Abstract
Vinegar is widely used in Chinese diet as a traditional condiment, and its functional component acetic acid has been proposed to prevent obesity, while its mechanism is still unclear. Bile acids (BAs) have been reported to have a protective effect on obesity. This study demonstrated that high-fat diet induced obesity (DIO) seriously disturbed BAs balance by significantly decreasing hepatic BAs synthesis and increasing fecal BAs excretion. However, acetate supplemented in the high-fat diet can restore BAs balance by mainly promoting hepatic taurine conjugated BAs (tauro-BAs) synthesis and decreasing fecal tauro-BAs excretion. The tauro-BAs, as the antagonists, inhibited the intestinal-liver farnesoid X receptor (FXR)-fibroblast growth factor 15 (FGF15)-FGF receptor 4 (FGFR4) signaling pathway, and negatively regulated the production of hepatic BAs. Present study provided important clues for further investigation of the mechanism of acetic acid inhibiting DIO.
Keywords: Obesity, Acetic acid, Bile acids, Antagonist, FXR
Graphical abstract
Highlights
-
•
Obesity disturbed Bile acids (BAs) balance by decreasing hepatic BAs synthesis.
-
•
Obesity disturbed BAs balance by increasing fecal BAs excretion.
-
•
Acetate restored BAs by promoting hepatic synthesis and decreasing fecal excretion.
-
•
The hepatic BAs inhibited FXR-SHP, negative feedback regulated BAs production.
-
•
The ileal taurine conjugated BAs inhibited FXR-FGF15-FGFR4, regulated BAs production.
1. Introduction
Currently, obesity, and its associated comorbidities carry a huge economic burden; therefore, improved strategies for obesity prevention and control urgently need. Acetic acid is an important component of vinegar in the human diet. The supplementation of exogenous acetic acid through dietary sources has proposed as a promising novel strategy for the prevention or management of obesity (Primec et al., 2017; Valdes et al., 2021). Previous studies mainly focused on the role of acetic acid in appetite regulation and lipid and glucose metabolism by epigenetically regulating related genes (Valdes et al., 2021). Colonic-derived or exogenous acetic acid reaches the liver via the portal vein, where it is converted to acetyl coenzyme A (Ac-CoA) as an intermediate, and further used as substrate for the synthesis of cholesterol or other materials (Valdes et al., 2021). At present, there was no research on acetic acid as an intermediate to trigger the synthesis of other substances, which was associated with the suppression of body weight.
It has been noticed that obesity and obesity-related diseases such as type 2 diabetes (T2D), non-alcoholic fatty liver disease (NAFLD) and cardiovascular diseases (CVD) are associated with altered BAs metabolism (Li et al., 2021); meanwhile BAs intervention could effectively control and prevent obesity and NAFLD (Jiao et al., 2018; Watanabe et al., 2006). Watanabe et al. reported that the administration of BAs to mice increased energy expenditure in brown adipose tissue (BAT), preventing obesity and resistance to insulin (Watanabe et al., 2006), which their effects were consistent with those of acetic acid supplemented in high-fat diet (Lu et al., 2016).
BAs are synthesized from cholesterol in liver through two pathways and secreted into the small intestine (Chiang and Ferrell, 2020). Cholesterol 7α-hydroxylase (CYP7A1) performs the initial and rate-limiting step in the classical pathway, which accounts for more than 90% of total BAs production. Sterol-27-hydroxylase (CYP27A1) initiates the hydroxylation of cholesterol in the alternative pathway (Daniel et al., 2019). Particularly, the most common BAs conjugate with taurine in the liver to form taurocholate, which renders BAs less hydrophobic and less cytotoxic, ready to exploit their physiological function in the intestine (Kim et al., 2007). BAs can also be used as signal molecules and endogenous ligands that activate or inhibit multiple nuclear receptors, such as FXR, to regulate cholesterol and BAs metabolism accordingly, e.g., taurocholic acid (TCA) is a FXR antagonist (Wahlström et al., 2016). Several studies reported that activation of FXR by BAs induces the negative nuclear receptor small heterodimer partner (SHP) to inhibit the transcription of BAs synthesis gene CYP7A1 and Sterol-12α-hydroxylase (CYP8B1) in hepatocytes (Antonio et al., 2004; Calkin and Tontonoz, 2012; Chiang et al., 2000; Zhang and Chiang, 2001). Pandak et al. reported that intraduodenal transfusion of taurocholate inhibited CYP7A1 enzyme activity and mRNA in biliary-diverted rats, but an intravenous infusion did not, suggesting that taurocholate might induce an intestinal factor in the ileum to regulate BAs synthesis in the liver (Pandak et al., 1995). Additionally, FGF15 was identified as an intestinal FXR-regulated hormone that activates hepatic membrane FGFR4 to inhibit CYP7A1 and CYP8B1 gene transcription (Inagaki et al., 2005).
It is noteworthy that BAs are cholesterol metabolites and maintain cholesterol homeostasis in the body (Li et al., 2017). Acetic acid is the substrate for cholesterol synthesis in the liver as well (Watanabe et al., 2006). Thus, we posed a hypothesis that acetic acid might promote the synthesis of BAs in mouse liver and further regulate FXR related signaling pathway in both mouse liver and gut, contributing suppression of body weight. We investigated whether suppression of DIO in mice by dietary acetic acid supplementation is associated with modulation of BAs metabolism. Furthermore, we demonstrated the relationship between tauro-BAs and FXR related signaling pathway in both mouse liver and gut.
2. Materials and methods
2.1. Animal details and dietary interventions
Three-to four-week- old C57BL/6J male mice were purchased from the specific-pathogen-free laboratory of animal technology Co., Ltd. (Beijing) and housed under a 12-h dark-light cycle and had free access to food and water in the National Institute of Occupational Health and Poison Control, China CDC. After one week of recovery from transportation, the mice were randomly divided into three groups: Control (C), high-fat diet (HFD) and HFD with acetate (HFD_A) (n = 6/group), fed with the low fat diet (LFD), HFD and HFD supplemented with 5% acetate (HFD_A) diets, respectively, for 16 weeks. The body weights and the diet intake were measured at 1st, 4th, 8th, 12th and 16th weeks. Fresh stool samples were collected at the end of the feeding interventions and stored at −80 °C.
After 16 h fasting, the mice were anesthetized by intraperitoneal injection of Avertin (125 mg/kg of 2,2,2-tribromoethanol, T-4840-2, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) to obtain blood samples by heart puncture, and then sacrificed by decapitation following administration of an overdose of Avertin (500 mg/kg). The serum was stored at −80 °C for later analysis of short chain fatty acids (SCFAs), BAs and other biochemical parameters. After sacrifice, the mouse epididymal fat, subcutaneous fat, subscapular BAT, liver and ileum were dissected free of the surrounding tissue, weighed and frozen in liquid N2 and finally were transferred to −80 °C until the further analysis.
