 2022. https://www.sciencedirect.com/journal/data-in-brief
- 17. Chemical Data Collections. Accessed September 19, 2022. https://www.sciencedirect.com/journal/chemical-data-collections
- 18. Bai X, Liu F, Liu Y, et al. Toward a systematic exploration of nano-bio interactions. Toxicol Appl Pharmacol. 2017;323:66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NBI Knowledgebase. Nanomaterial–Biological Interactions Knowlegebase. Accessed December 17, 2019. https://nbi.oregonstate.edu/
- 20. National Cancer Institute Center for Biomedical Informatics and Information Technology. caNanoLab. Accessed September 19, 2022. https://cananolab.nci.nih.gov/caNanoLab/
- 21. The Nanodatabase. About us. Accessed July 22, 2022. https://nanodb.dk/en/about-us/
- 22. Ostraat ML, Mills KC, Guzan KA, Murry D. The Nanomaterial Registry: facilitating the sharing and analysis of data in the diverse nanomaterial community. Int J Nanomed. 2013;8:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park HG, Yeo MK. Nanomaterial regulatory policy for human health and environment. Mol Cell Toxicol. 2016;12(3):223-236. [Google Scholar]
- 24. Justo-Hanani R, Dayan T. European risk governance of nanotechnology: explaining the emerging regulatory policy. Res Policy. 2015;44(8):1527-1536. [Google Scholar]
- 25. Trump BD, Keisler JM, Galaitsi SE, Palma-Oliveira JM, Linkov I. Safety-by-design as a governance problem. Nano Today. 2020;35:100989. [Google Scholar]
- 26. Stone V, Führ M, Feindt PH, et al. The essential elements of a risk governance framework for current and future nanotechnologies. Risk Anal. 2018;38(7):1321-1331. [DOI] [PubMed] [Google Scholar]
- 27. United States Environmental Protection Agency. Control of nanoscale materials under the Toxic Substances Control Act. Accessed September 19, 2022. https://www.epa.gov/reviewing-new-chemicals-under-toxic-substances-control-act-tsca/control-nanoscale-materials-under
- 28. Government of Canada. Canada–United States Regulatory Cooperation Council initiative on chemicals management. Updated October 6, 2017. Accessed September 19, 2022. https://www.canada.ca/en/health-canada/services/chemical-substances/chemicals-management-plan/canada-united-states-regulatory-cooperation-council.html
- 29. Organisation for Economic Co-operation and Development. Our global reach. Accessed August 4, 2022. https://www.oecd.org/about/members-and-partners/
- 30. International Organization for Standardization (ISO). ISO/TC 229 – Nanotechnologies. Accessed September 19, 2022. https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/committee/38/19/381983.html
- 31. ASTM International. Committee E56 on nanotechnology. Accessed September 19, 2022. https://www.astm.org/COMMITTEE/E56.htm
- 32. ASTM International. Committee E56 officers and staff support. Accessed August 4, 2022. https://www.astm.org/get-involved/technical-committees/committee-e56/officers-e56
- 33. Bundesanstalt für Materialforschung und -prüfung. About us. Accessed September 19, 2022. https://www.bam.de/Navigation/EN/About-us/about-us.html
- 34. International Electrotechnical Commission. TC 113 Nanotechnology for electrotechnical products and systems: TC 113 scope. Accessed September 19, 2022. https://www.iec.ch/dyn/www/f?p=103:7:16064114749424::::FSP_ORG_ID,FSP_LANG_ID:1315,25
- 35. International Electrotechnical Commission. TC 113 Nanotechnology for electrotechnical products and systems: TC 113 structure. Accessed August 4, 2022. https://www.iec.ch/dyn/www/f?p=103:29:615878171854764::::FSP_ORG_ID,FSP_LANG_ID:1315,25#3
- 36. IEEE NANO. IEEE Nanotechnology Council Advancing Nanotech for Humanity: about NTC. Accessed September 19, 2022. https://ieeenano.org/
- 37. IEEE NANO. IEEE Nanotechnology Council Advancing Nanotech for Humanity: officers. Accessed August 4, 2022. https://ieeenano.org/officers
- 38. American National Standards Institute (ANSI). ANSI Nanotechnology Standards Panel (ANSI-NSP). Accessed August 8, 2022. https://www.ansi.org/standards-coordination/collaboratives-activities/nanotechnology-panel
