Abstract
Background:
Oncogenic BRAF mutations are commonly found in advanced differentiated thyroid cancer (DTC), and reports have shown efficacy of BRAF inhibitors in these tumors. We investigated the difference in response between dabrafenib monotherapy and dabrafenib + trametinib therapy in patients with BRAF-mutated radioactive iodine refractory DTC.
Methods:
In this open-label randomized phase 2 multicenter trial, patients aged ≥18 years with BRAF-mutated radioactive iodine refractory DTC with progressive disease by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 within 13 months before enrollment were eligible. Patients were randomly assigned to receive dabrafenib alone or dabrafenib + trametinib. The primary endpoint was objective response rate by modified RECIST (minor response of −20% to −29%, partial and complete response) within the first 24 weeks of therapy. Trial Registration Number: NCT01723202.
Results:
A total of 53 patients were enrolled. The objective response rate (modified RECIST) was 42% (11/26 [95% confidence interval {CI} 23–63%]) with dabrafenib versus 48% (13/27 [CI 29–68%]) with dabrafenib + trametinib (p = 0.67). Objective response rate (RECIST 1.1) was 35% (9/26 [CI 17–56%]) with dabrafenib and 30% (8/27 [CI 14–51%]) with dabrafenib + trametinib. Most common treatment-related adverse events included skin and subcutaneous tissue disorders (17/26, 65%), fever (13/26, 50%), hyperglycemia (12/26, 46%) with dabrafenib alone and fever (16/27, 59%), nausea, chills, fatigue (14/27, 52% each) with dabrafenib + trametinib. There were no treatment-related deaths.
Conclusions:
Combination dabrafenib + trametinib was not superior in efficacy compared to dabrafenib monotherapy in patients with BRAF-mutated radioiodine refractory progressive DTC.
Keywords: thyroid cancer, targeted therapy, BRAF, kinase inhibitor
Introduction
Differentiated thyroid cancer (DTC) is a constellation of thyroid follicular epithelial cell-derived cancers that comprises papillary, follicular, hurthle cell and poorly DTC (1). Papillary thyroid cancer (PTC) is the most common histologic subtype (2), and v-Raf murine sarcoma viral oncogene homolog B (BRAFV600E) is the most common oncogenic mutation in PTC and is present in 60% of cases (2). The standard therapies for progressive metastatic radioiodine refractory DTC include sorafenib and lenvatinib. These multikinase inhibitors that target angiogenic pathways prolong progression-free survival but have several adverse events (3,4). Consequently, there have been efforts to identify personalized approaches for these patients, including kinase inhibitors targeting BRAFV600E and its principle downstream regulator, mitogen-activated extracellular signal regulated kinase (MEK). In preclinical studies using BRAFV600E-mutated thyroid cancer cell lines and xenograft tumors, BRAF and MEK inhibitors (5–7) demonstrated robust on-target effects and effectively reduced mitogen-activated protein kinase signaling and inhibited tumor growth (8).
Retrospective and prospective studies of single-agent BRAF inhibitors such as dabrafenib and vemurafenib in BRAFV600E-mutated PTC showed safety and clinical benefit (9,10). Dabrafenib and MEK inhibitor trametinib combination therapy is currently approved for use in BRAFV600E-mutated anaplastic thyroid cancer (11), melanoma (12), and nonsmall cell lung cancer (13).
We conducted an investigator-initiated randomized phase 2 clinical trial to test the hypothesis that dabrafenib + trametinib in patients with BRAF-mutant DTC will result in greater clinical efficacy than dabrafenib alone through vertical inhibition of the BRAF-mitogen-activated protein kinase pathway and mitigation of potential mechanisms of resistance.
Materials and Methods
Patients
Patients aged 18 years or older with BRAF-mutated (by local testing) DTC were eligible if they had measurable and progressive disease by the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) (14) criteria within 13 months before enrollment. Patients were required to be refractory to radioactive iodine as defined by: (a) ≥1 measurable lesion(s) without radioactive iodine uptake, (b) ≥1 measurable lesion(s) progressive by RECIST 1.1 (14) and/or the appearance of ≥1 new lesion(s) within 12 months of prior radioactive iodine therapy, (c) cumulative radioactive iodine dose of >600 mci, or (d) measurable disease that was Fludeoxyglucose (18F) positron emission tomography scan positive. Patients could have received up to three prior oral multikinase inhibitors, excluding selective BRAF or MEK inhibitors.
The full eligibility criteria are available in the protocol (Supplementary Data). All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and local laws. Peer review and funding for this trial were supported by the National Comprehensive Cancer Network. Experimental drugs were provided by GlaxoSmithKline and Novartis.
Study design and procedures
Patients were recruited from five academic centers in the United States and randomized 1:1 to single agent dabrafenib (150 mg orally twice daily) or combination therapy with dabrafenib (150 mg orally twice daily) + trametinib (2 mg orally daily). Stepwise dose reduction of dabrafenib to 100 mg, 75 mg, or 50 mg orally twice daily or trametinib to 1.5 mg or 1 mg orally daily was allowed per protocol to manage treatment-related adverse events. Patients received treatment until RECIST 1.1 (14) disease progression, development of an intolerable adverse event, voluntary withdrawal, or death. For those randomized to dabrafenib alone, crossover to dabrafenib + trametinib was allowed upon progression. Patients deriving clinical benefit from combination therapy were allowed to continue study treatment beyond RECIST progression. The period patients were on study treatment beyond progression was not counted toward the duration of response.
