Abstract
Pneumococcal infections are an important cause of morbidity and mortality in children with sickle-cell disease (SCD). Pneumococcal conjugate vaccines (PCVs) are immunogenic in healthy infants <2 years of age but have not been evaluated in young children with SCD. Infants with SCD were immunized with a 7-valent PCV (Wyeth-Lederle Vaccines & Pediatrics) at 2, 4, and 6 months of age. A booster dose of 23-valent pneumococcal polysaccharide vaccine (PPV; Pnu-Immune) was administered at 24 months of age. Antipneumococcal type 6B and 14 serum opsonic activity was measured to assess the biologic function of the antibody. Following the administration of three doses of PCV, opsonic activity against serotype 6B increased from 4.8% at 2 months to 33.5% at 7 months, with a subsequent decline to 8.1% at 12 months and 7.5% at 24 months and with an increase to 30.7% at 25 months after administration of a booster dose of PPV. Similar trends were seen with serotype 14 (opsonic activities were 9.4% at 2 months, 24.9% at 7 months, 16.5% at 12 months, and 12.6% at 24 months, and the opsonic activity was 27.3% 1 month after the administration of PPV). Serum opsonic activity correlated with antibody levels for both serotypes. PCV induces serum opsonic activity in infants with SCD. Antipneumococcal serum opsonic activity correlates with antibody levels.
Patients with sickle-cell disease (SCD) have an increased susceptibility to blood-borne Streptococcus pneumoniae infections such as sepsis and meningitis (1, 26, 38). In fact, their risk for invasive pneumococcal infection has been estimated to be as high as 300 to 600 times that of children of the same age without SCD (1, 38).
A variety of strategies have been used to protect children with SCD against pneumococcal infection. One such strategy has been to use penicillin prophylaxis (10). However, inconsistent compliance and the emergence of bacterial resistance may limit the protective effect of antibiotic prophylaxis (6, 33). Another strategy has been immunization with the currently available 23-valent capsular polysaccharide pneumococcal vaccine. However, that vaccine is not immunogenic in children younger than 2 years of age, the very age at which there is the highest incidence of pneumococcal sepsis (1, 6, 20).
A new pneumococcal polysaccharide protein conjugate vaccine has been shown to be immunogenic and effective in preventing invasive pneumococcal disease in healthy infants and children younger than 2 years of age (5, 25). This vaccine has the potential to be of particular benefit to infants and young children with SCD. Our previous studies demonstrated that this conjugate vaccine is immunogenic in infants younger than 1 year of age with SCD (19). We performed the current studies in order to determine whether the anticapsular antibody induced by this vaccine possesses opsonic activity, perhaps a better correlate of biologic activity than antibody level measured by enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
Immunization protocol and serum samples.
The infants in this study were from four medical centers and one private pediatric practice in the Baltimore Md., metropolitan area: the Johns Hopkins Children's Center, the University of Maryland Children's Hospital, Sinai Hospital, the Johns Hopkins Bayview Medical Center, and the Pediatric Center of Annapolis. The subjects in the first age group (28 patients with SCD and 7 age-matched African-American control patients without SCD) were immunized intramuscularly with three doses of heptavalent (serotypes 1, 4, 6B, 9V, 14, 18C, 19F, and 23) pneumococcal polysaccharide protein conjugate vaccine (lots 7-5018-003A and F37-4-I-02; Wyeth Lederle Vaccines & Pediatrics) at 2, 4, and 6 months of age. This vaccine was manufactured by covalently linking polysaccharide to the protein carrier CRM197. Each 0.5-ml dose contained 2 μg each of polysaccharide serotypes 4, 9V, 14, 19F, and 23F; 2 μg of oligosaccharide serotype 18C; 4 μg of polysaccharide serotype 6B; approximately 20 μg of CRM197; and approximately 0.5 mg of aluminum phosphate as an adjuvant. A booster dose of 23-valent polysaccharide pneumococcal vaccine (Pnu-Immune; Lederle Laboratories) was administered at 24 months. Serum samples were obtained at 2, 4, 6, 7, 12, 24, and 25 months of age. The children in the second age group (10 SCD patients and 3 age-matched African-American control patients without SCD) were immunized with a single dose of pneumococcal polysaccharide protein conjugate vaccine at 12 months of age. They received a booster dose of polysaccharide vaccine at 24 months, and serum samples were obtained at 12, 13, 24, and 25 months of age. Aliquots of serum were frozen at −70°C within 2 h of collection.
Our intention was to enroll equal numbers of 2-month-old infants with SCD, 12-month-old children with SCD, and healthy controls at each of these two ages. After initial enrollment into the 12-month-old SCD group, we concentrated our efforts on enrolling infants with SCD at age 2 months. These efforts were so successful that older children were thereafter not available for enrollment. The unbalanced recruitment of age-matched African-American infants without hemoglobinopathy reflects the difficulty in convincing parents to subject their healthy infants to additional series of intramuscular injections.