All animal procedures in this study were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of National Administration Regulations on Laboratory Animals of China. All experimental protocols (No. EAWE-2021-04) were approved by the Committee on the Ethics of Animal Experiments of the National Institute of Occupational Health and Poison Control, China CDC.
Two types of high-fat diet (HFD, 34.9% fat by wt., 60% kcal), including HFD and HFD_A, were designed according to the HFD formula (D12492) for the DIO mice from Research Diets, Inc. (New Brunswick, NJ). These HFDs contained the same amount of lard and soy oil as the main source of fat. Additionally, LFD (4.3% fat by wt., 10% kcal) was also designed based on the control diet formula (D12450B) from Research Diets, Inc. Sodium acetate (Sigma-Aldrich, St. Louis, MO) was added into the high-fat diet at a proportion of 5% (wt/wt) in the HFD_A group. The detailed information showed in the Table 1 and Table 2. The diets were prepared by Beijing Huafukang Bioscience Co. Inc. (Beijing, China) and stored at −20 °C before use.
Table 1.
Composition of mouse feed (a).
| Product | D12450B (LFD) |
D12492 (HFD) |
||
|---|---|---|---|---|
| gm% | kcal% | gm% | kcal% | |
| Protein | 19.2 | 20 | 26.2 | 20 |
| Carbohydrate | 67.3 | 70 | 26.3 | 20 |
| Fat | 4.3 | 10 | 34.9 | 60 |
Table 2.
Composition of mouse feed (b).
| Ingredient | LFD | HFD | HFD_A |
|---|---|---|---|
| Sodium acetate | 0 | 0 | 52.5 |
| Casein, 80 Mesh | 189.58 | 258.45 | 258.45 |
| L-Cystine | 2.84 | 3.88 | 3.88 |
| Corn Starch | 298.59 | 0 | 0 |
| Maltodextrin 10 | 33.18 | 161.53 | 161.53 |
| Sucrose | 331.77 | 88.91 | 88.91 |
| Cellulose, BW200 | 47.4 | 64.61 | 64.61 |
| Soybean Oil | 23.7 | 32.31 | 32.31 |
| Larda | 18.96 | 316.6 | 316.6 |
| Mineral Mix S10026 | 9.48 | 12.92 | 12.92 |
| DiCalcium Phosphate | 12.32 | 16.8 | 16.8 |
| Calcium Carbonate | 5.21 | 7.11 | 7.11 |
| Potassium Citrate, H2O | 15.64 | 21.32 | 21.32 |
| Vitamin Mix V10001 | 9.48 | 12.92 | 12.92 |
| Choline Bitartrate | 1.9 | 2.58 | 2.58 |
| FD&C Yellow Dye#5 | 0.047 | 0 | 0 |
| FD&C Blue Dye#5 | 0 | 0.065 | 0.065 |
| Total | 1000.10 | 999.94 | 1052.44 |
Formulated by E.A. Ulman, Ph.D., Research Diets, Inc., 8/26/98 and 3/11/99.
Typical analysis of cholesterol in lard = 0.95 mg/g.
2.2. Biochemical parameters
Serum and liver triglyceride (TG) concentrations, serum and liver total cholesterol (TC) concentrations were analyzed by an enzymatic procedure of gliseril phospo para amino phenazone (GPO-PAP) and cholesterol oxidase p-aminophenol (COD-CE-PAP) using a TG kit and a TC assay kit, respectively. Liver Ac-CoA concentrations were determined by an ultra high performance liquid chromatography-mass spectrometry (UHPLC-MS/MS) method, the detailed information was in the Supplementary material.
2.3. Measurement of SCFAs
Feces, serum and liver samples were collected from mice among the three assays. 50 mg liver tissue sample was mixed with 20 pre-chilled zirconium oxide beads and 100 μL of deionized water in 1.5 mL centrifuge tubes, and then the sample was homogenated for 3 min 100 μL samples of serum and liver homogenate were placed in 2 mL centrifuge tubes for the further analysis according to the previous method by gas chromatography-mass spectrometry (GC-MS, Agilent Technologies, St Clara, CA, USA) (Wang et al., 2020). About 50 mg fecal samples were mixed with 1 mL pure water vortexing for 3 min and centrifuging at 13,000 rpm for 8 min at 4 °C. Then, 900 μL liquid supernatant were transferred into brown glass adjusting the pH value to around 4.0 for the further analysis by GC-MS (Wang et al., 2020) and the details were shown in the Supplementary material.
2.4. Measurement of BAs
All of the BAs standards were purchased from Sigma-Aldrich (St. Louis, MO, USA) and TRC Chemicals (Toronto, ON, Canada). All the BAs and Isotopic internal standards were accurately weighed and prepared in methanol solution to obtain individual stock solution at a concentration of 5.0 mM. Appropriate amount of each BAs stock solution was mixed and stepwise diluted in bile-acid-free matrix (BAFM, serum) at the concentration of 2500, 500, 250, 50, 10, 2.5 and 1.0 nM to prepare standard solutions. In addition, appropriate amount of BAs stock solution was mixed and stepwise diluted in BAFM at the concentration of 1500, 150 and 5 nM to prepare quality control samples. Internal standard were added into all standard solutions and quality control samples to monitor data quality and to adjust for the matrix effect.
About 10 mg of fecal samples, 10 mg liver samples and 20 μL serum samples of mice were weighted and transferred to a 1.5 mL tube. Then 50 μL of water was added and the samples were homogenated with zirconium oxide beads for 3 min and 240 μL methanol containing internal standard was added to exact the BAs. 40 μL of freshly prepared derivative reagents was added to each sample at 30 °C for 60 min. After derivatization, 135 μL of supernatant was transferred to a 96-well plate and sealed for UHPLC-MS/MS analysis according to the previously reported methods (Takahashi et al., 2020; Xie et al., 2015, Xie et al., 2021). All separations were performed with an ACQUITY BEH C18 column (1.7 μm, 100 mm × 2.1 mm, Waters, USA). Data acquisition was performed using MassLynx version 4.1 and BAs quantification were carried out by the TargetLynx applications manager version 4.1 (Waters, USA).
2.5. RNA isolation and quantitative real-time PCR
Total RNA was extracted from liver and ileum using the RNAiso Plus (Takara Bio, Shiga, Japan), and then reverse transcribed to cDNA First-strand (cDNA) synthesis was performed using the All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (One-Step gDNA Removal) (TransGen Biotech, Beijing, China) according to the procedures provided by the manufacturer. Real-time PCR was performed using the Top Green qPCR SuperMix (TransGen Biotech, Beijing, China) and analyzed by the CFX96 Touch TM Real-Time PCR Detection System (Bio-Rad). Relative expression of mRNA was determined after normalization to ribosomal protein large P0 (RPLP0, also known as 36B4) levels using the 2-△Ct method (Fan et al., 2020). The oligonucleotide primers for the targeted genes were designed and verified by Primer-Blast (https://www.ncbi.nlm.nih.gov/tools/primer blast/), and showed in the Table S2.