Pharmacokinetic studies were performed as detailed in the protocol (Supplementary Data).
Tumor mutational sequencing was performed at baseline and at progression. DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissue scrolls using the Maxwell 16 FFPE DNA Purification Kit (Promega, Inc.) and quantitated using pico green on a Qubit (Life Technologies). Libraries were generated using the KAPA Hyper Kit (Roche Sequencing and Life Sciences) followed by hybridization with a custom targeted next generation sequencing panel that included the coding sequence of 407 commonly mutated genes in human cancers and the TERT promoter region. Pooled barcoded libraries underwent 150 bp paired-end sequencing on an Illumina HiSeq 4000 instrument (Illumina, Inc.) to an average depth of coverage >300 × . Raw sequence reads were processed and aligned to human reference genome using the Genome Analysis Toolkit (GATK) workflow. MuTect2 and VarScan 2 were used to identify tumor-specific variants, and Ensembl Variant Effect Predictor (VEP) was used to annotate and determine functional consequences of tumor specific variants.
Outcomes
The primary endpoint was objective response rate by modified RECIST, defined as the proportion of patients who had a complete response, partial response, or minor response within the first six cycles. Complete response and partial response were defined by RECIST 1.1 (14), and minor response was defined as 20–29% decrease in the sum of diameters of target lesions compared to baseline. With RECIST progression defined as at least a 20% increase in tumor measurements compared to nadir, we believe that 20–29% decrease represents true shrinkage that is clinically meaningful and not by chance. Secondary endpoints included duration of objective response, progression-free survival, overall survival, tolerability and safety, tumor mutation screening, and pharmacokinetics (PK) of the experimental drugs. Survival-related outcomes were measured from the date of study treatment initiation to the date of the event (death or disease progression) or the date of last follow-up. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (15).
Statistical analysis
This study utilized a flexible screening design developed by Sargent and Goldberg (16) to evaluate experimental treatment regimen activity in this patient population and to screen these regimens for the more promising one to be carried forward to phase 3 studies. Assuming that the true differential in the overall response rates between treatment arms was 0.20 (15% vs. 35%), this study design had a 90% power to identify the more efficacious regimen. Based on these assumptions and constraints, 26 evaluable patients were planned to accrue to each arm to evaluate the regimens under the parameters specified. As part of this design, we defined one treatment arm to be more promising than another if the actual observed overall response rate for each arm differed by 10% or more, in which case the regimen with the higher overall response rate would be recommended for further testing. If these rates differed by <10%, other factors would be considered. The objective response (minor + partial + complete response) rate was estimated for each treatment arm with the exact binomial 95% confidence intervals (CIs) and compared between arms using Chi-square test.
The median progression-free survival and duration of response were estimated using the Kaplan–Meier method with CIs and compared using log-rank test. Summary statistics were calculated for patient demographics and clinical characteristics. Toxicities were summarized by grade as per the NCI CTCAE v4.0 criteria using frequency and percentage. Statistical significance was concluded at p-value <0.05. These analyses were conducted in SAS version 9.04.
Results
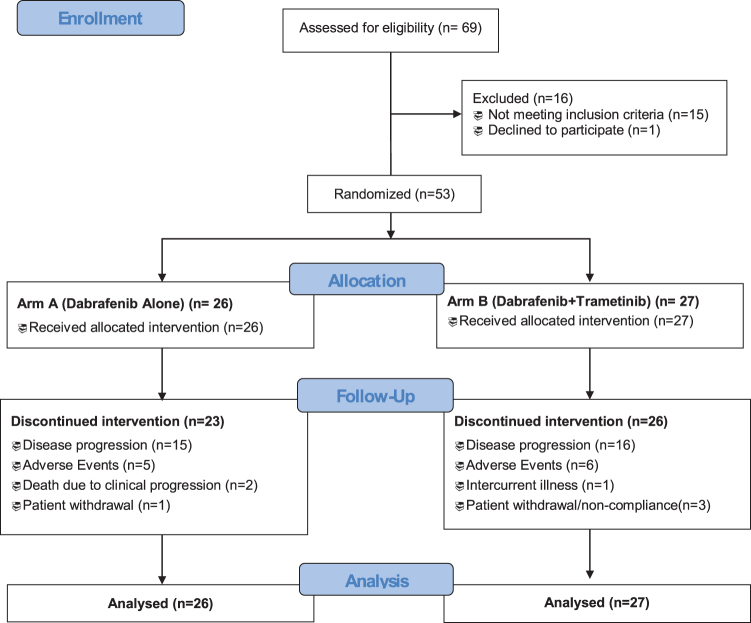
A total of 53 patients with BRAF-mutated radioiodine refractory progressive DTC were randomly assigned to receive either dabrafenib monotherapy (26 patients) or dabrafenib + trametinib (27 patients) (Fig. 1). Baseline characteristics were similar in both groups (Table 1). PTC was the most common histology overall (92%). Most patients (85%) had lung metastases, and most (77%) were systemic therapy naive. All patients had a BRAFV600E mutation except for one on dabrafenib monotherapy who had BRAFK601E mutated PTC. Of the two patients with hurthle cell carcinoma one had concomitant papillary microcarcinoma that was BRAFV600E mutated and the other had a subclonal BRAFV600E mutation.