The study was approved by the Institutional Review Boards of the Johns Hopkins University, the Maryland State Department of Health, the University of Maryland Medical School, and Sinai Hospital. Informed consent was obtained from the parents or legal guardians of the infants participating in the study.
Antipneumococcal antibody measurements.
Type-specific antipneumococcal immunoglobulin G (IgG) antibody levels were measured by ELISA, modified from the method of Koskela (14). All sera were premixed with pneumococcal C polysaccharide (2.0 μg/ml) to minimize nonspecific binding. For each serotype, eight 2.5-fold dilutions of serum were prepared and assayed in duplicate. Dilutions of Food and Drug Administration (FDA) pneumococcal reference serum lot 89-SF (24) were tested in parallel. Comparisons of patient and reference sera were performed by using a four-parameter logistic-log function (23). The coefficient of variation for antibody levels measured by ELISA was 33% for pneumococcal serotype (Pn) 6B and 28% for Pn 14.
Reference sera for opsonic assays.
A reference serum pool was composed of equal volumes of serum obtained from 13 healthy adult volunteers. It was aliquoted, frozen, and stored at −70°C. The serotype-specific anticapsular pneumococcal IgG antibody concentration was 2.46 μg/ml for serotype 14, as measured by ELISA, in comparison with that for FDA reference serum sample 89-SF. The reference serum pool was tested in each Pn 14 opsonization experiment to control for day-to-day variability in results. Because the reference serum pool had low opsonic activity for serotype 6B, in the experiments with that serotype, control serum from a single donor with a high (25.8 μg/ml) anti-Pn 6B anticapsular IgG titer was used as the reference serum sample.
Opsonic assay.
The opsonic assay was a modification of a previously published method (7, 29).
Preparation of PMNs.
Human polymorphonuclear cells (PMNs) were obtained daily from the peripheral venous blood of healthy adult volunteers by mixing equal volumes of blood and dextran solution (100 ml of 6% dextran 70 in 0.9% sodium chloride injection, 10 ml of 5% dextrose injection [USP; Abbott Laboratories, North Chicago, Ill.], 2 ml of heparin sodium injection [USP; 1,000 units/ml; Elkins-Sinn, Inc., Cherry Hill, N.J.]). The mixture was allowed to sediment at a 45-degree angle at room temperature for 45 min. PMNs were isolated from the resulting supernatant plasma by density centrifugation on lymphocyte separation medium (Cappel LSM; ICN Biomedicals, Aurora, Ohio) (7, 29). Both supernatant plasma and the Cappel LSM layer were carefully aspirated and discarded, leaving the pellet containing PMNs and a few erythrocytes. The PMN pellets from all tubes were pooled; the erythrocytes were lysed in hypotonic medium, and the PMNs were immediately restored to osmotic neutrality by the addition of hypertonic saline (36). PMNs were adjusted to a final concentration of 107 PMNs/ml in Veronal-buffered saline supplemented with 0.5% bovine serum albumin, MgCl2, and CaCl2 (7).
Preparation of radiolabeled pneumococci.
Pneumococci were obtained from the American Type Culture Collection (Manassas, Va.). They were passaged through mice, grown, and radiolabeled with [3H]thymidine by a previously published method (36). Serotype specificity was confirmed by the Quellung reaction. The bacteria were killed by heating to 56°C for 45 min and were stored in 0.1% formalin at 4°C. On the day of use an aliquot was washed twice with phosphate-buffered saline to remove the formalin and was resuspended to a concentration of 107/ml. An aliquot of the bacterial suspension was counted in the scintillation counter to determine the total bacterial input for each assay.
Opsonic assay performance.
The opsonic assay was performed by mixing 106 PMNs, the desired concentration of serum, and 106 bacteria in a total of 200 μl of Veronal-buffered saline-bovine serum albumin in a 1.5-ml Eppendorf plastic tube. Immediately after the bacteria were added, the mixture was rotated at 24 rpm at 37°C. After 70 min, incubation was stopped by placing the test tubes in ice water and the tubes were immediately spun for 7 min at 1,100 rpm (350 × g) at 4°C to separate the PMNs and PMN-associated bacteria. The supernatant was carefully aspirated, and the cell pellet was washed once with 950 μl of phosphate-buffered saline and was then resuspended in scintillation fluid (Aquasol). The scintillation vials were left to adapt to the dark for 30 min, and then the radioactivity was counted.
Since each sample was run in duplicate, final sample counts were calculated as an arithmetic mean from the two measurements. The serum opsonic activity was defined as the uptake of radiolabeled bacteria by PMNs, which was determined by the following formula: (PMN-associated bacteria counts/total bacterial input counts) × 100. In each experiment there were two controls: a positive control that consisted of the reference serum with PMNs and bacteria and a negative control that consisted of PMNs and bacteria but no serum.