2.6. Statistical analysis
All statistical and data analysis were carried out by the SPSS 19.0 statistical software and Origin 9.0 (Origin Lab, USA). The Kolmogorov-Smirnov test was used to evaluate whether the data was normally distributed. We used the unpaired t-test for the normally distributed data and the Mann-Whitney U test for the non-normally distributed data to calculate the difference between each two groups. P < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Acetate inhibited body weight gain in the DIO mice
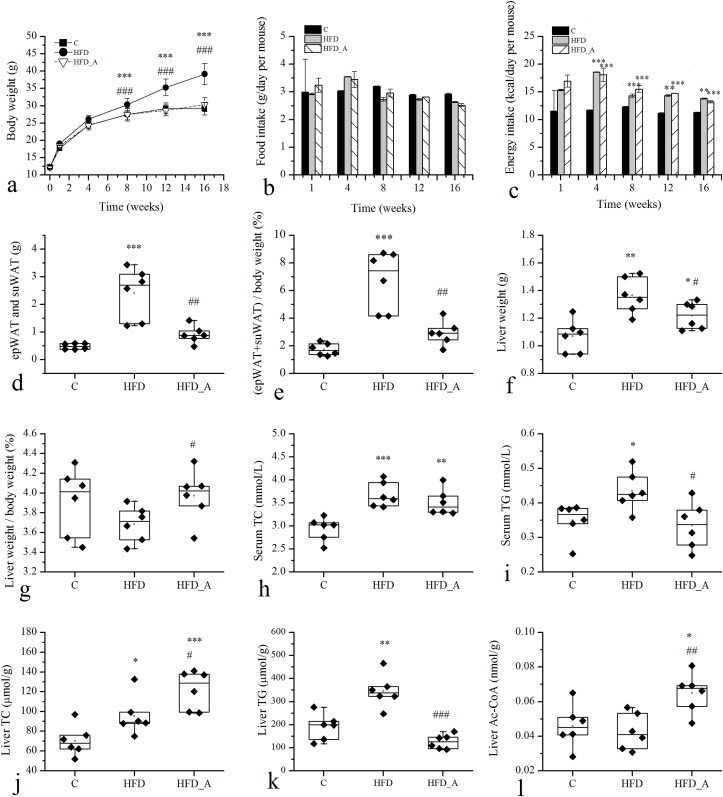
The effect of acetate on obesity was investigated using C57BL/6J male mice with HFD-induced obesity. The acetate-containing HFD was well tolerated and devoid of side effect for up to 16 weeks. The body weight of mice administered with LFD and HFD showed a significant difference, which those fed with HFD (39.14 g) gained around 34% body weight more than those fed with LFD (29.13 g) after 16 weeks of feeding (p < 0.01, Fig. 1a.). HFD supplemented with acetate (HFD_A) evidently suppressed mouse weight gain, which the body weight (30.3 g) decreased by 23% comparing to the HFD group (p < 0.05) and was closed to the C group. Food intake and energy intake were measured at the 1st, 4th, 8th, 12th and 16th weeks of the feeding intervention and the results showed in the Fig. 1b and c. There were no significant differences in food intake and energy intake between the HFD and the HFD_A groups during the feeding intervention. The collective data demonstrated that supplementation of acetate in the HFD group could prevent body weight gain from high fat dietary induced-obesity without affecting food and energy intakes.
Fig. 1.
Effects of acetate on body weight and metabolic indicators in mouse serum and liver. Note: d: the sum of the subcutaneous and epididymal fat; e: the body fat ratio, 100% * (subcutaneous fat weight + epididymal fat weight)/body weight; g: the liver index, 100% * liver weight/body weight; * Compared to the C group; # compared to the HFD group. p < 0.05 (*/#), p < 0.01 (**/##), p < 0.001 (***/###).
Effects of acetate on fat accumulation were studied by analyzing the weight of epididymal and subcutaneous fat and the size of adipocytes, and the results showed that acetate supplemented in HFD reduced fat accumulation, decreased the size of fat droplets, and inhibited BAT whitened, which the details were shown in the Supplementary material (Fig. 1 d, e and Fig. S1). In addition, effects of acetate on liver weights and histological changes were studied and the results suggested that HFD supplemented with acetate recovered the structure and morphology of mouse liver (Fig. S1). The effects of acetate on biochemical parameters were shown in the Fig. 1 h, i, j, k, l and the details were in the Supplementary material.
3.2. Effect of acetate on SCFAs levels of DIO mice
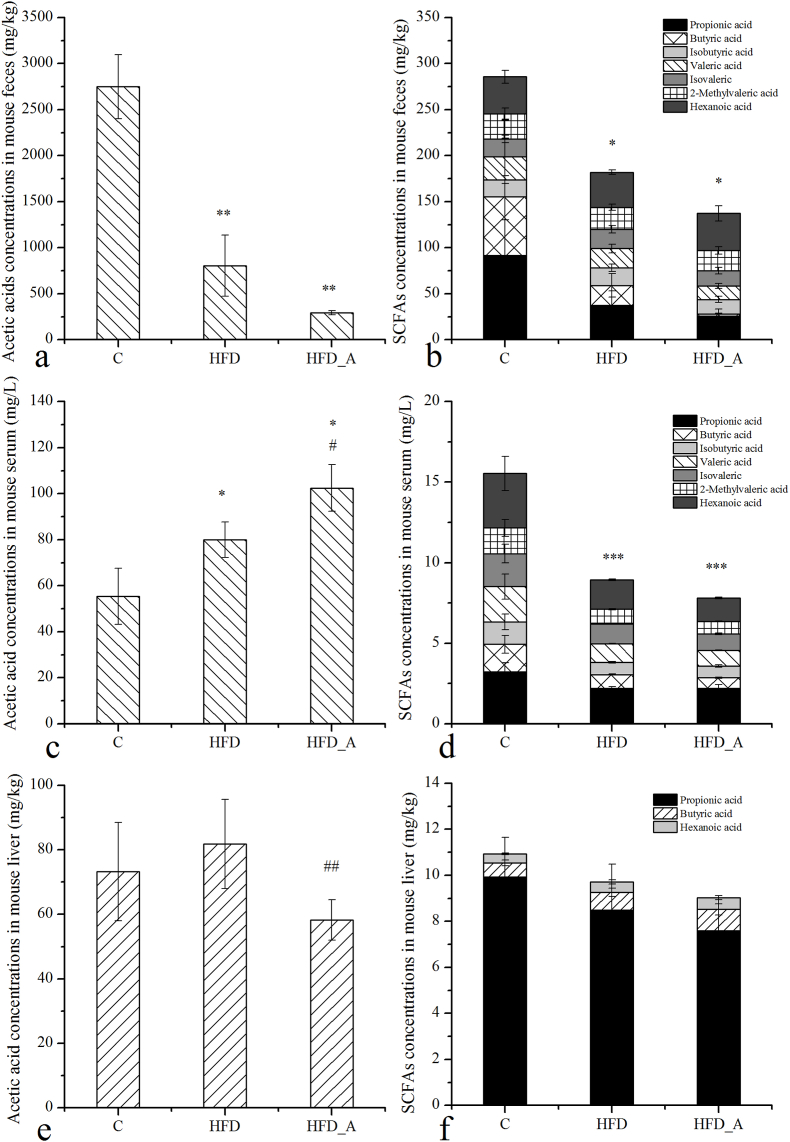
SCFAs in mouse feces, serum and livers in each group were detected and the results were shown in the Fig. 2. Acetic acid was the most abundant SCFAs in mouse feces, and its concentrations of the HFD group (805.18 mg/kg) and the HFD_A group (291.41 mg/kg) were much lower than that of the C group (C: 2749.38 mg/kg) (p < 0.01). In addition, the concentrations of acetic acid in mouse feces of the HFD_A group were slightly lower than that of the HFD group, although the difference was not significant (p > 0.05). The change trends of other SCFAs (propionic acid, butyric acid, valeric acid etc.) among the three groups were similar to that of acetic acid in mouse feces, which followed the order of C » HFD > HFD_A. In the pre experiment, the concentrations of acetic acid in mouse feces and colon contents were detected (Fig. S2). The results showed that there was no significant difference in acetic acid concentrations between the mouse feces and colon contents, which indicated that the concentrations of acetic acid in mouse feces could represent the concentrations of acetic acid in mouse colon contents. These results indicated that the supplementation of acetate in the HFD had no significant effect on the colonic acetic acid.
