Abstract
Significance:
Hypertrophic scarring is a challenging issue for patients and clinicians. The prevalence of hypertrophic scarring can be up to 70% after burns, and patients suffer from pain, itching, and loss of joint mobility. To date, the exact mechanisms underlying hypertrophic scar formation are unclear, and clinical options remain limited.
Recent Advances:
Several studies have demonstrated that pathological scars are a type of hyperactive vascular response to wounding. Scar regression has been found to be accompanied by microvessel occlusion, which causes severe hypoxia, malnutrition, and endothelial dysfunction, suggesting the essential roles of microvessels in scar regression. Therefore, interventions that target the vasculature, such as intense pulsed light, pulsed dye lasers, vascular endothelial growth factor antibodies, and Endostar, represent potential treatments. In addition, the mass of scar-associated collagen is usually not considered by current treatments. However, collagen-targeted therapies such as fractional CO2 laser and collagenase have shown promising outcomes in scar treatment.
Critical Issues:
Traditional modalities used in current clinical practice only partially target scar-associated microvessels or collagen. As a result, the effectiveness of current treatments is limited and is too often accompanied by undesirable side effects. The formation of scars in the early stage is mainly affected by microvessels, whereas the scars in later stages are mostly composed of residual collagen. Traditional therapies do not utilize specific targets for scars at different stages. Therefore, more precise treatment strategies are needed.
Future Directions:
Scars should be classified as either “vascular-dominant” or “collagen-dominant” before selecting a treatment. In this way, strategies that are vascular-targeted, collagen-targeted, or a combination thereof could be recommended to treat scars at different stages.
Keywords: hypertrophic scars, microvasculature, fibroblast, vascular target, collagen target

Xi-Qiao Wang, PhD

Chen Fan, PhD
Scope and Significance
Hypertrophic scars (HS) remain a challenging issue for both patients and clinicians. The outcomes of current therapies are not satisfactory. Herein, we discuss the interdependence of vascularization and collagen in the formation of HS and suggest that targeting vascular and collagen components is an effective strategy to improve the clinical management of HS.
Translational Relevance
Therapeutic interventions that target the vasculature, such as intense pulsed light (IPL), pulsed dye lasers (PDLs), and fractional CO2 lasers, all of which excite interstitial water molecules and disrupt fibrillar collagen, can achieve satisfactory clinical outcomes when managing HS. These results offer new insights for the development of future innovative interventions.
Clinical Relevance
Traditional approaches to managing HS lack specificity and effectiveness and partially target scar tissue microvessels and collagen. Further, most interventions are associated with various undesirable side effects. In clinics, combining therapeutic agents that target vascular and collagen elements is an effective modality for the clinical management of HS.
Background
Cutaneous wound healing
Cutaneous wound healing is the process of self-repair of the skin after trauma and/or lesions. Wound healing is generally considered to occur in four overlapping phases: hemostasis, inflammation, proliferation, and remodeling. Each of these overlapping processes involves the participation of different cell populations. In response to injury, platelets degranulate to initiate thrombogenesis, endothelial cells (ECs) lining blood and lymph vessels are activated, vessels become leaky and contract, and tissue-resident innate immune cells release bursts of hydrogen peroxide, triggering white cell infiltration and inflammation. These events stimulate fibroblasts and ECs to migrate into the wound bed and proliferate, generating proteoglycan- and collagen-rich granulation tissue and microvessels de novo. The newly synthesized granulation tissue provides a substrate that enables epidermal keratinocytes to migrate laterally and close the wound, which is a process termed re-epithelialization. The wound bed is largely hypoxic; thus, the de novo generation of granulation tissue is accompanied by a burst of angiogenesis to provide oxygen and nutrition, remove waste byproducts, and support subsequent tissue maturation and remodeling. During the remodeling process, excess fibroblasts and microvessels undergo programmed cell death (apoptosis), and tissue homeostasis is restored. The interruption of this process results in the formation of pathological scars, such as HS and keloids.
Pathological scars
HS and keloids are highly prevalent after burns and trauma. Clinically, HS is defined as a raised and pruritic lesion, but it remains confined to the boundaries of the original wound. In contrast, keloids grow beyond the boundary of the original wound.1 HS usually grows rapidly and tends to regress after a long time, but keloids rarely regress,2 usually grow without limitations, and are regarded as benign tumors.3 In addition, in the scar tissue, the architecture of the collagen fibers in HS and keloids is significantly different. Keloids possess thicker collagen bundles than HS.4 Evidence also suggests that the ratio between collagen type I and type III is also different and is significantly higher in keloids (17:1) than in HS (6:1).1 Due to their distinct mechanisms and characteristic features, clinical prevention and treatment methods also vary.1 In this review article, we particularly focus on the treatment of HS.
Roles of microvessels and collagen in HS formation
Although the exact mechanisms of HS formation are still not fully understood, it is well accepted that HS formation is involved in many factors, including cells and molecules, which are activated and secreted in a cascade reaction after wound healing. In essence, cells, vessels, and collagen are three major components in the scar. Cells, including fibroblasts, myofibroblasts, and inflammatory cells, communicate mutually through cytokines and growth factors. The historical literature discussing scar management has focused on the inhibition of fibroblasts and myofibroblasts, which are the key cells that produce growth factors and collagen. Myofibroblasts are transformed from fibroblasts, which express α-SMA, possess more powerful effects than fibroblasts, and promote scar hyperplasia and contracture. In addition, inflammation cell are also regarded as playing a crucial role in scar formation, and inflammatory cytokines stimulate fibroblasts to enhance their biology. Clinical application of steroid injections to inhibit inflammation improves scar reduction. However, the roles of vessels and collagen have been overlooked during this process.
In scar tissue, fibroblasts are adjacent to and intimately associated with vessels, communicating via nutrient transport and signal transmission.5 Vessels deliver oxygen and nutrition to fibroblasts via endothelial channels that allow the passage of water, solutes, and macromolecules, which form a microenvironment for fibroblast survival and proliferation. Fibroblasts are effector cells that are regulated by the microenvironment and then secrete collagen to form scars. In this sense, vessels and collagen are upstream and downstream of fibroblasts, respectively (Fig. 1). Therefore, here, we highlight the essential roles of microvessels and collagen in HS formation and call for therapies that target the vasculature and collagen.
Figure 1.
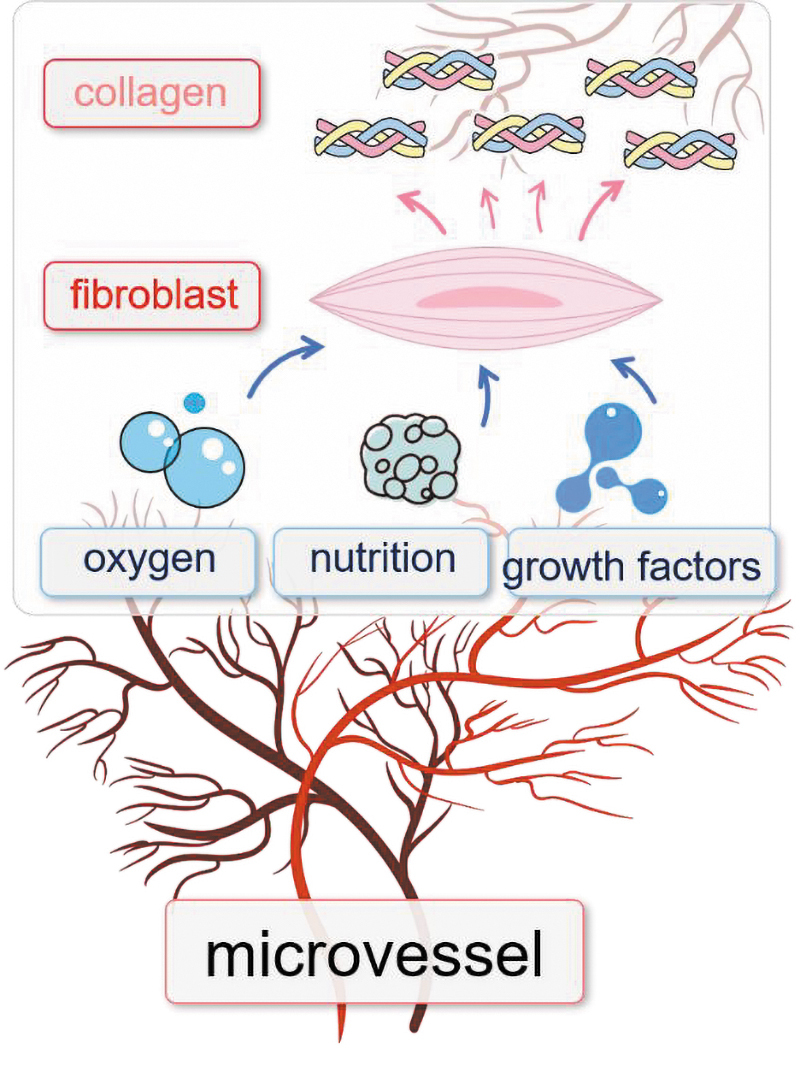
The relationship between microvessels, fibroblasts, and collagen during hypertrophic scar formation. Microvessels provide oxygen, nutrition, and EC-derived growth factors, all of which promote fibroblasts to produce collagen. EC, endothelial cell. Color images are available online.
A scar is a type of hyperactive vascular response to wounding
During scar progression, the scar initially appears as a red area with elevated thickness, which then rapidly develops into a purple–red color with further elevation. Subsequently, the scar ceases to grow and starts to slowly regress, appearing less red in color and eventually entering a mature stage. The scar color change indicates a change in blood supply in the scar tissue, which is correlated with scar formation and regression. Pathologically, Zheng et al. assessed the microvessel density of different scars and revealed prominent microvessel formation during scar formation, and most vessels were partially or completely occluded during scar regression (Fig. 2).6 The microvessel occlusion is correlated with the mechanical pressure of outer collagen on the microvessel and then causes them to be occluded. The microvessel change is consistent with another study, which found that the vessel number was higher, and the diameter was more dilated in HS than in normal scar and normal skin.7,8 In addition, laser Doppler blood flow revealed that HS also has elevated blood flow compared with that of normal scars and normal skin.9,10 Collectively, these findings indicate that a scar is a type of hyperactive vascular response to wounding, and much vascularization is involved in the development of HS,7 which shares some characteristics with tumor formation. In summary, no vessel formation and no scar hyperplasia were observed. Therefore, vascular targets can be used to prevent or treat HS formation.11,12 The evidence is as follows.