In order to adjust for the possibility of day-to-day variability in the assay, a correction factor was calculated for each experiment by using the following formula: opsonic activity of the reference serum sample in the first experiment/opsonic activity of the reference serum sample on a given day. This correction factor was used to standardize the results from all experiments according to the formula [(PMN-associated bacteria counts/total bacterial input counts) × 100] × correction factor. As a further precaution against day-to-day variability in the opsonic assay, all serum samples from an individual patient were run in the same daily assay, as much as possible. The coefficients of variation for the opsonic assay were 10% for Pn 6B and 20% for Pn 14.
In a preliminary experiment, we compared the radiolabeled assay simultaneously with a visual assay of opsonophagocytosis, in which bacteria were stained with methylene blue and the percentage of PMNs which had ingested bacteria was counted under a light microscope (36). The dose-response curves for serum opsonization activity were comparable, and the method with radiolabel was chosen for ease of processing of large numbers of specimens.
Data analysis and statistics.
Continuous variables were analyzed by the Wilcoxon signed-ranks test and the Mann-Whitney U test. Nonparametric correlations between antibody titers and opsonic activity were calculated with Spearman's correlation coefficient.
RESULTS
Optimization of assay conditions.
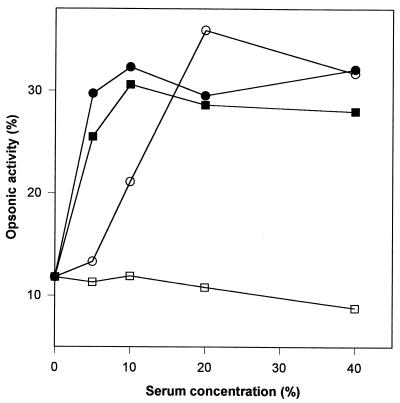
We performed initial experiments in order to determine the optimal assay conditions. As can be seen in Fig. 1, unheated sera, which contained both antibody and complement, had comparable opsonic activities for serotype 14 at all serum concentrations, whether the sera were from subjects with high (15.4 μg/ml) or low (0.5 μg/ml) antibody levels. In contrast, there were significant differences in the opsonic activities of heat-inactivated sera, which were depleted of complement activity but which still contained antibody, between subjects with high and low antibody levels. Comparable results were obtained for serotype 6B. In preliminary experiments the highest opsonic activity was achieved with 20 to 40% heat-inactivated serum (Fig. 1) and a PMN:bacteria ratio of 1:1. In all subsequent assays, 40% heat-inactivated serum and a PMN:bacteria ratio of 1:1 were used in order to measure the effects of antibody without the confounding effect of increasing complement function with age over the first 2 years of life.
FIG. 1.
Effect of serum concentration on opsonization of S. pneumoniae type 14. ●, unheated serum with high antibody level; ■, unheated serum with low antibody level; ○, heat-inactivated serum with high antibody level; □, heat-inactivated serum with low antibody level.
Serum opsonic activity for S. pneumoniae type 6B. (i) Two-month-old age group.
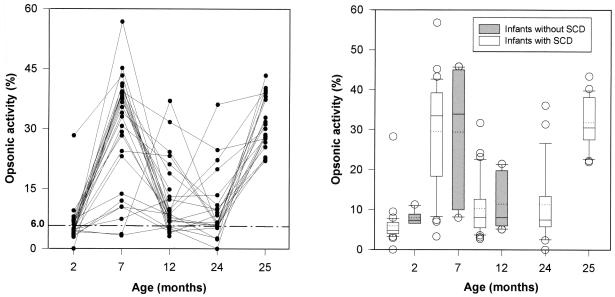
There was an increase in serum opsonic activity after the primary series of immunizations with three doses of pneumococcal polysaccharide protein conjugate vaccine in infants with SCD (Fig. 2A). The median opsonic activity increased from 4.8% (activity for negative control, 6.0%) at 2 months of age to 33.5% at 7 months of age (P < 0.001). There was a subsequent decline in the opsonic activity, which reached median values of 8.1% at 12 months of age and 7.5% at 24 months of age. However, there was a significant increase in opsonic activity to 30.7% at 25 months of age following administration of a booster dose of the 23-valent polysaccharide vaccine (P < 0.001 for comparison between 24 and 25 months of age).
FIG. 2.
(A) Serum opsonic activity for pneumococcus type 6B in infants with SCD immunized at 2, 4, and 6 months of age. Symbols represent individual patients (n = 28). The interrupted line indicates the data for negative controls (6.0%). (B) Comparison of serum opsonic activity for pneumococcus type 6B between infants with SCD and control infants immunized starting at 2 months of age. The lower and upper boundaries of the box indicate the 25th and 75th percentiles, respectively. A continuous line within the box marks the median; a dotted line marks the mean. Error bars indicate the 90th and 10th percentiles. Open symbols represent data outside the 10th and 90th percentiles.
Serum opsonic activity followed a similar course in the 2-month-old group of patients without SCD. Their preimmunization median opsonic activity was 7.4%; it rose to 34% at 7 months of age (P = 0.06) and subsequently declined to 8.1% at 12 months of age (P = 0.03).