- 39. United States Environmental Protection Agency. Research on nanomaterials. Updated June 1, 2022. Accessed September 19, 2022. https://www.epa.gov/chemical-research/research-nanomaterials
- 40. United States Environmental Protection Agency Office of Research and Development. Nanotechnology & nanomaterials research. Accessed August 8, 2022. https://www.epa.gov/sites/default/files/2013-12/documents/nanotechnology-fact-sheet.pdf
- 41. United States Environmental Protection Agency. Summary of the Toxic Substances Control Act. Updated October 22, 2021. Accessed September 19, 2022. https://www.epa.gov/laws-regulations/summary-toxic-substances-control-act
- 42. United States Environmental Protection Agency Office of Land and Emergency Management. Technical fact sheet – nanomaterials. Published November 2017. Accessed August 8, 2022. https://www.epa.gov/sites/default/files/2014-03/documents/ffrrofactsheet_emergingcontaminant_nanomaterials_jan2014_final.pdf
- 43. United States Environmental Protection Agency. Safe Drinking Water Act (SDWA). Updated July 14, 2022. Accessed September 19, 2022. https://www.epa.gov/sdwa
- 44. United States Environmental Protection Agency. Summary of the Federal Insecticide, Fungicide, and Rodenticide Act. Last updated September 12, 2022. Accessed September 19, 2022. https://www.epa.gov/laws-regulations/summary-federal-insecticide-fungicide-and-rodenticide-act
- 45. United States Environmental Protection Agency. Summary of the Comprehensive Environmental Response, Compensation, and Liability Act (Superfund). Updated September 12, 2022. Accessed September 19, 2022. https://www.epa.gov/laws-regulations/summary-comprehensive-environmental-response-compensation-and-liability-act
- 46. United States Environmental Protection Agency. Summary of the Resource Conservation and Recovery Act. Updated September 12, 2022. Accessed September 19, 2022. https://www.epa.gov/laws-regulations/summary-resource-conservation-and-recovery-act
- 47. United States Environmental Protection Agency. Summary of the Clean Water Act. Updated July 6, 2022. Accessed September 19, 2022. https://www.epa.gov/laws-regulations/summary-clean-water-act
- 48. United States Environmental Protection Agency. Summary of the Clean Air Act. Updated September 12, 2022. Accessed September 19, 2022. https://www.epa.gov/laws-regulations/summary-clean-air-act
- 49. US Food and Drug Administration. Nanotechnology Task Force. Updated February 23, 2021. Accessed August 8, 2022. https://www.fda.gov/science-research/nanotechnology-programs-fda/nanotechnology-task-force
- 50. US Food and Drug Administration. Center for Drug Evaluation and Research | CDER. Updated June 21, 2022. Accessed September 19, 2022. https://www.fda.gov/about-fda/fda-organization/center-drug-evaluation-and-research-cder
- 51. US Food and Drug Administration. Center for Biologics Evaluation and Research (CBER). Updated June 29, 2022. Accessed September 19, 2022. https://www.fda.gov/about-fda/fda-organization/center-biologics-evaluation-and-research-cber
- 52. US Food and Drug Administration. Center for Devices and Radiological Health. Updated February 3, 2022. Accessed September 19, 2022. https://www.fda.gov/about-fda/fda-organization/center-devices-and-radiological-health
- 53. National Institute for Occupational Safety and Health. Nanotechnology. Updated March 27, 2020. Accessed September 19, 2022. https://www.cdc.gov/niosh/topics/nanotech/default.html
- 54. International Organization for Standardization. Members. Accessed August 4, 2022. https://www.iso.org/members.html
- 55. International Electrotechnical Commission. National committees. Accessed August 4, 2022. https://www.iec.ch/national-committees#nclist
- 56. IEEE NANO. IEEE Nanotechnology Council Advancing Nanotech for Humanity: chapters & regional activities. Accessed August 8, 2022. https://ieeenano.org/technical-activities/chapters
- 57. Ridge S. A regulatory framework for nanotechnology. Homel Secur Aff. 2018;16. [Google Scholar]
- 58. Helmus MN. The need for rules and regulations. Nat Nanotechnol. 2007;2(6):333-334. [DOI] [PubMed] [Google Scholar]
- 59. Faunce T, Watal A. Nanosilver and global public health: international regulatory issues. Nanomed. 2010;5(4):617-632. [DOI] [PubMed] [Google Scholar]