FIG. 1.
CONSORT diagram.
Table 1.
Baseline Demographics and Clinical Characteristics of 53 Patients on Trial
| Characteristic | Dabrafenib alone (N = 26), n (%) | Dabrafenib + trametinib (N = 27), n (%) |
|---|---|---|
| Median age in years (range) | 60 (37–84) | 65 (43–84) |
| Female | 8 (31) | 7 (26) |
| Race | ||
| White | 25 (96) | 24 (89) |
| African American | 0 (0) | 2 (7) |
| Asian | 1 (4) | 1 (4) |
| ECOG performance status | ||
| 0 or 1 | 23 (88) | 26 (96) |
| 3 (12) | 1 (4) | |
| Histologic subtype | ||
| Papillary | 25 (96) | 24 (89) |
| Follicular | 1 (4) | 0 (0) |
| Hurthle cell | 0 (0) | 2 (8) |
| Poorly DTC | 0 (0) | 1 (4) |
| BRAF mutation | ||
| V600E | 25 (96) | 27 (100) |
| K601E | 1 (4) | 0 (0) |
| Site of metastases | ||
| Lung | 22 (85) | 23 (85) |
| Lymph node | 10 (38) | 15 (56) |
| Bone | 7 (27) | 3 (11) |
| Liver | 2 (8) | 2 (7) |
| Other | 4 (15) | 6 (22) |
| Prior therapy | ||
| Surgery | 26 (100) | 27 (100) |
| Radiotherapy | 16 (62) | 10 (37) |
| Radioactive iodine | 26 (100) | 27 (100) |
| Chemotherapy | 0 (0) | 1 (2) |
| Prior tyrosine kinase inhibitor# (up to 3 allowed) | 6 (23) | 5 (19) |
Dabrafenib single agent and dabrafenib + trametinib upfront combination. Crossover patients not included here.
DTC, differentiated thyroid cancer.
The response and safety data on the 53 patients are reported in this article. At the time of data cutoff for primary analysis for efficacy, the median duration of follow-up was 22.1 months (range: 1.0–74.0) on dabrafenib monotherapy arm and 25.0 months (range: 4.3–54.4) on dabrafenib + trametinib. The median duration of treatment was 14.8 months (range: 0.9–74.0) in patients who received dabrafenib versus 14.7 months (range: 1.4–50.6) in those who received dabrafenib + trametinib. Three patients on dabrafenib, one on upfront dabrafenib + trametinib, and one who crossed over to dabrafenib + trametinib were still on study treatment. Of the 26 patients randomized to dabrafenib, 14 (54%) crossed over to combination therapy at progression, of which one patient remains on study treatment without disease progression.
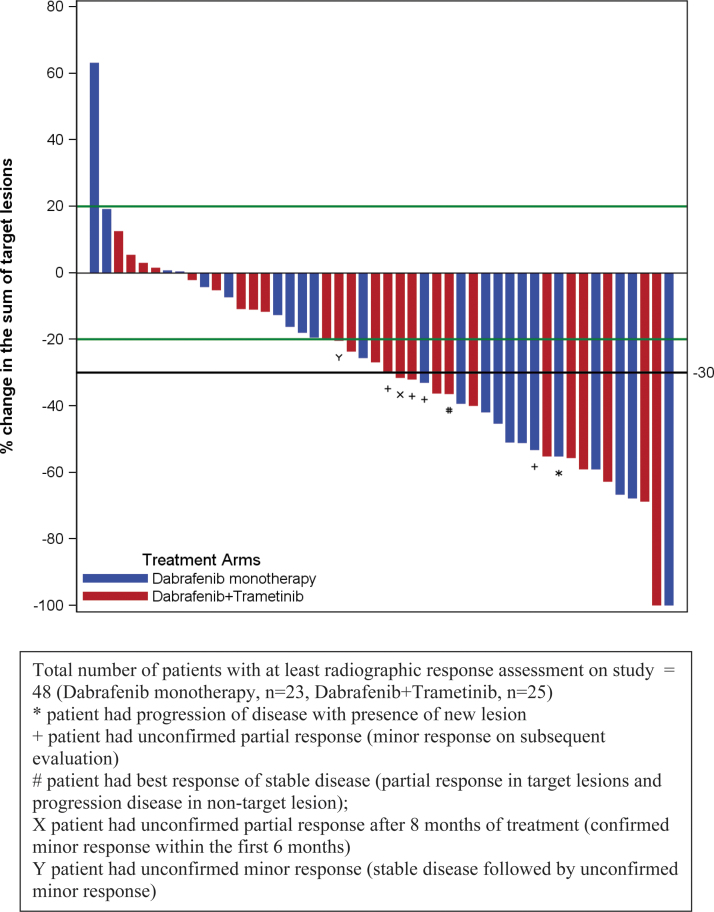
Of the 53 patients in the intent-to-treat population, 3 [death from clinical progression (1), dysphagia/stridor (1), encephalopathy (1)] on dabrafenib and 2 [cerebrovascular accident unrelated to study drugs (1), possible cardiac ischemia (1)] on dabrafenib + trametinib went off study treatment before first radiographic response assessment (Supplementary Fig. S1). Efficacy data are summarized in Table 2, Supplementary Tables S4 and S5. Protocol-defined objective response (minor + partial + complete response) rate within the first 6 cycles in the intent-to-treat population was 42% (11/26 [CI 23–63%]) with dabrafenib and 48% (13/27 [CI 29–68%]) with dabrafenib + trametinib (p = 0.67) (Fig. 2). The median time to response was 1.8 months. There were no complete responses. Confirmed partial response rate within the first 6 months of therapy was 35% (9/26 [CI 17–56%]) with dabrafenib and 30% (8/27 [CI 14–51%]) with dabrafenib + trametinib.