Figure 2B shows a comparison of infants with and without SCD who were immunized starting at 2 months of age. Median opsonic activities were not significantly different after immunization (by the Mann-Whitney U test, P = 0.9 at 7 months of age and P = 0.8 at 12 months of age).
(ii) Twelve-month-old age group.
Because of an initial concern that the conjugate vaccine would not be immunogenic in young infants, this vaccine was also tested in older children aged 12 months. It should be noted that when it became apparent that the infants immunized at 2, 4, and 6 months of age responded to the conjugate vaccine, we stopped enrollment of subjects in this study group. For the group of 10 infants with SCD who received a single dose of protein conjugate vaccine at 12 months of age, there was significant increase in opsonic activity from a preimmunization median level of 4.3% to a level of 14.4% (P = 0.03) 1 month after the immunization. It declined to 4.6% (P = 0.03) at 24 months of age but rose again to 35% (P = 0.03) after administration of a booster dose at 25 months of age.
For three infants without SCD the mean opsonic activity increased from 6.4% at 12 months of age to 16.2% (P = 0.11) at 13 months of age as a result of immunization with a single dose of protein conjugate vaccine.
(iii) Relationship of serum opsonic activity and antibody level.
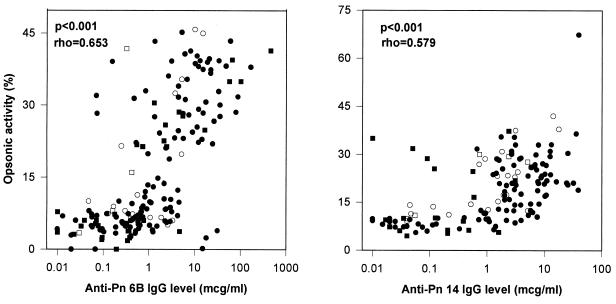
Geometric mean values of anti-Pn 6B IgG antibody levels and median opsonic activity in infants with SCD immunized starting at 2 months can be seen in Table 1. A comparison was also made between serum opsonic activity and antibody levels in all infants in this study. These data demonstrate that the serum opsonic activity correlated with antibody levels (P < 0.001; Spearman's rho = 0.653) in all patients in both age groups (Fig. 3A).
TABLE 1.
Antipneumococcal antibody levels and opsonic activity in SCD infants immunized starting at 2 months of age
| Type and parameter | 2 mo of age | 7 mo of age | 12 mo of age | 24 mo of age | 25 mo of age |
|---|---|---|---|---|---|
| Type 6B | |||||
| Geometric mean (range) anti-Pn 6B IgG level (μg/ml) | 0.11 (0.01–1.21) | 4.11 (0.007–33.7) | 1.37 (0.27–15.05) | 1.61 (0.22–46.05) | 25.49 (4.99–147.71) |
| Median (SD) opsonic activity (%) | 4.8 (4.79) | 33.5 (13.76) | 8.1 (7.22) | 7.5 (9.58) | 30.7 (6.52) |
| Type 14 | |||||
| Geometric mean (range) anti-Pn 14 IgG level (μg/ml) | 0.18 (0.01–8.75) | 6.09 (1.28–38.77) | 3.86 (0.89–12.81) | 1.91 (0.4–18.15) | 19.71 (1.87–65.76) |
| Median (SD) opsonic activity (%) | 9.4 (1.81) | 24.9 (16.68) | 16.5 (7.27) | 12.6 (8.52) | 27.3 (15.95) |
FIG. 3.
(A) Relationship between serum opsonic activity and level of anticapsular antibody against pneumococcus type 6B. Immunization was at 2, 4, and 6 months of age for infants with SCD (●) and control infants (○). Immunization was at 12 months of age for infants with SCD (■) and control infants (□). rho, Spearman's correlation coefficient. (B) Relationship between serum opsonic activity and level of anticapsular antibody against pneumococcus type 14. Immunization was at 2, 4, and 6 months for infants with SCD (●) and control infants. Immunization was at 12 months of age for infants with SCD (■) and control infants (□). rho, Spearman's correlation coefficient.
Serum opsonic activity for S. pneumoniae type 14. (i) Two-month-old age group.
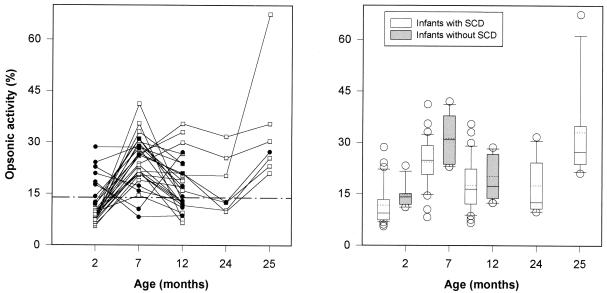
Figure 4A shows the changes in serum opsonic activity for Pn 14 induced by the immunization at 2, 4, and 6 months of age in infants with SCD. The median opsonic activity at 2 months of age was 9.4% (activity for negative control, 14.4%); it increased to 24.9% (P < 0.001) at 7 months of age, with subsequent declines to median values of 16.5 and 12.6% at 12 and 24 months of age, respectively. There was a strong response to a booster dose of polysaccharide vaccine at 24 months of age, with opsonic activity increasing to 27.3% at 25 months of age (P = 0.02).