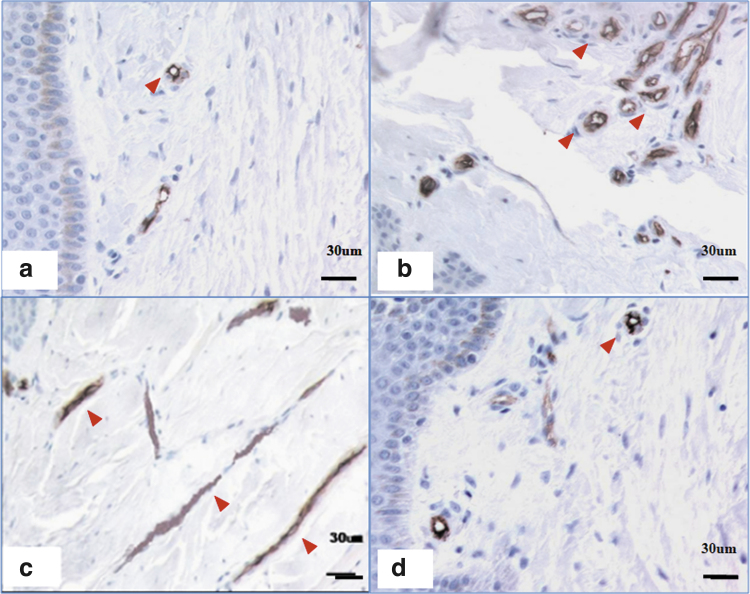
Figure 2.
CD34 staining of microvessel density in scars of different stages. (a) A few microvessels were apparent in normal skin. (b) Many microvessels were apparent in a proliferative scar. (c) Most microvessels were occluded in an RS. (d) The number of microvessels in a mature scar was comparable to that in normal skin. Scale bar = 30 μm. The red arrows indicate the microvessel. RS, regressive scar. Color images are available online.
Dynamic hypoxia and nutrition regulate scar formation and regression
Hypoxia resulting from disturbed vascularization is reported to be mainly responsible for the development of pathological scars.13,14 Using transcutaneous oximetry (TcpO2), Berry et al. measured the tissue oxygen values in HS of 16 patients before pressure therapy and found tissue oxygen values ranging from 2 to 66 mmHg,15 indicating that different scars had different TcpO2 values. Zheng et al. classified scars as early scars (1–2 months), proliferative scars (3–6 months), regressive scars (∼2 years), and mature scars (over 4 years) and found that the hypoxia is dynamic during scar progression. There was mild hypoxia in the early stage (51.2 ± 8.3 mmHg), but it increased in proliferative scars (30.2 ± 6.1 mmHg) and became severe in regressive scars (6.9 ± 2.1 mmHg), almost returning to normoxia in mature scars (71.1 ± 9.6 mmHg).6 During this process, it has been reported that hypoxia-inducible factor-1 (HIF-1), which senses hypoxia and regulates vascular endothelial growth factor (VEGF) transcription, is elevated in proliferative scars and reduced in the regressive stage.6,16 Lynam et al. simulated scar hypoxia and malnutrition in scars and found that moderate hypoxia and malnutrition enhance fibroblast proliferation and collagen production; however, severe hypoxia and malnutrition are associated with fibroblast inhibition and apoptosis.17 Therefore, scar formation and regression are regulated by dynamic hypoxia and nutrition changes, which are caused by microvessel change in tissue.
Endothelial dysfunction induces scar regression
Fibroblast biology is generally regulated by the surrounding tissue microenvironment,18 and capillaries play a key role in establishing this environment. The endothelium can be viewed as a modulatory interface between the microvessel lumen and neighboring cells.19 In addition to their critical role in transport, the endothelium of microvessels is also a significant endocrine organ, synthesizing and releasing numerous growth factors, including VEGF, platelet-derived growth factor (PDGF), transforming growth factor beta 1 (TGF-β1), and endothelin-1 (ET-1).20,21 Endothelium-derived growth factors are critical regulators of the development and maintenance of many organs, such as the liver, pancreas, and nervous system.22–24 Evidence supports a direct correlation between ECs and neurons in the brain.25 Indeed, ECs not only exert a protective effect on neurons,26 but they also initiate tissue repair processes after injury and support neurite outgrowth by secreting shared growth factors.24,27 Therefore, vascular ECs play important roles in regulating cell biology and tissue homeostasis. Recent studies have also demonstrated that endothelial dysfunction occurs during the formation of HS.28,29 In regressive scars, the microvascular lumen is almost completely occluded, and the ECs are atrophied and surrounded by a thick collagen layer (Fig. 3). In addition, the expression of EC-derived growth factors such as VEGF, PDGF, TGF-β1, and ET-1 in regressive scars is significantly decreased compared with that in uninvolved skin. In vitro experiments using media conditioned by ECs, isolated from regressive scars, showed inhibited cell viability and attenuated collagen biosynthesis and apoptosis induction in fibroblasts. These effects were found to be mediated via TGF-β1-, PDGF-, and basic fibroblast growth factor (bFGF)-related signaling pathways, suggesting that ECs within regressive scar tissue contribute to scar regression.29
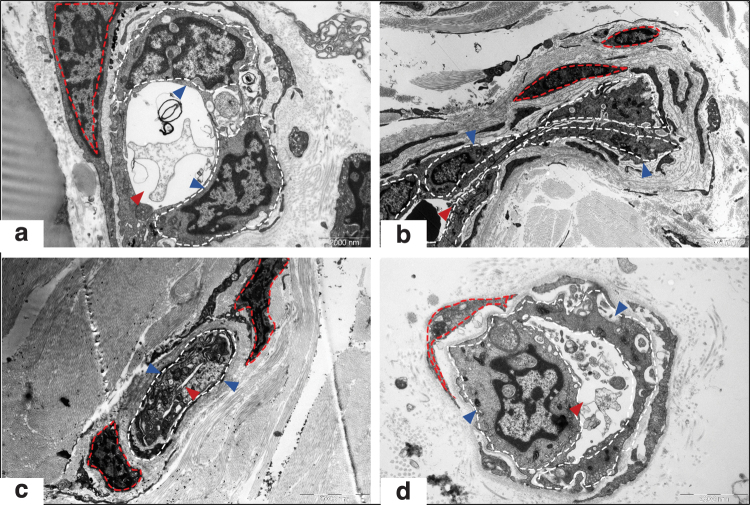
Figure 3.
Endothelial dysfunction in human hypertrophic scars examined by electron microscopy. Morphological changes in the microvessels and ECs in NS (a, × 9,700), PS (b, × 4,200), RS (c, × 5,800), and MS samples (d, × 13,500) are shown. The blue arrow indicates ECs; the red arrow indicates the lumen of microvessels; the white dotted line shows the edges of ECs; and the red dotted line shows smooth muscle cells. These images demonstrated that during scar development, the microvessel was gradually occluded and EC became atrophy, the basement membrane was replaced with thick collagen fibers, particularly in RSs. (This figure is cited from Mari et al.2). NS, normal skin; PS, proliferative scar; MS, mature scar. Color images are available online.
Chronic inflammation induced by vascular hyperpermeability causes scar formation
Excessive inflammation, as characterized by continuous and histologically localized inflammation in the reticular layer of HS, is believed to be one of the most important events precipitating HS fibrogenesis.30,31 Proinflammatory factors, such as interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor-α (TNF-α), are all upregulated in keloids, promoting chronic inflammation and likely also driving the invasive growth of keloids.32,33 The upregulated proinflammatory factors in pathological scars suggest that keloids and HS are a type of inflammatory disorder of the skin. Qian et al. used a rabbit ear model to demonstrate that prolonged inflammation triggered by heat-killed Pseudomonas aeruginosa impairs wound healing and increases scarring.30 The application of indomethacin and/or anti-inflammatory agents significantly reduced scar development in this model.30 Ogawa and Akaishi concluded that tissue inflammation in HS and keloids is associated with increased vascular permeability resulting from microvascular dilation during fibrogenesis.5 Increased vascular permeability allows inflammatory cell egress and migration into the interstitial space. According to the extent of inflammation, keloids are classified as strong inflammatory scars, whereas HS are mild inflammatory scars.5 Therefore, targeting vessels could reduce the permeability of inflammatory cells and then reduce scar formation.
Collectively, substantial evidence supports the interpretation that the formation of scar tissue is a type of hyperactive vascular response. Formation and regression are correlated with vascular changes, including oxygen/nutrition supply and EC-derived growth factors secretion and inflammation cells permeability; therefore, the vasculature offers a prospective target for scar management.
A scar is a type of abnormal collagen accumulation
In addition to microvessel formation, the excessive deposition of collagen is another characteristic feature of HS. A large amount of collagen deposits in the proliferative scar and has a little reduction in regressive scars, which almost goes to a normal level in mature scars (Fig. 4). It is well established that collagen constitutes approximately one-third of the proteinaceous mass in the human body; however, the proportion of collagen in HS tissues is largely higher than that.34 Several growth factors, including TGF-β1, PDGF, bFGF, and ET-1, contribute to increased collagen production; however, collagen homeostasis is also subject to the balance between matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinase-1 (TIMP-1),35–37 and increased collagen synthesis and reduced degradation cause collagen overdeposition. Supporting this perspective, Ghahary et al. found that the production of collagenase was reduced in fibroblasts from HS compared with that from normal skin.36 Thus, the overproduction and accumulation of collagen increases the scar volume, resulting in scar tissue that is elevated and firmer than adjacent tissue.
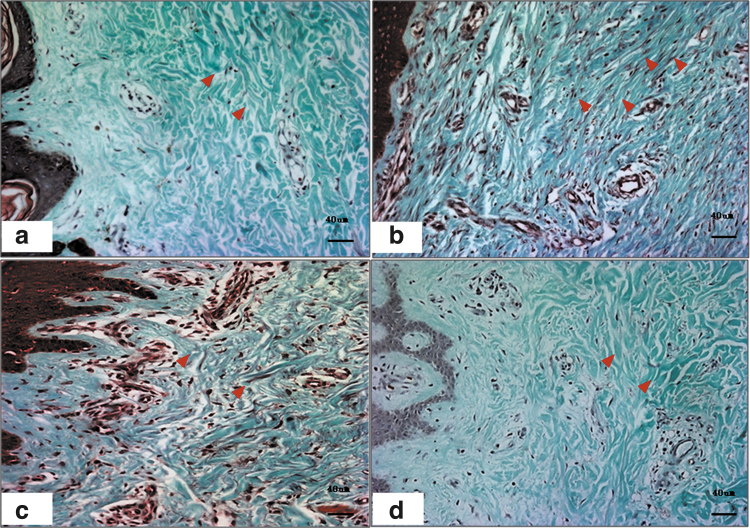
Figure 4.