- 60. Sarmento B. Have nanomedicines progressed as much as we'd hoped for in drug discovery and development? Expert Opin Drug Discov. 2019;14(8):723-725. [DOI] [PubMed] [Google Scholar]
- 61. Taylor AA, Schierz A, Freeman EL. New nanomaterial regulations require detailed information from industry. Exponent Environmental Perspectives Newsletter. Published June 2017. Accessed June 13, 2022. https://www.exponent.com/~/media/news-events-alerts/alerts/2017/06/new-nanomaterial-regulations/new-nanomaterial-regulations-require-detailed-information-from-industry.pdf
- 62. Hua S, de Matos MBC, Metselaar JM, Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heller DA, Jena PV, Pasquali M, et al. Banning carbon nanotubes would be scientifically unjustified and damaging to innovation. Nat Nanotechnol. 2020;15(3):164-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Richman EK, Hutchison JE. The nanomaterial characterization bottleneck. ACS Nano. 2009;3(9):2441-2446. [DOI] [PubMed] [Google Scholar]
- 65. Trump BD, Galaitsi SE, Appleton E, et al. Building biosecurity for synthetic biology. Mol Syst Biol. 2020;16(7):e9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stemerding D, Betten W, Rerimassie V, Robaey Z, Kupper F. Future making and responsible governance of innovation in synthetic biology. Futures. 2019;109:213-226. [Google Scholar]
- 67. Su H, Wang Y, Gu Y, Bowman L, Zhao J, Ding M. Potential applications and human biosafety of nanomaterials used in nanomedicine. J Appl Toxicol. 2018;38(1):3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Trump BD, Florin MV, Perkins E, Linkov I, eds. Emerging Threats of Synthetic Biology and Biotechnology: Addressing Security and Resilience Issues. Dordrecht, Netherlands: Springer Nature, 2021. [PubMed] [Google Scholar]
- 69. Linkov I, Trump BD, Anklam E, et al. Comparative, collaborative, and integrative risk governance for emerging technologies. Environ Syst Decis. 2018;38(2):170-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cha C, Shin SR, Annabi N, Dokmeci MR, Khademhosseini A. Carbon-based nanomaterials: multi-functional materials for biomedical engineering. ACS Nano. 2013;7(4):2891–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Loh KP, Ho D, Chiu GNC, Leong DT, Pastorin G, Chow EK-H. Clinical applications of carbon nanomaterials in diagnostics and therapy. Adv Mater. 2018;30(47):e1802368. [DOI] [PubMed] [Google Scholar]
- 72. d'Amora M, Giordani S. Carbon Nanomaterials for Nanomedicine. In: Ciofani G, ed. Smart Nanoparticles for Biomedicine. Amsterdam: Elsevier, 2019;103-113. [Google Scholar]
- 73. Cui X, Xu S, Wang X, Chen C. The nano-bio interaction and biomedical applications of carbon nanomaterials. Carbon. 2018;138:436-450. [Google Scholar]
- 74. Durairaj S, Sidhureddy B, Cirone J, Chen A. Nanomaterials-based electrochemical sensors for in vitro and in vivo analyses of neurotransmitters. Appl Sci. 2018;8(9):1504. [Google Scholar]
- 75. Rahmati M, Mozafari M. Biological response to carbon-family nanomaterials: interactions at the nano-bio interface. Front Bioeng Biotechnol. 2019;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Singh D, Singh S, Sahu J, Srivastava S, Singh MR. Ceramic nanoparticles: recompense, cellular uptake and toxicity concerns. Artif Cells Nanomedicine Biotechnol. 2016;44(1):401-409. [DOI] [PubMed] [Google Scholar]
- 77. Baino F. Bioactive glasses and glass-ceramics for ophthalmological applications. In: Kaur G, ed. Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses. Duxford, UK: Woodhead Publishing; 2019:357-382. [Google Scholar]
- 78. Madhumathi K, Rubaiya Y, Doble M, Venkateswari R, Sampath Kumar TS. Antibacterial, anti-inflammatory, and bone-regenerative dual-drug-loaded calcium phosphate nanocarriers—in vitro and in vivo studies. Drug Deliv Transl Res. 2018;8(5):1066-1077. [DOI] [PubMed] [Google Scholar]
- 79. Du X, Fu S, Zhu Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: an overview. J Mater Chem B. 2018;6(27):4397-4412. [DOI] [PubMed] [Google Scholar]