FIG. 4.
(A) Serum opsonic activity for pneumococcus type 14 in infants with SCD immunized at 2, 4, and 6 months of age. Open symbols represent infants with low (<1.0 μg/ml) preimmunization antibody levels. Closed symbols represent infants with high (>1.0 μg/ml) preimmunization antibody levels. The interrupted line indicates the data for negative controls (14.4%). (B) Comparison of serum opsonic activity for pneumococcus type 14 between infants with SCD and control infants immunized starting at 2 months of age. The lower and upper boundaries of the box indicate the 25th and 75th percentiles, respectively. A continuous line within the box marks the median; a dotted line marks the mean. Error bars indicate the 90th and 10th percentiles. Open symbols represent data outside 10th and 90th percentiles.
In contrast to the anti-Pn 6B IgG titers, nine infants had relatively high preimmunization anti-Pn 14 IgG levels (geometric mean level, 3.093 μg/ml). This high level was defined as ≥1.0 μg/ml on the basis of analogy to data obtained for Haemophilus influenzae type B, in which antibody levels of ≥1.0 μg/ml after immunization are associated with long-term immunity. Although differences in opsonic activity between patients with high and low preimmunization antibody levels were statistically significant (18.4 and 8.1% respectively; P < 0.001) at 2 months of age, median opsonic activities after immunization were comparable at 7 and 12 months of age (P = 0.4 and P = 0.8, respectively, by the Mann-Whitney U test).
The infants without SCD had similar patterns of responses to three doses of the vaccine, with median preimmunization opsonic activity of 14.1% that increased to 30.9% (P = 0.02) at 7 months of age and that subsequently fell to 17.3% (P = 0.02) at 12 months of age. The results of the comparison of infants with and without SCD is shown in Fig. 4B. The mean opsonic activity values were comparable in both groups (by the Mann-Whitney U test, P = 0.04 at 2 months of age, P = 0.06 at 7 months of age, and P = 0.3 at 12 months of age).
Twelve-month-old age group.
Among 10 infants with SCD who were immunized at 12 months of age, the median opsonic activity increased from 6.3% before immunization to 25.1% (P = 0.02) at 1 month postimmunization.
A similar response was observed in the infants without SCD, in whom the median opsonic activity at 12 months was 9.9% and in whom the activity rose to 29.4% (P = 0.1; only three subjects) at 13 months of age after administration of a single dose of the conjugate vaccine.
(iii) Relationship of serum opsonic activity and antibody levels.
Table 1 contains geometric mean anti-Pn 14 IgG antibody levels and median opsonic activity at selected time points in infants with SCD immunized starting at 2 months of age. Figure 3B shows the correlation between anti-Pn 14 IgG antibody levels and opsonic activity in all patients in both age groups (P< 0.001; Spearman's rho = 0.579).
DISCUSSION
Bacterial infection is the most common cause of hospitalization and death in children with SCD (1, 11, 16). Children under 3 years of age are at highest risk for invasive blood-borne bacterial infection, with the pneumococcus being the causative agent in 66% of those with bacteremia (38) and 70 to 85% of those with bacterial meningitis (1, 26).
Two abnormalities which contribute to the increased susceptibility of SCD patients to systemic pneumococcal infections have been described: defective splenic function (22) and deficient pneumococcal serum opsonizing activity (12, 36). Pearson et al. (22) demonstrated the absence of splenic clearance of intravenously injected radioactive colloid in children with SCD and splenomegaly, indicating that the spleen was inactive as a biologic filter for particles. It has also been shown that serum from patients with SCD does not enhance pneumococcal phagocytosis as well as serum from healthy subjects does (6, 12). A number of studies have suggested that the deficient pneumococcal opsonization activity in these patients results from defective antibody-mediated activation of the alternative complement pathway (2, 3, 12, 15, 34, 35).
There is strong experimental evidence that antibody against the pneumococcus may correct or compensate for both immunologic defects in patients with SCD. For instance, opsonic activity for the pneumococcus is restored by supplementation of sera from children with SCD with antibody against the pneumococcus (4, 12). Also, antibody provides protection against blood-borne pneumococcal infection in splenectomized animals (17). Therefore, immunization with pneumococcal vaccine should compensate for both immunologic defects in patients with SCD.