Fig. 2.
SCFAs concentrations of mouse feces, serum and liver. Note: * Compared to the C group; # compared to the HFD group. p < 0.05 (*/#), p < 0.01 (**/##), p < 0.001 (***/###).
We further detected the concentrations of SCFAs in mouse serum. The results showed that acetic acid concentrations in mouse serum of the HFD group (79.92 mg/L) was much higher than that of the C group (55.36 mg/L, p < 0.05), and that of the HFD_A group (102.44 mg/L) was much higher than that of the HFD group (p < 0.05). The change trend of acetic acid in mouse serum was totally opposite to that in mouse feces. Perry et al. reported that plasma acetic acid concentrations and whole-body acetic acid turnover in HFD-fed rats exhibited increased comparably to those in chow-fed rats (Perry et al., 2016), which were accordance with our results. From our data, we could also prove that more acetic acid was absorbed and released into the circulation in the HFD-fed mice, and most likely, acetate supplemented in the HFD had been absorbed via the upper gastrointestinal tract and had been released into circulation, rarely had reached the colon, which was accordance with the previous study (Valdes et al., 2021). The trends of other SCFAs in mouse serum were similar to those of mouse feces in each group, indicating that other SCFAs in mouse serum were mainly absorbed from colonic fermentation by microbiota (Koh et al., 2016).
The results of SCFAs in mouse liver showed that the concentrations of acetic acid had a slightly increase in the HFD group comparably to that in the C group, but the difference was not significant (p > 0.05). However, its concentrations decreased evidently in the HFD_A group comparably to that of the HFD group (p < 0.05), which was opposite to its change trend in mouse serum. Published studies demonstrated that acetic acid was the substrate for cholesterol synthesis through Ac-CoA in the liver (Besten et al., 2013; Wolever et al., 1991). In the HFD_A group, did the significantly reduced acetic acid in mouse liver use to synthesize Ac-CoA and further participate in the synthesis of cholesterol? The data from the biochemical parameters of mouse liver showed that the level of hepatic TC and Ac-CoA of mice fed with acetate-containing HFD was much higher than that of mice fed with normal HFD and LFD (Fig. 1j, l), indicating acetic acid indeed promoted hepatic cholesterol synthesis. Furthermore, cholesterol was the substrate of BAs synthesis in the liver, which has been proved to have an obviously protective effect on obesity induced by HFD (Watanabe et al., 2006).
3.3. Effect of acetate on BAs levels in DIO mice
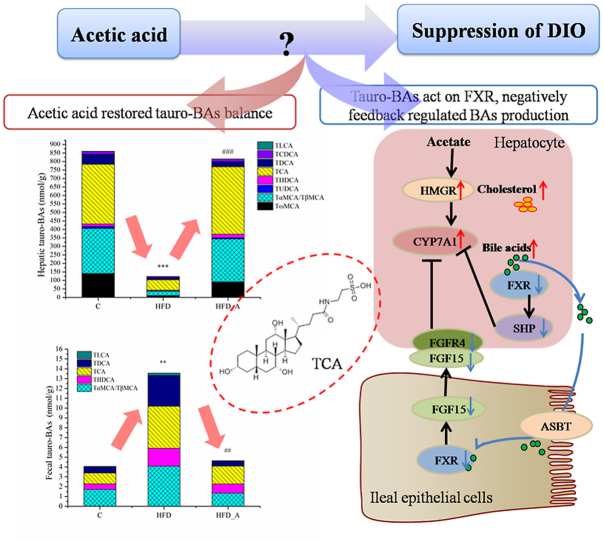
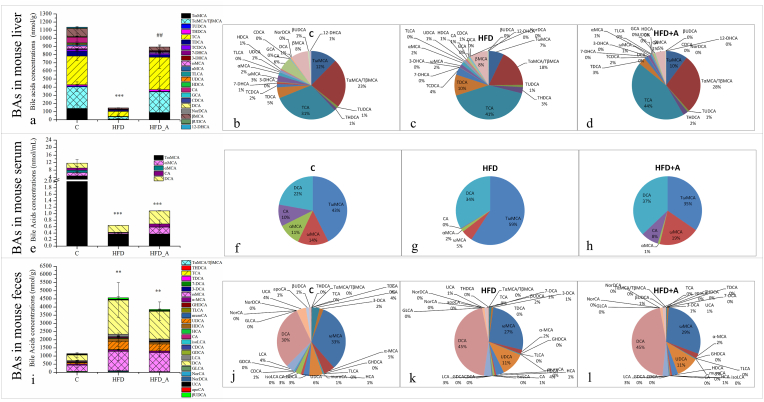
To investigate the effect of acetic acid on BAs levels, the concentrations of BAs in mouse livers, serum and feces were detected (Fig. 3). It was found that tauro-BAs were the dominant species in mouse liver, mainly including TCA and tauro-α-muricholic acid/tauro-β-muricholic acid (TαMCA/TβMCA), which accounted for 31%–44% and 18%–28% of the total BAs (Fig. 3 b, c, d). While non conjugated BAs were the dominant species in mouse feces, mainly including deoxycholic acid (DCA) and ω-muricholic acid (ωMCA), which accounted for 30%–45% and 27%–33% of the total BAs (Fig. 3 j, k, l). Different from the composition of hepatic and fecal BAs, the major BAs in mouse serum both included conjugated BAs (tauro-ω-muricholic acid (TωMCA), accounted for 35%–59%) and non conjugated BAs (DCA, accounted for 22%–37%) (Fig. 3 f, g, h).
Fig. 3.