Table 2.
Efficacy Data for Dabrafenib Alone, Dabrafenib + Trametinib, and Crossover
| Outcome | Dabrafenib alone (N = 26) | Dabrafenib + trametinib (N = 27) | p a | Crossover (N = 14) |
|---|---|---|---|---|
| Best response by week 24 | ||||
| Progressive disease | 2 | 0 | 2 | |
| Stable disease | 10 | 12 | 8 | |
| Minor response | 2 | 5 | 1 | |
| Partial response | 9 (35%, 17–56%) | 8 (30%, 14–51%) | 3 | |
| Objective responseb | 11 | 13 | 0.67 | 4 |
| = Partial + minor response (% [CI]) | (42% [23–63]) | (48% [29–68%]) | (29% [8–58%]) | |
| Duration of response (MR + PR) (months) median [CI] | 18.3 [5.0–NR] | 17.0 [9.7– 44.5] | 0.99 | 16.0 [6.3–25.6] |
| Duration of partial response | 18.3 (4.2–40.6) | 24.5 (9.7–NR) | 0.53 | * |
| Progression-free survival (months) median [CI] | 10.7 [3.8–34.7] | 15.1 [12.3–37.3] | 0.65 | 7.5 [2.8–38.5] |
| Overall survival (months) median [CI] | 37.9 [23.4–NR] | 47.5 [27.9–57.8] | 0.99 | 36 [23.4–NR] |
Subjects who progressed on dabrafenib alone and then had the opportunity to crossover into dabrafenib + trametinib arm.
One patient who crossed over to combination therapy had PR for a duration of 6.3 months. The other two patients were censored at 3.9 and 6.2 months for AEs.
Dabrafenib versus dabrafenib + trametinib.
Intent to treat analysis.
AEs, adverse events; CI, confidence interval; MR, minor response; NR, not reached; PR, partial response.
FIG. 2.
Waterfall plot demonstrating tumor response with dabrafenib alone (shown in blue) and with dabrafenib + trametinib (shown in red). Each bar represents a subject. Crossover not included here.
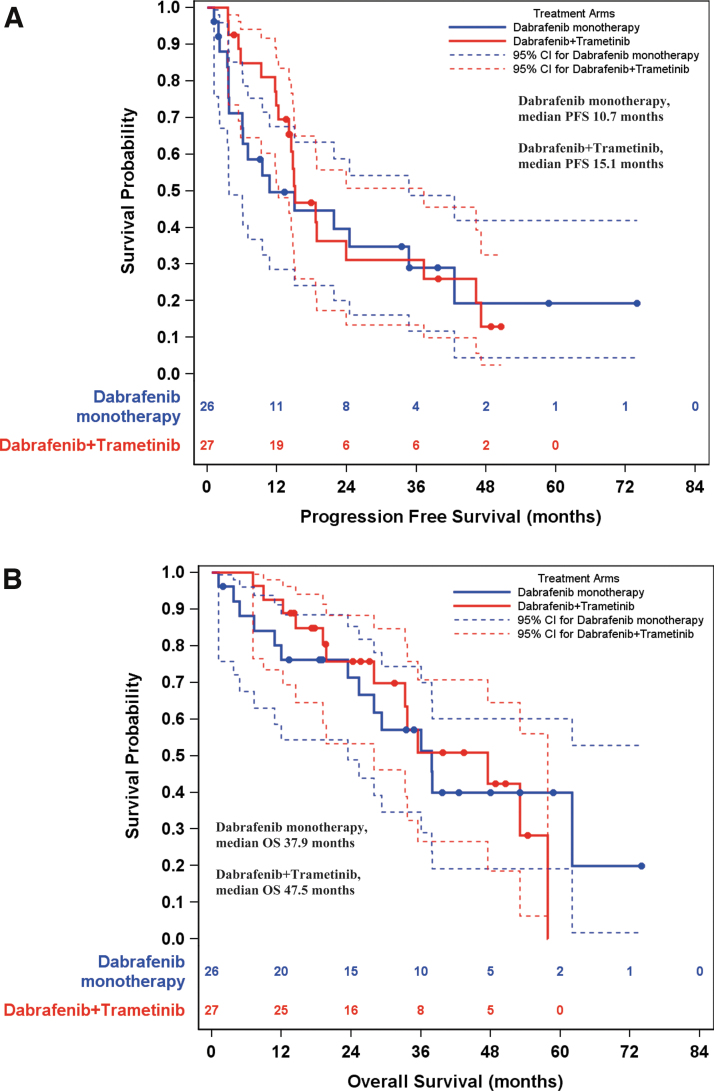
Median duration of objective response was 18.3 months [CI 5.0–not reached] with dabrafenib and 17.0 months [CI 9.7–44.5] with dabrafenib + trametinib (p = 0.99). At a median follow-up of 23.1 months (range: 1.0–74.0), 36 patients had disease progression or death (17 on dabrafenib, 19 on dabrafenib + trametinib). The median progression-free survival was 10.7 months [CI 3.8–34.7] with dabrafenib versus 15.1 months [CI 12.3–37.3] with dabrafenib + trametinib (p = 0.65) (Fig. 3A).