Pneumococcal capsular polysaccharide vaccine increases antibody levels and opsonic activity and also reduces the risk for pneumococcal infection in adults and older children with SCD. However, this vaccine is poorly immunogenic in children younger than 2 years of age (9, 21). Recently, a heptavalent pneumococcal polysaccharide protein conjugate vaccine has been shown to be more immunogenic than the polysaccharide vaccine in patients with SCD ages 2 years old or older (30). Moreover, it was immunogenic in healthy infants (25) and infants with SCD (19), both younger than 1 year of age. Even more important, it was highly effective (100%) in preventing invasive pneumococcal disease in healthy infants younger than 2 years of age (5). In addition, pneumococcal vaccine that contained only 6B polysaccharide conjugated with tetanus toxoid increased opsonic activity in infants and adults without SCD (28, 32).
We conducted the current studies in infants with SCD to establish whether the antibody induced by immunization with a heptavalent pneumococcal conjugate vaccine is opsonically active. Since the focus of our experiments was to investigate the opsonic functions of antibodies, we chose to deplete complement activity with heat. Thus, in our system we measured only heat-stable opsonic activity, which is attributed predominantly to serotype-specific anticapsular IgG antibodies (8, 31). This is a standard method that has been used by others to assess the opsonic role of antibody (27). Although measures of the opsonic activity that depend on antibody and complement may more closely resemble the situations that are present in vivo (9, 12, 36), we chose to use heat-inactivated serum to more directly measure the component of total serum opsonic activity attributable to antibody alone. Another advantage of heat inactivation is that it eliminates the confounding effect of increasing complement function with age over the first 2 years of life. We did not investigate other factors that might affect heat-stable serum opsonic activity, such as IgG subclass distribution (13, 18) or the presence of non-type-specific antipneumococcal antibodies (37).
Our results demonstrate that in infants with SCD, the pneumococcal polysaccharide protein conjugate vaccine causes a significant increase in serum opsonic activity for pneumococci of types 6B and 14. In most subjects, preimmunization serum opsonic activity was very low and was comparable to that for negative controls. Among infants in the 2-month-old age group, the maximum increase in serum opsonic activity occurred after the administration of three doses of the conjugate vaccine. It was followed by a gradual decrease at 12 and 24 months of age to values comparable to preimmunization levels. However, there was a strong anamnestic response to a booster dose of the polysaccharide pneumococcal vaccine at 24 months of age, with a significant rise in opsonic activity at 25 months of age. Nevertheless, it is not known whether these opsonic activities and antibody levels are sufficient to protect SCD infants from clinical disease.
Interestingly, infants with SCD and high preimmunization anti-Pn 14 IgG antibody levels had opsonic responses to the primary series of immunizations that were less than those of infants with low preimmunization antibody levels. However, the median opsonic activities at 7 and 12 months of age were comparable to those in the infants with low preimmunization antibody levels.
The alternative immunization schedule with a single dose of conjugate vaccine at 12 months of age was also effective. It induced significant increases in antibody levels and opsonic activity 1 month after vaccination in both healthy and SCD children. We stopped enrollment of subjects in the older age group after it became clear that immunizations starting at 2 months of age were effective. Therefore, insufficient numbers of patients were enrolled at 12 months to provide a meaningful statistical comparison. In any case, the primary immunization schedule of immunization at 2, 4, and 6 months of age is preferable since it offers protection at a younger age. Our results demonstrate that serum opsonic activity correlates with serotype-specific anticapsular IgG antibody levels for both Pn 6B and Pn 14. The relationship between opsonic activity and antibody levels is highly statistically significant (P < 0.001) for both serotypes. However, the correlation is not ideal. The lack of a perfect correlation may be attributed in part to the IgG subclass distribution (13, 18), non-type-specific antipneumococcal antibodies (37), or other heat-stable serum opsonins (e.g., mannose-binding lectin).
ACKNOWLEDGMENTS
This study was supported by Wyeth-Lederle Vaccines & Pediatrics, The Thrasher Research Fund, training grant T32 AI 07007 from the National Institutes of Health, and The Thomas Wilson Sanitarium.
The following members of the Pneumococcal Conjugate Vaccine Study Group participated in this study: Joseph Gootenberg, Georgetown University, Washington, D.C.; George Dover, Katherine O'Brien, Mathuram Santosham, Sally Snader, and Beth Stover, Johns Hopkins University, Baltimore, Md.; Kellie Hall, Susan Panny, and Sonya Ross, Maryland State Health Department, Baltimore, Md.; Kenneth M. Hoffman, Frank M. Kopack, Jeffrey T. Nold, and Perry S. Shelton, Pediatric Center of Annapolis, Annapolis, Md.; Ruth Luddy and Joan Marascuilo, Sinai Hospital, Baltimore, Md.; Allen Eskenazi and Kristen Sawyer, University of Maryland Children's Hospital, Baltimore, Md.; Allen Kimura and Frank Malinoski, Wyeth-Lederle Vaccines and Pediatrics.