- 80. Anderson SD, Gwenin VV, Gwenin CD. Magnetic functionalized nanoparticles for biomedical, drug delivery and imaging applications. Nanoscale Res Lett. 2019;14:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhao X, Zhou L, Rajoka MSR, et al. Fungal silver nanoparticles: synthesis, application and challenges. Crit Rev Biotechnol. 2018;38(6):817-835. [DOI] [PubMed] [Google Scholar]
- 82. Evans ER, Bugga P, Asthana V, Drezek R. Metallic nanoparticles for cancer immunotherapy. Mater Today. 2018;21(6):673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Singh P, Pandit S, Mokkapati VRSS, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7):1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thota S, Crans DC, eds. Metal Nanoparticles: Synthesis and Applications in Pharmaceutical Sciences. Weinheim, Germany: Wiley-VCH; 2018. [Google Scholar]
- 85. Granada-Ramírez DA, Arias-Cerón JS, Rodriguez-Fragoso P, et al. Quantum dots for biomedical applications. In: Narayan R, ed. Nanobiomaterials: Nanostructured Materials for Biomedical Applications. Duxford, UK: Woodhead Publishing; 2018:411-436. [Google Scholar]
- 86. Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr Polym. 2010;82(2):227-232. [Google Scholar]
- 87. Sun H, Hong Y, Xi Y, Zou Y, Gao J, Du J. Synthesis, self-assembly, and biomedical applications of antimicrobial peptide–polymer conjugates. Biomacromolecules. 2018;19(6):1701-1720. [DOI] [PubMed] [Google Scholar]
- 88. Kalantari K, Afifi A, Jahangirian H, Webster TJ. Biomedical applications of chitosan electrospun nanofibers as a green polymer – review. Carbohydr Polym. 2019;207:588-600. [DOI] [PubMed] [Google Scholar]
- 89. Aguilar MR, San Román J, eds. Smart Polymers and Their Applications. 2nd ed. Duxford, UK: Woodhead Publishing, 2019. [Google Scholar]
- 90. Kirillova A, Ionov L. Shape-changing polymers for biomedical applications. J Mater Chem B. 2019;7(10):1597-1624. [DOI] [PubMed] [Google Scholar]
- 91. Singh G, Faruk A, Bedi PMS. Technology overview and current biomedical application of polymeric nanoparticles. J Drug Deliv Ther. 2018;8(6):285-295. [Google Scholar]
- 92. Spicer CD, Jumeaux C, Gupta B, Stevens MM. Peptide and protein nanoparticle conjugates: versatile platforms for biomedical applications. Chem Soc Rev. 2018;47(10):3574-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Khan HA, Sakharkar MK, Nayak A, Kishore U, Khan A. Nanoparticles for biomedical applications: an overview. In: Narayan R, ed. Nanobiomaterials: Nanostructured Materials for Biomedical Applications. Duxford, UK: Woodhead Publishing; 2018:357-384. [Google Scholar]
- 94. Ball RL, Hajj KA, Vizelman J, Bajaj P, Whitehead KA. Lipid nanoparticle formulations for enhanced co-delivery of SiRNA and mRNA. Nano Lett. 2018;18(6):3814-3822. [DOI] [PubMed] [Google Scholar]
- 95. Zahin N, Anwar R, Tewari D, et al. Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res Int. 2020;27(16):19151-19168. [DOI] [PubMed] [Google Scholar]
- 96. Wallyn J, Anton N, Akram S, Vandamme TF. Biomedical imaging: principles, technologies, clinical aspects, contrast agents, limitations and future trends in nanomedicines. Pharm Res. 2019;36(6):78. [DOI] [PubMed] [Google Scholar]
- 97. Agrawal G, Agrawal R. Janus nanoparticles: recent advances in their interfacial and biomedical applications. ACS Appl Nano Mater. 2019;2(4):1738-1757. [Google Scholar]
- 98. Fan X, Yang J, Loh XJ, Li Z. Polymeric Janus nanoparticles: recent advances in synthetic strategies, materials properties, and applications. Macromol Rapid Commun. 2019;40(5):e1800203. [DOI] [PubMed] [Google Scholar]
- 99. Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9:1050-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ashby MF, Ferreira PJ, Schodek DL. The design context. In: Ashby MF, Ferreira PJ, Schodek DL, eds. Nanomaterials, Nanotechnologies and Design. Boston: Butterworth-Heinemann; 2009:41-86. [Google Scholar]