BAs concentrations and percentages in mouse liver, serum and feces. Note: b, c, d: BAs percentages in mouse liver; f, g, h: BAs percentages in mouse serum; j, k, l: BAs percentages in mouse feces. TDCA: taurodeoxycholic acid, 7-DCA: 7-dehydrocholic acid, 3-DCA: 3-dehydrocholic acid, muroCA: murocholic acid, HCA: hyocholic acid, NorDCA: nordeoxycholic acid, UCA: ursocholic acid, apoCA: apocholic acid, 7-DHCA: 7-dehydrocholic acid, 3-DHCA: 3-dehydrocholic acid, 12-DHCA: 12-dehydrocholic acid. * Compared to the C group; # compared to the HFD group. p < 0.05 (*/#), p < 0.01 (**/##), p < 0.001 (***/###).
The previous study reported that the primary BAs produced in humans were chenodeoxycholic acid (CDCA) and cholic acid (CA), while rodents, such as mouse, produced CA and muricholic acids (MCAs) (Wahlström et al., 2016). In this study, the hepatic dominant BAs TCA and TαMCA/TβMCA were taurine conjugated forms of CA and α-muricholic acid/β-muricholic acid (αMCA/βMCA). After aiding dietary fat absorption by acting as “intestinal soaps”, a large amount of the conjugated BAs was reabsorbed in the distal ileum by the apical sodium-dependent bile acids transporter (ASBT). While a small amount entered the colon to convert into the more hydrophobic secondary BAs by gut microbiota and reabsorbed from the colon. Approximately 95% of BAs are reabsorbed by the intestinal epithelium and are transported to hepatocytes, maintaining the BAs enterohepatic circulation. Only about 5% fecal BAs loss is balance by de novo synthesis of BAs in the liver to maintain BAs pool size (Wahlström et al., 2016). Therefore, the dominant fecal BAs were the secondary BAs and the major serum BAs not only included the primary BAs but the secondary BAs as well.
Interestingly, most of the hepatic dominant BAs in the HFD group were significantly lower than those in the C group (p < 0.05) (Fig. 3a), including TωMCA, TαMCA/TβMCA, tauroursodeoxycholic acid (TUDCA), taurohyodeoxycholic acid (THDCA), TCA, taurochenodeoxycholic acid (TCDCA), ωMCA, αMCA, βMCA and glycocholic acid (GCA) (Fig. S4). As a result, the total amount of the hepatic BAs in the HFD group (147.24 nmol/g) was very much lower than that in the C group (1137.96 nmol/g). HFD supplemented with acetate significantly increased the hepatic levels of total BAs (895.31 nmol/g, p < 0.01) comparably to that in the HFD group, especially the tauro-BAs, such as TωMCA, Tα/βMCA, TUDCA, THDCA, TCA, TCDCA (p < 0.05) (Fig. S4). The proportion of tauro-BAs of the total hepatic BAs increased from 83% (HFD) to 91% (HFD_A) in mouse liver; especially, the proportion of Tα/βMCA increased from 18% (HFD) to 28% (HFD_A).
The change trend of BAs excreted from mouse feces in each group seemed to be completely different from that synthesized by liver (Fig. 3i and Fig. S5). The concentrations of most fecal BAs in the HFD group were evidently higher than those in the C group, including THDCA, ursodeoxycholic acid (UDCA), glycolithocholic acid (GLCA), TCA, hyodeoxycholic acid (HDCA), norcholic acid (NorCA), ωMCA, isolithocholic acid (isoLCA), β-ursodeoxycholic acid (βUDCA), glycohyodeoxycholic acid (GHDCA), glycodeoxycholic acid (GDCA), taurolithocholic acid (TLCA), lithocholic acid (LCA) and DCA (p < 0.05, Fig. S5). The total amount of BAs excreted from mouse feces of the HFD group (4457.39 nmol/g) were extremely higher than that of the C group (C: 1148.88 nmol/g, p < 0.05), especially, the proportion of DCA (C: 30.43%; HFD: 44.60%; p < 0.05) (Fig. 3 j, k), which increased by around 14%. The overall profile of fecal BAs in the HFD_A group (3927.76 nmol/g) did not appear to be significantly different from that of the HFD group (4457.39 nmol/g). Only tauro-BAs of the HFD_A group showed a downward trend in mouse feces, especially THDCA, TCA and TLCA, which decreased evidently comparably to those of the HFD group (p < 0.05, Fig. S5). Thus, we inferred that these tauro-BAs were reabsorbed in the ileum of mice in the HFD_A group.
The change trend of the total BAs in mouse serum seemed to be somewhat similar to that in mouse liver among the three groups (Fig. 3e). The total BAs concentrations of the HFD group (0.64 nmol/mL) and HFD_A group (1.08 nmol/mL) were much lower than those of the C group (11.74 nmol/mL; p < 0.001). Although the total amount of BAs increased in the HFD_A group comparably to that in the HFD group, the difference was not significant. The change trends of DCA and TωMCA in mouse serum were similar to those in mouse feces. The percentage of DCA in the high-fat diet groups (HFD: 34%; HFD_A: 37%) were higher than that in the C group (22%; p < 0.05, Fig. 3). In addition, the percentage of TωMCA increased in the HFD group (C: 43%; HFD: 59%) and decrease in the HFD_A group (35%) comparably to that in the HFD group (Fig. S5). These results indicated that serum DCA and TωMCA might be mostly absorbed from the intestine into the circulation system. It has been recognized that serum BAs levels are sensitive indicators of hepatobiliary disease and are useful as one of reliable liver function tests. Serum BAs levels are dependent on several factors, including hepatic synthesis and elimination of BAs, intestinal absorption and renal elimination of BAs (Xie et al., 2015). In this study, it seemed that intestinal absorption played an important role in the serum BAs profile.
DCA is known to be associated with NAFLD, DNA damage, obesity and hepatocelluar carcinoma (Yoshimoto et al., 2013). A decrease in DCA concentration means an indication of a healthier BAs profile (Jiao et al., 2018); on the contrary, an increase in DCA concentration might be considered an indication of an unhealthier BAs profile. In this study, HFD resulted in an unhealthier fecal BAs profile. There were no significant differences in DCA concentrations and percentages in mouse feces and serum between the HFD_A and HFD groups. These data demonstrated that the HFD supplemented with acetate did not significant change the unhealthier fecal DCA level.