Collagen examination of human hypertrophic scars at different stages by Masson's staining. In normal skin, the collagen arrangement is loose (a). However, in hypertrophic scars, a large amount of collagen is deposited around the microvasculature (b). In RSs, the microvessels are partially or totally occluded, and the collagen density is decreased (c). In mature scars, the microvessels and collagen density are comparable to those of normal skin (d). The red arrows indicate the microvessels. Color images are available online.
Although targeting vascular elements can affect fibroblast activity and de novo collagen synthesis, it does not affect the collagen that is already deposited in situ. Indeed, residual collagen constitutes most of the scar volume, particularly late-stage scars. Thus, therapies targeting vessels are predicted to be more effective for early scars, and less effective for late scars with abundant collagen. Existing therapeutic approaches, such as the antimitotic drug fluorouracil (5-FU) and the injection of steroids, are intended to inhibit fibroblast collagen production rather than reduce the volume of existing collagen. Thus, targeting collagen is necessary for hard HS at the late stage.
Differences between the prevention and treatment of HS
Traditionally, a scar is often treated when it is formed. However, with a better understanding of wound healing, the prevention of scars has become increasingly important in the clinic. The most successful HS management is prevention. HS can be intervened during wound healing or at an early stage after wound healing, which constitutes the time window of scar pregnancy. Once a scar is formed, treatment is very difficult.38 Therefore, scar prevention is implemented in advance to inhibit scar occurrence. For instance, for surgical excision, techniques such as atraumatic closure, minimization of tension, and skin eversion are normally applied. After wound healing, silicon and paper tape are routinely used to prevent scar formation. For deep burn wounds or trauma, wound healing should be promoted. Usually, the earlier a wound heals, the less scarring there will be. Within 2 weeks of healing time, there was no or minor scar formation. After this, the longer a wound heals, the more severe the scar. Therefore, after healing, pressure therapy, silicone, and lasers should be used promptly, which is the best intervention window for scar prevention. Although scar hyperplasia can sometimes still not be controlled completely, this process will greatly reduce hyperplasia. Therefore, to enhance the effectiveness of scar treatment, optimizing the treatment modality is necessary.
Discussion of Findings and Relevant Literature
Traditional treatments for HS
It is well accepted that the primary cause of HS formation is the excessive accumulation of collagen secreted by fibroblasts; thus, most of the current therapies mainly focus on inhibiting the biosynthetic activities of fibroblasts. Some therapies also partially work on microvessels; however, the mechanisms of action of these therapies are, at best, partially understood. The side effects are common, contributing to patients' reluctance to access clinical scar management.
Silicone therapy
Silicone therapy, including silicone sheets and gels, is recommended as a noninvasive first-line prevention and treatment option for HS and keloids.39 For larger wound areas (such as joints) or wounds on exposed areas (such as the face and hands), the use of silicone is recommended owing to its convenient application. Silicone sheeting was shown to reduce the incidence of HS formation in high-risk patients.40 It has been reported that silicone therapy functions by reducing erythema and improving the pliability of HS.41 Although an exact mechanism has not been clarified, improving skin stretching, occlusion, and hydration may be the effects of silicone products on HS.42 Hydration of the stratum corneum reportedly results in increased oxygen permeability, thereby reducing hypoxia-induced angiogenesis and tissue growth.43 Another study showed that silicone application increases the surface temperature of HS, which might enhance collagenase activity; however, this theory requires further investigation.44 In addition, silicone gel or silicone gel sheeting can also be combined with other therapies to improve therapeutic outcomes. For example, a current study demonstrated that the combination of silicone gel with mixed extracts from Allium cepa, Centella asiatica, Aloe vera, and paper mulberry significantly inhibits the growth of HS in median sternotomy wounds.45 Further, given that the physical properties of silicone dressings are beneficial for scar management, they can also be used as dermal drug delivery vehicles.46
Silicone therapy often causes skin maceration due to the accumulation of water below the silicone product.47 Other side effects are also frequently observed, such as persistent pruritus, skin breakdown, rash, and a foul smell, which decrease patient compliance with the use of silicone.48 Notably, although silicone therapy is strongly recommended for HS management because it may have modes of action that complement other therapies,41 complications can arise when combining silicon use with other therapies.48
Compression therapy
Many burn centers around the world recommend compression therapy (pressure garments) as a standard first-line clinical intervention, particularly for the management of large-area burns. Compression therapy is intended to proactively manage the development and progression of scar tissue after burn injury, trauma, and surgical wounds. Compression is reported to be effective in 60–85% of patients, reducing scar volume and supporting the recovery of pliability.
It is clear that pressure exerted by compression garments limits the blood supply to the scar tissue. Compression therapy also markedly improves symptoms associated with hypertrophy, such as pruritis (itch), pain, and contraction. One study applied laser Doppler perfusion to measure the changes in scar blood perfusion after compression therapy and demonstrated that the application of 15–25 mmHg pressure was sufficient to reduce blood perfusion and attenuate nutrients and oxygen, which resulted in severe hypoxia and malnutrition within scar tissue.49 Hypoxia and malnutrition directly inhibit fibroblast activity, affecting collagen turnover and eventually precipitating scar regression.50 Compression was also found to induce dermal fibroblast apoptosis and stimulate IL-1β and TNF-α secretion in vitro.51 However, the role of compression-induced IL-1β and TNF-α expression in scar regression in vivo remains to be established. Compression has also been found to attenuate the accumulation of collagen by stimulating the expression of collagenases and MMP-952,53 and affecting the balance of collagen types in HS tissues.54
Nevertheless, the use of compression therapy is controversial. Many published studies conclude that compression therapy has limited effectiveness when managing burn wounds, and it is reportedly associated with adverse outcomes such as abnormal bone growth.55 Severe side effects associated with compression therapy are reportedly more prevalent among children. Apart from predictable discomfort and mechanical abrasions, other side effects include edema, eczema, and, in some children, growth retardation.56
Steroids
Intralesional corticosteroid injections have been used to treat pathological scars since the mid-1960s, and they continue to play a primary role in the clinical management of HS and keloids. Evidence suggests that keloids regress 50–100% after corticosteroid injection; however, the rate of recurrence can be up to 50% after 5 years.57,58 The Updated International Clinical Recommendations on Scar Management recommend that intralesional corticosteroids be used as a second-line treatment for HS but as a first-line treatment for keloids.58 For HS, intralesional corticosteroids are an adjuvant to silicone treatment if a scar fails to improve after 2 months of silicone gel treatment or when the scar is severe or pruritic.59 Therefore, the administration of these steroids has occurred more often in keloid studies.
In addition to their well-described anti-inflammatory activity, the administration of corticosteroids promotes vascular dysfunction and EC death. Wu et al. reported that in situ injection of the corticosteroid triamcinolone promoted HS regression by downregulating VEGF expression.60 This mechanism underlies the use of glucocorticoids as a first-line therapy for the management of hemangiomas. In vitro studies have confirmed that dexamethasone and cortisol antagonize the development of endothelial sprouts and tube formation stimulated by PGF2a and VEGF.61 Further, prolonged exposure to glucocorticoids is toxic to ECs and triggers caspase-regulated cell death pathways.62 It is thought that the direct injection of glucocorticoids into scar tissue is effective, because it induces tissue necrosis by the same mechanism—inducing caspase-regulated cell death of ECs and vascular collapse.63
There is currently a broad consensus that intralesional steroids are the preferred first-line therapy for treating isolated and small scars. However, due to the high incidence of recurrence, current clinical recommendations state that in situ steroid injections be combined with other modalities, such as PDL, irradiation, 5-FU, or cryotherapy. The rationale for this is to attenuate fibroblast proliferation and minimize recurrence.59
Radiotherapy
Exposure to ionizing irradiation effectively induces EC apoptosis in the tumor vasculature, resulting in tumor vessel disruption and the inhibition of tumor growth. Keloids are considered a type of benign tumor; therefore, radiotherapy is often used in combination with surgery for treating keloids. The use of radiotherapy to inhibit scar growth was first reported in 1906.64 Electron beam (β-ray) irradiation is considered to be the optimal radiotherapy approach, as it has better dose distribution and safety than other radiotherapy modalities.65 Current clinical recommendations state that the dose in each fraction should be >12 and <20 Gy to optimize the therapeutic effect while minimizing adverse effects.66
The current consensus is that radiotherapy primarily acts by suppressing fibroblast activity; however, recent evidence has shown that ECs are more sensitive to ionizing radiation than dermal fibroblasts, suggesting that radiotherapy may be effective due to inducing endothelial dysfunction.5 Irradiation induces the expression of p53 and p21, triggering apoptosis in ECs and preventing EC proliferation, migration, and sprouting activities in vitro.67 High-dose ionizing radiation suppresses VEGF- and FGF-2-induced angiogenesis in vivo; thus, it is postulated that radiotherapy largely acts by suppressing EC viability and physiological activity, which, in turn, prevents the de novo formation of blood vessels, leading to anoxia (and the accumulation of metabolic byproducts, acidosis) and the suppression of keloids. This interpretation is supported by clinical observations in which the color of keloids improved almost immediately after irradiation, and prominent scars were observed to gradually flatten after radiation therapy.68
Extensive clinical evidence supports the combined application of radiotherapy with surgical excision as the most effective prevention for keloids, with minimal recurrence.68 Low-dose fractionated radiotherapy delivered within 24 h of excision generally yields good results.69 The side effects of radiation therapy are usually well tolerated and include pigment change, pruritus, and erythema. The risk of carcinogenesis associated with radiation therapy is manageable and is typically very low when the surrounding tissue is adequately protected.70
Cryotherapy
Cryotherapy for treating scars can be applied through different approaches, for example, by direct contact, surface spray, and/or intralesional injection. Har-Shai et al. demonstrated that the intralesional needle cryoprobe method was more effective than methods using superficial contact or spray probes.71 Layton et al. found that smaller lesions responded to cryosurgery significantly better than larger lesions in the same patient.72 The combination of cryotherapy with intralesional injections of corticosteroid (triamcinolone) yields significant improvements in HS and keloids, and 33.3% of lesions were found to improve after one or two treatments. This increased to 78.9% in lesions exposed to three or more interventions. Interestingly, HS has a higher response rate to cryotherapy (76.3%) than keloids (50.9%).73
Cryotherapy has been found to stimulate the remodeling of collagen fibers in scar tissues into a more compact parallel organization compared with untreated scar tissues.71,74 Cryotherapy also reduces the myofibroblast population and the expression of TGF-β1 in scar tissue.74,75 Cryotherapy is believed to induce vascular damage, precipitating anoxia and tissue necrosis. The side effects of this treatment modality are manageable and include local pain, edema, and inflammation. Precooling the tissue activates cold-activated transient receptor potential sensory neurons and vasoconstriction. It should be noted, however, that prolonged cold-induced vasoconstriction carries a risk of tissue ischemia and nonfreezing cold injury, which can result in tissue necrosis and neuropathy. In addition, reestablishing tissue blood flow after prolonged periods of tissue ischemia carries a further risk of reperfusion injury. Despite these risks, cryotherapy can benefit scar regression.