- 102. Shen Z, Nieh MP, Li Y. Decorating nanoparticle surface for targeted drug delivery: opportunities and challenges. Polymers (Basel). 2016;8(3):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kluender EJ, Hedrick JL, Brown KA, et al. Catalyst discovery through megalibraries of nanomaterials. Proc Natl Acad Sci U S A. 2019;116(1):40-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Salley D, Keenan G, Grizou J, Sharma A, Martín S, Cronin L. A nanomaterials discovery robot for the Darwinian evolution of shape programmable gold nanoparticles. Nat Commun. 2020;11(1):2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lambert B, Gillen AJ, Schuergers N, Wu S-J, Boghossian AA. Directed evolution of the optoelectronic properties of synthetic nanomaterials. Chem Commun (Camb). 2019;55(22):3239-3242. [DOI] [PubMed] [Google Scholar]
- 106. Sago CD, Lokugamage MP, Islam FZ, Krupczak BR, Sato M, Dahlman JE. Nanoparticles that deliver RNA to bone marrow identified by in vivo directed evolution. J Am Chem Soc. 2018;140(49):17095-17105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chen P-C, Liu X, Hedrick JL, et al. Polyelemental nanoparticle libraries. Science. 2016;352(6293):1565-1569. [DOI] [PubMed] [Google Scholar]
- 108. Bourquin J, Milosevic A, Hauser D, et al. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv Mater. 2018;30(19):e1704307. [DOI] [PubMed] [Google Scholar]
- 109. Singh AV, Laux P, Luch A, et al. Review of emerging concepts in nanotoxicology: opportunities and challenges for safer nanomaterial design. Toxicol Mech Methods. 2019;29(5):378-387. [DOI] [PubMed] [Google Scholar]
- 110. Navya PN, Daima HK. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Holban A-M, Grumezescu AM, eds. Materials for Biomedical Engineering: Nanomaterials-Based Drug Delivery. Amsterdam: Elsevier; 2019. [Google Scholar]
- 112. Rizvi SAA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26(1):64-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhong H, Chan G, Hu Y, Hu H, Ouyang D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018;10(4):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Aguilar ZP. Targeted drug delivery. In: Aguilar ZP. Nanomaterials for Medical Applications. Amsterdam: Elsevier; 2013:181-234. [Google Scholar]
- 115. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Van Haute D, Berlin JM. Challenges in realizing selectivity for nanoparticle biodistribution and clearance: lessons from gold nanoparticles. Ther Deliv. 2017;8(9):763-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rabiee M, Rabiee N, Salarian R, Rabiee G. Introduction to Nanomaterials in Medicine. San Rafael, CA: Morgan & Claypool Publishers; 2019. [Google Scholar]
- 118. Yan L, Zhao F, Wang J, Zu Y, Gu Z, Zhao Y. A safe-by-design strategy towards safer nanomaterials in nanomedicines. Adv Mater. 2019;31(45):e1805391. [DOI] [PubMed] [Google Scholar]
- 119. Arnold AM, Holt BD, Tang C, Sydlik SA. Phosphate modified graphene oxide: long-term biodegradation and cytocompatibility. Carbon. 2019;154:342-349. [Google Scholar]
- 120. Chen F, Alphonse M, Liu Q. Strategies for nonviral nanoparticle-based delivery of CRISPR/Cas9 therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(3):e1609. [DOI] [PubMed] [Google Scholar]
- 121. Fajrial AK, He QQ, Wirusanti NI, Slansky JE, Ding X. A review of emerging physical transfection methods for CRISPR/Cas9-mediated gene editing. Theranostics. 2020;10(12):5532-5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Givens BE, Naguib YW, Geary SM, Devor EJ, Salem AK. Nanoparticle-based delivery of CRISPR/Cas9 genome-editing therapeutics. AAPS J. 2018;20(6):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rahimi H, Salehiabar M, Charmi J, et al. Harnessing nanoparticles for the efficient delivery of the CRISPR/Cas9 system. Nano Today. 2020;34:100895. [Google Scholar]
- 124. Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat Nanotechnol. 2020;15(4):313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Aghamiri S, Talaei S, Ghavidel AA, et al. Nanoparticles-mediated CRISPR/Cas9 delivery: recent advances in cancer treatment. J Drug Deliv Sci Technol. 2020;56:101533. [Google Scholar]