Why the total BAs excreted from mouse feces of the high-fat diet groups (HFD and HFD_A) were much higher than those of the C group, but there was no significant difference between the HFD and HFD_A groups? In fact, the main configuration of BAs has both hydrophilic and hydrophobic sides; their molecules have the characteristics of interfacial active molecules and are easier to bind to fat (Takahashi et al., 2020). HFD contains a large amount of fat (Table 2). Thus, we hypothesized that a large amount of BAs could be excreted with the undigested fat from the HFD and HFD_A groups. To investigate whether the amount of fat (calculated by fatty acids) make BAs easier to excrete with the feces of the high-fat diet groups, fecal fat amount was detected among the three groups. The results showed that the total amount of fat excreted from mouse feces feeding with high-fat diet (HFD and HFD_A groups) was indeed more than 10 times higher than that feeding with low-fat diet (Fig. S7). There was a significant positive correlation between the levels of fat and BAs in mouse feces (R2 = 0.822, p < 0.01) among the three groups. Other experimental studies in rodents and human epidemiological data also indicated that diets characterized by high intakes of fat and Western-style diet were strongly correlated with the incidence of obesity and metabolic syndrome with increased colonic and fecal BAs levels (Gadaleta et al., 2017). Based on these evident, we inferred that a large amount of undigested fat existed in the whole intestine of mice in the high-fat diet groups, tended to combine with BAs, which eventually lead to a large loss of BAs from the feces with fat.
Theoretically, the amount of BAs produced by liver should be equal to the amount of BAs excreted by feces, to achieve the metabolic balance of BAs. However, actually, only in the C group, the amount of BAs excreted from the mouse feces (1148.88 nmol/g) was almost equal to the amount of BAs synthesized by the liver (1137.96 nmol/g). The situations of the other two groups were very different (Fig. 3), which the amount of BAs excreted from the mouse feces (HFD: 4457.39 nmol/g; HFD_A: 3927.76 nmol/g) were much higher than those synthesized by the liver (HFD: 147.24 nmol/g; HFD_A: 895.31 nmol/g, p < 0.01). For HFD group, the decrease of BAs synthesized by the liver and the increase of BAs excreted by feces had “a double hit” to the BAs homeostasis in the obese mice, which seriously destroyed the BAs metabolic balance and leaded to a large loss of BAs. The physiological significance of BAs enterohepatic circulation is to reuse the limited BAs and promote digestion and absorption of lipid food. These findings indicate that the metabolic balance of BAs was disturbed by HFD. However, supplementation of HFD with acetate had a positive effect on the regulation of the metabolic balance of BAs, which significantly increased the amount of BAs synthesized by the liver and was even close to that in the C group (C and HFD_A groups: p > 0.05). These changes improved the BAs homeostasis in the obese mice, which finally might contribute to the decrease of the body weight in the HFD_A group.
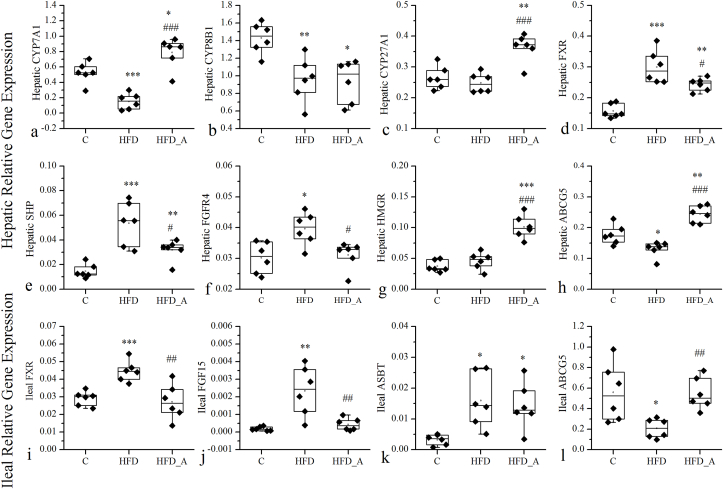
3.4. Effect of acetate on gene abundance in DIO mice
To further clarify the effect of acetic acid on the gene regulation of BAs metabolism, the expression of related genes in liver and ileum of mice in each group have been determined (Fig. 4). HFD significantly decreased the gene expression levels of hepatic BAs synthase genes CYP7A1 and CYP8B1 compared to the C group (Fig. 4a and b). Dietary supplementation of acetate to the HFD significantly increased the expression of CYP7A1 and CYP27A1 in liver by 5.1 and 1.5 fold respectively, compared with the HFD group (Fig. 4a and c). Pearson correlation analysis between BAs and their related genes in mouse liver showed that TωMCA, TαMCA/TβMCA, TCA, TCDCA, TUDCA and THDCA had significant positive correlations with the mRNA expression of CYP7A1 (R2 = 0.569, 0.581, 0.551, 0.572, 0.470, 0.547, p < 0.05). And TωMCA, βMCA, TUDCA, ωMCA, αMCA, CDCA, βUDCA had significant positive correlations with the mRNA expression of CYP8B1 (R2 = 0.481, 0.493, 0.500, 0.525, 0.482, 0.484, 0.473, p < 0.05). The mRNA expression of CYP27A1 had no significant correlations with the BAs in mouse liver. The above data indicated that HFD suppressed the mRNA expression of hepatic BAs synthase genes, and acetate supplementation could effectively restore them. Additionally, the increased hepatic expression of CYP7A1 mainly driven the increase of hepatic BAs synthesis in the acetate-containing HFD fed mice. Previous study reported that overexpression of CYP7A1 in mice enlarged the BAs pool and protected mice from HFD induced obesity and insulin resistance while maintaining cholesterol homeostasis (Chiang and Ferrell, 2020), which was accordance with our results.
Fig. 4.
Effects of acetate on the mRNA expression of hepatic and ileal related enzymes and transporters that involve in cholesterol and BAs metabolism. The expression of genes associated with BAs synthesis (CYP7A1, CYP8B1 and CYP27A1), negative feedback regulation of BAs synthesis (FXR, SHP and FGFR4), and cholesterol synthesis and secretion (HMGR and ABCG5) in the hepatic tissue (a–h). The expression of genes associated with negative feedback regulation of BAs synthesis (FXR and FGF15), BAs reabsorption (ASBT) and cholesterol secretion (ABCG5) in the ileum(i-l). * Compared to the C group; # compared to the HFD group. p < 0.05 (*/#), p < 0.01 (**/##), p < 0.001 (***/###).
Additionally, the synthesis of BAs is tightly regulated by negative feedback inhibition through the nuclear receptor FXR, which is mainly expressed in the liver and ileum (Li et al., 2017). In the liver, activated FXR induces a negative nuclear receptor SHP, which binds to the CYP7A1 promoter and represses its transcription through interactions with other transcription factors (Kir et al., 2012). In the intestine, activated ileum FXR induces the expression of FGF15, which passes through the portal vein to the liver, and activates the hepatic FGFR4, thereby inhibiting the CYP7A1 gene transcription (Shin and Osborne, 2009). The results showed that the hepatic FXR and its downstream target gene SHP mRNA expressions increased evidently in the HFD group compared to those in the C group (p < 0.001), while dietary supplementation of acetate to the HFD decreased the FXR and SHP mRNA expressions (p < 0.01) comparably to those in the HFD group. In ileum, compared with the C group, the mRNA expression of FXR and FGF15 significantly elevated in the HFD group (p < 0.01), while both of them evidently down-regulated by the acetate supplement comparably to those in the HFD group (p < 0.01). Furthermore, their downstream target gene, FGFR4 mRNA expression in mouse liver also significantly increased in the HFD group compared to that in the C group; while it evidently decreased in the HFD_A group compared with that in the HFD group (p < 0.05). Collectively these data suggested that the inhibition of hepatic BAs synthesis in the HFD group and the promotion of hepatic BAs synthesis in the HFD_A group were all related to the negative feedback regulation of BAs mediated by FXR. These results were in line with the results from the other studies on the negative feedback regulation of BAs synthesis via FXR (Calkin and Tontonoz, 2012; Eloranta and Kullak-Ublick, 2008; Lefebvre et al., 2009).