Unfortunately, poorly administered cryotherapy is not without side effects. These include permanent hypo- and/or hyperpigmentation, moderate skin atrophy, blistering, and postprocedural pain. It is, therefore, essential to establish appropriate protocols and procedures for cryotherapy to minimize adverse events and ensure patient benefit.
Occlusion therapy
It has been reported that an occlusive dressing (silicone-free) significantly reduces HS formation, indicating that occlusion itself might be the mechanism of action of HS treatments.76 Occlusion therapy has been demonstrated to attenuate collagen deposition by inhibiting the expression of TGF-β and other growth factors secreted by keratinocytes.77 In addition, severe hypoxia and/or malnutrition caused by microvessel occlusion suppresses fibroblast activities and triggers apoptosis, which facilitates scar regression. Transepidermal water loss, which is a measurement used to evaluate water loss after applying occlusive agents, was found to be decreased after occlusive therapy, supporting the role of occlusion in restoring barrier function. Occlusion therapy exerts an effect by establishing homeostasis of the epidermal barrier layer, that is, the stratum corneum.76 A further study demonstrated that occlusion increases keratin and antifibrotic cytokine TNF-α expression but decreases the expression of IL-1β, a profibrotic cytokine, resulting in a reduction in profibrotic signaling within the dermis through TGF-β family members.78 Occlusion therapy can yield some complications, such as eczema and an unpleasant odor.
Challenges of traditional therapies and the potential of vascular- and collagen-targeted therapies
Although the traditional therapies described earlier are widely used in clinical practice, other therapies are available, and an ideal treatment that can manage all types of scars has not been found. In addition to the lack of an evidence-based mechanism of action, we identified two major reasons limiting the clinical outcomes of current therapies: side effects and residual collagen.
Side effects of current treatments
Extreme conditions, such as severe hypoxia and/or malnutrition caused by microvessel occlusion, are required to inhibit fibroblast activity, induce cell apoptosis, and achieve scar regression. However, achieving such conditions is nontrivial while simultaneously minimizing off-target and unintended side effects. For example, under standard care compression therapy, it is recommended that a pressure of ∼25 mmHg should be maintained for at least 23 h per day, and the garment should be exchanged when it is no longer capable of maintaining this pressure. However, some patients are reluctant to exchange their compression garments with such frequency due to the discomfort of removing and redressing in new garments, as well as the financial cost of frequently purchasing new garments. Combination therapies, including glucocorticoid injection, laser therapy, and cryotherapy, are accompanied by pain in ∼88% of patients. Unsurprisingly, maintaining patient compliance is challenging and a barrier to the effective application of these treatments. Steroid injections and radiotherapy are also associated with an increased risk of carcinogenesis and ulceration development.79 The frequency and severity of side effects limit the therapeutic effectiveness of many current clinical modalities intended to occlude the microvasculature of pathological scars.
Residual collagen in scar tissues
Although severe hypoxia/malnutrition and endothelial dysfunction are effective inhibitors of collagen production, they have no impact on existing collagen deposits, which is true for steroid injection, radiotherapy, and cryotherapy. Although pressure therapy and occlusion therapy have an effect on local collagen to some extent,77,80 the effect is limited. Therefore, targeting collagen is necessary to remove the excess collagen in such scars.
The potential of vascular- and collagen-targeted therapies
To date, the rapid development of laser therapy and phototherapy has enabled these modalities to replace traditional treatments in many medical fields, including the clinical management of pathological scars. Several new and emerging therapies designed to disrupt the microvasculature in scar tissue have shown encouraging preliminary results. For example, IPL and PDLs are two widely used vascular-targeted therapies for scar treatment. Fractional CO2 lasers and collagenase are two major collagen-targeted therapies that provide improved clinical outcomes in late-stage scars, and they are discussed later.
Therapies targeting scar tissue vasculature
PDL and IPL
The PDL is a nonablative laser technology that has gained prominence owing to its effectiveness in treating vascular lesions and delivering high clinical efficiency with low risk.81 In recent years, 595 nm PDL has been combined with a fractional CO2 laser (10,600 nm) to yield beneficial clinical outcomes for recently developed erythemic HS.82 PDL selectively targets the oxygenated hemoglobin present in red blood cells, triggering hemolysis and coagulation, which occludes microvessels and causes microischemia.83 Microvascular necrosis reduces the density of blood vessels and subsequently reduces the objective and subjective symptoms of HS. In addition, PDL has been found to attenuate the expression of TGF-β1 and collagen III deposition in scar tissue.84 PDL is especially effective during the early stages of scar development, when microvessel formation is maximal. It is speculated that microischemia may also induce the release of collagenases and thus have indirect effects on collagen accumulation.85
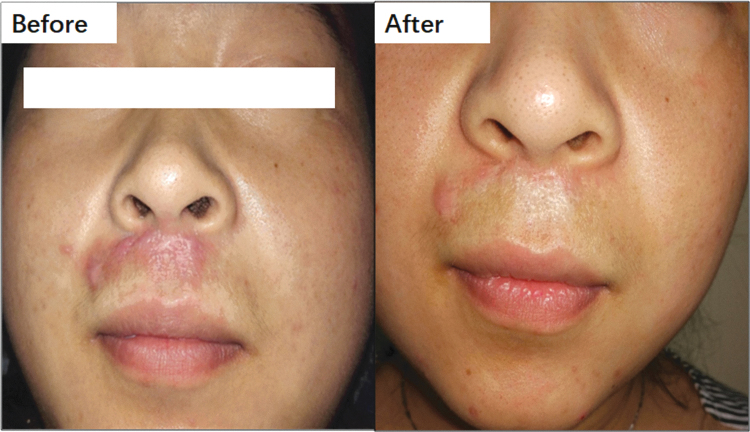
IPL is a nonlaser modality that is widely used to treat various dermatological conditions, including rosacea, disseminated parakeratosis, pilonidal cysts, seborrheic keratosis, HS, and keloids.86 IPL delivers low-energy light to the lower skin layers (dermis) without affecting the superficial layers (epidermis) of the skin. Unlike lasers, IPL delivers a spectrum of wavelengths (or colors) in each pulse of light, which can range from 500 to 1,300 nm. IPL effectively targets both oxyhemoglobin and pigment chromophores; therefore, it is suitable for treating hyperpigmented, erythematous, and proliferative scars. IPL has been demonstrated to improve scar appearance, texture, height, and color (Fig. 5).87 In one clinical study, keloids were 89.1% improved, as measured by the Vancouver Scar Scale (VSS), after IPL when combined with corticosteroid injection.88 Although IPL is not entirely free from side effects (e.g., incidental pain, blistering, and purpura), its prevalence is much lower than that of other interventions for HS.
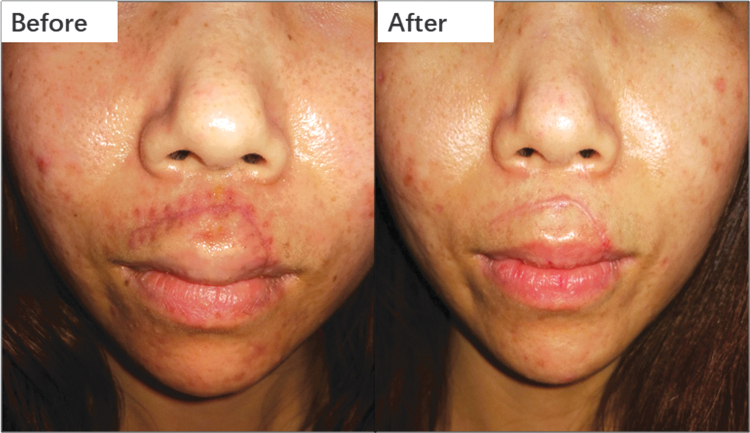
Figure 5.
This scar presented with redness. IPL, a vascular-targeting therapy, was applied once per month for 4 months, resulting in marked regression. IPL, intense pulsed light. Color images are available online.
Endostatin and Endostar
Endostatin is an endogenous peptide derived from the proteolytic cleavage of type XVIII collagen, is a potent inhibitor of EC proliferation and migration, and stimulates EC apoptosis.89 Through this mechanism, it is an effective antitumor therapy. Endostatin is highly specific for ECs and has thus been investigated for therapeutic benefits in many proliferative diseases.90 Endostar is a modified recombinant human endostatin that retains broad-spectrum antiangiogenic activity.91 In 2005, Endostar was licensed by the China State Food and Drug Administration for use in combination with vinorelbine-cisplatin as a first-line therapy for the clinical management of advanced non-small cell lung cancer.92
HS tissue shares several properties in common with carcinomas, such as rapid growth and enhanced angiogenesis. For these reasons, Wang et al. established a rabbit-ear HS model and treated HS with Endostar injection once per week for 3 weeks. The volume of microvessels and the number of viable cells were significantly decreased in the HS tissue after Endostar injection.93 The activity of Endostar is mediated by antagonizing the VEGF pathway.94,95 In addition, in rabbit scar models, Endostar reportedly attenuates the formation of HS by inhibiting the expression of VEGF and TIMP-194 and downregulates dermal fibroblast proliferation via G0/G1 and/or G2/M phase cell cycle arrest.96
Although a few publications have reported outcomes of the clinical application of Endostar for the management of HS, we look forward to the results of this promising therapeutic modality.