- 126. Rosenblum D, Gutkin A, Kedmi R, et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci Adv. 2020;6(47):eabc9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ricciardi AS, Bahal R, Farrelly JS, et al. In utero nanoparticle delivery for site-specific genome editing. Nat Commun. 2018;9:2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Varma LT, Singh N, Gorain B, et al. Recent advances in self-assembled nanoparticles for drug delivery. Curr Drug Deliv. 2020;17(4):279-291. [DOI] [PubMed] [Google Scholar]
- 129. Gisbert-Garzarán M, Berkmann JC, Giasafaki D, et al. Engineered pH-responsive mesoporous carbon nanoparticles for drug delivery. ACS Appl Mater Interfaces. 2020;12(13):14946-14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nagaraju GP, Srivani G, Dariya B, et al. Nanoparticles guided drug delivery and imaging in gastric cancer. Semin Cancer Biol. 2021;69:69-76. [DOI] [PubMed] [Google Scholar]
- 131. Hasnain MS, Nayak AK, Kurakula M, Hoda MN. Alginate nanoparticles in drug delivery. In: Nayak AK, Hasnain MS, eds. Alginates in Drug Delivery. London: Academic Press; 2020:129-152. [Google Scholar]
- 132. Begines B, Ortiz T, Pérez-Aranda M, et al. Polymeric nanoparticles for drug delivery: recent developments and future prospects. Nanomaterials (Basel). 2020;10(7):1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Alyassin Y, Sayed EG, Mehta P, et al. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov Today. 2020;25(8):1513-1520. [DOI] [PubMed] [Google Scholar]
- 134. Chung YH, Cai H, Steinmetz NF. Viral Nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv Drug Deliv Rev. 2020;156:214-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Manzano M, Vallet-Regí M. Mesoporous silica nanoparticles for drug delivery. Adv Funct Mater. 2020;30(2):1902634. [Google Scholar]
- 136. Åslund AKO, Berg S, Hak S, et al. Nanoparticle delivery to the brain—by focused ultrasound and self-assembled nanoparticle-stabilized microbubbles. J Control Release. 2015;220(Pt A):287-294. [DOI] [PubMed] [Google Scholar]
- 137. Zhao X, Shang T, Zhang X, Ye T, Wang D, Rei L. Passage of magnetic tat-conjugated Fe3O4@SiO2 nanoparticles across in vitro blood-brain barrier. Nanoscale Res Lett. 2016;11:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ohta S, Kikuchi E, Ishijima A, Azuma T, Sakuma I, Ito T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood–brain barrier opening. Sci Rep. 2020;10:18220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kaushik A, Yndart A, Atluri V, et al. Magnetically guided non-invasive CRISPR-Cas9/gRNA delivery across blood-brain barrier to eradicate latent HIV-1 infection. Sci Rep. 2019;9:3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Blood-brain delivery methods using nanotechnology. Pharmaceutics. 2018;10(4):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bors LA, Erdő F. Overcoming the blood–brain barrier: challenges and tricks for CNS drug delivery. Sci Pharm. 2019;87(1):6. [Google Scholar]
- 142. Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Oescheger F, Jenal U. Misuse potential and biosecurity in life sciences research. Swiss Academies Rep. 2017;12:3. [Google Scholar]
- 144. Kosal M. The security implications of nanotechnology. Bull At Sci. 2010;66(4):58-69. [Google Scholar]
- 145. Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339(6122):971-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. NovaVax. Pipeline—creating tomorrow's vaccines today. Accessed September 19, 2022. https://www.novavax.com/our-pipeline
- 147. Shinde V, Bhikha S, Hoosain Z, et al. A. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1899-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. White BD, Duan C, Townley HE. Nanoparticle activation methods in cancer treatment. Biomolecules. 2019;9(5):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kaur P, Aliru ML, Chadha AS, Asea A, Krishnan S. Hyperthermia using nanoparticles—promises and pitfalls. Int J Hyperth. 2016;32(1):76-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ma Y, Bao J, Zhang Y, et al. Mammalian near-infrared image vision through injectable and self-powered retinal nanoantennae. Cell. 2019;177(2):243-255.e15. [DOI] [PubMed] [Google Scholar]