BAs are synthesized from cholesterol in the liver. Thus, increased BAs biosynthesis in the HFD_A group means that more cholesterol need to be used as a substrate, given rise to a promotional demand of cholesterol in liver. 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), is a rate-limiting enzyme of cholesterol synthesis (Daniel et al., 2019). Interestingly, our study showed that hepatic HMGR mRNA levels significantly increased in the HFD_A group, compared to that in the C and HFD groups (p < 0.001). These findings indicated that acetate supplemented in the HFD increased the synthesis of cholesterol in hepatocyte, which was in line with the significant increase of hepatic TC in the HFD_A group (p < 0.05, Fig. 1k).
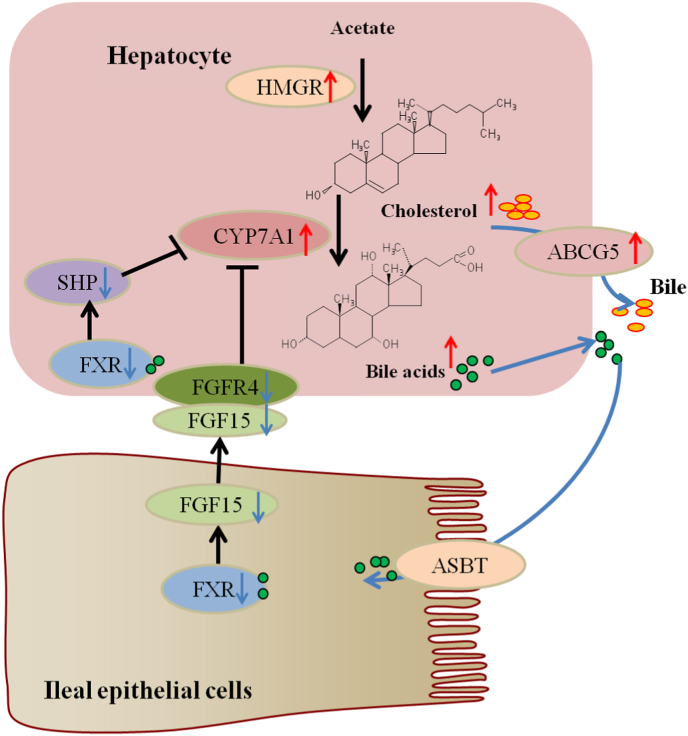
In fact, changes in the BAs pool size and composition might have metabolic regulatory consequences due to the FXR-antagonistic potencies of individual BAs species (Tan et al., 2019). In acetate-containing HFD fed mice, fecal reduced tauro-BAs were likely to be reabsorbed by the intestinal epithelium, which might act as antagonists and inhibit ileal-hepatic FXR-FGF15-FGFR4 signaling pathway, negatively feedback regulating the synthesis of BAs. In addition, most of the elevated hepatic tauro-BAs, such as TCA and Tα/βMCA, are also antagonists of FXR, which inhibited the hepatic FXR-SHP pathway and further negatively feedback regulated the synthesis of BAs via CYP7A1, and promoted the de novo BAs synthesis (Fig. 5).
Fig. 5.
FXR negatively feedback regulation of BAs synthesis. Hepatic cholesterol results from HMGR mediated conversion of acetate. BAs are synthesized from cholesterol by a series of enzymes including the initial and rate-limiting CYP7A1 in hepatocytes. BAs bind to FXR in the nucleus. In hepatocytes, FXR induces SHP, which inhibits transcription of CYP7A1 and BAs synthesis. Intestinal FXR induces FGF15, which circulates to the liver and binds to hepatic FGFR4, inhibiting transcription of CYP7A1 and BAs synthesis. ASBT expressed in the brush border of terminal ileal enterocytes mediate approximately 95% of BAs reabsorption. ABCG5/8 secretes cholesterol from hepatocytes into the bile. The cholesterol is transferred to the brush border membrane of enterocytes via bile salt micelles. Then, ABCG5/8 mediates biliary and cholesterol from the brush border membrane back into the gut lumen for excretion.
In conclusion, high-fat diet induced obesity had double hits on BAs balance, which significantly decreased hepatic BAs synthesis and significantly increased fecal BAs excretion. Acetate supplemented in the HFD promoted the synthesis of hepatic BAs and decreased fecal tauro-BAs excretion, which alleviated the negative effects of HFD on BAs balance. The elevated hepatic BAs synthesis and reduced fecal tauro-BAs excretion might act as the antagonist inhibiting the hepatic FXR-SHP signaling pathway and hepatic-ileal FXR-FGF15-FGFR4 signaling pathway, negative feedback regulating hepatic BAs production, further promoting the hepatic synthesis of BAs. The mechanism of acetic acid inhibiting DIO by BAs and hepatic-ileal FXR-FGF15-FGFR4 signaling pathway still needs further study.
CRediT authorship contribution statement
Rui Wang: Investigation, Validation, Writing – original draft, preparation, Writing – review & editing. Xiuqin Fan: Investigation, Validation, and, Writing – review & editing. Yuanyuan Lu: Investigation. Dawei Chen: Writing – review & editing, Funding acquisition. Yunfeng Zhao: Methodology, Supervision. Kemin Qi: Conceptualization, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 81803214; 81800750; 82003432) and the Natural Science Foundation of Beijing (No. 7212143).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.10.021.
Contributor Information
Rui Wang, Email: 15810948239@163.com.
Xiuqin Fan, Email: qincaifan@126.com.
Yuanyuan Lu, Email: luyuan1988220@163.com.
Dawei Chen, Email: dila2006@163.com.
Yunfeng Zhao, Email: zhaoyf@cfsa.net.cn.