VEGF monoclonal antibody
VEGF is known to be a key player in physiological and pathological angiogenesis. It is, therefore, unsurprising that VEGF has emerged as a key target for managing angiogenesis in cancer and other hyperproliferative diseases. Several new anti-VEGF antibodies are under development and are in the process of preclinical validation or have progressed to human clinical trials to assess their clinical effectiveness. Bevacizumab, which was the first anti-VEGF antibody drug, is widely used to treat various cancers, including colorectal cancer97 and cervical cancer.98
Pathological scars, including HS and keloids, are known to express high levels of VEGF, suggesting that targeting VEGF may benefit and improve scars in some individuals.99 Shen et al. established a rabbit HS model and injected a VEGF monoclonal antibody into scar tissue over the course of 3 weeks.100 Antibody injection triggered apoptosis in fibroblasts and ECs, causing the scar tissue volume to decrease.100 Subsequently, Kwak et al. applied the VEGF antibody to rabbit ear wounds, which significantly inhibited subsequent scar formation by preventing de novo angiogenesis.101 The precise mechanism by which VEGF reduces scar formation and the clinical validity of inhibiting VEGF pathways are currently under investigation. Shi et al. reported that a gel consisting of anti-VEGF antibody modified with paeonol liposomes significantly attenuated the scar hyperplasia index in a rabbit ear scar model by downregulating the expression of VEGF, TGF-β1, and TNF-α.102
Although in vitro and in vivo studies have provided encouraging data supporting the potential use of anti-VEGF antibodies in scar management, there is still insufficient evidence for clinical adoption. Therefore, clinical validation is required to support the use of this modality.
Photodynamic therapy
Photodynamic therapy (PDT) has a long application history in dermatology. A large-scale clinical study on the use of PDT in skin tumors was first reported in 1978.103
Vascular targeted PDT was originally used for treating port-wine stain (PWS), which is a discoloration of human skin caused by a vascular anomaly. The PDT can selectively eliminate abnormal PWS vessels, achieving more effective results than PDL.104,105 It has also been widely used for other vascular treatments, such as prostate cancer and brain cancer.106,107 The procedure initially involves intravenous infusion of a photosensitizer, which is then circulated systemically, and only the targeted area of the lesion is illuminated by laser light with fixed power and energy. The photosensitizer is then activated and induces irreversible damage to the vascular endothelium, which is quickly followed by vessel occlusion by thrombosis leading to tissue necrosis.108–110
A study compared PDT and PDL in patients with PWS, and the results demonstrated that PDL is more suitable in younger patients with superficial lesions, whereas PDT is more suitable for thicker lesions. The combination of PDL and PDT can reduce the total treatment duration as well as frequency.111 A clinical case study reported that HS softened and became more pliable after PDT thereapy.112 The number of elastin fibers was significantly increased in biopsies of these HS lesions.112 Similar results were also observed in another case report by Bruscino et al.113
Due to insufficient in vivo and clinical data, further validation is required before wide use for treating pathological scars.
Therapies targeting collagen
Fractional CO2 laser
CO2 lasers have a wavelength of 10,600 nm, which is well within the infrared portion of the electromagnetic spectrum. Due to their long wavelength and high-water absorption coefficient, CO2 lasers can penetrate into the deep dermal layers of human skin. Clinical studies have demonstrated that fractional CO2 lasers significantly improve HS within 6 months, according to the assessment by VSS and the Patient and Observer Scar Assessment Scale.114 A recent study reported that early intervention by fractional CO2 laser therapy combined with Z-plasty can be used as a potential treatment for HS after burns.115 The photothermal energy of CO2 lasers results in skin carbonization and vaporization, causing collagen denaturation and tissue dehydration.116 Significant tissue coagulation occurs after the skin temperature reaches 400°C during CO2 laser irradiation with three stacking pulses.117 Along with the increased dermal temperature caused by the CO2 laser, significantly more denatured and coagulated collagen is observed.118 This correlates with the improved clinical parameters of surface smoothness and skin pliability. Histological analysis indicated evident changes in the upper dermis, including newly formed dermal papilla.119 The expression of TGF-β1 in scar tissue was also found to be significantly decreased after CO2 laser treatment.120 In addition, CO2 laser treatment has been found to stimulate the expression of MMP-1, which plays key roles in collagen degradation.121
Compared with PDL lasers, fractional CO2 lasers are often applied to thickened scars and lesions with larger collagen deposits. CO2 lasers are usually applied as a part of a combination therapy. An initial application of PDL is used to target the microvasculature, and subsequently, a fractional CO2 laser is applied to target intralesional collagen (Fig. 6).
Figure 6.
This scar presented a raised height. A fractional CO2 laser, a collagen-targeting therapy, was applied once per month for 3 months, after which the scar was almost flat. Color images are available online.
Collagenase intervention
Collagenase is a metal-binding protease that is a member of the MMP protein family and it was first identified in 1962. Collagenase hydrolyzes the triple helical domain present in the four most abundant collagens (types I, II, III, and IV). Collagenase has been employed as a treatment for diseases caused by excessive collagen deposition, such as Dupuytren's contracture.122 Compared with surgical fasciotomy, collagenase injection is more efficient in treating Dupuytren's contracture, producing fewer side effects with a greater reduction in contracture.122 Collagenase administration is also widely used for the clinical management of Peyronie's disease, intervertebral disk herniation, and wound debridement.123
Using the popular rabbit ear scar wound-healing model, Jia et al. reported that collagenase administration significantly reduced the growth of scar tissue compared with the untreated control.124 Bae-Harboe et al. treated earlobe keloids in six patients by injecting a commercial collagenase preparation combined with compression therapy over a 12-month period and observed that the size of individual keloids was reduced by more than 50%; however, complete regression was not achieved.125 Notably, a number of adverse side effects were reported in this study and included injection site swelling, tenderness, and self-resolvable ulceration.125 However, Kang et al. observed the recurrence of keloids, and HS was observed in some patients within 6 months after receiving collagenase injection.126 The apparent inconsistency between these two studies might be attributed to the absence of compression therapy in the latter study; however, from a patient perspective, collagenase is an effective treatment for keloids. The potential mechanism underlying collagen disruption for scar remediation could be that changes in collagen stiffness have an effect on fibroblast activity. There is evidence that changes in matrix stiffness can regulate cell behavior.127
These findings also suggest that the combined application of vascular- and collagen-targeted therapies delivers improved therapeutic outcomes for patients. Several therapies that target collagen are now available, for example, MMPs such as collagenase, gelatinase, and stromalysin, as well as other proteases that hydrolyze components of the extracellular matrix. However, the clinical application of collagenase therapy remains largely overlooked and unutilized. It has yet to be included in standard recommendations and clinical guidelines for the management of pathological scars. Exploring clinical applications for these bioactive compounds holds promise for the management of mature scar tissue.
Novel collagen-targeted therapies
Connective tissue growth factor (CTGF), which is excreted by fibroblasts and ECs, functions as a crucial factor in hyperscarring formation after tissue injury. The peptide-mediated downregulation of CTGF by siRNA/KALA nanocomplexes modulates the collagen fibril organization and diameter in wounds and produces weave-like collagen arrangements that are comparable to normal skin.128 Pamrevlumab, which is a fully recombinant human monoclonal antibody against CTGF, has been demonstrated to attenuate the progression of idiopathic pulmonary fibrosis and is now in a phase 3 clinical trial.129 This antibody represents a novel therapy for treating skin fibrosis. Galunisertib, as a TGF-β receptor type I kinase inhibitor, is a potential candidate for the treatment of liver fibrosis. It exerts an antifibrotic action by blocking the production and maturation of collagens, as well as promoting their degradation.130 MicroRNA-29 (miR-29) negatively regulates fibrosis and is downregulated in multiple fibrotic organs and tissues, including the skin. Remlarsen, which is an miR-29b mimic, has been shown to repress collagen expression in incisional skin wounds and was demonstrated to have an effective therapeutic effect in preventing fibrotic scar formation.131 These novel therapies that target collagen have undergone preclinical studies and might be promising treatments for reducing collagen deposition.
Future Directions
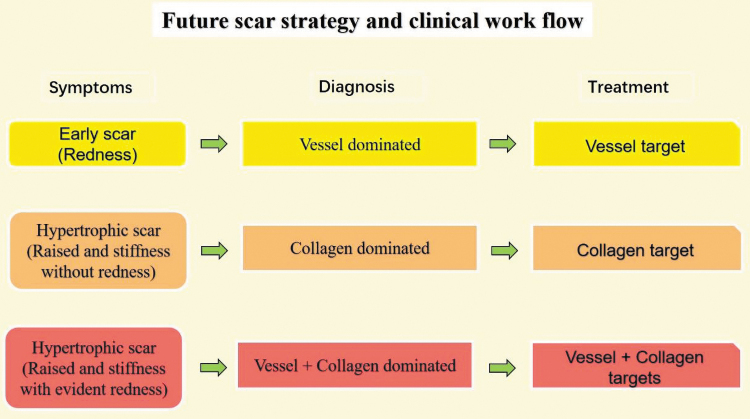
Past and current studies of scar formation have primarily focused on the biology and physiology of dermal fibroblasts. The roles of microvasculature, ECs, and the residual collagen in scar formation have been largely overlooked. Targeting the microvasculature and collagen are promising strategies for intervening in scar formation and maturation processes. We envision a new concept of classifying scars as “vascular-dominant” or “collagen-dominant” subtypes, which, in turn, will inform clinical interventions and management, delivering effective and improved outcomes for patients. For example, for “young” scars (≤2 months) that are flat with overt erythema, a vascular target is indicated as the primary therapy. For older scars (>2 months) with increased height and hardness and reduced erythema, a collagen target is indicated as the primary therapy. For scars with redness and significantly raised height, a combination of therapies that include vascular and collagen targets is indicated (Fig. 7). This proposed treatment strategy also matches the concept of precision medicine, that is, “feature analysis target-oriented treatment-specific outcomes with fewer side effects.”
Figure 7.
Future strategy and clinical work flow diagram for hypertrophic scars. The scar treatment is selected based on whether the scar is vessel- or collagen-dominant, and the corresponding targeted treatment will be applied. Color images are available online.
Summary
Herein, we have introduced and discussed a number of therapeutic options that are suitable for clinical intervention and the ongoing management of vascular- and collagen-dominant scar tissue. We believe that this alternative perspective of scar pathology offers insights to more appropriate treatment applications and the future development of therapeutic modalities with improved efficacy and fewer side effects for scar management. Of course, current therapies are still encouraged and could be combined for scar treatment.
Ethics Approval
The study protocol related to patients was approved by the Ethics Committee of Ruijin Hospital. Written informed consent was obtained from all participants before the intervention. Patients consented to the publication of their images.
Take-Home Messages
Current therapies for HS are often disappointing and often cause significant side effects.
Because HS are a type of hyperactive vascular response to wounding, vascular-targeted therapies are recommended for early-stage scars.
For late-stage scars, therapies targeting residual collagen are encouraged.
Classifying scars as vascular dominant or collagen dominant will improve treatment selection and application and produce better patient outcomes.
Combination treatment that includes vascular- and collagen-targeted therapies is advocated for use in future clinical practice.