- 151. Bar-Ilan University. Bar-Ilan University: researchers invent nano-drops that improve nearsightedness and farsightedness. PRNewswire. March 8, 2018. Accessed September 20, 2022. https://www.prnewswire.com/news-releases/bar-ilan-university--researchers-invent-nano-drops-that-improve-nearsightedness-and-farsightedness-300610963.html
- 152. Mannoor MS, Jiang Z, James T, et al. 3D printed bionic ears. Nano Lett. 2013;13(6):2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Schumann C, Nguyen DX, Norgard M, et al. Increasing lean muscle mass in mice via nanoparticle-mediated hepatic delivery of follistatin mRNA. Theranostics. 2018;8(19):5276-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Raimondo TM, Mooney DJ. Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice. Proc Natl Acad Sci U S A. 2018;115(42):10648-10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Leong J, Hong YT, Wu YF, et al. Surface tethering of inflammation-modulatory nanostimulators to stem cells for ischemic muscle repair. ACS Nano. 2020;14(5):5298-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Marino A, Arai S, Hou Y, et al. Gold nanoshell-mediated remote myotube activation. ACS Nano. 2017;11(3):2494-2508. [DOI] [PubMed] [Google Scholar]
- 157. Edelman BJ, Meng J, Suma D, et al. Noninvasive neuroimaging enhances continuous neural tracking for robotic device control. Sci Robot. 2019;4(31):eaaw6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Neuralink. Engineering with the brain. Accessed September 20, 2022. https://neuralink.com/applications/
- 159. Kim Y, Meade SM, Chen K, et al. Nano-architectural approaches for improved intracortical interface technologies. Front Neurosci. 2018;12:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Reardon S. Global summit reveals divergent views on human gene editing. Nature. 2015;528(7581):173. [DOI] [PubMed] [Google Scholar]
- 161. Liu S. Legal reflections on the case of genome-edited babies. Glob Health Res Policy. 2020;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Raposo VL. The first Chinese edited babies: a leap of faith in science. JBRA Assist Reprod. 2019;23(3):197-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wang H, Yang H. Gene-edited babies: what went wrong and what could go wrong. PLoS Biol. 2019;17(4):e3000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. World Health Organization (WHO). WHO Expert Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing: Report of the Sixth Meeting. Geneva: WHO; 2019:11. Accessed July 11, 2022. https://www.who.int/publications/i/item/who-expert-advisory-committee-on-developing-global-standards-for-governance-and-oversight-of-human-genome-editing-report-of-the-sixth-meeting
- 165. NanoEthics. Accessed May 3, 2022. https://www.springer.com/journal/11569
- 166. Zolnik BS, González-Fernández Á, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinol. 2010;151(2):458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Kandel ER. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 2012;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Marx G, Gilon C. The molecular basis of memory. ACS Chem Neurosci. 2012;3(8):633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ai L, Tan T, Tang Y, et al. Endogenous formaldehyde is a memory-related molecule in mice and humans. Commun Biol. 2019;2:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kassick AJ, Wu M, Luengas D, et al. Covalently loaded naloxone nanoparticles as a long-acting medical countermeasure to opioid poisoning. ACS Pharmacol Transl Sci. 2021;4(5):1654-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Kovaliov M, Li S, Korkmaz E, et al. Extended-release of opioids using fentanyl-based polymeric nanoparticles for enhanced pain management. RSC Adv. 2017;7(76):47904-47912. [Google Scholar]
- 172. Jimenez-Vargas NN, Gong J, Wisdom MJ, et al. Endosomal signaling of delta opioid receptors is an endogenous mechanism and therapeutic target for relief from inflammatory pain. Proc Natl Acad Sci U S A. 2020;117(26):15281-15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hempel G, Janosek DB, Raziano DB. Hacking humans: a case study and analysis of vulnerabilities in the advancing medical device landscape. Cyber Secur Peer-Rev J. 2020;3(4):351-362. [Google Scholar]