FIG. 3.
(A) Progression-free survival estimates for dabrafenib alone (shown in blue, median [CI] = 10.7 [3.8–34.7] months) and dabrafenib + trametinib (shown in red, 15.1 [12.3–37.3] months). (B) Overall survival estimates for dabrafenib alone (37.9 [23.4–NR] months) and dabrafenib + trametinib (47.5 [27.9–57.8] months). NR, not reached; PFS, progression free survival.
At data cutoff, 14 patients in the dabrafenib arm and 13 in the dabrafenib + trametinib arm had died. The median overall survival was 37.9 months [CI 23.4–not reached] with dabrafenib and 47.5 months [CI 27.9–57.8] with dabrafenib + trametinib (p = 0.99) (Fig. 3B).
One patient on combination therapy, who had stable disease within the first 6 cycles, had subsequent confirmed minor response 8 months into treatment. Eleven patients (6 on dabrafenib, 5 on dabrafenib + trametinib) had received prior multikinase inhibitor(s). Of these, 4 (1 on dabrafenib, 3 on dabrafenib + trametinib) had a partial response, and 5 (3 on dabrafenib, 2 on dabrafenib + trametinib) had stable disease. Overall, stable disease was attained in 38% (10/26) of patients on dabrafenib and 44% (12/27) on dabrafenib + trametinib. The only patient with BRAFK601E mutant PTC had progressive disease as best response. The patient with follicular thyroid cancer, on dabrafenib monotherapy, was not assessable as he died before first radiographic assessment (death was attributed to disease progression). A patient with hurthle cell cancer with subclonal mutant BRAFV600E on dabrafenib + trametinib had stable disease for 5.7 months before disease progression. The patient with poorly DTC had a partial response and remained on upfront dabrafenib + trametinib for 12.1 months before disease progression.
One-year progression-free survival rate was 50% on dabrafenib and 73% on dabrafenib + trametinib (p = 0.084). Among 42 patients who were multikinase inhibitor naive, the median progression-free survival was 24.5 months [CI 9.5–not reached] with dabrafenib and 14.9 months [CI 12.3–37.3] with dabrafenib + trametinib (p = 0.48). Of the 11 patients who had received at least one prior multikinase inhibitor, the median progression-free survival with dabrafenib was 3.7 months [CI 1.2–7.0] and 18.8 months [CI 3.7–not reached] with combination therapy (p = 0.01).
Of the 14 patients who crossed over at progression from dabrafenib to dabrafenib + trametinib, 4 (29%) patients had an objective response (partial and minor response) (Table 2). Stable disease was noted in 8 (57%) patients, with duration of stable disease >6 months in two patients. The median progression-free survival from the start of crossed-over combination therapy was 7.5 months [CI 2.8–38.5]. Median overall survival in this subset of patients was 36 months [CI 23.4–not reached] versus 47.5 months [CI 27.9–57.8] in patients who were randomized to combination therapy at study initiation (p = 0.42).
The median follow-up for adverse events was 14.1 months (range: 0.9–45.6). By data cutoff for adverse events, any grade treatment-related adverse events were noted in 100% of patients in each arm and were predominantly grade 1 or grade 2 (Table 3). Grade 3 treatment-related adverse events were noted in 15 patients (58%) on dabrafenib versus 13 patients (48%) on dabrafenib + trametinib. There were no grade 4 or 5 treatment-related adverse events. The number of treatment-related serious adverse events was greater with dabrafenib + trametinib (21 vs. 9) (Supplementary Tables S6 and S7).
Table 3.