REFERENCES
- 1.Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine. 1971;50:98–112. [PubMed] [Google Scholar]
- 2.Bjornson A B, Lobel J S, Harr K S. Relation between serum opsonic activity for Streptococcus pneumoniae and complement function in sickle cell disease. J Infect Dis. 1985;152:701–709. doi: 10.1093/infdis/152.4.701. [DOI] [PubMed] [Google Scholar]
- 3.Bjornson A B, Lobel J S. Direct evidence that decreased serum opsonization of Streptococcus pneumoniae via the alternative complement pathway in sickle cell disease is related to antibody deficiency. J Clin Investig. 1987;79:388–398. doi: 10.1172/JCI112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjornson A B, Lobel J S, Magnafici P I, Lampkin B C. Restoration by normal human immunoglobulin G of deficient serum opsonization for Streptococcus pneumoniae in sickle cell disease. Infect Immun. 1981;33:636–640. doi: 10.1128/iai.33.2.636-640.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black S, Shinefield H, Ray P, Lewis E, Fireman B, Austrian R, Siber G, Kohberger R, Chang I. Efficacy of heptavalent conjugate pneumococcal vaccine (Wyeth-Lederle) in 37,000 infants and children: results of the Northern California Kaiser Permanente efficacy trial. Pediatr Res. 1999;45:157A. [Google Scholar]
- 6.Buchanan G R, Smith S J. Pneumococcal septicemia despite pneumococcal vaccine and prescription of penicillin prophylaxis in children with sickle cell anemia. Am J Dis Child. 1986;140:428–432. doi: 10.1001/archpedi.1986.02140190038020. [DOI] [PubMed] [Google Scholar]
- 7.Cates K L, Marsh K H, Granoff D M. Serum opsonic activity after immunization of adults with Haemophilus influenzae type b-diphtheria toxoid conjugate vaccine. Infect Immun. 1985;48:183–189. doi: 10.1128/iai.48.1.183-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chudwin D S, Artirp S G, Korenblit A, Schiffman G, Rao S. Correlation of serum opsonins with in vitro phagocytosis of Streptococcus pneumoniae. Infect Immun. 1985;50:213–217. doi: 10.1128/iai.50.1.213-217.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chudwin D S, Wara D W, Matthay K K, Caulifield M H, Schiffman G, Mentzer W C, Ammann A J. Increased serum opsonic activity and antibody concentration in patients with sickle cell disease after pneumococcal polysaccharide immunization. J Pediatr. 1983;102:514. doi: 10.1016/s0022-3476(83)80285-6. [DOI] [PubMed] [Google Scholar]
- 10.Gaston M H, Verter J I, Woods G, Pegelow C, Kelleher J, Presbury G, Zarkovsky H, Vichinsky E, Iyer R, Lobel J S, Diamond S, Holbrook C T, Gill F M, Ritchey K, Falletta J M. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 11.Gill F M, Sleeper L A, Weiner S J, Brown A K, Bellevue R, Grover R, Pegelow C H, Vichinsky E. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative study of sickle cell disease. Blood. 1995;86:776–783. [PubMed] [Google Scholar]
- 12.Johnston R B, Newman S L, Struth A G. An abnormality of the alternate pathway of complement activation in sickle-cell disease. N Engl J Med. 1973;288:803–808. doi: 10.1056/NEJM197304192881601. [DOI] [PubMed] [Google Scholar]
- 13.Kaniuk A C, Lortan J E, Monteil M A. Specific IgG subclass antibody levels and phagocytosis of serotype 14 pneumococcus following immunization. Scand J Immunol. 1992;36:96–98. doi: 10.1111/j.1365-3083.1992.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 14.Koskela M. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr Infect Dis J. 1987;6:519–526. doi: 10.1097/00006454-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Larcher V F, Wyke R J, Davis L R, Stroud C E, Williams R. Defective yeast opsonization and functional deficiency of complement in sickle cell disease. Arch Dis Child. 1982;57:343–346. doi: 10.1136/adc.57.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leikin S L, Gallagher D, Kinney T R, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatrics. 1989;84:500–508. [PubMed] [Google Scholar]
- 17.Leung L S, Szal G J, Drachman R H. Increased susceptibility of splenectomized rats to infection with Diplococcus pneumoniae. J Infect Dis. 1972;126:507–513. doi: 10.1093/infdis/126.5.507. [DOI] [PubMed] [Google Scholar]
- 18.Lortan J E, Kaniuk A C, Monteil M A. Relationship of in vitro phagocytosis of serotype 14 Streptococcus pneumoniae to specific class and IgG subclass antibody levels in healthy adults. Clin Exp Immunol. 1993;91:54–57. doi: 10.1111/j.1365-2249.1993.tb03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien, K. L., A. J. Swift, J. A. Winkelstein, M. Santosham, B. Stover, R. Luddy, J. E. Gootenberg, J. T. Nold, A. Eskenazi, S .J. Snader, and H. M. Lederman. Immunogenicity of a pneumococcal protein conjugate vaccine in infants with sickle cell disease. Pediatrics, in press. [DOI] [PubMed]