- 174. Pycroft L, Aziz TZ. Security of implantable medical devices with wireless connections: the dangers of cyber-attacks. Expert Rev Med Devices. 2018;15(6):403-406. [DOI] [PubMed] [Google Scholar]
- 175. King NMP, Bishop CE. How should physicians help patients understand unknowns of nanoparticle-based medicines? AMA J Ethics. 2019;21(4):324-331. [DOI] [PubMed] [Google Scholar]
- 176. Uskoković V. Nanomedicine for the poor: a lost cause or an idea whose time has yet to come? Nanomedicine (Lond). 2021;16(14):1203-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bundschuh M, Filser J, Lüderwald S, et al. Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur. 2018;30(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zou Q. Nanoparticles in automotive applications. In: Wang QJ, Chung YW, eds. Encyclopedia of Tribology. Boston: Springer; 2013:2376-2381. [Google Scholar]
- 179. Mariappan N. Recent trends in nanotechnology applications in surgical specialties and orthopedic surgery. Biomed Pharmacol J. 2019;12(3):1095-1127. [Google Scholar]
- 180. Singh T, Shukla S, Kumar P, Wahla V, Bajpai VK. Application of nanotechnology in food science: perception and overview. Front Microbiol. 2017;8:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Manaia EB, Kaminski RCK, Corrêa MA, Chiavacci LA. Inorganic UV filters. Braz J Pharm Sci. 2013;49(2):201-209. [Google Scholar]
- 182. Schilling K, Bradford B, Castelli D, et al. Human safety review of “nano” titanium dioxide and zinc oxide. Photochem Photobiol Sci. 2010;9(4):495-509. [DOI] [PubMed] [Google Scholar]
- 183. Asztemborska M, Jakubiak M, Stęborowski R, Chajduk E, Bystrzejewska-Piotrowska G. Titanium dioxide nanoparticle circulation in an aquatic ecosystem. Water Air Soil Pollut. 2018;229(6):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Baranowska-Wójcik E, Szwajgier D, Oleszczuk P, Winiarska-Mieczan A. Effects of titanium dioxide nanoparticles exposure on human health—a review. Biol Trace Elem Res. 2020;193(1):118-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Guo Z, Martucci NJ, Moreno-Olivas F, Tako E, Mahler GJ. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact. 2017;5:70-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ziental D, Czarczynska-Goslinska B, Mlynarczyk DT, et al. Titanium dioxide nanoparticles: prospects and applications in medicine. Nanomaterials (Basel). 2020;10(2):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wiesmann N, Tremel W, Brieger J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J Mater Chem B. 2020;8(23):4973-4989. [DOI] [PubMed] [Google Scholar]
- 188. Greish K, Alqahtani AA, Alotaibi AF, et al. The effect of silver nanoparticles on learning, memory and social interaction in BALB/C mice. Int J Environ Res Public Health. 2019;16(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ferdous Z, Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci. 2020;21(7):2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Vazquez-Muñoz R, Borrego B, Juárez-Moreno K, et al. Toxicity of silver nanoparticles in biological systems: does the complexity of biological systems matter? Toxicol Lett. 2017;276:11-20. [DOI] [PubMed] [Google Scholar]
- 191. Mao BH, Chen ZY, Wang YJ, Yan SJ. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci Rep. 2018;8:2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. US Department of Food and Agriculture Foreign Agricultural Service. France: France bans titanium dioxide in food products by January 2020. Published May 8, 2019. Accessed June 10, 2022. https://www.fas.usda.gov/data/france-france-bans-titanium-dioxide-food-products-january-2020
- 193. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Accessed July 22, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02008R1272-20220301
- 194. Canadian General Standards Board (CGSB). Organic Production Systems: Permitted Substances Lists. Ottawa: CGSB; 2021. Accessed July 11, 2022. https://publications.gc.ca/collections/collection_2020/ongc-cgsb/P29-32-311-2020-eng.pdf