Treatment-Related Adverse Events (>15%) by Grade Seen with Dabrafenib Alone and Dabrafenib + trametinib
| Adverse event | Dabrafenib alone (n = 26) |
Dabrafenib + trametinib (N = 27) |
||
|---|---|---|---|---|
| Grade 3 (%) | Any grade (%) | Grade 3 (%) | Any grade (%) | |
| Skin and subcutaneous tissue disorders | 1 (4) | 17 (65) | 0 (0) | 9 (33) |
| Fever | 2 (8) | 13 (50) | 1 (4) | 16 (59) |
| Hyperglycemia | 3 (12) | 12 (46) | 1 (4) | 5 (19) |
| Anemia | 3 (12) | 11 (42) | 0 (0) | 8 (30) |
| Palmar-plantar erythrodysesthesia syndrome | 0 (0) | 11 (42) | 0 (0) | 6 (22) |
| Nausea | 0 (0) | 11 (42) | 1 (4) | 14 (52) |
| Alopecia | 0 (0) | 11 (42) | 0 (0) | 0 (0) |
| Chills | 1 (4) | 11 (42) | 0 (0) | 14 (52) |
| Fatigue | 1 (4) | 10 (38) | 1 (4) | 14 (52) |
| Hypophosphatemia | 2 (8) | 9 (35) | 3 (11) | 11 (41) |
| Vomiting | 0 (0) | 7 (27) | 1 (4) | 6 (22) |
| Rash maculo-papular | 0 (0) | 7 (27) | 0 (0) | 4 (15) |
| Weight loss | 1 (4) | 7 (27) | 0 (0) | 0 (0) |
| Anorexia | 1 (4) | 6 (23) | 0 (0) | 9 (33) |
| Pruritus | 0 (0) | 6 (23) | 0 (0) | 3 (11) |
| Arthralgia | 0 (0) | 6 (23) | 0 (0) | 0 (0) |
| Myalgia | 0 (0) | 5 (19) | 0 (0) | 6 (22) |
| Lymphocyte count decreased | 0 (0) | 5 (19) | 0 (0) | 0 (0) |
| Headache | 0 (0) | 5 (19) | 0 (0) | 0 (0) |
| Diarrhea | 0 (0) | 4 (15) | 0 (0) | 7 (26) |
| Edema limbs | 0 (0) | 3 (12) | 0 (0) | 5 (19) |
| Aspartate aminotransferase increased | 0 (0) | 0 (0) | 1 (4) | 10 (37) |
| Alanine aminotransferase increased | 0 (0) | 0 (0) | 1 (4) | 8 (30) |
| Alkaline phosphatase increased | 0 (0) | 0 (0) | 0 (0) | 5 (19) |
| Generalized muscle weakness | 0 (0) | 0 (0) | 0 (0) | 5 (19) |
Among patients who received at least one dose of a study drug, permanent discontinuations due to adverse events were reported in 5 of 26 patients (19%) on dabrafenib and 6 of 27 (22%) on dabrafenib + trametinib. Dose reductions were required in 6 (23%) and 15 (56%) patients, respectively.
Fifteen patients were included in the PK analysis, 7 on dabrafenib and 8 on dabrafenib + trametinib. One patient on dabrafenib + trametinib did not have drug levels collected for cycle 2. PK parameters for first-dose and steady-state dabrafenib and dabrafenib + trametinib (Supplementary Table S2) and mean dabrafenib plasma concentration–time profiles comparing the two arms for first dose and steady state (Supplementary Fig. S2A, B) and comparing first dose and steady state in each arm (Supplementary Fig. 3A, B) were consistent with previously published data (17,18). PK profiles of dabrafenib metabolites (hydroxy- and desmethyl-dabrafenib) are summarized in Supplementary Table S3 and Supplementary Figure S4. Supplementary Figure S5 depicts mean plasma concentration-time profiles for trametinib when given in combination with dabrafenib at first dose and steady state. Trametinib area under the curve (AUC) was comparable to previous single-dose studies and exhibited accumulation with repeat dosing with both AUC and maximum concentration (19).
Molecular profiling on 33 tumor samples at time of diagnosis using a targeted sequencing panel showed a median burden of 4 mutated genes (range: 1–9) per patient, including BRAFV600E (Supplementary Fig. S6). Expectedly, TERT promoter variants (C228T or C250T) were the most common co-occurring variants, detected in 26 out of 33 patients (79%). The only other genes found mutated in ≥3 patients in our cohort were CRLF2, NUP93 and SDHA, and STAG2, all of which are known recurrently mutated genes in PTC.
We were able to characterize molecular patterns during disease progression for seven patients with paired material available, of which four patients demonstrated molecular stability at time of progression, while two patients exhibited molecular evolution with gains of either 5 or 2 additional mutational gains at progression. One patient on dabrafenib alone had a clonal shift, with loss of a subclonal POLE mutation and gain of a new MRE11A splice-site variant at progression, while another patient on combination therapy acquired mutations in KRAS and CDKN2C. Another patient who received dabrafenib monotherapy and was biopsied at progression outside of the current protocol acquired an activating RAC1 (P34R) mutation and polyploidy of chromosome 7 with overexpression of RAC1 along with other genes (20). Correlative studies with respect to clinical associations or treatment response were precluded by the relatively small sample size.