Abbreviations and Acronyms
- 5-FU
fluorouracil
- bFGF
basic fibroblast growth factor
- CTGF
connective tissue growth factor
- ECs
endothelial cells
- ET-1
endothelin-1
- HS
hypertrophic scars
- IL
interleukin
- IPL
intense pulsed light
- miR-29
microRNA-29
- MMP
matrix metalloproteinase
- MS
mature scar
- NS
normal skin
- PDGF
platelet-derived growth factor
- PDL
pulsed dye laser
- PDT
photodynamic therapy
- PG
prostaglandin
- PS
proliferative scar
- PWS
port-wine stain
- RSs
regressive scars
- TcpO2
transcutaneous oximetry
- TGF-β1
transforming growth factor beta 1
- TIMP
tissue inhibitor of metalloproteinase
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- VSS
Vancouver Scar Scale
Authors' Contributions
B.Y.: searched the literature and wrote the article. Z.U. and D.L.: revised the article. C.F.: searched the literature and wrote the article. X.-Q.W.: designed the protocol, searched the literature, and wrote and revised the article.
Acknowledgments and Funding Sources
This work was supported by a grant from the National Natural Science Foundation of China (No. 81671914 and No. 81101433; B.Y., X.-Q.W.) and the Agency for Science, Technology and Research (A*STAR) under its Industry Alignment Fund—Prepositioning Programme (grant Nos. H17/01/a0/0B9 and H17/01/a0/0C9) as part of the Wound Care Innovation for the Tropics Programme (Z.U., D.L., C.F.).
Author Disclosure and Ghostwriting
The authors have no competing interests. The article was written solely by its authors.
About the Authors
Bo Yuan, PhD, is a burn surgeon in RuiJin Hospital, Shanghai Jiao Tong University School of Medicine. His research interests focus on adipose tissue and HS interactions, especially in the application of adipose stem cells for treating skin fibrosis. Zee Upton, PhD, is executive director of the Skin Research Institute of Singapore, Agency for Science Technology and Research (A*STAR). Her focus is creating affordable and fit-for-purpose solutions for skin health and disease that address unmet clinical needs and deliver better patient outcomes. David Leavesley, PhD, is a senior scientist at A*STAR. His focus is creating affordable and useable solutions to address the clinical challenges faced by patients and clinical caregivers. Chen Fan, PhD, is a research scientist at Wenzhou Institute, University of Chinese Academy of Sciences. His research area is skin tissue engineering and the application of natural products in wound healing and scar remediation. Xi-Qiao Wang, PhD, is an associate professor in the Burn Department of RuiJin Hospital affiliated with the Shanghai Jiao Tong University School of Medicine. He has specialized in wound healing and HS for 18 years, with special interests in scar regression and scar remediation.
References
- 1. Karppinen SM, Heljasvaara R, Gullberg D, Tasanen K, Pihlajaniemi T. Toward understanding scarless skin wound healing and pathological scarring. F1000Res 2019;8:F1000 Faculty Rev-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mari W, Alsabri SG, Tabal N, Younes S, Sherif A, Simman R. Novel insights on understanding of keloid scar: article review. J Am Coll Clin Wound Spec 2015;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan S, Khumalo N, Bayat A. Understanding keloid pathobiology from a quasi-neoplastic perspective: less of a scar and more of a chronic inflammatory disease with cancer-like tendencies. Front Immunol 2019;10:1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verhaegen PD, van Zuijlen PP, Pennings NM, et al. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: an objective histopathological analysis. Wound Repair Regen 2009;17:649–656. [DOI] [PubMed] [Google Scholar]
- 5. Ogawa R, Akaishi S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis - Keloids and hypertrophic scars may be vascular disorders. Med Hypotheses 2016;96:51–60. [DOI] [PubMed] [Google Scholar]
- 6. Zheng J, Song F, Lu SL, Wang XQ. Dynamic hypoxia in scar tissue during human hypertrophic scar progression. Dermatol Surg 2014;40:511–518. [DOI] [PubMed] [Google Scholar]
- 7. Amadeu T, Braune A, Mandarim-de-Lacerda C, Porto LC, Desmoulière A, Costa A. Vascularization pattern in hypertrophic scars and keloids: a stereological analysis. Pathol Res Pract 2003;199:469–473. [DOI] [PubMed] [Google Scholar]
- 8. Leung KS, Sher A, Clark JA, Cheng JC, Leung PC. Microcirculation in hypertrophic scars after burn injury. J Burn Care Rehabil 1989;10:436–444. [DOI] [PubMed] [Google Scholar]
- 9. Oliveira GV, Chinkes D, Mitchell C, Oliveras G, Hawkins HK, Herndon DN. Objective assessment of burn scar vascularity, erythema, pliability, thickness, and planimetry. Dermatol Surg 2005;31:48–58. [DOI] [PubMed] [Google Scholar]
- 10. Deng H, Li-Tsang C, Li J. Measuring vascularity of hypertrophic scars by dermoscopy: construct validity and predictive ability of scar thickness change. Skin Res Technol 2020;26:369–375. [DOI] [PubMed] [Google Scholar]
- 11. Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest 2008;88:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diao JS, Xia WS, Guo SZ. Bevacizumab: a potential agent for prevention and treatment of hypertrophic scar. Burns 2010;36:1136–1137. [DOI] [PubMed] [Google Scholar]
- 13. Kischer CW. The microvessels in hypertrophic scars, keloids and related lesions: a review. J Submicrosc Cytol Pathol 1992;24:281–296. [PubMed] [Google Scholar]
- 14. Kischer CW, Shetlar MR, Shetlar CL. Alteration of hypertrophic scars induced by mechanical pressure. Arch Dermatol 1975;111:60–64. [PubMed] [Google Scholar]
- 15. Berry RB, Tan OT, Cooke ED, et al. Transcutaneous oxygen tension as an index of maturity in hypertrophic scars treated by compression. Br J Plast Surg 1985;38:163–173. [DOI] [PubMed] [Google Scholar]
- 16. Xi-Qiao W, Ying-Kai L, Chun Q, Shu-Liang L. Hyperactivity of fibroblasts and functional regression of endothelial cells contribute to microvessel occlusion in hypertrophic scarring. Microvasc Res 2009;77:204–211. [DOI] [PubMed] [Google Scholar]
- 17. Lynam EC, Xie Y, Dawson R, Mcgovern J, Upton Z, Wang X. Severe hypoxia and malnutrition collectively contribute to scar fibroblast inhibition and cell apoptosis. Wound Repair Regen 2015;23:664–671. [DOI] [PubMed] [Google Scholar]
- 18. Soderblom C, Luo X, Blumenthal E, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 2013;33:13882–13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol 1996;50:335–362. [DOI] [PubMed] [Google Scholar]
- 20. Vane JR, Botting RM. Secretory functions of the vascular endothelium. J Physiol Pharmacol 1992;43:195–207. [PubMed] [Google Scholar]
- 21. Baumgartner-Parzer SM, Waldhäusl WK. The endothelium as a metabolic and endocrine organ: its relation with insulin resistance. Exp Clin Endocrinol Diabetes 2001;109(Suppl 2):S166–S179. [DOI] [PubMed] [Google Scholar]
- 22. Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 2001;294:559–563. [DOI] [PubMed] [Google Scholar]
- 23. Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science 2001;294:564–567. [DOI] [PubMed] [Google Scholar]
- 24. Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004;304:1338–1340. [DOI] [PubMed] [Google Scholar]
- 25. Li W, Li P, Hua Q, et al. The impact of paracrine signaling in brain microvascular endothelial cells on the survival of neurons. Brain Res 2009;1287:28–38. [DOI] [PubMed] [Google Scholar]
- 26. Park JA, Choi KS, Kim SY, Kim KW. Coordinated interaction of the vascular and nervous systems: from molecule- to cell-based approaches. Biochem Biophys Res Commun 2003;311:247–253. [DOI] [PubMed] [Google Scholar]
- 27. Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci 1999;13:450–464. [DOI] [PubMed] [Google Scholar]
- 28. Huang C, Liu L, You Z, et al. Endothelial dysfunction and mechanobiology in pathological cutaneous scarring: lessons learned from soft tissue fibrosis. Br J Dermatol 2017;177:1248–1255. [DOI] [PubMed] [Google Scholar]
- 29. Wang XQ, Song F, Liu YK. Hypertrophic scar regression is linked to the occurrence of endothelial dysfunction. PLoS One 2017;12:e0176681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qian LW, Fourcaudot AB, Yamane K, You T, Chan RK, Leung KP. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair Regen 2016;24:26–34. [DOI] [PubMed] [Google Scholar]
- 31. Wong VW, Rustad KC, Akaishi S, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 2011;18:148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong X, Mao S, Wen H. Upregulation of proinflammatory genes in skin lesions may be the cause of keloid formation (review). Biomed Rep 2013;1:833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen W, Fu X, Sun X, Sun T, Zhao Z, Sheng Z. Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray. J Surg Res 2003;113:208–216. [DOI] [PubMed] [Google Scholar]
- 34. Scott PG, Dodd CM, Tredget EE, Ghahary A, Rahemtulla F. Chemical characterization and quantification of proteoglycans in human post-burn hypertrophic and mature scars. Clin Sci (Lond) 1996;90:417–425. [DOI] [PubMed] [Google Scholar]
- 35. Subramaniam K, Pech CM, Stacey MC, Wallace HJ. Induction of MMP-1, MMP-3 and TIMP-1 in normal dermal fibroblasts by chronic venous leg ulcer wound fluid. Int Wound J 2008;5:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghahary A, Shen YJ, Nedelec B, Wang R, Scott PG, Tredget EE. Collagenase production is lower in post-burn hypertrophic scar fibroblasts than in normal fibroblasts and is reduced by insulin-like growth factor-1. J Invest Dermatol 1996;106:476–481. [DOI] [PubMed] [Google Scholar]
- 37. Mwaura B, Mahendran B, Hynes N, et al. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase-2, tissue inhibitor of matrix metalloproteinase-2 and PDGF-AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg 2006;31:306–310. [DOI] [PubMed] [Google Scholar]