- 20.Overturf G D, Powars D, Baraff L J. Bacterial meningitis and septicemia in sickle cell disease. Am J Dis Child. 1977;131:784–787. doi: 10.1001/archpedi.1977.02120200066014. [DOI] [PubMed] [Google Scholar]
- 21.Overturf G D, Selzer J W, Chan L, Weiss J, Field R, Rigau-Perez J G, Powars D, Uy C, Pang E J, Honig G, Steele R, Edmonds R, Portnoy B. Pneumococcal polysaccharide immunization of children with sickle cell disease. Am J Pediatr Hematol Oncol. 1982;4:25–35. [PubMed] [Google Scholar]
- 22.Pearson H A, Spencer R P, Cornelius E A. Functional asplenia in sickle-cell anemia. N Engl J Med. 1969;281:923–926. doi: 10.1056/NEJM196910232811703. [DOI] [PubMed] [Google Scholar]
- 23.Plikaytis B D, Carlone G M, Maslanka S E, Gheesling L L, Holder P F. Program ELISA user's manual. Atlanta, Ga: Centers for Disease Control and Prevention; 1993. [Google Scholar]
- 24.Quartaert S A, Kirch C S, Quackenbush Wiedl L J, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rennels M B, Edwards K M, Keyserling H L, Reisinger K S, Hogerman D A, Madore D V, Chang I, Paradiso P, Malinoski F J, Kimura A. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM sub 197 in United States in infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 26.Robinson M G, Watson R J. Pneumococcal meningitis in sickle-cell anemia. N Engl J Med. 1966;274:1006–1008. doi: 10.1056/NEJM196605052741806. [DOI] [PubMed] [Google Scholar]
- 27.Romer-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigurdardottir S T, Vidarsson G, Gudnason T, Gudnason T, Kjartansson S, Kristinson K G, Jonsson S, Valdimarsson H, Schiffman G, Schneerson R, Jonsdottir I. Immune responses of infants vaccinated with serotype 6B pneumococcal polysaccharide conjugated with tetanus toxoid. Pediatr Infect Dis J. 1997;16:667–674. doi: 10.1097/00006454-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Swift A J, Moxon E R, Zwahlen A, Winkelstein J A. Complement-mediated serum activities against genetically defined capsular transformants of Haemophilus influenzae. Microb Pathog. 1991;10:261–269. doi: 10.1016/0882-4010(91)90010-8. [DOI] [PubMed] [Google Scholar]
- 30.Vernacchio L, Neufeld E J, MacDonald K, Kurth S, Murakami S, Hohne C, King M, Molrine D. Combined schedule of 7-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal vaccine in children and young adults with sickle cell disease. J Pediatr. 1998;133:275–278. doi: 10.1016/s0022-3476(98)70235-5. [DOI] [PubMed] [Google Scholar]
- 31.Vidarsson G, Jonsdottir I, Jonsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]
- 32.Vidarsson G, Sigurdardottir S T, Gudnason T, Kjartansson S, Kristinsson K G, Ingolfsdottir G, Jonsson S, Valdimarsson H, Schiffman G, Schneerson R, Jonsdottir I. Isotypes and opsonophagocytosis of pneumococcus type 6b antibodies elicited in infants and adults by an experimental pneumococcus type 6B-tetanus toxoid vaccine. Infect Immun. 1998;66:2866–2870. doi: 10.1128/iai.66.6.2866-2870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W C, Wong W Y, Rogers Z R, Williams J A, Buchanan G R, Powars D R. Antibiotic-resistant pneumococcal infection in children with sickle cell disease in the United States. J Pediatr Hematol Oncol. 1996;18:140–144. doi: 10.1097/00043426-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Wilson W A, Hughes G R, Lachmann P J. Deficiency of factor B of the complement system in sickle cell anemia. BMJ. 1976;1:367–369. doi: 10.1136/bmj.1.6006.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson W A, Thomas E J, Sissons G P. Complement activation in asymptomatic patients with sickle cell anemia. Clin Exp Immunol. 1979;36:130–139. [PMC free article] [PubMed] [Google Scholar]
- 36.Winkelstein J A, Drachman R H. Deficiency of pneumococcal serum opsonizing activity in sickle-cell disease. N Engl J Med. 1968;279:459–466. doi: 10.1056/NEJM196808292790904. [DOI] [PubMed] [Google Scholar]
- 37.Yu X, Frasch C, Conception N, Nahm M H. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin Diagn Lab Immunol. 1999;6:519–523. doi: 10.1128/cdli.6.4.519-524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarkowsky H S, Gallagher D, Gill F M, Wang W C, Faletta J M, Lande W M, Levy P S, Verter J I, Wethers D. Bacteremia in sickle hemoglobinopathies. J Pediatr. 1986;109:579–585. doi: 10.1016/s0022-3476(86)80216-5. [DOI] [PubMed] [Google Scholar]