Discussion
Combination dabrafenib + trametinib did not offer statistically superior objective response rates compared to dabrafenib alone (48% vs. 42%, respectively, including minor responses, p = 0.67). Objective response rates per RECIST 1.1 were also similar at 30% and 35% with dabrafenib + trametinib and dabrafenib, respectively. There was no significant difference (p = 0.65) in median progression-free survival between dabrafenib + trametinib (15.1 months) and dabrafenib (10.7 months); however, this study was not adequately powered to detect a difference in progression-free survival. The majority (80%) of our patients was multikinase inhibitor therapy naive, and there was no difference in median progression-free survival between dabrafenib and dabrafenib + trametinib in this subset. While the combination therapy arm had longer median progression-free survival compared to dabrafenib monotherapy in previous multikinase inhibitor therapy recipients, this subset constituted only 19% of all patients, and therefore, this subgroup analysis is at best exploratory in nature. Of the patients who crossed over to dabrafenib + trametinib following progression on dabrafenib, 86% (12/14) had either an objective response or stable disease.
The adverse event profile revealed a higher incidence of skin-related side effects and hyperglycemia with dabrafenib and a higher incidence of fatigue, chills, and elevation of liver transaminases with combination therapy. Contrary to the expectation, combination therapy had a higher frequency of serious adverse events than monotherapy.
This is the first prospective randomized clinical trial in radioactive iodine refractory DTC that evaluates the efficacy of combination BRAF and MEK inhibition and compares this to BRAF inhibitor monotherapy. Recognizing the challenges of cross-trial comparisons, the efficacy of dabrafenib monotherapy seen in our study is comparable to earlier studies using single agent dabrafenib (9) and vemurafenib (10) in BRAF-mutated radioactive iodine refractory DTC. The lower response rate with either regimen compared to that in melanoma may be explained by upregulation of HER3 and subsequent reactivation of mitogen-activated protein kinase signaling by BRAF and MEK inhibitors in thyroid cancer (21).
The lack of superiority in efficacy of upfront combination BRAF and MEK inhibitor therapy over BRAF inhibitor therapy alone in advanced DTC contrasts with efficacy data in melanoma and nonsmall cell lung cancer, where combination therapy was significantly favored over BRAF inhibitor monotherapy (12,22,23). One reason may be that the “oncogene addiction” of PTC to BRAFV600E may be greater than melanoma. This concept may be supported by the relative genomic stability of PTCs (24), which we also see in our molecular data of the seven diagnosis/progression pairs, where 4 out of 7 patients showed disease progression with identical mutational profiles, and only one patient showed a minor clonal shift at progression. Thus, these tumors may not be as capable of mounting alternative mechanisms to support MEK activation. Another possibility is that the downstream reliance on MEK is greater in multikinase inhibitor-pretreated tumors due to clonal pressure from prior treatment. However, the subset of pretreated patients was too small to make definitive conclusions.
Although dabrafenib and trametinib have not been investigated in a head-to-head comparison against sorafenib and lenvatinib, the use of dabrafenib first line might be considered in a subset of patients with BRAF-mutated PTC. Examples include patients with tumors invading blood vessels or vital organs, those with difficult to control hypertension, or with significant clotting or bleeding tendencies, where use of strong antiangiogenic agents is problematic.
In summary, both dabrafenib and dabrafenib + trametinib had encouraging activity in progressive radioactive iodine refractory BRAF-mutated DTC. Upfront dabrafenib + trametinib monotherapy was not superior in efficacy to dabrafenib monotherapy, and the combination had a higher incidence of severe adverse events compared to dabrafenib alone. Considering the similar efficacy and better tolerability, dabrafenib monotherapy may be the preferred option in clinical practice.
Supplementary Material
Acknowledgments
The authors thank the Clinical Treatment Unit and the Clinical Trials Processing Laboratory Shared Resource, as well as the Pharmacoanalytical Shared Resource and the Solid Tumor Translational Science Shared Resource, at The Ohio State University Comprehensive Cancer Center, Columbus, OH for the support. The authors thank Jennifer L. Sexton, Mamdouh Beshara, Debra Nichols, and Norka Snyder for their tireless efforts in helping with patient and data acquisition and analysis. The authors also thank Fadi Nabhan, MD, Mimi Hu, MD, and Steven G. Waguespack, MD for helping with patient accrual for this study.
Authors' Contributions
N.L.B., B.K., L.W., and M.H.S. had access to and verified the data. N.L.B., B.K., L.W., L.J.W., C.D., G.A.D., J.A.D., M.P., N.D.S., M.E.C., J.A.S., M.D.R., A.-K.E., C.T., and M.H.S. were responsible for the decision to submit the article.
Author Disclosure Statement
N.L.B. receives consulting fees from Eisai and Loxo Oncology. B.K. has grant funding (to institution) from Eisai, Merck, Xencor, Eli-Lilly, Bristol-Myers Squibb. L.W., C.D., G.A.D., J.A.D., M.P., J.A.S., M.D.R., and C.T. have nothing to disclose. L.J.W. receives consulting fees from Bayer, Blueprint Medicines, Coherus, Eisai, Exelixis, Eli Lilly, Loxo Oncology, Merck, and Morphic Therapeutics and participates on the data safety monitoring board of PDS Biotech and Iovance Biotherapeutics. N.D.S. has grant funding (to institution) from GlaxoSmithKline. M.E.C. receives consulting fees from Exelixis, Blueprint, Ignyta, Bayer, and Loxo Oncology and has grant funding (to institution) from Eisai, Exelixis, Genentech, Merck, and Kura. Spouse of A.-K.E. has employment at Karyopharm. M.H.S. has grant funding (to institution) from Merck and Loxo Oncology.