- 38. Brewin MP, Lister TS. Prevention or treatment of hypertrophic burn scarring: a review of when and how to treat with the pulsed dye laser. Burns 2014;40:797–804. [DOI] [PubMed] [Google Scholar]
- 39. Monstrey S, Middelkoop E, Vranckx JJ, et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg 2014;67:1017–1025. [DOI] [PubMed] [Google Scholar]
- 40. O'Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2006;1:CD003826. [DOI] [PubMed] [Google Scholar]
- 41. Li-Tsang CW, Zheng YP, Lau JC. A randomized clinical trial to study the effect of silicone gel dressing and pressure therapy on posttraumatic hypertrophic scars. J Burn Care Res 2010;31:448–457. [DOI] [PubMed] [Google Scholar]
- 42. Hsu KC, Luan CW, Tsai YW. Review of silicone gel sheeting and silicone gel for the prevention of hypertrophic scars and keloids. Wounds 2017;29:154–158. [PubMed] [Google Scholar]
- 43. Gilman TH. Silicone sheet for treatment and prevention of hypertrophic scar: a new proposal for the mechanism of efficacy. Wound Repair Regen 2003;11:235–236. [DOI] [PubMed] [Google Scholar]
- 44. Musgrave MA, Umraw N, Fish JS, Gomez M, Cartotto RC. The effect of silicone gel sheets on perfusion of hypertrophic burn scars. J Burn Care Rehabil 2002;23:208–214. [DOI] [PubMed] [Google Scholar]
- 45. Surakunprapha P, Winaikosol K, Chowchuen B, Punyavong P, Jenwitheesuk K, Jenwitheesuk K. A prospective randomized double-blind study of silicone gel plus herbal extracts versus placebo in pre-sternal hypertrophic scar prevention and amelioration. Heliyon 2020;6:e03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mojsiewicz-Pieńkowska K, Jamrógiewicz M, Żebrowska M, Mikolaszek B, Sznitowska M. Double layer adhesive silicone dressing as a potential dermal drug delivery film in scar treatment. Int J Pharm 2015;481:18–26. [DOI] [PubMed] [Google Scholar]
- 47. Chan KY, Lau CL, Adeeb SM, Somasundaram S, Nasir-Zahari M. A randomized, placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast Reconstr Surg 2005;116:1013–1020; discussion 1021–1022. [DOI] [PubMed] [Google Scholar]
- 48. So K, Umraw N, Scott J, Campbell K, Musgrave M, Cartotto R. Effects of enhanced patient education on compliance with silicone gel sheeting and burn scar outcome: a randomized prospective study. J Burn Care Rehabil 2003;24:411–417; discussion 410. [DOI] [PubMed] [Google Scholar]
- 49. Li J, Bai YQ, Lü GL, Du YR, Zhao N. Influence of different pressure tension bandage on inhibiting scar proliferation. J Clin Rehabil Tissue Eng Res 2009;13:7583–7586. [Google Scholar]
- 50. DeBruler DM, Zbinden JC, Baumann ME, et al. Early cessation of pressure garment therapy results in scar contraction and thickening. PLoS One 2018;13:e0197558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Renò F, Sabbatini M, Lombardi F, et al. In vitro mechanical compression induces apoptosis and regulates cytokines release in hypertrophic scars. Wound Repair Regen 2003;11:331–336. [DOI] [PubMed] [Google Scholar]
- 52. Renò F, Grazianetti P, Cannas M. Effects of mechanical compression on hypertrophic scars: prostaglandin E2 release. Burns 2001;27:215–218. [DOI] [PubMed] [Google Scholar]
- 53. Renò F, Grazianetti P, Stella M, Magliacani G, Pezzuto C, Cannas M. Release and activation of matrix metalloproteinase-9 during in vitro mechanical compression in hypertrophic scars. Arch Dermatol 2002;138:475–478. [DOI] [PubMed] [Google Scholar]
- 54. Tejiram S, Zhang J, Travis TE, et al. Compression therapy affects collagen type balance in hypertrophic scar. J Surg Res 2016;201:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ai J, Liu J, Pei S, et al. The effectiveness of pressure therapy (15–25 mmHg) for hypertrophic burn scars: a systematic review and meta-analysis. Sci Rep 2017;7:40185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wiseman J, Simons M, Kimble R, Ware R, McPhail S, Tyack Z. Effectiveness of topical silicone gel and pressure garment therapy for burn scar prevention and management in children: study protocol for a randomised controlled trial. Trials 2017;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morelli Coppola M, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol 2018;11:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gold MH, Berman B, Clementoni MT, Gauglitz GG, Nahai F, Murcia C. Updated international clinical recommendations on scar management: part 1—evaluating the evidence. Dermatol Surg 2014;40:817–824. [DOI] [PubMed] [Google Scholar]
- 59. Del Toro D, Dedhia R, Tollefson TT. Advances in scar management: prevention and management of hypertrophic scars and keloids. Curr Opin Otolaryngol Head Neck Surg 2016;24:322–329. [DOI] [PubMed] [Google Scholar]
- 60. Wu WS, Wang FS, Yang KD, Huang CC, Kuo YR. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol 2006;126:1264–1271. [DOI] [PubMed] [Google Scholar]
- 61. Logie JJ, Ali S, Marshall KM, Heck MM, Walker BR, Hadoke PW. Glucocorticoid-mediated inhibition of angiogenic changes in human endothelial cells is not caused by reductions in cell proliferation or migration. PLoS One 2010;5:e14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Valamanesh F, Berdugo M, Sennlaub F, et al. Effects of triamcinolone acetonide on vessels of the posterior segment of the eye. Mol Vis 2009;15:2634–2648. [PMC free article] [PubMed] [Google Scholar]
- 63. El Zaoui I, Behar-Cohen F, Torriglia A. Glucocorticoids exert direct toxicity on microvasculature: analysis of cell death mechanisms. Toxicol Sci 2015;143:441–453. [DOI] [PubMed] [Google Scholar]
- 64. Xu J, Yang E, Yu N, Wang Y, Long X. The radiation therapy in keloids treatment: a comprehensive review of pathomechanism, damage mechanisms and cellular response. Plast Aesthet Res 2017;4:116. [Google Scholar]
- 65. Ogawa R, Tosa M, Dohi T, Akaishi S, Kuribayashi S. Surgical excision and postoperative radiotherapy for keloids. Scars Burn Heal 2019;5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu J, Yang E, Yu NZ, Long X. Radiation therapy in keloids treatment: history, strategy, effectiveness, and complication. Chin Med J (Engl) 2017;130:1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Imaizumi N, Monnier Y, Hegi M, Mirimanoff RO, Rüegg C. Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting. PLoS One 2010;5:e11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang LZ, Ding JP, Yang MY, Chen B. Forty-five cases of chest keloids treated with subcutaneous super-tension-reduction suture combined with postoperative electron-beam irradiation. Dermatol Surg 2014;40:1378–1384. [DOI] [PubMed] [Google Scholar]
- 69. Kuribayashi S, Miyashita T, Ozawa Y, et al. Post-keloidectomy irradiation using high-dose-rate superficial brachytherapy. J Radiat Res 2011;52:365–368. [DOI] [PubMed] [Google Scholar]
- 70. Khatri KA, Mahoney DL, McCartney MJ. Laser scar revision: a review. J Cosmet Laser Ther 2011;13:54–62. [DOI] [PubMed] [Google Scholar]
- 71. Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg 2003;111:1841–1852. [DOI] [PubMed] [Google Scholar]
- 72. Layton AM, Yip J, Cunliffe WJ. A comparison of intralesional triamcinolone and cryosurgery in the treatment of acne keloids. Br J Dermatol 1994;130:498–501. [DOI] [PubMed] [Google Scholar]
- 73. Zouboulis CC, Blume U, Büttner P, Orfanos CE. Outcomes of cryosurgery in keloids and hypertrophic scars. A prospective consecutive trial of case series. Arch Dermatol 1993;129:1146–1151. [PubMed] [Google Scholar]
- 74. Har-Shai Y, Mettanes I, Zilberstein Y, Genin O, Spector I, Pines M. Keloid histopathology after intralesional cryosurgery treatment. J Eur Acad Dermatol Venereol 2011;25:1027–1036. [DOI] [PubMed] [Google Scholar]
- 75. Awad SM, Ismail SA, Sayed DS, Refaiy AE, Makboul R. Suppression of transforming growth factor-beta1 expression in keloids after cryosurgery. Cryobiology 2017;75:151–153. [DOI] [PubMed] [Google Scholar]
- 76. O'Shaughnessy KD, De La Garza M, Roy NK, Mustoe TA. Homeostasis of the epidermal barrier layer: a theory of how occlusion reduces hypertrophic scarring. Wound Repair Regen 2009;17:700–708. [DOI] [PubMed] [Google Scholar]
- 77. Mustoe TA, Gurjala A. The role of the epidermis and the mechanism of action of occlusive dressings in scarring. Wound Repair Regen 2011;19(Suppl 1):s16–s21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gallant-Behm CL, Mustoe TA. Occlusion regulates epidermal cytokine production and inhibits scar formation. Wound Repair Regen 2010;18:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ma X, Jin Z, Li G, Yang W. Classification of chronic radiation-induced ulcers in the chest wall after surgery in breast cancers. Radiat Oncol 2017;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huang D, Shen KH, Wang HG. Pressure therapy upregulates matrix metalloproteinase expression and downregulates collagen expression in hypertrophic scar tissue. Chin Med J (Engl) 2013;126:3321–3324. [PubMed] [Google Scholar]
- 81. Geddes ER, Stout AB, Friedman PM. Retrospective analysis of the treatment of melasma lesions exhibiting increased vascularity with the 595-nm pulsed dye laser combined with the 1927-nm fractional low-powered diode laser. Lasers Surg Med 2017;49:20–26. [DOI] [PubMed] [Google Scholar]
- 82. Ouyang HW, Li GF, Lei Y, Gold MH, Tan J. Comparison of the effectiveness of pulsed dye laser vs pulsed dye laser combined with ultrapulse fractional CO2 laser in the treatment of immature red hypertrophic scars. J Cosmet Dermatol 2018;17:54–60. [DOI] [PubMed] [Google Scholar]
- 83. Kuehlmann B, Stern-Buchbinder Z, Wan DC, Friedstat JS, Gurtner GC. Beneath the surface: a review of laser remodeling of hypertrophic scars and burns. Adv Wound Care (New Rochelle) 2019;8:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kuo YR, Jeng SF, Wang FS, et al. Flashlamp pulsed dye laser (PDL) suppression of keloid proliferation through down-regulation of TGF-beta1 expression and extracellular matrix expression. Lasers Surg Med 2004;34:104–108. [DOI] [PubMed] [Google Scholar]