Kemin Qi, Email: qikemin@bch.com.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Castillo-Olivares Antonio del., Campos Jose A., Pandak Gregorio William M. The role of α1-fetoprotein transcription factor/LRH-1 in bile acid biosynthesis. J. Biol. Chem. 2004;279(16) doi: 10.1074/jbc.M400646200. [DOI] [PubMed] [Google Scholar]
- Besten G.D., Lange K., Havinga R., Dijk T.V., Gerding A., Eunen K.V., Muller M., Groen A.K., Hooiveld G.J., Bakker B.M. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305(12):G900–G910. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- Calkin A.C., Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012;13(4):213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J., Ferrell J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;318(3) doi: 10.1152/ajpgi.00223.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J.Y., Chen W., Zheng M., Wu Z., Kimmel R., Stroup D. Bile acids and FXR represses cholesterol 7A-hydroxylase (CYP7A1), sterol 12A-hydroxylase (CYP8B1) and sterol 27-hydroxylase (CYP27A1), but not oxysterol 7A-hydroxylase (CYP7B1) gene transcription. Gastroenterology. 2000;118(4) A1006-A1006. [Google Scholar]
- Daniel Rizzolo, Kyle Buckley, Bo Kong, Le Zhan, Jianliang Shen. Bile acid homeostasis in a cholesterol 7α-hydroxylase and sterol 27-hydroxylase double knockout mouse model. Hepatology. 2019;70:389–402. doi: 10.1002/hep.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Yao H., Liu X., Shi Q., Qi K. High-fat diet alters the expression of reference genes in male mice. Front. Nutr. 2020;7 doi: 10.3389/fnut.2020.589771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta R.M., Garcia-Irigoyen O., Moschetta A. Bile acids and colon cancer: is FXR the solution of the conundrum? Mol. Aspect. Med. 2017;56:66–74. doi: 10.1016/j.mam.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Inagaki T., Choi M., Moschetta A., Peng L., Cummins C.L., McDonald J.G., Luo G., Jones S.A., Goodwin B., Richardson J.A., Gerard R.D., Repa J.J., Mangelsdorf D.J., Kliewer S.A. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metabol. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Jiao N., Baker S.S., Chapa-Rodriguez A., Liu W., Nugent C.A., Tsompana M., Mastrandrea L., Buck M.J., Baker R.D., Genco R.J., Zhu R., Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67(10):1881–1891. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- Kim S.-K., Matsunari H., Takeuchi T., Yokoyama M., Murata Y., Ishihara K. Effect of different dietary taurine levels on the conjugated bile acid composition and growth performance of juvenile and fingerling Japanese flounder Paralichthys olivaceus. Aquaculture. 2007;273(4):595–601. [Google Scholar]
- Kir S., Zhang Y., Gerard R.D., Kliewer S.A., Mangelsdorf D.J. Nuclear receptors HNF4α and LRH-1 cooperate in regulating Cyp7a1 in vivo. J. Biol. Chem. 2012;287(49):41334–41341. doi: 10.1074/jbc.M112.421834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Lefebvre Philippe, Cariou Bertrand, Lien Fleur, Kuipers Folkert, Staels Bart. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009;89(1):147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- Li R., Andreu-Sánchez S., Kuipers F., Fu J. Gut microbiome and bile acids in obesity-related diseases. Best Pract. Res. Clin. Endocrinol. Metabol. 2021;35(3) doi: 10.1016/j.beem.2021.101493. [DOI] [PubMed] [Google Scholar]
- Li Y., Tang R., Leung P.S.C., Gershwin M.E., Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun. Rev. 2017;16(9):885–896. doi: 10.1016/j.autrev.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Lu Y., Fan C., Li P., Lu Y., Chang X., Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016;6 doi: 10.1038/srep37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandak W.M., Heuman D.M., Hylemon P.B., Chiang J., Vlahcevic Z.R. Failure of intravenous infusion of taurocholate to down-regulate cholesterol 7 alpha-hydroxylase in rats with biliary fistulas. Gastroenterology. 1995;108(2):533–544. doi: 10.1016/0016-5085(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Perry R.J., Peng L., Barry N.A., Cline G.W., Zhang D., Cardone R.L., Petersen K.F., Kibbey R.G., Goodman A.L., Shulman G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primec M., Mičetić-Turk D., Langerholc T. Analysis of short-chain fatty acids in human feces: a scoping review. Anal. Biochem. 2017;526:9–21. doi: 10.1016/j.ab.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Shin D.J., Osborne T.F. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J. Biol. Chem. 2009;284:11110–11120. doi: 10.1074/jbc.M808747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Luo Y., Ranjit S., Xie C., Libby A.E., Orlicky D.J., Dvornikov A., Wang X.X., Myakala K., Jones B.A., Bhasin K., Wang D., McManaman J.L., Krausz K.W., Gratton E., Ir D., Robertson C.E., Frank D.N., Gonzalez F.J., Levi M. Bile acid sequestration reverses liver injury and prevents progression of nonalcoholic steatohepatitis in Western diet–fed mice. J. Biol. Chem. 2020;295(14):4733–4747. doi: 10.1074/jbc.RA119.011913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Liu Y., Long J., Chen S., Liao G., Wu S., Li C., Wang L., Ling W., Zhu H. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol. Nutr. Food Res. 2019;63(17) doi: 10.1002/mnfr.201900257. [DOI] [PubMed] [Google Scholar]
- Valdes D.S., So D., Gill P.A., Kellow N.J. Effect of dietary acetic acid supplementation on plasma glucose, lipid profiles, and body mass index in human adults: a systematic review and meta-analysis. J. Acad. Nutr. Diet. 2021;121(5):895–914. doi: 10.1016/j.jand.2020.12.002. [DOI] [PubMed] [Google Scholar]
- Wahlström A., Sayin Sama I., Marschall H.-U., Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metabol. 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wang R., Fan C., Fan X., Zhao Y., Wang Y., Li P., Tang T., Yao H., Chen S., Chen D., Qi K. A fast and accurate way to determine short chain fatty acids in human serum by GC–MS and their distribution in children with digestive diseases. Chromatographia. 2020;83(2):273–286. [Google Scholar]
- Watanabe M., Houten S.M., Mataki C., Christoffolete M.A., Kim B.W., Sato H., Messaddeq N., Harney J.W., Ezaki O., Kodama T., Schoonjans K., Bianco A.C., Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- Wolever T., Spadafora P., Eshuis H. Interaction between colonic acetate and propionate in humans. Am. J. Clin. Nutr. 1991;(3):681–687. doi: 10.1093/ajcn/53.3.681. [DOI] [PubMed] [Google Scholar]
- Xie G., Wang L., Chen T., Zhou K., Jia W. A metabolite array technology for precision medicine. Anal. Chem. 2021;93(14):5709–5717. doi: 10.1021/acs.analchem.0c04686. [DOI] [PubMed] [Google Scholar]
- Xie G., Wang Y., Wang X., Zhao A., Chen T., Ni Y., Wong L., Zhang H., Zhang J., Liu C. Profiling of serum bile acids in a healthy Chinese population using UPLC-MS/MS. J. Proteome Res. 2015;14(2):850–859. doi: 10.1021/pr500920q. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S., Loo T.M., Atarashi K., Kanda H., Sato S., Oyadomari S., Iwakura Y., Oshima K., Morita H., Hattori M., Honda K., Ishikawa Y., Hara E., Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- Zhang M., Chiang J. Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1) J. Biol. Chem. 2001;276 doi: 10.1074/jbc.M105117200. [DOI] [PubMed] [Google Scholar]
- Eloranta, Jyrki, J., Kullak-Ublick, Gerd, A., 2008. The Role of FXR in Disorders of Bile Acid Homeostasis. Physiology. 10, 286-295. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.