Funding Information
The study was approved and funded by the National Comprehensive Cancer Network Oncology Research Program from financial support provided by Novartis Pharmaceuticals Corporation (formerly GlaxoSmithKline, LLC) and, in part, by the Ohio State University Cancer Center (OSUCCC) Support Grant (NCI Grant P30 CA016058), the OSUCCC Intramural Research Program, through Pelotonia funding, and by the MD Anderson Cancer Center Support Grant (NCI Grant P30 CA016672).
Supplementary Material
References
- 1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fagin JA, Wells SA Jr. 2016. Biologic and clinical perspectives on thyroid cancer. N Engl J Med 375:1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Pena C, Molnar I, Schlumberger MJ, investigators D. 2014. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Dutcus CE, Heras Bdl, Zhu J, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim S-B, Krzyzanowska MK, Sherman SI. 2014. A phase 3, multicenter, double-blind, placebo-controlled trial of lenvatinib (E7080) in patients with 131I-refractory differentiated thyroid cancer (SELECT). J Clin Oncol 32:LBA6008. [Google Scholar]
- 5. Xing J, Liu R, Xing M, Trink B. 2011. The BRAFT1799A mutation confers sensitivity of thyroid cancer cells to the BRAFV600E inhibitor PLX4032 (RG7204). Biochem Biophys Res Commun 404:958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leboeuf R, Baumgartner JE, Benezra M, Malaguarnera R, Solit D, Pratilas CA, Rosen N, Knauf JA, Fagin JA. 2008. BRAFV600E mutation is associated with preferential sensitivity to mitogen-activated protein kinase kinase inhibition in thyroid cancer cell lines. J Clin Endocrinol Metab 93:2194–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salerno P, De Falco V, Tamburrino A, Nappi TC, Vecchio G, Schweppe RE, Bollag G, Santoro M, Salvatore G. 2010. Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab 95:450–455. [DOI] [PubMed] [Google Scholar]
- 8. McFadden DG, Vernon A, Santiago PM, Martinez-McFaline R, Bhutkar A, Crowley DM, McMahon M, Sadow PM, Jacks T. 2014. p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc Natl Acad Sci U S A 111:E1600–E1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falchook GS, Millward M, Hong D, Naing A, Piha-Paul S, Waguespack SG, Cabanillas ME, Sherman SI, Ma B, Curtis M, Goodman V, Kurzrock R. 2015. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid 25:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, Sherman SI, Sherman EJ. 2016. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol 17:1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. 2018. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol 36:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JBAG, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Casey M, Ouellet D, Martin A-M, Le N, Patel K, Flaherty K. 2014. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877–1888. [DOI] [PubMed] [Google Scholar]
- 13. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, Giannone V, D'Amelio AM Jr., Zhang P, Mookerjee B, Johnson BE. 2017. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18:1307–1316. [DOI] [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. 2009. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. [DOI] [PubMed] [Google Scholar]
- 15. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Available at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 (accessed June 22, 2022).
- 16. Lui KJ 2002. A flexible design for multiple armed screening trials by Daniel J. Sargent and Richard M. Goldberg, Statistics in Medicine 2001; 20: 1051–1060. Stat Med 21:625–628. [DOI] [PubMed] [Google Scholar]
- 17. Food and Drug Administration 2020. Tafinlar (dabrafenib): clinical pharmacology and biopharmaceutics review(s). Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202806orig1s000clinpharmr.pdf (accessed June 22, 2022).
- 18. European Medicines Agency 2020. Tafinlar (dabrafenib): summary of product characteristics. Available at www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002604/WC500149671.pdf (accessed June 22, 2022).
- 19. Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, Cox DS, Xu Y, Morris SR, Peddareddigari VG, Le NT, Hart L, Bendell JC, Eckhardt G, Kurzrock R, Flaherty K, Burris HA, 3rd, Messersmith WA. 2012. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol 13:773–781. [DOI] [PubMed] [Google Scholar]
- 20. Bagheri-Yarmand R, Busaidy NL, McBeath E, Danysh BP, Evans KW, Moss TJ, Akcakanat A, Ng PKS, Knippler CM, Golden JA, Williams MD, Multani AS, Cabanillas ME, Shaw KR, Meric-Bernstam F, Shah MH, Ringel MD, Hofmann MC. 2021. RAC1 alterations induce acquired dabrafenib resistance in association with anaplastic transformation in a papillary thyroid cancer patient. Cancers (Basel) 13:4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA. 2013. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov 3:520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Planchard D, Besse B, Groen HJM, Souquet P-J, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S, Rigas JR, Upalawanna A, D'Amelio AM Jr., Zhang P, Mookerjee B, Johnson BE. 2016. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 17:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F, Souquet P-J, Smit EF, Groen HJM, Kelly RJ, Cho BC, Socinski MA, Pandite L, Nase C, Ma B, D'Amelio A, Mookerjee B, Curtis CM, Johnson BE. 2016. Dabrafenib in patients with BRAFV600E-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 17:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cancer Genome Atlas Research Network 2014. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.