- 85. Alster TS, Williams CM. Treatment of keloid sternotomy scars with 585 nm flashlamp-pumped pulsed-dye laser. Lancet 1995;345:1198–1200. [DOI] [PubMed] [Google Scholar]
- 86. Piccolo D, Di Marcantonio D, Crisman G, et al. Unconventional use of intense pulsed light. Biomed Res Int 2014;2014:618206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fu X, Dong J, Wang S, Yan M, Yao M. Advances in the treatment of traumatic scars with laser, intense pulsed light, radiofrequency, and ultrasound. Burns Trauma 2019;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shamsi Meymandi S, Rezazadeh A, Ekhlasi A. Studying intense pulsed light method along with corticosteroid injection in treating keloid scars. Iran Red Crescent Med J 2014;16:e12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Poluzzi C, Iozzo RV, Schaefer L. Endostatin and endorepellin: a common route of action for similar angiostatic cancer avengers. Adv Drug Deliv Rev 2016;97:156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dixelius J, Larsson H, Sasaki T, et al. Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis. Blood 2000;95:3403–3411. [PubMed] [Google Scholar]
- 91. Ye Q, Qin S, Liu Y, et al. Inhibitory effect of Endostar on specific angiogenesis induced by human hepatocellular carcinoma. Gastroenterol Res Pract 2015;2015:957574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ge W, Cao DD, Wang HM, Jie FF, Zheng YF, Chen Y. Endostar combined with chemotherapy versus chemotherapy alone for advanced NSCLCs: a meta-analysis. Asian Pac J Cancer Prev 2011;12:2705–2711. [PubMed] [Google Scholar]
- 93. ZhiYong W, Fei S, LianJu X, et al. Endostar injection inhibits rabbit ear hypertrophic scar formation. Int J Low Extrem Wounds 2012;11:271–276. [DOI] [PubMed] [Google Scholar]
- 94. Wang P, Jiang LZ, Xue B. Recombinant human endostatin reduces hypertrophic scar formation in rabbit ear model through down-regulation of VEGF and TIMP-1. Afr Health Sci 2016;16:542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ling Y, Yang Y, Lu N, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun 2007;361:79–84. [DOI] [PubMed] [Google Scholar]
- 96. Gong YF, Zhang XM, Liu F, et al. Inhibitory effect of recombinant human endostatin on the proliferation of hypertrophic scar fibroblasts in a rabbit ear model. Eur J Pharmacol 2016;791:647–654. [DOI] [PubMed] [Google Scholar]
- 97. Rosen LS, Jacobs IA, Burkes RL. Bevacizumab in colorectal cancer: current role in treatment and the potential of biosimilars. Target Oncol 2017;12:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pfaendler KS, Liu MC, Tewari KS. Bevacizumab in Cervical cancer: 5 years after. Cancer J 2018;24:187–192. [DOI] [PubMed] [Google Scholar]
- 99. Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle) 2014;3:647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shen R, Li TZ, Qi SH, et al. A research of endothelial cell-targeted therapy for cure of hypertrophic scar [in Chinese]. Zhonghua Zheng Xing Wai Ke Za Zhi 2003;19:254–257. [PubMed] [Google Scholar]
- 101. Kwak DH, Bae TH, Kim WS, Kim HK. Anti-vascular endothelial growth factor (bevacizumab) therapy reduces hypertrophic scar formation in a rabbit ear wounding model. Arch Plast Surg 2016;43:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shi J, Wu Y, Guo S, Zhang H, Chen G, Xu X. The efficacy of anti-VEGF antibody-modified liposomes loaded with paeonol in the prevention and treatment of hypertrophic scars. Drug Dev Ind Pharm 2019;45:439–455. [DOI] [PubMed] [Google Scholar]
- 103. Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res 1978;38:2628–2635. [PubMed] [Google Scholar]
- 104. Yuan KH, Li Q, Yu WL, Zeng D, Zhang C, Huang Z. Comparison of photodynamic therapy and pulsed dye laser in patients with port wine stain birthmarks: a retrospective analysis. Photodiagnosis Photodyn Ther 2008;5:50–57. [DOI] [PubMed] [Google Scholar]
- 105. Gao K, Huang Z, Yuan KH, Zhang B, Hu ZQ. Side-by-side comparison of photodynamic therapy and pulsed-dye laser treatment of port-wine stain birthmarks. Br J Dermatol 2013;168:1040–1046. [DOI] [PubMed] [Google Scholar]
- 106. Kawczyk-Krupka A, Wawrzyniec K, Musiol SK, Potempa M, Bugaj AM, Sieroń A. Treatment of localized prostate cancer using WST-09 and WST-11 mediated vascular targeted photodynamic therapy-a review. Photodiagnosis Photodyn Ther 2015;12:567–574. [DOI] [PubMed] [Google Scholar]
- 107. Vanaclocha V, Sureda M, Azinovic I, et al. Photodynamic therapy in the treatment of brain tumours. A feasibility study. Photodiagnosis Photodyn Ther 2015;12:422–427. [DOI] [PubMed] [Google Scholar]
- 108. Koudinova NV, Pinthus JH, Brandis A, et al. Photodynamic therapy with Pd-Bacteriopheophorbide (TOOKAD): successful in vivo treatment of human prostatic small cell carcinoma xenografts. Int J Cancer 2003;104:782–789. [DOI] [PubMed] [Google Scholar]
- 109. Kelleher DK, Thews O, Scherz A, Salomon Y, Vaupel P. Perfusion, oxygenation status and growth of experimental tumors upon photodynamic therapy with Pd-bacteriopheophorbide. Int J Oncol 2004;24:1505–1511. [PubMed] [Google Scholar]
- 110. Eymerit-Morin C, Zidane M, Lebdai S, Triau S, Azzouzi AR, Rousselet MC. Histopathology of prostate tissue after vascular-targeted photodynamic therapy for localized prostate cancer. Virchows Arch 2013;463:547–552. [DOI] [PubMed] [Google Scholar]
- 111. Zhang B, Zhang TH, Huang Z, Li Q, Yuan KH, Hu ZQ. Comparison of pulsed dye laser (PDL) and photodynamic therapy (PDT) for treatment of facial port-wine stain (PWS) birthmarks in pediatric patients. Photodiagnosis Photodyn Ther 2014;11:491–497. [DOI] [PubMed] [Google Scholar]
- 112. Campbell SM, Tyrrell J, Marshall R, Curnow A. Effect of MAL-photodynamic therapy on hypertrophic scarring. Photodiagnosis Photodyn Ther 2010;7:183–188. [DOI] [PubMed] [Google Scholar]
- 113. Bruscino N, Lotti T, Rossi R. Photodynamic therapy for a hypertrophic scarring: a promising choice. Photodermatol Photoimmunol Photomed 2011;27:334–335. [DOI] [PubMed] [Google Scholar]
- 114. Poetschke J, Dornseifer U, Clementoni MT, et al. Ultrapulsed fractional ablative carbon dioxide laser treatment of hypertrophic burn scars: evaluation of an in-patient controlled, standardized treatment approach. Lasers Med Sci 2017;32:1031–1040. [DOI] [PubMed] [Google Scholar]
- 115. Li N, Yang L, Cheng J, Han J, Hu D.. Early intervention by Z-plasty combined with fractional CO(2) laser therapy as a potential treatment for hypertrophic burn scars. J Plast Reconstr Aesthet Surg 2021. DOI: 10.1016/j.bjps.2021.03.079. [DOI] [PubMed] [Google Scholar]
- 116. Kirsch KM, Zelickson BD, Zachary CB, Tope WD. Ultrastructure of collagen thermally denatured by microsecond domain pulsed carbon dioxide laser. Arch Dermatol 1998;134:1255–1259. [DOI] [PubMed] [Google Scholar]
- 117. Stuzin JM, Baker TJ, Baker TM, Kligman AM. Histologic effects of the high-energy pulsed CO2 laser on photoaged facial skin. Plast Reconstr Surg 1997;99:2036–2050; discussion 2051–2055. [DOI] [PubMed] [Google Scholar]
- 118. Baleg SM, Bidin N, Suan LP, et al. The effect of CO2 laser treatment on skin tissue. J Cosmet Dermatol 2015;14:246–253. [DOI] [PubMed] [Google Scholar]
- 119. Lee SJ, Suh DH, Lee JM, Song KY, Ryu HJ. Dermal remodeling of burn scar by fractional CO2 laser. Aesthetic Plast Surg 2016;40:761–768. [DOI] [PubMed] [Google Scholar]
- 120. Makboul M, Makboul R, Abdelhafez AH, Hassan SS, Youssif SM. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-β1 expression in hypertrophic scar. J Cosmet Dermatol 2014;13:169–179. [DOI] [PubMed] [Google Scholar]
- 121. Qu L, Liu A, Zhou L, et al. Clinical and molecular effects on mature burn scars after treatment with a fractional CO(2) laser. Lasers Surg Med 2012;44:517–524. [DOI] [PubMed] [Google Scholar]
- 122. Zhou C, Hovius SE, Slijper HP, et al. Collagenase clostridium histolyticum versus limited fasciectomy for Dupuytren's contracture: outcomes from a multicenter propensity score matched study. Plast Reconstr Surg 2015;136:87–97. [DOI] [PubMed] [Google Scholar]
- 123. Patry J, Blanchette V. Enzymatic debridement with collagenase in wounds and ulcers: a systematic review and meta-analysis. Int Wound J 2017;14:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jia S, Zhao Y, Law M, Galiano R, Mustoe TA. The effects of collagenase ointment on the prevention of hypertrophic scarring in a rabbit ear scarring model: a pilot study. Wounds 2011;23:160–165. [PubMed] [Google Scholar]
- 125. Bae-Harboe YS, Harboe-Schmidt JE, Graber E, Gilchrest BA. Collagenase followed by compression for the treatment of earlobe keloids. Dermatol Surg 2014;40:519–524. [DOI] [PubMed] [Google Scholar]
- 126. Kang N, Sivakumar B, Sanders R, Nduka C, Gault D. Intra-lesional injections of collagenase are ineffective in the treatment of keloid and hypertrophic scars. J Plast Reconstr Aesthet Surg 2006;59:693–699. [DOI] [PubMed] [Google Scholar]
- 127. Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology 2008;47:1394–1400. [DOI] [PubMed] [Google Scholar]
- 128. Cho Lee AR, Woo I. Local silencing of connective tissue growth factor by siRNA/peptide improves dermal collagen arrangements. Tissue Eng Regen Med 2018;15:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Richeldi L, Fernández Pérez ER, Costabel U, et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2020;8:25–33. [DOI] [PubMed] [Google Scholar]
- 130. Luangmonkong T, Suriguga S, Bigaeva E, et al. Evaluating the antifibrotic potency of galunisertib in a human ex vivo model of liver fibrosis. Br J Pharmacol 2017;174:3107–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gallant-Behm CL, Piper J, Lynch JM, et al. A microRNA-29 mimic (remlarsen) represses extracellular matrix expression and fibroplasia in the skin. J Invest Dermatol 2019;139:1073–1081. [DOI] [PubMed] [Google Scholar]